User login
Is an IUD a good contraceptive choice for a never sexually active teen?
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
Hippocampal abnormalities seen in epilepsy subtypes may be congenital
NEW ORLEANS – , although to a lesser extent, based on findings from two studies presented at the annual meeting of the American Epilepsy Society.
While the studies suggest an imaging endophenotype associated with these disorders, it’s unclear if a larger degree of abnormality causes disease manifestation, or whether there are other predisposing actors at work.
“What our study tells us is that hippocampal abnormalities can occur in the absence of seizure,” Marian Galovic, MD, said in an interview. “It may be that, in some cases, hippocampal abnormalities could be the cause, rather than the consequence, of seizures.”
Dr. Galovic of University College London was on hand to discuss the work of his colleague, Lili Long, MD, PhD, of the Xiangya Hospital of Central South University, Changsha, China. Visa issues prevented her from attending the meeting.
The study included 18 sibling pairs in which the affected siblings had sporadic, nonlesional temporal lobe epilepsy (TLE), involving the right lobe in 12 and the left in 6. The patients, siblings, and 18 healthy, age-matched controls underwent clinical, electrophysiologic, and high-resolution structural neuroimaging.
The researchers compared overall hippocampal volumes between groups and determined the subregional extent of hippocampal abnormalities using shape analysis. They also looked at whole-brain differences in cortical thickness and folding complexity.
As expected, median hippocampal volumes were largest in the healthy controls (left = 2.82 mL, right = 2.94 mL), and smallest in patients. Patients with left TLE had a median left hippocampal volume of 2.23 mL, while those with right TLE had a median right hippocampal volume of 1.92 mL.
However, volume in the unaffected siblings was a surprise. Like the patients, these subjects also had significant reductions in hippocampal volume when compared with controls (left = 2.47 mL, right = 2.65 mL). “The atrophy was relatively similar in siblings and patients, although not as pronounced in siblings,” Dr. Galovic said. “It was mostly unilateral in the siblings and bilateral in the patients, but it was still more pronounced on the side where the epilepsy of the affected sibling was coming from.”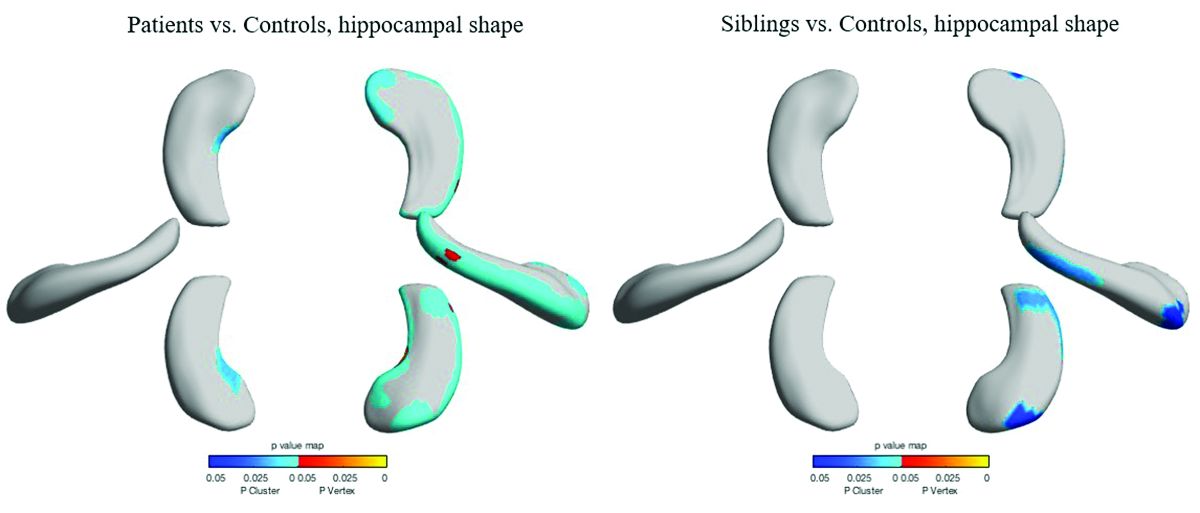
Patients and siblings also shared morphologic variations of the hippocampus, with atrophy more pronounced on the right than the left. The right lateral body and anterior head of the hippocampus were most affected, Dr. Galovic said, with reductions in the right cornu ammonis 1 subfield and subiculum.
Widespread cortical thinning was present in patients, including the pericentral, frontal, and temporal areas. Unaffected siblings also showed cortical thinning, but this was mostly restricted to the right postcentral gyrus. Patients and siblings also demonstrated increased cortical folding complexity, but in different areas: predominantly frontal in patients, but predominantly parieto-occipital in siblings. Both were significantly different than healthy control subjects.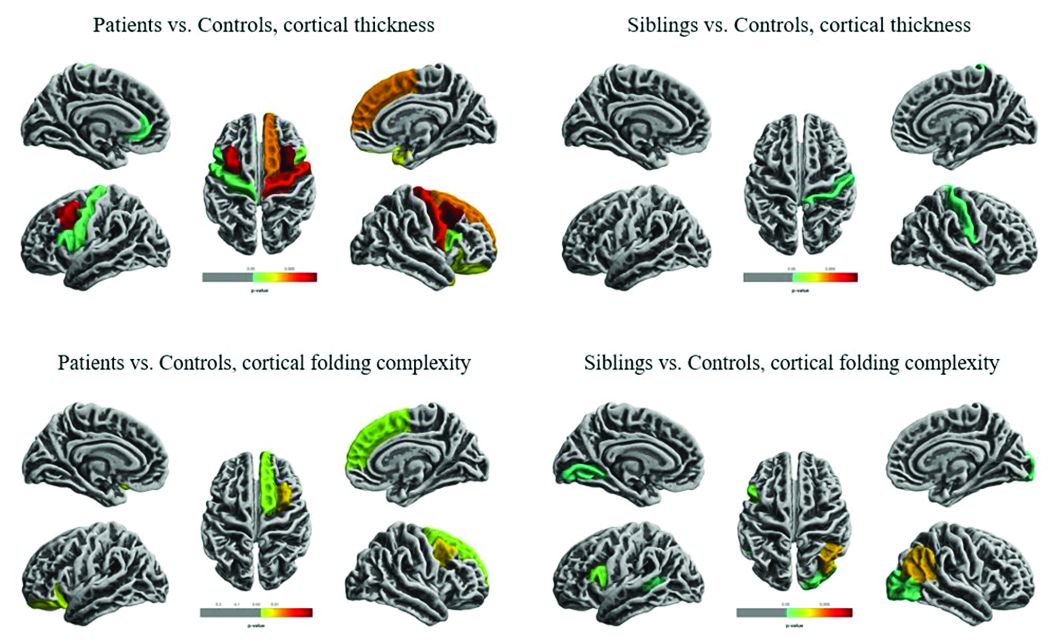
The study didn’t examine any association with memory, which is often impaired in patients with TLE. However, Dr. Galovic said, “We have just submitted for publication a study in which we did find an association between focal hippocampal atrophy and memory performance.”
A different study by a team at University College London looked at hippocampal structure and function in patients with juvenile myoclonic epilepsy (JME) and their unaffected siblings. The imaging study, lead by Lorenzo Caciagli, MD, of the university comprised 37 patients with JME, 16 unaffected siblings, and 20 healthy controls. It employed multimodal MRI and neuropsychological measures to examine the form and function of the mesiotemporal lobe.
The subjects were matched for age, sex, handedness, and hemispheric dominance, which was assessed with language lateralization indices. This measures the number of active voxels on functional MRI, showing which hemisphere is dominant for language.
Both patients and their siblings showed reductions in left hippocampal volume on the order of 5%-8%, significantly smaller than the volumes seen in healthy controls. About half of patients and half of siblings also showed either unilateral or bilateral hippocampal malrotation. This was present in just 15% of controls, another significant difference. The structural differences weren’t associated with seizure control or age at disease onset, or with any impairments in verbal or visual memory. But when the investigators performed functional mapping, they found unusual patterns of hippocampal activation in both patients and siblings, pointing to a dysfunction of verbal encoding. In patients, there appeared to be distinct patterns of underactivation along the hippocampal long axis, regardless of whether malrotation was present. But among patients who had malrotation, the left posterior hippocampus showed more activation during visual memory.
The team concluded that the hippocampal abnormalities in volume, shape, and positioning in patients with JME and their siblings are related to functional reorganization. The abnormalities probably occur during prenatal neurodevelopment, they noted.
“Cosegregation of imaging patterns in patients and their siblings is suggestive of genetic imaging phenotypes, and independent of disease activity,” Dr. Caciagli and his coinvestigators wrote in their abstract.
Funding for the TLE study came from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and Xiangya Hospital. Funding for the JME study came from a variety of U.K. charities and government agencies.
SOURCES: Long L et al. AES 2018, Abstract 2.183; Caciagli L et al. AES 2018, Abstract 2.166.
NEW ORLEANS – , although to a lesser extent, based on findings from two studies presented at the annual meeting of the American Epilepsy Society.
While the studies suggest an imaging endophenotype associated with these disorders, it’s unclear if a larger degree of abnormality causes disease manifestation, or whether there are other predisposing actors at work.
“What our study tells us is that hippocampal abnormalities can occur in the absence of seizure,” Marian Galovic, MD, said in an interview. “It may be that, in some cases, hippocampal abnormalities could be the cause, rather than the consequence, of seizures.”
Dr. Galovic of University College London was on hand to discuss the work of his colleague, Lili Long, MD, PhD, of the Xiangya Hospital of Central South University, Changsha, China. Visa issues prevented her from attending the meeting.
The study included 18 sibling pairs in which the affected siblings had sporadic, nonlesional temporal lobe epilepsy (TLE), involving the right lobe in 12 and the left in 6. The patients, siblings, and 18 healthy, age-matched controls underwent clinical, electrophysiologic, and high-resolution structural neuroimaging.
The researchers compared overall hippocampal volumes between groups and determined the subregional extent of hippocampal abnormalities using shape analysis. They also looked at whole-brain differences in cortical thickness and folding complexity.
As expected, median hippocampal volumes were largest in the healthy controls (left = 2.82 mL, right = 2.94 mL), and smallest in patients. Patients with left TLE had a median left hippocampal volume of 2.23 mL, while those with right TLE had a median right hippocampal volume of 1.92 mL.
However, volume in the unaffected siblings was a surprise. Like the patients, these subjects also had significant reductions in hippocampal volume when compared with controls (left = 2.47 mL, right = 2.65 mL). “The atrophy was relatively similar in siblings and patients, although not as pronounced in siblings,” Dr. Galovic said. “It was mostly unilateral in the siblings and bilateral in the patients, but it was still more pronounced on the side where the epilepsy of the affected sibling was coming from.”
Patients and siblings also shared morphologic variations of the hippocampus, with atrophy more pronounced on the right than the left. The right lateral body and anterior head of the hippocampus were most affected, Dr. Galovic said, with reductions in the right cornu ammonis 1 subfield and subiculum.
Widespread cortical thinning was present in patients, including the pericentral, frontal, and temporal areas. Unaffected siblings also showed cortical thinning, but this was mostly restricted to the right postcentral gyrus. Patients and siblings also demonstrated increased cortical folding complexity, but in different areas: predominantly frontal in patients, but predominantly parieto-occipital in siblings. Both were significantly different than healthy control subjects.
The study didn’t examine any association with memory, which is often impaired in patients with TLE. However, Dr. Galovic said, “We have just submitted for publication a study in which we did find an association between focal hippocampal atrophy and memory performance.”
A different study by a team at University College London looked at hippocampal structure and function in patients with juvenile myoclonic epilepsy (JME) and their unaffected siblings. The imaging study, lead by Lorenzo Caciagli, MD, of the university comprised 37 patients with JME, 16 unaffected siblings, and 20 healthy controls. It employed multimodal MRI and neuropsychological measures to examine the form and function of the mesiotemporal lobe.
The subjects were matched for age, sex, handedness, and hemispheric dominance, which was assessed with language lateralization indices. This measures the number of active voxels on functional MRI, showing which hemisphere is dominant for language.
Both patients and their siblings showed reductions in left hippocampal volume on the order of 5%-8%, significantly smaller than the volumes seen in healthy controls. About half of patients and half of siblings also showed either unilateral or bilateral hippocampal malrotation. This was present in just 15% of controls, another significant difference. The structural differences weren’t associated with seizure control or age at disease onset, or with any impairments in verbal or visual memory. But when the investigators performed functional mapping, they found unusual patterns of hippocampal activation in both patients and siblings, pointing to a dysfunction of verbal encoding. In patients, there appeared to be distinct patterns of underactivation along the hippocampal long axis, regardless of whether malrotation was present. But among patients who had malrotation, the left posterior hippocampus showed more activation during visual memory.
The team concluded that the hippocampal abnormalities in volume, shape, and positioning in patients with JME and their siblings are related to functional reorganization. The abnormalities probably occur during prenatal neurodevelopment, they noted.
“Cosegregation of imaging patterns in patients and their siblings is suggestive of genetic imaging phenotypes, and independent of disease activity,” Dr. Caciagli and his coinvestigators wrote in their abstract.
Funding for the TLE study came from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and Xiangya Hospital. Funding for the JME study came from a variety of U.K. charities and government agencies.
SOURCES: Long L et al. AES 2018, Abstract 2.183; Caciagli L et al. AES 2018, Abstract 2.166.
NEW ORLEANS – , although to a lesser extent, based on findings from two studies presented at the annual meeting of the American Epilepsy Society.
While the studies suggest an imaging endophenotype associated with these disorders, it’s unclear if a larger degree of abnormality causes disease manifestation, or whether there are other predisposing actors at work.
“What our study tells us is that hippocampal abnormalities can occur in the absence of seizure,” Marian Galovic, MD, said in an interview. “It may be that, in some cases, hippocampal abnormalities could be the cause, rather than the consequence, of seizures.”
Dr. Galovic of University College London was on hand to discuss the work of his colleague, Lili Long, MD, PhD, of the Xiangya Hospital of Central South University, Changsha, China. Visa issues prevented her from attending the meeting.
The study included 18 sibling pairs in which the affected siblings had sporadic, nonlesional temporal lobe epilepsy (TLE), involving the right lobe in 12 and the left in 6. The patients, siblings, and 18 healthy, age-matched controls underwent clinical, electrophysiologic, and high-resolution structural neuroimaging.
The researchers compared overall hippocampal volumes between groups and determined the subregional extent of hippocampal abnormalities using shape analysis. They also looked at whole-brain differences in cortical thickness and folding complexity.
As expected, median hippocampal volumes were largest in the healthy controls (left = 2.82 mL, right = 2.94 mL), and smallest in patients. Patients with left TLE had a median left hippocampal volume of 2.23 mL, while those with right TLE had a median right hippocampal volume of 1.92 mL.
However, volume in the unaffected siblings was a surprise. Like the patients, these subjects also had significant reductions in hippocampal volume when compared with controls (left = 2.47 mL, right = 2.65 mL). “The atrophy was relatively similar in siblings and patients, although not as pronounced in siblings,” Dr. Galovic said. “It was mostly unilateral in the siblings and bilateral in the patients, but it was still more pronounced on the side where the epilepsy of the affected sibling was coming from.”
Patients and siblings also shared morphologic variations of the hippocampus, with atrophy more pronounced on the right than the left. The right lateral body and anterior head of the hippocampus were most affected, Dr. Galovic said, with reductions in the right cornu ammonis 1 subfield and subiculum.
Widespread cortical thinning was present in patients, including the pericentral, frontal, and temporal areas. Unaffected siblings also showed cortical thinning, but this was mostly restricted to the right postcentral gyrus. Patients and siblings also demonstrated increased cortical folding complexity, but in different areas: predominantly frontal in patients, but predominantly parieto-occipital in siblings. Both were significantly different than healthy control subjects.
The study didn’t examine any association with memory, which is often impaired in patients with TLE. However, Dr. Galovic said, “We have just submitted for publication a study in which we did find an association between focal hippocampal atrophy and memory performance.”
A different study by a team at University College London looked at hippocampal structure and function in patients with juvenile myoclonic epilepsy (JME) and their unaffected siblings. The imaging study, lead by Lorenzo Caciagli, MD, of the university comprised 37 patients with JME, 16 unaffected siblings, and 20 healthy controls. It employed multimodal MRI and neuropsychological measures to examine the form and function of the mesiotemporal lobe.
The subjects were matched for age, sex, handedness, and hemispheric dominance, which was assessed with language lateralization indices. This measures the number of active voxels on functional MRI, showing which hemisphere is dominant for language.
Both patients and their siblings showed reductions in left hippocampal volume on the order of 5%-8%, significantly smaller than the volumes seen in healthy controls. About half of patients and half of siblings also showed either unilateral or bilateral hippocampal malrotation. This was present in just 15% of controls, another significant difference. The structural differences weren’t associated with seizure control or age at disease onset, or with any impairments in verbal or visual memory. But when the investigators performed functional mapping, they found unusual patterns of hippocampal activation in both patients and siblings, pointing to a dysfunction of verbal encoding. In patients, there appeared to be distinct patterns of underactivation along the hippocampal long axis, regardless of whether malrotation was present. But among patients who had malrotation, the left posterior hippocampus showed more activation during visual memory.
The team concluded that the hippocampal abnormalities in volume, shape, and positioning in patients with JME and their siblings are related to functional reorganization. The abnormalities probably occur during prenatal neurodevelopment, they noted.
“Cosegregation of imaging patterns in patients and their siblings is suggestive of genetic imaging phenotypes, and independent of disease activity,” Dr. Caciagli and his coinvestigators wrote in their abstract.
Funding for the TLE study came from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and Xiangya Hospital. Funding for the JME study came from a variety of U.K. charities and government agencies.
SOURCES: Long L et al. AES 2018, Abstract 2.183; Caciagli L et al. AES 2018, Abstract 2.166.
REPORTING FROM AES 2018
ECHELON-2: BV-CHP boosts survival in PTCL
SAN DIEGO – A newly approved treatment regimen provides a survival benefit over standard therapy for patients with CD30-positive peripheral T-cell lymphomas (PTCLs), according to new research presented at the annual meeting of the American Society of Hematology.
In the ECHELON-2 trial, patients who received brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (CHP) had superior progression-free survival (PFS) and overall survival (OS), compared with patients who received standard treatment with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results supported the recent U.S. approval of BV in combination with CHP for adults with previously untreated, systemic anaplastic large cell lymphoma or other CD30-expressing PTCLs.
“ECHELON-2 is the first prospective trial in peripheral T-cell lymphoma to show an overall survival benefit over CHOP,” said Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, with locations in New York and New Jersey.
Dr. Horwitz presented data from this trial at the ASH meeting. Results were simultaneously published in the Lancet (2018 Dec 3. doi: 10.1016/S0140-6736[18]32984-2).
ECHELON-2 (NCT01777152) enrolled 452 patients with previously untreated, CD30-positive PTCL. Subtypes included ALK-positive or ALK-negative systemic anaplastic large-cell lymphoma, PTCL not otherwise specified, angioimmunoblastic T-cell lymphoma, enteropathy-associated T-cell lymphoma, and adult T-cell leukemia/lymphoma.
Patients were randomized to receive BV-CHP plus placebo (n = 226) or CHOP plus placebo (n = 226) every 3 weeks for six to eight cycles.
At baseline, the median age was 58 in the BV-CHP arm and the CHOP arm. The majority of patients were male – 59% in the BV-CHP arm and 67% in the CHOP arm – and most patients had stage III/IV disease, 81% and 80%, respectively.
In all, 89% of patients in the BV-CHP arm and 81% in the CHOP arm completed six or more cycles of their assigned treatment.
The overall response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P = .0032). The complete response rates were 68% and 56%, respectively (P = .0066).
At a median follow-up of 36.2 months, the median PFS was 48.2 months in the BV-CHP arm and 20.8 months in the CHOP arm. The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011).
At a median follow-up of 42.1 months, the median OS was not reached in either treatment arm. The rate of death was 23% in the BV-CHP arm and 32% in the CHOP arm (HR = 0.66, P = .0244).
Dr. Horwitz noted that this study was not powered to determine differences in PFS or OS by PTCL subtypes.
BV-CHP had a safety profile comparable with that of CHOP, Dr. Horwitz said.
The rate of adverse events (AEs) was 99% in the BV-CHP arm and 98% in the CHOP arm. Grade 3 or higher AEs occurred in 66% and 65% of patients, respectively. Serious AEs occurred in 39% and 38%, respectively.
Three percent of patients in the BV-CHP arm and 4% of those in the CHOP arm had fatal AEs.
The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
SOURCE: Horwitz S et al. ASH 2018, Abstract 997.
SAN DIEGO – A newly approved treatment regimen provides a survival benefit over standard therapy for patients with CD30-positive peripheral T-cell lymphomas (PTCLs), according to new research presented at the annual meeting of the American Society of Hematology.
In the ECHELON-2 trial, patients who received brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (CHP) had superior progression-free survival (PFS) and overall survival (OS), compared with patients who received standard treatment with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results supported the recent U.S. approval of BV in combination with CHP for adults with previously untreated, systemic anaplastic large cell lymphoma or other CD30-expressing PTCLs.
“ECHELON-2 is the first prospective trial in peripheral T-cell lymphoma to show an overall survival benefit over CHOP,” said Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, with locations in New York and New Jersey.
Dr. Horwitz presented data from this trial at the ASH meeting. Results were simultaneously published in the Lancet (2018 Dec 3. doi: 10.1016/S0140-6736[18]32984-2).
ECHELON-2 (NCT01777152) enrolled 452 patients with previously untreated, CD30-positive PTCL. Subtypes included ALK-positive or ALK-negative systemic anaplastic large-cell lymphoma, PTCL not otherwise specified, angioimmunoblastic T-cell lymphoma, enteropathy-associated T-cell lymphoma, and adult T-cell leukemia/lymphoma.
Patients were randomized to receive BV-CHP plus placebo (n = 226) or CHOP plus placebo (n = 226) every 3 weeks for six to eight cycles.
At baseline, the median age was 58 in the BV-CHP arm and the CHOP arm. The majority of patients were male – 59% in the BV-CHP arm and 67% in the CHOP arm – and most patients had stage III/IV disease, 81% and 80%, respectively.
In all, 89% of patients in the BV-CHP arm and 81% in the CHOP arm completed six or more cycles of their assigned treatment.
The overall response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P = .0032). The complete response rates were 68% and 56%, respectively (P = .0066).
At a median follow-up of 36.2 months, the median PFS was 48.2 months in the BV-CHP arm and 20.8 months in the CHOP arm. The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011).
At a median follow-up of 42.1 months, the median OS was not reached in either treatment arm. The rate of death was 23% in the BV-CHP arm and 32% in the CHOP arm (HR = 0.66, P = .0244).
Dr. Horwitz noted that this study was not powered to determine differences in PFS or OS by PTCL subtypes.
BV-CHP had a safety profile comparable with that of CHOP, Dr. Horwitz said.
The rate of adverse events (AEs) was 99% in the BV-CHP arm and 98% in the CHOP arm. Grade 3 or higher AEs occurred in 66% and 65% of patients, respectively. Serious AEs occurred in 39% and 38%, respectively.
Three percent of patients in the BV-CHP arm and 4% of those in the CHOP arm had fatal AEs.
The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
SOURCE: Horwitz S et al. ASH 2018, Abstract 997.
SAN DIEGO – A newly approved treatment regimen provides a survival benefit over standard therapy for patients with CD30-positive peripheral T-cell lymphomas (PTCLs), according to new research presented at the annual meeting of the American Society of Hematology.
In the ECHELON-2 trial, patients who received brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (CHP) had superior progression-free survival (PFS) and overall survival (OS), compared with patients who received standard treatment with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results supported the recent U.S. approval of BV in combination with CHP for adults with previously untreated, systemic anaplastic large cell lymphoma or other CD30-expressing PTCLs.
“ECHELON-2 is the first prospective trial in peripheral T-cell lymphoma to show an overall survival benefit over CHOP,” said Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, with locations in New York and New Jersey.
Dr. Horwitz presented data from this trial at the ASH meeting. Results were simultaneously published in the Lancet (2018 Dec 3. doi: 10.1016/S0140-6736[18]32984-2).
ECHELON-2 (NCT01777152) enrolled 452 patients with previously untreated, CD30-positive PTCL. Subtypes included ALK-positive or ALK-negative systemic anaplastic large-cell lymphoma, PTCL not otherwise specified, angioimmunoblastic T-cell lymphoma, enteropathy-associated T-cell lymphoma, and adult T-cell leukemia/lymphoma.
Patients were randomized to receive BV-CHP plus placebo (n = 226) or CHOP plus placebo (n = 226) every 3 weeks for six to eight cycles.
At baseline, the median age was 58 in the BV-CHP arm and the CHOP arm. The majority of patients were male – 59% in the BV-CHP arm and 67% in the CHOP arm – and most patients had stage III/IV disease, 81% and 80%, respectively.
In all, 89% of patients in the BV-CHP arm and 81% in the CHOP arm completed six or more cycles of their assigned treatment.
The overall response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P = .0032). The complete response rates were 68% and 56%, respectively (P = .0066).
At a median follow-up of 36.2 months, the median PFS was 48.2 months in the BV-CHP arm and 20.8 months in the CHOP arm. The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011).
At a median follow-up of 42.1 months, the median OS was not reached in either treatment arm. The rate of death was 23% in the BV-CHP arm and 32% in the CHOP arm (HR = 0.66, P = .0244).
Dr. Horwitz noted that this study was not powered to determine differences in PFS or OS by PTCL subtypes.
BV-CHP had a safety profile comparable with that of CHOP, Dr. Horwitz said.
The rate of adverse events (AEs) was 99% in the BV-CHP arm and 98% in the CHOP arm. Grade 3 or higher AEs occurred in 66% and 65% of patients, respectively. Serious AEs occurred in 39% and 38%, respectively.
Three percent of patients in the BV-CHP arm and 4% of those in the CHOP arm had fatal AEs.
The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
SOURCE: Horwitz S et al. ASH 2018, Abstract 997.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The rate of death or progression was 42% in the BV-CHP arm and 55% in the CHOP arm (hazard ratio = 0.71, P = .011), while the rate of death alone was 23% and 32%, respectively (HR = 0.66, P = .0244).
Study details: A phase 3 trial of 452 patients with peripheral T-cell lymphoma.
Disclosures: The study was funded by Seattle Genetics, Millennium Pharmaceuticals, and the National Institutes of Health. Dr. Horwitz reported relationships with Seattle Genetics, Millennium Pharmaceuticals, and other companies.
Source: Horwitz S et al. ASH 2018, Abstract 997.
GO-8: Early promise for novel FVIII variant in hemophilia A
SAN DIEGO – A novel human factor VIII variant shows promise for the treatment of severe hemophilia A, according to preliminary findings from the ongoing Gene Therapy for Hemophilia A (GO-8) phase 1/2 dose-escalation study.
A single peripheral vein infusion of the factor VIII (FVIII) variant resulted in FVIII activity levels of about 6% versus levels of no more than 1% of normal at study entry in the first four patients, Pratima Chowdary, MD, reported at the annual meeting of the American Society of Hematology.
The variant, known as scAAV2/8-LP1-hFIXco, is being investigated for safety and efficacy in the GO-8 investigator-led, open-label, nonrandomized trial at a low, mid, and high dose (2 x 1011 vector genomes/kg, 6 x 1011 vector genomes/kg, and 2 x 1012 vector genomes/kg), said Dr. Chowdary, a consultant hematologist at Royal Free Hospital London.
The main study period is 6 months and 15 years of follow-up are offered.
The first patient received the low dose and achieved FVIII of about 6% within 1 week. That level persisted for about 6 weeks when the patient developed a transaminitis, which promptly responded to steroids.
His steady-state FVIII within a few weeks was 7% by one-stage assay and about 3% by chromogenic assay, Dr. Chowdary said.
The remaining patients received the mid dose and also achieved FVIII levels of about 6% within a week. Patient 2 started on prophylactic steroids at week 6, per protocol, and did not experience transaminitis, but also had no increase in FVIII level, compared with the low-dose patient, which may be explained by the potential drug half-life, she noted.
Patient 3 reached a FVIII level of about 30% by week 4. He developed transaminitis at that time, which was about 2 weeks before planned prophylactic drug administration, but the transaminitis was controlled by steroids over a period of about 8-10 weeks.
“His steady-state FVIII level by one stage was 34% and by chromogenic assay was 17%. He has not had any bleeds since his gene transfer and has not required any FVIII concentrate either,” she said.
Patient 4 reached a FVIII level of about 40% by week 4. He was given prophylactic steroids at that time because of the occurrence of transaminitis at week 4 in Patient 3.
The patient developed transaminitis during steroid taper about 4 weeks later, perhaps because of the rapid taper, Dr. Chowdary said, adding that the transaminitis was well controlled with steroids, but follow-up in this patient has only been about 12 weeks.
“The characteristics of FVIII expression in this patient are very similar to the previous patient. ... We suspect he will have a steady-state level of about 30%,” she said. “Again, he’s had no bleeds since his gene transfer and has not required any FVIII concentrate.”
The single infusion of this novel vector was well tolerated in each patient, with no evidence of infusion-related reactions, neutralizing anti-FVIII antibodies, or vector-related adverse events.
“The transgene expression was achieved in all patients and at both vector dosages,” Dr. Chowdary said. “What is very important is that the levels of less than 10% had only a modest impact on the bleed rates and FVIII usage, whereas an expression of more than 10% resulted in zero bleeds and the patient did not require any additional FVIII treatment.”
The data are “encouraging,” she said. “We look forward to escalating the dose in the next patient.”
Dr. Chowdary reported financial relationships with Bayer, CSL Behring, Baxalta, Baxter, Biogen, Freeline, Novo Nordisk, Pfizer, Roche, Shire, and SOBI.
SOURCE: Chowdary P et al. ASH 2018, Abstract 489.
SAN DIEGO – A novel human factor VIII variant shows promise for the treatment of severe hemophilia A, according to preliminary findings from the ongoing Gene Therapy for Hemophilia A (GO-8) phase 1/2 dose-escalation study.
A single peripheral vein infusion of the factor VIII (FVIII) variant resulted in FVIII activity levels of about 6% versus levels of no more than 1% of normal at study entry in the first four patients, Pratima Chowdary, MD, reported at the annual meeting of the American Society of Hematology.
The variant, known as scAAV2/8-LP1-hFIXco, is being investigated for safety and efficacy in the GO-8 investigator-led, open-label, nonrandomized trial at a low, mid, and high dose (2 x 1011 vector genomes/kg, 6 x 1011 vector genomes/kg, and 2 x 1012 vector genomes/kg), said Dr. Chowdary, a consultant hematologist at Royal Free Hospital London.
The main study period is 6 months and 15 years of follow-up are offered.
The first patient received the low dose and achieved FVIII of about 6% within 1 week. That level persisted for about 6 weeks when the patient developed a transaminitis, which promptly responded to steroids.
His steady-state FVIII within a few weeks was 7% by one-stage assay and about 3% by chromogenic assay, Dr. Chowdary said.
The remaining patients received the mid dose and also achieved FVIII levels of about 6% within a week. Patient 2 started on prophylactic steroids at week 6, per protocol, and did not experience transaminitis, but also had no increase in FVIII level, compared with the low-dose patient, which may be explained by the potential drug half-life, she noted.
Patient 3 reached a FVIII level of about 30% by week 4. He developed transaminitis at that time, which was about 2 weeks before planned prophylactic drug administration, but the transaminitis was controlled by steroids over a period of about 8-10 weeks.
“His steady-state FVIII level by one stage was 34% and by chromogenic assay was 17%. He has not had any bleeds since his gene transfer and has not required any FVIII concentrate either,” she said.
Patient 4 reached a FVIII level of about 40% by week 4. He was given prophylactic steroids at that time because of the occurrence of transaminitis at week 4 in Patient 3.
The patient developed transaminitis during steroid taper about 4 weeks later, perhaps because of the rapid taper, Dr. Chowdary said, adding that the transaminitis was well controlled with steroids, but follow-up in this patient has only been about 12 weeks.
“The characteristics of FVIII expression in this patient are very similar to the previous patient. ... We suspect he will have a steady-state level of about 30%,” she said. “Again, he’s had no bleeds since his gene transfer and has not required any FVIII concentrate.”
The single infusion of this novel vector was well tolerated in each patient, with no evidence of infusion-related reactions, neutralizing anti-FVIII antibodies, or vector-related adverse events.
“The transgene expression was achieved in all patients and at both vector dosages,” Dr. Chowdary said. “What is very important is that the levels of less than 10% had only a modest impact on the bleed rates and FVIII usage, whereas an expression of more than 10% resulted in zero bleeds and the patient did not require any additional FVIII treatment.”
The data are “encouraging,” she said. “We look forward to escalating the dose in the next patient.”
Dr. Chowdary reported financial relationships with Bayer, CSL Behring, Baxalta, Baxter, Biogen, Freeline, Novo Nordisk, Pfizer, Roche, Shire, and SOBI.
SOURCE: Chowdary P et al. ASH 2018, Abstract 489.
SAN DIEGO – A novel human factor VIII variant shows promise for the treatment of severe hemophilia A, according to preliminary findings from the ongoing Gene Therapy for Hemophilia A (GO-8) phase 1/2 dose-escalation study.
A single peripheral vein infusion of the factor VIII (FVIII) variant resulted in FVIII activity levels of about 6% versus levels of no more than 1% of normal at study entry in the first four patients, Pratima Chowdary, MD, reported at the annual meeting of the American Society of Hematology.
The variant, known as scAAV2/8-LP1-hFIXco, is being investigated for safety and efficacy in the GO-8 investigator-led, open-label, nonrandomized trial at a low, mid, and high dose (2 x 1011 vector genomes/kg, 6 x 1011 vector genomes/kg, and 2 x 1012 vector genomes/kg), said Dr. Chowdary, a consultant hematologist at Royal Free Hospital London.
The main study period is 6 months and 15 years of follow-up are offered.
The first patient received the low dose and achieved FVIII of about 6% within 1 week. That level persisted for about 6 weeks when the patient developed a transaminitis, which promptly responded to steroids.
His steady-state FVIII within a few weeks was 7% by one-stage assay and about 3% by chromogenic assay, Dr. Chowdary said.
The remaining patients received the mid dose and also achieved FVIII levels of about 6% within a week. Patient 2 started on prophylactic steroids at week 6, per protocol, and did not experience transaminitis, but also had no increase in FVIII level, compared with the low-dose patient, which may be explained by the potential drug half-life, she noted.
Patient 3 reached a FVIII level of about 30% by week 4. He developed transaminitis at that time, which was about 2 weeks before planned prophylactic drug administration, but the transaminitis was controlled by steroids over a period of about 8-10 weeks.
“His steady-state FVIII level by one stage was 34% and by chromogenic assay was 17%. He has not had any bleeds since his gene transfer and has not required any FVIII concentrate either,” she said.
Patient 4 reached a FVIII level of about 40% by week 4. He was given prophylactic steroids at that time because of the occurrence of transaminitis at week 4 in Patient 3.
The patient developed transaminitis during steroid taper about 4 weeks later, perhaps because of the rapid taper, Dr. Chowdary said, adding that the transaminitis was well controlled with steroids, but follow-up in this patient has only been about 12 weeks.
“The characteristics of FVIII expression in this patient are very similar to the previous patient. ... We suspect he will have a steady-state level of about 30%,” she said. “Again, he’s had no bleeds since his gene transfer and has not required any FVIII concentrate.”
The single infusion of this novel vector was well tolerated in each patient, with no evidence of infusion-related reactions, neutralizing anti-FVIII antibodies, or vector-related adverse events.
“The transgene expression was achieved in all patients and at both vector dosages,” Dr. Chowdary said. “What is very important is that the levels of less than 10% had only a modest impact on the bleed rates and FVIII usage, whereas an expression of more than 10% resulted in zero bleeds and the patient did not require any additional FVIII treatment.”
The data are “encouraging,” she said. “We look forward to escalating the dose in the next patient.”
Dr. Chowdary reported financial relationships with Bayer, CSL Behring, Baxalta, Baxter, Biogen, Freeline, Novo Nordisk, Pfizer, Roche, Shire, and SOBI.
SOURCE: Chowdary P et al. ASH 2018, Abstract 489.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: A single infusion of the factor VIII variant resulted in activity levels of about 6%, compared with 1% or less at baseline.
Study details: The findings were from the first four patients in a phase 1/2 dose-escalation study.
Disclosures: Dr. Chowdary reported financial relationships with Bayer, CSL Behring, Baxalta, Baxter, Biogen, Freeline, Novo Nordisk, Pfizer, Roche, Shire, and SOBI.
Source: Chowdary P et al. ASH 2018, Abstract 489.
Pembrolizumab approved for Merkel cell carcinoma
The Food and Drug Administration has , specifically for recurrent locally advanced or metastatic disease.
In a nonrandomized, open-label trial of 50 patients with recurrent locally advanced or metastatic Merkel cell carcinoma who had not received systemic treatment for the advanced disease, the overall response rate was 56% with a complete response rate of 24%; median response duration was not reached. But responses lasting more than 6 months were achieved by 96% and more than 12 months by 54%. The most common adverse reactions included fatigue, musculoskeletal pain, and decreased appetite.
Because it is an accelerated approval, “continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials,” according to the FDA press release announcing the approval.
Pembrolizumab is a programmed death receptor-1 (PD-1)-blocking antibody that was previously approved for treatment of unresectable or metastatic melanoma.
More about the latest approval, as well as full prescribing information, can be found on the FDA’s website.
The Food and Drug Administration has , specifically for recurrent locally advanced or metastatic disease.
In a nonrandomized, open-label trial of 50 patients with recurrent locally advanced or metastatic Merkel cell carcinoma who had not received systemic treatment for the advanced disease, the overall response rate was 56% with a complete response rate of 24%; median response duration was not reached. But responses lasting more than 6 months were achieved by 96% and more than 12 months by 54%. The most common adverse reactions included fatigue, musculoskeletal pain, and decreased appetite.
Because it is an accelerated approval, “continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials,” according to the FDA press release announcing the approval.
Pembrolizumab is a programmed death receptor-1 (PD-1)-blocking antibody that was previously approved for treatment of unresectable or metastatic melanoma.
More about the latest approval, as well as full prescribing information, can be found on the FDA’s website.
The Food and Drug Administration has , specifically for recurrent locally advanced or metastatic disease.
In a nonrandomized, open-label trial of 50 patients with recurrent locally advanced or metastatic Merkel cell carcinoma who had not received systemic treatment for the advanced disease, the overall response rate was 56% with a complete response rate of 24%; median response duration was not reached. But responses lasting more than 6 months were achieved by 96% and more than 12 months by 54%. The most common adverse reactions included fatigue, musculoskeletal pain, and decreased appetite.
Because it is an accelerated approval, “continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials,” according to the FDA press release announcing the approval.
Pembrolizumab is a programmed death receptor-1 (PD-1)-blocking antibody that was previously approved for treatment of unresectable or metastatic melanoma.
More about the latest approval, as well as full prescribing information, can be found on the FDA’s website.
Measuring the Impact of Developmental Encephalopathic Epilepsy
Developmental encephalopathic epilepsies (DEEs) are responsible for a disproportionate number of cases of drug resistance, early deaths, and disability, according to researchers from Northwestern-Feinberg School of Medicine and Yale School of Medicine.
- An analysis of 613 pediatric patients from the Connecticut Study of Epilepsy allowed researchers to classify patients into specific epilepsy syndromes and to reclassify them over a period of 9 years.
- Among these children, 58 were found to have DEEs (9.4%).
- DEEs were more resistant to drug therapy than other epilepsies (71% vs 18%), more likely to cause intellectual disability (84% vs 11%), and more likely to cause death (21% vs <1%).
- The analysis also revealed changes from the initial epilepsy diagnosis over time, eg, Lennox-Gastaut syndrome was initially diagnosed in only 4 children but by the end of 9 years, 22 had received the diagnosis.
Berg AT, Levy SR, Testa FM. Evolution and course of early life developmental encephalopathic epilepsies: Focus on Lennox‐Gastaut syndrome. Epilepsia. 2018;59:2096-2105.
Developmental encephalopathic epilepsies (DEEs) are responsible for a disproportionate number of cases of drug resistance, early deaths, and disability, according to researchers from Northwestern-Feinberg School of Medicine and Yale School of Medicine.
- An analysis of 613 pediatric patients from the Connecticut Study of Epilepsy allowed researchers to classify patients into specific epilepsy syndromes and to reclassify them over a period of 9 years.
- Among these children, 58 were found to have DEEs (9.4%).
- DEEs were more resistant to drug therapy than other epilepsies (71% vs 18%), more likely to cause intellectual disability (84% vs 11%), and more likely to cause death (21% vs <1%).
- The analysis also revealed changes from the initial epilepsy diagnosis over time, eg, Lennox-Gastaut syndrome was initially diagnosed in only 4 children but by the end of 9 years, 22 had received the diagnosis.
Berg AT, Levy SR, Testa FM. Evolution and course of early life developmental encephalopathic epilepsies: Focus on Lennox‐Gastaut syndrome. Epilepsia. 2018;59:2096-2105.
Developmental encephalopathic epilepsies (DEEs) are responsible for a disproportionate number of cases of drug resistance, early deaths, and disability, according to researchers from Northwestern-Feinberg School of Medicine and Yale School of Medicine.
- An analysis of 613 pediatric patients from the Connecticut Study of Epilepsy allowed researchers to classify patients into specific epilepsy syndromes and to reclassify them over a period of 9 years.
- Among these children, 58 were found to have DEEs (9.4%).
- DEEs were more resistant to drug therapy than other epilepsies (71% vs 18%), more likely to cause intellectual disability (84% vs 11%), and more likely to cause death (21% vs <1%).
- The analysis also revealed changes from the initial epilepsy diagnosis over time, eg, Lennox-Gastaut syndrome was initially diagnosed in only 4 children but by the end of 9 years, 22 had received the diagnosis.
Berg AT, Levy SR, Testa FM. Evolution and course of early life developmental encephalopathic epilepsies: Focus on Lennox‐Gastaut syndrome. Epilepsia. 2018;59:2096-2105.
Infraslow Envelope Analysis Holds Promise in Predicting Seizures
Analyzing infraslow amplitude modulations may help clinicians better understand changes in intracranial EEG readings over the long-term and help researchers develop better algorithms to forecast the onset of seizures, according to a report published in Epilepsia.
- Investigators from Yale-New Haven Hospital collected intracranial EEG readings from 13 adult patients with medically refractory epilepsy.
- To derive infraslow envelope correlations, they estimated magnitude-squared coherence at <0.15 Hz of EEG frequency band power time series for all electrode contact pairs.
- Infraslow envelope magnitude-squared coherence increased significantly after patients had their antiepileptic drugs tapered; it also increased at night and decreased during the day.
Joshi RB, Duckrow RB, Goncharova II, et al. Seizure susceptibility and infraslow modulatory activity in the intracranial electroencephalogram. Epilepsia. 2018;59:2075-2085.
Analyzing infraslow amplitude modulations may help clinicians better understand changes in intracranial EEG readings over the long-term and help researchers develop better algorithms to forecast the onset of seizures, according to a report published in Epilepsia.
- Investigators from Yale-New Haven Hospital collected intracranial EEG readings from 13 adult patients with medically refractory epilepsy.
- To derive infraslow envelope correlations, they estimated magnitude-squared coherence at <0.15 Hz of EEG frequency band power time series for all electrode contact pairs.
- Infraslow envelope magnitude-squared coherence increased significantly after patients had their antiepileptic drugs tapered; it also increased at night and decreased during the day.
Joshi RB, Duckrow RB, Goncharova II, et al. Seizure susceptibility and infraslow modulatory activity in the intracranial electroencephalogram. Epilepsia. 2018;59:2075-2085.
Analyzing infraslow amplitude modulations may help clinicians better understand changes in intracranial EEG readings over the long-term and help researchers develop better algorithms to forecast the onset of seizures, according to a report published in Epilepsia.
- Investigators from Yale-New Haven Hospital collected intracranial EEG readings from 13 adult patients with medically refractory epilepsy.
- To derive infraslow envelope correlations, they estimated magnitude-squared coherence at <0.15 Hz of EEG frequency band power time series for all electrode contact pairs.
- Infraslow envelope magnitude-squared coherence increased significantly after patients had their antiepileptic drugs tapered; it also increased at night and decreased during the day.
Joshi RB, Duckrow RB, Goncharova II, et al. Seizure susceptibility and infraslow modulatory activity in the intracranial electroencephalogram. Epilepsia. 2018;59:2075-2085.
Provocative Induction of PNES Doesn’t Require Placebos
Diagnosing psychogenic nonepileptic seizures (PNES) can prove challenging; using provocative induction is one way to detect the disorder. A recent experiment suggests that inducing seizures without the use of a placebo is just as effective as inducing them with one.
- Researchers compared 170 patients suspected of having PNES who underwent provocative induction plus placebo to 170 patients who underwent the same induction procedure without a saline solution placebo.
- Induction triggered a seizure in 79.4% of patients without the help of the placebo, compared to 73.5% with placebo, a non-significant difference.
- Investigators postulated that the greater success rate in the non-placebo group may have resulted from the greater cumulative induction experience of clinicians, which may have influenced the manner and presentation of how the induction was presented.
- The study concluded that experienced clinicians should opt for non-placebo based provocative induction.
Chen DK, Dave H, Gadelmola, K et al. Provocative induction of psychogenic nonepileptic seizures: Noninferiority of an induction technique without versus with placebo. Epilepsia. 2018; 59:e161-e165.
Diagnosing psychogenic nonepileptic seizures (PNES) can prove challenging; using provocative induction is one way to detect the disorder. A recent experiment suggests that inducing seizures without the use of a placebo is just as effective as inducing them with one.
- Researchers compared 170 patients suspected of having PNES who underwent provocative induction plus placebo to 170 patients who underwent the same induction procedure without a saline solution placebo.
- Induction triggered a seizure in 79.4% of patients without the help of the placebo, compared to 73.5% with placebo, a non-significant difference.
- Investigators postulated that the greater success rate in the non-placebo group may have resulted from the greater cumulative induction experience of clinicians, which may have influenced the manner and presentation of how the induction was presented.
- The study concluded that experienced clinicians should opt for non-placebo based provocative induction.
Chen DK, Dave H, Gadelmola, K et al. Provocative induction of psychogenic nonepileptic seizures: Noninferiority of an induction technique without versus with placebo. Epilepsia. 2018; 59:e161-e165.
Diagnosing psychogenic nonepileptic seizures (PNES) can prove challenging; using provocative induction is one way to detect the disorder. A recent experiment suggests that inducing seizures without the use of a placebo is just as effective as inducing them with one.
- Researchers compared 170 patients suspected of having PNES who underwent provocative induction plus placebo to 170 patients who underwent the same induction procedure without a saline solution placebo.
- Induction triggered a seizure in 79.4% of patients without the help of the placebo, compared to 73.5% with placebo, a non-significant difference.
- Investigators postulated that the greater success rate in the non-placebo group may have resulted from the greater cumulative induction experience of clinicians, which may have influenced the manner and presentation of how the induction was presented.
- The study concluded that experienced clinicians should opt for non-placebo based provocative induction.
Chen DK, Dave H, Gadelmola, K et al. Provocative induction of psychogenic nonepileptic seizures: Noninferiority of an induction technique without versus with placebo. Epilepsia. 2018; 59:e161-e165.
mRNA-based urine test performs well in bladder cancer surveillance
An mRNA-based assay for surveillance of patients with bladder cancer outperformed standard urine tests on certain measures in a validation study, investigators report.
The mRNA-based urine test (Xpert, Cepheid) had better sensitivity and negative predictive value compared to urine cytology and to UroVysion fluorescence in situ hybridization (FISH) testing, according to F. Johannes P. van Valenberg, of Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues.
The reported results suggest this mRNA test could partially replace white-light cystoscopy, helping urologists maintain the recommended follow-up schedules for patients with a history of bladder cancer, Dr. van Valenberg and his coauthors wrote. The report is in European Urology.
“If used in the follow-up of non–muscle invasive bladder cancer, a cystoscopy might be waived if the Xpert result is negative,” they wrote.
The negative predictive value for high-grade disease was 98%, suggesting the mRNA-based test could help urologists avoid invasive cystoscopies for intermediate- to high-risk patients, which could reduce costs and patient discomfort, they added.
The prospective, 19-center validation study enrolled 363 individuals with a history of non–muscle invasive bladder cancer who were scheduled for a standard cystoscopy. Voided urine specimens from a total of 259 patients were evaluated with all three methods: the mRNA test, FISH testing, and cytology.
In a comparison of the tests, the mRNA test identified more recurrent cancers correctly, and was more sensitive in detecting low-grade tumors than was FISH (P less than .001) or cytology (P = .021), they reported.
The mRNA test was more sensitive for detection of the most common recurrent tumors, independent of grade.
The sensitivity, specificity, and negative predictive values for mRNA testing were 74%, 80%, and 93%, respectively. By comparison the sensitivity, specificity, and negative predictive values were 51%, 80%, and 88% for FISH and 30%, 90%, and 86% for cytology.
Looking at high-grade disease only, the sensitivity, specificity, and negative predictive values were 83.3%, 75.8%, and 97.6% for mRNA testing, 75.0%, 79.5%, and 96.6% for FISH testing, and 50.0%, 90.7%, and 94.2% for cytology.
Bacillus Calmette-Guérin treatment in the past 90 days did not influence results of the mRNA test, one additional analysis showed.
The Xpert mRNA assay evaluates five mRNA targets: ABL1, ANXA10, UPK1B, CRH, and IGF2, results of which are combined to classify samples as either negative or positive. The test has a “hands-on time” of less than 2 minutes and provides results in 90 minutes, according to Dr. van Valenberg and his coinvestigators.
“Cytology requires a review by a pathologist, is not performed on the same day as a clinic visit, and has associated inter- and intraobserver variability,” the researchers wrote.
Dr. van Valenberg reported no financial disclosures related to the study. Several study coauthors reported employment or financial disclosures related to Cepheid, which provided funding and support for the conduct of the study. Study coauthors provided additional disclosures related to MDxHealth, PHotocure, Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, and Astellas, among others.
SOURCE: van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
An mRNA-based assay for surveillance of patients with bladder cancer outperformed standard urine tests on certain measures in a validation study, investigators report.
The mRNA-based urine test (Xpert, Cepheid) had better sensitivity and negative predictive value compared to urine cytology and to UroVysion fluorescence in situ hybridization (FISH) testing, according to F. Johannes P. van Valenberg, of Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues.
The reported results suggest this mRNA test could partially replace white-light cystoscopy, helping urologists maintain the recommended follow-up schedules for patients with a history of bladder cancer, Dr. van Valenberg and his coauthors wrote. The report is in European Urology.
“If used in the follow-up of non–muscle invasive bladder cancer, a cystoscopy might be waived if the Xpert result is negative,” they wrote.
The negative predictive value for high-grade disease was 98%, suggesting the mRNA-based test could help urologists avoid invasive cystoscopies for intermediate- to high-risk patients, which could reduce costs and patient discomfort, they added.
The prospective, 19-center validation study enrolled 363 individuals with a history of non–muscle invasive bladder cancer who were scheduled for a standard cystoscopy. Voided urine specimens from a total of 259 patients were evaluated with all three methods: the mRNA test, FISH testing, and cytology.
In a comparison of the tests, the mRNA test identified more recurrent cancers correctly, and was more sensitive in detecting low-grade tumors than was FISH (P less than .001) or cytology (P = .021), they reported.
The mRNA test was more sensitive for detection of the most common recurrent tumors, independent of grade.
The sensitivity, specificity, and negative predictive values for mRNA testing were 74%, 80%, and 93%, respectively. By comparison the sensitivity, specificity, and negative predictive values were 51%, 80%, and 88% for FISH and 30%, 90%, and 86% for cytology.
Looking at high-grade disease only, the sensitivity, specificity, and negative predictive values were 83.3%, 75.8%, and 97.6% for mRNA testing, 75.0%, 79.5%, and 96.6% for FISH testing, and 50.0%, 90.7%, and 94.2% for cytology.
Bacillus Calmette-Guérin treatment in the past 90 days did not influence results of the mRNA test, one additional analysis showed.
The Xpert mRNA assay evaluates five mRNA targets: ABL1, ANXA10, UPK1B, CRH, and IGF2, results of which are combined to classify samples as either negative or positive. The test has a “hands-on time” of less than 2 minutes and provides results in 90 minutes, according to Dr. van Valenberg and his coinvestigators.
“Cytology requires a review by a pathologist, is not performed on the same day as a clinic visit, and has associated inter- and intraobserver variability,” the researchers wrote.
Dr. van Valenberg reported no financial disclosures related to the study. Several study coauthors reported employment or financial disclosures related to Cepheid, which provided funding and support for the conduct of the study. Study coauthors provided additional disclosures related to MDxHealth, PHotocure, Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, and Astellas, among others.
SOURCE: van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
An mRNA-based assay for surveillance of patients with bladder cancer outperformed standard urine tests on certain measures in a validation study, investigators report.
The mRNA-based urine test (Xpert, Cepheid) had better sensitivity and negative predictive value compared to urine cytology and to UroVysion fluorescence in situ hybridization (FISH) testing, according to F. Johannes P. van Valenberg, of Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues.
The reported results suggest this mRNA test could partially replace white-light cystoscopy, helping urologists maintain the recommended follow-up schedules for patients with a history of bladder cancer, Dr. van Valenberg and his coauthors wrote. The report is in European Urology.
“If used in the follow-up of non–muscle invasive bladder cancer, a cystoscopy might be waived if the Xpert result is negative,” they wrote.
The negative predictive value for high-grade disease was 98%, suggesting the mRNA-based test could help urologists avoid invasive cystoscopies for intermediate- to high-risk patients, which could reduce costs and patient discomfort, they added.
The prospective, 19-center validation study enrolled 363 individuals with a history of non–muscle invasive bladder cancer who were scheduled for a standard cystoscopy. Voided urine specimens from a total of 259 patients were evaluated with all three methods: the mRNA test, FISH testing, and cytology.
In a comparison of the tests, the mRNA test identified more recurrent cancers correctly, and was more sensitive in detecting low-grade tumors than was FISH (P less than .001) or cytology (P = .021), they reported.
The mRNA test was more sensitive for detection of the most common recurrent tumors, independent of grade.
The sensitivity, specificity, and negative predictive values for mRNA testing were 74%, 80%, and 93%, respectively. By comparison the sensitivity, specificity, and negative predictive values were 51%, 80%, and 88% for FISH and 30%, 90%, and 86% for cytology.
Looking at high-grade disease only, the sensitivity, specificity, and negative predictive values were 83.3%, 75.8%, and 97.6% for mRNA testing, 75.0%, 79.5%, and 96.6% for FISH testing, and 50.0%, 90.7%, and 94.2% for cytology.
Bacillus Calmette-Guérin treatment in the past 90 days did not influence results of the mRNA test, one additional analysis showed.
The Xpert mRNA assay evaluates five mRNA targets: ABL1, ANXA10, UPK1B, CRH, and IGF2, results of which are combined to classify samples as either negative or positive. The test has a “hands-on time” of less than 2 minutes and provides results in 90 minutes, according to Dr. van Valenberg and his coinvestigators.
“Cytology requires a review by a pathologist, is not performed on the same day as a clinic visit, and has associated inter- and intraobserver variability,” the researchers wrote.
Dr. van Valenberg reported no financial disclosures related to the study. Several study coauthors reported employment or financial disclosures related to Cepheid, which provided funding and support for the conduct of the study. Study coauthors provided additional disclosures related to MDxHealth, PHotocure, Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, and Astellas, among others.
SOURCE: van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
FROM EUROPEAN UROLOGY
Key clinical point: A noninvasive mRNA-based urine test performed favorably compared with standard urine tests for surveillance of patients with bladder cancer.
Major finding: The mRNA test had a sensitivity of 74% and negative predictive value of 93%, superior to what was observed with fluorescence in situ hybridization (FISH) testing and cytology, according to investigators.
Study details: Prospective validation study including 363 patients with a history of non–muscle invasive bladder cancer.
Disclosures: Funding and trial support came from Cepheid. Several study coauthors reported employment or financial disclosures related to that company. Dr. van Valenberg reported no financial disclosures related to the study, while coauthors provided disclosures related to Bristol-Myers Squibb, Johnson & Johnson, Roche, Bayer, Astellas, and others.
Source: Van Valenberg FJP et al. Eur Urol. 2018 Dec 12. doi: 10.1016/j.eururo.2018.11.055.
FDA aims to boost safety of platelets for transfusion
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.


