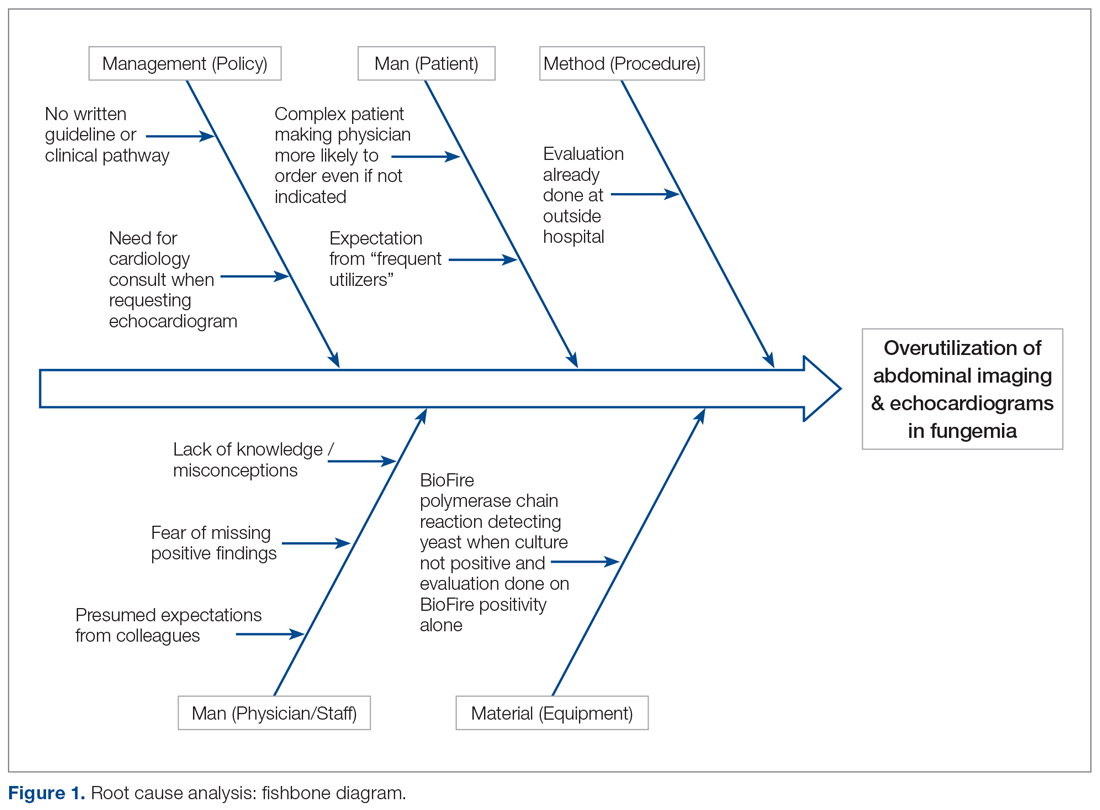
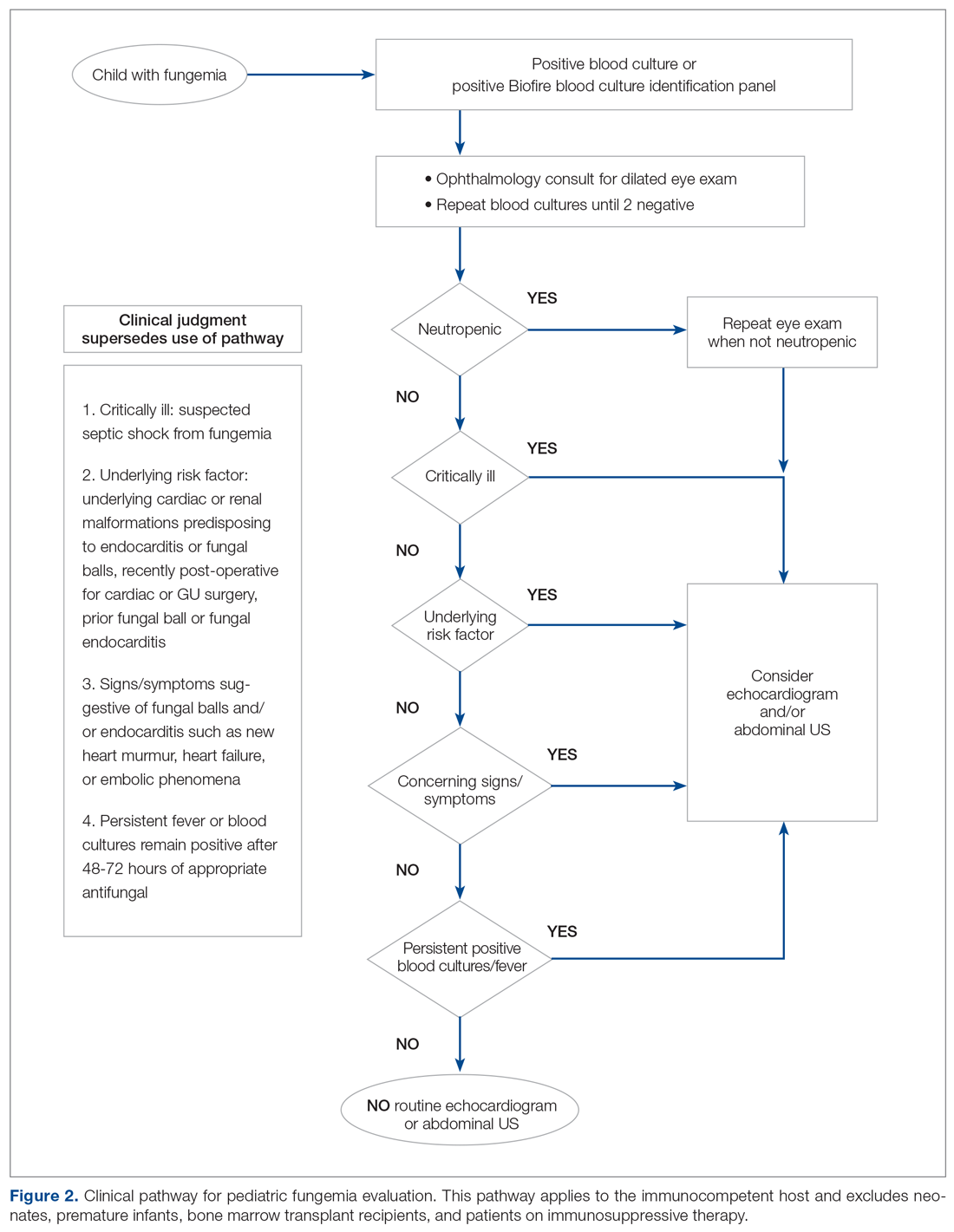
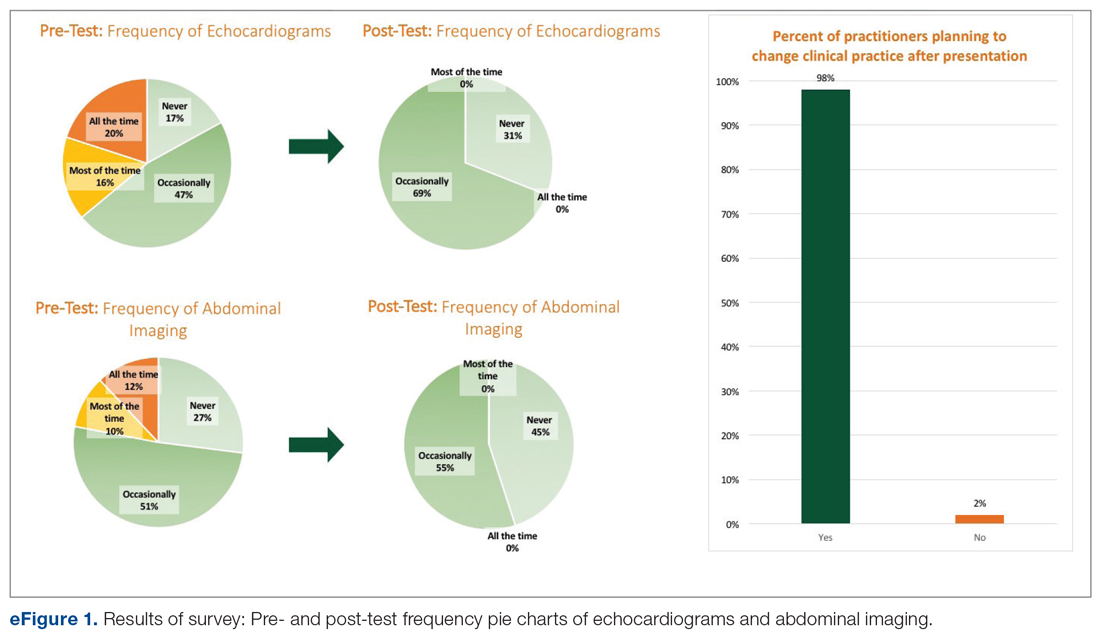
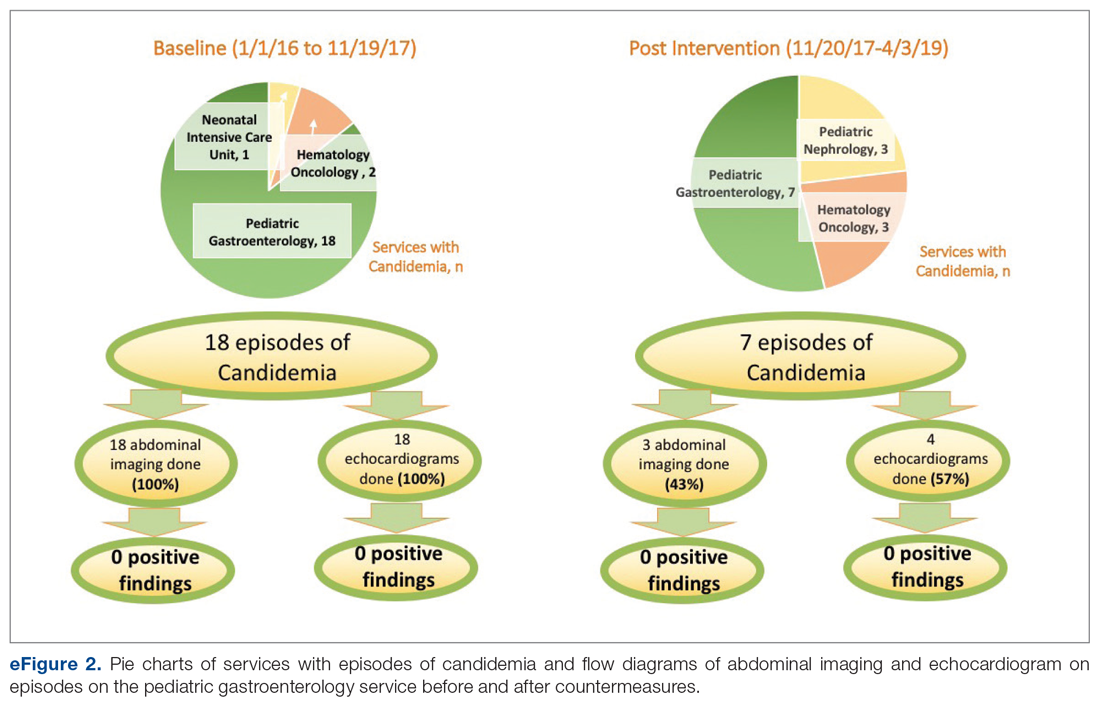
User login
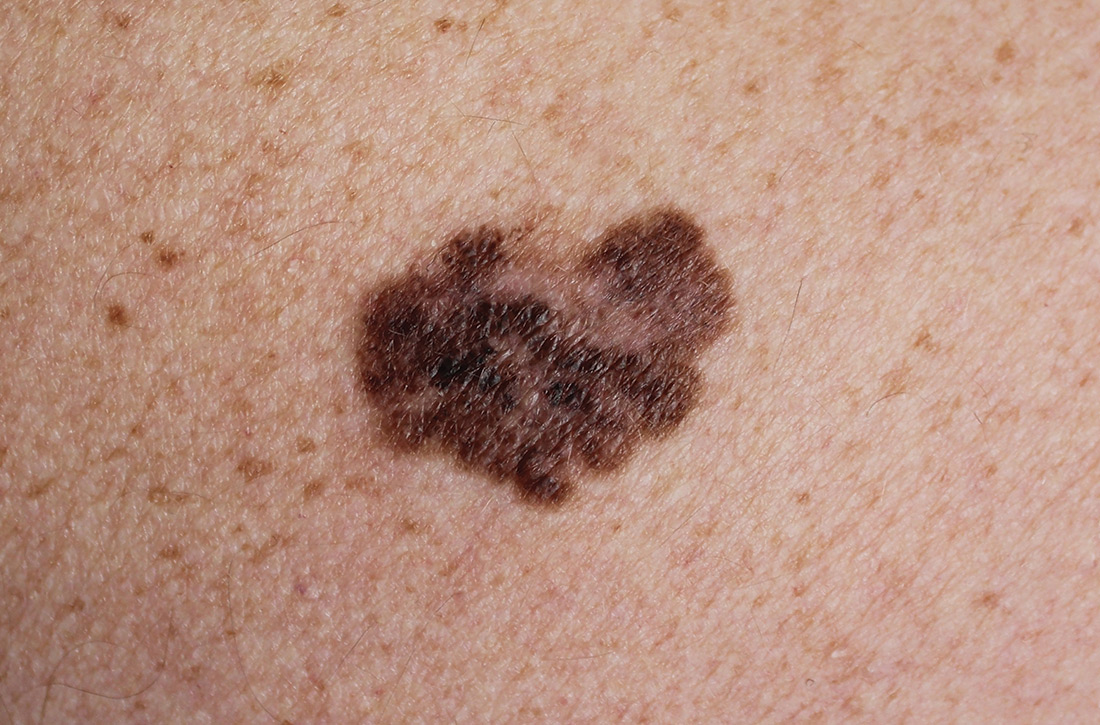
Does screening by primary care providers effectively detect melanoma and other skin cancers?
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE-BASED ANSWER:
Possibly. No trials have directly assessed detection of melanoma and other skin cancers by primary care providers.
Training a group comprised largely of primary care physicians to perform skin cancer screening was associated with a 41% increase in skin cancer diagnoses but no change in melanoma mortality.
Visual screening for melanoma by primary care physicians is 40% sensitive and 86% specific (compared with 49% and 98%, respectively, for dermatologists and plastic surgeons).
Melanomas found by visual screening are 38% more likely to be thin (≤ 0.75 mm) than melanomas discovered without screening, which correlates with improved outcomes.
Visual skin cancer screening overall is associated with false-positive rates as follows: 28 biopsies for each melanoma detected, 9 to 10 biopsies for each basal cell carcinoma, and 28 to 56 biopsies for squamous cell carcinoma. False-positive rates are higher for women—as much as double the rate for men—and younger patients—as much as 20-fold the rate for older patients (strength of recommendations for all foregoing statements: B, cohort studies).
Families as Care Partners: Implementing the Better Together Initiative Across a Large Health System
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken and Ms. Johnson), and Northwell Health, New Hyde Park, NY (Dr. Barden, Ms. Tuomey, and Ms. Giammarinaro).
Abstract
Objective: To describe the growth of Better Together: Partnering with Families, a campaign launched in 2014 to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care, and to discuss the processes involved in implementing the initiative in a large, integrated health system.
Methods: Descriptive report.
Results: In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the Better Together campaign to emphasize the importance of family presence and participation to the quality, experience, safety, and outcomes of care. Since then, this initiative has expanded in both the United States and Canada. With support from 2 funders in the United States, special attention was focused on acute care hospitals across New York State. Nearly 50 hospitals participated in 2 separate but related projects. Fifteen of the hospitals are part of Northwell Health, New York State’s largest health system. Over a 10-month period, these hospitals made significant progress in changing policy, practice, and communication to support family presence.
Conclusion: The Better Together initiative was implemented across a health system with strong support from leadership and the involvement of patient and family advisors. An intervention offering structured training, coaching, and resources, like IPFCC’s Better Together initiative, can facilitate the change process.
Keywords: family presence; visiting policies; patient-centered care; family-centered care; patient experience.
The presence of families at the bedside of patients is often restricted by hospital visiting hours. Hospitals that maintain these restrictive policies cite concerns about negative impacts on security, infection control, privacy, and staff workload. But there are no data to support these concerns, and the experience of hospitals that have successfully changed policy and practice to welcome families demonstrates the potential positive impacts of less restrictive policies on patient care and outcomes.1 For example, hospitalization can lead to reduced cognitive function in elderly patients. Family members would recognize the changes and could provide valuable information to hospital staff, potentially improving outcomes.2
In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families.3 The campaign is is grounded in patient- and family- centered care, an approach to care that supports partnerships among health care providers, patients, and families, and, among other core principles, advocates that patients define their “families” and how they will participate in care and decision-making.
Emphasizing the importance of family presence and participation to quality and safety, the Better Together campaign seeks to eliminate restrictive visiting policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference. As part of the campaign, IPFCC developed an extensive toolkit of resources that is available to hospitals and other organizations at no cost. The resources include sample policies; profiles of hospitals that have implemented family presence policies; educational materials for staff, patients, and families; and a template for hospital websites. This article, a follow-up to an article published in the January 2015 issue of JCOM,1 discusses the growth of the Better Together initiative as well as the processes involved in implementing the initiative across a large health system.
Growth of the Initiative
Since its launch in 2014, the Better Together initiative has continued to expand in the United States and Canada. In Canada, under the leadership of the Canadian Foundation for Healthcare Improvement (CFHI), more than 50 organizations have made a commitment to the Better Together program and family presence.4 Utilizing and adapting IPFCC’s Toolkit, CFHI developed a change package of free resources for Canadian organizations.5 Some of the materials, including the Pocket Guide for Families (Manuel des Familles), were translated into French.6
With support from 2 funders in the United States, the United Hospital Fund and the New York State Health (NYSHealth) Foundation, through a subcontract with the New York Public Interest Research Group (NYPIRG), IPFCC has been able to focus on hospitals in New York City, including public hospitals, and, more broadly, acute care hospitals across New York State. Nearly 50 hospitals participated in these 2 separate but related projects.
Education and Support for New York City Hospitals
Supported by the United Hospital Fund, an 18-month project that focused specifically on New York City hospitals was completed in June 2017. The project began with a 1-day intensive training event with representatives of 21 hospitals. Eighteen of those hospitals were eligible to participate in follow-up consultation provided by IPFCC, and 14 participated in some kind of follow-up. NYC Health + Hospitals (H+H), the system of public hospitals in NYC, participated most fully in these activities.
The outcomes of the Better Together initiative in New York City are summarized in the report Sick, Scared, & Separated From Loved Ones,2 which is based on a pre/post review of hospital visitation/family presence policies and website communications. According to the report, hospitals that participated in the IPFCC training and consultation program performed better, as a group, with respect to improved policy and website scores on post review than those that did not. Of the 10 hospitals whose scores improved during the review period, 8 had participated in the IPFCC training and 1 hospital was part of a hospital network that did so. (Six of these hospitals are part of the H+H public hospital system.) Those 9 hospitals saw an average increase in scores of 4.9 points (out of a possible 11).
A Learning Community for Hospitals in New York State
With support from the NYSHealth Foundation, IPFCC again collaborated with NYPIRG and New Yorkers for Patient & Family Empowerment on a 2-year initiative, completed in November 2019, that involved 26 hospitals: 15 from Northwell Health, New York State’s largest health system, and 11 hospitals from health systems throughout the state (Greater Hudson Valley Health System, now Garnet Health; Mohawk Valley Health System; Rochester Regional Health; and University of Vermont Health Network). An update of the report Sick, Scared, & Separated From Loved Onescompared pre/post reviews of policies and website communications regarding hospital visitation/family presence.7 Its findings confirm that hospitals that participated in the Better Together Learning Community improved both their policy and website scores to a greater degree than hospitals that did not participate and that a planned intervention can help facilitate change.
During the survey period, 28 out of 40 hospitals’ website navigability scores improved. Of those, hospitals that did not participate in the Better Together Learning Community saw an average increase in scores of 1.2 points, out of a possible 11, while the participating hospitals saw an average increase of 2.7 points, with the top 5 largest increases in scores belonging to hospitals that participated in the Better Together Learning Community.7
The Northwell Health Experience
Northwell Health is a large integrated health care organization comprising more than 69,000 employees, 23 hospitals, and more than 750 medical practices, located geographically across New York State. Embracing patient- and family-centered care, Northwell is dedicated to improving the quality, experience, and safety of care for patients and their families. Welcoming and including patients, families, and care partners as members of the health care team has always been a core element of Northwell’s organizational goal of providing world-class patient care and experience.
Four years ago, the organization reorganized and formalized a system-wide Patient & Family Partnership Council (PFPC).8 Representatives on the PFPC include a Northwell patient experience leader and patient/family co-chair from local councils that have been established in nearly all 23 hospitals as well as service lines. Modeling partnership, the PFPC is grounded in listening to the “voice” of patients and families and promoting collaboration, with the goal of driving change across varied aspects and experiences of health care delivery.
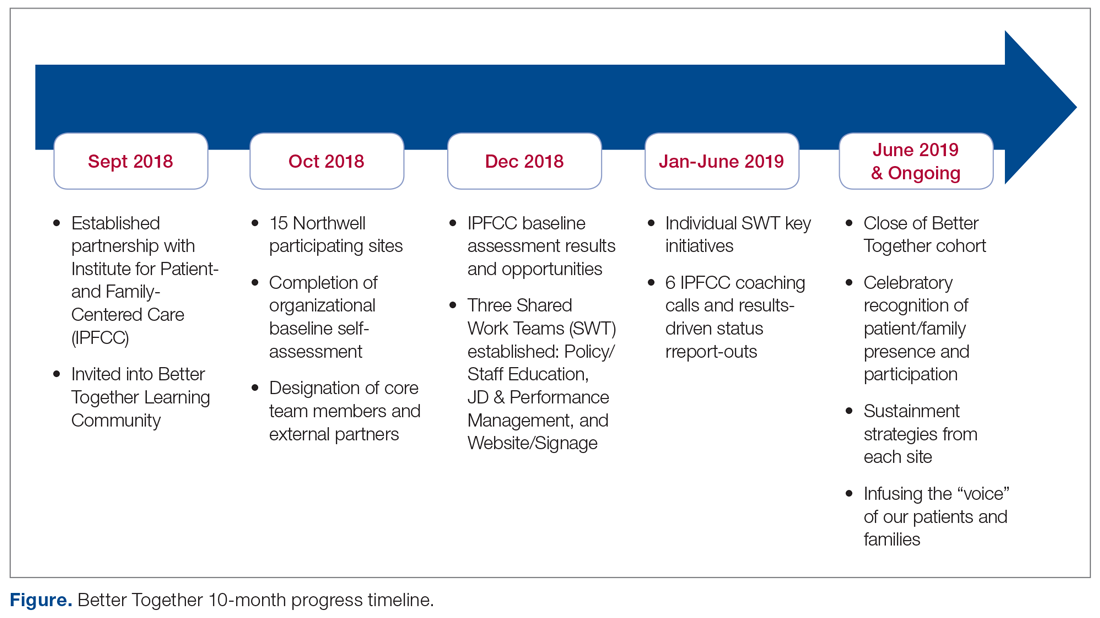
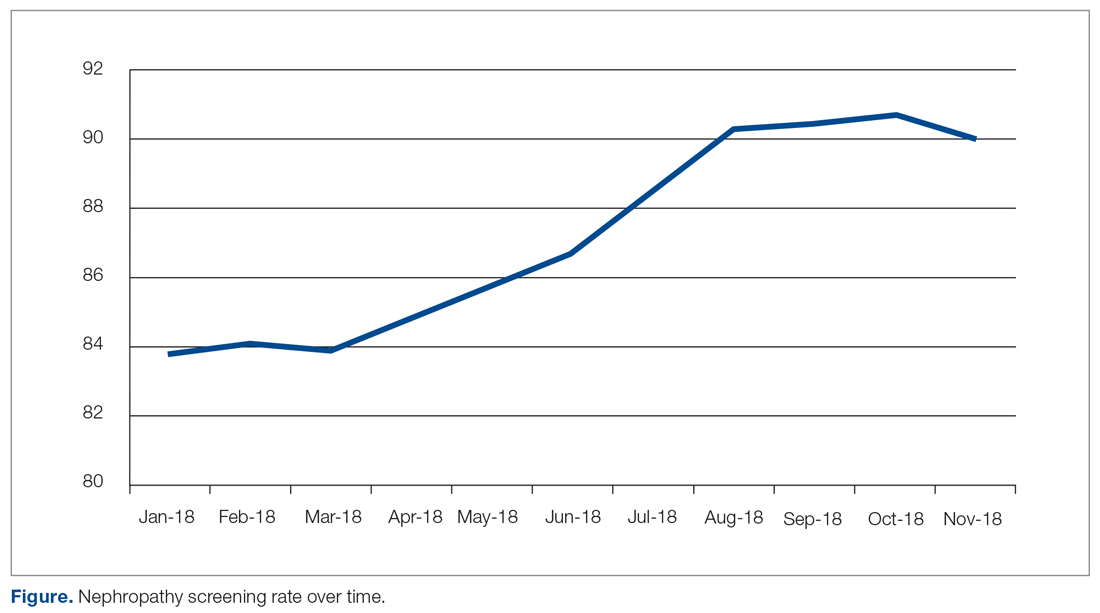
Through the Office of Patient and Customer Experience (OPCE), a partnership with IPFCC and the Better Together Learning Community for Hospitals in New York State was initiated as a fundamental next step in Northwell’s journey to enhance system-wide family presence and participation. Results from Better Together’s Organizational Self-Assessment Tool and process identified opportunities to influence 3 distinct areas: policy/staff education, position descriptions/performance management, and website/signage. Over a 10-month period (September 2018 through June 2019), 15 Northwell hospitals implemened significant patient- and family-centered improvements through multifaceted shared work teams (SWT) that partnered around the common goal of supporting the patient and family experience (Figure). Northwell’s SWT structure allowed teams to meet individually on specific tasks, led by a dedicated staff member of the OPCE to ensure progress, support, and accountability. Six monthly coaching calls or report-out meetings were attended by participating teams, where feedback and recommendations shared by IPFCC were discussed in order to maintain momentum and results.
Policy/Staff Education
The policy/staff education SWT focused on appraising and updating existing policies to ensure alignment with key patient- and family-centered concepts and Better Together principles (Table 1). By establishing representation on the System Policy and Procedure Committee, OPCE enabled patients and families to have a voice at the decision-making table. OPCE leaders presented the ideology and scope of the transformation to this committee. After reviewing all system-wide policies, 4 were identified as key opportunities for revision. One overarching policy titled “Visitation Guidelines” was reviewed and updated to reflect Northwell’s mission of patient- and family-centered care, retiring the reference to “families” as “visitors” in definitions, incorporating language of inclusion and partnership, and citing other related policies. The policy was vetted through a multilayer process of review and stakeholder feedback and was ultimately approved at a system
Three additional related policies were also updated to reflect core principles of inclusion and partnership. These included system policies focused on discharge planning; identification of health care proxy, agent, support person and caregiver; and standards of behavior not conducive in a health care setting. As a result of this work, OPCE was invited to remain an active member of the System Policy and Procedure Committee, adding meaningful new perspectives to the clinical and administrative policy management process. Once policies were updated and approved, the SWT focused on educating leaders and teams. Using a diversified strategy, education was provided through various modes, including weekly system-wide internal communication channels, patient experience huddle messages, yearly mandatory topics training, and the incorporation of essential concepts in existing educational courses (classroom and e-learning modalities).
Position Descriptions/Performance Management
The position descriptions/performance management SWT focused its efforts on incorporating patient- and family-centered concepts and language into position descriptions and the performance appraisal process (Table 2). Due to the complex nature of this work, the process required collaboration from key subject matter experts in human resources, talent management, corporate compensation, and labor management. In 2019, Northwell began an initiative focused on streamlining and standardizing job titles, roles, and developmental pathways across the system. The overarching goal was to create system-wide consistency and standardization. The SWT was successful in advising the leaders overseeing this job architecture initiative on the importance of including language of patient- and family-centered care, like partnership and collaboration, and of highlighting the critical role of family members as part of the care team in subsequent documents.
Northwell has 6 behavioral expectations, standards to which all team members are held accountable: Patient/Customer Focus, Teamwork, Execution, Organizational Awareness, Enable Change, and Develop Self. As a result of the SWT’s work, Patient/Customer Focus was revised to include “families” as essential care partners, demonstrating Northwell’s ongoing commitment to honoring the role of families as members of the care team. It also ensures that all employees are aligned around this priority, as these expectations are utilized to support areas such as recognition and performance. Collaborating with talent management and organizational development, the SWT reviewed yearly performance management and new-hire evaluations. In doing so, they identified an opportunity to refresh the anchored qualitative rating scales to include behavioral demonstrations of patient- and family-centered care, collaboration, respect, and partnership with family members.
Website/Signage
Websites make an important first impression on patients and families looking for information to best prepare for a hospital experience. Therefore, the website/signage SWT worked to redesign hospital websites, enhance digital signage, and perform a baseline assessment of physical signage across facilities. Initial feedback on Northwell’s websites identified opportunities to include more patient- and family-centered, care-partner-infused language; improve navigation; and streamline click levels for easier access. Content for the websites was carefully crafted in collaboration with Northwell’s internal web team, utilizing IPFCC’s best practice standards as a framework and guide.
Next, a multidisciplinary website shared-governance team was established by the OPCE to ensure that key stakeholders were represented and had the opportunity to review and make recommendations for appropriate language and messaging about family presence and participation. This 13-person team was comprised of patient/family partners, patient-experience culture leaders, quality, compliance, human resources, policy, a chief nursing officer, a medical director, and representation from the Institute for Nursing. After careful review and consideration from Northwell’s family partners and teams, all participating hospital websites were enhanced as of June 2019 to include prominent 1-click access from homepages to information for “patients, families and visitors,” as well as “your care partners” information on the important role of families and care partners.
Along with refreshing websites, another step in Northwell’s work to strengthen messaging about family presence and participation was to partner and collaborate with the system’s digital web team as well as local facility councils to understand the capacity to adjust digital signage across facilities. Opportunities were found to make simple yet effective enhancements to the language and imagery of digital signage upon entry, creating a warmer and more welcoming first impression for patients and families. With patient and family partner feedback, the team designed digital signage with inclusive messaging and images that would circulate appropriately based on the facility. Signage specifically welcomes families and refers to them as members of patients’ care teams.
Northwell’s website/signage SWT also directed a 2-phase physical signage assessment to determine ongoing opportunities to alter signs in areas that particularly impact patients and families, such as emergency departments, main lobbies, cafeterias, surgical waiting areas, and intensive care units. Each hospital’s local PFPC did a “walk-about”9 to make enhancements to physical signage, such as removing paper and overcrowded signs, adjusting negative language, ensuring alignment with brand guidelines, and including language that welcomed families. As a result of the team’s efforts around signage, collaboration began with the health system’s signage committee to help standardize signage terminology to reflect family inclusiveness, and to implement the recommendation for a standardized signage shared-governance team to ensure accountability and a patient- and family-centered structure.
Sustainment
Since implementing Better Together, Northwell has been able to infuse a more patient- and family-centered emphasis into its overall patient experience message of “Every role, every person, every moment matters.” As a strategic tool aimed at encouraging leaders, clinicians, and staff to pause and reflect about the “heart” of their work, patient and family stories are now included at the beginning of meetings, forums, and team huddles. Elements of the initiative have been integrated in current Patient and Family Partnership sustainment plans at participating hospitals. Some highlights include continued integration of patient/family partners on committees and councils that impact areas such as way finding, signage, recruitment, new-hire orientation, and community outreach; focus on enhancing partner retention and development programs; and inclusion of patient- and family-centered care and Better Together principles in ongoing leadership meetings.
Factors Contributing to Success
Health care is a complex, regulated, and often bureaucratic world that can be very difficult for patients and families to navigate. The system’s partnership with the Better Together Learning Community for Hospitals in New York State enhanced its efforts to improve family presence and participation and created powerful synergy. The success of this partnership was based on a number of important factors:
A solid foundation of support, structure, and accountability. The OPCE initiated the IPFCC Better Together partnership and established a synergistic collaboration inclusive of leadership, frontline teams, multiple departments, and patient and family partners. As a major strategic component of Northwell’s mission to deliver high-quality, patient- and family-centered care, OPCE was instrumental in connecting key areas and stakeholders and mobilizing the recommendations coming from patients and families.
A visible commitment of leadership at all levels. Partnering with leadership across Northwell’s system required a delineated vision, clear purpose and ownership, and comprehensive implementation and sustainment strategies. The existing format of Northwell’s PFPC provided the structure and framework needed for engaged patient and family input; the OPCE motivated and organized key areas of involvement and led communication efforts across the organization. The IPFCC coaching calls provided the underlying guidance and accountability needed to sustain momentum. As leadership and frontline teams became aware of the vision, they understood the larger connection to the system’s purpose, which ultimately created a clear path for positive change.
Meaningful involvement and input of patient and family partners. Throughout this project, Northwell’s patient/family partners were involved through the PFPC and local councils. For example, patient/family partners attended every IPFCC coaching call; members had a central voice in every decision made within each SWT; and local PFPCs actively participated in physical signage “walk-abouts” across facilities, making key recommendations for improvement. This multifaceted, supportive collaboration created a rejuvenated and purposeful focus for all council members involved. Some of their reactions include, “…I am so happy to be able to help other families in crisis, so that they don’t have to be alone, like I was,” and “I feel how important the patient and family’s voice is … it’s truly a partnership between patients, families, and staff.”
Regular access to IPFCC as a best practice coach and expert resource. Throughout the 10-month process, IPFCC’s Better Together Learning Community for Hospitals in New York State provided ongoing learning interventions for members of the SWT; multiple and varied resources from the Better Together toolkit for adaptation; and opportunities to share and reinforce new, learned expertise with colleagues within the Northwell Health system and beyond through IPFCC’s free online learning community, PFCC.Connect.
Conclusion
Family presence and participation are important to the quality, experience, safety, and outcomes of care. IPFCC’s campaign, Better Together: Partnering with Families, encourages hospitals to change restrictive visiting policies and, instead, to welcome families and caregivers 24 hours a day.
Two projects within Better Together involving almost 50 acute care hospitals in New York State confirm that change in policy, practice, and communication is particularly effective when implemented with strong support from leadership. An intervention like the Better Together Learning Community, offering structured training, coaching, and resources, can facilitate the change process.
Corresponding author: IPFCC, Deborah L. Dokken, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814; ddokken@ipfcc.org.
Funding disclosures: None.
1. Dokken DL, Kaufman J, Johnson BJ et al. Changing hospital visiting policies: from families as “visitors” to families as partners. J Clin Outcomes Manag. 2015; 22:29-36.
2. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. third edition: A pathway to improvement in New York City. New York: NYPIRG: 2018. www.nypirg.org/pubs/201801/NYPIRG_SICK_SCARED_FINAL.pdf. Accessed December 12, 2019.
3. Institute for Patient- and Family-Centered Care. Better Together: Partnering with Families. www.ipfcc.org/bestpractices/better-together.html. Accessed December 12, 2019.
4. Canadian Foundation for Healthcare Improvement. Better Together. www.cfhi-fcass.ca/WhatWeDo/better-together. Accessed December 12, 2019.
5. Canadian Foundation for Healthcare Improvement. Better Together: A change package to support the adoption of family presence and participation in acute care hospitals and accelerate healthcare improvement. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/better-together-change-package.pdf?sfvrsn=9656d044_4. Accessed December 12, 2019.
6. Canadian Foundation for Healthcare Improvement. L’Objectif santé: main dans la main avec les familles. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/families-pocket-screen_fr.pdf. Accessed December 12, 2019.
7. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. fourth edition: A pathway to improvement in New York. New York: NYPIRG: 2019. www.nypirg.org/pubs/201911/Sick_Scared_Separated_2019_web_FINAL.pdf. Accessed December 12, 2019.
8. Northwell Health. Patient and Family Partnership Councils. www.northwell.edu/about/commitment-to-excellence/patient-and-customer-experience/care-delivery-hospitality. Accessed December 12, 2019.
9 . Institute for Patient- and Family-Centered Care. How to conduct a “walk-about” from the patient and family perspective. www.ipfcc.org/resources/How_To_Conduct_A_Walk-About.pdf. Accessed December 12, 2019.
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken and Ms. Johnson), and Northwell Health, New Hyde Park, NY (Dr. Barden, Ms. Tuomey, and Ms. Giammarinaro).
Abstract
Objective: To describe the growth of Better Together: Partnering with Families, a campaign launched in 2014 to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care, and to discuss the processes involved in implementing the initiative in a large, integrated health system.
Methods: Descriptive report.
Results: In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the Better Together campaign to emphasize the importance of family presence and participation to the quality, experience, safety, and outcomes of care. Since then, this initiative has expanded in both the United States and Canada. With support from 2 funders in the United States, special attention was focused on acute care hospitals across New York State. Nearly 50 hospitals participated in 2 separate but related projects. Fifteen of the hospitals are part of Northwell Health, New York State’s largest health system. Over a 10-month period, these hospitals made significant progress in changing policy, practice, and communication to support family presence.
Conclusion: The Better Together initiative was implemented across a health system with strong support from leadership and the involvement of patient and family advisors. An intervention offering structured training, coaching, and resources, like IPFCC’s Better Together initiative, can facilitate the change process.
Keywords: family presence; visiting policies; patient-centered care; family-centered care; patient experience.
The presence of families at the bedside of patients is often restricted by hospital visiting hours. Hospitals that maintain these restrictive policies cite concerns about negative impacts on security, infection control, privacy, and staff workload. But there are no data to support these concerns, and the experience of hospitals that have successfully changed policy and practice to welcome families demonstrates the potential positive impacts of less restrictive policies on patient care and outcomes.1 For example, hospitalization can lead to reduced cognitive function in elderly patients. Family members would recognize the changes and could provide valuable information to hospital staff, potentially improving outcomes.2
In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families.3 The campaign is is grounded in patient- and family- centered care, an approach to care that supports partnerships among health care providers, patients, and families, and, among other core principles, advocates that patients define their “families” and how they will participate in care and decision-making.
Emphasizing the importance of family presence and participation to quality and safety, the Better Together campaign seeks to eliminate restrictive visiting policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference. As part of the campaign, IPFCC developed an extensive toolkit of resources that is available to hospitals and other organizations at no cost. The resources include sample policies; profiles of hospitals that have implemented family presence policies; educational materials for staff, patients, and families; and a template for hospital websites. This article, a follow-up to an article published in the January 2015 issue of JCOM,1 discusses the growth of the Better Together initiative as well as the processes involved in implementing the initiative across a large health system.
Growth of the Initiative
Since its launch in 2014, the Better Together initiative has continued to expand in the United States and Canada. In Canada, under the leadership of the Canadian Foundation for Healthcare Improvement (CFHI), more than 50 organizations have made a commitment to the Better Together program and family presence.4 Utilizing and adapting IPFCC’s Toolkit, CFHI developed a change package of free resources for Canadian organizations.5 Some of the materials, including the Pocket Guide for Families (Manuel des Familles), were translated into French.6
With support from 2 funders in the United States, the United Hospital Fund and the New York State Health (NYSHealth) Foundation, through a subcontract with the New York Public Interest Research Group (NYPIRG), IPFCC has been able to focus on hospitals in New York City, including public hospitals, and, more broadly, acute care hospitals across New York State. Nearly 50 hospitals participated in these 2 separate but related projects.
Education and Support for New York City Hospitals
Supported by the United Hospital Fund, an 18-month project that focused specifically on New York City hospitals was completed in June 2017. The project began with a 1-day intensive training event with representatives of 21 hospitals. Eighteen of those hospitals were eligible to participate in follow-up consultation provided by IPFCC, and 14 participated in some kind of follow-up. NYC Health + Hospitals (H+H), the system of public hospitals in NYC, participated most fully in these activities.
The outcomes of the Better Together initiative in New York City are summarized in the report Sick, Scared, & Separated From Loved Ones,2 which is based on a pre/post review of hospital visitation/family presence policies and website communications. According to the report, hospitals that participated in the IPFCC training and consultation program performed better, as a group, with respect to improved policy and website scores on post review than those that did not. Of the 10 hospitals whose scores improved during the review period, 8 had participated in the IPFCC training and 1 hospital was part of a hospital network that did so. (Six of these hospitals are part of the H+H public hospital system.) Those 9 hospitals saw an average increase in scores of 4.9 points (out of a possible 11).
A Learning Community for Hospitals in New York State
With support from the NYSHealth Foundation, IPFCC again collaborated with NYPIRG and New Yorkers for Patient & Family Empowerment on a 2-year initiative, completed in November 2019, that involved 26 hospitals: 15 from Northwell Health, New York State’s largest health system, and 11 hospitals from health systems throughout the state (Greater Hudson Valley Health System, now Garnet Health; Mohawk Valley Health System; Rochester Regional Health; and University of Vermont Health Network). An update of the report Sick, Scared, & Separated From Loved Onescompared pre/post reviews of policies and website communications regarding hospital visitation/family presence.7 Its findings confirm that hospitals that participated in the Better Together Learning Community improved both their policy and website scores to a greater degree than hospitals that did not participate and that a planned intervention can help facilitate change.
During the survey period, 28 out of 40 hospitals’ website navigability scores improved. Of those, hospitals that did not participate in the Better Together Learning Community saw an average increase in scores of 1.2 points, out of a possible 11, while the participating hospitals saw an average increase of 2.7 points, with the top 5 largest increases in scores belonging to hospitals that participated in the Better Together Learning Community.7
The Northwell Health Experience
Northwell Health is a large integrated health care organization comprising more than 69,000 employees, 23 hospitals, and more than 750 medical practices, located geographically across New York State. Embracing patient- and family-centered care, Northwell is dedicated to improving the quality, experience, and safety of care for patients and their families. Welcoming and including patients, families, and care partners as members of the health care team has always been a core element of Northwell’s organizational goal of providing world-class patient care and experience.
Four years ago, the organization reorganized and formalized a system-wide Patient & Family Partnership Council (PFPC).8 Representatives on the PFPC include a Northwell patient experience leader and patient/family co-chair from local councils that have been established in nearly all 23 hospitals as well as service lines. Modeling partnership, the PFPC is grounded in listening to the “voice” of patients and families and promoting collaboration, with the goal of driving change across varied aspects and experiences of health care delivery.
Through the Office of Patient and Customer Experience (OPCE), a partnership with IPFCC and the Better Together Learning Community for Hospitals in New York State was initiated as a fundamental next step in Northwell’s journey to enhance system-wide family presence and participation. Results from Better Together’s Organizational Self-Assessment Tool and process identified opportunities to influence 3 distinct areas: policy/staff education, position descriptions/performance management, and website/signage. Over a 10-month period (September 2018 through June 2019), 15 Northwell hospitals implemened significant patient- and family-centered improvements through multifaceted shared work teams (SWT) that partnered around the common goal of supporting the patient and family experience (Figure). Northwell’s SWT structure allowed teams to meet individually on specific tasks, led by a dedicated staff member of the OPCE to ensure progress, support, and accountability. Six monthly coaching calls or report-out meetings were attended by participating teams, where feedback and recommendations shared by IPFCC were discussed in order to maintain momentum and results.
Policy/Staff Education
The policy/staff education SWT focused on appraising and updating existing policies to ensure alignment with key patient- and family-centered concepts and Better Together principles (Table 1). By establishing representation on the System Policy and Procedure Committee, OPCE enabled patients and families to have a voice at the decision-making table. OPCE leaders presented the ideology and scope of the transformation to this committee. After reviewing all system-wide policies, 4 were identified as key opportunities for revision. One overarching policy titled “Visitation Guidelines” was reviewed and updated to reflect Northwell’s mission of patient- and family-centered care, retiring the reference to “families” as “visitors” in definitions, incorporating language of inclusion and partnership, and citing other related policies. The policy was vetted through a multilayer process of review and stakeholder feedback and was ultimately approved at a system
Three additional related policies were also updated to reflect core principles of inclusion and partnership. These included system policies focused on discharge planning; identification of health care proxy, agent, support person and caregiver; and standards of behavior not conducive in a health care setting. As a result of this work, OPCE was invited to remain an active member of the System Policy and Procedure Committee, adding meaningful new perspectives to the clinical and administrative policy management process. Once policies were updated and approved, the SWT focused on educating leaders and teams. Using a diversified strategy, education was provided through various modes, including weekly system-wide internal communication channels, patient experience huddle messages, yearly mandatory topics training, and the incorporation of essential concepts in existing educational courses (classroom and e-learning modalities).
Position Descriptions/Performance Management
The position descriptions/performance management SWT focused its efforts on incorporating patient- and family-centered concepts and language into position descriptions and the performance appraisal process (Table 2). Due to the complex nature of this work, the process required collaboration from key subject matter experts in human resources, talent management, corporate compensation, and labor management. In 2019, Northwell began an initiative focused on streamlining and standardizing job titles, roles, and developmental pathways across the system. The overarching goal was to create system-wide consistency and standardization. The SWT was successful in advising the leaders overseeing this job architecture initiative on the importance of including language of patient- and family-centered care, like partnership and collaboration, and of highlighting the critical role of family members as part of the care team in subsequent documents.
Northwell has 6 behavioral expectations, standards to which all team members are held accountable: Patient/Customer Focus, Teamwork, Execution, Organizational Awareness, Enable Change, and Develop Self. As a result of the SWT’s work, Patient/Customer Focus was revised to include “families” as essential care partners, demonstrating Northwell’s ongoing commitment to honoring the role of families as members of the care team. It also ensures that all employees are aligned around this priority, as these expectations are utilized to support areas such as recognition and performance. Collaborating with talent management and organizational development, the SWT reviewed yearly performance management and new-hire evaluations. In doing so, they identified an opportunity to refresh the anchored qualitative rating scales to include behavioral demonstrations of patient- and family-centered care, collaboration, respect, and partnership with family members.
Website/Signage
Websites make an important first impression on patients and families looking for information to best prepare for a hospital experience. Therefore, the website/signage SWT worked to redesign hospital websites, enhance digital signage, and perform a baseline assessment of physical signage across facilities. Initial feedback on Northwell’s websites identified opportunities to include more patient- and family-centered, care-partner-infused language; improve navigation; and streamline click levels for easier access. Content for the websites was carefully crafted in collaboration with Northwell’s internal web team, utilizing IPFCC’s best practice standards as a framework and guide.
Next, a multidisciplinary website shared-governance team was established by the OPCE to ensure that key stakeholders were represented and had the opportunity to review and make recommendations for appropriate language and messaging about family presence and participation. This 13-person team was comprised of patient/family partners, patient-experience culture leaders, quality, compliance, human resources, policy, a chief nursing officer, a medical director, and representation from the Institute for Nursing. After careful review and consideration from Northwell’s family partners and teams, all participating hospital websites were enhanced as of June 2019 to include prominent 1-click access from homepages to information for “patients, families and visitors,” as well as “your care partners” information on the important role of families and care partners.
Along with refreshing websites, another step in Northwell’s work to strengthen messaging about family presence and participation was to partner and collaborate with the system’s digital web team as well as local facility councils to understand the capacity to adjust digital signage across facilities. Opportunities were found to make simple yet effective enhancements to the language and imagery of digital signage upon entry, creating a warmer and more welcoming first impression for patients and families. With patient and family partner feedback, the team designed digital signage with inclusive messaging and images that would circulate appropriately based on the facility. Signage specifically welcomes families and refers to them as members of patients’ care teams.
Northwell’s website/signage SWT also directed a 2-phase physical signage assessment to determine ongoing opportunities to alter signs in areas that particularly impact patients and families, such as emergency departments, main lobbies, cafeterias, surgical waiting areas, and intensive care units. Each hospital’s local PFPC did a “walk-about”9 to make enhancements to physical signage, such as removing paper and overcrowded signs, adjusting negative language, ensuring alignment with brand guidelines, and including language that welcomed families. As a result of the team’s efforts around signage, collaboration began with the health system’s signage committee to help standardize signage terminology to reflect family inclusiveness, and to implement the recommendation for a standardized signage shared-governance team to ensure accountability and a patient- and family-centered structure.
Sustainment
Since implementing Better Together, Northwell has been able to infuse a more patient- and family-centered emphasis into its overall patient experience message of “Every role, every person, every moment matters.” As a strategic tool aimed at encouraging leaders, clinicians, and staff to pause and reflect about the “heart” of their work, patient and family stories are now included at the beginning of meetings, forums, and team huddles. Elements of the initiative have been integrated in current Patient and Family Partnership sustainment plans at participating hospitals. Some highlights include continued integration of patient/family partners on committees and councils that impact areas such as way finding, signage, recruitment, new-hire orientation, and community outreach; focus on enhancing partner retention and development programs; and inclusion of patient- and family-centered care and Better Together principles in ongoing leadership meetings.
Factors Contributing to Success
Health care is a complex, regulated, and often bureaucratic world that can be very difficult for patients and families to navigate. The system’s partnership with the Better Together Learning Community for Hospitals in New York State enhanced its efforts to improve family presence and participation and created powerful synergy. The success of this partnership was based on a number of important factors:
A solid foundation of support, structure, and accountability. The OPCE initiated the IPFCC Better Together partnership and established a synergistic collaboration inclusive of leadership, frontline teams, multiple departments, and patient and family partners. As a major strategic component of Northwell’s mission to deliver high-quality, patient- and family-centered care, OPCE was instrumental in connecting key areas and stakeholders and mobilizing the recommendations coming from patients and families.
A visible commitment of leadership at all levels. Partnering with leadership across Northwell’s system required a delineated vision, clear purpose and ownership, and comprehensive implementation and sustainment strategies. The existing format of Northwell’s PFPC provided the structure and framework needed for engaged patient and family input; the OPCE motivated and organized key areas of involvement and led communication efforts across the organization. The IPFCC coaching calls provided the underlying guidance and accountability needed to sustain momentum. As leadership and frontline teams became aware of the vision, they understood the larger connection to the system’s purpose, which ultimately created a clear path for positive change.
Meaningful involvement and input of patient and family partners. Throughout this project, Northwell’s patient/family partners were involved through the PFPC and local councils. For example, patient/family partners attended every IPFCC coaching call; members had a central voice in every decision made within each SWT; and local PFPCs actively participated in physical signage “walk-abouts” across facilities, making key recommendations for improvement. This multifaceted, supportive collaboration created a rejuvenated and purposeful focus for all council members involved. Some of their reactions include, “…I am so happy to be able to help other families in crisis, so that they don’t have to be alone, like I was,” and “I feel how important the patient and family’s voice is … it’s truly a partnership between patients, families, and staff.”
Regular access to IPFCC as a best practice coach and expert resource. Throughout the 10-month process, IPFCC’s Better Together Learning Community for Hospitals in New York State provided ongoing learning interventions for members of the SWT; multiple and varied resources from the Better Together toolkit for adaptation; and opportunities to share and reinforce new, learned expertise with colleagues within the Northwell Health system and beyond through IPFCC’s free online learning community, PFCC.Connect.
Conclusion
Family presence and participation are important to the quality, experience, safety, and outcomes of care. IPFCC’s campaign, Better Together: Partnering with Families, encourages hospitals to change restrictive visiting policies and, instead, to welcome families and caregivers 24 hours a day.
Two projects within Better Together involving almost 50 acute care hospitals in New York State confirm that change in policy, practice, and communication is particularly effective when implemented with strong support from leadership. An intervention like the Better Together Learning Community, offering structured training, coaching, and resources, can facilitate the change process.
Corresponding author: IPFCC, Deborah L. Dokken, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814; ddokken@ipfcc.org.
Funding disclosures: None.
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken and Ms. Johnson), and Northwell Health, New Hyde Park, NY (Dr. Barden, Ms. Tuomey, and Ms. Giammarinaro).
Abstract
Objective: To describe the growth of Better Together: Partnering with Families, a campaign launched in 2014 to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care, and to discuss the processes involved in implementing the initiative in a large, integrated health system.
Methods: Descriptive report.
Results: In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the Better Together campaign to emphasize the importance of family presence and participation to the quality, experience, safety, and outcomes of care. Since then, this initiative has expanded in both the United States and Canada. With support from 2 funders in the United States, special attention was focused on acute care hospitals across New York State. Nearly 50 hospitals participated in 2 separate but related projects. Fifteen of the hospitals are part of Northwell Health, New York State’s largest health system. Over a 10-month period, these hospitals made significant progress in changing policy, practice, and communication to support family presence.
Conclusion: The Better Together initiative was implemented across a health system with strong support from leadership and the involvement of patient and family advisors. An intervention offering structured training, coaching, and resources, like IPFCC’s Better Together initiative, can facilitate the change process.
Keywords: family presence; visiting policies; patient-centered care; family-centered care; patient experience.
The presence of families at the bedside of patients is often restricted by hospital visiting hours. Hospitals that maintain these restrictive policies cite concerns about negative impacts on security, infection control, privacy, and staff workload. But there are no data to support these concerns, and the experience of hospitals that have successfully changed policy and practice to welcome families demonstrates the potential positive impacts of less restrictive policies on patient care and outcomes.1 For example, hospitalization can lead to reduced cognitive function in elderly patients. Family members would recognize the changes and could provide valuable information to hospital staff, potentially improving outcomes.2
In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families.3 The campaign is is grounded in patient- and family- centered care, an approach to care that supports partnerships among health care providers, patients, and families, and, among other core principles, advocates that patients define their “families” and how they will participate in care and decision-making.
Emphasizing the importance of family presence and participation to quality and safety, the Better Together campaign seeks to eliminate restrictive visiting policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference. As part of the campaign, IPFCC developed an extensive toolkit of resources that is available to hospitals and other organizations at no cost. The resources include sample policies; profiles of hospitals that have implemented family presence policies; educational materials for staff, patients, and families; and a template for hospital websites. This article, a follow-up to an article published in the January 2015 issue of JCOM,1 discusses the growth of the Better Together initiative as well as the processes involved in implementing the initiative across a large health system.
Growth of the Initiative
Since its launch in 2014, the Better Together initiative has continued to expand in the United States and Canada. In Canada, under the leadership of the Canadian Foundation for Healthcare Improvement (CFHI), more than 50 organizations have made a commitment to the Better Together program and family presence.4 Utilizing and adapting IPFCC’s Toolkit, CFHI developed a change package of free resources for Canadian organizations.5 Some of the materials, including the Pocket Guide for Families (Manuel des Familles), were translated into French.6
With support from 2 funders in the United States, the United Hospital Fund and the New York State Health (NYSHealth) Foundation, through a subcontract with the New York Public Interest Research Group (NYPIRG), IPFCC has been able to focus on hospitals in New York City, including public hospitals, and, more broadly, acute care hospitals across New York State. Nearly 50 hospitals participated in these 2 separate but related projects.
Education and Support for New York City Hospitals
Supported by the United Hospital Fund, an 18-month project that focused specifically on New York City hospitals was completed in June 2017. The project began with a 1-day intensive training event with representatives of 21 hospitals. Eighteen of those hospitals were eligible to participate in follow-up consultation provided by IPFCC, and 14 participated in some kind of follow-up. NYC Health + Hospitals (H+H), the system of public hospitals in NYC, participated most fully in these activities.
The outcomes of the Better Together initiative in New York City are summarized in the report Sick, Scared, & Separated From Loved Ones,2 which is based on a pre/post review of hospital visitation/family presence policies and website communications. According to the report, hospitals that participated in the IPFCC training and consultation program performed better, as a group, with respect to improved policy and website scores on post review than those that did not. Of the 10 hospitals whose scores improved during the review period, 8 had participated in the IPFCC training and 1 hospital was part of a hospital network that did so. (Six of these hospitals are part of the H+H public hospital system.) Those 9 hospitals saw an average increase in scores of 4.9 points (out of a possible 11).
A Learning Community for Hospitals in New York State
With support from the NYSHealth Foundation, IPFCC again collaborated with NYPIRG and New Yorkers for Patient & Family Empowerment on a 2-year initiative, completed in November 2019, that involved 26 hospitals: 15 from Northwell Health, New York State’s largest health system, and 11 hospitals from health systems throughout the state (Greater Hudson Valley Health System, now Garnet Health; Mohawk Valley Health System; Rochester Regional Health; and University of Vermont Health Network). An update of the report Sick, Scared, & Separated From Loved Onescompared pre/post reviews of policies and website communications regarding hospital visitation/family presence.7 Its findings confirm that hospitals that participated in the Better Together Learning Community improved both their policy and website scores to a greater degree than hospitals that did not participate and that a planned intervention can help facilitate change.
During the survey period, 28 out of 40 hospitals’ website navigability scores improved. Of those, hospitals that did not participate in the Better Together Learning Community saw an average increase in scores of 1.2 points, out of a possible 11, while the participating hospitals saw an average increase of 2.7 points, with the top 5 largest increases in scores belonging to hospitals that participated in the Better Together Learning Community.7
The Northwell Health Experience
Northwell Health is a large integrated health care organization comprising more than 69,000 employees, 23 hospitals, and more than 750 medical practices, located geographically across New York State. Embracing patient- and family-centered care, Northwell is dedicated to improving the quality, experience, and safety of care for patients and their families. Welcoming and including patients, families, and care partners as members of the health care team has always been a core element of Northwell’s organizational goal of providing world-class patient care and experience.
Four years ago, the organization reorganized and formalized a system-wide Patient & Family Partnership Council (PFPC).8 Representatives on the PFPC include a Northwell patient experience leader and patient/family co-chair from local councils that have been established in nearly all 23 hospitals as well as service lines. Modeling partnership, the PFPC is grounded in listening to the “voice” of patients and families and promoting collaboration, with the goal of driving change across varied aspects and experiences of health care delivery.
Through the Office of Patient and Customer Experience (OPCE), a partnership with IPFCC and the Better Together Learning Community for Hospitals in New York State was initiated as a fundamental next step in Northwell’s journey to enhance system-wide family presence and participation. Results from Better Together’s Organizational Self-Assessment Tool and process identified opportunities to influence 3 distinct areas: policy/staff education, position descriptions/performance management, and website/signage. Over a 10-month period (September 2018 through June 2019), 15 Northwell hospitals implemened significant patient- and family-centered improvements through multifaceted shared work teams (SWT) that partnered around the common goal of supporting the patient and family experience (Figure). Northwell’s SWT structure allowed teams to meet individually on specific tasks, led by a dedicated staff member of the OPCE to ensure progress, support, and accountability. Six monthly coaching calls or report-out meetings were attended by participating teams, where feedback and recommendations shared by IPFCC were discussed in order to maintain momentum and results.
Policy/Staff Education
The policy/staff education SWT focused on appraising and updating existing policies to ensure alignment with key patient- and family-centered concepts and Better Together principles (Table 1). By establishing representation on the System Policy and Procedure Committee, OPCE enabled patients and families to have a voice at the decision-making table. OPCE leaders presented the ideology and scope of the transformation to this committee. After reviewing all system-wide policies, 4 were identified as key opportunities for revision. One overarching policy titled “Visitation Guidelines” was reviewed and updated to reflect Northwell’s mission of patient- and family-centered care, retiring the reference to “families” as “visitors” in definitions, incorporating language of inclusion and partnership, and citing other related policies. The policy was vetted through a multilayer process of review and stakeholder feedback and was ultimately approved at a system
Three additional related policies were also updated to reflect core principles of inclusion and partnership. These included system policies focused on discharge planning; identification of health care proxy, agent, support person and caregiver; and standards of behavior not conducive in a health care setting. As a result of this work, OPCE was invited to remain an active member of the System Policy and Procedure Committee, adding meaningful new perspectives to the clinical and administrative policy management process. Once policies were updated and approved, the SWT focused on educating leaders and teams. Using a diversified strategy, education was provided through various modes, including weekly system-wide internal communication channels, patient experience huddle messages, yearly mandatory topics training, and the incorporation of essential concepts in existing educational courses (classroom and e-learning modalities).
Position Descriptions/Performance Management
The position descriptions/performance management SWT focused its efforts on incorporating patient- and family-centered concepts and language into position descriptions and the performance appraisal process (Table 2). Due to the complex nature of this work, the process required collaboration from key subject matter experts in human resources, talent management, corporate compensation, and labor management. In 2019, Northwell began an initiative focused on streamlining and standardizing job titles, roles, and developmental pathways across the system. The overarching goal was to create system-wide consistency and standardization. The SWT was successful in advising the leaders overseeing this job architecture initiative on the importance of including language of patient- and family-centered care, like partnership and collaboration, and of highlighting the critical role of family members as part of the care team in subsequent documents.
Northwell has 6 behavioral expectations, standards to which all team members are held accountable: Patient/Customer Focus, Teamwork, Execution, Organizational Awareness, Enable Change, and Develop Self. As a result of the SWT’s work, Patient/Customer Focus was revised to include “families” as essential care partners, demonstrating Northwell’s ongoing commitment to honoring the role of families as members of the care team. It also ensures that all employees are aligned around this priority, as these expectations are utilized to support areas such as recognition and performance. Collaborating with talent management and organizational development, the SWT reviewed yearly performance management and new-hire evaluations. In doing so, they identified an opportunity to refresh the anchored qualitative rating scales to include behavioral demonstrations of patient- and family-centered care, collaboration, respect, and partnership with family members.
Website/Signage
Websites make an important first impression on patients and families looking for information to best prepare for a hospital experience. Therefore, the website/signage SWT worked to redesign hospital websites, enhance digital signage, and perform a baseline assessment of physical signage across facilities. Initial feedback on Northwell’s websites identified opportunities to include more patient- and family-centered, care-partner-infused language; improve navigation; and streamline click levels for easier access. Content for the websites was carefully crafted in collaboration with Northwell’s internal web team, utilizing IPFCC’s best practice standards as a framework and guide.
Next, a multidisciplinary website shared-governance team was established by the OPCE to ensure that key stakeholders were represented and had the opportunity to review and make recommendations for appropriate language and messaging about family presence and participation. This 13-person team was comprised of patient/family partners, patient-experience culture leaders, quality, compliance, human resources, policy, a chief nursing officer, a medical director, and representation from the Institute for Nursing. After careful review and consideration from Northwell’s family partners and teams, all participating hospital websites were enhanced as of June 2019 to include prominent 1-click access from homepages to information for “patients, families and visitors,” as well as “your care partners” information on the important role of families and care partners.
Along with refreshing websites, another step in Northwell’s work to strengthen messaging about family presence and participation was to partner and collaborate with the system’s digital web team as well as local facility councils to understand the capacity to adjust digital signage across facilities. Opportunities were found to make simple yet effective enhancements to the language and imagery of digital signage upon entry, creating a warmer and more welcoming first impression for patients and families. With patient and family partner feedback, the team designed digital signage with inclusive messaging and images that would circulate appropriately based on the facility. Signage specifically welcomes families and refers to them as members of patients’ care teams.
Northwell’s website/signage SWT also directed a 2-phase physical signage assessment to determine ongoing opportunities to alter signs in areas that particularly impact patients and families, such as emergency departments, main lobbies, cafeterias, surgical waiting areas, and intensive care units. Each hospital’s local PFPC did a “walk-about”9 to make enhancements to physical signage, such as removing paper and overcrowded signs, adjusting negative language, ensuring alignment with brand guidelines, and including language that welcomed families. As a result of the team’s efforts around signage, collaboration began with the health system’s signage committee to help standardize signage terminology to reflect family inclusiveness, and to implement the recommendation for a standardized signage shared-governance team to ensure accountability and a patient- and family-centered structure.
Sustainment
Since implementing Better Together, Northwell has been able to infuse a more patient- and family-centered emphasis into its overall patient experience message of “Every role, every person, every moment matters.” As a strategic tool aimed at encouraging leaders, clinicians, and staff to pause and reflect about the “heart” of their work, patient and family stories are now included at the beginning of meetings, forums, and team huddles. Elements of the initiative have been integrated in current Patient and Family Partnership sustainment plans at participating hospitals. Some highlights include continued integration of patient/family partners on committees and councils that impact areas such as way finding, signage, recruitment, new-hire orientation, and community outreach; focus on enhancing partner retention and development programs; and inclusion of patient- and family-centered care and Better Together principles in ongoing leadership meetings.
Factors Contributing to Success
Health care is a complex, regulated, and often bureaucratic world that can be very difficult for patients and families to navigate. The system’s partnership with the Better Together Learning Community for Hospitals in New York State enhanced its efforts to improve family presence and participation and created powerful synergy. The success of this partnership was based on a number of important factors:
A solid foundation of support, structure, and accountability. The OPCE initiated the IPFCC Better Together partnership and established a synergistic collaboration inclusive of leadership, frontline teams, multiple departments, and patient and family partners. As a major strategic component of Northwell’s mission to deliver high-quality, patient- and family-centered care, OPCE was instrumental in connecting key areas and stakeholders and mobilizing the recommendations coming from patients and families.
A visible commitment of leadership at all levels. Partnering with leadership across Northwell’s system required a delineated vision, clear purpose and ownership, and comprehensive implementation and sustainment strategies. The existing format of Northwell’s PFPC provided the structure and framework needed for engaged patient and family input; the OPCE motivated and organized key areas of involvement and led communication efforts across the organization. The IPFCC coaching calls provided the underlying guidance and accountability needed to sustain momentum. As leadership and frontline teams became aware of the vision, they understood the larger connection to the system’s purpose, which ultimately created a clear path for positive change.
Meaningful involvement and input of patient and family partners. Throughout this project, Northwell’s patient/family partners were involved through the PFPC and local councils. For example, patient/family partners attended every IPFCC coaching call; members had a central voice in every decision made within each SWT; and local PFPCs actively participated in physical signage “walk-abouts” across facilities, making key recommendations for improvement. This multifaceted, supportive collaboration created a rejuvenated and purposeful focus for all council members involved. Some of their reactions include, “…I am so happy to be able to help other families in crisis, so that they don’t have to be alone, like I was,” and “I feel how important the patient and family’s voice is … it’s truly a partnership between patients, families, and staff.”
Regular access to IPFCC as a best practice coach and expert resource. Throughout the 10-month process, IPFCC’s Better Together Learning Community for Hospitals in New York State provided ongoing learning interventions for members of the SWT; multiple and varied resources from the Better Together toolkit for adaptation; and opportunities to share and reinforce new, learned expertise with colleagues within the Northwell Health system and beyond through IPFCC’s free online learning community, PFCC.Connect.
Conclusion
Family presence and participation are important to the quality, experience, safety, and outcomes of care. IPFCC’s campaign, Better Together: Partnering with Families, encourages hospitals to change restrictive visiting policies and, instead, to welcome families and caregivers 24 hours a day.
Two projects within Better Together involving almost 50 acute care hospitals in New York State confirm that change in policy, practice, and communication is particularly effective when implemented with strong support from leadership. An intervention like the Better Together Learning Community, offering structured training, coaching, and resources, can facilitate the change process.
Corresponding author: IPFCC, Deborah L. Dokken, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814; ddokken@ipfcc.org.
Funding disclosures: None.
1. Dokken DL, Kaufman J, Johnson BJ et al. Changing hospital visiting policies: from families as “visitors” to families as partners. J Clin Outcomes Manag. 2015; 22:29-36.
2. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. third edition: A pathway to improvement in New York City. New York: NYPIRG: 2018. www.nypirg.org/pubs/201801/NYPIRG_SICK_SCARED_FINAL.pdf. Accessed December 12, 2019.
3. Institute for Patient- and Family-Centered Care. Better Together: Partnering with Families. www.ipfcc.org/bestpractices/better-together.html. Accessed December 12, 2019.
4. Canadian Foundation for Healthcare Improvement. Better Together. www.cfhi-fcass.ca/WhatWeDo/better-together. Accessed December 12, 2019.
5. Canadian Foundation for Healthcare Improvement. Better Together: A change package to support the adoption of family presence and participation in acute care hospitals and accelerate healthcare improvement. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/better-together-change-package.pdf?sfvrsn=9656d044_4. Accessed December 12, 2019.
6. Canadian Foundation for Healthcare Improvement. L’Objectif santé: main dans la main avec les familles. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/families-pocket-screen_fr.pdf. Accessed December 12, 2019.
7. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. fourth edition: A pathway to improvement in New York. New York: NYPIRG: 2019. www.nypirg.org/pubs/201911/Sick_Scared_Separated_2019_web_FINAL.pdf. Accessed December 12, 2019.
8. Northwell Health. Patient and Family Partnership Councils. www.northwell.edu/about/commitment-to-excellence/patient-and-customer-experience/care-delivery-hospitality. Accessed December 12, 2019.
9 . Institute for Patient- and Family-Centered Care. How to conduct a “walk-about” from the patient and family perspective. www.ipfcc.org/resources/How_To_Conduct_A_Walk-About.pdf. Accessed December 12, 2019.
1. Dokken DL, Kaufman J, Johnson BJ et al. Changing hospital visiting policies: from families as “visitors” to families as partners. J Clin Outcomes Manag. 2015; 22:29-36.
2. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. third edition: A pathway to improvement in New York City. New York: NYPIRG: 2018. www.nypirg.org/pubs/201801/NYPIRG_SICK_SCARED_FINAL.pdf. Accessed December 12, 2019.
3. Institute for Patient- and Family-Centered Care. Better Together: Partnering with Families. www.ipfcc.org/bestpractices/better-together.html. Accessed December 12, 2019.
4. Canadian Foundation for Healthcare Improvement. Better Together. www.cfhi-fcass.ca/WhatWeDo/better-together. Accessed December 12, 2019.
5. Canadian Foundation for Healthcare Improvement. Better Together: A change package to support the adoption of family presence and participation in acute care hospitals and accelerate healthcare improvement. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/better-together-change-package.pdf?sfvrsn=9656d044_4. Accessed December 12, 2019.
6. Canadian Foundation for Healthcare Improvement. L’Objectif santé: main dans la main avec les familles. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/families-pocket-screen_fr.pdf. Accessed December 12, 2019.
7. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. fourth edition: A pathway to improvement in New York. New York: NYPIRG: 2019. www.nypirg.org/pubs/201911/Sick_Scared_Separated_2019_web_FINAL.pdf. Accessed December 12, 2019.
8. Northwell Health. Patient and Family Partnership Councils. www.northwell.edu/about/commitment-to-excellence/patient-and-customer-experience/care-delivery-hospitality. Accessed December 12, 2019.
9 . Institute for Patient- and Family-Centered Care. How to conduct a “walk-about” from the patient and family perspective. www.ipfcc.org/resources/How_To_Conduct_A_Walk-About.pdf. Accessed December 12, 2019.
Improving Nephropathy Screening in Appalachian Patients With Diabetes Using Practice-Wide Outreach
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively.
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4).
Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.
Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.
Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; amashcraft@hsc.wvu.edu.
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively.
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4).
Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.
Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.
Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; amashcraft@hsc.wvu.edu.
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively.
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4).
Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.
Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.
Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; amashcraft@hsc.wvu.edu.
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.
Administrative data can help drive quality improvement in breast cancer care
Quality indicators of breast cancer care were successfully computed for more than 15,000 incident cases of breast cancer using electronic administrative databases in a project led by five regional oncology networks in Italy.
The project has shown that, despite some limitations in the use of administrative data to measure health care performance, “evaluating the quality of breast cancer care at a population level is possible,” investigators reported in the Journal of Oncology Practice.
The data obtained “from multiple administrative databases gathered in a real-world setting across five Italian regions” highlighted regional variations in breast cancer care and ways in which clinical guidelines were being overlooked, they wrote.
In doing so, the project confirmed that administrative data is “suitable” for measuring performance in health care and potentially useful for guiding quality improvement interventions. For instance, the project identified extensive use of blood tumor markers in breast cancer follow-up, wrote Valentina Guarneri, PhD, MD, of the University of Padova (Italy) and the Istituto Oncologico Veneto, also in Padova, and coauthors.
Oncologists and epidemiologists from the Italian regional oncology networks identified 46 clinically relevant indicators (9 structure indicators, 29 dealing with process, and 8 outcome indicators) by comparing pathways of care established by each network and identifying commonalities.
Of the 46 indicators, 22 were considered by the project leaders to be “potentially computable” from information retrieved by regional administrative databases. And of these 22 designed to be extractable, 9 (2 indicators of structure and 7 of process) were found to be actually evaluable for 15,342 cases of newly diagnosed invasive and/or in situ breast cancer diagnosed during 2016.
Blood tumor markers were tested in 44.2%-64.5% of patients in the first year after surgery – higher than the benchmark of 20% or less that was established to account for stage IV patients and other specific conditions in which markers might be indicated. National, international, and regional guidelines “discourage the use of blood tumor markers” in breast cancer follow-up, the investigators wrote.
The extensive use of these markers – observed across all five regions – is “a starting point to understanding how to improve clinical practice,” they added.
Other quality indicators that were evaluable included radiotherapy within 12 weeks after surgery if adjuvant chemotherapy is not administered (42%-83.8% in the project, compared with the benchmark of 90% or greater) and mammography 6-18 months after surgery (administered in 55.1%-72.6%, compared with the benchmark of 90% or greater), as well as the proportion of patients starting adjuvant systemic treatment (chemotherapy or endocrine therapy) within 60 days of surgery (for patients receiving systemic treatment).
To calculate the indicators, each regional cancer network used computerized sources of information including hospital discharge forms, outpatient records of diagnostic and therapeutic procedures, prescriptions of drugs reimbursed by the National Health Service in the hospital and outpatient settings, regional health registries, and the regional mortality registries.
All data used in the project came from regional repositories, which collect data from all National Health Service providers in the region, and not from single institutional repositories, the investigators noted.
More than half of the indicators expected to be assessable – but not found to be – were not computable as a result of data being unavailable (for example, pathology data) or incomplete, and as a result of data not being reliable for various reasons. The fact that examinations paid for directly by patients are not reported by the management systems of the National Health System was another complicating factor, they reported.
The authors disclosed funding and relationships with various pharmaceutical companies. The research was supported by the Periplo Association.
SOURCE: Guarneri V et al. J Oncol Pract. 2019 Dec 19. doi: 10.1200/JOP.19.00466.
Quality indicators of breast cancer care were successfully computed for more than 15,000 incident cases of breast cancer using electronic administrative databases in a project led by five regional oncology networks in Italy.
The project has shown that, despite some limitations in the use of administrative data to measure health care performance, “evaluating the quality of breast cancer care at a population level is possible,” investigators reported in the Journal of Oncology Practice.
The data obtained “from multiple administrative databases gathered in a real-world setting across five Italian regions” highlighted regional variations in breast cancer care and ways in which clinical guidelines were being overlooked, they wrote.
In doing so, the project confirmed that administrative data is “suitable” for measuring performance in health care and potentially useful for guiding quality improvement interventions. For instance, the project identified extensive use of blood tumor markers in breast cancer follow-up, wrote Valentina Guarneri, PhD, MD, of the University of Padova (Italy) and the Istituto Oncologico Veneto, also in Padova, and coauthors.
Oncologists and epidemiologists from the Italian regional oncology networks identified 46 clinically relevant indicators (9 structure indicators, 29 dealing with process, and 8 outcome indicators) by comparing pathways of care established by each network and identifying commonalities.
Of the 46 indicators, 22 were considered by the project leaders to be “potentially computable” from information retrieved by regional administrative databases. And of these 22 designed to be extractable, 9 (2 indicators of structure and 7 of process) were found to be actually evaluable for 15,342 cases of newly diagnosed invasive and/or in situ breast cancer diagnosed during 2016.
Blood tumor markers were tested in 44.2%-64.5% of patients in the first year after surgery – higher than the benchmark of 20% or less that was established to account for stage IV patients and other specific conditions in which markers might be indicated. National, international, and regional guidelines “discourage the use of blood tumor markers” in breast cancer follow-up, the investigators wrote.
The extensive use of these markers – observed across all five regions – is “a starting point to understanding how to improve clinical practice,” they added.
Other quality indicators that were evaluable included radiotherapy within 12 weeks after surgery if adjuvant chemotherapy is not administered (42%-83.8% in the project, compared with the benchmark of 90% or greater) and mammography 6-18 months after surgery (administered in 55.1%-72.6%, compared with the benchmark of 90% or greater), as well as the proportion of patients starting adjuvant systemic treatment (chemotherapy or endocrine therapy) within 60 days of surgery (for patients receiving systemic treatment).
To calculate the indicators, each regional cancer network used computerized sources of information including hospital discharge forms, outpatient records of diagnostic and therapeutic procedures, prescriptions of drugs reimbursed by the National Health Service in the hospital and outpatient settings, regional health registries, and the regional mortality registries.
All data used in the project came from regional repositories, which collect data from all National Health Service providers in the region, and not from single institutional repositories, the investigators noted.
More than half of the indicators expected to be assessable – but not found to be – were not computable as a result of data being unavailable (for example, pathology data) or incomplete, and as a result of data not being reliable for various reasons. The fact that examinations paid for directly by patients are not reported by the management systems of the National Health System was another complicating factor, they reported.
The authors disclosed funding and relationships with various pharmaceutical companies. The research was supported by the Periplo Association.
SOURCE: Guarneri V et al. J Oncol Pract. 2019 Dec 19. doi: 10.1200/JOP.19.00466.
Quality indicators of breast cancer care were successfully computed for more than 15,000 incident cases of breast cancer using electronic administrative databases in a project led by five regional oncology networks in Italy.
The project has shown that, despite some limitations in the use of administrative data to measure health care performance, “evaluating the quality of breast cancer care at a population level is possible,” investigators reported in the Journal of Oncology Practice.
The data obtained “from multiple administrative databases gathered in a real-world setting across five Italian regions” highlighted regional variations in breast cancer care and ways in which clinical guidelines were being overlooked, they wrote.
In doing so, the project confirmed that administrative data is “suitable” for measuring performance in health care and potentially useful for guiding quality improvement interventions. For instance, the project identified extensive use of blood tumor markers in breast cancer follow-up, wrote Valentina Guarneri, PhD, MD, of the University of Padova (Italy) and the Istituto Oncologico Veneto, also in Padova, and coauthors.
Oncologists and epidemiologists from the Italian regional oncology networks identified 46 clinically relevant indicators (9 structure indicators, 29 dealing with process, and 8 outcome indicators) by comparing pathways of care established by each network and identifying commonalities.
Of the 46 indicators, 22 were considered by the project leaders to be “potentially computable” from information retrieved by regional administrative databases. And of these 22 designed to be extractable, 9 (2 indicators of structure and 7 of process) were found to be actually evaluable for 15,342 cases of newly diagnosed invasive and/or in situ breast cancer diagnosed during 2016.
Blood tumor markers were tested in 44.2%-64.5% of patients in the first year after surgery – higher than the benchmark of 20% or less that was established to account for stage IV patients and other specific conditions in which markers might be indicated. National, international, and regional guidelines “discourage the use of blood tumor markers” in breast cancer follow-up, the investigators wrote.
The extensive use of these markers – observed across all five regions – is “a starting point to understanding how to improve clinical practice,” they added.
Other quality indicators that were evaluable included radiotherapy within 12 weeks after surgery if adjuvant chemotherapy is not administered (42%-83.8% in the project, compared with the benchmark of 90% or greater) and mammography 6-18 months after surgery (administered in 55.1%-72.6%, compared with the benchmark of 90% or greater), as well as the proportion of patients starting adjuvant systemic treatment (chemotherapy or endocrine therapy) within 60 days of surgery (for patients receiving systemic treatment).
To calculate the indicators, each regional cancer network used computerized sources of information including hospital discharge forms, outpatient records of diagnostic and therapeutic procedures, prescriptions of drugs reimbursed by the National Health Service in the hospital and outpatient settings, regional health registries, and the regional mortality registries.
All data used in the project came from regional repositories, which collect data from all National Health Service providers in the region, and not from single institutional repositories, the investigators noted.
More than half of the indicators expected to be assessable – but not found to be – were not computable as a result of data being unavailable (for example, pathology data) or incomplete, and as a result of data not being reliable for various reasons. The fact that examinations paid for directly by patients are not reported by the management systems of the National Health System was another complicating factor, they reported.
The authors disclosed funding and relationships with various pharmaceutical companies. The research was supported by the Periplo Association.
SOURCE: Guarneri V et al. J Oncol Pract. 2019 Dec 19. doi: 10.1200/JOP.19.00466.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Decreasing Overutilization of Echocardiograms and Abdominal Imaging in the Evaluation of Children with Fungemia
From the University of Miami, Department of Pediatrics and Department of Medicine, Miami, FL.
Abstract
- Objective: Pediatric fungemia is associated with a low risk of fungal endocarditis and renal infections. The majority of current guidelines do not recommend routine abdominal imaging/echocardiograms in the evaluation of fungemia, but such imaging has been routinely ordered for patients on the pediatric gastroenterology service at our institution. Our goals were to assess the financial impact of this deviation from current clinical guidelines and redefine the standard work to reduce overutilization of abdominal ultrasounds and echocardiograms. Specifically, our goal was to reduce imaging by 50% by 18 months.
- Methods: Root cause analysis showed a lack of familiarity with current evidence. Using this data, countermeasures were implemented, including practitioner education of guidelines and creation of a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Balancing measures were missed episodes of fungal endocarditis and renal infection.
- Results: During the period January 1, 2016 to November 19, 2017, 18 of 21 episodes of fungemia in our pediatric institution occurred in patients admitted to the pediatric gastroenterology service. Abdominal imaging and echocardiograms were done 100% of the time, with no positive findings and an estimated cost of approximately $58,000. Post-intervention from November 20, 2017 to April 3, 2019, 7 of 13 episodes of fungemia occurred on this service. Frequency of abdominal imaging and echocardiograms decreased to 43% and 57%, respectively. No episodes of fungal endocarditis or renal infection were identified.
- Conclusion: Overutilization of abdominal imaging and echocardiograms in pediatric fungemia evaluation can be safely decreased.
Keywords: guidelines; cost; candidemia; endocarditis.
Practitioners may remain under the impression that routine abdominal ultrasounds (US) and echocardiograms (echo) are indicated in fungemia to evaluate for fungal endocarditis and renal infection, although these conditions are rare and limited to a subset of the population.1-10 Risk factors include prematurity, immunosuppression, prior bacterial endocarditis, abnormal cardiac valves, and previous urogenital surgeries.11
The 2016 Infectious Diseases Society of America (IDSA) guidelines do not recommend routine US or echo but rather provide scenarios in which Candida endocarditis should be suspected, and these include: persistently positive blood cultures, persistent fevers despite appropriate therapy, and clinical signs that may suggest endocarditis, such as a new heart murmur, heart failure, or embolic phenomena.11 IDSA recommends abdominal imaging in neonates with persistently positive blood cultures to evaluate the urogenital system, in addition to the liver and spleen. They also recommend abdominal imaging in symptomatic ascending Candida pyelonephritis beyond the neonatal period and in chronic disseminated candidiasis; the latter is uncommon and seen almost exclusively in patients recovering from neutropenia with a hematologic malignancy.11
We also reviewed guidelines on fungemia originating outside the United States. The 2010 Canadian clinical guidelines on invasive candidiasis do not explicitly recommend routine imaging, but rather state that various imaging studies, including US and echo among others, may be helpful.12 The German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy published a joint recommendation against routine US and echo in uncomplicated candidemia in 2011.13
The European Society for Clinical Microbiology and Infectious Diseases is the only society that recommends routine echo. Their 2012 guidelines on candidiasis recommend transesophageal echo in adults14 and echocardiography in children,15 as well as abdominal imaging in the diagnosis of chronic disseminated candidiasis in adults with hematological malignancies/hematopoietic stem cell transplantation.16
The 2013 Brazilian guidelines explicitly recommend against routine abdominal imaging and echo because of the low frequency of visceral lesions in adults with candidemia and recommend reserving imaging for those with persistently positive blood cultures or with clinical signs/symptoms suggestive of endocarditis/abdominal infection or clinical deterioration.17 The 2014 Japanese guidelines recommend ruling out chronic disseminated candidiasis in these patients with symptoms during the neutrophil recovery phase, but do not mention routinely imaging other patients. They do not address the role of echocardiography.18
Although physicians in the United Sates typically follow IDSA guidelines, abdominal US and echo were ordered routinely for patients with fungemia on the pediatric gastroenterology service at our institution, leading to higher medical costs and waste of medical resources. Our goals were to assess the current standard work for fungemia evaluation on this service, assess the impact of its deviation from current clinical guidelines, and redefine the standard work by (1) presenting current evidence to practitioners taking care of patients on this service, (2) providing a clinical pathway that allowed for variations where appropriate, and (3) providing a plan for pediatric fungemia management. Our SMART (Specific, Measurable, Attainable, Relevant and Timely) goal was to reduce overutilization of abdominal US and echo in pediatric patients with fungemia on the pediatric gastroenterology service by 50%.
Methods
Study, Setting, and Participants
We executed this quality improvement project at a quaternary care pediatric hospital affiliated with a school of medicine. The project scope consisted of inpatient pediatric patients with fungemia on the pediatric gastroenterology service admitted to the wards or pediatric critical care unit at this institution, along with the practitioners caring for these patients. The project was part of an institutional quality improvement initiative program. The quality improvement team included quality improvement experts from the departments of medicine and pediatrics, a pediatric resident and student, and physicians from the divisions of pediatric infectious disease, pediatric critical care, and pediatric gastroenterology. This study qualified for Institutional Review Board (IRB) exemption based on the University’s IRB stipulations.
Current Condition
Root cause analysis was performed by creating a process map of the current standard work and a fishbone diagram (Figure 1). We incorporated feedback from voice of the customer in the root cause analysis. In this analysis, the voice of the customer came from the bedside floor nurses, ultrasound clerk and sonographer, echo technician, cardiology fellow, and microbiology medical technician. We got their feedback on our process map, its accuracy and ways to expand, their thoughts on the problem and why we have this problem, and any solutions they could offer to help improve the problem. Some of the key points obtained were: echos were not routinely done on the floors and were not considered urgent as they often did not change management; the sonographer and those from the cardiology department felt imaging was often overutilized because of misconceptions and lack of available hospital guidelines. Suggested solutions included provider education with reference to Duke’s criteria and establishing a clinical pathway approved by all concerned departments.
Prior to education, we surveyed current practices of practitioners on teams caring for these patients, which included physicians of all levels (attendings, fellows, residents) as well as nurse practitioners and medical students from the department of pediatrics and divisions of pediatric gastroenterology, pediatric infectious disease, and pediatric critical care medicine.
Countermeasures
Practitioner Education. In October 2017 practitioners were given a 20-minute presentation on the latest international guidelines on fungemia. Fifty-nine practitioners completed pre- and post-test surveys. Eight respondents were excluded due to incomplete surveys. We compared self-reported frequencies of ordering abdominal imaging and echo before the presentation with intention to order post education. Intention to change clinical practice after the presentation was also surveyed.
Clinical Pathway. Education alone may not result in sustainability, and thus we provided a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Inter-department buy-in was also necessary for success. It was important to get the input from the various teams (infectious disease, cardiology, gastroenterology, and critical care), which was done by incorporating members from those divisions in the project or getting their feedback through voice of the customer analysis.
We redefined standard work based on current evidence and created a clinical pathway during March 2018 that included variations when appropriate (Figure 2). We presented the clinical pathway to practitioners and distributed it via email. We also made it available to pediatric residents and fellows on their mobile institutional work resource application.
Electronic Order Set. We created an electronic order set for pediatric fungemia management and made it available in the electronic health record May 2018.
Measurement
Cases of fungemia were identified through the electronic health record pre-intervention (January 1, 2016 through November 19, 2017) and post-intervention (November 20, 2019 through April 3, 2019). An episode of fungemia was defined as an encounter with 1 or more positive blood culture(s) for Candida species or Cryptococcus species. We manually identified patients belonging to the pediatric gastroenterology service and reviewed these charts to determine the presenting complaint, organism isolated, transplant status, central lines status, risk factors, if abdominal imaging or echocardiography were done for the episode of fungemia, and their corresponding results. We calculated overall and per patient medical charges by using the average charges at our institution of US and echocardiography with a cardiology consult. These average charges were provided by patient financial services and the pediatric cardiology department, respectively. To address non-technical expenditures, we calculated the average time taken for transport to and from radiology and the echo suite for each identified patient. We identified missed fungal endocarditis and fungal balls as balancing measures.
Results
Survey
Among the 51 practitioners surveyed, 36% were performing routine echo and 22% self-reported performing routine abdominal imaging. After education, no respondents planned to routinely do echo or abdominal imaging. All but 1 respondent planned to change their practice for evaluation of fungemia patients based on the presentation (eFigure 1).
Baseline Data
Over the 23-month period from January 1, 2016 to November 19, 2017, there were 21 episodes of fungemia, 18 of which occurred in patients on the pediatric gastroenterology service (2 of the 18 were transplant recipients). For the 18 episodes on this service, abdominal imaging and echo were done 100% of the time, with 0 positive findings (eFigure 2).
Of those 18 episodes, the average age was 4.6 years, with two-thirds of the population being male. There were 3 patients with multiple episodes that accounted for 8 of the episodes (3, 3, and 2 episodes each). Fever was the most common presenting complaint. The most common organism was Candida parapsilosis (6 of the 18 episodes). All episodes but one involved a central line, and all central lines were removed when present except for one case. Of the risk factors, 3 episodes occurred in neutropenic patients, and for 1 episode the patient had a questionable history of fungal endocarditis (and was on fungal prophylaxis). There were no patients with recent cardiac/urogenital surgery or prior fungal balls. No episodes had clinical symptoms suggestive of fungal endocarditis or fungal balls.
Post-Intervention Data
Over the subsequent 17-month period (November 11, 2017 to April 3, 2019), there were 13 episodes of candidemia. There were no episodes of Cryptococcus fungemia. Seven episodes occurred in patients on the pediatric gastroenterology service (2 of the 7 occurred in transplant recipients). Abdominal imaging was done in 3 of these episodes (43%), and in 2 of these 3 episodes, imaging was done at an outside institution prior to arrival, with no positive results (eFigure 2).
Echocardiography was done 57% of the time (n = 4), with echo being done at an outside institution prior to arrival half of the time (n = 2), with no endocarditis identified. The cases of abdominal imaging and echo done at outside institutions prior to arrival were not impacted by the countermeasures. Excluding those 2 patients who had both abdominal imaging and echocardiography done prior to arrival, the overall rate of imaging (both abdominal imaging and echo) done after countermeasures were instituted was 30% (Figure 3).
Of those 7 episodes, the average age was 6.8 years (57% female). There were no patients with multiple episodes. The most common presenting complaint was fever. The most common organism was Candida albicans (3 of the 7 episodes). All episodes involved a central line, which was removed in all cases except for one. Of the risk factors, 2 episodes were in neutropenic patients, and 1 episode had a history of bacterial endocarditis (not related to fungemia). No episodes occurred in patients with prior fungal renal infection, urogenital malformations, or recent cardiac/urogenital surgery. No episodes had clinical symptoms suggestive of fungal endocarditis or renal infection. No episodes of fungal endocarditis or renal infection were identified.
On average, a patient at our institution undergoing abdominal US and echo with a cardiology consult results in medical waste of approximately $3200 per patient. This cost does not take into account other miscellaneous charges possibly incurred, such as the radiologist interpreting the findings and transportation. Baseline data calculations show that patients waste on average 55 minutes in physical transport, and this does not take into account wait times.
Discussion
Candidemia contributes to 10% of central-line associated blood stream infections (CLABSI).19 Increased usage of indwelling central catheters for administration of parenteral nutrition will inevitably result in practitioners encountering cases of candidemia when caring for this population. As seen from our results, the majority of episodes of candidemia at our institution occurred on the pediatric gastroenterology service, and thus redefining standard work on this service will be impactful.
Candida parapsilosis and Candida albicans were the most common causative agents before and after intervention, respectively, but overall the most common organism was Candida albicans, which is in keeping with that of CLABSI in the literature.19 Growth of Candida parapsilosis has been particularly linked to CLABSI.19 The third most common organism in our study was Candida glabrata, which is the second most common cause of candidemia in CLABSI.19
The cases of positive abdominal imaging in fungemia in the literature are limited to the neonatal population1-4 and chronic disseminated candidiasis in patients with hematologic malignancies/neutropenia/immunosuppression.5,6 In fungal endocarditis, the reported cases were generally in neonates,1,3,7 critically ill patients,8 patients with hematologic malignancies/neutropenia/immunosuppression,6,9 or those with a cardiac history.9,10 This population differs from the patient population on the pediatric gastroenterology service. Patients on this service may not need US or echo. Performing abdominal US and echo in fungemia patients in whom such imaging is not indicated may result in medical waste of approximately $3200 per patient. There is also a waste of medical resources and time.
We found almost all practitioners are willing to change clinical practice once provided with current guidelines. Face-to-face oral presentations allowed for questions and interaction, making this form of information dissemination better than e-mails or handouts.
Though the numbers were small over the short study period, we were able to decrease overutilization of abdominal imaging and echo after implementing countermeasures. Frequency decreased from 100% to 43% and 57% for abdominal imaging and echo, respectively. Imaging that was done after the countermeasures were implemented was mainly attributed to imaging patients underwent prior to presenting to our institution. This reinforces the need for education at other institutions as well. Of the balancing measures assessed, there were no missed cases of fungal balls or fungal endocarditis. Additionally,
The findings from this quality improvement project underscore current recommendations that, despite common misconceptions, routine abdominal US and echo are not indicated in all cases of fungemia. Case-by-case assessment based on the clinical scenario remains key to management of fungemia to avoid unnecessary medical interventions.
Corresponding author: Donna Cheung, MBBS, 200 Hawkins Drive, BT 1120-G, Iowa City, IA 52242; donna.ann.cheung@gmail.com.
Financial support: None.
1. Benjamin DK Jr, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634-640.
2. Wynn JL, Tan S, Gantz MG, et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis. 2012;54:331-339.
3. Noyola DE, Fernandez M, Moylett EH, et al. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32:1018-1023.
4. Phillips JR, Karlowicz MG Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190-194.
5. Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. Clinical features and outcome of 29 episodes. Haematologica. 2002;87:535-541.
6. Zaoutis TE, Greves HM, Lautenbach E, et al. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635-641.
7. Levy I, Shalit I, Birk E, et al. Candida endocarditis in neonates: report of five cases and review of the literature. Mycoses. 2006;49:43-48.
8. Aspesberro F, Beghetti M, Oberhansli I, et al. Fungal endocarditis in critically ill children. Eur J Pediatr. 1999;158:275-280.
9. Fernandez-Cruz A, Cruz Menarguez M, Munoz P, et al. The search for endocarditis in patients with candidemia: a systematic recommendation for echocardiography? A prospective cohort. Eur J Clin Microbiol Infect Dis. 2015;34:1543-1549.
10. Hernandez-Torres A, Garcia-Vazquez E, Laso-Ortiz A, et al. [Candida sp endocarditis. Experience in a third-level hospital and review of the literature]. Rev Esp Quimioter. 2013;26:51-55.
11. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50.
12. Bow EJ, Evans G, Fuller J, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21:e122-50.
13. Ruhnke M, Rickerts V, Cornely OA, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54:279-310.
14. Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
15. Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38-52.
16. Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18 Suppl 7:53-67.
17. Colombo AL, Guimaraes T, Camargo LF, et al. Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis. 2013;17:283-312.
18. Kohno S, Tamura K, Niki Y, et al. Executive Summary of Japanese Domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016;57:E117-E163.
19. Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiology Rev. 2004;17:255-267.
From the University of Miami, Department of Pediatrics and Department of Medicine, Miami, FL.
Abstract
- Objective: Pediatric fungemia is associated with a low risk of fungal endocarditis and renal infections. The majority of current guidelines do not recommend routine abdominal imaging/echocardiograms in the evaluation of fungemia, but such imaging has been routinely ordered for patients on the pediatric gastroenterology service at our institution. Our goals were to assess the financial impact of this deviation from current clinical guidelines and redefine the standard work to reduce overutilization of abdominal ultrasounds and echocardiograms. Specifically, our goal was to reduce imaging by 50% by 18 months.
- Methods: Root cause analysis showed a lack of familiarity with current evidence. Using this data, countermeasures were implemented, including practitioner education of guidelines and creation of a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Balancing measures were missed episodes of fungal endocarditis and renal infection.
- Results: During the period January 1, 2016 to November 19, 2017, 18 of 21 episodes of fungemia in our pediatric institution occurred in patients admitted to the pediatric gastroenterology service. Abdominal imaging and echocardiograms were done 100% of the time, with no positive findings and an estimated cost of approximately $58,000. Post-intervention from November 20, 2017 to April 3, 2019, 7 of 13 episodes of fungemia occurred on this service. Frequency of abdominal imaging and echocardiograms decreased to 43% and 57%, respectively. No episodes of fungal endocarditis or renal infection were identified.
- Conclusion: Overutilization of abdominal imaging and echocardiograms in pediatric fungemia evaluation can be safely decreased.
Keywords: guidelines; cost; candidemia; endocarditis.
Practitioners may remain under the impression that routine abdominal ultrasounds (US) and echocardiograms (echo) are indicated in fungemia to evaluate for fungal endocarditis and renal infection, although these conditions are rare and limited to a subset of the population.1-10 Risk factors include prematurity, immunosuppression, prior bacterial endocarditis, abnormal cardiac valves, and previous urogenital surgeries.11
The 2016 Infectious Diseases Society of America (IDSA) guidelines do not recommend routine US or echo but rather provide scenarios in which Candida endocarditis should be suspected, and these include: persistently positive blood cultures, persistent fevers despite appropriate therapy, and clinical signs that may suggest endocarditis, such as a new heart murmur, heart failure, or embolic phenomena.11 IDSA recommends abdominal imaging in neonates with persistently positive blood cultures to evaluate the urogenital system, in addition to the liver and spleen. They also recommend abdominal imaging in symptomatic ascending Candida pyelonephritis beyond the neonatal period and in chronic disseminated candidiasis; the latter is uncommon and seen almost exclusively in patients recovering from neutropenia with a hematologic malignancy.11
We also reviewed guidelines on fungemia originating outside the United States. The 2010 Canadian clinical guidelines on invasive candidiasis do not explicitly recommend routine imaging, but rather state that various imaging studies, including US and echo among others, may be helpful.12 The German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy published a joint recommendation against routine US and echo in uncomplicated candidemia in 2011.13
The European Society for Clinical Microbiology and Infectious Diseases is the only society that recommends routine echo. Their 2012 guidelines on candidiasis recommend transesophageal echo in adults14 and echocardiography in children,15 as well as abdominal imaging in the diagnosis of chronic disseminated candidiasis in adults with hematological malignancies/hematopoietic stem cell transplantation.16
The 2013 Brazilian guidelines explicitly recommend against routine abdominal imaging and echo because of the low frequency of visceral lesions in adults with candidemia and recommend reserving imaging for those with persistently positive blood cultures or with clinical signs/symptoms suggestive of endocarditis/abdominal infection or clinical deterioration.17 The 2014 Japanese guidelines recommend ruling out chronic disseminated candidiasis in these patients with symptoms during the neutrophil recovery phase, but do not mention routinely imaging other patients. They do not address the role of echocardiography.18
Although physicians in the United Sates typically follow IDSA guidelines, abdominal US and echo were ordered routinely for patients with fungemia on the pediatric gastroenterology service at our institution, leading to higher medical costs and waste of medical resources. Our goals were to assess the current standard work for fungemia evaluation on this service, assess the impact of its deviation from current clinical guidelines, and redefine the standard work by (1) presenting current evidence to practitioners taking care of patients on this service, (2) providing a clinical pathway that allowed for variations where appropriate, and (3) providing a plan for pediatric fungemia management. Our SMART (Specific, Measurable, Attainable, Relevant and Timely) goal was to reduce overutilization of abdominal US and echo in pediatric patients with fungemia on the pediatric gastroenterology service by 50%.
Methods
Study, Setting, and Participants
We executed this quality improvement project at a quaternary care pediatric hospital affiliated with a school of medicine. The project scope consisted of inpatient pediatric patients with fungemia on the pediatric gastroenterology service admitted to the wards or pediatric critical care unit at this institution, along with the practitioners caring for these patients. The project was part of an institutional quality improvement initiative program. The quality improvement team included quality improvement experts from the departments of medicine and pediatrics, a pediatric resident and student, and physicians from the divisions of pediatric infectious disease, pediatric critical care, and pediatric gastroenterology. This study qualified for Institutional Review Board (IRB) exemption based on the University’s IRB stipulations.
Current Condition
Root cause analysis was performed by creating a process map of the current standard work and a fishbone diagram (Figure 1). We incorporated feedback from voice of the customer in the root cause analysis. In this analysis, the voice of the customer came from the bedside floor nurses, ultrasound clerk and sonographer, echo technician, cardiology fellow, and microbiology medical technician. We got their feedback on our process map, its accuracy and ways to expand, their thoughts on the problem and why we have this problem, and any solutions they could offer to help improve the problem. Some of the key points obtained were: echos were not routinely done on the floors and were not considered urgent as they often did not change management; the sonographer and those from the cardiology department felt imaging was often overutilized because of misconceptions and lack of available hospital guidelines. Suggested solutions included provider education with reference to Duke’s criteria and establishing a clinical pathway approved by all concerned departments.
Prior to education, we surveyed current practices of practitioners on teams caring for these patients, which included physicians of all levels (attendings, fellows, residents) as well as nurse practitioners and medical students from the department of pediatrics and divisions of pediatric gastroenterology, pediatric infectious disease, and pediatric critical care medicine.
Countermeasures
Practitioner Education. In October 2017 practitioners were given a 20-minute presentation on the latest international guidelines on fungemia. Fifty-nine practitioners completed pre- and post-test surveys. Eight respondents were excluded due to incomplete surveys. We compared self-reported frequencies of ordering abdominal imaging and echo before the presentation with intention to order post education. Intention to change clinical practice after the presentation was also surveyed.
Clinical Pathway. Education alone may not result in sustainability, and thus we provided a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Inter-department buy-in was also necessary for success. It was important to get the input from the various teams (infectious disease, cardiology, gastroenterology, and critical care), which was done by incorporating members from those divisions in the project or getting their feedback through voice of the customer analysis.
We redefined standard work based on current evidence and created a clinical pathway during March 2018 that included variations when appropriate (Figure 2). We presented the clinical pathway to practitioners and distributed it via email. We also made it available to pediatric residents and fellows on their mobile institutional work resource application.
Electronic Order Set. We created an electronic order set for pediatric fungemia management and made it available in the electronic health record May 2018.
Measurement
Cases of fungemia were identified through the electronic health record pre-intervention (January 1, 2016 through November 19, 2017) and post-intervention (November 20, 2019 through April 3, 2019). An episode of fungemia was defined as an encounter with 1 or more positive blood culture(s) for Candida species or Cryptococcus species. We manually identified patients belonging to the pediatric gastroenterology service and reviewed these charts to determine the presenting complaint, organism isolated, transplant status, central lines status, risk factors, if abdominal imaging or echocardiography were done for the episode of fungemia, and their corresponding results. We calculated overall and per patient medical charges by using the average charges at our institution of US and echocardiography with a cardiology consult. These average charges were provided by patient financial services and the pediatric cardiology department, respectively. To address non-technical expenditures, we calculated the average time taken for transport to and from radiology and the echo suite for each identified patient. We identified missed fungal endocarditis and fungal balls as balancing measures.
Results
Survey
Among the 51 practitioners surveyed, 36% were performing routine echo and 22% self-reported performing routine abdominal imaging. After education, no respondents planned to routinely do echo or abdominal imaging. All but 1 respondent planned to change their practice for evaluation of fungemia patients based on the presentation (eFigure 1).
Baseline Data
Over the 23-month period from January 1, 2016 to November 19, 2017, there were 21 episodes of fungemia, 18 of which occurred in patients on the pediatric gastroenterology service (2 of the 18 were transplant recipients). For the 18 episodes on this service, abdominal imaging and echo were done 100% of the time, with 0 positive findings (eFigure 2).
Of those 18 episodes, the average age was 4.6 years, with two-thirds of the population being male. There were 3 patients with multiple episodes that accounted for 8 of the episodes (3, 3, and 2 episodes each). Fever was the most common presenting complaint. The most common organism was Candida parapsilosis (6 of the 18 episodes). All episodes but one involved a central line, and all central lines were removed when present except for one case. Of the risk factors, 3 episodes occurred in neutropenic patients, and for 1 episode the patient had a questionable history of fungal endocarditis (and was on fungal prophylaxis). There were no patients with recent cardiac/urogenital surgery or prior fungal balls. No episodes had clinical symptoms suggestive of fungal endocarditis or fungal balls.
Post-Intervention Data
Over the subsequent 17-month period (November 11, 2017 to April 3, 2019), there were 13 episodes of candidemia. There were no episodes of Cryptococcus fungemia. Seven episodes occurred in patients on the pediatric gastroenterology service (2 of the 7 occurred in transplant recipients). Abdominal imaging was done in 3 of these episodes (43%), and in 2 of these 3 episodes, imaging was done at an outside institution prior to arrival, with no positive results (eFigure 2).
Echocardiography was done 57% of the time (n = 4), with echo being done at an outside institution prior to arrival half of the time (n = 2), with no endocarditis identified. The cases of abdominal imaging and echo done at outside institutions prior to arrival were not impacted by the countermeasures. Excluding those 2 patients who had both abdominal imaging and echocardiography done prior to arrival, the overall rate of imaging (both abdominal imaging and echo) done after countermeasures were instituted was 30% (Figure 3).
Of those 7 episodes, the average age was 6.8 years (57% female). There were no patients with multiple episodes. The most common presenting complaint was fever. The most common organism was Candida albicans (3 of the 7 episodes). All episodes involved a central line, which was removed in all cases except for one. Of the risk factors, 2 episodes were in neutropenic patients, and 1 episode had a history of bacterial endocarditis (not related to fungemia). No episodes occurred in patients with prior fungal renal infection, urogenital malformations, or recent cardiac/urogenital surgery. No episodes had clinical symptoms suggestive of fungal endocarditis or renal infection. No episodes of fungal endocarditis or renal infection were identified.
On average, a patient at our institution undergoing abdominal US and echo with a cardiology consult results in medical waste of approximately $3200 per patient. This cost does not take into account other miscellaneous charges possibly incurred, such as the radiologist interpreting the findings and transportation. Baseline data calculations show that patients waste on average 55 minutes in physical transport, and this does not take into account wait times.
Discussion
Candidemia contributes to 10% of central-line associated blood stream infections (CLABSI).19 Increased usage of indwelling central catheters for administration of parenteral nutrition will inevitably result in practitioners encountering cases of candidemia when caring for this population. As seen from our results, the majority of episodes of candidemia at our institution occurred on the pediatric gastroenterology service, and thus redefining standard work on this service will be impactful.
Candida parapsilosis and Candida albicans were the most common causative agents before and after intervention, respectively, but overall the most common organism was Candida albicans, which is in keeping with that of CLABSI in the literature.19 Growth of Candida parapsilosis has been particularly linked to CLABSI.19 The third most common organism in our study was Candida glabrata, which is the second most common cause of candidemia in CLABSI.19
The cases of positive abdominal imaging in fungemia in the literature are limited to the neonatal population1-4 and chronic disseminated candidiasis in patients with hematologic malignancies/neutropenia/immunosuppression.5,6 In fungal endocarditis, the reported cases were generally in neonates,1,3,7 critically ill patients,8 patients with hematologic malignancies/neutropenia/immunosuppression,6,9 or those with a cardiac history.9,10 This population differs from the patient population on the pediatric gastroenterology service. Patients on this service may not need US or echo. Performing abdominal US and echo in fungemia patients in whom such imaging is not indicated may result in medical waste of approximately $3200 per patient. There is also a waste of medical resources and time.
We found almost all practitioners are willing to change clinical practice once provided with current guidelines. Face-to-face oral presentations allowed for questions and interaction, making this form of information dissemination better than e-mails or handouts.
Though the numbers were small over the short study period, we were able to decrease overutilization of abdominal imaging and echo after implementing countermeasures. Frequency decreased from 100% to 43% and 57% for abdominal imaging and echo, respectively. Imaging that was done after the countermeasures were implemented was mainly attributed to imaging patients underwent prior to presenting to our institution. This reinforces the need for education at other institutions as well. Of the balancing measures assessed, there were no missed cases of fungal balls or fungal endocarditis. Additionally,
The findings from this quality improvement project underscore current recommendations that, despite common misconceptions, routine abdominal US and echo are not indicated in all cases of fungemia. Case-by-case assessment based on the clinical scenario remains key to management of fungemia to avoid unnecessary medical interventions.
Corresponding author: Donna Cheung, MBBS, 200 Hawkins Drive, BT 1120-G, Iowa City, IA 52242; donna.ann.cheung@gmail.com.
Financial support: None.
From the University of Miami, Department of Pediatrics and Department of Medicine, Miami, FL.
Abstract
- Objective: Pediatric fungemia is associated with a low risk of fungal endocarditis and renal infections. The majority of current guidelines do not recommend routine abdominal imaging/echocardiograms in the evaluation of fungemia, but such imaging has been routinely ordered for patients on the pediatric gastroenterology service at our institution. Our goals were to assess the financial impact of this deviation from current clinical guidelines and redefine the standard work to reduce overutilization of abdominal ultrasounds and echocardiograms. Specifically, our goal was to reduce imaging by 50% by 18 months.
- Methods: Root cause analysis showed a lack of familiarity with current evidence. Using this data, countermeasures were implemented, including practitioner education of guidelines and creation of a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Balancing measures were missed episodes of fungal endocarditis and renal infection.
- Results: During the period January 1, 2016 to November 19, 2017, 18 of 21 episodes of fungemia in our pediatric institution occurred in patients admitted to the pediatric gastroenterology service. Abdominal imaging and echocardiograms were done 100% of the time, with no positive findings and an estimated cost of approximately $58,000. Post-intervention from November 20, 2017 to April 3, 2019, 7 of 13 episodes of fungemia occurred on this service. Frequency of abdominal imaging and echocardiograms decreased to 43% and 57%, respectively. No episodes of fungal endocarditis or renal infection were identified.
- Conclusion: Overutilization of abdominal imaging and echocardiograms in pediatric fungemia evaluation can be safely decreased.
Keywords: guidelines; cost; candidemia; endocarditis.
Practitioners may remain under the impression that routine abdominal ultrasounds (US) and echocardiograms (echo) are indicated in fungemia to evaluate for fungal endocarditis and renal infection, although these conditions are rare and limited to a subset of the population.1-10 Risk factors include prematurity, immunosuppression, prior bacterial endocarditis, abnormal cardiac valves, and previous urogenital surgeries.11
The 2016 Infectious Diseases Society of America (IDSA) guidelines do not recommend routine US or echo but rather provide scenarios in which Candida endocarditis should be suspected, and these include: persistently positive blood cultures, persistent fevers despite appropriate therapy, and clinical signs that may suggest endocarditis, such as a new heart murmur, heart failure, or embolic phenomena.11 IDSA recommends abdominal imaging in neonates with persistently positive blood cultures to evaluate the urogenital system, in addition to the liver and spleen. They also recommend abdominal imaging in symptomatic ascending Candida pyelonephritis beyond the neonatal period and in chronic disseminated candidiasis; the latter is uncommon and seen almost exclusively in patients recovering from neutropenia with a hematologic malignancy.11
We also reviewed guidelines on fungemia originating outside the United States. The 2010 Canadian clinical guidelines on invasive candidiasis do not explicitly recommend routine imaging, but rather state that various imaging studies, including US and echo among others, may be helpful.12 The German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy published a joint recommendation against routine US and echo in uncomplicated candidemia in 2011.13
The European Society for Clinical Microbiology and Infectious Diseases is the only society that recommends routine echo. Their 2012 guidelines on candidiasis recommend transesophageal echo in adults14 and echocardiography in children,15 as well as abdominal imaging in the diagnosis of chronic disseminated candidiasis in adults with hematological malignancies/hematopoietic stem cell transplantation.16
The 2013 Brazilian guidelines explicitly recommend against routine abdominal imaging and echo because of the low frequency of visceral lesions in adults with candidemia and recommend reserving imaging for those with persistently positive blood cultures or with clinical signs/symptoms suggestive of endocarditis/abdominal infection or clinical deterioration.17 The 2014 Japanese guidelines recommend ruling out chronic disseminated candidiasis in these patients with symptoms during the neutrophil recovery phase, but do not mention routinely imaging other patients. They do not address the role of echocardiography.18
Although physicians in the United Sates typically follow IDSA guidelines, abdominal US and echo were ordered routinely for patients with fungemia on the pediatric gastroenterology service at our institution, leading to higher medical costs and waste of medical resources. Our goals were to assess the current standard work for fungemia evaluation on this service, assess the impact of its deviation from current clinical guidelines, and redefine the standard work by (1) presenting current evidence to practitioners taking care of patients on this service, (2) providing a clinical pathway that allowed for variations where appropriate, and (3) providing a plan for pediatric fungemia management. Our SMART (Specific, Measurable, Attainable, Relevant and Timely) goal was to reduce overutilization of abdominal US and echo in pediatric patients with fungemia on the pediatric gastroenterology service by 50%.
Methods
Study, Setting, and Participants
We executed this quality improvement project at a quaternary care pediatric hospital affiliated with a school of medicine. The project scope consisted of inpatient pediatric patients with fungemia on the pediatric gastroenterology service admitted to the wards or pediatric critical care unit at this institution, along with the practitioners caring for these patients. The project was part of an institutional quality improvement initiative program. The quality improvement team included quality improvement experts from the departments of medicine and pediatrics, a pediatric resident and student, and physicians from the divisions of pediatric infectious disease, pediatric critical care, and pediatric gastroenterology. This study qualified for Institutional Review Board (IRB) exemption based on the University’s IRB stipulations.
Current Condition
Root cause analysis was performed by creating a process map of the current standard work and a fishbone diagram (Figure 1). We incorporated feedback from voice of the customer in the root cause analysis. In this analysis, the voice of the customer came from the bedside floor nurses, ultrasound clerk and sonographer, echo technician, cardiology fellow, and microbiology medical technician. We got their feedback on our process map, its accuracy and ways to expand, their thoughts on the problem and why we have this problem, and any solutions they could offer to help improve the problem. Some of the key points obtained were: echos were not routinely done on the floors and were not considered urgent as they often did not change management; the sonographer and those from the cardiology department felt imaging was often overutilized because of misconceptions and lack of available hospital guidelines. Suggested solutions included provider education with reference to Duke’s criteria and establishing a clinical pathway approved by all concerned departments.
Prior to education, we surveyed current practices of practitioners on teams caring for these patients, which included physicians of all levels (attendings, fellows, residents) as well as nurse practitioners and medical students from the department of pediatrics and divisions of pediatric gastroenterology, pediatric infectious disease, and pediatric critical care medicine.
Countermeasures
Practitioner Education. In October 2017 practitioners were given a 20-minute presentation on the latest international guidelines on fungemia. Fifty-nine practitioners completed pre- and post-test surveys. Eight respondents were excluded due to incomplete surveys. We compared self-reported frequencies of ordering abdominal imaging and echo before the presentation with intention to order post education. Intention to change clinical practice after the presentation was also surveyed.
Clinical Pathway. Education alone may not result in sustainability, and thus we provided a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Inter-department buy-in was also necessary for success. It was important to get the input from the various teams (infectious disease, cardiology, gastroenterology, and critical care), which was done by incorporating members from those divisions in the project or getting their feedback through voice of the customer analysis.
We redefined standard work based on current evidence and created a clinical pathway during March 2018 that included variations when appropriate (Figure 2). We presented the clinical pathway to practitioners and distributed it via email. We also made it available to pediatric residents and fellows on their mobile institutional work resource application.
Electronic Order Set. We created an electronic order set for pediatric fungemia management and made it available in the electronic health record May 2018.
Measurement
Cases of fungemia were identified through the electronic health record pre-intervention (January 1, 2016 through November 19, 2017) and post-intervention (November 20, 2019 through April 3, 2019). An episode of fungemia was defined as an encounter with 1 or more positive blood culture(s) for Candida species or Cryptococcus species. We manually identified patients belonging to the pediatric gastroenterology service and reviewed these charts to determine the presenting complaint, organism isolated, transplant status, central lines status, risk factors, if abdominal imaging or echocardiography were done for the episode of fungemia, and their corresponding results. We calculated overall and per patient medical charges by using the average charges at our institution of US and echocardiography with a cardiology consult. These average charges were provided by patient financial services and the pediatric cardiology department, respectively. To address non-technical expenditures, we calculated the average time taken for transport to and from radiology and the echo suite for each identified patient. We identified missed fungal endocarditis and fungal balls as balancing measures.
Results
Survey
Among the 51 practitioners surveyed, 36% were performing routine echo and 22% self-reported performing routine abdominal imaging. After education, no respondents planned to routinely do echo or abdominal imaging. All but 1 respondent planned to change their practice for evaluation of fungemia patients based on the presentation (eFigure 1).
Baseline Data
Over the 23-month period from January 1, 2016 to November 19, 2017, there were 21 episodes of fungemia, 18 of which occurred in patients on the pediatric gastroenterology service (2 of the 18 were transplant recipients). For the 18 episodes on this service, abdominal imaging and echo were done 100% of the time, with 0 positive findings (eFigure 2).
Of those 18 episodes, the average age was 4.6 years, with two-thirds of the population being male. There were 3 patients with multiple episodes that accounted for 8 of the episodes (3, 3, and 2 episodes each). Fever was the most common presenting complaint. The most common organism was Candida parapsilosis (6 of the 18 episodes). All episodes but one involved a central line, and all central lines were removed when present except for one case. Of the risk factors, 3 episodes occurred in neutropenic patients, and for 1 episode the patient had a questionable history of fungal endocarditis (and was on fungal prophylaxis). There were no patients with recent cardiac/urogenital surgery or prior fungal balls. No episodes had clinical symptoms suggestive of fungal endocarditis or fungal balls.
Post-Intervention Data
Over the subsequent 17-month period (November 11, 2017 to April 3, 2019), there were 13 episodes of candidemia. There were no episodes of Cryptococcus fungemia. Seven episodes occurred in patients on the pediatric gastroenterology service (2 of the 7 occurred in transplant recipients). Abdominal imaging was done in 3 of these episodes (43%), and in 2 of these 3 episodes, imaging was done at an outside institution prior to arrival, with no positive results (eFigure 2).
Echocardiography was done 57% of the time (n = 4), with echo being done at an outside institution prior to arrival half of the time (n = 2), with no endocarditis identified. The cases of abdominal imaging and echo done at outside institutions prior to arrival were not impacted by the countermeasures. Excluding those 2 patients who had both abdominal imaging and echocardiography done prior to arrival, the overall rate of imaging (both abdominal imaging and echo) done after countermeasures were instituted was 30% (Figure 3).
Of those 7 episodes, the average age was 6.8 years (57% female). There were no patients with multiple episodes. The most common presenting complaint was fever. The most common organism was Candida albicans (3 of the 7 episodes). All episodes involved a central line, which was removed in all cases except for one. Of the risk factors, 2 episodes were in neutropenic patients, and 1 episode had a history of bacterial endocarditis (not related to fungemia). No episodes occurred in patients with prior fungal renal infection, urogenital malformations, or recent cardiac/urogenital surgery. No episodes had clinical symptoms suggestive of fungal endocarditis or renal infection. No episodes of fungal endocarditis or renal infection were identified.
On average, a patient at our institution undergoing abdominal US and echo with a cardiology consult results in medical waste of approximately $3200 per patient. This cost does not take into account other miscellaneous charges possibly incurred, such as the radiologist interpreting the findings and transportation. Baseline data calculations show that patients waste on average 55 minutes in physical transport, and this does not take into account wait times.
Discussion
Candidemia contributes to 10% of central-line associated blood stream infections (CLABSI).19 Increased usage of indwelling central catheters for administration of parenteral nutrition will inevitably result in practitioners encountering cases of candidemia when caring for this population. As seen from our results, the majority of episodes of candidemia at our institution occurred on the pediatric gastroenterology service, and thus redefining standard work on this service will be impactful.
Candida parapsilosis and Candida albicans were the most common causative agents before and after intervention, respectively, but overall the most common organism was Candida albicans, which is in keeping with that of CLABSI in the literature.19 Growth of Candida parapsilosis has been particularly linked to CLABSI.19 The third most common organism in our study was Candida glabrata, which is the second most common cause of candidemia in CLABSI.19
The cases of positive abdominal imaging in fungemia in the literature are limited to the neonatal population1-4 and chronic disseminated candidiasis in patients with hematologic malignancies/neutropenia/immunosuppression.5,6 In fungal endocarditis, the reported cases were generally in neonates,1,3,7 critically ill patients,8 patients with hematologic malignancies/neutropenia/immunosuppression,6,9 or those with a cardiac history.9,10 This population differs from the patient population on the pediatric gastroenterology service. Patients on this service may not need US or echo. Performing abdominal US and echo in fungemia patients in whom such imaging is not indicated may result in medical waste of approximately $3200 per patient. There is also a waste of medical resources and time.
We found almost all practitioners are willing to change clinical practice once provided with current guidelines. Face-to-face oral presentations allowed for questions and interaction, making this form of information dissemination better than e-mails or handouts.
Though the numbers were small over the short study period, we were able to decrease overutilization of abdominal imaging and echo after implementing countermeasures. Frequency decreased from 100% to 43% and 57% for abdominal imaging and echo, respectively. Imaging that was done after the countermeasures were implemented was mainly attributed to imaging patients underwent prior to presenting to our institution. This reinforces the need for education at other institutions as well. Of the balancing measures assessed, there were no missed cases of fungal balls or fungal endocarditis. Additionally,
The findings from this quality improvement project underscore current recommendations that, despite common misconceptions, routine abdominal US and echo are not indicated in all cases of fungemia. Case-by-case assessment based on the clinical scenario remains key to management of fungemia to avoid unnecessary medical interventions.
Corresponding author: Donna Cheung, MBBS, 200 Hawkins Drive, BT 1120-G, Iowa City, IA 52242; donna.ann.cheung@gmail.com.
Financial support: None.
1. Benjamin DK Jr, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634-640.
2. Wynn JL, Tan S, Gantz MG, et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis. 2012;54:331-339.
3. Noyola DE, Fernandez M, Moylett EH, et al. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32:1018-1023.
4. Phillips JR, Karlowicz MG Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190-194.
5. Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. Clinical features and outcome of 29 episodes. Haematologica. 2002;87:535-541.
6. Zaoutis TE, Greves HM, Lautenbach E, et al. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635-641.
7. Levy I, Shalit I, Birk E, et al. Candida endocarditis in neonates: report of five cases and review of the literature. Mycoses. 2006;49:43-48.
8. Aspesberro F, Beghetti M, Oberhansli I, et al. Fungal endocarditis in critically ill children. Eur J Pediatr. 1999;158:275-280.
9. Fernandez-Cruz A, Cruz Menarguez M, Munoz P, et al. The search for endocarditis in patients with candidemia: a systematic recommendation for echocardiography? A prospective cohort. Eur J Clin Microbiol Infect Dis. 2015;34:1543-1549.
10. Hernandez-Torres A, Garcia-Vazquez E, Laso-Ortiz A, et al. [Candida sp endocarditis. Experience in a third-level hospital and review of the literature]. Rev Esp Quimioter. 2013;26:51-55.
11. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50.
12. Bow EJ, Evans G, Fuller J, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21:e122-50.
13. Ruhnke M, Rickerts V, Cornely OA, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54:279-310.
14. Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
15. Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38-52.
16. Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18 Suppl 7:53-67.
17. Colombo AL, Guimaraes T, Camargo LF, et al. Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis. 2013;17:283-312.
18. Kohno S, Tamura K, Niki Y, et al. Executive Summary of Japanese Domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016;57:E117-E163.
19. Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiology Rev. 2004;17:255-267.
1. Benjamin DK Jr, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634-640.
2. Wynn JL, Tan S, Gantz MG, et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis. 2012;54:331-339.
3. Noyola DE, Fernandez M, Moylett EH, et al. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32:1018-1023.
4. Phillips JR, Karlowicz MG Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190-194.
5. Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. Clinical features and outcome of 29 episodes. Haematologica. 2002;87:535-541.
6. Zaoutis TE, Greves HM, Lautenbach E, et al. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635-641.
7. Levy I, Shalit I, Birk E, et al. Candida endocarditis in neonates: report of five cases and review of the literature. Mycoses. 2006;49:43-48.
8. Aspesberro F, Beghetti M, Oberhansli I, et al. Fungal endocarditis in critically ill children. Eur J Pediatr. 1999;158:275-280.
9. Fernandez-Cruz A, Cruz Menarguez M, Munoz P, et al. The search for endocarditis in patients with candidemia: a systematic recommendation for echocardiography? A prospective cohort. Eur J Clin Microbiol Infect Dis. 2015;34:1543-1549.
10. Hernandez-Torres A, Garcia-Vazquez E, Laso-Ortiz A, et al. [Candida sp endocarditis. Experience in a third-level hospital and review of the literature]. Rev Esp Quimioter. 2013;26:51-55.
11. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50.
12. Bow EJ, Evans G, Fuller J, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21:e122-50.
13. Ruhnke M, Rickerts V, Cornely OA, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54:279-310.
14. Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
15. Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38-52.
16. Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18 Suppl 7:53-67.
17. Colombo AL, Guimaraes T, Camargo LF, et al. Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis. 2013;17:283-312.
18. Kohno S, Tamura K, Niki Y, et al. Executive Summary of Japanese Domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016;57:E117-E163.
19. Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiology Rev. 2004;17:255-267.
Applying Robust Process Improvement Techniques to the Voluntary Inpatient Psychiatry Admission Process
From the Wake Forest School of Medicine (Ms. Newman), and Wake Forest Baptist Health, Department of Psychiatry and Behavioral Medicine (Dr. Kramer), Winston-Salem, NC.
Abstract
- Background: Adults voluntarily admitted to inpatient behavioral health units can ask to sign a Request to Discharge (RTD) form if they would like to be discharged before the treatment team agrees that discharge is appropriate. This gives the team 72 hours to determine whether the patient is safe to discharge or to involuntarily commit the patient to the unit. At 1 medical center, patients who were offered voluntary admission often lacked complete understanding of the “72-hour rule” and the early discharge procedure.
- Methods: Robust Process Improvement® techniques were implemented to improve the admission process. Flow charts, standardized scripts, and pocket cards were distributed to relevant staff. The Request for Voluntary Admission form was revised to emphasize the “72-hour rule” and the process for requesting a RTD form.
- Results: The unit’s average overall Press Ganey score improved from 77.1 to 81.6 (P = 0.003), while the average discharge score improved from 83.0 to 87.5 (P = 0.023) following implementation of the new process.
- Conclusion: Incorporating strategies such as an opportunity to “teach back” important information about the voluntary admission process (ie, what the 72-hour rule is, what the request to discharge form is, and the possibility of involuntary commitment) allows clinicians to assess capacity while simultaneously giving patients realistic expectations of the admission. These changes can lead to improvement in patient satisfaction.
Keywords: behavioral health; communication; patient satisfaction.
Communication is paramount within medical teams to improve outcomes and strengthen rapport with patients, particularly with psychiatric patients in acute crisis. Studies
While some forms of communication are required to protect the safety of patients and others around them, other forms are required to build strong relationships with patients. However, these 2 goals do not have to be mutually exclusive in the psychiatric hospital environment. Hospitals aim to improve patient satisfaction while simultaneously providing effective communication about treatment. Studies have indicated that communication during graduate medical training may decline due to “emotional and physical brutality” associated with residency training programs.4 To ameliorate this and emphasize communication education, accredited psychiatry residency programs require residents to use structured communication tools to achieve a level 2 in the Accreditation Council for Graduate Medical Education milestone project for the category of patient safety and health care team.5 These standardized processes allow all patients to receive the same important information related to their care while minimizing human error. Such communication skills aim to improve health care outcomes and satisfaction for patients while also training better physicians.
For legal and ethical reasons, the adult inpatient behavioral health units at major hospitals are highly regulated. In most states, a patient who is admitted to an adult inpatient behavioral health unit on a voluntary basis can ask to sign a request to discharge (RTD) form if he or she would like to be discharged from the hospital before the treatment team sees fit.6 In most jurisdictions, this action gives the treatment team 72 hours to determine whether the patient is safe to discharge. Within that time frame, the physician must either discharge the patient, or, if it is not safe to do so, involuntarily commit him or her to the unit. In most jurisdictions, this process is commonly referred to as the “72-hour rule.”
In North Carolina, state legislation Chapter 122C, Article 5, Part 2(b) specifies: “In 24-hour facilities the application shall acknowledge that the applicant may be held by the facility for a period of 72 hours after any written request for release that the applicant may make, and shall acknowledge that the 24-hour facility may have the legal right to petition for involuntary commitment of the applicant during that period. At the time of application, the facility shall tell the applicant about procedures for discharge.”7 This requirement can be somewhat confusing for both medical team members and patients alike.
As formerly practiced on the behavioral health unit described in this report, patients offered voluntary admission status to the inpatient behavioral unit often lacked complete understanding of the 72-hour rule and the process for requesting early discharge from the facility. We hypothesized that this led to the observed patient frustration and hostility, lack of trust in the treatment team, poor attendance and participation in group therapy activities, medication refusal, and overall decreased patient satisfaction. To address this issue, this pilot project was conducted to improve the voluntary admission process on the adult inpatient unit of a major academic medical center in North Carolina.
In April 2008, The Joint Commission’s Center for Transforming Healthcare embarked on an enterprise-wide initiative called Robust Process Improvement (RPI). RPI was developed as a blended approach in applying Six Sigma, Lean, and Change Management techniques to improve medical processes and procedures. RPI techniques were applied in this study to better define the problems related to inpatient behavioral health unit admission and discharge by collecting data, obtaining staff involvement, creating a solution, and monitoring for lasting benefit.
Methods
This quality improvement project took place at an 885-bed tertiary care academic medical center with Level 1 Trauma Center designation. Institutional Review Board approval was not required because this was performed as a quality improvement project rather than an experimental clinical trial and was not designed to create new generalizable knowledge.
The techniques used to improve outcomes on the inpatient behavioral health unit included Active Listening, Elevator Speech, Statistics, Cause and Effect Diagrams, development of a Communication Plan, Brainstorming, and Standard Work. Through interviews with physician assistants, nurses, and resident physicians conducted over a 1-month period, it became clear that there was confusion among patients surrounding the voluntary admission process, the process for requesting discharge, and the possibility of a voluntary admission being converted to an involuntary one. Active listening was used to better understand the opportunities for improvement from multiple perspectives through varying stages of the admission—from the consent process in the emergency department, admission to the unit, throughout the hospital stay, to the time of discharge. The following elevator speech was used to highlight the areas of confusion and the importance of implementing change with the team involved in implementing the new admission procedures:
Our project is about improving patient understanding of the voluntary admission process to the Adult Psychiatry Unit and the 72-hour rule. This is important because the present process leads to patient misunderstanding, discontent on the unit, resistance to provided therapies, and low Press Ganey satisfaction scores. Success will look like reduced patient confusion about the 72-hour rule, increased group participation, decreased patient-staff conflict, and improved Press Ganey scores. What we are asking from you is to use a standardized, scripted informed consent process, flow chart, and pocket card during the voluntary admission process.
Additionally, brainstorming sessions were conducted with physician assistants, nurses, and residents to discuss options to improve the process and elicit a list of barriers.
Data Gathering
Several metrics were tracked to further understand the issue. Overall Press Ganey scores, in addition to admission and discharge subsection scores, were tracked for the 8 months prior to implementing the quality improvement procedures (March 2017-October 2017). Additionally, the treatment team members answered survey questions related to perceived patient understanding at the beginning of training sessions in the 5-week period immediately prior to implementing the quality improvement procedures. This survey was administered using a 6-point Likert scale, ranging from 1 (never) to 6 (frequently), and included the following questions:
- How often do you have to explain: (a) Request for Voluntary Admission form, (b) 72-Hour Rule, (c) Request to Discharge form?
- How often is there confusion about the 72-hour rule and RTD form once admitted to the adult inpatient psychiatric unit?
- How often do you need to re-explain the 72-hour rule and RTD form once admitted to the adult inpatient psychiatric unit?
In-depth interviews were conducted with 4 resident physicians, 4 physician assistants, and 3 nurses to identify specific shortcomings of the admission procedure. A key finding from these interviews was that some patients tended not to understand that the treatment team had 72 hours to respond to the RTD application. Instead, several patients had indicated that they thought that they could immediately discharge themselves since they were on the unit “voluntarily” or that they could categorically discharge themselves after 72 hours of being admitted. This feedback was crucial in determining the next steps that could be taken to minimize confusion.
Process Changes
In preparation for this quality improvement project, the language and layout of the Request for Voluntary Admission form was revised and approved internally by the hospital’s Forms Committee to emphasize the 72-hour rule and the process for completing a RTD form. Additionally, these interviews indicated that it was difficult to track patients who were admitted voluntarily versus involuntarily. To rectify this problem, a field was added to the electronic medical record system to include current psychiatric admission status, allowing the selection to be either “Voluntary” or “Involuntary.” This new field in the electronic medical record system gives nurses the ability to easily update legal status daily as appropriate, which minimizes the risk of information not being effectively communicated at shift changes, while also allowing various members of the treatment team to be updated on the admission status of each patient.
After reviewing data and obtaining staff involvement related to the problem, a new psychiatric admission and consent process was created. The new consent and admission process was characterized by a standardized procedure and scripted language to present to candidates for voluntary admission. The standardized procedure begins with the admitting staff member reading scripted consent language from a pocket card that includes 3 key points describing the voluntary admission procedure (see script in Figure 1). The first key point is to describe the 72-hour rule. Next, the staff member describes the purpose of the RTD form and shows an example to the patient. Finally, the staff member responsible for consenting describes the possibility of the patient being required to remain on the unit involuntarily in the event that he or she wants to leave before the treatment team sees fit and is deemed to be a danger to himself or herself or others.
On the reverse side of the pocket card, an example of the RTD form is available to show to the patient. The subsequent teach-back procedure is summarized using the flow chart in Figure 2, and this was made available to staff who participate in the admission and discharge processes. After reading the consent script, the consenting staff member must ask the patient to recall the 3 key points. For each key point that the patient cannot recall, the relevant section of the scripted language is re-read to the patient, who is asked to explain it again. If the patient recalls the 3 key points, then he or she is deemed to have cognitive capacity and thus can become a candidate for voluntary admission.
Flow charts, scripts, and pocket cards were created and distributed to relevant physicians, physician assistants, and nurses who participate in the admission or discharge process. Additional copies of pocket cards were made available within the department. In October 2017, an attending psychiatrist and medical student trained psychiatry physician assistants, nurses, and resident physicians who participated in the admission process in the ED or patient care on the unit on how to use the new materials. The new process was first implemented on November 1, 2017.
Measurements
Press Ganey scores were compared for 8 months before and 8 months after implementing the new process to monitor changes from the patients’ perspective. Additionally, the treatment team members answered survey questions related to perceived patient understanding at the beginning of training sessions in mid-September through mid-October and again 5 weeks after the new process was implemented.
Results
The behavioral health unit’s Press Ganey (overall and discharge) scores increased during the 8-month period following implementation of the quality improvement project (Figure 3). There was a notable upward trend of overall and discharge Press Ganey scores on a month-by-month basis from November through April. In total, 181 Press Ganey score reports were available for the 6-month period prior to the new process versus 157 score reports after (Figure 4). The average overall Press Ganey score for respondents improved from 77.1 to 81.6 (P = 0.003), while the average discharge score improved from 83.0 to 87.5 (P = 0.023).
In recent months, the behavioral health discharge satisfaction score has become one of the highest performing aspects of the department according to Press Ganey reports. From April through June 2018, the department has performed in the 98th percentile or higher in “information about patient’s rights” during admission and “discharge instructions if help is needed.”
The survey related to perceived patient satisfaction and confusion also indicated significant improvement. Survey respondents indicated that there was less confusion about the 72-hour rule and RTD form after the quality improvement procedure was implemented (P = 0.039) and that fewer attempts to re-explain these concepts were required as well (P = 0.035).
Discussion
The Press Ganey scores for this unit indicated an improvement in patient satisfaction, in particular with the discharge process. While the overall Press Ganey scores on the inpatient behavioral health unit showed a significant improvement, it remained stagnant, around 80, during the 8-month period after implementing the new standardized admission process. However, the discharge score consistently improved over the same 8 months, from 82 to 95 in the most recent month. Also, the overall and discharge scores indicated a brief spike/improvement in October, immediately preceding the implementation of the new scripted language. Given the timeline, this spike is likely related to the ongoing meetings, trainings, and awareness of the upcoming process improvement.
With hospital and health system reimbursements becoming increasingly tied with patient outcomes, quality improvement efforts to improve patient care and satisfaction are of the utmost importance. In order to develop the rapport with patients needed for a high level of cooperation and excellent outcomes on an inpatient psychiatric unit, it is essential that all patients receive specific information about what the admission entails and what the options are for being discharged from the unit. Since a voluntary admission can be converted to an involuntary admission if a patient is deemed a threat to himself or herself or others despite already signing a RTD form, it is essential that this is not only discussed prior to admission, but that these details are explicitly checked for understanding. This allows the treatment team to assess for capacity and the patient to demonstrate informed consent. Differing expectations or understanding in what the voluntary admission or discharge process entails can lead to patient frustration and hostility, lack of trust in the treatment team, poor attendance and participation in group therapy activities, and medication refusal. Altogether, this can lead to longer inpatient stays, increased costs, decreased outcomes, and decreased patient satisfaction.
These initiatives were relatively easy to implement and are backed by evidence that they ultimately increased patient satisfaction. These findings could be extended to other institutions to improve the voluntary admission process and, ultimately, the patient experience. Additionally, the methods could be applied to other patient care processes within psychiatric facilities, and to improve other aspects of the patient care experience that have room for improvement, as illustrated by the department’s Press Ganey subsection scores, or areas that the treatment team would like to focus on.
Limitations
There are several limitations in the design and evaluation of this project. The assessment of patient understanding, especially in psychiatric patients, is very difficult to quantify. The principal measure of assessing patient understanding was limited to health care professional survey results. This may have led to a slight social desirability bias. An objective assessment of understanding directly from the patients was not readily attainable in our study, but future studies could look at this metric in addition to health care professional survey results.
Additionally, the overall Press Ganey scores may be influenced by factors beyond the admission process and the applied improvement procedures. It is difficult to discern whether there were any other factors that also contributed to the overall increase. However, the discharge score was a more direct measure specifically related to the modified procedures, and the temporal association of the intervention with the increased scores suggests that the intervention was responsible.
Conclusion
Standardization of the consent process ensures that all patients receive the necessary information every time in busy clinical settings. Incorporating an opportunity to “teach back” specific important information about the voluntary admission process, specifically what the 72-hour rule is, what the RTD form is, and the possibility of involuntary commitment, allows clinicians to assess capacity, while simultaneously allowing patients to have realistic expectations of the admission. Concise, standardized answers regarding these points minimizes variation in information being dispersed and decreases the possibility of omitting important information. At a major academic medical center, easy-to-implement quality improvement techniques significantly decreased patient confusion surrounding the 72-hour rule and the RTD form, along with the frequency in which these policies needed to be re-explained on the adult inpatient psychiatric unit. These changes ultimately led to improvement in patient satisfaction, as indicated by significant improvement in both overall and discharge patient satisfaction scores.
Corresponding author: Jennifer F. Newman, 475 Vine St., Winston-Salem, NC, 27101; jnewman9@u.rochester.edu.
Financial disclosures: None.
1. Redfern E, Brown R, Vincent C. Improving communication in the emergency department. Emerg Med J. 2009;26:658-661.
2. Payne CE, Stein JM, Leong T, Dressler DD. Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21:925-932.
3. Weller J, Boyd M, Cumin D. Teams, tribes and patient safety: overcoming barriers to effective teamwork in healthcare. Postgrad Med J. 2014;90:149-154.
4. DiMatteo MR. The role of the physician in the emerging health care environment. Western J Med. 1998;168:328.
5. The Psychiatry Milestone Project. J Grad Med Educ. 2014;6(1 Suppl 1):284-304.
6. Garakani A, Shalenberg E, Burstin SC, et al. Voluntary psychiatric hospitalization and patient-driven requests for discharge: a statutory review and analysis of implications for the capacity to consent to voluntary hospitalization. Harv Rev Psychiatry. 2014;22:241-249.
7. Procedure for Admission and Discharge of Clients Act, 211 § 122C-211(2014).
From the Wake Forest School of Medicine (Ms. Newman), and Wake Forest Baptist Health, Department of Psychiatry and Behavioral Medicine (Dr. Kramer), Winston-Salem, NC.
Abstract
- Background: Adults voluntarily admitted to inpatient behavioral health units can ask to sign a Request to Discharge (RTD) form if they would like to be discharged before the treatment team agrees that discharge is appropriate. This gives the team 72 hours to determine whether the patient is safe to discharge or to involuntarily commit the patient to the unit. At 1 medical center, patients who were offered voluntary admission often lacked complete understanding of the “72-hour rule” and the early discharge procedure.
- Methods: Robust Process Improvement® techniques were implemented to improve the admission process. Flow charts, standardized scripts, and pocket cards were distributed to relevant staff. The Request for Voluntary Admission form was revised to emphasize the “72-hour rule” and the process for requesting a RTD form.
- Results: The unit’s average overall Press Ganey score improved from 77.1 to 81.6 (P = 0.003), while the average discharge score improved from 83.0 to 87.5 (P = 0.023) following implementation of the new process.
- Conclusion: Incorporating strategies such as an opportunity to “teach back” important information about the voluntary admission process (ie, what the 72-hour rule is, what the request to discharge form is, and the possibility of involuntary commitment) allows clinicians to assess capacity while simultaneously giving patients realistic expectations of the admission. These changes can lead to improvement in patient satisfaction.
Keywords: behavioral health; communication; patient satisfaction.
Communication is paramount within medical teams to improve outcomes and strengthen rapport with patients, particularly with psychiatric patients in acute crisis. Studies
While some forms of communication are required to protect the safety of patients and others around them, other forms are required to build strong relationships with patients. However, these 2 goals do not have to be mutually exclusive in the psychiatric hospital environment. Hospitals aim to improve patient satisfaction while simultaneously providing effective communication about treatment. Studies have indicated that communication during graduate medical training may decline due to “emotional and physical brutality” associated with residency training programs.4 To ameliorate this and emphasize communication education, accredited psychiatry residency programs require residents to use structured communication tools to achieve a level 2 in the Accreditation Council for Graduate Medical Education milestone project for the category of patient safety and health care team.5 These standardized processes allow all patients to receive the same important information related to their care while minimizing human error. Such communication skills aim to improve health care outcomes and satisfaction for patients while also training better physicians.
For legal and ethical reasons, the adult inpatient behavioral health units at major hospitals are highly regulated. In most states, a patient who is admitted to an adult inpatient behavioral health unit on a voluntary basis can ask to sign a request to discharge (RTD) form if he or she would like to be discharged from the hospital before the treatment team sees fit.6 In most jurisdictions, this action gives the treatment team 72 hours to determine whether the patient is safe to discharge. Within that time frame, the physician must either discharge the patient, or, if it is not safe to do so, involuntarily commit him or her to the unit. In most jurisdictions, this process is commonly referred to as the “72-hour rule.”
In North Carolina, state legislation Chapter 122C, Article 5, Part 2(b) specifies: “In 24-hour facilities the application shall acknowledge that the applicant may be held by the facility for a period of 72 hours after any written request for release that the applicant may make, and shall acknowledge that the 24-hour facility may have the legal right to petition for involuntary commitment of the applicant during that period. At the time of application, the facility shall tell the applicant about procedures for discharge.”7 This requirement can be somewhat confusing for both medical team members and patients alike.
As formerly practiced on the behavioral health unit described in this report, patients offered voluntary admission status to the inpatient behavioral unit often lacked complete understanding of the 72-hour rule and the process for requesting early discharge from the facility. We hypothesized that this led to the observed patient frustration and hostility, lack of trust in the treatment team, poor attendance and participation in group therapy activities, medication refusal, and overall decreased patient satisfaction. To address this issue, this pilot project was conducted to improve the voluntary admission process on the adult inpatient unit of a major academic medical center in North Carolina.
In April 2008, The Joint Commission’s Center for Transforming Healthcare embarked on an enterprise-wide initiative called Robust Process Improvement (RPI). RPI was developed as a blended approach in applying Six Sigma, Lean, and Change Management techniques to improve medical processes and procedures. RPI techniques were applied in this study to better define the problems related to inpatient behavioral health unit admission and discharge by collecting data, obtaining staff involvement, creating a solution, and monitoring for lasting benefit.
Methods
This quality improvement project took place at an 885-bed tertiary care academic medical center with Level 1 Trauma Center designation. Institutional Review Board approval was not required because this was performed as a quality improvement project rather than an experimental clinical trial and was not designed to create new generalizable knowledge.
The techniques used to improve outcomes on the inpatient behavioral health unit included Active Listening, Elevator Speech, Statistics, Cause and Effect Diagrams, development of a Communication Plan, Brainstorming, and Standard Work. Through interviews with physician assistants, nurses, and resident physicians conducted over a 1-month period, it became clear that there was confusion among patients surrounding the voluntary admission process, the process for requesting discharge, and the possibility of a voluntary admission being converted to an involuntary one. Active listening was used to better understand the opportunities for improvement from multiple perspectives through varying stages of the admission—from the consent process in the emergency department, admission to the unit, throughout the hospital stay, to the time of discharge. The following elevator speech was used to highlight the areas of confusion and the importance of implementing change with the team involved in implementing the new admission procedures:
Our project is about improving patient understanding of the voluntary admission process to the Adult Psychiatry Unit and the 72-hour rule. This is important because the present process leads to patient misunderstanding, discontent on the unit, resistance to provided therapies, and low Press Ganey satisfaction scores. Success will look like reduced patient confusion about the 72-hour rule, increased group participation, decreased patient-staff conflict, and improved Press Ganey scores. What we are asking from you is to use a standardized, scripted informed consent process, flow chart, and pocket card during the voluntary admission process.
Additionally, brainstorming sessions were conducted with physician assistants, nurses, and residents to discuss options to improve the process and elicit a list of barriers.
Data Gathering
Several metrics were tracked to further understand the issue. Overall Press Ganey scores, in addition to admission and discharge subsection scores, were tracked for the 8 months prior to implementing the quality improvement procedures (March 2017-October 2017). Additionally, the treatment team members answered survey questions related to perceived patient understanding at the beginning of training sessions in the 5-week period immediately prior to implementing the quality improvement procedures. This survey was administered using a 6-point Likert scale, ranging from 1 (never) to 6 (frequently), and included the following questions:
- How often do you have to explain: (a) Request for Voluntary Admission form, (b) 72-Hour Rule, (c) Request to Discharge form?
- How often is there confusion about the 72-hour rule and RTD form once admitted to the adult inpatient psychiatric unit?
- How often do you need to re-explain the 72-hour rule and RTD form once admitted to the adult inpatient psychiatric unit?
In-depth interviews were conducted with 4 resident physicians, 4 physician assistants, and 3 nurses to identify specific shortcomings of the admission procedure. A key finding from these interviews was that some patients tended not to understand that the treatment team had 72 hours to respond to the RTD application. Instead, several patients had indicated that they thought that they could immediately discharge themselves since they were on the unit “voluntarily” or that they could categorically discharge themselves after 72 hours of being admitted. This feedback was crucial in determining the next steps that could be taken to minimize confusion.
Process Changes
In preparation for this quality improvement project, the language and layout of the Request for Voluntary Admission form was revised and approved internally by the hospital’s Forms Committee to emphasize the 72-hour rule and the process for completing a RTD form. Additionally, these interviews indicated that it was difficult to track patients who were admitted voluntarily versus involuntarily. To rectify this problem, a field was added to the electronic medical record system to include current psychiatric admission status, allowing the selection to be either “Voluntary” or “Involuntary.” This new field in the electronic medical record system gives nurses the ability to easily update legal status daily as appropriate, which minimizes the risk of information not being effectively communicated at shift changes, while also allowing various members of the treatment team to be updated on the admission status of each patient.
After reviewing data and obtaining staff involvement related to the problem, a new psychiatric admission and consent process was created. The new consent and admission process was characterized by a standardized procedure and scripted language to present to candidates for voluntary admission. The standardized procedure begins with the admitting staff member reading scripted consent language from a pocket card that includes 3 key points describing the voluntary admission procedure (see script in Figure 1). The first key point is to describe the 72-hour rule. Next, the staff member describes the purpose of the RTD form and shows an example to the patient. Finally, the staff member responsible for consenting describes the possibility of the patient being required to remain on the unit involuntarily in the event that he or she wants to leave before the treatment team sees fit and is deemed to be a danger to himself or herself or others.
On the reverse side of the pocket card, an example of the RTD form is available to show to the patient. The subsequent teach-back procedure is summarized using the flow chart in Figure 2, and this was made available to staff who participate in the admission and discharge processes. After reading the consent script, the consenting staff member must ask the patient to recall the 3 key points. For each key point that the patient cannot recall, the relevant section of the scripted language is re-read to the patient, who is asked to explain it again. If the patient recalls the 3 key points, then he or she is deemed to have cognitive capacity and thus can become a candidate for voluntary admission.
Flow charts, scripts, and pocket cards were created and distributed to relevant physicians, physician assistants, and nurses who participate in the admission or discharge process. Additional copies of pocket cards were made available within the department. In October 2017, an attending psychiatrist and medical student trained psychiatry physician assistants, nurses, and resident physicians who participated in the admission process in the ED or patient care on the unit on how to use the new materials. The new process was first implemented on November 1, 2017.
Measurements
Press Ganey scores were compared for 8 months before and 8 months after implementing the new process to monitor changes from the patients’ perspective. Additionally, the treatment team members answered survey questions related to perceived patient understanding at the beginning of training sessions in mid-September through mid-October and again 5 weeks after the new process was implemented.
Results
The behavioral health unit’s Press Ganey (overall and discharge) scores increased during the 8-month period following implementation of the quality improvement project (Figure 3). There was a notable upward trend of overall and discharge Press Ganey scores on a month-by-month basis from November through April. In total, 181 Press Ganey score reports were available for the 6-month period prior to the new process versus 157 score reports after (Figure 4). The average overall Press Ganey score for respondents improved from 77.1 to 81.6 (P = 0.003), while the average discharge score improved from 83.0 to 87.5 (P = 0.023).
In recent months, the behavioral health discharge satisfaction score has become one of the highest performing aspects of the department according to Press Ganey reports. From April through June 2018, the department has performed in the 98th percentile or higher in “information about patient’s rights” during admission and “discharge instructions if help is needed.”
The survey related to perceived patient satisfaction and confusion also indicated significant improvement. Survey respondents indicated that there was less confusion about the 72-hour rule and RTD form after the quality improvement procedure was implemented (P = 0.039) and that fewer attempts to re-explain these concepts were required as well (P = 0.035).
Discussion
The Press Ganey scores for this unit indicated an improvement in patient satisfaction, in particular with the discharge process. While the overall Press Ganey scores on the inpatient behavioral health unit showed a significant improvement, it remained stagnant, around 80, during the 8-month period after implementing the new standardized admission process. However, the discharge score consistently improved over the same 8 months, from 82 to 95 in the most recent month. Also, the overall and discharge scores indicated a brief spike/improvement in October, immediately preceding the implementation of the new scripted language. Given the timeline, this spike is likely related to the ongoing meetings, trainings, and awareness of the upcoming process improvement.
With hospital and health system reimbursements becoming increasingly tied with patient outcomes, quality improvement efforts to improve patient care and satisfaction are of the utmost importance. In order to develop the rapport with patients needed for a high level of cooperation and excellent outcomes on an inpatient psychiatric unit, it is essential that all patients receive specific information about what the admission entails and what the options are for being discharged from the unit. Since a voluntary admission can be converted to an involuntary admission if a patient is deemed a threat to himself or herself or others despite already signing a RTD form, it is essential that this is not only discussed prior to admission, but that these details are explicitly checked for understanding. This allows the treatment team to assess for capacity and the patient to demonstrate informed consent. Differing expectations or understanding in what the voluntary admission or discharge process entails can lead to patient frustration and hostility, lack of trust in the treatment team, poor attendance and participation in group therapy activities, and medication refusal. Altogether, this can lead to longer inpatient stays, increased costs, decreased outcomes, and decreased patient satisfaction.
These initiatives were relatively easy to implement and are backed by evidence that they ultimately increased patient satisfaction. These findings could be extended to other institutions to improve the voluntary admission process and, ultimately, the patient experience. Additionally, the methods could be applied to other patient care processes within psychiatric facilities, and to improve other aspects of the patient care experience that have room for improvement, as illustrated by the department’s Press Ganey subsection scores, or areas that the treatment team would like to focus on.
Limitations
There are several limitations in the design and evaluation of this project. The assessment of patient understanding, especially in psychiatric patients, is very difficult to quantify. The principal measure of assessing patient understanding was limited to health care professional survey results. This may have led to a slight social desirability bias. An objective assessment of understanding directly from the patients was not readily attainable in our study, but future studies could look at this metric in addition to health care professional survey results.
Additionally, the overall Press Ganey scores may be influenced by factors beyond the admission process and the applied improvement procedures. It is difficult to discern whether there were any other factors that also contributed to the overall increase. However, the discharge score was a more direct measure specifically related to the modified procedures, and the temporal association of the intervention with the increased scores suggests that the intervention was responsible.
Conclusion
Standardization of the consent process ensures that all patients receive the necessary information every time in busy clinical settings. Incorporating an opportunity to “teach back” specific important information about the voluntary admission process, specifically what the 72-hour rule is, what the RTD form is, and the possibility of involuntary commitment, allows clinicians to assess capacity, while simultaneously allowing patients to have realistic expectations of the admission. Concise, standardized answers regarding these points minimizes variation in information being dispersed and decreases the possibility of omitting important information. At a major academic medical center, easy-to-implement quality improvement techniques significantly decreased patient confusion surrounding the 72-hour rule and the RTD form, along with the frequency in which these policies needed to be re-explained on the adult inpatient psychiatric unit. These changes ultimately led to improvement in patient satisfaction, as indicated by significant improvement in both overall and discharge patient satisfaction scores.
Corresponding author: Jennifer F. Newman, 475 Vine St., Winston-Salem, NC, 27101; jnewman9@u.rochester.edu.
Financial disclosures: None.
From the Wake Forest School of Medicine (Ms. Newman), and Wake Forest Baptist Health, Department of Psychiatry and Behavioral Medicine (Dr. Kramer), Winston-Salem, NC.
Abstract
- Background: Adults voluntarily admitted to inpatient behavioral health units can ask to sign a Request to Discharge (RTD) form if they would like to be discharged before the treatment team agrees that discharge is appropriate. This gives the team 72 hours to determine whether the patient is safe to discharge or to involuntarily commit the patient to the unit. At 1 medical center, patients who were offered voluntary admission often lacked complete understanding of the “72-hour rule” and the early discharge procedure.
- Methods: Robust Process Improvement® techniques were implemented to improve the admission process. Flow charts, standardized scripts, and pocket cards were distributed to relevant staff. The Request for Voluntary Admission form was revised to emphasize the “72-hour rule” and the process for requesting a RTD form.
- Results: The unit’s average overall Press Ganey score improved from 77.1 to 81.6 (P = 0.003), while the average discharge score improved from 83.0 to 87.5 (P = 0.023) following implementation of the new process.
- Conclusion: Incorporating strategies such as an opportunity to “teach back” important information about the voluntary admission process (ie, what the 72-hour rule is, what the request to discharge form is, and the possibility of involuntary commitment) allows clinicians to assess capacity while simultaneously giving patients realistic expectations of the admission. These changes can lead to improvement in patient satisfaction.
Keywords: behavioral health; communication; patient satisfaction.
Communication is paramount within medical teams to improve outcomes and strengthen rapport with patients, particularly with psychiatric patients in acute crisis. Studies
While some forms of communication are required to protect the safety of patients and others around them, other forms are required to build strong relationships with patients. However, these 2 goals do not have to be mutually exclusive in the psychiatric hospital environment. Hospitals aim to improve patient satisfaction while simultaneously providing effective communication about treatment. Studies have indicated that communication during graduate medical training may decline due to “emotional and physical brutality” associated with residency training programs.4 To ameliorate this and emphasize communication education, accredited psychiatry residency programs require residents to use structured communication tools to achieve a level 2 in the Accreditation Council for Graduate Medical Education milestone project for the category of patient safety and health care team.5 These standardized processes allow all patients to receive the same important information related to their care while minimizing human error. Such communication skills aim to improve health care outcomes and satisfaction for patients while also training better physicians.
For legal and ethical reasons, the adult inpatient behavioral health units at major hospitals are highly regulated. In most states, a patient who is admitted to an adult inpatient behavioral health unit on a voluntary basis can ask to sign a request to discharge (RTD) form if he or she would like to be discharged from the hospital before the treatment team sees fit.6 In most jurisdictions, this action gives the treatment team 72 hours to determine whether the patient is safe to discharge. Within that time frame, the physician must either discharge the patient, or, if it is not safe to do so, involuntarily commit him or her to the unit. In most jurisdictions, this process is commonly referred to as the “72-hour rule.”
In North Carolina, state legislation Chapter 122C, Article 5, Part 2(b) specifies: “In 24-hour facilities the application shall acknowledge that the applicant may be held by the facility for a period of 72 hours after any written request for release that the applicant may make, and shall acknowledge that the 24-hour facility may have the legal right to petition for involuntary commitment of the applicant during that period. At the time of application, the facility shall tell the applicant about procedures for discharge.”7 This requirement can be somewhat confusing for both medical team members and patients alike.
As formerly practiced on the behavioral health unit described in this report, patients offered voluntary admission status to the inpatient behavioral unit often lacked complete understanding of the 72-hour rule and the process for requesting early discharge from the facility. We hypothesized that this led to the observed patient frustration and hostility, lack of trust in the treatment team, poor attendance and participation in group therapy activities, medication refusal, and overall decreased patient satisfaction. To address this issue, this pilot project was conducted to improve the voluntary admission process on the adult inpatient unit of a major academic medical center in North Carolina.
In April 2008, The Joint Commission’s Center for Transforming Healthcare embarked on an enterprise-wide initiative called Robust Process Improvement (RPI). RPI was developed as a blended approach in applying Six Sigma, Lean, and Change Management techniques to improve medical processes and procedures. RPI techniques were applied in this study to better define the problems related to inpatient behavioral health unit admission and discharge by collecting data, obtaining staff involvement, creating a solution, and monitoring for lasting benefit.
Methods
This quality improvement project took place at an 885-bed tertiary care academic medical center with Level 1 Trauma Center designation. Institutional Review Board approval was not required because this was performed as a quality improvement project rather than an experimental clinical trial and was not designed to create new generalizable knowledge.
The techniques used to improve outcomes on the inpatient behavioral health unit included Active Listening, Elevator Speech, Statistics, Cause and Effect Diagrams, development of a Communication Plan, Brainstorming, and Standard Work. Through interviews with physician assistants, nurses, and resident physicians conducted over a 1-month period, it became clear that there was confusion among patients surrounding the voluntary admission process, the process for requesting discharge, and the possibility of a voluntary admission being converted to an involuntary one. Active listening was used to better understand the opportunities for improvement from multiple perspectives through varying stages of the admission—from the consent process in the emergency department, admission to the unit, throughout the hospital stay, to the time of discharge. The following elevator speech was used to highlight the areas of confusion and the importance of implementing change with the team involved in implementing the new admission procedures:
Our project is about improving patient understanding of the voluntary admission process to the Adult Psychiatry Unit and the 72-hour rule. This is important because the present process leads to patient misunderstanding, discontent on the unit, resistance to provided therapies, and low Press Ganey satisfaction scores. Success will look like reduced patient confusion about the 72-hour rule, increased group participation, decreased patient-staff conflict, and improved Press Ganey scores. What we are asking from you is to use a standardized, scripted informed consent process, flow chart, and pocket card during the voluntary admission process.
Additionally, brainstorming sessions were conducted with physician assistants, nurses, and residents to discuss options to improve the process and elicit a list of barriers.
Data Gathering
Several metrics were tracked to further understand the issue. Overall Press Ganey scores, in addition to admission and discharge subsection scores, were tracked for the 8 months prior to implementing the quality improvement procedures (March 2017-October 2017). Additionally, the treatment team members answered survey questions related to perceived patient understanding at the beginning of training sessions in the 5-week period immediately prior to implementing the quality improvement procedures. This survey was administered using a 6-point Likert scale, ranging from 1 (never) to 6 (frequently), and included the following questions:
- How often do you have to explain: (a) Request for Voluntary Admission form, (b) 72-Hour Rule, (c) Request to Discharge form?
- How often is there confusion about the 72-hour rule and RTD form once admitted to the adult inpatient psychiatric unit?
- How often do you need to re-explain the 72-hour rule and RTD form once admitted to the adult inpatient psychiatric unit?
In-depth interviews were conducted with 4 resident physicians, 4 physician assistants, and 3 nurses to identify specific shortcomings of the admission procedure. A key finding from these interviews was that some patients tended not to understand that the treatment team had 72 hours to respond to the RTD application. Instead, several patients had indicated that they thought that they could immediately discharge themselves since they were on the unit “voluntarily” or that they could categorically discharge themselves after 72 hours of being admitted. This feedback was crucial in determining the next steps that could be taken to minimize confusion.
Process Changes
In preparation for this quality improvement project, the language and layout of the Request for Voluntary Admission form was revised and approved internally by the hospital’s Forms Committee to emphasize the 72-hour rule and the process for completing a RTD form. Additionally, these interviews indicated that it was difficult to track patients who were admitted voluntarily versus involuntarily. To rectify this problem, a field was added to the electronic medical record system to include current psychiatric admission status, allowing the selection to be either “Voluntary” or “Involuntary.” This new field in the electronic medical record system gives nurses the ability to easily update legal status daily as appropriate, which minimizes the risk of information not being effectively communicated at shift changes, while also allowing various members of the treatment team to be updated on the admission status of each patient.
After reviewing data and obtaining staff involvement related to the problem, a new psychiatric admission and consent process was created. The new consent and admission process was characterized by a standardized procedure and scripted language to present to candidates for voluntary admission. The standardized procedure begins with the admitting staff member reading scripted consent language from a pocket card that includes 3 key points describing the voluntary admission procedure (see script in Figure 1). The first key point is to describe the 72-hour rule. Next, the staff member describes the purpose of the RTD form and shows an example to the patient. Finally, the staff member responsible for consenting describes the possibility of the patient being required to remain on the unit involuntarily in the event that he or she wants to leave before the treatment team sees fit and is deemed to be a danger to himself or herself or others.
On the reverse side of the pocket card, an example of the RTD form is available to show to the patient. The subsequent teach-back procedure is summarized using the flow chart in Figure 2, and this was made available to staff who participate in the admission and discharge processes. After reading the consent script, the consenting staff member must ask the patient to recall the 3 key points. For each key point that the patient cannot recall, the relevant section of the scripted language is re-read to the patient, who is asked to explain it again. If the patient recalls the 3 key points, then he or she is deemed to have cognitive capacity and thus can become a candidate for voluntary admission.
Flow charts, scripts, and pocket cards were created and distributed to relevant physicians, physician assistants, and nurses who participate in the admission or discharge process. Additional copies of pocket cards were made available within the department. In October 2017, an attending psychiatrist and medical student trained psychiatry physician assistants, nurses, and resident physicians who participated in the admission process in the ED or patient care on the unit on how to use the new materials. The new process was first implemented on November 1, 2017.
Measurements
Press Ganey scores were compared for 8 months before and 8 months after implementing the new process to monitor changes from the patients’ perspective. Additionally, the treatment team members answered survey questions related to perceived patient understanding at the beginning of training sessions in mid-September through mid-October and again 5 weeks after the new process was implemented.
Results
The behavioral health unit’s Press Ganey (overall and discharge) scores increased during the 8-month period following implementation of the quality improvement project (Figure 3). There was a notable upward trend of overall and discharge Press Ganey scores on a month-by-month basis from November through April. In total, 181 Press Ganey score reports were available for the 6-month period prior to the new process versus 157 score reports after (Figure 4). The average overall Press Ganey score for respondents improved from 77.1 to 81.6 (P = 0.003), while the average discharge score improved from 83.0 to 87.5 (P = 0.023).
In recent months, the behavioral health discharge satisfaction score has become one of the highest performing aspects of the department according to Press Ganey reports. From April through June 2018, the department has performed in the 98th percentile or higher in “information about patient’s rights” during admission and “discharge instructions if help is needed.”
The survey related to perceived patient satisfaction and confusion also indicated significant improvement. Survey respondents indicated that there was less confusion about the 72-hour rule and RTD form after the quality improvement procedure was implemented (P = 0.039) and that fewer attempts to re-explain these concepts were required as well (P = 0.035).
Discussion
The Press Ganey scores for this unit indicated an improvement in patient satisfaction, in particular with the discharge process. While the overall Press Ganey scores on the inpatient behavioral health unit showed a significant improvement, it remained stagnant, around 80, during the 8-month period after implementing the new standardized admission process. However, the discharge score consistently improved over the same 8 months, from 82 to 95 in the most recent month. Also, the overall and discharge scores indicated a brief spike/improvement in October, immediately preceding the implementation of the new scripted language. Given the timeline, this spike is likely related to the ongoing meetings, trainings, and awareness of the upcoming process improvement.
With hospital and health system reimbursements becoming increasingly tied with patient outcomes, quality improvement efforts to improve patient care and satisfaction are of the utmost importance. In order to develop the rapport with patients needed for a high level of cooperation and excellent outcomes on an inpatient psychiatric unit, it is essential that all patients receive specific information about what the admission entails and what the options are for being discharged from the unit. Since a voluntary admission can be converted to an involuntary admission if a patient is deemed a threat to himself or herself or others despite already signing a RTD form, it is essential that this is not only discussed prior to admission, but that these details are explicitly checked for understanding. This allows the treatment team to assess for capacity and the patient to demonstrate informed consent. Differing expectations or understanding in what the voluntary admission or discharge process entails can lead to patient frustration and hostility, lack of trust in the treatment team, poor attendance and participation in group therapy activities, and medication refusal. Altogether, this can lead to longer inpatient stays, increased costs, decreased outcomes, and decreased patient satisfaction.
These initiatives were relatively easy to implement and are backed by evidence that they ultimately increased patient satisfaction. These findings could be extended to other institutions to improve the voluntary admission process and, ultimately, the patient experience. Additionally, the methods could be applied to other patient care processes within psychiatric facilities, and to improve other aspects of the patient care experience that have room for improvement, as illustrated by the department’s Press Ganey subsection scores, or areas that the treatment team would like to focus on.
Limitations
There are several limitations in the design and evaluation of this project. The assessment of patient understanding, especially in psychiatric patients, is very difficult to quantify. The principal measure of assessing patient understanding was limited to health care professional survey results. This may have led to a slight social desirability bias. An objective assessment of understanding directly from the patients was not readily attainable in our study, but future studies could look at this metric in addition to health care professional survey results.
Additionally, the overall Press Ganey scores may be influenced by factors beyond the admission process and the applied improvement procedures. It is difficult to discern whether there were any other factors that also contributed to the overall increase. However, the discharge score was a more direct measure specifically related to the modified procedures, and the temporal association of the intervention with the increased scores suggests that the intervention was responsible.
Conclusion
Standardization of the consent process ensures that all patients receive the necessary information every time in busy clinical settings. Incorporating an opportunity to “teach back” specific important information about the voluntary admission process, specifically what the 72-hour rule is, what the RTD form is, and the possibility of involuntary commitment, allows clinicians to assess capacity, while simultaneously allowing patients to have realistic expectations of the admission. Concise, standardized answers regarding these points minimizes variation in information being dispersed and decreases the possibility of omitting important information. At a major academic medical center, easy-to-implement quality improvement techniques significantly decreased patient confusion surrounding the 72-hour rule and the RTD form, along with the frequency in which these policies needed to be re-explained on the adult inpatient psychiatric unit. These changes ultimately led to improvement in patient satisfaction, as indicated by significant improvement in both overall and discharge patient satisfaction scores.
Corresponding author: Jennifer F. Newman, 475 Vine St., Winston-Salem, NC, 27101; jnewman9@u.rochester.edu.
Financial disclosures: None.
1. Redfern E, Brown R, Vincent C. Improving communication in the emergency department. Emerg Med J. 2009;26:658-661.
2. Payne CE, Stein JM, Leong T, Dressler DD. Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21:925-932.
3. Weller J, Boyd M, Cumin D. Teams, tribes and patient safety: overcoming barriers to effective teamwork in healthcare. Postgrad Med J. 2014;90:149-154.
4. DiMatteo MR. The role of the physician in the emerging health care environment. Western J Med. 1998;168:328.
5. The Psychiatry Milestone Project. J Grad Med Educ. 2014;6(1 Suppl 1):284-304.
6. Garakani A, Shalenberg E, Burstin SC, et al. Voluntary psychiatric hospitalization and patient-driven requests for discharge: a statutory review and analysis of implications for the capacity to consent to voluntary hospitalization. Harv Rev Psychiatry. 2014;22:241-249.
7. Procedure for Admission and Discharge of Clients Act, 211 § 122C-211(2014).
1. Redfern E, Brown R, Vincent C. Improving communication in the emergency department. Emerg Med J. 2009;26:658-661.
2. Payne CE, Stein JM, Leong T, Dressler DD. Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21:925-932.
3. Weller J, Boyd M, Cumin D. Teams, tribes and patient safety: overcoming barriers to effective teamwork in healthcare. Postgrad Med J. 2014;90:149-154.
4. DiMatteo MR. The role of the physician in the emerging health care environment. Western J Med. 1998;168:328.
5. The Psychiatry Milestone Project. J Grad Med Educ. 2014;6(1 Suppl 1):284-304.
6. Garakani A, Shalenberg E, Burstin SC, et al. Voluntary psychiatric hospitalization and patient-driven requests for discharge: a statutory review and analysis of implications for the capacity to consent to voluntary hospitalization. Harv Rev Psychiatry. 2014;22:241-249.
7. Procedure for Admission and Discharge of Clients Act, 211 § 122C-211(2014).
Health policy Q&A: Oncology Care Model
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
Free HIV self-tests for at-risk groups can increase awareness, testing frequency
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Providing free HIV self-tests can lead to increased testing and more newly identified infections.
Major finding: About 77% of participants in the self-testing group tested three times or more in 12 months, compared with 22% of controls.
Study details: A 12-month longitudinal, two-group, randomized clinical trial of 2,665 men who have sex with men.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network.
Source: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
Optimizing a Total Joint Replacement Care Pathway to Reduce Skilled Nursing Facility Utilization
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.
Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.
The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).
Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; ggascon@ohiohealthgroup.com.
Financial disclosures: None.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.
Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.
The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).
Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; ggascon@ohiohealthgroup.com.
Financial disclosures: None.
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.
Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.
The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).
Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; ggascon@ohiohealthgroup.com.
Financial disclosures: None.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
Advanced team-based care: How we made it work
Leaders in health care and practicing physicians recognize the need for changes in how health care is delivered.1-3 Despite this awareness, though, barriers to meaningful change persist and the current practice environment wherein physicians must routinely spend 2 hours on electronic health records (EHRs) and desk work for every hour of direct face time with patients4 is driving trainees away from ambulatory specialties and is contributing to physicians’ decisions to reduce their practices to part-time, retire early, or leave medicine altogether.5,6 Those who persevere in this environment with heavy administrative burdens run the increasing risk of burnout.7
Some physicians and practices are responding by taking creative measures to reform the way patient care is delivered. Bellin Health—a 160-provider, multispecialty health system in northeast Wisconsin where one of the authors (JJ) works—introduced an advanced team-based care (aTBC) model between November 2014 and November 2018, starting with our primary care providers. The development and introduction of this new model arose from an iterative, multidisciplinary process driven by the desire to transform the Triple Aim—enhancing patient experience, improving population health, and reducing costs—into a Quadruple Aim8 by additionally focusing on improving the work life of health care providers, which, in turn, will help achieve the first 3 goals. In introducing an aTBC model, Bellin Health focused on 3 elements: office visit redesign, in-basket management redesign, and the use of extended care team members and system and community resources to assist in the care of complex and high-risk patients.
Herein we describe the 3 components of our aTBC model,1,9 identify the barriers that existed in the minds of multiple stakeholders (from patients to clinicians and Bellin executives), and describe the strategies that enabled us to overcome these barriers.
The impetus behind our move to aTBC
Bellin Health considered a move to an aTBC model to be critical in light of factors in the health care environment, in general, and at Bellin, in particular. The factors included
- an industry-wide shift to value-based payments, which requires new models for long-term financial viability.
- recognition that physician and medical staff burnout leads to lower productivity and, in some cases, workforce losses.5,6 Replacing a physician in a practice can be difficult and expensive, with cost estimates of $500,000 to more than $1 million per physician.10,11
- a belief that aTBC could help the Bellin Health leadership team meet its organizational goals of improved patient satisfaction, achieve gains in quality measures, enhance engagement and loyalty among patients and employees, and lower recruitment costs.
A 3-part aTBC initiative
■ Part 1: Redesign the office visit
We redesigned staffing and workflow for office visits to maximize the core skills of physicians, which required distributing ancillary tasks among support staff. We up-trained certified medical assistants (CMAs) and licensed practical nurses (LPNs) to take on the new role of care team coordinator (CTC) and optimized the direct clinical support ratio for busier physicians. For physicians who were seeing 15 to 19 patients a day, a ratio of 3 CTCs to 2 physicians was implemented; for those seeing 20 or more patients a day, we used a support ratio of 2:1.
The role of CTC was designed so that he or she would accompany a patient throughout the entire appointment. Responsibilities were broken out as follows:
Pre-visit. Before the physician enters the room, the CTC would now perform expanded rooming functions including pending orders, refill management, care gap closure using standing orders, agenda setting, and preliminary documentation.12
Visit. The CTC would now hand off the patient to the physician and stay in the room to document details of the visit and record new orders for consults, x-ray films, referrals, or prescriptions.13 This intensive EHR support was established to ensure that the physician could focus directly on the patient without the distraction of the computer.
Continue to: Post-visit
Post-visit. After a physician leaves a room, the CTC was now charged with finishing the pending orders, setting up the patient’s next appointment and pre-visit labs, reviewing details of the after-visit summary, and doing any basic health coaching with the patient. During this time, the physician would use the co-location space to review and edit the documentation, cosign the orders and prescriptions submitted by the CTC, and close the chart before going into the next room with the second CTC. The need to revisit these details after clinic hours was eliminated.
Another change … The role of our phone triage registered nurses (RN) was expanded. Care team RNs began providing diabetes counseling, blood pressure checks, annual wellness visits (AWV), and follow-up through the Centers for Medicare and Medicaid Services (CMS)'s Chronic Care Management and Transitional Care Management programs.
■ Part 2: Redesign between-visit in-basket management
Responding to an increasing number of inbox messages had become overwhelming for our physicians. Bellin Health’s management was aware that strategic delegation of inbox messages could save an hour or more of a physician’s time each day.14 Bellin implemented a procedure whereby inbox test results would be handled by the same CTC who saw the patient, thereby extending continuity. If the results were normal, the CTC would contact the patient. If the results were abnormal, the physician and the CTC would discuss them and develop a plan. Co-location of the RN, the CTC, and the physician would leverage face-to-face communication and make in-basket management more efficient.
■ Part 3: Redesign population health management
We developed an Extended Care Team (ECT), including social workers, clinical pharmacists, RN care coordinators, and diabetes educators, to assist with the care of patients with high-risk disorders or otherwise complex issues. These team members would work closely with the CTC, care team RN, and physician to review patients, develop plans of care, optimize management, and improve outcomes. Patients would be identified as candidates for potential ECT involvement based on the physician’s judgment in consultation with an EHR-based risk score for hospitalization or emergency department visit.
As we developed new processes, such as screening for determinants of health, we engaged additional system and community resources to help meet the needs of our patients.
Continue to: A look at stakeholder concerns and overcoming the barriers
A look at stakeholder concerns and overcoming the barriers
Critical to our success was being attentive to the concerns of our stakeholders and addressing them. Along the way, we gained valuable implementation insights, which we share here along with some specifics about how, exactly, we did things at Bellin.
Patients
Some patients expressed hesitation at having a person other than their physician in the exam room. They worried that the intimacy and privacy with their physician would be lost. In light of this, we gave patients the option not to have the CTC remain in the room. However, patients quickly saw the value of this team-based care approach and seldom asked to be seen without the CTC.
Throughout the process, we surveyed patients for feedback on their experiences. Comments indicated that the presence of the CTC in our team-based model led to positive patient experiences:
My physician is fully attentive. Patients appreciated that physicians were not distracted by the computer in the exam room. “I feel like I’ve got my doctor back” has been a common refrain.
The office staff is more responsive. The CTC, having been present during the appointment, has a deeper understanding of the care plan and can respond to calls or emails between visits, thereby reducing the time patients must wait for answers. One patient commented that, “I love [the doctor’s] team; his nurses are willing to answer every question I have.”
Continue to: I increasingly feel that I'm understood
I increasingly feel that I’m understood. We have seen patients develop meaningful relationships with other team members, confiding in them in ways that they hadn’t always done with physicians and advanced practice clinicians (APCs). Team members, in turn, have added valuable insights that help optimize patients’ care. In particular, the care of patients with multiple needs has been enhanced with the addition of ECT members who work with the core team and use their expertise to optimize the care of these patients.
Certified medical assistants and licensed practical nurses
Bellin’s leadership knew that team documentation could cause stress for the CMA, who, acting as a CTC, wanted to avoid misrepresenting details of the clinical encounter.13 Adding to the stress were other duties that would need to be learned, including agenda setting, refill management, care gap closure, and health coaching. With thorough training and preparation, many—but not all—of our CMAs and LPNs were able to successfully make the transition and flourish.
Implementation strategies
Provide thorough training. Our training process started 8 weeks before it was time to “go live.” There were weekly hour-long training sessions in population health basics, team culture and change management, documentation basics, and new roles and responsibilities. In the final week, the entire aTBC team sat together for 3 days of EHR training. All new teams shadowed existing teams to get a clear picture of the new processes.
Create a community of support. As our CMAs adapted to their new CTC roles, it was critical that they had support from experienced CTCs. Encouragement and patience from physicians were—and are—essential for CTCs to develop confidence in their new roles.
Enable ongoing feedback. We introduced weekly team meetings to enhance team communication and dynamics. Forums for all roles are held periodically to facilitate discussion, share learning, and enable support between teams.
Continue to: Use EHR tools to facilitate this work
Use EHR tools to facilitate this work. Using standard templates and documentation tools helped CTCs develop the confidence needed to thrive in their new role. Knowing these tools were available helped CTCs become effective in helping the team manage the between-visit work.
Monitor workload. As we developed more workflows and processes, we took care to monitor the amount of additional work for those in this role. We offloaded work whenever possible. For example, coordinated refill management at time of service, coupled with a back-up centralized refill system, can significantly decrease the number of refill requests made to CTCs. We continue to adjust staffing, where appropriate, to provide adequate support for those in this valuable role.
Be prepared for turnover. As CTCs became empowered in their new roles, some decided to advance their training into other roles. We developed a plan for replacing and training new staff. Higher pay can also be used to help attract and retain these staff members. Bellin uses LPNs in this role to ensure adequate staffing. Other health systems have developed a tier system for CMAs to improve retention.
Registered nurses
Before our move to an aTBC model, our office RNs primarily managed phone triage. Now the nurses were enlisted to play a more active role in patient care and team leadership. Although it was a dramatic departure from prior responsibilities, the majority of Bellin’s RNs have found increased satisfaction in taking on direct patient care.
Implementation strategies
Define new roles and provide training. In addition to participating in acute patient visits, consider ways that care team RNs can expand responsibilities as they pertain to disease counseling, population health management, and team leadership.15 At Bellin, the expanded role of the RN is evident in diabetes education and Medicare AWVs. Specifically, RNs now provide diabetes education to appropriate patients following a warm handoff from the physician at the time of the visit. RNs now also complete Medicare AWVs, which frees up physicians for other tasks and helps ensure sustainability for the new RN roles. Rates of completed AWVs at Bellin are now more than 70%, compared with reported national rates of less than 30%.16
Continue to: Maximize co-location
Maximize co-location. It is helpful to have the team members whose work is closely related—such as the CTCs and the RN for the team—to be situated near each other, rather than down a hall or in separate offices. Since the RN is co-located with the core teams at Bellin, there is now greater opportunity for verbal interaction, rather than just electronic communications, for matters such as triage calls and results management. RNs also provide a valuable resource for CMAs and LPNs, as well as help oversee team management of the in-basket.
Evaluate sustainability. Additional roles for the RNs required additional RN staffing. We assessed the new workload duties and balanced that against potential revenue from RN visits. This analysis indicated that an optimal ratio was 1 RN to every 3000 patients. This would allow an adequate number of RNs to fulfill additional roles and was financially sustainable with the goal of 4 billable RN visits per day.
Physicians
Bellin’s leadership recognized that some physicians might perceive team-based care as eroding their primary responsibility for patients’ care. Physicians have historically been trained in a model based on the primacy of the individual physician and that can be a hurdle to embracing team culture as a new paradigm of care. Several strategies helped us and can help others, too.
Implementation strategies
Cultivate trust. Thorough training of CTCs and RNs is critical to helping physicians develop trust and reliance in the team. The physician retains final authority over the team for cosigning orders, editing and finalizing documentation, and overseeing results management. Physicians invested in training and educating their staff will reap the rewards of a highly functioning, more satisfied team.
Encourage leadership. This can be a cultural shift for physicians, yet it is critical that they take a leadership role in this transformation.17 Physicians and their team leaders attended training sessions in team culture and change management. Prior to the go-live date, team leaders also met with the physician individually to explore their concerns and discuss ways to effectively lead and support their teams.
Continue to: Urge acceptance of support
Urge acceptance of support. The complexity of patient care today makes it difficult for a physician to manage all of a patient’s needs single-handedly. Complexity arises from the variety of plan co-pays and deductibles, the number of patients with chronic diseases, and the increased emphasis on improving quality measures.18 Enhanced support during any office visit and the extra support of an ECT for complex patients improves the ability of the physician to more effectively meet the needs of the patient.
Emphasize the benefit of an empowered team. The demands of the EHR on physicians and the resultant frustrations are well chronicled.4,19-22 Strategically delegating much of this work to other team members allows the physician to focus on the patient and perform physician-level work. At Bellin, we observed that our most successful care teams were those in which the physician fully accepted team-based care principles and empowered the staff to work at the top of their skill set.
Advanced practice clinicians
APCs in our system had traditionally practiced in 1 of 3 ways: independently handling defined panels with physician supervision; handling overflow or acute visits; or working collaboratively with a supervising physician to share a larger “team panel.” The third approach has become our preferred model. aTBC provides opportunities for APCs to thrive and collaborate with the physician to provide excellent care for patients.
APCs underwent the same process changes as physicians, including appropriate CTC support. Implementation strategies for APCs were similar to those that were useful for physicians.
Risk management professionals
At Bellin, we found that risk-management professionals had concerns about the scope of practice assigned to various team members, particularly regarding documentation. CMS allows for elements of a patient visit to be documented by CMAs and other members of the care team in real time as authorized by the physician.23,24 CTCs at Bellin also have other clinical duties in patient and EHR management. aTBC practices generally prefer the term team documentation over scribing, since it more accurately reflects the scope of the CTC’s work.
Continue to: Implementation strategies
Implementation strategies
Clarify regulatory issues. Extensive use of standing orders and protocols allowed us to increase involvement of various team members. State laws vary in what functions CMAs and LPNs are allowed to perform, so it is important to check your state guidelines.25 There is a tendency for some risk managers to overinterpret regulations. Challenge them to provide exact documentation from regulatory agencies to support their decisions.
Give assurances of physician oversight and processes. The physician assumes responsibility for standing orders, protocols, and documentation. We made sure that we had clear and consistent processes in place and worked closely with our risk managers as we developed our model. aTBC provides checks and balances to ensure accurate records, since team members are able to contribute and check for accuracy. A recent study suggested that CMAs perform documentation that is of equal or higher quality than that performed by the physician.26
Financial leadership
Like any organization adopting aTBC, Bellin’s leadership was concerned about the expense of adopting this approach. However, the leadership also recognized that the transition to aTBC could increase revenue by more than the increased staffing costs. In addition, we expected that capacity, access, continuity, and financial margins would increase.2,3,27,28 We also anticipated a decrease in downstream services, such as unnecessary tests, emergency department visits, and hospitalizations—a benefit of accountable care payment models.
Our efforts have been successful from a financial point of view. We attribute the financial sustainability that we have experienced to 4 factors:
1. Increased productivity. We knew that the increased efficiency of team-based care enables physicians to see 1 to 2 more patients per half day, and sometimes more.3,28,29 An increase of at least 1 patient visit per half-day was expected of our physicians and APCs on aTBC. In addition, they were expected to support the care team RN in achieving at least 4 billable visits per day. Our current level of RN visits is at 3.5 per nurse per day. There is significant variability in the increase of patients seen by a physician per day, ranging from 1 to 4 additional patients. These increased visits have helped us achieve financial viability, even in a predominantly fee-for-service environment.
2. More thorough service. The ability to keep patients in primary care and to focus on the patient’s full range of needs has led to higher levels of service and, consequently, to appropriately higher levels of billing codes. For example, Bellin’s revenue from billing increased by $724 per patient, related (in part) to higher rates of immunizations, cancer screenings with mammography, and colonoscopies.
Continue to: 3. New billable services
3. New billable services. Billing for RN blood pressure checks, AWVs, and extended care team services have helped make aTBC at Bellin financially feasible. Revenue from RN visits, for example, was $630,000 in 2018.
4. Improved access for patients. Of the 130 primary care providers now on aTBC, 15 (11.5%) had closed their practices to new patients before aTBC. Now, all of their practices are open to new patients, which has improved access to care. In a 2018 patient access survey, 96.6% of patients obtained an appointment as soon as they thought it was needed, compared with 70.7% of patients before the transition to aTBC.
Greater opportunity for financial sustainability. The combination of improved quality measures and decreased cost of care in the Bellin aTBC bodes well for future success in a value-based world. We have realized a significant increase in value-based payments for improved quality, and in our Next Gen Accountable Care Organization (ACO) patients, we have seen a decrease of $29 in per-member-per-month costs, likely due to the use of nonphysicians in expanded roles. In addition, hospital admissions have decreased by 5% due to the ability of ambulatory teams to manage more complex patients in the office setting. This model has also allowed physicians and APCs to increase their panel size, another key value-based metric. From 2016 to 2018, panel size for primary care providers increased by an average of 8%.
Enhanced ability to retain and recruit. Several of Bellin’s primary care recruits indicated that they had interviewed only at practices incorporating team-based care. This trend may increase as residencies transition to team-based models of care.
So how did we do?
Metrics of Bellin’s aTBC success
By the end of 2018, all 130 primary care physicians and APCs at Bellin had made the transition to this model, representing family medicine, internal medicine, and pediatrics. We have now begun the transition of our non-primary care specialties to team-based care.
Continue to: In the aTBC model...
In the aTBC model, the percentage of patients receiving age-appropriate screening is higher than before in every domain we measure (FIGURE 1). There has also been improvement in major quality metrics (FIGURE 2).
In a survey done in Spring 2018 by St. Norbert College Strategic Research Center, provider satisfaction increased, with 83% of physicians having made the transition to an aTBC practice moderately or very satisfied with their Bellin Health experience, compared with 70% in the traditional model. More recent 2019 survey data show a satisfaction rate of 90% for team-based care providers. Finally, in our aTBC model—in CMS’s Next-Gen ACO initiative—the cost per patient per month is significantly less than for those in a non-team-based care model ($796 vs $940).
CORRESPONDENCE
James Jerzak, MD, 1630 Commanche Ave, Green Bay, WI 54313; james.jerzak@bellin.org.
ACKNOWLEDGEMENTS
The authors would like to thank Lindsey E. Carlasare, MBA, from the American Medical Association, and Brad Wozney, MD, Kathy Kerscher, and Christopher Elfner from Bellin Health, for their contributions to the content and review of this manuscript.
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
3. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
4. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
5. Shanafelt TD, Mungo M, Schmitgen J, et al. Longitudinal study evaluating the association between physician burnout and changes in professional work effort. Mayo Clin Proc. 2016;91:422-431.
6. Sinsky CA, Dyrbye LN, West CP, et al. Professional satisfaction and the career plans of US physicians. Mayo Clin Proc. 2017;92:1625-1635.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90:1600-1613.
8. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
9. Sinsky CA, Sinsky TA, Althaus D, et al. Practice profile. ‘Core teams’: nurse-physician partnerships provide patient-centered care at an Iowa practice. Health Aff (Millwood). 2010;29:966-968.
10. Shanafelt T, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
11. Association for Advancing Physician and Provider Recruitment. Schutte L. What you don’t know can cost you: building a business case for recruitment and retention best practices. 2012. https://member.aappr.org/general/custom.asp?page=696. Accessed June 20, 2019.
12. American Medical Association. AMA STEPS Forward. Expanded rooming and discharge protocols. https://edhub.ama-assn.org/steps-forward/module/2702600. Accessed June 20, 2019.
13. American Medical Association. AMA STEPS Forward. Team documentation. https://edhub.ama-assn.org/steps-forward/module/2702598?resultClick=3&bypassSolrId=J_2702598. Accessed June 20, 2019.
14. American Medical Association. AMA STEPS Forward. EHR in-basket restructuring for improved efficiency. https://edhub.ama-assn.org/steps-forward/module/2702694?resultClick=3&bypassSolrId=J_2702694. Accessed June 20, 2019.
15. California Health Care Foundation. Bodenheimer T, Bauer L, Olayiwola JN. RN role reimagined: how empowering registered nurses can improve primary care. https://www.chcf.org/publication/rn-role-reimagined-how-empowering-registered-nurses-can-improve-primary-care/. Accessed June 20, 2019.
16. Chung S, Lesser LI, Lauderdale DS, et al. Medicare annual preventive care visits: use increased among fee-for-service patients, but many do not participate. Health Aff (Millwood). 2015;34:11-20.
17. American Medical Association. AMA Policy H-160.912. The structure and function of interprofessional health care teams. https://policysearch.ama-assn.org/policyfinder/detail/The%20Structure%20and%20Function%20of%20Interprofessional%20Health%20Care%20Teams?uri=%2FAMADoc%2FHOD.xml-0-727.xml. Accessed June 20, 2019.
18. Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128:337-343.
19. Hill RG Jr, Sears LM, Melanson SW. 4000 clicks: a productivity analysis of electronic medical records in a community hospital ED. Am J Emerg Med. 2013;31:1591-1594.
20. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21:e100-e106.
21. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
22. RAND Corporation. Friedberg MW, Chen PG, Ban Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. https://www.rand.org/pubs/research_reports/RR439.html. Accessed June 20, 2019.
23. Evaluation and Management (E/M) visit frequently asked questions (FAQs): physician fee schedule (PPS). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/E-M-Visit-FAQs-PFS.pdf. Accessed August 27, 2019.
24. Centers for Medicare & Medicaid Services. Scribe services signature requirements. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017-Transmittals-Items/R713PI.html. Accessed June 20, 2019.
25. American Association of Medical Assistants. State scope of practice laws. http://www.aama-ntl.org/employers/state-scope-of-practice-laws. Accessed June 20, 2019.
26. Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65:155-159.
27. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
28. Bank AJ, Obetz C, Konrardy A, et al. Impact of scribes on patient interaction, productivity, and revenue in a cardiology clinic: a prospective study. Clinicoecon Outcomes Res. 2013;5:399-406.
29. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
Leaders in health care and practicing physicians recognize the need for changes in how health care is delivered.1-3 Despite this awareness, though, barriers to meaningful change persist and the current practice environment wherein physicians must routinely spend 2 hours on electronic health records (EHRs) and desk work for every hour of direct face time with patients4 is driving trainees away from ambulatory specialties and is contributing to physicians’ decisions to reduce their practices to part-time, retire early, or leave medicine altogether.5,6 Those who persevere in this environment with heavy administrative burdens run the increasing risk of burnout.7
Some physicians and practices are responding by taking creative measures to reform the way patient care is delivered. Bellin Health—a 160-provider, multispecialty health system in northeast Wisconsin where one of the authors (JJ) works—introduced an advanced team-based care (aTBC) model between November 2014 and November 2018, starting with our primary care providers. The development and introduction of this new model arose from an iterative, multidisciplinary process driven by the desire to transform the Triple Aim—enhancing patient experience, improving population health, and reducing costs—into a Quadruple Aim8 by additionally focusing on improving the work life of health care providers, which, in turn, will help achieve the first 3 goals. In introducing an aTBC model, Bellin Health focused on 3 elements: office visit redesign, in-basket management redesign, and the use of extended care team members and system and community resources to assist in the care of complex and high-risk patients.
Herein we describe the 3 components of our aTBC model,1,9 identify the barriers that existed in the minds of multiple stakeholders (from patients to clinicians and Bellin executives), and describe the strategies that enabled us to overcome these barriers.
The impetus behind our move to aTBC
Bellin Health considered a move to an aTBC model to be critical in light of factors in the health care environment, in general, and at Bellin, in particular. The factors included
- an industry-wide shift to value-based payments, which requires new models for long-term financial viability.
- recognition that physician and medical staff burnout leads to lower productivity and, in some cases, workforce losses.5,6 Replacing a physician in a practice can be difficult and expensive, with cost estimates of $500,000 to more than $1 million per physician.10,11
- a belief that aTBC could help the Bellin Health leadership team meet its organizational goals of improved patient satisfaction, achieve gains in quality measures, enhance engagement and loyalty among patients and employees, and lower recruitment costs.
A 3-part aTBC initiative
■ Part 1: Redesign the office visit
We redesigned staffing and workflow for office visits to maximize the core skills of physicians, which required distributing ancillary tasks among support staff. We up-trained certified medical assistants (CMAs) and licensed practical nurses (LPNs) to take on the new role of care team coordinator (CTC) and optimized the direct clinical support ratio for busier physicians. For physicians who were seeing 15 to 19 patients a day, a ratio of 3 CTCs to 2 physicians was implemented; for those seeing 20 or more patients a day, we used a support ratio of 2:1.
The role of CTC was designed so that he or she would accompany a patient throughout the entire appointment. Responsibilities were broken out as follows:
Pre-visit. Before the physician enters the room, the CTC would now perform expanded rooming functions including pending orders, refill management, care gap closure using standing orders, agenda setting, and preliminary documentation.12
Visit. The CTC would now hand off the patient to the physician and stay in the room to document details of the visit and record new orders for consults, x-ray films, referrals, or prescriptions.13 This intensive EHR support was established to ensure that the physician could focus directly on the patient without the distraction of the computer.
Continue to: Post-visit
Post-visit. After a physician leaves a room, the CTC was now charged with finishing the pending orders, setting up the patient’s next appointment and pre-visit labs, reviewing details of the after-visit summary, and doing any basic health coaching with the patient. During this time, the physician would use the co-location space to review and edit the documentation, cosign the orders and prescriptions submitted by the CTC, and close the chart before going into the next room with the second CTC. The need to revisit these details after clinic hours was eliminated.
Another change … The role of our phone triage registered nurses (RN) was expanded. Care team RNs began providing diabetes counseling, blood pressure checks, annual wellness visits (AWV), and follow-up through the Centers for Medicare and Medicaid Services (CMS)'s Chronic Care Management and Transitional Care Management programs.
■ Part 2: Redesign between-visit in-basket management
Responding to an increasing number of inbox messages had become overwhelming for our physicians. Bellin Health’s management was aware that strategic delegation of inbox messages could save an hour or more of a physician’s time each day.14 Bellin implemented a procedure whereby inbox test results would be handled by the same CTC who saw the patient, thereby extending continuity. If the results were normal, the CTC would contact the patient. If the results were abnormal, the physician and the CTC would discuss them and develop a plan. Co-location of the RN, the CTC, and the physician would leverage face-to-face communication and make in-basket management more efficient.
■ Part 3: Redesign population health management
We developed an Extended Care Team (ECT), including social workers, clinical pharmacists, RN care coordinators, and diabetes educators, to assist with the care of patients with high-risk disorders or otherwise complex issues. These team members would work closely with the CTC, care team RN, and physician to review patients, develop plans of care, optimize management, and improve outcomes. Patients would be identified as candidates for potential ECT involvement based on the physician’s judgment in consultation with an EHR-based risk score for hospitalization or emergency department visit.
As we developed new processes, such as screening for determinants of health, we engaged additional system and community resources to help meet the needs of our patients.
Continue to: A look at stakeholder concerns and overcoming the barriers
A look at stakeholder concerns and overcoming the barriers
Critical to our success was being attentive to the concerns of our stakeholders and addressing them. Along the way, we gained valuable implementation insights, which we share here along with some specifics about how, exactly, we did things at Bellin.
Patients
Some patients expressed hesitation at having a person other than their physician in the exam room. They worried that the intimacy and privacy with their physician would be lost. In light of this, we gave patients the option not to have the CTC remain in the room. However, patients quickly saw the value of this team-based care approach and seldom asked to be seen without the CTC.
Throughout the process, we surveyed patients for feedback on their experiences. Comments indicated that the presence of the CTC in our team-based model led to positive patient experiences:
My physician is fully attentive. Patients appreciated that physicians were not distracted by the computer in the exam room. “I feel like I’ve got my doctor back” has been a common refrain.
The office staff is more responsive. The CTC, having been present during the appointment, has a deeper understanding of the care plan and can respond to calls or emails between visits, thereby reducing the time patients must wait for answers. One patient commented that, “I love [the doctor’s] team; his nurses are willing to answer every question I have.”
Continue to: I increasingly feel that I'm understood
I increasingly feel that I’m understood. We have seen patients develop meaningful relationships with other team members, confiding in them in ways that they hadn’t always done with physicians and advanced practice clinicians (APCs). Team members, in turn, have added valuable insights that help optimize patients’ care. In particular, the care of patients with multiple needs has been enhanced with the addition of ECT members who work with the core team and use their expertise to optimize the care of these patients.
Certified medical assistants and licensed practical nurses
Bellin’s leadership knew that team documentation could cause stress for the CMA, who, acting as a CTC, wanted to avoid misrepresenting details of the clinical encounter.13 Adding to the stress were other duties that would need to be learned, including agenda setting, refill management, care gap closure, and health coaching. With thorough training and preparation, many—but not all—of our CMAs and LPNs were able to successfully make the transition and flourish.
Implementation strategies
Provide thorough training. Our training process started 8 weeks before it was time to “go live.” There were weekly hour-long training sessions in population health basics, team culture and change management, documentation basics, and new roles and responsibilities. In the final week, the entire aTBC team sat together for 3 days of EHR training. All new teams shadowed existing teams to get a clear picture of the new processes.
Create a community of support. As our CMAs adapted to their new CTC roles, it was critical that they had support from experienced CTCs. Encouragement and patience from physicians were—and are—essential for CTCs to develop confidence in their new roles.
Enable ongoing feedback. We introduced weekly team meetings to enhance team communication and dynamics. Forums for all roles are held periodically to facilitate discussion, share learning, and enable support between teams.
Continue to: Use EHR tools to facilitate this work
Use EHR tools to facilitate this work. Using standard templates and documentation tools helped CTCs develop the confidence needed to thrive in their new role. Knowing these tools were available helped CTCs become effective in helping the team manage the between-visit work.
Monitor workload. As we developed more workflows and processes, we took care to monitor the amount of additional work for those in this role. We offloaded work whenever possible. For example, coordinated refill management at time of service, coupled with a back-up centralized refill system, can significantly decrease the number of refill requests made to CTCs. We continue to adjust staffing, where appropriate, to provide adequate support for those in this valuable role.
Be prepared for turnover. As CTCs became empowered in their new roles, some decided to advance their training into other roles. We developed a plan for replacing and training new staff. Higher pay can also be used to help attract and retain these staff members. Bellin uses LPNs in this role to ensure adequate staffing. Other health systems have developed a tier system for CMAs to improve retention.
Registered nurses
Before our move to an aTBC model, our office RNs primarily managed phone triage. Now the nurses were enlisted to play a more active role in patient care and team leadership. Although it was a dramatic departure from prior responsibilities, the majority of Bellin’s RNs have found increased satisfaction in taking on direct patient care.
Implementation strategies
Define new roles and provide training. In addition to participating in acute patient visits, consider ways that care team RNs can expand responsibilities as they pertain to disease counseling, population health management, and team leadership.15 At Bellin, the expanded role of the RN is evident in diabetes education and Medicare AWVs. Specifically, RNs now provide diabetes education to appropriate patients following a warm handoff from the physician at the time of the visit. RNs now also complete Medicare AWVs, which frees up physicians for other tasks and helps ensure sustainability for the new RN roles. Rates of completed AWVs at Bellin are now more than 70%, compared with reported national rates of less than 30%.16
Continue to: Maximize co-location
Maximize co-location. It is helpful to have the team members whose work is closely related—such as the CTCs and the RN for the team—to be situated near each other, rather than down a hall or in separate offices. Since the RN is co-located with the core teams at Bellin, there is now greater opportunity for verbal interaction, rather than just electronic communications, for matters such as triage calls and results management. RNs also provide a valuable resource for CMAs and LPNs, as well as help oversee team management of the in-basket.
Evaluate sustainability. Additional roles for the RNs required additional RN staffing. We assessed the new workload duties and balanced that against potential revenue from RN visits. This analysis indicated that an optimal ratio was 1 RN to every 3000 patients. This would allow an adequate number of RNs to fulfill additional roles and was financially sustainable with the goal of 4 billable RN visits per day.
Physicians
Bellin’s leadership recognized that some physicians might perceive team-based care as eroding their primary responsibility for patients’ care. Physicians have historically been trained in a model based on the primacy of the individual physician and that can be a hurdle to embracing team culture as a new paradigm of care. Several strategies helped us and can help others, too.
Implementation strategies
Cultivate trust. Thorough training of CTCs and RNs is critical to helping physicians develop trust and reliance in the team. The physician retains final authority over the team for cosigning orders, editing and finalizing documentation, and overseeing results management. Physicians invested in training and educating their staff will reap the rewards of a highly functioning, more satisfied team.
Encourage leadership. This can be a cultural shift for physicians, yet it is critical that they take a leadership role in this transformation.17 Physicians and their team leaders attended training sessions in team culture and change management. Prior to the go-live date, team leaders also met with the physician individually to explore their concerns and discuss ways to effectively lead and support their teams.
Continue to: Urge acceptance of support
Urge acceptance of support. The complexity of patient care today makes it difficult for a physician to manage all of a patient’s needs single-handedly. Complexity arises from the variety of plan co-pays and deductibles, the number of patients with chronic diseases, and the increased emphasis on improving quality measures.18 Enhanced support during any office visit and the extra support of an ECT for complex patients improves the ability of the physician to more effectively meet the needs of the patient.
Emphasize the benefit of an empowered team. The demands of the EHR on physicians and the resultant frustrations are well chronicled.4,19-22 Strategically delegating much of this work to other team members allows the physician to focus on the patient and perform physician-level work. At Bellin, we observed that our most successful care teams were those in which the physician fully accepted team-based care principles and empowered the staff to work at the top of their skill set.
Advanced practice clinicians
APCs in our system had traditionally practiced in 1 of 3 ways: independently handling defined panels with physician supervision; handling overflow or acute visits; or working collaboratively with a supervising physician to share a larger “team panel.” The third approach has become our preferred model. aTBC provides opportunities for APCs to thrive and collaborate with the physician to provide excellent care for patients.
APCs underwent the same process changes as physicians, including appropriate CTC support. Implementation strategies for APCs were similar to those that were useful for physicians.
Risk management professionals
At Bellin, we found that risk-management professionals had concerns about the scope of practice assigned to various team members, particularly regarding documentation. CMS allows for elements of a patient visit to be documented by CMAs and other members of the care team in real time as authorized by the physician.23,24 CTCs at Bellin also have other clinical duties in patient and EHR management. aTBC practices generally prefer the term team documentation over scribing, since it more accurately reflects the scope of the CTC’s work.
Continue to: Implementation strategies
Implementation strategies
Clarify regulatory issues. Extensive use of standing orders and protocols allowed us to increase involvement of various team members. State laws vary in what functions CMAs and LPNs are allowed to perform, so it is important to check your state guidelines.25 There is a tendency for some risk managers to overinterpret regulations. Challenge them to provide exact documentation from regulatory agencies to support their decisions.
Give assurances of physician oversight and processes. The physician assumes responsibility for standing orders, protocols, and documentation. We made sure that we had clear and consistent processes in place and worked closely with our risk managers as we developed our model. aTBC provides checks and balances to ensure accurate records, since team members are able to contribute and check for accuracy. A recent study suggested that CMAs perform documentation that is of equal or higher quality than that performed by the physician.26
Financial leadership
Like any organization adopting aTBC, Bellin’s leadership was concerned about the expense of adopting this approach. However, the leadership also recognized that the transition to aTBC could increase revenue by more than the increased staffing costs. In addition, we expected that capacity, access, continuity, and financial margins would increase.2,3,27,28 We also anticipated a decrease in downstream services, such as unnecessary tests, emergency department visits, and hospitalizations—a benefit of accountable care payment models.
Our efforts have been successful from a financial point of view. We attribute the financial sustainability that we have experienced to 4 factors:
1. Increased productivity. We knew that the increased efficiency of team-based care enables physicians to see 1 to 2 more patients per half day, and sometimes more.3,28,29 An increase of at least 1 patient visit per half-day was expected of our physicians and APCs on aTBC. In addition, they were expected to support the care team RN in achieving at least 4 billable visits per day. Our current level of RN visits is at 3.5 per nurse per day. There is significant variability in the increase of patients seen by a physician per day, ranging from 1 to 4 additional patients. These increased visits have helped us achieve financial viability, even in a predominantly fee-for-service environment.
2. More thorough service. The ability to keep patients in primary care and to focus on the patient’s full range of needs has led to higher levels of service and, consequently, to appropriately higher levels of billing codes. For example, Bellin’s revenue from billing increased by $724 per patient, related (in part) to higher rates of immunizations, cancer screenings with mammography, and colonoscopies.
Continue to: 3. New billable services
3. New billable services. Billing for RN blood pressure checks, AWVs, and extended care team services have helped make aTBC at Bellin financially feasible. Revenue from RN visits, for example, was $630,000 in 2018.
4. Improved access for patients. Of the 130 primary care providers now on aTBC, 15 (11.5%) had closed their practices to new patients before aTBC. Now, all of their practices are open to new patients, which has improved access to care. In a 2018 patient access survey, 96.6% of patients obtained an appointment as soon as they thought it was needed, compared with 70.7% of patients before the transition to aTBC.
Greater opportunity for financial sustainability. The combination of improved quality measures and decreased cost of care in the Bellin aTBC bodes well for future success in a value-based world. We have realized a significant increase in value-based payments for improved quality, and in our Next Gen Accountable Care Organization (ACO) patients, we have seen a decrease of $29 in per-member-per-month costs, likely due to the use of nonphysicians in expanded roles. In addition, hospital admissions have decreased by 5% due to the ability of ambulatory teams to manage more complex patients in the office setting. This model has also allowed physicians and APCs to increase their panel size, another key value-based metric. From 2016 to 2018, panel size for primary care providers increased by an average of 8%.
Enhanced ability to retain and recruit. Several of Bellin’s primary care recruits indicated that they had interviewed only at practices incorporating team-based care. This trend may increase as residencies transition to team-based models of care.
So how did we do?
Metrics of Bellin’s aTBC success
By the end of 2018, all 130 primary care physicians and APCs at Bellin had made the transition to this model, representing family medicine, internal medicine, and pediatrics. We have now begun the transition of our non-primary care specialties to team-based care.
Continue to: In the aTBC model...
In the aTBC model, the percentage of patients receiving age-appropriate screening is higher than before in every domain we measure (FIGURE 1). There has also been improvement in major quality metrics (FIGURE 2).
In a survey done in Spring 2018 by St. Norbert College Strategic Research Center, provider satisfaction increased, with 83% of physicians having made the transition to an aTBC practice moderately or very satisfied with their Bellin Health experience, compared with 70% in the traditional model. More recent 2019 survey data show a satisfaction rate of 90% for team-based care providers. Finally, in our aTBC model—in CMS’s Next-Gen ACO initiative—the cost per patient per month is significantly less than for those in a non-team-based care model ($796 vs $940).
CORRESPONDENCE
James Jerzak, MD, 1630 Commanche Ave, Green Bay, WI 54313; james.jerzak@bellin.org.
ACKNOWLEDGEMENTS
The authors would like to thank Lindsey E. Carlasare, MBA, from the American Medical Association, and Brad Wozney, MD, Kathy Kerscher, and Christopher Elfner from Bellin Health, for their contributions to the content and review of this manuscript.
Leaders in health care and practicing physicians recognize the need for changes in how health care is delivered.1-3 Despite this awareness, though, barriers to meaningful change persist and the current practice environment wherein physicians must routinely spend 2 hours on electronic health records (EHRs) and desk work for every hour of direct face time with patients4 is driving trainees away from ambulatory specialties and is contributing to physicians’ decisions to reduce their practices to part-time, retire early, or leave medicine altogether.5,6 Those who persevere in this environment with heavy administrative burdens run the increasing risk of burnout.7
Some physicians and practices are responding by taking creative measures to reform the way patient care is delivered. Bellin Health—a 160-provider, multispecialty health system in northeast Wisconsin where one of the authors (JJ) works—introduced an advanced team-based care (aTBC) model between November 2014 and November 2018, starting with our primary care providers. The development and introduction of this new model arose from an iterative, multidisciplinary process driven by the desire to transform the Triple Aim—enhancing patient experience, improving population health, and reducing costs—into a Quadruple Aim8 by additionally focusing on improving the work life of health care providers, which, in turn, will help achieve the first 3 goals. In introducing an aTBC model, Bellin Health focused on 3 elements: office visit redesign, in-basket management redesign, and the use of extended care team members and system and community resources to assist in the care of complex and high-risk patients.
Herein we describe the 3 components of our aTBC model,1,9 identify the barriers that existed in the minds of multiple stakeholders (from patients to clinicians and Bellin executives), and describe the strategies that enabled us to overcome these barriers.
The impetus behind our move to aTBC
Bellin Health considered a move to an aTBC model to be critical in light of factors in the health care environment, in general, and at Bellin, in particular. The factors included
- an industry-wide shift to value-based payments, which requires new models for long-term financial viability.
- recognition that physician and medical staff burnout leads to lower productivity and, in some cases, workforce losses.5,6 Replacing a physician in a practice can be difficult and expensive, with cost estimates of $500,000 to more than $1 million per physician.10,11
- a belief that aTBC could help the Bellin Health leadership team meet its organizational goals of improved patient satisfaction, achieve gains in quality measures, enhance engagement and loyalty among patients and employees, and lower recruitment costs.
A 3-part aTBC initiative
■ Part 1: Redesign the office visit
We redesigned staffing and workflow for office visits to maximize the core skills of physicians, which required distributing ancillary tasks among support staff. We up-trained certified medical assistants (CMAs) and licensed practical nurses (LPNs) to take on the new role of care team coordinator (CTC) and optimized the direct clinical support ratio for busier physicians. For physicians who were seeing 15 to 19 patients a day, a ratio of 3 CTCs to 2 physicians was implemented; for those seeing 20 or more patients a day, we used a support ratio of 2:1.
The role of CTC was designed so that he or she would accompany a patient throughout the entire appointment. Responsibilities were broken out as follows:
Pre-visit. Before the physician enters the room, the CTC would now perform expanded rooming functions including pending orders, refill management, care gap closure using standing orders, agenda setting, and preliminary documentation.12
Visit. The CTC would now hand off the patient to the physician and stay in the room to document details of the visit and record new orders for consults, x-ray films, referrals, or prescriptions.13 This intensive EHR support was established to ensure that the physician could focus directly on the patient without the distraction of the computer.
Continue to: Post-visit
Post-visit. After a physician leaves a room, the CTC was now charged with finishing the pending orders, setting up the patient’s next appointment and pre-visit labs, reviewing details of the after-visit summary, and doing any basic health coaching with the patient. During this time, the physician would use the co-location space to review and edit the documentation, cosign the orders and prescriptions submitted by the CTC, and close the chart before going into the next room with the second CTC. The need to revisit these details after clinic hours was eliminated.
Another change … The role of our phone triage registered nurses (RN) was expanded. Care team RNs began providing diabetes counseling, blood pressure checks, annual wellness visits (AWV), and follow-up through the Centers for Medicare and Medicaid Services (CMS)'s Chronic Care Management and Transitional Care Management programs.
■ Part 2: Redesign between-visit in-basket management
Responding to an increasing number of inbox messages had become overwhelming for our physicians. Bellin Health’s management was aware that strategic delegation of inbox messages could save an hour or more of a physician’s time each day.14 Bellin implemented a procedure whereby inbox test results would be handled by the same CTC who saw the patient, thereby extending continuity. If the results were normal, the CTC would contact the patient. If the results were abnormal, the physician and the CTC would discuss them and develop a plan. Co-location of the RN, the CTC, and the physician would leverage face-to-face communication and make in-basket management more efficient.
■ Part 3: Redesign population health management
We developed an Extended Care Team (ECT), including social workers, clinical pharmacists, RN care coordinators, and diabetes educators, to assist with the care of patients with high-risk disorders or otherwise complex issues. These team members would work closely with the CTC, care team RN, and physician to review patients, develop plans of care, optimize management, and improve outcomes. Patients would be identified as candidates for potential ECT involvement based on the physician’s judgment in consultation with an EHR-based risk score for hospitalization or emergency department visit.
As we developed new processes, such as screening for determinants of health, we engaged additional system and community resources to help meet the needs of our patients.
Continue to: A look at stakeholder concerns and overcoming the barriers
A look at stakeholder concerns and overcoming the barriers
Critical to our success was being attentive to the concerns of our stakeholders and addressing them. Along the way, we gained valuable implementation insights, which we share here along with some specifics about how, exactly, we did things at Bellin.
Patients
Some patients expressed hesitation at having a person other than their physician in the exam room. They worried that the intimacy and privacy with their physician would be lost. In light of this, we gave patients the option not to have the CTC remain in the room. However, patients quickly saw the value of this team-based care approach and seldom asked to be seen without the CTC.
Throughout the process, we surveyed patients for feedback on their experiences. Comments indicated that the presence of the CTC in our team-based model led to positive patient experiences:
My physician is fully attentive. Patients appreciated that physicians were not distracted by the computer in the exam room. “I feel like I’ve got my doctor back” has been a common refrain.
The office staff is more responsive. The CTC, having been present during the appointment, has a deeper understanding of the care plan and can respond to calls or emails between visits, thereby reducing the time patients must wait for answers. One patient commented that, “I love [the doctor’s] team; his nurses are willing to answer every question I have.”
Continue to: I increasingly feel that I'm understood
I increasingly feel that I’m understood. We have seen patients develop meaningful relationships with other team members, confiding in them in ways that they hadn’t always done with physicians and advanced practice clinicians (APCs). Team members, in turn, have added valuable insights that help optimize patients’ care. In particular, the care of patients with multiple needs has been enhanced with the addition of ECT members who work with the core team and use their expertise to optimize the care of these patients.
Certified medical assistants and licensed practical nurses
Bellin’s leadership knew that team documentation could cause stress for the CMA, who, acting as a CTC, wanted to avoid misrepresenting details of the clinical encounter.13 Adding to the stress were other duties that would need to be learned, including agenda setting, refill management, care gap closure, and health coaching. With thorough training and preparation, many—but not all—of our CMAs and LPNs were able to successfully make the transition and flourish.
Implementation strategies
Provide thorough training. Our training process started 8 weeks before it was time to “go live.” There were weekly hour-long training sessions in population health basics, team culture and change management, documentation basics, and new roles and responsibilities. In the final week, the entire aTBC team sat together for 3 days of EHR training. All new teams shadowed existing teams to get a clear picture of the new processes.
Create a community of support. As our CMAs adapted to their new CTC roles, it was critical that they had support from experienced CTCs. Encouragement and patience from physicians were—and are—essential for CTCs to develop confidence in their new roles.
Enable ongoing feedback. We introduced weekly team meetings to enhance team communication and dynamics. Forums for all roles are held periodically to facilitate discussion, share learning, and enable support between teams.
Continue to: Use EHR tools to facilitate this work
Use EHR tools to facilitate this work. Using standard templates and documentation tools helped CTCs develop the confidence needed to thrive in their new role. Knowing these tools were available helped CTCs become effective in helping the team manage the between-visit work.
Monitor workload. As we developed more workflows and processes, we took care to monitor the amount of additional work for those in this role. We offloaded work whenever possible. For example, coordinated refill management at time of service, coupled with a back-up centralized refill system, can significantly decrease the number of refill requests made to CTCs. We continue to adjust staffing, where appropriate, to provide adequate support for those in this valuable role.
Be prepared for turnover. As CTCs became empowered in their new roles, some decided to advance their training into other roles. We developed a plan for replacing and training new staff. Higher pay can also be used to help attract and retain these staff members. Bellin uses LPNs in this role to ensure adequate staffing. Other health systems have developed a tier system for CMAs to improve retention.
Registered nurses
Before our move to an aTBC model, our office RNs primarily managed phone triage. Now the nurses were enlisted to play a more active role in patient care and team leadership. Although it was a dramatic departure from prior responsibilities, the majority of Bellin’s RNs have found increased satisfaction in taking on direct patient care.
Implementation strategies
Define new roles and provide training. In addition to participating in acute patient visits, consider ways that care team RNs can expand responsibilities as they pertain to disease counseling, population health management, and team leadership.15 At Bellin, the expanded role of the RN is evident in diabetes education and Medicare AWVs. Specifically, RNs now provide diabetes education to appropriate patients following a warm handoff from the physician at the time of the visit. RNs now also complete Medicare AWVs, which frees up physicians for other tasks and helps ensure sustainability for the new RN roles. Rates of completed AWVs at Bellin are now more than 70%, compared with reported national rates of less than 30%.16
Continue to: Maximize co-location
Maximize co-location. It is helpful to have the team members whose work is closely related—such as the CTCs and the RN for the team—to be situated near each other, rather than down a hall or in separate offices. Since the RN is co-located with the core teams at Bellin, there is now greater opportunity for verbal interaction, rather than just electronic communications, for matters such as triage calls and results management. RNs also provide a valuable resource for CMAs and LPNs, as well as help oversee team management of the in-basket.
Evaluate sustainability. Additional roles for the RNs required additional RN staffing. We assessed the new workload duties and balanced that against potential revenue from RN visits. This analysis indicated that an optimal ratio was 1 RN to every 3000 patients. This would allow an adequate number of RNs to fulfill additional roles and was financially sustainable with the goal of 4 billable RN visits per day.
Physicians
Bellin’s leadership recognized that some physicians might perceive team-based care as eroding their primary responsibility for patients’ care. Physicians have historically been trained in a model based on the primacy of the individual physician and that can be a hurdle to embracing team culture as a new paradigm of care. Several strategies helped us and can help others, too.
Implementation strategies
Cultivate trust. Thorough training of CTCs and RNs is critical to helping physicians develop trust and reliance in the team. The physician retains final authority over the team for cosigning orders, editing and finalizing documentation, and overseeing results management. Physicians invested in training and educating their staff will reap the rewards of a highly functioning, more satisfied team.
Encourage leadership. This can be a cultural shift for physicians, yet it is critical that they take a leadership role in this transformation.17 Physicians and their team leaders attended training sessions in team culture and change management. Prior to the go-live date, team leaders also met with the physician individually to explore their concerns and discuss ways to effectively lead and support their teams.
Continue to: Urge acceptance of support
Urge acceptance of support. The complexity of patient care today makes it difficult for a physician to manage all of a patient’s needs single-handedly. Complexity arises from the variety of plan co-pays and deductibles, the number of patients with chronic diseases, and the increased emphasis on improving quality measures.18 Enhanced support during any office visit and the extra support of an ECT for complex patients improves the ability of the physician to more effectively meet the needs of the patient.
Emphasize the benefit of an empowered team. The demands of the EHR on physicians and the resultant frustrations are well chronicled.4,19-22 Strategically delegating much of this work to other team members allows the physician to focus on the patient and perform physician-level work. At Bellin, we observed that our most successful care teams were those in which the physician fully accepted team-based care principles and empowered the staff to work at the top of their skill set.
Advanced practice clinicians
APCs in our system had traditionally practiced in 1 of 3 ways: independently handling defined panels with physician supervision; handling overflow or acute visits; or working collaboratively with a supervising physician to share a larger “team panel.” The third approach has become our preferred model. aTBC provides opportunities for APCs to thrive and collaborate with the physician to provide excellent care for patients.
APCs underwent the same process changes as physicians, including appropriate CTC support. Implementation strategies for APCs were similar to those that were useful for physicians.
Risk management professionals
At Bellin, we found that risk-management professionals had concerns about the scope of practice assigned to various team members, particularly regarding documentation. CMS allows for elements of a patient visit to be documented by CMAs and other members of the care team in real time as authorized by the physician.23,24 CTCs at Bellin also have other clinical duties in patient and EHR management. aTBC practices generally prefer the term team documentation over scribing, since it more accurately reflects the scope of the CTC’s work.
Continue to: Implementation strategies
Implementation strategies
Clarify regulatory issues. Extensive use of standing orders and protocols allowed us to increase involvement of various team members. State laws vary in what functions CMAs and LPNs are allowed to perform, so it is important to check your state guidelines.25 There is a tendency for some risk managers to overinterpret regulations. Challenge them to provide exact documentation from regulatory agencies to support their decisions.
Give assurances of physician oversight and processes. The physician assumes responsibility for standing orders, protocols, and documentation. We made sure that we had clear and consistent processes in place and worked closely with our risk managers as we developed our model. aTBC provides checks and balances to ensure accurate records, since team members are able to contribute and check for accuracy. A recent study suggested that CMAs perform documentation that is of equal or higher quality than that performed by the physician.26
Financial leadership
Like any organization adopting aTBC, Bellin’s leadership was concerned about the expense of adopting this approach. However, the leadership also recognized that the transition to aTBC could increase revenue by more than the increased staffing costs. In addition, we expected that capacity, access, continuity, and financial margins would increase.2,3,27,28 We also anticipated a decrease in downstream services, such as unnecessary tests, emergency department visits, and hospitalizations—a benefit of accountable care payment models.
Our efforts have been successful from a financial point of view. We attribute the financial sustainability that we have experienced to 4 factors:
1. Increased productivity. We knew that the increased efficiency of team-based care enables physicians to see 1 to 2 more patients per half day, and sometimes more.3,28,29 An increase of at least 1 patient visit per half-day was expected of our physicians and APCs on aTBC. In addition, they were expected to support the care team RN in achieving at least 4 billable visits per day. Our current level of RN visits is at 3.5 per nurse per day. There is significant variability in the increase of patients seen by a physician per day, ranging from 1 to 4 additional patients. These increased visits have helped us achieve financial viability, even in a predominantly fee-for-service environment.
2. More thorough service. The ability to keep patients in primary care and to focus on the patient’s full range of needs has led to higher levels of service and, consequently, to appropriately higher levels of billing codes. For example, Bellin’s revenue from billing increased by $724 per patient, related (in part) to higher rates of immunizations, cancer screenings with mammography, and colonoscopies.
Continue to: 3. New billable services
3. New billable services. Billing for RN blood pressure checks, AWVs, and extended care team services have helped make aTBC at Bellin financially feasible. Revenue from RN visits, for example, was $630,000 in 2018.
4. Improved access for patients. Of the 130 primary care providers now on aTBC, 15 (11.5%) had closed their practices to new patients before aTBC. Now, all of their practices are open to new patients, which has improved access to care. In a 2018 patient access survey, 96.6% of patients obtained an appointment as soon as they thought it was needed, compared with 70.7% of patients before the transition to aTBC.
Greater opportunity for financial sustainability. The combination of improved quality measures and decreased cost of care in the Bellin aTBC bodes well for future success in a value-based world. We have realized a significant increase in value-based payments for improved quality, and in our Next Gen Accountable Care Organization (ACO) patients, we have seen a decrease of $29 in per-member-per-month costs, likely due to the use of nonphysicians in expanded roles. In addition, hospital admissions have decreased by 5% due to the ability of ambulatory teams to manage more complex patients in the office setting. This model has also allowed physicians and APCs to increase their panel size, another key value-based metric. From 2016 to 2018, panel size for primary care providers increased by an average of 8%.
Enhanced ability to retain and recruit. Several of Bellin’s primary care recruits indicated that they had interviewed only at practices incorporating team-based care. This trend may increase as residencies transition to team-based models of care.
So how did we do?
Metrics of Bellin’s aTBC success
By the end of 2018, all 130 primary care physicians and APCs at Bellin had made the transition to this model, representing family medicine, internal medicine, and pediatrics. We have now begun the transition of our non-primary care specialties to team-based care.
Continue to: In the aTBC model...
In the aTBC model, the percentage of patients receiving age-appropriate screening is higher than before in every domain we measure (FIGURE 1). There has also been improvement in major quality metrics (FIGURE 2).
In a survey done in Spring 2018 by St. Norbert College Strategic Research Center, provider satisfaction increased, with 83% of physicians having made the transition to an aTBC practice moderately or very satisfied with their Bellin Health experience, compared with 70% in the traditional model. More recent 2019 survey data show a satisfaction rate of 90% for team-based care providers. Finally, in our aTBC model—in CMS’s Next-Gen ACO initiative—the cost per patient per month is significantly less than for those in a non-team-based care model ($796 vs $940).
CORRESPONDENCE
James Jerzak, MD, 1630 Commanche Ave, Green Bay, WI 54313; james.jerzak@bellin.org.
ACKNOWLEDGEMENTS
The authors would like to thank Lindsey E. Carlasare, MBA, from the American Medical Association, and Brad Wozney, MD, Kathy Kerscher, and Christopher Elfner from Bellin Health, for their contributions to the content and review of this manuscript.
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
3. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
4. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
5. Shanafelt TD, Mungo M, Schmitgen J, et al. Longitudinal study evaluating the association between physician burnout and changes in professional work effort. Mayo Clin Proc. 2016;91:422-431.
6. Sinsky CA, Dyrbye LN, West CP, et al. Professional satisfaction and the career plans of US physicians. Mayo Clin Proc. 2017;92:1625-1635.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90:1600-1613.
8. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
9. Sinsky CA, Sinsky TA, Althaus D, et al. Practice profile. ‘Core teams’: nurse-physician partnerships provide patient-centered care at an Iowa practice. Health Aff (Millwood). 2010;29:966-968.
10. Shanafelt T, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
11. Association for Advancing Physician and Provider Recruitment. Schutte L. What you don’t know can cost you: building a business case for recruitment and retention best practices. 2012. https://member.aappr.org/general/custom.asp?page=696. Accessed June 20, 2019.
12. American Medical Association. AMA STEPS Forward. Expanded rooming and discharge protocols. https://edhub.ama-assn.org/steps-forward/module/2702600. Accessed June 20, 2019.
13. American Medical Association. AMA STEPS Forward. Team documentation. https://edhub.ama-assn.org/steps-forward/module/2702598?resultClick=3&bypassSolrId=J_2702598. Accessed June 20, 2019.
14. American Medical Association. AMA STEPS Forward. EHR in-basket restructuring for improved efficiency. https://edhub.ama-assn.org/steps-forward/module/2702694?resultClick=3&bypassSolrId=J_2702694. Accessed June 20, 2019.
15. California Health Care Foundation. Bodenheimer T, Bauer L, Olayiwola JN. RN role reimagined: how empowering registered nurses can improve primary care. https://www.chcf.org/publication/rn-role-reimagined-how-empowering-registered-nurses-can-improve-primary-care/. Accessed June 20, 2019.
16. Chung S, Lesser LI, Lauderdale DS, et al. Medicare annual preventive care visits: use increased among fee-for-service patients, but many do not participate. Health Aff (Millwood). 2015;34:11-20.
17. American Medical Association. AMA Policy H-160.912. The structure and function of interprofessional health care teams. https://policysearch.ama-assn.org/policyfinder/detail/The%20Structure%20and%20Function%20of%20Interprofessional%20Health%20Care%20Teams?uri=%2FAMADoc%2FHOD.xml-0-727.xml. Accessed June 20, 2019.
18. Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128:337-343.
19. Hill RG Jr, Sears LM, Melanson SW. 4000 clicks: a productivity analysis of electronic medical records in a community hospital ED. Am J Emerg Med. 2013;31:1591-1594.
20. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21:e100-e106.
21. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
22. RAND Corporation. Friedberg MW, Chen PG, Ban Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. https://www.rand.org/pubs/research_reports/RR439.html. Accessed June 20, 2019.
23. Evaluation and Management (E/M) visit frequently asked questions (FAQs): physician fee schedule (PPS). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/E-M-Visit-FAQs-PFS.pdf. Accessed August 27, 2019.
24. Centers for Medicare & Medicaid Services. Scribe services signature requirements. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017-Transmittals-Items/R713PI.html. Accessed June 20, 2019.
25. American Association of Medical Assistants. State scope of practice laws. http://www.aama-ntl.org/employers/state-scope-of-practice-laws. Accessed June 20, 2019.
26. Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65:155-159.
27. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
28. Bank AJ, Obetz C, Konrardy A, et al. Impact of scribes on patient interaction, productivity, and revenue in a cardiology clinic: a prospective study. Clinicoecon Outcomes Res. 2013;5:399-406.
29. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
3. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
4. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
5. Shanafelt TD, Mungo M, Schmitgen J, et al. Longitudinal study evaluating the association between physician burnout and changes in professional work effort. Mayo Clin Proc. 2016;91:422-431.
6. Sinsky CA, Dyrbye LN, West CP, et al. Professional satisfaction and the career plans of US physicians. Mayo Clin Proc. 2017;92:1625-1635.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90:1600-1613.
8. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
9. Sinsky CA, Sinsky TA, Althaus D, et al. Practice profile. ‘Core teams’: nurse-physician partnerships provide patient-centered care at an Iowa practice. Health Aff (Millwood). 2010;29:966-968.
10. Shanafelt T, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
11. Association for Advancing Physician and Provider Recruitment. Schutte L. What you don’t know can cost you: building a business case for recruitment and retention best practices. 2012. https://member.aappr.org/general/custom.asp?page=696. Accessed June 20, 2019.
12. American Medical Association. AMA STEPS Forward. Expanded rooming and discharge protocols. https://edhub.ama-assn.org/steps-forward/module/2702600. Accessed June 20, 2019.
13. American Medical Association. AMA STEPS Forward. Team documentation. https://edhub.ama-assn.org/steps-forward/module/2702598?resultClick=3&bypassSolrId=J_2702598. Accessed June 20, 2019.
14. American Medical Association. AMA STEPS Forward. EHR in-basket restructuring for improved efficiency. https://edhub.ama-assn.org/steps-forward/module/2702694?resultClick=3&bypassSolrId=J_2702694. Accessed June 20, 2019.
15. California Health Care Foundation. Bodenheimer T, Bauer L, Olayiwola JN. RN role reimagined: how empowering registered nurses can improve primary care. https://www.chcf.org/publication/rn-role-reimagined-how-empowering-registered-nurses-can-improve-primary-care/. Accessed June 20, 2019.
16. Chung S, Lesser LI, Lauderdale DS, et al. Medicare annual preventive care visits: use increased among fee-for-service patients, but many do not participate. Health Aff (Millwood). 2015;34:11-20.
17. American Medical Association. AMA Policy H-160.912. The structure and function of interprofessional health care teams. https://policysearch.ama-assn.org/policyfinder/detail/The%20Structure%20and%20Function%20of%20Interprofessional%20Health%20Care%20Teams?uri=%2FAMADoc%2FHOD.xml-0-727.xml. Accessed June 20, 2019.
18. Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128:337-343.
19. Hill RG Jr, Sears LM, Melanson SW. 4000 clicks: a productivity analysis of electronic medical records in a community hospital ED. Am J Emerg Med. 2013;31:1591-1594.
20. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21:e100-e106.
21. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
22. RAND Corporation. Friedberg MW, Chen PG, Ban Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. https://www.rand.org/pubs/research_reports/RR439.html. Accessed June 20, 2019.
23. Evaluation and Management (E/M) visit frequently asked questions (FAQs): physician fee schedule (PPS). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/E-M-Visit-FAQs-PFS.pdf. Accessed August 27, 2019.
24. Centers for Medicare & Medicaid Services. Scribe services signature requirements. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017-Transmittals-Items/R713PI.html. Accessed June 20, 2019.
25. American Association of Medical Assistants. State scope of practice laws. http://www.aama-ntl.org/employers/state-scope-of-practice-laws. Accessed June 20, 2019.
26. Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65:155-159.
27. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
28. Bank AJ, Obetz C, Konrardy A, et al. Impact of scribes on patient interaction, productivity, and revenue in a cardiology clinic: a prospective study. Clinicoecon Outcomes Res. 2013;5:399-406.
29. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
PRACTICE RECOMMENDATIONS
› Up-train staff to provide enhanced support for physicians during the office visit, such as handling most electronic health record work, including documentation. C
› Take a team approach to between-visit work, leveraging principles of team-based care (such as co-location) to optimize efficiency. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series