User login
OIG Finds More Problems in the VA’s Problem-Prone EHR Rollout
Scheduling delays. Disappearing lab orders. Bad links for telehealth appointments. Erroneous medication dispensing. Time-consuming workarounds.
The rollout of the $16 billion electronic health record (EHR) system at the US Department of Veterans Affair (VA) has met some fairly large bumps in the past few years. And now, the VA Office of the Inspector General (OIG) has pronounced on a “range of allegations” at the Mann-Grandstaff VA Medical Center in Spokane, Washington, the first of several hospitals and clinics in the Pacific Northwest set to implement the new system.
VA Inspector General Michael Missal issued 3 reports in mid-March on how the “go-live” process was faring: one on medication management deficiencies, one on care coordination deficiencies, and one on technical issues.
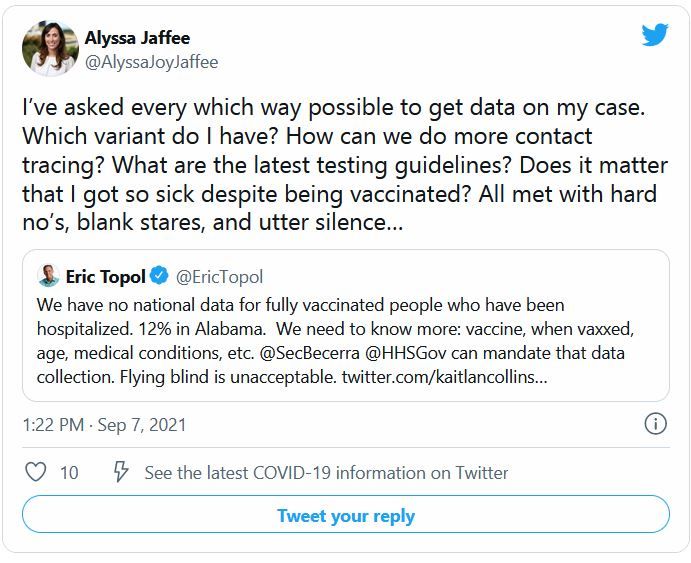
The reports document the OIG’s “concerns” with the new process. According to the technical report, for instance, between October 2020 and March 2021, new EHR users placed more than 38,700 requests for assistance. Of those, 33% were closed without a documented resolution. The OIG also reviewed 210 tickets related to care coordination and found that 1% were closed without a documented resolution.
The OIG said EHR implementation had “created difficulties” for end users in 8 areas:
- Patient record flags, including failures to transfer flags and information related to patients at high risk for suicide;
- Data migration errors leading to inaccurate name, sex, and contact information;
- Scheduling process issues, such as delays in primary care scheduling;
- VA Video Connect problems, including inoperable and misdirected links;
- Referral management deficiencies, including lost or unaddressed referrals;
- Laboratory orders “disappearing” that affected workflow and tracking, and delayed results;
- Patient portal and secure messaging being inaccessible; and
- Documentation processes, including creating additional work and limiting the ability to correctly code patient diagnoses.
The OIG’s technical report identified 5 factors that contributed to the headaches: EHR usability problems, training deficits, interoperability challenges, post–go-live fixes and refinement needs, and problem-resolution process challenges.
The OIG did not identify any associated patient deaths during the inspection but says “future deployment of the new EHR without resolving deficiencies can increase risks to patient safety.”
The technological overhaul has been handled by Cerner. The VA initially awarded Cerner $113 million for EHR modernization, and in 2018 the company secured a 10-year, $10 billion contract to help the VA rebuild its system, similar to the way it did for the US Department of Defense (DoD) with MHS GENESIS. The Cerner DoD project, which has been called “the most lucrative electronic health record contract in history” was launched at the Fairchild Air Force Base in Spokane, Washington, in 2017, and is expected to be operational in more than half of military hospitals and clinics by the end of this year. In 2021, Cerner received an 18-month, $134.1 million task order to deploy the company’s EHR platform at VA medical centers.
This isn’t the first time the VA/Cerner EHR project has hit snags. In 2021, the VA scrapped the schedule, trading it for a 6-month pause after a strategic review ordered by VA Secretary Denis McDonough found problems with governance and management. McDonough told the Senate Veterans’ Affairs Committee that a 3-month internal review had found too many structural problems to warrant continuing the rollout. The sole-source contract with Cerner also raised concerns, as did the influence of 3 confidants of Present Trump on the process. Moreover, cost estimates kept growing—from $10 billion to $16 billion—in part because VA leaders during the Trump administration did not budget for technology and hospital upgrades to allow the new platform to work, according to an article in The Washington Post.
During the senate hearing, committee chair Sen. Jon Tester (D-MT) said, “There’s been damn little accountability. I hope Cerner’s watching this. If they’re not open to making a user-friendly health medical record, they ought to admit it so we can get the money back and start all over.” He told McDonough that the failures were “not all your fault—I don’t know if any of it is your fault.”
“It’s a lot of money you’ve entrusted to us,” McDonough told the committee. The serious problems, he said, were “on us.” He added, “We are taking swift and decisive action to incorporate the management rigor and enterprise jointness required for this program to deliver on its intended purpose: seamless excellence in VA care for veterans. VA’s first implementation of the [project] did not live up to that promise, either for our veterans or for our providers.”
He said he had ordered an overhaul that will include better training for clinical staff, more reliable testing and oversight of Cerner, and a leadership shake-up. He also said he had installed a patient safety team at the Spokane hospital.
Terry Adirim, MD, formerly with the DoD, took over the EHR program in January. In an interview, she said, “[W]e’ve made a substantial number of changes,” such as a new round of training for the hospital’s medical staff. “These deployments are really complex and they’re really hard,” Adirim said, noting that about half of digital medical records programs at private hospitals fail at first. She pointed to the revamped DoD program, which also had its flaws but is running much more smoothly. One of the issues, she said, is that many physicians did not realize that the Cerner system would differ so dramatically from VistA, the system it’s replacing.
The first installment of the rocky rollout left hospital staff confused and worn out. Sen. Patty Murphy (D-WA) said in 2021 that the Spokane staff had filed hundreds of reports of patient safety issues caused by the new system. “Patients are not getting accurate meds. Meds are sent to the wrong address. What used to take a few clicks is now a lot more complicated. Providers are burning out.”
A year later, in a statement, echoing her earlier comments, she said, “We need to put a pause on this rollout right now.”
But Adirim has said the VA is moving ahead with the rollout. The VA has added extra support staff and plans to have physicians from outside the hospital on hand in case things go wrong. According to the Washington Post, Deputy VA Secretary Donald Remy told the OIG that the VA is working to address the outstanding issues and hopes to resolve them by mid-May.
Meanwhile, the beleaguered project ran into another obstacle in early March, when computers went down at Mann-Grandstaff, leading to 20 hours of yet more confusion about medications and surgeries. The VA said the IT system outage also happened at Columbus, Ohio (another of the planned pilot spots). The system was back online the next day, with no known patient safety issues.
Eastern Washington Congresswoman Cathy McMorris Rodgers released a statement saying, “The shutdown of Mann-Grandstaff VA yesterday is another event in a series of challenges that the new electronic health record has created for staff and veterans at the facility. My understanding is that an update made to help the VA’s database for demographic data better communicate with the Cerner system was not performed correctly. Mann-Grandstaff leadership rightly took the system offline until the scope of the problem was understood, so no patients were harmed.”
However, Sen. Murphy called the technical failure “absolutely unacceptable.” In a more recent statement about the rollout, she said, “This is about patient safety and it needs to get fixed—period. VA needs to be upfront about issues like this in real time—Congress absolutely requires transparency when it comes to failures as serious as this. I should be hearing about this from local reporting first.”
If the high-quality care veterans deserve is uncertain at any point, she added, “the rollout should be delayed.” Again.
Scheduling delays. Disappearing lab orders. Bad links for telehealth appointments. Erroneous medication dispensing. Time-consuming workarounds.
The rollout of the $16 billion electronic health record (EHR) system at the US Department of Veterans Affair (VA) has met some fairly large bumps in the past few years. And now, the VA Office of the Inspector General (OIG) has pronounced on a “range of allegations” at the Mann-Grandstaff VA Medical Center in Spokane, Washington, the first of several hospitals and clinics in the Pacific Northwest set to implement the new system.
VA Inspector General Michael Missal issued 3 reports in mid-March on how the “go-live” process was faring: one on medication management deficiencies, one on care coordination deficiencies, and one on technical issues.
The reports document the OIG’s “concerns” with the new process. According to the technical report, for instance, between October 2020 and March 2021, new EHR users placed more than 38,700 requests for assistance. Of those, 33% were closed without a documented resolution. The OIG also reviewed 210 tickets related to care coordination and found that 1% were closed without a documented resolution.
The OIG said EHR implementation had “created difficulties” for end users in 8 areas:
- Patient record flags, including failures to transfer flags and information related to patients at high risk for suicide;
- Data migration errors leading to inaccurate name, sex, and contact information;
- Scheduling process issues, such as delays in primary care scheduling;
- VA Video Connect problems, including inoperable and misdirected links;
- Referral management deficiencies, including lost or unaddressed referrals;
- Laboratory orders “disappearing” that affected workflow and tracking, and delayed results;
- Patient portal and secure messaging being inaccessible; and
- Documentation processes, including creating additional work and limiting the ability to correctly code patient diagnoses.
The OIG’s technical report identified 5 factors that contributed to the headaches: EHR usability problems, training deficits, interoperability challenges, post–go-live fixes and refinement needs, and problem-resolution process challenges.
The OIG did not identify any associated patient deaths during the inspection but says “future deployment of the new EHR without resolving deficiencies can increase risks to patient safety.”
The technological overhaul has been handled by Cerner. The VA initially awarded Cerner $113 million for EHR modernization, and in 2018 the company secured a 10-year, $10 billion contract to help the VA rebuild its system, similar to the way it did for the US Department of Defense (DoD) with MHS GENESIS. The Cerner DoD project, which has been called “the most lucrative electronic health record contract in history” was launched at the Fairchild Air Force Base in Spokane, Washington, in 2017, and is expected to be operational in more than half of military hospitals and clinics by the end of this year. In 2021, Cerner received an 18-month, $134.1 million task order to deploy the company’s EHR platform at VA medical centers.
This isn’t the first time the VA/Cerner EHR project has hit snags. In 2021, the VA scrapped the schedule, trading it for a 6-month pause after a strategic review ordered by VA Secretary Denis McDonough found problems with governance and management. McDonough told the Senate Veterans’ Affairs Committee that a 3-month internal review had found too many structural problems to warrant continuing the rollout. The sole-source contract with Cerner also raised concerns, as did the influence of 3 confidants of Present Trump on the process. Moreover, cost estimates kept growing—from $10 billion to $16 billion—in part because VA leaders during the Trump administration did not budget for technology and hospital upgrades to allow the new platform to work, according to an article in The Washington Post.
During the senate hearing, committee chair Sen. Jon Tester (D-MT) said, “There’s been damn little accountability. I hope Cerner’s watching this. If they’re not open to making a user-friendly health medical record, they ought to admit it so we can get the money back and start all over.” He told McDonough that the failures were “not all your fault—I don’t know if any of it is your fault.”
“It’s a lot of money you’ve entrusted to us,” McDonough told the committee. The serious problems, he said, were “on us.” He added, “We are taking swift and decisive action to incorporate the management rigor and enterprise jointness required for this program to deliver on its intended purpose: seamless excellence in VA care for veterans. VA’s first implementation of the [project] did not live up to that promise, either for our veterans or for our providers.”
He said he had ordered an overhaul that will include better training for clinical staff, more reliable testing and oversight of Cerner, and a leadership shake-up. He also said he had installed a patient safety team at the Spokane hospital.
Terry Adirim, MD, formerly with the DoD, took over the EHR program in January. In an interview, she said, “[W]e’ve made a substantial number of changes,” such as a new round of training for the hospital’s medical staff. “These deployments are really complex and they’re really hard,” Adirim said, noting that about half of digital medical records programs at private hospitals fail at first. She pointed to the revamped DoD program, which also had its flaws but is running much more smoothly. One of the issues, she said, is that many physicians did not realize that the Cerner system would differ so dramatically from VistA, the system it’s replacing.
The first installment of the rocky rollout left hospital staff confused and worn out. Sen. Patty Murphy (D-WA) said in 2021 that the Spokane staff had filed hundreds of reports of patient safety issues caused by the new system. “Patients are not getting accurate meds. Meds are sent to the wrong address. What used to take a few clicks is now a lot more complicated. Providers are burning out.”
A year later, in a statement, echoing her earlier comments, she said, “We need to put a pause on this rollout right now.”
But Adirim has said the VA is moving ahead with the rollout. The VA has added extra support staff and plans to have physicians from outside the hospital on hand in case things go wrong. According to the Washington Post, Deputy VA Secretary Donald Remy told the OIG that the VA is working to address the outstanding issues and hopes to resolve them by mid-May.
Meanwhile, the beleaguered project ran into another obstacle in early March, when computers went down at Mann-Grandstaff, leading to 20 hours of yet more confusion about medications and surgeries. The VA said the IT system outage also happened at Columbus, Ohio (another of the planned pilot spots). The system was back online the next day, with no known patient safety issues.
Eastern Washington Congresswoman Cathy McMorris Rodgers released a statement saying, “The shutdown of Mann-Grandstaff VA yesterday is another event in a series of challenges that the new electronic health record has created for staff and veterans at the facility. My understanding is that an update made to help the VA’s database for demographic data better communicate with the Cerner system was not performed correctly. Mann-Grandstaff leadership rightly took the system offline until the scope of the problem was understood, so no patients were harmed.”
However, Sen. Murphy called the technical failure “absolutely unacceptable.” In a more recent statement about the rollout, she said, “This is about patient safety and it needs to get fixed—period. VA needs to be upfront about issues like this in real time—Congress absolutely requires transparency when it comes to failures as serious as this. I should be hearing about this from local reporting first.”
If the high-quality care veterans deserve is uncertain at any point, she added, “the rollout should be delayed.” Again.
Scheduling delays. Disappearing lab orders. Bad links for telehealth appointments. Erroneous medication dispensing. Time-consuming workarounds.
The rollout of the $16 billion electronic health record (EHR) system at the US Department of Veterans Affair (VA) has met some fairly large bumps in the past few years. And now, the VA Office of the Inspector General (OIG) has pronounced on a “range of allegations” at the Mann-Grandstaff VA Medical Center in Spokane, Washington, the first of several hospitals and clinics in the Pacific Northwest set to implement the new system.
VA Inspector General Michael Missal issued 3 reports in mid-March on how the “go-live” process was faring: one on medication management deficiencies, one on care coordination deficiencies, and one on technical issues.
The reports document the OIG’s “concerns” with the new process. According to the technical report, for instance, between October 2020 and March 2021, new EHR users placed more than 38,700 requests for assistance. Of those, 33% were closed without a documented resolution. The OIG also reviewed 210 tickets related to care coordination and found that 1% were closed without a documented resolution.
The OIG said EHR implementation had “created difficulties” for end users in 8 areas:
- Patient record flags, including failures to transfer flags and information related to patients at high risk for suicide;
- Data migration errors leading to inaccurate name, sex, and contact information;
- Scheduling process issues, such as delays in primary care scheduling;
- VA Video Connect problems, including inoperable and misdirected links;
- Referral management deficiencies, including lost or unaddressed referrals;
- Laboratory orders “disappearing” that affected workflow and tracking, and delayed results;
- Patient portal and secure messaging being inaccessible; and
- Documentation processes, including creating additional work and limiting the ability to correctly code patient diagnoses.
The OIG’s technical report identified 5 factors that contributed to the headaches: EHR usability problems, training deficits, interoperability challenges, post–go-live fixes and refinement needs, and problem-resolution process challenges.
The OIG did not identify any associated patient deaths during the inspection but says “future deployment of the new EHR without resolving deficiencies can increase risks to patient safety.”
The technological overhaul has been handled by Cerner. The VA initially awarded Cerner $113 million for EHR modernization, and in 2018 the company secured a 10-year, $10 billion contract to help the VA rebuild its system, similar to the way it did for the US Department of Defense (DoD) with MHS GENESIS. The Cerner DoD project, which has been called “the most lucrative electronic health record contract in history” was launched at the Fairchild Air Force Base in Spokane, Washington, in 2017, and is expected to be operational in more than half of military hospitals and clinics by the end of this year. In 2021, Cerner received an 18-month, $134.1 million task order to deploy the company’s EHR platform at VA medical centers.
This isn’t the first time the VA/Cerner EHR project has hit snags. In 2021, the VA scrapped the schedule, trading it for a 6-month pause after a strategic review ordered by VA Secretary Denis McDonough found problems with governance and management. McDonough told the Senate Veterans’ Affairs Committee that a 3-month internal review had found too many structural problems to warrant continuing the rollout. The sole-source contract with Cerner also raised concerns, as did the influence of 3 confidants of Present Trump on the process. Moreover, cost estimates kept growing—from $10 billion to $16 billion—in part because VA leaders during the Trump administration did not budget for technology and hospital upgrades to allow the new platform to work, according to an article in The Washington Post.
During the senate hearing, committee chair Sen. Jon Tester (D-MT) said, “There’s been damn little accountability. I hope Cerner’s watching this. If they’re not open to making a user-friendly health medical record, they ought to admit it so we can get the money back and start all over.” He told McDonough that the failures were “not all your fault—I don’t know if any of it is your fault.”
“It’s a lot of money you’ve entrusted to us,” McDonough told the committee. The serious problems, he said, were “on us.” He added, “We are taking swift and decisive action to incorporate the management rigor and enterprise jointness required for this program to deliver on its intended purpose: seamless excellence in VA care for veterans. VA’s first implementation of the [project] did not live up to that promise, either for our veterans or for our providers.”
He said he had ordered an overhaul that will include better training for clinical staff, more reliable testing and oversight of Cerner, and a leadership shake-up. He also said he had installed a patient safety team at the Spokane hospital.
Terry Adirim, MD, formerly with the DoD, took over the EHR program in January. In an interview, she said, “[W]e’ve made a substantial number of changes,” such as a new round of training for the hospital’s medical staff. “These deployments are really complex and they’re really hard,” Adirim said, noting that about half of digital medical records programs at private hospitals fail at first. She pointed to the revamped DoD program, which also had its flaws but is running much more smoothly. One of the issues, she said, is that many physicians did not realize that the Cerner system would differ so dramatically from VistA, the system it’s replacing.
The first installment of the rocky rollout left hospital staff confused and worn out. Sen. Patty Murphy (D-WA) said in 2021 that the Spokane staff had filed hundreds of reports of patient safety issues caused by the new system. “Patients are not getting accurate meds. Meds are sent to the wrong address. What used to take a few clicks is now a lot more complicated. Providers are burning out.”
A year later, in a statement, echoing her earlier comments, she said, “We need to put a pause on this rollout right now.”
But Adirim has said the VA is moving ahead with the rollout. The VA has added extra support staff and plans to have physicians from outside the hospital on hand in case things go wrong. According to the Washington Post, Deputy VA Secretary Donald Remy told the OIG that the VA is working to address the outstanding issues and hopes to resolve them by mid-May.
Meanwhile, the beleaguered project ran into another obstacle in early March, when computers went down at Mann-Grandstaff, leading to 20 hours of yet more confusion about medications and surgeries. The VA said the IT system outage also happened at Columbus, Ohio (another of the planned pilot spots). The system was back online the next day, with no known patient safety issues.
Eastern Washington Congresswoman Cathy McMorris Rodgers released a statement saying, “The shutdown of Mann-Grandstaff VA yesterday is another event in a series of challenges that the new electronic health record has created for staff and veterans at the facility. My understanding is that an update made to help the VA’s database for demographic data better communicate with the Cerner system was not performed correctly. Mann-Grandstaff leadership rightly took the system offline until the scope of the problem was understood, so no patients were harmed.”
However, Sen. Murphy called the technical failure “absolutely unacceptable.” In a more recent statement about the rollout, she said, “This is about patient safety and it needs to get fixed—period. VA needs to be upfront about issues like this in real time—Congress absolutely requires transparency when it comes to failures as serious as this. I should be hearing about this from local reporting first.”
If the high-quality care veterans deserve is uncertain at any point, she added, “the rollout should be delayed.” Again.
Tech Glitches at One VA Site Raise Concerns About a Nationwide Rollout
Spokane, Washington, was supposed to be the center of the Department of Veterans Affairs’ tech reinvention, the first site in the agency’s decade-long project to change its medical records software. But one morning in early March, the latest system malfunction made some clinicians snap.
At Spokane’s Mann-Grandstaff VA Medical Center, the records system — developed by Cerner Corp., based in North Kansas City, Missouri — went down. Staffers, inside the hospital and its outpatient facilities, were back to relying on pen and paper. Computerized schedules were inaccessible. Physicians couldn’t enter new orders or change patients’ medications.
By the next day, the electronic health records were only partially available. Dozens of records remained “sequestered,” meaning that doctors and nurses struggled to update patient charts.
The snafu, the latest in a series at Mann-Grandstaff, heightened Spokane medical staff members’ frustration with a system that has been problematic since it was installed a year and a half ago. The VA said no patients had been harmed because of the problems.
But physicians on the ground there said it’s only a matter of time before serious safety problems — those causing injury or death — emerge, pointing to the program’s ongoing weaknesses amid VA leadership’s full-bore push toward implementation nationwide. One provider said she was glad she didn’t have a relative in Mann-Grandstaff.
The one-two punch of a dangerous outage and staff grievances is the latest setback in the VA’s more than $16 billion effort to upgrade its record-keeping technology. The issues have at times forced clinicians to see fewer patients and file tens of thousands of requests for help to Cerner with patient-safety problems, congressional and agency watchdog reviews show.
If those issues multiply over the vast VA system — which employs more than a quarter-million workers and serves 6.3 million active patients — it could create rampant patient-safety and productivity problems. Despite the VA’s goals of using the technology upgrade to provide seamless records for patients from enlistment in the military until discharge, the doctors and clinicians who spoke to KHN are convinced that the problems experienced in Spokane will be repeated again and again.
The records system, scheduled for deployment at multiple VA facilities in the Pacific Northwest in the coming weeks and months, most recently rolled out at Jonathan M. Wainwright Memorial VA Medical Center in Walla Walla, Washington.
Both Cerner and Mann-Grandstaff officials declined to comment and referred KHN to officials at VA headquarters in Washington, D.C.
“If it’s a total meltdown” in Walla Walla, said Katie Purswell, the American Legion’s director of veterans affairs and rehabilitation, it’s a problem for the department and its decade-long initiative. Initial impressions from staffers are positive, but the issues in Spokane appeared over the course of weeks after the system was turned on.
The distress was particularly acute in early March. On March 2, Cerner turned on a software update to a database containing patient identification information. Problems emerged the next morning. In the record of a patient who was checking in for surgery, staff members discovered incorrect data, including gender information for a different patient. About an hour later, a staffer from the national VA’s medical records office told some clinicians at Mann-Grandstaff to “log out.”
Robert Fischer, Mann-Grandstaff’s medical center director, sent a dire warning later that morning. “Assume all electronic data is corrupted/inaccurate,” Fischer said in an email. He urged clinicians to limit orders for lab work, imaging studies, and medications. The facility shifted to “downtime procedures,” meaning a reliance on paper.
Some staffers didn’t absorb the late-morning message and continued entering information in the mixed-up records, adding to the stew of erroneous data.
According to information provided to Congress later by the VA, Cerner had informed Mann-Grandstaff of a “complete degradation” the night before — leading to questions from staffers about why it took until late morning the next day to shut down the system. Agency spokesperson Erin Crowe told KHN there wasn’t any delay in notifying staff.
Problems stretched into the following week. Some records — 70 as of March 10, according to a briefing provided to Congress — remained unusable while auditors tried to ascertain what information had been mixed in from other charts. This left clinicians in some instances unable to keep track of patients’ care. Doctors said it became a confusing and chaotic environment. They couldn’t, for example, help patients refill prescriptions.
Members of Congress are concerned — not only about the outage but also VA’s explanations about it. In a letter to agency leadership, the leading Republicans on the House Veterans’ Affairs Committee and the subcommittee overseeing technology, Rep. Mike Bost of Illinois and Rep. Matt Rosendale of Montana, expressed worries the agency was “soft-pedal[ing]” its communications and argued that veterans were misled by assurances that records had been corrected.
The full committee plans to do a deeper examination soon. It has scheduled a closed-door roundtable with VA staffers from Spokane and Walla Walla on April 5.
The outage deepened unhappiness at Mann-Grandstaff. Clinicians there were already frazzled by a deeply buggy system. Downtime was common, a congressional aide told KHN.
A March 17 VA inspector general report documented nearly 39,000 requests for technical help or improvement since the October 2020 deployment of the new records system. Cerner employees often closed requests without resolving the underlying problems, the report said. Mann-Grandstaff staffers became disengaged or devised shortcuts to bypass the malfunctioning software, the inspector general wrote — each a potential root of patient-safety incidents.
The department said the shortcuts — or workarounds — aren’t its policy. “Workarounds are not authorized nor encouraged,” Crowe said.
The Biden administration tried to overhaul the software initiative, putting the program on hiatus before installing new leadership in the medical records office at the end of 2021. But by then, low morale had sunk in. “People in Spokane VA are … demoralized and unhappy,” Rep. Frank Mrvan (D-Ind.), chair of the House subcommittee focused on the VA’s technology modernization programs, told agency leaders during a November congressional hearing. He said staffers told him they felt as though they were beating their heads against a wall to make things function.
Other observers shared Mrvan’s concerns.
Purswell of the American Legion questioned whether appropriate steps are being taken to prepare the Walla Walla facility and its staff for the technology rollout. She asked whether staffers feel as if the Cerner system has been thrust upon them or are excited about the change.
Whether the VA has been persuasive about the benefits of the program is unclear. “I think it’s incumbent on us to demonstrate it’s not a loss,” said Dr. Terry Adirim, the leader of the VA office in charge of implementing the new records technology. “We might have dropped the ball on explaining what a benefit this is.”
Indeed, Adirim conducted a virtual town hall meeting March 21 for veterans in the Walla Walla area — where she was pressed about the problems in Spokane. “If Spokane has been a year figuring this out, why is this moving forward?” one questioner asked, expressing a point made frequently during the call. Adirim said the VA had made “thousands of changes” since the initial rollout.
Medical staffers at the Spokane and Walla Walla VA facilities are part of informal networks sharing their often-negative experiences about the program despite a perception among staff members that dissent will hurt their careers.
Adirim thinks negative feelings can be addressed by stepping up technical support. She also said training programs have been overhauled since the deployment in Spokane. Bottom line: The VA is proceeding.
“People want to revert back to what they did before,” Adirim said, but that’s not going to happen.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Subscribe to KHN's free Morning Briefing.
Spokane, Washington, was supposed to be the center of the Department of Veterans Affairs’ tech reinvention, the first site in the agency’s decade-long project to change its medical records software. But one morning in early March, the latest system malfunction made some clinicians snap.
At Spokane’s Mann-Grandstaff VA Medical Center, the records system — developed by Cerner Corp., based in North Kansas City, Missouri — went down. Staffers, inside the hospital and its outpatient facilities, were back to relying on pen and paper. Computerized schedules were inaccessible. Physicians couldn’t enter new orders or change patients’ medications.
By the next day, the electronic health records were only partially available. Dozens of records remained “sequestered,” meaning that doctors and nurses struggled to update patient charts.
The snafu, the latest in a series at Mann-Grandstaff, heightened Spokane medical staff members’ frustration with a system that has been problematic since it was installed a year and a half ago. The VA said no patients had been harmed because of the problems.
But physicians on the ground there said it’s only a matter of time before serious safety problems — those causing injury or death — emerge, pointing to the program’s ongoing weaknesses amid VA leadership’s full-bore push toward implementation nationwide. One provider said she was glad she didn’t have a relative in Mann-Grandstaff.
The one-two punch of a dangerous outage and staff grievances is the latest setback in the VA’s more than $16 billion effort to upgrade its record-keeping technology. The issues have at times forced clinicians to see fewer patients and file tens of thousands of requests for help to Cerner with patient-safety problems, congressional and agency watchdog reviews show.
If those issues multiply over the vast VA system — which employs more than a quarter-million workers and serves 6.3 million active patients — it could create rampant patient-safety and productivity problems. Despite the VA’s goals of using the technology upgrade to provide seamless records for patients from enlistment in the military until discharge, the doctors and clinicians who spoke to KHN are convinced that the problems experienced in Spokane will be repeated again and again.
The records system, scheduled for deployment at multiple VA facilities in the Pacific Northwest in the coming weeks and months, most recently rolled out at Jonathan M. Wainwright Memorial VA Medical Center in Walla Walla, Washington.
Both Cerner and Mann-Grandstaff officials declined to comment and referred KHN to officials at VA headquarters in Washington, D.C.
“If it’s a total meltdown” in Walla Walla, said Katie Purswell, the American Legion’s director of veterans affairs and rehabilitation, it’s a problem for the department and its decade-long initiative. Initial impressions from staffers are positive, but the issues in Spokane appeared over the course of weeks after the system was turned on.
The distress was particularly acute in early March. On March 2, Cerner turned on a software update to a database containing patient identification information. Problems emerged the next morning. In the record of a patient who was checking in for surgery, staff members discovered incorrect data, including gender information for a different patient. About an hour later, a staffer from the national VA’s medical records office told some clinicians at Mann-Grandstaff to “log out.”
Robert Fischer, Mann-Grandstaff’s medical center director, sent a dire warning later that morning. “Assume all electronic data is corrupted/inaccurate,” Fischer said in an email. He urged clinicians to limit orders for lab work, imaging studies, and medications. The facility shifted to “downtime procedures,” meaning a reliance on paper.
Some staffers didn’t absorb the late-morning message and continued entering information in the mixed-up records, adding to the stew of erroneous data.
According to information provided to Congress later by the VA, Cerner had informed Mann-Grandstaff of a “complete degradation” the night before — leading to questions from staffers about why it took until late morning the next day to shut down the system. Agency spokesperson Erin Crowe told KHN there wasn’t any delay in notifying staff.
Problems stretched into the following week. Some records — 70 as of March 10, according to a briefing provided to Congress — remained unusable while auditors tried to ascertain what information had been mixed in from other charts. This left clinicians in some instances unable to keep track of patients’ care. Doctors said it became a confusing and chaotic environment. They couldn’t, for example, help patients refill prescriptions.
Members of Congress are concerned — not only about the outage but also VA’s explanations about it. In a letter to agency leadership, the leading Republicans on the House Veterans’ Affairs Committee and the subcommittee overseeing technology, Rep. Mike Bost of Illinois and Rep. Matt Rosendale of Montana, expressed worries the agency was “soft-pedal[ing]” its communications and argued that veterans were misled by assurances that records had been corrected.
The full committee plans to do a deeper examination soon. It has scheduled a closed-door roundtable with VA staffers from Spokane and Walla Walla on April 5.
The outage deepened unhappiness at Mann-Grandstaff. Clinicians there were already frazzled by a deeply buggy system. Downtime was common, a congressional aide told KHN.
A March 17 VA inspector general report documented nearly 39,000 requests for technical help or improvement since the October 2020 deployment of the new records system. Cerner employees often closed requests without resolving the underlying problems, the report said. Mann-Grandstaff staffers became disengaged or devised shortcuts to bypass the malfunctioning software, the inspector general wrote — each a potential root of patient-safety incidents.
The department said the shortcuts — or workarounds — aren’t its policy. “Workarounds are not authorized nor encouraged,” Crowe said.
The Biden administration tried to overhaul the software initiative, putting the program on hiatus before installing new leadership in the medical records office at the end of 2021. But by then, low morale had sunk in. “People in Spokane VA are … demoralized and unhappy,” Rep. Frank Mrvan (D-Ind.), chair of the House subcommittee focused on the VA’s technology modernization programs, told agency leaders during a November congressional hearing. He said staffers told him they felt as though they were beating their heads against a wall to make things function.
Other observers shared Mrvan’s concerns.
Purswell of the American Legion questioned whether appropriate steps are being taken to prepare the Walla Walla facility and its staff for the technology rollout. She asked whether staffers feel as if the Cerner system has been thrust upon them or are excited about the change.
Whether the VA has been persuasive about the benefits of the program is unclear. “I think it’s incumbent on us to demonstrate it’s not a loss,” said Dr. Terry Adirim, the leader of the VA office in charge of implementing the new records technology. “We might have dropped the ball on explaining what a benefit this is.”
Indeed, Adirim conducted a virtual town hall meeting March 21 for veterans in the Walla Walla area — where she was pressed about the problems in Spokane. “If Spokane has been a year figuring this out, why is this moving forward?” one questioner asked, expressing a point made frequently during the call. Adirim said the VA had made “thousands of changes” since the initial rollout.
Medical staffers at the Spokane and Walla Walla VA facilities are part of informal networks sharing their often-negative experiences about the program despite a perception among staff members that dissent will hurt their careers.
Adirim thinks negative feelings can be addressed by stepping up technical support. She also said training programs have been overhauled since the deployment in Spokane. Bottom line: The VA is proceeding.
“People want to revert back to what they did before,” Adirim said, but that’s not going to happen.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Subscribe to KHN's free Morning Briefing.
Spokane, Washington, was supposed to be the center of the Department of Veterans Affairs’ tech reinvention, the first site in the agency’s decade-long project to change its medical records software. But one morning in early March, the latest system malfunction made some clinicians snap.
At Spokane’s Mann-Grandstaff VA Medical Center, the records system — developed by Cerner Corp., based in North Kansas City, Missouri — went down. Staffers, inside the hospital and its outpatient facilities, were back to relying on pen and paper. Computerized schedules were inaccessible. Physicians couldn’t enter new orders or change patients’ medications.
By the next day, the electronic health records were only partially available. Dozens of records remained “sequestered,” meaning that doctors and nurses struggled to update patient charts.
The snafu, the latest in a series at Mann-Grandstaff, heightened Spokane medical staff members’ frustration with a system that has been problematic since it was installed a year and a half ago. The VA said no patients had been harmed because of the problems.
But physicians on the ground there said it’s only a matter of time before serious safety problems — those causing injury or death — emerge, pointing to the program’s ongoing weaknesses amid VA leadership’s full-bore push toward implementation nationwide. One provider said she was glad she didn’t have a relative in Mann-Grandstaff.
The one-two punch of a dangerous outage and staff grievances is the latest setback in the VA’s more than $16 billion effort to upgrade its record-keeping technology. The issues have at times forced clinicians to see fewer patients and file tens of thousands of requests for help to Cerner with patient-safety problems, congressional and agency watchdog reviews show.
If those issues multiply over the vast VA system — which employs more than a quarter-million workers and serves 6.3 million active patients — it could create rampant patient-safety and productivity problems. Despite the VA’s goals of using the technology upgrade to provide seamless records for patients from enlistment in the military until discharge, the doctors and clinicians who spoke to KHN are convinced that the problems experienced in Spokane will be repeated again and again.
The records system, scheduled for deployment at multiple VA facilities in the Pacific Northwest in the coming weeks and months, most recently rolled out at Jonathan M. Wainwright Memorial VA Medical Center in Walla Walla, Washington.
Both Cerner and Mann-Grandstaff officials declined to comment and referred KHN to officials at VA headquarters in Washington, D.C.
“If it’s a total meltdown” in Walla Walla, said Katie Purswell, the American Legion’s director of veterans affairs and rehabilitation, it’s a problem for the department and its decade-long initiative. Initial impressions from staffers are positive, but the issues in Spokane appeared over the course of weeks after the system was turned on.
The distress was particularly acute in early March. On March 2, Cerner turned on a software update to a database containing patient identification information. Problems emerged the next morning. In the record of a patient who was checking in for surgery, staff members discovered incorrect data, including gender information for a different patient. About an hour later, a staffer from the national VA’s medical records office told some clinicians at Mann-Grandstaff to “log out.”
Robert Fischer, Mann-Grandstaff’s medical center director, sent a dire warning later that morning. “Assume all electronic data is corrupted/inaccurate,” Fischer said in an email. He urged clinicians to limit orders for lab work, imaging studies, and medications. The facility shifted to “downtime procedures,” meaning a reliance on paper.
Some staffers didn’t absorb the late-morning message and continued entering information in the mixed-up records, adding to the stew of erroneous data.
According to information provided to Congress later by the VA, Cerner had informed Mann-Grandstaff of a “complete degradation” the night before — leading to questions from staffers about why it took until late morning the next day to shut down the system. Agency spokesperson Erin Crowe told KHN there wasn’t any delay in notifying staff.
Problems stretched into the following week. Some records — 70 as of March 10, according to a briefing provided to Congress — remained unusable while auditors tried to ascertain what information had been mixed in from other charts. This left clinicians in some instances unable to keep track of patients’ care. Doctors said it became a confusing and chaotic environment. They couldn’t, for example, help patients refill prescriptions.
Members of Congress are concerned — not only about the outage but also VA’s explanations about it. In a letter to agency leadership, the leading Republicans on the House Veterans’ Affairs Committee and the subcommittee overseeing technology, Rep. Mike Bost of Illinois and Rep. Matt Rosendale of Montana, expressed worries the agency was “soft-pedal[ing]” its communications and argued that veterans were misled by assurances that records had been corrected.
The full committee plans to do a deeper examination soon. It has scheduled a closed-door roundtable with VA staffers from Spokane and Walla Walla on April 5.
The outage deepened unhappiness at Mann-Grandstaff. Clinicians there were already frazzled by a deeply buggy system. Downtime was common, a congressional aide told KHN.
A March 17 VA inspector general report documented nearly 39,000 requests for technical help or improvement since the October 2020 deployment of the new records system. Cerner employees often closed requests without resolving the underlying problems, the report said. Mann-Grandstaff staffers became disengaged or devised shortcuts to bypass the malfunctioning software, the inspector general wrote — each a potential root of patient-safety incidents.
The department said the shortcuts — or workarounds — aren’t its policy. “Workarounds are not authorized nor encouraged,” Crowe said.
The Biden administration tried to overhaul the software initiative, putting the program on hiatus before installing new leadership in the medical records office at the end of 2021. But by then, low morale had sunk in. “People in Spokane VA are … demoralized and unhappy,” Rep. Frank Mrvan (D-Ind.), chair of the House subcommittee focused on the VA’s technology modernization programs, told agency leaders during a November congressional hearing. He said staffers told him they felt as though they were beating their heads against a wall to make things function.
Other observers shared Mrvan’s concerns.
Purswell of the American Legion questioned whether appropriate steps are being taken to prepare the Walla Walla facility and its staff for the technology rollout. She asked whether staffers feel as if the Cerner system has been thrust upon them or are excited about the change.
Whether the VA has been persuasive about the benefits of the program is unclear. “I think it’s incumbent on us to demonstrate it’s not a loss,” said Dr. Terry Adirim, the leader of the VA office in charge of implementing the new records technology. “We might have dropped the ball on explaining what a benefit this is.”
Indeed, Adirim conducted a virtual town hall meeting March 21 for veterans in the Walla Walla area — where she was pressed about the problems in Spokane. “If Spokane has been a year figuring this out, why is this moving forward?” one questioner asked, expressing a point made frequently during the call. Adirim said the VA had made “thousands of changes” since the initial rollout.
Medical staffers at the Spokane and Walla Walla VA facilities are part of informal networks sharing their often-negative experiences about the program despite a perception among staff members that dissent will hurt their careers.
Adirim thinks negative feelings can be addressed by stepping up technical support. She also said training programs have been overhauled since the deployment in Spokane. Bottom line: The VA is proceeding.
“People want to revert back to what they did before,” Adirim said, but that’s not going to happen.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Subscribe to KHN's free Morning Briefing.
FDA panel slams Endologix response to stent-graft safety issues
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
Whistleblowers will play key role in enforcing workplace vaccine mandate
goes into effect in January.
The Occupational Safety and Health Administration (OSHA) doesn’t have enough workplace safety inspectors to cover the nation, the Associated Press reported, so the agency will count on people within organizations to identify violations.
“There is no army of OSHA inspectors that is going to be knocking on employers’ doors or even calling them,” Debbie Berkowitz, a former OSHA chief of staff who is a fellow at Georgetown University, told the news service.
“They’re going to rely on workers and their union representatives to file complaints where the company is totally flouting the law,” she said.
Last week, OSHA published the details of the Biden administration’s vaccine mandate. Companies with more than 100 employees must require their workers to get vaccinated or undergo weekly testing. Companies that don’t comply could face fines of $14,000 for each “serious” violation. Repeat violators could face 10 times that amount.
Employees who are concerned about workplace safety, unvaccinated co-workers, or people not being tested as required may report their employers, according to Reuters.
Jim Frederick, the acting chief for OSHA, told reporters that the agency will focus on job sites “where workers need assistance to have a safe and healthy workplace.”
“That typically comes through in the form of a complaint,” he said.
OSHA has jurisdiction in 29 states, the AP reported. OSHA is tasked with addressing violations of the Occupational Safety and Health Act of 1970, which is meant to create safe workplaces, and the agency has updated its guidance about COVID-19 safety in the workplace throughout this year.
Other states, such as California and Michigan, have their own workplace safety agencies, which will have until February to adopt their own version of a vaccine mandate, according to the AP.
OSHA and state counterparts will be tasked with enforcing the mandate, and their agencies are already short-staffed. About 1,850 inspectors will oversee 130 million workers at 8 million job sites.
OSHA has encouraged workers to first report complaints to employers “if possible.” Otherwise, employees can file a confidential safety complaint with OSHA or file a case through a representative, such as a lawyer or union leader, the AP reported.
But workplace experts have voiced caution about the potential risks of reporting. Whistleblowers tend to face retaliation and OSHA can’t always offer protection in these cases.
“Technically, the law says that companies can’t retaliate against a worker for raising a health and safety issue or filing an OSHA complaint or even reporting an injury,” Ms. Berkowitz said. “But retaliation is rampant.”
OSHA has some jurisdiction to pursue employers who punish workers for reporting unsafe working conditions, the AP reported. Last month, the agency sued a luxury car dealer in Texas for firing an employee who warned co-workers about potential coronavirus hazards.
But at the same time, Ms. Berkowitz and the National Employment Law Project found that OSHA dismissed more than half of the COVID-related complaints of retaliation that it received from whistleblowers. About 2% of complaints were resolved during a 5-month period last year, according to their report.
As the vaccine mandate deadline approaches, most companies are expected to comply, experts told the AP. Some employers wanted to require the shot but didn’t want to create their own rule, and others have said they’ll follow OSHA regulations as they always do.
“Most employers, they’re law abiding,” David Michaels, a former OSHA chief who is a public health professor at George Washington University, told the AP.
“They’re trying to make sure that they meet the requirements of every law and regulation,” he said. “Now OSHA will follow up. They’ll respond to complaints. They’ll do spot checks. They’ll issue citations and fines, and they’ll make a big deal of those.”
A version of this article first appeared on WebMD.com.
goes into effect in January.
The Occupational Safety and Health Administration (OSHA) doesn’t have enough workplace safety inspectors to cover the nation, the Associated Press reported, so the agency will count on people within organizations to identify violations.
“There is no army of OSHA inspectors that is going to be knocking on employers’ doors or even calling them,” Debbie Berkowitz, a former OSHA chief of staff who is a fellow at Georgetown University, told the news service.
“They’re going to rely on workers and their union representatives to file complaints where the company is totally flouting the law,” she said.
Last week, OSHA published the details of the Biden administration’s vaccine mandate. Companies with more than 100 employees must require their workers to get vaccinated or undergo weekly testing. Companies that don’t comply could face fines of $14,000 for each “serious” violation. Repeat violators could face 10 times that amount.
Employees who are concerned about workplace safety, unvaccinated co-workers, or people not being tested as required may report their employers, according to Reuters.
Jim Frederick, the acting chief for OSHA, told reporters that the agency will focus on job sites “where workers need assistance to have a safe and healthy workplace.”
“That typically comes through in the form of a complaint,” he said.
OSHA has jurisdiction in 29 states, the AP reported. OSHA is tasked with addressing violations of the Occupational Safety and Health Act of 1970, which is meant to create safe workplaces, and the agency has updated its guidance about COVID-19 safety in the workplace throughout this year.
Other states, such as California and Michigan, have their own workplace safety agencies, which will have until February to adopt their own version of a vaccine mandate, according to the AP.
OSHA and state counterparts will be tasked with enforcing the mandate, and their agencies are already short-staffed. About 1,850 inspectors will oversee 130 million workers at 8 million job sites.
OSHA has encouraged workers to first report complaints to employers “if possible.” Otherwise, employees can file a confidential safety complaint with OSHA or file a case through a representative, such as a lawyer or union leader, the AP reported.
But workplace experts have voiced caution about the potential risks of reporting. Whistleblowers tend to face retaliation and OSHA can’t always offer protection in these cases.
“Technically, the law says that companies can’t retaliate against a worker for raising a health and safety issue or filing an OSHA complaint or even reporting an injury,” Ms. Berkowitz said. “But retaliation is rampant.”
OSHA has some jurisdiction to pursue employers who punish workers for reporting unsafe working conditions, the AP reported. Last month, the agency sued a luxury car dealer in Texas for firing an employee who warned co-workers about potential coronavirus hazards.
But at the same time, Ms. Berkowitz and the National Employment Law Project found that OSHA dismissed more than half of the COVID-related complaints of retaliation that it received from whistleblowers. About 2% of complaints were resolved during a 5-month period last year, according to their report.
As the vaccine mandate deadline approaches, most companies are expected to comply, experts told the AP. Some employers wanted to require the shot but didn’t want to create their own rule, and others have said they’ll follow OSHA regulations as they always do.
“Most employers, they’re law abiding,” David Michaels, a former OSHA chief who is a public health professor at George Washington University, told the AP.
“They’re trying to make sure that they meet the requirements of every law and regulation,” he said. “Now OSHA will follow up. They’ll respond to complaints. They’ll do spot checks. They’ll issue citations and fines, and they’ll make a big deal of those.”
A version of this article first appeared on WebMD.com.
goes into effect in January.
The Occupational Safety and Health Administration (OSHA) doesn’t have enough workplace safety inspectors to cover the nation, the Associated Press reported, so the agency will count on people within organizations to identify violations.
“There is no army of OSHA inspectors that is going to be knocking on employers’ doors or even calling them,” Debbie Berkowitz, a former OSHA chief of staff who is a fellow at Georgetown University, told the news service.
“They’re going to rely on workers and their union representatives to file complaints where the company is totally flouting the law,” she said.
Last week, OSHA published the details of the Biden administration’s vaccine mandate. Companies with more than 100 employees must require their workers to get vaccinated or undergo weekly testing. Companies that don’t comply could face fines of $14,000 for each “serious” violation. Repeat violators could face 10 times that amount.
Employees who are concerned about workplace safety, unvaccinated co-workers, or people not being tested as required may report their employers, according to Reuters.
Jim Frederick, the acting chief for OSHA, told reporters that the agency will focus on job sites “where workers need assistance to have a safe and healthy workplace.”
“That typically comes through in the form of a complaint,” he said.
OSHA has jurisdiction in 29 states, the AP reported. OSHA is tasked with addressing violations of the Occupational Safety and Health Act of 1970, which is meant to create safe workplaces, and the agency has updated its guidance about COVID-19 safety in the workplace throughout this year.
Other states, such as California and Michigan, have their own workplace safety agencies, which will have until February to adopt their own version of a vaccine mandate, according to the AP.
OSHA and state counterparts will be tasked with enforcing the mandate, and their agencies are already short-staffed. About 1,850 inspectors will oversee 130 million workers at 8 million job sites.
OSHA has encouraged workers to first report complaints to employers “if possible.” Otherwise, employees can file a confidential safety complaint with OSHA or file a case through a representative, such as a lawyer or union leader, the AP reported.
But workplace experts have voiced caution about the potential risks of reporting. Whistleblowers tend to face retaliation and OSHA can’t always offer protection in these cases.
“Technically, the law says that companies can’t retaliate against a worker for raising a health and safety issue or filing an OSHA complaint or even reporting an injury,” Ms. Berkowitz said. “But retaliation is rampant.”
OSHA has some jurisdiction to pursue employers who punish workers for reporting unsafe working conditions, the AP reported. Last month, the agency sued a luxury car dealer in Texas for firing an employee who warned co-workers about potential coronavirus hazards.
But at the same time, Ms. Berkowitz and the National Employment Law Project found that OSHA dismissed more than half of the COVID-related complaints of retaliation that it received from whistleblowers. About 2% of complaints were resolved during a 5-month period last year, according to their report.
As the vaccine mandate deadline approaches, most companies are expected to comply, experts told the AP. Some employers wanted to require the shot but didn’t want to create their own rule, and others have said they’ll follow OSHA regulations as they always do.
“Most employers, they’re law abiding,” David Michaels, a former OSHA chief who is a public health professor at George Washington University, told the AP.
“They’re trying to make sure that they meet the requirements of every law and regulation,” he said. “Now OSHA will follow up. They’ll respond to complaints. They’ll do spot checks. They’ll issue citations and fines, and they’ll make a big deal of those.”
A version of this article first appeared on WebMD.com.
FDA posts new websites on accelerated approvals for cancer drugs
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
Updates to CDC’s STI guidelines relevant to midlife women too
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
FROM NAMS 2021
Want to see what COVID strain you have? The government says no
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Biden vaccine mandate rule could be ready within weeks
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
FDA inaction on hair loss drug’s suicide, depression, erectile dysfunction risk sparks lawsuit
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Military Medical Teams Deploy to Relieve COVID-Battered Hospitals
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”
Last summer, a team of US Department of Veterans Affairs (VA) health care professionals deployed to Alabama’s Bill Nichols State Veterans Home to help during the COVID-19 crisis. They were there as part of the “Fourth Mission”—supporting national, state, and local emergency management, public health, safety and homeland security efforts. “It was a really humbling experience,” said Mary Holloway, an RN with the Birmingham VA Health Care System. “Seeing the dedication of the staff there, some coming back to work after recovering from COVID themselves, was inspiring.”
But that turned out to be only one battle in a sadly long and drawn-out war. Since March 2020, more than 5,000 military medical personnel have deployed to 14 states and the Navajo Nation, 51 cities, 71 hospitals, all struggling to keep their heads above a cresting tsunami of new COVID patients.
Last year, the crisis spots for deployments included major metropolitan areas in coastal states: New York, California, and New Jersey. The urgency now is in the Southern states. Those tend to be reporting the highest numbers of new cases and deaths. Alabama, Arkansas, Florida, Louisiana, and Mississippi, for example, have all ranked among the highest rates of cases and hospitalizations per 100,000 people across the country in the last seven days.
This year, military teams have also deployed to support vaccination centers in 25 states and 42 cities. Nearly all—97%—of the new COVID patients in recent months are unvaccinated. And, again, they predominate in Southern states. In Alabama, for instance, only 37% of the population are fully vaccinated. In Louisiana, that number is 40%.
The at-risk states also tend to be the ones that are rapidly running out of space to put the patients in, ICU or otherwise. Where patients who might have been in the intensive care unit (ICU) are housed in the emergency department and in hallways, and where patients without COVID-19 who might have been hospitalized are being turned away. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
These states are at a breaking point. Take Alabama. On August 18, it was “negative 11.” It had 1,568 patients with COVID-19 who needed ICU beds. Only 1,557 beds were available. Patients “may even stay on the regular floor where you’re already stretched for capacity to take care of these people because so many of our staff are out with COVID,” Jeanne Marrazzo, director, Division of Infectious Diseases at the University of Alabama at Birmingham, told a CNN reporter. “It’s really just a domino effect that then clogs up our ERs, clogs up everything else. … It’s a very very tenuous situation.”
The state reported more than 4,000 new cases of COVID-19—“a new high for us,” Marrazzo said. “If you project these numbers out, you can expect that we will at some point, probably around Sept. 1, have at least 5,000 people in our hospitals. If the ratio of people who have to go to the ICU remains stable. That means that probably a third of those people are going to require ICU beds,” she continued. “That is frankly untenable, given the infrastructure, the resources, and really importantly, the staff that we have. I think it is basically apocalyptic. I do not use that word lightly.”
Thus, the US Defense Department (DoD) must once again rise to a sad and desperate occasion. At the request of Federal Emergency Management Agency and the state of Louisiana, the first of five teams of Navy doctors, nurses, and respiratory therapists were sent last week to Ochsner Lafayette General Medical Center in Lafayette, Louisiana.
The teams, consisting of approximately 20 members each, are coming from throughout the DoD’s universe, including the National Guard. US Army North, under US Northern Command’s oversight, is providing operational command of the active-duty military COVID-19 response. Lt. Gen. Laura J. Richardson, ARNORTH commander, noting that “[t]his is the second time Department of Defense medical assets have deployed to support Louisiana during the pandemic,” calls it a “whole-of-government fight against COVID-19.”
Why Louisiana and Mississippi, with so many states in dire need? “Our joint forces go where FEMA needs us,” Richardson says. “[R]ight now FEMA has determined the military’s unique surge capabilities are most needed in these two states.”
In a press briefing at the time, Pentagon Press Secretary Rear Adm. John Kirby said, “We expect that there could be additional requests from other states for other teams, so that’s why we’re being prepared to stand up five teams.” He was right: An Air Force team has now headed to Our Lady of the Lake Regional Medical Center in Baton Rouge. Mississippi also asked for assistance; an Air Force team will be supporting at University of Mississippi Medical Center in Jackson, and an Army team at North Mississippi Medical Center-Tupelo.
The support will likely include bolstering and extending the infrastructure. From July to December 2020, the Veterans Health Administration (VHA) Emergency Management Coordination Cell delivered Fold-Out Rigid Temporary Shelters (FORTS), C-FORTS (clinics), mobile ICUs and isolation units to locations across the US, such as North Chicago, El Paso, and Oklahoma City. In 2021, they’ll be needed in more hospitals unprepared to house the spiking numbers of patients. Some Louisiana hospitals, for instance, have been sending patients in ambulances to Texas for care.
The first go-round with COVID taught hard lessons that can help hone the Fourth Mission responses. One lesson, according to the VHA COVID-19 Response Report- Annex A, published this May, was the need to conduct due diligence, to be both efficient and effective. VHA, it says, now works to determine actual need before deploying resources. “For example, VHA might receive a request from a [State Veterans Home] for 50 RNs. But once VHA delved into the request and worked with the associated VISNs, it would find that 20 RNs or 10 LPNs could meet the needs of the request.”
Meeting the requests is, for the beleaguered hospitals, like answering letters to Santa. When the team of doctors, nurses, and respiratory therapists arrived at Ochsner Lafayette General Medical Center (OLGMC) last week the hospital staff greeted them with cheers and applause.
OLGMC CEO Al Patin said, "We're already in a nursing shortage, coupled with high numbers of this pandemic [which] creates a situation where we need additional support. We have patients boarding in our emergency rooms, patients in our ICU setting that can't transition out. That creates a bottleneck and does not allow us to continue to take in patients from our community."
That day, OLG posted on Twitter:
“Today, we received some much-needed assistance in the fight against COVID-19. Our team at Ochsner Lafayette General Medical Center is being expanded by four doctors, 14 nurses and two respiratory therapists – all highly trained personnel on loan from the U.S. Navy.
“These healthcare professionals are being onboarded in our facility today and are specially trained for the emergency department, ICU and Med Surg. Because of them, we’ll be able to staff an additional 16-18 beds – beds sorely needed as cases continue to rise in our area.
“We requested support from the Federal Emergency Management Agency and we were one of five U.S. cities to receive it.. We are most grateful and humbled.”