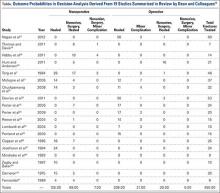
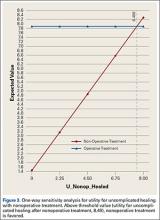
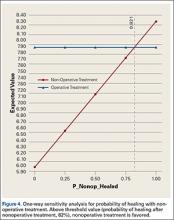
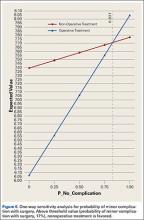
User login
Foot and Ankle Injuries in American Football
Foot and ankle injuries are common in American football, with injury rates significantly increasing over the past decade.1-5 Epidemiologic studies of collegiate football players have shown an annual incidence of foot and ankle injuries ranging from 9% to 39%,3,6 with as many as 72% of all collegiate players presenting to the National Football League (NFL) Combine with a history of a foot or ankle injury and 13% undergoing surgical treatment.5 Player position influences the rate and type of foot and ankle injury. Offensive and “skill position” players, including linemen, running backs, and wide receivers, are particularly susceptible to foot and ankle injuries due to high levels of force and torque placed on the distal extremity during running, cutting, and tackling. Shoe wear changes, playing field conditions, increasing player size and speed, and improved reporting of injuries are also contributing to increasing injury rates.
The interaction between player cleats and the playing surface is a central issue of foot and ankle injuries in football. Improved traction relates to performance, but increased subsequent torque on the lower extremity is associated with injury. While lateral ankle sprains are the most common foot and ankle injury experienced by football players,7 numerous other injuries can occur, including turf toe, Jones fractures, Lisfranc injuries, syndesmotic disruption, deltoid complex avulsion, and Achilles ruptures. It is important for physicians to be able to recognize, diagnose, and appropriately treat these injuries in players in order to expedite recovery, restore function, and help prevent future injury and long-term sequelae. This review focuses on updated treatment principles, surgical advances, and rehabilitation protocols for common football foot and ankle injuries.
Turf Toe
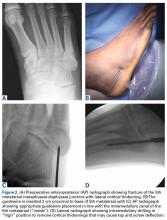
The term “turf toe” was first used in 1976 to refer to hyperextension injuries and plantar capsule-ligament sprains of the hallux metatarsophalangeal (MTP) joint that can lead to progressive cock-up deformity.8 While these injuries can occur on any surface and disrupt soft tissue balance with functional implications, predisposing factors include increasing playing surface hardness and decreasing shoe stiffness. In a classic scenario, the foot is fixed in equinus as an axial load is placed on the back of the heel, resulting in forced dorsiflexion of the hallux MTP joint.9 As the proximal phalanx extends, the sesamoids are drawn distally and the more dorsal portion of the metatarsal head articular surface bears the majority of the load, causing partial or complete tearing of the plantar plate with or without hallux MTP dislocation. Osteochondral lesions of the MTP joint and subchondral edema of the metatarsal head can occur concurrently as the proximal phalanx impacts or shears across the metatarsal head articular surface.
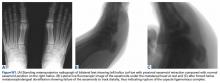
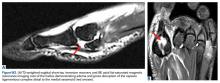
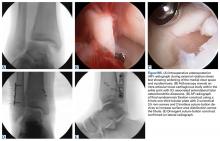
Clinical examination should focus on hallux swelling, alignment, and flexor hallucis longus (FHL) function along with vertical instability of the hallux MTP joint using a Lachman test. Radiographs should be evaluated for proximal migration of the sesamoids or diastasis (Figures W1A-W1C).
Indications for surgical intervention include loss of push-off strength, gross MTP instability, proximal migration of the sesamoids, and progressive hallux malalignment or clawing after immobilization. Cases can involve one or a combination of the following: (1) large capsular avulsion with unstable MTP joint; (2) diastasis of bipartite sesamoid; (3) diastasis of sesamoid fracture; (4) retraction of sesamoid; (5) traumatic hallux valgus deformity; (6) vertical instability (positive Lachman test); (7) loose body in MTP joint; or (8) chondral injury in MTP joint. The goal of surgery is the restoration of anatomy in order to restore normal function of the hallux MTP joint.
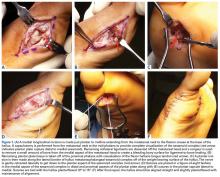
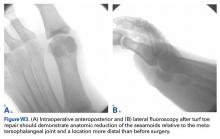
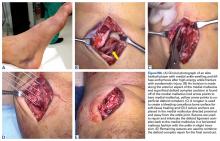
We have found that using dual medial and plantar incisions places less traction on the plantar medial cutaneous nerve, improves lateral exposure, and provides better wound healing. The medial capsulotomy extends from the metatarsal neck to the mid-phalanx to provide complete visualization of the sesamoid complex (Figures 1A-1F).
It is important to recognize that not all turf toe injuries involve pure hyperextension on artificial playing surfaces. In recent years, we have found an increasing rate of medial variant turf toe injuries in which a forceful valgus stress on the hallux leads to rupture of the medial collateral ligament, medial or plantar-medial capsule, and/or abductor halluces. Medial variant turf toe can lead to progressive hallux valgus and a traumatic bunion with a significant loss of push-off strength and difficulty with cutting maneuvers. Surgical treatment requires a modified McBride bunionectomy with adductor tenotomy and direct repair of the medial soft tissue defect.
Postoperative management is just as important as proper surgical technique for these injuries and involves a delicate balance between protecting the repair and starting early range of motion (ROM). Patients are immobilized non-weight-bearing (NWB) for 5 to 7 days maximum followed immediately with the initiation of passive hallux plantarflexion to keep the sesamoids moving. Active hallux plantarflexion is started at 4 weeks after surgery with active dorsiflexion from 6 to 8 weeks. Patients are transitioned to an accommodative shoe with stiff hallux insert 8 weeks postoperative with continued therapy focusing on hallux ROM. Running is initiated at 12 weeks and return to play (RTP) is typically allowed 4 months after surgery.
Jones Fractures
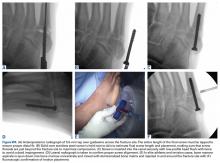
Jones fractures are fractures of the 5th metatarsal at the metaphyseal-diaphyseal junction, where there is a watershed area of decreased vascularity between the intramedullary nutrient and metaphyseal arteries. Current thought is that the rising rate of Jones fractures among football players is partially caused by the use of flexible, narrow cleats that do not provide enough stiffness and lateral support for the 5th metatarsal during running and cutting. Additionally, lateral overload from a baseline cavovarus foot posture with or without metatarsus adductus and/or skewfoot is thought to contribute to Jones fractures.10 Preoperative radiographs should be evaluated for fracture location, orientation, amount of cortical thickening, and overall geometry of the foot and 5th metatarsal. In elite athletes, the threshold for surgical intervention is decreasing in order to expedite healing and recovery and decrease re-fracture risk. This rationale is based on delayed union rates of 25% to 66%, nonunion rates of 7% to 28%,11 and re-fracture rates of up to 33% associated with nonoperative treatment.12 Nonoperative management is usually not feasible in the competitive athlete, as it typically involves a period of protected weight-bearing in a tall controlled ankle motion (CAM) boot for 6 to 8 weeks with serial radiographs to evaluate healing.
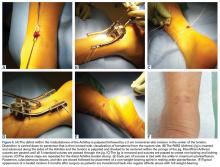
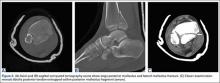
Our preference for surgical intervention involves percutaneous screw fixation with a “high and inside” starting point on fluoroscopy (Figures 2A-2D).
In career athletes, we augment the fracture site using a mixture of bone marrow aspirate concentrate (BMA) (Magellan, Arteriocyte Medical Systems) and demineralized bone matrix (DBM) (Mini Ignite, Wright Medical Technology) injected percutaneously in and around the fracture site under fluoroscopic guidance. Using this technique in a cohort of 25 NFL players treated operatively for Jones fractures, we found that 100% of athletes were able to RTP in the NFL in an average of 9.5 weeks.14 Two patients (7.5%) suffered re-fractures requiring revision surgery with iliac crest bone graft and repeat screw placement with a RTP after 15 weeks. We did not find an association between RTP and re-fracture rate.
The appropriate rehabilitation protocol for Jones fractures after surgery remains controversial and dependent on individual needs and abilities.15,16 For athletes in-season, we recommend a brief period of NWB for 1 to 2 weeks followed by toe-touch weight-bearing in a tall CAM boot for 2 to 4 weeks. After 4 weeks, patients begin gentle exercises on a stationary bike and pool therapy to reduce impact on the fracture site. Low-intensity pulsed ultrasound bone stimulators (Exogen, Bioventus) are frequently used directly over fracture site throughout the postoperative protocol as an adjuvant therapy. If clinically nontender over the fracture site, patients are allowed to begin running in modified protective shoe wear 4 weeks after surgery with an average RTP of 6 to 8 weeks. RTP is determined clinically, as radiographic union may not be evident for 12 to 16 weeks. Useful custom orthoses include turf toe plates with a cushioned lateral column and lateral heel wedge if hindfoot varus is present preoperatively.10 The solid intramedullary screw is left in place permanently.
In our experience, we have found the average re-fracture and nonunion rate to be approximately 8% across all athletes. Our observation that union rates do not appear to be related to RTP times suggests that underlying biology such as Vitamin D deficiency may play a larger role in union rates than previously thought. We have found that most Jones re-fractures occur in the first year after the initial injury, but can occur up to 2.5 years afterwards as well.14 For the management of symptomatic re-fractures and nonunions, the previous screw must be first removed. This can be difficult if the screw is bent or broken, and may require a lateral corticotomy of the metatarsal.
After hardware removal, we advocate open bone grafting of the fracture site using bone from the iliac crest retrieved with a small, percutaneous trephine. Re-fixation should be achieved using a larger, solid screw and postoperative adjuvants may include bone stimulators, Vitamin D and calcium supplemention, and possible teriparatide use (Forteo, Eli Lilly), depending on individual patient profile. In a cohort of 21 elite athletes treated for Jones fracture revision surgery with screw exchange and bone grafting, we found that 100% of patients had computed tomography (CT) evidence of union, with an average RTP of 12.3 weeks.17
Lisfranc Injuries
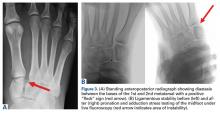
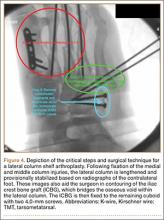
Lisfranc injuries include any bony or ligamentous damage that involves the tarsometatarsal (TMT) joints. While axial loading of a fixed, plantarflexed foot has traditionally been thought of as the most common mechanism of Lisfranc injury, we have found that noncontact twisting injuries leading to Lisfranc disruption are actually more common among NFL players. This mechanism is similar to noncontact turf toe and results in a purely ligamentous injury. We have found this to be particularly true in the case of defensive ends engaged with offensive linemen in which no axial loading or contact of the foot occurs. Clinically, patients often have painful weight-bearing, inability to perform a single limb heel rise, plantar ecchymosis, and swelling and point tenderness across the bases of the 1st and 2nd metatarsals.
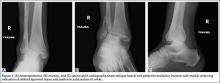
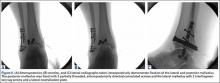
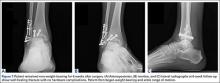
It is critical to obtain comparison weight-bearing radiographs of both feet during initial work-up to look for evidence of instability. Subtle radiographic findings of Lisfranc injury include a bony “fleck” sign, compression fracture of the cuboid, and diastasis between the base of the 1st and 2nd metatarsals and/or medial and middle cuneiforms (Figures 3A, 3B).
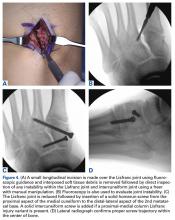
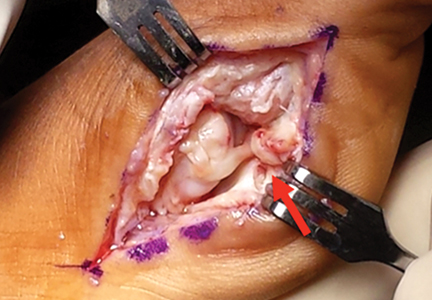
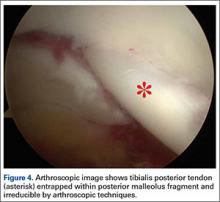
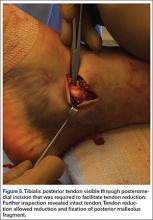
The goal of surgical intervention is to obtain and maintain anatomic reduction of all unstable joints in order to restore a normal foot posture. One of the difficulties with Lisfranc injuries is that there are no exact diastasis parameters and individuals should be treated based on symptoms, functional needs, and degree of instability. It has been shown that 5 mm of displacement can have good long-term clinical results in select cases without surgery.18 For surgery, we recommend open reduction to remove interposed soft tissue debris and directly assess the articular surfaces (Figures 4A-4D).
Proximal-medial column Lisfranc injury variants are increasingly common among football players.20 In these injuries, the force of injury extends through the intercuneiform joint and exits out the naviculocuneiform joint, thus causing instability at multiple joints and an unstable 1st ray. Patients often have minimal clinical findings and normal radiographs and stress radiographs. MRI of the foot often reveals edema at the naviculocuneiform joint. Often patients fail to improve with nonoperative immobilization with continued inability to push off from the hallux. Unrecognized or untreated instability will lead to rapid deterioration of the naviculocuneiform joint. Surgical intervention requires a homerun screw and intercuneiform screw. We do not recommend primary arthrodesis in athletes due to significant risk of malunion and nonunion unless severe articular damage is present.
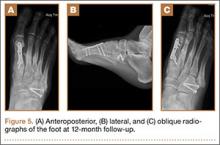
Patients are typically kept NWB in a splint for 2 weeks after surgery followed by NWB in a tall CAM from 3 to 4 weeks postoperative. Progressive weight-bearing and ROM exercises are initiated from 4 to 8 weeks, followed by return to accommodative shoe wear from 10 to 12 weeks. Hardware removal is performed 4 to 6 months after surgery, typically in the off-season to allow for 6 to 8 weeks or protected recovery afterwards. Premature hardware removal can lead to loss of reduction, particularly at the intercuneiform joints. All hardware crossing the TMT joints should be removed, while the homerun screw can be left in place in addition to the intercuneiform screw. RTP in football typically occurs 6 to 7 months after surgery. Final functional outcome is related to the adequacy of initial reduction and severity of the initial injury.21
Syndesmotic Disruption
Syndesmotic injuries comprise 1% to 18% of ankle sprains in the general population, but occur at much higher rates in football due to the increased rotation forces placed on the ankle during cutting and tackling. RTP after syndesmotic injury often takes twice as long when compared to isolated lateral ankle ligamentous injury.22 Missed injuries are common and if not treated properly can lead to chronic ankle instability and posttraumatic ankle arthritis.23 Syndesmotic injury can occur in isolation or with concomitant adjacent bony, cartilaginous, or ligamentous injuries. Therefore, clinical examination and imaging work-up are critical to successful management.
Syndesmotic injuries often result from an external rotation force applied to a hyperdorsiflexed ankle while the foot is planted. This mechanism causes the fibula to externally rotate while translating posteriorly and laterally, resulting in rupture of the anterior inferior tibiofibular ligament (AITFL) first, followed by the deep deltoid ligament, interosseous ligament (IOL), and lastly posterior talofibular ligament.24 Most syndesmotic injuries involve rupture of only the AITFL and IOL.25 Multiple clinical stress tests have been designed to assess syndesmotic stability, including the squeeze test, external rotation stress test, crossed-leg test, and fibula-translation test.26-29 However, no physical examination maneuver has been shown to reliably predict the presence or degree of syndesmotic injury and therefore imaging studies are necessary.30
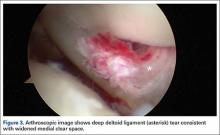
Initial imaging should include standing radiographs of the affected ankle. An increase in the medial clear space between the medial malleolus and talus can occur with a combined syndesmotic and deltoid disruption. In the case of subtle syndesmotic injuries, contralateral comparison weight-bearing radiographs are recommended. CT and MRI can provide additional information, but these static imaging tests cannot identify instability. Fluoroscopic stress evaluation is beneficial but has a high false-negative rate in low-grade injuries and may not detect partial rupture of the AITFL and IOL.31 It has been shown that malrotation of as much as 30° of external rotation can occur if relying on intraoperative fluoroscopy alone.32 It has been our practice to recommend surgical reduction and stabilization for any syndesmotic injury with documented diastasis or instability seen on imaging and/or arthroscopy.
Nonoperative treatment is indicated for truly stable grade I syndesmotic injuries. This involves rest and immobilization followed by a progressive rehabilitation program consisting of stretching, strengthening, and proprioceptive exercises.33 After 1 week of protected weight-bearing in a cast or tall CAM boot, progression to full weight-bearing should occur over the following week. Active-assisted ankle ROM exercises and light proprioceptive training should then be initiated followed by sport-specific exercises 2 to 3 weeks after injury.
Arthroscopy can be a valuable diagnostic tool in the setting of subtle syndesmotic injury with negative radiographs, positive MRI for edema, and a protracted recovery course with vague pain (Figures W5A-W5E).
Implants are placed above the true syndesmotic joint (at least 15 mm above the tibial plafond) angled 30° posterior to anterior to follow the normal relationship of the fibula to the distal tibia in the incisura. Typically 2 suture-buttons are used, with the devices placed in a divergent fashion. We highly recommend the use of a fibular buttress plate with button placement in individuals returning to contact activity. This construct increases surface area distribution while preventing stress risers and the risk of fibula fractures. In a cadaver model with deliberate syndesmotic malreduction, suture-button stabilization resulted in decreased postoperative displacement as opposed to conventional screw fixation.34 Therefore, dynamic syndesmotic fixation may help to decrease the negative sequelae of iatrogenic clamp malreduction.
Postoperative rehabilitation involves NWB in a cast or tall CAM boot for 4 weeks followed by ankle ROM exercises and progressive weight-bearing and physical therapy. Patients are transitioned to a lace-up ankle brace and athletic shoe from 6 to 12 weeks postoperative with increasing activity. Running and jumping is permitted 4 months after surgery with RTP typically at 6 to 7 months. Athletes who have had surgical stabilization for documented instability without any diastasis may engage in a more rapid recovery and RTP as symptoms and function allow.
Deltoid Complex Avulsion
Missed or neglected deltoid ligament injuries can lead to progressive chondral injury and joint degeneration. These injuries are often subtle and difficult to diagnose. An inability to perform a single limb heel rise, persistent pain with activity, and lack of normal functional improvement despite appropriate care are indicators of subtle ligament instability. These injuries often require an examination under anesthesia with combined ankle arthroscopy. Valgus stress testing of the ankle while directly visualizing the deltoid ligament from the anterolateral portal can reveal medial laxity in addition to potential osteochondral lesions along the anterolateral talar dome.
In American football players, we have observed that infolding and retraction of an avulsed superficial deltoid ligament complex after an ankle fracture, Maisonneuve injury, or severe high ankle sprain can be a source of persistent increased medial clear space, malreduction, and postoperative pain and medial instability. We have found that there is often complete avulsion of the superficial deltoid complex off the proximal aspect of the medial malleolus during high-energy ankle fractures in football players that is amenable to direct repair to bone (Figures W6A-W6E).
During surgical repair, an incision is made along the anterior aspect of the medial malleolus and the superficial deltoid ligament complex can often be found flipped and interposed in the medial gutter. A rongeur is used to create a bleeding cancellous bone surface for soft-tissue healing and 1 to 2 suture anchors are used to repair and imbricate the deltoid ligament complex back to the medial malleolus. The goal of these sutures is to repair the tibionavicular and tibial spring ligaments back to the medial malleolus. We believe that superficial deltoid complex avulsion during high-energy ankle fractures is a distinct injury pattern that should be recognized and may benefit from primary open repair.
We currently open explore every deltoid ligament complex in athletes with unstable syndesmotic injuries, as we believe that deltoid avulsion injuries are underrecognized and do not heal in an anatomic fashion if left alone. Postoperative recovery follows the same immobilization, progressive weight-bearing, and physical therapy protocol as that for syndesmotic disruption.
Achilles Ruptures
Acute midsubstance Achilles tendon ruptures are an increasingly common injury in patients 30 to 50 years of age, with more than 50% of all injuries occurring during basketball.36,37 Among NFL players, we have found that Achilles ruptures tend to occur at a higher rate during training camp, when athletes are deconditioned and quickly returning to explosive push-off activities. Physical examination should include a Thompson test, palpation of a gap within the tendon, and evaluation of resting ankle dorsiflexion in the affected extremity in the prone position with the knees bent. Lateral radiographs should be analyzed for the presence of a bony avulsion fragment indicative of an insertional avulsion injury or midsubstance calcium deposition reflecting chronic Achilles tendinosis, as both of these conditions will change surgical management. MRI is not recommended with acute midsubstance ruptures but may be helpful in the case of chronic ruptures or more proximal tears of the musculotendinous junction.
The management of acute midsubstance Achilles tendon ruptures is controversial, with no general consensus in the literature regarding nonoperative treatment, surgical repair, and ideal repair technique.36,38-42 American Academy of Orthopaedic Surgeons clinical practice guidelines report moderate evidence that nonoperative treatment of Achilles tendon ruptures has lower wound healing complications but higher rates of re-rupture.38,39 Additionally, limited incision approaches have been found to have fewer overall complications compared with traditional open repair. In an effort to reduce the incidence of postoperative wound complications while improving functional recovery, modern repair techniques focus on a limited incision repair using percutaneous suture insertion and management (PARS Achilles Jig System, Arthrex).36 The limited incision technique utilizes a 2-cm transverse incision and non-disposable jig with divergent needle passes and locking suture fixation options to secure and fixate both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. Limited incision repair is ideally performed within 2 weeks of the injury to ensure that both tendon ends are easy to identify, mobilize, and repair. An open repair is generally recommended for midsubstance ruptures more than 4 weeks old and cases of insertional rupture and Achilles tendinopathy.
In a cohort of 9 NFL players treated for midsubstance Achilles ruptures using the PARS technique, we found no re-ruptures, no wound complications, and no sural nerve issues after surgery.43 A comparative review of 270 cases of operatively treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) showed that the PARS group had significantly shorter operative times and a higher number of patients able to return to baseline physical activities by 5 months compared to open repair.36 Although not statistically significant, the overall PARS complication rate was 5% while the open complication rate was 11%. The PARS group had no cases of sural neuritis or deep infection requiring reoperation. We currently use a limited incision technique for all acute midsubstance Achilles ruptures in athletes regardless of sport, patient size, or position played.
During surgery, a 2-cm transverse incision is made over the gap in the Achilles tendon and dissection is carried down to the rupture site with minimal manipulation of the skin (Figures 5A-5F).
A key aspect of postoperative recovery is avoiding excessive ankle dorsiflexion while the tendon is healing during the first 4 weeks after surgery, as this can lead to an elongated tendon with loss of push-off strength. Patients are kept in a plantarflexion splint NWB for 2 weeks after surgery. If the incision is healed at 2 weeks, sutures are removed and patients are transitioned into a NWB tall CAM boot for 2 weeks with gentle ankle ROM exercises. If there is any concern regarding wound healing status, sutures are maintained for an additional 1 to 2 weeks.
From 4 to 8 weeks after surgery, progressive weight-bearing with continued ankle ROM exercises is initiated with peel-away heel lifts (~2 cm thick total, 3 layers). Each layer of the heel lift is gradually removed as pain allows every 2 to 3 days with the goal of being full weight-bearing with the foot flat at 6 weeks postoperative. Physical therapy focusing on ankle ROM and gentle Achilles stretching and strengthening is also started 6 weeks after surgery. From 8 to 12 weeks postoperative, patients are transitioned out of the tall CAM boot into normal, accommodative shoe wear with full weight-bearing. We avoid ankle dorsiflexion past neutral until 12 weeks after surgery, as overlengthening of the Achilles complex and the subsequent loss of push-off power can be devastating to running athletes. Activity levels are increased as tolerated, with no running or jumping from 12 to 16 weeks with full release to all activities after 16 weeks. RTP often takes 5 to 6 months after surgery, depending on the position played.
Am J Orthop. 2016;45(6):358-367. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Canale ST, Cantler ED Jr, Sisk TD, Freeman BL 3rd. A chronicle of injuries of an American intercollegiate football team. Am J Sports Med. 1981;9(6):384-389.2. Robey JM, Blyth CS, Mueller FO. Athletic injuries. Application of epidemiologic methods. JAMA. 1971;217(2):184-189.
3. Saal JA. Common American football injuries. Sports Med. 1991;12(2):132-147.
4. Thompson N, Halpern B, Curl WW, et al. High school football injuries: evaluation. Am J Sports Med. 1987;15(2):117-124.
5. Kaplan LD, Jost PW, Honkamp N, Norwig J, West R, Bradley JP. Incidence and variance of foot and ankle injuries in elite college football players. Am J Orthop. 2011;40(1):40-44.
6. DeLee JC, Farney WC. Incidence of injury in Texas high school football. Am J Sports Med. 1992;20(5):575-580.
7. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
8. Bowers KD Jr, Martin RB. Turf-toe: a shoe-surface related football injury. Med Sci Sports. 1976;8(2):81-83.
9. McCormick JJ, Anderson RB. Turf toe: anatomy, diagnosis, and treatment. Sports Health. 2010;2(6):487-494.
10. Raikin SM, Slenker N, Ratigan B. The association of a varus hindfoot and fracture of the fifth metatarsal metaphyseal-diaphyseal junction: the Jones fracture. Am J Sports Med. 2008;36(7):1367-1372.
11. Title CI, Katchis SD. Traumatic foot and ankle injuries in the athlete. Orthop Clin North Am. 2002;33(3):587-598.
12. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
13. Nunley JA, Glisson RR. A new option for intramedullary fixation of Jones fractures: the Charlotte Carolina Jones Fracture System. Foot Ankle Int. 2008;29(12):1216-1221.
14. Lareau CR, Hsu AR, Anderson RB. Return to play in National Football League players after operative Jones fracture treatment. Foot Ankle Int. 2016;37(1):8-16.
15. Larson CM, Almekinders LC, Taft TN, Garrett WE. Intramedullary screw fixation of Jones fractures. Analysis of failure. Am J Sports Med. 2002;30(1):55-60.
16. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
17. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
18. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
19. Alberta FG, Aronow MS, Barrero M, Diaz-Doran V, Sullivan RJ, Adams DJ. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int. 2005;26(6):462-473.
20. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle Surg. 2010;9(3):100-106.
21. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82-A(11):1609-1618.
22. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
23. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35(7):1197-1207.
24. Beumer A, Valstar ER, Garling EH, et al. Effects of ligament sectioning on the kinematics of the distal tibiofibular syndesmosis: a radiostereometric study of 10 cadaveric specimens based on presumed trauma mechanisms with suggestions for treatment. Acta Orthop. 2006;77(3):531-540.
25. McCollum GA, van den Bekerom MP, Kerkhoffs GM, Calder JD, van Dijk CN. Syndesmosis and deltoid ligament injuries in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1328-1337.
26. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
27. Nussbaum ED, Hosea TM, Sieler SD, Incremona BR, Kessler DE. Prospective evaluation of syndesmotic ankle sprains without diastasis. Am J Sports Med. 2001;29(1):31-35.
28. Kiter E, Bozkurt M. The crossed-leg test for examination of ankle syndesmosis injuries. Foot Ankle Int. 2005;26(2):187-188.
29. Beumer A, van Hemert WL, Swierstra BA, Jasper LE, Belkoff SM. A biomechanical evaluation of clinical stress tests for syndesmotic ankle instability. Foot Ankle Int. 2003;24(4):358-363.
30. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14(4):232-236.
31. Beumer A, Valstar ER, Garling EH, et al. External rotation stress imaging in syndesmotic injuries of the ankle: comparison of lateral radiography and radiostereometry in a cadaveric model. Acta Orthop Scand. 2003;74(2):201-205.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2(6):460-470.
34. Westermann RW, Rungprai C, Goetz JE, Femino J, Amendola A, Phisitkul P. The effect of suture-button fixation on simulated syndesmotic malreduction: a cadaveric study. J Bone Joint Surg Am. 2014;96(20):1732-1738.
35. Hsu AR, Lareau CR, Anderson RB. Repair of acute superficial deltoid complex avulsion during ankle fracture fixation in National Football League players. Foot Ankle Int. 2015;36(11):1272-1278.
36. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of percutaneous Achilles repair system versus open technique for acute achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
37. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
38. Chiodo CP, Glazebrook M, Bluman EM, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
39. Chiodo CP, Glazebrook M, Bluman EM, et al. Diagnosis and treatment of acute achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
40. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
41. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
42. Khan RJ, Carey Smith RL. Surgical interventions for treating acute achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
43. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of achilles rupture in the national football league. J Surg Orthop Adv. 2014;23(4):179-183.
Foot and ankle injuries are common in American football, with injury rates significantly increasing over the past decade.1-5 Epidemiologic studies of collegiate football players have shown an annual incidence of foot and ankle injuries ranging from 9% to 39%,3,6 with as many as 72% of all collegiate players presenting to the National Football League (NFL) Combine with a history of a foot or ankle injury and 13% undergoing surgical treatment.5 Player position influences the rate and type of foot and ankle injury. Offensive and “skill position” players, including linemen, running backs, and wide receivers, are particularly susceptible to foot and ankle injuries due to high levels of force and torque placed on the distal extremity during running, cutting, and tackling. Shoe wear changes, playing field conditions, increasing player size and speed, and improved reporting of injuries are also contributing to increasing injury rates.
The interaction between player cleats and the playing surface is a central issue of foot and ankle injuries in football. Improved traction relates to performance, but increased subsequent torque on the lower extremity is associated with injury. While lateral ankle sprains are the most common foot and ankle injury experienced by football players,7 numerous other injuries can occur, including turf toe, Jones fractures, Lisfranc injuries, syndesmotic disruption, deltoid complex avulsion, and Achilles ruptures. It is important for physicians to be able to recognize, diagnose, and appropriately treat these injuries in players in order to expedite recovery, restore function, and help prevent future injury and long-term sequelae. This review focuses on updated treatment principles, surgical advances, and rehabilitation protocols for common football foot and ankle injuries.
Turf Toe
The term “turf toe” was first used in 1976 to refer to hyperextension injuries and plantar capsule-ligament sprains of the hallux metatarsophalangeal (MTP) joint that can lead to progressive cock-up deformity.8 While these injuries can occur on any surface and disrupt soft tissue balance with functional implications, predisposing factors include increasing playing surface hardness and decreasing shoe stiffness. In a classic scenario, the foot is fixed in equinus as an axial load is placed on the back of the heel, resulting in forced dorsiflexion of the hallux MTP joint.9 As the proximal phalanx extends, the sesamoids are drawn distally and the more dorsal portion of the metatarsal head articular surface bears the majority of the load, causing partial or complete tearing of the plantar plate with or without hallux MTP dislocation. Osteochondral lesions of the MTP joint and subchondral edema of the metatarsal head can occur concurrently as the proximal phalanx impacts or shears across the metatarsal head articular surface.
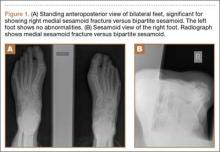
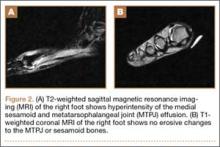
Clinical examination should focus on hallux swelling, alignment, and flexor hallucis longus (FHL) function along with vertical instability of the hallux MTP joint using a Lachman test. Radiographs should be evaluated for proximal migration of the sesamoids or diastasis (Figures W1A-W1C).
Indications for surgical intervention include loss of push-off strength, gross MTP instability, proximal migration of the sesamoids, and progressive hallux malalignment or clawing after immobilization. Cases can involve one or a combination of the following: (1) large capsular avulsion with unstable MTP joint; (2) diastasis of bipartite sesamoid; (3) diastasis of sesamoid fracture; (4) retraction of sesamoid; (5) traumatic hallux valgus deformity; (6) vertical instability (positive Lachman test); (7) loose body in MTP joint; or (8) chondral injury in MTP joint. The goal of surgery is the restoration of anatomy in order to restore normal function of the hallux MTP joint.
We have found that using dual medial and plantar incisions places less traction on the plantar medial cutaneous nerve, improves lateral exposure, and provides better wound healing. The medial capsulotomy extends from the metatarsal neck to the mid-phalanx to provide complete visualization of the sesamoid complex (Figures 1A-1F).
It is important to recognize that not all turf toe injuries involve pure hyperextension on artificial playing surfaces. In recent years, we have found an increasing rate of medial variant turf toe injuries in which a forceful valgus stress on the hallux leads to rupture of the medial collateral ligament, medial or plantar-medial capsule, and/or abductor halluces. Medial variant turf toe can lead to progressive hallux valgus and a traumatic bunion with a significant loss of push-off strength and difficulty with cutting maneuvers. Surgical treatment requires a modified McBride bunionectomy with adductor tenotomy and direct repair of the medial soft tissue defect.
Postoperative management is just as important as proper surgical technique for these injuries and involves a delicate balance between protecting the repair and starting early range of motion (ROM). Patients are immobilized non-weight-bearing (NWB) for 5 to 7 days maximum followed immediately with the initiation of passive hallux plantarflexion to keep the sesamoids moving. Active hallux plantarflexion is started at 4 weeks after surgery with active dorsiflexion from 6 to 8 weeks. Patients are transitioned to an accommodative shoe with stiff hallux insert 8 weeks postoperative with continued therapy focusing on hallux ROM. Running is initiated at 12 weeks and return to play (RTP) is typically allowed 4 months after surgery.
Jones Fractures
Jones fractures are fractures of the 5th metatarsal at the metaphyseal-diaphyseal junction, where there is a watershed area of decreased vascularity between the intramedullary nutrient and metaphyseal arteries. Current thought is that the rising rate of Jones fractures among football players is partially caused by the use of flexible, narrow cleats that do not provide enough stiffness and lateral support for the 5th metatarsal during running and cutting. Additionally, lateral overload from a baseline cavovarus foot posture with or without metatarsus adductus and/or skewfoot is thought to contribute to Jones fractures.10 Preoperative radiographs should be evaluated for fracture location, orientation, amount of cortical thickening, and overall geometry of the foot and 5th metatarsal. In elite athletes, the threshold for surgical intervention is decreasing in order to expedite healing and recovery and decrease re-fracture risk. This rationale is based on delayed union rates of 25% to 66%, nonunion rates of 7% to 28%,11 and re-fracture rates of up to 33% associated with nonoperative treatment.12 Nonoperative management is usually not feasible in the competitive athlete, as it typically involves a period of protected weight-bearing in a tall controlled ankle motion (CAM) boot for 6 to 8 weeks with serial radiographs to evaluate healing.
Our preference for surgical intervention involves percutaneous screw fixation with a “high and inside” starting point on fluoroscopy (Figures 2A-2D).
In career athletes, we augment the fracture site using a mixture of bone marrow aspirate concentrate (BMA) (Magellan, Arteriocyte Medical Systems) and demineralized bone matrix (DBM) (Mini Ignite, Wright Medical Technology) injected percutaneously in and around the fracture site under fluoroscopic guidance. Using this technique in a cohort of 25 NFL players treated operatively for Jones fractures, we found that 100% of athletes were able to RTP in the NFL in an average of 9.5 weeks.14 Two patients (7.5%) suffered re-fractures requiring revision surgery with iliac crest bone graft and repeat screw placement with a RTP after 15 weeks. We did not find an association between RTP and re-fracture rate.
The appropriate rehabilitation protocol for Jones fractures after surgery remains controversial and dependent on individual needs and abilities.15,16 For athletes in-season, we recommend a brief period of NWB for 1 to 2 weeks followed by toe-touch weight-bearing in a tall CAM boot for 2 to 4 weeks. After 4 weeks, patients begin gentle exercises on a stationary bike and pool therapy to reduce impact on the fracture site. Low-intensity pulsed ultrasound bone stimulators (Exogen, Bioventus) are frequently used directly over fracture site throughout the postoperative protocol as an adjuvant therapy. If clinically nontender over the fracture site, patients are allowed to begin running in modified protective shoe wear 4 weeks after surgery with an average RTP of 6 to 8 weeks. RTP is determined clinically, as radiographic union may not be evident for 12 to 16 weeks. Useful custom orthoses include turf toe plates with a cushioned lateral column and lateral heel wedge if hindfoot varus is present preoperatively.10 The solid intramedullary screw is left in place permanently.
In our experience, we have found the average re-fracture and nonunion rate to be approximately 8% across all athletes. Our observation that union rates do not appear to be related to RTP times suggests that underlying biology such as Vitamin D deficiency may play a larger role in union rates than previously thought. We have found that most Jones re-fractures occur in the first year after the initial injury, but can occur up to 2.5 years afterwards as well.14 For the management of symptomatic re-fractures and nonunions, the previous screw must be first removed. This can be difficult if the screw is bent or broken, and may require a lateral corticotomy of the metatarsal.
After hardware removal, we advocate open bone grafting of the fracture site using bone from the iliac crest retrieved with a small, percutaneous trephine. Re-fixation should be achieved using a larger, solid screw and postoperative adjuvants may include bone stimulators, Vitamin D and calcium supplemention, and possible teriparatide use (Forteo, Eli Lilly), depending on individual patient profile. In a cohort of 21 elite athletes treated for Jones fracture revision surgery with screw exchange and bone grafting, we found that 100% of patients had computed tomography (CT) evidence of union, with an average RTP of 12.3 weeks.17
Lisfranc Injuries
Lisfranc injuries include any bony or ligamentous damage that involves the tarsometatarsal (TMT) joints. While axial loading of a fixed, plantarflexed foot has traditionally been thought of as the most common mechanism of Lisfranc injury, we have found that noncontact twisting injuries leading to Lisfranc disruption are actually more common among NFL players. This mechanism is similar to noncontact turf toe and results in a purely ligamentous injury. We have found this to be particularly true in the case of defensive ends engaged with offensive linemen in which no axial loading or contact of the foot occurs. Clinically, patients often have painful weight-bearing, inability to perform a single limb heel rise, plantar ecchymosis, and swelling and point tenderness across the bases of the 1st and 2nd metatarsals.
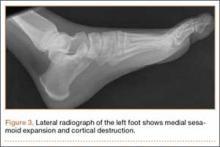
It is critical to obtain comparison weight-bearing radiographs of both feet during initial work-up to look for evidence of instability. Subtle radiographic findings of Lisfranc injury include a bony “fleck” sign, compression fracture of the cuboid, and diastasis between the base of the 1st and 2nd metatarsals and/or medial and middle cuneiforms (Figures 3A, 3B).
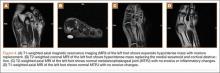
The goal of surgical intervention is to obtain and maintain anatomic reduction of all unstable joints in order to restore a normal foot posture. One of the difficulties with Lisfranc injuries is that there are no exact diastasis parameters and individuals should be treated based on symptoms, functional needs, and degree of instability. It has been shown that 5 mm of displacement can have good long-term clinical results in select cases without surgery.18 For surgery, we recommend open reduction to remove interposed soft tissue debris and directly assess the articular surfaces (Figures 4A-4D).
Proximal-medial column Lisfranc injury variants are increasingly common among football players.20 In these injuries, the force of injury extends through the intercuneiform joint and exits out the naviculocuneiform joint, thus causing instability at multiple joints and an unstable 1st ray. Patients often have minimal clinical findings and normal radiographs and stress radiographs. MRI of the foot often reveals edema at the naviculocuneiform joint. Often patients fail to improve with nonoperative immobilization with continued inability to push off from the hallux. Unrecognized or untreated instability will lead to rapid deterioration of the naviculocuneiform joint. Surgical intervention requires a homerun screw and intercuneiform screw. We do not recommend primary arthrodesis in athletes due to significant risk of malunion and nonunion unless severe articular damage is present.
Patients are typically kept NWB in a splint for 2 weeks after surgery followed by NWB in a tall CAM from 3 to 4 weeks postoperative. Progressive weight-bearing and ROM exercises are initiated from 4 to 8 weeks, followed by return to accommodative shoe wear from 10 to 12 weeks. Hardware removal is performed 4 to 6 months after surgery, typically in the off-season to allow for 6 to 8 weeks or protected recovery afterwards. Premature hardware removal can lead to loss of reduction, particularly at the intercuneiform joints. All hardware crossing the TMT joints should be removed, while the homerun screw can be left in place in addition to the intercuneiform screw. RTP in football typically occurs 6 to 7 months after surgery. Final functional outcome is related to the adequacy of initial reduction and severity of the initial injury.21
Syndesmotic Disruption
Syndesmotic injuries comprise 1% to 18% of ankle sprains in the general population, but occur at much higher rates in football due to the increased rotation forces placed on the ankle during cutting and tackling. RTP after syndesmotic injury often takes twice as long when compared to isolated lateral ankle ligamentous injury.22 Missed injuries are common and if not treated properly can lead to chronic ankle instability and posttraumatic ankle arthritis.23 Syndesmotic injury can occur in isolation or with concomitant adjacent bony, cartilaginous, or ligamentous injuries. Therefore, clinical examination and imaging work-up are critical to successful management.
Syndesmotic injuries often result from an external rotation force applied to a hyperdorsiflexed ankle while the foot is planted. This mechanism causes the fibula to externally rotate while translating posteriorly and laterally, resulting in rupture of the anterior inferior tibiofibular ligament (AITFL) first, followed by the deep deltoid ligament, interosseous ligament (IOL), and lastly posterior talofibular ligament.24 Most syndesmotic injuries involve rupture of only the AITFL and IOL.25 Multiple clinical stress tests have been designed to assess syndesmotic stability, including the squeeze test, external rotation stress test, crossed-leg test, and fibula-translation test.26-29 However, no physical examination maneuver has been shown to reliably predict the presence or degree of syndesmotic injury and therefore imaging studies are necessary.30
Initial imaging should include standing radiographs of the affected ankle. An increase in the medial clear space between the medial malleolus and talus can occur with a combined syndesmotic and deltoid disruption. In the case of subtle syndesmotic injuries, contralateral comparison weight-bearing radiographs are recommended. CT and MRI can provide additional information, but these static imaging tests cannot identify instability. Fluoroscopic stress evaluation is beneficial but has a high false-negative rate in low-grade injuries and may not detect partial rupture of the AITFL and IOL.31 It has been shown that malrotation of as much as 30° of external rotation can occur if relying on intraoperative fluoroscopy alone.32 It has been our practice to recommend surgical reduction and stabilization for any syndesmotic injury with documented diastasis or instability seen on imaging and/or arthroscopy.
Nonoperative treatment is indicated for truly stable grade I syndesmotic injuries. This involves rest and immobilization followed by a progressive rehabilitation program consisting of stretching, strengthening, and proprioceptive exercises.33 After 1 week of protected weight-bearing in a cast or tall CAM boot, progression to full weight-bearing should occur over the following week. Active-assisted ankle ROM exercises and light proprioceptive training should then be initiated followed by sport-specific exercises 2 to 3 weeks after injury.
Arthroscopy can be a valuable diagnostic tool in the setting of subtle syndesmotic injury with negative radiographs, positive MRI for edema, and a protracted recovery course with vague pain (Figures W5A-W5E).
Implants are placed above the true syndesmotic joint (at least 15 mm above the tibial plafond) angled 30° posterior to anterior to follow the normal relationship of the fibula to the distal tibia in the incisura. Typically 2 suture-buttons are used, with the devices placed in a divergent fashion. We highly recommend the use of a fibular buttress plate with button placement in individuals returning to contact activity. This construct increases surface area distribution while preventing stress risers and the risk of fibula fractures. In a cadaver model with deliberate syndesmotic malreduction, suture-button stabilization resulted in decreased postoperative displacement as opposed to conventional screw fixation.34 Therefore, dynamic syndesmotic fixation may help to decrease the negative sequelae of iatrogenic clamp malreduction.
Postoperative rehabilitation involves NWB in a cast or tall CAM boot for 4 weeks followed by ankle ROM exercises and progressive weight-bearing and physical therapy. Patients are transitioned to a lace-up ankle brace and athletic shoe from 6 to 12 weeks postoperative with increasing activity. Running and jumping is permitted 4 months after surgery with RTP typically at 6 to 7 months. Athletes who have had surgical stabilization for documented instability without any diastasis may engage in a more rapid recovery and RTP as symptoms and function allow.
Deltoid Complex Avulsion
Missed or neglected deltoid ligament injuries can lead to progressive chondral injury and joint degeneration. These injuries are often subtle and difficult to diagnose. An inability to perform a single limb heel rise, persistent pain with activity, and lack of normal functional improvement despite appropriate care are indicators of subtle ligament instability. These injuries often require an examination under anesthesia with combined ankle arthroscopy. Valgus stress testing of the ankle while directly visualizing the deltoid ligament from the anterolateral portal can reveal medial laxity in addition to potential osteochondral lesions along the anterolateral talar dome.
In American football players, we have observed that infolding and retraction of an avulsed superficial deltoid ligament complex after an ankle fracture, Maisonneuve injury, or severe high ankle sprain can be a source of persistent increased medial clear space, malreduction, and postoperative pain and medial instability. We have found that there is often complete avulsion of the superficial deltoid complex off the proximal aspect of the medial malleolus during high-energy ankle fractures in football players that is amenable to direct repair to bone (Figures W6A-W6E).
During surgical repair, an incision is made along the anterior aspect of the medial malleolus and the superficial deltoid ligament complex can often be found flipped and interposed in the medial gutter. A rongeur is used to create a bleeding cancellous bone surface for soft-tissue healing and 1 to 2 suture anchors are used to repair and imbricate the deltoid ligament complex back to the medial malleolus. The goal of these sutures is to repair the tibionavicular and tibial spring ligaments back to the medial malleolus. We believe that superficial deltoid complex avulsion during high-energy ankle fractures is a distinct injury pattern that should be recognized and may benefit from primary open repair.
We currently open explore every deltoid ligament complex in athletes with unstable syndesmotic injuries, as we believe that deltoid avulsion injuries are underrecognized and do not heal in an anatomic fashion if left alone. Postoperative recovery follows the same immobilization, progressive weight-bearing, and physical therapy protocol as that for syndesmotic disruption.
Achilles Ruptures
Acute midsubstance Achilles tendon ruptures are an increasingly common injury in patients 30 to 50 years of age, with more than 50% of all injuries occurring during basketball.36,37 Among NFL players, we have found that Achilles ruptures tend to occur at a higher rate during training camp, when athletes are deconditioned and quickly returning to explosive push-off activities. Physical examination should include a Thompson test, palpation of a gap within the tendon, and evaluation of resting ankle dorsiflexion in the affected extremity in the prone position with the knees bent. Lateral radiographs should be analyzed for the presence of a bony avulsion fragment indicative of an insertional avulsion injury or midsubstance calcium deposition reflecting chronic Achilles tendinosis, as both of these conditions will change surgical management. MRI is not recommended with acute midsubstance ruptures but may be helpful in the case of chronic ruptures or more proximal tears of the musculotendinous junction.
The management of acute midsubstance Achilles tendon ruptures is controversial, with no general consensus in the literature regarding nonoperative treatment, surgical repair, and ideal repair technique.36,38-42 American Academy of Orthopaedic Surgeons clinical practice guidelines report moderate evidence that nonoperative treatment of Achilles tendon ruptures has lower wound healing complications but higher rates of re-rupture.38,39 Additionally, limited incision approaches have been found to have fewer overall complications compared with traditional open repair. In an effort to reduce the incidence of postoperative wound complications while improving functional recovery, modern repair techniques focus on a limited incision repair using percutaneous suture insertion and management (PARS Achilles Jig System, Arthrex).36 The limited incision technique utilizes a 2-cm transverse incision and non-disposable jig with divergent needle passes and locking suture fixation options to secure and fixate both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. Limited incision repair is ideally performed within 2 weeks of the injury to ensure that both tendon ends are easy to identify, mobilize, and repair. An open repair is generally recommended for midsubstance ruptures more than 4 weeks old and cases of insertional rupture and Achilles tendinopathy.
In a cohort of 9 NFL players treated for midsubstance Achilles ruptures using the PARS technique, we found no re-ruptures, no wound complications, and no sural nerve issues after surgery.43 A comparative review of 270 cases of operatively treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) showed that the PARS group had significantly shorter operative times and a higher number of patients able to return to baseline physical activities by 5 months compared to open repair.36 Although not statistically significant, the overall PARS complication rate was 5% while the open complication rate was 11%. The PARS group had no cases of sural neuritis or deep infection requiring reoperation. We currently use a limited incision technique for all acute midsubstance Achilles ruptures in athletes regardless of sport, patient size, or position played.
During surgery, a 2-cm transverse incision is made over the gap in the Achilles tendon and dissection is carried down to the rupture site with minimal manipulation of the skin (Figures 5A-5F).
A key aspect of postoperative recovery is avoiding excessive ankle dorsiflexion while the tendon is healing during the first 4 weeks after surgery, as this can lead to an elongated tendon with loss of push-off strength. Patients are kept in a plantarflexion splint NWB for 2 weeks after surgery. If the incision is healed at 2 weeks, sutures are removed and patients are transitioned into a NWB tall CAM boot for 2 weeks with gentle ankle ROM exercises. If there is any concern regarding wound healing status, sutures are maintained for an additional 1 to 2 weeks.
From 4 to 8 weeks after surgery, progressive weight-bearing with continued ankle ROM exercises is initiated with peel-away heel lifts (~2 cm thick total, 3 layers). Each layer of the heel lift is gradually removed as pain allows every 2 to 3 days with the goal of being full weight-bearing with the foot flat at 6 weeks postoperative. Physical therapy focusing on ankle ROM and gentle Achilles stretching and strengthening is also started 6 weeks after surgery. From 8 to 12 weeks postoperative, patients are transitioned out of the tall CAM boot into normal, accommodative shoe wear with full weight-bearing. We avoid ankle dorsiflexion past neutral until 12 weeks after surgery, as overlengthening of the Achilles complex and the subsequent loss of push-off power can be devastating to running athletes. Activity levels are increased as tolerated, with no running or jumping from 12 to 16 weeks with full release to all activities after 16 weeks. RTP often takes 5 to 6 months after surgery, depending on the position played.
Am J Orthop. 2016;45(6):358-367. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Foot and ankle injuries are common in American football, with injury rates significantly increasing over the past decade.1-5 Epidemiologic studies of collegiate football players have shown an annual incidence of foot and ankle injuries ranging from 9% to 39%,3,6 with as many as 72% of all collegiate players presenting to the National Football League (NFL) Combine with a history of a foot or ankle injury and 13% undergoing surgical treatment.5 Player position influences the rate and type of foot and ankle injury. Offensive and “skill position” players, including linemen, running backs, and wide receivers, are particularly susceptible to foot and ankle injuries due to high levels of force and torque placed on the distal extremity during running, cutting, and tackling. Shoe wear changes, playing field conditions, increasing player size and speed, and improved reporting of injuries are also contributing to increasing injury rates.
The interaction between player cleats and the playing surface is a central issue of foot and ankle injuries in football. Improved traction relates to performance, but increased subsequent torque on the lower extremity is associated with injury. While lateral ankle sprains are the most common foot and ankle injury experienced by football players,7 numerous other injuries can occur, including turf toe, Jones fractures, Lisfranc injuries, syndesmotic disruption, deltoid complex avulsion, and Achilles ruptures. It is important for physicians to be able to recognize, diagnose, and appropriately treat these injuries in players in order to expedite recovery, restore function, and help prevent future injury and long-term sequelae. This review focuses on updated treatment principles, surgical advances, and rehabilitation protocols for common football foot and ankle injuries.
Turf Toe
The term “turf toe” was first used in 1976 to refer to hyperextension injuries and plantar capsule-ligament sprains of the hallux metatarsophalangeal (MTP) joint that can lead to progressive cock-up deformity.8 While these injuries can occur on any surface and disrupt soft tissue balance with functional implications, predisposing factors include increasing playing surface hardness and decreasing shoe stiffness. In a classic scenario, the foot is fixed in equinus as an axial load is placed on the back of the heel, resulting in forced dorsiflexion of the hallux MTP joint.9 As the proximal phalanx extends, the sesamoids are drawn distally and the more dorsal portion of the metatarsal head articular surface bears the majority of the load, causing partial or complete tearing of the plantar plate with or without hallux MTP dislocation. Osteochondral lesions of the MTP joint and subchondral edema of the metatarsal head can occur concurrently as the proximal phalanx impacts or shears across the metatarsal head articular surface.
Clinical examination should focus on hallux swelling, alignment, and flexor hallucis longus (FHL) function along with vertical instability of the hallux MTP joint using a Lachman test. Radiographs should be evaluated for proximal migration of the sesamoids or diastasis (Figures W1A-W1C).
Indications for surgical intervention include loss of push-off strength, gross MTP instability, proximal migration of the sesamoids, and progressive hallux malalignment or clawing after immobilization. Cases can involve one or a combination of the following: (1) large capsular avulsion with unstable MTP joint; (2) diastasis of bipartite sesamoid; (3) diastasis of sesamoid fracture; (4) retraction of sesamoid; (5) traumatic hallux valgus deformity; (6) vertical instability (positive Lachman test); (7) loose body in MTP joint; or (8) chondral injury in MTP joint. The goal of surgery is the restoration of anatomy in order to restore normal function of the hallux MTP joint.
We have found that using dual medial and plantar incisions places less traction on the plantar medial cutaneous nerve, improves lateral exposure, and provides better wound healing. The medial capsulotomy extends from the metatarsal neck to the mid-phalanx to provide complete visualization of the sesamoid complex (Figures 1A-1F).
It is important to recognize that not all turf toe injuries involve pure hyperextension on artificial playing surfaces. In recent years, we have found an increasing rate of medial variant turf toe injuries in which a forceful valgus stress on the hallux leads to rupture of the medial collateral ligament, medial or plantar-medial capsule, and/or abductor halluces. Medial variant turf toe can lead to progressive hallux valgus and a traumatic bunion with a significant loss of push-off strength and difficulty with cutting maneuvers. Surgical treatment requires a modified McBride bunionectomy with adductor tenotomy and direct repair of the medial soft tissue defect.
Postoperative management is just as important as proper surgical technique for these injuries and involves a delicate balance between protecting the repair and starting early range of motion (ROM). Patients are immobilized non-weight-bearing (NWB) for 5 to 7 days maximum followed immediately with the initiation of passive hallux plantarflexion to keep the sesamoids moving. Active hallux plantarflexion is started at 4 weeks after surgery with active dorsiflexion from 6 to 8 weeks. Patients are transitioned to an accommodative shoe with stiff hallux insert 8 weeks postoperative with continued therapy focusing on hallux ROM. Running is initiated at 12 weeks and return to play (RTP) is typically allowed 4 months after surgery.
Jones Fractures
Jones fractures are fractures of the 5th metatarsal at the metaphyseal-diaphyseal junction, where there is a watershed area of decreased vascularity between the intramedullary nutrient and metaphyseal arteries. Current thought is that the rising rate of Jones fractures among football players is partially caused by the use of flexible, narrow cleats that do not provide enough stiffness and lateral support for the 5th metatarsal during running and cutting. Additionally, lateral overload from a baseline cavovarus foot posture with or without metatarsus adductus and/or skewfoot is thought to contribute to Jones fractures.10 Preoperative radiographs should be evaluated for fracture location, orientation, amount of cortical thickening, and overall geometry of the foot and 5th metatarsal. In elite athletes, the threshold for surgical intervention is decreasing in order to expedite healing and recovery and decrease re-fracture risk. This rationale is based on delayed union rates of 25% to 66%, nonunion rates of 7% to 28%,11 and re-fracture rates of up to 33% associated with nonoperative treatment.12 Nonoperative management is usually not feasible in the competitive athlete, as it typically involves a period of protected weight-bearing in a tall controlled ankle motion (CAM) boot for 6 to 8 weeks with serial radiographs to evaluate healing.
Our preference for surgical intervention involves percutaneous screw fixation with a “high and inside” starting point on fluoroscopy (Figures 2A-2D).
In career athletes, we augment the fracture site using a mixture of bone marrow aspirate concentrate (BMA) (Magellan, Arteriocyte Medical Systems) and demineralized bone matrix (DBM) (Mini Ignite, Wright Medical Technology) injected percutaneously in and around the fracture site under fluoroscopic guidance. Using this technique in a cohort of 25 NFL players treated operatively for Jones fractures, we found that 100% of athletes were able to RTP in the NFL in an average of 9.5 weeks.14 Two patients (7.5%) suffered re-fractures requiring revision surgery with iliac crest bone graft and repeat screw placement with a RTP after 15 weeks. We did not find an association between RTP and re-fracture rate.
The appropriate rehabilitation protocol for Jones fractures after surgery remains controversial and dependent on individual needs and abilities.15,16 For athletes in-season, we recommend a brief period of NWB for 1 to 2 weeks followed by toe-touch weight-bearing in a tall CAM boot for 2 to 4 weeks. After 4 weeks, patients begin gentle exercises on a stationary bike and pool therapy to reduce impact on the fracture site. Low-intensity pulsed ultrasound bone stimulators (Exogen, Bioventus) are frequently used directly over fracture site throughout the postoperative protocol as an adjuvant therapy. If clinically nontender over the fracture site, patients are allowed to begin running in modified protective shoe wear 4 weeks after surgery with an average RTP of 6 to 8 weeks. RTP is determined clinically, as radiographic union may not be evident for 12 to 16 weeks. Useful custom orthoses include turf toe plates with a cushioned lateral column and lateral heel wedge if hindfoot varus is present preoperatively.10 The solid intramedullary screw is left in place permanently.
In our experience, we have found the average re-fracture and nonunion rate to be approximately 8% across all athletes. Our observation that union rates do not appear to be related to RTP times suggests that underlying biology such as Vitamin D deficiency may play a larger role in union rates than previously thought. We have found that most Jones re-fractures occur in the first year after the initial injury, but can occur up to 2.5 years afterwards as well.14 For the management of symptomatic re-fractures and nonunions, the previous screw must be first removed. This can be difficult if the screw is bent or broken, and may require a lateral corticotomy of the metatarsal.
After hardware removal, we advocate open bone grafting of the fracture site using bone from the iliac crest retrieved with a small, percutaneous trephine. Re-fixation should be achieved using a larger, solid screw and postoperative adjuvants may include bone stimulators, Vitamin D and calcium supplemention, and possible teriparatide use (Forteo, Eli Lilly), depending on individual patient profile. In a cohort of 21 elite athletes treated for Jones fracture revision surgery with screw exchange and bone grafting, we found that 100% of patients had computed tomography (CT) evidence of union, with an average RTP of 12.3 weeks.17
Lisfranc Injuries
Lisfranc injuries include any bony or ligamentous damage that involves the tarsometatarsal (TMT) joints. While axial loading of a fixed, plantarflexed foot has traditionally been thought of as the most common mechanism of Lisfranc injury, we have found that noncontact twisting injuries leading to Lisfranc disruption are actually more common among NFL players. This mechanism is similar to noncontact turf toe and results in a purely ligamentous injury. We have found this to be particularly true in the case of defensive ends engaged with offensive linemen in which no axial loading or contact of the foot occurs. Clinically, patients often have painful weight-bearing, inability to perform a single limb heel rise, plantar ecchymosis, and swelling and point tenderness across the bases of the 1st and 2nd metatarsals.
It is critical to obtain comparison weight-bearing radiographs of both feet during initial work-up to look for evidence of instability. Subtle radiographic findings of Lisfranc injury include a bony “fleck” sign, compression fracture of the cuboid, and diastasis between the base of the 1st and 2nd metatarsals and/or medial and middle cuneiforms (Figures 3A, 3B).
The goal of surgical intervention is to obtain and maintain anatomic reduction of all unstable joints in order to restore a normal foot posture. One of the difficulties with Lisfranc injuries is that there are no exact diastasis parameters and individuals should be treated based on symptoms, functional needs, and degree of instability. It has been shown that 5 mm of displacement can have good long-term clinical results in select cases without surgery.18 For surgery, we recommend open reduction to remove interposed soft tissue debris and directly assess the articular surfaces (Figures 4A-4D).
Proximal-medial column Lisfranc injury variants are increasingly common among football players.20 In these injuries, the force of injury extends through the intercuneiform joint and exits out the naviculocuneiform joint, thus causing instability at multiple joints and an unstable 1st ray. Patients often have minimal clinical findings and normal radiographs and stress radiographs. MRI of the foot often reveals edema at the naviculocuneiform joint. Often patients fail to improve with nonoperative immobilization with continued inability to push off from the hallux. Unrecognized or untreated instability will lead to rapid deterioration of the naviculocuneiform joint. Surgical intervention requires a homerun screw and intercuneiform screw. We do not recommend primary arthrodesis in athletes due to significant risk of malunion and nonunion unless severe articular damage is present.
Patients are typically kept NWB in a splint for 2 weeks after surgery followed by NWB in a tall CAM from 3 to 4 weeks postoperative. Progressive weight-bearing and ROM exercises are initiated from 4 to 8 weeks, followed by return to accommodative shoe wear from 10 to 12 weeks. Hardware removal is performed 4 to 6 months after surgery, typically in the off-season to allow for 6 to 8 weeks or protected recovery afterwards. Premature hardware removal can lead to loss of reduction, particularly at the intercuneiform joints. All hardware crossing the TMT joints should be removed, while the homerun screw can be left in place in addition to the intercuneiform screw. RTP in football typically occurs 6 to 7 months after surgery. Final functional outcome is related to the adequacy of initial reduction and severity of the initial injury.21
Syndesmotic Disruption
Syndesmotic injuries comprise 1% to 18% of ankle sprains in the general population, but occur at much higher rates in football due to the increased rotation forces placed on the ankle during cutting and tackling. RTP after syndesmotic injury often takes twice as long when compared to isolated lateral ankle ligamentous injury.22 Missed injuries are common and if not treated properly can lead to chronic ankle instability and posttraumatic ankle arthritis.23 Syndesmotic injury can occur in isolation or with concomitant adjacent bony, cartilaginous, or ligamentous injuries. Therefore, clinical examination and imaging work-up are critical to successful management.
Syndesmotic injuries often result from an external rotation force applied to a hyperdorsiflexed ankle while the foot is planted. This mechanism causes the fibula to externally rotate while translating posteriorly and laterally, resulting in rupture of the anterior inferior tibiofibular ligament (AITFL) first, followed by the deep deltoid ligament, interosseous ligament (IOL), and lastly posterior talofibular ligament.24 Most syndesmotic injuries involve rupture of only the AITFL and IOL.25 Multiple clinical stress tests have been designed to assess syndesmotic stability, including the squeeze test, external rotation stress test, crossed-leg test, and fibula-translation test.26-29 However, no physical examination maneuver has been shown to reliably predict the presence or degree of syndesmotic injury and therefore imaging studies are necessary.30
Initial imaging should include standing radiographs of the affected ankle. An increase in the medial clear space between the medial malleolus and talus can occur with a combined syndesmotic and deltoid disruption. In the case of subtle syndesmotic injuries, contralateral comparison weight-bearing radiographs are recommended. CT and MRI can provide additional information, but these static imaging tests cannot identify instability. Fluoroscopic stress evaluation is beneficial but has a high false-negative rate in low-grade injuries and may not detect partial rupture of the AITFL and IOL.31 It has been shown that malrotation of as much as 30° of external rotation can occur if relying on intraoperative fluoroscopy alone.32 It has been our practice to recommend surgical reduction and stabilization for any syndesmotic injury with documented diastasis or instability seen on imaging and/or arthroscopy.
Nonoperative treatment is indicated for truly stable grade I syndesmotic injuries. This involves rest and immobilization followed by a progressive rehabilitation program consisting of stretching, strengthening, and proprioceptive exercises.33 After 1 week of protected weight-bearing in a cast or tall CAM boot, progression to full weight-bearing should occur over the following week. Active-assisted ankle ROM exercises and light proprioceptive training should then be initiated followed by sport-specific exercises 2 to 3 weeks after injury.
Arthroscopy can be a valuable diagnostic tool in the setting of subtle syndesmotic injury with negative radiographs, positive MRI for edema, and a protracted recovery course with vague pain (Figures W5A-W5E).
Implants are placed above the true syndesmotic joint (at least 15 mm above the tibial plafond) angled 30° posterior to anterior to follow the normal relationship of the fibula to the distal tibia in the incisura. Typically 2 suture-buttons are used, with the devices placed in a divergent fashion. We highly recommend the use of a fibular buttress plate with button placement in individuals returning to contact activity. This construct increases surface area distribution while preventing stress risers and the risk of fibula fractures. In a cadaver model with deliberate syndesmotic malreduction, suture-button stabilization resulted in decreased postoperative displacement as opposed to conventional screw fixation.34 Therefore, dynamic syndesmotic fixation may help to decrease the negative sequelae of iatrogenic clamp malreduction.
Postoperative rehabilitation involves NWB in a cast or tall CAM boot for 4 weeks followed by ankle ROM exercises and progressive weight-bearing and physical therapy. Patients are transitioned to a lace-up ankle brace and athletic shoe from 6 to 12 weeks postoperative with increasing activity. Running and jumping is permitted 4 months after surgery with RTP typically at 6 to 7 months. Athletes who have had surgical stabilization for documented instability without any diastasis may engage in a more rapid recovery and RTP as symptoms and function allow.
Deltoid Complex Avulsion
Missed or neglected deltoid ligament injuries can lead to progressive chondral injury and joint degeneration. These injuries are often subtle and difficult to diagnose. An inability to perform a single limb heel rise, persistent pain with activity, and lack of normal functional improvement despite appropriate care are indicators of subtle ligament instability. These injuries often require an examination under anesthesia with combined ankle arthroscopy. Valgus stress testing of the ankle while directly visualizing the deltoid ligament from the anterolateral portal can reveal medial laxity in addition to potential osteochondral lesions along the anterolateral talar dome.
In American football players, we have observed that infolding and retraction of an avulsed superficial deltoid ligament complex after an ankle fracture, Maisonneuve injury, or severe high ankle sprain can be a source of persistent increased medial clear space, malreduction, and postoperative pain and medial instability. We have found that there is often complete avulsion of the superficial deltoid complex off the proximal aspect of the medial malleolus during high-energy ankle fractures in football players that is amenable to direct repair to bone (Figures W6A-W6E).
During surgical repair, an incision is made along the anterior aspect of the medial malleolus and the superficial deltoid ligament complex can often be found flipped and interposed in the medial gutter. A rongeur is used to create a bleeding cancellous bone surface for soft-tissue healing and 1 to 2 suture anchors are used to repair and imbricate the deltoid ligament complex back to the medial malleolus. The goal of these sutures is to repair the tibionavicular and tibial spring ligaments back to the medial malleolus. We believe that superficial deltoid complex avulsion during high-energy ankle fractures is a distinct injury pattern that should be recognized and may benefit from primary open repair.
We currently open explore every deltoid ligament complex in athletes with unstable syndesmotic injuries, as we believe that deltoid avulsion injuries are underrecognized and do not heal in an anatomic fashion if left alone. Postoperative recovery follows the same immobilization, progressive weight-bearing, and physical therapy protocol as that for syndesmotic disruption.
Achilles Ruptures
Acute midsubstance Achilles tendon ruptures are an increasingly common injury in patients 30 to 50 years of age, with more than 50% of all injuries occurring during basketball.36,37 Among NFL players, we have found that Achilles ruptures tend to occur at a higher rate during training camp, when athletes are deconditioned and quickly returning to explosive push-off activities. Physical examination should include a Thompson test, palpation of a gap within the tendon, and evaluation of resting ankle dorsiflexion in the affected extremity in the prone position with the knees bent. Lateral radiographs should be analyzed for the presence of a bony avulsion fragment indicative of an insertional avulsion injury or midsubstance calcium deposition reflecting chronic Achilles tendinosis, as both of these conditions will change surgical management. MRI is not recommended with acute midsubstance ruptures but may be helpful in the case of chronic ruptures or more proximal tears of the musculotendinous junction.
The management of acute midsubstance Achilles tendon ruptures is controversial, with no general consensus in the literature regarding nonoperative treatment, surgical repair, and ideal repair technique.36,38-42 American Academy of Orthopaedic Surgeons clinical practice guidelines report moderate evidence that nonoperative treatment of Achilles tendon ruptures has lower wound healing complications but higher rates of re-rupture.38,39 Additionally, limited incision approaches have been found to have fewer overall complications compared with traditional open repair. In an effort to reduce the incidence of postoperative wound complications while improving functional recovery, modern repair techniques focus on a limited incision repair using percutaneous suture insertion and management (PARS Achilles Jig System, Arthrex).36 The limited incision technique utilizes a 2-cm transverse incision and non-disposable jig with divergent needle passes and locking suture fixation options to secure and fixate both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. Limited incision repair is ideally performed within 2 weeks of the injury to ensure that both tendon ends are easy to identify, mobilize, and repair. An open repair is generally recommended for midsubstance ruptures more than 4 weeks old and cases of insertional rupture and Achilles tendinopathy.
In a cohort of 9 NFL players treated for midsubstance Achilles ruptures using the PARS technique, we found no re-ruptures, no wound complications, and no sural nerve issues after surgery.43 A comparative review of 270 cases of operatively treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) showed that the PARS group had significantly shorter operative times and a higher number of patients able to return to baseline physical activities by 5 months compared to open repair.36 Although not statistically significant, the overall PARS complication rate was 5% while the open complication rate was 11%. The PARS group had no cases of sural neuritis or deep infection requiring reoperation. We currently use a limited incision technique for all acute midsubstance Achilles ruptures in athletes regardless of sport, patient size, or position played.
During surgery, a 2-cm transverse incision is made over the gap in the Achilles tendon and dissection is carried down to the rupture site with minimal manipulation of the skin (Figures 5A-5F).
A key aspect of postoperative recovery is avoiding excessive ankle dorsiflexion while the tendon is healing during the first 4 weeks after surgery, as this can lead to an elongated tendon with loss of push-off strength. Patients are kept in a plantarflexion splint NWB for 2 weeks after surgery. If the incision is healed at 2 weeks, sutures are removed and patients are transitioned into a NWB tall CAM boot for 2 weeks with gentle ankle ROM exercises. If there is any concern regarding wound healing status, sutures are maintained for an additional 1 to 2 weeks.
From 4 to 8 weeks after surgery, progressive weight-bearing with continued ankle ROM exercises is initiated with peel-away heel lifts (~2 cm thick total, 3 layers). Each layer of the heel lift is gradually removed as pain allows every 2 to 3 days with the goal of being full weight-bearing with the foot flat at 6 weeks postoperative. Physical therapy focusing on ankle ROM and gentle Achilles stretching and strengthening is also started 6 weeks after surgery. From 8 to 12 weeks postoperative, patients are transitioned out of the tall CAM boot into normal, accommodative shoe wear with full weight-bearing. We avoid ankle dorsiflexion past neutral until 12 weeks after surgery, as overlengthening of the Achilles complex and the subsequent loss of push-off power can be devastating to running athletes. Activity levels are increased as tolerated, with no running or jumping from 12 to 16 weeks with full release to all activities after 16 weeks. RTP often takes 5 to 6 months after surgery, depending on the position played.
Am J Orthop. 2016;45(6):358-367. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Canale ST, Cantler ED Jr, Sisk TD, Freeman BL 3rd. A chronicle of injuries of an American intercollegiate football team. Am J Sports Med. 1981;9(6):384-389.2. Robey JM, Blyth CS, Mueller FO. Athletic injuries. Application of epidemiologic methods. JAMA. 1971;217(2):184-189.
3. Saal JA. Common American football injuries. Sports Med. 1991;12(2):132-147.
4. Thompson N, Halpern B, Curl WW, et al. High school football injuries: evaluation. Am J Sports Med. 1987;15(2):117-124.
5. Kaplan LD, Jost PW, Honkamp N, Norwig J, West R, Bradley JP. Incidence and variance of foot and ankle injuries in elite college football players. Am J Orthop. 2011;40(1):40-44.
6. DeLee JC, Farney WC. Incidence of injury in Texas high school football. Am J Sports Med. 1992;20(5):575-580.
7. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
8. Bowers KD Jr, Martin RB. Turf-toe: a shoe-surface related football injury. Med Sci Sports. 1976;8(2):81-83.
9. McCormick JJ, Anderson RB. Turf toe: anatomy, diagnosis, and treatment. Sports Health. 2010;2(6):487-494.
10. Raikin SM, Slenker N, Ratigan B. The association of a varus hindfoot and fracture of the fifth metatarsal metaphyseal-diaphyseal junction: the Jones fracture. Am J Sports Med. 2008;36(7):1367-1372.
11. Title CI, Katchis SD. Traumatic foot and ankle injuries in the athlete. Orthop Clin North Am. 2002;33(3):587-598.
12. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
13. Nunley JA, Glisson RR. A new option for intramedullary fixation of Jones fractures: the Charlotte Carolina Jones Fracture System. Foot Ankle Int. 2008;29(12):1216-1221.
14. Lareau CR, Hsu AR, Anderson RB. Return to play in National Football League players after operative Jones fracture treatment. Foot Ankle Int. 2016;37(1):8-16.
15. Larson CM, Almekinders LC, Taft TN, Garrett WE. Intramedullary screw fixation of Jones fractures. Analysis of failure. Am J Sports Med. 2002;30(1):55-60.
16. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
17. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
18. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
19. Alberta FG, Aronow MS, Barrero M, Diaz-Doran V, Sullivan RJ, Adams DJ. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int. 2005;26(6):462-473.
20. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle Surg. 2010;9(3):100-106.
21. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82-A(11):1609-1618.
22. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
23. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35(7):1197-1207.
24. Beumer A, Valstar ER, Garling EH, et al. Effects of ligament sectioning on the kinematics of the distal tibiofibular syndesmosis: a radiostereometric study of 10 cadaveric specimens based on presumed trauma mechanisms with suggestions for treatment. Acta Orthop. 2006;77(3):531-540.
25. McCollum GA, van den Bekerom MP, Kerkhoffs GM, Calder JD, van Dijk CN. Syndesmosis and deltoid ligament injuries in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1328-1337.
26. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
27. Nussbaum ED, Hosea TM, Sieler SD, Incremona BR, Kessler DE. Prospective evaluation of syndesmotic ankle sprains without diastasis. Am J Sports Med. 2001;29(1):31-35.
28. Kiter E, Bozkurt M. The crossed-leg test for examination of ankle syndesmosis injuries. Foot Ankle Int. 2005;26(2):187-188.
29. Beumer A, van Hemert WL, Swierstra BA, Jasper LE, Belkoff SM. A biomechanical evaluation of clinical stress tests for syndesmotic ankle instability. Foot Ankle Int. 2003;24(4):358-363.
30. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14(4):232-236.
31. Beumer A, Valstar ER, Garling EH, et al. External rotation stress imaging in syndesmotic injuries of the ankle: comparison of lateral radiography and radiostereometry in a cadaveric model. Acta Orthop Scand. 2003;74(2):201-205.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2(6):460-470.
34. Westermann RW, Rungprai C, Goetz JE, Femino J, Amendola A, Phisitkul P. The effect of suture-button fixation on simulated syndesmotic malreduction: a cadaveric study. J Bone Joint Surg Am. 2014;96(20):1732-1738.
35. Hsu AR, Lareau CR, Anderson RB. Repair of acute superficial deltoid complex avulsion during ankle fracture fixation in National Football League players. Foot Ankle Int. 2015;36(11):1272-1278.
36. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of percutaneous Achilles repair system versus open technique for acute achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
37. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
38. Chiodo CP, Glazebrook M, Bluman EM, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
39. Chiodo CP, Glazebrook M, Bluman EM, et al. Diagnosis and treatment of acute achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
40. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
41. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
42. Khan RJ, Carey Smith RL. Surgical interventions for treating acute achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
43. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of achilles rupture in the national football league. J Surg Orthop Adv. 2014;23(4):179-183.
1. Canale ST, Cantler ED Jr, Sisk TD, Freeman BL 3rd. A chronicle of injuries of an American intercollegiate football team. Am J Sports Med. 1981;9(6):384-389.2. Robey JM, Blyth CS, Mueller FO. Athletic injuries. Application of epidemiologic methods. JAMA. 1971;217(2):184-189.
3. Saal JA. Common American football injuries. Sports Med. 1991;12(2):132-147.
4. Thompson N, Halpern B, Curl WW, et al. High school football injuries: evaluation. Am J Sports Med. 1987;15(2):117-124.
5. Kaplan LD, Jost PW, Honkamp N, Norwig J, West R, Bradley JP. Incidence and variance of foot and ankle injuries in elite college football players. Am J Orthop. 2011;40(1):40-44.
6. DeLee JC, Farney WC. Incidence of injury in Texas high school football. Am J Sports Med. 1992;20(5):575-580.
7. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
8. Bowers KD Jr, Martin RB. Turf-toe: a shoe-surface related football injury. Med Sci Sports. 1976;8(2):81-83.
9. McCormick JJ, Anderson RB. Turf toe: anatomy, diagnosis, and treatment. Sports Health. 2010;2(6):487-494.
10. Raikin SM, Slenker N, Ratigan B. The association of a varus hindfoot and fracture of the fifth metatarsal metaphyseal-diaphyseal junction: the Jones fracture. Am J Sports Med. 2008;36(7):1367-1372.
11. Title CI, Katchis SD. Traumatic foot and ankle injuries in the athlete. Orthop Clin North Am. 2002;33(3):587-598.
12. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
13. Nunley JA, Glisson RR. A new option for intramedullary fixation of Jones fractures: the Charlotte Carolina Jones Fracture System. Foot Ankle Int. 2008;29(12):1216-1221.
14. Lareau CR, Hsu AR, Anderson RB. Return to play in National Football League players after operative Jones fracture treatment. Foot Ankle Int. 2016;37(1):8-16.
15. Larson CM, Almekinders LC, Taft TN, Garrett WE. Intramedullary screw fixation of Jones fractures. Analysis of failure. Am J Sports Med. 2002;30(1):55-60.
16. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
17. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
18. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
19. Alberta FG, Aronow MS, Barrero M, Diaz-Doran V, Sullivan RJ, Adams DJ. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int. 2005;26(6):462-473.
20. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle Surg. 2010;9(3):100-106.
21. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82-A(11):1609-1618.
22. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
23. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35(7):1197-1207.
24. Beumer A, Valstar ER, Garling EH, et al. Effects of ligament sectioning on the kinematics of the distal tibiofibular syndesmosis: a radiostereometric study of 10 cadaveric specimens based on presumed trauma mechanisms with suggestions for treatment. Acta Orthop. 2006;77(3):531-540.
25. McCollum GA, van den Bekerom MP, Kerkhoffs GM, Calder JD, van Dijk CN. Syndesmosis and deltoid ligament injuries in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1328-1337.
26. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
27. Nussbaum ED, Hosea TM, Sieler SD, Incremona BR, Kessler DE. Prospective evaluation of syndesmotic ankle sprains without diastasis. Am J Sports Med. 2001;29(1):31-35.
28. Kiter E, Bozkurt M. The crossed-leg test for examination of ankle syndesmosis injuries. Foot Ankle Int. 2005;26(2):187-188.
29. Beumer A, van Hemert WL, Swierstra BA, Jasper LE, Belkoff SM. A biomechanical evaluation of clinical stress tests for syndesmotic ankle instability. Foot Ankle Int. 2003;24(4):358-363.
30. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14(4):232-236.
31. Beumer A, Valstar ER, Garling EH, et al. External rotation stress imaging in syndesmotic injuries of the ankle: comparison of lateral radiography and radiostereometry in a cadaveric model. Acta Orthop Scand. 2003;74(2):201-205.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2(6):460-470.
34. Westermann RW, Rungprai C, Goetz JE, Femino J, Amendola A, Phisitkul P. The effect of suture-button fixation on simulated syndesmotic malreduction: a cadaveric study. J Bone Joint Surg Am. 2014;96(20):1732-1738.
35. Hsu AR, Lareau CR, Anderson RB. Repair of acute superficial deltoid complex avulsion during ankle fracture fixation in National Football League players. Foot Ankle Int. 2015;36(11):1272-1278.
36. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of percutaneous Achilles repair system versus open technique for acute achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
37. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
38. Chiodo CP, Glazebrook M, Bluman EM, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
39. Chiodo CP, Glazebrook M, Bluman EM, et al. Diagnosis and treatment of acute achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
40. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
41. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
42. Khan RJ, Carey Smith RL. Surgical interventions for treating acute achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
43. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of achilles rupture in the national football league. J Surg Orthop Adv. 2014;23(4):179-183.
No VTE prophylaxis needed after joint surgery in patients with hemophilia
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
ORLANDO – In patients with hemophilia who have therapeutic factor levels at the time of joint replacement surgery, prophylaxis against venous thromboembolism (VTE) may be unnecessary.
In a cohort study of patients with hemophilia A or B who underwent total joint replacement surgery while being in proper hemostasis with therapeutic factor levels, there were no clinically evident episodes of venous thromboembolism, even though none of the patients had received perioperative anticoagulant prophylaxis, reported investigators from the National Hemophilia Center and Institute of Thrombosis and Hemostasis at the Sheba Medical Center in Tel Hashomer, Israel.
The data should be reassuring to clinicians whose patients with hemophilia require major orthopedic procedures, said lead author Dr. Anna Seltser, an orthopedic resident at Sheba Medical Center, in an interview.
“We have a lot of hemophilia patients who are not well treated because they live in the desert or distant communities, and we also sometimes treat patients from the Palestinian side of the Gaza Strip who don’t have access to care and need this type of surgery,” she said.
“We collected what I think is the biggest series of patients until now, we didn’t give any of them VTE prophylaxis, and none of them had any DVT [deep vein thrombosis], PE [pulmonary embolism], or similar complication,” she said.
Skip the heparin?
VTE prophylaxis with low-molecular-weight heparin, warfarin, or other anticoagulant agents is a common practice following orthopedic surgery in patients without bleeding disorders. But for patients with severe hemophilia, who often require major joint replacement surgery following years of bleeding-induced arthropathy, it’s unclear whether perioperative anticoagulation is beneficial, the investigators noted in a scientific poster at the World Federation of Hemophilia World Congress.
Dr. Seltser and colleagues therefore conducted a prospective cohort study of 50 patients with hemophilia A or B treated with major joint surgery and subsequent revisions from 1988 through 2015 at their center. In all, 47 patients had severe hemophilia A, 2 had mild hemophilia A, and 1 had hemophilia B.
The authors analyzed data on demographics, comorbidities, type of surgery, use of factor concentrates therapy around the time of surgery, and complications during follow-up, including massive hemorrhage, infections, implant loosening, DVT, and PE.
The patients underwent a total of 74 primary joint replacements (16 hips, 52 knees, and 6 ankles) and 23 revision surgeries.
As noted, there were no episodes of either DVT or PE among any of the patients. All but one complication occurred among patients undergoing total knee replacement. These included three cases of hemarthrosis, three limited-range-of-motion cases requiring closed manipulations, four soft-tissue hematomas, and one case each of superficial wound infection, urinary tract infection, pneumonia, and Candida infection of the tongue.
The only other complication was a case of disseminated intravascular coagulation, sepsis, and hemorrhagic shock in a patient who had undergone a revision (original procedure unspecified).
“Despite the concern that proper replacement factor therapy, applied before and after the surgery, may increase the risk for thromboembolic complications in patients with hemophilia undergoing joint replacement, our data show that prophylactic anticoagulation in this group of patients is not necessary,” the investigators concluded.
The study was internally funded. The investigators reported no conflicts of interest.
AT WFH 2016 WORLD CONGRESS
Key clinical point: Prophylaxis against thromboembolic events after orthopedic surgery in patients with hemophilia may not be necessary.
Major finding: There were no thromboembolic events after joint surgery without anticoagulant prophylaxis in patients with hemophilia A or B.
Data source: Cohort study of 50 patients with hemophilia A or B undergoing major joint replacement surgery.
Disclosures: The study was internally funded. The investigators reported no conflicts of interest.
FDA alert: Canagliflozin use may be associated with toe, foot amputations
Interim safety results from an ongoing clinical trial found an increase in leg and foot amputations, mostly affecting the toes, in patients treated with the diabetes medicine canagliflozin, according to an FDA Drug Safety Communication on May 18, 2016.
The agency currently is investigating the safety issue but has yet to determine if taking canagliflozin is associated with an increased risk of leg and foot amputations. A sodium-glucose cotransporter 2 inhibitor, canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013.
“Patients should not stop or change their diabetes medicines without first talking to their health care professional,” the communication states. “Doing so can lead to uncontrolled blood sugar levels that can be harmful. Over time, this can cause serious problems, including blindness, nerve and kidney damage, and heart disease. Patients taking canagliflozin should notify their health care professionals right away if they notice any new pain or tenderness, sores or ulcers, or infections in their legs or feet.”
The agency advises health care professionals to follow the recommendations in the canagliflozin drug labels and to monitor patients for the signs and symptoms described above.
Upon its approval, the FDA required five postmarketing studies for canagliflozin: a cardiovascular outcomes trial; an enhanced pharmacovigilance program to monitor for malignancies, serious cases of pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, liver abnormalities, and adverse pregnancy outcomes; a bone safety study; and two pediatric studies under the Pediatric Research Equity Act (PREA), including a pharmacokinetic and pharmacodynamic study and a safety and efficacy study. In late 2015, investigators determined that the risk of bone fracture is increased with canagliflozin treatment.
Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Interim safety results from an ongoing clinical trial found an increase in leg and foot amputations, mostly affecting the toes, in patients treated with the diabetes medicine canagliflozin, according to an FDA Drug Safety Communication on May 18, 2016.
The agency currently is investigating the safety issue but has yet to determine if taking canagliflozin is associated with an increased risk of leg and foot amputations. A sodium-glucose cotransporter 2 inhibitor, canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013.
“Patients should not stop or change their diabetes medicines without first talking to their health care professional,” the communication states. “Doing so can lead to uncontrolled blood sugar levels that can be harmful. Over time, this can cause serious problems, including blindness, nerve and kidney damage, and heart disease. Patients taking canagliflozin should notify their health care professionals right away if they notice any new pain or tenderness, sores or ulcers, or infections in their legs or feet.”
The agency advises health care professionals to follow the recommendations in the canagliflozin drug labels and to monitor patients for the signs and symptoms described above.
Upon its approval, the FDA required five postmarketing studies for canagliflozin: a cardiovascular outcomes trial; an enhanced pharmacovigilance program to monitor for malignancies, serious cases of pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, liver abnormalities, and adverse pregnancy outcomes; a bone safety study; and two pediatric studies under the Pediatric Research Equity Act (PREA), including a pharmacokinetic and pharmacodynamic study and a safety and efficacy study. In late 2015, investigators determined that the risk of bone fracture is increased with canagliflozin treatment.
Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Interim safety results from an ongoing clinical trial found an increase in leg and foot amputations, mostly affecting the toes, in patients treated with the diabetes medicine canagliflozin, according to an FDA Drug Safety Communication on May 18, 2016.
The agency currently is investigating the safety issue but has yet to determine if taking canagliflozin is associated with an increased risk of leg and foot amputations. A sodium-glucose cotransporter 2 inhibitor, canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013.
“Patients should not stop or change their diabetes medicines without first talking to their health care professional,” the communication states. “Doing so can lead to uncontrolled blood sugar levels that can be harmful. Over time, this can cause serious problems, including blindness, nerve and kidney damage, and heart disease. Patients taking canagliflozin should notify their health care professionals right away if they notice any new pain or tenderness, sores or ulcers, or infections in their legs or feet.”
The agency advises health care professionals to follow the recommendations in the canagliflozin drug labels and to monitor patients for the signs and symptoms described above.
Upon its approval, the FDA required five postmarketing studies for canagliflozin: a cardiovascular outcomes trial; an enhanced pharmacovigilance program to monitor for malignancies, serious cases of pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, liver abnormalities, and adverse pregnancy outcomes; a bone safety study; and two pediatric studies under the Pediatric Research Equity Act (PREA), including a pharmacokinetic and pharmacodynamic study and a safety and efficacy study. In late 2015, investigators determined that the risk of bone fracture is increased with canagliflozin treatment.
Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Tibialis Posterior Tendon Entrapment Within Posterior Malleolar Fracture Fragment
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
Are Hook Plates Advantageous Compared to Antiglide Plates for Vertical Shear Malleolar Fractures?
Supination-adduction (SAD)-type fractures of the ankle comprise approximately 5% to 20% of ankle fractures.1-3 As the name describes, this fracture is caused by forceful adduction of the supinated foot. There are 2 stages of the fracture pattern: the injury usually occurs first on the lateral side of the ankle with injury to the soft tissues or a low transverse fracture of the distal fibula. With continued force, in the second stage, the talus causes a shearing of the medial malleolus, creating the vertical shear fracture pattern.4-7 The vertical shear medial malleolus fracture pattern is the subject of this investigation.
Several techniques have been traditionally recommended for fixation of SAD-type ankle fracture, including: a 2-screw construct without plate fixation, oriented perpendicular to the fracture; and an AG plate construct with variable positioning and numbers of screws for fixation. There have been, however, only 2 published articles about the biomechanical properties of fixation of vertical shear medial malleolar fractures, which reported conflicting results.8,9 The most recent of these studies argued that one-third tubular plate fixation offers significant mechanical advantage over screw-only fixation, supporting the use of AG plates for fixation of SAD ankle fractures.8
An additional design for fixation of medial malleolus fractures has been introduced, consisting of a hook plate (HP) contoured for the medial malleolus. To our knowledge, no studies have investigated HP’s biomechanical properties. Thus, the objective of this study was to investigate and compare the biomechanical properties of 3 constructs for fixation of SAD-ankle fractures: an antiglide (AG) plate, an AG plate with an additional lag-screw across the fracture, and a precontoured HP.
Materials and Methods
Thirty 4th-generation–composite polyurethane models of the left tibia were obtained (Sawbones, Pacific Research Laboratories, Inc.). Largely, our methods accorded with the precedent set by other studies on these fracture types.8,9
Prior to creation of the fractures, each model was individually evaluated for pretest stiffness by using the slope of the linear portion of the load-displacement curve during offset-axial loading. This demonstrated the baseline elasticity of the models during loading. Assessing pretest stiffness was performed to reduce potential variables in the stiffness of individual models in the analysis of the testing data.
The models were numbered 1 through 30 on the shaft and on the medial malleolus. A custom jig was constructed with a table saw to create identical vertical shear medial malleolar fracture patterns in each model. The jig created the vertical shear SAD fracture described by Lauge-Hansen.7 All models were randomly assigned to 1 of 3 groups; each group consisted of 10 models (Figures 1A, 1B).
The 10 specimens in group 1 were fixed with a 5-hole, 3.5-mm, one-third tubular plate (Smith & Nephew) in a traditional AG fashion. The plates were placed at the same location on all tibiae. The proximal hole and the hole closest to the fracture line were filled with 3.5-mm cortical screws, which were long enough to achieve bicortical fixation. No lag screws were placed in this specimen group. In group 2, specimens were fixed with the same plate used in group 1 (Smith & Nephew). In this modified AG (MAG) construct, specimens were fixed identically to group 1 for plate placement and fixation of the 2 proximal screws. In this group, an additional screw was placed perpendicular to the fracture and parallel to the distal tibial articular surface. In both groups (AG and MAG), the plates were not bent before application.
Group 3 consisted of specimens fixed with a 5-hole, precontoured medial malleolar HP (Arthrex). This HP construct was fixed with two 3.5-mm cortical screws long enough to achieve bicortical fixation. The plate also engaged the bone at the tip of the medial malleolus by using 2 sharp prongs. The screws were placed in the most proximal hole and the hole just proximal to the fracture line. No lag screws were placed in the HP construct.
All models were tested in offset-axial loading to replicate a SAD moment similar to previous studies. To test offset-axial loading, a vice held each model identically with a 17º angle from the longitudinal axis (Figure 2). Loading was performed with a material testing system; a material testing system plunger was directed at the inferior articulating cartilage surface of the medial malleolus. The specimens were loaded at a rate of 1 mm/sec until 2 mm of displacement was reached (Figure 3) or catastrophic failure occurred. The raw data analyzed consisted of the initial stiffness of the construct and the overall load-to-failure. The slope of the linear portion of the load-displacement curve of stiffness determined stiffness of the construct.
One-way analysis of variance with post hoc Tukey HSD data analysis was performed to determine if there were statistical differences among the different fixation constructs during load-to-failure. To prevent skewing of results by different values of model elasticity, pretest stiffness was accounted for by calculating a ratio of construct stiffness as a function of pretest model stiffness. Total force-to-failure was the recorded maximum force (in N) to cause failure. A P value of < .05 was set for significance. All data were analyzed using SPSS software (SPSS Version 15.0; SPSS Inc.).
Results
Analysis of pretest stiffness showed no significant difference among models (P = .490). All models failed by a gap of 2 mm at the distal fracture site except for 3 models in the MAG group. These 3 models failed at a much higher load than the remainder of the models and failed by fracture of the models.
The MAG group demonstrated significantly superior stiffness to the 2 other models tested (Figure 4). On average, this group required 753.5 N of force before failure. This was 530 N higher than the HP (P < .05) and 638 N higher than the AG constructs, respectively (P < .05). The HP and AG groups required forces of 223.2 N and 115.5 N for failure, respectively. These numbers were not significant (P= .063).
The absolute construct stiffness and construct stiffness as a function of pretest stiffness of the MAG group was the highest of all groups, 271.7 N/mm and 57.2%, respectively (Figure 5). These numbers showed significance when compared with the values of the HP group (P < .05 for both) and the AG group (P < .05 for both). The average stiffness of the HP group was 159.7 N/mm, which was 36.8% of pretest stiffness.
The AG group had the lowest construct stiffness and percent of pretest stiffness (128.1 N/mm and 29.6%). The HP and AG groups were not statistically different in these comparisons, P = .350 for construct stiffness and P = .395 for percent of pretest stiffness.
Discussion
These results support the use of a one-third tubular plate and lag-screw construct for fixation of vertical shear medial malleolus fractures. This is clinically important because one-third tubular plates with 3.5-mm screws are readily available and cost significantly less than a precountoured anatomic-specific type of fixation. These results are based on the biomechanical properties of the constructs tested in this study.
The previous 2 studies8,9showed conflicting results about the most biomechanically sound fixation for SAD medial malleolar fractures. The study by Toolan and colleagues9 reported that 2 screws placed perpendicular to the fracture demonstrated the strongest overall construct. This study compared 3 separate types of 2-screw–only fixations and 2 plate-and-screw fixations. One construct was similar to the AG group in our study, and the other construct had a lag screw at the apex of the fracture. This previous study,9 however, did not investigate a similar construct to the MAG group that was tested in our study.
According to Dumigan and associates,8 a construct that consisted of a 4-hole plate with 2 screws proximal to the fracture and 2 lag screws showed the strongest fixation. This study, however, did not include a group like our study’s AG group, which is the traditional AG form of fixation.
In our study, we examined the biomechanic properties of a traditional fixation (AG construct), a commonly used fixation (MAG construct), and a newer construct (HP construct). The HP group is unique to this study and, to our knowledge, there is no literature on its use as fixation for this fracture. We did not include a 2-screw–only group, which is a limitation, because this fixation type is not common for the SAD fracture. This study also did not include an HP construct with an additional lag screw, which is an available option as well.
The current investigation used synthetic bone models constructed for biomechanical testing. The models were thought to provide a consistent model for fixation as opposed to using potentially osteopenic cadaveric bone. Each model was the same size and laterality. The stiffness as determined by pretest stiffness was not significantly different among models. Because all models were similar in composition and size, this allowed for more consistent osteotomies and similarly sized malleolar fragments. Theoretically, this allowed a more uniform comparison of all specimens and constructs.
Using models, however, is a limit of this study. While the models were of similar biomechanical quality, it is possible that a model may not reproduce the biology of a cavaderic specimen or the physiology of a construct in vivo. Of the 2 studies that investigated SAD fractures, the Dumigan study8 used cadaveric specimens. The fact that these models were all mildly osteoporotic and were embalmed specimens were study limits. The Toolan study9 used synthetic models. Although these models were consistent, they were models of bones and not intended for biomechanical studies, thereby increasing the potential for skewed results.
Our study investigated loading only in the offset-axial direction, a difference when compared to the Dumigan and colleagues8 and Toolan and colleagues9 studies. The offest transverse loading previously investigated would most likely represent an external rotation moment. While fixation in vivo could experience an external rotation moment, the specific fracture pattern of interest fails in offset-axial loading. In the original discription of the SAD fracture, Lauge-Hanson7 stated that the talus causes the vertically oriented medial malleolar fracture in the extreme of ankle supination with an adduction moment. Considering this, we investigated failure with a force in the direction that causes this type of fracture.
There are some additional limitations. This study demonstrated superiority of a one-third tubular plate with 2 screws proximally and 1 lag screw. While this was shown in the laboratory under pure offset-axial loading conditions, this may not reproduce daily forces experienced by the constructs. Additionally, this study examined load-to-failure of the constructs and did not investigate cyclic loading that a construct would experience in vivo. Because the testing is not recognizably consistent with day-to-day stresses of these constructs in vivo, this confounds the clinical application of our study.
The stiffness required for clinical healing is undetermined and, therefore, all 3 types of fixation could be adequate clinically. Patients are typically instructed to adhere to weight-bearing limitations on the affected extremity, and casts or splints are applied postoperatively for extended periods of time. Clinical studies would have significant benefit in the evaluation of fixation of vertical shear medial malleolar fractures.
Conclusion
AG plating technique with lag-screw placement is biomechanically superior to the other 2 constructs investigated. The clinical applications of these results are not known, and clinical trials are suggested to determine the best type of fixation for SAD-type medial malleolar fractures.
1. Hak DJ, Egol KA, Gardner MJ, Haskell A. The “not so simple” ankle fracture: avoiding problems and pitfalls to improve patient outcomes. Instr Course Lect. 2011;60:73-88.
2. Hamilton WC. Supination-adduction injuries. In: Hamilton WC, ed. Traumatic Disorders of the Ankle. 1st ed. New York, NY: Springer-Verlag; 1984:101-112.
3. McConnell T, Tornetta P. Marginal plafond impaction in association with supination-adduction ankle fractures: a report of eight cases. J Orthop Trauma. 2001;15(6):447-449.
4. Arimoto HK, Forrester DM. Classification of ankle fractures: an algorithm. AJR Am J Roentgenol. 1980;135(5):1057-1063.
5. Carr JB. Malleolar fractures and soft tissue injuries of the ankle. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:2515-2584.
6. Davidovitch RI, Egol KA. Ankle fractures. In: Bucholz RW HJ, Court-Brown CM, Tornetta P III, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2010:1975-2021.
7. Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60(5):957-985.
8. Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691.
9. Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489.
Supination-adduction (SAD)-type fractures of the ankle comprise approximately 5% to 20% of ankle fractures.1-3 As the name describes, this fracture is caused by forceful adduction of the supinated foot. There are 2 stages of the fracture pattern: the injury usually occurs first on the lateral side of the ankle with injury to the soft tissues or a low transverse fracture of the distal fibula. With continued force, in the second stage, the talus causes a shearing of the medial malleolus, creating the vertical shear fracture pattern.4-7 The vertical shear medial malleolus fracture pattern is the subject of this investigation.
Several techniques have been traditionally recommended for fixation of SAD-type ankle fracture, including: a 2-screw construct without plate fixation, oriented perpendicular to the fracture; and an AG plate construct with variable positioning and numbers of screws for fixation. There have been, however, only 2 published articles about the biomechanical properties of fixation of vertical shear medial malleolar fractures, which reported conflicting results.8,9 The most recent of these studies argued that one-third tubular plate fixation offers significant mechanical advantage over screw-only fixation, supporting the use of AG plates for fixation of SAD ankle fractures.8
An additional design for fixation of medial malleolus fractures has been introduced, consisting of a hook plate (HP) contoured for the medial malleolus. To our knowledge, no studies have investigated HP’s biomechanical properties. Thus, the objective of this study was to investigate and compare the biomechanical properties of 3 constructs for fixation of SAD-ankle fractures: an antiglide (AG) plate, an AG plate with an additional lag-screw across the fracture, and a precontoured HP.
Materials and Methods
Thirty 4th-generation–composite polyurethane models of the left tibia were obtained (Sawbones, Pacific Research Laboratories, Inc.). Largely, our methods accorded with the precedent set by other studies on these fracture types.8,9
Prior to creation of the fractures, each model was individually evaluated for pretest stiffness by using the slope of the linear portion of the load-displacement curve during offset-axial loading. This demonstrated the baseline elasticity of the models during loading. Assessing pretest stiffness was performed to reduce potential variables in the stiffness of individual models in the analysis of the testing data.
The models were numbered 1 through 30 on the shaft and on the medial malleolus. A custom jig was constructed with a table saw to create identical vertical shear medial malleolar fracture patterns in each model. The jig created the vertical shear SAD fracture described by Lauge-Hansen.7 All models were randomly assigned to 1 of 3 groups; each group consisted of 10 models (Figures 1A, 1B).
The 10 specimens in group 1 were fixed with a 5-hole, 3.5-mm, one-third tubular plate (Smith & Nephew) in a traditional AG fashion. The plates were placed at the same location on all tibiae. The proximal hole and the hole closest to the fracture line were filled with 3.5-mm cortical screws, which were long enough to achieve bicortical fixation. No lag screws were placed in this specimen group. In group 2, specimens were fixed with the same plate used in group 1 (Smith & Nephew). In this modified AG (MAG) construct, specimens were fixed identically to group 1 for plate placement and fixation of the 2 proximal screws. In this group, an additional screw was placed perpendicular to the fracture and parallel to the distal tibial articular surface. In both groups (AG and MAG), the plates were not bent before application.
Group 3 consisted of specimens fixed with a 5-hole, precontoured medial malleolar HP (Arthrex). This HP construct was fixed with two 3.5-mm cortical screws long enough to achieve bicortical fixation. The plate also engaged the bone at the tip of the medial malleolus by using 2 sharp prongs. The screws were placed in the most proximal hole and the hole just proximal to the fracture line. No lag screws were placed in the HP construct.
All models were tested in offset-axial loading to replicate a SAD moment similar to previous studies. To test offset-axial loading, a vice held each model identically with a 17º angle from the longitudinal axis (Figure 2). Loading was performed with a material testing system; a material testing system plunger was directed at the inferior articulating cartilage surface of the medial malleolus. The specimens were loaded at a rate of 1 mm/sec until 2 mm of displacement was reached (Figure 3) or catastrophic failure occurred. The raw data analyzed consisted of the initial stiffness of the construct and the overall load-to-failure. The slope of the linear portion of the load-displacement curve of stiffness determined stiffness of the construct.
One-way analysis of variance with post hoc Tukey HSD data analysis was performed to determine if there were statistical differences among the different fixation constructs during load-to-failure. To prevent skewing of results by different values of model elasticity, pretest stiffness was accounted for by calculating a ratio of construct stiffness as a function of pretest model stiffness. Total force-to-failure was the recorded maximum force (in N) to cause failure. A P value of < .05 was set for significance. All data were analyzed using SPSS software (SPSS Version 15.0; SPSS Inc.).
Results
Analysis of pretest stiffness showed no significant difference among models (P = .490). All models failed by a gap of 2 mm at the distal fracture site except for 3 models in the MAG group. These 3 models failed at a much higher load than the remainder of the models and failed by fracture of the models.
The MAG group demonstrated significantly superior stiffness to the 2 other models tested (Figure 4). On average, this group required 753.5 N of force before failure. This was 530 N higher than the HP (P < .05) and 638 N higher than the AG constructs, respectively (P < .05). The HP and AG groups required forces of 223.2 N and 115.5 N for failure, respectively. These numbers were not significant (P= .063).
The absolute construct stiffness and construct stiffness as a function of pretest stiffness of the MAG group was the highest of all groups, 271.7 N/mm and 57.2%, respectively (Figure 5). These numbers showed significance when compared with the values of the HP group (P < .05 for both) and the AG group (P < .05 for both). The average stiffness of the HP group was 159.7 N/mm, which was 36.8% of pretest stiffness.
The AG group had the lowest construct stiffness and percent of pretest stiffness (128.1 N/mm and 29.6%). The HP and AG groups were not statistically different in these comparisons, P = .350 for construct stiffness and P = .395 for percent of pretest stiffness.
Discussion
These results support the use of a one-third tubular plate and lag-screw construct for fixation of vertical shear medial malleolus fractures. This is clinically important because one-third tubular plates with 3.5-mm screws are readily available and cost significantly less than a precountoured anatomic-specific type of fixation. These results are based on the biomechanical properties of the constructs tested in this study.
The previous 2 studies8,9showed conflicting results about the most biomechanically sound fixation for SAD medial malleolar fractures. The study by Toolan and colleagues9 reported that 2 screws placed perpendicular to the fracture demonstrated the strongest overall construct. This study compared 3 separate types of 2-screw–only fixations and 2 plate-and-screw fixations. One construct was similar to the AG group in our study, and the other construct had a lag screw at the apex of the fracture. This previous study,9 however, did not investigate a similar construct to the MAG group that was tested in our study.
According to Dumigan and associates,8 a construct that consisted of a 4-hole plate with 2 screws proximal to the fracture and 2 lag screws showed the strongest fixation. This study, however, did not include a group like our study’s AG group, which is the traditional AG form of fixation.
In our study, we examined the biomechanic properties of a traditional fixation (AG construct), a commonly used fixation (MAG construct), and a newer construct (HP construct). The HP group is unique to this study and, to our knowledge, there is no literature on its use as fixation for this fracture. We did not include a 2-screw–only group, which is a limitation, because this fixation type is not common for the SAD fracture. This study also did not include an HP construct with an additional lag screw, which is an available option as well.
The current investigation used synthetic bone models constructed for biomechanical testing. The models were thought to provide a consistent model for fixation as opposed to using potentially osteopenic cadaveric bone. Each model was the same size and laterality. The stiffness as determined by pretest stiffness was not significantly different among models. Because all models were similar in composition and size, this allowed for more consistent osteotomies and similarly sized malleolar fragments. Theoretically, this allowed a more uniform comparison of all specimens and constructs.
Using models, however, is a limit of this study. While the models were of similar biomechanical quality, it is possible that a model may not reproduce the biology of a cavaderic specimen or the physiology of a construct in vivo. Of the 2 studies that investigated SAD fractures, the Dumigan study8 used cadaveric specimens. The fact that these models were all mildly osteoporotic and were embalmed specimens were study limits. The Toolan study9 used synthetic models. Although these models were consistent, they were models of bones and not intended for biomechanical studies, thereby increasing the potential for skewed results.
Our study investigated loading only in the offset-axial direction, a difference when compared to the Dumigan and colleagues8 and Toolan and colleagues9 studies. The offest transverse loading previously investigated would most likely represent an external rotation moment. While fixation in vivo could experience an external rotation moment, the specific fracture pattern of interest fails in offset-axial loading. In the original discription of the SAD fracture, Lauge-Hanson7 stated that the talus causes the vertically oriented medial malleolar fracture in the extreme of ankle supination with an adduction moment. Considering this, we investigated failure with a force in the direction that causes this type of fracture.
There are some additional limitations. This study demonstrated superiority of a one-third tubular plate with 2 screws proximally and 1 lag screw. While this was shown in the laboratory under pure offset-axial loading conditions, this may not reproduce daily forces experienced by the constructs. Additionally, this study examined load-to-failure of the constructs and did not investigate cyclic loading that a construct would experience in vivo. Because the testing is not recognizably consistent with day-to-day stresses of these constructs in vivo, this confounds the clinical application of our study.
The stiffness required for clinical healing is undetermined and, therefore, all 3 types of fixation could be adequate clinically. Patients are typically instructed to adhere to weight-bearing limitations on the affected extremity, and casts or splints are applied postoperatively for extended periods of time. Clinical studies would have significant benefit in the evaluation of fixation of vertical shear medial malleolar fractures.
Conclusion
AG plating technique with lag-screw placement is biomechanically superior to the other 2 constructs investigated. The clinical applications of these results are not known, and clinical trials are suggested to determine the best type of fixation for SAD-type medial malleolar fractures.
Supination-adduction (SAD)-type fractures of the ankle comprise approximately 5% to 20% of ankle fractures.1-3 As the name describes, this fracture is caused by forceful adduction of the supinated foot. There are 2 stages of the fracture pattern: the injury usually occurs first on the lateral side of the ankle with injury to the soft tissues or a low transverse fracture of the distal fibula. With continued force, in the second stage, the talus causes a shearing of the medial malleolus, creating the vertical shear fracture pattern.4-7 The vertical shear medial malleolus fracture pattern is the subject of this investigation.
Several techniques have been traditionally recommended for fixation of SAD-type ankle fracture, including: a 2-screw construct without plate fixation, oriented perpendicular to the fracture; and an AG plate construct with variable positioning and numbers of screws for fixation. There have been, however, only 2 published articles about the biomechanical properties of fixation of vertical shear medial malleolar fractures, which reported conflicting results.8,9 The most recent of these studies argued that one-third tubular plate fixation offers significant mechanical advantage over screw-only fixation, supporting the use of AG plates for fixation of SAD ankle fractures.8
An additional design for fixation of medial malleolus fractures has been introduced, consisting of a hook plate (HP) contoured for the medial malleolus. To our knowledge, no studies have investigated HP’s biomechanical properties. Thus, the objective of this study was to investigate and compare the biomechanical properties of 3 constructs for fixation of SAD-ankle fractures: an antiglide (AG) plate, an AG plate with an additional lag-screw across the fracture, and a precontoured HP.
Materials and Methods
Thirty 4th-generation–composite polyurethane models of the left tibia were obtained (Sawbones, Pacific Research Laboratories, Inc.). Largely, our methods accorded with the precedent set by other studies on these fracture types.8,9
Prior to creation of the fractures, each model was individually evaluated for pretest stiffness by using the slope of the linear portion of the load-displacement curve during offset-axial loading. This demonstrated the baseline elasticity of the models during loading. Assessing pretest stiffness was performed to reduce potential variables in the stiffness of individual models in the analysis of the testing data.
The models were numbered 1 through 30 on the shaft and on the medial malleolus. A custom jig was constructed with a table saw to create identical vertical shear medial malleolar fracture patterns in each model. The jig created the vertical shear SAD fracture described by Lauge-Hansen.7 All models were randomly assigned to 1 of 3 groups; each group consisted of 10 models (Figures 1A, 1B).
The 10 specimens in group 1 were fixed with a 5-hole, 3.5-mm, one-third tubular plate (Smith & Nephew) in a traditional AG fashion. The plates were placed at the same location on all tibiae. The proximal hole and the hole closest to the fracture line were filled with 3.5-mm cortical screws, which were long enough to achieve bicortical fixation. No lag screws were placed in this specimen group. In group 2, specimens were fixed with the same plate used in group 1 (Smith & Nephew). In this modified AG (MAG) construct, specimens were fixed identically to group 1 for plate placement and fixation of the 2 proximal screws. In this group, an additional screw was placed perpendicular to the fracture and parallel to the distal tibial articular surface. In both groups (AG and MAG), the plates were not bent before application.
Group 3 consisted of specimens fixed with a 5-hole, precontoured medial malleolar HP (Arthrex). This HP construct was fixed with two 3.5-mm cortical screws long enough to achieve bicortical fixation. The plate also engaged the bone at the tip of the medial malleolus by using 2 sharp prongs. The screws were placed in the most proximal hole and the hole just proximal to the fracture line. No lag screws were placed in the HP construct.
All models were tested in offset-axial loading to replicate a SAD moment similar to previous studies. To test offset-axial loading, a vice held each model identically with a 17º angle from the longitudinal axis (Figure 2). Loading was performed with a material testing system; a material testing system plunger was directed at the inferior articulating cartilage surface of the medial malleolus. The specimens were loaded at a rate of 1 mm/sec until 2 mm of displacement was reached (Figure 3) or catastrophic failure occurred. The raw data analyzed consisted of the initial stiffness of the construct and the overall load-to-failure. The slope of the linear portion of the load-displacement curve of stiffness determined stiffness of the construct.
One-way analysis of variance with post hoc Tukey HSD data analysis was performed to determine if there were statistical differences among the different fixation constructs during load-to-failure. To prevent skewing of results by different values of model elasticity, pretest stiffness was accounted for by calculating a ratio of construct stiffness as a function of pretest model stiffness. Total force-to-failure was the recorded maximum force (in N) to cause failure. A P value of < .05 was set for significance. All data were analyzed using SPSS software (SPSS Version 15.0; SPSS Inc.).
Results
Analysis of pretest stiffness showed no significant difference among models (P = .490). All models failed by a gap of 2 mm at the distal fracture site except for 3 models in the MAG group. These 3 models failed at a much higher load than the remainder of the models and failed by fracture of the models.
The MAG group demonstrated significantly superior stiffness to the 2 other models tested (Figure 4). On average, this group required 753.5 N of force before failure. This was 530 N higher than the HP (P < .05) and 638 N higher than the AG constructs, respectively (P < .05). The HP and AG groups required forces of 223.2 N and 115.5 N for failure, respectively. These numbers were not significant (P= .063).
The absolute construct stiffness and construct stiffness as a function of pretest stiffness of the MAG group was the highest of all groups, 271.7 N/mm and 57.2%, respectively (Figure 5). These numbers showed significance when compared with the values of the HP group (P < .05 for both) and the AG group (P < .05 for both). The average stiffness of the HP group was 159.7 N/mm, which was 36.8% of pretest stiffness.
The AG group had the lowest construct stiffness and percent of pretest stiffness (128.1 N/mm and 29.6%). The HP and AG groups were not statistically different in these comparisons, P = .350 for construct stiffness and P = .395 for percent of pretest stiffness.
Discussion
These results support the use of a one-third tubular plate and lag-screw construct for fixation of vertical shear medial malleolus fractures. This is clinically important because one-third tubular plates with 3.5-mm screws are readily available and cost significantly less than a precountoured anatomic-specific type of fixation. These results are based on the biomechanical properties of the constructs tested in this study.
The previous 2 studies8,9showed conflicting results about the most biomechanically sound fixation for SAD medial malleolar fractures. The study by Toolan and colleagues9 reported that 2 screws placed perpendicular to the fracture demonstrated the strongest overall construct. This study compared 3 separate types of 2-screw–only fixations and 2 plate-and-screw fixations. One construct was similar to the AG group in our study, and the other construct had a lag screw at the apex of the fracture. This previous study,9 however, did not investigate a similar construct to the MAG group that was tested in our study.
According to Dumigan and associates,8 a construct that consisted of a 4-hole plate with 2 screws proximal to the fracture and 2 lag screws showed the strongest fixation. This study, however, did not include a group like our study’s AG group, which is the traditional AG form of fixation.
In our study, we examined the biomechanic properties of a traditional fixation (AG construct), a commonly used fixation (MAG construct), and a newer construct (HP construct). The HP group is unique to this study and, to our knowledge, there is no literature on its use as fixation for this fracture. We did not include a 2-screw–only group, which is a limitation, because this fixation type is not common for the SAD fracture. This study also did not include an HP construct with an additional lag screw, which is an available option as well.
The current investigation used synthetic bone models constructed for biomechanical testing. The models were thought to provide a consistent model for fixation as opposed to using potentially osteopenic cadaveric bone. Each model was the same size and laterality. The stiffness as determined by pretest stiffness was not significantly different among models. Because all models were similar in composition and size, this allowed for more consistent osteotomies and similarly sized malleolar fragments. Theoretically, this allowed a more uniform comparison of all specimens and constructs.
Using models, however, is a limit of this study. While the models were of similar biomechanical quality, it is possible that a model may not reproduce the biology of a cavaderic specimen or the physiology of a construct in vivo. Of the 2 studies that investigated SAD fractures, the Dumigan study8 used cadaveric specimens. The fact that these models were all mildly osteoporotic and were embalmed specimens were study limits. The Toolan study9 used synthetic models. Although these models were consistent, they were models of bones and not intended for biomechanical studies, thereby increasing the potential for skewed results.
Our study investigated loading only in the offset-axial direction, a difference when compared to the Dumigan and colleagues8 and Toolan and colleagues9 studies. The offest transverse loading previously investigated would most likely represent an external rotation moment. While fixation in vivo could experience an external rotation moment, the specific fracture pattern of interest fails in offset-axial loading. In the original discription of the SAD fracture, Lauge-Hanson7 stated that the talus causes the vertically oriented medial malleolar fracture in the extreme of ankle supination with an adduction moment. Considering this, we investigated failure with a force in the direction that causes this type of fracture.
There are some additional limitations. This study demonstrated superiority of a one-third tubular plate with 2 screws proximally and 1 lag screw. While this was shown in the laboratory under pure offset-axial loading conditions, this may not reproduce daily forces experienced by the constructs. Additionally, this study examined load-to-failure of the constructs and did not investigate cyclic loading that a construct would experience in vivo. Because the testing is not recognizably consistent with day-to-day stresses of these constructs in vivo, this confounds the clinical application of our study.
The stiffness required for clinical healing is undetermined and, therefore, all 3 types of fixation could be adequate clinically. Patients are typically instructed to adhere to weight-bearing limitations on the affected extremity, and casts or splints are applied postoperatively for extended periods of time. Clinical studies would have significant benefit in the evaluation of fixation of vertical shear medial malleolar fractures.
Conclusion
AG plating technique with lag-screw placement is biomechanically superior to the other 2 constructs investigated. The clinical applications of these results are not known, and clinical trials are suggested to determine the best type of fixation for SAD-type medial malleolar fractures.
1. Hak DJ, Egol KA, Gardner MJ, Haskell A. The “not so simple” ankle fracture: avoiding problems and pitfalls to improve patient outcomes. Instr Course Lect. 2011;60:73-88.
2. Hamilton WC. Supination-adduction injuries. In: Hamilton WC, ed. Traumatic Disorders of the Ankle. 1st ed. New York, NY: Springer-Verlag; 1984:101-112.
3. McConnell T, Tornetta P. Marginal plafond impaction in association with supination-adduction ankle fractures: a report of eight cases. J Orthop Trauma. 2001;15(6):447-449.
4. Arimoto HK, Forrester DM. Classification of ankle fractures: an algorithm. AJR Am J Roentgenol. 1980;135(5):1057-1063.
5. Carr JB. Malleolar fractures and soft tissue injuries of the ankle. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:2515-2584.
6. Davidovitch RI, Egol KA. Ankle fractures. In: Bucholz RW HJ, Court-Brown CM, Tornetta P III, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2010:1975-2021.
7. Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60(5):957-985.
8. Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691.
9. Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489.
1. Hak DJ, Egol KA, Gardner MJ, Haskell A. The “not so simple” ankle fracture: avoiding problems and pitfalls to improve patient outcomes. Instr Course Lect. 2011;60:73-88.
2. Hamilton WC. Supination-adduction injuries. In: Hamilton WC, ed. Traumatic Disorders of the Ankle. 1st ed. New York, NY: Springer-Verlag; 1984:101-112.
3. McConnell T, Tornetta P. Marginal plafond impaction in association with supination-adduction ankle fractures: a report of eight cases. J Orthop Trauma. 2001;15(6):447-449.
4. Arimoto HK, Forrester DM. Classification of ankle fractures: an algorithm. AJR Am J Roentgenol. 1980;135(5):1057-1063.
5. Carr JB. Malleolar fractures and soft tissue injuries of the ankle. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:2515-2584.
6. Davidovitch RI, Egol KA. Ankle fractures. In: Bucholz RW HJ, Court-Brown CM, Tornetta P III, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2010:1975-2021.
7. Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60(5):957-985.
8. Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691.
9. Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489.
Operative Versus Nonoperative Treatment of Jones Fractures: A Decision Analysis Model
The optimal management strategy for acute fractures of the metadiaphyseal fifth metatarsal (Jones fractures) is controversial. Patients can be successfully treated nonoperatively with non-weight-bearing and immobilization in a short leg cast1-7 or operatively with placement of an intramedullary screw.8-10 The primary advantage of nonoperative treatment is avoiding the risks and discomfort of surgery; disadvantages include the need for prolonged immobilization and protected weight-bearing as well as a decreased union rate.8,9 Advantages of operative treatment include accelerated functional recovery and an improved union rate; disadvantages include exposure to the risks, inconvenience, and discomfort of surgery. Clear, definitive evidence for guiding treatment is not available in the orthopedic literature, and treatment strategies vary substantially according to surgeon and patient preference.
Expected-value decision analysis, a research tool that helps guide decision-making in situations of uncertainty, has been effectively applied to other areas of uncertainty in the orthopedic literature.11-14 Borrowed from gaming theory, the technique involves creating a decision tree to define the clinical problem, determining outcome probabilities and utilities, performing a fold-back analysis to determine the optimal decision-making strategy, and performing a sensitivity analysis to model the effect of varying outcome probabilities and utilities on decision-making. Decision analysis may therefore allow the clinician and the patient to optimize decision-making based on best available evidence and patient preferences. It also helps determine the most important factors affecting management strategies and the decision-making process, which may not always be intuitive.
In the present study, we used expected-value decision analysis to determine the optimal management strategy, operative or nonoperative, for acute Jones fracture. We also explored factors with the most influence on the model and identified important questions for future research.
Materials and Methods
Institutional review board approval was obtained for this study. Analysis was performed with Treeage Pro statistical software (Treeage Software).
Outcome Probabilities
Outcome probabilities were determined by reviewing the literature for articles on Jones fractures. This body of literature was summarized in a comprehensive review by Dean and colleagues15, who extracted data from 19 studies: 1 randomized controlled trial, 1 prospective case series, and 17 retrospective case series.15 We used data from these studies to determine outcome probabilities (Table).
Outcome Utilities
Utilities represent patient preferences for various disease states. Outcome utility values were obtained from 32 adults (25 women, 7 men) with no history of foot injury. Mean age was 32.4 years (range, 20-69 years). The questionnaire presented scenarios for the different outcomes and asked patients to rate these outcomes on a scale ranging from 0 (worst possible outcome) to 10 (best possible outcome). The Sports subscale of the Foot and Ankle Ability Measure (FAAM) 16 was used to quantify patient activity level.
Decision Tree and Fold-Back Analysis
A decision tree was constructed with 1 decision node, 4 chance nodes, and 7 terminal nodes (Figure 1). The decision tree demonstrates 2 different strategies for managing a Jones fracture. The decision node divides the tree into 2 branches: initial operative or nonoperative treatment. Both branches are followed by various chance nodes, each terminating in a discrete clinical outcome. Per convention, utility data were placed to the right of the terminal nodes, and probability data were placed under the terminal nodes.
Fold-back analysis was performed to identify the optimal strategy. Fold-back analysis involves multiplying each outcome utility by its associated probability, thereby providing an “expected value” for each clinical endpoint. Then, the expected values for each endpoint can be summed for a given management strategy, and the ultimate expected values of the different strategies can be compared. The management strategy associated with the highest expected value is optimal for the given outcome utilities and probabilities.
Sensitivity Analysis
One-way sensitivity analysis was performed to model the effect on decision-making of changing the values for utility for uncomplicated surgery, utility for healing with nonoperative treatment, utility for uncomplicated treatment of nonunion, likelihood of healing with nonoperative treatment, likelihood of healing with surgery, and likelihood of minor complication with surgery. These were the variables found to affect the decision-making strategy within their clinically plausible ranges.
Results
Outcome Probabilities and Utilities
Outcome probabilities and utilities are illustrated in Figure 1. By convention, probabilities appear below the corresponding branches of the decision tree, and utilities appear at the end of each branch. Mean (SD) FAAM Sports subscale score was 84.6 (27.4). This subscale is scored as a percentage from 0% to 100%, with higher scores indicating a higher level of physical function.
Decision Analysis
The expected value for nonoperative treatment was 7.74, and the expected value for intramedullary screw fixation was 7.88 (Figure 1). Therefore, operative treatment was identified as the optimal treatment strategy.
Sensitivity analyses revealed that the optimal decision making strategy was very sensitive to small changes in several variables. Nonoperative treatment becomes the preferred strategy when the utility value for uncomplicated surgery falls below 8.04 (Figure 2), when the utility for healing with nonoperative treatment rises above 8.49 (Figure 3), when the likelihood of healing with nonoperative treatment rises above 82% (Figure 4), or when the probability of healing after surgery falls below 92% (Figure 5). Nonoperative treatment is also favored when the probability of minor complication with surgery is above 17% (Figure 6) and when utility for a successfully treated nonunion is higher than 6.9 (Figure 7).
Discussion
Optimal management of a metadiaphyseal fracture of the fifth metatarsal (Jones fracture) remains controversial. The decision between initial operative or nonoperative treatment lends itself to expected-value decision analysis because of well-defined treatment options and relatively discrete outcomes. The principal advantages of nonoperative treatment are that it allows the patient to avoid the risks and discomfort of surgery, and the principal advantages of operative treatment are that it maximizes the chance of fracture union and may accelerate functional recovery.
Our decision analysis determined that operative fixation is the optimal decision path, given the outcome probabilities derived from the literature and the utilities obtained from surveys. This finding is in accordance with several expert opinions in foot and ankle fracture surgery.17,18 However, the expected values of the operative and nonoperative treatment strategies differed by only 0.3 on a 10-point scale. Such similar expected values in our model are not surprising given the controversy surrounding clinical decision making in the treatment of these fractures.19
In addition, our analysis identified the important variables in the decision-making process. Patients averse to surgery, patients not averse to successful nonoperative treatment, and patients who view successful nonunion surgery after initial nonoperative treatment as a relatively positive outcome may be best treated nonoperatively. These findings emphasize the importance of patient preferences and shared decision-making. Higher rates of healing with nonoperative treatment, lower rates of healing with surgery, and higher complication rates with surgery also favor nonoperative management. It would therefore be valuable to identify risk factors for nonunion with nonoperative treatment and to identify the technical details of surgery that maximize rates of healing and minimize the risk of complications.
The limitations of decision analysis involve the methods by which probabilities and utilities are obtained. In general, the most accurate, stable, and robust estimates of outcome probabilities are derived from a meta-analytic synthesis of randomized clinical trials, the highest level of clinical evidence. In our model, data were extracted primarily from level IV studies; only 1 level III study20 and 1 level II study21 were available for analysis. Thus, as is the case with many foot and ankle disorders22, the information on treatment of Jones fractures is very limited in its level of clinical evidence.
Determination of outcome utility also has limitations. Utility is a subjective value that an individual places on a specific outcome. This can be very difficult to quantify. In general, the most robust estimates of patient-derived utilities are derived from complex qualitative methods, such as the standard reference gamble or time trade-offs, in which patients are asked to gamble or choose between health states usually referenced to death. In this study, we determined patient-derived utility values from a direct scaling method using a Likert scale because of the complexity of the standard reference gamble and the difficulty of referencing to death for metatarsal fracture. Although use of a direct scale to determine utility values is less rigorous than the standard reference gamble, this technique has been corroborated methodologically,23 is advantageous in terms of feasibility and reliability,24 and has been successfully used in other orthopedic decision analysis models.12,25,26 In our estimation, generally active patients without a history of foot pathology constituted a sample of convenience but also were representative of individuals at risk for Jones fracture. Although specific scenarios were presented, the patients who completed the questionnaire may not have had deep insights into the subtleties and implications of the various disease states and treatments. Regardless of how outcome probabilities and utilities are determined, they are considered point estimates in decision analysis, and sensitivity analyses are therefore performed to assess how decision making changes over a range of values.
Conclusion
The results of this study may help optimize the process of deciding between operative and nonoperative treatment for Jones fracture. For a given patient, the optimal strategy depends not only on the probabilities of the various outcomes but also on personal preference. Thus, there may not be one right answer for all patients. Patients who value a higher chance of fracture healing with initial treatment or an earlier return to sports are best treated operatively, whereas patients who are risk-averse and place a high value on fracture healing without surgery should be managed nonoperatively. We therefore advocate a model of shared medical decision-making in which the physician and the patient are jointly involved, considering both outcome probabilities and patient preferences. Ongoing research efforts should focus on predictors of nonunion with nonoperative treatment.
1. Dameron TB Jr. Fractures of the proximal fifth metatarsal: selecting the best treatment option. J Am Acad Orthop Surg. 1995;3(2):110-114.
2. Fetzer GB, Wright RW. Metatarsal shaft fractures and fractures of the proximal fifth metatarsal. Clin Sports Med. 2006;25(1):139-150, x.
3. Konkel KF, Menger AG, Retzlaff SA. Nonoperative treatment of fifth metatarsal fractures in an orthopaedic suburban private multispeciality practice. Foot Ankle Int. 2005;26(9):704-707.
4. Lawrence SJ, Botte MJ. Jones’ fractures and related fractures of the proximal fifth metatarsal. Foot Ankle. 1993;14(6):358-365.
5. Nunley JA. Fractures of the base of the fifth metatarsal: the Jones fracture. Orthop Clin North Am. 2001;32(1):171-180.
6. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
7. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. Classification and guidelines for non-surgical and surgical management. J Bone Joint Surg Am. 1984;66(2):209-214.
8. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
9. Kavanaugh JH, Brower TD, Mann RV. The Jones fracture revisited. J Bone Joint Surg Am. 1978;60(6):776-782.
10. Porter DA, Duncan M, Meyer SJ. Fifth metatarsal Jones fracture fixation with a 4.5-mm cannulated stainless steel screw in the competitive and recreational athlete: a clinical and radiographic evaluation. Am J Sports Med. 2005;33(5):726-733.
11. Aleem IS, Jalal H, Sheikh AA, Bhandari M. Clinical decision analysis: Incorporating the evidence with patient p. Patient Prefer Adherence. 2009;3:21-24.
12. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6):991-996.e1.
13. Chen NC, Shauver MJ, Chung KC. A primer on use of decision analysis methodology in hand surgery. J Hand Surg Am. 2009;34(6):983-990.
14. Kocher MS, Henley MB. It is money that matters: decision analysis and cost-effectiveness analysis. Clin Orthop Relat Res. 2003(413):106-116.
15. Dean BJ, Kothari A, Uppal H, Kankate R. The jones fracture classification, management, outcome, and complications: a systematic review. Foot Ankle Spec. 2012;5(4):256-259.
16. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26(11):968-983.
17. Roche AJ, Calder JD. Treatment and return to sport following a Jones fracture of the fifth metatarsal: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1307-1315.
18. Zwitser EW, Breederveld RS. Fractures of the fifth metatarsal; diagnosis and treatment. Injury. 2010;41(6):555-562.
19. McBryde AM Jr. The complicated Jones fracture, including revision and malalignment. Foot Ankle Clin. 2009;14(2):151-168.
20. Porter DA, Rund AM, Dobslaw R, Duncan M. Comparison of 4.5- and 5.5-mm cannulated stainless steel screws for fifth metatarsal Jones fracture fixation. Foot Ankle Int. 2009;30(1):27-33.
21. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med. 2005;33(7):970-975.
22. Hunt KJ, Hurwit D. Use of patient-reported outcome measures in foot and ankle research. J Bone Joint Surg Am. 2013;95(16):e118(1-9).
23. Stiggelbout AM, Eijkemans MJ, Kiebert GM, Kievit J, Leer JW, De Haes HJ. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off? International journal of technology assessment in health care. Spring. 1996;12(2):291-298.
24. Parkin D, Devlin N. Is there a case for using visual analogue scale valuations in cost-utility analysis? Health Econ. 2006;15(7):653-664.
25. Bishop JA, Crall TS, Kocher MS. Operative versus nonoperative treatment after primary traumatic anterior glenohumeral dislocation: expected-value decision analysis. J Shoulder Elbow Surg. 2011;20(7):1087-1094.
26. Kocher MS, Bishop J, Marshall R, Briggs KK, Hawkins RJ. Operative versus nonoperative management of acute Achilles tendon rupture: expected-value decision analysis. Am J Sports Med. 2002;30(6):783-790.
27. Nagao M, Saita Y, Kameda S, et al. Headless compression screw fixation of jones fractures: an outcomes study in Japanese athletes. Am J Sports Med. 2012;40(11):2578-2582.
28. Thomas JL, Davis BC. Treatment of Jones fracture nonunion with isolated intramedullary screw fixation. J Foot Ankle Surg. 2011;50(5):566-568.
29. Habbu RA, Marsh RS, Anderson JG, Bohay DR. Closed intramedullary screw fixation for nonunion of fifth metatarsal Jones fracture. Foot Ankle Int. 2011;32(6):603-608.
30. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
31. Chuckpaiwong B, Queen RM, Easley ME, Nunley JA. Distinguishing Jones and proximal diaphyseal fractures of the fifth metatarsal. Clin Orthop Relat Res. 2008;466(8):1966-1970.
32. DeVries JG, Cuttica DJ, Hyer CF. Cannulated screw fixation of Jones fifth metatarsal fractures: a comparison of titanium and stainless steel screw fixation. J Foot Ankle Surg. 2011;50(2):207-212.
33. Reese K, Litsky A, Kaeding C, Pedroza A, Shah N. Cannulated screw fixation of Jones fractures: a clinical and biomechanical study. Am J Sports Med. 2004;32(7):1736-1742.
34. Lombardi CM, Connolly FG, Silhanek AD. The use of external fixation for treatment of the acute Jones fracture: a retrospective review of 10 cases. J Foot Ankle Surg. 2004;43(3):173-178.
35. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
36. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal. Analysis of a fracture registry. Clin Orthop Relat Res. 1995(315):238-241.
37. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture. Good results in 40 cases after 11-26 years. Orthop Scand. 1994;65(5):545-547.
38. Mindrebo N, Shelbourne KD, Van Meter CD, Rettig AC. Outpatient percutaneous screw fixation of the acute Jones fracture. Am J Sports Med. 1993;21(5):720-723.
39. Zogby RG, Baker BE. A review of nonoperative treatment of Jones’ fracture. Am J Sports Med. 1987;15(4):304-307.
40. Dameron TB Jr. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am. 1975;57(6):788-792.
41. Fernandez Fairen M, Guillen J, Busto JM, Roura J. Fractures of the fifth metatarsal in basketball players. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):373-377.
The optimal management strategy for acute fractures of the metadiaphyseal fifth metatarsal (Jones fractures) is controversial. Patients can be successfully treated nonoperatively with non-weight-bearing and immobilization in a short leg cast1-7 or operatively with placement of an intramedullary screw.8-10 The primary advantage of nonoperative treatment is avoiding the risks and discomfort of surgery; disadvantages include the need for prolonged immobilization and protected weight-bearing as well as a decreased union rate.8,9 Advantages of operative treatment include accelerated functional recovery and an improved union rate; disadvantages include exposure to the risks, inconvenience, and discomfort of surgery. Clear, definitive evidence for guiding treatment is not available in the orthopedic literature, and treatment strategies vary substantially according to surgeon and patient preference.
Expected-value decision analysis, a research tool that helps guide decision-making in situations of uncertainty, has been effectively applied to other areas of uncertainty in the orthopedic literature.11-14 Borrowed from gaming theory, the technique involves creating a decision tree to define the clinical problem, determining outcome probabilities and utilities, performing a fold-back analysis to determine the optimal decision-making strategy, and performing a sensitivity analysis to model the effect of varying outcome probabilities and utilities on decision-making. Decision analysis may therefore allow the clinician and the patient to optimize decision-making based on best available evidence and patient preferences. It also helps determine the most important factors affecting management strategies and the decision-making process, which may not always be intuitive.
In the present study, we used expected-value decision analysis to determine the optimal management strategy, operative or nonoperative, for acute Jones fracture. We also explored factors with the most influence on the model and identified important questions for future research.
Materials and Methods
Institutional review board approval was obtained for this study. Analysis was performed with Treeage Pro statistical software (Treeage Software).
Outcome Probabilities
Outcome probabilities were determined by reviewing the literature for articles on Jones fractures. This body of literature was summarized in a comprehensive review by Dean and colleagues15, who extracted data from 19 studies: 1 randomized controlled trial, 1 prospective case series, and 17 retrospective case series.15 We used data from these studies to determine outcome probabilities (Table).
Outcome Utilities
Utilities represent patient preferences for various disease states. Outcome utility values were obtained from 32 adults (25 women, 7 men) with no history of foot injury. Mean age was 32.4 years (range, 20-69 years). The questionnaire presented scenarios for the different outcomes and asked patients to rate these outcomes on a scale ranging from 0 (worst possible outcome) to 10 (best possible outcome). The Sports subscale of the Foot and Ankle Ability Measure (FAAM) 16 was used to quantify patient activity level.
Decision Tree and Fold-Back Analysis
A decision tree was constructed with 1 decision node, 4 chance nodes, and 7 terminal nodes (Figure 1). The decision tree demonstrates 2 different strategies for managing a Jones fracture. The decision node divides the tree into 2 branches: initial operative or nonoperative treatment. Both branches are followed by various chance nodes, each terminating in a discrete clinical outcome. Per convention, utility data were placed to the right of the terminal nodes, and probability data were placed under the terminal nodes.
Fold-back analysis was performed to identify the optimal strategy. Fold-back analysis involves multiplying each outcome utility by its associated probability, thereby providing an “expected value” for each clinical endpoint. Then, the expected values for each endpoint can be summed for a given management strategy, and the ultimate expected values of the different strategies can be compared. The management strategy associated with the highest expected value is optimal for the given outcome utilities and probabilities.
Sensitivity Analysis
One-way sensitivity analysis was performed to model the effect on decision-making of changing the values for utility for uncomplicated surgery, utility for healing with nonoperative treatment, utility for uncomplicated treatment of nonunion, likelihood of healing with nonoperative treatment, likelihood of healing with surgery, and likelihood of minor complication with surgery. These were the variables found to affect the decision-making strategy within their clinically plausible ranges.
Results
Outcome Probabilities and Utilities
Outcome probabilities and utilities are illustrated in Figure 1. By convention, probabilities appear below the corresponding branches of the decision tree, and utilities appear at the end of each branch. Mean (SD) FAAM Sports subscale score was 84.6 (27.4). This subscale is scored as a percentage from 0% to 100%, with higher scores indicating a higher level of physical function.
Decision Analysis
The expected value for nonoperative treatment was 7.74, and the expected value for intramedullary screw fixation was 7.88 (Figure 1). Therefore, operative treatment was identified as the optimal treatment strategy.
Sensitivity analyses revealed that the optimal decision making strategy was very sensitive to small changes in several variables. Nonoperative treatment becomes the preferred strategy when the utility value for uncomplicated surgery falls below 8.04 (Figure 2), when the utility for healing with nonoperative treatment rises above 8.49 (Figure 3), when the likelihood of healing with nonoperative treatment rises above 82% (Figure 4), or when the probability of healing after surgery falls below 92% (Figure 5). Nonoperative treatment is also favored when the probability of minor complication with surgery is above 17% (Figure 6) and when utility for a successfully treated nonunion is higher than 6.9 (Figure 7).
Discussion
Optimal management of a metadiaphyseal fracture of the fifth metatarsal (Jones fracture) remains controversial. The decision between initial operative or nonoperative treatment lends itself to expected-value decision analysis because of well-defined treatment options and relatively discrete outcomes. The principal advantages of nonoperative treatment are that it allows the patient to avoid the risks and discomfort of surgery, and the principal advantages of operative treatment are that it maximizes the chance of fracture union and may accelerate functional recovery.
Our decision analysis determined that operative fixation is the optimal decision path, given the outcome probabilities derived from the literature and the utilities obtained from surveys. This finding is in accordance with several expert opinions in foot and ankle fracture surgery.17,18 However, the expected values of the operative and nonoperative treatment strategies differed by only 0.3 on a 10-point scale. Such similar expected values in our model are not surprising given the controversy surrounding clinical decision making in the treatment of these fractures.19
In addition, our analysis identified the important variables in the decision-making process. Patients averse to surgery, patients not averse to successful nonoperative treatment, and patients who view successful nonunion surgery after initial nonoperative treatment as a relatively positive outcome may be best treated nonoperatively. These findings emphasize the importance of patient preferences and shared decision-making. Higher rates of healing with nonoperative treatment, lower rates of healing with surgery, and higher complication rates with surgery also favor nonoperative management. It would therefore be valuable to identify risk factors for nonunion with nonoperative treatment and to identify the technical details of surgery that maximize rates of healing and minimize the risk of complications.
The limitations of decision analysis involve the methods by which probabilities and utilities are obtained. In general, the most accurate, stable, and robust estimates of outcome probabilities are derived from a meta-analytic synthesis of randomized clinical trials, the highest level of clinical evidence. In our model, data were extracted primarily from level IV studies; only 1 level III study20 and 1 level II study21 were available for analysis. Thus, as is the case with many foot and ankle disorders22, the information on treatment of Jones fractures is very limited in its level of clinical evidence.
Determination of outcome utility also has limitations. Utility is a subjective value that an individual places on a specific outcome. This can be very difficult to quantify. In general, the most robust estimates of patient-derived utilities are derived from complex qualitative methods, such as the standard reference gamble or time trade-offs, in which patients are asked to gamble or choose between health states usually referenced to death. In this study, we determined patient-derived utility values from a direct scaling method using a Likert scale because of the complexity of the standard reference gamble and the difficulty of referencing to death for metatarsal fracture. Although use of a direct scale to determine utility values is less rigorous than the standard reference gamble, this technique has been corroborated methodologically,23 is advantageous in terms of feasibility and reliability,24 and has been successfully used in other orthopedic decision analysis models.12,25,26 In our estimation, generally active patients without a history of foot pathology constituted a sample of convenience but also were representative of individuals at risk for Jones fracture. Although specific scenarios were presented, the patients who completed the questionnaire may not have had deep insights into the subtleties and implications of the various disease states and treatments. Regardless of how outcome probabilities and utilities are determined, they are considered point estimates in decision analysis, and sensitivity analyses are therefore performed to assess how decision making changes over a range of values.
Conclusion
The results of this study may help optimize the process of deciding between operative and nonoperative treatment for Jones fracture. For a given patient, the optimal strategy depends not only on the probabilities of the various outcomes but also on personal preference. Thus, there may not be one right answer for all patients. Patients who value a higher chance of fracture healing with initial treatment or an earlier return to sports are best treated operatively, whereas patients who are risk-averse and place a high value on fracture healing without surgery should be managed nonoperatively. We therefore advocate a model of shared medical decision-making in which the physician and the patient are jointly involved, considering both outcome probabilities and patient preferences. Ongoing research efforts should focus on predictors of nonunion with nonoperative treatment.
The optimal management strategy for acute fractures of the metadiaphyseal fifth metatarsal (Jones fractures) is controversial. Patients can be successfully treated nonoperatively with non-weight-bearing and immobilization in a short leg cast1-7 or operatively with placement of an intramedullary screw.8-10 The primary advantage of nonoperative treatment is avoiding the risks and discomfort of surgery; disadvantages include the need for prolonged immobilization and protected weight-bearing as well as a decreased union rate.8,9 Advantages of operative treatment include accelerated functional recovery and an improved union rate; disadvantages include exposure to the risks, inconvenience, and discomfort of surgery. Clear, definitive evidence for guiding treatment is not available in the orthopedic literature, and treatment strategies vary substantially according to surgeon and patient preference.
Expected-value decision analysis, a research tool that helps guide decision-making in situations of uncertainty, has been effectively applied to other areas of uncertainty in the orthopedic literature.11-14 Borrowed from gaming theory, the technique involves creating a decision tree to define the clinical problem, determining outcome probabilities and utilities, performing a fold-back analysis to determine the optimal decision-making strategy, and performing a sensitivity analysis to model the effect of varying outcome probabilities and utilities on decision-making. Decision analysis may therefore allow the clinician and the patient to optimize decision-making based on best available evidence and patient preferences. It also helps determine the most important factors affecting management strategies and the decision-making process, which may not always be intuitive.
In the present study, we used expected-value decision analysis to determine the optimal management strategy, operative or nonoperative, for acute Jones fracture. We also explored factors with the most influence on the model and identified important questions for future research.
Materials and Methods
Institutional review board approval was obtained for this study. Analysis was performed with Treeage Pro statistical software (Treeage Software).
Outcome Probabilities
Outcome probabilities were determined by reviewing the literature for articles on Jones fractures. This body of literature was summarized in a comprehensive review by Dean and colleagues15, who extracted data from 19 studies: 1 randomized controlled trial, 1 prospective case series, and 17 retrospective case series.15 We used data from these studies to determine outcome probabilities (Table).
Outcome Utilities
Utilities represent patient preferences for various disease states. Outcome utility values were obtained from 32 adults (25 women, 7 men) with no history of foot injury. Mean age was 32.4 years (range, 20-69 years). The questionnaire presented scenarios for the different outcomes and asked patients to rate these outcomes on a scale ranging from 0 (worst possible outcome) to 10 (best possible outcome). The Sports subscale of the Foot and Ankle Ability Measure (FAAM) 16 was used to quantify patient activity level.
Decision Tree and Fold-Back Analysis
A decision tree was constructed with 1 decision node, 4 chance nodes, and 7 terminal nodes (Figure 1). The decision tree demonstrates 2 different strategies for managing a Jones fracture. The decision node divides the tree into 2 branches: initial operative or nonoperative treatment. Both branches are followed by various chance nodes, each terminating in a discrete clinical outcome. Per convention, utility data were placed to the right of the terminal nodes, and probability data were placed under the terminal nodes.
Fold-back analysis was performed to identify the optimal strategy. Fold-back analysis involves multiplying each outcome utility by its associated probability, thereby providing an “expected value” for each clinical endpoint. Then, the expected values for each endpoint can be summed for a given management strategy, and the ultimate expected values of the different strategies can be compared. The management strategy associated with the highest expected value is optimal for the given outcome utilities and probabilities.
Sensitivity Analysis
One-way sensitivity analysis was performed to model the effect on decision-making of changing the values for utility for uncomplicated surgery, utility for healing with nonoperative treatment, utility for uncomplicated treatment of nonunion, likelihood of healing with nonoperative treatment, likelihood of healing with surgery, and likelihood of minor complication with surgery. These were the variables found to affect the decision-making strategy within their clinically plausible ranges.
Results
Outcome Probabilities and Utilities
Outcome probabilities and utilities are illustrated in Figure 1. By convention, probabilities appear below the corresponding branches of the decision tree, and utilities appear at the end of each branch. Mean (SD) FAAM Sports subscale score was 84.6 (27.4). This subscale is scored as a percentage from 0% to 100%, with higher scores indicating a higher level of physical function.
Decision Analysis
The expected value for nonoperative treatment was 7.74, and the expected value for intramedullary screw fixation was 7.88 (Figure 1). Therefore, operative treatment was identified as the optimal treatment strategy.
Sensitivity analyses revealed that the optimal decision making strategy was very sensitive to small changes in several variables. Nonoperative treatment becomes the preferred strategy when the utility value for uncomplicated surgery falls below 8.04 (Figure 2), when the utility for healing with nonoperative treatment rises above 8.49 (Figure 3), when the likelihood of healing with nonoperative treatment rises above 82% (Figure 4), or when the probability of healing after surgery falls below 92% (Figure 5). Nonoperative treatment is also favored when the probability of minor complication with surgery is above 17% (Figure 6) and when utility for a successfully treated nonunion is higher than 6.9 (Figure 7).
Discussion
Optimal management of a metadiaphyseal fracture of the fifth metatarsal (Jones fracture) remains controversial. The decision between initial operative or nonoperative treatment lends itself to expected-value decision analysis because of well-defined treatment options and relatively discrete outcomes. The principal advantages of nonoperative treatment are that it allows the patient to avoid the risks and discomfort of surgery, and the principal advantages of operative treatment are that it maximizes the chance of fracture union and may accelerate functional recovery.
Our decision analysis determined that operative fixation is the optimal decision path, given the outcome probabilities derived from the literature and the utilities obtained from surveys. This finding is in accordance with several expert opinions in foot and ankle fracture surgery.17,18 However, the expected values of the operative and nonoperative treatment strategies differed by only 0.3 on a 10-point scale. Such similar expected values in our model are not surprising given the controversy surrounding clinical decision making in the treatment of these fractures.19
In addition, our analysis identified the important variables in the decision-making process. Patients averse to surgery, patients not averse to successful nonoperative treatment, and patients who view successful nonunion surgery after initial nonoperative treatment as a relatively positive outcome may be best treated nonoperatively. These findings emphasize the importance of patient preferences and shared decision-making. Higher rates of healing with nonoperative treatment, lower rates of healing with surgery, and higher complication rates with surgery also favor nonoperative management. It would therefore be valuable to identify risk factors for nonunion with nonoperative treatment and to identify the technical details of surgery that maximize rates of healing and minimize the risk of complications.
The limitations of decision analysis involve the methods by which probabilities and utilities are obtained. In general, the most accurate, stable, and robust estimates of outcome probabilities are derived from a meta-analytic synthesis of randomized clinical trials, the highest level of clinical evidence. In our model, data were extracted primarily from level IV studies; only 1 level III study20 and 1 level II study21 were available for analysis. Thus, as is the case with many foot and ankle disorders22, the information on treatment of Jones fractures is very limited in its level of clinical evidence.
Determination of outcome utility also has limitations. Utility is a subjective value that an individual places on a specific outcome. This can be very difficult to quantify. In general, the most robust estimates of patient-derived utilities are derived from complex qualitative methods, such as the standard reference gamble or time trade-offs, in which patients are asked to gamble or choose between health states usually referenced to death. In this study, we determined patient-derived utility values from a direct scaling method using a Likert scale because of the complexity of the standard reference gamble and the difficulty of referencing to death for metatarsal fracture. Although use of a direct scale to determine utility values is less rigorous than the standard reference gamble, this technique has been corroborated methodologically,23 is advantageous in terms of feasibility and reliability,24 and has been successfully used in other orthopedic decision analysis models.12,25,26 In our estimation, generally active patients without a history of foot pathology constituted a sample of convenience but also were representative of individuals at risk for Jones fracture. Although specific scenarios were presented, the patients who completed the questionnaire may not have had deep insights into the subtleties and implications of the various disease states and treatments. Regardless of how outcome probabilities and utilities are determined, they are considered point estimates in decision analysis, and sensitivity analyses are therefore performed to assess how decision making changes over a range of values.
Conclusion
The results of this study may help optimize the process of deciding between operative and nonoperative treatment for Jones fracture. For a given patient, the optimal strategy depends not only on the probabilities of the various outcomes but also on personal preference. Thus, there may not be one right answer for all patients. Patients who value a higher chance of fracture healing with initial treatment or an earlier return to sports are best treated operatively, whereas patients who are risk-averse and place a high value on fracture healing without surgery should be managed nonoperatively. We therefore advocate a model of shared medical decision-making in which the physician and the patient are jointly involved, considering both outcome probabilities and patient preferences. Ongoing research efforts should focus on predictors of nonunion with nonoperative treatment.
1. Dameron TB Jr. Fractures of the proximal fifth metatarsal: selecting the best treatment option. J Am Acad Orthop Surg. 1995;3(2):110-114.
2. Fetzer GB, Wright RW. Metatarsal shaft fractures and fractures of the proximal fifth metatarsal. Clin Sports Med. 2006;25(1):139-150, x.
3. Konkel KF, Menger AG, Retzlaff SA. Nonoperative treatment of fifth metatarsal fractures in an orthopaedic suburban private multispeciality practice. Foot Ankle Int. 2005;26(9):704-707.
4. Lawrence SJ, Botte MJ. Jones’ fractures and related fractures of the proximal fifth metatarsal. Foot Ankle. 1993;14(6):358-365.
5. Nunley JA. Fractures of the base of the fifth metatarsal: the Jones fracture. Orthop Clin North Am. 2001;32(1):171-180.
6. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
7. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. Classification and guidelines for non-surgical and surgical management. J Bone Joint Surg Am. 1984;66(2):209-214.
8. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
9. Kavanaugh JH, Brower TD, Mann RV. The Jones fracture revisited. J Bone Joint Surg Am. 1978;60(6):776-782.
10. Porter DA, Duncan M, Meyer SJ. Fifth metatarsal Jones fracture fixation with a 4.5-mm cannulated stainless steel screw in the competitive and recreational athlete: a clinical and radiographic evaluation. Am J Sports Med. 2005;33(5):726-733.
11. Aleem IS, Jalal H, Sheikh AA, Bhandari M. Clinical decision analysis: Incorporating the evidence with patient p. Patient Prefer Adherence. 2009;3:21-24.
12. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6):991-996.e1.
13. Chen NC, Shauver MJ, Chung KC. A primer on use of decision analysis methodology in hand surgery. J Hand Surg Am. 2009;34(6):983-990.
14. Kocher MS, Henley MB. It is money that matters: decision analysis and cost-effectiveness analysis. Clin Orthop Relat Res. 2003(413):106-116.
15. Dean BJ, Kothari A, Uppal H, Kankate R. The jones fracture classification, management, outcome, and complications: a systematic review. Foot Ankle Spec. 2012;5(4):256-259.
16. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26(11):968-983.
17. Roche AJ, Calder JD. Treatment and return to sport following a Jones fracture of the fifth metatarsal: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1307-1315.
18. Zwitser EW, Breederveld RS. Fractures of the fifth metatarsal; diagnosis and treatment. Injury. 2010;41(6):555-562.
19. McBryde AM Jr. The complicated Jones fracture, including revision and malalignment. Foot Ankle Clin. 2009;14(2):151-168.
20. Porter DA, Rund AM, Dobslaw R, Duncan M. Comparison of 4.5- and 5.5-mm cannulated stainless steel screws for fifth metatarsal Jones fracture fixation. Foot Ankle Int. 2009;30(1):27-33.
21. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med. 2005;33(7):970-975.
22. Hunt KJ, Hurwit D. Use of patient-reported outcome measures in foot and ankle research. J Bone Joint Surg Am. 2013;95(16):e118(1-9).
23. Stiggelbout AM, Eijkemans MJ, Kiebert GM, Kievit J, Leer JW, De Haes HJ. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off? International journal of technology assessment in health care. Spring. 1996;12(2):291-298.
24. Parkin D, Devlin N. Is there a case for using visual analogue scale valuations in cost-utility analysis? Health Econ. 2006;15(7):653-664.
25. Bishop JA, Crall TS, Kocher MS. Operative versus nonoperative treatment after primary traumatic anterior glenohumeral dislocation: expected-value decision analysis. J Shoulder Elbow Surg. 2011;20(7):1087-1094.
26. Kocher MS, Bishop J, Marshall R, Briggs KK, Hawkins RJ. Operative versus nonoperative management of acute Achilles tendon rupture: expected-value decision analysis. Am J Sports Med. 2002;30(6):783-790.
27. Nagao M, Saita Y, Kameda S, et al. Headless compression screw fixation of jones fractures: an outcomes study in Japanese athletes. Am J Sports Med. 2012;40(11):2578-2582.
28. Thomas JL, Davis BC. Treatment of Jones fracture nonunion with isolated intramedullary screw fixation. J Foot Ankle Surg. 2011;50(5):566-568.
29. Habbu RA, Marsh RS, Anderson JG, Bohay DR. Closed intramedullary screw fixation for nonunion of fifth metatarsal Jones fracture. Foot Ankle Int. 2011;32(6):603-608.
30. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
31. Chuckpaiwong B, Queen RM, Easley ME, Nunley JA. Distinguishing Jones and proximal diaphyseal fractures of the fifth metatarsal. Clin Orthop Relat Res. 2008;466(8):1966-1970.
32. DeVries JG, Cuttica DJ, Hyer CF. Cannulated screw fixation of Jones fifth metatarsal fractures: a comparison of titanium and stainless steel screw fixation. J Foot Ankle Surg. 2011;50(2):207-212.
33. Reese K, Litsky A, Kaeding C, Pedroza A, Shah N. Cannulated screw fixation of Jones fractures: a clinical and biomechanical study. Am J Sports Med. 2004;32(7):1736-1742.
34. Lombardi CM, Connolly FG, Silhanek AD. The use of external fixation for treatment of the acute Jones fracture: a retrospective review of 10 cases. J Foot Ankle Surg. 2004;43(3):173-178.
35. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
36. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal. Analysis of a fracture registry. Clin Orthop Relat Res. 1995(315):238-241.
37. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture. Good results in 40 cases after 11-26 years. Orthop Scand. 1994;65(5):545-547.
38. Mindrebo N, Shelbourne KD, Van Meter CD, Rettig AC. Outpatient percutaneous screw fixation of the acute Jones fracture. Am J Sports Med. 1993;21(5):720-723.
39. Zogby RG, Baker BE. A review of nonoperative treatment of Jones’ fracture. Am J Sports Med. 1987;15(4):304-307.
40. Dameron TB Jr. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am. 1975;57(6):788-792.
41. Fernandez Fairen M, Guillen J, Busto JM, Roura J. Fractures of the fifth metatarsal in basketball players. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):373-377.
1. Dameron TB Jr. Fractures of the proximal fifth metatarsal: selecting the best treatment option. J Am Acad Orthop Surg. 1995;3(2):110-114.
2. Fetzer GB, Wright RW. Metatarsal shaft fractures and fractures of the proximal fifth metatarsal. Clin Sports Med. 2006;25(1):139-150, x.
3. Konkel KF, Menger AG, Retzlaff SA. Nonoperative treatment of fifth metatarsal fractures in an orthopaedic suburban private multispeciality practice. Foot Ankle Int. 2005;26(9):704-707.
4. Lawrence SJ, Botte MJ. Jones’ fractures and related fractures of the proximal fifth metatarsal. Foot Ankle. 1993;14(6):358-365.
5. Nunley JA. Fractures of the base of the fifth metatarsal: the Jones fracture. Orthop Clin North Am. 2001;32(1):171-180.
6. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
7. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. Classification and guidelines for non-surgical and surgical management. J Bone Joint Surg Am. 1984;66(2):209-214.
8. DeLee JC, Evans JP, Julian J. Stress fracture of the fifth metatarsal. Am J Sports Med. 1983;11(5):349-353.
9. Kavanaugh JH, Brower TD, Mann RV. The Jones fracture revisited. J Bone Joint Surg Am. 1978;60(6):776-782.
10. Porter DA, Duncan M, Meyer SJ. Fifth metatarsal Jones fracture fixation with a 4.5-mm cannulated stainless steel screw in the competitive and recreational athlete: a clinical and radiographic evaluation. Am J Sports Med. 2005;33(5):726-733.
11. Aleem IS, Jalal H, Sheikh AA, Bhandari M. Clinical decision analysis: Incorporating the evidence with patient p. Patient Prefer Adherence. 2009;3:21-24.
12. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6):991-996.e1.
13. Chen NC, Shauver MJ, Chung KC. A primer on use of decision analysis methodology in hand surgery. J Hand Surg Am. 2009;34(6):983-990.
14. Kocher MS, Henley MB. It is money that matters: decision analysis and cost-effectiveness analysis. Clin Orthop Relat Res. 2003(413):106-116.
15. Dean BJ, Kothari A, Uppal H, Kankate R. The jones fracture classification, management, outcome, and complications: a systematic review. Foot Ankle Spec. 2012;5(4):256-259.
16. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26(11):968-983.
17. Roche AJ, Calder JD. Treatment and return to sport following a Jones fracture of the fifth metatarsal: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1307-1315.
18. Zwitser EW, Breederveld RS. Fractures of the fifth metatarsal; diagnosis and treatment. Injury. 2010;41(6):555-562.
19. McBryde AM Jr. The complicated Jones fracture, including revision and malalignment. Foot Ankle Clin. 2009;14(2):151-168.
20. Porter DA, Rund AM, Dobslaw R, Duncan M. Comparison of 4.5- and 5.5-mm cannulated stainless steel screws for fifth metatarsal Jones fracture fixation. Foot Ankle Int. 2009;30(1):27-33.
21. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med. 2005;33(7):970-975.
22. Hunt KJ, Hurwit D. Use of patient-reported outcome measures in foot and ankle research. J Bone Joint Surg Am. 2013;95(16):e118(1-9).
23. Stiggelbout AM, Eijkemans MJ, Kiebert GM, Kievit J, Leer JW, De Haes HJ. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off? International journal of technology assessment in health care. Spring. 1996;12(2):291-298.
24. Parkin D, Devlin N. Is there a case for using visual analogue scale valuations in cost-utility analysis? Health Econ. 2006;15(7):653-664.
25. Bishop JA, Crall TS, Kocher MS. Operative versus nonoperative treatment after primary traumatic anterior glenohumeral dislocation: expected-value decision analysis. J Shoulder Elbow Surg. 2011;20(7):1087-1094.
26. Kocher MS, Bishop J, Marshall R, Briggs KK, Hawkins RJ. Operative versus nonoperative management of acute Achilles tendon rupture: expected-value decision analysis. Am J Sports Med. 2002;30(6):783-790.
27. Nagao M, Saita Y, Kameda S, et al. Headless compression screw fixation of jones fractures: an outcomes study in Japanese athletes. Am J Sports Med. 2012;40(11):2578-2582.
28. Thomas JL, Davis BC. Treatment of Jones fracture nonunion with isolated intramedullary screw fixation. J Foot Ankle Surg. 2011;50(5):566-568.
29. Habbu RA, Marsh RS, Anderson JG, Bohay DR. Closed intramedullary screw fixation for nonunion of fifth metatarsal Jones fracture. Foot Ankle Int. 2011;32(6):603-608.
30. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
31. Chuckpaiwong B, Queen RM, Easley ME, Nunley JA. Distinguishing Jones and proximal diaphyseal fractures of the fifth metatarsal. Clin Orthop Relat Res. 2008;466(8):1966-1970.
32. DeVries JG, Cuttica DJ, Hyer CF. Cannulated screw fixation of Jones fifth metatarsal fractures: a comparison of titanium and stainless steel screw fixation. J Foot Ankle Surg. 2011;50(2):207-212.
33. Reese K, Litsky A, Kaeding C, Pedroza A, Shah N. Cannulated screw fixation of Jones fractures: a clinical and biomechanical study. Am J Sports Med. 2004;32(7):1736-1742.
34. Lombardi CM, Connolly FG, Silhanek AD. The use of external fixation for treatment of the acute Jones fracture: a retrospective review of 10 cases. J Foot Ankle Surg. 2004;43(3):173-178.
35. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
36. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal. Analysis of a fracture registry. Clin Orthop Relat Res. 1995(315):238-241.
37. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture. Good results in 40 cases after 11-26 years. Orthop Scand. 1994;65(5):545-547.
38. Mindrebo N, Shelbourne KD, Van Meter CD, Rettig AC. Outpatient percutaneous screw fixation of the acute Jones fracture. Am J Sports Med. 1993;21(5):720-723.
39. Zogby RG, Baker BE. A review of nonoperative treatment of Jones’ fracture. Am J Sports Med. 1987;15(4):304-307.
40. Dameron TB Jr. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am. 1975;57(6):788-792.
41. Fernandez Fairen M, Guillen J, Busto JM, Roura J. Fractures of the fifth metatarsal in basketball players. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):373-377.
Reconstructive Shelf Arthroplasty as a Salvage Procedure for Complex Fifth Tarsometatarsal Joint Complex Injuries: A Case Review and Discussion
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Osteoid Osteoma of the Talar Neck With Subacute Presentation
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
Gout Causing Isolated Sesamoid Destruction Mimicking a Neoplastic Process
The sesamoid bones are a major contributor to normal gait, with more than 50% of body weight transmitted through the hallux metatarsophalangeal joint (MTPJ) complex. There are varying amounts of stress on the sesamoids, dependent on the gait cycle.1,2 The sesamoids act as a fulcrum to increase the mechanical force of the flexor hallucis brevis tendon.3 Sesamoid pathology can be a source of significant morbidity in patients, especially young athletes or laborers who spend long hours on their feet. More common causes of isolated sesamoid discomfort include sesamoiditis, fracture, and avascular necrosis, with neoplastic, infectious, and inflammatory conditions rarely isolated to the sesamoid.
Gout is a systemic disorder of uric acid metabolism characterized by deposition of monosodium urate crystals in soft tissues and joints.1 This deposition leads to tophus formation with an accompanying inflammatory response. Gout progresses through 3 stages, beginning with acute gout, which may end with chronic, recurrent, and tophaceous gouty arthritis. The hallux MTPJ is the most common joint affected by gout, with few case reports of primary sesamoid gout.1-2,4 We present a case of gout, with radiographic findings isolated to the medial sesamoid, that mimicked a neoplastic process in a patient with no known history of gout. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old laborer presented for evaluation of a right sesamoid injury he sustained 4 months earlier when he fell off a ladder and had acute onset plantar hallux MTPJ pain and swelling. He was treated by an outside physician for a presumptive diagnosis of a medial sesamoid fracture with rest and controlled ankle movement (CAM) boot immobilization that resulted in slowly improving symptoms. In discussion of the patient’s history, he reported that 1 year earlier he had a traumatic event with similar symptoms of MTPJ pain and swelling. At that time, treatment with a CAM boot resulted in complete resolution of pain. His outside physician performed a hematologic workup for gout, which showed a normal uric acid level.
On examination, the patient presented with edema to the right hallux MTPJ and mild tenderness to palpation of the medial sesamoid. He had no pain with motion of the hallux MTPJ or with palpation of the lateral sesamoid. His radiographs showed a bipartite versus fractured sesamoid (Figures 1A, 1B) and serial magnetic resonance imaging (MRI) showed an MTPJ effusion and hyperintense signal in the medial sesamoid, but no erosive findings or soft-tissue masses (Figures 2A, 2B).
The patient was treated with wedge-sandal forefoot offloading, leading to resolution of symptoms over 6 weeks, at which point he was transitioned to normal shoe wear and allowed to progress in his activity as dictated by his symptoms. He presented for reevaluation approximately 2 weeks later with acute, atraumatic onset of plantar left hallux pain and swelling. His examination showed diffuse hallux MTPJ swelling and tenderness isolated to the medial sesamoid. An attempt at aspiration of the MTPJ yielded no fluid, and the patient again was placed in a forefoot-offloading sandal.
Radiographs of the left foot showed an expansile destructive lesion of the medial sesamoid with interval change from his previous imaging approximately 3 months earlier, obtained as part of his contralateral foot evaluation (Figure 3). MRI with and without contrast showed an expansile process isolated to the medial sesamoid with cortical thinning and marrow replacement (Figures 4A-4D).
Because of continued discomfort and lack of a definitive diagnosis, an excisional biopsy of the sesamoid was performed. Intraoperatively, the sesamoid was extensively fragmented with near complete replacement by a chalky tophus, as well as chalky deposition throughout the hallux MTPJ. No significant degenerative changes were observed. Surgical pathology showed bone and fibroconnective tissue with deposits of negative birefringement needle-shaped crystals consistent with monosodium urate deposition and foreign body histocytic reaction, as well as repair reaction of bone (Figures 5A, 5B).
Postoperatively, the patient was again placed in a forefoot-offloading wedge sandal for 6 weeks, followed by progression of activity as dictated by his symptoms. He was also evaluated by a rheumatologist and started on medical treatment for gout, with complete resolution of his bilateral hallux pain. He has been able to return to his previous employment.
Discussion
The sesamoid bones are an important component of the hallux MTPJ complex, giving a mechanical advantage to the flexor hallucis brevis tendons in plantar flexion of the hallux.5 Many pathologic conditions have been well described in the literature, including fracture, sesamoiditis, nonunion, avascular necrosis, and plantar keratosis. There is also a 10% incidence of bipartite sesamoids, most commonly isolated to the medial sesamoid, with up to 25% of patients presenting with bilateral bipartite sesamoids.5 Neoplastic processes of the sesamoid are rare, with a paucity of reports in the literature.6,7 Gout is a condition in which hyperuricemia, due to an imbalance in uric acid production and excretion, leads to deposition of monosodium urate crystals in joints, bones, and soft tissues, causing an inflammatory reaction. Risk factors for gout are male sex, advanced age, and ethnicity, as well as obesity, high protein diet, alcohol use, hypertension, and certain medications. Precipitation of acute attacks has been associated with acute trauma, and the first MTPJ is the most common location for an acute attack.8
Isolated sesamoid lesions are rare, with few isolated case reports in the literature. Benign and malignant lesions appear most often in the metatarsals, with the calcaneus being the second most commonly afflicted site.9 The typical differential diagnosis for isolated lytic bone lesions includes fibrous dysplasia, osteoblastoma, giant cell tumor, metastatic lesion, multiple myeloma, aneurysmal bone cyst, chondroblastoma, brown tumor, infection, eosinophilic granuloma, enchondroma, and bone cyst, with no reports in the literature to our knowledge of these entities presenting in the hallux MTPJ sesamoid. In contrast, gout typically begins with normal radiographic findings, and later leads to erosive, “punched out” lesions on either side of the MTPJ.2
Hyperuricemia is an essential part of the pathophysiology of gout, but not all patients with an acute gouty attack have elevated uric acid levels and, in contrast, may actually have normal or low levels in 12% to 43% of cases.8 The most accurate time frame for assessment of serum uric acid levels is 2 weeks or more after subsidence of an acute event.8 The normal uric acid levels seen in our patient were most likely due to the fact that the workup was undertaken during an acute attack. The difficulty with establishing the diagnosis was compounded by bilateral involvement, history of trauma, negative joint aspiration, and atypical radiographic findings. A number of reports have described patients with tophus deposits prior to or in the absence of gouty arthritis or a gouty attack.10 Risk factors for this presentation include female sex, the predominant or exclusive involvement of fingers, chronic kidney disease, and treatment with a diuretic or anti-inflammatory drug.10
Conclusion
Our case report illustrates the difficulty in diagnosing an acute gouty attack in a patient with a history of trauma and atypical radiographic findings. The hallux MTPJ is the most common location of acute gouty attacks, but the medial sesamoid as an isolated location is a rare site of presentation. The combination of pain isolated to palpation of the sesamoid and radiographs that showed an aggressive and rapidly expansile lesion of the medial sesamoid raised concerns about a neoplastic lesion. Practitioners should consider acute gout in patients with sesamoid pain and with radiographs showing an expansile sesamoid lesion.
1. Mair SD, Coogan AC, Speer KP, Hall RL. Gout as a source of sesamoid pain. Foot Ankle Int. 1995;16(10):613-616.
2. Reber PU, Patel AG, Noesberger B. Gout: rare cause of hallucal sesamoid pain: a case report. Foot Ankle Int. 1997;12(18):818-820.
3. Van Hal ME, Kenne JS, Lange TA, Clancy WG Jr. Stress fractures of the great toe sesamoids. Am J Sports Med. 1982;10(2):122-128.
4. Liu S-Z, Yeh L, Chou Y, Chen CK, Pan HB. Isolated intraosseous gout in hallux sesamoid mimicking a bone tumor in a teenaged patient. Skeletal Radiol. 2003;32(11):647-650.
5. Cohen BE. Hallux sesamoid disorders. Foot Ankle Clin. 2009;14(1):91-104.
6. Harty JA, Kelly P, Niall D, O’Keane JC, Stephens MM. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the sesamoid: a case report. Foot Ankle Int. 2000;21(5):408-412.
7. Noguchi M, Ikoma K, Matsumoto N, Nagasawa K. Bizarre parosteal osteochondromatous proliferation of the sesamoid: an unusual hallux valgus deformity. Foot Ankle Int. 2004;25(7):503-506.
8. Becker MA. Clinical manifestations and diagnosis of gout. Up to Date website. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-gout. Updated June 20, 2015. Accessed August 19, 2015.
9. Bos GD, Esther RJ, Woll TS. Foot tumors: diagnosis and treatment. J Am Acad Orthop Surg. 2002;10(4):259-270.
10. Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. Report of six cases and review of the literature. Arch Intern Med. 1992;152(4):873-876.
The sesamoid bones are a major contributor to normal gait, with more than 50% of body weight transmitted through the hallux metatarsophalangeal joint (MTPJ) complex. There are varying amounts of stress on the sesamoids, dependent on the gait cycle.1,2 The sesamoids act as a fulcrum to increase the mechanical force of the flexor hallucis brevis tendon.3 Sesamoid pathology can be a source of significant morbidity in patients, especially young athletes or laborers who spend long hours on their feet. More common causes of isolated sesamoid discomfort include sesamoiditis, fracture, and avascular necrosis, with neoplastic, infectious, and inflammatory conditions rarely isolated to the sesamoid.
Gout is a systemic disorder of uric acid metabolism characterized by deposition of monosodium urate crystals in soft tissues and joints.1 This deposition leads to tophus formation with an accompanying inflammatory response. Gout progresses through 3 stages, beginning with acute gout, which may end with chronic, recurrent, and tophaceous gouty arthritis. The hallux MTPJ is the most common joint affected by gout, with few case reports of primary sesamoid gout.1-2,4 We present a case of gout, with radiographic findings isolated to the medial sesamoid, that mimicked a neoplastic process in a patient with no known history of gout. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old laborer presented for evaluation of a right sesamoid injury he sustained 4 months earlier when he fell off a ladder and had acute onset plantar hallux MTPJ pain and swelling. He was treated by an outside physician for a presumptive diagnosis of a medial sesamoid fracture with rest and controlled ankle movement (CAM) boot immobilization that resulted in slowly improving symptoms. In discussion of the patient’s history, he reported that 1 year earlier he had a traumatic event with similar symptoms of MTPJ pain and swelling. At that time, treatment with a CAM boot resulted in complete resolution of pain. His outside physician performed a hematologic workup for gout, which showed a normal uric acid level.
On examination, the patient presented with edema to the right hallux MTPJ and mild tenderness to palpation of the medial sesamoid. He had no pain with motion of the hallux MTPJ or with palpation of the lateral sesamoid. His radiographs showed a bipartite versus fractured sesamoid (Figures 1A, 1B) and serial magnetic resonance imaging (MRI) showed an MTPJ effusion and hyperintense signal in the medial sesamoid, but no erosive findings or soft-tissue masses (Figures 2A, 2B).
The patient was treated with wedge-sandal forefoot offloading, leading to resolution of symptoms over 6 weeks, at which point he was transitioned to normal shoe wear and allowed to progress in his activity as dictated by his symptoms. He presented for reevaluation approximately 2 weeks later with acute, atraumatic onset of plantar left hallux pain and swelling. His examination showed diffuse hallux MTPJ swelling and tenderness isolated to the medial sesamoid. An attempt at aspiration of the MTPJ yielded no fluid, and the patient again was placed in a forefoot-offloading sandal.
Radiographs of the left foot showed an expansile destructive lesion of the medial sesamoid with interval change from his previous imaging approximately 3 months earlier, obtained as part of his contralateral foot evaluation (Figure 3). MRI with and without contrast showed an expansile process isolated to the medial sesamoid with cortical thinning and marrow replacement (Figures 4A-4D).
Because of continued discomfort and lack of a definitive diagnosis, an excisional biopsy of the sesamoid was performed. Intraoperatively, the sesamoid was extensively fragmented with near complete replacement by a chalky tophus, as well as chalky deposition throughout the hallux MTPJ. No significant degenerative changes were observed. Surgical pathology showed bone and fibroconnective tissue with deposits of negative birefringement needle-shaped crystals consistent with monosodium urate deposition and foreign body histocytic reaction, as well as repair reaction of bone (Figures 5A, 5B).
Postoperatively, the patient was again placed in a forefoot-offloading wedge sandal for 6 weeks, followed by progression of activity as dictated by his symptoms. He was also evaluated by a rheumatologist and started on medical treatment for gout, with complete resolution of his bilateral hallux pain. He has been able to return to his previous employment.
Discussion
The sesamoid bones are an important component of the hallux MTPJ complex, giving a mechanical advantage to the flexor hallucis brevis tendons in plantar flexion of the hallux.5 Many pathologic conditions have been well described in the literature, including fracture, sesamoiditis, nonunion, avascular necrosis, and plantar keratosis. There is also a 10% incidence of bipartite sesamoids, most commonly isolated to the medial sesamoid, with up to 25% of patients presenting with bilateral bipartite sesamoids.5 Neoplastic processes of the sesamoid are rare, with a paucity of reports in the literature.6,7 Gout is a condition in which hyperuricemia, due to an imbalance in uric acid production and excretion, leads to deposition of monosodium urate crystals in joints, bones, and soft tissues, causing an inflammatory reaction. Risk factors for gout are male sex, advanced age, and ethnicity, as well as obesity, high protein diet, alcohol use, hypertension, and certain medications. Precipitation of acute attacks has been associated with acute trauma, and the first MTPJ is the most common location for an acute attack.8
Isolated sesamoid lesions are rare, with few isolated case reports in the literature. Benign and malignant lesions appear most often in the metatarsals, with the calcaneus being the second most commonly afflicted site.9 The typical differential diagnosis for isolated lytic bone lesions includes fibrous dysplasia, osteoblastoma, giant cell tumor, metastatic lesion, multiple myeloma, aneurysmal bone cyst, chondroblastoma, brown tumor, infection, eosinophilic granuloma, enchondroma, and bone cyst, with no reports in the literature to our knowledge of these entities presenting in the hallux MTPJ sesamoid. In contrast, gout typically begins with normal radiographic findings, and later leads to erosive, “punched out” lesions on either side of the MTPJ.2
Hyperuricemia is an essential part of the pathophysiology of gout, but not all patients with an acute gouty attack have elevated uric acid levels and, in contrast, may actually have normal or low levels in 12% to 43% of cases.8 The most accurate time frame for assessment of serum uric acid levels is 2 weeks or more after subsidence of an acute event.8 The normal uric acid levels seen in our patient were most likely due to the fact that the workup was undertaken during an acute attack. The difficulty with establishing the diagnosis was compounded by bilateral involvement, history of trauma, negative joint aspiration, and atypical radiographic findings. A number of reports have described patients with tophus deposits prior to or in the absence of gouty arthritis or a gouty attack.10 Risk factors for this presentation include female sex, the predominant or exclusive involvement of fingers, chronic kidney disease, and treatment with a diuretic or anti-inflammatory drug.10
Conclusion
Our case report illustrates the difficulty in diagnosing an acute gouty attack in a patient with a history of trauma and atypical radiographic findings. The hallux MTPJ is the most common location of acute gouty attacks, but the medial sesamoid as an isolated location is a rare site of presentation. The combination of pain isolated to palpation of the sesamoid and radiographs that showed an aggressive and rapidly expansile lesion of the medial sesamoid raised concerns about a neoplastic lesion. Practitioners should consider acute gout in patients with sesamoid pain and with radiographs showing an expansile sesamoid lesion.
The sesamoid bones are a major contributor to normal gait, with more than 50% of body weight transmitted through the hallux metatarsophalangeal joint (MTPJ) complex. There are varying amounts of stress on the sesamoids, dependent on the gait cycle.1,2 The sesamoids act as a fulcrum to increase the mechanical force of the flexor hallucis brevis tendon.3 Sesamoid pathology can be a source of significant morbidity in patients, especially young athletes or laborers who spend long hours on their feet. More common causes of isolated sesamoid discomfort include sesamoiditis, fracture, and avascular necrosis, with neoplastic, infectious, and inflammatory conditions rarely isolated to the sesamoid.
Gout is a systemic disorder of uric acid metabolism characterized by deposition of monosodium urate crystals in soft tissues and joints.1 This deposition leads to tophus formation with an accompanying inflammatory response. Gout progresses through 3 stages, beginning with acute gout, which may end with chronic, recurrent, and tophaceous gouty arthritis. The hallux MTPJ is the most common joint affected by gout, with few case reports of primary sesamoid gout.1-2,4 We present a case of gout, with radiographic findings isolated to the medial sesamoid, that mimicked a neoplastic process in a patient with no known history of gout. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old laborer presented for evaluation of a right sesamoid injury he sustained 4 months earlier when he fell off a ladder and had acute onset plantar hallux MTPJ pain and swelling. He was treated by an outside physician for a presumptive diagnosis of a medial sesamoid fracture with rest and controlled ankle movement (CAM) boot immobilization that resulted in slowly improving symptoms. In discussion of the patient’s history, he reported that 1 year earlier he had a traumatic event with similar symptoms of MTPJ pain and swelling. At that time, treatment with a CAM boot resulted in complete resolution of pain. His outside physician performed a hematologic workup for gout, which showed a normal uric acid level.
On examination, the patient presented with edema to the right hallux MTPJ and mild tenderness to palpation of the medial sesamoid. He had no pain with motion of the hallux MTPJ or with palpation of the lateral sesamoid. His radiographs showed a bipartite versus fractured sesamoid (Figures 1A, 1B) and serial magnetic resonance imaging (MRI) showed an MTPJ effusion and hyperintense signal in the medial sesamoid, but no erosive findings or soft-tissue masses (Figures 2A, 2B).
The patient was treated with wedge-sandal forefoot offloading, leading to resolution of symptoms over 6 weeks, at which point he was transitioned to normal shoe wear and allowed to progress in his activity as dictated by his symptoms. He presented for reevaluation approximately 2 weeks later with acute, atraumatic onset of plantar left hallux pain and swelling. His examination showed diffuse hallux MTPJ swelling and tenderness isolated to the medial sesamoid. An attempt at aspiration of the MTPJ yielded no fluid, and the patient again was placed in a forefoot-offloading sandal.
Radiographs of the left foot showed an expansile destructive lesion of the medial sesamoid with interval change from his previous imaging approximately 3 months earlier, obtained as part of his contralateral foot evaluation (Figure 3). MRI with and without contrast showed an expansile process isolated to the medial sesamoid with cortical thinning and marrow replacement (Figures 4A-4D).
Because of continued discomfort and lack of a definitive diagnosis, an excisional biopsy of the sesamoid was performed. Intraoperatively, the sesamoid was extensively fragmented with near complete replacement by a chalky tophus, as well as chalky deposition throughout the hallux MTPJ. No significant degenerative changes were observed. Surgical pathology showed bone and fibroconnective tissue with deposits of negative birefringement needle-shaped crystals consistent with monosodium urate deposition and foreign body histocytic reaction, as well as repair reaction of bone (Figures 5A, 5B).
Postoperatively, the patient was again placed in a forefoot-offloading wedge sandal for 6 weeks, followed by progression of activity as dictated by his symptoms. He was also evaluated by a rheumatologist and started on medical treatment for gout, with complete resolution of his bilateral hallux pain. He has been able to return to his previous employment.
Discussion
The sesamoid bones are an important component of the hallux MTPJ complex, giving a mechanical advantage to the flexor hallucis brevis tendons in plantar flexion of the hallux.5 Many pathologic conditions have been well described in the literature, including fracture, sesamoiditis, nonunion, avascular necrosis, and plantar keratosis. There is also a 10% incidence of bipartite sesamoids, most commonly isolated to the medial sesamoid, with up to 25% of patients presenting with bilateral bipartite sesamoids.5 Neoplastic processes of the sesamoid are rare, with a paucity of reports in the literature.6,7 Gout is a condition in which hyperuricemia, due to an imbalance in uric acid production and excretion, leads to deposition of monosodium urate crystals in joints, bones, and soft tissues, causing an inflammatory reaction. Risk factors for gout are male sex, advanced age, and ethnicity, as well as obesity, high protein diet, alcohol use, hypertension, and certain medications. Precipitation of acute attacks has been associated with acute trauma, and the first MTPJ is the most common location for an acute attack.8
Isolated sesamoid lesions are rare, with few isolated case reports in the literature. Benign and malignant lesions appear most often in the metatarsals, with the calcaneus being the second most commonly afflicted site.9 The typical differential diagnosis for isolated lytic bone lesions includes fibrous dysplasia, osteoblastoma, giant cell tumor, metastatic lesion, multiple myeloma, aneurysmal bone cyst, chondroblastoma, brown tumor, infection, eosinophilic granuloma, enchondroma, and bone cyst, with no reports in the literature to our knowledge of these entities presenting in the hallux MTPJ sesamoid. In contrast, gout typically begins with normal radiographic findings, and later leads to erosive, “punched out” lesions on either side of the MTPJ.2
Hyperuricemia is an essential part of the pathophysiology of gout, but not all patients with an acute gouty attack have elevated uric acid levels and, in contrast, may actually have normal or low levels in 12% to 43% of cases.8 The most accurate time frame for assessment of serum uric acid levels is 2 weeks or more after subsidence of an acute event.8 The normal uric acid levels seen in our patient were most likely due to the fact that the workup was undertaken during an acute attack. The difficulty with establishing the diagnosis was compounded by bilateral involvement, history of trauma, negative joint aspiration, and atypical radiographic findings. A number of reports have described patients with tophus deposits prior to or in the absence of gouty arthritis or a gouty attack.10 Risk factors for this presentation include female sex, the predominant or exclusive involvement of fingers, chronic kidney disease, and treatment with a diuretic or anti-inflammatory drug.10
Conclusion
Our case report illustrates the difficulty in diagnosing an acute gouty attack in a patient with a history of trauma and atypical radiographic findings. The hallux MTPJ is the most common location of acute gouty attacks, but the medial sesamoid as an isolated location is a rare site of presentation. The combination of pain isolated to palpation of the sesamoid and radiographs that showed an aggressive and rapidly expansile lesion of the medial sesamoid raised concerns about a neoplastic lesion. Practitioners should consider acute gout in patients with sesamoid pain and with radiographs showing an expansile sesamoid lesion.
1. Mair SD, Coogan AC, Speer KP, Hall RL. Gout as a source of sesamoid pain. Foot Ankle Int. 1995;16(10):613-616.
2. Reber PU, Patel AG, Noesberger B. Gout: rare cause of hallucal sesamoid pain: a case report. Foot Ankle Int. 1997;12(18):818-820.
3. Van Hal ME, Kenne JS, Lange TA, Clancy WG Jr. Stress fractures of the great toe sesamoids. Am J Sports Med. 1982;10(2):122-128.
4. Liu S-Z, Yeh L, Chou Y, Chen CK, Pan HB. Isolated intraosseous gout in hallux sesamoid mimicking a bone tumor in a teenaged patient. Skeletal Radiol. 2003;32(11):647-650.
5. Cohen BE. Hallux sesamoid disorders. Foot Ankle Clin. 2009;14(1):91-104.
6. Harty JA, Kelly P, Niall D, O’Keane JC, Stephens MM. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the sesamoid: a case report. Foot Ankle Int. 2000;21(5):408-412.
7. Noguchi M, Ikoma K, Matsumoto N, Nagasawa K. Bizarre parosteal osteochondromatous proliferation of the sesamoid: an unusual hallux valgus deformity. Foot Ankle Int. 2004;25(7):503-506.
8. Becker MA. Clinical manifestations and diagnosis of gout. Up to Date website. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-gout. Updated June 20, 2015. Accessed August 19, 2015.
9. Bos GD, Esther RJ, Woll TS. Foot tumors: diagnosis and treatment. J Am Acad Orthop Surg. 2002;10(4):259-270.
10. Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. Report of six cases and review of the literature. Arch Intern Med. 1992;152(4):873-876.
1. Mair SD, Coogan AC, Speer KP, Hall RL. Gout as a source of sesamoid pain. Foot Ankle Int. 1995;16(10):613-616.
2. Reber PU, Patel AG, Noesberger B. Gout: rare cause of hallucal sesamoid pain: a case report. Foot Ankle Int. 1997;12(18):818-820.
3. Van Hal ME, Kenne JS, Lange TA, Clancy WG Jr. Stress fractures of the great toe sesamoids. Am J Sports Med. 1982;10(2):122-128.
4. Liu S-Z, Yeh L, Chou Y, Chen CK, Pan HB. Isolated intraosseous gout in hallux sesamoid mimicking a bone tumor in a teenaged patient. Skeletal Radiol. 2003;32(11):647-650.
5. Cohen BE. Hallux sesamoid disorders. Foot Ankle Clin. 2009;14(1):91-104.
6. Harty JA, Kelly P, Niall D, O’Keane JC, Stephens MM. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the sesamoid: a case report. Foot Ankle Int. 2000;21(5):408-412.
7. Noguchi M, Ikoma K, Matsumoto N, Nagasawa K. Bizarre parosteal osteochondromatous proliferation of the sesamoid: an unusual hallux valgus deformity. Foot Ankle Int. 2004;25(7):503-506.
8. Becker MA. Clinical manifestations and diagnosis of gout. Up to Date website. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-gout. Updated June 20, 2015. Accessed August 19, 2015.
9. Bos GD, Esther RJ, Woll TS. Foot tumors: diagnosis and treatment. J Am Acad Orthop Surg. 2002;10(4):259-270.
10. Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. Report of six cases and review of the literature. Arch Intern Med. 1992;152(4):873-876.
Risk Factors for Thromboembolic Events After Surgery for Ankle Fractures
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.