User login
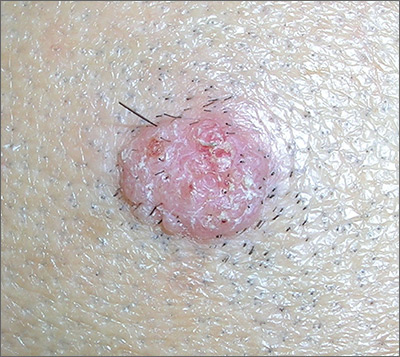
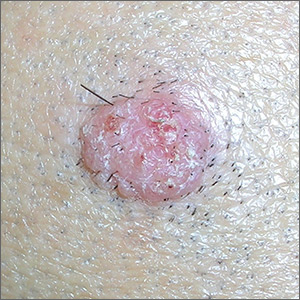
Wart on scalp
The FP had seen many recalcitrant warts before, but rather than repeat the cryotherapy, he performed a shave biopsy to get a definitive diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The biopsy revealed a well-differentiated squamous cell carcinoma (SCC).
This lesion may have started with a wart, as human papillomavirus (HPV) is both the cause of warts and a risk factor for cutaneous SCC. The FP referred the patient to a Mohs surgeon for complete excision of the SCC. He also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP had seen many recalcitrant warts before, but rather than repeat the cryotherapy, he performed a shave biopsy to get a definitive diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The biopsy revealed a well-differentiated squamous cell carcinoma (SCC).
This lesion may have started with a wart, as human papillomavirus (HPV) is both the cause of warts and a risk factor for cutaneous SCC. The FP referred the patient to a Mohs surgeon for complete excision of the SCC. He also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP had seen many recalcitrant warts before, but rather than repeat the cryotherapy, he performed a shave biopsy to get a definitive diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The biopsy revealed a well-differentiated squamous cell carcinoma (SCC).
This lesion may have started with a wart, as human papillomavirus (HPV) is both the cause of warts and a risk factor for cutaneous SCC. The FP referred the patient to a Mohs surgeon for complete excision of the SCC. He also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Cute aggression, a soused super spy, and hospital holiday discharges
I could just eat your little toes!
You know how people are weird around babies and act like they want to squeeze them to death? Yeah, that’s a real phenomenon that goes by the name “cute aggression.” It’s a neural response to adorable stimuli that makes us want to hug and pinch and even bite cute things – baby animals included. A researcher from the University of California, Riverside, took a look at why we do this weird, weird thing.
Using electrophysiology, Katherine Stavropoulos determined that the brain’s reward system plays an integral part in our inexplicable urge to nibble on babies and puppies. She hypothesizes that this may be an evolutionary adaptation, as a way of tempering the feelings of being positively overwhelmed by cuteness. Instead of becoming wholly incapacitated by the sight of your own progeny, your brain responds with some light aggression to snap you out of it and spur you to continue to provide for your young. Reason #12,849 that the human mind is one of the most bizarre things in this universe.
Shaken, not stirred
“No, Mr. Bond, I expect you to imbibe.” A new study published in the Medical Journal of Australia has taken a close look at James Bond’s drinking habits over the past 60 years. Unsurprisingly, researchers reported that 007 “has drunk heavily and consistently across 6 decades.” At least he’s consistent.
Study authors estimated that the secret agent’s peak blood alcohol content reached 0.36 g/dL, high enough to kill some people. Maybe weaker men perhaps, but not our Bond. His postdrinking activities include “fights, driving vehicles, gambling, sex, athletic extremes, and operating complex machinery, or devices.” He’s very good at multitasking. Researchers concluded that Bond has a severe drinking problem, according to DSM-5 criteria for alcohol use disorder. They recommend he seek professional help, and they also suggest that MI6 could be a more responsible employer by offering services for his drinking problem and likely PTSD.
“Home Alone,” with life-threatening injuries
The cinematic holiday hit “Home Alone” features an intrepid 8-year-old left alone to defend his house from the depredations of the two notorious Wet Bandits. While some say “intrepid,” others say “sadistic.” Young Kevin McCallister visits a veritable Spanish Inquisition’s worth of torments upon the iniquitous heads, torsos, and extremities of the larcenous duo: BB gunshots, falling steam irons, paint cans to the brain bucket, and everyone’s favorite theft deterrent, the old blowtorch-to-the-scalp routine.
The slapstick Wet Bandits survived their ordeal to burgle again in an equally painful sequel. But would Marv and Harry’s on-screen survival be possible in the world of real-life Sherman-Williams cans? Dr. Ryan St. Clair of the Weill Cornell Medical College in New York examined their celluloid injuries and offered some real-world diagnoses.
Close-range BB gunshots to the head and groin? They’d break the skin, sure. But the Wet Bandits’ skulls and scrotums would likely remain intact. Ah, but what about a steam iron to the face? Marv could expect a “blowout fracture,” leading to serious disfigurement and debilitating double vision. Paint can to the head? Ten pounds of paint can at the end of a 10-foot rope equals a roughly 2-kN blow to the face. Not only are the pair both out cold, they’re probably sporting toothless grins. And that blowtorch to the scalp? Harry’s now the victim of a full-thickness burn likely to cause skull bone necrosis that demands a transplant.
Dr. St. Clair’s expert medical summation? “This movie was way more believable when I was 8.”
Discharged for the holidays
No one wants to be in the hospital during the Christmas holiday, but the most wonderful time of the year could be the most dangerous time to leave the hospital. Canadian investigators compared a group of patients who were discharged during the 2-week holiday period with patients released during control periods before and after the holiday season.
The analysis revealed that the risk of death or readmission was higher within the next 7 days (odds ratio, 1.16), 14 days (OR, 1.14), and 30 days (OR, 1.09) for the group discharged during the holiday period (BMJ. 2018 Dec 10;363. doi: 10.1136/bmj.k4481). “The holiday period might be a time of reduced access to outpatient care” as caregivers take time off, the investigators suggested, or “patients might prefer to postpone their follow-up visit until their usual physician is available, or until the end of the holiday festivities or travel commitments.”
This hospital-related holiday danger is new information, of course, but maybe it’s not such a surprise. The beloved Christmas characters are, after all, a rather unhealthy bunch: Santa is obese, Rudolf has a stunningly severe case of rosacea, Charlie Brown is depressed, and don’t even get us started on the Grinch and his rapidly expanding heart.
I could just eat your little toes!
You know how people are weird around babies and act like they want to squeeze them to death? Yeah, that’s a real phenomenon that goes by the name “cute aggression.” It’s a neural response to adorable stimuli that makes us want to hug and pinch and even bite cute things – baby animals included. A researcher from the University of California, Riverside, took a look at why we do this weird, weird thing.
Using electrophysiology, Katherine Stavropoulos determined that the brain’s reward system plays an integral part in our inexplicable urge to nibble on babies and puppies. She hypothesizes that this may be an evolutionary adaptation, as a way of tempering the feelings of being positively overwhelmed by cuteness. Instead of becoming wholly incapacitated by the sight of your own progeny, your brain responds with some light aggression to snap you out of it and spur you to continue to provide for your young. Reason #12,849 that the human mind is one of the most bizarre things in this universe.
Shaken, not stirred
“No, Mr. Bond, I expect you to imbibe.” A new study published in the Medical Journal of Australia has taken a close look at James Bond’s drinking habits over the past 60 years. Unsurprisingly, researchers reported that 007 “has drunk heavily and consistently across 6 decades.” At least he’s consistent.
Study authors estimated that the secret agent’s peak blood alcohol content reached 0.36 g/dL, high enough to kill some people. Maybe weaker men perhaps, but not our Bond. His postdrinking activities include “fights, driving vehicles, gambling, sex, athletic extremes, and operating complex machinery, or devices.” He’s very good at multitasking. Researchers concluded that Bond has a severe drinking problem, according to DSM-5 criteria for alcohol use disorder. They recommend he seek professional help, and they also suggest that MI6 could be a more responsible employer by offering services for his drinking problem and likely PTSD.
“Home Alone,” with life-threatening injuries
The cinematic holiday hit “Home Alone” features an intrepid 8-year-old left alone to defend his house from the depredations of the two notorious Wet Bandits. While some say “intrepid,” others say “sadistic.” Young Kevin McCallister visits a veritable Spanish Inquisition’s worth of torments upon the iniquitous heads, torsos, and extremities of the larcenous duo: BB gunshots, falling steam irons, paint cans to the brain bucket, and everyone’s favorite theft deterrent, the old blowtorch-to-the-scalp routine.
The slapstick Wet Bandits survived their ordeal to burgle again in an equally painful sequel. But would Marv and Harry’s on-screen survival be possible in the world of real-life Sherman-Williams cans? Dr. Ryan St. Clair of the Weill Cornell Medical College in New York examined their celluloid injuries and offered some real-world diagnoses.
Close-range BB gunshots to the head and groin? They’d break the skin, sure. But the Wet Bandits’ skulls and scrotums would likely remain intact. Ah, but what about a steam iron to the face? Marv could expect a “blowout fracture,” leading to serious disfigurement and debilitating double vision. Paint can to the head? Ten pounds of paint can at the end of a 10-foot rope equals a roughly 2-kN blow to the face. Not only are the pair both out cold, they’re probably sporting toothless grins. And that blowtorch to the scalp? Harry’s now the victim of a full-thickness burn likely to cause skull bone necrosis that demands a transplant.
Dr. St. Clair’s expert medical summation? “This movie was way more believable when I was 8.”
Discharged for the holidays
No one wants to be in the hospital during the Christmas holiday, but the most wonderful time of the year could be the most dangerous time to leave the hospital. Canadian investigators compared a group of patients who were discharged during the 2-week holiday period with patients released during control periods before and after the holiday season.
The analysis revealed that the risk of death or readmission was higher within the next 7 days (odds ratio, 1.16), 14 days (OR, 1.14), and 30 days (OR, 1.09) for the group discharged during the holiday period (BMJ. 2018 Dec 10;363. doi: 10.1136/bmj.k4481). “The holiday period might be a time of reduced access to outpatient care” as caregivers take time off, the investigators suggested, or “patients might prefer to postpone their follow-up visit until their usual physician is available, or until the end of the holiday festivities or travel commitments.”
This hospital-related holiday danger is new information, of course, but maybe it’s not such a surprise. The beloved Christmas characters are, after all, a rather unhealthy bunch: Santa is obese, Rudolf has a stunningly severe case of rosacea, Charlie Brown is depressed, and don’t even get us started on the Grinch and his rapidly expanding heart.
I could just eat your little toes!
You know how people are weird around babies and act like they want to squeeze them to death? Yeah, that’s a real phenomenon that goes by the name “cute aggression.” It’s a neural response to adorable stimuli that makes us want to hug and pinch and even bite cute things – baby animals included. A researcher from the University of California, Riverside, took a look at why we do this weird, weird thing.
Using electrophysiology, Katherine Stavropoulos determined that the brain’s reward system plays an integral part in our inexplicable urge to nibble on babies and puppies. She hypothesizes that this may be an evolutionary adaptation, as a way of tempering the feelings of being positively overwhelmed by cuteness. Instead of becoming wholly incapacitated by the sight of your own progeny, your brain responds with some light aggression to snap you out of it and spur you to continue to provide for your young. Reason #12,849 that the human mind is one of the most bizarre things in this universe.
Shaken, not stirred
“No, Mr. Bond, I expect you to imbibe.” A new study published in the Medical Journal of Australia has taken a close look at James Bond’s drinking habits over the past 60 years. Unsurprisingly, researchers reported that 007 “has drunk heavily and consistently across 6 decades.” At least he’s consistent.
Study authors estimated that the secret agent’s peak blood alcohol content reached 0.36 g/dL, high enough to kill some people. Maybe weaker men perhaps, but not our Bond. His postdrinking activities include “fights, driving vehicles, gambling, sex, athletic extremes, and operating complex machinery, or devices.” He’s very good at multitasking. Researchers concluded that Bond has a severe drinking problem, according to DSM-5 criteria for alcohol use disorder. They recommend he seek professional help, and they also suggest that MI6 could be a more responsible employer by offering services for his drinking problem and likely PTSD.
“Home Alone,” with life-threatening injuries
The cinematic holiday hit “Home Alone” features an intrepid 8-year-old left alone to defend his house from the depredations of the two notorious Wet Bandits. While some say “intrepid,” others say “sadistic.” Young Kevin McCallister visits a veritable Spanish Inquisition’s worth of torments upon the iniquitous heads, torsos, and extremities of the larcenous duo: BB gunshots, falling steam irons, paint cans to the brain bucket, and everyone’s favorite theft deterrent, the old blowtorch-to-the-scalp routine.
The slapstick Wet Bandits survived their ordeal to burgle again in an equally painful sequel. But would Marv and Harry’s on-screen survival be possible in the world of real-life Sherman-Williams cans? Dr. Ryan St. Clair of the Weill Cornell Medical College in New York examined their celluloid injuries and offered some real-world diagnoses.
Close-range BB gunshots to the head and groin? They’d break the skin, sure. But the Wet Bandits’ skulls and scrotums would likely remain intact. Ah, but what about a steam iron to the face? Marv could expect a “blowout fracture,” leading to serious disfigurement and debilitating double vision. Paint can to the head? Ten pounds of paint can at the end of a 10-foot rope equals a roughly 2-kN blow to the face. Not only are the pair both out cold, they’re probably sporting toothless grins. And that blowtorch to the scalp? Harry’s now the victim of a full-thickness burn likely to cause skull bone necrosis that demands a transplant.
Dr. St. Clair’s expert medical summation? “This movie was way more believable when I was 8.”
Discharged for the holidays
No one wants to be in the hospital during the Christmas holiday, but the most wonderful time of the year could be the most dangerous time to leave the hospital. Canadian investigators compared a group of patients who were discharged during the 2-week holiday period with patients released during control periods before and after the holiday season.
The analysis revealed that the risk of death or readmission was higher within the next 7 days (odds ratio, 1.16), 14 days (OR, 1.14), and 30 days (OR, 1.09) for the group discharged during the holiday period (BMJ. 2018 Dec 10;363. doi: 10.1136/bmj.k4481). “The holiday period might be a time of reduced access to outpatient care” as caregivers take time off, the investigators suggested, or “patients might prefer to postpone their follow-up visit until their usual physician is available, or until the end of the holiday festivities or travel commitments.”
This hospital-related holiday danger is new information, of course, but maybe it’s not such a surprise. The beloved Christmas characters are, after all, a rather unhealthy bunch: Santa is obese, Rudolf has a stunningly severe case of rosacea, Charlie Brown is depressed, and don’t even get us started on the Grinch and his rapidly expanding heart.
Guest editorial: Best of both worlds
Within 3 minutes of the car engine rumbling to a roar in the morning air, cruise control is set, freshly ground coffee is in hand and NPR is playing on WOSU 90.5. I settle in for the morning news on my 45-minute commute to the hospital. Sure, I could’ve found a hospital closer to shorten my commute, especially since I live in the 14th largest metropolitan city in the country.
If I’d wanted, I could be knocking out carotid endarterectomies at a level 1 trauma center, three blocks away from my front door. But no, that’s not what does it for me. What does? It’s having the opportunity to be my own boss and care for salt-of-the-earth folks in rural America.
You see, 5 years ago when I finished my vascular surgery fellowship at Good Samaritan Hospital in Cincinnati, I opened my own solo private practice in a rural community: population 30,000. Yep, that’s right, you heard it. I hung a shingle and went old school. And now as I reflect over the lessons learned during the first half-decade of my practice at Ohio Vein & Vascular Inc., I can tell you it has been a hell of a ride, and boy have we learned a lot.
The better half of the ‘we’ is my wife, Crystal, who doubles as my practice administrator, with her own solid foundation coming from a doctorate in physical therapy. We have successfully built a small company with four full-time employees, one contract registered vascular technologist, and two therapy dogs who serve more than 3,500 patients to date.
From the first day I opened my doors to this small-town rural community, I realized that it’s not what you know, but rather who you know. Well frankly, I didn’t know a soul!
Fortunately, my front office manager was born, raised, and still lives in Wilmington and knows everyone’s mother, brother, sister, niece, and grandchild in what felt like a 60-mile radius. She gave this young, slick city kid from Columbus instant street cred despite all the fancy credentials behind my name. I ditched the tie and fancy shoes and embraced my new ‘work’ home with open arms.
In a community such as Wilmington, Ohio, it’s the little things that count. I wear my own scrubs on days when I operate. Not only do they have my practice logo embroidered on the chest pocket, but they are also adorned with the brown leather symbol for Carhart, a clothing brand. In rural America, Carhart denim clothing – overall bibs, jackets, gloves, etc., are considered king. When my patients see that symbol, there is an instant point of mutual appreciation and it almost always results in some good laughs – who knew Carhart made scrubs?
As a result, I’ve been offered opportunities to ride combines, go drag racing, and go hunting for the infamous morel mushrooms. Just to be clear, I haven’t found a morel yet, so I guess I will stick to my day job as a surgeon.
Having a good laugh and connecting with my patients was something I was not accustomed to in my training. I was there to operate, and rarely participated in office days. At times this routine left me feeling unappreciated by my patients and their families. I was just a surgeon delivering bad news. I now find myself fortunate to have the opportunity to get to know my patients and participate in their health care, and I know they appreciate me for it.
A recent malpractice survey cited a finding that the more patients ‘like’ their physicians, the less likely they are to file malpractice lawsuits against them.1 Other reports have suggested that the relationship a physician has with a patient is a critical factor, more so than any single medical mistake, in determining whether or not a lawsuit is filed.2,3
While I feel appreciated and ‘liked’ by my patients, I’ve learned that I am not necessarily their favorite employee in the office. This honor is most often bestowed upon Claire and Whitney, aka “The Girls” – our two, miniature Labradoodles who serve in the capacity of therapy dogs and have perfected the ability to nap in nearly any situation. Try as I may to convince patients that what I am saying is important, they never lose focus on The Girls. They are the first thing patients ask about, I swear they receive more gifts than I do, and they always are on the receiving end of some good ‘pets’ as my patients leave the office. Despite any bad news they may have been told, very rarely do patients leave my office without smiles on their faces. It keeps me humble, as I think most of my patients aren’t really here to see me; after all I am just a fancy plumber.
Speaking of plumbing, I could’ve sworn that the ginormous two-volume Rutherford edition always gave me the impression that vascular disease is composed of 75% venous disease and 25% arterial disease. However, our fellowship training in the United States makes Rutherford seem like he had his numbers flipped – 99.8% was arterial with a splash of venous as an afterthought. Truth be told though, I see roughly 55% venous, 25% dialysis, and 20% arterial. I guess that wasn’t made up after all.
If my practice name, Ohio Vein & Vascular, didn’t give it away, I admit that I focus marketing efforts toward venous pathology. This has significantly improved my work-life balance. Let’s face it, not everything we do as a surgeon is fun and can certainly carry a large amount of stress. I devote an honest amount of time to developing what ‘type’ of practice I desire. I communicate regularly with my referring docs about the types of disease I focus on, write press releases to the local paper, and always have my elevator speech handy when speaking with fellow physicians and potential patients about what I do as a surgical subspecialist.
In such a small community, the more my vascular surgery practice grows, the more likely the podiatrist and his wife (also a podiatrist) across the hall will grow their practice. Same holds true of the cardiologist upstairs and the nephrologist down the hall. It’s not rocket science that the more I help their businesses thrive, the more likely they are to do the same for mine. We are all one large family working together with the common goal to stay independent, a rarity these days amongst the conglomerate of hospitals taking over.
Wait, did I mention that I have never run a business before? Well, let me tell the most important lesson I have learned ... some days it is really hard. I remember having to let go my first medical assistant after her 90-day review. All of my medical training never prepared me for a how hard that conversation was going to be, and she wasn’t even losing her leg. My wife, a trained physical therapist, jumped right in until we eventually got the gusto to hire another MA. Fortunately, we found a remarkable individual who is worth her weight in gold. The same holds true for our other employees and we aren’t about to let them leave so we pay them well, fund 80% of their health insurance premiums, established a 401K with matching funds, and profit share with each employee. We foster an environment that makes our employees want to work hard, although like my patients, sometimes I think they come to work just to see The Girls.
All in all, we treat our staff with respect and provide a significant monetary carrot to each of them at the holidays; this is unmatched in our area. Happy employees are instrumental to my work life and have a direct impact on the success of my practice. All boats rise with the rising tide, and we are sailing smoothly.
Despite all the challenges and hard work, nothing is better than being your own boss. Nothing. I don’t know a single physician whose desire was to trek through grueling medical school and years of residency and fellowship to ultimately become an employee of an overly glorified postgraduate degree holder in health care administration. I cannot recall having had a single conversation with any surgeon or physician who is 100% happy with his or her working situation who isn’t self-employed. Do I work now more than I ever thought I would? Absolutely. But the work I am doing isn’t simply waking up at all hours to operate or trudge through countless hours in a lab or clinic. No, the work I do is running a successful small business – and even better yet, it is great!
Here I am on a Saturday morning writing a guest editorial for publication in the official newspaper of the Society for Vascular Surgery and I am loving it. Life is short and I’m trying to enjoy every minute that I have on this planet by spending my time working in a manner that I find enjoyable.
Being the fancy plumber in rural America provides me that opportunity. I hope others realize that it is still possible to navigate health care’s oftentimes unknown waters as a solo private practitioner and be successful. That they too could be taking the bull by the horns and changing up their work-life balance for the betterment of themselves, the care they provide to patients, and their families.
And in the meantime, I’ll jump back in my car and head due north for a 45-minute decompressing drive, chat with family and friends, dictate the last case of the day, and continue to enjoy the best of both worlds living in the big city and working with the most genuine folks in rural America. You should try it.
Dr. Santin is a vascular surgeon in private practice at Ohio Vein & Vascular, Wilmington.
References
1. Medscape Malpractice Report 2015: Why Most Doctors Get Sued, by Carol Peckham, Dec. 9, 2015.
2. Decrease Your Risk of Being Successfully Sued, by Nancy Young, Crozer-Chester Medical Center.
3. To Be Sued Less, Doctors Should Consider Talking to Patients More, by Aaron E. Carroll, New York Times, June 1, 2015.
Within 3 minutes of the car engine rumbling to a roar in the morning air, cruise control is set, freshly ground coffee is in hand and NPR is playing on WOSU 90.5. I settle in for the morning news on my 45-minute commute to the hospital. Sure, I could’ve found a hospital closer to shorten my commute, especially since I live in the 14th largest metropolitan city in the country.
If I’d wanted, I could be knocking out carotid endarterectomies at a level 1 trauma center, three blocks away from my front door. But no, that’s not what does it for me. What does? It’s having the opportunity to be my own boss and care for salt-of-the-earth folks in rural America.
You see, 5 years ago when I finished my vascular surgery fellowship at Good Samaritan Hospital in Cincinnati, I opened my own solo private practice in a rural community: population 30,000. Yep, that’s right, you heard it. I hung a shingle and went old school. And now as I reflect over the lessons learned during the first half-decade of my practice at Ohio Vein & Vascular Inc., I can tell you it has been a hell of a ride, and boy have we learned a lot.
The better half of the ‘we’ is my wife, Crystal, who doubles as my practice administrator, with her own solid foundation coming from a doctorate in physical therapy. We have successfully built a small company with four full-time employees, one contract registered vascular technologist, and two therapy dogs who serve more than 3,500 patients to date.
From the first day I opened my doors to this small-town rural community, I realized that it’s not what you know, but rather who you know. Well frankly, I didn’t know a soul!
Fortunately, my front office manager was born, raised, and still lives in Wilmington and knows everyone’s mother, brother, sister, niece, and grandchild in what felt like a 60-mile radius. She gave this young, slick city kid from Columbus instant street cred despite all the fancy credentials behind my name. I ditched the tie and fancy shoes and embraced my new ‘work’ home with open arms.
In a community such as Wilmington, Ohio, it’s the little things that count. I wear my own scrubs on days when I operate. Not only do they have my practice logo embroidered on the chest pocket, but they are also adorned with the brown leather symbol for Carhart, a clothing brand. In rural America, Carhart denim clothing – overall bibs, jackets, gloves, etc., are considered king. When my patients see that symbol, there is an instant point of mutual appreciation and it almost always results in some good laughs – who knew Carhart made scrubs?
As a result, I’ve been offered opportunities to ride combines, go drag racing, and go hunting for the infamous morel mushrooms. Just to be clear, I haven’t found a morel yet, so I guess I will stick to my day job as a surgeon.
Having a good laugh and connecting with my patients was something I was not accustomed to in my training. I was there to operate, and rarely participated in office days. At times this routine left me feeling unappreciated by my patients and their families. I was just a surgeon delivering bad news. I now find myself fortunate to have the opportunity to get to know my patients and participate in their health care, and I know they appreciate me for it.
A recent malpractice survey cited a finding that the more patients ‘like’ their physicians, the less likely they are to file malpractice lawsuits against them.1 Other reports have suggested that the relationship a physician has with a patient is a critical factor, more so than any single medical mistake, in determining whether or not a lawsuit is filed.2,3
While I feel appreciated and ‘liked’ by my patients, I’ve learned that I am not necessarily their favorite employee in the office. This honor is most often bestowed upon Claire and Whitney, aka “The Girls” – our two, miniature Labradoodles who serve in the capacity of therapy dogs and have perfected the ability to nap in nearly any situation. Try as I may to convince patients that what I am saying is important, they never lose focus on The Girls. They are the first thing patients ask about, I swear they receive more gifts than I do, and they always are on the receiving end of some good ‘pets’ as my patients leave the office. Despite any bad news they may have been told, very rarely do patients leave my office without smiles on their faces. It keeps me humble, as I think most of my patients aren’t really here to see me; after all I am just a fancy plumber.
Speaking of plumbing, I could’ve sworn that the ginormous two-volume Rutherford edition always gave me the impression that vascular disease is composed of 75% venous disease and 25% arterial disease. However, our fellowship training in the United States makes Rutherford seem like he had his numbers flipped – 99.8% was arterial with a splash of venous as an afterthought. Truth be told though, I see roughly 55% venous, 25% dialysis, and 20% arterial. I guess that wasn’t made up after all.
If my practice name, Ohio Vein & Vascular, didn’t give it away, I admit that I focus marketing efforts toward venous pathology. This has significantly improved my work-life balance. Let’s face it, not everything we do as a surgeon is fun and can certainly carry a large amount of stress. I devote an honest amount of time to developing what ‘type’ of practice I desire. I communicate regularly with my referring docs about the types of disease I focus on, write press releases to the local paper, and always have my elevator speech handy when speaking with fellow physicians and potential patients about what I do as a surgical subspecialist.
In such a small community, the more my vascular surgery practice grows, the more likely the podiatrist and his wife (also a podiatrist) across the hall will grow their practice. Same holds true of the cardiologist upstairs and the nephrologist down the hall. It’s not rocket science that the more I help their businesses thrive, the more likely they are to do the same for mine. We are all one large family working together with the common goal to stay independent, a rarity these days amongst the conglomerate of hospitals taking over.
Wait, did I mention that I have never run a business before? Well, let me tell the most important lesson I have learned ... some days it is really hard. I remember having to let go my first medical assistant after her 90-day review. All of my medical training never prepared me for a how hard that conversation was going to be, and she wasn’t even losing her leg. My wife, a trained physical therapist, jumped right in until we eventually got the gusto to hire another MA. Fortunately, we found a remarkable individual who is worth her weight in gold. The same holds true for our other employees and we aren’t about to let them leave so we pay them well, fund 80% of their health insurance premiums, established a 401K with matching funds, and profit share with each employee. We foster an environment that makes our employees want to work hard, although like my patients, sometimes I think they come to work just to see The Girls.
All in all, we treat our staff with respect and provide a significant monetary carrot to each of them at the holidays; this is unmatched in our area. Happy employees are instrumental to my work life and have a direct impact on the success of my practice. All boats rise with the rising tide, and we are sailing smoothly.
Despite all the challenges and hard work, nothing is better than being your own boss. Nothing. I don’t know a single physician whose desire was to trek through grueling medical school and years of residency and fellowship to ultimately become an employee of an overly glorified postgraduate degree holder in health care administration. I cannot recall having had a single conversation with any surgeon or physician who is 100% happy with his or her working situation who isn’t self-employed. Do I work now more than I ever thought I would? Absolutely. But the work I am doing isn’t simply waking up at all hours to operate or trudge through countless hours in a lab or clinic. No, the work I do is running a successful small business – and even better yet, it is great!
Here I am on a Saturday morning writing a guest editorial for publication in the official newspaper of the Society for Vascular Surgery and I am loving it. Life is short and I’m trying to enjoy every minute that I have on this planet by spending my time working in a manner that I find enjoyable.
Being the fancy plumber in rural America provides me that opportunity. I hope others realize that it is still possible to navigate health care’s oftentimes unknown waters as a solo private practitioner and be successful. That they too could be taking the bull by the horns and changing up their work-life balance for the betterment of themselves, the care they provide to patients, and their families.
And in the meantime, I’ll jump back in my car and head due north for a 45-minute decompressing drive, chat with family and friends, dictate the last case of the day, and continue to enjoy the best of both worlds living in the big city and working with the most genuine folks in rural America. You should try it.
Dr. Santin is a vascular surgeon in private practice at Ohio Vein & Vascular, Wilmington.
References
1. Medscape Malpractice Report 2015: Why Most Doctors Get Sued, by Carol Peckham, Dec. 9, 2015.
2. Decrease Your Risk of Being Successfully Sued, by Nancy Young, Crozer-Chester Medical Center.
3. To Be Sued Less, Doctors Should Consider Talking to Patients More, by Aaron E. Carroll, New York Times, June 1, 2015.
Within 3 minutes of the car engine rumbling to a roar in the morning air, cruise control is set, freshly ground coffee is in hand and NPR is playing on WOSU 90.5. I settle in for the morning news on my 45-minute commute to the hospital. Sure, I could’ve found a hospital closer to shorten my commute, especially since I live in the 14th largest metropolitan city in the country.
If I’d wanted, I could be knocking out carotid endarterectomies at a level 1 trauma center, three blocks away from my front door. But no, that’s not what does it for me. What does? It’s having the opportunity to be my own boss and care for salt-of-the-earth folks in rural America.
You see, 5 years ago when I finished my vascular surgery fellowship at Good Samaritan Hospital in Cincinnati, I opened my own solo private practice in a rural community: population 30,000. Yep, that’s right, you heard it. I hung a shingle and went old school. And now as I reflect over the lessons learned during the first half-decade of my practice at Ohio Vein & Vascular Inc., I can tell you it has been a hell of a ride, and boy have we learned a lot.
The better half of the ‘we’ is my wife, Crystal, who doubles as my practice administrator, with her own solid foundation coming from a doctorate in physical therapy. We have successfully built a small company with four full-time employees, one contract registered vascular technologist, and two therapy dogs who serve more than 3,500 patients to date.
From the first day I opened my doors to this small-town rural community, I realized that it’s not what you know, but rather who you know. Well frankly, I didn’t know a soul!
Fortunately, my front office manager was born, raised, and still lives in Wilmington and knows everyone’s mother, brother, sister, niece, and grandchild in what felt like a 60-mile radius. She gave this young, slick city kid from Columbus instant street cred despite all the fancy credentials behind my name. I ditched the tie and fancy shoes and embraced my new ‘work’ home with open arms.
In a community such as Wilmington, Ohio, it’s the little things that count. I wear my own scrubs on days when I operate. Not only do they have my practice logo embroidered on the chest pocket, but they are also adorned with the brown leather symbol for Carhart, a clothing brand. In rural America, Carhart denim clothing – overall bibs, jackets, gloves, etc., are considered king. When my patients see that symbol, there is an instant point of mutual appreciation and it almost always results in some good laughs – who knew Carhart made scrubs?
As a result, I’ve been offered opportunities to ride combines, go drag racing, and go hunting for the infamous morel mushrooms. Just to be clear, I haven’t found a morel yet, so I guess I will stick to my day job as a surgeon.
Having a good laugh and connecting with my patients was something I was not accustomed to in my training. I was there to operate, and rarely participated in office days. At times this routine left me feeling unappreciated by my patients and their families. I was just a surgeon delivering bad news. I now find myself fortunate to have the opportunity to get to know my patients and participate in their health care, and I know they appreciate me for it.
A recent malpractice survey cited a finding that the more patients ‘like’ their physicians, the less likely they are to file malpractice lawsuits against them.1 Other reports have suggested that the relationship a physician has with a patient is a critical factor, more so than any single medical mistake, in determining whether or not a lawsuit is filed.2,3
While I feel appreciated and ‘liked’ by my patients, I’ve learned that I am not necessarily their favorite employee in the office. This honor is most often bestowed upon Claire and Whitney, aka “The Girls” – our two, miniature Labradoodles who serve in the capacity of therapy dogs and have perfected the ability to nap in nearly any situation. Try as I may to convince patients that what I am saying is important, they never lose focus on The Girls. They are the first thing patients ask about, I swear they receive more gifts than I do, and they always are on the receiving end of some good ‘pets’ as my patients leave the office. Despite any bad news they may have been told, very rarely do patients leave my office without smiles on their faces. It keeps me humble, as I think most of my patients aren’t really here to see me; after all I am just a fancy plumber.
Speaking of plumbing, I could’ve sworn that the ginormous two-volume Rutherford edition always gave me the impression that vascular disease is composed of 75% venous disease and 25% arterial disease. However, our fellowship training in the United States makes Rutherford seem like he had his numbers flipped – 99.8% was arterial with a splash of venous as an afterthought. Truth be told though, I see roughly 55% venous, 25% dialysis, and 20% arterial. I guess that wasn’t made up after all.
If my practice name, Ohio Vein & Vascular, didn’t give it away, I admit that I focus marketing efforts toward venous pathology. This has significantly improved my work-life balance. Let’s face it, not everything we do as a surgeon is fun and can certainly carry a large amount of stress. I devote an honest amount of time to developing what ‘type’ of practice I desire. I communicate regularly with my referring docs about the types of disease I focus on, write press releases to the local paper, and always have my elevator speech handy when speaking with fellow physicians and potential patients about what I do as a surgical subspecialist.
In such a small community, the more my vascular surgery practice grows, the more likely the podiatrist and his wife (also a podiatrist) across the hall will grow their practice. Same holds true of the cardiologist upstairs and the nephrologist down the hall. It’s not rocket science that the more I help their businesses thrive, the more likely they are to do the same for mine. We are all one large family working together with the common goal to stay independent, a rarity these days amongst the conglomerate of hospitals taking over.
Wait, did I mention that I have never run a business before? Well, let me tell the most important lesson I have learned ... some days it is really hard. I remember having to let go my first medical assistant after her 90-day review. All of my medical training never prepared me for a how hard that conversation was going to be, and she wasn’t even losing her leg. My wife, a trained physical therapist, jumped right in until we eventually got the gusto to hire another MA. Fortunately, we found a remarkable individual who is worth her weight in gold. The same holds true for our other employees and we aren’t about to let them leave so we pay them well, fund 80% of their health insurance premiums, established a 401K with matching funds, and profit share with each employee. We foster an environment that makes our employees want to work hard, although like my patients, sometimes I think they come to work just to see The Girls.
All in all, we treat our staff with respect and provide a significant monetary carrot to each of them at the holidays; this is unmatched in our area. Happy employees are instrumental to my work life and have a direct impact on the success of my practice. All boats rise with the rising tide, and we are sailing smoothly.
Despite all the challenges and hard work, nothing is better than being your own boss. Nothing. I don’t know a single physician whose desire was to trek through grueling medical school and years of residency and fellowship to ultimately become an employee of an overly glorified postgraduate degree holder in health care administration. I cannot recall having had a single conversation with any surgeon or physician who is 100% happy with his or her working situation who isn’t self-employed. Do I work now more than I ever thought I would? Absolutely. But the work I am doing isn’t simply waking up at all hours to operate or trudge through countless hours in a lab or clinic. No, the work I do is running a successful small business – and even better yet, it is great!
Here I am on a Saturday morning writing a guest editorial for publication in the official newspaper of the Society for Vascular Surgery and I am loving it. Life is short and I’m trying to enjoy every minute that I have on this planet by spending my time working in a manner that I find enjoyable.
Being the fancy plumber in rural America provides me that opportunity. I hope others realize that it is still possible to navigate health care’s oftentimes unknown waters as a solo private practitioner and be successful. That they too could be taking the bull by the horns and changing up their work-life balance for the betterment of themselves, the care they provide to patients, and their families.
And in the meantime, I’ll jump back in my car and head due north for a 45-minute decompressing drive, chat with family and friends, dictate the last case of the day, and continue to enjoy the best of both worlds living in the big city and working with the most genuine folks in rural America. You should try it.
Dr. Santin is a vascular surgeon in private practice at Ohio Vein & Vascular, Wilmington.
References
1. Medscape Malpractice Report 2015: Why Most Doctors Get Sued, by Carol Peckham, Dec. 9, 2015.
2. Decrease Your Risk of Being Successfully Sued, by Nancy Young, Crozer-Chester Medical Center.
3. To Be Sued Less, Doctors Should Consider Talking to Patients More, by Aaron E. Carroll, New York Times, June 1, 2015.
Surgeon general takes on teen vaping; medical groups show support
U.S. Surgeon General Jerome Adams, MD, responded Dec. 18 to recent data showing a sharp increase in the use of nicotine-based e-cigarette products among American teens with an urgent call to action.
“I, Surgeon General of the United States Public Health Service, [Vice Admiral] Jerome Adams, am emphasizing the importance of protecting our children from a lifetime of nicotine addiction and associated health risks by immediately addressing the epidemic of youth e-cigarette use,” Dr. Adams said in an advisory. “The recent surge in e-cigarette use among youth, which has been fueled by new types of e-cigarettes that have recently entered the market, is a cause for great concern. We must take action now to protect the health of our nation’s young people.”
The surgeon general’s advisory emphasized that e-cigarette products are not harmless and that, in addition to potentially addictive nicotine, many products contain other dangerous substances, including heavy metals, volatile organic compounds, and ultrafine particles that can affect the lungs. In addition, some e-cigarette products contain potentially harmful chemicals used to add flavoring.
The complete advisory includes information for parents, teachers, and clinicians about the details of current e-cigarette products and strategies for working to reduce their use among teens.
The American Medical Association expressed support of the surgeon general’s call to action.
“The only way to prevent another generation from developing nicotine dependence is to continue to raise awareness that e-cigarettes are harmful, powerfully addictive, and can often lead young people to smoke conventional cigarettes. E-cigarettes have the potential to undermine the public health gains that have been made over the years in combating the smoking epidemic,” Barbara L. McAneny, MD, president of the AMA, said in the statement.
“Recognizing the use of e-cigarettes and vaping as an urgent public health epidemic, the AMA has adopted numerous policies in recent years aimed at preventing youth access to these harmful tobacco products. In line with the surgeon general’s advisory, the AMA also has existing policy urging physicians to educate themselves about e-cigarettes and be prepared to counsel patients about e-cigarette usage and the potential for nicotine addiction,” Dr. McAneny said. she added.
The American Heart Association expressed support for the surgeon general’s call as well.
“That the U.S. Surgeon General is calling teen vaping an ‘epidemic’ should seize the attention of elected officials and the community of organizations working to protect the health of our nation’s children. We commend Surgeon General Adams, Commissioner [Adam] Gottlieb and Secretary [Alex M.] Azar for marshaling parents, educators, health providers, and communities to apply proven methods to overcome the epidemic of e-cigarette use,” Nancy Brown, CEO of the AHA, said in a Dec. 18 statement.
“But more must be done in the face of rapidly rising e-cigarette use among youth. The FDA’s recent announcement that it plans to restrict marketing and sales of flavored tobacco products must be followed by immediate, concrete action that sends an unmistakable message that the tobacco industry’s relentless targeting of our nation’s children will no longer be tolerated,” she emphasized.
View the surgeon general’s video message and access additional e-cigarette information here.
U.S. Surgeon General Jerome Adams, MD, responded Dec. 18 to recent data showing a sharp increase in the use of nicotine-based e-cigarette products among American teens with an urgent call to action.
“I, Surgeon General of the United States Public Health Service, [Vice Admiral] Jerome Adams, am emphasizing the importance of protecting our children from a lifetime of nicotine addiction and associated health risks by immediately addressing the epidemic of youth e-cigarette use,” Dr. Adams said in an advisory. “The recent surge in e-cigarette use among youth, which has been fueled by new types of e-cigarettes that have recently entered the market, is a cause for great concern. We must take action now to protect the health of our nation’s young people.”
The surgeon general’s advisory emphasized that e-cigarette products are not harmless and that, in addition to potentially addictive nicotine, many products contain other dangerous substances, including heavy metals, volatile organic compounds, and ultrafine particles that can affect the lungs. In addition, some e-cigarette products contain potentially harmful chemicals used to add flavoring.
The complete advisory includes information for parents, teachers, and clinicians about the details of current e-cigarette products and strategies for working to reduce their use among teens.
The American Medical Association expressed support of the surgeon general’s call to action.
“The only way to prevent another generation from developing nicotine dependence is to continue to raise awareness that e-cigarettes are harmful, powerfully addictive, and can often lead young people to smoke conventional cigarettes. E-cigarettes have the potential to undermine the public health gains that have been made over the years in combating the smoking epidemic,” Barbara L. McAneny, MD, president of the AMA, said in the statement.
“Recognizing the use of e-cigarettes and vaping as an urgent public health epidemic, the AMA has adopted numerous policies in recent years aimed at preventing youth access to these harmful tobacco products. In line with the surgeon general’s advisory, the AMA also has existing policy urging physicians to educate themselves about e-cigarettes and be prepared to counsel patients about e-cigarette usage and the potential for nicotine addiction,” Dr. McAneny said. she added.
The American Heart Association expressed support for the surgeon general’s call as well.
“That the U.S. Surgeon General is calling teen vaping an ‘epidemic’ should seize the attention of elected officials and the community of organizations working to protect the health of our nation’s children. We commend Surgeon General Adams, Commissioner [Adam] Gottlieb and Secretary [Alex M.] Azar for marshaling parents, educators, health providers, and communities to apply proven methods to overcome the epidemic of e-cigarette use,” Nancy Brown, CEO of the AHA, said in a Dec. 18 statement.
“But more must be done in the face of rapidly rising e-cigarette use among youth. The FDA’s recent announcement that it plans to restrict marketing and sales of flavored tobacco products must be followed by immediate, concrete action that sends an unmistakable message that the tobacco industry’s relentless targeting of our nation’s children will no longer be tolerated,” she emphasized.
View the surgeon general’s video message and access additional e-cigarette information here.
U.S. Surgeon General Jerome Adams, MD, responded Dec. 18 to recent data showing a sharp increase in the use of nicotine-based e-cigarette products among American teens with an urgent call to action.
“I, Surgeon General of the United States Public Health Service, [Vice Admiral] Jerome Adams, am emphasizing the importance of protecting our children from a lifetime of nicotine addiction and associated health risks by immediately addressing the epidemic of youth e-cigarette use,” Dr. Adams said in an advisory. “The recent surge in e-cigarette use among youth, which has been fueled by new types of e-cigarettes that have recently entered the market, is a cause for great concern. We must take action now to protect the health of our nation’s young people.”
The surgeon general’s advisory emphasized that e-cigarette products are not harmless and that, in addition to potentially addictive nicotine, many products contain other dangerous substances, including heavy metals, volatile organic compounds, and ultrafine particles that can affect the lungs. In addition, some e-cigarette products contain potentially harmful chemicals used to add flavoring.
The complete advisory includes information for parents, teachers, and clinicians about the details of current e-cigarette products and strategies for working to reduce their use among teens.
The American Medical Association expressed support of the surgeon general’s call to action.
“The only way to prevent another generation from developing nicotine dependence is to continue to raise awareness that e-cigarettes are harmful, powerfully addictive, and can often lead young people to smoke conventional cigarettes. E-cigarettes have the potential to undermine the public health gains that have been made over the years in combating the smoking epidemic,” Barbara L. McAneny, MD, president of the AMA, said in the statement.
“Recognizing the use of e-cigarettes and vaping as an urgent public health epidemic, the AMA has adopted numerous policies in recent years aimed at preventing youth access to these harmful tobacco products. In line with the surgeon general’s advisory, the AMA also has existing policy urging physicians to educate themselves about e-cigarettes and be prepared to counsel patients about e-cigarette usage and the potential for nicotine addiction,” Dr. McAneny said. she added.
The American Heart Association expressed support for the surgeon general’s call as well.
“That the U.S. Surgeon General is calling teen vaping an ‘epidemic’ should seize the attention of elected officials and the community of organizations working to protect the health of our nation’s children. We commend Surgeon General Adams, Commissioner [Adam] Gottlieb and Secretary [Alex M.] Azar for marshaling parents, educators, health providers, and communities to apply proven methods to overcome the epidemic of e-cigarette use,” Nancy Brown, CEO of the AHA, said in a Dec. 18 statement.
“But more must be done in the face of rapidly rising e-cigarette use among youth. The FDA’s recent announcement that it plans to restrict marketing and sales of flavored tobacco products must be followed by immediate, concrete action that sends an unmistakable message that the tobacco industry’s relentless targeting of our nation’s children will no longer be tolerated,” she emphasized.
View the surgeon general’s video message and access additional e-cigarette information here.
Unintentional injuries top killer of U.S. children
Unintentional injuries accounted for more than half of all deaths among U.S. children aged 1-19 years in 2016, according to a new study based on data from the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (WONDER) database. 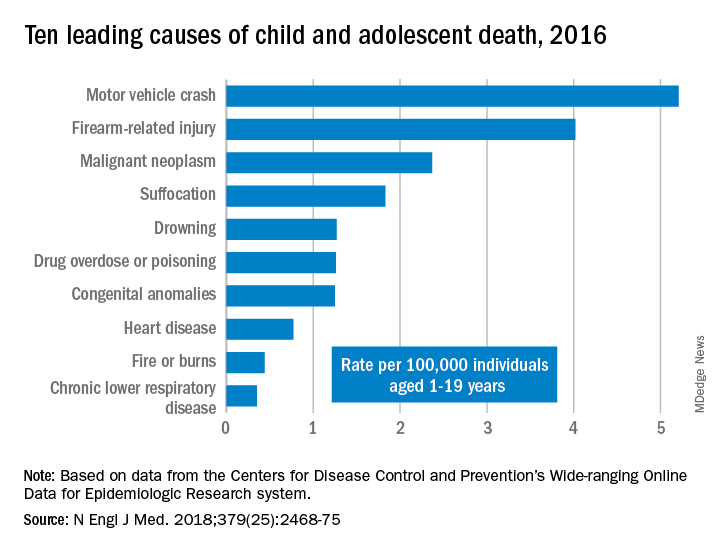
WONDER collects data from U.S. death certificates for 57 vital-statistics jurisdictions, and the 2016 data included 20,360 deaths. Injuries accounted for 12,336 deaths; unintentional injuries accounted for 57% or 7,057 deaths. Approximately one in five U.S. youth deaths (21%) were suicides, and another one in five (20%) were homicides.
Motor vehicle accidents, also responsible for one in five (20%) of all deaths, were the leading cause of accidental deaths, followed by firearm-related injuries, which accounted for 15% of all deaths. Of the firearm-related deaths, 59% were homicides, 35% suicides, 4% accidental, and 2% undetermined.
The only high-ranking noninjury cause of death overall was neoplasms, yet childhood cancer accounted for just 9% of all deaths. Suffocation was the cause of 7% of deaths, and included homicides, suicides and unintentional injuries.
The remaining causes included drowning (5.9%), drug overdose or poisoning (4.8%), congenital anomalies (4.8%), heart disease (2.9%), fire or burns (1.7%) and chronic lower respiratory disease (1.3%).
“Progress toward further reducing deaths among children and adolescents will require a shift in public perceptions so that injury deaths are viewed not as ‘accidents,’ but rather as social ecologic phenomena that are amenable to prevention,” wrote Rebecca M. Cunningham, MD, and her colleagues at the University of Michigan, Ann Arbor (N Engl J Med. 2018 Dec 20. doi: 10.1056/NEJMsr1804754). The findings “highlight the need to implement public health strategies that are tailored according to age, underlying developmental factors, and injury-related intent” to reduce the risk for death in children.”
“The sad fact is that a child or adolescent in the United States is 57% more likely to die by the age of 19 years than those in other wealthy nations,” Edward W. Campion, MD, executive editor and online editor of the New England Journal of Medicine, wrote in an editorial that accompanied the study (N Engl J Med. 2018 Dec. 20;379[25]:2466-7. doi: 10.1056/NEJMe1814600). “Children in America are dying or being killed at rates that are shameful.
“Our country has led the way in so much medical research, but the facts summarized by Cunningham et al. reveal a need to invest far more in research on the prevention of the injuries that threaten the lives of children and adolescents,” he said.
In an interview, Ben Hoffman, MD, professor of pediatrics at Oregon Health and Science University, Portland, said the only thing surprising in this report is that nothing is surprising.
“This is the stuff that those of us in injury prevention have been screaming about for decades,” said Dr. Hoffman, also medical director of the Tom Sargent Safety Center at OHSU Doernbecher Children’s Hospital.
“Unintentional injuries are what kill kids. We have made such tremendous progress in other areas, and we’ve made progress in terms of preventing injuries, but what we see is unacceptable,” he said. “The fact that [injuries] remain such an issue is a testament to the fact that our collective will [to address these issues] has failed us.”
Among children aged 1-4, drowning was the leading cause of death, followed by congenital anomalies and motor vehicle crashes.
Mandated four-sided fencing around pools is a highly effective intervention for reducing drowning risks, Dr. Hoffman said.
Children aged 5-9 represented the smallest proportion of all youth deaths (12%) and were the only age group not to have injuries as the leading cause of death. Malignant neoplasms led the causes of death in this group, followed by car accidents and congenital anomalies.
Adolescents aged 10-19, the widest age range, comprised 68% of all youth deaths, led by motor vehicle accidents, firearms, and suffocation.
“These findings reflect social and developmental factors that are associated with adolescence, including increased risk-taking behavior, differential peer and parental influence, and initiation of substance use,” Dr. Cunningham and her colleagues wrote.
The most concerning trends, according to Dr. Hoffman, were the upticks in motor vehicle deaths, suffocation, and poisonings, the latter driven largely by opioid overdoses, which were responsible for more than half of all overdoses in adolescents.
Addressing these issues “will require an investment in kids, which is not something that our society does really well,” Dr. Hoffman said. “We talk about it, we tiptoe around it, but when push comes to shove, nobody is really willing to support and fund the efforts to do it.”
In his editorial, Dr. Campion observed that despite a decades-long trend of decreasing mortality from car accidents, these deaths began steadily increasing from 2013 to 2016.
Previous gains in this area came from “the widespread adoption of seat belts and appropriate child safety seats, the production of cars with improved safety standards, better constructed roads, graduated driver-licensing programs, and a focus on reducing teen drinking and driving,” the authors stated. Multiple reasons likely account for the reversal, including distracted driving and possibly marijuana use, though the latter requires more data.
Firearm deaths increased by 28% from 2013 to 2016, driven by suicides (a 26% increase) and homicides (a 32% increase), including increasing school shootings.
Dr. Hoffman acknowledged the complexities of addressing firearm deaths, but “there are effective common sense interventions that could be made ... there’s just not the will.” An example is passing child access prevention (CAP) laws, such as mandating safe storage of guns and imposing criminal liability when children negligently acquire access to firearms. While a variety of small groups address child injury issues, a large, coordinated, centralized national advocacy for kids is lacking, he added.
“The approach to this underrecognized public health problem has to be social as well as technological, and the risks are highest in areas of poverty and social isolation,” Dr. Campion wrote. “We are living in a divisive era in which there are few areas of consensus and agreement. Perhaps one of the few core beliefs that all can agree on is that deaths in childhood and adolescence are tragedies that we must find ways to prevent.”
“Every day, 10 babies die in their sleep, 1.7 kids under age 4 drown, and 4 kids over the age of 1 die in car crashes,” Dr. Hoffman said. “We need to acknowledge the impact of unintentional and intentional injuries and recognize that there are things we can do, that we’re complicit in all of those deaths because in every circumstance, there is something we as a society could have done.”
SOURCE: Cunningham et al. N Engl J Med. 2018 Dec 20;379(25):2468-75. doi: 10.1056/NEJMsr1804754.
Unintentional injuries accounted for more than half of all deaths among U.S. children aged 1-19 years in 2016, according to a new study based on data from the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (WONDER) database. 
WONDER collects data from U.S. death certificates for 57 vital-statistics jurisdictions, and the 2016 data included 20,360 deaths. Injuries accounted for 12,336 deaths; unintentional injuries accounted for 57% or 7,057 deaths. Approximately one in five U.S. youth deaths (21%) were suicides, and another one in five (20%) were homicides.
Motor vehicle accidents, also responsible for one in five (20%) of all deaths, were the leading cause of accidental deaths, followed by firearm-related injuries, which accounted for 15% of all deaths. Of the firearm-related deaths, 59% were homicides, 35% suicides, 4% accidental, and 2% undetermined.
The only high-ranking noninjury cause of death overall was neoplasms, yet childhood cancer accounted for just 9% of all deaths. Suffocation was the cause of 7% of deaths, and included homicides, suicides and unintentional injuries.
The remaining causes included drowning (5.9%), drug overdose or poisoning (4.8%), congenital anomalies (4.8%), heart disease (2.9%), fire or burns (1.7%) and chronic lower respiratory disease (1.3%).
“Progress toward further reducing deaths among children and adolescents will require a shift in public perceptions so that injury deaths are viewed not as ‘accidents,’ but rather as social ecologic phenomena that are amenable to prevention,” wrote Rebecca M. Cunningham, MD, and her colleagues at the University of Michigan, Ann Arbor (N Engl J Med. 2018 Dec 20. doi: 10.1056/NEJMsr1804754). The findings “highlight the need to implement public health strategies that are tailored according to age, underlying developmental factors, and injury-related intent” to reduce the risk for death in children.”
“The sad fact is that a child or adolescent in the United States is 57% more likely to die by the age of 19 years than those in other wealthy nations,” Edward W. Campion, MD, executive editor and online editor of the New England Journal of Medicine, wrote in an editorial that accompanied the study (N Engl J Med. 2018 Dec. 20;379[25]:2466-7. doi: 10.1056/NEJMe1814600). “Children in America are dying or being killed at rates that are shameful.
“Our country has led the way in so much medical research, but the facts summarized by Cunningham et al. reveal a need to invest far more in research on the prevention of the injuries that threaten the lives of children and adolescents,” he said.
In an interview, Ben Hoffman, MD, professor of pediatrics at Oregon Health and Science University, Portland, said the only thing surprising in this report is that nothing is surprising.
“This is the stuff that those of us in injury prevention have been screaming about for decades,” said Dr. Hoffman, also medical director of the Tom Sargent Safety Center at OHSU Doernbecher Children’s Hospital.
“Unintentional injuries are what kill kids. We have made such tremendous progress in other areas, and we’ve made progress in terms of preventing injuries, but what we see is unacceptable,” he said. “The fact that [injuries] remain such an issue is a testament to the fact that our collective will [to address these issues] has failed us.”
Among children aged 1-4, drowning was the leading cause of death, followed by congenital anomalies and motor vehicle crashes.
Mandated four-sided fencing around pools is a highly effective intervention for reducing drowning risks, Dr. Hoffman said.
Children aged 5-9 represented the smallest proportion of all youth deaths (12%) and were the only age group not to have injuries as the leading cause of death. Malignant neoplasms led the causes of death in this group, followed by car accidents and congenital anomalies.
Adolescents aged 10-19, the widest age range, comprised 68% of all youth deaths, led by motor vehicle accidents, firearms, and suffocation.
“These findings reflect social and developmental factors that are associated with adolescence, including increased risk-taking behavior, differential peer and parental influence, and initiation of substance use,” Dr. Cunningham and her colleagues wrote.
The most concerning trends, according to Dr. Hoffman, were the upticks in motor vehicle deaths, suffocation, and poisonings, the latter driven largely by opioid overdoses, which were responsible for more than half of all overdoses in adolescents.
Addressing these issues “will require an investment in kids, which is not something that our society does really well,” Dr. Hoffman said. “We talk about it, we tiptoe around it, but when push comes to shove, nobody is really willing to support and fund the efforts to do it.”
In his editorial, Dr. Campion observed that despite a decades-long trend of decreasing mortality from car accidents, these deaths began steadily increasing from 2013 to 2016.
Previous gains in this area came from “the widespread adoption of seat belts and appropriate child safety seats, the production of cars with improved safety standards, better constructed roads, graduated driver-licensing programs, and a focus on reducing teen drinking and driving,” the authors stated. Multiple reasons likely account for the reversal, including distracted driving and possibly marijuana use, though the latter requires more data.
Firearm deaths increased by 28% from 2013 to 2016, driven by suicides (a 26% increase) and homicides (a 32% increase), including increasing school shootings.
Dr. Hoffman acknowledged the complexities of addressing firearm deaths, but “there are effective common sense interventions that could be made ... there’s just not the will.” An example is passing child access prevention (CAP) laws, such as mandating safe storage of guns and imposing criminal liability when children negligently acquire access to firearms. While a variety of small groups address child injury issues, a large, coordinated, centralized national advocacy for kids is lacking, he added.
“The approach to this underrecognized public health problem has to be social as well as technological, and the risks are highest in areas of poverty and social isolation,” Dr. Campion wrote. “We are living in a divisive era in which there are few areas of consensus and agreement. Perhaps one of the few core beliefs that all can agree on is that deaths in childhood and adolescence are tragedies that we must find ways to prevent.”
“Every day, 10 babies die in their sleep, 1.7 kids under age 4 drown, and 4 kids over the age of 1 die in car crashes,” Dr. Hoffman said. “We need to acknowledge the impact of unintentional and intentional injuries and recognize that there are things we can do, that we’re complicit in all of those deaths because in every circumstance, there is something we as a society could have done.”
SOURCE: Cunningham et al. N Engl J Med. 2018 Dec 20;379(25):2468-75. doi: 10.1056/NEJMsr1804754.
Unintentional injuries accounted for more than half of all deaths among U.S. children aged 1-19 years in 2016, according to a new study based on data from the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (WONDER) database. 
WONDER collects data from U.S. death certificates for 57 vital-statistics jurisdictions, and the 2016 data included 20,360 deaths. Injuries accounted for 12,336 deaths; unintentional injuries accounted for 57% or 7,057 deaths. Approximately one in five U.S. youth deaths (21%) were suicides, and another one in five (20%) were homicides.
Motor vehicle accidents, also responsible for one in five (20%) of all deaths, were the leading cause of accidental deaths, followed by firearm-related injuries, which accounted for 15% of all deaths. Of the firearm-related deaths, 59% were homicides, 35% suicides, 4% accidental, and 2% undetermined.
The only high-ranking noninjury cause of death overall was neoplasms, yet childhood cancer accounted for just 9% of all deaths. Suffocation was the cause of 7% of deaths, and included homicides, suicides and unintentional injuries.
The remaining causes included drowning (5.9%), drug overdose or poisoning (4.8%), congenital anomalies (4.8%), heart disease (2.9%), fire or burns (1.7%) and chronic lower respiratory disease (1.3%).
“Progress toward further reducing deaths among children and adolescents will require a shift in public perceptions so that injury deaths are viewed not as ‘accidents,’ but rather as social ecologic phenomena that are amenable to prevention,” wrote Rebecca M. Cunningham, MD, and her colleagues at the University of Michigan, Ann Arbor (N Engl J Med. 2018 Dec 20. doi: 10.1056/NEJMsr1804754). The findings “highlight the need to implement public health strategies that are tailored according to age, underlying developmental factors, and injury-related intent” to reduce the risk for death in children.”
“The sad fact is that a child or adolescent in the United States is 57% more likely to die by the age of 19 years than those in other wealthy nations,” Edward W. Campion, MD, executive editor and online editor of the New England Journal of Medicine, wrote in an editorial that accompanied the study (N Engl J Med. 2018 Dec. 20;379[25]:2466-7. doi: 10.1056/NEJMe1814600). “Children in America are dying or being killed at rates that are shameful.
“Our country has led the way in so much medical research, but the facts summarized by Cunningham et al. reveal a need to invest far more in research on the prevention of the injuries that threaten the lives of children and adolescents,” he said.
In an interview, Ben Hoffman, MD, professor of pediatrics at Oregon Health and Science University, Portland, said the only thing surprising in this report is that nothing is surprising.
“This is the stuff that those of us in injury prevention have been screaming about for decades,” said Dr. Hoffman, also medical director of the Tom Sargent Safety Center at OHSU Doernbecher Children’s Hospital.
“Unintentional injuries are what kill kids. We have made such tremendous progress in other areas, and we’ve made progress in terms of preventing injuries, but what we see is unacceptable,” he said. “The fact that [injuries] remain such an issue is a testament to the fact that our collective will [to address these issues] has failed us.”
Among children aged 1-4, drowning was the leading cause of death, followed by congenital anomalies and motor vehicle crashes.
Mandated four-sided fencing around pools is a highly effective intervention for reducing drowning risks, Dr. Hoffman said.
Children aged 5-9 represented the smallest proportion of all youth deaths (12%) and were the only age group not to have injuries as the leading cause of death. Malignant neoplasms led the causes of death in this group, followed by car accidents and congenital anomalies.
Adolescents aged 10-19, the widest age range, comprised 68% of all youth deaths, led by motor vehicle accidents, firearms, and suffocation.
“These findings reflect social and developmental factors that are associated with adolescence, including increased risk-taking behavior, differential peer and parental influence, and initiation of substance use,” Dr. Cunningham and her colleagues wrote.
The most concerning trends, according to Dr. Hoffman, were the upticks in motor vehicle deaths, suffocation, and poisonings, the latter driven largely by opioid overdoses, which were responsible for more than half of all overdoses in adolescents.
Addressing these issues “will require an investment in kids, which is not something that our society does really well,” Dr. Hoffman said. “We talk about it, we tiptoe around it, but when push comes to shove, nobody is really willing to support and fund the efforts to do it.”
In his editorial, Dr. Campion observed that despite a decades-long trend of decreasing mortality from car accidents, these deaths began steadily increasing from 2013 to 2016.
Previous gains in this area came from “the widespread adoption of seat belts and appropriate child safety seats, the production of cars with improved safety standards, better constructed roads, graduated driver-licensing programs, and a focus on reducing teen drinking and driving,” the authors stated. Multiple reasons likely account for the reversal, including distracted driving and possibly marijuana use, though the latter requires more data.
Firearm deaths increased by 28% from 2013 to 2016, driven by suicides (a 26% increase) and homicides (a 32% increase), including increasing school shootings.
Dr. Hoffman acknowledged the complexities of addressing firearm deaths, but “there are effective common sense interventions that could be made ... there’s just not the will.” An example is passing child access prevention (CAP) laws, such as mandating safe storage of guns and imposing criminal liability when children negligently acquire access to firearms. While a variety of small groups address child injury issues, a large, coordinated, centralized national advocacy for kids is lacking, he added.
“The approach to this underrecognized public health problem has to be social as well as technological, and the risks are highest in areas of poverty and social isolation,” Dr. Campion wrote. “We are living in a divisive era in which there are few areas of consensus and agreement. Perhaps one of the few core beliefs that all can agree on is that deaths in childhood and adolescence are tragedies that we must find ways to prevent.”
“Every day, 10 babies die in their sleep, 1.7 kids under age 4 drown, and 4 kids over the age of 1 die in car crashes,” Dr. Hoffman said. “We need to acknowledge the impact of unintentional and intentional injuries and recognize that there are things we can do, that we’re complicit in all of those deaths because in every circumstance, there is something we as a society could have done.”
SOURCE: Cunningham et al. N Engl J Med. 2018 Dec 20;379(25):2468-75. doi: 10.1056/NEJMsr1804754.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Injury prevention efforts are needed to address unintentional injuries, the leading cause of death in U.S. children.
Major finding: Unintentional injuries were the cause of death for 57% of U.S. children aged 1-19 in 2016.
Study details: The findings are based on an analysis of the CDC WONDER database mortality data on 20,360 deaths of U.S. youth aged 1-19.
Disclosures: No external funding was noted. The authors and Dr. Hoffman had no relevant financial disclosures. Dr. Campion is executive editor of the New England Journal of Medicine.
Source: Cunningham et al. N Engl J Med. 2018 Dec 20;379(25):2468-75. doi: 10.1056/NEJMsr1804754.
Adjuvant modified FOLFIRINOX improves survival of pancreatic cancer
For patients with completely or near-completely resected pancreatic ductal adenocarcinoma, adjuvant therapy with a modified FOLFIRINOX regimen was associated with significantly better 3-year disease-free survival and overall survival compared with gemcitabine, results of the phase 3 randomized PRODIGE 24 trial showed.
At a median follow-up of 33.6 months, median disease-free survival was 21.6 months for 247 patients assigned to receive adjuvant modified FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), compared with 12.8 months for 246 patients randomized to receive gemcitabine.
The median overall survival was 54.4 months for patients treated with FOLFIRINOX, vs. 35 months for patients treated with gemcitabine, a difference that translated into a hazard ratio (HR) of 0.65 (P = .003) for the combination regimen, reported Thierry Conroy, MD, of Institut de Cancérologie de Lorraine in Vandoeuvre-lès-Nancy, France, and colleagues in the Canadian Cancer Trials Group and Unicancer-GI-PRODIGE Group.
“The disease-free survival benefit with modified FOLFIRINOX was significant in the majority of subgroups, including subgroups of patients with adverse prognostic factors (i.e., T3 or T4 tumor status, positive lymph nodes, or R1 resection),” they wrote. The report is in The New England Journal of Medicine.
The survival advantage with FOLFIRINOX came at the cost of more frequent adverse events, however, although the only treatment-related death occurred in a patient treated with gemcitabine.
A previous randomized trial by the PRODIGE group showed a survival advantage of FOLFIRINOX over gemcitabine in patients with metastatic pancreatic cancer, prompting the investigators to look at the same two regimens as adjuvant therapy for patients with pancreatic cancer following R0 (clear surgical margins) or RI (minimal residual disease) resections. In the current trial, FOLFIRINOX was modified by the elimination of a bolus dose of fluorouracil to decrease hematologic toxicities and diarrhea without compromising efficacy.
In the intention-to-treat analysis, 493 patients were randomly assigned to receive either modified FOLFIRINOX, consisting of oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, which was reduced to 150 mg/m2 after a protocol-specified safety analysis, leucovorin 400 mg/m2, and fluorouracil 2,400 mg/m2 every 2 weeks, or gemcitabine 1,000 mg/m2 on days 1, 8, and 15 every 4 weeks for 24 weeks.
The disease-free survival primary endpoint and overall survival secondary endpoints were as reported before.
The 3-year overall survival rates were 63.4% with modified FOLFIRINOX, compared with 48.6% with gemcitabine.
Additional secondary endpoints also favored FOLFIRINOX, including metastasis-free survival at a median 30.4 month vs. 17.7 months, respectively, translating into a stratified HR of 0.59 (P less than .001). The 3-year metastasis-free survival rates were 48.2% vs. 30.9% for gemcitabine.
The median cancer-specific survival was not reached in the combination therapy group compared with 36.4 months with gemcitabine monotherapy, a difference that translated into a stratified HR for death from the treated cancer or treatment-related complications of 0.63 (P = .003).
The safety analysis included data on 238 patients treated with FOLFIRINOX and 241 with gemcitabine.
Grade 3 or 4 adverse events occurred in 75.9% of patients treated with modified FOLFIRINOX and 52.9% of patients treated with gemcitabine. The single treatment-related death was from interstitial pneumonitis in a patient treated with gemcitabine.
Grade 3 or 4 diarrhea, increase in the gamma-glutamyltransferase level, paresthesia, fatigue, sensory peripheral neuropathy, nausea, vomiting, abdominal pain, and mucositis were all significantly more frequent with modified FOLFIRINOX.
Slightly more than half (56.8%) of patients in the FOLFIRINOX arm received granulocyte-colony stimulating factor support as either primary prophylaxis or therapy for uncomplicated neutropenia, with no delays in treatment.
The investigators acknowledged that although “disease-free survival is not validated as a surrogate endpoint for overall survival in trials of adjuvant therapy for pancreatic cancer, this criterion was robust and correlated with overall survival.”
The study was supported by R&D Unicancer, which received a grant from Chugai Pharmaceutical the French Ministry of Health and the Institut National du Cancer, and by the French National League against Cancer. The Canadian Cancer Trials Group Pancreatic Adenocarcinoma part of the trial was supported by a Program Grant from the Canadian Cancer Society and by grants from 7 Days in May. Dr. Conroy disclosed receiving travel grants form Roche.
SOURCE: Conroy T et al. N Engl J Med. 2018 Dec 19;379:2395-406.
Is surgery followed by 6 months of adjuvant chemotherapy the appropriate sequence in patients with a disease that may be systemic at diagnosis? Can we further improve outcomes by delivering modified FOLFIRINOX before surgery? The administration of preoperative chemotherapy has several theoretical advantages, including maximizing the potential for an R0 resection by downstaging tumor; treating micrometastatic disease early, thus selecting patients whose disease responds to therapy, while sparing from surgery those whose disease will inevitably progress; and administering chemotherapy when patients are more likely to have fewer or less serious side effects. Retrospective data suggest that neoadjuvant treatment may lead to results that are superior to those with a surgery-first approach, and prospective, randomized trials ought to evaluate this further.
Is there a role for radiation therapy in this context? Will it decrease the incidence of local recurrence among patients with a positive surgical margin who have systemic control with modified FOLFIRINOX therapy? Can preoperative radiotherapy increase the likelihood of an R0 resection? These, too, are questions for future randomized trials.
This trial represents the culmination of more than a decade of careful work that initially established FOLFIRINOX as a standard treatment for advanced pancreatic cancer. The remarkable results that have been achieved with adjuvant modified FOLFIRINOX therapy in the PRODIGE 24 trial have now changed the standard of care for many patients with resectable tumors. However, the majority of patients with pancreatic cancer present with far more advanced disease. For them, this remains a recalcitrant cancer.
Hedy L. Kindler, MD, is with the Section of Hematology/Oncology at the University of Chicago. Her remarks are adapted and condensed from an editorial accompanying the study. She disclosed consulting/advisory board activities for 14 companies, and institutional research funding from six companies.
Is surgery followed by 6 months of adjuvant chemotherapy the appropriate sequence in patients with a disease that may be systemic at diagnosis? Can we further improve outcomes by delivering modified FOLFIRINOX before surgery? The administration of preoperative chemotherapy has several theoretical advantages, including maximizing the potential for an R0 resection by downstaging tumor; treating micrometastatic disease early, thus selecting patients whose disease responds to therapy, while sparing from surgery those whose disease will inevitably progress; and administering chemotherapy when patients are more likely to have fewer or less serious side effects. Retrospective data suggest that neoadjuvant treatment may lead to results that are superior to those with a surgery-first approach, and prospective, randomized trials ought to evaluate this further.
Is there a role for radiation therapy in this context? Will it decrease the incidence of local recurrence among patients with a positive surgical margin who have systemic control with modified FOLFIRINOX therapy? Can preoperative radiotherapy increase the likelihood of an R0 resection? These, too, are questions for future randomized trials.
This trial represents the culmination of more than a decade of careful work that initially established FOLFIRINOX as a standard treatment for advanced pancreatic cancer. The remarkable results that have been achieved with adjuvant modified FOLFIRINOX therapy in the PRODIGE 24 trial have now changed the standard of care for many patients with resectable tumors. However, the majority of patients with pancreatic cancer present with far more advanced disease. For them, this remains a recalcitrant cancer.
Hedy L. Kindler, MD, is with the Section of Hematology/Oncology at the University of Chicago. Her remarks are adapted and condensed from an editorial accompanying the study. She disclosed consulting/advisory board activities for 14 companies, and institutional research funding from six companies.
Is surgery followed by 6 months of adjuvant chemotherapy the appropriate sequence in patients with a disease that may be systemic at diagnosis? Can we further improve outcomes by delivering modified FOLFIRINOX before surgery? The administration of preoperative chemotherapy has several theoretical advantages, including maximizing the potential for an R0 resection by downstaging tumor; treating micrometastatic disease early, thus selecting patients whose disease responds to therapy, while sparing from surgery those whose disease will inevitably progress; and administering chemotherapy when patients are more likely to have fewer or less serious side effects. Retrospective data suggest that neoadjuvant treatment may lead to results that are superior to those with a surgery-first approach, and prospective, randomized trials ought to evaluate this further.
Is there a role for radiation therapy in this context? Will it decrease the incidence of local recurrence among patients with a positive surgical margin who have systemic control with modified FOLFIRINOX therapy? Can preoperative radiotherapy increase the likelihood of an R0 resection? These, too, are questions for future randomized trials.
This trial represents the culmination of more than a decade of careful work that initially established FOLFIRINOX as a standard treatment for advanced pancreatic cancer. The remarkable results that have been achieved with adjuvant modified FOLFIRINOX therapy in the PRODIGE 24 trial have now changed the standard of care for many patients with resectable tumors. However, the majority of patients with pancreatic cancer present with far more advanced disease. For them, this remains a recalcitrant cancer.
Hedy L. Kindler, MD, is with the Section of Hematology/Oncology at the University of Chicago. Her remarks are adapted and condensed from an editorial accompanying the study. She disclosed consulting/advisory board activities for 14 companies, and institutional research funding from six companies.
For patients with completely or near-completely resected pancreatic ductal adenocarcinoma, adjuvant therapy with a modified FOLFIRINOX regimen was associated with significantly better 3-year disease-free survival and overall survival compared with gemcitabine, results of the phase 3 randomized PRODIGE 24 trial showed.
At a median follow-up of 33.6 months, median disease-free survival was 21.6 months for 247 patients assigned to receive adjuvant modified FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), compared with 12.8 months for 246 patients randomized to receive gemcitabine.
The median overall survival was 54.4 months for patients treated with FOLFIRINOX, vs. 35 months for patients treated with gemcitabine, a difference that translated into a hazard ratio (HR) of 0.65 (P = .003) for the combination regimen, reported Thierry Conroy, MD, of Institut de Cancérologie de Lorraine in Vandoeuvre-lès-Nancy, France, and colleagues in the Canadian Cancer Trials Group and Unicancer-GI-PRODIGE Group.
“The disease-free survival benefit with modified FOLFIRINOX was significant in the majority of subgroups, including subgroups of patients with adverse prognostic factors (i.e., T3 or T4 tumor status, positive lymph nodes, or R1 resection),” they wrote. The report is in The New England Journal of Medicine.
The survival advantage with FOLFIRINOX came at the cost of more frequent adverse events, however, although the only treatment-related death occurred in a patient treated with gemcitabine.
A previous randomized trial by the PRODIGE group showed a survival advantage of FOLFIRINOX over gemcitabine in patients with metastatic pancreatic cancer, prompting the investigators to look at the same two regimens as adjuvant therapy for patients with pancreatic cancer following R0 (clear surgical margins) or RI (minimal residual disease) resections. In the current trial, FOLFIRINOX was modified by the elimination of a bolus dose of fluorouracil to decrease hematologic toxicities and diarrhea without compromising efficacy.
In the intention-to-treat analysis, 493 patients were randomly assigned to receive either modified FOLFIRINOX, consisting of oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, which was reduced to 150 mg/m2 after a protocol-specified safety analysis, leucovorin 400 mg/m2, and fluorouracil 2,400 mg/m2 every 2 weeks, or gemcitabine 1,000 mg/m2 on days 1, 8, and 15 every 4 weeks for 24 weeks.
The disease-free survival primary endpoint and overall survival secondary endpoints were as reported before.
The 3-year overall survival rates were 63.4% with modified FOLFIRINOX, compared with 48.6% with gemcitabine.
Additional secondary endpoints also favored FOLFIRINOX, including metastasis-free survival at a median 30.4 month vs. 17.7 months, respectively, translating into a stratified HR of 0.59 (P less than .001). The 3-year metastasis-free survival rates were 48.2% vs. 30.9% for gemcitabine.
The median cancer-specific survival was not reached in the combination therapy group compared with 36.4 months with gemcitabine monotherapy, a difference that translated into a stratified HR for death from the treated cancer or treatment-related complications of 0.63 (P = .003).
The safety analysis included data on 238 patients treated with FOLFIRINOX and 241 with gemcitabine.
Grade 3 or 4 adverse events occurred in 75.9% of patients treated with modified FOLFIRINOX and 52.9% of patients treated with gemcitabine. The single treatment-related death was from interstitial pneumonitis in a patient treated with gemcitabine.
Grade 3 or 4 diarrhea, increase in the gamma-glutamyltransferase level, paresthesia, fatigue, sensory peripheral neuropathy, nausea, vomiting, abdominal pain, and mucositis were all significantly more frequent with modified FOLFIRINOX.
Slightly more than half (56.8%) of patients in the FOLFIRINOX arm received granulocyte-colony stimulating factor support as either primary prophylaxis or therapy for uncomplicated neutropenia, with no delays in treatment.
The investigators acknowledged that although “disease-free survival is not validated as a surrogate endpoint for overall survival in trials of adjuvant therapy for pancreatic cancer, this criterion was robust and correlated with overall survival.”
The study was supported by R&D Unicancer, which received a grant from Chugai Pharmaceutical the French Ministry of Health and the Institut National du Cancer, and by the French National League against Cancer. The Canadian Cancer Trials Group Pancreatic Adenocarcinoma part of the trial was supported by a Program Grant from the Canadian Cancer Society and by grants from 7 Days in May. Dr. Conroy disclosed receiving travel grants form Roche.
SOURCE: Conroy T et al. N Engl J Med. 2018 Dec 19;379:2395-406.
For patients with completely or near-completely resected pancreatic ductal adenocarcinoma, adjuvant therapy with a modified FOLFIRINOX regimen was associated with significantly better 3-year disease-free survival and overall survival compared with gemcitabine, results of the phase 3 randomized PRODIGE 24 trial showed.
At a median follow-up of 33.6 months, median disease-free survival was 21.6 months for 247 patients assigned to receive adjuvant modified FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), compared with 12.8 months for 246 patients randomized to receive gemcitabine.
The median overall survival was 54.4 months for patients treated with FOLFIRINOX, vs. 35 months for patients treated with gemcitabine, a difference that translated into a hazard ratio (HR) of 0.65 (P = .003) for the combination regimen, reported Thierry Conroy, MD, of Institut de Cancérologie de Lorraine in Vandoeuvre-lès-Nancy, France, and colleagues in the Canadian Cancer Trials Group and Unicancer-GI-PRODIGE Group.
“The disease-free survival benefit with modified FOLFIRINOX was significant in the majority of subgroups, including subgroups of patients with adverse prognostic factors (i.e., T3 or T4 tumor status, positive lymph nodes, or R1 resection),” they wrote. The report is in The New England Journal of Medicine.
The survival advantage with FOLFIRINOX came at the cost of more frequent adverse events, however, although the only treatment-related death occurred in a patient treated with gemcitabine.
A previous randomized trial by the PRODIGE group showed a survival advantage of FOLFIRINOX over gemcitabine in patients with metastatic pancreatic cancer, prompting the investigators to look at the same two regimens as adjuvant therapy for patients with pancreatic cancer following R0 (clear surgical margins) or RI (minimal residual disease) resections. In the current trial, FOLFIRINOX was modified by the elimination of a bolus dose of fluorouracil to decrease hematologic toxicities and diarrhea without compromising efficacy.
In the intention-to-treat analysis, 493 patients were randomly assigned to receive either modified FOLFIRINOX, consisting of oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, which was reduced to 150 mg/m2 after a protocol-specified safety analysis, leucovorin 400 mg/m2, and fluorouracil 2,400 mg/m2 every 2 weeks, or gemcitabine 1,000 mg/m2 on days 1, 8, and 15 every 4 weeks for 24 weeks.
The disease-free survival primary endpoint and overall survival secondary endpoints were as reported before.
The 3-year overall survival rates were 63.4% with modified FOLFIRINOX, compared with 48.6% with gemcitabine.
Additional secondary endpoints also favored FOLFIRINOX, including metastasis-free survival at a median 30.4 month vs. 17.7 months, respectively, translating into a stratified HR of 0.59 (P less than .001). The 3-year metastasis-free survival rates were 48.2% vs. 30.9% for gemcitabine.
The median cancer-specific survival was not reached in the combination therapy group compared with 36.4 months with gemcitabine monotherapy, a difference that translated into a stratified HR for death from the treated cancer or treatment-related complications of 0.63 (P = .003).
The safety analysis included data on 238 patients treated with FOLFIRINOX and 241 with gemcitabine.
Grade 3 or 4 adverse events occurred in 75.9% of patients treated with modified FOLFIRINOX and 52.9% of patients treated with gemcitabine. The single treatment-related death was from interstitial pneumonitis in a patient treated with gemcitabine.
Grade 3 or 4 diarrhea, increase in the gamma-glutamyltransferase level, paresthesia, fatigue, sensory peripheral neuropathy, nausea, vomiting, abdominal pain, and mucositis were all significantly more frequent with modified FOLFIRINOX.
Slightly more than half (56.8%) of patients in the FOLFIRINOX arm received granulocyte-colony stimulating factor support as either primary prophylaxis or therapy for uncomplicated neutropenia, with no delays in treatment.
The investigators acknowledged that although “disease-free survival is not validated as a surrogate endpoint for overall survival in trials of adjuvant therapy for pancreatic cancer, this criterion was robust and correlated with overall survival.”
The study was supported by R&D Unicancer, which received a grant from Chugai Pharmaceutical the French Ministry of Health and the Institut National du Cancer, and by the French National League against Cancer. The Canadian Cancer Trials Group Pancreatic Adenocarcinoma part of the trial was supported by a Program Grant from the Canadian Cancer Society and by grants from 7 Days in May. Dr. Conroy disclosed receiving travel grants form Roche.
SOURCE: Conroy T et al. N Engl J Med. 2018 Dec 19;379:2395-406.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Modified FOLFIRINOX resulted in significantly better disease-free and overall survival compared with gemcitabine as adjuvant therapy following complete/near complete resection of pancreatic ductal adenocarcinoma.
Major finding: Median disease-free survival was 21.6 months with modified FOLFIRINOX vs. 12.8 months with gemcitabine.
Study details: Randomized phase 3 trial of 493 patients with resected pancreatic cancer.
Disclosures: The study was supported by R&D Unicancer, which received a grant from Chugai Pharmaceutical the French Ministry of Health and the Institut National du Cancer, and by the French National League against Cancer. The Canadian Cancer Trials Group Pancreatic Adenocarcinoma part of the trial was supported by a Program Grant from the Canadian Cancer Society and by grants from 7 Days in May. Dr. Conroy disclosed receiving travel grants form Roche.
Source: Conroy T et al. N Engl J Med. 2018 Dec 19 379:2395-406.
Sorafenib extends PFS for refractory desmoid tumors
For patients with progressive, refractory, or symptomatic desmoid tumors – also known as aggressive fibromatosis – treatment with daily sorafenib (Nexavar) was associated with durable responses and a significant improvement in progression-free survival.
After a median follow-up of 27.2 months, the 2-year progression-free survival (PFS) rate for patients randomly assigned to receive 400 mg sorafenib daily was 81%, compared with 36% for patients assigned to placebo (P less than .001), reported Mrinal M. Gounder, MD, from Memorial Sloan Kettering Cancer Center in New York City, and his colleagues.
“Other agents that are used to treat these tumors include anthracyclines [e.g., pegylated liposomal doxorubicin], vinca alkaloids, and pazopanib. On the basis of the predictable toxic-effects profile and substantial progression-free survival advantage conferred by sorafenib, the drug has antitumor activity as first-line therapy or as subsequent therapy for desmoid tumors,” they wrote in the New England Journal of Medicine.
There is no accepted standard of care for the systemic treatment for desmoid tumors, with options ranging from hormonal blockade, cytotoxic chemotherapy, and targeted agents such as tyrosine kinase inhibitors (TKIs).
Based on a retrospective study showing that the multitargeting oral TKI sorafenib was associated with a 25% response rate and acceptable safety in patients with desmoid tumors, the investigators initiated a phase 3, randomized trial to evaluate the efficacy and safety of sorafenib in this population.
They enrolled 87 patients aged 18 years or older with a histologically documented desmoid tumor that showed clinical and radiographic progression of at least 10% in maximum unidimensional measurement within the last 6 months, symptomatic disease, or recurrent or primary disease that was either inoperable or deemed to require extensive surgery.
The patients were randomized in double-blinded fashion on a 2:1 basis to receive either sorafenib 400 mg daily or placebo until progression. Crossover to sorafenib was allowed for patients assigned to placebo who experienced disease progressions.
As noted before, investigator-assessed PFS, the primary endpoint, clearly favored sorafenib.
Objective response rates before crossover were 33% in the sorafenib arm, consisting of 1 complete and 15 partial responses, and 20% in the placebo arm, consisting of 7 partial responses. The respective median times to objective response were 9.6 months versus 13.3 months. The earliest response, defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, occurred at 2.2 months in the sorafenib arm versus 8.8 months in the placebo arm.
The authors also performed an exploratory analysis looking at MRI as a measure of response evaluation and found that “changes in T2-weighted signal intensity and volumetric measurements may be better measures of treatment effect than RECIST. This is particularly evident when the best response according to RECIST is stable disease.”
The most frequently reported adverse events among patients treated with sorafenib were grade 1 or 2 rash in 73%, fatigue in 67%, hypertension in 55%, and diarrhea in 51%. The most frequent treatment-emergent adverse events in the placebo group were rash of any kind in 42% and palmar-plantar erythrodysesthesia syndrome in 22%.
The investigators acknowledged that the mechanism of action of sorafenib in desmoid tumors is unknown, but noted that they are looking for clues in 25 sets of paired biopsy samples.
The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
SOURCE: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
For patients with progressive, refractory, or symptomatic desmoid tumors – also known as aggressive fibromatosis – treatment with daily sorafenib (Nexavar) was associated with durable responses and a significant improvement in progression-free survival.
After a median follow-up of 27.2 months, the 2-year progression-free survival (PFS) rate for patients randomly assigned to receive 400 mg sorafenib daily was 81%, compared with 36% for patients assigned to placebo (P less than .001), reported Mrinal M. Gounder, MD, from Memorial Sloan Kettering Cancer Center in New York City, and his colleagues.
“Other agents that are used to treat these tumors include anthracyclines [e.g., pegylated liposomal doxorubicin], vinca alkaloids, and pazopanib. On the basis of the predictable toxic-effects profile and substantial progression-free survival advantage conferred by sorafenib, the drug has antitumor activity as first-line therapy or as subsequent therapy for desmoid tumors,” they wrote in the New England Journal of Medicine.
There is no accepted standard of care for the systemic treatment for desmoid tumors, with options ranging from hormonal blockade, cytotoxic chemotherapy, and targeted agents such as tyrosine kinase inhibitors (TKIs).
Based on a retrospective study showing that the multitargeting oral TKI sorafenib was associated with a 25% response rate and acceptable safety in patients with desmoid tumors, the investigators initiated a phase 3, randomized trial to evaluate the efficacy and safety of sorafenib in this population.
They enrolled 87 patients aged 18 years or older with a histologically documented desmoid tumor that showed clinical and radiographic progression of at least 10% in maximum unidimensional measurement within the last 6 months, symptomatic disease, or recurrent or primary disease that was either inoperable or deemed to require extensive surgery.
The patients were randomized in double-blinded fashion on a 2:1 basis to receive either sorafenib 400 mg daily or placebo until progression. Crossover to sorafenib was allowed for patients assigned to placebo who experienced disease progressions.
As noted before, investigator-assessed PFS, the primary endpoint, clearly favored sorafenib.
Objective response rates before crossover were 33% in the sorafenib arm, consisting of 1 complete and 15 partial responses, and 20% in the placebo arm, consisting of 7 partial responses. The respective median times to objective response were 9.6 months versus 13.3 months. The earliest response, defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, occurred at 2.2 months in the sorafenib arm versus 8.8 months in the placebo arm.
The authors also performed an exploratory analysis looking at MRI as a measure of response evaluation and found that “changes in T2-weighted signal intensity and volumetric measurements may be better measures of treatment effect than RECIST. This is particularly evident when the best response according to RECIST is stable disease.”
The most frequently reported adverse events among patients treated with sorafenib were grade 1 or 2 rash in 73%, fatigue in 67%, hypertension in 55%, and diarrhea in 51%. The most frequent treatment-emergent adverse events in the placebo group were rash of any kind in 42% and palmar-plantar erythrodysesthesia syndrome in 22%.
The investigators acknowledged that the mechanism of action of sorafenib in desmoid tumors is unknown, but noted that they are looking for clues in 25 sets of paired biopsy samples.
The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
SOURCE: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
For patients with progressive, refractory, or symptomatic desmoid tumors – also known as aggressive fibromatosis – treatment with daily sorafenib (Nexavar) was associated with durable responses and a significant improvement in progression-free survival.
After a median follow-up of 27.2 months, the 2-year progression-free survival (PFS) rate for patients randomly assigned to receive 400 mg sorafenib daily was 81%, compared with 36% for patients assigned to placebo (P less than .001), reported Mrinal M. Gounder, MD, from Memorial Sloan Kettering Cancer Center in New York City, and his colleagues.
“Other agents that are used to treat these tumors include anthracyclines [e.g., pegylated liposomal doxorubicin], vinca alkaloids, and pazopanib. On the basis of the predictable toxic-effects profile and substantial progression-free survival advantage conferred by sorafenib, the drug has antitumor activity as first-line therapy or as subsequent therapy for desmoid tumors,” they wrote in the New England Journal of Medicine.
There is no accepted standard of care for the systemic treatment for desmoid tumors, with options ranging from hormonal blockade, cytotoxic chemotherapy, and targeted agents such as tyrosine kinase inhibitors (TKIs).
Based on a retrospective study showing that the multitargeting oral TKI sorafenib was associated with a 25% response rate and acceptable safety in patients with desmoid tumors, the investigators initiated a phase 3, randomized trial to evaluate the efficacy and safety of sorafenib in this population.
They enrolled 87 patients aged 18 years or older with a histologically documented desmoid tumor that showed clinical and radiographic progression of at least 10% in maximum unidimensional measurement within the last 6 months, symptomatic disease, or recurrent or primary disease that was either inoperable or deemed to require extensive surgery.
The patients were randomized in double-blinded fashion on a 2:1 basis to receive either sorafenib 400 mg daily or placebo until progression. Crossover to sorafenib was allowed for patients assigned to placebo who experienced disease progressions.
As noted before, investigator-assessed PFS, the primary endpoint, clearly favored sorafenib.
Objective response rates before crossover were 33% in the sorafenib arm, consisting of 1 complete and 15 partial responses, and 20% in the placebo arm, consisting of 7 partial responses. The respective median times to objective response were 9.6 months versus 13.3 months. The earliest response, defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, occurred at 2.2 months in the sorafenib arm versus 8.8 months in the placebo arm.
The authors also performed an exploratory analysis looking at MRI as a measure of response evaluation and found that “changes in T2-weighted signal intensity and volumetric measurements may be better measures of treatment effect than RECIST. This is particularly evident when the best response according to RECIST is stable disease.”
The most frequently reported adverse events among patients treated with sorafenib were grade 1 or 2 rash in 73%, fatigue in 67%, hypertension in 55%, and diarrhea in 51%. The most frequent treatment-emergent adverse events in the placebo group were rash of any kind in 42% and palmar-plantar erythrodysesthesia syndrome in 22%.
The investigators acknowledged that the mechanism of action of sorafenib in desmoid tumors is unknown, but noted that they are looking for clues in 25 sets of paired biopsy samples.
The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
SOURCE: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: There is no accepted standard of systemic therapy for recurrent, refractory, or symptomatic desmoid tumors.
Major finding: Median progression-free survival with sorafenib after a median follow-up of 27.2 months was 81% versus 36% for placebo.
Study details: A double-blind, phase 3 trial with 2:1 randomization of sorafenib to placebo in 87 patients.
Disclosures: The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
Source: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
Rising microbiome investigator: Lea Ann Chen, MD
We spoke with Dr. Chen, assistant professor of medicine at New York University and the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S.*, Chen L.A.*, Grigoryan Z., et al. Microbiome changes associated with sustained eradication of Clostridium difficile after fecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
We spoke with Dr. Chen, assistant professor of medicine at New York University and the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S.*, Chen L.A.*, Grigoryan Z., et al. Microbiome changes associated with sustained eradication of Clostridium difficile after fecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
We spoke with Dr. Chen, assistant professor of medicine at New York University and the recipient of the AGA Research Foundation’s 2016 Research Scholar Award, to learn about her work on the gut microbiome and inflammatory bowel disease (IBD).
How would you sum up your research in one sentence?
I study longitudinal changes of the gut microbiome as it relates to gastrointestinal illnesses, particularly IBD.
What impact do you hope your research will have on patients?
I hope that my research will provide greater insights into the role of gut microbes in disease pathogenesis and activity to ultimately inform the development of new diagnostics and treatments.
What inspired you to focus your research career on the gut microbiome?
I’ve long been fascinated by ecological systems and host-microbe interactions. As technologies to study the gut microbiome became more readily available, I was eager, and somewhat relieved, to be able to combine my research interests with my clinical interest in gastroenterology.
What recent publication from your lab best represents your work, if anyone wants to learn more?
In this study, we show that gut bacterial disturbances are resolved after fecal transplantation in children without IBD but are only transiently resolved in those with IBD.
Hourigan S.*, Chen L.A.*, Grigoryan Z., et al. Microbiome changes associated with sustained eradication of Clostridium difficile after fecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:741-52.
You’re involved with several AGA initiatives including the Future Leaders Program and the FMT National Registry. How has being an AGA member impacted your career?
AGA has provided key mentorship and training opportunities that have been instrumental in my career development. It has further helped me discover a diverse community of clinicians and scientists who are amazing role models, resources and colleagues. I really had no inkling what was in store when I first joined AGA as a trainee, but I feel very lucky that I did and am grateful for how AGA membership has really enriched my life as a gastroenterologist.
Differences in gut bacteria can distinguish IBD from IBS
Thanks to shotgun metagenomic sequencing of gut microbiota, physicians are on track to more easily distinguish inflammatory bowel disease (IBD) from irritable bowel syndrome (IBS), according to an analysis of stool samples from patients with the two common gastrointestinal diseases.
“The integration of these datasets allowed us to pinpoint key species as targets for functional studies in IBD and IBS and to connect knowledge of the etiology and pathogenesis of IBD and IBS with the gut microbiome to provide potential new targets for treatment,” wrote Arnau Vich Vila, of the University of Groningen, the Netherlands, and his coauthors. The report is in Science Translational Medicine.
Stool samples from 1,792 participants were analyzed: 355 from patients with IBD, 412 from patients with IBS, and 1,025 from the control group. The researchers found 24 bacterial taxa associated with both IBD and IBS and specific species that accompanied specific diseases, such as an abundance of Bacteroides in patients with IBD and Firmicutes in patients with IBS. In addition, their predictive model to distinguish IBD from IBS via gut microbial composition data [area under the curve (AUC) mean = 0.91 (0.81 to 0.99)] proved more accurate than did current fecal biomarker calprotectin [AUC mean = 0.80 (0.71 to 0.88); P = .002].
The authors acknowledged additional evidence that will be needed before these results can be translated to clinical practice, including supporting their described microbial pathways with metatranscriptomics and metabolomics data as well as functional experiments. They also observed that their predictive model will need to be validated through replication of their findings in patients with other gastrointestinal disorders or prediagnostic groups. They noted that their analysis benefited from being able to correct for confounding factors such as medication use, which is “essential for identifying disease-associated microbial features and avoiding false-positive associations due to changes in GI acidity or bowel mobility.”
One author reported receiving speaker fees from AbbVie and was a shareholder of the health care IT company Aceso BV and of Floris Medical Holding BV. Another author declared unrestricted research grants from AbbVie, Takeda, and Ferring Pharmaceuticals, is on the advisory boards for Mundipharma and Pharmacosmos, and has received speaker fees from Takeda and Janssen Pharmaceuticals. A third author declared consulting work for Takeda. The others reported no conflicts of interest.
SOURCE: Vich Vila A et al. Sci Transl Med. 2018 Dec 19. doi: 10.1126/scitranslmed.aap8914.
Thanks to shotgun metagenomic sequencing of gut microbiota, physicians are on track to more easily distinguish inflammatory bowel disease (IBD) from irritable bowel syndrome (IBS), according to an analysis of stool samples from patients with the two common gastrointestinal diseases.
“The integration of these datasets allowed us to pinpoint key species as targets for functional studies in IBD and IBS and to connect knowledge of the etiology and pathogenesis of IBD and IBS with the gut microbiome to provide potential new targets for treatment,” wrote Arnau Vich Vila, of the University of Groningen, the Netherlands, and his coauthors. The report is in Science Translational Medicine.
Stool samples from 1,792 participants were analyzed: 355 from patients with IBD, 412 from patients with IBS, and 1,025 from the control group. The researchers found 24 bacterial taxa associated with both IBD and IBS and specific species that accompanied specific diseases, such as an abundance of Bacteroides in patients with IBD and Firmicutes in patients with IBS. In addition, their predictive model to distinguish IBD from IBS via gut microbial composition data [area under the curve (AUC) mean = 0.91 (0.81 to 0.99)] proved more accurate than did current fecal biomarker calprotectin [AUC mean = 0.80 (0.71 to 0.88); P = .002].
The authors acknowledged additional evidence that will be needed before these results can be translated to clinical practice, including supporting their described microbial pathways with metatranscriptomics and metabolomics data as well as functional experiments. They also observed that their predictive model will need to be validated through replication of their findings in patients with other gastrointestinal disorders or prediagnostic groups. They noted that their analysis benefited from being able to correct for confounding factors such as medication use, which is “essential for identifying disease-associated microbial features and avoiding false-positive associations due to changes in GI acidity or bowel mobility.”
One author reported receiving speaker fees from AbbVie and was a shareholder of the health care IT company Aceso BV and of Floris Medical Holding BV. Another author declared unrestricted research grants from AbbVie, Takeda, and Ferring Pharmaceuticals, is on the advisory boards for Mundipharma and Pharmacosmos, and has received speaker fees from Takeda and Janssen Pharmaceuticals. A third author declared consulting work for Takeda. The others reported no conflicts of interest.
SOURCE: Vich Vila A et al. Sci Transl Med. 2018 Dec 19. doi: 10.1126/scitranslmed.aap8914.
Thanks to shotgun metagenomic sequencing of gut microbiota, physicians are on track to more easily distinguish inflammatory bowel disease (IBD) from irritable bowel syndrome (IBS), according to an analysis of stool samples from patients with the two common gastrointestinal diseases.
“The integration of these datasets allowed us to pinpoint key species as targets for functional studies in IBD and IBS and to connect knowledge of the etiology and pathogenesis of IBD and IBS with the gut microbiome to provide potential new targets for treatment,” wrote Arnau Vich Vila, of the University of Groningen, the Netherlands, and his coauthors. The report is in Science Translational Medicine.
Stool samples from 1,792 participants were analyzed: 355 from patients with IBD, 412 from patients with IBS, and 1,025 from the control group. The researchers found 24 bacterial taxa associated with both IBD and IBS and specific species that accompanied specific diseases, such as an abundance of Bacteroides in patients with IBD and Firmicutes in patients with IBS. In addition, their predictive model to distinguish IBD from IBS via gut microbial composition data [area under the curve (AUC) mean = 0.91 (0.81 to 0.99)] proved more accurate than did current fecal biomarker calprotectin [AUC mean = 0.80 (0.71 to 0.88); P = .002].
The authors acknowledged additional evidence that will be needed before these results can be translated to clinical practice, including supporting their described microbial pathways with metatranscriptomics and metabolomics data as well as functional experiments. They also observed that their predictive model will need to be validated through replication of their findings in patients with other gastrointestinal disorders or prediagnostic groups. They noted that their analysis benefited from being able to correct for confounding factors such as medication use, which is “essential for identifying disease-associated microbial features and avoiding false-positive associations due to changes in GI acidity or bowel mobility.”
One author reported receiving speaker fees from AbbVie and was a shareholder of the health care IT company Aceso BV and of Floris Medical Holding BV. Another author declared unrestricted research grants from AbbVie, Takeda, and Ferring Pharmaceuticals, is on the advisory boards for Mundipharma and Pharmacosmos, and has received speaker fees from Takeda and Janssen Pharmaceuticals. A third author declared consulting work for Takeda. The others reported no conflicts of interest.
SOURCE: Vich Vila A et al. Sci Transl Med. 2018 Dec 19. doi: 10.1126/scitranslmed.aap8914.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Shotgun metagenomic sequencing data revealed key differences in gut microbiome composition between patients with inflammatory bowel disease and patients with irritable bowel syndrome.
Major finding: A predictive model to distinguish IBD from IBS based on gut microbial composition data [area under the curve (AUC) mean = 0.91 (0.81-0.99)] proved more accurate than did fecal biomarker calprotectin [AUC mean = 0.80 (0.71-0.88); P = .002].
Study details: A case-control analysis using shotgun metagenomic sequencing of stool samples from 1,792 individuals with IBD, IBS, or neither.
Disclosures: One author reported receiving speaker fees from AbbVie and was a shareholder of the health care IT company Aceso BV and of Floris Medical Holding BV. Another author declared unrestricted research grants from AbbVie, Takeda, and Ferring Pharmaceuticals, is on the advisory boards for Mundipharma and Pharmacosmos, and has received speaker fees from Takeda and Janssen Pharmaceuticals. A third author declared consulting work for Takeda. The others reported no conflicts of interest.
Source: Vich Vila A et al. Sci Transl Med. 2018 Dec 19. doi: 10.1126/scitranslmed.aap8914.
Ingredients for effective team-based care
Changing times for U.S. health care
The current health care environment is undergoing a rapid transformation. In evolutionary biology, a theory exists called punctuated equilibrium. This theory suggests there are long periods of little or no morphological change amongst species and then, geologically speaking, short periods of rapid change in response to pressures within the environment. This rapid period of change adds significant diversity to the landscape of existing species. In health care, we are undergoing a period of “punctuation.”
A testament to the degree of change is a scan of the various consolidation activities occurring across the health care space. Some are more traditional, such as mergers of health systems with different or competing geographical footprints or hospitalist management companies that provide similar services and desire to increase their market share. Others that are more interesting are those that include mergers of seemingly different business lines or offerings, like CVS Health and Aetna; Humana and Kindred; or even organizations such as Amazon, Berkshire Hathaway, and JP Morgan hiring Atul Gawande as the CEO of their newly formed health care venture. The latter examples serve as an illustration of the reorganization that is occurring within health care delivery. This represents, at the very least, a blurring of the lines – if not a deconstruction and complete rebuild – of traditional lines of separation between payers, providers, employers, and retailers.
In other words, the silos are coming down, significant diversity in the landscape of existing species. A common theme across these changes is that most – if not all – participants will share some portion of the financial risk associated with these evolving models. High-deductible health plans, alternative payment models (APMs), and advanced APMs are examples of tactics and models that distribute the financial risk. The consolidations referenced above will likely continue to encourage distribution of the financial risk across patients, providers, employers, and payers.
A key theme coming into focus is that the evolving care delivery system will not be defined by bricks and mortar. Rather, it will follow the patient and go wherever he or she goes to meet his or her specific needs. This is why we’re seeing mergers comprised of a variety of assets, including personnel, technology, critical supplies (such as pharmaceuticals), and funding resources. This very purposeful and deliberate melting pot phenomenon will restructure and reformat the care delivery model.
To be successful within this new landscape, there will need to be a renewed focus on working within a collaborative model. The days of a single entity or provider being able to serve as the “be all” or “do all” is over, and the days of practicing medicine as the Lone Ranger are anachronistic. Instead, there is a need for health care providers to embrace and lead a team-based care model. Team-based care should have the patient at the center of the care delivery model and leverage the expertise of the various team members to practice at the “top of their expertise.”
In hospital medicine, this includes a variety of team members – from physicians, nurse practitioners, physician assistants, and clinical pharmacists to case managers, physical therapists, subject matter experts in quality improvement, and analysts – who identify operational priorities from the data rather than reporting predefined goals on dashboards. Although possibly a good start, this is by no means an exhaustive list of team members. The team will be defined by the goals the health care team aspires to achieve. These goals may include closer alignment with payers, employers, and post-acute partners; the goals will influence the composition of the team. Once the team is defined, the challenge will be to effectively integrate team members, so they are contributing their expertise to the patient care being delivered.
Some ingredients for effective team-based care include the following:
- Developing an effective process for engagement and providing a voice for all team members. Interdisciplinary team rounds where there is an established time for team members to plan and operationalize their plans around patient care can serve as an example of this type of structured process.
- Creating well-defined roles and responsibilities with key performance indicators to promote accountability. The team will have outcomes they are measuring and striving to impact, and each team member will have a role in achieving those goals. Being able to parse out and measure how each team member contributes to the overall outcome can be beneficial. This provides an opportunity for each team member to play a meaningful role in accomplishing the overall goal and allows for a measurement process to track success. For example, an overall team goal may be to have a specific percentage of eligible discharges completed by 11:00 a.m. To accomplish this goal, there may be specific objectives for the clinicians to have discharge orders in the chart by 9:30 a.m. and for case management to have communicated with any post-acute services the day before discharge. These specific accountability measures facilitate accomplishing the larger team goal.
- Developing a culture of safety and transparency. Effective teams promote an environment where all members are empowered and encouraged to speak and share their perspective and knowledge. Communication is based on the value it provides to accomplishing the team’s goals rather than based on a hierarchy which determines who contributes and when.
- Defining and then redefining the competencies required of the team to promote continued development and growth. In this time of dynamic change, the skill sets that helped us get where we are today may be different then the skill sets that are needed for success in the future. There will continue to be a need for functional and knowledge-based competencies in addition to the need to focus on competencies that engender a culture of team-based care. For example, hospitalist leaders will need to understand evidence-based medicine to support appropriate management of a septic patient and simultaneously understand evidence-based management/leadership to affect sepsis care across his or her health care system.
With this change in the health care environment come new and exciting opportunities. Hospital medicine has always elected to assume a leadership role in these times of change, these periods of “punctuation.” Development of effective team-based care is a great place for those of us working in hospital medicine to demonstrate our leadership as we care for our patients.
Dr. Frost is national medical director, hospital-based services, at LifePoint Health, Brentwood, Tenn. He is president-elect of the Society of Hospital Medicine.
Changing times for U.S. health care
Changing times for U.S. health care
The current health care environment is undergoing a rapid transformation. In evolutionary biology, a theory exists called punctuated equilibrium. This theory suggests there are long periods of little or no morphological change amongst species and then, geologically speaking, short periods of rapid change in response to pressures within the environment. This rapid period of change adds significant diversity to the landscape of existing species. In health care, we are undergoing a period of “punctuation.”
A testament to the degree of change is a scan of the various consolidation activities occurring across the health care space. Some are more traditional, such as mergers of health systems with different or competing geographical footprints or hospitalist management companies that provide similar services and desire to increase their market share. Others that are more interesting are those that include mergers of seemingly different business lines or offerings, like CVS Health and Aetna; Humana and Kindred; or even organizations such as Amazon, Berkshire Hathaway, and JP Morgan hiring Atul Gawande as the CEO of their newly formed health care venture. The latter examples serve as an illustration of the reorganization that is occurring within health care delivery. This represents, at the very least, a blurring of the lines – if not a deconstruction and complete rebuild – of traditional lines of separation between payers, providers, employers, and retailers.
In other words, the silos are coming down, significant diversity in the landscape of existing species. A common theme across these changes is that most – if not all – participants will share some portion of the financial risk associated with these evolving models. High-deductible health plans, alternative payment models (APMs), and advanced APMs are examples of tactics and models that distribute the financial risk. The consolidations referenced above will likely continue to encourage distribution of the financial risk across patients, providers, employers, and payers.
A key theme coming into focus is that the evolving care delivery system will not be defined by bricks and mortar. Rather, it will follow the patient and go wherever he or she goes to meet his or her specific needs. This is why we’re seeing mergers comprised of a variety of assets, including personnel, technology, critical supplies (such as pharmaceuticals), and funding resources. This very purposeful and deliberate melting pot phenomenon will restructure and reformat the care delivery model.
To be successful within this new landscape, there will need to be a renewed focus on working within a collaborative model. The days of a single entity or provider being able to serve as the “be all” or “do all” is over, and the days of practicing medicine as the Lone Ranger are anachronistic. Instead, there is a need for health care providers to embrace and lead a team-based care model. Team-based care should have the patient at the center of the care delivery model and leverage the expertise of the various team members to practice at the “top of their expertise.”
In hospital medicine, this includes a variety of team members – from physicians, nurse practitioners, physician assistants, and clinical pharmacists to case managers, physical therapists, subject matter experts in quality improvement, and analysts – who identify operational priorities from the data rather than reporting predefined goals on dashboards. Although possibly a good start, this is by no means an exhaustive list of team members. The team will be defined by the goals the health care team aspires to achieve. These goals may include closer alignment with payers, employers, and post-acute partners; the goals will influence the composition of the team. Once the team is defined, the challenge will be to effectively integrate team members, so they are contributing their expertise to the patient care being delivered.
Some ingredients for effective team-based care include the following:
- Developing an effective process for engagement and providing a voice for all team members. Interdisciplinary team rounds where there is an established time for team members to plan and operationalize their plans around patient care can serve as an example of this type of structured process.
- Creating well-defined roles and responsibilities with key performance indicators to promote accountability. The team will have outcomes they are measuring and striving to impact, and each team member will have a role in achieving those goals. Being able to parse out and measure how each team member contributes to the overall outcome can be beneficial. This provides an opportunity for each team member to play a meaningful role in accomplishing the overall goal and allows for a measurement process to track success. For example, an overall team goal may be to have a specific percentage of eligible discharges completed by 11:00 a.m. To accomplish this goal, there may be specific objectives for the clinicians to have discharge orders in the chart by 9:30 a.m. and for case management to have communicated with any post-acute services the day before discharge. These specific accountability measures facilitate accomplishing the larger team goal.
- Developing a culture of safety and transparency. Effective teams promote an environment where all members are empowered and encouraged to speak and share their perspective and knowledge. Communication is based on the value it provides to accomplishing the team’s goals rather than based on a hierarchy which determines who contributes and when.
- Defining and then redefining the competencies required of the team to promote continued development and growth. In this time of dynamic change, the skill sets that helped us get where we are today may be different then the skill sets that are needed for success in the future. There will continue to be a need for functional and knowledge-based competencies in addition to the need to focus on competencies that engender a culture of team-based care. For example, hospitalist leaders will need to understand evidence-based medicine to support appropriate management of a septic patient and simultaneously understand evidence-based management/leadership to affect sepsis care across his or her health care system.
With this change in the health care environment come new and exciting opportunities. Hospital medicine has always elected to assume a leadership role in these times of change, these periods of “punctuation.” Development of effective team-based care is a great place for those of us working in hospital medicine to demonstrate our leadership as we care for our patients.
Dr. Frost is national medical director, hospital-based services, at LifePoint Health, Brentwood, Tenn. He is president-elect of the Society of Hospital Medicine.
The current health care environment is undergoing a rapid transformation. In evolutionary biology, a theory exists called punctuated equilibrium. This theory suggests there are long periods of little or no morphological change amongst species and then, geologically speaking, short periods of rapid change in response to pressures within the environment. This rapid period of change adds significant diversity to the landscape of existing species. In health care, we are undergoing a period of “punctuation.”
A testament to the degree of change is a scan of the various consolidation activities occurring across the health care space. Some are more traditional, such as mergers of health systems with different or competing geographical footprints or hospitalist management companies that provide similar services and desire to increase their market share. Others that are more interesting are those that include mergers of seemingly different business lines or offerings, like CVS Health and Aetna; Humana and Kindred; or even organizations such as Amazon, Berkshire Hathaway, and JP Morgan hiring Atul Gawande as the CEO of their newly formed health care venture. The latter examples serve as an illustration of the reorganization that is occurring within health care delivery. This represents, at the very least, a blurring of the lines – if not a deconstruction and complete rebuild – of traditional lines of separation between payers, providers, employers, and retailers.
In other words, the silos are coming down, significant diversity in the landscape of existing species. A common theme across these changes is that most – if not all – participants will share some portion of the financial risk associated with these evolving models. High-deductible health plans, alternative payment models (APMs), and advanced APMs are examples of tactics and models that distribute the financial risk. The consolidations referenced above will likely continue to encourage distribution of the financial risk across patients, providers, employers, and payers.
A key theme coming into focus is that the evolving care delivery system will not be defined by bricks and mortar. Rather, it will follow the patient and go wherever he or she goes to meet his or her specific needs. This is why we’re seeing mergers comprised of a variety of assets, including personnel, technology, critical supplies (such as pharmaceuticals), and funding resources. This very purposeful and deliberate melting pot phenomenon will restructure and reformat the care delivery model.
To be successful within this new landscape, there will need to be a renewed focus on working within a collaborative model. The days of a single entity or provider being able to serve as the “be all” or “do all” is over, and the days of practicing medicine as the Lone Ranger are anachronistic. Instead, there is a need for health care providers to embrace and lead a team-based care model. Team-based care should have the patient at the center of the care delivery model and leverage the expertise of the various team members to practice at the “top of their expertise.”
In hospital medicine, this includes a variety of team members – from physicians, nurse practitioners, physician assistants, and clinical pharmacists to case managers, physical therapists, subject matter experts in quality improvement, and analysts – who identify operational priorities from the data rather than reporting predefined goals on dashboards. Although possibly a good start, this is by no means an exhaustive list of team members. The team will be defined by the goals the health care team aspires to achieve. These goals may include closer alignment with payers, employers, and post-acute partners; the goals will influence the composition of the team. Once the team is defined, the challenge will be to effectively integrate team members, so they are contributing their expertise to the patient care being delivered.
Some ingredients for effective team-based care include the following:
- Developing an effective process for engagement and providing a voice for all team members. Interdisciplinary team rounds where there is an established time for team members to plan and operationalize their plans around patient care can serve as an example of this type of structured process.
- Creating well-defined roles and responsibilities with key performance indicators to promote accountability. The team will have outcomes they are measuring and striving to impact, and each team member will have a role in achieving those goals. Being able to parse out and measure how each team member contributes to the overall outcome can be beneficial. This provides an opportunity for each team member to play a meaningful role in accomplishing the overall goal and allows for a measurement process to track success. For example, an overall team goal may be to have a specific percentage of eligible discharges completed by 11:00 a.m. To accomplish this goal, there may be specific objectives for the clinicians to have discharge orders in the chart by 9:30 a.m. and for case management to have communicated with any post-acute services the day before discharge. These specific accountability measures facilitate accomplishing the larger team goal.
- Developing a culture of safety and transparency. Effective teams promote an environment where all members are empowered and encouraged to speak and share their perspective and knowledge. Communication is based on the value it provides to accomplishing the team’s goals rather than based on a hierarchy which determines who contributes and when.
- Defining and then redefining the competencies required of the team to promote continued development and growth. In this time of dynamic change, the skill sets that helped us get where we are today may be different then the skill sets that are needed for success in the future. There will continue to be a need for functional and knowledge-based competencies in addition to the need to focus on competencies that engender a culture of team-based care. For example, hospitalist leaders will need to understand evidence-based medicine to support appropriate management of a septic patient and simultaneously understand evidence-based management/leadership to affect sepsis care across his or her health care system.
With this change in the health care environment come new and exciting opportunities. Hospital medicine has always elected to assume a leadership role in these times of change, these periods of “punctuation.” Development of effective team-based care is a great place for those of us working in hospital medicine to demonstrate our leadership as we care for our patients.
Dr. Frost is national medical director, hospital-based services, at LifePoint Health, Brentwood, Tenn. He is president-elect of the Society of Hospital Medicine.