User login
Underlying Mental Illness and Risk of Severe Outcomes Associated With COVID-19
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
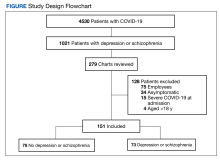
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
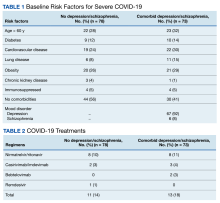
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
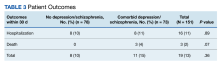
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
Trauma-Informed Telehealth in the COVID-19 Era and Beyond
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
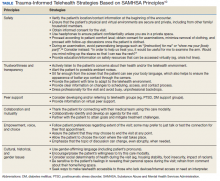
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
Methylenetetrahydrofolate Reductase Screening in Treatment-Resistant Depression
Therapeutic response to antidepressant drugs is often partial. Multiple trials of medications may be prescribed before a patient achieves remission of symptoms. Further, no universally accepted definition for treatment-resistant depression (TRD) has been established. The most commonly proposed definition (and the definition used in this article) is the failure to achieve remission with 2 or more adequate antidepressant treatments.1
About 20% to 30% of patients with depression are treatment resistant. The overall Canada-wide prevalence of TRD in primary care was 21.7%.2 In the US, about 15.7 million adults have had at least 1 major depressive episode in the past year, and 10% to 15% of major depressive disorder (MDD) cases can be classified as treatment resistant.3,4 In a retrospective, longitudinal cohort analysis in a Medicaid population, 25.9% of pharmacologically treated adults with MDD met criteria for TRD.5 Similarly, TRD in this review was defined as starting a third treatment regimen after 2 adequate regimens of antidepressants.
Why is this important? Treatment resistance is often associated with high rates of disability and comorbidity. Given the significant prevalence and impact of TRD, research into better understanding and treating these patients is paramount. Pharmacogenetics has been proposed for tailoring therapy and theoretically circumventing treatment resistance to achieve better outcomes.
Methylenetetrahydrofolate reductase (MTHFR) is a gene that encodes an enzyme similarly called MTHFR. The enzyme converts 5,10-MTHF to 5-MTHF. 5-MTHF then donates a methyl group in the conversion of homocysteine to methionine. Decreased or absent expression of MTHFR leads to decreased levels of 5-MTHF, which then leads to high levels of homocysteine. This results in suboptimal production of monoamines, including serotonin, dopamine, and norepinephrine as well as subsequent abnormalities in neural and vascular pathways.6
Screening for MTHFR polymorphisms has been proposed in past years due to weak associations with conditions such as cardiac disease, poor pregnancy outcomes, and colorectal cancer.7 Recently, an increasing number of studies suggest screening for MTHFR polymorphisms in patients with depression. This proposal is based on demonstrated links between abnormal folate metabolism and high levels of homocysteine and an increased risk for MDD and reduced antidepressant effectiveness.
In a meta-analysis by Wu and colleagues of 26 published studies, including 4,992 depression cases and 17,082 controls, MTHFR C677T polymorphism was associated with an increased risk of depression especially in Asian populations. This relationship was not observed in the elderly.8 A more recent article reviewing 6 small studies from 2005 to 2016 suggested that the MTHFR A1298C polymorphism (via abnormal homocysteine metabolism and folate cycles) may play a role in identifying those at risk of developing MDD particularly women in white populations.9
As the proposed mechanism of treatment resistance associated with the MTHFR polymorphisms seems to be related to folate metabolism, L-methylfolate supplementation has been recommended. In a 60-day randomized trial of a selective serotonin reuptake inhibitor (SSRI) and L-methylfolate vs SSRI and placebo, patients prescribed an SSRI with L-methylfolate had a greater response rate (reduction of baseline symptoms by at least 50%) that was statistically significant (P = .04) vs patients taking the placebo.10
In primary care and specialty settings, screening patients with TRD for MTHFR polymorphisms has been proposed. LabCorp (Burlington, NC) and Quest Diagnostics (Secaucus, NJ) have a DNA assay that detects C677T and A1298C mutations in the MTHFR gene, using whole blood samples; however, the cost is high. In the DC/Maryland/Virginia region, test cost varies from $390 if the patient requests it from the lab to $325 if requested through an institution that has an account with LabCorp. Although there are little data regarding false positive and false negative rates, 1 source suggested an analytic sensitivity and specificity of 99% for the tests.11
Once obtained, positive screening results may assist in directing next steps in terms of adjunctive or next-line therapies. Given the high price of the test and positive responses with L-methylfolate supplementation thus far, the question remains: Why not supplement patients with TRD with folate and forego screening? For these 2 reasons: The treatment dosage in the studies referenced is 15 mg of L-methylfolate. This dosage is often unavailable over-the-counter and can cost as much as $75 for 90 capsules. Additionally, the high dosage of methylfolate may increase the risk of colon cancer in certain subpopulations, such as those with precancerous lesions.12Although the current data seem promising, further research is needed to explore the benefits of folate supplementation in larger study samples and perhaps other targeted treatment options for patients with TRD with MTHFR gene polymorphisms.
1. McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1-7.
2. Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349-357.
3. Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739-744.
4. Little A. Treatment-resistant depression. Am Fam Physician. 2009;80(2):167-172.
5. Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of Medicaid beneficiaries with treatment resistant depression. J Manag Care Spec Pharm. 2018;24(3):226-236.
6. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228-232.
7. Long S, Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Aust Fam Physician. 2016;45(4):237-240.
8. Wu YL, Ding XX, Sun YH, et al. Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:78-85.
9. Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Methylenetetrahydrogolate reductase A1298C polymorphism and major depressive disorder. Cureus. 2017;9(10):e1734.
10. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
11. Hickey SE, Curry CJ, Toriello HV. ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013;15(2):153-156.
12. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
Therapeutic response to antidepressant drugs is often partial. Multiple trials of medications may be prescribed before a patient achieves remission of symptoms. Further, no universally accepted definition for treatment-resistant depression (TRD) has been established. The most commonly proposed definition (and the definition used in this article) is the failure to achieve remission with 2 or more adequate antidepressant treatments.1
About 20% to 30% of patients with depression are treatment resistant. The overall Canada-wide prevalence of TRD in primary care was 21.7%.2 In the US, about 15.7 million adults have had at least 1 major depressive episode in the past year, and 10% to 15% of major depressive disorder (MDD) cases can be classified as treatment resistant.3,4 In a retrospective, longitudinal cohort analysis in a Medicaid population, 25.9% of pharmacologically treated adults with MDD met criteria for TRD.5 Similarly, TRD in this review was defined as starting a third treatment regimen after 2 adequate regimens of antidepressants.
Why is this important? Treatment resistance is often associated with high rates of disability and comorbidity. Given the significant prevalence and impact of TRD, research into better understanding and treating these patients is paramount. Pharmacogenetics has been proposed for tailoring therapy and theoretically circumventing treatment resistance to achieve better outcomes.
Methylenetetrahydrofolate reductase (MTHFR) is a gene that encodes an enzyme similarly called MTHFR. The enzyme converts 5,10-MTHF to 5-MTHF. 5-MTHF then donates a methyl group in the conversion of homocysteine to methionine. Decreased or absent expression of MTHFR leads to decreased levels of 5-MTHF, which then leads to high levels of homocysteine. This results in suboptimal production of monoamines, including serotonin, dopamine, and norepinephrine as well as subsequent abnormalities in neural and vascular pathways.6
Screening for MTHFR polymorphisms has been proposed in past years due to weak associations with conditions such as cardiac disease, poor pregnancy outcomes, and colorectal cancer.7 Recently, an increasing number of studies suggest screening for MTHFR polymorphisms in patients with depression. This proposal is based on demonstrated links between abnormal folate metabolism and high levels of homocysteine and an increased risk for MDD and reduced antidepressant effectiveness.
In a meta-analysis by Wu and colleagues of 26 published studies, including 4,992 depression cases and 17,082 controls, MTHFR C677T polymorphism was associated with an increased risk of depression especially in Asian populations. This relationship was not observed in the elderly.8 A more recent article reviewing 6 small studies from 2005 to 2016 suggested that the MTHFR A1298C polymorphism (via abnormal homocysteine metabolism and folate cycles) may play a role in identifying those at risk of developing MDD particularly women in white populations.9
As the proposed mechanism of treatment resistance associated with the MTHFR polymorphisms seems to be related to folate metabolism, L-methylfolate supplementation has been recommended. In a 60-day randomized trial of a selective serotonin reuptake inhibitor (SSRI) and L-methylfolate vs SSRI and placebo, patients prescribed an SSRI with L-methylfolate had a greater response rate (reduction of baseline symptoms by at least 50%) that was statistically significant (P = .04) vs patients taking the placebo.10
In primary care and specialty settings, screening patients with TRD for MTHFR polymorphisms has been proposed. LabCorp (Burlington, NC) and Quest Diagnostics (Secaucus, NJ) have a DNA assay that detects C677T and A1298C mutations in the MTHFR gene, using whole blood samples; however, the cost is high. In the DC/Maryland/Virginia region, test cost varies from $390 if the patient requests it from the lab to $325 if requested through an institution that has an account with LabCorp. Although there are little data regarding false positive and false negative rates, 1 source suggested an analytic sensitivity and specificity of 99% for the tests.11
Once obtained, positive screening results may assist in directing next steps in terms of adjunctive or next-line therapies. Given the high price of the test and positive responses with L-methylfolate supplementation thus far, the question remains: Why not supplement patients with TRD with folate and forego screening? For these 2 reasons: The treatment dosage in the studies referenced is 15 mg of L-methylfolate. This dosage is often unavailable over-the-counter and can cost as much as $75 for 90 capsules. Additionally, the high dosage of methylfolate may increase the risk of colon cancer in certain subpopulations, such as those with precancerous lesions.12Although the current data seem promising, further research is needed to explore the benefits of folate supplementation in larger study samples and perhaps other targeted treatment options for patients with TRD with MTHFR gene polymorphisms.
Therapeutic response to antidepressant drugs is often partial. Multiple trials of medications may be prescribed before a patient achieves remission of symptoms. Further, no universally accepted definition for treatment-resistant depression (TRD) has been established. The most commonly proposed definition (and the definition used in this article) is the failure to achieve remission with 2 or more adequate antidepressant treatments.1
About 20% to 30% of patients with depression are treatment resistant. The overall Canada-wide prevalence of TRD in primary care was 21.7%.2 In the US, about 15.7 million adults have had at least 1 major depressive episode in the past year, and 10% to 15% of major depressive disorder (MDD) cases can be classified as treatment resistant.3,4 In a retrospective, longitudinal cohort analysis in a Medicaid population, 25.9% of pharmacologically treated adults with MDD met criteria for TRD.5 Similarly, TRD in this review was defined as starting a third treatment regimen after 2 adequate regimens of antidepressants.
Why is this important? Treatment resistance is often associated with high rates of disability and comorbidity. Given the significant prevalence and impact of TRD, research into better understanding and treating these patients is paramount. Pharmacogenetics has been proposed for tailoring therapy and theoretically circumventing treatment resistance to achieve better outcomes.
Methylenetetrahydrofolate reductase (MTHFR) is a gene that encodes an enzyme similarly called MTHFR. The enzyme converts 5,10-MTHF to 5-MTHF. 5-MTHF then donates a methyl group in the conversion of homocysteine to methionine. Decreased or absent expression of MTHFR leads to decreased levels of 5-MTHF, which then leads to high levels of homocysteine. This results in suboptimal production of monoamines, including serotonin, dopamine, and norepinephrine as well as subsequent abnormalities in neural and vascular pathways.6
Screening for MTHFR polymorphisms has been proposed in past years due to weak associations with conditions such as cardiac disease, poor pregnancy outcomes, and colorectal cancer.7 Recently, an increasing number of studies suggest screening for MTHFR polymorphisms in patients with depression. This proposal is based on demonstrated links between abnormal folate metabolism and high levels of homocysteine and an increased risk for MDD and reduced antidepressant effectiveness.
In a meta-analysis by Wu and colleagues of 26 published studies, including 4,992 depression cases and 17,082 controls, MTHFR C677T polymorphism was associated with an increased risk of depression especially in Asian populations. This relationship was not observed in the elderly.8 A more recent article reviewing 6 small studies from 2005 to 2016 suggested that the MTHFR A1298C polymorphism (via abnormal homocysteine metabolism and folate cycles) may play a role in identifying those at risk of developing MDD particularly women in white populations.9
As the proposed mechanism of treatment resistance associated with the MTHFR polymorphisms seems to be related to folate metabolism, L-methylfolate supplementation has been recommended. In a 60-day randomized trial of a selective serotonin reuptake inhibitor (SSRI) and L-methylfolate vs SSRI and placebo, patients prescribed an SSRI with L-methylfolate had a greater response rate (reduction of baseline symptoms by at least 50%) that was statistically significant (P = .04) vs patients taking the placebo.10
In primary care and specialty settings, screening patients with TRD for MTHFR polymorphisms has been proposed. LabCorp (Burlington, NC) and Quest Diagnostics (Secaucus, NJ) have a DNA assay that detects C677T and A1298C mutations in the MTHFR gene, using whole blood samples; however, the cost is high. In the DC/Maryland/Virginia region, test cost varies from $390 if the patient requests it from the lab to $325 if requested through an institution that has an account with LabCorp. Although there are little data regarding false positive and false negative rates, 1 source suggested an analytic sensitivity and specificity of 99% for the tests.11
Once obtained, positive screening results may assist in directing next steps in terms of adjunctive or next-line therapies. Given the high price of the test and positive responses with L-methylfolate supplementation thus far, the question remains: Why not supplement patients with TRD with folate and forego screening? For these 2 reasons: The treatment dosage in the studies referenced is 15 mg of L-methylfolate. This dosage is often unavailable over-the-counter and can cost as much as $75 for 90 capsules. Additionally, the high dosage of methylfolate may increase the risk of colon cancer in certain subpopulations, such as those with precancerous lesions.12Although the current data seem promising, further research is needed to explore the benefits of folate supplementation in larger study samples and perhaps other targeted treatment options for patients with TRD with MTHFR gene polymorphisms.
1. McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1-7.
2. Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349-357.
3. Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739-744.
4. Little A. Treatment-resistant depression. Am Fam Physician. 2009;80(2):167-172.
5. Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of Medicaid beneficiaries with treatment resistant depression. J Manag Care Spec Pharm. 2018;24(3):226-236.
6. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228-232.
7. Long S, Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Aust Fam Physician. 2016;45(4):237-240.
8. Wu YL, Ding XX, Sun YH, et al. Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:78-85.
9. Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Methylenetetrahydrogolate reductase A1298C polymorphism and major depressive disorder. Cureus. 2017;9(10):e1734.
10. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
11. Hickey SE, Curry CJ, Toriello HV. ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013;15(2):153-156.
12. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
1. McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1-7.
2. Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349-357.
3. Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739-744.
4. Little A. Treatment-resistant depression. Am Fam Physician. 2009;80(2):167-172.
5. Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of Medicaid beneficiaries with treatment resistant depression. J Manag Care Spec Pharm. 2018;24(3):226-236.
6. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228-232.
7. Long S, Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Aust Fam Physician. 2016;45(4):237-240.
8. Wu YL, Ding XX, Sun YH, et al. Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:78-85.
9. Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Methylenetetrahydrogolate reductase A1298C polymorphism and major depressive disorder. Cureus. 2017;9(10):e1734.
10. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
11. Hickey SE, Curry CJ, Toriello HV. ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013;15(2):153-156.
12. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
Overemphasizing Communities in the National Strategy for Preventing Veteran Suicide Could Undercut VA Successes
In June 2018, the US Department of Veterans Affairs (VA) issued its National Strategy for Preventing Veteran Suicide, 2018-2028. Its 14 goals—many highly innovative—are “to provide a framework for identifying priorities, organizing efforts, and contributing to a national focus on Veteran suicide prevention.”1
The National Strategy recognizes that suicide prevention requires a 3-pronged approach that includes universal, selective, and targeted strategies because “suicide cannot be prevented by any single strategy.”1 Even so, the National Strategy does not heed this core tenet. It focuses exclusively on universal, non-VA community-based priorities and efforts. That focus causes a problem because it neglects the other strategies. It also is precarious because in the current era of VA zero sum budgets, increases in 1 domain come from decreases in another. Thus, sole prioritizing of universal community components could divert funds from extant effective VA suicide prevention programs.
Community-based engagement is unquestionably necessary to prevent suicide among all veterans. Even so, a 10-year prospective strategy should build up, not compromise, VA initiatives. The plan would be improved by explicitly bolstering VA programs that are making a vital difference.
Undercutting VA Suicide Prevention
As my recent review in Federal Practitioner documented, VA’s multiple levels of evidenced-based suicide prevention practices are pre-eminent in the field.2 The VA’s innovative use of predictive analytics to identify and intervene with at-risk individuals is more advanced than anything available in the community. For older veterans who constitute the majority of veterans and the majority of veteran suicides, the VA has more comprehensive and integrated mental health care services than those found in community-based care systems. The embedding of suicide prevention coordinators at every VA facility is unparalleled.
But one would never know about such quality from the National Strategy document: The VA is barely mentioned. The report never advocates for strengthening—or even maintaining—VA’s resources, programs, and efforts. It never recommends that eligible veterans be connected to VA mental health services.
The strategy observes that employment and housing are keys that protect against suicide risk. It does not, however, call for boosting and resourcing VA’s integrated approach that wraps in social services better than does any other program. Similarly, it acknowledges the role of family involvement in mitigating risk but does not propose expanding VA treatments to improve relationship well-being, leaving these services to the private sector.
The National Strategy expands on the recent suicide prevention executive order (EO) for supporting veterans during their transition from military to civilian life. Yet the EO has no funding allocated to this critical initiative. The National Strategy has the same shortcoming. In failing to advocate for more funds to pay for vastly enhanced outreach and intervention, the plan could drain the VA of existing resources needed to maintain its high-quality, suicide prevention services.
First Step: Define the Problem
The National Strategy wisely specifies that the initial step in any suicide prevention effort should be to “define the problem. This involves collecting data to determine the ‘who,’ ‘what,’ ‘where,’ ‘when,’ and ‘how’ of suicide deaths.” Then, “identify risk and protective factors.”
Yet the report doesn’t follow its own advice. Although little is known about the 14 of 20 veterans who die by suicide daily who are not recent users of VA health services, the National Strategy foregoes the necessity of first ascertaining crucial factors, including whether those veterans were (a) eligible for VA care; (b) receiving any mental health or substance use treatment; and (c) going through life crises, etc. What’s needed before reallocating funds to community-based programs is for Congress to finance a post-suicide, case-by-case study of these veteran decedents who did not use VA.
Proceeding in this manner has 2 benefits. First, it would allow initiatives to be targeted. Second, it could preserve funds for successful VA programs that otherwise might be cut to pay for private sector programs.
A Positive Starting Point
There are many positive components of the National Strategy for Preventing Veteran Suicide that will make a difference. That said, they fall short of their potential. The following are suggestions that could strengthen the VA’s plan.
First, given the overwhelming use of firearms by veterans who die by suicide, the National Strategy acknowledges that an effective prevention policy must attend to this factor. It prudently calls for expansion of firearm safety/suicide prevention collaboration with firearm owners, firearm dealers, shooting clubs, and gun/hunting organizations. This will help ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and has no antifirearm bias.
Nothing would be more useful in diminishing suicide than correcting the false belief among many veterans that “the VA wants to take away our guns.” If that misperception were replaced with an accurate message, not only would more at-risk veterans seek out VA mental health care, more veterans/families/friends would adopt a new cultural norm akin to buddies talk to vets in crisis about safely storing guns. Establishing a workgroup with gun constituency collaborators could spearhead such a shift.
Second, although, the National Strategy emphasizes the benefits of using peer supports, peers currently express qualms that they have too little expertise intervening with this vulnerable population. Peers could be given extensive training and continued supervision in suicide prevention techniques.
Third, the National Strategy calls for expanded use of big data predictive analytics, whose initial implementation has shown great promise. However, it fails to mention that this approach depends on linked electronic health records and therefore best succeeds for at-risk veterans within VA but not in insulated community care.
Fourth, the National Strategy recognizes that reshaping media and entertainment portrayals could help prevent veteran suicide. Yet it ignores the importance of correcting the sullied narrative about the VA. The disproportionate negative image contributes to veterans’ reticence to seek VA health care. One simple solution would be to require that service members readying to transition to civilian life be informed about the superior nature of VA mental health care. Another is to provide the media with positive VA stories more routinely.
Fifth, the National Strategy suggests that enhanced community care guidelines be developed, but it never recommends that community partners should equal VA’s standards. Those providers should be mandated to conduct the same root cause analyses and comprehensive documentation of suicide risk assessments that VA does.
Conclusion
With zero sum department budgets, the National Strategy’s exclusive priority on public health, community-based initiatives could undercut VA successes. An amended plan that explicitly supports and further strengthens successful VA suicide prevention programs is warranted.
1. US Department of Veterans Affairs. National Strategy for Preventing Veteran Suicide, 2018-2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published June 2018. Accessed November 6, 2018.
2. Lemle RB. Choice program expansion jeopardizes high-quality VHA mental health Services. Fed Pract. 2018;35(3):18-24.
In June 2018, the US Department of Veterans Affairs (VA) issued its National Strategy for Preventing Veteran Suicide, 2018-2028. Its 14 goals—many highly innovative—are “to provide a framework for identifying priorities, organizing efforts, and contributing to a national focus on Veteran suicide prevention.”1
The National Strategy recognizes that suicide prevention requires a 3-pronged approach that includes universal, selective, and targeted strategies because “suicide cannot be prevented by any single strategy.”1 Even so, the National Strategy does not heed this core tenet. It focuses exclusively on universal, non-VA community-based priorities and efforts. That focus causes a problem because it neglects the other strategies. It also is precarious because in the current era of VA zero sum budgets, increases in 1 domain come from decreases in another. Thus, sole prioritizing of universal community components could divert funds from extant effective VA suicide prevention programs.
Community-based engagement is unquestionably necessary to prevent suicide among all veterans. Even so, a 10-year prospective strategy should build up, not compromise, VA initiatives. The plan would be improved by explicitly bolstering VA programs that are making a vital difference.
Undercutting VA Suicide Prevention
As my recent review in Federal Practitioner documented, VA’s multiple levels of evidenced-based suicide prevention practices are pre-eminent in the field.2 The VA’s innovative use of predictive analytics to identify and intervene with at-risk individuals is more advanced than anything available in the community. For older veterans who constitute the majority of veterans and the majority of veteran suicides, the VA has more comprehensive and integrated mental health care services than those found in community-based care systems. The embedding of suicide prevention coordinators at every VA facility is unparalleled.
But one would never know about such quality from the National Strategy document: The VA is barely mentioned. The report never advocates for strengthening—or even maintaining—VA’s resources, programs, and efforts. It never recommends that eligible veterans be connected to VA mental health services.
The strategy observes that employment and housing are keys that protect against suicide risk. It does not, however, call for boosting and resourcing VA’s integrated approach that wraps in social services better than does any other program. Similarly, it acknowledges the role of family involvement in mitigating risk but does not propose expanding VA treatments to improve relationship well-being, leaving these services to the private sector.
The National Strategy expands on the recent suicide prevention executive order (EO) for supporting veterans during their transition from military to civilian life. Yet the EO has no funding allocated to this critical initiative. The National Strategy has the same shortcoming. In failing to advocate for more funds to pay for vastly enhanced outreach and intervention, the plan could drain the VA of existing resources needed to maintain its high-quality, suicide prevention services.
First Step: Define the Problem
The National Strategy wisely specifies that the initial step in any suicide prevention effort should be to “define the problem. This involves collecting data to determine the ‘who,’ ‘what,’ ‘where,’ ‘when,’ and ‘how’ of suicide deaths.” Then, “identify risk and protective factors.”
Yet the report doesn’t follow its own advice. Although little is known about the 14 of 20 veterans who die by suicide daily who are not recent users of VA health services, the National Strategy foregoes the necessity of first ascertaining crucial factors, including whether those veterans were (a) eligible for VA care; (b) receiving any mental health or substance use treatment; and (c) going through life crises, etc. What’s needed before reallocating funds to community-based programs is for Congress to finance a post-suicide, case-by-case study of these veteran decedents who did not use VA.
Proceeding in this manner has 2 benefits. First, it would allow initiatives to be targeted. Second, it could preserve funds for successful VA programs that otherwise might be cut to pay for private sector programs.
A Positive Starting Point
There are many positive components of the National Strategy for Preventing Veteran Suicide that will make a difference. That said, they fall short of their potential. The following are suggestions that could strengthen the VA’s plan.
First, given the overwhelming use of firearms by veterans who die by suicide, the National Strategy acknowledges that an effective prevention policy must attend to this factor. It prudently calls for expansion of firearm safety/suicide prevention collaboration with firearm owners, firearm dealers, shooting clubs, and gun/hunting organizations. This will help ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and has no antifirearm bias.
Nothing would be more useful in diminishing suicide than correcting the false belief among many veterans that “the VA wants to take away our guns.” If that misperception were replaced with an accurate message, not only would more at-risk veterans seek out VA mental health care, more veterans/families/friends would adopt a new cultural norm akin to buddies talk to vets in crisis about safely storing guns. Establishing a workgroup with gun constituency collaborators could spearhead such a shift.
Second, although, the National Strategy emphasizes the benefits of using peer supports, peers currently express qualms that they have too little expertise intervening with this vulnerable population. Peers could be given extensive training and continued supervision in suicide prevention techniques.
Third, the National Strategy calls for expanded use of big data predictive analytics, whose initial implementation has shown great promise. However, it fails to mention that this approach depends on linked electronic health records and therefore best succeeds for at-risk veterans within VA but not in insulated community care.
Fourth, the National Strategy recognizes that reshaping media and entertainment portrayals could help prevent veteran suicide. Yet it ignores the importance of correcting the sullied narrative about the VA. The disproportionate negative image contributes to veterans’ reticence to seek VA health care. One simple solution would be to require that service members readying to transition to civilian life be informed about the superior nature of VA mental health care. Another is to provide the media with positive VA stories more routinely.
Fifth, the National Strategy suggests that enhanced community care guidelines be developed, but it never recommends that community partners should equal VA’s standards. Those providers should be mandated to conduct the same root cause analyses and comprehensive documentation of suicide risk assessments that VA does.
Conclusion
With zero sum department budgets, the National Strategy’s exclusive priority on public health, community-based initiatives could undercut VA successes. An amended plan that explicitly supports and further strengthens successful VA suicide prevention programs is warranted.
In June 2018, the US Department of Veterans Affairs (VA) issued its National Strategy for Preventing Veteran Suicide, 2018-2028. Its 14 goals—many highly innovative—are “to provide a framework for identifying priorities, organizing efforts, and contributing to a national focus on Veteran suicide prevention.”1
The National Strategy recognizes that suicide prevention requires a 3-pronged approach that includes universal, selective, and targeted strategies because “suicide cannot be prevented by any single strategy.”1 Even so, the National Strategy does not heed this core tenet. It focuses exclusively on universal, non-VA community-based priorities and efforts. That focus causes a problem because it neglects the other strategies. It also is precarious because in the current era of VA zero sum budgets, increases in 1 domain come from decreases in another. Thus, sole prioritizing of universal community components could divert funds from extant effective VA suicide prevention programs.
Community-based engagement is unquestionably necessary to prevent suicide among all veterans. Even so, a 10-year prospective strategy should build up, not compromise, VA initiatives. The plan would be improved by explicitly bolstering VA programs that are making a vital difference.
Undercutting VA Suicide Prevention
As my recent review in Federal Practitioner documented, VA’s multiple levels of evidenced-based suicide prevention practices are pre-eminent in the field.2 The VA’s innovative use of predictive analytics to identify and intervene with at-risk individuals is more advanced than anything available in the community. For older veterans who constitute the majority of veterans and the majority of veteran suicides, the VA has more comprehensive and integrated mental health care services than those found in community-based care systems. The embedding of suicide prevention coordinators at every VA facility is unparalleled.
But one would never know about such quality from the National Strategy document: The VA is barely mentioned. The report never advocates for strengthening—or even maintaining—VA’s resources, programs, and efforts. It never recommends that eligible veterans be connected to VA mental health services.
The strategy observes that employment and housing are keys that protect against suicide risk. It does not, however, call for boosting and resourcing VA’s integrated approach that wraps in social services better than does any other program. Similarly, it acknowledges the role of family involvement in mitigating risk but does not propose expanding VA treatments to improve relationship well-being, leaving these services to the private sector.
The National Strategy expands on the recent suicide prevention executive order (EO) for supporting veterans during their transition from military to civilian life. Yet the EO has no funding allocated to this critical initiative. The National Strategy has the same shortcoming. In failing to advocate for more funds to pay for vastly enhanced outreach and intervention, the plan could drain the VA of existing resources needed to maintain its high-quality, suicide prevention services.
First Step: Define the Problem
The National Strategy wisely specifies that the initial step in any suicide prevention effort should be to “define the problem. This involves collecting data to determine the ‘who,’ ‘what,’ ‘where,’ ‘when,’ and ‘how’ of suicide deaths.” Then, “identify risk and protective factors.”
Yet the report doesn’t follow its own advice. Although little is known about the 14 of 20 veterans who die by suicide daily who are not recent users of VA health services, the National Strategy foregoes the necessity of first ascertaining crucial factors, including whether those veterans were (a) eligible for VA care; (b) receiving any mental health or substance use treatment; and (c) going through life crises, etc. What’s needed before reallocating funds to community-based programs is for Congress to finance a post-suicide, case-by-case study of these veteran decedents who did not use VA.
Proceeding in this manner has 2 benefits. First, it would allow initiatives to be targeted. Second, it could preserve funds for successful VA programs that otherwise might be cut to pay for private sector programs.
A Positive Starting Point
There are many positive components of the National Strategy for Preventing Veteran Suicide that will make a difference. That said, they fall short of their potential. The following are suggestions that could strengthen the VA’s plan.
First, given the overwhelming use of firearms by veterans who die by suicide, the National Strategy acknowledges that an effective prevention policy must attend to this factor. It prudently calls for expansion of firearm safety/suicide prevention collaboration with firearm owners, firearm dealers, shooting clubs, and gun/hunting organizations. This will help ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and has no antifirearm bias.
Nothing would be more useful in diminishing suicide than correcting the false belief among many veterans that “the VA wants to take away our guns.” If that misperception were replaced with an accurate message, not only would more at-risk veterans seek out VA mental health care, more veterans/families/friends would adopt a new cultural norm akin to buddies talk to vets in crisis about safely storing guns. Establishing a workgroup with gun constituency collaborators could spearhead such a shift.
Second, although, the National Strategy emphasizes the benefits of using peer supports, peers currently express qualms that they have too little expertise intervening with this vulnerable population. Peers could be given extensive training and continued supervision in suicide prevention techniques.
Third, the National Strategy calls for expanded use of big data predictive analytics, whose initial implementation has shown great promise. However, it fails to mention that this approach depends on linked electronic health records and therefore best succeeds for at-risk veterans within VA but not in insulated community care.
Fourth, the National Strategy recognizes that reshaping media and entertainment portrayals could help prevent veteran suicide. Yet it ignores the importance of correcting the sullied narrative about the VA. The disproportionate negative image contributes to veterans’ reticence to seek VA health care. One simple solution would be to require that service members readying to transition to civilian life be informed about the superior nature of VA mental health care. Another is to provide the media with positive VA stories more routinely.
Fifth, the National Strategy suggests that enhanced community care guidelines be developed, but it never recommends that community partners should equal VA’s standards. Those providers should be mandated to conduct the same root cause analyses and comprehensive documentation of suicide risk assessments that VA does.
Conclusion
With zero sum department budgets, the National Strategy’s exclusive priority on public health, community-based initiatives could undercut VA successes. An amended plan that explicitly supports and further strengthens successful VA suicide prevention programs is warranted.
1. US Department of Veterans Affairs. National Strategy for Preventing Veteran Suicide, 2018-2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published June 2018. Accessed November 6, 2018.
2. Lemle RB. Choice program expansion jeopardizes high-quality VHA mental health Services. Fed Pract. 2018;35(3):18-24.
1. US Department of Veterans Affairs. National Strategy for Preventing Veteran Suicide, 2018-2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published June 2018. Accessed November 6, 2018.
2. Lemle RB. Choice program expansion jeopardizes high-quality VHA mental health Services. Fed Pract. 2018;35(3):18-24.
Choice Program Expansion Jeopardizes High-Quality VHA Mental Health Services
Last summer, the Department of Veteran Affairs (VA) published the most comprehensive analysis of veteran suicide in our nation’s history. That study examined 55 million records from every state and revealed that in 2014, an average of 20 veterans died by suicide each day.1 Six of the 20 were recent users of Veterans Health Administration (VHA) services; the other 14 had not used VHA services in the prior 2 years.
Policy makers are currently deliberating whether expanding the Veterans Choice Program (VCP) is a judicious way to prevent these tragic deaths, especially for veterans who do not use the VHA. One proposal, presented at a congressional committee hearing in October 2017, advocates expanding the VCP.2 Its core tenet—allowing veterans to seek mental health care from VCP providers without needing VHA preauthorization—is similar to provisions in other subsequent VCP bills regarding Access to Walk-In Carefor episodic physical and mental health care.3
The original Veterans Choice Act of 2014 was enacted with $10 billion supplemental funding for the VCP as well as $5 billion to augment VHA staffing. In contrast, these recent proposals include no supplemental allocations. Veterans could bypass VHA approval, obtain VCP services on their own; the VHA would be sent the bill and payment would be taken from the VHA facility’s budgets.
The set of proposals serves as a reminder of the need for further reflection and discussion about how the nation can best address the crisis of veteran suicide and, more broadly, how to optimize access to evidence-based, integrated mental health care services.
This article critiques the myths underlying the proposals’ rationale, gives factual evidence on veterans’ suicide prevention and comprehensive mental health care issues, and concludes with a cautionary warning about the risk of VCP expansion adversely impacting veterans.
Preventing Veteran Suicides
For veterans in VHA care who are at risk for suicide, mental health policies include regular screening, follow-ups to missed appointments, and safety planning. For high-risk veterans, suicide prevention policies also involve a medical record flagging and monitoring system with mandatory mental health appointments.
The 2010 National Strategy for Suicide Prevention report extolled VHA’s multiple levels of evidenced-based suicide prevention practices and recommended that other health care systems emulate the practices.5 Despite this, few community health care providers or systems have adopted a similar approach. As the Congressional Research Service observed in 2016, “Outside the VA, the use of suicide prevention coordinators has not been widely adopted.”6
Since its launch a decade ago, the 24-hour VCL has answered > 3 million calls from veterans and their family/friends, with > 500,000 follow-up referrals to local VA SPCs. Because the VCL links directly to VHA facilities, care coordination is more effective when a veteran’s provider is in the VHA. When the veteran is not a VHA patient, coordinating with his/her community provider is laden with logistic impediments.
Comprehensive Mental Health Care
By contrast, a RAND Corporation study of therapists who treat PTSD and major depressive disorder found that when compared with providers affiliated with the VHA or DoD, “a psychotherapist selected from the community is unlikely to have the skills necessary to deliver high-quality mental health care to service members or veterans with these conditions.”23 Only 13% of community therapists were trained in and used an EBP and had veteran/military cultural competency. A separate 2017 study of community providers who treat veterans found that only a minority reported prior training in, or use of, any EBP for PTSD.24 Also, as the industry leader in telemental health, the VHA’s delivery of EBPs to veterans in remote locations and/or having difficulty accessing clinic-based care is far beyond that of the private sector.
VHA providers proactively screen veterans for PTSD, alcohol misuse, depression, military sexual trauma (MST), and traumatic brain injury. When problems are identified, primary care providers are able to deliver a warm handoff to mental health team members for further evaluation and intervention as needed. Such integration of services, required by VHA policy since 2008, appears related to increased detection and treatment of mental illness among older veterans.36 Referred older veterans have shown significant reduction in depressive symptoms with antidepressant medication treatment.37 Veterans with chronic obstructive pulmonary disease receiving brief cognitive behavioral psychotherapy in primary care clinics had decreased symptoms of depression and anxiety maintained at 12 months.38
As the Commission on Care Final Report recognized, “Veterans who receive health care exclusively through VA generally receive well-coordinated care, yet care is often highly fragmented among those combining VHA care with care secured through private health plans, Medicare, and TRICARE. This fragmentation often results in lower quality, threatens patient safety, and shifts cost among payers.”39
Given that many survivors never talk about their MST experience unless asked directly, the VHA’s routine screening, culturally competent sensitivity, and unflagging efforts to engage veterans are crucial ways to proactively reach survivors who might not otherwise seek care. Each VHA facility has a dedicated MST coordinator, mandatory MST training for all primary and mental health care providers, free MST-related treatment, and MST outreach efforts. All veterans enrolled in the VHA are screened for experiences of MST, and tailored treatment plans are created for survivors who need care. More than 1 million outpatient MST-related mental health visits were provided to veterans with a positive MST screen in fiscal year (FY) 2015, a 13% increase from the prior year.15 Widespread screening and treatment programs do not exist in the community-based care, where mental health care providers are less likely to have relevant experience or recognize that it is important to ask veterans about MST.
The DoD recently indicated that lesbian, gay bisexual, transgender (LGBT) service members experience disproportionately higher rates of MST, reporting sexual assault 5 times and harassment 3 times as often as non-LGBT service members.45 Civilian research consistently identifies LGBT individuals as being at greater risk for suicide.46 Although exact rates of LGBT veteran suicides are unknown, one study found that 47% of lesbian, gay, and bisexual veterans reported lifetime suicidal ideation compared with that of 22% of heterosexual veterans.47 Each VHA facility has a dedicated LGBT care coordinator who works closely with the MST coordinator and mental health treatment teams to ensure timely referrals to appropriate care. Comparable care coordination does not exist in the community, where providers also are less likely to have relevant experience and training to address veteran-specific correlates of trauma for LGBT individuals.
In the VHA’s Intensive Community Mental Health Recovery (ICMHR) program, mental health staff visit veterans with SMI at least weekly to provide recovery-oriented interventions, typically in the veteran’s place of residence, which ensures more routine follow-up and alleviates the burden of having to go to a medical facility. In fiscal year 2016, veterans enrolled in ICMHR services had an average of 12 to 27 fewer hospital days after admission to the program.15
Preliminary results show decreases in pain, opioid risk, and opioid use as well as improved provider perception of pain care delivered in primary care.52,53 For those veterans who require a higher level of care, the VHA has mandated the creation of tertiary pain programs, based on well-established models of more intensive, comprehensive treatment shown to be effective in the treatment of chronic pain.54
Although interdisciplinary pain management continues to grow in the VHA, it very rare in the U.S. private sector where health care tends to be fragmented and truncated. The VHA accounts for 40% of the U.S. interdisciplinary pain programs even though it serves 8% of the adult population.55 The importance of effective pain management, including behavioral interventions, is further highlighted by the fact that pain is the most commonly identified risk factor in VHA users whose suicides are reported to central office.56
Anecdotal instances do arise where veterans express discontent about VA mental health services. That is no surprise in a large pool of millions of patients. One example was a 2016 VA Center for Innovation report, quoted during the October 2017 Congressional hearing, which asked about 40 veterans and 5 family members for their criticisms.58 Using an unrepresentative sampling method, the report found that some of the veterans desired more privacy and easier access to mental health care. The report also noted that the VA’s 300 Vet Centers and 80 mobile Vet Centers would provide such quick, confidential access, but many veterans did not know about that resource.
Conclusion
As VA Secretary David J. Shulkin, MD, has underscored, preventing suicide among all our nation’s veterans, is a sacred VA responsibility. The VHA must identify areas for improvement and mitigate obstacles that impede veterans receiving quality mental health care. When prompt access to VHA mental health care for enrolled veterans isn’t feasible, the VHA should continue to purchase services from VCP providers. For all veterans, their families, and non-VA professionals, the VHA should continue to share its educational and clinical expertise (as it has successfully done in efforts such as the Be There Campaign, VA Community Provider Toolkit, VA Campus Toolkit, PTSD Consultation Program and Suicide Risk Management Consultation Program.)
Nevertheless, in crafting policies, it is essential to ensure that there is no collateral damage to the overall superior quality, unique advantages, and cost-effectiveness of VHA mental health care. The guiding principle for all health care systems and providers, “first, do no harm,” must be heeded.
The VCP is intended to supplement not supplant the VHA, but the recent proposals would do the opposite. Furnishing vouchers to veterans that bypass VA preauthorization will weaken veterans’ mental health care and suicide prevention efforts. It sets in motion a gradual, persistent hollowing out of VHA care. In zero-sum budgets, VHA facilities will receive less money, vacant positions will not be filled, and mental health services will be cut. As the availability of VHA services diminishes, many veterans will be placed into VCP, leading to a vicious cycle of further VHA cuts. In the name of freedom of choice, veterans, especially the most vulnerable who depend on the VHA, will ultimately have fewer quality choices.
The stand-alone mental health clinic model runs completely counter to the VHA’s best practice interprofessional and integrated care approach. Veterans have more complex comorbidities and need greater, not less, integration of mental health services across the continuum, including primary care, specialty care, and geriatric/extended care programs.59
Implementing unrestrained choice, even as a pilot for newly transitioning service members or other groups of veterans, would be the initial step on a slippery slope to vouchers for the entire VHA system. Once mental health services are privatized, the remainder of VHA services, whose overall quality also has been determined to be equal or better than that delivered in the community, would follow in quick succession.11 In January 2018, the National Academies of Science, Engineering and Medicine published an exhaustive evaluation of VHA mental health care and hailed it as the preeminent system that is “positioned to inform and influence how mental health care services are provided more broadly in the United States.”60 It was decisive confirmation that, first and foremost, we must guarantee that VHA mental health care is fully funded and staffed and remains the coordinator and authorizer of care.
Acknowledgments
The considerations offered in this article are those of the author. The Association of VA Psychologist Leaders provided substantial help for the manuscript as well as for a prior white paper. Special thanks for factual assistance go to Heather Kelly, PhD, and the American Psychological Association; Suzanne Gordon; Ben Emmert-Aronson, PhD; Jennifer Boyd, PhD; Kaela Joseph, PhD; Tracey Smith, PhD; Jen Manuel, PhD; Brian Borsari, PhD; Jan Bowman, PhD; Gareth Loy, PhD; Karen Seal, MD; Ron Gironda, PhD; Sarah Palyo, PhD; Victoria Lemle Beckner, PhD, Joel Schmidt, PhD, Terri Huh, PhD, and Thomas Horvath, MD.
About this column
Mental Health Care Practice column is edited and occasionally authored by COL (Ret) Elspeth Ritchie, MD, MPH. Proposals for articles are encouraged and can be sent to elspethcameronritchie@gmail.com.
1. U.S. Department of Veterans Affairs, Office of Suicide Prevention. Suicide among veterans and other Americans, 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Updated August 2017. Accessed February 6, 2018.
2. Provision of Mental Health Care to Veterans by Certain Mental Health Providers Participating in the Veterans Choice Program, 115th Cong, 1st Sess (2017). http://docs.house.gov/meetings/VR/VR00/20171024/106521/BILLS-1152pih-DRAFTtodirectVAtofurnishMHcaretoveteransatcommunityornon-profitMHprovidersparticipatinginChoice.pdf. Accessed February 5, 2018.
3. Caring for Our Veterans Act of 2017, S 2193, 115th Cong, 1st Sess (2017).
4. Farmer CM, Hosek SD, Adamson DM; RAND Corporation. Balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. 2016. https://www.rand.org/pubs/research_reports/RR1165z4.html. Published February 2016. Accessed February 5, 2018.
5. Suicide Prevention Resource Center, SPAN USA; Litts D, ed. Charting the future of suicide prevention: a 2010 progress review of the national strategy and recommendations for the decade ahead. http://www.sprc.org/sites/default/files/migrate /library/ChartingTheFuture_Fullbook.pdf. Published August 2010. Accessed February 5, 2018.
6. Bagalman E; Congressional Research Service. Health care for veterans: suicide prevention. https://fas.org/sgp/crs/misc/R42340.pdf. Published February 23, 2016.Accessed January 5, 2018.
7. McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942.
8. Kaplan MS, McFarland BH, Huguet N, Valenstein M. Suicide risk and precipitating circumstances among young, middle-aged, and older male veterans. Am J Public Health. 2012;102(suppl 1):S131-S137.
9. Louzon SA, Bossarte R, McCarthy JF, Katz IR. Does suicidal ideation as measured by the PHQ-9 predict suicide among VA patients? Psychiatr Serv. 2016;67(5):517-522.
10. Mills PD, Watts BV, Huh TJ, Boar S, Kemp J. Helping elderly patients to avoid suicide: a review of case reports from a national veterans affairs database. J Nerv Ment Dis. 2013;201(1):12-16.
11. Smith EG, Kim HM, Ganoczy D, Stano C, Pfeiffer PN, Valenstein M. Suicide risk assessment received prior to suicide death by Veterans Health Administration patients with a history of depression. J Clin Psychiatry. 2013;74(3):226-232.
12. Blais RK, Tsai J, Southwick SM, Pietrzak RH. Barriers and facilitators related to mental health care use among older veterans in the United States. Psychiatr Serv. 2015;66(5):500-506.
13. Gonçalves DC, Coelho CM, Byrne GJ. The use of healthcare services for mental health problems by middle-aged and older adults. Arch Gerontol Geriatr. 2014;59(2):393-397.
14. Gasper J, Jiu H, Kim S, May L. 2015 survey of veteran enrollees’ health and use of health care. https://va.gov/HEALTHPOLICYPLANNING/SoE2015/2015_VHA_SoE_Full _Findings_Report.pdf. Published December 11, 2015. Accessed February 5, 2019.
15. U.S. Department of Veterans Affairs. VA’s unparalleled integrated mental health services. https://www.scribd.com/document/356011769/U-S-Department-of-Veterans-Affairs -mental-health-fact-sheet. Published May 2017. Accessed January 26, 2018.
16. Karlin BE, Ruzek JI, Chard KM, et al. Dissemination of evidence-based psychological treatments for posttraumatic stress disorder in the Veterans Health Administration. J Trauma Stress. 2010;23(6):663-673.
17. Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum MA, Karlin BE. Effectiveness of national implementation of prolonged exposure therapy in Veterans Affairs care. JAMA Psychiatry. 2013;70(9):949-955.
18. Chard KM, Ricksecker EG, Healy ET, Karlin BE, Resick PA. Dissemination and experience with cognitive processing therapy. J Rehabil Res Dev. 2012;49(5):667-678.
19. Karlin BE, Walser RD, Yesavage J, Zhang A, Trockel M, Taylor CB. Effectiveness of acceptance and commitment therapy for depression: comparison among older and younger veterans. Aging Ment Health. 2013;17(5):555-563.
20. Karlin BE, Trockel M, Brown GK, Gordienko M, Yesavage J, Taylor CB. Comparison of the effectiveness of cognitive behavioral therapy for depression among older versus younger veterans: results of a national evaluation. J Gerontol B Psychol Sci Soc Sci. 2015;70(1):3-12.
21. Stewart MO, Raffa SD, Steele JL, et al. National dissemination of interpersonal psychotherapy for depression in veterans: therapist and patient-level outcomes. J Consult Clin Psychol. 2014;82(6):1201-1206.
22. Walser RD, Karlin BE, Trockel M, Mazina B, Barr Taylor C. Training in and implementation of Acceptance and Commitment Therapy for depression in the Veterans Health Administration: therapist and patient outcomes. Behav Res Ther. 2013;51(9):555-563.
23. Tanielian T, Farris C, Batka C, et al; Rand Corporation. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. https://www.rand.org/pubs/research_reports/RR806.html. Published November 2014. Accessed February 5, 2018.
24. Finley EP, Noël PH, Lee S, et al. Psychotherapy practices for veterans with PTSD among community-based providers in Texas. Psychol Serv. 2017. [Epub ahead of print.]
25. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2015;67(4):391-396.
26. Barry CN, Bowe TR, Suneja A. An update on the quality of medication treatment for mental disorders in the VA. Psychiatr Serv. 2016;67(8):930.
27. Mechanic D. More people than ever before are receiving behavioral health care in the United States, but gaps and challenges remain. Health Aff (Millwood). 2014;33(8):1416-1424.
28. Karlin B, Zeiss AM. Transforming mental health care for older veterans in the Veterans Health Administration. Generations. 2010;34(2):74-83.
29. Karlin BE, Karel MJ. National integration of mental health providers in VA home-based primary care: an innovative model for mental health care delivery with older adults. Gerontologist. 2014;54(5):868-879.
30. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
31. DiNapoli EA, Cully JA, Wayde E, Sansgiry S, Yu HJ, Kunik ME. Age as a predictive factor of mental health service use among adults with depression and/or anxiety disorder receiving care through the Veterans Health Administration. Int J Geriatr Psychiatry. 2016;31(6):575-582.
32. Shepardson RL, Funderburk JS. Likelihood of attending treatment for anxiety among veteran primary care patients: patient preferences for treatment attributes. J Clin Psychol Med Settings. 2016;23(3):225-239.
33. Johnson-Lawrence VD, Szymanski BR, Zivin K, McCarthy JF, Valenstein M, Pfeiffer PN. Primary care-mental health integration programs in the Veterans Affairs health system serve a different patient population than specialty mental health clinics. Prim Care Companion CNS Disord. 2012;14(3):pii.
34. Mackenzie CS, Gekoski WL, Knox VJ. Age, gender, and the underutilization of mental health services: the influence of help-seeking attitudes. Aging Ment Health. 2006;10(6):574-582.
35. Bartels SJ, Coakley EH, Zubritsky C, et al; PRISM-E Investigators. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry. 2004;161(8):1455-1462.
36. Wiechers IR, Karel MJ, Hoff R, Karlin BE. Growing use of mental and general health care services among older veterans with mental illness. Psychiatr Serv. 2015;66(11):1242-1244.
37. Mavandadi S, Klaus JR, Oslin DW. Age group differences among veterans enrolled in a clinical service for behavioral health issues in primary care. Am J Geriatr Psychiatry. 2012;20(3):205-214.
38. Cully JA, Stanley MA, Petersen NJ, et al. Delivery of brief cognitive behavioral therapy for medically ill patients in primary care: a pragmatic randomized clinical trial. J Gen Intern Med. 2017;32(9):1014-1024.
39. Commission on Care. Commission on care: final report.https://s3.amazonaws.com/sitesusa/wp-content/uploads/sites/912/2016/07/Commission-on-Care_Final-Report_063016_FOR-WEB.pdf. Published June 30, 2016. Accessed February 5, 2018.
40. U.S. Department of Veterans Affairs, Military Sexual Trauma Support Team. Military sexual trauma. https://www.menta lhealth.va.gov/docs/mst_general_factsheet.pdf. Published May 2015. Accessed February 13, 2018.
41. Kimerling R, Gima K, Smith MW, Street A, Frayne S. The Veterans Health Administration and military sexual trauma. Am J Public Health. 2007;97(12):2160-2166.
42. Schry AR, Hibberd R, Wagner HR, et al; Veterans Affairs Mid-Atlantic Medical Research Education and Clinical Center Workgroup, Brancu M. Functional correlates of military sexual assault in male veterans. Psychol Serv. 2015;12(4):384-393.
43. Millegan J, Milburn EK, LeardMann CA, et al. Recent sexual trauma and adverse health and occupational outcomes among U.S. service women. J Trauma Stress. 2015;28(4):298-306.
44. Kimerling R, Makin-Byrd K, Louzon S, Ignacio RV, McCarthy JF. Military sexual trauma and suicide mortality. Am J Prev Med. 2016;50(6):684-691.
45. U.S. Department of Defense. Department of Defense Fiscal Year 2016 Annual Report on Sexual Assault in the Military. Washington DC: U.S. Office of the Under Secretary of Defense; 2017.
46. Haas AP, Eliason M, Mays VM, et al. Suicide and suicide risk in lesbian, gay, bisexual, and transgender populations: review and recommendations. J Homosex. 2011;58(1):10-51.
47. Blosnich JR, Mays VM, Cochran SD. Suicidality among veterans: implications of sexual minority status. Am J Public Health. 2014;104(suppl 4):S535-S537.
48. Kilbourne AM, Ignacio RV, Kim HM, Blow FC. Datapoints: are VA patients with serious mental illness dying younger? Psychiatr Serv. 2009;60(5):589.
49. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79.
50. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans’ reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
51. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(11):1-49.
52. Seal K, Becker W, Tighe J, Li Y, Rife T. Managing chronic pain in primary care: it really does take a village. J Gen Intern Med. 2017;32(8):931-934.
53. Dorflinger LM, Ruser C, Sellinger J, Edens EL, Kerns RD, Becker WC. Integrating interdisciplinary pain management into primary care: development and implementation of a novel clinical program. Pain Med. 2014;15(12):2046-2054.
54. Gatchel RJ, Okifuji A. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J Pain. 2006;7(11):779-793.
55. Schatman ME. Interdisciplinary chronic pain management: international perspectives. Pain Clin Updates. 2012;20(7):1-5.
56. U.S. Department of Veterans Affairs. The US Department of Veterans Affairs Behavioral Health Autopsy Program (BHAP) Report, December 1, 2012-June 30, 2015. https://catalog .data.gov/dataset/behavioral-health-autopsy-program-bhap. Accessed November 3, 2017.
57. Veterans of Foreign Wars. Our care 2017: a report evaluating veterans health care. March 2017. https://vfw-cdn .azureedge.net/-/media/VFWSite/Files/Advocacy/VFW-Our -Care-2017--Executive-Summary.pdf. Published March 2017. Accessed February 6, 2018.
58. U.S. Department of Veterans Affairs, VA Center for Innovation and the Public Policy Lab. Veteran access to mental health services. https://www.innovation.va.gov/docs/Ve teranAccessToMentalHealthServices.pdf. Published 2016. Accessed February 6, 2017.
59. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25-64: United States, 2007-2010. NCHS Data Brief. 2012;(101):1-8.
60. National Academies of Sciences, Engineering, and Medicine. Evaluation of the Department of Veterans Affairs Mental Health Services. Washington D.C.: The National Academies Press; 2018.
Last summer, the Department of Veteran Affairs (VA) published the most comprehensive analysis of veteran suicide in our nation’s history. That study examined 55 million records from every state and revealed that in 2014, an average of 20 veterans died by suicide each day.1 Six of the 20 were recent users of Veterans Health Administration (VHA) services; the other 14 had not used VHA services in the prior 2 years.
Policy makers are currently deliberating whether expanding the Veterans Choice Program (VCP) is a judicious way to prevent these tragic deaths, especially for veterans who do not use the VHA. One proposal, presented at a congressional committee hearing in October 2017, advocates expanding the VCP.2 Its core tenet—allowing veterans to seek mental health care from VCP providers without needing VHA preauthorization—is similar to provisions in other subsequent VCP bills regarding Access to Walk-In Carefor episodic physical and mental health care.3
The original Veterans Choice Act of 2014 was enacted with $10 billion supplemental funding for the VCP as well as $5 billion to augment VHA staffing. In contrast, these recent proposals include no supplemental allocations. Veterans could bypass VHA approval, obtain VCP services on their own; the VHA would be sent the bill and payment would be taken from the VHA facility’s budgets.
The set of proposals serves as a reminder of the need for further reflection and discussion about how the nation can best address the crisis of veteran suicide and, more broadly, how to optimize access to evidence-based, integrated mental health care services.
This article critiques the myths underlying the proposals’ rationale, gives factual evidence on veterans’ suicide prevention and comprehensive mental health care issues, and concludes with a cautionary warning about the risk of VCP expansion adversely impacting veterans.
Preventing Veteran Suicides
For veterans in VHA care who are at risk for suicide, mental health policies include regular screening, follow-ups to missed appointments, and safety planning. For high-risk veterans, suicide prevention policies also involve a medical record flagging and monitoring system with mandatory mental health appointments.
The 2010 National Strategy for Suicide Prevention report extolled VHA’s multiple levels of evidenced-based suicide prevention practices and recommended that other health care systems emulate the practices.5 Despite this, few community health care providers or systems have adopted a similar approach. As the Congressional Research Service observed in 2016, “Outside the VA, the use of suicide prevention coordinators has not been widely adopted.”6
Since its launch a decade ago, the 24-hour VCL has answered > 3 million calls from veterans and their family/friends, with > 500,000 follow-up referrals to local VA SPCs. Because the VCL links directly to VHA facilities, care coordination is more effective when a veteran’s provider is in the VHA. When the veteran is not a VHA patient, coordinating with his/her community provider is laden with logistic impediments.
Comprehensive Mental Health Care
By contrast, a RAND Corporation study of therapists who treat PTSD and major depressive disorder found that when compared with providers affiliated with the VHA or DoD, “a psychotherapist selected from the community is unlikely to have the skills necessary to deliver high-quality mental health care to service members or veterans with these conditions.”23 Only 13% of community therapists were trained in and used an EBP and had veteran/military cultural competency. A separate 2017 study of community providers who treat veterans found that only a minority reported prior training in, or use of, any EBP for PTSD.24 Also, as the industry leader in telemental health, the VHA’s delivery of EBPs to veterans in remote locations and/or having difficulty accessing clinic-based care is far beyond that of the private sector.
VHA providers proactively screen veterans for PTSD, alcohol misuse, depression, military sexual trauma (MST), and traumatic brain injury. When problems are identified, primary care providers are able to deliver a warm handoff to mental health team members for further evaluation and intervention as needed. Such integration of services, required by VHA policy since 2008, appears related to increased detection and treatment of mental illness among older veterans.36 Referred older veterans have shown significant reduction in depressive symptoms with antidepressant medication treatment.37 Veterans with chronic obstructive pulmonary disease receiving brief cognitive behavioral psychotherapy in primary care clinics had decreased symptoms of depression and anxiety maintained at 12 months.38
As the Commission on Care Final Report recognized, “Veterans who receive health care exclusively through VA generally receive well-coordinated care, yet care is often highly fragmented among those combining VHA care with care secured through private health plans, Medicare, and TRICARE. This fragmentation often results in lower quality, threatens patient safety, and shifts cost among payers.”39
Given that many survivors never talk about their MST experience unless asked directly, the VHA’s routine screening, culturally competent sensitivity, and unflagging efforts to engage veterans are crucial ways to proactively reach survivors who might not otherwise seek care. Each VHA facility has a dedicated MST coordinator, mandatory MST training for all primary and mental health care providers, free MST-related treatment, and MST outreach efforts. All veterans enrolled in the VHA are screened for experiences of MST, and tailored treatment plans are created for survivors who need care. More than 1 million outpatient MST-related mental health visits were provided to veterans with a positive MST screen in fiscal year (FY) 2015, a 13% increase from the prior year.15 Widespread screening and treatment programs do not exist in the community-based care, where mental health care providers are less likely to have relevant experience or recognize that it is important to ask veterans about MST.
The DoD recently indicated that lesbian, gay bisexual, transgender (LGBT) service members experience disproportionately higher rates of MST, reporting sexual assault 5 times and harassment 3 times as often as non-LGBT service members.45 Civilian research consistently identifies LGBT individuals as being at greater risk for suicide.46 Although exact rates of LGBT veteran suicides are unknown, one study found that 47% of lesbian, gay, and bisexual veterans reported lifetime suicidal ideation compared with that of 22% of heterosexual veterans.47 Each VHA facility has a dedicated LGBT care coordinator who works closely with the MST coordinator and mental health treatment teams to ensure timely referrals to appropriate care. Comparable care coordination does not exist in the community, where providers also are less likely to have relevant experience and training to address veteran-specific correlates of trauma for LGBT individuals.
In the VHA’s Intensive Community Mental Health Recovery (ICMHR) program, mental health staff visit veterans with SMI at least weekly to provide recovery-oriented interventions, typically in the veteran’s place of residence, which ensures more routine follow-up and alleviates the burden of having to go to a medical facility. In fiscal year 2016, veterans enrolled in ICMHR services had an average of 12 to 27 fewer hospital days after admission to the program.15
Preliminary results show decreases in pain, opioid risk, and opioid use as well as improved provider perception of pain care delivered in primary care.52,53 For those veterans who require a higher level of care, the VHA has mandated the creation of tertiary pain programs, based on well-established models of more intensive, comprehensive treatment shown to be effective in the treatment of chronic pain.54
Although interdisciplinary pain management continues to grow in the VHA, it very rare in the U.S. private sector where health care tends to be fragmented and truncated. The VHA accounts for 40% of the U.S. interdisciplinary pain programs even though it serves 8% of the adult population.55 The importance of effective pain management, including behavioral interventions, is further highlighted by the fact that pain is the most commonly identified risk factor in VHA users whose suicides are reported to central office.56
Anecdotal instances do arise where veterans express discontent about VA mental health services. That is no surprise in a large pool of millions of patients. One example was a 2016 VA Center for Innovation report, quoted during the October 2017 Congressional hearing, which asked about 40 veterans and 5 family members for their criticisms.58 Using an unrepresentative sampling method, the report found that some of the veterans desired more privacy and easier access to mental health care. The report also noted that the VA’s 300 Vet Centers and 80 mobile Vet Centers would provide such quick, confidential access, but many veterans did not know about that resource.
Conclusion
As VA Secretary David J. Shulkin, MD, has underscored, preventing suicide among all our nation’s veterans, is a sacred VA responsibility. The VHA must identify areas for improvement and mitigate obstacles that impede veterans receiving quality mental health care. When prompt access to VHA mental health care for enrolled veterans isn’t feasible, the VHA should continue to purchase services from VCP providers. For all veterans, their families, and non-VA professionals, the VHA should continue to share its educational and clinical expertise (as it has successfully done in efforts such as the Be There Campaign, VA Community Provider Toolkit, VA Campus Toolkit, PTSD Consultation Program and Suicide Risk Management Consultation Program.)
Nevertheless, in crafting policies, it is essential to ensure that there is no collateral damage to the overall superior quality, unique advantages, and cost-effectiveness of VHA mental health care. The guiding principle for all health care systems and providers, “first, do no harm,” must be heeded.
The VCP is intended to supplement not supplant the VHA, but the recent proposals would do the opposite. Furnishing vouchers to veterans that bypass VA preauthorization will weaken veterans’ mental health care and suicide prevention efforts. It sets in motion a gradual, persistent hollowing out of VHA care. In zero-sum budgets, VHA facilities will receive less money, vacant positions will not be filled, and mental health services will be cut. As the availability of VHA services diminishes, many veterans will be placed into VCP, leading to a vicious cycle of further VHA cuts. In the name of freedom of choice, veterans, especially the most vulnerable who depend on the VHA, will ultimately have fewer quality choices.
The stand-alone mental health clinic model runs completely counter to the VHA’s best practice interprofessional and integrated care approach. Veterans have more complex comorbidities and need greater, not less, integration of mental health services across the continuum, including primary care, specialty care, and geriatric/extended care programs.59
Implementing unrestrained choice, even as a pilot for newly transitioning service members or other groups of veterans, would be the initial step on a slippery slope to vouchers for the entire VHA system. Once mental health services are privatized, the remainder of VHA services, whose overall quality also has been determined to be equal or better than that delivered in the community, would follow in quick succession.11 In January 2018, the National Academies of Science, Engineering and Medicine published an exhaustive evaluation of VHA mental health care and hailed it as the preeminent system that is “positioned to inform and influence how mental health care services are provided more broadly in the United States.”60 It was decisive confirmation that, first and foremost, we must guarantee that VHA mental health care is fully funded and staffed and remains the coordinator and authorizer of care.
Acknowledgments
The considerations offered in this article are those of the author. The Association of VA Psychologist Leaders provided substantial help for the manuscript as well as for a prior white paper. Special thanks for factual assistance go to Heather Kelly, PhD, and the American Psychological Association; Suzanne Gordon; Ben Emmert-Aronson, PhD; Jennifer Boyd, PhD; Kaela Joseph, PhD; Tracey Smith, PhD; Jen Manuel, PhD; Brian Borsari, PhD; Jan Bowman, PhD; Gareth Loy, PhD; Karen Seal, MD; Ron Gironda, PhD; Sarah Palyo, PhD; Victoria Lemle Beckner, PhD, Joel Schmidt, PhD, Terri Huh, PhD, and Thomas Horvath, MD.
About this column
Mental Health Care Practice column is edited and occasionally authored by COL (Ret) Elspeth Ritchie, MD, MPH. Proposals for articles are encouraged and can be sent to elspethcameronritchie@gmail.com.
Last summer, the Department of Veteran Affairs (VA) published the most comprehensive analysis of veteran suicide in our nation’s history. That study examined 55 million records from every state and revealed that in 2014, an average of 20 veterans died by suicide each day.1 Six of the 20 were recent users of Veterans Health Administration (VHA) services; the other 14 had not used VHA services in the prior 2 years.
Policy makers are currently deliberating whether expanding the Veterans Choice Program (VCP) is a judicious way to prevent these tragic deaths, especially for veterans who do not use the VHA. One proposal, presented at a congressional committee hearing in October 2017, advocates expanding the VCP.2 Its core tenet—allowing veterans to seek mental health care from VCP providers without needing VHA preauthorization—is similar to provisions in other subsequent VCP bills regarding Access to Walk-In Carefor episodic physical and mental health care.3
The original Veterans Choice Act of 2014 was enacted with $10 billion supplemental funding for the VCP as well as $5 billion to augment VHA staffing. In contrast, these recent proposals include no supplemental allocations. Veterans could bypass VHA approval, obtain VCP services on their own; the VHA would be sent the bill and payment would be taken from the VHA facility’s budgets.
The set of proposals serves as a reminder of the need for further reflection and discussion about how the nation can best address the crisis of veteran suicide and, more broadly, how to optimize access to evidence-based, integrated mental health care services.
This article critiques the myths underlying the proposals’ rationale, gives factual evidence on veterans’ suicide prevention and comprehensive mental health care issues, and concludes with a cautionary warning about the risk of VCP expansion adversely impacting veterans.
Preventing Veteran Suicides
For veterans in VHA care who are at risk for suicide, mental health policies include regular screening, follow-ups to missed appointments, and safety planning. For high-risk veterans, suicide prevention policies also involve a medical record flagging and monitoring system with mandatory mental health appointments.
The 2010 National Strategy for Suicide Prevention report extolled VHA’s multiple levels of evidenced-based suicide prevention practices and recommended that other health care systems emulate the practices.5 Despite this, few community health care providers or systems have adopted a similar approach. As the Congressional Research Service observed in 2016, “Outside the VA, the use of suicide prevention coordinators has not been widely adopted.”6
Since its launch a decade ago, the 24-hour VCL has answered > 3 million calls from veterans and their family/friends, with > 500,000 follow-up referrals to local VA SPCs. Because the VCL links directly to VHA facilities, care coordination is more effective when a veteran’s provider is in the VHA. When the veteran is not a VHA patient, coordinating with his/her community provider is laden with logistic impediments.
Comprehensive Mental Health Care
By contrast, a RAND Corporation study of therapists who treat PTSD and major depressive disorder found that when compared with providers affiliated with the VHA or DoD, “a psychotherapist selected from the community is unlikely to have the skills necessary to deliver high-quality mental health care to service members or veterans with these conditions.”23 Only 13% of community therapists were trained in and used an EBP and had veteran/military cultural competency. A separate 2017 study of community providers who treat veterans found that only a minority reported prior training in, or use of, any EBP for PTSD.24 Also, as the industry leader in telemental health, the VHA’s delivery of EBPs to veterans in remote locations and/or having difficulty accessing clinic-based care is far beyond that of the private sector.
VHA providers proactively screen veterans for PTSD, alcohol misuse, depression, military sexual trauma (MST), and traumatic brain injury. When problems are identified, primary care providers are able to deliver a warm handoff to mental health team members for further evaluation and intervention as needed. Such integration of services, required by VHA policy since 2008, appears related to increased detection and treatment of mental illness among older veterans.36 Referred older veterans have shown significant reduction in depressive symptoms with antidepressant medication treatment.37 Veterans with chronic obstructive pulmonary disease receiving brief cognitive behavioral psychotherapy in primary care clinics had decreased symptoms of depression and anxiety maintained at 12 months.38
As the Commission on Care Final Report recognized, “Veterans who receive health care exclusively through VA generally receive well-coordinated care, yet care is often highly fragmented among those combining VHA care with care secured through private health plans, Medicare, and TRICARE. This fragmentation often results in lower quality, threatens patient safety, and shifts cost among payers.”39
Given that many survivors never talk about their MST experience unless asked directly, the VHA’s routine screening, culturally competent sensitivity, and unflagging efforts to engage veterans are crucial ways to proactively reach survivors who might not otherwise seek care. Each VHA facility has a dedicated MST coordinator, mandatory MST training for all primary and mental health care providers, free MST-related treatment, and MST outreach efforts. All veterans enrolled in the VHA are screened for experiences of MST, and tailored treatment plans are created for survivors who need care. More than 1 million outpatient MST-related mental health visits were provided to veterans with a positive MST screen in fiscal year (FY) 2015, a 13% increase from the prior year.15 Widespread screening and treatment programs do not exist in the community-based care, where mental health care providers are less likely to have relevant experience or recognize that it is important to ask veterans about MST.
The DoD recently indicated that lesbian, gay bisexual, transgender (LGBT) service members experience disproportionately higher rates of MST, reporting sexual assault 5 times and harassment 3 times as often as non-LGBT service members.45 Civilian research consistently identifies LGBT individuals as being at greater risk for suicide.46 Although exact rates of LGBT veteran suicides are unknown, one study found that 47% of lesbian, gay, and bisexual veterans reported lifetime suicidal ideation compared with that of 22% of heterosexual veterans.47 Each VHA facility has a dedicated LGBT care coordinator who works closely with the MST coordinator and mental health treatment teams to ensure timely referrals to appropriate care. Comparable care coordination does not exist in the community, where providers also are less likely to have relevant experience and training to address veteran-specific correlates of trauma for LGBT individuals.
In the VHA’s Intensive Community Mental Health Recovery (ICMHR) program, mental health staff visit veterans with SMI at least weekly to provide recovery-oriented interventions, typically in the veteran’s place of residence, which ensures more routine follow-up and alleviates the burden of having to go to a medical facility. In fiscal year 2016, veterans enrolled in ICMHR services had an average of 12 to 27 fewer hospital days after admission to the program.15
Preliminary results show decreases in pain, opioid risk, and opioid use as well as improved provider perception of pain care delivered in primary care.52,53 For those veterans who require a higher level of care, the VHA has mandated the creation of tertiary pain programs, based on well-established models of more intensive, comprehensive treatment shown to be effective in the treatment of chronic pain.54
Although interdisciplinary pain management continues to grow in the VHA, it very rare in the U.S. private sector where health care tends to be fragmented and truncated. The VHA accounts for 40% of the U.S. interdisciplinary pain programs even though it serves 8% of the adult population.55 The importance of effective pain management, including behavioral interventions, is further highlighted by the fact that pain is the most commonly identified risk factor in VHA users whose suicides are reported to central office.56
Anecdotal instances do arise where veterans express discontent about VA mental health services. That is no surprise in a large pool of millions of patients. One example was a 2016 VA Center for Innovation report, quoted during the October 2017 Congressional hearing, which asked about 40 veterans and 5 family members for their criticisms.58 Using an unrepresentative sampling method, the report found that some of the veterans desired more privacy and easier access to mental health care. The report also noted that the VA’s 300 Vet Centers and 80 mobile Vet Centers would provide such quick, confidential access, but many veterans did not know about that resource.
Conclusion
As VA Secretary David J. Shulkin, MD, has underscored, preventing suicide among all our nation’s veterans, is a sacred VA responsibility. The VHA must identify areas for improvement and mitigate obstacles that impede veterans receiving quality mental health care. When prompt access to VHA mental health care for enrolled veterans isn’t feasible, the VHA should continue to purchase services from VCP providers. For all veterans, their families, and non-VA professionals, the VHA should continue to share its educational and clinical expertise (as it has successfully done in efforts such as the Be There Campaign, VA Community Provider Toolkit, VA Campus Toolkit, PTSD Consultation Program and Suicide Risk Management Consultation Program.)
Nevertheless, in crafting policies, it is essential to ensure that there is no collateral damage to the overall superior quality, unique advantages, and cost-effectiveness of VHA mental health care. The guiding principle for all health care systems and providers, “first, do no harm,” must be heeded.
The VCP is intended to supplement not supplant the VHA, but the recent proposals would do the opposite. Furnishing vouchers to veterans that bypass VA preauthorization will weaken veterans’ mental health care and suicide prevention efforts. It sets in motion a gradual, persistent hollowing out of VHA care. In zero-sum budgets, VHA facilities will receive less money, vacant positions will not be filled, and mental health services will be cut. As the availability of VHA services diminishes, many veterans will be placed into VCP, leading to a vicious cycle of further VHA cuts. In the name of freedom of choice, veterans, especially the most vulnerable who depend on the VHA, will ultimately have fewer quality choices.
The stand-alone mental health clinic model runs completely counter to the VHA’s best practice interprofessional and integrated care approach. Veterans have more complex comorbidities and need greater, not less, integration of mental health services across the continuum, including primary care, specialty care, and geriatric/extended care programs.59
Implementing unrestrained choice, even as a pilot for newly transitioning service members or other groups of veterans, would be the initial step on a slippery slope to vouchers for the entire VHA system. Once mental health services are privatized, the remainder of VHA services, whose overall quality also has been determined to be equal or better than that delivered in the community, would follow in quick succession.11 In January 2018, the National Academies of Science, Engineering and Medicine published an exhaustive evaluation of VHA mental health care and hailed it as the preeminent system that is “positioned to inform and influence how mental health care services are provided more broadly in the United States.”60 It was decisive confirmation that, first and foremost, we must guarantee that VHA mental health care is fully funded and staffed and remains the coordinator and authorizer of care.
Acknowledgments
The considerations offered in this article are those of the author. The Association of VA Psychologist Leaders provided substantial help for the manuscript as well as for a prior white paper. Special thanks for factual assistance go to Heather Kelly, PhD, and the American Psychological Association; Suzanne Gordon; Ben Emmert-Aronson, PhD; Jennifer Boyd, PhD; Kaela Joseph, PhD; Tracey Smith, PhD; Jen Manuel, PhD; Brian Borsari, PhD; Jan Bowman, PhD; Gareth Loy, PhD; Karen Seal, MD; Ron Gironda, PhD; Sarah Palyo, PhD; Victoria Lemle Beckner, PhD, Joel Schmidt, PhD, Terri Huh, PhD, and Thomas Horvath, MD.
About this column
Mental Health Care Practice column is edited and occasionally authored by COL (Ret) Elspeth Ritchie, MD, MPH. Proposals for articles are encouraged and can be sent to elspethcameronritchie@gmail.com.
1. U.S. Department of Veterans Affairs, Office of Suicide Prevention. Suicide among veterans and other Americans, 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Updated August 2017. Accessed February 6, 2018.
2. Provision of Mental Health Care to Veterans by Certain Mental Health Providers Participating in the Veterans Choice Program, 115th Cong, 1st Sess (2017). http://docs.house.gov/meetings/VR/VR00/20171024/106521/BILLS-1152pih-DRAFTtodirectVAtofurnishMHcaretoveteransatcommunityornon-profitMHprovidersparticipatinginChoice.pdf. Accessed February 5, 2018.
3. Caring for Our Veterans Act of 2017, S 2193, 115th Cong, 1st Sess (2017).
4. Farmer CM, Hosek SD, Adamson DM; RAND Corporation. Balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. 2016. https://www.rand.org/pubs/research_reports/RR1165z4.html. Published February 2016. Accessed February 5, 2018.
5. Suicide Prevention Resource Center, SPAN USA; Litts D, ed. Charting the future of suicide prevention: a 2010 progress review of the national strategy and recommendations for the decade ahead. http://www.sprc.org/sites/default/files/migrate /library/ChartingTheFuture_Fullbook.pdf. Published August 2010. Accessed February 5, 2018.
6. Bagalman E; Congressional Research Service. Health care for veterans: suicide prevention. https://fas.org/sgp/crs/misc/R42340.pdf. Published February 23, 2016.Accessed January 5, 2018.
7. McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942.
8. Kaplan MS, McFarland BH, Huguet N, Valenstein M. Suicide risk and precipitating circumstances among young, middle-aged, and older male veterans. Am J Public Health. 2012;102(suppl 1):S131-S137.
9. Louzon SA, Bossarte R, McCarthy JF, Katz IR. Does suicidal ideation as measured by the PHQ-9 predict suicide among VA patients? Psychiatr Serv. 2016;67(5):517-522.
10. Mills PD, Watts BV, Huh TJ, Boar S, Kemp J. Helping elderly patients to avoid suicide: a review of case reports from a national veterans affairs database. J Nerv Ment Dis. 2013;201(1):12-16.
11. Smith EG, Kim HM, Ganoczy D, Stano C, Pfeiffer PN, Valenstein M. Suicide risk assessment received prior to suicide death by Veterans Health Administration patients with a history of depression. J Clin Psychiatry. 2013;74(3):226-232.
12. Blais RK, Tsai J, Southwick SM, Pietrzak RH. Barriers and facilitators related to mental health care use among older veterans in the United States. Psychiatr Serv. 2015;66(5):500-506.
13. Gonçalves DC, Coelho CM, Byrne GJ. The use of healthcare services for mental health problems by middle-aged and older adults. Arch Gerontol Geriatr. 2014;59(2):393-397.
14. Gasper J, Jiu H, Kim S, May L. 2015 survey of veteran enrollees’ health and use of health care. https://va.gov/HEALTHPOLICYPLANNING/SoE2015/2015_VHA_SoE_Full _Findings_Report.pdf. Published December 11, 2015. Accessed February 5, 2019.
15. U.S. Department of Veterans Affairs. VA’s unparalleled integrated mental health services. https://www.scribd.com/document/356011769/U-S-Department-of-Veterans-Affairs -mental-health-fact-sheet. Published May 2017. Accessed January 26, 2018.
16. Karlin BE, Ruzek JI, Chard KM, et al. Dissemination of evidence-based psychological treatments for posttraumatic stress disorder in the Veterans Health Administration. J Trauma Stress. 2010;23(6):663-673.
17. Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum MA, Karlin BE. Effectiveness of national implementation of prolonged exposure therapy in Veterans Affairs care. JAMA Psychiatry. 2013;70(9):949-955.
18. Chard KM, Ricksecker EG, Healy ET, Karlin BE, Resick PA. Dissemination and experience with cognitive processing therapy. J Rehabil Res Dev. 2012;49(5):667-678.
19. Karlin BE, Walser RD, Yesavage J, Zhang A, Trockel M, Taylor CB. Effectiveness of acceptance and commitment therapy for depression: comparison among older and younger veterans. Aging Ment Health. 2013;17(5):555-563.
20. Karlin BE, Trockel M, Brown GK, Gordienko M, Yesavage J, Taylor CB. Comparison of the effectiveness of cognitive behavioral therapy for depression among older versus younger veterans: results of a national evaluation. J Gerontol B Psychol Sci Soc Sci. 2015;70(1):3-12.
21. Stewart MO, Raffa SD, Steele JL, et al. National dissemination of interpersonal psychotherapy for depression in veterans: therapist and patient-level outcomes. J Consult Clin Psychol. 2014;82(6):1201-1206.
22. Walser RD, Karlin BE, Trockel M, Mazina B, Barr Taylor C. Training in and implementation of Acceptance and Commitment Therapy for depression in the Veterans Health Administration: therapist and patient outcomes. Behav Res Ther. 2013;51(9):555-563.
23. Tanielian T, Farris C, Batka C, et al; Rand Corporation. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. https://www.rand.org/pubs/research_reports/RR806.html. Published November 2014. Accessed February 5, 2018.
24. Finley EP, Noël PH, Lee S, et al. Psychotherapy practices for veterans with PTSD among community-based providers in Texas. Psychol Serv. 2017. [Epub ahead of print.]
25. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2015;67(4):391-396.
26. Barry CN, Bowe TR, Suneja A. An update on the quality of medication treatment for mental disorders in the VA. Psychiatr Serv. 2016;67(8):930.
27. Mechanic D. More people than ever before are receiving behavioral health care in the United States, but gaps and challenges remain. Health Aff (Millwood). 2014;33(8):1416-1424.
28. Karlin B, Zeiss AM. Transforming mental health care for older veterans in the Veterans Health Administration. Generations. 2010;34(2):74-83.
29. Karlin BE, Karel MJ. National integration of mental health providers in VA home-based primary care: an innovative model for mental health care delivery with older adults. Gerontologist. 2014;54(5):868-879.
30. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
31. DiNapoli EA, Cully JA, Wayde E, Sansgiry S, Yu HJ, Kunik ME. Age as a predictive factor of mental health service use among adults with depression and/or anxiety disorder receiving care through the Veterans Health Administration. Int J Geriatr Psychiatry. 2016;31(6):575-582.
32. Shepardson RL, Funderburk JS. Likelihood of attending treatment for anxiety among veteran primary care patients: patient preferences for treatment attributes. J Clin Psychol Med Settings. 2016;23(3):225-239.
33. Johnson-Lawrence VD, Szymanski BR, Zivin K, McCarthy JF, Valenstein M, Pfeiffer PN. Primary care-mental health integration programs in the Veterans Affairs health system serve a different patient population than specialty mental health clinics. Prim Care Companion CNS Disord. 2012;14(3):pii.
34. Mackenzie CS, Gekoski WL, Knox VJ. Age, gender, and the underutilization of mental health services: the influence of help-seeking attitudes. Aging Ment Health. 2006;10(6):574-582.
35. Bartels SJ, Coakley EH, Zubritsky C, et al; PRISM-E Investigators. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry. 2004;161(8):1455-1462.
36. Wiechers IR, Karel MJ, Hoff R, Karlin BE. Growing use of mental and general health care services among older veterans with mental illness. Psychiatr Serv. 2015;66(11):1242-1244.
37. Mavandadi S, Klaus JR, Oslin DW. Age group differences among veterans enrolled in a clinical service for behavioral health issues in primary care. Am J Geriatr Psychiatry. 2012;20(3):205-214.
38. Cully JA, Stanley MA, Petersen NJ, et al. Delivery of brief cognitive behavioral therapy for medically ill patients in primary care: a pragmatic randomized clinical trial. J Gen Intern Med. 2017;32(9):1014-1024.
39. Commission on Care. Commission on care: final report.https://s3.amazonaws.com/sitesusa/wp-content/uploads/sites/912/2016/07/Commission-on-Care_Final-Report_063016_FOR-WEB.pdf. Published June 30, 2016. Accessed February 5, 2018.
40. U.S. Department of Veterans Affairs, Military Sexual Trauma Support Team. Military sexual trauma. https://www.menta lhealth.va.gov/docs/mst_general_factsheet.pdf. Published May 2015. Accessed February 13, 2018.
41. Kimerling R, Gima K, Smith MW, Street A, Frayne S. The Veterans Health Administration and military sexual trauma. Am J Public Health. 2007;97(12):2160-2166.
42. Schry AR, Hibberd R, Wagner HR, et al; Veterans Affairs Mid-Atlantic Medical Research Education and Clinical Center Workgroup, Brancu M. Functional correlates of military sexual assault in male veterans. Psychol Serv. 2015;12(4):384-393.
43. Millegan J, Milburn EK, LeardMann CA, et al. Recent sexual trauma and adverse health and occupational outcomes among U.S. service women. J Trauma Stress. 2015;28(4):298-306.
44. Kimerling R, Makin-Byrd K, Louzon S, Ignacio RV, McCarthy JF. Military sexual trauma and suicide mortality. Am J Prev Med. 2016;50(6):684-691.
45. U.S. Department of Defense. Department of Defense Fiscal Year 2016 Annual Report on Sexual Assault in the Military. Washington DC: U.S. Office of the Under Secretary of Defense; 2017.
46. Haas AP, Eliason M, Mays VM, et al. Suicide and suicide risk in lesbian, gay, bisexual, and transgender populations: review and recommendations. J Homosex. 2011;58(1):10-51.
47. Blosnich JR, Mays VM, Cochran SD. Suicidality among veterans: implications of sexual minority status. Am J Public Health. 2014;104(suppl 4):S535-S537.
48. Kilbourne AM, Ignacio RV, Kim HM, Blow FC. Datapoints: are VA patients with serious mental illness dying younger? Psychiatr Serv. 2009;60(5):589.
49. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79.
50. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans’ reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
51. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(11):1-49.
52. Seal K, Becker W, Tighe J, Li Y, Rife T. Managing chronic pain in primary care: it really does take a village. J Gen Intern Med. 2017;32(8):931-934.
53. Dorflinger LM, Ruser C, Sellinger J, Edens EL, Kerns RD, Becker WC. Integrating interdisciplinary pain management into primary care: development and implementation of a novel clinical program. Pain Med. 2014;15(12):2046-2054.
54. Gatchel RJ, Okifuji A. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J Pain. 2006;7(11):779-793.
55. Schatman ME. Interdisciplinary chronic pain management: international perspectives. Pain Clin Updates. 2012;20(7):1-5.
56. U.S. Department of Veterans Affairs. The US Department of Veterans Affairs Behavioral Health Autopsy Program (BHAP) Report, December 1, 2012-June 30, 2015. https://catalog .data.gov/dataset/behavioral-health-autopsy-program-bhap. Accessed November 3, 2017.
57. Veterans of Foreign Wars. Our care 2017: a report evaluating veterans health care. March 2017. https://vfw-cdn .azureedge.net/-/media/VFWSite/Files/Advocacy/VFW-Our -Care-2017--Executive-Summary.pdf. Published March 2017. Accessed February 6, 2018.
58. U.S. Department of Veterans Affairs, VA Center for Innovation and the Public Policy Lab. Veteran access to mental health services. https://www.innovation.va.gov/docs/Ve teranAccessToMentalHealthServices.pdf. Published 2016. Accessed February 6, 2017.
59. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25-64: United States, 2007-2010. NCHS Data Brief. 2012;(101):1-8.
60. National Academies of Sciences, Engineering, and Medicine. Evaluation of the Department of Veterans Affairs Mental Health Services. Washington D.C.: The National Academies Press; 2018.
1. U.S. Department of Veterans Affairs, Office of Suicide Prevention. Suicide among veterans and other Americans, 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Updated August 2017. Accessed February 6, 2018.
2. Provision of Mental Health Care to Veterans by Certain Mental Health Providers Participating in the Veterans Choice Program, 115th Cong, 1st Sess (2017). http://docs.house.gov/meetings/VR/VR00/20171024/106521/BILLS-1152pih-DRAFTtodirectVAtofurnishMHcaretoveteransatcommunityornon-profitMHprovidersparticipatinginChoice.pdf. Accessed February 5, 2018.
3. Caring for Our Veterans Act of 2017, S 2193, 115th Cong, 1st Sess (2017).
4. Farmer CM, Hosek SD, Adamson DM; RAND Corporation. Balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. 2016. https://www.rand.org/pubs/research_reports/RR1165z4.html. Published February 2016. Accessed February 5, 2018.
5. Suicide Prevention Resource Center, SPAN USA; Litts D, ed. Charting the future of suicide prevention: a 2010 progress review of the national strategy and recommendations for the decade ahead. http://www.sprc.org/sites/default/files/migrate /library/ChartingTheFuture_Fullbook.pdf. Published August 2010. Accessed February 5, 2018.
6. Bagalman E; Congressional Research Service. Health care for veterans: suicide prevention. https://fas.org/sgp/crs/misc/R42340.pdf. Published February 23, 2016.Accessed January 5, 2018.
7. McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942.
8. Kaplan MS, McFarland BH, Huguet N, Valenstein M. Suicide risk and precipitating circumstances among young, middle-aged, and older male veterans. Am J Public Health. 2012;102(suppl 1):S131-S137.
9. Louzon SA, Bossarte R, McCarthy JF, Katz IR. Does suicidal ideation as measured by the PHQ-9 predict suicide among VA patients? Psychiatr Serv. 2016;67(5):517-522.
10. Mills PD, Watts BV, Huh TJ, Boar S, Kemp J. Helping elderly patients to avoid suicide: a review of case reports from a national veterans affairs database. J Nerv Ment Dis. 2013;201(1):12-16.
11. Smith EG, Kim HM, Ganoczy D, Stano C, Pfeiffer PN, Valenstein M. Suicide risk assessment received prior to suicide death by Veterans Health Administration patients with a history of depression. J Clin Psychiatry. 2013;74(3):226-232.
12. Blais RK, Tsai J, Southwick SM, Pietrzak RH. Barriers and facilitators related to mental health care use among older veterans in the United States. Psychiatr Serv. 2015;66(5):500-506.
13. Gonçalves DC, Coelho CM, Byrne GJ. The use of healthcare services for mental health problems by middle-aged and older adults. Arch Gerontol Geriatr. 2014;59(2):393-397.
14. Gasper J, Jiu H, Kim S, May L. 2015 survey of veteran enrollees’ health and use of health care. https://va.gov/HEALTHPOLICYPLANNING/SoE2015/2015_VHA_SoE_Full _Findings_Report.pdf. Published December 11, 2015. Accessed February 5, 2019.
15. U.S. Department of Veterans Affairs. VA’s unparalleled integrated mental health services. https://www.scribd.com/document/356011769/U-S-Department-of-Veterans-Affairs -mental-health-fact-sheet. Published May 2017. Accessed January 26, 2018.
16. Karlin BE, Ruzek JI, Chard KM, et al. Dissemination of evidence-based psychological treatments for posttraumatic stress disorder in the Veterans Health Administration. J Trauma Stress. 2010;23(6):663-673.
17. Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum MA, Karlin BE. Effectiveness of national implementation of prolonged exposure therapy in Veterans Affairs care. JAMA Psychiatry. 2013;70(9):949-955.
18. Chard KM, Ricksecker EG, Healy ET, Karlin BE, Resick PA. Dissemination and experience with cognitive processing therapy. J Rehabil Res Dev. 2012;49(5):667-678.
19. Karlin BE, Walser RD, Yesavage J, Zhang A, Trockel M, Taylor CB. Effectiveness of acceptance and commitment therapy for depression: comparison among older and younger veterans. Aging Ment Health. 2013;17(5):555-563.
20. Karlin BE, Trockel M, Brown GK, Gordienko M, Yesavage J, Taylor CB. Comparison of the effectiveness of cognitive behavioral therapy for depression among older versus younger veterans: results of a national evaluation. J Gerontol B Psychol Sci Soc Sci. 2015;70(1):3-12.
21. Stewart MO, Raffa SD, Steele JL, et al. National dissemination of interpersonal psychotherapy for depression in veterans: therapist and patient-level outcomes. J Consult Clin Psychol. 2014;82(6):1201-1206.
22. Walser RD, Karlin BE, Trockel M, Mazina B, Barr Taylor C. Training in and implementation of Acceptance and Commitment Therapy for depression in the Veterans Health Administration: therapist and patient outcomes. Behav Res Ther. 2013;51(9):555-563.
23. Tanielian T, Farris C, Batka C, et al; Rand Corporation. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. https://www.rand.org/pubs/research_reports/RR806.html. Published November 2014. Accessed February 5, 2018.
24. Finley EP, Noël PH, Lee S, et al. Psychotherapy practices for veterans with PTSD among community-based providers in Texas. Psychol Serv. 2017. [Epub ahead of print.]
25. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2015;67(4):391-396.
26. Barry CN, Bowe TR, Suneja A. An update on the quality of medication treatment for mental disorders in the VA. Psychiatr Serv. 2016;67(8):930.
27. Mechanic D. More people than ever before are receiving behavioral health care in the United States, but gaps and challenges remain. Health Aff (Millwood). 2014;33(8):1416-1424.
28. Karlin B, Zeiss AM. Transforming mental health care for older veterans in the Veterans Health Administration. Generations. 2010;34(2):74-83.
29. Karlin BE, Karel MJ. National integration of mental health providers in VA home-based primary care: an innovative model for mental health care delivery with older adults. Gerontologist. 2014;54(5):868-879.
30. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
31. DiNapoli EA, Cully JA, Wayde E, Sansgiry S, Yu HJ, Kunik ME. Age as a predictive factor of mental health service use among adults with depression and/or anxiety disorder receiving care through the Veterans Health Administration. Int J Geriatr Psychiatry. 2016;31(6):575-582.
32. Shepardson RL, Funderburk JS. Likelihood of attending treatment for anxiety among veteran primary care patients: patient preferences for treatment attributes. J Clin Psychol Med Settings. 2016;23(3):225-239.
33. Johnson-Lawrence VD, Szymanski BR, Zivin K, McCarthy JF, Valenstein M, Pfeiffer PN. Primary care-mental health integration programs in the Veterans Affairs health system serve a different patient population than specialty mental health clinics. Prim Care Companion CNS Disord. 2012;14(3):pii.
34. Mackenzie CS, Gekoski WL, Knox VJ. Age, gender, and the underutilization of mental health services: the influence of help-seeking attitudes. Aging Ment Health. 2006;10(6):574-582.
35. Bartels SJ, Coakley EH, Zubritsky C, et al; PRISM-E Investigators. Improving access to geriatric mental health services: a randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. Am J Psychiatry. 2004;161(8):1455-1462.
36. Wiechers IR, Karel MJ, Hoff R, Karlin BE. Growing use of mental and general health care services among older veterans with mental illness. Psychiatr Serv. 2015;66(11):1242-1244.
37. Mavandadi S, Klaus JR, Oslin DW. Age group differences among veterans enrolled in a clinical service for behavioral health issues in primary care. Am J Geriatr Psychiatry. 2012;20(3):205-214.
38. Cully JA, Stanley MA, Petersen NJ, et al. Delivery of brief cognitive behavioral therapy for medically ill patients in primary care: a pragmatic randomized clinical trial. J Gen Intern Med. 2017;32(9):1014-1024.
39. Commission on Care. Commission on care: final report.https://s3.amazonaws.com/sitesusa/wp-content/uploads/sites/912/2016/07/Commission-on-Care_Final-Report_063016_FOR-WEB.pdf. Published June 30, 2016. Accessed February 5, 2018.
40. U.S. Department of Veterans Affairs, Military Sexual Trauma Support Team. Military sexual trauma. https://www.menta lhealth.va.gov/docs/mst_general_factsheet.pdf. Published May 2015. Accessed February 13, 2018.
41. Kimerling R, Gima K, Smith MW, Street A, Frayne S. The Veterans Health Administration and military sexual trauma. Am J Public Health. 2007;97(12):2160-2166.
42. Schry AR, Hibberd R, Wagner HR, et al; Veterans Affairs Mid-Atlantic Medical Research Education and Clinical Center Workgroup, Brancu M. Functional correlates of military sexual assault in male veterans. Psychol Serv. 2015;12(4):384-393.
43. Millegan J, Milburn EK, LeardMann CA, et al. Recent sexual trauma and adverse health and occupational outcomes among U.S. service women. J Trauma Stress. 2015;28(4):298-306.
44. Kimerling R, Makin-Byrd K, Louzon S, Ignacio RV, McCarthy JF. Military sexual trauma and suicide mortality. Am J Prev Med. 2016;50(6):684-691.
45. U.S. Department of Defense. Department of Defense Fiscal Year 2016 Annual Report on Sexual Assault in the Military. Washington DC: U.S. Office of the Under Secretary of Defense; 2017.
46. Haas AP, Eliason M, Mays VM, et al. Suicide and suicide risk in lesbian, gay, bisexual, and transgender populations: review and recommendations. J Homosex. 2011;58(1):10-51.
47. Blosnich JR, Mays VM, Cochran SD. Suicidality among veterans: implications of sexual minority status. Am J Public Health. 2014;104(suppl 4):S535-S537.
48. Kilbourne AM, Ignacio RV, Kim HM, Blow FC. Datapoints: are VA patients with serious mental illness dying younger? Psychiatr Serv. 2009;60(5):589.
49. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79.
50. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans’ reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
51. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(11):1-49.
52. Seal K, Becker W, Tighe J, Li Y, Rife T. Managing chronic pain in primary care: it really does take a village. J Gen Intern Med. 2017;32(8):931-934.
53. Dorflinger LM, Ruser C, Sellinger J, Edens EL, Kerns RD, Becker WC. Integrating interdisciplinary pain management into primary care: development and implementation of a novel clinical program. Pain Med. 2014;15(12):2046-2054.
54. Gatchel RJ, Okifuji A. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J Pain. 2006;7(11):779-793.
55. Schatman ME. Interdisciplinary chronic pain management: international perspectives. Pain Clin Updates. 2012;20(7):1-5.
56. U.S. Department of Veterans Affairs. The US Department of Veterans Affairs Behavioral Health Autopsy Program (BHAP) Report, December 1, 2012-June 30, 2015. https://catalog .data.gov/dataset/behavioral-health-autopsy-program-bhap. Accessed November 3, 2017.
57. Veterans of Foreign Wars. Our care 2017: a report evaluating veterans health care. March 2017. https://vfw-cdn .azureedge.net/-/media/VFWSite/Files/Advocacy/VFW-Our -Care-2017--Executive-Summary.pdf. Published March 2017. Accessed February 6, 2018.
58. U.S. Department of Veterans Affairs, VA Center for Innovation and the Public Policy Lab. Veteran access to mental health services. https://www.innovation.va.gov/docs/Ve teranAccessToMentalHealthServices.pdf. Published 2016. Accessed February 6, 2017.
59. Kramarow EA, Pastor PN. The health of male veterans and nonveterans aged 25-64: United States, 2007-2010. NCHS Data Brief. 2012;(101):1-8.
60. National Academies of Sciences, Engineering, and Medicine. Evaluation of the Department of Veterans Affairs Mental Health Services. Washington D.C.: The National Academies Press; 2018.
The Clinicians’ Role in Building a System of Care: Army Behavioral Health Since 2001
The Military Health System has changed in countless ways during the era of the Global War on Terror, but no clinical area has been challenged as thoroughly and transformed as extensively as U.S. Army behavioral health care. Through 2011, the wars in Iraq and Afghanistan required soldiers to spend more than 1.5 million years in theater and contributed to significant increases in the incidence of posttraumatic stress disorder (PTSD), depression, and other behavioral health conditions in soldiers and family members.1,2
Unfortunately, the Army’s behavioral health treatment system, which had sufficiently met Army beneficiaries’ needs during peacetime, w
as not adequately resourced or structured to provide care on the scale that was required, and major problems in access, quality, continuity, and safety emerged.3 Army behavioral health and primary care providers experienced these challenges within their own practices, and many developed local solutions. Realizing that a near-complete system transformation was necessary, medical leaders turned to clinicians drawn from the field to develop a strategy to identify the best clinical practices and to build a standardized, cohesive system of care around them.
Although many challenges remain, the Army’s system of behavioral health care has substantially improved and now better supports clinicians in the field as they care for soldiers and their family members. Clinicians at the headquarters and local hospital levels played key roles in this transformation; however, very little literature on the topic exists. Clinicians engaging in current or planning for future health care system changes would benefit from a description of the process.
Unprecedented Challenges
As the wars in Iraq and Afghanistan intensified in the mid-2000s and the demand for behavioral health care grew, the full picture of the shortfalls in the Army’s system of behavioral health care came to the forefront. Provider shortages contributed to long waits for initial appointments, disrupted continuity, and reduced effectiveness of care.4 Even after an infusion of more than $1 billion in congressionally directed funding increased the number of staff and multiplied the clinical and nonclinical programs across the force, significant problems remained.5,6
Army hospitals divided behavioral health care between psychiatry, psychology, and social work fiefdoms, whose stovepipes often fractured communication between providers treating the same patient and prevented effective care coordination. Behavioral health clinics on each post offered different clinical services. Confused soldiers, leaders, and family members were forced to navigate various versions of behavioral health care each time they moved. Inaccurate, locally developed information systems produced little data on the effectiveness of local programs and hindered clinicians and leaders seeking to improve care. Without sufficient clinical capacity on the outpatient side, outpatient providers frequently admitted their patients to inpatient settings because it presented the only option to deliver needed services in a safe setting.
The turning point for improving care occurred in 2012 when senior medical leaders designated a behavioral health leadership team of clinicians, administrators, and analysts at the Army’s Office of the Surgeon General (OTSG) as its first service line. The move consolidated authority over behavioral health-related policy, programs, and funding and clearly communicated that Army Medicine was committed to improving behavioral health care. While behavioral health leaders had operated at OTSG for several years prior, they did not have the authority or resources to make widespread changes. Fortunately, the Army Surgeon General also adopted a command philosophy that emphasized the Operating Company Model, which sought to reduce variance by replicating successful practices across the enterprise. The Operating Company Model also limited each hospital commander’s authority to design their own unique clinical structure and shifted that responsibility to the clinicians in each service line at the headquarters level.
Forming a System of Care
The Behavioral Health Service Line partnered with the U.S. Army Public Health Command and systems engineers at the Massachusetts Institute of Technology to map the existing system of care and identify innovative clinical programs that represented best practices. In addition to 2 programs mandated by the DoD (Behavioral Health in Primary Care Medical Homes7 and Family Advocacy Programs),8 other programs (Embedded Behavioral Health, Multi-Disciplinary Behavioral Health clinics, Intensive Outpatient Program, inpatient care, residential care for substance use disorders, Connect Care, Tele-Behavioral Health, and the Child and Family Behavioral Health System) were selected for replication throughout the Army because they successfully demonstrated promising outcomes and filled a critical need.
To run the emerging standardized clinical programs, the Behavioral Health Service Line streamlined the leadership structure by eliminating all departments organized around provider discipline (ie, psychiatry, psychology, and social work) and created a single department of Behavioral Health at each Army hospital. For the first time, staff were organized into clinical teams based on the needs of the patients, not professional background. This change created new leadership opportunities across disciplines, reduced infighting, and eliminated a major source of confusion for patients and line leaders. The group of standard clinical programs, managed through integrated behavioral health departments in each Army hospital, was dubbed the Behavioral Health System of Care.
Change to department organizations and clinical programs created the need to reconfigure administrative data systems to provide accurate and timely information about system performance. Administrative teams revised Medical Expense Reporting and Performance System codes to provide visibility on important data, such as patient encounters and Revenue Value Units, down to the clinic and provider levels. To improve the reliability of existing data, OTSG staff led a multiyear effort to “clean” several data sources, such as those specifying provider type and work center.
Analysts used the refined workload information to build new tools, such as one to display individual provider productivity and one to predict the number and type of clinical staff required to meet the needs of the population at each Army installation. The administrative reorganization better informed clinical leaders at all levels by supplying meaningful data that enabled comparisons of performance within one or more Army hospitals.8
Translating System Changes Into Better Care
Despite transformative changes to Army behavioral health care, clinicians and leaders still had little insight into what mattered most: measuirng patient response to the treatment. To solve this issue, a team of Army clinical and information technology innovators developed the Behavioral Health Data Portal (BHDP), which used validated scales to collect input from patients at each outpatient visit about their current and recent symptoms. Clinicians use the information to complement their assessment, refine diagnoses, individualize treatment plans, and follow their patients’ progress.9
The BHDP also aggregates information on each patient’s progress into clinical outcome metrics that inform leaders about the effectiveness of the care their clinics provide. For the first time, analysts can establish correlations between specific actions at the clinic level and positive clinical outcomes. For example, the relationship between the use of evidence-based psychotherapies, regular follow-up, a strong therapeutic alliance, and provider use of BHDP have been clearly linked to more rapid resolution of PTSD symptoms. These insights provide opportunities to act on specific processes at the clinic level with high confidence that by doing so, clinicians are improving the effectiveness of the care delivered in their clinics.
Conclusion
The transformation of Army behavioral health care has encompassed all aspects of the treatment system, but it has been led by clinicians working at the local and headquarters levels (Figure). Although many challenges remain, today’s outpatient system is more efficient and effective. For example, Army medical facilities are now able to meet more of the total demand for outpatient behavioral health care of its beneficiaries; 77% in September 2017 compared with a low of 59% in January 2013, based on Army Strategic Management System data as of December 1, 2017. With
The Army continues to improve its system of care to better inform and enable clinicians to deliver evidence-based care. The clinician-led process that advanced this area of military medicine is applicable to others. A core group of dedicated clinical professionals committed to multiyear processes can implement large-scale changes if they are empowered and resourced by senior medical leaders. A standardized system of care built on clinical best practices and guided by clinicians using accurate data, including clinical outcomes, would benefit any component of the MHS.
1. Baiocchi D. Measuring Army deployments to Iraq and Afghanistan. https://www.rand.org/pubs/research_reports/RR145.html. Published 2013. Accessed December 1, 2017.
2. Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman D. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
3. Tanielian T, Jaycox LH (eds). Invisible wounds of war. RAND Corporation. https://www.rand.org/pubs/monographs/MG720.html. Published 2008. Accessed December 1, 2017.
4. Prine C. Army’s mental health programs swamped, understaffed. Pittsburgh Tribune-Review. http://triblive .com/x/pittsburghtrib/news/s_721604.html. Published February 7, 2011. Accessed December 1, 2017.
5. Weinick R, Beckjord E, Farmer C, et al. Programs addressing psychological health and traumatic brain injury among U.S. military servicemembers and their families. https://www.rand.org/pubs/technical_reports/TR950 .html. Published 2011. Accessed December 1, 2017.
6. Hoge CW, Ivany CG, Brusher EA, et al. Transformation of mental health care for U.S. soldiers and families during the Iraq and Afghanistan wars: where science and politics intersect. Am J Psychiatry. 2016;173(4):334-343.
7. Hunter CL, Goodie JL. Operational and clinical components for integrated-collaborative behavioral healthcare in the patient-centered medical home. Fam Syst Health. 2010;28(4):308-321.
8. Srinivasan J, Ivany CG, Sarmiento D, Woodson J. How the U.S. Army redesigned its mental health system. Harvard Business Review. https://hbr.org/2017/10/how-the-u-s-army-redesigned-its-mental-health-system. Published October 16, 2017. Accessed December 1, 2017.
9. Srinivasan J, Brown MD, Ivany CG, Woodson J. How the U.S. Army personalized its mental health care. Harvard Business Review. https://hbr.org/2016/12/how-the-u-s-army-personalized-its-mental-health-care. Published December 7, 2016. Accessed December 1, 2017.
The Military Health System has changed in countless ways during the era of the Global War on Terror, but no clinical area has been challenged as thoroughly and transformed as extensively as U.S. Army behavioral health care. Through 2011, the wars in Iraq and Afghanistan required soldiers to spend more than 1.5 million years in theater and contributed to significant increases in the incidence of posttraumatic stress disorder (PTSD), depression, and other behavioral health conditions in soldiers and family members.1,2
Unfortunately, the Army’s behavioral health treatment system, which had sufficiently met Army beneficiaries’ needs during peacetime, w
as not adequately resourced or structured to provide care on the scale that was required, and major problems in access, quality, continuity, and safety emerged.3 Army behavioral health and primary care providers experienced these challenges within their own practices, and many developed local solutions. Realizing that a near-complete system transformation was necessary, medical leaders turned to clinicians drawn from the field to develop a strategy to identify the best clinical practices and to build a standardized, cohesive system of care around them.
Although many challenges remain, the Army’s system of behavioral health care has substantially improved and now better supports clinicians in the field as they care for soldiers and their family members. Clinicians at the headquarters and local hospital levels played key roles in this transformation; however, very little literature on the topic exists. Clinicians engaging in current or planning for future health care system changes would benefit from a description of the process.
Unprecedented Challenges
As the wars in Iraq and Afghanistan intensified in the mid-2000s and the demand for behavioral health care grew, the full picture of the shortfalls in the Army’s system of behavioral health care came to the forefront. Provider shortages contributed to long waits for initial appointments, disrupted continuity, and reduced effectiveness of care.4 Even after an infusion of more than $1 billion in congressionally directed funding increased the number of staff and multiplied the clinical and nonclinical programs across the force, significant problems remained.5,6
Army hospitals divided behavioral health care between psychiatry, psychology, and social work fiefdoms, whose stovepipes often fractured communication between providers treating the same patient and prevented effective care coordination. Behavioral health clinics on each post offered different clinical services. Confused soldiers, leaders, and family members were forced to navigate various versions of behavioral health care each time they moved. Inaccurate, locally developed information systems produced little data on the effectiveness of local programs and hindered clinicians and leaders seeking to improve care. Without sufficient clinical capacity on the outpatient side, outpatient providers frequently admitted their patients to inpatient settings because it presented the only option to deliver needed services in a safe setting.
The turning point for improving care occurred in 2012 when senior medical leaders designated a behavioral health leadership team of clinicians, administrators, and analysts at the Army’s Office of the Surgeon General (OTSG) as its first service line. The move consolidated authority over behavioral health-related policy, programs, and funding and clearly communicated that Army Medicine was committed to improving behavioral health care. While behavioral health leaders had operated at OTSG for several years prior, they did not have the authority or resources to make widespread changes. Fortunately, the Army Surgeon General also adopted a command philosophy that emphasized the Operating Company Model, which sought to reduce variance by replicating successful practices across the enterprise. The Operating Company Model also limited each hospital commander’s authority to design their own unique clinical structure and shifted that responsibility to the clinicians in each service line at the headquarters level.
Forming a System of Care
The Behavioral Health Service Line partnered with the U.S. Army Public Health Command and systems engineers at the Massachusetts Institute of Technology to map the existing system of care and identify innovative clinical programs that represented best practices. In addition to 2 programs mandated by the DoD (Behavioral Health in Primary Care Medical Homes7 and Family Advocacy Programs),8 other programs (Embedded Behavioral Health, Multi-Disciplinary Behavioral Health clinics, Intensive Outpatient Program, inpatient care, residential care for substance use disorders, Connect Care, Tele-Behavioral Health, and the Child and Family Behavioral Health System) were selected for replication throughout the Army because they successfully demonstrated promising outcomes and filled a critical need.
To run the emerging standardized clinical programs, the Behavioral Health Service Line streamlined the leadership structure by eliminating all departments organized around provider discipline (ie, psychiatry, psychology, and social work) and created a single department of Behavioral Health at each Army hospital. For the first time, staff were organized into clinical teams based on the needs of the patients, not professional background. This change created new leadership opportunities across disciplines, reduced infighting, and eliminated a major source of confusion for patients and line leaders. The group of standard clinical programs, managed through integrated behavioral health departments in each Army hospital, was dubbed the Behavioral Health System of Care.
Change to department organizations and clinical programs created the need to reconfigure administrative data systems to provide accurate and timely information about system performance. Administrative teams revised Medical Expense Reporting and Performance System codes to provide visibility on important data, such as patient encounters and Revenue Value Units, down to the clinic and provider levels. To improve the reliability of existing data, OTSG staff led a multiyear effort to “clean” several data sources, such as those specifying provider type and work center.
Analysts used the refined workload information to build new tools, such as one to display individual provider productivity and one to predict the number and type of clinical staff required to meet the needs of the population at each Army installation. The administrative reorganization better informed clinical leaders at all levels by supplying meaningful data that enabled comparisons of performance within one or more Army hospitals.8
Translating System Changes Into Better Care
Despite transformative changes to Army behavioral health care, clinicians and leaders still had little insight into what mattered most: measuirng patient response to the treatment. To solve this issue, a team of Army clinical and information technology innovators developed the Behavioral Health Data Portal (BHDP), which used validated scales to collect input from patients at each outpatient visit about their current and recent symptoms. Clinicians use the information to complement their assessment, refine diagnoses, individualize treatment plans, and follow their patients’ progress.9
The BHDP also aggregates information on each patient’s progress into clinical outcome metrics that inform leaders about the effectiveness of the care their clinics provide. For the first time, analysts can establish correlations between specific actions at the clinic level and positive clinical outcomes. For example, the relationship between the use of evidence-based psychotherapies, regular follow-up, a strong therapeutic alliance, and provider use of BHDP have been clearly linked to more rapid resolution of PTSD symptoms. These insights provide opportunities to act on specific processes at the clinic level with high confidence that by doing so, clinicians are improving the effectiveness of the care delivered in their clinics.
Conclusion
The transformation of Army behavioral health care has encompassed all aspects of the treatment system, but it has been led by clinicians working at the local and headquarters levels (Figure). Although many challenges remain, today’s outpatient system is more efficient and effective. For example, Army medical facilities are now able to meet more of the total demand for outpatient behavioral health care of its beneficiaries; 77% in September 2017 compared with a low of 59% in January 2013, based on Army Strategic Management System data as of December 1, 2017. With
The Army continues to improve its system of care to better inform and enable clinicians to deliver evidence-based care. The clinician-led process that advanced this area of military medicine is applicable to others. A core group of dedicated clinical professionals committed to multiyear processes can implement large-scale changes if they are empowered and resourced by senior medical leaders. A standardized system of care built on clinical best practices and guided by clinicians using accurate data, including clinical outcomes, would benefit any component of the MHS.
The Military Health System has changed in countless ways during the era of the Global War on Terror, but no clinical area has been challenged as thoroughly and transformed as extensively as U.S. Army behavioral health care. Through 2011, the wars in Iraq and Afghanistan required soldiers to spend more than 1.5 million years in theater and contributed to significant increases in the incidence of posttraumatic stress disorder (PTSD), depression, and other behavioral health conditions in soldiers and family members.1,2
Unfortunately, the Army’s behavioral health treatment system, which had sufficiently met Army beneficiaries’ needs during peacetime, w
as not adequately resourced or structured to provide care on the scale that was required, and major problems in access, quality, continuity, and safety emerged.3 Army behavioral health and primary care providers experienced these challenges within their own practices, and many developed local solutions. Realizing that a near-complete system transformation was necessary, medical leaders turned to clinicians drawn from the field to develop a strategy to identify the best clinical practices and to build a standardized, cohesive system of care around them.
Although many challenges remain, the Army’s system of behavioral health care has substantially improved and now better supports clinicians in the field as they care for soldiers and their family members. Clinicians at the headquarters and local hospital levels played key roles in this transformation; however, very little literature on the topic exists. Clinicians engaging in current or planning for future health care system changes would benefit from a description of the process.
Unprecedented Challenges
As the wars in Iraq and Afghanistan intensified in the mid-2000s and the demand for behavioral health care grew, the full picture of the shortfalls in the Army’s system of behavioral health care came to the forefront. Provider shortages contributed to long waits for initial appointments, disrupted continuity, and reduced effectiveness of care.4 Even after an infusion of more than $1 billion in congressionally directed funding increased the number of staff and multiplied the clinical and nonclinical programs across the force, significant problems remained.5,6
Army hospitals divided behavioral health care between psychiatry, psychology, and social work fiefdoms, whose stovepipes often fractured communication between providers treating the same patient and prevented effective care coordination. Behavioral health clinics on each post offered different clinical services. Confused soldiers, leaders, and family members were forced to navigate various versions of behavioral health care each time they moved. Inaccurate, locally developed information systems produced little data on the effectiveness of local programs and hindered clinicians and leaders seeking to improve care. Without sufficient clinical capacity on the outpatient side, outpatient providers frequently admitted their patients to inpatient settings because it presented the only option to deliver needed services in a safe setting.
The turning point for improving care occurred in 2012 when senior medical leaders designated a behavioral health leadership team of clinicians, administrators, and analysts at the Army’s Office of the Surgeon General (OTSG) as its first service line. The move consolidated authority over behavioral health-related policy, programs, and funding and clearly communicated that Army Medicine was committed to improving behavioral health care. While behavioral health leaders had operated at OTSG for several years prior, they did not have the authority or resources to make widespread changes. Fortunately, the Army Surgeon General also adopted a command philosophy that emphasized the Operating Company Model, which sought to reduce variance by replicating successful practices across the enterprise. The Operating Company Model also limited each hospital commander’s authority to design their own unique clinical structure and shifted that responsibility to the clinicians in each service line at the headquarters level.
Forming a System of Care
The Behavioral Health Service Line partnered with the U.S. Army Public Health Command and systems engineers at the Massachusetts Institute of Technology to map the existing system of care and identify innovative clinical programs that represented best practices. In addition to 2 programs mandated by the DoD (Behavioral Health in Primary Care Medical Homes7 and Family Advocacy Programs),8 other programs (Embedded Behavioral Health, Multi-Disciplinary Behavioral Health clinics, Intensive Outpatient Program, inpatient care, residential care for substance use disorders, Connect Care, Tele-Behavioral Health, and the Child and Family Behavioral Health System) were selected for replication throughout the Army because they successfully demonstrated promising outcomes and filled a critical need.
To run the emerging standardized clinical programs, the Behavioral Health Service Line streamlined the leadership structure by eliminating all departments organized around provider discipline (ie, psychiatry, psychology, and social work) and created a single department of Behavioral Health at each Army hospital. For the first time, staff were organized into clinical teams based on the needs of the patients, not professional background. This change created new leadership opportunities across disciplines, reduced infighting, and eliminated a major source of confusion for patients and line leaders. The group of standard clinical programs, managed through integrated behavioral health departments in each Army hospital, was dubbed the Behavioral Health System of Care.
Change to department organizations and clinical programs created the need to reconfigure administrative data systems to provide accurate and timely information about system performance. Administrative teams revised Medical Expense Reporting and Performance System codes to provide visibility on important data, such as patient encounters and Revenue Value Units, down to the clinic and provider levels. To improve the reliability of existing data, OTSG staff led a multiyear effort to “clean” several data sources, such as those specifying provider type and work center.
Analysts used the refined workload information to build new tools, such as one to display individual provider productivity and one to predict the number and type of clinical staff required to meet the needs of the population at each Army installation. The administrative reorganization better informed clinical leaders at all levels by supplying meaningful data that enabled comparisons of performance within one or more Army hospitals.8
Translating System Changes Into Better Care
Despite transformative changes to Army behavioral health care, clinicians and leaders still had little insight into what mattered most: measuirng patient response to the treatment. To solve this issue, a team of Army clinical and information technology innovators developed the Behavioral Health Data Portal (BHDP), which used validated scales to collect input from patients at each outpatient visit about their current and recent symptoms. Clinicians use the information to complement their assessment, refine diagnoses, individualize treatment plans, and follow their patients’ progress.9
The BHDP also aggregates information on each patient’s progress into clinical outcome metrics that inform leaders about the effectiveness of the care their clinics provide. For the first time, analysts can establish correlations between specific actions at the clinic level and positive clinical outcomes. For example, the relationship between the use of evidence-based psychotherapies, regular follow-up, a strong therapeutic alliance, and provider use of BHDP have been clearly linked to more rapid resolution of PTSD symptoms. These insights provide opportunities to act on specific processes at the clinic level with high confidence that by doing so, clinicians are improving the effectiveness of the care delivered in their clinics.
Conclusion
The transformation of Army behavioral health care has encompassed all aspects of the treatment system, but it has been led by clinicians working at the local and headquarters levels (Figure). Although many challenges remain, today’s outpatient system is more efficient and effective. For example, Army medical facilities are now able to meet more of the total demand for outpatient behavioral health care of its beneficiaries; 77% in September 2017 compared with a low of 59% in January 2013, based on Army Strategic Management System data as of December 1, 2017. With
The Army continues to improve its system of care to better inform and enable clinicians to deliver evidence-based care. The clinician-led process that advanced this area of military medicine is applicable to others. A core group of dedicated clinical professionals committed to multiyear processes can implement large-scale changes if they are empowered and resourced by senior medical leaders. A standardized system of care built on clinical best practices and guided by clinicians using accurate data, including clinical outcomes, would benefit any component of the MHS.
1. Baiocchi D. Measuring Army deployments to Iraq and Afghanistan. https://www.rand.org/pubs/research_reports/RR145.html. Published 2013. Accessed December 1, 2017.
2. Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman D. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
3. Tanielian T, Jaycox LH (eds). Invisible wounds of war. RAND Corporation. https://www.rand.org/pubs/monographs/MG720.html. Published 2008. Accessed December 1, 2017.
4. Prine C. Army’s mental health programs swamped, understaffed. Pittsburgh Tribune-Review. http://triblive .com/x/pittsburghtrib/news/s_721604.html. Published February 7, 2011. Accessed December 1, 2017.
5. Weinick R, Beckjord E, Farmer C, et al. Programs addressing psychological health and traumatic brain injury among U.S. military servicemembers and their families. https://www.rand.org/pubs/technical_reports/TR950 .html. Published 2011. Accessed December 1, 2017.
6. Hoge CW, Ivany CG, Brusher EA, et al. Transformation of mental health care for U.S. soldiers and families during the Iraq and Afghanistan wars: where science and politics intersect. Am J Psychiatry. 2016;173(4):334-343.
7. Hunter CL, Goodie JL. Operational and clinical components for integrated-collaborative behavioral healthcare in the patient-centered medical home. Fam Syst Health. 2010;28(4):308-321.
8. Srinivasan J, Ivany CG, Sarmiento D, Woodson J. How the U.S. Army redesigned its mental health system. Harvard Business Review. https://hbr.org/2017/10/how-the-u-s-army-redesigned-its-mental-health-system. Published October 16, 2017. Accessed December 1, 2017.
9. Srinivasan J, Brown MD, Ivany CG, Woodson J. How the U.S. Army personalized its mental health care. Harvard Business Review. https://hbr.org/2016/12/how-the-u-s-army-personalized-its-mental-health-care. Published December 7, 2016. Accessed December 1, 2017.
1. Baiocchi D. Measuring Army deployments to Iraq and Afghanistan. https://www.rand.org/pubs/research_reports/RR145.html. Published 2013. Accessed December 1, 2017.
2. Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman D. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
3. Tanielian T, Jaycox LH (eds). Invisible wounds of war. RAND Corporation. https://www.rand.org/pubs/monographs/MG720.html. Published 2008. Accessed December 1, 2017.
4. Prine C. Army’s mental health programs swamped, understaffed. Pittsburgh Tribune-Review. http://triblive .com/x/pittsburghtrib/news/s_721604.html. Published February 7, 2011. Accessed December 1, 2017.
5. Weinick R, Beckjord E, Farmer C, et al. Programs addressing psychological health and traumatic brain injury among U.S. military servicemembers and their families. https://www.rand.org/pubs/technical_reports/TR950 .html. Published 2011. Accessed December 1, 2017.
6. Hoge CW, Ivany CG, Brusher EA, et al. Transformation of mental health care for U.S. soldiers and families during the Iraq and Afghanistan wars: where science and politics intersect. Am J Psychiatry. 2016;173(4):334-343.
7. Hunter CL, Goodie JL. Operational and clinical components for integrated-collaborative behavioral healthcare in the patient-centered medical home. Fam Syst Health. 2010;28(4):308-321.
8. Srinivasan J, Ivany CG, Sarmiento D, Woodson J. How the U.S. Army redesigned its mental health system. Harvard Business Review. https://hbr.org/2017/10/how-the-u-s-army-redesigned-its-mental-health-system. Published October 16, 2017. Accessed December 1, 2017.
9. Srinivasan J, Brown MD, Ivany CG, Woodson J. How the U.S. Army personalized its mental health care. Harvard Business Review. https://hbr.org/2016/12/how-the-u-s-army-personalized-its-mental-health-care. Published December 7, 2016. Accessed December 1, 2017.
Psychological Consequences of Detainee Operations: What DoD and VA Health Care Providers Need to Know
Detainee operations is a dark topic and one that often is avoided in the welcome home of veterans participating in detainee operations. Many veterans who have been involved have hidden these missions, fearing that they would be tarnished by past scandals. However, the burden of these detainee missions may contribute to depression, moral injury, and suicidal behaviors.
The recent conflicts in Afghanistan and Iraq have produced many opportunities for lessons on detainee operations. Unfortunately, often the lessons learned from one conflict have not been carried forward to the next. The scandals at the Abu Ghraib prison in Iraq and the continuing controversy over practices at Guantanamo Bay (GTMO) in Cuba illustrate these lapses. This column will not dwell on these issues but on what has been the psychological effects on U.S. service members of guarding and caring for detainees.
Background
Since 9/11, military service members have been involved in detainee operations in many roles, including at the point of capture (when the detainee is taken into custody), guarding the detainee; interrogating the detainee, and providing medical and psychological care.
Our service members have been woefully underprepared for these missions, and as a result may have faced adverse psychological consequences. The work is often dangerous and tedious. The following dangerous or frustrating examples are from my experiences:
- Correctional staff at GTMO had feces thrown at them;
- U.S. staff were targeted by rocket propelled grenades at Abu Ghraib and moved into jail cells for protection;
- Medical personnel at Camp Bucca in Iraq were attacked by the detainees who used hand sanitizer and latex gloves to make miniature fire balls;
- Insufficient medical equipment at Abu Ghraib and other facilities to care for detainees;
- An overall lack of perceived support from the medical and correctional chain of commands; and
- Numerous different chains of commands with different priorities, leading to a sense of chaos.
Corrections Overlap
There is overlap with traditional correctional medical care, including the care of prisoners in traditional jails and prisons. Likewise, there are similar issues with migrants from Central America and other regions who enter the country illegally and often are put into makeshift camps or overcrowded jails. However, there are key differences when treating detainees in facilities outside the U.S.
These differences include the cultural aspects in caring for detainees from the Middle East and elsewhere, large holding areas with 200 to 300 detainees, such as Camp Bucca and Abhu Ghraib, indefinite terms of confinement, such as at GTMO, and high-visibility political implications especially suicide attempts and interrogations.
There are many challenges to providing medical support in detainee operations that may have adverse psychological consequences. These include the following:
- Care for detainees when first captured;
- Fear of infectious diseases in detainees;
- Care for detainees in the correctional facilities in Abu Ghraib, GTMO, the Theater Internment Facility at Bagram Air Base Afghanistan; and other facilities;
- Involvement in force-feeding and during hunger strikes;
- Avoiding abuse of detainees by military staff;
- Providing psychological support to interrogation efforts often known as Behavioral Science Consultation Teams; and
- Challenges in treatment of complex medical and psychiatric conditions among detainees, which may include a limited formulary, lack of full medical equipment, and austere conditions.
Psychological Reactions
These challenges contribute to the development of negative psychological reactions, including posttramatic stress syndrome (PTSD) and moral injury, in medical and corrections staff. The negative view of the American public toward these correctional issues contributes to a sense of shame among those who have guarded or treated detainees.
One veteran who worked at GTMO and the jail in Bagram reported that 10 of his colleagues killed themselves. Although there is robust literature on suicides in army and other military personnel, I do not know of any studies that examine suicides specifically in detainee’s operational staff.1,2 There have been analyses of the relationship between suicide and military occupational specialty (MOS).1 However, personnel in detainee operations may come from many different disciplines, including medical, correctional, and others.
Again, data here are anecdotal, since there is no public information available. However, I was sent to Abu Ghraib and Camp Bucca in 2004 to evaluate these issues. I have been to GTMO 5 times. Thus, I have personal experience that in forms this column.
Often the veteran will not bring up these experiences because of the shame and stigma. Therefore, clinicians need to ask about the veteran’s experience and whether he or she served at GTMO, Abu Ghraib, Camp Bucca, the Theater Facility in Bagram, or other points of capture.
Although there are many books and manuals about treating PTSD and some literature about providing mental health care to detainees, there is little published about providing care to staff involved with detainee’s operations. I postulate that many staff will have shame, guilt, or moral injury, which leads to suicidal thoughts. Treatment thus needs to be supportive, whether with medications or psychotherapy.
Conclusion
All these issues should be considered by clinical staff who are caring for service members who have been involved in detainee operations. Veterans may not volunteer that they have been involved in these operations; it is important to ask.
Guilt and shame may be large components of the presenting psychological presentation. This may lead to moral injury. Careful exploration of depressive and suicidal thoughts with these patients is needed. An understanding of these challenges will help with clinical care.
1. Black SA, Galloway MS, Bell MR, Ritchie EC. Prevalence and risk factors associated with suicides of army soldiers 2001-2009. Milit Psychol. 2011;23(4):433-451.
2. Ritchie EC. Suicides and the United States Army: perspectives from the former psychiatry consultant to the army surgeon general. Cerebrum. 2012;1.
Detainee operations is a dark topic and one that often is avoided in the welcome home of veterans participating in detainee operations. Many veterans who have been involved have hidden these missions, fearing that they would be tarnished by past scandals. However, the burden of these detainee missions may contribute to depression, moral injury, and suicidal behaviors.
The recent conflicts in Afghanistan and Iraq have produced many opportunities for lessons on detainee operations. Unfortunately, often the lessons learned from one conflict have not been carried forward to the next. The scandals at the Abu Ghraib prison in Iraq and the continuing controversy over practices at Guantanamo Bay (GTMO) in Cuba illustrate these lapses. This column will not dwell on these issues but on what has been the psychological effects on U.S. service members of guarding and caring for detainees.
Background
Since 9/11, military service members have been involved in detainee operations in many roles, including at the point of capture (when the detainee is taken into custody), guarding the detainee; interrogating the detainee, and providing medical and psychological care.
Our service members have been woefully underprepared for these missions, and as a result may have faced adverse psychological consequences. The work is often dangerous and tedious. The following dangerous or frustrating examples are from my experiences:
- Correctional staff at GTMO had feces thrown at them;
- U.S. staff were targeted by rocket propelled grenades at Abu Ghraib and moved into jail cells for protection;
- Medical personnel at Camp Bucca in Iraq were attacked by the detainees who used hand sanitizer and latex gloves to make miniature fire balls;
- Insufficient medical equipment at Abu Ghraib and other facilities to care for detainees;
- An overall lack of perceived support from the medical and correctional chain of commands; and
- Numerous different chains of commands with different priorities, leading to a sense of chaos.
Corrections Overlap
There is overlap with traditional correctional medical care, including the care of prisoners in traditional jails and prisons. Likewise, there are similar issues with migrants from Central America and other regions who enter the country illegally and often are put into makeshift camps or overcrowded jails. However, there are key differences when treating detainees in facilities outside the U.S.
These differences include the cultural aspects in caring for detainees from the Middle East and elsewhere, large holding areas with 200 to 300 detainees, such as Camp Bucca and Abhu Ghraib, indefinite terms of confinement, such as at GTMO, and high-visibility political implications especially suicide attempts and interrogations.
There are many challenges to providing medical support in detainee operations that may have adverse psychological consequences. These include the following:
- Care for detainees when first captured;
- Fear of infectious diseases in detainees;
- Care for detainees in the correctional facilities in Abu Ghraib, GTMO, the Theater Internment Facility at Bagram Air Base Afghanistan; and other facilities;
- Involvement in force-feeding and during hunger strikes;
- Avoiding abuse of detainees by military staff;
- Providing psychological support to interrogation efforts often known as Behavioral Science Consultation Teams; and
- Challenges in treatment of complex medical and psychiatric conditions among detainees, which may include a limited formulary, lack of full medical equipment, and austere conditions.
Psychological Reactions
These challenges contribute to the development of negative psychological reactions, including posttramatic stress syndrome (PTSD) and moral injury, in medical and corrections staff. The negative view of the American public toward these correctional issues contributes to a sense of shame among those who have guarded or treated detainees.
One veteran who worked at GTMO and the jail in Bagram reported that 10 of his colleagues killed themselves. Although there is robust literature on suicides in army and other military personnel, I do not know of any studies that examine suicides specifically in detainee’s operational staff.1,2 There have been analyses of the relationship between suicide and military occupational specialty (MOS).1 However, personnel in detainee operations may come from many different disciplines, including medical, correctional, and others.
Again, data here are anecdotal, since there is no public information available. However, I was sent to Abu Ghraib and Camp Bucca in 2004 to evaluate these issues. I have been to GTMO 5 times. Thus, I have personal experience that in forms this column.
Often the veteran will not bring up these experiences because of the shame and stigma. Therefore, clinicians need to ask about the veteran’s experience and whether he or she served at GTMO, Abu Ghraib, Camp Bucca, the Theater Facility in Bagram, or other points of capture.
Although there are many books and manuals about treating PTSD and some literature about providing mental health care to detainees, there is little published about providing care to staff involved with detainee’s operations. I postulate that many staff will have shame, guilt, or moral injury, which leads to suicidal thoughts. Treatment thus needs to be supportive, whether with medications or psychotherapy.
Conclusion
All these issues should be considered by clinical staff who are caring for service members who have been involved in detainee operations. Veterans may not volunteer that they have been involved in these operations; it is important to ask.
Guilt and shame may be large components of the presenting psychological presentation. This may lead to moral injury. Careful exploration of depressive and suicidal thoughts with these patients is needed. An understanding of these challenges will help with clinical care.
Detainee operations is a dark topic and one that often is avoided in the welcome home of veterans participating in detainee operations. Many veterans who have been involved have hidden these missions, fearing that they would be tarnished by past scandals. However, the burden of these detainee missions may contribute to depression, moral injury, and suicidal behaviors.
The recent conflicts in Afghanistan and Iraq have produced many opportunities for lessons on detainee operations. Unfortunately, often the lessons learned from one conflict have not been carried forward to the next. The scandals at the Abu Ghraib prison in Iraq and the continuing controversy over practices at Guantanamo Bay (GTMO) in Cuba illustrate these lapses. This column will not dwell on these issues but on what has been the psychological effects on U.S. service members of guarding and caring for detainees.
Background
Since 9/11, military service members have been involved in detainee operations in many roles, including at the point of capture (when the detainee is taken into custody), guarding the detainee; interrogating the detainee, and providing medical and psychological care.
Our service members have been woefully underprepared for these missions, and as a result may have faced adverse psychological consequences. The work is often dangerous and tedious. The following dangerous or frustrating examples are from my experiences:
- Correctional staff at GTMO had feces thrown at them;
- U.S. staff were targeted by rocket propelled grenades at Abu Ghraib and moved into jail cells for protection;
- Medical personnel at Camp Bucca in Iraq were attacked by the detainees who used hand sanitizer and latex gloves to make miniature fire balls;
- Insufficient medical equipment at Abu Ghraib and other facilities to care for detainees;
- An overall lack of perceived support from the medical and correctional chain of commands; and
- Numerous different chains of commands with different priorities, leading to a sense of chaos.
Corrections Overlap
There is overlap with traditional correctional medical care, including the care of prisoners in traditional jails and prisons. Likewise, there are similar issues with migrants from Central America and other regions who enter the country illegally and often are put into makeshift camps or overcrowded jails. However, there are key differences when treating detainees in facilities outside the U.S.
These differences include the cultural aspects in caring for detainees from the Middle East and elsewhere, large holding areas with 200 to 300 detainees, such as Camp Bucca and Abhu Ghraib, indefinite terms of confinement, such as at GTMO, and high-visibility political implications especially suicide attempts and interrogations.
There are many challenges to providing medical support in detainee operations that may have adverse psychological consequences. These include the following:
- Care for detainees when first captured;
- Fear of infectious diseases in detainees;
- Care for detainees in the correctional facilities in Abu Ghraib, GTMO, the Theater Internment Facility at Bagram Air Base Afghanistan; and other facilities;
- Involvement in force-feeding and during hunger strikes;
- Avoiding abuse of detainees by military staff;
- Providing psychological support to interrogation efforts often known as Behavioral Science Consultation Teams; and
- Challenges in treatment of complex medical and psychiatric conditions among detainees, which may include a limited formulary, lack of full medical equipment, and austere conditions.
Psychological Reactions
These challenges contribute to the development of negative psychological reactions, including posttramatic stress syndrome (PTSD) and moral injury, in medical and corrections staff. The negative view of the American public toward these correctional issues contributes to a sense of shame among those who have guarded or treated detainees.
One veteran who worked at GTMO and the jail in Bagram reported that 10 of his colleagues killed themselves. Although there is robust literature on suicides in army and other military personnel, I do not know of any studies that examine suicides specifically in detainee’s operational staff.1,2 There have been analyses of the relationship between suicide and military occupational specialty (MOS).1 However, personnel in detainee operations may come from many different disciplines, including medical, correctional, and others.
Again, data here are anecdotal, since there is no public information available. However, I was sent to Abu Ghraib and Camp Bucca in 2004 to evaluate these issues. I have been to GTMO 5 times. Thus, I have personal experience that in forms this column.
Often the veteran will not bring up these experiences because of the shame and stigma. Therefore, clinicians need to ask about the veteran’s experience and whether he or she served at GTMO, Abu Ghraib, Camp Bucca, the Theater Facility in Bagram, or other points of capture.
Although there are many books and manuals about treating PTSD and some literature about providing mental health care to detainees, there is little published about providing care to staff involved with detainee’s operations. I postulate that many staff will have shame, guilt, or moral injury, which leads to suicidal thoughts. Treatment thus needs to be supportive, whether with medications or psychotherapy.
Conclusion
All these issues should be considered by clinical staff who are caring for service members who have been involved in detainee operations. Veterans may not volunteer that they have been involved in these operations; it is important to ask.
Guilt and shame may be large components of the presenting psychological presentation. This may lead to moral injury. Careful exploration of depressive and suicidal thoughts with these patients is needed. An understanding of these challenges will help with clinical care.
1. Black SA, Galloway MS, Bell MR, Ritchie EC. Prevalence and risk factors associated with suicides of army soldiers 2001-2009. Milit Psychol. 2011;23(4):433-451.
2. Ritchie EC. Suicides and the United States Army: perspectives from the former psychiatry consultant to the army surgeon general. Cerebrum. 2012;1.
1. Black SA, Galloway MS, Bell MR, Ritchie EC. Prevalence and risk factors associated with suicides of army soldiers 2001-2009. Milit Psychol. 2011;23(4):433-451.
2. Ritchie EC. Suicides and the United States Army: perspectives from the former psychiatry consultant to the army surgeon general. Cerebrum. 2012;1.
Improving Veteran Engagement With Mental Health Care
The VA is intent on reducing and preventing veteran deaths by suicide. Most veteran who die by suicide, however, did not get treatment from the VHA, emphasizing the need for improved outreach to those veterans who are not part of the VA health care system.
I will begin by reviewing some reasons why veterans do not go to the VHA or to other mental health treatment centers and how we can change that. I am well aware that the health care providers at the DoD and VHA—including myself—feel overwhelmed by the influx of patients already at their doorstep. Thus, many providers are ambivalent about bringing in more patients when timely access remains a challenge. However, it is critical to engage patients in care to try to improve their lives and, hopefully, bring down the suicide rate.
Another critical issue then is hiring additional clinical providers and administrative staff. More providers are essential for timely patient care. If phones are not answered and patients cannot make appointments, they become frustrated and give up, especially if they already are ambivalent about seeking treatment.
Mental Health Service Experiences
Active-duty service members’ experience of the mental health service ranges from helpful to humiliating. Why is this? The helpful part is easy. The military has hundreds of finely trained professional mental health care providers who try their best to help the soldiers, marines, airmen, and sailors recover from the stress of combat or from providing humanitarian assistance. They use up-to-date therapeutic techniques and medication.
At the humiliating end of the spectrum, many service members are sent to behavioral health for “clearance” before they are administratively separated, ie, discharged without benefits. The separation may be for a variety of administrative discharges, such as a personality disorder; other mental health or medical disorders; or less than honorable discharges. The labels of the discharge vary in the different services, but the disappointment remains.
If the service member is enrolled in a drug and alcohol program (eg, the Army Substance Alcohol Program), the standard is total abstinence. If a service member fails to achieve abstinence, he or she may be discharged without benefits. The denial of benefits is controversial but is still the standard. I recommend a harm reduction model, eg, less drinking or drug use, which may be more achievable in many cases.
The waiting room of a military mental health or behavioral health clinic usually is occupied with service members who are facing a variety of discharges from the military (considered “losers”). Sitting in a crowded waiting room, sometimes for hours, can be humiliating.
To be clear, the clinic experience is not always humiliating. At many Wounded Warrior clinics, the environment is more welcoming. For example, at the National Intrepid Center of Excellence and other specialty clinics, the atmosphere is much more therapeutic.
Significantly, the negative feelings from the routine military clinics often translate into reluctance to go to a mental health clinic at the VHA or elsewhere. To reduce the stigma, the military has switched from using the term mental health to behavioral health, but a name change does not really change the stigma.
Ending the Stigma
To reduce the stigma, DoD has deployed many public service announcements (PSAs). These often have positive messages, such as: It is a sign of strength to accept help; Being mentally injured is like having a broken leg; I am a 3-star general, I have PTSD, and I got help—you can too. Unfortunately, these messages do not resonate with many service members: They have seen many of their friends discharged after seeking mental health services (although that may not have been the actual reason for their discharge). Hoping for a promotion may lead to avoidance of care out of fear that treatment will lead to being passed over for promotion.
Reluctance
When service members come to the VA, it is often with a defeatist attitude. “My wife said I need to come, or she will divorce me.” “I cannot concentrate in school, and I am failing classes.” “My girlfriend threw me out, and I have no place to live.” There is an initial interview with a mental health provider—often after a long waiting period. Often the veteran does not return for a follow-up.
Unquestionably, psychiatric and psychological treatments benefit service members—but the treatments also have drawbacks. Psychiatric medications, although potentially helpful often cause weight gain and sexual adverse effects. Trauma-based therapies deliberately force service members to reexperience the trauma. Reliving the traumatic experience is inherently painful. Additionally, there may be practical concerns, such as getting to the clinic, traffic, and parking.
Solutions
So how do we engage the veteran? There are several well-established practices. I am a big supporter of all veteran outreach. The veteran service organizations (VSOs) are well established but traditionally appeal to older veterans. However, VSOs are working to reach younger veterans in the context of outreach or sporting activities. Peer outreach also works well with veterans in or out of the VA system connecting with their fellow veterans. I favor engaging veterans through baseball games, kayaking, picnics, and other athletic/social activities. These are nonthreatening ways to engage the veteran and his or her family. Using animals, especially dogs and horses, also is a good way to connect.
Clinical Strategies
When I treat veterans who are ambivalent—which the younger ones usually are—I ask where they live, then when or where did they serve, and what was their military occupational specialty. In other words, I ask them about their strengths.
Besides the standard depression and PTSD symptoms, I ask about sexual health, knowing that it often is a major concern. I describe the wide range of PTSD treatments, using the “3 buckets” model to describe them. The 3 buckets are psychiatric medication, talking therapies, and everything else. The last bucket includes exercise, yoga, meditation, animal-assisted therapies, and others, such as transcranial magnetic stimulation and stellate ganglion block.
Veterans often are more comfortable with the last bucket, as it allows them more options. With this knowledge the service members have more tools, so they feel less helpless and more in charge of their health care.
Conclusion
There are many reasons why service members do not seek mental health care. Stigma is one that is often cited. Also, they often associate mental health treatment with humiliation. We have a duty to change that paradigm.
The VA is intent on reducing and preventing veteran deaths by suicide. Most veteran who die by suicide, however, did not get treatment from the VHA, emphasizing the need for improved outreach to those veterans who are not part of the VA health care system.
I will begin by reviewing some reasons why veterans do not go to the VHA or to other mental health treatment centers and how we can change that. I am well aware that the health care providers at the DoD and VHA—including myself—feel overwhelmed by the influx of patients already at their doorstep. Thus, many providers are ambivalent about bringing in more patients when timely access remains a challenge. However, it is critical to engage patients in care to try to improve their lives and, hopefully, bring down the suicide rate.
Another critical issue then is hiring additional clinical providers and administrative staff. More providers are essential for timely patient care. If phones are not answered and patients cannot make appointments, they become frustrated and give up, especially if they already are ambivalent about seeking treatment.
Mental Health Service Experiences
Active-duty service members’ experience of the mental health service ranges from helpful to humiliating. Why is this? The helpful part is easy. The military has hundreds of finely trained professional mental health care providers who try their best to help the soldiers, marines, airmen, and sailors recover from the stress of combat or from providing humanitarian assistance. They use up-to-date therapeutic techniques and medication.
At the humiliating end of the spectrum, many service members are sent to behavioral health for “clearance” before they are administratively separated, ie, discharged without benefits. The separation may be for a variety of administrative discharges, such as a personality disorder; other mental health or medical disorders; or less than honorable discharges. The labels of the discharge vary in the different services, but the disappointment remains.
If the service member is enrolled in a drug and alcohol program (eg, the Army Substance Alcohol Program), the standard is total abstinence. If a service member fails to achieve abstinence, he or she may be discharged without benefits. The denial of benefits is controversial but is still the standard. I recommend a harm reduction model, eg, less drinking or drug use, which may be more achievable in many cases.
The waiting room of a military mental health or behavioral health clinic usually is occupied with service members who are facing a variety of discharges from the military (considered “losers”). Sitting in a crowded waiting room, sometimes for hours, can be humiliating.
To be clear, the clinic experience is not always humiliating. At many Wounded Warrior clinics, the environment is more welcoming. For example, at the National Intrepid Center of Excellence and other specialty clinics, the atmosphere is much more therapeutic.
Significantly, the negative feelings from the routine military clinics often translate into reluctance to go to a mental health clinic at the VHA or elsewhere. To reduce the stigma, the military has switched from using the term mental health to behavioral health, but a name change does not really change the stigma.
Ending the Stigma
To reduce the stigma, DoD has deployed many public service announcements (PSAs). These often have positive messages, such as: It is a sign of strength to accept help; Being mentally injured is like having a broken leg; I am a 3-star general, I have PTSD, and I got help—you can too. Unfortunately, these messages do not resonate with many service members: They have seen many of their friends discharged after seeking mental health services (although that may not have been the actual reason for their discharge). Hoping for a promotion may lead to avoidance of care out of fear that treatment will lead to being passed over for promotion.
Reluctance
When service members come to the VA, it is often with a defeatist attitude. “My wife said I need to come, or she will divorce me.” “I cannot concentrate in school, and I am failing classes.” “My girlfriend threw me out, and I have no place to live.” There is an initial interview with a mental health provider—often after a long waiting period. Often the veteran does not return for a follow-up.
Unquestionably, psychiatric and psychological treatments benefit service members—but the treatments also have drawbacks. Psychiatric medications, although potentially helpful often cause weight gain and sexual adverse effects. Trauma-based therapies deliberately force service members to reexperience the trauma. Reliving the traumatic experience is inherently painful. Additionally, there may be practical concerns, such as getting to the clinic, traffic, and parking.
Solutions
So how do we engage the veteran? There are several well-established practices. I am a big supporter of all veteran outreach. The veteran service organizations (VSOs) are well established but traditionally appeal to older veterans. However, VSOs are working to reach younger veterans in the context of outreach or sporting activities. Peer outreach also works well with veterans in or out of the VA system connecting with their fellow veterans. I favor engaging veterans through baseball games, kayaking, picnics, and other athletic/social activities. These are nonthreatening ways to engage the veteran and his or her family. Using animals, especially dogs and horses, also is a good way to connect.
Clinical Strategies
When I treat veterans who are ambivalent—which the younger ones usually are—I ask where they live, then when or where did they serve, and what was their military occupational specialty. In other words, I ask them about their strengths.
Besides the standard depression and PTSD symptoms, I ask about sexual health, knowing that it often is a major concern. I describe the wide range of PTSD treatments, using the “3 buckets” model to describe them. The 3 buckets are psychiatric medication, talking therapies, and everything else. The last bucket includes exercise, yoga, meditation, animal-assisted therapies, and others, such as transcranial magnetic stimulation and stellate ganglion block.
Veterans often are more comfortable with the last bucket, as it allows them more options. With this knowledge the service members have more tools, so they feel less helpless and more in charge of their health care.
Conclusion
There are many reasons why service members do not seek mental health care. Stigma is one that is often cited. Also, they often associate mental health treatment with humiliation. We have a duty to change that paradigm.
The VA is intent on reducing and preventing veteran deaths by suicide. Most veteran who die by suicide, however, did not get treatment from the VHA, emphasizing the need for improved outreach to those veterans who are not part of the VA health care system.
I will begin by reviewing some reasons why veterans do not go to the VHA or to other mental health treatment centers and how we can change that. I am well aware that the health care providers at the DoD and VHA—including myself—feel overwhelmed by the influx of patients already at their doorstep. Thus, many providers are ambivalent about bringing in more patients when timely access remains a challenge. However, it is critical to engage patients in care to try to improve their lives and, hopefully, bring down the suicide rate.
Another critical issue then is hiring additional clinical providers and administrative staff. More providers are essential for timely patient care. If phones are not answered and patients cannot make appointments, they become frustrated and give up, especially if they already are ambivalent about seeking treatment.
Mental Health Service Experiences
Active-duty service members’ experience of the mental health service ranges from helpful to humiliating. Why is this? The helpful part is easy. The military has hundreds of finely trained professional mental health care providers who try their best to help the soldiers, marines, airmen, and sailors recover from the stress of combat or from providing humanitarian assistance. They use up-to-date therapeutic techniques and medication.
At the humiliating end of the spectrum, many service members are sent to behavioral health for “clearance” before they are administratively separated, ie, discharged without benefits. The separation may be for a variety of administrative discharges, such as a personality disorder; other mental health or medical disorders; or less than honorable discharges. The labels of the discharge vary in the different services, but the disappointment remains.
If the service member is enrolled in a drug and alcohol program (eg, the Army Substance Alcohol Program), the standard is total abstinence. If a service member fails to achieve abstinence, he or she may be discharged without benefits. The denial of benefits is controversial but is still the standard. I recommend a harm reduction model, eg, less drinking or drug use, which may be more achievable in many cases.
The waiting room of a military mental health or behavioral health clinic usually is occupied with service members who are facing a variety of discharges from the military (considered “losers”). Sitting in a crowded waiting room, sometimes for hours, can be humiliating.
To be clear, the clinic experience is not always humiliating. At many Wounded Warrior clinics, the environment is more welcoming. For example, at the National Intrepid Center of Excellence and other specialty clinics, the atmosphere is much more therapeutic.
Significantly, the negative feelings from the routine military clinics often translate into reluctance to go to a mental health clinic at the VHA or elsewhere. To reduce the stigma, the military has switched from using the term mental health to behavioral health, but a name change does not really change the stigma.
Ending the Stigma
To reduce the stigma, DoD has deployed many public service announcements (PSAs). These often have positive messages, such as: It is a sign of strength to accept help; Being mentally injured is like having a broken leg; I am a 3-star general, I have PTSD, and I got help—you can too. Unfortunately, these messages do not resonate with many service members: They have seen many of their friends discharged after seeking mental health services (although that may not have been the actual reason for their discharge). Hoping for a promotion may lead to avoidance of care out of fear that treatment will lead to being passed over for promotion.
Reluctance
When service members come to the VA, it is often with a defeatist attitude. “My wife said I need to come, or she will divorce me.” “I cannot concentrate in school, and I am failing classes.” “My girlfriend threw me out, and I have no place to live.” There is an initial interview with a mental health provider—often after a long waiting period. Often the veteran does not return for a follow-up.
Unquestionably, psychiatric and psychological treatments benefit service members—but the treatments also have drawbacks. Psychiatric medications, although potentially helpful often cause weight gain and sexual adverse effects. Trauma-based therapies deliberately force service members to reexperience the trauma. Reliving the traumatic experience is inherently painful. Additionally, there may be practical concerns, such as getting to the clinic, traffic, and parking.
Solutions
So how do we engage the veteran? There are several well-established practices. I am a big supporter of all veteran outreach. The veteran service organizations (VSOs) are well established but traditionally appeal to older veterans. However, VSOs are working to reach younger veterans in the context of outreach or sporting activities. Peer outreach also works well with veterans in or out of the VA system connecting with their fellow veterans. I favor engaging veterans through baseball games, kayaking, picnics, and other athletic/social activities. These are nonthreatening ways to engage the veteran and his or her family. Using animals, especially dogs and horses, also is a good way to connect.
Clinical Strategies
When I treat veterans who are ambivalent—which the younger ones usually are—I ask where they live, then when or where did they serve, and what was their military occupational specialty. In other words, I ask them about their strengths.
Besides the standard depression and PTSD symptoms, I ask about sexual health, knowing that it often is a major concern. I describe the wide range of PTSD treatments, using the “3 buckets” model to describe them. The 3 buckets are psychiatric medication, talking therapies, and everything else. The last bucket includes exercise, yoga, meditation, animal-assisted therapies, and others, such as transcranial magnetic stimulation and stellate ganglion block.
Veterans often are more comfortable with the last bucket, as it allows them more options. With this knowledge the service members have more tools, so they feel less helpless and more in charge of their health care.
Conclusion
There are many reasons why service members do not seek mental health care. Stigma is one that is often cited. Also, they often associate mental health treatment with humiliation. We have a duty to change that paradigm.
Need for Mental Health Providers in Progressive Tinnitus Management
Hearing loss and tinnitus (ringing or other noises in the ears or head) have been problematic for military service members and veterans for many years. Military personnel are exposed to high levels of noise in operational and training settings. In spite of hearing conservation efforts, hearing loss and auditory injuries (including tinnitus) continue to occur. Although current military leadership teaches the importance of hearing protection, that was not usually the case until the past few decades. Military leadership provides the means for hearing protection and monitors risk through conservation and hearing readiness programs. Unfortunately, the need for hearing during battle often overrides the expediency of using hearing protective devices.
Military members often equate hearing protection with increased vulnerability, widening the gap between preventive efforts and hearing preservation. It is therefore not surprising that tinnitus and hearing loss have been the 2 most common service-connected disabilities for veterans for a decade.1 These conditions are irreversible; affected service members and veterans need strategies to cope with distress associated with these chronic conditions. Clinical care often is essential to manage the associated distress and mental health (MH) symptoms, such as sleep disturbance, irritability, isolation, tension, and low mood.
There is no cure for tinnitus, meaning there is no proven method to permanently eliminate or even reduce the perception of tinnitus. Intervention for tinnitus therefore is limited to methods intended to mitigate reactions to tinnitus, with the ultimate goal to facilitate good quality of life in spite of the perception of this unwanted auditory anomaly. These methods include numerous means of utilizing therapeutic sound.2 Sound therapy, however, has been shown in controlled trials to be effective only when accompanied by counseling, which often focuses on teaching different coping skills.3 In such instances, MH providers can become an integral part of the hearing health team to assist patients in the management of their tinnitus.
Evidence-Based Practice
Evidence-based research should guide clinical services that are offered for tinnitus. Randomized controlled trials (RCTs) comprise the most important source for such evidence.4 Cochrane Reviews uses meta-analyses to examine rigorous RCTs to determine which methods have credible evidence. One of these reviews conducted in 2007 and updated in 2010 concluded that cognitive behavioral therapy (CBT) can improve depression scores and reduce distress for many people with bothersome tinnitus.5,6 Another Cochrane Review concluded that sound therapy combined with counseling can be beneficial, but on its own, sound therapy has not been shown to result in significant benefit.3 Yet another Cochrane Review focused on using hearing aids with patients who have both hearing loss and bothersome tinnitus; the researchers concluded that “there is currently no evidence to support or refute their use as a more routine intervention for tinnitus.”7 However, many patients and clinicians report hearing aids are helpful for coping with tinnitus.
The American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) published a clinical practice guideline (CPG) for the management of tinnitus.8 Developing the CPG involved a comprehensive evaluation of the peer-reviewed literature, including the available Cochrane Reviews, to identify appropriate RCTs to inform evidence-based recommendations. Cognitive behavioral therapy was the only intervention for tinnitus recommended in the CPG. Cognitive behavioral therapy targets emotional response by identifying behaviors, thoughts, and beliefs that may be altered.9 For tinnitus, CBT typically includes stress management including relaxation exercises, purposeful distraction, and changing how individuals view and appraise their tinnitus.
Both the CPG and Cochrane Reviews concluded that CBT has the strongest evidence base for reducing effects of tinnitus. It should be noted that the CPG recommended teaching patients basic information about tinnitus management and stated that it was optional (due to limited research evidence) to use sound therapy to augment coping skills training.
Progressive Tinnitus Management
Tinnitus research at the VA National Center for Rehabilitative Auditory Research (NCRAR) has led to the development and refinement of an interdisciplinary program called Progressive Tinnitus Management (PTM). Audiologists and MH providers work together to deliver portions of the protocol. In addition, otolaryngologists are important for patients requiring a medical examination. Audiologists, MH providers, and otolaryngologists comprise the hearing health team for tinnitus management. The PTM program involves 5 stepped-care levels of management, and patients receive only the levels they need.
Level 1 is the referral level, which specifies guidelines for any clinician who encounters patients experiencing tinnitus. The “standard” referral is to audiology for a hearing evaluation (PTM level 2)—every patient reporting tinnitus should have a hearing evaluation and brief tinnitus assessment. Less typical would be an urgent referral to a different provider for certain symptoms such as referral to ENT for sudden hearing loss.
Patients who desire intervention for bothersome tinnitus are offered PTM skills education (level 3). At this level, patients are taught facts and skills that they need to self-manage their tinnitus-related problems. Ideally, the audiologist and MH provider collaborate to deliver the level 3 intervention, which utilizes a 5-session (2 with an audiologist and 3 with a MH provider) problem-solving method. Audiologists explain different forms of sound therapy, and MH providers deliver brief CBT. The research studies and clinics that use PTM have shown that the majority of patients who receive the level 3 skills education interventions have their tinnitus needs met to the degree that they do not desire further services.
Those relatively few patients who desire further services are invited for a PTM interdisciplinary evaluation (level 4), which involves a more in-depth needs evaluation by both an audiologist and a MH provider. Based on the outcome of the level 4 evaluation, clear treatment goals are discussed with the patient. If the patient and providers mutually agree that further intervention is needed, then the patient is offered PTM individualized support (level 5), which involves one-on-one services by an audiologist and/or a MH provider. The providers then build on the lessons taught during level 3 and address barriers to enacting the already discussed skills. The MH provider also may expand on CBT skills that were provided in level 3, offering care such as CBT for insomnia during level 5, depending on the specific needs and desires of the patient.
At the NCRAR, a pilot study and 2 RCTs of PTM have been completed.10 The first of these 2 RCTs was a clinical effectiveness study of PTM that was conducted in 2 VA audiology clinics: Memphis, Tennessee, and West Haven, Connecticut.11 Patients who came to the clinics signed up for the study if they felt that the PTM level 3 intervention might be helpful. Half of the 300 veterans in the study were enrolled to receive PTM right away, and half were put on a 6-month wait list. The PTM group showed significantly greater benefit than that of the wait-list group.
The second RCT of PTM was motivated by the high number of service members and veterans with a history of traumatic brain injury (TBI), which is strongly associated with tinnitus.12 The PTM level 3 skills education was administered to participants individually over the telephone by both an audiologist and a psychologist. Participants, located all over the U.S., had bothersome tinnitus, and some had experienced ≥ 1 TBI. They were randomized to receive either Tele-PTM immediately for 6 months or to be put on a 6-month wait list. The Tele-PTM group showed much greater improvement than that of the wait-list group.
Both of these recent RCTs have validated the effectiveness of PTM and demonstrated that PTM should be considered for the practice of evidence-based tinnitus management. PTM is mostly consistent with the AAO-HNSF CPG and provides a structured and defined framework for implementing both assessment and intervention services for patients who report tinnitus. As such, VA Central Office has endorsed PTM as an effective intervention for tinnitus management and has recommended its use at VAMCs. The NCRAR researchers have provided PTM training to hundreds of VA audiologists and MH providers, yet the level of implementation across the VA system of care varies widely.
VA Survey
In 2015, in partnership with the VA Offices of Audiology and Speech Pathology and Mental Health Services, and the Health Services Research & Development/Quality Enhancement Research Initiative (HSR&D/QUERI), the NCRAR conducted a study to examine PTM variation across sites via surveys and/or interviews of VA Audiology and MH programs nationwide.13,14 The objectives of this study were to: (1) describe current tinnitus-management practices in VAMCs; (2) identify barriers and facilitators to PTM program implementation based on clinics that have fully, partially, or not implemented PTM; and (3) determine readiness to implement PTM within VISN 20 (Northwest states and Alaska).
Clinicians at VAMCs nationwide were surveyed regarding current provision of tinnitus clinical services. Requests were sent to audiology programs and MH programs at 142 major VAMCs along with instructions to complete the online survey. Responses were received from 87 audiologists and 66 MH providers. Clinicians at VAMCs with full PTM, partial PTM, and no-PTM (based on survey results) were then interviewed regarding site-specific barriers and facilitators to implementing and providing PTM, readiness to adopt PTM, and strategies for full PTM implementation.
Key findings from the study demonstrated the following: (1) There is considerable between-site variability in how PTM is implemented, particularly with the delivery of the MH portion of the protocol; (2) audiologists show higher levels of readiness to provide tinnitus services than do MH providers (7% of MH survey respondents vs 62% of audiologists reported their site implementing PTM); (3) 66% of MH survey respondents were interested in receiving training in tinnitus management (note that online PTM training for MH does not yet exist); (4) PTM implementation barriers include audio-visual technology issues, room scheduling, as well as lack of collaboration and colocation between MH and audiology departments, administrative time/support, group facilitator skills, and availability of PTM materials.
Overall, results of this HSR&D/QUERI-funded study suggested the need to develop MH-specific training to support the necessary interdisciplinary engagement. Although a patient workbook is available to order and visual presentation aids may be accessed online, it became clear that lack of MH participation in the inherently interdisciplinary PTM skills education was the most common deviation from PTM.
DoD an VA Questionnaire
In 2014 the DoD Hearing Center of Excellence (HCE) conducted the DoD and VA Tinnitus Evaluation, Management, and Treatment Assessment.13 The HCE conducted this questionnaire under the Tinnitus Care Quality Improvement, Process Development, and Implementation Plan, to develop, establish, and implement an interdisciplinary and ongoing process to continually assess and improve the quality and continuum of tinnitus care delivered to service members and veterans at a consistent, enterprise-wide level. The HCE developed the questionnaire to: (1) identify DoD and VA audiologists and otolaryngologists and their institutions providing comprehensive tinnitus care; (2) assess current tinnitus evaluation and management/treatment protocols used; (3) disseminate common practice improvements to all providers for enhancing overall tinnitus evaluation and management/treatment; and (4) evaluate implementation of improvements to include efficiency of implementation and efficacy of improvements.
The questionnaire was administered using SurveyMonkey (San Mateo, CA) and was disseminated by the otolaryngology and audiology consultants to the Army, Navy, and Air Force surgeons general and specialty leaders as well as through VA specialty leaders. Also, the HCE posted the link for the questionnaire on its website for 11 months. A total of 200 providers responded to the questionnaire, of which 13 did not indicate their specialty (eg, otolaryngology) or classification (eg, DoD active duty) and were excluded from data analysis. The 187 qualified respondents included 66 DoD audiologists, 120 VA audiologists, and 1 DoD otolaryngologist.
The questionnaire results indicated that DoD and VA respondents provided tinnitus services for their patients at similar rates (72% of DoD providers and 79% of VA providers). The use of PTM by those same providers, however, was far more widespread in VA (66%) than it was in DoD (37%). Of the providers indicating they did not offer tinnitus clinical services, the main reasons given were lack of necessary training/expertise, lack of time, and insufficient clinical support. The majority of respondents indicated they had training on tinnitus evaluation and/or management and that they were comfortable providing these services; despite this, most providers indicated a need or desire for tinnitus-specific training and education. These results suggested that more support and education for hearing health care providers were needed to implement PTM in VA and, especially, in DoD.
About half of the respondents indicated that psychological/behavioral treatment services, which would correspond to PTM levels 3 and 5, are available for patients at their facility who have tinnitus. It is encouraging to know that some patients with problematic tinnitus are receiving MH services. However, it is essential that patients with any degree of bothersome tinnitus have access to evidence-based clinical services, which would require CBT delivered by a qualified MH provider.
Conclusion
Numerous VA and DoD clinics have begun providing PTM. Individual sites, however, typically adapt the program during the process of implementation.13,14 The most common adaptation that sites make to PTM is to proceed with level 3 skills education without the assistance of MH, and thus CBT, due to the lack of provider availability. It is unknown what impact this has on the effectiveness of PTM. Skills education forms the heart of PTM and addresses the needs of the majority of patients who seek intervention.
Collaboration with MH is integral to the delivery of PTM. Mental health providers partner in PTM levels 3 and 5 by providing CBT, which has the strongest evidence for reducing tinnitus distress among all interventions and always will be critical to the provision of PTM. Clearly VA MH programs need to increase involvement in veterans’ tinnitus management. Increased involvement may be accomplished by (1) developing training or other materials that increase understanding of MH’s role in addressing tinnitus; (2) developing pathways for coordination of care between audiology and MH providers, including different models of coordination based on individual site needs; and (3) documenting the prevalence of tinnitus-MH comorbidities to empirically justify the need for such coordination between audiology and MH providers.
To address gaps identified in the VA survey and in a similar questionnaire conducted by HCE regarding tinnitus care in VA and DoD, the NCRAR, HCE, and Walter Reed National Military Medical Center are collaborating on several initiatives to improve tinnitus services for service members and veterans.13-15 These efforts include enhancing service member and veteran access to VA and DoD MH services in PTM.
1. U.S. Department of Veterans Affairs. Veterans Benefits Administration reports: annual benefits report. http://www.benefits.va.gov/REPORTS/abr/index.asp. Updated December 19, 2016. Accessed April 13, 2017.
2. Hoare DJ, Searchfield GD, El Refaie A, Henry JA. Sound therapy for tinnitus management: practicable options. J Am Acad Audiol. 2014;25(1):62-75.
3. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
4. Keech A, Gebski V, Pike R. Interpreting and Reporting Clinical Trials. A Guide to the CONSORT Statement and the Principles of Randomised Controlled Trials. Sydney: MJA Books, Australasian Medical Publishing Company; 2007.
5. Martinez Devesa P, Waddell A, Perera R, Theodoulou M. Cognitive behavioural therapy for tinnitus (review). Cochrane Database Syst Rev. 2007;(1):CD005233.
6. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233.
7. Hoare DJ, Edmondson-Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst Rev. 2014;(1):CD010151.
8. Tunkel DE, Bauer CA, Rosenfeld RM, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
9. Beck JS, Beck AT. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. New York, New York: Guilford Press; 2011.
10. Henry JA, Zaugg TL, Myers PJ, et al. Pilot study to develop telehealth tinnitus management for persons with and without traumatic brain injury. J Rehab Res Dev. 2012;49(7):1025-1042.
11. Henry JA, Thielman EJ, Zaugg TL, et al. Randomized controlled trial in clinical settings to evaluate effectiveness of coping skills education used with progressive tinnitus management. J Speech Lang Hear Res. 2017;1-20. [Epub ahead of print]
12. Henry JA, Griest S, Thielman E, McMillan G, Kaelin C, Carlson K. The tinnitus functional index: development, validation, outcomes research, and clinical application. Hear Res. 2016;334:58-64.
13. Boudin A, Carlson KC, Elnitsky C, et al. Online Surveys of Tinnitus Management Practices in VA and DoD: Results and Clinical Implications. Joint Defense Veterans Audiology Conference (JDVAC), St Louis, MO, February 22-24, 2016.
14. Carlson KC, Thielman E, Zaugg TL, Elnitsky C, Tuepker A, Kaelin C, Henry JA. “VA Clinician Surveys and Interviews Reveal Need for Increased Mental Health Involvement in Tinnitus Management.” Joint Defense Veterans Audiology Conference (JDVAC), St Louis, MO, February 22-24, 2016.
15. Carlson K, Thielman E, Zaugg T, et al. Factors affecting the provision of evidence-based progressive tinnitus management in Department of Veterans Affairs medical centers. Paper presented at: Academy Health Annual Research Meeting; June 26-28, 2016;
Hearing loss and tinnitus (ringing or other noises in the ears or head) have been problematic for military service members and veterans for many years. Military personnel are exposed to high levels of noise in operational and training settings. In spite of hearing conservation efforts, hearing loss and auditory injuries (including tinnitus) continue to occur. Although current military leadership teaches the importance of hearing protection, that was not usually the case until the past few decades. Military leadership provides the means for hearing protection and monitors risk through conservation and hearing readiness programs. Unfortunately, the need for hearing during battle often overrides the expediency of using hearing protective devices.
Military members often equate hearing protection with increased vulnerability, widening the gap between preventive efforts and hearing preservation. It is therefore not surprising that tinnitus and hearing loss have been the 2 most common service-connected disabilities for veterans for a decade.1 These conditions are irreversible; affected service members and veterans need strategies to cope with distress associated with these chronic conditions. Clinical care often is essential to manage the associated distress and mental health (MH) symptoms, such as sleep disturbance, irritability, isolation, tension, and low mood.
There is no cure for tinnitus, meaning there is no proven method to permanently eliminate or even reduce the perception of tinnitus. Intervention for tinnitus therefore is limited to methods intended to mitigate reactions to tinnitus, with the ultimate goal to facilitate good quality of life in spite of the perception of this unwanted auditory anomaly. These methods include numerous means of utilizing therapeutic sound.2 Sound therapy, however, has been shown in controlled trials to be effective only when accompanied by counseling, which often focuses on teaching different coping skills.3 In such instances, MH providers can become an integral part of the hearing health team to assist patients in the management of their tinnitus.
Evidence-Based Practice
Evidence-based research should guide clinical services that are offered for tinnitus. Randomized controlled trials (RCTs) comprise the most important source for such evidence.4 Cochrane Reviews uses meta-analyses to examine rigorous RCTs to determine which methods have credible evidence. One of these reviews conducted in 2007 and updated in 2010 concluded that cognitive behavioral therapy (CBT) can improve depression scores and reduce distress for many people with bothersome tinnitus.5,6 Another Cochrane Review concluded that sound therapy combined with counseling can be beneficial, but on its own, sound therapy has not been shown to result in significant benefit.3 Yet another Cochrane Review focused on using hearing aids with patients who have both hearing loss and bothersome tinnitus; the researchers concluded that “there is currently no evidence to support or refute their use as a more routine intervention for tinnitus.”7 However, many patients and clinicians report hearing aids are helpful for coping with tinnitus.
The American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) published a clinical practice guideline (CPG) for the management of tinnitus.8 Developing the CPG involved a comprehensive evaluation of the peer-reviewed literature, including the available Cochrane Reviews, to identify appropriate RCTs to inform evidence-based recommendations. Cognitive behavioral therapy was the only intervention for tinnitus recommended in the CPG. Cognitive behavioral therapy targets emotional response by identifying behaviors, thoughts, and beliefs that may be altered.9 For tinnitus, CBT typically includes stress management including relaxation exercises, purposeful distraction, and changing how individuals view and appraise their tinnitus.
Both the CPG and Cochrane Reviews concluded that CBT has the strongest evidence base for reducing effects of tinnitus. It should be noted that the CPG recommended teaching patients basic information about tinnitus management and stated that it was optional (due to limited research evidence) to use sound therapy to augment coping skills training.
Progressive Tinnitus Management
Tinnitus research at the VA National Center for Rehabilitative Auditory Research (NCRAR) has led to the development and refinement of an interdisciplinary program called Progressive Tinnitus Management (PTM). Audiologists and MH providers work together to deliver portions of the protocol. In addition, otolaryngologists are important for patients requiring a medical examination. Audiologists, MH providers, and otolaryngologists comprise the hearing health team for tinnitus management. The PTM program involves 5 stepped-care levels of management, and patients receive only the levels they need.
Level 1 is the referral level, which specifies guidelines for any clinician who encounters patients experiencing tinnitus. The “standard” referral is to audiology for a hearing evaluation (PTM level 2)—every patient reporting tinnitus should have a hearing evaluation and brief tinnitus assessment. Less typical would be an urgent referral to a different provider for certain symptoms such as referral to ENT for sudden hearing loss.
Patients who desire intervention for bothersome tinnitus are offered PTM skills education (level 3). At this level, patients are taught facts and skills that they need to self-manage their tinnitus-related problems. Ideally, the audiologist and MH provider collaborate to deliver the level 3 intervention, which utilizes a 5-session (2 with an audiologist and 3 with a MH provider) problem-solving method. Audiologists explain different forms of sound therapy, and MH providers deliver brief CBT. The research studies and clinics that use PTM have shown that the majority of patients who receive the level 3 skills education interventions have their tinnitus needs met to the degree that they do not desire further services.
Those relatively few patients who desire further services are invited for a PTM interdisciplinary evaluation (level 4), which involves a more in-depth needs evaluation by both an audiologist and a MH provider. Based on the outcome of the level 4 evaluation, clear treatment goals are discussed with the patient. If the patient and providers mutually agree that further intervention is needed, then the patient is offered PTM individualized support (level 5), which involves one-on-one services by an audiologist and/or a MH provider. The providers then build on the lessons taught during level 3 and address barriers to enacting the already discussed skills. The MH provider also may expand on CBT skills that were provided in level 3, offering care such as CBT for insomnia during level 5, depending on the specific needs and desires of the patient.
At the NCRAR, a pilot study and 2 RCTs of PTM have been completed.10 The first of these 2 RCTs was a clinical effectiveness study of PTM that was conducted in 2 VA audiology clinics: Memphis, Tennessee, and West Haven, Connecticut.11 Patients who came to the clinics signed up for the study if they felt that the PTM level 3 intervention might be helpful. Half of the 300 veterans in the study were enrolled to receive PTM right away, and half were put on a 6-month wait list. The PTM group showed significantly greater benefit than that of the wait-list group.
The second RCT of PTM was motivated by the high number of service members and veterans with a history of traumatic brain injury (TBI), which is strongly associated with tinnitus.12 The PTM level 3 skills education was administered to participants individually over the telephone by both an audiologist and a psychologist. Participants, located all over the U.S., had bothersome tinnitus, and some had experienced ≥ 1 TBI. They were randomized to receive either Tele-PTM immediately for 6 months or to be put on a 6-month wait list. The Tele-PTM group showed much greater improvement than that of the wait-list group.
Both of these recent RCTs have validated the effectiveness of PTM and demonstrated that PTM should be considered for the practice of evidence-based tinnitus management. PTM is mostly consistent with the AAO-HNSF CPG and provides a structured and defined framework for implementing both assessment and intervention services for patients who report tinnitus. As such, VA Central Office has endorsed PTM as an effective intervention for tinnitus management and has recommended its use at VAMCs. The NCRAR researchers have provided PTM training to hundreds of VA audiologists and MH providers, yet the level of implementation across the VA system of care varies widely.
VA Survey
In 2015, in partnership with the VA Offices of Audiology and Speech Pathology and Mental Health Services, and the Health Services Research & Development/Quality Enhancement Research Initiative (HSR&D/QUERI), the NCRAR conducted a study to examine PTM variation across sites via surveys and/or interviews of VA Audiology and MH programs nationwide.13,14 The objectives of this study were to: (1) describe current tinnitus-management practices in VAMCs; (2) identify barriers and facilitators to PTM program implementation based on clinics that have fully, partially, or not implemented PTM; and (3) determine readiness to implement PTM within VISN 20 (Northwest states and Alaska).
Clinicians at VAMCs nationwide were surveyed regarding current provision of tinnitus clinical services. Requests were sent to audiology programs and MH programs at 142 major VAMCs along with instructions to complete the online survey. Responses were received from 87 audiologists and 66 MH providers. Clinicians at VAMCs with full PTM, partial PTM, and no-PTM (based on survey results) were then interviewed regarding site-specific barriers and facilitators to implementing and providing PTM, readiness to adopt PTM, and strategies for full PTM implementation.
Key findings from the study demonstrated the following: (1) There is considerable between-site variability in how PTM is implemented, particularly with the delivery of the MH portion of the protocol; (2) audiologists show higher levels of readiness to provide tinnitus services than do MH providers (7% of MH survey respondents vs 62% of audiologists reported their site implementing PTM); (3) 66% of MH survey respondents were interested in receiving training in tinnitus management (note that online PTM training for MH does not yet exist); (4) PTM implementation barriers include audio-visual technology issues, room scheduling, as well as lack of collaboration and colocation between MH and audiology departments, administrative time/support, group facilitator skills, and availability of PTM materials.
Overall, results of this HSR&D/QUERI-funded study suggested the need to develop MH-specific training to support the necessary interdisciplinary engagement. Although a patient workbook is available to order and visual presentation aids may be accessed online, it became clear that lack of MH participation in the inherently interdisciplinary PTM skills education was the most common deviation from PTM.
DoD an VA Questionnaire
In 2014 the DoD Hearing Center of Excellence (HCE) conducted the DoD and VA Tinnitus Evaluation, Management, and Treatment Assessment.13 The HCE conducted this questionnaire under the Tinnitus Care Quality Improvement, Process Development, and Implementation Plan, to develop, establish, and implement an interdisciplinary and ongoing process to continually assess and improve the quality and continuum of tinnitus care delivered to service members and veterans at a consistent, enterprise-wide level. The HCE developed the questionnaire to: (1) identify DoD and VA audiologists and otolaryngologists and their institutions providing comprehensive tinnitus care; (2) assess current tinnitus evaluation and management/treatment protocols used; (3) disseminate common practice improvements to all providers for enhancing overall tinnitus evaluation and management/treatment; and (4) evaluate implementation of improvements to include efficiency of implementation and efficacy of improvements.
The questionnaire was administered using SurveyMonkey (San Mateo, CA) and was disseminated by the otolaryngology and audiology consultants to the Army, Navy, and Air Force surgeons general and specialty leaders as well as through VA specialty leaders. Also, the HCE posted the link for the questionnaire on its website for 11 months. A total of 200 providers responded to the questionnaire, of which 13 did not indicate their specialty (eg, otolaryngology) or classification (eg, DoD active duty) and were excluded from data analysis. The 187 qualified respondents included 66 DoD audiologists, 120 VA audiologists, and 1 DoD otolaryngologist.
The questionnaire results indicated that DoD and VA respondents provided tinnitus services for their patients at similar rates (72% of DoD providers and 79% of VA providers). The use of PTM by those same providers, however, was far more widespread in VA (66%) than it was in DoD (37%). Of the providers indicating they did not offer tinnitus clinical services, the main reasons given were lack of necessary training/expertise, lack of time, and insufficient clinical support. The majority of respondents indicated they had training on tinnitus evaluation and/or management and that they were comfortable providing these services; despite this, most providers indicated a need or desire for tinnitus-specific training and education. These results suggested that more support and education for hearing health care providers were needed to implement PTM in VA and, especially, in DoD.
About half of the respondents indicated that psychological/behavioral treatment services, which would correspond to PTM levels 3 and 5, are available for patients at their facility who have tinnitus. It is encouraging to know that some patients with problematic tinnitus are receiving MH services. However, it is essential that patients with any degree of bothersome tinnitus have access to evidence-based clinical services, which would require CBT delivered by a qualified MH provider.
Conclusion
Numerous VA and DoD clinics have begun providing PTM. Individual sites, however, typically adapt the program during the process of implementation.13,14 The most common adaptation that sites make to PTM is to proceed with level 3 skills education without the assistance of MH, and thus CBT, due to the lack of provider availability. It is unknown what impact this has on the effectiveness of PTM. Skills education forms the heart of PTM and addresses the needs of the majority of patients who seek intervention.
Collaboration with MH is integral to the delivery of PTM. Mental health providers partner in PTM levels 3 and 5 by providing CBT, which has the strongest evidence for reducing tinnitus distress among all interventions and always will be critical to the provision of PTM. Clearly VA MH programs need to increase involvement in veterans’ tinnitus management. Increased involvement may be accomplished by (1) developing training or other materials that increase understanding of MH’s role in addressing tinnitus; (2) developing pathways for coordination of care between audiology and MH providers, including different models of coordination based on individual site needs; and (3) documenting the prevalence of tinnitus-MH comorbidities to empirically justify the need for such coordination between audiology and MH providers.
To address gaps identified in the VA survey and in a similar questionnaire conducted by HCE regarding tinnitus care in VA and DoD, the NCRAR, HCE, and Walter Reed National Military Medical Center are collaborating on several initiatives to improve tinnitus services for service members and veterans.13-15 These efforts include enhancing service member and veteran access to VA and DoD MH services in PTM.
Hearing loss and tinnitus (ringing or other noises in the ears or head) have been problematic for military service members and veterans for many years. Military personnel are exposed to high levels of noise in operational and training settings. In spite of hearing conservation efforts, hearing loss and auditory injuries (including tinnitus) continue to occur. Although current military leadership teaches the importance of hearing protection, that was not usually the case until the past few decades. Military leadership provides the means for hearing protection and monitors risk through conservation and hearing readiness programs. Unfortunately, the need for hearing during battle often overrides the expediency of using hearing protective devices.
Military members often equate hearing protection with increased vulnerability, widening the gap between preventive efforts and hearing preservation. It is therefore not surprising that tinnitus and hearing loss have been the 2 most common service-connected disabilities for veterans for a decade.1 These conditions are irreversible; affected service members and veterans need strategies to cope with distress associated with these chronic conditions. Clinical care often is essential to manage the associated distress and mental health (MH) symptoms, such as sleep disturbance, irritability, isolation, tension, and low mood.
There is no cure for tinnitus, meaning there is no proven method to permanently eliminate or even reduce the perception of tinnitus. Intervention for tinnitus therefore is limited to methods intended to mitigate reactions to tinnitus, with the ultimate goal to facilitate good quality of life in spite of the perception of this unwanted auditory anomaly. These methods include numerous means of utilizing therapeutic sound.2 Sound therapy, however, has been shown in controlled trials to be effective only when accompanied by counseling, which often focuses on teaching different coping skills.3 In such instances, MH providers can become an integral part of the hearing health team to assist patients in the management of their tinnitus.
Evidence-Based Practice
Evidence-based research should guide clinical services that are offered for tinnitus. Randomized controlled trials (RCTs) comprise the most important source for such evidence.4 Cochrane Reviews uses meta-analyses to examine rigorous RCTs to determine which methods have credible evidence. One of these reviews conducted in 2007 and updated in 2010 concluded that cognitive behavioral therapy (CBT) can improve depression scores and reduce distress for many people with bothersome tinnitus.5,6 Another Cochrane Review concluded that sound therapy combined with counseling can be beneficial, but on its own, sound therapy has not been shown to result in significant benefit.3 Yet another Cochrane Review focused on using hearing aids with patients who have both hearing loss and bothersome tinnitus; the researchers concluded that “there is currently no evidence to support or refute their use as a more routine intervention for tinnitus.”7 However, many patients and clinicians report hearing aids are helpful for coping with tinnitus.
The American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) published a clinical practice guideline (CPG) for the management of tinnitus.8 Developing the CPG involved a comprehensive evaluation of the peer-reviewed literature, including the available Cochrane Reviews, to identify appropriate RCTs to inform evidence-based recommendations. Cognitive behavioral therapy was the only intervention for tinnitus recommended in the CPG. Cognitive behavioral therapy targets emotional response by identifying behaviors, thoughts, and beliefs that may be altered.9 For tinnitus, CBT typically includes stress management including relaxation exercises, purposeful distraction, and changing how individuals view and appraise their tinnitus.
Both the CPG and Cochrane Reviews concluded that CBT has the strongest evidence base for reducing effects of tinnitus. It should be noted that the CPG recommended teaching patients basic information about tinnitus management and stated that it was optional (due to limited research evidence) to use sound therapy to augment coping skills training.
Progressive Tinnitus Management
Tinnitus research at the VA National Center for Rehabilitative Auditory Research (NCRAR) has led to the development and refinement of an interdisciplinary program called Progressive Tinnitus Management (PTM). Audiologists and MH providers work together to deliver portions of the protocol. In addition, otolaryngologists are important for patients requiring a medical examination. Audiologists, MH providers, and otolaryngologists comprise the hearing health team for tinnitus management. The PTM program involves 5 stepped-care levels of management, and patients receive only the levels they need.
Level 1 is the referral level, which specifies guidelines for any clinician who encounters patients experiencing tinnitus. The “standard” referral is to audiology for a hearing evaluation (PTM level 2)—every patient reporting tinnitus should have a hearing evaluation and brief tinnitus assessment. Less typical would be an urgent referral to a different provider for certain symptoms such as referral to ENT for sudden hearing loss.
Patients who desire intervention for bothersome tinnitus are offered PTM skills education (level 3). At this level, patients are taught facts and skills that they need to self-manage their tinnitus-related problems. Ideally, the audiologist and MH provider collaborate to deliver the level 3 intervention, which utilizes a 5-session (2 with an audiologist and 3 with a MH provider) problem-solving method. Audiologists explain different forms of sound therapy, and MH providers deliver brief CBT. The research studies and clinics that use PTM have shown that the majority of patients who receive the level 3 skills education interventions have their tinnitus needs met to the degree that they do not desire further services.
Those relatively few patients who desire further services are invited for a PTM interdisciplinary evaluation (level 4), which involves a more in-depth needs evaluation by both an audiologist and a MH provider. Based on the outcome of the level 4 evaluation, clear treatment goals are discussed with the patient. If the patient and providers mutually agree that further intervention is needed, then the patient is offered PTM individualized support (level 5), which involves one-on-one services by an audiologist and/or a MH provider. The providers then build on the lessons taught during level 3 and address barriers to enacting the already discussed skills. The MH provider also may expand on CBT skills that were provided in level 3, offering care such as CBT for insomnia during level 5, depending on the specific needs and desires of the patient.
At the NCRAR, a pilot study and 2 RCTs of PTM have been completed.10 The first of these 2 RCTs was a clinical effectiveness study of PTM that was conducted in 2 VA audiology clinics: Memphis, Tennessee, and West Haven, Connecticut.11 Patients who came to the clinics signed up for the study if they felt that the PTM level 3 intervention might be helpful. Half of the 300 veterans in the study were enrolled to receive PTM right away, and half were put on a 6-month wait list. The PTM group showed significantly greater benefit than that of the wait-list group.
The second RCT of PTM was motivated by the high number of service members and veterans with a history of traumatic brain injury (TBI), which is strongly associated with tinnitus.12 The PTM level 3 skills education was administered to participants individually over the telephone by both an audiologist and a psychologist. Participants, located all over the U.S., had bothersome tinnitus, and some had experienced ≥ 1 TBI. They were randomized to receive either Tele-PTM immediately for 6 months or to be put on a 6-month wait list. The Tele-PTM group showed much greater improvement than that of the wait-list group.
Both of these recent RCTs have validated the effectiveness of PTM and demonstrated that PTM should be considered for the practice of evidence-based tinnitus management. PTM is mostly consistent with the AAO-HNSF CPG and provides a structured and defined framework for implementing both assessment and intervention services for patients who report tinnitus. As such, VA Central Office has endorsed PTM as an effective intervention for tinnitus management and has recommended its use at VAMCs. The NCRAR researchers have provided PTM training to hundreds of VA audiologists and MH providers, yet the level of implementation across the VA system of care varies widely.
VA Survey
In 2015, in partnership with the VA Offices of Audiology and Speech Pathology and Mental Health Services, and the Health Services Research & Development/Quality Enhancement Research Initiative (HSR&D/QUERI), the NCRAR conducted a study to examine PTM variation across sites via surveys and/or interviews of VA Audiology and MH programs nationwide.13,14 The objectives of this study were to: (1) describe current tinnitus-management practices in VAMCs; (2) identify barriers and facilitators to PTM program implementation based on clinics that have fully, partially, or not implemented PTM; and (3) determine readiness to implement PTM within VISN 20 (Northwest states and Alaska).
Clinicians at VAMCs nationwide were surveyed regarding current provision of tinnitus clinical services. Requests were sent to audiology programs and MH programs at 142 major VAMCs along with instructions to complete the online survey. Responses were received from 87 audiologists and 66 MH providers. Clinicians at VAMCs with full PTM, partial PTM, and no-PTM (based on survey results) were then interviewed regarding site-specific barriers and facilitators to implementing and providing PTM, readiness to adopt PTM, and strategies for full PTM implementation.
Key findings from the study demonstrated the following: (1) There is considerable between-site variability in how PTM is implemented, particularly with the delivery of the MH portion of the protocol; (2) audiologists show higher levels of readiness to provide tinnitus services than do MH providers (7% of MH survey respondents vs 62% of audiologists reported their site implementing PTM); (3) 66% of MH survey respondents were interested in receiving training in tinnitus management (note that online PTM training for MH does not yet exist); (4) PTM implementation barriers include audio-visual technology issues, room scheduling, as well as lack of collaboration and colocation between MH and audiology departments, administrative time/support, group facilitator skills, and availability of PTM materials.
Overall, results of this HSR&D/QUERI-funded study suggested the need to develop MH-specific training to support the necessary interdisciplinary engagement. Although a patient workbook is available to order and visual presentation aids may be accessed online, it became clear that lack of MH participation in the inherently interdisciplinary PTM skills education was the most common deviation from PTM.
DoD an VA Questionnaire
In 2014 the DoD Hearing Center of Excellence (HCE) conducted the DoD and VA Tinnitus Evaluation, Management, and Treatment Assessment.13 The HCE conducted this questionnaire under the Tinnitus Care Quality Improvement, Process Development, and Implementation Plan, to develop, establish, and implement an interdisciplinary and ongoing process to continually assess and improve the quality and continuum of tinnitus care delivered to service members and veterans at a consistent, enterprise-wide level. The HCE developed the questionnaire to: (1) identify DoD and VA audiologists and otolaryngologists and their institutions providing comprehensive tinnitus care; (2) assess current tinnitus evaluation and management/treatment protocols used; (3) disseminate common practice improvements to all providers for enhancing overall tinnitus evaluation and management/treatment; and (4) evaluate implementation of improvements to include efficiency of implementation and efficacy of improvements.
The questionnaire was administered using SurveyMonkey (San Mateo, CA) and was disseminated by the otolaryngology and audiology consultants to the Army, Navy, and Air Force surgeons general and specialty leaders as well as through VA specialty leaders. Also, the HCE posted the link for the questionnaire on its website for 11 months. A total of 200 providers responded to the questionnaire, of which 13 did not indicate their specialty (eg, otolaryngology) or classification (eg, DoD active duty) and were excluded from data analysis. The 187 qualified respondents included 66 DoD audiologists, 120 VA audiologists, and 1 DoD otolaryngologist.
The questionnaire results indicated that DoD and VA respondents provided tinnitus services for their patients at similar rates (72% of DoD providers and 79% of VA providers). The use of PTM by those same providers, however, was far more widespread in VA (66%) than it was in DoD (37%). Of the providers indicating they did not offer tinnitus clinical services, the main reasons given were lack of necessary training/expertise, lack of time, and insufficient clinical support. The majority of respondents indicated they had training on tinnitus evaluation and/or management and that they were comfortable providing these services; despite this, most providers indicated a need or desire for tinnitus-specific training and education. These results suggested that more support and education for hearing health care providers were needed to implement PTM in VA and, especially, in DoD.
About half of the respondents indicated that psychological/behavioral treatment services, which would correspond to PTM levels 3 and 5, are available for patients at their facility who have tinnitus. It is encouraging to know that some patients with problematic tinnitus are receiving MH services. However, it is essential that patients with any degree of bothersome tinnitus have access to evidence-based clinical services, which would require CBT delivered by a qualified MH provider.
Conclusion
Numerous VA and DoD clinics have begun providing PTM. Individual sites, however, typically adapt the program during the process of implementation.13,14 The most common adaptation that sites make to PTM is to proceed with level 3 skills education without the assistance of MH, and thus CBT, due to the lack of provider availability. It is unknown what impact this has on the effectiveness of PTM. Skills education forms the heart of PTM and addresses the needs of the majority of patients who seek intervention.
Collaboration with MH is integral to the delivery of PTM. Mental health providers partner in PTM levels 3 and 5 by providing CBT, which has the strongest evidence for reducing tinnitus distress among all interventions and always will be critical to the provision of PTM. Clearly VA MH programs need to increase involvement in veterans’ tinnitus management. Increased involvement may be accomplished by (1) developing training or other materials that increase understanding of MH’s role in addressing tinnitus; (2) developing pathways for coordination of care between audiology and MH providers, including different models of coordination based on individual site needs; and (3) documenting the prevalence of tinnitus-MH comorbidities to empirically justify the need for such coordination between audiology and MH providers.
To address gaps identified in the VA survey and in a similar questionnaire conducted by HCE regarding tinnitus care in VA and DoD, the NCRAR, HCE, and Walter Reed National Military Medical Center are collaborating on several initiatives to improve tinnitus services for service members and veterans.13-15 These efforts include enhancing service member and veteran access to VA and DoD MH services in PTM.
1. U.S. Department of Veterans Affairs. Veterans Benefits Administration reports: annual benefits report. http://www.benefits.va.gov/REPORTS/abr/index.asp. Updated December 19, 2016. Accessed April 13, 2017.
2. Hoare DJ, Searchfield GD, El Refaie A, Henry JA. Sound therapy for tinnitus management: practicable options. J Am Acad Audiol. 2014;25(1):62-75.
3. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
4. Keech A, Gebski V, Pike R. Interpreting and Reporting Clinical Trials. A Guide to the CONSORT Statement and the Principles of Randomised Controlled Trials. Sydney: MJA Books, Australasian Medical Publishing Company; 2007.
5. Martinez Devesa P, Waddell A, Perera R, Theodoulou M. Cognitive behavioural therapy for tinnitus (review). Cochrane Database Syst Rev. 2007;(1):CD005233.
6. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233.
7. Hoare DJ, Edmondson-Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst Rev. 2014;(1):CD010151.
8. Tunkel DE, Bauer CA, Rosenfeld RM, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
9. Beck JS, Beck AT. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. New York, New York: Guilford Press; 2011.
10. Henry JA, Zaugg TL, Myers PJ, et al. Pilot study to develop telehealth tinnitus management for persons with and without traumatic brain injury. J Rehab Res Dev. 2012;49(7):1025-1042.
11. Henry JA, Thielman EJ, Zaugg TL, et al. Randomized controlled trial in clinical settings to evaluate effectiveness of coping skills education used with progressive tinnitus management. J Speech Lang Hear Res. 2017;1-20. [Epub ahead of print]
12. Henry JA, Griest S, Thielman E, McMillan G, Kaelin C, Carlson K. The tinnitus functional index: development, validation, outcomes research, and clinical application. Hear Res. 2016;334:58-64.
13. Boudin A, Carlson KC, Elnitsky C, et al. Online Surveys of Tinnitus Management Practices in VA and DoD: Results and Clinical Implications. Joint Defense Veterans Audiology Conference (JDVAC), St Louis, MO, February 22-24, 2016.
14. Carlson KC, Thielman E, Zaugg TL, Elnitsky C, Tuepker A, Kaelin C, Henry JA. “VA Clinician Surveys and Interviews Reveal Need for Increased Mental Health Involvement in Tinnitus Management.” Joint Defense Veterans Audiology Conference (JDVAC), St Louis, MO, February 22-24, 2016.
15. Carlson K, Thielman E, Zaugg T, et al. Factors affecting the provision of evidence-based progressive tinnitus management in Department of Veterans Affairs medical centers. Paper presented at: Academy Health Annual Research Meeting; June 26-28, 2016;
1. U.S. Department of Veterans Affairs. Veterans Benefits Administration reports: annual benefits report. http://www.benefits.va.gov/REPORTS/abr/index.asp. Updated December 19, 2016. Accessed April 13, 2017.
2. Hoare DJ, Searchfield GD, El Refaie A, Henry JA. Sound therapy for tinnitus management: practicable options. J Am Acad Audiol. 2014;25(1):62-75.
3. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
4. Keech A, Gebski V, Pike R. Interpreting and Reporting Clinical Trials. A Guide to the CONSORT Statement and the Principles of Randomised Controlled Trials. Sydney: MJA Books, Australasian Medical Publishing Company; 2007.
5. Martinez Devesa P, Waddell A, Perera R, Theodoulou M. Cognitive behavioural therapy for tinnitus (review). Cochrane Database Syst Rev. 2007;(1):CD005233.
6. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233.
7. Hoare DJ, Edmondson-Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst Rev. 2014;(1):CD010151.
8. Tunkel DE, Bauer CA, Rosenfeld RM, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(suppl 2):S1-S40.
9. Beck JS, Beck AT. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. New York, New York: Guilford Press; 2011.
10. Henry JA, Zaugg TL, Myers PJ, et al. Pilot study to develop telehealth tinnitus management for persons with and without traumatic brain injury. J Rehab Res Dev. 2012;49(7):1025-1042.
11. Henry JA, Thielman EJ, Zaugg TL, et al. Randomized controlled trial in clinical settings to evaluate effectiveness of coping skills education used with progressive tinnitus management. J Speech Lang Hear Res. 2017;1-20. [Epub ahead of print]
12. Henry JA, Griest S, Thielman E, McMillan G, Kaelin C, Carlson K. The tinnitus functional index: development, validation, outcomes research, and clinical application. Hear Res. 2016;334:58-64.
13. Boudin A, Carlson KC, Elnitsky C, et al. Online Surveys of Tinnitus Management Practices in VA and DoD: Results and Clinical Implications. Joint Defense Veterans Audiology Conference (JDVAC), St Louis, MO, February 22-24, 2016.
14. Carlson KC, Thielman E, Zaugg TL, Elnitsky C, Tuepker A, Kaelin C, Henry JA. “VA Clinician Surveys and Interviews Reveal Need for Increased Mental Health Involvement in Tinnitus Management.” Joint Defense Veterans Audiology Conference (JDVAC), St Louis, MO, February 22-24, 2016.
15. Carlson K, Thielman E, Zaugg T, et al. Factors affecting the provision of evidence-based progressive tinnitus management in Department of Veterans Affairs medical centers. Paper presented at: Academy Health Annual Research Meeting; June 26-28, 2016;
Screening for Symptomatic Mefloquine Exposure Among Veterans With Chronic Psychiatric Symptoms
Mefloquine is an antimalarial drug that is associated with a significant risk of chronic neuropsychiatric adverse effects (AEs). The drug was licensed by the FDA in 1989 after development by scientists affiliated with Walter Reed Army Institute of Research (WRAIR). By the early 1990s, mefloquine had become the U.S. military’s drug of choice both for treatment of uncomplicated malaria and for antimalarial prophylaxis and was administered as a convenient weekly dose. Mefloquine was prescribed widely to U.S. military personnel beginning with operations in Somalia in 1992 and over the next 2 decades during certain deployments to Iraq and Afghanistan and to other malaria-endemic areas.1
In 2013, following a decline of U.S. military use, the FDA added a boxed warning to the mefloquine product documentation to caution that neuropsychiatric AEs from the drug could last years after use and even be permanent. The U.S. military subsequently deemed mefloquine to be a prophylactic “drug of last resort.”2 Recently, researchers at WRAIR have acknowledged that chronic neuropsychiatric AEs attributable to mefloquine, including nightmares, insomnia, anxiety, irritability, and cognitive dysfunction, may confound the diagnosis of posttraumatic stress disorder (PTSD).3 The VA has awarded at least 1 disability claim for service-connected psychiatric conditions that it attributed to mefloquine exposure, and it is likely that in the coming years such claims will increase.2
Susceptibility to Chronic Neuropsychiatric AEs
Why mefloquine seems to cause chronic neuropsychiatric AEs in only certain individuals is unclear, although genetic susceptibility to drug-induced toxic encephalopathy and neurotoxicity are suspected.1 There is no screening test for susceptibility to AEs before mefloquine use, so the current U.S. product documentation cautiously warns that when used for prophylaxis, mefloquine should be discontinued at the onset of any neurologic or psychiatric symptom, many of which are considered prodromal to more serious AEs that may occur with continued dosing.4
Although chronic neuropsychiatric AEs have been reported to develop after only a single weekly dose, most clinically significant chronic AEs seem to occur among those who developed at least 1 prodromal neuropsychiatric symptom during early use but who continued weekly use despite these symptoms in a manner contrary to current product documentation guidance.4 In contrast, when mefloquine is administered for the treatment of malaria, typically at 5 times the weekly prophylactic dose and commonly in split doses over 8 to 12 hours, dosing is often complete by the time prodromal symptoms develop. Consequently, when mefloquine is used for treatment of malaria, the risks of more serious AEs are significantly higher than when the drug is used as directed in prophylaxis.5
Screening for Symptomatic Mefloquine Exposure
As the boxed warning indicates, certain psychiatric symptoms that occur with mefloquine use may become chronic and may confound psychiatric diagnosis. Particularly among veterans, these symptoms risk being misattributed, potentially affecting treatment decisions.6 Clinicians caring for veterans with persistent psychiatric symptoms should therefore screen for prior symptomatic mefloquine exposure and consider the possible AEs of the drug when formulating a differential diagnosis and treatment plan.
For example, a veteran with a history of symptomatic mefloquine exposure who later is diagnosed with PTSD may experience 1 or more symptoms, such as insomnia or cognitive dysfunction, which may be primarily attributable to the chronic AEs of the drug. The origins of the symptoms may be distinct from exposure to trauma and may not respond as effectively to certain conventional therapies for PTSD, requiring consideration of alternate therapies.3 The confounding role of psychiatric symptoms attributable to mefloquine exposure may explain failed response to medications and psychotherapy. Multidisciplinary evaluation and management may be appropriate for such patients.
If symptomatic mefloquine exposure is suspected, a clinician must establish evidence of exposure to the drug and the veteran’s development of neuropsychiatric symptoms associated with such exposure. The following sections provide guidance to aid in screening both for exposure to the drug and for the development of specific neuropsychiatric symptoms during prophylaxis or following the treatment of malaria.
Mefloquine Exposure
Mefloquine was licensed in the U.S. as a branded medication (Lariam) from 1989 to 2011, and the drug also has been available in a variety of generic equivalents from 2003 to the present. All versions of mefloquine approved in the U.S. have been formulated as a white/slightly-off-white, smaller than dime-sized round tablet, containing 250 mg of mefloquine hydrochloride.
When used for prophylaxis in military settings, the drug was often dispensed informally without documentation, sometimes including directly observed therapy under command direction.1,2 Therefore, even in the absence of prescribing documentation, a veteran who endorses a consistent history of malaria prophylaxis with mefloquine should be considered as having evidence of exposure.2
Exposure to mefloquine is unlikely if the veteran reports taking a daily antimalarial medication—more likely it was doxycycline or atovaquone/proguanil (marketed as Malarone). In rare cases, the drug may have been erroneously prescribed or been mistakenly taken daily for prophylaxis or, in more common cases, a prophylactic “loading dose” (typically 1 tablet daily for 3 days prior to weekly dosing) was used.7,8
Exposure also was unlikely if the veteran reports taking an antimalarial that was dosed weekly with a tablet that was not of the appropriate color, shape, and size. More likely that drug was chloroquine. Although most prophylactic use of mefloquine among U.S. veterans followed its licensing by the FDA in 1989, the drug is known to have been administered to a small number of U.S. military personnel prior to its licensing during clinical trials, including personnel deployed on certain operations during the 1980s.1
For treatment of malaria, mefloquine was used widely until better-tolerated drugs became available, beginning in the early 2000s, although some use of mefloquine in the military continues to this day. In most cases, clinicians should rely on records of hospitalization to identify whether mefloquine was administered. In rare cases where documentation is unavailable, exposure should be assumed if the veteran reports a reliable history of taking about 5 tablets (corresponding to the usual treatment dose of 1,250 mg) of appropriate color, shape, and size in response to confirmed or suspected malaria infection, either in 1 dose, or in split doses over 8 to 12 hours.
Symptoms During Prophylaxis
If prophylactic exposure to the drug has been established, the clinician should confirm the presence of neuropsychiatric symptoms during the exposure. Particularly among veterans deploying to malaria-endemic combat areas, such symptoms may have occurred during a period of heightened stress coincident with their initial deployment, and the veterans may have misattributed these symptoms to nonmefloquine factors. The clinician should therefore take a careful history to identify specific symptoms listed in the mefloquine product documentation. Many AEs will commonly manifest following the first 3 doses, and the clinician may find that focusing on this period is useful.9
When mefloquine is used for prophylaxis, anxiety and depression each affect between 1% and 10% of users. Other AEs that may develop include panic attacks; severe mood swings; behavioral AEs, including agitation, aggression, restlessness, and mania; symptoms of psychosis, including paranoia, delusions, and hallucinations; dissociative symptoms, including depersonalization; suicidal ideation; and cognitive AEs, including confusion.
The common symptoms of insomnia and abnormal dreaming affect > 10% of users. Particularly if multiple symptoms occur or if any of these symptoms occur following or coincident with symptoms of disturbed sleep, these should be considered strong evidence of symptomatic exposure.4 Veterans who report a history of continued mefloquine use despite the onset of such symptoms may be at particularly increased risk of chronic AEs.
The clinician should consider as evidence of symptomatic exposure information provided by others, including reports of obvious signs of nightmares or psychosis affecting the veteran. Clinicians should be aware that confusion and other psychiatric AEs caused by mefloquine during prophylactic use may limit the validity of self-reported history. Similarly, a history of seizure with mefloquine use or of the development of specific neurologic symptoms, particularly visual disturbances, dizziness, vertigo, disequilibrium, and paresthesias, also should be considered strong evidence of symptomatic exposure and indication of an increased risk of chronic psychiatric AEs.4
Posttreatment Adverse Effects
Although chronic psychiatric AEs following malaria infection have long been attributed to cerebral involvement, recognition that mefloquine may independently cause chronic neuropsychiatric AEs may require that individual cases be reexamined to properly assign causation.10 Particularly in uncomplicated cases of malaria, neuropsychiatric symptoms that develop only after treatment with mefloquine should be considered plausibly to be due to the drug and as evidence of symptomatic exposure.
As with use of mefloquine in prophylaxis, these neuropsychiatric symptoms may evolve in the weeks to months following exposure. They also may contribute to lasting and significant changes in personality, mood, cognition, thought, sleep, and behavior.6
Conclusion
Chronic AEs from mefloquine may provide a parsimonious explanation for the onset and persistence of a veteran’s psychiatric symptoms, particularly in cases where these may have failed to respond to treatment. Clinicians evaluating veterans who are seeking care for lasting psychiatric symptoms should ensure that they screen for prior symptomatic mefloquine exposure. As recognition grows of the drug’s chronic AEs, symptomatic mefloquine exposure is likely to emerge as a significant known confounder in the diagnosis of psychiatric disorders, including PTSD, among the current generation of U.S. veterans.
1. Nevin RL. Mefloquine and posttraumatic stress disorder. In: Ritchie EC, ed. Textbook of Military Medicine. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: Borden Institute; 2015:277-296.
2. Nevin RL, Ritchie EC. FDA black box, VA red ink? A successful service-connected disability claim for chronic neuropsychiatric adverse effects from mefloquine. Fed Pract. 2016;33(10):20-24.
3. Livezey J, Oliver T, Cantilena L. Prolonged neuropsychiatric symptoms in a military service member exposed to mefloquine. Drug Saf Case Rep. 2016;3(1):7.
4. Nevin RL, Byrd AM. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol Ther. 2016;5(1):69-83.
5. Rendi-Wagner P, Noedl H, Wernsdorfer WH, Wiedermann G, Mikolasek A, Kollaritsch H. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81(2):167-173.
6. Nevin RL, Ritchie E. The mefloquine intoxication syndrome: a significant potential confounder in the diagnosis and management of PTSD and other chronic deployment-related neuropsychiatric disorders. In: Ritchie EC, ed. Posttraumatic Stress Disorder and Related Diseases in Combat Veterans. Cham, Switzerland: Springer International Publishing; 2015:257-278.
7. Cohen MR, Smetzer JL. FDA advise-ERR: mefloquine—not the same as Malarone; zoster vaccine is not for the immunosuppressed; TXA mistaken as tenecteplase; guidelines for adult IV push medications. Hosp Pharm. 2015;50(11):961-964.
8. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol. 1993;44(3):257-265.
9. Stürchler D, Handschin J, Kaiser D, et al. Neuropsychiatric side effects of mefloquine. N Engl J Med. 1990;322(24):1752-1753.
10. Nevin RL, Croft AM. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332.
Mefloquine is an antimalarial drug that is associated with a significant risk of chronic neuropsychiatric adverse effects (AEs). The drug was licensed by the FDA in 1989 after development by scientists affiliated with Walter Reed Army Institute of Research (WRAIR). By the early 1990s, mefloquine had become the U.S. military’s drug of choice both for treatment of uncomplicated malaria and for antimalarial prophylaxis and was administered as a convenient weekly dose. Mefloquine was prescribed widely to U.S. military personnel beginning with operations in Somalia in 1992 and over the next 2 decades during certain deployments to Iraq and Afghanistan and to other malaria-endemic areas.1
In 2013, following a decline of U.S. military use, the FDA added a boxed warning to the mefloquine product documentation to caution that neuropsychiatric AEs from the drug could last years after use and even be permanent. The U.S. military subsequently deemed mefloquine to be a prophylactic “drug of last resort.”2 Recently, researchers at WRAIR have acknowledged that chronic neuropsychiatric AEs attributable to mefloquine, including nightmares, insomnia, anxiety, irritability, and cognitive dysfunction, may confound the diagnosis of posttraumatic stress disorder (PTSD).3 The VA has awarded at least 1 disability claim for service-connected psychiatric conditions that it attributed to mefloquine exposure, and it is likely that in the coming years such claims will increase.2
Susceptibility to Chronic Neuropsychiatric AEs
Why mefloquine seems to cause chronic neuropsychiatric AEs in only certain individuals is unclear, although genetic susceptibility to drug-induced toxic encephalopathy and neurotoxicity are suspected.1 There is no screening test for susceptibility to AEs before mefloquine use, so the current U.S. product documentation cautiously warns that when used for prophylaxis, mefloquine should be discontinued at the onset of any neurologic or psychiatric symptom, many of which are considered prodromal to more serious AEs that may occur with continued dosing.4
Although chronic neuropsychiatric AEs have been reported to develop after only a single weekly dose, most clinically significant chronic AEs seem to occur among those who developed at least 1 prodromal neuropsychiatric symptom during early use but who continued weekly use despite these symptoms in a manner contrary to current product documentation guidance.4 In contrast, when mefloquine is administered for the treatment of malaria, typically at 5 times the weekly prophylactic dose and commonly in split doses over 8 to 12 hours, dosing is often complete by the time prodromal symptoms develop. Consequently, when mefloquine is used for treatment of malaria, the risks of more serious AEs are significantly higher than when the drug is used as directed in prophylaxis.5
Screening for Symptomatic Mefloquine Exposure
As the boxed warning indicates, certain psychiatric symptoms that occur with mefloquine use may become chronic and may confound psychiatric diagnosis. Particularly among veterans, these symptoms risk being misattributed, potentially affecting treatment decisions.6 Clinicians caring for veterans with persistent psychiatric symptoms should therefore screen for prior symptomatic mefloquine exposure and consider the possible AEs of the drug when formulating a differential diagnosis and treatment plan.
For example, a veteran with a history of symptomatic mefloquine exposure who later is diagnosed with PTSD may experience 1 or more symptoms, such as insomnia or cognitive dysfunction, which may be primarily attributable to the chronic AEs of the drug. The origins of the symptoms may be distinct from exposure to trauma and may not respond as effectively to certain conventional therapies for PTSD, requiring consideration of alternate therapies.3 The confounding role of psychiatric symptoms attributable to mefloquine exposure may explain failed response to medications and psychotherapy. Multidisciplinary evaluation and management may be appropriate for such patients.
If symptomatic mefloquine exposure is suspected, a clinician must establish evidence of exposure to the drug and the veteran’s development of neuropsychiatric symptoms associated with such exposure. The following sections provide guidance to aid in screening both for exposure to the drug and for the development of specific neuropsychiatric symptoms during prophylaxis or following the treatment of malaria.
Mefloquine Exposure
Mefloquine was licensed in the U.S. as a branded medication (Lariam) from 1989 to 2011, and the drug also has been available in a variety of generic equivalents from 2003 to the present. All versions of mefloquine approved in the U.S. have been formulated as a white/slightly-off-white, smaller than dime-sized round tablet, containing 250 mg of mefloquine hydrochloride.
When used for prophylaxis in military settings, the drug was often dispensed informally without documentation, sometimes including directly observed therapy under command direction.1,2 Therefore, even in the absence of prescribing documentation, a veteran who endorses a consistent history of malaria prophylaxis with mefloquine should be considered as having evidence of exposure.2
Exposure to mefloquine is unlikely if the veteran reports taking a daily antimalarial medication—more likely it was doxycycline or atovaquone/proguanil (marketed as Malarone). In rare cases, the drug may have been erroneously prescribed or been mistakenly taken daily for prophylaxis or, in more common cases, a prophylactic “loading dose” (typically 1 tablet daily for 3 days prior to weekly dosing) was used.7,8
Exposure also was unlikely if the veteran reports taking an antimalarial that was dosed weekly with a tablet that was not of the appropriate color, shape, and size. More likely that drug was chloroquine. Although most prophylactic use of mefloquine among U.S. veterans followed its licensing by the FDA in 1989, the drug is known to have been administered to a small number of U.S. military personnel prior to its licensing during clinical trials, including personnel deployed on certain operations during the 1980s.1
For treatment of malaria, mefloquine was used widely until better-tolerated drugs became available, beginning in the early 2000s, although some use of mefloquine in the military continues to this day. In most cases, clinicians should rely on records of hospitalization to identify whether mefloquine was administered. In rare cases where documentation is unavailable, exposure should be assumed if the veteran reports a reliable history of taking about 5 tablets (corresponding to the usual treatment dose of 1,250 mg) of appropriate color, shape, and size in response to confirmed or suspected malaria infection, either in 1 dose, or in split doses over 8 to 12 hours.
Symptoms During Prophylaxis
If prophylactic exposure to the drug has been established, the clinician should confirm the presence of neuropsychiatric symptoms during the exposure. Particularly among veterans deploying to malaria-endemic combat areas, such symptoms may have occurred during a period of heightened stress coincident with their initial deployment, and the veterans may have misattributed these symptoms to nonmefloquine factors. The clinician should therefore take a careful history to identify specific symptoms listed in the mefloquine product documentation. Many AEs will commonly manifest following the first 3 doses, and the clinician may find that focusing on this period is useful.9
When mefloquine is used for prophylaxis, anxiety and depression each affect between 1% and 10% of users. Other AEs that may develop include panic attacks; severe mood swings; behavioral AEs, including agitation, aggression, restlessness, and mania; symptoms of psychosis, including paranoia, delusions, and hallucinations; dissociative symptoms, including depersonalization; suicidal ideation; and cognitive AEs, including confusion.
The common symptoms of insomnia and abnormal dreaming affect > 10% of users. Particularly if multiple symptoms occur or if any of these symptoms occur following or coincident with symptoms of disturbed sleep, these should be considered strong evidence of symptomatic exposure.4 Veterans who report a history of continued mefloquine use despite the onset of such symptoms may be at particularly increased risk of chronic AEs.
The clinician should consider as evidence of symptomatic exposure information provided by others, including reports of obvious signs of nightmares or psychosis affecting the veteran. Clinicians should be aware that confusion and other psychiatric AEs caused by mefloquine during prophylactic use may limit the validity of self-reported history. Similarly, a history of seizure with mefloquine use or of the development of specific neurologic symptoms, particularly visual disturbances, dizziness, vertigo, disequilibrium, and paresthesias, also should be considered strong evidence of symptomatic exposure and indication of an increased risk of chronic psychiatric AEs.4
Posttreatment Adverse Effects
Although chronic psychiatric AEs following malaria infection have long been attributed to cerebral involvement, recognition that mefloquine may independently cause chronic neuropsychiatric AEs may require that individual cases be reexamined to properly assign causation.10 Particularly in uncomplicated cases of malaria, neuropsychiatric symptoms that develop only after treatment with mefloquine should be considered plausibly to be due to the drug and as evidence of symptomatic exposure.
As with use of mefloquine in prophylaxis, these neuropsychiatric symptoms may evolve in the weeks to months following exposure. They also may contribute to lasting and significant changes in personality, mood, cognition, thought, sleep, and behavior.6
Conclusion
Chronic AEs from mefloquine may provide a parsimonious explanation for the onset and persistence of a veteran’s psychiatric symptoms, particularly in cases where these may have failed to respond to treatment. Clinicians evaluating veterans who are seeking care for lasting psychiatric symptoms should ensure that they screen for prior symptomatic mefloquine exposure. As recognition grows of the drug’s chronic AEs, symptomatic mefloquine exposure is likely to emerge as a significant known confounder in the diagnosis of psychiatric disorders, including PTSD, among the current generation of U.S. veterans.
Mefloquine is an antimalarial drug that is associated with a significant risk of chronic neuropsychiatric adverse effects (AEs). The drug was licensed by the FDA in 1989 after development by scientists affiliated with Walter Reed Army Institute of Research (WRAIR). By the early 1990s, mefloquine had become the U.S. military’s drug of choice both for treatment of uncomplicated malaria and for antimalarial prophylaxis and was administered as a convenient weekly dose. Mefloquine was prescribed widely to U.S. military personnel beginning with operations in Somalia in 1992 and over the next 2 decades during certain deployments to Iraq and Afghanistan and to other malaria-endemic areas.1
In 2013, following a decline of U.S. military use, the FDA added a boxed warning to the mefloquine product documentation to caution that neuropsychiatric AEs from the drug could last years after use and even be permanent. The U.S. military subsequently deemed mefloquine to be a prophylactic “drug of last resort.”2 Recently, researchers at WRAIR have acknowledged that chronic neuropsychiatric AEs attributable to mefloquine, including nightmares, insomnia, anxiety, irritability, and cognitive dysfunction, may confound the diagnosis of posttraumatic stress disorder (PTSD).3 The VA has awarded at least 1 disability claim for service-connected psychiatric conditions that it attributed to mefloquine exposure, and it is likely that in the coming years such claims will increase.2
Susceptibility to Chronic Neuropsychiatric AEs
Why mefloquine seems to cause chronic neuropsychiatric AEs in only certain individuals is unclear, although genetic susceptibility to drug-induced toxic encephalopathy and neurotoxicity are suspected.1 There is no screening test for susceptibility to AEs before mefloquine use, so the current U.S. product documentation cautiously warns that when used for prophylaxis, mefloquine should be discontinued at the onset of any neurologic or psychiatric symptom, many of which are considered prodromal to more serious AEs that may occur with continued dosing.4
Although chronic neuropsychiatric AEs have been reported to develop after only a single weekly dose, most clinically significant chronic AEs seem to occur among those who developed at least 1 prodromal neuropsychiatric symptom during early use but who continued weekly use despite these symptoms in a manner contrary to current product documentation guidance.4 In contrast, when mefloquine is administered for the treatment of malaria, typically at 5 times the weekly prophylactic dose and commonly in split doses over 8 to 12 hours, dosing is often complete by the time prodromal symptoms develop. Consequently, when mefloquine is used for treatment of malaria, the risks of more serious AEs are significantly higher than when the drug is used as directed in prophylaxis.5
Screening for Symptomatic Mefloquine Exposure
As the boxed warning indicates, certain psychiatric symptoms that occur with mefloquine use may become chronic and may confound psychiatric diagnosis. Particularly among veterans, these symptoms risk being misattributed, potentially affecting treatment decisions.6 Clinicians caring for veterans with persistent psychiatric symptoms should therefore screen for prior symptomatic mefloquine exposure and consider the possible AEs of the drug when formulating a differential diagnosis and treatment plan.
For example, a veteran with a history of symptomatic mefloquine exposure who later is diagnosed with PTSD may experience 1 or more symptoms, such as insomnia or cognitive dysfunction, which may be primarily attributable to the chronic AEs of the drug. The origins of the symptoms may be distinct from exposure to trauma and may not respond as effectively to certain conventional therapies for PTSD, requiring consideration of alternate therapies.3 The confounding role of psychiatric symptoms attributable to mefloquine exposure may explain failed response to medications and psychotherapy. Multidisciplinary evaluation and management may be appropriate for such patients.
If symptomatic mefloquine exposure is suspected, a clinician must establish evidence of exposure to the drug and the veteran’s development of neuropsychiatric symptoms associated with such exposure. The following sections provide guidance to aid in screening both for exposure to the drug and for the development of specific neuropsychiatric symptoms during prophylaxis or following the treatment of malaria.
Mefloquine Exposure
Mefloquine was licensed in the U.S. as a branded medication (Lariam) from 1989 to 2011, and the drug also has been available in a variety of generic equivalents from 2003 to the present. All versions of mefloquine approved in the U.S. have been formulated as a white/slightly-off-white, smaller than dime-sized round tablet, containing 250 mg of mefloquine hydrochloride.
When used for prophylaxis in military settings, the drug was often dispensed informally without documentation, sometimes including directly observed therapy under command direction.1,2 Therefore, even in the absence of prescribing documentation, a veteran who endorses a consistent history of malaria prophylaxis with mefloquine should be considered as having evidence of exposure.2
Exposure to mefloquine is unlikely if the veteran reports taking a daily antimalarial medication—more likely it was doxycycline or atovaquone/proguanil (marketed as Malarone). In rare cases, the drug may have been erroneously prescribed or been mistakenly taken daily for prophylaxis or, in more common cases, a prophylactic “loading dose” (typically 1 tablet daily for 3 days prior to weekly dosing) was used.7,8
Exposure also was unlikely if the veteran reports taking an antimalarial that was dosed weekly with a tablet that was not of the appropriate color, shape, and size. More likely that drug was chloroquine. Although most prophylactic use of mefloquine among U.S. veterans followed its licensing by the FDA in 1989, the drug is known to have been administered to a small number of U.S. military personnel prior to its licensing during clinical trials, including personnel deployed on certain operations during the 1980s.1
For treatment of malaria, mefloquine was used widely until better-tolerated drugs became available, beginning in the early 2000s, although some use of mefloquine in the military continues to this day. In most cases, clinicians should rely on records of hospitalization to identify whether mefloquine was administered. In rare cases where documentation is unavailable, exposure should be assumed if the veteran reports a reliable history of taking about 5 tablets (corresponding to the usual treatment dose of 1,250 mg) of appropriate color, shape, and size in response to confirmed or suspected malaria infection, either in 1 dose, or in split doses over 8 to 12 hours.
Symptoms During Prophylaxis
If prophylactic exposure to the drug has been established, the clinician should confirm the presence of neuropsychiatric symptoms during the exposure. Particularly among veterans deploying to malaria-endemic combat areas, such symptoms may have occurred during a period of heightened stress coincident with their initial deployment, and the veterans may have misattributed these symptoms to nonmefloquine factors. The clinician should therefore take a careful history to identify specific symptoms listed in the mefloquine product documentation. Many AEs will commonly manifest following the first 3 doses, and the clinician may find that focusing on this period is useful.9
When mefloquine is used for prophylaxis, anxiety and depression each affect between 1% and 10% of users. Other AEs that may develop include panic attacks; severe mood swings; behavioral AEs, including agitation, aggression, restlessness, and mania; symptoms of psychosis, including paranoia, delusions, and hallucinations; dissociative symptoms, including depersonalization; suicidal ideation; and cognitive AEs, including confusion.
The common symptoms of insomnia and abnormal dreaming affect > 10% of users. Particularly if multiple symptoms occur or if any of these symptoms occur following or coincident with symptoms of disturbed sleep, these should be considered strong evidence of symptomatic exposure.4 Veterans who report a history of continued mefloquine use despite the onset of such symptoms may be at particularly increased risk of chronic AEs.
The clinician should consider as evidence of symptomatic exposure information provided by others, including reports of obvious signs of nightmares or psychosis affecting the veteran. Clinicians should be aware that confusion and other psychiatric AEs caused by mefloquine during prophylactic use may limit the validity of self-reported history. Similarly, a history of seizure with mefloquine use or of the development of specific neurologic symptoms, particularly visual disturbances, dizziness, vertigo, disequilibrium, and paresthesias, also should be considered strong evidence of symptomatic exposure and indication of an increased risk of chronic psychiatric AEs.4
Posttreatment Adverse Effects
Although chronic psychiatric AEs following malaria infection have long been attributed to cerebral involvement, recognition that mefloquine may independently cause chronic neuropsychiatric AEs may require that individual cases be reexamined to properly assign causation.10 Particularly in uncomplicated cases of malaria, neuropsychiatric symptoms that develop only after treatment with mefloquine should be considered plausibly to be due to the drug and as evidence of symptomatic exposure.
As with use of mefloquine in prophylaxis, these neuropsychiatric symptoms may evolve in the weeks to months following exposure. They also may contribute to lasting and significant changes in personality, mood, cognition, thought, sleep, and behavior.6
Conclusion
Chronic AEs from mefloquine may provide a parsimonious explanation for the onset and persistence of a veteran’s psychiatric symptoms, particularly in cases where these may have failed to respond to treatment. Clinicians evaluating veterans who are seeking care for lasting psychiatric symptoms should ensure that they screen for prior symptomatic mefloquine exposure. As recognition grows of the drug’s chronic AEs, symptomatic mefloquine exposure is likely to emerge as a significant known confounder in the diagnosis of psychiatric disorders, including PTSD, among the current generation of U.S. veterans.
1. Nevin RL. Mefloquine and posttraumatic stress disorder. In: Ritchie EC, ed. Textbook of Military Medicine. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: Borden Institute; 2015:277-296.
2. Nevin RL, Ritchie EC. FDA black box, VA red ink? A successful service-connected disability claim for chronic neuropsychiatric adverse effects from mefloquine. Fed Pract. 2016;33(10):20-24.
3. Livezey J, Oliver T, Cantilena L. Prolonged neuropsychiatric symptoms in a military service member exposed to mefloquine. Drug Saf Case Rep. 2016;3(1):7.
4. Nevin RL, Byrd AM. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol Ther. 2016;5(1):69-83.
5. Rendi-Wagner P, Noedl H, Wernsdorfer WH, Wiedermann G, Mikolasek A, Kollaritsch H. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81(2):167-173.
6. Nevin RL, Ritchie E. The mefloquine intoxication syndrome: a significant potential confounder in the diagnosis and management of PTSD and other chronic deployment-related neuropsychiatric disorders. In: Ritchie EC, ed. Posttraumatic Stress Disorder and Related Diseases in Combat Veterans. Cham, Switzerland: Springer International Publishing; 2015:257-278.
7. Cohen MR, Smetzer JL. FDA advise-ERR: mefloquine—not the same as Malarone; zoster vaccine is not for the immunosuppressed; TXA mistaken as tenecteplase; guidelines for adult IV push medications. Hosp Pharm. 2015;50(11):961-964.
8. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol. 1993;44(3):257-265.
9. Stürchler D, Handschin J, Kaiser D, et al. Neuropsychiatric side effects of mefloquine. N Engl J Med. 1990;322(24):1752-1753.
10. Nevin RL, Croft AM. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332.
1. Nevin RL. Mefloquine and posttraumatic stress disorder. In: Ritchie EC, ed. Textbook of Military Medicine. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: Borden Institute; 2015:277-296.
2. Nevin RL, Ritchie EC. FDA black box, VA red ink? A successful service-connected disability claim for chronic neuropsychiatric adverse effects from mefloquine. Fed Pract. 2016;33(10):20-24.
3. Livezey J, Oliver T, Cantilena L. Prolonged neuropsychiatric symptoms in a military service member exposed to mefloquine. Drug Saf Case Rep. 2016;3(1):7.
4. Nevin RL, Byrd AM. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol Ther. 2016;5(1):69-83.
5. Rendi-Wagner P, Noedl H, Wernsdorfer WH, Wiedermann G, Mikolasek A, Kollaritsch H. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81(2):167-173.
6. Nevin RL, Ritchie E. The mefloquine intoxication syndrome: a significant potential confounder in the diagnosis and management of PTSD and other chronic deployment-related neuropsychiatric disorders. In: Ritchie EC, ed. Posttraumatic Stress Disorder and Related Diseases in Combat Veterans. Cham, Switzerland: Springer International Publishing; 2015:257-278.
7. Cohen MR, Smetzer JL. FDA advise-ERR: mefloquine—not the same as Malarone; zoster vaccine is not for the immunosuppressed; TXA mistaken as tenecteplase; guidelines for adult IV push medications. Hosp Pharm. 2015;50(11):961-964.
8. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol. 1993;44(3):257-265.
9. Stürchler D, Handschin J, Kaiser D, et al. Neuropsychiatric side effects of mefloquine. N Engl J Med. 1990;322(24):1752-1753.
10. Nevin RL, Croft AM. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332.