User login
FDA chief calls for release of all data tracking problems with medical devices
Food and Drug Administration Commissioner Scott Gottlieb, MD, announced in a tweet Wednesday that the agency plans to
“We’re now prioritizing making ALL of this data available,” Dr. Gottlieb tweeted.
A recent Kaiser Health News investigation revealed the scope of a hidden reporting pathway for device makers, with the agency accepting more than 1.1 million such reports since the start of 2016.
Device makers for nearly 20 years were able to quietly seek an “exemption” from standard, public harm-reporting rules. Devices with such exemptions have included surgical staplers and balloon pumps used in the vessels of heart-surgery patients.
Dr. Gottlieb’s tweet also referenced the challenge in opening the database, saying it “wasn’t easily accessible electronically owing to the system’s age. But it’s imperative that all safety information be available to the public.”
The agency made changes to the “alternative summary reporting” program in mid-2017 to require a public report summarizing data filed within the FDA. But nearly two decades of data remained cordoned off from doctors, patients, and device-safety researchers who say they could use it to detect problems.
Dr. Gottlieb’s announcement was welcomed by Madris Tomes, who has testified to FDA device-review panels about the importance of making summary data on patient harm open to the public.
“That’s the best news I’ve heard in years,” said Ms. Tomes, president of Device Events, which makes the FDA device-harm data more user-friendly. “I’m really happy that they’re taking notice and realizing that physicians who couldn’t see this data before were using devices that they wouldn’t have used if they had this data in front of them.”
Since September, KHN has filed Freedom of Information Act requests for parts or all of the “alternative summary reporting” database and for other special “exemption” reports, to little effect. A request to expedite delivery of those records was denied, and the FDA cited the lack of “compelling need” for the public to have the information. Officials noted that it might take up to 2 years to get such records through the FOIA process.
As recently as March 22, though, the agency began publishing previously undisclosed reports of harm, suddenly updating the numbers of breast implant malfunctions or injuries submitted over the years. The new data was presented to an FDA advisory panel, which is reviewing the safety of such devices. The panel, which met March 25 and 26, saw a chart showing hundreds of thousands more accounts of harm or malfunctions than had previously been acknowledged.
Michael Carome, MD, director of Public Citizen’s health research group, said his initial reaction to the news is “better late than never.”
“If [Dr. Gottlieb] follows through with his pledge to make all this data public, then that’s certainly a positive development,” he said. “But this is safety information that should have been made available years ago.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Food and Drug Administration Commissioner Scott Gottlieb, MD, announced in a tweet Wednesday that the agency plans to
“We’re now prioritizing making ALL of this data available,” Dr. Gottlieb tweeted.
A recent Kaiser Health News investigation revealed the scope of a hidden reporting pathway for device makers, with the agency accepting more than 1.1 million such reports since the start of 2016.
Device makers for nearly 20 years were able to quietly seek an “exemption” from standard, public harm-reporting rules. Devices with such exemptions have included surgical staplers and balloon pumps used in the vessels of heart-surgery patients.
Dr. Gottlieb’s tweet also referenced the challenge in opening the database, saying it “wasn’t easily accessible electronically owing to the system’s age. But it’s imperative that all safety information be available to the public.”
The agency made changes to the “alternative summary reporting” program in mid-2017 to require a public report summarizing data filed within the FDA. But nearly two decades of data remained cordoned off from doctors, patients, and device-safety researchers who say they could use it to detect problems.
Dr. Gottlieb’s announcement was welcomed by Madris Tomes, who has testified to FDA device-review panels about the importance of making summary data on patient harm open to the public.
“That’s the best news I’ve heard in years,” said Ms. Tomes, president of Device Events, which makes the FDA device-harm data more user-friendly. “I’m really happy that they’re taking notice and realizing that physicians who couldn’t see this data before were using devices that they wouldn’t have used if they had this data in front of them.”
Since September, KHN has filed Freedom of Information Act requests for parts or all of the “alternative summary reporting” database and for other special “exemption” reports, to little effect. A request to expedite delivery of those records was denied, and the FDA cited the lack of “compelling need” for the public to have the information. Officials noted that it might take up to 2 years to get such records through the FOIA process.
As recently as March 22, though, the agency began publishing previously undisclosed reports of harm, suddenly updating the numbers of breast implant malfunctions or injuries submitted over the years. The new data was presented to an FDA advisory panel, which is reviewing the safety of such devices. The panel, which met March 25 and 26, saw a chart showing hundreds of thousands more accounts of harm or malfunctions than had previously been acknowledged.
Michael Carome, MD, director of Public Citizen’s health research group, said his initial reaction to the news is “better late than never.”
“If [Dr. Gottlieb] follows through with his pledge to make all this data public, then that’s certainly a positive development,” he said. “But this is safety information that should have been made available years ago.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Food and Drug Administration Commissioner Scott Gottlieb, MD, announced in a tweet Wednesday that the agency plans to
“We’re now prioritizing making ALL of this data available,” Dr. Gottlieb tweeted.
A recent Kaiser Health News investigation revealed the scope of a hidden reporting pathway for device makers, with the agency accepting more than 1.1 million such reports since the start of 2016.
Device makers for nearly 20 years were able to quietly seek an “exemption” from standard, public harm-reporting rules. Devices with such exemptions have included surgical staplers and balloon pumps used in the vessels of heart-surgery patients.
Dr. Gottlieb’s tweet also referenced the challenge in opening the database, saying it “wasn’t easily accessible electronically owing to the system’s age. But it’s imperative that all safety information be available to the public.”
The agency made changes to the “alternative summary reporting” program in mid-2017 to require a public report summarizing data filed within the FDA. But nearly two decades of data remained cordoned off from doctors, patients, and device-safety researchers who say they could use it to detect problems.
Dr. Gottlieb’s announcement was welcomed by Madris Tomes, who has testified to FDA device-review panels about the importance of making summary data on patient harm open to the public.
“That’s the best news I’ve heard in years,” said Ms. Tomes, president of Device Events, which makes the FDA device-harm data more user-friendly. “I’m really happy that they’re taking notice and realizing that physicians who couldn’t see this data before were using devices that they wouldn’t have used if they had this data in front of them.”
Since September, KHN has filed Freedom of Information Act requests for parts or all of the “alternative summary reporting” database and for other special “exemption” reports, to little effect. A request to expedite delivery of those records was denied, and the FDA cited the lack of “compelling need” for the public to have the information. Officials noted that it might take up to 2 years to get such records through the FOIA process.
As recently as March 22, though, the agency began publishing previously undisclosed reports of harm, suddenly updating the numbers of breast implant malfunctions or injuries submitted over the years. The new data was presented to an FDA advisory panel, which is reviewing the safety of such devices. The panel, which met March 25 and 26, saw a chart showing hundreds of thousands more accounts of harm or malfunctions than had previously been acknowledged.
Michael Carome, MD, director of Public Citizen’s health research group, said his initial reaction to the news is “better late than never.”
“If [Dr. Gottlieb] follows through with his pledge to make all this data public, then that’s certainly a positive development,” he said. “But this is safety information that should have been made available years ago.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
VA Community Living Centers Health Care Reports Are Now Public
Although VA nursing homes, on the whole, have sicker patients than do those in private sector nursing homes, they compare closely in terms of quality of care—and in some cases, VA health care gets higher marks. VA has more higher performing facilities (17% vs 11%) and fewer low-performing facilities (17% vs 20%).
Those figures come from the health care inspection reports and staffing data for its 134 community living centers (CLCs) that the VA is, for the first time, posting publicly. So far, VA has posted 101 health inspection reports; the remainder are scheduled for later this year. The reports cover April 2018 to the present.
The VA reports are based on yearly, unannounced inspections conducted by an outside contracted agency. The survey teams assess a variety of aspects of life at VA nursing homes, such as the care of residents and the processes used to give that care, how the staff and residents interact, and the nursing home environment. The surveyors also review residents’ clinical records and interview residents, family members, caregivers, and staff.
VA nursing homes also had a significantly lower percentage (6%) of 1-star (lowest rated) nursing homes compared with 15,487 private sector nursing homes rated by the Centers for Medicare and Medicaid Services. Both Medicare-certified skilled nursing facilities and VA CLCs must meet federal standards, such as having enough staff to provide adequate care. “There is significant evidence of a relationship between resident outcomes and staffing levels in nursing homes,” the VA says in its description of survey criteria.
Many VA nursing home residents are being treated for conditions rarely seen in private sector nursing homes, the VA says, including veteran-specific conditions, such as posttraumatic stress disorder (12% vs 0.5%) and traumatic brain injury (2% vs 0.8%). In 2018, 42% of 41,076 VA CLC residents had a service-connected disability rating of ≥ 50%. CLCs also provide more hospice care and care for conditions related to homelessness.
However, the VA notes that “quality measures are not the same as quality standards.” According to Medicare Nursing Home Compare, the quality of resident care measures are not benchmarks, thresholds, guidelines, or standards of care—they are a “snapshot at a point in time” of the average condition of residents. For instance, individual CLCs may serve special populations and have a higher rate of certain conditions. A CLC that specializes in complex skin and wound care may admit veterans with severe pressure ulcers that occurred at home or another hospital.
Detailed information on individual quality measures and how VA facilities compare with others in their areas are available at www.accesstocare.va.gov/healthcare/qualityofcare. That site also has an interactive searchable map that can be used to locate CLCs by zip code or distance. The health inspection reports are available at www.va.gov/qualityofcare/apps/aspire/clcsurvey.aspx.
Although VA nursing homes, on the whole, have sicker patients than do those in private sector nursing homes, they compare closely in terms of quality of care—and in some cases, VA health care gets higher marks. VA has more higher performing facilities (17% vs 11%) and fewer low-performing facilities (17% vs 20%).
Those figures come from the health care inspection reports and staffing data for its 134 community living centers (CLCs) that the VA is, for the first time, posting publicly. So far, VA has posted 101 health inspection reports; the remainder are scheduled for later this year. The reports cover April 2018 to the present.
The VA reports are based on yearly, unannounced inspections conducted by an outside contracted agency. The survey teams assess a variety of aspects of life at VA nursing homes, such as the care of residents and the processes used to give that care, how the staff and residents interact, and the nursing home environment. The surveyors also review residents’ clinical records and interview residents, family members, caregivers, and staff.
VA nursing homes also had a significantly lower percentage (6%) of 1-star (lowest rated) nursing homes compared with 15,487 private sector nursing homes rated by the Centers for Medicare and Medicaid Services. Both Medicare-certified skilled nursing facilities and VA CLCs must meet federal standards, such as having enough staff to provide adequate care. “There is significant evidence of a relationship between resident outcomes and staffing levels in nursing homes,” the VA says in its description of survey criteria.
Many VA nursing home residents are being treated for conditions rarely seen in private sector nursing homes, the VA says, including veteran-specific conditions, such as posttraumatic stress disorder (12% vs 0.5%) and traumatic brain injury (2% vs 0.8%). In 2018, 42% of 41,076 VA CLC residents had a service-connected disability rating of ≥ 50%. CLCs also provide more hospice care and care for conditions related to homelessness.
However, the VA notes that “quality measures are not the same as quality standards.” According to Medicare Nursing Home Compare, the quality of resident care measures are not benchmarks, thresholds, guidelines, or standards of care—they are a “snapshot at a point in time” of the average condition of residents. For instance, individual CLCs may serve special populations and have a higher rate of certain conditions. A CLC that specializes in complex skin and wound care may admit veterans with severe pressure ulcers that occurred at home or another hospital.
Detailed information on individual quality measures and how VA facilities compare with others in their areas are available at www.accesstocare.va.gov/healthcare/qualityofcare. That site also has an interactive searchable map that can be used to locate CLCs by zip code or distance. The health inspection reports are available at www.va.gov/qualityofcare/apps/aspire/clcsurvey.aspx.
Although VA nursing homes, on the whole, have sicker patients than do those in private sector nursing homes, they compare closely in terms of quality of care—and in some cases, VA health care gets higher marks. VA has more higher performing facilities (17% vs 11%) and fewer low-performing facilities (17% vs 20%).
Those figures come from the health care inspection reports and staffing data for its 134 community living centers (CLCs) that the VA is, for the first time, posting publicly. So far, VA has posted 101 health inspection reports; the remainder are scheduled for later this year. The reports cover April 2018 to the present.
The VA reports are based on yearly, unannounced inspections conducted by an outside contracted agency. The survey teams assess a variety of aspects of life at VA nursing homes, such as the care of residents and the processes used to give that care, how the staff and residents interact, and the nursing home environment. The surveyors also review residents’ clinical records and interview residents, family members, caregivers, and staff.
VA nursing homes also had a significantly lower percentage (6%) of 1-star (lowest rated) nursing homes compared with 15,487 private sector nursing homes rated by the Centers for Medicare and Medicaid Services. Both Medicare-certified skilled nursing facilities and VA CLCs must meet federal standards, such as having enough staff to provide adequate care. “There is significant evidence of a relationship between resident outcomes and staffing levels in nursing homes,” the VA says in its description of survey criteria.
Many VA nursing home residents are being treated for conditions rarely seen in private sector nursing homes, the VA says, including veteran-specific conditions, such as posttraumatic stress disorder (12% vs 0.5%) and traumatic brain injury (2% vs 0.8%). In 2018, 42% of 41,076 VA CLC residents had a service-connected disability rating of ≥ 50%. CLCs also provide more hospice care and care for conditions related to homelessness.
However, the VA notes that “quality measures are not the same as quality standards.” According to Medicare Nursing Home Compare, the quality of resident care measures are not benchmarks, thresholds, guidelines, or standards of care—they are a “snapshot at a point in time” of the average condition of residents. For instance, individual CLCs may serve special populations and have a higher rate of certain conditions. A CLC that specializes in complex skin and wound care may admit veterans with severe pressure ulcers that occurred at home or another hospital.
Detailed information on individual quality measures and how VA facilities compare with others in their areas are available at www.accesstocare.va.gov/healthcare/qualityofcare. That site also has an interactive searchable map that can be used to locate CLCs by zip code or distance. The health inspection reports are available at www.va.gov/qualityofcare/apps/aspire/clcsurvey.aspx.
AAP, AHA push for policies limiting children’s sugary beverage consumption
Physicians should actively advocate for policies that will reduce children’s consumption of sugar-sweetened beverages and subsequently reduce their risk of obesity, type 2 diabetes and other chronic diseases, urges a new policy statement from the American Academy of Pediatrics and the American Heart Association.
As “the leading source of added sugars in the U.S. diet,” sugary drinks “provide little to no nutritional value, are high in energy density, and do little to increase feelings of satiety,” wrote Natalie D. Muth, MD, MPH, of the University of California, Los Angeles, and the Children’s Primary Care Medical Group in Carlsbad, Calif., and her associates from the AAP’s Section on Obesity, the AAP’s Committee on Nutrition, and the American Heart Association.
Added sugars include sucrose, glucose, high-fructose corn syrup and processed fruit juice (but not naturally occurring fructose or lactose) that are added as sweeteners to food and drink products. In addition to contributing to childhood obesity, consuming drinks with added sugars increases children’s risk for “dental decay, cardiovascular disease, hypertension, dyslipidemia, insulin resistance, type 2 diabetes mellitus, fatty liver disease and all-cause mortality,” the statement noted.
Although less than 10% of individuals’ total calories should come from added sugars, according to the 2015–2020 Dietary Guidelines for Americans, added sugars make up an estimated 17% of U.S. children’s and teens’ average total caloric intake, and sugary drinks comprise almost half of this excess sugar, the statement noted. Consumption is higher and therefore more harmful in several subgroups, particularly those from minority and economically disadvantaged communities.
Previous AAP policy statements have urged doctors to encourage children and teens to drink water instead of high-calorie drinks, such as fruit juice, energy drinks and high-carbohydrate “sports drinks.” But this statement takes a page from success with tobacco control measures and focuses on the need for policy changes at local, state, and federal levels to help to reduce children’s intake of sugary drinks.
The statement makes six major recommendations:
• Excise tax: The AAP and AHA support the use of excise taxes or other means of increasing the cost of sugar-sweetened beverages – alongside an educational campaign to explain the rationale – and apportioning some of the subsequent revenue to addressing health and socioeconomic disparities.
Evidence shows how tobacco and alcohol consumption declined as their prices rose from taxes, and several places that have already implemented a sugary beverage tax have seen similar declines in consumption. Research models suggest an excise tax would potentially prevent 575,000 cases of childhood obesity.
• Reducing advertising/marketing: Research shows that teens, especially low-income and minority teens, are exposed to high amounts of advertising for soft drinks, fruit drinks, energy drinks, and sports drinks. Though such advertising cannot be outright banned without violating free speech, policies can disincentivize such marketing, such as “eliminating the advertising subsidy for nutritionally poor foods and beverages marketed to children” and not allowing in-school marketing.
• Improve existing nutritional assistance programs: WIC, the Child and Adult Care Food Program, school breakfast and lunch programs, and the Supplemental Nutrition Assistance Program (SNAP) vary in how well they discourage sugary drink consumption and can be improved through legislative changes.
• Consumer risk information: The AAP and AHA promote use of clear nutrition labels and menu labeling that explain the sugar content and risks of products, “including warning labels of the health harms of consumption of added sugars” not unlike those on cigarette packages.
• Changing the default: Physicians should advocate for policies that reduce access to sugary drinks and prioritize access to water and similarly healthier options.
• Norm-changing medical industry: Hospitals should implement policies that reduce consumption and/or access to sugary beverages, such as higher prices, less availability and risk-related labeling.
Pediatricians should continue to advise families about the risks of consuming added sugars, the substantial contribution that sugary drinks make to excess sugars in one’s diet, and the need to replace sugar-sweetened beverages with drinking water. Yet pediatricians can go beyond individual recommendations by advocating “for policy change through school boards, school health councils, hospital and medical group boards and committees, outreach to elected representatives and public comment opportunities.”
The statement used no external funding, and the authors had no disclosures or conflicts of interest.
SOURCE: Muth ND et al. Pediatrics. 2019;143(4):e20190282. doi: 10.1542/peds.2019-0282
Make counseling on healthy eating and exercise a priority
Pediatricians daily bear witness to the impact of poor nutrition on the health and quality of life of children. Healthy eating and drinking has long been an expected domain of conversation with children, adolescents and their families. In recent years, counseling regarding the consumption of added sugars, especially those in sugar-sweetened beverages (SSB), has gained traction. Pediatricians are aware of the health risks associated with over-consumption of SSB and that these risks, and consumption of sugary drinks, are more prevalent among the most vulnerable of children, including minorities and those living in low-income communities.
Primary care pediatricians face many demands that compete for time and attention, and time spent counseling on healthy eating and exercise needs to continue to be a priority. AAP-supported initiatives, such as the 5-3-2-1-0 rule – five vegetables and fruits per day, three structured meals, 2 hours or less of screen time, 1 hour or more of physical activity and zero sugary drinks most days – provide simple, clear messaging about limiting of sugary drinks. We should promote drinking of water as first choice, including during sports activities.
Messaging with community partners and on social media maximizes the impact of these important messages. The new AAP guideline “Public Policies to Reduce Sugary Drink Consumption in Children and Adolescents” also advocates for decreasing sugary drink marketing to children and adolescents. Pediatricians are trusted, credible voices who are well-positioned to advocate for an ad-free childhood, including SSB ads.
Melinda Clark, MD, is an associate professor of pediatrics at the Albany (N.Y.) Medical Center, and a MDedge Pediatrics editorial advisory board member. Dr. Clark had no disclosures.
Make counseling on healthy eating and exercise a priority
Pediatricians daily bear witness to the impact of poor nutrition on the health and quality of life of children. Healthy eating and drinking has long been an expected domain of conversation with children, adolescents and their families. In recent years, counseling regarding the consumption of added sugars, especially those in sugar-sweetened beverages (SSB), has gained traction. Pediatricians are aware of the health risks associated with over-consumption of SSB and that these risks, and consumption of sugary drinks, are more prevalent among the most vulnerable of children, including minorities and those living in low-income communities.
Primary care pediatricians face many demands that compete for time and attention, and time spent counseling on healthy eating and exercise needs to continue to be a priority. AAP-supported initiatives, such as the 5-3-2-1-0 rule – five vegetables and fruits per day, three structured meals, 2 hours or less of screen time, 1 hour or more of physical activity and zero sugary drinks most days – provide simple, clear messaging about limiting of sugary drinks. We should promote drinking of water as first choice, including during sports activities.
Messaging with community partners and on social media maximizes the impact of these important messages. The new AAP guideline “Public Policies to Reduce Sugary Drink Consumption in Children and Adolescents” also advocates for decreasing sugary drink marketing to children and adolescents. Pediatricians are trusted, credible voices who are well-positioned to advocate for an ad-free childhood, including SSB ads.
Melinda Clark, MD, is an associate professor of pediatrics at the Albany (N.Y.) Medical Center, and a MDedge Pediatrics editorial advisory board member. Dr. Clark had no disclosures.
Make counseling on healthy eating and exercise a priority
Pediatricians daily bear witness to the impact of poor nutrition on the health and quality of life of children. Healthy eating and drinking has long been an expected domain of conversation with children, adolescents and their families. In recent years, counseling regarding the consumption of added sugars, especially those in sugar-sweetened beverages (SSB), has gained traction. Pediatricians are aware of the health risks associated with over-consumption of SSB and that these risks, and consumption of sugary drinks, are more prevalent among the most vulnerable of children, including minorities and those living in low-income communities.
Primary care pediatricians face many demands that compete for time and attention, and time spent counseling on healthy eating and exercise needs to continue to be a priority. AAP-supported initiatives, such as the 5-3-2-1-0 rule – five vegetables and fruits per day, three structured meals, 2 hours or less of screen time, 1 hour or more of physical activity and zero sugary drinks most days – provide simple, clear messaging about limiting of sugary drinks. We should promote drinking of water as first choice, including during sports activities.
Messaging with community partners and on social media maximizes the impact of these important messages. The new AAP guideline “Public Policies to Reduce Sugary Drink Consumption in Children and Adolescents” also advocates for decreasing sugary drink marketing to children and adolescents. Pediatricians are trusted, credible voices who are well-positioned to advocate for an ad-free childhood, including SSB ads.
Melinda Clark, MD, is an associate professor of pediatrics at the Albany (N.Y.) Medical Center, and a MDedge Pediatrics editorial advisory board member. Dr. Clark had no disclosures.
Physicians should actively advocate for policies that will reduce children’s consumption of sugar-sweetened beverages and subsequently reduce their risk of obesity, type 2 diabetes and other chronic diseases, urges a new policy statement from the American Academy of Pediatrics and the American Heart Association.
As “the leading source of added sugars in the U.S. diet,” sugary drinks “provide little to no nutritional value, are high in energy density, and do little to increase feelings of satiety,” wrote Natalie D. Muth, MD, MPH, of the University of California, Los Angeles, and the Children’s Primary Care Medical Group in Carlsbad, Calif., and her associates from the AAP’s Section on Obesity, the AAP’s Committee on Nutrition, and the American Heart Association.
Added sugars include sucrose, glucose, high-fructose corn syrup and processed fruit juice (but not naturally occurring fructose or lactose) that are added as sweeteners to food and drink products. In addition to contributing to childhood obesity, consuming drinks with added sugars increases children’s risk for “dental decay, cardiovascular disease, hypertension, dyslipidemia, insulin resistance, type 2 diabetes mellitus, fatty liver disease and all-cause mortality,” the statement noted.
Although less than 10% of individuals’ total calories should come from added sugars, according to the 2015–2020 Dietary Guidelines for Americans, added sugars make up an estimated 17% of U.S. children’s and teens’ average total caloric intake, and sugary drinks comprise almost half of this excess sugar, the statement noted. Consumption is higher and therefore more harmful in several subgroups, particularly those from minority and economically disadvantaged communities.
Previous AAP policy statements have urged doctors to encourage children and teens to drink water instead of high-calorie drinks, such as fruit juice, energy drinks and high-carbohydrate “sports drinks.” But this statement takes a page from success with tobacco control measures and focuses on the need for policy changes at local, state, and federal levels to help to reduce children’s intake of sugary drinks.
The statement makes six major recommendations:
• Excise tax: The AAP and AHA support the use of excise taxes or other means of increasing the cost of sugar-sweetened beverages – alongside an educational campaign to explain the rationale – and apportioning some of the subsequent revenue to addressing health and socioeconomic disparities.
Evidence shows how tobacco and alcohol consumption declined as their prices rose from taxes, and several places that have already implemented a sugary beverage tax have seen similar declines in consumption. Research models suggest an excise tax would potentially prevent 575,000 cases of childhood obesity.
• Reducing advertising/marketing: Research shows that teens, especially low-income and minority teens, are exposed to high amounts of advertising for soft drinks, fruit drinks, energy drinks, and sports drinks. Though such advertising cannot be outright banned without violating free speech, policies can disincentivize such marketing, such as “eliminating the advertising subsidy for nutritionally poor foods and beverages marketed to children” and not allowing in-school marketing.
• Improve existing nutritional assistance programs: WIC, the Child and Adult Care Food Program, school breakfast and lunch programs, and the Supplemental Nutrition Assistance Program (SNAP) vary in how well they discourage sugary drink consumption and can be improved through legislative changes.
• Consumer risk information: The AAP and AHA promote use of clear nutrition labels and menu labeling that explain the sugar content and risks of products, “including warning labels of the health harms of consumption of added sugars” not unlike those on cigarette packages.
• Changing the default: Physicians should advocate for policies that reduce access to sugary drinks and prioritize access to water and similarly healthier options.
• Norm-changing medical industry: Hospitals should implement policies that reduce consumption and/or access to sugary beverages, such as higher prices, less availability and risk-related labeling.
Pediatricians should continue to advise families about the risks of consuming added sugars, the substantial contribution that sugary drinks make to excess sugars in one’s diet, and the need to replace sugar-sweetened beverages with drinking water. Yet pediatricians can go beyond individual recommendations by advocating “for policy change through school boards, school health councils, hospital and medical group boards and committees, outreach to elected representatives and public comment opportunities.”
The statement used no external funding, and the authors had no disclosures or conflicts of interest.
SOURCE: Muth ND et al. Pediatrics. 2019;143(4):e20190282. doi: 10.1542/peds.2019-0282
Physicians should actively advocate for policies that will reduce children’s consumption of sugar-sweetened beverages and subsequently reduce their risk of obesity, type 2 diabetes and other chronic diseases, urges a new policy statement from the American Academy of Pediatrics and the American Heart Association.
As “the leading source of added sugars in the U.S. diet,” sugary drinks “provide little to no nutritional value, are high in energy density, and do little to increase feelings of satiety,” wrote Natalie D. Muth, MD, MPH, of the University of California, Los Angeles, and the Children’s Primary Care Medical Group in Carlsbad, Calif., and her associates from the AAP’s Section on Obesity, the AAP’s Committee on Nutrition, and the American Heart Association.
Added sugars include sucrose, glucose, high-fructose corn syrup and processed fruit juice (but not naturally occurring fructose or lactose) that are added as sweeteners to food and drink products. In addition to contributing to childhood obesity, consuming drinks with added sugars increases children’s risk for “dental decay, cardiovascular disease, hypertension, dyslipidemia, insulin resistance, type 2 diabetes mellitus, fatty liver disease and all-cause mortality,” the statement noted.
Although less than 10% of individuals’ total calories should come from added sugars, according to the 2015–2020 Dietary Guidelines for Americans, added sugars make up an estimated 17% of U.S. children’s and teens’ average total caloric intake, and sugary drinks comprise almost half of this excess sugar, the statement noted. Consumption is higher and therefore more harmful in several subgroups, particularly those from minority and economically disadvantaged communities.
Previous AAP policy statements have urged doctors to encourage children and teens to drink water instead of high-calorie drinks, such as fruit juice, energy drinks and high-carbohydrate “sports drinks.” But this statement takes a page from success with tobacco control measures and focuses on the need for policy changes at local, state, and federal levels to help to reduce children’s intake of sugary drinks.
The statement makes six major recommendations:
• Excise tax: The AAP and AHA support the use of excise taxes or other means of increasing the cost of sugar-sweetened beverages – alongside an educational campaign to explain the rationale – and apportioning some of the subsequent revenue to addressing health and socioeconomic disparities.
Evidence shows how tobacco and alcohol consumption declined as their prices rose from taxes, and several places that have already implemented a sugary beverage tax have seen similar declines in consumption. Research models suggest an excise tax would potentially prevent 575,000 cases of childhood obesity.
• Reducing advertising/marketing: Research shows that teens, especially low-income and minority teens, are exposed to high amounts of advertising for soft drinks, fruit drinks, energy drinks, and sports drinks. Though such advertising cannot be outright banned without violating free speech, policies can disincentivize such marketing, such as “eliminating the advertising subsidy for nutritionally poor foods and beverages marketed to children” and not allowing in-school marketing.
• Improve existing nutritional assistance programs: WIC, the Child and Adult Care Food Program, school breakfast and lunch programs, and the Supplemental Nutrition Assistance Program (SNAP) vary in how well they discourage sugary drink consumption and can be improved through legislative changes.
• Consumer risk information: The AAP and AHA promote use of clear nutrition labels and menu labeling that explain the sugar content and risks of products, “including warning labels of the health harms of consumption of added sugars” not unlike those on cigarette packages.
• Changing the default: Physicians should advocate for policies that reduce access to sugary drinks and prioritize access to water and similarly healthier options.
• Norm-changing medical industry: Hospitals should implement policies that reduce consumption and/or access to sugary beverages, such as higher prices, less availability and risk-related labeling.
Pediatricians should continue to advise families about the risks of consuming added sugars, the substantial contribution that sugary drinks make to excess sugars in one’s diet, and the need to replace sugar-sweetened beverages with drinking water. Yet pediatricians can go beyond individual recommendations by advocating “for policy change through school boards, school health councils, hospital and medical group boards and committees, outreach to elected representatives and public comment opportunities.”
The statement used no external funding, and the authors had no disclosures or conflicts of interest.
SOURCE: Muth ND et al. Pediatrics. 2019;143(4):e20190282. doi: 10.1542/peds.2019-0282
FROM PEDIATRICS
FDA chief calls for stricter scrutiny of electronic health records
“What we really need is a much more tailored approach, so that we have appropriate oversight of EHRs when they’re doing things that could create risk for patients,” Dr. Gottlieb said in an interview with Kaiser Health News.
Dr. Gottlieb was responding to “Botched Operation,” a report published March 18 by KHN and Fortune magazine. The investigation found that the federal government has spent more than $36 billion over the past 10 years to switch doctors and hospitals from paper to digital records systems. In that time, thousands of reports of deaths, injuries, and near misses linked to EHRs have piled up in databases – including at least one run by the FDA.
Dr. Gottlieb said Congress would need to enact legislation to define when an EHR would require government oversight. He said that the digital records systems, which store a patient’s medical history, don’t fit neatly under the agency’s existing mandate to regulate items such as drugs and medical devices.
Dr. Gottlieb said the best approach might be to say that an EHR that has a certain capability becomes a medical device. He called EHRs a “unique tool,” noting that the risks posed by their use aren’t the same as for a traditional medical device implanted in a patient. “You need a much different regulatory scheme,” he said.
The 21st Century Cures Act of 2016 excludes the FDA from having oversight over EHRs as a medical device.
Dr. Gottlieb said that health IT companies could add new functions that would improve EHRs, but they have been reluctant to do so because they didn’t want their products to fall under FDA jurisdiction. He added that he was “not calling” for FDA to take over such a duty, however, and suggested that any new approach could be years away. Proponents have long argued that widespread use of EHRs can make medicine safer by alerting doctors to potential medical errors, though critics counter that software glitches and user errors may cause new varieties of medical mistakes.
How closely the FDA should watch over the digital medical record revolution has been controversial for years. The agency’s interest in the issue perked up after Congress decided in February 2009 to spend billions of dollars on digital medical records as part of an economic stimulus program.
At the time, many industry groups argued that FDA regulation would “stifle innovation” and stall the national drive to bring medicine into the modern era. Federal officials responsible for doling out billions in subsidies to doctors and hospitals generally sympathized with that view and were skeptical of allowing the FDA to play a role.
The debate became public in February 2010, when Jeffrey Shuren, MD, an FDA official, testified at a public hearing that the agency had tied 6 deaths and more than 200 injuries to health information technology. In all, the FDA said, it had logged 260 reports in the previous 2 years of “malfunctions with the potential for patient harm.”
The agency said the findings were based largely on reports voluntarily submitted to the FDA and suggested “significant clinical implications and public safety issues.” In one case cited, lab tests done in a hospital emergency department were sent to the wrong patient’s file. Since then, several government and private repositories have associated thousands of injuries, near misses, and deaths to EHR technology.
Dr. Shuren said in 2010 that the agency recognized that health information technology had great potential to improve patient care, but also needed oversight to “assure patient safety.”
While some safety proponents agree that EHRs offer tremendous benefits, they also see a greater opportunities to improve their safety.
Dean Sittig, PhD, a professor of bioinformatics and bioengineering at the University of Texas, Houston, said EHRs have improved safety within the health care system, but they have not eliminated errors to the extent that he would have expected. Federal officials were initially pushing for rapid adoption and ‘there wasn’t a lot of interest in talking about things that could go wrong,’ ” Dr. Sittig told KHN and Fortune.
Earlier in March, Gottlieb announced his resignation from the FDA. His last day is scheduled to be April 5.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN correspondents Sarah Jane Tribble, Sydney Lupkin, and Julie Rovner contributed to this report.
“What we really need is a much more tailored approach, so that we have appropriate oversight of EHRs when they’re doing things that could create risk for patients,” Dr. Gottlieb said in an interview with Kaiser Health News.
Dr. Gottlieb was responding to “Botched Operation,” a report published March 18 by KHN and Fortune magazine. The investigation found that the federal government has spent more than $36 billion over the past 10 years to switch doctors and hospitals from paper to digital records systems. In that time, thousands of reports of deaths, injuries, and near misses linked to EHRs have piled up in databases – including at least one run by the FDA.
Dr. Gottlieb said Congress would need to enact legislation to define when an EHR would require government oversight. He said that the digital records systems, which store a patient’s medical history, don’t fit neatly under the agency’s existing mandate to regulate items such as drugs and medical devices.
Dr. Gottlieb said the best approach might be to say that an EHR that has a certain capability becomes a medical device. He called EHRs a “unique tool,” noting that the risks posed by their use aren’t the same as for a traditional medical device implanted in a patient. “You need a much different regulatory scheme,” he said.
The 21st Century Cures Act of 2016 excludes the FDA from having oversight over EHRs as a medical device.
Dr. Gottlieb said that health IT companies could add new functions that would improve EHRs, but they have been reluctant to do so because they didn’t want their products to fall under FDA jurisdiction. He added that he was “not calling” for FDA to take over such a duty, however, and suggested that any new approach could be years away. Proponents have long argued that widespread use of EHRs can make medicine safer by alerting doctors to potential medical errors, though critics counter that software glitches and user errors may cause new varieties of medical mistakes.
How closely the FDA should watch over the digital medical record revolution has been controversial for years. The agency’s interest in the issue perked up after Congress decided in February 2009 to spend billions of dollars on digital medical records as part of an economic stimulus program.
At the time, many industry groups argued that FDA regulation would “stifle innovation” and stall the national drive to bring medicine into the modern era. Federal officials responsible for doling out billions in subsidies to doctors and hospitals generally sympathized with that view and were skeptical of allowing the FDA to play a role.
The debate became public in February 2010, when Jeffrey Shuren, MD, an FDA official, testified at a public hearing that the agency had tied 6 deaths and more than 200 injuries to health information technology. In all, the FDA said, it had logged 260 reports in the previous 2 years of “malfunctions with the potential for patient harm.”
The agency said the findings were based largely on reports voluntarily submitted to the FDA and suggested “significant clinical implications and public safety issues.” In one case cited, lab tests done in a hospital emergency department were sent to the wrong patient’s file. Since then, several government and private repositories have associated thousands of injuries, near misses, and deaths to EHR technology.
Dr. Shuren said in 2010 that the agency recognized that health information technology had great potential to improve patient care, but also needed oversight to “assure patient safety.”
While some safety proponents agree that EHRs offer tremendous benefits, they also see a greater opportunities to improve their safety.
Dean Sittig, PhD, a professor of bioinformatics and bioengineering at the University of Texas, Houston, said EHRs have improved safety within the health care system, but they have not eliminated errors to the extent that he would have expected. Federal officials were initially pushing for rapid adoption and ‘there wasn’t a lot of interest in talking about things that could go wrong,’ ” Dr. Sittig told KHN and Fortune.
Earlier in March, Gottlieb announced his resignation from the FDA. His last day is scheduled to be April 5.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN correspondents Sarah Jane Tribble, Sydney Lupkin, and Julie Rovner contributed to this report.
“What we really need is a much more tailored approach, so that we have appropriate oversight of EHRs when they’re doing things that could create risk for patients,” Dr. Gottlieb said in an interview with Kaiser Health News.
Dr. Gottlieb was responding to “Botched Operation,” a report published March 18 by KHN and Fortune magazine. The investigation found that the federal government has spent more than $36 billion over the past 10 years to switch doctors and hospitals from paper to digital records systems. In that time, thousands of reports of deaths, injuries, and near misses linked to EHRs have piled up in databases – including at least one run by the FDA.
Dr. Gottlieb said Congress would need to enact legislation to define when an EHR would require government oversight. He said that the digital records systems, which store a patient’s medical history, don’t fit neatly under the agency’s existing mandate to regulate items such as drugs and medical devices.
Dr. Gottlieb said the best approach might be to say that an EHR that has a certain capability becomes a medical device. He called EHRs a “unique tool,” noting that the risks posed by their use aren’t the same as for a traditional medical device implanted in a patient. “You need a much different regulatory scheme,” he said.
The 21st Century Cures Act of 2016 excludes the FDA from having oversight over EHRs as a medical device.
Dr. Gottlieb said that health IT companies could add new functions that would improve EHRs, but they have been reluctant to do so because they didn’t want their products to fall under FDA jurisdiction. He added that he was “not calling” for FDA to take over such a duty, however, and suggested that any new approach could be years away. Proponents have long argued that widespread use of EHRs can make medicine safer by alerting doctors to potential medical errors, though critics counter that software glitches and user errors may cause new varieties of medical mistakes.
How closely the FDA should watch over the digital medical record revolution has been controversial for years. The agency’s interest in the issue perked up after Congress decided in February 2009 to spend billions of dollars on digital medical records as part of an economic stimulus program.
At the time, many industry groups argued that FDA regulation would “stifle innovation” and stall the national drive to bring medicine into the modern era. Federal officials responsible for doling out billions in subsidies to doctors and hospitals generally sympathized with that view and were skeptical of allowing the FDA to play a role.
The debate became public in February 2010, when Jeffrey Shuren, MD, an FDA official, testified at a public hearing that the agency had tied 6 deaths and more than 200 injuries to health information technology. In all, the FDA said, it had logged 260 reports in the previous 2 years of “malfunctions with the potential for patient harm.”
The agency said the findings were based largely on reports voluntarily submitted to the FDA and suggested “significant clinical implications and public safety issues.” In one case cited, lab tests done in a hospital emergency department were sent to the wrong patient’s file. Since then, several government and private repositories have associated thousands of injuries, near misses, and deaths to EHR technology.
Dr. Shuren said in 2010 that the agency recognized that health information technology had great potential to improve patient care, but also needed oversight to “assure patient safety.”
While some safety proponents agree that EHRs offer tremendous benefits, they also see a greater opportunities to improve their safety.
Dean Sittig, PhD, a professor of bioinformatics and bioengineering at the University of Texas, Houston, said EHRs have improved safety within the health care system, but they have not eliminated errors to the extent that he would have expected. Federal officials were initially pushing for rapid adoption and ‘there wasn’t a lot of interest in talking about things that could go wrong,’ ” Dr. Sittig told KHN and Fortune.
Earlier in March, Gottlieb announced his resignation from the FDA. His last day is scheduled to be April 5.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN correspondents Sarah Jane Tribble, Sydney Lupkin, and Julie Rovner contributed to this report.
For patients with end-stage liver disease, acute care incurs steep costs
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
MedPAC puts Part B reference pricing, binding arbitration on the table
Much of the presentation, offered during the commission’s March meeting, was general ideas with more work to come in terms of fleshing out the details. An ambitious goal of having something ready for the commission’s June 2019 report to Congress was set.
The policy recommendations for reference pricing, to be used when multiple similar drugs are available, and binding arbitration, to be used on new entrants to the market with limited or no competition, are being designed to work with the previously recommended drug value program, but could be implemented on their own.
In general, the reference pricing policy would set a maximum payment rate for a group of drugs with similar health effects based on the minimum, median, or other point along the range of prices for all drugs in that group. Providers would be incentivized to choose a lower-cost alternative when clinically appropriate.
Beneficiaries who still want access to a higher-cost drug would be on the hook for the difference through cost-sharing mechanisms.
MedPAC staff presented two options for setting the reference price. One would be to establish the price based on internal Medicare data. The other would take international pricing into consideration.
Binding arbitration, which is already a component of the drug value program, would be expanded. In the program described by staff, Medicare and the manufacturer would each come to the table with a price and the arbitrator (either an individual or a panel) would set one price.
Potential cost savings from one or both programs was not addressed
“It seems like an important thing for us to understand in order to know the potential impact ... through these two levers that work on different parts of the spend problem,” said Commissioner Dana Safran, head of measurement for the health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
Staff said it would work on making that determination.
Commissioners raised additional questions on operational details.
Marjorie Ginsburg, founding executive director of the Center for Healthcare Decisions Inc. in Sacramento, Calif., questioned what would happen if a manufacturer declined to participate in the arbitration process and whether that would mean Medicare would not cover a drug in that circumstance.
Jay Crosson, MD, noted that “Congress would have to … figure out how to deal with that circumstance. ... We would not want to end up with a system that would deny coverage” of effective medications for Medicare beneficiaries.
Another area affecting both issues was the potential for cross subsidization of drugs.
Jonathan Perlin, MD, president of clinical services and chief medical officer of HCA Healthcare of Nashville, Tenn., questioned whether this could open a door for a provider buying at a cheaper government price and using the drugs across patients not from Medicare or whether it could lead to higher prices being charged to commercial payers.
MedPAC staff member Kim Neuman said that “there would need to be some back end reconciliations that would happen to ensure that the stock that was then administered to Medicare patients was provided at a price that was no higher than that ceiling. ... We haven’t scoped out implications for other payers.”
Commissioner Kathy Buto, independent consultant and former vice president of global health policy at Johnson & Johnson, inquired about whether a drug would be made available upon launch while reference pricing or arbitration processes were in progress.
Commissioners also inquired as to how the reference pricing aspects will be operationalized into conversations between the doctor and the patient.
Ms. Buto also cautioned that using a reference pricing scheme could alter the dynamic of pricing competition that has companies competing against a reference price rather than doing what they can to lower prices beyond that.
Much of the presentation, offered during the commission’s March meeting, was general ideas with more work to come in terms of fleshing out the details. An ambitious goal of having something ready for the commission’s June 2019 report to Congress was set.
The policy recommendations for reference pricing, to be used when multiple similar drugs are available, and binding arbitration, to be used on new entrants to the market with limited or no competition, are being designed to work with the previously recommended drug value program, but could be implemented on their own.
In general, the reference pricing policy would set a maximum payment rate for a group of drugs with similar health effects based on the minimum, median, or other point along the range of prices for all drugs in that group. Providers would be incentivized to choose a lower-cost alternative when clinically appropriate.
Beneficiaries who still want access to a higher-cost drug would be on the hook for the difference through cost-sharing mechanisms.
MedPAC staff presented two options for setting the reference price. One would be to establish the price based on internal Medicare data. The other would take international pricing into consideration.
Binding arbitration, which is already a component of the drug value program, would be expanded. In the program described by staff, Medicare and the manufacturer would each come to the table with a price and the arbitrator (either an individual or a panel) would set one price.
Potential cost savings from one or both programs was not addressed
“It seems like an important thing for us to understand in order to know the potential impact ... through these two levers that work on different parts of the spend problem,” said Commissioner Dana Safran, head of measurement for the health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
Staff said it would work on making that determination.
Commissioners raised additional questions on operational details.
Marjorie Ginsburg, founding executive director of the Center for Healthcare Decisions Inc. in Sacramento, Calif., questioned what would happen if a manufacturer declined to participate in the arbitration process and whether that would mean Medicare would not cover a drug in that circumstance.
Jay Crosson, MD, noted that “Congress would have to … figure out how to deal with that circumstance. ... We would not want to end up with a system that would deny coverage” of effective medications for Medicare beneficiaries.
Another area affecting both issues was the potential for cross subsidization of drugs.
Jonathan Perlin, MD, president of clinical services and chief medical officer of HCA Healthcare of Nashville, Tenn., questioned whether this could open a door for a provider buying at a cheaper government price and using the drugs across patients not from Medicare or whether it could lead to higher prices being charged to commercial payers.
MedPAC staff member Kim Neuman said that “there would need to be some back end reconciliations that would happen to ensure that the stock that was then administered to Medicare patients was provided at a price that was no higher than that ceiling. ... We haven’t scoped out implications for other payers.”
Commissioner Kathy Buto, independent consultant and former vice president of global health policy at Johnson & Johnson, inquired about whether a drug would be made available upon launch while reference pricing or arbitration processes were in progress.
Commissioners also inquired as to how the reference pricing aspects will be operationalized into conversations between the doctor and the patient.
Ms. Buto also cautioned that using a reference pricing scheme could alter the dynamic of pricing competition that has companies competing against a reference price rather than doing what they can to lower prices beyond that.
Much of the presentation, offered during the commission’s March meeting, was general ideas with more work to come in terms of fleshing out the details. An ambitious goal of having something ready for the commission’s June 2019 report to Congress was set.
The policy recommendations for reference pricing, to be used when multiple similar drugs are available, and binding arbitration, to be used on new entrants to the market with limited or no competition, are being designed to work with the previously recommended drug value program, but could be implemented on their own.
In general, the reference pricing policy would set a maximum payment rate for a group of drugs with similar health effects based on the minimum, median, or other point along the range of prices for all drugs in that group. Providers would be incentivized to choose a lower-cost alternative when clinically appropriate.
Beneficiaries who still want access to a higher-cost drug would be on the hook for the difference through cost-sharing mechanisms.
MedPAC staff presented two options for setting the reference price. One would be to establish the price based on internal Medicare data. The other would take international pricing into consideration.
Binding arbitration, which is already a component of the drug value program, would be expanded. In the program described by staff, Medicare and the manufacturer would each come to the table with a price and the arbitrator (either an individual or a panel) would set one price.
Potential cost savings from one or both programs was not addressed
“It seems like an important thing for us to understand in order to know the potential impact ... through these two levers that work on different parts of the spend problem,” said Commissioner Dana Safran, head of measurement for the health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
Staff said it would work on making that determination.
Commissioners raised additional questions on operational details.
Marjorie Ginsburg, founding executive director of the Center for Healthcare Decisions Inc. in Sacramento, Calif., questioned what would happen if a manufacturer declined to participate in the arbitration process and whether that would mean Medicare would not cover a drug in that circumstance.
Jay Crosson, MD, noted that “Congress would have to … figure out how to deal with that circumstance. ... We would not want to end up with a system that would deny coverage” of effective medications for Medicare beneficiaries.
Another area affecting both issues was the potential for cross subsidization of drugs.
Jonathan Perlin, MD, president of clinical services and chief medical officer of HCA Healthcare of Nashville, Tenn., questioned whether this could open a door for a provider buying at a cheaper government price and using the drugs across patients not from Medicare or whether it could lead to higher prices being charged to commercial payers.
MedPAC staff member Kim Neuman said that “there would need to be some back end reconciliations that would happen to ensure that the stock that was then administered to Medicare patients was provided at a price that was no higher than that ceiling. ... We haven’t scoped out implications for other payers.”
Commissioner Kathy Buto, independent consultant and former vice president of global health policy at Johnson & Johnson, inquired about whether a drug would be made available upon launch while reference pricing or arbitration processes were in progress.
Commissioners also inquired as to how the reference pricing aspects will be operationalized into conversations between the doctor and the patient.
Ms. Buto also cautioned that using a reference pricing scheme could alter the dynamic of pricing competition that has companies competing against a reference price rather than doing what they can to lower prices beyond that.
REPORTING FROM A MEDPAC MEETING
Will patient rewards for lower-cost choices impact physicians?
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
FROM HEALTH AFFAIRS
Survey: Americans support regulation of vaping products
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.
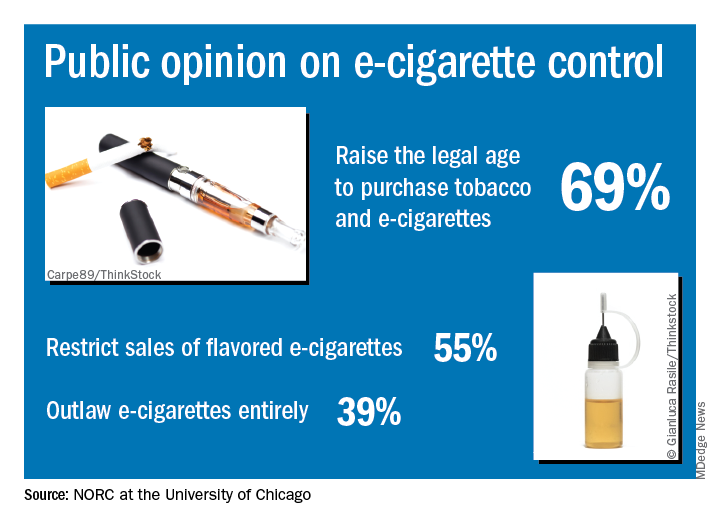
“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
VEText 1 Year Later—Still Growing
Nearly 6 million veterans get health care scheduling reminders via VEText, an interactive mobile program launched a year ago. More than 70 million text messages later, how is VEText doing? Apparently, well. The VA says an “overwhelming majority” of veterans like the enhanced access. Only 4% have opted out.
The VA has worked to improve the user experience along the way. The latest enhancement allows the VA to send facility and clinic location in the unsecured text message appointment reminders. One user, James Preston, interviewed for a VA article, says at the Loma Linda VA Medical Center veterans still had to use the information desk to find out exactly where they needed to go. “Now it’s almost perfect,” he says, “because it provides all the necessary information.” Deanna Callahan, innovation specialist and National Program Manager for VEText, says before VEText, the VA was relying on phone calls, robocalls, and mail. “We wanted to modernize our efforts to not only bring text message appointment reminders but go above and beyond and positively affect the No Show rate.” It worked—the No Show rate has dropped from 13.7% to 11.7% in the year the program has been active.
The system automatically enrolls veterans based on phone information already on file, but they can opt out by replying STOP to a reminder. Accidentally opting out is easily reversed by replying START to a previous reminder. Reminders are sent for clinical appointments at local medical centers and outpatient clinics, but not for Lab, Community Care, Research, Telephone Clinics, or Home-based Primary Care. (The reminders are additional—they do not replace letters, postcards, or automated phone call reminders.) VEText itself does not cost the veteran anything, but text messaging rates may apply, depending on individual cell phone plans. For more information, go to www.va.gov/HEALTH/vetext_faqs.asp.
Nearly 6 million veterans get health care scheduling reminders via VEText, an interactive mobile program launched a year ago. More than 70 million text messages later, how is VEText doing? Apparently, well. The VA says an “overwhelming majority” of veterans like the enhanced access. Only 4% have opted out.
The VA has worked to improve the user experience along the way. The latest enhancement allows the VA to send facility and clinic location in the unsecured text message appointment reminders. One user, James Preston, interviewed for a VA article, says at the Loma Linda VA Medical Center veterans still had to use the information desk to find out exactly where they needed to go. “Now it’s almost perfect,” he says, “because it provides all the necessary information.” Deanna Callahan, innovation specialist and National Program Manager for VEText, says before VEText, the VA was relying on phone calls, robocalls, and mail. “We wanted to modernize our efforts to not only bring text message appointment reminders but go above and beyond and positively affect the No Show rate.” It worked—the No Show rate has dropped from 13.7% to 11.7% in the year the program has been active.
The system automatically enrolls veterans based on phone information already on file, but they can opt out by replying STOP to a reminder. Accidentally opting out is easily reversed by replying START to a previous reminder. Reminders are sent for clinical appointments at local medical centers and outpatient clinics, but not for Lab, Community Care, Research, Telephone Clinics, or Home-based Primary Care. (The reminders are additional—they do not replace letters, postcards, or automated phone call reminders.) VEText itself does not cost the veteran anything, but text messaging rates may apply, depending on individual cell phone plans. For more information, go to www.va.gov/HEALTH/vetext_faqs.asp.
Nearly 6 million veterans get health care scheduling reminders via VEText, an interactive mobile program launched a year ago. More than 70 million text messages later, how is VEText doing? Apparently, well. The VA says an “overwhelming majority” of veterans like the enhanced access. Only 4% have opted out.
The VA has worked to improve the user experience along the way. The latest enhancement allows the VA to send facility and clinic location in the unsecured text message appointment reminders. One user, James Preston, interviewed for a VA article, says at the Loma Linda VA Medical Center veterans still had to use the information desk to find out exactly where they needed to go. “Now it’s almost perfect,” he says, “because it provides all the necessary information.” Deanna Callahan, innovation specialist and National Program Manager for VEText, says before VEText, the VA was relying on phone calls, robocalls, and mail. “We wanted to modernize our efforts to not only bring text message appointment reminders but go above and beyond and positively affect the No Show rate.” It worked—the No Show rate has dropped from 13.7% to 11.7% in the year the program has been active.
The system automatically enrolls veterans based on phone information already on file, but they can opt out by replying STOP to a reminder. Accidentally opting out is easily reversed by replying START to a previous reminder. Reminders are sent for clinical appointments at local medical centers and outpatient clinics, but not for Lab, Community Care, Research, Telephone Clinics, or Home-based Primary Care. (The reminders are additional—they do not replace letters, postcards, or automated phone call reminders.) VEText itself does not cost the veteran anything, but text messaging rates may apply, depending on individual cell phone plans. For more information, go to www.va.gov/HEALTH/vetext_faqs.asp.
Access to abortion care: Facts matter
In 1973, the Supreme Court of the United States recognized a constitutional right to abortion in the landmark case of Roe v Wade. The Court held that states may regulate, but not ban, abortion after the first trimester, for the purpose of protecting the woman’s health. The Court further indicated that states’ interest in “potential life” could be the basis for abortion regulations only after the point of viability, at which point states may ban abortion except when necessary to preserve the life or health of the woman.1 In 1992, the Court decided Planned Parenthood v Casey and eliminated the trimester framework while upholding women’s right to abortion.2 As with Roe v Wade, the Casey decision held that there must be an exception for the woman’s health and life.
Fast forward to 2019
New York passed a law in 2019,3 and Virginia had a proposed law that was recently tabled by the House of Delegates,4 both related to abortions performed past the first trimester.
New York. The New York law supports legal abortion by a licensed practitioner within 24 weeks of pregnancy commencement. After 24 weeks’ gestation, if there is “an absence of fetal viability, or the abortion is necessary to protect the patient’s life or health” then termination is permissible.3
Virginia. Previously, Virginia had abortion laws that required significant measures to approve a third-trimester abortion, including certification by 3 physicians that the procedure is necessary to “save mother’s life or [prevent] substantial and irremediable impairment of mental or physical health of the mother.”5 Violation included potential for jail time and a significant monetary fine.
The proposed bill, now tabled, was introduced by delegate Kathy Tran (House Bill 2491) and would have rolled back many requirements of the old law, including the 24-hour waiting period and mandate for second-trimester abortions to occur in a hospital.
The controversy centered on a provision concerning third-trimester abortions. Specifically, the proposed bill would only have required 1 doctor to deem the abortion necessary and would have removed the “substantially and irremediably” qualifier. Thus, abortions would be allowed in cases in which the woman’s mental or physical health was threatened, even in cases in which the potential damage may be reversible.5
The facts
Misconceptions about abortion care can be dangerous and work to further stigmatize our patients who may need an abortion or who have had an abortion in the past. The American College of Obstetricians and Gynecologists (ACOG) recently published a document discussing facts regarding abortion care later in pregnancy. The document (aptly named “Facts are Important”) enforces that policy be based on medical science and facts, and not simply driven by political beliefs.6
Fact. The majority of abortions occur prior to 21 weeks, before viability:
- 91.1% of abortions occur at or before 13 weeks’ gestation7
- only 1.3% of abortions occur at or after 21 weeks’ gestation7
- abortions occurring later in the second trimester or in the third trimester are very uncommon.
Fact. The language “late-term abortion” has no medical definition, is not used in a clinical setting or to describe the delivery of abortion care later in pregnancy in any medical institution.6
Fact. Many of the abortions occurring later in pregnancy are due to fetal anomalies incompatible with life. Anomalies can include lack of a major portion of the brain (anencephaly), bilateral renal agenesis, some skeletal dysplasias, and other chromosomal abnormalities. These are cases in which death is likely before or shortly after birth, with great potential for suffering of both the fetus and the family.
Fact. The need for abortion also may be due to serious complications that will likely cause significant morbidity or mortality to the woman. These complications, in turn, reduce the likelihood of survival of the fetus.
It is thus vital for women to have the freedom to evaluate their medical circumstance with their provider and, using evidence, make informed health care decisions—which may include abortion, induction of labor, or cesarean delivery in some circumstances. Access to accurate, complete information and care is a right bestowed amongst all women and “must never be constrained by politicians.”6 We must focus on medically appropriate and compassionate care for both the family and the fetus.
Use your voice
As clinicians, we are trusted members of our communities. The New York law and the prior proposed Virginia law emphasize important access to care for women and their families. Abortions at a later gestational age are a rare event but are most often performed when the health or life of the mother is at risk or the fetus has an anomaly incompatible with life.
We urge you to use your voice to correct misconceptions, whether in your office with your patients or colleagues or in your communities, locally and nationally. Email your friends and colleagues about ACOG’s “Facts are Important” document, organize a grand rounds on the topic, and utilize social media to share facts about abortion care. These actions support our patients and can make an impact by spreading factual information.
For more facts and figures about abortion laws, visit the website of the Guttmacher Institute.
- Roe v Wade, 410 US 113 (1973).
- Planned Parenthood v Casey, 505 US 833 (1992).
- New York abortion laws. FindLaw website. https://statelaws.findlaw.com/new-york-law/new-york-abortion-laws.html. Accessed March 7, 2019.
- North A. The controversy around Virginia’s new abortion bill, explained. https://www.vox.com/2019/2/1/18205428/virginia-abortion-bill-kathy-tran-ralph-northam Accessed March 13, 2019.
- Virginia abortion laws. FindLaw website. https://statelaws.findlaw.com/virginia-law/virginia-abortion-laws.html. Accessed March 7, 2019.
- Facts are important. The American College of Obstetricians and Gynecologists website. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/Facts-Are-Important_Abortion-Care-Later-In-Pregnancy-February-2019-College.pdf?dmc=1&ts=20190214T2242210541. Accessed March 7, 2019.
- Jatlaoui TC, Boutot ME, Mandel MG, et al. Abortion surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(13):1-45.
In 1973, the Supreme Court of the United States recognized a constitutional right to abortion in the landmark case of Roe v Wade. The Court held that states may regulate, but not ban, abortion after the first trimester, for the purpose of protecting the woman’s health. The Court further indicated that states’ interest in “potential life” could be the basis for abortion regulations only after the point of viability, at which point states may ban abortion except when necessary to preserve the life or health of the woman.1 In 1992, the Court decided Planned Parenthood v Casey and eliminated the trimester framework while upholding women’s right to abortion.2 As with Roe v Wade, the Casey decision held that there must be an exception for the woman’s health and life.
Fast forward to 2019
New York passed a law in 2019,3 and Virginia had a proposed law that was recently tabled by the House of Delegates,4 both related to abortions performed past the first trimester.
New York. The New York law supports legal abortion by a licensed practitioner within 24 weeks of pregnancy commencement. After 24 weeks’ gestation, if there is “an absence of fetal viability, or the abortion is necessary to protect the patient’s life or health” then termination is permissible.3
Virginia. Previously, Virginia had abortion laws that required significant measures to approve a third-trimester abortion, including certification by 3 physicians that the procedure is necessary to “save mother’s life or [prevent] substantial and irremediable impairment of mental or physical health of the mother.”5 Violation included potential for jail time and a significant monetary fine.
The proposed bill, now tabled, was introduced by delegate Kathy Tran (House Bill 2491) and would have rolled back many requirements of the old law, including the 24-hour waiting period and mandate for second-trimester abortions to occur in a hospital.
The controversy centered on a provision concerning third-trimester abortions. Specifically, the proposed bill would only have required 1 doctor to deem the abortion necessary and would have removed the “substantially and irremediably” qualifier. Thus, abortions would be allowed in cases in which the woman’s mental or physical health was threatened, even in cases in which the potential damage may be reversible.5
The facts
Misconceptions about abortion care can be dangerous and work to further stigmatize our patients who may need an abortion or who have had an abortion in the past. The American College of Obstetricians and Gynecologists (ACOG) recently published a document discussing facts regarding abortion care later in pregnancy. The document (aptly named “Facts are Important”) enforces that policy be based on medical science and facts, and not simply driven by political beliefs.6
Fact. The majority of abortions occur prior to 21 weeks, before viability:
- 91.1% of abortions occur at or before 13 weeks’ gestation7
- only 1.3% of abortions occur at or after 21 weeks’ gestation7
- abortions occurring later in the second trimester or in the third trimester are very uncommon.
Fact. The language “late-term abortion” has no medical definition, is not used in a clinical setting or to describe the delivery of abortion care later in pregnancy in any medical institution.6
Fact. Many of the abortions occurring later in pregnancy are due to fetal anomalies incompatible with life. Anomalies can include lack of a major portion of the brain (anencephaly), bilateral renal agenesis, some skeletal dysplasias, and other chromosomal abnormalities. These are cases in which death is likely before or shortly after birth, with great potential for suffering of both the fetus and the family.
Fact. The need for abortion also may be due to serious complications that will likely cause significant morbidity or mortality to the woman. These complications, in turn, reduce the likelihood of survival of the fetus.
It is thus vital for women to have the freedom to evaluate their medical circumstance with their provider and, using evidence, make informed health care decisions—which may include abortion, induction of labor, or cesarean delivery in some circumstances. Access to accurate, complete information and care is a right bestowed amongst all women and “must never be constrained by politicians.”6 We must focus on medically appropriate and compassionate care for both the family and the fetus.
Use your voice
As clinicians, we are trusted members of our communities. The New York law and the prior proposed Virginia law emphasize important access to care for women and their families. Abortions at a later gestational age are a rare event but are most often performed when the health or life of the mother is at risk or the fetus has an anomaly incompatible with life.
We urge you to use your voice to correct misconceptions, whether in your office with your patients or colleagues or in your communities, locally and nationally. Email your friends and colleagues about ACOG’s “Facts are Important” document, organize a grand rounds on the topic, and utilize social media to share facts about abortion care. These actions support our patients and can make an impact by spreading factual information.
For more facts and figures about abortion laws, visit the website of the Guttmacher Institute.
In 1973, the Supreme Court of the United States recognized a constitutional right to abortion in the landmark case of Roe v Wade. The Court held that states may regulate, but not ban, abortion after the first trimester, for the purpose of protecting the woman’s health. The Court further indicated that states’ interest in “potential life” could be the basis for abortion regulations only after the point of viability, at which point states may ban abortion except when necessary to preserve the life or health of the woman.1 In 1992, the Court decided Planned Parenthood v Casey and eliminated the trimester framework while upholding women’s right to abortion.2 As with Roe v Wade, the Casey decision held that there must be an exception for the woman’s health and life.
Fast forward to 2019
New York passed a law in 2019,3 and Virginia had a proposed law that was recently tabled by the House of Delegates,4 both related to abortions performed past the first trimester.
New York. The New York law supports legal abortion by a licensed practitioner within 24 weeks of pregnancy commencement. After 24 weeks’ gestation, if there is “an absence of fetal viability, or the abortion is necessary to protect the patient’s life or health” then termination is permissible.3
Virginia. Previously, Virginia had abortion laws that required significant measures to approve a third-trimester abortion, including certification by 3 physicians that the procedure is necessary to “save mother’s life or [prevent] substantial and irremediable impairment of mental or physical health of the mother.”5 Violation included potential for jail time and a significant monetary fine.
The proposed bill, now tabled, was introduced by delegate Kathy Tran (House Bill 2491) and would have rolled back many requirements of the old law, including the 24-hour waiting period and mandate for second-trimester abortions to occur in a hospital.
The controversy centered on a provision concerning third-trimester abortions. Specifically, the proposed bill would only have required 1 doctor to deem the abortion necessary and would have removed the “substantially and irremediably” qualifier. Thus, abortions would be allowed in cases in which the woman’s mental or physical health was threatened, even in cases in which the potential damage may be reversible.5
The facts
Misconceptions about abortion care can be dangerous and work to further stigmatize our patients who may need an abortion or who have had an abortion in the past. The American College of Obstetricians and Gynecologists (ACOG) recently published a document discussing facts regarding abortion care later in pregnancy. The document (aptly named “Facts are Important”) enforces that policy be based on medical science and facts, and not simply driven by political beliefs.6
Fact. The majority of abortions occur prior to 21 weeks, before viability:
- 91.1% of abortions occur at or before 13 weeks’ gestation7
- only 1.3% of abortions occur at or after 21 weeks’ gestation7
- abortions occurring later in the second trimester or in the third trimester are very uncommon.
Fact. The language “late-term abortion” has no medical definition, is not used in a clinical setting or to describe the delivery of abortion care later in pregnancy in any medical institution.6
Fact. Many of the abortions occurring later in pregnancy are due to fetal anomalies incompatible with life. Anomalies can include lack of a major portion of the brain (anencephaly), bilateral renal agenesis, some skeletal dysplasias, and other chromosomal abnormalities. These are cases in which death is likely before or shortly after birth, with great potential for suffering of both the fetus and the family.
Fact. The need for abortion also may be due to serious complications that will likely cause significant morbidity or mortality to the woman. These complications, in turn, reduce the likelihood of survival of the fetus.
It is thus vital for women to have the freedom to evaluate their medical circumstance with their provider and, using evidence, make informed health care decisions—which may include abortion, induction of labor, or cesarean delivery in some circumstances. Access to accurate, complete information and care is a right bestowed amongst all women and “must never be constrained by politicians.”6 We must focus on medically appropriate and compassionate care for both the family and the fetus.
Use your voice
As clinicians, we are trusted members of our communities. The New York law and the prior proposed Virginia law emphasize important access to care for women and their families. Abortions at a later gestational age are a rare event but are most often performed when the health or life of the mother is at risk or the fetus has an anomaly incompatible with life.
We urge you to use your voice to correct misconceptions, whether in your office with your patients or colleagues or in your communities, locally and nationally. Email your friends and colleagues about ACOG’s “Facts are Important” document, organize a grand rounds on the topic, and utilize social media to share facts about abortion care. These actions support our patients and can make an impact by spreading factual information.
For more facts and figures about abortion laws, visit the website of the Guttmacher Institute.
- Roe v Wade, 410 US 113 (1973).
- Planned Parenthood v Casey, 505 US 833 (1992).
- New York abortion laws. FindLaw website. https://statelaws.findlaw.com/new-york-law/new-york-abortion-laws.html. Accessed March 7, 2019.
- North A. The controversy around Virginia’s new abortion bill, explained. https://www.vox.com/2019/2/1/18205428/virginia-abortion-bill-kathy-tran-ralph-northam Accessed March 13, 2019.
- Virginia abortion laws. FindLaw website. https://statelaws.findlaw.com/virginia-law/virginia-abortion-laws.html. Accessed March 7, 2019.
- Facts are important. The American College of Obstetricians and Gynecologists website. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/Facts-Are-Important_Abortion-Care-Later-In-Pregnancy-February-2019-College.pdf?dmc=1&ts=20190214T2242210541. Accessed March 7, 2019.
- Jatlaoui TC, Boutot ME, Mandel MG, et al. Abortion surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(13):1-45.
- Roe v Wade, 410 US 113 (1973).
- Planned Parenthood v Casey, 505 US 833 (1992).
- New York abortion laws. FindLaw website. https://statelaws.findlaw.com/new-york-law/new-york-abortion-laws.html. Accessed March 7, 2019.
- North A. The controversy around Virginia’s new abortion bill, explained. https://www.vox.com/2019/2/1/18205428/virginia-abortion-bill-kathy-tran-ralph-northam Accessed March 13, 2019.
- Virginia abortion laws. FindLaw website. https://statelaws.findlaw.com/virginia-law/virginia-abortion-laws.html. Accessed March 7, 2019.
- Facts are important. The American College of Obstetricians and Gynecologists website. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/Facts-Are-Important_Abortion-Care-Later-In-Pregnancy-February-2019-College.pdf?dmc=1&ts=20190214T2242210541. Accessed March 7, 2019.
- Jatlaoui TC, Boutot ME, Mandel MG, et al. Abortion surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(13):1-45.