User login
Unusual Form and Location of a Tumor: Multiosseous Ewing Sarcoma in the Foot
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
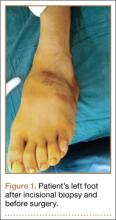
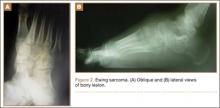
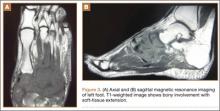
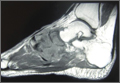
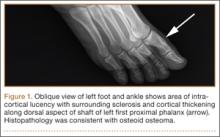
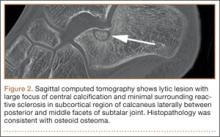
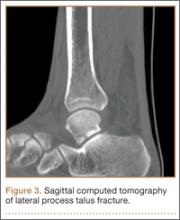
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
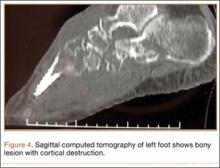
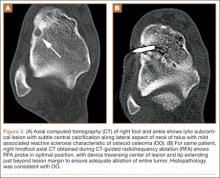
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
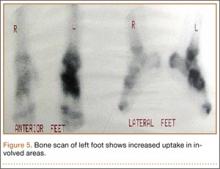
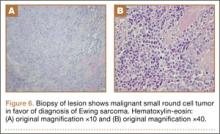
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
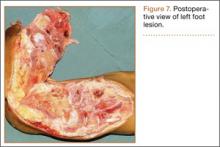
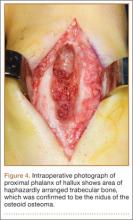
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
Improved Function and Joint Kinematics After Correction of Tibial Malalignment
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
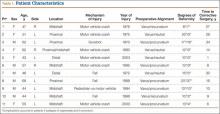
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
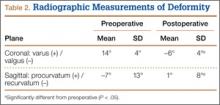
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
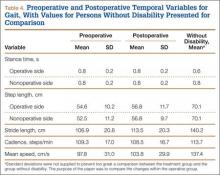
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
Midfoot Sprains in the National Football League
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Osteoid Osteomas of the Foot and Ankle: A Study of Patients Over a 20-Year Period
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Lower Extremity Injuries in Snowboarders
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
Epidemiology
The several studies of lower extremity injuries sustained while skiing and snowboarding have differed markedly with respect to patient demographics. Kim and colleagues1 compared snowboarding and skiing injuries over 18 seasons at a Vermont ski resort and found that the injury rate, assessed as mean number of days between injuries, was 400 for snowboarders and 345 for skiers. However, most snowboarding injuries were wrist injuries and generally of the upper extremity, whereas skiing injuries were mainly lower extremity injuries. Overall, young and inexperienced snowboarders had the highest injury rate. In a study on skiing and snowboarding injuries through 4 Utah seasons, Wasden and colleagues2 found that mean age at injury was 41 years for skiers and 23 years for snowboarders. This corroborates the finding from several studies1-3 that snowboarders tend to be younger. Snowboarding is a newer sport with many beginners. However, Ishimaru and colleagues4 found that lower extremity injuries may be associated with experienced snowboarders, who may be prone to take more risks and tackle more challenging slopes. Experienced snowboarders are also likely to sustain lower extremity injuries from falling, because of their risk-taking behavior.5
Although upper extremity injuries account for most snowboarding injuries, lower extremity injuries are a significant issue.6 Modern equipment and more challenging slopes have allowed snowboarders to attain great speeds going down slopes—leading to a surge in lower extremity injuries.7 Lower extremity injuries sustained during snowboarding are more likely to be on the leading side4; the ankle is the most frequent fracture site. Unlike snowboard equipment, modern ski equipment, including new boots and binding systems, is designed to reduce ankle injuries and lower leg fractures.6 The decline in foot, ankle, and tibia fractures can be attributed to taller and stiffer boots, which offer the lower extremities more protection.8
Mechanism of Injury
Talus Fractures
An increasingly common injury among snowboarders is a fracture of the lateral process of the talus; this injury accounts for 32% of snowboarders’ ankle fractures.6 The lateral process of the talus—wedge-shaped and covered in articular cartilage—is involved in the subtalar and ankle joints.9 A fracture here is often misdiagnosed as an ankle sprain (Figures 1–3).6,9,10 The exact mechanism of injury remains controversial, and several biomechanical factors seem to be involved. Funk and colleagues11 conducted a cadaveric study and concluded that eversion of an axially loaded, dorsiflexed ankle may be the primary injury mechanism for fracture. Furthermore, snowboarders have their feet in a position perpendicular to the board, and a fall parallel to the board could increase the eversion force on the ankle of the leading leg. Valderrabano and colleagues9 conducted a clinical study of 26 patients who sustained this injury from snowboarding. All the patients reported they had felt an axial impact from falling, jumping, or unexpectedly hitting a ground object, and 80% reported a rotational movement in the lower leg during the impact. The authors concluded that axial loading and dorsiflexion were not the only factors involved in lateral process talus fractures, and an external moment is necessary to cause this injury from a forward fall.9
Anterior Cruciate Ligament Injuries
Although snowboarders’ lower extremity injuries are primarily ankle injuries, snowboarders are also at risk for serious knee issues when landing from jumps. In skiers, anterior cruciate ligament (ACL) injuries have 5 well-established mechanisms, all involving separation of the feet and a twisting force in the knee (Figures 4, 5): boot-induced anterior drawer mechanism, phantom-foot mechanism, valgus-external rotation, forceful quadriceps muscle contraction, and a combination of internal rotation and extension.8,12 A valgus–external rotation mechanism of knee injury occurs when external rotation of the tibia results from the skier catching the inside edge of the front of the ski. A valgus force acts on the knee as the lower leg is abducted during forward momentum. The torque created on the knee joint is amplified by the length of the knee and commonly results in an ACL injury or medial collateral ligament injury.6 Reports indicate that the phantom-foot mechanism is the most common mechanism of ACL injury among skiers.6,13,14 In this situation, internal rotation of the knee results when an off-balance skier falls backward, which causes the knee to hyperflex. The skier catches an inside edge on the snow, which creates a torque that rotates the tibia relative to the femur and results in injury to the ACL.6,14 A boot-induced anterior drawer mechanism occurs during a landing, when the tail of the ski lands first and in an off-balance position, resulting in a load transmitted through the skis to the skier; this load causes an anterior drawer of the ski boot and tibia relative to the femur, straining the ACL and causing ACL rupture.6,13,14 In the forceful quadriceps muscle contraction mechanism of ACL injury, a forceful quadriceps contraction occurs after a jump to prevent a backward fall. With the knee in flexion, this quadriceps contraction causes an anterior translation of the tibia, resulting in ACL rupture.13,14
The mechanism of injury differs in snowboarding, in which both feet remain attached to the board. Davies and colleagues15 examined 35 snowboarders who sustained ACL injuries after a flat landing from a jump and concluded that snowboarders preparing for a landing exhibit more quadriceps contraction, which increases the loading force on the ACL during landing. Furthermore, the snowboarder’s stance on the board, with the front foot slightly rotated relative to the board, results in a slight internal tibial rotation of the knee and establishes a posture that makes the snowboarder susceptible to injury. However, the lower incidence of knee injuries among snowboarders compared with skiers may be attributable to the fact that there is a limited amount of torque that can be generated on either knee as both feet are fixed to the board.16
The increased quadriceps force in anticipation of a landing, combined with the internal tibial rotation of the knee caused by the snowboarder’s stance, may be the primary mechanism of ACL rupture in snowboarders.15
Injury Prevention Strategies
Prevention strategies require an identification of injury risk factors for snowboarders. Hasler and colleagues7 conducted a study with 306 patients to identify variables that presented a risk for snowboarders. Low readiness for speed, bad weather, and bad visibility, as well as snow conditions, were found to be significant risk factors.
Skiers’ overall injury rate has decreased over the past 60 years, and this decrease has been attributed in part to improved ski technique and instruction.17,18 Improperly adjusted ski bindings are the culprit in many equipment-related lower extremity injuries, and beginners are at much higher risk for such injuries. Lessons and comprehensive safety training could reduce this injury rate.17,19 Several awareness video and training programs focusing on injury prevention have reduced knee sprains in ski patrollers compared with controls by 62% in 1 study; a similar program reduced injury by 30% in nonprofessional skiers.17 A study of injured snowboarders during a winter in Scotland found that 37% of the patients had no formal instruction or training in correct snowboarding and falling technique.20 Training programs for snowboarders could yield meaningful results in injury prevention and avoidance of risk-taking behavior among snowboarders.
Advances in equipment have also had an impact on the incidence of skiing injuries. Ski bindings protect skiers in 2 ways. First, the binding keeps the boot attached to the ski and prevents unintended release on difficult terrain. Second, the binding releases the boot from the ski during extreme conditions to prevent the skier from experiencing extreme forces or moments that could result in injury. Functional failure in ski bindings has been implicated in increased incidence of knee injuries and ligament rupture. In a study of injuries sustained by recreational alpine skiers in Japan, Urabe and colleagues21 found that 96% of those injured stated that the ski bindings had not released at time of incident. The effects of binding adjustment and maintenance among snowboarders have not been fully investigated, and there are no set guidelines for individual snowboarders on appropriate binding level. However, as there is a range of binding adjustment options available, snowboarders may have an optimum level that maximizes both mobility and protection from injury.22
Soft-shelled boots may also increase injury risk for snowboarders. Such boots allow for a wider range of ankle motion and offer little protection from extreme joint movements. Soft boots are generally preferred among snowboarders because they allow for increased mobility for sharp turns and maneuvers. However, modification of the stiffness of boots that limit ankle and foot joint mobility could reduce the incidence of ankle fractures and sprains among snowboarders.22
Summary
Snowboarding has become increasingly popular worldwide. It attracts a loyal group of amateur athletes and has developed into a billion-dollar industry with a growing rank of professionals. Although most snowboarding injuries are upper extremity injuries, the foot, ankle, and knee represent commonly injured areas among recreational and experienced snowboarders. Advances in ski equipment have significantly reduced the incidence of ankle injuries, but rising knee ligament injuries continue to pose a challenge. Foot and ankle injuries remain an issue in snowboarders despite advances in equipment and safety. New snowboard designs and boot and binding modifications may hold promise in decreasing the risk for injury in these athletes.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
1. Kim S, Endres NK, Johnson RJ, Ettlinger CF, Shealy JE. Snowboarding injuries: trends over time and comparisons with alpine skiing injuries. Am J Sports Med. 2012;40(4):770-776.
2. Wasden CC, McIntosh SE, Keith DS, McCowan C. An analysis of skiing and snowboarding injuries on Utah slopes. J Trauma. 2009;67(5):1022-1026.
3. Rust DA, Gilmore CJ, Treme G. Injury patterns at a large western United States ski resort with and without snowboarders: the Taos experience. Am J Sports Med. 2013;41(3):652-656.
4. Ishimaru D, Ogawa H, Sumi H, Sumi Y, Shimizu K. Lower extremity injuries in snowboarding. J Trauma. 2011;70(3):E48-E52.
5. Torjussen J, Bahr R. Injuries among competitive snowboarders at the national elite level. Am J Sports Med. 2005;33(3):370-377.
6. Deady LH, Salonen D. Skiing and snowboarding injuries: a review with a focus on mechanism of injury. Radiol Clin North Am. 2010;48(6):1113-1124.
7. Hasler RM, Berov S, Banneker L, et al. Are there risk factors for snowboard injuries? A case–control multicentre study of 559 snowboarders. Br J Sports Med. 2010;44(11):816-821.
8. St-Onge N, Chevalier Y, Hagemeister N, Van De Putte M, De Guise J. Effect of ski binding parameters on knee biomechanics: a three-dimensional computational study. Med Sci Sports Exerc. 2004;36(7):1218-1225.
9. Valderrabano V, Perren T, Ryf C, Rillmann P, Hintermann B. Snowboarder’s talus fracture: treatment outcome of 20 cases after 3.5 years. Am J Sports Med. 2005;33(6):871-880.
10. von Knoch F, Reckord U, von Knoch M, Sommer C. Fracture of the lateral process of the talus in snowboarders. J Bone Joint Surg Br. 2007;89(6):772-777.
11. Funk JR, Srinivasan SC, Crandall JR. Snowboarder’s talus fractures experimentally produced by eversion and dorsiflexion. Am J Sports Med. 2003;31(6):921-928.
12. Pujol N, Blanchi MP, Chambat P. The incidence of anterior cruciate ligament injuries among competitive alpine skiers: a 25-year investigation. Am J Sports Med. 2007;35(7):1070-1074.
13. Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537-540.
14. Bere T, Flørenes TW, Krosshaug T, Nordsletten L, Bahr R. Events leading to anterior cruciate ligament injury in World Cup alpine skiing: a systematic video analysis of 20 cases. Br J Sports Med. 2011;45(16):1294-1302.
15. Davies H, Tietjens B, Van Sterkenburg M, Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
16. Bladin C, McCrory P, Pogorzelski A. Snowboarding injuries: current trends and future directions. Sports Med. 2004;34(2):133-139.
17. Rossi MJ, Lubowitz JH, Guttmann D. The skier’s knee. Arthroscopy. 2003;19(1):75-84.
18. Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194-202.
19. Hildebrandt C, Mildner E, Hotter B, Kirschner W, Höbenreich C, Raschner C. Accident prevention on ski slopes—perceptions of safety and knowledge of existing rules. Accid Anal Prev. 2011;43(4):1421-1426.
20. Langran M, Selvaraj S. Increased injury risk among first-day skiers, snowboarders, and skiboarders. Am J Sports Med. 2004;32(1):96-103.
21. Urabe Y, Ochi M, Onari K, Ikuta Y. Anterior cruciate ligament injury in recreational alpine skiers: analysis of mechanisms and strategy for prevention. J Orthop Sci. 2002;7(1):1-5.
22. McAlpine PR. Biomechanical Analysis of Snowboard Jump Landings: A Focus on the Ankle Joint Complex [doctoral thesis]. Auckland, New Zealand: University of Auckland; 2010.
Acute Achilles Tendon Ruptures: A Comparison of Minimally Invasive and Open Approach Repairs Followed by Early Rehabilitation
Advancing Orthopedic Postsurgical Pain Management & Multimodal Care Pathways: Improving Clinical & Economic Outcomes
Commentary to "5 Points on Total Ankle Arthroplasty"
There are considerable differences in the design and implantation technique of the current total ankle implants available in the United States, eg, mobile vs. fixed bearing, intramedullary vs. extramedullary guidance, anterior vs. lateral surgical approach, flat vs. curved bone cuts, natural articular design with minimal bone resection (Zimmer Trabecular Metal Total Ankle; Zimmer, Warsaw, Indiana) vs. larger implant construct with more bone resection (Inbone II; Figure 2). There is no evidence that one implant design is superior, and, as the authors conclude, “Direct comparisons between TAA [total ankle arthroplasty] implant systems are needed to determine what clinical benefits are achieved with each design and what contributes to these differences.”
Hsu AR, Anderson RB, Cohen BE. Total Ankle Arthroplasty. Am J Orthop. 2014;43(10):451-457.
There are considerable differences in the design and implantation technique of the current total ankle implants available in the United States, eg, mobile vs. fixed bearing, intramedullary vs. extramedullary guidance, anterior vs. lateral surgical approach, flat vs. curved bone cuts, natural articular design with minimal bone resection (Zimmer Trabecular Metal Total Ankle; Zimmer, Warsaw, Indiana) vs. larger implant construct with more bone resection (Inbone II; Figure 2). There is no evidence that one implant design is superior, and, as the authors conclude, “Direct comparisons between TAA [total ankle arthroplasty] implant systems are needed to determine what clinical benefits are achieved with each design and what contributes to these differences.”
Hsu AR, Anderson RB, Cohen BE. Total Ankle Arthroplasty. Am J Orthop. 2014;43(10):451-457.
There are considerable differences in the design and implantation technique of the current total ankle implants available in the United States, eg, mobile vs. fixed bearing, intramedullary vs. extramedullary guidance, anterior vs. lateral surgical approach, flat vs. curved bone cuts, natural articular design with minimal bone resection (Zimmer Trabecular Metal Total Ankle; Zimmer, Warsaw, Indiana) vs. larger implant construct with more bone resection (Inbone II; Figure 2). There is no evidence that one implant design is superior, and, as the authors conclude, “Direct comparisons between TAA [total ankle arthroplasty] implant systems are needed to determine what clinical benefits are achieved with each design and what contributes to these differences.”
Hsu AR, Anderson RB, Cohen BE. Total Ankle Arthroplasty. Am J Orthop. 2014;43(10):451-457.