User login
Things We Do for No Reason™: Discontinuing Urate-Lowering Therapy on Admission
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
© 2021 Society of Hospital Medicine
Things We Do For No Reason™: Ultrasonography After an Initial Negative CT in Patients Presenting With Acute Abdominal or Pelvic Pain
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
A 70-year-old woman presented to the emergency department (ED) with diffuse abdominal pain, nausea, and vomiting with normal liver function tests and lipase. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast revealed no acute intraabdominal pathology except for an incidentally noted, mildly enlarged but nondistended gallbladder without evident cholelithiasis, pericholecystic fluid, or gallbladder wall edema. The hospitalist orders an abdominal ultrasound to evaluate for acute biliary pathology potentially missed by CT.
Why You Might Consider Ordering an Abdominal Ultrasound After a Negative CT
Guidelines and expert opinion recommend an “ultrasound-first” approach when patients present with right upper quadrant (RUQ) abdominal pain or pelvic pain of suspected gynecologic origin.1-3 When evaluating suspected biliary disease, experts recommend beginning with ultrasonography based on the speed of obtaining results, absence of radiation exposure, reduced cost, and good diagnostic accuracy.1 Ultrasound has superior sensitivity, of 98%,4 in identifying radiolucent gallstones, compared to CT’s 79% sensitivity.5 Ultrasonography also differentiates gallbladder sludge from cholelithiasis, evaluates the extrahepatic and intrahepatic bile ducts, and can identify alternate causes of RUQ pain.1,3 Since ultrasound has important advantages, a negative initial CT may lead the clinician to consider an ultrasound to evaluate for gallbladder diseases.
Additionally, ultrasound provides improved anatomic detail of pelvic structures when diagnosing endometrial or ovarian pathology2 and improves diagnostic accuracy when the initial CT reveals an abnormal pelvic finding (eg, defining an enlarged ovary on CT as ovarian torsion, a cyst, or an adnexal mass).6 While CT excludes emergent surgical diagnoses, ultrasound may add value in elucidating a cause of the pain, even when urgent surgical management is not necessary.7
Many providers believe that a CT lacks sensitivity for acute biliary or pelvic pathology and will order an ultrasound to avoid missing an important diagnosis.7 Within 6 months at a single center, clinicians ordered 614 abdominal ultrasounds within 72 hours of an abdominal CT; 227 of these orders were to evaluate the gallbladder. Clinicians documented a discussion with a radiologist in only 19% of cases.8
Why Ordering an Ultrasound After a Negative CT Is Unnecessary
While ultrasound is more sensitive for detecting gallstones, the data do not indicate that it is more sensitive than CT for detecting acute cholecystitis. Abdominal ultrasound has a sensitivity for the diagnosis of acute cholecystitis of 81%, with a specificity of 83%,9 while CT has a comparable 85% to 94%9,10 sensitivity and specificity ranging from 59% to 99%.9,11 A recent study using more stringent radiographic criteria (two or more abnormal features) for diagnosing acute cholecystitis found ultrasound and CT had near equivalent sensitivities of 61% and 55%, respectively.12 Even with these stringent criteria, CT had a negative predictive value of 90% and approached 95% when applying a less strict (one feature) criterion.12 As a result, an abdominal ultrasound will rarely diagnose cholecystitis after a normal CT.
A 2020 study evaluated the diagnostic yield and clinical impact of ordering an abdominal or pelvic ultrasound within 24 hours of a negative abdominal CT.7
As with abdominal CT and ultrasound, the recommendation for an initial pelvic ultrasound when evaluating female pelvic pain also stems from the reduced cost, absence of radiation exposure, and superior anatomic visualization of the pelvic organs when compared with pelvic CT.2,13 However, as with the results of studies investigating the use of abdominal ultrasound after negative CT, a study of pelvic ultrasound after a negative CT revealed that only 4/126 (3.2%) follow-up ultrasounds had an abnormal finding not identified on CT.13 Pelvic ultrasound found four endometrial abnormalities that did not alter acute management.13 Notably, in 58% of the cases, the indication for ordering the subsequent ultrasound was “rule out ovarian torsion.” However, CT almost always finds a morphologically abnormal ovary in the case of torsion.6 One study and literature review found that all 28 patients studied and all 85 patients from previous studies with proven ovarian torsion had either an adnexal mass or an enlarged ovary on pelvic CT.6 Harfouch et al found that 0 out of 199 pelvic ultrasounds ordered after a negative CT revealed acute surgical pathology, but pelvic ultrasound did identify nonsurgical uterine and ovarian abnormalities.7 In conclusion, when clinicians order CT as the first study to diagnose acute, surgical biliary or gynecologic causes of pain, follow-up ultrasound has a low probability of affecting diagnosis or management if the CT is normal.
When You Should Consider Ultrasound After CT
The previous discussion only applies if hospitalists order an ultrasound within 24 to 48 hours of the initial CT. Time and clinical course are critical diagnostic tools during an admission for abdominal pain. Consider pelvic or abdominal ultrasound based on guideline recommendations if a patient develops new or evolving RUQ or pelvic pain.1,2 The rationale for obtaining the initial negative CT may no longer apply, and the clinician must consider the changing characteristics of the patient’s symptoms. For example, initial CT imaging may miss cholelithiasis in a patient presenting for biliary colic. Under observation, the patient may develop acute cholecystitis, potentially requiring an abdominal ultrasound. Also, the data for pelvic ultrasound apply to a normal CT of the abdomen and pelvis. Ultrasound may help to further evaluate indeterminate findings present on initial CT or if recommended by radiology.
What You Should Do Instead
When the hospitalist assumes care for a patient with abdominal pain and a negative CT, appropriate next steps include taking time to reexamine the differential diagnosis, repeating the history and physical, and communicating directly with a radiologist. These steps ensure the highest diagnostic yield and the lowest cost and help prevent diagnostic error arising from anchoring on the initial negative ED evaluation. Prior research demonstrates that the initial history alone can lead to the correct diagnosis in up to 76% of cases of abdominal pain.14 If repeat evaluation determines that additional imaging is necessary, the American College of Radiology provides evidence-based guidelines to help clinicians determine the correct imaging test based on the clinical situation (Appendix Table).1,2 For example, an equivocal ultrasound or CT exam with continued suspicion for acute cholecystitis or an alternate diagnosis, such as acalculous cholecystitis or choledocholithiasis, merits alternative tests with improved sensitivity and specificity profiles (Tc 99 m hepatobiliary iminodiacetic acid scan, also known as cholescintigraphy, for cholecystitis and acalculous cholecystitis, or magnetic resonance cholangiopancreatography for choledocholithiasis).1
Remember to communicate with the radiologist to rule out “can’t miss” diagnoses, increase mutual understanding of the radiographic test characteristics for specific disease processes, and improve the radiologist’s understanding of the patient’s history and clinical question.15 Collaboration with the radiologist can also determine the need for follow-up imaging and its timing. One single-center study found that surgeons’ diagnostic impression and management changed in 35/100 (35%) cases after an in-person review with the radiologist.15 Observing patients in the hospital with a nondiagnostic initial evaluation but concerning clinical features often allows for either a trial of cure or for the disease process to “declare itself.”14 This allows clinicians to target additional testing to a specific diagnosis and avoid reflexive ordering of additional radiographic studies.
Recommendations
- Order an ultrasound for initial imaging of RUQ and female pelvic pain.
- Do not reflexively order an ultrasound within 24 to 48 hours of a negative CT scan to pursue biliary or pelvic pathology.
- Only order repeat abdominal imaging if clinical circumstances evolve or discussions with a radiologist conclude it will answer a more specific diagnostic question.
Conclusion
In our clinical scenario involving a patient with diffuse abdominal pain and a negative CT, the hospitalist should reevaluate the history, exam, and differential diagnosis before pursuing further diagnostic imaging. Based on the evidence presented, CT has similar diagnostic accuracy to ultrasound for biliary and gynecologic pathologies necessitating urgent surgical management (eg, acute cholecystitis, ovarian torsion), and a follow-up ultrasound adds little. If the utility of imaging remains in question, hospitalist consultation with a radiologist can clarify whether prior imaging answered the clinical question and the diagnostic utility of repeat abdominal imaging. With thoughtful reevaluation of the history and physical, and communication with radiology, hospitalists can reduce unnecessary, low-yield imaging and reduce healthcare costs when evaluating patients with abdominal pain.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
1. Expert Panel on Gastrointestinal Imaging; Peterson CM, McNamara MM, Kamel IR, et al. ACR Appropriateness Criteria® Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243. https://doi.org/10.1016/j.jacr.2019.02.013
2. Bhosale PR, Javitt MC, Atri M, et al. ACR Appropriateness Criteria® Acute Pelvic Pain in the Reproductive Age Group. Ultrasound Q. 2016;32(2):108-115. https://doi.org/10.1097/RUQ.0000000000000200
3. Revzin MV, Scoutt LM, Garner JG, Moore CL. Right upper quadrant pain: ultrasound first! J Ultrasound Med. 2017;36(10):1975-1985. https://doi.org/10.1002/jum.14274
4. Cooperberg PL, Burhenne HJ. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N Engl J Med. 1980;302(23):1277-1279. https://doi.org/10.1056/NEJM198006053022303
5. Barakos JA, Ralls PW, Lapin SA, et al. Cholelithiasis: evaluation with CT. Radiology. 1987;162(2):415-418. https://doi.org/10.1148/radiology.162.2.3797654
6. Moore C, Meyers AB, Capotasto J, Bokhari J. Prevalence of abnormal CT findings in patients with proven ovarian torsion and a proposed triage schema. Emerg Radiol. 2009;16(2):115-120. https://doi.org/10.1007/s10140-008-0754-x
7. Harfouch N, Stern J, Chowdhary V, et al. Utility of ultrasound after a negative CT abdomen and pelvis in the emergency department. Clin Imaging. 2020;68:29-35. https://doi.org/10.1016/j.clinimag.2020.06.007
8. Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing duplicative imaging: inpatient and emergency medicine abdominal ultrasound within 72 hours of abdominal CT. J Am Coll Radiol. 2020;17(5):590-596. https://doi.org/10.1016/j.jacr.2020.03.010
9. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720. https://doi.org/10.1148/radiol.12111561
10. Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the diagnostic accuracy of ultrasound and CT in evaluating acute cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97. https://doi.org/10.2214/AJR.17.18884
11. Bennett GL, Rusinek H, Lisi V, et al. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178(2):275-281. https://doi.org/10.2214/ajr.178.2.1780275
12. Hiatt KD, Ou JJ, Childs DD. Role of ultrasound and CT in the workup of right upper quadrant pain in adults in the emergency department: a retrospective review of more than 2800 cases. AJR Am J Roentgenol. 2020;214(6):1305-1310. https://doi.org/10.2214/AJR.19.22188
13. Gao Y, Lee K, Camacho M. Utility of pelvic ultrasound following negative abdominal and pelvic CT in the emergency room. Clin Radiol. 2013;68(11):e586-e592. https://doi.org/10.1016/j.crad.2013.05.101
14. Natesan S, Lee J, Volkamer H, Thoureen T. Evidence-based medicine approach to abdominal pain. Emerg Med Clin North Am. 2016;34(2):165-190. https://doi.org/10.1016/j.emc.2015.12.008.
15. Dickerson EC, Alam HB, Brown RK, Stojanovska J, Davenport MS; Michigan Radiology Quality Collaborative. In-person communication between radiologists and acute care surgeons leads to significant alterations in surgical decision making. J Am Coll Radiol. 2016;13(8):943-949. https://doi.org/10.1016/j.jacr.2016.02.005
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Routine Use of Corticosteroids for the Treatment of Anaphylaxis
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11
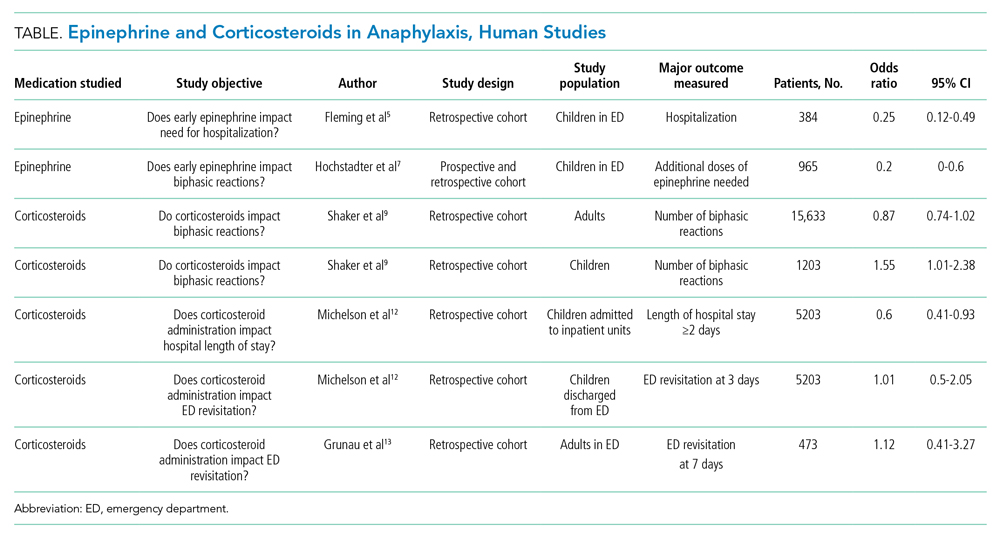
The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Prescribing Appetite Stimulants to Hospitalized Older Adults With Unintentional Weight Loss
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Prescribing Thiamine, Folate and Multivitamins on Discharge for Patients With Alcohol Use Disorder
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
© 2021 Society of Hospital Medicine
Things We Do for No Reason:™ Prescribing Tramadol for Inpatients in Pain
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2
Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2
Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2
Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Emergent Hemodialysis After Intravascular Iodinated Contrast Exposure in Chronic Hemodialysis Patients
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™" (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 56-year-old anuric man with end-stage renal disease (ESRD) on maintenance hemodialysis (HD) for an acute coronary syndrome. He received his regularly scheduled HD the day before admission. Cardiology delays his coronary catheterization until nephrology can arrange for HD immediately after angiography. After angiography, the patient receives emergent HD even though he had acceptable metabolic parameters and did not show signs or symptoms of volume overload. The hospitalist wonders whether arranging emergent HD after the procedure with intravascular (IV) contrast was necessary for this patient.
BACKGROUND
Of the approximately 600 million radiological examinations performed annually, 75 million require iodinated contrast material (ICM).1 ICM are small, highly diffusible, minimally protein-bound molecules. They are not metabolized by humans, with healthy kidneys excreting approximately 99.8% of the administered dose within 24 hours.2 ICM has been associated with acute kidney injury (AKI), but its deleterious effects have not been thoroughly described, and the incidence and severity of contrast-associated nephropathy vary among studies.3 Not surprisingly, the strongest independent patient-related risk factor for developing contrast-induced AKI is preexisting chronic kidney disease.4 In patients with ESRD, the biliary system slowly clears the contrast, leading to long-standing retention. Newer low- or iso-osmolar contrast material is now used rather than older, conventional high-osmolality agents. These agents are less likely to lead to AKI.5
Recent studies have challenged the association between AKI and ICM administration.6-8 In 2015, the American College of Radiology endorsed the terms contrast-associated acute kidney injury and contrast-induced acute kidney injury, instead of the contrast-induced nephropathy, to avoid the uncertainty about the causal relationship between AKI and ICM.9 ESRD patients have little or no functional renal tissue and are on renal replacement therapy, either HD or peritoneal dialysis. However, physicians apprehensive about the renal and cardiovascular toxicity caused by retained ICM might request postprocedural HD to promote quicker contrast clearance in patients with ESRD.
WHY YOU MIGHT THINK PERFORMING EMERGENT HEMODIALYSIS AFTER IV CONTRAST IS NECESSARY
Clinicians divide patients with ESRD into two groups depending on their ability to produce urine. Those who produce urine have residual renal function (RRF), which independently predicts survival.10 Among a cohort of peritoneal and HD patients, Maiorca et al described a 40% reduction in the risk of death for each 1 mL/min increase in glomerular filtration rate (GFR).10 Therefore, patients on maintenance dialysis who have RRF are considered similar to patients with AKI and eGFR <30 mL/min/1.73 m2.9 Clinicians might worry that contrast retention could reduce RRF by inducing AKI.2,4,11
Volume overload is a second concern with ICM administration in ESRD patients. In mice, higher-osmolality ICM produced acute pulmonary edema, leading to death.12 A rapid bolus of diatrizoate caused transient intravascular expansion as reflected by an average decrease in hemoglobin of 0.5 to 0.8 g/dL, depending on the osmolality of the agent.12
Conventional high-osmolar ICM also depresses myocardial contractile force, sinoatrial automaticity, and atrioventricular nodal conduction, resulting in bradycardia, transient heart blocks, and increased risk of ventricular fibrillation.12 High-osmolar calcium-binding ICM transiently reduces systemic vascular resistance, resulting in transient hypotension and increased cardiac output. Researchers linked these adverse cardiac effects to the high-osmolality ionic ICM, not newer agents.12 In one study of adverse outcomes linked to ICM, 36% of patients with normal kidney function exposed to contrast developed an adverse reaction; 2% of patients developed level 4 (severe) adverse reactions.13 The study noted a significantly increased risk of bradycardia (relative risk [RR], 17.9), hypotension (RR, 6.3), and angina (RR, 3.4) among those who received high-osmolality contrast agents.
HD removes 72% to 82% of ICM at 4 hours.14 Armed with data from mice or small-population studies that demonstrated the toxic effects of conventional high-osmolar ICM, many radiologists and clinicians recommend post-contrast HD for patients at high risk for contrast-induced AKI and chronic HD patients.2 Moon et al suggested prophylactic HD for quicker removal of the iodinated contrast medium to prevent reduction in renal function among high-risk patients after angiographic interventions.15
WHY THERE IS LITTLE REASON TO HEMODIALYZE AFTER CONTRAST EXPOSURE
Over the last 3 decades, we have transitioned from conventional radiocontrast to low-osmolality agents that are not directly toxic to the kidneys. Iodixanol, iohexol, and iopromide exposure during intravascular radiological procedures did not result in a decline of RRF among well-hydrated peritoneal dialysis patients with RRF.16,17 The limited analysis of HD trials in the systematic review by Cruz et al concluded that periprocedural HD in patients with chronic kidney disease did not decrease the incidence of radiocontrast-associated nephropathy.18 A meta-analysis of nine studies (434 patients) concluded that ICM administration does not cause significant reduction of residual function in dialysis patients.19 Because anuric ESRD patients have no salvageable renal function and are on HD, managing AKI seems irrelevant.
Although volume overload is an important consideration, the theoretical increase in intravascular volume with administration of 100 mL of 1500 mOsm/L of conventional ICM to a 70 kg-patient is only 120 mL.14 More importantly, use of low-osmolar ICM substantially reduces any significant volume shifts.
Studies have not associated low-osmolality ICM with cardiovascular adverse effects.20-23 A retrospective study by Takebayashi et al showed an absence of serious adverse reactions to low-osmolar contrast media when HD was performed on their regular HD schedule.22 Older, smaller prospective trials did not show a need for periprocedural HD after ICM exposure.20,21,23 In a prospective study of 10 ESRD patients, Younathan et al assessed for postprocedural adverse effects of non-ionic contrast material and found that none required emergent HD.23 Similarly, Hamani et al and Harasawa et al did not observe hemodynamic and cardiopulmonary effects of IV contrast in chronic HD patients (Table).20,21 Injection of non-ionic contrast material in patients on chronic HD did not produce significant changes in blood pressure, electrocardiogram results, osmolality, extracellular fluid volume, or body weight.23 Finally, the vasoconstrictor-mediated ischemic injury of ICM occurs within minutes of administration, making dialysis performed hours later of little benefit.
HD is associated with adverse effects, including hypotension, which can jeopardize cardiovascular recovery after a myocardial infarction.24 The retrospective study performed by Fujimoto et al demonstrated dialytic complications in 24% of patients dialyzed the day of angiography.25 They noted that the amount of contrast agent administered independently predicted intradialytic hypotension.25,26
Delays in performing cardiac revascularizations are associated with an increase in 30-day mortality. The 30-day mortality rates of patients diagnosed with ST-elevation myocardial infarction who underwent revascularization in <60 minutes, 61 to 75 minutes, 76 to 90 minutes, and >90 minutes from study enrollment were 1%, 3.7%, 4%, and 6.7%, respectively.27 Delayed diagnosis of pulmonary embolism or acute limb ischemia was associated with increased rates of complications and mortality.28,29 The benefits of essential radiocontrast procedures outweigh the potential cardiovascular and cerebrovascular complications for HD patients. Considering the evidence, the American College of Radiology’s 2020 Manual on Contrast Media and the European Society for Urogenital Radiology’s 2018 guidelines on contrast medium administration in patients on HD concluded that an extra session or a change in the usual timing of HD is unnecessary.13,30
WHAT YOU SHOULD DO INSTEAD
HD performed post-contrast exposure does not provide any protective benefit, regardless of the degree of RRF (anuric ESRD or otherwise), making the timing of HD irrelevant. Do not delay studies that provide essential information for clinical management of high-risk conditions. The decision to perform HD in a patient who needs contrast-enhanced studies should be made independent of whether they will receive contrast.
RECOMMENDATIONS
- Immediate post-procedural HD after ICM exposure in ESRD patients is not required.
- Do not delay vital diagnostic or therapeutic procedures requiring ICM in ESRD patients.
- The indication for HD is independent of contrast exposure in ESRD patients.
CONCLUSION
The hospitalist did not need to arrange emergent post-procedural HD because it does not improve clinical outcomes. Delaying potentially lifesaving diagnostic and therapeutic measures involving the use of radiocontrast to secure post-radiocontrast HD could lead to worse outcomes.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Christiansen C. X-ray contrast media--an overview. Toxicology. 2005;209(2):185-187. https://doi.org/10.1016/j.tox.2004.12.020
2. Deray G. Dialysis and iodinated contrast media. Kidney Int Suppl. 2006(100):S25-29. https://doi.org/ 10.1038/sj.ki.5000371
3. American College of Radiology. ACR manual on contrast media. Published 2020. Accessed July 18, 2021. https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf
4. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-2155. https://doi.org/10.1056/NEJMra1805256
5. Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis. 2020;75(1):105-113. https://doi.org/10.1053/j.ajkd.2019.05.022
6. Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med. 2018;44(1):104-106. https://doi.org/10.1007/s00134-017-4950-6
7. Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med. 2018;44(1):110-114. https://doi.org/10.1007/s00134-017-4970-2
8. Weisbord SD, du Cheryon D. Contrast-associated acute kidney injury is a myth: no. Intensive Care Med. 2018;44(1):107-109. https://doi.org/10.1007/s00134-017-5015-6
9. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660-668. https://doi.org/10.1148/radiol.2019192094
10. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53(6):1068-1081. https://doi.org/10.1053/j.ajkd.2009.02.012
11. Hsieh MS, Chiu CS, How CK, et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: a nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore). 2016;95(16):e3388. https://doi.org/10.1097/MD.0000000000003388
12. Hirshfeld JW, Jr. Cardiovascular effects of iodinate contrast agents. Am J Cardiol. 1990;66(14):9F-17F. https://doi.org/10.1016/0002-9149(90)90635-e
13. Steinberg EP, Moore RD, Powe NR, et al. Safety and cost effectiveness of high-osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med. 1992;326(7):425-430. https://doi.org/10.1056/NEJM199202133260701
14. Rodby RA. Preventing complications of radiographic contrast media: Is there a role for dialysis? Sem Dial. 2007;20(1):19-23. https://doi.org/10.1111/j.1525-139X.2007.00233.x
15. Moon SS, Bäck SE, Kurkus J, Nilsson-Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70(4):430-437. https://doi.org/10.1159/000188641
16. Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21(5):1334-1339. https://doi.org/10.1093/ndt/gfi023
17. Moranne O, Willoteaux S, Pagniez D, Dequiedt P, Boulanger E. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21(4):1040-1045. https://doi.org/10.1093/ndt/gfi327
18. Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48(3):361-371. https://doi.org/10.1053/j.ajkd.2006.05.023
19. Oloko A, Talreja H, Davis A, et al. Does iodinated contrast affect residual renal function in dialysis patients? a systematic review and meta-analysis. Nephron. 2020;144(4):176-184. https://doi.org/10.1159/000505576
20. Hamani A, Petitclerc T, Jacobs C, Deray G. Is dialysis indicated immediately after administration of iodinated contrast agents in patients on haemodialysis? Nephrol Dial Transplant. 1998;13:1051-1052.
21. Harasawa H, Yamazaki C, Masuko K. Side effects and pharmacokinetics of nonionic iodinated contrast medium in hemodialized patients. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50(12):1524-1531.
22. Takebayashi S, Hidai H, Chiba T. No need for immediate dialysis after administration of low-osmolarity contrast medium in patients undergoing hemodialysis. Am J Kidney Dis. 2000;36(1):226. https://doi.org/10.1053/ajkd.2000.8301
23. Younathan CM, Kaude JV, Cook MD, Shaw GS, Peterson JC. Dialysis not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR. Am J Roentgenol. 1994;163:969-971. https://doi.org/10.2214/ajr.163.4.8092045
24. Coritsidis G, Sutariya D, Stern A, et al. Does timing of dialysis in patients with ESRD and acute myocardial infarcts affect morbidity or mortality? Clin J Am Soc Nephrol. 2009;4(8):1324-1330. https://doi.org/10.2215/CJN.04470908
25. Fujimoto M, Ishikawa E, Haruki A, et al. Hemodialysis complications after angiography and its risk factors. Nihon Toseki Igakkai Zasshi. 2015;48(5):269-274. https://doi.org/10.4009/jsdt.48.269
26. Tachibana K, Kida H, Uenoyama M, Nakamura T, Yamada T, Hayahi T. Risk factors for intradialytic hypotension after percutaneous coronary interventions. Nihon Toseki Igakkai Zasshi. 2019;52(4):227-232. https://doi.org/10.4009/jsdt.52.227
27. Berger PB, Ellis SG, Holmes DR Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction. Circulation. 1999;100(1):14-20. https://doi.org/10.1161/01.cir.100.1.14
28. Nagasheth K, Nassiri N, Shafritz R, Rahimi S. Delayed revascularization for acute lower extremity ischemia leads to increased mortality. J Vasc Surg. 2016;63(6S):121S-122S.
29. Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA Jr. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592-598. https://doi.org/10.1197/j.aem.2007.03.1356
30. European Society of Urogenital Radiology. ESUR guidelines on contrast agents. Accessed July 20, 2021. http://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™" (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 56-year-old anuric man with end-stage renal disease (ESRD) on maintenance hemodialysis (HD) for an acute coronary syndrome. He received his regularly scheduled HD the day before admission. Cardiology delays his coronary catheterization until nephrology can arrange for HD immediately after angiography. After angiography, the patient receives emergent HD even though he had acceptable metabolic parameters and did not show signs or symptoms of volume overload. The hospitalist wonders whether arranging emergent HD after the procedure with intravascular (IV) contrast was necessary for this patient.
BACKGROUND
Of the approximately 600 million radiological examinations performed annually, 75 million require iodinated contrast material (ICM).1 ICM are small, highly diffusible, minimally protein-bound molecules. They are not metabolized by humans, with healthy kidneys excreting approximately 99.8% of the administered dose within 24 hours.2 ICM has been associated with acute kidney injury (AKI), but its deleterious effects have not been thoroughly described, and the incidence and severity of contrast-associated nephropathy vary among studies.3 Not surprisingly, the strongest independent patient-related risk factor for developing contrast-induced AKI is preexisting chronic kidney disease.4 In patients with ESRD, the biliary system slowly clears the contrast, leading to long-standing retention. Newer low- or iso-osmolar contrast material is now used rather than older, conventional high-osmolality agents. These agents are less likely to lead to AKI.5
Recent studies have challenged the association between AKI and ICM administration.6-8 In 2015, the American College of Radiology endorsed the terms contrast-associated acute kidney injury and contrast-induced acute kidney injury, instead of the contrast-induced nephropathy, to avoid the uncertainty about the causal relationship between AKI and ICM.9 ESRD patients have little or no functional renal tissue and are on renal replacement therapy, either HD or peritoneal dialysis. However, physicians apprehensive about the renal and cardiovascular toxicity caused by retained ICM might request postprocedural HD to promote quicker contrast clearance in patients with ESRD.
WHY YOU MIGHT THINK PERFORMING EMERGENT HEMODIALYSIS AFTER IV CONTRAST IS NECESSARY
Clinicians divide patients with ESRD into two groups depending on their ability to produce urine. Those who produce urine have residual renal function (RRF), which independently predicts survival.10 Among a cohort of peritoneal and HD patients, Maiorca et al described a 40% reduction in the risk of death for each 1 mL/min increase in glomerular filtration rate (GFR).10 Therefore, patients on maintenance dialysis who have RRF are considered similar to patients with AKI and eGFR <30 mL/min/1.73 m2.9 Clinicians might worry that contrast retention could reduce RRF by inducing AKI.2,4,11
Volume overload is a second concern with ICM administration in ESRD patients. In mice, higher-osmolality ICM produced acute pulmonary edema, leading to death.12 A rapid bolus of diatrizoate caused transient intravascular expansion as reflected by an average decrease in hemoglobin of 0.5 to 0.8 g/dL, depending on the osmolality of the agent.12
Conventional high-osmolar ICM also depresses myocardial contractile force, sinoatrial automaticity, and atrioventricular nodal conduction, resulting in bradycardia, transient heart blocks, and increased risk of ventricular fibrillation.12 High-osmolar calcium-binding ICM transiently reduces systemic vascular resistance, resulting in transient hypotension and increased cardiac output. Researchers linked these adverse cardiac effects to the high-osmolality ionic ICM, not newer agents.12 In one study of adverse outcomes linked to ICM, 36% of patients with normal kidney function exposed to contrast developed an adverse reaction; 2% of patients developed level 4 (severe) adverse reactions.13 The study noted a significantly increased risk of bradycardia (relative risk [RR], 17.9), hypotension (RR, 6.3), and angina (RR, 3.4) among those who received high-osmolality contrast agents.
HD removes 72% to 82% of ICM at 4 hours.14 Armed with data from mice or small-population studies that demonstrated the toxic effects of conventional high-osmolar ICM, many radiologists and clinicians recommend post-contrast HD for patients at high risk for contrast-induced AKI and chronic HD patients.2 Moon et al suggested prophylactic HD for quicker removal of the iodinated contrast medium to prevent reduction in renal function among high-risk patients after angiographic interventions.15
WHY THERE IS LITTLE REASON TO HEMODIALYZE AFTER CONTRAST EXPOSURE
Over the last 3 decades, we have transitioned from conventional radiocontrast to low-osmolality agents that are not directly toxic to the kidneys. Iodixanol, iohexol, and iopromide exposure during intravascular radiological procedures did not result in a decline of RRF among well-hydrated peritoneal dialysis patients with RRF.16,17 The limited analysis of HD trials in the systematic review by Cruz et al concluded that periprocedural HD in patients with chronic kidney disease did not decrease the incidence of radiocontrast-associated nephropathy.18 A meta-analysis of nine studies (434 patients) concluded that ICM administration does not cause significant reduction of residual function in dialysis patients.19 Because anuric ESRD patients have no salvageable renal function and are on HD, managing AKI seems irrelevant.
Although volume overload is an important consideration, the theoretical increase in intravascular volume with administration of 100 mL of 1500 mOsm/L of conventional ICM to a 70 kg-patient is only 120 mL.14 More importantly, use of low-osmolar ICM substantially reduces any significant volume shifts.
Studies have not associated low-osmolality ICM with cardiovascular adverse effects.20-23 A retrospective study by Takebayashi et al showed an absence of serious adverse reactions to low-osmolar contrast media when HD was performed on their regular HD schedule.22 Older, smaller prospective trials did not show a need for periprocedural HD after ICM exposure.20,21,23 In a prospective study of 10 ESRD patients, Younathan et al assessed for postprocedural adverse effects of non-ionic contrast material and found that none required emergent HD.23 Similarly, Hamani et al and Harasawa et al did not observe hemodynamic and cardiopulmonary effects of IV contrast in chronic HD patients (Table).20,21 Injection of non-ionic contrast material in patients on chronic HD did not produce significant changes in blood pressure, electrocardiogram results, osmolality, extracellular fluid volume, or body weight.23 Finally, the vasoconstrictor-mediated ischemic injury of ICM occurs within minutes of administration, making dialysis performed hours later of little benefit.
HD is associated with adverse effects, including hypotension, which can jeopardize cardiovascular recovery after a myocardial infarction.24 The retrospective study performed by Fujimoto et al demonstrated dialytic complications in 24% of patients dialyzed the day of angiography.25 They noted that the amount of contrast agent administered independently predicted intradialytic hypotension.25,26
Delays in performing cardiac revascularizations are associated with an increase in 30-day mortality. The 30-day mortality rates of patients diagnosed with ST-elevation myocardial infarction who underwent revascularization in <60 minutes, 61 to 75 minutes, 76 to 90 minutes, and >90 minutes from study enrollment were 1%, 3.7%, 4%, and 6.7%, respectively.27 Delayed diagnosis of pulmonary embolism or acute limb ischemia was associated with increased rates of complications and mortality.28,29 The benefits of essential radiocontrast procedures outweigh the potential cardiovascular and cerebrovascular complications for HD patients. Considering the evidence, the American College of Radiology’s 2020 Manual on Contrast Media and the European Society for Urogenital Radiology’s 2018 guidelines on contrast medium administration in patients on HD concluded that an extra session or a change in the usual timing of HD is unnecessary.13,30
WHAT YOU SHOULD DO INSTEAD
HD performed post-contrast exposure does not provide any protective benefit, regardless of the degree of RRF (anuric ESRD or otherwise), making the timing of HD irrelevant. Do not delay studies that provide essential information for clinical management of high-risk conditions. The decision to perform HD in a patient who needs contrast-enhanced studies should be made independent of whether they will receive contrast.
RECOMMENDATIONS
- Immediate post-procedural HD after ICM exposure in ESRD patients is not required.
- Do not delay vital diagnostic or therapeutic procedures requiring ICM in ESRD patients.
- The indication for HD is independent of contrast exposure in ESRD patients.
CONCLUSION
The hospitalist did not need to arrange emergent post-procedural HD because it does not improve clinical outcomes. Delaying potentially lifesaving diagnostic and therapeutic measures involving the use of radiocontrast to secure post-radiocontrast HD could lead to worse outcomes.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™" (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 56-year-old anuric man with end-stage renal disease (ESRD) on maintenance hemodialysis (HD) for an acute coronary syndrome. He received his regularly scheduled HD the day before admission. Cardiology delays his coronary catheterization until nephrology can arrange for HD immediately after angiography. After angiography, the patient receives emergent HD even though he had acceptable metabolic parameters and did not show signs or symptoms of volume overload. The hospitalist wonders whether arranging emergent HD after the procedure with intravascular (IV) contrast was necessary for this patient.
BACKGROUND
Of the approximately 600 million radiological examinations performed annually, 75 million require iodinated contrast material (ICM).1 ICM are small, highly diffusible, minimally protein-bound molecules. They are not metabolized by humans, with healthy kidneys excreting approximately 99.8% of the administered dose within 24 hours.2 ICM has been associated with acute kidney injury (AKI), but its deleterious effects have not been thoroughly described, and the incidence and severity of contrast-associated nephropathy vary among studies.3 Not surprisingly, the strongest independent patient-related risk factor for developing contrast-induced AKI is preexisting chronic kidney disease.4 In patients with ESRD, the biliary system slowly clears the contrast, leading to long-standing retention. Newer low- or iso-osmolar contrast material is now used rather than older, conventional high-osmolality agents. These agents are less likely to lead to AKI.5
Recent studies have challenged the association between AKI and ICM administration.6-8 In 2015, the American College of Radiology endorsed the terms contrast-associated acute kidney injury and contrast-induced acute kidney injury, instead of the contrast-induced nephropathy, to avoid the uncertainty about the causal relationship between AKI and ICM.9 ESRD patients have little or no functional renal tissue and are on renal replacement therapy, either HD or peritoneal dialysis. However, physicians apprehensive about the renal and cardiovascular toxicity caused by retained ICM might request postprocedural HD to promote quicker contrast clearance in patients with ESRD.
WHY YOU MIGHT THINK PERFORMING EMERGENT HEMODIALYSIS AFTER IV CONTRAST IS NECESSARY
Clinicians divide patients with ESRD into two groups depending on their ability to produce urine. Those who produce urine have residual renal function (RRF), which independently predicts survival.10 Among a cohort of peritoneal and HD patients, Maiorca et al described a 40% reduction in the risk of death for each 1 mL/min increase in glomerular filtration rate (GFR).10 Therefore, patients on maintenance dialysis who have RRF are considered similar to patients with AKI and eGFR <30 mL/min/1.73 m2.9 Clinicians might worry that contrast retention could reduce RRF by inducing AKI.2,4,11
Volume overload is a second concern with ICM administration in ESRD patients. In mice, higher-osmolality ICM produced acute pulmonary edema, leading to death.12 A rapid bolus of diatrizoate caused transient intravascular expansion as reflected by an average decrease in hemoglobin of 0.5 to 0.8 g/dL, depending on the osmolality of the agent.12
Conventional high-osmolar ICM also depresses myocardial contractile force, sinoatrial automaticity, and atrioventricular nodal conduction, resulting in bradycardia, transient heart blocks, and increased risk of ventricular fibrillation.12 High-osmolar calcium-binding ICM transiently reduces systemic vascular resistance, resulting in transient hypotension and increased cardiac output. Researchers linked these adverse cardiac effects to the high-osmolality ionic ICM, not newer agents.12 In one study of adverse outcomes linked to ICM, 36% of patients with normal kidney function exposed to contrast developed an adverse reaction; 2% of patients developed level 4 (severe) adverse reactions.13 The study noted a significantly increased risk of bradycardia (relative risk [RR], 17.9), hypotension (RR, 6.3), and angina (RR, 3.4) among those who received high-osmolality contrast agents.
HD removes 72% to 82% of ICM at 4 hours.14 Armed with data from mice or small-population studies that demonstrated the toxic effects of conventional high-osmolar ICM, many radiologists and clinicians recommend post-contrast HD for patients at high risk for contrast-induced AKI and chronic HD patients.2 Moon et al suggested prophylactic HD for quicker removal of the iodinated contrast medium to prevent reduction in renal function among high-risk patients after angiographic interventions.15
WHY THERE IS LITTLE REASON TO HEMODIALYZE AFTER CONTRAST EXPOSURE
Over the last 3 decades, we have transitioned from conventional radiocontrast to low-osmolality agents that are not directly toxic to the kidneys. Iodixanol, iohexol, and iopromide exposure during intravascular radiological procedures did not result in a decline of RRF among well-hydrated peritoneal dialysis patients with RRF.16,17 The limited analysis of HD trials in the systematic review by Cruz et al concluded that periprocedural HD in patients with chronic kidney disease did not decrease the incidence of radiocontrast-associated nephropathy.18 A meta-analysis of nine studies (434 patients) concluded that ICM administration does not cause significant reduction of residual function in dialysis patients.19 Because anuric ESRD patients have no salvageable renal function and are on HD, managing AKI seems irrelevant.
Although volume overload is an important consideration, the theoretical increase in intravascular volume with administration of 100 mL of 1500 mOsm/L of conventional ICM to a 70 kg-patient is only 120 mL.14 More importantly, use of low-osmolar ICM substantially reduces any significant volume shifts.
Studies have not associated low-osmolality ICM with cardiovascular adverse effects.20-23 A retrospective study by Takebayashi et al showed an absence of serious adverse reactions to low-osmolar contrast media when HD was performed on their regular HD schedule.22 Older, smaller prospective trials did not show a need for periprocedural HD after ICM exposure.20,21,23 In a prospective study of 10 ESRD patients, Younathan et al assessed for postprocedural adverse effects of non-ionic contrast material and found that none required emergent HD.23 Similarly, Hamani et al and Harasawa et al did not observe hemodynamic and cardiopulmonary effects of IV contrast in chronic HD patients (Table).20,21 Injection of non-ionic contrast material in patients on chronic HD did not produce significant changes in blood pressure, electrocardiogram results, osmolality, extracellular fluid volume, or body weight.23 Finally, the vasoconstrictor-mediated ischemic injury of ICM occurs within minutes of administration, making dialysis performed hours later of little benefit.
HD is associated with adverse effects, including hypotension, which can jeopardize cardiovascular recovery after a myocardial infarction.24 The retrospective study performed by Fujimoto et al demonstrated dialytic complications in 24% of patients dialyzed the day of angiography.25 They noted that the amount of contrast agent administered independently predicted intradialytic hypotension.25,26
Delays in performing cardiac revascularizations are associated with an increase in 30-day mortality. The 30-day mortality rates of patients diagnosed with ST-elevation myocardial infarction who underwent revascularization in <60 minutes, 61 to 75 minutes, 76 to 90 minutes, and >90 minutes from study enrollment were 1%, 3.7%, 4%, and 6.7%, respectively.27 Delayed diagnosis of pulmonary embolism or acute limb ischemia was associated with increased rates of complications and mortality.28,29 The benefits of essential radiocontrast procedures outweigh the potential cardiovascular and cerebrovascular complications for HD patients. Considering the evidence, the American College of Radiology’s 2020 Manual on Contrast Media and the European Society for Urogenital Radiology’s 2018 guidelines on contrast medium administration in patients on HD concluded that an extra session or a change in the usual timing of HD is unnecessary.13,30
WHAT YOU SHOULD DO INSTEAD
HD performed post-contrast exposure does not provide any protective benefit, regardless of the degree of RRF (anuric ESRD or otherwise), making the timing of HD irrelevant. Do not delay studies that provide essential information for clinical management of high-risk conditions. The decision to perform HD in a patient who needs contrast-enhanced studies should be made independent of whether they will receive contrast.
RECOMMENDATIONS
- Immediate post-procedural HD after ICM exposure in ESRD patients is not required.
- Do not delay vital diagnostic or therapeutic procedures requiring ICM in ESRD patients.
- The indication for HD is independent of contrast exposure in ESRD patients.
CONCLUSION
The hospitalist did not need to arrange emergent post-procedural HD because it does not improve clinical outcomes. Delaying potentially lifesaving diagnostic and therapeutic measures involving the use of radiocontrast to secure post-radiocontrast HD could lead to worse outcomes.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Christiansen C. X-ray contrast media--an overview. Toxicology. 2005;209(2):185-187. https://doi.org/10.1016/j.tox.2004.12.020
2. Deray G. Dialysis and iodinated contrast media. Kidney Int Suppl. 2006(100):S25-29. https://doi.org/ 10.1038/sj.ki.5000371
3. American College of Radiology. ACR manual on contrast media. Published 2020. Accessed July 18, 2021. https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf
4. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-2155. https://doi.org/10.1056/NEJMra1805256
5. Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis. 2020;75(1):105-113. https://doi.org/10.1053/j.ajkd.2019.05.022
6. Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med. 2018;44(1):104-106. https://doi.org/10.1007/s00134-017-4950-6
7. Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med. 2018;44(1):110-114. https://doi.org/10.1007/s00134-017-4970-2
8. Weisbord SD, du Cheryon D. Contrast-associated acute kidney injury is a myth: no. Intensive Care Med. 2018;44(1):107-109. https://doi.org/10.1007/s00134-017-5015-6
9. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660-668. https://doi.org/10.1148/radiol.2019192094
10. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53(6):1068-1081. https://doi.org/10.1053/j.ajkd.2009.02.012
11. Hsieh MS, Chiu CS, How CK, et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: a nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore). 2016;95(16):e3388. https://doi.org/10.1097/MD.0000000000003388
12. Hirshfeld JW, Jr. Cardiovascular effects of iodinate contrast agents. Am J Cardiol. 1990;66(14):9F-17F. https://doi.org/10.1016/0002-9149(90)90635-e
13. Steinberg EP, Moore RD, Powe NR, et al. Safety and cost effectiveness of high-osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med. 1992;326(7):425-430. https://doi.org/10.1056/NEJM199202133260701
14. Rodby RA. Preventing complications of radiographic contrast media: Is there a role for dialysis? Sem Dial. 2007;20(1):19-23. https://doi.org/10.1111/j.1525-139X.2007.00233.x
15. Moon SS, Bäck SE, Kurkus J, Nilsson-Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70(4):430-437. https://doi.org/10.1159/000188641
16. Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21(5):1334-1339. https://doi.org/10.1093/ndt/gfi023
17. Moranne O, Willoteaux S, Pagniez D, Dequiedt P, Boulanger E. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21(4):1040-1045. https://doi.org/10.1093/ndt/gfi327
18. Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48(3):361-371. https://doi.org/10.1053/j.ajkd.2006.05.023
19. Oloko A, Talreja H, Davis A, et al. Does iodinated contrast affect residual renal function in dialysis patients? a systematic review and meta-analysis. Nephron. 2020;144(4):176-184. https://doi.org/10.1159/000505576
20. Hamani A, Petitclerc T, Jacobs C, Deray G. Is dialysis indicated immediately after administration of iodinated contrast agents in patients on haemodialysis? Nephrol Dial Transplant. 1998;13:1051-1052.
21. Harasawa H, Yamazaki C, Masuko K. Side effects and pharmacokinetics of nonionic iodinated contrast medium in hemodialized patients. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50(12):1524-1531.
22. Takebayashi S, Hidai H, Chiba T. No need for immediate dialysis after administration of low-osmolarity contrast medium in patients undergoing hemodialysis. Am J Kidney Dis. 2000;36(1):226. https://doi.org/10.1053/ajkd.2000.8301
23. Younathan CM, Kaude JV, Cook MD, Shaw GS, Peterson JC. Dialysis not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR. Am J Roentgenol. 1994;163:969-971. https://doi.org/10.2214/ajr.163.4.8092045
24. Coritsidis G, Sutariya D, Stern A, et al. Does timing of dialysis in patients with ESRD and acute myocardial infarcts affect morbidity or mortality? Clin J Am Soc Nephrol. 2009;4(8):1324-1330. https://doi.org/10.2215/CJN.04470908
25. Fujimoto M, Ishikawa E, Haruki A, et al. Hemodialysis complications after angiography and its risk factors. Nihon Toseki Igakkai Zasshi. 2015;48(5):269-274. https://doi.org/10.4009/jsdt.48.269
26. Tachibana K, Kida H, Uenoyama M, Nakamura T, Yamada T, Hayahi T. Risk factors for intradialytic hypotension after percutaneous coronary interventions. Nihon Toseki Igakkai Zasshi. 2019;52(4):227-232. https://doi.org/10.4009/jsdt.52.227
27. Berger PB, Ellis SG, Holmes DR Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction. Circulation. 1999;100(1):14-20. https://doi.org/10.1161/01.cir.100.1.14
28. Nagasheth K, Nassiri N, Shafritz R, Rahimi S. Delayed revascularization for acute lower extremity ischemia leads to increased mortality. J Vasc Surg. 2016;63(6S):121S-122S.
29. Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA Jr. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592-598. https://doi.org/10.1197/j.aem.2007.03.1356
30. European Society of Urogenital Radiology. ESUR guidelines on contrast agents. Accessed July 20, 2021. http://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
1. Christiansen C. X-ray contrast media--an overview. Toxicology. 2005;209(2):185-187. https://doi.org/10.1016/j.tox.2004.12.020
2. Deray G. Dialysis and iodinated contrast media. Kidney Int Suppl. 2006(100):S25-29. https://doi.org/ 10.1038/sj.ki.5000371
3. American College of Radiology. ACR manual on contrast media. Published 2020. Accessed July 18, 2021. https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf
4. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-2155. https://doi.org/10.1056/NEJMra1805256
5. Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis. 2020;75(1):105-113. https://doi.org/10.1053/j.ajkd.2019.05.022
6. Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med. 2018;44(1):104-106. https://doi.org/10.1007/s00134-017-4950-6
7. Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med. 2018;44(1):110-114. https://doi.org/10.1007/s00134-017-4970-2
8. Weisbord SD, du Cheryon D. Contrast-associated acute kidney injury is a myth: no. Intensive Care Med. 2018;44(1):107-109. https://doi.org/10.1007/s00134-017-5015-6
9. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660-668. https://doi.org/10.1148/radiol.2019192094
10. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53(6):1068-1081. https://doi.org/10.1053/j.ajkd.2009.02.012
11. Hsieh MS, Chiu CS, How CK, et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: a nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore). 2016;95(16):e3388. https://doi.org/10.1097/MD.0000000000003388
12. Hirshfeld JW, Jr. Cardiovascular effects of iodinate contrast agents. Am J Cardiol. 1990;66(14):9F-17F. https://doi.org/10.1016/0002-9149(90)90635-e
13. Steinberg EP, Moore RD, Powe NR, et al. Safety and cost effectiveness of high-osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med. 1992;326(7):425-430. https://doi.org/10.1056/NEJM199202133260701
14. Rodby RA. Preventing complications of radiographic contrast media: Is there a role for dialysis? Sem Dial. 2007;20(1):19-23. https://doi.org/10.1111/j.1525-139X.2007.00233.x
15. Moon SS, Bäck SE, Kurkus J, Nilsson-Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70(4):430-437. https://doi.org/10.1159/000188641
16. Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21(5):1334-1339. https://doi.org/10.1093/ndt/gfi023
17. Moranne O, Willoteaux S, Pagniez D, Dequiedt P, Boulanger E. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21(4):1040-1045. https://doi.org/10.1093/ndt/gfi327
18. Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48(3):361-371. https://doi.org/10.1053/j.ajkd.2006.05.023
19. Oloko A, Talreja H, Davis A, et al. Does iodinated contrast affect residual renal function in dialysis patients? a systematic review and meta-analysis. Nephron. 2020;144(4):176-184. https://doi.org/10.1159/000505576
20. Hamani A, Petitclerc T, Jacobs C, Deray G. Is dialysis indicated immediately after administration of iodinated contrast agents in patients on haemodialysis? Nephrol Dial Transplant. 1998;13:1051-1052.
21. Harasawa H, Yamazaki C, Masuko K. Side effects and pharmacokinetics of nonionic iodinated contrast medium in hemodialized patients. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50(12):1524-1531.
22. Takebayashi S, Hidai H, Chiba T. No need for immediate dialysis after administration of low-osmolarity contrast medium in patients undergoing hemodialysis. Am J Kidney Dis. 2000;36(1):226. https://doi.org/10.1053/ajkd.2000.8301
23. Younathan CM, Kaude JV, Cook MD, Shaw GS, Peterson JC. Dialysis not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR. Am J Roentgenol. 1994;163:969-971. https://doi.org/10.2214/ajr.163.4.8092045
24. Coritsidis G, Sutariya D, Stern A, et al. Does timing of dialysis in patients with ESRD and acute myocardial infarcts affect morbidity or mortality? Clin J Am Soc Nephrol. 2009;4(8):1324-1330. https://doi.org/10.2215/CJN.04470908
25. Fujimoto M, Ishikawa E, Haruki A, et al. Hemodialysis complications after angiography and its risk factors. Nihon Toseki Igakkai Zasshi. 2015;48(5):269-274. https://doi.org/10.4009/jsdt.48.269
26. Tachibana K, Kida H, Uenoyama M, Nakamura T, Yamada T, Hayahi T. Risk factors for intradialytic hypotension after percutaneous coronary interventions. Nihon Toseki Igakkai Zasshi. 2019;52(4):227-232. https://doi.org/10.4009/jsdt.52.227
27. Berger PB, Ellis SG, Holmes DR Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction. Circulation. 1999;100(1):14-20. https://doi.org/10.1161/01.cir.100.1.14
28. Nagasheth K, Nassiri N, Shafritz R, Rahimi S. Delayed revascularization for acute lower extremity ischemia leads to increased mortality. J Vasc Surg. 2016;63(6S):121S-122S.
29. Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA Jr. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592-598. https://doi.org/10.1197/j.aem.2007.03.1356
30. European Society of Urogenital Radiology. ESUR guidelines on contrast agents. Accessed July 20, 2021. http://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Routine Inclusion of Race in the History of Present Illness
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
On teaching rounds, a medical student presents the following case to the attending hospitalist: “Mrs. L is a 54-year-old Black female with chronic kidney disease who was admitted with community-acquired pneumonia. She continues to improve symptomatically on ceftriaxone. Currently, she is afebrile and her vitals are stable. Supplemental oxygen has been weaned to 2 L/min by nasal cannula. Exam reveals improved crackles in the left lower chest without dullness to percussion. Labs are notable for down-trending leukocytosis and a stable serum creatinine of 2.8 mg/dL.” The hospitalist considers how including racial descriptors in clinical presentations may influence the care of the patient.
WHY YOU MIGHT THINK INCLUDING RACE IN THE HISTORY OF PRESENT ILLNESS IS HELPFUL
For decades, medical educators have taught learners to include sociopolitical constructs such as race in the opening sentence of the history of present illness (HPI). This practice presumably stems from the assumption that race accurately reflects biogenetic information about patients and serves as a key attribute in problem representations.1 Proponents of including race in the HPI suggest doing so aids the clinical assessment of patients’ risks for particular diseases and may inform the selection of race-appropriate therapies.2
The construct of race does sometimes correlate with the risk of disease or response to therapies. For example, sickle cell disease (SCD) occurs more commonly among patients who identify as Black rather than White. Specifically, ancestry from African nations such as Nigeria or the Democratic Republic of Congo increases the likelihood of having the disease-associated hemoglobin gene variant HbS.1 Popular genomic ancestry tests often report ancestral groupings that map to racial categories and may reinforce the perception that race has a genetic basis.3
WHY IT IS NOT HELPFUL TO INCLUDE RACE IN THE HPI
Race, a construct of sociopolitical origins, incorrectly conflates skin color with genetic variation. Associations between race and disease have the potential to cause diagnostic and therapeutic errors and inequitable allocation of resources. Increased illness burden in minority populations results primarily from social factors such as environment, access to care, housing instability, food insecurity, and experiences of discrimination, rather than genetic differences. The resulting chronic and recurrent physiologic stress—known as allostatic load—also contributes to the inequitable health outcomes observed in vulnerable populations, including patients who identify as Black.4
Historically, race evolved as a sociopolitical framework stemming from colonialism, discrimination, and exploitation.5 Numerous studies reveal a lack of genetic precision in racial categories. In fact, genetic data compared across major continental groups found greater variation of microsatellite loci and restriction fragment length polymorphisms within racial groups than between them.6 The evidence indicates that racial categories do not reflect homogenous population groups but rather “arbitrary division[s] of continuous variation” that cannot serve as a surrogate to genetic diversity.5 Not only are racial categories genetically inaccurate, but data on race within the electronic health record often differ from patients’ self-description of race, underscoring the problematic nature of even identifying race.7 In one study, up to 41% of patients self-reported identification with at least one other racial or ethnic group than the race or ethnicity documented in their electronic health record.7
Additionally, conflating race with genetic variation can lead to diagnostic errors. As an example, the incidence of cystic fibrosis (CF) varies widely across populations of European ancestry. The primary focus on CF’s occurrence in patients of European descent may divert attention from the identification of mutations causing CF in populations of African descent or the decreased survival observed in the United States among CF patients of Hispanic descent.8,9 Similarly, India represents one of the countries largely affected by SCD, suggesting that a myopic focus on SCD among those identifying as Black can lead to underdiagnosis of SCD among those with Indian ancestry.
Perhaps more insidiously, linking disease to race or other social constructs can result in differential support for affected individuals. SCD offers a striking illustration of this point. Reflecting the legacy of transatlantic slave trading, the majority of people with SCD in the United States are Black and face interpersonal and structural racism within society and healthcare that amplify the effects of this devastating illness.10 Compulsory screening programs for sickle cell trait introduced by many states in the 1970s targeted Black Americans and resulted in stigmatization and the denial of insurance, educational opportunities, and jobs for many identified with sickle cell trait. Federal funding for SCD research remains low, particularly in comparison to the tenfold higher funding for CF, which afflicts fewer, but primarily White, Americans.10
The incorporation of race into risk models and guidelines—alongside biologically relevant variables such as age and comorbid conditions—has received increasing attention for its potential to compound racial disparities in health outcomes. The American Heart Association Heart Failure Risk Score, for instance, may lead to the exclusion of some Black patients from necessary care because “Black” race, for no clear physiologic reason, serves as a protective factor against heart failure mortality.11 Likewise, race adjustments in pulmonary function tests, breast cancer risk models, and estimated glomerular filtration rate calculations, among others, have limited biological basis and the potential to divert care disproportionately from minority populations.11
Researchers have even called into question the application of race to pharmacotherapies. A 2001 investigation on geographic patterns of genetic variation in drug response concluded that common racial and ethnic labels were “insufficient and inaccurate representations” of the individual genetic clusters.12 Further, numerous experts have criticized two landmark studies of vasodilators and angiotensin-converting enzyme inhibitors in Black patients with heart failure for inconsistent results and nonsignificant associations between race and major outcomes, such as the development of heart failure or death.13
Race-based labels can also divert attention from true causes of health inequities. The National Academy of Sciences concluded that social determinants of health and structural racism are the root causes of health inequities, rather than genetics.14 Medical professionals may perpetuate these disparities: Most US physicians demonstrate an unconscious preference—or implicit bias—for White Americans over Black Americans.15 Beyond obscuring the role of social determinants of health and structural racism in health outcomes, race-based labels may exacerbate the ways in which physicians’ implicit biases contribute to racial and ethnic health disparities, primarily affecting Black Americans.2 In a recent study, clinicians documented race in the HPI for 33% of Black patients compared with 16% of White patients, and White clinicians were twice as likely to document race as Black physicians.16 Moreover, training medical students to view race as an independent risk factor of disease without discussing structural inequities can pathologize race and reinforce implicit biases linking race and disease.15
Based on the current evidence, we believe routine use of race-based labels in clinical presentations confuses providers at a minimum and potentially produces far more damage by obscuring or perpetuating the role of racism in health inequities.
WHAT YOU SHOULD DO INSTEAD
Instead of routinely presenting race in the HPI, we recommend including racial or ethnic information in the social history only when the patient reports it as a meaningful identity or when it informs health disparities stemming from structural or interpersonal racism. Clinicians should include physical characteristics pertaining to race, such as skin tone, in the physical exam only if required to describe exam findings accurately. When presenting race, clinicians should explicitly justify its use and take care to avoid obfuscatory, inaccurate, or stigmatizing mention of associations between race and disease. Clinicians should not use race in clinical algorithms. Medical educators should emphasize the role of social determinants of health and structural racism in health outcomes to inform the use of race in medicine, in hopes that doing so will help students minimize implicit biases and learn to mitigate racial inequities in healthcare.2,16 In short, clinicians and medical educators alike should ensure that clinical care and the medical curriculum avoid presenting race as a proxy for pathology.
There is little evidence to guide proper inclusion of race in clinical interviews. In the absence of clear guidance about how to approach patients about race, we suggest not asking about it unless there is a reasonable probability that doing so will improve clinical care. If a clinician decides to ask about race, it is important to provide a rationale—such as explaining that the information can be used to assure high-quality care for all patients—since many patients are uncomfortable with questions about race.17 If clinicians report information about race in the social history, we advise using the patient’s description of race rather than traditional racial categories.
Clinicians who ask their patients about race should approach every patient in a uniform manner to avoid perpetuating biases. We hope future studies will inform equitable, inclusive, and person-centered approaches to discussing race with patients and promote a shared understanding of how racism contributes to illness.
RECOMMENDATIONS
- Avoid using racial descriptors in the HPI.
- Include racial and ethnic information in the social history only when it serves as a meaningful identity or it informs disparities stemming from racism.
- If racial or ethnic information is asked for, explain to patients why and how it will be used.
- Mention physical characteristics such as skin tone, rather than race, in the physical exam if required to describe findings accurately.
- Advocate for the replacement of race or race-adjusted algorithms in patient care.
- Expand the medical curriculum in the social determinants of health and structural racism, and develop systems to avoid the use of stigmatizing, race-based labels.
CONCLUSION
Race, a sociopolitical construct, does not accurately represent genetic variation. The routine use of race in the HPI can perpetuate racial biases and muddle both diagnoses and treatment. Only mention race in the social history if it is meaningful to the patient’s self-identity or explains health disparities arising from racism. All documentation and presentations should avoid the use of stigmatizing, race-based labels.
In the clinical scenario mentioned earlier, the attending hospitalist raises the issue of race-based labels in patient care in a nonjudgmental fashion. To provide illustrative specificity, she notes how the incorporation of race in formulas of glomerular filtration rate can lead to under-referral for renal transplant. The hospitalist then facilitates an open and inclusive discussion with the team regarding the use of race in clinical presentations and its potential impact on health disparities.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason™” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348(12):1170-1175. https://doi.org/10.1056/NEJMsb025007
2. Tsai J, Ucik L, Baldwin N, et al. Race matters? Examining and rethinking race portrayal in preclinical medical education. Acad Med. 2016;91(7):916-920. https://doi.org/10.1097/ACM.0000000000001232
3. Roth WD, Yaylacı S, Jaffe K, et al. Do genetic ancestry tests increase racial essentialism? Findings from a randomized controlled trial. PLoS One. 2020;15(1):e0227399. https://doi.org/10.1371/journal.pone.0227399
4. Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14(4):311-346. https://doi.org/10.1177/1099800412455688
5. Fuentes A, Ackermann RR, Athreya S, et al. AAPA Statement on race and racism. Am J Phys Anthropol. 2019;169(3):400-402. https://doi.org/10.1002/ajpa.23882
6. Barbujani G, Magagni A, Minch E, et al. An apportionment of human DNA diversity. Proc Natl Acad Sci U S A. 1997;94(9):4516-4519. https://doi.org/10.1073/pnas.94.9.4516
7. Klinger EV, Carlini SV, Gonzalez I, et al. Accuracy of race, ethnicity, and language preference in an electronic health record. J Gen Intern Med. 2015;30(6):719-723. https://doi.org/10.1007/s11606-014-3102-8
8. Stewart C, Pepper MS. Cystic fibrosis in the African diaspora. Ann Am Thorac Soc. 2017;14(1):1-7. https://doi.org/10.1513/AnnalsATS.201606-481FR
9. Rho J, Ahn C, Gao A, et al. Disparities in mortality of Hispanic patients with cystic fibrosis in the United States. A national and regional cohort study. Am J Respir Crit Care Med. 2018;198(8):1055-1063. https://doi.org/10.1164/rccm.201711-2357OC
10. Power-Hays A, McGann PT. When actions speak louder than words—racism and sickle cell disease. N Engl J Med. 2020;383(20):1902-1903. https://doi.org/10.1056/NEJMp2022125
11. Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874-882. https://doi.org/10.1056/NEJMms2004740
12. Wilson JF, Weale ME, Smith AC, et al. Population genetic structure of variable drug response. Nat Genet. 2001;29(3):265-269. https://doi.org/10.1038/ng761
13. Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348(12):1166-1170. https://doi.org/10.1056/NEJMsb022863
14. National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to Health Equity. National Academies Press; 2017.
15. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
16. Balderston JR, Gertz ZM, Seedat R, et al. Differential documentation of race in the first line of the history of present illness. JAMA Intern Med. 2021;181(3):386-388. https://doi.org/10.1001/jamainternmed.2020.5792
17. Baker DW, Hasnain-Wynia R, Kandula NR, Thompson JA, Brown ER. Attitudes toward health care providers, collecting information about patients’ race, ethnicity, and language. Med Care. 2007;45(11):1034-1042. https://doi.org/10.1097/MLR.0b013e318127148f
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
On teaching rounds, a medical student presents the following case to the attending hospitalist: “Mrs. L is a 54-year-old Black female with chronic kidney disease who was admitted with community-acquired pneumonia. She continues to improve symptomatically on ceftriaxone. Currently, she is afebrile and her vitals are stable. Supplemental oxygen has been weaned to 2 L/min by nasal cannula. Exam reveals improved crackles in the left lower chest without dullness to percussion. Labs are notable for down-trending leukocytosis and a stable serum creatinine of 2.8 mg/dL.” The hospitalist considers how including racial descriptors in clinical presentations may influence the care of the patient.
WHY YOU MIGHT THINK INCLUDING RACE IN THE HISTORY OF PRESENT ILLNESS IS HELPFUL
For decades, medical educators have taught learners to include sociopolitical constructs such as race in the opening sentence of the history of present illness (HPI). This practice presumably stems from the assumption that race accurately reflects biogenetic information about patients and serves as a key attribute in problem representations.1 Proponents of including race in the HPI suggest doing so aids the clinical assessment of patients’ risks for particular diseases and may inform the selection of race-appropriate therapies.2
The construct of race does sometimes correlate with the risk of disease or response to therapies. For example, sickle cell disease (SCD) occurs more commonly among patients who identify as Black rather than White. Specifically, ancestry from African nations such as Nigeria or the Democratic Republic of Congo increases the likelihood of having the disease-associated hemoglobin gene variant HbS.1 Popular genomic ancestry tests often report ancestral groupings that map to racial categories and may reinforce the perception that race has a genetic basis.3
WHY IT IS NOT HELPFUL TO INCLUDE RACE IN THE HPI
Race, a construct of sociopolitical origins, incorrectly conflates skin color with genetic variation. Associations between race and disease have the potential to cause diagnostic and therapeutic errors and inequitable allocation of resources. Increased illness burden in minority populations results primarily from social factors such as environment, access to care, housing instability, food insecurity, and experiences of discrimination, rather than genetic differences. The resulting chronic and recurrent physiologic stress—known as allostatic load—also contributes to the inequitable health outcomes observed in vulnerable populations, including patients who identify as Black.4
Historically, race evolved as a sociopolitical framework stemming from colonialism, discrimination, and exploitation.5 Numerous studies reveal a lack of genetic precision in racial categories. In fact, genetic data compared across major continental groups found greater variation of microsatellite loci and restriction fragment length polymorphisms within racial groups than between them.6 The evidence indicates that racial categories do not reflect homogenous population groups but rather “arbitrary division[s] of continuous variation” that cannot serve as a surrogate to genetic diversity.5 Not only are racial categories genetically inaccurate, but data on race within the electronic health record often differ from patients’ self-description of race, underscoring the problematic nature of even identifying race.7 In one study, up to 41% of patients self-reported identification with at least one other racial or ethnic group than the race or ethnicity documented in their electronic health record.7
Additionally, conflating race with genetic variation can lead to diagnostic errors. As an example, the incidence of cystic fibrosis (CF) varies widely across populations of European ancestry. The primary focus on CF’s occurrence in patients of European descent may divert attention from the identification of mutations causing CF in populations of African descent or the decreased survival observed in the United States among CF patients of Hispanic descent.8,9 Similarly, India represents one of the countries largely affected by SCD, suggesting that a myopic focus on SCD among those identifying as Black can lead to underdiagnosis of SCD among those with Indian ancestry.
Perhaps more insidiously, linking disease to race or other social constructs can result in differential support for affected individuals. SCD offers a striking illustration of this point. Reflecting the legacy of transatlantic slave trading, the majority of people with SCD in the United States are Black and face interpersonal and structural racism within society and healthcare that amplify the effects of this devastating illness.10 Compulsory screening programs for sickle cell trait introduced by many states in the 1970s targeted Black Americans and resulted in stigmatization and the denial of insurance, educational opportunities, and jobs for many identified with sickle cell trait. Federal funding for SCD research remains low, particularly in comparison to the tenfold higher funding for CF, which afflicts fewer, but primarily White, Americans.10
The incorporation of race into risk models and guidelines—alongside biologically relevant variables such as age and comorbid conditions—has received increasing attention for its potential to compound racial disparities in health outcomes. The American Heart Association Heart Failure Risk Score, for instance, may lead to the exclusion of some Black patients from necessary care because “Black” race, for no clear physiologic reason, serves as a protective factor against heart failure mortality.11 Likewise, race adjustments in pulmonary function tests, breast cancer risk models, and estimated glomerular filtration rate calculations, among others, have limited biological basis and the potential to divert care disproportionately from minority populations.11
Researchers have even called into question the application of race to pharmacotherapies. A 2001 investigation on geographic patterns of genetic variation in drug response concluded that common racial and ethnic labels were “insufficient and inaccurate representations” of the individual genetic clusters.12 Further, numerous experts have criticized two landmark studies of vasodilators and angiotensin-converting enzyme inhibitors in Black patients with heart failure for inconsistent results and nonsignificant associations between race and major outcomes, such as the development of heart failure or death.13
Race-based labels can also divert attention from true causes of health inequities. The National Academy of Sciences concluded that social determinants of health and structural racism are the root causes of health inequities, rather than genetics.14 Medical professionals may perpetuate these disparities: Most US physicians demonstrate an unconscious preference—or implicit bias—for White Americans over Black Americans.15 Beyond obscuring the role of social determinants of health and structural racism in health outcomes, race-based labels may exacerbate the ways in which physicians’ implicit biases contribute to racial and ethnic health disparities, primarily affecting Black Americans.2 In a recent study, clinicians documented race in the HPI for 33% of Black patients compared with 16% of White patients, and White clinicians were twice as likely to document race as Black physicians.16 Moreover, training medical students to view race as an independent risk factor of disease without discussing structural inequities can pathologize race and reinforce implicit biases linking race and disease.15
Based on the current evidence, we believe routine use of race-based labels in clinical presentations confuses providers at a minimum and potentially produces far more damage by obscuring or perpetuating the role of racism in health inequities.
WHAT YOU SHOULD DO INSTEAD
Instead of routinely presenting race in the HPI, we recommend including racial or ethnic information in the social history only when the patient reports it as a meaningful identity or when it informs health disparities stemming from structural or interpersonal racism. Clinicians should include physical characteristics pertaining to race, such as skin tone, in the physical exam only if required to describe exam findings accurately. When presenting race, clinicians should explicitly justify its use and take care to avoid obfuscatory, inaccurate, or stigmatizing mention of associations between race and disease. Clinicians should not use race in clinical algorithms. Medical educators should emphasize the role of social determinants of health and structural racism in health outcomes to inform the use of race in medicine, in hopes that doing so will help students minimize implicit biases and learn to mitigate racial inequities in healthcare.2,16 In short, clinicians and medical educators alike should ensure that clinical care and the medical curriculum avoid presenting race as a proxy for pathology.
There is little evidence to guide proper inclusion of race in clinical interviews. In the absence of clear guidance about how to approach patients about race, we suggest not asking about it unless there is a reasonable probability that doing so will improve clinical care. If a clinician decides to ask about race, it is important to provide a rationale—such as explaining that the information can be used to assure high-quality care for all patients—since many patients are uncomfortable with questions about race.17 If clinicians report information about race in the social history, we advise using the patient’s description of race rather than traditional racial categories.
Clinicians who ask their patients about race should approach every patient in a uniform manner to avoid perpetuating biases. We hope future studies will inform equitable, inclusive, and person-centered approaches to discussing race with patients and promote a shared understanding of how racism contributes to illness.
RECOMMENDATIONS
- Avoid using racial descriptors in the HPI.
- Include racial and ethnic information in the social history only when it serves as a meaningful identity or it informs disparities stemming from racism.
- If racial or ethnic information is asked for, explain to patients why and how it will be used.
- Mention physical characteristics such as skin tone, rather than race, in the physical exam if required to describe findings accurately.
- Advocate for the replacement of race or race-adjusted algorithms in patient care.
- Expand the medical curriculum in the social determinants of health and structural racism, and develop systems to avoid the use of stigmatizing, race-based labels.
CONCLUSION
Race, a sociopolitical construct, does not accurately represent genetic variation. The routine use of race in the HPI can perpetuate racial biases and muddle both diagnoses and treatment. Only mention race in the social history if it is meaningful to the patient’s self-identity or explains health disparities arising from racism. All documentation and presentations should avoid the use of stigmatizing, race-based labels.
In the clinical scenario mentioned earlier, the attending hospitalist raises the issue of race-based labels in patient care in a nonjudgmental fashion. To provide illustrative specificity, she notes how the incorporation of race in formulas of glomerular filtration rate can lead to under-referral for renal transplant. The hospitalist then facilitates an open and inclusive discussion with the team regarding the use of race in clinical presentations and its potential impact on health disparities.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason™” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
On teaching rounds, a medical student presents the following case to the attending hospitalist: “Mrs. L is a 54-year-old Black female with chronic kidney disease who was admitted with community-acquired pneumonia. She continues to improve symptomatically on ceftriaxone. Currently, she is afebrile and her vitals are stable. Supplemental oxygen has been weaned to 2 L/min by nasal cannula. Exam reveals improved crackles in the left lower chest without dullness to percussion. Labs are notable for down-trending leukocytosis and a stable serum creatinine of 2.8 mg/dL.” The hospitalist considers how including racial descriptors in clinical presentations may influence the care of the patient.
WHY YOU MIGHT THINK INCLUDING RACE IN THE HISTORY OF PRESENT ILLNESS IS HELPFUL
For decades, medical educators have taught learners to include sociopolitical constructs such as race in the opening sentence of the history of present illness (HPI). This practice presumably stems from the assumption that race accurately reflects biogenetic information about patients and serves as a key attribute in problem representations.1 Proponents of including race in the HPI suggest doing so aids the clinical assessment of patients’ risks for particular diseases and may inform the selection of race-appropriate therapies.2
The construct of race does sometimes correlate with the risk of disease or response to therapies. For example, sickle cell disease (SCD) occurs more commonly among patients who identify as Black rather than White. Specifically, ancestry from African nations such as Nigeria or the Democratic Republic of Congo increases the likelihood of having the disease-associated hemoglobin gene variant HbS.1 Popular genomic ancestry tests often report ancestral groupings that map to racial categories and may reinforce the perception that race has a genetic basis.3
WHY IT IS NOT HELPFUL TO INCLUDE RACE IN THE HPI
Race, a construct of sociopolitical origins, incorrectly conflates skin color with genetic variation. Associations between race and disease have the potential to cause diagnostic and therapeutic errors and inequitable allocation of resources. Increased illness burden in minority populations results primarily from social factors such as environment, access to care, housing instability, food insecurity, and experiences of discrimination, rather than genetic differences. The resulting chronic and recurrent physiologic stress—known as allostatic load—also contributes to the inequitable health outcomes observed in vulnerable populations, including patients who identify as Black.4
Historically, race evolved as a sociopolitical framework stemming from colonialism, discrimination, and exploitation.5 Numerous studies reveal a lack of genetic precision in racial categories. In fact, genetic data compared across major continental groups found greater variation of microsatellite loci and restriction fragment length polymorphisms within racial groups than between them.6 The evidence indicates that racial categories do not reflect homogenous population groups but rather “arbitrary division[s] of continuous variation” that cannot serve as a surrogate to genetic diversity.5 Not only are racial categories genetically inaccurate, but data on race within the electronic health record often differ from patients’ self-description of race, underscoring the problematic nature of even identifying race.7 In one study, up to 41% of patients self-reported identification with at least one other racial or ethnic group than the race or ethnicity documented in their electronic health record.7
Additionally, conflating race with genetic variation can lead to diagnostic errors. As an example, the incidence of cystic fibrosis (CF) varies widely across populations of European ancestry. The primary focus on CF’s occurrence in patients of European descent may divert attention from the identification of mutations causing CF in populations of African descent or the decreased survival observed in the United States among CF patients of Hispanic descent.8,9 Similarly, India represents one of the countries largely affected by SCD, suggesting that a myopic focus on SCD among those identifying as Black can lead to underdiagnosis of SCD among those with Indian ancestry.
Perhaps more insidiously, linking disease to race or other social constructs can result in differential support for affected individuals. SCD offers a striking illustration of this point. Reflecting the legacy of transatlantic slave trading, the majority of people with SCD in the United States are Black and face interpersonal and structural racism within society and healthcare that amplify the effects of this devastating illness.10 Compulsory screening programs for sickle cell trait introduced by many states in the 1970s targeted Black Americans and resulted in stigmatization and the denial of insurance, educational opportunities, and jobs for many identified with sickle cell trait. Federal funding for SCD research remains low, particularly in comparison to the tenfold higher funding for CF, which afflicts fewer, but primarily White, Americans.10
The incorporation of race into risk models and guidelines—alongside biologically relevant variables such as age and comorbid conditions—has received increasing attention for its potential to compound racial disparities in health outcomes. The American Heart Association Heart Failure Risk Score, for instance, may lead to the exclusion of some Black patients from necessary care because “Black” race, for no clear physiologic reason, serves as a protective factor against heart failure mortality.11 Likewise, race adjustments in pulmonary function tests, breast cancer risk models, and estimated glomerular filtration rate calculations, among others, have limited biological basis and the potential to divert care disproportionately from minority populations.11
Researchers have even called into question the application of race to pharmacotherapies. A 2001 investigation on geographic patterns of genetic variation in drug response concluded that common racial and ethnic labels were “insufficient and inaccurate representations” of the individual genetic clusters.12 Further, numerous experts have criticized two landmark studies of vasodilators and angiotensin-converting enzyme inhibitors in Black patients with heart failure for inconsistent results and nonsignificant associations between race and major outcomes, such as the development of heart failure or death.13
Race-based labels can also divert attention from true causes of health inequities. The National Academy of Sciences concluded that social determinants of health and structural racism are the root causes of health inequities, rather than genetics.14 Medical professionals may perpetuate these disparities: Most US physicians demonstrate an unconscious preference—or implicit bias—for White Americans over Black Americans.15 Beyond obscuring the role of social determinants of health and structural racism in health outcomes, race-based labels may exacerbate the ways in which physicians’ implicit biases contribute to racial and ethnic health disparities, primarily affecting Black Americans.2 In a recent study, clinicians documented race in the HPI for 33% of Black patients compared with 16% of White patients, and White clinicians were twice as likely to document race as Black physicians.16 Moreover, training medical students to view race as an independent risk factor of disease without discussing structural inequities can pathologize race and reinforce implicit biases linking race and disease.15
Based on the current evidence, we believe routine use of race-based labels in clinical presentations confuses providers at a minimum and potentially produces far more damage by obscuring or perpetuating the role of racism in health inequities.
WHAT YOU SHOULD DO INSTEAD
Instead of routinely presenting race in the HPI, we recommend including racial or ethnic information in the social history only when the patient reports it as a meaningful identity or when it informs health disparities stemming from structural or interpersonal racism. Clinicians should include physical characteristics pertaining to race, such as skin tone, in the physical exam only if required to describe exam findings accurately. When presenting race, clinicians should explicitly justify its use and take care to avoid obfuscatory, inaccurate, or stigmatizing mention of associations between race and disease. Clinicians should not use race in clinical algorithms. Medical educators should emphasize the role of social determinants of health and structural racism in health outcomes to inform the use of race in medicine, in hopes that doing so will help students minimize implicit biases and learn to mitigate racial inequities in healthcare.2,16 In short, clinicians and medical educators alike should ensure that clinical care and the medical curriculum avoid presenting race as a proxy for pathology.
There is little evidence to guide proper inclusion of race in clinical interviews. In the absence of clear guidance about how to approach patients about race, we suggest not asking about it unless there is a reasonable probability that doing so will improve clinical care. If a clinician decides to ask about race, it is important to provide a rationale—such as explaining that the information can be used to assure high-quality care for all patients—since many patients are uncomfortable with questions about race.17 If clinicians report information about race in the social history, we advise using the patient’s description of race rather than traditional racial categories.
Clinicians who ask their patients about race should approach every patient in a uniform manner to avoid perpetuating biases. We hope future studies will inform equitable, inclusive, and person-centered approaches to discussing race with patients and promote a shared understanding of how racism contributes to illness.
RECOMMENDATIONS
- Avoid using racial descriptors in the HPI.
- Include racial and ethnic information in the social history only when it serves as a meaningful identity or it informs disparities stemming from racism.
- If racial or ethnic information is asked for, explain to patients why and how it will be used.
- Mention physical characteristics such as skin tone, rather than race, in the physical exam if required to describe findings accurately.
- Advocate for the replacement of race or race-adjusted algorithms in patient care.
- Expand the medical curriculum in the social determinants of health and structural racism, and develop systems to avoid the use of stigmatizing, race-based labels.
CONCLUSION
Race, a sociopolitical construct, does not accurately represent genetic variation. The routine use of race in the HPI can perpetuate racial biases and muddle both diagnoses and treatment. Only mention race in the social history if it is meaningful to the patient’s self-identity or explains health disparities arising from racism. All documentation and presentations should avoid the use of stigmatizing, race-based labels.
In the clinical scenario mentioned earlier, the attending hospitalist raises the issue of race-based labels in patient care in a nonjudgmental fashion. To provide illustrative specificity, she notes how the incorporation of race in formulas of glomerular filtration rate can lead to under-referral for renal transplant. The hospitalist then facilitates an open and inclusive discussion with the team regarding the use of race in clinical presentations and its potential impact on health disparities.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason™” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348(12):1170-1175. https://doi.org/10.1056/NEJMsb025007
2. Tsai J, Ucik L, Baldwin N, et al. Race matters? Examining and rethinking race portrayal in preclinical medical education. Acad Med. 2016;91(7):916-920. https://doi.org/10.1097/ACM.0000000000001232
3. Roth WD, Yaylacı S, Jaffe K, et al. Do genetic ancestry tests increase racial essentialism? Findings from a randomized controlled trial. PLoS One. 2020;15(1):e0227399. https://doi.org/10.1371/journal.pone.0227399
4. Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14(4):311-346. https://doi.org/10.1177/1099800412455688
5. Fuentes A, Ackermann RR, Athreya S, et al. AAPA Statement on race and racism. Am J Phys Anthropol. 2019;169(3):400-402. https://doi.org/10.1002/ajpa.23882
6. Barbujani G, Magagni A, Minch E, et al. An apportionment of human DNA diversity. Proc Natl Acad Sci U S A. 1997;94(9):4516-4519. https://doi.org/10.1073/pnas.94.9.4516
7. Klinger EV, Carlini SV, Gonzalez I, et al. Accuracy of race, ethnicity, and language preference in an electronic health record. J Gen Intern Med. 2015;30(6):719-723. https://doi.org/10.1007/s11606-014-3102-8
8. Stewart C, Pepper MS. Cystic fibrosis in the African diaspora. Ann Am Thorac Soc. 2017;14(1):1-7. https://doi.org/10.1513/AnnalsATS.201606-481FR
9. Rho J, Ahn C, Gao A, et al. Disparities in mortality of Hispanic patients with cystic fibrosis in the United States. A national and regional cohort study. Am J Respir Crit Care Med. 2018;198(8):1055-1063. https://doi.org/10.1164/rccm.201711-2357OC
10. Power-Hays A, McGann PT. When actions speak louder than words—racism and sickle cell disease. N Engl J Med. 2020;383(20):1902-1903. https://doi.org/10.1056/NEJMp2022125
11. Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874-882. https://doi.org/10.1056/NEJMms2004740
12. Wilson JF, Weale ME, Smith AC, et al. Population genetic structure of variable drug response. Nat Genet. 2001;29(3):265-269. https://doi.org/10.1038/ng761
13. Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348(12):1166-1170. https://doi.org/10.1056/NEJMsb022863
14. National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to Health Equity. National Academies Press; 2017.
15. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
16. Balderston JR, Gertz ZM, Seedat R, et al. Differential documentation of race in the first line of the history of present illness. JAMA Intern Med. 2021;181(3):386-388. https://doi.org/10.1001/jamainternmed.2020.5792
17. Baker DW, Hasnain-Wynia R, Kandula NR, Thompson JA, Brown ER. Attitudes toward health care providers, collecting information about patients’ race, ethnicity, and language. Med Care. 2007;45(11):1034-1042. https://doi.org/10.1097/MLR.0b013e318127148f
1. Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348(12):1170-1175. https://doi.org/10.1056/NEJMsb025007
2. Tsai J, Ucik L, Baldwin N, et al. Race matters? Examining and rethinking race portrayal in preclinical medical education. Acad Med. 2016;91(7):916-920. https://doi.org/10.1097/ACM.0000000000001232
3. Roth WD, Yaylacı S, Jaffe K, et al. Do genetic ancestry tests increase racial essentialism? Findings from a randomized controlled trial. PLoS One. 2020;15(1):e0227399. https://doi.org/10.1371/journal.pone.0227399
4. Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14(4):311-346. https://doi.org/10.1177/1099800412455688
5. Fuentes A, Ackermann RR, Athreya S, et al. AAPA Statement on race and racism. Am J Phys Anthropol. 2019;169(3):400-402. https://doi.org/10.1002/ajpa.23882
6. Barbujani G, Magagni A, Minch E, et al. An apportionment of human DNA diversity. Proc Natl Acad Sci U S A. 1997;94(9):4516-4519. https://doi.org/10.1073/pnas.94.9.4516
7. Klinger EV, Carlini SV, Gonzalez I, et al. Accuracy of race, ethnicity, and language preference in an electronic health record. J Gen Intern Med. 2015;30(6):719-723. https://doi.org/10.1007/s11606-014-3102-8
8. Stewart C, Pepper MS. Cystic fibrosis in the African diaspora. Ann Am Thorac Soc. 2017;14(1):1-7. https://doi.org/10.1513/AnnalsATS.201606-481FR
9. Rho J, Ahn C, Gao A, et al. Disparities in mortality of Hispanic patients with cystic fibrosis in the United States. A national and regional cohort study. Am J Respir Crit Care Med. 2018;198(8):1055-1063. https://doi.org/10.1164/rccm.201711-2357OC
10. Power-Hays A, McGann PT. When actions speak louder than words—racism and sickle cell disease. N Engl J Med. 2020;383(20):1902-1903. https://doi.org/10.1056/NEJMp2022125
11. Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874-882. https://doi.org/10.1056/NEJMms2004740
12. Wilson JF, Weale ME, Smith AC, et al. Population genetic structure of variable drug response. Nat Genet. 2001;29(3):265-269. https://doi.org/10.1038/ng761
13. Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348(12):1166-1170. https://doi.org/10.1056/NEJMsb022863
14. National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to Health Equity. National Academies Press; 2017.
15. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
16. Balderston JR, Gertz ZM, Seedat R, et al. Differential documentation of race in the first line of the history of present illness. JAMA Intern Med. 2021;181(3):386-388. https://doi.org/10.1001/jamainternmed.2020.5792
17. Baker DW, Hasnain-Wynia R, Kandula NR, Thompson JA, Brown ER. Attitudes toward health care providers, collecting information about patients’ race, ethnicity, and language. Med Care. 2007;45(11):1034-1042. https://doi.org/10.1097/MLR.0b013e318127148f
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Fluid Restriction for the Management of Acute Decompensated Heart Failure in Patients With Reduced Ejection Fraction
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist enters admission orders for an 80-year-old woman with hypertension, coronary artery disease, and heart failure with reduced ejection fraction who presented to the emergency department with weight gain, lower extremity edema, and dyspnea on exertion. She has an elevated jugular venous pressure, crackles on pulmonary exam, and bilateral pitting edema with warm extremities. Labs show a sodium of 140 mmol/L and creatinine of 1.4 mg/dL. After ordering intravenous furosemide for management of acute decompensated heart failure (ADHF), the hospitalist arrives at the nutrition section of the CHF Admission Order Set and reflexively picks an option for a fluid-restricted diet.
BACKGROUND
Patients with ADHF, the leading cause of hospitalization for patients older than 65 years,1 may present with signs and symptoms of volume overload: shortness of breath, lower-extremity swelling, and end-organ dysfunction. Before the 1980s, treatment of ADHF relied on loop diuretics, bedrest, and fluid restriction to minimize congestive symptoms.2 Clinicians based this practice on early theories framing heart failure as primarily an issue of salt and water retention that could be counterbalanced by sodium and fluid restriction.2
Today, hospitalists understand heart failure with reduced ejection fraction (HFrEF) as a heterogenous disease with a shared pathophysiology in which reduced cardiac output, elevated systemic venous pressures, and/or shunting of blood away from the kidneys may all lead to decreased renal perfusion. These phenomena trigger the activation of the renin-angiotensin-aldosterone system (RAAS), leading to sodium and water retention and fluid redistribution.2 As part of the modern day treatment regimen, providers continue to place patients on fluid-restricted diets. Guidelines support this practice.3,4
Since most of the existing literature on the topic of fluid restriction in ADHF relates to HFrEF (left ventricular ejection fraction [LVEF] <40%), as opposed to heart failure with a preserved ejection fraction (HFpEF, LVEF ≥50%), this review will focus on HFrEF patients. Limited existing data support extrapolating these arguments to HFpEF patients as well.5
WHY YOU MIGHT THINK FLUID RESTRICTION IS IMPORTANT IN THE MANAGEMENT OF ADHF IN HFREF PATIENTS
Longstanding conventional wisdom and data extrapolation from the chronic heart failure population has undergirded the practice of fluid restriction for ADHF. Current iterations of the American and European heart failure guidelines recommend fluid restriction of 1.5 to 2.0 L/day in severe ADHF as a management strategy.3,4 The American guidelines recommend considering restricting fluid intake to 2 L/day for most hospitalized ADHF patients without hyponatremia or diuretic resistance. The guidelines base the recommendation on clinical experience and data from a single randomized trial evaluating the effects of sodium restriction on heart failure outcomes in outpatients recently admitted for ADHF.4,6 This trial randomly assigned 232 patients with compensated HFrEF to either a normal or low-sodium diet plus oral furosemide. Researchers instructed both groups to adhere to a 1000 mL/day fluid restriction. The authors found a high incidence of readmissions for worsening congestive heart failure among a cohort of patients (n = 54) with a normal sodium diet who were excluded from randomization due to inability to adhere to the prescribed fluid restriction.6 Notably, this study did not evaluate patients receiving treatment for ADHF and was not designed to investigate the role of fluid restriction for the treatment of ADHF.
A subsequent study by the same investigators looked more deliberately, although not singularly, at outpatient fluid restriction. This study randomly assigned 410 patients with compensated HFrEF into eight groups by fluid intake (1 L vs 2 L), salt intake (80 mmol vs 120 mmol), and furosemide dose (125 mg twice daily vs 250 mg twice daily). At 180 days, the group receiving the fluid-restricted diet with higher sodium intake and higher diuretic dose had the lowest risk of hospital readmission.7Results from these studies of the chronic, compensated heart failure population, in conjunction with longstanding conventional wisdom, have influenced the management of patients hospitalized with ADHF.
WHY FLUID RESTRICTION IN THE MANAGEMENT OF ADHF IN HFREF PATIENTS MIGHT NOT BE HELPFUL
From a pathophysiologic perspective, fluid restriction in ADHF may counterproductively lead to RAAS activation.8 Congestion develops when arterial underfilling leads to RAAS activation, triggering sodium and water retention.2 Furthermore, RAAS activation, as measured by plasma levels of renin, angiotensin II, and aldosterone, correlates with prognosis and mortality in chronic HFrEF.9 Analyses from one of the largest databases of biomarkers from ADHF suggest that RAAS is further upregulated during decongestive therapy.10 While researchers have not studied the effects of fluid restriction on RAAS activation in ADHF patients, extrapolating from these data one may question whether fluid restriction in ADHF patients may further drive RAAS activation. Further activation may contribute to adverse incident outcomes such as worsening renal function.
The most relevant and compelling evidence against fluid restriction to date comes from Travers et al,11 who conducted the first randomized controlled trial examining fluid restriction in ADHF patients. Their small study compared restricted (1 L fluid restriction) vs liberal (free fluid) intake in hospitalized patients with ADHF and demonstrated no difference in duration or daily dose of intravenous diuretics, time to symptomatic improvement, total daily fluid output, or average hospitalization weight loss between the two arms. Furthermore, researchers withdrew more patients in the fluid-restricted arm due to a sustained rise in serum creatinine, suggesting potential harm of this intervention.11 The sample size (N = 67) and fluid-intake difference of only 400 mL between the two groups limited the study results.
In a subsequent randomized controlled trial, Aliti et al12 examined the clinical outcomes of even more aggressive fluid restriction (800 mL/day) and sodium restriction (800 mg/day) versus liberal intake (at least 2.5 L fluid/day and approximately 3-5 g sodium/day) in hospitalized patients with ADHF (N = 75). While this study evaluated both fluid and sodium restriction, it produced relevant results. The study demonstrated no significant difference in weight loss, use of diuretics, or rehospitalization between the study arms.12 At 30-day follow-up, researchers found that patients in the intervention group had more congestion and an increased likelihood of having a B-type natriuretic peptide (BNP) level greater than 700 pg/mL. In the subset of all patients with an elevated BNP level greater than 700 pg/mL at the end of the study, patients in the intervention group had a significantly higher rate of readmission (7 out of 22) compared with controls (1 of 20). Moreover, the fluid-restricted group had 50% higher perceived thirst values compared to the control group.12 The sensation of thirst not only reduces quality of life, but, given that angiotensin II stimulates thirst, it may reflect RAAS activation.13 For these reasons, clinicians should consider this side effect seriously, especially when the literature lacks evidence of the benefits from fluid restriction.
WHEN FLUID RESTRICTION IS HELPFUL IN THE MANAGEMENT OF DECOMPENSATED HEART FAILURE IN HFREF PATIENTS
Fluid-restrict patients who have chronic hyponatremia (Na <135 mmol/L) due to end-stage HFrEF in select circumstances. Hyponatremia develops in heart failure primarily because of the body’s inability to excrete free water due to non-osmotic arginine vasopressin secretion.4 Other processes contribute to hyponatremia, including increased free water intake due to angiotensin II stimulating thirst and decreased glomerular filtration rate limiting the kidney’s ability to excrete free water. Since hyponatremia in heart failure primarily occurs due to derangements of free water regulation, limiting free water intake may help; the American College of Cardiology/American Heart Association and European heart failure guidelines explicitly recommend this strategy for patients with stage D heart failure.3,4 However, no available randomized data support this practice, and observational data suggest that fluid restriction has limited impact on hyponatremia in ADHF.14 Guidelines also suggest employing fluid restriction in patients with diuretic resistance as an adjunctive therapy.
Twenty-nine percent of patients with ADHF have comorbid chronic kidney disease (CKD).15 Providers often prescribe patients with advanced CKD salt- and fluid-restrictive diets due to more limited abilities in sodium and free water excretion. However, no studies have examined the effects of fluid restriction alone without salt restriction in the CKD/ADHF population.
WHAT YOU SHOULD DO INSTEAD
In the present day of evidence-based pharmacologic therapies, research indicates that fluid-restriction does not help and potentially may harm. Instead, treat hospitalized HFrEF patients with ADHF with modern, evidence-based pharmacologic therapies and allow the patients to drink when thirsty.
RECOMMENDATIONS
- Treat patients with ADHF and reduced ejection fraction with evidence-based neurohormonal blockade and initiate loop diuretics to alleviate congestion.
- Allow patients with ADHF and reduced ejection fraction to drink when thirsty in the absence of hyponatremia.
- Consider initiating fluid restriction in patients with ADHF and concurrent hyponatremia and/or diuretic resistance. There is little evidence to guide setting specific limits on fluid intake.
CONCLUSION
The hospitalist starts the patient admitted for ADHF on an intravenous loop diuretic, continues her home beta blocker and angiotensin-converting enzyme inhibitor, and does not impose any fluid restriction. Her symptoms of congestion resolve, and she is discharged.
Hospitalists often treat patients with ADHF and reduced ejection fraction with fluid restriction. However, limited evidence supports this practice as part of the management of ADHF. Fluid restriction may have unintended adverse effects of increasing thirst and worsening renal function and quality of life.
What do you do? Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-322. https://doi.org/10.1161/cir.0000000000000152
2. Arrigo M, Parissis JT, Akiyama E, Mebazaa A. Understanding acute heart failure: pathophysiology and diagnosis. Eur Heart J Suppl. 2016;18(Suppl G):G11-G18. https://doi.org/10.1093/eurheartj/suw044
3. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18(8):891-975. https://doi.org/10.1002/ejhf.592
4. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019
5. Machado d’Almeida KS, Rabelo-Silva ER, Souza GC, et al. Aggressive fluid and sodium restriction in decompensated heart failure with preserved ejection fraction: results from a randomized clinical trial. Nutrition. 2018;54:111-117. https://doi.org/10.1016/j.nut.2018.02.007
6. Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond). 2008;114(3):221-230. https://doi.org/10.1042/cs20070193
7. Paterna S, Parrinello G, Cannizzaro S, et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103(1):93-102. https://doi.org/10.1016/j.amjcard.2008.08.043
8. Shore AC, Markandu ND, Sagnella GA, et al. Endocrine and renal response to water loading and water restriction in normal man. Clin Sci (Lond). 1988;75(2):171-177. https://doi.org/10.1042/cs0750171
9. Oliveros E, Oni ET, Shahzad A, et al. Benefits and risks of continuing angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and mineralocorticoid receptor antagonists during hospitalizations for acute heart failure. Cardiorenal Med. 2020;10(2):69-84. https://doi.org/10.1159/000504167
10. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail. 2015;3(2):97-107. https://doi.org/10.1016/j.jchf.2014.09.003
11. Travers B, O’Loughlin C, Murphy NF, et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13(2):128-132. https://doi.org/10.1016/j.cardfail.2006.10.012
12. Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med. 2013;173(12):1058-1064. https://doi.org/10.1001/jamainternmed.2013.552
13. Jao GT, Chiong JR. Hyponatremia in acute decompensated heart failure: mechanisms, prognosis, and treatment options. Clin Cardiol. 2010;33(11):666-671. https://doi.org/10.1002/clc.20822
14. Nagler EV, Haller MC, Van Biesen W, Vanholder R, Craig JC, Webster AC. Interventions for chronic non-hypovolaemic hypotonic hyponatraemia. Cochrane Database Syst Rev. 2018;28(6):CD010965. https://doi.org/10.1002/14651858.cd010965.pub2
15. Fonarow GC; ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(Suppl 7):S21-S30.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist enters admission orders for an 80-year-old woman with hypertension, coronary artery disease, and heart failure with reduced ejection fraction who presented to the emergency department with weight gain, lower extremity edema, and dyspnea on exertion. She has an elevated jugular venous pressure, crackles on pulmonary exam, and bilateral pitting edema with warm extremities. Labs show a sodium of 140 mmol/L and creatinine of 1.4 mg/dL. After ordering intravenous furosemide for management of acute decompensated heart failure (ADHF), the hospitalist arrives at the nutrition section of the CHF Admission Order Set and reflexively picks an option for a fluid-restricted diet.
BACKGROUND
Patients with ADHF, the leading cause of hospitalization for patients older than 65 years,1 may present with signs and symptoms of volume overload: shortness of breath, lower-extremity swelling, and end-organ dysfunction. Before the 1980s, treatment of ADHF relied on loop diuretics, bedrest, and fluid restriction to minimize congestive symptoms.2 Clinicians based this practice on early theories framing heart failure as primarily an issue of salt and water retention that could be counterbalanced by sodium and fluid restriction.2
Today, hospitalists understand heart failure with reduced ejection fraction (HFrEF) as a heterogenous disease with a shared pathophysiology in which reduced cardiac output, elevated systemic venous pressures, and/or shunting of blood away from the kidneys may all lead to decreased renal perfusion. These phenomena trigger the activation of the renin-angiotensin-aldosterone system (RAAS), leading to sodium and water retention and fluid redistribution.2 As part of the modern day treatment regimen, providers continue to place patients on fluid-restricted diets. Guidelines support this practice.3,4
Since most of the existing literature on the topic of fluid restriction in ADHF relates to HFrEF (left ventricular ejection fraction [LVEF] <40%), as opposed to heart failure with a preserved ejection fraction (HFpEF, LVEF ≥50%), this review will focus on HFrEF patients. Limited existing data support extrapolating these arguments to HFpEF patients as well.5
WHY YOU MIGHT THINK FLUID RESTRICTION IS IMPORTANT IN THE MANAGEMENT OF ADHF IN HFREF PATIENTS
Longstanding conventional wisdom and data extrapolation from the chronic heart failure population has undergirded the practice of fluid restriction for ADHF. Current iterations of the American and European heart failure guidelines recommend fluid restriction of 1.5 to 2.0 L/day in severe ADHF as a management strategy.3,4 The American guidelines recommend considering restricting fluid intake to 2 L/day for most hospitalized ADHF patients without hyponatremia or diuretic resistance. The guidelines base the recommendation on clinical experience and data from a single randomized trial evaluating the effects of sodium restriction on heart failure outcomes in outpatients recently admitted for ADHF.4,6 This trial randomly assigned 232 patients with compensated HFrEF to either a normal or low-sodium diet plus oral furosemide. Researchers instructed both groups to adhere to a 1000 mL/day fluid restriction. The authors found a high incidence of readmissions for worsening congestive heart failure among a cohort of patients (n = 54) with a normal sodium diet who were excluded from randomization due to inability to adhere to the prescribed fluid restriction.6 Notably, this study did not evaluate patients receiving treatment for ADHF and was not designed to investigate the role of fluid restriction for the treatment of ADHF.
A subsequent study by the same investigators looked more deliberately, although not singularly, at outpatient fluid restriction. This study randomly assigned 410 patients with compensated HFrEF into eight groups by fluid intake (1 L vs 2 L), salt intake (80 mmol vs 120 mmol), and furosemide dose (125 mg twice daily vs 250 mg twice daily). At 180 days, the group receiving the fluid-restricted diet with higher sodium intake and higher diuretic dose had the lowest risk of hospital readmission.7Results from these studies of the chronic, compensated heart failure population, in conjunction with longstanding conventional wisdom, have influenced the management of patients hospitalized with ADHF.
WHY FLUID RESTRICTION IN THE MANAGEMENT OF ADHF IN HFREF PATIENTS MIGHT NOT BE HELPFUL
From a pathophysiologic perspective, fluid restriction in ADHF may counterproductively lead to RAAS activation.8 Congestion develops when arterial underfilling leads to RAAS activation, triggering sodium and water retention.2 Furthermore, RAAS activation, as measured by plasma levels of renin, angiotensin II, and aldosterone, correlates with prognosis and mortality in chronic HFrEF.9 Analyses from one of the largest databases of biomarkers from ADHF suggest that RAAS is further upregulated during decongestive therapy.10 While researchers have not studied the effects of fluid restriction on RAAS activation in ADHF patients, extrapolating from these data one may question whether fluid restriction in ADHF patients may further drive RAAS activation. Further activation may contribute to adverse incident outcomes such as worsening renal function.
The most relevant and compelling evidence against fluid restriction to date comes from Travers et al,11 who conducted the first randomized controlled trial examining fluid restriction in ADHF patients. Their small study compared restricted (1 L fluid restriction) vs liberal (free fluid) intake in hospitalized patients with ADHF and demonstrated no difference in duration or daily dose of intravenous diuretics, time to symptomatic improvement, total daily fluid output, or average hospitalization weight loss between the two arms. Furthermore, researchers withdrew more patients in the fluid-restricted arm due to a sustained rise in serum creatinine, suggesting potential harm of this intervention.11 The sample size (N = 67) and fluid-intake difference of only 400 mL between the two groups limited the study results.
In a subsequent randomized controlled trial, Aliti et al12 examined the clinical outcomes of even more aggressive fluid restriction (800 mL/day) and sodium restriction (800 mg/day) versus liberal intake (at least 2.5 L fluid/day and approximately 3-5 g sodium/day) in hospitalized patients with ADHF (N = 75). While this study evaluated both fluid and sodium restriction, it produced relevant results. The study demonstrated no significant difference in weight loss, use of diuretics, or rehospitalization between the study arms.12 At 30-day follow-up, researchers found that patients in the intervention group had more congestion and an increased likelihood of having a B-type natriuretic peptide (BNP) level greater than 700 pg/mL. In the subset of all patients with an elevated BNP level greater than 700 pg/mL at the end of the study, patients in the intervention group had a significantly higher rate of readmission (7 out of 22) compared with controls (1 of 20). Moreover, the fluid-restricted group had 50% higher perceived thirst values compared to the control group.12 The sensation of thirst not only reduces quality of life, but, given that angiotensin II stimulates thirst, it may reflect RAAS activation.13 For these reasons, clinicians should consider this side effect seriously, especially when the literature lacks evidence of the benefits from fluid restriction.
WHEN FLUID RESTRICTION IS HELPFUL IN THE MANAGEMENT OF DECOMPENSATED HEART FAILURE IN HFREF PATIENTS
Fluid-restrict patients who have chronic hyponatremia (Na <135 mmol/L) due to end-stage HFrEF in select circumstances. Hyponatremia develops in heart failure primarily because of the body’s inability to excrete free water due to non-osmotic arginine vasopressin secretion.4 Other processes contribute to hyponatremia, including increased free water intake due to angiotensin II stimulating thirst and decreased glomerular filtration rate limiting the kidney’s ability to excrete free water. Since hyponatremia in heart failure primarily occurs due to derangements of free water regulation, limiting free water intake may help; the American College of Cardiology/American Heart Association and European heart failure guidelines explicitly recommend this strategy for patients with stage D heart failure.3,4 However, no available randomized data support this practice, and observational data suggest that fluid restriction has limited impact on hyponatremia in ADHF.14 Guidelines also suggest employing fluid restriction in patients with diuretic resistance as an adjunctive therapy.
Twenty-nine percent of patients with ADHF have comorbid chronic kidney disease (CKD).15 Providers often prescribe patients with advanced CKD salt- and fluid-restrictive diets due to more limited abilities in sodium and free water excretion. However, no studies have examined the effects of fluid restriction alone without salt restriction in the CKD/ADHF population.
WHAT YOU SHOULD DO INSTEAD
In the present day of evidence-based pharmacologic therapies, research indicates that fluid-restriction does not help and potentially may harm. Instead, treat hospitalized HFrEF patients with ADHF with modern, evidence-based pharmacologic therapies and allow the patients to drink when thirsty.
RECOMMENDATIONS
- Treat patients with ADHF and reduced ejection fraction with evidence-based neurohormonal blockade and initiate loop diuretics to alleviate congestion.
- Allow patients with ADHF and reduced ejection fraction to drink when thirsty in the absence of hyponatremia.
- Consider initiating fluid restriction in patients with ADHF and concurrent hyponatremia and/or diuretic resistance. There is little evidence to guide setting specific limits on fluid intake.
CONCLUSION
The hospitalist starts the patient admitted for ADHF on an intravenous loop diuretic, continues her home beta blocker and angiotensin-converting enzyme inhibitor, and does not impose any fluid restriction. Her symptoms of congestion resolve, and she is discharged.
Hospitalists often treat patients with ADHF and reduced ejection fraction with fluid restriction. However, limited evidence supports this practice as part of the management of ADHF. Fluid restriction may have unintended adverse effects of increasing thirst and worsening renal function and quality of life.
What do you do? Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist enters admission orders for an 80-year-old woman with hypertension, coronary artery disease, and heart failure with reduced ejection fraction who presented to the emergency department with weight gain, lower extremity edema, and dyspnea on exertion. She has an elevated jugular venous pressure, crackles on pulmonary exam, and bilateral pitting edema with warm extremities. Labs show a sodium of 140 mmol/L and creatinine of 1.4 mg/dL. After ordering intravenous furosemide for management of acute decompensated heart failure (ADHF), the hospitalist arrives at the nutrition section of the CHF Admission Order Set and reflexively picks an option for a fluid-restricted diet.
BACKGROUND
Patients with ADHF, the leading cause of hospitalization for patients older than 65 years,1 may present with signs and symptoms of volume overload: shortness of breath, lower-extremity swelling, and end-organ dysfunction. Before the 1980s, treatment of ADHF relied on loop diuretics, bedrest, and fluid restriction to minimize congestive symptoms.2 Clinicians based this practice on early theories framing heart failure as primarily an issue of salt and water retention that could be counterbalanced by sodium and fluid restriction.2
Today, hospitalists understand heart failure with reduced ejection fraction (HFrEF) as a heterogenous disease with a shared pathophysiology in which reduced cardiac output, elevated systemic venous pressures, and/or shunting of blood away from the kidneys may all lead to decreased renal perfusion. These phenomena trigger the activation of the renin-angiotensin-aldosterone system (RAAS), leading to sodium and water retention and fluid redistribution.2 As part of the modern day treatment regimen, providers continue to place patients on fluid-restricted diets. Guidelines support this practice.3,4
Since most of the existing literature on the topic of fluid restriction in ADHF relates to HFrEF (left ventricular ejection fraction [LVEF] <40%), as opposed to heart failure with a preserved ejection fraction (HFpEF, LVEF ≥50%), this review will focus on HFrEF patients. Limited existing data support extrapolating these arguments to HFpEF patients as well.5
WHY YOU MIGHT THINK FLUID RESTRICTION IS IMPORTANT IN THE MANAGEMENT OF ADHF IN HFREF PATIENTS
Longstanding conventional wisdom and data extrapolation from the chronic heart failure population has undergirded the practice of fluid restriction for ADHF. Current iterations of the American and European heart failure guidelines recommend fluid restriction of 1.5 to 2.0 L/day in severe ADHF as a management strategy.3,4 The American guidelines recommend considering restricting fluid intake to 2 L/day for most hospitalized ADHF patients without hyponatremia or diuretic resistance. The guidelines base the recommendation on clinical experience and data from a single randomized trial evaluating the effects of sodium restriction on heart failure outcomes in outpatients recently admitted for ADHF.4,6 This trial randomly assigned 232 patients with compensated HFrEF to either a normal or low-sodium diet plus oral furosemide. Researchers instructed both groups to adhere to a 1000 mL/day fluid restriction. The authors found a high incidence of readmissions for worsening congestive heart failure among a cohort of patients (n = 54) with a normal sodium diet who were excluded from randomization due to inability to adhere to the prescribed fluid restriction.6 Notably, this study did not evaluate patients receiving treatment for ADHF and was not designed to investigate the role of fluid restriction for the treatment of ADHF.
A subsequent study by the same investigators looked more deliberately, although not singularly, at outpatient fluid restriction. This study randomly assigned 410 patients with compensated HFrEF into eight groups by fluid intake (1 L vs 2 L), salt intake (80 mmol vs 120 mmol), and furosemide dose (125 mg twice daily vs 250 mg twice daily). At 180 days, the group receiving the fluid-restricted diet with higher sodium intake and higher diuretic dose had the lowest risk of hospital readmission.7Results from these studies of the chronic, compensated heart failure population, in conjunction with longstanding conventional wisdom, have influenced the management of patients hospitalized with ADHF.
WHY FLUID RESTRICTION IN THE MANAGEMENT OF ADHF IN HFREF PATIENTS MIGHT NOT BE HELPFUL
From a pathophysiologic perspective, fluid restriction in ADHF may counterproductively lead to RAAS activation.8 Congestion develops when arterial underfilling leads to RAAS activation, triggering sodium and water retention.2 Furthermore, RAAS activation, as measured by plasma levels of renin, angiotensin II, and aldosterone, correlates with prognosis and mortality in chronic HFrEF.9 Analyses from one of the largest databases of biomarkers from ADHF suggest that RAAS is further upregulated during decongestive therapy.10 While researchers have not studied the effects of fluid restriction on RAAS activation in ADHF patients, extrapolating from these data one may question whether fluid restriction in ADHF patients may further drive RAAS activation. Further activation may contribute to adverse incident outcomes such as worsening renal function.
The most relevant and compelling evidence against fluid restriction to date comes from Travers et al,11 who conducted the first randomized controlled trial examining fluid restriction in ADHF patients. Their small study compared restricted (1 L fluid restriction) vs liberal (free fluid) intake in hospitalized patients with ADHF and demonstrated no difference in duration or daily dose of intravenous diuretics, time to symptomatic improvement, total daily fluid output, or average hospitalization weight loss between the two arms. Furthermore, researchers withdrew more patients in the fluid-restricted arm due to a sustained rise in serum creatinine, suggesting potential harm of this intervention.11 The sample size (N = 67) and fluid-intake difference of only 400 mL between the two groups limited the study results.
In a subsequent randomized controlled trial, Aliti et al12 examined the clinical outcomes of even more aggressive fluid restriction (800 mL/day) and sodium restriction (800 mg/day) versus liberal intake (at least 2.5 L fluid/day and approximately 3-5 g sodium/day) in hospitalized patients with ADHF (N = 75). While this study evaluated both fluid and sodium restriction, it produced relevant results. The study demonstrated no significant difference in weight loss, use of diuretics, or rehospitalization between the study arms.12 At 30-day follow-up, researchers found that patients in the intervention group had more congestion and an increased likelihood of having a B-type natriuretic peptide (BNP) level greater than 700 pg/mL. In the subset of all patients with an elevated BNP level greater than 700 pg/mL at the end of the study, patients in the intervention group had a significantly higher rate of readmission (7 out of 22) compared with controls (1 of 20). Moreover, the fluid-restricted group had 50% higher perceived thirst values compared to the control group.12 The sensation of thirst not only reduces quality of life, but, given that angiotensin II stimulates thirst, it may reflect RAAS activation.13 For these reasons, clinicians should consider this side effect seriously, especially when the literature lacks evidence of the benefits from fluid restriction.
WHEN FLUID RESTRICTION IS HELPFUL IN THE MANAGEMENT OF DECOMPENSATED HEART FAILURE IN HFREF PATIENTS
Fluid-restrict patients who have chronic hyponatremia (Na <135 mmol/L) due to end-stage HFrEF in select circumstances. Hyponatremia develops in heart failure primarily because of the body’s inability to excrete free water due to non-osmotic arginine vasopressin secretion.4 Other processes contribute to hyponatremia, including increased free water intake due to angiotensin II stimulating thirst and decreased glomerular filtration rate limiting the kidney’s ability to excrete free water. Since hyponatremia in heart failure primarily occurs due to derangements of free water regulation, limiting free water intake may help; the American College of Cardiology/American Heart Association and European heart failure guidelines explicitly recommend this strategy for patients with stage D heart failure.3,4 However, no available randomized data support this practice, and observational data suggest that fluid restriction has limited impact on hyponatremia in ADHF.14 Guidelines also suggest employing fluid restriction in patients with diuretic resistance as an adjunctive therapy.
Twenty-nine percent of patients with ADHF have comorbid chronic kidney disease (CKD).15 Providers often prescribe patients with advanced CKD salt- and fluid-restrictive diets due to more limited abilities in sodium and free water excretion. However, no studies have examined the effects of fluid restriction alone without salt restriction in the CKD/ADHF population.
WHAT YOU SHOULD DO INSTEAD
In the present day of evidence-based pharmacologic therapies, research indicates that fluid-restriction does not help and potentially may harm. Instead, treat hospitalized HFrEF patients with ADHF with modern, evidence-based pharmacologic therapies and allow the patients to drink when thirsty.
RECOMMENDATIONS
- Treat patients with ADHF and reduced ejection fraction with evidence-based neurohormonal blockade and initiate loop diuretics to alleviate congestion.
- Allow patients with ADHF and reduced ejection fraction to drink when thirsty in the absence of hyponatremia.
- Consider initiating fluid restriction in patients with ADHF and concurrent hyponatremia and/or diuretic resistance. There is little evidence to guide setting specific limits on fluid intake.
CONCLUSION
The hospitalist starts the patient admitted for ADHF on an intravenous loop diuretic, continues her home beta blocker and angiotensin-converting enzyme inhibitor, and does not impose any fluid restriction. Her symptoms of congestion resolve, and she is discharged.
Hospitalists often treat patients with ADHF and reduced ejection fraction with fluid restriction. However, limited evidence supports this practice as part of the management of ADHF. Fluid restriction may have unintended adverse effects of increasing thirst and worsening renal function and quality of life.
What do you do? Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-322. https://doi.org/10.1161/cir.0000000000000152
2. Arrigo M, Parissis JT, Akiyama E, Mebazaa A. Understanding acute heart failure: pathophysiology and diagnosis. Eur Heart J Suppl. 2016;18(Suppl G):G11-G18. https://doi.org/10.1093/eurheartj/suw044
3. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18(8):891-975. https://doi.org/10.1002/ejhf.592
4. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019
5. Machado d’Almeida KS, Rabelo-Silva ER, Souza GC, et al. Aggressive fluid and sodium restriction in decompensated heart failure with preserved ejection fraction: results from a randomized clinical trial. Nutrition. 2018;54:111-117. https://doi.org/10.1016/j.nut.2018.02.007
6. Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond). 2008;114(3):221-230. https://doi.org/10.1042/cs20070193
7. Paterna S, Parrinello G, Cannizzaro S, et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103(1):93-102. https://doi.org/10.1016/j.amjcard.2008.08.043
8. Shore AC, Markandu ND, Sagnella GA, et al. Endocrine and renal response to water loading and water restriction in normal man. Clin Sci (Lond). 1988;75(2):171-177. https://doi.org/10.1042/cs0750171
9. Oliveros E, Oni ET, Shahzad A, et al. Benefits and risks of continuing angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and mineralocorticoid receptor antagonists during hospitalizations for acute heart failure. Cardiorenal Med. 2020;10(2):69-84. https://doi.org/10.1159/000504167
10. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail. 2015;3(2):97-107. https://doi.org/10.1016/j.jchf.2014.09.003
11. Travers B, O’Loughlin C, Murphy NF, et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13(2):128-132. https://doi.org/10.1016/j.cardfail.2006.10.012
12. Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med. 2013;173(12):1058-1064. https://doi.org/10.1001/jamainternmed.2013.552
13. Jao GT, Chiong JR. Hyponatremia in acute decompensated heart failure: mechanisms, prognosis, and treatment options. Clin Cardiol. 2010;33(11):666-671. https://doi.org/10.1002/clc.20822
14. Nagler EV, Haller MC, Van Biesen W, Vanholder R, Craig JC, Webster AC. Interventions for chronic non-hypovolaemic hypotonic hyponatraemia. Cochrane Database Syst Rev. 2018;28(6):CD010965. https://doi.org/10.1002/14651858.cd010965.pub2
15. Fonarow GC; ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(Suppl 7):S21-S30.
1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-322. https://doi.org/10.1161/cir.0000000000000152
2. Arrigo M, Parissis JT, Akiyama E, Mebazaa A. Understanding acute heart failure: pathophysiology and diagnosis. Eur Heart J Suppl. 2016;18(Suppl G):G11-G18. https://doi.org/10.1093/eurheartj/suw044
3. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18(8):891-975. https://doi.org/10.1002/ejhf.592
4. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019
5. Machado d’Almeida KS, Rabelo-Silva ER, Souza GC, et al. Aggressive fluid and sodium restriction in decompensated heart failure with preserved ejection fraction: results from a randomized clinical trial. Nutrition. 2018;54:111-117. https://doi.org/10.1016/j.nut.2018.02.007
6. Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond). 2008;114(3):221-230. https://doi.org/10.1042/cs20070193
7. Paterna S, Parrinello G, Cannizzaro S, et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103(1):93-102. https://doi.org/10.1016/j.amjcard.2008.08.043
8. Shore AC, Markandu ND, Sagnella GA, et al. Endocrine and renal response to water loading and water restriction in normal man. Clin Sci (Lond). 1988;75(2):171-177. https://doi.org/10.1042/cs0750171
9. Oliveros E, Oni ET, Shahzad A, et al. Benefits and risks of continuing angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and mineralocorticoid receptor antagonists during hospitalizations for acute heart failure. Cardiorenal Med. 2020;10(2):69-84. https://doi.org/10.1159/000504167
10. Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail. 2015;3(2):97-107. https://doi.org/10.1016/j.jchf.2014.09.003
11. Travers B, O’Loughlin C, Murphy NF, et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13(2):128-132. https://doi.org/10.1016/j.cardfail.2006.10.012
12. Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med. 2013;173(12):1058-1064. https://doi.org/10.1001/jamainternmed.2013.552
13. Jao GT, Chiong JR. Hyponatremia in acute decompensated heart failure: mechanisms, prognosis, and treatment options. Clin Cardiol. 2010;33(11):666-671. https://doi.org/10.1002/clc.20822
14. Nagler EV, Haller MC, Van Biesen W, Vanholder R, Craig JC, Webster AC. Interventions for chronic non-hypovolaemic hypotonic hyponatraemia. Cochrane Database Syst Rev. 2018;28(6):CD010965. https://doi.org/10.1002/14651858.cd010965.pub2
15. Fonarow GC; ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(Suppl 7):S21-S30.
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Tumor Markers CA125, CA19-9, and CEA in the Initial Diagnosis of Malignancy
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
© 2021 Society of Hospital Medicine