User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
Veterans Found Relief From Chronic Pain Through Telehealth Mindfulness
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized clinical trial of 811 veterans who had moderate to severe chronic pain and were recruited from three Veterans Affairs facilities in the United States.
- Participants were divided into three groups: Group MBI (270), self-paced MBI (271), and usual care (270), with interventions lasting 8 weeks.
- The primary outcome was pain-related function measured using a scale on interference from pain in areas like mood, walking, work, relationships, and sleep at 10 weeks, 6 months, and 1 year.
- Secondary outcomes included pain intensity, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, and posttraumatic stress disorder (PTSD).
TAKEAWAY:
- Pain-related function significantly improved in participants in both the MBI groups versus usual care group, with a mean difference of −0.4 (95% CI, −0.7 to −0.2) for group MBI and −0.7 (95% CI, −1.0 to −0.4) for self-paced MBI (P < .001).
- Compared with the usual care group, both the MBI groups had significantly improved secondary outcomes, including pain intensity, depression, and PTSD.
- The probability of achieving 30% improvement in pain-related function was higher for group MBI at 10 weeks and 6 months and for self-paced MBI at all three timepoints.
- No significant differences were found between the MBI groups for primary and secondary outcomes.
IN PRACTICE:
“The viability and similarity of both these approaches for delivering MBIs increase patient options for meeting their individual needs and could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems,” the study authors wrote.
SOURCE:
The study was led by Diana J. Burgess, PhD, of the Center for Care Delivery and Outcomes Research, VA Health Systems Research in Minneapolis, Minnesota, and published online in JAMA Internal Medicine.
LIMITATIONS:
The trial was not designed to compare less resource-intensive MBIs with more intensive mindfulness-based stress reduction programs or in-person MBIs. The study did not address cost-effectiveness or control for time, attention, and other contextual factors. The high nonresponse rate (81%) to initial recruitment may have affected the generalizability of the findings.
DISCLOSURES:
The study was supported by the Pain Management Collaboratory–Pragmatic Clinical Trials Demonstration. Various authors reported grants from the National Center for Complementary and Integrative Health and the National Institute of Nursing Research.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized clinical trial of 811 veterans who had moderate to severe chronic pain and were recruited from three Veterans Affairs facilities in the United States.
- Participants were divided into three groups: Group MBI (270), self-paced MBI (271), and usual care (270), with interventions lasting 8 weeks.
- The primary outcome was pain-related function measured using a scale on interference from pain in areas like mood, walking, work, relationships, and sleep at 10 weeks, 6 months, and 1 year.
- Secondary outcomes included pain intensity, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, and posttraumatic stress disorder (PTSD).
TAKEAWAY:
- Pain-related function significantly improved in participants in both the MBI groups versus usual care group, with a mean difference of −0.4 (95% CI, −0.7 to −0.2) for group MBI and −0.7 (95% CI, −1.0 to −0.4) for self-paced MBI (P < .001).
- Compared with the usual care group, both the MBI groups had significantly improved secondary outcomes, including pain intensity, depression, and PTSD.
- The probability of achieving 30% improvement in pain-related function was higher for group MBI at 10 weeks and 6 months and for self-paced MBI at all three timepoints.
- No significant differences were found between the MBI groups for primary and secondary outcomes.
IN PRACTICE:
“The viability and similarity of both these approaches for delivering MBIs increase patient options for meeting their individual needs and could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems,” the study authors wrote.
SOURCE:
The study was led by Diana J. Burgess, PhD, of the Center for Care Delivery and Outcomes Research, VA Health Systems Research in Minneapolis, Minnesota, and published online in JAMA Internal Medicine.
LIMITATIONS:
The trial was not designed to compare less resource-intensive MBIs with more intensive mindfulness-based stress reduction programs or in-person MBIs. The study did not address cost-effectiveness or control for time, attention, and other contextual factors. The high nonresponse rate (81%) to initial recruitment may have affected the generalizability of the findings.
DISCLOSURES:
The study was supported by the Pain Management Collaboratory–Pragmatic Clinical Trials Demonstration. Various authors reported grants from the National Center for Complementary and Integrative Health and the National Institute of Nursing Research.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a randomized clinical trial of 811 veterans who had moderate to severe chronic pain and were recruited from three Veterans Affairs facilities in the United States.
- Participants were divided into three groups: Group MBI (270), self-paced MBI (271), and usual care (270), with interventions lasting 8 weeks.
- The primary outcome was pain-related function measured using a scale on interference from pain in areas like mood, walking, work, relationships, and sleep at 10 weeks, 6 months, and 1 year.
- Secondary outcomes included pain intensity, anxiety, fatigue, sleep disturbance, participation in social roles and activities, depression, and posttraumatic stress disorder (PTSD).
TAKEAWAY:
- Pain-related function significantly improved in participants in both the MBI groups versus usual care group, with a mean difference of −0.4 (95% CI, −0.7 to −0.2) for group MBI and −0.7 (95% CI, −1.0 to −0.4) for self-paced MBI (P < .001).
- Compared with the usual care group, both the MBI groups had significantly improved secondary outcomes, including pain intensity, depression, and PTSD.
- The probability of achieving 30% improvement in pain-related function was higher for group MBI at 10 weeks and 6 months and for self-paced MBI at all three timepoints.
- No significant differences were found between the MBI groups for primary and secondary outcomes.
IN PRACTICE:
“The viability and similarity of both these approaches for delivering MBIs increase patient options for meeting their individual needs and could help accelerate and improve the implementation of nonpharmacological pain treatment in health care systems,” the study authors wrote.
SOURCE:
The study was led by Diana J. Burgess, PhD, of the Center for Care Delivery and Outcomes Research, VA Health Systems Research in Minneapolis, Minnesota, and published online in JAMA Internal Medicine.
LIMITATIONS:
The trial was not designed to compare less resource-intensive MBIs with more intensive mindfulness-based stress reduction programs or in-person MBIs. The study did not address cost-effectiveness or control for time, attention, and other contextual factors. The high nonresponse rate (81%) to initial recruitment may have affected the generalizability of the findings.
DISCLOSURES:
The study was supported by the Pain Management Collaboratory–Pragmatic Clinical Trials Demonstration. Various authors reported grants from the National Center for Complementary and Integrative Health and the National Institute of Nursing Research.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
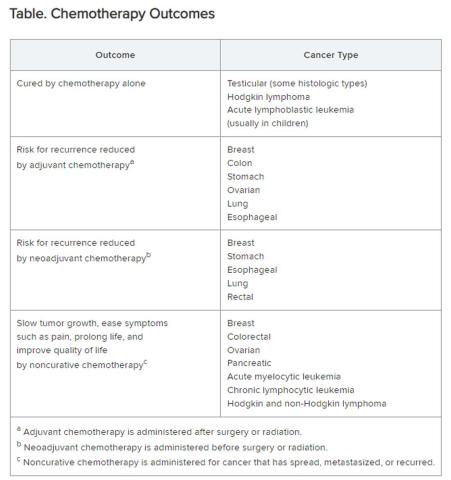
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
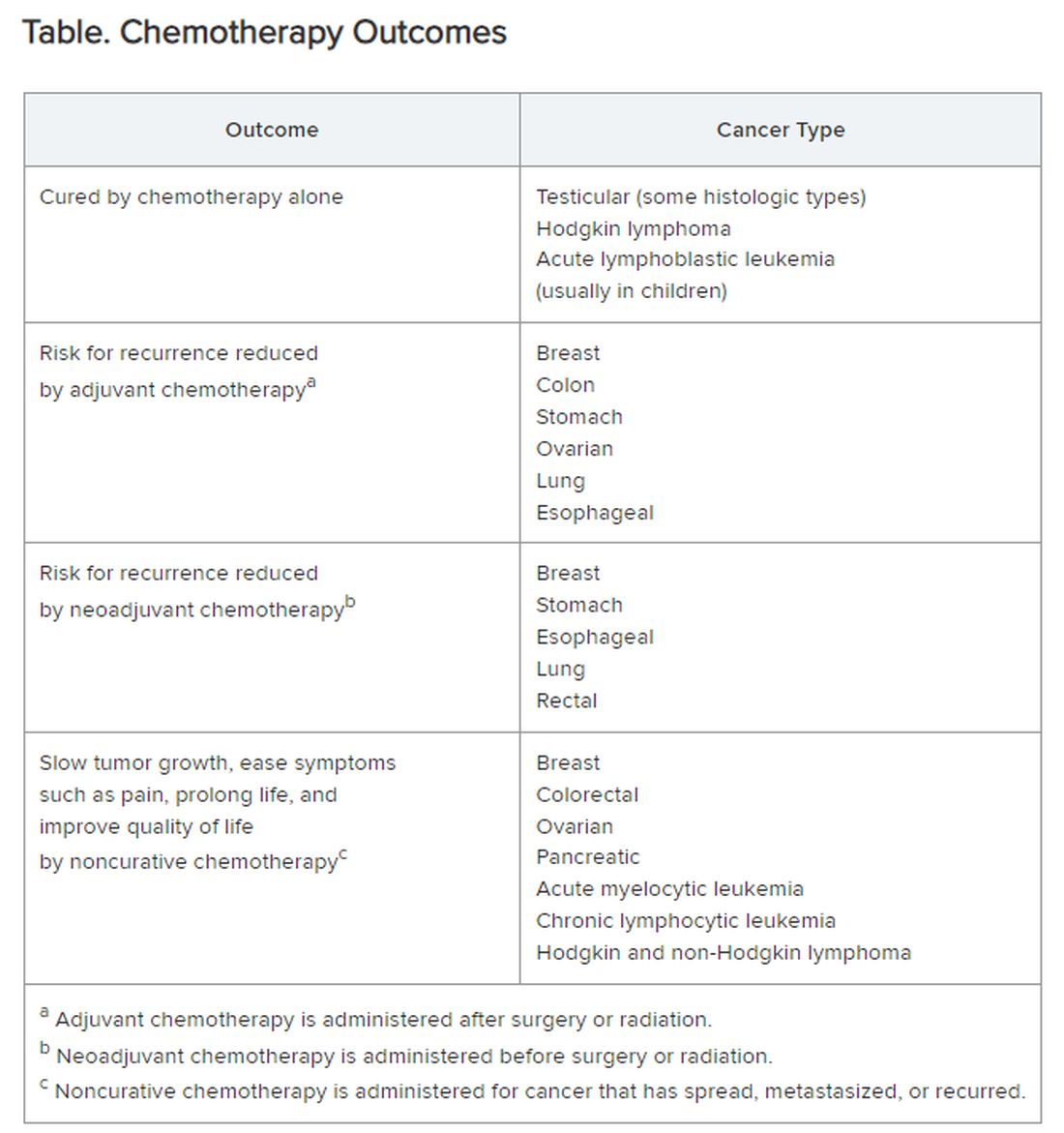
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Severe COVID-19 Tied to Increased Risk for Mental Illness
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Hearing Loss, Neuropathy Cut Survival in Older Adults
TOPLINE:
METHODOLOGY:
- Researchers analyzed 793 older adults recruited from primary care practices participating in the OKLAHOMA Studies in 1999.
- Participants completed a questionnaire and underwent a physical examination; timed gait assessments (50 ft); and tests for peripheral nerve function, balance, and hearing.
- Hearing thresholds were tested at 20, 25, and 40 dB, respectively, and at sound frequencies of 500, 1000, 2000, and 4000 Hz.
- Researchers tracked mortality data over 22 years.
TAKEAWAY:
- Overall, 83% participants experienced hearing loss. Regular use of hearing aids was low, reported in 19% and 55% of those with moderate and severe hearing loss, respectively.
- Hearing loss was linked to impaired balance (P = .0014), slower walking (P = .0024), and reduced survival time (P = .0001). Moderate to severe hearing loss was strongly associated with reduced survival time (odds ratio, 1.36; P = .001), independent of the use of hearing aids.
- Peripheral neuropathy was present in 32% participants. The condition also increased the risk for death over the study period (hazard ratio [HR], 1.32; P = .003). Participants with both hearing loss and peripheral neuropathy showed reduced balance and survival time compared with people with either condition alone (HR, 1.55; P < .0001).
IN PRACTICE:
“Like peripheral neuropathy, advanced-age hearing loss is associated with reduced life expectancy, probably mediated in part through an adverse impact on balance,” the authors wrote. “Greater appreciation for the serious impacts of hearing loss and peripheral neuropathy could lead to further efforts to understand their causes and improve prevention and treatment strategies.”
SOURCE:
The study was led by James W. Mold, MD, MPH, of the University of Oklahoma Health Sciences Center, Oklahoma City. It was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
The dataset was collected in 1999 and may not entirely represent the current cohorts of older primary care patients. The absence of soundproof rooms and the exclusion of some components of the standard audiometric evaluation may have affected low-frequency sound measurements. Furthermore, physical examination was a less accurate measure of peripheral neuropathy. Information on the duration or severity of predictors and causes of death was not available.
DISCLOSURES:
The study was funded by the Presbyterian Health Foundation. The authors did not disclose any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed 793 older adults recruited from primary care practices participating in the OKLAHOMA Studies in 1999.
- Participants completed a questionnaire and underwent a physical examination; timed gait assessments (50 ft); and tests for peripheral nerve function, balance, and hearing.
- Hearing thresholds were tested at 20, 25, and 40 dB, respectively, and at sound frequencies of 500, 1000, 2000, and 4000 Hz.
- Researchers tracked mortality data over 22 years.
TAKEAWAY:
- Overall, 83% participants experienced hearing loss. Regular use of hearing aids was low, reported in 19% and 55% of those with moderate and severe hearing loss, respectively.
- Hearing loss was linked to impaired balance (P = .0014), slower walking (P = .0024), and reduced survival time (P = .0001). Moderate to severe hearing loss was strongly associated with reduced survival time (odds ratio, 1.36; P = .001), independent of the use of hearing aids.
- Peripheral neuropathy was present in 32% participants. The condition also increased the risk for death over the study period (hazard ratio [HR], 1.32; P = .003). Participants with both hearing loss and peripheral neuropathy showed reduced balance and survival time compared with people with either condition alone (HR, 1.55; P < .0001).
IN PRACTICE:
“Like peripheral neuropathy, advanced-age hearing loss is associated with reduced life expectancy, probably mediated in part through an adverse impact on balance,” the authors wrote. “Greater appreciation for the serious impacts of hearing loss and peripheral neuropathy could lead to further efforts to understand their causes and improve prevention and treatment strategies.”
SOURCE:
The study was led by James W. Mold, MD, MPH, of the University of Oklahoma Health Sciences Center, Oklahoma City. It was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
The dataset was collected in 1999 and may not entirely represent the current cohorts of older primary care patients. The absence of soundproof rooms and the exclusion of some components of the standard audiometric evaluation may have affected low-frequency sound measurements. Furthermore, physical examination was a less accurate measure of peripheral neuropathy. Information on the duration or severity of predictors and causes of death was not available.
DISCLOSURES:
The study was funded by the Presbyterian Health Foundation. The authors did not disclose any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed 793 older adults recruited from primary care practices participating in the OKLAHOMA Studies in 1999.
- Participants completed a questionnaire and underwent a physical examination; timed gait assessments (50 ft); and tests for peripheral nerve function, balance, and hearing.
- Hearing thresholds were tested at 20, 25, and 40 dB, respectively, and at sound frequencies of 500, 1000, 2000, and 4000 Hz.
- Researchers tracked mortality data over 22 years.
TAKEAWAY:
- Overall, 83% participants experienced hearing loss. Regular use of hearing aids was low, reported in 19% and 55% of those with moderate and severe hearing loss, respectively.
- Hearing loss was linked to impaired balance (P = .0014), slower walking (P = .0024), and reduced survival time (P = .0001). Moderate to severe hearing loss was strongly associated with reduced survival time (odds ratio, 1.36; P = .001), independent of the use of hearing aids.
- Peripheral neuropathy was present in 32% participants. The condition also increased the risk for death over the study period (hazard ratio [HR], 1.32; P = .003). Participants with both hearing loss and peripheral neuropathy showed reduced balance and survival time compared with people with either condition alone (HR, 1.55; P < .0001).
IN PRACTICE:
“Like peripheral neuropathy, advanced-age hearing loss is associated with reduced life expectancy, probably mediated in part through an adverse impact on balance,” the authors wrote. “Greater appreciation for the serious impacts of hearing loss and peripheral neuropathy could lead to further efforts to understand their causes and improve prevention and treatment strategies.”
SOURCE:
The study was led by James W. Mold, MD, MPH, of the University of Oklahoma Health Sciences Center, Oklahoma City. It was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
The dataset was collected in 1999 and may not entirely represent the current cohorts of older primary care patients. The absence of soundproof rooms and the exclusion of some components of the standard audiometric evaluation may have affected low-frequency sound measurements. Furthermore, physical examination was a less accurate measure of peripheral neuropathy. Information on the duration or severity of predictors and causes of death was not available.
DISCLOSURES:
The study was funded by the Presbyterian Health Foundation. The authors did not disclose any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Hearing Loss, Hearing Aids, and Dementia Risk: What to Tell Your Patients
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Mobile App Shows Promise in Managing Fibromyalgia Symptoms
TOPLINE:
A smartphone app that delivers acceptance and commitment therapy (ACT), a type of cognitive behavioral therapy, improves overall well-being and reduces the severity of pain, fatigue, sleep issues, and depression to a greater extent than daily symptom tracking in patients with fibromyalgia.
METHODOLOGY:
- Researchers conducted the phase 3 PROSPER-FM trial at 25 community sites in the United States to assess the efficacy and safety of digital ACT for patients with fibromyalgia.
- A total of 275 adult patients aged 22-75 years with fibromyalgia were randomly assigned to either the digital ACT group (n = 140) or the active control group (n = 135) for 12 weeks.
- Patients in the digital ACT group received a self-guided, smartphone-delivered program in which they learned and practiced the core ACT skills of acceptance, values, mindfulness, defusion, self as context, and willingness and committed action to build psychological flexibility, while the control group underwent daily symptom tracking and received educational materials.
- The primary endpoint was the response rate on the Patient Global Impression of Change (PGIC) at week 12, which is an indicator of patient well-being.
- The secondary endpoints included changes in the Revised Fibromyalgia Impact Questionnaire (FIQ-R) total score and pain intensity, pain interference, and sleep interference scores.
TAKEAWAY:
- At week 12, 71% of the patients in the digital ACT group responded with a minimally improved or better change in the PGIC response, compared with only 22% of the patients in the control group (P < .0001).
- The digital ACT group showed a significant reduction in the impact of fibromyalgia, with a between-group effect size of d = 0.65 (P < .0001) at week 12. The FIQ-R total score significantly improved within 3 weeks of using the self-guided digital ACT app.
- The use of digital ACT also demonstrated positive effects on the levels of weekly pain intensity (P = .001) and depression (P < .0001), compared with the control group.
- No serious adverse effects related to the app were reported, and both groups demonstrated high rates of adherence, with most (72%) participants in the digital ACT group completing at least 42 sessions.
IN PRACTICE:
“The results found in the study are essential for professionals who care for patients with fibromyalgia as they present a new viable treatment alternative,” Guilherme Torres Vilarino, PhD, Santa Catarina State University, Florianópolis, Brazil, wrote in an accompanying editorial.
SOURCE:
This study was led by R. Michael Gendreau, MD, PhD, Gendreau Consulting, Poway, California. It was published online in The Lancet.
LIMITATIONS:
The study population predominantly consisted of women and White individuals, which may limit the generalizability of the findings to more diverse populations. Additionally, the study was conducted in the United States, and the results may thus not be applicable to other countries with different racial, ethnic, educational, and economic characteristics. The study duration was 12 weeks, and the long-term benefits of digital ACT have not yet been shown.
DISCLOSURES:
This study was funded by Swing Therapeutics. Seven authors declared having stock options and/or receiving salary from Swing Therapeutics. Other authors reported having many ties with several sources, including Swing Therapeutics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A smartphone app that delivers acceptance and commitment therapy (ACT), a type of cognitive behavioral therapy, improves overall well-being and reduces the severity of pain, fatigue, sleep issues, and depression to a greater extent than daily symptom tracking in patients with fibromyalgia.
METHODOLOGY:
- Researchers conducted the phase 3 PROSPER-FM trial at 25 community sites in the United States to assess the efficacy and safety of digital ACT for patients with fibromyalgia.
- A total of 275 adult patients aged 22-75 years with fibromyalgia were randomly assigned to either the digital ACT group (n = 140) or the active control group (n = 135) for 12 weeks.
- Patients in the digital ACT group received a self-guided, smartphone-delivered program in which they learned and practiced the core ACT skills of acceptance, values, mindfulness, defusion, self as context, and willingness and committed action to build psychological flexibility, while the control group underwent daily symptom tracking and received educational materials.
- The primary endpoint was the response rate on the Patient Global Impression of Change (PGIC) at week 12, which is an indicator of patient well-being.
- The secondary endpoints included changes in the Revised Fibromyalgia Impact Questionnaire (FIQ-R) total score and pain intensity, pain interference, and sleep interference scores.
TAKEAWAY:
- At week 12, 71% of the patients in the digital ACT group responded with a minimally improved or better change in the PGIC response, compared with only 22% of the patients in the control group (P < .0001).
- The digital ACT group showed a significant reduction in the impact of fibromyalgia, with a between-group effect size of d = 0.65 (P < .0001) at week 12. The FIQ-R total score significantly improved within 3 weeks of using the self-guided digital ACT app.
- The use of digital ACT also demonstrated positive effects on the levels of weekly pain intensity (P = .001) and depression (P < .0001), compared with the control group.
- No serious adverse effects related to the app were reported, and both groups demonstrated high rates of adherence, with most (72%) participants in the digital ACT group completing at least 42 sessions.
IN PRACTICE:
“The results found in the study are essential for professionals who care for patients with fibromyalgia as they present a new viable treatment alternative,” Guilherme Torres Vilarino, PhD, Santa Catarina State University, Florianópolis, Brazil, wrote in an accompanying editorial.
SOURCE:
This study was led by R. Michael Gendreau, MD, PhD, Gendreau Consulting, Poway, California. It was published online in The Lancet.
LIMITATIONS:
The study population predominantly consisted of women and White individuals, which may limit the generalizability of the findings to more diverse populations. Additionally, the study was conducted in the United States, and the results may thus not be applicable to other countries with different racial, ethnic, educational, and economic characteristics. The study duration was 12 weeks, and the long-term benefits of digital ACT have not yet been shown.
DISCLOSURES:
This study was funded by Swing Therapeutics. Seven authors declared having stock options and/or receiving salary from Swing Therapeutics. Other authors reported having many ties with several sources, including Swing Therapeutics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A smartphone app that delivers acceptance and commitment therapy (ACT), a type of cognitive behavioral therapy, improves overall well-being and reduces the severity of pain, fatigue, sleep issues, and depression to a greater extent than daily symptom tracking in patients with fibromyalgia.
METHODOLOGY:
- Researchers conducted the phase 3 PROSPER-FM trial at 25 community sites in the United States to assess the efficacy and safety of digital ACT for patients with fibromyalgia.
- A total of 275 adult patients aged 22-75 years with fibromyalgia were randomly assigned to either the digital ACT group (n = 140) or the active control group (n = 135) for 12 weeks.
- Patients in the digital ACT group received a self-guided, smartphone-delivered program in which they learned and practiced the core ACT skills of acceptance, values, mindfulness, defusion, self as context, and willingness and committed action to build psychological flexibility, while the control group underwent daily symptom tracking and received educational materials.
- The primary endpoint was the response rate on the Patient Global Impression of Change (PGIC) at week 12, which is an indicator of patient well-being.
- The secondary endpoints included changes in the Revised Fibromyalgia Impact Questionnaire (FIQ-R) total score and pain intensity, pain interference, and sleep interference scores.
TAKEAWAY:
- At week 12, 71% of the patients in the digital ACT group responded with a minimally improved or better change in the PGIC response, compared with only 22% of the patients in the control group (P < .0001).
- The digital ACT group showed a significant reduction in the impact of fibromyalgia, with a between-group effect size of d = 0.65 (P < .0001) at week 12. The FIQ-R total score significantly improved within 3 weeks of using the self-guided digital ACT app.
- The use of digital ACT also demonstrated positive effects on the levels of weekly pain intensity (P = .001) and depression (P < .0001), compared with the control group.
- No serious adverse effects related to the app were reported, and both groups demonstrated high rates of adherence, with most (72%) participants in the digital ACT group completing at least 42 sessions.
IN PRACTICE:
“The results found in the study are essential for professionals who care for patients with fibromyalgia as they present a new viable treatment alternative,” Guilherme Torres Vilarino, PhD, Santa Catarina State University, Florianópolis, Brazil, wrote in an accompanying editorial.
SOURCE:
This study was led by R. Michael Gendreau, MD, PhD, Gendreau Consulting, Poway, California. It was published online in The Lancet.
LIMITATIONS:
The study population predominantly consisted of women and White individuals, which may limit the generalizability of the findings to more diverse populations. Additionally, the study was conducted in the United States, and the results may thus not be applicable to other countries with different racial, ethnic, educational, and economic characteristics. The study duration was 12 weeks, and the long-term benefits of digital ACT have not yet been shown.
DISCLOSURES:
This study was funded by Swing Therapeutics. Seven authors declared having stock options and/or receiving salary from Swing Therapeutics. Other authors reported having many ties with several sources, including Swing Therapeutics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
AI Matches Expert Interpretation of Routine EEGs
, according to investigators.
These findings suggest that SCORE-AI, the model tested, can reliably interpret common EEGs in real-world practice, supporting its recent FDA approval, reported lead author Daniel Mansilla, MD, a neurologist at Montreal Neurological Institute and Hospital, and colleagues.
“Overinterpretation of clinical EEG is the most common cause of misdiagnosing epilepsy,” the investigators wrote in Epilepsia. “AI tools may be a solution for this challenge, both as an additional resource for confirmation and classification of epilepsy, and as an aid for the interpretation of EEG in critical care medicine.”
To date, however, AI tools have struggled with the variability encountered in real-world neurology practice.“When tested on external data from different centers and diverse patient populations, and using equipment distinct from the initial study, medical AI models frequently exhibit modest performance, and only a few AI tools have successfully transitioned into medical practice,” the investigators wrote.
SCORE-AI Matches Expert Interpretation of Routine EEGs
The present study put SCORE-AI to the test with EEGs from 104 patients between 16 and 91 years. These individuals hailed from “geographically distinct” regions, while recording equipment and conditions also varied widely, according to Dr. Mansilla and colleagues.
To set an external gold-standard for comparison, EEGs were first interpreted by three human expert raters, who were blinded to all case information except the EEGs themselves. The dataset comprised 50% normal and 50% abnormal EEGs. Four major classes of EEG abnormalities were included: focal epileptiform, generalized epileptiform, focal nonepileptiform, and diffuse nonepileptiform.
Comparing SCORE-AI interpretations with the experts’ interpretations revealed no significant difference in any metric or category. The AI tool had an overall accuracy of 92%, compared with 94% for the human experts. Of note, SCORE-AI maintained this level of performance regardless of vigilance state or normal variants.
“SCORE-AI has obtained FDA approval for routine clinical EEGs and is presently being integrated into broadly available EEG software (Natus NeuroWorks),” the investigators wrote.
Further Validation May Be Needed
Wesley T. Kerr, MD, PhD, functional (nonepileptic) seizures clinic lead epileptologist at the University of Pittsburgh Medical Center, and handling associate editor for this study in Epilepsia, said the present findings are important because they show that SCORE-AI can perform in scenarios beyond the one in which it was developed.
Still, it may be premature for broad commercial rollout.
In a written comment, Dr. Kerr called for “much larger studies” to validate SCORE-AI, noting that seizures can be caused by “many rare conditions,” and some patients have multiple EEG abnormalities.
Since SCORE-AI has not yet demonstrated accuracy in those situations, he predicted that the tool will remain exactly that – a tool – before it replaces human experts.
“They have only looked at SCORE-AI by itself,” Dr. Kerr said. “Practically, SCORE-AI is going to be used in combination with a neurologist for a long time before SCORE-AI can operate semi-independently or independently. They need to do studies looking at this combination to see how this tool impacts the clinical practice of EEG interpretation.”
Daniel Friedman, MD, an epileptologist and associate clinical professor of neurology at NYU Langone, pointed out another limitation of the present study: The EEGs were collected at specialty centers.
“The technical standards of data collection were, therefore, pretty high,” Dr. Friedman said in a written comment. “The majority of EEGs performed in the world are not collected by highly skilled EEG technologists and the performance of AI classification algorithms under less-than-ideal technical conditions is unknown.”
AI-Assisted EEG Interpretation Is Here to Stay
When asked about the long-term future of AI-assisted EEG interpretation, Dr. Friedman predicted that it will be “critical” for helping improve the accuracy of epilepsy diagnoses, particularly because most EEGs worldwide are interpreted by non-experts, leading to the known issue with epilepsy misdiagnosis.
“However,” he added, “it is important to note that epilepsy is a clinical diagnosis ... [EEG] is only one piece of evidence in neurologic decision making. History and accurate eyewitness description of the events of concern are extremely critical to the diagnosis and cannot be replaced by AI yet.”
Dr. Kerr offered a similar view, highlighting the potential for SCORE-AI to raise the game of non-epileptologists.
“My anticipation is that neurologists who don’t use SCORE-AI will be replaced by neurologists who use SCORE-AI well,” he said. “Neurologists who use it well will be able to read more EEGs in less time without sacrificing quality. This will allow the neurologist to spend more time talking with the patient about the interpretation of the tests and how that impacts clinical care.”
Then again, that time spent talking with the patient may also one day be delegated to a machine.
“It is certainly imaginable that AI chatbots using large language models to interact with patients and family could be developed to extract consistent epilepsy histories for diagnostic support,” Dr. Wesley said.
This work was supported by a project grant from the Canadian Institutes of Health Research and Duke Neurology start-up funding. The investigators and interviewees reported no relevant conflicts of interest.
, according to investigators.
These findings suggest that SCORE-AI, the model tested, can reliably interpret common EEGs in real-world practice, supporting its recent FDA approval, reported lead author Daniel Mansilla, MD, a neurologist at Montreal Neurological Institute and Hospital, and colleagues.
“Overinterpretation of clinical EEG is the most common cause of misdiagnosing epilepsy,” the investigators wrote in Epilepsia. “AI tools may be a solution for this challenge, both as an additional resource for confirmation and classification of epilepsy, and as an aid for the interpretation of EEG in critical care medicine.”
To date, however, AI tools have struggled with the variability encountered in real-world neurology practice.“When tested on external data from different centers and diverse patient populations, and using equipment distinct from the initial study, medical AI models frequently exhibit modest performance, and only a few AI tools have successfully transitioned into medical practice,” the investigators wrote.
SCORE-AI Matches Expert Interpretation of Routine EEGs
The present study put SCORE-AI to the test with EEGs from 104 patients between 16 and 91 years. These individuals hailed from “geographically distinct” regions, while recording equipment and conditions also varied widely, according to Dr. Mansilla and colleagues.
To set an external gold-standard for comparison, EEGs were first interpreted by three human expert raters, who were blinded to all case information except the EEGs themselves. The dataset comprised 50% normal and 50% abnormal EEGs. Four major classes of EEG abnormalities were included: focal epileptiform, generalized epileptiform, focal nonepileptiform, and diffuse nonepileptiform.
Comparing SCORE-AI interpretations with the experts’ interpretations revealed no significant difference in any metric or category. The AI tool had an overall accuracy of 92%, compared with 94% for the human experts. Of note, SCORE-AI maintained this level of performance regardless of vigilance state or normal variants.
“SCORE-AI has obtained FDA approval for routine clinical EEGs and is presently being integrated into broadly available EEG software (Natus NeuroWorks),” the investigators wrote.
Further Validation May Be Needed
Wesley T. Kerr, MD, PhD, functional (nonepileptic) seizures clinic lead epileptologist at the University of Pittsburgh Medical Center, and handling associate editor for this study in Epilepsia, said the present findings are important because they show that SCORE-AI can perform in scenarios beyond the one in which it was developed.
Still, it may be premature for broad commercial rollout.
In a written comment, Dr. Kerr called for “much larger studies” to validate SCORE-AI, noting that seizures can be caused by “many rare conditions,” and some patients have multiple EEG abnormalities.
Since SCORE-AI has not yet demonstrated accuracy in those situations, he predicted that the tool will remain exactly that – a tool – before it replaces human experts.
“They have only looked at SCORE-AI by itself,” Dr. Kerr said. “Practically, SCORE-AI is going to be used in combination with a neurologist for a long time before SCORE-AI can operate semi-independently or independently. They need to do studies looking at this combination to see how this tool impacts the clinical practice of EEG interpretation.”
Daniel Friedman, MD, an epileptologist and associate clinical professor of neurology at NYU Langone, pointed out another limitation of the present study: The EEGs were collected at specialty centers.
“The technical standards of data collection were, therefore, pretty high,” Dr. Friedman said in a written comment. “The majority of EEGs performed in the world are not collected by highly skilled EEG technologists and the performance of AI classification algorithms under less-than-ideal technical conditions is unknown.”
AI-Assisted EEG Interpretation Is Here to Stay
When asked about the long-term future of AI-assisted EEG interpretation, Dr. Friedman predicted that it will be “critical” for helping improve the accuracy of epilepsy diagnoses, particularly because most EEGs worldwide are interpreted by non-experts, leading to the known issue with epilepsy misdiagnosis.
“However,” he added, “it is important to note that epilepsy is a clinical diagnosis ... [EEG] is only one piece of evidence in neurologic decision making. History and accurate eyewitness description of the events of concern are extremely critical to the diagnosis and cannot be replaced by AI yet.”
Dr. Kerr offered a similar view, highlighting the potential for SCORE-AI to raise the game of non-epileptologists.
“My anticipation is that neurologists who don’t use SCORE-AI will be replaced by neurologists who use SCORE-AI well,” he said. “Neurologists who use it well will be able to read more EEGs in less time without sacrificing quality. This will allow the neurologist to spend more time talking with the patient about the interpretation of the tests and how that impacts clinical care.”
Then again, that time spent talking with the patient may also one day be delegated to a machine.
“It is certainly imaginable that AI chatbots using large language models to interact with patients and family could be developed to extract consistent epilepsy histories for diagnostic support,” Dr. Wesley said.
This work was supported by a project grant from the Canadian Institutes of Health Research and Duke Neurology start-up funding. The investigators and interviewees reported no relevant conflicts of interest.
, according to investigators.
These findings suggest that SCORE-AI, the model tested, can reliably interpret common EEGs in real-world practice, supporting its recent FDA approval, reported lead author Daniel Mansilla, MD, a neurologist at Montreal Neurological Institute and Hospital, and colleagues.
“Overinterpretation of clinical EEG is the most common cause of misdiagnosing epilepsy,” the investigators wrote in Epilepsia. “AI tools may be a solution for this challenge, both as an additional resource for confirmation and classification of epilepsy, and as an aid for the interpretation of EEG in critical care medicine.”
To date, however, AI tools have struggled with the variability encountered in real-world neurology practice.“When tested on external data from different centers and diverse patient populations, and using equipment distinct from the initial study, medical AI models frequently exhibit modest performance, and only a few AI tools have successfully transitioned into medical practice,” the investigators wrote.
SCORE-AI Matches Expert Interpretation of Routine EEGs
The present study put SCORE-AI to the test with EEGs from 104 patients between 16 and 91 years. These individuals hailed from “geographically distinct” regions, while recording equipment and conditions also varied widely, according to Dr. Mansilla and colleagues.
To set an external gold-standard for comparison, EEGs were first interpreted by three human expert raters, who were blinded to all case information except the EEGs themselves. The dataset comprised 50% normal and 50% abnormal EEGs. Four major classes of EEG abnormalities were included: focal epileptiform, generalized epileptiform, focal nonepileptiform, and diffuse nonepileptiform.
Comparing SCORE-AI interpretations with the experts’ interpretations revealed no significant difference in any metric or category. The AI tool had an overall accuracy of 92%, compared with 94% for the human experts. Of note, SCORE-AI maintained this level of performance regardless of vigilance state or normal variants.
“SCORE-AI has obtained FDA approval for routine clinical EEGs and is presently being integrated into broadly available EEG software (Natus NeuroWorks),” the investigators wrote.
Further Validation May Be Needed
Wesley T. Kerr, MD, PhD, functional (nonepileptic) seizures clinic lead epileptologist at the University of Pittsburgh Medical Center, and handling associate editor for this study in Epilepsia, said the present findings are important because they show that SCORE-AI can perform in scenarios beyond the one in which it was developed.
Still, it may be premature for broad commercial rollout.
In a written comment, Dr. Kerr called for “much larger studies” to validate SCORE-AI, noting that seizures can be caused by “many rare conditions,” and some patients have multiple EEG abnormalities.
Since SCORE-AI has not yet demonstrated accuracy in those situations, he predicted that the tool will remain exactly that – a tool – before it replaces human experts.
“They have only looked at SCORE-AI by itself,” Dr. Kerr said. “Practically, SCORE-AI is going to be used in combination with a neurologist for a long time before SCORE-AI can operate semi-independently or independently. They need to do studies looking at this combination to see how this tool impacts the clinical practice of EEG interpretation.”
Daniel Friedman, MD, an epileptologist and associate clinical professor of neurology at NYU Langone, pointed out another limitation of the present study: The EEGs were collected at specialty centers.
“The technical standards of data collection were, therefore, pretty high,” Dr. Friedman said in a written comment. “The majority of EEGs performed in the world are not collected by highly skilled EEG technologists and the performance of AI classification algorithms under less-than-ideal technical conditions is unknown.”
AI-Assisted EEG Interpretation Is Here to Stay
When asked about the long-term future of AI-assisted EEG interpretation, Dr. Friedman predicted that it will be “critical” for helping improve the accuracy of epilepsy diagnoses, particularly because most EEGs worldwide are interpreted by non-experts, leading to the known issue with epilepsy misdiagnosis.
“However,” he added, “it is important to note that epilepsy is a clinical diagnosis ... [EEG] is only one piece of evidence in neurologic decision making. History and accurate eyewitness description of the events of concern are extremely critical to the diagnosis and cannot be replaced by AI yet.”
Dr. Kerr offered a similar view, highlighting the potential for SCORE-AI to raise the game of non-epileptologists.
“My anticipation is that neurologists who don’t use SCORE-AI will be replaced by neurologists who use SCORE-AI well,” he said. “Neurologists who use it well will be able to read more EEGs in less time without sacrificing quality. This will allow the neurologist to spend more time talking with the patient about the interpretation of the tests and how that impacts clinical care.”
Then again, that time spent talking with the patient may also one day be delegated to a machine.
“It is certainly imaginable that AI chatbots using large language models to interact with patients and family could be developed to extract consistent epilepsy histories for diagnostic support,” Dr. Wesley said.
This work was supported by a project grant from the Canadian Institutes of Health Research and Duke Neurology start-up funding. The investigators and interviewees reported no relevant conflicts of interest.
FROM EPILEPSIA
When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Which Medications Can Cause Edema?
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Is Vision Loss a New Dementia Risk Factor? What Do the Data Say?
In 2019, 57 million people worldwide were living with dementia, a figure expected to soar to 153 million by 2050. A recent Lancet Commission report suggests that nearly half of dementia cases could be prevented or delayed by addressing 14 modifiable risk factors, including impaired vision.
The report’s authors recommend that vision-loss screening and treatment be universally available. But are these recommendations warranted? What is the evidence? What is the potential mechanism? And what are the potential implications for clinical practice?
Worldwide, the prevalence of avoidable vision loss and blindness in adults aged 50 years or older is estimated to hover around 13%.
“There is now overwhelming evidence that vision impairment in later life is associated with more rapid cognitive decline and an increased risk of dementia,” said Joshua Ehrlich, MD, MPH, associate professor in ophthalmology and visual sciences, the Institute for Social Research at the University of Michigan, Ann Arbor.
The evidence includes a meta-analysis of 14 prospective cohort studies with roughly 6.2 million older adults who were cognitively intact at baseline. Over the course of up to 14 years, 171,888 developed dementia. Vision loss was associated with a pooled relative risk (RR) for dementia of 1.47.
A separate meta-analysis also identified an increased risk for dementia (RR, 1.38) with visual loss. When broken down into different eye conditions, an increased dementia risk was associated with cataracts and diabetic retinopathy but not with glaucoma or age-related macular degeneration.
A US study that followed roughly 3000 older adults with cataracts and normal cognition at baseline for more than 20 years found that those who had cataract extraction had significantly reduced risk for dementia compared with those who did not have cataract extraction (hazard ratio, 0.71), after controlling for age, race, APOE genotype, education, smoking, and an extensive list of comorbidities.
Causation or Coincidence?
The mechanisms behind these associations might be related to underlying illness, such as diabetes, which is a risk factor for dementia; vision loss itself, as might be suggested by a possible effect of cataract surgery; or shared neuropathologic processes in the retina and the brain.
A longitudinal study from Korea that included roughly 6 million adults showed that dementia risk increased with severity of visual loss, which supports the hypothesis that vision loss in itself might be causal or that there is a dose-response effect to a shared causal factor.
“Work is still needed to sort out” exactly how visual deficits may raise dementia risk, although several hypotheses exist, Dr. Ehrlich said.
For example, “decreased input to the brain via the visual pathways may directly induce brain changes. Also, consequences of vision loss, like social isolation, physical inactivity, and depression, are themselves risk factors for dementia and may explain the pathways through which vision impairment increases risk,” he said.
Is the link causal? “We’ll never know definitively because we can’t randomize people to not get cataract surgery versus getting cataract surgery, because we know that improving vision improves quality of life, so we’d never want to do that. But the new evidence that’s come in over the last 5 years or so is pretty promising,” said Esme Fuller-Thomson, PhD, director of the Institute for Life Course and Aging and professor, Department of Family and Community Medicine and Faculty of Nursing, at the University of Toronto, Ontario, Canada.
She noted that results of two studies that have looked at this “seem to indicate that those who have cataract surgery are not nearly at as high risk of dementia as those who have cataracts but don’t have the surgery. That’s leaning towards causality.”
A study published in July suggests that cataracts increase dementia risk through vascular and non–Alzheimer’s disease mechanisms.
Clear Clinical Implications
Dr. Ehrlich said that evidence for an association between untreated vision loss and dementia risk and potential modification by treatment has clear implications for care.
“Loss of vision impacts so many aspects of people’s lives beyond just how they see the world and losing vision in later life is not a normal part of aging. Thus, when older adults experience vision loss, this should be a cause for concern and prompt an immediate referral to an eye care professional,” he noted.
Dr. Fuller-Thomson agrees. “Addressing vision loss will certainly help people see better and function at a higher level and improve quality of life, and it seems probable that it might decrease dementia risk so it’s a win-win,” she said.
In her own research, Dr. Fuller-Thomson has found that the combination of hearing loss and vision loss is linked to an eightfold increased risk for cognitive impairment.
“The idea is that vision and/or hearing loss makes it harder for you to be physically active, to be socially engaged, to be mentally stimulated. They are equally important in terms of social isolation, which could lead to loneliness, and we know that loneliness is not good for dementia,” she said.
“With dual sensory impairment, you don’t have as much information coming in — your brain is not engaged as much — and having an engaged brain, doing hobbies, having intellectually stimulating conversation, all of those are factors are associated with lowering risk of dementia,” Dr. Fuller-Thomson said.
The latest Lancet Commission report noted that treatment for visual loss is “effective and cost-effective” for an estimated 90% of people. However, across the world, particularly in low- and middle-income countries, visual loss often goes untreated.
the report concluded.
Dr. Ehrlich and Dr. Fuller-Thomson have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
In 2019, 57 million people worldwide were living with dementia, a figure expected to soar to 153 million by 2050. A recent Lancet Commission report suggests that nearly half of dementia cases could be prevented or delayed by addressing 14 modifiable risk factors, including impaired vision.
The report’s authors recommend that vision-loss screening and treatment be universally available. But are these recommendations warranted? What is the evidence? What is the potential mechanism? And what are the potential implications for clinical practice?
Worldwide, the prevalence of avoidable vision loss and blindness in adults aged 50 years or older is estimated to hover around 13%.
“There is now overwhelming evidence that vision impairment in later life is associated with more rapid cognitive decline and an increased risk of dementia,” said Joshua Ehrlich, MD, MPH, associate professor in ophthalmology and visual sciences, the Institute for Social Research at the University of Michigan, Ann Arbor.
The evidence includes a meta-analysis of 14 prospective cohort studies with roughly 6.2 million older adults who were cognitively intact at baseline. Over the course of up to 14 years, 171,888 developed dementia. Vision loss was associated with a pooled relative risk (RR) for dementia of 1.47.
A separate meta-analysis also identified an increased risk for dementia (RR, 1.38) with visual loss. When broken down into different eye conditions, an increased dementia risk was associated with cataracts and diabetic retinopathy but not with glaucoma or age-related macular degeneration.
A US study that followed roughly 3000 older adults with cataracts and normal cognition at baseline for more than 20 years found that those who had cataract extraction had significantly reduced risk for dementia compared with those who did not have cataract extraction (hazard ratio, 0.71), after controlling for age, race, APOE genotype, education, smoking, and an extensive list of comorbidities.
Causation or Coincidence?
The mechanisms behind these associations might be related to underlying illness, such as diabetes, which is a risk factor for dementia; vision loss itself, as might be suggested by a possible effect of cataract surgery; or shared neuropathologic processes in the retina and the brain.
A longitudinal study from Korea that included roughly 6 million adults showed that dementia risk increased with severity of visual loss, which supports the hypothesis that vision loss in itself might be causal or that there is a dose-response effect to a shared causal factor.
“Work is still needed to sort out” exactly how visual deficits may raise dementia risk, although several hypotheses exist, Dr. Ehrlich said.
For example, “decreased input to the brain via the visual pathways may directly induce brain changes. Also, consequences of vision loss, like social isolation, physical inactivity, and depression, are themselves risk factors for dementia and may explain the pathways through which vision impairment increases risk,” he said.
Is the link causal? “We’ll never know definitively because we can’t randomize people to not get cataract surgery versus getting cataract surgery, because we know that improving vision improves quality of life, so we’d never want to do that. But the new evidence that’s come in over the last 5 years or so is pretty promising,” said Esme Fuller-Thomson, PhD, director of the Institute for Life Course and Aging and professor, Department of Family and Community Medicine and Faculty of Nursing, at the University of Toronto, Ontario, Canada.
She noted that results of two studies that have looked at this “seem to indicate that those who have cataract surgery are not nearly at as high risk of dementia as those who have cataracts but don’t have the surgery. That’s leaning towards causality.”
A study published in July suggests that cataracts increase dementia risk through vascular and non–Alzheimer’s disease mechanisms.
Clear Clinical Implications
Dr. Ehrlich said that evidence for an association between untreated vision loss and dementia risk and potential modification by treatment has clear implications for care.
“Loss of vision impacts so many aspects of people’s lives beyond just how they see the world and losing vision in later life is not a normal part of aging. Thus, when older adults experience vision loss, this should be a cause for concern and prompt an immediate referral to an eye care professional,” he noted.
Dr. Fuller-Thomson agrees. “Addressing vision loss will certainly help people see better and function at a higher level and improve quality of life, and it seems probable that it might decrease dementia risk so it’s a win-win,” she said.
In her own research, Dr. Fuller-Thomson has found that the combination of hearing loss and vision loss is linked to an eightfold increased risk for cognitive impairment.
“The idea is that vision and/or hearing loss makes it harder for you to be physically active, to be socially engaged, to be mentally stimulated. They are equally important in terms of social isolation, which could lead to loneliness, and we know that loneliness is not good for dementia,” she said.
“With dual sensory impairment, you don’t have as much information coming in — your brain is not engaged as much — and having an engaged brain, doing hobbies, having intellectually stimulating conversation, all of those are factors are associated with lowering risk of dementia,” Dr. Fuller-Thomson said.
The latest Lancet Commission report noted that treatment for visual loss is “effective and cost-effective” for an estimated 90% of people. However, across the world, particularly in low- and middle-income countries, visual loss often goes untreated.
the report concluded.
Dr. Ehrlich and Dr. Fuller-Thomson have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
In 2019, 57 million people worldwide were living with dementia, a figure expected to soar to 153 million by 2050. A recent Lancet Commission report suggests that nearly half of dementia cases could be prevented or delayed by addressing 14 modifiable risk factors, including impaired vision.
The report’s authors recommend that vision-loss screening and treatment be universally available. But are these recommendations warranted? What is the evidence? What is the potential mechanism? And what are the potential implications for clinical practice?
Worldwide, the prevalence of avoidable vision loss and blindness in adults aged 50 years or older is estimated to hover around 13%.
“There is now overwhelming evidence that vision impairment in later life is associated with more rapid cognitive decline and an increased risk of dementia,” said Joshua Ehrlich, MD, MPH, associate professor in ophthalmology and visual sciences, the Institute for Social Research at the University of Michigan, Ann Arbor.
The evidence includes a meta-analysis of 14 prospective cohort studies with roughly 6.2 million older adults who were cognitively intact at baseline. Over the course of up to 14 years, 171,888 developed dementia. Vision loss was associated with a pooled relative risk (RR) for dementia of 1.47.
A separate meta-analysis also identified an increased risk for dementia (RR, 1.38) with visual loss. When broken down into different eye conditions, an increased dementia risk was associated with cataracts and diabetic retinopathy but not with glaucoma or age-related macular degeneration.
A US study that followed roughly 3000 older adults with cataracts and normal cognition at baseline for more than 20 years found that those who had cataract extraction had significantly reduced risk for dementia compared with those who did not have cataract extraction (hazard ratio, 0.71), after controlling for age, race, APOE genotype, education, smoking, and an extensive list of comorbidities.
Causation or Coincidence?
The mechanisms behind these associations might be related to underlying illness, such as diabetes, which is a risk factor for dementia; vision loss itself, as might be suggested by a possible effect of cataract surgery; or shared neuropathologic processes in the retina and the brain.
A longitudinal study from Korea that included roughly 6 million adults showed that dementia risk increased with severity of visual loss, which supports the hypothesis that vision loss in itself might be causal or that there is a dose-response effect to a shared causal factor.
“Work is still needed to sort out” exactly how visual deficits may raise dementia risk, although several hypotheses exist, Dr. Ehrlich said.
For example, “decreased input to the brain via the visual pathways may directly induce brain changes. Also, consequences of vision loss, like social isolation, physical inactivity, and depression, are themselves risk factors for dementia and may explain the pathways through which vision impairment increases risk,” he said.
Is the link causal? “We’ll never know definitively because we can’t randomize people to not get cataract surgery versus getting cataract surgery, because we know that improving vision improves quality of life, so we’d never want to do that. But the new evidence that’s come in over the last 5 years or so is pretty promising,” said Esme Fuller-Thomson, PhD, director of the Institute for Life Course and Aging and professor, Department of Family and Community Medicine and Faculty of Nursing, at the University of Toronto, Ontario, Canada.
She noted that results of two studies that have looked at this “seem to indicate that those who have cataract surgery are not nearly at as high risk of dementia as those who have cataracts but don’t have the surgery. That’s leaning towards causality.”
A study published in July suggests that cataracts increase dementia risk through vascular and non–Alzheimer’s disease mechanisms.
Clear Clinical Implications
Dr. Ehrlich said that evidence for an association between untreated vision loss and dementia risk and potential modification by treatment has clear implications for care.
“Loss of vision impacts so many aspects of people’s lives beyond just how they see the world and losing vision in later life is not a normal part of aging. Thus, when older adults experience vision loss, this should be a cause for concern and prompt an immediate referral to an eye care professional,” he noted.
Dr. Fuller-Thomson agrees. “Addressing vision loss will certainly help people see better and function at a higher level and improve quality of life, and it seems probable that it might decrease dementia risk so it’s a win-win,” she said.
In her own research, Dr. Fuller-Thomson has found that the combination of hearing loss and vision loss is linked to an eightfold increased risk for cognitive impairment.
“The idea is that vision and/or hearing loss makes it harder for you to be physically active, to be socially engaged, to be mentally stimulated. They are equally important in terms of social isolation, which could lead to loneliness, and we know that loneliness is not good for dementia,” she said.
“With dual sensory impairment, you don’t have as much information coming in — your brain is not engaged as much — and having an engaged brain, doing hobbies, having intellectually stimulating conversation, all of those are factors are associated with lowering risk of dementia,” Dr. Fuller-Thomson said.
The latest Lancet Commission report noted that treatment for visual loss is “effective and cost-effective” for an estimated 90% of people. However, across the world, particularly in low- and middle-income countries, visual loss often goes untreated.
the report concluded.
Dr. Ehrlich and Dr. Fuller-Thomson have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.