User login
Studying in Dermatology Residency
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
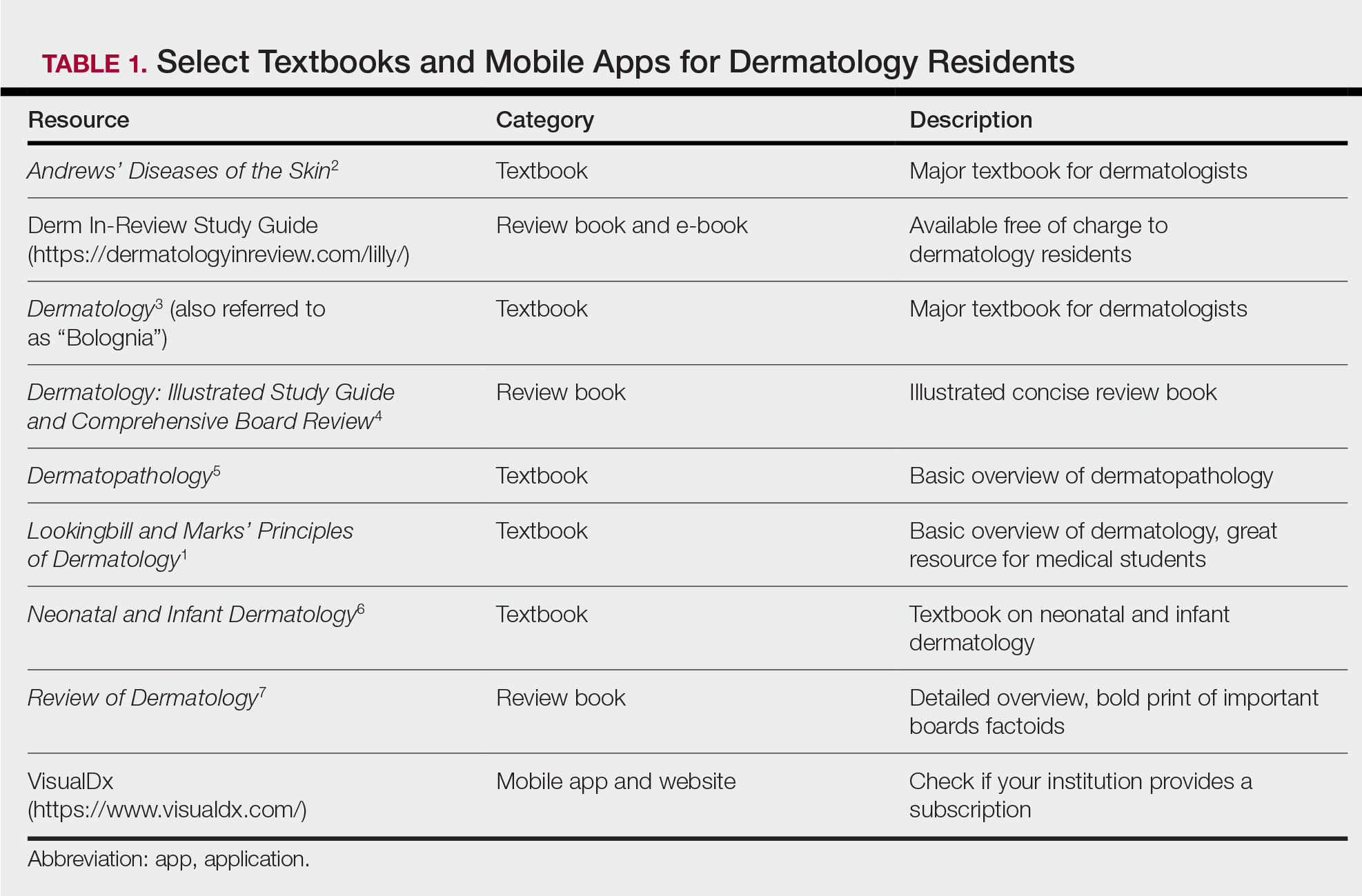
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.
First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
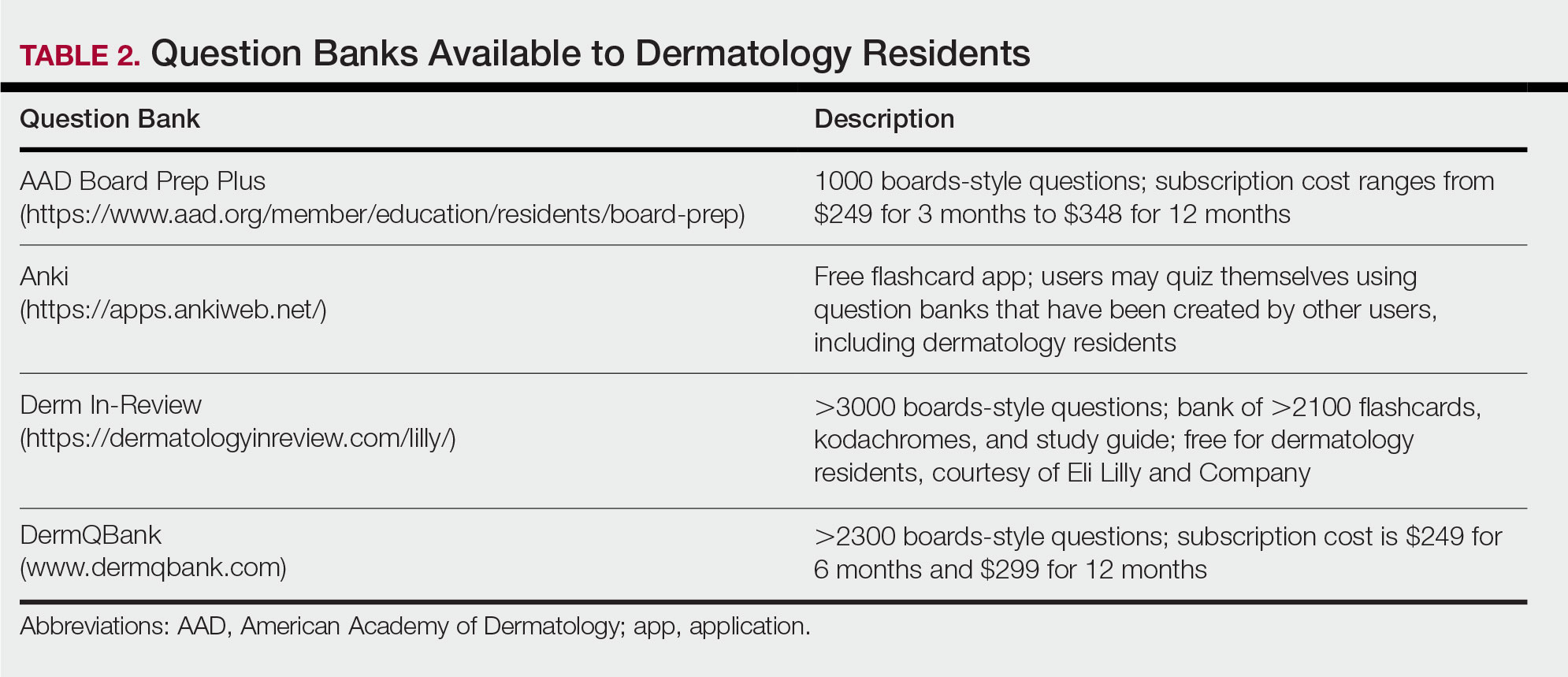
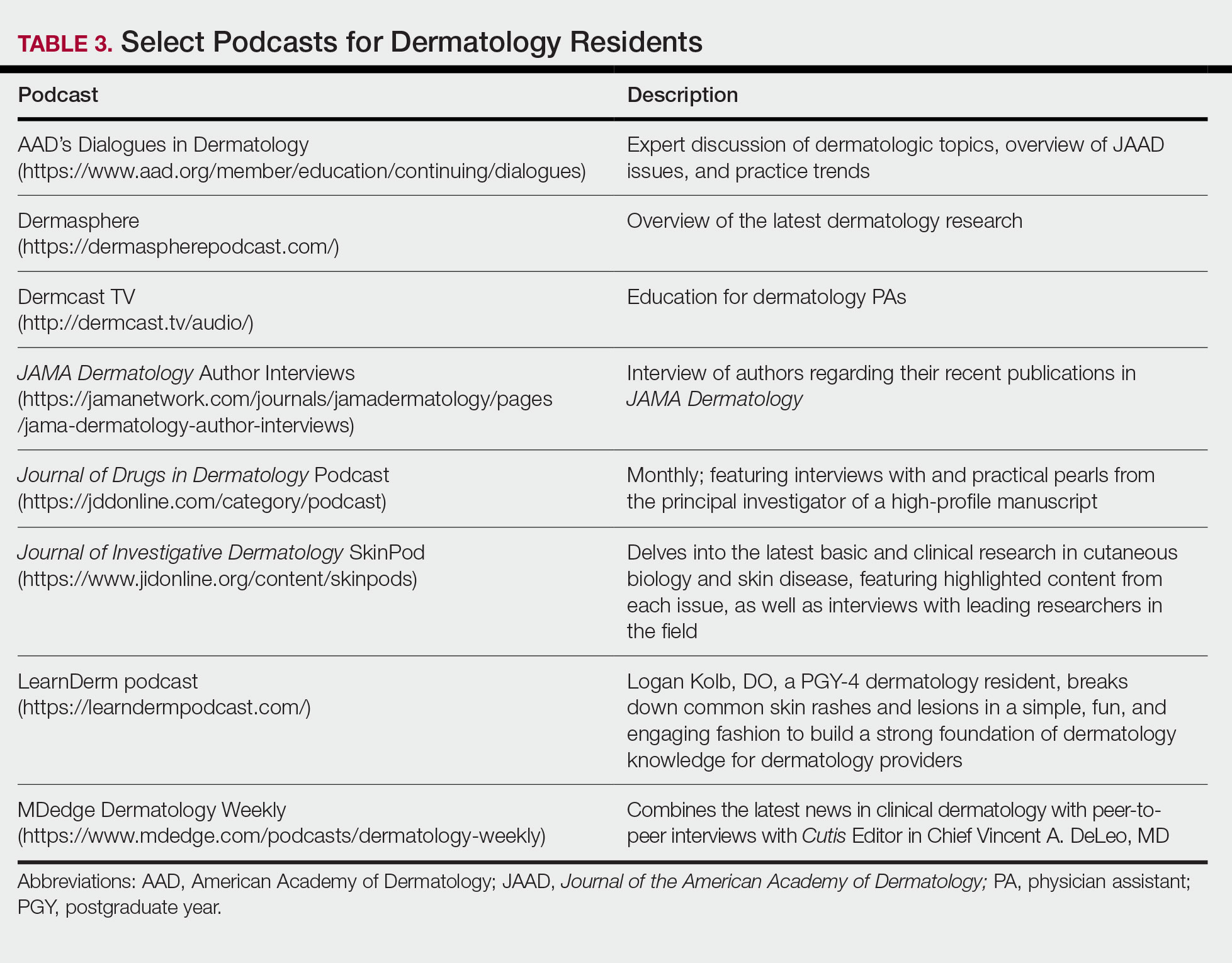
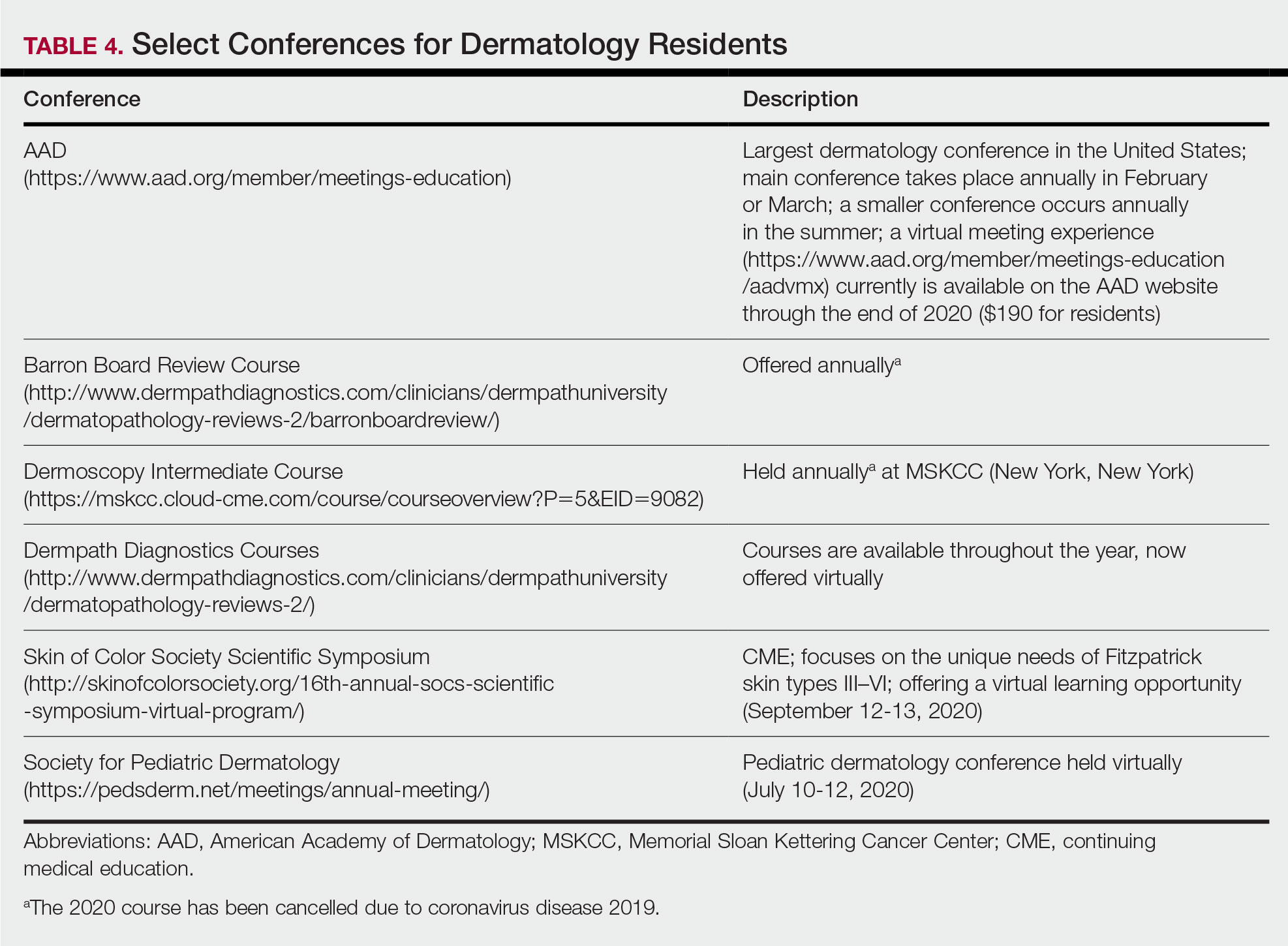
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.
Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
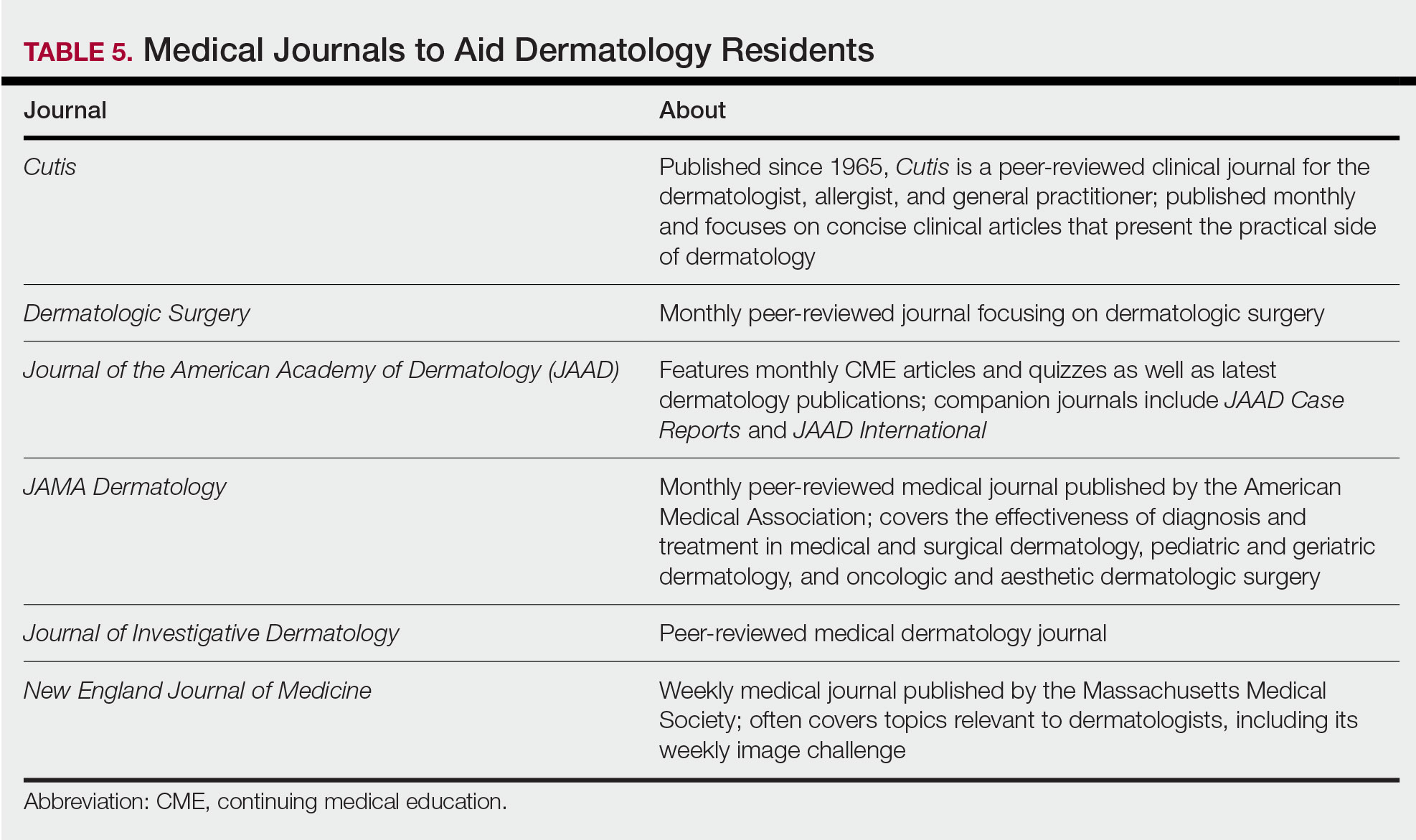
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.
Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.

First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.



Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.

Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
Dermatology residency can feel like drinking from a firehose, in which one is bombarded with so much information that it is impossible to retain any content. This article provides an overview of available resources and a guide on how to tailor studying throughout one’s training.
Prior to Residency
There are several resources that provide an introduction to dermatology and are appropriate for all medical students, regardless of intended specialty. The American Academy of Dermatology offers a free basic dermatology curriculum (https://www.aad.org/member/education/residents/bdc), with a choice of a 2- or 4-week course consisting of modules such as skin examination, basic science of the skin, dermatologic therapies, and specific dermatologic conditions. VisualDx offers LearnDerm (https://www.visualdx.com/learnderm/), which includes a 5-part tutorial and quiz focused on the skin examination, morphology, and lesion distribution. Lookingbill and Marks’ Principles of Dermatology1 is a book at an appropriate level for a medical student to learn about the fundamentals of dermatology. These resources may be helpful for residents to review immediately before starting dermatology residency (toward the end of intern year for most).
First Year
During the beginning of dermatology residency (postgraduate year [PGY] 2 for most), the fire hose of information feels most daunting. During this time, studying should focus on engendering a broad overview of dermatology. Most residencies maintain a textbook reading schedule, which provides a framework from which residents may structure their studying. Selection of a textbook tends to be program dependent. Even if the details of reading the textbook do not stick when reading it the first time, benefits include becoming familiar with what information one is expected to learn as a dermatologist and developing a strong foundation upon which one may continue to build. Based on my informal discussions with current residents, some reported that reading the textbook did not work well for them, citing too much minutiae in the textbooks and/or a preference for a more active learning approach. These residents instead focused on reading a review book for a broad overview, accompanied by a textbook or VisualDx when a more detailed reference is necessary. Table 1 provides a list of textbooks and mobile applications (apps) that residents may find helpful.

First-year residents may begin their efforts in synthesizing this new knowledge base toward the end of the year in preparation for the BASIC examination. The American Board of Dermatology provides a content outline as well as sample questions on their website (https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx#content), which may be used to guide more focused studying efforts during the weeks leading up to the examination.
Second Year
For second-year residents (PGY-3 for most) studying should focus on deepening and consolidating the broad foundation that was established during their first year. For many, this pursuit involves rereading the textbook chapters alongside more active learning measures, such as taking notes and quizzing oneself using flashcard apps and question banks (Table 2). Others may benefit from listening to podcasts (Table 3) or other sources utilizing audiovisual content, including attending conferences and other lectures virtually, which is becoming increasingly available in the setting of the coronavirus disease 2019 pandemic (Table 4). Because there are so many resources available to support these efforts, residents should be encouraged to try out a variety to determine what works best.



Toward the end of second year, studying may be tailored to preparing for the CORE examinations using the resources of one’s choice. Based on my discussions with current residents, a combination of reading review books, reviewing one’s personal notes, and quizzing through question banks and/or flashcard apps could be used.
In addition to maintaining a consistent and organized study schedule, second-year residents should continue to read in depth on topics related to patients for whom they are caring and stay on top of the dermatology literature. Table 5 provides a list of medical journals that dermatology residents should aim to read. The Journal of the American Academy of Dermatology’s continuing medical education series (https://www.jaad.org/content/collection-cme) may be particularly helpful to residents. In this series, experts review a variety of dermatologic topics in depth paired with quiz questions.

Third Year
As a third-year resident (PGY-4 for most), studying should focus on deepening one’s knowledge base and beginning preparation for the boards examination. At this point, residents should stick to a limited selection of resources (ie, 1 textbook, 1 review book, 1 question bank) for in-depth study. More time should be spent on active learning, such as note-taking and question banks. Boards review courses historically have been available to dermatology residents, namely the Barron Board Review course and a plenary session at the American Academy of Dermatology Annual Conference (Table 4).
Consistent Habits
Studying strategies can and should differ throughout dermatology residency, though consistency is necessary throughout. It is helpful to plan study schedules in advance—yearly, monthly, weekly—and aim to stick to them as much as possible. Finding what works for each individual may take trial and error. For some, it may mean waking up early to study before work, whereas others may do better in the evenings. It also is helpful to utilize a combination of longer blocks of studying (ie, weekend days), with consistent shorter blocks of time during the week. Many residents also learn to take advantage of time spent commuting by listening to podcasts in the car or reading while on public transportation.
Final Thoughts
There are many resources available to support residents in their learning such as textbooks, journals, podcasts, flashcards, question banks, and more. The path to mastery will be individualized for each resident, likely using a unique combination of resources. The beginning of residency is a good time to explore a variety of resources to see what works best, whereas at the end studying becomes more targeted.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
- Marks Jr JG, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019.
- James WD, Elston DM, Treat JR. Andrews’ Diseases of the Skin. 13th ed. China: Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018.
- Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York, NY: Springer; 2012.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. China: Elsevier Saunders; 2014.
- Eichenfield LF, Frieden IJ, eds. Neonatal and Infant Dermatology. London, England: Saunders; 2015.
- Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
Resident Pearls
- Independent study is a large component of dermatology residency.
- Consistent habits and a tailored approach will support optimal learning for each dermatology resident.
- The beginning of residency is a good time to explore a variety of resources to see what works best. Toward the end of residency, as studying becomes more targeted, residents may benefit from sticking to the resources with which they are most comfortable.
APPlying Knowledge: Evidence for and Regulation of Mobile Apps for Dermatologists
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Practice Points
- Physicians who are selecting an app for self-education or patient care should take into consideration the strength of the evidence supporting the app as well as the rigor of any approval process the app had to undergo.
- Only a minority of health-related apps are regulated by the government. This regulation has not kept up with the evolution of app software and may become more indirect.
Wellness for the Dermatology Resident
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident Pearls
- Resident wellness is an important issue affecting resident physicians of all specialties, including dermatology.
- To improve wellness, changes can be made by targeting the following 4 areas of well-being: professional, physical, psychological, and social.
Applications for the CUTIS 2021 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
PD-1 Signaling in Extramammary Paget Disease
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Resident Pearls
- Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts, while secondary EMPD is an extension of malignant cells from an underlying internal neoplasm.
- Surgical margin clearance in EMPD often is problematic, with high recurrence rates indicating the need for additional treatment modalities.
- Programmed cell death receptor 1 (PD-1) signaling can serve as a potential target in EMPD. Further studies and clinical trials are needed to test the efficacy of PD-1 inhibitors in unresectable or invasive/metastatic EMPD.
Multiethnic Training in Residency: A Survey of Dermatology Residents
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Practice Points
- To treat the ever-changing demographics of patients in the United States, dermatologists must receive adequate exposure and education regarding dermatologic conditions in patients from various ethnic backgrounds.
- Dermatology residents from less diverse regions are more likely to agree that dedicated clinics and rotations are important to gain competence compared to those from more diverse regions.
- In areas with less diversity, dedicated multiethnic skin clinics and faculty may be more important for assuring an adequate residency experience.
Compounding Topicals in Dermatology
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
Resident Pearls
- Compounding topical medications provides dermatologists with the ability to create custom formulations that cater to the individual needs of each patient.
- Dermatologists must keep in mind that data are limited regarding both safety and efficacy of compounded medications.
Over-the-counter Topical Products in Dermatology
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
Resident Pearls
- Several branches of the US Food and Drug Administration are responsible for regulation of overthe-counter (OTC) topical products with both direct and indirect oversight.
- There are several excellent resources available to dermatologists in training who are interested in learning about pharmacology and tolerability of OTC products.
- Firsthand experience in personally sampling a variety of products also helps clinicians provide practical recommendations to patients.
More on How to Decrease Dermatology Interview Costs
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP (aamir.nav.hussain@gmail.com).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP (aamir.nav.hussain@gmail.com).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP (aamir.nav.hussain@gmail.com).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
The DNA Mismatch Repair System in Sebaceous Tumors: An Update on the Genetics and Workup of Muir-Torre Syndrome
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
Resident Pearls
- When patients present with a solitary sebaceous tumor, there is a high likelihood they have Muir-Torre syndrome (MTS) and thus are at a high risk to develop visceral malignancies.
- It is important to perform further testing using immunohistochemistry for DNA mismatch repair proteins and microsatellite instability gene analysis in some cases to confirm the diagnosis of MTS and to perform the appropriate cancer screening tests.




