User login
Hospitalist Value in an ACO World
The accountable care organization (ACO) concept, elucidated in 2006 as the development of partnerships between hospitals and physicians to coordinate and deliver efficient care,1 seeks to remove existing barriers to improving value.2 Some advocate this concept as a promising payment model that could successfully realign the current payment system to financially reward improvements in quality and efficiency that bend the cost curve.3,4 Hospitalists fit well with this philosophy. As the fastest growing medical specialty in the history of American medicine, from a couple of thousand hospitalists in the mid-1990s to more than 50,000, the remarkable progression of hospitalists has ostensibly been driven partially by hospitals’ efforts to improve the value equation through enhanced efficiency in inpatient care. Importantly, hospitalists probably provide care for more than half of all hospitalized Medicare beneficiaries and increasingly patients in skilled nursing facilities (ie, SNFists).5 Along with primary care physicians, hospitalists thus represent an essential group of physicians needed to transform care delivery.
RAPID GROWTH AND THE FUTURE OF ACOs
When the Affordable Care Act (ACA) established the Medicare Shared Savings Program (MSSP), ACOs leaped from being an intellectual concept1,2 into a pragmatic health system strategy.3,4 Following Medicare, various private health insurance plans and some state Medicaid programs entered into contracts with groups of healthcare providers (hospitals, physicians, or health systems) to serve as ACOs for their insured enrollees.6 Leavitt Partners’ ACO tracking database showed that the number of ACOs increased from 157 in March of 2012 to 782 in December of 2015.7
Until recently, the federal government’s commitment to having 50% of total Medicare spending via value-based payment models by 2018, coupled with endorsement from state Medicaid programs and commercial insurers, demonstrated strong support for continuation of ACOs. Unexpectedly on August 15, 2017, the Centers for Medicare & Medicaid Services (CMS) outlined a plan in its proposed rulemaking to cancel the Episode Payment Models and the Cardiac Rehabilitation incentive payment model, which were scheduled to commence on January 1, 2018. CMS also plans to scale back the mandatory Comprehensive Care for Joint Replacement (CCJR) bundled payment model from 67 selected geographic areas to 34. Although this proposed rulemaking created some equipoise in the healthcare industry regarding the future of value-based reimbursement approaches, cost containment and improved efficiency remain as major focuses of the federal government’s healthcare effort. Notably, CMS offers providers that are newly excluded from the CCJR model the opportunity to voluntarily participate in the program and is expected to increase opportunities for providers to participate in voluntary rather than large-scale mandatory episode payment model initiatives. In 2018, the agency also plans to develop new voluntary bundled payment models that will meet criteria to be considered an advanced alternative payment model for Quality Payment Program purposes.
Importantly, the value-based reimbursement movement was well underway before ACA legislation. Through ACA health reform, value-based reimbursement efforts were expanded through ACOs, bundled payments, value-based purchasing, the CMS Innovation Center and other initiatives. With health systems having an overflowing plate of activities, a wait-and-see attitude might seem reasonable at first. However, being unprepared for the inevitable shift to value-based reimbursement and reduced fee-for-service revenue places an organization at risk. A successful ACO requires system-level transformation, especially cultural and structural changes to achieve clinical integration. Being embedded in health system delivery, hospitalists can help shape a team-oriented culture and foster success in value-based payment models. This requires hospitalists to take a more active role in assessing and striking a balance between high-quality, cost-efficient care and financial risk inherent in ACO models.
WHAT HOSPITALISTS NEED TO KNOW ABOUT ACOs
The key to hospitalists fulfilling their value creation potential and becoming enablers for ACO success lies in developing a thorough understanding of the aspects of an ACO that promote efficient and effective care, while accounting for financial factors. Fundamentally, the ACO concept combines provider payment and delivery system reforms. Specifically, the definition of an ACO contains 3 factors: (1) a local healthcare organization (eg, hospital or multispecialty group of physicians) with a related set of providers that (2) can be held accountable for the cost and quality of care delivered to (3) a defined population. While the notion of accountability is not new, the locus of accountability is changed in the ACO model—emphasizing accountability at the level of actual care delivery with documentation of quality and cost outcomes. The ACO approach aims to address multiple, frequent, and recurring problems, including lack of financial incentives to improve quality and reduce cost, as well as the negative consequences of a pay-for-volume system—uncoordinated and fragmented care, overutilization of unnecessary tests and treatments, and poor patient experience all manifested as unwarranted geographic variation in practice patterns, clinical outcomes, and health spending. Participants in an ACO are rewarded financially if they can slow the growth of their patients’ healthcare costs while maintaining or improving the quality of care delivered. To succeed in this ACO world, hospitalists must assume greater prudence in the use of healthcare services while improving (or at a minimum, maintaining) patient outcomes, thus excising avoidable waste across the continuum of care.
More than half of ACOs include a hospital.8 However, whether hospital-led ACOs possess an advantage remains to be elucidated. Early reports indicated that physician-led ACOs saved more money.9,10 However, others argue that hospitals11 are better capitalized, have greater capacity for data sharing, and possess economies of scale that allow them to invest in more advanced technology, such as predictive modeling and/or simulation software. Such analytics can identify high-cost patients (ie, multiple comorbidities), super utilizers and populations lacking care, allowing ACOs to implement preventive measures to reduce unnecessary utilization. Recently released CMS MSSP 2016 performance data12 showed that nearly half (45%) of physician-only ACOs earned shared savings, whereas 23% of ACOs that include hospitals earned shared savings. However, among all the ACOs that achieved savings, ACO entities that include hospitals generated the highest amount of shared savings (eg, Advocate, Hackensack Alliance, Cleveland Clinic, and AMITA Health). Notably, hospital-led ACOs tend to have much larger beneficiary populations than physician-led ACOs, which may create a scenario of higher risk but higher potential reward.
HOW HOSPITALISTS CONTRIBUTE VALUE TO ACO SUCCESS
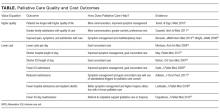
The emphasis on value over volume inherent in the development of ACOs occurs through employing care strategies implemented through changes in policies, and eventual structural and cultural changes. These changes require participating organizations to possess certain key competencies, including the following: 1) leadership that facilitates change; 2) organizational culture of teamwork; 3) collaborative relationships among providers; 4) information technology infrastructure for population management and care coordination; 5) infrastructure for monitoring, managing, and reporting quality; 6) ability to manage financial risk; 7) ability to receive and distribute payments or savings; and 8) resources for patient education and support.2,3,13-16 Table 1 summarizes the broad range of roles that hospitalists can serve in delivering care to ACO populations.17-19
Hospitalists’ active pursuit of nonclinical training and selection for administrative positions demonstrate their proclivity to provide these competencies. In addition to full-time clinician hospitalists, who can directly influence the delivery of high-value care to patients, hospitalists serve many other roles in hospitals and each can contribute differently based on their specialized expertise. Examples include the success of the Society of Hospital Medicine’s Leadership Academy; the acknowledged expertise of hospitalists in quality improvement (QI), informatics, teamwork, patient experience, care coordination and utilization; and advancement of hospitalists to senior leadership positions (eg, CQO, CMO, CEO). Given that nearly a third of healthcare expenditures are for hospital care,20 hospitalists are in a unique position to foster ACO competencies while impacting the quality of care episodes associated with an index hospital stay.
Importantly, hospitalists cannot act as gatekeepers to restrict care. Managed care organizations and health maintenance organizations use of this approach in the 1990s to limit access to services in order to reduce costs led to unacceptable outcomes and numerous malpractice lawsuits. ACOs should aspire to deliver the most cost-effective high-quality care, and their performance should be monitored to ensure that they provide recommended services and timely access. The Medicare ACO contract holds the provider accountable for meeting 34 different quality measures (Supplemental Table 1), and hospitalists can influence outcomes for the majority. Especially through hospital and health system QI initiatives, hospitalists can directly impact and share accountability for measures ranging from care coordination to implementation of evidence-based care (eg, ACE inhibitors and beta blockers for heart failure) to patient and family caregiver experience.
Aligned with Medicare ACO quality measures, 5 high-impact target areas were identified for ACOs21: (1) Prevention and wellness; (2) Chronic conditions/care management; (3) Reduced hospitalizations; (4) Care transitions across the fragmented system; and (5) Multispecialty care coordination of complex patients. One essential element of a successful ACO is the ability to implement evidence-based medical guidelines and/or practices across the continuum of care for selected targeted initiatives. Optimizing care coordination/continuum requires team-based care, and hospitalists already routinely collaborate with nurses, social workers, case managers, pharmacists, and other stakeholders such as dieticians and physical therapists on inpatient care. Hospitalists are also experienced in facilitating communication and improving integration and coordination efficiencies among primary care providers and specialists, and between hospital care and post-acute care, as they coordinate post-hospital care and follow-up. This provides an opportunity to lead health system care coordination efforts, especially for complex and/or high-risk patients.22,23 CMS MSSP 2016 performance data12 showed that ACOs achieving shared savings had a decline in inpatient expenditures and utilization across several facility types (hospital, SNF, rehabilitation, long term). Postacute care management is critical to earning shared savings; SNF and Home Health expenditures fell by 18.3% and 9.7%, respectively, on average. We believe that hospitalists can have more influence over these cost areas by influencing treatment of hospitalized patients in a timely manner, discharge coordination, and selection of appropriate disposition locations. Hospitalists also play an integral role in ensuring the hospital performs well on quality metrics, including 30-day readmissions, hospital acquired conditions, and patient satisfaction. Examples below document the effectiveness of hospitalists in this new ACO era.
Care Transitions/Coordination
Before the Hospital Readmission Reduction Program (HRRP) delineated in the ACA, hospitalists developed Project BOOST (Better Outcomes by Optimizing Care Transitions) to improve hospital discharge care transition. The evidence-based foundation of this project led CMS to list Project BOOST as an example program that can reduce readmissions.24 Through the dissemination and mentored implementation of Project BOOST to over 200 hospitals across the United States,25 hospitalists contributed to the marked reduction in hospital readmission occurring since 2010.26 Although hospital medicine began as a practice specific to the hospital setting, hospitalists’ skills generated growing demand for them in postacute facilities. SNF residents commonly come from hospitals postdischarge and suffer from multiple comorbidities and limitations in activities of daily living. Not surprisingly, SNF residents experience high rates of rehospitalizations.27 Hospitalists can serve as a bridge between hospitals and SNFs and optimize this transition process to yield improved outcomes. Industry experts endorse this approach.28 A recent study demonstrated a significant reduction in readmissions in 1 SNF (32.3% to 16.1%, odds ratio = 0.403, P < .001), by having a hospitalist-led team follow patients discharged from the hospital.29
Chronic Conditions Management/High-Risk Patients
Interest in patients with multiple chronic comorbidities and social issues intensifies as healthcare systems focus limited resources on these high-risk patients to prevent the unnecessary use of costly services.30,31 As health systems assume financial risk for health outcomes and costs of designated patient groups, they undertake efforts to understand the population they serve. Such efforts aim to identify patients with established high utilization patterns (or those at risk for high utilization). This knowledge enables targeted actions to provide access, treatment, and preventive interventions to avoid unneeded emergency and hospital services. Hospitalists commonly care for these patients and are positioned to lead the implementation of patient risk assessment and stratification, develop patient-centered care models across care settings, and act as a liaison with primary care. For frail elderly and seriously ill patients, the integration of hospitalists into palliative care provides several opportunities for improving the quality of care at the end of life.32 As patients and their family caregivers commonly do not address goals of care until faced with a life-threatening condition in the hospital, hospitalists represent ideal primary palliative care physicians to initiate these conversations.33 A hospitalist communicating with a patient and/or their family caregiver about alleviating symptoms and clarifying patients’ preferences for care often yields decreases in ineffective healthcare utilization and better patient outcomes. The hospitalists’ ability to communicate with other providers within the hospital setting also allows them to better coordinate interdisciplinary care and prevent unnecessary and ineffective treatments and procedures.
De-Implementation/Waste Reduction
The largest inefficiencies in healthcare noted in the National Academy of Medicine report, Demanding Value from Our Health Care (2012), are failure to deliver known beneficial therapies or providing unnecessary or nonevidenced based services that do not improve outcomes, but come with associated risk and cost.34 “De-implementation” of unnecessary diagnostic tests or ineffective or even harmful treatments by hospitalists represents a significant opportunity to reduce costs while maintaining or even improving the quality of care. The Society of Hospital Medicine joined the Choosing Wisely® campaign and made 5 recommendations in adult care as an explicit starting point for eliminating waste in the hospital in 2013.35 Since then, hospitalists have participated in multiple successful efforts to address overutilization of care; some published results include the following:
- decreased frequency of unnecessary common labs through a multifaceted hospitalist QI intervention;36
- reduced length of stay and cost by appropriate use of telemetry;37 and
- reduced unnecessary radiology testing by providing physicians with individualized audit and feedback reports.38
CONCLUSION
Hundreds of ACOs now exist across the US, formed by a variety of providers including hospitals, physician groups, and integrated delivery systems. Provider groups range in size from primary care-focused physician groups with a handful of offices to large, multistate integrated delivery systems with dozens of hospitals and hundreds of office locations. Evaluations of ACO outcomes reveal mixed results.9,39-53 Admittedly, assessments attempting to compare the magnitude of savings across ACO models are difficult given the variation in size, variability in specific efforts to influence utilization, and substantial turnover among participating beneficiaries.54 Nonetheless, a newly published Office of Inspector General report55 showed that most Medicare ACOs reduced spending and improved care quality (82% of the individual quality measures) over the first 3 years of the program, and savings increased with duration of an ACO program. The report also noted that considerable time and managerial resources are required to implement changes to improve quality and lower costs. While the political terrain ostensibly supports value-based care and the need to diminish the proportion of our nation’s gross domestic product dedicated to healthcare, health systems are navigating an environment that still largely rewards volume. Hospitalists may be ideal facilitators for this transitional period as they possess the clinical experience caring for complex patients with multiple comorbidities and quality improvement skills to lead efforts in this new ACO era.
Disclosures
The authors have nothing to disclose.
1. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff(Project Hope). 2007;26(1):w44-w57. PubMed
2. Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in medicare. Health Aff(Project Hope). 2009;28(2):w219-w231. PubMed
3. McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Aff(Project Hope). 2010;29(5):982-990. PubMed
4. Berwick DM. Making good on ACOs’ promise--the final rule for the Medicare shared savings program. N Engl J Med. 2011;365(19):1753-1756. PubMed
5. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
6. Kennedy K. Health Care Providers Embracing Cost-saving Groups. USA Today, July 24, 2011.
7. Leavitt Partners. Available at http://leavittpartners.com, April 2016.
8. Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals Participating In ACOs Tend To Be Large And Urban, Allowing Access To Capital And Data. Health Aff(Millwood). 2016;35(3):431-439. PubMed
9. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early Performance of Accountable Care Organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. PubMed
10. Muhlestein D, Saunders R, McClellan M. Medicare Accountable Care Organization Results For 2015: The Journey To Better Quality And Lower Costs Continues. In. Health Affairs Blog. Bethesda, MD 2016.
11. Chernew ME. New Health Care Symposium: Building An ACO---What Services Do You Need And How Are Physicians Impacted? In Health Affairs Blog. Bethesda, MD 2016.
12. Centers for Medicare & Medicaid Services. Performance Year 2016 Quality Performance and Financial Reconciliation Results for ACOs with 2012-2016 Start Dates. Available at https://strategichealthcare.net/wp-content/uploads/2017/10/CMS-Slides-on-ACOs.pdf. 2017.
13. Shortell SM, Casalino LP. Implementing qualifications criteria and technical assistance for accountable care organizations. JAMA. 2010;303(17):1747-1748. PubMed
14. Shortell SM, Casalino LP, Fisher ES. How the center for Medicare and Medicaid innovation should test accountable care organizations. Health Aff (Project Hope). 2010;29(7):1293-1298. PubMed
15. Medicare Payment Advisory Commission. Accountable Care Organizations Payment Systems October 2015. Available at http://www.medpac.gov/documents/payment-basics/accountable-care-organization-payment-systems-15.pdf?sfvrsn=0.
16. American Hospital Association. 2010 Committee on Research. AHA Research Synthesis Report: Accountable Care Organization.
17. D’Aunno T, Broffman L, Sparer M, Kumar SR. Factors That Distinguish High-Performing Accountable Care Organizations in the Medicare Shared Savings Program. Health Serv. Res. 2016. PubMed
18. Peiris D, Phipps-Taylor MC, Stachowski CA, et al. ACOs Holding Commercial Contracts Are Larger And More Efficient Than Noncommercial ACOs. Health Aff (Project Hope). 2016;35(10):1849-1856. PubMed
19. Ouayogode MH, Colla CH, Lewis VA. Determinants of success in Shared Savings Programs: An analysis of ACO and market characteristics. Healthcare (Amsterdam, Netherlands). 2017;5(1-2):53-61. PubMed
20. National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. In: Hyattsville, MD.2017. PubMed
21. Gbemudu JN. Larson BK, Van Citters AD, Kreindler SA, Nelson EC, Shortell SM, Fisher ES. Norton Healthcare: A Strong Payer–Provider Partnership for the Journey to Accountable Care. January 2012. Available at http://www.commonwealthfund.org/~/media/files/publications/case-study/2012/jan/1574_gbemudu_norton_case-study_01_12_2012.pdf.
22. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. PubMed
23. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J. Hosp. Med.. 2013;8(8):421-427. PubMed
24. Centers for Medicare and Medicaid Services. Solicitation for Applications: Community-based Care Transitions Program. Available at https://innovation.cms.gov/Files/Migrated-Medicare-Demonstration-x/CCTP-Solicitation.pdf. September 7, 2017.
25. Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90(3):303-310. PubMed
26. Williams MV, Li J, Hansen LO, et al. Project BOOST implementation: lessons learned. South Med J. 2014;107(7):455-465. PubMed
27. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761]. J Am Geriatr Soc. 2010;58(4):627-635. PubMed
28. Pittman D. SNFs: New Turf for Hospitalists? 2013. Available at https://www.medpagetoday.com/hospitalbasedmedicine/hospitalists/39401.
29. Petigara S, Krishnamurthy M, Livert D. Necessity is the mother of invention: an innovative hospitalist-resident initiative for improving quality and reducing readmissions from skilled nursing facilities. J Community Hosp Intern Med Perspect. 2017;7(2):66-69. PubMed
30. Silow-Carroll S, Edwards J. Early Adopters of the Accountable Care Model: A Field Report on Improvements in Health Care Delivery. New York, NY: The Commonwealth Fund;March 2013.
31. Hasselman D. Super-Utilizer Summit: Common Themes from Innovative Complex Care Management Programs. Hamilton, NJ: Center for Health Care Strategies;October 2013.
32. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
33. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
34. O’Kane M, Buto K, Alteras T, et. al. Demanding Value from Our Health Care: Motivating Patient Action to Reduce Waste in Health Care. Institute of Medicine of the National Academies. July 2012. https://nam.edu/wp-content/uploads/2015/06/VSRT-DemandingValue.pdf. Accessed Accessed June 18, 2017.
35. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
36. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. PubMed
37. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
38. Neeman N, Quinn K, Soni K, Mourad M, Sehgal NL. Reducing radiology use on an inpatient medical service: choosing wisely. JAMA Intern Med. 2012;172(20):1606-1608. PubMed
39. Abrams M, Nuzum R, Zezza M, Ryan J, Kiszla J, Guterman S. The Affordable Care Act’s Payment and Delivery System Reforms: A Progress Report at Five Years. Bipartisan Policy Center, May 2015. Available at http://www.commonwealthfund.org/publications/issue-briefs/2015/may/aca-payment-and-delivery-system-reforms-at-5-years.
40. Kocot SL, White R, Katikaneni P, McClellan MB. A More Complete Picture of Pioneer ACO Results. The Brookings Institution, October 13, 2014. Available at http://www.brookings.edu/blogs/up-front/posts/2014/10/09-pioneer-aco-results-mcclellan/#recent_rr/
41. Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 Years. N Engl J Med. 2015;372(25):2451-2458. PubMed
42. Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association Between Medicare Accountable Care Organization Implementation and Spending Among Clinically Vulnerable Beneficiaries. JAMA Intern Med. 2016;176(8):1167-1175. PubMed
43. Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Project Hope). 2014;33(1):95-102. PubMed
44. Fullerton CA, Henke RM, Crable E, Hohlbauch A, Cummings N. The Impact Of Medicare ACOs On Improving Integration And Coordination Of Physical And Behavioral Health Care. Health Aff (Project Hope). 2016;35(7):1257-1265. PubMed
45. Herrel LA, Norton EC, Hawken SR, Ye Z, Hollenbeck BK, Miller DC. Early impact of Medicare accountable care organizations on cancer surgery outcomes. Cancer. 2016;122(17):2739-2746. PubMed
46. McConnell KJ, Renfro S, Chan BK, et al. Early Performance in Medicaid Accountable Care Organizations: A Comparison of Oregon and Colorado. JAMA Intern Med. 2017;177(4):538-545. PubMed
47. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer Accountable Care Organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
48. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center--a five-year self-assessment. N Engl J Med. 2015;372(21):1981-1983. PubMed
49. Rose S, Zaslavsky AM, McWilliams JM. Variation In Accountable Care Organization Spending And Sensitivity To Risk Adjustment: Implications For Benchmarking. Health affairs (Project Hope). 2016;35(3):440-448. PubMed
50. Shortell SM, Poon BY, Ramsay PP, et al. A Multilevel Analysis of Patient Engagement and Patient-Reported Outcomes in Primary Care Practices of Accountable Care Organizations. J Gen Intern Med. 2017;32(6):640-647. PubMed
51. Winblad U, Mor V, McHugh JP, Rahman M. ACO-Affiliated Hospitals Reduced Rehospitalizations From Skilled Nursing Facilities Faster Than Other Hospitals. Health Aff (Project Hope). 2017;36(1):67-73. PubMed
52. Zhang Y, Caines KJ, Powers CA. Evaluating the Effects of Pioneer Accountable Care Organizations on Medicare Part D Drug Spending and Utilization. Med Care. 2017;55(5):470-475. PubMed
53. Muhlestein D. Medicare ACOs: Mixed Initial Results and Cautious Optimism. Health Affairs Blog, February 4, 2014. Available at http://healthaffairs.org/blog/2014/02/04/medicare-acos-mixed-initial-results-and-cautious-optimism/.
54. Hsu J, Price M, Vogeli C, et al. Bending The Spending Curve By Altering Care Delivery Patterns: The Role Of Care Management Within A Pioneer ACO. Health Aff (Project Hope). 2017;36(5):876-884. PubMed
55. Medicare Shared Savings Program Accountable Care Organizations Have Shown Potential For Reducing Spending And Improving Quality. Office of Inspector General;August 2017.
The accountable care organization (ACO) concept, elucidated in 2006 as the development of partnerships between hospitals and physicians to coordinate and deliver efficient care,1 seeks to remove existing barriers to improving value.2 Some advocate this concept as a promising payment model that could successfully realign the current payment system to financially reward improvements in quality and efficiency that bend the cost curve.3,4 Hospitalists fit well with this philosophy. As the fastest growing medical specialty in the history of American medicine, from a couple of thousand hospitalists in the mid-1990s to more than 50,000, the remarkable progression of hospitalists has ostensibly been driven partially by hospitals’ efforts to improve the value equation through enhanced efficiency in inpatient care. Importantly, hospitalists probably provide care for more than half of all hospitalized Medicare beneficiaries and increasingly patients in skilled nursing facilities (ie, SNFists).5 Along with primary care physicians, hospitalists thus represent an essential group of physicians needed to transform care delivery.
RAPID GROWTH AND THE FUTURE OF ACOs
When the Affordable Care Act (ACA) established the Medicare Shared Savings Program (MSSP), ACOs leaped from being an intellectual concept1,2 into a pragmatic health system strategy.3,4 Following Medicare, various private health insurance plans and some state Medicaid programs entered into contracts with groups of healthcare providers (hospitals, physicians, or health systems) to serve as ACOs for their insured enrollees.6 Leavitt Partners’ ACO tracking database showed that the number of ACOs increased from 157 in March of 2012 to 782 in December of 2015.7
Until recently, the federal government’s commitment to having 50% of total Medicare spending via value-based payment models by 2018, coupled with endorsement from state Medicaid programs and commercial insurers, demonstrated strong support for continuation of ACOs. Unexpectedly on August 15, 2017, the Centers for Medicare & Medicaid Services (CMS) outlined a plan in its proposed rulemaking to cancel the Episode Payment Models and the Cardiac Rehabilitation incentive payment model, which were scheduled to commence on January 1, 2018. CMS also plans to scale back the mandatory Comprehensive Care for Joint Replacement (CCJR) bundled payment model from 67 selected geographic areas to 34. Although this proposed rulemaking created some equipoise in the healthcare industry regarding the future of value-based reimbursement approaches, cost containment and improved efficiency remain as major focuses of the federal government’s healthcare effort. Notably, CMS offers providers that are newly excluded from the CCJR model the opportunity to voluntarily participate in the program and is expected to increase opportunities for providers to participate in voluntary rather than large-scale mandatory episode payment model initiatives. In 2018, the agency also plans to develop new voluntary bundled payment models that will meet criteria to be considered an advanced alternative payment model for Quality Payment Program purposes.
Importantly, the value-based reimbursement movement was well underway before ACA legislation. Through ACA health reform, value-based reimbursement efforts were expanded through ACOs, bundled payments, value-based purchasing, the CMS Innovation Center and other initiatives. With health systems having an overflowing plate of activities, a wait-and-see attitude might seem reasonable at first. However, being unprepared for the inevitable shift to value-based reimbursement and reduced fee-for-service revenue places an organization at risk. A successful ACO requires system-level transformation, especially cultural and structural changes to achieve clinical integration. Being embedded in health system delivery, hospitalists can help shape a team-oriented culture and foster success in value-based payment models. This requires hospitalists to take a more active role in assessing and striking a balance between high-quality, cost-efficient care and financial risk inherent in ACO models.
WHAT HOSPITALISTS NEED TO KNOW ABOUT ACOs
The key to hospitalists fulfilling their value creation potential and becoming enablers for ACO success lies in developing a thorough understanding of the aspects of an ACO that promote efficient and effective care, while accounting for financial factors. Fundamentally, the ACO concept combines provider payment and delivery system reforms. Specifically, the definition of an ACO contains 3 factors: (1) a local healthcare organization (eg, hospital or multispecialty group of physicians) with a related set of providers that (2) can be held accountable for the cost and quality of care delivered to (3) a defined population. While the notion of accountability is not new, the locus of accountability is changed in the ACO model—emphasizing accountability at the level of actual care delivery with documentation of quality and cost outcomes. The ACO approach aims to address multiple, frequent, and recurring problems, including lack of financial incentives to improve quality and reduce cost, as well as the negative consequences of a pay-for-volume system—uncoordinated and fragmented care, overutilization of unnecessary tests and treatments, and poor patient experience all manifested as unwarranted geographic variation in practice patterns, clinical outcomes, and health spending. Participants in an ACO are rewarded financially if they can slow the growth of their patients’ healthcare costs while maintaining or improving the quality of care delivered. To succeed in this ACO world, hospitalists must assume greater prudence in the use of healthcare services while improving (or at a minimum, maintaining) patient outcomes, thus excising avoidable waste across the continuum of care.
More than half of ACOs include a hospital.8 However, whether hospital-led ACOs possess an advantage remains to be elucidated. Early reports indicated that physician-led ACOs saved more money.9,10 However, others argue that hospitals11 are better capitalized, have greater capacity for data sharing, and possess economies of scale that allow them to invest in more advanced technology, such as predictive modeling and/or simulation software. Such analytics can identify high-cost patients (ie, multiple comorbidities), super utilizers and populations lacking care, allowing ACOs to implement preventive measures to reduce unnecessary utilization. Recently released CMS MSSP 2016 performance data12 showed that nearly half (45%) of physician-only ACOs earned shared savings, whereas 23% of ACOs that include hospitals earned shared savings. However, among all the ACOs that achieved savings, ACO entities that include hospitals generated the highest amount of shared savings (eg, Advocate, Hackensack Alliance, Cleveland Clinic, and AMITA Health). Notably, hospital-led ACOs tend to have much larger beneficiary populations than physician-led ACOs, which may create a scenario of higher risk but higher potential reward.
HOW HOSPITALISTS CONTRIBUTE VALUE TO ACO SUCCESS
The emphasis on value over volume inherent in the development of ACOs occurs through employing care strategies implemented through changes in policies, and eventual structural and cultural changes. These changes require participating organizations to possess certain key competencies, including the following: 1) leadership that facilitates change; 2) organizational culture of teamwork; 3) collaborative relationships among providers; 4) information technology infrastructure for population management and care coordination; 5) infrastructure for monitoring, managing, and reporting quality; 6) ability to manage financial risk; 7) ability to receive and distribute payments or savings; and 8) resources for patient education and support.2,3,13-16 Table 1 summarizes the broad range of roles that hospitalists can serve in delivering care to ACO populations.17-19
Hospitalists’ active pursuit of nonclinical training and selection for administrative positions demonstrate their proclivity to provide these competencies. In addition to full-time clinician hospitalists, who can directly influence the delivery of high-value care to patients, hospitalists serve many other roles in hospitals and each can contribute differently based on their specialized expertise. Examples include the success of the Society of Hospital Medicine’s Leadership Academy; the acknowledged expertise of hospitalists in quality improvement (QI), informatics, teamwork, patient experience, care coordination and utilization; and advancement of hospitalists to senior leadership positions (eg, CQO, CMO, CEO). Given that nearly a third of healthcare expenditures are for hospital care,20 hospitalists are in a unique position to foster ACO competencies while impacting the quality of care episodes associated with an index hospital stay.
Importantly, hospitalists cannot act as gatekeepers to restrict care. Managed care organizations and health maintenance organizations use of this approach in the 1990s to limit access to services in order to reduce costs led to unacceptable outcomes and numerous malpractice lawsuits. ACOs should aspire to deliver the most cost-effective high-quality care, and their performance should be monitored to ensure that they provide recommended services and timely access. The Medicare ACO contract holds the provider accountable for meeting 34 different quality measures (Supplemental Table 1), and hospitalists can influence outcomes for the majority. Especially through hospital and health system QI initiatives, hospitalists can directly impact and share accountability for measures ranging from care coordination to implementation of evidence-based care (eg, ACE inhibitors and beta blockers for heart failure) to patient and family caregiver experience.
Aligned with Medicare ACO quality measures, 5 high-impact target areas were identified for ACOs21: (1) Prevention and wellness; (2) Chronic conditions/care management; (3) Reduced hospitalizations; (4) Care transitions across the fragmented system; and (5) Multispecialty care coordination of complex patients. One essential element of a successful ACO is the ability to implement evidence-based medical guidelines and/or practices across the continuum of care for selected targeted initiatives. Optimizing care coordination/continuum requires team-based care, and hospitalists already routinely collaborate with nurses, social workers, case managers, pharmacists, and other stakeholders such as dieticians and physical therapists on inpatient care. Hospitalists are also experienced in facilitating communication and improving integration and coordination efficiencies among primary care providers and specialists, and between hospital care and post-acute care, as they coordinate post-hospital care and follow-up. This provides an opportunity to lead health system care coordination efforts, especially for complex and/or high-risk patients.22,23 CMS MSSP 2016 performance data12 showed that ACOs achieving shared savings had a decline in inpatient expenditures and utilization across several facility types (hospital, SNF, rehabilitation, long term). Postacute care management is critical to earning shared savings; SNF and Home Health expenditures fell by 18.3% and 9.7%, respectively, on average. We believe that hospitalists can have more influence over these cost areas by influencing treatment of hospitalized patients in a timely manner, discharge coordination, and selection of appropriate disposition locations. Hospitalists also play an integral role in ensuring the hospital performs well on quality metrics, including 30-day readmissions, hospital acquired conditions, and patient satisfaction. Examples below document the effectiveness of hospitalists in this new ACO era.
Care Transitions/Coordination
Before the Hospital Readmission Reduction Program (HRRP) delineated in the ACA, hospitalists developed Project BOOST (Better Outcomes by Optimizing Care Transitions) to improve hospital discharge care transition. The evidence-based foundation of this project led CMS to list Project BOOST as an example program that can reduce readmissions.24 Through the dissemination and mentored implementation of Project BOOST to over 200 hospitals across the United States,25 hospitalists contributed to the marked reduction in hospital readmission occurring since 2010.26 Although hospital medicine began as a practice specific to the hospital setting, hospitalists’ skills generated growing demand for them in postacute facilities. SNF residents commonly come from hospitals postdischarge and suffer from multiple comorbidities and limitations in activities of daily living. Not surprisingly, SNF residents experience high rates of rehospitalizations.27 Hospitalists can serve as a bridge between hospitals and SNFs and optimize this transition process to yield improved outcomes. Industry experts endorse this approach.28 A recent study demonstrated a significant reduction in readmissions in 1 SNF (32.3% to 16.1%, odds ratio = 0.403, P < .001), by having a hospitalist-led team follow patients discharged from the hospital.29
Chronic Conditions Management/High-Risk Patients
Interest in patients with multiple chronic comorbidities and social issues intensifies as healthcare systems focus limited resources on these high-risk patients to prevent the unnecessary use of costly services.30,31 As health systems assume financial risk for health outcomes and costs of designated patient groups, they undertake efforts to understand the population they serve. Such efforts aim to identify patients with established high utilization patterns (or those at risk for high utilization). This knowledge enables targeted actions to provide access, treatment, and preventive interventions to avoid unneeded emergency and hospital services. Hospitalists commonly care for these patients and are positioned to lead the implementation of patient risk assessment and stratification, develop patient-centered care models across care settings, and act as a liaison with primary care. For frail elderly and seriously ill patients, the integration of hospitalists into palliative care provides several opportunities for improving the quality of care at the end of life.32 As patients and their family caregivers commonly do not address goals of care until faced with a life-threatening condition in the hospital, hospitalists represent ideal primary palliative care physicians to initiate these conversations.33 A hospitalist communicating with a patient and/or their family caregiver about alleviating symptoms and clarifying patients’ preferences for care often yields decreases in ineffective healthcare utilization and better patient outcomes. The hospitalists’ ability to communicate with other providers within the hospital setting also allows them to better coordinate interdisciplinary care and prevent unnecessary and ineffective treatments and procedures.
De-Implementation/Waste Reduction
The largest inefficiencies in healthcare noted in the National Academy of Medicine report, Demanding Value from Our Health Care (2012), are failure to deliver known beneficial therapies or providing unnecessary or nonevidenced based services that do not improve outcomes, but come with associated risk and cost.34 “De-implementation” of unnecessary diagnostic tests or ineffective or even harmful treatments by hospitalists represents a significant opportunity to reduce costs while maintaining or even improving the quality of care. The Society of Hospital Medicine joined the Choosing Wisely® campaign and made 5 recommendations in adult care as an explicit starting point for eliminating waste in the hospital in 2013.35 Since then, hospitalists have participated in multiple successful efforts to address overutilization of care; some published results include the following:
- decreased frequency of unnecessary common labs through a multifaceted hospitalist QI intervention;36
- reduced length of stay and cost by appropriate use of telemetry;37 and
- reduced unnecessary radiology testing by providing physicians with individualized audit and feedback reports.38
CONCLUSION
Hundreds of ACOs now exist across the US, formed by a variety of providers including hospitals, physician groups, and integrated delivery systems. Provider groups range in size from primary care-focused physician groups with a handful of offices to large, multistate integrated delivery systems with dozens of hospitals and hundreds of office locations. Evaluations of ACO outcomes reveal mixed results.9,39-53 Admittedly, assessments attempting to compare the magnitude of savings across ACO models are difficult given the variation in size, variability in specific efforts to influence utilization, and substantial turnover among participating beneficiaries.54 Nonetheless, a newly published Office of Inspector General report55 showed that most Medicare ACOs reduced spending and improved care quality (82% of the individual quality measures) over the first 3 years of the program, and savings increased with duration of an ACO program. The report also noted that considerable time and managerial resources are required to implement changes to improve quality and lower costs. While the political terrain ostensibly supports value-based care and the need to diminish the proportion of our nation’s gross domestic product dedicated to healthcare, health systems are navigating an environment that still largely rewards volume. Hospitalists may be ideal facilitators for this transitional period as they possess the clinical experience caring for complex patients with multiple comorbidities and quality improvement skills to lead efforts in this new ACO era.
Disclosures
The authors have nothing to disclose.
The accountable care organization (ACO) concept, elucidated in 2006 as the development of partnerships between hospitals and physicians to coordinate and deliver efficient care,1 seeks to remove existing barriers to improving value.2 Some advocate this concept as a promising payment model that could successfully realign the current payment system to financially reward improvements in quality and efficiency that bend the cost curve.3,4 Hospitalists fit well with this philosophy. As the fastest growing medical specialty in the history of American medicine, from a couple of thousand hospitalists in the mid-1990s to more than 50,000, the remarkable progression of hospitalists has ostensibly been driven partially by hospitals’ efforts to improve the value equation through enhanced efficiency in inpatient care. Importantly, hospitalists probably provide care for more than half of all hospitalized Medicare beneficiaries and increasingly patients in skilled nursing facilities (ie, SNFists).5 Along with primary care physicians, hospitalists thus represent an essential group of physicians needed to transform care delivery.
RAPID GROWTH AND THE FUTURE OF ACOs
When the Affordable Care Act (ACA) established the Medicare Shared Savings Program (MSSP), ACOs leaped from being an intellectual concept1,2 into a pragmatic health system strategy.3,4 Following Medicare, various private health insurance plans and some state Medicaid programs entered into contracts with groups of healthcare providers (hospitals, physicians, or health systems) to serve as ACOs for their insured enrollees.6 Leavitt Partners’ ACO tracking database showed that the number of ACOs increased from 157 in March of 2012 to 782 in December of 2015.7
Until recently, the federal government’s commitment to having 50% of total Medicare spending via value-based payment models by 2018, coupled with endorsement from state Medicaid programs and commercial insurers, demonstrated strong support for continuation of ACOs. Unexpectedly on August 15, 2017, the Centers for Medicare & Medicaid Services (CMS) outlined a plan in its proposed rulemaking to cancel the Episode Payment Models and the Cardiac Rehabilitation incentive payment model, which were scheduled to commence on January 1, 2018. CMS also plans to scale back the mandatory Comprehensive Care for Joint Replacement (CCJR) bundled payment model from 67 selected geographic areas to 34. Although this proposed rulemaking created some equipoise in the healthcare industry regarding the future of value-based reimbursement approaches, cost containment and improved efficiency remain as major focuses of the federal government’s healthcare effort. Notably, CMS offers providers that are newly excluded from the CCJR model the opportunity to voluntarily participate in the program and is expected to increase opportunities for providers to participate in voluntary rather than large-scale mandatory episode payment model initiatives. In 2018, the agency also plans to develop new voluntary bundled payment models that will meet criteria to be considered an advanced alternative payment model for Quality Payment Program purposes.
Importantly, the value-based reimbursement movement was well underway before ACA legislation. Through ACA health reform, value-based reimbursement efforts were expanded through ACOs, bundled payments, value-based purchasing, the CMS Innovation Center and other initiatives. With health systems having an overflowing plate of activities, a wait-and-see attitude might seem reasonable at first. However, being unprepared for the inevitable shift to value-based reimbursement and reduced fee-for-service revenue places an organization at risk. A successful ACO requires system-level transformation, especially cultural and structural changes to achieve clinical integration. Being embedded in health system delivery, hospitalists can help shape a team-oriented culture and foster success in value-based payment models. This requires hospitalists to take a more active role in assessing and striking a balance between high-quality, cost-efficient care and financial risk inherent in ACO models.
WHAT HOSPITALISTS NEED TO KNOW ABOUT ACOs
The key to hospitalists fulfilling their value creation potential and becoming enablers for ACO success lies in developing a thorough understanding of the aspects of an ACO that promote efficient and effective care, while accounting for financial factors. Fundamentally, the ACO concept combines provider payment and delivery system reforms. Specifically, the definition of an ACO contains 3 factors: (1) a local healthcare organization (eg, hospital or multispecialty group of physicians) with a related set of providers that (2) can be held accountable for the cost and quality of care delivered to (3) a defined population. While the notion of accountability is not new, the locus of accountability is changed in the ACO model—emphasizing accountability at the level of actual care delivery with documentation of quality and cost outcomes. The ACO approach aims to address multiple, frequent, and recurring problems, including lack of financial incentives to improve quality and reduce cost, as well as the negative consequences of a pay-for-volume system—uncoordinated and fragmented care, overutilization of unnecessary tests and treatments, and poor patient experience all manifested as unwarranted geographic variation in practice patterns, clinical outcomes, and health spending. Participants in an ACO are rewarded financially if they can slow the growth of their patients’ healthcare costs while maintaining or improving the quality of care delivered. To succeed in this ACO world, hospitalists must assume greater prudence in the use of healthcare services while improving (or at a minimum, maintaining) patient outcomes, thus excising avoidable waste across the continuum of care.
More than half of ACOs include a hospital.8 However, whether hospital-led ACOs possess an advantage remains to be elucidated. Early reports indicated that physician-led ACOs saved more money.9,10 However, others argue that hospitals11 are better capitalized, have greater capacity for data sharing, and possess economies of scale that allow them to invest in more advanced technology, such as predictive modeling and/or simulation software. Such analytics can identify high-cost patients (ie, multiple comorbidities), super utilizers and populations lacking care, allowing ACOs to implement preventive measures to reduce unnecessary utilization. Recently released CMS MSSP 2016 performance data12 showed that nearly half (45%) of physician-only ACOs earned shared savings, whereas 23% of ACOs that include hospitals earned shared savings. However, among all the ACOs that achieved savings, ACO entities that include hospitals generated the highest amount of shared savings (eg, Advocate, Hackensack Alliance, Cleveland Clinic, and AMITA Health). Notably, hospital-led ACOs tend to have much larger beneficiary populations than physician-led ACOs, which may create a scenario of higher risk but higher potential reward.
HOW HOSPITALISTS CONTRIBUTE VALUE TO ACO SUCCESS
The emphasis on value over volume inherent in the development of ACOs occurs through employing care strategies implemented through changes in policies, and eventual structural and cultural changes. These changes require participating organizations to possess certain key competencies, including the following: 1) leadership that facilitates change; 2) organizational culture of teamwork; 3) collaborative relationships among providers; 4) information technology infrastructure for population management and care coordination; 5) infrastructure for monitoring, managing, and reporting quality; 6) ability to manage financial risk; 7) ability to receive and distribute payments or savings; and 8) resources for patient education and support.2,3,13-16 Table 1 summarizes the broad range of roles that hospitalists can serve in delivering care to ACO populations.17-19
Hospitalists’ active pursuit of nonclinical training and selection for administrative positions demonstrate their proclivity to provide these competencies. In addition to full-time clinician hospitalists, who can directly influence the delivery of high-value care to patients, hospitalists serve many other roles in hospitals and each can contribute differently based on their specialized expertise. Examples include the success of the Society of Hospital Medicine’s Leadership Academy; the acknowledged expertise of hospitalists in quality improvement (QI), informatics, teamwork, patient experience, care coordination and utilization; and advancement of hospitalists to senior leadership positions (eg, CQO, CMO, CEO). Given that nearly a third of healthcare expenditures are for hospital care,20 hospitalists are in a unique position to foster ACO competencies while impacting the quality of care episodes associated with an index hospital stay.
Importantly, hospitalists cannot act as gatekeepers to restrict care. Managed care organizations and health maintenance organizations use of this approach in the 1990s to limit access to services in order to reduce costs led to unacceptable outcomes and numerous malpractice lawsuits. ACOs should aspire to deliver the most cost-effective high-quality care, and their performance should be monitored to ensure that they provide recommended services and timely access. The Medicare ACO contract holds the provider accountable for meeting 34 different quality measures (Supplemental Table 1), and hospitalists can influence outcomes for the majority. Especially through hospital and health system QI initiatives, hospitalists can directly impact and share accountability for measures ranging from care coordination to implementation of evidence-based care (eg, ACE inhibitors and beta blockers for heart failure) to patient and family caregiver experience.
Aligned with Medicare ACO quality measures, 5 high-impact target areas were identified for ACOs21: (1) Prevention and wellness; (2) Chronic conditions/care management; (3) Reduced hospitalizations; (4) Care transitions across the fragmented system; and (5) Multispecialty care coordination of complex patients. One essential element of a successful ACO is the ability to implement evidence-based medical guidelines and/or practices across the continuum of care for selected targeted initiatives. Optimizing care coordination/continuum requires team-based care, and hospitalists already routinely collaborate with nurses, social workers, case managers, pharmacists, and other stakeholders such as dieticians and physical therapists on inpatient care. Hospitalists are also experienced in facilitating communication and improving integration and coordination efficiencies among primary care providers and specialists, and between hospital care and post-acute care, as they coordinate post-hospital care and follow-up. This provides an opportunity to lead health system care coordination efforts, especially for complex and/or high-risk patients.22,23 CMS MSSP 2016 performance data12 showed that ACOs achieving shared savings had a decline in inpatient expenditures and utilization across several facility types (hospital, SNF, rehabilitation, long term). Postacute care management is critical to earning shared savings; SNF and Home Health expenditures fell by 18.3% and 9.7%, respectively, on average. We believe that hospitalists can have more influence over these cost areas by influencing treatment of hospitalized patients in a timely manner, discharge coordination, and selection of appropriate disposition locations. Hospitalists also play an integral role in ensuring the hospital performs well on quality metrics, including 30-day readmissions, hospital acquired conditions, and patient satisfaction. Examples below document the effectiveness of hospitalists in this new ACO era.
Care Transitions/Coordination
Before the Hospital Readmission Reduction Program (HRRP) delineated in the ACA, hospitalists developed Project BOOST (Better Outcomes by Optimizing Care Transitions) to improve hospital discharge care transition. The evidence-based foundation of this project led CMS to list Project BOOST as an example program that can reduce readmissions.24 Through the dissemination and mentored implementation of Project BOOST to over 200 hospitals across the United States,25 hospitalists contributed to the marked reduction in hospital readmission occurring since 2010.26 Although hospital medicine began as a practice specific to the hospital setting, hospitalists’ skills generated growing demand for them in postacute facilities. SNF residents commonly come from hospitals postdischarge and suffer from multiple comorbidities and limitations in activities of daily living. Not surprisingly, SNF residents experience high rates of rehospitalizations.27 Hospitalists can serve as a bridge between hospitals and SNFs and optimize this transition process to yield improved outcomes. Industry experts endorse this approach.28 A recent study demonstrated a significant reduction in readmissions in 1 SNF (32.3% to 16.1%, odds ratio = 0.403, P < .001), by having a hospitalist-led team follow patients discharged from the hospital.29
Chronic Conditions Management/High-Risk Patients
Interest in patients with multiple chronic comorbidities and social issues intensifies as healthcare systems focus limited resources on these high-risk patients to prevent the unnecessary use of costly services.30,31 As health systems assume financial risk for health outcomes and costs of designated patient groups, they undertake efforts to understand the population they serve. Such efforts aim to identify patients with established high utilization patterns (or those at risk for high utilization). This knowledge enables targeted actions to provide access, treatment, and preventive interventions to avoid unneeded emergency and hospital services. Hospitalists commonly care for these patients and are positioned to lead the implementation of patient risk assessment and stratification, develop patient-centered care models across care settings, and act as a liaison with primary care. For frail elderly and seriously ill patients, the integration of hospitalists into palliative care provides several opportunities for improving the quality of care at the end of life.32 As patients and their family caregivers commonly do not address goals of care until faced with a life-threatening condition in the hospital, hospitalists represent ideal primary palliative care physicians to initiate these conversations.33 A hospitalist communicating with a patient and/or their family caregiver about alleviating symptoms and clarifying patients’ preferences for care often yields decreases in ineffective healthcare utilization and better patient outcomes. The hospitalists’ ability to communicate with other providers within the hospital setting also allows them to better coordinate interdisciplinary care and prevent unnecessary and ineffective treatments and procedures.
De-Implementation/Waste Reduction
The largest inefficiencies in healthcare noted in the National Academy of Medicine report, Demanding Value from Our Health Care (2012), are failure to deliver known beneficial therapies or providing unnecessary or nonevidenced based services that do not improve outcomes, but come with associated risk and cost.34 “De-implementation” of unnecessary diagnostic tests or ineffective or even harmful treatments by hospitalists represents a significant opportunity to reduce costs while maintaining or even improving the quality of care. The Society of Hospital Medicine joined the Choosing Wisely® campaign and made 5 recommendations in adult care as an explicit starting point for eliminating waste in the hospital in 2013.35 Since then, hospitalists have participated in multiple successful efforts to address overutilization of care; some published results include the following:
- decreased frequency of unnecessary common labs through a multifaceted hospitalist QI intervention;36
- reduced length of stay and cost by appropriate use of telemetry;37 and
- reduced unnecessary radiology testing by providing physicians with individualized audit and feedback reports.38
CONCLUSION
Hundreds of ACOs now exist across the US, formed by a variety of providers including hospitals, physician groups, and integrated delivery systems. Provider groups range in size from primary care-focused physician groups with a handful of offices to large, multistate integrated delivery systems with dozens of hospitals and hundreds of office locations. Evaluations of ACO outcomes reveal mixed results.9,39-53 Admittedly, assessments attempting to compare the magnitude of savings across ACO models are difficult given the variation in size, variability in specific efforts to influence utilization, and substantial turnover among participating beneficiaries.54 Nonetheless, a newly published Office of Inspector General report55 showed that most Medicare ACOs reduced spending and improved care quality (82% of the individual quality measures) over the first 3 years of the program, and savings increased with duration of an ACO program. The report also noted that considerable time and managerial resources are required to implement changes to improve quality and lower costs. While the political terrain ostensibly supports value-based care and the need to diminish the proportion of our nation’s gross domestic product dedicated to healthcare, health systems are navigating an environment that still largely rewards volume. Hospitalists may be ideal facilitators for this transitional period as they possess the clinical experience caring for complex patients with multiple comorbidities and quality improvement skills to lead efforts in this new ACO era.
Disclosures
The authors have nothing to disclose.
1. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff(Project Hope). 2007;26(1):w44-w57. PubMed
2. Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in medicare. Health Aff(Project Hope). 2009;28(2):w219-w231. PubMed
3. McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Aff(Project Hope). 2010;29(5):982-990. PubMed
4. Berwick DM. Making good on ACOs’ promise--the final rule for the Medicare shared savings program. N Engl J Med. 2011;365(19):1753-1756. PubMed
5. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
6. Kennedy K. Health Care Providers Embracing Cost-saving Groups. USA Today, July 24, 2011.
7. Leavitt Partners. Available at http://leavittpartners.com, April 2016.
8. Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals Participating In ACOs Tend To Be Large And Urban, Allowing Access To Capital And Data. Health Aff(Millwood). 2016;35(3):431-439. PubMed
9. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early Performance of Accountable Care Organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. PubMed
10. Muhlestein D, Saunders R, McClellan M. Medicare Accountable Care Organization Results For 2015: The Journey To Better Quality And Lower Costs Continues. In. Health Affairs Blog. Bethesda, MD 2016.
11. Chernew ME. New Health Care Symposium: Building An ACO---What Services Do You Need And How Are Physicians Impacted? In Health Affairs Blog. Bethesda, MD 2016.
12. Centers for Medicare & Medicaid Services. Performance Year 2016 Quality Performance and Financial Reconciliation Results for ACOs with 2012-2016 Start Dates. Available at https://strategichealthcare.net/wp-content/uploads/2017/10/CMS-Slides-on-ACOs.pdf. 2017.
13. Shortell SM, Casalino LP. Implementing qualifications criteria and technical assistance for accountable care organizations. JAMA. 2010;303(17):1747-1748. PubMed
14. Shortell SM, Casalino LP, Fisher ES. How the center for Medicare and Medicaid innovation should test accountable care organizations. Health Aff (Project Hope). 2010;29(7):1293-1298. PubMed
15. Medicare Payment Advisory Commission. Accountable Care Organizations Payment Systems October 2015. Available at http://www.medpac.gov/documents/payment-basics/accountable-care-organization-payment-systems-15.pdf?sfvrsn=0.
16. American Hospital Association. 2010 Committee on Research. AHA Research Synthesis Report: Accountable Care Organization.
17. D’Aunno T, Broffman L, Sparer M, Kumar SR. Factors That Distinguish High-Performing Accountable Care Organizations in the Medicare Shared Savings Program. Health Serv. Res. 2016. PubMed
18. Peiris D, Phipps-Taylor MC, Stachowski CA, et al. ACOs Holding Commercial Contracts Are Larger And More Efficient Than Noncommercial ACOs. Health Aff (Project Hope). 2016;35(10):1849-1856. PubMed
19. Ouayogode MH, Colla CH, Lewis VA. Determinants of success in Shared Savings Programs: An analysis of ACO and market characteristics. Healthcare (Amsterdam, Netherlands). 2017;5(1-2):53-61. PubMed
20. National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. In: Hyattsville, MD.2017. PubMed
21. Gbemudu JN. Larson BK, Van Citters AD, Kreindler SA, Nelson EC, Shortell SM, Fisher ES. Norton Healthcare: A Strong Payer–Provider Partnership for the Journey to Accountable Care. January 2012. Available at http://www.commonwealthfund.org/~/media/files/publications/case-study/2012/jan/1574_gbemudu_norton_case-study_01_12_2012.pdf.
22. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. PubMed
23. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J. Hosp. Med.. 2013;8(8):421-427. PubMed
24. Centers for Medicare and Medicaid Services. Solicitation for Applications: Community-based Care Transitions Program. Available at https://innovation.cms.gov/Files/Migrated-Medicare-Demonstration-x/CCTP-Solicitation.pdf. September 7, 2017.
25. Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90(3):303-310. PubMed
26. Williams MV, Li J, Hansen LO, et al. Project BOOST implementation: lessons learned. South Med J. 2014;107(7):455-465. PubMed
27. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761]. J Am Geriatr Soc. 2010;58(4):627-635. PubMed
28. Pittman D. SNFs: New Turf for Hospitalists? 2013. Available at https://www.medpagetoday.com/hospitalbasedmedicine/hospitalists/39401.
29. Petigara S, Krishnamurthy M, Livert D. Necessity is the mother of invention: an innovative hospitalist-resident initiative for improving quality and reducing readmissions from skilled nursing facilities. J Community Hosp Intern Med Perspect. 2017;7(2):66-69. PubMed
30. Silow-Carroll S, Edwards J. Early Adopters of the Accountable Care Model: A Field Report on Improvements in Health Care Delivery. New York, NY: The Commonwealth Fund;March 2013.
31. Hasselman D. Super-Utilizer Summit: Common Themes from Innovative Complex Care Management Programs. Hamilton, NJ: Center for Health Care Strategies;October 2013.
32. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
33. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
34. O’Kane M, Buto K, Alteras T, et. al. Demanding Value from Our Health Care: Motivating Patient Action to Reduce Waste in Health Care. Institute of Medicine of the National Academies. July 2012. https://nam.edu/wp-content/uploads/2015/06/VSRT-DemandingValue.pdf. Accessed Accessed June 18, 2017.
35. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
36. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. PubMed
37. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
38. Neeman N, Quinn K, Soni K, Mourad M, Sehgal NL. Reducing radiology use on an inpatient medical service: choosing wisely. JAMA Intern Med. 2012;172(20):1606-1608. PubMed
39. Abrams M, Nuzum R, Zezza M, Ryan J, Kiszla J, Guterman S. The Affordable Care Act’s Payment and Delivery System Reforms: A Progress Report at Five Years. Bipartisan Policy Center, May 2015. Available at http://www.commonwealthfund.org/publications/issue-briefs/2015/may/aca-payment-and-delivery-system-reforms-at-5-years.
40. Kocot SL, White R, Katikaneni P, McClellan MB. A More Complete Picture of Pioneer ACO Results. The Brookings Institution, October 13, 2014. Available at http://www.brookings.edu/blogs/up-front/posts/2014/10/09-pioneer-aco-results-mcclellan/#recent_rr/
41. Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 Years. N Engl J Med. 2015;372(25):2451-2458. PubMed
42. Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association Between Medicare Accountable Care Organization Implementation and Spending Among Clinically Vulnerable Beneficiaries. JAMA Intern Med. 2016;176(8):1167-1175. PubMed
43. Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Project Hope). 2014;33(1):95-102. PubMed
44. Fullerton CA, Henke RM, Crable E, Hohlbauch A, Cummings N. The Impact Of Medicare ACOs On Improving Integration And Coordination Of Physical And Behavioral Health Care. Health Aff (Project Hope). 2016;35(7):1257-1265. PubMed
45. Herrel LA, Norton EC, Hawken SR, Ye Z, Hollenbeck BK, Miller DC. Early impact of Medicare accountable care organizations on cancer surgery outcomes. Cancer. 2016;122(17):2739-2746. PubMed
46. McConnell KJ, Renfro S, Chan BK, et al. Early Performance in Medicaid Accountable Care Organizations: A Comparison of Oregon and Colorado. JAMA Intern Med. 2017;177(4):538-545. PubMed
47. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer Accountable Care Organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
48. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center--a five-year self-assessment. N Engl J Med. 2015;372(21):1981-1983. PubMed
49. Rose S, Zaslavsky AM, McWilliams JM. Variation In Accountable Care Organization Spending And Sensitivity To Risk Adjustment: Implications For Benchmarking. Health affairs (Project Hope). 2016;35(3):440-448. PubMed
50. Shortell SM, Poon BY, Ramsay PP, et al. A Multilevel Analysis of Patient Engagement and Patient-Reported Outcomes in Primary Care Practices of Accountable Care Organizations. J Gen Intern Med. 2017;32(6):640-647. PubMed
51. Winblad U, Mor V, McHugh JP, Rahman M. ACO-Affiliated Hospitals Reduced Rehospitalizations From Skilled Nursing Facilities Faster Than Other Hospitals. Health Aff (Project Hope). 2017;36(1):67-73. PubMed
52. Zhang Y, Caines KJ, Powers CA. Evaluating the Effects of Pioneer Accountable Care Organizations on Medicare Part D Drug Spending and Utilization. Med Care. 2017;55(5):470-475. PubMed
53. Muhlestein D. Medicare ACOs: Mixed Initial Results and Cautious Optimism. Health Affairs Blog, February 4, 2014. Available at http://healthaffairs.org/blog/2014/02/04/medicare-acos-mixed-initial-results-and-cautious-optimism/.
54. Hsu J, Price M, Vogeli C, et al. Bending The Spending Curve By Altering Care Delivery Patterns: The Role Of Care Management Within A Pioneer ACO. Health Aff (Project Hope). 2017;36(5):876-884. PubMed
55. Medicare Shared Savings Program Accountable Care Organizations Have Shown Potential For Reducing Spending And Improving Quality. Office of Inspector General;August 2017.
1. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff(Project Hope). 2007;26(1):w44-w57. PubMed
2. Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in medicare. Health Aff(Project Hope). 2009;28(2):w219-w231. PubMed
3. McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Aff(Project Hope). 2010;29(5):982-990. PubMed
4. Berwick DM. Making good on ACOs’ promise--the final rule for the Medicare shared savings program. N Engl J Med. 2011;365(19):1753-1756. PubMed
5. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
6. Kennedy K. Health Care Providers Embracing Cost-saving Groups. USA Today, July 24, 2011.
7. Leavitt Partners. Available at http://leavittpartners.com, April 2016.
8. Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals Participating In ACOs Tend To Be Large And Urban, Allowing Access To Capital And Data. Health Aff(Millwood). 2016;35(3):431-439. PubMed
9. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early Performance of Accountable Care Organizations in Medicare. N Engl J Med. 2016;374(24):2357-2366. PubMed
10. Muhlestein D, Saunders R, McClellan M. Medicare Accountable Care Organization Results For 2015: The Journey To Better Quality And Lower Costs Continues. In. Health Affairs Blog. Bethesda, MD 2016.
11. Chernew ME. New Health Care Symposium: Building An ACO---What Services Do You Need And How Are Physicians Impacted? In Health Affairs Blog. Bethesda, MD 2016.
12. Centers for Medicare & Medicaid Services. Performance Year 2016 Quality Performance and Financial Reconciliation Results for ACOs with 2012-2016 Start Dates. Available at https://strategichealthcare.net/wp-content/uploads/2017/10/CMS-Slides-on-ACOs.pdf. 2017.
13. Shortell SM, Casalino LP. Implementing qualifications criteria and technical assistance for accountable care organizations. JAMA. 2010;303(17):1747-1748. PubMed
14. Shortell SM, Casalino LP, Fisher ES. How the center for Medicare and Medicaid innovation should test accountable care organizations. Health Aff (Project Hope). 2010;29(7):1293-1298. PubMed
15. Medicare Payment Advisory Commission. Accountable Care Organizations Payment Systems October 2015. Available at http://www.medpac.gov/documents/payment-basics/accountable-care-organization-payment-systems-15.pdf?sfvrsn=0.
16. American Hospital Association. 2010 Committee on Research. AHA Research Synthesis Report: Accountable Care Organization.
17. D’Aunno T, Broffman L, Sparer M, Kumar SR. Factors That Distinguish High-Performing Accountable Care Organizations in the Medicare Shared Savings Program. Health Serv. Res. 2016. PubMed
18. Peiris D, Phipps-Taylor MC, Stachowski CA, et al. ACOs Holding Commercial Contracts Are Larger And More Efficient Than Noncommercial ACOs. Health Aff (Project Hope). 2016;35(10):1849-1856. PubMed
19. Ouayogode MH, Colla CH, Lewis VA. Determinants of success in Shared Savings Programs: An analysis of ACO and market characteristics. Healthcare (Amsterdam, Netherlands). 2017;5(1-2):53-61. PubMed
20. National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. In: Hyattsville, MD.2017. PubMed
21. Gbemudu JN. Larson BK, Van Citters AD, Kreindler SA, Nelson EC, Shortell SM, Fisher ES. Norton Healthcare: A Strong Payer–Provider Partnership for the Journey to Accountable Care. January 2012. Available at http://www.commonwealthfund.org/~/media/files/publications/case-study/2012/jan/1574_gbemudu_norton_case-study_01_12_2012.pdf.
22. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. PubMed
23. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J. Hosp. Med.. 2013;8(8):421-427. PubMed
24. Centers for Medicare and Medicaid Services. Solicitation for Applications: Community-based Care Transitions Program. Available at https://innovation.cms.gov/Files/Migrated-Medicare-Demonstration-x/CCTP-Solicitation.pdf. September 7, 2017.
25. Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90(3):303-310. PubMed
26. Williams MV, Li J, Hansen LO, et al. Project BOOST implementation: lessons learned. South Med J. 2014;107(7):455-465. PubMed
27. Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761]. J Am Geriatr Soc. 2010;58(4):627-635. PubMed
28. Pittman D. SNFs: New Turf for Hospitalists? 2013. Available at https://www.medpagetoday.com/hospitalbasedmedicine/hospitalists/39401.
29. Petigara S, Krishnamurthy M, Livert D. Necessity is the mother of invention: an innovative hospitalist-resident initiative for improving quality and reducing readmissions from skilled nursing facilities. J Community Hosp Intern Med Perspect. 2017;7(2):66-69. PubMed
30. Silow-Carroll S, Edwards J. Early Adopters of the Accountable Care Model: A Field Report on Improvements in Health Care Delivery. New York, NY: The Commonwealth Fund;March 2013.
31. Hasselman D. Super-Utilizer Summit: Common Themes from Innovative Complex Care Management Programs. Hamilton, NJ: Center for Health Care Strategies;October 2013.
32. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
33. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. PubMed
34. O’Kane M, Buto K, Alteras T, et. al. Demanding Value from Our Health Care: Motivating Patient Action to Reduce Waste in Health Care. Institute of Medicine of the National Academies. July 2012. https://nam.edu/wp-content/uploads/2015/06/VSRT-DemandingValue.pdf. Accessed Accessed June 18, 2017.
35. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
36. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. PubMed
37. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
38. Neeman N, Quinn K, Soni K, Mourad M, Sehgal NL. Reducing radiology use on an inpatient medical service: choosing wisely. JAMA Intern Med. 2012;172(20):1606-1608. PubMed
39. Abrams M, Nuzum R, Zezza M, Ryan J, Kiszla J, Guterman S. The Affordable Care Act’s Payment and Delivery System Reforms: A Progress Report at Five Years. Bipartisan Policy Center, May 2015. Available at http://www.commonwealthfund.org/publications/issue-briefs/2015/may/aca-payment-and-delivery-system-reforms-at-5-years.
40. Kocot SL, White R, Katikaneni P, McClellan MB. A More Complete Picture of Pioneer ACO Results. The Brookings Institution, October 13, 2014. Available at http://www.brookings.edu/blogs/up-front/posts/2014/10/09-pioneer-aco-results-mcclellan/#recent_rr/
41. Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 Years. N Engl J Med. 2015;372(25):2451-2458. PubMed
42. Colla CH, Lewis VA, Kao LS, O’Malley AJ, Chang CH, Fisher ES. Association Between Medicare Accountable Care Organization Implementation and Spending Among Clinically Vulnerable Beneficiaries. JAMA Intern Med. 2016;176(8):1167-1175. PubMed
43. Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Aff (Project Hope). 2014;33(1):95-102. PubMed
44. Fullerton CA, Henke RM, Crable E, Hohlbauch A, Cummings N. The Impact Of Medicare ACOs On Improving Integration And Coordination Of Physical And Behavioral Health Care. Health Aff (Project Hope). 2016;35(7):1257-1265. PubMed
45. Herrel LA, Norton EC, Hawken SR, Ye Z, Hollenbeck BK, Miller DC. Early impact of Medicare accountable care organizations on cancer surgery outcomes. Cancer. 2016;122(17):2739-2746. PubMed
46. McConnell KJ, Renfro S, Chan BK, et al. Early Performance in Medicaid Accountable Care Organizations: A Comparison of Oregon and Colorado. JAMA Intern Med. 2017;177(4):538-545. PubMed
47. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer Accountable Care Organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
48. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center--a five-year self-assessment. N Engl J Med. 2015;372(21):1981-1983. PubMed
49. Rose S, Zaslavsky AM, McWilliams JM. Variation In Accountable Care Organization Spending And Sensitivity To Risk Adjustment: Implications For Benchmarking. Health affairs (Project Hope). 2016;35(3):440-448. PubMed
50. Shortell SM, Poon BY, Ramsay PP, et al. A Multilevel Analysis of Patient Engagement and Patient-Reported Outcomes in Primary Care Practices of Accountable Care Organizations. J Gen Intern Med. 2017;32(6):640-647. PubMed
51. Winblad U, Mor V, McHugh JP, Rahman M. ACO-Affiliated Hospitals Reduced Rehospitalizations From Skilled Nursing Facilities Faster Than Other Hospitals. Health Aff (Project Hope). 2017;36(1):67-73. PubMed
52. Zhang Y, Caines KJ, Powers CA. Evaluating the Effects of Pioneer Accountable Care Organizations on Medicare Part D Drug Spending and Utilization. Med Care. 2017;55(5):470-475. PubMed
53. Muhlestein D. Medicare ACOs: Mixed Initial Results and Cautious Optimism. Health Affairs Blog, February 4, 2014. Available at http://healthaffairs.org/blog/2014/02/04/medicare-acos-mixed-initial-results-and-cautious-optimism/.
54. Hsu J, Price M, Vogeli C, et al. Bending The Spending Curve By Altering Care Delivery Patterns: The Role Of Care Management Within A Pioneer ACO. Health Aff (Project Hope). 2017;36(5):876-884. PubMed
55. Medicare Shared Savings Program Accountable Care Organizations Have Shown Potential For Reducing Spending And Improving Quality. Office of Inspector General;August 2017.
© Society of Hospital Medicine
Improving Quality of Care for Seriously Ill Patients: Opportunities for Hospitalists
Palliative care is specialized medical care focused on providing relief from the symptoms, pain, and stress of a serious illness. The goal is to improve the quality of life for both the patient and the family. In all settings, palliative care has been found to improve patients’ quality of life,1,2 improve family satisfaction and well-being,3 reduce resource utilization and costs,4 and, in some studies, increase the length of life for seriously ill patients.5
Given the frequency with which seriously ill patients are hospitalized, hospitalists are well positioned to identify those who could benefit from palliative care interventions.6 Hospitalists routinely use primary palliative care skills, including pain and symptom management and skilled care planning conversations. For complex cases, such as patients with intractable symptoms or major family conflict, hospitalists may refer to specialist palliative care teams for consultation.
The Society of Hospital Medicine (SHM) defines the key primary palliative care responsibilities for hospitalists as (1) leading discussions on the goals of care and advance care planning with patients and families, (2) screening and treating common physical symptoms, and (3) referring patients to community services to provide support postdischarge.7 According to data in the National Palliative Care Registry,8 48% of all palliative care referrals in 2015 came from hospitalists, which is more than double the percentage of referrals from any other specialty.9
In a recent survey conducted by SHM about serious illness communication, 53% of hospitalists reported concerns about a patient or family’s understanding of their prognosis, and 50% indicated that they do not feel confident managing family conflict.10
IMPROVING VALUE
Context
Patients with multiple serious chronic conditions are often forced to rely on emergency services when crises, such as uncontrolled pain or dyspnea exacerbation, occur after hours, resulting in the revolving-door hospitalizations that typically characterize their care.11 As the prevalence of serious illness rises and the shift to value-based payment accelerates, hospitals are under increasing pressure to deliver efficient and high-quality services that meet the needs of seriously ill patients. The integration of standardized palliative care screening and assessment enables hospitalists and other providers to identify high-need individuals and match services and delivery models to needs, whether it be respite care for an exhausted and overwhelmed family caregiver or a home protocol for managing recurrent dyspnea crises for a patient with chronic obstructive pulmonary disease (COPD). This process improves the quality of care and quality of life, and in doing so, prevents the need for costly crisis care.
Reducing Readmissions
By identifying patients in need of extra symptom management support, or those at a turning point requiring discussion about achievable priorities for care, hospitalists can avert crises for patients earlier in the disease trajectory either by managing the patient’s palliative needs themselves or by connecting patients with specialty palliative care services as needed. This leads to a better quality of life (and survival in some studies) for both patients and their families1,3,5 and reduces unnecessary emergency department (ED) and hospital use.12 Hospitalists providing palliative care can also reduce readmissions by improving care coordination, including clinical communication and medication reconciliation after discharge.13
A 2015 Harvard Business Review study found that the quality of communication in the hospital is the strongest independent predictor of readmissions when combined with process-of-care improvements, such as standardized patient screening and assessment of family caregiver capacity.14 While medical education prepares physicians to deliver evidence-based medical care, it currently offers little to no training in communication skills, despite mounting evidence that this is a critical component of quality healthcare.
Cost Savings
Hospital palliative care teams are associated with significant hospital cost savings that result from aligning care with patient priorities, leading, in turn, to reduced nonbeneficial hospital imaging, medications, procedures, and length of stay.15 See the table16,17 for examples of cost and quality outcomes of specialist palliative care provision and evidence supporting each outcome.18-25
Multiple studies consistently demonstrate that inpatient palliative care teams reduce hospital costs.26 One randomized controlled trial investigating the impact of an inpatient palliative care service found that patients who received care from the palliative care team reported greater satisfaction with their care, had fewer intensive care unit admissions, had more advanced directives at hospital discharge, longer hospice length of stay, and lower total healthcare costs (a net difference of $6766 per patient).23
Research shows that the earlier palliative care is provided, the greater the impact on the subsequent course of care,27 suggesting that hospitalists who provide frontline palliative care interventions as early as possible in a seriously ill patient’s stay will be able to provide higher quality care with lower overall costs. Notably, the majority of research on cost savings associated with palliative care has focused on the impact of specialist palliative care teams, and further research is needed to understand the economic impact of primary palliative care provision.
Improving Satisfaction
Shifting to value-based payment means that the patient and family experience determine an increasingly large percentage of hospital and provider reimbursement. Palliative care approaches, such as family caregiver assessment and support, access to 24/7 assistance after discharge, and person-centered care by an interdisciplinary team, improve performance in all of these measures. Communication skills training improves patient satisfaction scores, and skilled discussions about achievable priorities for care are associated with better quality of life, reduced nonbeneficial and burdensome treatments, and an increase in goal-concordant care.19 Communication skills training has also been shown to reduce burnout and improve empathy among physicians.28,29
SKILLS TRAINING OPPORTUNITIES
Though more evidence is needed to understand the impact of primary palliative care provision by hospitalists, the strong evidence on the benefits of specialty palliative care suggests that the skilled provision of primary palliative care by hospitalists will result in higher quality, higher value care. A number of training options exist for midcareer hospital medicine clinicians, including both in-person and online training in communication and other palliative care skills.
- The Center to Advance Palliative Care (CAPC) is a membership organization that offers online continuing education unit and continuing medical education courses on communication skills, pain and symptom management, caregiver support, and care coordination. CAPC also offers courses on palliative interventions for patients with dementia, COPD, and heart failure.
- SHM is actively invested in engaging hospitalists in palliative care skills training. SHM provides free toolkits on a variety of topics within the palliative care domain, including pain management, postacute care transitions, and opioid safety. The recently released Serious Illness Communication toolkit offers background on the role of hospitalists in palliative care provision, a pathway for fitting goals-of-care conversations into hospitalist workflow and recommended metrics and training resources. SHM also uses a mentored implementation model in which expert physicians mentor hospital team members on best practices in palliative care. SHM’s Palliative Care Task Force seeks to identify educational activities for hospitalists and create opportunities to integrate palliative care in hospital medicine.30
- The Serious Illness Care Program at Ariadne Labs in Boston aims to facilitate conversations between clinicians and seriously ill patients through its Serious Illness Conversation Guide, combined with technical assistance on workflow redesign to help clinicians conduct and document serious illness conversations.
- VitalTalk specializes in clinical communication education. Through online and in-person train-the-trainer programs, VitalTalk equips clinicians to lead communication training programs at their home institutions.
- The Education in Palliative and End-of-Life Care Program and End-of-Life Nursing Education Consortium (ELNEC) uses a train-the-trainer approach to educate providers in palliative care clinical competencies and increase the reach of primary palliative care provision. ELNEC workshops are complemented by a curriculum of online clinical training modules.
CULTURE CHANGE
Though palliative care skills training is a necessary first step, hospitalists also cite lack of time, difficulty finding records of previous patient discussions, and frequent handoffs as among the barriers to integrating palliative care into their practice.10 Studies examining the process of palliative care and hospital culture change have found that barriers to palliative care integration include a culture of aggressive care in EDs, lack of standardized patient identification criteria, and limited knowledge about and staffing for palliative care.31 These data indicate the need for system changes that enable hospitalists to operationalize palliative care principles.
Health systems must implement systems and processes that routinize palliative care, making it part of the mainstream course of care for seriously ill patients and their caregivers. This includes developing systems for the identification of patients with palliative care needs, embedding palliative care assessment and referral into clinical workflows, and enabling standardized palliative care documentation in electronic medical records. While palliative care skills training is essential, investment in systems change is no less critical to embedding palliative care practices in clinical norms across specialties.
CONCLUSION
Hospitalists can use a palliative approach to improve care quality and quality of life for seriously ill patients while helping to avoid preventable and unnecessary 911 calls, ED visits, and hospitalizations. The shift towards value-based payment is a strong incentive for hospitals and hospitalists to direct resources toward practices that improve the quality of life and care for the highest-need patients and their families. When equipped with the tools they need to provide palliative care, either themselves or in collaboration with palliative care teams, hospitalists have the opportunity to profoundly redirect the experience of care for seriously ill patients and their families.
Disclosure
The authors declared no conflicts of interest.
1. Casarett D, Pickard A, Bailey FA, et al. Do Palliative Consultations Improve Patient Outcomes? J Am Geriatr Soc. 2008;56(4):595-599. PubMed
2. Delgado-Guay MO, Parsons HA, Zhijun LM, Palmer LJ, Bruera E. Symptom distress, interventions, and outcomes of intensive care unit cancer patients referred to a palliative care consult team. Cancer. 2009;115(2):437-445. PubMed
3. Gelfman LP, Meier DE, Morrison SR. Does Palliative Care Improve Quality? A Survey of Bereaved Family Members. J Pain Symptom Manage. 2008;36(1):22-28. PubMed
4. Morrison SR, Dietrich J, Ladwig S, et al. Palliative Care Consultation Teams Cut Hospital Costs for Medicaid Beneficiaries. Health Aff. 2011;30:454-463. PubMed
5. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363(8):733-742. PubMed
6. Lin RJ, Adelman RD, Diamond RR, Evans AT. The Sentinel Hospitalization and the Role of Palliative Care. J Hosp Med. 2014;9(5):320-323. PubMed
7. Palliative care. J Hosp Med. 2006;1:80-81. doi:10.1002/jhm.54.
8. Center to Advance Palliative Care. National palliative care registry. https://registry.capc.org/. Accessed May 26, 2017.
9. Rogers M, Dumanovsky T. How We Work: Trends and Insights in Hospital Palliative Care. New York: The Center to Advance Palliative Care and the National Palliative Care Research Center. https://registry.capc.org/wp-content/uploads/2017/02/How-We-Work-Trends-and-Insights-in-Hospital-Palliative-Care-2009-2015.pdf; 2017. Accessed May 26, 2017.
10. Rosenberg LB, Greenwald J, Caponi B, et al. Confidence with and Barriers to Serious Illness Communication: A National Survey of Hospitalists. J Palliat Med. 2017;20(9):1013-1019. PubMed
11. Aldridge MD, Kelley AS. The Myth Regarding the High Cost of End-of-Life Care. Am J Public Health. 2015;105(12):2411-2415. PubMed
12. Enguidanos S, Vesper E, Lorenz K. 30-Day Readmissions among Seriously Ill Older Adults. J Palliat Med. 2012;15(12):1356-1361. PubMed
13. Kripalani S, Theobald C, Anctil B, Vasilevskis E. Reducing Hospital Readmission Rates: Current Strategies and Future Directions. Annu Rev Med. 2014;65:471-485. PubMed
14. Senot C, Chandrasekaran A. What Has the Biggest Impact on Hospital Readmission Rates. Harvard Business Review. September 23, 2015. https://hbr.org/2015/09/what-has-the-biggest-impact-on-hospital-readmission-rates. Accessed March 29, 2017.
15. Morrison RS, Penrod JD, Cassel JB, et al. Cost Savings Associated with US Hospital Palliative Care Consultation Programs. Arch Intern Med. 2008;168(16):1783-1790. PubMed
16. Cassel JB. Palliative Care’s Impact on Utilization and Costs: Implications for Health Services Research and Policy. In: Kelley AS, Meier DE, editors. Meeting the Needs of Older Adults with Serious Illness: Challenges and Opportunities in the Age of Health Care Reform. New York: Springer Science+Business Media; 2014:109-126.
17. Meier D, Silvers A. Serious Illness Strategies for Health Plans and Accountable Care Organizations. https://media.capc.org/filer_public/2c/69/2c69a0f0-c90f-43ac-893e-e90cd0438482/serious_illness_strategies_web.pdf. 2017. Accessed August 10, 2017.
18. Casarett D, Johnson M, Smith D, Richardson D. The Optimal Delivery of Palliative Care: A National Comparison of the Outcomes of Consultation Teams vs Inpatient Units. Arch Intern Med. 2011;171(7):649-655. PubMed
19. Bernacki RE, Block SD. Communication About Serious Illness Care Goals: A Review and Synthesis of Best Practices. JAMA Intern Med. 2014;174(12):1994-2003. PubMed
20. Wright AA, Zhang B, Ray A, et al. Associations Between End-of-Life Discussions, Patient Mental Health, Medical Care Near Death, and Caregiver Bereavement Adjustment. JAMA. 2008;300(14):1665-1673. PubMed
21. May P, Garrido M, Cassel JB, et al. Cost Analysis of a Prospective Multi-Site Cohort Study of Palliative Care Consultation Teams for Adults with Advanced Cancer: Where Do Cost Savings Come From? Palliat Med. 2017;31(4):378-386. PubMed
22. Norton SA, Hogan LA, Holloway RG, et al. Proactive Palliative Care in the Medical Intensive Care Unit: Effects of Length of Stay for Selected High-Risk Patients. Crit Care Med. 2007;35(6):1530-1535. PubMed
23. Gade G, Venohr I, Conner D, et al. Impact of an Inpatient Palliative Care Team: A Randomized Controlled Trial. J Palliat Med. 2008;11(2):180-201. PubMed
24. Adelson K, Paris J, Horton J, et al. Standardized Criteria for Palliative Care Consultation on a Solid Tumor Oncology Service Reduces Downstream Health Care use. J Oncol Pract. 2017;13(5):e431-e440. PubMed
25. Lustbader D, Mitchell M, Carole R, et al. The Impact of a Home-Based Palliative Care Program in an Accountable Care Organization. J Palliat Med. 2017;20(1):23-28. PubMed
26. May P, Normand C, Morrison R. Economic Impact of Hospital Inpatient Palliative Care Consultation: Review of Current Evidence and Directions for Future Research. J Palliat Med. 2014;17(9):1054-1063. PubMed
27. May P, Garrido MM, Bassel JB, et al. Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients with Advanced Cancer: Earlier Consultation Is Associated with Larger Cost-Saving Effect. J Clin Oncol. 2015;33(25):2745-2752. PubMed
28. Boissy A, Windover A, Bokar D, et al. Communication Skills Training for Physicians Improves Patient Satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
29. Kirkland KB. Finding Joy in Practice Cocreation in Palliative Care. JAMA. 2017;317(20):2065-2066. PubMed
30. Whelan C. SHM Establishes Palliative Care Task Force. The Hospitalist. 2005;11. http://www.the-hospitalist.org/hospitalist/article/123027/hospice-palliative-medicine/shm-establishes-palliative-care-task-force. Accessed July 31, 2017.
31. Grudzen CR, Richardson LD, Major-Monfried H, et al. Hospital Administrators’ Views on Barriers and Opportunities to Delivering Palliative Care in the Emergency Department. Ann Emerg Med. 2013;61(6):654-660. PubMed
Palliative care is specialized medical care focused on providing relief from the symptoms, pain, and stress of a serious illness. The goal is to improve the quality of life for both the patient and the family. In all settings, palliative care has been found to improve patients’ quality of life,1,2 improve family satisfaction and well-being,3 reduce resource utilization and costs,4 and, in some studies, increase the length of life for seriously ill patients.5
Given the frequency with which seriously ill patients are hospitalized, hospitalists are well positioned to identify those who could benefit from palliative care interventions.6 Hospitalists routinely use primary palliative care skills, including pain and symptom management and skilled care planning conversations. For complex cases, such as patients with intractable symptoms or major family conflict, hospitalists may refer to specialist palliative care teams for consultation.
The Society of Hospital Medicine (SHM) defines the key primary palliative care responsibilities for hospitalists as (1) leading discussions on the goals of care and advance care planning with patients and families, (2) screening and treating common physical symptoms, and (3) referring patients to community services to provide support postdischarge.7 According to data in the National Palliative Care Registry,8 48% of all palliative care referrals in 2015 came from hospitalists, which is more than double the percentage of referrals from any other specialty.9
In a recent survey conducted by SHM about serious illness communication, 53% of hospitalists reported concerns about a patient or family’s understanding of their prognosis, and 50% indicated that they do not feel confident managing family conflict.10
IMPROVING VALUE
Context
Patients with multiple serious chronic conditions are often forced to rely on emergency services when crises, such as uncontrolled pain or dyspnea exacerbation, occur after hours, resulting in the revolving-door hospitalizations that typically characterize their care.11 As the prevalence of serious illness rises and the shift to value-based payment accelerates, hospitals are under increasing pressure to deliver efficient and high-quality services that meet the needs of seriously ill patients. The integration of standardized palliative care screening and assessment enables hospitalists and other providers to identify high-need individuals and match services and delivery models to needs, whether it be respite care for an exhausted and overwhelmed family caregiver or a home protocol for managing recurrent dyspnea crises for a patient with chronic obstructive pulmonary disease (COPD). This process improves the quality of care and quality of life, and in doing so, prevents the need for costly crisis care.
Reducing Readmissions
By identifying patients in need of extra symptom management support, or those at a turning point requiring discussion about achievable priorities for care, hospitalists can avert crises for patients earlier in the disease trajectory either by managing the patient’s palliative needs themselves or by connecting patients with specialty palliative care services as needed. This leads to a better quality of life (and survival in some studies) for both patients and their families1,3,5 and reduces unnecessary emergency department (ED) and hospital use.12 Hospitalists providing palliative care can also reduce readmissions by improving care coordination, including clinical communication and medication reconciliation after discharge.13
A 2015 Harvard Business Review study found that the quality of communication in the hospital is the strongest independent predictor of readmissions when combined with process-of-care improvements, such as standardized patient screening and assessment of family caregiver capacity.14 While medical education prepares physicians to deliver evidence-based medical care, it currently offers little to no training in communication skills, despite mounting evidence that this is a critical component of quality healthcare.
Cost Savings
Hospital palliative care teams are associated with significant hospital cost savings that result from aligning care with patient priorities, leading, in turn, to reduced nonbeneficial hospital imaging, medications, procedures, and length of stay.15 See the table16,17 for examples of cost and quality outcomes of specialist palliative care provision and evidence supporting each outcome.18-25
Multiple studies consistently demonstrate that inpatient palliative care teams reduce hospital costs.26 One randomized controlled trial investigating the impact of an inpatient palliative care service found that patients who received care from the palliative care team reported greater satisfaction with their care, had fewer intensive care unit admissions, had more advanced directives at hospital discharge, longer hospice length of stay, and lower total healthcare costs (a net difference of $6766 per patient).23
Research shows that the earlier palliative care is provided, the greater the impact on the subsequent course of care,27 suggesting that hospitalists who provide frontline palliative care interventions as early as possible in a seriously ill patient’s stay will be able to provide higher quality care with lower overall costs. Notably, the majority of research on cost savings associated with palliative care has focused on the impact of specialist palliative care teams, and further research is needed to understand the economic impact of primary palliative care provision.
Improving Satisfaction
Shifting to value-based payment means that the patient and family experience determine an increasingly large percentage of hospital and provider reimbursement. Palliative care approaches, such as family caregiver assessment and support, access to 24/7 assistance after discharge, and person-centered care by an interdisciplinary team, improve performance in all of these measures. Communication skills training improves patient satisfaction scores, and skilled discussions about achievable priorities for care are associated with better quality of life, reduced nonbeneficial and burdensome treatments, and an increase in goal-concordant care.19 Communication skills training has also been shown to reduce burnout and improve empathy among physicians.28,29
SKILLS TRAINING OPPORTUNITIES
Though more evidence is needed to understand the impact of primary palliative care provision by hospitalists, the strong evidence on the benefits of specialty palliative care suggests that the skilled provision of primary palliative care by hospitalists will result in higher quality, higher value care. A number of training options exist for midcareer hospital medicine clinicians, including both in-person and online training in communication and other palliative care skills.
- The Center to Advance Palliative Care (CAPC) is a membership organization that offers online continuing education unit and continuing medical education courses on communication skills, pain and symptom management, caregiver support, and care coordination. CAPC also offers courses on palliative interventions for patients with dementia, COPD, and heart failure.
- SHM is actively invested in engaging hospitalists in palliative care skills training. SHM provides free toolkits on a variety of topics within the palliative care domain, including pain management, postacute care transitions, and opioid safety. The recently released Serious Illness Communication toolkit offers background on the role of hospitalists in palliative care provision, a pathway for fitting goals-of-care conversations into hospitalist workflow and recommended metrics and training resources. SHM also uses a mentored implementation model in which expert physicians mentor hospital team members on best practices in palliative care. SHM’s Palliative Care Task Force seeks to identify educational activities for hospitalists and create opportunities to integrate palliative care in hospital medicine.30
- The Serious Illness Care Program at Ariadne Labs in Boston aims to facilitate conversations between clinicians and seriously ill patients through its Serious Illness Conversation Guide, combined with technical assistance on workflow redesign to help clinicians conduct and document serious illness conversations.
- VitalTalk specializes in clinical communication education. Through online and in-person train-the-trainer programs, VitalTalk equips clinicians to lead communication training programs at their home institutions.
- The Education in Palliative and End-of-Life Care Program and End-of-Life Nursing Education Consortium (ELNEC) uses a train-the-trainer approach to educate providers in palliative care clinical competencies and increase the reach of primary palliative care provision. ELNEC workshops are complemented by a curriculum of online clinical training modules.
CULTURE CHANGE
Though palliative care skills training is a necessary first step, hospitalists also cite lack of time, difficulty finding records of previous patient discussions, and frequent handoffs as among the barriers to integrating palliative care into their practice.10 Studies examining the process of palliative care and hospital culture change have found that barriers to palliative care integration include a culture of aggressive care in EDs, lack of standardized patient identification criteria, and limited knowledge about and staffing for palliative care.31 These data indicate the need for system changes that enable hospitalists to operationalize palliative care principles.
Health systems must implement systems and processes that routinize palliative care, making it part of the mainstream course of care for seriously ill patients and their caregivers. This includes developing systems for the identification of patients with palliative care needs, embedding palliative care assessment and referral into clinical workflows, and enabling standardized palliative care documentation in electronic medical records. While palliative care skills training is essential, investment in systems change is no less critical to embedding palliative care practices in clinical norms across specialties.
CONCLUSION
Hospitalists can use a palliative approach to improve care quality and quality of life for seriously ill patients while helping to avoid preventable and unnecessary 911 calls, ED visits, and hospitalizations. The shift towards value-based payment is a strong incentive for hospitals and hospitalists to direct resources toward practices that improve the quality of life and care for the highest-need patients and their families. When equipped with the tools they need to provide palliative care, either themselves or in collaboration with palliative care teams, hospitalists have the opportunity to profoundly redirect the experience of care for seriously ill patients and their families.
Disclosure
The authors declared no conflicts of interest.
Palliative care is specialized medical care focused on providing relief from the symptoms, pain, and stress of a serious illness. The goal is to improve the quality of life for both the patient and the family. In all settings, palliative care has been found to improve patients’ quality of life,1,2 improve family satisfaction and well-being,3 reduce resource utilization and costs,4 and, in some studies, increase the length of life for seriously ill patients.5
Given the frequency with which seriously ill patients are hospitalized, hospitalists are well positioned to identify those who could benefit from palliative care interventions.6 Hospitalists routinely use primary palliative care skills, including pain and symptom management and skilled care planning conversations. For complex cases, such as patients with intractable symptoms or major family conflict, hospitalists may refer to specialist palliative care teams for consultation.
The Society of Hospital Medicine (SHM) defines the key primary palliative care responsibilities for hospitalists as (1) leading discussions on the goals of care and advance care planning with patients and families, (2) screening and treating common physical symptoms, and (3) referring patients to community services to provide support postdischarge.7 According to data in the National Palliative Care Registry,8 48% of all palliative care referrals in 2015 came from hospitalists, which is more than double the percentage of referrals from any other specialty.9
In a recent survey conducted by SHM about serious illness communication, 53% of hospitalists reported concerns about a patient or family’s understanding of their prognosis, and 50% indicated that they do not feel confident managing family conflict.10
IMPROVING VALUE
Context
Patients with multiple serious chronic conditions are often forced to rely on emergency services when crises, such as uncontrolled pain or dyspnea exacerbation, occur after hours, resulting in the revolving-door hospitalizations that typically characterize their care.11 As the prevalence of serious illness rises and the shift to value-based payment accelerates, hospitals are under increasing pressure to deliver efficient and high-quality services that meet the needs of seriously ill patients. The integration of standardized palliative care screening and assessment enables hospitalists and other providers to identify high-need individuals and match services and delivery models to needs, whether it be respite care for an exhausted and overwhelmed family caregiver or a home protocol for managing recurrent dyspnea crises for a patient with chronic obstructive pulmonary disease (COPD). This process improves the quality of care and quality of life, and in doing so, prevents the need for costly crisis care.
Reducing Readmissions
By identifying patients in need of extra symptom management support, or those at a turning point requiring discussion about achievable priorities for care, hospitalists can avert crises for patients earlier in the disease trajectory either by managing the patient’s palliative needs themselves or by connecting patients with specialty palliative care services as needed. This leads to a better quality of life (and survival in some studies) for both patients and their families1,3,5 and reduces unnecessary emergency department (ED) and hospital use.12 Hospitalists providing palliative care can also reduce readmissions by improving care coordination, including clinical communication and medication reconciliation after discharge.13
A 2015 Harvard Business Review study found that the quality of communication in the hospital is the strongest independent predictor of readmissions when combined with process-of-care improvements, such as standardized patient screening and assessment of family caregiver capacity.14 While medical education prepares physicians to deliver evidence-based medical care, it currently offers little to no training in communication skills, despite mounting evidence that this is a critical component of quality healthcare.
Cost Savings
Hospital palliative care teams are associated with significant hospital cost savings that result from aligning care with patient priorities, leading, in turn, to reduced nonbeneficial hospital imaging, medications, procedures, and length of stay.15 See the table16,17 for examples of cost and quality outcomes of specialist palliative care provision and evidence supporting each outcome.18-25
Multiple studies consistently demonstrate that inpatient palliative care teams reduce hospital costs.26 One randomized controlled trial investigating the impact of an inpatient palliative care service found that patients who received care from the palliative care team reported greater satisfaction with their care, had fewer intensive care unit admissions, had more advanced directives at hospital discharge, longer hospice length of stay, and lower total healthcare costs (a net difference of $6766 per patient).23
Research shows that the earlier palliative care is provided, the greater the impact on the subsequent course of care,27 suggesting that hospitalists who provide frontline palliative care interventions as early as possible in a seriously ill patient’s stay will be able to provide higher quality care with lower overall costs. Notably, the majority of research on cost savings associated with palliative care has focused on the impact of specialist palliative care teams, and further research is needed to understand the economic impact of primary palliative care provision.
Improving Satisfaction
Shifting to value-based payment means that the patient and family experience determine an increasingly large percentage of hospital and provider reimbursement. Palliative care approaches, such as family caregiver assessment and support, access to 24/7 assistance after discharge, and person-centered care by an interdisciplinary team, improve performance in all of these measures. Communication skills training improves patient satisfaction scores, and skilled discussions about achievable priorities for care are associated with better quality of life, reduced nonbeneficial and burdensome treatments, and an increase in goal-concordant care.19 Communication skills training has also been shown to reduce burnout and improve empathy among physicians.28,29
SKILLS TRAINING OPPORTUNITIES
Though more evidence is needed to understand the impact of primary palliative care provision by hospitalists, the strong evidence on the benefits of specialty palliative care suggests that the skilled provision of primary palliative care by hospitalists will result in higher quality, higher value care. A number of training options exist for midcareer hospital medicine clinicians, including both in-person and online training in communication and other palliative care skills.
- The Center to Advance Palliative Care (CAPC) is a membership organization that offers online continuing education unit and continuing medical education courses on communication skills, pain and symptom management, caregiver support, and care coordination. CAPC also offers courses on palliative interventions for patients with dementia, COPD, and heart failure.
- SHM is actively invested in engaging hospitalists in palliative care skills training. SHM provides free toolkits on a variety of topics within the palliative care domain, including pain management, postacute care transitions, and opioid safety. The recently released Serious Illness Communication toolkit offers background on the role of hospitalists in palliative care provision, a pathway for fitting goals-of-care conversations into hospitalist workflow and recommended metrics and training resources. SHM also uses a mentored implementation model in which expert physicians mentor hospital team members on best practices in palliative care. SHM’s Palliative Care Task Force seeks to identify educational activities for hospitalists and create opportunities to integrate palliative care in hospital medicine.30
- The Serious Illness Care Program at Ariadne Labs in Boston aims to facilitate conversations between clinicians and seriously ill patients through its Serious Illness Conversation Guide, combined with technical assistance on workflow redesign to help clinicians conduct and document serious illness conversations.
- VitalTalk specializes in clinical communication education. Through online and in-person train-the-trainer programs, VitalTalk equips clinicians to lead communication training programs at their home institutions.
- The Education in Palliative and End-of-Life Care Program and End-of-Life Nursing Education Consortium (ELNEC) uses a train-the-trainer approach to educate providers in palliative care clinical competencies and increase the reach of primary palliative care provision. ELNEC workshops are complemented by a curriculum of online clinical training modules.
CULTURE CHANGE
Though palliative care skills training is a necessary first step, hospitalists also cite lack of time, difficulty finding records of previous patient discussions, and frequent handoffs as among the barriers to integrating palliative care into their practice.10 Studies examining the process of palliative care and hospital culture change have found that barriers to palliative care integration include a culture of aggressive care in EDs, lack of standardized patient identification criteria, and limited knowledge about and staffing for palliative care.31 These data indicate the need for system changes that enable hospitalists to operationalize palliative care principles.
Health systems must implement systems and processes that routinize palliative care, making it part of the mainstream course of care for seriously ill patients and their caregivers. This includes developing systems for the identification of patients with palliative care needs, embedding palliative care assessment and referral into clinical workflows, and enabling standardized palliative care documentation in electronic medical records. While palliative care skills training is essential, investment in systems change is no less critical to embedding palliative care practices in clinical norms across specialties.
CONCLUSION
Hospitalists can use a palliative approach to improve care quality and quality of life for seriously ill patients while helping to avoid preventable and unnecessary 911 calls, ED visits, and hospitalizations. The shift towards value-based payment is a strong incentive for hospitals and hospitalists to direct resources toward practices that improve the quality of life and care for the highest-need patients and their families. When equipped with the tools they need to provide palliative care, either themselves or in collaboration with palliative care teams, hospitalists have the opportunity to profoundly redirect the experience of care for seriously ill patients and their families.
Disclosure
The authors declared no conflicts of interest.
1. Casarett D, Pickard A, Bailey FA, et al. Do Palliative Consultations Improve Patient Outcomes? J Am Geriatr Soc. 2008;56(4):595-599. PubMed
2. Delgado-Guay MO, Parsons HA, Zhijun LM, Palmer LJ, Bruera E. Symptom distress, interventions, and outcomes of intensive care unit cancer patients referred to a palliative care consult team. Cancer. 2009;115(2):437-445. PubMed
3. Gelfman LP, Meier DE, Morrison SR. Does Palliative Care Improve Quality? A Survey of Bereaved Family Members. J Pain Symptom Manage. 2008;36(1):22-28. PubMed
4. Morrison SR, Dietrich J, Ladwig S, et al. Palliative Care Consultation Teams Cut Hospital Costs for Medicaid Beneficiaries. Health Aff. 2011;30:454-463. PubMed
5. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363(8):733-742. PubMed
6. Lin RJ, Adelman RD, Diamond RR, Evans AT. The Sentinel Hospitalization and the Role of Palliative Care. J Hosp Med. 2014;9(5):320-323. PubMed
7. Palliative care. J Hosp Med. 2006;1:80-81. doi:10.1002/jhm.54.
8. Center to Advance Palliative Care. National palliative care registry. https://registry.capc.org/. Accessed May 26, 2017.
9. Rogers M, Dumanovsky T. How We Work: Trends and Insights in Hospital Palliative Care. New York: The Center to Advance Palliative Care and the National Palliative Care Research Center. https://registry.capc.org/wp-content/uploads/2017/02/How-We-Work-Trends-and-Insights-in-Hospital-Palliative-Care-2009-2015.pdf; 2017. Accessed May 26, 2017.
10. Rosenberg LB, Greenwald J, Caponi B, et al. Confidence with and Barriers to Serious Illness Communication: A National Survey of Hospitalists. J Palliat Med. 2017;20(9):1013-1019. PubMed
11. Aldridge MD, Kelley AS. The Myth Regarding the High Cost of End-of-Life Care. Am J Public Health. 2015;105(12):2411-2415. PubMed
12. Enguidanos S, Vesper E, Lorenz K. 30-Day Readmissions among Seriously Ill Older Adults. J Palliat Med. 2012;15(12):1356-1361. PubMed
13. Kripalani S, Theobald C, Anctil B, Vasilevskis E. Reducing Hospital Readmission Rates: Current Strategies and Future Directions. Annu Rev Med. 2014;65:471-485. PubMed
14. Senot C, Chandrasekaran A. What Has the Biggest Impact on Hospital Readmission Rates. Harvard Business Review. September 23, 2015. https://hbr.org/2015/09/what-has-the-biggest-impact-on-hospital-readmission-rates. Accessed March 29, 2017.
15. Morrison RS, Penrod JD, Cassel JB, et al. Cost Savings Associated with US Hospital Palliative Care Consultation Programs. Arch Intern Med. 2008;168(16):1783-1790. PubMed
16. Cassel JB. Palliative Care’s Impact on Utilization and Costs: Implications for Health Services Research and Policy. In: Kelley AS, Meier DE, editors. Meeting the Needs of Older Adults with Serious Illness: Challenges and Opportunities in the Age of Health Care Reform. New York: Springer Science+Business Media; 2014:109-126.
17. Meier D, Silvers A. Serious Illness Strategies for Health Plans and Accountable Care Organizations. https://media.capc.org/filer_public/2c/69/2c69a0f0-c90f-43ac-893e-e90cd0438482/serious_illness_strategies_web.pdf. 2017. Accessed August 10, 2017.
18. Casarett D, Johnson M, Smith D, Richardson D. The Optimal Delivery of Palliative Care: A National Comparison of the Outcomes of Consultation Teams vs Inpatient Units. Arch Intern Med. 2011;171(7):649-655. PubMed
19. Bernacki RE, Block SD. Communication About Serious Illness Care Goals: A Review and Synthesis of Best Practices. JAMA Intern Med. 2014;174(12):1994-2003. PubMed
20. Wright AA, Zhang B, Ray A, et al. Associations Between End-of-Life Discussions, Patient Mental Health, Medical Care Near Death, and Caregiver Bereavement Adjustment. JAMA. 2008;300(14):1665-1673. PubMed
21. May P, Garrido M, Cassel JB, et al. Cost Analysis of a Prospective Multi-Site Cohort Study of Palliative Care Consultation Teams for Adults with Advanced Cancer: Where Do Cost Savings Come From? Palliat Med. 2017;31(4):378-386. PubMed
22. Norton SA, Hogan LA, Holloway RG, et al. Proactive Palliative Care in the Medical Intensive Care Unit: Effects of Length of Stay for Selected High-Risk Patients. Crit Care Med. 2007;35(6):1530-1535. PubMed
23. Gade G, Venohr I, Conner D, et al. Impact of an Inpatient Palliative Care Team: A Randomized Controlled Trial. J Palliat Med. 2008;11(2):180-201. PubMed
24. Adelson K, Paris J, Horton J, et al. Standardized Criteria for Palliative Care Consultation on a Solid Tumor Oncology Service Reduces Downstream Health Care use. J Oncol Pract. 2017;13(5):e431-e440. PubMed
25. Lustbader D, Mitchell M, Carole R, et al. The Impact of a Home-Based Palliative Care Program in an Accountable Care Organization. J Palliat Med. 2017;20(1):23-28. PubMed
26. May P, Normand C, Morrison R. Economic Impact of Hospital Inpatient Palliative Care Consultation: Review of Current Evidence and Directions for Future Research. J Palliat Med. 2014;17(9):1054-1063. PubMed
27. May P, Garrido MM, Bassel JB, et al. Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients with Advanced Cancer: Earlier Consultation Is Associated with Larger Cost-Saving Effect. J Clin Oncol. 2015;33(25):2745-2752. PubMed
28. Boissy A, Windover A, Bokar D, et al. Communication Skills Training for Physicians Improves Patient Satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
29. Kirkland KB. Finding Joy in Practice Cocreation in Palliative Care. JAMA. 2017;317(20):2065-2066. PubMed
30. Whelan C. SHM Establishes Palliative Care Task Force. The Hospitalist. 2005;11. http://www.the-hospitalist.org/hospitalist/article/123027/hospice-palliative-medicine/shm-establishes-palliative-care-task-force. Accessed July 31, 2017.
31. Grudzen CR, Richardson LD, Major-Monfried H, et al. Hospital Administrators’ Views on Barriers and Opportunities to Delivering Palliative Care in the Emergency Department. Ann Emerg Med. 2013;61(6):654-660. PubMed
1. Casarett D, Pickard A, Bailey FA, et al. Do Palliative Consultations Improve Patient Outcomes? J Am Geriatr Soc. 2008;56(4):595-599. PubMed
2. Delgado-Guay MO, Parsons HA, Zhijun LM, Palmer LJ, Bruera E. Symptom distress, interventions, and outcomes of intensive care unit cancer patients referred to a palliative care consult team. Cancer. 2009;115(2):437-445. PubMed
3. Gelfman LP, Meier DE, Morrison SR. Does Palliative Care Improve Quality? A Survey of Bereaved Family Members. J Pain Symptom Manage. 2008;36(1):22-28. PubMed
4. Morrison SR, Dietrich J, Ladwig S, et al. Palliative Care Consultation Teams Cut Hospital Costs for Medicaid Beneficiaries. Health Aff. 2011;30:454-463. PubMed
5. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363(8):733-742. PubMed
6. Lin RJ, Adelman RD, Diamond RR, Evans AT. The Sentinel Hospitalization and the Role of Palliative Care. J Hosp Med. 2014;9(5):320-323. PubMed
7. Palliative care. J Hosp Med. 2006;1:80-81. doi:10.1002/jhm.54.
8. Center to Advance Palliative Care. National palliative care registry. https://registry.capc.org/. Accessed May 26, 2017.
9. Rogers M, Dumanovsky T. How We Work: Trends and Insights in Hospital Palliative Care. New York: The Center to Advance Palliative Care and the National Palliative Care Research Center. https://registry.capc.org/wp-content/uploads/2017/02/How-We-Work-Trends-and-Insights-in-Hospital-Palliative-Care-2009-2015.pdf; 2017. Accessed May 26, 2017.
10. Rosenberg LB, Greenwald J, Caponi B, et al. Confidence with and Barriers to Serious Illness Communication: A National Survey of Hospitalists. J Palliat Med. 2017;20(9):1013-1019. PubMed
11. Aldridge MD, Kelley AS. The Myth Regarding the High Cost of End-of-Life Care. Am J Public Health. 2015;105(12):2411-2415. PubMed
12. Enguidanos S, Vesper E, Lorenz K. 30-Day Readmissions among Seriously Ill Older Adults. J Palliat Med. 2012;15(12):1356-1361. PubMed
13. Kripalani S, Theobald C, Anctil B, Vasilevskis E. Reducing Hospital Readmission Rates: Current Strategies and Future Directions. Annu Rev Med. 2014;65:471-485. PubMed
14. Senot C, Chandrasekaran A. What Has the Biggest Impact on Hospital Readmission Rates. Harvard Business Review. September 23, 2015. https://hbr.org/2015/09/what-has-the-biggest-impact-on-hospital-readmission-rates. Accessed March 29, 2017.
15. Morrison RS, Penrod JD, Cassel JB, et al. Cost Savings Associated with US Hospital Palliative Care Consultation Programs. Arch Intern Med. 2008;168(16):1783-1790. PubMed
16. Cassel JB. Palliative Care’s Impact on Utilization and Costs: Implications for Health Services Research and Policy. In: Kelley AS, Meier DE, editors. Meeting the Needs of Older Adults with Serious Illness: Challenges and Opportunities in the Age of Health Care Reform. New York: Springer Science+Business Media; 2014:109-126.
17. Meier D, Silvers A. Serious Illness Strategies for Health Plans and Accountable Care Organizations. https://media.capc.org/filer_public/2c/69/2c69a0f0-c90f-43ac-893e-e90cd0438482/serious_illness_strategies_web.pdf. 2017. Accessed August 10, 2017.
18. Casarett D, Johnson M, Smith D, Richardson D. The Optimal Delivery of Palliative Care: A National Comparison of the Outcomes of Consultation Teams vs Inpatient Units. Arch Intern Med. 2011;171(7):649-655. PubMed
19. Bernacki RE, Block SD. Communication About Serious Illness Care Goals: A Review and Synthesis of Best Practices. JAMA Intern Med. 2014;174(12):1994-2003. PubMed
20. Wright AA, Zhang B, Ray A, et al. Associations Between End-of-Life Discussions, Patient Mental Health, Medical Care Near Death, and Caregiver Bereavement Adjustment. JAMA. 2008;300(14):1665-1673. PubMed
21. May P, Garrido M, Cassel JB, et al. Cost Analysis of a Prospective Multi-Site Cohort Study of Palliative Care Consultation Teams for Adults with Advanced Cancer: Where Do Cost Savings Come From? Palliat Med. 2017;31(4):378-386. PubMed
22. Norton SA, Hogan LA, Holloway RG, et al. Proactive Palliative Care in the Medical Intensive Care Unit: Effects of Length of Stay for Selected High-Risk Patients. Crit Care Med. 2007;35(6):1530-1535. PubMed
23. Gade G, Venohr I, Conner D, et al. Impact of an Inpatient Palliative Care Team: A Randomized Controlled Trial. J Palliat Med. 2008;11(2):180-201. PubMed
24. Adelson K, Paris J, Horton J, et al. Standardized Criteria for Palliative Care Consultation on a Solid Tumor Oncology Service Reduces Downstream Health Care use. J Oncol Pract. 2017;13(5):e431-e440. PubMed
25. Lustbader D, Mitchell M, Carole R, et al. The Impact of a Home-Based Palliative Care Program in an Accountable Care Organization. J Palliat Med. 2017;20(1):23-28. PubMed
26. May P, Normand C, Morrison R. Economic Impact of Hospital Inpatient Palliative Care Consultation: Review of Current Evidence and Directions for Future Research. J Palliat Med. 2014;17(9):1054-1063. PubMed
27. May P, Garrido MM, Bassel JB, et al. Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients with Advanced Cancer: Earlier Consultation Is Associated with Larger Cost-Saving Effect. J Clin Oncol. 2015;33(25):2745-2752. PubMed
28. Boissy A, Windover A, Bokar D, et al. Communication Skills Training for Physicians Improves Patient Satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
29. Kirkland KB. Finding Joy in Practice Cocreation in Palliative Care. JAMA. 2017;317(20):2065-2066. PubMed
30. Whelan C. SHM Establishes Palliative Care Task Force. The Hospitalist. 2005;11. http://www.the-hospitalist.org/hospitalist/article/123027/hospice-palliative-medicine/shm-establishes-palliative-care-task-force. Accessed July 31, 2017.
31. Grudzen CR, Richardson LD, Major-Monfried H, et al. Hospital Administrators’ Views on Barriers and Opportunities to Delivering Palliative Care in the Emergency Department. Ann Emerg Med. 2013;61(6):654-660. PubMed
© 2018 Society of Hospital Medicine
Caring Wisely: A Program to Support Frontline Clinicians and Staff in Improving Healthcare Delivery and Reducing Costs
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
© 2017 Society of Hospital Medicine
Strategies are needed to empower frontline clinicians to work with organizational leadership to reduce healthcare costs and improve high-value care. Caring Wisely® is a program developed by the University of California, San Francisco’s (UCSF) Center for Healthcare Value (CHV), aimed at engaging frontline clinicians and staff, connecting them with implementation experts, and supporting the development of targeted interventions to improve value. Financial savings from the program more than cover program costs. Caring Wisely® provides an institutional model for implementing robust interventions to address areas of low-value care.
Launched in 2013, the annual Caring Wisely® program consists of 3 stages for identifying projects that meet the following criteria:
- Potential to measurably reduce UCSF Health’s costs of care without transferring costs to patients, insurers, or other providers
- Plan for ensuring that health outcomes are maintained or improved
- Envision disseminating the intervention within and beyond UCSF
- Demonstrate commitment and engagement of clinical leadership and frontline staff.
The first stage is the Ideas Contest, a UCSF Health-wide call (to learn more about UCSF Health: https://www.ucsf.edu/sites/default/files/052516_About_UCSF.pdf) to identify areas that may be targeted to reduce unnecessary services, inefficiencies, and healthcare costs. We use a crowdsourcing platform—Open Proposals—to solicit the best ideas from frontline clinicians and staff.1 Open Proposals is a secure, web-based platform for transparent and collaborative proposal development that displays threads of comments, responses, and revisions, and allows submissions to be “liked.” Open Proposals is managed by the UCSF Clinical and Translational Science Institute, funded by the National Center for Advancing Translational Sciences (Grant Number UL1 TR000004) at the National Institutes of Health. Using institutional e-mail lists for faculty, staff and residents, as well as described at monthly managers and directors meetings, the Ideas Contest is announced each year by the Chief Medical Officer and the CHV leadership. The Caring Wisely® Executive Steering Committee, which consists of CHV and senior UCSF Health system leaders, selects the top 5-10 ideas based on the above criteria. Each winning idea receives a $100 gift certificate for a popular restaurant in San Francisco, and the list of winners is announced to the entire UCSF community.
The second stage is the Request for Proposals. The Caring Wisely® program solicits proposals that outline implementation plans to target specific areas identified through the Ideas Contest. Finalists from the Ideas Contest are encouraged to submit proposals that address the problem they identified, but anyone affiliated with UCSF Health may submit a proposal on a winning idea. There is an approximately 4-week open submission period during which applicants submit brief 2-page proposals on the Open Proposal platform. This is followed by a period of optimization that leverages the social media aspect of the Open Proposals platform in which the UCSF Health community asks clarifying questions, make suggestions, and modifications can be made to the proposals. All submissions receive written feedback from at least one Steering Committee member. In addition, the Caring Wisely® Director directly invites relevant UCSF colleagues, administrators, or program leaders to comment on proposals and make suggestions for improvement. Plans for assessing financial and health care delivery impacts are developed in collaboration with the UCSF Health Finance department. UCSF Health managers and leaders who are stakeholders in project proposal areas are consulted to provide input and finalize proposal plans, including the identification of existing personnel who can support and drive the project forward. Proposers use this feedback to revise their applications throughout this stage.
The third stage is Project Implementation. The Caring Wisely® Executive Steering Committee selects up to 3 winners from the submitted proposals. Using the program criteria above, each project is scored independently, discussed in committee, and rescored to identify the top proposals. Each selected project receives a maximum budget of $50,000 that can be used for project materials, activities, and salary support for project leaders or staff. In addition to funding, each project team receives input from the implementation science team to co-develop and implement the intervention with a goal of creating a first-test-of-change within 3-6 months. A key feature of Caring Wisely® is the partnership between project teams and the Caring Wisely® implementation team, which includes a director, program manager, data analysts, and implementation scientists (Table 1).
The $150,000 administrative budget for the Caring Wisely® program provides 20% support of the medical director, 50% support of a program manager/analyst, and 10% support of an implementation scientist. Approximately 5% support is donated from additional senior implementation scientists and various UCSF Health experts based on project needs. To make most efficient use of the Caring Wisely® program staff time with the project teams, there is a weekly 60-90 minute works-in-progress session attended by all 3 teams with a rotating schedule for lead presenter during the first 6 months; these meetings occur every 2-3 weeks during the second 6 months. Caring Wisely® program staff and the implementation scientist are also available for 1:1 meetings as needed. The Caring Wisely® Executive Steering Committee is not paid and meets for 90 minutes quarterly. Custom reports and modifications of the electronic health record are provided by the UCSF Health clinical informatics department as part of their operating budget.
The collaboration between the project teams and the implementation science team is guided by the Consolidated Framework for Implementation Research (CFIR)2 and PRECEDE-PROCEED model—a logic model and evaluation tool that is based on a composite of individual behavior change theory and social ecology.3 Table 2 illustrates how we weave PRECEDE-PROCEED and Plan-Do-Study-Act frameworks into project design and strategy. Each funded team is required to submit an end-of-year progress report.
Cost and cost savings estimates were based on administrative financial data obtained through the assistance of the Decision Support Services unit of the Finance Department of UCSF Health. All costs reflect direct institutional costs, rather than charges. For some projects, costs are directly available through computerized dashboards that provide year-to-year comparisons of specific costs of materials, supplies, and services (eg, blood transfusion reduction, surgical supplies project, OR efficiency program). This same dashboard also allows calculation of CMI-adjusted direct costs of hospital care by service line, as used in the perioperative pathways program evaluation. In other cases, the Decision Support Services and/or Caring Wisely® program manager created custom cost reports based on the key performance indicator (eg, nebulizer therapy costs consist of medication costs plus respiratory therapist time; CT scan utilization for suspected pulmonary embolus in emergency department; and antimicrobial utilization for suspected neonatal sepsis).
Ongoing monitoring and sustainability of Caring Wisely® projects is supported by the Caring Wisely® program leaders. Monitoring of ongoing cost savings is based on automated service-line level dashboards related to cost, utilization, and quality outcomes with quarterly updates provided to the Caring Wisely® Steering Committee. Depending on the project or program, appropriate UCSF Health senior leaders determine the level of support within their departments that is required to sustain the program(s). Ongoing monitoring of each program is also included in the strategic deployment visibility room with regular rounding by senior health system executives.
Since 2013, there have been 3 complete Caring Wisely® cycles. The Ideas Contest generated more than 75 ideas in each of the past 3 cycles, ranging from eliminating redundant laboratory or radiological studies to reducing linen and food waste. We received between 13-20 full proposals in each of the request for proposal stages, and 9 projects have been implemented, 3 in each year. Funded projects have been led by a variety of individuals including physicians, nurses, pharmacists, administrators and residents, and topics have ranged from reducing overutilization of tests, supplies and treatments, to improving patient throughput during the perioperative period (Table 3). Estimated cumulative savings to date from Caring Wisely® projects has exceeded $4 million, based on the four projects shown in Table 4. The IV-to-PO switch program and the neonatal sepsis risk prediction project (Table 3) have been successful in reducing unnecessary utilization, but cost and savings estimates are not yet finalized. Three funded projects were equivocal in cost savings but were successful in their primary aims: (1) increasing the appropriateness of CT scan ordering for suspected pulmonary embolus; (2) shortening operating room turnover times; and (3) implementing a postoperative debrief program for the systematic documentation of safety events, waste, and inefficiencies related to surgery.
We developed an innovative program that reduces hospital costs through crowdsourcing of ideas from frontline clinicians and staff, and by connecting these ideas to project and implementation science teams. At a time when healthcare costs have reached unsustainable levels, the Caring Wisely® program provides a process for healthcare personnel to make a positive impact on healthcare costs in areas under their direct control. Through the Open Proposals platform, we have tapped a growing desire among frontline providers to reduce medical waste.
A key criterion for the Caring Wisely® program is to propose changes that reduce cost without adversely affect healthcare quality or outcomes. While this is an important consideration in selecting projects, there is limited power to detect many of the most clinically relevant outcomes. We find this acceptable because many of the sponsored Caring Wisely® project goals were to increase compliance with evidence-based practice guidelines and reduce harms associated with unnecessary treatments (eg, blood transfusion, nebulizer therapy, CT scan, antimicrobial therapy). Selected balancing metrics for each project are reported by established quality and safety programs at UCSF Health, but we acknowledge that many factors that can affect these clinical outcomes are not related to the cost-reduction intervention and are not possible to control outside of a clinical research study. Therefore, any response to changes in these outcome and balancing measures requires further analysis beyond the Caring Wisely® project alone.
We believe one of the key factors in the success of the Caring Wisely® program is the application of implementation science principles to the intervention design strategies (Table 1). These principles included stakeholder engagement, behavior change theory, market (target audience) segmentation, and process measurement and feedback. Because we are conducting this program in an academic health center, resident and fellow education and engagement are also critical to success. In each project, we utilize the PRECEDE model as a guide to ensure that each intervention design includes complementary elements of effective behavior change, intended to increase awareness and motivation to change, to make change “easy,” and to reinforce change(Table 2).3
The Caring Wisely® program—itself a multifaceted intervention—embodies the same PRECEDE dimensions we apply to each specific project. The Ideas Contest serves as a tool for increasing awareness, attitudes, and motivation across the clinical enterprise for reducing healthcare costs. The support provided to the project teams by the Caring Wisely® program is an enabling factor that makes it “easier” for frontline teams to design and implement interventions with a greater likelihood of achieving early success. Timely measurement and feedback of results to the hospital leadership and broadcasting to the larger community reinforces the support of the program at both the leadership and frontline levels.
Collaboration between project teams and the Caring Wisely® program also provides frontline clinicians and staff with practical experience and lessons that they can apply to future improvement work. Project teams learn implementation science principles such as constructing a pragmatic theoretical framework to guide implementation design using CFIR model.2 Incorporating multiple, rapid-cycle tests of change allows teams to modify and adapt final interventions as they learn how the target audience and environment responds to specific intervention components. Access to real-time, actionable data and a data analyst is essential to rapid cycle adaptation that allows teams to focus on specific units or providers. We also find that cross-fertilization between project teams working in different areas helps to share resources and minimize duplication of efforts from the clinical and staff champions. Partnering with UCSF Health system leaders at every phase of project development—from proposal selection, development, and final evaluation of results—enhances sustainable transition of successful projects into clinical operations.
The costs and coordination for the first cycle of Caring Wisely® were supported by the UCSF Center for Healthcare Value. Upon completion of the evaluation of the first cycle, UCSF Health agreed to fund the program going forward, with the expectation that Caring Wisely would continue to achieve direct cost-savings for the organization. The Caring Wisely team provides a final report each year detailing the impact of each project on utilization and associated costs. Currently, program costs are approximately $150,000 for the Caring Wisely program leaders, staff, and other resources, and $50,000 for each of 3 projects for a total program cost of $300,000 per year. Projects included in the first three cycles have already saved more than $4 million, representing a strong return on investment. This program could be a model for other academic health centers to engage frontline clinicians and staff in addressing healthcare costs, and lends itself to being scaled-up into a multi-system collaborative.
LIST OF ABBREVIATIONS
UCSF—University of California, San Francisco; PRECEDE—Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation; PROCEED—Policy, Regulatory and Organizational Constructs in Educational and Environmental Development
Acknowledgments
Other participants in blood transfusion reduction project (D. Johnson, K. Curcione); IV-to-PO Switch (C. Tsourounis, A. Pollock); Surgical Supply Cost Reduction (C. Zygourakis); Perioperative Efficiency (L. Hampson); CT for PE Risk Prediction (E. Weber); ERAS Pathways (L. Chen); Neonatal Sepsis Risk Prediction (T. Newman); Post-Operative Debrief (S. Imershein). Caring Wisely Executive Steering Committee (J. Adler, S. Antrum, A Auerbach, J. Bennan, M. Blum, C. Ritchie, C. Tsourounis). This Center for Healthcare Value is funded in part by a grant from the Grove Foundation. We appreciate additional review and comments to the manuscript provided by George Sawaya and Adams Dudley.
Disclosures
Christopher Moriates has accepted royalties from McGraw-Hill for textbook, Understanding Value-Based Healthcare. Alvin Rajkomar has received fees as a research adviser from Google, Inc.
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
1. Kahlon M, Yuan L, Gologorskaya O, Johnston SC. Crowdsourcing the CTSA innovation mission. Clin Transl Sci. 2014;7:89-92. PubMed
2. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. PubMed
3. Green LW and Kreuter. Health Program Planning: An Educational and Ecological Approach. 4th Ed. McGraw-Hill. New York, NY. 2005.
4. Zygourakis CC, Valencia V, Moriates C et al. Association between surgeon scorecard use and operating room costs. JAMA Surg. 2016 Dec 7. doi: 10.1001/jamasurg.2016.4674. [Epub ahead of print] PubMed
AcademyHealth’s Delivery System Science Fellowship: training embedded researchers to design, implement, and evaluate new models of care
For over 3 decades, AcademyHealth and its predecessor organizations and members have been studying how the healthcare system works and ways to improve health outcomes. The value of health services research (HSR) training programs that emphasize learning opportunities in delivery system settings was initially articulated at the 2009 AcademyHealth Summit on the Future of HSR Data and Methods.1 Two years later, the need for such programs was reiterated as a priority for AcademyHealth’s HSR Learning Consortium in their strategic plan.2 While HSR methods have become increasingly sophisticated, historical approaches largely relied on extant (usually academic-based) researchers.
To realize the goal of building learning health systems (considered here to be entities where applied, operationally relevant research is systematically designed, generated, and translated into high-quality care delivery), many healthcare organizations have begun to use researchers as an internal resource to inform and support higher quality and more efficient care delivery operations. However, there is a current dearth of scientists trained in research disciplines (eg, comparative effectiveness research, patient-centered outcomes research, implementation science) more directly applicable to operational settings.3,4 Conducting research within these “real-world” environments is challenging for a variety of well-documented reasons,5,6 and many important questions cannot be answered using traditional study designs and/or methodologies. Until more researchers are trained in research approaches that align better with care delivery needs, the field will continue to fall short of addressing topics identified by the National Academy of Medicine (NAM; formerly the Institute of Medicine) as priorities for real system improvement.
While researchers in academic and consulting settings play critical roles in knowledge generation, a substantial area for expansion is support for “embedded researchers” who work more directly with operational leaders and understand local context, data, and organizational-level goals of delivery systems. AcademyHealth’s Delivery System Science Fellowship (DSSF) was developed to forge a stronger link between rigorous research practice and pragmatic aspects of care delivery.
Since inception of the DSSF, national attention has further emphasized the need for specialized, experiential learning with specific competencies that extend formal HSR training. The Agency for Healthcare Research and Quality (AHRQ) is currently convening a Technical Expert Panel to guide Training the Next Generation of Learning Health System Researchers as part of its larger effort to provide support for evidence generation and uptake in these applied settings (see http://www.ahrq.gov/news/blog/ahrqviews/supporting-learning-health-systems.html for more information). This work is reinforced by an ongoing project at AcademyHealth focused on Understanding the Current Health Services Research Workforce and Maximizing Its Future,7 partially sponsored by AHRQ. These efforts aim to inform AHRQ-funded career and training program requirements in order to build a workforce in partnership with academia and delivery organizations in support of developing high-functioning researchers directly positioned to drive progress toward learning healthcare systems.
ACADEMYHEALTH’S DSSF
The AcademyHealth DSSF Program is a pioneering effort designed to meet the needs of learning healthcare systems for a human capital resource capable of generating insights from operational data and deploying this knowledge effectively. Established in 2012 in partnership with 3 initial host sites, the DSSF provides a paid, postdoctoral training opportunity to help highly qualified, early-career researchers gain applied experience in delivery system settings.8 The goal is to provide hands-on experience and professional leadership opportunities to enhance the array of skills needed to generate and apply evidence in delivery systems. The long-term program objective is for graduates to employ the methods and training garnered during the DSSF to produce new, practical insights required to transform healthcare delivery and achieve the “Triple Aim.”9
Sixteen delivery systems have participated in the DSSF program over the course of 5 years. These organizations represent a diverse group of innovative, high-performing systems that serve populations across the rural-urban continuum. Host site preceptors are nationally recognized experts in areas such as health economics, comparative effectiveness, pragmatic trials, clinical decision support, and implementation science. The fellowship is guided by an advisory committee that provides strategic direction and plays a key role in selecting fellows. AcademyHealth partners with 8 to 10 health systems annually, accepting new partnerships as interest and availability allows. The Figure summarizes program inputs, elements, antecedents, outputs, and intended outcomes. As a relatively new and evolving program, the DSSF is using a disciplined approach to assess both mid- and long-term outcomes. The Table is a comparative matrix that depicts the range in areas of investment offered by a sample of host sites during the fellowship program. Complementing development of the common core competencies and exposures presented in the Table, specific project choice at a host site is mutually determined according to system needs and the fellow’s interests.
To apply for DSSF, researchers must hold a doctoral degree in any relevant aspect of clinical medicine, HSR, or a related field. The review committee evaluates applicants based on their qualifications, a clear statement of professional goals, appropriateness for placement, the quality of a writing sample, and letters of recommendation. Host sites select a fellow based on their individual preferences and recommendations from the review committee. The minimum duration of the fellowship is 1 year, during which the fellow works full time at the host site. Host sites provide the fellows’ salary and benefits, financial support to attend AcademyHealth’s Annual Research Meeting, and mentorship and/or training. To ensure general continuity across sites, each fellow’s work focuses on “delivery system science (DSS),” with a significant part of the work intended for public dissemination (eg, a conference presentation) or publication in a peer-reviewed journal.
Host sites additionally provide AcademyHealth financial support to manage the process of recruiting promising candidates. AcademyHealth manages and convenes the advisory committee proceedings, facilitates the application cycle (including peer review of applicants), markets the fellowship, guides the interview and match process, and promotes placement of fellows upon program completion. AcademyHealth also convenes active and graduate fellows to foster engagement and professional development.
To date, 118 individuals have applied to the program and 25 fellows have been accepted. Nineteen have completed the fellowship, 2 are continuing as second-year fellows, and 4 started their fellowship in the fall of 2016. Fellows have a wide range of expertise in areas such as epidemiology, exercise physiology, health psychology, anthropology, clinical medicine, qualitative methods, organizational behavior, and systems engineering. Once individuals complete their fellowship, they become DSSF “alumni” and remain involved in program activities and as peer mentors.
EARLY EXPERIENCE WITH OUTCOMES
Program Level
AcademyHealth administers an annual evaluation to assess the program, understand impact on the fellow’s professional development and growth, track publications, and inform programmatic goals. To date, we have identified over 50 peer-reviewed publications resulting from work conducted through the fellowship (visit http://www.academyhealth.org/dssfpublications for a full listing of publications stemming from the DSSF program). As an example of continuous program improvement, this year staff implemented a fellow-led monthly call in response to requests to connect fellows. This has proven to be a useful response for fellows to understand how to enhance their own experience by learning from fellows in other systems to incorporate focused areas of development via cross-system sharing. As an indicator of continued value, of the 16 sites that have participated to date, 10 have participated in the DSSF for more than 1 cycle; 3 are new host sites currently participating in their first year of the program; and 3 host sites participated for 1 year. The 3 inaugural host sites that helped launch the DSSF continue to serve as host sites to date.
As a marker of longitudinal success, staff will continue to follow up with preceptors and fellows to understand fellows’ contributions to the host site and the field, as well as impact on the fellow career trajectory. Of the 19 fellow alumni to date, 8 have moved on to academic or research positions, and 11 have remained in care delivery systems to provide local expertise in study design, execution, and evidence uptake.
The program has also made some general contributions to advance the discipline of delivery system science, including:
1. Defining DSS and clarifying training needs for “embedded researchers” and health system analysts. To characterize the fellowship, AcademyHealth and the program advisory committee jointly developed the following definition for delivery system science (DSS):
“DSS includes research that seeks to understand how delivery systems operate, influence, change, and respond to external stimulus, among other topics. DSS may include efforts to examine how and under what circumstances interventions work and how delivery systems effectively implement evidence-based innovations. For the DSSF, DSS is conducted by researchers who are ‘embedded’ in delivery systems and respond to the decision-making needs of those systems.”
Additionally, closer connections with DSS leaders have led to a better understanding of challenges, opportunities, and needs of delivery systems.10,11
2. Cultivating a network of delivery systems and system leaders interested in expanding the cadre of embedded researchers, and trainees who intend to build careers in DSS. The DSSF also aims to enhance fellows’ skills and knowledge base, career opportunities, and professional network. To extend these relationships and support delivery system analytics, AcademyHealth worked with preceptors and fellows to inform creation of a new Community of Practice supported by AcademyHealth’s EDM Forum and guide planning for AcademyHealth’s Concordium conference to provide a national meeting to showcase DSS.
3. Strategic planning to ensure sustainable support for embedded research within delivery systems. Substantial interest in the program developed quickly, with rapid learning over the first few cycles to refine the program to meet host sites’ and fellows’ needs. Both efforts were critical to demonstrate that the DSSF fulfills an important need for our health system partners and members. As indicated previously, strong, sustained interest from prospective host sites and applicants demonstrates the program has created a win-win to jointly assess fit while building skills and supporting continuous learning.
Likewise, Lisa Simpson, President and CEO of AcademyHealth, and Lucy Savitz, DSSF host site preceptor at Intermountain Healthcare, participated in the Canadian Institutes of Health Research (CIHR) Invitational Workshop, “Modernizing Health Services and Policy Research Training in Canada” in March 2016. Shared learning largely informed by the DSSF led to CIHR creating a similar fellowship program with initial awards to be made in 2017 (see https://www.researchnet-recherchenet.ca/rnr16/vwOpprtntyDtls.do?prog=2540&view=browseActive&sponsor=CIHR-8&type=EXACT&resultCount=25 for more information). We are working to thread these efforts together in a way to leverage our learning community of government agencies, academia, and employers as a long-term funding stream for training in delivery science.
Participant Host Site Level
Two selected examples of how DSSF researchers have engaged high-priority topics that contributed to health system operations are provided here.
Kaiser Permanente Southern California: Assessing adherence with “Choosing Wisely” recommendations in oncology. In partnership with preceptor Dr. Michael Gould, 2013-2014 DSSF fellow Dr. Erin Hahn worked with the Kaiser Permanente Southern California (KPSC) Care Improvement Research Team to lead a project addressing several KPSC priority areas. Focusing on “Choosing Wisely” recommendations from the American Society of Clinical Oncology,12 the project evaluated appropriateness of imaging and laboratory services for early-stage cancer patients and survivors between and within 2 integrated health systems, Kaiser Permanente (KP) and Intermountain Healthcare.13,14 Results were presented to KP national leaders, including an external health policy advisory board. In close collaboration with clinical and operational leaders in medical oncology, this multiregional, multisystem project is contributing to targeted quality improvement efforts and improved healthcare value, including audit and feedback of nonrecommended labs.
Dr. Hahn subsequently received a KPSC Incubator Award, a competitive internal grant, to further study factors associated with use of nonrecommended surveillance lab tests for early-stage breast cancer patients. The study focused on medical oncologists within KPSC, categorizing them as high or low utilizers of the tests.15 Results indicate that high utilizers perceive that the tests help manage patient anxiety about recurrence, while acknowledging that the tests do not provide clinical utility. These findings are contributing to the development of targeted survivorship services across the organization.
Intermountain Healthcare: Formative evaluation of large-scale implementation of shared decision-making. Preceptor Dr. Lucy Savitz assembled a team to conduct a formative evaluation of Intermountain Healthcare’s efforts to implement shared decision-making (SDM) as part of its Center for Medicare and Medicaid Innovation Challenge Award. The 2015-2017 DSSF fellow at Intermountain Healthcare, Dr. Kim Brunisholz, served as a core member of the project team, focusing primarily on a mixed-methods evaluation of the SDM program.
Dr. Brunisholz engaged operational leads, clinical teams, patient and family advisory councils, and senior executives to conduct the program assessment. Results demonstrated significant variation in invited participation in SDM among eligible patient populations: preference sensitive conditions (1 in 30 patients), oncology-related diagnosis (1 in 3 patients), and chronic conditions (1 in 74 patients). Provisional analysis of patient-level clinical outcomes demonstrated that among those invited to the SDM program compared to those that were not, total joint replacement was decreased (10.1% vs 17.3%; P < 0.001) and a trend towards breast conservation emerged (61.8% vs 56.4%; P = 0.10). No difference in treatment choice for lower back pain was observed. Qualitative program analysis suggested need for improvements in the areas of (1) routine and continuous staff training, (2) workflow standardization, and (3) active data monitoring with meaningful, actionable feedback to caregivers. In response to these results, a chartered SDM Steering Committee was created (Dr. Brunisholz is a member of that group) to develop a strategic plan for SDM, with an accompanying organizational response to reimplement SDM in a targeted manner. Learning from this program is being leveraged to support a subproject analysis on a large scale using data from the High Value Healthcare Collaborative as part of an AHRQ-funded Center of Excellence award. (See https://www.ahrq.gov/news/newsroom/press-releases/2015/pcorawards.html. For more information on the High Value Healthcare Collaborative, please visit: https://www.highvaluehealthcare.org.)
CONCLUSION
Moving forward, the DSSF will continue working with progressive delivery systems. Partnerships between organizations that are interested in integrating rigorous research practice to drive continuous system improvement and maximize the value of care will have substantial need for technical skills and analytic capacity. They will also need to ensure that researchers working in their systems have sufficient understanding of cultural and political context within the organization to be effective leaders who can manage change.
AcademyHealth created the DSSF in response to the field’s request to build a research workforce that reflects the vision for a 21st Century Health System, as laid out by the NAM.16 We anticipate that as the US Department of Health & Human Services’ goals for payment reform and new measures to promote quality and high-value care are implemented, the DSSF trainees’ skill set will be increasingly valuable and will provide needed thought leadership on strategies to generate and apply evidence in practice.
Disclosure
Ms. Kanani received funding from Intermountain Healthcare, Kaiser Permanente Southern California for support for the Delivery System Science Fellowship. Drs. Hahn, Gould, and Brunisholz have no conflicts to disclose. Dr. Savitz has received funding from HRQ COE, PCORI LHSNet; received funding for lectures from the Institute from Healthcare Improvement, Department of Epidemiology, University of Utah; received funding for travel, accommodations, and meeting expenses from AHRQ NAC, EDM Forum, AH CAPP, AARP NPC, and PROM TEP; and received additional funding from Dartmouth University. Dr. Holve received funding from Intermountain Healthcare, Kaiser Permanente Southern California for support for the Delivery System Science Fellowship, provided by our delivery system partners, several of whom are coauthors on this manuscript.
1. AcademyHealth. Health Services Research in 2020: Summit on the Future of HSR Data and Methods. http://www.academyhealth.org/About/content.cfm?ItemNumber=2529. Accessed March 21, 2016.
2. AcademyHealth. Health Services Research (HSR) Learning Consortium Strategic Plan. http://www.academyhealth.org/files/ProfDev/Files/HSRstrategicplan2011FINAL.pdf. Accessed March 21, 2016.
3. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2009/ComparativeEffectivenessResearchPriorities/CER%20report%20brief%2008-13-09.pdf. Accessed March 21, 2016.
4. Bonham A, Rich E, Davis D, Longnecker D, Heinig S. Putting evidence to work: an expanded research agenda for academic medicine in the era of health care reform. Acad Med. 2010;85(10):1551-1553. PubMed
5. Zerhouni E. Translational and clinical science—time for a new vision. N Engl J Med. 2005;353(15):1621-1623. PubMed
6. AcademyHealth. “Getting Answers We Can Believe In: Methodological Considerations When Using Electronic Clinical Data for Research,” EDM Forum, December 2012.
7. Rich G, Collins A. Current and Future Demand for Health Services Researchers. Funded by the Agency for Healthcare Research and Quality (AHRQ). Presented at the AcademyHealth HSR Workforce Conference, Understanding the Current Health Services Research Workforce and Maximizing Its Future, funded by AHRQ, Patient-Centered Outcomes Research Institute, and the Robert Wood Johnson Foundation. October 2016.
8. AcademyHealth. Delivery System Science Fellowship. http://www.academyhealth.org/dssf. Accessed March 21, 2016.
9. Institute for Healthcare Improvement. IHI Triple Aim Initiative. http://www.ihi.org/engage/initiatives/tripleaim/Pages/default.aspx. Accessed March 21, 2016.
10. Psek W, Stametz R, Bailey-Davis L, et al. Operationalizing the learning health care system in an integrated delivery system. eGEMs. 2015;3(1):1122. PubMed
11. Thompson C, Kurian A, Luft H. Linking electronic health records to better understand breast cancer patient pathways within and between two health systems. eGEMs. 2015;3(1):1127. PubMed
12. Schnipper L, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30(14):1715-1724. PubMed
13. Hahn E, Tang T, Lee JS, et al. Use of posttreatment imaging and biomarkers in survivors of early‐stage breast cancer: Inappropriate surveillance or necessary care? Cancer. 2015;122(6):908-916. PubMed
14. Hahn E, Tang T, Lee JS, et al. Use of imaging for staging of early-stage breast cancer in two integrated health care systems: Adherence with a choosing wisely recommendation. J Oncol Pract. 2015;11(3):e320-e328. PubMed
15. Hahn EE, Munoz-Plaza C, Wang J, et al. Anxiety, culture, expectations: Oncologist-perceived factors associated with use of non-recommended serum tumor marker tests for surveillance of early stage breast cancer. J Oncol Pract. 2016;13(1):e77-e290. PubMed
16. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2000.
For over 3 decades, AcademyHealth and its predecessor organizations and members have been studying how the healthcare system works and ways to improve health outcomes. The value of health services research (HSR) training programs that emphasize learning opportunities in delivery system settings was initially articulated at the 2009 AcademyHealth Summit on the Future of HSR Data and Methods.1 Two years later, the need for such programs was reiterated as a priority for AcademyHealth’s HSR Learning Consortium in their strategic plan.2 While HSR methods have become increasingly sophisticated, historical approaches largely relied on extant (usually academic-based) researchers.
To realize the goal of building learning health systems (considered here to be entities where applied, operationally relevant research is systematically designed, generated, and translated into high-quality care delivery), many healthcare organizations have begun to use researchers as an internal resource to inform and support higher quality and more efficient care delivery operations. However, there is a current dearth of scientists trained in research disciplines (eg, comparative effectiveness research, patient-centered outcomes research, implementation science) more directly applicable to operational settings.3,4 Conducting research within these “real-world” environments is challenging for a variety of well-documented reasons,5,6 and many important questions cannot be answered using traditional study designs and/or methodologies. Until more researchers are trained in research approaches that align better with care delivery needs, the field will continue to fall short of addressing topics identified by the National Academy of Medicine (NAM; formerly the Institute of Medicine) as priorities for real system improvement.
While researchers in academic and consulting settings play critical roles in knowledge generation, a substantial area for expansion is support for “embedded researchers” who work more directly with operational leaders and understand local context, data, and organizational-level goals of delivery systems. AcademyHealth’s Delivery System Science Fellowship (DSSF) was developed to forge a stronger link between rigorous research practice and pragmatic aspects of care delivery.
Since inception of the DSSF, national attention has further emphasized the need for specialized, experiential learning with specific competencies that extend formal HSR training. The Agency for Healthcare Research and Quality (AHRQ) is currently convening a Technical Expert Panel to guide Training the Next Generation of Learning Health System Researchers as part of its larger effort to provide support for evidence generation and uptake in these applied settings (see http://www.ahrq.gov/news/blog/ahrqviews/supporting-learning-health-systems.html for more information). This work is reinforced by an ongoing project at AcademyHealth focused on Understanding the Current Health Services Research Workforce and Maximizing Its Future,7 partially sponsored by AHRQ. These efforts aim to inform AHRQ-funded career and training program requirements in order to build a workforce in partnership with academia and delivery organizations in support of developing high-functioning researchers directly positioned to drive progress toward learning healthcare systems.
ACADEMYHEALTH’S DSSF
The AcademyHealth DSSF Program is a pioneering effort designed to meet the needs of learning healthcare systems for a human capital resource capable of generating insights from operational data and deploying this knowledge effectively. Established in 2012 in partnership with 3 initial host sites, the DSSF provides a paid, postdoctoral training opportunity to help highly qualified, early-career researchers gain applied experience in delivery system settings.8 The goal is to provide hands-on experience and professional leadership opportunities to enhance the array of skills needed to generate and apply evidence in delivery systems. The long-term program objective is for graduates to employ the methods and training garnered during the DSSF to produce new, practical insights required to transform healthcare delivery and achieve the “Triple Aim.”9
Sixteen delivery systems have participated in the DSSF program over the course of 5 years. These organizations represent a diverse group of innovative, high-performing systems that serve populations across the rural-urban continuum. Host site preceptors are nationally recognized experts in areas such as health economics, comparative effectiveness, pragmatic trials, clinical decision support, and implementation science. The fellowship is guided by an advisory committee that provides strategic direction and plays a key role in selecting fellows. AcademyHealth partners with 8 to 10 health systems annually, accepting new partnerships as interest and availability allows. The Figure summarizes program inputs, elements, antecedents, outputs, and intended outcomes. As a relatively new and evolving program, the DSSF is using a disciplined approach to assess both mid- and long-term outcomes. The Table is a comparative matrix that depicts the range in areas of investment offered by a sample of host sites during the fellowship program. Complementing development of the common core competencies and exposures presented in the Table, specific project choice at a host site is mutually determined according to system needs and the fellow’s interests.
To apply for DSSF, researchers must hold a doctoral degree in any relevant aspect of clinical medicine, HSR, or a related field. The review committee evaluates applicants based on their qualifications, a clear statement of professional goals, appropriateness for placement, the quality of a writing sample, and letters of recommendation. Host sites select a fellow based on their individual preferences and recommendations from the review committee. The minimum duration of the fellowship is 1 year, during which the fellow works full time at the host site. Host sites provide the fellows’ salary and benefits, financial support to attend AcademyHealth’s Annual Research Meeting, and mentorship and/or training. To ensure general continuity across sites, each fellow’s work focuses on “delivery system science (DSS),” with a significant part of the work intended for public dissemination (eg, a conference presentation) or publication in a peer-reviewed journal.
Host sites additionally provide AcademyHealth financial support to manage the process of recruiting promising candidates. AcademyHealth manages and convenes the advisory committee proceedings, facilitates the application cycle (including peer review of applicants), markets the fellowship, guides the interview and match process, and promotes placement of fellows upon program completion. AcademyHealth also convenes active and graduate fellows to foster engagement and professional development.
To date, 118 individuals have applied to the program and 25 fellows have been accepted. Nineteen have completed the fellowship, 2 are continuing as second-year fellows, and 4 started their fellowship in the fall of 2016. Fellows have a wide range of expertise in areas such as epidemiology, exercise physiology, health psychology, anthropology, clinical medicine, qualitative methods, organizational behavior, and systems engineering. Once individuals complete their fellowship, they become DSSF “alumni” and remain involved in program activities and as peer mentors.
EARLY EXPERIENCE WITH OUTCOMES
Program Level
AcademyHealth administers an annual evaluation to assess the program, understand impact on the fellow’s professional development and growth, track publications, and inform programmatic goals. To date, we have identified over 50 peer-reviewed publications resulting from work conducted through the fellowship (visit http://www.academyhealth.org/dssfpublications for a full listing of publications stemming from the DSSF program). As an example of continuous program improvement, this year staff implemented a fellow-led monthly call in response to requests to connect fellows. This has proven to be a useful response for fellows to understand how to enhance their own experience by learning from fellows in other systems to incorporate focused areas of development via cross-system sharing. As an indicator of continued value, of the 16 sites that have participated to date, 10 have participated in the DSSF for more than 1 cycle; 3 are new host sites currently participating in their first year of the program; and 3 host sites participated for 1 year. The 3 inaugural host sites that helped launch the DSSF continue to serve as host sites to date.
As a marker of longitudinal success, staff will continue to follow up with preceptors and fellows to understand fellows’ contributions to the host site and the field, as well as impact on the fellow career trajectory. Of the 19 fellow alumni to date, 8 have moved on to academic or research positions, and 11 have remained in care delivery systems to provide local expertise in study design, execution, and evidence uptake.
The program has also made some general contributions to advance the discipline of delivery system science, including:
1. Defining DSS and clarifying training needs for “embedded researchers” and health system analysts. To characterize the fellowship, AcademyHealth and the program advisory committee jointly developed the following definition for delivery system science (DSS):
“DSS includes research that seeks to understand how delivery systems operate, influence, change, and respond to external stimulus, among other topics. DSS may include efforts to examine how and under what circumstances interventions work and how delivery systems effectively implement evidence-based innovations. For the DSSF, DSS is conducted by researchers who are ‘embedded’ in delivery systems and respond to the decision-making needs of those systems.”
Additionally, closer connections with DSS leaders have led to a better understanding of challenges, opportunities, and needs of delivery systems.10,11
2. Cultivating a network of delivery systems and system leaders interested in expanding the cadre of embedded researchers, and trainees who intend to build careers in DSS. The DSSF also aims to enhance fellows’ skills and knowledge base, career opportunities, and professional network. To extend these relationships and support delivery system analytics, AcademyHealth worked with preceptors and fellows to inform creation of a new Community of Practice supported by AcademyHealth’s EDM Forum and guide planning for AcademyHealth’s Concordium conference to provide a national meeting to showcase DSS.
3. Strategic planning to ensure sustainable support for embedded research within delivery systems. Substantial interest in the program developed quickly, with rapid learning over the first few cycles to refine the program to meet host sites’ and fellows’ needs. Both efforts were critical to demonstrate that the DSSF fulfills an important need for our health system partners and members. As indicated previously, strong, sustained interest from prospective host sites and applicants demonstrates the program has created a win-win to jointly assess fit while building skills and supporting continuous learning.
Likewise, Lisa Simpson, President and CEO of AcademyHealth, and Lucy Savitz, DSSF host site preceptor at Intermountain Healthcare, participated in the Canadian Institutes of Health Research (CIHR) Invitational Workshop, “Modernizing Health Services and Policy Research Training in Canada” in March 2016. Shared learning largely informed by the DSSF led to CIHR creating a similar fellowship program with initial awards to be made in 2017 (see https://www.researchnet-recherchenet.ca/rnr16/vwOpprtntyDtls.do?prog=2540&view=browseActive&sponsor=CIHR-8&type=EXACT&resultCount=25 for more information). We are working to thread these efforts together in a way to leverage our learning community of government agencies, academia, and employers as a long-term funding stream for training in delivery science.
Participant Host Site Level
Two selected examples of how DSSF researchers have engaged high-priority topics that contributed to health system operations are provided here.
Kaiser Permanente Southern California: Assessing adherence with “Choosing Wisely” recommendations in oncology. In partnership with preceptor Dr. Michael Gould, 2013-2014 DSSF fellow Dr. Erin Hahn worked with the Kaiser Permanente Southern California (KPSC) Care Improvement Research Team to lead a project addressing several KPSC priority areas. Focusing on “Choosing Wisely” recommendations from the American Society of Clinical Oncology,12 the project evaluated appropriateness of imaging and laboratory services for early-stage cancer patients and survivors between and within 2 integrated health systems, Kaiser Permanente (KP) and Intermountain Healthcare.13,14 Results were presented to KP national leaders, including an external health policy advisory board. In close collaboration with clinical and operational leaders in medical oncology, this multiregional, multisystem project is contributing to targeted quality improvement efforts and improved healthcare value, including audit and feedback of nonrecommended labs.
Dr. Hahn subsequently received a KPSC Incubator Award, a competitive internal grant, to further study factors associated with use of nonrecommended surveillance lab tests for early-stage breast cancer patients. The study focused on medical oncologists within KPSC, categorizing them as high or low utilizers of the tests.15 Results indicate that high utilizers perceive that the tests help manage patient anxiety about recurrence, while acknowledging that the tests do not provide clinical utility. These findings are contributing to the development of targeted survivorship services across the organization.
Intermountain Healthcare: Formative evaluation of large-scale implementation of shared decision-making. Preceptor Dr. Lucy Savitz assembled a team to conduct a formative evaluation of Intermountain Healthcare’s efforts to implement shared decision-making (SDM) as part of its Center for Medicare and Medicaid Innovation Challenge Award. The 2015-2017 DSSF fellow at Intermountain Healthcare, Dr. Kim Brunisholz, served as a core member of the project team, focusing primarily on a mixed-methods evaluation of the SDM program.
Dr. Brunisholz engaged operational leads, clinical teams, patient and family advisory councils, and senior executives to conduct the program assessment. Results demonstrated significant variation in invited participation in SDM among eligible patient populations: preference sensitive conditions (1 in 30 patients), oncology-related diagnosis (1 in 3 patients), and chronic conditions (1 in 74 patients). Provisional analysis of patient-level clinical outcomes demonstrated that among those invited to the SDM program compared to those that were not, total joint replacement was decreased (10.1% vs 17.3%; P < 0.001) and a trend towards breast conservation emerged (61.8% vs 56.4%; P = 0.10). No difference in treatment choice for lower back pain was observed. Qualitative program analysis suggested need for improvements in the areas of (1) routine and continuous staff training, (2) workflow standardization, and (3) active data monitoring with meaningful, actionable feedback to caregivers. In response to these results, a chartered SDM Steering Committee was created (Dr. Brunisholz is a member of that group) to develop a strategic plan for SDM, with an accompanying organizational response to reimplement SDM in a targeted manner. Learning from this program is being leveraged to support a subproject analysis on a large scale using data from the High Value Healthcare Collaborative as part of an AHRQ-funded Center of Excellence award. (See https://www.ahrq.gov/news/newsroom/press-releases/2015/pcorawards.html. For more information on the High Value Healthcare Collaborative, please visit: https://www.highvaluehealthcare.org.)
CONCLUSION
Moving forward, the DSSF will continue working with progressive delivery systems. Partnerships between organizations that are interested in integrating rigorous research practice to drive continuous system improvement and maximize the value of care will have substantial need for technical skills and analytic capacity. They will also need to ensure that researchers working in their systems have sufficient understanding of cultural and political context within the organization to be effective leaders who can manage change.
AcademyHealth created the DSSF in response to the field’s request to build a research workforce that reflects the vision for a 21st Century Health System, as laid out by the NAM.16 We anticipate that as the US Department of Health & Human Services’ goals for payment reform and new measures to promote quality and high-value care are implemented, the DSSF trainees’ skill set will be increasingly valuable and will provide needed thought leadership on strategies to generate and apply evidence in practice.
Disclosure
Ms. Kanani received funding from Intermountain Healthcare, Kaiser Permanente Southern California for support for the Delivery System Science Fellowship. Drs. Hahn, Gould, and Brunisholz have no conflicts to disclose. Dr. Savitz has received funding from HRQ COE, PCORI LHSNet; received funding for lectures from the Institute from Healthcare Improvement, Department of Epidemiology, University of Utah; received funding for travel, accommodations, and meeting expenses from AHRQ NAC, EDM Forum, AH CAPP, AARP NPC, and PROM TEP; and received additional funding from Dartmouth University. Dr. Holve received funding from Intermountain Healthcare, Kaiser Permanente Southern California for support for the Delivery System Science Fellowship, provided by our delivery system partners, several of whom are coauthors on this manuscript.
For over 3 decades, AcademyHealth and its predecessor organizations and members have been studying how the healthcare system works and ways to improve health outcomes. The value of health services research (HSR) training programs that emphasize learning opportunities in delivery system settings was initially articulated at the 2009 AcademyHealth Summit on the Future of HSR Data and Methods.1 Two years later, the need for such programs was reiterated as a priority for AcademyHealth’s HSR Learning Consortium in their strategic plan.2 While HSR methods have become increasingly sophisticated, historical approaches largely relied on extant (usually academic-based) researchers.
To realize the goal of building learning health systems (considered here to be entities where applied, operationally relevant research is systematically designed, generated, and translated into high-quality care delivery), many healthcare organizations have begun to use researchers as an internal resource to inform and support higher quality and more efficient care delivery operations. However, there is a current dearth of scientists trained in research disciplines (eg, comparative effectiveness research, patient-centered outcomes research, implementation science) more directly applicable to operational settings.3,4 Conducting research within these “real-world” environments is challenging for a variety of well-documented reasons,5,6 and many important questions cannot be answered using traditional study designs and/or methodologies. Until more researchers are trained in research approaches that align better with care delivery needs, the field will continue to fall short of addressing topics identified by the National Academy of Medicine (NAM; formerly the Institute of Medicine) as priorities for real system improvement.
While researchers in academic and consulting settings play critical roles in knowledge generation, a substantial area for expansion is support for “embedded researchers” who work more directly with operational leaders and understand local context, data, and organizational-level goals of delivery systems. AcademyHealth’s Delivery System Science Fellowship (DSSF) was developed to forge a stronger link between rigorous research practice and pragmatic aspects of care delivery.
Since inception of the DSSF, national attention has further emphasized the need for specialized, experiential learning with specific competencies that extend formal HSR training. The Agency for Healthcare Research and Quality (AHRQ) is currently convening a Technical Expert Panel to guide Training the Next Generation of Learning Health System Researchers as part of its larger effort to provide support for evidence generation and uptake in these applied settings (see http://www.ahrq.gov/news/blog/ahrqviews/supporting-learning-health-systems.html for more information). This work is reinforced by an ongoing project at AcademyHealth focused on Understanding the Current Health Services Research Workforce and Maximizing Its Future,7 partially sponsored by AHRQ. These efforts aim to inform AHRQ-funded career and training program requirements in order to build a workforce in partnership with academia and delivery organizations in support of developing high-functioning researchers directly positioned to drive progress toward learning healthcare systems.
ACADEMYHEALTH’S DSSF
The AcademyHealth DSSF Program is a pioneering effort designed to meet the needs of learning healthcare systems for a human capital resource capable of generating insights from operational data and deploying this knowledge effectively. Established in 2012 in partnership with 3 initial host sites, the DSSF provides a paid, postdoctoral training opportunity to help highly qualified, early-career researchers gain applied experience in delivery system settings.8 The goal is to provide hands-on experience and professional leadership opportunities to enhance the array of skills needed to generate and apply evidence in delivery systems. The long-term program objective is for graduates to employ the methods and training garnered during the DSSF to produce new, practical insights required to transform healthcare delivery and achieve the “Triple Aim.”9
Sixteen delivery systems have participated in the DSSF program over the course of 5 years. These organizations represent a diverse group of innovative, high-performing systems that serve populations across the rural-urban continuum. Host site preceptors are nationally recognized experts in areas such as health economics, comparative effectiveness, pragmatic trials, clinical decision support, and implementation science. The fellowship is guided by an advisory committee that provides strategic direction and plays a key role in selecting fellows. AcademyHealth partners with 8 to 10 health systems annually, accepting new partnerships as interest and availability allows. The Figure summarizes program inputs, elements, antecedents, outputs, and intended outcomes. As a relatively new and evolving program, the DSSF is using a disciplined approach to assess both mid- and long-term outcomes. The Table is a comparative matrix that depicts the range in areas of investment offered by a sample of host sites during the fellowship program. Complementing development of the common core competencies and exposures presented in the Table, specific project choice at a host site is mutually determined according to system needs and the fellow’s interests.
To apply for DSSF, researchers must hold a doctoral degree in any relevant aspect of clinical medicine, HSR, or a related field. The review committee evaluates applicants based on their qualifications, a clear statement of professional goals, appropriateness for placement, the quality of a writing sample, and letters of recommendation. Host sites select a fellow based on their individual preferences and recommendations from the review committee. The minimum duration of the fellowship is 1 year, during which the fellow works full time at the host site. Host sites provide the fellows’ salary and benefits, financial support to attend AcademyHealth’s Annual Research Meeting, and mentorship and/or training. To ensure general continuity across sites, each fellow’s work focuses on “delivery system science (DSS),” with a significant part of the work intended for public dissemination (eg, a conference presentation) or publication in a peer-reviewed journal.
Host sites additionally provide AcademyHealth financial support to manage the process of recruiting promising candidates. AcademyHealth manages and convenes the advisory committee proceedings, facilitates the application cycle (including peer review of applicants), markets the fellowship, guides the interview and match process, and promotes placement of fellows upon program completion. AcademyHealth also convenes active and graduate fellows to foster engagement and professional development.
To date, 118 individuals have applied to the program and 25 fellows have been accepted. Nineteen have completed the fellowship, 2 are continuing as second-year fellows, and 4 started their fellowship in the fall of 2016. Fellows have a wide range of expertise in areas such as epidemiology, exercise physiology, health psychology, anthropology, clinical medicine, qualitative methods, organizational behavior, and systems engineering. Once individuals complete their fellowship, they become DSSF “alumni” and remain involved in program activities and as peer mentors.
EARLY EXPERIENCE WITH OUTCOMES
Program Level
AcademyHealth administers an annual evaluation to assess the program, understand impact on the fellow’s professional development and growth, track publications, and inform programmatic goals. To date, we have identified over 50 peer-reviewed publications resulting from work conducted through the fellowship (visit http://www.academyhealth.org/dssfpublications for a full listing of publications stemming from the DSSF program). As an example of continuous program improvement, this year staff implemented a fellow-led monthly call in response to requests to connect fellows. This has proven to be a useful response for fellows to understand how to enhance their own experience by learning from fellows in other systems to incorporate focused areas of development via cross-system sharing. As an indicator of continued value, of the 16 sites that have participated to date, 10 have participated in the DSSF for more than 1 cycle; 3 are new host sites currently participating in their first year of the program; and 3 host sites participated for 1 year. The 3 inaugural host sites that helped launch the DSSF continue to serve as host sites to date.
As a marker of longitudinal success, staff will continue to follow up with preceptors and fellows to understand fellows’ contributions to the host site and the field, as well as impact on the fellow career trajectory. Of the 19 fellow alumni to date, 8 have moved on to academic or research positions, and 11 have remained in care delivery systems to provide local expertise in study design, execution, and evidence uptake.
The program has also made some general contributions to advance the discipline of delivery system science, including:
1. Defining DSS and clarifying training needs for “embedded researchers” and health system analysts. To characterize the fellowship, AcademyHealth and the program advisory committee jointly developed the following definition for delivery system science (DSS):
“DSS includes research that seeks to understand how delivery systems operate, influence, change, and respond to external stimulus, among other topics. DSS may include efforts to examine how and under what circumstances interventions work and how delivery systems effectively implement evidence-based innovations. For the DSSF, DSS is conducted by researchers who are ‘embedded’ in delivery systems and respond to the decision-making needs of those systems.”
Additionally, closer connections with DSS leaders have led to a better understanding of challenges, opportunities, and needs of delivery systems.10,11
2. Cultivating a network of delivery systems and system leaders interested in expanding the cadre of embedded researchers, and trainees who intend to build careers in DSS. The DSSF also aims to enhance fellows’ skills and knowledge base, career opportunities, and professional network. To extend these relationships and support delivery system analytics, AcademyHealth worked with preceptors and fellows to inform creation of a new Community of Practice supported by AcademyHealth’s EDM Forum and guide planning for AcademyHealth’s Concordium conference to provide a national meeting to showcase DSS.
3. Strategic planning to ensure sustainable support for embedded research within delivery systems. Substantial interest in the program developed quickly, with rapid learning over the first few cycles to refine the program to meet host sites’ and fellows’ needs. Both efforts were critical to demonstrate that the DSSF fulfills an important need for our health system partners and members. As indicated previously, strong, sustained interest from prospective host sites and applicants demonstrates the program has created a win-win to jointly assess fit while building skills and supporting continuous learning.
Likewise, Lisa Simpson, President and CEO of AcademyHealth, and Lucy Savitz, DSSF host site preceptor at Intermountain Healthcare, participated in the Canadian Institutes of Health Research (CIHR) Invitational Workshop, “Modernizing Health Services and Policy Research Training in Canada” in March 2016. Shared learning largely informed by the DSSF led to CIHR creating a similar fellowship program with initial awards to be made in 2017 (see https://www.researchnet-recherchenet.ca/rnr16/vwOpprtntyDtls.do?prog=2540&view=browseActive&sponsor=CIHR-8&type=EXACT&resultCount=25 for more information). We are working to thread these efforts together in a way to leverage our learning community of government agencies, academia, and employers as a long-term funding stream for training in delivery science.
Participant Host Site Level
Two selected examples of how DSSF researchers have engaged high-priority topics that contributed to health system operations are provided here.
Kaiser Permanente Southern California: Assessing adherence with “Choosing Wisely” recommendations in oncology. In partnership with preceptor Dr. Michael Gould, 2013-2014 DSSF fellow Dr. Erin Hahn worked with the Kaiser Permanente Southern California (KPSC) Care Improvement Research Team to lead a project addressing several KPSC priority areas. Focusing on “Choosing Wisely” recommendations from the American Society of Clinical Oncology,12 the project evaluated appropriateness of imaging and laboratory services for early-stage cancer patients and survivors between and within 2 integrated health systems, Kaiser Permanente (KP) and Intermountain Healthcare.13,14 Results were presented to KP national leaders, including an external health policy advisory board. In close collaboration with clinical and operational leaders in medical oncology, this multiregional, multisystem project is contributing to targeted quality improvement efforts and improved healthcare value, including audit and feedback of nonrecommended labs.
Dr. Hahn subsequently received a KPSC Incubator Award, a competitive internal grant, to further study factors associated with use of nonrecommended surveillance lab tests for early-stage breast cancer patients. The study focused on medical oncologists within KPSC, categorizing them as high or low utilizers of the tests.15 Results indicate that high utilizers perceive that the tests help manage patient anxiety about recurrence, while acknowledging that the tests do not provide clinical utility. These findings are contributing to the development of targeted survivorship services across the organization.
Intermountain Healthcare: Formative evaluation of large-scale implementation of shared decision-making. Preceptor Dr. Lucy Savitz assembled a team to conduct a formative evaluation of Intermountain Healthcare’s efforts to implement shared decision-making (SDM) as part of its Center for Medicare and Medicaid Innovation Challenge Award. The 2015-2017 DSSF fellow at Intermountain Healthcare, Dr. Kim Brunisholz, served as a core member of the project team, focusing primarily on a mixed-methods evaluation of the SDM program.
Dr. Brunisholz engaged operational leads, clinical teams, patient and family advisory councils, and senior executives to conduct the program assessment. Results demonstrated significant variation in invited participation in SDM among eligible patient populations: preference sensitive conditions (1 in 30 patients), oncology-related diagnosis (1 in 3 patients), and chronic conditions (1 in 74 patients). Provisional analysis of patient-level clinical outcomes demonstrated that among those invited to the SDM program compared to those that were not, total joint replacement was decreased (10.1% vs 17.3%; P < 0.001) and a trend towards breast conservation emerged (61.8% vs 56.4%; P = 0.10). No difference in treatment choice for lower back pain was observed. Qualitative program analysis suggested need for improvements in the areas of (1) routine and continuous staff training, (2) workflow standardization, and (3) active data monitoring with meaningful, actionable feedback to caregivers. In response to these results, a chartered SDM Steering Committee was created (Dr. Brunisholz is a member of that group) to develop a strategic plan for SDM, with an accompanying organizational response to reimplement SDM in a targeted manner. Learning from this program is being leveraged to support a subproject analysis on a large scale using data from the High Value Healthcare Collaborative as part of an AHRQ-funded Center of Excellence award. (See https://www.ahrq.gov/news/newsroom/press-releases/2015/pcorawards.html. For more information on the High Value Healthcare Collaborative, please visit: https://www.highvaluehealthcare.org.)
CONCLUSION
Moving forward, the DSSF will continue working with progressive delivery systems. Partnerships between organizations that are interested in integrating rigorous research practice to drive continuous system improvement and maximize the value of care will have substantial need for technical skills and analytic capacity. They will also need to ensure that researchers working in their systems have sufficient understanding of cultural and political context within the organization to be effective leaders who can manage change.
AcademyHealth created the DSSF in response to the field’s request to build a research workforce that reflects the vision for a 21st Century Health System, as laid out by the NAM.16 We anticipate that as the US Department of Health & Human Services’ goals for payment reform and new measures to promote quality and high-value care are implemented, the DSSF trainees’ skill set will be increasingly valuable and will provide needed thought leadership on strategies to generate and apply evidence in practice.
Disclosure
Ms. Kanani received funding from Intermountain Healthcare, Kaiser Permanente Southern California for support for the Delivery System Science Fellowship. Drs. Hahn, Gould, and Brunisholz have no conflicts to disclose. Dr. Savitz has received funding from HRQ COE, PCORI LHSNet; received funding for lectures from the Institute from Healthcare Improvement, Department of Epidemiology, University of Utah; received funding for travel, accommodations, and meeting expenses from AHRQ NAC, EDM Forum, AH CAPP, AARP NPC, and PROM TEP; and received additional funding from Dartmouth University. Dr. Holve received funding from Intermountain Healthcare, Kaiser Permanente Southern California for support for the Delivery System Science Fellowship, provided by our delivery system partners, several of whom are coauthors on this manuscript.
1. AcademyHealth. Health Services Research in 2020: Summit on the Future of HSR Data and Methods. http://www.academyhealth.org/About/content.cfm?ItemNumber=2529. Accessed March 21, 2016.
2. AcademyHealth. Health Services Research (HSR) Learning Consortium Strategic Plan. http://www.academyhealth.org/files/ProfDev/Files/HSRstrategicplan2011FINAL.pdf. Accessed March 21, 2016.
3. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2009/ComparativeEffectivenessResearchPriorities/CER%20report%20brief%2008-13-09.pdf. Accessed March 21, 2016.
4. Bonham A, Rich E, Davis D, Longnecker D, Heinig S. Putting evidence to work: an expanded research agenda for academic medicine in the era of health care reform. Acad Med. 2010;85(10):1551-1553. PubMed
5. Zerhouni E. Translational and clinical science—time for a new vision. N Engl J Med. 2005;353(15):1621-1623. PubMed
6. AcademyHealth. “Getting Answers We Can Believe In: Methodological Considerations When Using Electronic Clinical Data for Research,” EDM Forum, December 2012.
7. Rich G, Collins A. Current and Future Demand for Health Services Researchers. Funded by the Agency for Healthcare Research and Quality (AHRQ). Presented at the AcademyHealth HSR Workforce Conference, Understanding the Current Health Services Research Workforce and Maximizing Its Future, funded by AHRQ, Patient-Centered Outcomes Research Institute, and the Robert Wood Johnson Foundation. October 2016.
8. AcademyHealth. Delivery System Science Fellowship. http://www.academyhealth.org/dssf. Accessed March 21, 2016.
9. Institute for Healthcare Improvement. IHI Triple Aim Initiative. http://www.ihi.org/engage/initiatives/tripleaim/Pages/default.aspx. Accessed March 21, 2016.
10. Psek W, Stametz R, Bailey-Davis L, et al. Operationalizing the learning health care system in an integrated delivery system. eGEMs. 2015;3(1):1122. PubMed
11. Thompson C, Kurian A, Luft H. Linking electronic health records to better understand breast cancer patient pathways within and between two health systems. eGEMs. 2015;3(1):1127. PubMed
12. Schnipper L, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30(14):1715-1724. PubMed
13. Hahn E, Tang T, Lee JS, et al. Use of posttreatment imaging and biomarkers in survivors of early‐stage breast cancer: Inappropriate surveillance or necessary care? Cancer. 2015;122(6):908-916. PubMed
14. Hahn E, Tang T, Lee JS, et al. Use of imaging for staging of early-stage breast cancer in two integrated health care systems: Adherence with a choosing wisely recommendation. J Oncol Pract. 2015;11(3):e320-e328. PubMed
15. Hahn EE, Munoz-Plaza C, Wang J, et al. Anxiety, culture, expectations: Oncologist-perceived factors associated with use of non-recommended serum tumor marker tests for surveillance of early stage breast cancer. J Oncol Pract. 2016;13(1):e77-e290. PubMed
16. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2000.
1. AcademyHealth. Health Services Research in 2020: Summit on the Future of HSR Data and Methods. http://www.academyhealth.org/About/content.cfm?ItemNumber=2529. Accessed March 21, 2016.
2. AcademyHealth. Health Services Research (HSR) Learning Consortium Strategic Plan. http://www.academyhealth.org/files/ProfDev/Files/HSRstrategicplan2011FINAL.pdf. Accessed March 21, 2016.
3. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2009/ComparativeEffectivenessResearchPriorities/CER%20report%20brief%2008-13-09.pdf. Accessed March 21, 2016.
4. Bonham A, Rich E, Davis D, Longnecker D, Heinig S. Putting evidence to work: an expanded research agenda for academic medicine in the era of health care reform. Acad Med. 2010;85(10):1551-1553. PubMed
5. Zerhouni E. Translational and clinical science—time for a new vision. N Engl J Med. 2005;353(15):1621-1623. PubMed
6. AcademyHealth. “Getting Answers We Can Believe In: Methodological Considerations When Using Electronic Clinical Data for Research,” EDM Forum, December 2012.
7. Rich G, Collins A. Current and Future Demand for Health Services Researchers. Funded by the Agency for Healthcare Research and Quality (AHRQ). Presented at the AcademyHealth HSR Workforce Conference, Understanding the Current Health Services Research Workforce and Maximizing Its Future, funded by AHRQ, Patient-Centered Outcomes Research Institute, and the Robert Wood Johnson Foundation. October 2016.
8. AcademyHealth. Delivery System Science Fellowship. http://www.academyhealth.org/dssf. Accessed March 21, 2016.
9. Institute for Healthcare Improvement. IHI Triple Aim Initiative. http://www.ihi.org/engage/initiatives/tripleaim/Pages/default.aspx. Accessed March 21, 2016.
10. Psek W, Stametz R, Bailey-Davis L, et al. Operationalizing the learning health care system in an integrated delivery system. eGEMs. 2015;3(1):1122. PubMed
11. Thompson C, Kurian A, Luft H. Linking electronic health records to better understand breast cancer patient pathways within and between two health systems. eGEMs. 2015;3(1):1127. PubMed
12. Schnipper L, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30(14):1715-1724. PubMed
13. Hahn E, Tang T, Lee JS, et al. Use of posttreatment imaging and biomarkers in survivors of early‐stage breast cancer: Inappropriate surveillance or necessary care? Cancer. 2015;122(6):908-916. PubMed
14. Hahn E, Tang T, Lee JS, et al. Use of imaging for staging of early-stage breast cancer in two integrated health care systems: Adherence with a choosing wisely recommendation. J Oncol Pract. 2015;11(3):e320-e328. PubMed
15. Hahn EE, Munoz-Plaza C, Wang J, et al. Anxiety, culture, expectations: Oncologist-perceived factors associated with use of non-recommended serum tumor marker tests for surveillance of early stage breast cancer. J Oncol Pract. 2016;13(1):e77-e290. PubMed
16. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2000.
© 2017 Society of Hospital Medicine
A practical framework for understanding and reducing medical overuse: Conceptualizing overuse through the patient-clinician interaction
Medical services overuse is the provision of healthcare services for which there is no medical basis or for which harms equal or exceed benefits.1 This overuse drives poor-quality care and unnecessary cost.2,3 The high prevalence of overuse is recognized by patients,4 clinicians,5 and policymakers.6 Initiatives to reduce overuse have targeted physicians,7 the public,8 and medical educators9,10 but have had limited impact.11,12 Few studies have addressed methods for reducing overuse, and de-implementation of nonbeneficial practices has proved challenging.1,13,14 Models for reducing overuse are only theoretical15 or are focused on administrative decisions.16,17 We think a practical framework is needed. We used an iterative process, informed by expert opinion and discussion, to design such a framework.
METHODS
The authors, who have expertise in overuse, value, medical education, evidence-based medicine, and implementation science, reviewed related conceptual frameworks18 and evidence regarding drivers of overuse. We organized these drivers into domains to create a draft framework, which we presented at Preventing Overdiagnosis 2015, a meeting of clinicians, patients, and policymakers interested in overuse. We incorporated feedback from meeting attendees to modify framework domains, and we performed structured searches (using key words in Pubmed) to explore, and estimate the strength of, evidence supporting items within each domain. We rated supporting evidence as strong (studies found a clear correlation between a factor and overuse), moderate (evidence suggests such a correlation or demonstrates a correlation between a particular factor and utilization but not overuse per se), weak (only indirect evidence exists), or absent (no studies identified evaluating a particular factor). All authors reached consensus on ratings.
Framework Principles and Evidence
Patient-centered definition of overuse. During framework development, defining clinical appropriateness emerged as the primary challenge to identifying and reducing overuse. Although some care generally is appropriate based on strong evidence of benefit, and some is inappropriate given a clear lack of benefit or harm, much care is of unclear or variable benefit. Practice guidelines can help identify overuse, but their utility may be limited by lack of evidence in specific clinical situations,19 and their recommendations may apply poorly to an individual patient. This presents challenges to using guidelines to identify and reduce overuse.
Despite limitations, the scope of overuse has been estimated by applying broad, often guideline-based, criteria for care appropriateness to administrative data.20 Unfortunately, these estimates provide little direction to clinicians and patients partnering to make usage decisions. During framework development, we identified the importance of a patient-level, patient-specific definition of overuse. This approach reinforces the importance of meeting patient needs while standardizing treatments to reduce overuse. A patient-centered approach may also assist professional societies and advocacy groups in developing actionable campaigns and may uncover evidence gaps.
Centrality of patient-clinician interaction. During framework development, the patient–clinician interaction emerged as the nexus through which drivers of overuse exert influence. The centrality of this interaction has been demonstrated in studies of the relationship between care continuity and overuse21 or utilization,22,23 by evidence that communication and patient–clinician relationships affect utilization,24 and by the observation that clinician training in shared decision-making reduces overuse.25 A patient-centered framework assumes that, at least in the weighing of clinically reasonable options, a patient-centered approach optimizes outcomes for that patient.
Incorporating drivers of overuse. We incorporated drivers of overuse into domains and related them to the patient–clinician interaction.26 Domains included the culture of healthcare consumption, patient factors and experiences, the practice environment, the culture of professional medicine, and clinician attitudes and beliefs.
We characterized the evidence illustrating how drivers within each domain influence healthcare use. The evidence for each domain is listed in Table 1.
RESULTS
The final framework is shown in the Figure. Within the healthcare system, patients are influenced by the culture of healthcare consumption, which varies within and among countries.27 Clinicians are influenced by the culture of medical care, which varies by practice setting,28 and by their training environment.29 Both clinicians and patients are influenced by the practice environment and by personal experiences. Ultimately, clinical decisions occur within the specific patient–clinician interaction.24 Table 1 lists each domain’s components, likely impact on overuse, and estimated strength of supporting evidence. Interventions can be conceptualized within appropriate domains or through the interaction between patient and clinician.
DISCUSSION
We developed a novel and practical conceptual framework for characterizing drivers of overuse and potential intervention points. To our knowledge, this is the first framework incorporating a patient-specific approach to overuse and emphasizing the patient–clinician interaction. Key strengths of framework development are inclusion of a range of perspectives and characterization of the evidence within each domain. Limitations include lack of a formal systematic review and broad, qualitative assessments of evidence strength. However, we believe this framework provides an important conceptual foundation for the study of overuse and interventions to reduce overuse.
Framework Applications
This framework, which highlights the many drivers of overuse, can facilitate understanding of overuse and help conceptualize change, prioritize research goals, and inform specific interventions. For policymakers, the framework can inform efforts to reduce overuse by emphasizing the need for complex interventions and by clarifying the likely impact of interventions targeting specific domains. Similarly, for clinicians and quality improvement professionals, the framework can ground root cause analyses of overuse-related problems and inform allocation of limited resources. Finally, the relatively weak evidence on the role of most acknowledged drivers of overuse suggests an important research agenda. Specifically, several pressing needs have been identified: defining relevant physician and patient cultural factors, investigating interventions to impact culture, defining practice environment features that optimize care appropriateness, and describing specific patient–clinician interaction practices that minimize overuse while providing needed care.
Targeting Interventions
Domains within the framework are influenced by different types of interventions, and different stakeholders may target different domains. For example:
- The culture of healthcare consumption may be influenced through public education (eg, Choosing Wisely® patient resources)30-32 and public health campaigns.
- The practice environment may be influenced by initiatives to align clinician incentives,33 team care,34 electronic health record interventions,35 and improved access.36
- Clinician attitudes and beliefs may be influenced by audit and feedback,37-40 reflection,41 role modeling,42 and education.43-45
- Patient attitudes and beliefs may be influenced by education, access to price and quality information, and increased engagement in care.46,47
- For clinicians, the patient–clinician interaction can be improved through training in communication and shared decision-making,25 through access to information (eg, costs) that can be easily shared with patients,48,49 and through novel visit structures (eg, scribes).50
- On the patient side, this interaction can be optimized with improved access (eg, through telemedicine)51,52 or with patient empowerment during hospitalization.
- The culture of medicine is difficult to influence. Change likely will occur through:
○ Regulatory interventions (eg, Transforming Clinical Practice Initiative of Center for Medicare & Medicaid Innovation).
○ Educational initiatives (eg, high-value care curricula of Alliance for Academic Internal Medicine/American College of Physicians53).
○ Medical journal features (eg, “Less Is More” in JAMA Internal Medicine54 and “Things We Do for No Reason” in Journal of Hospital Medicine).
○ Professional organizations (eg, Choosing Wisely®).
As organizations implement quality improvement initiatives to reduce overuse of services, the framework can be used to target interventions to relevant domains. For example, a hospital leader who wants to reduce opioid prescribing may use the framework to identify the factors that encourage prescribing in each domain—poor understanding of pain treatment (a clinician factor), desire for early discharge encouraging overly aggressive pain management (an environmental factor), patient demand for opioids combined with poor understanding of harms (patient factors), and poor communication regarding pain (a patient–clinician interaction factor). Although not all relevant factors can be addressed, their classification by domain facilitates intervention, in this case perhaps leading to a focus on clinician and patient education on opioids and development of a practical communication tool that targets 3 domains. Table 2 lists ways in which the framework informs approaches to this and other overused services in the hospital setting. Note that some drivers can be acknowledged without identifying targeted interventions.
Moving Forward
Through a multi-stakeholder iterative process, we developed a practical framework for understanding medical overuse and interventions to reduce it. Centered on the patient–clinician interaction, this framework explains overuse as the product of medical and patient culture, the practice environment and incentives, and other clinician and patient factors. Ultimately, care is implemented during the patient–clinician interaction, though few interventions to reduce overuse have focused on that domain.
Conceptualizing overuse through the patient–clinician interaction maintains focus on patients while promoting population health that is both better and lower in cost. This framework can guide interventions to reduce overuse in important parts of the healthcare system while ensuring the final goal of high-quality individualized patient care.
Acknowledgments
The authors thank Valerie Pocus for helping with the artistic design of Framework. An early version of Framework was presented at the 2015 Preventing Overdiagnosis meeting in Bethesda, Maryland.
Disclosures
Dr. Morgan received research support from the VA Health Services Research (CRE 12-307), Agency for Healthcare Research and Quality (AHRQ) (K08- HS18111). Dr. Leppin’s work was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Dr. Korenstein’s work on this paper was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). Dr. Morgan provided a self-developed lecture in a 3M-sponsored series on hospital epidemiology and has received honoraria for serving as a book and journal editor for Springer Publishing. Dr. Smith is employed by the American College of Physicians and owns stock in Merck, where her husband is employed. The other authors report no potential conflicts of interest.
1. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. PubMed
2. Hood VL, Weinberger SE. High value, cost-conscious care: an international imperative. Eur J Intern Med. 2012;23(6):495-498. PubMed
3. Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171-178. PubMed
4. How SKH, Shih A, Lau J, Schoen C. Public Views on U.S. Health System Organization: A Call for New Directions. http://www.commonwealthfund.org/publications/data-briefs/2008/aug/public-views-on-u-s--health-system-organization--a-call-for-new-directions. Published August 1, 2008. Accessed December 11, 2015.
5. Sirovich BE, Woloshin S, Schwartz LM. Too little? Too much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med. 2011;171(17):1582-1585. PubMed
6. Joint Commission, American Medical Association–Convened Physician Consortium for Performance Improvement. Proceedings From the National Summit on Overuse. https://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf. Published September 24, 2012. Accessed July 8, 2016.
7. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. PubMed
8. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the Choosing Wisely campaign. Acad Med. 2014;89(7):990-995. PubMed
9. Smith CD, Levinson WS. A commitment to high-value care education from the internal medicine community. Ann Int Med. 2015;162(9):639-640. PubMed
10. Korenstein D, Kale M, Levinson W. Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671-1672. PubMed
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142-148. PubMed
12. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
13. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. PubMed
14. Ubel PA, Asch DA. Creating value in health by understanding and overcoming resistance to de-innovation. Health Aff (Millwood). 2015;34(2):239-244. PubMed
15. Powell AA, Bloomfield HE, Burgess DJ, Wilt TJ, Partin MR. A conceptual framework for understanding and reducing overuse by primary care providers. Med Care Res Rev. 2013;70(5):451-472. PubMed
16. Nassery N, Segal JB, Chang E, Bridges JF. Systematic overuse of healthcare services: a conceptual model. Appl Health Econ Health Policy. 2015;13(1):1-6. PubMed
17. Segal JB, Nassery N, Chang HY, Chang E, Chan K, Bridges JF. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. PubMed
18. Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(suppl 3):S555-S561. PubMed
19. Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine.” Am J Med. 1997;103(6):529-535. PubMed
20. Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1(2):185-194. PubMed
21. Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148-1154. PubMed
22. Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Ann Fam Med. 2015;13(2):123-129. PubMed
23. Chaiyachati KH, Gordon K, Long T, et al. Continuity in a VA patient-centered medical home reduces emergency department visits. PloS One. 2014;9(5):e96356. PubMed
24. Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39(5):555-563. PubMed
25. Legare F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ. 2012;184(13):E726-E734. PubMed
26. PerryUndum Research/Communication; for ABIM Foundation. Unnecessary Tests and Procedures in the Health Care System: What Physicians Say About the Problem, the Causes, and the Solutions: Results From a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2015/04/Final-Choosing-Wisely-Survey-Report.pdf. Published May 1, 2014. Accessed July 8, 2016.
27. Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA. A systematic review of medical practice variation in OECD countries. Health Policy. 2014;114(1):5-14. PubMed
28. Cutler D, Skinner JS, Stern AD, Wennberg DE. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. NBER Working Paper No. 19320. Cambridge, MA: National Bureau of Economic Research; 2013. http://www.nber.org/papers/w19320. Published August 2013. Accessed July 8, 2016.
29. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. PubMed
30. Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10(1):17-31. PubMed
31. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103-3109. PubMed
32. Sabuncu E, David J, Bernede-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009;6(6):e1000084. PubMed
33. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. 2011;(7):CD009255. PubMed
34. Yoon J, Rose DE, Canelo I, et al. Medical home features of VHA primary care clinics and avoidable hospitalizations. J Gen Intern Med. 2013;28(9):1188-1194. PubMed
35. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267-273. PubMed
36. Davis MM, Balasubramanian BA, Cifuentes M, et al. Clinician staffing, scheduling, and engagement strategies among primary care practices delivering integrated care. J Am Board Fam Med. 2015;28(suppl 1):S32-S40. PubMed
37. Dine CJ, Miller J, Fuld A, Bellini LM, Iwashyna TJ. Educating physicians-in-training about resource utilization and their own outcomes of care in the inpatient setting. J Grad Med Educ. 2010;2(2):175-180. PubMed
38. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(4):354-361. PubMed
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345-2352. PubMed
40. Taggart LR, Leung E, Muller MP, Matukas LM, Daneman N. Differential outcome of an antimicrobial stewardship audit and feedback program in two intensive care units: a controlled interrupted time series study. BMC Infect Dis. 2015;15:480. PubMed
41. Hughes DR, Sunshine JH, Bhargavan M, Forman H. Physician self-referral for imaging and the cost of chronic care for Medicare beneficiaries. Med Care. 2011;49(9):857-864. PubMed
42. Ryskina KL, Pesko MF, Gossey JT, Caesar EP, Bishop TF. Brand name statin prescribing in a resident ambulatory practice: implications for teaching cost-conscious medicine. J Grad Med Educ. 2014;6(3):484-488. PubMed
43. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. PubMed
44. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72. PubMed
45. Wilson I, Cowin LS, Johnson M, Young H. Professional identity in medical students: pedagogical challenges to medical education. Teach Learn Med. 2013;25(4):369-373. PubMed
46. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
47. Dykes PC, Stade D, Chang F, et al. Participatory design and development of a patient-centered toolkit to engage hospitalized patients and care partners in their plan of care. AMIA Annu Symp Proc. 2014;2014:486-495. PubMed
48. Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;(11):CD010907. PubMed
49. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. PubMed
50. Bank AJ, Gage RM. Annual impact of scribes on physician productivity and revenue in a cardiology clinic. Clinicoecon Outcomes Res. 2015;7:489-495. PubMed
51. Lyles CR, Sarkar U, Schillinger D, et al. Refilling medications through an online patient portal: consistent improvements in adherence across racial/ethnic groups. J Am Med Inform Assoc. 2016;23(e1):e28-e33. PubMed
52. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res. 2015;17(2):e44. PubMed
53. Smith CD. Teaching high-value, cost-conscious care to residents: the Alliance for Academic Internal Medicine-American College of Physicians curriculum. Ann Intern Med. 2012;157(4):284-286. PubMed
54. Redberg RF. Less is more. Arch Intern Med. 2010;170(7):584. PubMed
65. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121-1129. PubMed
66. Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10(10):557-564. PubMed
67. Tubbs EP, Elrod JA, Flum DR. Risk taking and tolerance of uncertainty: implications for surgeons. J Surg Res. 2006;131(1):1-6. PubMed
68. Zaat JO, van Eijk JT. General practitioners’ uncertainty, risk preference, and use of laboratory tests. Med Care. 1992;30(9):846-854. PubMed
69. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
70. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362. PubMed
71. Yasaitis LC, Bynum JP, Skinner JS. Association between physician supply, local practice norms, and outpatient visit rates. Med Care. 2013;51(6):524-531. PubMed
72. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. PubMed
73. Ryskina KL, Smith CD, Weissman A, et al. U.S. internal medicine residents’ knowledge and practice of high-value care: a national survey. Acad Med. 2015;90(10):1373-1379. PubMed
74. Khullar D, Chokshi DA, Kocher R, et al. Behavioral economics and physician compensation—promise and challenges. N Engl J Med. 2015;372(24):2281-2283. PubMed
75. Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39(8):889-905. PubMed
76. Fanari Z, Abraham N, Kolm P, et al. Aggressive measures to decrease “door to balloon” time and incidence of unnecessary cardiac catheterization: potential risks and role of quality improvement. Mayo Clin Proc. 2015;90(12):1614-1622. PubMed
77. Kerr EA, Lucatorto MA, Holleman R, Hogan MM, Klamerus ML, Hofer TP. Monitoring performance for blood pressure management among patients with diabetes mellitus: too much of a good thing? Arch Intern Med. 2012;172(12):938-945. PubMed
78. Verhofstede R, Smets T, Cohen J, Costantini M, Van Den Noortgate N, Deliens L. Implementing the care programme for the last days of life in an acute geriatric hospital ward: a phase 2 mixed method study. BMC Palliat Care. 2016;15:27. PubMed
Medical services overuse is the provision of healthcare services for which there is no medical basis or for which harms equal or exceed benefits.1 This overuse drives poor-quality care and unnecessary cost.2,3 The high prevalence of overuse is recognized by patients,4 clinicians,5 and policymakers.6 Initiatives to reduce overuse have targeted physicians,7 the public,8 and medical educators9,10 but have had limited impact.11,12 Few studies have addressed methods for reducing overuse, and de-implementation of nonbeneficial practices has proved challenging.1,13,14 Models for reducing overuse are only theoretical15 or are focused on administrative decisions.16,17 We think a practical framework is needed. We used an iterative process, informed by expert opinion and discussion, to design such a framework.
METHODS
The authors, who have expertise in overuse, value, medical education, evidence-based medicine, and implementation science, reviewed related conceptual frameworks18 and evidence regarding drivers of overuse. We organized these drivers into domains to create a draft framework, which we presented at Preventing Overdiagnosis 2015, a meeting of clinicians, patients, and policymakers interested in overuse. We incorporated feedback from meeting attendees to modify framework domains, and we performed structured searches (using key words in Pubmed) to explore, and estimate the strength of, evidence supporting items within each domain. We rated supporting evidence as strong (studies found a clear correlation between a factor and overuse), moderate (evidence suggests such a correlation or demonstrates a correlation between a particular factor and utilization but not overuse per se), weak (only indirect evidence exists), or absent (no studies identified evaluating a particular factor). All authors reached consensus on ratings.
Framework Principles and Evidence
Patient-centered definition of overuse. During framework development, defining clinical appropriateness emerged as the primary challenge to identifying and reducing overuse. Although some care generally is appropriate based on strong evidence of benefit, and some is inappropriate given a clear lack of benefit or harm, much care is of unclear or variable benefit. Practice guidelines can help identify overuse, but their utility may be limited by lack of evidence in specific clinical situations,19 and their recommendations may apply poorly to an individual patient. This presents challenges to using guidelines to identify and reduce overuse.
Despite limitations, the scope of overuse has been estimated by applying broad, often guideline-based, criteria for care appropriateness to administrative data.20 Unfortunately, these estimates provide little direction to clinicians and patients partnering to make usage decisions. During framework development, we identified the importance of a patient-level, patient-specific definition of overuse. This approach reinforces the importance of meeting patient needs while standardizing treatments to reduce overuse. A patient-centered approach may also assist professional societies and advocacy groups in developing actionable campaigns and may uncover evidence gaps.
Centrality of patient-clinician interaction. During framework development, the patient–clinician interaction emerged as the nexus through which drivers of overuse exert influence. The centrality of this interaction has been demonstrated in studies of the relationship between care continuity and overuse21 or utilization,22,23 by evidence that communication and patient–clinician relationships affect utilization,24 and by the observation that clinician training in shared decision-making reduces overuse.25 A patient-centered framework assumes that, at least in the weighing of clinically reasonable options, a patient-centered approach optimizes outcomes for that patient.
Incorporating drivers of overuse. We incorporated drivers of overuse into domains and related them to the patient–clinician interaction.26 Domains included the culture of healthcare consumption, patient factors and experiences, the practice environment, the culture of professional medicine, and clinician attitudes and beliefs.
We characterized the evidence illustrating how drivers within each domain influence healthcare use. The evidence for each domain is listed in Table 1.
RESULTS
The final framework is shown in the Figure. Within the healthcare system, patients are influenced by the culture of healthcare consumption, which varies within and among countries.27 Clinicians are influenced by the culture of medical care, which varies by practice setting,28 and by their training environment.29 Both clinicians and patients are influenced by the practice environment and by personal experiences. Ultimately, clinical decisions occur within the specific patient–clinician interaction.24 Table 1 lists each domain’s components, likely impact on overuse, and estimated strength of supporting evidence. Interventions can be conceptualized within appropriate domains or through the interaction between patient and clinician.
DISCUSSION
We developed a novel and practical conceptual framework for characterizing drivers of overuse and potential intervention points. To our knowledge, this is the first framework incorporating a patient-specific approach to overuse and emphasizing the patient–clinician interaction. Key strengths of framework development are inclusion of a range of perspectives and characterization of the evidence within each domain. Limitations include lack of a formal systematic review and broad, qualitative assessments of evidence strength. However, we believe this framework provides an important conceptual foundation for the study of overuse and interventions to reduce overuse.
Framework Applications
This framework, which highlights the many drivers of overuse, can facilitate understanding of overuse and help conceptualize change, prioritize research goals, and inform specific interventions. For policymakers, the framework can inform efforts to reduce overuse by emphasizing the need for complex interventions and by clarifying the likely impact of interventions targeting specific domains. Similarly, for clinicians and quality improvement professionals, the framework can ground root cause analyses of overuse-related problems and inform allocation of limited resources. Finally, the relatively weak evidence on the role of most acknowledged drivers of overuse suggests an important research agenda. Specifically, several pressing needs have been identified: defining relevant physician and patient cultural factors, investigating interventions to impact culture, defining practice environment features that optimize care appropriateness, and describing specific patient–clinician interaction practices that minimize overuse while providing needed care.
Targeting Interventions
Domains within the framework are influenced by different types of interventions, and different stakeholders may target different domains. For example:
- The culture of healthcare consumption may be influenced through public education (eg, Choosing Wisely® patient resources)30-32 and public health campaigns.
- The practice environment may be influenced by initiatives to align clinician incentives,33 team care,34 electronic health record interventions,35 and improved access.36
- Clinician attitudes and beliefs may be influenced by audit and feedback,37-40 reflection,41 role modeling,42 and education.43-45
- Patient attitudes and beliefs may be influenced by education, access to price and quality information, and increased engagement in care.46,47
- For clinicians, the patient–clinician interaction can be improved through training in communication and shared decision-making,25 through access to information (eg, costs) that can be easily shared with patients,48,49 and through novel visit structures (eg, scribes).50
- On the patient side, this interaction can be optimized with improved access (eg, through telemedicine)51,52 or with patient empowerment during hospitalization.
- The culture of medicine is difficult to influence. Change likely will occur through:
○ Regulatory interventions (eg, Transforming Clinical Practice Initiative of Center for Medicare & Medicaid Innovation).
○ Educational initiatives (eg, high-value care curricula of Alliance for Academic Internal Medicine/American College of Physicians53).
○ Medical journal features (eg, “Less Is More” in JAMA Internal Medicine54 and “Things We Do for No Reason” in Journal of Hospital Medicine).
○ Professional organizations (eg, Choosing Wisely®).
As organizations implement quality improvement initiatives to reduce overuse of services, the framework can be used to target interventions to relevant domains. For example, a hospital leader who wants to reduce opioid prescribing may use the framework to identify the factors that encourage prescribing in each domain—poor understanding of pain treatment (a clinician factor), desire for early discharge encouraging overly aggressive pain management (an environmental factor), patient demand for opioids combined with poor understanding of harms (patient factors), and poor communication regarding pain (a patient–clinician interaction factor). Although not all relevant factors can be addressed, their classification by domain facilitates intervention, in this case perhaps leading to a focus on clinician and patient education on opioids and development of a practical communication tool that targets 3 domains. Table 2 lists ways in which the framework informs approaches to this and other overused services in the hospital setting. Note that some drivers can be acknowledged without identifying targeted interventions.
Moving Forward
Through a multi-stakeholder iterative process, we developed a practical framework for understanding medical overuse and interventions to reduce it. Centered on the patient–clinician interaction, this framework explains overuse as the product of medical and patient culture, the practice environment and incentives, and other clinician and patient factors. Ultimately, care is implemented during the patient–clinician interaction, though few interventions to reduce overuse have focused on that domain.
Conceptualizing overuse through the patient–clinician interaction maintains focus on patients while promoting population health that is both better and lower in cost. This framework can guide interventions to reduce overuse in important parts of the healthcare system while ensuring the final goal of high-quality individualized patient care.
Acknowledgments
The authors thank Valerie Pocus for helping with the artistic design of Framework. An early version of Framework was presented at the 2015 Preventing Overdiagnosis meeting in Bethesda, Maryland.
Disclosures
Dr. Morgan received research support from the VA Health Services Research (CRE 12-307), Agency for Healthcare Research and Quality (AHRQ) (K08- HS18111). Dr. Leppin’s work was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Dr. Korenstein’s work on this paper was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). Dr. Morgan provided a self-developed lecture in a 3M-sponsored series on hospital epidemiology and has received honoraria for serving as a book and journal editor for Springer Publishing. Dr. Smith is employed by the American College of Physicians and owns stock in Merck, where her husband is employed. The other authors report no potential conflicts of interest.
Medical services overuse is the provision of healthcare services for which there is no medical basis or for which harms equal or exceed benefits.1 This overuse drives poor-quality care and unnecessary cost.2,3 The high prevalence of overuse is recognized by patients,4 clinicians,5 and policymakers.6 Initiatives to reduce overuse have targeted physicians,7 the public,8 and medical educators9,10 but have had limited impact.11,12 Few studies have addressed methods for reducing overuse, and de-implementation of nonbeneficial practices has proved challenging.1,13,14 Models for reducing overuse are only theoretical15 or are focused on administrative decisions.16,17 We think a practical framework is needed. We used an iterative process, informed by expert opinion and discussion, to design such a framework.
METHODS
The authors, who have expertise in overuse, value, medical education, evidence-based medicine, and implementation science, reviewed related conceptual frameworks18 and evidence regarding drivers of overuse. We organized these drivers into domains to create a draft framework, which we presented at Preventing Overdiagnosis 2015, a meeting of clinicians, patients, and policymakers interested in overuse. We incorporated feedback from meeting attendees to modify framework domains, and we performed structured searches (using key words in Pubmed) to explore, and estimate the strength of, evidence supporting items within each domain. We rated supporting evidence as strong (studies found a clear correlation between a factor and overuse), moderate (evidence suggests such a correlation or demonstrates a correlation between a particular factor and utilization but not overuse per se), weak (only indirect evidence exists), or absent (no studies identified evaluating a particular factor). All authors reached consensus on ratings.
Framework Principles and Evidence
Patient-centered definition of overuse. During framework development, defining clinical appropriateness emerged as the primary challenge to identifying and reducing overuse. Although some care generally is appropriate based on strong evidence of benefit, and some is inappropriate given a clear lack of benefit or harm, much care is of unclear or variable benefit. Practice guidelines can help identify overuse, but their utility may be limited by lack of evidence in specific clinical situations,19 and their recommendations may apply poorly to an individual patient. This presents challenges to using guidelines to identify and reduce overuse.
Despite limitations, the scope of overuse has been estimated by applying broad, often guideline-based, criteria for care appropriateness to administrative data.20 Unfortunately, these estimates provide little direction to clinicians and patients partnering to make usage decisions. During framework development, we identified the importance of a patient-level, patient-specific definition of overuse. This approach reinforces the importance of meeting patient needs while standardizing treatments to reduce overuse. A patient-centered approach may also assist professional societies and advocacy groups in developing actionable campaigns and may uncover evidence gaps.
Centrality of patient-clinician interaction. During framework development, the patient–clinician interaction emerged as the nexus through which drivers of overuse exert influence. The centrality of this interaction has been demonstrated in studies of the relationship between care continuity and overuse21 or utilization,22,23 by evidence that communication and patient–clinician relationships affect utilization,24 and by the observation that clinician training in shared decision-making reduces overuse.25 A patient-centered framework assumes that, at least in the weighing of clinically reasonable options, a patient-centered approach optimizes outcomes for that patient.
Incorporating drivers of overuse. We incorporated drivers of overuse into domains and related them to the patient–clinician interaction.26 Domains included the culture of healthcare consumption, patient factors and experiences, the practice environment, the culture of professional medicine, and clinician attitudes and beliefs.
We characterized the evidence illustrating how drivers within each domain influence healthcare use. The evidence for each domain is listed in Table 1.
RESULTS
The final framework is shown in the Figure. Within the healthcare system, patients are influenced by the culture of healthcare consumption, which varies within and among countries.27 Clinicians are influenced by the culture of medical care, which varies by practice setting,28 and by their training environment.29 Both clinicians and patients are influenced by the practice environment and by personal experiences. Ultimately, clinical decisions occur within the specific patient–clinician interaction.24 Table 1 lists each domain’s components, likely impact on overuse, and estimated strength of supporting evidence. Interventions can be conceptualized within appropriate domains or through the interaction between patient and clinician.
DISCUSSION
We developed a novel and practical conceptual framework for characterizing drivers of overuse and potential intervention points. To our knowledge, this is the first framework incorporating a patient-specific approach to overuse and emphasizing the patient–clinician interaction. Key strengths of framework development are inclusion of a range of perspectives and characterization of the evidence within each domain. Limitations include lack of a formal systematic review and broad, qualitative assessments of evidence strength. However, we believe this framework provides an important conceptual foundation for the study of overuse and interventions to reduce overuse.
Framework Applications
This framework, which highlights the many drivers of overuse, can facilitate understanding of overuse and help conceptualize change, prioritize research goals, and inform specific interventions. For policymakers, the framework can inform efforts to reduce overuse by emphasizing the need for complex interventions and by clarifying the likely impact of interventions targeting specific domains. Similarly, for clinicians and quality improvement professionals, the framework can ground root cause analyses of overuse-related problems and inform allocation of limited resources. Finally, the relatively weak evidence on the role of most acknowledged drivers of overuse suggests an important research agenda. Specifically, several pressing needs have been identified: defining relevant physician and patient cultural factors, investigating interventions to impact culture, defining practice environment features that optimize care appropriateness, and describing specific patient–clinician interaction practices that minimize overuse while providing needed care.
Targeting Interventions
Domains within the framework are influenced by different types of interventions, and different stakeholders may target different domains. For example:
- The culture of healthcare consumption may be influenced through public education (eg, Choosing Wisely® patient resources)30-32 and public health campaigns.
- The practice environment may be influenced by initiatives to align clinician incentives,33 team care,34 electronic health record interventions,35 and improved access.36
- Clinician attitudes and beliefs may be influenced by audit and feedback,37-40 reflection,41 role modeling,42 and education.43-45
- Patient attitudes and beliefs may be influenced by education, access to price and quality information, and increased engagement in care.46,47
- For clinicians, the patient–clinician interaction can be improved through training in communication and shared decision-making,25 through access to information (eg, costs) that can be easily shared with patients,48,49 and through novel visit structures (eg, scribes).50
- On the patient side, this interaction can be optimized with improved access (eg, through telemedicine)51,52 or with patient empowerment during hospitalization.
- The culture of medicine is difficult to influence. Change likely will occur through:
○ Regulatory interventions (eg, Transforming Clinical Practice Initiative of Center for Medicare & Medicaid Innovation).
○ Educational initiatives (eg, high-value care curricula of Alliance for Academic Internal Medicine/American College of Physicians53).
○ Medical journal features (eg, “Less Is More” in JAMA Internal Medicine54 and “Things We Do for No Reason” in Journal of Hospital Medicine).
○ Professional organizations (eg, Choosing Wisely®).
As organizations implement quality improvement initiatives to reduce overuse of services, the framework can be used to target interventions to relevant domains. For example, a hospital leader who wants to reduce opioid prescribing may use the framework to identify the factors that encourage prescribing in each domain—poor understanding of pain treatment (a clinician factor), desire for early discharge encouraging overly aggressive pain management (an environmental factor), patient demand for opioids combined with poor understanding of harms (patient factors), and poor communication regarding pain (a patient–clinician interaction factor). Although not all relevant factors can be addressed, their classification by domain facilitates intervention, in this case perhaps leading to a focus on clinician and patient education on opioids and development of a practical communication tool that targets 3 domains. Table 2 lists ways in which the framework informs approaches to this and other overused services in the hospital setting. Note that some drivers can be acknowledged without identifying targeted interventions.
Moving Forward
Through a multi-stakeholder iterative process, we developed a practical framework for understanding medical overuse and interventions to reduce it. Centered on the patient–clinician interaction, this framework explains overuse as the product of medical and patient culture, the practice environment and incentives, and other clinician and patient factors. Ultimately, care is implemented during the patient–clinician interaction, though few interventions to reduce overuse have focused on that domain.
Conceptualizing overuse through the patient–clinician interaction maintains focus on patients while promoting population health that is both better and lower in cost. This framework can guide interventions to reduce overuse in important parts of the healthcare system while ensuring the final goal of high-quality individualized patient care.
Acknowledgments
The authors thank Valerie Pocus for helping with the artistic design of Framework. An early version of Framework was presented at the 2015 Preventing Overdiagnosis meeting in Bethesda, Maryland.
Disclosures
Dr. Morgan received research support from the VA Health Services Research (CRE 12-307), Agency for Healthcare Research and Quality (AHRQ) (K08- HS18111). Dr. Leppin’s work was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Dr. Korenstein’s work on this paper was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). Dr. Morgan provided a self-developed lecture in a 3M-sponsored series on hospital epidemiology and has received honoraria for serving as a book and journal editor for Springer Publishing. Dr. Smith is employed by the American College of Physicians and owns stock in Merck, where her husband is employed. The other authors report no potential conflicts of interest.
1. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. PubMed
2. Hood VL, Weinberger SE. High value, cost-conscious care: an international imperative. Eur J Intern Med. 2012;23(6):495-498. PubMed
3. Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171-178. PubMed
4. How SKH, Shih A, Lau J, Schoen C. Public Views on U.S. Health System Organization: A Call for New Directions. http://www.commonwealthfund.org/publications/data-briefs/2008/aug/public-views-on-u-s--health-system-organization--a-call-for-new-directions. Published August 1, 2008. Accessed December 11, 2015.
5. Sirovich BE, Woloshin S, Schwartz LM. Too little? Too much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med. 2011;171(17):1582-1585. PubMed
6. Joint Commission, American Medical Association–Convened Physician Consortium for Performance Improvement. Proceedings From the National Summit on Overuse. https://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf. Published September 24, 2012. Accessed July 8, 2016.
7. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. PubMed
8. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the Choosing Wisely campaign. Acad Med. 2014;89(7):990-995. PubMed
9. Smith CD, Levinson WS. A commitment to high-value care education from the internal medicine community. Ann Int Med. 2015;162(9):639-640. PubMed
10. Korenstein D, Kale M, Levinson W. Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671-1672. PubMed
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142-148. PubMed
12. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
13. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. PubMed
14. Ubel PA, Asch DA. Creating value in health by understanding and overcoming resistance to de-innovation. Health Aff (Millwood). 2015;34(2):239-244. PubMed
15. Powell AA, Bloomfield HE, Burgess DJ, Wilt TJ, Partin MR. A conceptual framework for understanding and reducing overuse by primary care providers. Med Care Res Rev. 2013;70(5):451-472. PubMed
16. Nassery N, Segal JB, Chang E, Bridges JF. Systematic overuse of healthcare services: a conceptual model. Appl Health Econ Health Policy. 2015;13(1):1-6. PubMed
17. Segal JB, Nassery N, Chang HY, Chang E, Chan K, Bridges JF. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. PubMed
18. Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(suppl 3):S555-S561. PubMed
19. Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine.” Am J Med. 1997;103(6):529-535. PubMed
20. Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1(2):185-194. PubMed
21. Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148-1154. PubMed
22. Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Ann Fam Med. 2015;13(2):123-129. PubMed
23. Chaiyachati KH, Gordon K, Long T, et al. Continuity in a VA patient-centered medical home reduces emergency department visits. PloS One. 2014;9(5):e96356. PubMed
24. Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39(5):555-563. PubMed
25. Legare F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ. 2012;184(13):E726-E734. PubMed
26. PerryUndum Research/Communication; for ABIM Foundation. Unnecessary Tests and Procedures in the Health Care System: What Physicians Say About the Problem, the Causes, and the Solutions: Results From a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2015/04/Final-Choosing-Wisely-Survey-Report.pdf. Published May 1, 2014. Accessed July 8, 2016.
27. Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA. A systematic review of medical practice variation in OECD countries. Health Policy. 2014;114(1):5-14. PubMed
28. Cutler D, Skinner JS, Stern AD, Wennberg DE. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. NBER Working Paper No. 19320. Cambridge, MA: National Bureau of Economic Research; 2013. http://www.nber.org/papers/w19320. Published August 2013. Accessed July 8, 2016.
29. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. PubMed
30. Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10(1):17-31. PubMed
31. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103-3109. PubMed
32. Sabuncu E, David J, Bernede-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009;6(6):e1000084. PubMed
33. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. 2011;(7):CD009255. PubMed
34. Yoon J, Rose DE, Canelo I, et al. Medical home features of VHA primary care clinics and avoidable hospitalizations. J Gen Intern Med. 2013;28(9):1188-1194. PubMed
35. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267-273. PubMed
36. Davis MM, Balasubramanian BA, Cifuentes M, et al. Clinician staffing, scheduling, and engagement strategies among primary care practices delivering integrated care. J Am Board Fam Med. 2015;28(suppl 1):S32-S40. PubMed
37. Dine CJ, Miller J, Fuld A, Bellini LM, Iwashyna TJ. Educating physicians-in-training about resource utilization and their own outcomes of care in the inpatient setting. J Grad Med Educ. 2010;2(2):175-180. PubMed
38. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(4):354-361. PubMed
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345-2352. PubMed
40. Taggart LR, Leung E, Muller MP, Matukas LM, Daneman N. Differential outcome of an antimicrobial stewardship audit and feedback program in two intensive care units: a controlled interrupted time series study. BMC Infect Dis. 2015;15:480. PubMed
41. Hughes DR, Sunshine JH, Bhargavan M, Forman H. Physician self-referral for imaging and the cost of chronic care for Medicare beneficiaries. Med Care. 2011;49(9):857-864. PubMed
42. Ryskina KL, Pesko MF, Gossey JT, Caesar EP, Bishop TF. Brand name statin prescribing in a resident ambulatory practice: implications for teaching cost-conscious medicine. J Grad Med Educ. 2014;6(3):484-488. PubMed
43. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. PubMed
44. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72. PubMed
45. Wilson I, Cowin LS, Johnson M, Young H. Professional identity in medical students: pedagogical challenges to medical education. Teach Learn Med. 2013;25(4):369-373. PubMed
46. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
47. Dykes PC, Stade D, Chang F, et al. Participatory design and development of a patient-centered toolkit to engage hospitalized patients and care partners in their plan of care. AMIA Annu Symp Proc. 2014;2014:486-495. PubMed
48. Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;(11):CD010907. PubMed
49. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. PubMed
50. Bank AJ, Gage RM. Annual impact of scribes on physician productivity and revenue in a cardiology clinic. Clinicoecon Outcomes Res. 2015;7:489-495. PubMed
51. Lyles CR, Sarkar U, Schillinger D, et al. Refilling medications through an online patient portal: consistent improvements in adherence across racial/ethnic groups. J Am Med Inform Assoc. 2016;23(e1):e28-e33. PubMed
52. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res. 2015;17(2):e44. PubMed
53. Smith CD. Teaching high-value, cost-conscious care to residents: the Alliance for Academic Internal Medicine-American College of Physicians curriculum. Ann Intern Med. 2012;157(4):284-286. PubMed
54. Redberg RF. Less is more. Arch Intern Med. 2010;170(7):584. PubMed
65. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121-1129. PubMed
66. Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10(10):557-564. PubMed
67. Tubbs EP, Elrod JA, Flum DR. Risk taking and tolerance of uncertainty: implications for surgeons. J Surg Res. 2006;131(1):1-6. PubMed
68. Zaat JO, van Eijk JT. General practitioners’ uncertainty, risk preference, and use of laboratory tests. Med Care. 1992;30(9):846-854. PubMed
69. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
70. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362. PubMed
71. Yasaitis LC, Bynum JP, Skinner JS. Association between physician supply, local practice norms, and outpatient visit rates. Med Care. 2013;51(6):524-531. PubMed
72. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. PubMed
73. Ryskina KL, Smith CD, Weissman A, et al. U.S. internal medicine residents’ knowledge and practice of high-value care: a national survey. Acad Med. 2015;90(10):1373-1379. PubMed
74. Khullar D, Chokshi DA, Kocher R, et al. Behavioral economics and physician compensation—promise and challenges. N Engl J Med. 2015;372(24):2281-2283. PubMed
75. Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39(8):889-905. PubMed
76. Fanari Z, Abraham N, Kolm P, et al. Aggressive measures to decrease “door to balloon” time and incidence of unnecessary cardiac catheterization: potential risks and role of quality improvement. Mayo Clin Proc. 2015;90(12):1614-1622. PubMed
77. Kerr EA, Lucatorto MA, Holleman R, Hogan MM, Klamerus ML, Hofer TP. Monitoring performance for blood pressure management among patients with diabetes mellitus: too much of a good thing? Arch Intern Med. 2012;172(12):938-945. PubMed
78. Verhofstede R, Smets T, Cohen J, Costantini M, Van Den Noortgate N, Deliens L. Implementing the care programme for the last days of life in an acute geriatric hospital ward: a phase 2 mixed method study. BMC Palliat Care. 2016;15:27. PubMed
1. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. PubMed
2. Hood VL, Weinberger SE. High value, cost-conscious care: an international imperative. Eur J Intern Med. 2012;23(6):495-498. PubMed
3. Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171-178. PubMed
4. How SKH, Shih A, Lau J, Schoen C. Public Views on U.S. Health System Organization: A Call for New Directions. http://www.commonwealthfund.org/publications/data-briefs/2008/aug/public-views-on-u-s--health-system-organization--a-call-for-new-directions. Published August 1, 2008. Accessed December 11, 2015.
5. Sirovich BE, Woloshin S, Schwartz LM. Too little? Too much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med. 2011;171(17):1582-1585. PubMed
6. Joint Commission, American Medical Association–Convened Physician Consortium for Performance Improvement. Proceedings From the National Summit on Overuse. https://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf. Published September 24, 2012. Accessed July 8, 2016.
7. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. PubMed
8. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the Choosing Wisely campaign. Acad Med. 2014;89(7):990-995. PubMed
9. Smith CD, Levinson WS. A commitment to high-value care education from the internal medicine community. Ann Int Med. 2015;162(9):639-640. PubMed
10. Korenstein D, Kale M, Levinson W. Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671-1672. PubMed
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142-148. PubMed
12. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
13. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. PubMed
14. Ubel PA, Asch DA. Creating value in health by understanding and overcoming resistance to de-innovation. Health Aff (Millwood). 2015;34(2):239-244. PubMed
15. Powell AA, Bloomfield HE, Burgess DJ, Wilt TJ, Partin MR. A conceptual framework for understanding and reducing overuse by primary care providers. Med Care Res Rev. 2013;70(5):451-472. PubMed
16. Nassery N, Segal JB, Chang E, Bridges JF. Systematic overuse of healthcare services: a conceptual model. Appl Health Econ Health Policy. 2015;13(1):1-6. PubMed
17. Segal JB, Nassery N, Chang HY, Chang E, Chan K, Bridges JF. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. PubMed
18. Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(suppl 3):S555-S561. PubMed
19. Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine.” Am J Med. 1997;103(6):529-535. PubMed
20. Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1(2):185-194. PubMed
21. Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148-1154. PubMed
22. Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Ann Fam Med. 2015;13(2):123-129. PubMed
23. Chaiyachati KH, Gordon K, Long T, et al. Continuity in a VA patient-centered medical home reduces emergency department visits. PloS One. 2014;9(5):e96356. PubMed
24. Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39(5):555-563. PubMed
25. Legare F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ. 2012;184(13):E726-E734. PubMed
26. PerryUndum Research/Communication; for ABIM Foundation. Unnecessary Tests and Procedures in the Health Care System: What Physicians Say About the Problem, the Causes, and the Solutions: Results From a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2015/04/Final-Choosing-Wisely-Survey-Report.pdf. Published May 1, 2014. Accessed July 8, 2016.
27. Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA. A systematic review of medical practice variation in OECD countries. Health Policy. 2014;114(1):5-14. PubMed
28. Cutler D, Skinner JS, Stern AD, Wennberg DE. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. NBER Working Paper No. 19320. Cambridge, MA: National Bureau of Economic Research; 2013. http://www.nber.org/papers/w19320. Published August 2013. Accessed July 8, 2016.
29. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. PubMed
30. Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10(1):17-31. PubMed
31. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103-3109. PubMed
32. Sabuncu E, David J, Bernede-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009;6(6):e1000084. PubMed
33. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. 2011;(7):CD009255. PubMed
34. Yoon J, Rose DE, Canelo I, et al. Medical home features of VHA primary care clinics and avoidable hospitalizations. J Gen Intern Med. 2013;28(9):1188-1194. PubMed
35. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267-273. PubMed
36. Davis MM, Balasubramanian BA, Cifuentes M, et al. Clinician staffing, scheduling, and engagement strategies among primary care practices delivering integrated care. J Am Board Fam Med. 2015;28(suppl 1):S32-S40. PubMed
37. Dine CJ, Miller J, Fuld A, Bellini LM, Iwashyna TJ. Educating physicians-in-training about resource utilization and their own outcomes of care in the inpatient setting. J Grad Med Educ. 2010;2(2):175-180. PubMed
38. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(4):354-361. PubMed
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345-2352. PubMed
40. Taggart LR, Leung E, Muller MP, Matukas LM, Daneman N. Differential outcome of an antimicrobial stewardship audit and feedback program in two intensive care units: a controlled interrupted time series study. BMC Infect Dis. 2015;15:480. PubMed
41. Hughes DR, Sunshine JH, Bhargavan M, Forman H. Physician self-referral for imaging and the cost of chronic care for Medicare beneficiaries. Med Care. 2011;49(9):857-864. PubMed
42. Ryskina KL, Pesko MF, Gossey JT, Caesar EP, Bishop TF. Brand name statin prescribing in a resident ambulatory practice: implications for teaching cost-conscious medicine. J Grad Med Educ. 2014;6(3):484-488. PubMed
43. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. PubMed
44. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72. PubMed
45. Wilson I, Cowin LS, Johnson M, Young H. Professional identity in medical students: pedagogical challenges to medical education. Teach Learn Med. 2013;25(4):369-373. PubMed
46. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
47. Dykes PC, Stade D, Chang F, et al. Participatory design and development of a patient-centered toolkit to engage hospitalized patients and care partners in their plan of care. AMIA Annu Symp Proc. 2014;2014:486-495. PubMed
48. Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;(11):CD010907. PubMed
49. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. PubMed
50. Bank AJ, Gage RM. Annual impact of scribes on physician productivity and revenue in a cardiology clinic. Clinicoecon Outcomes Res. 2015;7:489-495. PubMed
51. Lyles CR, Sarkar U, Schillinger D, et al. Refilling medications through an online patient portal: consistent improvements in adherence across racial/ethnic groups. J Am Med Inform Assoc. 2016;23(e1):e28-e33. PubMed
52. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res. 2015;17(2):e44. PubMed
53. Smith CD. Teaching high-value, cost-conscious care to residents: the Alliance for Academic Internal Medicine-American College of Physicians curriculum. Ann Intern Med. 2012;157(4):284-286. PubMed
54. Redberg RF. Less is more. Arch Intern Med. 2010;170(7):584. PubMed
65. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121-1129. PubMed
66. Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10(10):557-564. PubMed
67. Tubbs EP, Elrod JA, Flum DR. Risk taking and tolerance of uncertainty: implications for surgeons. J Surg Res. 2006;131(1):1-6. PubMed
68. Zaat JO, van Eijk JT. General practitioners’ uncertainty, risk preference, and use of laboratory tests. Med Care. 1992;30(9):846-854. PubMed
69. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
70. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362. PubMed
71. Yasaitis LC, Bynum JP, Skinner JS. Association between physician supply, local practice norms, and outpatient visit rates. Med Care. 2013;51(6):524-531. PubMed
72. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. PubMed
73. Ryskina KL, Smith CD, Weissman A, et al. U.S. internal medicine residents’ knowledge and practice of high-value care: a national survey. Acad Med. 2015;90(10):1373-1379. PubMed
74. Khullar D, Chokshi DA, Kocher R, et al. Behavioral economics and physician compensation—promise and challenges. N Engl J Med. 2015;372(24):2281-2283. PubMed
75. Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39(8):889-905. PubMed
76. Fanari Z, Abraham N, Kolm P, et al. Aggressive measures to decrease “door to balloon” time and incidence of unnecessary cardiac catheterization: potential risks and role of quality improvement. Mayo Clin Proc. 2015;90(12):1614-1622. PubMed
77. Kerr EA, Lucatorto MA, Holleman R, Hogan MM, Klamerus ML, Hofer TP. Monitoring performance for blood pressure management among patients with diabetes mellitus: too much of a good thing? Arch Intern Med. 2012;172(12):938-945. PubMed
78. Verhofstede R, Smets T, Cohen J, Costantini M, Van Den Noortgate N, Deliens L. Implementing the care programme for the last days of life in an acute geriatric hospital ward: a phase 2 mixed method study. BMC Palliat Care. 2016;15:27. PubMed
© 2017 Society of Hospital Medicine
Post–Acute Care Reform Implications
The landscape of post–acute care (PAC), which is predominantly provided by inpatient rehabilitation facilities (IRFs), skilled nursing facilities (SNFs), and home healthcare (HHC) providers, is rapidly changing. As hospitalizations shorten, PAC utilization is rising, resulting in rapidly increasing costs.1-5 However, patient outcomes in PAC are characterized by high rates of readmission and low rates of return to the community.6,7 Emerging evidence suggests these outcomes could be substantially improved through use of better in-hospital and transitional care processes.8-10
Legislators took notice of the spiraling costs, potential quality concerns, and undesirable patient outcomes in PAC. Provisions in the Patient Protection and Affordable Care Act of 2010 (ACA), the Protecting Access to Medicare Act of 2014 (PAMA), and the Improving Medicare Post–Acute Care Transformation (IMPACT) Act of 2014 affect patient selection, payment, and quality measurement in PAC. As older adults are increasingly being cared for by hospitalists,11 hospitalists must be aware of the implications of these reforms.
IMPLICATIONS FOR HOSPITALISTS
Choosing Patients Wisely for PAC
Because PAC-related decision making is not standardized, referral rates vary significantly.12 The variability in PAC use accounts for 79% of all regional variation in Medicare spending in the United States.13,14 Compared with other physicians, hospitalists are more likely to use PAC15 but typically receive little exposure to PAC during training.16
The IMPACT Act proposes 2 major changes to patient selection: a uniform assessment tool for patients being discharged to PAC and “site-neutral” payments for PAC. Starting in 2018, the Continuity Assessment Record and Evaluation (CARE) tool must be completed before a hospital discharge in order to better match PAC resources to patient needs. The current 26-page CARE tool includes questions about demographics and home support, medical complexity, physical function, cognitive status, and “transition items,” including discharge plans and advance directives. In pilot testing, significant amounts of missing data and average completion times of up to 60 minutes raised concerns about feasibility.17 CARE tool assessments accurately predicted what form of PAC patients actually received, but further testing is planned to validate whether the type of PAC selected was optimal for patient outcomes.. A plan for using CARE tool assessments to determine site-neutral payments is due to Congress by 2020. In the site-neutral payment system, the PAC provider will be reimbursed according to patient needs (identified by the CARE tool), regardless of PAC setting—a radical change from the current system, in which IRF, SNF, and HHC episodes show major differences in median costs (Table 1).18
Hospitalists may be concerned that use of the CARE tool will supplant clinical judgment about patients’ PAC needs. The burden of completing the CARE tool could inadvertently reduce the amount of attention hospitalists give to other aspects of a safe discharge rather than lead to the improvement desired.19-21 Hospitalists will benefit from developing interdisciplinary, iterative workflows to complete the tool, improving accuracy and reducing the burden.
A potential unintended consequence of the site-neutral payment system may be increased difficulty discharging elderly patients who have limited rehabilitation potential but are lacking sufficient social support to return home. In the current system, these patients are commonly discharged to SNFs as a bridge to long-term nursing home care. Hospitalists will need to become increasingly familiar with novel alternatives to nursing home–based care, such as home-based primary care, medical foster homes, and Medicare/Medicaid’s Program of All-Inclusive Care of the Elderly (PACE).22-25
Choosing PAC Providers Wisely
Medicare’s Nursing Home Compare tool (https://www.medicare.gov/nursinghomecompare/search.html) provides a “5-star” system for rating SNFs on several quality metrics; these metrics, however, are not correlated with readmission or mortality rates.26,27 Improving quality measurement in PAC and tying payment to quality and outcomes are major emphases of the IMPACT Act and PAMA, respectively. PAC providers must publicly report an expanded list of quality measures and outcomes by 2018. In 2017, SNFs will begin reporting rates of “potentially preventable” readmissions, and starting in 2019 they will face penalties for having high
risk-adjusted rates.
These reforms coincide with an increased emphasis on hospitals and PAC providers sharing responsibility for costs and outcomes. One model of the Bundled Payments for Care Improvement (BPCI) initiative includes a single payment for an acute hospitalization and PAC up to 90 days after hospital discharge for select conditions. The Medicare Spending Per Beneficiary (MSPB) measure compares hospitals on their spending for Medicare beneficiaries from 3 days before hospital admission to 30 days after hospital discharge, and penalizes outliers with high costs.28 PAC spending is the main driver of costs in both BPCI and MSPB.29 One way that hospitals have responded to the BPCI is by drastically reducing their referrals to SNFs and increasing their referrals to HHC providers; unfortunately, this response has resulted in increases in post-discharge emergency department visits.29,30 Taking a novel step in November 2015, the Centers for Medicare & Medicaid Services (CMS) ruled that hospitals in more than 67 metropolitan service areas will be involuntarily enrolled in the BPCI initiative, using elective lower extremity joint replacement as the sample condition.31 This ruling signaled that these reforms are not meant solely for “high-performing” hospital and PAC systems able to volunteer for novel models of payment.
These changes have direct implications for hospitalists. Bundled payments incentivize hospitalists to reduce hospital length of stay and choose PAC alternatives with lower costs. SNFs may start accepting fewer “high-risk” patients in order to avoid readmission penalties. Hospitals will need to identify and partner with high-performing PAC providers in their community to maximize outcomes for their patients. On their websites, the Society of Post-Acute and Long-Term Care Medicine (AMDA) lists its state chapters,32 and the National Association for Home Care & Hospice lists national HHC agencies.33 Reviewing early lessons learned in the evaluation of PAC providers as potential hospital partners in Pioneer accountable care organizations may be helpful,34 though the PAC cost savings in these organizations largely resulted from redirecting patients from SNFs to HHC providers.35,36 In many markets, the relationships between hospitals and PAC providers may become more formalized, leading to vertical integration.37 Hospitalists may increasingly be asked to work with, or even in, SNFs.38 For hospitalists who begin working in PAC, the AMDA is developing an educational curriculum to maximize efficacy in a new practice setting.39 In other markets, hospitals may turn to for-profit entities that provide “integrated post-acute care services,”40 taking over PAC decision making from inpatient teams and sharing any resulting profits
from bundled payments.
OPPORTUNITIES FOR HOSPITALISTS
Improve Hospital and Transitional Care to Ensure Successful Early Outcomes in PAC
Payment reform ensures hospitalists will increasingly have a stake in these matters, as joint responsibility for costs and outcomes increases for patients discharged to PAC. Hospitalists play a major role in these outcomes by deciding when and where to discharge patients and ensuring that optimal transition-of-care processes are used.8-10,41-45 Although no single intervention has been prospectively found to improve hospital-to-PAC transitional care outcomes, areas in need of improvement are known. Table 2 lists these within 9 of the Ideal Transition of Care Framework domains.43,46
Advocate Patient-Centered PAC Placement That Maximizes Long-Term Outcomes
Payment reforms could reinforce the cynical view that the optimal PAC setting is the least costly one that avoids hospital readmission. This view does not incorporate evidence that, in some cases, placement in a more costly PAC setting results in better long-term outcomes (eg, community discharge rates).47,48 It is also incongruent with a holistic view of the patient’s needs, particularly for patients who may otherwise be suitable for home-based PAC but have limited social support.49 Finally, it does not acknowledge the reality that patients who are inadequately rehabilitated often transition to long-term nursing home care,50 which could result in significant cost-shifting from Medicare to Medicaid, the predominant payer for long-term care.51 Given the extraordinary cost of long-term nursing home care, attending only to short-term costs and outcomes could increase national healthcare expenditures.
With most PAC-related decisions being made in the hospital, hospitalists find themselves at the center of a care team that must advocate the PAC that is best for the patient over the long term. This endeavor requires that hospitalists and others work for improvements in at least 3 aspects of in-hospital care. First, systems for accurately and reliably identifying patient factors that could substantially affect ability to rehabilitate (eg delirium) must be developed or enhanced.52-54 Second, more formal evaluation of the ability of patients and their caregivers to succeed at home is needed.55-60 Patients and caregivers may not understand their home needs without first “testing” the experience prior to discharge.61 Third, hospitalists must understand PAC in order to provide safe transitions.16 It is logistically challenging to expose practicing hospitalists to PAC, and it is unclear which exposures are most effective in improving decision making.62 An alternative approach that provides hospitalists with feedback about the short- and long-term outcomes of patients they have discharged to PAC may iteratively improve decision making. However, despite the high rate of discharges to PAC, there are anecdotal reports that few hospitalists receive feedback on patient outcomes.
As these reforms are tested and implemented, advocacy at regional and national levels is needed. The American Geriatrics Society (AGS), the AMDA, and the American Academy of Home Care Medicine all have well-developed advocacy platforms hospitalists can access.63-65
Share Expertise to Improve Quality in a Constrained Environment
There are opportunities for synergy between robust quality improvement (QI) efforts in PAC (often as part of Quality Assurance and Performance Improvement programs) and similarly robust hospital QI efforts led by hospitalists.66-70 These efforts have largely occurred in parallel, but now some important bridging QI interventions (eg, collaborative root cause analyses for patients readmitted after PAC) are starting at some sites, and these may drive improvement across the care spectrum.45 The Society of Hospital Medicine, the AGS, and the AMDA have written White Papers on care transitions that may serve as starting points for
discussion.41,71,72
CONCLUSION
PAC is rapidly changing in response to reform legislation that is intended to address poor outcomes and high costs. Hospitalists will increasingly feel the effects of these reforms in their day-to-day practices. To continue to deliver high-value care, hospitalists should review their in-hospital and transitional care practices and start building relationships with high-quality PAC providers in their community.
Disclosures: Dr. Burke was supported by a VA Health Services Research and Development Service career development award and by National Institute on Aging grant R03 AG050885. The funders had no role in the design, conduct, interpretation, or presentation of the data. The other authors have nothing to report. The views represented here are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. Kosecoff J, Kahn KL, Rogers WH, et al. Prospective payment system and impairment at discharge. The ‘quicker-and-sicker’ story revisited. JAMA. 1990;264(15):1980-1983. PubMed
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. PubMed
3. Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc. 2015;63(6):1265-1266. PubMed
4. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. PubMed
5. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff (Millwood). 2013;32(5):864-872. PubMed
6. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57-64. PubMed
7. Kramer A, Fish R, Min S. Community Discharge and Rehospitalization Outcome Measures (Fiscal Year 2011): Final Report. Denver, CO: Providigm. http://67.59.137.244/documents/Apr13_CommunityDischarge_CONTRACTOR.pdf. Published April 15, 2013. Accessed March 24, 2016.
8. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255. PubMed
9. Levinson DR. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Washington, DC: Office of the Inspector General, US Dept of Health and Human Services. Report OEI-06-11-00370. http://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf. Published February 2014. Accessed March 5, 2014.
10. Ouslander JG, Naharci I, Engstrom G, et al. Lessons learned from root cause analyses of transfers of skilled nursing facility (SNF) patients to acute hospitals: transfers rated as preventable versus nonpreventable by SNF staff. J Am Med Dir Assoc. 2016;17(7):596-601. PubMed
11. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
12. Kane RL. Finding the right level of posthospital care: “We didn’t realize there was any other option for him.” JAMA. 2011;305(3):284-293. PubMed
13. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. PubMed
14. Kane RL, Lin WC, Blewett LA. Geographic variation in the use of post-acute care. Health Serv Res. 2002;37(3):667-682. PubMed
15. Kuo YF, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152-159. PubMed
16. Ward KT, Eslami MS, Garcia MB, McCreath HE. Do internal medicine residents know enough about skilled nursing facilities to orchestrate a good care transition? J Am Med Dir Assoc. 2014;15(11):841-843. PubMed
17. Centers for Medicare & Medicaid Services. CARE Item Set and B-CARE. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/CARE-Item-Set-and-B-CARE.html. Published January 13, 2015. Accessed November 2, 2015.
18. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Washington, DC: Medicare Payment Advisory Commission. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf. Published March 13, 2015. Accessed December 8, 2015.
19. Block L, Morgan-Gouveia M, Levine RB, Cayea D. We could have done a better job: a qualitative study of medical student reflections on safe hospital discharge.
J Am Geriatr Soc. 2014;62(6):1147-1154. PubMed
20. Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley EH. “Out of sight, out of mind”: housestaff perceptions of quality-limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376-381. PubMed
21. Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354-360. PubMed
22. Hughes SL, Weaver FM, Giobbie-Hurder A, et al.; Department of Veterans Affairs Cooperative Study Group on Home-Based Primary Care. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877-2885. PubMed
23. Kinosian B, Taler G, Boling P, Gilden D; Independence at Home Learning Collaborative Writing Group. Projected savings and workforce transformation from converting Independence at Home to a Medicare benefit. J Am Geriatr Soc. 2016;64(8):1531-1536. PubMed
24. Segelman M, Szydlowski J, Kinosian B, et al. Hospitalizations in the Program of All-Inclusive Care for the Elderly. J Am Geriatr Soc. 2014;62(2):320-324. PubMed
25. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. PubMed
26. Neuman MD, Wirtalla C, Werner RM. Association between skilled nursing facility quality indicators and hospital readmissions. JAMA. 2014;312(15):1542-1551. PubMed
27. Unroe KT, Greiner MA, Colón-Emeric C, Peterson ED, Curtis LH. Associations between published quality ratings of skilled nursing facilities and outcomes of Medicare beneficiaries with heart failure. J Am Med Dir Assoc. 2012;13(2):
188.e1-e6. PubMed
28. Schumacher DN, Dobkin ED. Medicare spending per beneficiary. Health Aff (Millwood). 2014;33(10):1878. PubMed
29. Das A, Norton EC, Miller DC, Chen LM. Association of postdischarge spending and performance on new episode-based spending measure. JAMA Intern Med. 2016;176(1):117-119. PubMed
30. Jubelt LE, Goldfeld KS, Chung WY, Blecker SB, Horwitz LI. Changes in discharge location and readmission rates under Medicare bundled payment. JAMA Intern Med. 2016;176(1):115-117. PubMed
31. Federal Register: Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services. https://www.gpo.gov/fdsys/pkg/FR-2015-11-24/pdf/2015-29438.pdf. Accessed June 20, 2016.
32. Society for Post-Acute and Long-Term Care Medicine. State chapters; AMDA state chapter network. http://www.paltc.org/state-chapters. Accessed June 6, 2016.
33. National Association for Home Care & Hospice. National agency location service. https://agencylocator.nahc.org. Accessed June 6, 2016.
34. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. PubMed
35. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer accountable care organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
36. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of Pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927-1936. PubMed
37. Afendulis CC, Kessler DP. Vertical integration and optimal reimbursement policy. Int J Health Care Finance Econ. 2011;11(3):165-179. PubMed
38. IPC Healthcare. Post-acute care services. http://www.ipchealthcare.com/advantage/post-acute-care. Accessed January 11, 2016.
39. Society for Post-Acute and Long-Term Care Medicine. Competencies curriculum for post-acute and long-term care medicine. http://www.paltc.org/competencies-curriculum-post-acute-and-long-term-care-medicine. Accessed May 26, 2016.
40. NaviHealth. Leading the way in a rapidly changing PAC marketplace. http://www.navihealth.us/home/solutions/services. Accessed January 11, 2016.
41. Arbaje AI, Kansagara DL, Salanitro AH, et al. Regardless of age: incorporating principles from geriatric medicine to improve care transitions for patients with complex needs. J Gen Intern Med. 2014;29(6):932-939. PubMed
42. Schoenborn NL, Arbaje AI, Eubank KJ, Maynor K, Carrese JA. Clinician roles and responsibilities during care transitions of older adults. J Am Geriatr Soc. 2013;61(2):231-236. PubMed
43. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. PubMed
44. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
45. Ouslander JG, Naharci I, Engstrom G, et al. Root cause analyses of transfers of skilled nursing facility patients to acute hospitals: lessons learned for reducing unnecessary hospitalizations. J Am Med Dir Assoc. 2016;17(3):256-262. PubMed
46. Burke RE, Guo R, Prochazka AV, Misky GJ. Identifying keys to success in reducing readmissions using the ideal transitions in care framework. BMC Health Serv Res. 2014;14:423. PubMed
47. Kane RL, Chen Q, Finch M, Blewett L, Burns R, Moskowitz M. Functional outcomes of posthospital care for stroke and hip fracture patients under Medicare. J Am Geriatr Soc. 1998;46(12):1525-1533. PubMed
48. Kane RL, Chen Q, Finch M, Blewett L, Burns R, Moskowitz M. The optimal outcomes of post-hospital care under Medicare. Health Serv Res. 2000;35(3):615-661. PubMed
49. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study [published online January 14, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2015-004570. PubMed
50. Goodwin JS, Howrey B, Zhang DD, Kuo YF. Risk of continued institutionalization after hospitalization in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(12):1321-1327. PubMed
51. Reaves EL, Musumeci M. Medicaid and Long-Term Services and Supports: A Primer. Washington, DC: Kaiser Commission on Medicaid and the Uninsured. Report 8617-02. Henry J. Kaiser Family Foundation website. http://files.kff.org/attachment/report-medicaid-and-long-term-services-and-supports-a-primer. Published December 15, 2015. Accessed May 18, 2016.
52. Bell SP, Vasilevskis EE, Saraf AA, et al. Geriatric syndromes in hospitalized older adults discharged to skilled nursing facilities. J Am Geriatr Soc. 2016;64(4):
715-722. PubMed
53. Cawthon C, Mion LC, Willens DE, Roumie CL, Kripalani S. Implementing routine health literacy assessment in hospital and primary care patients. Jt Comm J Qual Patient Saf. 2014;40(2):68-76. PubMed
54. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
55. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. PubMed
56. Coleman EA, Min SJ. Patients’ and family caregivers’ goals for care during transitions out of the hospital. Home Health Care Serv Q. 2015;34(3-4):173-184. PubMed
57. Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52(11):1817-1825. PubMed
58. Coleman EA, Ground KL, Maul A. The Family Caregiver Activation in Transitions (FCAT) tool: a new measure of family caregiver self-efficacy. Jt Comm J Qual Patient Saf. 2015;41(11):502-507. PubMed
59. Cain CH, Neuwirth E, Bellows J, Zuber C, Green J. Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. PubMed
J Hosp Med. 2012;7(5):382-387.
60. Burke RE, Jones J, Ho PM, Bekelman DB. Caregivers’ perceived roles in caring for patients with heart failure: what do clinicians need to know? J Card Fail. 2014;20(10):731-738. PubMed
61. Coleman EA. Extending simulation learning experiences to patients with chronic health conditions. JAMA. 2014;311(3):243-244. PubMed
62. Meade LB, Hall SL, Kleppel RW, Hinchey KT. TRACER: an ‘eye-opener’ to the patient experience across the transition of care in an internal medicine resident program. J Community Hosp Intern Med Perspect. 2015;5(2):26230. PubMed
63. American Geriatrics Society. Public policy & advocacy. http://www.americangeriatrics.org/advocacy_public_policy. Accessed May 26, 2016.
64. Society for Post-Acute and Long-Term Care Medicine. Public policy. http://www.paltc.org/public-policy. Accessed May 26, 2016.
65. American Academy of Home Care Medicine. Public policy. http://www.aahcm.org/?page=Public_Policy. Accessed May 26, 2016.
66. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
67. Unroe KT, Nazir A, Holtz LR, et al. The Optimizing Patient Transfers, Impacting Medical Quality, and Improving Symptoms: Transforming Institutional Care approach: preliminary data from the implementation of a Centers for Medicare and Medicaid Services nursing facility demonstration project. J Am Geriatr Soc. 2015;63(1):165-169. PubMed
68. Meehan TP Sr, Qazi DJ, Van Hoof TJ, et al. Process evaluation of a quality improvement project to decrease hospital readmissions from skilled nursing facilities. J Am Med Dir Assoc. 2015;16(8):648-653. PubMed
69. Gillespie SM, Olsan T, Liebel D, et al. Pioneering a nursing home quality improvement learning collaborative: a case study of method and lessons learned. J Am Med Dir Assoc. 2016;17(2):136-141. PubMed
70. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
71. Snow V, Beck D, Budnitz T, et al. Transitions of Care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364-370. PubMed
72. Lett JE 2nd. AMDA national engagement in care transitions. J Am Med Dir Assoc. 2011;12(5):387. PubMed
73. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 suppl):I49-I61. PubMed
74. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. PubMed
75. Bowles KH, Ratcliffe SJ, Holmes JH, Liberatore M, Nydick R, Naylor MD. Post-acute referral decisions made by multidisciplinary experts compared to hospital clinicians and the patients’ 12-week outcomes. Med Care. 2008;46(2):158-166. PubMed
76. Kane RL, Bershadsky B, Bershadsky J. Who recommends long-term care matters. Gerontologist. 2006;46(4):474-482. PubMed
77. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
78. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
79. Falvey JR, Burke RE, Malone D, Ridgeway KJ, McManus BM, Stevens-Lapsley JE. Role of physical therapists in reducing hospital readmissions: optimizing outcomes for older adults during care transitions from hospital to community. Phys Ther. 2016;96(8):1125-1134. PubMed
80. Levy CR, Fish R, Kramer A. Do-not-resuscitate and do-not-hospitalize directives of persons admitted to skilled nursing facilities under the Medicare benefit. J Am Geriatr Soc. 2005;53(12):2060-2068. PubMed
81. Boockvar KS, Fridman B, Marturano C. Ineffective communication of mental status information during care transfer of older adults. J Gen Intern Med. 2005;20(12):1146-1150. PubMed
82. Kiely DK, Bergmann MA, Murphy KM, Jones RN, Orav EJ, Marcantonio ER. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003;58(5):M441-M445. PubMed
83. Kind AJ, Thorpe CT, Sattin JA, Walz SE, Smith MA. Provider characteristics, clinical-work processes and their relationship to discharge summary quality for sub-acute care patients. J Gen Intern Med. 2012;27(1):78-84. PubMed
84. King BJ, Gilmore-Bykovskyi AL, Roiland RA, Polnaszek BE, Bowers BJ, Kind AJ. The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095-1102. PubMed
85. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. PubMed
86. Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med. 2009;24(5):630-635. PubMed
87. Vogelsmeier A. Identifying medication order discrepancies during medication reconciliation: perceptions of nursing home leaders and staff. J Nurs Manag. 2014;22(3):362-372. PubMed
88. Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med. 2004;164(5):545-550. PubMed
89. Sinvani LD, Beizer J, Akerman M, et al. Medication reconciliation in continuum of care transitions: a moving target. J Am Med Dir Assoc. 2013;14(9):668-672. PubMed
90. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834. PubMed
91. Marcantonio ER, Bergmann MA, Kiely DK, Orav EJ, Jones RN. Randomized trial of a delirium abatement program for postacute skilled nursing facilities. J Am Geriatr Soc. 2010;58(6):1019-1026. PubMed
92. Callahan CM, Tu W, Unroe KT, LaMantia MA, Stump TE, Clark DO. Transitions in care in a nationally representative sample of older Americans with dementia. PubMed
J Am Geriatr Soc. 2015;63(8):1495-1502.
93. Givens JL, Mitchell SL, Kuo S, Gozalo P, Mor V, Teno J. Skilled nursing facility admissions of nursing home residents with advanced dementia. J Am Geriatr Soc. 2013;61(10):1645-1650. PubMed
94. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. PubMed
95. Ottenbacher KJ, Karmarkar A, Graham JE, et al. Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA. 2014;311(6):604-614. PubMed
96. Konetzka RT, Grabowski DC, Perraillon MC, Werner RM. Nursing home 5-star rating system exacerbates disparities in quality, by payer source. Health Aff (Millwood). 2015;34(5):819-827. PubMed
97. Williams A, Straker JK, Applebaum R. The nursing home five star rating: how does it compare to resident and family views of care? Gerontologist. 2016;56(2):234-242. PubMed
98. Caplan GA, Meller A, Squires B, Chan S, Willett W. Advance care planning and hospital in the nursing home. Age Ageing. 2006;35(6):581-585. PubMed
99. Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190. PubMed
100. Gill TM, Gahbauer EA, Han L, Allore HG. The role of intervening hospital admissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ. 2015;350:h2361. PubMed
101. Levy C, Morris M, Kramer A. Improving end-of-life outcomes in nursing homes by targeting residents at high-risk of mortality for palliative care: program description and evaluation. J Palliat Med. 2008;11(2):217-225. PubMed
102. Miller SC, Lima JC, Looze J, Mitchell SL. Dying in U.S. nursing homes with advanced dementia: how does health care use differ for residents with, versus without, end-of-life Medicare skilled nursing facility care? J Palliat Med. 2012;15(1):
43-50. PubMed
103. Halm EA, Magaziner J, Hannan EL, et al. Frequency and impact of active clinical issues and new impairments on hospital discharge in patients with hip fracture. Arch Intern Med. 2003;163(1):108-113. PubMed
104. Thomas KS, Mor V, Tyler DA, Hyer K. The relationships among licensed nurse turnover, retention, and rehospitalization of nursing home residents. Gerontologist. 2013;53(2):211-221. PubMed
The landscape of post–acute care (PAC), which is predominantly provided by inpatient rehabilitation facilities (IRFs), skilled nursing facilities (SNFs), and home healthcare (HHC) providers, is rapidly changing. As hospitalizations shorten, PAC utilization is rising, resulting in rapidly increasing costs.1-5 However, patient outcomes in PAC are characterized by high rates of readmission and low rates of return to the community.6,7 Emerging evidence suggests these outcomes could be substantially improved through use of better in-hospital and transitional care processes.8-10
Legislators took notice of the spiraling costs, potential quality concerns, and undesirable patient outcomes in PAC. Provisions in the Patient Protection and Affordable Care Act of 2010 (ACA), the Protecting Access to Medicare Act of 2014 (PAMA), and the Improving Medicare Post–Acute Care Transformation (IMPACT) Act of 2014 affect patient selection, payment, and quality measurement in PAC. As older adults are increasingly being cared for by hospitalists,11 hospitalists must be aware of the implications of these reforms.
IMPLICATIONS FOR HOSPITALISTS
Choosing Patients Wisely for PAC
Because PAC-related decision making is not standardized, referral rates vary significantly.12 The variability in PAC use accounts for 79% of all regional variation in Medicare spending in the United States.13,14 Compared with other physicians, hospitalists are more likely to use PAC15 but typically receive little exposure to PAC during training.16
The IMPACT Act proposes 2 major changes to patient selection: a uniform assessment tool for patients being discharged to PAC and “site-neutral” payments for PAC. Starting in 2018, the Continuity Assessment Record and Evaluation (CARE) tool must be completed before a hospital discharge in order to better match PAC resources to patient needs. The current 26-page CARE tool includes questions about demographics and home support, medical complexity, physical function, cognitive status, and “transition items,” including discharge plans and advance directives. In pilot testing, significant amounts of missing data and average completion times of up to 60 minutes raised concerns about feasibility.17 CARE tool assessments accurately predicted what form of PAC patients actually received, but further testing is planned to validate whether the type of PAC selected was optimal for patient outcomes.. A plan for using CARE tool assessments to determine site-neutral payments is due to Congress by 2020. In the site-neutral payment system, the PAC provider will be reimbursed according to patient needs (identified by the CARE tool), regardless of PAC setting—a radical change from the current system, in which IRF, SNF, and HHC episodes show major differences in median costs (Table 1).18
Hospitalists may be concerned that use of the CARE tool will supplant clinical judgment about patients’ PAC needs. The burden of completing the CARE tool could inadvertently reduce the amount of attention hospitalists give to other aspects of a safe discharge rather than lead to the improvement desired.19-21 Hospitalists will benefit from developing interdisciplinary, iterative workflows to complete the tool, improving accuracy and reducing the burden.
A potential unintended consequence of the site-neutral payment system may be increased difficulty discharging elderly patients who have limited rehabilitation potential but are lacking sufficient social support to return home. In the current system, these patients are commonly discharged to SNFs as a bridge to long-term nursing home care. Hospitalists will need to become increasingly familiar with novel alternatives to nursing home–based care, such as home-based primary care, medical foster homes, and Medicare/Medicaid’s Program of All-Inclusive Care of the Elderly (PACE).22-25
Choosing PAC Providers Wisely
Medicare’s Nursing Home Compare tool (https://www.medicare.gov/nursinghomecompare/search.html) provides a “5-star” system for rating SNFs on several quality metrics; these metrics, however, are not correlated with readmission or mortality rates.26,27 Improving quality measurement in PAC and tying payment to quality and outcomes are major emphases of the IMPACT Act and PAMA, respectively. PAC providers must publicly report an expanded list of quality measures and outcomes by 2018. In 2017, SNFs will begin reporting rates of “potentially preventable” readmissions, and starting in 2019 they will face penalties for having high
risk-adjusted rates.
These reforms coincide with an increased emphasis on hospitals and PAC providers sharing responsibility for costs and outcomes. One model of the Bundled Payments for Care Improvement (BPCI) initiative includes a single payment for an acute hospitalization and PAC up to 90 days after hospital discharge for select conditions. The Medicare Spending Per Beneficiary (MSPB) measure compares hospitals on their spending for Medicare beneficiaries from 3 days before hospital admission to 30 days after hospital discharge, and penalizes outliers with high costs.28 PAC spending is the main driver of costs in both BPCI and MSPB.29 One way that hospitals have responded to the BPCI is by drastically reducing their referrals to SNFs and increasing their referrals to HHC providers; unfortunately, this response has resulted in increases in post-discharge emergency department visits.29,30 Taking a novel step in November 2015, the Centers for Medicare & Medicaid Services (CMS) ruled that hospitals in more than 67 metropolitan service areas will be involuntarily enrolled in the BPCI initiative, using elective lower extremity joint replacement as the sample condition.31 This ruling signaled that these reforms are not meant solely for “high-performing” hospital and PAC systems able to volunteer for novel models of payment.
These changes have direct implications for hospitalists. Bundled payments incentivize hospitalists to reduce hospital length of stay and choose PAC alternatives with lower costs. SNFs may start accepting fewer “high-risk” patients in order to avoid readmission penalties. Hospitals will need to identify and partner with high-performing PAC providers in their community to maximize outcomes for their patients. On their websites, the Society of Post-Acute and Long-Term Care Medicine (AMDA) lists its state chapters,32 and the National Association for Home Care & Hospice lists national HHC agencies.33 Reviewing early lessons learned in the evaluation of PAC providers as potential hospital partners in Pioneer accountable care organizations may be helpful,34 though the PAC cost savings in these organizations largely resulted from redirecting patients from SNFs to HHC providers.35,36 In many markets, the relationships between hospitals and PAC providers may become more formalized, leading to vertical integration.37 Hospitalists may increasingly be asked to work with, or even in, SNFs.38 For hospitalists who begin working in PAC, the AMDA is developing an educational curriculum to maximize efficacy in a new practice setting.39 In other markets, hospitals may turn to for-profit entities that provide “integrated post-acute care services,”40 taking over PAC decision making from inpatient teams and sharing any resulting profits
from bundled payments.
OPPORTUNITIES FOR HOSPITALISTS
Improve Hospital and Transitional Care to Ensure Successful Early Outcomes in PAC
Payment reform ensures hospitalists will increasingly have a stake in these matters, as joint responsibility for costs and outcomes increases for patients discharged to PAC. Hospitalists play a major role in these outcomes by deciding when and where to discharge patients and ensuring that optimal transition-of-care processes are used.8-10,41-45 Although no single intervention has been prospectively found to improve hospital-to-PAC transitional care outcomes, areas in need of improvement are known. Table 2 lists these within 9 of the Ideal Transition of Care Framework domains.43,46
Advocate Patient-Centered PAC Placement That Maximizes Long-Term Outcomes
Payment reforms could reinforce the cynical view that the optimal PAC setting is the least costly one that avoids hospital readmission. This view does not incorporate evidence that, in some cases, placement in a more costly PAC setting results in better long-term outcomes (eg, community discharge rates).47,48 It is also incongruent with a holistic view of the patient’s needs, particularly for patients who may otherwise be suitable for home-based PAC but have limited social support.49 Finally, it does not acknowledge the reality that patients who are inadequately rehabilitated often transition to long-term nursing home care,50 which could result in significant cost-shifting from Medicare to Medicaid, the predominant payer for long-term care.51 Given the extraordinary cost of long-term nursing home care, attending only to short-term costs and outcomes could increase national healthcare expenditures.
With most PAC-related decisions being made in the hospital, hospitalists find themselves at the center of a care team that must advocate the PAC that is best for the patient over the long term. This endeavor requires that hospitalists and others work for improvements in at least 3 aspects of in-hospital care. First, systems for accurately and reliably identifying patient factors that could substantially affect ability to rehabilitate (eg delirium) must be developed or enhanced.52-54 Second, more formal evaluation of the ability of patients and their caregivers to succeed at home is needed.55-60 Patients and caregivers may not understand their home needs without first “testing” the experience prior to discharge.61 Third, hospitalists must understand PAC in order to provide safe transitions.16 It is logistically challenging to expose practicing hospitalists to PAC, and it is unclear which exposures are most effective in improving decision making.62 An alternative approach that provides hospitalists with feedback about the short- and long-term outcomes of patients they have discharged to PAC may iteratively improve decision making. However, despite the high rate of discharges to PAC, there are anecdotal reports that few hospitalists receive feedback on patient outcomes.
As these reforms are tested and implemented, advocacy at regional and national levels is needed. The American Geriatrics Society (AGS), the AMDA, and the American Academy of Home Care Medicine all have well-developed advocacy platforms hospitalists can access.63-65
Share Expertise to Improve Quality in a Constrained Environment
There are opportunities for synergy between robust quality improvement (QI) efforts in PAC (often as part of Quality Assurance and Performance Improvement programs) and similarly robust hospital QI efforts led by hospitalists.66-70 These efforts have largely occurred in parallel, but now some important bridging QI interventions (eg, collaborative root cause analyses for patients readmitted after PAC) are starting at some sites, and these may drive improvement across the care spectrum.45 The Society of Hospital Medicine, the AGS, and the AMDA have written White Papers on care transitions that may serve as starting points for
discussion.41,71,72
CONCLUSION
PAC is rapidly changing in response to reform legislation that is intended to address poor outcomes and high costs. Hospitalists will increasingly feel the effects of these reforms in their day-to-day practices. To continue to deliver high-value care, hospitalists should review their in-hospital and transitional care practices and start building relationships with high-quality PAC providers in their community.
Disclosures: Dr. Burke was supported by a VA Health Services Research and Development Service career development award and by National Institute on Aging grant R03 AG050885. The funders had no role in the design, conduct, interpretation, or presentation of the data. The other authors have nothing to report. The views represented here are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
The landscape of post–acute care (PAC), which is predominantly provided by inpatient rehabilitation facilities (IRFs), skilled nursing facilities (SNFs), and home healthcare (HHC) providers, is rapidly changing. As hospitalizations shorten, PAC utilization is rising, resulting in rapidly increasing costs.1-5 However, patient outcomes in PAC are characterized by high rates of readmission and low rates of return to the community.6,7 Emerging evidence suggests these outcomes could be substantially improved through use of better in-hospital and transitional care processes.8-10
Legislators took notice of the spiraling costs, potential quality concerns, and undesirable patient outcomes in PAC. Provisions in the Patient Protection and Affordable Care Act of 2010 (ACA), the Protecting Access to Medicare Act of 2014 (PAMA), and the Improving Medicare Post–Acute Care Transformation (IMPACT) Act of 2014 affect patient selection, payment, and quality measurement in PAC. As older adults are increasingly being cared for by hospitalists,11 hospitalists must be aware of the implications of these reforms.
IMPLICATIONS FOR HOSPITALISTS
Choosing Patients Wisely for PAC
Because PAC-related decision making is not standardized, referral rates vary significantly.12 The variability in PAC use accounts for 79% of all regional variation in Medicare spending in the United States.13,14 Compared with other physicians, hospitalists are more likely to use PAC15 but typically receive little exposure to PAC during training.16
The IMPACT Act proposes 2 major changes to patient selection: a uniform assessment tool for patients being discharged to PAC and “site-neutral” payments for PAC. Starting in 2018, the Continuity Assessment Record and Evaluation (CARE) tool must be completed before a hospital discharge in order to better match PAC resources to patient needs. The current 26-page CARE tool includes questions about demographics and home support, medical complexity, physical function, cognitive status, and “transition items,” including discharge plans and advance directives. In pilot testing, significant amounts of missing data and average completion times of up to 60 minutes raised concerns about feasibility.17 CARE tool assessments accurately predicted what form of PAC patients actually received, but further testing is planned to validate whether the type of PAC selected was optimal for patient outcomes.. A plan for using CARE tool assessments to determine site-neutral payments is due to Congress by 2020. In the site-neutral payment system, the PAC provider will be reimbursed according to patient needs (identified by the CARE tool), regardless of PAC setting—a radical change from the current system, in which IRF, SNF, and HHC episodes show major differences in median costs (Table 1).18
Hospitalists may be concerned that use of the CARE tool will supplant clinical judgment about patients’ PAC needs. The burden of completing the CARE tool could inadvertently reduce the amount of attention hospitalists give to other aspects of a safe discharge rather than lead to the improvement desired.19-21 Hospitalists will benefit from developing interdisciplinary, iterative workflows to complete the tool, improving accuracy and reducing the burden.
A potential unintended consequence of the site-neutral payment system may be increased difficulty discharging elderly patients who have limited rehabilitation potential but are lacking sufficient social support to return home. In the current system, these patients are commonly discharged to SNFs as a bridge to long-term nursing home care. Hospitalists will need to become increasingly familiar with novel alternatives to nursing home–based care, such as home-based primary care, medical foster homes, and Medicare/Medicaid’s Program of All-Inclusive Care of the Elderly (PACE).22-25
Choosing PAC Providers Wisely
Medicare’s Nursing Home Compare tool (https://www.medicare.gov/nursinghomecompare/search.html) provides a “5-star” system for rating SNFs on several quality metrics; these metrics, however, are not correlated with readmission or mortality rates.26,27 Improving quality measurement in PAC and tying payment to quality and outcomes are major emphases of the IMPACT Act and PAMA, respectively. PAC providers must publicly report an expanded list of quality measures and outcomes by 2018. In 2017, SNFs will begin reporting rates of “potentially preventable” readmissions, and starting in 2019 they will face penalties for having high
risk-adjusted rates.
These reforms coincide with an increased emphasis on hospitals and PAC providers sharing responsibility for costs and outcomes. One model of the Bundled Payments for Care Improvement (BPCI) initiative includes a single payment for an acute hospitalization and PAC up to 90 days after hospital discharge for select conditions. The Medicare Spending Per Beneficiary (MSPB) measure compares hospitals on their spending for Medicare beneficiaries from 3 days before hospital admission to 30 days after hospital discharge, and penalizes outliers with high costs.28 PAC spending is the main driver of costs in both BPCI and MSPB.29 One way that hospitals have responded to the BPCI is by drastically reducing their referrals to SNFs and increasing their referrals to HHC providers; unfortunately, this response has resulted in increases in post-discharge emergency department visits.29,30 Taking a novel step in November 2015, the Centers for Medicare & Medicaid Services (CMS) ruled that hospitals in more than 67 metropolitan service areas will be involuntarily enrolled in the BPCI initiative, using elective lower extremity joint replacement as the sample condition.31 This ruling signaled that these reforms are not meant solely for “high-performing” hospital and PAC systems able to volunteer for novel models of payment.
These changes have direct implications for hospitalists. Bundled payments incentivize hospitalists to reduce hospital length of stay and choose PAC alternatives with lower costs. SNFs may start accepting fewer “high-risk” patients in order to avoid readmission penalties. Hospitals will need to identify and partner with high-performing PAC providers in their community to maximize outcomes for their patients. On their websites, the Society of Post-Acute and Long-Term Care Medicine (AMDA) lists its state chapters,32 and the National Association for Home Care & Hospice lists national HHC agencies.33 Reviewing early lessons learned in the evaluation of PAC providers as potential hospital partners in Pioneer accountable care organizations may be helpful,34 though the PAC cost savings in these organizations largely resulted from redirecting patients from SNFs to HHC providers.35,36 In many markets, the relationships between hospitals and PAC providers may become more formalized, leading to vertical integration.37 Hospitalists may increasingly be asked to work with, or even in, SNFs.38 For hospitalists who begin working in PAC, the AMDA is developing an educational curriculum to maximize efficacy in a new practice setting.39 In other markets, hospitals may turn to for-profit entities that provide “integrated post-acute care services,”40 taking over PAC decision making from inpatient teams and sharing any resulting profits
from bundled payments.
OPPORTUNITIES FOR HOSPITALISTS
Improve Hospital and Transitional Care to Ensure Successful Early Outcomes in PAC
Payment reform ensures hospitalists will increasingly have a stake in these matters, as joint responsibility for costs and outcomes increases for patients discharged to PAC. Hospitalists play a major role in these outcomes by deciding when and where to discharge patients and ensuring that optimal transition-of-care processes are used.8-10,41-45 Although no single intervention has been prospectively found to improve hospital-to-PAC transitional care outcomes, areas in need of improvement are known. Table 2 lists these within 9 of the Ideal Transition of Care Framework domains.43,46
Advocate Patient-Centered PAC Placement That Maximizes Long-Term Outcomes
Payment reforms could reinforce the cynical view that the optimal PAC setting is the least costly one that avoids hospital readmission. This view does not incorporate evidence that, in some cases, placement in a more costly PAC setting results in better long-term outcomes (eg, community discharge rates).47,48 It is also incongruent with a holistic view of the patient’s needs, particularly for patients who may otherwise be suitable for home-based PAC but have limited social support.49 Finally, it does not acknowledge the reality that patients who are inadequately rehabilitated often transition to long-term nursing home care,50 which could result in significant cost-shifting from Medicare to Medicaid, the predominant payer for long-term care.51 Given the extraordinary cost of long-term nursing home care, attending only to short-term costs and outcomes could increase national healthcare expenditures.
With most PAC-related decisions being made in the hospital, hospitalists find themselves at the center of a care team that must advocate the PAC that is best for the patient over the long term. This endeavor requires that hospitalists and others work for improvements in at least 3 aspects of in-hospital care. First, systems for accurately and reliably identifying patient factors that could substantially affect ability to rehabilitate (eg delirium) must be developed or enhanced.52-54 Second, more formal evaluation of the ability of patients and their caregivers to succeed at home is needed.55-60 Patients and caregivers may not understand their home needs without first “testing” the experience prior to discharge.61 Third, hospitalists must understand PAC in order to provide safe transitions.16 It is logistically challenging to expose practicing hospitalists to PAC, and it is unclear which exposures are most effective in improving decision making.62 An alternative approach that provides hospitalists with feedback about the short- and long-term outcomes of patients they have discharged to PAC may iteratively improve decision making. However, despite the high rate of discharges to PAC, there are anecdotal reports that few hospitalists receive feedback on patient outcomes.
As these reforms are tested and implemented, advocacy at regional and national levels is needed. The American Geriatrics Society (AGS), the AMDA, and the American Academy of Home Care Medicine all have well-developed advocacy platforms hospitalists can access.63-65
Share Expertise to Improve Quality in a Constrained Environment
There are opportunities for synergy between robust quality improvement (QI) efforts in PAC (often as part of Quality Assurance and Performance Improvement programs) and similarly robust hospital QI efforts led by hospitalists.66-70 These efforts have largely occurred in parallel, but now some important bridging QI interventions (eg, collaborative root cause analyses for patients readmitted after PAC) are starting at some sites, and these may drive improvement across the care spectrum.45 The Society of Hospital Medicine, the AGS, and the AMDA have written White Papers on care transitions that may serve as starting points for
discussion.41,71,72
CONCLUSION
PAC is rapidly changing in response to reform legislation that is intended to address poor outcomes and high costs. Hospitalists will increasingly feel the effects of these reforms in their day-to-day practices. To continue to deliver high-value care, hospitalists should review their in-hospital and transitional care practices and start building relationships with high-quality PAC providers in their community.
Disclosures: Dr. Burke was supported by a VA Health Services Research and Development Service career development award and by National Institute on Aging grant R03 AG050885. The funders had no role in the design, conduct, interpretation, or presentation of the data. The other authors have nothing to report. The views represented here are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. Kosecoff J, Kahn KL, Rogers WH, et al. Prospective payment system and impairment at discharge. The ‘quicker-and-sicker’ story revisited. JAMA. 1990;264(15):1980-1983. PubMed
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. PubMed
3. Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc. 2015;63(6):1265-1266. PubMed
4. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. PubMed
5. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff (Millwood). 2013;32(5):864-872. PubMed
6. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57-64. PubMed
7. Kramer A, Fish R, Min S. Community Discharge and Rehospitalization Outcome Measures (Fiscal Year 2011): Final Report. Denver, CO: Providigm. http://67.59.137.244/documents/Apr13_CommunityDischarge_CONTRACTOR.pdf. Published April 15, 2013. Accessed March 24, 2016.
8. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255. PubMed
9. Levinson DR. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Washington, DC: Office of the Inspector General, US Dept of Health and Human Services. Report OEI-06-11-00370. http://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf. Published February 2014. Accessed March 5, 2014.
10. Ouslander JG, Naharci I, Engstrom G, et al. Lessons learned from root cause analyses of transfers of skilled nursing facility (SNF) patients to acute hospitals: transfers rated as preventable versus nonpreventable by SNF staff. J Am Med Dir Assoc. 2016;17(7):596-601. PubMed
11. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
12. Kane RL. Finding the right level of posthospital care: “We didn’t realize there was any other option for him.” JAMA. 2011;305(3):284-293. PubMed
13. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. PubMed
14. Kane RL, Lin WC, Blewett LA. Geographic variation in the use of post-acute care. Health Serv Res. 2002;37(3):667-682. PubMed
15. Kuo YF, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152-159. PubMed
16. Ward KT, Eslami MS, Garcia MB, McCreath HE. Do internal medicine residents know enough about skilled nursing facilities to orchestrate a good care transition? J Am Med Dir Assoc. 2014;15(11):841-843. PubMed
17. Centers for Medicare & Medicaid Services. CARE Item Set and B-CARE. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/CARE-Item-Set-and-B-CARE.html. Published January 13, 2015. Accessed November 2, 2015.
18. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Washington, DC: Medicare Payment Advisory Commission. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf. Published March 13, 2015. Accessed December 8, 2015.
19. Block L, Morgan-Gouveia M, Levine RB, Cayea D. We could have done a better job: a qualitative study of medical student reflections on safe hospital discharge.
J Am Geriatr Soc. 2014;62(6):1147-1154. PubMed
20. Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley EH. “Out of sight, out of mind”: housestaff perceptions of quality-limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376-381. PubMed
21. Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354-360. PubMed
22. Hughes SL, Weaver FM, Giobbie-Hurder A, et al.; Department of Veterans Affairs Cooperative Study Group on Home-Based Primary Care. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877-2885. PubMed
23. Kinosian B, Taler G, Boling P, Gilden D; Independence at Home Learning Collaborative Writing Group. Projected savings and workforce transformation from converting Independence at Home to a Medicare benefit. J Am Geriatr Soc. 2016;64(8):1531-1536. PubMed
24. Segelman M, Szydlowski J, Kinosian B, et al. Hospitalizations in the Program of All-Inclusive Care for the Elderly. J Am Geriatr Soc. 2014;62(2):320-324. PubMed
25. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. PubMed
26. Neuman MD, Wirtalla C, Werner RM. Association between skilled nursing facility quality indicators and hospital readmissions. JAMA. 2014;312(15):1542-1551. PubMed
27. Unroe KT, Greiner MA, Colón-Emeric C, Peterson ED, Curtis LH. Associations between published quality ratings of skilled nursing facilities and outcomes of Medicare beneficiaries with heart failure. J Am Med Dir Assoc. 2012;13(2):
188.e1-e6. PubMed
28. Schumacher DN, Dobkin ED. Medicare spending per beneficiary. Health Aff (Millwood). 2014;33(10):1878. PubMed
29. Das A, Norton EC, Miller DC, Chen LM. Association of postdischarge spending and performance on new episode-based spending measure. JAMA Intern Med. 2016;176(1):117-119. PubMed
30. Jubelt LE, Goldfeld KS, Chung WY, Blecker SB, Horwitz LI. Changes in discharge location and readmission rates under Medicare bundled payment. JAMA Intern Med. 2016;176(1):115-117. PubMed
31. Federal Register: Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services. https://www.gpo.gov/fdsys/pkg/FR-2015-11-24/pdf/2015-29438.pdf. Accessed June 20, 2016.
32. Society for Post-Acute and Long-Term Care Medicine. State chapters; AMDA state chapter network. http://www.paltc.org/state-chapters. Accessed June 6, 2016.
33. National Association for Home Care & Hospice. National agency location service. https://agencylocator.nahc.org. Accessed June 6, 2016.
34. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. PubMed
35. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer accountable care organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
36. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of Pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927-1936. PubMed
37. Afendulis CC, Kessler DP. Vertical integration and optimal reimbursement policy. Int J Health Care Finance Econ. 2011;11(3):165-179. PubMed
38. IPC Healthcare. Post-acute care services. http://www.ipchealthcare.com/advantage/post-acute-care. Accessed January 11, 2016.
39. Society for Post-Acute and Long-Term Care Medicine. Competencies curriculum for post-acute and long-term care medicine. http://www.paltc.org/competencies-curriculum-post-acute-and-long-term-care-medicine. Accessed May 26, 2016.
40. NaviHealth. Leading the way in a rapidly changing PAC marketplace. http://www.navihealth.us/home/solutions/services. Accessed January 11, 2016.
41. Arbaje AI, Kansagara DL, Salanitro AH, et al. Regardless of age: incorporating principles from geriatric medicine to improve care transitions for patients with complex needs. J Gen Intern Med. 2014;29(6):932-939. PubMed
42. Schoenborn NL, Arbaje AI, Eubank KJ, Maynor K, Carrese JA. Clinician roles and responsibilities during care transitions of older adults. J Am Geriatr Soc. 2013;61(2):231-236. PubMed
43. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. PubMed
44. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
45. Ouslander JG, Naharci I, Engstrom G, et al. Root cause analyses of transfers of skilled nursing facility patients to acute hospitals: lessons learned for reducing unnecessary hospitalizations. J Am Med Dir Assoc. 2016;17(3):256-262. PubMed
46. Burke RE, Guo R, Prochazka AV, Misky GJ. Identifying keys to success in reducing readmissions using the ideal transitions in care framework. BMC Health Serv Res. 2014;14:423. PubMed
47. Kane RL, Chen Q, Finch M, Blewett L, Burns R, Moskowitz M. Functional outcomes of posthospital care for stroke and hip fracture patients under Medicare. J Am Geriatr Soc. 1998;46(12):1525-1533. PubMed
48. Kane RL, Chen Q, Finch M, Blewett L, Burns R, Moskowitz M. The optimal outcomes of post-hospital care under Medicare. Health Serv Res. 2000;35(3):615-661. PubMed
49. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study [published online January 14, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2015-004570. PubMed
50. Goodwin JS, Howrey B, Zhang DD, Kuo YF. Risk of continued institutionalization after hospitalization in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(12):1321-1327. PubMed
51. Reaves EL, Musumeci M. Medicaid and Long-Term Services and Supports: A Primer. Washington, DC: Kaiser Commission on Medicaid and the Uninsured. Report 8617-02. Henry J. Kaiser Family Foundation website. http://files.kff.org/attachment/report-medicaid-and-long-term-services-and-supports-a-primer. Published December 15, 2015. Accessed May 18, 2016.
52. Bell SP, Vasilevskis EE, Saraf AA, et al. Geriatric syndromes in hospitalized older adults discharged to skilled nursing facilities. J Am Geriatr Soc. 2016;64(4):
715-722. PubMed
53. Cawthon C, Mion LC, Willens DE, Roumie CL, Kripalani S. Implementing routine health literacy assessment in hospital and primary care patients. Jt Comm J Qual Patient Saf. 2014;40(2):68-76. PubMed
54. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
55. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. PubMed
56. Coleman EA, Min SJ. Patients’ and family caregivers’ goals for care during transitions out of the hospital. Home Health Care Serv Q. 2015;34(3-4):173-184. PubMed
57. Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52(11):1817-1825. PubMed
58. Coleman EA, Ground KL, Maul A. The Family Caregiver Activation in Transitions (FCAT) tool: a new measure of family caregiver self-efficacy. Jt Comm J Qual Patient Saf. 2015;41(11):502-507. PubMed
59. Cain CH, Neuwirth E, Bellows J, Zuber C, Green J. Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. PubMed
J Hosp Med. 2012;7(5):382-387.
60. Burke RE, Jones J, Ho PM, Bekelman DB. Caregivers’ perceived roles in caring for patients with heart failure: what do clinicians need to know? J Card Fail. 2014;20(10):731-738. PubMed
61. Coleman EA. Extending simulation learning experiences to patients with chronic health conditions. JAMA. 2014;311(3):243-244. PubMed
62. Meade LB, Hall SL, Kleppel RW, Hinchey KT. TRACER: an ‘eye-opener’ to the patient experience across the transition of care in an internal medicine resident program. J Community Hosp Intern Med Perspect. 2015;5(2):26230. PubMed
63. American Geriatrics Society. Public policy & advocacy. http://www.americangeriatrics.org/advocacy_public_policy. Accessed May 26, 2016.
64. Society for Post-Acute and Long-Term Care Medicine. Public policy. http://www.paltc.org/public-policy. Accessed May 26, 2016.
65. American Academy of Home Care Medicine. Public policy. http://www.aahcm.org/?page=Public_Policy. Accessed May 26, 2016.
66. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
67. Unroe KT, Nazir A, Holtz LR, et al. The Optimizing Patient Transfers, Impacting Medical Quality, and Improving Symptoms: Transforming Institutional Care approach: preliminary data from the implementation of a Centers for Medicare and Medicaid Services nursing facility demonstration project. J Am Geriatr Soc. 2015;63(1):165-169. PubMed
68. Meehan TP Sr, Qazi DJ, Van Hoof TJ, et al. Process evaluation of a quality improvement project to decrease hospital readmissions from skilled nursing facilities. J Am Med Dir Assoc. 2015;16(8):648-653. PubMed
69. Gillespie SM, Olsan T, Liebel D, et al. Pioneering a nursing home quality improvement learning collaborative: a case study of method and lessons learned. J Am Med Dir Assoc. 2016;17(2):136-141. PubMed
70. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
71. Snow V, Beck D, Budnitz T, et al. Transitions of Care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364-370. PubMed
72. Lett JE 2nd. AMDA national engagement in care transitions. J Am Med Dir Assoc. 2011;12(5):387. PubMed
73. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 suppl):I49-I61. PubMed
74. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. PubMed
75. Bowles KH, Ratcliffe SJ, Holmes JH, Liberatore M, Nydick R, Naylor MD. Post-acute referral decisions made by multidisciplinary experts compared to hospital clinicians and the patients’ 12-week outcomes. Med Care. 2008;46(2):158-166. PubMed
76. Kane RL, Bershadsky B, Bershadsky J. Who recommends long-term care matters. Gerontologist. 2006;46(4):474-482. PubMed
77. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
78. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
79. Falvey JR, Burke RE, Malone D, Ridgeway KJ, McManus BM, Stevens-Lapsley JE. Role of physical therapists in reducing hospital readmissions: optimizing outcomes for older adults during care transitions from hospital to community. Phys Ther. 2016;96(8):1125-1134. PubMed
80. Levy CR, Fish R, Kramer A. Do-not-resuscitate and do-not-hospitalize directives of persons admitted to skilled nursing facilities under the Medicare benefit. J Am Geriatr Soc. 2005;53(12):2060-2068. PubMed
81. Boockvar KS, Fridman B, Marturano C. Ineffective communication of mental status information during care transfer of older adults. J Gen Intern Med. 2005;20(12):1146-1150. PubMed
82. Kiely DK, Bergmann MA, Murphy KM, Jones RN, Orav EJ, Marcantonio ER. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003;58(5):M441-M445. PubMed
83. Kind AJ, Thorpe CT, Sattin JA, Walz SE, Smith MA. Provider characteristics, clinical-work processes and their relationship to discharge summary quality for sub-acute care patients. J Gen Intern Med. 2012;27(1):78-84. PubMed
84. King BJ, Gilmore-Bykovskyi AL, Roiland RA, Polnaszek BE, Bowers BJ, Kind AJ. The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095-1102. PubMed
85. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. PubMed
86. Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med. 2009;24(5):630-635. PubMed
87. Vogelsmeier A. Identifying medication order discrepancies during medication reconciliation: perceptions of nursing home leaders and staff. J Nurs Manag. 2014;22(3):362-372. PubMed
88. Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med. 2004;164(5):545-550. PubMed
89. Sinvani LD, Beizer J, Akerman M, et al. Medication reconciliation in continuum of care transitions: a moving target. J Am Med Dir Assoc. 2013;14(9):668-672. PubMed
90. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834. PubMed
91. Marcantonio ER, Bergmann MA, Kiely DK, Orav EJ, Jones RN. Randomized trial of a delirium abatement program for postacute skilled nursing facilities. J Am Geriatr Soc. 2010;58(6):1019-1026. PubMed
92. Callahan CM, Tu W, Unroe KT, LaMantia MA, Stump TE, Clark DO. Transitions in care in a nationally representative sample of older Americans with dementia. PubMed
J Am Geriatr Soc. 2015;63(8):1495-1502.
93. Givens JL, Mitchell SL, Kuo S, Gozalo P, Mor V, Teno J. Skilled nursing facility admissions of nursing home residents with advanced dementia. J Am Geriatr Soc. 2013;61(10):1645-1650. PubMed
94. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. PubMed
95. Ottenbacher KJ, Karmarkar A, Graham JE, et al. Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA. 2014;311(6):604-614. PubMed
96. Konetzka RT, Grabowski DC, Perraillon MC, Werner RM. Nursing home 5-star rating system exacerbates disparities in quality, by payer source. Health Aff (Millwood). 2015;34(5):819-827. PubMed
97. Williams A, Straker JK, Applebaum R. The nursing home five star rating: how does it compare to resident and family views of care? Gerontologist. 2016;56(2):234-242. PubMed
98. Caplan GA, Meller A, Squires B, Chan S, Willett W. Advance care planning and hospital in the nursing home. Age Ageing. 2006;35(6):581-585. PubMed
99. Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190. PubMed
100. Gill TM, Gahbauer EA, Han L, Allore HG. The role of intervening hospital admissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ. 2015;350:h2361. PubMed
101. Levy C, Morris M, Kramer A. Improving end-of-life outcomes in nursing homes by targeting residents at high-risk of mortality for palliative care: program description and evaluation. J Palliat Med. 2008;11(2):217-225. PubMed
102. Miller SC, Lima JC, Looze J, Mitchell SL. Dying in U.S. nursing homes with advanced dementia: how does health care use differ for residents with, versus without, end-of-life Medicare skilled nursing facility care? J Palliat Med. 2012;15(1):
43-50. PubMed
103. Halm EA, Magaziner J, Hannan EL, et al. Frequency and impact of active clinical issues and new impairments on hospital discharge in patients with hip fracture. Arch Intern Med. 2003;163(1):108-113. PubMed
104. Thomas KS, Mor V, Tyler DA, Hyer K. The relationships among licensed nurse turnover, retention, and rehospitalization of nursing home residents. Gerontologist. 2013;53(2):211-221. PubMed
1. Kosecoff J, Kahn KL, Rogers WH, et al. Prospective payment system and impairment at discharge. The ‘quicker-and-sicker’ story revisited. JAMA. 1990;264(15):1980-1983. PubMed
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. PubMed
3. Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc. 2015;63(6):1265-1266. PubMed
4. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. PubMed
5. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff (Millwood). 2013;32(5):864-872. PubMed
6. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57-64. PubMed
7. Kramer A, Fish R, Min S. Community Discharge and Rehospitalization Outcome Measures (Fiscal Year 2011): Final Report. Denver, CO: Providigm. http://67.59.137.244/documents/Apr13_CommunityDischarge_CONTRACTOR.pdf. Published April 15, 2013. Accessed March 24, 2016.
8. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255. PubMed
9. Levinson DR. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Washington, DC: Office of the Inspector General, US Dept of Health and Human Services. Report OEI-06-11-00370. http://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf. Published February 2014. Accessed March 5, 2014.
10. Ouslander JG, Naharci I, Engstrom G, et al. Lessons learned from root cause analyses of transfers of skilled nursing facility (SNF) patients to acute hospitals: transfers rated as preventable versus nonpreventable by SNF staff. J Am Med Dir Assoc. 2016;17(7):596-601. PubMed
11. Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
12. Kane RL. Finding the right level of posthospital care: “We didn’t realize there was any other option for him.” JAMA. 2011;305(3):284-293. PubMed
13. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. PubMed
14. Kane RL, Lin WC, Blewett LA. Geographic variation in the use of post-acute care. Health Serv Res. 2002;37(3):667-682. PubMed
15. Kuo YF, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152-159. PubMed
16. Ward KT, Eslami MS, Garcia MB, McCreath HE. Do internal medicine residents know enough about skilled nursing facilities to orchestrate a good care transition? J Am Med Dir Assoc. 2014;15(11):841-843. PubMed
17. Centers for Medicare & Medicaid Services. CARE Item Set and B-CARE. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/CARE-Item-Set-and-B-CARE.html. Published January 13, 2015. Accessed November 2, 2015.
18. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Washington, DC: Medicare Payment Advisory Commission. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf. Published March 13, 2015. Accessed December 8, 2015.
19. Block L, Morgan-Gouveia M, Levine RB, Cayea D. We could have done a better job: a qualitative study of medical student reflections on safe hospital discharge.
J Am Geriatr Soc. 2014;62(6):1147-1154. PubMed
20. Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley EH. “Out of sight, out of mind”: housestaff perceptions of quality-limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376-381. PubMed
21. Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354-360. PubMed
22. Hughes SL, Weaver FM, Giobbie-Hurder A, et al.; Department of Veterans Affairs Cooperative Study Group on Home-Based Primary Care. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877-2885. PubMed
23. Kinosian B, Taler G, Boling P, Gilden D; Independence at Home Learning Collaborative Writing Group. Projected savings and workforce transformation from converting Independence at Home to a Medicare benefit. J Am Geriatr Soc. 2016;64(8):1531-1536. PubMed
24. Segelman M, Szydlowski J, Kinosian B, et al. Hospitalizations in the Program of All-Inclusive Care for the Elderly. J Am Geriatr Soc. 2014;62(2):320-324. PubMed
25. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. PubMed
26. Neuman MD, Wirtalla C, Werner RM. Association between skilled nursing facility quality indicators and hospital readmissions. JAMA. 2014;312(15):1542-1551. PubMed
27. Unroe KT, Greiner MA, Colón-Emeric C, Peterson ED, Curtis LH. Associations between published quality ratings of skilled nursing facilities and outcomes of Medicare beneficiaries with heart failure. J Am Med Dir Assoc. 2012;13(2):
188.e1-e6. PubMed
28. Schumacher DN, Dobkin ED. Medicare spending per beneficiary. Health Aff (Millwood). 2014;33(10):1878. PubMed
29. Das A, Norton EC, Miller DC, Chen LM. Association of postdischarge spending and performance on new episode-based spending measure. JAMA Intern Med. 2016;176(1):117-119. PubMed
30. Jubelt LE, Goldfeld KS, Chung WY, Blecker SB, Horwitz LI. Changes in discharge location and readmission rates under Medicare bundled payment. JAMA Intern Med. 2016;176(1):115-117. PubMed
31. Federal Register: Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services. https://www.gpo.gov/fdsys/pkg/FR-2015-11-24/pdf/2015-29438.pdf. Accessed June 20, 2016.
32. Society for Post-Acute and Long-Term Care Medicine. State chapters; AMDA state chapter network. http://www.paltc.org/state-chapters. Accessed June 6, 2016.
33. National Association for Home Care & Hospice. National agency location service. https://agencylocator.nahc.org. Accessed June 6, 2016.
34. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. PubMed
35. Nyweide DJ, Lee W, Cuerdon TT, et al. Association of Pioneer accountable care organizations vs traditional Medicare fee for service with spending, utilization, and patient experience. JAMA. 2015;313(21):2152-2161. PubMed
36. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of Pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927-1936. PubMed
37. Afendulis CC, Kessler DP. Vertical integration and optimal reimbursement policy. Int J Health Care Finance Econ. 2011;11(3):165-179. PubMed
38. IPC Healthcare. Post-acute care services. http://www.ipchealthcare.com/advantage/post-acute-care. Accessed January 11, 2016.
39. Society for Post-Acute and Long-Term Care Medicine. Competencies curriculum for post-acute and long-term care medicine. http://www.paltc.org/competencies-curriculum-post-acute-and-long-term-care-medicine. Accessed May 26, 2016.
40. NaviHealth. Leading the way in a rapidly changing PAC marketplace. http://www.navihealth.us/home/solutions/services. Accessed January 11, 2016.
41. Arbaje AI, Kansagara DL, Salanitro AH, et al. Regardless of age: incorporating principles from geriatric medicine to improve care transitions for patients with complex needs. J Gen Intern Med. 2014;29(6):932-939. PubMed
42. Schoenborn NL, Arbaje AI, Eubank KJ, Maynor K, Carrese JA. Clinician roles and responsibilities during care transitions of older adults. J Am Geriatr Soc. 2013;61(2):231-236. PubMed
43. Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102-109. PubMed
44. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
45. Ouslander JG, Naharci I, Engstrom G, et al. Root cause analyses of transfers of skilled nursing facility patients to acute hospitals: lessons learned for reducing unnecessary hospitalizations. J Am Med Dir Assoc. 2016;17(3):256-262. PubMed
46. Burke RE, Guo R, Prochazka AV, Misky GJ. Identifying keys to success in reducing readmissions using the ideal transitions in care framework. BMC Health Serv Res. 2014;14:423. PubMed
47. Kane RL, Chen Q, Finch M, Blewett L, Burns R, Moskowitz M. Functional outcomes of posthospital care for stroke and hip fracture patients under Medicare. J Am Geriatr Soc. 1998;46(12):1525-1533. PubMed
48. Kane RL, Chen Q, Finch M, Blewett L, Burns R, Moskowitz M. The optimal outcomes of post-hospital care under Medicare. Health Serv Res. 2000;35(3):615-661. PubMed
49. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study [published online January 14, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2015-004570. PubMed
50. Goodwin JS, Howrey B, Zhang DD, Kuo YF. Risk of continued institutionalization after hospitalization in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(12):1321-1327. PubMed
51. Reaves EL, Musumeci M. Medicaid and Long-Term Services and Supports: A Primer. Washington, DC: Kaiser Commission on Medicaid and the Uninsured. Report 8617-02. Henry J. Kaiser Family Foundation website. http://files.kff.org/attachment/report-medicaid-and-long-term-services-and-supports-a-primer. Published December 15, 2015. Accessed May 18, 2016.
52. Bell SP, Vasilevskis EE, Saraf AA, et al. Geriatric syndromes in hospitalized older adults discharged to skilled nursing facilities. J Am Geriatr Soc. 2016;64(4):
715-722. PubMed
53. Cawthon C, Mion LC, Willens DE, Roumie CL, Kripalani S. Implementing routine health literacy assessment in hospital and primary care patients. Jt Comm J Qual Patient Saf. 2014;40(2):68-76. PubMed
54. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
55. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. PubMed
56. Coleman EA, Min SJ. Patients’ and family caregivers’ goals for care during transitions out of the hospital. Home Health Care Serv Q. 2015;34(3-4):173-184. PubMed
57. Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52(11):1817-1825. PubMed
58. Coleman EA, Ground KL, Maul A. The Family Caregiver Activation in Transitions (FCAT) tool: a new measure of family caregiver self-efficacy. Jt Comm J Qual Patient Saf. 2015;41(11):502-507. PubMed
59. Cain CH, Neuwirth E, Bellows J, Zuber C, Green J. Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. PubMed
J Hosp Med. 2012;7(5):382-387.
60. Burke RE, Jones J, Ho PM, Bekelman DB. Caregivers’ perceived roles in caring for patients with heart failure: what do clinicians need to know? J Card Fail. 2014;20(10):731-738. PubMed
61. Coleman EA. Extending simulation learning experiences to patients with chronic health conditions. JAMA. 2014;311(3):243-244. PubMed
62. Meade LB, Hall SL, Kleppel RW, Hinchey KT. TRACER: an ‘eye-opener’ to the patient experience across the transition of care in an internal medicine resident program. J Community Hosp Intern Med Perspect. 2015;5(2):26230. PubMed
63. American Geriatrics Society. Public policy & advocacy. http://www.americangeriatrics.org/advocacy_public_policy. Accessed May 26, 2016.
64. Society for Post-Acute and Long-Term Care Medicine. Public policy. http://www.paltc.org/public-policy. Accessed May 26, 2016.
65. American Academy of Home Care Medicine. Public policy. http://www.aahcm.org/?page=Public_Policy. Accessed May 26, 2016.
66. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
67. Unroe KT, Nazir A, Holtz LR, et al. The Optimizing Patient Transfers, Impacting Medical Quality, and Improving Symptoms: Transforming Institutional Care approach: preliminary data from the implementation of a Centers for Medicare and Medicaid Services nursing facility demonstration project. J Am Geriatr Soc. 2015;63(1):165-169. PubMed
68. Meehan TP Sr, Qazi DJ, Van Hoof TJ, et al. Process evaluation of a quality improvement project to decrease hospital readmissions from skilled nursing facilities. J Am Med Dir Assoc. 2015;16(8):648-653. PubMed
69. Gillespie SM, Olsan T, Liebel D, et al. Pioneering a nursing home quality improvement learning collaborative: a case study of method and lessons learned. J Am Med Dir Assoc. 2016;17(2):136-141. PubMed
70. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
71. Snow V, Beck D, Budnitz T, et al. Transitions of Care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364-370. PubMed
72. Lett JE 2nd. AMDA national engagement in care transitions. J Am Med Dir Assoc. 2011;12(5):387. PubMed
73. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 suppl):I49-I61. PubMed
74. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. PubMed
75. Bowles KH, Ratcliffe SJ, Holmes JH, Liberatore M, Nydick R, Naylor MD. Post-acute referral decisions made by multidisciplinary experts compared to hospital clinicians and the patients’ 12-week outcomes. Med Care. 2008;46(2):158-166. PubMed
76. Kane RL, Bershadsky B, Bershadsky J. Who recommends long-term care matters. Gerontologist. 2006;46(4):474-482. PubMed
77. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med. 2011;6(6):313-321. PubMed
78. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
79. Falvey JR, Burke RE, Malone D, Ridgeway KJ, McManus BM, Stevens-Lapsley JE. Role of physical therapists in reducing hospital readmissions: optimizing outcomes for older adults during care transitions from hospital to community. Phys Ther. 2016;96(8):1125-1134. PubMed
80. Levy CR, Fish R, Kramer A. Do-not-resuscitate and do-not-hospitalize directives of persons admitted to skilled nursing facilities under the Medicare benefit. J Am Geriatr Soc. 2005;53(12):2060-2068. PubMed
81. Boockvar KS, Fridman B, Marturano C. Ineffective communication of mental status information during care transfer of older adults. J Gen Intern Med. 2005;20(12):1146-1150. PubMed
82. Kiely DK, Bergmann MA, Murphy KM, Jones RN, Orav EJ, Marcantonio ER. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003;58(5):M441-M445. PubMed
83. Kind AJ, Thorpe CT, Sattin JA, Walz SE, Smith MA. Provider characteristics, clinical-work processes and their relationship to discharge summary quality for sub-acute care patients. J Gen Intern Med. 2012;27(1):78-84. PubMed
84. King BJ, Gilmore-Bykovskyi AL, Roiland RA, Polnaszek BE, Bowers BJ, Kind AJ. The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095-1102. PubMed
85. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. PubMed
86. Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med. 2009;24(5):630-635. PubMed
87. Vogelsmeier A. Identifying medication order discrepancies during medication reconciliation: perceptions of nursing home leaders and staff. J Nurs Manag. 2014;22(3):362-372. PubMed
88. Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med. 2004;164(5):545-550. PubMed
89. Sinvani LD, Beizer J, Akerman M, et al. Medication reconciliation in continuum of care transitions: a moving target. J Am Med Dir Assoc. 2013;14(9):668-672. PubMed
90. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834. PubMed
91. Marcantonio ER, Bergmann MA, Kiely DK, Orav EJ, Jones RN. Randomized trial of a delirium abatement program for postacute skilled nursing facilities. J Am Geriatr Soc. 2010;58(6):1019-1026. PubMed
92. Callahan CM, Tu W, Unroe KT, LaMantia MA, Stump TE, Clark DO. Transitions in care in a nationally representative sample of older Americans with dementia. PubMed
J Am Geriatr Soc. 2015;63(8):1495-1502.
93. Givens JL, Mitchell SL, Kuo S, Gozalo P, Mor V, Teno J. Skilled nursing facility admissions of nursing home residents with advanced dementia. J Am Geriatr Soc. 2013;61(10):1645-1650. PubMed
94. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. PubMed
95. Ottenbacher KJ, Karmarkar A, Graham JE, et al. Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA. 2014;311(6):604-614. PubMed
96. Konetzka RT, Grabowski DC, Perraillon MC, Werner RM. Nursing home 5-star rating system exacerbates disparities in quality, by payer source. Health Aff (Millwood). 2015;34(5):819-827. PubMed
97. Williams A, Straker JK, Applebaum R. The nursing home five star rating: how does it compare to resident and family views of care? Gerontologist. 2016;56(2):234-242. PubMed
98. Caplan GA, Meller A, Squires B, Chan S, Willett W. Advance care planning and hospital in the nursing home. Age Ageing. 2006;35(6):581-585. PubMed
99. Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190. PubMed
100. Gill TM, Gahbauer EA, Han L, Allore HG. The role of intervening hospital admissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ. 2015;350:h2361. PubMed
101. Levy C, Morris M, Kramer A. Improving end-of-life outcomes in nursing homes by targeting residents at high-risk of mortality for palliative care: program description and evaluation. J Palliat Med. 2008;11(2):217-225. PubMed
102. Miller SC, Lima JC, Looze J, Mitchell SL. Dying in U.S. nursing homes with advanced dementia: how does health care use differ for residents with, versus without, end-of-life Medicare skilled nursing facility care? J Palliat Med. 2012;15(1):
43-50. PubMed
103. Halm EA, Magaziner J, Hannan EL, et al. Frequency and impact of active clinical issues and new impairments on hospital discharge in patients with hip fracture. Arch Intern Med. 2003;163(1):108-113. PubMed
104. Thomas KS, Mor V, Tyler DA, Hyer K. The relationships among licensed nurse turnover, retention, and rehospitalization of nursing home residents. Gerontologist. 2013;53(2):211-221. PubMed
© 2017 Society of Hospital Medicine
Why and How ACOs Must Evolve
The Centers for Medicare & Medicaid Services (CMS) triumphantly announced in January 2016 that 121 new participants had joined 1 of its 3 accountable care organization (ACO) programs, and that the various ACOs had together saved the federal government $411 million in 2014 while improving on various quality metrics.[1] Yet even as the ACO model has gathered political momentum and shown promise in reducing payer spending in the short term, it is facing growing scrutiny that it may be insufficient to support meaningful care delivery transformation.
Different ACO models differ in their financial details, but the fundamental theme is the same: healthcare organizations earn bonuses, or shared savings, if they bill payers less than their projected fee‐for‐service revenue (known as the spending benchmark) and meet quality measures. In double‐sided ACO models, if fee‐for‐service revenue exceeds the benchmark, ACOs are also fined. In contrast with traditional fee‐for‐service, in which payments are tied directly to price and volume of services, ACOs were envisioned as a business model that would enable health systems to thrive by improving the care efficiency and clinical outcomes of their patient populations.
However, because health systems only earn shared savings bonuses if they reduce their fee‐for‐service revenue, ACOs do not have a clear business case for moving toward value‐oriented care. ThedaCare, the best performing Pioneer ACO in the first year of the program, reported that successful reduction of preventable hospital admissions led to shared savings payments. However, those payments did not cover the reduced fee‐for‐service revenue, leading to diminished overall financial performance.[2] Only 5% of the 434 Shared Savings Program ACOs have agreed to double‐sided contracts, suggesting discomfort with the structure of the model.[1] Although ACOs have continued to grow in overall number, the programs have experienced significant churn, with over two thirds of Pioneer ACOs leaving over the last 3 years. This suggests widespread commitment to the principle of population health but a struggle by health systems to make the specific models financially viable.[3]
How can payers reshape the ACO model to better support value‐oriented care delivery transformation while maintaining its key cost control elements? One strategy is for CMS to establish a pathway for adding care delivery interventions to the fee‐for‐service schedule for ACOs, a concept we call population health billing. Payers have shown some interest in supporting population health efforts via fee‐for‐service: for example, in 2015 CMS established the chronic care management fee, which pays a per patient per month fee for care coordination of Medicare beneficiaries with 2 or more chronic conditions, and the transitional care management fee, which reimburses a postdischarge office visit focused on managing a patient's transition out of the hospital.[4] UnitedHealthcare has begun reimbursing virtual physician visits for some self‐insured employers.[5]
These isolated efforts should be rolled up into a systematic pathway for population health interventions to become billable under fee‐for‐service for organizations in Medicare ACO contracts. CMS and institutional provider associations, whose technical committees generate the majority of billing codes, could together adopt a formal set of criteria to grade the evidence basis of population health interventions in terms of their impact on clinical outcomes, care quality, and care value, similar to the National Quality Forum's work with quality metrics, and establish a formal process by which interventions with demonstrated efficacy can be assigned billing values in a timely, rigorous, and transparent manner. Candidates for population health billing codes could include high‐risk case management, virtual visits, and home‐based primary care. ACOs would be free to determine which, if any, particular interventions to adopt.
ACOs must currently invest in population health programs with out‐of‐pocket funds and bet that reductions in preventable healthcare utilization result in sufficient shared savings to compensate for both program costs and lost fee‐for‐service profit. Even clinically successful programs are often unable to reach this high threshold.[6] A recent New England Journal of Medicine article lamented a common theme among studies of population health interventions: clinical success, but financial unsustainability.[7] CMS' Comprehensive Primary Care Initiative, which provides about 500 primary care practices with case management fees to redesign their care delivery, resulted in a reduced volume of primary care visits and improved patient communication but no cost savings to Medicare after accounting for program fees (14). Congressional analysis found that although case management programs with substantial physician interaction reduced Medicare expenditures by an average of 6%, only 1 out of 34 programs achieved statistically significant savings to Medicare relative to their program fees alone.[8] E‐consults, when a specialist physician provides recommendations to a primary doctor by reviewing a patient's chart electronically, are associated with decreased wait times, high primary provider satisfaction, and lower costs compared to traditional care, yet adoption among ACOs has been limited by the opportunity cost of fee‐for‐service revenue.[9] In reducing Medicare costs and hence their own fee‐for‐service revenue by $300 million in their first year, Pioneer ACOs were only collectively granted bonuses of about $77 million against the significant operating and capital costs of population health.[10] The financial challenges of ACOs are a reflection of the difficulty in consistently developing a fiscally and clinically successful set of population health interventions under current ACO financial rules.
In Figure 1, we use high‐risk case management as an example to demonstrate how population billing could work. Population health billing could provide a per patient, per month fee‐for‐service payment to ACOs for high‐risk case management services. This payment would count against ACOs' spending benchmarks at the end of the year. By helping cover the operating costs of these programs, population health billing would make value‐oriented interventions significantly more sustainable compared to the current ACO models.
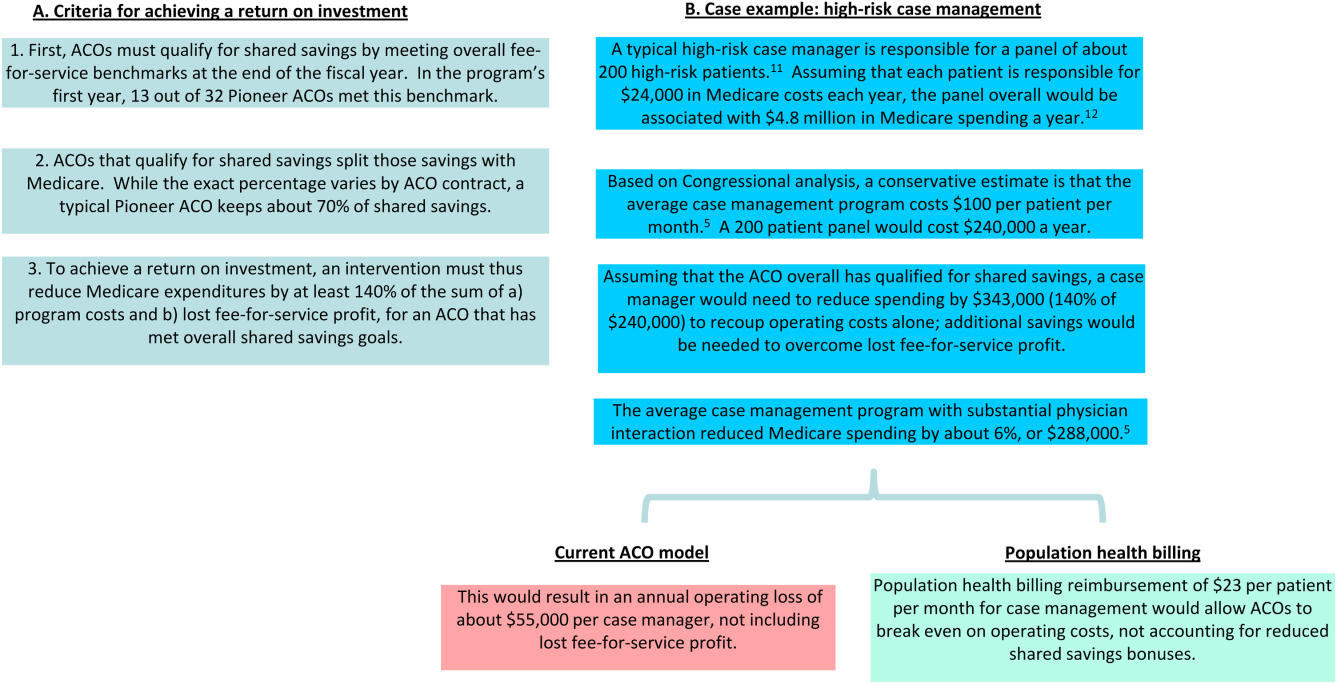
In providing fee‐for‐service revenue for population health interventions, population health billing would break the inherent tension between fee‐for‐service and shared savings bonuses. It would allow ACOs to transition ever‐greater portions of revenue from traditional transactional‐based sources toward shared savings, without requiring success in accountable care to mean fee‐for‐service losses. This is an important threshold for operational leaders who must integrate population health, which currently represent loss centers on balance sheets, within existing profitable fee‐for‐service business lines.
Some observers may argue that allowing billable care delivery interventions may encourage practices to roll out interventions that meet billing requirements but have little meaningful impact on population health; the efficacy of care delivery interventions is clearly dependent on the context of the health system and quality of execution. This concern is the same fundamental concern of fee‐for‐service reimbursement as a whole. However, because ACOs are paid bonuses for reducing fee‐for‐service revenue, they would have an incentive to only develop and bill for population health interventions they believe would have a meaningful return on investment in reducing healthcare costs. The fundamental incentives of ACOs would remain the same‐ to reduce healthcare spending by better managing the costs of their patient populations. Others may argue that population health billing would build upon our fee‐for‐service system that many have advocated we must move past. But ACO initiatives and bundled payments are similarly built upon a foundation of fee‐for‐service.
Whereas a greater number of billable services will likely reduce CMS' short‐term savings from ACO programs, the ACO model must offer a sustainable business case for care delivery reform to ultimately bend long‐term healthcare costs. Payers are not obligated to ensure that providers maintain historical income levels, but over the long term providers will not make the sizable infrastructure investments, such as integrated information technology platforms, data analytics, and risk management, required to deliver value‐based care without a sustainable business case. To limit the costs of population health billing, Medicare should restrict it to ACO contracts that allow for penalties. The fee‐for‐service reimbursement rates under population health billing could also be tied to performance on quality metrics, similar to how Medicare fee‐for‐service hospital reimbursement is linked to performance on value‐based metrics.[13]
In addition, this reduction in short‐term cost savings may actually improve the sustainability of the ACO model. Every year, each ACO's spending benchmark is re‐based, or recalculated based on the most recent spending data. This means that ACOs that successfully reduce their fee‐for‐service revenue below their spending benchmark will face an even lower benchmark the next year and have to reduce their costs even further, creating an unsustainable trend. Because population health billing would count against the spending benchmark, it would help slow down this race to the bottom while driving forward value‐oriented care delivery transformation.
ACOs have a number of other design problems, including high rates of patient churn, imperfect quality metrics that do not adequately capture the scope of population‐level health, and lags in data access.[14] The Next Generation ACO model addresses some of these concerns. For example, it allows ACOs to prospectively define their patient populations. Yet many challenges remain. Population health billing does not solve all of these problems, but it will improve the ability of health systems to meaningfully pivot toward a value‐oriented strategy.
As physicians and ACO operational leaders, we believe in the clinical and policy vision behind the ACO model but have also struggled with the limitations of the model to meaningfully support care delivery transformation. If CMS truly wants to meaningfully transform US healthcare from volume‐based to value‐based, it must invest in the needed care redesign even at the expense of short‐term cost savings.
Disclosures: Dr. Chen was formerly a consultant for Partners HealthCare, a Pioneer ACO, and a physician fellow on the Pioneer ACO Team at the Center for Medicare & Medicaid Innovation. He currently serves on the advisory board of Radial Analytics and is a resident physician at Massachusetts General Hospital. Dr. Ackerly was formerly the associate medical director for Population Health and Continuing Care at Partners HealthCare and an Innovation Advisor to the Center for Medicare & Medicaid Innovation. He currently serves as the Chief Clinical Officer of naviHealth. Dr. Gottlieb was formerly the President and Chief Executive Officer of Partners HealthCare and currently serves as the Chief Executive Officer of Partners In Health. The views represented here are those of the authors' and do not represent the views of Partners HealthCare, Massachusetts General Hospital, naviHealth, or Partners In Health.
- U.S. Department of Health 310:1341–1342.
- , , , . Performance differences in year 1 of the Pioneer accountable care organizations. N Engl J Med. 2015;372:1927–1936.
- , , , , . Medicare chronic care management payments and financial returns to primary care practice: a modeling study. Ann Intern Med. 2015;163:580–588.
- UnitedHealthcare. UnitedHealthcare covers virtual care physician visits, expanding consumers' access to affordable health care options. Available at: http://www.uhc.com/news‐room/2015‐news‐release‐archive/unitedhealthcare‐covers‐virtual‐care‐physician‐visits. Published April 30, 2015. Accessed February 6, 2016.
- , , . Toward increased adoption of complex care management. N Engl J Med. 2014;371:491–493.
- , , . Asymmetric thinking about return on investment. N Engl J Med. 2016;374(7):606–608.
- . Lessons from Medicare's demonstration projects on disease management and care coordination. Washington, D.C.: Congressional Budget Office, Health and Human Resources Division, working paper 2012‐01, 2012. Available at: http://www.cbo.gov/sites/default/files/cbofiles/attachments/WP2012‐01_Nelson_Medicare_DMCC_Demonstrations.pdf. Published January 2012. Accessed June 15, 2015.
- , , , . A safety‐net system gains efficiencies through ‘eReferrals’ to specialists. Health Aff (Millwood). 2010;29:969–971.
- Centers for Medicare MGH Medicare Demonstration Project for High-Cost Beneficiaries. Available at: http://www.massgeneral.org/News/assets/pdf/CMS_project_phase1FactSheet.pdf. Accessed April 2, 2016.
- , , , et al. Two-Year Costs and Quality in the Comprehensive Primary Care Initiative. N Engl J Med. 2016; DOI: 10.1056/NEJMsa1414953.
- , . Beyond ACOs and bundled payments: Medicare's shift toward accountability in fee‐for‐service. JAMA. 2014;311:673–674.
- , , , , . ACO model should encourage efficient care delivery. Healthc (Amst). 2015;3(3):150–152.
The Centers for Medicare & Medicaid Services (CMS) triumphantly announced in January 2016 that 121 new participants had joined 1 of its 3 accountable care organization (ACO) programs, and that the various ACOs had together saved the federal government $411 million in 2014 while improving on various quality metrics.[1] Yet even as the ACO model has gathered political momentum and shown promise in reducing payer spending in the short term, it is facing growing scrutiny that it may be insufficient to support meaningful care delivery transformation.
Different ACO models differ in their financial details, but the fundamental theme is the same: healthcare organizations earn bonuses, or shared savings, if they bill payers less than their projected fee‐for‐service revenue (known as the spending benchmark) and meet quality measures. In double‐sided ACO models, if fee‐for‐service revenue exceeds the benchmark, ACOs are also fined. In contrast with traditional fee‐for‐service, in which payments are tied directly to price and volume of services, ACOs were envisioned as a business model that would enable health systems to thrive by improving the care efficiency and clinical outcomes of their patient populations.
However, because health systems only earn shared savings bonuses if they reduce their fee‐for‐service revenue, ACOs do not have a clear business case for moving toward value‐oriented care. ThedaCare, the best performing Pioneer ACO in the first year of the program, reported that successful reduction of preventable hospital admissions led to shared savings payments. However, those payments did not cover the reduced fee‐for‐service revenue, leading to diminished overall financial performance.[2] Only 5% of the 434 Shared Savings Program ACOs have agreed to double‐sided contracts, suggesting discomfort with the structure of the model.[1] Although ACOs have continued to grow in overall number, the programs have experienced significant churn, with over two thirds of Pioneer ACOs leaving over the last 3 years. This suggests widespread commitment to the principle of population health but a struggle by health systems to make the specific models financially viable.[3]
How can payers reshape the ACO model to better support value‐oriented care delivery transformation while maintaining its key cost control elements? One strategy is for CMS to establish a pathway for adding care delivery interventions to the fee‐for‐service schedule for ACOs, a concept we call population health billing. Payers have shown some interest in supporting population health efforts via fee‐for‐service: for example, in 2015 CMS established the chronic care management fee, which pays a per patient per month fee for care coordination of Medicare beneficiaries with 2 or more chronic conditions, and the transitional care management fee, which reimburses a postdischarge office visit focused on managing a patient's transition out of the hospital.[4] UnitedHealthcare has begun reimbursing virtual physician visits for some self‐insured employers.[5]
These isolated efforts should be rolled up into a systematic pathway for population health interventions to become billable under fee‐for‐service for organizations in Medicare ACO contracts. CMS and institutional provider associations, whose technical committees generate the majority of billing codes, could together adopt a formal set of criteria to grade the evidence basis of population health interventions in terms of their impact on clinical outcomes, care quality, and care value, similar to the National Quality Forum's work with quality metrics, and establish a formal process by which interventions with demonstrated efficacy can be assigned billing values in a timely, rigorous, and transparent manner. Candidates for population health billing codes could include high‐risk case management, virtual visits, and home‐based primary care. ACOs would be free to determine which, if any, particular interventions to adopt.
ACOs must currently invest in population health programs with out‐of‐pocket funds and bet that reductions in preventable healthcare utilization result in sufficient shared savings to compensate for both program costs and lost fee‐for‐service profit. Even clinically successful programs are often unable to reach this high threshold.[6] A recent New England Journal of Medicine article lamented a common theme among studies of population health interventions: clinical success, but financial unsustainability.[7] CMS' Comprehensive Primary Care Initiative, which provides about 500 primary care practices with case management fees to redesign their care delivery, resulted in a reduced volume of primary care visits and improved patient communication but no cost savings to Medicare after accounting for program fees (14). Congressional analysis found that although case management programs with substantial physician interaction reduced Medicare expenditures by an average of 6%, only 1 out of 34 programs achieved statistically significant savings to Medicare relative to their program fees alone.[8] E‐consults, when a specialist physician provides recommendations to a primary doctor by reviewing a patient's chart electronically, are associated with decreased wait times, high primary provider satisfaction, and lower costs compared to traditional care, yet adoption among ACOs has been limited by the opportunity cost of fee‐for‐service revenue.[9] In reducing Medicare costs and hence their own fee‐for‐service revenue by $300 million in their first year, Pioneer ACOs were only collectively granted bonuses of about $77 million against the significant operating and capital costs of population health.[10] The financial challenges of ACOs are a reflection of the difficulty in consistently developing a fiscally and clinically successful set of population health interventions under current ACO financial rules.
In Figure 1, we use high‐risk case management as an example to demonstrate how population billing could work. Population health billing could provide a per patient, per month fee‐for‐service payment to ACOs for high‐risk case management services. This payment would count against ACOs' spending benchmarks at the end of the year. By helping cover the operating costs of these programs, population health billing would make value‐oriented interventions significantly more sustainable compared to the current ACO models.

In providing fee‐for‐service revenue for population health interventions, population health billing would break the inherent tension between fee‐for‐service and shared savings bonuses. It would allow ACOs to transition ever‐greater portions of revenue from traditional transactional‐based sources toward shared savings, without requiring success in accountable care to mean fee‐for‐service losses. This is an important threshold for operational leaders who must integrate population health, which currently represent loss centers on balance sheets, within existing profitable fee‐for‐service business lines.
Some observers may argue that allowing billable care delivery interventions may encourage practices to roll out interventions that meet billing requirements but have little meaningful impact on population health; the efficacy of care delivery interventions is clearly dependent on the context of the health system and quality of execution. This concern is the same fundamental concern of fee‐for‐service reimbursement as a whole. However, because ACOs are paid bonuses for reducing fee‐for‐service revenue, they would have an incentive to only develop and bill for population health interventions they believe would have a meaningful return on investment in reducing healthcare costs. The fundamental incentives of ACOs would remain the same‐ to reduce healthcare spending by better managing the costs of their patient populations. Others may argue that population health billing would build upon our fee‐for‐service system that many have advocated we must move past. But ACO initiatives and bundled payments are similarly built upon a foundation of fee‐for‐service.
Whereas a greater number of billable services will likely reduce CMS' short‐term savings from ACO programs, the ACO model must offer a sustainable business case for care delivery reform to ultimately bend long‐term healthcare costs. Payers are not obligated to ensure that providers maintain historical income levels, but over the long term providers will not make the sizable infrastructure investments, such as integrated information technology platforms, data analytics, and risk management, required to deliver value‐based care without a sustainable business case. To limit the costs of population health billing, Medicare should restrict it to ACO contracts that allow for penalties. The fee‐for‐service reimbursement rates under population health billing could also be tied to performance on quality metrics, similar to how Medicare fee‐for‐service hospital reimbursement is linked to performance on value‐based metrics.[13]
In addition, this reduction in short‐term cost savings may actually improve the sustainability of the ACO model. Every year, each ACO's spending benchmark is re‐based, or recalculated based on the most recent spending data. This means that ACOs that successfully reduce their fee‐for‐service revenue below their spending benchmark will face an even lower benchmark the next year and have to reduce their costs even further, creating an unsustainable trend. Because population health billing would count against the spending benchmark, it would help slow down this race to the bottom while driving forward value‐oriented care delivery transformation.
ACOs have a number of other design problems, including high rates of patient churn, imperfect quality metrics that do not adequately capture the scope of population‐level health, and lags in data access.[14] The Next Generation ACO model addresses some of these concerns. For example, it allows ACOs to prospectively define their patient populations. Yet many challenges remain. Population health billing does not solve all of these problems, but it will improve the ability of health systems to meaningfully pivot toward a value‐oriented strategy.
As physicians and ACO operational leaders, we believe in the clinical and policy vision behind the ACO model but have also struggled with the limitations of the model to meaningfully support care delivery transformation. If CMS truly wants to meaningfully transform US healthcare from volume‐based to value‐based, it must invest in the needed care redesign even at the expense of short‐term cost savings.
Disclosures: Dr. Chen was formerly a consultant for Partners HealthCare, a Pioneer ACO, and a physician fellow on the Pioneer ACO Team at the Center for Medicare & Medicaid Innovation. He currently serves on the advisory board of Radial Analytics and is a resident physician at Massachusetts General Hospital. Dr. Ackerly was formerly the associate medical director for Population Health and Continuing Care at Partners HealthCare and an Innovation Advisor to the Center for Medicare & Medicaid Innovation. He currently serves as the Chief Clinical Officer of naviHealth. Dr. Gottlieb was formerly the President and Chief Executive Officer of Partners HealthCare and currently serves as the Chief Executive Officer of Partners In Health. The views represented here are those of the authors' and do not represent the views of Partners HealthCare, Massachusetts General Hospital, naviHealth, or Partners In Health.
The Centers for Medicare & Medicaid Services (CMS) triumphantly announced in January 2016 that 121 new participants had joined 1 of its 3 accountable care organization (ACO) programs, and that the various ACOs had together saved the federal government $411 million in 2014 while improving on various quality metrics.[1] Yet even as the ACO model has gathered political momentum and shown promise in reducing payer spending in the short term, it is facing growing scrutiny that it may be insufficient to support meaningful care delivery transformation.
Different ACO models differ in their financial details, but the fundamental theme is the same: healthcare organizations earn bonuses, or shared savings, if they bill payers less than their projected fee‐for‐service revenue (known as the spending benchmark) and meet quality measures. In double‐sided ACO models, if fee‐for‐service revenue exceeds the benchmark, ACOs are also fined. In contrast with traditional fee‐for‐service, in which payments are tied directly to price and volume of services, ACOs were envisioned as a business model that would enable health systems to thrive by improving the care efficiency and clinical outcomes of their patient populations.
However, because health systems only earn shared savings bonuses if they reduce their fee‐for‐service revenue, ACOs do not have a clear business case for moving toward value‐oriented care. ThedaCare, the best performing Pioneer ACO in the first year of the program, reported that successful reduction of preventable hospital admissions led to shared savings payments. However, those payments did not cover the reduced fee‐for‐service revenue, leading to diminished overall financial performance.[2] Only 5% of the 434 Shared Savings Program ACOs have agreed to double‐sided contracts, suggesting discomfort with the structure of the model.[1] Although ACOs have continued to grow in overall number, the programs have experienced significant churn, with over two thirds of Pioneer ACOs leaving over the last 3 years. This suggests widespread commitment to the principle of population health but a struggle by health systems to make the specific models financially viable.[3]
How can payers reshape the ACO model to better support value‐oriented care delivery transformation while maintaining its key cost control elements? One strategy is for CMS to establish a pathway for adding care delivery interventions to the fee‐for‐service schedule for ACOs, a concept we call population health billing. Payers have shown some interest in supporting population health efforts via fee‐for‐service: for example, in 2015 CMS established the chronic care management fee, which pays a per patient per month fee for care coordination of Medicare beneficiaries with 2 or more chronic conditions, and the transitional care management fee, which reimburses a postdischarge office visit focused on managing a patient's transition out of the hospital.[4] UnitedHealthcare has begun reimbursing virtual physician visits for some self‐insured employers.[5]
These isolated efforts should be rolled up into a systematic pathway for population health interventions to become billable under fee‐for‐service for organizations in Medicare ACO contracts. CMS and institutional provider associations, whose technical committees generate the majority of billing codes, could together adopt a formal set of criteria to grade the evidence basis of population health interventions in terms of their impact on clinical outcomes, care quality, and care value, similar to the National Quality Forum's work with quality metrics, and establish a formal process by which interventions with demonstrated efficacy can be assigned billing values in a timely, rigorous, and transparent manner. Candidates for population health billing codes could include high‐risk case management, virtual visits, and home‐based primary care. ACOs would be free to determine which, if any, particular interventions to adopt.
ACOs must currently invest in population health programs with out‐of‐pocket funds and bet that reductions in preventable healthcare utilization result in sufficient shared savings to compensate for both program costs and lost fee‐for‐service profit. Even clinically successful programs are often unable to reach this high threshold.[6] A recent New England Journal of Medicine article lamented a common theme among studies of population health interventions: clinical success, but financial unsustainability.[7] CMS' Comprehensive Primary Care Initiative, which provides about 500 primary care practices with case management fees to redesign their care delivery, resulted in a reduced volume of primary care visits and improved patient communication but no cost savings to Medicare after accounting for program fees (14). Congressional analysis found that although case management programs with substantial physician interaction reduced Medicare expenditures by an average of 6%, only 1 out of 34 programs achieved statistically significant savings to Medicare relative to their program fees alone.[8] E‐consults, when a specialist physician provides recommendations to a primary doctor by reviewing a patient's chart electronically, are associated with decreased wait times, high primary provider satisfaction, and lower costs compared to traditional care, yet adoption among ACOs has been limited by the opportunity cost of fee‐for‐service revenue.[9] In reducing Medicare costs and hence their own fee‐for‐service revenue by $300 million in their first year, Pioneer ACOs were only collectively granted bonuses of about $77 million against the significant operating and capital costs of population health.[10] The financial challenges of ACOs are a reflection of the difficulty in consistently developing a fiscally and clinically successful set of population health interventions under current ACO financial rules.
In Figure 1, we use high‐risk case management as an example to demonstrate how population billing could work. Population health billing could provide a per patient, per month fee‐for‐service payment to ACOs for high‐risk case management services. This payment would count against ACOs' spending benchmarks at the end of the year. By helping cover the operating costs of these programs, population health billing would make value‐oriented interventions significantly more sustainable compared to the current ACO models.

In providing fee‐for‐service revenue for population health interventions, population health billing would break the inherent tension between fee‐for‐service and shared savings bonuses. It would allow ACOs to transition ever‐greater portions of revenue from traditional transactional‐based sources toward shared savings, without requiring success in accountable care to mean fee‐for‐service losses. This is an important threshold for operational leaders who must integrate population health, which currently represent loss centers on balance sheets, within existing profitable fee‐for‐service business lines.
Some observers may argue that allowing billable care delivery interventions may encourage practices to roll out interventions that meet billing requirements but have little meaningful impact on population health; the efficacy of care delivery interventions is clearly dependent on the context of the health system and quality of execution. This concern is the same fundamental concern of fee‐for‐service reimbursement as a whole. However, because ACOs are paid bonuses for reducing fee‐for‐service revenue, they would have an incentive to only develop and bill for population health interventions they believe would have a meaningful return on investment in reducing healthcare costs. The fundamental incentives of ACOs would remain the same‐ to reduce healthcare spending by better managing the costs of their patient populations. Others may argue that population health billing would build upon our fee‐for‐service system that many have advocated we must move past. But ACO initiatives and bundled payments are similarly built upon a foundation of fee‐for‐service.
Whereas a greater number of billable services will likely reduce CMS' short‐term savings from ACO programs, the ACO model must offer a sustainable business case for care delivery reform to ultimately bend long‐term healthcare costs. Payers are not obligated to ensure that providers maintain historical income levels, but over the long term providers will not make the sizable infrastructure investments, such as integrated information technology platforms, data analytics, and risk management, required to deliver value‐based care without a sustainable business case. To limit the costs of population health billing, Medicare should restrict it to ACO contracts that allow for penalties. The fee‐for‐service reimbursement rates under population health billing could also be tied to performance on quality metrics, similar to how Medicare fee‐for‐service hospital reimbursement is linked to performance on value‐based metrics.[13]
In addition, this reduction in short‐term cost savings may actually improve the sustainability of the ACO model. Every year, each ACO's spending benchmark is re‐based, or recalculated based on the most recent spending data. This means that ACOs that successfully reduce their fee‐for‐service revenue below their spending benchmark will face an even lower benchmark the next year and have to reduce their costs even further, creating an unsustainable trend. Because population health billing would count against the spending benchmark, it would help slow down this race to the bottom while driving forward value‐oriented care delivery transformation.
ACOs have a number of other design problems, including high rates of patient churn, imperfect quality metrics that do not adequately capture the scope of population‐level health, and lags in data access.[14] The Next Generation ACO model addresses some of these concerns. For example, it allows ACOs to prospectively define their patient populations. Yet many challenges remain. Population health billing does not solve all of these problems, but it will improve the ability of health systems to meaningfully pivot toward a value‐oriented strategy.
As physicians and ACO operational leaders, we believe in the clinical and policy vision behind the ACO model but have also struggled with the limitations of the model to meaningfully support care delivery transformation. If CMS truly wants to meaningfully transform US healthcare from volume‐based to value‐based, it must invest in the needed care redesign even at the expense of short‐term cost savings.
Disclosures: Dr. Chen was formerly a consultant for Partners HealthCare, a Pioneer ACO, and a physician fellow on the Pioneer ACO Team at the Center for Medicare & Medicaid Innovation. He currently serves on the advisory board of Radial Analytics and is a resident physician at Massachusetts General Hospital. Dr. Ackerly was formerly the associate medical director for Population Health and Continuing Care at Partners HealthCare and an Innovation Advisor to the Center for Medicare & Medicaid Innovation. He currently serves as the Chief Clinical Officer of naviHealth. Dr. Gottlieb was formerly the President and Chief Executive Officer of Partners HealthCare and currently serves as the Chief Executive Officer of Partners In Health. The views represented here are those of the authors' and do not represent the views of Partners HealthCare, Massachusetts General Hospital, naviHealth, or Partners In Health.
- U.S. Department of Health 310:1341–1342.
- , , , . Performance differences in year 1 of the Pioneer accountable care organizations. N Engl J Med. 2015;372:1927–1936.
- , , , , . Medicare chronic care management payments and financial returns to primary care practice: a modeling study. Ann Intern Med. 2015;163:580–588.
- UnitedHealthcare. UnitedHealthcare covers virtual care physician visits, expanding consumers' access to affordable health care options. Available at: http://www.uhc.com/news‐room/2015‐news‐release‐archive/unitedhealthcare‐covers‐virtual‐care‐physician‐visits. Published April 30, 2015. Accessed February 6, 2016.
- , , . Toward increased adoption of complex care management. N Engl J Med. 2014;371:491–493.
- , , . Asymmetric thinking about return on investment. N Engl J Med. 2016;374(7):606–608.
- . Lessons from Medicare's demonstration projects on disease management and care coordination. Washington, D.C.: Congressional Budget Office, Health and Human Resources Division, working paper 2012‐01, 2012. Available at: http://www.cbo.gov/sites/default/files/cbofiles/attachments/WP2012‐01_Nelson_Medicare_DMCC_Demonstrations.pdf. Published January 2012. Accessed June 15, 2015.
- , , , . A safety‐net system gains efficiencies through ‘eReferrals’ to specialists. Health Aff (Millwood). 2010;29:969–971.
- Centers for Medicare MGH Medicare Demonstration Project for High-Cost Beneficiaries. Available at: http://www.massgeneral.org/News/assets/pdf/CMS_project_phase1FactSheet.pdf. Accessed April 2, 2016.
- , , , et al. Two-Year Costs and Quality in the Comprehensive Primary Care Initiative. N Engl J Med. 2016; DOI: 10.1056/NEJMsa1414953.
- , . Beyond ACOs and bundled payments: Medicare's shift toward accountability in fee‐for‐service. JAMA. 2014;311:673–674.
- , , , , . ACO model should encourage efficient care delivery. Healthc (Amst). 2015;3(3):150–152.
- U.S. Department of Health 310:1341–1342.
- , , , . Performance differences in year 1 of the Pioneer accountable care organizations. N Engl J Med. 2015;372:1927–1936.
- , , , , . Medicare chronic care management payments and financial returns to primary care practice: a modeling study. Ann Intern Med. 2015;163:580–588.
- UnitedHealthcare. UnitedHealthcare covers virtual care physician visits, expanding consumers' access to affordable health care options. Available at: http://www.uhc.com/news‐room/2015‐news‐release‐archive/unitedhealthcare‐covers‐virtual‐care‐physician‐visits. Published April 30, 2015. Accessed February 6, 2016.
- , , . Toward increased adoption of complex care management. N Engl J Med. 2014;371:491–493.
- , , . Asymmetric thinking about return on investment. N Engl J Med. 2016;374(7):606–608.
- . Lessons from Medicare's demonstration projects on disease management and care coordination. Washington, D.C.: Congressional Budget Office, Health and Human Resources Division, working paper 2012‐01, 2012. Available at: http://www.cbo.gov/sites/default/files/cbofiles/attachments/WP2012‐01_Nelson_Medicare_DMCC_Demonstrations.pdf. Published January 2012. Accessed June 15, 2015.
- , , , . A safety‐net system gains efficiencies through ‘eReferrals’ to specialists. Health Aff (Millwood). 2010;29:969–971.
- Centers for Medicare MGH Medicare Demonstration Project for High-Cost Beneficiaries. Available at: http://www.massgeneral.org/News/assets/pdf/CMS_project_phase1FactSheet.pdf. Accessed April 2, 2016.
- , , , et al. Two-Year Costs and Quality in the Comprehensive Primary Care Initiative. N Engl J Med. 2016; DOI: 10.1056/NEJMsa1414953.
- , . Beyond ACOs and bundled payments: Medicare's shift toward accountability in fee‐for‐service. JAMA. 2014;311:673–674.
- , , , , . ACO model should encourage efficient care delivery. Healthc (Amst). 2015;3(3):150–152.