User login
How to Prevent and Manage Hospital-Based Infections During Coronavirus Outbreaks: Five Lessons from Taiwan
During the severe acute respiratory syndrome (SARS) outbreak in 2003, Taiwan reported 346 confirmed cases and 73 deaths.1 Of all known infections, 94% were transmitted inside hospitals. Nine major hospitals were fully or partially shut down, and many doctors and nurses quit for fear of becoming infected. The Taipei Municipal Ho-Ping Hospital was most severely affected. Its index patient, a 42-year-old undocumented hospital laundry worker who interacted with staff and patients for 6 days before being hospitalized, became a superspreader, infecting at least 20 other patients and 10 staff members.2,3 The entire 450-bed hospital was ordered to shut down, and all 930 staff and 240 patients were quarantined within the hospital. The central government appointed the previous Minister of Health as head of the Anti-SARS Taskforce. Ultimately the hospital was evacuated; the outbreak resulted in 26 deaths.2 Events surrounding the hospital’s evacuation offer important lessons for hospitals struggling to cope with the COVID-19 pandemic, which has been caused by spread of a similar coronavirus.
LESSON 1: DIAGNOSIS
Flexibility about case definition is important, as is use of clinical criteria for diagnosis when reliable laboratory tests are not available.
The laundry worker of Ho-Ping Hospital was initially misdiagnosed with infectious enteritis, which delayed proper management and, crucially, isolation from other patients. The low index of suspicion for SARS reflected the initial World Health Organization diagnostic criteria for SARS, which included travel to or residence in an area with recent local transmission of SARS within 10 days of symptom onset.4 The laundry worker did not have a recent travel history.3 Additionally, SARS manifested as a lower respiratory tract infection, so many patients were hospitalized for pneumonia before being diagnosed with SARS. Similarly, the Wuhan Municipal Health Commission initially issued diagnostic criteria for COVID-19 that, in addition to fever and symptoms of respiratory infections, emphasized direct exposure to the Huanan Seafood Wholesale Market.5 As a result, many cases of COVID-19 were not identified.
Diagnosing SARS was challenging. Early symptoms such as fever and malaise were nonspecific. Polymerase chain reaction tests, although available, were unreliable especially in early stages of the disease and had a high false-negative rate. As cases of SARS increased rapidly, Taiwan began using fever alone for early detection.6 Patients and hospital staff received temperature measurements twice daily. Despite the late start to SARS screening, the fever criterion identified many suspected patients, which ensured widespread detection and containment.
For COVID-19, symptoms such as fever, dry cough, and shortness of breath can be used as clinical criteria to triage patients for quarantine in endemic areas when reliable diagnostic tests are not readily available, but all frontline clinical staff should receive daily temperature checks and/or COVID-19 tests, if available, to protect their families and the public.
LESSON 2: COORDINATION
Ineffective coordination between central and local governments can delay response, but this can be remedied.
During the SARS outbreak, the Taipei City Government and the Taiwan central government were controlled by opposing political parties. Responses to SARS were initially impeded by political skirmishes, which hindered implementation of policies regarding criteria for diagnosis, tracking of suspected cases and their contacts, duration of quarantine, and allocation of resources and facilities for confirmed cases. To avoid further delays, the central government acted swiftly to create the nonpartisan Anti-SARS Taskforce and appointed leaders who could work cordially with both local and central government agencies. To help to deal with the crisis, the central government also designated a new Minister of Health, an epidemiologist, who became the first nonphysician to hold this position.
LESSON 3: EVACUATION
Treatment in place vs evacuation during hospital infections is a critical decision.
The surge of SARS cases at Ho-Ping Hospital led to confusion and panic among patients and hospital staff. Whether to treat its SARS patients on site or to evacuate the hospital was a complex decision and reflected many concerns, including the following: How many wards had been infected? Was there sufficient equipment (eg, respirators) to monitor or treat infected patients? How many isolation beds were available? How many hospital staff were already infected and quarantined? Were they in different wards? Were there neighboring facilities (eg, hospitals, military camps, dorms) available for quarantine?
If a hospital has sufficient capacity to isolate persons under investigation and to treat confirmed patients, on-site treatment is possible. However, evacuation should be considered when there is widespread infection involving different hospital wards and hospital staff. In such cases, patients should be transferred to different facilities based on clinical severity: Patients with new onset fever or respiratory symptoms but who are relatively healthy should be sent to community or regional hospitals with isolation rooms for monitoring; sicker infected patients should be sent to medical centers; and other hospitalized patients (eg, admitted for heart failure) without infection risk or symptoms should seek care elsewhere.
So far, there has been only one instance of hospital-based COVID-19 infections in Taiwan, and the spread of infection was quickly contained within one ward. All nine confirmed cases (including the index patient, one patient in the same ward but a different room, three nurses, one laundry worker, and three members of patients’ families) and their known contacts were identified, then isolated or quarantined individually. Because the affected hospital is part of a complex with more than 3,000 beds, it was big enough to accommodate all infected patients and no evacuation measures were needed. To further reduce potential nodes in the chain of transmission, interns and many other healthcare workers were temporarily relieved of their duties, elective surgeries were canceled, and hospital visitation was limited to immediate family members. The clear communication of intervention measures ensured rapid cooperation and staved off both social panic and further spread of the disease.
LESSON 4: PATIENT FLOW
Hospitals should establish different flows for different patients.
Having learned from the SARS experience in 2003, hospitals in Taiwan have designated specific pathways to manage patient flow during the COVID-19 outbreak, in addition to checking all patients for travel history and fever: Patients with fever were quickly triaged to a designated fever clinic so they did not mingle with other patients, patients visiting the hospital to obtain chronic disease medications were directed to a “drive-through” lane, patients needing emergent care went through the emergency department, all other regular outpatients were seen in outpatient departments, and visitors of patients were restricted to one visitor per patient at a given time.
LESSON 5: ORGANIZATION
Healthcare providers should be organized into blocks and modular teams to avoid hospital-wide infection.
After SARS, Taiwan learned that one way to reduce the spread of something like COVID-19 among healthcare providers and from providers to patients is to divide providers’ work areas into discrete blocks and organize providers into modular teams. This approach was inspired by the design of watertight compartments in ships: Should the hull be breached, flooding is restricted to the breached compartments. Under this organizational strategy, movements of physicians and nurses would be restricted to their designated locations: They would be routinely exposed only to other staff and patients within their division. Doctors and nurses would be asked to practice in modular teams within their blocked locations, reducing the likelihood that infection in one team would spread to another, which could lead to hospital-wide infections. Movement of senior hospital executives would be similarly restricted. Common areas such as cafeterias where people mingle would be closed. Owing to these stringent initiatives, aside from the hospital-based infection mentioned in Lesson 3, no other hospital-based infections have been reported in Taiwan so far.
CONCLUSION
Lessons from previous hospital-based coronavirus infections can be used to minimize future infections.
Acknowledgments
The authors would like to thank Dr Lee Ming-Liang, former Health Minister of Taiwan and director of that central government’s Anti-SARS Taskforce during the 2003 outbreak, for providing valuable recommendations to this work.
1. Hsieh YH, King CC, Chen CWS, et al. Quarantine for SARS, Taiwan. Emerg Infect Dis. 2005;11(2):278-282. https://doi.org/10.3201/eid1102.040190.
2. From the Centers for Disease Control and Prevention. Severe Acute Respiratory Syndrome—Taiwan, 2003. JAMA. 2003;289(22):2930-2932. https://doi.org/10.1001/jama.289.22.2930.
3. McNeil DG. The SARS epidemic: the virus; most Taiwan SARS cases spread by one misdiagnosis. New York Times. May 8, 2003. https://www.nytimes.com/2003/05/08/world/the-sars-epidemic-the-virus-most-taiwan-sars-cases-spread-by-one-misdiagnosis.html. Accessed March 28, 2020.
4. Hui DSC, Chan MCH, Wu AK, Ng PC. Severe acute respiratory syndrome (SARS): epidemiology and clinical features. Postgrad Med J. 2004;80(945):373-381. https://doi.org/10.1136/pgmj.2004.020263.
5. Yang DL. Wuhan officials tried to cover up covid-19 — and sent it careening outward. Washington Post. March 10, 2020. https://www.washingtonpost.com/politics/2020/03/10/wuhan-officials-tried-cover-up-covid-19-sent-it-careening-outward/. Accessed March 28, 2020.
6. Lin EC, Peng YC, Hung Tsai JC. Lessons learned from the anti-SARS quarantine experience in a hospital-based fever screening station in Taiwan. Am J Infect Control. 2010;38(4):302-307. https://doi.org/10.1016/j.ajic.2009.09.008.
During the severe acute respiratory syndrome (SARS) outbreak in 2003, Taiwan reported 346 confirmed cases and 73 deaths.1 Of all known infections, 94% were transmitted inside hospitals. Nine major hospitals were fully or partially shut down, and many doctors and nurses quit for fear of becoming infected. The Taipei Municipal Ho-Ping Hospital was most severely affected. Its index patient, a 42-year-old undocumented hospital laundry worker who interacted with staff and patients for 6 days before being hospitalized, became a superspreader, infecting at least 20 other patients and 10 staff members.2,3 The entire 450-bed hospital was ordered to shut down, and all 930 staff and 240 patients were quarantined within the hospital. The central government appointed the previous Minister of Health as head of the Anti-SARS Taskforce. Ultimately the hospital was evacuated; the outbreak resulted in 26 deaths.2 Events surrounding the hospital’s evacuation offer important lessons for hospitals struggling to cope with the COVID-19 pandemic, which has been caused by spread of a similar coronavirus.
LESSON 1: DIAGNOSIS
Flexibility about case definition is important, as is use of clinical criteria for diagnosis when reliable laboratory tests are not available.
The laundry worker of Ho-Ping Hospital was initially misdiagnosed with infectious enteritis, which delayed proper management and, crucially, isolation from other patients. The low index of suspicion for SARS reflected the initial World Health Organization diagnostic criteria for SARS, which included travel to or residence in an area with recent local transmission of SARS within 10 days of symptom onset.4 The laundry worker did not have a recent travel history.3 Additionally, SARS manifested as a lower respiratory tract infection, so many patients were hospitalized for pneumonia before being diagnosed with SARS. Similarly, the Wuhan Municipal Health Commission initially issued diagnostic criteria for COVID-19 that, in addition to fever and symptoms of respiratory infections, emphasized direct exposure to the Huanan Seafood Wholesale Market.5 As a result, many cases of COVID-19 were not identified.
Diagnosing SARS was challenging. Early symptoms such as fever and malaise were nonspecific. Polymerase chain reaction tests, although available, were unreliable especially in early stages of the disease and had a high false-negative rate. As cases of SARS increased rapidly, Taiwan began using fever alone for early detection.6 Patients and hospital staff received temperature measurements twice daily. Despite the late start to SARS screening, the fever criterion identified many suspected patients, which ensured widespread detection and containment.
For COVID-19, symptoms such as fever, dry cough, and shortness of breath can be used as clinical criteria to triage patients for quarantine in endemic areas when reliable diagnostic tests are not readily available, but all frontline clinical staff should receive daily temperature checks and/or COVID-19 tests, if available, to protect their families and the public.
LESSON 2: COORDINATION
Ineffective coordination between central and local governments can delay response, but this can be remedied.
During the SARS outbreak, the Taipei City Government and the Taiwan central government were controlled by opposing political parties. Responses to SARS were initially impeded by political skirmishes, which hindered implementation of policies regarding criteria for diagnosis, tracking of suspected cases and their contacts, duration of quarantine, and allocation of resources and facilities for confirmed cases. To avoid further delays, the central government acted swiftly to create the nonpartisan Anti-SARS Taskforce and appointed leaders who could work cordially with both local and central government agencies. To help to deal with the crisis, the central government also designated a new Minister of Health, an epidemiologist, who became the first nonphysician to hold this position.
LESSON 3: EVACUATION
Treatment in place vs evacuation during hospital infections is a critical decision.
The surge of SARS cases at Ho-Ping Hospital led to confusion and panic among patients and hospital staff. Whether to treat its SARS patients on site or to evacuate the hospital was a complex decision and reflected many concerns, including the following: How many wards had been infected? Was there sufficient equipment (eg, respirators) to monitor or treat infected patients? How many isolation beds were available? How many hospital staff were already infected and quarantined? Were they in different wards? Were there neighboring facilities (eg, hospitals, military camps, dorms) available for quarantine?
If a hospital has sufficient capacity to isolate persons under investigation and to treat confirmed patients, on-site treatment is possible. However, evacuation should be considered when there is widespread infection involving different hospital wards and hospital staff. In such cases, patients should be transferred to different facilities based on clinical severity: Patients with new onset fever or respiratory symptoms but who are relatively healthy should be sent to community or regional hospitals with isolation rooms for monitoring; sicker infected patients should be sent to medical centers; and other hospitalized patients (eg, admitted for heart failure) without infection risk or symptoms should seek care elsewhere.
So far, there has been only one instance of hospital-based COVID-19 infections in Taiwan, and the spread of infection was quickly contained within one ward. All nine confirmed cases (including the index patient, one patient in the same ward but a different room, three nurses, one laundry worker, and three members of patients’ families) and their known contacts were identified, then isolated or quarantined individually. Because the affected hospital is part of a complex with more than 3,000 beds, it was big enough to accommodate all infected patients and no evacuation measures were needed. To further reduce potential nodes in the chain of transmission, interns and many other healthcare workers were temporarily relieved of their duties, elective surgeries were canceled, and hospital visitation was limited to immediate family members. The clear communication of intervention measures ensured rapid cooperation and staved off both social panic and further spread of the disease.
LESSON 4: PATIENT FLOW
Hospitals should establish different flows for different patients.
Having learned from the SARS experience in 2003, hospitals in Taiwan have designated specific pathways to manage patient flow during the COVID-19 outbreak, in addition to checking all patients for travel history and fever: Patients with fever were quickly triaged to a designated fever clinic so they did not mingle with other patients, patients visiting the hospital to obtain chronic disease medications were directed to a “drive-through” lane, patients needing emergent care went through the emergency department, all other regular outpatients were seen in outpatient departments, and visitors of patients were restricted to one visitor per patient at a given time.
LESSON 5: ORGANIZATION
Healthcare providers should be organized into blocks and modular teams to avoid hospital-wide infection.
After SARS, Taiwan learned that one way to reduce the spread of something like COVID-19 among healthcare providers and from providers to patients is to divide providers’ work areas into discrete blocks and organize providers into modular teams. This approach was inspired by the design of watertight compartments in ships: Should the hull be breached, flooding is restricted to the breached compartments. Under this organizational strategy, movements of physicians and nurses would be restricted to their designated locations: They would be routinely exposed only to other staff and patients within their division. Doctors and nurses would be asked to practice in modular teams within their blocked locations, reducing the likelihood that infection in one team would spread to another, which could lead to hospital-wide infections. Movement of senior hospital executives would be similarly restricted. Common areas such as cafeterias where people mingle would be closed. Owing to these stringent initiatives, aside from the hospital-based infection mentioned in Lesson 3, no other hospital-based infections have been reported in Taiwan so far.
CONCLUSION
Lessons from previous hospital-based coronavirus infections can be used to minimize future infections.
Acknowledgments
The authors would like to thank Dr Lee Ming-Liang, former Health Minister of Taiwan and director of that central government’s Anti-SARS Taskforce during the 2003 outbreak, for providing valuable recommendations to this work.
During the severe acute respiratory syndrome (SARS) outbreak in 2003, Taiwan reported 346 confirmed cases and 73 deaths.1 Of all known infections, 94% were transmitted inside hospitals. Nine major hospitals were fully or partially shut down, and many doctors and nurses quit for fear of becoming infected. The Taipei Municipal Ho-Ping Hospital was most severely affected. Its index patient, a 42-year-old undocumented hospital laundry worker who interacted with staff and patients for 6 days before being hospitalized, became a superspreader, infecting at least 20 other patients and 10 staff members.2,3 The entire 450-bed hospital was ordered to shut down, and all 930 staff and 240 patients were quarantined within the hospital. The central government appointed the previous Minister of Health as head of the Anti-SARS Taskforce. Ultimately the hospital was evacuated; the outbreak resulted in 26 deaths.2 Events surrounding the hospital’s evacuation offer important lessons for hospitals struggling to cope with the COVID-19 pandemic, which has been caused by spread of a similar coronavirus.
LESSON 1: DIAGNOSIS
Flexibility about case definition is important, as is use of clinical criteria for diagnosis when reliable laboratory tests are not available.
The laundry worker of Ho-Ping Hospital was initially misdiagnosed with infectious enteritis, which delayed proper management and, crucially, isolation from other patients. The low index of suspicion for SARS reflected the initial World Health Organization diagnostic criteria for SARS, which included travel to or residence in an area with recent local transmission of SARS within 10 days of symptom onset.4 The laundry worker did not have a recent travel history.3 Additionally, SARS manifested as a lower respiratory tract infection, so many patients were hospitalized for pneumonia before being diagnosed with SARS. Similarly, the Wuhan Municipal Health Commission initially issued diagnostic criteria for COVID-19 that, in addition to fever and symptoms of respiratory infections, emphasized direct exposure to the Huanan Seafood Wholesale Market.5 As a result, many cases of COVID-19 were not identified.
Diagnosing SARS was challenging. Early symptoms such as fever and malaise were nonspecific. Polymerase chain reaction tests, although available, were unreliable especially in early stages of the disease and had a high false-negative rate. As cases of SARS increased rapidly, Taiwan began using fever alone for early detection.6 Patients and hospital staff received temperature measurements twice daily. Despite the late start to SARS screening, the fever criterion identified many suspected patients, which ensured widespread detection and containment.
For COVID-19, symptoms such as fever, dry cough, and shortness of breath can be used as clinical criteria to triage patients for quarantine in endemic areas when reliable diagnostic tests are not readily available, but all frontline clinical staff should receive daily temperature checks and/or COVID-19 tests, if available, to protect their families and the public.
LESSON 2: COORDINATION
Ineffective coordination between central and local governments can delay response, but this can be remedied.
During the SARS outbreak, the Taipei City Government and the Taiwan central government were controlled by opposing political parties. Responses to SARS were initially impeded by political skirmishes, which hindered implementation of policies regarding criteria for diagnosis, tracking of suspected cases and their contacts, duration of quarantine, and allocation of resources and facilities for confirmed cases. To avoid further delays, the central government acted swiftly to create the nonpartisan Anti-SARS Taskforce and appointed leaders who could work cordially with both local and central government agencies. To help to deal with the crisis, the central government also designated a new Minister of Health, an epidemiologist, who became the first nonphysician to hold this position.
LESSON 3: EVACUATION
Treatment in place vs evacuation during hospital infections is a critical decision.
The surge of SARS cases at Ho-Ping Hospital led to confusion and panic among patients and hospital staff. Whether to treat its SARS patients on site or to evacuate the hospital was a complex decision and reflected many concerns, including the following: How many wards had been infected? Was there sufficient equipment (eg, respirators) to monitor or treat infected patients? How many isolation beds were available? How many hospital staff were already infected and quarantined? Were they in different wards? Were there neighboring facilities (eg, hospitals, military camps, dorms) available for quarantine?
If a hospital has sufficient capacity to isolate persons under investigation and to treat confirmed patients, on-site treatment is possible. However, evacuation should be considered when there is widespread infection involving different hospital wards and hospital staff. In such cases, patients should be transferred to different facilities based on clinical severity: Patients with new onset fever or respiratory symptoms but who are relatively healthy should be sent to community or regional hospitals with isolation rooms for monitoring; sicker infected patients should be sent to medical centers; and other hospitalized patients (eg, admitted for heart failure) without infection risk or symptoms should seek care elsewhere.
So far, there has been only one instance of hospital-based COVID-19 infections in Taiwan, and the spread of infection was quickly contained within one ward. All nine confirmed cases (including the index patient, one patient in the same ward but a different room, three nurses, one laundry worker, and three members of patients’ families) and their known contacts were identified, then isolated or quarantined individually. Because the affected hospital is part of a complex with more than 3,000 beds, it was big enough to accommodate all infected patients and no evacuation measures were needed. To further reduce potential nodes in the chain of transmission, interns and many other healthcare workers were temporarily relieved of their duties, elective surgeries were canceled, and hospital visitation was limited to immediate family members. The clear communication of intervention measures ensured rapid cooperation and staved off both social panic and further spread of the disease.
LESSON 4: PATIENT FLOW
Hospitals should establish different flows for different patients.
Having learned from the SARS experience in 2003, hospitals in Taiwan have designated specific pathways to manage patient flow during the COVID-19 outbreak, in addition to checking all patients for travel history and fever: Patients with fever were quickly triaged to a designated fever clinic so they did not mingle with other patients, patients visiting the hospital to obtain chronic disease medications were directed to a “drive-through” lane, patients needing emergent care went through the emergency department, all other regular outpatients were seen in outpatient departments, and visitors of patients were restricted to one visitor per patient at a given time.
LESSON 5: ORGANIZATION
Healthcare providers should be organized into blocks and modular teams to avoid hospital-wide infection.
After SARS, Taiwan learned that one way to reduce the spread of something like COVID-19 among healthcare providers and from providers to patients is to divide providers’ work areas into discrete blocks and organize providers into modular teams. This approach was inspired by the design of watertight compartments in ships: Should the hull be breached, flooding is restricted to the breached compartments. Under this organizational strategy, movements of physicians and nurses would be restricted to their designated locations: They would be routinely exposed only to other staff and patients within their division. Doctors and nurses would be asked to practice in modular teams within their blocked locations, reducing the likelihood that infection in one team would spread to another, which could lead to hospital-wide infections. Movement of senior hospital executives would be similarly restricted. Common areas such as cafeterias where people mingle would be closed. Owing to these stringent initiatives, aside from the hospital-based infection mentioned in Lesson 3, no other hospital-based infections have been reported in Taiwan so far.
CONCLUSION
Lessons from previous hospital-based coronavirus infections can be used to minimize future infections.
Acknowledgments
The authors would like to thank Dr Lee Ming-Liang, former Health Minister of Taiwan and director of that central government’s Anti-SARS Taskforce during the 2003 outbreak, for providing valuable recommendations to this work.
1. Hsieh YH, King CC, Chen CWS, et al. Quarantine for SARS, Taiwan. Emerg Infect Dis. 2005;11(2):278-282. https://doi.org/10.3201/eid1102.040190.
2. From the Centers for Disease Control and Prevention. Severe Acute Respiratory Syndrome—Taiwan, 2003. JAMA. 2003;289(22):2930-2932. https://doi.org/10.1001/jama.289.22.2930.
3. McNeil DG. The SARS epidemic: the virus; most Taiwan SARS cases spread by one misdiagnosis. New York Times. May 8, 2003. https://www.nytimes.com/2003/05/08/world/the-sars-epidemic-the-virus-most-taiwan-sars-cases-spread-by-one-misdiagnosis.html. Accessed March 28, 2020.
4. Hui DSC, Chan MCH, Wu AK, Ng PC. Severe acute respiratory syndrome (SARS): epidemiology and clinical features. Postgrad Med J. 2004;80(945):373-381. https://doi.org/10.1136/pgmj.2004.020263.
5. Yang DL. Wuhan officials tried to cover up covid-19 — and sent it careening outward. Washington Post. March 10, 2020. https://www.washingtonpost.com/politics/2020/03/10/wuhan-officials-tried-cover-up-covid-19-sent-it-careening-outward/. Accessed March 28, 2020.
6. Lin EC, Peng YC, Hung Tsai JC. Lessons learned from the anti-SARS quarantine experience in a hospital-based fever screening station in Taiwan. Am J Infect Control. 2010;38(4):302-307. https://doi.org/10.1016/j.ajic.2009.09.008.
1. Hsieh YH, King CC, Chen CWS, et al. Quarantine for SARS, Taiwan. Emerg Infect Dis. 2005;11(2):278-282. https://doi.org/10.3201/eid1102.040190.
2. From the Centers for Disease Control and Prevention. Severe Acute Respiratory Syndrome—Taiwan, 2003. JAMA. 2003;289(22):2930-2932. https://doi.org/10.1001/jama.289.22.2930.
3. McNeil DG. The SARS epidemic: the virus; most Taiwan SARS cases spread by one misdiagnosis. New York Times. May 8, 2003. https://www.nytimes.com/2003/05/08/world/the-sars-epidemic-the-virus-most-taiwan-sars-cases-spread-by-one-misdiagnosis.html. Accessed March 28, 2020.
4. Hui DSC, Chan MCH, Wu AK, Ng PC. Severe acute respiratory syndrome (SARS): epidemiology and clinical features. Postgrad Med J. 2004;80(945):373-381. https://doi.org/10.1136/pgmj.2004.020263.
5. Yang DL. Wuhan officials tried to cover up covid-19 — and sent it careening outward. Washington Post. March 10, 2020. https://www.washingtonpost.com/politics/2020/03/10/wuhan-officials-tried-cover-up-covid-19-sent-it-careening-outward/. Accessed March 28, 2020.
6. Lin EC, Peng YC, Hung Tsai JC. Lessons learned from the anti-SARS quarantine experience in a hospital-based fever screening station in Taiwan. Am J Infect Control. 2010;38(4):302-307. https://doi.org/10.1016/j.ajic.2009.09.008.
© 2020 Society of Hospital Medicine
A Transdisciplinary COVID-19 Early Respiratory Intervention Protocol: An Implementation Story
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.
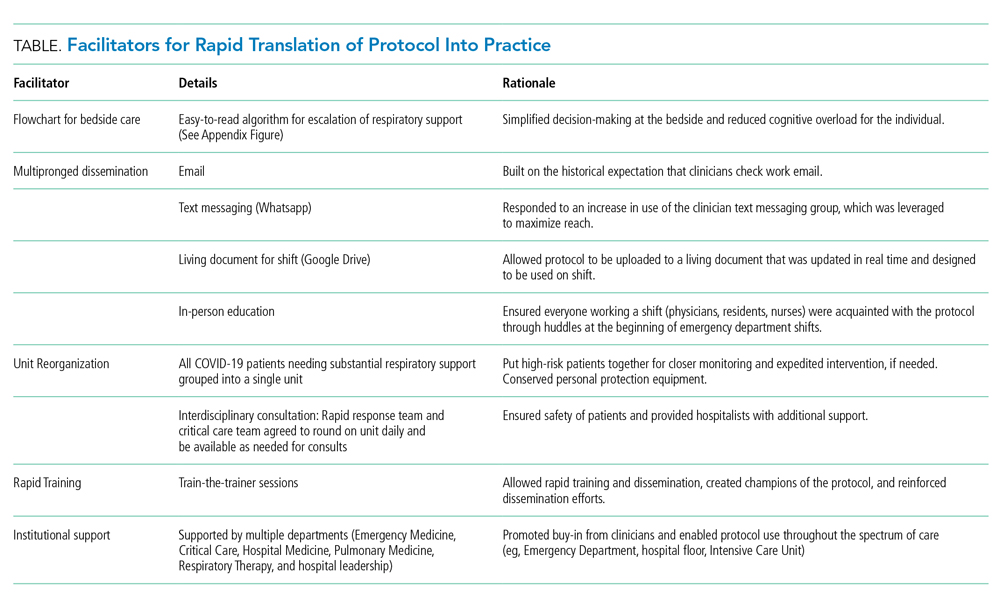
For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
© 2020 Society of Hospital Medicine
The Role of Hospitalists in Biocontainment Units: A Perspective
In 2015, and in response to the Ebola virus outbreak in West Africa, the United States Department of Health and Human Services (HHS) designated 10 health departments and associated partner hospitals to become regional treatment centers for patients with highly infectious diseases, such as the Ebola virus and other highly infectious special pathogens (HISPs), and reinforce the nation’s infectious disease response capability. These efforts catalyzed the creation and/or expansion of a network of biocontainment units (BCUs) to safely care for patients diagnosed with highly infectious diseases. These units are designed as special care units with environmental/engineering controls, laboratory capabilities, simple imaging testing, and dedicated staff to allow for the uninterrupted care of patients.1,2 The HHS approach closely resembled the tiered structure of trauma center levels familiar to the healthcare system. The regional framework identified four types of facilities (frontline healthcare facilities, assessment hospitals, treatment centers, and regional Ebola and other special pathogens treatment centers [RESPTCs]) with increasing levels of capabilities and responsibilities.
There are over 4,845 frontline healthcare facilities across the United States, which are able to identify and isolate a patient suspected of a HISP infection and inform local and state partners. The facility provides stabilizing treatment while coordinating the transport of the patient to a specialized center. An assessment hospital can identify and isolate a patient with a HISP, inform partnering agencies, and provide care at the facility for up to 96 hours. There are over 217 hospitals with this designation in the United States. Treatment centers are designated as state or jurisdiction treatment centers and have the capacity to care for HISP-infected patients for the entirety of their care plan, as well as serve as a partner in caring for a potential surge in high-risk patients if their partner RESPTC is unable to care for a patient because of capacity limits. Patients may receive care at a treatment center if and when it is determined to be more appropriate (eg, clinical purview, logistics, resources) than sending them to a RESPTC. There are currently 63 designated treatment centers in the United States.
As outlined by HHS, the RESPTCs3 must be ready to receive a HISP-infected patient within their HHS region, domestically, or internationally within 8 hours. RESPTCs provide care for the entirety of the patient care plan. The 10 regional Departments of Public Health representatives are: Massachusetts (Region 1); New York (Region 2); Maryland (Region 3); Georgia (Region 4); Minnesota (Region 5); Texas (Region 6); Nebraska (Region 7); Colorado in partnership with Denver Health Hospital Authority (DHHA; Region 8); California (Region 9); and Washington State (Region 10).
BCU PHYSICIAN STAFFING MODELS
Most RESPCTs BCUs are staffed by a self-selected group of core providers with expertise in infectious diseases (ID) and critical care (CC). Teams are interdisciplinary and committed to a culture of safety.4-7 ID physicians are experts in HISP disease processes and epidemiology, which enables expert guidance on patient care and infection control. CC physicians are trained to provide care to patients requiring life-saving interventions.
DHHA STAFFING MODEL
As specialized units are shifting to include high-risk infectious diseases beyond Ebola, hospitals are developing innovative ways to manage a potential surge of patients with respiratory pathogens, expanding care far beyond the biocontainment unit. With the potential for an influx of HISPs in the healthcare setting, identifying stakeholders uniquely equipped to provide care in all areas of the hospital is ideal. At DHHA, the BCU physician staffing model transitioned from ID and CC physicians to a selected group of ten hospitalists as the primary managing service in 2018.
When DHHA received the RESPTC nomination, it developed a fully voluntary multidisciplinary high-risk infection team (HITeam; Table) with specialized training in personal protective equipment (PPE) donning and doffing, as well as BCU protocols. Our HITeam members participate in every-6-weeks mandatory team drills that involve practicing team dynamics and dexterity while wearing PPE. Team members participate in regular hospital and/or BCU-focused exercises to simulate and practice real-world experiences. They also participate in HISP Journal Club, where members from different disciplines discuss pertinent articles in the field, as well as every-other-month team-building meetings. Our unit’s main challenges included maintaining competencies and staff retention. We developed a unique staffing model aimed at mitigating some of these challenges through a multidisciplinary team approach utilizing different levels of physician involvement. The first group comprises physicians providing primary, direct care to patients, which which consists our specially trained group of 10 hospitalists—including 2 pediatricians, as well as 3 CC and 4 ID consultants. The second group involves consultants from specialties such as nephrology, anesthesia, general surgery, radiology and gynecology; just–in-time training is available for them.
WHY HOSPITALISTS?
Hospitalists are uniquely positioned to care for this distinctive set of patients because they are comfortable with the care of acutely ill patients, many maintain bedside procedural skills, and many have acquired point-of-care-ultrasonography (POCUS) skills. Furthermore, hospitalists usually outnumber available specialists who may be needed to maintain consultative and critical care services for non-BCU patients. We were able to develop a feasible physician staffing model, as described below, and validate hospital medicine’s commitment in addressing institutional needs.
In order to provide ideal unit coverage, 2 hospitalists are scheduled daily and available to respond in case of unit activation. BCU hospitalists are scheduled to cover the BCU when already scheduled to work a clinical shift. In the very infrequent event of BCU activation, BCU hospitalists would move their clinical work to the BCU and a back-up hospitalist would be called in to cover the “other” clinical shift; by overlapping coverage, we ensure BCU hospitalists’ work-life balance and job satisfaction remain intact. Because of expected postexposure monitoring, individual physician’s planned international travel is considered while generating the call schedule. When the unit is activated, the hospital provides payment for extra hours worked by both the BCU hospitalist and the back-up hospitalist, with anticipated revenue recapture through critical care billing by the BCU hospitalist. There are 10 HITeam hospitalists, who are trained and credentialed in bedside procedures and with different levels of POCUS expertise (from literacy to expert level). Team members are expected to attend training in bedside procedures while in PPE 2 times a year. The hospital also provides financial support for hospitalists seeking to further their BCU-related skills and training. It is anticipated that hospitalists will require consultative services from CC and ID in some selected cases to provide a well-rounded care approach to the biocontained patient.
WHAT IT TAKES TO BE A TEAM
Eligibility criteria for HITeam hospitalists include complete initial and quarterly maintenance PPE training; possession of active bedside procedure credentials for at least central line, paracentesis, thoracentesis, and arterial line; demonstration of basic POCUS skills, such as having enough POCUS verbiage literacy to be able to follow a radiologist’s instructions on probe management (ie, a radiologist outside anteroom); and possibility of completing additional training and credentialing on conscious sedation and/or advanced airway management.
Similarly, hospitalists who join the team commit to attendance of at least one National Ebola Training and Education Center (NETEC) provider’s course, representing the Region 8 RESPTC for educational presentations and research at regional and national levels, participation in quarterly HISP multidisciplinary meetings, attendance at quarterly donning and doffing sessions, as well as HITeam training sessions and drills, and active participation in the every-other-month HISP Journal Club/Grand Rounds to maintain competence and knowledge on management of many different pathogens, including Ebola and other filoviruses, MERS-CoV, SARS-CoV, Arenaviruses causing hemorrhagic fevers, Hantavirus, and novel influenzas or coronaviruses.
All of these commitments provide HITeam hospitalists with multiple opportunities for professional growth and development, such as augmenting scholarship venues by participating in collaborative national research projects, participating in national topic networks discussion groups and committees, becoming topic experts, and engaging in diversified training such as advanced airway training and conscious sedation. A new bedside ultrasound machine was purchased for the unit and housed within the division of hospital medicine with the intent to provide hospitalists the means necessary to achieve POCUS proficiency. Above all, by fostering a highly motivated and collegial multidisciplinary team, our model helps develop lasting partnerships at an institutional, regional, and national level.
This multidisciplinary team—with its skillfully trained and engaged nurses, physicians, respiratory therapists, pharmacists, infection control and laboratory specialists—works, learns, trains, and thrives collectively with the aim of providing excellent clinical care to our patients while assuring the safety of the team. DHHA has pioneered a RESPTC physician staffing model led by hospitalists.
We live in an ever-changing landscape of emerging diseases with blurred borders of disease geography. Hospitalists are versatile, capable of managing patients of varying acuity, able to perform many bedside procedures and POCUS; they are champions of interdisciplinary and teamwork disposition. By utilizing the resourcefulness of hospital medicine while helping to ease some of the burden that might otherwise be placed on a smaller numbers of physician groups, this approach provides a unique, cost-effective, and viable physician staffing model, which could be implemented in other BCUs in the United States.
1. Smith PW, Anderson AO, Christopher GW, et al. Designing a biocontainment unit to care for patients with serious communicable diseases: a consensus statement. Biosecur Bioterror. 2006;4(4):351-65. https://doi.org/10.1089/bsp.2006.4.351.
2. Bannister B, Puro V, Fusco FM, Heptonstall J, Ippolito G; EUNID Working Group. Framework for the design and operation of high-level isolation units: consensus of the European Network of Infectious Diseases. Lancet Infect Dis. 2009;9(1):45-56. https://doi.org/10.1016/S1473-3099(08)70304-9.
3. US Department of Health and Human Services. Office of the Assistant Secretary for preparedness and Response Regional Treatment Network for Ebola and Other Special Pathogens. November 2017 Report. https://www.phe.gov/Preparedness/planning/hpp/reports/Documents/RETN-Ebola-Report-508.pdf Accessed August 17, 2019.
4. Vasa A, Schwedhelm M, Johnson, D. Critical care for the patient with Ebola virus disease: the Nebraska perspective. J Intensive Crit Care. 2015;1(8):1-5.
5. Garibaldi BT, Kelen GD, Brower RG, et al. The creation of a biocontainment unit at a tertiary care hospital. The Johns Hopkins medicine experience. Ann Am Thorac Soc. 2016;13(5):600-608. https://doi.org/10.1513/AnnalsATS.201509-587PS.
6. Beam EL, Boulter KC, Freihaut F, Schwedhelm S, Smith PW. The Nebraska experience in biocontainment patient care. Public Health Nurs. 2010 Mar-Apr;27(2):140-7. https://doi.org/10.1111/j.1525-1446.2010.00837.x.
7. Hewlett AL, Varkey JB, Smith PW, Ribner BS. Ebola virus disease: preparedness and infection control lessons learned from two biocontainment units. Curr Opin Infect Dis. 2015 Aug;28(4):343-8. https://doi.org/10.1097/QCO.0000000000000176.
In 2015, and in response to the Ebola virus outbreak in West Africa, the United States Department of Health and Human Services (HHS) designated 10 health departments and associated partner hospitals to become regional treatment centers for patients with highly infectious diseases, such as the Ebola virus and other highly infectious special pathogens (HISPs), and reinforce the nation’s infectious disease response capability. These efforts catalyzed the creation and/or expansion of a network of biocontainment units (BCUs) to safely care for patients diagnosed with highly infectious diseases. These units are designed as special care units with environmental/engineering controls, laboratory capabilities, simple imaging testing, and dedicated staff to allow for the uninterrupted care of patients.1,2 The HHS approach closely resembled the tiered structure of trauma center levels familiar to the healthcare system. The regional framework identified four types of facilities (frontline healthcare facilities, assessment hospitals, treatment centers, and regional Ebola and other special pathogens treatment centers [RESPTCs]) with increasing levels of capabilities and responsibilities.
There are over 4,845 frontline healthcare facilities across the United States, which are able to identify and isolate a patient suspected of a HISP infection and inform local and state partners. The facility provides stabilizing treatment while coordinating the transport of the patient to a specialized center. An assessment hospital can identify and isolate a patient with a HISP, inform partnering agencies, and provide care at the facility for up to 96 hours. There are over 217 hospitals with this designation in the United States. Treatment centers are designated as state or jurisdiction treatment centers and have the capacity to care for HISP-infected patients for the entirety of their care plan, as well as serve as a partner in caring for a potential surge in high-risk patients if their partner RESPTC is unable to care for a patient because of capacity limits. Patients may receive care at a treatment center if and when it is determined to be more appropriate (eg, clinical purview, logistics, resources) than sending them to a RESPTC. There are currently 63 designated treatment centers in the United States.
As outlined by HHS, the RESPTCs3 must be ready to receive a HISP-infected patient within their HHS region, domestically, or internationally within 8 hours. RESPTCs provide care for the entirety of the patient care plan. The 10 regional Departments of Public Health representatives are: Massachusetts (Region 1); New York (Region 2); Maryland (Region 3); Georgia (Region 4); Minnesota (Region 5); Texas (Region 6); Nebraska (Region 7); Colorado in partnership with Denver Health Hospital Authority (DHHA; Region 8); California (Region 9); and Washington State (Region 10).
BCU PHYSICIAN STAFFING MODELS
Most RESPCTs BCUs are staffed by a self-selected group of core providers with expertise in infectious diseases (ID) and critical care (CC). Teams are interdisciplinary and committed to a culture of safety.4-7 ID physicians are experts in HISP disease processes and epidemiology, which enables expert guidance on patient care and infection control. CC physicians are trained to provide care to patients requiring life-saving interventions.
DHHA STAFFING MODEL
As specialized units are shifting to include high-risk infectious diseases beyond Ebola, hospitals are developing innovative ways to manage a potential surge of patients with respiratory pathogens, expanding care far beyond the biocontainment unit. With the potential for an influx of HISPs in the healthcare setting, identifying stakeholders uniquely equipped to provide care in all areas of the hospital is ideal. At DHHA, the BCU physician staffing model transitioned from ID and CC physicians to a selected group of ten hospitalists as the primary managing service in 2018.
When DHHA received the RESPTC nomination, it developed a fully voluntary multidisciplinary high-risk infection team (HITeam; Table) with specialized training in personal protective equipment (PPE) donning and doffing, as well as BCU protocols. Our HITeam members participate in every-6-weeks mandatory team drills that involve practicing team dynamics and dexterity while wearing PPE. Team members participate in regular hospital and/or BCU-focused exercises to simulate and practice real-world experiences. They also participate in HISP Journal Club, where members from different disciplines discuss pertinent articles in the field, as well as every-other-month team-building meetings. Our unit’s main challenges included maintaining competencies and staff retention. We developed a unique staffing model aimed at mitigating some of these challenges through a multidisciplinary team approach utilizing different levels of physician involvement. The first group comprises physicians providing primary, direct care to patients, which which consists our specially trained group of 10 hospitalists—including 2 pediatricians, as well as 3 CC and 4 ID consultants. The second group involves consultants from specialties such as nephrology, anesthesia, general surgery, radiology and gynecology; just–in-time training is available for them.
WHY HOSPITALISTS?
Hospitalists are uniquely positioned to care for this distinctive set of patients because they are comfortable with the care of acutely ill patients, many maintain bedside procedural skills, and many have acquired point-of-care-ultrasonography (POCUS) skills. Furthermore, hospitalists usually outnumber available specialists who may be needed to maintain consultative and critical care services for non-BCU patients. We were able to develop a feasible physician staffing model, as described below, and validate hospital medicine’s commitment in addressing institutional needs.
In order to provide ideal unit coverage, 2 hospitalists are scheduled daily and available to respond in case of unit activation. BCU hospitalists are scheduled to cover the BCU when already scheduled to work a clinical shift. In the very infrequent event of BCU activation, BCU hospitalists would move their clinical work to the BCU and a back-up hospitalist would be called in to cover the “other” clinical shift; by overlapping coverage, we ensure BCU hospitalists’ work-life balance and job satisfaction remain intact. Because of expected postexposure monitoring, individual physician’s planned international travel is considered while generating the call schedule. When the unit is activated, the hospital provides payment for extra hours worked by both the BCU hospitalist and the back-up hospitalist, with anticipated revenue recapture through critical care billing by the BCU hospitalist. There are 10 HITeam hospitalists, who are trained and credentialed in bedside procedures and with different levels of POCUS expertise (from literacy to expert level). Team members are expected to attend training in bedside procedures while in PPE 2 times a year. The hospital also provides financial support for hospitalists seeking to further their BCU-related skills and training. It is anticipated that hospitalists will require consultative services from CC and ID in some selected cases to provide a well-rounded care approach to the biocontained patient.
WHAT IT TAKES TO BE A TEAM
Eligibility criteria for HITeam hospitalists include complete initial and quarterly maintenance PPE training; possession of active bedside procedure credentials for at least central line, paracentesis, thoracentesis, and arterial line; demonstration of basic POCUS skills, such as having enough POCUS verbiage literacy to be able to follow a radiologist’s instructions on probe management (ie, a radiologist outside anteroom); and possibility of completing additional training and credentialing on conscious sedation and/or advanced airway management.
Similarly, hospitalists who join the team commit to attendance of at least one National Ebola Training and Education Center (NETEC) provider’s course, representing the Region 8 RESPTC for educational presentations and research at regional and national levels, participation in quarterly HISP multidisciplinary meetings, attendance at quarterly donning and doffing sessions, as well as HITeam training sessions and drills, and active participation in the every-other-month HISP Journal Club/Grand Rounds to maintain competence and knowledge on management of many different pathogens, including Ebola and other filoviruses, MERS-CoV, SARS-CoV, Arenaviruses causing hemorrhagic fevers, Hantavirus, and novel influenzas or coronaviruses.
All of these commitments provide HITeam hospitalists with multiple opportunities for professional growth and development, such as augmenting scholarship venues by participating in collaborative national research projects, participating in national topic networks discussion groups and committees, becoming topic experts, and engaging in diversified training such as advanced airway training and conscious sedation. A new bedside ultrasound machine was purchased for the unit and housed within the division of hospital medicine with the intent to provide hospitalists the means necessary to achieve POCUS proficiency. Above all, by fostering a highly motivated and collegial multidisciplinary team, our model helps develop lasting partnerships at an institutional, regional, and national level.
This multidisciplinary team—with its skillfully trained and engaged nurses, physicians, respiratory therapists, pharmacists, infection control and laboratory specialists—works, learns, trains, and thrives collectively with the aim of providing excellent clinical care to our patients while assuring the safety of the team. DHHA has pioneered a RESPTC physician staffing model led by hospitalists.
We live in an ever-changing landscape of emerging diseases with blurred borders of disease geography. Hospitalists are versatile, capable of managing patients of varying acuity, able to perform many bedside procedures and POCUS; they are champions of interdisciplinary and teamwork disposition. By utilizing the resourcefulness of hospital medicine while helping to ease some of the burden that might otherwise be placed on a smaller numbers of physician groups, this approach provides a unique, cost-effective, and viable physician staffing model, which could be implemented in other BCUs in the United States.
In 2015, and in response to the Ebola virus outbreak in West Africa, the United States Department of Health and Human Services (HHS) designated 10 health departments and associated partner hospitals to become regional treatment centers for patients with highly infectious diseases, such as the Ebola virus and other highly infectious special pathogens (HISPs), and reinforce the nation’s infectious disease response capability. These efforts catalyzed the creation and/or expansion of a network of biocontainment units (BCUs) to safely care for patients diagnosed with highly infectious diseases. These units are designed as special care units with environmental/engineering controls, laboratory capabilities, simple imaging testing, and dedicated staff to allow for the uninterrupted care of patients.1,2 The HHS approach closely resembled the tiered structure of trauma center levels familiar to the healthcare system. The regional framework identified four types of facilities (frontline healthcare facilities, assessment hospitals, treatment centers, and regional Ebola and other special pathogens treatment centers [RESPTCs]) with increasing levels of capabilities and responsibilities.
There are over 4,845 frontline healthcare facilities across the United States, which are able to identify and isolate a patient suspected of a HISP infection and inform local and state partners. The facility provides stabilizing treatment while coordinating the transport of the patient to a specialized center. An assessment hospital can identify and isolate a patient with a HISP, inform partnering agencies, and provide care at the facility for up to 96 hours. There are over 217 hospitals with this designation in the United States. Treatment centers are designated as state or jurisdiction treatment centers and have the capacity to care for HISP-infected patients for the entirety of their care plan, as well as serve as a partner in caring for a potential surge in high-risk patients if their partner RESPTC is unable to care for a patient because of capacity limits. Patients may receive care at a treatment center if and when it is determined to be more appropriate (eg, clinical purview, logistics, resources) than sending them to a RESPTC. There are currently 63 designated treatment centers in the United States.
As outlined by HHS, the RESPTCs3 must be ready to receive a HISP-infected patient within their HHS region, domestically, or internationally within 8 hours. RESPTCs provide care for the entirety of the patient care plan. The 10 regional Departments of Public Health representatives are: Massachusetts (Region 1); New York (Region 2); Maryland (Region 3); Georgia (Region 4); Minnesota (Region 5); Texas (Region 6); Nebraska (Region 7); Colorado in partnership with Denver Health Hospital Authority (DHHA; Region 8); California (Region 9); and Washington State (Region 10).
BCU PHYSICIAN STAFFING MODELS
Most RESPCTs BCUs are staffed by a self-selected group of core providers with expertise in infectious diseases (ID) and critical care (CC). Teams are interdisciplinary and committed to a culture of safety.4-7 ID physicians are experts in HISP disease processes and epidemiology, which enables expert guidance on patient care and infection control. CC physicians are trained to provide care to patients requiring life-saving interventions.
DHHA STAFFING MODEL
As specialized units are shifting to include high-risk infectious diseases beyond Ebola, hospitals are developing innovative ways to manage a potential surge of patients with respiratory pathogens, expanding care far beyond the biocontainment unit. With the potential for an influx of HISPs in the healthcare setting, identifying stakeholders uniquely equipped to provide care in all areas of the hospital is ideal. At DHHA, the BCU physician staffing model transitioned from ID and CC physicians to a selected group of ten hospitalists as the primary managing service in 2018.
When DHHA received the RESPTC nomination, it developed a fully voluntary multidisciplinary high-risk infection team (HITeam; Table) with specialized training in personal protective equipment (PPE) donning and doffing, as well as BCU protocols. Our HITeam members participate in every-6-weeks mandatory team drills that involve practicing team dynamics and dexterity while wearing PPE. Team members participate in regular hospital and/or BCU-focused exercises to simulate and practice real-world experiences. They also participate in HISP Journal Club, where members from different disciplines discuss pertinent articles in the field, as well as every-other-month team-building meetings. Our unit’s main challenges included maintaining competencies and staff retention. We developed a unique staffing model aimed at mitigating some of these challenges through a multidisciplinary team approach utilizing different levels of physician involvement. The first group comprises physicians providing primary, direct care to patients, which which consists our specially trained group of 10 hospitalists—including 2 pediatricians, as well as 3 CC and 4 ID consultants. The second group involves consultants from specialties such as nephrology, anesthesia, general surgery, radiology and gynecology; just–in-time training is available for them.
WHY HOSPITALISTS?
Hospitalists are uniquely positioned to care for this distinctive set of patients because they are comfortable with the care of acutely ill patients, many maintain bedside procedural skills, and many have acquired point-of-care-ultrasonography (POCUS) skills. Furthermore, hospitalists usually outnumber available specialists who may be needed to maintain consultative and critical care services for non-BCU patients. We were able to develop a feasible physician staffing model, as described below, and validate hospital medicine’s commitment in addressing institutional needs.
In order to provide ideal unit coverage, 2 hospitalists are scheduled daily and available to respond in case of unit activation. BCU hospitalists are scheduled to cover the BCU when already scheduled to work a clinical shift. In the very infrequent event of BCU activation, BCU hospitalists would move their clinical work to the BCU and a back-up hospitalist would be called in to cover the “other” clinical shift; by overlapping coverage, we ensure BCU hospitalists’ work-life balance and job satisfaction remain intact. Because of expected postexposure monitoring, individual physician’s planned international travel is considered while generating the call schedule. When the unit is activated, the hospital provides payment for extra hours worked by both the BCU hospitalist and the back-up hospitalist, with anticipated revenue recapture through critical care billing by the BCU hospitalist. There are 10 HITeam hospitalists, who are trained and credentialed in bedside procedures and with different levels of POCUS expertise (from literacy to expert level). Team members are expected to attend training in bedside procedures while in PPE 2 times a year. The hospital also provides financial support for hospitalists seeking to further their BCU-related skills and training. It is anticipated that hospitalists will require consultative services from CC and ID in some selected cases to provide a well-rounded care approach to the biocontained patient.
WHAT IT TAKES TO BE A TEAM
Eligibility criteria for HITeam hospitalists include complete initial and quarterly maintenance PPE training; possession of active bedside procedure credentials for at least central line, paracentesis, thoracentesis, and arterial line; demonstration of basic POCUS skills, such as having enough POCUS verbiage literacy to be able to follow a radiologist’s instructions on probe management (ie, a radiologist outside anteroom); and possibility of completing additional training and credentialing on conscious sedation and/or advanced airway management.
Similarly, hospitalists who join the team commit to attendance of at least one National Ebola Training and Education Center (NETEC) provider’s course, representing the Region 8 RESPTC for educational presentations and research at regional and national levels, participation in quarterly HISP multidisciplinary meetings, attendance at quarterly donning and doffing sessions, as well as HITeam training sessions and drills, and active participation in the every-other-month HISP Journal Club/Grand Rounds to maintain competence and knowledge on management of many different pathogens, including Ebola and other filoviruses, MERS-CoV, SARS-CoV, Arenaviruses causing hemorrhagic fevers, Hantavirus, and novel influenzas or coronaviruses.
All of these commitments provide HITeam hospitalists with multiple opportunities for professional growth and development, such as augmenting scholarship venues by participating in collaborative national research projects, participating in national topic networks discussion groups and committees, becoming topic experts, and engaging in diversified training such as advanced airway training and conscious sedation. A new bedside ultrasound machine was purchased for the unit and housed within the division of hospital medicine with the intent to provide hospitalists the means necessary to achieve POCUS proficiency. Above all, by fostering a highly motivated and collegial multidisciplinary team, our model helps develop lasting partnerships at an institutional, regional, and national level.
This multidisciplinary team—with its skillfully trained and engaged nurses, physicians, respiratory therapists, pharmacists, infection control and laboratory specialists—works, learns, trains, and thrives collectively with the aim of providing excellent clinical care to our patients while assuring the safety of the team. DHHA has pioneered a RESPTC physician staffing model led by hospitalists.
We live in an ever-changing landscape of emerging diseases with blurred borders of disease geography. Hospitalists are versatile, capable of managing patients of varying acuity, able to perform many bedside procedures and POCUS; they are champions of interdisciplinary and teamwork disposition. By utilizing the resourcefulness of hospital medicine while helping to ease some of the burden that might otherwise be placed on a smaller numbers of physician groups, this approach provides a unique, cost-effective, and viable physician staffing model, which could be implemented in other BCUs in the United States.
1. Smith PW, Anderson AO, Christopher GW, et al. Designing a biocontainment unit to care for patients with serious communicable diseases: a consensus statement. Biosecur Bioterror. 2006;4(4):351-65. https://doi.org/10.1089/bsp.2006.4.351.
2. Bannister B, Puro V, Fusco FM, Heptonstall J, Ippolito G; EUNID Working Group. Framework for the design and operation of high-level isolation units: consensus of the European Network of Infectious Diseases. Lancet Infect Dis. 2009;9(1):45-56. https://doi.org/10.1016/S1473-3099(08)70304-9.
3. US Department of Health and Human Services. Office of the Assistant Secretary for preparedness and Response Regional Treatment Network for Ebola and Other Special Pathogens. November 2017 Report. https://www.phe.gov/Preparedness/planning/hpp/reports/Documents/RETN-Ebola-Report-508.pdf Accessed August 17, 2019.
4. Vasa A, Schwedhelm M, Johnson, D. Critical care for the patient with Ebola virus disease: the Nebraska perspective. J Intensive Crit Care. 2015;1(8):1-5.
5. Garibaldi BT, Kelen GD, Brower RG, et al. The creation of a biocontainment unit at a tertiary care hospital. The Johns Hopkins medicine experience. Ann Am Thorac Soc. 2016;13(5):600-608. https://doi.org/10.1513/AnnalsATS.201509-587PS.
6. Beam EL, Boulter KC, Freihaut F, Schwedhelm S, Smith PW. The Nebraska experience in biocontainment patient care. Public Health Nurs. 2010 Mar-Apr;27(2):140-7. https://doi.org/10.1111/j.1525-1446.2010.00837.x.
7. Hewlett AL, Varkey JB, Smith PW, Ribner BS. Ebola virus disease: preparedness and infection control lessons learned from two biocontainment units. Curr Opin Infect Dis. 2015 Aug;28(4):343-8. https://doi.org/10.1097/QCO.0000000000000176.
1. Smith PW, Anderson AO, Christopher GW, et al. Designing a biocontainment unit to care for patients with serious communicable diseases: a consensus statement. Biosecur Bioterror. 2006;4(4):351-65. https://doi.org/10.1089/bsp.2006.4.351.
2. Bannister B, Puro V, Fusco FM, Heptonstall J, Ippolito G; EUNID Working Group. Framework for the design and operation of high-level isolation units: consensus of the European Network of Infectious Diseases. Lancet Infect Dis. 2009;9(1):45-56. https://doi.org/10.1016/S1473-3099(08)70304-9.
3. US Department of Health and Human Services. Office of the Assistant Secretary for preparedness and Response Regional Treatment Network for Ebola and Other Special Pathogens. November 2017 Report. https://www.phe.gov/Preparedness/planning/hpp/reports/Documents/RETN-Ebola-Report-508.pdf Accessed August 17, 2019.
4. Vasa A, Schwedhelm M, Johnson, D. Critical care for the patient with Ebola virus disease: the Nebraska perspective. J Intensive Crit Care. 2015;1(8):1-5.
5. Garibaldi BT, Kelen GD, Brower RG, et al. The creation of a biocontainment unit at a tertiary care hospital. The Johns Hopkins medicine experience. Ann Am Thorac Soc. 2016;13(5):600-608. https://doi.org/10.1513/AnnalsATS.201509-587PS.
6. Beam EL, Boulter KC, Freihaut F, Schwedhelm S, Smith PW. The Nebraska experience in biocontainment patient care. Public Health Nurs. 2010 Mar-Apr;27(2):140-7. https://doi.org/10.1111/j.1525-1446.2010.00837.x.
7. Hewlett AL, Varkey JB, Smith PW, Ribner BS. Ebola virus disease: preparedness and infection control lessons learned from two biocontainment units. Curr Opin Infect Dis. 2015 Aug;28(4):343-8. https://doi.org/10.1097/QCO.0000000000000176.
© 2020 Society of Hospital Medicine
Secure Text Messaging in Healthcare: Latent Threats and Opportunities to Improve Patient Safety
UNINTENDED CONSEQUENCES
Over the past two decades, physicians and nurses practicing in hospital settings have faced an onslaught of challenges in communication, an area frequently cited as critical to providing safe and effective care to patients.1-3 Communication needs have increased significantly as hospitalized patients have become more acute, complex, and technology-dependent, requiring larger healthcare teams comprising subspecialists across multiple disciplines spread across increasingly larger inpatient facilities.4 During this same period, the evolution of mobile phones has led to dramatic shifts in personal communication patterns, with asynchronous text messaging replacing verbal communication.5-7
In response to both the changing communication needs of clinicians and shifting cultural conventions, healthcare systems and providers alike have viewed text messaging as a solution to these growing communication problems. In fact, an entire industry has developed around “secure” and “Health Insurance Portability and Accountability Act (HIPAA)-compliant” text messaging platforms, which we will refer to below as secure text messaging systems (STMS). These systems offer benefits over carrier-based text messaging given their focus on the healthcare environment and HIPAA compliance. However, hospitals’ rapid adoption of these systems has outpaced our abilities to surveil, recognize, and understand the unintended consequences of transitioning to STMS communication in the hospital setting where failures in communication can be catastrophic. Below, we highlight three critical areas of concern encountered at our institutions and offer five potential mitigating strategies (Table).
CRITICAL AREAS OF CONCERN
Text Messaging is a Form of Alarm Fatigue
Text messaging renders clinicians vulnerable to a unique form of alarm fatigue. The burden of alarm fatigue has been well described in the literature and applies to interruptions to workflow in the electronic medical record and sensory alerts in clinical settings.8,9 Text messaging serves as yet another interruption for healthcare providers. Without a framework to triage urgent versus nonurgent messages, a clinician can become inundated with information and miss critical messages. This can lead to delayed or incorrect responses and impede patient care. System design and implementation can also contribute to this phenomenon. For example, a text message analysis at one center identified how system and workflow design resulted in all messages to an intensive care unit team being routed to a single physician’s phone.10 This design left the singular physician at risk of information and task overload and at the mercy of endless interruptive alerts. Although this can occur with any communication system, it has been well demonstrated that adopting STMS correlates with an increased frequency of messaging, leading to an increase in interruptive alerts, which may have implications for patient safety.11 This type of systems failure is silent unless proactively identified or revealed through a retrospective review of a resulting safety event.
Text Messaging Inappropriately Replaces Critical Communications that Should Happen in Person or by Phone
Text messaging has de-emphasized interpersonal communication skills and behaviors critical for quality and safety in hospital-based care. This concern emerges alongside evidence suggesting that new generations of physician trainees have profoundly different communication habits, preferences, and skillsets based on their experience in a text-heavy, asynchronous world of communication.12 There is reason to worry that reliance on text messaging in healthcare leads to similar alterations in relationships and collaboration as it has in our broader cultural context.13 Academic medical centers in particular should attempt to mitigate the loss of profound and formative learning that occurs during face-to-face encounters between providers of different disciplines, experience levels, and specialties.
Text Messaging Increases the Risk of Communication Error
Finally, text messaging appears to be highly vulnerable to communication errors in the healthcare setting. Prior work emphasizes the importance of nonverbal communication in face-to-face and even voice-to-voice interactions, highlighting the loss of fidelity when using text-only methods to communicate.1 Furthermore, the asynchronous nature of text messaging grants little room for clarification of minor misunderstandings that often arise in text-only communication through minor alterations in punctuation or automatic spelling corrections, a frequent occurrence when using medical terminology. Although a seasoned physician may be able to piece together the issues that deserve further clarification, young residents may be more hesitant to ask clarifying questions and determine the right course of action due to clinical inexperience.
PROPOSED SOLUTIONS
Deliberate Design and Implementation
A recent systematic review identified a lack of high-quality evidence evaluating the impact of mobile technologies on communication and teamwork in hospital settings.14 This paucity of understanding renders communication via STMS in the healthcare setting uniquely vulnerable to latent safety threats unless the design and implementation of these systems are purposeful and proactive.
These concerns led us to postulate that deliberate and proactive implementation of these systems, rather than passive adoption, is needed in the healthcare environment. We propose a number of approaches and interventions that may guide institutions as they seek to implement STMS or redesign communication in the inpatient setting. At the core of these proposals lies an important tension: can implementation of STMS occur in isolation or should the arrival of these systems prompt an overhaul of an institution’s clinical communication system and culture?15
Proactive Surveillance
Surveillance is one proactive method for healthcare systems to understand where and how the implementation of STMS might lead to safety threats. From a quantitative standpoint, understanding the burden of messaging for each user across the system can reveal the clinical roles in the system that are particularly vulnerable to alert fatigue or information overload. Quality assurance monitoring of critical roles in the hospital (ie, airway emergency team, rapid response teams) could be conducted to ensure accurate directory listings at all times. Associating conversations with events, from serious safety events to near misses, could help leaders understand when and how text messaging contributes to safety events and create actionable learnings for safety learning systems.
Standardized Communication
A standardized language eliminates the burden of individuals to parse and translate each individual text message. A standardized algorithm for language, urgency, and expectations (ie, response before escalation) would help define the interaction in the clinical setting.16 Moving toward standardized, meaningful “quick messages,” one of our centers has implemented a campaign to “stick to the FACS,” where the following four standard quick messages are available for users: (1) “FYI no response needed,” (2) “ACTION needed within X min,” (3) “CONCERN can we talk or meet,” and (4) “STAT immediate response required.” These quick messages, developed with frontline stakeholders, represent the majority of requests exchanged by providers, and help standardize expectations and task prioritization.
Targeted Training
Targeted training and culture change efforts might help institutions counteract the broader impact of asynchronous messaging on communication skills and behaviors. Highlighting the contrast between clinical and casual communication with an emphasis on examples, scenarios, or role-playing has the potential to emphasize why and how clinical communication with STMS requires a careful, deliberate approach. For instance, safety culture training at one of our institutions features a scenario that illustrates the potential for miscommunication and missed connection between a nurse and a physician on the wards. The scenario gives way to discussion between participants about the shortcomings of text messaging and allows the facilitator to segue into the “dos and don’ts” of text messaging and when a phone call might be more appropriate.
Innovate
Finally, creatively harnessing the technology and data underlying these STMS may uncover methods to identify and mitigate communication errors in real time. For instance, using trigger methods to create a “ripple in the pond,” whereby a floor nurse reaching out with an urgent text automatically loops in the charge nurse of the unit. Building a chatbot or a virtual assistant functionality by leveraging user behavior patterns and natural language processing to provide text-based guidance to users might help busy clinicians connect to the key decision-makers on their team. For example, in response to an unanswered text, a virtual assistant might reach out to the waiting provider as follows: “you texted the resident 20 minutes ago and they haven’t replied, would you like to call the fellow instead?” The data-rich nature of these systems implies that they are ripe for automated solutions that can respond to behavioral- or text-based patterns to augment the existing operation and safety infrastructure.
CONCLUSION
The transition of healthcare communication systems toward STMS is already well underway. These systems, despite their flaws, are undoubtedly an improvement over legacy paging systems and, if properly implemented, offer several benefits to large healthcare systems. However, the communication needs in the healthcare setting are vastly different from the personal communication needs in everyday text messaging. As clinicians at the forefront of these transitions, we have the opportunity to critically assess the unique communication requirements in our hospital settings and help shape the way STMS are implemented in our hospitals. Pausing to deliberate about the limitations and the vulnerabilities of the current messaging systems for our acute clinical needs, including how they impact training and education, will allow us to proactively design and implement better communication systems that improve patient safety.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019.
2. Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: The nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11(2):104-112. https://doi.org/10.1197/jamia.M1471.
3. Coiera E. When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277-286. https://doi.org/10.1136/jamia.2000.0070277.
4. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. https://doi.org/10.1542/peds.2009-3266.
5. The Nielsen Company. In U.S., SMS Text Messaging Tops Mobile Phone Calling. https://www.nielsen.com/us/en/insights/article/2008/in-us-text-messaging-tops-mobile-phone-calling/. Accessed July 22, 2019.
6. The Nielsen Company. New Mobile Obsession in U.S. Teens Triple Data Usage. The Nielsen Company. Published 2011. Accessed July 22, 2019.
7. The Nielsen Company. U.S. Teen Mobile Report Calling Yesterday, Texting Today, Using Apps Tomorrow. The Nielsen Company. https://www.nielsen.com/us/en/insights/article/2010/u-s-teen-mobile-report-calling-yesterday-texting-today-using-apps-tomorrow/. Accessed July 22, 2019.
8. Sendelbach S, Funk M. Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378-386; quiz 387-378.
9. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136-144. https://doi.org/10.1002/jhm.2520.
10. Hagedorn PA, Kirkendall ES, Spooner SA, Mohan V. Inpatient communication networks: leveraging secure text-messaging platforms to gain insight into inpatient communication systems. Appl Clin Inform. 2019;10(3):471-478. https://doi.org/10.1055/s-0039-1692401.
11. Westbrook JI, Coiera E, Dunsmuir WT, et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284-289. https://doi.org/10.1136/qshc.2009.039255.
12. Castells M. The Rise of the Network Society. 2nd ed. Malden, MA: Wiley-Blackwell; 2010.
13. Lo V, Wu RC, Morra D, Lee L, Reeves S. The use of smartphones in general and internal medicine units: A boon or a bane to the promotion of interprofessional collaboration? J Interprof Care. 2012;26(4):276-282. https://doi.org/10.3109/13561820.2012.663013.
14. Martin G, Khajuria A, Arora S, King D, Ashrafian H, Darzi A. The impact of mobile technology on teamwork and communication in hospitals: a systematic review. J Am Med Inform Assn. 2019;26(4):339-355. https://doi.org/10.1093/jamia/ocy175.
15. Liu X, Sutton PR, McKenna R, et al. Evaluation of secure messaging applications for a health care system: a case study. Appl Clin Inform. 2019;10(1):140-150. https://doi.org/10.1055/s-0039-1678607.
16. Weigert RM, Schmitz AH, Soung PJ, Porada K, Weisgerber MC. Improving standardization of paging communication using quality improvement methodology. Pediatrics. 2019;143(4). https://doi.org/10.1542/peds.2018-1362.
UNINTENDED CONSEQUENCES
Over the past two decades, physicians and nurses practicing in hospital settings have faced an onslaught of challenges in communication, an area frequently cited as critical to providing safe and effective care to patients.1-3 Communication needs have increased significantly as hospitalized patients have become more acute, complex, and technology-dependent, requiring larger healthcare teams comprising subspecialists across multiple disciplines spread across increasingly larger inpatient facilities.4 During this same period, the evolution of mobile phones has led to dramatic shifts in personal communication patterns, with asynchronous text messaging replacing verbal communication.5-7
In response to both the changing communication needs of clinicians and shifting cultural conventions, healthcare systems and providers alike have viewed text messaging as a solution to these growing communication problems. In fact, an entire industry has developed around “secure” and “Health Insurance Portability and Accountability Act (HIPAA)-compliant” text messaging platforms, which we will refer to below as secure text messaging systems (STMS). These systems offer benefits over carrier-based text messaging given their focus on the healthcare environment and HIPAA compliance. However, hospitals’ rapid adoption of these systems has outpaced our abilities to surveil, recognize, and understand the unintended consequences of transitioning to STMS communication in the hospital setting where failures in communication can be catastrophic. Below, we highlight three critical areas of concern encountered at our institutions and offer five potential mitigating strategies (Table).
CRITICAL AREAS OF CONCERN
Text Messaging is a Form of Alarm Fatigue
Text messaging renders clinicians vulnerable to a unique form of alarm fatigue. The burden of alarm fatigue has been well described in the literature and applies to interruptions to workflow in the electronic medical record and sensory alerts in clinical settings.8,9 Text messaging serves as yet another interruption for healthcare providers. Without a framework to triage urgent versus nonurgent messages, a clinician can become inundated with information and miss critical messages. This can lead to delayed or incorrect responses and impede patient care. System design and implementation can also contribute to this phenomenon. For example, a text message analysis at one center identified how system and workflow design resulted in all messages to an intensive care unit team being routed to a single physician’s phone.10 This design left the singular physician at risk of information and task overload and at the mercy of endless interruptive alerts. Although this can occur with any communication system, it has been well demonstrated that adopting STMS correlates with an increased frequency of messaging, leading to an increase in interruptive alerts, which may have implications for patient safety.11 This type of systems failure is silent unless proactively identified or revealed through a retrospective review of a resulting safety event.
Text Messaging Inappropriately Replaces Critical Communications that Should Happen in Person or by Phone
Text messaging has de-emphasized interpersonal communication skills and behaviors critical for quality and safety in hospital-based care. This concern emerges alongside evidence suggesting that new generations of physician trainees have profoundly different communication habits, preferences, and skillsets based on their experience in a text-heavy, asynchronous world of communication.12 There is reason to worry that reliance on text messaging in healthcare leads to similar alterations in relationships and collaboration as it has in our broader cultural context.13 Academic medical centers in particular should attempt to mitigate the loss of profound and formative learning that occurs during face-to-face encounters between providers of different disciplines, experience levels, and specialties.
Text Messaging Increases the Risk of Communication Error
Finally, text messaging appears to be highly vulnerable to communication errors in the healthcare setting. Prior work emphasizes the importance of nonverbal communication in face-to-face and even voice-to-voice interactions, highlighting the loss of fidelity when using text-only methods to communicate.1 Furthermore, the asynchronous nature of text messaging grants little room for clarification of minor misunderstandings that often arise in text-only communication through minor alterations in punctuation or automatic spelling corrections, a frequent occurrence when using medical terminology. Although a seasoned physician may be able to piece together the issues that deserve further clarification, young residents may be more hesitant to ask clarifying questions and determine the right course of action due to clinical inexperience.
PROPOSED SOLUTIONS
Deliberate Design and Implementation
A recent systematic review identified a lack of high-quality evidence evaluating the impact of mobile technologies on communication and teamwork in hospital settings.14 This paucity of understanding renders communication via STMS in the healthcare setting uniquely vulnerable to latent safety threats unless the design and implementation of these systems are purposeful and proactive.
These concerns led us to postulate that deliberate and proactive implementation of these systems, rather than passive adoption, is needed in the healthcare environment. We propose a number of approaches and interventions that may guide institutions as they seek to implement STMS or redesign communication in the inpatient setting. At the core of these proposals lies an important tension: can implementation of STMS occur in isolation or should the arrival of these systems prompt an overhaul of an institution’s clinical communication system and culture?15
Proactive Surveillance
Surveillance is one proactive method for healthcare systems to understand where and how the implementation of STMS might lead to safety threats. From a quantitative standpoint, understanding the burden of messaging for each user across the system can reveal the clinical roles in the system that are particularly vulnerable to alert fatigue or information overload. Quality assurance monitoring of critical roles in the hospital (ie, airway emergency team, rapid response teams) could be conducted to ensure accurate directory listings at all times. Associating conversations with events, from serious safety events to near misses, could help leaders understand when and how text messaging contributes to safety events and create actionable learnings for safety learning systems.
Standardized Communication
A standardized language eliminates the burden of individuals to parse and translate each individual text message. A standardized algorithm for language, urgency, and expectations (ie, response before escalation) would help define the interaction in the clinical setting.16 Moving toward standardized, meaningful “quick messages,” one of our centers has implemented a campaign to “stick to the FACS,” where the following four standard quick messages are available for users: (1) “FYI no response needed,” (2) “ACTION needed within X min,” (3) “CONCERN can we talk or meet,” and (4) “STAT immediate response required.” These quick messages, developed with frontline stakeholders, represent the majority of requests exchanged by providers, and help standardize expectations and task prioritization.
Targeted Training
Targeted training and culture change efforts might help institutions counteract the broader impact of asynchronous messaging on communication skills and behaviors. Highlighting the contrast between clinical and casual communication with an emphasis on examples, scenarios, or role-playing has the potential to emphasize why and how clinical communication with STMS requires a careful, deliberate approach. For instance, safety culture training at one of our institutions features a scenario that illustrates the potential for miscommunication and missed connection between a nurse and a physician on the wards. The scenario gives way to discussion between participants about the shortcomings of text messaging and allows the facilitator to segue into the “dos and don’ts” of text messaging and when a phone call might be more appropriate.
Innovate
Finally, creatively harnessing the technology and data underlying these STMS may uncover methods to identify and mitigate communication errors in real time. For instance, using trigger methods to create a “ripple in the pond,” whereby a floor nurse reaching out with an urgent text automatically loops in the charge nurse of the unit. Building a chatbot or a virtual assistant functionality by leveraging user behavior patterns and natural language processing to provide text-based guidance to users might help busy clinicians connect to the key decision-makers on their team. For example, in response to an unanswered text, a virtual assistant might reach out to the waiting provider as follows: “you texted the resident 20 minutes ago and they haven’t replied, would you like to call the fellow instead?” The data-rich nature of these systems implies that they are ripe for automated solutions that can respond to behavioral- or text-based patterns to augment the existing operation and safety infrastructure.
CONCLUSION
The transition of healthcare communication systems toward STMS is already well underway. These systems, despite their flaws, are undoubtedly an improvement over legacy paging systems and, if properly implemented, offer several benefits to large healthcare systems. However, the communication needs in the healthcare setting are vastly different from the personal communication needs in everyday text messaging. As clinicians at the forefront of these transitions, we have the opportunity to critically assess the unique communication requirements in our hospital settings and help shape the way STMS are implemented in our hospitals. Pausing to deliberate about the limitations and the vulnerabilities of the current messaging systems for our acute clinical needs, including how they impact training and education, will allow us to proactively design and implement better communication systems that improve patient safety.
UNINTENDED CONSEQUENCES
Over the past two decades, physicians and nurses practicing in hospital settings have faced an onslaught of challenges in communication, an area frequently cited as critical to providing safe and effective care to patients.1-3 Communication needs have increased significantly as hospitalized patients have become more acute, complex, and technology-dependent, requiring larger healthcare teams comprising subspecialists across multiple disciplines spread across increasingly larger inpatient facilities.4 During this same period, the evolution of mobile phones has led to dramatic shifts in personal communication patterns, with asynchronous text messaging replacing verbal communication.5-7
In response to both the changing communication needs of clinicians and shifting cultural conventions, healthcare systems and providers alike have viewed text messaging as a solution to these growing communication problems. In fact, an entire industry has developed around “secure” and “Health Insurance Portability and Accountability Act (HIPAA)-compliant” text messaging platforms, which we will refer to below as secure text messaging systems (STMS). These systems offer benefits over carrier-based text messaging given their focus on the healthcare environment and HIPAA compliance. However, hospitals’ rapid adoption of these systems has outpaced our abilities to surveil, recognize, and understand the unintended consequences of transitioning to STMS communication in the hospital setting where failures in communication can be catastrophic. Below, we highlight three critical areas of concern encountered at our institutions and offer five potential mitigating strategies (Table).
CRITICAL AREAS OF CONCERN
Text Messaging is a Form of Alarm Fatigue
Text messaging renders clinicians vulnerable to a unique form of alarm fatigue. The burden of alarm fatigue has been well described in the literature and applies to interruptions to workflow in the electronic medical record and sensory alerts in clinical settings.8,9 Text messaging serves as yet another interruption for healthcare providers. Without a framework to triage urgent versus nonurgent messages, a clinician can become inundated with information and miss critical messages. This can lead to delayed or incorrect responses and impede patient care. System design and implementation can also contribute to this phenomenon. For example, a text message analysis at one center identified how system and workflow design resulted in all messages to an intensive care unit team being routed to a single physician’s phone.10 This design left the singular physician at risk of information and task overload and at the mercy of endless interruptive alerts. Although this can occur with any communication system, it has been well demonstrated that adopting STMS correlates with an increased frequency of messaging, leading to an increase in interruptive alerts, which may have implications for patient safety.11 This type of systems failure is silent unless proactively identified or revealed through a retrospective review of a resulting safety event.
Text Messaging Inappropriately Replaces Critical Communications that Should Happen in Person or by Phone
Text messaging has de-emphasized interpersonal communication skills and behaviors critical for quality and safety in hospital-based care. This concern emerges alongside evidence suggesting that new generations of physician trainees have profoundly different communication habits, preferences, and skillsets based on their experience in a text-heavy, asynchronous world of communication.12 There is reason to worry that reliance on text messaging in healthcare leads to similar alterations in relationships and collaboration as it has in our broader cultural context.13 Academic medical centers in particular should attempt to mitigate the loss of profound and formative learning that occurs during face-to-face encounters between providers of different disciplines, experience levels, and specialties.
Text Messaging Increases the Risk of Communication Error
Finally, text messaging appears to be highly vulnerable to communication errors in the healthcare setting. Prior work emphasizes the importance of nonverbal communication in face-to-face and even voice-to-voice interactions, highlighting the loss of fidelity when using text-only methods to communicate.1 Furthermore, the asynchronous nature of text messaging grants little room for clarification of minor misunderstandings that often arise in text-only communication through minor alterations in punctuation or automatic spelling corrections, a frequent occurrence when using medical terminology. Although a seasoned physician may be able to piece together the issues that deserve further clarification, young residents may be more hesitant to ask clarifying questions and determine the right course of action due to clinical inexperience.
PROPOSED SOLUTIONS
Deliberate Design and Implementation
A recent systematic review identified a lack of high-quality evidence evaluating the impact of mobile technologies on communication and teamwork in hospital settings.14 This paucity of understanding renders communication via STMS in the healthcare setting uniquely vulnerable to latent safety threats unless the design and implementation of these systems are purposeful and proactive.
These concerns led us to postulate that deliberate and proactive implementation of these systems, rather than passive adoption, is needed in the healthcare environment. We propose a number of approaches and interventions that may guide institutions as they seek to implement STMS or redesign communication in the inpatient setting. At the core of these proposals lies an important tension: can implementation of STMS occur in isolation or should the arrival of these systems prompt an overhaul of an institution’s clinical communication system and culture?15
Proactive Surveillance
Surveillance is one proactive method for healthcare systems to understand where and how the implementation of STMS might lead to safety threats. From a quantitative standpoint, understanding the burden of messaging for each user across the system can reveal the clinical roles in the system that are particularly vulnerable to alert fatigue or information overload. Quality assurance monitoring of critical roles in the hospital (ie, airway emergency team, rapid response teams) could be conducted to ensure accurate directory listings at all times. Associating conversations with events, from serious safety events to near misses, could help leaders understand when and how text messaging contributes to safety events and create actionable learnings for safety learning systems.
Standardized Communication
A standardized language eliminates the burden of individuals to parse and translate each individual text message. A standardized algorithm for language, urgency, and expectations (ie, response before escalation) would help define the interaction in the clinical setting.16 Moving toward standardized, meaningful “quick messages,” one of our centers has implemented a campaign to “stick to the FACS,” where the following four standard quick messages are available for users: (1) “FYI no response needed,” (2) “ACTION needed within X min,” (3) “CONCERN can we talk or meet,” and (4) “STAT immediate response required.” These quick messages, developed with frontline stakeholders, represent the majority of requests exchanged by providers, and help standardize expectations and task prioritization.
Targeted Training
Targeted training and culture change efforts might help institutions counteract the broader impact of asynchronous messaging on communication skills and behaviors. Highlighting the contrast between clinical and casual communication with an emphasis on examples, scenarios, or role-playing has the potential to emphasize why and how clinical communication with STMS requires a careful, deliberate approach. For instance, safety culture training at one of our institutions features a scenario that illustrates the potential for miscommunication and missed connection between a nurse and a physician on the wards. The scenario gives way to discussion between participants about the shortcomings of text messaging and allows the facilitator to segue into the “dos and don’ts” of text messaging and when a phone call might be more appropriate.
Innovate
Finally, creatively harnessing the technology and data underlying these STMS may uncover methods to identify and mitigate communication errors in real time. For instance, using trigger methods to create a “ripple in the pond,” whereby a floor nurse reaching out with an urgent text automatically loops in the charge nurse of the unit. Building a chatbot or a virtual assistant functionality by leveraging user behavior patterns and natural language processing to provide text-based guidance to users might help busy clinicians connect to the key decision-makers on their team. For example, in response to an unanswered text, a virtual assistant might reach out to the waiting provider as follows: “you texted the resident 20 minutes ago and they haven’t replied, would you like to call the fellow instead?” The data-rich nature of these systems implies that they are ripe for automated solutions that can respond to behavioral- or text-based patterns to augment the existing operation and safety infrastructure.
CONCLUSION
The transition of healthcare communication systems toward STMS is already well underway. These systems, despite their flaws, are undoubtedly an improvement over legacy paging systems and, if properly implemented, offer several benefits to large healthcare systems. However, the communication needs in the healthcare setting are vastly different from the personal communication needs in everyday text messaging. As clinicians at the forefront of these transitions, we have the opportunity to critically assess the unique communication requirements in our hospital settings and help shape the way STMS are implemented in our hospitals. Pausing to deliberate about the limitations and the vulnerabilities of the current messaging systems for our acute clinical needs, including how they impact training and education, will allow us to proactively design and implement better communication systems that improve patient safety.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019.
2. Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: The nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11(2):104-112. https://doi.org/10.1197/jamia.M1471.
3. Coiera E. When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277-286. https://doi.org/10.1136/jamia.2000.0070277.
4. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. https://doi.org/10.1542/peds.2009-3266.
5. The Nielsen Company. In U.S., SMS Text Messaging Tops Mobile Phone Calling. https://www.nielsen.com/us/en/insights/article/2008/in-us-text-messaging-tops-mobile-phone-calling/. Accessed July 22, 2019.
6. The Nielsen Company. New Mobile Obsession in U.S. Teens Triple Data Usage. The Nielsen Company. Published 2011. Accessed July 22, 2019.
7. The Nielsen Company. U.S. Teen Mobile Report Calling Yesterday, Texting Today, Using Apps Tomorrow. The Nielsen Company. https://www.nielsen.com/us/en/insights/article/2010/u-s-teen-mobile-report-calling-yesterday-texting-today-using-apps-tomorrow/. Accessed July 22, 2019.
8. Sendelbach S, Funk M. Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378-386; quiz 387-378.
9. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136-144. https://doi.org/10.1002/jhm.2520.
10. Hagedorn PA, Kirkendall ES, Spooner SA, Mohan V. Inpatient communication networks: leveraging secure text-messaging platforms to gain insight into inpatient communication systems. Appl Clin Inform. 2019;10(3):471-478. https://doi.org/10.1055/s-0039-1692401.
11. Westbrook JI, Coiera E, Dunsmuir WT, et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284-289. https://doi.org/10.1136/qshc.2009.039255.
12. Castells M. The Rise of the Network Society. 2nd ed. Malden, MA: Wiley-Blackwell; 2010.
13. Lo V, Wu RC, Morra D, Lee L, Reeves S. The use of smartphones in general and internal medicine units: A boon or a bane to the promotion of interprofessional collaboration? J Interprof Care. 2012;26(4):276-282. https://doi.org/10.3109/13561820.2012.663013.
14. Martin G, Khajuria A, Arora S, King D, Ashrafian H, Darzi A. The impact of mobile technology on teamwork and communication in hospitals: a systematic review. J Am Med Inform Assn. 2019;26(4):339-355. https://doi.org/10.1093/jamia/ocy175.
15. Liu X, Sutton PR, McKenna R, et al. Evaluation of secure messaging applications for a health care system: a case study. Appl Clin Inform. 2019;10(1):140-150. https://doi.org/10.1055/s-0039-1678607.
16. Weigert RM, Schmitz AH, Soung PJ, Porada K, Weisgerber MC. Improving standardization of paging communication using quality improvement methodology. Pediatrics. 2019;143(4). https://doi.org/10.1542/peds.2018-1362.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019.
2. Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: The nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11(2):104-112. https://doi.org/10.1197/jamia.M1471.
3. Coiera E. When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277-286. https://doi.org/10.1136/jamia.2000.0070277.
4. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. https://doi.org/10.1542/peds.2009-3266.
5. The Nielsen Company. In U.S., SMS Text Messaging Tops Mobile Phone Calling. https://www.nielsen.com/us/en/insights/article/2008/in-us-text-messaging-tops-mobile-phone-calling/. Accessed July 22, 2019.
6. The Nielsen Company. New Mobile Obsession in U.S. Teens Triple Data Usage. The Nielsen Company. Published 2011. Accessed July 22, 2019.
7. The Nielsen Company. U.S. Teen Mobile Report Calling Yesterday, Texting Today, Using Apps Tomorrow. The Nielsen Company. https://www.nielsen.com/us/en/insights/article/2010/u-s-teen-mobile-report-calling-yesterday-texting-today-using-apps-tomorrow/. Accessed July 22, 2019.
8. Sendelbach S, Funk M. Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378-386; quiz 387-378.
9. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136-144. https://doi.org/10.1002/jhm.2520.
10. Hagedorn PA, Kirkendall ES, Spooner SA, Mohan V. Inpatient communication networks: leveraging secure text-messaging platforms to gain insight into inpatient communication systems. Appl Clin Inform. 2019;10(3):471-478. https://doi.org/10.1055/s-0039-1692401.
11. Westbrook JI, Coiera E, Dunsmuir WT, et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284-289. https://doi.org/10.1136/qshc.2009.039255.
12. Castells M. The Rise of the Network Society. 2nd ed. Malden, MA: Wiley-Blackwell; 2010.
13. Lo V, Wu RC, Morra D, Lee L, Reeves S. The use of smartphones in general and internal medicine units: A boon or a bane to the promotion of interprofessional collaboration? J Interprof Care. 2012;26(4):276-282. https://doi.org/10.3109/13561820.2012.663013.
14. Martin G, Khajuria A, Arora S, King D, Ashrafian H, Darzi A. The impact of mobile technology on teamwork and communication in hospitals: a systematic review. J Am Med Inform Assn. 2019;26(4):339-355. https://doi.org/10.1093/jamia/ocy175.
15. Liu X, Sutton PR, McKenna R, et al. Evaluation of secure messaging applications for a health care system: a case study. Appl Clin Inform. 2019;10(1):140-150. https://doi.org/10.1055/s-0039-1678607.
16. Weigert RM, Schmitz AH, Soung PJ, Porada K, Weisgerber MC. Improving standardization of paging communication using quality improvement methodology. Pediatrics. 2019;143(4). https://doi.org/10.1542/peds.2018-1362.
© 2019 Society of Hospital Medicine
Should the Pendulum Swing Back? More Transfers to the ICU After Implementing Ward-Based High-Flow Nasal Cannula Initiation Protocols for Bronchiolitis
As an appealing, physiologically plausible treatment, humidified oxygen delivery via high-flow nasal cannula (HFNC) has been rapidly adopted for the treatment of bronchiolitis despite weak evidence supporting its routine and early use in hypoxemic infants.1 Although HFNC use has been associated with decreased work of breathing and lower rates of progression to invasive ventilation in some studies, the one large trial published on the topic found no difference between early HFNC and standard oxygen therapy on length of stay in hospital, duration of oxygen therapy, or rates of intubation.2,3 No adequately powered studies have examined the effect of ward-based HFNC initiation on ICU transfer, an outcome that it is designed to prevent.
In this month’s issue of the Journal of Hospital Medicine, Coon et al examine the association between the implementation of ward-based HFNC initiation protocols and subsequent ICU transfer rates.4 Hospitals enrolled in the Pediatric Health Information System database were surveyed about their HFNC use and protocol implementation, with 41 (93% response rate) hospitals replying, 12 of which implemented ward-based HFNC initiation protocols during 2010 to 2016. Administrative data for bronchiolitis encounters were obtained with use of International Classification of Diseases, 9th and 10th Revisions, coding of children aged 3 to 24 months discharged during the respiratory seasons of the study period. The authors used an interrupted time series analysis to study the association between ward-based HFNC protocol initiation and several outcomes, revealing a small but significant increase in ICU transfers (absolute difference, 3.1%; 95% CI, 2.8%-3.4%) and ICU length of stay (absolute difference, 9.1 days per 100 patients; 95% CI 5.1-13.2), but not overall length of stay or use of mechanical ventilation. Modifications to the analysis that account for a learning period during the first season of implementation at each hospital, and for trends among nonadopting hospitals, did not substantially affect the findings.
The authors acknowledged many of the study’s limitations, including its retrospective design, presumption of bronchiolitis discharge code validity, restriction to tertiary care hospitals, and analysis of hospital-level rather than patient-level variables and outcomes. Because the data source does not capture patient-level HFNC use, the number and characteristics of patients receiving HFNC at the centers are unknown. It is also important to note that the 12 included protocols are quite heterogeneous, with differing exclusion criteria, maximum flow rates, and indications for ICU transfer. Given the rapid evolution of ward-based HFNC use for bronchiolitis, these protocols from 2010 to 2016 are already out of date. All of the protocols allowed much lower maximum flow rates (4-10 L/min) than would typically be expected today (usually 2 L/kg per minute, which translates to 10 L/min of flow for a 5-kg child or 20 L/min for a 10-kg child). Many also had time-based criteria prompting ICU transfer (eg, 24 hours without improvement) that are not typically included in more recent protocols. Few had instructions for weaning or discontinuation of HFNC.
In spite of the above limitations, the results of this large, multicenter study advance our understanding of the consequences of ward-based protocols for HFNC initiation. However, it is important to contextualize this work as an examination of the implementation of a technology to a broad population in a specific era, not necessarily a study of the effectiveness of the technology itself.
The pediatric hospital medicine community has long recognized the need for more evidence regarding HFNC use.5-7 Coon et al have highlighted possible unintended consequences, notably increased ICU use, that may be associated with ward-based HFNC implementation on a population basis. This finding mirrors evidence from a recent similarly designed study analyzing Canadian tertiary care centers implementing HFNC administration during 2009 to 2014, though not specifically limited to ward use.8
More recently there has been discussion of how we might deimplement ward-based HFNC protocols. Although it is increasingly clear that HFNC is not a panacea for bronchiolitis, there is not necessarily a problem with the technology; the problem that this study so clearly demonstrates is how we have applied it. We need pragmatic trials of HFNC protocols to understand what parameters should guide HFNC initiation as a rescue treatment; what oxygen and flow settings might prevent ICU transfer; how it should be used in populations that have been largely excluded from trials (ie, children with medical complexity); and how to optimally wean it. With that information we could construct evidence-based, utilitarian HFNC initiation and treatment protocols to maximize benefit and minimize harm and cost.
It is understandable that our desire to help patients has led us to hear the “siren’s call” for this therapy, and indeed we should work on putting some of the “horses back in the barn.”5,6 Until new evidence guides how to best use this technology, institutional practice guidelines for HFNC initiation in ward settings should target children for whom ICU transfer seems very likely (eg, having oxygen saturations not maintained on maximum low-flow oxygen therapy) so that HFNC is not used routinely and that we maximize its cost to benefit ratio. It is important to approach this shift in a thoughtful manner to prevent a pendulum swing to premature universal deimplementation.
1. Piper L, Stalets EL, Statile AM. Clinical practice update: high flow nasal cannula therapy for bronchiolitis outside the ICU in infants. J Hosp Med. 2019;14:E1-E3. https://doi.org/10.12788/jhm.3328.
2. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/nejmoa1714855.
3. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019;104(6):564-576. https://doi.org/10.1136/archdischild-2018-315846.
4. Coon ER, G. S, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3456.
5. de Benedictis FM. The Effectiveness of high-flow oxygen therapy and the fascinating song of the sirens. JAMA Pediatr. 2019;173(2):125-126. https://doi.org/10.1001/jamapediatrics.2018.3831.
6. Ralston SL. High-flow nasal cannula therapy for pediatric patients with bronchiolitis: time to put the horse back in the barn [online first]. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.0040.
7. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2015-2862.
8. Garland H, Miller MR, Gunz AC, Lim RK. High-flow nasal cannula implementation has not reduced intubation rates for bronchiolitis in Canada [online first]. Paediatr Child Health. 2020. https://doi.org/10.1093/pch/pxaa023.
As an appealing, physiologically plausible treatment, humidified oxygen delivery via high-flow nasal cannula (HFNC) has been rapidly adopted for the treatment of bronchiolitis despite weak evidence supporting its routine and early use in hypoxemic infants.1 Although HFNC use has been associated with decreased work of breathing and lower rates of progression to invasive ventilation in some studies, the one large trial published on the topic found no difference between early HFNC and standard oxygen therapy on length of stay in hospital, duration of oxygen therapy, or rates of intubation.2,3 No adequately powered studies have examined the effect of ward-based HFNC initiation on ICU transfer, an outcome that it is designed to prevent.
In this month’s issue of the Journal of Hospital Medicine, Coon et al examine the association between the implementation of ward-based HFNC initiation protocols and subsequent ICU transfer rates.4 Hospitals enrolled in the Pediatric Health Information System database were surveyed about their HFNC use and protocol implementation, with 41 (93% response rate) hospitals replying, 12 of which implemented ward-based HFNC initiation protocols during 2010 to 2016. Administrative data for bronchiolitis encounters were obtained with use of International Classification of Diseases, 9th and 10th Revisions, coding of children aged 3 to 24 months discharged during the respiratory seasons of the study period. The authors used an interrupted time series analysis to study the association between ward-based HFNC protocol initiation and several outcomes, revealing a small but significant increase in ICU transfers (absolute difference, 3.1%; 95% CI, 2.8%-3.4%) and ICU length of stay (absolute difference, 9.1 days per 100 patients; 95% CI 5.1-13.2), but not overall length of stay or use of mechanical ventilation. Modifications to the analysis that account for a learning period during the first season of implementation at each hospital, and for trends among nonadopting hospitals, did not substantially affect the findings.
The authors acknowledged many of the study’s limitations, including its retrospective design, presumption of bronchiolitis discharge code validity, restriction to tertiary care hospitals, and analysis of hospital-level rather than patient-level variables and outcomes. Because the data source does not capture patient-level HFNC use, the number and characteristics of patients receiving HFNC at the centers are unknown. It is also important to note that the 12 included protocols are quite heterogeneous, with differing exclusion criteria, maximum flow rates, and indications for ICU transfer. Given the rapid evolution of ward-based HFNC use for bronchiolitis, these protocols from 2010 to 2016 are already out of date. All of the protocols allowed much lower maximum flow rates (4-10 L/min) than would typically be expected today (usually 2 L/kg per minute, which translates to 10 L/min of flow for a 5-kg child or 20 L/min for a 10-kg child). Many also had time-based criteria prompting ICU transfer (eg, 24 hours without improvement) that are not typically included in more recent protocols. Few had instructions for weaning or discontinuation of HFNC.
In spite of the above limitations, the results of this large, multicenter study advance our understanding of the consequences of ward-based protocols for HFNC initiation. However, it is important to contextualize this work as an examination of the implementation of a technology to a broad population in a specific era, not necessarily a study of the effectiveness of the technology itself.
The pediatric hospital medicine community has long recognized the need for more evidence regarding HFNC use.5-7 Coon et al have highlighted possible unintended consequences, notably increased ICU use, that may be associated with ward-based HFNC implementation on a population basis. This finding mirrors evidence from a recent similarly designed study analyzing Canadian tertiary care centers implementing HFNC administration during 2009 to 2014, though not specifically limited to ward use.8
More recently there has been discussion of how we might deimplement ward-based HFNC protocols. Although it is increasingly clear that HFNC is not a panacea for bronchiolitis, there is not necessarily a problem with the technology; the problem that this study so clearly demonstrates is how we have applied it. We need pragmatic trials of HFNC protocols to understand what parameters should guide HFNC initiation as a rescue treatment; what oxygen and flow settings might prevent ICU transfer; how it should be used in populations that have been largely excluded from trials (ie, children with medical complexity); and how to optimally wean it. With that information we could construct evidence-based, utilitarian HFNC initiation and treatment protocols to maximize benefit and minimize harm and cost.
It is understandable that our desire to help patients has led us to hear the “siren’s call” for this therapy, and indeed we should work on putting some of the “horses back in the barn.”5,6 Until new evidence guides how to best use this technology, institutional practice guidelines for HFNC initiation in ward settings should target children for whom ICU transfer seems very likely (eg, having oxygen saturations not maintained on maximum low-flow oxygen therapy) so that HFNC is not used routinely and that we maximize its cost to benefit ratio. It is important to approach this shift in a thoughtful manner to prevent a pendulum swing to premature universal deimplementation.
As an appealing, physiologically plausible treatment, humidified oxygen delivery via high-flow nasal cannula (HFNC) has been rapidly adopted for the treatment of bronchiolitis despite weak evidence supporting its routine and early use in hypoxemic infants.1 Although HFNC use has been associated with decreased work of breathing and lower rates of progression to invasive ventilation in some studies, the one large trial published on the topic found no difference between early HFNC and standard oxygen therapy on length of stay in hospital, duration of oxygen therapy, or rates of intubation.2,3 No adequately powered studies have examined the effect of ward-based HFNC initiation on ICU transfer, an outcome that it is designed to prevent.
In this month’s issue of the Journal of Hospital Medicine, Coon et al examine the association between the implementation of ward-based HFNC initiation protocols and subsequent ICU transfer rates.4 Hospitals enrolled in the Pediatric Health Information System database were surveyed about their HFNC use and protocol implementation, with 41 (93% response rate) hospitals replying, 12 of which implemented ward-based HFNC initiation protocols during 2010 to 2016. Administrative data for bronchiolitis encounters were obtained with use of International Classification of Diseases, 9th and 10th Revisions, coding of children aged 3 to 24 months discharged during the respiratory seasons of the study period. The authors used an interrupted time series analysis to study the association between ward-based HFNC protocol initiation and several outcomes, revealing a small but significant increase in ICU transfers (absolute difference, 3.1%; 95% CI, 2.8%-3.4%) and ICU length of stay (absolute difference, 9.1 days per 100 patients; 95% CI 5.1-13.2), but not overall length of stay or use of mechanical ventilation. Modifications to the analysis that account for a learning period during the first season of implementation at each hospital, and for trends among nonadopting hospitals, did not substantially affect the findings.
The authors acknowledged many of the study’s limitations, including its retrospective design, presumption of bronchiolitis discharge code validity, restriction to tertiary care hospitals, and analysis of hospital-level rather than patient-level variables and outcomes. Because the data source does not capture patient-level HFNC use, the number and characteristics of patients receiving HFNC at the centers are unknown. It is also important to note that the 12 included protocols are quite heterogeneous, with differing exclusion criteria, maximum flow rates, and indications for ICU transfer. Given the rapid evolution of ward-based HFNC use for bronchiolitis, these protocols from 2010 to 2016 are already out of date. All of the protocols allowed much lower maximum flow rates (4-10 L/min) than would typically be expected today (usually 2 L/kg per minute, which translates to 10 L/min of flow for a 5-kg child or 20 L/min for a 10-kg child). Many also had time-based criteria prompting ICU transfer (eg, 24 hours without improvement) that are not typically included in more recent protocols. Few had instructions for weaning or discontinuation of HFNC.
In spite of the above limitations, the results of this large, multicenter study advance our understanding of the consequences of ward-based protocols for HFNC initiation. However, it is important to contextualize this work as an examination of the implementation of a technology to a broad population in a specific era, not necessarily a study of the effectiveness of the technology itself.
The pediatric hospital medicine community has long recognized the need for more evidence regarding HFNC use.5-7 Coon et al have highlighted possible unintended consequences, notably increased ICU use, that may be associated with ward-based HFNC implementation on a population basis. This finding mirrors evidence from a recent similarly designed study analyzing Canadian tertiary care centers implementing HFNC administration during 2009 to 2014, though not specifically limited to ward use.8
More recently there has been discussion of how we might deimplement ward-based HFNC protocols. Although it is increasingly clear that HFNC is not a panacea for bronchiolitis, there is not necessarily a problem with the technology; the problem that this study so clearly demonstrates is how we have applied it. We need pragmatic trials of HFNC protocols to understand what parameters should guide HFNC initiation as a rescue treatment; what oxygen and flow settings might prevent ICU transfer; how it should be used in populations that have been largely excluded from trials (ie, children with medical complexity); and how to optimally wean it. With that information we could construct evidence-based, utilitarian HFNC initiation and treatment protocols to maximize benefit and minimize harm and cost.
It is understandable that our desire to help patients has led us to hear the “siren’s call” for this therapy, and indeed we should work on putting some of the “horses back in the barn.”5,6 Until new evidence guides how to best use this technology, institutional practice guidelines for HFNC initiation in ward settings should target children for whom ICU transfer seems very likely (eg, having oxygen saturations not maintained on maximum low-flow oxygen therapy) so that HFNC is not used routinely and that we maximize its cost to benefit ratio. It is important to approach this shift in a thoughtful manner to prevent a pendulum swing to premature universal deimplementation.
1. Piper L, Stalets EL, Statile AM. Clinical practice update: high flow nasal cannula therapy for bronchiolitis outside the ICU in infants. J Hosp Med. 2019;14:E1-E3. https://doi.org/10.12788/jhm.3328.
2. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/nejmoa1714855.
3. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019;104(6):564-576. https://doi.org/10.1136/archdischild-2018-315846.
4. Coon ER, G. S, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3456.
5. de Benedictis FM. The Effectiveness of high-flow oxygen therapy and the fascinating song of the sirens. JAMA Pediatr. 2019;173(2):125-126. https://doi.org/10.1001/jamapediatrics.2018.3831.
6. Ralston SL. High-flow nasal cannula therapy for pediatric patients with bronchiolitis: time to put the horse back in the barn [online first]. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.0040.
7. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2015-2862.
8. Garland H, Miller MR, Gunz AC, Lim RK. High-flow nasal cannula implementation has not reduced intubation rates for bronchiolitis in Canada [online first]. Paediatr Child Health. 2020. https://doi.org/10.1093/pch/pxaa023.
1. Piper L, Stalets EL, Statile AM. Clinical practice update: high flow nasal cannula therapy for bronchiolitis outside the ICU in infants. J Hosp Med. 2019;14:E1-E3. https://doi.org/10.12788/jhm.3328.
2. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/nejmoa1714855.
3. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019;104(6):564-576. https://doi.org/10.1136/archdischild-2018-315846.
4. Coon ER, G. S, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3456.
5. de Benedictis FM. The Effectiveness of high-flow oxygen therapy and the fascinating song of the sirens. JAMA Pediatr. 2019;173(2):125-126. https://doi.org/10.1001/jamapediatrics.2018.3831.
6. Ralston SL. High-flow nasal cannula therapy for pediatric patients with bronchiolitis: time to put the horse back in the barn [online first]. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.0040.
7. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2015-2862.
8. Garland H, Miller MR, Gunz AC, Lim RK. High-flow nasal cannula implementation has not reduced intubation rates for bronchiolitis in Canada [online first]. Paediatr Child Health. 2020. https://doi.org/10.1093/pch/pxaa023.
© 2020 Society of Hospital Medicine
Setting an Agenda for Hospital Medicine Research: Making Sure the Right People Are at the Table
Unlike other service industries, US healthcare has been slower to adopt an approach of asking users (patients) how to make things better. However, patient engagement in systems of healthcare (eg, Patient and Family Advisory Councils [PFAC]) and health system-based research (eg, Patient Centered Outcomes Research Institute [PCORI]) are gaining currency in the United States.1,2
Increasing patient/family involvement in health systems research design, especially in terms of setting research priorities, may lead to improved patient outcomes and experience. Patients and investigators have coproduced research agendas,1 typically for specific diagnoses or with a focus on ambulatory care.3 To date, few efforts have actively engaged patients/families as true partners in identifying research gaps in the inpatient setting.3,4
In their prospective study, Harrison et al5 used a systematic approach and methods established by PCORI and the James Lind Alliance to establish a patient-centered research agenda for improving care of hospitalized adult patients. They formed a national steering committee of clinical researchers, patients and caregivers, administrators, and stakeholder organizations. A survey was distributed to about 500 similar stakeholders to generate a list of potential research questions, which were sorted, analyzed, ranked, and prioritized based on frequency. The steering committee ultimately identified an agenda of 11 system of care–related research questions. The highest priority questions focused on ensuring shared decision making (SDM) and transitions of care.
This study has several strengths. Patients served as coleads on the steering committee and were engaged early and often throughout the process, considered a Tier 1, or deliberative, engagement approach.1 This is in contrast to a consultative, or Tier 2, approach in which patients serve as consultants and comment later in the process.1 As Harrison et al. demonstrate, including patients impacted the breadth and depth of results. An emphasis on patient perspectives seems to have led to recognition of topics that clinical researchers did not develop a priori. Some patient-proposed research topics, such as best modes to navigate the hospital and visiting hours, suggest a bigger question beyond patient experience: How might attention to details minimize disorientation, which likely detracts from ability to engage in care?
The most highly ranked research question regarded study of interventions that would ensure SDM among patients and physicians. SDM-based interventions in pediatrics have led to significantly improved knowledge and lower decisional conflict.6
More than half of the research questions ranked by the investigators related to transitions of care, including ensuring proper comprehension of and adherence to postdischarge care plans, medical provider handoffs, and mechanisms for communication after discharge.
Moving from understanding to execution is another gap recognized in this study. Improving resources and care in the home after discharge also would likely improve outcomes. Industry, with use of rapid-cycle improvement methods, has already implemented comprehensive, home-based approaches focusing on enhanced presence of care team members (including physicians, nurses, and social workers) in the home. Team tasks include verifying that prescriptions are filled and medications are taken properly and ensuring that social needs are met, which could possibly lead to decreased healthcare utilization.8 Additional innovative strategies that leverage technology to optimize information exchange and facilitate postdischarge communication when questions arise (eg, telemedicine as suggested by stakeholders in this study) may also be beneficial. Such strategies, as well as models established by industry, should be further studied as part of interventions that also incorporate the perspective of patients, caregivers, and other stakeholders.
The study had a few limitations. This study, while national in scope, did not provide patient/caregiver demographics or preferred language, so it is unclear if participation was inclusive of all populations. Use of qualitative methods, including this study’s apparently modified Delphi approach, is important to ensuring equal consideration is given to all suggestions—but this only works if the stakeholders are representative. Patients and caregivers were primarily recruited from PFAC, which represent a more activated constituency and often lacks demographic diversity.9 Given that “care of vulnerable populations” was an infrequently proposed question category, future work would benefit from oversampling from marginalized, underrepresented groups.
While the study’s aim was development of a research agenda for adult patients, children, especially those who are medically complex, and their caregivers may experience similar issues. There may be barriers related to hospitalizations and transitions unique to children given their inherent dependent status.
1. Manafò E, Petermann L, Vandall-Walker V, Mason-Lai P. Patient and public engagement in priority setting: a systematic rapid review of the literature. PLoS One. 2018;13(3):1-18. https://doi.org/10.1371/journal.pone.0193579.
2. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25(7):509-517. https://doi.org/10.1136/bmjqs-2015-004315.
3. Bombard Y, Baker GR, Orlando E, et al. Engaging patients to improve quality of care: a systematic review. Implement Sci. 2018;13(1):98. https://doi.org/10.1186/s13012-018-0784-z.
4. Liang L, Cako A, Urquhart R, et al. Patient engagement in hospital health service planning and improvement: a scoping review. BMJ Open. 2018;8(1):1-8. https://doi.org/10.1136/bmjopen-2017-018263.
5. Harrison J, Archuleta M, Avitia E, et al. Developing a patient & family centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337 https://doi.org/10.12788/jhm.3386.
6. Wyatt KD, List B, Brinkman WB, et al. Shared decision making in pediatrics: a systematic review and meta-analysis. Acad Pediatr. 2015;15(6):573-583. https://doi.org/10.1016/j.acap.2015.03.011.
7. Glick AF, Brach C, Yin HS, Dreyer BP. Health literacy in the inpatient setting: implications for patient care and patient safety. Pediatr Clin North Am. 2019;66(4):805-826. https://doi.org/10.1016/j.pcl.2019.03.007.
8. Di Capua P, Mathur J, Garg V, Jain SH. How home-based primary care can reduce expensive hospitalizations. Harvard Business Review. https://hbr.org/2019/05/how-home-care-can-reduce-expensive-hospitalizations. Accessed January 30, 2020.
9. New York Health Foundation. Strategically advancing patient and family advisory councils in New York State hospitals. https://nyshealthfoundation.org/wp-content/uploads/2018/06/strategically-advancing-patient-and-family-advisory-councils.pdf. Accessed January 26, 2020.
Unlike other service industries, US healthcare has been slower to adopt an approach of asking users (patients) how to make things better. However, patient engagement in systems of healthcare (eg, Patient and Family Advisory Councils [PFAC]) and health system-based research (eg, Patient Centered Outcomes Research Institute [PCORI]) are gaining currency in the United States.1,2
Increasing patient/family involvement in health systems research design, especially in terms of setting research priorities, may lead to improved patient outcomes and experience. Patients and investigators have coproduced research agendas,1 typically for specific diagnoses or with a focus on ambulatory care.3 To date, few efforts have actively engaged patients/families as true partners in identifying research gaps in the inpatient setting.3,4
In their prospective study, Harrison et al5 used a systematic approach and methods established by PCORI and the James Lind Alliance to establish a patient-centered research agenda for improving care of hospitalized adult patients. They formed a national steering committee of clinical researchers, patients and caregivers, administrators, and stakeholder organizations. A survey was distributed to about 500 similar stakeholders to generate a list of potential research questions, which were sorted, analyzed, ranked, and prioritized based on frequency. The steering committee ultimately identified an agenda of 11 system of care–related research questions. The highest priority questions focused on ensuring shared decision making (SDM) and transitions of care.
This study has several strengths. Patients served as coleads on the steering committee and were engaged early and often throughout the process, considered a Tier 1, or deliberative, engagement approach.1 This is in contrast to a consultative, or Tier 2, approach in which patients serve as consultants and comment later in the process.1 As Harrison et al. demonstrate, including patients impacted the breadth and depth of results. An emphasis on patient perspectives seems to have led to recognition of topics that clinical researchers did not develop a priori. Some patient-proposed research topics, such as best modes to navigate the hospital and visiting hours, suggest a bigger question beyond patient experience: How might attention to details minimize disorientation, which likely detracts from ability to engage in care?
The most highly ranked research question regarded study of interventions that would ensure SDM among patients and physicians. SDM-based interventions in pediatrics have led to significantly improved knowledge and lower decisional conflict.6
More than half of the research questions ranked by the investigators related to transitions of care, including ensuring proper comprehension of and adherence to postdischarge care plans, medical provider handoffs, and mechanisms for communication after discharge.
Moving from understanding to execution is another gap recognized in this study. Improving resources and care in the home after discharge also would likely improve outcomes. Industry, with use of rapid-cycle improvement methods, has already implemented comprehensive, home-based approaches focusing on enhanced presence of care team members (including physicians, nurses, and social workers) in the home. Team tasks include verifying that prescriptions are filled and medications are taken properly and ensuring that social needs are met, which could possibly lead to decreased healthcare utilization.8 Additional innovative strategies that leverage technology to optimize information exchange and facilitate postdischarge communication when questions arise (eg, telemedicine as suggested by stakeholders in this study) may also be beneficial. Such strategies, as well as models established by industry, should be further studied as part of interventions that also incorporate the perspective of patients, caregivers, and other stakeholders.
The study had a few limitations. This study, while national in scope, did not provide patient/caregiver demographics or preferred language, so it is unclear if participation was inclusive of all populations. Use of qualitative methods, including this study’s apparently modified Delphi approach, is important to ensuring equal consideration is given to all suggestions—but this only works if the stakeholders are representative. Patients and caregivers were primarily recruited from PFAC, which represent a more activated constituency and often lacks demographic diversity.9 Given that “care of vulnerable populations” was an infrequently proposed question category, future work would benefit from oversampling from marginalized, underrepresented groups.
While the study’s aim was development of a research agenda for adult patients, children, especially those who are medically complex, and their caregivers may experience similar issues. There may be barriers related to hospitalizations and transitions unique to children given their inherent dependent status.
Unlike other service industries, US healthcare has been slower to adopt an approach of asking users (patients) how to make things better. However, patient engagement in systems of healthcare (eg, Patient and Family Advisory Councils [PFAC]) and health system-based research (eg, Patient Centered Outcomes Research Institute [PCORI]) are gaining currency in the United States.1,2
Increasing patient/family involvement in health systems research design, especially in terms of setting research priorities, may lead to improved patient outcomes and experience. Patients and investigators have coproduced research agendas,1 typically for specific diagnoses or with a focus on ambulatory care.3 To date, few efforts have actively engaged patients/families as true partners in identifying research gaps in the inpatient setting.3,4
In their prospective study, Harrison et al5 used a systematic approach and methods established by PCORI and the James Lind Alliance to establish a patient-centered research agenda for improving care of hospitalized adult patients. They formed a national steering committee of clinical researchers, patients and caregivers, administrators, and stakeholder organizations. A survey was distributed to about 500 similar stakeholders to generate a list of potential research questions, which were sorted, analyzed, ranked, and prioritized based on frequency. The steering committee ultimately identified an agenda of 11 system of care–related research questions. The highest priority questions focused on ensuring shared decision making (SDM) and transitions of care.
This study has several strengths. Patients served as coleads on the steering committee and were engaged early and often throughout the process, considered a Tier 1, or deliberative, engagement approach.1 This is in contrast to a consultative, or Tier 2, approach in which patients serve as consultants and comment later in the process.1 As Harrison et al. demonstrate, including patients impacted the breadth and depth of results. An emphasis on patient perspectives seems to have led to recognition of topics that clinical researchers did not develop a priori. Some patient-proposed research topics, such as best modes to navigate the hospital and visiting hours, suggest a bigger question beyond patient experience: How might attention to details minimize disorientation, which likely detracts from ability to engage in care?
The most highly ranked research question regarded study of interventions that would ensure SDM among patients and physicians. SDM-based interventions in pediatrics have led to significantly improved knowledge and lower decisional conflict.6
More than half of the research questions ranked by the investigators related to transitions of care, including ensuring proper comprehension of and adherence to postdischarge care plans, medical provider handoffs, and mechanisms for communication after discharge.
Moving from understanding to execution is another gap recognized in this study. Improving resources and care in the home after discharge also would likely improve outcomes. Industry, with use of rapid-cycle improvement methods, has already implemented comprehensive, home-based approaches focusing on enhanced presence of care team members (including physicians, nurses, and social workers) in the home. Team tasks include verifying that prescriptions are filled and medications are taken properly and ensuring that social needs are met, which could possibly lead to decreased healthcare utilization.8 Additional innovative strategies that leverage technology to optimize information exchange and facilitate postdischarge communication when questions arise (eg, telemedicine as suggested by stakeholders in this study) may also be beneficial. Such strategies, as well as models established by industry, should be further studied as part of interventions that also incorporate the perspective of patients, caregivers, and other stakeholders.
The study had a few limitations. This study, while national in scope, did not provide patient/caregiver demographics or preferred language, so it is unclear if participation was inclusive of all populations. Use of qualitative methods, including this study’s apparently modified Delphi approach, is important to ensuring equal consideration is given to all suggestions—but this only works if the stakeholders are representative. Patients and caregivers were primarily recruited from PFAC, which represent a more activated constituency and often lacks demographic diversity.9 Given that “care of vulnerable populations” was an infrequently proposed question category, future work would benefit from oversampling from marginalized, underrepresented groups.
While the study’s aim was development of a research agenda for adult patients, children, especially those who are medically complex, and their caregivers may experience similar issues. There may be barriers related to hospitalizations and transitions unique to children given their inherent dependent status.
1. Manafò E, Petermann L, Vandall-Walker V, Mason-Lai P. Patient and public engagement in priority setting: a systematic rapid review of the literature. PLoS One. 2018;13(3):1-18. https://doi.org/10.1371/journal.pone.0193579.
2. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25(7):509-517. https://doi.org/10.1136/bmjqs-2015-004315.
3. Bombard Y, Baker GR, Orlando E, et al. Engaging patients to improve quality of care: a systematic review. Implement Sci. 2018;13(1):98. https://doi.org/10.1186/s13012-018-0784-z.
4. Liang L, Cako A, Urquhart R, et al. Patient engagement in hospital health service planning and improvement: a scoping review. BMJ Open. 2018;8(1):1-8. https://doi.org/10.1136/bmjopen-2017-018263.
5. Harrison J, Archuleta M, Avitia E, et al. Developing a patient & family centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337 https://doi.org/10.12788/jhm.3386.
6. Wyatt KD, List B, Brinkman WB, et al. Shared decision making in pediatrics: a systematic review and meta-analysis. Acad Pediatr. 2015;15(6):573-583. https://doi.org/10.1016/j.acap.2015.03.011.
7. Glick AF, Brach C, Yin HS, Dreyer BP. Health literacy in the inpatient setting: implications for patient care and patient safety. Pediatr Clin North Am. 2019;66(4):805-826. https://doi.org/10.1016/j.pcl.2019.03.007.
8. Di Capua P, Mathur J, Garg V, Jain SH. How home-based primary care can reduce expensive hospitalizations. Harvard Business Review. https://hbr.org/2019/05/how-home-care-can-reduce-expensive-hospitalizations. Accessed January 30, 2020.
9. New York Health Foundation. Strategically advancing patient and family advisory councils in New York State hospitals. https://nyshealthfoundation.org/wp-content/uploads/2018/06/strategically-advancing-patient-and-family-advisory-councils.pdf. Accessed January 26, 2020.
1. Manafò E, Petermann L, Vandall-Walker V, Mason-Lai P. Patient and public engagement in priority setting: a systematic rapid review of the literature. PLoS One. 2018;13(3):1-18. https://doi.org/10.1371/journal.pone.0193579.
2. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25(7):509-517. https://doi.org/10.1136/bmjqs-2015-004315.
3. Bombard Y, Baker GR, Orlando E, et al. Engaging patients to improve quality of care: a systematic review. Implement Sci. 2018;13(1):98. https://doi.org/10.1186/s13012-018-0784-z.
4. Liang L, Cako A, Urquhart R, et al. Patient engagement in hospital health service planning and improvement: a scoping review. BMJ Open. 2018;8(1):1-8. https://doi.org/10.1136/bmjopen-2017-018263.
5. Harrison J, Archuleta M, Avitia E, et al. Developing a patient & family centered research agenda for hospital medicine: the Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study. J Hosp Med. 2020;15(6):331-337 https://doi.org/10.12788/jhm.3386.
6. Wyatt KD, List B, Brinkman WB, et al. Shared decision making in pediatrics: a systematic review and meta-analysis. Acad Pediatr. 2015;15(6):573-583. https://doi.org/10.1016/j.acap.2015.03.011.
7. Glick AF, Brach C, Yin HS, Dreyer BP. Health literacy in the inpatient setting: implications for patient care and patient safety. Pediatr Clin North Am. 2019;66(4):805-826. https://doi.org/10.1016/j.pcl.2019.03.007.
8. Di Capua P, Mathur J, Garg V, Jain SH. How home-based primary care can reduce expensive hospitalizations. Harvard Business Review. https://hbr.org/2019/05/how-home-care-can-reduce-expensive-hospitalizations. Accessed January 30, 2020.
9. New York Health Foundation. Strategically advancing patient and family advisory councils in New York State hospitals. https://nyshealthfoundation.org/wp-content/uploads/2018/06/strategically-advancing-patient-and-family-advisory-councils.pdf. Accessed January 26, 2020.
© 2020 Society of Hospital Medicine
4.16 Healthcare Systems: Research
Introduction
Research is a rapidly growing aspect of inpatient medicine. The practice of evidence-based medicine and the acute need for more evidence on inpatient conditions require that pediatric hospitalists understand and participate in research related activities. Pediatric hospitalists’ role in research will vary depending on their setting and job description. This role may include many facets, from reviewing relevant patient-based articles, to participating in multi-institutional studies requiring enrollment of patients, to leading local or national studies. Pediatric hospitalists should have a basic understanding of research methods and processes in order to participate in and benefit from research. Pediatric hospitalists are well positioned to promote research to patients, the family/caregivers, colleagues, and other healthcare providers and through this, to contribute to the effective care of hospitalized patients.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the advantages and disadvantages of experimental (such as randomized control trials) and observational (such as descriptive, cohort, or case control) study designs, including meta-analyses and systematic reviews.
- Define common sources of bias, including information bias, selection bias, and uncontrolled confounding, and describe how each may impact a study.
- Define basic statistical terms such as sample, discrete and continuous data variables, measures of central tendency (mean, median, and mode), and variability (variance, standard deviation, range).
- List resources available to access current or proposed studies including The Pediatric Health Information System (PHIS), the Healthcare Cost and Utilization Project (HCUP), the Kids’ Inpatient Database (KID), clinicaltrials.gov, and others.
- Name potential research funding sources, such as the Agency for Healthcare Research and Quality (AHRQ), the National Institutes of Health (NIH), the Patient-Centered Outcomes Research Institute (PCORI), the Robert Wood Johnson Foundation, local and state funding sources, and others.
- Summarize the goals of pediatric hospital medicine-specific research networks, including the Pediatric Research in the Inpatient Setting (PRIS) network and the Value in Pediatrics (VIP) network.
- Discuss the basic resources commonly required to support research components, including data collection, data analysis, abstract and manuscript preparation, grant funding, and others.
- Review the aspects of the research process that relate to protection of participants, including informed consent and/or assent, the institutional review boards (IRB) review, and HIPAA (Health Insurance Portability and Accountability Act) forms.
- Discuss special protections needed when conducting research with vulnerable populations.
- Define “minimal risk” for a healthy child and for a child with an illness.
- Discuss why common training that addresses ethics, vulnerable populations, consenting, data safety, and other items is required prior to participating as a research team member for a research study.
- Compare and contrast the goals, intent, study focus, and IRB requirements for quality improvement studies from those of traditional clinical research.
- Cite the steps needed to obtain approval for a QI study within the local context.
- Compare and contrast the goals, intent, study focus, and IRB requirements for education studies to those of traditional clinical research.
- Cite the steps needed to obtain approval for a study focused on educational outcomes.
- List common barriers to implementation of clinical studies and describe the pediatric hospitalist’s role in overcoming these barriers.
Skills
Pediatric hospitalists should be able to:
- Utilize a format such as PICO (Population, Intervention, Comparison, Outcome) to generate an answerable patient-centered clinical question that is relevant to improving patient care.
- Demonstrate proficiency in systematic searching of the primary medical literature using online search engines.
- Perform critical appraisal of the literature, including identifying threats to study validity, determining if study subjects were similar to local patients, and determining if all clinically important outcomes were considered.
- Apply and integrate the results of studies to clinical practice.
- Determine if the likely benefits noted in a treatment study are worth the potential harm and cost.
- Determine whether a test noted in a diagnostic study is available, affordable, accurate, and precise in the present clinical setting and determine whether the results of the test will change the management of patients being treated.
- Determine if the magnitude of risk warrants an attempt to stop the exposure for a given study on harm.
- Identify if the results of a given study on disease prognosis will lead directly to selecting therapy and/or are useful for counseling patients.
- Participate in educating learners and junior faculty about research and research methodologies, within the local context.
- Determine the relevance of potential research studies with regards to impact on patient care.
- Perform effective informed consent or assent for patients participating in research studies, as appropriate.
- Identify and resolve conflict of interest or potential conflict of interest when participating in research studies.
- Demonstrate basic skills in acquiring, managing, and sharing data collected for research purposes in a responsible and professional manner.
- Adhere to standards for protecting confidentiality, avoiding unjustified exclusions, sharing data, and adhering to copyright law.
- Perform peer-review of a manuscript, abstract, or other research-based work, in collaboration with colleagues as appropriate.
- Demonstrate basic skills in communicating about research opportunities with patients and the family/caregivers within the local context.
Attitudes
Pediatric hospitalists should be able to:
- Recognize the value of seeking the research that supports clinical care decisions and how research fills knowledge gaps and challenges the field to advance.
- Realize the importance of informed consent for patient participation in clinical research.
- Reflect on the importance of patient assent, even in the presence of legal guardian informed consent, when involving children in clinical research.
- Exemplify highly ethical behaviors when promoting or participating in research studies.
- Realize the value of and exemplify a willingness to perform journal-requested peer review of manuscripts, conference abstracts, or other research-based work.
- Reflect on and provide support and education for patients and the family/caregivers on the benefits of research for hospitalized children.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in interdisciplinary initiatives to develop and sustain participation of interdisciplinary teams in performance of research.
- Collaborate with colleagues, hospital administration, and community leaders for thoughtful application of research findings to improve systems of healthcare delivery.
- Lead, coordinate, or participate in national multi-center research efforts that improve the evidence base in inpatient pediatrics, within local context.
- Collaborate with leaders in the university department of pediatrics and school of medicine, hospital administration, and medical staff to encourage local hospital participation in national multi-center research efforts.
- Collaborate with research team members to educate colleagues, hospital staff, and others on the importance of research in improving child health outcomes.
1. Hulley SB, Cummins SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research, 4th ed. Philadelphia, PA: Wolters Kluwer; 2013.
Introduction
Research is a rapidly growing aspect of inpatient medicine. The practice of evidence-based medicine and the acute need for more evidence on inpatient conditions require that pediatric hospitalists understand and participate in research related activities. Pediatric hospitalists’ role in research will vary depending on their setting and job description. This role may include many facets, from reviewing relevant patient-based articles, to participating in multi-institutional studies requiring enrollment of patients, to leading local or national studies. Pediatric hospitalists should have a basic understanding of research methods and processes in order to participate in and benefit from research. Pediatric hospitalists are well positioned to promote research to patients, the family/caregivers, colleagues, and other healthcare providers and through this, to contribute to the effective care of hospitalized patients.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the advantages and disadvantages of experimental (such as randomized control trials) and observational (such as descriptive, cohort, or case control) study designs, including meta-analyses and systematic reviews.
- Define common sources of bias, including information bias, selection bias, and uncontrolled confounding, and describe how each may impact a study.
- Define basic statistical terms such as sample, discrete and continuous data variables, measures of central tendency (mean, median, and mode), and variability (variance, standard deviation, range).
- List resources available to access current or proposed studies including The Pediatric Health Information System (PHIS), the Healthcare Cost and Utilization Project (HCUP), the Kids’ Inpatient Database (KID), clinicaltrials.gov, and others.
- Name potential research funding sources, such as the Agency for Healthcare Research and Quality (AHRQ), the National Institutes of Health (NIH), the Patient-Centered Outcomes Research Institute (PCORI), the Robert Wood Johnson Foundation, local and state funding sources, and others.
- Summarize the goals of pediatric hospital medicine-specific research networks, including the Pediatric Research in the Inpatient Setting (PRIS) network and the Value in Pediatrics (VIP) network.
- Discuss the basic resources commonly required to support research components, including data collection, data analysis, abstract and manuscript preparation, grant funding, and others.
- Review the aspects of the research process that relate to protection of participants, including informed consent and/or assent, the institutional review boards (IRB) review, and HIPAA (Health Insurance Portability and Accountability Act) forms.
- Discuss special protections needed when conducting research with vulnerable populations.
- Define “minimal risk” for a healthy child and for a child with an illness.
- Discuss why common training that addresses ethics, vulnerable populations, consenting, data safety, and other items is required prior to participating as a research team member for a research study.
- Compare and contrast the goals, intent, study focus, and IRB requirements for quality improvement studies from those of traditional clinical research.
- Cite the steps needed to obtain approval for a QI study within the local context.
- Compare and contrast the goals, intent, study focus, and IRB requirements for education studies to those of traditional clinical research.
- Cite the steps needed to obtain approval for a study focused on educational outcomes.
- List common barriers to implementation of clinical studies and describe the pediatric hospitalist’s role in overcoming these barriers.
Skills
Pediatric hospitalists should be able to:
- Utilize a format such as PICO (Population, Intervention, Comparison, Outcome) to generate an answerable patient-centered clinical question that is relevant to improving patient care.
- Demonstrate proficiency in systematic searching of the primary medical literature using online search engines.
- Perform critical appraisal of the literature, including identifying threats to study validity, determining if study subjects were similar to local patients, and determining if all clinically important outcomes were considered.
- Apply and integrate the results of studies to clinical practice.
- Determine if the likely benefits noted in a treatment study are worth the potential harm and cost.
- Determine whether a test noted in a diagnostic study is available, affordable, accurate, and precise in the present clinical setting and determine whether the results of the test will change the management of patients being treated.
- Determine if the magnitude of risk warrants an attempt to stop the exposure for a given study on harm.
- Identify if the results of a given study on disease prognosis will lead directly to selecting therapy and/or are useful for counseling patients.
- Participate in educating learners and junior faculty about research and research methodologies, within the local context.
- Determine the relevance of potential research studies with regards to impact on patient care.
- Perform effective informed consent or assent for patients participating in research studies, as appropriate.
- Identify and resolve conflict of interest or potential conflict of interest when participating in research studies.
- Demonstrate basic skills in acquiring, managing, and sharing data collected for research purposes in a responsible and professional manner.
- Adhere to standards for protecting confidentiality, avoiding unjustified exclusions, sharing data, and adhering to copyright law.
- Perform peer-review of a manuscript, abstract, or other research-based work, in collaboration with colleagues as appropriate.
- Demonstrate basic skills in communicating about research opportunities with patients and the family/caregivers within the local context.
Attitudes
Pediatric hospitalists should be able to:
- Recognize the value of seeking the research that supports clinical care decisions and how research fills knowledge gaps and challenges the field to advance.
- Realize the importance of informed consent for patient participation in clinical research.
- Reflect on the importance of patient assent, even in the presence of legal guardian informed consent, when involving children in clinical research.
- Exemplify highly ethical behaviors when promoting or participating in research studies.
- Realize the value of and exemplify a willingness to perform journal-requested peer review of manuscripts, conference abstracts, or other research-based work.
- Reflect on and provide support and education for patients and the family/caregivers on the benefits of research for hospitalized children.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in interdisciplinary initiatives to develop and sustain participation of interdisciplinary teams in performance of research.
- Collaborate with colleagues, hospital administration, and community leaders for thoughtful application of research findings to improve systems of healthcare delivery.
- Lead, coordinate, or participate in national multi-center research efforts that improve the evidence base in inpatient pediatrics, within local context.
- Collaborate with leaders in the university department of pediatrics and school of medicine, hospital administration, and medical staff to encourage local hospital participation in national multi-center research efforts.
- Collaborate with research team members to educate colleagues, hospital staff, and others on the importance of research in improving child health outcomes.
Introduction
Research is a rapidly growing aspect of inpatient medicine. The practice of evidence-based medicine and the acute need for more evidence on inpatient conditions require that pediatric hospitalists understand and participate in research related activities. Pediatric hospitalists’ role in research will vary depending on their setting and job description. This role may include many facets, from reviewing relevant patient-based articles, to participating in multi-institutional studies requiring enrollment of patients, to leading local or national studies. Pediatric hospitalists should have a basic understanding of research methods and processes in order to participate in and benefit from research. Pediatric hospitalists are well positioned to promote research to patients, the family/caregivers, colleagues, and other healthcare providers and through this, to contribute to the effective care of hospitalized patients.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the advantages and disadvantages of experimental (such as randomized control trials) and observational (such as descriptive, cohort, or case control) study designs, including meta-analyses and systematic reviews.
- Define common sources of bias, including information bias, selection bias, and uncontrolled confounding, and describe how each may impact a study.
- Define basic statistical terms such as sample, discrete and continuous data variables, measures of central tendency (mean, median, and mode), and variability (variance, standard deviation, range).
- List resources available to access current or proposed studies including The Pediatric Health Information System (PHIS), the Healthcare Cost and Utilization Project (HCUP), the Kids’ Inpatient Database (KID), clinicaltrials.gov, and others.
- Name potential research funding sources, such as the Agency for Healthcare Research and Quality (AHRQ), the National Institutes of Health (NIH), the Patient-Centered Outcomes Research Institute (PCORI), the Robert Wood Johnson Foundation, local and state funding sources, and others.
- Summarize the goals of pediatric hospital medicine-specific research networks, including the Pediatric Research in the Inpatient Setting (PRIS) network and the Value in Pediatrics (VIP) network.
- Discuss the basic resources commonly required to support research components, including data collection, data analysis, abstract and manuscript preparation, grant funding, and others.
- Review the aspects of the research process that relate to protection of participants, including informed consent and/or assent, the institutional review boards (IRB) review, and HIPAA (Health Insurance Portability and Accountability Act) forms.
- Discuss special protections needed when conducting research with vulnerable populations.
- Define “minimal risk” for a healthy child and for a child with an illness.
- Discuss why common training that addresses ethics, vulnerable populations, consenting, data safety, and other items is required prior to participating as a research team member for a research study.
- Compare and contrast the goals, intent, study focus, and IRB requirements for quality improvement studies from those of traditional clinical research.
- Cite the steps needed to obtain approval for a QI study within the local context.
- Compare and contrast the goals, intent, study focus, and IRB requirements for education studies to those of traditional clinical research.
- Cite the steps needed to obtain approval for a study focused on educational outcomes.
- List common barriers to implementation of clinical studies and describe the pediatric hospitalist’s role in overcoming these barriers.
Skills
Pediatric hospitalists should be able to:
- Utilize a format such as PICO (Population, Intervention, Comparison, Outcome) to generate an answerable patient-centered clinical question that is relevant to improving patient care.
- Demonstrate proficiency in systematic searching of the primary medical literature using online search engines.
- Perform critical appraisal of the literature, including identifying threats to study validity, determining if study subjects were similar to local patients, and determining if all clinically important outcomes were considered.
- Apply and integrate the results of studies to clinical practice.
- Determine if the likely benefits noted in a treatment study are worth the potential harm and cost.
- Determine whether a test noted in a diagnostic study is available, affordable, accurate, and precise in the present clinical setting and determine whether the results of the test will change the management of patients being treated.
- Determine if the magnitude of risk warrants an attempt to stop the exposure for a given study on harm.
- Identify if the results of a given study on disease prognosis will lead directly to selecting therapy and/or are useful for counseling patients.
- Participate in educating learners and junior faculty about research and research methodologies, within the local context.
- Determine the relevance of potential research studies with regards to impact on patient care.
- Perform effective informed consent or assent for patients participating in research studies, as appropriate.
- Identify and resolve conflict of interest or potential conflict of interest when participating in research studies.
- Demonstrate basic skills in acquiring, managing, and sharing data collected for research purposes in a responsible and professional manner.
- Adhere to standards for protecting confidentiality, avoiding unjustified exclusions, sharing data, and adhering to copyright law.
- Perform peer-review of a manuscript, abstract, or other research-based work, in collaboration with colleagues as appropriate.
- Demonstrate basic skills in communicating about research opportunities with patients and the family/caregivers within the local context.
Attitudes
Pediatric hospitalists should be able to:
- Recognize the value of seeking the research that supports clinical care decisions and how research fills knowledge gaps and challenges the field to advance.
- Realize the importance of informed consent for patient participation in clinical research.
- Reflect on the importance of patient assent, even in the presence of legal guardian informed consent, when involving children in clinical research.
- Exemplify highly ethical behaviors when promoting or participating in research studies.
- Realize the value of and exemplify a willingness to perform journal-requested peer review of manuscripts, conference abstracts, or other research-based work.
- Reflect on and provide support and education for patients and the family/caregivers on the benefits of research for hospitalized children.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in interdisciplinary initiatives to develop and sustain participation of interdisciplinary teams in performance of research.
- Collaborate with colleagues, hospital administration, and community leaders for thoughtful application of research findings to improve systems of healthcare delivery.
- Lead, coordinate, or participate in national multi-center research efforts that improve the evidence base in inpatient pediatrics, within local context.
- Collaborate with leaders in the university department of pediatrics and school of medicine, hospital administration, and medical staff to encourage local hospital participation in national multi-center research efforts.
- Collaborate with research team members to educate colleagues, hospital staff, and others on the importance of research in improving child health outcomes.
1. Hulley SB, Cummins SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research, 4th ed. Philadelphia, PA: Wolters Kluwer; 2013.
1. Hulley SB, Cummins SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research, 4th ed. Philadelphia, PA: Wolters Kluwer; 2013.
4.15 Healthcare Systems: Quality Improvement
Introduction
Quality Improvement (QI) in healthcare involves planning, implementation, and ongoing assessment of care to proactively improve healthcare outcomes. Hospitals use QI programs to optimize care, streamline systems operations, meet regulatory requirements, and enhance customer service quality. Since the publication of Crossing the Quality Chasm decades ago by the Institutes of Medicine (now the National Academies of Medicine), even greater attention has been focused on improving use and assessing outcomes of evidence-based practices. Proving that “quality of care” and healthcare “value” (quality achieved relative to cost) has been achieved is critical for individual hospitals as well as the national healthcare system. The challenge is to maintain fiscal viability while delivering appropriate healthcare. Healthcare leaders therefore consider QI programs integral to system operations as a means to assure that resources are used wisely and delivery of consistent outcomes that improve the health of the populations served occurs. Pediatric hospitalists work on the front lines of clinical care and are aware of opportunities to improve acute care management, address gaps in chronic care needs, and identify opportunities for system-wide enhancements. Pediatric hospitalists are well positioned to act as influential change agents to promote, champion, and lead QI projects to ensure the highest value of healthcare for hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast between Quality Assurance (focus on individual compliance with standards) and Quality Improvement (proactive systems improvement via integration of best practices).
- Define the “Model for Improvement.”
- Summarize the steps of the Shewart-Deming Plan Do Study Act (PDSA) cycle of improvement.
- Explain the value of demonstrating small gains and identifying failures for correction through rapid cycle improvement.
- Describe how lean methodology attempts to eliminate waste and Six Sigma attempts to reduce variation and defects within a process.
- Define commonly used QI tools and terms such as common cause and special cause variation, run charts, cumulative proportion charts, process map, and others.
- Cite examples of structure, process, outcome, and balancing metrics, attending to areas such as clinical, financial, resource use, and perceptions of care improvement.
- Summarize how QI supports effective development of care standardization, best practices, and practice guidelines in order to improve clinical outcomes.
- Discuss the importance of integrating evidence-based medicine into the planning stage of QI projects affecting patient care.
- Explain how QI can be effectively used for both clinical and system operations improvements using examples such as clinical care guidelines and hospital procedures.
- Describe the business case for quality and review why quality should drive cost and resource allocation.
- Define the role of the patient and family in QI and illustrate how their involvement or perspectives are central to QI project success.
- Discuss how interprofessional teams and a culture of commitment to QI impact the success of QI Programs.
- Explain the role of human factors in implementing healthcare improvements.
- List the attributes necessary to moderate, facilitate, and lead QI initiatives and discuss the importance of team building methods.
- Summarize how regulatory, accrediting, advocacy, research funders, and insurers impact QI initiatives and outcomes reporting for hospitalized children, attending to the Centers for Medicare and Medicaid, The Joint Commission, Agency for Healthcare Research and Quality, Leapfrog, and the National Quality Forum.
- Discuss the value of national, state, and local comparative quality data reporting and the clinical, educational, and research utility of national sources such as the Pediatric Health Information Dataset (PHIS).
- Review how reporting quality outcomes to external sources and posting on local hospital websites can affect the patient experience and community trust.
- Summarize the value of continuous participation in QI activities, noting the expectations from medical school through American Board of Pediatrics initial and ongoing certification.
Skills
Pediatric hospitalists should be able to:
- Identify processes in need of improvement and engage the appropriate personnel to gain approval for a QI project.
- Demonstrate proficiency in performing each step in a basic QI project.
- Demonstrate proficiency in utilizing basic QI tools such as a process map, key driver diagram, and fishbone diagram.
- Perform review of quality data, including basic data analysis, interpretation, and development of recommendations from the data.
- Serve as a liaison between physician staff and hospital administrative staff when interpreting physician-specific information and clinical care outliers.
- Utilize communication and leadership skills to participate effectively on an interdisciplinary team.
- Educate trainees, nursing staff, ancillary staff, and peers on the basic principles of QI.
- Assist with development of practice guidelines to assure delivery of standardized high value care in the hospital setting.
- Use best practice guidelines effectively and consistently.
- Demonstrate facility with the use of common computer applications, including spreadsheet and database management for information retrieval and analysis.
Attitudes
Pediatric hospitalists should be able to:
- Realize the value of leading as an “early adopter” and “change agent” by building an awareness of and consensus for changes needed to make patient care quality a high priority.
- Recognize the importance of team building, leadership, and family centeredness in performing effective QI.
- Acknowledge the importance of collaboration with healthcare providers critical to QI efforts, such as clinical team members, information technology staff, data analysts, and others.
- Seek opportunities to initiate or actively participate in QI projects.
- Work collaboratively to help create and maintain a QI culture within the institution.
- Exemplify professional behavior when reviewing and interpreting data.
- Recognize how value is defined by the patient and family/caregivers and support QI efforts to increase this value.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Engage hospital, medical group, and medical staff leadership in creating, implementing, and sustaining short- and long-term QI goals that add value for all customers.
- Participate on QI committees and seek opportunities to serve as QI officers or consultants.
- Advocate for the necessary information systems and other infrastructure to secure accurate data and assure success in the QI process.
1. Agency for Healthcare Research and Quality. Toolkit for Using the AHRQ Quality Indicators. 2017 Edition. https://www.ahrq.gov/patient-safety/settings/hospital/resource/qitool/index.html. Accessed August 21, 2019.
2. Department of Health and Human Services Health Resources and Services Administration. Quality Improvement Toolkit. April 2011 Edition. https://www.hrsa.gov/sites/default/files/quality/toolbox/508pdfs/qualityimprovement.pdf. Accessed August 21, 2019.
3. Langley GL, Moen R, Nolan KM, Norman CL, Provost LP. The Improvement Guide - A Practical Approach to Enhancing Organizational Performance, 2nd ed. San Francisco, CA: Jossey-Bass; 2009.
Introduction
Quality Improvement (QI) in healthcare involves planning, implementation, and ongoing assessment of care to proactively improve healthcare outcomes. Hospitals use QI programs to optimize care, streamline systems operations, meet regulatory requirements, and enhance customer service quality. Since the publication of Crossing the Quality Chasm decades ago by the Institutes of Medicine (now the National Academies of Medicine), even greater attention has been focused on improving use and assessing outcomes of evidence-based practices. Proving that “quality of care” and healthcare “value” (quality achieved relative to cost) has been achieved is critical for individual hospitals as well as the national healthcare system. The challenge is to maintain fiscal viability while delivering appropriate healthcare. Healthcare leaders therefore consider QI programs integral to system operations as a means to assure that resources are used wisely and delivery of consistent outcomes that improve the health of the populations served occurs. Pediatric hospitalists work on the front lines of clinical care and are aware of opportunities to improve acute care management, address gaps in chronic care needs, and identify opportunities for system-wide enhancements. Pediatric hospitalists are well positioned to act as influential change agents to promote, champion, and lead QI projects to ensure the highest value of healthcare for hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast between Quality Assurance (focus on individual compliance with standards) and Quality Improvement (proactive systems improvement via integration of best practices).
- Define the “Model for Improvement.”
- Summarize the steps of the Shewart-Deming Plan Do Study Act (PDSA) cycle of improvement.
- Explain the value of demonstrating small gains and identifying failures for correction through rapid cycle improvement.
- Describe how lean methodology attempts to eliminate waste and Six Sigma attempts to reduce variation and defects within a process.
- Define commonly used QI tools and terms such as common cause and special cause variation, run charts, cumulative proportion charts, process map, and others.
- Cite examples of structure, process, outcome, and balancing metrics, attending to areas such as clinical, financial, resource use, and perceptions of care improvement.
- Summarize how QI supports effective development of care standardization, best practices, and practice guidelines in order to improve clinical outcomes.
- Discuss the importance of integrating evidence-based medicine into the planning stage of QI projects affecting patient care.
- Explain how QI can be effectively used for both clinical and system operations improvements using examples such as clinical care guidelines and hospital procedures.
- Describe the business case for quality and review why quality should drive cost and resource allocation.
- Define the role of the patient and family in QI and illustrate how their involvement or perspectives are central to QI project success.
- Discuss how interprofessional teams and a culture of commitment to QI impact the success of QI Programs.
- Explain the role of human factors in implementing healthcare improvements.
- List the attributes necessary to moderate, facilitate, and lead QI initiatives and discuss the importance of team building methods.
- Summarize how regulatory, accrediting, advocacy, research funders, and insurers impact QI initiatives and outcomes reporting for hospitalized children, attending to the Centers for Medicare and Medicaid, The Joint Commission, Agency for Healthcare Research and Quality, Leapfrog, and the National Quality Forum.
- Discuss the value of national, state, and local comparative quality data reporting and the clinical, educational, and research utility of national sources such as the Pediatric Health Information Dataset (PHIS).
- Review how reporting quality outcomes to external sources and posting on local hospital websites can affect the patient experience and community trust.
- Summarize the value of continuous participation in QI activities, noting the expectations from medical school through American Board of Pediatrics initial and ongoing certification.
Skills
Pediatric hospitalists should be able to:
- Identify processes in need of improvement and engage the appropriate personnel to gain approval for a QI project.
- Demonstrate proficiency in performing each step in a basic QI project.
- Demonstrate proficiency in utilizing basic QI tools such as a process map, key driver diagram, and fishbone diagram.
- Perform review of quality data, including basic data analysis, interpretation, and development of recommendations from the data.
- Serve as a liaison between physician staff and hospital administrative staff when interpreting physician-specific information and clinical care outliers.
- Utilize communication and leadership skills to participate effectively on an interdisciplinary team.
- Educate trainees, nursing staff, ancillary staff, and peers on the basic principles of QI.
- Assist with development of practice guidelines to assure delivery of standardized high value care in the hospital setting.
- Use best practice guidelines effectively and consistently.
- Demonstrate facility with the use of common computer applications, including spreadsheet and database management for information retrieval and analysis.
Attitudes
Pediatric hospitalists should be able to:
- Realize the value of leading as an “early adopter” and “change agent” by building an awareness of and consensus for changes needed to make patient care quality a high priority.
- Recognize the importance of team building, leadership, and family centeredness in performing effective QI.
- Acknowledge the importance of collaboration with healthcare providers critical to QI efforts, such as clinical team members, information technology staff, data analysts, and others.
- Seek opportunities to initiate or actively participate in QI projects.
- Work collaboratively to help create and maintain a QI culture within the institution.
- Exemplify professional behavior when reviewing and interpreting data.
- Recognize how value is defined by the patient and family/caregivers and support QI efforts to increase this value.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Engage hospital, medical group, and medical staff leadership in creating, implementing, and sustaining short- and long-term QI goals that add value for all customers.
- Participate on QI committees and seek opportunities to serve as QI officers or consultants.
- Advocate for the necessary information systems and other infrastructure to secure accurate data and assure success in the QI process.
Introduction
Quality Improvement (QI) in healthcare involves planning, implementation, and ongoing assessment of care to proactively improve healthcare outcomes. Hospitals use QI programs to optimize care, streamline systems operations, meet regulatory requirements, and enhance customer service quality. Since the publication of Crossing the Quality Chasm decades ago by the Institutes of Medicine (now the National Academies of Medicine), even greater attention has been focused on improving use and assessing outcomes of evidence-based practices. Proving that “quality of care” and healthcare “value” (quality achieved relative to cost) has been achieved is critical for individual hospitals as well as the national healthcare system. The challenge is to maintain fiscal viability while delivering appropriate healthcare. Healthcare leaders therefore consider QI programs integral to system operations as a means to assure that resources are used wisely and delivery of consistent outcomes that improve the health of the populations served occurs. Pediatric hospitalists work on the front lines of clinical care and are aware of opportunities to improve acute care management, address gaps in chronic care needs, and identify opportunities for system-wide enhancements. Pediatric hospitalists are well positioned to act as influential change agents to promote, champion, and lead QI projects to ensure the highest value of healthcare for hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast between Quality Assurance (focus on individual compliance with standards) and Quality Improvement (proactive systems improvement via integration of best practices).
- Define the “Model for Improvement.”
- Summarize the steps of the Shewart-Deming Plan Do Study Act (PDSA) cycle of improvement.
- Explain the value of demonstrating small gains and identifying failures for correction through rapid cycle improvement.
- Describe how lean methodology attempts to eliminate waste and Six Sigma attempts to reduce variation and defects within a process.
- Define commonly used QI tools and terms such as common cause and special cause variation, run charts, cumulative proportion charts, process map, and others.
- Cite examples of structure, process, outcome, and balancing metrics, attending to areas such as clinical, financial, resource use, and perceptions of care improvement.
- Summarize how QI supports effective development of care standardization, best practices, and practice guidelines in order to improve clinical outcomes.
- Discuss the importance of integrating evidence-based medicine into the planning stage of QI projects affecting patient care.
- Explain how QI can be effectively used for both clinical and system operations improvements using examples such as clinical care guidelines and hospital procedures.
- Describe the business case for quality and review why quality should drive cost and resource allocation.
- Define the role of the patient and family in QI and illustrate how their involvement or perspectives are central to QI project success.
- Discuss how interprofessional teams and a culture of commitment to QI impact the success of QI Programs.
- Explain the role of human factors in implementing healthcare improvements.
- List the attributes necessary to moderate, facilitate, and lead QI initiatives and discuss the importance of team building methods.
- Summarize how regulatory, accrediting, advocacy, research funders, and insurers impact QI initiatives and outcomes reporting for hospitalized children, attending to the Centers for Medicare and Medicaid, The Joint Commission, Agency for Healthcare Research and Quality, Leapfrog, and the National Quality Forum.
- Discuss the value of national, state, and local comparative quality data reporting and the clinical, educational, and research utility of national sources such as the Pediatric Health Information Dataset (PHIS).
- Review how reporting quality outcomes to external sources and posting on local hospital websites can affect the patient experience and community trust.
- Summarize the value of continuous participation in QI activities, noting the expectations from medical school through American Board of Pediatrics initial and ongoing certification.
Skills
Pediatric hospitalists should be able to:
- Identify processes in need of improvement and engage the appropriate personnel to gain approval for a QI project.
- Demonstrate proficiency in performing each step in a basic QI project.
- Demonstrate proficiency in utilizing basic QI tools such as a process map, key driver diagram, and fishbone diagram.
- Perform review of quality data, including basic data analysis, interpretation, and development of recommendations from the data.
- Serve as a liaison between physician staff and hospital administrative staff when interpreting physician-specific information and clinical care outliers.
- Utilize communication and leadership skills to participate effectively on an interdisciplinary team.
- Educate trainees, nursing staff, ancillary staff, and peers on the basic principles of QI.
- Assist with development of practice guidelines to assure delivery of standardized high value care in the hospital setting.
- Use best practice guidelines effectively and consistently.
- Demonstrate facility with the use of common computer applications, including spreadsheet and database management for information retrieval and analysis.
Attitudes
Pediatric hospitalists should be able to:
- Realize the value of leading as an “early adopter” and “change agent” by building an awareness of and consensus for changes needed to make patient care quality a high priority.
- Recognize the importance of team building, leadership, and family centeredness in performing effective QI.
- Acknowledge the importance of collaboration with healthcare providers critical to QI efforts, such as clinical team members, information technology staff, data analysts, and others.
- Seek opportunities to initiate or actively participate in QI projects.
- Work collaboratively to help create and maintain a QI culture within the institution.
- Exemplify professional behavior when reviewing and interpreting data.
- Recognize how value is defined by the patient and family/caregivers and support QI efforts to increase this value.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Engage hospital, medical group, and medical staff leadership in creating, implementing, and sustaining short- and long-term QI goals that add value for all customers.
- Participate on QI committees and seek opportunities to serve as QI officers or consultants.
- Advocate for the necessary information systems and other infrastructure to secure accurate data and assure success in the QI process.
1. Agency for Healthcare Research and Quality. Toolkit for Using the AHRQ Quality Indicators. 2017 Edition. https://www.ahrq.gov/patient-safety/settings/hospital/resource/qitool/index.html. Accessed August 21, 2019.
2. Department of Health and Human Services Health Resources and Services Administration. Quality Improvement Toolkit. April 2011 Edition. https://www.hrsa.gov/sites/default/files/quality/toolbox/508pdfs/qualityimprovement.pdf. Accessed August 21, 2019.
3. Langley GL, Moen R, Nolan KM, Norman CL, Provost LP. The Improvement Guide - A Practical Approach to Enhancing Organizational Performance, 2nd ed. San Francisco, CA: Jossey-Bass; 2009.
1. Agency for Healthcare Research and Quality. Toolkit for Using the AHRQ Quality Indicators. 2017 Edition. https://www.ahrq.gov/patient-safety/settings/hospital/resource/qitool/index.html. Accessed August 21, 2019.
2. Department of Health and Human Services Health Resources and Services Administration. Quality Improvement Toolkit. April 2011 Edition. https://www.hrsa.gov/sites/default/files/quality/toolbox/508pdfs/qualityimprovement.pdf. Accessed August 21, 2019.
3. Langley GL, Moen R, Nolan KM, Norman CL, Provost LP. The Improvement Guide - A Practical Approach to Enhancing Organizational Performance, 2nd ed. San Francisco, CA: Jossey-Bass; 2009.
4.14 Healthcare Systems: Patient Safety
Introduction
Patient safety is defined as freedom from accidental injury caused by medical care, such as harm or death attributable to adverse drug events, patient misidentifications, or health care-acquired infections. In 1999 the Institute of Medicine (IOM; now the National Academy of Medicine) published the “To Err is Human” report, which challenged United States healthcare systems and providers to recognize, report, and mitigate error and harm to patients. Children, as a vulnerable population, are at particular risk for medical errors and specifically medication errors. Pediatric hospitalists work in the acute care hospital setting where high-risk diagnostic decision-making, transitions of care, medication safety, and handoffs are commonly performed. Pediatric hospitalists therefore have a duty to promote patient safety and help develop and implement systems to reduce both error and harm to hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Review the basic principles of patient safety, including systems redesign and the prevention, identification, and mitigation of preventable adverse events.
- Review the difference between error and harm including different types of errors.
- Cite the key components of a culture of safety.
- Review the fundamental components of a “Just Culture” and describe how organizations can achieve them.
- Discuss why errors are multifactorial and more often the result of systems failures rather than individual failures.
- Define the concept of “second victim” and review steps to support colleagues, trainees, and other providers when they become a second victim.
- Define common features of a “High Reliability Organization” and explain how high reliability principles apply to clinical care and work on patient safety initiatives.
- Review common patient safety interventions to reduce errors, including electronic order sets, practice guidelines, checklists, clinical decision support, double checks, bar coding, lock-out drawers, and others.
- Discuss factors unique to children that lead to increased risk for medication errors.
- Describe how using structured communication techniques, such as standardized handoffs, closed loop communication, active listening, and critical language are critical to safety.
- Describe the role of patient/family engagement in patient safety.
- Describe the safety components of hospital accreditation and how pediatric hospitalists can help ensure these standards are met.
- Describe common types of cognitive biases, such as premature closure, anchoring, and others, and review how they contribute to diagnostic error.
- Discuss the goals of national safety collaboratives, such as Solutions for Patient Safety (SPS) and describe safety bundles for common hospital-acquired conditions (HACs).
- Review the role of pediatric hospitalists in maintaining national safety goals required by common key accrediting organizations, such as The Joint Commission (TJC) and others.
Skills
Pediatric hospitalists should be able to:
- Demonstrate skill in creating an environment that reflects a high reliability organization.
- Facilitate safe and efficient hospital admissions and discharges.
- Identify and order the level of nursing care needed for safe patient care.
- Engage and educate patients and the family/caregivers on their role in ensuring patient safety.
- Utilize and participate in optimizing patient safety features of health information technology.
- Educate trainees, colleagues, and other healthcare providers on basic safety principles.
- Demonstrate proficiency in reporting errors using safety reporting systems.
- Work effectively and collaboratively with patient safety teams.
- Engage in patient safety event reviews, including (root) causal analyses, Morbidity and Mortality committees, and sentinel event reviews.
- Disclose medical errors clearly, concisely, and completely to patients and the family/caregivers.
- Participate in continuous readiness for accreditation agencies by consistently adhering to patient safety practices.
Attitudes
Pediatric hospitalists should be able to:
- Reflect on the importance of creating and sustaining a culture of patient safety.
- Role model behaviors that exemplify a “Just Culture,” accountability, and learning from failure.
- Recognize that patient safety improvements come from consistently reporting near misses as well as medical errors.
- Promote an awareness of the need for and will for change to make patient safety a high and consistent priority.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in multidisciplinary broad strategies to positively impact patient safety in the organization.
- Collaborate with hospital administration and community leaders for the necessary information systems and other infrastructure to ensure success with pediatric patient safety initiatives.
- Lead, coordinate, or participate in multidisciplinary initiatives to develop and implement patient safety interventions where possible.
- Actively participate in hospital-wide safety committees and seek to become leaders in pediatric patient safety in their institutions.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ Solutions for Patient Safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://pediatrics.aappublications.org/content/140/3/e20163494.long. Accessed August 28, 2019.
2. Muething SE, Goudie A, Schoettker PJ, et al. Quality improvement initiative to reduce serious safety events and improve patient safety culture. Pediatrics. 2012;130(2): e423-e431. https://pediatrics.aappublications.org/content/130/2/e423.long. Accessed August 28, 2019.
3. Mueller BU, Neuspiel DR, Fisher ER. Principles of pediatric patient safety: Reducing harm due to medical care. Pediatrics. 2019;143(2):e20183649. https://doi.org/10.1542/peds.2018-3649.
Introduction
Patient safety is defined as freedom from accidental injury caused by medical care, such as harm or death attributable to adverse drug events, patient misidentifications, or health care-acquired infections. In 1999 the Institute of Medicine (IOM; now the National Academy of Medicine) published the “To Err is Human” report, which challenged United States healthcare systems and providers to recognize, report, and mitigate error and harm to patients. Children, as a vulnerable population, are at particular risk for medical errors and specifically medication errors. Pediatric hospitalists work in the acute care hospital setting where high-risk diagnostic decision-making, transitions of care, medication safety, and handoffs are commonly performed. Pediatric hospitalists therefore have a duty to promote patient safety and help develop and implement systems to reduce both error and harm to hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Review the basic principles of patient safety, including systems redesign and the prevention, identification, and mitigation of preventable adverse events.
- Review the difference between error and harm including different types of errors.
- Cite the key components of a culture of safety.
- Review the fundamental components of a “Just Culture” and describe how organizations can achieve them.
- Discuss why errors are multifactorial and more often the result of systems failures rather than individual failures.
- Define the concept of “second victim” and review steps to support colleagues, trainees, and other providers when they become a second victim.
- Define common features of a “High Reliability Organization” and explain how high reliability principles apply to clinical care and work on patient safety initiatives.
- Review common patient safety interventions to reduce errors, including electronic order sets, practice guidelines, checklists, clinical decision support, double checks, bar coding, lock-out drawers, and others.
- Discuss factors unique to children that lead to increased risk for medication errors.
- Describe how using structured communication techniques, such as standardized handoffs, closed loop communication, active listening, and critical language are critical to safety.
- Describe the role of patient/family engagement in patient safety.
- Describe the safety components of hospital accreditation and how pediatric hospitalists can help ensure these standards are met.
- Describe common types of cognitive biases, such as premature closure, anchoring, and others, and review how they contribute to diagnostic error.
- Discuss the goals of national safety collaboratives, such as Solutions for Patient Safety (SPS) and describe safety bundles for common hospital-acquired conditions (HACs).
- Review the role of pediatric hospitalists in maintaining national safety goals required by common key accrediting organizations, such as The Joint Commission (TJC) and others.
Skills
Pediatric hospitalists should be able to:
- Demonstrate skill in creating an environment that reflects a high reliability organization.
- Facilitate safe and efficient hospital admissions and discharges.
- Identify and order the level of nursing care needed for safe patient care.
- Engage and educate patients and the family/caregivers on their role in ensuring patient safety.
- Utilize and participate in optimizing patient safety features of health information technology.
- Educate trainees, colleagues, and other healthcare providers on basic safety principles.
- Demonstrate proficiency in reporting errors using safety reporting systems.
- Work effectively and collaboratively with patient safety teams.
- Engage in patient safety event reviews, including (root) causal analyses, Morbidity and Mortality committees, and sentinel event reviews.
- Disclose medical errors clearly, concisely, and completely to patients and the family/caregivers.
- Participate in continuous readiness for accreditation agencies by consistently adhering to patient safety practices.
Attitudes
Pediatric hospitalists should be able to:
- Reflect on the importance of creating and sustaining a culture of patient safety.
- Role model behaviors that exemplify a “Just Culture,” accountability, and learning from failure.
- Recognize that patient safety improvements come from consistently reporting near misses as well as medical errors.
- Promote an awareness of the need for and will for change to make patient safety a high and consistent priority.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in multidisciplinary broad strategies to positively impact patient safety in the organization.
- Collaborate with hospital administration and community leaders for the necessary information systems and other infrastructure to ensure success with pediatric patient safety initiatives.
- Lead, coordinate, or participate in multidisciplinary initiatives to develop and implement patient safety interventions where possible.
- Actively participate in hospital-wide safety committees and seek to become leaders in pediatric patient safety in their institutions.
Introduction
Patient safety is defined as freedom from accidental injury caused by medical care, such as harm or death attributable to adverse drug events, patient misidentifications, or health care-acquired infections. In 1999 the Institute of Medicine (IOM; now the National Academy of Medicine) published the “To Err is Human” report, which challenged United States healthcare systems and providers to recognize, report, and mitigate error and harm to patients. Children, as a vulnerable population, are at particular risk for medical errors and specifically medication errors. Pediatric hospitalists work in the acute care hospital setting where high-risk diagnostic decision-making, transitions of care, medication safety, and handoffs are commonly performed. Pediatric hospitalists therefore have a duty to promote patient safety and help develop and implement systems to reduce both error and harm to hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Review the basic principles of patient safety, including systems redesign and the prevention, identification, and mitigation of preventable adverse events.
- Review the difference between error and harm including different types of errors.
- Cite the key components of a culture of safety.
- Review the fundamental components of a “Just Culture” and describe how organizations can achieve them.
- Discuss why errors are multifactorial and more often the result of systems failures rather than individual failures.
- Define the concept of “second victim” and review steps to support colleagues, trainees, and other providers when they become a second victim.
- Define common features of a “High Reliability Organization” and explain how high reliability principles apply to clinical care and work on patient safety initiatives.
- Review common patient safety interventions to reduce errors, including electronic order sets, practice guidelines, checklists, clinical decision support, double checks, bar coding, lock-out drawers, and others.
- Discuss factors unique to children that lead to increased risk for medication errors.
- Describe how using structured communication techniques, such as standardized handoffs, closed loop communication, active listening, and critical language are critical to safety.
- Describe the role of patient/family engagement in patient safety.
- Describe the safety components of hospital accreditation and how pediatric hospitalists can help ensure these standards are met.
- Describe common types of cognitive biases, such as premature closure, anchoring, and others, and review how they contribute to diagnostic error.
- Discuss the goals of national safety collaboratives, such as Solutions for Patient Safety (SPS) and describe safety bundles for common hospital-acquired conditions (HACs).
- Review the role of pediatric hospitalists in maintaining national safety goals required by common key accrediting organizations, such as The Joint Commission (TJC) and others.
Skills
Pediatric hospitalists should be able to:
- Demonstrate skill in creating an environment that reflects a high reliability organization.
- Facilitate safe and efficient hospital admissions and discharges.
- Identify and order the level of nursing care needed for safe patient care.
- Engage and educate patients and the family/caregivers on their role in ensuring patient safety.
- Utilize and participate in optimizing patient safety features of health information technology.
- Educate trainees, colleagues, and other healthcare providers on basic safety principles.
- Demonstrate proficiency in reporting errors using safety reporting systems.
- Work effectively and collaboratively with patient safety teams.
- Engage in patient safety event reviews, including (root) causal analyses, Morbidity and Mortality committees, and sentinel event reviews.
- Disclose medical errors clearly, concisely, and completely to patients and the family/caregivers.
- Participate in continuous readiness for accreditation agencies by consistently adhering to patient safety practices.
Attitudes
Pediatric hospitalists should be able to:
- Reflect on the importance of creating and sustaining a culture of patient safety.
- Role model behaviors that exemplify a “Just Culture,” accountability, and learning from failure.
- Recognize that patient safety improvements come from consistently reporting near misses as well as medical errors.
- Promote an awareness of the need for and will for change to make patient safety a high and consistent priority.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in multidisciplinary broad strategies to positively impact patient safety in the organization.
- Collaborate with hospital administration and community leaders for the necessary information systems and other infrastructure to ensure success with pediatric patient safety initiatives.
- Lead, coordinate, or participate in multidisciplinary initiatives to develop and implement patient safety interventions where possible.
- Actively participate in hospital-wide safety committees and seek to become leaders in pediatric patient safety in their institutions.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ Solutions for Patient Safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://pediatrics.aappublications.org/content/140/3/e20163494.long. Accessed August 28, 2019.
2. Muething SE, Goudie A, Schoettker PJ, et al. Quality improvement initiative to reduce serious safety events and improve patient safety culture. Pediatrics. 2012;130(2): e423-e431. https://pediatrics.aappublications.org/content/130/2/e423.long. Accessed August 28, 2019.
3. Mueller BU, Neuspiel DR, Fisher ER. Principles of pediatric patient safety: Reducing harm due to medical care. Pediatrics. 2019;143(2):e20183649. https://doi.org/10.1542/peds.2018-3649.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ Solutions for Patient Safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://pediatrics.aappublications.org/content/140/3/e20163494.long. Accessed August 28, 2019.
2. Muething SE, Goudie A, Schoettker PJ, et al. Quality improvement initiative to reduce serious safety events and improve patient safety culture. Pediatrics. 2012;130(2): e423-e431. https://pediatrics.aappublications.org/content/130/2/e423.long. Accessed August 28, 2019.
3. Mueller BU, Neuspiel DR, Fisher ER. Principles of pediatric patient safety: Reducing harm due to medical care. Pediatrics. 2019;143(2):e20183649. https://doi.org/10.1542/peds.2018-3649.
4.13 Healthcare Systems: Legal Issues and Risk Management
Introduction
Risk Management is a discipline commonly perceived to be the domain of institutional personnel and committees who are called upon to address adverse events that have already occurred. However, consequence management is far from the most effective utilization of such resources, as they are most efficiently and ethically deployed in preventive programs. Risk management therefore prospectively draws upon the disciplines of patient safety, performance improvement, systems management (including engineering and technology), ethics, and human factors in addition to medicine, in an effort to eliminate or ameliorate the undesirable consequences of delivering healthcare services. Hospitalized children are a highly vulnerable population due to social dependencies and developmental needs and have unique legal regulations that may impact care delivery. Pediatric hospitalists deliver care in this acute, high-risk healthcare environment and should be knowledgeable about legal and regulatory requirements, prevention strategies, and ways in which to collaborate with other professionals in management of hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Summarize the role of common entities that accredit and license organizations, including The Joint Commission (TJC), the Centers for Medicare and Medicaid Services (CMS), and state health departments.
- Cite examples of how interfacility transfer of patients may be affected by the Emergency Medical Treatment and Active Labor Act (EMTALA).
- Summarize the basic regulatory and legal stipulations that may impact pediatric hospitalist contracting and practice, as noted in the anti-kickback regulations (Stark Rules) and anti-trust regulations (Sherman Act).
- Discuss the importance of fraud and abuse regulations for billing, coding, documentation, collections, utilization review, and managed care operations.
- Describe the common features of privacy regulations, such as the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
- Review the role of physician licensing and oversight agencies such as the state Medical Board, National Practitioner Data Bank, and Drug Enforcement Agency.
- Define “medical liability,” “standard of practice,” and “negligence” and discuss the role of state malpractice statutes of limitation for children.
- Discuss the role of behavior and attitudes in generating patient and family/caregiver complaints.
- Describe the behavioral and physical characteristics of the impaired practitioner, including fatigue, substance abuse, and disruptive behavior.
- Summarize the role of the hospital medical staff in granting clinical privileges and initiating disciplinary actions through peer review process.
- List responsibilities associated with maintaining malpractice insurance, including documentation and disclosure requirements.
- Define the terms “assent” and “consent” and describe the circumstances in which informed assent or consent is needed.
- Give an example of legal issues that can arise in various clinical scenarios, such as end of life care, “no code” discussions (do-not-resuscitate or allow-natural-death), organ donation, guardianship, and newborn resuscitation.
- Describe the role of pediatric hospitalists in appropriate and timely notification to risk management or hospital counsel when medical errors or preventable events occur.
- Describe the role of pediatric hospitalists in recognizing and reporting family violence for the child, spouse, or elder.
- Provide examples of potential errors related to devices and technology, including Electronic Health Record (EHR) data entry, use, and documentation, privacy, device alert fatigue, and others.
- Review the relationship between human factors, design factors, risk management, patient safety, and quality improvement.
Skills
Pediatric hospitalists should be able to:
- Obtain informed assent and/or consent from patients and/or the family/caregivers.
- Disclose medical errors clearly, concisely, and completely to patients and the family/caregivers.
- Communicate in difficult situations and when delivering sensitive information, with compassion and a professional attitude.
- Support and communicate end-of-life decisions and planning.
- Transfer patient information concisely and precisely to other healthcare providers during all transitions of care.
- Prescribe treatments using safe medication prescribing practices.
- Document in the medical record with accuracy and appropriate detail.
- Identify when legal and risk management notification and/or expert consultation is indicated and initiate the escalation process.
- Demonstrate basic skills in utilizing risk reduction strategies, in partnership with local legal and risk management experts.
Attitudes
Pediatric hospitalists should be able to:
- Role model professional behavior.
- Recognize the importance of responding to complaints in a compassionate and sensitive manner.
- Reflect on the importance of collaborating with legal and risk management experts to learn and practice risk reduction strategies, such as failure modes and effects analysis (FMEA) and others.
- Reflect on and provide support and education for trainees in discussions on the importance of communication and documentation from the legal and risk management perspective.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in organizational risk management efforts and promote risk prevention by active participation in appropriate hospital committees.
- Collaborate with hospital administration and other colleagues to advocate for and modify systems and processes that help risk reduction.
- Lead, coordinate, or participate in healthcare information systems related initiatives that enhance the ease and accuracy of documentation and prescribing.
- Lead, coordinate, or participate in efforts to create a comprehensive risk reduction program encompassing education for hospital staff, medical staff, and trainees.
1. Dickson G. Principles of risk management. Qual Health Care. 1995;4(2):75-79. https://doi.org/10.1136/qshc.4.2.75.
2. Vincent C, Taylor-Adams S, Chapman EJ, et al. How to investigate and analyze clinical incidents: Clinical risk unit and association of litigation and risk management protocol. BMJ. 2000;320(7237):777–781. https://doi.org/ 10.1136/bmj.320.7237.777.
Introduction
Risk Management is a discipline commonly perceived to be the domain of institutional personnel and committees who are called upon to address adverse events that have already occurred. However, consequence management is far from the most effective utilization of such resources, as they are most efficiently and ethically deployed in preventive programs. Risk management therefore prospectively draws upon the disciplines of patient safety, performance improvement, systems management (including engineering and technology), ethics, and human factors in addition to medicine, in an effort to eliminate or ameliorate the undesirable consequences of delivering healthcare services. Hospitalized children are a highly vulnerable population due to social dependencies and developmental needs and have unique legal regulations that may impact care delivery. Pediatric hospitalists deliver care in this acute, high-risk healthcare environment and should be knowledgeable about legal and regulatory requirements, prevention strategies, and ways in which to collaborate with other professionals in management of hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Summarize the role of common entities that accredit and license organizations, including The Joint Commission (TJC), the Centers for Medicare and Medicaid Services (CMS), and state health departments.
- Cite examples of how interfacility transfer of patients may be affected by the Emergency Medical Treatment and Active Labor Act (EMTALA).
- Summarize the basic regulatory and legal stipulations that may impact pediatric hospitalist contracting and practice, as noted in the anti-kickback regulations (Stark Rules) and anti-trust regulations (Sherman Act).
- Discuss the importance of fraud and abuse regulations for billing, coding, documentation, collections, utilization review, and managed care operations.
- Describe the common features of privacy regulations, such as the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
- Review the role of physician licensing and oversight agencies such as the state Medical Board, National Practitioner Data Bank, and Drug Enforcement Agency.
- Define “medical liability,” “standard of practice,” and “negligence” and discuss the role of state malpractice statutes of limitation for children.
- Discuss the role of behavior and attitudes in generating patient and family/caregiver complaints.
- Describe the behavioral and physical characteristics of the impaired practitioner, including fatigue, substance abuse, and disruptive behavior.
- Summarize the role of the hospital medical staff in granting clinical privileges and initiating disciplinary actions through peer review process.
- List responsibilities associated with maintaining malpractice insurance, including documentation and disclosure requirements.
- Define the terms “assent” and “consent” and describe the circumstances in which informed assent or consent is needed.
- Give an example of legal issues that can arise in various clinical scenarios, such as end of life care, “no code” discussions (do-not-resuscitate or allow-natural-death), organ donation, guardianship, and newborn resuscitation.
- Describe the role of pediatric hospitalists in appropriate and timely notification to risk management or hospital counsel when medical errors or preventable events occur.
- Describe the role of pediatric hospitalists in recognizing and reporting family violence for the child, spouse, or elder.
- Provide examples of potential errors related to devices and technology, including Electronic Health Record (EHR) data entry, use, and documentation, privacy, device alert fatigue, and others.
- Review the relationship between human factors, design factors, risk management, patient safety, and quality improvement.
Skills
Pediatric hospitalists should be able to:
- Obtain informed assent and/or consent from patients and/or the family/caregivers.
- Disclose medical errors clearly, concisely, and completely to patients and the family/caregivers.
- Communicate in difficult situations and when delivering sensitive information, with compassion and a professional attitude.
- Support and communicate end-of-life decisions and planning.
- Transfer patient information concisely and precisely to other healthcare providers during all transitions of care.
- Prescribe treatments using safe medication prescribing practices.
- Document in the medical record with accuracy and appropriate detail.
- Identify when legal and risk management notification and/or expert consultation is indicated and initiate the escalation process.
- Demonstrate basic skills in utilizing risk reduction strategies, in partnership with local legal and risk management experts.
Attitudes
Pediatric hospitalists should be able to:
- Role model professional behavior.
- Recognize the importance of responding to complaints in a compassionate and sensitive manner.
- Reflect on the importance of collaborating with legal and risk management experts to learn and practice risk reduction strategies, such as failure modes and effects analysis (FMEA) and others.
- Reflect on and provide support and education for trainees in discussions on the importance of communication and documentation from the legal and risk management perspective.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in organizational risk management efforts and promote risk prevention by active participation in appropriate hospital committees.
- Collaborate with hospital administration and other colleagues to advocate for and modify systems and processes that help risk reduction.
- Lead, coordinate, or participate in healthcare information systems related initiatives that enhance the ease and accuracy of documentation and prescribing.
- Lead, coordinate, or participate in efforts to create a comprehensive risk reduction program encompassing education for hospital staff, medical staff, and trainees.
Introduction
Risk Management is a discipline commonly perceived to be the domain of institutional personnel and committees who are called upon to address adverse events that have already occurred. However, consequence management is far from the most effective utilization of such resources, as they are most efficiently and ethically deployed in preventive programs. Risk management therefore prospectively draws upon the disciplines of patient safety, performance improvement, systems management (including engineering and technology), ethics, and human factors in addition to medicine, in an effort to eliminate or ameliorate the undesirable consequences of delivering healthcare services. Hospitalized children are a highly vulnerable population due to social dependencies and developmental needs and have unique legal regulations that may impact care delivery. Pediatric hospitalists deliver care in this acute, high-risk healthcare environment and should be knowledgeable about legal and regulatory requirements, prevention strategies, and ways in which to collaborate with other professionals in management of hospitalized children.
Knowledge
Pediatric hospitalists should be able to:
- Summarize the role of common entities that accredit and license organizations, including The Joint Commission (TJC), the Centers for Medicare and Medicaid Services (CMS), and state health departments.
- Cite examples of how interfacility transfer of patients may be affected by the Emergency Medical Treatment and Active Labor Act (EMTALA).
- Summarize the basic regulatory and legal stipulations that may impact pediatric hospitalist contracting and practice, as noted in the anti-kickback regulations (Stark Rules) and anti-trust regulations (Sherman Act).
- Discuss the importance of fraud and abuse regulations for billing, coding, documentation, collections, utilization review, and managed care operations.
- Describe the common features of privacy regulations, such as the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
- Review the role of physician licensing and oversight agencies such as the state Medical Board, National Practitioner Data Bank, and Drug Enforcement Agency.
- Define “medical liability,” “standard of practice,” and “negligence” and discuss the role of state malpractice statutes of limitation for children.
- Discuss the role of behavior and attitudes in generating patient and family/caregiver complaints.
- Describe the behavioral and physical characteristics of the impaired practitioner, including fatigue, substance abuse, and disruptive behavior.
- Summarize the role of the hospital medical staff in granting clinical privileges and initiating disciplinary actions through peer review process.
- List responsibilities associated with maintaining malpractice insurance, including documentation and disclosure requirements.
- Define the terms “assent” and “consent” and describe the circumstances in which informed assent or consent is needed.
- Give an example of legal issues that can arise in various clinical scenarios, such as end of life care, “no code” discussions (do-not-resuscitate or allow-natural-death), organ donation, guardianship, and newborn resuscitation.
- Describe the role of pediatric hospitalists in appropriate and timely notification to risk management or hospital counsel when medical errors or preventable events occur.
- Describe the role of pediatric hospitalists in recognizing and reporting family violence for the child, spouse, or elder.
- Provide examples of potential errors related to devices and technology, including Electronic Health Record (EHR) data entry, use, and documentation, privacy, device alert fatigue, and others.
- Review the relationship between human factors, design factors, risk management, patient safety, and quality improvement.
Skills
Pediatric hospitalists should be able to:
- Obtain informed assent and/or consent from patients and/or the family/caregivers.
- Disclose medical errors clearly, concisely, and completely to patients and the family/caregivers.
- Communicate in difficult situations and when delivering sensitive information, with compassion and a professional attitude.
- Support and communicate end-of-life decisions and planning.
- Transfer patient information concisely and precisely to other healthcare providers during all transitions of care.
- Prescribe treatments using safe medication prescribing practices.
- Document in the medical record with accuracy and appropriate detail.
- Identify when legal and risk management notification and/or expert consultation is indicated and initiate the escalation process.
- Demonstrate basic skills in utilizing risk reduction strategies, in partnership with local legal and risk management experts.
Attitudes
Pediatric hospitalists should be able to:
- Role model professional behavior.
- Recognize the importance of responding to complaints in a compassionate and sensitive manner.
- Reflect on the importance of collaborating with legal and risk management experts to learn and practice risk reduction strategies, such as failure modes and effects analysis (FMEA) and others.
- Reflect on and provide support and education for trainees in discussions on the importance of communication and documentation from the legal and risk management perspective.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in organizational risk management efforts and promote risk prevention by active participation in appropriate hospital committees.
- Collaborate with hospital administration and other colleagues to advocate for and modify systems and processes that help risk reduction.
- Lead, coordinate, or participate in healthcare information systems related initiatives that enhance the ease and accuracy of documentation and prescribing.
- Lead, coordinate, or participate in efforts to create a comprehensive risk reduction program encompassing education for hospital staff, medical staff, and trainees.
1. Dickson G. Principles of risk management. Qual Health Care. 1995;4(2):75-79. https://doi.org/10.1136/qshc.4.2.75.
2. Vincent C, Taylor-Adams S, Chapman EJ, et al. How to investigate and analyze clinical incidents: Clinical risk unit and association of litigation and risk management protocol. BMJ. 2000;320(7237):777–781. https://doi.org/ 10.1136/bmj.320.7237.777.
1. Dickson G. Principles of risk management. Qual Health Care. 1995;4(2):75-79. https://doi.org/10.1136/qshc.4.2.75.
2. Vincent C, Taylor-Adams S, Chapman EJ, et al. How to investigate and analyze clinical incidents: Clinical risk unit and association of litigation and risk management protocol. BMJ. 2000;320(7237):777–781. https://doi.org/ 10.1136/bmj.320.7237.777.

