User login
Telerehabilitation may be effective in MS
Telerehabilitation also saves time and travel cost, compared with outpatient rehabilitation.
“This model of home-based telerehabilitation offers a safe and cost-effective method for improving function and quality of life for MS patients with mobility deficits,” said Heather Barksdale, DPT, a neurological clinical specialist at UF Health Jacksonville (Florida).
The study was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The Centers for Medicare & Medicaid Services do not reimburse for telerehabilitation services. Patients with MS have difficulty accessing rehabilitation specialists because of impaired mobility and lack of access to transportation. “We are based in Jacksonville, Fla., and often have patients who have to travel from Tallahassee, Panama City, Daytona Beach, and Brunswick, Ga., to receive specialty services,” said Dr. Barksdale. “Telerehabilitation would allow these patients to get access to high-quality rehab services with clinicians that specialize in MS.”
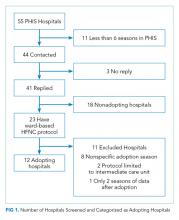
Dr. Barksdale and colleagues conducted a pilot study to evaluate the feasibility of a physical therapy–guided telerehabilitation program for people with mobility impairments resulting from confirmed MS. The investigators enrolled patients at the MS Center of Excellence at University of Florida Health Jacksonville into a telerehabilitation group. A board-certified neurologist and a physical therapist specializing in MS examined participants in person at baseline. The latter underwent an 8-week program of physical therapy–guided telerehabilitation that used the Jintronix software platform and a kinetic tracking system.
By reviewing charts during January 2018–September 2019, Dr. Barksdale and colleagues selected patients with MS who were seen on an outpatient basis by the same physical therapists who were administering telerehabilitation. This outpatient comparison group was matched to the telerehabilitation group on duration of treatment and outcome measures completed. Dr. Barksdale and colleagues reviewed the data for the effects of the two interventions on mobility and travel.
Eight patients completed the telerehabilitation program, and all had improvements in fatigue, quality of life, or mobility measures. The investigators did not observe any adverse events during or after the intervention. The total savings in projected travel costs for all eight participants was $8,487.23, compared with the outpatient group. Participants in the telerehabilitation and outpatient groups achieved minimal detectable changes in the outcome measures examined at equivalent rates.
“The game-based model with virtual visits by a physical therapist can be modified to include exercises specific for other motor, coordination, spasticity, and movement dysfunctions and may be useful for other chronic and progressive dysfunction seen in Parkinson’s disease, stroke, and other movement and neuromuscular disorders,” said Dr. Barksdale.
“Future studies are needed to further establish guidelines for patient selection and mode of delivery, as well as design of future telerehabilitation programs,” she added. “Duration of treatment and types of exercises to be included should also be examined. Further research into use of telerehabilitation for the treatment of upper-extremity, cognitive, speech, and swallowing dysfunction should also be examined.”
The investigators conducted their study without outside funding and reported no disclosures.
SOURCE: Barksdale H et al. CMSC 2020. Abstract REH11.
Telerehabilitation also saves time and travel cost, compared with outpatient rehabilitation.
“This model of home-based telerehabilitation offers a safe and cost-effective method for improving function and quality of life for MS patients with mobility deficits,” said Heather Barksdale, DPT, a neurological clinical specialist at UF Health Jacksonville (Florida).
The study was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The Centers for Medicare & Medicaid Services do not reimburse for telerehabilitation services. Patients with MS have difficulty accessing rehabilitation specialists because of impaired mobility and lack of access to transportation. “We are based in Jacksonville, Fla., and often have patients who have to travel from Tallahassee, Panama City, Daytona Beach, and Brunswick, Ga., to receive specialty services,” said Dr. Barksdale. “Telerehabilitation would allow these patients to get access to high-quality rehab services with clinicians that specialize in MS.”
Dr. Barksdale and colleagues conducted a pilot study to evaluate the feasibility of a physical therapy–guided telerehabilitation program for people with mobility impairments resulting from confirmed MS. The investigators enrolled patients at the MS Center of Excellence at University of Florida Health Jacksonville into a telerehabilitation group. A board-certified neurologist and a physical therapist specializing in MS examined participants in person at baseline. The latter underwent an 8-week program of physical therapy–guided telerehabilitation that used the Jintronix software platform and a kinetic tracking system.
By reviewing charts during January 2018–September 2019, Dr. Barksdale and colleagues selected patients with MS who were seen on an outpatient basis by the same physical therapists who were administering telerehabilitation. This outpatient comparison group was matched to the telerehabilitation group on duration of treatment and outcome measures completed. Dr. Barksdale and colleagues reviewed the data for the effects of the two interventions on mobility and travel.
Eight patients completed the telerehabilitation program, and all had improvements in fatigue, quality of life, or mobility measures. The investigators did not observe any adverse events during or after the intervention. The total savings in projected travel costs for all eight participants was $8,487.23, compared with the outpatient group. Participants in the telerehabilitation and outpatient groups achieved minimal detectable changes in the outcome measures examined at equivalent rates.
“The game-based model with virtual visits by a physical therapist can be modified to include exercises specific for other motor, coordination, spasticity, and movement dysfunctions and may be useful for other chronic and progressive dysfunction seen in Parkinson’s disease, stroke, and other movement and neuromuscular disorders,” said Dr. Barksdale.
“Future studies are needed to further establish guidelines for patient selection and mode of delivery, as well as design of future telerehabilitation programs,” she added. “Duration of treatment and types of exercises to be included should also be examined. Further research into use of telerehabilitation for the treatment of upper-extremity, cognitive, speech, and swallowing dysfunction should also be examined.”
The investigators conducted their study without outside funding and reported no disclosures.
SOURCE: Barksdale H et al. CMSC 2020. Abstract REH11.
Telerehabilitation also saves time and travel cost, compared with outpatient rehabilitation.
“This model of home-based telerehabilitation offers a safe and cost-effective method for improving function and quality of life for MS patients with mobility deficits,” said Heather Barksdale, DPT, a neurological clinical specialist at UF Health Jacksonville (Florida).
The study was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The Centers for Medicare & Medicaid Services do not reimburse for telerehabilitation services. Patients with MS have difficulty accessing rehabilitation specialists because of impaired mobility and lack of access to transportation. “We are based in Jacksonville, Fla., and often have patients who have to travel from Tallahassee, Panama City, Daytona Beach, and Brunswick, Ga., to receive specialty services,” said Dr. Barksdale. “Telerehabilitation would allow these patients to get access to high-quality rehab services with clinicians that specialize in MS.”
Dr. Barksdale and colleagues conducted a pilot study to evaluate the feasibility of a physical therapy–guided telerehabilitation program for people with mobility impairments resulting from confirmed MS. The investigators enrolled patients at the MS Center of Excellence at University of Florida Health Jacksonville into a telerehabilitation group. A board-certified neurologist and a physical therapist specializing in MS examined participants in person at baseline. The latter underwent an 8-week program of physical therapy–guided telerehabilitation that used the Jintronix software platform and a kinetic tracking system.
By reviewing charts during January 2018–September 2019, Dr. Barksdale and colleagues selected patients with MS who were seen on an outpatient basis by the same physical therapists who were administering telerehabilitation. This outpatient comparison group was matched to the telerehabilitation group on duration of treatment and outcome measures completed. Dr. Barksdale and colleagues reviewed the data for the effects of the two interventions on mobility and travel.
Eight patients completed the telerehabilitation program, and all had improvements in fatigue, quality of life, or mobility measures. The investigators did not observe any adverse events during or after the intervention. The total savings in projected travel costs for all eight participants was $8,487.23, compared with the outpatient group. Participants in the telerehabilitation and outpatient groups achieved minimal detectable changes in the outcome measures examined at equivalent rates.
“The game-based model with virtual visits by a physical therapist can be modified to include exercises specific for other motor, coordination, spasticity, and movement dysfunctions and may be useful for other chronic and progressive dysfunction seen in Parkinson’s disease, stroke, and other movement and neuromuscular disorders,” said Dr. Barksdale.
“Future studies are needed to further establish guidelines for patient selection and mode of delivery, as well as design of future telerehabilitation programs,” she added. “Duration of treatment and types of exercises to be included should also be examined. Further research into use of telerehabilitation for the treatment of upper-extremity, cognitive, speech, and swallowing dysfunction should also be examined.”
The investigators conducted their study without outside funding and reported no disclosures.
SOURCE: Barksdale H et al. CMSC 2020. Abstract REH11.
REPORTING FROM CMSC 2020
FDA approves ixekizumab for nonradiographic axSpA
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
No benefit of three commonly used medications for MS fatigue
The TRIUMPHANT study found no difference between the effects of amantadine, modafinil, methylphenidate, and placebo in the Modified Fatigue Impact Scale (MFIS) in a study involving 141 patients with MS.
There was also no difference between any of the drugs and placebo in any of the preplanned subgroups which included different Expanded Disability Status Scale scores, depressive scores, use of disease-modifying therapy, or type of MS (relapsing remitting or progressive).
The research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
“These three drugs are used very commonly used for MS fatigue by neurologists, psychiatrists, and primary care doctors, but they don’t seem to be any better than placebo. They were all associated with increased side effects compared with placebo even with short-term use,” said lead investigator Bardia Nourbakhsh, MD, assistant professor of neurology at Johns Hopkins University, Baltimore.
However, in a post hoc analysis there was an improvement in daytime sleepiness with two of the drugs – methylphenidate and modafinil. “These two agents reduced daytime sleepiness in patients with high daytime sleepiness scores at baseline, with about a 4-point difference versus placebo, which was significant. But as this was not a preplanned analysis, we have to be cautious in its interpretation,” Dr. Nourbakhsh said. “However, this finding may not be too surprising as both these drugs are licensed as stimulants for use in narcolepsy patients with excessive daytime sleepiness.”
“Our recommendations are that as amantadine was not better than placebo in any subgroup its use should be discouraged in MS fatigue,” Dr. Nourbakhsh commented. “Modafinil and methylphenidate may possibly be considered for MS patients with excessive daytime sleepiness, but this should really be confirmed in further studies.”
Fatigue is a common and debilitating symptom of MS, occurring in about 70%-80% of patients with MS. There is no approved drug treatment. However nonpharmacologic therapies have shown some success: studies of exercise and cognitive-behavioral therapy (CBT) have shown these may be effective without causing side effects, Dr. Nourbakhsh noted. “So we should be getting patients to try exercise and CBT before jumping to medication.”
Dr. Nourbakhsh said he was disappointed with the results of the study but not terribly surprised. “We use these three medications frequently in the clinic and we have not been seeing great benefits so we wondered whether they were actually effective.”
He said that the trial was adequately powered and the question has been answered. “These are valuable results – they will hopefully encourage doctors to think twice before prescribing these medications that could be harmful and have no clear benefit,” Dr. Nourbakhsh concluded.
For the randomized, double-blind, placebo-controlled, four-sequence, four-period crossover trial, 141 patients with MS and fatigue received twice-daily oral amantadine (maximum 200 mg/day), modafinil (maximum 200 mg/day), methylphenidate (maximum 20 mg/day), or placebo, each given for up to 6 weeks with a 2-week washout between each medication.
Patients had a mean baseline MFIS score of 51.3 and were randomly assigned to one of four medication administration sequences. Data from 136 participants were available for the analysis of the primary outcome (change in MFIS score), and 111 participants completed all four medication periods.
In the intent-to-treat analysis, the least-squares means of total MFIS scores at the maximally tolerated dose were as follows: 40.7 with placebo, 41.2 with amantadine, 39.0 with modafinil, and 38.7 with methylphenidate (P = .20 for the overall medication effect; P > .05 for all pairwise comparisons). “All medications and placebo reduced the MS fatigue score by 10-12 points from baseline, so there was quite a substantial placebo effect,” Dr. Nourbakhsh noted. There was no statistically significant difference in the physical and cognitive subscales of MFIS and quality of life measures between any of the study medications and placebo. All three drugs were associated with an increase in adverse effects versus placebo.
Dr. Nourbakhsh says he is hopeful that this negative study may stimulate further research into new targets and medications for MS fatigue.
His group has recently conducted a pilot study of intravenous ketamine in MS fatigue with some encouraging results, but he stressed it needs to be tested in a larger study before it can be recommended for use in clinical practice. “While an IV medication is not ideal, the effect did seem to be quite long-lived with a difference still evident at 28 days, so it could perhaps be dosed once a month, which could be feasible,” he said.
Commenting on the TRIUMPHANT study, Jeffrey Cohen, MD, of the Cleveland Clinic, said that “fatigue is a common, often disabling, symptom of MS. It is poorly understood and probably encompasses several mechanisms. There currently is no generally effective treatment for MS-related fatigue.”
“These results are not surprising and confirm previous studies,” Dr. Cohen said. “Despite no benefit from these medicines for patients as a group, they are occasionally helpful for individual patients, so they are frequently tried empirically.
“It also is important to address any factors besides MS that may be causing or contributing to fatigue, for example, sleep disruption, medication side effects, depression, other medical conditions such as anemia or hypothyroidism,” he added.
Dr. Nourbakhsh has reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities for Jazz Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
The TRIUMPHANT study found no difference between the effects of amantadine, modafinil, methylphenidate, and placebo in the Modified Fatigue Impact Scale (MFIS) in a study involving 141 patients with MS.
There was also no difference between any of the drugs and placebo in any of the preplanned subgroups which included different Expanded Disability Status Scale scores, depressive scores, use of disease-modifying therapy, or type of MS (relapsing remitting or progressive).
The research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
“These three drugs are used very commonly used for MS fatigue by neurologists, psychiatrists, and primary care doctors, but they don’t seem to be any better than placebo. They were all associated with increased side effects compared with placebo even with short-term use,” said lead investigator Bardia Nourbakhsh, MD, assistant professor of neurology at Johns Hopkins University, Baltimore.
However, in a post hoc analysis there was an improvement in daytime sleepiness with two of the drugs – methylphenidate and modafinil. “These two agents reduced daytime sleepiness in patients with high daytime sleepiness scores at baseline, with about a 4-point difference versus placebo, which was significant. But as this was not a preplanned analysis, we have to be cautious in its interpretation,” Dr. Nourbakhsh said. “However, this finding may not be too surprising as both these drugs are licensed as stimulants for use in narcolepsy patients with excessive daytime sleepiness.”
“Our recommendations are that as amantadine was not better than placebo in any subgroup its use should be discouraged in MS fatigue,” Dr. Nourbakhsh commented. “Modafinil and methylphenidate may possibly be considered for MS patients with excessive daytime sleepiness, but this should really be confirmed in further studies.”
Fatigue is a common and debilitating symptom of MS, occurring in about 70%-80% of patients with MS. There is no approved drug treatment. However nonpharmacologic therapies have shown some success: studies of exercise and cognitive-behavioral therapy (CBT) have shown these may be effective without causing side effects, Dr. Nourbakhsh noted. “So we should be getting patients to try exercise and CBT before jumping to medication.”
Dr. Nourbakhsh said he was disappointed with the results of the study but not terribly surprised. “We use these three medications frequently in the clinic and we have not been seeing great benefits so we wondered whether they were actually effective.”
He said that the trial was adequately powered and the question has been answered. “These are valuable results – they will hopefully encourage doctors to think twice before prescribing these medications that could be harmful and have no clear benefit,” Dr. Nourbakhsh concluded.
For the randomized, double-blind, placebo-controlled, four-sequence, four-period crossover trial, 141 patients with MS and fatigue received twice-daily oral amantadine (maximum 200 mg/day), modafinil (maximum 200 mg/day), methylphenidate (maximum 20 mg/day), or placebo, each given for up to 6 weeks with a 2-week washout between each medication.
Patients had a mean baseline MFIS score of 51.3 and were randomly assigned to one of four medication administration sequences. Data from 136 participants were available for the analysis of the primary outcome (change in MFIS score), and 111 participants completed all four medication periods.
In the intent-to-treat analysis, the least-squares means of total MFIS scores at the maximally tolerated dose were as follows: 40.7 with placebo, 41.2 with amantadine, 39.0 with modafinil, and 38.7 with methylphenidate (P = .20 for the overall medication effect; P > .05 for all pairwise comparisons). “All medications and placebo reduced the MS fatigue score by 10-12 points from baseline, so there was quite a substantial placebo effect,” Dr. Nourbakhsh noted. There was no statistically significant difference in the physical and cognitive subscales of MFIS and quality of life measures between any of the study medications and placebo. All three drugs were associated with an increase in adverse effects versus placebo.
Dr. Nourbakhsh says he is hopeful that this negative study may stimulate further research into new targets and medications for MS fatigue.
His group has recently conducted a pilot study of intravenous ketamine in MS fatigue with some encouraging results, but he stressed it needs to be tested in a larger study before it can be recommended for use in clinical practice. “While an IV medication is not ideal, the effect did seem to be quite long-lived with a difference still evident at 28 days, so it could perhaps be dosed once a month, which could be feasible,” he said.
Commenting on the TRIUMPHANT study, Jeffrey Cohen, MD, of the Cleveland Clinic, said that “fatigue is a common, often disabling, symptom of MS. It is poorly understood and probably encompasses several mechanisms. There currently is no generally effective treatment for MS-related fatigue.”
“These results are not surprising and confirm previous studies,” Dr. Cohen said. “Despite no benefit from these medicines for patients as a group, they are occasionally helpful for individual patients, so they are frequently tried empirically.
“It also is important to address any factors besides MS that may be causing or contributing to fatigue, for example, sleep disruption, medication side effects, depression, other medical conditions such as anemia or hypothyroidism,” he added.
Dr. Nourbakhsh has reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities for Jazz Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
The TRIUMPHANT study found no difference between the effects of amantadine, modafinil, methylphenidate, and placebo in the Modified Fatigue Impact Scale (MFIS) in a study involving 141 patients with MS.
There was also no difference between any of the drugs and placebo in any of the preplanned subgroups which included different Expanded Disability Status Scale scores, depressive scores, use of disease-modifying therapy, or type of MS (relapsing remitting or progressive).
The research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
“These three drugs are used very commonly used for MS fatigue by neurologists, psychiatrists, and primary care doctors, but they don’t seem to be any better than placebo. They were all associated with increased side effects compared with placebo even with short-term use,” said lead investigator Bardia Nourbakhsh, MD, assistant professor of neurology at Johns Hopkins University, Baltimore.
However, in a post hoc analysis there was an improvement in daytime sleepiness with two of the drugs – methylphenidate and modafinil. “These two agents reduced daytime sleepiness in patients with high daytime sleepiness scores at baseline, with about a 4-point difference versus placebo, which was significant. But as this was not a preplanned analysis, we have to be cautious in its interpretation,” Dr. Nourbakhsh said. “However, this finding may not be too surprising as both these drugs are licensed as stimulants for use in narcolepsy patients with excessive daytime sleepiness.”
“Our recommendations are that as amantadine was not better than placebo in any subgroup its use should be discouraged in MS fatigue,” Dr. Nourbakhsh commented. “Modafinil and methylphenidate may possibly be considered for MS patients with excessive daytime sleepiness, but this should really be confirmed in further studies.”
Fatigue is a common and debilitating symptom of MS, occurring in about 70%-80% of patients with MS. There is no approved drug treatment. However nonpharmacologic therapies have shown some success: studies of exercise and cognitive-behavioral therapy (CBT) have shown these may be effective without causing side effects, Dr. Nourbakhsh noted. “So we should be getting patients to try exercise and CBT before jumping to medication.”
Dr. Nourbakhsh said he was disappointed with the results of the study but not terribly surprised. “We use these three medications frequently in the clinic and we have not been seeing great benefits so we wondered whether they were actually effective.”
He said that the trial was adequately powered and the question has been answered. “These are valuable results – they will hopefully encourage doctors to think twice before prescribing these medications that could be harmful and have no clear benefit,” Dr. Nourbakhsh concluded.
For the randomized, double-blind, placebo-controlled, four-sequence, four-period crossover trial, 141 patients with MS and fatigue received twice-daily oral amantadine (maximum 200 mg/day), modafinil (maximum 200 mg/day), methylphenidate (maximum 20 mg/day), or placebo, each given for up to 6 weeks with a 2-week washout between each medication.
Patients had a mean baseline MFIS score of 51.3 and were randomly assigned to one of four medication administration sequences. Data from 136 participants were available for the analysis of the primary outcome (change in MFIS score), and 111 participants completed all four medication periods.
In the intent-to-treat analysis, the least-squares means of total MFIS scores at the maximally tolerated dose were as follows: 40.7 with placebo, 41.2 with amantadine, 39.0 with modafinil, and 38.7 with methylphenidate (P = .20 for the overall medication effect; P > .05 for all pairwise comparisons). “All medications and placebo reduced the MS fatigue score by 10-12 points from baseline, so there was quite a substantial placebo effect,” Dr. Nourbakhsh noted. There was no statistically significant difference in the physical and cognitive subscales of MFIS and quality of life measures between any of the study medications and placebo. All three drugs were associated with an increase in adverse effects versus placebo.
Dr. Nourbakhsh says he is hopeful that this negative study may stimulate further research into new targets and medications for MS fatigue.
His group has recently conducted a pilot study of intravenous ketamine in MS fatigue with some encouraging results, but he stressed it needs to be tested in a larger study before it can be recommended for use in clinical practice. “While an IV medication is not ideal, the effect did seem to be quite long-lived with a difference still evident at 28 days, so it could perhaps be dosed once a month, which could be feasible,” he said.
Commenting on the TRIUMPHANT study, Jeffrey Cohen, MD, of the Cleveland Clinic, said that “fatigue is a common, often disabling, symptom of MS. It is poorly understood and probably encompasses several mechanisms. There currently is no generally effective treatment for MS-related fatigue.”
“These results are not surprising and confirm previous studies,” Dr. Cohen said. “Despite no benefit from these medicines for patients as a group, they are occasionally helpful for individual patients, so they are frequently tried empirically.
“It also is important to address any factors besides MS that may be causing or contributing to fatigue, for example, sleep disruption, medication side effects, depression, other medical conditions such as anemia or hypothyroidism,” he added.
Dr. Nourbakhsh has reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities for Jazz Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
‘After Life’ and before good treatment
Portrayal of psychiatry in Netflix series could deter people from getting help
While many across the world who have access to Netflix and other streaming services have been on lockdown, the second season of Ricky Gervais’s dark comedy series, “After Life,” was released. The show will also return for a third season.
The setup of the show is that Lisa, the wife of Gervais’s protagonist, Tony, has died of breast cancer. Knowing that he would need help after, she made him a video guide to life without her, ranging from the mundane of a garbage day or house alarm to feeding their dog Brandy, tidying the house, and constantly reminding him to take care of himself.
When we first see Tony, he is not doing great on self-care, and he has turned his grief into a “super power” allowing himself to do or say whatever he wants to – from pretending to reprimand his dog for calling a man (who had just told him his dog should be on a lead) a “fat hairy nosy !#$%&” to getting into a name-calling exchange with a primary school child. He later (jokingly) threatens this same child with a hammer, so that the child will stop bullying his nephew.
Tony works as the head of features for the Tambury Gazette, the free local paper. The comedy is full of the hometown charm with Tony and the photographer, Lenny, visiting the homes of the interesting personalities who have called into the paper with their small-town newsworthy stories.
Colorful characters abound in his town, including Postman Pat, who pops in and helps himself to a bath. Tony develops an unlikely friendship with a sex worker whom he hires to clean his house – since she said that she would do “anything for 50 quid.”
Tony, in the midst of an existential crisis, visits his wife’s grave frequently. While there, he meets an older widow, Anne, who befriends him and offers good advice. (Anne is played by Penelope Wilton of The Best Exotic Marigold Hotel and Downton Abbey.)
Tony also dutifully visits his father daily at the Autumnal Leaves Care Home. His father has dementia and keeps asking about Lisa, forgetting that she is dead. Tony comments that if his father were a dog, he would euthanize him. In actuality, Tony’s dog, Brandy, stops Tony’s potential suicide throughout the series.
Matt, who is Tony’s brother-in-law (and boss at the paper) describes Tony as “devastated, suicidal.” Tony explains that he can do and say what he wants, and “then when it all gets too much, I can always kill myself.” By season 2, Matt’s wife has left him, and he, too, needs to see the psychiatrist.
The problem is the Tambury psychiatrist (played by Paul Kaye). General psychiatrists in film have been described in various ways by the late Irving Schneider, MD, including Dr. Evil, Dr. Wonderful, and Dr. Dippy types. “Dr. Dippy’s Sanitarium” was a 1906 silent film in which Dr. Dippy is seen lacking in common sense but being harmless overall. Based on the behaviors displayed in and out of therapy, the Tambury psychiatrist could never be described as Dr. Wonderful, leading to the Dr. Evil or the Dr. Dippy options. He is certainly using patients for his own personal gratification (like a Dr. Evil might) and is certainly lacking in common sense and acting “crazier or more foolish than his patients”1 (like a Dr. Dippy). However, this psychiatrist may need a category all to himself.
Tony sought out the psychiatrist at a desperate time in his life. The dark but comical way he expresses himself: “A good day is one where I don’t go around wanting to shoot random strangers in the face and then turn the gun on myself” is not met with compassion, but unfortunately by inappropriate chuckles. Instead of offering solace, the psychiatrist revealed confidential doctor-patient information about other patients. When pressed, the psychiatrist insists, “I didn’t say his name.” The psychiatrist also explains he is telling Tony privileged information to “let you know you’re not … the only mental case out there.” The psychiatrist is also blatantly tweeting on his phone during the session. He tells his patient that it is ridiculous to want a soul mate and explains that other species might rape their sexual conquest. He yawns loudly in a session with Tony. These are just some of the many cringe-worthy behaviors displayed by this (unnamed) fictional embarrassment to our field.
By season 2, the psychiatrist begins seeing Tony’s brother-in-law, Matt, in treatment, the first of his boundary violations with Matt since Matt is Tony’s close friend and relative. The psychiatrist soon makes the crass self-disclosure to Matt that, “I was bleeding from the anus for a month last year, and I never went to the doctor,” implying Matt is a wimp for coming in. The psychiatrist invites him to go out with him and his friends, and gives him a beer in a session. The psychiatrist tells Matt stories of his sex life and complains about why people are bothered about toxic masculinity. When there is no way it can get worse, Tony and Matt run into the psychiatrist and his mates in a pub. The psychiatrist tells his comrades: “That’s the suicidal one with the dead wife I was telling you about.” When asked about confidentiality, he again protests: “I didn’t say your name mate,” Gestures are made, and the patients are mocked and laughed at. Unfathomably, Matt still returns for therapy, but is told by the psychiatrist to “lie, cheat, just be a man,” and about lesbians using dildos. The psychiatrist complains to Matt he is “sick of this @#!&, hearing people winge all day.”
Dr. Dippy or Dr. Evil – or somewhere in between – Tambury’s psychiatrist is not anyone who should be seeing humans, let alone a vulnerable population seeking help. These satirical behaviors and comments perhaps suggest worries of the general population about what happens behind the closed doors of psychotherapy and the concern that there may not be such a thing as a “safe space.” Even though this character is meant to be funny, there is a concern that, in this difficult time, this portrayal could deter even one person from getting the help that they need.
In spite of this unfortunate characterization of psychiatry, “After Life” is a brilliant, dark portrayal of grief after loss, the comfort of pets, grief while losing someone to dementia, and even growth after loss. The theme of grief is especially poignant during this time of collective grief.
The difficulty is the portrayal of psychiatry and therapy – released at a time when in the real world, we are coping with a pandemic and expecting massive mental health fallout. Negative portrayals of psychiatry and therapy in this and other shows could potentially deter people from taking care of their own mental health in this traumatic time in our collective history when we all need to be vigilant about mental health.
Reference
1. Schneider I. Am J Psychiatry. 1987 Aug;144(8):966-1002.
Portrayal of psychiatry in Netflix series could deter people from getting help
Portrayal of psychiatry in Netflix series could deter people from getting help
While many across the world who have access to Netflix and other streaming services have been on lockdown, the second season of Ricky Gervais’s dark comedy series, “After Life,” was released. The show will also return for a third season.
The setup of the show is that Lisa, the wife of Gervais’s protagonist, Tony, has died of breast cancer. Knowing that he would need help after, she made him a video guide to life without her, ranging from the mundane of a garbage day or house alarm to feeding their dog Brandy, tidying the house, and constantly reminding him to take care of himself.
When we first see Tony, he is not doing great on self-care, and he has turned his grief into a “super power” allowing himself to do or say whatever he wants to – from pretending to reprimand his dog for calling a man (who had just told him his dog should be on a lead) a “fat hairy nosy !#$%&” to getting into a name-calling exchange with a primary school child. He later (jokingly) threatens this same child with a hammer, so that the child will stop bullying his nephew.
Tony works as the head of features for the Tambury Gazette, the free local paper. The comedy is full of the hometown charm with Tony and the photographer, Lenny, visiting the homes of the interesting personalities who have called into the paper with their small-town newsworthy stories.
Colorful characters abound in his town, including Postman Pat, who pops in and helps himself to a bath. Tony develops an unlikely friendship with a sex worker whom he hires to clean his house – since she said that she would do “anything for 50 quid.”
Tony, in the midst of an existential crisis, visits his wife’s grave frequently. While there, he meets an older widow, Anne, who befriends him and offers good advice. (Anne is played by Penelope Wilton of The Best Exotic Marigold Hotel and Downton Abbey.)
Tony also dutifully visits his father daily at the Autumnal Leaves Care Home. His father has dementia and keeps asking about Lisa, forgetting that she is dead. Tony comments that if his father were a dog, he would euthanize him. In actuality, Tony’s dog, Brandy, stops Tony’s potential suicide throughout the series.
Matt, who is Tony’s brother-in-law (and boss at the paper) describes Tony as “devastated, suicidal.” Tony explains that he can do and say what he wants, and “then when it all gets too much, I can always kill myself.” By season 2, Matt’s wife has left him, and he, too, needs to see the psychiatrist.
The problem is the Tambury psychiatrist (played by Paul Kaye). General psychiatrists in film have been described in various ways by the late Irving Schneider, MD, including Dr. Evil, Dr. Wonderful, and Dr. Dippy types. “Dr. Dippy’s Sanitarium” was a 1906 silent film in which Dr. Dippy is seen lacking in common sense but being harmless overall. Based on the behaviors displayed in and out of therapy, the Tambury psychiatrist could never be described as Dr. Wonderful, leading to the Dr. Evil or the Dr. Dippy options. He is certainly using patients for his own personal gratification (like a Dr. Evil might) and is certainly lacking in common sense and acting “crazier or more foolish than his patients”1 (like a Dr. Dippy). However, this psychiatrist may need a category all to himself.
Tony sought out the psychiatrist at a desperate time in his life. The dark but comical way he expresses himself: “A good day is one where I don’t go around wanting to shoot random strangers in the face and then turn the gun on myself” is not met with compassion, but unfortunately by inappropriate chuckles. Instead of offering solace, the psychiatrist revealed confidential doctor-patient information about other patients. When pressed, the psychiatrist insists, “I didn’t say his name.” The psychiatrist also explains he is telling Tony privileged information to “let you know you’re not … the only mental case out there.” The psychiatrist is also blatantly tweeting on his phone during the session. He tells his patient that it is ridiculous to want a soul mate and explains that other species might rape their sexual conquest. He yawns loudly in a session with Tony. These are just some of the many cringe-worthy behaviors displayed by this (unnamed) fictional embarrassment to our field.
By season 2, the psychiatrist begins seeing Tony’s brother-in-law, Matt, in treatment, the first of his boundary violations with Matt since Matt is Tony’s close friend and relative. The psychiatrist soon makes the crass self-disclosure to Matt that, “I was bleeding from the anus for a month last year, and I never went to the doctor,” implying Matt is a wimp for coming in. The psychiatrist invites him to go out with him and his friends, and gives him a beer in a session. The psychiatrist tells Matt stories of his sex life and complains about why people are bothered about toxic masculinity. When there is no way it can get worse, Tony and Matt run into the psychiatrist and his mates in a pub. The psychiatrist tells his comrades: “That’s the suicidal one with the dead wife I was telling you about.” When asked about confidentiality, he again protests: “I didn’t say your name mate,” Gestures are made, and the patients are mocked and laughed at. Unfathomably, Matt still returns for therapy, but is told by the psychiatrist to “lie, cheat, just be a man,” and about lesbians using dildos. The psychiatrist complains to Matt he is “sick of this @#!&, hearing people winge all day.”
Dr. Dippy or Dr. Evil – or somewhere in between – Tambury’s psychiatrist is not anyone who should be seeing humans, let alone a vulnerable population seeking help. These satirical behaviors and comments perhaps suggest worries of the general population about what happens behind the closed doors of psychotherapy and the concern that there may not be such a thing as a “safe space.” Even though this character is meant to be funny, there is a concern that, in this difficult time, this portrayal could deter even one person from getting the help that they need.
In spite of this unfortunate characterization of psychiatry, “After Life” is a brilliant, dark portrayal of grief after loss, the comfort of pets, grief while losing someone to dementia, and even growth after loss. The theme of grief is especially poignant during this time of collective grief.
The difficulty is the portrayal of psychiatry and therapy – released at a time when in the real world, we are coping with a pandemic and expecting massive mental health fallout. Negative portrayals of psychiatry and therapy in this and other shows could potentially deter people from taking care of their own mental health in this traumatic time in our collective history when we all need to be vigilant about mental health.
Reference
1. Schneider I. Am J Psychiatry. 1987 Aug;144(8):966-1002.
While many across the world who have access to Netflix and other streaming services have been on lockdown, the second season of Ricky Gervais’s dark comedy series, “After Life,” was released. The show will also return for a third season.
The setup of the show is that Lisa, the wife of Gervais’s protagonist, Tony, has died of breast cancer. Knowing that he would need help after, she made him a video guide to life without her, ranging from the mundane of a garbage day or house alarm to feeding their dog Brandy, tidying the house, and constantly reminding him to take care of himself.
When we first see Tony, he is not doing great on self-care, and he has turned his grief into a “super power” allowing himself to do or say whatever he wants to – from pretending to reprimand his dog for calling a man (who had just told him his dog should be on a lead) a “fat hairy nosy !#$%&” to getting into a name-calling exchange with a primary school child. He later (jokingly) threatens this same child with a hammer, so that the child will stop bullying his nephew.
Tony works as the head of features for the Tambury Gazette, the free local paper. The comedy is full of the hometown charm with Tony and the photographer, Lenny, visiting the homes of the interesting personalities who have called into the paper with their small-town newsworthy stories.
Colorful characters abound in his town, including Postman Pat, who pops in and helps himself to a bath. Tony develops an unlikely friendship with a sex worker whom he hires to clean his house – since she said that she would do “anything for 50 quid.”
Tony, in the midst of an existential crisis, visits his wife’s grave frequently. While there, he meets an older widow, Anne, who befriends him and offers good advice. (Anne is played by Penelope Wilton of The Best Exotic Marigold Hotel and Downton Abbey.)
Tony also dutifully visits his father daily at the Autumnal Leaves Care Home. His father has dementia and keeps asking about Lisa, forgetting that she is dead. Tony comments that if his father were a dog, he would euthanize him. In actuality, Tony’s dog, Brandy, stops Tony’s potential suicide throughout the series.
Matt, who is Tony’s brother-in-law (and boss at the paper) describes Tony as “devastated, suicidal.” Tony explains that he can do and say what he wants, and “then when it all gets too much, I can always kill myself.” By season 2, Matt’s wife has left him, and he, too, needs to see the psychiatrist.
The problem is the Tambury psychiatrist (played by Paul Kaye). General psychiatrists in film have been described in various ways by the late Irving Schneider, MD, including Dr. Evil, Dr. Wonderful, and Dr. Dippy types. “Dr. Dippy’s Sanitarium” was a 1906 silent film in which Dr. Dippy is seen lacking in common sense but being harmless overall. Based on the behaviors displayed in and out of therapy, the Tambury psychiatrist could never be described as Dr. Wonderful, leading to the Dr. Evil or the Dr. Dippy options. He is certainly using patients for his own personal gratification (like a Dr. Evil might) and is certainly lacking in common sense and acting “crazier or more foolish than his patients”1 (like a Dr. Dippy). However, this psychiatrist may need a category all to himself.
Tony sought out the psychiatrist at a desperate time in his life. The dark but comical way he expresses himself: “A good day is one where I don’t go around wanting to shoot random strangers in the face and then turn the gun on myself” is not met with compassion, but unfortunately by inappropriate chuckles. Instead of offering solace, the psychiatrist revealed confidential doctor-patient information about other patients. When pressed, the psychiatrist insists, “I didn’t say his name.” The psychiatrist also explains he is telling Tony privileged information to “let you know you’re not … the only mental case out there.” The psychiatrist is also blatantly tweeting on his phone during the session. He tells his patient that it is ridiculous to want a soul mate and explains that other species might rape their sexual conquest. He yawns loudly in a session with Tony. These are just some of the many cringe-worthy behaviors displayed by this (unnamed) fictional embarrassment to our field.
By season 2, the psychiatrist begins seeing Tony’s brother-in-law, Matt, in treatment, the first of his boundary violations with Matt since Matt is Tony’s close friend and relative. The psychiatrist soon makes the crass self-disclosure to Matt that, “I was bleeding from the anus for a month last year, and I never went to the doctor,” implying Matt is a wimp for coming in. The psychiatrist invites him to go out with him and his friends, and gives him a beer in a session. The psychiatrist tells Matt stories of his sex life and complains about why people are bothered about toxic masculinity. When there is no way it can get worse, Tony and Matt run into the psychiatrist and his mates in a pub. The psychiatrist tells his comrades: “That’s the suicidal one with the dead wife I was telling you about.” When asked about confidentiality, he again protests: “I didn’t say your name mate,” Gestures are made, and the patients are mocked and laughed at. Unfathomably, Matt still returns for therapy, but is told by the psychiatrist to “lie, cheat, just be a man,” and about lesbians using dildos. The psychiatrist complains to Matt he is “sick of this @#!&, hearing people winge all day.”
Dr. Dippy or Dr. Evil – or somewhere in between – Tambury’s psychiatrist is not anyone who should be seeing humans, let alone a vulnerable population seeking help. These satirical behaviors and comments perhaps suggest worries of the general population about what happens behind the closed doors of psychotherapy and the concern that there may not be such a thing as a “safe space.” Even though this character is meant to be funny, there is a concern that, in this difficult time, this portrayal could deter even one person from getting the help that they need.
In spite of this unfortunate characterization of psychiatry, “After Life” is a brilliant, dark portrayal of grief after loss, the comfort of pets, grief while losing someone to dementia, and even growth after loss. The theme of grief is especially poignant during this time of collective grief.
The difficulty is the portrayal of psychiatry and therapy – released at a time when in the real world, we are coping with a pandemic and expecting massive mental health fallout. Negative portrayals of psychiatry and therapy in this and other shows could potentially deter people from taking care of their own mental health in this traumatic time in our collective history when we all need to be vigilant about mental health.
Reference
1. Schneider I. Am J Psychiatry. 1987 Aug;144(8):966-1002.
Short medication regimen noninferior to long regimen for rifampin-resistant TB
Background: Multidrug-resistant TB is more difficult to treat than is drug-susceptible TB. The 2011 World Health Organization (WHO) recommendations for the treatment of multidrug-resistant TB, based on very-low-quality and conditional evidence, consists of an intensive treatment phase of 8 months and total treatment duration of 20 months. Although cohort studies have shown promising cure rates among patients with multidrug-resistant TB who received existing drugs in regimens shorter than that recommended by the WHO, data from phase 3 randomized trials were lacking.
Study design: Randomized phase 3 noninferior trial.
Setting: Multisite, international; countries were selected based on background disease burden of TB, multidrug-resistant TB, and TB-HIV coinfection (Ethiopia, Mongolia, South Africa, Vietnam).
Synopsis: 424 patients were randomized to the short and long medication regimen groups with 369 included in the modified intention-to-treat analysis and 310 included in the final per protocol efficacy analysis. The short regimen included IV moxifloxacin, clofazimine, ethambutol, and pyrazinamide administered over a 40-week period, supplemented by kanamycin, isoniazid, and prothionamide in the first 16 weeks, compared with 8 months of intense treatment and total 20 months of treatment in the long regimen. At 132 weeks after randomization, cultures were negative for Mycobacterium tuberculosis in more than 78 % patients in both long- and short-regimen group. Unfavorable bacteriologic outcome (10.6%), cardiac conduction defects (9.9%), and hepatobiliary problems (8.9%) were more common in the short-regimen group whereas patients in long-regimen group were lost to follow-up more frequently (2.4%) and had more metabolic disorders (7.1%). More deaths were reported in the short-regimen group, especially in those with HIV coinfections (17.5%). Although the results of this trial are encouraging, further studies will be needed to find a short, simple regimen for multidrug-resistant tuberculosis with improved safety outcomes.
Bottom line: Short medication regimen (9-11 months) is noninferior to the traditional WHO-recommended long regimen (20 months) for treating rifampin-resistant tuberculosis.
Citation: Nunn AJ et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019 Mar 28; 380:1201-13.
Dr. Kamath is an assistant professor of medicine at Duke University.
Background: Multidrug-resistant TB is more difficult to treat than is drug-susceptible TB. The 2011 World Health Organization (WHO) recommendations for the treatment of multidrug-resistant TB, based on very-low-quality and conditional evidence, consists of an intensive treatment phase of 8 months and total treatment duration of 20 months. Although cohort studies have shown promising cure rates among patients with multidrug-resistant TB who received existing drugs in regimens shorter than that recommended by the WHO, data from phase 3 randomized trials were lacking.
Study design: Randomized phase 3 noninferior trial.
Setting: Multisite, international; countries were selected based on background disease burden of TB, multidrug-resistant TB, and TB-HIV coinfection (Ethiopia, Mongolia, South Africa, Vietnam).
Synopsis: 424 patients were randomized to the short and long medication regimen groups with 369 included in the modified intention-to-treat analysis and 310 included in the final per protocol efficacy analysis. The short regimen included IV moxifloxacin, clofazimine, ethambutol, and pyrazinamide administered over a 40-week period, supplemented by kanamycin, isoniazid, and prothionamide in the first 16 weeks, compared with 8 months of intense treatment and total 20 months of treatment in the long regimen. At 132 weeks after randomization, cultures were negative for Mycobacterium tuberculosis in more than 78 % patients in both long- and short-regimen group. Unfavorable bacteriologic outcome (10.6%), cardiac conduction defects (9.9%), and hepatobiliary problems (8.9%) were more common in the short-regimen group whereas patients in long-regimen group were lost to follow-up more frequently (2.4%) and had more metabolic disorders (7.1%). More deaths were reported in the short-regimen group, especially in those with HIV coinfections (17.5%). Although the results of this trial are encouraging, further studies will be needed to find a short, simple regimen for multidrug-resistant tuberculosis with improved safety outcomes.
Bottom line: Short medication regimen (9-11 months) is noninferior to the traditional WHO-recommended long regimen (20 months) for treating rifampin-resistant tuberculosis.
Citation: Nunn AJ et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019 Mar 28; 380:1201-13.
Dr. Kamath is an assistant professor of medicine at Duke University.
Background: Multidrug-resistant TB is more difficult to treat than is drug-susceptible TB. The 2011 World Health Organization (WHO) recommendations for the treatment of multidrug-resistant TB, based on very-low-quality and conditional evidence, consists of an intensive treatment phase of 8 months and total treatment duration of 20 months. Although cohort studies have shown promising cure rates among patients with multidrug-resistant TB who received existing drugs in regimens shorter than that recommended by the WHO, data from phase 3 randomized trials were lacking.
Study design: Randomized phase 3 noninferior trial.
Setting: Multisite, international; countries were selected based on background disease burden of TB, multidrug-resistant TB, and TB-HIV coinfection (Ethiopia, Mongolia, South Africa, Vietnam).
Synopsis: 424 patients were randomized to the short and long medication regimen groups with 369 included in the modified intention-to-treat analysis and 310 included in the final per protocol efficacy analysis. The short regimen included IV moxifloxacin, clofazimine, ethambutol, and pyrazinamide administered over a 40-week period, supplemented by kanamycin, isoniazid, and prothionamide in the first 16 weeks, compared with 8 months of intense treatment and total 20 months of treatment in the long regimen. At 132 weeks after randomization, cultures were negative for Mycobacterium tuberculosis in more than 78 % patients in both long- and short-regimen group. Unfavorable bacteriologic outcome (10.6%), cardiac conduction defects (9.9%), and hepatobiliary problems (8.9%) were more common in the short-regimen group whereas patients in long-regimen group were lost to follow-up more frequently (2.4%) and had more metabolic disorders (7.1%). More deaths were reported in the short-regimen group, especially in those with HIV coinfections (17.5%). Although the results of this trial are encouraging, further studies will be needed to find a short, simple regimen for multidrug-resistant tuberculosis with improved safety outcomes.
Bottom line: Short medication regimen (9-11 months) is noninferior to the traditional WHO-recommended long regimen (20 months) for treating rifampin-resistant tuberculosis.
Citation: Nunn AJ et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019 Mar 28; 380:1201-13.
Dr. Kamath is an assistant professor of medicine at Duke University.
COVID-19: An opportunity to rehumanize psychiatry
Prior to the current crisis of COVID-19, I had a critical view of the direction of our psychiatric field. We have given up on complicated psychotherapies in favor of dispensing medications. We have given up on complicated diagnostic assessments in favor of simple self-rated symptoms questionnaires. Many of us even chose to give up on seeing patients face to face in favor of practicing telepsychiatry in the comfort of our homes. Some even promoted a future of psychiatry in which psychiatrists treated patients through large spreadsheets of evidence-based rating tools following evidence-based algorithms without even ever meeting the patients.
I do not view this problem as unique to psychiatry but rather as part of a larger trend in society. For the past couple of years, Vivek Murthy, MD, the former U.S. surgeon general, has popularized the idea that we are in a loneliness epidemic, saying, “We live in the most technologically connected age in the history of civilization, yet rates of loneliness have doubled since the 1980s.” Despite having enumerable means to reach other human beings, so many of us feel distant and out of touch with others. This loneliness has a measurable impact on our well-being with one study that states, “Actual and perceived social isolation are both associated with increased risk for early mortality.”
Then, seemingly out of nowhere, we were confronted with the largest challenge to our sense of connectedness in my lifetime. Throughout the past months, we have been asked to meet each other less frequently, do so through sterile means, and certainly not shake hands, hug, or embrace. The COVID-19 crisis has quickly made us all experts in telepsychiatry, remote work, and doing more with less. The COVID-19 crisis has asked many of us to put aside some of our human rituals like eating together, enjoying artistic experiences as a group, and touching, for the sake of saving lives.
For many, socially distancing has been a considerable added stressor – a stressor that continues to test humanity’s ability to be resilient. I am saddened by prior patients reaching out to seek comfort in these difficult times. I am touched by their desire to reconnect with someone they know, someone who feels familiar. I am surprised by the power of connection through phone and video calls. For some patients, despite the added burden, the current crisis has been an opportunity for their mental health and a reminder of the things that are important, including calling old friends and staying in touch with those who matter the most.
Yet, Checking in on others can become a chore. The social norm to partake in fashion, and self-care, become harder to find. In some cases, even hygiene and our health take a side role. The weekly phone visits with a therapist can feel just as mundane and repetitive as life. Sleep becomes harder to find, and food loses its taste. At this point, we realize the humanity that we lost in all this.
In the past couple of months, we have all become much more aware of the fragility of connectedness. However, we should recognize that the impact was well on its way before the COVID-19 crisis. It is my opinion that psychiatry should champion the issue of human relations. I do not think that we need to wait for a new DSM diagnosis, an evidence-based paradigm, or a Food and Drug Administration–approved medication to do so. The COVID-19 crisis has rendered us all cognizant of the importance of relationships.
While it may be that psychiatry continues to foray in electronic means of communication, use of impersonal scales and diagnosis, as well as anonymized algorithmic treatment plans, we should also promote as much humanity as society and public health safety will permit. Getting dressed to see your psychiatrist, face to face, to have an open-ended conversation about the nature of one’s life has clearly become something precious and powerful that should be cherished and protected. My hope is the rules and mandates we are required to use during the pandemic today do not become a continued habit that result in further loneliness and disconnect. If we chose to, the lessons we learn today can, in fact, strengthen our appreciation and pursuit of human connection.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019). He has no disclosures.
Prior to the current crisis of COVID-19, I had a critical view of the direction of our psychiatric field. We have given up on complicated psychotherapies in favor of dispensing medications. We have given up on complicated diagnostic assessments in favor of simple self-rated symptoms questionnaires. Many of us even chose to give up on seeing patients face to face in favor of practicing telepsychiatry in the comfort of our homes. Some even promoted a future of psychiatry in which psychiatrists treated patients through large spreadsheets of evidence-based rating tools following evidence-based algorithms without even ever meeting the patients.
I do not view this problem as unique to psychiatry but rather as part of a larger trend in society. For the past couple of years, Vivek Murthy, MD, the former U.S. surgeon general, has popularized the idea that we are in a loneliness epidemic, saying, “We live in the most technologically connected age in the history of civilization, yet rates of loneliness have doubled since the 1980s.” Despite having enumerable means to reach other human beings, so many of us feel distant and out of touch with others. This loneliness has a measurable impact on our well-being with one study that states, “Actual and perceived social isolation are both associated with increased risk for early mortality.”
Then, seemingly out of nowhere, we were confronted with the largest challenge to our sense of connectedness in my lifetime. Throughout the past months, we have been asked to meet each other less frequently, do so through sterile means, and certainly not shake hands, hug, or embrace. The COVID-19 crisis has quickly made us all experts in telepsychiatry, remote work, and doing more with less. The COVID-19 crisis has asked many of us to put aside some of our human rituals like eating together, enjoying artistic experiences as a group, and touching, for the sake of saving lives.
For many, socially distancing has been a considerable added stressor – a stressor that continues to test humanity’s ability to be resilient. I am saddened by prior patients reaching out to seek comfort in these difficult times. I am touched by their desire to reconnect with someone they know, someone who feels familiar. I am surprised by the power of connection through phone and video calls. For some patients, despite the added burden, the current crisis has been an opportunity for their mental health and a reminder of the things that are important, including calling old friends and staying in touch with those who matter the most.
Yet, Checking in on others can become a chore. The social norm to partake in fashion, and self-care, become harder to find. In some cases, even hygiene and our health take a side role. The weekly phone visits with a therapist can feel just as mundane and repetitive as life. Sleep becomes harder to find, and food loses its taste. At this point, we realize the humanity that we lost in all this.
In the past couple of months, we have all become much more aware of the fragility of connectedness. However, we should recognize that the impact was well on its way before the COVID-19 crisis. It is my opinion that psychiatry should champion the issue of human relations. I do not think that we need to wait for a new DSM diagnosis, an evidence-based paradigm, or a Food and Drug Administration–approved medication to do so. The COVID-19 crisis has rendered us all cognizant of the importance of relationships.
While it may be that psychiatry continues to foray in electronic means of communication, use of impersonal scales and diagnosis, as well as anonymized algorithmic treatment plans, we should also promote as much humanity as society and public health safety will permit. Getting dressed to see your psychiatrist, face to face, to have an open-ended conversation about the nature of one’s life has clearly become something precious and powerful that should be cherished and protected. My hope is the rules and mandates we are required to use during the pandemic today do not become a continued habit that result in further loneliness and disconnect. If we chose to, the lessons we learn today can, in fact, strengthen our appreciation and pursuit of human connection.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019). He has no disclosures.
Prior to the current crisis of COVID-19, I had a critical view of the direction of our psychiatric field. We have given up on complicated psychotherapies in favor of dispensing medications. We have given up on complicated diagnostic assessments in favor of simple self-rated symptoms questionnaires. Many of us even chose to give up on seeing patients face to face in favor of practicing telepsychiatry in the comfort of our homes. Some even promoted a future of psychiatry in which psychiatrists treated patients through large spreadsheets of evidence-based rating tools following evidence-based algorithms without even ever meeting the patients.
I do not view this problem as unique to psychiatry but rather as part of a larger trend in society. For the past couple of years, Vivek Murthy, MD, the former U.S. surgeon general, has popularized the idea that we are in a loneliness epidemic, saying, “We live in the most technologically connected age in the history of civilization, yet rates of loneliness have doubled since the 1980s.” Despite having enumerable means to reach other human beings, so many of us feel distant and out of touch with others. This loneliness has a measurable impact on our well-being with one study that states, “Actual and perceived social isolation are both associated with increased risk for early mortality.”
Then, seemingly out of nowhere, we were confronted with the largest challenge to our sense of connectedness in my lifetime. Throughout the past months, we have been asked to meet each other less frequently, do so through sterile means, and certainly not shake hands, hug, or embrace. The COVID-19 crisis has quickly made us all experts in telepsychiatry, remote work, and doing more with less. The COVID-19 crisis has asked many of us to put aside some of our human rituals like eating together, enjoying artistic experiences as a group, and touching, for the sake of saving lives.
For many, socially distancing has been a considerable added stressor – a stressor that continues to test humanity’s ability to be resilient. I am saddened by prior patients reaching out to seek comfort in these difficult times. I am touched by their desire to reconnect with someone they know, someone who feels familiar. I am surprised by the power of connection through phone and video calls. For some patients, despite the added burden, the current crisis has been an opportunity for their mental health and a reminder of the things that are important, including calling old friends and staying in touch with those who matter the most.
Yet, Checking in on others can become a chore. The social norm to partake in fashion, and self-care, become harder to find. In some cases, even hygiene and our health take a side role. The weekly phone visits with a therapist can feel just as mundane and repetitive as life. Sleep becomes harder to find, and food loses its taste. At this point, we realize the humanity that we lost in all this.
In the past couple of months, we have all become much more aware of the fragility of connectedness. However, we should recognize that the impact was well on its way before the COVID-19 crisis. It is my opinion that psychiatry should champion the issue of human relations. I do not think that we need to wait for a new DSM diagnosis, an evidence-based paradigm, or a Food and Drug Administration–approved medication to do so. The COVID-19 crisis has rendered us all cognizant of the importance of relationships.
While it may be that psychiatry continues to foray in electronic means of communication, use of impersonal scales and diagnosis, as well as anonymized algorithmic treatment plans, we should also promote as much humanity as society and public health safety will permit. Getting dressed to see your psychiatrist, face to face, to have an open-ended conversation about the nature of one’s life has clearly become something precious and powerful that should be cherished and protected. My hope is the rules and mandates we are required to use during the pandemic today do not become a continued habit that result in further loneliness and disconnect. If we chose to, the lessons we learn today can, in fact, strengthen our appreciation and pursuit of human connection.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019). He has no disclosures.
Treating primary tumor doesn’t improve OS in stage IV breast cancer
In patients with newly diagnosed stage IV breast cancer and an intact primary tumor, locoregional therapy after optimal systemic therapy does not improve survival or quality of life, results of the phase 3 E2108 trial suggest.
Among 256 patients with stage IV breast cancer with intact primary tumors who had no disease progression for 4-8 months after the start of optimal systemic therapy, there were no significant differences in overall survival or progression-free survival between patients randomized to receive locoregional therapy and those who did not receive the locoregional treatment.
Although patients who did not receive locoregional treatment had a 150% higher rate of local recurrence/progression, health-related quality of life (HRQOL) was actually worse at 18 months among the patients who underwent locoregional therapy. There were no HRQOL differences at 6 months, 12 months, or 30 months of follow-up.
Seema A. Khan, MD, of Northwestern University, Chicago, reported these results during a plenary session broadcast as a part of the American Society of Clinical Oncology virtual scientific program.
“There is no hint here of an advantage in terms of survival with the use of early locoregional therapy for the primary site,” Dr. Khan said.
Although neither the E2108 trial nor similar trials showed an overall survival advantage for locoregional therapy, as many as 20% of patients who are treated with systemic therapy alone may need locoregional therapy with surgery and/or radiation at some point for palliation or progression, said invited discussant Julia R. White, MD, professor of radiation oncology at the Ohio State University, Columbus.
“Locoregional therapy should be reserved for these patients that become symptomatic or progress locally. There may be a role for routine locoregional therapy for de novo oligometastatic breast cancer in combination with systemic therapy plus ablative therapy” to secure long-term remission or cure, questions that are being addressed in ongoing clinical trials, Dr. White said.
Past data
An estimated 6% of newly diagnosed breast cancer patients present with stage IV disease and an intact primary tumor.
The rationale for locoregional therapy of the primary tumor in patients with metastatic disease is based on retrospective data suggesting a survival advantage. However, the studies were biased because of younger patient populations with small tumors, a higher proportion of estrogen receptor–positive disease, and a generally lower metastatic burden than that seen in the E2108 population, according to Dr. Khan.
She went on to cite two randomized trials with differing outcomes. One trial showed no survival advantage with locoregional therapy at 2 years (Lancet Oncol. 2015 Oct;16[13]:1380-8). The other showed an improvement in survival with locoregional therapy at 5 years (Ann Surg Oncol. 2018 Oct;25[11]:3141-9).
E2108 details
In the E2108 trial, patients first received optimal systemic therapy based on individual patient and disease features. Patients who had no disease progression or distant disease for at least 4-8 months of therapy were then randomized to additional therapy.
In one randomized arm, patients received continued systemic therapy alone. The other arm received early local therapy, which included complete tumor resection with free surgical margins and postoperative radiotherapy according to the standard of care.
A total of 390 patients were registered, and 256 went on to randomization. Of those subjects, 131 were randomized to the continued systemic therapy arm and 125 to the early local therapy arm. All patients in each arm were included in the efficacy analysis.
In all, 59.6% of randomized patients had hormone receptor–positive/HER2-negative disease, 8.2% had triple-negative disease, and 32.2% had HER2-positive disease. Metastases included bone-only disease in 37.9% of patients, visceral-only disease in 24.2%, and 40.9% in both sites.
Among the patients randomized to early local therapy, 14 did not have surgery for personal, clinical, or insurance reasons. Of the 109 who went on to surgery, 87 had clear surgical margins, and 74 received locoregional radiation therapy.
Survival, progression, and HRQOL
At a median follow-up of 53 months, the median overall survival was 54 months in each arm. There was no significant difference in survival between the study arms, with superimposable survival curves (hazard ratio, 1.09; P = .63).
An analysis of overall survival by tumor type showed that, for the 20 women with triple-negative disease, survival was worse with early local therapy (HR, 3.50). There were no differences in survival either for the 79 patients with HER2-positive disease or for the 137 patients with hormone receptor–positive/HER2-negative disease.
Locoregional progression occurred in 25.6% of patients assigned to continued systemic therapy, compared with 10.2% assigned to early local therapy. However, progression-free survival was virtually identical between the study arms (P = .40).
At most time points, there were no significant between-arm differences in HRQOL. The exception was at 18 months of follow-up, when the HRQOL was significantly lower among patients who had undergone early local therapy (P = .001).
“Based on available data, locoregional therapy for the primary tumor should not be offered to women with stage IV breast cancer with the expectation of a survival benefit. When systemic disease is well controlled with systemic therapy but the primary site is progressing, as does happen occasionally, locoregional treatment can be considered,” Dr. Khan concluded.
She noted there is an ongoing trial of similar design in Japan (JCOG-1017), with results expected in 2022.
The current trial was supported by the National Cancer Institute and Canadian Cancer Society. Dr. Khan reported no conflicts of interest. Dr. White reported institutional research funding from Intraop Medical.
SOURCE: Khan SA et al. ASCO 2020, Abstract LBA2.
In patients with newly diagnosed stage IV breast cancer and an intact primary tumor, locoregional therapy after optimal systemic therapy does not improve survival or quality of life, results of the phase 3 E2108 trial suggest.
Among 256 patients with stage IV breast cancer with intact primary tumors who had no disease progression for 4-8 months after the start of optimal systemic therapy, there were no significant differences in overall survival or progression-free survival between patients randomized to receive locoregional therapy and those who did not receive the locoregional treatment.
Although patients who did not receive locoregional treatment had a 150% higher rate of local recurrence/progression, health-related quality of life (HRQOL) was actually worse at 18 months among the patients who underwent locoregional therapy. There were no HRQOL differences at 6 months, 12 months, or 30 months of follow-up.
Seema A. Khan, MD, of Northwestern University, Chicago, reported these results during a plenary session broadcast as a part of the American Society of Clinical Oncology virtual scientific program.
“There is no hint here of an advantage in terms of survival with the use of early locoregional therapy for the primary site,” Dr. Khan said.
Although neither the E2108 trial nor similar trials showed an overall survival advantage for locoregional therapy, as many as 20% of patients who are treated with systemic therapy alone may need locoregional therapy with surgery and/or radiation at some point for palliation or progression, said invited discussant Julia R. White, MD, professor of radiation oncology at the Ohio State University, Columbus.
“Locoregional therapy should be reserved for these patients that become symptomatic or progress locally. There may be a role for routine locoregional therapy for de novo oligometastatic breast cancer in combination with systemic therapy plus ablative therapy” to secure long-term remission or cure, questions that are being addressed in ongoing clinical trials, Dr. White said.
Past data
An estimated 6% of newly diagnosed breast cancer patients present with stage IV disease and an intact primary tumor.
The rationale for locoregional therapy of the primary tumor in patients with metastatic disease is based on retrospective data suggesting a survival advantage. However, the studies were biased because of younger patient populations with small tumors, a higher proportion of estrogen receptor–positive disease, and a generally lower metastatic burden than that seen in the E2108 population, according to Dr. Khan.
She went on to cite two randomized trials with differing outcomes. One trial showed no survival advantage with locoregional therapy at 2 years (Lancet Oncol. 2015 Oct;16[13]:1380-8). The other showed an improvement in survival with locoregional therapy at 5 years (Ann Surg Oncol. 2018 Oct;25[11]:3141-9).
E2108 details
In the E2108 trial, patients first received optimal systemic therapy based on individual patient and disease features. Patients who had no disease progression or distant disease for at least 4-8 months of therapy were then randomized to additional therapy.
In one randomized arm, patients received continued systemic therapy alone. The other arm received early local therapy, which included complete tumor resection with free surgical margins and postoperative radiotherapy according to the standard of care.
A total of 390 patients were registered, and 256 went on to randomization. Of those subjects, 131 were randomized to the continued systemic therapy arm and 125 to the early local therapy arm. All patients in each arm were included in the efficacy analysis.
In all, 59.6% of randomized patients had hormone receptor–positive/HER2-negative disease, 8.2% had triple-negative disease, and 32.2% had HER2-positive disease. Metastases included bone-only disease in 37.9% of patients, visceral-only disease in 24.2%, and 40.9% in both sites.
Among the patients randomized to early local therapy, 14 did not have surgery for personal, clinical, or insurance reasons. Of the 109 who went on to surgery, 87 had clear surgical margins, and 74 received locoregional radiation therapy.
Survival, progression, and HRQOL
At a median follow-up of 53 months, the median overall survival was 54 months in each arm. There was no significant difference in survival between the study arms, with superimposable survival curves (hazard ratio, 1.09; P = .63).
An analysis of overall survival by tumor type showed that, for the 20 women with triple-negative disease, survival was worse with early local therapy (HR, 3.50). There were no differences in survival either for the 79 patients with HER2-positive disease or for the 137 patients with hormone receptor–positive/HER2-negative disease.
Locoregional progression occurred in 25.6% of patients assigned to continued systemic therapy, compared with 10.2% assigned to early local therapy. However, progression-free survival was virtually identical between the study arms (P = .40).
At most time points, there were no significant between-arm differences in HRQOL. The exception was at 18 months of follow-up, when the HRQOL was significantly lower among patients who had undergone early local therapy (P = .001).
“Based on available data, locoregional therapy for the primary tumor should not be offered to women with stage IV breast cancer with the expectation of a survival benefit. When systemic disease is well controlled with systemic therapy but the primary site is progressing, as does happen occasionally, locoregional treatment can be considered,” Dr. Khan concluded.
She noted there is an ongoing trial of similar design in Japan (JCOG-1017), with results expected in 2022.
The current trial was supported by the National Cancer Institute and Canadian Cancer Society. Dr. Khan reported no conflicts of interest. Dr. White reported institutional research funding from Intraop Medical.
SOURCE: Khan SA et al. ASCO 2020, Abstract LBA2.
In patients with newly diagnosed stage IV breast cancer and an intact primary tumor, locoregional therapy after optimal systemic therapy does not improve survival or quality of life, results of the phase 3 E2108 trial suggest.
Among 256 patients with stage IV breast cancer with intact primary tumors who had no disease progression for 4-8 months after the start of optimal systemic therapy, there were no significant differences in overall survival or progression-free survival between patients randomized to receive locoregional therapy and those who did not receive the locoregional treatment.
Although patients who did not receive locoregional treatment had a 150% higher rate of local recurrence/progression, health-related quality of life (HRQOL) was actually worse at 18 months among the patients who underwent locoregional therapy. There were no HRQOL differences at 6 months, 12 months, or 30 months of follow-up.
Seema A. Khan, MD, of Northwestern University, Chicago, reported these results during a plenary session broadcast as a part of the American Society of Clinical Oncology virtual scientific program.
“There is no hint here of an advantage in terms of survival with the use of early locoregional therapy for the primary site,” Dr. Khan said.
Although neither the E2108 trial nor similar trials showed an overall survival advantage for locoregional therapy, as many as 20% of patients who are treated with systemic therapy alone may need locoregional therapy with surgery and/or radiation at some point for palliation or progression, said invited discussant Julia R. White, MD, professor of radiation oncology at the Ohio State University, Columbus.
“Locoregional therapy should be reserved for these patients that become symptomatic or progress locally. There may be a role for routine locoregional therapy for de novo oligometastatic breast cancer in combination with systemic therapy plus ablative therapy” to secure long-term remission or cure, questions that are being addressed in ongoing clinical trials, Dr. White said.
Past data
An estimated 6% of newly diagnosed breast cancer patients present with stage IV disease and an intact primary tumor.
The rationale for locoregional therapy of the primary tumor in patients with metastatic disease is based on retrospective data suggesting a survival advantage. However, the studies were biased because of younger patient populations with small tumors, a higher proportion of estrogen receptor–positive disease, and a generally lower metastatic burden than that seen in the E2108 population, according to Dr. Khan.
She went on to cite two randomized trials with differing outcomes. One trial showed no survival advantage with locoregional therapy at 2 years (Lancet Oncol. 2015 Oct;16[13]:1380-8). The other showed an improvement in survival with locoregional therapy at 5 years (Ann Surg Oncol. 2018 Oct;25[11]:3141-9).
E2108 details
In the E2108 trial, patients first received optimal systemic therapy based on individual patient and disease features. Patients who had no disease progression or distant disease for at least 4-8 months of therapy were then randomized to additional therapy.
In one randomized arm, patients received continued systemic therapy alone. The other arm received early local therapy, which included complete tumor resection with free surgical margins and postoperative radiotherapy according to the standard of care.
A total of 390 patients were registered, and 256 went on to randomization. Of those subjects, 131 were randomized to the continued systemic therapy arm and 125 to the early local therapy arm. All patients in each arm were included in the efficacy analysis.
In all, 59.6% of randomized patients had hormone receptor–positive/HER2-negative disease, 8.2% had triple-negative disease, and 32.2% had HER2-positive disease. Metastases included bone-only disease in 37.9% of patients, visceral-only disease in 24.2%, and 40.9% in both sites.
Among the patients randomized to early local therapy, 14 did not have surgery for personal, clinical, or insurance reasons. Of the 109 who went on to surgery, 87 had clear surgical margins, and 74 received locoregional radiation therapy.
Survival, progression, and HRQOL
At a median follow-up of 53 months, the median overall survival was 54 months in each arm. There was no significant difference in survival between the study arms, with superimposable survival curves (hazard ratio, 1.09; P = .63).
An analysis of overall survival by tumor type showed that, for the 20 women with triple-negative disease, survival was worse with early local therapy (HR, 3.50). There were no differences in survival either for the 79 patients with HER2-positive disease or for the 137 patients with hormone receptor–positive/HER2-negative disease.
Locoregional progression occurred in 25.6% of patients assigned to continued systemic therapy, compared with 10.2% assigned to early local therapy. However, progression-free survival was virtually identical between the study arms (P = .40).
At most time points, there were no significant between-arm differences in HRQOL. The exception was at 18 months of follow-up, when the HRQOL was significantly lower among patients who had undergone early local therapy (P = .001).
“Based on available data, locoregional therapy for the primary tumor should not be offered to women with stage IV breast cancer with the expectation of a survival benefit. When systemic disease is well controlled with systemic therapy but the primary site is progressing, as does happen occasionally, locoregional treatment can be considered,” Dr. Khan concluded.
She noted there is an ongoing trial of similar design in Japan (JCOG-1017), with results expected in 2022.
The current trial was supported by the National Cancer Institute and Canadian Cancer Society. Dr. Khan reported no conflicts of interest. Dr. White reported institutional research funding from Intraop Medical.
SOURCE: Khan SA et al. ASCO 2020, Abstract LBA2.
FROM ASCO 2020
Latest from ISCHEMIA: Worse outcomes in patients with intermediate left main disease on CCTA
Patients in the landmark ISCHEMIA trial with intermediate left main disease had a greater extent of coronary artery disease on invasive angiography, indicating greater atherosclerotic burden. They also had worse prognosis with a higher risk of cardiovascular events.
“Many times, we are looking at results as to whether patients have left main disease or not,” Sripal Bangalore, MD, said during the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “Here, we are showing that it’s not black and white; there are shades of gray. If a patient has intermediate left main disease, the prognosis is worse. That’s very important information we need to convey to our referrals also, because many times they may just look at the bottom line and say, ‘there is no left main disease.’ But here, we’re seeing that even having intermediate left main disease has significantly worse prognosis. We need to take that seriously.”
Prior studies show that patients with significant left main disease (LMD; defined as 50% or greater stenosis on coronary CT angiography [CCTA]) have a high risk of cardiovascular events and guidelines recommend revascularization to improve survival, said Dr. Bangalore, an interventional cardiologist at New York University Langone Health. However, the impact of intermediate LMD (defined as 25%-49% stenosis on CCTA) on outcomes is unclear.
Members of the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) research group randomized 5,179 participants to an initial invasive or conservative strategy. The main results showed that immediate revascularization in patients with stable ischemic heart disease provided no reduction in cardiovascular endpoints through 4 years of follow-up, compared with initial optimal medical therapy alone.
‘Discordance’ revealed in imaging modalities
For the current analysis, named the ISCHEMIA Intermediate LM Substudy, those who underwent coronary CCTA comprise the LMD substudy cohort. The objective was to evaluate clinical and quality of life outcomes in patients with and without intermediate left main disease on coronary CT and to evaluate the impact of treatment strategy on those outcomes across subgroups.
At baseline, these patients were categorized into those with and without intermediate LMD as determined by a core lab. Patients with LMD of 50% or greater, those with prior coronary artery bypass graft surgery, and those with nonevaluable or missing data on LM stenosis were excluded.
Among the 3,913 ISCHEMIA participants who underwent CCTA, 3,699 satisfied the inclusion criteria. Of these patients, 962 (26%) had intermediate LMD and 2,737 (74%) did not.
The researchers observed no significant differences in baseline characteristics between patients with and without LMD. However, patients with intermediate LMD tended to be older, and a greater proportion had hypertension and diabetes. Stress test characteristics were also similar between patients with and without LMD. However, patients with intermediate LMD tended toward a greater severity of severe ischemia.
This was also true for anatomic disease on CCTA. A higher proportion of patients with intermediate LMD had triple-vessel disease (61%-62%, compared with 36%-40% along those without intermediate LMD). In addition, a higher proportion of patients with intermediate LMD had stenosis in the proximal left anterior artery descending (LAD) artery (65% vs. 39% among those without intermediate LMD).
On analysis limited to 1,846 patients who underwent invasive angiography treatment in the main ISCHEMIA trial, 7% of those who were categorized into the intermediate LMD group were found to have LMD disease of 50% or greater, compared with 1.4% of patients who were categorized as not having intermediate LMD. “This goes to show this discordance between the two modalities [CCTA and coronary angiography], and I think we have to be careful,” said Dr. Bangalore, who also directs NYU Langone’s Cardiac Catheterization Laboratory. “There may be patients with left main disease, even if the CCTA says it’s not at 25%-29% [stenosis].”
The researchers found that, among patients who underwent invasive angiography, a greater proportion of those who were categorized into the LMD group had proximal LAD disease (43% vs. 33% among those who were categorized into the nonintermediate LMD group), triple-vessel disease (47% vs. 35%), a greater extent of coronary artery disease as denoted by a higher SYNTAX score (21 vs. 15), and a higher proportion underwent coronary artery bypass graft surgery (32% vs. 18%).
Intermediate LMD linked to worse outcomes
After the researchers adjusted for baseline differences between the two groups in overall substudy cohort, they found that intermediate LMD severity was an independent predictor of the primary composite endpoint of cardiovascular death, MI, hospitalization for unstable angina, heart failure, and resuscitated cardiac arrest (hazard ratio, 1.31; P = .0123); cardiovascular death/MI/stroke (HR, 1.30; P = .0143); procedural primary MI (HR, 1.64; P = .0487); heart failure (HR, 2.06; P = .0239); and stroke (HR, 1.82, P = .0362).
“We then looked to see if there is a treatment difference, a treatment effect based on whether patients had intermediate LMD,” Dr. Bangalore said. “Most of the P values were not significant. The results are very consistent with what we saw in the main analysis: not a significant difference between invasive and conservative strategy. We do see some differences, though. An invasive strategy was associated with a significantly higher risk of procedural MI [2.9% vs. 1.5%], but a significantly lower risk of nonprocedural MI [–6.4% vs. –2%].”
Dr. Bangalore added that there was significant benefit of the invasive strategy in reducing angina and improving quality of life based on the Seattle Angina Questionnaire-7. “This result was durable up to 48 months of follow-up, whether the patient had intermediate left main disease or not. These results were dependent on baseline angina status. The benefit of invasive strategy was mainly in patients who had daily, weekly, and monthly angina, and no benefit in patients with no angina; there was no interaction based on intermediate left main status.”
Dr. Bangalore emphasized that the original ISCHEMIA trial excluded patients with severe left main disease by design. “But patients with intermediate left main disease in ISCHEMIA tended to have a greater extent of coronary artery disease, indicating greater atherosclerotic burden. I don’t think that’s any surprise. They had a worse prognosis with higher risk of cardiovascular events but similar quality of life, including angina-specific quality of life.”
The key clinical message, he said, is that patients with intermediate LMD face an increased risk of cardiovascular events. “I think we have to be aggressive in trying to reduce their risk with medical therapy, etc.,” he said. “If they are symptomatic, ISCHEMIA tells us that patients have two options. They can choose an invasive strategy, because clearly there is a benefit. You have a significant benefit at making you feel better and potentially reducing the risk of spontaneous MI over a period of time. Or, you can try medical therapy first. If you do see some left main disease, it’s showing the general burden of atherosclerosis disease in those patients. I think that’s the critical message, that we have to be very aggressive with these patients.”
A call for more imaging studies
An invited panelist, Timothy D. Henry, MD, said that the results of the ISCHEMIA substudy should stimulate further research. “With an intermediate lesion, clearly the interventional group did better, and it wasn’t symptom related,” said Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research and Education at the Christ Hospital in Cincinnati. “So even if you do medical therapy, you’re not going to really find it out. In my mind, this should stimulate us to do more imaging of the left main that are moderate lesions, and follow this up as an independent study. I think this is a really important finding.”
ISCHEMIA was supported by grants from the National Heart, Lung, and Blood Institute. Dr. Bangalore disclosed that he is a member of the advisory board and/or a board member for Meril, SMT, Pfizer, Amgen, Biotronik, and Abbott. He also is a consultant for Reata Pharmaceuticals.
SOURCE: Bangalore S et al. SCAI 2020, Abstract 11656.
Patients in the landmark ISCHEMIA trial with intermediate left main disease had a greater extent of coronary artery disease on invasive angiography, indicating greater atherosclerotic burden. They also had worse prognosis with a higher risk of cardiovascular events.
“Many times, we are looking at results as to whether patients have left main disease or not,” Sripal Bangalore, MD, said during the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “Here, we are showing that it’s not black and white; there are shades of gray. If a patient has intermediate left main disease, the prognosis is worse. That’s very important information we need to convey to our referrals also, because many times they may just look at the bottom line and say, ‘there is no left main disease.’ But here, we’re seeing that even having intermediate left main disease has significantly worse prognosis. We need to take that seriously.”
Prior studies show that patients with significant left main disease (LMD; defined as 50% or greater stenosis on coronary CT angiography [CCTA]) have a high risk of cardiovascular events and guidelines recommend revascularization to improve survival, said Dr. Bangalore, an interventional cardiologist at New York University Langone Health. However, the impact of intermediate LMD (defined as 25%-49% stenosis on CCTA) on outcomes is unclear.
Members of the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) research group randomized 5,179 participants to an initial invasive or conservative strategy. The main results showed that immediate revascularization in patients with stable ischemic heart disease provided no reduction in cardiovascular endpoints through 4 years of follow-up, compared with initial optimal medical therapy alone.
‘Discordance’ revealed in imaging modalities
For the current analysis, named the ISCHEMIA Intermediate LM Substudy, those who underwent coronary CCTA comprise the LMD substudy cohort. The objective was to evaluate clinical and quality of life outcomes in patients with and without intermediate left main disease on coronary CT and to evaluate the impact of treatment strategy on those outcomes across subgroups.
At baseline, these patients were categorized into those with and without intermediate LMD as determined by a core lab. Patients with LMD of 50% or greater, those with prior coronary artery bypass graft surgery, and those with nonevaluable or missing data on LM stenosis were excluded.
Among the 3,913 ISCHEMIA participants who underwent CCTA, 3,699 satisfied the inclusion criteria. Of these patients, 962 (26%) had intermediate LMD and 2,737 (74%) did not.
The researchers observed no significant differences in baseline characteristics between patients with and without LMD. However, patients with intermediate LMD tended to be older, and a greater proportion had hypertension and diabetes. Stress test characteristics were also similar between patients with and without LMD. However, patients with intermediate LMD tended toward a greater severity of severe ischemia.
This was also true for anatomic disease on CCTA. A higher proportion of patients with intermediate LMD had triple-vessel disease (61%-62%, compared with 36%-40% along those without intermediate LMD). In addition, a higher proportion of patients with intermediate LMD had stenosis in the proximal left anterior artery descending (LAD) artery (65% vs. 39% among those without intermediate LMD).
On analysis limited to 1,846 patients who underwent invasive angiography treatment in the main ISCHEMIA trial, 7% of those who were categorized into the intermediate LMD group were found to have LMD disease of 50% or greater, compared with 1.4% of patients who were categorized as not having intermediate LMD. “This goes to show this discordance between the two modalities [CCTA and coronary angiography], and I think we have to be careful,” said Dr. Bangalore, who also directs NYU Langone’s Cardiac Catheterization Laboratory. “There may be patients with left main disease, even if the CCTA says it’s not at 25%-29% [stenosis].”
The researchers found that, among patients who underwent invasive angiography, a greater proportion of those who were categorized into the LMD group had proximal LAD disease (43% vs. 33% among those who were categorized into the nonintermediate LMD group), triple-vessel disease (47% vs. 35%), a greater extent of coronary artery disease as denoted by a higher SYNTAX score (21 vs. 15), and a higher proportion underwent coronary artery bypass graft surgery (32% vs. 18%).
Intermediate LMD linked to worse outcomes
After the researchers adjusted for baseline differences between the two groups in overall substudy cohort, they found that intermediate LMD severity was an independent predictor of the primary composite endpoint of cardiovascular death, MI, hospitalization for unstable angina, heart failure, and resuscitated cardiac arrest (hazard ratio, 1.31; P = .0123); cardiovascular death/MI/stroke (HR, 1.30; P = .0143); procedural primary MI (HR, 1.64; P = .0487); heart failure (HR, 2.06; P = .0239); and stroke (HR, 1.82, P = .0362).
“We then looked to see if there is a treatment difference, a treatment effect based on whether patients had intermediate LMD,” Dr. Bangalore said. “Most of the P values were not significant. The results are very consistent with what we saw in the main analysis: not a significant difference between invasive and conservative strategy. We do see some differences, though. An invasive strategy was associated with a significantly higher risk of procedural MI [2.9% vs. 1.5%], but a significantly lower risk of nonprocedural MI [–6.4% vs. –2%].”
Dr. Bangalore added that there was significant benefit of the invasive strategy in reducing angina and improving quality of life based on the Seattle Angina Questionnaire-7. “This result was durable up to 48 months of follow-up, whether the patient had intermediate left main disease or not. These results were dependent on baseline angina status. The benefit of invasive strategy was mainly in patients who had daily, weekly, and monthly angina, and no benefit in patients with no angina; there was no interaction based on intermediate left main status.”
Dr. Bangalore emphasized that the original ISCHEMIA trial excluded patients with severe left main disease by design. “But patients with intermediate left main disease in ISCHEMIA tended to have a greater extent of coronary artery disease, indicating greater atherosclerotic burden. I don’t think that’s any surprise. They had a worse prognosis with higher risk of cardiovascular events but similar quality of life, including angina-specific quality of life.”
The key clinical message, he said, is that patients with intermediate LMD face an increased risk of cardiovascular events. “I think we have to be aggressive in trying to reduce their risk with medical therapy, etc.,” he said. “If they are symptomatic, ISCHEMIA tells us that patients have two options. They can choose an invasive strategy, because clearly there is a benefit. You have a significant benefit at making you feel better and potentially reducing the risk of spontaneous MI over a period of time. Or, you can try medical therapy first. If you do see some left main disease, it’s showing the general burden of atherosclerosis disease in those patients. I think that’s the critical message, that we have to be very aggressive with these patients.”
A call for more imaging studies
An invited panelist, Timothy D. Henry, MD, said that the results of the ISCHEMIA substudy should stimulate further research. “With an intermediate lesion, clearly the interventional group did better, and it wasn’t symptom related,” said Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research and Education at the Christ Hospital in Cincinnati. “So even if you do medical therapy, you’re not going to really find it out. In my mind, this should stimulate us to do more imaging of the left main that are moderate lesions, and follow this up as an independent study. I think this is a really important finding.”
ISCHEMIA was supported by grants from the National Heart, Lung, and Blood Institute. Dr. Bangalore disclosed that he is a member of the advisory board and/or a board member for Meril, SMT, Pfizer, Amgen, Biotronik, and Abbott. He also is a consultant for Reata Pharmaceuticals.
SOURCE: Bangalore S et al. SCAI 2020, Abstract 11656.
Patients in the landmark ISCHEMIA trial with intermediate left main disease had a greater extent of coronary artery disease on invasive angiography, indicating greater atherosclerotic burden. They also had worse prognosis with a higher risk of cardiovascular events.
“Many times, we are looking at results as to whether patients have left main disease or not,” Sripal Bangalore, MD, said during the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “Here, we are showing that it’s not black and white; there are shades of gray. If a patient has intermediate left main disease, the prognosis is worse. That’s very important information we need to convey to our referrals also, because many times they may just look at the bottom line and say, ‘there is no left main disease.’ But here, we’re seeing that even having intermediate left main disease has significantly worse prognosis. We need to take that seriously.”
Prior studies show that patients with significant left main disease (LMD; defined as 50% or greater stenosis on coronary CT angiography [CCTA]) have a high risk of cardiovascular events and guidelines recommend revascularization to improve survival, said Dr. Bangalore, an interventional cardiologist at New York University Langone Health. However, the impact of intermediate LMD (defined as 25%-49% stenosis on CCTA) on outcomes is unclear.
Members of the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) research group randomized 5,179 participants to an initial invasive or conservative strategy. The main results showed that immediate revascularization in patients with stable ischemic heart disease provided no reduction in cardiovascular endpoints through 4 years of follow-up, compared with initial optimal medical therapy alone.
‘Discordance’ revealed in imaging modalities
For the current analysis, named the ISCHEMIA Intermediate LM Substudy, those who underwent coronary CCTA comprise the LMD substudy cohort. The objective was to evaluate clinical and quality of life outcomes in patients with and without intermediate left main disease on coronary CT and to evaluate the impact of treatment strategy on those outcomes across subgroups.
At baseline, these patients were categorized into those with and without intermediate LMD as determined by a core lab. Patients with LMD of 50% or greater, those with prior coronary artery bypass graft surgery, and those with nonevaluable or missing data on LM stenosis were excluded.
Among the 3,913 ISCHEMIA participants who underwent CCTA, 3,699 satisfied the inclusion criteria. Of these patients, 962 (26%) had intermediate LMD and 2,737 (74%) did not.
The researchers observed no significant differences in baseline characteristics between patients with and without LMD. However, patients with intermediate LMD tended to be older, and a greater proportion had hypertension and diabetes. Stress test characteristics were also similar between patients with and without LMD. However, patients with intermediate LMD tended toward a greater severity of severe ischemia.
This was also true for anatomic disease on CCTA. A higher proportion of patients with intermediate LMD had triple-vessel disease (61%-62%, compared with 36%-40% along those without intermediate LMD). In addition, a higher proportion of patients with intermediate LMD had stenosis in the proximal left anterior artery descending (LAD) artery (65% vs. 39% among those without intermediate LMD).
On analysis limited to 1,846 patients who underwent invasive angiography treatment in the main ISCHEMIA trial, 7% of those who were categorized into the intermediate LMD group were found to have LMD disease of 50% or greater, compared with 1.4% of patients who were categorized as not having intermediate LMD. “This goes to show this discordance between the two modalities [CCTA and coronary angiography], and I think we have to be careful,” said Dr. Bangalore, who also directs NYU Langone’s Cardiac Catheterization Laboratory. “There may be patients with left main disease, even if the CCTA says it’s not at 25%-29% [stenosis].”
The researchers found that, among patients who underwent invasive angiography, a greater proportion of those who were categorized into the LMD group had proximal LAD disease (43% vs. 33% among those who were categorized into the nonintermediate LMD group), triple-vessel disease (47% vs. 35%), a greater extent of coronary artery disease as denoted by a higher SYNTAX score (21 vs. 15), and a higher proportion underwent coronary artery bypass graft surgery (32% vs. 18%).
Intermediate LMD linked to worse outcomes
After the researchers adjusted for baseline differences between the two groups in overall substudy cohort, they found that intermediate LMD severity was an independent predictor of the primary composite endpoint of cardiovascular death, MI, hospitalization for unstable angina, heart failure, and resuscitated cardiac arrest (hazard ratio, 1.31; P = .0123); cardiovascular death/MI/stroke (HR, 1.30; P = .0143); procedural primary MI (HR, 1.64; P = .0487); heart failure (HR, 2.06; P = .0239); and stroke (HR, 1.82, P = .0362).
“We then looked to see if there is a treatment difference, a treatment effect based on whether patients had intermediate LMD,” Dr. Bangalore said. “Most of the P values were not significant. The results are very consistent with what we saw in the main analysis: not a significant difference between invasive and conservative strategy. We do see some differences, though. An invasive strategy was associated with a significantly higher risk of procedural MI [2.9% vs. 1.5%], but a significantly lower risk of nonprocedural MI [–6.4% vs. –2%].”
Dr. Bangalore added that there was significant benefit of the invasive strategy in reducing angina and improving quality of life based on the Seattle Angina Questionnaire-7. “This result was durable up to 48 months of follow-up, whether the patient had intermediate left main disease or not. These results were dependent on baseline angina status. The benefit of invasive strategy was mainly in patients who had daily, weekly, and monthly angina, and no benefit in patients with no angina; there was no interaction based on intermediate left main status.”
Dr. Bangalore emphasized that the original ISCHEMIA trial excluded patients with severe left main disease by design. “But patients with intermediate left main disease in ISCHEMIA tended to have a greater extent of coronary artery disease, indicating greater atherosclerotic burden. I don’t think that’s any surprise. They had a worse prognosis with higher risk of cardiovascular events but similar quality of life, including angina-specific quality of life.”
The key clinical message, he said, is that patients with intermediate LMD face an increased risk of cardiovascular events. “I think we have to be aggressive in trying to reduce their risk with medical therapy, etc.,” he said. “If they are symptomatic, ISCHEMIA tells us that patients have two options. They can choose an invasive strategy, because clearly there is a benefit. You have a significant benefit at making you feel better and potentially reducing the risk of spontaneous MI over a period of time. Or, you can try medical therapy first. If you do see some left main disease, it’s showing the general burden of atherosclerosis disease in those patients. I think that’s the critical message, that we have to be very aggressive with these patients.”
A call for more imaging studies
An invited panelist, Timothy D. Henry, MD, said that the results of the ISCHEMIA substudy should stimulate further research. “With an intermediate lesion, clearly the interventional group did better, and it wasn’t symptom related,” said Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research and Education at the Christ Hospital in Cincinnati. “So even if you do medical therapy, you’re not going to really find it out. In my mind, this should stimulate us to do more imaging of the left main that are moderate lesions, and follow this up as an independent study. I think this is a really important finding.”
ISCHEMIA was supported by grants from the National Heart, Lung, and Blood Institute. Dr. Bangalore disclosed that he is a member of the advisory board and/or a board member for Meril, SMT, Pfizer, Amgen, Biotronik, and Abbott. He also is a consultant for Reata Pharmaceuticals.
SOURCE: Bangalore S et al. SCAI 2020, Abstract 11656.
FROM SCAI 2020
Pembrolizumab prolonged PFS vs. brentuximab vedotin in r/r Hodgkin lymphoma
Pembrolizumab treatment significantly improved progression-free survival versus brentuximab vedotin in a randomized, phase 3 trial including patients with relapsed or refractory classical Hodgkin lymphoma, an investigator has reported.
Median progression-free survival (PFS) was 13.2 versus 8.3 months in favor of pembrolizumab, according to the report on the KEYNOTE-204 trial, which included patients with classical Hodgkin lymphoma who either had relapsed after autologous stem cell transplant (SCT) or were ineligible for autologous SCT.
of Princess Margaret Cancer Centre in Toronto.
“This PFS benefit extended to key subgroups, including those ineligible for autologous transplant, patients with primary refractory disease, and patients who were brentuximab-vedotin naive,” Dr. Kuruvilla added in his presentation, which was part of the American Society of Clinical Oncology virtual scientific program.
Pneumonitis was more frequent in the pembrolizumab arm, but “appeared in general to be quite well managed” among patients who experienced this adverse event, according to Dr. Kuruvilla, who said that treatment with the programmed death–1 inhibitor should be considered “the preferred treatment option and the new standard of care” for patients with relapsed/refractory classic Hodgkin lymphoma who have relapsed after autologous SCT or are ineligible for it.
Although the pneumonitis findings are important to keep in mind, results of KEYNOTE-204 are indeed “practice defining” and immediately impactful, said Mark J. Roschewski, MD, clinical investigator in the lymphoid malignancies branch at the Center for Cancer Research, part of the National Cancer Institute, Bethesda, Md.
“I would select pembrolizumab over brentuximab for this patient population, particularly those that are refractory to chemotherapy,” he said in a commentary on the study also included in the virtual ASCO proceedings.
“There may be specific patient populations that I’d reconsider, such as those that might be at high risk for lung toxicity,” he added. “They may not be suitable for this, but it’s something to at least to be aware of.”
Although the antibody-drug conjugate brentuximab vedotin has been considered the standard of care for patients with relapse after autologous SCT, there has historically been no standard of care for patients who are ineligible for transplant because of chemorefractory disease, advanced age, or comorbidities, Dr. Kuruvilla said in his presentation.
In the KEYNOTE-204 study, 304 patients with relapsed/refractory classic Hodgkin lymphoma were randomized to receive either pembrolizumab 200 mg or brentuximab at 1.8 mg/kg intravenously every 3 weeks for up to 35 cycles.
The median age of patients was 36 years in the pembrolizumab arm and 35 years in the brentuximab vedotin arm, according to the report. Approximately 37% of the patients had previously undergone autologous SCT. About 40% had been refractory to frontline therapy, while 28% relapsed within 12 months of therapy and 32% relapsed later than 12 months.
Median PFS by blinded independent central review was 13.2 versus 8.3 months in the pembrolizumab and brentuximab arms, respectively (hazard ratio, 0.65; 95% confidence interval, 0.48-0.88; P = .00271), Dr. Kuruvilla reported.
The benefit extended to “key subgroups” in the trial, he added, including those who were ineligible for autologous SCT, those with primary refractory disease, and those who were naive to brentuximab vedotin, with HRs of 0.61, 0.52, and 0.67, respectively.
Pembrolizumab was also associated with more durable responses versus brentuximab vedotin, according to the investigator.
The overall response rate was 65.6% and 54.2%, respectively, for pembrolizumab and brentuximab, although this difference of approximately 11 percentage points did not meet criteria for statistical significance, he said. Duration of response was 20.7 months or pembrolizumab and 13.8 months for brentuximab.
The rate of serious treatment-related adverse events was similar between groups, according to Dr. Kuruvilla, who reported grade 3-5 events occurring in 19.6% and 25.0% of the pembrolizumab and brentuximab arms. Serious treatment-related adverse events were numerically more frequent in the pembrolizumab arm (16.2% vs. 10.5%) and there was one treatment-related death caused by pneumonia, seen in the pembrolizumab arm.
Pneumonitis occurred in 2.6% of the brentuximab-treated patients and in 10.8% of pembrolizumab-treated patients, of which half of cases were grade 3-4, according to the report.
In the pembrolizumab arm, pneumonitis was felt to be drug-related in 15 of 16 cases, according to Dr. Kuruvilla, who added that 15 of 16 patients required corticosteroid therapy. “This has led to the resolution of the pneumonitis in 12 of 16 patients, with ongoing resolution in one further patient.”
Research funding for KEYNOTE-204 came from Merck Sharp & Dohme. Dr. Kuruvilla provided disclosures related to Merck and a variety of other pharmaceutical companies. Dr. Roschewski said he had no relationships to disclose.
SOURCE: Kuruvilla J et al. ASCO 2020, Abstract 8005.
Pembrolizumab treatment significantly improved progression-free survival versus brentuximab vedotin in a randomized, phase 3 trial including patients with relapsed or refractory classical Hodgkin lymphoma, an investigator has reported.
Median progression-free survival (PFS) was 13.2 versus 8.3 months in favor of pembrolizumab, according to the report on the KEYNOTE-204 trial, which included patients with classical Hodgkin lymphoma who either had relapsed after autologous stem cell transplant (SCT) or were ineligible for autologous SCT.
of Princess Margaret Cancer Centre in Toronto.
“This PFS benefit extended to key subgroups, including those ineligible for autologous transplant, patients with primary refractory disease, and patients who were brentuximab-vedotin naive,” Dr. Kuruvilla added in his presentation, which was part of the American Society of Clinical Oncology virtual scientific program.
Pneumonitis was more frequent in the pembrolizumab arm, but “appeared in general to be quite well managed” among patients who experienced this adverse event, according to Dr. Kuruvilla, who said that treatment with the programmed death–1 inhibitor should be considered “the preferred treatment option and the new standard of care” for patients with relapsed/refractory classic Hodgkin lymphoma who have relapsed after autologous SCT or are ineligible for it.
Although the pneumonitis findings are important to keep in mind, results of KEYNOTE-204 are indeed “practice defining” and immediately impactful, said Mark J. Roschewski, MD, clinical investigator in the lymphoid malignancies branch at the Center for Cancer Research, part of the National Cancer Institute, Bethesda, Md.
“I would select pembrolizumab over brentuximab for this patient population, particularly those that are refractory to chemotherapy,” he said in a commentary on the study also included in the virtual ASCO proceedings.
“There may be specific patient populations that I’d reconsider, such as those that might be at high risk for lung toxicity,” he added. “They may not be suitable for this, but it’s something to at least to be aware of.”
Although the antibody-drug conjugate brentuximab vedotin has been considered the standard of care for patients with relapse after autologous SCT, there has historically been no standard of care for patients who are ineligible for transplant because of chemorefractory disease, advanced age, or comorbidities, Dr. Kuruvilla said in his presentation.
In the KEYNOTE-204 study, 304 patients with relapsed/refractory classic Hodgkin lymphoma were randomized to receive either pembrolizumab 200 mg or brentuximab at 1.8 mg/kg intravenously every 3 weeks for up to 35 cycles.
The median age of patients was 36 years in the pembrolizumab arm and 35 years in the brentuximab vedotin arm, according to the report. Approximately 37% of the patients had previously undergone autologous SCT. About 40% had been refractory to frontline therapy, while 28% relapsed within 12 months of therapy and 32% relapsed later than 12 months.
Median PFS by blinded independent central review was 13.2 versus 8.3 months in the pembrolizumab and brentuximab arms, respectively (hazard ratio, 0.65; 95% confidence interval, 0.48-0.88; P = .00271), Dr. Kuruvilla reported.
The benefit extended to “key subgroups” in the trial, he added, including those who were ineligible for autologous SCT, those with primary refractory disease, and those who were naive to brentuximab vedotin, with HRs of 0.61, 0.52, and 0.67, respectively.
Pembrolizumab was also associated with more durable responses versus brentuximab vedotin, according to the investigator.
The overall response rate was 65.6% and 54.2%, respectively, for pembrolizumab and brentuximab, although this difference of approximately 11 percentage points did not meet criteria for statistical significance, he said. Duration of response was 20.7 months or pembrolizumab and 13.8 months for brentuximab.
The rate of serious treatment-related adverse events was similar between groups, according to Dr. Kuruvilla, who reported grade 3-5 events occurring in 19.6% and 25.0% of the pembrolizumab and brentuximab arms. Serious treatment-related adverse events were numerically more frequent in the pembrolizumab arm (16.2% vs. 10.5%) and there was one treatment-related death caused by pneumonia, seen in the pembrolizumab arm.
Pneumonitis occurred in 2.6% of the brentuximab-treated patients and in 10.8% of pembrolizumab-treated patients, of which half of cases were grade 3-4, according to the report.
In the pembrolizumab arm, pneumonitis was felt to be drug-related in 15 of 16 cases, according to Dr. Kuruvilla, who added that 15 of 16 patients required corticosteroid therapy. “This has led to the resolution of the pneumonitis in 12 of 16 patients, with ongoing resolution in one further patient.”
Research funding for KEYNOTE-204 came from Merck Sharp & Dohme. Dr. Kuruvilla provided disclosures related to Merck and a variety of other pharmaceutical companies. Dr. Roschewski said he had no relationships to disclose.
SOURCE: Kuruvilla J et al. ASCO 2020, Abstract 8005.
Pembrolizumab treatment significantly improved progression-free survival versus brentuximab vedotin in a randomized, phase 3 trial including patients with relapsed or refractory classical Hodgkin lymphoma, an investigator has reported.
Median progression-free survival (PFS) was 13.2 versus 8.3 months in favor of pembrolizumab, according to the report on the KEYNOTE-204 trial, which included patients with classical Hodgkin lymphoma who either had relapsed after autologous stem cell transplant (SCT) or were ineligible for autologous SCT.
of Princess Margaret Cancer Centre in Toronto.
“This PFS benefit extended to key subgroups, including those ineligible for autologous transplant, patients with primary refractory disease, and patients who were brentuximab-vedotin naive,” Dr. Kuruvilla added in his presentation, which was part of the American Society of Clinical Oncology virtual scientific program.
Pneumonitis was more frequent in the pembrolizumab arm, but “appeared in general to be quite well managed” among patients who experienced this adverse event, according to Dr. Kuruvilla, who said that treatment with the programmed death–1 inhibitor should be considered “the preferred treatment option and the new standard of care” for patients with relapsed/refractory classic Hodgkin lymphoma who have relapsed after autologous SCT or are ineligible for it.
Although the pneumonitis findings are important to keep in mind, results of KEYNOTE-204 are indeed “practice defining” and immediately impactful, said Mark J. Roschewski, MD, clinical investigator in the lymphoid malignancies branch at the Center for Cancer Research, part of the National Cancer Institute, Bethesda, Md.
“I would select pembrolizumab over brentuximab for this patient population, particularly those that are refractory to chemotherapy,” he said in a commentary on the study also included in the virtual ASCO proceedings.
“There may be specific patient populations that I’d reconsider, such as those that might be at high risk for lung toxicity,” he added. “They may not be suitable for this, but it’s something to at least to be aware of.”
Although the antibody-drug conjugate brentuximab vedotin has been considered the standard of care for patients with relapse after autologous SCT, there has historically been no standard of care for patients who are ineligible for transplant because of chemorefractory disease, advanced age, or comorbidities, Dr. Kuruvilla said in his presentation.
In the KEYNOTE-204 study, 304 patients with relapsed/refractory classic Hodgkin lymphoma were randomized to receive either pembrolizumab 200 mg or brentuximab at 1.8 mg/kg intravenously every 3 weeks for up to 35 cycles.
The median age of patients was 36 years in the pembrolizumab arm and 35 years in the brentuximab vedotin arm, according to the report. Approximately 37% of the patients had previously undergone autologous SCT. About 40% had been refractory to frontline therapy, while 28% relapsed within 12 months of therapy and 32% relapsed later than 12 months.
Median PFS by blinded independent central review was 13.2 versus 8.3 months in the pembrolizumab and brentuximab arms, respectively (hazard ratio, 0.65; 95% confidence interval, 0.48-0.88; P = .00271), Dr. Kuruvilla reported.
The benefit extended to “key subgroups” in the trial, he added, including those who were ineligible for autologous SCT, those with primary refractory disease, and those who were naive to brentuximab vedotin, with HRs of 0.61, 0.52, and 0.67, respectively.
Pembrolizumab was also associated with more durable responses versus brentuximab vedotin, according to the investigator.
The overall response rate was 65.6% and 54.2%, respectively, for pembrolizumab and brentuximab, although this difference of approximately 11 percentage points did not meet criteria for statistical significance, he said. Duration of response was 20.7 months or pembrolizumab and 13.8 months for brentuximab.
The rate of serious treatment-related adverse events was similar between groups, according to Dr. Kuruvilla, who reported grade 3-5 events occurring in 19.6% and 25.0% of the pembrolizumab and brentuximab arms. Serious treatment-related adverse events were numerically more frequent in the pembrolizumab arm (16.2% vs. 10.5%) and there was one treatment-related death caused by pneumonia, seen in the pembrolizumab arm.
Pneumonitis occurred in 2.6% of the brentuximab-treated patients and in 10.8% of pembrolizumab-treated patients, of which half of cases were grade 3-4, according to the report.
In the pembrolizumab arm, pneumonitis was felt to be drug-related in 15 of 16 cases, according to Dr. Kuruvilla, who added that 15 of 16 patients required corticosteroid therapy. “This has led to the resolution of the pneumonitis in 12 of 16 patients, with ongoing resolution in one further patient.”
Research funding for KEYNOTE-204 came from Merck Sharp & Dohme. Dr. Kuruvilla provided disclosures related to Merck and a variety of other pharmaceutical companies. Dr. Roschewski said he had no relationships to disclose.
SOURCE: Kuruvilla J et al. ASCO 2020, Abstract 8005.
FROM ASCO 2020
Intensive Care Unit Utilization After Adoption of a Ward-Based High-Flow Nasal Cannula Protocol
Children hospitalized for bronchiolitis frequently require admission to the intensive care unit (ICU), with estimates as high as 18%1,2 and 35%3 in two prospective, multicenter studies. The indication for ICU admission is nearly always a need for advanced respiratory support, which historically consisted of continuous or bilevel positive airway pressure (CPAP and BiPAP, respectively) or mechanical ventilation. High-flow nasal cannula (HFNC) is a recent addition to the respiratory support armamentarium, delivering heated and humidified oxygen at rates of up to 60 L/min and allowing for clinicians to titrate both flow rate and fraction of inspired oxygen (FiO2).4
Several studies have demonstrated that HFNC is capable of decreasing a child’s work of breathing,5-8 and it has the potential advantage of being better tolerated than other forms of advanced respiratory support.9,10 These case-series physiologic studies informed early ward-based HFNC protocols for bronchiolitis, which were adopted to decrease ICU utilization. Since then, single center observational studies examining the association between ward-based HFNC protocols and subsequent ICU utilization have come to discordant conclusions.11-14 Studying the effect of employing HFNC outside of the ICU is challenging in the context of a randomized, controlled trial (RCT) because it is difficult to blind healthcare providers to the intervention and because crossover from the control group to HFNC is frequent. Two unblinded RCTs published in 2017 and 2018 found that children randomized to conventional nasal cannula were frequently escalated to HFNC (flow rates of 1-2 L/kg per minute), but neither trial found a difference in ICU admission.15,16 Sample sizes substantially larger than those present in currently published or registered RCTs would be required to evaluate the impact of ward-based HFNC protocols on the outcome that inspired the protocols in the first place, namely ICU utilization.17
Children’s hospitals have adopted ward-based HFNC protocols at different time points over the last decade, which allows for a natural experiment—a promising alternative study design that avoids the challenges of blinding, crossover, and modest sample sizes. In order to have sufficient postadoption data for analyses, the present study is limited to ward-based HFNC protocols adopted prior to 2016, which we have termed “early” ward-based HFNC protocols. Among children with bronchiolitis, our objective was to measure the association between hospital-level adoption of a ward-based HFNC protocol and subsequent ICU utilization, using a multicenter network of children’s hospitals.
METHODS
We conducted a multicenter retrospective cohort study using the Pediatric Health Information System (PHIS) database. The PHIS database is operated by the Children’s Hospital Association (Lenexa, Kansas) and provides deidentified patient-level information for children who receive hospital care at 55 US children’s hospitals. Available data elements include patient demographic data, discharge diagnosis and procedure codes, and detailed billing information, such as laboratory, imaging, pharmacy, and supply charges. At the patient level, the use of HFNC vs standard oxygen therapy circuits cannot be discriminated.
Exposure
The study exposure was a hospital’s first ward-based HFNC protocol, with adoption measured at the hospital level at each PHIS site via direct communication with leaders in hospital medicine. In most cases, first contact was made with the pediatric hospital medicine division chief or fellowship program director, who then, if necessary, connected study investigators to local HFNC champions aware of site-specific historical HFNC protocol details. Contact with a hospital was made only if the hospital had contributed at least 6 consecutive years of data to PHIS. Hospitals were classified as “adopting” hospitals if their HFNC protocol met all of the following criteria: (a) allows initiation of HFNC outside of the ICU (on the floor or in the ED), (b) allows continued care outside of the ICU (on the floor), (c) not limited to a small unit like an intermediate care unit, and (d) adopted during a specific, known respiratory season. Hospitals for which ward-based HFNC protocols were adopted but did not meet these criteria were excluded from further analysis. Our intent was to identify large scale, programmatic protocol launches and exclude hospitals with exceptions that might preclude a sizable portion of our cohort from being eligible for the protocol. Hospitals for which inpatient use of HFNC remains limited to the ICU were defined as “nonadopting” hospitals. Respondents at adopting hospitals were asked to share details about their protocol, including patient eligibility criteria and maximum HFNC rates of flow permitted outside of the ICU.
Patient Characteristics
Patients aged 3 to 24 months who were hospitalized at adopting and nonadopting hospitals were included if an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis code for bronchiolitis (466.XX) was present in any position (not limited to a primary diagnosis). The lower age limit of 3 months was chosen to match the most restrictive age eligibility criteria of provided HFNC protocols (Appendix 1). A crosswalk available from the Centers for Medicare & Medicaid Services18 was used to convert ICD-10 diagnosis and procedure codes from recent years to ICD-9 diagnosis and procedure codes. Patients were excluded if their encounter contained a diagnosis or procedure code signifying a complex chronic condition,19 if their hospitalization involved care in the neonatal ICU, or if their admission date occurred outside of the respiratory season. Respiratory season was defined as November 1 through April 30.
Outcomes
Outcomes were measured during three respiratory seasons leading up to adoption and during three respiratory seasons after adoption. The primary outcome was ICU utilization, including the proportion of patients admitted to the ICU and ICU length of stay, expressed as ICU days per 100 patients. Secondary outcomes included mean total length of stay and the proportion of patients who received mechanical ventilation. Lengths of stay were measured in days, the most granular unit of time provided in PHIS over the entire study period. As such, partial days of care are rounded up to 1 full day. A previously published strict definition for mechanical ventilation that limits false positives was used, requiring that patients have a procedure or supply code for mechanical ventilation and a pharmacy charge for a neuromuscular blocking agent.20
Primary Analysis
The primary analysis was restricted to adopting hospitals. An interrupted time series approach was used to measure two possible types of change associated with HFNC protocol adoption: an immediate intervention effect and a change in the slope of an outcome.21 The immediate intervention effect represents the change in the level of the outcome that occurs in the period immediately following the introduction of the protocol. The change in slope is the extent to which the outcome changes on a per season basis, attributable to the protocol. Interrupted time series estimates were adjusted for patient age, gender, race, ethnicity, and insurance type; linear regression was used for continuous outcomes and logistic regression for dichotomous outcomes. An ordinary least squares time series model was used to adjust for autocorrelation and Newey-West standard errors were employed.22 Analyses were performed using STATA version 14 (Stata-Corp, College Station, Texas).
Supplementary Analyses
Two preplanned supplementary analyses were conducted. Supplementary analysis 1 was identical to the primary analysis, with the exception that the first season after adoption was censored. The rationale for censoring the first adoption season was to account for a potential learning effect and/or delayed start to full protocol implementation. Supplementary analysis 2 used the nonadopting hospitals as a control group and subtracted the effects measured from an interrupted time series analysis among nonadopting hospitals from the effects measured among adopting hospitals. The rationale for this approach was to control for unmeasured secular (eg, availability of ICU beds) and temporal (eg, severity of a given bronchiolitis season) factors that may have coincidentally occurred with HFNC adoption seasons. The only modification to the interrupted time series approach for supplementary analysis 2 was to provide the nonadopting hospitals with an artificial interruption point because nonadopting hospitals, by definition, did not have an adoption season that could be used in an interrupted time series approach. The interruption point for nonadopting hospitals was set at the median adoption season for adopting hospitals.
RESULTS
Exposure
Leaders at 44 hospitals were contacted regarding their hospital’s use of HFNC outside of the ICU (Figure 1). Responses were obtained for 41 hospitals (93% response rate), 18 of which were classified as nonadopting hospitals. Of the 23 hospitals where the presence of ward-based HFNC protocols were reported, 12 met inclusion criteria and were classified as adopting hospitals. HFNC protocols were adopted at these hospitals in a staggered fashion between the 2010-2011 and 2015-2016 respiratory seasons (Figure 2). The median adoption season was the 2013-14 respiratory season.
Nine adopting hospitals were able to provide details about their first HFNC protocols (Appendix 1). No two protocols were identical, but they shared many similarities. Minimum age requirements ranged from birth to a few months of age. Exclusion criteria were particularly variable, with a history of chronic lung disease or apnea being the most common criteria. Maximum allowed rates of flow ranged from 4 to 10 liters per minute. Criteria for transfer to the ICU were consistently based on an elevated FiO2 and duration of HFNC exposure.
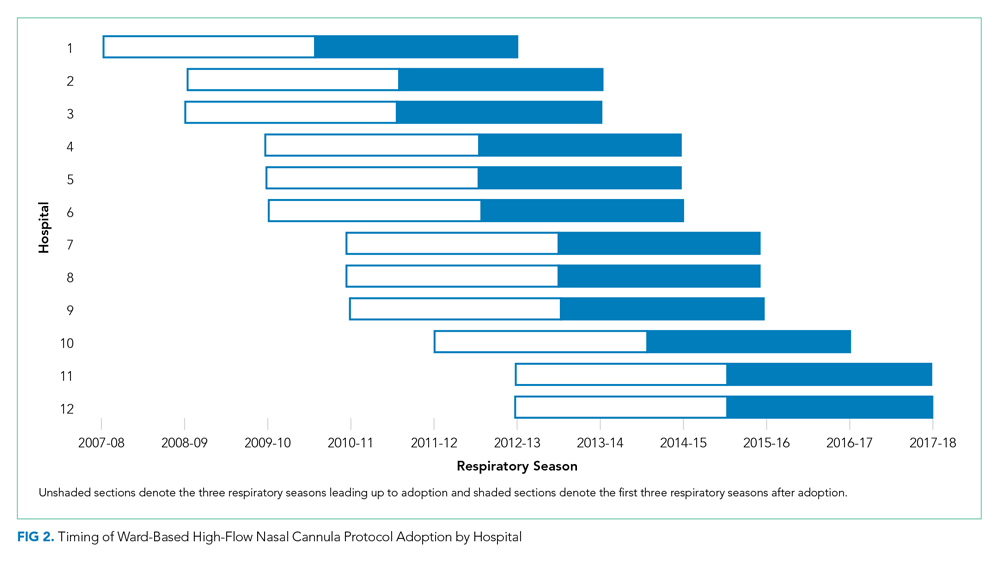
Patient Characteristics
A total of 32,809 bronchiolitis encounters occurred at adopting hospitals during qualifying respiratory seasons, of which 6,556 (20%) involved patients with a complex chronic condition and were excluded. Of the 26,253 included bronchiolitis encounters, 12,495 encounters occurred prior to ward-based HFNC protocol adoption and 13,758 encounters occurred after adoption. The median age of patients was 8 months (interquartile range, 5-14 months). Most patients were on government insurance (64%), male (58%), of white (56%) or black (18%) race, and of non-Hispanic ethnicity (72%). Pre- and postadoption patient demographics were similar (Appendix 2).
Primary Analysis
Shifts in the level of ICU use and ICU length of stay were observed at the time of adoption of a ward-based HFNC protocol (Figure 3). Specifically, ward-based HFNC protocol adoption was associated with an immediate 3.1% absolute increase (95% CI, 2.8%-3.4%) in the proportion of patients admitted to the ICU and a 9.1 days per 100 patients increase (95% CI, 5.1-13.2) in ICU length of stay (Table). The slope of ICU admissions per season was increasing after HFNC protocol adoption (1.0% increase per season; 95% CI, 0.8%-1.1%). When examined at the individual-hospital level (Appendix 3), seven hospitals were found to have significant increases in ICU admissions (immediate intervention effect or change in slope) after adoption, and one hospital was found to have a significant decrease in ICU admissions (change in slope only). Neither immediate intervention effects nor changes in the slopes of total length of stay and mechanical ventilation were observed, with mean total length of stay approximately 3 days and just over 1% of patients receiving mechanical ventilation (Figure 3).

Supplementary Analyses
Supplementary analyses were largely consistent with the primary analysis. Associations with increased ICU utilization were again observed, although the immediate change in ICU length of stay for supplementary analysis 1 was not significant and the slope for ICU length of stay in supplementary analysis 2 was down trending (Table). Changes in total length of stay and mechanical ventilation were not observed in either supplementary analysis, with the lone exception being an increase in the proportion of patients receiving mechanical ventilation per season (increase in slope) in supplementary analysis 1.
DISCUSSION
This is the largest multicenter study to date evaluating ICU utilization after adoption of a ward-based HFNC protocol for patients with bronchiolitis. While a principal goal of allowing HFNC use outside of the ICU is to reduce the time that patients with bronchiolitis spend in the ICU, we found that early protocols were, paradoxically, associated with increased ICU utilization. Ward-based HFNC protocols were not associated with changes in hospital length of stay or need for mechanical ventilation. Our findings are particularly relevant given that the majority of children’s hospitals in our sample have adopted ward-based HFNC protocols to care for patients with bronchiolitis.
The increase in ICU utilization measured in our study is a novel finding, seemingly in contradiction to existing literature. Early pilot studies inspired hope that employing HFNC on the general ward might prevent a portion of children from needing ICU care.11,12 Subsequent larger observational studies did not demonstrate decreases in ICU utilization after adoption of ward-based HFNC protocols.13,14 The two RCTs comparing low-flow and high-flow nasal cannula use outside of the ICU did not measure a statistically significant effect on ICU utilization, an exploratory outcome in both trials.15,16 However, the reported point estimates for absolute differences in ICU admission were 2% to 3% higher among patients randomized to HFNC, which is consistent with the 2% to 4% increase in ICU admission measured in the present study.
What might explain this surprising finding? While our observational study cannot speak to mechanism, the protocol details examined in the present study suggest that initial adoption of a ward-based HFNC protocol is often coupled with specific ICU transfer criteria that were unlikely in place prior to protocol initiation. For example, most protocols recommended consideration of ICU transfer for elevated FiO2 or prolonged duration of HFNC. Transfer to the ICU for prolonged HFNC duration is only possible in the setting of a ward-based HFNC protocol and transfer for elevated FiO2 was probably unnecessary prior to protocol adoption given that low-flow nasal cannula generally delivers 100% FiO2. It is also possible that with HFNC comes a perception of increased acuity. For example, medical providers may see patients on HFNC as sicker than patients with the same amount of work of breathing but off HFNC, which makes providers more likely to seek ICU admission for patients on HFNC. The combination of unchanged total length of stay and increased ICU utilization suggests that early ward-based HFNC protocols were an ineffective instrument to improve hospital bed availability during the peak census times that often occur in bronchiolitis season.
The large sample size afforded our study by its multicenter, retrospective design also allowed for a meaningful assessment of the association between a ward-based HFNC protocol and the need for mechanical ventilation. Early indications suggested a lack of substantial association between HFNC use outside of the ICU and rates of mechanical ventilation, but prior studies were limited by small numbers of patients receiving mechanical ventilation (<30 patients in each study).13,14,16 The present study, in which 783 patients received mechanical ventilation, supports the lack of association between early ward-based HFNC protocols and the need for mechanical ventilation. It should be noted that other studies have measured decreases in mechanical ventilation in association with ICU-based HFNC use.23-26 In addition to examining HFNC use in a different clinical context, decreases in mechanical ventilation measured after HFNC implementation in the ICU could be explained by preexisting practice trends to limit invasive ventilation and/or selection bias resulting from an increase in less severely ill patients being admitted to the ICU over time. The interrupted time series approach and the staggered adoption of HFNC protocols make the present study less susceptible to biases from preexisting trends and the inclusion of patients cared for both on the ward and within the ICU reduces selection bias.
Our study has several important limitations. First, all hospitals included in the analysis were US children’s hospitals and these findings may not generalize to other practice environments, including community hospitals and other countries. Second, our cohort and outcomes were defined using administrative billing data, which have been incompletely validated, making some degree of misclassification likely. Third, we measured HFNC exposure at the hospital level, but could not examine the extent to which individual patients were exposed to HFNC because such data are not present in PHIS. Even if we had access to patient-level HFNC exposure data, we would have still compared outcomes among all patients with bronchiolitis (not just those who received HFNC), to avoid selection bias. However, knowing HFNC exposure status at the patient level would have allowed for weighting of the effects measured at each hospital according to the extent of HFNC exposure. Fourth, there are likely other, unmeasured secular and temporal factors that could affect study outcomes. To some degree, the interrupted time series approach, observed staggered adoption of protocols, and nonadopting hospital supplementary analysis mitigate this risk of bias. Fifth, while the pre- and postadoption populations appeared demographically similar, it is possible that the populations might have differed by other unmeasured factors. Finally, early ward-based HFNC protocols have likely undergone iterative changes since adoption. We compared pre- and postadoption outcome slopes and censored the first adoption season in a supplementary analysis to attempt to account for this potential limitation.
In conclusion, our findings suggest that initial implementation of ward-based HFNC protocols were not successful at reducing ICU utilization for children with bronchiolitis. Future research should examine whether more evolved HFNC protocols that use higher flow rates, more generous ICU transfer criteria, and more rapid weaning criteria can reduce ICU utilization.
Acknowledgments
We thank Dr Vineeta Mittal (University of Texas Southwestern Medical Center) for providing feedback regarding the manuscript.
1. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700-706. https://doi.org/10.1001/archpediatrics.2011.1669.
2. Hasegawa K, Pate BM, Mansbach JM, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr. 2015;15(1):77-81. https://doi.org/10.1016/j.acap.2014.06.008.
3. Schroeder AR, Destino LA, Brooks R, Wang CJ, Coon ER. Outcomes of follow-up visits after bronchiolitis hospitalizations. JAMA Pediatr. 2018;172(3):296-297. https://doi.org/10.1001/jamapediatrics.2017.4002.
4. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15(2):145-155. https://doi.org/10.1513/AnnalsATS.201707-548FR.
5. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15(1):1-6. https://doi.org/10.1097/PCC.0000000000000011.
6. Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15(5):e214-e219. https://doi.org/10.1097/PCC.0000000000000112.
7. Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713-720. https://doi.org/10.1002/ppul.23060.
8. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71.e63. https://doi.org/10.1016/j.jpeds.2017.06.006.
9. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014(3):CD009850. https://doi.org/10.1002/14651858.CD009850.pub2.
10. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408-413.
11. Kallappa C, Hufton M, Millen G, Ninan TK. Use of high flow nasal cannula oxygen (HFNCO) in infants with bronchiolitis on a paediatric ward: a 3-year experience. Arch Dis Child. 2014;99(8):790-791. https://doi.org/10.1136/archdischild-2014-306637.
12. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
13. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
16. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
17. Coon ER, Mittal V, Brady PW. High flow nasal cannula-just expensive paracetamol? Lancet Child Adolesc Health. 2019;3(9):593-595. https://doi.org/10.1016/S2352-4642(19)30235-4.
18. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. 2012. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html. Accessed November 19, 2016.
19. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
20. Shein SL, Slain K, Wilson-Costello D, McKee B, Rotta AT. Temporal changes in prescription of neuropharmacologic drugs and utilization of resources related to neurologic morbidity in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2017;18(12):e606-e614. https://doi.org/10.1097/PCC.0000000000001351.
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002.
22. Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703-708.
23. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
24. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
25. Kawaguchi A, Yasui Y, deCaen A, Garros D. The clinical impact of heated humidified high-flow nasal cannula on pediatric respiratory distress. Pediatr Crit Care Med. 2017;18(2):112-119. https://doi.org/10.1097/PCC.0000000000000985.
26. Schlapbach LJ, Straney L, Gelbart B, et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017;49(6):1601648. https://doi.org/10.1183/13993003.01648-2016.
Children hospitalized for bronchiolitis frequently require admission to the intensive care unit (ICU), with estimates as high as 18%1,2 and 35%3 in two prospective, multicenter studies. The indication for ICU admission is nearly always a need for advanced respiratory support, which historically consisted of continuous or bilevel positive airway pressure (CPAP and BiPAP, respectively) or mechanical ventilation. High-flow nasal cannula (HFNC) is a recent addition to the respiratory support armamentarium, delivering heated and humidified oxygen at rates of up to 60 L/min and allowing for clinicians to titrate both flow rate and fraction of inspired oxygen (FiO2).4
Several studies have demonstrated that HFNC is capable of decreasing a child’s work of breathing,5-8 and it has the potential advantage of being better tolerated than other forms of advanced respiratory support.9,10 These case-series physiologic studies informed early ward-based HFNC protocols for bronchiolitis, which were adopted to decrease ICU utilization. Since then, single center observational studies examining the association between ward-based HFNC protocols and subsequent ICU utilization have come to discordant conclusions.11-14 Studying the effect of employing HFNC outside of the ICU is challenging in the context of a randomized, controlled trial (RCT) because it is difficult to blind healthcare providers to the intervention and because crossover from the control group to HFNC is frequent. Two unblinded RCTs published in 2017 and 2018 found that children randomized to conventional nasal cannula were frequently escalated to HFNC (flow rates of 1-2 L/kg per minute), but neither trial found a difference in ICU admission.15,16 Sample sizes substantially larger than those present in currently published or registered RCTs would be required to evaluate the impact of ward-based HFNC protocols on the outcome that inspired the protocols in the first place, namely ICU utilization.17
Children’s hospitals have adopted ward-based HFNC protocols at different time points over the last decade, which allows for a natural experiment—a promising alternative study design that avoids the challenges of blinding, crossover, and modest sample sizes. In order to have sufficient postadoption data for analyses, the present study is limited to ward-based HFNC protocols adopted prior to 2016, which we have termed “early” ward-based HFNC protocols. Among children with bronchiolitis, our objective was to measure the association between hospital-level adoption of a ward-based HFNC protocol and subsequent ICU utilization, using a multicenter network of children’s hospitals.
METHODS
We conducted a multicenter retrospective cohort study using the Pediatric Health Information System (PHIS) database. The PHIS database is operated by the Children’s Hospital Association (Lenexa, Kansas) and provides deidentified patient-level information for children who receive hospital care at 55 US children’s hospitals. Available data elements include patient demographic data, discharge diagnosis and procedure codes, and detailed billing information, such as laboratory, imaging, pharmacy, and supply charges. At the patient level, the use of HFNC vs standard oxygen therapy circuits cannot be discriminated.
Exposure
The study exposure was a hospital’s first ward-based HFNC protocol, with adoption measured at the hospital level at each PHIS site via direct communication with leaders in hospital medicine. In most cases, first contact was made with the pediatric hospital medicine division chief or fellowship program director, who then, if necessary, connected study investigators to local HFNC champions aware of site-specific historical HFNC protocol details. Contact with a hospital was made only if the hospital had contributed at least 6 consecutive years of data to PHIS. Hospitals were classified as “adopting” hospitals if their HFNC protocol met all of the following criteria: (a) allows initiation of HFNC outside of the ICU (on the floor or in the ED), (b) allows continued care outside of the ICU (on the floor), (c) not limited to a small unit like an intermediate care unit, and (d) adopted during a specific, known respiratory season. Hospitals for which ward-based HFNC protocols were adopted but did not meet these criteria were excluded from further analysis. Our intent was to identify large scale, programmatic protocol launches and exclude hospitals with exceptions that might preclude a sizable portion of our cohort from being eligible for the protocol. Hospitals for which inpatient use of HFNC remains limited to the ICU were defined as “nonadopting” hospitals. Respondents at adopting hospitals were asked to share details about their protocol, including patient eligibility criteria and maximum HFNC rates of flow permitted outside of the ICU.
Patient Characteristics
Patients aged 3 to 24 months who were hospitalized at adopting and nonadopting hospitals were included if an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis code for bronchiolitis (466.XX) was present in any position (not limited to a primary diagnosis). The lower age limit of 3 months was chosen to match the most restrictive age eligibility criteria of provided HFNC protocols (Appendix 1). A crosswalk available from the Centers for Medicare & Medicaid Services18 was used to convert ICD-10 diagnosis and procedure codes from recent years to ICD-9 diagnosis and procedure codes. Patients were excluded if their encounter contained a diagnosis or procedure code signifying a complex chronic condition,19 if their hospitalization involved care in the neonatal ICU, or if their admission date occurred outside of the respiratory season. Respiratory season was defined as November 1 through April 30.
Outcomes
Outcomes were measured during three respiratory seasons leading up to adoption and during three respiratory seasons after adoption. The primary outcome was ICU utilization, including the proportion of patients admitted to the ICU and ICU length of stay, expressed as ICU days per 100 patients. Secondary outcomes included mean total length of stay and the proportion of patients who received mechanical ventilation. Lengths of stay were measured in days, the most granular unit of time provided in PHIS over the entire study period. As such, partial days of care are rounded up to 1 full day. A previously published strict definition for mechanical ventilation that limits false positives was used, requiring that patients have a procedure or supply code for mechanical ventilation and a pharmacy charge for a neuromuscular blocking agent.20
Primary Analysis
The primary analysis was restricted to adopting hospitals. An interrupted time series approach was used to measure two possible types of change associated with HFNC protocol adoption: an immediate intervention effect and a change in the slope of an outcome.21 The immediate intervention effect represents the change in the level of the outcome that occurs in the period immediately following the introduction of the protocol. The change in slope is the extent to which the outcome changes on a per season basis, attributable to the protocol. Interrupted time series estimates were adjusted for patient age, gender, race, ethnicity, and insurance type; linear regression was used for continuous outcomes and logistic regression for dichotomous outcomes. An ordinary least squares time series model was used to adjust for autocorrelation and Newey-West standard errors were employed.22 Analyses were performed using STATA version 14 (Stata-Corp, College Station, Texas).
Supplementary Analyses
Two preplanned supplementary analyses were conducted. Supplementary analysis 1 was identical to the primary analysis, with the exception that the first season after adoption was censored. The rationale for censoring the first adoption season was to account for a potential learning effect and/or delayed start to full protocol implementation. Supplementary analysis 2 used the nonadopting hospitals as a control group and subtracted the effects measured from an interrupted time series analysis among nonadopting hospitals from the effects measured among adopting hospitals. The rationale for this approach was to control for unmeasured secular (eg, availability of ICU beds) and temporal (eg, severity of a given bronchiolitis season) factors that may have coincidentally occurred with HFNC adoption seasons. The only modification to the interrupted time series approach for supplementary analysis 2 was to provide the nonadopting hospitals with an artificial interruption point because nonadopting hospitals, by definition, did not have an adoption season that could be used in an interrupted time series approach. The interruption point for nonadopting hospitals was set at the median adoption season for adopting hospitals.
RESULTS
Exposure
Leaders at 44 hospitals were contacted regarding their hospital’s use of HFNC outside of the ICU (Figure 1). Responses were obtained for 41 hospitals (93% response rate), 18 of which were classified as nonadopting hospitals. Of the 23 hospitals where the presence of ward-based HFNC protocols were reported, 12 met inclusion criteria and were classified as adopting hospitals. HFNC protocols were adopted at these hospitals in a staggered fashion between the 2010-2011 and 2015-2016 respiratory seasons (Figure 2). The median adoption season was the 2013-14 respiratory season.
Nine adopting hospitals were able to provide details about their first HFNC protocols (Appendix 1). No two protocols were identical, but they shared many similarities. Minimum age requirements ranged from birth to a few months of age. Exclusion criteria were particularly variable, with a history of chronic lung disease or apnea being the most common criteria. Maximum allowed rates of flow ranged from 4 to 10 liters per minute. Criteria for transfer to the ICU were consistently based on an elevated FiO2 and duration of HFNC exposure.

Patient Characteristics
A total of 32,809 bronchiolitis encounters occurred at adopting hospitals during qualifying respiratory seasons, of which 6,556 (20%) involved patients with a complex chronic condition and were excluded. Of the 26,253 included bronchiolitis encounters, 12,495 encounters occurred prior to ward-based HFNC protocol adoption and 13,758 encounters occurred after adoption. The median age of patients was 8 months (interquartile range, 5-14 months). Most patients were on government insurance (64%), male (58%), of white (56%) or black (18%) race, and of non-Hispanic ethnicity (72%). Pre- and postadoption patient demographics were similar (Appendix 2).
Primary Analysis
Shifts in the level of ICU use and ICU length of stay were observed at the time of adoption of a ward-based HFNC protocol (Figure 3). Specifically, ward-based HFNC protocol adoption was associated with an immediate 3.1% absolute increase (95% CI, 2.8%-3.4%) in the proportion of patients admitted to the ICU and a 9.1 days per 100 patients increase (95% CI, 5.1-13.2) in ICU length of stay (Table). The slope of ICU admissions per season was increasing after HFNC protocol adoption (1.0% increase per season; 95% CI, 0.8%-1.1%). When examined at the individual-hospital level (Appendix 3), seven hospitals were found to have significant increases in ICU admissions (immediate intervention effect or change in slope) after adoption, and one hospital was found to have a significant decrease in ICU admissions (change in slope only). Neither immediate intervention effects nor changes in the slopes of total length of stay and mechanical ventilation were observed, with mean total length of stay approximately 3 days and just over 1% of patients receiving mechanical ventilation (Figure 3).

Supplementary Analyses
Supplementary analyses were largely consistent with the primary analysis. Associations with increased ICU utilization were again observed, although the immediate change in ICU length of stay for supplementary analysis 1 was not significant and the slope for ICU length of stay in supplementary analysis 2 was down trending (Table). Changes in total length of stay and mechanical ventilation were not observed in either supplementary analysis, with the lone exception being an increase in the proportion of patients receiving mechanical ventilation per season (increase in slope) in supplementary analysis 1.
DISCUSSION
This is the largest multicenter study to date evaluating ICU utilization after adoption of a ward-based HFNC protocol for patients with bronchiolitis. While a principal goal of allowing HFNC use outside of the ICU is to reduce the time that patients with bronchiolitis spend in the ICU, we found that early protocols were, paradoxically, associated with increased ICU utilization. Ward-based HFNC protocols were not associated with changes in hospital length of stay or need for mechanical ventilation. Our findings are particularly relevant given that the majority of children’s hospitals in our sample have adopted ward-based HFNC protocols to care for patients with bronchiolitis.
The increase in ICU utilization measured in our study is a novel finding, seemingly in contradiction to existing literature. Early pilot studies inspired hope that employing HFNC on the general ward might prevent a portion of children from needing ICU care.11,12 Subsequent larger observational studies did not demonstrate decreases in ICU utilization after adoption of ward-based HFNC protocols.13,14 The two RCTs comparing low-flow and high-flow nasal cannula use outside of the ICU did not measure a statistically significant effect on ICU utilization, an exploratory outcome in both trials.15,16 However, the reported point estimates for absolute differences in ICU admission were 2% to 3% higher among patients randomized to HFNC, which is consistent with the 2% to 4% increase in ICU admission measured in the present study.
What might explain this surprising finding? While our observational study cannot speak to mechanism, the protocol details examined in the present study suggest that initial adoption of a ward-based HFNC protocol is often coupled with specific ICU transfer criteria that were unlikely in place prior to protocol initiation. For example, most protocols recommended consideration of ICU transfer for elevated FiO2 or prolonged duration of HFNC. Transfer to the ICU for prolonged HFNC duration is only possible in the setting of a ward-based HFNC protocol and transfer for elevated FiO2 was probably unnecessary prior to protocol adoption given that low-flow nasal cannula generally delivers 100% FiO2. It is also possible that with HFNC comes a perception of increased acuity. For example, medical providers may see patients on HFNC as sicker than patients with the same amount of work of breathing but off HFNC, which makes providers more likely to seek ICU admission for patients on HFNC. The combination of unchanged total length of stay and increased ICU utilization suggests that early ward-based HFNC protocols were an ineffective instrument to improve hospital bed availability during the peak census times that often occur in bronchiolitis season.
The large sample size afforded our study by its multicenter, retrospective design also allowed for a meaningful assessment of the association between a ward-based HFNC protocol and the need for mechanical ventilation. Early indications suggested a lack of substantial association between HFNC use outside of the ICU and rates of mechanical ventilation, but prior studies were limited by small numbers of patients receiving mechanical ventilation (<30 patients in each study).13,14,16 The present study, in which 783 patients received mechanical ventilation, supports the lack of association between early ward-based HFNC protocols and the need for mechanical ventilation. It should be noted that other studies have measured decreases in mechanical ventilation in association with ICU-based HFNC use.23-26 In addition to examining HFNC use in a different clinical context, decreases in mechanical ventilation measured after HFNC implementation in the ICU could be explained by preexisting practice trends to limit invasive ventilation and/or selection bias resulting from an increase in less severely ill patients being admitted to the ICU over time. The interrupted time series approach and the staggered adoption of HFNC protocols make the present study less susceptible to biases from preexisting trends and the inclusion of patients cared for both on the ward and within the ICU reduces selection bias.
Our study has several important limitations. First, all hospitals included in the analysis were US children’s hospitals and these findings may not generalize to other practice environments, including community hospitals and other countries. Second, our cohort and outcomes were defined using administrative billing data, which have been incompletely validated, making some degree of misclassification likely. Third, we measured HFNC exposure at the hospital level, but could not examine the extent to which individual patients were exposed to HFNC because such data are not present in PHIS. Even if we had access to patient-level HFNC exposure data, we would have still compared outcomes among all patients with bronchiolitis (not just those who received HFNC), to avoid selection bias. However, knowing HFNC exposure status at the patient level would have allowed for weighting of the effects measured at each hospital according to the extent of HFNC exposure. Fourth, there are likely other, unmeasured secular and temporal factors that could affect study outcomes. To some degree, the interrupted time series approach, observed staggered adoption of protocols, and nonadopting hospital supplementary analysis mitigate this risk of bias. Fifth, while the pre- and postadoption populations appeared demographically similar, it is possible that the populations might have differed by other unmeasured factors. Finally, early ward-based HFNC protocols have likely undergone iterative changes since adoption. We compared pre- and postadoption outcome slopes and censored the first adoption season in a supplementary analysis to attempt to account for this potential limitation.
In conclusion, our findings suggest that initial implementation of ward-based HFNC protocols were not successful at reducing ICU utilization for children with bronchiolitis. Future research should examine whether more evolved HFNC protocols that use higher flow rates, more generous ICU transfer criteria, and more rapid weaning criteria can reduce ICU utilization.
Acknowledgments
We thank Dr Vineeta Mittal (University of Texas Southwestern Medical Center) for providing feedback regarding the manuscript.
Children hospitalized for bronchiolitis frequently require admission to the intensive care unit (ICU), with estimates as high as 18%1,2 and 35%3 in two prospective, multicenter studies. The indication for ICU admission is nearly always a need for advanced respiratory support, which historically consisted of continuous or bilevel positive airway pressure (CPAP and BiPAP, respectively) or mechanical ventilation. High-flow nasal cannula (HFNC) is a recent addition to the respiratory support armamentarium, delivering heated and humidified oxygen at rates of up to 60 L/min and allowing for clinicians to titrate both flow rate and fraction of inspired oxygen (FiO2).4
Several studies have demonstrated that HFNC is capable of decreasing a child’s work of breathing,5-8 and it has the potential advantage of being better tolerated than other forms of advanced respiratory support.9,10 These case-series physiologic studies informed early ward-based HFNC protocols for bronchiolitis, which were adopted to decrease ICU utilization. Since then, single center observational studies examining the association between ward-based HFNC protocols and subsequent ICU utilization have come to discordant conclusions.11-14 Studying the effect of employing HFNC outside of the ICU is challenging in the context of a randomized, controlled trial (RCT) because it is difficult to blind healthcare providers to the intervention and because crossover from the control group to HFNC is frequent. Two unblinded RCTs published in 2017 and 2018 found that children randomized to conventional nasal cannula were frequently escalated to HFNC (flow rates of 1-2 L/kg per minute), but neither trial found a difference in ICU admission.15,16 Sample sizes substantially larger than those present in currently published or registered RCTs would be required to evaluate the impact of ward-based HFNC protocols on the outcome that inspired the protocols in the first place, namely ICU utilization.17
Children’s hospitals have adopted ward-based HFNC protocols at different time points over the last decade, which allows for a natural experiment—a promising alternative study design that avoids the challenges of blinding, crossover, and modest sample sizes. In order to have sufficient postadoption data for analyses, the present study is limited to ward-based HFNC protocols adopted prior to 2016, which we have termed “early” ward-based HFNC protocols. Among children with bronchiolitis, our objective was to measure the association between hospital-level adoption of a ward-based HFNC protocol and subsequent ICU utilization, using a multicenter network of children’s hospitals.
METHODS
We conducted a multicenter retrospective cohort study using the Pediatric Health Information System (PHIS) database. The PHIS database is operated by the Children’s Hospital Association (Lenexa, Kansas) and provides deidentified patient-level information for children who receive hospital care at 55 US children’s hospitals. Available data elements include patient demographic data, discharge diagnosis and procedure codes, and detailed billing information, such as laboratory, imaging, pharmacy, and supply charges. At the patient level, the use of HFNC vs standard oxygen therapy circuits cannot be discriminated.
Exposure
The study exposure was a hospital’s first ward-based HFNC protocol, with adoption measured at the hospital level at each PHIS site via direct communication with leaders in hospital medicine. In most cases, first contact was made with the pediatric hospital medicine division chief or fellowship program director, who then, if necessary, connected study investigators to local HFNC champions aware of site-specific historical HFNC protocol details. Contact with a hospital was made only if the hospital had contributed at least 6 consecutive years of data to PHIS. Hospitals were classified as “adopting” hospitals if their HFNC protocol met all of the following criteria: (a) allows initiation of HFNC outside of the ICU (on the floor or in the ED), (b) allows continued care outside of the ICU (on the floor), (c) not limited to a small unit like an intermediate care unit, and (d) adopted during a specific, known respiratory season. Hospitals for which ward-based HFNC protocols were adopted but did not meet these criteria were excluded from further analysis. Our intent was to identify large scale, programmatic protocol launches and exclude hospitals with exceptions that might preclude a sizable portion of our cohort from being eligible for the protocol. Hospitals for which inpatient use of HFNC remains limited to the ICU were defined as “nonadopting” hospitals. Respondents at adopting hospitals were asked to share details about their protocol, including patient eligibility criteria and maximum HFNC rates of flow permitted outside of the ICU.
Patient Characteristics
Patients aged 3 to 24 months who were hospitalized at adopting and nonadopting hospitals were included if an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis code for bronchiolitis (466.XX) was present in any position (not limited to a primary diagnosis). The lower age limit of 3 months was chosen to match the most restrictive age eligibility criteria of provided HFNC protocols (Appendix 1). A crosswalk available from the Centers for Medicare & Medicaid Services18 was used to convert ICD-10 diagnosis and procedure codes from recent years to ICD-9 diagnosis and procedure codes. Patients were excluded if their encounter contained a diagnosis or procedure code signifying a complex chronic condition,19 if their hospitalization involved care in the neonatal ICU, or if their admission date occurred outside of the respiratory season. Respiratory season was defined as November 1 through April 30.
Outcomes
Outcomes were measured during three respiratory seasons leading up to adoption and during three respiratory seasons after adoption. The primary outcome was ICU utilization, including the proportion of patients admitted to the ICU and ICU length of stay, expressed as ICU days per 100 patients. Secondary outcomes included mean total length of stay and the proportion of patients who received mechanical ventilation. Lengths of stay were measured in days, the most granular unit of time provided in PHIS over the entire study period. As such, partial days of care are rounded up to 1 full day. A previously published strict definition for mechanical ventilation that limits false positives was used, requiring that patients have a procedure or supply code for mechanical ventilation and a pharmacy charge for a neuromuscular blocking agent.20
Primary Analysis
The primary analysis was restricted to adopting hospitals. An interrupted time series approach was used to measure two possible types of change associated with HFNC protocol adoption: an immediate intervention effect and a change in the slope of an outcome.21 The immediate intervention effect represents the change in the level of the outcome that occurs in the period immediately following the introduction of the protocol. The change in slope is the extent to which the outcome changes on a per season basis, attributable to the protocol. Interrupted time series estimates were adjusted for patient age, gender, race, ethnicity, and insurance type; linear regression was used for continuous outcomes and logistic regression for dichotomous outcomes. An ordinary least squares time series model was used to adjust for autocorrelation and Newey-West standard errors were employed.22 Analyses were performed using STATA version 14 (Stata-Corp, College Station, Texas).
Supplementary Analyses
Two preplanned supplementary analyses were conducted. Supplementary analysis 1 was identical to the primary analysis, with the exception that the first season after adoption was censored. The rationale for censoring the first adoption season was to account for a potential learning effect and/or delayed start to full protocol implementation. Supplementary analysis 2 used the nonadopting hospitals as a control group and subtracted the effects measured from an interrupted time series analysis among nonadopting hospitals from the effects measured among adopting hospitals. The rationale for this approach was to control for unmeasured secular (eg, availability of ICU beds) and temporal (eg, severity of a given bronchiolitis season) factors that may have coincidentally occurred with HFNC adoption seasons. The only modification to the interrupted time series approach for supplementary analysis 2 was to provide the nonadopting hospitals with an artificial interruption point because nonadopting hospitals, by definition, did not have an adoption season that could be used in an interrupted time series approach. The interruption point for nonadopting hospitals was set at the median adoption season for adopting hospitals.
RESULTS
Exposure
Leaders at 44 hospitals were contacted regarding their hospital’s use of HFNC outside of the ICU (Figure 1). Responses were obtained for 41 hospitals (93% response rate), 18 of which were classified as nonadopting hospitals. Of the 23 hospitals where the presence of ward-based HFNC protocols were reported, 12 met inclusion criteria and were classified as adopting hospitals. HFNC protocols were adopted at these hospitals in a staggered fashion between the 2010-2011 and 2015-2016 respiratory seasons (Figure 2). The median adoption season was the 2013-14 respiratory season.
Nine adopting hospitals were able to provide details about their first HFNC protocols (Appendix 1). No two protocols were identical, but they shared many similarities. Minimum age requirements ranged from birth to a few months of age. Exclusion criteria were particularly variable, with a history of chronic lung disease or apnea being the most common criteria. Maximum allowed rates of flow ranged from 4 to 10 liters per minute. Criteria for transfer to the ICU were consistently based on an elevated FiO2 and duration of HFNC exposure.

Patient Characteristics
A total of 32,809 bronchiolitis encounters occurred at adopting hospitals during qualifying respiratory seasons, of which 6,556 (20%) involved patients with a complex chronic condition and were excluded. Of the 26,253 included bronchiolitis encounters, 12,495 encounters occurred prior to ward-based HFNC protocol adoption and 13,758 encounters occurred after adoption. The median age of patients was 8 months (interquartile range, 5-14 months). Most patients were on government insurance (64%), male (58%), of white (56%) or black (18%) race, and of non-Hispanic ethnicity (72%). Pre- and postadoption patient demographics were similar (Appendix 2).
Primary Analysis
Shifts in the level of ICU use and ICU length of stay were observed at the time of adoption of a ward-based HFNC protocol (Figure 3). Specifically, ward-based HFNC protocol adoption was associated with an immediate 3.1% absolute increase (95% CI, 2.8%-3.4%) in the proportion of patients admitted to the ICU and a 9.1 days per 100 patients increase (95% CI, 5.1-13.2) in ICU length of stay (Table). The slope of ICU admissions per season was increasing after HFNC protocol adoption (1.0% increase per season; 95% CI, 0.8%-1.1%). When examined at the individual-hospital level (Appendix 3), seven hospitals were found to have significant increases in ICU admissions (immediate intervention effect or change in slope) after adoption, and one hospital was found to have a significant decrease in ICU admissions (change in slope only). Neither immediate intervention effects nor changes in the slopes of total length of stay and mechanical ventilation were observed, with mean total length of stay approximately 3 days and just over 1% of patients receiving mechanical ventilation (Figure 3).

Supplementary Analyses
Supplementary analyses were largely consistent with the primary analysis. Associations with increased ICU utilization were again observed, although the immediate change in ICU length of stay for supplementary analysis 1 was not significant and the slope for ICU length of stay in supplementary analysis 2 was down trending (Table). Changes in total length of stay and mechanical ventilation were not observed in either supplementary analysis, with the lone exception being an increase in the proportion of patients receiving mechanical ventilation per season (increase in slope) in supplementary analysis 1.
DISCUSSION
This is the largest multicenter study to date evaluating ICU utilization after adoption of a ward-based HFNC protocol for patients with bronchiolitis. While a principal goal of allowing HFNC use outside of the ICU is to reduce the time that patients with bronchiolitis spend in the ICU, we found that early protocols were, paradoxically, associated with increased ICU utilization. Ward-based HFNC protocols were not associated with changes in hospital length of stay or need for mechanical ventilation. Our findings are particularly relevant given that the majority of children’s hospitals in our sample have adopted ward-based HFNC protocols to care for patients with bronchiolitis.
The increase in ICU utilization measured in our study is a novel finding, seemingly in contradiction to existing literature. Early pilot studies inspired hope that employing HFNC on the general ward might prevent a portion of children from needing ICU care.11,12 Subsequent larger observational studies did not demonstrate decreases in ICU utilization after adoption of ward-based HFNC protocols.13,14 The two RCTs comparing low-flow and high-flow nasal cannula use outside of the ICU did not measure a statistically significant effect on ICU utilization, an exploratory outcome in both trials.15,16 However, the reported point estimates for absolute differences in ICU admission were 2% to 3% higher among patients randomized to HFNC, which is consistent with the 2% to 4% increase in ICU admission measured in the present study.
What might explain this surprising finding? While our observational study cannot speak to mechanism, the protocol details examined in the present study suggest that initial adoption of a ward-based HFNC protocol is often coupled with specific ICU transfer criteria that were unlikely in place prior to protocol initiation. For example, most protocols recommended consideration of ICU transfer for elevated FiO2 or prolonged duration of HFNC. Transfer to the ICU for prolonged HFNC duration is only possible in the setting of a ward-based HFNC protocol and transfer for elevated FiO2 was probably unnecessary prior to protocol adoption given that low-flow nasal cannula generally delivers 100% FiO2. It is also possible that with HFNC comes a perception of increased acuity. For example, medical providers may see patients on HFNC as sicker than patients with the same amount of work of breathing but off HFNC, which makes providers more likely to seek ICU admission for patients on HFNC. The combination of unchanged total length of stay and increased ICU utilization suggests that early ward-based HFNC protocols were an ineffective instrument to improve hospital bed availability during the peak census times that often occur in bronchiolitis season.
The large sample size afforded our study by its multicenter, retrospective design also allowed for a meaningful assessment of the association between a ward-based HFNC protocol and the need for mechanical ventilation. Early indications suggested a lack of substantial association between HFNC use outside of the ICU and rates of mechanical ventilation, but prior studies were limited by small numbers of patients receiving mechanical ventilation (<30 patients in each study).13,14,16 The present study, in which 783 patients received mechanical ventilation, supports the lack of association between early ward-based HFNC protocols and the need for mechanical ventilation. It should be noted that other studies have measured decreases in mechanical ventilation in association with ICU-based HFNC use.23-26 In addition to examining HFNC use in a different clinical context, decreases in mechanical ventilation measured after HFNC implementation in the ICU could be explained by preexisting practice trends to limit invasive ventilation and/or selection bias resulting from an increase in less severely ill patients being admitted to the ICU over time. The interrupted time series approach and the staggered adoption of HFNC protocols make the present study less susceptible to biases from preexisting trends and the inclusion of patients cared for both on the ward and within the ICU reduces selection bias.
Our study has several important limitations. First, all hospitals included in the analysis were US children’s hospitals and these findings may not generalize to other practice environments, including community hospitals and other countries. Second, our cohort and outcomes were defined using administrative billing data, which have been incompletely validated, making some degree of misclassification likely. Third, we measured HFNC exposure at the hospital level, but could not examine the extent to which individual patients were exposed to HFNC because such data are not present in PHIS. Even if we had access to patient-level HFNC exposure data, we would have still compared outcomes among all patients with bronchiolitis (not just those who received HFNC), to avoid selection bias. However, knowing HFNC exposure status at the patient level would have allowed for weighting of the effects measured at each hospital according to the extent of HFNC exposure. Fourth, there are likely other, unmeasured secular and temporal factors that could affect study outcomes. To some degree, the interrupted time series approach, observed staggered adoption of protocols, and nonadopting hospital supplementary analysis mitigate this risk of bias. Fifth, while the pre- and postadoption populations appeared demographically similar, it is possible that the populations might have differed by other unmeasured factors. Finally, early ward-based HFNC protocols have likely undergone iterative changes since adoption. We compared pre- and postadoption outcome slopes and censored the first adoption season in a supplementary analysis to attempt to account for this potential limitation.
In conclusion, our findings suggest that initial implementation of ward-based HFNC protocols were not successful at reducing ICU utilization for children with bronchiolitis. Future research should examine whether more evolved HFNC protocols that use higher flow rates, more generous ICU transfer criteria, and more rapid weaning criteria can reduce ICU utilization.
Acknowledgments
We thank Dr Vineeta Mittal (University of Texas Southwestern Medical Center) for providing feedback regarding the manuscript.
1. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700-706. https://doi.org/10.1001/archpediatrics.2011.1669.
2. Hasegawa K, Pate BM, Mansbach JM, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr. 2015;15(1):77-81. https://doi.org/10.1016/j.acap.2014.06.008.
3. Schroeder AR, Destino LA, Brooks R, Wang CJ, Coon ER. Outcomes of follow-up visits after bronchiolitis hospitalizations. JAMA Pediatr. 2018;172(3):296-297. https://doi.org/10.1001/jamapediatrics.2017.4002.
4. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15(2):145-155. https://doi.org/10.1513/AnnalsATS.201707-548FR.
5. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15(1):1-6. https://doi.org/10.1097/PCC.0000000000000011.
6. Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15(5):e214-e219. https://doi.org/10.1097/PCC.0000000000000112.
7. Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713-720. https://doi.org/10.1002/ppul.23060.
8. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71.e63. https://doi.org/10.1016/j.jpeds.2017.06.006.
9. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014(3):CD009850. https://doi.org/10.1002/14651858.CD009850.pub2.
10. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408-413.
11. Kallappa C, Hufton M, Millen G, Ninan TK. Use of high flow nasal cannula oxygen (HFNCO) in infants with bronchiolitis on a paediatric ward: a 3-year experience. Arch Dis Child. 2014;99(8):790-791. https://doi.org/10.1136/archdischild-2014-306637.
12. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
13. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
16. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
17. Coon ER, Mittal V, Brady PW. High flow nasal cannula-just expensive paracetamol? Lancet Child Adolesc Health. 2019;3(9):593-595. https://doi.org/10.1016/S2352-4642(19)30235-4.
18. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. 2012. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html. Accessed November 19, 2016.
19. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
20. Shein SL, Slain K, Wilson-Costello D, McKee B, Rotta AT. Temporal changes in prescription of neuropharmacologic drugs and utilization of resources related to neurologic morbidity in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2017;18(12):e606-e614. https://doi.org/10.1097/PCC.0000000000001351.
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002.
22. Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703-708.
23. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
24. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
25. Kawaguchi A, Yasui Y, deCaen A, Garros D. The clinical impact of heated humidified high-flow nasal cannula on pediatric respiratory distress. Pediatr Crit Care Med. 2017;18(2):112-119. https://doi.org/10.1097/PCC.0000000000000985.
26. Schlapbach LJ, Straney L, Gelbart B, et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017;49(6):1601648. https://doi.org/10.1183/13993003.01648-2016.
1. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700-706. https://doi.org/10.1001/archpediatrics.2011.1669.
2. Hasegawa K, Pate BM, Mansbach JM, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr. 2015;15(1):77-81. https://doi.org/10.1016/j.acap.2014.06.008.
3. Schroeder AR, Destino LA, Brooks R, Wang CJ, Coon ER. Outcomes of follow-up visits after bronchiolitis hospitalizations. JAMA Pediatr. 2018;172(3):296-297. https://doi.org/10.1001/jamapediatrics.2017.4002.
4. Drake MG. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018;15(2):145-155. https://doi.org/10.1513/AnnalsATS.201707-548FR.
5. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15(1):1-6. https://doi.org/10.1097/PCC.0000000000000011.
6. Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15(5):e214-e219. https://doi.org/10.1097/PCC.0000000000000112.
7. Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713-720. https://doi.org/10.1002/ppul.23060.
8. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71.e63. https://doi.org/10.1016/j.jpeds.2017.06.006.
9. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014(3):CD009850. https://doi.org/10.1002/14651858.CD009850.pub2.
10. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408-413.
11. Kallappa C, Hufton M, Millen G, Ninan TK. Use of high flow nasal cannula oxygen (HFNCO) in infants with bronchiolitis on a paediatric ward: a 3-year experience. Arch Dis Child. 2014;99(8):790-791. https://doi.org/10.1136/archdischild-2014-306637.
12. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
13. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
16. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
17. Coon ER, Mittal V, Brady PW. High flow nasal cannula-just expensive paracetamol? Lancet Child Adolesc Health. 2019;3(9):593-595. https://doi.org/10.1016/S2352-4642(19)30235-4.
18. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. 2012. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html. Accessed November 19, 2016.
19. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
20. Shein SL, Slain K, Wilson-Costello D, McKee B, Rotta AT. Temporal changes in prescription of neuropharmacologic drugs and utilization of resources related to neurologic morbidity in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2017;18(12):e606-e614. https://doi.org/10.1097/PCC.0000000000001351.
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002.
22. Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703-708.
23. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
24. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
25. Kawaguchi A, Yasui Y, deCaen A, Garros D. The clinical impact of heated humidified high-flow nasal cannula on pediatric respiratory distress. Pediatr Crit Care Med. 2017;18(2):112-119. https://doi.org/10.1097/PCC.0000000000000985.
26. Schlapbach LJ, Straney L, Gelbart B, et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J. 2017;49(6):1601648. https://doi.org/10.1183/13993003.01648-2016.
© 2020 Society of Hospital Medicine