User login
Retrograde Reamer/Irrigator/Aspirator Technique for Autologous Bone Graft Harvesting With the Patient in the Prone Position
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
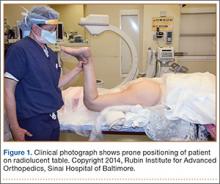
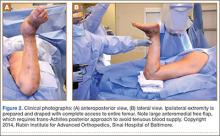
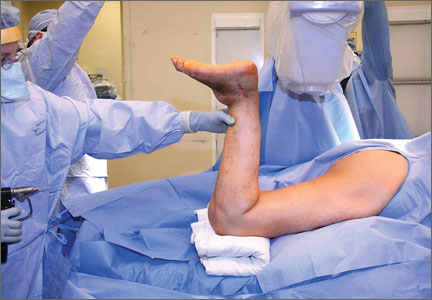
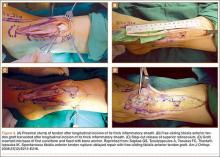
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
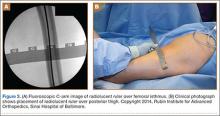
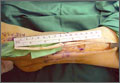
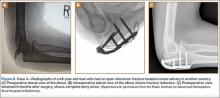
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
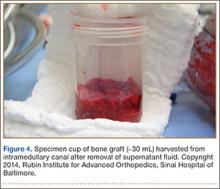
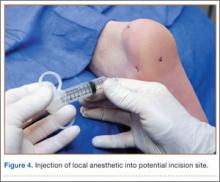
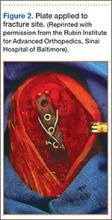
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
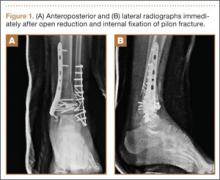
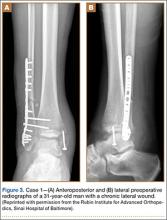
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
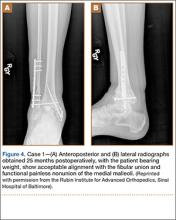
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
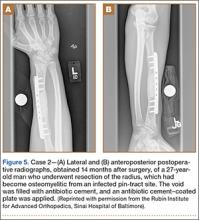
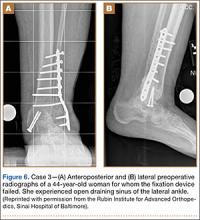
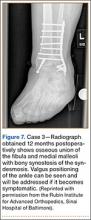
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
Successful Surgical Treatment of an Intraneural Ganglion of the Common Peroneal Nerve
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
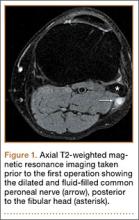
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
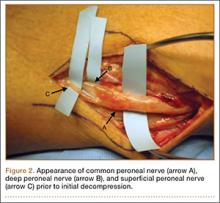
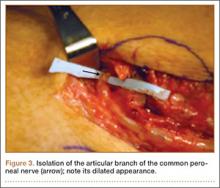
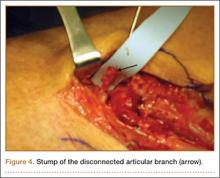
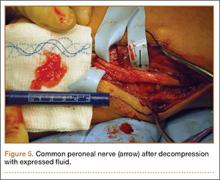
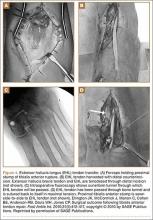
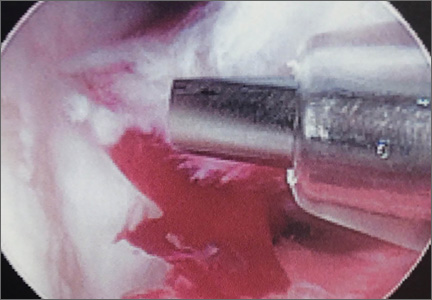
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
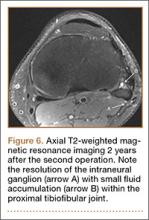
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Lumbar Degenerative Disc Disease and Tibiotalar Joint Arthritis: A 710-Specimen Postmortem Study
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
A Systematic Review of Tibialis Anterior Tendon Rupture Treatments and Outcomes
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
Running Barefoot May Increase Injury Risk in Older, More Experienced Athletes
LAS VEGAS─In recent years there has been an explosion in barefoot running, as well as the purchase and use of “minimalist” running shoes that more closely resemble barefoot running by encouraging the balls of the feet, between the arch and toes, to hit the pavement first. A new study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) found that a significant number of experienced runners, age 30 years and older (40% of men and 20% of women), maintained a heel-first running pattern—which naturally occurs when wearing a shoe with an elevated heel—when running without shoes. Maintaining a heel-toe pattern while running barefoot or in a minimalist shoe may lead to more frequent injuries.
“Previous studies have demonstrated that an adolescent runner’s foot strike is heavily influenced by their running shoe,” said orthopedic surgeon Scott Mullen, MD, the lead author of the study. “Young runners quickly adapt to a forefoot strike pattern when running barefoot, whereas a heel strike is normally associated with wearing large-heeled training shoes.”
In this study, a team of researchers from the University of Kansas Department of Orthopedics and Sports Medicine measured the heel-to-toe drop of 26 runners, all age 30 years or older with at least 10 years of running experience, when each ran in a traditional running shoe, and again when barefoot. The heel and forefoot thickness was measured at running speeds of 6, 7, and 8 miles per hour (mph) for women, and 7, 8, and 9 mph for men. A motion capture system was utilized to analyze foot strikes by a single blinded examiner skilled in the use of the camera system and running mechanics.
Heel-to-toe thickness of the running shoe did not significantly correlate with a change in heel strike, nor did alterations in speed. Running barefoot resulted in a significant drop in percent heel strike at all speeds; however, 40% of the men and 20% of the women persisted with consistent strike patterns across all speeds with and without shoes.
“Our study indicates that older runners (age 30 years and older) are not able to adapt as quickly to running barefoot,” said Dr. Mullen. “The inability to adapt the foot strike to the change in shoe type may put these runners at increased risk of injury. Older runners should be cautious when transitioning to a more minimalist type of shoe.”
LAS VEGAS─In recent years there has been an explosion in barefoot running, as well as the purchase and use of “minimalist” running shoes that more closely resemble barefoot running by encouraging the balls of the feet, between the arch and toes, to hit the pavement first. A new study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) found that a significant number of experienced runners, age 30 years and older (40% of men and 20% of women), maintained a heel-first running pattern—which naturally occurs when wearing a shoe with an elevated heel—when running without shoes. Maintaining a heel-toe pattern while running barefoot or in a minimalist shoe may lead to more frequent injuries.
“Previous studies have demonstrated that an adolescent runner’s foot strike is heavily influenced by their running shoe,” said orthopedic surgeon Scott Mullen, MD, the lead author of the study. “Young runners quickly adapt to a forefoot strike pattern when running barefoot, whereas a heel strike is normally associated with wearing large-heeled training shoes.”
In this study, a team of researchers from the University of Kansas Department of Orthopedics and Sports Medicine measured the heel-to-toe drop of 26 runners, all age 30 years or older with at least 10 years of running experience, when each ran in a traditional running shoe, and again when barefoot. The heel and forefoot thickness was measured at running speeds of 6, 7, and 8 miles per hour (mph) for women, and 7, 8, and 9 mph for men. A motion capture system was utilized to analyze foot strikes by a single blinded examiner skilled in the use of the camera system and running mechanics.
Heel-to-toe thickness of the running shoe did not significantly correlate with a change in heel strike, nor did alterations in speed. Running barefoot resulted in a significant drop in percent heel strike at all speeds; however, 40% of the men and 20% of the women persisted with consistent strike patterns across all speeds with and without shoes.
“Our study indicates that older runners (age 30 years and older) are not able to adapt as quickly to running barefoot,” said Dr. Mullen. “The inability to adapt the foot strike to the change in shoe type may put these runners at increased risk of injury. Older runners should be cautious when transitioning to a more minimalist type of shoe.”
LAS VEGAS─In recent years there has been an explosion in barefoot running, as well as the purchase and use of “minimalist” running shoes that more closely resemble barefoot running by encouraging the balls of the feet, between the arch and toes, to hit the pavement first. A new study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) found that a significant number of experienced runners, age 30 years and older (40% of men and 20% of women), maintained a heel-first running pattern—which naturally occurs when wearing a shoe with an elevated heel—when running without shoes. Maintaining a heel-toe pattern while running barefoot or in a minimalist shoe may lead to more frequent injuries.
“Previous studies have demonstrated that an adolescent runner’s foot strike is heavily influenced by their running shoe,” said orthopedic surgeon Scott Mullen, MD, the lead author of the study. “Young runners quickly adapt to a forefoot strike pattern when running barefoot, whereas a heel strike is normally associated with wearing large-heeled training shoes.”
In this study, a team of researchers from the University of Kansas Department of Orthopedics and Sports Medicine measured the heel-to-toe drop of 26 runners, all age 30 years or older with at least 10 years of running experience, when each ran in a traditional running shoe, and again when barefoot. The heel and forefoot thickness was measured at running speeds of 6, 7, and 8 miles per hour (mph) for women, and 7, 8, and 9 mph for men. A motion capture system was utilized to analyze foot strikes by a single blinded examiner skilled in the use of the camera system and running mechanics.
Heel-to-toe thickness of the running shoe did not significantly correlate with a change in heel strike, nor did alterations in speed. Running barefoot resulted in a significant drop in percent heel strike at all speeds; however, 40% of the men and 20% of the women persisted with consistent strike patterns across all speeds with and without shoes.
“Our study indicates that older runners (age 30 years and older) are not able to adapt as quickly to running barefoot,” said Dr. Mullen. “The inability to adapt the foot strike to the change in shoe type may put these runners at increased risk of injury. Older runners should be cautious when transitioning to a more minimalist type of shoe.”
Atypical Presentation of Fat Embolism Syndrome After Gunshot Wound to the Foot
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
Use of Cross-Leg Flap for Wound Complications Resulting From Open Pilon Fracture
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Complications of Open Reduction and Internal Fixation of Ankle Fractures in Patients With Positive Urine Drug Screen
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
Open treatment of ankle fractures is one of the most common procedures performed by orthopedic surgeons.1 Among the younger patient population, ankle fractures represent a significant proportion of orthopedic injuries.2 The reported incidence of illicit drug and alcohol use in the urban trauma population ranges from 36% to 86%,2 and medical and anesthetic complications associated with illicit drug use have been well documented in surgical patients.2 However, patients with a recent history of drug abuse may be subject to a separate but related set of complications of open treatment of ankle fractures.
The perioperative complications associated with open treatment of ankle fractures in patients with diabetes mellitus have been well described.3-6 Similarly, previous studies have suggested that peripheral vascular disease, complicated diabetes, and smoking are risk factors for poor outcomes in patients who require open reduction and internal fixation (ORIF) in lower extremity trauma.7-9 However, there are few data on the complications specifically associated with illicit drug use and orthopedic surgery. Properly identifying these high-risk groups and being cognizant of commonly associated complications are likely important in ensuring proper perioperative care and may alter follow-up protocols in these patients.
We conducted a study to identify the complications associated with open treatment of ankle fractures in patients who tested positive for illicit drugs on urine drug screen (UDS). We hypothesized that patients who had a history of positive UDS and underwent ORIF of an ankle fracture would have a higher incidence of major and minor complications.
Materials and Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 142 patients who underwent open treatment of an ankle fracture between 2006 and 2010. Data sources included patient demographic information, radiographs, preoperative UDS, attending surgeons’ clinical office notes, and clinical laboratory data. Our institution’s standard protocol for ankle fractures was followed for all patients in the study. All patients were evaluated by an orthopedic physician, in either the emergency department or the office, during application of a well-padded Jones splint before surgery. Oral narcotic pain medication was routinely prescribed. All patients were seen, within 10 days of injury, for surgery planning. A board-certified orthopedic surgeon surgically stabilized the ankle fractures. The postoperative treatment regimen, per protocol, included non-weight-bearing in a padded Jones splint dressing; oral narcotic pain medication; physical therapy; and routine scheduled follow-up. In open fracture cases, patients were taken urgently to the operating room for irrigation and débridement with stabilization. Which treatment would be initially used—external fixation or ORIF—was determined on a case-by-case basis.
The sample consisted of adults (age, >18 years) who had undergone definitive ORIF of a lateral malleolar, bimalleolar, or trimalleolar ankle fracture during the study period. Polytrauma patients, patients with external fixation as definitive treatment, and patients with nonoperative treatment were excluded. Before surgical management, all patients were tested for recent illicit drug use by UDS (standard protocol at our institution). UDS, measured for cocaine, marijuana, PCP (phencyclidine), opiates, and barbiturates, was obtained in the office setting or emergency department or on day of surgery. The patients were divided into 2 groups, positive and negative UDS. Patients with documented receipt of narcotic pain medication before UDS were excluded.
The outcomes identified as dependent variables included nonunion, malunion, superficial or deep infection, amputation, delay in treatment, days to healing, repeat surgery, long-term bracing, and loss to follow-up. A nonunion was defined as lasting longer than 9 months and not showing radiographic signs of progression toward healing for 3 consecutive months. These complications were identified with use of attending surgeon clinical progress notes, laboratory values, radiographic parameters, and inpatient readmissions/surgeries associated with these outcomes. Nonunion, malunion, superficial or deep infection, and amputation were then grouped as major complications and analyzed as pooled major complications.
The Fisher exact test was used to analyze categorical variables with respect to UDS. The Wilcoxon rank sum test was used to determine statistical significance for continuous variables. Univariate logistic regression examined both continuous and categorical variables to evaluate predictors for a selected outcome. Statistical significance was set a priori at P ≤ .05, with significant factors indicating an increase (or decrease) in the outcome variable being tested.
Results
We retrospectively reviewed the cases of 142 patients. Table 1 lists the number of cases by fracture type. Bimalleolar fractures were most common, accounting for 99 (69.8%) of the 142 cases. Isolated lateral malleolar fractures accounted for 16 cases (11.2%), and trimalleolar fractures accounted for 27 cases (19%).
Twenty-five (18%) of the 142 patients tested positive for illicit drugs. Mean age was 45.2 years for positive UDS patients and 41.5 years for negative UDS patients. Open fracture cases represented 4.3% of negative UDS patients and 16% of positive UDS patients. Fifty-two percent of positive UDS patients and 32% of negative UDS patients were also tobacco users. These data were statistically significant (P = .003) There were no significant differences in age, sex, incidence of diabetes, incidence of open fracture, or time to surgery between the groups (Table 2).
Incidence of nonunion was higher in positive UDS patients (n = 5; P = .01), as was incidence of deep infection (n = 4; P = .05) (Table 3).
Mean time to radiographic healing was 50.7 days in negative UDS patients and 82.8 days in positive UDS patients (P > .99). Incidence of nonunion was 3.5% in negative UDS patients and 20% in positive UDS patients (P = .01). There were no malunions in negative UDS patients and 2 malunions in positive UDS patients. Incidence of deep infections was 2.5% in negative UDS patients and 16% in positive UDS patients (P = .04). No significant differences were found in incidence of malunions, superficial infections, amputations, need for repeat surgery, continued bracing, or loss to follow-up.
Major complications were defined as superficial or deep infections, amputations, malunions, and nonunions. The rate of major complication was significantly (P = .03) higher in positive UDS patients (24.24%) than in negative UDS patients (7.69%) (Table 4).
Discussion
In the present study, we retrospectively reviewed the cases of patients treated with ORIF for varying types of ankle fractures. Important major and minor complications were analyzed. The overall incidence of major complications in negative UDS patients was only 7.69%, consistent with previously reported results in patients with ankle fractures.6,10 However, a statistically significant (P = .03) increased incidence of major complications—an alarmingly high rate of almost 1 in 4—was found in positive UDS patients. Our results also demonstrated a significantly higher rate of nonunion and deep infection in positive UDS patients. Calculated odds ratios were 7.37 and 4.27 for nonunion and deep infection, respectively—arguably 2 of the most devastating postoperative complications in positive UDS patients.
Previous studies have found that open fractures, age, and medical comorbidities are significant predictors of short-term complications, such as wound healing, infection, persistent pain, and delayed union.3-6 Levy and colleagues11 examined the incidence of orthopedic trauma in positive UDS patients. These patients had orthopedic injuries that were more severe and required longer hospitalization. However, the study did not address patients with ankle fractures or the incidence of major complications. Diabetes and peripheral vascular disease are significant risk factors for many surgical procedures in orthopedic surgery.3,7-9,12,13 Tight glycemic control and optimization of medical comorbidities decrease postoperative complications.12,13 SooHoo and colleagues6 found that history of diabetes and history of peripheral vascular disease were significant predictors of short-term complications of mortality, infection, reoperation, and amputation. The rate of infection in the complicated diabetes group was statistically higher as well. The effect of illicit drug use was not analyzed in that study. We think the findings of the present study highlight the importance of screening for high-risk populations (eg, patients with diabetes, patients with peripheral vascular disease, drug abusers) before orthopedic surgery, especially during definitive treatment of ankle fracture.
Recently, Nåsell and colleagues10 found that a well-implemented smoking cessation program was associated with a statistically significant reduction in complications 6 and 12 weeks after surgery. The target treatment groups were patients who underwent major lower extremity and upper extremity orthopedic surgery. The most common surgery performed in the study was ORIF of ankle fractures. The authors concluded that a smoking cessation intervention program during the first 6 weeks after acute fracture surgery decreases the risk for postoperative complications. However, no recommendations were made for treating patients with other addictions, such as alcohol and illicit drug addictions.
To our knowledge, our study is the first to critically examine postoperative complications in ankle fracture patients with a history of illicit drug abuse as determined by preoperative positive UDS. These data suggest the importance of critically evaluating this patient population. The rates of deep infection, nonunion, and pooled major complications were all notable. Furthermore, compared with negative UDS patients, positive UDS patients were more than 7 times likely to develop a nonunion and more than 4 times likely to develop a deep infection. The reasons are likely multifactorial but may involve factors such as injury severity, poor nutrition, suboptimal living conditions, difficulty complying with weight-bearing restrictions, and, possibly, poor compliance with wound-care recommendations. Determining the influence of each factor was beyond the scope of this study. However, further investigation is warranted.
The difference in incidence of smoking between the 2 groups was statistically significant. As smoking has been well documented as contributing to poor wound and bone healing,14-16 it is likely to have been a contributory factor. However, nicotine levels are not routinely part of UDS, and people who quit smoking typically take 7 to 10 days to demonstrate a measurable drop in cotinine levels. On the other hand, screening for drugs takes only a few minutes and can provide useful information during the preoperative period. It was suggested that positive UDS patients were significantly likely to be tobacco users as well.
The 2 groups were not significantly different with respect to mean follow-up time or loss to follow-up. Although mean follow-up was longer in negative UDS patients, the standard deviation was large in both groups. Given the positive UDS patients’ higher incidence of deep infection and nonunion, both of which typically prolong the course of treatment, the results were likely deceptive. Patients with a history of illicit drug use have confounding variables (eg, psychiatric disorders, financial strife) that make treatment compliance and follow-up difficult.17
Some of the weaknesses of this study are inherent to its retrospective design and limited sample size. Furthermore, patient satisfaction scores and ankle-specific outcome measures, such as AOFAS (American Orthopaedic Foot and Ankle Society) scores, were not considered. Prospective collection of data that include patient satisfaction scores and ankle-specific outcome measures would be optimal. Our current recommendation is to obtain preoperative UDS and illicit drug use history for all trauma patients. In addition, operating surgeons should exercise caution when caring for patients who test positive for illicit drugs.
Conclusion
We evaluated the incidence of complications experienced by positive UDS patients undergoing surgical treatment of ankle fractures. It is well documented that illicit drug users who receive general anesthesia have complications. However, little is known about the untoward effects of illicit drugs on postoperative complications. Furthermore, the efficacy of drug cessation programs in minimizing these complications has not been fully explored.
In conclusion, similar to patients with diabetes, patients with a history of recent illicit drug use, as evidenced by preoperative positive UDS, are at increased risk for complications during treatment for ankle fracture. These data suggest that practicing orthopedists should be more vigilant when caring for ankle fracture patients with preoperative positive UDS.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
1. Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77(1):142-152.
2. Culver JL, Walker JR. Anesthetic implications of illicit drug use. J Perianesth Nurs. 1999;14(2):82-90.
3. Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32(1):113-133.
4. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2);288-293.
5. Clark RF, Harchelroad F. Toxicology screening of the trauma patient: a changing profile. Ann Emerg Med. 1991;20(2):151-153.
6. SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91(5):1042-1049.
7. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570-1578.
8. Egol KA, Tejwani NC, Walsh MG, Capla EL, Koval KJ. Predictors of short-term functional outcome following ankle fracture surgery. J Bone Joint Surg Am. 2006;88(5):974-979.
9. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus J Bone Joint Surg Br. 2005;87(4):489-495.
10. Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010;92(6):1335-1342.
11. Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21-27.
12. Flynn JM, Rodriguez-del Rio F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21(4):311-319.
13. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375-380.
14. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1-5.
15. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85(2):178-181.
16. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
17. Torrens M, Gilchrist G, Domingo-Salvany A; PsyCoBarcelona Group. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2010;113(2-3):147-156.
A Novel Treatment for Refractory Plantar Fasciitis
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
Chronic plantar fasciitis is a major health care problem worldwide and affects nearly 10% of the US population. Plantar fasciitis presents as heel pain in the mornings and usually gets better and then gets worse. Inflammation at the plantar fascia attachment causes acute and sometimes disabling pain. Chronic pain at the site can develop as time goes on because of long-standing inflammatory changes. Fibrotic tissues may develop at the site. On a continuum, symptoms may begin in an insidious phase and progress to chronic pain. Although most cases resolve with conservative care, the numerous treatments for refractory plantar fasciitis attest to the lack of consensus regarding these cases. The condition frustrates patient and physician alike.
Treatments for refractory plantar fasciitis include conservative measures, including rest, analgesics, walking orthosis, heel cup, night splint, walking boot, and then, in a standard and logical progression, cortisone or platelet-rich plasma injections. Improved magnetic resonance imaging and ultrasonographic imaging allow accurate localization of the pathologic process,1-3 and this localization in turn provides an opportunity to deliver a more reliable and focused intervention, as in needle-guided therapy.4 Surgical procedures for plantar fasciitis have included open or endoscopically assisted plantar fasciectomies with or without gastrocnemius recession; these procedures have had varying results. The emerging goals for this condition are a minimally invasive percutaneous intervention that is safe, effective, and well-tolerated and has minimal morbidity and a low complication rate.
We conducted a prospective study in which patients were allowed either to continue with noninvasive treatment or to undergo focal aspiration and partial fasciotomy with an ultrasonic probe. Study inclusion criteria were plantar fasciitis symptoms lasting 12 months or longer. Exclusion criteria were unwillingness to participate in the study. Prior treatments, even surgeries, were not exclusionary.
Twelve patients with refractory plantar fasciitis lasting a mean of 19 months (minimum, 12 months; range, 12-24 months) chose the procedure. They all had failed conservative care, including physical therapy, casting, shockwave therapy, and invasive procedures such as injections and endoscopic partial releases. Four of the 12 had undergone an open or endoscopic partial release at a different institution but had experienced no improvement in symptoms.
Based on the study protocol, patients continued noninvasive care (night splint, stretching exercises) for 2 to 6 weeks after the initial visit. When this conservative care failed, they were offered focal partial fasciectomy with a percutaneous ultrasonic probe. American Orthopaedic Foot and Ankle Society (AOFAS) scores were obtained before and after surgery. Follow-up consisted of clinic visits 2 weeks after surgery and monthly thereafter. I saw all 12 patients 3 months after surgery (range, 11-14 weeks), and all 12 underwent postoperative physical therapy.
Technique
The TX1 Tissue Removal System (Tenex Health, Lake Forest, California) (Figure 1) consists of an energy module, a pump/suction cassette that provides irrigation and suction through a probe, and the probe itself, the TX1, which is the size of an 18-gauge needle and delivers ultrasonic energy. The cassette is inserted into the energy module, and the ultrasonic energy probe is primed so it will deliver the irrigation fluid, normal saline. The safety features of the energy module are such that no energy is expended unless the system is properly irrigating and aspirating the diseased tissue. Ultrasonic treatment may be performed in a clinical or ambulatory surgical center. The patient is placed supine on an operating table, on a clinical examining table, or, if in a cast room, on a cart. A pillow is placed under the distal tibia so the knees can flex slightly, and the patient is positioned so the feet are free of the edge of the bed or gurney (Figure 2).
The pathology is first confirmed by ultrasonography (Figures 3–5). The first step is to identify the calcaneus with the sensor along the long axis of the foot. Then the plantar fascia is visualized and followed along its long axis to the site of attachment at the medial tubercle. As the pathologic process involves the medial site of attachment, a transverse image may also be obtained to better understand the medial/lateral extent of the disease process. The ultrasonographic image of plantar fasciitis has been well characterized.2,5 The pathology is visualized as an area of edema or of disruption of the linear appearance of the fascia as it attaches to the calcaneus. While the diagnosis is being confirmed, the optimal site for probe insertion should be considered based on the location of the pain and the localization of the pathology by the 2 orthogonal images.
The area is prepared as if for an injection and is squared off with sterile towels. Then the sensor is placed in the sterile sleeve. The area of maximum tenderness is again confirmed. Determining the location of the probe insertion site is a crucial step. We use the ultrasonic sensor in the longitudinal and transverse planes to direct the injection of a fast-acting local anesthetic to the medial aspect of the calcaneus. A skin wheal is created, and the fast-acting local anesthetic (3-4 mL) is injected into the region of the fascia pathology.
An 11-blade knife is used to create a site for the probe through the skin wheal at the medial aspect of the heel, in line with the pathology (Figure 6). The probe is then introduced through the puncture site and is identified, along with the pathology, with the sensor, which may be oriented transverse or longitudinal to the long axis of the foot.
Once the pathologic area is identified, the ultrasonic energy is delivered to the region by the probe, which is activated with a foot pedal, effectively releasing the pathologic tissue from its insertion at the medial tubercle of the calcaneus. The probe is moved in a linear fashion medially and laterally within the lesion across the site of attachment. Treatment continues until the entire soft-tissue lesion is addressed.
Postoperative Care
The wound or wounds are closed with a nylon stitch and Steri-Strip (3M, St. Paul, Minnesota) and covered with Tegaderm (3M) or similar dressing (Figure 7). A compressive dressing is applied. The dressing is removed in 2 to 3 days; the Steri-Strip and stitch are removed in 10 to 14 days. A walking boot is put on immediately after the procedure (most patients in this study already have a boot) and is worn for a few days, or until the symptoms have resolved. How long the boot is used is very much based on patient preference. Patients may continue stretching exercises at home, but there should be no high-impact activity. As-needed ice and analgesics are recommended for the first few days.
The 12 patients had a mean preoperative AOFAS score of 30 (range, 17-46) and a mean postoperative score of 88 (range, 25-92). By the 3-month postoperative visit, symptoms were resolved in 11 patients (no activity restricted by plantar fascia pain). On physical examination, 11 patients had no palpable tenderness at the site of preoperative pain. Pain relief was documented as having occurred between 5 and 13 weeks after treatment. One patient had bilateral procedures. One foot was treated, pain resolved by the 3-month postoperative visit, and the patient asked for the other foot to be treated. Three months after the second procedure, he had minimal non-activity-restricting pain. There were no postoperative infections or wound complications.
I phoned my patients during postoperative month 24. All 12 patients (13 feet) indicated they were essentially pain-free. None admitted to activity restriction or required over-the-counter pain medication. All indicated they were satisfied with the procedure and would have it again.
The refractory nature of plantar fasciitis, and the resistance to and unpredictability of current treatment options, is well known. Considerable efforts have been made to develop treatment guidelines and algorithms.6 A standard and logical treatment plan involves initial attempts with rest, analgesics, and a walking orthosis and then, if those fail, cortisone or platelet-rich plasma injections. Reluctance to perform surgery is well justified because of the unpredictability of the intervention. As might be expected, the utility of ultrasonography has been on the rise. The diagnostic value of ultrasonography, first recognized in the early 1970s, is of increasing importance.7,8 Subsequent use of ultrasonographic imaging as guidance for various treatments, including percutaneous release, has also been recognized and documented.4,9-12 The present article is the first to describe and document the outcome of using ultrasonic energy for percutaneous release of the diseased attachment of the plantar fascia.
This report is preliminary and was designed to alert the orthopedic community to a safe and promising treatment for a chronic, refractory condition. The safety and efficacy of this treatment are reflected in our experience and have been documented for tennis elbow as well.13
This study was limited by its single-surgeon and relatively small clinical experience. Nevertheless, the benefits of this novel technique—effectiveness, safety, tolerability, and rapid recovery—are encouraging enough to share at this time. Prospective randomized controlled studies are needed.
Conclusion
This is the first report of a plantar fascia partial release guided by ultrasonic energy delivered by a percutaneously inserted probe under local anesthesia. The procedure appears to be a safe, effective, well-tolerated treatment for a condition that is refractory to other options. More studies are needed to further validate the safety and efficacy of this innovative treatment modality.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
1. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465-470.
2. Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med. 1987;21(4):158-162.
3. Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341-355.
4. Royall NA, Farrin E, Bahner DP, Stawicki SP. Ultrasound-assisted musculoskeletal procedures: a practical overview of current literature. World J Orthop. 2011;2(7):57-66.
5. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255-259.
6. Thomas JL, Christensen JC, Kravitz SR, et al; American College of Foot and Ankle Surgeons Heel Pain Committee. The diagnosis and treatment of heel pain: a clinical practice guideline—revision 2010. J Foot Ankle Surg. 2010;49(3 suppl):S1-S19.
7. McDonald DG, Leopold GR. Ultrasound B–scanning in the differentiation of Baker’s cyst and thrombophlebitis. Br J Radiol. 1972;45(538):729-732.
8. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
9. Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16(12):831-842.
10. Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34(4):573-580.
11. Debrule MB. Ultrasound-guided Weil percutaneous plantar fasciotomy. J Am Podiatr Med Assoc. 2010;100(2):146-148.
12. Vohra PK, Japour CJ. Ultrasound-guided plantar fascia release technique: a retrospective study of 46 feet. J Am Podiatr Med Assoc. 2009;99(3):183-190.
13. Koh JS, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
Antibiotic Cement-Coated Plates for Management of Infected Fractures
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.