User login
Myocarditis higher with Moderna COVID vax in young men
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Bariatric surgery cuts cardiovascular events, even in seniors
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Combo of SGLT2 inhibitor + GLP-1 RA boosts diabetes survival
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
AT ACC 2022
New smart device shows highly accurate AFib detection: mAFA II
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Empagliflozin rapidly improves acute heart failure symptoms in hospitalized patients
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
AT ACC 2022
TAVI device shows less deterioration than surgery 5 years out
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
FROM ACC 2022
Surgeons in China ‘are the executioners,’ procuring organs before brain death
In a deep dive into obscure Chinese language transplant journals, a pair of researchers from Australia and Israel have added a new layer of horror to what’s already known about forced organ harvesting in China.
Searching for documentation that vital organs are being harvested from nonconsenting executed prisoners, a practice that the China Tribunal confirmed “beyond any reasonable doubt” in 2020, Jacob Lavee, MD, an Israeli heart transplant surgeon, and Matthew Roberston, a PhD student at Australian National University, uncovered something even more shocking: that vital organs are being explanted from patients who are still alive.
“We have shown for the first time that the transplant surgeons are the executioners – that the mode of execution is organ procurement. These are self-admissions of executing the patient,” Dr. Lavee told this news organization. “Up until now, there has been what we call circumstantial evidence of this, but our paper is what you’d call the smoking gun, because it’s in the words of the physicians themselves that they are doing it. In the words of these surgeons, intubation was done only after the beginning of surgery, which means the patients were breathing spontaneously up until the moment the operation started ... meaning they were not brain dead.”
The research, published in the American Journal of Transplantation, involved intricate analysis of thousands of Chinese language transplant articles and identified 71 articles in which transplant surgeons describe starting organ procurement surgery before declaring their patients brain dead.
“What we found were improper, illegitimate, nonexistent, or false declarations of brain death,” Mr. Robertson said in an interview. He explained that this violates what’s known as the dead donor rule, which is fundamental in transplant ethics. “The surgeons wrote that the donor was brain dead, but according to everything we know about medical science, they could not possibly have been brain dead because there was no apnea test performed. Brain death is not just something you say, there’s this whole battery of tests, and the key is the apnea test, [in which] the patient is already intubated and ventilated, they turn the machine off, and they’re looking for carbon dioxide in the blood above a certain level.”
Mr. Robertson and Dr. Lavee have painstakingly documented “incriminating sentences” in each of the 71 articles proving that brain death had not occurred before the organ explantation procedure began. “There were two criteria by which we claimed a problematic brain death declaration,” said Mr. Robertson, who translated the Chinese. “One was where the patient was not ventilated and was only intubated after they were declared brain dead; the other was that the intubation took place immediately prior to the surgery beginning.”
“It was mind-boggling,” said Dr. Lavee, from Tel Aviv University. “When I first started reading, my initial reaction is, ‘This can’t be.’ I read it once, and again, and I insisted that Matt get another independent translation of the Chinese just to be sure. I told him, ‘There’s no way a physician, a surgeon could write this – it doesn’t make sense.’ But the more of these papers we read, we saw it was a pattern – and they didn’t come out of a single medical center, they are spread all over China.”
For the analysis, Mr. Robertson wrote code and customized an algorithm to examine 124,770 medical articles from official Chinese databases between 1980 and 2020. The 71 articles revealing cases involving problematic brain death came from 56 hospitals (of which 12 were military) in 33 cities across 15 provinces, they report. In total, 348 surgeons, nurses, anesthesiologists, and other medical workers or researchers were listed as authors of these publications.
Why would these medical personnel write such self-incriminating evidence? The researchers say it’s unclear. “They don’t think anyone’s reading this stuff,” Mr. Robertson suggests. “Sometimes it’s revealed in just five or six characters in a paper of eight pages.” Dr. Lavee wonders if it’s also ignorance. “If this has been a practice for 20 or 30 years in China, I guess nobody at that time was aware they were doing something wrong, although how to declare brain death is something that is known in China. They’ve published a lot about it.”
The article is “evidence that this barbarity continues and is a very valuable contribution that continues to bring attention to an enormous human rights violation,” said Arthur Caplan, PhD, head of the Division of Medical Ethics at New York University’s Grossman School of Medicine. “What they’ve reported has been going on for many, many years, the data are very clear that China’s doing many more transplants than they have cadaver organ donors,” he said, adding that the country’s well-documented and lucrative involvement in transplant tourism “means you have to have a donor ready when the would-be recipient appears; you have to have a matched organ available, and that’s hard to do waiting on a cadaver donor.”
Although the researchers found no incriminating publications after 2015, they speculate that this is likely due to growing awareness among Chinese surgeons that publishing the information would attract international condemnation. “We think these practices are continuing to go on,” said Dr. Lavee. He acknowledged that a voluntary organ donation program is slowly developing in parallel to this. He said, given China’s place as the world’s second largest transplant country behind the U.S., as well as its low rate of voluntary donation, it’s reasonable to conclude that the main source of organs remains prisoners on death row.
Dr. Caplan and the researchers have called for academic institutions and medical journals to resume their previous boycotts of Chinese transplant publications and speakers, but as long as China denies the practices, economic and political leaders will turn a blind eye. “In the past, I don’t think the question of China’s medical professional involvement in the execution of donors has been taken as seriously as it should have,” said Mr. Robertson. “I certainly hope that with the publication of this paper in the leading journal in the field, this will change.”
The study was supported by the Google Cloud Research Credits program, the Australian Government Research Training Program Scholarship, and the Victims of Communism Memorial Foundation. Mr. Robertson, Dr. Lavee, and Dr. Caplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a deep dive into obscure Chinese language transplant journals, a pair of researchers from Australia and Israel have added a new layer of horror to what’s already known about forced organ harvesting in China.
Searching for documentation that vital organs are being harvested from nonconsenting executed prisoners, a practice that the China Tribunal confirmed “beyond any reasonable doubt” in 2020, Jacob Lavee, MD, an Israeli heart transplant surgeon, and Matthew Roberston, a PhD student at Australian National University, uncovered something even more shocking: that vital organs are being explanted from patients who are still alive.
“We have shown for the first time that the transplant surgeons are the executioners – that the mode of execution is organ procurement. These are self-admissions of executing the patient,” Dr. Lavee told this news organization. “Up until now, there has been what we call circumstantial evidence of this, but our paper is what you’d call the smoking gun, because it’s in the words of the physicians themselves that they are doing it. In the words of these surgeons, intubation was done only after the beginning of surgery, which means the patients were breathing spontaneously up until the moment the operation started ... meaning they were not brain dead.”
The research, published in the American Journal of Transplantation, involved intricate analysis of thousands of Chinese language transplant articles and identified 71 articles in which transplant surgeons describe starting organ procurement surgery before declaring their patients brain dead.
“What we found were improper, illegitimate, nonexistent, or false declarations of brain death,” Mr. Robertson said in an interview. He explained that this violates what’s known as the dead donor rule, which is fundamental in transplant ethics. “The surgeons wrote that the donor was brain dead, but according to everything we know about medical science, they could not possibly have been brain dead because there was no apnea test performed. Brain death is not just something you say, there’s this whole battery of tests, and the key is the apnea test, [in which] the patient is already intubated and ventilated, they turn the machine off, and they’re looking for carbon dioxide in the blood above a certain level.”
Mr. Robertson and Dr. Lavee have painstakingly documented “incriminating sentences” in each of the 71 articles proving that brain death had not occurred before the organ explantation procedure began. “There were two criteria by which we claimed a problematic brain death declaration,” said Mr. Robertson, who translated the Chinese. “One was where the patient was not ventilated and was only intubated after they were declared brain dead; the other was that the intubation took place immediately prior to the surgery beginning.”
“It was mind-boggling,” said Dr. Lavee, from Tel Aviv University. “When I first started reading, my initial reaction is, ‘This can’t be.’ I read it once, and again, and I insisted that Matt get another independent translation of the Chinese just to be sure. I told him, ‘There’s no way a physician, a surgeon could write this – it doesn’t make sense.’ But the more of these papers we read, we saw it was a pattern – and they didn’t come out of a single medical center, they are spread all over China.”
For the analysis, Mr. Robertson wrote code and customized an algorithm to examine 124,770 medical articles from official Chinese databases between 1980 and 2020. The 71 articles revealing cases involving problematic brain death came from 56 hospitals (of which 12 were military) in 33 cities across 15 provinces, they report. In total, 348 surgeons, nurses, anesthesiologists, and other medical workers or researchers were listed as authors of these publications.
Why would these medical personnel write such self-incriminating evidence? The researchers say it’s unclear. “They don’t think anyone’s reading this stuff,” Mr. Robertson suggests. “Sometimes it’s revealed in just five or six characters in a paper of eight pages.” Dr. Lavee wonders if it’s also ignorance. “If this has been a practice for 20 or 30 years in China, I guess nobody at that time was aware they were doing something wrong, although how to declare brain death is something that is known in China. They’ve published a lot about it.”
The article is “evidence that this barbarity continues and is a very valuable contribution that continues to bring attention to an enormous human rights violation,” said Arthur Caplan, PhD, head of the Division of Medical Ethics at New York University’s Grossman School of Medicine. “What they’ve reported has been going on for many, many years, the data are very clear that China’s doing many more transplants than they have cadaver organ donors,” he said, adding that the country’s well-documented and lucrative involvement in transplant tourism “means you have to have a donor ready when the would-be recipient appears; you have to have a matched organ available, and that’s hard to do waiting on a cadaver donor.”
Although the researchers found no incriminating publications after 2015, they speculate that this is likely due to growing awareness among Chinese surgeons that publishing the information would attract international condemnation. “We think these practices are continuing to go on,” said Dr. Lavee. He acknowledged that a voluntary organ donation program is slowly developing in parallel to this. He said, given China’s place as the world’s second largest transplant country behind the U.S., as well as its low rate of voluntary donation, it’s reasonable to conclude that the main source of organs remains prisoners on death row.
Dr. Caplan and the researchers have called for academic institutions and medical journals to resume their previous boycotts of Chinese transplant publications and speakers, but as long as China denies the practices, economic and political leaders will turn a blind eye. “In the past, I don’t think the question of China’s medical professional involvement in the execution of donors has been taken as seriously as it should have,” said Mr. Robertson. “I certainly hope that with the publication of this paper in the leading journal in the field, this will change.”
The study was supported by the Google Cloud Research Credits program, the Australian Government Research Training Program Scholarship, and the Victims of Communism Memorial Foundation. Mr. Robertson, Dr. Lavee, and Dr. Caplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a deep dive into obscure Chinese language transplant journals, a pair of researchers from Australia and Israel have added a new layer of horror to what’s already known about forced organ harvesting in China.
Searching for documentation that vital organs are being harvested from nonconsenting executed prisoners, a practice that the China Tribunal confirmed “beyond any reasonable doubt” in 2020, Jacob Lavee, MD, an Israeli heart transplant surgeon, and Matthew Roberston, a PhD student at Australian National University, uncovered something even more shocking: that vital organs are being explanted from patients who are still alive.
“We have shown for the first time that the transplant surgeons are the executioners – that the mode of execution is organ procurement. These are self-admissions of executing the patient,” Dr. Lavee told this news organization. “Up until now, there has been what we call circumstantial evidence of this, but our paper is what you’d call the smoking gun, because it’s in the words of the physicians themselves that they are doing it. In the words of these surgeons, intubation was done only after the beginning of surgery, which means the patients were breathing spontaneously up until the moment the operation started ... meaning they were not brain dead.”
The research, published in the American Journal of Transplantation, involved intricate analysis of thousands of Chinese language transplant articles and identified 71 articles in which transplant surgeons describe starting organ procurement surgery before declaring their patients brain dead.
“What we found were improper, illegitimate, nonexistent, or false declarations of brain death,” Mr. Robertson said in an interview. He explained that this violates what’s known as the dead donor rule, which is fundamental in transplant ethics. “The surgeons wrote that the donor was brain dead, but according to everything we know about medical science, they could not possibly have been brain dead because there was no apnea test performed. Brain death is not just something you say, there’s this whole battery of tests, and the key is the apnea test, [in which] the patient is already intubated and ventilated, they turn the machine off, and they’re looking for carbon dioxide in the blood above a certain level.”
Mr. Robertson and Dr. Lavee have painstakingly documented “incriminating sentences” in each of the 71 articles proving that brain death had not occurred before the organ explantation procedure began. “There were two criteria by which we claimed a problematic brain death declaration,” said Mr. Robertson, who translated the Chinese. “One was where the patient was not ventilated and was only intubated after they were declared brain dead; the other was that the intubation took place immediately prior to the surgery beginning.”
“It was mind-boggling,” said Dr. Lavee, from Tel Aviv University. “When I first started reading, my initial reaction is, ‘This can’t be.’ I read it once, and again, and I insisted that Matt get another independent translation of the Chinese just to be sure. I told him, ‘There’s no way a physician, a surgeon could write this – it doesn’t make sense.’ But the more of these papers we read, we saw it was a pattern – and they didn’t come out of a single medical center, they are spread all over China.”
For the analysis, Mr. Robertson wrote code and customized an algorithm to examine 124,770 medical articles from official Chinese databases between 1980 and 2020. The 71 articles revealing cases involving problematic brain death came from 56 hospitals (of which 12 were military) in 33 cities across 15 provinces, they report. In total, 348 surgeons, nurses, anesthesiologists, and other medical workers or researchers were listed as authors of these publications.
Why would these medical personnel write such self-incriminating evidence? The researchers say it’s unclear. “They don’t think anyone’s reading this stuff,” Mr. Robertson suggests. “Sometimes it’s revealed in just five or six characters in a paper of eight pages.” Dr. Lavee wonders if it’s also ignorance. “If this has been a practice for 20 or 30 years in China, I guess nobody at that time was aware they were doing something wrong, although how to declare brain death is something that is known in China. They’ve published a lot about it.”
The article is “evidence that this barbarity continues and is a very valuable contribution that continues to bring attention to an enormous human rights violation,” said Arthur Caplan, PhD, head of the Division of Medical Ethics at New York University’s Grossman School of Medicine. “What they’ve reported has been going on for many, many years, the data are very clear that China’s doing many more transplants than they have cadaver organ donors,” he said, adding that the country’s well-documented and lucrative involvement in transplant tourism “means you have to have a donor ready when the would-be recipient appears; you have to have a matched organ available, and that’s hard to do waiting on a cadaver donor.”
Although the researchers found no incriminating publications after 2015, they speculate that this is likely due to growing awareness among Chinese surgeons that publishing the information would attract international condemnation. “We think these practices are continuing to go on,” said Dr. Lavee. He acknowledged that a voluntary organ donation program is slowly developing in parallel to this. He said, given China’s place as the world’s second largest transplant country behind the U.S., as well as its low rate of voluntary donation, it’s reasonable to conclude that the main source of organs remains prisoners on death row.
Dr. Caplan and the researchers have called for academic institutions and medical journals to resume their previous boycotts of Chinese transplant publications and speakers, but as long as China denies the practices, economic and political leaders will turn a blind eye. “In the past, I don’t think the question of China’s medical professional involvement in the execution of donors has been taken as seriously as it should have,” said Mr. Robertson. “I certainly hope that with the publication of this paper in the leading journal in the field, this will change.”
The study was supported by the Google Cloud Research Credits program, the Australian Government Research Training Program Scholarship, and the Victims of Communism Memorial Foundation. Mr. Robertson, Dr. Lavee, and Dr. Caplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DIAMOND: Adding patiromer helps optimize HF meds, foils hyperkalemia
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.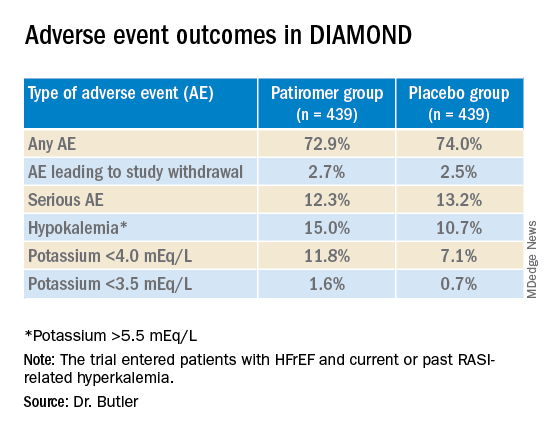
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Extraction of infected implanted cardiac devices rare, despite guidelines
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
FROM ACC 2022
Flu vaccines cut seasonal death in heart failure patients
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
AT ACC 2022