User login
Oropouche Virus
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
Predicting RSV’s Role in the Upcoming Winter Respiratory Season
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
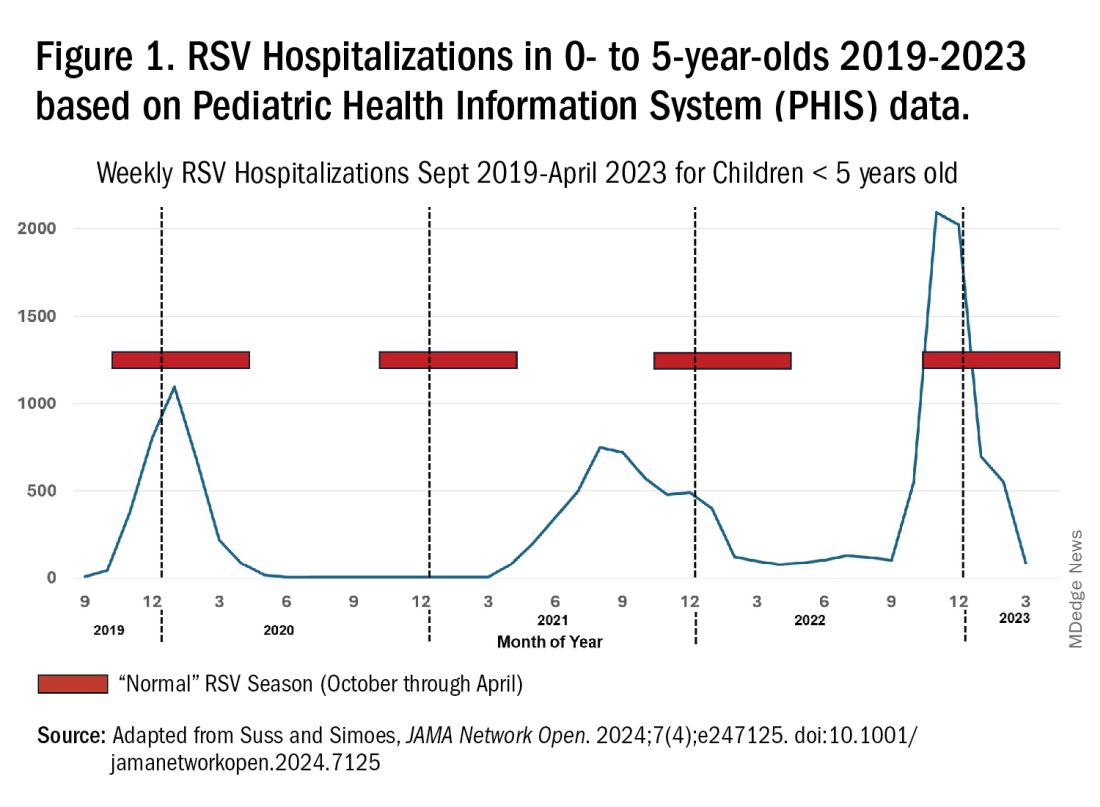
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
Summer Is Not Over: Let's Talk About Recreational Water–Associated Illnesses
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Predicting and Understanding Vaccine Response Determinants
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
Highly Pathogenic Avian Influenza (HPAI)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Worldwide Uptick in Invasive Group A Streptococcus Disease Post Pandemic — What Should We Know?
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Summertime and Mosquitoes Are Breeding
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia.Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases.Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia.Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases.Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia.Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases.Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
Microbiome Impacts Vaccine Responses
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
A Tale of Two Babies and the ‘Family Tragedy’ of Congenital Syphilis
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Laissez-faire
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.