User login
Steroids during late preterm labor: Better later than never
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
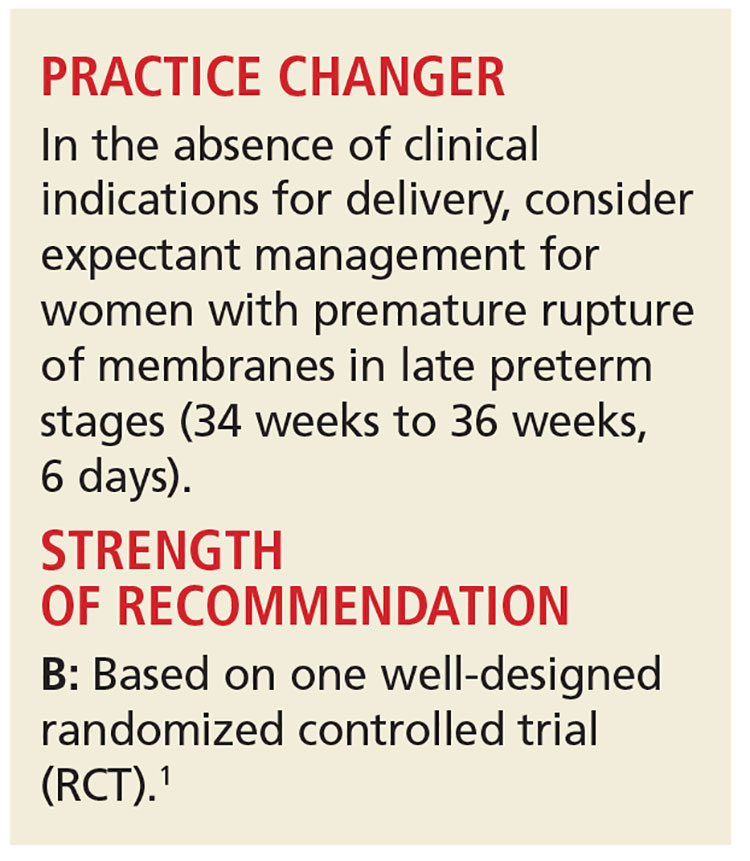
PRACTICE CHANGER
Use steroids in women at risk of preterm delivery, even if they are 36 weeks, 6 days’ pregnant, because steroids may reduce respiratory complications in the newborn with minimal risk for neonatal or maternal complications.
Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.1
STRENGTH OF RECOMMENDATION
A: Based on a good quality randomized controlled trial and consistent with a meta-analysis.
Oral Rehydration Therapy for KidsA More Palatable Alternative
A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
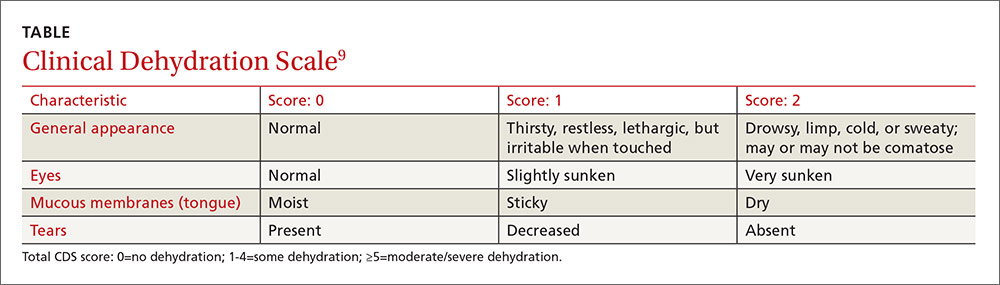
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.
Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.
Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
A 3-year-old boy is brought in by his mother for vomiting and diarrhea that started in the middle of the night. On examination, he is slightly dehydrated but does not have an acute abdomen or other source of infection. He is drinking from a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, resulting in 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost, taste, availability, and caregiver and professional preference for IV hydration have all been barriers to the use of ORT.2,4-8 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that less than half of the children would voluntarily drink the ORT again.5
This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, noninferiority RCT conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lb), and had vomiting and/or diarrhea for less than 96 hours (with ≥ 3 episodes over the past 24 hours). They also had a Clinical Dehydration Scale (CDS) score < 5 and a capillary refill of < 2 seconds (see Table).9 Of the total, 68% of the children had a CDS score of 0; 25.5%, of 1 to 2; and 6.4%, of 3 to 4. Exclusion criteria included chronic gastrointestinal disease or other significant comorbidities (eg, diabetes) that could affect the clinical state and potential acute abdominal pathology.
Children were randomly assigned to receive half-strength apple juice (intervention group, n = 323) or an apple-flavored sucralose-sweetened electrolyte maintenance solution (EMS; control group, n = 324). Immediately on triage, each child received 2 L of their assigned fluid, to be used while in the ED and then at home. The children received 5 mL of fluid every two to five minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the juice group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency, as well as any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure, defined as the occurrence of any of the following within seven days of the ED visit: hospitalization, IV rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥ 3 episodes of vomiting or diarrhea within a 24-hour period occurring > 7 days after enrollment), 3% or greater weight loss, or CDS score ≥ 5 at follow-up.
Treatment failure occurred in 16.7% of the juice group, compared to 25% of the EMS group (difference, 8.3 percentage points; number needed to treat [NNT], 12), consistent with noninferior effectiveness. The benefit was seen primarily in children ≥ 24 months of age. In children < 24 months, the treatment failure for juice was 23.9% and for EMS, 24.1%. In older children (those ≥ 24 months to 5 years), the treatment failure with juice was 9.8% and with EMS, 25.9% (difference, 16.2 percentage points; NNT, 6.2).
IV rehydration in the ED or within seven days of the initial visit was needed in 2.5% of the juice group and in 9% of the EMS group (difference, 6.5 percentage points; NNT, 15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥ 24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit; ondansetron played a role
Children in this study were only mildly dehydrated. The study did not include infants younger than 6 months of age, and the greatest benefit was seen in children ≥ 24 months of age.
Also noteworthy was that most of the children (67.4%) received an oral dose of ondansetron (0.1 mg/kg). Although ondansetron is expensive, it would be considered cost-effective if one dose prevents a hospitalization. Previous studies of oral ondansetron show it reduces vomiting (NNT, 5); lowers the rate of IV hydration in the ED (NNT, 5); and reduces the hospitalization rate from the ED (NNT, 17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for ORT, there are no foreseen barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(12): 924-926.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. World Health Organization. New formula oral rehydration salts. WHO Drug Information. 2002;16(2). http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed December 5, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011; CD005506.
Need an add-on to metformin? Consider this
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 58-year-old woman with type 2 diabetes mellitus (T2DM) and heart failure returns to your office for follow-up of her T2DM. She has been on the maximum dose of metformin alone for the past 6 months, but her HbA1c is now 7.8%. She is keen to avoid injections. What do you recommend next?
There is surprisingly little consensus about what to add to metformin for patients with T2DM who require a second agent to achieve their glycemic goal. Attainment of glycemic control earlier in the course of the disease may lead to reduced overall cardiovascular risk, so the choice of a second drug is an important one.2 While metformin is well established as initial pharmacotherapy because of its proven mortality benefit, wide availability, and low cost, no second-choice drug has amassed enough evidence of benefit to emerge as the add-on therapy of choice.
Furthermore, the professional societies and associations are of little assistance. Dual therapy recommendations from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes do not denote a specific preference, and while the American Association of Clinical Endocrinologists/American College of Endocrinology do suggest a hierarchy of choices, it is based upon expert consensus recommendation.3,4
Sulfonylureas can cause hypoglycemia and weight gain
Options for add-on therapy include sulfonylureas, thiazolidines, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) agonists, and insulin. Providers have frequently prescribed a sulfonylurea after metformin because such agents are low in cost, have long-term safety data, and are effective at lowering HbA1c. Sulfonylureas work by directly stimulating insulin secretion by pancreatic beta cells in a glucose-independent manner. But as a 2010 meta-analysis revealed, they carry significant risks of hypoglycemia (relative risk [RR]=4.57; 95% confidence interval [CI], 2.11-11.45) and weight gain (2.06 kg; 95% CI, 1.15-2.96) compared to placebo.5
DPP-4 inhibitors, on the other hand, work by inducing insulin secretion in a glucose-dependent manner through an incretin mechanism. Combined with metformin, they provide glucose control similar to that achieved with the combination of a sulfonylurea and metformin.6 DPP-4 inhibitors were initially found to be associated with fewer cardiovascular events and less hypoglycemia than sulfonylureas, but were subsequently linked to an increased risk of hospitalization for heart failure.7
This latest large observational study provides more evidence on the effects of DPP-4s when added to metformin.1
STUDY SUMMARY
DPP-4s as effective as sulfonylureas with no increased risks
This population-based observational cohort study compared DPP-4 inhibitors and sulfonylureas when added to metformin for the treatment of T2DM.1 Outcomes were all-cause mortality, major adverse cardiovascular events (MACEs; defined as hospitalization for ischemic stroke or myocardial infarction [MI]), and hospitalizations for either heart failure or hypoglycemia. Using the National Health Insurance Research Database in Taiwan, the study included data on over 70,000 patients ages 20 years and older with a diagnosis of T2DM. Individuals adherent to metformin were considered to be enrolled into the cohort on the day they began using either a DPP-4 inhibitor or a sulfonylurea, in addition to metformin.
The researchers collected additional data on the enrolled individuals regarding socioeconomic factors, urbanization, robustness of the local health care system, Charlson Comorbidity Index, adapted Diabetes Complications Severity Index, and other comorbidities and medications that could affect the outcomes of interest. Using these data, enrollees were matched by propensity score into 10,089 pairs consisting of a DPP-4 inhibitor user and a sulfonylurea user.
After a mean follow-up period of 2.8 years, the authors of the study used Cox regression analysis to evaluate the relative hazards of the outcomes. Subgroup analysis performed by age, sex, Charlson Comorbidity Index, hypertension, chronic kidney disease, hospitalization for heart failure, MI, and cerebrovascular disease yielded results similar to those of the primary analysis for each outcome. Additionally, similar results were obtained when the data were analyzed without propensity-score matching.
The researchers found that users of DPP-4 inhibitors—when compared to users of sulfonylureas—had a lower risk of all-cause mortality (366 vs 488 deaths; hazard ratio [HR]=0.63; 95% CI, 0.55-0.72; number needed to treat [NNT]=117), MACE (209 vs 282 events; HR=0.68; 95% CI, 0.55-0.83; NNT=191), ischemic stroke (144 vs 203 strokes; HR 0.64; 95% CI, 0.51-0.81; NNT=246), and hypoglycemia (89 vs 170 events; HR=0.43; 95% CI, 0.33-0.56; NNT=201). Further, there were no significant differences in either the number of MIs that occurred (69 vs 88 MIs; HR=0.75; 95% CI, 0.52-1.07) or in the number of hospitalizations for heart failure (100 vs 100 events; HR=0.78; 95% CI, 0.57-1.06) between users of DPP-4 inhibitors and those of sulfonylureas.
WHAT’S NEW
Lower risks of death, CV events, and hypoglycemia
This study found that when added to metformin, DPP-4 inhibitors were associated with lower risks for all-cause mortality, cardiovascular events, and hypoglycemia when compared to sulfonylureas. Additionally, DPP-4 inhibitors did not increase the risk of hospitalization for heart failure. A recent multicenter observational study of nearly 1.5 million patients on the effects of incretin-based treatments, including both DPP-4 inhibitors and GLP-1 agonists, similarly found no increased risk of hospitalization for heart failure, with DPP-4 inhibitors compared to other combinations of oral T2DM agents.8
CAVEATS
Did unmeasured confounders play a role?
Unmeasured confounders potentially bias all observational population cohort results. In this study, in particular, there may have been unmeasured, but significant, patient factors that providers used to choose diabetes medications. Also, the study did not evaluate diabetes control, although previous studies have shown similar glucose control between sulfonylureas and DPP-4 inhibitors when they were added to metformin.6
Another caveat is that the results from this study group may not be fully generalizable to other populations due to physiologic differences. People of Asian ancestry are at risk of developing T2DM at a lower body mass index than people of European ancestry, which could affect the outcomes of interest.9
Furthermore, the study did not evaluate outcomes based on whether patients were taking first-, second-, or third-generation sulfonylureas. Some sulfonylureas, such as glyburide, carry a higher risk of hypoglycemia, which could bias the results if a large number of patients were taking them.10
Lastly, the study only provides guidance when choosing between a sulfonylurea and a DPP-4 inhibitor for second-line pharmacotherapy. The GRADE trial, due to be completed in 2023, is comparing sulfonylureas, DPP-4 inhibitors, GLP-1 agonists, and insulin as add-on medications to metformin, and may provide more data on which to base treatment decisions.11
CHALLENGES TO IMPLEMENTATION
DPP-4s have a higher price tag than sulfonylureas
Sulfonylureas and DPP-4 inhibitors are both available as generic medications, but the cost of DPP-4 inhibitors remains significantly higher.12 Higher copays and deductibles could affect patient preference. Furthermore, for patients without health insurance, sulfonylureas are available on the discounted drug lists of many major retailers, while DPP-4 inhibitors are not.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
1. Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.
2. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197-2206.
3. American Diabetes Association. Approaches to glycemic treatment. Sec 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016; 39:e88-e89.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes 4. Management Algorithm—2016 Executive Summary. Endocr Pract. 2016;22:84-113.
5. Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410-1418.
6. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475-483.
7. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
8. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145-1154.
9. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, pathophysiology. JAMA. 2009;301:2129-2140.
10. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
11. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254-2261.
12. GoodRx. Gliptins. Available at: http://www.goodrx.com/gliptins. Accessed August 31, 2016.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Consider a dipeptidyl peptidase-4 inhibitor before a sulfonylurea for patients with type 2 diabetes mellitus who require therapy in addition to metformin.
Ou SM, Shih CJ, Chao PW, et al. Effects of clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663-672.1
STRENGTH OF RECOMMENDATION
B: Based on limited-quality, patient-oriented data from a high-quality, population-based cohort study.
Deliver or Wait with Late Preterm Membrane Rupture?
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the past two hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, from 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery.
In the absence of these factors, delivery versus expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended if there are no signs of infection or maternal or fetal compromise. This is because of the significant morbidity and mortality risk associated with births before 34 weeks’ gestation.4
Currently, the American College of Obstetricians and Gynecologists (ACOG) recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis
The Preterm Pre-labour Rupture of the Membranes close to Term (PPROMT) trial was a multicenter RCT that included 1,839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Participants were randomized to either expectant management or immediate delivery by induction. Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and two additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (ie, chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the two groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (ie, sepsis, mechanical ventilation ≥ 24 h, stillbirth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, low birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR], 0.8). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR, 1.2). However, infants born in the immediate delivery group had significantly lower birth weights (2,574.7 g vs 2,673.2 g; absolute difference, –125 g), a higher incidence of respiratory distress (RR, 1.6; number needed to treat [NNT], 32), and spent more time in the NICU/special care nursery (four days vs two days).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR, 0.6; number needed to harm [NNH], 50) and intrapartum fever (RR, 0.4; NNH, 100). Of the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% of the expectant management group (RR, 1.4; NNT, 14). Six percent of the women assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent, with no significant differences between the two groups.
WHAT’S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (involving 736 women) evaluated expectant management versus induction in the late preterm stage of pregnancy. No increased risk for neonatal sepsis with expectant management was found in either study.8,9
However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes. The PPROMT study is the largest to indicate that immediate birth increases infant risk for respiratory distress and duration of NICU/special care stay and increases the mother’s risk for cesarean section. It also showed that risk for neonatal sepsis was not higher in the expectant management group.
CAVEATS
Singleton pregnancies only
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. This created variation in fetal and maternal monitoring. The majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage. If one of these occurs, immediate delivery may be necessary.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, as well.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines (updated October 2016) recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(11):820-822.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387: 444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3: CD004735.
5. American College of Obstetricians and Gynecologists. Practice Bulletin No 172: Premature rupture of membranes [interim update]. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012; 207:276.
A more palatable alternative to oral rehydration therapy for kids
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.
Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.
Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.
Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Recommend that parents give half-strength apple juice to children ≥24 months of age who are minimally dehydrated following a case of simple viral gastroenteritis. The juice reduces the need for further interventions better than oral hydration therapy.
Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.1
STRENGTH OF RECOMMENDATION
B: Based on a single, good quality randomized controlled trial.
Monitoring Home BP Readings Just Got Easier
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Deliver or wait with late preterm membrane rupture?
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
In the absence of clinical indications for delivery, consider expectant management in women with premature rupture of membranes in late preterm stages (34 weeks to 36 weeks, 6 days).
Strength of recommendation
B: Based on one well-designed randomized controlled trial.1
Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
ILLUSTRATIVE CASE
A 26-year-old G2P1001 at 35 weeks, 2 days of gestation presents with leakage of clear fluid for the last 2 hours. There is obvious pooling in the vaginal vault, and rupture of membranes is confirmed with appropriate testing. Her cervix is closed, she is not in labor, and tests of fetal well-being are reassuring. She had an uncomplicated vaginal delivery with her first child. How should you manage this situation?
Preterm premature rupture of membranes (PPROM)—when rupture of membranes occurs before 37 weeks’ gestation—affects about 3% of all pregnancies in the United States, and is a major contributor to perinatal morbidity and mortality.2,3 PPROM management remains controversial, especially during the late preterm stage (ie, 34 weeks to 36 weeks, 6 days). Non-reassuring fetal status, clinical chorioamnionitis, cord prolapse, and significant placental abruption are clear indications for delivery. In the absence of those factors, delivery vs expectant management is determined by gestational age. Between 23 and 34 weeks’ gestation, when the fetus is at or close to viability, expectant management is recommended, provided there are no signs of infection or maternal or fetal compromise.4 This is because of the significant morbidity and mortality associated with births before 34 weeks’ gestation.4
The American College of Obstetricians and Gynecologists (ACOG) currently recommends delivery for all women with rupture of membranes after 34 weeks’ gestation, while acknowledging that this recommendation is based on “limited and inconsistent scientific evidence.”5 The recommendation for delivery after 34 weeks is predicated on the belief that disability-free survival is high in late preterm infants. However, there is a growing body of evidence that shows negative short- and long-term effects for these children, including medical concerns, academic difficulties, and more frequent hospital admissions in early childhood.6,7
STUDY SUMMARY
Higher birth weights, fewer C-sections, and no increased sepsis with wait-and-see
The Preterm Pre-labour Rupture Of the Membranes close to Term (PPROMT) trial was a multicenter (65 institutions across 11 countries), randomized controlled trial (RCT) that included 1839 women with singleton pregnancies and confirmed rupture of membranes between 34 weeks and 36 weeks, 6 days’ gestation.1 Conducted from May 2004 to June 2013, participants were randomized to expectant management (915 women) vs immediate delivery by induction (924 women). Patients and care providers were not masked to treatment allocation, but those determining the primary outcome were masked to group allocation.
One woman in each group was lost to follow-up, and 2 additional women withdrew from the immediate birth group. Women already in active labor or with clinical indications for delivery (chorioamnionitis, abruption, cord prolapse, fetal distress) were excluded. The baseline characteristics of the 2 groups were similar.
Women in the induction group had delivery scheduled as soon as possible after randomization. Women in the expectant management group were allowed to go into spontaneous labor and were only induced if they reached term or the clinician identified other indications for immediate delivery.
The primary outcome was probable or confirmed neonatal sepsis. Secondary infant outcomes included a composite neonatal morbidity and mortality indicator (sepsis, mechanical ventilation ≥24 hours, still birth, or neonatal death), respiratory distress syndrome, any mechanical ventilation, birth weight, and duration of stay in a neonatal intensive care unit (NICU) or special care nursery. Secondary maternal outcomes included antepartum or intrapartum hemorrhage, intrapartum fever, mode of delivery, duration of hospital stay, and development of chorioamnionitis in the expectant management group.
The primary outcome of neonatal sepsis occurred in 2% of the neonates assigned to immediate delivery and 3% of neonates assigned to expectant management (relative risk [RR]=0.8; 95% confidence interval [CI], 0.5-1.3; P=.37). There was also no statistically significant difference in composite neonatal morbidity and mortality (RR=1.2; 95% CI, 0.9-1.6; P=.32). However, infants born in the immediate delivery group had significantly lower birth weights (2574.7 g vs 2673.2 g; absolute difference= -125 g; P<.0001), a higher incidence of respiratory distress (RR=1.6; 95% CI, 1.1-2.3; P=.008; number needed to treat [NNT]=32), and spent more time in the NICU/special care nursery (4 days vs 2 days; P<.0001).
Compared to immediate delivery, expectant management was associated with a higher likelihood of antepartum or intrapartum hemorrhage (RR=0.6; 95% CI, 0.4-0.9; P=.02; number needed to harm [NNH]=50) and intrapartum fever (RR=0.4; 95% CI, 0.2-0.9; P=.02; NNH=100). In the women assigned to immediate delivery, 26% had a cesarean section, compared to 19% in the expectant management group (RR=1.4; 95% CI, 1.2-1.7, P=.0001; NNT=14). A total of 56 women (6%) assigned to the expectant management group developed clinically significant chorioamnionitis requiring delivery. All other secondary maternal and neonatal outcomes were equivalent with no significant differences between the 2 groups.
WHAT'S NEW?
Largest study to show no increased sepsis with expectant management
Two prior RCTs (the PPROMEXIL trial8 and PPROMEXIL-29), involving a total of 736 women, evaluated expectant management vs induction in the late preterm stage of pregnancy. There was no increased risk of neonatal sepsis with expectant management in either study. However, those studies did not have sufficient power to show a statistically significant change in any of the outcomes.
The PPROMT study is the largest one to show that immediate birth increases the risk of respiratory distress and duration of NICU/special care stay for the baby and increases the risk of cesarean section for the mother. It also showed that the risk of neonatal sepsis was not higher in the expectant management group.
CAVEATS
Findings only apply to singleton pregnancies
Delivery of the infants in the expectant management group was not by specified protocol; each birth was managed according to the policies of the local center and clinician judgment. Because of this, there was variation in fetal and maternal monitoring. The vast majority of women in both groups (92% to 93%) received intrapartum antibiotics. Expectant management should include careful monitoring for infection and hemorrhage and may need to be changed to immediate delivery if one of these occurs.
The study participants all had singleton pregnancies; this recommendation cannot be extended to non-singleton pregnancies. However, a prior cesarean section was not an exclusion criterion for the study, and these recommendations would be valid for that group of women, too.
CHALLENGES TO IMPLEMENTATION
Going against the tide of ACOG
The most recent ACOG guidelines, updated October 2016, recommend induction of labor for women with ruptured membranes in the late preterm stages.5 This may present a challenge to widespread acceptance of expectant management for PPROM.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
1. Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
2. Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307-312.
3. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1-72.
4. Buchanan SL, Crowther CA, Levett KM, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst Rev. 2010;3:CD004735.
5. Practice Bulletin Summary. Interim update. Premature rupture of membranes. Number 172, October 2016. Obstet Gynecol. 2016;128:934-936.
6. McGowan JE, Alderdice FA, Holmes VA, et al. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127:1111-1124.
7. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205:374.
8. van der Ham DP, Vijgen SM, Nijhuis JG, et al; PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 2012;9:e1001208.
9. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207:276.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.