User login
Real-world weight loss with meds approximates RCT results
NASHVILLE, TENN. –
The results seen with the medication combo – a mean 15.5% total body weight loss at 12 months – bested the 8%-11% seen in randomized controlled trials (RCTs), said Gerardo Calderon, MD, in an interview during a poster session at Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. The combination was also the most commonly prescribed weight loss medication at the Mayo Clinic, where Dr. Calderon is a gastroenterology and hepatology research fellow.
Patients taking lorcaserin at the Mayo Clinic also lost more weight loss than RCT participants (8.8% vs. 5%-6%, respectively). Notably, they also had a higher probability of losing at least 10% of their baseline total body weight (40% vs. 17%-23% in clinical trials). In RCTs, 37%-48% of patients taking phentermine/topiramate-ER had a total body weight loss of at least 10%, similar to the Mayo Clinic’s figure of 49%.
The rate of reported adverse events – 23.8% – exceeded that reported in RCTs, noted Dr. Calderon. Gastrointestinal symptoms, such as nausea, vomiting, diarrhea, and constipation, were reported by 2%-20% of patients across the various drugs prescribed. Insomnia and mood changes, along with dizziness or lightheadedness, were reported by 2%-6% of patients. Almost 12% of patients taking phentermine/topiramate-SR reported paresthesias. No patients stopped taking their medication because of side effects, however.
The study was a review of patients seen at the Mayo Clinic during January 2016 – June 2018. Patients were included if they were prescribed weight loss medications and had a body mass index of at least 25 kg/m2 with comorbidities related to adiposity or with a BMI of at least 30 without such comorbidities. To be included, patients had to be followed for at least 3 months and see a Mayo Clinic physician at least twice.
Patients with previous bariatric surgery or other major gastrointestinal surgery, those who didn’t use their medications because of insurance problems or drug costs, and those who were on weight loss medication before being seen for the first time were excluded from the study.
Patients were a mean 49 years old, and most were female (86/118; 72.9%). Mean BMI at enrollment was 41.7, with a mean weight of 117.6 kg.
Of 118 patients, 76 (64.4%) had dyslipidemia. About half of patients reported obstructive sleep apnea, and the same amount had hypertension. About 40% had diabetes, and the same number had degenerative joint disease.
Phentermine/topiramate was prescribed the most frequently, with 43.2% of patients on this medication. Liraglutide was taken by 34.7% of patients, bupropion/naltrexone-SR by 16.1%, and lorcaserin by 5.9%.
Patients taking liraglutide had similar weight loss (7.1%) to that seen in RCTs (6%-8%). For this medication, the real-world Mayo Clinic experience showed less chance of hitting the 10% total body weight loss mark (12% vs. 26%-33% in RCTs).
For bupropion/naltrexone-SR, weight loss was similar among the Mayo Clinic patients (7.2%) and RCT participants (5%-8%), and probability of achieving at least 10% total body weight loss was similar as well (32% vs. 34%).
Weight loss medication was a component of a multidisciplinary weight loss approach at Mayo Clinic. Physicians, dietitians, and psychologists worked together to care for patients with overweight and obesity at his facility, Dr. Calderon said. Overall, patients were followed for a mean 6.7 months, and patients had a mean 3 follow-up visits, with over half of patients attending at least one follow-up appointment in study months 6-12. At 12 months, though, the attrition rate was 57.9%. “We notice an attrition rate of almost 60% at 1 year. People are not coming to their follow-up. ... Definitely, this is something we are concerned about, and we would like to bring these attrition rates lower,” he said.
Most patients (63.6%) saw a dietitian, but on average, patients had just one appointment. “At the Mayo Clinic, we provide dietitians and psychological support. But it’s up to the patients if they want to have it or not,” said Dr. Calderon. “Most of them, they just went once to a dietitian.”
“Overall, these outcomes are similar to those in RCTs and support the concept that weight loss medications can achieve clinically significant weight loss in a multidisciplinary weight loss program,” noted Dr. Calderon and his coauthors.
Dr. Calderon reported no outside sources of funding and no conflicts of interest.
SOURCE: Calderon G et al. Obesity Week, Abstract T-P-3425.
NASHVILLE, TENN. –
The results seen with the medication combo – a mean 15.5% total body weight loss at 12 months – bested the 8%-11% seen in randomized controlled trials (RCTs), said Gerardo Calderon, MD, in an interview during a poster session at Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. The combination was also the most commonly prescribed weight loss medication at the Mayo Clinic, where Dr. Calderon is a gastroenterology and hepatology research fellow.
Patients taking lorcaserin at the Mayo Clinic also lost more weight loss than RCT participants (8.8% vs. 5%-6%, respectively). Notably, they also had a higher probability of losing at least 10% of their baseline total body weight (40% vs. 17%-23% in clinical trials). In RCTs, 37%-48% of patients taking phentermine/topiramate-ER had a total body weight loss of at least 10%, similar to the Mayo Clinic’s figure of 49%.
The rate of reported adverse events – 23.8% – exceeded that reported in RCTs, noted Dr. Calderon. Gastrointestinal symptoms, such as nausea, vomiting, diarrhea, and constipation, were reported by 2%-20% of patients across the various drugs prescribed. Insomnia and mood changes, along with dizziness or lightheadedness, were reported by 2%-6% of patients. Almost 12% of patients taking phentermine/topiramate-SR reported paresthesias. No patients stopped taking their medication because of side effects, however.
The study was a review of patients seen at the Mayo Clinic during January 2016 – June 2018. Patients were included if they were prescribed weight loss medications and had a body mass index of at least 25 kg/m2 with comorbidities related to adiposity or with a BMI of at least 30 without such comorbidities. To be included, patients had to be followed for at least 3 months and see a Mayo Clinic physician at least twice.
Patients with previous bariatric surgery or other major gastrointestinal surgery, those who didn’t use their medications because of insurance problems or drug costs, and those who were on weight loss medication before being seen for the first time were excluded from the study.
Patients were a mean 49 years old, and most were female (86/118; 72.9%). Mean BMI at enrollment was 41.7, with a mean weight of 117.6 kg.
Of 118 patients, 76 (64.4%) had dyslipidemia. About half of patients reported obstructive sleep apnea, and the same amount had hypertension. About 40% had diabetes, and the same number had degenerative joint disease.
Phentermine/topiramate was prescribed the most frequently, with 43.2% of patients on this medication. Liraglutide was taken by 34.7% of patients, bupropion/naltrexone-SR by 16.1%, and lorcaserin by 5.9%.
Patients taking liraglutide had similar weight loss (7.1%) to that seen in RCTs (6%-8%). For this medication, the real-world Mayo Clinic experience showed less chance of hitting the 10% total body weight loss mark (12% vs. 26%-33% in RCTs).
For bupropion/naltrexone-SR, weight loss was similar among the Mayo Clinic patients (7.2%) and RCT participants (5%-8%), and probability of achieving at least 10% total body weight loss was similar as well (32% vs. 34%).
Weight loss medication was a component of a multidisciplinary weight loss approach at Mayo Clinic. Physicians, dietitians, and psychologists worked together to care for patients with overweight and obesity at his facility, Dr. Calderon said. Overall, patients were followed for a mean 6.7 months, and patients had a mean 3 follow-up visits, with over half of patients attending at least one follow-up appointment in study months 6-12. At 12 months, though, the attrition rate was 57.9%. “We notice an attrition rate of almost 60% at 1 year. People are not coming to their follow-up. ... Definitely, this is something we are concerned about, and we would like to bring these attrition rates lower,” he said.
Most patients (63.6%) saw a dietitian, but on average, patients had just one appointment. “At the Mayo Clinic, we provide dietitians and psychological support. But it’s up to the patients if they want to have it or not,” said Dr. Calderon. “Most of them, they just went once to a dietitian.”
“Overall, these outcomes are similar to those in RCTs and support the concept that weight loss medications can achieve clinically significant weight loss in a multidisciplinary weight loss program,” noted Dr. Calderon and his coauthors.
Dr. Calderon reported no outside sources of funding and no conflicts of interest.
SOURCE: Calderon G et al. Obesity Week, Abstract T-P-3425.
NASHVILLE, TENN. –
The results seen with the medication combo – a mean 15.5% total body weight loss at 12 months – bested the 8%-11% seen in randomized controlled trials (RCTs), said Gerardo Calderon, MD, in an interview during a poster session at Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. The combination was also the most commonly prescribed weight loss medication at the Mayo Clinic, where Dr. Calderon is a gastroenterology and hepatology research fellow.
Patients taking lorcaserin at the Mayo Clinic also lost more weight loss than RCT participants (8.8% vs. 5%-6%, respectively). Notably, they also had a higher probability of losing at least 10% of their baseline total body weight (40% vs. 17%-23% in clinical trials). In RCTs, 37%-48% of patients taking phentermine/topiramate-ER had a total body weight loss of at least 10%, similar to the Mayo Clinic’s figure of 49%.
The rate of reported adverse events – 23.8% – exceeded that reported in RCTs, noted Dr. Calderon. Gastrointestinal symptoms, such as nausea, vomiting, diarrhea, and constipation, were reported by 2%-20% of patients across the various drugs prescribed. Insomnia and mood changes, along with dizziness or lightheadedness, were reported by 2%-6% of patients. Almost 12% of patients taking phentermine/topiramate-SR reported paresthesias. No patients stopped taking their medication because of side effects, however.
The study was a review of patients seen at the Mayo Clinic during January 2016 – June 2018. Patients were included if they were prescribed weight loss medications and had a body mass index of at least 25 kg/m2 with comorbidities related to adiposity or with a BMI of at least 30 without such comorbidities. To be included, patients had to be followed for at least 3 months and see a Mayo Clinic physician at least twice.
Patients with previous bariatric surgery or other major gastrointestinal surgery, those who didn’t use their medications because of insurance problems or drug costs, and those who were on weight loss medication before being seen for the first time were excluded from the study.
Patients were a mean 49 years old, and most were female (86/118; 72.9%). Mean BMI at enrollment was 41.7, with a mean weight of 117.6 kg.
Of 118 patients, 76 (64.4%) had dyslipidemia. About half of patients reported obstructive sleep apnea, and the same amount had hypertension. About 40% had diabetes, and the same number had degenerative joint disease.
Phentermine/topiramate was prescribed the most frequently, with 43.2% of patients on this medication. Liraglutide was taken by 34.7% of patients, bupropion/naltrexone-SR by 16.1%, and lorcaserin by 5.9%.
Patients taking liraglutide had similar weight loss (7.1%) to that seen in RCTs (6%-8%). For this medication, the real-world Mayo Clinic experience showed less chance of hitting the 10% total body weight loss mark (12% vs. 26%-33% in RCTs).
For bupropion/naltrexone-SR, weight loss was similar among the Mayo Clinic patients (7.2%) and RCT participants (5%-8%), and probability of achieving at least 10% total body weight loss was similar as well (32% vs. 34%).
Weight loss medication was a component of a multidisciplinary weight loss approach at Mayo Clinic. Physicians, dietitians, and psychologists worked together to care for patients with overweight and obesity at his facility, Dr. Calderon said. Overall, patients were followed for a mean 6.7 months, and patients had a mean 3 follow-up visits, with over half of patients attending at least one follow-up appointment in study months 6-12. At 12 months, though, the attrition rate was 57.9%. “We notice an attrition rate of almost 60% at 1 year. People are not coming to their follow-up. ... Definitely, this is something we are concerned about, and we would like to bring these attrition rates lower,” he said.
Most patients (63.6%) saw a dietitian, but on average, patients had just one appointment. “At the Mayo Clinic, we provide dietitians and psychological support. But it’s up to the patients if they want to have it or not,” said Dr. Calderon. “Most of them, they just went once to a dietitian.”
“Overall, these outcomes are similar to those in RCTs and support the concept that weight loss medications can achieve clinically significant weight loss in a multidisciplinary weight loss program,” noted Dr. Calderon and his coauthors.
Dr. Calderon reported no outside sources of funding and no conflicts of interest.
SOURCE: Calderon G et al. Obesity Week, Abstract T-P-3425.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Patients taking weight loss medications saw real-world results comparable to those seen in RCTs
Major finding: Patients on phertermine-topiramate-ER lost over 15% of their body weight at 12 months.
Study details: Single-center retrospective cohort study of 188 patients taking weight loss medications.
Disclosures: The authors reported no conflicts of interest and no outside sources of funding.
Source: Calderon G et al. Obesity Week 2018, Abstract T-P-3425.
Apply for a VRIC Travel Scholarship
The Vascular Research Initiatives Conference emphasizes emerging vascular science and encourages interactive participation of attendees, including trainees who are aspiring academic vascular surgeons. The SVS Foundation offers the Vascular Research Initiatives Conference Trainee Travel Scholarship for trainees to attend the conference. The trainee authors of top-scoring abstracts will be considered for the award, which includes complimentary registration to VRIC as well as the American Heart Association's Vascular Discover Scientific Sessions, plus $1,000 for conference travel. Applications are accepted until Jan 15, 2019.
The Vascular Research Initiatives Conference emphasizes emerging vascular science and encourages interactive participation of attendees, including trainees who are aspiring academic vascular surgeons. The SVS Foundation offers the Vascular Research Initiatives Conference Trainee Travel Scholarship for trainees to attend the conference. The trainee authors of top-scoring abstracts will be considered for the award, which includes complimentary registration to VRIC as well as the American Heart Association's Vascular Discover Scientific Sessions, plus $1,000 for conference travel. Applications are accepted until Jan 15, 2019.
The Vascular Research Initiatives Conference emphasizes emerging vascular science and encourages interactive participation of attendees, including trainees who are aspiring academic vascular surgeons. The SVS Foundation offers the Vascular Research Initiatives Conference Trainee Travel Scholarship for trainees to attend the conference. The trainee authors of top-scoring abstracts will be considered for the award, which includes complimentary registration to VRIC as well as the American Heart Association's Vascular Discover Scientific Sessions, plus $1,000 for conference travel. Applications are accepted until Jan 15, 2019.
Learn about Workings of Washington
The workings of Congress and other governmental agencies frequently seem obscure and the impact of their decisions unclear. Yet collectively, these entities in Washington, D.C., wield huge power over vascular surgeons’ careers and lives. Do you have questions about what goes on in Washington and the efforts of SVS advocacy staff to shape legislation and policies on your behalf? Email questions to Mindi Walker at mwalker@vascularsociety.org.
The workings of Congress and other governmental agencies frequently seem obscure and the impact of their decisions unclear. Yet collectively, these entities in Washington, D.C., wield huge power over vascular surgeons’ careers and lives. Do you have questions about what goes on in Washington and the efforts of SVS advocacy staff to shape legislation and policies on your behalf? Email questions to Mindi Walker at mwalker@vascularsociety.org.
The workings of Congress and other governmental agencies frequently seem obscure and the impact of their decisions unclear. Yet collectively, these entities in Washington, D.C., wield huge power over vascular surgeons’ careers and lives. Do you have questions about what goes on in Washington and the efforts of SVS advocacy staff to shape legislation and policies on your behalf? Email questions to Mindi Walker at mwalker@vascularsociety.org.
Complete Survey on Regulatory Burdens
The SVS is working to ascertain members' experiences in meeting federal regulatory requirements, with responses due by Dec. 31. Approximately 135 members have already responded – join them. Read more and find the survey link here.
The SVS is working to ascertain members' experiences in meeting federal regulatory requirements, with responses due by Dec. 31. Approximately 135 members have already responded – join them. Read more and find the survey link here.
The SVS is working to ascertain members' experiences in meeting federal regulatory requirements, with responses due by Dec. 31. Approximately 135 members have already responded – join them. Read more and find the survey link here.
Beware “The Great Mimicker” that can lurk in the vulva
LAS VEGAS – Officially a type of precancerous lesion is known as vulvar intraepithelial neoplasia (VIN); unofficially, an obstetrician-gynecologist calls it something else: “The Great Mimicker.” That’s because symptoms of VIN can fool physicians into thinking they’re seeing other vulvar conditions. The good news: A biopsy can offer crucial insight and should be performed on any dysplastic or unusual lesion on the vulva.
Amanda Nickles Fader, MD, of Johns Hopkins Hospital in Baltimore, offered this advice and other tips about this type of precancerous vulvar lesion in a presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium.
According to Dr. Nickles Fader, vulvar cancer accounts for 5% of all gynecologic malignancies, and it appears most in women aged 65-75 years. However, about 15% of all vulvar cancers appear in women under the age of 40 years. “We’re seeing a greater number of premenopausal women with this condition, probably due to HPV [human papillomavirus],” she said, adding that HPV vaccines are crucial to prevention.
The VIN form of precancerous lesion is most common in premenopausal women (75%) and – like vulvar cancer – is linked to HPV infection, HIV infection, cigarette smoking, and weakened or suppressed immune systems, Dr. Nickles Faber said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
VIN presents with symptoms such as pruritus, altered vulvar appearance at the site of the lesion, palpable abnormality, and perineal pain or burning. About 40% of cases do not show symptoms and are diagnosed by gynecologists at annual visits.
It’s important to biopsy these lesions, she said, because they can mimic other conditions such as vulvar cancer, condyloma acuminatum (genital warts), lichen sclerosus, lichen planus, and condyloma latum (a lesion linked to syphilis).
“Biopsy, biopsy, biopsy,” she urged.
In fact, one form of VIN – differentiated VIN – is associated with dermatologic conditions such as lichen sclerosus, and treatment of these conditions can prevent development of this VIN type.
As for treatment, Dr. Nickles Faber said surgery is the mainstay. About 90% of the time, wide local excision is the “go-to” approach, although the skinning vulvectomy procedure may be appropriate in lesions that are more extensive or multifocal and confluent. “It’s a lot more disfiguring.”
Laser ablation is a “very reasonable” option when cancer has been eliminated as a possibility, she said. It may be appropriate in multifocal or extensive lesions and can have important cosmetic advantages when excision would be inappropriate.
Off-label use of imiquimod 5%, a topical immune response modifier, can be appropriate in multifocal high-grade VINs, but it’s crucial to exclude invasive squamous cell carcinoma. As she noted, imiquimod is Food and Drug Administration–approved for anogenital warts but not for VIN. Beware of toxicity over the long term.
Dr. Nickles Fader reported no relevant financial disclosures.
LAS VEGAS – Officially a type of precancerous lesion is known as vulvar intraepithelial neoplasia (VIN); unofficially, an obstetrician-gynecologist calls it something else: “The Great Mimicker.” That’s because symptoms of VIN can fool physicians into thinking they’re seeing other vulvar conditions. The good news: A biopsy can offer crucial insight and should be performed on any dysplastic or unusual lesion on the vulva.
Amanda Nickles Fader, MD, of Johns Hopkins Hospital in Baltimore, offered this advice and other tips about this type of precancerous vulvar lesion in a presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium.
According to Dr. Nickles Fader, vulvar cancer accounts for 5% of all gynecologic malignancies, and it appears most in women aged 65-75 years. However, about 15% of all vulvar cancers appear in women under the age of 40 years. “We’re seeing a greater number of premenopausal women with this condition, probably due to HPV [human papillomavirus],” she said, adding that HPV vaccines are crucial to prevention.
The VIN form of precancerous lesion is most common in premenopausal women (75%) and – like vulvar cancer – is linked to HPV infection, HIV infection, cigarette smoking, and weakened or suppressed immune systems, Dr. Nickles Faber said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
VIN presents with symptoms such as pruritus, altered vulvar appearance at the site of the lesion, palpable abnormality, and perineal pain or burning. About 40% of cases do not show symptoms and are diagnosed by gynecologists at annual visits.
It’s important to biopsy these lesions, she said, because they can mimic other conditions such as vulvar cancer, condyloma acuminatum (genital warts), lichen sclerosus, lichen planus, and condyloma latum (a lesion linked to syphilis).
“Biopsy, biopsy, biopsy,” she urged.
In fact, one form of VIN – differentiated VIN – is associated with dermatologic conditions such as lichen sclerosus, and treatment of these conditions can prevent development of this VIN type.
As for treatment, Dr. Nickles Faber said surgery is the mainstay. About 90% of the time, wide local excision is the “go-to” approach, although the skinning vulvectomy procedure may be appropriate in lesions that are more extensive or multifocal and confluent. “It’s a lot more disfiguring.”
Laser ablation is a “very reasonable” option when cancer has been eliminated as a possibility, she said. It may be appropriate in multifocal or extensive lesions and can have important cosmetic advantages when excision would be inappropriate.
Off-label use of imiquimod 5%, a topical immune response modifier, can be appropriate in multifocal high-grade VINs, but it’s crucial to exclude invasive squamous cell carcinoma. As she noted, imiquimod is Food and Drug Administration–approved for anogenital warts but not for VIN. Beware of toxicity over the long term.
Dr. Nickles Fader reported no relevant financial disclosures.
LAS VEGAS – Officially a type of precancerous lesion is known as vulvar intraepithelial neoplasia (VIN); unofficially, an obstetrician-gynecologist calls it something else: “The Great Mimicker.” That’s because symptoms of VIN can fool physicians into thinking they’re seeing other vulvar conditions. The good news: A biopsy can offer crucial insight and should be performed on any dysplastic or unusual lesion on the vulva.
Amanda Nickles Fader, MD, of Johns Hopkins Hospital in Baltimore, offered this advice and other tips about this type of precancerous vulvar lesion in a presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium.
According to Dr. Nickles Fader, vulvar cancer accounts for 5% of all gynecologic malignancies, and it appears most in women aged 65-75 years. However, about 15% of all vulvar cancers appear in women under the age of 40 years. “We’re seeing a greater number of premenopausal women with this condition, probably due to HPV [human papillomavirus],” she said, adding that HPV vaccines are crucial to prevention.
The VIN form of precancerous lesion is most common in premenopausal women (75%) and – like vulvar cancer – is linked to HPV infection, HIV infection, cigarette smoking, and weakened or suppressed immune systems, Dr. Nickles Faber said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
VIN presents with symptoms such as pruritus, altered vulvar appearance at the site of the lesion, palpable abnormality, and perineal pain or burning. About 40% of cases do not show symptoms and are diagnosed by gynecologists at annual visits.
It’s important to biopsy these lesions, she said, because they can mimic other conditions such as vulvar cancer, condyloma acuminatum (genital warts), lichen sclerosus, lichen planus, and condyloma latum (a lesion linked to syphilis).
“Biopsy, biopsy, biopsy,” she urged.
In fact, one form of VIN – differentiated VIN – is associated with dermatologic conditions such as lichen sclerosus, and treatment of these conditions can prevent development of this VIN type.
As for treatment, Dr. Nickles Faber said surgery is the mainstay. About 90% of the time, wide local excision is the “go-to” approach, although the skinning vulvectomy procedure may be appropriate in lesions that are more extensive or multifocal and confluent. “It’s a lot more disfiguring.”
Laser ablation is a “very reasonable” option when cancer has been eliminated as a possibility, she said. It may be appropriate in multifocal or extensive lesions and can have important cosmetic advantages when excision would be inappropriate.
Off-label use of imiquimod 5%, a topical immune response modifier, can be appropriate in multifocal high-grade VINs, but it’s crucial to exclude invasive squamous cell carcinoma. As she noted, imiquimod is Food and Drug Administration–approved for anogenital warts but not for VIN. Beware of toxicity over the long term.
Dr. Nickles Fader reported no relevant financial disclosures.
EXPERT ANALYSIS FROM PAGS
How does CBD compare and interact with other AEDs?
according to a review published in Developmental Medicine & Child Neurology. “Careful down-titration of benzodiazepines is essential to minimize sedation with adjunctive CBD,” the authors said.
Although CBD’s antiepileptic mechanisms “are not fully elucidated, it is clear that administration of CBD as adjunct therapy decreases seizure frequency in patients with Dravet syndrome and Lennox-Gastaut syndrome,” wrote Shayma Ali, a doctoral student in the department of pediatrics and child health at the University of Otago in Wellington, New Zealand, and her colleagues. “Contrary to public expectation of miraculous results, CBD has a similar antiepileptic and side effect profile to other AEDs. Nevertheless, as individual children with these developmental and epileptic encephalopathies are often refractory to available AEDs, the addition of another potentially effective therapeutic medicine will be warmly welcomed by families and physicians.”
The FDA approved Epidiolex, a pharmaceutical-grade oral solution that is 98% CBD, in June of 2018. In September of 2018, the Drug Enforcement Administration classified it as a Schedule V controlled substance. Patients’ use of nonpharmaceutical grade CBD products, including those combined with tetrahydrocannabinol (THC), “raises concerns about the use of products with THC on the developing brain,” the review authors said.
Randomized trials
Three randomized, controlled, double-blind trials in patients with Dravet syndrome and Lennox-Gastaut syndrome found that CBD, compared with placebo, results in greater median seizure reductions (38%-41% vs. 13%-19%) and responder rates (i.e., the proportion of patients with 50% reductions in convulsive or drop seizures; 39%-46% vs. 14%-27%).
Common adverse effects include somnolence, diarrhea, decreased appetite, fatigue, lethargy, pyrexia, and vomiting. Hepatic transaminases became elevated in some patients, and this result occurred more often in patients taking valproate.
No phase 2 or phase 3 trials have assessed the efficacy of CBD without coadministration of other AEDs, and CBD’s efficacy may relate to its impact on the pharmacokinetics of coadministered AEDs. “The most important clinical interaction is between CBD and clobazam, as [the dose of] clobazam often needs to be lowered because of excessive sedation,” wrote Ms. Ali and her colleagues. CBD inhibits CYP2C19 and CYP3A4 – enzymes that are involved in clobazam metabolism – which results in high plasma concentrations of clobazam’s active metabolite, norclobazam. Plasma levels of topiramate, rufinamide, zonisamide, and eslicarbazepine also may increase when these drugs are taken with CBD.
Challenges and opportunities
Of the hundreds of compounds in the marijuana plant, CBD “has the most evidence of antiepileptic efficacy and does not have the psychoactive effects” of THC, the authors said. Little evidence supports the combination of THC and CBD for the treatment of epilepsy. In addition, research indicates that THC can have a proconvulsive effect in animal models and harm the development of the human brain.
Investigators are evaluating alternative routes of CBD delivery to avoid first-pass metabolism, such as oromucosal sprays, transdermal gels, eye drops, intranasal sprays, and rectal suppositories. “Alternative methods of administration ... deserve consideration, particularly for the developmental and epileptic encephalopathies population, as administration of oral medication can be challenging,” they said.
SOURCE: Ali S et al. Dev Med Child Neurol. 2018. doi: 10.1111/dmcn.14087.
according to a review published in Developmental Medicine & Child Neurology. “Careful down-titration of benzodiazepines is essential to minimize sedation with adjunctive CBD,” the authors said.
Although CBD’s antiepileptic mechanisms “are not fully elucidated, it is clear that administration of CBD as adjunct therapy decreases seizure frequency in patients with Dravet syndrome and Lennox-Gastaut syndrome,” wrote Shayma Ali, a doctoral student in the department of pediatrics and child health at the University of Otago in Wellington, New Zealand, and her colleagues. “Contrary to public expectation of miraculous results, CBD has a similar antiepileptic and side effect profile to other AEDs. Nevertheless, as individual children with these developmental and epileptic encephalopathies are often refractory to available AEDs, the addition of another potentially effective therapeutic medicine will be warmly welcomed by families and physicians.”
The FDA approved Epidiolex, a pharmaceutical-grade oral solution that is 98% CBD, in June of 2018. In September of 2018, the Drug Enforcement Administration classified it as a Schedule V controlled substance. Patients’ use of nonpharmaceutical grade CBD products, including those combined with tetrahydrocannabinol (THC), “raises concerns about the use of products with THC on the developing brain,” the review authors said.
Randomized trials
Three randomized, controlled, double-blind trials in patients with Dravet syndrome and Lennox-Gastaut syndrome found that CBD, compared with placebo, results in greater median seizure reductions (38%-41% vs. 13%-19%) and responder rates (i.e., the proportion of patients with 50% reductions in convulsive or drop seizures; 39%-46% vs. 14%-27%).
Common adverse effects include somnolence, diarrhea, decreased appetite, fatigue, lethargy, pyrexia, and vomiting. Hepatic transaminases became elevated in some patients, and this result occurred more often in patients taking valproate.
No phase 2 or phase 3 trials have assessed the efficacy of CBD without coadministration of other AEDs, and CBD’s efficacy may relate to its impact on the pharmacokinetics of coadministered AEDs. “The most important clinical interaction is between CBD and clobazam, as [the dose of] clobazam often needs to be lowered because of excessive sedation,” wrote Ms. Ali and her colleagues. CBD inhibits CYP2C19 and CYP3A4 – enzymes that are involved in clobazam metabolism – which results in high plasma concentrations of clobazam’s active metabolite, norclobazam. Plasma levels of topiramate, rufinamide, zonisamide, and eslicarbazepine also may increase when these drugs are taken with CBD.
Challenges and opportunities
Of the hundreds of compounds in the marijuana plant, CBD “has the most evidence of antiepileptic efficacy and does not have the psychoactive effects” of THC, the authors said. Little evidence supports the combination of THC and CBD for the treatment of epilepsy. In addition, research indicates that THC can have a proconvulsive effect in animal models and harm the development of the human brain.
Investigators are evaluating alternative routes of CBD delivery to avoid first-pass metabolism, such as oromucosal sprays, transdermal gels, eye drops, intranasal sprays, and rectal suppositories. “Alternative methods of administration ... deserve consideration, particularly for the developmental and epileptic encephalopathies population, as administration of oral medication can be challenging,” they said.
SOURCE: Ali S et al. Dev Med Child Neurol. 2018. doi: 10.1111/dmcn.14087.
according to a review published in Developmental Medicine & Child Neurology. “Careful down-titration of benzodiazepines is essential to minimize sedation with adjunctive CBD,” the authors said.
Although CBD’s antiepileptic mechanisms “are not fully elucidated, it is clear that administration of CBD as adjunct therapy decreases seizure frequency in patients with Dravet syndrome and Lennox-Gastaut syndrome,” wrote Shayma Ali, a doctoral student in the department of pediatrics and child health at the University of Otago in Wellington, New Zealand, and her colleagues. “Contrary to public expectation of miraculous results, CBD has a similar antiepileptic and side effect profile to other AEDs. Nevertheless, as individual children with these developmental and epileptic encephalopathies are often refractory to available AEDs, the addition of another potentially effective therapeutic medicine will be warmly welcomed by families and physicians.”
The FDA approved Epidiolex, a pharmaceutical-grade oral solution that is 98% CBD, in June of 2018. In September of 2018, the Drug Enforcement Administration classified it as a Schedule V controlled substance. Patients’ use of nonpharmaceutical grade CBD products, including those combined with tetrahydrocannabinol (THC), “raises concerns about the use of products with THC on the developing brain,” the review authors said.
Randomized trials
Three randomized, controlled, double-blind trials in patients with Dravet syndrome and Lennox-Gastaut syndrome found that CBD, compared with placebo, results in greater median seizure reductions (38%-41% vs. 13%-19%) and responder rates (i.e., the proportion of patients with 50% reductions in convulsive or drop seizures; 39%-46% vs. 14%-27%).
Common adverse effects include somnolence, diarrhea, decreased appetite, fatigue, lethargy, pyrexia, and vomiting. Hepatic transaminases became elevated in some patients, and this result occurred more often in patients taking valproate.
No phase 2 or phase 3 trials have assessed the efficacy of CBD without coadministration of other AEDs, and CBD’s efficacy may relate to its impact on the pharmacokinetics of coadministered AEDs. “The most important clinical interaction is between CBD and clobazam, as [the dose of] clobazam often needs to be lowered because of excessive sedation,” wrote Ms. Ali and her colleagues. CBD inhibits CYP2C19 and CYP3A4 – enzymes that are involved in clobazam metabolism – which results in high plasma concentrations of clobazam’s active metabolite, norclobazam. Plasma levels of topiramate, rufinamide, zonisamide, and eslicarbazepine also may increase when these drugs are taken with CBD.
Challenges and opportunities
Of the hundreds of compounds in the marijuana plant, CBD “has the most evidence of antiepileptic efficacy and does not have the psychoactive effects” of THC, the authors said. Little evidence supports the combination of THC and CBD for the treatment of epilepsy. In addition, research indicates that THC can have a proconvulsive effect in animal models and harm the development of the human brain.
Investigators are evaluating alternative routes of CBD delivery to avoid first-pass metabolism, such as oromucosal sprays, transdermal gels, eye drops, intranasal sprays, and rectal suppositories. “Alternative methods of administration ... deserve consideration, particularly for the developmental and epileptic encephalopathies population, as administration of oral medication can be challenging,” they said.
SOURCE: Ali S et al. Dev Med Child Neurol. 2018. doi: 10.1111/dmcn.14087.
FROM DEVELOPMENTAL MEDICINE & CHILD NEUROLOGY
Key clinical point: Cannabidiol’s efficacy is similar to that of other antiepileptic drugs.
Major finding: Cannabidiol inhibits CYP2C19 and CYP3A4, which are involved in clobazam metabolism.
Study details: An invited review.
Disclosures: No disclosures were reported.
Source: Ali S et al. Dev Med Child Neurol. 2018. doi: 10.1111/dmcn.14087.
Frontline veliparib/cisplatin/etoposide shows efficacy in advanced SCLC
For patients with extensive-stage small-cell lung cancer (ES-SCLC), the addition of the poly (ADP ribose) polymerase (PARP) inhibitor veliparib to frontline chemotherapy with cisplatin and etoposide resulted in a slight but significant improvement in progression-free survival but not overall survival, compared with cisplatin/etoposide alone, investigators reported in the Journal of Clinical Oncology.
Among 128 patients with newly diagnosed ES-SCLC, the median progression-free survival (PFS; the primary endpoint) for those randomized to veliparib/cisplatin/etoposide was 6.1 months, compared with 5.5 months for patients randomized to cisplatin/etoposide alone.
This translated into an unstratified hazard ratio for PFS with veliparib of 0.75 (one-sided P = .06) and a stratified HR of 0.63 (one-sided P = .01), reported Taofeek K. Owonikoko, MD, PhD, and his colleagues at Emory University in Atlanta.
“Although the initial result of our study is promising, additional confirmation in a larger definitive study is warranted, given the mixed results reported by other studies of PARP inhibitors in this patient population,” they wrote.
Median overall survival (OS) with cisplatin/etoposide in ES-SCLC is approximately 9-11 months, and fewer than 5% of patients survive out to 5 years. To see whether the addition of a PARP inhibitor to the standard of care could improve outcomes, the investigators first demonstrated in a phase 1 trial that the combination of veliparib with a platinum doublet of cisplatin and etoposide was safe (Lung Cancer. 2015 Jul;89[1]:66-70), and in the current study, they evaluated efficacy.
A total of 128 eligible patients (median age, 66 years; 52% men) with ES-SCLC were included in the analysis. Extensive-stage disease was defined as the presence of extrathoracic metastatic disease, malignant pleural effusion, and bilateral or contralateral supraclavicular adenopathy.
The patients were stratified by sex and serum lactate dehydrogenase (LDH) levels and then randomized to receive a maximum of four 3-week cycles of of cisplatin 75 mg/m2 intravenously on day 1 and etoposide 100 mg/m2 on days 1 through 3, plus either oral veliparib 100 mg twice daily on days 1 through 7 or placebo.
The primary endpoint of PFS was as noted before. Median overall survival was 10.3 months in the veliparib arm versus 8.9 months in the cisplatin/etoposide alone arm, a difference that was not statistically significant. The respective overall response rates were 71.9% vs. 65.6%, but this difference was also not significant.
Looking at the treatment effect by strata, the investigators found that men with high serum LDH levels had a significant PFS benefit with veliparib (HR, 0.34; one-sided P less than .001), but no significant benefit was seen in men with normal LDH or in women regardless of LDH status.
Grade 3 or greater hematologic toxicities that occurred more frequently in the veliparib arm included CD4 lymphopenia in 8% vs. 0% and neutropenia in 49% vs. 32%.
The investigators noted that the discrepancy between the magnitude of the risk reduction as measured by the hazard ratio and the actual, modest difference in median PFS between the study arms may be explained by the fact that men with elevated LDH represented the largest patient strata.
“Therefore, we hypothesize that this cohort probably contained a sufficient proportion of patients with SCLC who harbored some biologic vulnerability to this therapeutic strategy,” they wrote.
They acknowledged that although toxicities were higher with veliparib combination, the ability to deliver chemotherapy was equal between the arms.
Further exploration of the combination of veliparib/cisplatin/etoposide may be justified if results of another ongoing phase 2 study (NCT02289690) of a carboplatin-based chemotherapy doublet plus veliparib has similar efficacy results, Dr. Owonikoko and his associates concluded.
The study was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group and supported by the National Cancer Institute. Dr. Owonikoko disclosed a consulting or advisory role with AbbVie, developer of veliparib, and other companies, as well as institutional research funding from AbbVie and others.
SOURCE: Owonikoko TK et al. J Clin Oncol. 2018 Dec 5. doi: 10.1200/JCO.18.00264.
For patients with extensive-stage small-cell lung cancer (ES-SCLC), the addition of the poly (ADP ribose) polymerase (PARP) inhibitor veliparib to frontline chemotherapy with cisplatin and etoposide resulted in a slight but significant improvement in progression-free survival but not overall survival, compared with cisplatin/etoposide alone, investigators reported in the Journal of Clinical Oncology.
Among 128 patients with newly diagnosed ES-SCLC, the median progression-free survival (PFS; the primary endpoint) for those randomized to veliparib/cisplatin/etoposide was 6.1 months, compared with 5.5 months for patients randomized to cisplatin/etoposide alone.
This translated into an unstratified hazard ratio for PFS with veliparib of 0.75 (one-sided P = .06) and a stratified HR of 0.63 (one-sided P = .01), reported Taofeek K. Owonikoko, MD, PhD, and his colleagues at Emory University in Atlanta.
“Although the initial result of our study is promising, additional confirmation in a larger definitive study is warranted, given the mixed results reported by other studies of PARP inhibitors in this patient population,” they wrote.
Median overall survival (OS) with cisplatin/etoposide in ES-SCLC is approximately 9-11 months, and fewer than 5% of patients survive out to 5 years. To see whether the addition of a PARP inhibitor to the standard of care could improve outcomes, the investigators first demonstrated in a phase 1 trial that the combination of veliparib with a platinum doublet of cisplatin and etoposide was safe (Lung Cancer. 2015 Jul;89[1]:66-70), and in the current study, they evaluated efficacy.
A total of 128 eligible patients (median age, 66 years; 52% men) with ES-SCLC were included in the analysis. Extensive-stage disease was defined as the presence of extrathoracic metastatic disease, malignant pleural effusion, and bilateral or contralateral supraclavicular adenopathy.
The patients were stratified by sex and serum lactate dehydrogenase (LDH) levels and then randomized to receive a maximum of four 3-week cycles of of cisplatin 75 mg/m2 intravenously on day 1 and etoposide 100 mg/m2 on days 1 through 3, plus either oral veliparib 100 mg twice daily on days 1 through 7 or placebo.
The primary endpoint of PFS was as noted before. Median overall survival was 10.3 months in the veliparib arm versus 8.9 months in the cisplatin/etoposide alone arm, a difference that was not statistically significant. The respective overall response rates were 71.9% vs. 65.6%, but this difference was also not significant.
Looking at the treatment effect by strata, the investigators found that men with high serum LDH levels had a significant PFS benefit with veliparib (HR, 0.34; one-sided P less than .001), but no significant benefit was seen in men with normal LDH or in women regardless of LDH status.
Grade 3 or greater hematologic toxicities that occurred more frequently in the veliparib arm included CD4 lymphopenia in 8% vs. 0% and neutropenia in 49% vs. 32%.
The investigators noted that the discrepancy between the magnitude of the risk reduction as measured by the hazard ratio and the actual, modest difference in median PFS between the study arms may be explained by the fact that men with elevated LDH represented the largest patient strata.
“Therefore, we hypothesize that this cohort probably contained a sufficient proportion of patients with SCLC who harbored some biologic vulnerability to this therapeutic strategy,” they wrote.
They acknowledged that although toxicities were higher with veliparib combination, the ability to deliver chemotherapy was equal between the arms.
Further exploration of the combination of veliparib/cisplatin/etoposide may be justified if results of another ongoing phase 2 study (NCT02289690) of a carboplatin-based chemotherapy doublet plus veliparib has similar efficacy results, Dr. Owonikoko and his associates concluded.
The study was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group and supported by the National Cancer Institute. Dr. Owonikoko disclosed a consulting or advisory role with AbbVie, developer of veliparib, and other companies, as well as institutional research funding from AbbVie and others.
SOURCE: Owonikoko TK et al. J Clin Oncol. 2018 Dec 5. doi: 10.1200/JCO.18.00264.
For patients with extensive-stage small-cell lung cancer (ES-SCLC), the addition of the poly (ADP ribose) polymerase (PARP) inhibitor veliparib to frontline chemotherapy with cisplatin and etoposide resulted in a slight but significant improvement in progression-free survival but not overall survival, compared with cisplatin/etoposide alone, investigators reported in the Journal of Clinical Oncology.
Among 128 patients with newly diagnosed ES-SCLC, the median progression-free survival (PFS; the primary endpoint) for those randomized to veliparib/cisplatin/etoposide was 6.1 months, compared with 5.5 months for patients randomized to cisplatin/etoposide alone.
This translated into an unstratified hazard ratio for PFS with veliparib of 0.75 (one-sided P = .06) and a stratified HR of 0.63 (one-sided P = .01), reported Taofeek K. Owonikoko, MD, PhD, and his colleagues at Emory University in Atlanta.
“Although the initial result of our study is promising, additional confirmation in a larger definitive study is warranted, given the mixed results reported by other studies of PARP inhibitors in this patient population,” they wrote.
Median overall survival (OS) with cisplatin/etoposide in ES-SCLC is approximately 9-11 months, and fewer than 5% of patients survive out to 5 years. To see whether the addition of a PARP inhibitor to the standard of care could improve outcomes, the investigators first demonstrated in a phase 1 trial that the combination of veliparib with a platinum doublet of cisplatin and etoposide was safe (Lung Cancer. 2015 Jul;89[1]:66-70), and in the current study, they evaluated efficacy.
A total of 128 eligible patients (median age, 66 years; 52% men) with ES-SCLC were included in the analysis. Extensive-stage disease was defined as the presence of extrathoracic metastatic disease, malignant pleural effusion, and bilateral or contralateral supraclavicular adenopathy.
The patients were stratified by sex and serum lactate dehydrogenase (LDH) levels and then randomized to receive a maximum of four 3-week cycles of of cisplatin 75 mg/m2 intravenously on day 1 and etoposide 100 mg/m2 on days 1 through 3, plus either oral veliparib 100 mg twice daily on days 1 through 7 or placebo.
The primary endpoint of PFS was as noted before. Median overall survival was 10.3 months in the veliparib arm versus 8.9 months in the cisplatin/etoposide alone arm, a difference that was not statistically significant. The respective overall response rates were 71.9% vs. 65.6%, but this difference was also not significant.
Looking at the treatment effect by strata, the investigators found that men with high serum LDH levels had a significant PFS benefit with veliparib (HR, 0.34; one-sided P less than .001), but no significant benefit was seen in men with normal LDH or in women regardless of LDH status.
Grade 3 or greater hematologic toxicities that occurred more frequently in the veliparib arm included CD4 lymphopenia in 8% vs. 0% and neutropenia in 49% vs. 32%.
The investigators noted that the discrepancy between the magnitude of the risk reduction as measured by the hazard ratio and the actual, modest difference in median PFS between the study arms may be explained by the fact that men with elevated LDH represented the largest patient strata.
“Therefore, we hypothesize that this cohort probably contained a sufficient proportion of patients with SCLC who harbored some biologic vulnerability to this therapeutic strategy,” they wrote.
They acknowledged that although toxicities were higher with veliparib combination, the ability to deliver chemotherapy was equal between the arms.
Further exploration of the combination of veliparib/cisplatin/etoposide may be justified if results of another ongoing phase 2 study (NCT02289690) of a carboplatin-based chemotherapy doublet plus veliparib has similar efficacy results, Dr. Owonikoko and his associates concluded.
The study was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group and supported by the National Cancer Institute. Dr. Owonikoko disclosed a consulting or advisory role with AbbVie, developer of veliparib, and other companies, as well as institutional research funding from AbbVie and others.
SOURCE: Owonikoko TK et al. J Clin Oncol. 2018 Dec 5. doi: 10.1200/JCO.18.00264.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Adding a PARP inhibitor to standard chemotherapy may improve progression-free survival in patients with extensive-stage small-cell lung cancer (ES-SCLC).
Major finding: The stratified hazard ratio for PFS with veliparib/cisplatin/etoposide was 0.63 (one-sided P = .01).
Study details: Randomized phase 2 trial in 128 patients with newly diagnosed ES-SCLC.
Disclosures: The study was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group and supported by the National Cancer Institute. Dr. Owonikoko disclosed a consulting or advisory role with AbbVie, the developer of veliparib, and other companies, as well as institutional research funding from AbbVie and others.
Source: Owonikoko TK et al. J Clin Oncol. 2018 Dec 5. doi: 10.1200/JCO.18.00264.
CLL at ASH: A ‘mountain of data’ for targeted therapies
SAN DIEGO – There was a mountain of data presented at the annual meeting of the American Society of Hematology on the use of novel agents – both as frontline therapy and in combination – for the treatment of chronic lymphocytic leukemia (CLL).
In a video interview at the meeting, Brian T. Hill, MD, PhD, of the Cleveland Clinic and Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center, New York, summed up the key studies and what they mean in practice. They also looked ahead at what data are still missing that could aid in making important treatment decisions.
Dr. Hill highlighted the late-breaking abstract on the ECOG-ACRIN Cancer Research Group E1912 trial comparing ibrutinib-rituximab to a chemotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR) in previously untreated patients under age 70 years (Abstract LBA-4). Not only was there a progression-free survival benefit with the use of the ibrutinib regimen, but there was an overall survival benefit as well, he noted.
Dr. Mato pointed to notable results from the Alliance A041202 trial of older patients with previously untreated disease that compared ibrutinib alone or in combination with rituximab, with bendamustine plus rituximab (Abstract #6). The ibrutinib-containing regimens resulted in superior progression-free survival.
The two trials taken together show a movement away from chemotherapy in the frontline setting and toward targeted agents for CLL, Dr. Mato said. “What that agent or combination of agents will be, remains to be seen,” he said. “We have now a real message about the fact that we’re ending, potentially, the era of chemotherapy for patients with CLL, which is a very welcome change.”
Dr. Mato and Dr. Hill will be discussing these trials and more CLL data during a Twitter chat on Jan. 31, 2019, from 7 p.m. to 8 p.m. EST. Join in the conversation by using and following #MDedgeChats.
SAN DIEGO – There was a mountain of data presented at the annual meeting of the American Society of Hematology on the use of novel agents – both as frontline therapy and in combination – for the treatment of chronic lymphocytic leukemia (CLL).
In a video interview at the meeting, Brian T. Hill, MD, PhD, of the Cleveland Clinic and Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center, New York, summed up the key studies and what they mean in practice. They also looked ahead at what data are still missing that could aid in making important treatment decisions.
Dr. Hill highlighted the late-breaking abstract on the ECOG-ACRIN Cancer Research Group E1912 trial comparing ibrutinib-rituximab to a chemotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR) in previously untreated patients under age 70 years (Abstract LBA-4). Not only was there a progression-free survival benefit with the use of the ibrutinib regimen, but there was an overall survival benefit as well, he noted.
Dr. Mato pointed to notable results from the Alliance A041202 trial of older patients with previously untreated disease that compared ibrutinib alone or in combination with rituximab, with bendamustine plus rituximab (Abstract #6). The ibrutinib-containing regimens resulted in superior progression-free survival.
The two trials taken together show a movement away from chemotherapy in the frontline setting and toward targeted agents for CLL, Dr. Mato said. “What that agent or combination of agents will be, remains to be seen,” he said. “We have now a real message about the fact that we’re ending, potentially, the era of chemotherapy for patients with CLL, which is a very welcome change.”
Dr. Mato and Dr. Hill will be discussing these trials and more CLL data during a Twitter chat on Jan. 31, 2019, from 7 p.m. to 8 p.m. EST. Join in the conversation by using and following #MDedgeChats.
SAN DIEGO – There was a mountain of data presented at the annual meeting of the American Society of Hematology on the use of novel agents – both as frontline therapy and in combination – for the treatment of chronic lymphocytic leukemia (CLL).
In a video interview at the meeting, Brian T. Hill, MD, PhD, of the Cleveland Clinic and Anthony Mato, MD, of Memorial Sloan Kettering Cancer Center, New York, summed up the key studies and what they mean in practice. They also looked ahead at what data are still missing that could aid in making important treatment decisions.
Dr. Hill highlighted the late-breaking abstract on the ECOG-ACRIN Cancer Research Group E1912 trial comparing ibrutinib-rituximab to a chemotherapy regimen of fludarabine, cyclophosphamide, and rituximab (FCR) in previously untreated patients under age 70 years (Abstract LBA-4). Not only was there a progression-free survival benefit with the use of the ibrutinib regimen, but there was an overall survival benefit as well, he noted.
Dr. Mato pointed to notable results from the Alliance A041202 trial of older patients with previously untreated disease that compared ibrutinib alone or in combination with rituximab, with bendamustine plus rituximab (Abstract #6). The ibrutinib-containing regimens resulted in superior progression-free survival.
The two trials taken together show a movement away from chemotherapy in the frontline setting and toward targeted agents for CLL, Dr. Mato said. “What that agent or combination of agents will be, remains to be seen,” he said. “We have now a real message about the fact that we’re ending, potentially, the era of chemotherapy for patients with CLL, which is a very welcome change.”
Dr. Mato and Dr. Hill will be discussing these trials and more CLL data during a Twitter chat on Jan. 31, 2019, from 7 p.m. to 8 p.m. EST. Join in the conversation by using and following #MDedgeChats.
REPORTING FROM ASH 2018
For weight-loss apps, the evidence base is still small
NASHVILLE –
Beginning with a pool of 1,380 publications, Christina Hopkins and her colleagues at Duke University, Durham, N.C., eventually identified just nine trials of all-digital interventions for weight loss that met their inclusion criteria.
Presenting their findings at a late-breaking poster session during Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery, Ms. Hopkins, a clinical psychology graduate student at Duke, and her colleagues found that three of the nine studies showed statistically significant weight loss, compared with a control state. Absolute weight loss in these three trials ranged from 3 kg to about 7 kg (between-group differences, P less than .001 for all).
Participants in another trial didn’t lose a statistically significant amount of weight, compared with the control arm of the study. However, the mean 5 kg lost by those in the intervention arm was enough to be clinically significant, so Ms. Hopkins and her colleagues included this study in a subanalysis that looked for effective modalities and interventions among the studies with significant results.
The duration of the studies ranged from 6 to 24 months, though five of the trials were less than 1 year long. Women made up the majority of participants in all but one trial.
“There is limited evidence that standalone digital weight-loss interventions produce clinically meaningful outcomes,” wrote Ms. Hopkins and her coauthors. “Absolute magnitude of weight loss was low, and the short intervention lengths call into question the sustainability of these weight losses.”
The systematic review cast a broad net to include digital modalities such as wireless scales, text messaging, email, and web-based interventions, as well as the use of smartphone apps and tracking devices. All interventions used multiple digital modalities.
The most frequently used technologies were the use of a website, used in six (67%) of the trials, followed by text messaging and smartphone apps, each used in five (56%) of the trials. Tracking devices, email, message boards, and gamification of some sort were all used in three (33%) of the trials.
In terms of the specific interventions used in the trials, weight, diet, and activity were all tracked in eight trials (89%). Similarly, all but one trial gave feedback and weight and health education to participants. Behavior change education, as well as calorie goals, were each used in six trials (67%).
Ms. Hopkins and her colleagues looked at which trials incorporated which modalities and interventions, finding that “trials that integrated components unique to digital interventions, such as gamification, podcasts, or interactive features, yielded significantly greater and more clinically meaningful weight losses.”
To be included in the systematic review, trials had to include adult participants with a body mass index of at least 25 kg/m2 and use a standalone digital intervention of at least 6 months’ duration. The primary outcome of interest in the review was the change in participant weight from baseline to the end of the minimum 6-month follow-up period. Randomized, controlled trials and feasibility trials were included, so long as participants were allocated randomly.
Of the 126 trials reviewed at the full text level, the most frequent reason for exclusion was the inclusion of human coaching. Also, 30 of the trials didn’t report weight change as an outcome, the investigators said.
Future directions should include comparing digital interventions that “utilize features unique to digital delivery” with those that more closely resemble in-person weight-loss management interventions, suggested Ms. Hopkins and her collaborators.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Hopkins C et al. Obesity Week 2018, Abstract T-P-LB-3640.
NASHVILLE –
Beginning with a pool of 1,380 publications, Christina Hopkins and her colleagues at Duke University, Durham, N.C., eventually identified just nine trials of all-digital interventions for weight loss that met their inclusion criteria.
Presenting their findings at a late-breaking poster session during Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery, Ms. Hopkins, a clinical psychology graduate student at Duke, and her colleagues found that three of the nine studies showed statistically significant weight loss, compared with a control state. Absolute weight loss in these three trials ranged from 3 kg to about 7 kg (between-group differences, P less than .001 for all).
Participants in another trial didn’t lose a statistically significant amount of weight, compared with the control arm of the study. However, the mean 5 kg lost by those in the intervention arm was enough to be clinically significant, so Ms. Hopkins and her colleagues included this study in a subanalysis that looked for effective modalities and interventions among the studies with significant results.
The duration of the studies ranged from 6 to 24 months, though five of the trials were less than 1 year long. Women made up the majority of participants in all but one trial.
“There is limited evidence that standalone digital weight-loss interventions produce clinically meaningful outcomes,” wrote Ms. Hopkins and her coauthors. “Absolute magnitude of weight loss was low, and the short intervention lengths call into question the sustainability of these weight losses.”
The systematic review cast a broad net to include digital modalities such as wireless scales, text messaging, email, and web-based interventions, as well as the use of smartphone apps and tracking devices. All interventions used multiple digital modalities.
The most frequently used technologies were the use of a website, used in six (67%) of the trials, followed by text messaging and smartphone apps, each used in five (56%) of the trials. Tracking devices, email, message boards, and gamification of some sort were all used in three (33%) of the trials.
In terms of the specific interventions used in the trials, weight, diet, and activity were all tracked in eight trials (89%). Similarly, all but one trial gave feedback and weight and health education to participants. Behavior change education, as well as calorie goals, were each used in six trials (67%).
Ms. Hopkins and her colleagues looked at which trials incorporated which modalities and interventions, finding that “trials that integrated components unique to digital interventions, such as gamification, podcasts, or interactive features, yielded significantly greater and more clinically meaningful weight losses.”
To be included in the systematic review, trials had to include adult participants with a body mass index of at least 25 kg/m2 and use a standalone digital intervention of at least 6 months’ duration. The primary outcome of interest in the review was the change in participant weight from baseline to the end of the minimum 6-month follow-up period. Randomized, controlled trials and feasibility trials were included, so long as participants were allocated randomly.
Of the 126 trials reviewed at the full text level, the most frequent reason for exclusion was the inclusion of human coaching. Also, 30 of the trials didn’t report weight change as an outcome, the investigators said.
Future directions should include comparing digital interventions that “utilize features unique to digital delivery” with those that more closely resemble in-person weight-loss management interventions, suggested Ms. Hopkins and her collaborators.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Hopkins C et al. Obesity Week 2018, Abstract T-P-LB-3640.
NASHVILLE –
Beginning with a pool of 1,380 publications, Christina Hopkins and her colleagues at Duke University, Durham, N.C., eventually identified just nine trials of all-digital interventions for weight loss that met their inclusion criteria.
Presenting their findings at a late-breaking poster session during Obesity Week, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery, Ms. Hopkins, a clinical psychology graduate student at Duke, and her colleagues found that three of the nine studies showed statistically significant weight loss, compared with a control state. Absolute weight loss in these three trials ranged from 3 kg to about 7 kg (between-group differences, P less than .001 for all).
Participants in another trial didn’t lose a statistically significant amount of weight, compared with the control arm of the study. However, the mean 5 kg lost by those in the intervention arm was enough to be clinically significant, so Ms. Hopkins and her colleagues included this study in a subanalysis that looked for effective modalities and interventions among the studies with significant results.
The duration of the studies ranged from 6 to 24 months, though five of the trials were less than 1 year long. Women made up the majority of participants in all but one trial.
“There is limited evidence that standalone digital weight-loss interventions produce clinically meaningful outcomes,” wrote Ms. Hopkins and her coauthors. “Absolute magnitude of weight loss was low, and the short intervention lengths call into question the sustainability of these weight losses.”
The systematic review cast a broad net to include digital modalities such as wireless scales, text messaging, email, and web-based interventions, as well as the use of smartphone apps and tracking devices. All interventions used multiple digital modalities.
The most frequently used technologies were the use of a website, used in six (67%) of the trials, followed by text messaging and smartphone apps, each used in five (56%) of the trials. Tracking devices, email, message boards, and gamification of some sort were all used in three (33%) of the trials.
In terms of the specific interventions used in the trials, weight, diet, and activity were all tracked in eight trials (89%). Similarly, all but one trial gave feedback and weight and health education to participants. Behavior change education, as well as calorie goals, were each used in six trials (67%).
Ms. Hopkins and her colleagues looked at which trials incorporated which modalities and interventions, finding that “trials that integrated components unique to digital interventions, such as gamification, podcasts, or interactive features, yielded significantly greater and more clinically meaningful weight losses.”
To be included in the systematic review, trials had to include adult participants with a body mass index of at least 25 kg/m2 and use a standalone digital intervention of at least 6 months’ duration. The primary outcome of interest in the review was the change in participant weight from baseline to the end of the minimum 6-month follow-up period. Randomized, controlled trials and feasibility trials were included, so long as participants were allocated randomly.
Of the 126 trials reviewed at the full text level, the most frequent reason for exclusion was the inclusion of human coaching. Also, 30 of the trials didn’t report weight change as an outcome, the investigators said.
Future directions should include comparing digital interventions that “utilize features unique to digital delivery” with those that more closely resemble in-person weight-loss management interventions, suggested Ms. Hopkins and her collaborators.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Hopkins C et al. Obesity Week 2018, Abstract T-P-LB-3640.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Three of nine studies found statistically significant weight loss with digital interventions.
Major finding: The largest effect was seen in one study showing 7 kg of long-term weight loss (P less than .001).
Study details: A systematic review of nine studies of digital-only interventions for weight loss.
Disclosures: The authors reported no outside sources of funding and no conflicts of interest.
Source: Hopkins C et al. Obesity Week 2018, Abstract T-P-PB-3640.
How I Became a Derm Guru (And How You Can, Too)
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).
Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
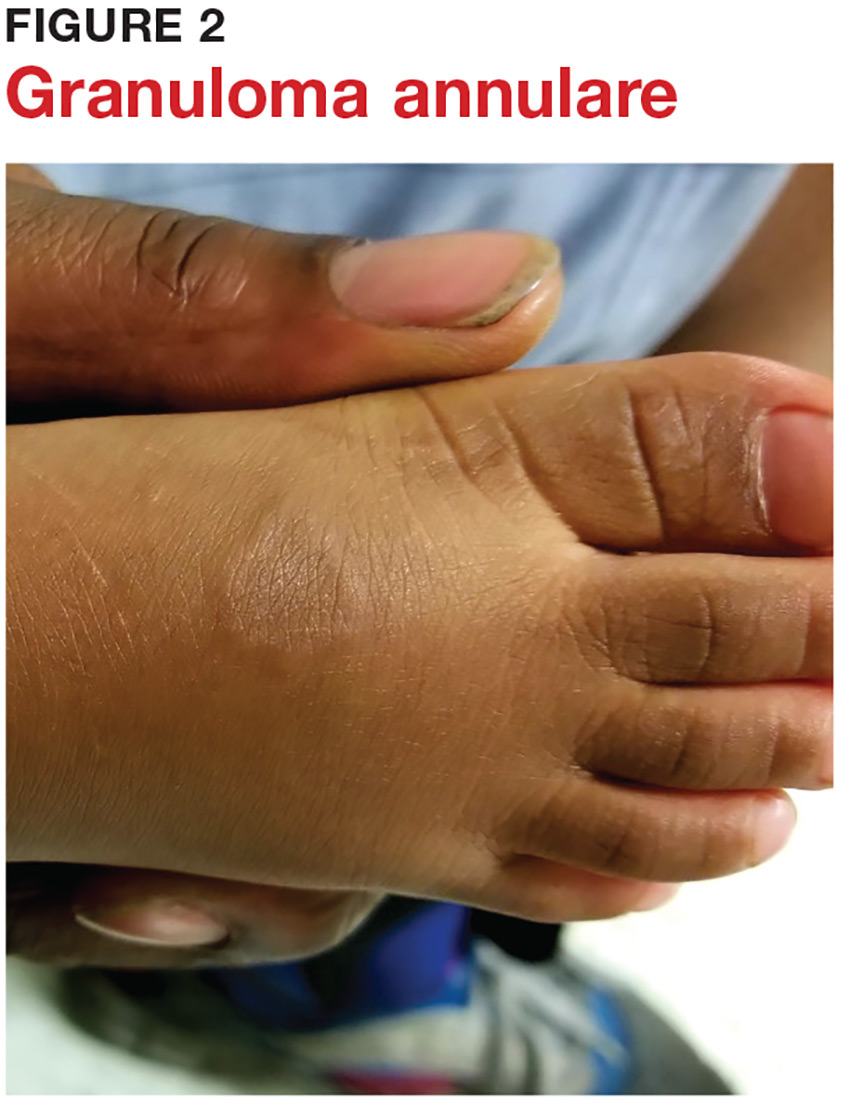
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).
4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
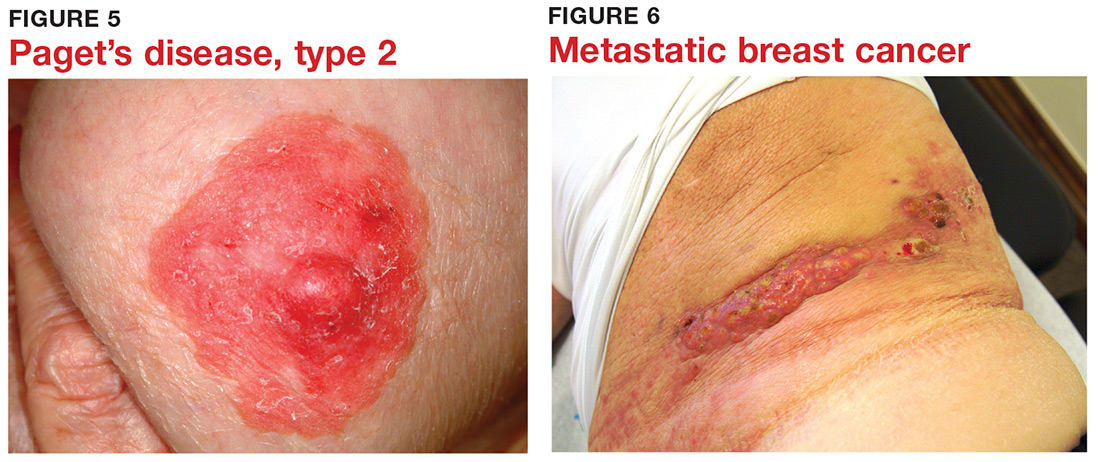
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.
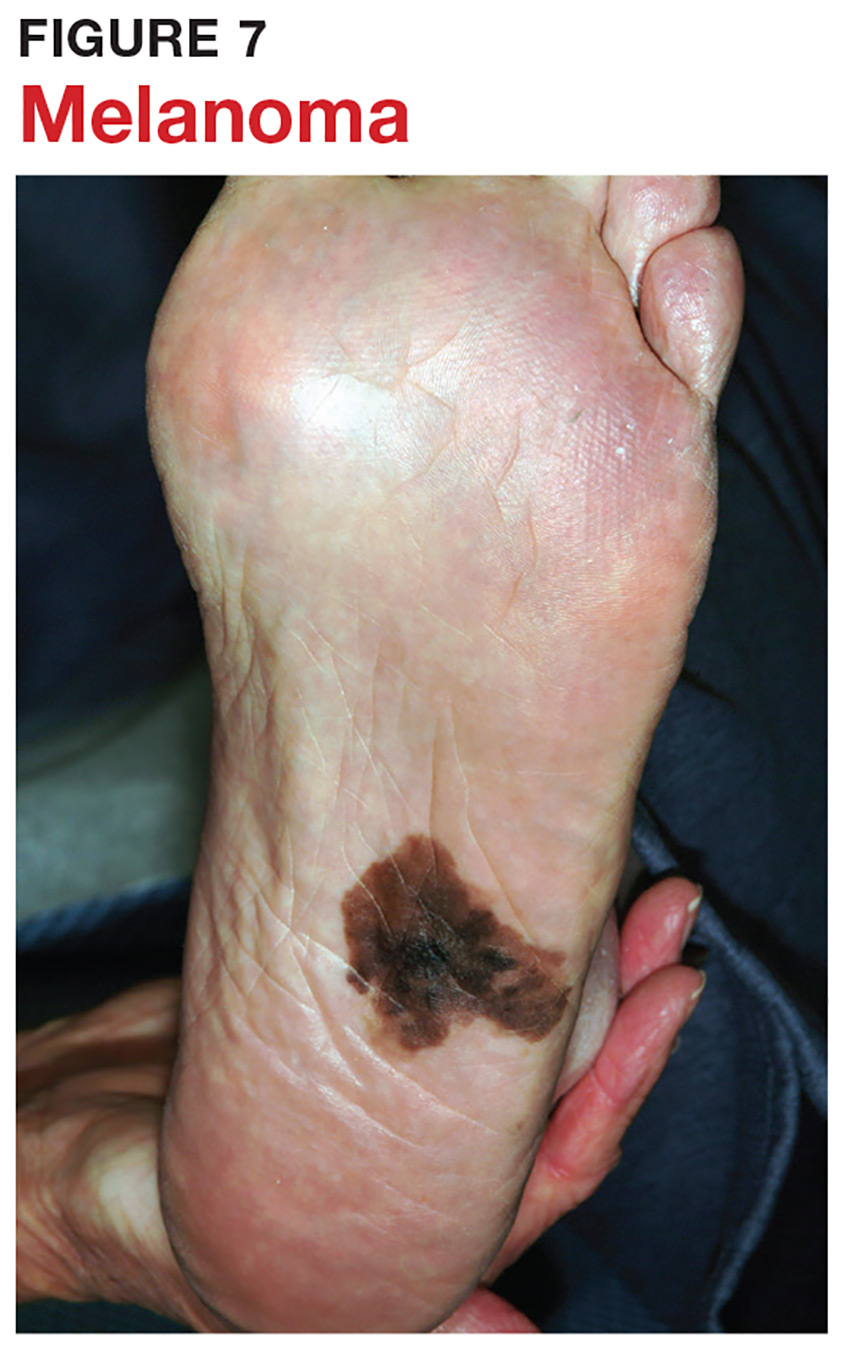
6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).
Continue to: #7...
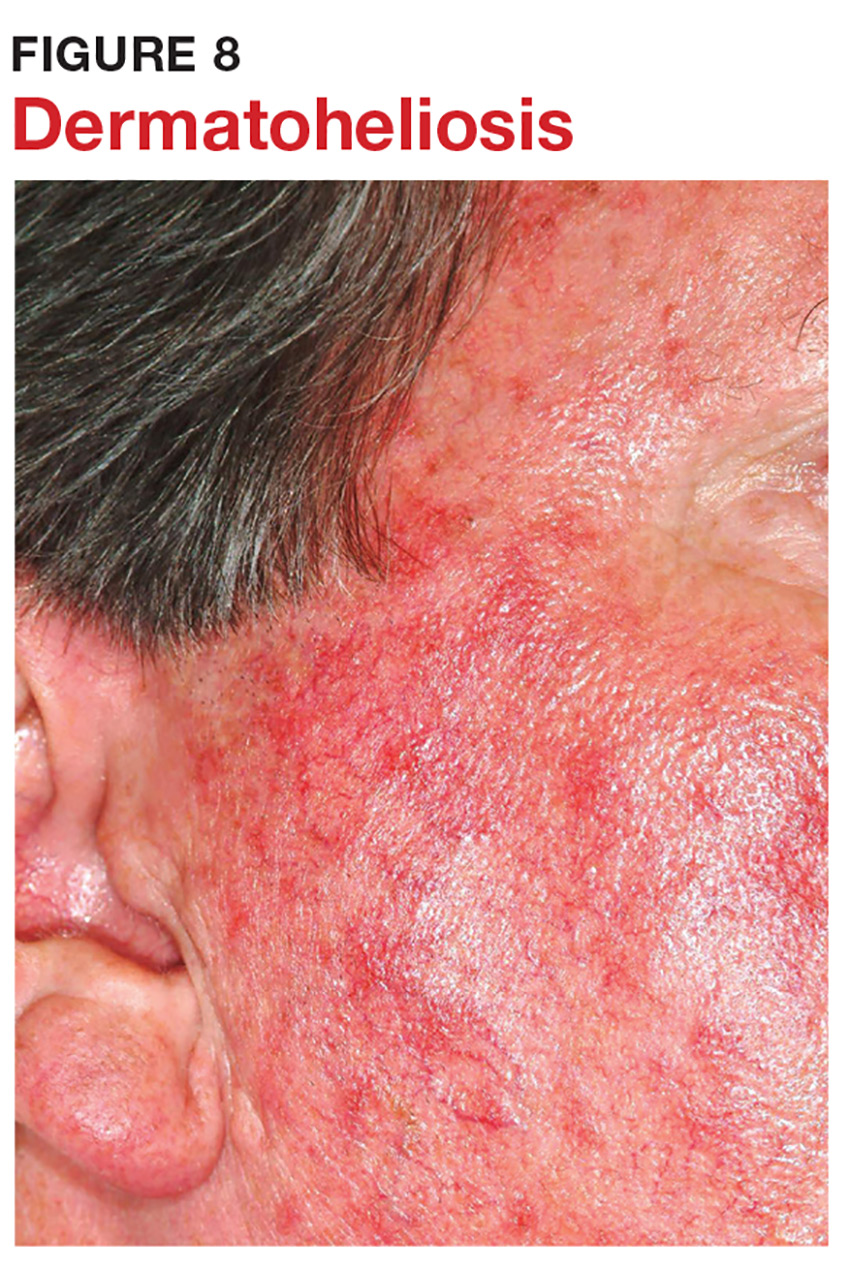
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).
8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.
9 “Infections” are not always what they seem
10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).
Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).
4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.
6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).
Continue to: #7...
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).
8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.
9 “Infections” are not always what they seem
10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).
Many years ago, when I was still in primary care (internal medicine), I thought I knew a bit about the practice of medicine. I was totally comfortable in the hospital (in those days, we saw our own patients twice a day in the hospital), including the ER, the OR, even obstetrics. MIs, shootings, stab wounds, renal failure—I would never say I had mastered them, but I was comfortable with most of what I saw. Deliveries, assisting with C-sections, performing lumbar punctures, performing and interpreting exercise tolerance tests, performing flexible sigmoidoscopies—no problem.
But the one thing that nearly always stopped me in my tracks was … you guessed it: dermatology complaints. Rashes, lesions, or any other skin complaint the least bit out of the ordinary were completely baffling to me. I still remember that feeling after all these years (and I still occasionally experience it!).
I felt like saying to those patients: What in the world would make you think I’d have any idea what that is? But of course, I couldn’t say that, so I’d mumble something, throw some cream at it, then quickly change the subject. Mind you, this was in a setting where a derm referral from us would take 4 to 6 months. And in case you’re wondering, the other providers in my department were as bad at derm as I was.
Long story short, it got to the point that I would scan my schedule every morning, praying I wouldn’t see the word “rash” or “skin.” But, of course, they still came—often just as my hand touched the doorknob to leave: “Oh, by the way, what about this …?” You get the picture. Many of you, if not most, live that picture.
I finally got up the nerve to go to our dermatology department to ask if I could follow one of the docs while he saw patients. Little did I know that practically every provider in the building had already done the same, and had been dismissed with words that essentially meant, “You? A mere PA? You can’t get there from here. Just send ’em to us.”
For a short time, I bought that line—but in the meantime, my patients were not getting the care they needed. So, driven in part by anger at the notion that a mere PA was simply unable to learn dermatology, I bought a decent textbook, Fitzpatrick’s Color Atlas of Dermatology, and started reading it. I also started collecting all the derm articles I could find in the journals, and read about those cases.
I won’t bore you with the grimy details, but what I did differently was work at learning derm (what a concept!). I started going to derm conferences, bought a good camera and started taking pictures with it, and continued to buy books (this was in the pre-computer days of the ’80s) and actually read them.
Continue to: And a funny thing happened...
And a funny thing happened: The more I read, the more diagnoses I recognized on my patients. My colleagues and the clinic schedulers took note of this and began sending me their problem cases. Even the derm department, beleaguered as usual by huge backlogs of patients, started sending patients to me. By 1985, even though I was in the internal medicine department, I had transitioned to doing derm fulltime. And that’s what I’ve been doing since.
Around 1992, I discovered that I was one of 6 dermatology PAs in this country. Last time I checked, our numbers were approaching 4,000. So, yes, derm is indeed difficult, but rocket science it isn’t.
Being the pedantic sort that I am, and finding that whole experience so enlightening, I resolved to make it my mission to foster the use of PAs in dermatology—part of which involves the education of those PAs, by means of taking students but also by writing articles (several hundred at last count) and lecturing at conferences and at PA programs. Nearing retirement, I only practice two days a week, but I write and publish at least 5 clinical articles a month, all of which are based on real cases: my cases, using my photos, doing new research on each case. This keeps my knowledge fresh and my 75-year-old mind sharp, helps ward off burnout, and, most importantly, saves lives while reducing patient discomfort.
What follows are 10 dermatology pearls that I have gleaned along the way. My apologies to my former students and attendees at my lectures who’ve heard all this before:
1 If the treatment for your diagnosis isn’t working, consider another diagnosis. Here’s an example (Figure 1): A man in his 50s was sent to dermatology for psoriasis that wasn’t responding to a biologic. Was it really psoriasis? A KOH prep quickly showed it to be tinea corporis, which cleared completely with a month’s worth of oral terbinafine (250 mg qid).
Continue to: #2...
2 The correct diagnosis dictates correct treatment. This may sound obvious, but in primary care, the emphasis is often on “let’s try this” or “let’s try that,” an understandable approach to a symptomatic patient with an uncertain diagnosis. But by the time he finally gets to dermatology, the patient has tried a whole bag full of prescription and OTC products given for numerous, totally different diagnoses. A better approach might be to expedite an urgent referral to dermatology, when possible.
3 Cutaneous fungal infections (ie, dermatophytosis) are vastly overdiagnosed, especially by novices. If you truly suspect it, ask about a potential source; one doesn’t acquire a fungal infection out of thin air. It must come from a person, animal, or occasionally, the soil. It also helps if the victim has been rendered susceptible by the injudicious use of steroids. Better yet, find the fungus with a microscopic examination (KOH prep) or culture. Finally, remember, not everything round and scaly is fungal (see Figure 2).
4 Remember these ancient words of wisdom regarding skin complaints: (a) A diagnosis is seldom made if not entertained, (b) you won’t entertain it if you’ve never heard of it, (c) you will not see it if you’re not looking for it, and (d) even if you did see it, you would not “see” it because you’re not looking for it. Dermatology is far deeper and wider than most imagine it to be. The trick is to expose yourself to as many different diagnoses as possible, by reading and attending lectures, ahead of the possible sighting. Figures 3 and 4 offer examples of common conditions that are seldom recognized outside dermatology.
5 Skin cancer can present as a rash. Examples abound, such as mammary and extramammary Paget’s disease (Figure 5), mycosis fungoides, metastatic breast cancer (Figure 6), and superficial basal cell carcinoma. A biopsy is usually required to diagnose these, but you wouldn’t think to do that if you’d never heard of the condition.
6 Melanoma doesn’t typically arise from a mole or other pre-existing lesion. Far more often, it arises “de novo,” out of nothing. So, in general, we’re not worried about “moles” (nevi) unless there’s a history of change (see Figure 7).
Continue to: #7...
7 When looking for skin cancer, pay as much attention to the owner as to the lesion. The most common skin cancers—basal cell and squamous cell carcinoma—usually occur on sun-damaged, fair-skinned, blue-eyed older patients. Though there are certainly exceptions to this paradigm, it pays to be generally suspicious of any odd lesion seen on these patients (Figure 8).
8 It’s practically impossible to overstate the role of atopy when evaluating pediatric skin complaints. These children—20% of all newborns!—are born with thin, dry, sensitive, overreactive skin that is prone to eczema and urticaria. They will also have a marked tendency to develop seasonal allergies, allergic rhinitis, and asthma. Parents find it difficult to accept the genetic basis for atopic dermatitis (Figure 9), preferring instead to blame everything on laundry detergent or food. Education (of oneself first!) is the key.
9 “Infections” are not always what they seem
10 Overcome your fear of steroids by educating yourself about their safe use. Glucocorticoids (eg, triamcinolone, prednisone, betamethasone) are extremely useful in treating common derm conditions. We see patients every day who are so frightened of steroids, they won’t even consider using them because some well-meaning medical provider scared them to death. The proper use of these miraculous products could easily be the subject of an entire article. For now, I’ll advise you to read about their safe use in any number of dermatology texts (including online publications).