User login
Most effective meds for alcohol use disorder flagged
TOPLINE:
In conjunction with psychosocial interventions, oral naltrexone and acamprosate are both effective first-line drug therapies for alcohol use disorder (AUD), results of a systematic review and meta-analysis found.
METHODOLOGY:
- Researchers evaluated efficacy and comparative efficacy of three therapies for AUD that are approved in the United States (acamprosate, naltrexone, and disulfiram) and six that are commonly used off-label (baclofen, gabapentin, varenicline, topiramate, prazosin, and ondansetron).
- Data came from 118 randomized clinical trials lasting at least 12 weeks with 20,976 participants.
- 74% of these studies included psychosocial co-interventions, and the primary outcome was alcohol consumption.
- Numbers needed to treat (NNT) were calculated for medications with at least moderate strength of evidence for benefit.
TAKEAWAY:
- Acamprosate (NNT = 11) and naltrexone (50 mg/day; NNT = 18) had the highest strength of evidence and were both associated with statistically significant improvement in drinking outcomes.
- Oral naltrexone but not acamprosate was also associated with lower rates of return to heavy drinking (NNT = 11), compared with placebo.
- Injectable naltrexone was not associated with return to any or heavy drinking but was associated with fewer drinking days over the 30-day treatment period (weighted mean difference, –4.99 days).
- The four trials that directly compared acamprosate with oral naltrexone did not consistently establish superiority of either medication for alcohol use outcomes, and among off-label drugs, only topiramate had moderate strength of evidence for benefit.
IN PRACTICE:
“Alcohol use disorder affects more than 28.3 million people in the United States and is associated with increased rates of morbidity and mortality. In conjunction with psychosocial interventions, these findings support the use of oral naltrexone, 50 mg/day, and acamprosate as first-line pharmacotherapies for alcohol use disorder,” the authors write.
SOURCE:
The study, with first author Melissa McPheeters, PhD, MPH, RTI International, Research Triangle Park, North Carolina, was published online in JAMA.
LIMITATIONS:
Most study participants had moderate to severe AUD, and the applicability of the findings to people with mild AUD is uncertain. The mean age of participants was typically between ages 40 and 49 years, and it’s unclear whether the medications have similar efficacy for older or younger age groups. Information on adverse effects was limited.
DISCLOSURES:
Funding for the study was provided by the Agency for Healthcare Research and Quality of the U.S. Department of Health & Human Services. The authors have disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
In conjunction with psychosocial interventions, oral naltrexone and acamprosate are both effective first-line drug therapies for alcohol use disorder (AUD), results of a systematic review and meta-analysis found.
METHODOLOGY:
- Researchers evaluated efficacy and comparative efficacy of three therapies for AUD that are approved in the United States (acamprosate, naltrexone, and disulfiram) and six that are commonly used off-label (baclofen, gabapentin, varenicline, topiramate, prazosin, and ondansetron).
- Data came from 118 randomized clinical trials lasting at least 12 weeks with 20,976 participants.
- 74% of these studies included psychosocial co-interventions, and the primary outcome was alcohol consumption.
- Numbers needed to treat (NNT) were calculated for medications with at least moderate strength of evidence for benefit.
TAKEAWAY:
- Acamprosate (NNT = 11) and naltrexone (50 mg/day; NNT = 18) had the highest strength of evidence and were both associated with statistically significant improvement in drinking outcomes.
- Oral naltrexone but not acamprosate was also associated with lower rates of return to heavy drinking (NNT = 11), compared with placebo.
- Injectable naltrexone was not associated with return to any or heavy drinking but was associated with fewer drinking days over the 30-day treatment period (weighted mean difference, –4.99 days).
- The four trials that directly compared acamprosate with oral naltrexone did not consistently establish superiority of either medication for alcohol use outcomes, and among off-label drugs, only topiramate had moderate strength of evidence for benefit.
IN PRACTICE:
“Alcohol use disorder affects more than 28.3 million people in the United States and is associated with increased rates of morbidity and mortality. In conjunction with psychosocial interventions, these findings support the use of oral naltrexone, 50 mg/day, and acamprosate as first-line pharmacotherapies for alcohol use disorder,” the authors write.
SOURCE:
The study, with first author Melissa McPheeters, PhD, MPH, RTI International, Research Triangle Park, North Carolina, was published online in JAMA.
LIMITATIONS:
Most study participants had moderate to severe AUD, and the applicability of the findings to people with mild AUD is uncertain. The mean age of participants was typically between ages 40 and 49 years, and it’s unclear whether the medications have similar efficacy for older or younger age groups. Information on adverse effects was limited.
DISCLOSURES:
Funding for the study was provided by the Agency for Healthcare Research and Quality of the U.S. Department of Health & Human Services. The authors have disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
In conjunction with psychosocial interventions, oral naltrexone and acamprosate are both effective first-line drug therapies for alcohol use disorder (AUD), results of a systematic review and meta-analysis found.
METHODOLOGY:
- Researchers evaluated efficacy and comparative efficacy of three therapies for AUD that are approved in the United States (acamprosate, naltrexone, and disulfiram) and six that are commonly used off-label (baclofen, gabapentin, varenicline, topiramate, prazosin, and ondansetron).
- Data came from 118 randomized clinical trials lasting at least 12 weeks with 20,976 participants.
- 74% of these studies included psychosocial co-interventions, and the primary outcome was alcohol consumption.
- Numbers needed to treat (NNT) were calculated for medications with at least moderate strength of evidence for benefit.
TAKEAWAY:
- Acamprosate (NNT = 11) and naltrexone (50 mg/day; NNT = 18) had the highest strength of evidence and were both associated with statistically significant improvement in drinking outcomes.
- Oral naltrexone but not acamprosate was also associated with lower rates of return to heavy drinking (NNT = 11), compared with placebo.
- Injectable naltrexone was not associated with return to any or heavy drinking but was associated with fewer drinking days over the 30-day treatment period (weighted mean difference, –4.99 days).
- The four trials that directly compared acamprosate with oral naltrexone did not consistently establish superiority of either medication for alcohol use outcomes, and among off-label drugs, only topiramate had moderate strength of evidence for benefit.
IN PRACTICE:
“Alcohol use disorder affects more than 28.3 million people in the United States and is associated with increased rates of morbidity and mortality. In conjunction with psychosocial interventions, these findings support the use of oral naltrexone, 50 mg/day, and acamprosate as first-line pharmacotherapies for alcohol use disorder,” the authors write.
SOURCE:
The study, with first author Melissa McPheeters, PhD, MPH, RTI International, Research Triangle Park, North Carolina, was published online in JAMA.
LIMITATIONS:
Most study participants had moderate to severe AUD, and the applicability of the findings to people with mild AUD is uncertain. The mean age of participants was typically between ages 40 and 49 years, and it’s unclear whether the medications have similar efficacy for older or younger age groups. Information on adverse effects was limited.
DISCLOSURES:
Funding for the study was provided by the Agency for Healthcare Research and Quality of the U.S. Department of Health & Human Services. The authors have disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FDA approves first tx for rare, deadly clotting disorder
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Dropping aspirin cuts bleeding in LVAD patients: ARIES-HM3
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
AT AHA 2023
Popular liver supplements lack data supporting efficacy, study shows
The 10 best-selling liver health supplements on Amazon bring in an estimated $2.5 million each month. But none of them contain ingredients recommended by major groups of doctors who treat liver issues in the United States or Europe.
Like many supplements, popular online liver products are unregulated, meaning they do not have to meet the same safety and effectiveness standards as prescription medications.
Sales of liver supplements are growing, particularly in the last few years, said Ahmed Eltelbany, MD, MPH, a first-year gastrointestinal fellow at the University of New Mexico. One reason could be increased alcohol use during the COVID-19 pandemic.
Some manufacturers make bold claims on Amazon, said Dr. Eltelbany, author of a study that looked into the supplements. “The most recurrent claims were that their supplements maintain normal liver function, are scientifically formulated, and – my personal favorite – are a highly effective liver detox formulation developed according to the latest scientific findings.”
Does natural mean safe?
Many supplements are marketed as “liver cleansing,” for “liver detox,” or for “liver support,” Dr. Eltelbany said as he presented the study results at the annual meeting of the American College of Gastroenterology.
“People take these supplements because they believe they’re natural and therefore they’re safe,” said Paul Y. Kwo, MD, who moderated a session on the study at the meeting, when asked to comment. “As I tell every patient in clinic, a great white shark is natural, a scorpion is natural, and so is a hurricane. So just because they’re natural doesn’t mean they’re safe.”
At the same time, “it’s not that every supplement is bad for you. Nonetheless, there’s just a dizzying array of these out there” said Dr. Kwo, a professor of medicine at Stanford Medicine in Redwood, Calif.
“We have to be very cautious,” he said. For example, some people might believe that “if a little bit of a supplement is good, a tremendous amount must be really good.” The antioxidant turmeric, for example, has a pretty good safety record, he said. But this past year, some liver toxicity concerns arose about preparations with “very, very high concentrations” of turmeric.
The top 10 sellers
Dr. Eltelbany and colleagues studied prices for 1-month supplies, monthly sales, and revenue for the top 10 liver supplements sold on Amazon on June 3, 2023:
Ranking by sales:
- 1. Liver Cleanse Detox & Repair Formula – Herbal Liver Supplement with Milk Thistle, Dandelion Root, Organic Turmeric and Artichoke Extract for Liver Health – Silymarin Milk Thistle Detox Capsules
- 2. Ancestral Supplements Grass Fed Beef Liver Capsules. Supports Energy Production, Detoxification, Digestion, Immunity and Full Body Wellness, Non-GMO, Freeze Dried Liver Health Supplement, 180 Capsules
- 3. Bronson Milk Thistle 1,000 mg Silymarin Marianum & Dandelion Root Liver Health Support, 120 Capsules
- 4. PUREHEALTH RESEARCH Liver Supplement – Herbal Liver Cleanse Detox & Repair with Milk Thistle, Artichoke Extract, Dandelion Root, Turmeric, Berberine to Healthy Liver Renew with 11 Natural Nutrients
- 5. TUDCA Bile Salts Liver Support Supplement, 500-mg Servings, Liver and Gallbladder Cleanse Supplement (60 Capsules 250 mg) Genuine Bile Acid. TUDCA Strong Bitter Taste by Double Wood
- 6. 28-in-1 Liver Cleanse Detox & Repair Fatty Liver Formula, Milk Thistle Silymarin, Artichoke Extract, Dandelion & Apple Cider Vinegar – Liver Health Supplement Support Pills – Vegan Capsules
- 7. Vita-Liver Liver Health Supplement – Support Liver Cleanse & Detox – Liquid Delivery for Absorption – Milk Thistle, Artichoke, Chanca Piedra, Dandelion & More
- 8. Liver Supplement with Milk Thistle, Liver Detox Formula, Artichoke and Turmeric. Supports Liver Health Defense & Liver Renew. Liver Cleanse Detox & Repair for Fatty Liver Support. 60 Capsules
- 9. Liver Cleanse Detox & Repair. Milk Thistle Extract with Silymarin 80%, Artichoke Extract, Dandelion Root, Chicory, 25+ Herbs – Premium Liver Health Formula, Liver Support Detox Health Formula – Liver Support Detox Cleanse Supplement
- 10. Arazo Nutrition Liver Cleanse Detox & Repair Formula – Milk Thistle Herbal Support Supplement: Silymarin, Beet, Artichoke, Dandelion, Chicory Root
The investigators found a total of 65 unique ingredients. “Most of these ingredients have historical uses linked to liver health. But our research revealed that strong scientific evidence supporting the efficacy of any of these supplements is currently lacking,” Dr. Eltelbany said. They started the study by creating a new account on Amazon to make sure the search would not be influenced by prior shopping or purchases. They next searched for supplements using the keywords “liver” and “cleanse.” To figure out sales numbers, they used the AMZScout proprietary analytics software that Amazon sellers use to track sales.
Reviewing the reviews
The researchers discovered an average 11,526 reviews for each supplement product. The average rating was 4.42 stars out of 5.
Using Fakespot.com, proprietary Amazon customer review software that analyzes the timing and language of reviews, they found that only 65% of product reviews were genuine.
“We felt it was crucial to vet the authenticity of customer feedback,” Dr. Eltelbany said.
Few other options?
Liver disease remains a persistent and significant global health burden. Despite advances in many areas of gastroenterology, there remains no curative treatment for liver cirrhosis, Dr. Eltelbany said.
The primary option for people with end-stage liver disease is a liver transplant. “However, given the scarcity of donors and the vast number of patients in need, many individuals, unfortunately, do not get a timely transplant,” he said. “This void of treatment options and the desperation to find relief often drives patients towards alternative therapies.”
Also, online shopping has made getting these supplements “as simple as a click away. But what’s more concerning is the trust placed in these products by our patients,” Dr. Eltelbany said.
“There’s a strong need for rigorous scientific investigation into the actual health benefits of any liver detox supplements,” he said. “Above all, patient education remains paramount, warning them of potential risks and unknowns of these supplements.”
A version of this article appeared on WebMD.com.
The 10 best-selling liver health supplements on Amazon bring in an estimated $2.5 million each month. But none of them contain ingredients recommended by major groups of doctors who treat liver issues in the United States or Europe.
Like many supplements, popular online liver products are unregulated, meaning they do not have to meet the same safety and effectiveness standards as prescription medications.
Sales of liver supplements are growing, particularly in the last few years, said Ahmed Eltelbany, MD, MPH, a first-year gastrointestinal fellow at the University of New Mexico. One reason could be increased alcohol use during the COVID-19 pandemic.
Some manufacturers make bold claims on Amazon, said Dr. Eltelbany, author of a study that looked into the supplements. “The most recurrent claims were that their supplements maintain normal liver function, are scientifically formulated, and – my personal favorite – are a highly effective liver detox formulation developed according to the latest scientific findings.”
Does natural mean safe?
Many supplements are marketed as “liver cleansing,” for “liver detox,” or for “liver support,” Dr. Eltelbany said as he presented the study results at the annual meeting of the American College of Gastroenterology.
“People take these supplements because they believe they’re natural and therefore they’re safe,” said Paul Y. Kwo, MD, who moderated a session on the study at the meeting, when asked to comment. “As I tell every patient in clinic, a great white shark is natural, a scorpion is natural, and so is a hurricane. So just because they’re natural doesn’t mean they’re safe.”
At the same time, “it’s not that every supplement is bad for you. Nonetheless, there’s just a dizzying array of these out there” said Dr. Kwo, a professor of medicine at Stanford Medicine in Redwood, Calif.
“We have to be very cautious,” he said. For example, some people might believe that “if a little bit of a supplement is good, a tremendous amount must be really good.” The antioxidant turmeric, for example, has a pretty good safety record, he said. But this past year, some liver toxicity concerns arose about preparations with “very, very high concentrations” of turmeric.
The top 10 sellers
Dr. Eltelbany and colleagues studied prices for 1-month supplies, monthly sales, and revenue for the top 10 liver supplements sold on Amazon on June 3, 2023:
Ranking by sales:
- 1. Liver Cleanse Detox & Repair Formula – Herbal Liver Supplement with Milk Thistle, Dandelion Root, Organic Turmeric and Artichoke Extract for Liver Health – Silymarin Milk Thistle Detox Capsules
- 2. Ancestral Supplements Grass Fed Beef Liver Capsules. Supports Energy Production, Detoxification, Digestion, Immunity and Full Body Wellness, Non-GMO, Freeze Dried Liver Health Supplement, 180 Capsules
- 3. Bronson Milk Thistle 1,000 mg Silymarin Marianum & Dandelion Root Liver Health Support, 120 Capsules
- 4. PUREHEALTH RESEARCH Liver Supplement – Herbal Liver Cleanse Detox & Repair with Milk Thistle, Artichoke Extract, Dandelion Root, Turmeric, Berberine to Healthy Liver Renew with 11 Natural Nutrients
- 5. TUDCA Bile Salts Liver Support Supplement, 500-mg Servings, Liver and Gallbladder Cleanse Supplement (60 Capsules 250 mg) Genuine Bile Acid. TUDCA Strong Bitter Taste by Double Wood
- 6. 28-in-1 Liver Cleanse Detox & Repair Fatty Liver Formula, Milk Thistle Silymarin, Artichoke Extract, Dandelion & Apple Cider Vinegar – Liver Health Supplement Support Pills – Vegan Capsules
- 7. Vita-Liver Liver Health Supplement – Support Liver Cleanse & Detox – Liquid Delivery for Absorption – Milk Thistle, Artichoke, Chanca Piedra, Dandelion & More
- 8. Liver Supplement with Milk Thistle, Liver Detox Formula, Artichoke and Turmeric. Supports Liver Health Defense & Liver Renew. Liver Cleanse Detox & Repair for Fatty Liver Support. 60 Capsules
- 9. Liver Cleanse Detox & Repair. Milk Thistle Extract with Silymarin 80%, Artichoke Extract, Dandelion Root, Chicory, 25+ Herbs – Premium Liver Health Formula, Liver Support Detox Health Formula – Liver Support Detox Cleanse Supplement
- 10. Arazo Nutrition Liver Cleanse Detox & Repair Formula – Milk Thistle Herbal Support Supplement: Silymarin, Beet, Artichoke, Dandelion, Chicory Root
The investigators found a total of 65 unique ingredients. “Most of these ingredients have historical uses linked to liver health. But our research revealed that strong scientific evidence supporting the efficacy of any of these supplements is currently lacking,” Dr. Eltelbany said. They started the study by creating a new account on Amazon to make sure the search would not be influenced by prior shopping or purchases. They next searched for supplements using the keywords “liver” and “cleanse.” To figure out sales numbers, they used the AMZScout proprietary analytics software that Amazon sellers use to track sales.
Reviewing the reviews
The researchers discovered an average 11,526 reviews for each supplement product. The average rating was 4.42 stars out of 5.
Using Fakespot.com, proprietary Amazon customer review software that analyzes the timing and language of reviews, they found that only 65% of product reviews were genuine.
“We felt it was crucial to vet the authenticity of customer feedback,” Dr. Eltelbany said.
Few other options?
Liver disease remains a persistent and significant global health burden. Despite advances in many areas of gastroenterology, there remains no curative treatment for liver cirrhosis, Dr. Eltelbany said.
The primary option for people with end-stage liver disease is a liver transplant. “However, given the scarcity of donors and the vast number of patients in need, many individuals, unfortunately, do not get a timely transplant,” he said. “This void of treatment options and the desperation to find relief often drives patients towards alternative therapies.”
Also, online shopping has made getting these supplements “as simple as a click away. But what’s more concerning is the trust placed in these products by our patients,” Dr. Eltelbany said.
“There’s a strong need for rigorous scientific investigation into the actual health benefits of any liver detox supplements,” he said. “Above all, patient education remains paramount, warning them of potential risks and unknowns of these supplements.”
A version of this article appeared on WebMD.com.
The 10 best-selling liver health supplements on Amazon bring in an estimated $2.5 million each month. But none of them contain ingredients recommended by major groups of doctors who treat liver issues in the United States or Europe.
Like many supplements, popular online liver products are unregulated, meaning they do not have to meet the same safety and effectiveness standards as prescription medications.
Sales of liver supplements are growing, particularly in the last few years, said Ahmed Eltelbany, MD, MPH, a first-year gastrointestinal fellow at the University of New Mexico. One reason could be increased alcohol use during the COVID-19 pandemic.
Some manufacturers make bold claims on Amazon, said Dr. Eltelbany, author of a study that looked into the supplements. “The most recurrent claims were that their supplements maintain normal liver function, are scientifically formulated, and – my personal favorite – are a highly effective liver detox formulation developed according to the latest scientific findings.”
Does natural mean safe?
Many supplements are marketed as “liver cleansing,” for “liver detox,” or for “liver support,” Dr. Eltelbany said as he presented the study results at the annual meeting of the American College of Gastroenterology.
“People take these supplements because they believe they’re natural and therefore they’re safe,” said Paul Y. Kwo, MD, who moderated a session on the study at the meeting, when asked to comment. “As I tell every patient in clinic, a great white shark is natural, a scorpion is natural, and so is a hurricane. So just because they’re natural doesn’t mean they’re safe.”
At the same time, “it’s not that every supplement is bad for you. Nonetheless, there’s just a dizzying array of these out there” said Dr. Kwo, a professor of medicine at Stanford Medicine in Redwood, Calif.
“We have to be very cautious,” he said. For example, some people might believe that “if a little bit of a supplement is good, a tremendous amount must be really good.” The antioxidant turmeric, for example, has a pretty good safety record, he said. But this past year, some liver toxicity concerns arose about preparations with “very, very high concentrations” of turmeric.
The top 10 sellers
Dr. Eltelbany and colleagues studied prices for 1-month supplies, monthly sales, and revenue for the top 10 liver supplements sold on Amazon on June 3, 2023:
Ranking by sales:
- 1. Liver Cleanse Detox & Repair Formula – Herbal Liver Supplement with Milk Thistle, Dandelion Root, Organic Turmeric and Artichoke Extract for Liver Health – Silymarin Milk Thistle Detox Capsules
- 2. Ancestral Supplements Grass Fed Beef Liver Capsules. Supports Energy Production, Detoxification, Digestion, Immunity and Full Body Wellness, Non-GMO, Freeze Dried Liver Health Supplement, 180 Capsules
- 3. Bronson Milk Thistle 1,000 mg Silymarin Marianum & Dandelion Root Liver Health Support, 120 Capsules
- 4. PUREHEALTH RESEARCH Liver Supplement – Herbal Liver Cleanse Detox & Repair with Milk Thistle, Artichoke Extract, Dandelion Root, Turmeric, Berberine to Healthy Liver Renew with 11 Natural Nutrients
- 5. TUDCA Bile Salts Liver Support Supplement, 500-mg Servings, Liver and Gallbladder Cleanse Supplement (60 Capsules 250 mg) Genuine Bile Acid. TUDCA Strong Bitter Taste by Double Wood
- 6. 28-in-1 Liver Cleanse Detox & Repair Fatty Liver Formula, Milk Thistle Silymarin, Artichoke Extract, Dandelion & Apple Cider Vinegar – Liver Health Supplement Support Pills – Vegan Capsules
- 7. Vita-Liver Liver Health Supplement – Support Liver Cleanse & Detox – Liquid Delivery for Absorption – Milk Thistle, Artichoke, Chanca Piedra, Dandelion & More
- 8. Liver Supplement with Milk Thistle, Liver Detox Formula, Artichoke and Turmeric. Supports Liver Health Defense & Liver Renew. Liver Cleanse Detox & Repair for Fatty Liver Support. 60 Capsules
- 9. Liver Cleanse Detox & Repair. Milk Thistle Extract with Silymarin 80%, Artichoke Extract, Dandelion Root, Chicory, 25+ Herbs – Premium Liver Health Formula, Liver Support Detox Health Formula – Liver Support Detox Cleanse Supplement
- 10. Arazo Nutrition Liver Cleanse Detox & Repair Formula – Milk Thistle Herbal Support Supplement: Silymarin, Beet, Artichoke, Dandelion, Chicory Root
The investigators found a total of 65 unique ingredients. “Most of these ingredients have historical uses linked to liver health. But our research revealed that strong scientific evidence supporting the efficacy of any of these supplements is currently lacking,” Dr. Eltelbany said. They started the study by creating a new account on Amazon to make sure the search would not be influenced by prior shopping or purchases. They next searched for supplements using the keywords “liver” and “cleanse.” To figure out sales numbers, they used the AMZScout proprietary analytics software that Amazon sellers use to track sales.
Reviewing the reviews
The researchers discovered an average 11,526 reviews for each supplement product. The average rating was 4.42 stars out of 5.
Using Fakespot.com, proprietary Amazon customer review software that analyzes the timing and language of reviews, they found that only 65% of product reviews were genuine.
“We felt it was crucial to vet the authenticity of customer feedback,” Dr. Eltelbany said.
Few other options?
Liver disease remains a persistent and significant global health burden. Despite advances in many areas of gastroenterology, there remains no curative treatment for liver cirrhosis, Dr. Eltelbany said.
The primary option for people with end-stage liver disease is a liver transplant. “However, given the scarcity of donors and the vast number of patients in need, many individuals, unfortunately, do not get a timely transplant,” he said. “This void of treatment options and the desperation to find relief often drives patients towards alternative therapies.”
Also, online shopping has made getting these supplements “as simple as a click away. But what’s more concerning is the trust placed in these products by our patients,” Dr. Eltelbany said.
“There’s a strong need for rigorous scientific investigation into the actual health benefits of any liver detox supplements,” he said. “Above all, patient education remains paramount, warning them of potential risks and unknowns of these supplements.”
A version of this article appeared on WebMD.com.
FROM ACG 2023
Elective Hand Surgery and Antithrombotic Use in Veterans
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
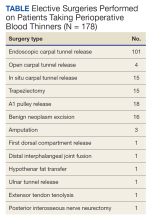
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Insulin appears less heat-sensitive than previously thought
The review included 17 studies in 22 published articles and additional unpublished information from major insulin manufacturers. The data suggest it is possible to store unopened short- and intermediate-acting human insulin vials, pens/cartridges or prefilled plastic syringes at temperatures up to 25 °C (77 °F) for a maximum of 6 months and up to 37 °C (98.6 °F) for a maximum of 2 months without a clinically relevant loss of insulin potency.
Two studies found small decrements in potency at higher temperatures and/or longer durations unrefrigerated, but the rest did not.
This contrasts with current guidance and labeling that advises storing unopened human insulin at temperatures between 2 °C (35.6 °F) and 8°C (46.4 °F), necessitating refrigeration. Once the vial or pen cartridge is opened, the guidance is to store at “room temperature” and use within about 4-6 weeks.
The recommendations vary, however, and there is no clear consensus on how human insulin should be stored in settings where reliable refrigeration can’t be guaranteed, such as low-income countries, those affected by extreme heat, or areas of conflict or natural disasters. Such areas are home to growing numbers of people with diabetes, according to the Cochrane Database of Systematic Reviews report, published online.
The review also found that oscillating temperatures between 25 °C (77 °F) and 37 °C (98.6 °F), typical of daytime and nighttime fluctuations in tropical countries, for up to 3 months do not result in clinically relevant loss of insulin activity for short-acting, intermediate-acting, or mixed human insulin.
“Our study opens up new possibilities for individuals living in challenging environments, where access to refrigeration is limited. By understanding the thermal stability of insulin and exploring innovative storage solutions, we can make a significant impact on the lives of those who depend on insulin for their well-being,” the study’s lead author, Bernd Richter, MD, of the Institute of General Practice, Medical Faculty of the Heinrich-Heine-University, Düsseldorf, Germany, said in a statement.
In addition, one small pilot clinical study showed that human insulin stored for 6 weeks in an unglazed clay pot with temperatures ranging between 25 °C (77 °F) and 27°C (80.6 °F) did not result in differences in plasma glucose–lowering in eight healthy volunteers, compared with refrigerator-stored insulin. “With the help of simple cooling devices for insulin storage such as clay pots, it is possible to effectively reduce high outside temperatures in many high-temperature regions of the world,” wrote Dr. Richter and colleagues Brenda Bongaerts, PhD, and Maria-Inti Metzendorf, also from Heinrich-Heine-University.
Asked to comment, Leonardo Scapozza, PhD, of the School of Pharmaceutical Sciences at the University of Geneva, Switzerland, said that these findings align with a study he published in 2021.
“Indeed ... we have done a small-scale study by analyzing the insulin coming back from the field and showing that insulin potency and stability was conserved. An extended clinical study where the insulin is submitted to varying controlled condition is not possible and ethically debatable. But an extended study where patients are given a log tag to monitor their real storage condition and the remaining samples are collected back and sent back for analysis in a specialized lab would be very good to further confirm the [conclusions],” said Dr. Scapozza, who was not part of Dr. Richter’s team on this review.
While the issue of insulin refrigeration is less urgent in higher-income countries, it does arise, as in situations where people accidentally leave their unopened insulin out of the refrigerator or when they carry backup insulin while traveling.
The Cochrane Review excluded studies of insulin analogs, used in most developed countries, but Dr. Scapozza’s study had included them. “We observed the same stability as the ones used in low-income countries.” He added that his data combined with those in the new report provide evidence that would make it “possible to better and optimally use the available insulin that is becoming more and more costly.”
Dr. Scapozza also told this news organization that after he presented his data to Médecins Sans Frontières (Doctors Without Borders), he heard from a collaborator with that group who has diabetes. “He always used his insulin for his own treatment when he was in the field working and his insulin pen was in his backpack or pocket and his treatment was working. After hearing the data, he was very happy because he got a scientific explanation why his treatment was working, whether he was in Switzerland or during his mission in the camps submitted to the same condition of storage as any other patient in low income countries.”
Dr. Richter and Dr. Scapozza report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The review included 17 studies in 22 published articles and additional unpublished information from major insulin manufacturers. The data suggest it is possible to store unopened short- and intermediate-acting human insulin vials, pens/cartridges or prefilled plastic syringes at temperatures up to 25 °C (77 °F) for a maximum of 6 months and up to 37 °C (98.6 °F) for a maximum of 2 months without a clinically relevant loss of insulin potency.
Two studies found small decrements in potency at higher temperatures and/or longer durations unrefrigerated, but the rest did not.
This contrasts with current guidance and labeling that advises storing unopened human insulin at temperatures between 2 °C (35.6 °F) and 8°C (46.4 °F), necessitating refrigeration. Once the vial or pen cartridge is opened, the guidance is to store at “room temperature” and use within about 4-6 weeks.
The recommendations vary, however, and there is no clear consensus on how human insulin should be stored in settings where reliable refrigeration can’t be guaranteed, such as low-income countries, those affected by extreme heat, or areas of conflict or natural disasters. Such areas are home to growing numbers of people with diabetes, according to the Cochrane Database of Systematic Reviews report, published online.
The review also found that oscillating temperatures between 25 °C (77 °F) and 37 °C (98.6 °F), typical of daytime and nighttime fluctuations in tropical countries, for up to 3 months do not result in clinically relevant loss of insulin activity for short-acting, intermediate-acting, or mixed human insulin.
“Our study opens up new possibilities for individuals living in challenging environments, where access to refrigeration is limited. By understanding the thermal stability of insulin and exploring innovative storage solutions, we can make a significant impact on the lives of those who depend on insulin for their well-being,” the study’s lead author, Bernd Richter, MD, of the Institute of General Practice, Medical Faculty of the Heinrich-Heine-University, Düsseldorf, Germany, said in a statement.
In addition, one small pilot clinical study showed that human insulin stored for 6 weeks in an unglazed clay pot with temperatures ranging between 25 °C (77 °F) and 27°C (80.6 °F) did not result in differences in plasma glucose–lowering in eight healthy volunteers, compared with refrigerator-stored insulin. “With the help of simple cooling devices for insulin storage such as clay pots, it is possible to effectively reduce high outside temperatures in many high-temperature regions of the world,” wrote Dr. Richter and colleagues Brenda Bongaerts, PhD, and Maria-Inti Metzendorf, also from Heinrich-Heine-University.
Asked to comment, Leonardo Scapozza, PhD, of the School of Pharmaceutical Sciences at the University of Geneva, Switzerland, said that these findings align with a study he published in 2021.
“Indeed ... we have done a small-scale study by analyzing the insulin coming back from the field and showing that insulin potency and stability was conserved. An extended clinical study where the insulin is submitted to varying controlled condition is not possible and ethically debatable. But an extended study where patients are given a log tag to monitor their real storage condition and the remaining samples are collected back and sent back for analysis in a specialized lab would be very good to further confirm the [conclusions],” said Dr. Scapozza, who was not part of Dr. Richter’s team on this review.
While the issue of insulin refrigeration is less urgent in higher-income countries, it does arise, as in situations where people accidentally leave their unopened insulin out of the refrigerator or when they carry backup insulin while traveling.
The Cochrane Review excluded studies of insulin analogs, used in most developed countries, but Dr. Scapozza’s study had included them. “We observed the same stability as the ones used in low-income countries.” He added that his data combined with those in the new report provide evidence that would make it “possible to better and optimally use the available insulin that is becoming more and more costly.”
Dr. Scapozza also told this news organization that after he presented his data to Médecins Sans Frontières (Doctors Without Borders), he heard from a collaborator with that group who has diabetes. “He always used his insulin for his own treatment when he was in the field working and his insulin pen was in his backpack or pocket and his treatment was working. After hearing the data, he was very happy because he got a scientific explanation why his treatment was working, whether he was in Switzerland or during his mission in the camps submitted to the same condition of storage as any other patient in low income countries.”
Dr. Richter and Dr. Scapozza report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The review included 17 studies in 22 published articles and additional unpublished information from major insulin manufacturers. The data suggest it is possible to store unopened short- and intermediate-acting human insulin vials, pens/cartridges or prefilled plastic syringes at temperatures up to 25 °C (77 °F) for a maximum of 6 months and up to 37 °C (98.6 °F) for a maximum of 2 months without a clinically relevant loss of insulin potency.
Two studies found small decrements in potency at higher temperatures and/or longer durations unrefrigerated, but the rest did not.
This contrasts with current guidance and labeling that advises storing unopened human insulin at temperatures between 2 °C (35.6 °F) and 8°C (46.4 °F), necessitating refrigeration. Once the vial or pen cartridge is opened, the guidance is to store at “room temperature” and use within about 4-6 weeks.
The recommendations vary, however, and there is no clear consensus on how human insulin should be stored in settings where reliable refrigeration can’t be guaranteed, such as low-income countries, those affected by extreme heat, or areas of conflict or natural disasters. Such areas are home to growing numbers of people with diabetes, according to the Cochrane Database of Systematic Reviews report, published online.
The review also found that oscillating temperatures between 25 °C (77 °F) and 37 °C (98.6 °F), typical of daytime and nighttime fluctuations in tropical countries, for up to 3 months do not result in clinically relevant loss of insulin activity for short-acting, intermediate-acting, or mixed human insulin.
“Our study opens up new possibilities for individuals living in challenging environments, where access to refrigeration is limited. By understanding the thermal stability of insulin and exploring innovative storage solutions, we can make a significant impact on the lives of those who depend on insulin for their well-being,” the study’s lead author, Bernd Richter, MD, of the Institute of General Practice, Medical Faculty of the Heinrich-Heine-University, Düsseldorf, Germany, said in a statement.
In addition, one small pilot clinical study showed that human insulin stored for 6 weeks in an unglazed clay pot with temperatures ranging between 25 °C (77 °F) and 27°C (80.6 °F) did not result in differences in plasma glucose–lowering in eight healthy volunteers, compared with refrigerator-stored insulin. “With the help of simple cooling devices for insulin storage such as clay pots, it is possible to effectively reduce high outside temperatures in many high-temperature regions of the world,” wrote Dr. Richter and colleagues Brenda Bongaerts, PhD, and Maria-Inti Metzendorf, also from Heinrich-Heine-University.
Asked to comment, Leonardo Scapozza, PhD, of the School of Pharmaceutical Sciences at the University of Geneva, Switzerland, said that these findings align with a study he published in 2021.
“Indeed ... we have done a small-scale study by analyzing the insulin coming back from the field and showing that insulin potency and stability was conserved. An extended clinical study where the insulin is submitted to varying controlled condition is not possible and ethically debatable. But an extended study where patients are given a log tag to monitor their real storage condition and the remaining samples are collected back and sent back for analysis in a specialized lab would be very good to further confirm the [conclusions],” said Dr. Scapozza, who was not part of Dr. Richter’s team on this review.
While the issue of insulin refrigeration is less urgent in higher-income countries, it does arise, as in situations where people accidentally leave their unopened insulin out of the refrigerator or when they carry backup insulin while traveling.
The Cochrane Review excluded studies of insulin analogs, used in most developed countries, but Dr. Scapozza’s study had included them. “We observed the same stability as the ones used in low-income countries.” He added that his data combined with those in the new report provide evidence that would make it “possible to better and optimally use the available insulin that is becoming more and more costly.”
Dr. Scapozza also told this news organization that after he presented his data to Médecins Sans Frontières (Doctors Without Borders), he heard from a collaborator with that group who has diabetes. “He always used his insulin for his own treatment when he was in the field working and his insulin pen was in his backpack or pocket and his treatment was working. After hearing the data, he was very happy because he got a scientific explanation why his treatment was working, whether he was in Switzerland or during his mission in the camps submitted to the same condition of storage as any other patient in low income countries.”
Dr. Richter and Dr. Scapozza report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Older adults at risk from inappropriate prescribing
Roughly 2% of prescriptions to older patients appear to be inappropriate – but the figure does not appear to differ between physicians and nurse practitioners, according to a study published in Annals of Internal Medicine.
Older adults are “especially vulnerable to adverse drug events from inappropriate prescribing due to comorbidities and aging-related physiological changes,” said Johnny Huynh, MA, doctoral candidate in economics at UCLA and lead author of the study. “Considering the volume of prescriptions for older adults, even a small percentage can translate to a big impact on adverse drug events and spending.”
In recent years, more states have granted prescriptive authority to NPs, while professional medical organizations have opposed the reforms and made claims about differences in quality of care.
The medical community must focus on the prescribing performance of individual clinicians rather than whether an NP has prescriptive authority, said David Studdert, LLB, ScD, MPH, professor of health policy at Stanford (Calif.) University and a co-author of the study.
“Don’t fixate on whether nurse practitioners have prescriptive authority or don’t,” said Mr. Studdert. “Just try to identify those practitioners who need to boost their performance.”
The investigators found that rates of potentially inappropriate prescribing were “virtually identical.” Adjusted rates were 1.66 per 100 prescriptions for NPs versus 1.68 per 100 prescriptions for physicians (adjusted odds ratio, 0.99; 95% confidence interval, 0.97-1.01).
“Older adults often have more than one chronic condition and are prescribed multiple medications to manage these conditions, putting them at risk for adverse events,” said Paula Rochon, MD, MPH, founding director of the Women’s Age Lab and professor in the Division of Geriatric Medicine at Dalla Lana School of Public Health in Toronto. “Furthermore, older women are more likely than men to have multiple medical problems and experience adverse drug events.”
Dr. Rochon led a 2021 research review on polypharmacy and inappropriate prescribing among older adults in both the United States and abroad. She and her team noted that while women are physiologically more susceptible to drug-related harm, rates of inappropriate prescribing also tend to be higher for women, such as in the case of senior U.S. veterans and older adults in Canada.
The researchers analyzed data over a 7-year period starting in 2013 from 23,669 primary care NPs and 50,060 physicians who wrote prescriptions for at least 100 patients with Medicare Part D coverage. Data from 29 states, which had all expanded prescriptive authority to NPs, was included.
Prescriptive quality was defined by the American Geriatrics Society’s Beers Criteria, a list of potentially inappropriate medications (PIMs) for adults ages 65 and over. Mr. Studdert said it’s important to note the nuance in the Beers Criteria.
“It’s not to say that there may not be certain clinical circumstances where it’s appropriate to” prescribe these drugs, Mr. Studdert said, “But generally, it’s not appropriate.”
Ten medications accounted for 99.5% of the PIMs prescribed, including drugs that were antidepressants, muscle relaxants, hypnotics, antihistamines (generation 1), antispasmodics, sulfonylureas, barbiturates, antineoplastics, thyroid medications, and nonsteroidal anti-inflammatory drugs.
The top three most frequently potentially inappropriately prescribed were antidepressants (0.393 NPs vs. 0.481 PCPs per 100 prescriptions), muscle relaxants (0.372 NPs vs. 0.305 PCPs per 100), and hypnotics (0.364 NPs vs. 0.440 PCPs per 100). Both antidepressants and hypnotics are associated with an increased risk for falls and fractures among older adults, while muscle relaxants have been shown to increase the risk for hospitalization in this population.
Despite the overall similar PIM rates, NPs were more present in the “tails,” or highest and lowest end of the quality bell curve. The higher variation among NPs means these patients are at a higher risk of receiving a prescription for an inappropriate medication, said David Chan, MD, PhD, associate professor of health policy at Stanford (Calif.) School of Medicine, and a co-author of the study.
Other studies have shown “high-intensity prescribers” were more likely to dispense drugs like benzodiazepines and opioids, which can be harmful to older patients.
According to Dr. Rochon, clinicians should use the Beers Criteria and STOPP/START Criteria to guide decision-making, along with the DRUGS framework, which follows a geriatric medicine approach that advises clinicians to discuss goals of care with their patients and conduct routine reviews of medications.
Prescribers should also avoid prescribing cascades, which “occur when a drug is prescribed, an adverse event occurs that is misinterpreted as a new medical condition, and a further drug is prescribed to treat that medical condition,” Dr. Rochon said.
To reduce cascades, “it’s important to document when a medication was started, why it was started, and who started it so that this information is available when evaluating if a medication continues to be needed,” she said.
The study was funded by grants from Robert Wood Johnson Foundation and National Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 2% of prescriptions to older patients appear to be inappropriate – but the figure does not appear to differ between physicians and nurse practitioners, according to a study published in Annals of Internal Medicine.
Older adults are “especially vulnerable to adverse drug events from inappropriate prescribing due to comorbidities and aging-related physiological changes,” said Johnny Huynh, MA, doctoral candidate in economics at UCLA and lead author of the study. “Considering the volume of prescriptions for older adults, even a small percentage can translate to a big impact on adverse drug events and spending.”
In recent years, more states have granted prescriptive authority to NPs, while professional medical organizations have opposed the reforms and made claims about differences in quality of care.
The medical community must focus on the prescribing performance of individual clinicians rather than whether an NP has prescriptive authority, said David Studdert, LLB, ScD, MPH, professor of health policy at Stanford (Calif.) University and a co-author of the study.
“Don’t fixate on whether nurse practitioners have prescriptive authority or don’t,” said Mr. Studdert. “Just try to identify those practitioners who need to boost their performance.”
The investigators found that rates of potentially inappropriate prescribing were “virtually identical.” Adjusted rates were 1.66 per 100 prescriptions for NPs versus 1.68 per 100 prescriptions for physicians (adjusted odds ratio, 0.99; 95% confidence interval, 0.97-1.01).
“Older adults often have more than one chronic condition and are prescribed multiple medications to manage these conditions, putting them at risk for adverse events,” said Paula Rochon, MD, MPH, founding director of the Women’s Age Lab and professor in the Division of Geriatric Medicine at Dalla Lana School of Public Health in Toronto. “Furthermore, older women are more likely than men to have multiple medical problems and experience adverse drug events.”
Dr. Rochon led a 2021 research review on polypharmacy and inappropriate prescribing among older adults in both the United States and abroad. She and her team noted that while women are physiologically more susceptible to drug-related harm, rates of inappropriate prescribing also tend to be higher for women, such as in the case of senior U.S. veterans and older adults in Canada.
The researchers analyzed data over a 7-year period starting in 2013 from 23,669 primary care NPs and 50,060 physicians who wrote prescriptions for at least 100 patients with Medicare Part D coverage. Data from 29 states, which had all expanded prescriptive authority to NPs, was included.
Prescriptive quality was defined by the American Geriatrics Society’s Beers Criteria, a list of potentially inappropriate medications (PIMs) for adults ages 65 and over. Mr. Studdert said it’s important to note the nuance in the Beers Criteria.
“It’s not to say that there may not be certain clinical circumstances where it’s appropriate to” prescribe these drugs, Mr. Studdert said, “But generally, it’s not appropriate.”
Ten medications accounted for 99.5% of the PIMs prescribed, including drugs that were antidepressants, muscle relaxants, hypnotics, antihistamines (generation 1), antispasmodics, sulfonylureas, barbiturates, antineoplastics, thyroid medications, and nonsteroidal anti-inflammatory drugs.
The top three most frequently potentially inappropriately prescribed were antidepressants (0.393 NPs vs. 0.481 PCPs per 100 prescriptions), muscle relaxants (0.372 NPs vs. 0.305 PCPs per 100), and hypnotics (0.364 NPs vs. 0.440 PCPs per 100). Both antidepressants and hypnotics are associated with an increased risk for falls and fractures among older adults, while muscle relaxants have been shown to increase the risk for hospitalization in this population.
Despite the overall similar PIM rates, NPs were more present in the “tails,” or highest and lowest end of the quality bell curve. The higher variation among NPs means these patients are at a higher risk of receiving a prescription for an inappropriate medication, said David Chan, MD, PhD, associate professor of health policy at Stanford (Calif.) School of Medicine, and a co-author of the study.
Other studies have shown “high-intensity prescribers” were more likely to dispense drugs like benzodiazepines and opioids, which can be harmful to older patients.
According to Dr. Rochon, clinicians should use the Beers Criteria and STOPP/START Criteria to guide decision-making, along with the DRUGS framework, which follows a geriatric medicine approach that advises clinicians to discuss goals of care with their patients and conduct routine reviews of medications.
Prescribers should also avoid prescribing cascades, which “occur when a drug is prescribed, an adverse event occurs that is misinterpreted as a new medical condition, and a further drug is prescribed to treat that medical condition,” Dr. Rochon said.
To reduce cascades, “it’s important to document when a medication was started, why it was started, and who started it so that this information is available when evaluating if a medication continues to be needed,” she said.
The study was funded by grants from Robert Wood Johnson Foundation and National Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 2% of prescriptions to older patients appear to be inappropriate – but the figure does not appear to differ between physicians and nurse practitioners, according to a study published in Annals of Internal Medicine.
Older adults are “especially vulnerable to adverse drug events from inappropriate prescribing due to comorbidities and aging-related physiological changes,” said Johnny Huynh, MA, doctoral candidate in economics at UCLA and lead author of the study. “Considering the volume of prescriptions for older adults, even a small percentage can translate to a big impact on adverse drug events and spending.”
In recent years, more states have granted prescriptive authority to NPs, while professional medical organizations have opposed the reforms and made claims about differences in quality of care.
The medical community must focus on the prescribing performance of individual clinicians rather than whether an NP has prescriptive authority, said David Studdert, LLB, ScD, MPH, professor of health policy at Stanford (Calif.) University and a co-author of the study.
“Don’t fixate on whether nurse practitioners have prescriptive authority or don’t,” said Mr. Studdert. “Just try to identify those practitioners who need to boost their performance.”
The investigators found that rates of potentially inappropriate prescribing were “virtually identical.” Adjusted rates were 1.66 per 100 prescriptions for NPs versus 1.68 per 100 prescriptions for physicians (adjusted odds ratio, 0.99; 95% confidence interval, 0.97-1.01).
“Older adults often have more than one chronic condition and are prescribed multiple medications to manage these conditions, putting them at risk for adverse events,” said Paula Rochon, MD, MPH, founding director of the Women’s Age Lab and professor in the Division of Geriatric Medicine at Dalla Lana School of Public Health in Toronto. “Furthermore, older women are more likely than men to have multiple medical problems and experience adverse drug events.”
Dr. Rochon led a 2021 research review on polypharmacy and inappropriate prescribing among older adults in both the United States and abroad. She and her team noted that while women are physiologically more susceptible to drug-related harm, rates of inappropriate prescribing also tend to be higher for women, such as in the case of senior U.S. veterans and older adults in Canada.
The researchers analyzed data over a 7-year period starting in 2013 from 23,669 primary care NPs and 50,060 physicians who wrote prescriptions for at least 100 patients with Medicare Part D coverage. Data from 29 states, which had all expanded prescriptive authority to NPs, was included.
Prescriptive quality was defined by the American Geriatrics Society’s Beers Criteria, a list of potentially inappropriate medications (PIMs) for adults ages 65 and over. Mr. Studdert said it’s important to note the nuance in the Beers Criteria.
“It’s not to say that there may not be certain clinical circumstances where it’s appropriate to” prescribe these drugs, Mr. Studdert said, “But generally, it’s not appropriate.”
Ten medications accounted for 99.5% of the PIMs prescribed, including drugs that were antidepressants, muscle relaxants, hypnotics, antihistamines (generation 1), antispasmodics, sulfonylureas, barbiturates, antineoplastics, thyroid medications, and nonsteroidal anti-inflammatory drugs.
The top three most frequently potentially inappropriately prescribed were antidepressants (0.393 NPs vs. 0.481 PCPs per 100 prescriptions), muscle relaxants (0.372 NPs vs. 0.305 PCPs per 100), and hypnotics (0.364 NPs vs. 0.440 PCPs per 100). Both antidepressants and hypnotics are associated with an increased risk for falls and fractures among older adults, while muscle relaxants have been shown to increase the risk for hospitalization in this population.
Despite the overall similar PIM rates, NPs were more present in the “tails,” or highest and lowest end of the quality bell curve. The higher variation among NPs means these patients are at a higher risk of receiving a prescription for an inappropriate medication, said David Chan, MD, PhD, associate professor of health policy at Stanford (Calif.) School of Medicine, and a co-author of the study.
Other studies have shown “high-intensity prescribers” were more likely to dispense drugs like benzodiazepines and opioids, which can be harmful to older patients.
According to Dr. Rochon, clinicians should use the Beers Criteria and STOPP/START Criteria to guide decision-making, along with the DRUGS framework, which follows a geriatric medicine approach that advises clinicians to discuss goals of care with their patients and conduct routine reviews of medications.
Prescribers should also avoid prescribing cascades, which “occur when a drug is prescribed, an adverse event occurs that is misinterpreted as a new medical condition, and a further drug is prescribed to treat that medical condition,” Dr. Rochon said.
To reduce cascades, “it’s important to document when a medication was started, why it was started, and who started it so that this information is available when evaluating if a medication continues to be needed,” she said.
The study was funded by grants from Robert Wood Johnson Foundation and National Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ketamine no better for depression than placebo?
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
Another study ties statins to T2D: Should practice change?
Studies have shown links between statin use and type 2 diabetes (T2D) for more than a decade. A U.S. Food and Drug Administration label change for the drugs warned in 2012 about reports of increased risks of high blood glucose and glycosylated hemoglobin (A1c) levels. However, in the same warning, the FDA said it “continues to believe that the cardiovascular benefits of statins outweigh these small increased risks.”
Indeed, although the warning triggered much discussion at the time and a number of meta-analyses and other observational studies in more recent years, that conclusion seems to hold among clinicians and society guidelines.
For example, in a recent practice pointer on the risk of diabetes with statins published in the BMJ, Ishak Mansi, MD, of the Orlando VA Health Care System, and colleagues write, “This potential adverse effect of diabetes with statin use should not be a barrier to starting statin treatment when indicated.”
They also called for further research to answer such questions as, “Is statin-associated diabetes reversible upon statin discontinuation? Would intermittent use minimize this risk while maintaining cardiovascular benefits?”
An earlier study among individuals at high risk for diabetes found significantly higher rates of incident diabetes at 10 years among patients on placebo, metformin, or lifestyle intervention who also initiated statin therapy. Jill Crandall, MD, Albert Einstein College of Medicine, New York, and colleagues conclude, “For individual patients, a potential modest increase in diabetes risk clearly needs to be balanced against the consistent and highly significant reductions in myocardial infarction, stroke, and cardiovascular death associated with statin treatment.”
In the same vein, a recent review by Byron Hoogwerf, MD, Emeritus, department of endocrinology, diabetes, and metabolism, Cleveland Clinic, is titled, “Statins may increase diabetes, but benefit still outweighs risk.”
Rosuvastatin versus Atorvastatin
The latest study in this arena is an analysis of the LODESTAR randomized controlled trial of 4,400 patients with coronary artery disease in 12 hospitals in Korea which compares the risks associated with individual statins.
Senior author Myeong-Ki Hong, MD, PhD, Yonsei University College of Medicine, Severance Cardiovascular Hospital, Seoul, South Korea, said in an interview that the study was prompted by the “limited” studies evaluating clinical outcomes, including diabetes risk, according to statin type.
Dr. Hong and colleagues compared the risk of developing diabetes among those taking rosuvastatin (mean daily dose, 17.1 mg) or atorvastatin (mean daily dose 36 mg) for 3 years. While both statins effectively prevented myocardial infarction, stroke, and death, (2.5% vs. 1.5%; HR, 1.66).
Overall, the HR of new-onset T2D was 1.29 (95% confidence interval, 1.01-1.63; P = .04).
“The percentages of new-onset diabetes and cataract are in line with previous studies regarding statin therapy in patients with atherosclerotic cardiovascular disease,” Dr. Hong said. “Additional research specifically focusing on these outcomes is required, with more frequent measurement of glucose and A1c levels to detect new-onset diabetes and regular ophthalmologic examinations to detect cataracts.”
“However,” he added, “when using rosuvastatin over atorvastatin, we ... emphasize the importance of meticulous monitoring and appropriate lifestyle interventions to mitigate the risk of new-onset diabetes or cataracts.”
Steven Nissen, MD, chief academic officer of Cleveland Clinic’s Heart and Vascular Institute, was not convinced, and said the study “does not provide useful insights into the use of these drugs.”
The investigators used whatever dose they wanted, “and the authors report only the median dose after 3 years,” he said in an interview. “Because there was a slightly greater reduction in low-density lipoprotein (LDL) cholesterol with rosuvastatin, the relative dose was actually higher.”
“We know that new-onset diabetes with statins is dose-dependent,” he said. “The P-values for diabetes incidence were marginal (very close to P = .05). Accordingly, the diabetes data are unconvincing. ... The similar efficacy is not surprising given the open-label dosing with relatively similar effects on lipids.”
Seth Shay Martin, MD, MHS, director of the Advanced Lipid Disorders Program and Digital Health Lab, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Medicine, Baltimore, also commented on the results. The findings are “in line with existing knowledge and current guidelines,” he said. “Therefore, the study should not influence prescribing.”
“Although the study suggests that rosuvastatin was associated with a higher risk of new-onset diabetes mellitus requiring antidiabetics and cataract surgery, compared with atorvastatin, these findings should be interpreted with caution given the open-label nature of the study and require further investigation,” he said.
“The mean daily doses of statins were somewhat below target for secondary prevention,” he noted. “Ideally, patients with coronary artery disease (CAD) take 20-40 mg daily of rosuvastatin or 40-80 mg daily of atorvastatin.”
“Furthermore, the LDL cholesterol levels were not optimized in the patients,” he said. “The mean LDL-C was 1.8-1.9 mmol/L, which is equivalent to 70-73 mg/dL. In the current treatment era, we generally treat to LDL-C levels less than 70 mg/dL and often less than 55 mg/dL in CAD patients.”
“The cataracts finding is particularly odd,” he added. “There was historic concern for cataracts with statin therapy, initially because of studies in beagle dogs. However, high-quality evidence from statin trials has not shown a risk for cataracts.”
So which statin has the lowest risk of triggering new-onset diabetes? As Dr. Hong noted, the literature is sparse when it comes to comparing the risk among specific statins. Some studies suggest that the risk may depend on the individual and their specific risk factors, as well as the dose and intensity of the prescribed statin.
One recent study suggests that while the overall chance of developing diabetes is small, when looking at risk by years of exposure, atorvastatin, rosuvastatin, and lovastatin carried the largest risk, whereas the risk was lower with pravastatin and simvastatin.
Risks also seemed lower with fluvastatin and pitavastatin, but there were too few study patients taking those drugs long-term to include in the subanalysis.
With input from the latest guidelines from the American Heart Association and the American Diabetes Association, as well as findings from a clinical guide on statin-associated diabetes, Dr. Hoogwerf suggests in his review that shared decision-making before starting statin therapy of any type include the following considerations/discussion points:
- For all patients: Screening to determine baseline glycemic status; nonstatin therapies to lower cholesterol; and variables associated with an increased risk of diabetes, including antihypertensive drugs.
- For patients without T2D: The possibility of developing T2D, types and doses of statins, and the fact that statin benefits “generally far outweigh” risks of developing diabetes.
- For patients with T2D: Possible small adverse effects on glycemic control; statin benefits in reducing risk for atherosclerotic cardiovascular disease, which “significantly outweigh” the small increase in A1c; and mitigation of adverse glycemic effects of statins with glucose-lowering therapies.
It’s worth noting that the AHA and ADA guidelines, among others, also emphasize that such discussions should include the importance of weight loss, regular exercise, and adhering to a healthy lifestyle to mitigate risks of both diabetes and heart disease, with or without statins.
Dr. Hong, Dr. Nissen, and Dr. Martin report no relevant financial relationships. Dr. Hoogwerf has disclosed ownership interest in Eli Lilly and consulting for MannKind and Zealand Pharmaceuticals.
A version of this article appeared on Medscape.com.
Studies have shown links between statin use and type 2 diabetes (T2D) for more than a decade. A U.S. Food and Drug Administration label change for the drugs warned in 2012 about reports of increased risks of high blood glucose and glycosylated hemoglobin (A1c) levels. However, in the same warning, the FDA said it “continues to believe that the cardiovascular benefits of statins outweigh these small increased risks.”
Indeed, although the warning triggered much discussion at the time and a number of meta-analyses and other observational studies in more recent years, that conclusion seems to hold among clinicians and society guidelines.
For example, in a recent practice pointer on the risk of diabetes with statins published in the BMJ, Ishak Mansi, MD, of the Orlando VA Health Care System, and colleagues write, “This potential adverse effect of diabetes with statin use should not be a barrier to starting statin treatment when indicated.”
They also called for further research to answer such questions as, “Is statin-associated diabetes reversible upon statin discontinuation? Would intermittent use minimize this risk while maintaining cardiovascular benefits?”
An earlier study among individuals at high risk for diabetes found significantly higher rates of incident diabetes at 10 years among patients on placebo, metformin, or lifestyle intervention who also initiated statin therapy. Jill Crandall, MD, Albert Einstein College of Medicine, New York, and colleagues conclude, “For individual patients, a potential modest increase in diabetes risk clearly needs to be balanced against the consistent and highly significant reductions in myocardial infarction, stroke, and cardiovascular death associated with statin treatment.”
In the same vein, a recent review by Byron Hoogwerf, MD, Emeritus, department of endocrinology, diabetes, and metabolism, Cleveland Clinic, is titled, “Statins may increase diabetes, but benefit still outweighs risk.”
Rosuvastatin versus Atorvastatin
The latest study in this arena is an analysis of the LODESTAR randomized controlled trial of 4,400 patients with coronary artery disease in 12 hospitals in Korea which compares the risks associated with individual statins.
Senior author Myeong-Ki Hong, MD, PhD, Yonsei University College of Medicine, Severance Cardiovascular Hospital, Seoul, South Korea, said in an interview that the study was prompted by the “limited” studies evaluating clinical outcomes, including diabetes risk, according to statin type.
Dr. Hong and colleagues compared the risk of developing diabetes among those taking rosuvastatin (mean daily dose, 17.1 mg) or atorvastatin (mean daily dose 36 mg) for 3 years. While both statins effectively prevented myocardial infarction, stroke, and death, (2.5% vs. 1.5%; HR, 1.66).
Overall, the HR of new-onset T2D was 1.29 (95% confidence interval, 1.01-1.63; P = .04).
“The percentages of new-onset diabetes and cataract are in line with previous studies regarding statin therapy in patients with atherosclerotic cardiovascular disease,” Dr. Hong said. “Additional research specifically focusing on these outcomes is required, with more frequent measurement of glucose and A1c levels to detect new-onset diabetes and regular ophthalmologic examinations to detect cataracts.”
“However,” he added, “when using rosuvastatin over atorvastatin, we ... emphasize the importance of meticulous monitoring and appropriate lifestyle interventions to mitigate the risk of new-onset diabetes or cataracts.”
Steven Nissen, MD, chief academic officer of Cleveland Clinic’s Heart and Vascular Institute, was not convinced, and said the study “does not provide useful insights into the use of these drugs.”
The investigators used whatever dose they wanted, “and the authors report only the median dose after 3 years,” he said in an interview. “Because there was a slightly greater reduction in low-density lipoprotein (LDL) cholesterol with rosuvastatin, the relative dose was actually higher.”
“We know that new-onset diabetes with statins is dose-dependent,” he said. “The P-values for diabetes incidence were marginal (very close to P = .05). Accordingly, the diabetes data are unconvincing. ... The similar efficacy is not surprising given the open-label dosing with relatively similar effects on lipids.”
Seth Shay Martin, MD, MHS, director of the Advanced Lipid Disorders Program and Digital Health Lab, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Medicine, Baltimore, also commented on the results. The findings are “in line with existing knowledge and current guidelines,” he said. “Therefore, the study should not influence prescribing.”
“Although the study suggests that rosuvastatin was associated with a higher risk of new-onset diabetes mellitus requiring antidiabetics and cataract surgery, compared with atorvastatin, these findings should be interpreted with caution given the open-label nature of the study and require further investigation,” he said.
“The mean daily doses of statins were somewhat below target for secondary prevention,” he noted. “Ideally, patients with coronary artery disease (CAD) take 20-40 mg daily of rosuvastatin or 40-80 mg daily of atorvastatin.”
“Furthermore, the LDL cholesterol levels were not optimized in the patients,” he said. “The mean LDL-C was 1.8-1.9 mmol/L, which is equivalent to 70-73 mg/dL. In the current treatment era, we generally treat to LDL-C levels less than 70 mg/dL and often less than 55 mg/dL in CAD patients.”
“The cataracts finding is particularly odd,” he added. “There was historic concern for cataracts with statin therapy, initially because of studies in beagle dogs. However, high-quality evidence from statin trials has not shown a risk for cataracts.”
So which statin has the lowest risk of triggering new-onset diabetes? As Dr. Hong noted, the literature is sparse when it comes to comparing the risk among specific statins. Some studies suggest that the risk may depend on the individual and their specific risk factors, as well as the dose and intensity of the prescribed statin.
One recent study suggests that while the overall chance of developing diabetes is small, when looking at risk by years of exposure, atorvastatin, rosuvastatin, and lovastatin carried the largest risk, whereas the risk was lower with pravastatin and simvastatin.
Risks also seemed lower with fluvastatin and pitavastatin, but there were too few study patients taking those drugs long-term to include in the subanalysis.
With input from the latest guidelines from the American Heart Association and the American Diabetes Association, as well as findings from a clinical guide on statin-associated diabetes, Dr. Hoogwerf suggests in his review that shared decision-making before starting statin therapy of any type include the following considerations/discussion points:
- For all patients: Screening to determine baseline glycemic status; nonstatin therapies to lower cholesterol; and variables associated with an increased risk of diabetes, including antihypertensive drugs.
- For patients without T2D: The possibility of developing T2D, types and doses of statins, and the fact that statin benefits “generally far outweigh” risks of developing diabetes.
- For patients with T2D: Possible small adverse effects on glycemic control; statin benefits in reducing risk for atherosclerotic cardiovascular disease, which “significantly outweigh” the small increase in A1c; and mitigation of adverse glycemic effects of statins with glucose-lowering therapies.
It’s worth noting that the AHA and ADA guidelines, among others, also emphasize that such discussions should include the importance of weight loss, regular exercise, and adhering to a healthy lifestyle to mitigate risks of both diabetes and heart disease, with or without statins.
Dr. Hong, Dr. Nissen, and Dr. Martin report no relevant financial relationships. Dr. Hoogwerf has disclosed ownership interest in Eli Lilly and consulting for MannKind and Zealand Pharmaceuticals.
A version of this article appeared on Medscape.com.
Studies have shown links between statin use and type 2 diabetes (T2D) for more than a decade. A U.S. Food and Drug Administration label change for the drugs warned in 2012 about reports of increased risks of high blood glucose and glycosylated hemoglobin (A1c) levels. However, in the same warning, the FDA said it “continues to believe that the cardiovascular benefits of statins outweigh these small increased risks.”
Indeed, although the warning triggered much discussion at the time and a number of meta-analyses and other observational studies in more recent years, that conclusion seems to hold among clinicians and society guidelines.
For example, in a recent practice pointer on the risk of diabetes with statins published in the BMJ, Ishak Mansi, MD, of the Orlando VA Health Care System, and colleagues write, “This potential adverse effect of diabetes with statin use should not be a barrier to starting statin treatment when indicated.”
They also called for further research to answer such questions as, “Is statin-associated diabetes reversible upon statin discontinuation? Would intermittent use minimize this risk while maintaining cardiovascular benefits?”
An earlier study among individuals at high risk for diabetes found significantly higher rates of incident diabetes at 10 years among patients on placebo, metformin, or lifestyle intervention who also initiated statin therapy. Jill Crandall, MD, Albert Einstein College of Medicine, New York, and colleagues conclude, “For individual patients, a potential modest increase in diabetes risk clearly needs to be balanced against the consistent and highly significant reductions in myocardial infarction, stroke, and cardiovascular death associated with statin treatment.”
In the same vein, a recent review by Byron Hoogwerf, MD, Emeritus, department of endocrinology, diabetes, and metabolism, Cleveland Clinic, is titled, “Statins may increase diabetes, but benefit still outweighs risk.”
Rosuvastatin versus Atorvastatin
The latest study in this arena is an analysis of the LODESTAR randomized controlled trial of 4,400 patients with coronary artery disease in 12 hospitals in Korea which compares the risks associated with individual statins.
Senior author Myeong-Ki Hong, MD, PhD, Yonsei University College of Medicine, Severance Cardiovascular Hospital, Seoul, South Korea, said in an interview that the study was prompted by the “limited” studies evaluating clinical outcomes, including diabetes risk, according to statin type.
Dr. Hong and colleagues compared the risk of developing diabetes among those taking rosuvastatin (mean daily dose, 17.1 mg) or atorvastatin (mean daily dose 36 mg) for 3 years. While both statins effectively prevented myocardial infarction, stroke, and death, (2.5% vs. 1.5%; HR, 1.66).
Overall, the HR of new-onset T2D was 1.29 (95% confidence interval, 1.01-1.63; P = .04).
“The percentages of new-onset diabetes and cataract are in line with previous studies regarding statin therapy in patients with atherosclerotic cardiovascular disease,” Dr. Hong said. “Additional research specifically focusing on these outcomes is required, with more frequent measurement of glucose and A1c levels to detect new-onset diabetes and regular ophthalmologic examinations to detect cataracts.”
“However,” he added, “when using rosuvastatin over atorvastatin, we ... emphasize the importance of meticulous monitoring and appropriate lifestyle interventions to mitigate the risk of new-onset diabetes or cataracts.”
Steven Nissen, MD, chief academic officer of Cleveland Clinic’s Heart and Vascular Institute, was not convinced, and said the study “does not provide useful insights into the use of these drugs.”
The investigators used whatever dose they wanted, “and the authors report only the median dose after 3 years,” he said in an interview. “Because there was a slightly greater reduction in low-density lipoprotein (LDL) cholesterol with rosuvastatin, the relative dose was actually higher.”
“We know that new-onset diabetes with statins is dose-dependent,” he said. “The P-values for diabetes incidence were marginal (very close to P = .05). Accordingly, the diabetes data are unconvincing. ... The similar efficacy is not surprising given the open-label dosing with relatively similar effects on lipids.”
Seth Shay Martin, MD, MHS, director of the Advanced Lipid Disorders Program and Digital Health Lab, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Medicine, Baltimore, also commented on the results. The findings are “in line with existing knowledge and current guidelines,” he said. “Therefore, the study should not influence prescribing.”
“Although the study suggests that rosuvastatin was associated with a higher risk of new-onset diabetes mellitus requiring antidiabetics and cataract surgery, compared with atorvastatin, these findings should be interpreted with caution given the open-label nature of the study and require further investigation,” he said.
“The mean daily doses of statins were somewhat below target for secondary prevention,” he noted. “Ideally, patients with coronary artery disease (CAD) take 20-40 mg daily of rosuvastatin or 40-80 mg daily of atorvastatin.”
“Furthermore, the LDL cholesterol levels were not optimized in the patients,” he said. “The mean LDL-C was 1.8-1.9 mmol/L, which is equivalent to 70-73 mg/dL. In the current treatment era, we generally treat to LDL-C levels less than 70 mg/dL and often less than 55 mg/dL in CAD patients.”
“The cataracts finding is particularly odd,” he added. “There was historic concern for cataracts with statin therapy, initially because of studies in beagle dogs. However, high-quality evidence from statin trials has not shown a risk for cataracts.”
So which statin has the lowest risk of triggering new-onset diabetes? As Dr. Hong noted, the literature is sparse when it comes to comparing the risk among specific statins. Some studies suggest that the risk may depend on the individual and their specific risk factors, as well as the dose and intensity of the prescribed statin.
One recent study suggests that while the overall chance of developing diabetes is small, when looking at risk by years of exposure, atorvastatin, rosuvastatin, and lovastatin carried the largest risk, whereas the risk was lower with pravastatin and simvastatin.
Risks also seemed lower with fluvastatin and pitavastatin, but there were too few study patients taking those drugs long-term to include in the subanalysis.
With input from the latest guidelines from the American Heart Association and the American Diabetes Association, as well as findings from a clinical guide on statin-associated diabetes, Dr. Hoogwerf suggests in his review that shared decision-making before starting statin therapy of any type include the following considerations/discussion points:
- For all patients: Screening to determine baseline glycemic status; nonstatin therapies to lower cholesterol; and variables associated with an increased risk of diabetes, including antihypertensive drugs.
- For patients without T2D: The possibility of developing T2D, types and doses of statins, and the fact that statin benefits “generally far outweigh” risks of developing diabetes.
- For patients with T2D: Possible small adverse effects on glycemic control; statin benefits in reducing risk for atherosclerotic cardiovascular disease, which “significantly outweigh” the small increase in A1c; and mitigation of adverse glycemic effects of statins with glucose-lowering therapies.
It’s worth noting that the AHA and ADA guidelines, among others, also emphasize that such discussions should include the importance of weight loss, regular exercise, and adhering to a healthy lifestyle to mitigate risks of both diabetes and heart disease, with or without statins.
Dr. Hong, Dr. Nissen, and Dr. Martin report no relevant financial relationships. Dr. Hoogwerf has disclosed ownership interest in Eli Lilly and consulting for MannKind and Zealand Pharmaceuticals.
A version of this article appeared on Medscape.com.
Antidepressants ‘don’t blunt’ semaglutide and weight loss
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
FROM OBESITYWEEK® 2023