User login
EHR stress linked to burnout
Physicians who experience stress related to the use of health information technology are twice as likely to experience burnout.
Rebekah Gardner, MD, of Brown University in Providence, R.I., and her colleagues surveyed all 4,197 Rhode Island physicians in 2017 to learn how the use of electronic health records affected their practices and their job satisfaction.
Just over a quarter (25.0%) of 1,792 respondents reported burnout. Among electronic health record users (91% of respondents), 70% reported health IT-related stress (J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145).
“After adjustment, physicians reporting poor/marginal time for documentation had 2.8 times the odds of burnout (95% confidence interval, 2.0-4.1; P less than .0001) compared to those reporting sufficient time,” according to the researchers.
The team looked at three stress-related variables: whether the EHR adds to the frustration of one’s day; whether physicians felt they had sufficient time for documentation; and the amount of time spent on the EHR at home. Variables were measured on a four- or five-point scale depending on the question related to the specific stress variable.
Almost two-thirds (64.2%) of respondents “agreed” or “strongly agreed” that EHRs add to the frustration of their day.
“It was the most commonly cited HIT-related stress measure in almost every specialty, with the highest prevalence among emergency physicians (77.6%),” the investigators wrote.
More than a third of physicians (37.7%) reported “moderately high” or “excessive” time spent on EHRs at home; this metric was the most commonly cited stress measure among pediatricians (63.6%).
Nearly half (46.4%) of physicians reported “poor” or “marginal” sufficiency of time for documentation.
“Presence of any 1 of the HIT-related stress measures was associated with approximately twice the odds of burnout among physician respondents,” Dr. Gardner and her colleagues noted, adding that “measuring and addressing HIT-related stress is an important step in reducing workforce burden and improving the care of our patients.”
To alleviate burnout, the authors recommended increased use of scribes, use of medical assistants to help create a more team-based documentation function, improved EHR training, providing more time during the day for documentation, and streamlining documentation expectations, with certain culture shifts needed in some cases (i.e., banning work-related email and clinical tasks for vacationing physicians).
SOURCE: Gardner R et al. J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145.
Physicians who experience stress related to the use of health information technology are twice as likely to experience burnout.
Rebekah Gardner, MD, of Brown University in Providence, R.I., and her colleagues surveyed all 4,197 Rhode Island physicians in 2017 to learn how the use of electronic health records affected their practices and their job satisfaction.
Just over a quarter (25.0%) of 1,792 respondents reported burnout. Among electronic health record users (91% of respondents), 70% reported health IT-related stress (J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145).
“After adjustment, physicians reporting poor/marginal time for documentation had 2.8 times the odds of burnout (95% confidence interval, 2.0-4.1; P less than .0001) compared to those reporting sufficient time,” according to the researchers.
The team looked at three stress-related variables: whether the EHR adds to the frustration of one’s day; whether physicians felt they had sufficient time for documentation; and the amount of time spent on the EHR at home. Variables were measured on a four- or five-point scale depending on the question related to the specific stress variable.
Almost two-thirds (64.2%) of respondents “agreed” or “strongly agreed” that EHRs add to the frustration of their day.
“It was the most commonly cited HIT-related stress measure in almost every specialty, with the highest prevalence among emergency physicians (77.6%),” the investigators wrote.
More than a third of physicians (37.7%) reported “moderately high” or “excessive” time spent on EHRs at home; this metric was the most commonly cited stress measure among pediatricians (63.6%).
Nearly half (46.4%) of physicians reported “poor” or “marginal” sufficiency of time for documentation.
“Presence of any 1 of the HIT-related stress measures was associated with approximately twice the odds of burnout among physician respondents,” Dr. Gardner and her colleagues noted, adding that “measuring and addressing HIT-related stress is an important step in reducing workforce burden and improving the care of our patients.”
To alleviate burnout, the authors recommended increased use of scribes, use of medical assistants to help create a more team-based documentation function, improved EHR training, providing more time during the day for documentation, and streamlining documentation expectations, with certain culture shifts needed in some cases (i.e., banning work-related email and clinical tasks for vacationing physicians).
SOURCE: Gardner R et al. J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145.
Physicians who experience stress related to the use of health information technology are twice as likely to experience burnout.
Rebekah Gardner, MD, of Brown University in Providence, R.I., and her colleagues surveyed all 4,197 Rhode Island physicians in 2017 to learn how the use of electronic health records affected their practices and their job satisfaction.
Just over a quarter (25.0%) of 1,792 respondents reported burnout. Among electronic health record users (91% of respondents), 70% reported health IT-related stress (J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145).
“After adjustment, physicians reporting poor/marginal time for documentation had 2.8 times the odds of burnout (95% confidence interval, 2.0-4.1; P less than .0001) compared to those reporting sufficient time,” according to the researchers.
The team looked at three stress-related variables: whether the EHR adds to the frustration of one’s day; whether physicians felt they had sufficient time for documentation; and the amount of time spent on the EHR at home. Variables were measured on a four- or five-point scale depending on the question related to the specific stress variable.
Almost two-thirds (64.2%) of respondents “agreed” or “strongly agreed” that EHRs add to the frustration of their day.
“It was the most commonly cited HIT-related stress measure in almost every specialty, with the highest prevalence among emergency physicians (77.6%),” the investigators wrote.
More than a third of physicians (37.7%) reported “moderately high” or “excessive” time spent on EHRs at home; this metric was the most commonly cited stress measure among pediatricians (63.6%).
Nearly half (46.4%) of physicians reported “poor” or “marginal” sufficiency of time for documentation.
“Presence of any 1 of the HIT-related stress measures was associated with approximately twice the odds of burnout among physician respondents,” Dr. Gardner and her colleagues noted, adding that “measuring and addressing HIT-related stress is an important step in reducing workforce burden and improving the care of our patients.”
To alleviate burnout, the authors recommended increased use of scribes, use of medical assistants to help create a more team-based documentation function, improved EHR training, providing more time during the day for documentation, and streamlining documentation expectations, with certain culture shifts needed in some cases (i.e., banning work-related email and clinical tasks for vacationing physicians).
SOURCE: Gardner R et al. J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145.
FROM JAMIA
Key clinical point: EHR-related stress is a measurable predictor of physician burnout.
Major finding: Seventy percent of EHR users who responded to a survey reported stress related to the use of health information technology.
Study details: Survey of all 4,197 physicians in Rhode Island; 1,792 (43%) responded.
Disclosures: The Rhode Island Department of Health funded the study. The authors reported no conflicts of interest.
Source: Gardner R et al. J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145.
2018-2019 flu season starts in earnest
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.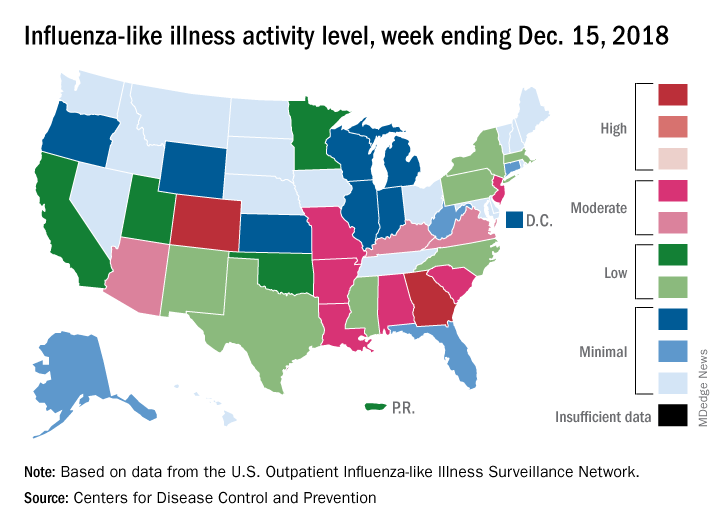
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
Michigan police receive training to recognize mental illness
Responding to a police call can prove dangerous. In those kinds of high-pressure situations, agitation or other manifestations of mental illness might be mistaken for violent intent – with disastrous results.
In Kalamazoo, Mich., crime response training now includes subduing suspects without using violent force. “Through training and education, and scenarios that we use in the training, [the officers] start to detect the different cues or indicators where they start to see that this is really a crisis event. And we treat it as a medical issue and get that person the help that they need,” said Rafael Diaz, executive lieutenant with the Kalamazoo Department of Public Safety in an interview on Michigan NPR.
In the training, called the Crisis Intervention Team model, the goal is to slow down the pace of the interaction and keep some distance between themselves and the suspect after officers recognize signs of mental illness. Both responses can lower the chances of a lash-out response.
“The number of injuries to officers goes down, the number of injuries to the person in crisis goes down, and there is a huge benefit to society there if you don’t have to use physical force,” Mr. Diaz said.
Animal neglect and mental health
Images of neglected and abused livestock on farms can inspire thoughts of how someone could mistreat the animals in their care. “Frankly, if you can’t understand that, it’s probably a good thing. It means you haven’t been in the depths of low, low mental health, depression, and anxiety,” Andria Jones-Bitton, DVM, PhD, said in an interview with the Western Producer.
Dr. Jones-Bitton is a veterinarian and epidemiologist at the Ontario Veterinary College in Guelph. She is studying the mental health and mental resilience of farmers and veterinarians.
“If farmers are struggling with their own well-being and motivation, they’re likely going to find it difficult to invest in improving animal welfare. When we’re mentally unwell, it’s hard to care for ourselves, let alone to care for others, even when those others are really important to us,” she said.
A national survey of Canadian farmers by Dr. Jones-Bitton showed high levels of stress and diminished ability to cope with the pressures that come with running a livestock farm. “What makes me the most upset is I have everything I’ve ever dreamed of – love, family, and a farm, and all I feel is overwhelmed out of control and sad,” one respondent said.
The problems are not unique to Canada. Studies from Ireland, for example, documented an association between animal neglect cases and the mental health, drug/alcohol addiction, and social problems of farmers.
“Even if you didn’t care about the humans that were struggling and you only cared about the animal welfare, you’d be wise to address the issue of farmer stress,” Dr. Jones-Britton said.
Depression and rural America
A recent “Farming in Tough Times” workshop that convened in Minnesota focused on the mental health of farmers. Making a living is challenging for many reasons. One is that prices for commodities are set by others.
“I realized that I can’t change the situation that we’re in. I can’t change milk prices. I can’t stop farms from going bankrupt. But I can change how we are. And we are together, and that really does matter,” said dairy farmer Brenda Rudolph during the workshop, according to a report from the St. Cloud Times, which is part of the USA Today network.
“There is a conversation you people have to have in America, rural America, that says, depression is part of your life. It is not a sign of weakness. It’s a sign of reality,” said Dennis Hoiberg, a farming consultant based in Australia who spoke at the workshop. He added that, from his perspective, the United States still tends to be more repressed about mental health issues than elsewhere in the world – with the focus on stress and not on resiliency.
“Most of you folk are proud folk, and most you folk are very proud of what you do,” Mr. Hoiberg said. “You’re also psychologically exposed because you are a true believer [in what you do].”
Advice offered to lessen the tough times included noticing the beauty in the world, breaking down problems into small chunks that are more easily dealt with and then moving on to the next, sleep, and a good diet.
People with mental illness languishing
Public defenders in Colorado are seeking to have dozens of people diagnosed with mental illness who are in jail awaiting trial set free until their court date. The usual scenario in Colorado for someone charged with a crime and jailed who is deemed mentally incompetent is treatment within 28 days. However, this system is broken and wait times are far longer – in one instance 270 days.
“Many of them are there for very, very low level offenses and they’re holding in jail for way longer than a person who did not suffer from mental illness would be in custody,” said Maureen Cain, policy liaison for the public defender’s office in an interview with the Denver Post. “They are being incarcerated for their mental illness, not really because of the crime they committed.” Responses from judges have ranged from immediate release to finding the incarcerated person guilty of contempt and sending them back to jail.
The Colorado Department of Human Services is in charge of people who have been jailed but have been found to be incompetent to stand trial. Officials there have say they do not have enough bed space or capacity to get people moved out of jail within 28 days.
“We are in a situation where [the human services department] is in breach, and I need to know what efforts are being made to bring it back into compliance,” said federal Judge Nina Y. Wang. “These individuals are not being served, and frankly, the state is not being served.”
“Cruel” practice confined youth
A federal class action lawsuit filed against the Departmental of Children and Family Services (DCFS) in the Chicago area alleges that, from 2015 to 2017, more than 800 youth were being confined to psychiatric hospitals even when they were cleared for discharge. The problem goes back decades and is getting worse, the lawsuit contends.
“I spent Thanksgiving, Christmas, New Year’s, Easter, and my 16th birthday in the hospital,” said Skylar, who’s now 19 years old. “I only got to go outside one time. I felt like a prisoner; I felt very depressed.”
As reported on Chicago’s WGN9 News, the delay between clearance for discharge and actual freedom is a month or more in many of the cases. Acting Cook County Public Guardian Charles Golbert said the practice is “cruel, unusual, and illegal. It’s a violation of the children’s civil and most basic human rights.”
Many of the youth had been incarcerated for setting fires and self-harm and had been rejected by foster parents and other providers, in some cases their own families, who were concerned with the possible behavior of the youth after their release.
“Blame the children is the wrong response from DCFS,” said attorney Russell Ainsworth. “DCFS should be apologizing for not addressing this issue and for violating the Constitution.”
Responding to a police call can prove dangerous. In those kinds of high-pressure situations, agitation or other manifestations of mental illness might be mistaken for violent intent – with disastrous results.
In Kalamazoo, Mich., crime response training now includes subduing suspects without using violent force. “Through training and education, and scenarios that we use in the training, [the officers] start to detect the different cues or indicators where they start to see that this is really a crisis event. And we treat it as a medical issue and get that person the help that they need,” said Rafael Diaz, executive lieutenant with the Kalamazoo Department of Public Safety in an interview on Michigan NPR.
In the training, called the Crisis Intervention Team model, the goal is to slow down the pace of the interaction and keep some distance between themselves and the suspect after officers recognize signs of mental illness. Both responses can lower the chances of a lash-out response.
“The number of injuries to officers goes down, the number of injuries to the person in crisis goes down, and there is a huge benefit to society there if you don’t have to use physical force,” Mr. Diaz said.
Animal neglect and mental health
Images of neglected and abused livestock on farms can inspire thoughts of how someone could mistreat the animals in their care. “Frankly, if you can’t understand that, it’s probably a good thing. It means you haven’t been in the depths of low, low mental health, depression, and anxiety,” Andria Jones-Bitton, DVM, PhD, said in an interview with the Western Producer.
Dr. Jones-Bitton is a veterinarian and epidemiologist at the Ontario Veterinary College in Guelph. She is studying the mental health and mental resilience of farmers and veterinarians.
“If farmers are struggling with their own well-being and motivation, they’re likely going to find it difficult to invest in improving animal welfare. When we’re mentally unwell, it’s hard to care for ourselves, let alone to care for others, even when those others are really important to us,” she said.
A national survey of Canadian farmers by Dr. Jones-Bitton showed high levels of stress and diminished ability to cope with the pressures that come with running a livestock farm. “What makes me the most upset is I have everything I’ve ever dreamed of – love, family, and a farm, and all I feel is overwhelmed out of control and sad,” one respondent said.
The problems are not unique to Canada. Studies from Ireland, for example, documented an association between animal neglect cases and the mental health, drug/alcohol addiction, and social problems of farmers.
“Even if you didn’t care about the humans that were struggling and you only cared about the animal welfare, you’d be wise to address the issue of farmer stress,” Dr. Jones-Britton said.
Depression and rural America
A recent “Farming in Tough Times” workshop that convened in Minnesota focused on the mental health of farmers. Making a living is challenging for many reasons. One is that prices for commodities are set by others.
“I realized that I can’t change the situation that we’re in. I can’t change milk prices. I can’t stop farms from going bankrupt. But I can change how we are. And we are together, and that really does matter,” said dairy farmer Brenda Rudolph during the workshop, according to a report from the St. Cloud Times, which is part of the USA Today network.
“There is a conversation you people have to have in America, rural America, that says, depression is part of your life. It is not a sign of weakness. It’s a sign of reality,” said Dennis Hoiberg, a farming consultant based in Australia who spoke at the workshop. He added that, from his perspective, the United States still tends to be more repressed about mental health issues than elsewhere in the world – with the focus on stress and not on resiliency.
“Most of you folk are proud folk, and most you folk are very proud of what you do,” Mr. Hoiberg said. “You’re also psychologically exposed because you are a true believer [in what you do].”
Advice offered to lessen the tough times included noticing the beauty in the world, breaking down problems into small chunks that are more easily dealt with and then moving on to the next, sleep, and a good diet.
People with mental illness languishing
Public defenders in Colorado are seeking to have dozens of people diagnosed with mental illness who are in jail awaiting trial set free until their court date. The usual scenario in Colorado for someone charged with a crime and jailed who is deemed mentally incompetent is treatment within 28 days. However, this system is broken and wait times are far longer – in one instance 270 days.
“Many of them are there for very, very low level offenses and they’re holding in jail for way longer than a person who did not suffer from mental illness would be in custody,” said Maureen Cain, policy liaison for the public defender’s office in an interview with the Denver Post. “They are being incarcerated for their mental illness, not really because of the crime they committed.” Responses from judges have ranged from immediate release to finding the incarcerated person guilty of contempt and sending them back to jail.
The Colorado Department of Human Services is in charge of people who have been jailed but have been found to be incompetent to stand trial. Officials there have say they do not have enough bed space or capacity to get people moved out of jail within 28 days.
“We are in a situation where [the human services department] is in breach, and I need to know what efforts are being made to bring it back into compliance,” said federal Judge Nina Y. Wang. “These individuals are not being served, and frankly, the state is not being served.”
“Cruel” practice confined youth
A federal class action lawsuit filed against the Departmental of Children and Family Services (DCFS) in the Chicago area alleges that, from 2015 to 2017, more than 800 youth were being confined to psychiatric hospitals even when they were cleared for discharge. The problem goes back decades and is getting worse, the lawsuit contends.
“I spent Thanksgiving, Christmas, New Year’s, Easter, and my 16th birthday in the hospital,” said Skylar, who’s now 19 years old. “I only got to go outside one time. I felt like a prisoner; I felt very depressed.”
As reported on Chicago’s WGN9 News, the delay between clearance for discharge and actual freedom is a month or more in many of the cases. Acting Cook County Public Guardian Charles Golbert said the practice is “cruel, unusual, and illegal. It’s a violation of the children’s civil and most basic human rights.”
Many of the youth had been incarcerated for setting fires and self-harm and had been rejected by foster parents and other providers, in some cases their own families, who were concerned with the possible behavior of the youth after their release.
“Blame the children is the wrong response from DCFS,” said attorney Russell Ainsworth. “DCFS should be apologizing for not addressing this issue and for violating the Constitution.”
Responding to a police call can prove dangerous. In those kinds of high-pressure situations, agitation or other manifestations of mental illness might be mistaken for violent intent – with disastrous results.
In Kalamazoo, Mich., crime response training now includes subduing suspects without using violent force. “Through training and education, and scenarios that we use in the training, [the officers] start to detect the different cues or indicators where they start to see that this is really a crisis event. And we treat it as a medical issue and get that person the help that they need,” said Rafael Diaz, executive lieutenant with the Kalamazoo Department of Public Safety in an interview on Michigan NPR.
In the training, called the Crisis Intervention Team model, the goal is to slow down the pace of the interaction and keep some distance between themselves and the suspect after officers recognize signs of mental illness. Both responses can lower the chances of a lash-out response.
“The number of injuries to officers goes down, the number of injuries to the person in crisis goes down, and there is a huge benefit to society there if you don’t have to use physical force,” Mr. Diaz said.
Animal neglect and mental health
Images of neglected and abused livestock on farms can inspire thoughts of how someone could mistreat the animals in their care. “Frankly, if you can’t understand that, it’s probably a good thing. It means you haven’t been in the depths of low, low mental health, depression, and anxiety,” Andria Jones-Bitton, DVM, PhD, said in an interview with the Western Producer.
Dr. Jones-Bitton is a veterinarian and epidemiologist at the Ontario Veterinary College in Guelph. She is studying the mental health and mental resilience of farmers and veterinarians.
“If farmers are struggling with their own well-being and motivation, they’re likely going to find it difficult to invest in improving animal welfare. When we’re mentally unwell, it’s hard to care for ourselves, let alone to care for others, even when those others are really important to us,” she said.
A national survey of Canadian farmers by Dr. Jones-Bitton showed high levels of stress and diminished ability to cope with the pressures that come with running a livestock farm. “What makes me the most upset is I have everything I’ve ever dreamed of – love, family, and a farm, and all I feel is overwhelmed out of control and sad,” one respondent said.
The problems are not unique to Canada. Studies from Ireland, for example, documented an association between animal neglect cases and the mental health, drug/alcohol addiction, and social problems of farmers.
“Even if you didn’t care about the humans that were struggling and you only cared about the animal welfare, you’d be wise to address the issue of farmer stress,” Dr. Jones-Britton said.
Depression and rural America
A recent “Farming in Tough Times” workshop that convened in Minnesota focused on the mental health of farmers. Making a living is challenging for many reasons. One is that prices for commodities are set by others.
“I realized that I can’t change the situation that we’re in. I can’t change milk prices. I can’t stop farms from going bankrupt. But I can change how we are. And we are together, and that really does matter,” said dairy farmer Brenda Rudolph during the workshop, according to a report from the St. Cloud Times, which is part of the USA Today network.
“There is a conversation you people have to have in America, rural America, that says, depression is part of your life. It is not a sign of weakness. It’s a sign of reality,” said Dennis Hoiberg, a farming consultant based in Australia who spoke at the workshop. He added that, from his perspective, the United States still tends to be more repressed about mental health issues than elsewhere in the world – with the focus on stress and not on resiliency.
“Most of you folk are proud folk, and most you folk are very proud of what you do,” Mr. Hoiberg said. “You’re also psychologically exposed because you are a true believer [in what you do].”
Advice offered to lessen the tough times included noticing the beauty in the world, breaking down problems into small chunks that are more easily dealt with and then moving on to the next, sleep, and a good diet.
People with mental illness languishing
Public defenders in Colorado are seeking to have dozens of people diagnosed with mental illness who are in jail awaiting trial set free until their court date. The usual scenario in Colorado for someone charged with a crime and jailed who is deemed mentally incompetent is treatment within 28 days. However, this system is broken and wait times are far longer – in one instance 270 days.
“Many of them are there for very, very low level offenses and they’re holding in jail for way longer than a person who did not suffer from mental illness would be in custody,” said Maureen Cain, policy liaison for the public defender’s office in an interview with the Denver Post. “They are being incarcerated for their mental illness, not really because of the crime they committed.” Responses from judges have ranged from immediate release to finding the incarcerated person guilty of contempt and sending them back to jail.
The Colorado Department of Human Services is in charge of people who have been jailed but have been found to be incompetent to stand trial. Officials there have say they do not have enough bed space or capacity to get people moved out of jail within 28 days.
“We are in a situation where [the human services department] is in breach, and I need to know what efforts are being made to bring it back into compliance,” said federal Judge Nina Y. Wang. “These individuals are not being served, and frankly, the state is not being served.”
“Cruel” practice confined youth
A federal class action lawsuit filed against the Departmental of Children and Family Services (DCFS) in the Chicago area alleges that, from 2015 to 2017, more than 800 youth were being confined to psychiatric hospitals even when they were cleared for discharge. The problem goes back decades and is getting worse, the lawsuit contends.
“I spent Thanksgiving, Christmas, New Year’s, Easter, and my 16th birthday in the hospital,” said Skylar, who’s now 19 years old. “I only got to go outside one time. I felt like a prisoner; I felt very depressed.”
As reported on Chicago’s WGN9 News, the delay between clearance for discharge and actual freedom is a month or more in many of the cases. Acting Cook County Public Guardian Charles Golbert said the practice is “cruel, unusual, and illegal. It’s a violation of the children’s civil and most basic human rights.”
Many of the youth had been incarcerated for setting fires and self-harm and had been rejected by foster parents and other providers, in some cases their own families, who were concerned with the possible behavior of the youth after their release.
“Blame the children is the wrong response from DCFS,” said attorney Russell Ainsworth. “DCFS should be apologizing for not addressing this issue and for violating the Constitution.”
December 2018
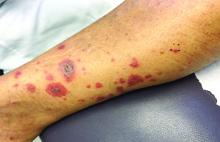
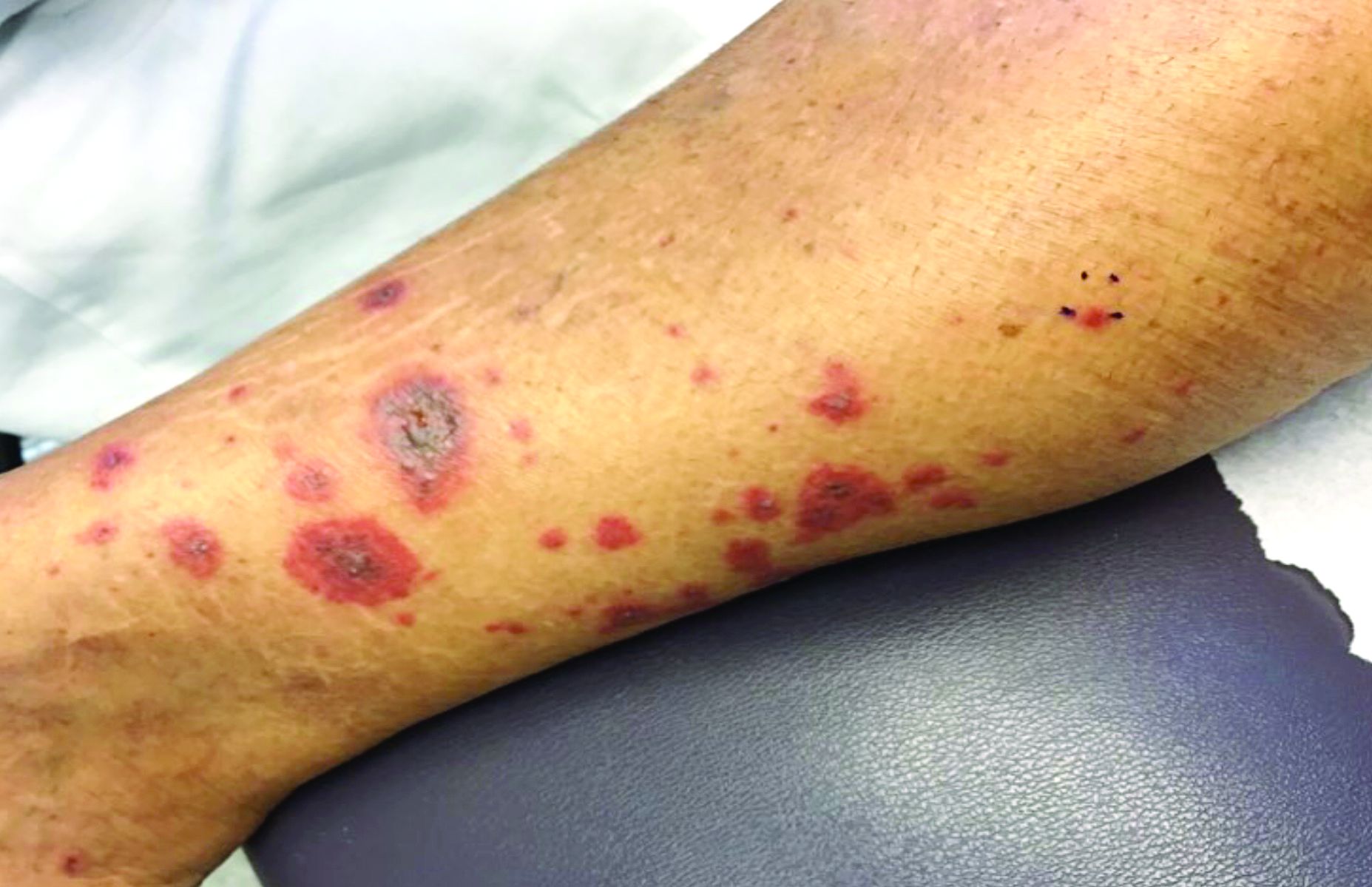
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small-vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. In addition to skin, organs such as joints, kidneys, and intestines can be involved.
where immunoglobulin A (IgA) is deposited in the vessel walls. It is the most common form of vasculitis in children (usually aged 4-8 years). The incidence is higher in the winter. Some patients experience a prodrome of fever, colicky abdominal pain, and joint pain prior to the development of cutaneous symptoms. Disease in children tends to be self-limited. Adults may present with HSP as well, and often exhibit more severe disease that may become chronic with relapses and is more difficult to treat. In both children and adults, infectious causes, such as streptococcus pharyngitis, are the most common trigger. In adults, malignancy may be associated with HSP. A literature search revealed medications implicated in HSP such as antibiotics (vancomycin, penicillin, cephalosporins, clarithromycin), ACE inhibitors, and nonsteroidal anti-inflammatories. Many cases of HSP are idiopathic.
Patients present with erythematous macules that progress to purpura on the extremities. Lesions may be vesicular or bullous and may become necrotic and ulcerate. Arthralgias, often of lower-extremity joints, may be present. Abdominal pain and renal disease may occur in both children and adults. Adults are more likely to develop chronic kidney disease and must be followed carefully with serial blood work and urinalysis to evaluate for hematuria and proteinuria. Severe abdominal pain is an emergency as intussusception may occur.
Histologically, leukocytoclastic vasculitis of small vessels is present. On direct immunofluorescence of perilesional skin, IgA, C3, and fibrin deposits can be seen. Serum IgA is unreliable and may be seen in healthy adults as well.
Treatment is generally supportive as the disease is self-limited. The use of corticosteroids is controversial. This may be effective for joint inflammation, abdominal disease, active nephritis, and ulcerated skin lesions, but doesn’t prevent the recurrence of skin lesions. Dapsone or colchicine can be used for resistant cutaneous lesions. In severe cases, intravenous immunoglobulin may be warranted.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
A 63-year-old white female presented with a 2-week history of hemorrhagic purpuric lesions and necrotic vesicles on the bilateral lower extremities.
Nearly 1 year prior to presentation, the patient underwent surgical resection for lung cancer. The patient also complained of joint swelling and pain in her ankles. She denied abdominal pain. She denied recent illness, including sore throat and upper respiratory infection. Skin biopsies were performed, including for direct immunofluorescence.
FDA approves first treatment for BPDCN
The U.S. Food and Drug Administration (FDA) has approved tagraxofusp-erzs (Elzonris) to treat patients age 2 and older who have blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Tagraxofusp-erzs (formerly SL-401) is a CD123-directed cytotoxin that is the first FDA-approved treatment for BPDCN.
Tagraxofusp-erzs will be commercially available in early 2019, according to Stemline Therapeutics, makers of the drug.
The prescribing information for tagraxofusp-erzs contains a boxed warning noting that the drug is associated with an increased risk of capillary leak syndrome (CLS), which may be life-threatening or fatal.
The FDA previously granted tagraxofusp-erzs breakthrough therapy and orphan drug designations and assessed the drug under priority review.
The FDA’s approval of tagraxofusp-erzs was based on a phase 1 trial (STML-401-0114; NCT02113982).
The trial enrolled 47 patients with BPDCN, including 32 who were treatment-naïve and 15 who were previously treated.
Patients received tagraxofusp-erzs intravenously on days 1-5 of a 21-day cycle for multiple consecutive cycles. The trial had a dose-escalation stage (stage 1), an expansion stage (stage 2), a confirmatory stage (stage 3), and a stage that enabled uninterrupted access to tagraxofusp-erzs (stage 4).
In the confirmatory stage, 13 patients with treatment-naïve BPDCN received tagraxofusp-erzs at the recommended dose and schedule—12 mcg/kg daily for 5 days of a 21-day cycle.
Efficacy was based on the rate of complete response (CR) or clinical complete response (CRc). CRc was defined as CR with residual skin abnormality not indicative of active disease.
The CR/CRc rate was 53.8% (7/13), and the median duration of CR/CRc was not reached (range, 3.9 to 12.2 months).
The safety of tagraxofusp-erzs was assessed in 94 adults with treatment-naïve or previously treated myeloid malignancies, including 58 patients with BPDCN, who were treated at the recommended dose and schedule.
There were two fatal adverse events—both CLS. Eleven percent of patients discontinued treatment with tagraxofusp-erzs due to an adverse event. The most common of these were hepatic toxicities and CLS.
The most common adverse events overall were CLS (55%), nausea (49%), fatigue (45%), peripheral edema (43%), pyrexia (43%), and weight increase (31%).
The most common laboratory abnormalities were decreases in albumin (77%), platelets (67%), hemoglobin (60%), calcium (57%), and sodium (50%), as well as increases in glucose (87%), alanine aminotransferase (82%), and aspartate aminotransferase (79%).
The U.S. Food and Drug Administration (FDA) has approved tagraxofusp-erzs (Elzonris) to treat patients age 2 and older who have blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Tagraxofusp-erzs (formerly SL-401) is a CD123-directed cytotoxin that is the first FDA-approved treatment for BPDCN.
Tagraxofusp-erzs will be commercially available in early 2019, according to Stemline Therapeutics, makers of the drug.
The prescribing information for tagraxofusp-erzs contains a boxed warning noting that the drug is associated with an increased risk of capillary leak syndrome (CLS), which may be life-threatening or fatal.
The FDA previously granted tagraxofusp-erzs breakthrough therapy and orphan drug designations and assessed the drug under priority review.
The FDA’s approval of tagraxofusp-erzs was based on a phase 1 trial (STML-401-0114; NCT02113982).
The trial enrolled 47 patients with BPDCN, including 32 who were treatment-naïve and 15 who were previously treated.
Patients received tagraxofusp-erzs intravenously on days 1-5 of a 21-day cycle for multiple consecutive cycles. The trial had a dose-escalation stage (stage 1), an expansion stage (stage 2), a confirmatory stage (stage 3), and a stage that enabled uninterrupted access to tagraxofusp-erzs (stage 4).
In the confirmatory stage, 13 patients with treatment-naïve BPDCN received tagraxofusp-erzs at the recommended dose and schedule—12 mcg/kg daily for 5 days of a 21-day cycle.
Efficacy was based on the rate of complete response (CR) or clinical complete response (CRc). CRc was defined as CR with residual skin abnormality not indicative of active disease.
The CR/CRc rate was 53.8% (7/13), and the median duration of CR/CRc was not reached (range, 3.9 to 12.2 months).
The safety of tagraxofusp-erzs was assessed in 94 adults with treatment-naïve or previously treated myeloid malignancies, including 58 patients with BPDCN, who were treated at the recommended dose and schedule.
There were two fatal adverse events—both CLS. Eleven percent of patients discontinued treatment with tagraxofusp-erzs due to an adverse event. The most common of these were hepatic toxicities and CLS.
The most common adverse events overall were CLS (55%), nausea (49%), fatigue (45%), peripheral edema (43%), pyrexia (43%), and weight increase (31%).
The most common laboratory abnormalities were decreases in albumin (77%), platelets (67%), hemoglobin (60%), calcium (57%), and sodium (50%), as well as increases in glucose (87%), alanine aminotransferase (82%), and aspartate aminotransferase (79%).
The U.S. Food and Drug Administration (FDA) has approved tagraxofusp-erzs (Elzonris) to treat patients age 2 and older who have blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Tagraxofusp-erzs (formerly SL-401) is a CD123-directed cytotoxin that is the first FDA-approved treatment for BPDCN.
Tagraxofusp-erzs will be commercially available in early 2019, according to Stemline Therapeutics, makers of the drug.
The prescribing information for tagraxofusp-erzs contains a boxed warning noting that the drug is associated with an increased risk of capillary leak syndrome (CLS), which may be life-threatening or fatal.
The FDA previously granted tagraxofusp-erzs breakthrough therapy and orphan drug designations and assessed the drug under priority review.
The FDA’s approval of tagraxofusp-erzs was based on a phase 1 trial (STML-401-0114; NCT02113982).
The trial enrolled 47 patients with BPDCN, including 32 who were treatment-naïve and 15 who were previously treated.
Patients received tagraxofusp-erzs intravenously on days 1-5 of a 21-day cycle for multiple consecutive cycles. The trial had a dose-escalation stage (stage 1), an expansion stage (stage 2), a confirmatory stage (stage 3), and a stage that enabled uninterrupted access to tagraxofusp-erzs (stage 4).
In the confirmatory stage, 13 patients with treatment-naïve BPDCN received tagraxofusp-erzs at the recommended dose and schedule—12 mcg/kg daily for 5 days of a 21-day cycle.
Efficacy was based on the rate of complete response (CR) or clinical complete response (CRc). CRc was defined as CR with residual skin abnormality not indicative of active disease.
The CR/CRc rate was 53.8% (7/13), and the median duration of CR/CRc was not reached (range, 3.9 to 12.2 months).
The safety of tagraxofusp-erzs was assessed in 94 adults with treatment-naïve or previously treated myeloid malignancies, including 58 patients with BPDCN, who were treated at the recommended dose and schedule.
There were two fatal adverse events—both CLS. Eleven percent of patients discontinued treatment with tagraxofusp-erzs due to an adverse event. The most common of these were hepatic toxicities and CLS.
The most common adverse events overall were CLS (55%), nausea (49%), fatigue (45%), peripheral edema (43%), pyrexia (43%), and weight increase (31%).
The most common laboratory abnormalities were decreases in albumin (77%), platelets (67%), hemoglobin (60%), calcium (57%), and sodium (50%), as well as increases in glucose (87%), alanine aminotransferase (82%), and aspartate aminotransferase (79%).
FDA approves ravulizumab for PNH
The U.S. Food and Drug Administration (FDA) has approved ravulizumab-cwvz (Ultomiris) to treat adults with paroxysmal nocturnal hemoglobinuria (PNH).
Ravulizumab is a long-acting C5 complement inhibitor, administered every 8 weeks, that has been shown to prevent hemolysis.
The prescribing information for ravulizumab includes a boxed warning stating that meningococcal infections/sepsis have occurred in patients treated with the drug, and these adverse effects can become life-threatening or fatal if not recognized and treated early.
Ravulizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy.
The FDA previously granted the application for ravulizumab priority review, and the product received orphan drug designation from the FDA.
The FDA granted the approval of ravulizumab to Alexion Pharmaceuticals.
The FDA’s approval of ravulizumab is based on results from two phase 3 studies, one in patients who had previously received treatment with a complement inhibitor and one in patients who were complement-inhibitor-naïve. Both studies were recently published in Blood.
Efficacy in inhibitor-experienced patients
In one study (NCT03056040), researchers compared ravulizumab administered every 8 weeks to eculizumab administered every 2 weeks in complement-inhibitor-experienced patients.
The trial included 195 PNH patients who were taking eculizumab for more than 6 months. They were randomized to switch to ravulizumab (n=97) or continue on eculizumab (n=98).
Ravulizumab proved noninferior to eculizumab for all endpoints studied (P<0.0006), including:
- Percentage change in lactate dehydrogenase (LDH): difference, 9.21% (95% CI: -0.42 to 18.84; P=0.058 for superiority)
- Breakthrough hemolysis: difference, 5.1 (95% CI: -8.89 to 18.99)
- Change in FACIT-Fatigue score: difference, 1.47 (95% CI: -0.21 to 3.15)
- Transfusion avoidance: difference, 5.5 (95% CI: -4.27 to 15.68)
- Stabilized hemoglobin: difference, 1.4 (95% CI: -10.41 to 13.31).
Efficacy in inhibitor-naïve patients
In another study (NCT02946463), researchers compared ravulizumab and eculizumab in 246 PNH patients who had not previously received a complement inhibitor.
Ravulizumab was noninferior to eculizumab for all endpoints (P<0.0001), including:
- Transfusion avoidance: 73.6% vs 66.1%; difference of 6.8% (95% CI: -4.66 to 18.14)
- LDH normalization: 53.6% vs 49.4%; odds ratio=1.19 (95% CI: 0.80 to 1.77)
- Percent reduction in LDH: -76.8% vs -76.0%; difference of -0.83% (95% CI: -5.21 to 3.56)
- Change in FACIT-Fatigue score: 7.07 vs 6.40; difference of 0.67 (95% CI: -1.21 to 2.55)
- Breakthrough hemolysis: 4.0% vs 10.7%; difference of -6.7% (95% CI: -14.21 to 0.18)
- Stabilized hemoglobin: 68.0% vs 64.5%; difference of 2.9 (95% CI: -8.80 to 14.64).
Safety in both trials
The safety data from both trials included 441 adults who received ravulizumab (n=222) or eculizumab (n=219) for a median of 6 months.
The most frequent adverse events in both arms (ravulizumab and eculizumab, respectively) were upper respiratory tract infection (39% and 39%) and headache (32% and 26%).
Serious adverse events occurred in 15 (6.8%) patients treated with ravulizumab. These events included hyperthermia and pyrexia.
There was one fatal case of sepsis in a patient treated with ravulizumab.
The U.S. Food and Drug Administration (FDA) has approved ravulizumab-cwvz (Ultomiris) to treat adults with paroxysmal nocturnal hemoglobinuria (PNH).
Ravulizumab is a long-acting C5 complement inhibitor, administered every 8 weeks, that has been shown to prevent hemolysis.
The prescribing information for ravulizumab includes a boxed warning stating that meningococcal infections/sepsis have occurred in patients treated with the drug, and these adverse effects can become life-threatening or fatal if not recognized and treated early.
Ravulizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy.
The FDA previously granted the application for ravulizumab priority review, and the product received orphan drug designation from the FDA.
The FDA granted the approval of ravulizumab to Alexion Pharmaceuticals.
The FDA’s approval of ravulizumab is based on results from two phase 3 studies, one in patients who had previously received treatment with a complement inhibitor and one in patients who were complement-inhibitor-naïve. Both studies were recently published in Blood.
Efficacy in inhibitor-experienced patients
In one study (NCT03056040), researchers compared ravulizumab administered every 8 weeks to eculizumab administered every 2 weeks in complement-inhibitor-experienced patients.
The trial included 195 PNH patients who were taking eculizumab for more than 6 months. They were randomized to switch to ravulizumab (n=97) or continue on eculizumab (n=98).
Ravulizumab proved noninferior to eculizumab for all endpoints studied (P<0.0006), including:
- Percentage change in lactate dehydrogenase (LDH): difference, 9.21% (95% CI: -0.42 to 18.84; P=0.058 for superiority)
- Breakthrough hemolysis: difference, 5.1 (95% CI: -8.89 to 18.99)
- Change in FACIT-Fatigue score: difference, 1.47 (95% CI: -0.21 to 3.15)
- Transfusion avoidance: difference, 5.5 (95% CI: -4.27 to 15.68)
- Stabilized hemoglobin: difference, 1.4 (95% CI: -10.41 to 13.31).
Efficacy in inhibitor-naïve patients
In another study (NCT02946463), researchers compared ravulizumab and eculizumab in 246 PNH patients who had not previously received a complement inhibitor.
Ravulizumab was noninferior to eculizumab for all endpoints (P<0.0001), including:
- Transfusion avoidance: 73.6% vs 66.1%; difference of 6.8% (95% CI: -4.66 to 18.14)
- LDH normalization: 53.6% vs 49.4%; odds ratio=1.19 (95% CI: 0.80 to 1.77)
- Percent reduction in LDH: -76.8% vs -76.0%; difference of -0.83% (95% CI: -5.21 to 3.56)
- Change in FACIT-Fatigue score: 7.07 vs 6.40; difference of 0.67 (95% CI: -1.21 to 2.55)
- Breakthrough hemolysis: 4.0% vs 10.7%; difference of -6.7% (95% CI: -14.21 to 0.18)
- Stabilized hemoglobin: 68.0% vs 64.5%; difference of 2.9 (95% CI: -8.80 to 14.64).
Safety in both trials
The safety data from both trials included 441 adults who received ravulizumab (n=222) or eculizumab (n=219) for a median of 6 months.
The most frequent adverse events in both arms (ravulizumab and eculizumab, respectively) were upper respiratory tract infection (39% and 39%) and headache (32% and 26%).
Serious adverse events occurred in 15 (6.8%) patients treated with ravulizumab. These events included hyperthermia and pyrexia.
There was one fatal case of sepsis in a patient treated with ravulizumab.
The U.S. Food and Drug Administration (FDA) has approved ravulizumab-cwvz (Ultomiris) to treat adults with paroxysmal nocturnal hemoglobinuria (PNH).
Ravulizumab is a long-acting C5 complement inhibitor, administered every 8 weeks, that has been shown to prevent hemolysis.
The prescribing information for ravulizumab includes a boxed warning stating that meningococcal infections/sepsis have occurred in patients treated with the drug, and these adverse effects can become life-threatening or fatal if not recognized and treated early.
Ravulizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy.
The FDA previously granted the application for ravulizumab priority review, and the product received orphan drug designation from the FDA.
The FDA granted the approval of ravulizumab to Alexion Pharmaceuticals.
The FDA’s approval of ravulizumab is based on results from two phase 3 studies, one in patients who had previously received treatment with a complement inhibitor and one in patients who were complement-inhibitor-naïve. Both studies were recently published in Blood.
Efficacy in inhibitor-experienced patients
In one study (NCT03056040), researchers compared ravulizumab administered every 8 weeks to eculizumab administered every 2 weeks in complement-inhibitor-experienced patients.
The trial included 195 PNH patients who were taking eculizumab for more than 6 months. They were randomized to switch to ravulizumab (n=97) or continue on eculizumab (n=98).
Ravulizumab proved noninferior to eculizumab for all endpoints studied (P<0.0006), including:
- Percentage change in lactate dehydrogenase (LDH): difference, 9.21% (95% CI: -0.42 to 18.84; P=0.058 for superiority)
- Breakthrough hemolysis: difference, 5.1 (95% CI: -8.89 to 18.99)
- Change in FACIT-Fatigue score: difference, 1.47 (95% CI: -0.21 to 3.15)
- Transfusion avoidance: difference, 5.5 (95% CI: -4.27 to 15.68)
- Stabilized hemoglobin: difference, 1.4 (95% CI: -10.41 to 13.31).
Efficacy in inhibitor-naïve patients
In another study (NCT02946463), researchers compared ravulizumab and eculizumab in 246 PNH patients who had not previously received a complement inhibitor.
Ravulizumab was noninferior to eculizumab for all endpoints (P<0.0001), including:
- Transfusion avoidance: 73.6% vs 66.1%; difference of 6.8% (95% CI: -4.66 to 18.14)
- LDH normalization: 53.6% vs 49.4%; odds ratio=1.19 (95% CI: 0.80 to 1.77)
- Percent reduction in LDH: -76.8% vs -76.0%; difference of -0.83% (95% CI: -5.21 to 3.56)
- Change in FACIT-Fatigue score: 7.07 vs 6.40; difference of 0.67 (95% CI: -1.21 to 2.55)
- Breakthrough hemolysis: 4.0% vs 10.7%; difference of -6.7% (95% CI: -14.21 to 0.18)
- Stabilized hemoglobin: 68.0% vs 64.5%; difference of 2.9 (95% CI: -8.80 to 14.64).
Safety in both trials
The safety data from both trials included 441 adults who received ravulizumab (n=222) or eculizumab (n=219) for a median of 6 months.
The most frequent adverse events in both arms (ravulizumab and eculizumab, respectively) were upper respiratory tract infection (39% and 39%) and headache (32% and 26%).
Serious adverse events occurred in 15 (6.8%) patients treated with ravulizumab. These events included hyperthermia and pyrexia.
There was one fatal case of sepsis in a patient treated with ravulizumab.
Black women more likely to have open hysterectomies
Black women were more likely than white women to undergo open hysterectomy, according to a recent analysis of national surgical data by Amy L. Alexander, MD, MS, of Northwestern University, Chicago, and her colleagues.
Even after the researchers controlled for many factors that might influence surgical approach, such as comorbidities and body mass index, black women had an odds ratio of 2.02 to receive open, rather than laparoscopic, hysterectomy (95% confidence interval, 1.85-2.20), according to a study in Obstetrics & Gynecology.
The analysis of the targeted hysterectomy file in the National Surgical Quality Improvement Program (NSQIP) database showed that, of 15,316 women who had hysterectomy for nonmalignant indications, the 25% who were black also were more likely to have major complications with open procedures. Such complications as sepsis, wound dehiscence, prolonged intubation, and death were seen in 4% of the black women, versus 2% of the white women receiving open hysterectomy (P less than .001). Minor complications, such as urinary tract infections, superficial wound infections, and blood transfusions, also were more common for black women having open procedures (11% vs. 7%; P less than .001).
The study used a large national database with detailed information about comorbidities and patient characteristics to look at racial disparities in surgical route and complications for the second-most-common surgical procedure women receive on the United States. The results, said Dr. Alexander and her coauthors, confirm and extend previous work showing these disparities.
Black women are known to have more diabetes and hypertension, as well as higher rates of obesity, compared with white women, wrote Dr. Alexander and her coauthors. Even after they controlled for all these variables, black women still had significantly higher odds of having complications from hysterectomy: The odds ratios for major and minor complications were 1.56 and 1.27, respectively.
Uterine weight was included and tracked as a binary variable, with large uteri considered those weighing 250 g or more. Making uterine weight a binary, rather than continuous or categorical variable, didn’t significantly change results, and realistically mirrors a surgeon’s assessment of a uterus as “large” or “small” when making treatment decisions, Dr. Alexander and her coauthors said.
Because the median weight of uteri from black women was more than double the weight of those from white women (262 g vs. 123 g), the investigators also performed an analysis looking just at women with uterine weight less than 250 g, to ensure that uterine weight alone was not accounting for much of the disparity. In this analysis, the black patients still had an adjusted odds ratio of 1.84 for receiving an open procedure.
“Some of the postoperative complications experienced by black women are likely attributable to the fact that black women are more likely to undergo an open hysterectomy,” noted Dr. Alexander and her colleagues. “However, because black race is still associated with a higher odds of complications, even when adjusting for hysterectomy route, there are other contributing factors that warrant further investigation.” Among these factors, they said, may be access to care and quality of care while hospitalized.
The study’s strengths include the use of the NSQIP database’s prospectively collected data to construct the cohort study; the data base included “important patient-level factors such as uterine size, obesity, and comorbidities not previously available in other secondary data set studies,” noted Dr. Alexander and her colleagues. But the possibility of unmeasured bias persists, they said, and such variables as regional practice patterns and surgeon experience and procedure volume could not be detected from the NSQIP data on hand.
“This study suggests that an important step to reduce the disparity in route of surgery and postoperative complications is to increase access to and use of minimally invasive surgery,” wrote Dr. Alexander and her coauthors.
The study was funded by the National Institutes of Health. Dr. Alexander and her colleagues reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG.0000000000002990
The discrepancies reported by Dr. Alexander and her colleagues between black and white women undergoing hysterectomy for nonmalignant reasons mirror discrepancies found for other oncologic procedures, including colectomy and prostatectomy.
Findings of the current study show that black women continue to be less likely to receive more modern, minimally invasive procedures, and that outcomes are worse for these women, even after controlling for factors that may contribute to complications.
The American College of Obstetricians and Gynecologists (ACOG) has looked at this issue. In a 2015 opinion, an ACOG committee looked at three categories of factors contributing to racial disparities in women’s health care. Patient-level factors include such things as genetics, medical comorbidities, and patient preferences and adherence. Health care system–level factors include insurance status and geographic barriers to accessing care, while stereotyping and implicit bias on the part of practitioners constitute the third set of factors.
All these factors are likely in play for the discrepancies seen in hysterectomy rates. Those who care for black women need to understand the long, shameful history of how black Americans were mistreated, undertreated, and used for medical experimentation without consent. This past shapes present care and contributes to the difficulty black patients have trusting today’s health care systems.
The important question today is how individual health care providers will address their own biases, learn from the past, and move forward to do better for our black patients.
Shanna N. Wingo, MD , is an ob.gyn. and a gynecologic oncologist in private practice in Phoenix. She had no potential conflicts of interest. These remarks were drawn from an editorial accompanying the study by Dr. Alexander et al.( Obstet Gynecol. 2018 Dec 4;133[1]:4-5 ).
The discrepancies reported by Dr. Alexander and her colleagues between black and white women undergoing hysterectomy for nonmalignant reasons mirror discrepancies found for other oncologic procedures, including colectomy and prostatectomy.
Findings of the current study show that black women continue to be less likely to receive more modern, minimally invasive procedures, and that outcomes are worse for these women, even after controlling for factors that may contribute to complications.
The American College of Obstetricians and Gynecologists (ACOG) has looked at this issue. In a 2015 opinion, an ACOG committee looked at three categories of factors contributing to racial disparities in women’s health care. Patient-level factors include such things as genetics, medical comorbidities, and patient preferences and adherence. Health care system–level factors include insurance status and geographic barriers to accessing care, while stereotyping and implicit bias on the part of practitioners constitute the third set of factors.
All these factors are likely in play for the discrepancies seen in hysterectomy rates. Those who care for black women need to understand the long, shameful history of how black Americans were mistreated, undertreated, and used for medical experimentation without consent. This past shapes present care and contributes to the difficulty black patients have trusting today’s health care systems.
The important question today is how individual health care providers will address their own biases, learn from the past, and move forward to do better for our black patients.
Shanna N. Wingo, MD , is an ob.gyn. and a gynecologic oncologist in private practice in Phoenix. She had no potential conflicts of interest. These remarks were drawn from an editorial accompanying the study by Dr. Alexander et al.( Obstet Gynecol. 2018 Dec 4;133[1]:4-5 ).
The discrepancies reported by Dr. Alexander and her colleagues between black and white women undergoing hysterectomy for nonmalignant reasons mirror discrepancies found for other oncologic procedures, including colectomy and prostatectomy.
Findings of the current study show that black women continue to be less likely to receive more modern, minimally invasive procedures, and that outcomes are worse for these women, even after controlling for factors that may contribute to complications.
The American College of Obstetricians and Gynecologists (ACOG) has looked at this issue. In a 2015 opinion, an ACOG committee looked at three categories of factors contributing to racial disparities in women’s health care. Patient-level factors include such things as genetics, medical comorbidities, and patient preferences and adherence. Health care system–level factors include insurance status and geographic barriers to accessing care, while stereotyping and implicit bias on the part of practitioners constitute the third set of factors.
All these factors are likely in play for the discrepancies seen in hysterectomy rates. Those who care for black women need to understand the long, shameful history of how black Americans were mistreated, undertreated, and used for medical experimentation without consent. This past shapes present care and contributes to the difficulty black patients have trusting today’s health care systems.
The important question today is how individual health care providers will address their own biases, learn from the past, and move forward to do better for our black patients.
Shanna N. Wingo, MD , is an ob.gyn. and a gynecologic oncologist in private practice in Phoenix. She had no potential conflicts of interest. These remarks were drawn from an editorial accompanying the study by Dr. Alexander et al.( Obstet Gynecol. 2018 Dec 4;133[1]:4-5 ).
Black women were more likely than white women to undergo open hysterectomy, according to a recent analysis of national surgical data by Amy L. Alexander, MD, MS, of Northwestern University, Chicago, and her colleagues.
Even after the researchers controlled for many factors that might influence surgical approach, such as comorbidities and body mass index, black women had an odds ratio of 2.02 to receive open, rather than laparoscopic, hysterectomy (95% confidence interval, 1.85-2.20), according to a study in Obstetrics & Gynecology.
The analysis of the targeted hysterectomy file in the National Surgical Quality Improvement Program (NSQIP) database showed that, of 15,316 women who had hysterectomy for nonmalignant indications, the 25% who were black also were more likely to have major complications with open procedures. Such complications as sepsis, wound dehiscence, prolonged intubation, and death were seen in 4% of the black women, versus 2% of the white women receiving open hysterectomy (P less than .001). Minor complications, such as urinary tract infections, superficial wound infections, and blood transfusions, also were more common for black women having open procedures (11% vs. 7%; P less than .001).
The study used a large national database with detailed information about comorbidities and patient characteristics to look at racial disparities in surgical route and complications for the second-most-common surgical procedure women receive on the United States. The results, said Dr. Alexander and her coauthors, confirm and extend previous work showing these disparities.
Black women are known to have more diabetes and hypertension, as well as higher rates of obesity, compared with white women, wrote Dr. Alexander and her coauthors. Even after they controlled for all these variables, black women still had significantly higher odds of having complications from hysterectomy: The odds ratios for major and minor complications were 1.56 and 1.27, respectively.
Uterine weight was included and tracked as a binary variable, with large uteri considered those weighing 250 g or more. Making uterine weight a binary, rather than continuous or categorical variable, didn’t significantly change results, and realistically mirrors a surgeon’s assessment of a uterus as “large” or “small” when making treatment decisions, Dr. Alexander and her coauthors said.
Because the median weight of uteri from black women was more than double the weight of those from white women (262 g vs. 123 g), the investigators also performed an analysis looking just at women with uterine weight less than 250 g, to ensure that uterine weight alone was not accounting for much of the disparity. In this analysis, the black patients still had an adjusted odds ratio of 1.84 for receiving an open procedure.
“Some of the postoperative complications experienced by black women are likely attributable to the fact that black women are more likely to undergo an open hysterectomy,” noted Dr. Alexander and her colleagues. “However, because black race is still associated with a higher odds of complications, even when adjusting for hysterectomy route, there are other contributing factors that warrant further investigation.” Among these factors, they said, may be access to care and quality of care while hospitalized.
The study’s strengths include the use of the NSQIP database’s prospectively collected data to construct the cohort study; the data base included “important patient-level factors such as uterine size, obesity, and comorbidities not previously available in other secondary data set studies,” noted Dr. Alexander and her colleagues. But the possibility of unmeasured bias persists, they said, and such variables as regional practice patterns and surgeon experience and procedure volume could not be detected from the NSQIP data on hand.
“This study suggests that an important step to reduce the disparity in route of surgery and postoperative complications is to increase access to and use of minimally invasive surgery,” wrote Dr. Alexander and her coauthors.
The study was funded by the National Institutes of Health. Dr. Alexander and her colleagues reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG.0000000000002990
Black women were more likely than white women to undergo open hysterectomy, according to a recent analysis of national surgical data by Amy L. Alexander, MD, MS, of Northwestern University, Chicago, and her colleagues.
Even after the researchers controlled for many factors that might influence surgical approach, such as comorbidities and body mass index, black women had an odds ratio of 2.02 to receive open, rather than laparoscopic, hysterectomy (95% confidence interval, 1.85-2.20), according to a study in Obstetrics & Gynecology.
The analysis of the targeted hysterectomy file in the National Surgical Quality Improvement Program (NSQIP) database showed that, of 15,316 women who had hysterectomy for nonmalignant indications, the 25% who were black also were more likely to have major complications with open procedures. Such complications as sepsis, wound dehiscence, prolonged intubation, and death were seen in 4% of the black women, versus 2% of the white women receiving open hysterectomy (P less than .001). Minor complications, such as urinary tract infections, superficial wound infections, and blood transfusions, also were more common for black women having open procedures (11% vs. 7%; P less than .001).
The study used a large national database with detailed information about comorbidities and patient characteristics to look at racial disparities in surgical route and complications for the second-most-common surgical procedure women receive on the United States. The results, said Dr. Alexander and her coauthors, confirm and extend previous work showing these disparities.
Black women are known to have more diabetes and hypertension, as well as higher rates of obesity, compared with white women, wrote Dr. Alexander and her coauthors. Even after they controlled for all these variables, black women still had significantly higher odds of having complications from hysterectomy: The odds ratios for major and minor complications were 1.56 and 1.27, respectively.
Uterine weight was included and tracked as a binary variable, with large uteri considered those weighing 250 g or more. Making uterine weight a binary, rather than continuous or categorical variable, didn’t significantly change results, and realistically mirrors a surgeon’s assessment of a uterus as “large” or “small” when making treatment decisions, Dr. Alexander and her coauthors said.
Because the median weight of uteri from black women was more than double the weight of those from white women (262 g vs. 123 g), the investigators also performed an analysis looking just at women with uterine weight less than 250 g, to ensure that uterine weight alone was not accounting for much of the disparity. In this analysis, the black patients still had an adjusted odds ratio of 1.84 for receiving an open procedure.
“Some of the postoperative complications experienced by black women are likely attributable to the fact that black women are more likely to undergo an open hysterectomy,” noted Dr. Alexander and her colleagues. “However, because black race is still associated with a higher odds of complications, even when adjusting for hysterectomy route, there are other contributing factors that warrant further investigation.” Among these factors, they said, may be access to care and quality of care while hospitalized.
The study’s strengths include the use of the NSQIP database’s prospectively collected data to construct the cohort study; the data base included “important patient-level factors such as uterine size, obesity, and comorbidities not previously available in other secondary data set studies,” noted Dr. Alexander and her colleagues. But the possibility of unmeasured bias persists, they said, and such variables as regional practice patterns and surgeon experience and procedure volume could not be detected from the NSQIP data on hand.
“This study suggests that an important step to reduce the disparity in route of surgery and postoperative complications is to increase access to and use of minimally invasive surgery,” wrote Dr. Alexander and her coauthors.
The study was funded by the National Institutes of Health. Dr. Alexander and her colleagues reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG.0000000000002990
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Black women were more likely than white women were to have open hysterectomies.
Major finding:
Study details: Analysis of prospectively collected hysterectomy data of 15,136 women in the NSQIP database.
Disclosures: The study was funded by the National Institutes of Health. Dr. Alexander and her coauthors reported no conflicts of interest.
Source: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG0000000000002990.
FDA approves calaspargase pegol-mknl for ALL
The U.S. Food and Drug Administration (FDA) has approved calaspargase pegol-mknl (Asparlas) as a component of a multi-agent chemotherapeutic regimen to treat acute lymphoblastic leukemia (ALL) in pediatric and young adult patients age 1 month to 21 years.
Calaspargase pegol-mknl is an asparagine-specific enzyme intended to provide a longer interval between doses compared to other available pegaspargase products.
The recommended dosage of calaspargase pegol-mknl is 2,500 U/m2 given no more frequently than every 21 days.
The FDA said it approved calaspargase pegol-mknl because the drug maintained nadir serum asparaginase activity above the level of 0.1 U/mL when given at 2,500 U/m2 every 3 weeks.
Calaspargase pegol-mknl was evaluated in Study DFCI 11-001, a trial of 237 children and adolescents with newly diagnosed ALL or lymphoblastic lymphoma. The patients’ median age was 5 years (range, 1 to 20 years).
They received calaspargase pegol-mknl at 2,500 U/m2 (n=118) or pegaspargase at 2,500 U/m2 (n=119) as part of a Dana-Farber Cancer Institute (DFCI) ALL Consortium backbone therapy.
The median duration of exposure was 8 months for both calaspargase pegol-mknl and pegaspargase.
Among the patients with B-cell lineage ALL, the complete remission rate was 98% in the calaspargase pegol-mknl arm and 99% in the pegaspargase arm. Estimated overall survival rates were comparable between the arms.
Common grade 3 or higher adverse events (in the calaspargase pegol-mknl and pegaspargase arms, respectively) included elevated transaminase (52% and 66%), bilirubin increase (20% and 25%), pancreatitis (18% and 24%), and abnormal clotting studies (14% and 21%).
There was one fatal adverse event among patients on calaspargase pegol-mknl—multi-organ failure in the setting of chronic pancreatitis associated with a pancreatic pseudocyst.
The safety of calaspargase pegol-mknl was also evaluated in Study AALL07P4, a trial of patients with newly diagnosed, high-risk B-precursor ALL.
The patients received calaspargase pegol-mknl at 2,500 U/m2 (n=43) or 2,100 U/m2 (n=68) or pegaspargase at 2,500 U/m2 (n=52) as a component of an augmented Berlin-Frankfurt-Münster regimen.
The patients’ median age was 11 years (range, 1 to 26 years). The median duration of exposure was 7 months for both calaspargase pegol-mknl and pegaspargase.
There were three induction deaths among the 111 patients who received calaspargase pegol-mknl (2.8%) but no induction deaths among the 52 patients treated with pegaspargase.
Additional details on these studies and calaspargase pegol-mknl can be found in the drug’s prescribing information.
Calaspargase pegol-mknl is a product of Servier Pharmaceuticals LLC.
The U.S. Food and Drug Administration (FDA) has approved calaspargase pegol-mknl (Asparlas) as a component of a multi-agent chemotherapeutic regimen to treat acute lymphoblastic leukemia (ALL) in pediatric and young adult patients age 1 month to 21 years.
Calaspargase pegol-mknl is an asparagine-specific enzyme intended to provide a longer interval between doses compared to other available pegaspargase products.
The recommended dosage of calaspargase pegol-mknl is 2,500 U/m2 given no more frequently than every 21 days.
The FDA said it approved calaspargase pegol-mknl because the drug maintained nadir serum asparaginase activity above the level of 0.1 U/mL when given at 2,500 U/m2 every 3 weeks.
Calaspargase pegol-mknl was evaluated in Study DFCI 11-001, a trial of 237 children and adolescents with newly diagnosed ALL or lymphoblastic lymphoma. The patients’ median age was 5 years (range, 1 to 20 years).
They received calaspargase pegol-mknl at 2,500 U/m2 (n=118) or pegaspargase at 2,500 U/m2 (n=119) as part of a Dana-Farber Cancer Institute (DFCI) ALL Consortium backbone therapy.
The median duration of exposure was 8 months for both calaspargase pegol-mknl and pegaspargase.
Among the patients with B-cell lineage ALL, the complete remission rate was 98% in the calaspargase pegol-mknl arm and 99% in the pegaspargase arm. Estimated overall survival rates were comparable between the arms.
Common grade 3 or higher adverse events (in the calaspargase pegol-mknl and pegaspargase arms, respectively) included elevated transaminase (52% and 66%), bilirubin increase (20% and 25%), pancreatitis (18% and 24%), and abnormal clotting studies (14% and 21%).
There was one fatal adverse event among patients on calaspargase pegol-mknl—multi-organ failure in the setting of chronic pancreatitis associated with a pancreatic pseudocyst.
The safety of calaspargase pegol-mknl was also evaluated in Study AALL07P4, a trial of patients with newly diagnosed, high-risk B-precursor ALL.
The patients received calaspargase pegol-mknl at 2,500 U/m2 (n=43) or 2,100 U/m2 (n=68) or pegaspargase at 2,500 U/m2 (n=52) as a component of an augmented Berlin-Frankfurt-Münster regimen.
The patients’ median age was 11 years (range, 1 to 26 years). The median duration of exposure was 7 months for both calaspargase pegol-mknl and pegaspargase.
There were three induction deaths among the 111 patients who received calaspargase pegol-mknl (2.8%) but no induction deaths among the 52 patients treated with pegaspargase.
Additional details on these studies and calaspargase pegol-mknl can be found in the drug’s prescribing information.
Calaspargase pegol-mknl is a product of Servier Pharmaceuticals LLC.
The U.S. Food and Drug Administration (FDA) has approved calaspargase pegol-mknl (Asparlas) as a component of a multi-agent chemotherapeutic regimen to treat acute lymphoblastic leukemia (ALL) in pediatric and young adult patients age 1 month to 21 years.
Calaspargase pegol-mknl is an asparagine-specific enzyme intended to provide a longer interval between doses compared to other available pegaspargase products.
The recommended dosage of calaspargase pegol-mknl is 2,500 U/m2 given no more frequently than every 21 days.
The FDA said it approved calaspargase pegol-mknl because the drug maintained nadir serum asparaginase activity above the level of 0.1 U/mL when given at 2,500 U/m2 every 3 weeks.
Calaspargase pegol-mknl was evaluated in Study DFCI 11-001, a trial of 237 children and adolescents with newly diagnosed ALL or lymphoblastic lymphoma. The patients’ median age was 5 years (range, 1 to 20 years).
They received calaspargase pegol-mknl at 2,500 U/m2 (n=118) or pegaspargase at 2,500 U/m2 (n=119) as part of a Dana-Farber Cancer Institute (DFCI) ALL Consortium backbone therapy.
The median duration of exposure was 8 months for both calaspargase pegol-mknl and pegaspargase.
Among the patients with B-cell lineage ALL, the complete remission rate was 98% in the calaspargase pegol-mknl arm and 99% in the pegaspargase arm. Estimated overall survival rates were comparable between the arms.
Common grade 3 or higher adverse events (in the calaspargase pegol-mknl and pegaspargase arms, respectively) included elevated transaminase (52% and 66%), bilirubin increase (20% and 25%), pancreatitis (18% and 24%), and abnormal clotting studies (14% and 21%).
There was one fatal adverse event among patients on calaspargase pegol-mknl—multi-organ failure in the setting of chronic pancreatitis associated with a pancreatic pseudocyst.
The safety of calaspargase pegol-mknl was also evaluated in Study AALL07P4, a trial of patients with newly diagnosed, high-risk B-precursor ALL.
The patients received calaspargase pegol-mknl at 2,500 U/m2 (n=43) or 2,100 U/m2 (n=68) or pegaspargase at 2,500 U/m2 (n=52) as a component of an augmented Berlin-Frankfurt-Münster regimen.
The patients’ median age was 11 years (range, 1 to 26 years). The median duration of exposure was 7 months for both calaspargase pegol-mknl and pegaspargase.
There were three induction deaths among the 111 patients who received calaspargase pegol-mknl (2.8%) but no induction deaths among the 52 patients treated with pegaspargase.
Additional details on these studies and calaspargase pegol-mknl can be found in the drug’s prescribing information.
Calaspargase pegol-mknl is a product of Servier Pharmaceuticals LLC.
FDA expands Essure’s postmarketing surveillance study
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
With hemp now legal, FDA reaffirms rules for cannabis compounds
The newly enacted Agriculture Improvement Act of 2018 legalizes hemp production and use, but the Food and Drug Administration’s regulation of cannabis and cannabis-derived products remains unchanged.
The act (H.R. 2) revamps federal authorities’ regulatory approach to hemp production. The law removes hemp from the Controlled Substances Act, which means it is no longer an illegal substance. Hemp is now defined as cannabis and derivatives of cannabis that have extremely low concentrations (less than 0.3%, on a dry weight basis) of the psychoactive compound delta-9-tetrahydrocannabinol (THC).
Despite hemp’s new legal status, FDA Commissioner Scott Gottlieb, MD, said the FDA’s regulation of cannabis and cannabis-derived products remains the same.
“In short, we treat products containing cannabis or cannabis-derived compounds as we do any other FDA-regulated products,” FDA Commissioner Scott Gottlieb, MD, said in a Dec. 20 statement published on the FDA website. That means “they’re subject to the same authorities and requirements as FDA-regulated products containing any other substance.”
The regulation of those products will be the same regardless of the source of the substance, including from plants classified as hemp. The FDA will require cannabis and cannabis-derived products to undergo testing similar to other drug products, given the concern regarding medical claims made about those products.
“Cannabis and cannabis-derived products claiming in their marketing and promotional materials that they’re intended for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases (such as cancer, Alzheimer’s disease, psychiatric disorders and diabetes) are considered new drugs or new animal drugs,” Dr. Gottlieb explained. And they “must go through the FDA drug approval process for human or animal use before they are marketed in the United States.”
Selling unapproved products with unsubstantiated claims is a “violation of the law.”
In addition, “it’s unlawful under the [Food, Drug & Cosmetics Act] to introduce food containing added CBD [cannabidiol] or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp derived,” Dr. Gottlieb noted. That’s because both CBD and THC are active ingredients in FDA-approved drugs.
Commissioner Gottlieb also noted that “pathways remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement.” The FDA announced plans for a future meeting to discuss the lawful marketing of hemp-derived foods that do not contain CBD or THC.
The newly enacted Agriculture Improvement Act of 2018 legalizes hemp production and use, but the Food and Drug Administration’s regulation of cannabis and cannabis-derived products remains unchanged.
The act (H.R. 2) revamps federal authorities’ regulatory approach to hemp production. The law removes hemp from the Controlled Substances Act, which means it is no longer an illegal substance. Hemp is now defined as cannabis and derivatives of cannabis that have extremely low concentrations (less than 0.3%, on a dry weight basis) of the psychoactive compound delta-9-tetrahydrocannabinol (THC).
Despite hemp’s new legal status, FDA Commissioner Scott Gottlieb, MD, said the FDA’s regulation of cannabis and cannabis-derived products remains the same.
“In short, we treat products containing cannabis or cannabis-derived compounds as we do any other FDA-regulated products,” FDA Commissioner Scott Gottlieb, MD, said in a Dec. 20 statement published on the FDA website. That means “they’re subject to the same authorities and requirements as FDA-regulated products containing any other substance.”
The regulation of those products will be the same regardless of the source of the substance, including from plants classified as hemp. The FDA will require cannabis and cannabis-derived products to undergo testing similar to other drug products, given the concern regarding medical claims made about those products.
“Cannabis and cannabis-derived products claiming in their marketing and promotional materials that they’re intended for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases (such as cancer, Alzheimer’s disease, psychiatric disorders and diabetes) are considered new drugs or new animal drugs,” Dr. Gottlieb explained. And they “must go through the FDA drug approval process for human or animal use before they are marketed in the United States.”
Selling unapproved products with unsubstantiated claims is a “violation of the law.”
In addition, “it’s unlawful under the [Food, Drug & Cosmetics Act] to introduce food containing added CBD [cannabidiol] or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp derived,” Dr. Gottlieb noted. That’s because both CBD and THC are active ingredients in FDA-approved drugs.
Commissioner Gottlieb also noted that “pathways remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement.” The FDA announced plans for a future meeting to discuss the lawful marketing of hemp-derived foods that do not contain CBD or THC.
The newly enacted Agriculture Improvement Act of 2018 legalizes hemp production and use, but the Food and Drug Administration’s regulation of cannabis and cannabis-derived products remains unchanged.
The act (H.R. 2) revamps federal authorities’ regulatory approach to hemp production. The law removes hemp from the Controlled Substances Act, which means it is no longer an illegal substance. Hemp is now defined as cannabis and derivatives of cannabis that have extremely low concentrations (less than 0.3%, on a dry weight basis) of the psychoactive compound delta-9-tetrahydrocannabinol (THC).
Despite hemp’s new legal status, FDA Commissioner Scott Gottlieb, MD, said the FDA’s regulation of cannabis and cannabis-derived products remains the same.
“In short, we treat products containing cannabis or cannabis-derived compounds as we do any other FDA-regulated products,” FDA Commissioner Scott Gottlieb, MD, said in a Dec. 20 statement published on the FDA website. That means “they’re subject to the same authorities and requirements as FDA-regulated products containing any other substance.”
The regulation of those products will be the same regardless of the source of the substance, including from plants classified as hemp. The FDA will require cannabis and cannabis-derived products to undergo testing similar to other drug products, given the concern regarding medical claims made about those products.
“Cannabis and cannabis-derived products claiming in their marketing and promotional materials that they’re intended for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases (such as cancer, Alzheimer’s disease, psychiatric disorders and diabetes) are considered new drugs or new animal drugs,” Dr. Gottlieb explained. And they “must go through the FDA drug approval process for human or animal use before they are marketed in the United States.”
Selling unapproved products with unsubstantiated claims is a “violation of the law.”
In addition, “it’s unlawful under the [Food, Drug & Cosmetics Act] to introduce food containing added CBD [cannabidiol] or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp derived,” Dr. Gottlieb noted. That’s because both CBD and THC are active ingredients in FDA-approved drugs.
Commissioner Gottlieb also noted that “pathways remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement.” The FDA announced plans for a future meeting to discuss the lawful marketing of hemp-derived foods that do not contain CBD or THC.