User login
Children and COVID: New cases increase for third straight week
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.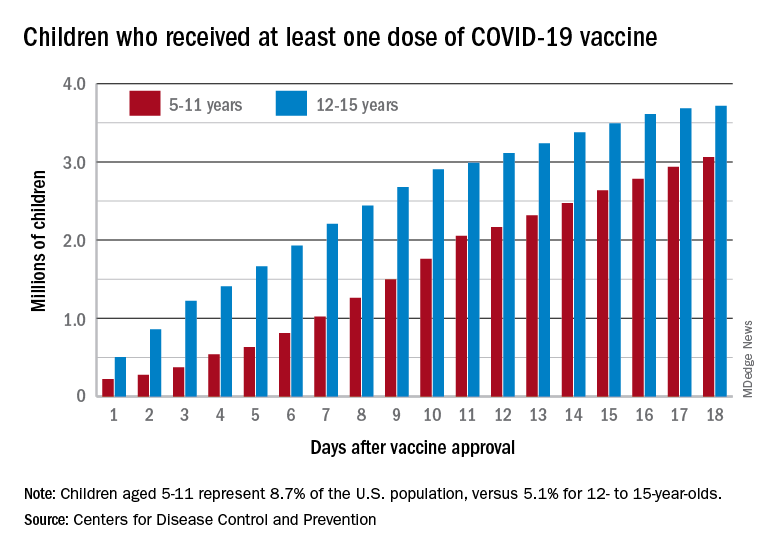
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
Short-acting opioids needed for withdrawal in U.S. hospitals, say experts
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
‘Misleading’ results in colchicine COVID-19 trials meta-analysis
A new meta-analysis appears to show that colchicine has no benefit as a treatment for COVID-19, but its inclusion of trials studying differing patient populations and testing different outcomes led to “misleading” results, says a researcher involved in one of the trials.
The meta-analysis, which includes data from the recent Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, was published Nov. 22 in RMD Open.
Kedar Gautambhai Mehta, MBBS, MD, of the GMERS Medical College Gotri in Vadodara, India, and colleagues included outcomes from six studies of 16,148 patients with COVID-19 who received colchicine or supportive care. They evaluated the efficacy outcomes of mortality, need for ventilation, intensive care unit admission, and length of stay in hospital, as well as safety outcomes of adverse events, serious adverse events, and diarrhea.
The studies in the meta-analysis included a randomized, controlled trial (RCT) of 105 patients hospitalized with COVID-19 in Greece, the international, open-label RECOVERY RCT of 11,340 patients hospitalized with COVID-19, an RCT of 72 hospitalized patients with moderate or severe COVID-19 in Brazil, an RCT of 100 patients hospitalized with COVID-19 in Iran, the international COLCORONA trial of 4,488 patients with COVID-19 who were treated with colchicine or placebo on an outpatient basis, and the randomized COLORIT trial of 43 patients hospitalized with COVID-19 in Russia.
Studies “asked very different questions” about colchicine
Commenting on the meta-analysis, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology with New York University, said the authors combined studies “that are not comparable and that asked very different questions.” Two of the studies in the meta-analysis are very large, and four are very small, which skews the results, he explained.
“The larger studies therefore drive the outcome, and while the small studies are potentially insight providing, the large studies are the only ones worth giving our attention to in the context of the meta-analysis,” he said. The two largest studies – RECOVERY and COLCORONA – taken together show no benefit for colchicine as a treatment, even though the former demonstrated no benefit and the latter did show a benefit, explained Dr. Pillinger, a co–principal investigator for the COLCORONA trial in the United States.
The studies were designed differently and should not have been included in the same analysis, Dr. Pillinger argued. In the case of COLCORONA, early treatment with colchicine was the intervention, whereas RECOVERY focused on hospitalized patients.
“In designing [COLCORONA], the author group (of whom I was a member) expressly rejected the idea that colchicine might be useful for the sicker hospitalized patients, based on the long experience with colchicine of some of us as rheumatologists,” Dr. Pillinger said.
“In short, COLCORONA proved a benefit of colchicine in outpatient COVID-19, and its authors presumed there would be no inpatient benefit; RECOVERY went ahead and proved a lack of inpatient benefit, at least when high-dose steroids were also given,” he said. “While there is no conflict between these results, the combination of the two studies in this meta-analysis suggests there might be no benefit for colchicine overall, which is misleading and can lead physicians to reject the potential of outpatient colchicine, even for future studies.”
Dr. Pillinger said he still believes colchicine has potential value as a COVID-19 treatment option for patients with mild disease, “especially for low–vaccine rate, resource-starved countries.
“It would be unfortunate if meta-analyses such as this one would put a stop to colchicine’s use, or at least its further investigation,” he said.
Study details
The authors of the study assessed heterogeneity of the trials’ data across the outcomes using an I2 test. They evaluated the quality of the evidence for the outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).
The results of their meta-analysis showed that colchicine offered no significant improvement in mortality in six studies (risk difference, –0.0; 95% confidence interval, –0.01 to 0.01; I2 = 15%). It showed no benefit with respect to requiring ventilatory support in five studies of 15,519 patients (risk ratio, 0.67; 95% CI, 0.38-1.21; I2 = 47%); being admitted to the ICU in three studies with 220 patients (RR, 0.49; 95% CI, 0.19-1.25; I2 = 34%); and length of stay while in the hospital in four studies of 11,560 patients (mean difference, –1.17; 95% CI, –3.02 to 0.67; I2 = 77%).
There was no difference in serious adverse events in three studies with 4,665 patients (RD, –0.01; 95% CI, –0.02 to 0.00; I2 = 28%) for patients who received colchicine, compared with supportive care alone. Patients who received colchicine were more likely to have a higher rate of adverse events (RR, 1.58; 95% CI, 1.07-2.33; I2 = 81%) and to experience diarrhea (RR, 1.93; 95% CI, 1.62-2.29; I2 = 0%) than were patients who received supportive care alone. The researchers note that for most outcomes, the GRADE quality of evidence was moderate.
“Our findings on colchicine should be interpreted cautiously due to the inclusion of open-labeled, randomized clinical trials,” Dr. Mehta and colleagues write. “The analysis of efficacy and safety outcomes are based on a small number of RCTs in control interventions.”
The authors reported no relevant financial relationships. Dr. Pillinger is co–principal investigator of the U.S. component of the COLCORONA trial; he reported no other relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis appears to show that colchicine has no benefit as a treatment for COVID-19, but its inclusion of trials studying differing patient populations and testing different outcomes led to “misleading” results, says a researcher involved in one of the trials.
The meta-analysis, which includes data from the recent Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, was published Nov. 22 in RMD Open.
Kedar Gautambhai Mehta, MBBS, MD, of the GMERS Medical College Gotri in Vadodara, India, and colleagues included outcomes from six studies of 16,148 patients with COVID-19 who received colchicine or supportive care. They evaluated the efficacy outcomes of mortality, need for ventilation, intensive care unit admission, and length of stay in hospital, as well as safety outcomes of adverse events, serious adverse events, and diarrhea.
The studies in the meta-analysis included a randomized, controlled trial (RCT) of 105 patients hospitalized with COVID-19 in Greece, the international, open-label RECOVERY RCT of 11,340 patients hospitalized with COVID-19, an RCT of 72 hospitalized patients with moderate or severe COVID-19 in Brazil, an RCT of 100 patients hospitalized with COVID-19 in Iran, the international COLCORONA trial of 4,488 patients with COVID-19 who were treated with colchicine or placebo on an outpatient basis, and the randomized COLORIT trial of 43 patients hospitalized with COVID-19 in Russia.
Studies “asked very different questions” about colchicine
Commenting on the meta-analysis, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology with New York University, said the authors combined studies “that are not comparable and that asked very different questions.” Two of the studies in the meta-analysis are very large, and four are very small, which skews the results, he explained.
“The larger studies therefore drive the outcome, and while the small studies are potentially insight providing, the large studies are the only ones worth giving our attention to in the context of the meta-analysis,” he said. The two largest studies – RECOVERY and COLCORONA – taken together show no benefit for colchicine as a treatment, even though the former demonstrated no benefit and the latter did show a benefit, explained Dr. Pillinger, a co–principal investigator for the COLCORONA trial in the United States.
The studies were designed differently and should not have been included in the same analysis, Dr. Pillinger argued. In the case of COLCORONA, early treatment with colchicine was the intervention, whereas RECOVERY focused on hospitalized patients.
“In designing [COLCORONA], the author group (of whom I was a member) expressly rejected the idea that colchicine might be useful for the sicker hospitalized patients, based on the long experience with colchicine of some of us as rheumatologists,” Dr. Pillinger said.
“In short, COLCORONA proved a benefit of colchicine in outpatient COVID-19, and its authors presumed there would be no inpatient benefit; RECOVERY went ahead and proved a lack of inpatient benefit, at least when high-dose steroids were also given,” he said. “While there is no conflict between these results, the combination of the two studies in this meta-analysis suggests there might be no benefit for colchicine overall, which is misleading and can lead physicians to reject the potential of outpatient colchicine, even for future studies.”
Dr. Pillinger said he still believes colchicine has potential value as a COVID-19 treatment option for patients with mild disease, “especially for low–vaccine rate, resource-starved countries.
“It would be unfortunate if meta-analyses such as this one would put a stop to colchicine’s use, or at least its further investigation,” he said.
Study details
The authors of the study assessed heterogeneity of the trials’ data across the outcomes using an I2 test. They evaluated the quality of the evidence for the outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).
The results of their meta-analysis showed that colchicine offered no significant improvement in mortality in six studies (risk difference, –0.0; 95% confidence interval, –0.01 to 0.01; I2 = 15%). It showed no benefit with respect to requiring ventilatory support in five studies of 15,519 patients (risk ratio, 0.67; 95% CI, 0.38-1.21; I2 = 47%); being admitted to the ICU in three studies with 220 patients (RR, 0.49; 95% CI, 0.19-1.25; I2 = 34%); and length of stay while in the hospital in four studies of 11,560 patients (mean difference, –1.17; 95% CI, –3.02 to 0.67; I2 = 77%).
There was no difference in serious adverse events in three studies with 4,665 patients (RD, –0.01; 95% CI, –0.02 to 0.00; I2 = 28%) for patients who received colchicine, compared with supportive care alone. Patients who received colchicine were more likely to have a higher rate of adverse events (RR, 1.58; 95% CI, 1.07-2.33; I2 = 81%) and to experience diarrhea (RR, 1.93; 95% CI, 1.62-2.29; I2 = 0%) than were patients who received supportive care alone. The researchers note that for most outcomes, the GRADE quality of evidence was moderate.
“Our findings on colchicine should be interpreted cautiously due to the inclusion of open-labeled, randomized clinical trials,” Dr. Mehta and colleagues write. “The analysis of efficacy and safety outcomes are based on a small number of RCTs in control interventions.”
The authors reported no relevant financial relationships. Dr. Pillinger is co–principal investigator of the U.S. component of the COLCORONA trial; he reported no other relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis appears to show that colchicine has no benefit as a treatment for COVID-19, but its inclusion of trials studying differing patient populations and testing different outcomes led to “misleading” results, says a researcher involved in one of the trials.
The meta-analysis, which includes data from the recent Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, was published Nov. 22 in RMD Open.
Kedar Gautambhai Mehta, MBBS, MD, of the GMERS Medical College Gotri in Vadodara, India, and colleagues included outcomes from six studies of 16,148 patients with COVID-19 who received colchicine or supportive care. They evaluated the efficacy outcomes of mortality, need for ventilation, intensive care unit admission, and length of stay in hospital, as well as safety outcomes of adverse events, serious adverse events, and diarrhea.
The studies in the meta-analysis included a randomized, controlled trial (RCT) of 105 patients hospitalized with COVID-19 in Greece, the international, open-label RECOVERY RCT of 11,340 patients hospitalized with COVID-19, an RCT of 72 hospitalized patients with moderate or severe COVID-19 in Brazil, an RCT of 100 patients hospitalized with COVID-19 in Iran, the international COLCORONA trial of 4,488 patients with COVID-19 who were treated with colchicine or placebo on an outpatient basis, and the randomized COLORIT trial of 43 patients hospitalized with COVID-19 in Russia.
Studies “asked very different questions” about colchicine
Commenting on the meta-analysis, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology with New York University, said the authors combined studies “that are not comparable and that asked very different questions.” Two of the studies in the meta-analysis are very large, and four are very small, which skews the results, he explained.
“The larger studies therefore drive the outcome, and while the small studies are potentially insight providing, the large studies are the only ones worth giving our attention to in the context of the meta-analysis,” he said. The two largest studies – RECOVERY and COLCORONA – taken together show no benefit for colchicine as a treatment, even though the former demonstrated no benefit and the latter did show a benefit, explained Dr. Pillinger, a co–principal investigator for the COLCORONA trial in the United States.
The studies were designed differently and should not have been included in the same analysis, Dr. Pillinger argued. In the case of COLCORONA, early treatment with colchicine was the intervention, whereas RECOVERY focused on hospitalized patients.
“In designing [COLCORONA], the author group (of whom I was a member) expressly rejected the idea that colchicine might be useful for the sicker hospitalized patients, based on the long experience with colchicine of some of us as rheumatologists,” Dr. Pillinger said.
“In short, COLCORONA proved a benefit of colchicine in outpatient COVID-19, and its authors presumed there would be no inpatient benefit; RECOVERY went ahead and proved a lack of inpatient benefit, at least when high-dose steroids were also given,” he said. “While there is no conflict between these results, the combination of the two studies in this meta-analysis suggests there might be no benefit for colchicine overall, which is misleading and can lead physicians to reject the potential of outpatient colchicine, even for future studies.”
Dr. Pillinger said he still believes colchicine has potential value as a COVID-19 treatment option for patients with mild disease, “especially for low–vaccine rate, resource-starved countries.
“It would be unfortunate if meta-analyses such as this one would put a stop to colchicine’s use, or at least its further investigation,” he said.
Study details
The authors of the study assessed heterogeneity of the trials’ data across the outcomes using an I2 test. They evaluated the quality of the evidence for the outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).
The results of their meta-analysis showed that colchicine offered no significant improvement in mortality in six studies (risk difference, –0.0; 95% confidence interval, –0.01 to 0.01; I2 = 15%). It showed no benefit with respect to requiring ventilatory support in five studies of 15,519 patients (risk ratio, 0.67; 95% CI, 0.38-1.21; I2 = 47%); being admitted to the ICU in three studies with 220 patients (RR, 0.49; 95% CI, 0.19-1.25; I2 = 34%); and length of stay while in the hospital in four studies of 11,560 patients (mean difference, –1.17; 95% CI, –3.02 to 0.67; I2 = 77%).
There was no difference in serious adverse events in three studies with 4,665 patients (RD, –0.01; 95% CI, –0.02 to 0.00; I2 = 28%) for patients who received colchicine, compared with supportive care alone. Patients who received colchicine were more likely to have a higher rate of adverse events (RR, 1.58; 95% CI, 1.07-2.33; I2 = 81%) and to experience diarrhea (RR, 1.93; 95% CI, 1.62-2.29; I2 = 0%) than were patients who received supportive care alone. The researchers note that for most outcomes, the GRADE quality of evidence was moderate.
“Our findings on colchicine should be interpreted cautiously due to the inclusion of open-labeled, randomized clinical trials,” Dr. Mehta and colleagues write. “The analysis of efficacy and safety outcomes are based on a small number of RCTs in control interventions.”
The authors reported no relevant financial relationships. Dr. Pillinger is co–principal investigator of the U.S. component of the COLCORONA trial; he reported no other relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Oakland score identifies patients with lower GI bleed at low risk for adverse events
Background: The Oakland score was initially designed to be used in patients presenting with LGIB in the urgent, emergent, or primary care setting to help predict risk of readmission and determine if outpatient management is feasible. National guidelines in the United Kingdom have recommended use of the Oakland score despite limited external validation for the triage of patients with acute LGIB. This study aimed to externally validate the Oakland score in a large population in the United States and compare the performance at two thresholds.
Study design: Retrospective observational study.
Setting: 140 hospitals across the United States.
Synopsis: In this prognostic study, 38,067 patients were identified retrospectively using ICD-10 codes that were consistent with a diagnosis of LGIB and were admitted to the hospital. The Oakland score consisted of seven variables, including age, sex, prior hospitalization with LGIB, digital rectal exam results, heart rate, systolic blood pressure, and hemoglobin concentration. The primary outcome was safe discharge from the hospital, defined as absence of in-hospital rebleeding, RBC transfusion, therapeutic colonoscopy, mesenteric embolization or laparotomy for bleeding, in-hospital death, or readmission with subsequent LGIB in 28 days. In total, 47.9% of the identified patients experienced no adverse outcomes and were classified as meeting criteria for safe discharge. In addition, 8.7% of patients scored 8 points or fewer with a sensitivity of 98.4% and specificity of 16.0% for safe discharge. A sensitivity of 96% was maintained after increasing the threshold to 10 points or fewer with a specificity of 31.9%, suggesting the threshold can be increased while still maintaining adequate sensitivity. The study suggests that, by using the Oakland score threshold of 8, hospital admission may be avoided in low-risk patients leading to a savings of at least $44.5 million and even more if the threshold is increased to 10. Low specificity does present limitation of the score as some patients considered to be at risk for adverse events may have been safely discharged and managed as an outpatient, avoiding hospitalization.
Bottom line: The Oakland score was externally validated for use in assessing risk of adverse outcomes in patients with LGIB and had a high sensitivity but low specificity for identifying low-risk patients.
Citation: Oakland K et al. External validation of the Oakland score to assess safe hospital discharge among adult patients with acute lower gastrointestinal bleeding in the US. JAMA Netw Open. 2020 Jul 1;3:e209630. doi:
Dr. Steker is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: The Oakland score was initially designed to be used in patients presenting with LGIB in the urgent, emergent, or primary care setting to help predict risk of readmission and determine if outpatient management is feasible. National guidelines in the United Kingdom have recommended use of the Oakland score despite limited external validation for the triage of patients with acute LGIB. This study aimed to externally validate the Oakland score in a large population in the United States and compare the performance at two thresholds.
Study design: Retrospective observational study.
Setting: 140 hospitals across the United States.
Synopsis: In this prognostic study, 38,067 patients were identified retrospectively using ICD-10 codes that were consistent with a diagnosis of LGIB and were admitted to the hospital. The Oakland score consisted of seven variables, including age, sex, prior hospitalization with LGIB, digital rectal exam results, heart rate, systolic blood pressure, and hemoglobin concentration. The primary outcome was safe discharge from the hospital, defined as absence of in-hospital rebleeding, RBC transfusion, therapeutic colonoscopy, mesenteric embolization or laparotomy for bleeding, in-hospital death, or readmission with subsequent LGIB in 28 days. In total, 47.9% of the identified patients experienced no adverse outcomes and were classified as meeting criteria for safe discharge. In addition, 8.7% of patients scored 8 points or fewer with a sensitivity of 98.4% and specificity of 16.0% for safe discharge. A sensitivity of 96% was maintained after increasing the threshold to 10 points or fewer with a specificity of 31.9%, suggesting the threshold can be increased while still maintaining adequate sensitivity. The study suggests that, by using the Oakland score threshold of 8, hospital admission may be avoided in low-risk patients leading to a savings of at least $44.5 million and even more if the threshold is increased to 10. Low specificity does present limitation of the score as some patients considered to be at risk for adverse events may have been safely discharged and managed as an outpatient, avoiding hospitalization.
Bottom line: The Oakland score was externally validated for use in assessing risk of adverse outcomes in patients with LGIB and had a high sensitivity but low specificity for identifying low-risk patients.
Citation: Oakland K et al. External validation of the Oakland score to assess safe hospital discharge among adult patients with acute lower gastrointestinal bleeding in the US. JAMA Netw Open. 2020 Jul 1;3:e209630. doi:
Dr. Steker is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: The Oakland score was initially designed to be used in patients presenting with LGIB in the urgent, emergent, or primary care setting to help predict risk of readmission and determine if outpatient management is feasible. National guidelines in the United Kingdom have recommended use of the Oakland score despite limited external validation for the triage of patients with acute LGIB. This study aimed to externally validate the Oakland score in a large population in the United States and compare the performance at two thresholds.
Study design: Retrospective observational study.
Setting: 140 hospitals across the United States.
Synopsis: In this prognostic study, 38,067 patients were identified retrospectively using ICD-10 codes that were consistent with a diagnosis of LGIB and were admitted to the hospital. The Oakland score consisted of seven variables, including age, sex, prior hospitalization with LGIB, digital rectal exam results, heart rate, systolic blood pressure, and hemoglobin concentration. The primary outcome was safe discharge from the hospital, defined as absence of in-hospital rebleeding, RBC transfusion, therapeutic colonoscopy, mesenteric embolization or laparotomy for bleeding, in-hospital death, or readmission with subsequent LGIB in 28 days. In total, 47.9% of the identified patients experienced no adverse outcomes and were classified as meeting criteria for safe discharge. In addition, 8.7% of patients scored 8 points or fewer with a sensitivity of 98.4% and specificity of 16.0% for safe discharge. A sensitivity of 96% was maintained after increasing the threshold to 10 points or fewer with a specificity of 31.9%, suggesting the threshold can be increased while still maintaining adequate sensitivity. The study suggests that, by using the Oakland score threshold of 8, hospital admission may be avoided in low-risk patients leading to a savings of at least $44.5 million and even more if the threshold is increased to 10. Low specificity does present limitation of the score as some patients considered to be at risk for adverse events may have been safely discharged and managed as an outpatient, avoiding hospitalization.
Bottom line: The Oakland score was externally validated for use in assessing risk of adverse outcomes in patients with LGIB and had a high sensitivity but low specificity for identifying low-risk patients.
Citation: Oakland K et al. External validation of the Oakland score to assess safe hospital discharge among adult patients with acute lower gastrointestinal bleeding in the US. JAMA Netw Open. 2020 Jul 1;3:e209630. doi:
Dr. Steker is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Predicting cardiac shock mortality in the ICU
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
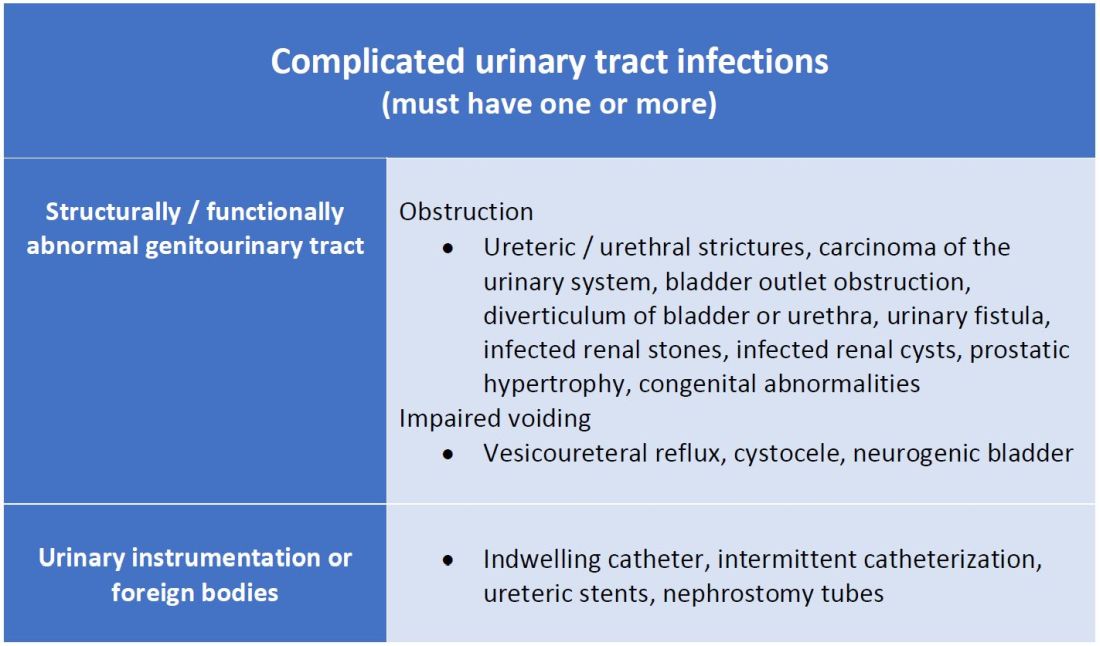
What makes a urinary tract infection complicated?
Consider anatomical and severity risk factors
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.
This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
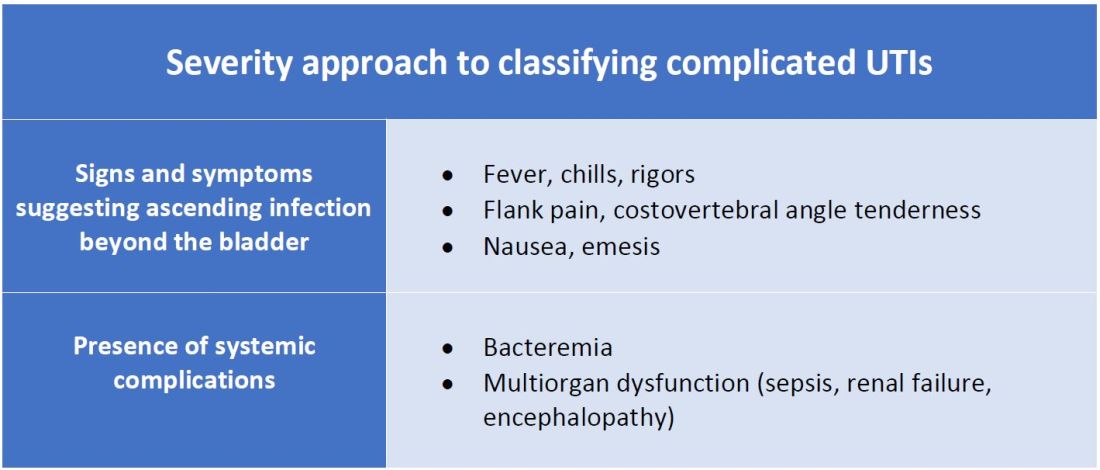
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.
The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Consider anatomical and severity risk factors
Consider anatomical and severity risk factors
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.
This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.
The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.
This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.
The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
COVID surge in Europe: A preview of what’s ahead for the U.S.?
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Is ERCP indicated in gallstone pancreatitis without cholangitis?
Background: The timing and need for ERCP in the setting of gallstone pancreatitis without acute cholangitis has been debated widely. Guidelines recommend urgent ERCP for patients with gallstone pancreatitis with concurrent cholangitis, severe cholestasis, or a visualized stone in the duct, but it is unclear if ERCP benefits those with gallstone pancreatitis without those clear indicators.
Study design: Prospective randomized controlled superiority trial.
Setting: 26 hospitals in the Netherlands.
Synopsis: Of patients with severe gallstone pancreatitis without cholangitis, 232 were randomized 1:1 to undergo urgent ERCP with biliary sphincterotomy (less than 24 hours after presentation) or conservative therapy (analgesia, intravenous fluids, with selective ERCP for cholangitis or persistent cholestasis). The primary endpoint was a composite score of mortality or major complications within 6 months of randomization. There was no difference in the primary endpoint, which occurred in 38% of the urgent-ERCP group and 44% of the conservative-therapy group (P = .37). In a subgroup of patients with cholestasis suggestive of biliary obstruction, the primary endpoint occurred in 32% of the urgent-ERCP group and 42% in the conservative group (P = .18). Similar rates of adverse events were observed between both groups. Limitations included difficulty in diagnosis of cholangitis, moderate positive predictive value of scoring tools to isolate those with severe pancreatitis, and lack of endoscopic ultrasound to determine the presence of ductal stones or sludge.
Bottom line: Conservative management was equal to ERCP with sphincterotomy in patients with severe gallstone pancreatitis without cholangitis, and ERCP may be best reserved for patients with persistent cholestasis or later-developed cholangitis.
Citation: Schepers NJ et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): A multicentre randomised controlled trial. Lancet. 2020;396:167-76. doi: 10.1016/S0140-6736(20)30539-0.
Dr. Reddy is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: The timing and need for ERCP in the setting of gallstone pancreatitis without acute cholangitis has been debated widely. Guidelines recommend urgent ERCP for patients with gallstone pancreatitis with concurrent cholangitis, severe cholestasis, or a visualized stone in the duct, but it is unclear if ERCP benefits those with gallstone pancreatitis without those clear indicators.
Study design: Prospective randomized controlled superiority trial.
Setting: 26 hospitals in the Netherlands.
Synopsis: Of patients with severe gallstone pancreatitis without cholangitis, 232 were randomized 1:1 to undergo urgent ERCP with biliary sphincterotomy (less than 24 hours after presentation) or conservative therapy (analgesia, intravenous fluids, with selective ERCP for cholangitis or persistent cholestasis). The primary endpoint was a composite score of mortality or major complications within 6 months of randomization. There was no difference in the primary endpoint, which occurred in 38% of the urgent-ERCP group and 44% of the conservative-therapy group (P = .37). In a subgroup of patients with cholestasis suggestive of biliary obstruction, the primary endpoint occurred in 32% of the urgent-ERCP group and 42% in the conservative group (P = .18). Similar rates of adverse events were observed between both groups. Limitations included difficulty in diagnosis of cholangitis, moderate positive predictive value of scoring tools to isolate those with severe pancreatitis, and lack of endoscopic ultrasound to determine the presence of ductal stones or sludge.
Bottom line: Conservative management was equal to ERCP with sphincterotomy in patients with severe gallstone pancreatitis without cholangitis, and ERCP may be best reserved for patients with persistent cholestasis or later-developed cholangitis.
Citation: Schepers NJ et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): A multicentre randomised controlled trial. Lancet. 2020;396:167-76. doi: 10.1016/S0140-6736(20)30539-0.
Dr. Reddy is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: The timing and need for ERCP in the setting of gallstone pancreatitis without acute cholangitis has been debated widely. Guidelines recommend urgent ERCP for patients with gallstone pancreatitis with concurrent cholangitis, severe cholestasis, or a visualized stone in the duct, but it is unclear if ERCP benefits those with gallstone pancreatitis without those clear indicators.
Study design: Prospective randomized controlled superiority trial.
Setting: 26 hospitals in the Netherlands.
Synopsis: Of patients with severe gallstone pancreatitis without cholangitis, 232 were randomized 1:1 to undergo urgent ERCP with biliary sphincterotomy (less than 24 hours after presentation) or conservative therapy (analgesia, intravenous fluids, with selective ERCP for cholangitis or persistent cholestasis). The primary endpoint was a composite score of mortality or major complications within 6 months of randomization. There was no difference in the primary endpoint, which occurred in 38% of the urgent-ERCP group and 44% of the conservative-therapy group (P = .37). In a subgroup of patients with cholestasis suggestive of biliary obstruction, the primary endpoint occurred in 32% of the urgent-ERCP group and 42% in the conservative group (P = .18). Similar rates of adverse events were observed between both groups. Limitations included difficulty in diagnosis of cholangitis, moderate positive predictive value of scoring tools to isolate those with severe pancreatitis, and lack of endoscopic ultrasound to determine the presence of ductal stones or sludge.
Bottom line: Conservative management was equal to ERCP with sphincterotomy in patients with severe gallstone pancreatitis without cholangitis, and ERCP may be best reserved for patients with persistent cholestasis or later-developed cholangitis.
Citation: Schepers NJ et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): A multicentre randomised controlled trial. Lancet. 2020;396:167-76. doi: 10.1016/S0140-6736(20)30539-0.
Dr. Reddy is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
CDC unveils mental health protection plan for health care workers
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
CDC: Thirty percent of hospital workers in U.S. still unvaccinated
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.