User login
FDA approves topical antiandrogen for acne
Clascoterone is a topical androgen receptor inhibitor indicated for treatment of acne vulgaris in patients aged 12 years and older, according to the labeling from manufacturer Cassiopea. Clascoterone, which will be marketed as Winlevi, targets the androgen hormones that contribute to acne by inhibiting serum production and inflammation, according to a company press release.
“Although clascoterone’s exact mechanism of action is unknown, laboratory studies suggest clascoterone competes with androgens, specifically dihydrotestosterone, for binding to the androgen receptors within the sebaceous gland and hair follicles,” according to the release.
Approval was based in part on a pair of phase 3, double-blind, vehicle-controlled, 12-week, randomized trials including 1,440 patients aged 9 years and older with moderate to severe facial acne. The findings were published in April, in JAMA Dermatology .
Participants were randomized to twice-daily application of clascoterone or a control vehicle; treatment success was defined as having an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear), as well as at least a 2-grade improvement from baseline, and absolute change in noninflammatory and inflammatory lesion counts at week 12.
At 12 weeks, treatment success rates were 18.4% and 20.3% among those on clascoterone, compared with 9% and 6.5%, respectively, among controls. There were also significant reductions in noninflammatory and inflammatory lesions from baseline at 12 weeks, compared with controls.
In the studies, treatment was well tolerated, with a safety profile similar to safety in controls. Adverse events thought to be related to clascoterone in the studies (a total of 13) included application-site pain; erythema; oropharyngeal pain; hypersensitivity, dryness, or hypertrichosis at the application site; eye irritation; headache; and hair color changes. “Clascoterone targets androgen receptors at the site of application and is quickly metabolized to an inactive form, thus limiting systemic activity,” the authors of the study wrote.
Clascoterone is expected to be available in the United States in early 2021, according to the manufacturer.
Clascoterone is a topical androgen receptor inhibitor indicated for treatment of acne vulgaris in patients aged 12 years and older, according to the labeling from manufacturer Cassiopea. Clascoterone, which will be marketed as Winlevi, targets the androgen hormones that contribute to acne by inhibiting serum production and inflammation, according to a company press release.
“Although clascoterone’s exact mechanism of action is unknown, laboratory studies suggest clascoterone competes with androgens, specifically dihydrotestosterone, for binding to the androgen receptors within the sebaceous gland and hair follicles,” according to the release.
Approval was based in part on a pair of phase 3, double-blind, vehicle-controlled, 12-week, randomized trials including 1,440 patients aged 9 years and older with moderate to severe facial acne. The findings were published in April, in JAMA Dermatology .
Participants were randomized to twice-daily application of clascoterone or a control vehicle; treatment success was defined as having an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear), as well as at least a 2-grade improvement from baseline, and absolute change in noninflammatory and inflammatory lesion counts at week 12.
At 12 weeks, treatment success rates were 18.4% and 20.3% among those on clascoterone, compared with 9% and 6.5%, respectively, among controls. There were also significant reductions in noninflammatory and inflammatory lesions from baseline at 12 weeks, compared with controls.
In the studies, treatment was well tolerated, with a safety profile similar to safety in controls. Adverse events thought to be related to clascoterone in the studies (a total of 13) included application-site pain; erythema; oropharyngeal pain; hypersensitivity, dryness, or hypertrichosis at the application site; eye irritation; headache; and hair color changes. “Clascoterone targets androgen receptors at the site of application and is quickly metabolized to an inactive form, thus limiting systemic activity,” the authors of the study wrote.
Clascoterone is expected to be available in the United States in early 2021, according to the manufacturer.
Clascoterone is a topical androgen receptor inhibitor indicated for treatment of acne vulgaris in patients aged 12 years and older, according to the labeling from manufacturer Cassiopea. Clascoterone, which will be marketed as Winlevi, targets the androgen hormones that contribute to acne by inhibiting serum production and inflammation, according to a company press release.
“Although clascoterone’s exact mechanism of action is unknown, laboratory studies suggest clascoterone competes with androgens, specifically dihydrotestosterone, for binding to the androgen receptors within the sebaceous gland and hair follicles,” according to the release.
Approval was based in part on a pair of phase 3, double-blind, vehicle-controlled, 12-week, randomized trials including 1,440 patients aged 9 years and older with moderate to severe facial acne. The findings were published in April, in JAMA Dermatology .
Participants were randomized to twice-daily application of clascoterone or a control vehicle; treatment success was defined as having an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear), as well as at least a 2-grade improvement from baseline, and absolute change in noninflammatory and inflammatory lesion counts at week 12.
At 12 weeks, treatment success rates were 18.4% and 20.3% among those on clascoterone, compared with 9% and 6.5%, respectively, among controls. There were also significant reductions in noninflammatory and inflammatory lesions from baseline at 12 weeks, compared with controls.
In the studies, treatment was well tolerated, with a safety profile similar to safety in controls. Adverse events thought to be related to clascoterone in the studies (a total of 13) included application-site pain; erythema; oropharyngeal pain; hypersensitivity, dryness, or hypertrichosis at the application site; eye irritation; headache; and hair color changes. “Clascoterone targets androgen receptors at the site of application and is quickly metabolized to an inactive form, thus limiting systemic activity,” the authors of the study wrote.
Clascoterone is expected to be available in the United States in early 2021, according to the manufacturer.
Variations in Preference for Topical Vehicles Among Demographic Groups
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
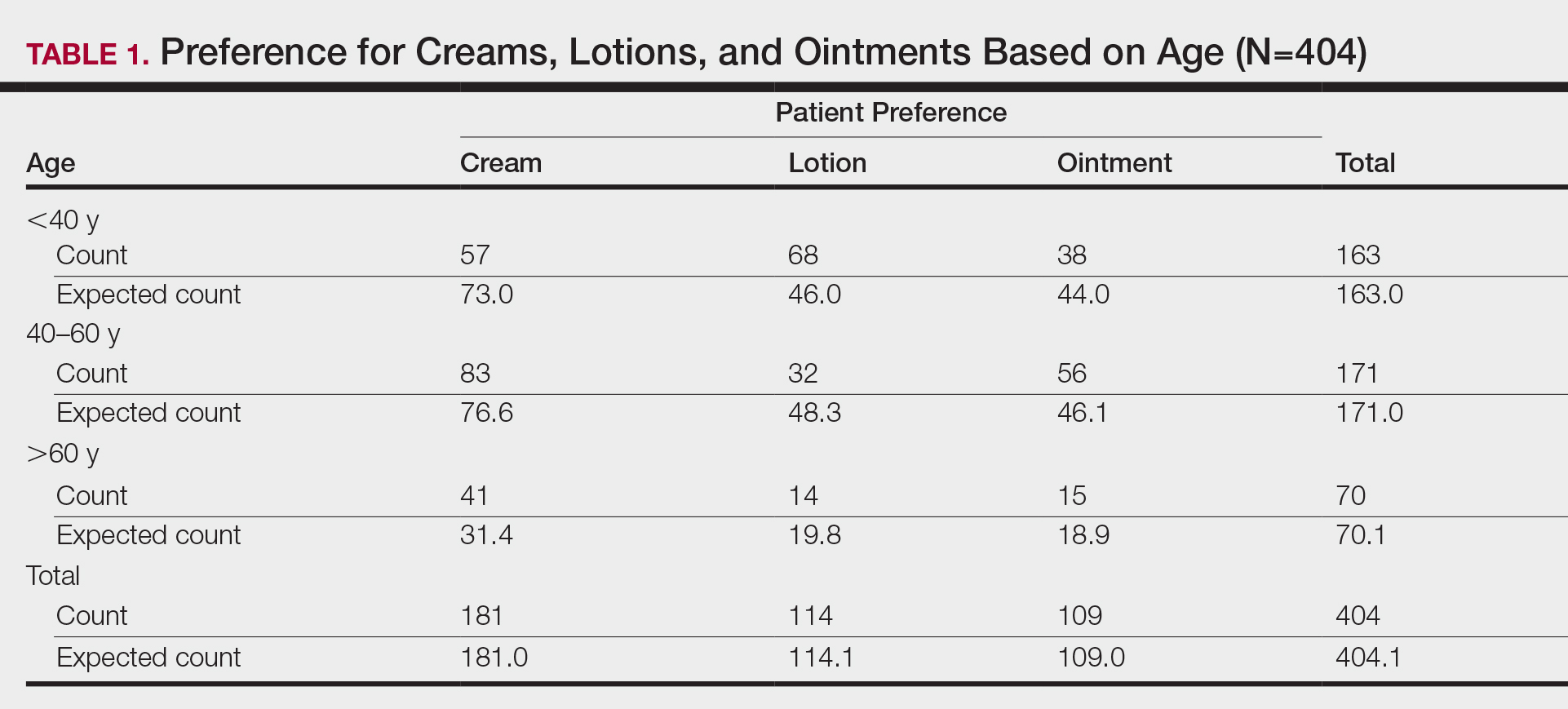
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).
Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Practice Points
- Variations exist in preference for topical vehicles by age group, gender, and ethnicity.
- Identifying and utilizing preferred treatment options can help maximize patient outcomes.
Tolerability of Tretinoin Lotion 0.05% for Moderate to Severe Acne Vulgaris: A Post Hoc Analysis in a Black Population
Acne vulgaris (acne) is the most common dermatologic condition in black patients.1,2 However, among outpatient visits, racial disparities exist in both the likelihood of seeing a dermatologist and being treated.3 Black patients are less likely to visit a dermatologist or receive any acne medication. Acne in black skin is frequently associated with postinflammatory hyperpigmentation (PIH), an important consideration in treatment choice and maintenance.
There is a paucity of clinical studies that specifically evaluate acne treatment in this patient population. An 8-week, vehicle-controlled study with tretinoin cream 0.025% in 27 black patients with acne reported notable decreases in papules, pustules, and hyperpigmented macules in 83% of patients treated with tretinoin compared to only 13% receiving vehicle.4 However, irritation and inflammation were problematic. An open-label study of adapalene gel 0.1% in 65 black South Africans also demonstrated significant improvement in inflammatory and noninflammatory lesions and PIH (P<.01), with seemingly better tolerability.5,6 A meta-analysis of 5 randomized studies from the United States and Europe (N=655) compared the efficacy and safety of adapalene gel 0.1% in black (n=46) and white patients.7 There was no significant difference in percentage reduction in comedonal (44%) or total (42%) lesion counts. The percentage reduction in inflammatory lesion counts (53%) was significantly greater in black patients (P=.012). Tolerability also was better; black patients experienced significantly less erythema and scaling (P<.001 and P=.026, respectively), though erythema can be underestimated in darker skin tones because of the masking effects of melanin.5,7 Dryness was more common, though a smaller percentage of black patients reported moderate or severe dryness compared to white patients (7% vs 18%).7
Black patients also are less likely to receive combination therapy, and again clinical data are limited.3 A more recent subgroup analysis evaluated the safety and efficacy of adapalene 0.1%–benzoyl peroxide 2.5% gel in black patients with moderate acne from 3 studies (n=238 out of a total of 3855 patients).8 Similar results were obtained as in the overall study populations, with 64.3% and 48.5% reductions in inflammatory and noninflammatory lesion counts, respectively, at week 12. The most common treatment-related adverse event (AE) in both treatment groups was dry skin (11.3%).8
Extensive clinical data in a predominantly white population have shown that topical retinoids (eg, tretinoin, adapalene, tazarotene) are highly effective in treating acne, and they are recommended as the cornerstone of topical therapy.9 However, there is a common perception that they are primarily effective in comedonal acne10 and that their use is associated with notable cutaneous irritation.11,12 Several attempts have been made to alleviate the tolerability issue using novel delivery systems. A new lotion formulation of tretinoin recently was developed and leveraged polymeric emulsion technology with the aim to improve both efficacy and tolerability of tretinoin. Herein, we performed a post hoc analysis of 2 large phase 3 clinical studies13 in patients with moderate or severe acne treated with tretinoin lotion 0.05% to evaluate its safety and tolerability in a black population.
METHODS
Study Design
We conducted a post hoc analysis of 2 identical multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical studies13 in black patients with moderate or severe acne. Protocols received approval from the appropriate institutional review board for each center before patient enrollment, and the studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as well as in compliance with local regulatory requirements. All patients were informed of the study details and provided written consent before entering the studies.
Patients were enrolled with an evaluator global severity score (EGSS) of 3 (moderate) or 4 (severe). Participants were randomized (1:1) to receive tretinoin lotion 0.05% or vehicle applied to the face once daily for 12 weeks.
Study Population
Eligible patients for the post hoc analysis included male and female patients with black skin who were 9 years and older and presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 noninflammatory lesions (open and closed comedones), and 2 or fewer nodules. A washout period of up to 1 month was required for patients who previously used prescription and over-the-counter acne treatments, and a washout period of 6 months was required for systemic retinoids.
Safety Evaluation
Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated on a 4-point scale (0=none; 3=severe). Severity of hypopigmentation and hyperpigmentation also was assessed using this 4-point scale. The investigator assessed erythema and scaling at the time of each study visit. Reports of itching, burning, and stinging were solicited from participants and recorded as an average score of their symptoms during the period since the prior visit.
Adverse events were evaluated throughout and summarized by treatment group, severity, and relationship to study medication.
Statistical Analysis
The safety analysis set comprised all randomized patients who were presumed to have used the study drug at least once and who provided at least 1 postbaseline evaluation. All AEs occurring during the studies were recorded and coded using the Medical Dictionary for Regulatory Activities version 18.0. Treatment group comparisons were made by tabulating the frequency of participants reporting 1 or more AEs during the study.
Cutaneous safety (scaling, erythema, hypopigmentation, and hyperpigmentation) and tolerability (itching, burning, and stinging) scores were presented by treatment group with descriptive statistics at baseline and weeks 4, 8, and 12. Frequencies and percentages for each outcome category were included in the statistics.
RESULTS
Baseline Characteristics
A total of 308 patients were included in the post hoc analysis. Overall, 257 (83.4%) patients completed the studies, including 138 (83.6%) patients receiving tretinoin lotion 0.05% and 119 (83.2%) receiving vehicle (Figure 1). Completion rates were similar in the female and male subgroups (83.3% and 83.8%, respectively). The most common reasons for study discontinuations were lost to follow-up (n=32; 10.4%) or participant request (n=13; 4.2%) and were similar irrespective of treatment or sex. There were no study discontinuations due to AEs.

Demographic data (Table) were similar across the 2 treatment arms. The mean age (standard deviation [SD]) of the participants was 22.1 (8.35) years (range, 9–58 years). Participants were predominantly female (209/308 [67.9%]) and tended to be a little older than the males (mean age, 23.6 vs 18.8 years).
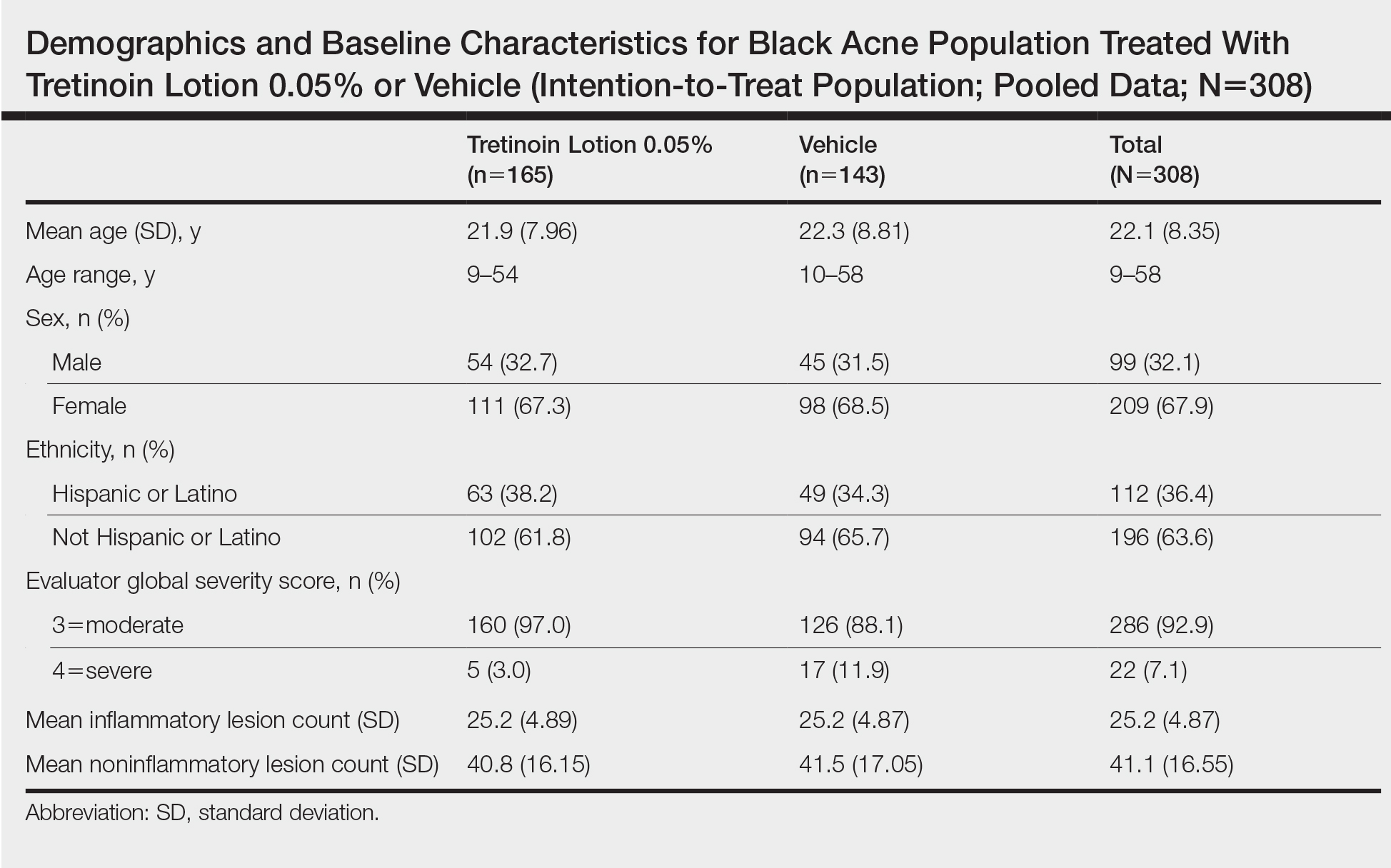
There were no noticeable differences between treatment groups regarding baseline lesion counts or EGSS. At baseline, the mean number (SD) of inflammatory and noninflammatory lesions was 25.2 (4.87) and 41.1 (16.55), respectively. At baseline, 286 (92.9%) participants had moderate acne (EGSS=3). A higher proportion of male participants (10.1%) had severe acne (EGSS=4) at baseline compared to female participants (5.7%).
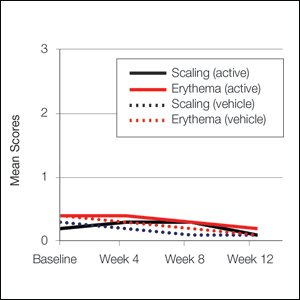
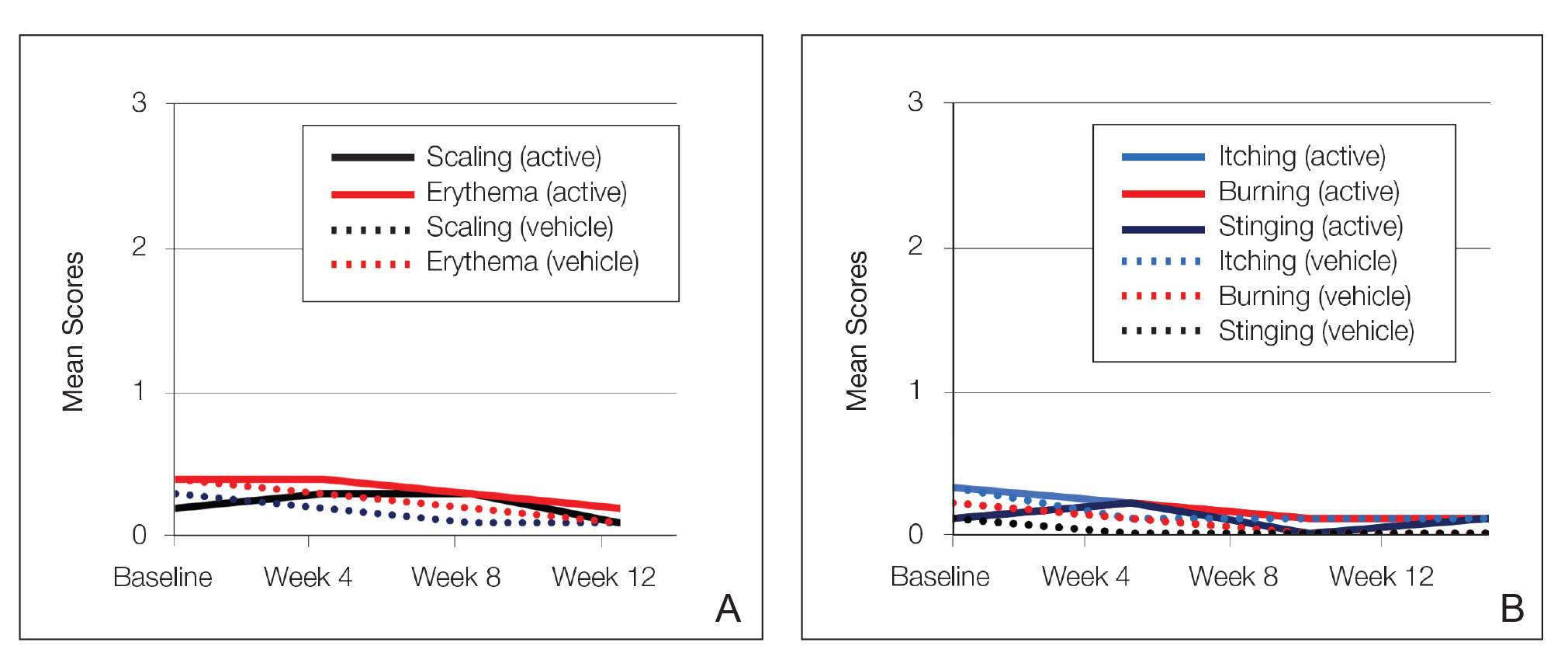
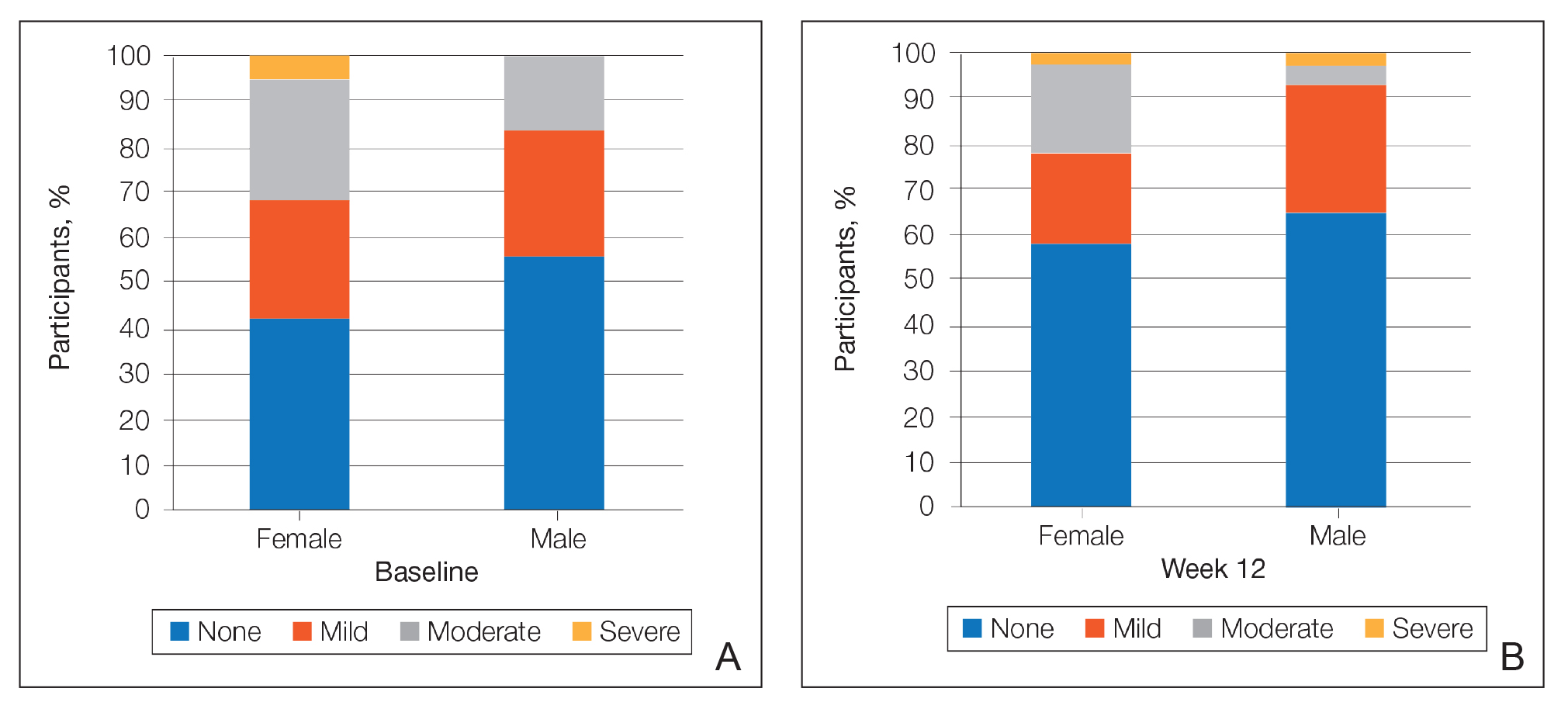
At baseline, the mean score (SD) for scaling, erythema, itching, burning, and stinging in those participants that were subsequently treated with tretinoin lotion 0.05% was 0.2 (0.42), 0.4 (0.68), 0.3 (0.60), 0.1 (0.28), and 0.1 (0.32), respectively (where 1=mild)(Figure 2). There were no differences in mean baseline scores between active and vehicle treatment groups for hyperpigmentation (0.8 each) and hypopigmentation (0.1 each) in the active and vehicle treatment groups. Mean baseline scores were slightly higher in the female participants (0.9) compared to male participants (0.6). Baseline moderate or severe hyperpigmentation was reported in 23.2% and 3.2% of participants, respectively, who were subsequently treated with tretinoin lotion 0.05%, which also was more commonly reported in female participants (33/105 [31.5%]) than male participants (8/50 [16.0%]).
Safety
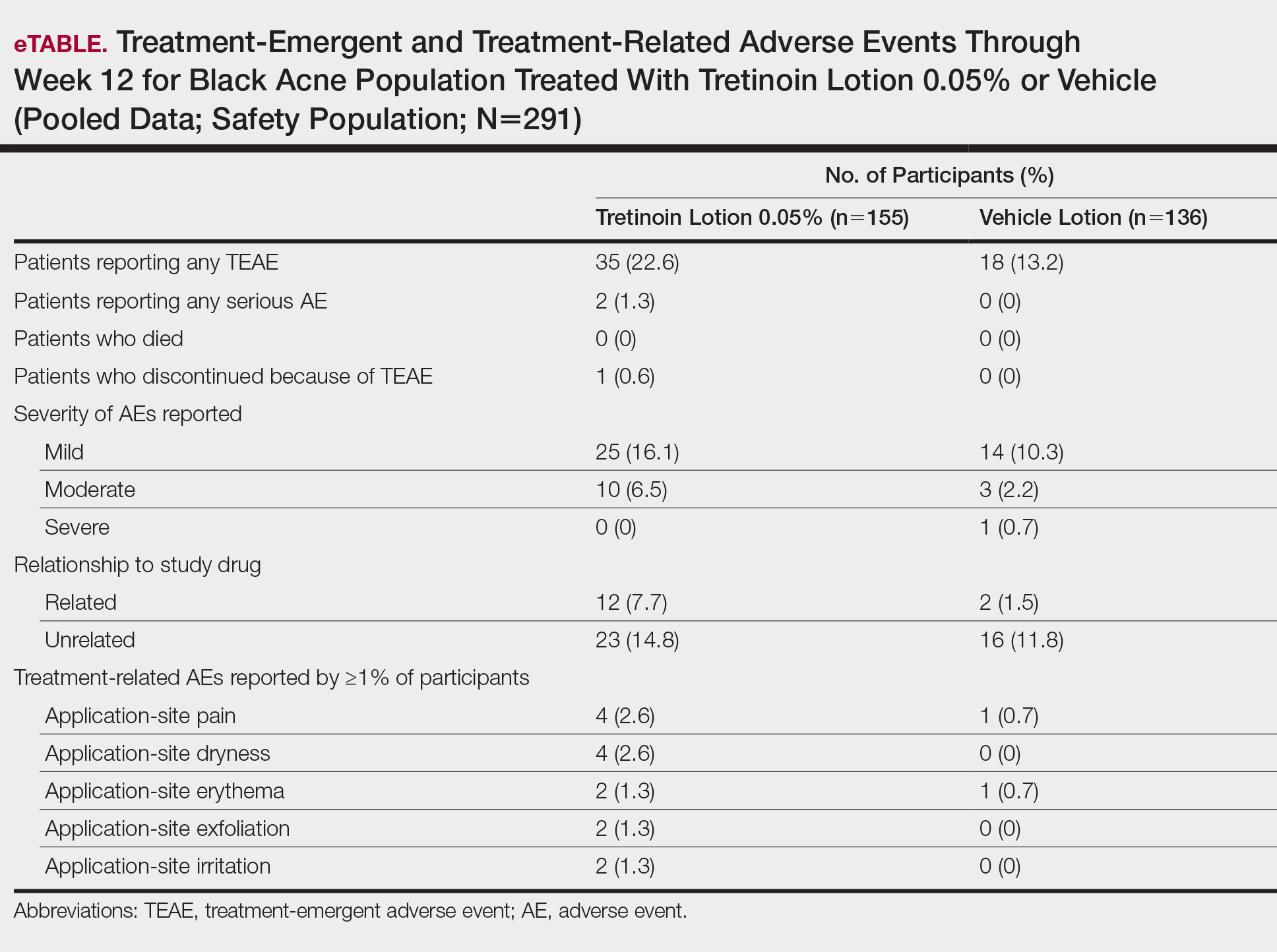
Treatment-Related AEs
More participants treated with tretinoin lotion 0.05% reported treatment-emergent AEs (TEAEs) compared to vehicle (35 vs 18). The majority of participants reporting TEAEs were female (24 of 35). There were 2 (1.3%) serious AEs with tretinoin lotion 0.05% (both female), and 1 female participant (0.6%) discontinued the study drug because of a TEAE (eTable).
Overall, there were 12 (7.7%) treatment-related AEs; all were mild (n=10) or moderate (n=2). Treatment-related AEs reported by more than 1% of participants treated with tretinoin lotion 0.05% included application-site pain (n=4; 2.6%), dryness (n=4; 2.6%), irritation (n=2; 1.3%), exfoliation (n=2; 1.3%), or erythema (n=2; 1.3%). The majority of treatment-related AEs (10/12) were reported in the female subgroup. Although application-site pain (3.4%) and dryness (3.8%) were more commonly reported in the white population (unpublished data, Ortho Dermatologics) in the 2 studies, differences between the 2 racial groups were not significant.
Cutaneous Safety and Tolerability
Erythema and scaling were recorded by the investigator. Mild to moderate erythema was noted in 31% of participants at baseline, with 21% reporting mild to moderate scaling. Both improved over the study period following treatment with tretinoin lotion 0.05%, with 79% of participants having no erythema and 88% having no scaling by week 12. Mean scores for erythema and scaling remained less than 0.5 throughout the study (1=mild). There were slight transient increases in the mean baseline score for scaling (from 0.2 to 0.3) at week 4 in the active treatment group. By week 12, mean scores were half those reported at baseline (Figure 2).
Severity of itching, burning, and stinging was reported by participants. Overall, 23% reported mild to moderate itching at baseline. Only 7 participants (5%) reported any itching by week 12 in the tretinoin lotion 0.05% group. Reports of burning and stinging were both rare and mild at baseline. Mean scores for itching, burning, and stinging at baseline for those participants who were subsequently treated with tretinoin lotion 0.05% were 0.3, 0.1, and 0.1, respectively (1=mild). Itching severity reduced progressively with treatment. There were slight transient increases in mean scores for burning (from 0.1 to 0.2) and stinging (from 0.1 to 0.2) at week 4, returning to baseline levels or below by week 12.
Hyperpigmentation and Hypopigmentation
There was a progressive improvement in baseline hyperpigmentation severity in participants treated with tretinoin lotion 0.05%; mean scores reduced from 0.8 at baseline to 0.6 by week 12 (Figure 3), with a similar improvement in both sexes (Figure 4). Moderate to severe hyperpigmentation was reported in 24 (17.3%) participants by week 12 compared to 41 (26.4%) at baseline; the majority (n=21) were female at week 12. Moderate to severe hyperpigmentation was reported in 24 (19.7%) participants treated with vehicle at week 12.
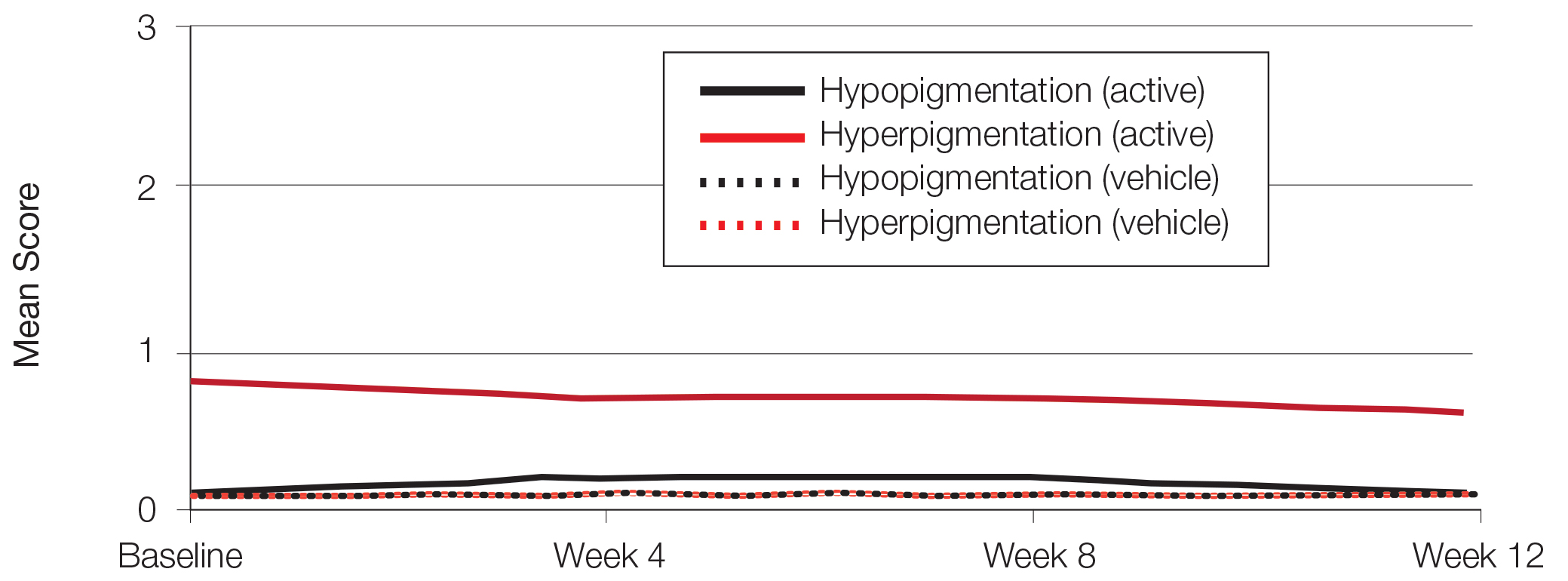
Hypopigmentation at baseline was rare and mild, and again most common in female patients. There was no increase in hypopigmentation over the course of the study.
COMMENT
Topical retinoids (eg, tretinoin, adapalene, tazarotene) are recommended as the cornerstone of topical acne treatment, with safety and efficacy well documented in large pivotal trials.14 However, data in black patients are lacking. Acne is the most common dermatologic condition in these patients, and yet investigation into this important population is limited to small study populations or subgroup analyses.
Tretinoin lotion 0.05% is a novel topical treatment for moderate to severe acne that leverages polymeric emulsion technology. The development rationale was to provide a tretinoin formulation with improved efficacy and tolerability, features that could be especially suited to black patients with acne.
In our post hoc analysis of black patients with acne, tretinoin lotion 0.05% generally was considered safe and well tolerated. The most commonly reported treatment-related AEs were of low incidence and included application-site reactions and skin-related events attributed to the known properties of tretinoin. Most noteworthy was the extremely low irritation potential of this novel tretinoin formulation. Treatment-related AEs generally were mild, and interestingly, the majority occurred in female patients. The incidence of the most common treatment-related AEs—application-site dryness (2.6%) and application-site pain (2.6%)—was lower than that reported in the white populations in the 2 studies (3.8% and 3.4%, respectively).(unpublished data, Ortho Dermatologics), though the differences were not significant (P=.625 and P=.799).
Approximately one-quarter of participants had mild to moderate erythema, scaling, itching, and stinging at baseline. All of these cutaneous symptoms improved with treatment. There were slight transient increases in scaling and stinging at week 4, with stinging more noticeable in the female population. There were no noticeable changes in mild to moderate burning during the study.
Postinflammatory hyperpigmentation is an important consideration in black patients with acne. It can arise from either acne-induced inflammation or injury. It can be of greater concern to the patient than the acne itself and often is the main reason black patients seek a dermatologist consultation. In a survey of adult female acne, nonwhite women experienced substantially more PIH than white women. In addition, clearance of PIH was most important for these nonwhite women (42% vs 8% for white women), whereas lesion clearance was the most important aspect for white women (58% vs 32% for nonwhite women).15 Erring on the side of increased tolerability is appropriate in black patients with acne, given that any irritant reactions can lead to pigmentary alterations—hyperpigmentation or hypopigmentation—that can cause considerable patient anxiety. The psychologic impact of PIH can be devastating, and an ideal acne treatment in these patients would be one that is effective against both PIH and acne. Tretinoin cream 0.1% monotherapy has been shown to be effective in reducing PIH.16 Postinflammatory hyperpigmentation lesions and normal skin were assessed by clinical and colorimetric evaluations and by analysis of biopsy specimens. Although facial PIH lesions in the 24 tretinoin-treated patients were significantly lighter after 40 weeks of treatment compared to vehicle in this study (P<.001), overall improvement was first noted after 4 weeks (P=.009). Normal skin also was minimally lightened by tretinoin; however, exuberant local skin reactions, including peeling, developed in 50% of patients. Mild to moderate PIH was present in the majority of tretinoin-treated patients at baseline in our post hoc analysis, severe in 3.2% of cases, and both more common and severe in females. Mean scores reduced over the 12-week study period, from 0.6 to 0.4 in male patients and 0.9 to 0.7 in female patients. Hypopigmentation was rare and mild at baseline and did not increase over the course of the study. A pilot study with a cream formulation of tazarotene in patients with acne from darker racial groups showed the retinoid to be effective in treating PIH following 18 weeks of once-daily application.17 Further longer-term studies on treating PIH with tretinoin lotion 0.05% are warranted given its tolerability profile.
CONCLUSION
This novel tretinoin lotion 0.05% formulation is a safe and well-tolerated topical treatment for moderate to severe comedonal and inflammatory acne in black patients. Tretinoin lotion 0.05% does not appear to induce PIH and may afford an effective, well-tolerated, dual-treatment option.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for medical writing support. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388,390.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(suppl 2):S98-S106.
- Rogers AT, Semenov YR, Kwatra SG, et al. Racial disparities in the management of acne: evidence from the National Ambulatory Medial Care Survey, 2005-2014. J Dermatolog Treat. 2018;29:287-289.
- Halder RM. The role of retinoids in the management of cutaneous conditions in blacks. J Am Acad Dermatol. 1998;39(suppl 2):S98-S103.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Jacyk WK, Mpofu P. Adapalene gel 0.1% for topical treatment of acne vulgaris in African patients. Cutis. 2001;68(suppl 4):48-54.
- Czernielewski J, Poncet M, Mizzi F. Efficacy and cutaneous safety of adapalene in black patients versus white patients with acne vulgaris. Cutis. 2002;70:243-248.
- Alexis AF, Johnson LA, Kerrouche N, et al. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Dermatol. 2014;13:170-174.
- Leyden JJ, Shalita A, Thiboutot D, et al. Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther. 2005;27:216-224.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids?J Cutan Med Surg. 2015;19:530-538.
- Kircik LH. Evaluating tretinoin formulations in the treatment of acne. J Drugs Dermatol. 2014;13:466-470.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Leyden J, Stein-Gold l, Weiss J. Why topical retionoids are the mainstay of therapy for acne. Dermatol Ther (Heidelb) 2017;7:293-304.
- Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
Acne vulgaris (acne) is the most common dermatologic condition in black patients.1,2 However, among outpatient visits, racial disparities exist in both the likelihood of seeing a dermatologist and being treated.3 Black patients are less likely to visit a dermatologist or receive any acne medication. Acne in black skin is frequently associated with postinflammatory hyperpigmentation (PIH), an important consideration in treatment choice and maintenance.
There is a paucity of clinical studies that specifically evaluate acne treatment in this patient population. An 8-week, vehicle-controlled study with tretinoin cream 0.025% in 27 black patients with acne reported notable decreases in papules, pustules, and hyperpigmented macules in 83% of patients treated with tretinoin compared to only 13% receiving vehicle.4 However, irritation and inflammation were problematic. An open-label study of adapalene gel 0.1% in 65 black South Africans also demonstrated significant improvement in inflammatory and noninflammatory lesions and PIH (P<.01), with seemingly better tolerability.5,6 A meta-analysis of 5 randomized studies from the United States and Europe (N=655) compared the efficacy and safety of adapalene gel 0.1% in black (n=46) and white patients.7 There was no significant difference in percentage reduction in comedonal (44%) or total (42%) lesion counts. The percentage reduction in inflammatory lesion counts (53%) was significantly greater in black patients (P=.012). Tolerability also was better; black patients experienced significantly less erythema and scaling (P<.001 and P=.026, respectively), though erythema can be underestimated in darker skin tones because of the masking effects of melanin.5,7 Dryness was more common, though a smaller percentage of black patients reported moderate or severe dryness compared to white patients (7% vs 18%).7
Black patients also are less likely to receive combination therapy, and again clinical data are limited.3 A more recent subgroup analysis evaluated the safety and efficacy of adapalene 0.1%–benzoyl peroxide 2.5% gel in black patients with moderate acne from 3 studies (n=238 out of a total of 3855 patients).8 Similar results were obtained as in the overall study populations, with 64.3% and 48.5% reductions in inflammatory and noninflammatory lesion counts, respectively, at week 12. The most common treatment-related adverse event (AE) in both treatment groups was dry skin (11.3%).8
Extensive clinical data in a predominantly white population have shown that topical retinoids (eg, tretinoin, adapalene, tazarotene) are highly effective in treating acne, and they are recommended as the cornerstone of topical therapy.9 However, there is a common perception that they are primarily effective in comedonal acne10 and that their use is associated with notable cutaneous irritation.11,12 Several attempts have been made to alleviate the tolerability issue using novel delivery systems. A new lotion formulation of tretinoin recently was developed and leveraged polymeric emulsion technology with the aim to improve both efficacy and tolerability of tretinoin. Herein, we performed a post hoc analysis of 2 large phase 3 clinical studies13 in patients with moderate or severe acne treated with tretinoin lotion 0.05% to evaluate its safety and tolerability in a black population.
METHODS
Study Design
We conducted a post hoc analysis of 2 identical multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical studies13 in black patients with moderate or severe acne. Protocols received approval from the appropriate institutional review board for each center before patient enrollment, and the studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as well as in compliance with local regulatory requirements. All patients were informed of the study details and provided written consent before entering the studies.
Patients were enrolled with an evaluator global severity score (EGSS) of 3 (moderate) or 4 (severe). Participants were randomized (1:1) to receive tretinoin lotion 0.05% or vehicle applied to the face once daily for 12 weeks.
Study Population
Eligible patients for the post hoc analysis included male and female patients with black skin who were 9 years and older and presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 noninflammatory lesions (open and closed comedones), and 2 or fewer nodules. A washout period of up to 1 month was required for patients who previously used prescription and over-the-counter acne treatments, and a washout period of 6 months was required for systemic retinoids.
Safety Evaluation
Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated on a 4-point scale (0=none; 3=severe). Severity of hypopigmentation and hyperpigmentation also was assessed using this 4-point scale. The investigator assessed erythema and scaling at the time of each study visit. Reports of itching, burning, and stinging were solicited from participants and recorded as an average score of their symptoms during the period since the prior visit.
Adverse events were evaluated throughout and summarized by treatment group, severity, and relationship to study medication.
Statistical Analysis
The safety analysis set comprised all randomized patients who were presumed to have used the study drug at least once and who provided at least 1 postbaseline evaluation. All AEs occurring during the studies were recorded and coded using the Medical Dictionary for Regulatory Activities version 18.0. Treatment group comparisons were made by tabulating the frequency of participants reporting 1 or more AEs during the study.
Cutaneous safety (scaling, erythema, hypopigmentation, and hyperpigmentation) and tolerability (itching, burning, and stinging) scores were presented by treatment group with descriptive statistics at baseline and weeks 4, 8, and 12. Frequencies and percentages for each outcome category were included in the statistics.
RESULTS
Baseline Characteristics
A total of 308 patients were included in the post hoc analysis. Overall, 257 (83.4%) patients completed the studies, including 138 (83.6%) patients receiving tretinoin lotion 0.05% and 119 (83.2%) receiving vehicle (Figure 1). Completion rates were similar in the female and male subgroups (83.3% and 83.8%, respectively). The most common reasons for study discontinuations were lost to follow-up (n=32; 10.4%) or participant request (n=13; 4.2%) and were similar irrespective of treatment or sex. There were no study discontinuations due to AEs.

Demographic data (Table) were similar across the 2 treatment arms. The mean age (standard deviation [SD]) of the participants was 22.1 (8.35) years (range, 9–58 years). Participants were predominantly female (209/308 [67.9%]) and tended to be a little older than the males (mean age, 23.6 vs 18.8 years).

There were no noticeable differences between treatment groups regarding baseline lesion counts or EGSS. At baseline, the mean number (SD) of inflammatory and noninflammatory lesions was 25.2 (4.87) and 41.1 (16.55), respectively. At baseline, 286 (92.9%) participants had moderate acne (EGSS=3). A higher proportion of male participants (10.1%) had severe acne (EGSS=4) at baseline compared to female participants (5.7%).
At baseline, the mean score (SD) for scaling, erythema, itching, burning, and stinging in those participants that were subsequently treated with tretinoin lotion 0.05% was 0.2 (0.42), 0.4 (0.68), 0.3 (0.60), 0.1 (0.28), and 0.1 (0.32), respectively (where 1=mild)(Figure 2). There were no differences in mean baseline scores between active and vehicle treatment groups for hyperpigmentation (0.8 each) and hypopigmentation (0.1 each) in the active and vehicle treatment groups. Mean baseline scores were slightly higher in the female participants (0.9) compared to male participants (0.6). Baseline moderate or severe hyperpigmentation was reported in 23.2% and 3.2% of participants, respectively, who were subsequently treated with tretinoin lotion 0.05%, which also was more commonly reported in female participants (33/105 [31.5%]) than male participants (8/50 [16.0%]).

Safety
Treatment-Related AEs
More participants treated with tretinoin lotion 0.05% reported treatment-emergent AEs (TEAEs) compared to vehicle (35 vs 18). The majority of participants reporting TEAEs were female (24 of 35). There were 2 (1.3%) serious AEs with tretinoin lotion 0.05% (both female), and 1 female participant (0.6%) discontinued the study drug because of a TEAE (eTable).

Overall, there were 12 (7.7%) treatment-related AEs; all were mild (n=10) or moderate (n=2). Treatment-related AEs reported by more than 1% of participants treated with tretinoin lotion 0.05% included application-site pain (n=4; 2.6%), dryness (n=4; 2.6%), irritation (n=2; 1.3%), exfoliation (n=2; 1.3%), or erythema (n=2; 1.3%). The majority of treatment-related AEs (10/12) were reported in the female subgroup. Although application-site pain (3.4%) and dryness (3.8%) were more commonly reported in the white population (unpublished data, Ortho Dermatologics) in the 2 studies, differences between the 2 racial groups were not significant.
Cutaneous Safety and Tolerability
Erythema and scaling were recorded by the investigator. Mild to moderate erythema was noted in 31% of participants at baseline, with 21% reporting mild to moderate scaling. Both improved over the study period following treatment with tretinoin lotion 0.05%, with 79% of participants having no erythema and 88% having no scaling by week 12. Mean scores for erythema and scaling remained less than 0.5 throughout the study (1=mild). There were slight transient increases in the mean baseline score for scaling (from 0.2 to 0.3) at week 4 in the active treatment group. By week 12, mean scores were half those reported at baseline (Figure 2).
Severity of itching, burning, and stinging was reported by participants. Overall, 23% reported mild to moderate itching at baseline. Only 7 participants (5%) reported any itching by week 12 in the tretinoin lotion 0.05% group. Reports of burning and stinging were both rare and mild at baseline. Mean scores for itching, burning, and stinging at baseline for those participants who were subsequently treated with tretinoin lotion 0.05% were 0.3, 0.1, and 0.1, respectively (1=mild). Itching severity reduced progressively with treatment. There were slight transient increases in mean scores for burning (from 0.1 to 0.2) and stinging (from 0.1 to 0.2) at week 4, returning to baseline levels or below by week 12.
Hyperpigmentation and Hypopigmentation
There was a progressive improvement in baseline hyperpigmentation severity in participants treated with tretinoin lotion 0.05%; mean scores reduced from 0.8 at baseline to 0.6 by week 12 (Figure 3), with a similar improvement in both sexes (Figure 4). Moderate to severe hyperpigmentation was reported in 24 (17.3%) participants by week 12 compared to 41 (26.4%) at baseline; the majority (n=21) were female at week 12. Moderate to severe hyperpigmentation was reported in 24 (19.7%) participants treated with vehicle at week 12.


Hypopigmentation at baseline was rare and mild, and again most common in female patients. There was no increase in hypopigmentation over the course of the study.
COMMENT
Topical retinoids (eg, tretinoin, adapalene, tazarotene) are recommended as the cornerstone of topical acne treatment, with safety and efficacy well documented in large pivotal trials.14 However, data in black patients are lacking. Acne is the most common dermatologic condition in these patients, and yet investigation into this important population is limited to small study populations or subgroup analyses.
Tretinoin lotion 0.05% is a novel topical treatment for moderate to severe acne that leverages polymeric emulsion technology. The development rationale was to provide a tretinoin formulation with improved efficacy and tolerability, features that could be especially suited to black patients with acne.
In our post hoc analysis of black patients with acne, tretinoin lotion 0.05% generally was considered safe and well tolerated. The most commonly reported treatment-related AEs were of low incidence and included application-site reactions and skin-related events attributed to the known properties of tretinoin. Most noteworthy was the extremely low irritation potential of this novel tretinoin formulation. Treatment-related AEs generally were mild, and interestingly, the majority occurred in female patients. The incidence of the most common treatment-related AEs—application-site dryness (2.6%) and application-site pain (2.6%)—was lower than that reported in the white populations in the 2 studies (3.8% and 3.4%, respectively).(unpublished data, Ortho Dermatologics), though the differences were not significant (P=.625 and P=.799).
Approximately one-quarter of participants had mild to moderate erythema, scaling, itching, and stinging at baseline. All of these cutaneous symptoms improved with treatment. There were slight transient increases in scaling and stinging at week 4, with stinging more noticeable in the female population. There were no noticeable changes in mild to moderate burning during the study.
Postinflammatory hyperpigmentation is an important consideration in black patients with acne. It can arise from either acne-induced inflammation or injury. It can be of greater concern to the patient than the acne itself and often is the main reason black patients seek a dermatologist consultation. In a survey of adult female acne, nonwhite women experienced substantially more PIH than white women. In addition, clearance of PIH was most important for these nonwhite women (42% vs 8% for white women), whereas lesion clearance was the most important aspect for white women (58% vs 32% for nonwhite women).15 Erring on the side of increased tolerability is appropriate in black patients with acne, given that any irritant reactions can lead to pigmentary alterations—hyperpigmentation or hypopigmentation—that can cause considerable patient anxiety. The psychologic impact of PIH can be devastating, and an ideal acne treatment in these patients would be one that is effective against both PIH and acne. Tretinoin cream 0.1% monotherapy has been shown to be effective in reducing PIH.16 Postinflammatory hyperpigmentation lesions and normal skin were assessed by clinical and colorimetric evaluations and by analysis of biopsy specimens. Although facial PIH lesions in the 24 tretinoin-treated patients were significantly lighter after 40 weeks of treatment compared to vehicle in this study (P<.001), overall improvement was first noted after 4 weeks (P=.009). Normal skin also was minimally lightened by tretinoin; however, exuberant local skin reactions, including peeling, developed in 50% of patients. Mild to moderate PIH was present in the majority of tretinoin-treated patients at baseline in our post hoc analysis, severe in 3.2% of cases, and both more common and severe in females. Mean scores reduced over the 12-week study period, from 0.6 to 0.4 in male patients and 0.9 to 0.7 in female patients. Hypopigmentation was rare and mild at baseline and did not increase over the course of the study. A pilot study with a cream formulation of tazarotene in patients with acne from darker racial groups showed the retinoid to be effective in treating PIH following 18 weeks of once-daily application.17 Further longer-term studies on treating PIH with tretinoin lotion 0.05% are warranted given its tolerability profile.
CONCLUSION
This novel tretinoin lotion 0.05% formulation is a safe and well-tolerated topical treatment for moderate to severe comedonal and inflammatory acne in black patients. Tretinoin lotion 0.05% does not appear to induce PIH and may afford an effective, well-tolerated, dual-treatment option.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for medical writing support. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
Acne vulgaris (acne) is the most common dermatologic condition in black patients.1,2 However, among outpatient visits, racial disparities exist in both the likelihood of seeing a dermatologist and being treated.3 Black patients are less likely to visit a dermatologist or receive any acne medication. Acne in black skin is frequently associated with postinflammatory hyperpigmentation (PIH), an important consideration in treatment choice and maintenance.
There is a paucity of clinical studies that specifically evaluate acne treatment in this patient population. An 8-week, vehicle-controlled study with tretinoin cream 0.025% in 27 black patients with acne reported notable decreases in papules, pustules, and hyperpigmented macules in 83% of patients treated with tretinoin compared to only 13% receiving vehicle.4 However, irritation and inflammation were problematic. An open-label study of adapalene gel 0.1% in 65 black South Africans also demonstrated significant improvement in inflammatory and noninflammatory lesions and PIH (P<.01), with seemingly better tolerability.5,6 A meta-analysis of 5 randomized studies from the United States and Europe (N=655) compared the efficacy and safety of adapalene gel 0.1% in black (n=46) and white patients.7 There was no significant difference in percentage reduction in comedonal (44%) or total (42%) lesion counts. The percentage reduction in inflammatory lesion counts (53%) was significantly greater in black patients (P=.012). Tolerability also was better; black patients experienced significantly less erythema and scaling (P<.001 and P=.026, respectively), though erythema can be underestimated in darker skin tones because of the masking effects of melanin.5,7 Dryness was more common, though a smaller percentage of black patients reported moderate or severe dryness compared to white patients (7% vs 18%).7
Black patients also are less likely to receive combination therapy, and again clinical data are limited.3 A more recent subgroup analysis evaluated the safety and efficacy of adapalene 0.1%–benzoyl peroxide 2.5% gel in black patients with moderate acne from 3 studies (n=238 out of a total of 3855 patients).8 Similar results were obtained as in the overall study populations, with 64.3% and 48.5% reductions in inflammatory and noninflammatory lesion counts, respectively, at week 12. The most common treatment-related adverse event (AE) in both treatment groups was dry skin (11.3%).8
Extensive clinical data in a predominantly white population have shown that topical retinoids (eg, tretinoin, adapalene, tazarotene) are highly effective in treating acne, and they are recommended as the cornerstone of topical therapy.9 However, there is a common perception that they are primarily effective in comedonal acne10 and that their use is associated with notable cutaneous irritation.11,12 Several attempts have been made to alleviate the tolerability issue using novel delivery systems. A new lotion formulation of tretinoin recently was developed and leveraged polymeric emulsion technology with the aim to improve both efficacy and tolerability of tretinoin. Herein, we performed a post hoc analysis of 2 large phase 3 clinical studies13 in patients with moderate or severe acne treated with tretinoin lotion 0.05% to evaluate its safety and tolerability in a black population.
METHODS
Study Design
We conducted a post hoc analysis of 2 identical multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical studies13 in black patients with moderate or severe acne. Protocols received approval from the appropriate institutional review board for each center before patient enrollment, and the studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as well as in compliance with local regulatory requirements. All patients were informed of the study details and provided written consent before entering the studies.
Patients were enrolled with an evaluator global severity score (EGSS) of 3 (moderate) or 4 (severe). Participants were randomized (1:1) to receive tretinoin lotion 0.05% or vehicle applied to the face once daily for 12 weeks.
Study Population
Eligible patients for the post hoc analysis included male and female patients with black skin who were 9 years and older and presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 noninflammatory lesions (open and closed comedones), and 2 or fewer nodules. A washout period of up to 1 month was required for patients who previously used prescription and over-the-counter acne treatments, and a washout period of 6 months was required for systemic retinoids.
Safety Evaluation
Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated on a 4-point scale (0=none; 3=severe). Severity of hypopigmentation and hyperpigmentation also was assessed using this 4-point scale. The investigator assessed erythema and scaling at the time of each study visit. Reports of itching, burning, and stinging were solicited from participants and recorded as an average score of their symptoms during the period since the prior visit.
Adverse events were evaluated throughout and summarized by treatment group, severity, and relationship to study medication.
Statistical Analysis
The safety analysis set comprised all randomized patients who were presumed to have used the study drug at least once and who provided at least 1 postbaseline evaluation. All AEs occurring during the studies were recorded and coded using the Medical Dictionary for Regulatory Activities version 18.0. Treatment group comparisons were made by tabulating the frequency of participants reporting 1 or more AEs during the study.
Cutaneous safety (scaling, erythema, hypopigmentation, and hyperpigmentation) and tolerability (itching, burning, and stinging) scores were presented by treatment group with descriptive statistics at baseline and weeks 4, 8, and 12. Frequencies and percentages for each outcome category were included in the statistics.
RESULTS
Baseline Characteristics
A total of 308 patients were included in the post hoc analysis. Overall, 257 (83.4%) patients completed the studies, including 138 (83.6%) patients receiving tretinoin lotion 0.05% and 119 (83.2%) receiving vehicle (Figure 1). Completion rates were similar in the female and male subgroups (83.3% and 83.8%, respectively). The most common reasons for study discontinuations were lost to follow-up (n=32; 10.4%) or participant request (n=13; 4.2%) and were similar irrespective of treatment or sex. There were no study discontinuations due to AEs.

Demographic data (Table) were similar across the 2 treatment arms. The mean age (standard deviation [SD]) of the participants was 22.1 (8.35) years (range, 9–58 years). Participants were predominantly female (209/308 [67.9%]) and tended to be a little older than the males (mean age, 23.6 vs 18.8 years).

There were no noticeable differences between treatment groups regarding baseline lesion counts or EGSS. At baseline, the mean number (SD) of inflammatory and noninflammatory lesions was 25.2 (4.87) and 41.1 (16.55), respectively. At baseline, 286 (92.9%) participants had moderate acne (EGSS=3). A higher proportion of male participants (10.1%) had severe acne (EGSS=4) at baseline compared to female participants (5.7%).
At baseline, the mean score (SD) for scaling, erythema, itching, burning, and stinging in those participants that were subsequently treated with tretinoin lotion 0.05% was 0.2 (0.42), 0.4 (0.68), 0.3 (0.60), 0.1 (0.28), and 0.1 (0.32), respectively (where 1=mild)(Figure 2). There were no differences in mean baseline scores between active and vehicle treatment groups for hyperpigmentation (0.8 each) and hypopigmentation (0.1 each) in the active and vehicle treatment groups. Mean baseline scores were slightly higher in the female participants (0.9) compared to male participants (0.6). Baseline moderate or severe hyperpigmentation was reported in 23.2% and 3.2% of participants, respectively, who were subsequently treated with tretinoin lotion 0.05%, which also was more commonly reported in female participants (33/105 [31.5%]) than male participants (8/50 [16.0%]).

Safety
Treatment-Related AEs
More participants treated with tretinoin lotion 0.05% reported treatment-emergent AEs (TEAEs) compared to vehicle (35 vs 18). The majority of participants reporting TEAEs were female (24 of 35). There were 2 (1.3%) serious AEs with tretinoin lotion 0.05% (both female), and 1 female participant (0.6%) discontinued the study drug because of a TEAE (eTable).

Overall, there were 12 (7.7%) treatment-related AEs; all were mild (n=10) or moderate (n=2). Treatment-related AEs reported by more than 1% of participants treated with tretinoin lotion 0.05% included application-site pain (n=4; 2.6%), dryness (n=4; 2.6%), irritation (n=2; 1.3%), exfoliation (n=2; 1.3%), or erythema (n=2; 1.3%). The majority of treatment-related AEs (10/12) were reported in the female subgroup. Although application-site pain (3.4%) and dryness (3.8%) were more commonly reported in the white population (unpublished data, Ortho Dermatologics) in the 2 studies, differences between the 2 racial groups were not significant.
Cutaneous Safety and Tolerability
Erythema and scaling were recorded by the investigator. Mild to moderate erythema was noted in 31% of participants at baseline, with 21% reporting mild to moderate scaling. Both improved over the study period following treatment with tretinoin lotion 0.05%, with 79% of participants having no erythema and 88% having no scaling by week 12. Mean scores for erythema and scaling remained less than 0.5 throughout the study (1=mild). There were slight transient increases in the mean baseline score for scaling (from 0.2 to 0.3) at week 4 in the active treatment group. By week 12, mean scores were half those reported at baseline (Figure 2).
Severity of itching, burning, and stinging was reported by participants. Overall, 23% reported mild to moderate itching at baseline. Only 7 participants (5%) reported any itching by week 12 in the tretinoin lotion 0.05% group. Reports of burning and stinging were both rare and mild at baseline. Mean scores for itching, burning, and stinging at baseline for those participants who were subsequently treated with tretinoin lotion 0.05% were 0.3, 0.1, and 0.1, respectively (1=mild). Itching severity reduced progressively with treatment. There were slight transient increases in mean scores for burning (from 0.1 to 0.2) and stinging (from 0.1 to 0.2) at week 4, returning to baseline levels or below by week 12.
Hyperpigmentation and Hypopigmentation
There was a progressive improvement in baseline hyperpigmentation severity in participants treated with tretinoin lotion 0.05%; mean scores reduced from 0.8 at baseline to 0.6 by week 12 (Figure 3), with a similar improvement in both sexes (Figure 4). Moderate to severe hyperpigmentation was reported in 24 (17.3%) participants by week 12 compared to 41 (26.4%) at baseline; the majority (n=21) were female at week 12. Moderate to severe hyperpigmentation was reported in 24 (19.7%) participants treated with vehicle at week 12.


Hypopigmentation at baseline was rare and mild, and again most common in female patients. There was no increase in hypopigmentation over the course of the study.
COMMENT
Topical retinoids (eg, tretinoin, adapalene, tazarotene) are recommended as the cornerstone of topical acne treatment, with safety and efficacy well documented in large pivotal trials.14 However, data in black patients are lacking. Acne is the most common dermatologic condition in these patients, and yet investigation into this important population is limited to small study populations or subgroup analyses.
Tretinoin lotion 0.05% is a novel topical treatment for moderate to severe acne that leverages polymeric emulsion technology. The development rationale was to provide a tretinoin formulation with improved efficacy and tolerability, features that could be especially suited to black patients with acne.
In our post hoc analysis of black patients with acne, tretinoin lotion 0.05% generally was considered safe and well tolerated. The most commonly reported treatment-related AEs were of low incidence and included application-site reactions and skin-related events attributed to the known properties of tretinoin. Most noteworthy was the extremely low irritation potential of this novel tretinoin formulation. Treatment-related AEs generally were mild, and interestingly, the majority occurred in female patients. The incidence of the most common treatment-related AEs—application-site dryness (2.6%) and application-site pain (2.6%)—was lower than that reported in the white populations in the 2 studies (3.8% and 3.4%, respectively).(unpublished data, Ortho Dermatologics), though the differences were not significant (P=.625 and P=.799).
Approximately one-quarter of participants had mild to moderate erythema, scaling, itching, and stinging at baseline. All of these cutaneous symptoms improved with treatment. There were slight transient increases in scaling and stinging at week 4, with stinging more noticeable in the female population. There were no noticeable changes in mild to moderate burning during the study.
Postinflammatory hyperpigmentation is an important consideration in black patients with acne. It can arise from either acne-induced inflammation or injury. It can be of greater concern to the patient than the acne itself and often is the main reason black patients seek a dermatologist consultation. In a survey of adult female acne, nonwhite women experienced substantially more PIH than white women. In addition, clearance of PIH was most important for these nonwhite women (42% vs 8% for white women), whereas lesion clearance was the most important aspect for white women (58% vs 32% for nonwhite women).15 Erring on the side of increased tolerability is appropriate in black patients with acne, given that any irritant reactions can lead to pigmentary alterations—hyperpigmentation or hypopigmentation—that can cause considerable patient anxiety. The psychologic impact of PIH can be devastating, and an ideal acne treatment in these patients would be one that is effective against both PIH and acne. Tretinoin cream 0.1% monotherapy has been shown to be effective in reducing PIH.16 Postinflammatory hyperpigmentation lesions and normal skin were assessed by clinical and colorimetric evaluations and by analysis of biopsy specimens. Although facial PIH lesions in the 24 tretinoin-treated patients were significantly lighter after 40 weeks of treatment compared to vehicle in this study (P<.001), overall improvement was first noted after 4 weeks (P=.009). Normal skin also was minimally lightened by tretinoin; however, exuberant local skin reactions, including peeling, developed in 50% of patients. Mild to moderate PIH was present in the majority of tretinoin-treated patients at baseline in our post hoc analysis, severe in 3.2% of cases, and both more common and severe in females. Mean scores reduced over the 12-week study period, from 0.6 to 0.4 in male patients and 0.9 to 0.7 in female patients. Hypopigmentation was rare and mild at baseline and did not increase over the course of the study. A pilot study with a cream formulation of tazarotene in patients with acne from darker racial groups showed the retinoid to be effective in treating PIH following 18 weeks of once-daily application.17 Further longer-term studies on treating PIH with tretinoin lotion 0.05% are warranted given its tolerability profile.
CONCLUSION
This novel tretinoin lotion 0.05% formulation is a safe and well-tolerated topical treatment for moderate to severe comedonal and inflammatory acne in black patients. Tretinoin lotion 0.05% does not appear to induce PIH and may afford an effective, well-tolerated, dual-treatment option.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for medical writing support. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388,390.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(suppl 2):S98-S106.
- Rogers AT, Semenov YR, Kwatra SG, et al. Racial disparities in the management of acne: evidence from the National Ambulatory Medial Care Survey, 2005-2014. J Dermatolog Treat. 2018;29:287-289.
- Halder RM. The role of retinoids in the management of cutaneous conditions in blacks. J Am Acad Dermatol. 1998;39(suppl 2):S98-S103.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Jacyk WK, Mpofu P. Adapalene gel 0.1% for topical treatment of acne vulgaris in African patients. Cutis. 2001;68(suppl 4):48-54.
- Czernielewski J, Poncet M, Mizzi F. Efficacy and cutaneous safety of adapalene in black patients versus white patients with acne vulgaris. Cutis. 2002;70:243-248.
- Alexis AF, Johnson LA, Kerrouche N, et al. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Dermatol. 2014;13:170-174.
- Leyden JJ, Shalita A, Thiboutot D, et al. Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther. 2005;27:216-224.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids?J Cutan Med Surg. 2015;19:530-538.
- Kircik LH. Evaluating tretinoin formulations in the treatment of acne. J Drugs Dermatol. 2014;13:466-470.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Leyden J, Stein-Gold l, Weiss J. Why topical retionoids are the mainstay of therapy for acne. Dermatol Ther (Heidelb) 2017;7:293-304.
- Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388,390.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(suppl 2):S98-S106.
- Rogers AT, Semenov YR, Kwatra SG, et al. Racial disparities in the management of acne: evidence from the National Ambulatory Medial Care Survey, 2005-2014. J Dermatolog Treat. 2018;29:287-289.
- Halder RM. The role of retinoids in the management of cutaneous conditions in blacks. J Am Acad Dermatol. 1998;39(suppl 2):S98-S103.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Jacyk WK, Mpofu P. Adapalene gel 0.1% for topical treatment of acne vulgaris in African patients. Cutis. 2001;68(suppl 4):48-54.
- Czernielewski J, Poncet M, Mizzi F. Efficacy and cutaneous safety of adapalene in black patients versus white patients with acne vulgaris. Cutis. 2002;70:243-248.
- Alexis AF, Johnson LA, Kerrouche N, et al. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Dermatol. 2014;13:170-174.
- Leyden JJ, Shalita A, Thiboutot D, et al. Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther. 2005;27:216-224.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids?J Cutan Med Surg. 2015;19:530-538.
- Kircik LH. Evaluating tretinoin formulations in the treatment of acne. J Drugs Dermatol. 2014;13:466-470.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Leyden J, Stein-Gold l, Weiss J. Why topical retionoids are the mainstay of therapy for acne. Dermatol Ther (Heidelb) 2017;7:293-304.
- Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
Practice Points
- Acne vulgaris is the most common dermatologic disorder seen in black patients, though data on treatment effects is lacking.
- Postinflammatory hyperpigmentation (PIH) frequently coexists with acne, and retinoids are known to treat both.
- Tretinoin lotion 0.05% is effective in treating both inflammatory and noninflammatory lesions in black patients with acne and reducing PIH without the irritant contact dermatitis seen with other retinoid formulations.
Fighting Acne for the Fighting Forces
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.
The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.

The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
Acne treatment presents unique challenges in the active-duty military population. Lesions on the face may interfere with proper fit and seal of protective masks and helmets, while those involving the shoulders or back may cause considerable discomfort beneath safety restraints, parachute harnesses, or flak jackets. Therefore, untreated acne may limit servicemembers from performing their assigned duties. Treatments themselves also may be limiting; for instance, aircrew members who are taking oral doxycycline, tetracycline, or erythromycin may be grounded (ie, temporarily removed from duty) during and after therapy to monitor for side effects. Minocycline is considered unacceptable for aviators and is completely restricted for use due to risk for central nervous system side effects. Isotretinoin is restricted in aircrew members, submariners, and divers. If initiated, isotretinoin requires grounding for the entire duration of therapy and up to 3 months following treatment. Normalization of triglyceride levels and slit-lamp ocular examination also must take place prior to return to full duty, which may lead to additional grounding time. Well-established topical and oral treatments not impacting military duty are omitted from this review.
Antibiotics
Minocycline
Minocycline carries a small risk for development of systemic lupus erythematosus and other autoimmune treatment-emergent adverse effects. It has known gastrointestinal tract side effects, and long-term use also can lead to bluish discoloration of the skin.1 Systemic minocycline is restricted in aircrew members due to its risk for central nervous system side effects, including light-headedness, dizziness, and vertigo.2-5
A topical formulation of minocycline recently was developed and approved by the US Food and Drug Administration as a means to reduce systemic adverse effects. This 4% minocycline foam has thus far been safe and well tolerated, with adverse events reported in less than 1% of study participants.1,6 In addition, topical minocycline was shown in a recent phase 3 study to notably reduce inflammatory lesion counts when compared to control vehicles at as early as 3 weeks.7 Topical minocycline may emerge as a viable treatment option for active-duty servicemembers in the future.
Doxycycline
Doxycycline is not medically disqualifying. Even so, it may still necessitate grounding for a period of time while monitoring for side effects.4 Doxycycline can lead to photosensitivity, which could be difficult to tolerate for active-duty personnel training in sunny climates. Fortunately, uniform regulations and personal protective equipment requirements provide cover for most of the body surfaces aside from the face, which is protected by various forms of covers. If the patient tolerates the medication well without considerable side effects, he/she may be returned to full duty, making doxycycline an acceptable alternative to minocycline in the military population.
Sarecycline
This novel compound is a tetracycline-class antibiotic with a narrower spectrum of activity, with reduced activity against enteric gram-negative bacteria. It has shown efficacy in reducing inflammatory and noninflammatory acne lesions, including lesions on the face, back, and chest. Common adverse side effects are nausea, headache, nasopharyngitis, and vomiting. Vestibular and phototoxic adverse effects were reported in less than 1% of patients.1,8 The US Food and Drug Administration approved sarecycline as a once-daily oral formulation for moderate to severe acne vulgaris, the first new antibiotic to be approved for the disease in the last 40 years. Sarecycline is not mentioned in any US military guidelines with regard to medical readiness and duty status; however, given its lack of vestibular side effects and narrower activity spectrum, it may become another acceptable treatment option in the military population.
Isotretinoin
Isotretinoin is well established as an excellent treatment of acne and stands alone as the only currently available medication that alters the disease course and prevents relapse in many patients. Nearly all patients on isotretinoin experience considerable mucocutaneous dryness, and up to 25% of patients on high-dose isotretinoin develop myalgia.9 Isotretinoin causes serious retinoid embryopathy, requiring all patients to be enrolled in the iPLEDGE program (https://www.ipledgeprogram.com/iPledgeUI/home.u) and to use 2 methods of contraception during treatment. Although it is uncommon to have notable elevations in lipids and transaminases during treatment with isotretinoin, routine laboratory monitoring generally is performed until the patient reaches steady dosing.
Isotretinoin is not permitted for use in active aircrew members, submariners, or divers. Servicemembers pursuing isotretinoin therapy are removed from their duty and are nondeployable for the entirety of their treatment course and several months after completion.4,5
Photodynamic Therapy
Aminolevulinic acid and photodynamic therapy (ALA-PDT) has been successfully used in the management of acne.10 In addition to inducing selective damage to sebaceous glands, it has been proposed that PDT also destroys Propionibacterium acnes and reduces keratinocyte shedding and immunologic changes that play key roles in the development of acne.10
A recent randomized controlled trial comparing the efficacy of ALA-PDT vs adapalene gel plus oral doxycycline for treatment of moderate acne included 46 patients with moderate inflammatory acne.10 Twenty-three participants received 2 sessions (spaced 2 weeks apart) of 20% ALA incubated for 90 minutes before red light irradiation with a fluence of 37 J/cm2, and the other 23 received 100 mg/d of oral doxycycline plus adapalene gel 0.1%. By 6-week follow-up, there was a significantly higher reduction in total lesions within the PDT group (P=.038), which was sustained at the secondary 12-week follow-up (P=.026). There was a 79% total reduction of lesions in the ALA-PDT group vs 67% in the doxycycline plus adapalene group.10
Although some studies have shown promise for PDT as an emerging treatment option for acne, further research is needed. A 2016 systematic review of the related literature determined that although 20% ALA-PDT with red light was more effective than lower concentrations of ALA and also more effective than ALA-PDT with blue light—which offered no additional benefit when compared with blue light alone—high-quality evidence on the use of PDT for acne is lacking overall.11 At the time of the review, there was little certainty as to the usefulness of ALA-PDT with red or blue light as a standard treatment for individuals with moderate to severe acne. A 2019 review by Marson and Baldwin12 echoed this sentiment, recommending more stringently designed studies to elucidate the true role of PDT as a monotherapy or adjunctive treatment of acne.
Pulsed Dye Laser
Pulsed dye laser (PDL) was first shown to be a potential therapy for acne by Seaton et al,13 who conducted a small-scale, randomized, controlled trial with 41 patients, each assigned to either a single PDL treatment or a sham treatment. Patients were re-evaluated at 12 weeks, measuring acne severity by the Leeds revised acne grading system and taking total lesion counts. Acne severity (P=.007) and total lesion counts (P=.023) were significantly improved in the treatment group, with a 53% reduction in total lesion count following a single PDL treatment.13
In 2007, a Spanish study described use of PDL every 4 weeks for a total of 12 weeks in 36 patients with mild to moderate acne. Using lesion counts as their primary outcome measure, the investigators found results similar to those from Seaton et al,13 with a 57% decrease in active lesions.14 Others still have found similar outcomes. A 2009 study of 45 patients with mild to moderate acne compared patients treated with PDL every 2 weeks for 12 weeks to patients receiving either topical therapy or chemical peels with 25% trichloroacetic acid. At 12 weeks, they noted the best results were in the PDL group.15
Karsai et al16 compared PDL as an adjuvant treatment of acne to proven treatment with clindamycin plus benzoyl peroxide gel. Eighty patients were randomized to topical therapy plus PDL or topical therapy alone and were followed at 2 and 4 weeks after the initial treatment. Although both groups showed improvement as measured by inflammatory lesion count and dermatology life quality index, there was no statistically significant difference noted between groups.16
Case Report
A 24-year-old active-duty male servicemember was referred to the dermatology department for evaluation of treatment-resistant nodulocystic scarring acne. Prior to his arrival to dermatology, he had completed 2 weeks of isotretinoin before discontinuation due to notable mood alteration. Following the isotretinoin, he was then switched to doxycycline 100 mg twice daily, which he trialed for 3 months. Even on the antibiotic, the patient continued to develop new pustules and cysts, prompting referral to dermatology for additional treatment options (Figure, A). All of the previous topical and oral medications had been discontinued at the current presentation.

The patient received 3 treatments with the 595-nm PDL (spot size, 10 mm; fluence, 7 J/cm2; pulse width, 6 milliseconds) spaced 4 weeks apart. At each treatment, fewer than 10 total inflammatory lesions were treated, including inflammatory papules, pustules, and nodules. Nodular lesions were treated with 2 pulses. After each treatment, the patient reported that all treated lesions resolved within 2 days (Figure, B). Subsequent treated lesions all occurred at previously uninvolved sites.
Final Thoughts
Antibiotic resistance is a known and growing problem throughout the medical community. In 2013, the US Centers for Disease Control and Prevention reported that dermatologists prescribe more antibiotics than any other specialty.17 Aside from antibiotic stewardship, systemic antibiotics come with various considerations when selecting ideal acne treatment regimens in military populations, as they are either medically disqualifying or lead to temporary grounding status. Numerous guidelines on acne have recommended limiting the use of antibiotics, instead pursuing alternative therapies such as spironolactone, oral contraceptives, or isotretinoin.9,18 Both spironolactone and oral contraceptives work well via antiandrogenic and antisebogenic properties; however, these therapies are limited to female patients only, who make up a minority of patients in the active-duty military setting. Isotretinoin is highly effective in the treatment of acne, but it requires grounding for the entirety of treatment and for months afterward, which comes at great personal and financial costs to servicemembers and their commanders due to limited-duty status and inability to deploy.
Given the operational constraints with isotretinoin and the continual rise of antibiotic resistance, PDL appears to be a safe and effective alternative therapy for acne. In our case, the patient had complete resolution of active inflammatory lesions after each of his treatments. He had no adverse effects and tolerated the treatments well. We report this case here to highlight the use of PDL as an effective therapy for spot treatment in patients limited by personal or operational constraints and as a means to reduce antibiotic use in the face of a growing tide of antibiotic resistance.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- Kircik LH. What’s new in the management of acne vulgaris. Cutis. 2019;104:48-52.
- US Department of the Army. Standards of medical fitness. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf. Published June 27, 2019. Accessed June 23, 2020.
- US Department of the Air Force. Medical examinations and standards. http://aangfs.com/wp-content/uploads/2012/10/AFI-48-123-Medical-Examination-Standards.pdf. Published January 29, 2013. Accessed June 23, 2020.
- US Navy Aeromedical Reference and Waiver Guide. Navy Medicine website. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed June 17, 2020.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with skin disease. Cutis. 2019:103:329-332.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;30:168-177.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris; results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments.J Am Acad Dermatol. 2019;80:538-549.
- Nicklas C, Rubio R, Cardenas C, et al. Comparison of efficacy of aminolaevulinic acid photodynamic therapy vs. adapalene gel plus oral doxycycline for treatment of moderate acne vulgaris—a simple, blind, randomized, and controlled trial. Photodermatol Photoimmunol Photomed. 2019;35:3-10.
- Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne [published online September 27, 2016]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007917.pub2.
- Marson JW, Baldwin HE. New concepts, concerns, and creations in acne. Dermatol Clin. 2019;37:1-9.
- Seaton ED, Charakida A, Mouser PE, et al. Pulsed-dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet Lond Engl. 2003;362:1347-1352.
- Harto A, Garcia-Morales I, Belmar P, et al. Pulsed dye laser treatment of acne. study of clinical efficacy and mechanism of action. Actas Dermosifiliogr. 2007;98:415-419.
- Leheta TM. Role of the 585-nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. J Cosmet Laser Ther Off Publ Eur Soc Laser Dermatol. 2009;11:118-124.
- Karsai S, Schmitt L, Raulin C. The pulsed-dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single-blinded trial. Br J Dermatol. 2010;163:395-401.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States. annual report 2013.https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2013.pdf. Accessed June 23, 2020.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
Practice Points
- Acne is a common disease that may cause considerable physical and psychological morbidity. Numerous therapies are available, each with their respective risks and benefits.
- Military servicemembers face unique challenges in the management of acne due to operational and medical readiness considerations.
- Less conventional treatments such as photodynamic therapy and pulsed dye laser may be available to military servicemembers.
- Pulsed dye laser is an effective alternative treatment of acne, especially in an age of growing antibiotic resistance.
Why Is It That the Biggest Resistance With Fighting the Battle Against Bacterial Resistance Seems to Fall on Dermatology Clinicians?
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
This discussion focuses on antibiotic resistance in acne therapy but also includes general principles related to this subject. “Seeing is believing” is a concept we have all heard many times, and we generally can all agree with and relate to what this is saying to us. However, it is harder to get a consensus of agreement on concepts that are happening beneath the surface but are not visibly apparent. Antibiotic resistance is a concept that falls into this latter category, especially in acne treatment. Many clinicians are not convinced antibiotic resistance is clinically relevant, exclaiming “I just do not see it in my practice.” The problem is—especially in the case of acne where oral tetracycline agents commonly are prescribed—how does the clinician “see” antibiotic resistance? Clinicians do not obtain bacterial cultures or perform sensitivity testing as they might do when evaluating a suspected cutaneous infection such as folliculitis, an inflamed postsurgical wound, a purulent leg ulcer, or an abscess. Additionally, if the selected therapy is not as effective as anticipated, it may be attributed to the patient needing another type of treatment or something “stronger,” or maybe they are not fully compliant. In fact, a very possible reason for inadequate therapeutic response may be that the predominant Cutibacterium acnes strains in a particular case are proinflammatory, and many of the strains are not highly sensitive to the chosen antibiotic.1
In the United States, antibiotic resistance in C acnes is most prevalent with erythromycin, followed by clindamycin, tetracycline, doxycycline, and minocycline, respectively.2 The relative patterns of antibiotic resistance in specific geographic regions correlate with the magnitude of specific antibiotic use, and that consistent reduction in use of a given antibiotic in a community can reverse the prevalence of resistance to that antibiotic progressively over time.3 Combination therapy approaches to mitigate emergence of resistant bacteria during acne treatment with an exit plan explained up-front with the patient are important to reduce prolonged use or repeated cycles of antibiotic use and in some cases to circumvent antibiotic use and incorporate a different therapeutic approach.1-3 Interestingly, in a retrospective chart review of acne patients who were eventually treated with oral isotretinoin at dermatology practices within a major university health system, approximately two-thirds received oral antibiotics for 6 months or longer and one-third for 1 year or longer.4 It is easy for all of us to have good intentions; however, in reality it is not always easy, practical, or in the best interest of the patient to stringently enforce recommendations that are determined not to be the best option at that time. Patients get a vote, too, as long as they are fully informed of benefits vs risks.
The concern about emergence of less-sensitive bacteria during acne antibiotic treatment is not limited to discussion of C acnes resistance. Use of both oral and topical antibiotics creates “ecologic mischief,” which is the emergence of less-sensitive strains of other bacteria exposed to the antibiotic—both commensal and opportunistic—especially at anatomic sites such as the skin, nasopharyngeal region, and gastrointestinal and genitourinary tracts.5-7 Application of topical erythromycin to the face can induce erythromycin-resistant bacteria such as staphylococci on the face as well as at remote sites such as the nares (nasal vestibule) and the back.6 Antibiotics used to treat acne, predominantly oral tetracyclines, showed positive oropharyngeal cultures for Streptococcus pyogenes in 33% (13/39) of treated patients; among these positive cultures, 85% (11/13) were resistant to at least one tetracycline antibiotic.7 Importantly, the streptococcal colonization of the oropharynx in individuals taking an oral antibiotic for acne may not induce a clinically apparent pharyngitis in that individual, but that person can carry and spread that streptococcal pathogen to others. In either case, the dermatology clinician, even if he/she suspects the connection related to antibiotic selection pressure and resistance, would not “see” the antibiotic resistance, as the individuals who develop a “sore throat” or strep throat do not seek care for this problem through a dermatology office.
The first formally organized and independent group in dermatology to address antibiotic use and resistance issues was the Scientific Panel on Antibiotic Use in Dermatology, which I put together in 2004 with James J. Leyden, MD (Philadelphia, Pennsylvania) and Guy F. Webster, MD (Hockessin, Delaware), and was comprised mostly of interested dermatologists with contributions from microbiologists and infectious disease specialists. A series of meetings, publications, and presentations have emerged from this group, which now falls under the auspices of the American Acne & Rosacea Society. Through the efforts of these organizations and other groups and companies with a strong interest in combating antibiotic resistance, we continue to see slow but steady progress in enlightening dermatology clinicians to think about if and when antibiotic therapy is needed and for how long. The subject of when antibiotics are not necessary also has been addressed, including both oral and topical antibiotics in many common scenarios encountered in dermatology practice.8 Examples include incision and drainage of an inflamed epidermal cyst without antibiotic therapy and use of white petrolatum instead of a topical antibiotic after most dermatologic procedures such as biopsies, tangential procedures, and closures after excisional procedures. Overall, the potential for topical antibiotics containing bacitracin and/or neomycin to induce allergic contact dermatitis is higher than the risk for postoperative wound infection. A major reason to avoid facilitating the emergence of antibiotic-resistant bacteria is that these organisms are efficient in packaging their resistance genes along with those from other bacteria, thus creating multidrug-resistant bacterial strains. This situation creates bigger challenges with trying to select effective therapies.
A cross-sectional analysis of antibiotics prescribed by dermatologists from January 1, 2008, to December 31, 2016, performed via a large commercial prescription claims database showed that among almost 1 million courses of oral antibiotics prescribed by approximately 12,000 unique dermatology prescribers, overall antibiotic prescribing decreased 36.6%, reflecting a drop of 1.23 courses per 100 visits, with much of the reduction occurring among extended antibiotic courses for acne and rosacea.9 Dermatology clinicians appear to be increasing their consideration of treatment alternatives such as oral spironolactone in adult female patients or earlier transition to oral isotretinoin therapy before starting another cycle with the same or a different oral antibiotic. Some have increased the use of physical device therapies. Importantly, we do not want to throw out the baby with the bathwater. Oral antibiotics remain important agents for treatment of moderate to severe inflammatory acne and in rosacea when subantibiotic-dose doxycycline is not accessible or is not effective after an adequate trial of therapy. Last but not least, a full-court press with an optimal topical regimen is the foundation of acne therapy, as monotherapy with an oral antibiotic for acne is considered dermatologic heresy and for good reason.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol Clin. 2009;27:1-15.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Del Rosso JQ, Kim GK. Topical antibiotics: therapeutic value or ecologic mischief? Dermatol Ther. 2009;22:398-406.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, England: Informa Healthcare; 2011:125-133.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139:467-471.
- Hirschmann JV. When antibiotics are unnecessary. Dermatol Clin. 2009;27:75-83.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
Treat acne aggressively upfront, expert advises
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
FROM PEDIATRIC DERMATOLOGY 2020
Trifarotene sails through 52-week acne trial
James Q. Del Rosso, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The study is noteworthy because, even though roughly half of patients with facial acne also have truncal acne, there is actually very little clinical trial data on the treatment of truncal acne other than this new long-term study and the two earlier pivotal phase 3, 12-week trials which led to the October 2019 approval of trifarotene 50 mcg/g cream (Aklief) as the first novel retinoid for acne to reach the market in 20 years, observed Dr. Del Rosso, research director at JDR Research in Las Vegas and a member of the dermatology faculty at Touro University in Henderson, Nev.
The 52-week study, known as SATISFY, began with 454 patients with moderate facial and truncal acne who treated themselves with trifarotene once daily. Among the 348 patients who completed the full year, 67% achieved a score of 0 or 1 – clear or almost clear – with at least a 2-grade improvement from baseline by Investigator’s Global Assessment on their facial acne, and 65% met the same measure of success on the trunk. Moreover, 58% of patients met that standard at both acne sites.
The IGA success rate rose throughout the study period without ever reaching a plateau. However, it should be noted that 23% of participants dropped out of the study over the course of the year.
Mean tolerability scores reflecting redness, scaling, stinging or burning, and skin dryness remained well below the threshold for mild severity, peaking at weeks 2-4 of the study. The most common treatment-related adverse events were mild to moderate itching and irritation, each occurring in less than 5% of subjects.
Trifarotene is a first-in-class retinoid that specifically targets the retinoic acid receptor gamma, the most common cutaneous retinoic acid receptor.
Dr. Del Rosso reported serving as an investigator and consultant for Galderma, which sponsored the study and markets trifarotene cream.
James Q. Del Rosso, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The study is noteworthy because, even though roughly half of patients with facial acne also have truncal acne, there is actually very little clinical trial data on the treatment of truncal acne other than this new long-term study and the two earlier pivotal phase 3, 12-week trials which led to the October 2019 approval of trifarotene 50 mcg/g cream (Aklief) as the first novel retinoid for acne to reach the market in 20 years, observed Dr. Del Rosso, research director at JDR Research in Las Vegas and a member of the dermatology faculty at Touro University in Henderson, Nev.
The 52-week study, known as SATISFY, began with 454 patients with moderate facial and truncal acne who treated themselves with trifarotene once daily. Among the 348 patients who completed the full year, 67% achieved a score of 0 or 1 – clear or almost clear – with at least a 2-grade improvement from baseline by Investigator’s Global Assessment on their facial acne, and 65% met the same measure of success on the trunk. Moreover, 58% of patients met that standard at both acne sites.
The IGA success rate rose throughout the study period without ever reaching a plateau. However, it should be noted that 23% of participants dropped out of the study over the course of the year.
Mean tolerability scores reflecting redness, scaling, stinging or burning, and skin dryness remained well below the threshold for mild severity, peaking at weeks 2-4 of the study. The most common treatment-related adverse events were mild to moderate itching and irritation, each occurring in less than 5% of subjects.
Trifarotene is a first-in-class retinoid that specifically targets the retinoic acid receptor gamma, the most common cutaneous retinoic acid receptor.
Dr. Del Rosso reported serving as an investigator and consultant for Galderma, which sponsored the study and markets trifarotene cream.
James Q. Del Rosso, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The study is noteworthy because, even though roughly half of patients with facial acne also have truncal acne, there is actually very little clinical trial data on the treatment of truncal acne other than this new long-term study and the two earlier pivotal phase 3, 12-week trials which led to the October 2019 approval of trifarotene 50 mcg/g cream (Aklief) as the first novel retinoid for acne to reach the market in 20 years, observed Dr. Del Rosso, research director at JDR Research in Las Vegas and a member of the dermatology faculty at Touro University in Henderson, Nev.
The 52-week study, known as SATISFY, began with 454 patients with moderate facial and truncal acne who treated themselves with trifarotene once daily. Among the 348 patients who completed the full year, 67% achieved a score of 0 or 1 – clear or almost clear – with at least a 2-grade improvement from baseline by Investigator’s Global Assessment on their facial acne, and 65% met the same measure of success on the trunk. Moreover, 58% of patients met that standard at both acne sites.
The IGA success rate rose throughout the study period without ever reaching a plateau. However, it should be noted that 23% of participants dropped out of the study over the course of the year.
Mean tolerability scores reflecting redness, scaling, stinging or burning, and skin dryness remained well below the threshold for mild severity, peaking at weeks 2-4 of the study. The most common treatment-related adverse events were mild to moderate itching and irritation, each occurring in less than 5% of subjects.
Trifarotene is a first-in-class retinoid that specifically targets the retinoic acid receptor gamma, the most common cutaneous retinoic acid receptor.
Dr. Del Rosso reported serving as an investigator and consultant for Galderma, which sponsored the study and markets trifarotene cream.
FROM AAD 2020
Daily Recap 6/17
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
FROM JAMA DERMATOLOGY
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness