User login
Diet and Skin: A Primer
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
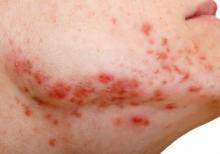
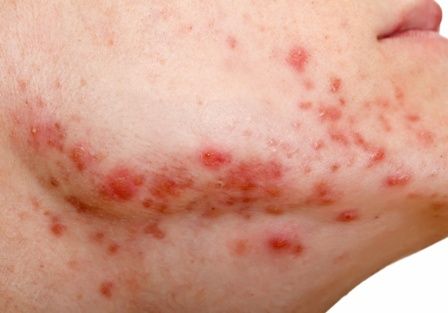
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
Resident Pearls
- There are strong data on the relationship between dietary patterns and skin conditions.
- High glycemic index foods (eg, skim milk, whey protein, sugary beverages, fatty foods) are associated with acne vulgaris.
- Obesity is a risk factor for psoriasis; weight loss interventions such as improved dietary patterns can improve psoriasis.
- Children with atopic dermatitis (AD) are at higher risk for food allergies (both IgE and non–IgE-mediated allergies). A small subset may experience flares in their AD in relation to non–IgE-mediated food allergies.
Racial Disparities in Dermatology Training: The Impact on Black Patients
Although physicians commit themselves to providing equitable treatment to all patients, significant disparities remain in the dermatologic care of Black patients, who constitute 13% of the US population, which continues to grow increasingly diverse.1 Despite these changes in the population, the literature demonstrates that dermatologic training does not adequately focus on unique presentations of cutaneous pathology in the Black population.2,3 Accordingly, medical students lack proper training in how skin disorders manifest in people of color. Compounding the problem, only 3% of dermatologists are Black, creating a cultural barrier that can compromise care for Black patients.2,4 Racial disparities in dermatology training can compromise treatment, patient satisfaction, and outcomes.3
Issues in Medical Education Training and Resources
Lack of diversity in the resources used for dermatology training in medical schools affects diagnosis and treatment, as skin manifestations such as hypersensitivity reactions, rashes, and cancer can appear differently on different skin tones.5 A study of medical students’ ability to diagnose common dermatologic pathologies found that when trainees were presented with photographs of dark skin, their accuracy in identifying urticaria, squamous cell carcinoma, and even atopic dermatitis was reduced, despite these diseases being more prevalent in children of African American ancestry.4,6
Dermatologic diseases also can have different distributions in different races; for example, on non–sun-exposed sites, squamous cell carcinoma in Black patients occurs at 8.5 times the frequency of White patients.7 Failure to identify diseases accurately due to insufficient training can have grave consequences for patients. Although skin cancer is less common in individuals with skin of color, it is associated with greater morbidity and mortality, in part due to delayed diagnosis.7
Inadequate research, reporting, and instruction on dermatologic findings in patients with darker complexions further compound racial disparities in dermatology. A 2006 study of the representation of darker skin in major dermatology educational resources found that only 2% of teaching events at American Academy of Dermatology annual meetings focused on skin of color. Furthermore, the study determined that many common diseases in patients with dark skin, such as acne vulgaris and pityriasis rosea, were completely absent or limited in dermatology textbooks.8
Impact on the Black Patient Experience
Patients’ therapeutic relationship with their physician also is damaged by limitations in training in diverse skin color. A study that assessed Black patients seen in a skin of color clinic (SOCC) compared to Black patients seen in a non-SOCC found that non-SOCC patients reported a lower degree of respect, dignity, understanding, and trust compared to the patients seen in a SOCC. Black patients expressed specific concerns about non-SOCC dermatologists’ knowledge of abnormalities that present in darker skin and Black hair.3 These findings are compounded by reports suggesting that, independent of care, structural racism contributes to dermatologic disease severity by influencing patient education level, household income, and degree of exposure to harmful environmental irritants.6
Racial disparities continue to be seen in the makeup of the universe of dermatologists and skin researchers. As of 2016, only 3% of dermatologists were Black, making dermatology one of the least diverse medical specialties.2 Increasing the diversity of the dermatology workforce is important to improve patient satisfaction and treatment, both for minority and nonminority patients. Compared to race-discordant medical visits, race-concordant visits were shown to have a higher rate of satisfaction and better shared decision-making.9 Also, minority physicians are more likely to practice health care in areas that are traditionally underserved and to care for patients who do not have health insurance, making their participation essential in addressing some of the baseline disparities Black patients face in securing quality dermatologic care.1
Structural Racism in Medicine
Changing dermatology training to ensure improved treatment of Black patients requires not only increased attention to differences in disease presentation but also heightened awareness of underlying genetic, environmental, and structural factors that contribute to the disease course.6 For example, there is evidence suggesting that structural racism in the form of residential segregation, lower socioeconomic status, and lower educational attainment contribute to disease severity in conditions such as atopic dermatitis. There is additional evidence suggesting that White patients are more readily offered therapeutic options than Black patients. A study of racial disparities in psoriasis treatment found that Black patients with moderate to severe psoriasis were 70% less likely to receive treatment with a biologic than White patients, independent of socioeconomic factors, comorbidities, and insurance plans.10
Moving Forward
Although research continues to underscore racial disparities in dermatology, some leaders in the field are actively combating these problems. A recent study that looked at representations of dark skin images in medical educational resources found far greater representation of dark pigmented skin in web-based resources than in traditional printed texts. Specifically, the online resource VisualDx (https://www.visualdx.com/) features 28.5% dark skin images compared to 10.3% (on average) in printed dermatology books.11 There also is increasing public awareness of these issues, with organizations such as the Skin of Color Society (http://skinofcolorsociety.org/) helping to promote interest in racial disparities in dermatology. Physicians also have created textbooks and social media accounts focused on dermatologic manifestations in skin of color.12 The Instagram account Brown Skin Matters (@brownskinmatters) has created a publicly accessible online resource where physicians and patients can see and post dermatologic diseases in skin of color.5
Final Thoughts
It is critical that physicians be trained to identify skin and hair manifestations of disease and disorders in Black patients. Training can be improved by including more images of skin manifestations in dark skin, both in medical school curricula and in new editions of dermatology textbooks. Training also must teach students about hair in Black individuals and how to properly treat it as well as related conditions of the hair and scalp.13 More research also is needed to better understand how dermatologists can improve the patient experience for Black patients. Residency programs must work to increase diversity among dermatology trainees.
Lastly, dermatology education should increasingly be supplemented with newer, web-based resources that show dermatologic manifestations across the spectrum of skin tones. Dermatology training must be adapted to better account for diverse patient populations and increase its focus on the systems that produce baseline disparities in disease morbidity and mortality.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Gallegos A. Dermatology lacks diversity. Dermatology News. June 1, 2016. Accessed November 18, 2020. https://www.mdedge.com/dermatology/article/108920/practice-management/dermatology-lacks-diversity.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958.
- Prichep D. Diagnostic gaps: skin comes in many shades and so do rashes. NPR website. November 14, 2019. Accessed November 19, 2020. https://www.npr.org/sections/health-shots/2019/11/04/774910915/diagnostic-gaps-skin-comes-in-many-shades-and-so-do-rashes.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Rabin RC. Dermatology has a problem with skin color. The New York Times. August 30, 2020. http://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed November 19, 2020.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80.
Although physicians commit themselves to providing equitable treatment to all patients, significant disparities remain in the dermatologic care of Black patients, who constitute 13% of the US population, which continues to grow increasingly diverse.1 Despite these changes in the population, the literature demonstrates that dermatologic training does not adequately focus on unique presentations of cutaneous pathology in the Black population.2,3 Accordingly, medical students lack proper training in how skin disorders manifest in people of color. Compounding the problem, only 3% of dermatologists are Black, creating a cultural barrier that can compromise care for Black patients.2,4 Racial disparities in dermatology training can compromise treatment, patient satisfaction, and outcomes.3
Issues in Medical Education Training and Resources
Lack of diversity in the resources used for dermatology training in medical schools affects diagnosis and treatment, as skin manifestations such as hypersensitivity reactions, rashes, and cancer can appear differently on different skin tones.5 A study of medical students’ ability to diagnose common dermatologic pathologies found that when trainees were presented with photographs of dark skin, their accuracy in identifying urticaria, squamous cell carcinoma, and even atopic dermatitis was reduced, despite these diseases being more prevalent in children of African American ancestry.4,6
Dermatologic diseases also can have different distributions in different races; for example, on non–sun-exposed sites, squamous cell carcinoma in Black patients occurs at 8.5 times the frequency of White patients.7 Failure to identify diseases accurately due to insufficient training can have grave consequences for patients. Although skin cancer is less common in individuals with skin of color, it is associated with greater morbidity and mortality, in part due to delayed diagnosis.7
Inadequate research, reporting, and instruction on dermatologic findings in patients with darker complexions further compound racial disparities in dermatology. A 2006 study of the representation of darker skin in major dermatology educational resources found that only 2% of teaching events at American Academy of Dermatology annual meetings focused on skin of color. Furthermore, the study determined that many common diseases in patients with dark skin, such as acne vulgaris and pityriasis rosea, were completely absent or limited in dermatology textbooks.8
Impact on the Black Patient Experience
Patients’ therapeutic relationship with their physician also is damaged by limitations in training in diverse skin color. A study that assessed Black patients seen in a skin of color clinic (SOCC) compared to Black patients seen in a non-SOCC found that non-SOCC patients reported a lower degree of respect, dignity, understanding, and trust compared to the patients seen in a SOCC. Black patients expressed specific concerns about non-SOCC dermatologists’ knowledge of abnormalities that present in darker skin and Black hair.3 These findings are compounded by reports suggesting that, independent of care, structural racism contributes to dermatologic disease severity by influencing patient education level, household income, and degree of exposure to harmful environmental irritants.6
Racial disparities continue to be seen in the makeup of the universe of dermatologists and skin researchers. As of 2016, only 3% of dermatologists were Black, making dermatology one of the least diverse medical specialties.2 Increasing the diversity of the dermatology workforce is important to improve patient satisfaction and treatment, both for minority and nonminority patients. Compared to race-discordant medical visits, race-concordant visits were shown to have a higher rate of satisfaction and better shared decision-making.9 Also, minority physicians are more likely to practice health care in areas that are traditionally underserved and to care for patients who do not have health insurance, making their participation essential in addressing some of the baseline disparities Black patients face in securing quality dermatologic care.1
Structural Racism in Medicine
Changing dermatology training to ensure improved treatment of Black patients requires not only increased attention to differences in disease presentation but also heightened awareness of underlying genetic, environmental, and structural factors that contribute to the disease course.6 For example, there is evidence suggesting that structural racism in the form of residential segregation, lower socioeconomic status, and lower educational attainment contribute to disease severity in conditions such as atopic dermatitis. There is additional evidence suggesting that White patients are more readily offered therapeutic options than Black patients. A study of racial disparities in psoriasis treatment found that Black patients with moderate to severe psoriasis were 70% less likely to receive treatment with a biologic than White patients, independent of socioeconomic factors, comorbidities, and insurance plans.10
Moving Forward
Although research continues to underscore racial disparities in dermatology, some leaders in the field are actively combating these problems. A recent study that looked at representations of dark skin images in medical educational resources found far greater representation of dark pigmented skin in web-based resources than in traditional printed texts. Specifically, the online resource VisualDx (https://www.visualdx.com/) features 28.5% dark skin images compared to 10.3% (on average) in printed dermatology books.11 There also is increasing public awareness of these issues, with organizations such as the Skin of Color Society (http://skinofcolorsociety.org/) helping to promote interest in racial disparities in dermatology. Physicians also have created textbooks and social media accounts focused on dermatologic manifestations in skin of color.12 The Instagram account Brown Skin Matters (@brownskinmatters) has created a publicly accessible online resource where physicians and patients can see and post dermatologic diseases in skin of color.5
Final Thoughts
It is critical that physicians be trained to identify skin and hair manifestations of disease and disorders in Black patients. Training can be improved by including more images of skin manifestations in dark skin, both in medical school curricula and in new editions of dermatology textbooks. Training also must teach students about hair in Black individuals and how to properly treat it as well as related conditions of the hair and scalp.13 More research also is needed to better understand how dermatologists can improve the patient experience for Black patients. Residency programs must work to increase diversity among dermatology trainees.
Lastly, dermatology education should increasingly be supplemented with newer, web-based resources that show dermatologic manifestations across the spectrum of skin tones. Dermatology training must be adapted to better account for diverse patient populations and increase its focus on the systems that produce baseline disparities in disease morbidity and mortality.
Although physicians commit themselves to providing equitable treatment to all patients, significant disparities remain in the dermatologic care of Black patients, who constitute 13% of the US population, which continues to grow increasingly diverse.1 Despite these changes in the population, the literature demonstrates that dermatologic training does not adequately focus on unique presentations of cutaneous pathology in the Black population.2,3 Accordingly, medical students lack proper training in how skin disorders manifest in people of color. Compounding the problem, only 3% of dermatologists are Black, creating a cultural barrier that can compromise care for Black patients.2,4 Racial disparities in dermatology training can compromise treatment, patient satisfaction, and outcomes.3
Issues in Medical Education Training and Resources
Lack of diversity in the resources used for dermatology training in medical schools affects diagnosis and treatment, as skin manifestations such as hypersensitivity reactions, rashes, and cancer can appear differently on different skin tones.5 A study of medical students’ ability to diagnose common dermatologic pathologies found that when trainees were presented with photographs of dark skin, their accuracy in identifying urticaria, squamous cell carcinoma, and even atopic dermatitis was reduced, despite these diseases being more prevalent in children of African American ancestry.4,6
Dermatologic diseases also can have different distributions in different races; for example, on non–sun-exposed sites, squamous cell carcinoma in Black patients occurs at 8.5 times the frequency of White patients.7 Failure to identify diseases accurately due to insufficient training can have grave consequences for patients. Although skin cancer is less common in individuals with skin of color, it is associated with greater morbidity and mortality, in part due to delayed diagnosis.7
Inadequate research, reporting, and instruction on dermatologic findings in patients with darker complexions further compound racial disparities in dermatology. A 2006 study of the representation of darker skin in major dermatology educational resources found that only 2% of teaching events at American Academy of Dermatology annual meetings focused on skin of color. Furthermore, the study determined that many common diseases in patients with dark skin, such as acne vulgaris and pityriasis rosea, were completely absent or limited in dermatology textbooks.8
Impact on the Black Patient Experience
Patients’ therapeutic relationship with their physician also is damaged by limitations in training in diverse skin color. A study that assessed Black patients seen in a skin of color clinic (SOCC) compared to Black patients seen in a non-SOCC found that non-SOCC patients reported a lower degree of respect, dignity, understanding, and trust compared to the patients seen in a SOCC. Black patients expressed specific concerns about non-SOCC dermatologists’ knowledge of abnormalities that present in darker skin and Black hair.3 These findings are compounded by reports suggesting that, independent of care, structural racism contributes to dermatologic disease severity by influencing patient education level, household income, and degree of exposure to harmful environmental irritants.6
Racial disparities continue to be seen in the makeup of the universe of dermatologists and skin researchers. As of 2016, only 3% of dermatologists were Black, making dermatology one of the least diverse medical specialties.2 Increasing the diversity of the dermatology workforce is important to improve patient satisfaction and treatment, both for minority and nonminority patients. Compared to race-discordant medical visits, race-concordant visits were shown to have a higher rate of satisfaction and better shared decision-making.9 Also, minority physicians are more likely to practice health care in areas that are traditionally underserved and to care for patients who do not have health insurance, making their participation essential in addressing some of the baseline disparities Black patients face in securing quality dermatologic care.1
Structural Racism in Medicine
Changing dermatology training to ensure improved treatment of Black patients requires not only increased attention to differences in disease presentation but also heightened awareness of underlying genetic, environmental, and structural factors that contribute to the disease course.6 For example, there is evidence suggesting that structural racism in the form of residential segregation, lower socioeconomic status, and lower educational attainment contribute to disease severity in conditions such as atopic dermatitis. There is additional evidence suggesting that White patients are more readily offered therapeutic options than Black patients. A study of racial disparities in psoriasis treatment found that Black patients with moderate to severe psoriasis were 70% less likely to receive treatment with a biologic than White patients, independent of socioeconomic factors, comorbidities, and insurance plans.10
Moving Forward
Although research continues to underscore racial disparities in dermatology, some leaders in the field are actively combating these problems. A recent study that looked at representations of dark skin images in medical educational resources found far greater representation of dark pigmented skin in web-based resources than in traditional printed texts. Specifically, the online resource VisualDx (https://www.visualdx.com/) features 28.5% dark skin images compared to 10.3% (on average) in printed dermatology books.11 There also is increasing public awareness of these issues, with organizations such as the Skin of Color Society (http://skinofcolorsociety.org/) helping to promote interest in racial disparities in dermatology. Physicians also have created textbooks and social media accounts focused on dermatologic manifestations in skin of color.12 The Instagram account Brown Skin Matters (@brownskinmatters) has created a publicly accessible online resource where physicians and patients can see and post dermatologic diseases in skin of color.5
Final Thoughts
It is critical that physicians be trained to identify skin and hair manifestations of disease and disorders in Black patients. Training can be improved by including more images of skin manifestations in dark skin, both in medical school curricula and in new editions of dermatology textbooks. Training also must teach students about hair in Black individuals and how to properly treat it as well as related conditions of the hair and scalp.13 More research also is needed to better understand how dermatologists can improve the patient experience for Black patients. Residency programs must work to increase diversity among dermatology trainees.
Lastly, dermatology education should increasingly be supplemented with newer, web-based resources that show dermatologic manifestations across the spectrum of skin tones. Dermatology training must be adapted to better account for diverse patient populations and increase its focus on the systems that produce baseline disparities in disease morbidity and mortality.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Gallegos A. Dermatology lacks diversity. Dermatology News. June 1, 2016. Accessed November 18, 2020. https://www.mdedge.com/dermatology/article/108920/practice-management/dermatology-lacks-diversity.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958.
- Prichep D. Diagnostic gaps: skin comes in many shades and so do rashes. NPR website. November 14, 2019. Accessed November 19, 2020. https://www.npr.org/sections/health-shots/2019/11/04/774910915/diagnostic-gaps-skin-comes-in-many-shades-and-so-do-rashes.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Rabin RC. Dermatology has a problem with skin color. The New York Times. August 30, 2020. http://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed November 19, 2020.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Gallegos A. Dermatology lacks diversity. Dermatology News. June 1, 2016. Accessed November 18, 2020. https://www.mdedge.com/dermatology/article/108920/practice-management/dermatology-lacks-diversity.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958.
- Prichep D. Diagnostic gaps: skin comes in many shades and so do rashes. NPR website. November 14, 2019. Accessed November 19, 2020. https://www.npr.org/sections/health-shots/2019/11/04/774910915/diagnostic-gaps-skin-comes-in-many-shades-and-so-do-rashes.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Rabin RC. Dermatology has a problem with skin color. The New York Times. August 30, 2020. http://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed November 19, 2020.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80.
Practice Points
- Dermatologists should be aware of the existing health disparities in dermatology training, including lack of representation among dermatologists, treatment, patient satisfaction, and outcomes.
- Dermatologic diseases can present differently in different skin tones, and current dermatology training does not reflect these differences.
- We must continue to work toward increasing diversity of the dermatology workforce, including a diverse range of skin tones in images used in dermatology training, and teaching trainees how diseases present differently in different skin tones.
Novel topical acne combo hits marks in phase 3 trials
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
bjancin@mdedge.com
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
bjancin@mdedge.com
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
bjancin@mdedge.com
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Embrace new and classic acne treatments
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Dermatologists and the history of skin care and beauty devices: Part 4
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
Who’s at risk for depression on isotretinoin?
A Sanaa Butt, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
This was, however, the sole identifiable risk factor for treatment-limiting depressive symptoms in acne patients on isotretinoin in the study of 3,151 consecutive acne patients taking isotretinoin. There was no significant difference between those who did or did not develop depression on the oral retinoid in terms of age, gender, or daily dose of the drug at the time it was discontinued.
“Depressive symptoms occurred at any time from the date of initiation of isotretinoin up to 6 months into therapy, with no identifiable peak time period,” said Dr. Butt, a dermatologist with the U.K. National Health Service Tayside district at Ninewells Hospital, Dundee, Scotland. “Lower doses appear not to be protective,” she added.
The Tayside district has a catchment of roughly 450,000 people. The local population tends to stay put because Tayside is an economically disadvantaged and remote part of Scotland. There are very few private practice dermatologists in the area, so Dr. Butt and coinvestigators are confident their observational study of NHS patients captured the great majority of isotretinoin users in northern Scotland.
The investigators utilized software to analyze the contents of more than 8,000 digitized letters exchanged between NHS Tayside dermatologists and general practitioners during 2005-2018, zeroing in on 3,151 consecutive patients on isotretinoin for acne and 158 on the drug for other conditions, most often rosacea or folliculitis. They then drilled down further through the letters, electronically searching for key words such as suicide, depression, and anxiety. In this way, they ultimately identified 30 patients who discontinued the drug because they developed depressive symptoms. All 30 were on the drug for acne.
The annual incidence of treatment-limiting depressive mood changes was 0.96%, a figure that remained steady over the 13-year study period, even though prescribing of isotretinoin increased over time. This flat incidence rate effectively rules out the potential for confounding because of assessor bias, especially since many different NHS dermatologists were prescribing the drug, Dr. Butt said.
Half of acne patients prescribed isotretinoin were female and 50% were male. And 15 cases of treatment discontinuation caused by development of depressive symptoms occurred in females, 15 in males. A history of past depressive illness was present in 9.3% of females who started on isotretinoin and in 4.5% of the males. The relative risk of treatment-limiting depressive mood changes was increased 790% among females with a prior history of depressive illness and 440% in males with such a history.
Dr. Butt reported having no financial conflicts regarding her NHS-funded study.
A Sanaa Butt, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
This was, however, the sole identifiable risk factor for treatment-limiting depressive symptoms in acne patients on isotretinoin in the study of 3,151 consecutive acne patients taking isotretinoin. There was no significant difference between those who did or did not develop depression on the oral retinoid in terms of age, gender, or daily dose of the drug at the time it was discontinued.
“Depressive symptoms occurred at any time from the date of initiation of isotretinoin up to 6 months into therapy, with no identifiable peak time period,” said Dr. Butt, a dermatologist with the U.K. National Health Service Tayside district at Ninewells Hospital, Dundee, Scotland. “Lower doses appear not to be protective,” she added.
The Tayside district has a catchment of roughly 450,000 people. The local population tends to stay put because Tayside is an economically disadvantaged and remote part of Scotland. There are very few private practice dermatologists in the area, so Dr. Butt and coinvestigators are confident their observational study of NHS patients captured the great majority of isotretinoin users in northern Scotland.
The investigators utilized software to analyze the contents of more than 8,000 digitized letters exchanged between NHS Tayside dermatologists and general practitioners during 2005-2018, zeroing in on 3,151 consecutive patients on isotretinoin for acne and 158 on the drug for other conditions, most often rosacea or folliculitis. They then drilled down further through the letters, electronically searching for key words such as suicide, depression, and anxiety. In this way, they ultimately identified 30 patients who discontinued the drug because they developed depressive symptoms. All 30 were on the drug for acne.
The annual incidence of treatment-limiting depressive mood changes was 0.96%, a figure that remained steady over the 13-year study period, even though prescribing of isotretinoin increased over time. This flat incidence rate effectively rules out the potential for confounding because of assessor bias, especially since many different NHS dermatologists were prescribing the drug, Dr. Butt said.
Half of acne patients prescribed isotretinoin were female and 50% were male. And 15 cases of treatment discontinuation caused by development of depressive symptoms occurred in females, 15 in males. A history of past depressive illness was present in 9.3% of females who started on isotretinoin and in 4.5% of the males. The relative risk of treatment-limiting depressive mood changes was increased 790% among females with a prior history of depressive illness and 440% in males with such a history.
Dr. Butt reported having no financial conflicts regarding her NHS-funded study.
A Sanaa Butt, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
This was, however, the sole identifiable risk factor for treatment-limiting depressive symptoms in acne patients on isotretinoin in the study of 3,151 consecutive acne patients taking isotretinoin. There was no significant difference between those who did or did not develop depression on the oral retinoid in terms of age, gender, or daily dose of the drug at the time it was discontinued.
“Depressive symptoms occurred at any time from the date of initiation of isotretinoin up to 6 months into therapy, with no identifiable peak time period,” said Dr. Butt, a dermatologist with the U.K. National Health Service Tayside district at Ninewells Hospital, Dundee, Scotland. “Lower doses appear not to be protective,” she added.
The Tayside district has a catchment of roughly 450,000 people. The local population tends to stay put because Tayside is an economically disadvantaged and remote part of Scotland. There are very few private practice dermatologists in the area, so Dr. Butt and coinvestigators are confident their observational study of NHS patients captured the great majority of isotretinoin users in northern Scotland.
The investigators utilized software to analyze the contents of more than 8,000 digitized letters exchanged between NHS Tayside dermatologists and general practitioners during 2005-2018, zeroing in on 3,151 consecutive patients on isotretinoin for acne and 158 on the drug for other conditions, most often rosacea or folliculitis. They then drilled down further through the letters, electronically searching for key words such as suicide, depression, and anxiety. In this way, they ultimately identified 30 patients who discontinued the drug because they developed depressive symptoms. All 30 were on the drug for acne.
The annual incidence of treatment-limiting depressive mood changes was 0.96%, a figure that remained steady over the 13-year study period, even though prescribing of isotretinoin increased over time. This flat incidence rate effectively rules out the potential for confounding because of assessor bias, especially since many different NHS dermatologists were prescribing the drug, Dr. Butt said.
Half of acne patients prescribed isotretinoin were female and 50% were male. And 15 cases of treatment discontinuation caused by development of depressive symptoms occurred in females, 15 in males. A history of past depressive illness was present in 9.3% of females who started on isotretinoin and in 4.5% of the males. The relative risk of treatment-limiting depressive mood changes was increased 790% among females with a prior history of depressive illness and 440% in males with such a history.
Dr. Butt reported having no financial conflicts regarding her NHS-funded study.
FROM THE EADV CONGRESS
For acne in darker skin, judicious use of peeling agents can speed resolution
according to an expert, who cited both published data and empirical experience at the virtual Skin of Color Update 2020.
Because of the risk of exacerbating hyperpigmentation, superficial peels must be used judiciously, but “peels do add some benefit in terms of resolving the hyperpigmentation more rapidly,” Andrew Alexis, MD, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said at the meeting.
Addressing hyperpigmentation in skin of color is a critical goal. For many patients, the postinflammatory hyperpigmentation (PIH) that accompanies acne in Fitzpatrick skin types IV or higher imposes a greater burden than the acne itself.
“PIH is one of the driving forces among patients with darker skin coming to a dermatologist,” said Dr. Alexis, who is also professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York. “Patients often describe these hyperpigmented macules as scars, and they are concerned that they are not reversible.”
In darker skin, the combination of treatments used for acne should address the pathogenic factors that contribute to acne and PIH at the same time, according to Dr. Alexis. He advised describing the goals and the timeline of acne and PIH resolution at the very first visit.
Of these two goals, resolution of PIH is often the more challenging. First-line topical retinoids have anti-inflammatory effects, but Dr. Alexis suggested that additional agents, such as topical antibiotics, topical dapsone, and benzoyl peroxide, are commonly needed to fully control inflammation.
“Topical retinoids serve as the foundation of acne treatment, especially in skin of color due to their dual action on acne and PIH,” he said. However, he added that this needs support with a “well-rounded combination therapy to address as many pathogenic factors as possible.”
One of these factors is subclinical inflammation. Citing studies first initiated at Howard University, Washington, Dr. Alexis said there are now compelling data showing T lymphocyte infiltration and increased expression of proinflammatory cytokines even in clinically uninvolved skin in acne patients with darker skin.
In patients with significant PIH, he considers oral antibiotics for their systemic anti-inflammatory effects, singling out sarecycline as a narrow-spectrum agent with a potent effect on Cutibacterium acnes. This tetracycline, a relatively recent addition to acne treatment options, has specifically been shown to be “superior to placebo across a diverse patient population” that includes those with darker skin tones.
“Another addition that can be leveraged for anti-inflammatory effects is topical minocycline foam. This has also been studied in diverse patient populations and shown to be superior to vehicle,” Dr. Alexis said.
For acne, the response to most of these therapies is relatively rapid, but control of PIH takes longer. After resolution of acne, he considers superficial chemical peels to speed the healing of PIH.
In a small randomized trial he cited, superficial glycolic acid peel added to a modified Kligman formula (hydroquinone 2%, tretinoin 0.05%, and hydrocortisone 1%) provided significantly lower scores in the mean Hyperpigmentation Area and Severity Index at 12 weeks (P = .004) and 21 weeks (P < .001 relative to the Kligman formula alone). Dr. Alexis said he has had the same clinical experience with chemical peels
For many acne patients with darker skin, good results are achieved after four weeks on a multidrug combination with a topical retinoid backbone. One week after stopping the combination, the superficial chemical peel can be started at a very low dose on an every-other-night schedule. If tolerated, the dose can be slowly increased.
Slow up-titration of all topical agents in skin of color, not just superficial chemical peels, is prudent, according to Dr. Alexis. For patients new to retinoids, he also recommended every-other-night dosing to avoid the irritation that might exacerbate PIH. He said the risks of adverse reactions come early. “We need to hold the hands of our patients through the first 2 weeks. Warn of dryness and pealing. Recommend moisturizers and keep the doses low.”
The benefits and risks of acne treatment are different in dark relative to light skin, Dr. Alexis emphasized. He added that a measured approach that includes specific strategies for PIH delivers results.
Providing treatment with a strategy that addresses both acne and PIH, he said, “we can have excellent outcomes time and time again for acne in patients with darker skin types.”
There is an evidence basis for making effective treatment of PIH a specific goal in the treatment of acne. In a study that evaluated the psychosocial impact of PIH in 50 patients with acne, 54% responded that PIH was a source of embarrassment. The study was one of the first to evaluate the impact of PIH as a separate source of impaired quality of life in acne patients.
“To improve the patient’s quality of life, the dermatologist should treat acne and postinflammatory hyperpigmentation at the same time,” said Katlein Franca, MD, PhD, assistant professor of dermatology, University of Miami.
In particular, Dr. Franca, who led the PIH study, suggested that PIH, like acne, is a source of low self-esteem. In regard to PIH, “most patients feel embarrassed about the spots,” she said in an interview.
“Strategies to hide the hyperpigmented spots include the use of makeup and even different hairstyles to cover the affected areas,” she added, indicating that treatments provided to clear PIH as well as acne can remove a source of stress and threat to a sense of well-being.
Dr. Alexis reports financial relationships with many pharmaceutical companies, including those that make acne drugs.
according to an expert, who cited both published data and empirical experience at the virtual Skin of Color Update 2020.
Because of the risk of exacerbating hyperpigmentation, superficial peels must be used judiciously, but “peels do add some benefit in terms of resolving the hyperpigmentation more rapidly,” Andrew Alexis, MD, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said at the meeting.
Addressing hyperpigmentation in skin of color is a critical goal. For many patients, the postinflammatory hyperpigmentation (PIH) that accompanies acne in Fitzpatrick skin types IV or higher imposes a greater burden than the acne itself.
“PIH is one of the driving forces among patients with darker skin coming to a dermatologist,” said Dr. Alexis, who is also professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York. “Patients often describe these hyperpigmented macules as scars, and they are concerned that they are not reversible.”
In darker skin, the combination of treatments used for acne should address the pathogenic factors that contribute to acne and PIH at the same time, according to Dr. Alexis. He advised describing the goals and the timeline of acne and PIH resolution at the very first visit.
Of these two goals, resolution of PIH is often the more challenging. First-line topical retinoids have anti-inflammatory effects, but Dr. Alexis suggested that additional agents, such as topical antibiotics, topical dapsone, and benzoyl peroxide, are commonly needed to fully control inflammation.
“Topical retinoids serve as the foundation of acne treatment, especially in skin of color due to their dual action on acne and PIH,” he said. However, he added that this needs support with a “well-rounded combination therapy to address as many pathogenic factors as possible.”
One of these factors is subclinical inflammation. Citing studies first initiated at Howard University, Washington, Dr. Alexis said there are now compelling data showing T lymphocyte infiltration and increased expression of proinflammatory cytokines even in clinically uninvolved skin in acne patients with darker skin.
In patients with significant PIH, he considers oral antibiotics for their systemic anti-inflammatory effects, singling out sarecycline as a narrow-spectrum agent with a potent effect on Cutibacterium acnes. This tetracycline, a relatively recent addition to acne treatment options, has specifically been shown to be “superior to placebo across a diverse patient population” that includes those with darker skin tones.
“Another addition that can be leveraged for anti-inflammatory effects is topical minocycline foam. This has also been studied in diverse patient populations and shown to be superior to vehicle,” Dr. Alexis said.
For acne, the response to most of these therapies is relatively rapid, but control of PIH takes longer. After resolution of acne, he considers superficial chemical peels to speed the healing of PIH.
In a small randomized trial he cited, superficial glycolic acid peel added to a modified Kligman formula (hydroquinone 2%, tretinoin 0.05%, and hydrocortisone 1%) provided significantly lower scores in the mean Hyperpigmentation Area and Severity Index at 12 weeks (P = .004) and 21 weeks (P < .001 relative to the Kligman formula alone). Dr. Alexis said he has had the same clinical experience with chemical peels
For many acne patients with darker skin, good results are achieved after four weeks on a multidrug combination with a topical retinoid backbone. One week after stopping the combination, the superficial chemical peel can be started at a very low dose on an every-other-night schedule. If tolerated, the dose can be slowly increased.
Slow up-titration of all topical agents in skin of color, not just superficial chemical peels, is prudent, according to Dr. Alexis. For patients new to retinoids, he also recommended every-other-night dosing to avoid the irritation that might exacerbate PIH. He said the risks of adverse reactions come early. “We need to hold the hands of our patients through the first 2 weeks. Warn of dryness and pealing. Recommend moisturizers and keep the doses low.”
The benefits and risks of acne treatment are different in dark relative to light skin, Dr. Alexis emphasized. He added that a measured approach that includes specific strategies for PIH delivers results.
Providing treatment with a strategy that addresses both acne and PIH, he said, “we can have excellent outcomes time and time again for acne in patients with darker skin types.”
There is an evidence basis for making effective treatment of PIH a specific goal in the treatment of acne. In a study that evaluated the psychosocial impact of PIH in 50 patients with acne, 54% responded that PIH was a source of embarrassment. The study was one of the first to evaluate the impact of PIH as a separate source of impaired quality of life in acne patients.
“To improve the patient’s quality of life, the dermatologist should treat acne and postinflammatory hyperpigmentation at the same time,” said Katlein Franca, MD, PhD, assistant professor of dermatology, University of Miami.
In particular, Dr. Franca, who led the PIH study, suggested that PIH, like acne, is a source of low self-esteem. In regard to PIH, “most patients feel embarrassed about the spots,” she said in an interview.
“Strategies to hide the hyperpigmented spots include the use of makeup and even different hairstyles to cover the affected areas,” she added, indicating that treatments provided to clear PIH as well as acne can remove a source of stress and threat to a sense of well-being.
Dr. Alexis reports financial relationships with many pharmaceutical companies, including those that make acne drugs.
according to an expert, who cited both published data and empirical experience at the virtual Skin of Color Update 2020.
Because of the risk of exacerbating hyperpigmentation, superficial peels must be used judiciously, but “peels do add some benefit in terms of resolving the hyperpigmentation more rapidly,” Andrew Alexis, MD, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said at the meeting.
Addressing hyperpigmentation in skin of color is a critical goal. For many patients, the postinflammatory hyperpigmentation (PIH) that accompanies acne in Fitzpatrick skin types IV or higher imposes a greater burden than the acne itself.
“PIH is one of the driving forces among patients with darker skin coming to a dermatologist,” said Dr. Alexis, who is also professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York. “Patients often describe these hyperpigmented macules as scars, and they are concerned that they are not reversible.”
In darker skin, the combination of treatments used for acne should address the pathogenic factors that contribute to acne and PIH at the same time, according to Dr. Alexis. He advised describing the goals and the timeline of acne and PIH resolution at the very first visit.
Of these two goals, resolution of PIH is often the more challenging. First-line topical retinoids have anti-inflammatory effects, but Dr. Alexis suggested that additional agents, such as topical antibiotics, topical dapsone, and benzoyl peroxide, are commonly needed to fully control inflammation.
“Topical retinoids serve as the foundation of acne treatment, especially in skin of color due to their dual action on acne and PIH,” he said. However, he added that this needs support with a “well-rounded combination therapy to address as many pathogenic factors as possible.”
One of these factors is subclinical inflammation. Citing studies first initiated at Howard University, Washington, Dr. Alexis said there are now compelling data showing T lymphocyte infiltration and increased expression of proinflammatory cytokines even in clinically uninvolved skin in acne patients with darker skin.
In patients with significant PIH, he considers oral antibiotics for their systemic anti-inflammatory effects, singling out sarecycline as a narrow-spectrum agent with a potent effect on Cutibacterium acnes. This tetracycline, a relatively recent addition to acne treatment options, has specifically been shown to be “superior to placebo across a diverse patient population” that includes those with darker skin tones.
“Another addition that can be leveraged for anti-inflammatory effects is topical minocycline foam. This has also been studied in diverse patient populations and shown to be superior to vehicle,” Dr. Alexis said.
For acne, the response to most of these therapies is relatively rapid, but control of PIH takes longer. After resolution of acne, he considers superficial chemical peels to speed the healing of PIH.
In a small randomized trial he cited, superficial glycolic acid peel added to a modified Kligman formula (hydroquinone 2%, tretinoin 0.05%, and hydrocortisone 1%) provided significantly lower scores in the mean Hyperpigmentation Area and Severity Index at 12 weeks (P = .004) and 21 weeks (P < .001 relative to the Kligman formula alone). Dr. Alexis said he has had the same clinical experience with chemical peels
For many acne patients with darker skin, good results are achieved after four weeks on a multidrug combination with a topical retinoid backbone. One week after stopping the combination, the superficial chemical peel can be started at a very low dose on an every-other-night schedule. If tolerated, the dose can be slowly increased.
Slow up-titration of all topical agents in skin of color, not just superficial chemical peels, is prudent, according to Dr. Alexis. For patients new to retinoids, he also recommended every-other-night dosing to avoid the irritation that might exacerbate PIH. He said the risks of adverse reactions come early. “We need to hold the hands of our patients through the first 2 weeks. Warn of dryness and pealing. Recommend moisturizers and keep the doses low.”
The benefits and risks of acne treatment are different in dark relative to light skin, Dr. Alexis emphasized. He added that a measured approach that includes specific strategies for PIH delivers results.
Providing treatment with a strategy that addresses both acne and PIH, he said, “we can have excellent outcomes time and time again for acne in patients with darker skin types.”
There is an evidence basis for making effective treatment of PIH a specific goal in the treatment of acne. In a study that evaluated the psychosocial impact of PIH in 50 patients with acne, 54% responded that PIH was a source of embarrassment. The study was one of the first to evaluate the impact of PIH as a separate source of impaired quality of life in acne patients.
“To improve the patient’s quality of life, the dermatologist should treat acne and postinflammatory hyperpigmentation at the same time,” said Katlein Franca, MD, PhD, assistant professor of dermatology, University of Miami.
In particular, Dr. Franca, who led the PIH study, suggested that PIH, like acne, is a source of low self-esteem. In regard to PIH, “most patients feel embarrassed about the spots,” she said in an interview.
“Strategies to hide the hyperpigmented spots include the use of makeup and even different hairstyles to cover the affected areas,” she added, indicating that treatments provided to clear PIH as well as acne can remove a source of stress and threat to a sense of well-being.
Dr. Alexis reports financial relationships with many pharmaceutical companies, including those that make acne drugs.
FROM SOC 2020
Expert spotlights recent advances in the medical treatment of acne
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
FROM MOA 2020
Active Comparator Trial Designs Used to Promote Development of Innovative New Medications
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Practice Points
- When evaluating a new treatment, it is important to consider not only whether it is effective but also whether it provides additional value compared to existing treatment options.
- Encouraging active comparator trials will provide clinicians and patients with important data to guide decision-making regarding the most appropriate treatment options.
Post-acne nasal papules described in a series of patients
, but researchers believe the condition could be an underrecognized problem, affecting patients with skin of color in particular, according to the authors of a case series published in Pediatric Dermatology.
Jorge Roman, MD, and coauthors in the department of dermatology at New York (N.Y.) University identified 20 patients with a history of acne who had nasal papules, in a retrospective review of electronic medical records at NYU over 1 year (April 2018 to April 2019). The presentation ranged from “a few, small skin-colored papules to large, dome-shaped papulonodules, to more extensive rhinophymatous-like” changes with some patients having papular lesions on the chin in addition to the nose, they wrote in the report.
These papules greatly resembled angiofibromas, but appear to be a sequela of acne, according to the authors. In five patients who had biopsies, the results showed “a dome-shaped proliferation of spindle and stellate-shaped cells with thickened collagen bundles and dilated thin-walled blood vessels,” the authors wrote. “The histopathological findings of these nasal papules were indistinguishable from those of a conventional angiofibroma.”
In addition, the patients did not have evidence of underlying genetic conditions that could explain the angiofibroma-like lesions. “Although acne has not previously been implicated in the development of angiofibromas, based on the data available for our patients, it seems extremely unlikely that the lesions would be related to anything else,” Dr. Roman, a dermatology resident at New York University, said in an interview.
He said he first recognized the nasal papules in clinic as a first-year resident, but was surprised to find a lack of information on the condition. “Dermatology has a name for just about every skin disease imaginable, so I found it very odd when I couldn’t find much describing this condition,” he said. “There was a large disparity between what we were seeing in clinic and what was reported in the literature.”
Nearly all the patients were Hispanic (17 of 20) and adolescent males (17 patients), with a median age of 16 years at the time of presentation. There were two Black patients and one Asian patient. Race and ethnicity were not mentioned in two previous reports describing papular acne scarring, but Dr. Roman and colleagues noted that in their clinic, the condition appeared to affect adolescent patients with skin of color predominantly.
Reasons why nasal papules may be underreported are unclear, Dr. Roman noted. One possible explanation is lower use of dermatologic care among patients with skin of color. “Interestingly, previous research has shown that racial minorities are lower utilizers of dermatologic care. It is possible that the patient demographic most afflicted by this condition face significant barriers when seeking care,” he said.
Due to a low level of awareness of acne-related nasal papules, “clinicians may not recognize it as an acne-related scarring process. This is significant, as early recognition and treatment can prevent the development or progression of these potentially disfiguring sequelae,” Dr. Roman said.
Although the results are from a small case series at a single center, Dr. Roman said this condition may be more prevalent than realized. “Having been raised in a predominately Latino community in Texas, I can easily recall seeing people with these papules growing up. I don’t think it would be surprising for dermatologists reading our paper to say, ‘I’ve seen this in clinic before,’ ” he said.
Regarding treatment, there is an ongoing investigation into what treatments are effective for the acne-related nasal papules. “Physical treatment modalities such as ablative laser or surgical removal seem to be the most efficacious,” Dr. Roman said. “In the future, a prospective clinical study will help to better define the prevalence and risk factors for the condition,” he said.
He and coauthors reported no conflicts of interest. No funding source was listed.
SOURCE: Roman J et al. Pediatr Dermatol. 2020 Aug 7. doi: 10.1111/pde.14319.
, but researchers believe the condition could be an underrecognized problem, affecting patients with skin of color in particular, according to the authors of a case series published in Pediatric Dermatology.
Jorge Roman, MD, and coauthors in the department of dermatology at New York (N.Y.) University identified 20 patients with a history of acne who had nasal papules, in a retrospective review of electronic medical records at NYU over 1 year (April 2018 to April 2019). The presentation ranged from “a few, small skin-colored papules to large, dome-shaped papulonodules, to more extensive rhinophymatous-like” changes with some patients having papular lesions on the chin in addition to the nose, they wrote in the report.
These papules greatly resembled angiofibromas, but appear to be a sequela of acne, according to the authors. In five patients who had biopsies, the results showed “a dome-shaped proliferation of spindle and stellate-shaped cells with thickened collagen bundles and dilated thin-walled blood vessels,” the authors wrote. “The histopathological findings of these nasal papules were indistinguishable from those of a conventional angiofibroma.”
In addition, the patients did not have evidence of underlying genetic conditions that could explain the angiofibroma-like lesions. “Although acne has not previously been implicated in the development of angiofibromas, based on the data available for our patients, it seems extremely unlikely that the lesions would be related to anything else,” Dr. Roman, a dermatology resident at New York University, said in an interview.
He said he first recognized the nasal papules in clinic as a first-year resident, but was surprised to find a lack of information on the condition. “Dermatology has a name for just about every skin disease imaginable, so I found it very odd when I couldn’t find much describing this condition,” he said. “There was a large disparity between what we were seeing in clinic and what was reported in the literature.”
Nearly all the patients were Hispanic (17 of 20) and adolescent males (17 patients), with a median age of 16 years at the time of presentation. There were two Black patients and one Asian patient. Race and ethnicity were not mentioned in two previous reports describing papular acne scarring, but Dr. Roman and colleagues noted that in their clinic, the condition appeared to affect adolescent patients with skin of color predominantly.
Reasons why nasal papules may be underreported are unclear, Dr. Roman noted. One possible explanation is lower use of dermatologic care among patients with skin of color. “Interestingly, previous research has shown that racial minorities are lower utilizers of dermatologic care. It is possible that the patient demographic most afflicted by this condition face significant barriers when seeking care,” he said.
Due to a low level of awareness of acne-related nasal papules, “clinicians may not recognize it as an acne-related scarring process. This is significant, as early recognition and treatment can prevent the development or progression of these potentially disfiguring sequelae,” Dr. Roman said.
Although the results are from a small case series at a single center, Dr. Roman said this condition may be more prevalent than realized. “Having been raised in a predominately Latino community in Texas, I can easily recall seeing people with these papules growing up. I don’t think it would be surprising for dermatologists reading our paper to say, ‘I’ve seen this in clinic before,’ ” he said.
Regarding treatment, there is an ongoing investigation into what treatments are effective for the acne-related nasal papules. “Physical treatment modalities such as ablative laser or surgical removal seem to be the most efficacious,” Dr. Roman said. “In the future, a prospective clinical study will help to better define the prevalence and risk factors for the condition,” he said.
He and coauthors reported no conflicts of interest. No funding source was listed.
SOURCE: Roman J et al. Pediatr Dermatol. 2020 Aug 7. doi: 10.1111/pde.14319.
, but researchers believe the condition could be an underrecognized problem, affecting patients with skin of color in particular, according to the authors of a case series published in Pediatric Dermatology.
Jorge Roman, MD, and coauthors in the department of dermatology at New York (N.Y.) University identified 20 patients with a history of acne who had nasal papules, in a retrospective review of electronic medical records at NYU over 1 year (April 2018 to April 2019). The presentation ranged from “a few, small skin-colored papules to large, dome-shaped papulonodules, to more extensive rhinophymatous-like” changes with some patients having papular lesions on the chin in addition to the nose, they wrote in the report.
These papules greatly resembled angiofibromas, but appear to be a sequela of acne, according to the authors. In five patients who had biopsies, the results showed “a dome-shaped proliferation of spindle and stellate-shaped cells with thickened collagen bundles and dilated thin-walled blood vessels,” the authors wrote. “The histopathological findings of these nasal papules were indistinguishable from those of a conventional angiofibroma.”
In addition, the patients did not have evidence of underlying genetic conditions that could explain the angiofibroma-like lesions. “Although acne has not previously been implicated in the development of angiofibromas, based on the data available for our patients, it seems extremely unlikely that the lesions would be related to anything else,” Dr. Roman, a dermatology resident at New York University, said in an interview.
He said he first recognized the nasal papules in clinic as a first-year resident, but was surprised to find a lack of information on the condition. “Dermatology has a name for just about every skin disease imaginable, so I found it very odd when I couldn’t find much describing this condition,” he said. “There was a large disparity between what we were seeing in clinic and what was reported in the literature.”
Nearly all the patients were Hispanic (17 of 20) and adolescent males (17 patients), with a median age of 16 years at the time of presentation. There were two Black patients and one Asian patient. Race and ethnicity were not mentioned in two previous reports describing papular acne scarring, but Dr. Roman and colleagues noted that in their clinic, the condition appeared to affect adolescent patients with skin of color predominantly.
Reasons why nasal papules may be underreported are unclear, Dr. Roman noted. One possible explanation is lower use of dermatologic care among patients with skin of color. “Interestingly, previous research has shown that racial minorities are lower utilizers of dermatologic care. It is possible that the patient demographic most afflicted by this condition face significant barriers when seeking care,” he said.
Due to a low level of awareness of acne-related nasal papules, “clinicians may not recognize it as an acne-related scarring process. This is significant, as early recognition and treatment can prevent the development or progression of these potentially disfiguring sequelae,” Dr. Roman said.
Although the results are from a small case series at a single center, Dr. Roman said this condition may be more prevalent than realized. “Having been raised in a predominately Latino community in Texas, I can easily recall seeing people with these papules growing up. I don’t think it would be surprising for dermatologists reading our paper to say, ‘I’ve seen this in clinic before,’ ” he said.
Regarding treatment, there is an ongoing investigation into what treatments are effective for the acne-related nasal papules. “Physical treatment modalities such as ablative laser or surgical removal seem to be the most efficacious,” Dr. Roman said. “In the future, a prospective clinical study will help to better define the prevalence and risk factors for the condition,” he said.
He and coauthors reported no conflicts of interest. No funding source was listed.
SOURCE: Roman J et al. Pediatr Dermatol. 2020 Aug 7. doi: 10.1111/pde.14319.
FROM PEDIATRIC DERMATOLOGY