User login
Decreasing Treatment of Asymptomatic Bacteriuria: An Interprofessional Approach to Antibiotic Stewardship
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
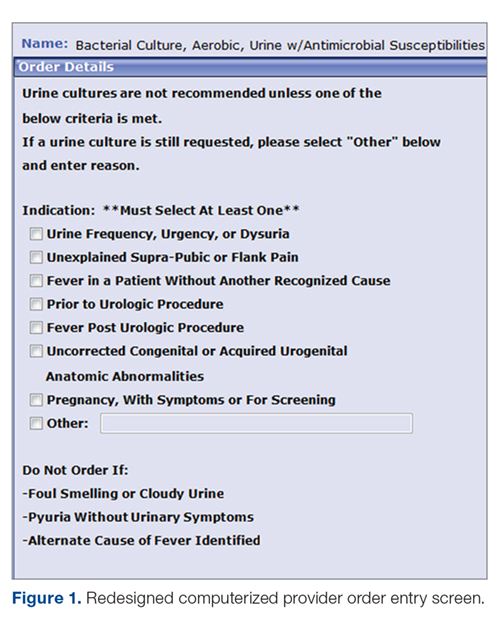
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
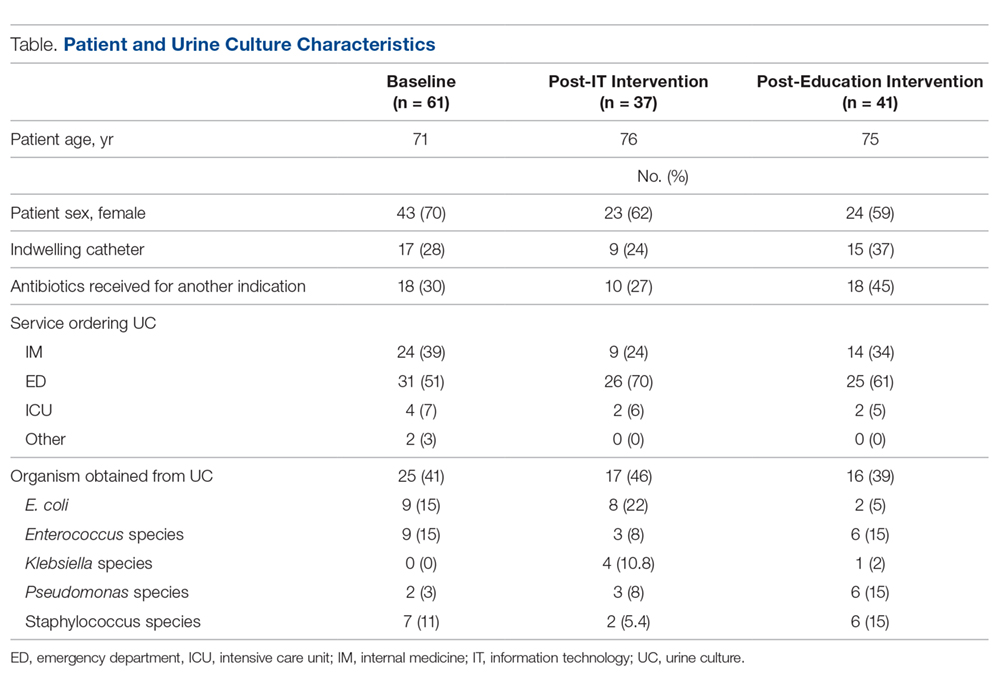
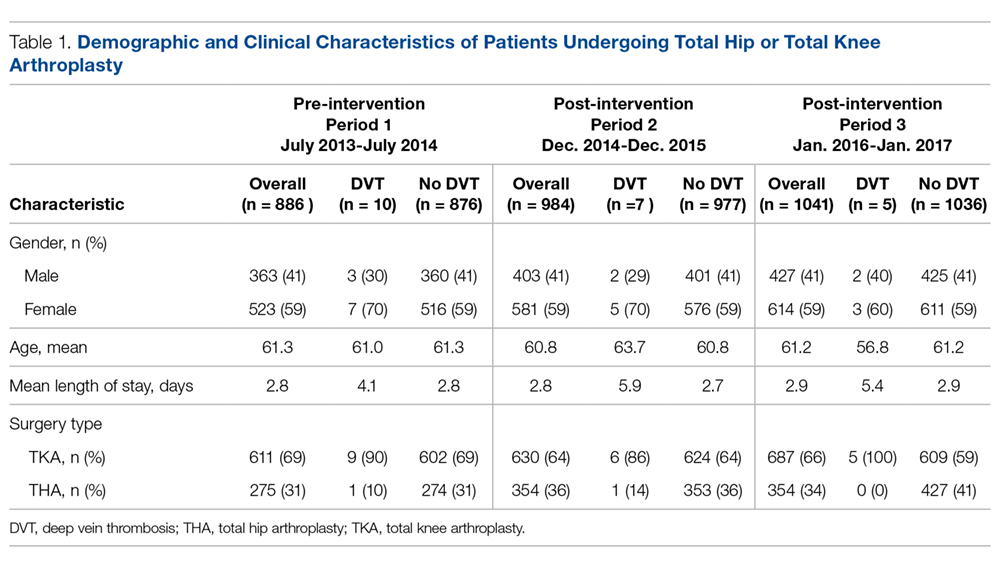
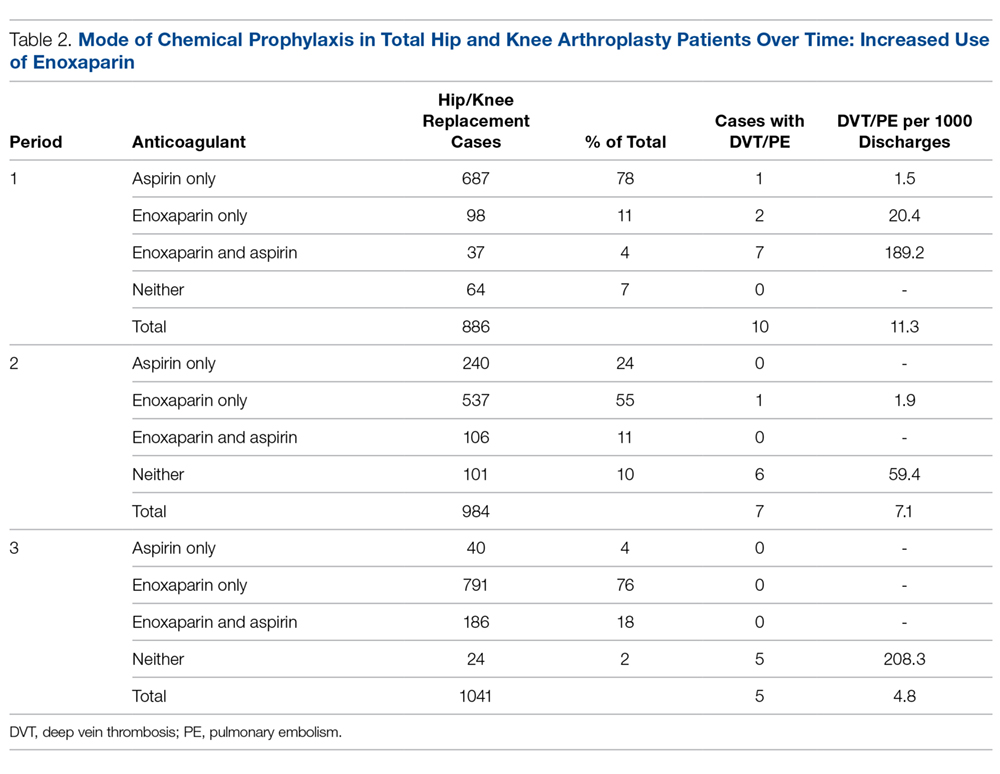
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
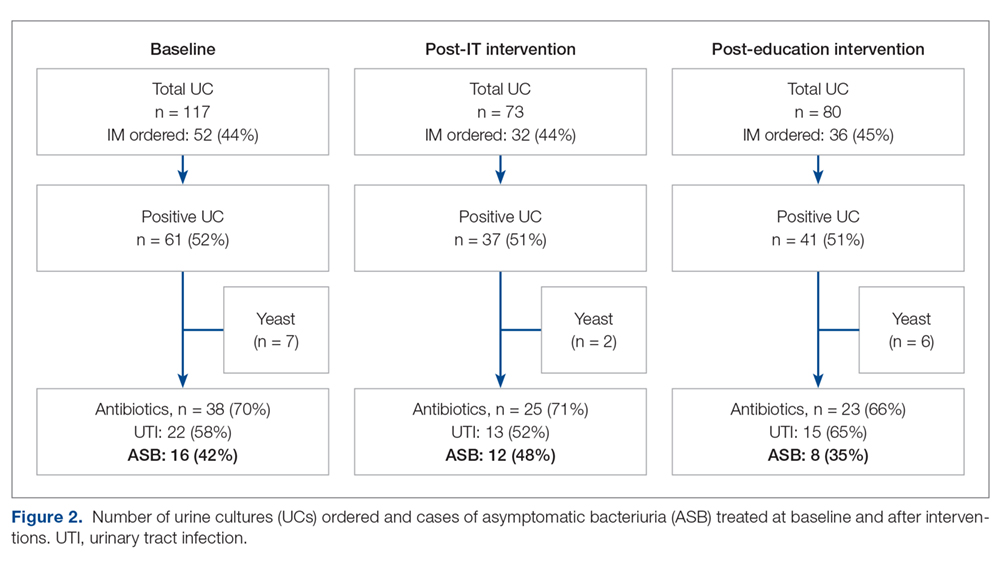
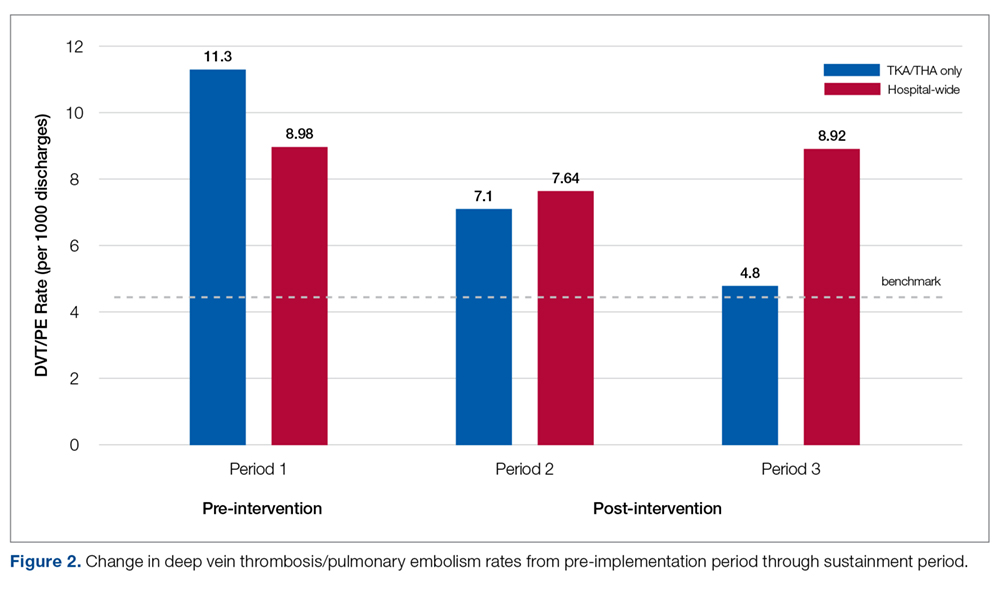
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; narayanan.prasanna@mayo.edu.
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; narayanan.prasanna@mayo.edu.
Financial disclosures: None.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; narayanan.prasanna@mayo.edu.
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
Elevating Critical Care Pharmacy Services in a Resource-Limited Environment Through Establishment of a Pharmacist Team
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
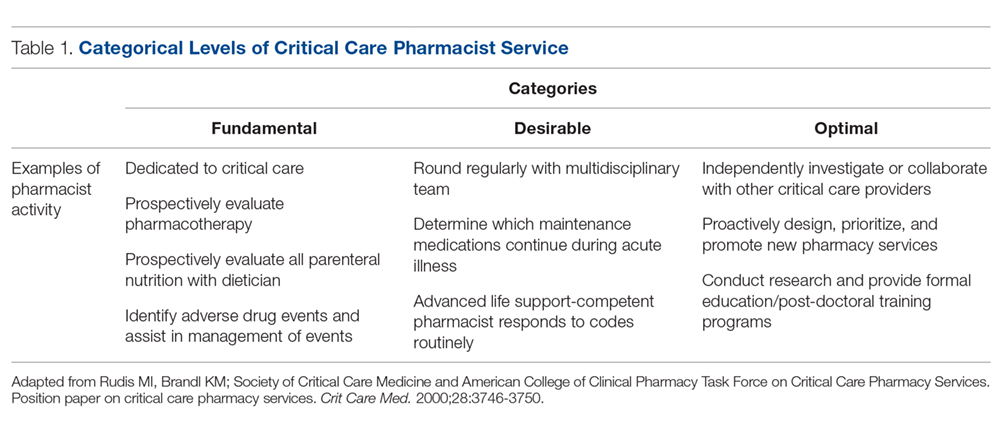
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4
Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
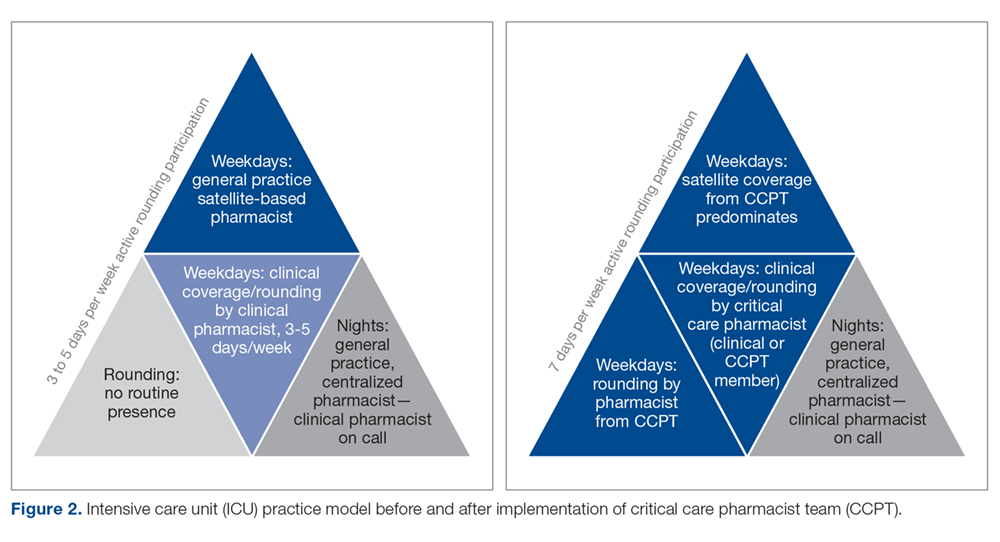
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
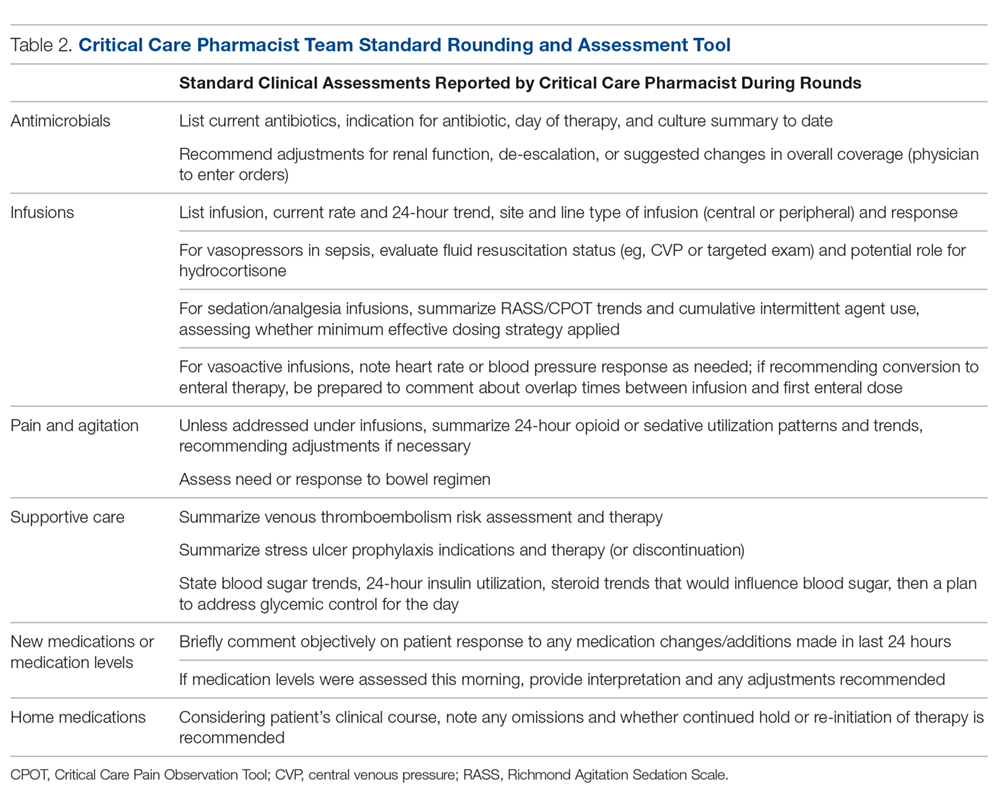
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.
Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
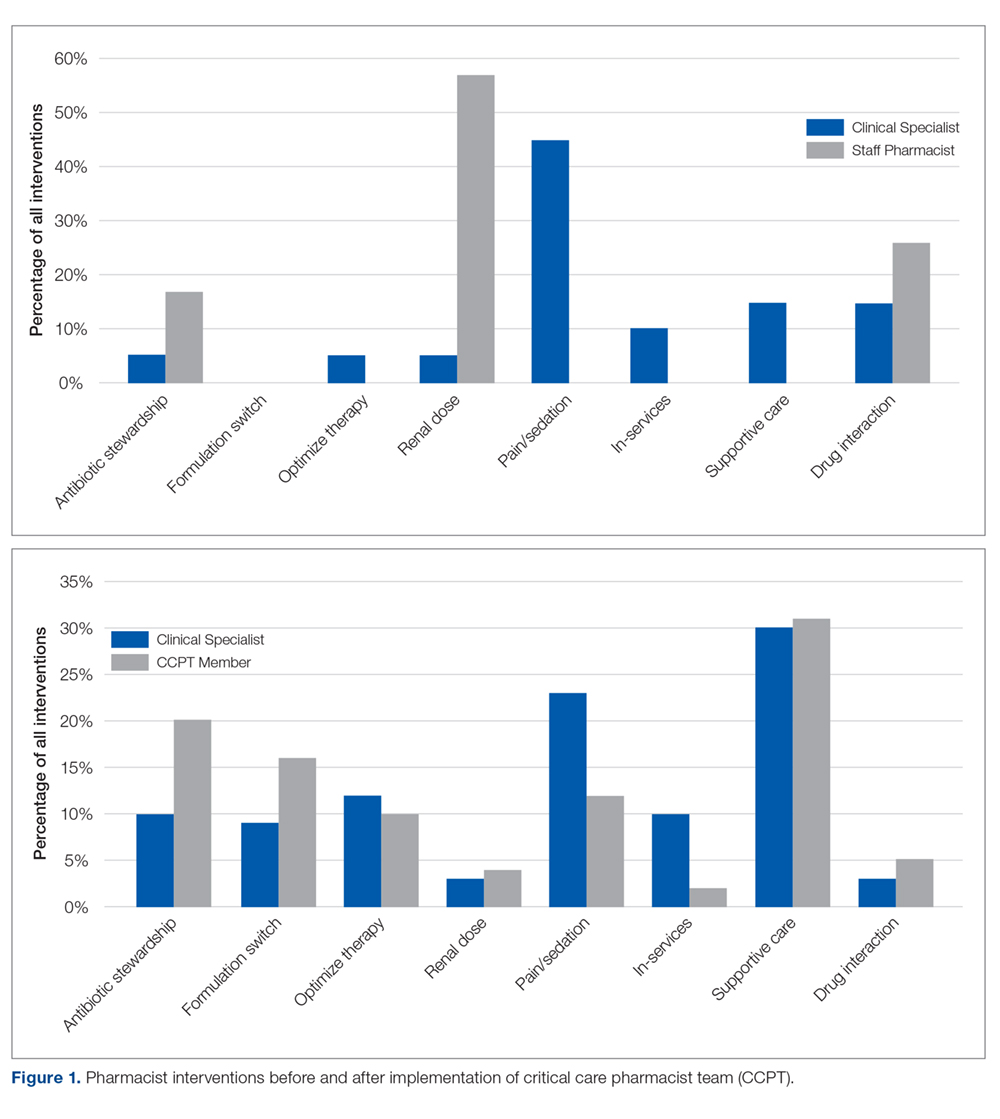
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.
When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.
Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; lbarbarello@pharmacy.rutgers.edu.
Financial disclosures: None.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4
Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.
Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.
When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.
Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; lbarbarello@pharmacy.rutgers.edu.
Financial disclosures: None.
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4
Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.
Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.
When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.
Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; lbarbarello@pharmacy.rutgers.edu.
Financial disclosures: None.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
Study eyes narcolepsy’s impact on patient quality of life
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
REPORTING FROM SLEEP 2019
Reducing Rates of Perioperative Deep Vein Thrombosis and Pulmonary Emboli in Hip and Knee Arthroplasty Patients: A Quality Improvement Project
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.
From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.
From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.
From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
Early intervention initiative cut oncology patient hospitalizations
, according to a report from a large, independent, community-based oncology practice.
The program saved nearly $3.2 million in Medicare costs over the course of the year, said Molly Mendenhall, RN, of Oncology Hematology Care in Cincinnati.
“By keeping those patients out of the hospital, we were able to improve patient quality of life, and increase patient satisfaction by treating them in their home clinic instead of the hospital,” Ms. Mendenhall said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
The campaign was developed in anticipation of participating in the Oncology Care Model (OCM), a program focused on providing coordinated and high-quality care for Medicare oncology patients at the same or lower cost.
Prior to participating in OCM, Ms. Mendenhall and her colleagues set up an after-hours phone triage system, proactive chemotherapy follow-up calls, and a Saturday-Sunday urgent care clinic designed to help avoid any unnecessary hospitalizations over the weekend.
They also set up a 2-hour structured OCM treatment planning visit to prioritize shared decision making between the patient and the clinical team regarding diagnosis, symptom management, financial assistance, and other aspects of care.
The most influential part of the initiative, according to Ms. Mendenhall, was a patient-directed “Call Us Early – Call Us First” campaign that included symptom management teaching sheets, a 34-page teaching book, and branded buttons, pens, and magnets all designed to emphasize the patient responsibility to use the phone.
“All of those previous steps really wouldn’t make a difference if the patients still weren’t calling us,” Ms. Mendenhall explained.
Over the first year of participation in the OCM program, the oncology practice saw a 16% statistically significant reduction in hospital admissions (P = .005). The number of inpatient admissions per 100 patients dropped from 26.8 at baseline to 22.6 at the most recent follow-up in a report published simultaneously in the Journal of Oncology Practice.
Reduced admissions translated into a drop of $798,000 in inpatient costs per quarter over 1,600 patients, or $3.129 million in savings for the Centers for Medicare & Medicaid Services over the first year of participation in OCM, according to the researchers.
Patient satisfaction scores trended positively over the course of that year based on a review of blinded surveys that asked patients to rate clinical care, communication, access, information exchange, and other aspects of their experience.
Scores on those surveys were 8.03 on a scale of 0-10 (low to high) during the baseline period of January to September 2016, the researchers said. Scores were 8.29 and 8.26 for two follow-up surveys.
Ms. Mendenhall had no disclosures to report. Coauthors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, and Lilly. Two coauthors reported leadership, stock, or other ownership interests in Oncology Hematology Care/US Oncology.
SOURCE: Mendenhall M et al. Quality Care Symposium, Abstract 30.
, according to a report from a large, independent, community-based oncology practice.
The program saved nearly $3.2 million in Medicare costs over the course of the year, said Molly Mendenhall, RN, of Oncology Hematology Care in Cincinnati.
“By keeping those patients out of the hospital, we were able to improve patient quality of life, and increase patient satisfaction by treating them in their home clinic instead of the hospital,” Ms. Mendenhall said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
The campaign was developed in anticipation of participating in the Oncology Care Model (OCM), a program focused on providing coordinated and high-quality care for Medicare oncology patients at the same or lower cost.
Prior to participating in OCM, Ms. Mendenhall and her colleagues set up an after-hours phone triage system, proactive chemotherapy follow-up calls, and a Saturday-Sunday urgent care clinic designed to help avoid any unnecessary hospitalizations over the weekend.
They also set up a 2-hour structured OCM treatment planning visit to prioritize shared decision making between the patient and the clinical team regarding diagnosis, symptom management, financial assistance, and other aspects of care.
The most influential part of the initiative, according to Ms. Mendenhall, was a patient-directed “Call Us Early – Call Us First” campaign that included symptom management teaching sheets, a 34-page teaching book, and branded buttons, pens, and magnets all designed to emphasize the patient responsibility to use the phone.
“All of those previous steps really wouldn’t make a difference if the patients still weren’t calling us,” Ms. Mendenhall explained.
Over the first year of participation in the OCM program, the oncology practice saw a 16% statistically significant reduction in hospital admissions (P = .005). The number of inpatient admissions per 100 patients dropped from 26.8 at baseline to 22.6 at the most recent follow-up in a report published simultaneously in the Journal of Oncology Practice.
Reduced admissions translated into a drop of $798,000 in inpatient costs per quarter over 1,600 patients, or $3.129 million in savings for the Centers for Medicare & Medicaid Services over the first year of participation in OCM, according to the researchers.
Patient satisfaction scores trended positively over the course of that year based on a review of blinded surveys that asked patients to rate clinical care, communication, access, information exchange, and other aspects of their experience.
Scores on those surveys were 8.03 on a scale of 0-10 (low to high) during the baseline period of January to September 2016, the researchers said. Scores were 8.29 and 8.26 for two follow-up surveys.
Ms. Mendenhall had no disclosures to report. Coauthors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, and Lilly. Two coauthors reported leadership, stock, or other ownership interests in Oncology Hematology Care/US Oncology.
SOURCE: Mendenhall M et al. Quality Care Symposium, Abstract 30.
, according to a report from a large, independent, community-based oncology practice.
The program saved nearly $3.2 million in Medicare costs over the course of the year, said Molly Mendenhall, RN, of Oncology Hematology Care in Cincinnati.
“By keeping those patients out of the hospital, we were able to improve patient quality of life, and increase patient satisfaction by treating them in their home clinic instead of the hospital,” Ms. Mendenhall said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
The campaign was developed in anticipation of participating in the Oncology Care Model (OCM), a program focused on providing coordinated and high-quality care for Medicare oncology patients at the same or lower cost.
Prior to participating in OCM, Ms. Mendenhall and her colleagues set up an after-hours phone triage system, proactive chemotherapy follow-up calls, and a Saturday-Sunday urgent care clinic designed to help avoid any unnecessary hospitalizations over the weekend.
They also set up a 2-hour structured OCM treatment planning visit to prioritize shared decision making between the patient and the clinical team regarding diagnosis, symptom management, financial assistance, and other aspects of care.
The most influential part of the initiative, according to Ms. Mendenhall, was a patient-directed “Call Us Early – Call Us First” campaign that included symptom management teaching sheets, a 34-page teaching book, and branded buttons, pens, and magnets all designed to emphasize the patient responsibility to use the phone.
“All of those previous steps really wouldn’t make a difference if the patients still weren’t calling us,” Ms. Mendenhall explained.
Over the first year of participation in the OCM program, the oncology practice saw a 16% statistically significant reduction in hospital admissions (P = .005). The number of inpatient admissions per 100 patients dropped from 26.8 at baseline to 22.6 at the most recent follow-up in a report published simultaneously in the Journal of Oncology Practice.
Reduced admissions translated into a drop of $798,000 in inpatient costs per quarter over 1,600 patients, or $3.129 million in savings for the Centers for Medicare & Medicaid Services over the first year of participation in OCM, according to the researchers.
Patient satisfaction scores trended positively over the course of that year based on a review of blinded surveys that asked patients to rate clinical care, communication, access, information exchange, and other aspects of their experience.
Scores on those surveys were 8.03 on a scale of 0-10 (low to high) during the baseline period of January to September 2016, the researchers said. Scores were 8.29 and 8.26 for two follow-up surveys.
Ms. Mendenhall had no disclosures to report. Coauthors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, and Lilly. Two coauthors reported leadership, stock, or other ownership interests in Oncology Hematology Care/US Oncology.
SOURCE: Mendenhall M et al. Quality Care Symposium, Abstract 30.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: An initiative designed to reduce avoidable emergency room visits and hospitalizations reduced both admissions and inpatient costs.
Major finding: The program cut admissions by 16% and saved nearly $3.2 million in Medicare costs savings over the course of a year.
Study details: Analysis of first-year experience including 1,600 patients per quarter for a large, independent, community-based oncology practice participating in the Oncology Care Model (OCM) of the Centers for Medicare and Medicaid Services.
Disclosures: Authors on the study provided disclosures related to Janssen Oncology, Pfizer, Amgen, Abbvie, Merck, Pharmacyclics, Bristol-Myers Squibb, Celgene, Genentech/Roche, AZTherapies, Lilly, and Oncology Hematology Care/US Oncology.
Source: Mendenhall M et al. Quality Care Symposium, Abstract 30.
Screening for Lynch Syndrome Among Patients with Colorectal Cancer: Experiences from a Multihospital Health System
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
Patterns of malignancies in patients with HIV-AIDS: a single institution observational study
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
Atopic dermatitis hits mental health, quality of life
Atopic dermatitis (AD) places a considerable burden on mental health and quality of life for patients with disease of even moderate severity, according to a cross-sectional study of data from the Atopic Dermatitis in America survey.
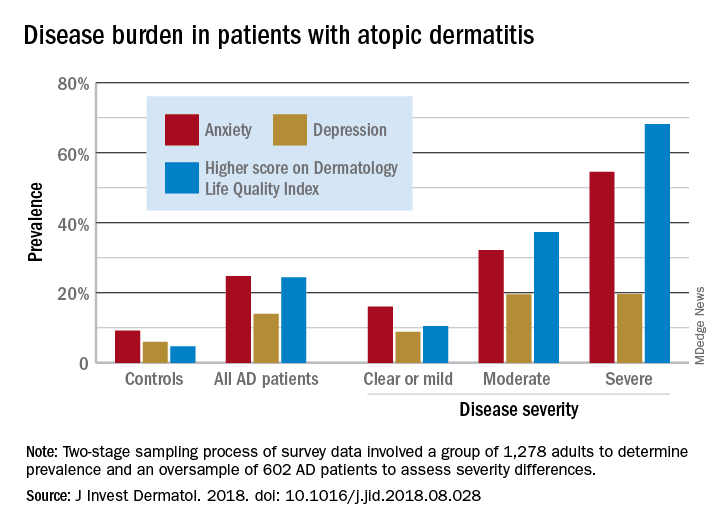
Among adults with severe AD, the mean score on the Dermatology Life Quality Index was 11.4, with a score of 6-30 representing a moderate to large effect on quality of life. The mean for those with moderate disease, 5.9, was just below that range, but 37% of that group did have scores between 6 and 30, Zelma C. Chiesa Fuxench, MD, of the University of Pennsylvania, Philadelphia, and her associates said in the Journal of Investigative Dermatology.
The mean on the Dermatology Life Quality Index for all AD patients was 4.1, with 24% falling into the moderate to large effect range, compared with 1% and 5% for controls. Results were similar on the mental health measure used, the Hospital Anxiety and Depression Scale (HADS). Mean HADS-anxiety scores were 7.0 for all AD patients and 4.7 for controls, and HADS-depression means were 5.8 for AD patients and 3.6 for controls, the investigators reported.
Analysis by disease severity found that 32% of those with moderate AD and almost 56% with severe AD had clinical anxiety (HADS-A score of 11-21), while somewhat lower prevalences were seen for clinical depression (HADS-D score of 11-21): 19.5% for those with moderate AD and 19.7% for patients with severe AD, Dr. Chiesa Fuxench and her associates said.
“An increasing number of studies provide evidence that AD is associated with marked [quality of life] impairment and increased health care costs with higher burden and costs in those with more severe disease. Additional studies should center on exploring those factors associated with AD, and AD disease severity, which lead to increased disease burden in this population,” they wrote.
Respondents to the Atopic Dermatitis in America survey were part of the GfK Knowledge Panel. The study involved a two-stage sampling process: one group of 1,278 adults determined prevalence ,and an oversample of 602 AD patients assessed severity differences.
Dr. Chiesa Fuxench has received research grants from Regeneron, Sanofi, Tioga, and Vanda for work related to atopic dermatitis and has received honoraria for CME work in atopic dermatitis sponsored by educational grants from Regeneron and Sanofi.
SOURCE: J Invest Dermatol. 2018. doi: 10.1016/j.jid.2018.08.028.
Atopic dermatitis (AD) places a considerable burden on mental health and quality of life for patients with disease of even moderate severity, according to a cross-sectional study of data from the Atopic Dermatitis in America survey.

Among adults with severe AD, the mean score on the Dermatology Life Quality Index was 11.4, with a score of 6-30 representing a moderate to large effect on quality of life. The mean for those with moderate disease, 5.9, was just below that range, but 37% of that group did have scores between 6 and 30, Zelma C. Chiesa Fuxench, MD, of the University of Pennsylvania, Philadelphia, and her associates said in the Journal of Investigative Dermatology.
The mean on the Dermatology Life Quality Index for all AD patients was 4.1, with 24% falling into the moderate to large effect range, compared with 1% and 5% for controls. Results were similar on the mental health measure used, the Hospital Anxiety and Depression Scale (HADS). Mean HADS-anxiety scores were 7.0 for all AD patients and 4.7 for controls, and HADS-depression means were 5.8 for AD patients and 3.6 for controls, the investigators reported.
Analysis by disease severity found that 32% of those with moderate AD and almost 56% with severe AD had clinical anxiety (HADS-A score of 11-21), while somewhat lower prevalences were seen for clinical depression (HADS-D score of 11-21): 19.5% for those with moderate AD and 19.7% for patients with severe AD, Dr. Chiesa Fuxench and her associates said.
“An increasing number of studies provide evidence that AD is associated with marked [quality of life] impairment and increased health care costs with higher burden and costs in those with more severe disease. Additional studies should center on exploring those factors associated with AD, and AD disease severity, which lead to increased disease burden in this population,” they wrote.
Respondents to the Atopic Dermatitis in America survey were part of the GfK Knowledge Panel. The study involved a two-stage sampling process: one group of 1,278 adults determined prevalence ,and an oversample of 602 AD patients assessed severity differences.
Dr. Chiesa Fuxench has received research grants from Regeneron, Sanofi, Tioga, and Vanda for work related to atopic dermatitis and has received honoraria for CME work in atopic dermatitis sponsored by educational grants from Regeneron and Sanofi.
SOURCE: J Invest Dermatol. 2018. doi: 10.1016/j.jid.2018.08.028.
Atopic dermatitis (AD) places a considerable burden on mental health and quality of life for patients with disease of even moderate severity, according to a cross-sectional study of data from the Atopic Dermatitis in America survey.

Among adults with severe AD, the mean score on the Dermatology Life Quality Index was 11.4, with a score of 6-30 representing a moderate to large effect on quality of life. The mean for those with moderate disease, 5.9, was just below that range, but 37% of that group did have scores between 6 and 30, Zelma C. Chiesa Fuxench, MD, of the University of Pennsylvania, Philadelphia, and her associates said in the Journal of Investigative Dermatology.
The mean on the Dermatology Life Quality Index for all AD patients was 4.1, with 24% falling into the moderate to large effect range, compared with 1% and 5% for controls. Results were similar on the mental health measure used, the Hospital Anxiety and Depression Scale (HADS). Mean HADS-anxiety scores were 7.0 for all AD patients and 4.7 for controls, and HADS-depression means were 5.8 for AD patients and 3.6 for controls, the investigators reported.
Analysis by disease severity found that 32% of those with moderate AD and almost 56% with severe AD had clinical anxiety (HADS-A score of 11-21), while somewhat lower prevalences were seen for clinical depression (HADS-D score of 11-21): 19.5% for those with moderate AD and 19.7% for patients with severe AD, Dr. Chiesa Fuxench and her associates said.
“An increasing number of studies provide evidence that AD is associated with marked [quality of life] impairment and increased health care costs with higher burden and costs in those with more severe disease. Additional studies should center on exploring those factors associated with AD, and AD disease severity, which lead to increased disease burden in this population,” they wrote.
Respondents to the Atopic Dermatitis in America survey were part of the GfK Knowledge Panel. The study involved a two-stage sampling process: one group of 1,278 adults determined prevalence ,and an oversample of 602 AD patients assessed severity differences.
Dr. Chiesa Fuxench has received research grants from Regeneron, Sanofi, Tioga, and Vanda for work related to atopic dermatitis and has received honoraria for CME work in atopic dermatitis sponsored by educational grants from Regeneron and Sanofi.
SOURCE: J Invest Dermatol. 2018. doi: 10.1016/j.jid.2018.08.028.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
EHR-guided strategy reduces postop VTE events
BOSTON – Avoiding could result in a reduction in VTE rates, a speaker said at the annual clinical congress of the American College of Surgeons.
The VTE rate dropped by about one-quarter in the trauma care pathway at the University of Pittsburgh Medical Center (UPMC) after implementation of algorithms to risk-stratify patients and guide nursing staff, said Matthew D. Neal, MD, FACS, the Roberta G. Simmons Assistant Professor of Surgery at the University of Pittsburgh.
By incorporating algorithms into the electronic health record (EHR), UPMC was able to realize a “dramatic” 72% reduction in missed doses, from 4,331 missed doses in 2014 to 1,193 in 2015, Dr. Neal told attendees in a session focused on hot topics in surgical patient safety.
That decrease in missed doses has translated into a decreased rate of VTE, from an already relatively low rate of 1.5% in 2015, to 1.1% in 2017, representing a 26.7% reduction, according to data Dr. Neal shared in his podium presentation.
“This has been a sustainable event for us, largely linked to the implementation of an EHR-guided risk assessment pathway to guide the implementation of VTE prophylaxis,” he said.
The change was safe, he added, noting that, since utilization of this pathway, there have been no significant increases in the rate of bleeding events among patients who have mandatory orders.
These results corroborate those of some previous investigations, including one key study from the Johns Hopkins Hospital that described the adoption of a mandatory computerized clinical decision support tool to improve adherence to best practices for VTE prophylaxis.
After incorporation of the tool in the computerized order entry system, there was a significant increase in VTE prophylaxis, translating into a significant drop in preventable harm from VTE, from 1.0% to 0.17% (P = .04), investigators reported in JAMA Surgery.
Reducing missed doses is one of the major contributing factors to decreased VTE rates, according to Dr. Neal.
Missed doses of enoxaparin correlate with increased incidence of deep vein thrombosis (DVT) in trauma and general surgery patients, according to results of one prospective study Dr. Neal described. In that study of 202 patients, reported in JAMA Surgery, DVTs were seen in 23.5% of patients with missed doses, compared with 4.8 for patients with no missed doses (P < .01).
“We need to understand how to risk assess and how to utilize our EHR as a tool,” Dr. Neal told attendees.
Dr. Neal reported disclosures related to Janssen Pharmaceuticals, CSL Behring, Accriva Diagnostics, and Haemonetics, as well as a U.S. patent for a treatment of infectious and inflammatory disorders, and laboratory funding from the National Institutes of Health, Department of Defense, and the Biomedical Advanced Research and Development Authority.
SOURCE: Neal MD. Presentation at the American College of Surgeons Clinical Congress. 2018 Oct 25.
BOSTON – Avoiding could result in a reduction in VTE rates, a speaker said at the annual clinical congress of the American College of Surgeons.
The VTE rate dropped by about one-quarter in the trauma care pathway at the University of Pittsburgh Medical Center (UPMC) after implementation of algorithms to risk-stratify patients and guide nursing staff, said Matthew D. Neal, MD, FACS, the Roberta G. Simmons Assistant Professor of Surgery at the University of Pittsburgh.
By incorporating algorithms into the electronic health record (EHR), UPMC was able to realize a “dramatic” 72% reduction in missed doses, from 4,331 missed doses in 2014 to 1,193 in 2015, Dr. Neal told attendees in a session focused on hot topics in surgical patient safety.
That decrease in missed doses has translated into a decreased rate of VTE, from an already relatively low rate of 1.5% in 2015, to 1.1% in 2017, representing a 26.7% reduction, according to data Dr. Neal shared in his podium presentation.
“This has been a sustainable event for us, largely linked to the implementation of an EHR-guided risk assessment pathway to guide the implementation of VTE prophylaxis,” he said.
The change was safe, he added, noting that, since utilization of this pathway, there have been no significant increases in the rate of bleeding events among patients who have mandatory orders.
These results corroborate those of some previous investigations, including one key study from the Johns Hopkins Hospital that described the adoption of a mandatory computerized clinical decision support tool to improve adherence to best practices for VTE prophylaxis.
After incorporation of the tool in the computerized order entry system, there was a significant increase in VTE prophylaxis, translating into a significant drop in preventable harm from VTE, from 1.0% to 0.17% (P = .04), investigators reported in JAMA Surgery.
Reducing missed doses is one of the major contributing factors to decreased VTE rates, according to Dr. Neal.
Missed doses of enoxaparin correlate with increased incidence of deep vein thrombosis (DVT) in trauma and general surgery patients, according to results of one prospective study Dr. Neal described. In that study of 202 patients, reported in JAMA Surgery, DVTs were seen in 23.5% of patients with missed doses, compared with 4.8 for patients with no missed doses (P < .01).
“We need to understand how to risk assess and how to utilize our EHR as a tool,” Dr. Neal told attendees.
Dr. Neal reported disclosures related to Janssen Pharmaceuticals, CSL Behring, Accriva Diagnostics, and Haemonetics, as well as a U.S. patent for a treatment of infectious and inflammatory disorders, and laboratory funding from the National Institutes of Health, Department of Defense, and the Biomedical Advanced Research and Development Authority.
SOURCE: Neal MD. Presentation at the American College of Surgeons Clinical Congress. 2018 Oct 25.
BOSTON – Avoiding could result in a reduction in VTE rates, a speaker said at the annual clinical congress of the American College of Surgeons.
The VTE rate dropped by about one-quarter in the trauma care pathway at the University of Pittsburgh Medical Center (UPMC) after implementation of algorithms to risk-stratify patients and guide nursing staff, said Matthew D. Neal, MD, FACS, the Roberta G. Simmons Assistant Professor of Surgery at the University of Pittsburgh.
By incorporating algorithms into the electronic health record (EHR), UPMC was able to realize a “dramatic” 72% reduction in missed doses, from 4,331 missed doses in 2014 to 1,193 in 2015, Dr. Neal told attendees in a session focused on hot topics in surgical patient safety.
That decrease in missed doses has translated into a decreased rate of VTE, from an already relatively low rate of 1.5% in 2015, to 1.1% in 2017, representing a 26.7% reduction, according to data Dr. Neal shared in his podium presentation.
“This has been a sustainable event for us, largely linked to the implementation of an EHR-guided risk assessment pathway to guide the implementation of VTE prophylaxis,” he said.
The change was safe, he added, noting that, since utilization of this pathway, there have been no significant increases in the rate of bleeding events among patients who have mandatory orders.
These results corroborate those of some previous investigations, including one key study from the Johns Hopkins Hospital that described the adoption of a mandatory computerized clinical decision support tool to improve adherence to best practices for VTE prophylaxis.
After incorporation of the tool in the computerized order entry system, there was a significant increase in VTE prophylaxis, translating into a significant drop in preventable harm from VTE, from 1.0% to 0.17% (P = .04), investigators reported in JAMA Surgery.
Reducing missed doses is one of the major contributing factors to decreased VTE rates, according to Dr. Neal.
Missed doses of enoxaparin correlate with increased incidence of deep vein thrombosis (DVT) in trauma and general surgery patients, according to results of one prospective study Dr. Neal described. In that study of 202 patients, reported in JAMA Surgery, DVTs were seen in 23.5% of patients with missed doses, compared with 4.8 for patients with no missed doses (P < .01).
“We need to understand how to risk assess and how to utilize our EHR as a tool,” Dr. Neal told attendees.
Dr. Neal reported disclosures related to Janssen Pharmaceuticals, CSL Behring, Accriva Diagnostics, and Haemonetics, as well as a U.S. patent for a treatment of infectious and inflammatory disorders, and laboratory funding from the National Institutes of Health, Department of Defense, and the Biomedical Advanced Research and Development Authority.
SOURCE: Neal MD. Presentation at the American College of Surgeons Clinical Congress. 2018 Oct 25.
AT THE ACS CLINICAL CONGRESS
Pharmacist-led clinic boosted hypertension control after discharge
SAN DIEGO – Pharmacist-physician collaboration on hypertension management was effective for controlling blood pressure in patients discharged from an urban ED, results from a pilot study showed.
“Hypertension is the leading risk factor for cardiovascular events and stroke in this country,” Brittany Stewart, RD, PharmD, said at the annual meeting of the American College of Emergency Physicians. “The good news is that we’re not trying to figure out how to treat this disease, but the bad news is that we have 50% of people with uncontrolled high blood pressure.”
“Pharmacists are highly accessible in the community in outpatient settings,” Dr. Stewart of the department of pharmacy practice at Wayne State University, Detroit, said. “This is where ED providers can really integrate and work on a team to refer patients for their chronic diseases on an outpatient basis.”
In a prospective pilot trial, Dr. Stewart and her colleagues recruited 89 patients with uncontrolled hypertension who presented to the ED at Wayne State during May 24, 2017–May 18, 2018. Their average age was 43 years, 51% were male, 94% were black, 51% were current smokers, the mean body mass index was 34.5 kg/m2, and 18% had no health insurance.
“In Detroit, we have significant health disparities with our patient population,” she said. “They are high utilizers of the emergency department, not only for their acute illness but for chronic illness as well. There are several studies showing that team-based care has improved hypertension and blood pressure control. However, the adoption and sustainability of these models haven’t really taken off in our health care landscape yet.”
Over a period of 1.5 years, Dr. Stewart and two physicians developed a transitional care clinic, based on a collaborative practice agreement with emergency physicians. Per protocol, five follow-up visits were planned in an outpatient pharmacy clinic, where Dr. Stewart initiated and titrated antihypertensive medications and handled refills.
“The physician does not have to physically be at the clinic,” she said. “We work closely over the phone to make the best decisions, but it’s not typical ambulatory care where the physician has to be sitting right next to the pharmacist to make the best decisions for the patients.” The primary outcome was the transitional care clinic’s impact on blood pressure.
Dr. Stewart reported results from 47 medication interventions that were provided over 97 follow-up visits. The researchers found that the median blood pressure dropped from an initial reading of 160/102 mm Hg to 130/93 mm Hg by the fifth transitional care clinic visit. Across all patients, systolic blood pressure decreased by an average of 48 mm Hg. “I don’t think we were surprised by these results; we are getting people very much in need down to their blood pressure goals,” she said. “By the end of the study, patients were on an average of three antihypertensive medications.”
She and her colleagues plan to conduct a larger randomized, clinical trial of this care model. The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.
SAN DIEGO – Pharmacist-physician collaboration on hypertension management was effective for controlling blood pressure in patients discharged from an urban ED, results from a pilot study showed.
“Hypertension is the leading risk factor for cardiovascular events and stroke in this country,” Brittany Stewart, RD, PharmD, said at the annual meeting of the American College of Emergency Physicians. “The good news is that we’re not trying to figure out how to treat this disease, but the bad news is that we have 50% of people with uncontrolled high blood pressure.”
“Pharmacists are highly accessible in the community in outpatient settings,” Dr. Stewart of the department of pharmacy practice at Wayne State University, Detroit, said. “This is where ED providers can really integrate and work on a team to refer patients for their chronic diseases on an outpatient basis.”
In a prospective pilot trial, Dr. Stewart and her colleagues recruited 89 patients with uncontrolled hypertension who presented to the ED at Wayne State during May 24, 2017–May 18, 2018. Their average age was 43 years, 51% were male, 94% were black, 51% were current smokers, the mean body mass index was 34.5 kg/m2, and 18% had no health insurance.
“In Detroit, we have significant health disparities with our patient population,” she said. “They are high utilizers of the emergency department, not only for their acute illness but for chronic illness as well. There are several studies showing that team-based care has improved hypertension and blood pressure control. However, the adoption and sustainability of these models haven’t really taken off in our health care landscape yet.”
Over a period of 1.5 years, Dr. Stewart and two physicians developed a transitional care clinic, based on a collaborative practice agreement with emergency physicians. Per protocol, five follow-up visits were planned in an outpatient pharmacy clinic, where Dr. Stewart initiated and titrated antihypertensive medications and handled refills.
“The physician does not have to physically be at the clinic,” she said. “We work closely over the phone to make the best decisions, but it’s not typical ambulatory care where the physician has to be sitting right next to the pharmacist to make the best decisions for the patients.” The primary outcome was the transitional care clinic’s impact on blood pressure.
Dr. Stewart reported results from 47 medication interventions that were provided over 97 follow-up visits. The researchers found that the median blood pressure dropped from an initial reading of 160/102 mm Hg to 130/93 mm Hg by the fifth transitional care clinic visit. Across all patients, systolic blood pressure decreased by an average of 48 mm Hg. “I don’t think we were surprised by these results; we are getting people very much in need down to their blood pressure goals,” she said. “By the end of the study, patients were on an average of three antihypertensive medications.”
She and her colleagues plan to conduct a larger randomized, clinical trial of this care model. The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.
SAN DIEGO – Pharmacist-physician collaboration on hypertension management was effective for controlling blood pressure in patients discharged from an urban ED, results from a pilot study showed.
“Hypertension is the leading risk factor for cardiovascular events and stroke in this country,” Brittany Stewart, RD, PharmD, said at the annual meeting of the American College of Emergency Physicians. “The good news is that we’re not trying to figure out how to treat this disease, but the bad news is that we have 50% of people with uncontrolled high blood pressure.”
“Pharmacists are highly accessible in the community in outpatient settings,” Dr. Stewart of the department of pharmacy practice at Wayne State University, Detroit, said. “This is where ED providers can really integrate and work on a team to refer patients for their chronic diseases on an outpatient basis.”
In a prospective pilot trial, Dr. Stewart and her colleagues recruited 89 patients with uncontrolled hypertension who presented to the ED at Wayne State during May 24, 2017–May 18, 2018. Their average age was 43 years, 51% were male, 94% were black, 51% were current smokers, the mean body mass index was 34.5 kg/m2, and 18% had no health insurance.
“In Detroit, we have significant health disparities with our patient population,” she said. “They are high utilizers of the emergency department, not only for their acute illness but for chronic illness as well. There are several studies showing that team-based care has improved hypertension and blood pressure control. However, the adoption and sustainability of these models haven’t really taken off in our health care landscape yet.”
Over a period of 1.5 years, Dr. Stewart and two physicians developed a transitional care clinic, based on a collaborative practice agreement with emergency physicians. Per protocol, five follow-up visits were planned in an outpatient pharmacy clinic, where Dr. Stewart initiated and titrated antihypertensive medications and handled refills.
“The physician does not have to physically be at the clinic,” she said. “We work closely over the phone to make the best decisions, but it’s not typical ambulatory care where the physician has to be sitting right next to the pharmacist to make the best decisions for the patients.” The primary outcome was the transitional care clinic’s impact on blood pressure.
Dr. Stewart reported results from 47 medication interventions that were provided over 97 follow-up visits. The researchers found that the median blood pressure dropped from an initial reading of 160/102 mm Hg to 130/93 mm Hg by the fifth transitional care clinic visit. Across all patients, systolic blood pressure decreased by an average of 48 mm Hg. “I don’t think we were surprised by these results; we are getting people very much in need down to their blood pressure goals,” she said. “By the end of the study, patients were on an average of three antihypertensive medications.”
She and her colleagues plan to conduct a larger randomized, clinical trial of this care model. The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.
REPORTING FROM ACEP18
Key clinical point: Hypertensive patients discharged from an urban ED benefited from a pharmacist-led clinic.
Major finding: By the last follow-up visit, patients’ mean systolic blood pressure had decreased by 48 mm Hg.
Study details: A pilot study of 89 patients with uncontrolled blood pressure.
Disclosures: The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.