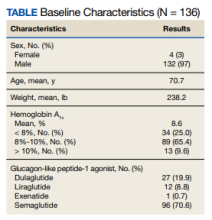
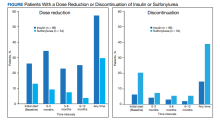
User login
Selenium Supplementation Shows Thyroid Benefits
TOPLINE:
, research from a new meta-analysis showed.
METHODOLOGY:
- For the systematic review and meta-analysis, 35 randomized controlled trials were identified that included evaluation of selenium supplementation’s effects on thyroid function.
- The studies focused on a variety of key thyroid function measures, including thyroid-stimulating hormone (TSH), free and total thyroxine (fT4, T4), free and total triiodothyronine (fT3, T3), thyroid antibodies, safety, and other factors.
- Stratified analyses were conducted to evaluate key factors including the dose and duration of selenium supplementation; patients’ thyroid status, age, gender, treatment with hormone replacement, and selenium status, such as deficiency or sufficiency; and other factors.
- While patients’ selenium levels at baseline were reported in only about half of the studies, among those that did have the data, the vast majority — 89% of cohorts — were selenium deficient.
- The study populations ranged from 31 to 364 and included children, adolescents, and adults.
TAKEAWAY:
- The analysis showed selenium supplementation to be significantly associated with decreased TSH in patients who were not treated with thyroid hormone replacement therapy (standardized mean difference [SMD], −0.21 in seven cohorts, involving 869 participants).
- Improvements associated with selenium replacement were also observed regardless of whether patients were on thyroid hormone replacement therapy in terms of decreases in thyroid peroxidase antibodies (TPOAb) (SMD, −0.96 in 29 cohorts, involving 2358 participants) and malondialdehyde (SMD, −1.16 in three cohorts, involving 248 participants).
- Overall, selenium supplementation had no significant effects on other notable thyroid measures, including fT4, T4, fT3, T3, thyroglobulin antibody (TGAb), thyroid volume, interleukin 2, or interleukin 10. However, when the analysis only included adults aged 18 and older, the selenium supplementation was linked to reductions in TSH and TPOAb, as well as increases in fT4 levels.
- Importantly, no significant differences were observed in terms of adverse effects between the studies’ intervention and control groups at selenium supplementation doses ranging from 80 to 400 μg/d for up to 12 months (odds ratio, 0.89 in 16 cohorts, involving 1339 participants).
- The authors determined that the certainty of evidence, overall, was moderate.
IN PRACTICE:
The results regarding effects of selenium on TSH “add to the existing knowledge in this field by demonstrating an effect of selenium supplementation on lowering TSH levels exclusively in Hashimoto thyroiditis patients without thyroid hormone replacement therapy,” the authors wrote. Furthermore, “our study reaffirmed the results of six prior meta-analyses reporting an effect of selenium in reducing TPOAb levels,” they added. “The inclusion of 31 cohorts enhanced statistical power compared to the previous meta-analyses, which included a maximum of nine cohorts.” “Our study suggests that selenium supplementation is safe and holds potential as a disease-modifying factor for Hashimoto thyroiditis–associated hypothyroidism,” the authors reported. “Further research is needed to confirm its efficacy, fully understand its mechanism of action, and elucidate its cost-effectiveness.”
SOURCE:
The study’s first author was Valentina V. Huwiler, MSc, of the Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland. The study was published in Thyroid.
LIMITATIONS:
Due to variations in assays used in the different studies for measures including TPOAb and TGAb, the authors used SMD instead of the mean difference typically recommended when varying assays are used; however, only the effect size can be interpreted and not the clinical significance, the authors noted. Serum selenium concentrations may vary based on the analytical technique. Data on participants’ dietary habits and compliance with study regimens were not available.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, research from a new meta-analysis showed.
METHODOLOGY:
- For the systematic review and meta-analysis, 35 randomized controlled trials were identified that included evaluation of selenium supplementation’s effects on thyroid function.
- The studies focused on a variety of key thyroid function measures, including thyroid-stimulating hormone (TSH), free and total thyroxine (fT4, T4), free and total triiodothyronine (fT3, T3), thyroid antibodies, safety, and other factors.
- Stratified analyses were conducted to evaluate key factors including the dose and duration of selenium supplementation; patients’ thyroid status, age, gender, treatment with hormone replacement, and selenium status, such as deficiency or sufficiency; and other factors.
- While patients’ selenium levels at baseline were reported in only about half of the studies, among those that did have the data, the vast majority — 89% of cohorts — were selenium deficient.
- The study populations ranged from 31 to 364 and included children, adolescents, and adults.
TAKEAWAY:
- The analysis showed selenium supplementation to be significantly associated with decreased TSH in patients who were not treated with thyroid hormone replacement therapy (standardized mean difference [SMD], −0.21 in seven cohorts, involving 869 participants).
- Improvements associated with selenium replacement were also observed regardless of whether patients were on thyroid hormone replacement therapy in terms of decreases in thyroid peroxidase antibodies (TPOAb) (SMD, −0.96 in 29 cohorts, involving 2358 participants) and malondialdehyde (SMD, −1.16 in three cohorts, involving 248 participants).
- Overall, selenium supplementation had no significant effects on other notable thyroid measures, including fT4, T4, fT3, T3, thyroglobulin antibody (TGAb), thyroid volume, interleukin 2, or interleukin 10. However, when the analysis only included adults aged 18 and older, the selenium supplementation was linked to reductions in TSH and TPOAb, as well as increases in fT4 levels.
- Importantly, no significant differences were observed in terms of adverse effects between the studies’ intervention and control groups at selenium supplementation doses ranging from 80 to 400 μg/d for up to 12 months (odds ratio, 0.89 in 16 cohorts, involving 1339 participants).
- The authors determined that the certainty of evidence, overall, was moderate.
IN PRACTICE:
The results regarding effects of selenium on TSH “add to the existing knowledge in this field by demonstrating an effect of selenium supplementation on lowering TSH levels exclusively in Hashimoto thyroiditis patients without thyroid hormone replacement therapy,” the authors wrote. Furthermore, “our study reaffirmed the results of six prior meta-analyses reporting an effect of selenium in reducing TPOAb levels,” they added. “The inclusion of 31 cohorts enhanced statistical power compared to the previous meta-analyses, which included a maximum of nine cohorts.” “Our study suggests that selenium supplementation is safe and holds potential as a disease-modifying factor for Hashimoto thyroiditis–associated hypothyroidism,” the authors reported. “Further research is needed to confirm its efficacy, fully understand its mechanism of action, and elucidate its cost-effectiveness.”
SOURCE:
The study’s first author was Valentina V. Huwiler, MSc, of the Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland. The study was published in Thyroid.
LIMITATIONS:
Due to variations in assays used in the different studies for measures including TPOAb and TGAb, the authors used SMD instead of the mean difference typically recommended when varying assays are used; however, only the effect size can be interpreted and not the clinical significance, the authors noted. Serum selenium concentrations may vary based on the analytical technique. Data on participants’ dietary habits and compliance with study regimens were not available.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, research from a new meta-analysis showed.
METHODOLOGY:
- For the systematic review and meta-analysis, 35 randomized controlled trials were identified that included evaluation of selenium supplementation’s effects on thyroid function.
- The studies focused on a variety of key thyroid function measures, including thyroid-stimulating hormone (TSH), free and total thyroxine (fT4, T4), free and total triiodothyronine (fT3, T3), thyroid antibodies, safety, and other factors.
- Stratified analyses were conducted to evaluate key factors including the dose and duration of selenium supplementation; patients’ thyroid status, age, gender, treatment with hormone replacement, and selenium status, such as deficiency or sufficiency; and other factors.
- While patients’ selenium levels at baseline were reported in only about half of the studies, among those that did have the data, the vast majority — 89% of cohorts — were selenium deficient.
- The study populations ranged from 31 to 364 and included children, adolescents, and adults.
TAKEAWAY:
- The analysis showed selenium supplementation to be significantly associated with decreased TSH in patients who were not treated with thyroid hormone replacement therapy (standardized mean difference [SMD], −0.21 in seven cohorts, involving 869 participants).
- Improvements associated with selenium replacement were also observed regardless of whether patients were on thyroid hormone replacement therapy in terms of decreases in thyroid peroxidase antibodies (TPOAb) (SMD, −0.96 in 29 cohorts, involving 2358 participants) and malondialdehyde (SMD, −1.16 in three cohorts, involving 248 participants).
- Overall, selenium supplementation had no significant effects on other notable thyroid measures, including fT4, T4, fT3, T3, thyroglobulin antibody (TGAb), thyroid volume, interleukin 2, or interleukin 10. However, when the analysis only included adults aged 18 and older, the selenium supplementation was linked to reductions in TSH and TPOAb, as well as increases in fT4 levels.
- Importantly, no significant differences were observed in terms of adverse effects between the studies’ intervention and control groups at selenium supplementation doses ranging from 80 to 400 μg/d for up to 12 months (odds ratio, 0.89 in 16 cohorts, involving 1339 participants).
- The authors determined that the certainty of evidence, overall, was moderate.
IN PRACTICE:
The results regarding effects of selenium on TSH “add to the existing knowledge in this field by demonstrating an effect of selenium supplementation on lowering TSH levels exclusively in Hashimoto thyroiditis patients without thyroid hormone replacement therapy,” the authors wrote. Furthermore, “our study reaffirmed the results of six prior meta-analyses reporting an effect of selenium in reducing TPOAb levels,” they added. “The inclusion of 31 cohorts enhanced statistical power compared to the previous meta-analyses, which included a maximum of nine cohorts.” “Our study suggests that selenium supplementation is safe and holds potential as a disease-modifying factor for Hashimoto thyroiditis–associated hypothyroidism,” the authors reported. “Further research is needed to confirm its efficacy, fully understand its mechanism of action, and elucidate its cost-effectiveness.”
SOURCE:
The study’s first author was Valentina V. Huwiler, MSc, of the Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland. The study was published in Thyroid.
LIMITATIONS:
Due to variations in assays used in the different studies for measures including TPOAb and TGAb, the authors used SMD instead of the mean difference typically recommended when varying assays are used; however, only the effect size can be interpreted and not the clinical significance, the authors noted. Serum selenium concentrations may vary based on the analytical technique. Data on participants’ dietary habits and compliance with study regimens were not available.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
New Antibiotic Promising for Complicated UTIs
TOPLINE:
, according to a study published in The New England Journal of Medicine.
METHODOLOGY:
- Cefepime-taniborbactam is an antibiotic currently being explored as a treatment for antibiotic-resistant bacteria.
- The phase 3, double-blind, randomized trial included participants from 15 countries, including a safety group of 657 patients who were studied for adverse events and 436 in the micro intention-to-treat group who were studied for drug effectiveness.
- Each drug’s efficacy was measured as a combination of reduced bacteria levels and a resolution of symptoms and signs of infection.
- Patients in the study were over age 18; had a diagnosis of either complicated UTI or acute pyelonephritis; and had pyuria, at least one systemic sign, and at least one local sign or symptom. People were excluded if they had already received antibacterial drug therapy for more than 24 hours before randomization or had an infection with a meropenem-resistant pathogen.
TAKEAWAY:
- At days 19-23, 70.6% of patients in the cefepime-taniborbactam group showed a successful reduction in bacteria and symptoms compared with 58.0% in the meropenem group.
- Cefepime-taniborbactam was more effective than meropenem during follow-up, with 89.1% efficacy less than 24 hours after the last dose, compared to meropenem’s 86%. Cefepime-taniborbactam continued to have 63.8% efficacy up to 35 days after starting treatment, while meropenem was 51.7% during that timeframe.
- In the cefepime-taniborbactam group, 35.5% of patients experienced adverse effects that were mild to moderate, including headache, diarrhea, constipation, hypertension, and nausea, compared to 29% in the meropenem group.
- Overall, 3% of participants discontinued cefepime-taniborbactam and 1.8% discontinued meropenem, but reasons were heterogeneous.
IN PRACTICE:
“Cefepime-taniborbactam was superior to meropenem for the treatment of complicated UTI that included acute pyelonephritis, with a safety profile similar to that of meropenem,” the study authors wrote.
SOURCE:
Paul McGovern, MD, infectious disease specialist and senior vice president of Venatorx Pharmaceuticals, was the corresponding author of the study.
LIMITATIONS:
The authors reported no limitations.
DISCLOSURES:
The study was funded by Venatorx Pharmaceuticals, which received funding from the US Department of Health and Human Services, the Administration for Strategic Preparedness and Response, the Biomedical Advanced Research and Development Authority, the Global Antibiotic Research and Development Partnership, and Everest Medicines.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a study published in The New England Journal of Medicine.
METHODOLOGY:
- Cefepime-taniborbactam is an antibiotic currently being explored as a treatment for antibiotic-resistant bacteria.
- The phase 3, double-blind, randomized trial included participants from 15 countries, including a safety group of 657 patients who were studied for adverse events and 436 in the micro intention-to-treat group who were studied for drug effectiveness.
- Each drug’s efficacy was measured as a combination of reduced bacteria levels and a resolution of symptoms and signs of infection.
- Patients in the study were over age 18; had a diagnosis of either complicated UTI or acute pyelonephritis; and had pyuria, at least one systemic sign, and at least one local sign or symptom. People were excluded if they had already received antibacterial drug therapy for more than 24 hours before randomization or had an infection with a meropenem-resistant pathogen.
TAKEAWAY:
- At days 19-23, 70.6% of patients in the cefepime-taniborbactam group showed a successful reduction in bacteria and symptoms compared with 58.0% in the meropenem group.
- Cefepime-taniborbactam was more effective than meropenem during follow-up, with 89.1% efficacy less than 24 hours after the last dose, compared to meropenem’s 86%. Cefepime-taniborbactam continued to have 63.8% efficacy up to 35 days after starting treatment, while meropenem was 51.7% during that timeframe.
- In the cefepime-taniborbactam group, 35.5% of patients experienced adverse effects that were mild to moderate, including headache, diarrhea, constipation, hypertension, and nausea, compared to 29% in the meropenem group.
- Overall, 3% of participants discontinued cefepime-taniborbactam and 1.8% discontinued meropenem, but reasons were heterogeneous.
IN PRACTICE:
“Cefepime-taniborbactam was superior to meropenem for the treatment of complicated UTI that included acute pyelonephritis, with a safety profile similar to that of meropenem,” the study authors wrote.
SOURCE:
Paul McGovern, MD, infectious disease specialist and senior vice president of Venatorx Pharmaceuticals, was the corresponding author of the study.
LIMITATIONS:
The authors reported no limitations.
DISCLOSURES:
The study was funded by Venatorx Pharmaceuticals, which received funding from the US Department of Health and Human Services, the Administration for Strategic Preparedness and Response, the Biomedical Advanced Research and Development Authority, the Global Antibiotic Research and Development Partnership, and Everest Medicines.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a study published in The New England Journal of Medicine.
METHODOLOGY:
- Cefepime-taniborbactam is an antibiotic currently being explored as a treatment for antibiotic-resistant bacteria.
- The phase 3, double-blind, randomized trial included participants from 15 countries, including a safety group of 657 patients who were studied for adverse events and 436 in the micro intention-to-treat group who were studied for drug effectiveness.
- Each drug’s efficacy was measured as a combination of reduced bacteria levels and a resolution of symptoms and signs of infection.
- Patients in the study were over age 18; had a diagnosis of either complicated UTI or acute pyelonephritis; and had pyuria, at least one systemic sign, and at least one local sign or symptom. People were excluded if they had already received antibacterial drug therapy for more than 24 hours before randomization or had an infection with a meropenem-resistant pathogen.
TAKEAWAY:
- At days 19-23, 70.6% of patients in the cefepime-taniborbactam group showed a successful reduction in bacteria and symptoms compared with 58.0% in the meropenem group.
- Cefepime-taniborbactam was more effective than meropenem during follow-up, with 89.1% efficacy less than 24 hours after the last dose, compared to meropenem’s 86%. Cefepime-taniborbactam continued to have 63.8% efficacy up to 35 days after starting treatment, while meropenem was 51.7% during that timeframe.
- In the cefepime-taniborbactam group, 35.5% of patients experienced adverse effects that were mild to moderate, including headache, diarrhea, constipation, hypertension, and nausea, compared to 29% in the meropenem group.
- Overall, 3% of participants discontinued cefepime-taniborbactam and 1.8% discontinued meropenem, but reasons were heterogeneous.
IN PRACTICE:
“Cefepime-taniborbactam was superior to meropenem for the treatment of complicated UTI that included acute pyelonephritis, with a safety profile similar to that of meropenem,” the study authors wrote.
SOURCE:
Paul McGovern, MD, infectious disease specialist and senior vice president of Venatorx Pharmaceuticals, was the corresponding author of the study.
LIMITATIONS:
The authors reported no limitations.
DISCLOSURES:
The study was funded by Venatorx Pharmaceuticals, which received funding from the US Department of Health and Human Services, the Administration for Strategic Preparedness and Response, the Biomedical Advanced Research and Development Authority, the Global Antibiotic Research and Development Partnership, and Everest Medicines.
A version of this article appeared on Medscape.com.
FDA Approves First Cellular Therapy for Metastatic Melanoma
The US Food and Drug Administration (FDA) has approved lifileucel (Amtagvi, Iovance Biotherapeutics) for the treatment of certain adults with unresectable or metastatic melanoma, marking the first approval of a cellular therapy in the solid tumor setting.
Specifically, the tumor-derived autologous T-cell immunotherapy is indicated for adult patients previously treated with a programmed cell death protein 1 (PD-1)–blocking antibody, and if BRAF V600–positive, a BRAF inhibitor with or without an MEK inhibitor.
,” Samantha R. Guild, JD, president, AIM at Melanoma Foundation, stated in a press release. “This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
The approval was based on findings from the open-label single-arm global C-144-01 clinical trial, which showed an objective response rate of 31.5% in 73 patients treated within the recommended dosing rage of 7.5 x 109 to 72 x 109 viable cells. Complete responses occurred in three patients (4.1%) and partial responses occurred in 20 patients (27.4%)
Median duration of response was not reached at 18.6 months of follow-up. The median time to initial response to the therapy was 1.5 months, according to an FDA press release.
“Unresectable or metastatic melanoma is an aggressive form of cancer that can be fatal,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research stated in the FDA release. “The approval of Amtagvi represents the culmination of scientific and clinical research efforts leading to a novel T cell immunotherapy for patients with limited treatment options.”
“The melanoma community is so grateful to the patients, caregivers, and clinicians who have made the clinical trials of this therapy possible and got lifileucel to approval,” Allison Betof Warner, MD, PhD, director of Melanoma Medical Oncology at Stanford Medicine, wrote on X. “We are very excited to bring this life-saving therapy to patients ASAP! Available immediately at @StanfordCancer!!!”
For the C-144-01 trial, lifileucel was administered after a lymphodepletion regimen of 60 mg/kg/d of cyclophosphamide for 2 days followed by 25 mg/m2/d of fludarabine for 5 days. Between 3 and 34 hours after infusion, patients received 600,000 IU/Kg of the interleukin 2 aldesleukin every 8-12 hours for up to six doses to support cell expansion in vivo.
The full prescribing information for lifileucel contains a boxed warning for treatment-related mortality, prolonged severe cytopenia, severe infection, cardiopulmonary, and renal impairment. The most common adverse reactions, which occurred in at least 20% of patients, were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash hypotension, alopecia, infection, hypoxia, and dyspnea.
“Patients receiving this product should be closely monitored before and after infusion for signs and symptoms of adverse reactions. Treatment should be withheld or discontinued in the presence of these symptoms, as indicated,” according to the FDA statement.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved lifileucel (Amtagvi, Iovance Biotherapeutics) for the treatment of certain adults with unresectable or metastatic melanoma, marking the first approval of a cellular therapy in the solid tumor setting.
Specifically, the tumor-derived autologous T-cell immunotherapy is indicated for adult patients previously treated with a programmed cell death protein 1 (PD-1)–blocking antibody, and if BRAF V600–positive, a BRAF inhibitor with or without an MEK inhibitor.
,” Samantha R. Guild, JD, president, AIM at Melanoma Foundation, stated in a press release. “This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
The approval was based on findings from the open-label single-arm global C-144-01 clinical trial, which showed an objective response rate of 31.5% in 73 patients treated within the recommended dosing rage of 7.5 x 109 to 72 x 109 viable cells. Complete responses occurred in three patients (4.1%) and partial responses occurred in 20 patients (27.4%)
Median duration of response was not reached at 18.6 months of follow-up. The median time to initial response to the therapy was 1.5 months, according to an FDA press release.
“Unresectable or metastatic melanoma is an aggressive form of cancer that can be fatal,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research stated in the FDA release. “The approval of Amtagvi represents the culmination of scientific and clinical research efforts leading to a novel T cell immunotherapy for patients with limited treatment options.”
“The melanoma community is so grateful to the patients, caregivers, and clinicians who have made the clinical trials of this therapy possible and got lifileucel to approval,” Allison Betof Warner, MD, PhD, director of Melanoma Medical Oncology at Stanford Medicine, wrote on X. “We are very excited to bring this life-saving therapy to patients ASAP! Available immediately at @StanfordCancer!!!”
For the C-144-01 trial, lifileucel was administered after a lymphodepletion regimen of 60 mg/kg/d of cyclophosphamide for 2 days followed by 25 mg/m2/d of fludarabine for 5 days. Between 3 and 34 hours after infusion, patients received 600,000 IU/Kg of the interleukin 2 aldesleukin every 8-12 hours for up to six doses to support cell expansion in vivo.
The full prescribing information for lifileucel contains a boxed warning for treatment-related mortality, prolonged severe cytopenia, severe infection, cardiopulmonary, and renal impairment. The most common adverse reactions, which occurred in at least 20% of patients, were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash hypotension, alopecia, infection, hypoxia, and dyspnea.
“Patients receiving this product should be closely monitored before and after infusion for signs and symptoms of adverse reactions. Treatment should be withheld or discontinued in the presence of these symptoms, as indicated,” according to the FDA statement.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved lifileucel (Amtagvi, Iovance Biotherapeutics) for the treatment of certain adults with unresectable or metastatic melanoma, marking the first approval of a cellular therapy in the solid tumor setting.
Specifically, the tumor-derived autologous T-cell immunotherapy is indicated for adult patients previously treated with a programmed cell death protein 1 (PD-1)–blocking antibody, and if BRAF V600–positive, a BRAF inhibitor with or without an MEK inhibitor.
,” Samantha R. Guild, JD, president, AIM at Melanoma Foundation, stated in a press release. “This one-time cell therapy represents a promising innovation for the melanoma community, and we are excited by its potential to transform care for patients who are in dire need of additional therapeutic options.”
The approval was based on findings from the open-label single-arm global C-144-01 clinical trial, which showed an objective response rate of 31.5% in 73 patients treated within the recommended dosing rage of 7.5 x 109 to 72 x 109 viable cells. Complete responses occurred in three patients (4.1%) and partial responses occurred in 20 patients (27.4%)
Median duration of response was not reached at 18.6 months of follow-up. The median time to initial response to the therapy was 1.5 months, according to an FDA press release.
“Unresectable or metastatic melanoma is an aggressive form of cancer that can be fatal,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research stated in the FDA release. “The approval of Amtagvi represents the culmination of scientific and clinical research efforts leading to a novel T cell immunotherapy for patients with limited treatment options.”
“The melanoma community is so grateful to the patients, caregivers, and clinicians who have made the clinical trials of this therapy possible and got lifileucel to approval,” Allison Betof Warner, MD, PhD, director of Melanoma Medical Oncology at Stanford Medicine, wrote on X. “We are very excited to bring this life-saving therapy to patients ASAP! Available immediately at @StanfordCancer!!!”
For the C-144-01 trial, lifileucel was administered after a lymphodepletion regimen of 60 mg/kg/d of cyclophosphamide for 2 days followed by 25 mg/m2/d of fludarabine for 5 days. Between 3 and 34 hours after infusion, patients received 600,000 IU/Kg of the interleukin 2 aldesleukin every 8-12 hours for up to six doses to support cell expansion in vivo.
The full prescribing information for lifileucel contains a boxed warning for treatment-related mortality, prolonged severe cytopenia, severe infection, cardiopulmonary, and renal impairment. The most common adverse reactions, which occurred in at least 20% of patients, were chills, pyrexia, fatigue, tachycardia, diarrhea, febrile neutropenia, edema, rash hypotension, alopecia, infection, hypoxia, and dyspnea.
“Patients receiving this product should be closely monitored before and after infusion for signs and symptoms of adverse reactions. Treatment should be withheld or discontinued in the presence of these symptoms, as indicated,” according to the FDA statement.
A version of this article appeared on Medscape.com.
FDA Approves Drug to Reduce Accidental Food Allergies
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
Prednisolone May Improve MOH Withdrawal
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
, an observational study out of South Korea has found.
The study, a post-hoc analysis of the RELEASE multicenter observational cohort study of MOH patients in South Korea, found that patients who took prednisolone as a bridge therapy in the early phase of withdrawal from headache medications, or detoxification, had statistically significant higher rates of MOH reversal at 3 months after enrollment than those who did not, 73.8% versus 57.8% (P = .034)
The reversal trend also was noted at 1 month after treatment, the study authors, led by Mi Ji Lee, MD, PhD, an assistant professor at Seoul National University Hospital, Seoul, South Korea, wrote. “Although an observational study cannot draw a definitive conclusion, our study supports the use of prednisolone for the treatment of MOH in a real-world setting,” Dr. Lee and colleagues wrote.
Study methods
The study was a post hoc analysis of the RELEASE study, which stands for Registry for Load and Management of Medication Overuse Headache. RELEASE is a multicenter observational cohort study that has been ongoing in South Korea since April 2020. The post hoc analysis included 309 patients, 59 of whom received prednisolone at a varying dose of 10-40 mg a day, with a varying course of 5-14 days. About 74% of patients (228 of 309) completed the 3-month follow-up period, including 41 in the prednisolone group.
The study used three different forms of medication withdrawal before the patients started prednisolone therapy: abrupt discontinuation; gradual discontinuation concurrent with starting prednisolone; and no withdrawal.
Because of the observational nature of the RELEASE study, participating physicians prescribed prednisolone at their own discretion. The study authors noted prednisolone use was neither randomized nor controlled, which they acknowledged as a limitation.
Dr. Lee and colleagues also acknowledged that newer calcitonin gene–related peptide (CGRP) receptor antagonists may not require detoxification to reverse MOH, but that those therapies are not always available for a variety of reasons, such as reimbursement restrictions, regional distribution issues, and financial issues.
The study also evaluated a number of secondary outcomes. For example, 72% of prednisolone patients achieved MOH reversal 1 month after starting treatment versus 54.9% of the nonprednisolone patients. (P = .33). Prednisolone users also had greater reductions in acute medication days (AMD) at 1 month and scores on headache impact test-6 (HIT-6) at 6 months.
Dr. Lee and colleagues noted that the concept of detoxification, or discontinuing medication overuse, as a treatment for MOH has been controversial due to a lack of high-quality evidence to support the approach. “Nevertheless,” they wrote, “several experts still put withdrawal of medication overuse as an important step of MOH treatment in clinical practice despite limited evidence.”
Commentary
Alan Rapoport, MD, a clinical professor of neurology at the David Geffen School of Medicine at University of California, Los Angeles, noted a number of limitations with the study. “It wasn’t a unified population of patients,” he said, “which makes it a little harder to say this medicine worked — worked on whom?” The lack of a treatment regimen — the varied dosing and treatment durations, along with the different withdrawal approaches — are further limitations, Dr. Rapoport said.
Nonetheless, the study is an important addition to the evidence on how to manage medication withdrawal in MOH, said Dr. Rapoport, a past president of the International Headache Society and founder and director emeritus of the New England Center for Headache in Stamford, Connecticut, who has a keen interest in MOH research.
“I think this shows to some extent, although it doesn’t prove it because it’s a whole mixture of patients who were all treated differently by different doctors, but when you put them all together the patients who took steroids did better than the patients who did not,” he said. “The study authors did the best they could with the information they had.”
He termed the study “well-done by well-known authors in South Korea.” As medications such as CGRP receptor antagonists and monoclonal antibodies that target CGRP and its receptors become more available, MOH patients “may not need actual detoxification or steroids in their treatment,” Dr. Rapoport said.
Dr. Lee and co-authors have no disclosures. Dr. Rapoport is editor-in-chief of Neurology Reviews. He disclosed relationships with AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica.
FROM HEADACHE
Utility of NSAID Response Called Into Question for Longstanding AxSpA
TOPLINE:
Adults with axial spondyloarthritis (axSpA) with longstanding back pain symptoms had response rates to nonsteroidal anti-inflammatory drugs (NSAIDs) that were no different from patients with non-axSpA back pain of similar duration, according to findings from a prospective study.
METHODOLOGY:
The researchers recruited 233 consecutive outpatients with chronic back pain, including 68 with axSpA and 165 with non-axSpA back pain.
The mean ages of the participants in the axSpA and non-axSpA groups were 42.7 years and 49.3 years, respectively; symptom durations were approximately 15 years in both groups.
Participants were given NSAIDs and “any response” was defined as back pain improvement of more than two units on the Numerical Rating Scale, while “good response” was defined as an improvement of > 50% compared with baseline.
TAKEAWAY:
The proportion of patients showing improvement ranged from 19% to 31% in both groups after 4 weeks of treatment.
No significant differences in response appeared in subgroups of patients based on inflammatory back pain stage or in different axSpA stages.
IN PRACTICE:
“We think that this information has an effect on clinical practice since a response to NSAIDs is an important criterion in the ASAS [Assessment of SpondyloArthritis international Society]/European Alliance of Associations for Rheumatology treatment recommendations that may influence decisions to initiate treatment with biologic or targeted-synthetic DMARDs [disease-modifying antirheumatic drugs]. Further, a good response to NSAIDs is also an important clinical feature in the ASAS classification criteria,” the researchers wrote.
SOURCE:
The lead author on the study was Xenofon Baraliakos, MD, of Ruhr University Bochum, Germany. The study was published online on January 15, 2024, in The Journal of Rheumatology.
LIMITATIONS:
The uneven sex match in the diagnoses and the history of NSAID treatment among patients in both groups were potential limiting factors. The researchers also noted that a similarly conducted study in patients with early disease could have findings that are “much different.”
DISCLOSURES:
The study was sponsored in part by Novartis. The researchers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Adults with axial spondyloarthritis (axSpA) with longstanding back pain symptoms had response rates to nonsteroidal anti-inflammatory drugs (NSAIDs) that were no different from patients with non-axSpA back pain of similar duration, according to findings from a prospective study.
METHODOLOGY:
The researchers recruited 233 consecutive outpatients with chronic back pain, including 68 with axSpA and 165 with non-axSpA back pain.
The mean ages of the participants in the axSpA and non-axSpA groups were 42.7 years and 49.3 years, respectively; symptom durations were approximately 15 years in both groups.
Participants were given NSAIDs and “any response” was defined as back pain improvement of more than two units on the Numerical Rating Scale, while “good response” was defined as an improvement of > 50% compared with baseline.
TAKEAWAY:
The proportion of patients showing improvement ranged from 19% to 31% in both groups after 4 weeks of treatment.
No significant differences in response appeared in subgroups of patients based on inflammatory back pain stage or in different axSpA stages.
IN PRACTICE:
“We think that this information has an effect on clinical practice since a response to NSAIDs is an important criterion in the ASAS [Assessment of SpondyloArthritis international Society]/European Alliance of Associations for Rheumatology treatment recommendations that may influence decisions to initiate treatment with biologic or targeted-synthetic DMARDs [disease-modifying antirheumatic drugs]. Further, a good response to NSAIDs is also an important clinical feature in the ASAS classification criteria,” the researchers wrote.
SOURCE:
The lead author on the study was Xenofon Baraliakos, MD, of Ruhr University Bochum, Germany. The study was published online on January 15, 2024, in The Journal of Rheumatology.
LIMITATIONS:
The uneven sex match in the diagnoses and the history of NSAID treatment among patients in both groups were potential limiting factors. The researchers also noted that a similarly conducted study in patients with early disease could have findings that are “much different.”
DISCLOSURES:
The study was sponsored in part by Novartis. The researchers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Adults with axial spondyloarthritis (axSpA) with longstanding back pain symptoms had response rates to nonsteroidal anti-inflammatory drugs (NSAIDs) that were no different from patients with non-axSpA back pain of similar duration, according to findings from a prospective study.
METHODOLOGY:
The researchers recruited 233 consecutive outpatients with chronic back pain, including 68 with axSpA and 165 with non-axSpA back pain.
The mean ages of the participants in the axSpA and non-axSpA groups were 42.7 years and 49.3 years, respectively; symptom durations were approximately 15 years in both groups.
Participants were given NSAIDs and “any response” was defined as back pain improvement of more than two units on the Numerical Rating Scale, while “good response” was defined as an improvement of > 50% compared with baseline.
TAKEAWAY:
The proportion of patients showing improvement ranged from 19% to 31% in both groups after 4 weeks of treatment.
No significant differences in response appeared in subgroups of patients based on inflammatory back pain stage or in different axSpA stages.
IN PRACTICE:
“We think that this information has an effect on clinical practice since a response to NSAIDs is an important criterion in the ASAS [Assessment of SpondyloArthritis international Society]/European Alliance of Associations for Rheumatology treatment recommendations that may influence decisions to initiate treatment with biologic or targeted-synthetic DMARDs [disease-modifying antirheumatic drugs]. Further, a good response to NSAIDs is also an important clinical feature in the ASAS classification criteria,” the researchers wrote.
SOURCE:
The lead author on the study was Xenofon Baraliakos, MD, of Ruhr University Bochum, Germany. The study was published online on January 15, 2024, in The Journal of Rheumatology.
LIMITATIONS:
The uneven sex match in the diagnoses and the history of NSAID treatment among patients in both groups were potential limiting factors. The researchers also noted that a similarly conducted study in patients with early disease could have findings that are “much different.”
DISCLOSURES:
The study was sponsored in part by Novartis. The researchers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Gabapentinoids Increase Exacerbation in COPD
TOPLINE:
Gabapentinoid use significantly increased the risk for exacerbations in adults with chronic obstructive pulmonary disease (COPD).
METHODOLOGY:
- Previous research has prompted warnings from North American and European health agencies of severe exacerbations associated with gabapentinoid use by patients with COPD.
- The researchers compared data from patients with COPD in Canadian databases between 1994 and 2015 who were new to gabapentinoids and matched them to patients who did not use gabapentinoids.
- The primary outcome was exacerbation of COPD that required hospitalization in a propensity score-matched study.
TAKEAWAY:
- The study population included 356 epilepsy patients, 9411 neuropathic pain patients, and 3737 patients with other chronic pain.
- Use of gabapentinoids was significantly associated with an overall increased risk for severe COPD exacerbation (hazard ratio, 1.49) compared with nonuse.
- Gabapentinoid use was associated with a significantly increased COPD exacerbation risk for each group of users compared with nonusers, with hazard ratios of 1.58, 1.35, and 1.49 for epilepsy, neuropathic pain, and other chronic pain, respectively.
IN PRACTICE:
“This study supports the warnings from regulatory agencies and highlights the importance of considering this potential risk when prescribing gabapentin and pregabalin to patients with COPD,” the researchers wrote.
SOURCE:
The lead author on the study was Alvi A. Rahman, MSc, of Jewish General Hospital, Montreal. The study was published online on January 16, 2024, in Annals of Internal Medicine.
LIMITATIONS:
A lack of data on smoking status and other residual confounding factors limited the study findings.
DISCLOSURES:
The study was supported by the Canadian Institutes of Health Research and the Canadian Lung Association. Mr. Rahman had no financial conflicts to disclose, but some coauthors disclosed consulting and advisory relationships with various companies, including Merck, Pfizer, Seqirus, Boehringer-Ingelheim, and Novartis outside of the current work.
A version of this article appeared on Medscape.com.
TOPLINE:
Gabapentinoid use significantly increased the risk for exacerbations in adults with chronic obstructive pulmonary disease (COPD).
METHODOLOGY:
- Previous research has prompted warnings from North American and European health agencies of severe exacerbations associated with gabapentinoid use by patients with COPD.
- The researchers compared data from patients with COPD in Canadian databases between 1994 and 2015 who were new to gabapentinoids and matched them to patients who did not use gabapentinoids.
- The primary outcome was exacerbation of COPD that required hospitalization in a propensity score-matched study.
TAKEAWAY:
- The study population included 356 epilepsy patients, 9411 neuropathic pain patients, and 3737 patients with other chronic pain.
- Use of gabapentinoids was significantly associated with an overall increased risk for severe COPD exacerbation (hazard ratio, 1.49) compared with nonuse.
- Gabapentinoid use was associated with a significantly increased COPD exacerbation risk for each group of users compared with nonusers, with hazard ratios of 1.58, 1.35, and 1.49 for epilepsy, neuropathic pain, and other chronic pain, respectively.
IN PRACTICE:
“This study supports the warnings from regulatory agencies and highlights the importance of considering this potential risk when prescribing gabapentin and pregabalin to patients with COPD,” the researchers wrote.
SOURCE:
The lead author on the study was Alvi A. Rahman, MSc, of Jewish General Hospital, Montreal. The study was published online on January 16, 2024, in Annals of Internal Medicine.
LIMITATIONS:
A lack of data on smoking status and other residual confounding factors limited the study findings.
DISCLOSURES:
The study was supported by the Canadian Institutes of Health Research and the Canadian Lung Association. Mr. Rahman had no financial conflicts to disclose, but some coauthors disclosed consulting and advisory relationships with various companies, including Merck, Pfizer, Seqirus, Boehringer-Ingelheim, and Novartis outside of the current work.
A version of this article appeared on Medscape.com.
TOPLINE:
Gabapentinoid use significantly increased the risk for exacerbations in adults with chronic obstructive pulmonary disease (COPD).
METHODOLOGY:
- Previous research has prompted warnings from North American and European health agencies of severe exacerbations associated with gabapentinoid use by patients with COPD.
- The researchers compared data from patients with COPD in Canadian databases between 1994 and 2015 who were new to gabapentinoids and matched them to patients who did not use gabapentinoids.
- The primary outcome was exacerbation of COPD that required hospitalization in a propensity score-matched study.
TAKEAWAY:
- The study population included 356 epilepsy patients, 9411 neuropathic pain patients, and 3737 patients with other chronic pain.
- Use of gabapentinoids was significantly associated with an overall increased risk for severe COPD exacerbation (hazard ratio, 1.49) compared with nonuse.
- Gabapentinoid use was associated with a significantly increased COPD exacerbation risk for each group of users compared with nonusers, with hazard ratios of 1.58, 1.35, and 1.49 for epilepsy, neuropathic pain, and other chronic pain, respectively.
IN PRACTICE:
“This study supports the warnings from regulatory agencies and highlights the importance of considering this potential risk when prescribing gabapentin and pregabalin to patients with COPD,” the researchers wrote.
SOURCE:
The lead author on the study was Alvi A. Rahman, MSc, of Jewish General Hospital, Montreal. The study was published online on January 16, 2024, in Annals of Internal Medicine.
LIMITATIONS:
A lack of data on smoking status and other residual confounding factors limited the study findings.
DISCLOSURES:
The study was supported by the Canadian Institutes of Health Research and the Canadian Lung Association. Mr. Rahman had no financial conflicts to disclose, but some coauthors disclosed consulting and advisory relationships with various companies, including Merck, Pfizer, Seqirus, Boehringer-Ingelheim, and Novartis outside of the current work.
A version of this article appeared on Medscape.com.
Top US Oncology Regulator Seeks Changes in Drug Studies
Richard Pazdur, MD, who leads the cancer division at the US Food and Drug Administration (FDA), said there’s a need to simplify the paperwork involved in clinical trials. Before joining the FDA in 1999, Dr. Pazdur participated in and published cancer research. He says the informed consent forms used for studies have grown too elaborate over the years, such that they can intimidate even experts.
“When I read informed consents now in clinical trials, folks, it gives me a headache. Okay, I can’t follow them,” Dr. Pazdur said.
Dr. Pazdur said informed consent forms can be “mind-boggling” these days.
“They’re so damn complicated with so many damn questions being answered,” he said. “So our point is what’s the essential question that you need answered and what’s the quickest way of answering that question with the least amount of data that can be collected?”
Dr. Pazdur made these comments during a joint meeting of the FDA and the European Medicines Agency (EMA).
The meeting was a broad discussion about how to build on the successes seen in treatment of blood cancers in the past two decades. No formal recommendations were introduced or considered at the meeting. Instead, the meeting served as a chance for oncologists and patients to discuss ways to more quickly and efficiently address the key questions in drug research: Do medicines deliver a significant benefit to patients?
Dr. Pazdur also said at the meeting that there needs to be a way to attract more people to enroll in clinical trials.
“When I started in oncology, it was about 5%. When I’m sitting here now, 40 years later, it’s 5%. Basically it hasn’t moved,” he said at the discussion, held on February 1.
Ellin Berman, MD, of Memorial Sloan Kettering Cancer Center in New York, spoke at the meeting about the changes she has witnessed in her career in oncology. Until 2001, there were limited drug options, and physicians tried to get patients to transplant teams as possible. Then the FDA in 2001 approved imatinib to treat patients with chronic myelogenous leukemia (CML) that has the Philadelphia chromosome. That set the stage, Dr. Berman said, for a sea change in treatment of CML.
“The fellows now have no idea what it is like to talk to a CML patient about transplant and the question is which among the treasures we have of drugs do we start people on? And that’s always a conversation,” Dr. Berman said.
She noted that advances in treatment have also let some female patients get pregnant and have children.
“We have at least half a dozen women who bring their kids to clinic. And boy, if that doesn’t bring tears to our eyes, our collective eyes, I don’t know what does,” she said.
Dr. Pazdur also recalled his experience treating patients in the 1970s and 1980s for cancers for which “you had nothing so to speak” in terms of effective treatment.
“So then ask yourself the question, what would their stories be now?” with the many options available, Dr. Pazdur said.
Seeking clinician feedback
To try to improve the development and testing of cancer drugs, the FDA is seeking to get more feedback from clinicians about which questions trials should address, Dr. Pazdur said.
The agency is considering a way to poll clinicians on what their most crucial questions are about the medicines, he said. Better design of trial questions might serve to improve enrollment in studies.
“What we’re thinking of doing is taking the common disease areas and asking clinicians what are the five basic questions that you want answered in the next 5 years,” he said.
He cited PD-1 drugs as a possible example of a class where regulators could consider new approaches. There could be a discussion about the safety data collection for this class of drugs, which has been used by millions of patients.
Dr. Pazdur said he has been discussing these kinds of themes with his European and Japanese counterparts, who also are interested in simplifying clinical trials.
The goal is to have trials better represent real-world experiences rather than “artificial” ones created when patients must meet extensive eligibility requirements. Improved use of emerging technologies could aid in the needed streamlining, Dr. Pazdur said.
“As an oncology community, we have made our lives somewhat too complicated and need to draw back and ask the basic questions,” Dr. Pazdur said.
Richard Pazdur, MD, who leads the cancer division at the US Food and Drug Administration (FDA), said there’s a need to simplify the paperwork involved in clinical trials. Before joining the FDA in 1999, Dr. Pazdur participated in and published cancer research. He says the informed consent forms used for studies have grown too elaborate over the years, such that they can intimidate even experts.
“When I read informed consents now in clinical trials, folks, it gives me a headache. Okay, I can’t follow them,” Dr. Pazdur said.
Dr. Pazdur said informed consent forms can be “mind-boggling” these days.
“They’re so damn complicated with so many damn questions being answered,” he said. “So our point is what’s the essential question that you need answered and what’s the quickest way of answering that question with the least amount of data that can be collected?”
Dr. Pazdur made these comments during a joint meeting of the FDA and the European Medicines Agency (EMA).
The meeting was a broad discussion about how to build on the successes seen in treatment of blood cancers in the past two decades. No formal recommendations were introduced or considered at the meeting. Instead, the meeting served as a chance for oncologists and patients to discuss ways to more quickly and efficiently address the key questions in drug research: Do medicines deliver a significant benefit to patients?
Dr. Pazdur also said at the meeting that there needs to be a way to attract more people to enroll in clinical trials.
“When I started in oncology, it was about 5%. When I’m sitting here now, 40 years later, it’s 5%. Basically it hasn’t moved,” he said at the discussion, held on February 1.
Ellin Berman, MD, of Memorial Sloan Kettering Cancer Center in New York, spoke at the meeting about the changes she has witnessed in her career in oncology. Until 2001, there were limited drug options, and physicians tried to get patients to transplant teams as possible. Then the FDA in 2001 approved imatinib to treat patients with chronic myelogenous leukemia (CML) that has the Philadelphia chromosome. That set the stage, Dr. Berman said, for a sea change in treatment of CML.
“The fellows now have no idea what it is like to talk to a CML patient about transplant and the question is which among the treasures we have of drugs do we start people on? And that’s always a conversation,” Dr. Berman said.
She noted that advances in treatment have also let some female patients get pregnant and have children.
“We have at least half a dozen women who bring their kids to clinic. And boy, if that doesn’t bring tears to our eyes, our collective eyes, I don’t know what does,” she said.
Dr. Pazdur also recalled his experience treating patients in the 1970s and 1980s for cancers for which “you had nothing so to speak” in terms of effective treatment.
“So then ask yourself the question, what would their stories be now?” with the many options available, Dr. Pazdur said.
Seeking clinician feedback
To try to improve the development and testing of cancer drugs, the FDA is seeking to get more feedback from clinicians about which questions trials should address, Dr. Pazdur said.
The agency is considering a way to poll clinicians on what their most crucial questions are about the medicines, he said. Better design of trial questions might serve to improve enrollment in studies.
“What we’re thinking of doing is taking the common disease areas and asking clinicians what are the five basic questions that you want answered in the next 5 years,” he said.
He cited PD-1 drugs as a possible example of a class where regulators could consider new approaches. There could be a discussion about the safety data collection for this class of drugs, which has been used by millions of patients.
Dr. Pazdur said he has been discussing these kinds of themes with his European and Japanese counterparts, who also are interested in simplifying clinical trials.
The goal is to have trials better represent real-world experiences rather than “artificial” ones created when patients must meet extensive eligibility requirements. Improved use of emerging technologies could aid in the needed streamlining, Dr. Pazdur said.
“As an oncology community, we have made our lives somewhat too complicated and need to draw back and ask the basic questions,” Dr. Pazdur said.
Richard Pazdur, MD, who leads the cancer division at the US Food and Drug Administration (FDA), said there’s a need to simplify the paperwork involved in clinical trials. Before joining the FDA in 1999, Dr. Pazdur participated in and published cancer research. He says the informed consent forms used for studies have grown too elaborate over the years, such that they can intimidate even experts.
“When I read informed consents now in clinical trials, folks, it gives me a headache. Okay, I can’t follow them,” Dr. Pazdur said.
Dr. Pazdur said informed consent forms can be “mind-boggling” these days.
“They’re so damn complicated with so many damn questions being answered,” he said. “So our point is what’s the essential question that you need answered and what’s the quickest way of answering that question with the least amount of data that can be collected?”
Dr. Pazdur made these comments during a joint meeting of the FDA and the European Medicines Agency (EMA).
The meeting was a broad discussion about how to build on the successes seen in treatment of blood cancers in the past two decades. No formal recommendations were introduced or considered at the meeting. Instead, the meeting served as a chance for oncologists and patients to discuss ways to more quickly and efficiently address the key questions in drug research: Do medicines deliver a significant benefit to patients?
Dr. Pazdur also said at the meeting that there needs to be a way to attract more people to enroll in clinical trials.
“When I started in oncology, it was about 5%. When I’m sitting here now, 40 years later, it’s 5%. Basically it hasn’t moved,” he said at the discussion, held on February 1.
Ellin Berman, MD, of Memorial Sloan Kettering Cancer Center in New York, spoke at the meeting about the changes she has witnessed in her career in oncology. Until 2001, there were limited drug options, and physicians tried to get patients to transplant teams as possible. Then the FDA in 2001 approved imatinib to treat patients with chronic myelogenous leukemia (CML) that has the Philadelphia chromosome. That set the stage, Dr. Berman said, for a sea change in treatment of CML.
“The fellows now have no idea what it is like to talk to a CML patient about transplant and the question is which among the treasures we have of drugs do we start people on? And that’s always a conversation,” Dr. Berman said.
She noted that advances in treatment have also let some female patients get pregnant and have children.
“We have at least half a dozen women who bring their kids to clinic. And boy, if that doesn’t bring tears to our eyes, our collective eyes, I don’t know what does,” she said.
Dr. Pazdur also recalled his experience treating patients in the 1970s and 1980s for cancers for which “you had nothing so to speak” in terms of effective treatment.
“So then ask yourself the question, what would their stories be now?” with the many options available, Dr. Pazdur said.
Seeking clinician feedback
To try to improve the development and testing of cancer drugs, the FDA is seeking to get more feedback from clinicians about which questions trials should address, Dr. Pazdur said.
The agency is considering a way to poll clinicians on what their most crucial questions are about the medicines, he said. Better design of trial questions might serve to improve enrollment in studies.
“What we’re thinking of doing is taking the common disease areas and asking clinicians what are the five basic questions that you want answered in the next 5 years,” he said.
He cited PD-1 drugs as a possible example of a class where regulators could consider new approaches. There could be a discussion about the safety data collection for this class of drugs, which has been used by millions of patients.
Dr. Pazdur said he has been discussing these kinds of themes with his European and Japanese counterparts, who also are interested in simplifying clinical trials.
The goal is to have trials better represent real-world experiences rather than “artificial” ones created when patients must meet extensive eligibility requirements. Improved use of emerging technologies could aid in the needed streamlining, Dr. Pazdur said.
“As an oncology community, we have made our lives somewhat too complicated and need to draw back and ask the basic questions,” Dr. Pazdur said.
Thiazide-Induced Hyponatremia Presenting as a Fall in an Older Adult
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Reducing or Discontinuing Insulin or Sulfonylurea When Initiating a Glucagon-like Peptide-1 Agonist
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2