User login
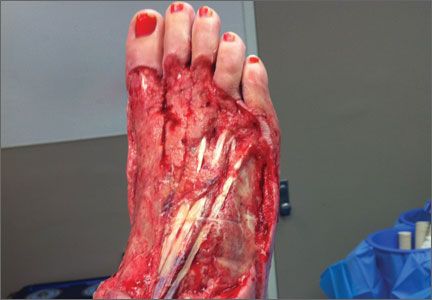
Necrotizing Fasciitis of Lower Extremity Caused by Haemophilus influenzae in a Healthy Adult With a Closed Lisfranc Injury
Failed First Metatarsophalangeal Arthroplasty Salvaged by Hamstring Interposition Arthroplasty: Metallic Debris From Grommets
joint, arthroplasty, implant, metallic debris, grommets, titanium, synovitis, foot
joint, arthroplasty, implant, metallic debris, grommets, titanium, synovitis, foot
joint, arthroplasty, implant, metallic debris, grommets, titanium, synovitis, foot
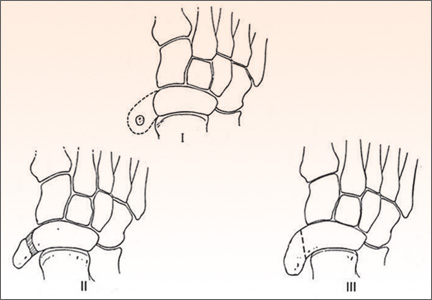
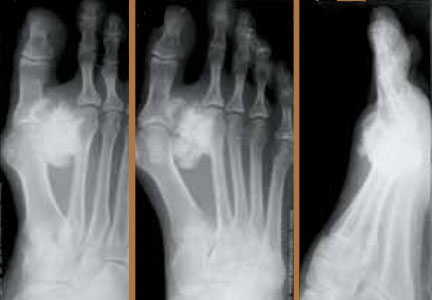
Surgical Treatment of Symptomatic Accessory Navicular in Children and Adolescents
The Orthopedic Stepchild
Throughout antiquity, physicians and surgeons have concerned themselves with maladies of the foot and ankle. The literature is rife with articles describing management of clubfoot deformities and traumatic amputations of feet and legs. Authors have described tenotomies and manipulation for clubfeet as well as optimal techniques and levels for amputations to promote healing and functional outcomes. In progressive and aggressive surgical centers in Austria and Germany, techniques for correction of the deformities created by disease and trauma formed the basis for today’s reconstructive methodologies.
During my orthopedic residency in the 1960s, we managed pediatric versions of clubfoot, vertical talus, and neuromuscular conditions of the lower extremity (myelomeningocele, muscular dystrophy, cerebral palsy); adolescent bunions and pathologic flat feet; and, in adults, residual polio, arthritis, bunions, lesser toe deformities, ankle disorders, and trauma. Then along came the excitement of total joint arthroplasty, with its spectacular results, and the thrill in devoting careers to athletes and their myriad problems. Other interesting subspecialties emerged, and the orthopedic focus on a significant part of our heritage, the issues of foot and ankle, was lost for decades. Care for these problems was left to a small cadre of pediatric doctors, and soon-to-retire orthopedists who tended to view the field as
less demanding. Dynamic young practitioners showed little or no interest in caring for foot and ankle patients, and no progress was made in clinical care, research, and development of orthopedic technology and devices.
In the late 1960s, a small group of middle-aged and senior devotees of the specialty met in New York to form the American Orthopaedic Foot Society (AOFS), later to become the American Orthopaedic Foot and Ankle Society (AOFAS). The group’s goal was to renew interest in the foot and ankle specialty among orthopedic surgeons. As everyone knows, AOFAS has flourished and become one of the most progressive, innovative, and dynamic of all the orthopedic subspecialty
groups. In 1985, John Gartland, president of the American Academy of Orthopaedic Surgeons, called together the leaders in the foot and ankle field to formulate a long-range plan to reclaim foot and ankle from the morass of substandard care
and to advance the subspecialty in every quarter. As AOFS (and later AOFAS) president, I was part of Gartland’s team. I recall we aimed to convince orthopedic chairs, the Residency Review Committee for Orthopaedic Surgery, and the Board
of Orthopaedic Surgery to increase training requirements, to develop foot and ankle educators, and to promote the area in training programs. Orthopedic educators developed fellowships (essentially nonexistent up until then) and organized and taught beginner and advanced continuing education courses at annual meetings and throughout the year.
There were other needs to be addressed. One was to educate nonorthopedic doctors to appreciate that foot and ankle problems had good nonoperative and surgical solutions, that there was an orthopedic subspecialty for these conditions, and that foot and ankle patients should be referred to its practitioners. Second, these patients’ public advocacy groups needed to know what knowledgeable orthopedists could provide and needed to be encouraged to seek care from these physicians rather than from less qualified providers and nonspecialists.
To an extraordinary degree, the goal of educating orthopedists has been achieved, and the field is now populated with young, energetic leaders, teachers, and practitioners. We have been less successful in educating potential referring physicians, the public, public advocacy groups, and third-party payers, including the US government. Progress has been made with private insurers and, as advisors, with the Centers for Medicare and Medicaid Services and state government health committees.
Driven by emerging market opportunities, the orthopedic device industry has made unanticipated and enormous advances in the distal lower extremity realm. Small
companies have been founded, and larger companies have dedicated entire divisions to making fixation devices and prosthetic implants for every procedure involving the foot or the ankle. Biomedical engineers, metallurgists, orthopedic
consultant researchers, and well-funded projects have led the surge to develop the best foot and ankle technology. In addition, industry courses and scholarships for residents, fellows, teaching programs, and young physicians have been generating interest in these advances. Although it may be argued that entrepreneurship brings enormous bias, it must be conceded that interest in the foot and ankle field has increased tremendously. Outreach programs for foot and ankle care in the Third World have emerged as an additional humanitarian benefit of the expansion of the field.
From its strong start as a medical specialty to its fall into ignorance and neglect, the foot and ankle field, the unwanted stepchild of medicine and orthopedics, has made a dramatic recovery and has become a premier example of what medicine can achieve through focused effort. As leaders in orthopedic medicine in North America, we must also acknowledge the huge contributions made by a sterling array of international researchers, educators, and practitioners.
Some journals in the United States and other countries now concentrate solely on foot and ankle. Nevertheless, it is appropriate that The American Journal of Orthopedics and other general orthopedic surgery publications focus on foot and
ankle (and other specialties) in an annual issue. As each orthopedist tends mainly to his or her own area of interest, it is essential that we all stay current on the field as a whole. The basic science, innovations, and concepts of one specialty are often applicable to the entire field, and a casual notation of an idea from such a focused issue may have unimagined benefits for the readership and their patients. ◾
Throughout antiquity, physicians and surgeons have concerned themselves with maladies of the foot and ankle. The literature is rife with articles describing management of clubfoot deformities and traumatic amputations of feet and legs. Authors have described tenotomies and manipulation for clubfeet as well as optimal techniques and levels for amputations to promote healing and functional outcomes. In progressive and aggressive surgical centers in Austria and Germany, techniques for correction of the deformities created by disease and trauma formed the basis for today’s reconstructive methodologies.
During my orthopedic residency in the 1960s, we managed pediatric versions of clubfoot, vertical talus, and neuromuscular conditions of the lower extremity (myelomeningocele, muscular dystrophy, cerebral palsy); adolescent bunions and pathologic flat feet; and, in adults, residual polio, arthritis, bunions, lesser toe deformities, ankle disorders, and trauma. Then along came the excitement of total joint arthroplasty, with its spectacular results, and the thrill in devoting careers to athletes and their myriad problems. Other interesting subspecialties emerged, and the orthopedic focus on a significant part of our heritage, the issues of foot and ankle, was lost for decades. Care for these problems was left to a small cadre of pediatric doctors, and soon-to-retire orthopedists who tended to view the field as
less demanding. Dynamic young practitioners showed little or no interest in caring for foot and ankle patients, and no progress was made in clinical care, research, and development of orthopedic technology and devices.
In the late 1960s, a small group of middle-aged and senior devotees of the specialty met in New York to form the American Orthopaedic Foot Society (AOFS), later to become the American Orthopaedic Foot and Ankle Society (AOFAS). The group’s goal was to renew interest in the foot and ankle specialty among orthopedic surgeons. As everyone knows, AOFAS has flourished and become one of the most progressive, innovative, and dynamic of all the orthopedic subspecialty
groups. In 1985, John Gartland, president of the American Academy of Orthopaedic Surgeons, called together the leaders in the foot and ankle field to formulate a long-range plan to reclaim foot and ankle from the morass of substandard care
and to advance the subspecialty in every quarter. As AOFS (and later AOFAS) president, I was part of Gartland’s team. I recall we aimed to convince orthopedic chairs, the Residency Review Committee for Orthopaedic Surgery, and the Board
of Orthopaedic Surgery to increase training requirements, to develop foot and ankle educators, and to promote the area in training programs. Orthopedic educators developed fellowships (essentially nonexistent up until then) and organized and taught beginner and advanced continuing education courses at annual meetings and throughout the year.
There were other needs to be addressed. One was to educate nonorthopedic doctors to appreciate that foot and ankle problems had good nonoperative and surgical solutions, that there was an orthopedic subspecialty for these conditions, and that foot and ankle patients should be referred to its practitioners. Second, these patients’ public advocacy groups needed to know what knowledgeable orthopedists could provide and needed to be encouraged to seek care from these physicians rather than from less qualified providers and nonspecialists.
To an extraordinary degree, the goal of educating orthopedists has been achieved, and the field is now populated with young, energetic leaders, teachers, and practitioners. We have been less successful in educating potential referring physicians, the public, public advocacy groups, and third-party payers, including the US government. Progress has been made with private insurers and, as advisors, with the Centers for Medicare and Medicaid Services and state government health committees.
Driven by emerging market opportunities, the orthopedic device industry has made unanticipated and enormous advances in the distal lower extremity realm. Small
companies have been founded, and larger companies have dedicated entire divisions to making fixation devices and prosthetic implants for every procedure involving the foot or the ankle. Biomedical engineers, metallurgists, orthopedic
consultant researchers, and well-funded projects have led the surge to develop the best foot and ankle technology. In addition, industry courses and scholarships for residents, fellows, teaching programs, and young physicians have been generating interest in these advances. Although it may be argued that entrepreneurship brings enormous bias, it must be conceded that interest in the foot and ankle field has increased tremendously. Outreach programs for foot and ankle care in the Third World have emerged as an additional humanitarian benefit of the expansion of the field.
From its strong start as a medical specialty to its fall into ignorance and neglect, the foot and ankle field, the unwanted stepchild of medicine and orthopedics, has made a dramatic recovery and has become a premier example of what medicine can achieve through focused effort. As leaders in orthopedic medicine in North America, we must also acknowledge the huge contributions made by a sterling array of international researchers, educators, and practitioners.
Some journals in the United States and other countries now concentrate solely on foot and ankle. Nevertheless, it is appropriate that The American Journal of Orthopedics and other general orthopedic surgery publications focus on foot and
ankle (and other specialties) in an annual issue. As each orthopedist tends mainly to his or her own area of interest, it is essential that we all stay current on the field as a whole. The basic science, innovations, and concepts of one specialty are often applicable to the entire field, and a casual notation of an idea from such a focused issue may have unimagined benefits for the readership and their patients. ◾
Throughout antiquity, physicians and surgeons have concerned themselves with maladies of the foot and ankle. The literature is rife with articles describing management of clubfoot deformities and traumatic amputations of feet and legs. Authors have described tenotomies and manipulation for clubfeet as well as optimal techniques and levels for amputations to promote healing and functional outcomes. In progressive and aggressive surgical centers in Austria and Germany, techniques for correction of the deformities created by disease and trauma formed the basis for today’s reconstructive methodologies.
During my orthopedic residency in the 1960s, we managed pediatric versions of clubfoot, vertical talus, and neuromuscular conditions of the lower extremity (myelomeningocele, muscular dystrophy, cerebral palsy); adolescent bunions and pathologic flat feet; and, in adults, residual polio, arthritis, bunions, lesser toe deformities, ankle disorders, and trauma. Then along came the excitement of total joint arthroplasty, with its spectacular results, and the thrill in devoting careers to athletes and their myriad problems. Other interesting subspecialties emerged, and the orthopedic focus on a significant part of our heritage, the issues of foot and ankle, was lost for decades. Care for these problems was left to a small cadre of pediatric doctors, and soon-to-retire orthopedists who tended to view the field as
less demanding. Dynamic young practitioners showed little or no interest in caring for foot and ankle patients, and no progress was made in clinical care, research, and development of orthopedic technology and devices.
In the late 1960s, a small group of middle-aged and senior devotees of the specialty met in New York to form the American Orthopaedic Foot Society (AOFS), later to become the American Orthopaedic Foot and Ankle Society (AOFAS). The group’s goal was to renew interest in the foot and ankle specialty among orthopedic surgeons. As everyone knows, AOFAS has flourished and become one of the most progressive, innovative, and dynamic of all the orthopedic subspecialty
groups. In 1985, John Gartland, president of the American Academy of Orthopaedic Surgeons, called together the leaders in the foot and ankle field to formulate a long-range plan to reclaim foot and ankle from the morass of substandard care
and to advance the subspecialty in every quarter. As AOFS (and later AOFAS) president, I was part of Gartland’s team. I recall we aimed to convince orthopedic chairs, the Residency Review Committee for Orthopaedic Surgery, and the Board
of Orthopaedic Surgery to increase training requirements, to develop foot and ankle educators, and to promote the area in training programs. Orthopedic educators developed fellowships (essentially nonexistent up until then) and organized and taught beginner and advanced continuing education courses at annual meetings and throughout the year.
There were other needs to be addressed. One was to educate nonorthopedic doctors to appreciate that foot and ankle problems had good nonoperative and surgical solutions, that there was an orthopedic subspecialty for these conditions, and that foot and ankle patients should be referred to its practitioners. Second, these patients’ public advocacy groups needed to know what knowledgeable orthopedists could provide and needed to be encouraged to seek care from these physicians rather than from less qualified providers and nonspecialists.
To an extraordinary degree, the goal of educating orthopedists has been achieved, and the field is now populated with young, energetic leaders, teachers, and practitioners. We have been less successful in educating potential referring physicians, the public, public advocacy groups, and third-party payers, including the US government. Progress has been made with private insurers and, as advisors, with the Centers for Medicare and Medicaid Services and state government health committees.
Driven by emerging market opportunities, the orthopedic device industry has made unanticipated and enormous advances in the distal lower extremity realm. Small
companies have been founded, and larger companies have dedicated entire divisions to making fixation devices and prosthetic implants for every procedure involving the foot or the ankle. Biomedical engineers, metallurgists, orthopedic
consultant researchers, and well-funded projects have led the surge to develop the best foot and ankle technology. In addition, industry courses and scholarships for residents, fellows, teaching programs, and young physicians have been generating interest in these advances. Although it may be argued that entrepreneurship brings enormous bias, it must be conceded that interest in the foot and ankle field has increased tremendously. Outreach programs for foot and ankle care in the Third World have emerged as an additional humanitarian benefit of the expansion of the field.
From its strong start as a medical specialty to its fall into ignorance and neglect, the foot and ankle field, the unwanted stepchild of medicine and orthopedics, has made a dramatic recovery and has become a premier example of what medicine can achieve through focused effort. As leaders in orthopedic medicine in North America, we must also acknowledge the huge contributions made by a sterling array of international researchers, educators, and practitioners.
Some journals in the United States and other countries now concentrate solely on foot and ankle. Nevertheless, it is appropriate that The American Journal of Orthopedics and other general orthopedic surgery publications focus on foot and
ankle (and other specialties) in an annual issue. As each orthopedist tends mainly to his or her own area of interest, it is essential that we all stay current on the field as a whole. The basic science, innovations, and concepts of one specialty are often applicable to the entire field, and a casual notation of an idea from such a focused issue may have unimagined benefits for the readership and their patients. ◾
Parosteal Osteosarcoma of the 2nd Metatarsal
Antirheumatic drugs don’t boost surgical infection risk
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
AT THE ACR ANNUAL MEETING
Major finding: Rheumatoid arthritis patients who remained on their antirheumatic medication while they underwent various types of surgery did not have a significantly different 30-day wound infection rate than those who stopped treatment temporarily prior to surgery.
Data source: This was a retrospective observational cohort study involving 6,548 rheumatoid arthritis patients undergoing various types of surgery.
Disclosures: The study was funded by the Department of Veterans Affairs. The presenters reported having no financial conflicts.
Irreducible Longitudinal Distraction-Dislocation of the Hallux Interphalangeal Joint
FDA approves denosumab for giant cell tumors
Denosumab is approved for the treatment of giant cell tumors of the bone, the Food and Drug Administration announced June 13.
Rare and usually noncancerous, giant cell tumors of the bone generally affect 20- to 40-year-olds. Most of these tumors destroy growing bone, causing pain, limited range of motion, and bone fractures. Rarely, the tumors become cancerous and spread to the lungs.
Denosumab (Xgeva) is indicated for use in patients who are not candidates for surgical resection of their tumors or when surgery is likely to result in severe morbidity, such as loss of limbs or joint removal. It should be used only in adolescents whose bones have matured, the FDA said in a statement.
"Today’s approval of Xgeva provides a needed treatment option for patients with GCTB who are not surgical candidates or who would otherwise have to undergo extensive, life-altering surgery," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA reviewed denosumab under its priority review program as the drug was granted orphan product designation because GCTB is a rare disease.
The safety and effectiveness of denosumab for GCTB were established in two clinical trials that enrolled 305 adult or adolescent patients with confirmed GCTB that was recurrent, unresectable, or would be associated with severe morbidity if surgically managed.
After an average of 3 months, tumors reduced in size among 47 of 187 patients whose tumors could be measured. Over an average follow-up of 20 months, GCTBs regrew in three patients whose tumors originally became smaller during treatment.
Common side effects of denosumab included joint pain, headache, nausea, fatigue, back pain, and extremity pain. The most common serious side effects were osteonecrosis of the jaw and osteomyelitis. Women of reproductive potential should use highly effective contraception while taking denosumab because of potential fetal harm, according to the FDA.
Denosumab was approved in 2010 to prevent fractures when cancer has spread to the bones. It is marketed by Amgen.
On Twitter @maryjodales
Denosumab is approved for the treatment of giant cell tumors of the bone, the Food and Drug Administration announced June 13.
Rare and usually noncancerous, giant cell tumors of the bone generally affect 20- to 40-year-olds. Most of these tumors destroy growing bone, causing pain, limited range of motion, and bone fractures. Rarely, the tumors become cancerous and spread to the lungs.
Denosumab (Xgeva) is indicated for use in patients who are not candidates for surgical resection of their tumors or when surgery is likely to result in severe morbidity, such as loss of limbs or joint removal. It should be used only in adolescents whose bones have matured, the FDA said in a statement.
"Today’s approval of Xgeva provides a needed treatment option for patients with GCTB who are not surgical candidates or who would otherwise have to undergo extensive, life-altering surgery," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA reviewed denosumab under its priority review program as the drug was granted orphan product designation because GCTB is a rare disease.
The safety and effectiveness of denosumab for GCTB were established in two clinical trials that enrolled 305 adult or adolescent patients with confirmed GCTB that was recurrent, unresectable, or would be associated with severe morbidity if surgically managed.
After an average of 3 months, tumors reduced in size among 47 of 187 patients whose tumors could be measured. Over an average follow-up of 20 months, GCTBs regrew in three patients whose tumors originally became smaller during treatment.
Common side effects of denosumab included joint pain, headache, nausea, fatigue, back pain, and extremity pain. The most common serious side effects were osteonecrosis of the jaw and osteomyelitis. Women of reproductive potential should use highly effective contraception while taking denosumab because of potential fetal harm, according to the FDA.
Denosumab was approved in 2010 to prevent fractures when cancer has spread to the bones. It is marketed by Amgen.
On Twitter @maryjodales
Denosumab is approved for the treatment of giant cell tumors of the bone, the Food and Drug Administration announced June 13.
Rare and usually noncancerous, giant cell tumors of the bone generally affect 20- to 40-year-olds. Most of these tumors destroy growing bone, causing pain, limited range of motion, and bone fractures. Rarely, the tumors become cancerous and spread to the lungs.
Denosumab (Xgeva) is indicated for use in patients who are not candidates for surgical resection of their tumors or when surgery is likely to result in severe morbidity, such as loss of limbs or joint removal. It should be used only in adolescents whose bones have matured, the FDA said in a statement.
"Today’s approval of Xgeva provides a needed treatment option for patients with GCTB who are not surgical candidates or who would otherwise have to undergo extensive, life-altering surgery," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA reviewed denosumab under its priority review program as the drug was granted orphan product designation because GCTB is a rare disease.
The safety and effectiveness of denosumab for GCTB were established in two clinical trials that enrolled 305 adult or adolescent patients with confirmed GCTB that was recurrent, unresectable, or would be associated with severe morbidity if surgically managed.
After an average of 3 months, tumors reduced in size among 47 of 187 patients whose tumors could be measured. Over an average follow-up of 20 months, GCTBs regrew in three patients whose tumors originally became smaller during treatment.
Common side effects of denosumab included joint pain, headache, nausea, fatigue, back pain, and extremity pain. The most common serious side effects were osteonecrosis of the jaw and osteomyelitis. Women of reproductive potential should use highly effective contraception while taking denosumab because of potential fetal harm, according to the FDA.
Denosumab was approved in 2010 to prevent fractures when cancer has spread to the bones. It is marketed by Amgen.
On Twitter @maryjodales