User login
Tips for addressing uptick in mental health visits: Primary care providers collaborate, innovate
This growth in the number of patients needing behavioral health–related care is likely driven by multiple factors, including a shortage of mental health care providers, an increasing incidence of psychiatric illness, and destigmatization of mental health in general, suggested Swetha P. Iruku, MD, MPH, associate professor of family medicine and community health at the University of Pennsylvania and Penn Medicine family physician in Philadelphia.
The Centers for Disease Control and Prevention noted that “the COVID-19 pandemic has been associated with mental health challenges related to the morbidity and mortality caused by the disease and to mitigation activities, including the impact of physical distancing and stay-at-home orders,” in a Morbidity and Mortality Weekly Report.
From June 24 to 30, 2020, U.S. adults reported considerably elevated adverse mental health conditions associated with COVID-19, and symptoms of anxiety disorder and depressive disorder climbed during the months of April through June of the same year, compared with the same period in 2019, they wrote.
Even before the pandemic got underway, multiple studies of national data published this year suggested mental issues were on the rise in the United States. For example, the proportion of adult patient visits to primary care providers that addressed mental health concerns rose from 10.7% to 15.9% from 2006 to 2018, according to research published in Health Affairs. Plus, the number and proportion of pediatric acute care hospitalizations because of mental health diagnoses increased significantly between 2009 and 2019, according to a paper published in JAMA.
“I truly believe that we can’t, as primary care physicians, take care of someone’s physical health without also taking care of their mental health,” Dr. Iruku said in an interview. “It’s all intertwined.”
To rise to this challenge, PCPs first need a collaborative mindset, she suggested, as well as familiarity with available resources, both locally and virtually.
This article examines strategies for managing mental illness in primary care, outlines clinical resources, and reviews related educational opportunities.
In addition, clinical pearls are shared by Dr. Iruku and five other clinicians who provide or have provided mental health care to primary care patients or work in close collaboration with a primary care practice, including a clinical psychologist, a nurse practitioner licensed in psychiatric health, a pediatrician, and a licensed clinical social worker.
Build a network
Most of the providers interviewed cited the importance of collaboration in mental health care, particularly for complex cases.
“I would recommend [that primary care providers get] to know the psychiatric providers [in their area],” said Jessica Viton, DNP, FNP, PMHNP, who delivers mental health care through a community-based primary care practice in Colorado which she requested remain anonymous.
Dr. Iruku suggested making an in-person connection first, if possible.
“So much of what we do is ‘see one, do one, teach one,’ so learn a little bit, then go off and trial,” she said. “[It can be valuable] having someone in your back pocket that you can contact in the case of an emergency, or in a situation where you just don’t know how to tackle it.”
Screen for depression and anxiety
William J. Sieber, PhD, a clinical psychologist, director of integrated behavioral health, and professor in the department of family medicine and public health and the department of psychiatry at the University of California, San Diego, said primary care providers should screen all adult patients for depression and anxiety with the Patient Health Questionnaire (PHQ-9) and General Anxiety Disorder Assessment (GAD-7), respectively.
To save time, he suggested a cascading approach.
“In primary care, everybody’s in a hurry,” Dr. Sieber said. “[With the cascading approach,] the first two items [from each questionnaire] are given, and if a person endorses either of those items … then they are asked to complete the other items.”
Jennifer Mullally, MD, a pediatrician at Sanford Health in Fargo, N.D., uses this cascading approach to depression and anxiety screening with all her patients aged 13-18. For younger kids, she screens only those who present with signs or symptoms of mental health issues, or if the parent shares a concern.
This approach differs slightly from U.S. Preventive Services Task Force recommendations, which suggest screening for anxiety in patients aged 8-18 years and depression in patients aged 12-18 years.
Use other screening tools only as needed
Dr. Sieber, the research director for the division of family medicine at UC San Diego, collaborates regularly with primary care providers via hallway consultations, by sharing cases, and through providing oversight of psychiatric care at 13 primary care practices within the UC San Diego network. He recommended against routine screening beyond depression and anxiety in the primary care setting.
“There are a lot of screening tools,” Dr. Sieber said. “It depends on what you’re presented with. The challenge in primary care is you’re going to see all kinds of things. It’s not like running a depression clinic.”
Other than the PHQ-9 and GAD-7, he suggested primary care providers establish familiarity with screening tools for posttraumatic stress disorder and attention-deficit/hyperactivity disorder, noting again that these should be used only when one of the conditions is already suspected.
Dr. Mullally follows a similar approach with her pediatric population. In addition to the GAD-7, she investigates whether a patient has anxiety with the Screen for Child Anxiety Related Disorders (SCARED). For depression, she couples the PHQ-9 with the Columbia Suicide Severity Rating Scale.
While additional screening tools like these are readily available online, Dr. Viton suggested that they should be employed only if the provider is trained to interpret and respond to those findings, and only if they know which tool to use, and when.
For example, she has recently observed PCPs diagnosing adults with ADHD using a three-question test, when in fact a full-length, standardized instrument should be administered by a provider with necessary training.
She also pointed out that bipolar disorder continues to be underdiagnosed, possibly because of providers detecting depression using a questionnaire like the PHQ-9, while failing to inquire about manic episodes.
Leverage online resources
If depression is confirmed, Dr. Iruku often directs the patient to the Mayo Clinic Depression Medication Choice Decision Aid. This website steers patients through medication options based on their answers to a questionnaire. Choices are listed alongside possible adverse effects.
For clinician use, Dr. Iruku recommended The Waco Guide to Psychopharmacology in Primary Care, which aids clinical decision-making for mental illness and substance abuse. The app processes case details to suggest first-, second-, and third-line pharmacotherapies, as well as modifications based on patient needs.
Even with tools like these, however, a referral may be needed.
“[Primary care providers] may not be the best fit for what the patient is looking for, from a mental health or behavioral standpoint,” Dr. Sieber said.
In this case, he encourages patients to visit Psychology Today, a “quite popular portal” that helps patients locate a suitable provider based on location, insurance, driving radius, and mental health concern. This usually generates 10-20 options, Dr. Sieber said, although results can vary.
“It may be discouraging, because maybe only three [providers] pop up based on your criteria, and the closest one is miles away,” he said.
Consider virtual support
If no local psychiatric help is available, Dr. Sieber suggested virtual support, highlighting that “it’s much easier now than it was 3 or 4 years ago” to connect patients with external mental health care.
But this strategy should be reserved for cases of actual need instead of pure convenience, cautioned Dr. Viton, who noted that virtual visits may fail to capture the nuance of an in-person meeting, as body language, mode of dress, and other clues can provide insights into mental health status.
“Occasionally, I think you do have to have an in-person visit, especially when you’re developing a rapport with someone,” Dr. Viton said.
Claire McArdle, a licensed clinical social worker in Fort Collins, Colo., noted that virtual care from an outside provider may also impede the collaboration needed to effectively address mental illness.
In her 11 years in primary care at Associates in Family Medicine, Ms. McArdle had countless interactions with colleagues seeking support when managing a complex case. “I’m coaching providers, front desk staff, and nursing staff on how to interact with patients [with] behavioral health needs,” she said, citing the multitude of nonmedical factors that need to be considered, such as family relationships and patient preferences.
These unscheduled conversations with colleagues throughout the day are impossible to have when sharing a case with an unknown, remote peer.
Ms. McArdle speaks from experience. She recently resigned from Associates in Family Medicine to start her own private therapy practice after her former employer was acquired by VillageMD, a national provider that terminated employment of most other social workers in the practice and began outsourcing mental health care to Mindoula Health, a virtual provider.
Dr. Sieber offered a similar perspective on in-person collaboration as the psychiatric specialist at his center. He routinely offers on-site support for both providers and patients, serving as “another set of eyes and ears” when there is a concern about patient safety or directly managing care when a patient is hospitalized for mental illness.
While virtual solutions may fall short of in-person management, they can offer care at a scale and cost impossible through traditional practice.
This could even be free. Zero-cost, automated software now allows individuals who are uninsured or unable to afford care at least one avenue to manage their mental health concerns.
For example, Bliss is a free, 8-session, interactive online therapy program for depression that was created by the Centre for Interactive Mental Health Solutions. The program offers a tool for monitoring mood and quizzes to test understanding of personal mental health management, among other features.
More advanced programs are emerging as artificial intelligence (AI) enables dialogues between humans and machines. This is the case with Woebot, an app that asks the user about their mood throughout the day, and responds with evidence-based strategies for managing concerns, all for free at press time.
Keep learning
A range of educational options and professional resources are available for primary care providers who would like to improve their knowledge of mental health care. These include formal fellowships in primary care psychiatry/behavioral health integration, free mental health webinars, and various other opportunities.
Eric Eschweiler, DNP, APRN, FNP-C, PHN, completed the University of California, Irvine, Train New Trainers (TNT) Primary Care Psychiatry (PCP) Fellowship in 2016, when he was working as a solo nurse practitioner.
“I was drowning in practice,” said Dr. Eschweiler, director of nursing and public health outreach services at Riverside-San Bernardino County Indian Health, Grand Terrace, Calif., in an interview. “I was a solo NP. There was no physician on site. We were seeing a lot of [individuals with] schizoaffective [disorder] in downtown San Bernardino, the homeless, unhoused – a lot of substance use. I felt I needed to have the skills to be able to treat them effectively. That’s what the fellowship did.”
The skills Dr. Eschweiler learned from participating in his fellowship allowed him to manage more cases of mental illness without need for referral. When a referral was needed for a complex or severe case, he had the confidence to bridge care and collaborate more effectively with psychiatric specialists.
“It was awesome, because we were able to communicate using the same language,” Dr. Eschweiler said of these collaborations. “It’s [about] talking that same language, starting those initial treatments, and then moving forward with specialty care, and vice versa. [Psychiatric specialists] would send me patients that needed medical care because of the types of medications they were taking. And I was then very well aware of those side effects and other issues that might come up from those treatments. So it’s a two-way street.”
Dr. Eschweiler was so impressed by his fellowship that he has since ushered multiple providers through the program since transitioning to an administrative role as director of nursing.
In Fargo, where psychiatric care is sparse and wait times for referral can be months long, Dr. Mullally, like Dr. Eschweiler, knew that she needed more training in mental health.
“I don’t feel like we get enough training in residency,” Dr. Mullally said. “So you do need to look at your options for further CME.”
Out of several CME courses she has taken to further her understanding of pediatric psychiatry, Dr. Mullally recommended The Reach Institute above all others, as their courses involve in-depth discussions and valuable handouts, particularly for medication selection.
“I think that a lot of the other CMEs tend to involve a lot more PowerPoint presentations,” Dr. Mullally said. “And you don’t necessarily leave with a lot of good documents. I still use my Reach handouts. I have them sitting right next to me. I use them every single day.”
Providers interested in The Reach Institute, however, should be prepared to invest both time and money, she added, citing a 2-3 day commitment, and calling it “not cheap.” To overcome these barriers, she suggested that providers get their institution to support their attendance.
For a lighter commitment, Dr. Iruku recommended the American Academy of Family Physicians CME portal, as this offers 13 online, accredited courses covering a range of topics, from adolescent health to substance abuse disorders.
Dr. Sieber suggested that primary care providers join the Collaborative Family Healthcare Association, which aims to integrate physical and behavioral health in routine practice. CFHA, of which he is a member, offers a “bevy of different resources” for interested providers, including a conference in Phoenix this October.
The interviewees disclosed no conflicts of interest.
This growth in the number of patients needing behavioral health–related care is likely driven by multiple factors, including a shortage of mental health care providers, an increasing incidence of psychiatric illness, and destigmatization of mental health in general, suggested Swetha P. Iruku, MD, MPH, associate professor of family medicine and community health at the University of Pennsylvania and Penn Medicine family physician in Philadelphia.
The Centers for Disease Control and Prevention noted that “the COVID-19 pandemic has been associated with mental health challenges related to the morbidity and mortality caused by the disease and to mitigation activities, including the impact of physical distancing and stay-at-home orders,” in a Morbidity and Mortality Weekly Report.
From June 24 to 30, 2020, U.S. adults reported considerably elevated adverse mental health conditions associated with COVID-19, and symptoms of anxiety disorder and depressive disorder climbed during the months of April through June of the same year, compared with the same period in 2019, they wrote.
Even before the pandemic got underway, multiple studies of national data published this year suggested mental issues were on the rise in the United States. For example, the proportion of adult patient visits to primary care providers that addressed mental health concerns rose from 10.7% to 15.9% from 2006 to 2018, according to research published in Health Affairs. Plus, the number and proportion of pediatric acute care hospitalizations because of mental health diagnoses increased significantly between 2009 and 2019, according to a paper published in JAMA.
“I truly believe that we can’t, as primary care physicians, take care of someone’s physical health without also taking care of their mental health,” Dr. Iruku said in an interview. “It’s all intertwined.”
To rise to this challenge, PCPs first need a collaborative mindset, she suggested, as well as familiarity with available resources, both locally and virtually.
This article examines strategies for managing mental illness in primary care, outlines clinical resources, and reviews related educational opportunities.
In addition, clinical pearls are shared by Dr. Iruku and five other clinicians who provide or have provided mental health care to primary care patients or work in close collaboration with a primary care practice, including a clinical psychologist, a nurse practitioner licensed in psychiatric health, a pediatrician, and a licensed clinical social worker.
Build a network
Most of the providers interviewed cited the importance of collaboration in mental health care, particularly for complex cases.
“I would recommend [that primary care providers get] to know the psychiatric providers [in their area],” said Jessica Viton, DNP, FNP, PMHNP, who delivers mental health care through a community-based primary care practice in Colorado which she requested remain anonymous.
Dr. Iruku suggested making an in-person connection first, if possible.
“So much of what we do is ‘see one, do one, teach one,’ so learn a little bit, then go off and trial,” she said. “[It can be valuable] having someone in your back pocket that you can contact in the case of an emergency, or in a situation where you just don’t know how to tackle it.”
Screen for depression and anxiety
William J. Sieber, PhD, a clinical psychologist, director of integrated behavioral health, and professor in the department of family medicine and public health and the department of psychiatry at the University of California, San Diego, said primary care providers should screen all adult patients for depression and anxiety with the Patient Health Questionnaire (PHQ-9) and General Anxiety Disorder Assessment (GAD-7), respectively.
To save time, he suggested a cascading approach.
“In primary care, everybody’s in a hurry,” Dr. Sieber said. “[With the cascading approach,] the first two items [from each questionnaire] are given, and if a person endorses either of those items … then they are asked to complete the other items.”
Jennifer Mullally, MD, a pediatrician at Sanford Health in Fargo, N.D., uses this cascading approach to depression and anxiety screening with all her patients aged 13-18. For younger kids, she screens only those who present with signs or symptoms of mental health issues, or if the parent shares a concern.
This approach differs slightly from U.S. Preventive Services Task Force recommendations, which suggest screening for anxiety in patients aged 8-18 years and depression in patients aged 12-18 years.
Use other screening tools only as needed
Dr. Sieber, the research director for the division of family medicine at UC San Diego, collaborates regularly with primary care providers via hallway consultations, by sharing cases, and through providing oversight of psychiatric care at 13 primary care practices within the UC San Diego network. He recommended against routine screening beyond depression and anxiety in the primary care setting.
“There are a lot of screening tools,” Dr. Sieber said. “It depends on what you’re presented with. The challenge in primary care is you’re going to see all kinds of things. It’s not like running a depression clinic.”
Other than the PHQ-9 and GAD-7, he suggested primary care providers establish familiarity with screening tools for posttraumatic stress disorder and attention-deficit/hyperactivity disorder, noting again that these should be used only when one of the conditions is already suspected.
Dr. Mullally follows a similar approach with her pediatric population. In addition to the GAD-7, she investigates whether a patient has anxiety with the Screen for Child Anxiety Related Disorders (SCARED). For depression, she couples the PHQ-9 with the Columbia Suicide Severity Rating Scale.
While additional screening tools like these are readily available online, Dr. Viton suggested that they should be employed only if the provider is trained to interpret and respond to those findings, and only if they know which tool to use, and when.
For example, she has recently observed PCPs diagnosing adults with ADHD using a three-question test, when in fact a full-length, standardized instrument should be administered by a provider with necessary training.
She also pointed out that bipolar disorder continues to be underdiagnosed, possibly because of providers detecting depression using a questionnaire like the PHQ-9, while failing to inquire about manic episodes.
Leverage online resources
If depression is confirmed, Dr. Iruku often directs the patient to the Mayo Clinic Depression Medication Choice Decision Aid. This website steers patients through medication options based on their answers to a questionnaire. Choices are listed alongside possible adverse effects.
For clinician use, Dr. Iruku recommended The Waco Guide to Psychopharmacology in Primary Care, which aids clinical decision-making for mental illness and substance abuse. The app processes case details to suggest first-, second-, and third-line pharmacotherapies, as well as modifications based on patient needs.
Even with tools like these, however, a referral may be needed.
“[Primary care providers] may not be the best fit for what the patient is looking for, from a mental health or behavioral standpoint,” Dr. Sieber said.
In this case, he encourages patients to visit Psychology Today, a “quite popular portal” that helps patients locate a suitable provider based on location, insurance, driving radius, and mental health concern. This usually generates 10-20 options, Dr. Sieber said, although results can vary.
“It may be discouraging, because maybe only three [providers] pop up based on your criteria, and the closest one is miles away,” he said.
Consider virtual support
If no local psychiatric help is available, Dr. Sieber suggested virtual support, highlighting that “it’s much easier now than it was 3 or 4 years ago” to connect patients with external mental health care.
But this strategy should be reserved for cases of actual need instead of pure convenience, cautioned Dr. Viton, who noted that virtual visits may fail to capture the nuance of an in-person meeting, as body language, mode of dress, and other clues can provide insights into mental health status.
“Occasionally, I think you do have to have an in-person visit, especially when you’re developing a rapport with someone,” Dr. Viton said.
Claire McArdle, a licensed clinical social worker in Fort Collins, Colo., noted that virtual care from an outside provider may also impede the collaboration needed to effectively address mental illness.
In her 11 years in primary care at Associates in Family Medicine, Ms. McArdle had countless interactions with colleagues seeking support when managing a complex case. “I’m coaching providers, front desk staff, and nursing staff on how to interact with patients [with] behavioral health needs,” she said, citing the multitude of nonmedical factors that need to be considered, such as family relationships and patient preferences.
These unscheduled conversations with colleagues throughout the day are impossible to have when sharing a case with an unknown, remote peer.
Ms. McArdle speaks from experience. She recently resigned from Associates in Family Medicine to start her own private therapy practice after her former employer was acquired by VillageMD, a national provider that terminated employment of most other social workers in the practice and began outsourcing mental health care to Mindoula Health, a virtual provider.
Dr. Sieber offered a similar perspective on in-person collaboration as the psychiatric specialist at his center. He routinely offers on-site support for both providers and patients, serving as “another set of eyes and ears” when there is a concern about patient safety or directly managing care when a patient is hospitalized for mental illness.
While virtual solutions may fall short of in-person management, they can offer care at a scale and cost impossible through traditional practice.
This could even be free. Zero-cost, automated software now allows individuals who are uninsured or unable to afford care at least one avenue to manage their mental health concerns.
For example, Bliss is a free, 8-session, interactive online therapy program for depression that was created by the Centre for Interactive Mental Health Solutions. The program offers a tool for monitoring mood and quizzes to test understanding of personal mental health management, among other features.
More advanced programs are emerging as artificial intelligence (AI) enables dialogues between humans and machines. This is the case with Woebot, an app that asks the user about their mood throughout the day, and responds with evidence-based strategies for managing concerns, all for free at press time.
Keep learning
A range of educational options and professional resources are available for primary care providers who would like to improve their knowledge of mental health care. These include formal fellowships in primary care psychiatry/behavioral health integration, free mental health webinars, and various other opportunities.
Eric Eschweiler, DNP, APRN, FNP-C, PHN, completed the University of California, Irvine, Train New Trainers (TNT) Primary Care Psychiatry (PCP) Fellowship in 2016, when he was working as a solo nurse practitioner.
“I was drowning in practice,” said Dr. Eschweiler, director of nursing and public health outreach services at Riverside-San Bernardino County Indian Health, Grand Terrace, Calif., in an interview. “I was a solo NP. There was no physician on site. We were seeing a lot of [individuals with] schizoaffective [disorder] in downtown San Bernardino, the homeless, unhoused – a lot of substance use. I felt I needed to have the skills to be able to treat them effectively. That’s what the fellowship did.”
The skills Dr. Eschweiler learned from participating in his fellowship allowed him to manage more cases of mental illness without need for referral. When a referral was needed for a complex or severe case, he had the confidence to bridge care and collaborate more effectively with psychiatric specialists.
“It was awesome, because we were able to communicate using the same language,” Dr. Eschweiler said of these collaborations. “It’s [about] talking that same language, starting those initial treatments, and then moving forward with specialty care, and vice versa. [Psychiatric specialists] would send me patients that needed medical care because of the types of medications they were taking. And I was then very well aware of those side effects and other issues that might come up from those treatments. So it’s a two-way street.”
Dr. Eschweiler was so impressed by his fellowship that he has since ushered multiple providers through the program since transitioning to an administrative role as director of nursing.
In Fargo, where psychiatric care is sparse and wait times for referral can be months long, Dr. Mullally, like Dr. Eschweiler, knew that she needed more training in mental health.
“I don’t feel like we get enough training in residency,” Dr. Mullally said. “So you do need to look at your options for further CME.”
Out of several CME courses she has taken to further her understanding of pediatric psychiatry, Dr. Mullally recommended The Reach Institute above all others, as their courses involve in-depth discussions and valuable handouts, particularly for medication selection.
“I think that a lot of the other CMEs tend to involve a lot more PowerPoint presentations,” Dr. Mullally said. “And you don’t necessarily leave with a lot of good documents. I still use my Reach handouts. I have them sitting right next to me. I use them every single day.”
Providers interested in The Reach Institute, however, should be prepared to invest both time and money, she added, citing a 2-3 day commitment, and calling it “not cheap.” To overcome these barriers, she suggested that providers get their institution to support their attendance.
For a lighter commitment, Dr. Iruku recommended the American Academy of Family Physicians CME portal, as this offers 13 online, accredited courses covering a range of topics, from adolescent health to substance abuse disorders.
Dr. Sieber suggested that primary care providers join the Collaborative Family Healthcare Association, which aims to integrate physical and behavioral health in routine practice. CFHA, of which he is a member, offers a “bevy of different resources” for interested providers, including a conference in Phoenix this October.
The interviewees disclosed no conflicts of interest.
This growth in the number of patients needing behavioral health–related care is likely driven by multiple factors, including a shortage of mental health care providers, an increasing incidence of psychiatric illness, and destigmatization of mental health in general, suggested Swetha P. Iruku, MD, MPH, associate professor of family medicine and community health at the University of Pennsylvania and Penn Medicine family physician in Philadelphia.
The Centers for Disease Control and Prevention noted that “the COVID-19 pandemic has been associated with mental health challenges related to the morbidity and mortality caused by the disease and to mitigation activities, including the impact of physical distancing and stay-at-home orders,” in a Morbidity and Mortality Weekly Report.
From June 24 to 30, 2020, U.S. adults reported considerably elevated adverse mental health conditions associated with COVID-19, and symptoms of anxiety disorder and depressive disorder climbed during the months of April through June of the same year, compared with the same period in 2019, they wrote.
Even before the pandemic got underway, multiple studies of national data published this year suggested mental issues were on the rise in the United States. For example, the proportion of adult patient visits to primary care providers that addressed mental health concerns rose from 10.7% to 15.9% from 2006 to 2018, according to research published in Health Affairs. Plus, the number and proportion of pediatric acute care hospitalizations because of mental health diagnoses increased significantly between 2009 and 2019, according to a paper published in JAMA.
“I truly believe that we can’t, as primary care physicians, take care of someone’s physical health without also taking care of their mental health,” Dr. Iruku said in an interview. “It’s all intertwined.”
To rise to this challenge, PCPs first need a collaborative mindset, she suggested, as well as familiarity with available resources, both locally and virtually.
This article examines strategies for managing mental illness in primary care, outlines clinical resources, and reviews related educational opportunities.
In addition, clinical pearls are shared by Dr. Iruku and five other clinicians who provide or have provided mental health care to primary care patients or work in close collaboration with a primary care practice, including a clinical psychologist, a nurse practitioner licensed in psychiatric health, a pediatrician, and a licensed clinical social worker.
Build a network
Most of the providers interviewed cited the importance of collaboration in mental health care, particularly for complex cases.
“I would recommend [that primary care providers get] to know the psychiatric providers [in their area],” said Jessica Viton, DNP, FNP, PMHNP, who delivers mental health care through a community-based primary care practice in Colorado which she requested remain anonymous.
Dr. Iruku suggested making an in-person connection first, if possible.
“So much of what we do is ‘see one, do one, teach one,’ so learn a little bit, then go off and trial,” she said. “[It can be valuable] having someone in your back pocket that you can contact in the case of an emergency, or in a situation where you just don’t know how to tackle it.”
Screen for depression and anxiety
William J. Sieber, PhD, a clinical psychologist, director of integrated behavioral health, and professor in the department of family medicine and public health and the department of psychiatry at the University of California, San Diego, said primary care providers should screen all adult patients for depression and anxiety with the Patient Health Questionnaire (PHQ-9) and General Anxiety Disorder Assessment (GAD-7), respectively.
To save time, he suggested a cascading approach.
“In primary care, everybody’s in a hurry,” Dr. Sieber said. “[With the cascading approach,] the first two items [from each questionnaire] are given, and if a person endorses either of those items … then they are asked to complete the other items.”
Jennifer Mullally, MD, a pediatrician at Sanford Health in Fargo, N.D., uses this cascading approach to depression and anxiety screening with all her patients aged 13-18. For younger kids, she screens only those who present with signs or symptoms of mental health issues, or if the parent shares a concern.
This approach differs slightly from U.S. Preventive Services Task Force recommendations, which suggest screening for anxiety in patients aged 8-18 years and depression in patients aged 12-18 years.
Use other screening tools only as needed
Dr. Sieber, the research director for the division of family medicine at UC San Diego, collaborates regularly with primary care providers via hallway consultations, by sharing cases, and through providing oversight of psychiatric care at 13 primary care practices within the UC San Diego network. He recommended against routine screening beyond depression and anxiety in the primary care setting.
“There are a lot of screening tools,” Dr. Sieber said. “It depends on what you’re presented with. The challenge in primary care is you’re going to see all kinds of things. It’s not like running a depression clinic.”
Other than the PHQ-9 and GAD-7, he suggested primary care providers establish familiarity with screening tools for posttraumatic stress disorder and attention-deficit/hyperactivity disorder, noting again that these should be used only when one of the conditions is already suspected.
Dr. Mullally follows a similar approach with her pediatric population. In addition to the GAD-7, she investigates whether a patient has anxiety with the Screen for Child Anxiety Related Disorders (SCARED). For depression, she couples the PHQ-9 with the Columbia Suicide Severity Rating Scale.
While additional screening tools like these are readily available online, Dr. Viton suggested that they should be employed only if the provider is trained to interpret and respond to those findings, and only if they know which tool to use, and when.
For example, she has recently observed PCPs diagnosing adults with ADHD using a three-question test, when in fact a full-length, standardized instrument should be administered by a provider with necessary training.
She also pointed out that bipolar disorder continues to be underdiagnosed, possibly because of providers detecting depression using a questionnaire like the PHQ-9, while failing to inquire about manic episodes.
Leverage online resources
If depression is confirmed, Dr. Iruku often directs the patient to the Mayo Clinic Depression Medication Choice Decision Aid. This website steers patients through medication options based on their answers to a questionnaire. Choices are listed alongside possible adverse effects.
For clinician use, Dr. Iruku recommended The Waco Guide to Psychopharmacology in Primary Care, which aids clinical decision-making for mental illness and substance abuse. The app processes case details to suggest first-, second-, and third-line pharmacotherapies, as well as modifications based on patient needs.
Even with tools like these, however, a referral may be needed.
“[Primary care providers] may not be the best fit for what the patient is looking for, from a mental health or behavioral standpoint,” Dr. Sieber said.
In this case, he encourages patients to visit Psychology Today, a “quite popular portal” that helps patients locate a suitable provider based on location, insurance, driving radius, and mental health concern. This usually generates 10-20 options, Dr. Sieber said, although results can vary.
“It may be discouraging, because maybe only three [providers] pop up based on your criteria, and the closest one is miles away,” he said.
Consider virtual support
If no local psychiatric help is available, Dr. Sieber suggested virtual support, highlighting that “it’s much easier now than it was 3 or 4 years ago” to connect patients with external mental health care.
But this strategy should be reserved for cases of actual need instead of pure convenience, cautioned Dr. Viton, who noted that virtual visits may fail to capture the nuance of an in-person meeting, as body language, mode of dress, and other clues can provide insights into mental health status.
“Occasionally, I think you do have to have an in-person visit, especially when you’re developing a rapport with someone,” Dr. Viton said.
Claire McArdle, a licensed clinical social worker in Fort Collins, Colo., noted that virtual care from an outside provider may also impede the collaboration needed to effectively address mental illness.
In her 11 years in primary care at Associates in Family Medicine, Ms. McArdle had countless interactions with colleagues seeking support when managing a complex case. “I’m coaching providers, front desk staff, and nursing staff on how to interact with patients [with] behavioral health needs,” she said, citing the multitude of nonmedical factors that need to be considered, such as family relationships and patient preferences.
These unscheduled conversations with colleagues throughout the day are impossible to have when sharing a case with an unknown, remote peer.
Ms. McArdle speaks from experience. She recently resigned from Associates in Family Medicine to start her own private therapy practice after her former employer was acquired by VillageMD, a national provider that terminated employment of most other social workers in the practice and began outsourcing mental health care to Mindoula Health, a virtual provider.
Dr. Sieber offered a similar perspective on in-person collaboration as the psychiatric specialist at his center. He routinely offers on-site support for both providers and patients, serving as “another set of eyes and ears” when there is a concern about patient safety or directly managing care when a patient is hospitalized for mental illness.
While virtual solutions may fall short of in-person management, they can offer care at a scale and cost impossible through traditional practice.
This could even be free. Zero-cost, automated software now allows individuals who are uninsured or unable to afford care at least one avenue to manage their mental health concerns.
For example, Bliss is a free, 8-session, interactive online therapy program for depression that was created by the Centre for Interactive Mental Health Solutions. The program offers a tool for monitoring mood and quizzes to test understanding of personal mental health management, among other features.
More advanced programs are emerging as artificial intelligence (AI) enables dialogues between humans and machines. This is the case with Woebot, an app that asks the user about their mood throughout the day, and responds with evidence-based strategies for managing concerns, all for free at press time.
Keep learning
A range of educational options and professional resources are available for primary care providers who would like to improve their knowledge of mental health care. These include formal fellowships in primary care psychiatry/behavioral health integration, free mental health webinars, and various other opportunities.
Eric Eschweiler, DNP, APRN, FNP-C, PHN, completed the University of California, Irvine, Train New Trainers (TNT) Primary Care Psychiatry (PCP) Fellowship in 2016, when he was working as a solo nurse practitioner.
“I was drowning in practice,” said Dr. Eschweiler, director of nursing and public health outreach services at Riverside-San Bernardino County Indian Health, Grand Terrace, Calif., in an interview. “I was a solo NP. There was no physician on site. We were seeing a lot of [individuals with] schizoaffective [disorder] in downtown San Bernardino, the homeless, unhoused – a lot of substance use. I felt I needed to have the skills to be able to treat them effectively. That’s what the fellowship did.”
The skills Dr. Eschweiler learned from participating in his fellowship allowed him to manage more cases of mental illness without need for referral. When a referral was needed for a complex or severe case, he had the confidence to bridge care and collaborate more effectively with psychiatric specialists.
“It was awesome, because we were able to communicate using the same language,” Dr. Eschweiler said of these collaborations. “It’s [about] talking that same language, starting those initial treatments, and then moving forward with specialty care, and vice versa. [Psychiatric specialists] would send me patients that needed medical care because of the types of medications they were taking. And I was then very well aware of those side effects and other issues that might come up from those treatments. So it’s a two-way street.”
Dr. Eschweiler was so impressed by his fellowship that he has since ushered multiple providers through the program since transitioning to an administrative role as director of nursing.
In Fargo, where psychiatric care is sparse and wait times for referral can be months long, Dr. Mullally, like Dr. Eschweiler, knew that she needed more training in mental health.
“I don’t feel like we get enough training in residency,” Dr. Mullally said. “So you do need to look at your options for further CME.”
Out of several CME courses she has taken to further her understanding of pediatric psychiatry, Dr. Mullally recommended The Reach Institute above all others, as their courses involve in-depth discussions and valuable handouts, particularly for medication selection.
“I think that a lot of the other CMEs tend to involve a lot more PowerPoint presentations,” Dr. Mullally said. “And you don’t necessarily leave with a lot of good documents. I still use my Reach handouts. I have them sitting right next to me. I use them every single day.”
Providers interested in The Reach Institute, however, should be prepared to invest both time and money, she added, citing a 2-3 day commitment, and calling it “not cheap.” To overcome these barriers, she suggested that providers get their institution to support their attendance.
For a lighter commitment, Dr. Iruku recommended the American Academy of Family Physicians CME portal, as this offers 13 online, accredited courses covering a range of topics, from adolescent health to substance abuse disorders.
Dr. Sieber suggested that primary care providers join the Collaborative Family Healthcare Association, which aims to integrate physical and behavioral health in routine practice. CFHA, of which he is a member, offers a “bevy of different resources” for interested providers, including a conference in Phoenix this October.
The interviewees disclosed no conflicts of interest.
Link between bipolar disorder and CVD mortality explained?
in new findings that may explain the “excessive and premature mortality” related to heart disease in this patient population.
The investigators found that higher reactive hyperemia index (RHI) scores, a measure of endothelial function, were tied to mood severity in patients with higher mania, but not depression scores. These findings persisted even after accounting for medications, obesity, and other cardiovascular risk factors (CVRFs).
“From a clinical perspective, these findings highlight the potential value of integrating vascular health in the assessment and management of youth with BD, and from a scientific perspective, these findings call for additional research focused on shared biological mechanisms linking vascular health and mood symptoms of BD,” senior investigator Benjamin Goldstein, MD, PhD, full professor of psychiatry, pharmacology, and psychological clinical science, University of Toronto, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
‘Excessively present’
BD is associated with “excessive and premature cardiovascular mortality” and CVD is “excessively present” in BD, exceeding what can be explained by traditional cardiovascular risk factors, psychiatric medications, and substance use, the researchers noted.
“In adults, more severe mood symptoms increase the risk of future CVD. Our focus on endothelial function rose due to the fact that CVD is rare in youth, whereas endothelial dysfunction – considered a precursor of CVD – can be assessed in youth,” said Dr. Goldstein, who holds the RBC Investments Chair in children’s mental health and developmental psychopathology at the Centre for Addiction and Mental Health, Toronto, where he is director of the Centre for Youth Bipolar Disorder.
For this reason, he and his colleagues were “interested in researching whether endothelial dysfunction is associated with mood symptoms in youth with BD.” Ultimately, the motivation was to “inspire new therapeutic opportunities that may improve both cardiovascular and mental health simultaneously.”
To investigate the question, the researchers studied 209 youth aged 13-20 years (n = 114 with BD and 94 healthy controls [HCs]).
In the BD group, there were 34 BD-euthymia, 36 BD-depressed, and 44 BD-hypomanic/mixed; and within the groups who had depression or hypomania/mixed features, 72 were experiencing clinically significant depression.
Participants had to be free of chronic inflammatory illness, use of medications that might be addressing traditional CVRFs, recent infectious diseases, or neurologic conditions.
Participants’ bipolar symptoms, psychosocial functioning, and family history were assessed. In addition, they were asked about treatment, physical and/or sexual abuse, smoking status, and socioeconomic status. Height, weight, waist circumference, blood pressure, and blood tests to assess CVRFs, including C-reactive protein (CRP), were also assessed. RHI was measured via pulse amplitude tonometry, with lower values indicating poorer endothelial function.
Positive affect beneficial?
Compared with HCs, there were fewer White participants in the BD group (78% vs. 55%; P < .001). The BD group also had higher Tanner stage development scores (stage 5: 65% vs. 35%; P = .03; V = 0.21), higher body mass index (BMI, 24.4 ± 4.6 vs. 22.0 ± 4.2; P < .001; d = 0.53), and higher CRP (1.94 ± 3.99 vs. 0.76 ± 0.86; P = .009; d = –0.40).
After controlling for age, sex, and BMI (F3,202 = 4.47; P = .005; np2 = 0.06), the researchers found significant between-group differences in RHI.
Post hoc pairwise comparisons showed RHI to be significantly lower in the BD-depressed versus the HC group (P = .04; d = 0.4). Moreover, the BD-hypomanic/mixed group had significantly higher RHI, compared with the other BD groups and the HC group.
RHI was associated with higher mania scores (beta, 0.26; P = .006), but there was no similar significant association with depression mood scores (beta, 0.01; P = .90).
The mood state differences in RHI and the RHI-mania association remained significant in sensitivity analyses examining the effect of current medication use as well as CVRFs, including lipids, CRP, and blood pressure on RHI.
“We found that youth with BD experiencing a depressive episode had lower endothelial function, whereas youth with BD experiencing a hypomanic/mixed episode had higher endothelial function, as compared to healthy youth,” Dr. Goldstein said.
There are several mechanisms potentially underlying the association between endothelial function and hypomania, the investigators noted. For example, positive affect is associated with increased endothelial function in normative samples, so hypomanic symptoms, including elation, may have similar beneficial associations, although those benefits likely do not extend to mania, which has been associated with cardiovascular risk.
They also point to several limitations in the study. The cross-sectional design “precludes making inferences regarding the temporal relationship between RHI and mood.” Moreover, the study focused only on hypomania, so “we cannot draw conclusions about mania.” In addition, the HC group had a “significantly higher proportion” of White participants, and a lower Tanner stage, so it “may not be a representative control sample.”
Nevertheless, the researchers concluded that the study “adds to the existing evidence for the potential value of integrating cardiovascular-related therapeutic approaches in BD,” noting that further research is needed to elucidate the mechanisms of the association.
Observable changes in youth
In a comment, Jess G Fiedorowicz, MD, PhD, head and chief, department of mental health, Ottawa Hospital Research Institute, noted that individuals with BD “have a much higher risk of CVD, which tends to develop earlier and shortens life expectancy by more than a decade.”
This cardiovascular risk “appears to be acquired over the long-term course of illness and proportionate to the persistence and severity of mood symptoms, which implies that mood syndromes, such as depression and mania, themselves may induce changes in the body relevant to CVD,” said Dr. Fiedorowicz, who is also a professor in the department of psychiatry and senior research chair in adult psychiatry at the Brain and Mind Research Institute, University of Ottawa, and was not involved with the study.
The study “adds to a growing body of evidence that mood syndromes may enact physiological changes that may be relevant to risk of CVD. One important aspect of this study is that this can even be observed in young sample,” he said.
This study was funded by the Canadian Institutes of Health Research and a Miner’s Lamp Innovation Fund from the University of Toronto. Dr. Goldstein and coauthors declare no relevant financial relationships. Dr. Fiedorowicz receives an honorarium from Elsevier for his work as editor-in-chief of the Journal of Psychosomatic Research.
A version of this article first appeared on Medscape.com.
in new findings that may explain the “excessive and premature mortality” related to heart disease in this patient population.
The investigators found that higher reactive hyperemia index (RHI) scores, a measure of endothelial function, were tied to mood severity in patients with higher mania, but not depression scores. These findings persisted even after accounting for medications, obesity, and other cardiovascular risk factors (CVRFs).
“From a clinical perspective, these findings highlight the potential value of integrating vascular health in the assessment and management of youth with BD, and from a scientific perspective, these findings call for additional research focused on shared biological mechanisms linking vascular health and mood symptoms of BD,” senior investigator Benjamin Goldstein, MD, PhD, full professor of psychiatry, pharmacology, and psychological clinical science, University of Toronto, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
‘Excessively present’
BD is associated with “excessive and premature cardiovascular mortality” and CVD is “excessively present” in BD, exceeding what can be explained by traditional cardiovascular risk factors, psychiatric medications, and substance use, the researchers noted.
“In adults, more severe mood symptoms increase the risk of future CVD. Our focus on endothelial function rose due to the fact that CVD is rare in youth, whereas endothelial dysfunction – considered a precursor of CVD – can be assessed in youth,” said Dr. Goldstein, who holds the RBC Investments Chair in children’s mental health and developmental psychopathology at the Centre for Addiction and Mental Health, Toronto, where he is director of the Centre for Youth Bipolar Disorder.
For this reason, he and his colleagues were “interested in researching whether endothelial dysfunction is associated with mood symptoms in youth with BD.” Ultimately, the motivation was to “inspire new therapeutic opportunities that may improve both cardiovascular and mental health simultaneously.”
To investigate the question, the researchers studied 209 youth aged 13-20 years (n = 114 with BD and 94 healthy controls [HCs]).
In the BD group, there were 34 BD-euthymia, 36 BD-depressed, and 44 BD-hypomanic/mixed; and within the groups who had depression or hypomania/mixed features, 72 were experiencing clinically significant depression.
Participants had to be free of chronic inflammatory illness, use of medications that might be addressing traditional CVRFs, recent infectious diseases, or neurologic conditions.
Participants’ bipolar symptoms, psychosocial functioning, and family history were assessed. In addition, they were asked about treatment, physical and/or sexual abuse, smoking status, and socioeconomic status. Height, weight, waist circumference, blood pressure, and blood tests to assess CVRFs, including C-reactive protein (CRP), were also assessed. RHI was measured via pulse amplitude tonometry, with lower values indicating poorer endothelial function.
Positive affect beneficial?
Compared with HCs, there were fewer White participants in the BD group (78% vs. 55%; P < .001). The BD group also had higher Tanner stage development scores (stage 5: 65% vs. 35%; P = .03; V = 0.21), higher body mass index (BMI, 24.4 ± 4.6 vs. 22.0 ± 4.2; P < .001; d = 0.53), and higher CRP (1.94 ± 3.99 vs. 0.76 ± 0.86; P = .009; d = –0.40).
After controlling for age, sex, and BMI (F3,202 = 4.47; P = .005; np2 = 0.06), the researchers found significant between-group differences in RHI.
Post hoc pairwise comparisons showed RHI to be significantly lower in the BD-depressed versus the HC group (P = .04; d = 0.4). Moreover, the BD-hypomanic/mixed group had significantly higher RHI, compared with the other BD groups and the HC group.
RHI was associated with higher mania scores (beta, 0.26; P = .006), but there was no similar significant association with depression mood scores (beta, 0.01; P = .90).
The mood state differences in RHI and the RHI-mania association remained significant in sensitivity analyses examining the effect of current medication use as well as CVRFs, including lipids, CRP, and blood pressure on RHI.
“We found that youth with BD experiencing a depressive episode had lower endothelial function, whereas youth with BD experiencing a hypomanic/mixed episode had higher endothelial function, as compared to healthy youth,” Dr. Goldstein said.
There are several mechanisms potentially underlying the association between endothelial function and hypomania, the investigators noted. For example, positive affect is associated with increased endothelial function in normative samples, so hypomanic symptoms, including elation, may have similar beneficial associations, although those benefits likely do not extend to mania, which has been associated with cardiovascular risk.
They also point to several limitations in the study. The cross-sectional design “precludes making inferences regarding the temporal relationship between RHI and mood.” Moreover, the study focused only on hypomania, so “we cannot draw conclusions about mania.” In addition, the HC group had a “significantly higher proportion” of White participants, and a lower Tanner stage, so it “may not be a representative control sample.”
Nevertheless, the researchers concluded that the study “adds to the existing evidence for the potential value of integrating cardiovascular-related therapeutic approaches in BD,” noting that further research is needed to elucidate the mechanisms of the association.
Observable changes in youth
In a comment, Jess G Fiedorowicz, MD, PhD, head and chief, department of mental health, Ottawa Hospital Research Institute, noted that individuals with BD “have a much higher risk of CVD, which tends to develop earlier and shortens life expectancy by more than a decade.”
This cardiovascular risk “appears to be acquired over the long-term course of illness and proportionate to the persistence and severity of mood symptoms, which implies that mood syndromes, such as depression and mania, themselves may induce changes in the body relevant to CVD,” said Dr. Fiedorowicz, who is also a professor in the department of psychiatry and senior research chair in adult psychiatry at the Brain and Mind Research Institute, University of Ottawa, and was not involved with the study.
The study “adds to a growing body of evidence that mood syndromes may enact physiological changes that may be relevant to risk of CVD. One important aspect of this study is that this can even be observed in young sample,” he said.
This study was funded by the Canadian Institutes of Health Research and a Miner’s Lamp Innovation Fund from the University of Toronto. Dr. Goldstein and coauthors declare no relevant financial relationships. Dr. Fiedorowicz receives an honorarium from Elsevier for his work as editor-in-chief of the Journal of Psychosomatic Research.
A version of this article first appeared on Medscape.com.
in new findings that may explain the “excessive and premature mortality” related to heart disease in this patient population.
The investigators found that higher reactive hyperemia index (RHI) scores, a measure of endothelial function, were tied to mood severity in patients with higher mania, but not depression scores. These findings persisted even after accounting for medications, obesity, and other cardiovascular risk factors (CVRFs).
“From a clinical perspective, these findings highlight the potential value of integrating vascular health in the assessment and management of youth with BD, and from a scientific perspective, these findings call for additional research focused on shared biological mechanisms linking vascular health and mood symptoms of BD,” senior investigator Benjamin Goldstein, MD, PhD, full professor of psychiatry, pharmacology, and psychological clinical science, University of Toronto, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
‘Excessively present’
BD is associated with “excessive and premature cardiovascular mortality” and CVD is “excessively present” in BD, exceeding what can be explained by traditional cardiovascular risk factors, psychiatric medications, and substance use, the researchers noted.
“In adults, more severe mood symptoms increase the risk of future CVD. Our focus on endothelial function rose due to the fact that CVD is rare in youth, whereas endothelial dysfunction – considered a precursor of CVD – can be assessed in youth,” said Dr. Goldstein, who holds the RBC Investments Chair in children’s mental health and developmental psychopathology at the Centre for Addiction and Mental Health, Toronto, where he is director of the Centre for Youth Bipolar Disorder.
For this reason, he and his colleagues were “interested in researching whether endothelial dysfunction is associated with mood symptoms in youth with BD.” Ultimately, the motivation was to “inspire new therapeutic opportunities that may improve both cardiovascular and mental health simultaneously.”
To investigate the question, the researchers studied 209 youth aged 13-20 years (n = 114 with BD and 94 healthy controls [HCs]).
In the BD group, there were 34 BD-euthymia, 36 BD-depressed, and 44 BD-hypomanic/mixed; and within the groups who had depression or hypomania/mixed features, 72 were experiencing clinically significant depression.
Participants had to be free of chronic inflammatory illness, use of medications that might be addressing traditional CVRFs, recent infectious diseases, or neurologic conditions.
Participants’ bipolar symptoms, psychosocial functioning, and family history were assessed. In addition, they were asked about treatment, physical and/or sexual abuse, smoking status, and socioeconomic status. Height, weight, waist circumference, blood pressure, and blood tests to assess CVRFs, including C-reactive protein (CRP), were also assessed. RHI was measured via pulse amplitude tonometry, with lower values indicating poorer endothelial function.
Positive affect beneficial?
Compared with HCs, there were fewer White participants in the BD group (78% vs. 55%; P < .001). The BD group also had higher Tanner stage development scores (stage 5: 65% vs. 35%; P = .03; V = 0.21), higher body mass index (BMI, 24.4 ± 4.6 vs. 22.0 ± 4.2; P < .001; d = 0.53), and higher CRP (1.94 ± 3.99 vs. 0.76 ± 0.86; P = .009; d = –0.40).
After controlling for age, sex, and BMI (F3,202 = 4.47; P = .005; np2 = 0.06), the researchers found significant between-group differences in RHI.
Post hoc pairwise comparisons showed RHI to be significantly lower in the BD-depressed versus the HC group (P = .04; d = 0.4). Moreover, the BD-hypomanic/mixed group had significantly higher RHI, compared with the other BD groups and the HC group.
RHI was associated with higher mania scores (beta, 0.26; P = .006), but there was no similar significant association with depression mood scores (beta, 0.01; P = .90).
The mood state differences in RHI and the RHI-mania association remained significant in sensitivity analyses examining the effect of current medication use as well as CVRFs, including lipids, CRP, and blood pressure on RHI.
“We found that youth with BD experiencing a depressive episode had lower endothelial function, whereas youth with BD experiencing a hypomanic/mixed episode had higher endothelial function, as compared to healthy youth,” Dr. Goldstein said.
There are several mechanisms potentially underlying the association between endothelial function and hypomania, the investigators noted. For example, positive affect is associated with increased endothelial function in normative samples, so hypomanic symptoms, including elation, may have similar beneficial associations, although those benefits likely do not extend to mania, which has been associated with cardiovascular risk.
They also point to several limitations in the study. The cross-sectional design “precludes making inferences regarding the temporal relationship between RHI and mood.” Moreover, the study focused only on hypomania, so “we cannot draw conclusions about mania.” In addition, the HC group had a “significantly higher proportion” of White participants, and a lower Tanner stage, so it “may not be a representative control sample.”
Nevertheless, the researchers concluded that the study “adds to the existing evidence for the potential value of integrating cardiovascular-related therapeutic approaches in BD,” noting that further research is needed to elucidate the mechanisms of the association.
Observable changes in youth
In a comment, Jess G Fiedorowicz, MD, PhD, head and chief, department of mental health, Ottawa Hospital Research Institute, noted that individuals with BD “have a much higher risk of CVD, which tends to develop earlier and shortens life expectancy by more than a decade.”
This cardiovascular risk “appears to be acquired over the long-term course of illness and proportionate to the persistence and severity of mood symptoms, which implies that mood syndromes, such as depression and mania, themselves may induce changes in the body relevant to CVD,” said Dr. Fiedorowicz, who is also a professor in the department of psychiatry and senior research chair in adult psychiatry at the Brain and Mind Research Institute, University of Ottawa, and was not involved with the study.
The study “adds to a growing body of evidence that mood syndromes may enact physiological changes that may be relevant to risk of CVD. One important aspect of this study is that this can even be observed in young sample,” he said.
This study was funded by the Canadian Institutes of Health Research and a Miner’s Lamp Innovation Fund from the University of Toronto. Dr. Goldstein and coauthors declare no relevant financial relationships. Dr. Fiedorowicz receives an honorarium from Elsevier for his work as editor-in-chief of the Journal of Psychosomatic Research.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Quick medication, better communication linked to less violence at inpatient psych unit
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
AT APA 2023
Widespread prescribing of stimulants with other CNS-active meds
Investigators analyzed prescription drug claims for over 9.1 million U.S. adults over a 1-year period and found that 276,223 (3%) had used a schedule II stimulant, such as methylphenidate and amphetamines, during that time. Of these 276,223 patients, 45% combined these agents with one or more additional CNS-active drugs and almost 25% were simultaneously using two or more additional CNS-active drugs.
Close to half of the stimulant users were taking an antidepressant, while close to one-third filled prescriptions for anxiolytic/sedative/hypnotic meditations, and one-fifth received opioid prescriptions.
The widespread, often off-label use of these stimulants in combination therapy with antidepressants, anxiolytics, opioids, and other psychoactive drugs, “reveals new patterns of utilization beyond the approved use of stimulants as monotherapy for ADHD, but because there are so few studies of these kinds of combination therapy, both the advantages and additional risks [of this type of prescribing] remain unknown,” study investigator Thomas J. Moore, AB, faculty associate in epidemiology, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins Medicine, Baltimore, told this news organization.
The study was published online in BMJ Open.
‘Dangerous’ substances
Amphetamines and methylphenidate are CNS stimulants that have been in use for almost a century. Like opioids and barbiturates, they’re considered “dangerous” and classified as schedule II Controlled Substances because of their high potential for abuse.
Over many years, these stimulants have been used for multiple purposes, including nasal congestion, narcolepsy, appetite suppression, binge eating, depression, senile behavior, lethargy, and ADHD, the researchers note.
Observational studies suggest medical use of these agents has been increasing in the United States. The investigators conducted previous research that revealed a 79% increase from 2013 to 2018 in the number of adults who self-report their use. The current study, said Mr. Moore, explores how these stimulants are being used.
For the study, data was extracted from the MarketScan 2019 and 2020 Commercial Claims and Encounters Databases, focusing on 9.1 million adults aged 19-64 years who were continuously enrolled in an included commercial benefit plan from Oct. 1, 2019 to Dec. 31, 2020.
The primary outcome consisted of an outpatient prescription claim, service date, and days’ supply for the CNS-active drugs.
The researchers defined “combination-2” therapy as 60 or more days of combination treatment with a schedule II stimulant and at least one additional CNS-active drug. “Combination-3” therapy was defined as the addition of at least two additional CNS-active drugs.
The researchers used service date and days’ supply to examine the number of stimulant and other CNS-active drugs for each of the days of 2020.
CNS-active drug classes included antidepressants, anxiolytics/sedatives/hypnotics, antipsychotics, opioids, anticonvulsants, and other CNS-active drugs.
Prescribing cascade
Of the total number of adults enrolled, 3% (n = 276,223) were taking schedule II stimulants during 2020, with a median of 8 (interquartile range, 4-11) prescriptions. These drugs provided 227 (IQR, 110-322) treatment days of exposure.
Among those taking stimulants 45.5% combined the use of at least one additional CNS-active drug for a median of 213 (IQR, 126-301) treatment days; and 24.3% used at least two additional CNS-active drugs for a median of 182 (IQR, 108-276) days.
“Clinicians should beware of the prescribing cascade. Sometimes it begins with an antidepressant that causes too much sedation, so a stimulant gets added, which leads to insomnia, so alprazolam gets added to the mix,” Mr. Moore said.
He cautioned that this “leaves a patient with multiple drugs, all with discontinuation effects of different kinds and clashing effects.”
These new findings, the investigators note, “add new public health concerns to those raised by our previous study. ... this more-detailed profile reveals several new patterns.”
Most patients become “long-term users” once treatment has started, with 75% continuing for a 1-year period.
“This underscores the possible risks of nonmedical use and dependence that have warranted the classification of these drugs as having high potential for psychological or physical dependence and their prominent appearance in toxicology drug rankings of fatal overdose cases,” they write.
They note that the data “do not indicate which intervention may have come first – a stimulant added to compensate for excess sedation from the benzodiazepine, or the alprazolam added to calm excessive CNS stimulation and/or insomnia from the stimulants or other drugs.”
Several limitations cited by the authors include the fact that, although the population encompassed 9.1 million people, it “may not represent all commercially insured adults,” and it doesn’t include people who aren’t covered by commercial insurance.
Moreover, the MarketScan dataset included up to four diagnosis codes for each outpatient and emergency department encounter; therefore, it was not possible to directly link the diagnoses to specific prescription drug claims, and thus the diagnoses were not evaluated.
“Since many providers will not accept a drug claim for a schedule II stimulant without an on-label diagnosis of ADHD,” the authors suspect that “large numbers of this diagnosis were present.”
Complex prescribing regimens
Mark Olfson, MD, MPH, professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said the report “highlights the pharmacological complexity of adults who are treated with stimulants.”
Dr. Olfson, who is a research psychiatrist at the New York State Psychiatric Institute, New York, and was not involved with the study, observed there is “evidence to support stimulants as an adjunctive therapy for treatment-resistant unipolar depression in older adults.”
However, he added, “this indication is unlikely to fully explain the high proportion of nonelderly, stimulant-treated adults who also receive antidepressants.”
These new findings “call for research to increase our understanding of the clinical contexts that motivate these complex prescribing regimens as well as their effectiveness and safety,” said Dr. Olfson.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. Mr. Moore declares no relevant financial relationships. Coauthor G. Caleb Alexander, MD, is past chair and a current member of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. Dr. Olfson declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed prescription drug claims for over 9.1 million U.S. adults over a 1-year period and found that 276,223 (3%) had used a schedule II stimulant, such as methylphenidate and amphetamines, during that time. Of these 276,223 patients, 45% combined these agents with one or more additional CNS-active drugs and almost 25% were simultaneously using two or more additional CNS-active drugs.
Close to half of the stimulant users were taking an antidepressant, while close to one-third filled prescriptions for anxiolytic/sedative/hypnotic meditations, and one-fifth received opioid prescriptions.
The widespread, often off-label use of these stimulants in combination therapy with antidepressants, anxiolytics, opioids, and other psychoactive drugs, “reveals new patterns of utilization beyond the approved use of stimulants as monotherapy for ADHD, but because there are so few studies of these kinds of combination therapy, both the advantages and additional risks [of this type of prescribing] remain unknown,” study investigator Thomas J. Moore, AB, faculty associate in epidemiology, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins Medicine, Baltimore, told this news organization.
The study was published online in BMJ Open.
‘Dangerous’ substances
Amphetamines and methylphenidate are CNS stimulants that have been in use for almost a century. Like opioids and barbiturates, they’re considered “dangerous” and classified as schedule II Controlled Substances because of their high potential for abuse.
Over many years, these stimulants have been used for multiple purposes, including nasal congestion, narcolepsy, appetite suppression, binge eating, depression, senile behavior, lethargy, and ADHD, the researchers note.
Observational studies suggest medical use of these agents has been increasing in the United States. The investigators conducted previous research that revealed a 79% increase from 2013 to 2018 in the number of adults who self-report their use. The current study, said Mr. Moore, explores how these stimulants are being used.
For the study, data was extracted from the MarketScan 2019 and 2020 Commercial Claims and Encounters Databases, focusing on 9.1 million adults aged 19-64 years who were continuously enrolled in an included commercial benefit plan from Oct. 1, 2019 to Dec. 31, 2020.
The primary outcome consisted of an outpatient prescription claim, service date, and days’ supply for the CNS-active drugs.
The researchers defined “combination-2” therapy as 60 or more days of combination treatment with a schedule II stimulant and at least one additional CNS-active drug. “Combination-3” therapy was defined as the addition of at least two additional CNS-active drugs.
The researchers used service date and days’ supply to examine the number of stimulant and other CNS-active drugs for each of the days of 2020.
CNS-active drug classes included antidepressants, anxiolytics/sedatives/hypnotics, antipsychotics, opioids, anticonvulsants, and other CNS-active drugs.
Prescribing cascade
Of the total number of adults enrolled, 3% (n = 276,223) were taking schedule II stimulants during 2020, with a median of 8 (interquartile range, 4-11) prescriptions. These drugs provided 227 (IQR, 110-322) treatment days of exposure.
Among those taking stimulants 45.5% combined the use of at least one additional CNS-active drug for a median of 213 (IQR, 126-301) treatment days; and 24.3% used at least two additional CNS-active drugs for a median of 182 (IQR, 108-276) days.
“Clinicians should beware of the prescribing cascade. Sometimes it begins with an antidepressant that causes too much sedation, so a stimulant gets added, which leads to insomnia, so alprazolam gets added to the mix,” Mr. Moore said.
He cautioned that this “leaves a patient with multiple drugs, all with discontinuation effects of different kinds and clashing effects.”
These new findings, the investigators note, “add new public health concerns to those raised by our previous study. ... this more-detailed profile reveals several new patterns.”
Most patients become “long-term users” once treatment has started, with 75% continuing for a 1-year period.
“This underscores the possible risks of nonmedical use and dependence that have warranted the classification of these drugs as having high potential for psychological or physical dependence and their prominent appearance in toxicology drug rankings of fatal overdose cases,” they write.
They note that the data “do not indicate which intervention may have come first – a stimulant added to compensate for excess sedation from the benzodiazepine, or the alprazolam added to calm excessive CNS stimulation and/or insomnia from the stimulants or other drugs.”
Several limitations cited by the authors include the fact that, although the population encompassed 9.1 million people, it “may not represent all commercially insured adults,” and it doesn’t include people who aren’t covered by commercial insurance.
Moreover, the MarketScan dataset included up to four diagnosis codes for each outpatient and emergency department encounter; therefore, it was not possible to directly link the diagnoses to specific prescription drug claims, and thus the diagnoses were not evaluated.
“Since many providers will not accept a drug claim for a schedule II stimulant without an on-label diagnosis of ADHD,” the authors suspect that “large numbers of this diagnosis were present.”
Complex prescribing regimens
Mark Olfson, MD, MPH, professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said the report “highlights the pharmacological complexity of adults who are treated with stimulants.”
Dr. Olfson, who is a research psychiatrist at the New York State Psychiatric Institute, New York, and was not involved with the study, observed there is “evidence to support stimulants as an adjunctive therapy for treatment-resistant unipolar depression in older adults.”
However, he added, “this indication is unlikely to fully explain the high proportion of nonelderly, stimulant-treated adults who also receive antidepressants.”
These new findings “call for research to increase our understanding of the clinical contexts that motivate these complex prescribing regimens as well as their effectiveness and safety,” said Dr. Olfson.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. Mr. Moore declares no relevant financial relationships. Coauthor G. Caleb Alexander, MD, is past chair and a current member of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. Dr. Olfson declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed prescription drug claims for over 9.1 million U.S. adults over a 1-year period and found that 276,223 (3%) had used a schedule II stimulant, such as methylphenidate and amphetamines, during that time. Of these 276,223 patients, 45% combined these agents with one or more additional CNS-active drugs and almost 25% were simultaneously using two or more additional CNS-active drugs.
Close to half of the stimulant users were taking an antidepressant, while close to one-third filled prescriptions for anxiolytic/sedative/hypnotic meditations, and one-fifth received opioid prescriptions.
The widespread, often off-label use of these stimulants in combination therapy with antidepressants, anxiolytics, opioids, and other psychoactive drugs, “reveals new patterns of utilization beyond the approved use of stimulants as monotherapy for ADHD, but because there are so few studies of these kinds of combination therapy, both the advantages and additional risks [of this type of prescribing] remain unknown,” study investigator Thomas J. Moore, AB, faculty associate in epidemiology, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins Medicine, Baltimore, told this news organization.
The study was published online in BMJ Open.
‘Dangerous’ substances
Amphetamines and methylphenidate are CNS stimulants that have been in use for almost a century. Like opioids and barbiturates, they’re considered “dangerous” and classified as schedule II Controlled Substances because of their high potential for abuse.
Over many years, these stimulants have been used for multiple purposes, including nasal congestion, narcolepsy, appetite suppression, binge eating, depression, senile behavior, lethargy, and ADHD, the researchers note.
Observational studies suggest medical use of these agents has been increasing in the United States. The investigators conducted previous research that revealed a 79% increase from 2013 to 2018 in the number of adults who self-report their use. The current study, said Mr. Moore, explores how these stimulants are being used.
For the study, data was extracted from the MarketScan 2019 and 2020 Commercial Claims and Encounters Databases, focusing on 9.1 million adults aged 19-64 years who were continuously enrolled in an included commercial benefit plan from Oct. 1, 2019 to Dec. 31, 2020.
The primary outcome consisted of an outpatient prescription claim, service date, and days’ supply for the CNS-active drugs.
The researchers defined “combination-2” therapy as 60 or more days of combination treatment with a schedule II stimulant and at least one additional CNS-active drug. “Combination-3” therapy was defined as the addition of at least two additional CNS-active drugs.
The researchers used service date and days’ supply to examine the number of stimulant and other CNS-active drugs for each of the days of 2020.
CNS-active drug classes included antidepressants, anxiolytics/sedatives/hypnotics, antipsychotics, opioids, anticonvulsants, and other CNS-active drugs.
Prescribing cascade
Of the total number of adults enrolled, 3% (n = 276,223) were taking schedule II stimulants during 2020, with a median of 8 (interquartile range, 4-11) prescriptions. These drugs provided 227 (IQR, 110-322) treatment days of exposure.
Among those taking stimulants 45.5% combined the use of at least one additional CNS-active drug for a median of 213 (IQR, 126-301) treatment days; and 24.3% used at least two additional CNS-active drugs for a median of 182 (IQR, 108-276) days.
“Clinicians should beware of the prescribing cascade. Sometimes it begins with an antidepressant that causes too much sedation, so a stimulant gets added, which leads to insomnia, so alprazolam gets added to the mix,” Mr. Moore said.
He cautioned that this “leaves a patient with multiple drugs, all with discontinuation effects of different kinds and clashing effects.”
These new findings, the investigators note, “add new public health concerns to those raised by our previous study. ... this more-detailed profile reveals several new patterns.”
Most patients become “long-term users” once treatment has started, with 75% continuing for a 1-year period.
“This underscores the possible risks of nonmedical use and dependence that have warranted the classification of these drugs as having high potential for psychological or physical dependence and their prominent appearance in toxicology drug rankings of fatal overdose cases,” they write.
They note that the data “do not indicate which intervention may have come first – a stimulant added to compensate for excess sedation from the benzodiazepine, or the alprazolam added to calm excessive CNS stimulation and/or insomnia from the stimulants or other drugs.”
Several limitations cited by the authors include the fact that, although the population encompassed 9.1 million people, it “may not represent all commercially insured adults,” and it doesn’t include people who aren’t covered by commercial insurance.
Moreover, the MarketScan dataset included up to four diagnosis codes for each outpatient and emergency department encounter; therefore, it was not possible to directly link the diagnoses to specific prescription drug claims, and thus the diagnoses were not evaluated.
“Since many providers will not accept a drug claim for a schedule II stimulant without an on-label diagnosis of ADHD,” the authors suspect that “large numbers of this diagnosis were present.”
Complex prescribing regimens
Mark Olfson, MD, MPH, professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said the report “highlights the pharmacological complexity of adults who are treated with stimulants.”
Dr. Olfson, who is a research psychiatrist at the New York State Psychiatric Institute, New York, and was not involved with the study, observed there is “evidence to support stimulants as an adjunctive therapy for treatment-resistant unipolar depression in older adults.”
However, he added, “this indication is unlikely to fully explain the high proportion of nonelderly, stimulant-treated adults who also receive antidepressants.”
These new findings “call for research to increase our understanding of the clinical contexts that motivate these complex prescribing regimens as well as their effectiveness and safety,” said Dr. Olfson.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. Mr. Moore declares no relevant financial relationships. Coauthor G. Caleb Alexander, MD, is past chair and a current member of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. Dr. Olfson declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
New tool accurately predicts suicide risk in serious mental illness
The 17-question Oxford Mental Illness and Suicide Tool (OxMIS) assessment is designed to predict 12-month suicide risk in people with schizophrenia spectrum disorders and bipolar disorder based on risk factors such as familial traits, antisocial traits, and information about self-harm.
“We have demonstrated the clinical utility of OxMIS in two separate studies and countries. As with any clinical risk prediction tool, it will not improve outcomes unless coupled with effective interventions,” lead investigator Amir Sariaslan, PhD, a senior research fellow in psychiatric epidemiology at the University of Oxford, England, told this news organization.
The findings were published online in Translational Psychiatry.
Twice validated
Dr. Sariaslan and his team originally developed and validated the OxMIS in a cohort of 75,000 people with SMI in Sweden. Recognizing the lack of externally validated prognostic models in the mental health field, the team wanted to validate the instrument in a new, population-based sample in Finland.
The investigators accessed information about patient diagnosis and treatment from the Finnish Care Register for Health Care, which contains de-identified information for all individuals between ages 15 and 65 years diagnosed with an SMI between Jan. 1, 1996, and Dec. 31, 2017.
They included 137,000 patients with somatic symptom disorder or bipolar disorder for a total of more than 5 million episodes of inpatient or outpatient treatment. Investigators linked the cohort to the Causes of Death Register to identify those who had died by suicide within 12 months of an index treatment episode, which investigators randomly selected for each person.
The investigators found that 1,475 individuals in the sample died by suicide within 1 year of their index episode (1.1%).
Each patient was assigned a clinical suicide risk score based on their clinical information, familial traits, prescription information, and comorbid conditions. Using OxMIS, the investigators found that the instrument accurately predicted suicide with an area under the curve of 0.70.
In other words, in 70% of the instances where the investigators randomly selected two people from the sample, one of whom died by suicide and the other of whom did not, the individual who died by suicide had a higher OxMIS risk score.
The investigators note the model overestimated the risk for patients who were at extremely high risk for suicide (those with a predicted suicide risk of > 5%). “In our complementary sensitivity analysis, we observed improved calibration in these patients when we assigned them a suicide risk prediction of no more than 5%,” they write.
Dr. Sariaslan said that the findings highlight the importance of safety planning interventions. “It is also essential to remember that OxMIS is not intended to replace clinical decision-making, but rather to support it,” he said.
As to whether the tool could be used in other populations, such as in the United States, Dr. Sariaslan said, “there is no good evidence that the contribution of risk factors to suicide in this population is different in the U.S. than in northern Europe, so there is no a priori reason to have to do multiple external validations before it can be used for research or clinical purposes.”
One size does not fit all
Commenting on the study, Ronald Kessler, PhD, McNeil Family Professor, department of health care policy at Harvard Medical School, Boston, said that he’d be “surprised” if OxMIS was adopted in the United States because there is already an existing tool that is “slightly more accurate,” which he helped develop.
“In addition, when we start thinking about uses for such scales, it becomes clear that different scales should be used for different segments of the population, depending on intervention options,” Dr. Kessler said.
“So, for example, a different scale would probably be optimal in deciding how to manage psychiatric inpatients in the transition back to the community after hospital discharge than [it would be], say, in deciding how to respond to suicidality among patients presenting at an emergency department. No one scale will fit for all the scenarios in which prediction is desired,” he added.
The study was funded by the Academy of Finland. Dr. Kessler receives funding from the National Institute of Mental Health, Department of Defense, and Veterans Administration to develop suicide prediction models. Dr. Sariaslan has no disclosures to report.
A version of this article first appeared on Medscape.com.
The 17-question Oxford Mental Illness and Suicide Tool (OxMIS) assessment is designed to predict 12-month suicide risk in people with schizophrenia spectrum disorders and bipolar disorder based on risk factors such as familial traits, antisocial traits, and information about self-harm.
“We have demonstrated the clinical utility of OxMIS in two separate studies and countries. As with any clinical risk prediction tool, it will not improve outcomes unless coupled with effective interventions,” lead investigator Amir Sariaslan, PhD, a senior research fellow in psychiatric epidemiology at the University of Oxford, England, told this news organization.
The findings were published online in Translational Psychiatry.
Twice validated
Dr. Sariaslan and his team originally developed and validated the OxMIS in a cohort of 75,000 people with SMI in Sweden. Recognizing the lack of externally validated prognostic models in the mental health field, the team wanted to validate the instrument in a new, population-based sample in Finland.
The investigators accessed information about patient diagnosis and treatment from the Finnish Care Register for Health Care, which contains de-identified information for all individuals between ages 15 and 65 years diagnosed with an SMI between Jan. 1, 1996, and Dec. 31, 2017.
They included 137,000 patients with somatic symptom disorder or bipolar disorder for a total of more than 5 million episodes of inpatient or outpatient treatment. Investigators linked the cohort to the Causes of Death Register to identify those who had died by suicide within 12 months of an index treatment episode, which investigators randomly selected for each person.
The investigators found that 1,475 individuals in the sample died by suicide within 1 year of their index episode (1.1%).
Each patient was assigned a clinical suicide risk score based on their clinical information, familial traits, prescription information, and comorbid conditions. Using OxMIS, the investigators found that the instrument accurately predicted suicide with an area under the curve of 0.70.
In other words, in 70% of the instances where the investigators randomly selected two people from the sample, one of whom died by suicide and the other of whom did not, the individual who died by suicide had a higher OxMIS risk score.
The investigators note the model overestimated the risk for patients who were at extremely high risk for suicide (those with a predicted suicide risk of > 5%). “In our complementary sensitivity analysis, we observed improved calibration in these patients when we assigned them a suicide risk prediction of no more than 5%,” they write.
Dr. Sariaslan said that the findings highlight the importance of safety planning interventions. “It is also essential to remember that OxMIS is not intended to replace clinical decision-making, but rather to support it,” he said.
As to whether the tool could be used in other populations, such as in the United States, Dr. Sariaslan said, “there is no good evidence that the contribution of risk factors to suicide in this population is different in the U.S. than in northern Europe, so there is no a priori reason to have to do multiple external validations before it can be used for research or clinical purposes.”
One size does not fit all
Commenting on the study, Ronald Kessler, PhD, McNeil Family Professor, department of health care policy at Harvard Medical School, Boston, said that he’d be “surprised” if OxMIS was adopted in the United States because there is already an existing tool that is “slightly more accurate,” which he helped develop.
“In addition, when we start thinking about uses for such scales, it becomes clear that different scales should be used for different segments of the population, depending on intervention options,” Dr. Kessler said.
“So, for example, a different scale would probably be optimal in deciding how to manage psychiatric inpatients in the transition back to the community after hospital discharge than [it would be], say, in deciding how to respond to suicidality among patients presenting at an emergency department. No one scale will fit for all the scenarios in which prediction is desired,” he added.
The study was funded by the Academy of Finland. Dr. Kessler receives funding from the National Institute of Mental Health, Department of Defense, and Veterans Administration to develop suicide prediction models. Dr. Sariaslan has no disclosures to report.
A version of this article first appeared on Medscape.com.
The 17-question Oxford Mental Illness and Suicide Tool (OxMIS) assessment is designed to predict 12-month suicide risk in people with schizophrenia spectrum disorders and bipolar disorder based on risk factors such as familial traits, antisocial traits, and information about self-harm.
“We have demonstrated the clinical utility of OxMIS in two separate studies and countries. As with any clinical risk prediction tool, it will not improve outcomes unless coupled with effective interventions,” lead investigator Amir Sariaslan, PhD, a senior research fellow in psychiatric epidemiology at the University of Oxford, England, told this news organization.
The findings were published online in Translational Psychiatry.
Twice validated
Dr. Sariaslan and his team originally developed and validated the OxMIS in a cohort of 75,000 people with SMI in Sweden. Recognizing the lack of externally validated prognostic models in the mental health field, the team wanted to validate the instrument in a new, population-based sample in Finland.
The investigators accessed information about patient diagnosis and treatment from the Finnish Care Register for Health Care, which contains de-identified information for all individuals between ages 15 and 65 years diagnosed with an SMI between Jan. 1, 1996, and Dec. 31, 2017.
They included 137,000 patients with somatic symptom disorder or bipolar disorder for a total of more than 5 million episodes of inpatient or outpatient treatment. Investigators linked the cohort to the Causes of Death Register to identify those who had died by suicide within 12 months of an index treatment episode, which investigators randomly selected for each person.
The investigators found that 1,475 individuals in the sample died by suicide within 1 year of their index episode (1.1%).
Each patient was assigned a clinical suicide risk score based on their clinical information, familial traits, prescription information, and comorbid conditions. Using OxMIS, the investigators found that the instrument accurately predicted suicide with an area under the curve of 0.70.
In other words, in 70% of the instances where the investigators randomly selected two people from the sample, one of whom died by suicide and the other of whom did not, the individual who died by suicide had a higher OxMIS risk score.
The investigators note the model overestimated the risk for patients who were at extremely high risk for suicide (those with a predicted suicide risk of > 5%). “In our complementary sensitivity analysis, we observed improved calibration in these patients when we assigned them a suicide risk prediction of no more than 5%,” they write.
Dr. Sariaslan said that the findings highlight the importance of safety planning interventions. “It is also essential to remember that OxMIS is not intended to replace clinical decision-making, but rather to support it,” he said.
As to whether the tool could be used in other populations, such as in the United States, Dr. Sariaslan said, “there is no good evidence that the contribution of risk factors to suicide in this population is different in the U.S. than in northern Europe, so there is no a priori reason to have to do multiple external validations before it can be used for research or clinical purposes.”
One size does not fit all
Commenting on the study, Ronald Kessler, PhD, McNeil Family Professor, department of health care policy at Harvard Medical School, Boston, said that he’d be “surprised” if OxMIS was adopted in the United States because there is already an existing tool that is “slightly more accurate,” which he helped develop.
“In addition, when we start thinking about uses for such scales, it becomes clear that different scales should be used for different segments of the population, depending on intervention options,” Dr. Kessler said.
“So, for example, a different scale would probably be optimal in deciding how to manage psychiatric inpatients in the transition back to the community after hospital discharge than [it would be], say, in deciding how to respond to suicidality among patients presenting at an emergency department. No one scale will fit for all the scenarios in which prediction is desired,” he added.
The study was funded by the Academy of Finland. Dr. Kessler receives funding from the National Institute of Mental Health, Department of Defense, and Veterans Administration to develop suicide prediction models. Dr. Sariaslan has no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM TRANSLATIONAL PSYCHIATRY
Bipolar disorder: The foundational role of mood stabilizers
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
Predicting BPD vs. bipolar treatment response: New imaging data
A new study identifies specific brain regions involved in treatment response in bipolar disorder (BD) and borderline personality disorder (BPD), potentially paving the way for more targeted treatment.
In a meta-analysis of 34 studies that used neuroimaging to investigate changes in brain activation following psychotherapy and pharmacotherapy for BD and BPD, investigators found most brain regions showing abnormal activation in both conditions improved after treatment. In particular, changes in brain activity after psychotherapy were found primarily in the frontal areas, whereas pharmacotherapy largely altered the limbic areas.
“,” senior investigator Xiaoming Li, PhD, professor, department of medical psychology, Anhui Medical University, Hefei, China, told this news organization.
“It may also contribute to the identification of more accurate neuroimaging biomarkers for treatment of the two disorders and to the finding of more effective therapy,” Dr. Li said.
The study was published online in the Journal of Clinical Psychiatry.
Blurred boundary
Dr. Li called BDs and BPDs “difficult to diagnose and differentiate,” noting that the comorbidity rate is “very high.” Underestimating the boundary between BD and BPD “increases the risk of improper or harmful drug exposure,” since mood stabilizing drugs are “considered to be the key therapeutic intervention for BD, while psychotherapy is the key treatment for BPD.”
The “blurred boundary between BD and BPD is one of the reasons it is important to study the relationship between these two diseases,” the authors said.
Previous studies comparing the relationship between BD and BPD “did not explore the similarities and differences in brain mechanisms between these two disorders after treatment,” they pointed out.
Patients with BD have a different disease course and response to therapy, compared to patient with BPD patients. “Misdiagnosis may result in the patients receiving ineffective treatment, so it is particularly important to explore the neural mechanisms of the treatment of these two diseases,” Dr. Li said.
To investigate, the researchers used activation likelihood estimation (ALE) – a technique that examines coordinates of neuroimaging data gleaned from published studies – after searching several databases from inception until June 2021.
This approach was used to “evaluate the similarities and differences in the activation of different brain regions in patients with BD and BPD after treatment with psychotherapy and drug therapy.”
Studies were required to focus on patients with a clinical diagnosis of BD or BPD; neuroimaging studies using functional MRI; coordinates of the peak activations in the stereotactic space of the Montreal Neurologic Institute or Talairach; treatment (pharmacologic or psychological) for patients with BD or BPD; and results of changes in brain activation after treatment, relative to a before-treatment condition.
Of 1,592 records, 34 studies (n = 912 subjects) met inclusion criteria and were selected and used in extracting the activation coordinates. The researchers extracted a total of 186 activity increase points and 90 activity decrease points. After combining these calculations, they found 12 increased activation clusters and 2 decreased activation clusters.
Of the studies, 23 focused on BD and 11 on BPD; 14 used psychotherapy, 18 used drug therapy, and 2 used a combination of both approaches.
Normalizing activation levels
Both treatments were associated with convergent activity increases and decreases in several brain regions: the anterior cingulate cortex, medial frontal gyrus, inferior frontal gyrus, cingulate gyrus, parahippocampal gyrus, and the posterior cingulate cortex.
The researchers then examined studies based on treatment method – psychotherapy or pharmacotherapy and the effect on the two disorders.
“After psychotherapy, the frontal lobe and temporal lobe were the primary brain regions in which activation changed, indicating a top-down effect of this therapy type, while after drug therapy, the limbic area was the region in which activation changed, indicating a ‘bottom-up’ effect,” said Dr. Li.
Dr. Li cited previous research pointing to functional and structural abnormalities in both disorders – especially in the default mode network (DMN) and frontolimbic network.
In particular, alterations in the amygdala and the parahippocampal gyrus are reported more frequently in BPD than in BD, whereas dysfunctional frontolimbic brain regions seem to underlie the emotional dysfunction in BPD. Several studies have also associated the impulsivity of BD with dysfunctions in the interplay of cortical-limbic circuits.
Dr. Li said the study findings suggest “that treatment may change these brain activation levels by acting on the abnormal brain circuit, such as the DMN and the frontolimbic network so as to ‘normalize’ its activity and improve symptoms.”
Specifically, brain regions with abnormally increased activation “showed decreased activation after treatment, and brain regions with abnormally decreased activation showed increased activation after treatment.”
Discrete, overlapping mechanisms
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study “provides additional support for the underlying neurobiological signature of bipolar disorder and a commonly encountered co-occurring condition – borderline personality disorder – having both discrete yet overlapping mechanisms.”
He found it interesting that “medications have a different principal target than psychosocial interventions, which has both academic and clinical implications.
“The academic implication is that we have reasons to believe that we will be in a position to parse the neurobiology of bipolar disorder or borderline personality disorder when we take an approach that isolates specific domains of psychopathology, which is what they [the authors] appear to be doing,” said Dr. McIntyre, who wasn’t associated with this research.
In addition, “from the clinical perspective, this provides a rationale for why we should be integrating pharmacotherapy with psychotherapy in people who have comorbid conditions like borderline personality disorder, which affects 20% of people living with bipolar disorder and 60% to 70% have borderline traits,” he added.
The research was supported by the Anhui Natural Science Foundation and Grants for Scientific Research from Anhui Medical University. Dr. Li and coauthors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
A new study identifies specific brain regions involved in treatment response in bipolar disorder (BD) and borderline personality disorder (BPD), potentially paving the way for more targeted treatment.
In a meta-analysis of 34 studies that used neuroimaging to investigate changes in brain activation following psychotherapy and pharmacotherapy for BD and BPD, investigators found most brain regions showing abnormal activation in both conditions improved after treatment. In particular, changes in brain activity after psychotherapy were found primarily in the frontal areas, whereas pharmacotherapy largely altered the limbic areas.
“,” senior investigator Xiaoming Li, PhD, professor, department of medical psychology, Anhui Medical University, Hefei, China, told this news organization.
“It may also contribute to the identification of more accurate neuroimaging biomarkers for treatment of the two disorders and to the finding of more effective therapy,” Dr. Li said.
The study was published online in the Journal of Clinical Psychiatry.
Blurred boundary
Dr. Li called BDs and BPDs “difficult to diagnose and differentiate,” noting that the comorbidity rate is “very high.” Underestimating the boundary between BD and BPD “increases the risk of improper or harmful drug exposure,” since mood stabilizing drugs are “considered to be the key therapeutic intervention for BD, while psychotherapy is the key treatment for BPD.”
The “blurred boundary between BD and BPD is one of the reasons it is important to study the relationship between these two diseases,” the authors said.
Previous studies comparing the relationship between BD and BPD “did not explore the similarities and differences in brain mechanisms between these two disorders after treatment,” they pointed out.
Patients with BD have a different disease course and response to therapy, compared to patient with BPD patients. “Misdiagnosis may result in the patients receiving ineffective treatment, so it is particularly important to explore the neural mechanisms of the treatment of these two diseases,” Dr. Li said.
To investigate, the researchers used activation likelihood estimation (ALE) – a technique that examines coordinates of neuroimaging data gleaned from published studies – after searching several databases from inception until June 2021.
This approach was used to “evaluate the similarities and differences in the activation of different brain regions in patients with BD and BPD after treatment with psychotherapy and drug therapy.”
Studies were required to focus on patients with a clinical diagnosis of BD or BPD; neuroimaging studies using functional MRI; coordinates of the peak activations in the stereotactic space of the Montreal Neurologic Institute or Talairach; treatment (pharmacologic or psychological) for patients with BD or BPD; and results of changes in brain activation after treatment, relative to a before-treatment condition.
Of 1,592 records, 34 studies (n = 912 subjects) met inclusion criteria and were selected and used in extracting the activation coordinates. The researchers extracted a total of 186 activity increase points and 90 activity decrease points. After combining these calculations, they found 12 increased activation clusters and 2 decreased activation clusters.
Of the studies, 23 focused on BD and 11 on BPD; 14 used psychotherapy, 18 used drug therapy, and 2 used a combination of both approaches.
Normalizing activation levels
Both treatments were associated with convergent activity increases and decreases in several brain regions: the anterior cingulate cortex, medial frontal gyrus, inferior frontal gyrus, cingulate gyrus, parahippocampal gyrus, and the posterior cingulate cortex.
The researchers then examined studies based on treatment method – psychotherapy or pharmacotherapy and the effect on the two disorders.
“After psychotherapy, the frontal lobe and temporal lobe were the primary brain regions in which activation changed, indicating a top-down effect of this therapy type, while after drug therapy, the limbic area was the region in which activation changed, indicating a ‘bottom-up’ effect,” said Dr. Li.
Dr. Li cited previous research pointing to functional and structural abnormalities in both disorders – especially in the default mode network (DMN) and frontolimbic network.
In particular, alterations in the amygdala and the parahippocampal gyrus are reported more frequently in BPD than in BD, whereas dysfunctional frontolimbic brain regions seem to underlie the emotional dysfunction in BPD. Several studies have also associated the impulsivity of BD with dysfunctions in the interplay of cortical-limbic circuits.
Dr. Li said the study findings suggest “that treatment may change these brain activation levels by acting on the abnormal brain circuit, such as the DMN and the frontolimbic network so as to ‘normalize’ its activity and improve symptoms.”
Specifically, brain regions with abnormally increased activation “showed decreased activation after treatment, and brain regions with abnormally decreased activation showed increased activation after treatment.”
Discrete, overlapping mechanisms
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study “provides additional support for the underlying neurobiological signature of bipolar disorder and a commonly encountered co-occurring condition – borderline personality disorder – having both discrete yet overlapping mechanisms.”
He found it interesting that “medications have a different principal target than psychosocial interventions, which has both academic and clinical implications.
“The academic implication is that we have reasons to believe that we will be in a position to parse the neurobiology of bipolar disorder or borderline personality disorder when we take an approach that isolates specific domains of psychopathology, which is what they [the authors] appear to be doing,” said Dr. McIntyre, who wasn’t associated with this research.
In addition, “from the clinical perspective, this provides a rationale for why we should be integrating pharmacotherapy with psychotherapy in people who have comorbid conditions like borderline personality disorder, which affects 20% of people living with bipolar disorder and 60% to 70% have borderline traits,” he added.
The research was supported by the Anhui Natural Science Foundation and Grants for Scientific Research from Anhui Medical University. Dr. Li and coauthors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
A new study identifies specific brain regions involved in treatment response in bipolar disorder (BD) and borderline personality disorder (BPD), potentially paving the way for more targeted treatment.
In a meta-analysis of 34 studies that used neuroimaging to investigate changes in brain activation following psychotherapy and pharmacotherapy for BD and BPD, investigators found most brain regions showing abnormal activation in both conditions improved after treatment. In particular, changes in brain activity after psychotherapy were found primarily in the frontal areas, whereas pharmacotherapy largely altered the limbic areas.
“,” senior investigator Xiaoming Li, PhD, professor, department of medical psychology, Anhui Medical University, Hefei, China, told this news organization.
“It may also contribute to the identification of more accurate neuroimaging biomarkers for treatment of the two disorders and to the finding of more effective therapy,” Dr. Li said.
The study was published online in the Journal of Clinical Psychiatry.
Blurred boundary
Dr. Li called BDs and BPDs “difficult to diagnose and differentiate,” noting that the comorbidity rate is “very high.” Underestimating the boundary between BD and BPD “increases the risk of improper or harmful drug exposure,” since mood stabilizing drugs are “considered to be the key therapeutic intervention for BD, while psychotherapy is the key treatment for BPD.”
The “blurred boundary between BD and BPD is one of the reasons it is important to study the relationship between these two diseases,” the authors said.
Previous studies comparing the relationship between BD and BPD “did not explore the similarities and differences in brain mechanisms between these two disorders after treatment,” they pointed out.
Patients with BD have a different disease course and response to therapy, compared to patient with BPD patients. “Misdiagnosis may result in the patients receiving ineffective treatment, so it is particularly important to explore the neural mechanisms of the treatment of these two diseases,” Dr. Li said.
To investigate, the researchers used activation likelihood estimation (ALE) – a technique that examines coordinates of neuroimaging data gleaned from published studies – after searching several databases from inception until June 2021.
This approach was used to “evaluate the similarities and differences in the activation of different brain regions in patients with BD and BPD after treatment with psychotherapy and drug therapy.”
Studies were required to focus on patients with a clinical diagnosis of BD or BPD; neuroimaging studies using functional MRI; coordinates of the peak activations in the stereotactic space of the Montreal Neurologic Institute or Talairach; treatment (pharmacologic or psychological) for patients with BD or BPD; and results of changes in brain activation after treatment, relative to a before-treatment condition.
Of 1,592 records, 34 studies (n = 912 subjects) met inclusion criteria and were selected and used in extracting the activation coordinates. The researchers extracted a total of 186 activity increase points and 90 activity decrease points. After combining these calculations, they found 12 increased activation clusters and 2 decreased activation clusters.
Of the studies, 23 focused on BD and 11 on BPD; 14 used psychotherapy, 18 used drug therapy, and 2 used a combination of both approaches.
Normalizing activation levels
Both treatments were associated with convergent activity increases and decreases in several brain regions: the anterior cingulate cortex, medial frontal gyrus, inferior frontal gyrus, cingulate gyrus, parahippocampal gyrus, and the posterior cingulate cortex.
The researchers then examined studies based on treatment method – psychotherapy or pharmacotherapy and the effect on the two disorders.
“After psychotherapy, the frontal lobe and temporal lobe were the primary brain regions in which activation changed, indicating a top-down effect of this therapy type, while after drug therapy, the limbic area was the region in which activation changed, indicating a ‘bottom-up’ effect,” said Dr. Li.
Dr. Li cited previous research pointing to functional and structural abnormalities in both disorders – especially in the default mode network (DMN) and frontolimbic network.
In particular, alterations in the amygdala and the parahippocampal gyrus are reported more frequently in BPD than in BD, whereas dysfunctional frontolimbic brain regions seem to underlie the emotional dysfunction in BPD. Several studies have also associated the impulsivity of BD with dysfunctions in the interplay of cortical-limbic circuits.
Dr. Li said the study findings suggest “that treatment may change these brain activation levels by acting on the abnormal brain circuit, such as the DMN and the frontolimbic network so as to ‘normalize’ its activity and improve symptoms.”
Specifically, brain regions with abnormally increased activation “showed decreased activation after treatment, and brain regions with abnormally decreased activation showed increased activation after treatment.”
Discrete, overlapping mechanisms
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study “provides additional support for the underlying neurobiological signature of bipolar disorder and a commonly encountered co-occurring condition – borderline personality disorder – having both discrete yet overlapping mechanisms.”
He found it interesting that “medications have a different principal target than psychosocial interventions, which has both academic and clinical implications.
“The academic implication is that we have reasons to believe that we will be in a position to parse the neurobiology of bipolar disorder or borderline personality disorder when we take an approach that isolates specific domains of psychopathology, which is what they [the authors] appear to be doing,” said Dr. McIntyre, who wasn’t associated with this research.
In addition, “from the clinical perspective, this provides a rationale for why we should be integrating pharmacotherapy with psychotherapy in people who have comorbid conditions like borderline personality disorder, which affects 20% of people living with bipolar disorder and 60% to 70% have borderline traits,” he added.
The research was supported by the Anhui Natural Science Foundation and Grants for Scientific Research from Anhui Medical University. Dr. Li and coauthors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Lithium-induced diabetes insipidus: Pathophysiology and treatment
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10
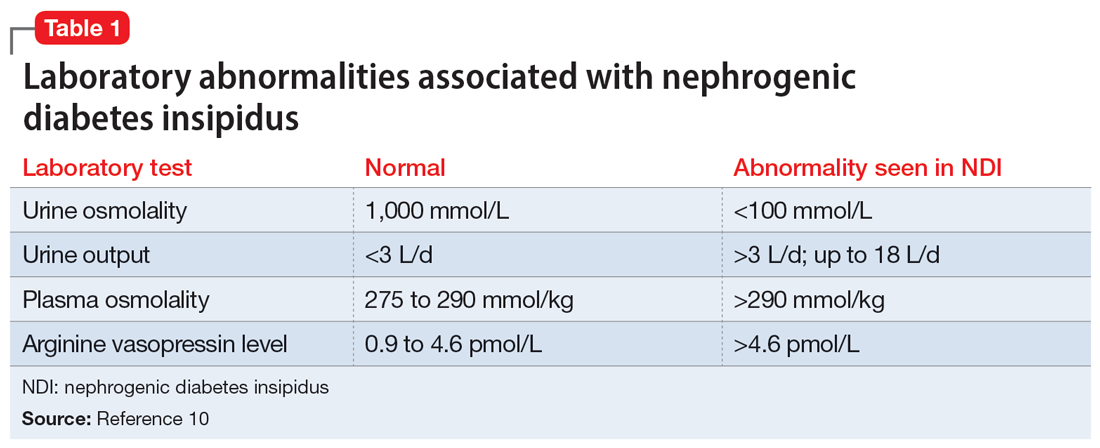
Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.
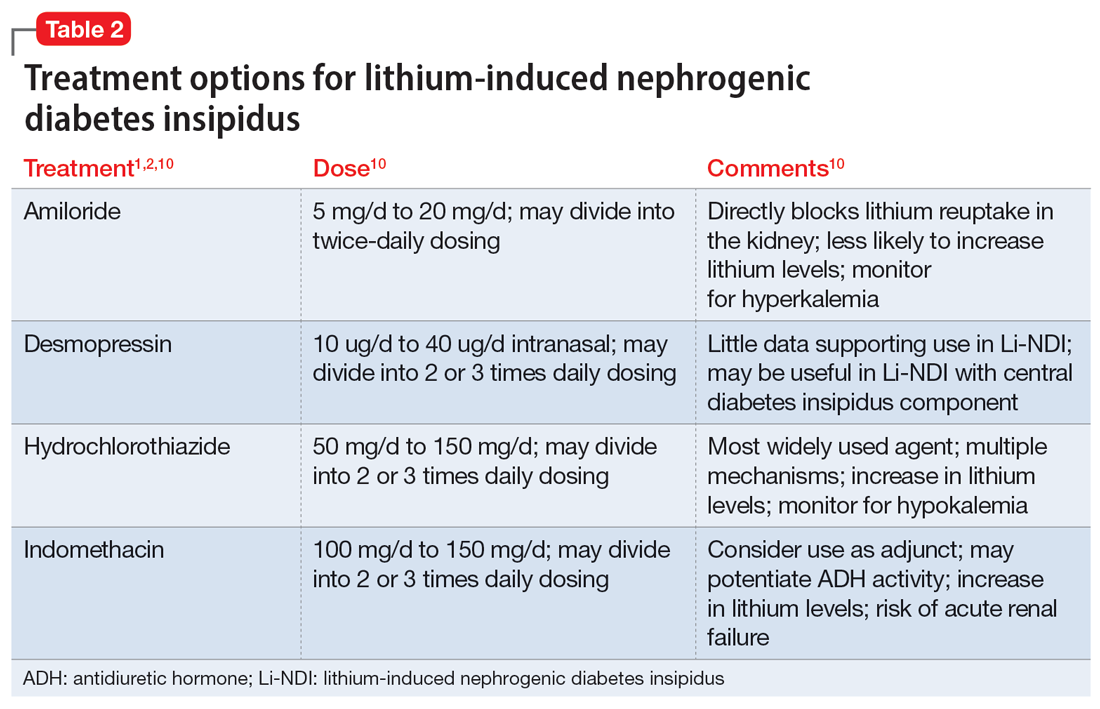
Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
Psychiatric comorbidities predict complex polypharmacy in bipolar disorder
Patients with bipolar disorder (BD) often receive prescriptions for multiple medications to manage a range of medical and psychiatric symptoms, but the definition of polypharmacy in these patients is inconsistent, and characteristics associated with complex polypharmacy have not been well studied, wrote Andrea Aguglia, MD, of the University of Genoa, Italy, and colleagues.
Previous studies have shown an increased risk for comorbid medical and psychiatric illnesses in BD patients, the researchers noted, and changes in prescribing trends have prompted greater use of combination therapies such as mood stabilizers with or without antipsychotics.
In a study published in Psychiatry Research, the investigators reviewed data from 556 adults with BD. Participants were aged 18 and older with a primary diagnosis of BD type I or II based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria. The mean age of the participants was 49.17 years, 43.7% were male, and 34.2% were employed. A total of 327 patients (58.8%) had a medical comorbidity, and 193 (34.7%) used an illicit substance.
A total of 225 patients (40.5%) met the criteria for complex polypharmacy by taking at least 4 medications.
BD patients with complex polypharmacy were significantly more likely than those without complex polypharmacy to be single (50.7% vs. 37.8%, P = .025) and unemployed (25.3% vs. 40.2%, P < .001).
On the clinical side, complex polypharmacy in BD patients was significantly associated with a higher prevalence of both medical and psychiatric comorbidities (65.3% vs. 54.4%, P = .010; and 50.7% vs. 34.1%, P < .001, respectively). The association with medical comorbidities and complex polypharmacy in BD was unexpected, the researchers said, “as psychotropic medications should be used with cautiousness in patients suffering from medical conditions.”
BD patients with complex polypharmacy also had a significantly earlier age of onset, longer duration of illness, and increased number of hospitalizations than those without complex polypharmacy.
Rates of at least one substance including alcohol, cannabinoids, and cocaine/amphetamines were significantly higher among BD patients with complex polypharmacy, compared with those without, but no differences in heroin use were noted between the groups.
In a logistic regression analysis, single status, older age, number of hospitalizations, and the presence of psychiatric comorbidities were significantly associated with complex polypharmacy.
The study findings were limited by several factors including the focus on an inpatient population, inability to consider clinical factors such as type of mood episode and bipolar cycle, and the cross-sectional design that prevented conclusions of causality, the researchers noted.
However, the study is the first known to focus on both sociodemographic and clinical factors associated with polypharmacy in BD, and the results suggest that implementing complementary psychosocial strategies might help reduce medication use in these patients, they concluded. Data from further longitudinal studies may help guide long-term management of BD, “especially when pharmacological discontinuation is needed,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Patients with bipolar disorder (BD) often receive prescriptions for multiple medications to manage a range of medical and psychiatric symptoms, but the definition of polypharmacy in these patients is inconsistent, and characteristics associated with complex polypharmacy have not been well studied, wrote Andrea Aguglia, MD, of the University of Genoa, Italy, and colleagues.
Previous studies have shown an increased risk for comorbid medical and psychiatric illnesses in BD patients, the researchers noted, and changes in prescribing trends have prompted greater use of combination therapies such as mood stabilizers with or without antipsychotics.
In a study published in Psychiatry Research, the investigators reviewed data from 556 adults with BD. Participants were aged 18 and older with a primary diagnosis of BD type I or II based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria. The mean age of the participants was 49.17 years, 43.7% were male, and 34.2% were employed. A total of 327 patients (58.8%) had a medical comorbidity, and 193 (34.7%) used an illicit substance.
A total of 225 patients (40.5%) met the criteria for complex polypharmacy by taking at least 4 medications.
BD patients with complex polypharmacy were significantly more likely than those without complex polypharmacy to be single (50.7% vs. 37.8%, P = .025) and unemployed (25.3% vs. 40.2%, P < .001).
On the clinical side, complex polypharmacy in BD patients was significantly associated with a higher prevalence of both medical and psychiatric comorbidities (65.3% vs. 54.4%, P = .010; and 50.7% vs. 34.1%, P < .001, respectively). The association with medical comorbidities and complex polypharmacy in BD was unexpected, the researchers said, “as psychotropic medications should be used with cautiousness in patients suffering from medical conditions.”
BD patients with complex polypharmacy also had a significantly earlier age of onset, longer duration of illness, and increased number of hospitalizations than those without complex polypharmacy.
Rates of at least one substance including alcohol, cannabinoids, and cocaine/amphetamines were significantly higher among BD patients with complex polypharmacy, compared with those without, but no differences in heroin use were noted between the groups.
In a logistic regression analysis, single status, older age, number of hospitalizations, and the presence of psychiatric comorbidities were significantly associated with complex polypharmacy.
The study findings were limited by several factors including the focus on an inpatient population, inability to consider clinical factors such as type of mood episode and bipolar cycle, and the cross-sectional design that prevented conclusions of causality, the researchers noted.
However, the study is the first known to focus on both sociodemographic and clinical factors associated with polypharmacy in BD, and the results suggest that implementing complementary psychosocial strategies might help reduce medication use in these patients, they concluded. Data from further longitudinal studies may help guide long-term management of BD, “especially when pharmacological discontinuation is needed,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Patients with bipolar disorder (BD) often receive prescriptions for multiple medications to manage a range of medical and psychiatric symptoms, but the definition of polypharmacy in these patients is inconsistent, and characteristics associated with complex polypharmacy have not been well studied, wrote Andrea Aguglia, MD, of the University of Genoa, Italy, and colleagues.
Previous studies have shown an increased risk for comorbid medical and psychiatric illnesses in BD patients, the researchers noted, and changes in prescribing trends have prompted greater use of combination therapies such as mood stabilizers with or without antipsychotics.
In a study published in Psychiatry Research, the investigators reviewed data from 556 adults with BD. Participants were aged 18 and older with a primary diagnosis of BD type I or II based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria. The mean age of the participants was 49.17 years, 43.7% were male, and 34.2% were employed. A total of 327 patients (58.8%) had a medical comorbidity, and 193 (34.7%) used an illicit substance.
A total of 225 patients (40.5%) met the criteria for complex polypharmacy by taking at least 4 medications.
BD patients with complex polypharmacy were significantly more likely than those without complex polypharmacy to be single (50.7% vs. 37.8%, P = .025) and unemployed (25.3% vs. 40.2%, P < .001).
On the clinical side, complex polypharmacy in BD patients was significantly associated with a higher prevalence of both medical and psychiatric comorbidities (65.3% vs. 54.4%, P = .010; and 50.7% vs. 34.1%, P < .001, respectively). The association with medical comorbidities and complex polypharmacy in BD was unexpected, the researchers said, “as psychotropic medications should be used with cautiousness in patients suffering from medical conditions.”
BD patients with complex polypharmacy also had a significantly earlier age of onset, longer duration of illness, and increased number of hospitalizations than those without complex polypharmacy.
Rates of at least one substance including alcohol, cannabinoids, and cocaine/amphetamines were significantly higher among BD patients with complex polypharmacy, compared with those without, but no differences in heroin use were noted between the groups.
In a logistic regression analysis, single status, older age, number of hospitalizations, and the presence of psychiatric comorbidities were significantly associated with complex polypharmacy.
The study findings were limited by several factors including the focus on an inpatient population, inability to consider clinical factors such as type of mood episode and bipolar cycle, and the cross-sectional design that prevented conclusions of causality, the researchers noted.
However, the study is the first known to focus on both sociodemographic and clinical factors associated with polypharmacy in BD, and the results suggest that implementing complementary psychosocial strategies might help reduce medication use in these patients, they concluded. Data from further longitudinal studies may help guide long-term management of BD, “especially when pharmacological discontinuation is needed,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
Cultivating strength: Psychological well-being after nonfatal suicide attempts
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.