User login
Genomic sequencing sheds light on development of pediatric cancer
Genome sequencing technologies are providing a valuable new window into the development and progression of pediatric cancers, according to the authors of a review.
In contrast to adult cancers, which are frequently driven by oncogenic mutations, many pediatric cancers have a low burden of somatic mutations, wrote E. Alejandro Sweet-Cordero, MD, from the University of California, San Francisco, and Jaclyn A. Biegel, MD, from the University of Southern California in Science. Instead, large-scale sequencing studies have found that childhood cancers have a much higher likelihood of being caused by germline mutations in genes that predispose development of cancer.
“Particularly surprising was the observation that even high-risk, highly aggressive cancers in many cases had no identifiable driver gene or pathway,” the authors wrote.
Some pediatric cancers do have identified driver genes, but even these are often different to those seen in adult cancers. The authors gave the example of one study of 1,699 patients and six types of cancer: This study identified 142 likely oncogenes, but only 45% of these matched those seen in the adult cancers.
Many pediatric cancers also have unique genetic features, such as the age-dependent gene fusion events, in which two genes join to form an oncogenic hybrid, and focal areas of gene deletion, which are often seen in pediatric acute myeloid leukemia but less so in adult forms of this cancer.
“In some instances, the fusion events involve genes that are known to be cancer drivers; this raises the intriguing possibility that some pediatric cancers are driven by ‘private’ oncogenic fusions,” the authors wrote, pointing out that this has daunting implications for the development of precision medicine. However they also noted that the presence of common gene fusion events could hold significance for choice of therapies; for example, central nervous system gliomas with the common BRAF V600E mutation may respond to specific BRAF inhibitors.
The authors drew particular attention to the role that genomic analysis could play in studying cancer during treatment and relapse, but they said few studies have explored this in pediatric patients.
“Such studies are critical given what we have learned from adult cancers, which show a capacity to evolve rapidly and acquire new driver mutations,” they wrote. One study found that only one-third of tumors with a potentially targetable genetic mutation had retained that target when analyzed at a later time.
On the issue of targeted therapy, the authors noted that no prospective study has yet looked at the use of sequencing approaches to define new therapies for pediatric cancer. However, they did refer to the Pediatric MATCH clinical trial, which is currently evaluating targeted therapies for relapsed solid tumors in children.
They also identified a need for research on predictors of treatment response in pediatric cancer.
“As the genetic variants that are associated with drug response are, by nature and design, variants present in the normal population, they are typically not included in DNA sequencing panels and are filtered out in WES [whole-exome sequencing] or WGS [whole-genome sequencing] bioinformatics pipelines,” they wrote.
They addressed the question of when to do germline testing in pediatric cancer, saying that, for most pediatric cancer patients, germline testing was indicated by the presence of a pathogenic genetic alternative affecting a gene known to be associated with a predisposition for germline cancer.
The authors suggested that data sharing was important to advancing genomic analysis in pediatric cancers because most of the studies so far had been relatively small. However, they highlighted emerging resources for large-scale analysis of pediatric cancer data, such as public portals for investigating discovery genomic data sets and data repositories of clinical-grade sequencing data.
The review was funded by the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sweet-Cordero A et al. Science 2019;363:1170-5.
Genome sequencing technologies are providing a valuable new window into the development and progression of pediatric cancers, according to the authors of a review.
In contrast to adult cancers, which are frequently driven by oncogenic mutations, many pediatric cancers have a low burden of somatic mutations, wrote E. Alejandro Sweet-Cordero, MD, from the University of California, San Francisco, and Jaclyn A. Biegel, MD, from the University of Southern California in Science. Instead, large-scale sequencing studies have found that childhood cancers have a much higher likelihood of being caused by germline mutations in genes that predispose development of cancer.
“Particularly surprising was the observation that even high-risk, highly aggressive cancers in many cases had no identifiable driver gene or pathway,” the authors wrote.
Some pediatric cancers do have identified driver genes, but even these are often different to those seen in adult cancers. The authors gave the example of one study of 1,699 patients and six types of cancer: This study identified 142 likely oncogenes, but only 45% of these matched those seen in the adult cancers.
Many pediatric cancers also have unique genetic features, such as the age-dependent gene fusion events, in which two genes join to form an oncogenic hybrid, and focal areas of gene deletion, which are often seen in pediatric acute myeloid leukemia but less so in adult forms of this cancer.
“In some instances, the fusion events involve genes that are known to be cancer drivers; this raises the intriguing possibility that some pediatric cancers are driven by ‘private’ oncogenic fusions,” the authors wrote, pointing out that this has daunting implications for the development of precision medicine. However they also noted that the presence of common gene fusion events could hold significance for choice of therapies; for example, central nervous system gliomas with the common BRAF V600E mutation may respond to specific BRAF inhibitors.
The authors drew particular attention to the role that genomic analysis could play in studying cancer during treatment and relapse, but they said few studies have explored this in pediatric patients.
“Such studies are critical given what we have learned from adult cancers, which show a capacity to evolve rapidly and acquire new driver mutations,” they wrote. One study found that only one-third of tumors with a potentially targetable genetic mutation had retained that target when analyzed at a later time.
On the issue of targeted therapy, the authors noted that no prospective study has yet looked at the use of sequencing approaches to define new therapies for pediatric cancer. However, they did refer to the Pediatric MATCH clinical trial, which is currently evaluating targeted therapies for relapsed solid tumors in children.
They also identified a need for research on predictors of treatment response in pediatric cancer.
“As the genetic variants that are associated with drug response are, by nature and design, variants present in the normal population, they are typically not included in DNA sequencing panels and are filtered out in WES [whole-exome sequencing] or WGS [whole-genome sequencing] bioinformatics pipelines,” they wrote.
They addressed the question of when to do germline testing in pediatric cancer, saying that, for most pediatric cancer patients, germline testing was indicated by the presence of a pathogenic genetic alternative affecting a gene known to be associated with a predisposition for germline cancer.
The authors suggested that data sharing was important to advancing genomic analysis in pediatric cancers because most of the studies so far had been relatively small. However, they highlighted emerging resources for large-scale analysis of pediatric cancer data, such as public portals for investigating discovery genomic data sets and data repositories of clinical-grade sequencing data.
The review was funded by the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sweet-Cordero A et al. Science 2019;363:1170-5.
Genome sequencing technologies are providing a valuable new window into the development and progression of pediatric cancers, according to the authors of a review.
In contrast to adult cancers, which are frequently driven by oncogenic mutations, many pediatric cancers have a low burden of somatic mutations, wrote E. Alejandro Sweet-Cordero, MD, from the University of California, San Francisco, and Jaclyn A. Biegel, MD, from the University of Southern California in Science. Instead, large-scale sequencing studies have found that childhood cancers have a much higher likelihood of being caused by germline mutations in genes that predispose development of cancer.
“Particularly surprising was the observation that even high-risk, highly aggressive cancers in many cases had no identifiable driver gene or pathway,” the authors wrote.
Some pediatric cancers do have identified driver genes, but even these are often different to those seen in adult cancers. The authors gave the example of one study of 1,699 patients and six types of cancer: This study identified 142 likely oncogenes, but only 45% of these matched those seen in the adult cancers.
Many pediatric cancers also have unique genetic features, such as the age-dependent gene fusion events, in which two genes join to form an oncogenic hybrid, and focal areas of gene deletion, which are often seen in pediatric acute myeloid leukemia but less so in adult forms of this cancer.
“In some instances, the fusion events involve genes that are known to be cancer drivers; this raises the intriguing possibility that some pediatric cancers are driven by ‘private’ oncogenic fusions,” the authors wrote, pointing out that this has daunting implications for the development of precision medicine. However they also noted that the presence of common gene fusion events could hold significance for choice of therapies; for example, central nervous system gliomas with the common BRAF V600E mutation may respond to specific BRAF inhibitors.
The authors drew particular attention to the role that genomic analysis could play in studying cancer during treatment and relapse, but they said few studies have explored this in pediatric patients.
“Such studies are critical given what we have learned from adult cancers, which show a capacity to evolve rapidly and acquire new driver mutations,” they wrote. One study found that only one-third of tumors with a potentially targetable genetic mutation had retained that target when analyzed at a later time.
On the issue of targeted therapy, the authors noted that no prospective study has yet looked at the use of sequencing approaches to define new therapies for pediatric cancer. However, they did refer to the Pediatric MATCH clinical trial, which is currently evaluating targeted therapies for relapsed solid tumors in children.
They also identified a need for research on predictors of treatment response in pediatric cancer.
“As the genetic variants that are associated with drug response are, by nature and design, variants present in the normal population, they are typically not included in DNA sequencing panels and are filtered out in WES [whole-exome sequencing] or WGS [whole-genome sequencing] bioinformatics pipelines,” they wrote.
They addressed the question of when to do germline testing in pediatric cancer, saying that, for most pediatric cancer patients, germline testing was indicated by the presence of a pathogenic genetic alternative affecting a gene known to be associated with a predisposition for germline cancer.
The authors suggested that data sharing was important to advancing genomic analysis in pediatric cancers because most of the studies so far had been relatively small. However, they highlighted emerging resources for large-scale analysis of pediatric cancer data, such as public portals for investigating discovery genomic data sets and data repositories of clinical-grade sequencing data.
The review was funded by the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sweet-Cordero A et al. Science 2019;363:1170-5.
FROM SCIENCE
Key clinical point: Genome sequencing is providing valuable information on pediatric cancer development and progression.
Major finding: Many pediatric cancers have very different oncogenic drivers to adult cancers.
Study details: Review.
Disclosures: The review was funded by the National Cancer Institute. No conflicts of interest were declared.
Source: Sweet-Cordero EA et al. Science. 2019;363:1170-5.
MRD status at transplant predicts outcomes in ALL patients
HOUSTON – Acute lymphoblastic leukemia patients with measurable residual disease (MRD) negativity prior to hematopoietic cell transplantation achieve better outcomes than do those who are MRD positive, particularly when total body irradiation (TBI)–based conditioning is used, a large retrospective study suggests.
Of 2,780 ALL patients who underwent hematopoietic cell transplantation (HCT) in first or second complete remission (CR), and who were included in the study, 1,816 were MRD negative before transplantation and 964 were MRD positive.
Overall, with follow-up of 40-44 months, MRD positivity was a significant independent predictor of lower overall survival (OS; hazard ratio, 1.19), leukemia-free survival (LFS; HR, 1.26), and higher relapse incidence (RI; 1.51), Arnon Nagler, MD, reported at the Transplantation & Cellular Therapy Meetings.
Conditioning was TBI-based in 76% of the patients; when these patients were compared with those who received chemotherapy-based conditioning, they were found to have better OS, LFS, and RI (HRs, 0.75, 0.70, and 0.60, respectively), said Dr. Nagler, director of both the division of hematology and the bone marrow transplantation and cord blood bank at the Chaim Sheba Medical Center, Tel-Hashomer, and professor of medicine at Tel Aviv University, both in Israel.
“There was no significant interaction between the MRD status and the conditioning,” he said.
On multivariate analysis, MRD positivity was found to be associated with lower OS and LFS (HRs, 1.26 and 1.3), and higher RI (HR, 1.53) in the TBI group, and with higher RI (HR 1.58) in the chemotherapy group, he said. There was no significant association between MRD and other outcomes in this last cohort, he added, noting that TBI-based conditioning was associated with improved OS, LFS, and RI in both MRD-negative and MRD-positive patients.
“MRD is an extremely important prognostic factor for ALL,” he said, noting that its prognostic value in this setting has been established in multiple studies, and that MRD measured at the end of induction is increasingly used to guide further therapy.
However, although MRD detectable immediately before HCT is known to be associated with poor outcomes, it has been unclear if – or to what extent – this differs with different types of conditioning, he added.
“So the aim of this study was to explore if MRD detectable before allogeneic HCT for ALL is associated with different outcomes in adult patients receiving myeloablative conditioning, either TBI or chemotherapy based,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Patients included in the analysis had a median age of 38 years and underwent HCT between 2000 and 2017 using sibling or unrelated 9/10 or 10/10 matched donors. None received blinatumomab or inotuzumab, Dr. Nagler said, adding that more patients are likely to achieve MRD negativity with these agents.
It will be interesting to see if the prognostic value of MRD will remain as strong with the new agents, and if TBI will be “a strong factor in overall survival and disease-free survival” with modern immunotherapy, he concluded.
The study was conducted on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT).
Dr. Nagler reported having no relevant financial disclosures.
SOURCE: Nagler A et al. TCT 2019, Abstract 7.
HOUSTON – Acute lymphoblastic leukemia patients with measurable residual disease (MRD) negativity prior to hematopoietic cell transplantation achieve better outcomes than do those who are MRD positive, particularly when total body irradiation (TBI)–based conditioning is used, a large retrospective study suggests.
Of 2,780 ALL patients who underwent hematopoietic cell transplantation (HCT) in first or second complete remission (CR), and who were included in the study, 1,816 were MRD negative before transplantation and 964 were MRD positive.
Overall, with follow-up of 40-44 months, MRD positivity was a significant independent predictor of lower overall survival (OS; hazard ratio, 1.19), leukemia-free survival (LFS; HR, 1.26), and higher relapse incidence (RI; 1.51), Arnon Nagler, MD, reported at the Transplantation & Cellular Therapy Meetings.
Conditioning was TBI-based in 76% of the patients; when these patients were compared with those who received chemotherapy-based conditioning, they were found to have better OS, LFS, and RI (HRs, 0.75, 0.70, and 0.60, respectively), said Dr. Nagler, director of both the division of hematology and the bone marrow transplantation and cord blood bank at the Chaim Sheba Medical Center, Tel-Hashomer, and professor of medicine at Tel Aviv University, both in Israel.
“There was no significant interaction between the MRD status and the conditioning,” he said.
On multivariate analysis, MRD positivity was found to be associated with lower OS and LFS (HRs, 1.26 and 1.3), and higher RI (HR, 1.53) in the TBI group, and with higher RI (HR 1.58) in the chemotherapy group, he said. There was no significant association between MRD and other outcomes in this last cohort, he added, noting that TBI-based conditioning was associated with improved OS, LFS, and RI in both MRD-negative and MRD-positive patients.
“MRD is an extremely important prognostic factor for ALL,” he said, noting that its prognostic value in this setting has been established in multiple studies, and that MRD measured at the end of induction is increasingly used to guide further therapy.
However, although MRD detectable immediately before HCT is known to be associated with poor outcomes, it has been unclear if – or to what extent – this differs with different types of conditioning, he added.
“So the aim of this study was to explore if MRD detectable before allogeneic HCT for ALL is associated with different outcomes in adult patients receiving myeloablative conditioning, either TBI or chemotherapy based,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Patients included in the analysis had a median age of 38 years and underwent HCT between 2000 and 2017 using sibling or unrelated 9/10 or 10/10 matched donors. None received blinatumomab or inotuzumab, Dr. Nagler said, adding that more patients are likely to achieve MRD negativity with these agents.
It will be interesting to see if the prognostic value of MRD will remain as strong with the new agents, and if TBI will be “a strong factor in overall survival and disease-free survival” with modern immunotherapy, he concluded.
The study was conducted on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT).
Dr. Nagler reported having no relevant financial disclosures.
SOURCE: Nagler A et al. TCT 2019, Abstract 7.
HOUSTON – Acute lymphoblastic leukemia patients with measurable residual disease (MRD) negativity prior to hematopoietic cell transplantation achieve better outcomes than do those who are MRD positive, particularly when total body irradiation (TBI)–based conditioning is used, a large retrospective study suggests.
Of 2,780 ALL patients who underwent hematopoietic cell transplantation (HCT) in first or second complete remission (CR), and who were included in the study, 1,816 were MRD negative before transplantation and 964 were MRD positive.
Overall, with follow-up of 40-44 months, MRD positivity was a significant independent predictor of lower overall survival (OS; hazard ratio, 1.19), leukemia-free survival (LFS; HR, 1.26), and higher relapse incidence (RI; 1.51), Arnon Nagler, MD, reported at the Transplantation & Cellular Therapy Meetings.
Conditioning was TBI-based in 76% of the patients; when these patients were compared with those who received chemotherapy-based conditioning, they were found to have better OS, LFS, and RI (HRs, 0.75, 0.70, and 0.60, respectively), said Dr. Nagler, director of both the division of hematology and the bone marrow transplantation and cord blood bank at the Chaim Sheba Medical Center, Tel-Hashomer, and professor of medicine at Tel Aviv University, both in Israel.
“There was no significant interaction between the MRD status and the conditioning,” he said.
On multivariate analysis, MRD positivity was found to be associated with lower OS and LFS (HRs, 1.26 and 1.3), and higher RI (HR, 1.53) in the TBI group, and with higher RI (HR 1.58) in the chemotherapy group, he said. There was no significant association between MRD and other outcomes in this last cohort, he added, noting that TBI-based conditioning was associated with improved OS, LFS, and RI in both MRD-negative and MRD-positive patients.
“MRD is an extremely important prognostic factor for ALL,” he said, noting that its prognostic value in this setting has been established in multiple studies, and that MRD measured at the end of induction is increasingly used to guide further therapy.
However, although MRD detectable immediately before HCT is known to be associated with poor outcomes, it has been unclear if – or to what extent – this differs with different types of conditioning, he added.
“So the aim of this study was to explore if MRD detectable before allogeneic HCT for ALL is associated with different outcomes in adult patients receiving myeloablative conditioning, either TBI or chemotherapy based,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Patients included in the analysis had a median age of 38 years and underwent HCT between 2000 and 2017 using sibling or unrelated 9/10 or 10/10 matched donors. None received blinatumomab or inotuzumab, Dr. Nagler said, adding that more patients are likely to achieve MRD negativity with these agents.
It will be interesting to see if the prognostic value of MRD will remain as strong with the new agents, and if TBI will be “a strong factor in overall survival and disease-free survival” with modern immunotherapy, he concluded.
The study was conducted on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT).
Dr. Nagler reported having no relevant financial disclosures.
SOURCE: Nagler A et al. TCT 2019, Abstract 7.
REPORTING FROM TCT 2019
Ovarian cancer survivors carry burden of severe long-term fatigue
Women who have survived epithelial ovarian cancer (EOC) more often report severe long-term fatigue than healthy women, according to a case-control study involving more than 600 individuals.
Ovarian cancer survivors had a higher rate of sleep disturbance, neuropathy, and depression, reported lead author Florence Joly, MD, of Centre François Baclesse in Caen, France, and her colleagues. These factors likely contribute to severe long-term fatigue; a condition that has been minimally researched in EOC survivors.
“Long-term fatigue has been described as one of the most common and distressing adverse effects of cancer and its treatment,” the investigators wrote in Annals of Oncology. However, “Little is known about the prevalence of long-term fatigue in EOC survivors several years after treatment in comparison with age-matched healthy women.”
The study involved 318 EOC survivors who had not relapsed for at least 3 years, and 318 age-matched, healthy women. Survivors were 63 years old, on average, and split almost evenly between cases of early and advanced disease (50% stage I/II vs. 48% stage III/IV). Almost all patients had received platinum/taxane chemotherapy (99%). Average follow-up was 6 years.
Participants self-reported through questionnaires about physical activity (International Physical Activity Questionnaire), sleep disturbance ( Insomnia Severity Index), anxiety/depression (Hospital Anxiety and Depression Scale), neuropathy (Functional Assessment of Cancer Therapy-Taxane Neurotoxicity [FACT-Ntx]), quality of life ( Functional Assessment of Cancer Therapy-General/Ovarian), and fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Severe long-term fatigue was defined as a FACIT-F score of less than 37. Analysis was performed to find rates of severe long-term fatigue and contributing factors.
Although sociodemographic measures and global quality of life were similar between groups, 26% of EOC survivors reported severe long-term fatigue, compared with 13% of healthy women (P = .0004). Multivariable analysis revealed that three main factors contributed to this trend; worse neuropathy scores (FACT-Ntx 35 vs. 39), higher rates of depression (22% vs. 13%), and lower sleep quality (63% vs. 47%).
“These results highlight the need for continuous screening of sleep disturbance and depression as soon as the diagnosis of EOC is made, and for sleep disturbance interventions in EOC survivors,” the investigators wrote. “As pharmacological treatment seems to have limited efficacy, behavioral interventions should be offered to improve sleep quality and/or depressive symptoms.”
“Fewer than 20% of our EOC survivors and controls exercised regularly, a finding consistent with a recent study conducted in long-term EOC survivors,” the investigators noted. “Personalized clinical exercise programs were effective in improving fatigue and depression in a heterogeneous population of cancer survivors, so they should be promoted in EOC survivors [too].”
The study was funded by Fondation de France. The investigators disclosed financial relationships with AstraZeneca, Janssen, Sanofi, Novartis, and others.
SOURCE: Joly F et al. Ann Onc. 2019 Mar 9. doi: 10.1093/annonc/mdz074.
Women who have survived epithelial ovarian cancer (EOC) more often report severe long-term fatigue than healthy women, according to a case-control study involving more than 600 individuals.
Ovarian cancer survivors had a higher rate of sleep disturbance, neuropathy, and depression, reported lead author Florence Joly, MD, of Centre François Baclesse in Caen, France, and her colleagues. These factors likely contribute to severe long-term fatigue; a condition that has been minimally researched in EOC survivors.
“Long-term fatigue has been described as one of the most common and distressing adverse effects of cancer and its treatment,” the investigators wrote in Annals of Oncology. However, “Little is known about the prevalence of long-term fatigue in EOC survivors several years after treatment in comparison with age-matched healthy women.”
The study involved 318 EOC survivors who had not relapsed for at least 3 years, and 318 age-matched, healthy women. Survivors were 63 years old, on average, and split almost evenly between cases of early and advanced disease (50% stage I/II vs. 48% stage III/IV). Almost all patients had received platinum/taxane chemotherapy (99%). Average follow-up was 6 years.
Participants self-reported through questionnaires about physical activity (International Physical Activity Questionnaire), sleep disturbance ( Insomnia Severity Index), anxiety/depression (Hospital Anxiety and Depression Scale), neuropathy (Functional Assessment of Cancer Therapy-Taxane Neurotoxicity [FACT-Ntx]), quality of life ( Functional Assessment of Cancer Therapy-General/Ovarian), and fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Severe long-term fatigue was defined as a FACIT-F score of less than 37. Analysis was performed to find rates of severe long-term fatigue and contributing factors.
Although sociodemographic measures and global quality of life were similar between groups, 26% of EOC survivors reported severe long-term fatigue, compared with 13% of healthy women (P = .0004). Multivariable analysis revealed that three main factors contributed to this trend; worse neuropathy scores (FACT-Ntx 35 vs. 39), higher rates of depression (22% vs. 13%), and lower sleep quality (63% vs. 47%).
“These results highlight the need for continuous screening of sleep disturbance and depression as soon as the diagnosis of EOC is made, and for sleep disturbance interventions in EOC survivors,” the investigators wrote. “As pharmacological treatment seems to have limited efficacy, behavioral interventions should be offered to improve sleep quality and/or depressive symptoms.”
“Fewer than 20% of our EOC survivors and controls exercised regularly, a finding consistent with a recent study conducted in long-term EOC survivors,” the investigators noted. “Personalized clinical exercise programs were effective in improving fatigue and depression in a heterogeneous population of cancer survivors, so they should be promoted in EOC survivors [too].”
The study was funded by Fondation de France. The investigators disclosed financial relationships with AstraZeneca, Janssen, Sanofi, Novartis, and others.
SOURCE: Joly F et al. Ann Onc. 2019 Mar 9. doi: 10.1093/annonc/mdz074.
Women who have survived epithelial ovarian cancer (EOC) more often report severe long-term fatigue than healthy women, according to a case-control study involving more than 600 individuals.
Ovarian cancer survivors had a higher rate of sleep disturbance, neuropathy, and depression, reported lead author Florence Joly, MD, of Centre François Baclesse in Caen, France, and her colleagues. These factors likely contribute to severe long-term fatigue; a condition that has been minimally researched in EOC survivors.
“Long-term fatigue has been described as one of the most common and distressing adverse effects of cancer and its treatment,” the investigators wrote in Annals of Oncology. However, “Little is known about the prevalence of long-term fatigue in EOC survivors several years after treatment in comparison with age-matched healthy women.”
The study involved 318 EOC survivors who had not relapsed for at least 3 years, and 318 age-matched, healthy women. Survivors were 63 years old, on average, and split almost evenly between cases of early and advanced disease (50% stage I/II vs. 48% stage III/IV). Almost all patients had received platinum/taxane chemotherapy (99%). Average follow-up was 6 years.
Participants self-reported through questionnaires about physical activity (International Physical Activity Questionnaire), sleep disturbance ( Insomnia Severity Index), anxiety/depression (Hospital Anxiety and Depression Scale), neuropathy (Functional Assessment of Cancer Therapy-Taxane Neurotoxicity [FACT-Ntx]), quality of life ( Functional Assessment of Cancer Therapy-General/Ovarian), and fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Severe long-term fatigue was defined as a FACIT-F score of less than 37. Analysis was performed to find rates of severe long-term fatigue and contributing factors.
Although sociodemographic measures and global quality of life were similar between groups, 26% of EOC survivors reported severe long-term fatigue, compared with 13% of healthy women (P = .0004). Multivariable analysis revealed that three main factors contributed to this trend; worse neuropathy scores (FACT-Ntx 35 vs. 39), higher rates of depression (22% vs. 13%), and lower sleep quality (63% vs. 47%).
“These results highlight the need for continuous screening of sleep disturbance and depression as soon as the diagnosis of EOC is made, and for sleep disturbance interventions in EOC survivors,” the investigators wrote. “As pharmacological treatment seems to have limited efficacy, behavioral interventions should be offered to improve sleep quality and/or depressive symptoms.”
“Fewer than 20% of our EOC survivors and controls exercised regularly, a finding consistent with a recent study conducted in long-term EOC survivors,” the investigators noted. “Personalized clinical exercise programs were effective in improving fatigue and depression in a heterogeneous population of cancer survivors, so they should be promoted in EOC survivors [too].”
The study was funded by Fondation de France. The investigators disclosed financial relationships with AstraZeneca, Janssen, Sanofi, Novartis, and others.
SOURCE: Joly F et al. Ann Onc. 2019 Mar 9. doi: 10.1093/annonc/mdz074.
FROM ANNALS OF ONCOLOGY
Secondary AML in first remission predicts outcomes
HOUSTON – Secondary acute myeloid leukemia (sAML) predicts outcomes after stem cell transplantation in first complete remission, whereas factors such as age, cytogenetics, and performance status are more relevant predictors of outcomes in patients with de novo AML, according to a large, registry-based analysis.
Of 11,439 patients with de novo AML and 1,325 with sAML identified in the registry, 7,691 and 909, respectively, underwent a stem cell transplant (SCT) in first complete remission (CR1), Bipin Savani, MD, said at the Transplantation & Cellular Therapies Meetings.
The 3-year cumulative incidence of relapse (CIR) and nonrelapse mortality (NRM) rates in those who underwent SCT in CR1 were higher in the sAML versus de novo AML groups (35% vs. 28.5% for CIR and 23.4% vs. 16.4% for NRM, respectively), said Dr. Savani, professor of medicine, director of the Long-Term Transplant Clinic, and medical director of the Stem Cell Transplant Processing Laboratory at Vanderbilt University Medical Center & Veterans Affairs Medical Center, Nashville, Tenn.
The 3-year overall survival (OS), leukemia-free survival (LFS), and graft-versus-host disease/relapse-free survival (GRFS) were significantly lower in the sAML group versus the de novo AML group (46.7% vs. 60.8% for OS; 41.6% vs. 55.1% for LFS; and 28.4% vs. 28.6% for GRFS).
Multivariate analysis controlling for risk factors and stratified by disease stage at SCT showed that sAML in CR1 was significantly associated with higher NRM (hazard ratio, 1.32) and CIR (HR, 1.28), and with lower LFS (HR, 1.30), OS (HR, 1.32) and GRFS (HR, 1.20).
Other significant predictors of OS in the model were age, cytogenetics, patient/donor sex combination, Karnofsky performance status (KPS), and donor, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
In the patients who underwent SCT for primary refractory AML (607 with de novo AML and 199 with sAML) or relapsed AML (1,009 with de novo AML and 124 with sAML), the outcomes were generally inferior to those seen with SCT in CR1. However, sAML in those patients did not predict outcomes, Dr. Savani said, noting that outcome in those cases were predicted by age, cytogenetics, and KPS.
In an analysis of 877 pairs matched for age, disease stage at SCT, KPS, conditioning, in vivo/ex vivo T-cell depletion, donor, donor/recipient sex and cytomegalovirus-status combination, cytogenetics, and graft source, the finding that sAML was associated with significantly higher NRM, and lower LFS, OS, and GRFS overall was confirmed.
However, stratification by stage at the time of SCT again showed that the differences between groups were only seen among those transplanted in CR1, and not in those with advanced disease at the time of transplant.
Patients included in the study were adults aged 18 years and older who underwent SCT for de novo or sAML from a matched related, unrelated, or T-cell replete haploidentical donor between 2000 and 2016.
The findings confirm the general belief that the prognosis in AML secondary to another hematologic neoplasia or malignant disease is poorer than that for de novo AML, and clarify the role of this difference for SCT, Dr. Savani said.
“These data may help to improve risk stratification and prognostic estimates after allogeneic hematopoietic cell transplantation for acute myeloid leukemia,” he concluded.
Dr. Savani reported having no financial disclosures.
SOURCE: Savani B et al. TCT 2019, Abstract 12.
HOUSTON – Secondary acute myeloid leukemia (sAML) predicts outcomes after stem cell transplantation in first complete remission, whereas factors such as age, cytogenetics, and performance status are more relevant predictors of outcomes in patients with de novo AML, according to a large, registry-based analysis.
Of 11,439 patients with de novo AML and 1,325 with sAML identified in the registry, 7,691 and 909, respectively, underwent a stem cell transplant (SCT) in first complete remission (CR1), Bipin Savani, MD, said at the Transplantation & Cellular Therapies Meetings.
The 3-year cumulative incidence of relapse (CIR) and nonrelapse mortality (NRM) rates in those who underwent SCT in CR1 were higher in the sAML versus de novo AML groups (35% vs. 28.5% for CIR and 23.4% vs. 16.4% for NRM, respectively), said Dr. Savani, professor of medicine, director of the Long-Term Transplant Clinic, and medical director of the Stem Cell Transplant Processing Laboratory at Vanderbilt University Medical Center & Veterans Affairs Medical Center, Nashville, Tenn.
The 3-year overall survival (OS), leukemia-free survival (LFS), and graft-versus-host disease/relapse-free survival (GRFS) were significantly lower in the sAML group versus the de novo AML group (46.7% vs. 60.8% for OS; 41.6% vs. 55.1% for LFS; and 28.4% vs. 28.6% for GRFS).
Multivariate analysis controlling for risk factors and stratified by disease stage at SCT showed that sAML in CR1 was significantly associated with higher NRM (hazard ratio, 1.32) and CIR (HR, 1.28), and with lower LFS (HR, 1.30), OS (HR, 1.32) and GRFS (HR, 1.20).
Other significant predictors of OS in the model were age, cytogenetics, patient/donor sex combination, Karnofsky performance status (KPS), and donor, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
In the patients who underwent SCT for primary refractory AML (607 with de novo AML and 199 with sAML) or relapsed AML (1,009 with de novo AML and 124 with sAML), the outcomes were generally inferior to those seen with SCT in CR1. However, sAML in those patients did not predict outcomes, Dr. Savani said, noting that outcome in those cases were predicted by age, cytogenetics, and KPS.
In an analysis of 877 pairs matched for age, disease stage at SCT, KPS, conditioning, in vivo/ex vivo T-cell depletion, donor, donor/recipient sex and cytomegalovirus-status combination, cytogenetics, and graft source, the finding that sAML was associated with significantly higher NRM, and lower LFS, OS, and GRFS overall was confirmed.
However, stratification by stage at the time of SCT again showed that the differences between groups were only seen among those transplanted in CR1, and not in those with advanced disease at the time of transplant.
Patients included in the study were adults aged 18 years and older who underwent SCT for de novo or sAML from a matched related, unrelated, or T-cell replete haploidentical donor between 2000 and 2016.
The findings confirm the general belief that the prognosis in AML secondary to another hematologic neoplasia or malignant disease is poorer than that for de novo AML, and clarify the role of this difference for SCT, Dr. Savani said.
“These data may help to improve risk stratification and prognostic estimates after allogeneic hematopoietic cell transplantation for acute myeloid leukemia,” he concluded.
Dr. Savani reported having no financial disclosures.
SOURCE: Savani B et al. TCT 2019, Abstract 12.
HOUSTON – Secondary acute myeloid leukemia (sAML) predicts outcomes after stem cell transplantation in first complete remission, whereas factors such as age, cytogenetics, and performance status are more relevant predictors of outcomes in patients with de novo AML, according to a large, registry-based analysis.
Of 11,439 patients with de novo AML and 1,325 with sAML identified in the registry, 7,691 and 909, respectively, underwent a stem cell transplant (SCT) in first complete remission (CR1), Bipin Savani, MD, said at the Transplantation & Cellular Therapies Meetings.
The 3-year cumulative incidence of relapse (CIR) and nonrelapse mortality (NRM) rates in those who underwent SCT in CR1 were higher in the sAML versus de novo AML groups (35% vs. 28.5% for CIR and 23.4% vs. 16.4% for NRM, respectively), said Dr. Savani, professor of medicine, director of the Long-Term Transplant Clinic, and medical director of the Stem Cell Transplant Processing Laboratory at Vanderbilt University Medical Center & Veterans Affairs Medical Center, Nashville, Tenn.
The 3-year overall survival (OS), leukemia-free survival (LFS), and graft-versus-host disease/relapse-free survival (GRFS) were significantly lower in the sAML group versus the de novo AML group (46.7% vs. 60.8% for OS; 41.6% vs. 55.1% for LFS; and 28.4% vs. 28.6% for GRFS).
Multivariate analysis controlling for risk factors and stratified by disease stage at SCT showed that sAML in CR1 was significantly associated with higher NRM (hazard ratio, 1.32) and CIR (HR, 1.28), and with lower LFS (HR, 1.30), OS (HR, 1.32) and GRFS (HR, 1.20).
Other significant predictors of OS in the model were age, cytogenetics, patient/donor sex combination, Karnofsky performance status (KPS), and donor, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
In the patients who underwent SCT for primary refractory AML (607 with de novo AML and 199 with sAML) or relapsed AML (1,009 with de novo AML and 124 with sAML), the outcomes were generally inferior to those seen with SCT in CR1. However, sAML in those patients did not predict outcomes, Dr. Savani said, noting that outcome in those cases were predicted by age, cytogenetics, and KPS.
In an analysis of 877 pairs matched for age, disease stage at SCT, KPS, conditioning, in vivo/ex vivo T-cell depletion, donor, donor/recipient sex and cytomegalovirus-status combination, cytogenetics, and graft source, the finding that sAML was associated with significantly higher NRM, and lower LFS, OS, and GRFS overall was confirmed.
However, stratification by stage at the time of SCT again showed that the differences between groups were only seen among those transplanted in CR1, and not in those with advanced disease at the time of transplant.
Patients included in the study were adults aged 18 years and older who underwent SCT for de novo or sAML from a matched related, unrelated, or T-cell replete haploidentical donor between 2000 and 2016.
The findings confirm the general belief that the prognosis in AML secondary to another hematologic neoplasia or malignant disease is poorer than that for de novo AML, and clarify the role of this difference for SCT, Dr. Savani said.
“These data may help to improve risk stratification and prognostic estimates after allogeneic hematopoietic cell transplantation for acute myeloid leukemia,” he concluded.
Dr. Savani reported having no financial disclosures.
SOURCE: Savani B et al. TCT 2019, Abstract 12.
REPORTING FROM TCT 2019
New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
WASHINGTON – compared with placebo, according to the results of two trials presented at the annual meeting of the American Academy of Dermatology.
VP-102, a drug-device combination, was well tolerated and was not associated with serious adverse events.
No Food and Drug Administration–approved treatment is available for treating molluscum contagiosum, which is routinely treated with cantharidin, a naturally occurring vesicant.
VP-102 is a novel formulation of 0.7% cantharidin solution, provided in a single-use applicator, to provide consistent delivery and long-term drug stability.
To test the efficacy and safety of VP-102, Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and his associates conducted the CAMP-1 (Cantharidin Application in Molluscum Patients) and CAMP-2 phase 3 studies, which had similar designs. The studies enrolled patients with molluscum contagiosum aged 2 years and older who had not received any treatment in the 2 weeks before enrollment. Patients were randomized to VP-102 or vehicle for 12 weeks. Treatment was administered topically to each lesion every 3 weeks for a maximum of four applications, and washed off with soap and warm water 24 hours after application.
The trials’ primary endpoint was the percentage of patients with complete clearance of their lesions. Secondary endpoints were the percentage of patients with complete clearance at 3, 6, and 9 weeks, and decrease in lesions over time. The researchers also assessed safety and tolerability.
In the two studies, 528 patients aged 2-60 years (mean age, approximately 7 years) were randomized to treatment or vehicle. About 30% of participants had prior treatment. The baseline lesion count ranged from 1 to 184.
At day 84, the proportion of patients in the VP-102 arm who achieved complete clearance of lesions was 46% in CAMP-1 and 54% in CAMP-2, compared with 18% and 13%, respectively, among controls (P less than .0001). By day 84, among treated patients, the lesion count had decreased by a mean of 69% in CAMP-1 and 83% in CAMP-2, compared with 20% and 19%, respectively, among controls. Results among controls were “probably consistent with natural history,” Dr. Eichenfield observed.
The researchers observed a high incidence of treatment-emergent adverse events among patients receiving VP-102. “Any crust or vesiculation was considered to be a treatment-emergent adverse event,” Dr. Eichenfield said. Most adverse events were mild, although five patients discontinued the studies because of treatment-emergent adverse events. Vesiculation was a common adverse event in the VP-102 group; pruritus and application-site pain were reported as well.
Verrica Pharmaceuticals developed VP-102 and funded the study. Dr. Eichenfield reported receiving no funding from the company; several other investigators are employees of Verrica, which plans to submit for FDA approval in the second half of 2019.
SOURCE: Eichenfield L et al. AAD 19, Abstract 11251.
WASHINGTON – compared with placebo, according to the results of two trials presented at the annual meeting of the American Academy of Dermatology.
VP-102, a drug-device combination, was well tolerated and was not associated with serious adverse events.
No Food and Drug Administration–approved treatment is available for treating molluscum contagiosum, which is routinely treated with cantharidin, a naturally occurring vesicant.
VP-102 is a novel formulation of 0.7% cantharidin solution, provided in a single-use applicator, to provide consistent delivery and long-term drug stability.
To test the efficacy and safety of VP-102, Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and his associates conducted the CAMP-1 (Cantharidin Application in Molluscum Patients) and CAMP-2 phase 3 studies, which had similar designs. The studies enrolled patients with molluscum contagiosum aged 2 years and older who had not received any treatment in the 2 weeks before enrollment. Patients were randomized to VP-102 or vehicle for 12 weeks. Treatment was administered topically to each lesion every 3 weeks for a maximum of four applications, and washed off with soap and warm water 24 hours after application.
The trials’ primary endpoint was the percentage of patients with complete clearance of their lesions. Secondary endpoints were the percentage of patients with complete clearance at 3, 6, and 9 weeks, and decrease in lesions over time. The researchers also assessed safety and tolerability.
In the two studies, 528 patients aged 2-60 years (mean age, approximately 7 years) were randomized to treatment or vehicle. About 30% of participants had prior treatment. The baseline lesion count ranged from 1 to 184.
At day 84, the proportion of patients in the VP-102 arm who achieved complete clearance of lesions was 46% in CAMP-1 and 54% in CAMP-2, compared with 18% and 13%, respectively, among controls (P less than .0001). By day 84, among treated patients, the lesion count had decreased by a mean of 69% in CAMP-1 and 83% in CAMP-2, compared with 20% and 19%, respectively, among controls. Results among controls were “probably consistent with natural history,” Dr. Eichenfield observed.
The researchers observed a high incidence of treatment-emergent adverse events among patients receiving VP-102. “Any crust or vesiculation was considered to be a treatment-emergent adverse event,” Dr. Eichenfield said. Most adverse events were mild, although five patients discontinued the studies because of treatment-emergent adverse events. Vesiculation was a common adverse event in the VP-102 group; pruritus and application-site pain were reported as well.
Verrica Pharmaceuticals developed VP-102 and funded the study. Dr. Eichenfield reported receiving no funding from the company; several other investigators are employees of Verrica, which plans to submit for FDA approval in the second half of 2019.
SOURCE: Eichenfield L et al. AAD 19, Abstract 11251.
WASHINGTON – compared with placebo, according to the results of two trials presented at the annual meeting of the American Academy of Dermatology.
VP-102, a drug-device combination, was well tolerated and was not associated with serious adverse events.
No Food and Drug Administration–approved treatment is available for treating molluscum contagiosum, which is routinely treated with cantharidin, a naturally occurring vesicant.
VP-102 is a novel formulation of 0.7% cantharidin solution, provided in a single-use applicator, to provide consistent delivery and long-term drug stability.
To test the efficacy and safety of VP-102, Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and his associates conducted the CAMP-1 (Cantharidin Application in Molluscum Patients) and CAMP-2 phase 3 studies, which had similar designs. The studies enrolled patients with molluscum contagiosum aged 2 years and older who had not received any treatment in the 2 weeks before enrollment. Patients were randomized to VP-102 or vehicle for 12 weeks. Treatment was administered topically to each lesion every 3 weeks for a maximum of four applications, and washed off with soap and warm water 24 hours after application.
The trials’ primary endpoint was the percentage of patients with complete clearance of their lesions. Secondary endpoints were the percentage of patients with complete clearance at 3, 6, and 9 weeks, and decrease in lesions over time. The researchers also assessed safety and tolerability.
In the two studies, 528 patients aged 2-60 years (mean age, approximately 7 years) were randomized to treatment or vehicle. About 30% of participants had prior treatment. The baseline lesion count ranged from 1 to 184.
At day 84, the proportion of patients in the VP-102 arm who achieved complete clearance of lesions was 46% in CAMP-1 and 54% in CAMP-2, compared with 18% and 13%, respectively, among controls (P less than .0001). By day 84, among treated patients, the lesion count had decreased by a mean of 69% in CAMP-1 and 83% in CAMP-2, compared with 20% and 19%, respectively, among controls. Results among controls were “probably consistent with natural history,” Dr. Eichenfield observed.
The researchers observed a high incidence of treatment-emergent adverse events among patients receiving VP-102. “Any crust or vesiculation was considered to be a treatment-emergent adverse event,” Dr. Eichenfield said. Most adverse events were mild, although five patients discontinued the studies because of treatment-emergent adverse events. Vesiculation was a common adverse event in the VP-102 group; pruritus and application-site pain were reported as well.
Verrica Pharmaceuticals developed VP-102 and funded the study. Dr. Eichenfield reported receiving no funding from the company; several other investigators are employees of Verrica, which plans to submit for FDA approval in the second half of 2019.
SOURCE: Eichenfield L et al. AAD 19, Abstract 11251.
REPORTING FROM AAD 2019
Key clinical point: VP-102 is an effective treatment for molluscum contagiosum.
Major finding: In two studies, 46% and 54% of actively treated patients had complete resolution, compared with 13% and 18% of controls, respectively.
Study details: Two phase 3, randomized, double-blind, placebo-controlled trials of 528 patients with molluscum contagiosum.
Disclosures: Verrica Pharmaceuticals sponsored the study. Dr. Eichenfield reported receiving no funding from the company; several other investigators are employees of Verrica Pharmaceuticals.Source: Eichenfield L et al. AAD 19, Abstract 11251.
Up-close view of climate change proves sobering
Dr. Carl Bell steps away from American College of Psychiatrists meeting and gets a jolt
It used to be difficult to conceive of writing about climate change in light of the illnesses we psychiatrists treat. But talking about climate change has become unavoidable. Sometimes, it seems that things weigh heavy on my heart, and I have to write about them – especially when it is serious.
David Alan Pollack, MD, has been talking about climate change for some years now, and while I understood his concern, I had yet to see the psychological effects up close and personal. After all, I live in Chicago, and we are surrounded by concrete and asphalt.
Thankfully, I also travel, and I get a chance to get into nature. While in Hawaii at the American College of Psychiatrists annual meeting in February, I went snorkeling in Hanauma Bay. I saw coral and fish. The problem is I have a very vivid memories of snorkeling in that exact same nature preserve, which also was a Marine Life Conservation District in 1972 while I was attending the American Psychiatric Association annual meeting.
The contrast between the two experiences leaves me with a glum, sad, disappointed, heart-broken feeling because it was an intimate and personal experience with climate change. In 1972, I saw every type of coral imaginable: brain coral, club finger coral, elk coral, great star coral, pillar coral, staghorn coral, table coral, and tube coral. If I remember correctly, there were corky sea fingers and sea fans, but not sea turtles. In 1972, I saw bigeyefish, damselfish, doctorfish, filefish, goatfish, gobies, hogfish, lemon butterflyfish, lizardfish, parrottfish, porcupinefish, pufferfish, queen angelfish, rock beauties, sergeant majors, soldierfish, spot-tail spot-tail butterflyfish, Spanish hogfish, squirrelfish, tangs, trunkfish, or any bluehead or yellowhead wrasses.
In 2019, I saw two pieces of coral less that 9 inches in diameter and not a single sea urchin. There were maybe three types of tropical fish that I was unfamiliar with seeing. The difference between what I saw in 1972 and what I saw in 2019 was like the difference between the rain forest in Puerto Rico and the dunes of the Sahara Desert.
Sure, I have heard David talk about the mental health effects of climate change on stress, anxiety, and depression, and I have always thought that he was right. But to see climate change up close and personal is a sobering experience. I apologize to them for being part of the system and process that is destroying the planet – and leaving them with a hot mess.
At this point, it seems to me that we cannot just try to save the planet by being better stewards of our garbage and pointing out measurable indicators of climate change. We need to actively rather than passively try to save the planet. Of course, the question is who will pay for the active efforts to depollute Earth. From what I saw for myself in Hanauma Bay, I don’t think we have much time. So I am hoping that more people will take the issue of climate change seriously.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago. Check out Dr. Bell’s new book, “Fetal Alcohol Exposure in the African-American Community,” at https://thirdworldpressfoundation.org/product/pre-order-fetal-alcohol-exposure-in-the-african-american-community/.
Dr. Carl Bell steps away from American College of Psychiatrists meeting and gets a jolt
Dr. Carl Bell steps away from American College of Psychiatrists meeting and gets a jolt
It used to be difficult to conceive of writing about climate change in light of the illnesses we psychiatrists treat. But talking about climate change has become unavoidable. Sometimes, it seems that things weigh heavy on my heart, and I have to write about them – especially when it is serious.
David Alan Pollack, MD, has been talking about climate change for some years now, and while I understood his concern, I had yet to see the psychological effects up close and personal. After all, I live in Chicago, and we are surrounded by concrete and asphalt.
Thankfully, I also travel, and I get a chance to get into nature. While in Hawaii at the American College of Psychiatrists annual meeting in February, I went snorkeling in Hanauma Bay. I saw coral and fish. The problem is I have a very vivid memories of snorkeling in that exact same nature preserve, which also was a Marine Life Conservation District in 1972 while I was attending the American Psychiatric Association annual meeting.
The contrast between the two experiences leaves me with a glum, sad, disappointed, heart-broken feeling because it was an intimate and personal experience with climate change. In 1972, I saw every type of coral imaginable: brain coral, club finger coral, elk coral, great star coral, pillar coral, staghorn coral, table coral, and tube coral. If I remember correctly, there were corky sea fingers and sea fans, but not sea turtles. In 1972, I saw bigeyefish, damselfish, doctorfish, filefish, goatfish, gobies, hogfish, lemon butterflyfish, lizardfish, parrottfish, porcupinefish, pufferfish, queen angelfish, rock beauties, sergeant majors, soldierfish, spot-tail spot-tail butterflyfish, Spanish hogfish, squirrelfish, tangs, trunkfish, or any bluehead or yellowhead wrasses.
In 2019, I saw two pieces of coral less that 9 inches in diameter and not a single sea urchin. There were maybe three types of tropical fish that I was unfamiliar with seeing. The difference between what I saw in 1972 and what I saw in 2019 was like the difference between the rain forest in Puerto Rico and the dunes of the Sahara Desert.
Sure, I have heard David talk about the mental health effects of climate change on stress, anxiety, and depression, and I have always thought that he was right. But to see climate change up close and personal is a sobering experience. I apologize to them for being part of the system and process that is destroying the planet – and leaving them with a hot mess.
At this point, it seems to me that we cannot just try to save the planet by being better stewards of our garbage and pointing out measurable indicators of climate change. We need to actively rather than passively try to save the planet. Of course, the question is who will pay for the active efforts to depollute Earth. From what I saw for myself in Hanauma Bay, I don’t think we have much time. So I am hoping that more people will take the issue of climate change seriously.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago. Check out Dr. Bell’s new book, “Fetal Alcohol Exposure in the African-American Community,” at https://thirdworldpressfoundation.org/product/pre-order-fetal-alcohol-exposure-in-the-african-american-community/.
It used to be difficult to conceive of writing about climate change in light of the illnesses we psychiatrists treat. But talking about climate change has become unavoidable. Sometimes, it seems that things weigh heavy on my heart, and I have to write about them – especially when it is serious.
David Alan Pollack, MD, has been talking about climate change for some years now, and while I understood his concern, I had yet to see the psychological effects up close and personal. After all, I live in Chicago, and we are surrounded by concrete and asphalt.
Thankfully, I also travel, and I get a chance to get into nature. While in Hawaii at the American College of Psychiatrists annual meeting in February, I went snorkeling in Hanauma Bay. I saw coral and fish. The problem is I have a very vivid memories of snorkeling in that exact same nature preserve, which also was a Marine Life Conservation District in 1972 while I was attending the American Psychiatric Association annual meeting.
The contrast between the two experiences leaves me with a glum, sad, disappointed, heart-broken feeling because it was an intimate and personal experience with climate change. In 1972, I saw every type of coral imaginable: brain coral, club finger coral, elk coral, great star coral, pillar coral, staghorn coral, table coral, and tube coral. If I remember correctly, there were corky sea fingers and sea fans, but not sea turtles. In 1972, I saw bigeyefish, damselfish, doctorfish, filefish, goatfish, gobies, hogfish, lemon butterflyfish, lizardfish, parrottfish, porcupinefish, pufferfish, queen angelfish, rock beauties, sergeant majors, soldierfish, spot-tail spot-tail butterflyfish, Spanish hogfish, squirrelfish, tangs, trunkfish, or any bluehead or yellowhead wrasses.
In 2019, I saw two pieces of coral less that 9 inches in diameter and not a single sea urchin. There were maybe three types of tropical fish that I was unfamiliar with seeing. The difference between what I saw in 1972 and what I saw in 2019 was like the difference between the rain forest in Puerto Rico and the dunes of the Sahara Desert.
Sure, I have heard David talk about the mental health effects of climate change on stress, anxiety, and depression, and I have always thought that he was right. But to see climate change up close and personal is a sobering experience. I apologize to them for being part of the system and process that is destroying the planet – and leaving them with a hot mess.
At this point, it seems to me that we cannot just try to save the planet by being better stewards of our garbage and pointing out measurable indicators of climate change. We need to actively rather than passively try to save the planet. Of course, the question is who will pay for the active efforts to depollute Earth. From what I saw for myself in Hanauma Bay, I don’t think we have much time. So I am hoping that more people will take the issue of climate change seriously.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital’s Medical/Surgical-Psychiatry Inpatient Unit in Chicago, clinical psychiatrist emeritus in the department of psychiatry at the University of Illinois at Chicago, former president/CEO of Community Mental Health Council, and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago. Check out Dr. Bell’s new book, “Fetal Alcohol Exposure in the African-American Community,” at https://thirdworldpressfoundation.org/product/pre-order-fetal-alcohol-exposure-in-the-african-american-community/.
A Vital Clue to the Problem
ANSWER
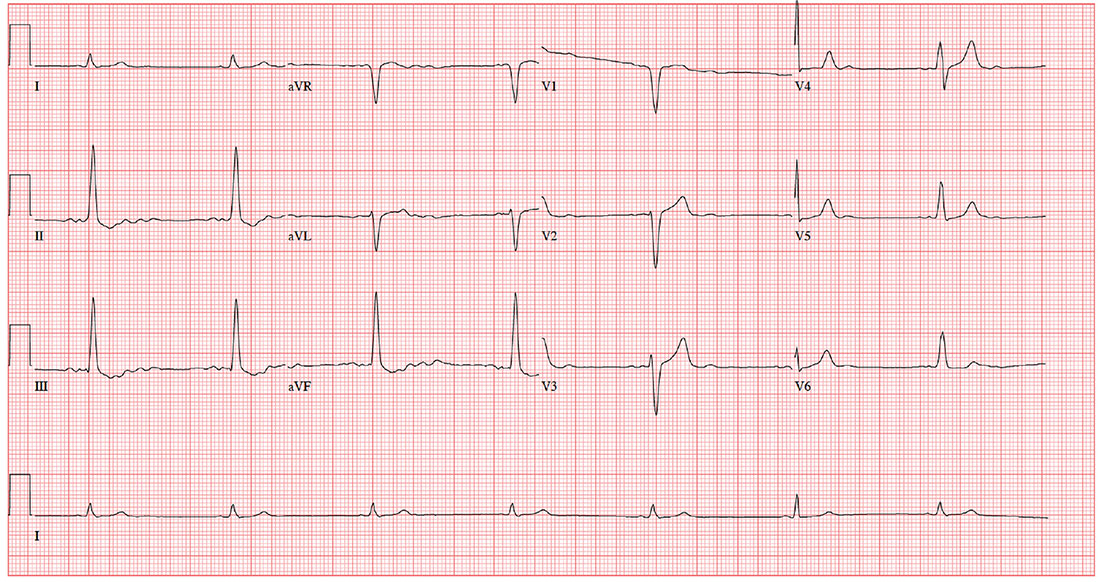
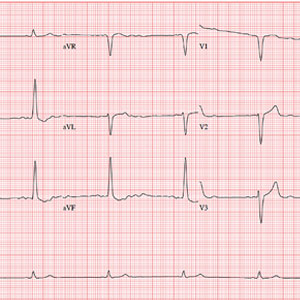
The correct interpretation is a bit challenging. You’ve confirmed the electrodes were placed correctly; however, the P waves are unusual (best seen in lead II) and are suggestive of an ectopic atrial rhythm. In lead II, there are two P waves for each QRS complex, consistent with second-degree atrioventricular (AV) block with 2:1 AV conduction. The QRS complex is wide at 130 ms without a right or left bundle branch pattern, consistent with a nonspecific intraventricular conduction block, and there is evidence of nonspecific ST-T wave abnormality.
These changes were new compared with the patient’s preoperative ECG, which showed sinus rhythm and no evidence of heart block.
ANSWER
The correct interpretation is a bit challenging. You’ve confirmed the electrodes were placed correctly; however, the P waves are unusual (best seen in lead II) and are suggestive of an ectopic atrial rhythm. In lead II, there are two P waves for each QRS complex, consistent with second-degree atrioventricular (AV) block with 2:1 AV conduction. The QRS complex is wide at 130 ms without a right or left bundle branch pattern, consistent with a nonspecific intraventricular conduction block, and there is evidence of nonspecific ST-T wave abnormality.
These changes were new compared with the patient’s preoperative ECG, which showed sinus rhythm and no evidence of heart block.
ANSWER
The correct interpretation is a bit challenging. You’ve confirmed the electrodes were placed correctly; however, the P waves are unusual (best seen in lead II) and are suggestive of an ectopic atrial rhythm. In lead II, there are two P waves for each QRS complex, consistent with second-degree atrioventricular (AV) block with 2:1 AV conduction. The QRS complex is wide at 130 ms without a right or left bundle branch pattern, consistent with a nonspecific intraventricular conduction block, and there is evidence of nonspecific ST-T wave abnormality.
These changes were new compared with the patient’s preoperative ECG, which showed sinus rhythm and no evidence of heart block.
Two days ago, a 76-year-old man underwent surgical repair of a left femoral fracture sustained in an automobile accident. Last night, his vital signs were concerning for asymptomatic bradycardia, prompting an order for ECG prior to morning rounds. His postoperative surgical course has been otherwise uneventful, and he is using minimal narcotic analgesia.
The patient has a long history of mild hypertension, which is well controlled with hydrochlorothiazide (50 mg/d). Otherwise, his medical history shows no angina, dyspnea, shortness of breath, or palpitations. There is no history of thyroid dysfunction or renal disease.
A pack-a-day smoker since age 15, the patient has been using a nicotine patch during his hospital stay. In the past 36 hours, he has taken 4 acetaminophen/oxycodone tablets (325/5 mg).
The patient works on a 400-acre ranch with about 100 head of cattle. His alcohol consumption was heavy until about 12 years ago, when he “blacked out” and gave up drinking altogether. He attends Alcoholics Anonymous meetings and leads sessions at his church. He denies recreational or illicit drug use.
Family history is positive for prostate cancer in his father and lung cancer in two brothers. His mother died of a hemorrhagic stroke at age 84.
The review of systems is remarkable only for discomfort due to an indwelling Foley catheter placed at the time of the surgery. He has no other specific complaints.
This morning, his vital signs include a blood pressure of 108/60 mm Hg; pulse, 42 beats/min; and O2 saturation, 100% on 2L of oxygen via nasal prongs.
When you arrive at the patient’s room, the ECG is in progress. The technician expresses concern about the tracing and shows you that the electrodes are placed properly. Looking at the ECG, you see a ventricular rate of 42 beats/min; PR interval, unmeasured; QRS duration, 130 ms; QT/QTc interval, 514/429 ms; P axis, +83°; R axis, +84°; and T axis, –43°. What is your interpretation?
Hope for hyperhidrosis
Hyperhidrosis – excessive sweating – is one of those diagnoses that make most physicians cringe because we know that the side effects of most of the common treatments are very limiting and patients continue to be frustrated. New treatments are emerging, and although oxybutynin is not Food and Drug Administration–approved for hyperhidrosis, it does show promise.
Primary hyperhidrosis is the excessive sweating from the axilla, palms, soles, or cranial-facial area. It is a clinical diagnosis that has been occurring for more than 6 months and meets at least four1 of the following criteria:
1. It occurs in eccrine dense areas (axilla, soles, palms or head).
2. It is bilateral and symmetrical.
3. It is absent nocturnally.
4. Its onset should be before age 25 years.
5. It occurs at least weekly.
6. There is a positive family history.
7. It impairs daily activities.
If signs of underlying disease are apparent – such as palpitations, night sweats, weight loss, unilateral symptoms, anxiety, or hypertension – further workup is needed to rule out disorders such as diabetes, hyperthyroidism, pheochromocytoma, or peripheral nerve injury.1
The pathophysiology of hyperhidrosis is not clearly understood. It is believed to be due to increased cholinergic stimulation given that there is no hypertrophy or hyperplasia of the sweat gland.2 Genetics appear to play a role as there is usually a family history of the disorder.2
Topical treatments are usually first line, starting with aluminum chloride antiperspirants, or anticholinergic creams such as glycopyrrolate or glycopyrronium. Unfortunately, many patients complain of the skin irritation so they discontinue their use.1
Botulinum toxin type A is a very safe and effective way of treating hyperhidrosis and is FDA approved for that purpose.3 Its drawbacks are that it is an injection (approximately 25 in each armpit), and it is very costly, usually $1,000-1,500 per session for both underarms. There are no major side effects, and reduction in sweating lasts for 4-12 months, with a median of 6 months.3
Oral treatment of hyperhidrosis, with medications such as glycopyrrolate and benztropine, has been reserved for second- or third-line treatment because of the unwanted side effects of dry mouth and drowsiness. But more recent studies are showing favorable outcomes with oxybutynin.1
Oxybutynin is well known and FDA approved for treatment of urinary frequency, incontinence, and enuresis. Recent studies have shown great success for use to control generalized hyperhidrosis. The mechanism of action is blocking the binding of acetylcholine and numerous other neurotransmitters.2
The literature does not give clear-cut dosing because it is not approved for hyperhidrosis, but gradually increasing doses starting at 2.5 mg daily for a week, then increasing to twice daily for 2 weeks, and then to 5 mg twice daily as a continued dose appears to be the most effective regimen with few side effects. The dosage can be increased, but increased side effects are noted with doses reaching 15 mg/day.1,2
Oxybutynin is not FDA approved for the treatment of hyperhidrosis, but it is an inexpensive drug, which makes it a viable option for use off label given all of the current research with positive outcomes.
It should be noted that if patients have any urinary retention, gastric motility issues, or narrow angle glaucoma, oxybutynin is contraindicated.
More studies are on the horizon, but finally there is hope for hyperhidrosis.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Skin Appendage Disord. 2015 Mar;1(1):6-13.
2. An Bras Dermatol. 2017 Mar-Apr;92(2):217-20.
3. ISRN Dermatol. 2012. doi: 10.5402/2012/702714.
Hyperhidrosis – excessive sweating – is one of those diagnoses that make most physicians cringe because we know that the side effects of most of the common treatments are very limiting and patients continue to be frustrated. New treatments are emerging, and although oxybutynin is not Food and Drug Administration–approved for hyperhidrosis, it does show promise.
Primary hyperhidrosis is the excessive sweating from the axilla, palms, soles, or cranial-facial area. It is a clinical diagnosis that has been occurring for more than 6 months and meets at least four1 of the following criteria:
1. It occurs in eccrine dense areas (axilla, soles, palms or head).
2. It is bilateral and symmetrical.
3. It is absent nocturnally.
4. Its onset should be before age 25 years.
5. It occurs at least weekly.
6. There is a positive family history.
7. It impairs daily activities.
If signs of underlying disease are apparent – such as palpitations, night sweats, weight loss, unilateral symptoms, anxiety, or hypertension – further workup is needed to rule out disorders such as diabetes, hyperthyroidism, pheochromocytoma, or peripheral nerve injury.1
The pathophysiology of hyperhidrosis is not clearly understood. It is believed to be due to increased cholinergic stimulation given that there is no hypertrophy or hyperplasia of the sweat gland.2 Genetics appear to play a role as there is usually a family history of the disorder.2
Topical treatments are usually first line, starting with aluminum chloride antiperspirants, or anticholinergic creams such as glycopyrrolate or glycopyrronium. Unfortunately, many patients complain of the skin irritation so they discontinue their use.1
Botulinum toxin type A is a very safe and effective way of treating hyperhidrosis and is FDA approved for that purpose.3 Its drawbacks are that it is an injection (approximately 25 in each armpit), and it is very costly, usually $1,000-1,500 per session for both underarms. There are no major side effects, and reduction in sweating lasts for 4-12 months, with a median of 6 months.3
Oral treatment of hyperhidrosis, with medications such as glycopyrrolate and benztropine, has been reserved for second- or third-line treatment because of the unwanted side effects of dry mouth and drowsiness. But more recent studies are showing favorable outcomes with oxybutynin.1
Oxybutynin is well known and FDA approved for treatment of urinary frequency, incontinence, and enuresis. Recent studies have shown great success for use to control generalized hyperhidrosis. The mechanism of action is blocking the binding of acetylcholine and numerous other neurotransmitters.2
The literature does not give clear-cut dosing because it is not approved for hyperhidrosis, but gradually increasing doses starting at 2.5 mg daily for a week, then increasing to twice daily for 2 weeks, and then to 5 mg twice daily as a continued dose appears to be the most effective regimen with few side effects. The dosage can be increased, but increased side effects are noted with doses reaching 15 mg/day.1,2
Oxybutynin is not FDA approved for the treatment of hyperhidrosis, but it is an inexpensive drug, which makes it a viable option for use off label given all of the current research with positive outcomes.
It should be noted that if patients have any urinary retention, gastric motility issues, or narrow angle glaucoma, oxybutynin is contraindicated.
More studies are on the horizon, but finally there is hope for hyperhidrosis.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Skin Appendage Disord. 2015 Mar;1(1):6-13.
2. An Bras Dermatol. 2017 Mar-Apr;92(2):217-20.
3. ISRN Dermatol. 2012. doi: 10.5402/2012/702714.
Hyperhidrosis – excessive sweating – is one of those diagnoses that make most physicians cringe because we know that the side effects of most of the common treatments are very limiting and patients continue to be frustrated. New treatments are emerging, and although oxybutynin is not Food and Drug Administration–approved for hyperhidrosis, it does show promise.
Primary hyperhidrosis is the excessive sweating from the axilla, palms, soles, or cranial-facial area. It is a clinical diagnosis that has been occurring for more than 6 months and meets at least four1 of the following criteria:
1. It occurs in eccrine dense areas (axilla, soles, palms or head).
2. It is bilateral and symmetrical.
3. It is absent nocturnally.
4. Its onset should be before age 25 years.
5. It occurs at least weekly.
6. There is a positive family history.
7. It impairs daily activities.
If signs of underlying disease are apparent – such as palpitations, night sweats, weight loss, unilateral symptoms, anxiety, or hypertension – further workup is needed to rule out disorders such as diabetes, hyperthyroidism, pheochromocytoma, or peripheral nerve injury.1
The pathophysiology of hyperhidrosis is not clearly understood. It is believed to be due to increased cholinergic stimulation given that there is no hypertrophy or hyperplasia of the sweat gland.2 Genetics appear to play a role as there is usually a family history of the disorder.2
Topical treatments are usually first line, starting with aluminum chloride antiperspirants, or anticholinergic creams such as glycopyrrolate or glycopyrronium. Unfortunately, many patients complain of the skin irritation so they discontinue their use.1
Botulinum toxin type A is a very safe and effective way of treating hyperhidrosis and is FDA approved for that purpose.3 Its drawbacks are that it is an injection (approximately 25 in each armpit), and it is very costly, usually $1,000-1,500 per session for both underarms. There are no major side effects, and reduction in sweating lasts for 4-12 months, with a median of 6 months.3
Oral treatment of hyperhidrosis, with medications such as glycopyrrolate and benztropine, has been reserved for second- or third-line treatment because of the unwanted side effects of dry mouth and drowsiness. But more recent studies are showing favorable outcomes with oxybutynin.1
Oxybutynin is well known and FDA approved for treatment of urinary frequency, incontinence, and enuresis. Recent studies have shown great success for use to control generalized hyperhidrosis. The mechanism of action is blocking the binding of acetylcholine and numerous other neurotransmitters.2
The literature does not give clear-cut dosing because it is not approved for hyperhidrosis, but gradually increasing doses starting at 2.5 mg daily for a week, then increasing to twice daily for 2 weeks, and then to 5 mg twice daily as a continued dose appears to be the most effective regimen with few side effects. The dosage can be increased, but increased side effects are noted with doses reaching 15 mg/day.1,2
Oxybutynin is not FDA approved for the treatment of hyperhidrosis, but it is an inexpensive drug, which makes it a viable option for use off label given all of the current research with positive outcomes.
It should be noted that if patients have any urinary retention, gastric motility issues, or narrow angle glaucoma, oxybutynin is contraindicated.
More studies are on the horizon, but finally there is hope for hyperhidrosis.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Skin Appendage Disord. 2015 Mar;1(1):6-13.
2. An Bras Dermatol. 2017 Mar-Apr;92(2):217-20.
3. ISRN Dermatol. 2012. doi: 10.5402/2012/702714.
Clinical trials unavailable for more than half of all cancer patients
More than half of all cancer patients do not participate in clinical trials because none are available for their cancer type or stage at their institution, according to a meta-analysis of cancer clinical trials that examined the trial decision-making pathway.
“This is the first effort to systematically both define and quantify domains of clinical trial barriers using a meta-analytic approach,” wrote lead author Joseph M. Unger, PhD, of the Fred Hutchinson Cancer Research Center in Seattle and his coauthors in the Journal of the National Cancer Institute.
To identify trials that addressed barriers to enrollment, Dr. Unger and his colleagues conducted a literature search using the PubMed, Google Scholar, Web of Science, and Ovid Medline databases. The search returned 7,576 unique results, of which they reviewed 38 full articles and eventually decided on 13 studies comprising 8,883 patients. Nine of the studies were focused on academic care settings, and four were focused on community care settings; seven examined patient decision-making patterns in all types of cancers, while the others focused on breast cancer only (n = 2), lung cancer only (n = 2), prostate cancer only (n = 1), and cervix/uterine cancers (n = 1).
Their analysis found that, for 55.6% of patients, no trial was available for their cancer type and stage (95% confidence interval, 43.7%-67.3%). In addition, 21.5% (95% CI, 10.9%-34.6%) were not eligible for an available trial, and 14.8% (95% CI, 9.0%-21.7%) did not enroll; only 8.1% (95% CI, 6.3%-10.0%) enrolled in a trial. Academic sites (15.9%, 95% CI, 13.8%-18.2%) saw much higher rates of participation than community sites (7.0%, 95% CI, 5.1%-9.1%; P less than .001).
The authors acknowledged their study’s limitations, including details on trial availability not being available for all analyzed studies. In addition, several of the studies relied on selected cancer types instead of sampling a representative set of cancers. Finally, these studies may have oversampled research-oriented sites, which would mean “the actual overall trial participation rate may be lower than we estimated.”
The study was supported by the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Unger JM et al. J Natl Cancer Inst. 2019 Feb 19. doi: 10.1093/jnci/djy221.
More than half of all cancer patients do not participate in clinical trials because none are available for their cancer type or stage at their institution, according to a meta-analysis of cancer clinical trials that examined the trial decision-making pathway.
“This is the first effort to systematically both define and quantify domains of clinical trial barriers using a meta-analytic approach,” wrote lead author Joseph M. Unger, PhD, of the Fred Hutchinson Cancer Research Center in Seattle and his coauthors in the Journal of the National Cancer Institute.
To identify trials that addressed barriers to enrollment, Dr. Unger and his colleagues conducted a literature search using the PubMed, Google Scholar, Web of Science, and Ovid Medline databases. The search returned 7,576 unique results, of which they reviewed 38 full articles and eventually decided on 13 studies comprising 8,883 patients. Nine of the studies were focused on academic care settings, and four were focused on community care settings; seven examined patient decision-making patterns in all types of cancers, while the others focused on breast cancer only (n = 2), lung cancer only (n = 2), prostate cancer only (n = 1), and cervix/uterine cancers (n = 1).
Their analysis found that, for 55.6% of patients, no trial was available for their cancer type and stage (95% confidence interval, 43.7%-67.3%). In addition, 21.5% (95% CI, 10.9%-34.6%) were not eligible for an available trial, and 14.8% (95% CI, 9.0%-21.7%) did not enroll; only 8.1% (95% CI, 6.3%-10.0%) enrolled in a trial. Academic sites (15.9%, 95% CI, 13.8%-18.2%) saw much higher rates of participation than community sites (7.0%, 95% CI, 5.1%-9.1%; P less than .001).
The authors acknowledged their study’s limitations, including details on trial availability not being available for all analyzed studies. In addition, several of the studies relied on selected cancer types instead of sampling a representative set of cancers. Finally, these studies may have oversampled research-oriented sites, which would mean “the actual overall trial participation rate may be lower than we estimated.”
The study was supported by the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Unger JM et al. J Natl Cancer Inst. 2019 Feb 19. doi: 10.1093/jnci/djy221.
More than half of all cancer patients do not participate in clinical trials because none are available for their cancer type or stage at their institution, according to a meta-analysis of cancer clinical trials that examined the trial decision-making pathway.
“This is the first effort to systematically both define and quantify domains of clinical trial barriers using a meta-analytic approach,” wrote lead author Joseph M. Unger, PhD, of the Fred Hutchinson Cancer Research Center in Seattle and his coauthors in the Journal of the National Cancer Institute.
To identify trials that addressed barriers to enrollment, Dr. Unger and his colleagues conducted a literature search using the PubMed, Google Scholar, Web of Science, and Ovid Medline databases. The search returned 7,576 unique results, of which they reviewed 38 full articles and eventually decided on 13 studies comprising 8,883 patients. Nine of the studies were focused on academic care settings, and four were focused on community care settings; seven examined patient decision-making patterns in all types of cancers, while the others focused on breast cancer only (n = 2), lung cancer only (n = 2), prostate cancer only (n = 1), and cervix/uterine cancers (n = 1).
Their analysis found that, for 55.6% of patients, no trial was available for their cancer type and stage (95% confidence interval, 43.7%-67.3%). In addition, 21.5% (95% CI, 10.9%-34.6%) were not eligible for an available trial, and 14.8% (95% CI, 9.0%-21.7%) did not enroll; only 8.1% (95% CI, 6.3%-10.0%) enrolled in a trial. Academic sites (15.9%, 95% CI, 13.8%-18.2%) saw much higher rates of participation than community sites (7.0%, 95% CI, 5.1%-9.1%; P less than .001).
The authors acknowledged their study’s limitations, including details on trial availability not being available for all analyzed studies. In addition, several of the studies relied on selected cancer types instead of sampling a representative set of cancers. Finally, these studies may have oversampled research-oriented sites, which would mean “the actual overall trial participation rate may be lower than we estimated.”
The study was supported by the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Unger JM et al. J Natl Cancer Inst. 2019 Feb 19. doi: 10.1093/jnci/djy221.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
‘The birth of a mother is a complex process’
Softening the blow to women and families of severe perinatal, postpartum psychiatric disorders
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
“But this was not what I expected!” That’s a statement I have heard from countless new mothers.
Women often envision pregnancy and the postpartum period as a time of pure joy. The glow of an expectant woman and the excitement of the arrival of a new baby masks the reality that many women struggle emotionally when transitioning to motherhood. Like the birth of a child, the birth of a mother is a complex process. Upholding the myth that all women seamlessly transform into mothers can have devastating effects and hinder access to mental health care.
As a psychiatrist working on a women’s inpatient unit with a perinatal program, I treat women at times of crisis. What may have begun as mild anxiety or depression sometimes quickly spirals into severe psychiatric illness. The sheer force of these severe perinatal and postpartum psychiatric disorders often leaves women and families shocked and confused, wondering what happened to their crumbled dreams of early motherhood.
What must general psychiatrists know about perinatal and postpartum psychiatric disorders? Why is maternal mental health so important? What are the barriers to treatment for these women? How can general psychiatrists best support and treat these new mothers and their families?
What data show
Maternal depression is now known to be one of the most common complications of pregnancy. Studies have suggested that about 11% of women experience depression during pregnancy1 and approximately 17% of women are depressed in the postpartum period.2 Perinatal generalized anxiety disorder has been shown to have a prevalence of 8.5%-10.5% during pregnancy with a wider variance post partum.3 Approximately 3% of women in the general community develop PTSD symptoms following childbirth.4 Research suggests that about 2% of women develop obsessive-compulsive disorder symptoms in the postpartum period.5 Postpartum psychosis, a rare but potentially devastating illness, occurs after 0.1%-0.2% of births.6
Importance of maternal mental health
There is a growing body of literature supporting both obstetric and pediatric adverse outcomes related to untreated psychiatric illness. Untreated maternal depression has been associated with obstetric complications, such as preterm delivery, preeclampsia, low birth weight, as well as the child’s developing cognitive function.7 Anxiety during pregnancy has been associated with both a shorter gestational period and adverse implications for fetal neurodevelopment.
These adverse effects were found to be even more potent in “pregnancy anxiety,” or anxiety specifically focused on the pregnancy, the birth experience, and the transition to motherhood.8 The psychotic symptoms occurring during postpartum psychosis can jeopardize the lives of both a woman and her child and carries a 4% risk of infanticide.9 Although there are limited data about the long-term effects of postpartum obsessive-compulsive disorders and PTSD, it is reasonable to assume that they might carry negative long-term implications for the mother and possibly her child.
Barriers to treatment
Despite the significant rates of mental illness, pregnant and new mothers often face barriers to receiving treatment. Many psychiatrists are hesitant to prescribe psychiatric medication to pregnant women because of concerns about teratogenic potential of psychiatric medications; similar concerns exist for newborn babies when prescribing medications to lactating mothers. In addition, the field of reproductive psychiatry is evolving at a rapid pace, making it difficult for busy psychiatrists to keep up with the ever-growing literature.
Also, it is hard to imagine a population that has more barriers to attending outpatient appointments. For many new mothers, the exhaustion and all-consuming work involved with taking care of a newborn are insurmountable barriers to obtaining mental health care. In addition, despite the awareness that new mothers often are more emotional, families can be slow to recognize the developing severity of a psychiatric illness during the peripartum and postpartum periods.
Supporting and treating new mothers
As general psychiatrists, there are several ways to directly help these women.
1. Expect the expected. Even in women with no prior psychiatric history, a significant percentage of expectant and postpartum women will develop acute psychiatric symptoms. Learn about the different presentations and treatments of perinatal and postpartum psychiatric disorders. For example, a woman might have thoughts of harming her baby in both postpartum psychosis and obsessive-compulsive disorder. However, the acuity and treatment of these two conditions drastically differ.
2. Learn more about psychiatric medications. Several apps and websites are available to psychiatrists to learn about the safety profile of psychiatric medications, such as Reprotox.org, mothertobaby.org, lactmed, and womensmentalhealth.org. Many medications are considered to be relatively safe during pregnancy and breastfeeding. It is important for psychiatrists to appreciate the risks when choosing not to prescribe to pregnant and postpartum women. Sometimes a known risk of a specific medication may be preferable to the unknown risk of leaving a woman susceptible to a severe psychiatric decompensation.
3. Involve all members of the family. A mother’s mental health has significant implications for the entire family. Psychoeducation for the family as well as frequent family sessions are key tools when treating this population. In addition, prescribing to pregnant women provides the opportunity for a psychiatrist to refine skills in joint decision making; it is crucial to involve both a patient and her spouse when discussing psychiatric medications.
4. Provide ready access and collaborate care. It is important to understand the potential rapid onset of psychiatric symptoms during the pregnancy and postpartum period. Psychiatrists should be prepared to collaborate care with other specialties. It is important to establish relationships with community psychotherapists specializing in maternal mental health, pediatricians, as well as obstetricians.
5. Learn when to seek a higher level of care. Although many women with perinatal and postpartum psychiatric symptoms can be managed as outpatients, women at times need a higher level of care. Similar to general psychiatry, women who are acutely suicidal or homicidal or have a sudden onset of psychotic and manic symptoms all should be evaluated immediately for inpatient hospitalization. Women with less severe symptoms but who require a higher level of care than typically offered in standard outpatient treatment should be candidates for partial hospitalization programs.
General intensive programs usually can accommodate these women, but it is ideal to refer this population to perinatal intensive programs. Postpartum Support International (postpartum.net) lists the nationwide inpatient and partial perinatal programs as well as regional and local services. An example of inpatient perinatal care is the women’s unit at Zucker Hillside Hospital (Northwell Health System, Glen Oaks, N.Y.), which houses an inpatient perinatal program. As a psychiatrist on the unit, I treat acute symptoms such as depression, anxiety, psychosis, mania, and catatonia that occur during the perinatal and postpartum periods. Given the severity of symptoms, I use a wide range of psychiatric medications with the possibility of electroconvulsive therapy when indicated. Psychotherapy staff on the unit offer specialized perinatal, mothers, and dialectical behavioral therapy groups. Breast pumps are available for women who wish to breastfeed. Accommodations are made for babies and children to visit their mother when clinically appropriate. Once discharged, women often are referred to Zucker Hillside’s own perinatal outpatient clinic for continued treatment. Similar models exist in select inpatient units as well as an increasing number of partial programs across the United States.
Conclusion
Psychiatric care for pregnant and new mothers can be challenging, but it is also immensely rewarding. Restoring a mother’s mental health usually leads to increased emotional stability for her entire family. Given the prevalence of maternal mental health disorders, psychiatrists in nearly every setting will encounter this population of women. With dedicated time devoted to reviewing the literature and learning about local resources, psychiatrists can feel comfortable treating women throughout the childbearing experience.
References
1. J Affect Disord. 2017 Sep;219:86-92.
2. J Psychiatr Res. 2018 Sep;104:235-48.
3. J Womens Health. (Larchmt). 2015 Sep;24(9):762-70.
4. Clin Psychol Rev. 2014 Jul;34(5):389-401.
5. Compr Psychiatry. 2009 Nov-Dec;50(6):503-9.
6. Int Rev Psychiatry. 2003 Aug;15(3):231-42.
7. Clin Obstet Gynecol. 2018 Sep;61(3):533-43.
8. Curr Opinion Psychiatry. 2012 Mar;25(2):141-8.
9. Am J Psychiatry. 2009 Apr;166(4):405-8.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine, New York, after graduating from the Albert Einstein College of Medicine and Yeshiva University, New York, with a BA in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
Softening the blow to women and families of severe perinatal, postpartum psychiatric disorders
Softening the blow to women and families of severe perinatal, postpartum psychiatric disorders
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
“But this was not what I expected!” That’s a statement I have heard from countless new mothers.
Women often envision pregnancy and the postpartum period as a time of pure joy. The glow of an expectant woman and the excitement of the arrival of a new baby masks the reality that many women struggle emotionally when transitioning to motherhood. Like the birth of a child, the birth of a mother is a complex process. Upholding the myth that all women seamlessly transform into mothers can have devastating effects and hinder access to mental health care.
As a psychiatrist working on a women’s inpatient unit with a perinatal program, I treat women at times of crisis. What may have begun as mild anxiety or depression sometimes quickly spirals into severe psychiatric illness. The sheer force of these severe perinatal and postpartum psychiatric disorders often leaves women and families shocked and confused, wondering what happened to their crumbled dreams of early motherhood.
What must general psychiatrists know about perinatal and postpartum psychiatric disorders? Why is maternal mental health so important? What are the barriers to treatment for these women? How can general psychiatrists best support and treat these new mothers and their families?
What data show
Maternal depression is now known to be one of the most common complications of pregnancy. Studies have suggested that about 11% of women experience depression during pregnancy1 and approximately 17% of women are depressed in the postpartum period.2 Perinatal generalized anxiety disorder has been shown to have a prevalence of 8.5%-10.5% during pregnancy with a wider variance post partum.3 Approximately 3% of women in the general community develop PTSD symptoms following childbirth.4 Research suggests that about 2% of women develop obsessive-compulsive disorder symptoms in the postpartum period.5 Postpartum psychosis, a rare but potentially devastating illness, occurs after 0.1%-0.2% of births.6
Importance of maternal mental health
There is a growing body of literature supporting both obstetric and pediatric adverse outcomes related to untreated psychiatric illness. Untreated maternal depression has been associated with obstetric complications, such as preterm delivery, preeclampsia, low birth weight, as well as the child’s developing cognitive function.7 Anxiety during pregnancy has been associated with both a shorter gestational period and adverse implications for fetal neurodevelopment.
These adverse effects were found to be even more potent in “pregnancy anxiety,” or anxiety specifically focused on the pregnancy, the birth experience, and the transition to motherhood.8 The psychotic symptoms occurring during postpartum psychosis can jeopardize the lives of both a woman and her child and carries a 4% risk of infanticide.9 Although there are limited data about the long-term effects of postpartum obsessive-compulsive disorders and PTSD, it is reasonable to assume that they might carry negative long-term implications for the mother and possibly her child.
Barriers to treatment
Despite the significant rates of mental illness, pregnant and new mothers often face barriers to receiving treatment. Many psychiatrists are hesitant to prescribe psychiatric medication to pregnant women because of concerns about teratogenic potential of psychiatric medications; similar concerns exist for newborn babies when prescribing medications to lactating mothers. In addition, the field of reproductive psychiatry is evolving at a rapid pace, making it difficult for busy psychiatrists to keep up with the ever-growing literature.
Also, it is hard to imagine a population that has more barriers to attending outpatient appointments. For many new mothers, the exhaustion and all-consuming work involved with taking care of a newborn are insurmountable barriers to obtaining mental health care. In addition, despite the awareness that new mothers often are more emotional, families can be slow to recognize the developing severity of a psychiatric illness during the peripartum and postpartum periods.
Supporting and treating new mothers
As general psychiatrists, there are several ways to directly help these women.
1. Expect the expected. Even in women with no prior psychiatric history, a significant percentage of expectant and postpartum women will develop acute psychiatric symptoms. Learn about the different presentations and treatments of perinatal and postpartum psychiatric disorders. For example, a woman might have thoughts of harming her baby in both postpartum psychosis and obsessive-compulsive disorder. However, the acuity and treatment of these two conditions drastically differ.
2. Learn more about psychiatric medications. Several apps and websites are available to psychiatrists to learn about the safety profile of psychiatric medications, such as Reprotox.org, mothertobaby.org, lactmed, and womensmentalhealth.org. Many medications are considered to be relatively safe during pregnancy and breastfeeding. It is important for psychiatrists to appreciate the risks when choosing not to prescribe to pregnant and postpartum women. Sometimes a known risk of a specific medication may be preferable to the unknown risk of leaving a woman susceptible to a severe psychiatric decompensation.
3. Involve all members of the family. A mother’s mental health has significant implications for the entire family. Psychoeducation for the family as well as frequent family sessions are key tools when treating this population. In addition, prescribing to pregnant women provides the opportunity for a psychiatrist to refine skills in joint decision making; it is crucial to involve both a patient and her spouse when discussing psychiatric medications.
4. Provide ready access and collaborate care. It is important to understand the potential rapid onset of psychiatric symptoms during the pregnancy and postpartum period. Psychiatrists should be prepared to collaborate care with other specialties. It is important to establish relationships with community psychotherapists specializing in maternal mental health, pediatricians, as well as obstetricians.
5. Learn when to seek a higher level of care. Although many women with perinatal and postpartum psychiatric symptoms can be managed as outpatients, women at times need a higher level of care. Similar to general psychiatry, women who are acutely suicidal or homicidal or have a sudden onset of psychotic and manic symptoms all should be evaluated immediately for inpatient hospitalization. Women with less severe symptoms but who require a higher level of care than typically offered in standard outpatient treatment should be candidates for partial hospitalization programs.
General intensive programs usually can accommodate these women, but it is ideal to refer this population to perinatal intensive programs. Postpartum Support International (postpartum.net) lists the nationwide inpatient and partial perinatal programs as well as regional and local services. An example of inpatient perinatal care is the women’s unit at Zucker Hillside Hospital (Northwell Health System, Glen Oaks, N.Y.), which houses an inpatient perinatal program. As a psychiatrist on the unit, I treat acute symptoms such as depression, anxiety, psychosis, mania, and catatonia that occur during the perinatal and postpartum periods. Given the severity of symptoms, I use a wide range of psychiatric medications with the possibility of electroconvulsive therapy when indicated. Psychotherapy staff on the unit offer specialized perinatal, mothers, and dialectical behavioral therapy groups. Breast pumps are available for women who wish to breastfeed. Accommodations are made for babies and children to visit their mother when clinically appropriate. Once discharged, women often are referred to Zucker Hillside’s own perinatal outpatient clinic for continued treatment. Similar models exist in select inpatient units as well as an increasing number of partial programs across the United States.
Conclusion
Psychiatric care for pregnant and new mothers can be challenging, but it is also immensely rewarding. Restoring a mother’s mental health usually leads to increased emotional stability for her entire family. Given the prevalence of maternal mental health disorders, psychiatrists in nearly every setting will encounter this population of women. With dedicated time devoted to reviewing the literature and learning about local resources, psychiatrists can feel comfortable treating women throughout the childbearing experience.
References
1. J Affect Disord. 2017 Sep;219:86-92.
2. J Psychiatr Res. 2018 Sep;104:235-48.
3. J Womens Health. (Larchmt). 2015 Sep;24(9):762-70.
4. Clin Psychol Rev. 2014 Jul;34(5):389-401.
5. Compr Psychiatry. 2009 Nov-Dec;50(6):503-9.
6. Int Rev Psychiatry. 2003 Aug;15(3):231-42.
7. Clin Obstet Gynecol. 2018 Sep;61(3):533-43.
8. Curr Opinion Psychiatry. 2012 Mar;25(2):141-8.
9. Am J Psychiatry. 2009 Apr;166(4):405-8.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine, New York, after graduating from the Albert Einstein College of Medicine and Yeshiva University, New York, with a BA in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
“But this was not what I expected!” That’s a statement I have heard from countless new mothers.
Women often envision pregnancy and the postpartum period as a time of pure joy. The glow of an expectant woman and the excitement of the arrival of a new baby masks the reality that many women struggle emotionally when transitioning to motherhood. Like the birth of a child, the birth of a mother is a complex process. Upholding the myth that all women seamlessly transform into mothers can have devastating effects and hinder access to mental health care.
As a psychiatrist working on a women’s inpatient unit with a perinatal program, I treat women at times of crisis. What may have begun as mild anxiety or depression sometimes quickly spirals into severe psychiatric illness. The sheer force of these severe perinatal and postpartum psychiatric disorders often leaves women and families shocked and confused, wondering what happened to their crumbled dreams of early motherhood.
What must general psychiatrists know about perinatal and postpartum psychiatric disorders? Why is maternal mental health so important? What are the barriers to treatment for these women? How can general psychiatrists best support and treat these new mothers and their families?
What data show
Maternal depression is now known to be one of the most common complications of pregnancy. Studies have suggested that about 11% of women experience depression during pregnancy1 and approximately 17% of women are depressed in the postpartum period.2 Perinatal generalized anxiety disorder has been shown to have a prevalence of 8.5%-10.5% during pregnancy with a wider variance post partum.3 Approximately 3% of women in the general community develop PTSD symptoms following childbirth.4 Research suggests that about 2% of women develop obsessive-compulsive disorder symptoms in the postpartum period.5 Postpartum psychosis, a rare but potentially devastating illness, occurs after 0.1%-0.2% of births.6
Importance of maternal mental health
There is a growing body of literature supporting both obstetric and pediatric adverse outcomes related to untreated psychiatric illness. Untreated maternal depression has been associated with obstetric complications, such as preterm delivery, preeclampsia, low birth weight, as well as the child’s developing cognitive function.7 Anxiety during pregnancy has been associated with both a shorter gestational period and adverse implications for fetal neurodevelopment.
These adverse effects were found to be even more potent in “pregnancy anxiety,” or anxiety specifically focused on the pregnancy, the birth experience, and the transition to motherhood.8 The psychotic symptoms occurring during postpartum psychosis can jeopardize the lives of both a woman and her child and carries a 4% risk of infanticide.9 Although there are limited data about the long-term effects of postpartum obsessive-compulsive disorders and PTSD, it is reasonable to assume that they might carry negative long-term implications for the mother and possibly her child.
Barriers to treatment
Despite the significant rates of mental illness, pregnant and new mothers often face barriers to receiving treatment. Many psychiatrists are hesitant to prescribe psychiatric medication to pregnant women because of concerns about teratogenic potential of psychiatric medications; similar concerns exist for newborn babies when prescribing medications to lactating mothers. In addition, the field of reproductive psychiatry is evolving at a rapid pace, making it difficult for busy psychiatrists to keep up with the ever-growing literature.
Also, it is hard to imagine a population that has more barriers to attending outpatient appointments. For many new mothers, the exhaustion and all-consuming work involved with taking care of a newborn are insurmountable barriers to obtaining mental health care. In addition, despite the awareness that new mothers often are more emotional, families can be slow to recognize the developing severity of a psychiatric illness during the peripartum and postpartum periods.
Supporting and treating new mothers
As general psychiatrists, there are several ways to directly help these women.
1. Expect the expected. Even in women with no prior psychiatric history, a significant percentage of expectant and postpartum women will develop acute psychiatric symptoms. Learn about the different presentations and treatments of perinatal and postpartum psychiatric disorders. For example, a woman might have thoughts of harming her baby in both postpartum psychosis and obsessive-compulsive disorder. However, the acuity and treatment of these two conditions drastically differ.
2. Learn more about psychiatric medications. Several apps and websites are available to psychiatrists to learn about the safety profile of psychiatric medications, such as Reprotox.org, mothertobaby.org, lactmed, and womensmentalhealth.org. Many medications are considered to be relatively safe during pregnancy and breastfeeding. It is important for psychiatrists to appreciate the risks when choosing not to prescribe to pregnant and postpartum women. Sometimes a known risk of a specific medication may be preferable to the unknown risk of leaving a woman susceptible to a severe psychiatric decompensation.
3. Involve all members of the family. A mother’s mental health has significant implications for the entire family. Psychoeducation for the family as well as frequent family sessions are key tools when treating this population. In addition, prescribing to pregnant women provides the opportunity for a psychiatrist to refine skills in joint decision making; it is crucial to involve both a patient and her spouse when discussing psychiatric medications.
4. Provide ready access and collaborate care. It is important to understand the potential rapid onset of psychiatric symptoms during the pregnancy and postpartum period. Psychiatrists should be prepared to collaborate care with other specialties. It is important to establish relationships with community psychotherapists specializing in maternal mental health, pediatricians, as well as obstetricians.
5. Learn when to seek a higher level of care. Although many women with perinatal and postpartum psychiatric symptoms can be managed as outpatients, women at times need a higher level of care. Similar to general psychiatry, women who are acutely suicidal or homicidal or have a sudden onset of psychotic and manic symptoms all should be evaluated immediately for inpatient hospitalization. Women with less severe symptoms but who require a higher level of care than typically offered in standard outpatient treatment should be candidates for partial hospitalization programs.
General intensive programs usually can accommodate these women, but it is ideal to refer this population to perinatal intensive programs. Postpartum Support International (postpartum.net) lists the nationwide inpatient and partial perinatal programs as well as regional and local services. An example of inpatient perinatal care is the women’s unit at Zucker Hillside Hospital (Northwell Health System, Glen Oaks, N.Y.), which houses an inpatient perinatal program. As a psychiatrist on the unit, I treat acute symptoms such as depression, anxiety, psychosis, mania, and catatonia that occur during the perinatal and postpartum periods. Given the severity of symptoms, I use a wide range of psychiatric medications with the possibility of electroconvulsive therapy when indicated. Psychotherapy staff on the unit offer specialized perinatal, mothers, and dialectical behavioral therapy groups. Breast pumps are available for women who wish to breastfeed. Accommodations are made for babies and children to visit their mother when clinically appropriate. Once discharged, women often are referred to Zucker Hillside’s own perinatal outpatient clinic for continued treatment. Similar models exist in select inpatient units as well as an increasing number of partial programs across the United States.
Conclusion
Psychiatric care for pregnant and new mothers can be challenging, but it is also immensely rewarding. Restoring a mother’s mental health usually leads to increased emotional stability for her entire family. Given the prevalence of maternal mental health disorders, psychiatrists in nearly every setting will encounter this population of women. With dedicated time devoted to reviewing the literature and learning about local resources, psychiatrists can feel comfortable treating women throughout the childbearing experience.
References
1. J Affect Disord. 2017 Sep;219:86-92.
2. J Psychiatr Res. 2018 Sep;104:235-48.
3. J Womens Health. (Larchmt). 2015 Sep;24(9):762-70.
4. Clin Psychol Rev. 2014 Jul;34(5):389-401.
5. Compr Psychiatry. 2009 Nov-Dec;50(6):503-9.
6. Int Rev Psychiatry. 2003 Aug;15(3):231-42.
7. Clin Obstet Gynecol. 2018 Sep;61(3):533-43.
8. Curr Opinion Psychiatry. 2012 Mar;25(2):141-8.
9. Am J Psychiatry. 2009 Apr;166(4):405-8.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine, New York, after graduating from the Albert Einstein College of Medicine and Yeshiva University, New York, with a BA in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.