User login
Cardiovascular complications most common with carfilzomib in relapsed myeloma
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
NSAIDs a significant mediator of cardiovascular risk in osteoarthritis
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
FROM ARTHRITIS & RHEUMATOLOGY
Entresto, inpatient therapy, and surrogate markers
The recently published PIONEER-HF study attempts to move sacubitril/valsartan (Entresto) therapy to the inpatient environment to improve patient and physician acceptance of this therapy for patients with heart failure (N Engl J Med. 2019 Feb 7;380;539-48).
When given to outpatients in the PARADIGM-HF trial, the combination was superior to enalapril for reducing the risks of death and hospitalization for heart failure (N Engl J Med 2014;371:993-1004.) Specifically, sacubitril/valsartan decreased mortality by 15% and hospitalization by 21% as an outpatient therapy for patients with systolic heart failure. Nevertheless, there has not been widespread adoption of this approach. It is well known that , one of the first drugs shown to be effective in heart failure therapy (Entresto costs more than $4,000 per year; enalapril costs about $120 per year).
The investigators in the PIONEER-HF study compared Entresto to enalapril over a 2-month period in patients hospitalized with systolic heart failure. To accelerate the trial, the investigators used the proportional change in patients’ N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels as the primary endpoint rather than the traditional outcome of morbidity and mortality. In the short term, no significant clinical benefits were observed, but there was a significant decrease in NT-proBNP of about 30% (P less than .001).
The investigators suggested that this finding extended the previous benefit observed with Entresto during outpatient initiation and could be used as a rationale for initiating Entresto therapy in the hospital. This earlier application of the therapy could make the drug more widely acceptable.
Considerable investigation in BNP measurement has occurred over the last few years, and although it is clear that BNP is elevated in heart failure patients, there is no evidence to confirm that the decrease in BNP is associated with improved outcome. BNP will fall with decrease in ventricular volume, which may have significant physiologic mechanisms but ventricular volume could decrease with fall in blood pressure that may have occurred in this population since hypotension tended to be more frequent with Entresto than with enalapril. The traditional measure of heart failure benefit with beta-blockers, ACE inhibitors, and aldosterone antagonists in the inpatient and early postdischarge period has depended on clinical outcomes.
Regardless of the physiologic explanation of this fall in BNP, we must pause in our assumptions when a surrogate measure is used to assess clinical benefit as inpatient therapy. The Food and Drug Administration has long given up using surrogate measures as proof of efficacy, and rightly so. Clinical medicine is replete with dubious drug benefits based on surrogate measures. Let’s not forget that only a few years ago suppression of premature ventricular contractions was considered to be a measure of the pharmacologic prevention of sudden death. We have come a long way from that and other clinical missteps to use BNP, an uncertain marker at best of clinical improvement, as a surrogate for the improvement in heart failure.
There is a substantial amount of data supporting the benefit of Entresto in the clinical management of outpatients with heart failure without using the PIONEER-HF trial results as a pretense to initiate therapy when patients are hospitalized. One might suggest that if Novartis is concerned about introducing the drug in the clinical management of heart failure, the company might consider the possibility of decreasing its price.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
The recently published PIONEER-HF study attempts to move sacubitril/valsartan (Entresto) therapy to the inpatient environment to improve patient and physician acceptance of this therapy for patients with heart failure (N Engl J Med. 2019 Feb 7;380;539-48).
When given to outpatients in the PARADIGM-HF trial, the combination was superior to enalapril for reducing the risks of death and hospitalization for heart failure (N Engl J Med 2014;371:993-1004.) Specifically, sacubitril/valsartan decreased mortality by 15% and hospitalization by 21% as an outpatient therapy for patients with systolic heart failure. Nevertheless, there has not been widespread adoption of this approach. It is well known that , one of the first drugs shown to be effective in heart failure therapy (Entresto costs more than $4,000 per year; enalapril costs about $120 per year).
The investigators in the PIONEER-HF study compared Entresto to enalapril over a 2-month period in patients hospitalized with systolic heart failure. To accelerate the trial, the investigators used the proportional change in patients’ N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels as the primary endpoint rather than the traditional outcome of morbidity and mortality. In the short term, no significant clinical benefits were observed, but there was a significant decrease in NT-proBNP of about 30% (P less than .001).
The investigators suggested that this finding extended the previous benefit observed with Entresto during outpatient initiation and could be used as a rationale for initiating Entresto therapy in the hospital. This earlier application of the therapy could make the drug more widely acceptable.
Considerable investigation in BNP measurement has occurred over the last few years, and although it is clear that BNP is elevated in heart failure patients, there is no evidence to confirm that the decrease in BNP is associated with improved outcome. BNP will fall with decrease in ventricular volume, which may have significant physiologic mechanisms but ventricular volume could decrease with fall in blood pressure that may have occurred in this population since hypotension tended to be more frequent with Entresto than with enalapril. The traditional measure of heart failure benefit with beta-blockers, ACE inhibitors, and aldosterone antagonists in the inpatient and early postdischarge period has depended on clinical outcomes.
Regardless of the physiologic explanation of this fall in BNP, we must pause in our assumptions when a surrogate measure is used to assess clinical benefit as inpatient therapy. The Food and Drug Administration has long given up using surrogate measures as proof of efficacy, and rightly so. Clinical medicine is replete with dubious drug benefits based on surrogate measures. Let’s not forget that only a few years ago suppression of premature ventricular contractions was considered to be a measure of the pharmacologic prevention of sudden death. We have come a long way from that and other clinical missteps to use BNP, an uncertain marker at best of clinical improvement, as a surrogate for the improvement in heart failure.
There is a substantial amount of data supporting the benefit of Entresto in the clinical management of outpatients with heart failure without using the PIONEER-HF trial results as a pretense to initiate therapy when patients are hospitalized. One might suggest that if Novartis is concerned about introducing the drug in the clinical management of heart failure, the company might consider the possibility of decreasing its price.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
The recently published PIONEER-HF study attempts to move sacubitril/valsartan (Entresto) therapy to the inpatient environment to improve patient and physician acceptance of this therapy for patients with heart failure (N Engl J Med. 2019 Feb 7;380;539-48).
When given to outpatients in the PARADIGM-HF trial, the combination was superior to enalapril for reducing the risks of death and hospitalization for heart failure (N Engl J Med 2014;371:993-1004.) Specifically, sacubitril/valsartan decreased mortality by 15% and hospitalization by 21% as an outpatient therapy for patients with systolic heart failure. Nevertheless, there has not been widespread adoption of this approach. It is well known that , one of the first drugs shown to be effective in heart failure therapy (Entresto costs more than $4,000 per year; enalapril costs about $120 per year).
The investigators in the PIONEER-HF study compared Entresto to enalapril over a 2-month period in patients hospitalized with systolic heart failure. To accelerate the trial, the investigators used the proportional change in patients’ N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels as the primary endpoint rather than the traditional outcome of morbidity and mortality. In the short term, no significant clinical benefits were observed, but there was a significant decrease in NT-proBNP of about 30% (P less than .001).
The investigators suggested that this finding extended the previous benefit observed with Entresto during outpatient initiation and could be used as a rationale for initiating Entresto therapy in the hospital. This earlier application of the therapy could make the drug more widely acceptable.
Considerable investigation in BNP measurement has occurred over the last few years, and although it is clear that BNP is elevated in heart failure patients, there is no evidence to confirm that the decrease in BNP is associated with improved outcome. BNP will fall with decrease in ventricular volume, which may have significant physiologic mechanisms but ventricular volume could decrease with fall in blood pressure that may have occurred in this population since hypotension tended to be more frequent with Entresto than with enalapril. The traditional measure of heart failure benefit with beta-blockers, ACE inhibitors, and aldosterone antagonists in the inpatient and early postdischarge period has depended on clinical outcomes.
Regardless of the physiologic explanation of this fall in BNP, we must pause in our assumptions when a surrogate measure is used to assess clinical benefit as inpatient therapy. The Food and Drug Administration has long given up using surrogate measures as proof of efficacy, and rightly so. Clinical medicine is replete with dubious drug benefits based on surrogate measures. Let’s not forget that only a few years ago suppression of premature ventricular contractions was considered to be a measure of the pharmacologic prevention of sudden death. We have come a long way from that and other clinical missteps to use BNP, an uncertain marker at best of clinical improvement, as a surrogate for the improvement in heart failure.
There is a substantial amount of data supporting the benefit of Entresto in the clinical management of outpatients with heart failure without using the PIONEER-HF trial results as a pretense to initiate therapy when patients are hospitalized. One might suggest that if Novartis is concerned about introducing the drug in the clinical management of heart failure, the company might consider the possibility of decreasing its price.
Dr. Goldstein is professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit.
Meeting just 2 of 7 ‘simple’ goals lowers HF risk
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
FROM JACC: HEART FAILURE
Higher omega-3 fatty acid levels cut heart failure risk
Higher levels of eicosapentaenoic acid, a type of omega-3 polyunsaturated fatty acid, were associated with a significantly reduced risk of heart failure in a large, multi-ethnic cohort of adults in the United States.
Despite the potential benefits of omega-3s eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) for heart health, their use has been controversial, although data in a mouse model showed that dietary EPA was protective against heart failure, wrote Robert C. Block, MD, of the University of Rochester (N.Y.), and colleagues. Their report is in the Journal of the American College of Cardiology.
To examine the impact of EPA on heart failure in humans, the researchers used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a longitudinal cohort study of U.S. adults, including those who are African American, Hispanic, Asian, and white.
The researchers included 6,562 MESA participants aged 45-84 years from six communities. Participants underwent a baseline exam between July 2000 and July 2002 that included phospholipid measurements used to identify plasma EPA percentage, and they completed study visits approximately every other year for a median follow-up of 13 years.
A total of 292 heart failure events occurred during the follow-up period: 128 with reduced ejection fraction (EF less than 45%), 110 with preserved ejection fraction (EF at least 45%), and 54 with unknown EF status.
The percent EPA for individuals without heart failure was significantly higher compared with those with heart failure (0.76% vs. 0.69%, P =.005). The association remained significant after the researchers controlled for age, sex, race, body mass index, smoking, diabetes, blood pressure, lipids and lipid-lowering drugs, albuminuria, and the lead fatty acid (defined as the fatty acid with the largest in-cluster correlation).
An EPA level greater than 2.5% was considered sufficient to prevent heart failure based on prior definitions. A total of 73% of the participants had insufficient EPA (less than 1.0%), 2.4% had marginal levels (1.0%-2.5%), and 4.5% had sufficient levels. However, given that EPA levels can be easily and safely increased with the consumption of seafood or fish oil capsules, increasing EPA is a feasible heart failure prevention strategy, the researchers said.
The study included 2,532 white, 1,794 black, 1,442 Hispanic, and 794 Chinese participants. Overall, the fewest Hispanic participants met the criteria for sufficient EPA (1.4%), followed by black (4.4%), white (4.9%), and Chinese participants (9.8%).
The study findings were limited by several factors, including relatively few participants with preserved ejection fractions and sufficient EPA levels, as well as the inability to account for changes in omega-3 levels and other risk factors over time, the researchers noted.
“We consider this study to strongly determine a benefit of EPA exists, but insufficient to determine whether a threshold for %EPA exists near 3%,” they said. They proposed a follow-up study including individuals with higher levels of EPA to better detect a protective effect.
Lead author Dr. Block had no financial conflicts to disclose. Several coauthors received honoraria from Amarin Pharmaceuticals. The study was funded in part by the National Heart, Lung, and Blood Institute.
The study findings suggest that revisiting omega-3 fatty acids to improve outcomes in patients with or at risk of cardiovascular disease may be worthwhile. Not only did the study predict heart failure in a range of ethnicities, but the same authors showed previously in animal models that these dietary supplements can preserve left ventricular function and reduce interstitial fibrosis.
The question is: Is it sufficient to give dietary recommendations of an increased fish consumption, or do we need to take purified pharmaceutical supplements such as those tested in trials? In other words, shall we have to go to the fish market or to the pharmacy to elevate our circulating levels of omega-3 fatty acids and, in this way, to try to prevent (or treat) HF?
The answer, at least in part, lies in additional large, randomized clinical trials that test high doses of omega-3 fatty acids along and combined with pharmacological and nonpharmacological treatments. Considering the very favorable tolerability and safety profile of this therapeutic approach, any positive results of these trials could provide us with an additional strategy to improve the outcomes of patients with HF or at high risk to develop it.
Aldo P. Maggioni, MD, of the ANMCO Research Center Heart Care Foundation, in Florence, Italy, made these remarks in an editorial. He disclosed honoraria for participation in committees of studies sponsored by Bayer, Novartis, and Fresenius.
The study findings suggest that revisiting omega-3 fatty acids to improve outcomes in patients with or at risk of cardiovascular disease may be worthwhile. Not only did the study predict heart failure in a range of ethnicities, but the same authors showed previously in animal models that these dietary supplements can preserve left ventricular function and reduce interstitial fibrosis.
The question is: Is it sufficient to give dietary recommendations of an increased fish consumption, or do we need to take purified pharmaceutical supplements such as those tested in trials? In other words, shall we have to go to the fish market or to the pharmacy to elevate our circulating levels of omega-3 fatty acids and, in this way, to try to prevent (or treat) HF?
The answer, at least in part, lies in additional large, randomized clinical trials that test high doses of omega-3 fatty acids along and combined with pharmacological and nonpharmacological treatments. Considering the very favorable tolerability and safety profile of this therapeutic approach, any positive results of these trials could provide us with an additional strategy to improve the outcomes of patients with HF or at high risk to develop it.
Aldo P. Maggioni, MD, of the ANMCO Research Center Heart Care Foundation, in Florence, Italy, made these remarks in an editorial. He disclosed honoraria for participation in committees of studies sponsored by Bayer, Novartis, and Fresenius.
The study findings suggest that revisiting omega-3 fatty acids to improve outcomes in patients with or at risk of cardiovascular disease may be worthwhile. Not only did the study predict heart failure in a range of ethnicities, but the same authors showed previously in animal models that these dietary supplements can preserve left ventricular function and reduce interstitial fibrosis.
The question is: Is it sufficient to give dietary recommendations of an increased fish consumption, or do we need to take purified pharmaceutical supplements such as those tested in trials? In other words, shall we have to go to the fish market or to the pharmacy to elevate our circulating levels of omega-3 fatty acids and, in this way, to try to prevent (or treat) HF?
The answer, at least in part, lies in additional large, randomized clinical trials that test high doses of omega-3 fatty acids along and combined with pharmacological and nonpharmacological treatments. Considering the very favorable tolerability and safety profile of this therapeutic approach, any positive results of these trials could provide us with an additional strategy to improve the outcomes of patients with HF or at high risk to develop it.
Aldo P. Maggioni, MD, of the ANMCO Research Center Heart Care Foundation, in Florence, Italy, made these remarks in an editorial. He disclosed honoraria for participation in committees of studies sponsored by Bayer, Novartis, and Fresenius.
Higher levels of eicosapentaenoic acid, a type of omega-3 polyunsaturated fatty acid, were associated with a significantly reduced risk of heart failure in a large, multi-ethnic cohort of adults in the United States.
Despite the potential benefits of omega-3s eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) for heart health, their use has been controversial, although data in a mouse model showed that dietary EPA was protective against heart failure, wrote Robert C. Block, MD, of the University of Rochester (N.Y.), and colleagues. Their report is in the Journal of the American College of Cardiology.
To examine the impact of EPA on heart failure in humans, the researchers used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a longitudinal cohort study of U.S. adults, including those who are African American, Hispanic, Asian, and white.
The researchers included 6,562 MESA participants aged 45-84 years from six communities. Participants underwent a baseline exam between July 2000 and July 2002 that included phospholipid measurements used to identify plasma EPA percentage, and they completed study visits approximately every other year for a median follow-up of 13 years.
A total of 292 heart failure events occurred during the follow-up period: 128 with reduced ejection fraction (EF less than 45%), 110 with preserved ejection fraction (EF at least 45%), and 54 with unknown EF status.
The percent EPA for individuals without heart failure was significantly higher compared with those with heart failure (0.76% vs. 0.69%, P =.005). The association remained significant after the researchers controlled for age, sex, race, body mass index, smoking, diabetes, blood pressure, lipids and lipid-lowering drugs, albuminuria, and the lead fatty acid (defined as the fatty acid with the largest in-cluster correlation).
An EPA level greater than 2.5% was considered sufficient to prevent heart failure based on prior definitions. A total of 73% of the participants had insufficient EPA (less than 1.0%), 2.4% had marginal levels (1.0%-2.5%), and 4.5% had sufficient levels. However, given that EPA levels can be easily and safely increased with the consumption of seafood or fish oil capsules, increasing EPA is a feasible heart failure prevention strategy, the researchers said.
The study included 2,532 white, 1,794 black, 1,442 Hispanic, and 794 Chinese participants. Overall, the fewest Hispanic participants met the criteria for sufficient EPA (1.4%), followed by black (4.4%), white (4.9%), and Chinese participants (9.8%).
The study findings were limited by several factors, including relatively few participants with preserved ejection fractions and sufficient EPA levels, as well as the inability to account for changes in omega-3 levels and other risk factors over time, the researchers noted.
“We consider this study to strongly determine a benefit of EPA exists, but insufficient to determine whether a threshold for %EPA exists near 3%,” they said. They proposed a follow-up study including individuals with higher levels of EPA to better detect a protective effect.
Lead author Dr. Block had no financial conflicts to disclose. Several coauthors received honoraria from Amarin Pharmaceuticals. The study was funded in part by the National Heart, Lung, and Blood Institute.
Higher levels of eicosapentaenoic acid, a type of omega-3 polyunsaturated fatty acid, were associated with a significantly reduced risk of heart failure in a large, multi-ethnic cohort of adults in the United States.
Despite the potential benefits of omega-3s eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) for heart health, their use has been controversial, although data in a mouse model showed that dietary EPA was protective against heart failure, wrote Robert C. Block, MD, of the University of Rochester (N.Y.), and colleagues. Their report is in the Journal of the American College of Cardiology.
To examine the impact of EPA on heart failure in humans, the researchers used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a longitudinal cohort study of U.S. adults, including those who are African American, Hispanic, Asian, and white.
The researchers included 6,562 MESA participants aged 45-84 years from six communities. Participants underwent a baseline exam between July 2000 and July 2002 that included phospholipid measurements used to identify plasma EPA percentage, and they completed study visits approximately every other year for a median follow-up of 13 years.
A total of 292 heart failure events occurred during the follow-up period: 128 with reduced ejection fraction (EF less than 45%), 110 with preserved ejection fraction (EF at least 45%), and 54 with unknown EF status.
The percent EPA for individuals without heart failure was significantly higher compared with those with heart failure (0.76% vs. 0.69%, P =.005). The association remained significant after the researchers controlled for age, sex, race, body mass index, smoking, diabetes, blood pressure, lipids and lipid-lowering drugs, albuminuria, and the lead fatty acid (defined as the fatty acid with the largest in-cluster correlation).
An EPA level greater than 2.5% was considered sufficient to prevent heart failure based on prior definitions. A total of 73% of the participants had insufficient EPA (less than 1.0%), 2.4% had marginal levels (1.0%-2.5%), and 4.5% had sufficient levels. However, given that EPA levels can be easily and safely increased with the consumption of seafood or fish oil capsules, increasing EPA is a feasible heart failure prevention strategy, the researchers said.
The study included 2,532 white, 1,794 black, 1,442 Hispanic, and 794 Chinese participants. Overall, the fewest Hispanic participants met the criteria for sufficient EPA (1.4%), followed by black (4.4%), white (4.9%), and Chinese participants (9.8%).
The study findings were limited by several factors, including relatively few participants with preserved ejection fractions and sufficient EPA levels, as well as the inability to account for changes in omega-3 levels and other risk factors over time, the researchers noted.
“We consider this study to strongly determine a benefit of EPA exists, but insufficient to determine whether a threshold for %EPA exists near 3%,” they said. They proposed a follow-up study including individuals with higher levels of EPA to better detect a protective effect.
Lead author Dr. Block had no financial conflicts to disclose. Several coauthors received honoraria from Amarin Pharmaceuticals. The study was funded in part by the National Heart, Lung, and Blood Institute.
FROM JACC
Key clinical point: Adults with high levels of eicosapentaenoic acid had significantly lower risk of heart failure than did those with lower levels of EPA.
Major finding: The percent EPA was 0.76% for individuals without heart failure vs. 0.69% for those who suffered heart failure (P = .005).
Study details: An analysis of 6,562 adults aged 45-84 years in the Multi-Ethnic Study of Atherosclerosis.
Disclosures: Lead author Dr. Block had no financial conflicts to disclose. Several coauthors received honoraria from Amarin Pharmaceuticals. The study was funded in part by the National Heart, Lung, and Blood Institute.
Medicare may best Medicare Advantage at reducing readmissions
Although earlier research may suggest otherwise, traditional new research suggests.
Researchers used what they described as “a novel data linkage” comparing 30-day readmission rates after hospitalization for three major conditions in the Hospital Readmissions Reduction Program for patients using traditional Medicare versus Medicare Advantage. Those conditions included acute MI, heart failure, and pneumonia.
“Our results contrast with those of previous studies that have reported lower or statistically similar readmission rates for Medicare Advantage beneficiaries,” Orestis A. Panagiotou, MD, of Brown University, Providence, R.I., and colleagues wrote in a research report published in Annals of Internal Medicine.
In this retrospective cohort study, the researchers linked data from 2011 to 2014 from the Medicare Provider Analysis and Review (MedPAR) file to the Healthcare Effectiveness Data and Information Set (HEDIS).
The novel linkage found that HEDIS data underreported hospital admissions for acute MI, heart failure, and pneumonia, the researchers stated. “Plans incorrectly excluded hospitalizations that should have qualified for the readmission measure, and readmission rates were substantially higher among incorrectly excluded hospitalizations.”
Despite this, in analyses using the linkage of HEDIS and MedPAR, “Medicare Advantage beneficiaries had higher 30-day risk-adjusted readmission rates after [acute MI, heart failure, and pneumonia] than did traditional Medicare beneficiaries,” the investigators noted.
Patients in Medicare Advantage had lower unadjusted readmission rates compared with those in traditional Medicare (16.6% vs. 17.1% for acute MI; 21.4% vs. 21.7% for heart failure; and 16.3% vs. 16.4% for pneumonia). After standardization, Medicare Advantage patients had higher readmission rates, compared with those in traditional Medicare (17.2% vs. 16.9% for acute MI; 21.7% vs. 21.4% for heart failure; and 16.5% vs. 16.0% for pneumonia).
The study authors added that, while unadjusted readmission rates were higher for traditional Medicare beneficiaries, “the direction of the difference reversed after standardization. This occurred because Medicare Advantage beneficiaries have, on average, a lower expected readmission risk [that is, they are ‘healthier’].” Prior studies have documented that Medicare Advantage plans enroll beneficiaries with fewer comorbid conditions and that high-cost beneficiaries switch out of Medicare Advantage and into traditional Medicare.
The researchers suggested four reasons for the differences between the results in this study versus others that compared patients using Medicare with those using Medicare Advantage. These were that the new study included a more comprehensive data set, analyses with comorbid conditions “from a well-validated model applied by CMS [Centers for Medicare & Medicaid Services],” national data focused on three conditions included in the Hospital Readmissions Reduction Program, and patients discharged to places other than skilled nursing facilities and inpatient rehabilitation facilities.
Authors of an accompanying editorial called for caution to be used in interpreting Medicare Advantage enrollment as causing an increased readmission risk.
“[The] results are sensitive to adjustment for case mix,” wrote Peter Huckfeldt, PhD, of the University of Minnesota, Minneapolis, and Neeraj Sood, PhD, of the University of Southern California, Los Angeles, in the editorial published in Annals of Internal Medicine (2019 June 25. doi:10.7326/M19-1599) “Using diagnosis codes on hospital claims for case-mix adjustments may be increasingly perilous. ... To our knowledge, there is no recent evidence comparing the intensity of diagnostic coding between clinically similar [traditional Medicare] and [Medicare Advantage] hospital admissions, but if [traditional Medicare] enrollees were coded more intensively than [Medicare Advantage] enrollees, this could lead to [traditional Medicare] enrollees having lower risk-adjusted readmission rares due to coding practices.”
The editorialists added that using a cross-sectional comparison of Medicare Advantage and traditional Medicare patients is concerning because a “key challenge in estimating the effect of [Medicare Advantage] is that enrollment is voluntary,” which can lead to a number of analytical concerns.
The researchers concluded that their findings “are concerning because CMS uses HEDIS performance to construct composite quality ratings and assign payment bonuses to Medicare Advantage plans.
“Our study suggests a need for improved monitoring of the accuracy of HEDIS data,” they noted.
The National Institute on Aging provided the primary funding for this study. A number of the authors received grants from the National Institutes of Health during the conduct of the study. No other relevant disclosures were reported.
SOURCE: Panagiotou OA et al. Ann Intern Med. 2019 Jun 25. doi: 10.7326/M18-1795.
Although earlier research may suggest otherwise, traditional new research suggests.
Researchers used what they described as “a novel data linkage” comparing 30-day readmission rates after hospitalization for three major conditions in the Hospital Readmissions Reduction Program for patients using traditional Medicare versus Medicare Advantage. Those conditions included acute MI, heart failure, and pneumonia.
“Our results contrast with those of previous studies that have reported lower or statistically similar readmission rates for Medicare Advantage beneficiaries,” Orestis A. Panagiotou, MD, of Brown University, Providence, R.I., and colleagues wrote in a research report published in Annals of Internal Medicine.
In this retrospective cohort study, the researchers linked data from 2011 to 2014 from the Medicare Provider Analysis and Review (MedPAR) file to the Healthcare Effectiveness Data and Information Set (HEDIS).
The novel linkage found that HEDIS data underreported hospital admissions for acute MI, heart failure, and pneumonia, the researchers stated. “Plans incorrectly excluded hospitalizations that should have qualified for the readmission measure, and readmission rates were substantially higher among incorrectly excluded hospitalizations.”
Despite this, in analyses using the linkage of HEDIS and MedPAR, “Medicare Advantage beneficiaries had higher 30-day risk-adjusted readmission rates after [acute MI, heart failure, and pneumonia] than did traditional Medicare beneficiaries,” the investigators noted.
Patients in Medicare Advantage had lower unadjusted readmission rates compared with those in traditional Medicare (16.6% vs. 17.1% for acute MI; 21.4% vs. 21.7% for heart failure; and 16.3% vs. 16.4% for pneumonia). After standardization, Medicare Advantage patients had higher readmission rates, compared with those in traditional Medicare (17.2% vs. 16.9% for acute MI; 21.7% vs. 21.4% for heart failure; and 16.5% vs. 16.0% for pneumonia).
The study authors added that, while unadjusted readmission rates were higher for traditional Medicare beneficiaries, “the direction of the difference reversed after standardization. This occurred because Medicare Advantage beneficiaries have, on average, a lower expected readmission risk [that is, they are ‘healthier’].” Prior studies have documented that Medicare Advantage plans enroll beneficiaries with fewer comorbid conditions and that high-cost beneficiaries switch out of Medicare Advantage and into traditional Medicare.
The researchers suggested four reasons for the differences between the results in this study versus others that compared patients using Medicare with those using Medicare Advantage. These were that the new study included a more comprehensive data set, analyses with comorbid conditions “from a well-validated model applied by CMS [Centers for Medicare & Medicaid Services],” national data focused on three conditions included in the Hospital Readmissions Reduction Program, and patients discharged to places other than skilled nursing facilities and inpatient rehabilitation facilities.
Authors of an accompanying editorial called for caution to be used in interpreting Medicare Advantage enrollment as causing an increased readmission risk.
“[The] results are sensitive to adjustment for case mix,” wrote Peter Huckfeldt, PhD, of the University of Minnesota, Minneapolis, and Neeraj Sood, PhD, of the University of Southern California, Los Angeles, in the editorial published in Annals of Internal Medicine (2019 June 25. doi:10.7326/M19-1599) “Using diagnosis codes on hospital claims for case-mix adjustments may be increasingly perilous. ... To our knowledge, there is no recent evidence comparing the intensity of diagnostic coding between clinically similar [traditional Medicare] and [Medicare Advantage] hospital admissions, but if [traditional Medicare] enrollees were coded more intensively than [Medicare Advantage] enrollees, this could lead to [traditional Medicare] enrollees having lower risk-adjusted readmission rares due to coding practices.”
The editorialists added that using a cross-sectional comparison of Medicare Advantage and traditional Medicare patients is concerning because a “key challenge in estimating the effect of [Medicare Advantage] is that enrollment is voluntary,” which can lead to a number of analytical concerns.
The researchers concluded that their findings “are concerning because CMS uses HEDIS performance to construct composite quality ratings and assign payment bonuses to Medicare Advantage plans.
“Our study suggests a need for improved monitoring of the accuracy of HEDIS data,” they noted.
The National Institute on Aging provided the primary funding for this study. A number of the authors received grants from the National Institutes of Health during the conduct of the study. No other relevant disclosures were reported.
SOURCE: Panagiotou OA et al. Ann Intern Med. 2019 Jun 25. doi: 10.7326/M18-1795.
Although earlier research may suggest otherwise, traditional new research suggests.
Researchers used what they described as “a novel data linkage” comparing 30-day readmission rates after hospitalization for three major conditions in the Hospital Readmissions Reduction Program for patients using traditional Medicare versus Medicare Advantage. Those conditions included acute MI, heart failure, and pneumonia.
“Our results contrast with those of previous studies that have reported lower or statistically similar readmission rates for Medicare Advantage beneficiaries,” Orestis A. Panagiotou, MD, of Brown University, Providence, R.I., and colleagues wrote in a research report published in Annals of Internal Medicine.
In this retrospective cohort study, the researchers linked data from 2011 to 2014 from the Medicare Provider Analysis and Review (MedPAR) file to the Healthcare Effectiveness Data and Information Set (HEDIS).
The novel linkage found that HEDIS data underreported hospital admissions for acute MI, heart failure, and pneumonia, the researchers stated. “Plans incorrectly excluded hospitalizations that should have qualified for the readmission measure, and readmission rates were substantially higher among incorrectly excluded hospitalizations.”
Despite this, in analyses using the linkage of HEDIS and MedPAR, “Medicare Advantage beneficiaries had higher 30-day risk-adjusted readmission rates after [acute MI, heart failure, and pneumonia] than did traditional Medicare beneficiaries,” the investigators noted.
Patients in Medicare Advantage had lower unadjusted readmission rates compared with those in traditional Medicare (16.6% vs. 17.1% for acute MI; 21.4% vs. 21.7% for heart failure; and 16.3% vs. 16.4% for pneumonia). After standardization, Medicare Advantage patients had higher readmission rates, compared with those in traditional Medicare (17.2% vs. 16.9% for acute MI; 21.7% vs. 21.4% for heart failure; and 16.5% vs. 16.0% for pneumonia).
The study authors added that, while unadjusted readmission rates were higher for traditional Medicare beneficiaries, “the direction of the difference reversed after standardization. This occurred because Medicare Advantage beneficiaries have, on average, a lower expected readmission risk [that is, they are ‘healthier’].” Prior studies have documented that Medicare Advantage plans enroll beneficiaries with fewer comorbid conditions and that high-cost beneficiaries switch out of Medicare Advantage and into traditional Medicare.
The researchers suggested four reasons for the differences between the results in this study versus others that compared patients using Medicare with those using Medicare Advantage. These were that the new study included a more comprehensive data set, analyses with comorbid conditions “from a well-validated model applied by CMS [Centers for Medicare & Medicaid Services],” national data focused on three conditions included in the Hospital Readmissions Reduction Program, and patients discharged to places other than skilled nursing facilities and inpatient rehabilitation facilities.
Authors of an accompanying editorial called for caution to be used in interpreting Medicare Advantage enrollment as causing an increased readmission risk.
“[The] results are sensitive to adjustment for case mix,” wrote Peter Huckfeldt, PhD, of the University of Minnesota, Minneapolis, and Neeraj Sood, PhD, of the University of Southern California, Los Angeles, in the editorial published in Annals of Internal Medicine (2019 June 25. doi:10.7326/M19-1599) “Using diagnosis codes on hospital claims for case-mix adjustments may be increasingly perilous. ... To our knowledge, there is no recent evidence comparing the intensity of diagnostic coding between clinically similar [traditional Medicare] and [Medicare Advantage] hospital admissions, but if [traditional Medicare] enrollees were coded more intensively than [Medicare Advantage] enrollees, this could lead to [traditional Medicare] enrollees having lower risk-adjusted readmission rares due to coding practices.”
The editorialists added that using a cross-sectional comparison of Medicare Advantage and traditional Medicare patients is concerning because a “key challenge in estimating the effect of [Medicare Advantage] is that enrollment is voluntary,” which can lead to a number of analytical concerns.
The researchers concluded that their findings “are concerning because CMS uses HEDIS performance to construct composite quality ratings and assign payment bonuses to Medicare Advantage plans.
“Our study suggests a need for improved monitoring of the accuracy of HEDIS data,” they noted.
The National Institute on Aging provided the primary funding for this study. A number of the authors received grants from the National Institutes of Health during the conduct of the study. No other relevant disclosures were reported.
SOURCE: Panagiotou OA et al. Ann Intern Med. 2019 Jun 25. doi: 10.7326/M18-1795.
FROM ANNALS OF INTERNAL MEDICINE
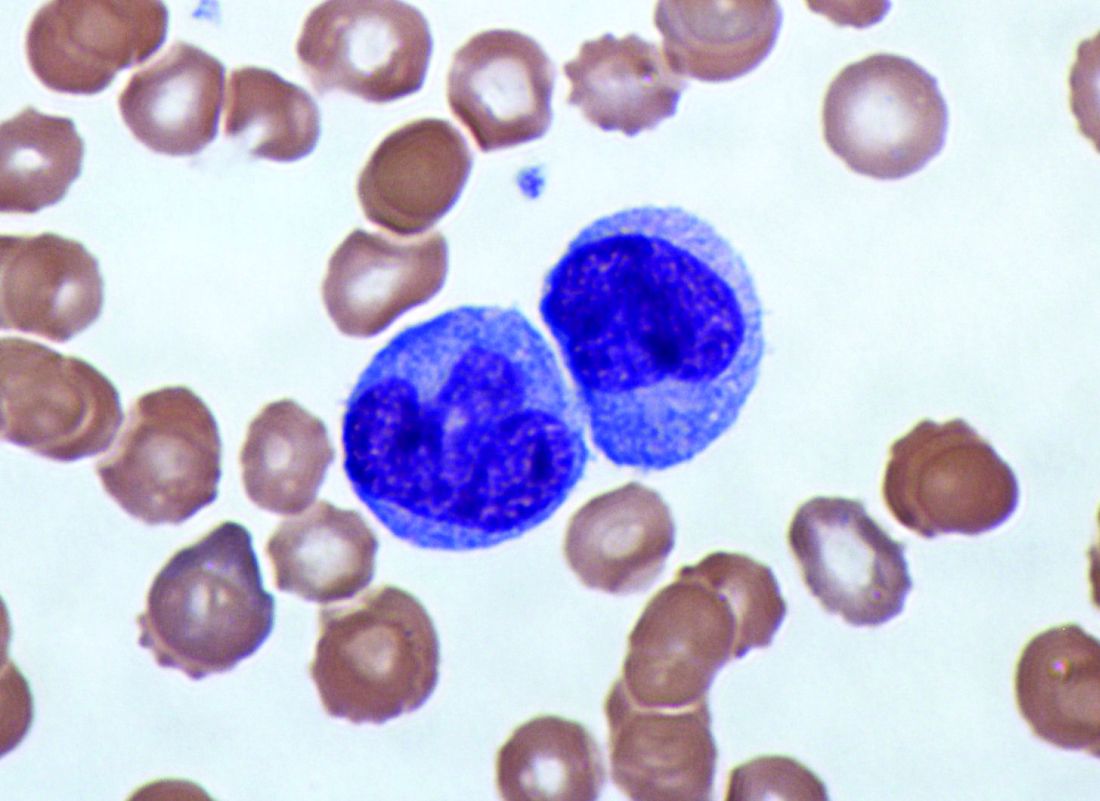
Elevated monocyte count predicts poor outcomes in idiopathic pulmonary fibrosis
, including hypertrophic cardiomyopathy, systemic sclerosis, and myelofibrosis, according to research published in The Lancet Respiratory Medicine.
The data indicate that “a single threshold value of absolute monocyte counts of 0.95 K/mcL could be used to identify high-risk patients with a fibrotic disease,” said Madeleine K. D. Scott, a researcher at Stanford (Calif.) University, and coauthors. The results “suggest that monocyte count should be incorporated into the clinical assessment” and may “enable more conscientious allocation of scarce resources, including lung transplantations,” they said.
While other published biomarkers – including gene panels and multicytokine signatures – may be expensive and not readily available, “absolute monocyte count is routinely measured as part of a complete blood count, an inexpensive test used in clinical practice worldwide,” the authors said.
Further study of monocytes’ mechanistic role in fibrosis ultimately could point to new treatment approaches.
A retrospective multicenter cohort study
To assess whether immune cells may identify patients with idiopathic pulmonary fibrosis at greater risk of poor outcomes, Ms. Scott and her collaborators conducted a retrospective multicenter cohort study.
They first analyzed transcriptome data from 120 peripheral blood mononuclear cell samples of patients with idiopathic pulmonary fibrosis, which they obtained from the Gene Expression Omnibus at the National Center for Biotechnology Information. They used statistical deconvolution to estimate percentages of 13 immune cell types and examined their associations with transplant-free survival. Their discovery analysis found that estimated CD14+ classical monocyte percentages above the mean correlated with shorter transplant-free survival times (hazard ratio, 1.82), but percentages of T cells and B cells did not.
The researchers then validated these results using samples from patients with idiopathic pulmonary fibrosis in two independent cohorts. In the COMET validation cohort, which included 45 patients with idiopathic pulmonary fibrosis whose monocyte counts were measured using flow cytometry, higher monocyte counts were significantly associated with greater risk of disease progression. In the Yale cohort, which included 15 patients with idiopathic pulmonary fibrosis, the 6 patients who were classified as high risk on the basis of a 52-gene signature had more CD14+ monocytes than the 9 low-risk patients did.
In addition, Ms. Scott and her collaborators looked at complete blood count values in the electronic health records of 45,068 patients with idiopathic pulmonary fibrosis, systemic sclerosis, hypertrophic cardiomyopathy, or myelofibrosis in Stanford, Northwestern, Vanderbilt, and Optum Clinformatics Data Mart cohorts.
Among patients in the COMET, Stanford, and Northwestern datasets, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (HR, 2.47) and the gender, age, and physiology index (HR, 2.06). Data from 7,459 patients with idiopathic pulmonary fibrosis “showed that patients with monocyte counts of 0.95 K/mcL or greater were at increased risk of mortality with lung transplantation as a censoring event, after adjusting for age at diagnosis and sex” in the Stanford (HR, 2.30), Vanderbilt (HR, 1.52), and Optum (HR, 1.74) cohorts. “Likewise, higher absolute monocyte count was associated with shortened survival in patients with hypertrophic cardiomyopathy across all three cohorts, and in patients with systemic sclerosis or myelofibrosis in two of the three cohorts,” the researchers said.
The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
SOURCE: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
The study by Scott et al. provides evidence that monocyte count may be a “novel, simple, and inexpensive prognostic biomarker in idiopathic pulmonary fibrosis,” according to an accompanying editorial.
Progress has been made in the treatment of idiopathic pulmonary fibrosis, but patient prognosis remains “challenging to predict,” wrote Michael Kreuter, MD, of University of Heidelberg, Germany, and Toby M. Maher, MB, MSc, PhD, of Royal Brompton Hospital in London and Imperial College London. “One lesson that can be learned from other respiratory disorders is that routinely measured cellular biomarkers, such as blood eosinophil counts in chronic obstructive pulmonary disease (COPD), can predict treatment responses” (Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600[19]30050-5).
Increased blood monocyte counts in idiopathic pulmonary fibrosis may reflect disease activity, which “could explain the outcome differences,” said Dr. Kreuter and Dr. Maher. “As highlighted by the investigators themselves, before introducing assessment of monocyte counts as part of routine clinical care for individuals with idiopathic pulmonary fibrosis, the limitations of this research should be taken into account. These include uncertainty around diagnosis and disease severity in a substantial subset of the patients, and the unknown effect of medical therapies (including corticosteroids and immunosuppressant and antifibrotic drugs) on monocyte counts and prognosis.” Researchers should validate the clinical value of blood monocyte counts in existing and future cohorts and evaluate the biomarker in clinical trials.
The editorialists have received compensation and funding from various pharmaceutical companies.
The study by Scott et al. provides evidence that monocyte count may be a “novel, simple, and inexpensive prognostic biomarker in idiopathic pulmonary fibrosis,” according to an accompanying editorial.
Progress has been made in the treatment of idiopathic pulmonary fibrosis, but patient prognosis remains “challenging to predict,” wrote Michael Kreuter, MD, of University of Heidelberg, Germany, and Toby M. Maher, MB, MSc, PhD, of Royal Brompton Hospital in London and Imperial College London. “One lesson that can be learned from other respiratory disorders is that routinely measured cellular biomarkers, such as blood eosinophil counts in chronic obstructive pulmonary disease (COPD), can predict treatment responses” (Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600[19]30050-5).
Increased blood monocyte counts in idiopathic pulmonary fibrosis may reflect disease activity, which “could explain the outcome differences,” said Dr. Kreuter and Dr. Maher. “As highlighted by the investigators themselves, before introducing assessment of monocyte counts as part of routine clinical care for individuals with idiopathic pulmonary fibrosis, the limitations of this research should be taken into account. These include uncertainty around diagnosis and disease severity in a substantial subset of the patients, and the unknown effect of medical therapies (including corticosteroids and immunosuppressant and antifibrotic drugs) on monocyte counts and prognosis.” Researchers should validate the clinical value of blood monocyte counts in existing and future cohorts and evaluate the biomarker in clinical trials.
The editorialists have received compensation and funding from various pharmaceutical companies.
The study by Scott et al. provides evidence that monocyte count may be a “novel, simple, and inexpensive prognostic biomarker in idiopathic pulmonary fibrosis,” according to an accompanying editorial.
Progress has been made in the treatment of idiopathic pulmonary fibrosis, but patient prognosis remains “challenging to predict,” wrote Michael Kreuter, MD, of University of Heidelberg, Germany, and Toby M. Maher, MB, MSc, PhD, of Royal Brompton Hospital in London and Imperial College London. “One lesson that can be learned from other respiratory disorders is that routinely measured cellular biomarkers, such as blood eosinophil counts in chronic obstructive pulmonary disease (COPD), can predict treatment responses” (Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600[19]30050-5).
Increased blood monocyte counts in idiopathic pulmonary fibrosis may reflect disease activity, which “could explain the outcome differences,” said Dr. Kreuter and Dr. Maher. “As highlighted by the investigators themselves, before introducing assessment of monocyte counts as part of routine clinical care for individuals with idiopathic pulmonary fibrosis, the limitations of this research should be taken into account. These include uncertainty around diagnosis and disease severity in a substantial subset of the patients, and the unknown effect of medical therapies (including corticosteroids and immunosuppressant and antifibrotic drugs) on monocyte counts and prognosis.” Researchers should validate the clinical value of blood monocyte counts in existing and future cohorts and evaluate the biomarker in clinical trials.
The editorialists have received compensation and funding from various pharmaceutical companies.
, including hypertrophic cardiomyopathy, systemic sclerosis, and myelofibrosis, according to research published in The Lancet Respiratory Medicine.
The data indicate that “a single threshold value of absolute monocyte counts of 0.95 K/mcL could be used to identify high-risk patients with a fibrotic disease,” said Madeleine K. D. Scott, a researcher at Stanford (Calif.) University, and coauthors. The results “suggest that monocyte count should be incorporated into the clinical assessment” and may “enable more conscientious allocation of scarce resources, including lung transplantations,” they said.
While other published biomarkers – including gene panels and multicytokine signatures – may be expensive and not readily available, “absolute monocyte count is routinely measured as part of a complete blood count, an inexpensive test used in clinical practice worldwide,” the authors said.
Further study of monocytes’ mechanistic role in fibrosis ultimately could point to new treatment approaches.
A retrospective multicenter cohort study
To assess whether immune cells may identify patients with idiopathic pulmonary fibrosis at greater risk of poor outcomes, Ms. Scott and her collaborators conducted a retrospective multicenter cohort study.
They first analyzed transcriptome data from 120 peripheral blood mononuclear cell samples of patients with idiopathic pulmonary fibrosis, which they obtained from the Gene Expression Omnibus at the National Center for Biotechnology Information. They used statistical deconvolution to estimate percentages of 13 immune cell types and examined their associations with transplant-free survival. Their discovery analysis found that estimated CD14+ classical monocyte percentages above the mean correlated with shorter transplant-free survival times (hazard ratio, 1.82), but percentages of T cells and B cells did not.
The researchers then validated these results using samples from patients with idiopathic pulmonary fibrosis in two independent cohorts. In the COMET validation cohort, which included 45 patients with idiopathic pulmonary fibrosis whose monocyte counts were measured using flow cytometry, higher monocyte counts were significantly associated with greater risk of disease progression. In the Yale cohort, which included 15 patients with idiopathic pulmonary fibrosis, the 6 patients who were classified as high risk on the basis of a 52-gene signature had more CD14+ monocytes than the 9 low-risk patients did.
In addition, Ms. Scott and her collaborators looked at complete blood count values in the electronic health records of 45,068 patients with idiopathic pulmonary fibrosis, systemic sclerosis, hypertrophic cardiomyopathy, or myelofibrosis in Stanford, Northwestern, Vanderbilt, and Optum Clinformatics Data Mart cohorts.
Among patients in the COMET, Stanford, and Northwestern datasets, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (HR, 2.47) and the gender, age, and physiology index (HR, 2.06). Data from 7,459 patients with idiopathic pulmonary fibrosis “showed that patients with monocyte counts of 0.95 K/mcL or greater were at increased risk of mortality with lung transplantation as a censoring event, after adjusting for age at diagnosis and sex” in the Stanford (HR, 2.30), Vanderbilt (HR, 1.52), and Optum (HR, 1.74) cohorts. “Likewise, higher absolute monocyte count was associated with shortened survival in patients with hypertrophic cardiomyopathy across all three cohorts, and in patients with systemic sclerosis or myelofibrosis in two of the three cohorts,” the researchers said.
The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
SOURCE: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
, including hypertrophic cardiomyopathy, systemic sclerosis, and myelofibrosis, according to research published in The Lancet Respiratory Medicine.
The data indicate that “a single threshold value of absolute monocyte counts of 0.95 K/mcL could be used to identify high-risk patients with a fibrotic disease,” said Madeleine K. D. Scott, a researcher at Stanford (Calif.) University, and coauthors. The results “suggest that monocyte count should be incorporated into the clinical assessment” and may “enable more conscientious allocation of scarce resources, including lung transplantations,” they said.
While other published biomarkers – including gene panels and multicytokine signatures – may be expensive and not readily available, “absolute monocyte count is routinely measured as part of a complete blood count, an inexpensive test used in clinical practice worldwide,” the authors said.
Further study of monocytes’ mechanistic role in fibrosis ultimately could point to new treatment approaches.
A retrospective multicenter cohort study
To assess whether immune cells may identify patients with idiopathic pulmonary fibrosis at greater risk of poor outcomes, Ms. Scott and her collaborators conducted a retrospective multicenter cohort study.
They first analyzed transcriptome data from 120 peripheral blood mononuclear cell samples of patients with idiopathic pulmonary fibrosis, which they obtained from the Gene Expression Omnibus at the National Center for Biotechnology Information. They used statistical deconvolution to estimate percentages of 13 immune cell types and examined their associations with transplant-free survival. Their discovery analysis found that estimated CD14+ classical monocyte percentages above the mean correlated with shorter transplant-free survival times (hazard ratio, 1.82), but percentages of T cells and B cells did not.
The researchers then validated these results using samples from patients with idiopathic pulmonary fibrosis in two independent cohorts. In the COMET validation cohort, which included 45 patients with idiopathic pulmonary fibrosis whose monocyte counts were measured using flow cytometry, higher monocyte counts were significantly associated with greater risk of disease progression. In the Yale cohort, which included 15 patients with idiopathic pulmonary fibrosis, the 6 patients who were classified as high risk on the basis of a 52-gene signature had more CD14+ monocytes than the 9 low-risk patients did.
In addition, Ms. Scott and her collaborators looked at complete blood count values in the electronic health records of 45,068 patients with idiopathic pulmonary fibrosis, systemic sclerosis, hypertrophic cardiomyopathy, or myelofibrosis in Stanford, Northwestern, Vanderbilt, and Optum Clinformatics Data Mart cohorts.
Among patients in the COMET, Stanford, and Northwestern datasets, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (HR, 2.47) and the gender, age, and physiology index (HR, 2.06). Data from 7,459 patients with idiopathic pulmonary fibrosis “showed that patients with monocyte counts of 0.95 K/mcL or greater were at increased risk of mortality with lung transplantation as a censoring event, after adjusting for age at diagnosis and sex” in the Stanford (HR, 2.30), Vanderbilt (HR, 1.52), and Optum (HR, 1.74) cohorts. “Likewise, higher absolute monocyte count was associated with shortened survival in patients with hypertrophic cardiomyopathy across all three cohorts, and in patients with systemic sclerosis or myelofibrosis in two of the three cohorts,” the researchers said.
The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
SOURCE: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: An increased monocyte count predicts poor outcomes among patients with idiopathic pulmonary fibrosis and other fibrotic diseases.
Major finding: Among patients in three cohorts, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (hazard ratio, 2.47) and the gender, age, and physiology index (HR, 2.06).
Study details: A retrospective analysis of data from 7,000 patients with idiopathic pulmonary fibrosis from five independent cohorts.
Disclosures: The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
Source: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
Which antidiabetic for elderly patients? It depends on their CV risk
SAN FRANCISCO – SGLT2 inhibitors did a better job than GLP-1 receptor agonists at preventing heart failure hospitalizations in elderly patients with type 2 diabetes, but at the cost of more strokes, myocardial infarctions, and deaths among those without preexisting cardiovascular disease, according to Harvard University investigators.
Using Medicare claims data and propensity scoring, they matched 43,609 elderly patients who started a sodium-glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes, 77% of whom were taking canagliflozin (Invokana), to 43,609 who started a glucagonlike peptide–1 (GLP-1)–receptor agonist, 60% of whom were taking liraglutide (Victoza).
Patients were paired by age, comorbidities, diabetes severity, and dozens of other variables, more than 120 in all. The data window ran from April 2013 through December 2016.
The idea was to compare the drugs directly in order to help clinicians decide which class to choose for older patients as second-line therapy, an important consideration at a time when there’s not much guidance specifically for the elderly, and manufacturers are issuing dueling placebo-controlled trials.
Both classes have shown cardiovascular benefits, but studies were mostly in younger people with preexisting cardiovascular disease (CVD). “The comparative impact of these agents in the older population has not yet been established,” lead investigator Elisabetta Patorno, MD, DrPH, of Harvard University, Boston, said at the annual scientific sessions of the American Diabetes Association.
General themes are emerging from Dr. Patorno’s work; it seems that deciding between the two classes has a lot to do with whether the main concern is heart failure or cardiovascular events. Even so, she said, it’s too early to incorporate the observations into guidelines. The analysis is ongoing, and there are plans to compare impacts on renal disease and other problems.
In the meantime, she and her colleagues found that initiating an SGLT2 inhibitor versus a GLP-1 receptor agonist in the elderly was associated with a 34% decreased risk of heart failure hospitalization (2.5 fewer hospitalizations per 1,000 patient years), with an even larger drop among people who had preexisting CVD.
There was, however, a 41% increased risk of lower limb amputations (0.8 more events per 1,000 patient years) and a 62% increase in diabetic ketoacidosis (DKA, 1 more event), problems previously associated with the class.
Results were comparable – fewer heart failure hospitalizations but more amputations and DKA – when SGLT2 initiation was compared to initiation with dipeptidyl peptidase-4 (DPP-4) inhibitors, another second-line option for type 2 diabetes that includes sitagliptin (Januvia), among others.
There was a 25% increased relative risk of the composite primary outcome of myocardial infarction, stroke, and all-cause mortality when patients without baseline CVD were started on an SGLT2 inhibitor instead of a GLP-1 receptor agonist (3.7 more events per 1,000 patient years). There was no increased risk among patients who already had CVD.
SGLT2 initiation actually had a protective effect, compared with dipeptidyl peptidase-4 inhibitors, with a 23% decreased risk of the composite outcome (6.5 fewer events) among patients both with and without baseline CVD. The findings were all statistically significant.
The average age in the study was 71.5 years; 45% of the subjects were men; 40% had a history of cardiovascular disease; and 60% were on metformin and 24% on insulin at study entry.
The work was funded by the National Institutes of Health. Dr. Patorno disclosed research grants form Boehringer Ingelheim and GlaxoSmithKline. Other investigators reported relationships with numerous pharmaceutical companies.
SAN FRANCISCO – SGLT2 inhibitors did a better job than GLP-1 receptor agonists at preventing heart failure hospitalizations in elderly patients with type 2 diabetes, but at the cost of more strokes, myocardial infarctions, and deaths among those without preexisting cardiovascular disease, according to Harvard University investigators.
Using Medicare claims data and propensity scoring, they matched 43,609 elderly patients who started a sodium-glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes, 77% of whom were taking canagliflozin (Invokana), to 43,609 who started a glucagonlike peptide–1 (GLP-1)–receptor agonist, 60% of whom were taking liraglutide (Victoza).
Patients were paired by age, comorbidities, diabetes severity, and dozens of other variables, more than 120 in all. The data window ran from April 2013 through December 2016.
The idea was to compare the drugs directly in order to help clinicians decide which class to choose for older patients as second-line therapy, an important consideration at a time when there’s not much guidance specifically for the elderly, and manufacturers are issuing dueling placebo-controlled trials.
Both classes have shown cardiovascular benefits, but studies were mostly in younger people with preexisting cardiovascular disease (CVD). “The comparative impact of these agents in the older population has not yet been established,” lead investigator Elisabetta Patorno, MD, DrPH, of Harvard University, Boston, said at the annual scientific sessions of the American Diabetes Association.
General themes are emerging from Dr. Patorno’s work; it seems that deciding between the two classes has a lot to do with whether the main concern is heart failure or cardiovascular events. Even so, she said, it’s too early to incorporate the observations into guidelines. The analysis is ongoing, and there are plans to compare impacts on renal disease and other problems.
In the meantime, she and her colleagues found that initiating an SGLT2 inhibitor versus a GLP-1 receptor agonist in the elderly was associated with a 34% decreased risk of heart failure hospitalization (2.5 fewer hospitalizations per 1,000 patient years), with an even larger drop among people who had preexisting CVD.
There was, however, a 41% increased risk of lower limb amputations (0.8 more events per 1,000 patient years) and a 62% increase in diabetic ketoacidosis (DKA, 1 more event), problems previously associated with the class.
Results were comparable – fewer heart failure hospitalizations but more amputations and DKA – when SGLT2 initiation was compared to initiation with dipeptidyl peptidase-4 (DPP-4) inhibitors, another second-line option for type 2 diabetes that includes sitagliptin (Januvia), among others.
There was a 25% increased relative risk of the composite primary outcome of myocardial infarction, stroke, and all-cause mortality when patients without baseline CVD were started on an SGLT2 inhibitor instead of a GLP-1 receptor agonist (3.7 more events per 1,000 patient years). There was no increased risk among patients who already had CVD.
SGLT2 initiation actually had a protective effect, compared with dipeptidyl peptidase-4 inhibitors, with a 23% decreased risk of the composite outcome (6.5 fewer events) among patients both with and without baseline CVD. The findings were all statistically significant.
The average age in the study was 71.5 years; 45% of the subjects were men; 40% had a history of cardiovascular disease; and 60% were on metformin and 24% on insulin at study entry.
The work was funded by the National Institutes of Health. Dr. Patorno disclosed research grants form Boehringer Ingelheim and GlaxoSmithKline. Other investigators reported relationships with numerous pharmaceutical companies.
SAN FRANCISCO – SGLT2 inhibitors did a better job than GLP-1 receptor agonists at preventing heart failure hospitalizations in elderly patients with type 2 diabetes, but at the cost of more strokes, myocardial infarctions, and deaths among those without preexisting cardiovascular disease, according to Harvard University investigators.
Using Medicare claims data and propensity scoring, they matched 43,609 elderly patients who started a sodium-glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes, 77% of whom were taking canagliflozin (Invokana), to 43,609 who started a glucagonlike peptide–1 (GLP-1)–receptor agonist, 60% of whom were taking liraglutide (Victoza).
Patients were paired by age, comorbidities, diabetes severity, and dozens of other variables, more than 120 in all. The data window ran from April 2013 through December 2016.
The idea was to compare the drugs directly in order to help clinicians decide which class to choose for older patients as second-line therapy, an important consideration at a time when there’s not much guidance specifically for the elderly, and manufacturers are issuing dueling placebo-controlled trials.
Both classes have shown cardiovascular benefits, but studies were mostly in younger people with preexisting cardiovascular disease (CVD). “The comparative impact of these agents in the older population has not yet been established,” lead investigator Elisabetta Patorno, MD, DrPH, of Harvard University, Boston, said at the annual scientific sessions of the American Diabetes Association.
General themes are emerging from Dr. Patorno’s work; it seems that deciding between the two classes has a lot to do with whether the main concern is heart failure or cardiovascular events. Even so, she said, it’s too early to incorporate the observations into guidelines. The analysis is ongoing, and there are plans to compare impacts on renal disease and other problems.
In the meantime, she and her colleagues found that initiating an SGLT2 inhibitor versus a GLP-1 receptor agonist in the elderly was associated with a 34% decreased risk of heart failure hospitalization (2.5 fewer hospitalizations per 1,000 patient years), with an even larger drop among people who had preexisting CVD.
There was, however, a 41% increased risk of lower limb amputations (0.8 more events per 1,000 patient years) and a 62% increase in diabetic ketoacidosis (DKA, 1 more event), problems previously associated with the class.
Results were comparable – fewer heart failure hospitalizations but more amputations and DKA – when SGLT2 initiation was compared to initiation with dipeptidyl peptidase-4 (DPP-4) inhibitors, another second-line option for type 2 diabetes that includes sitagliptin (Januvia), among others.
There was a 25% increased relative risk of the composite primary outcome of myocardial infarction, stroke, and all-cause mortality when patients without baseline CVD were started on an SGLT2 inhibitor instead of a GLP-1 receptor agonist (3.7 more events per 1,000 patient years). There was no increased risk among patients who already had CVD.
SGLT2 initiation actually had a protective effect, compared with dipeptidyl peptidase-4 inhibitors, with a 23% decreased risk of the composite outcome (6.5 fewer events) among patients both with and without baseline CVD. The findings were all statistically significant.
The average age in the study was 71.5 years; 45% of the subjects were men; 40% had a history of cardiovascular disease; and 60% were on metformin and 24% on insulin at study entry.
The work was funded by the National Institutes of Health. Dr. Patorno disclosed research grants form Boehringer Ingelheim and GlaxoSmithKline. Other investigators reported relationships with numerous pharmaceutical companies.
REPORTING FROM ADA 2019
Pivotal trial shows HFrEF benefits from baroreceptor stimulation
SAN FRANCISCO – Baroreflex activation therapy met all four of its primary endpoints in its U.S. pivotal trial of 264 patients with advanced heart failure with reduced ejection fraction who were ineligible for cardiac resynchronization therapy.
The results showed that ongoing baroreflex activation therapy (BAT) via a single, stimulating electrode surgically placed on a patient’s carotid artery led to statistically significant and clinically meaningful improvements in quality of life and functional capacity while also reducing the level of a biomarker of heart failure severity in patients already on guideline-directed medical therapy, Michael R. Zile, MD, said at the annual scientific sessions of the Heart Rhythm Society. He estimated that the device is appropriate for perhaps a third or more of patients with heart failure with reduced ejection fraction (HFrEF), specifically patients with New York Heart Association functional class III disease who are not candidates for treatment with cardiac resynchronization therapy (CRT) and with a blood level of N-terminal pro–brain natriuretic peptide (NT-proBNP) of less than 1,600 pg/mL, a cutoff that excludes patients with very severe class III HFrEF and focuses on those who benefited in the study.
“To our knowledge, this is the first successful pivotal trial of device-based neuromodulation therapy in HFrEF patients,” said Dr. Zile, professor of medicine at the Medical University of South Carolina in Charleston. “We think that BAT fills an unmet need” in a large number of HFrEF patients. He stressed that the placement of the single, 2-mm, unilateral electrode on the baroreceptor-containing carotid sinus is an “extremely safe and simple” surgery. The electrode attaches to a small, subcutaneously placed generator.
Dr. Zile attributed the treatment’s success, in contrast to a prior, failed attempt to treat HFrEF by vagus nerve stimulation (J Am Coll Cardiol. 2016 Jul 12;68[2]:147-56) to BAT’s action via the patient’s brain, which processes the afferent signal it receives from stimulation to in turn inhibit sympathetic activation and upregulate parasympathetic innervation, with both actions benefiting HFrEF patients. “The integrated autonomic balance is the real difference with this device,” he said. Other helpful effects from BAT are reduced heart rate, reduced cardiac remodeling, increased vasodilation, a decrease in elevated blood pressure, increased diuresis, and a drop in renin secretion. The pivotal trial built on findings from a phase 2 study (JACC Heart Fail. 2015 Jun;3[6]:487-96).
The BeAT-HF (Barostim Neo - Baroreflex Activation Therapy for Heart Failure) trial enrolled patients with class III HFrEF with a left ventricular ejection fraction of 35% or less and a 6-minute walk distance of 150-400 m, who were ineligible for CRT, on optimal medical therapy, and who had an elevated NT-proBNP level. After the study randomized 271 patients to either BAT or ongoing medical therapy only (without use of a sham procedure or sham BAT), the results showed a statistically significant benefit for three of the four primary endpoints. Patients treated with BAT for 6 months had statistically significant and clinically meaningful improvements in their quality of life scores as measured on the Minnesota Living With Heart Failure Questionnaire, in their function as measured by the 6-min walk distance, and in the treatment’s safety, based on the combined rate of major adverse neurological and cardiovascular events, which occurred in 6% of patients treated with BAT, which was significantly better than the study’s prespecified performance goal of 15%.
However, for the fourth primary endpoint – reduction in blood levels of NT-proBNP – the BAT-treated patients showed no significant improvement, compared with the controls. The design of BeAT-HF called for consultation with the Food and Drug Administration in such a situation, and further data analysis showed that the problem may have been that some enrolled patients entered with extremely elevated levels of this biomarker. The agency authorized an added protocol that randomized 102 additional patients that matched the initial cohort but had a requirement for an NT-proBNP level of less than 1,600 pg/mL. The 6-month outcomes of these patients were combined with the previously determined outcomes for 162 of the original 271 patients who entered with NT-proBNP levels within the specified limit, producing a total, final study group of 264 patients, of whom 120 received BAT and completed 6-month follow-up, and 125 received medical therapy only and had 6-month follow-up. These patients averaged about 63 years of age, and 20% were women. On average they were on four heart failure medications, and more than three-quarters also had an implanted cardiac device.
The results from an analysis of this cohort showed a statistically significant, 25% relative reduction in blood levels of NT-proBNP in the BAT patients, compared with the controls, and it also confirmed statistically significant and meaningful improvements in quality of life and function on BAT, compared with controls. The 14-point average improvement in the quality of life score in BAT patients, compared with the controls, on the Minnesota Living With Heart Failure Questionnaire was nearly triple the point improvement that’s considered clinically meaningful and hence was “very convincing” about the treatment’s efficacy, noted Dr. Zile, who is also director of cardiology at the VA Medical Center in Charleston. The 25% drop in average NT-proBNP levels “predicts a marked reduction in morbidity and mortality.” He added that researchers have developed a percutaneous, transcatheter method for placing the carotid electrode that will soon undergo clinical testing.
These results “reconfirm the safety of BAT,” but are limited by a relatively short follow-up of 6 months, no data on survival benefit, and by not having echocardiographic data on possible cardiac remodeling, commented Sanjeev Saksena, MD, medical director of the Electrophysiology Research Foundation in Warren, N.J.
BeAT-HF was sponsored by CVRx, the company developing the baroreflex activation device. Dr. Zile has been a consultant to CVRx and to Abbott, AstraZeneca, Bayer, Bristol-Myers Squibb, Lilly, Merck, and Novartis. Dr. Saksena had no disclosures.
SOURCE: Zile MR. Heart Rhythm 2019, Abstract, S-LBCT01-04.
The results that Dr. Zile reported are obviously very promising. It was a huge step forward when researchers identified medical treatments that can safely manipulate the autonomic nervous system in patients with heart failure with reduced ejection fraction. Now we are asking what else we can do because we have run into limits on what we can accomplish with drugs alone. The BeAT-HF study is a step in that direction.
Andrew D. Krahn, MD, is professor of medicine and head of cardiology at the University of British Columbia and St. Paul’s Hospital in Vancouver. He has been a consultant to Medtronic and he has received research funding from Boston Scientific and Medtronic. He made these comments as a discussant for BeAT-HF.
The results that Dr. Zile reported are obviously very promising. It was a huge step forward when researchers identified medical treatments that can safely manipulate the autonomic nervous system in patients with heart failure with reduced ejection fraction. Now we are asking what else we can do because we have run into limits on what we can accomplish with drugs alone. The BeAT-HF study is a step in that direction.
Andrew D. Krahn, MD, is professor of medicine and head of cardiology at the University of British Columbia and St. Paul’s Hospital in Vancouver. He has been a consultant to Medtronic and he has received research funding from Boston Scientific and Medtronic. He made these comments as a discussant for BeAT-HF.
The results that Dr. Zile reported are obviously very promising. It was a huge step forward when researchers identified medical treatments that can safely manipulate the autonomic nervous system in patients with heart failure with reduced ejection fraction. Now we are asking what else we can do because we have run into limits on what we can accomplish with drugs alone. The BeAT-HF study is a step in that direction.
Andrew D. Krahn, MD, is professor of medicine and head of cardiology at the University of British Columbia and St. Paul’s Hospital in Vancouver. He has been a consultant to Medtronic and he has received research funding from Boston Scientific and Medtronic. He made these comments as a discussant for BeAT-HF.
SAN FRANCISCO – Baroreflex activation therapy met all four of its primary endpoints in its U.S. pivotal trial of 264 patients with advanced heart failure with reduced ejection fraction who were ineligible for cardiac resynchronization therapy.
The results showed that ongoing baroreflex activation therapy (BAT) via a single, stimulating electrode surgically placed on a patient’s carotid artery led to statistically significant and clinically meaningful improvements in quality of life and functional capacity while also reducing the level of a biomarker of heart failure severity in patients already on guideline-directed medical therapy, Michael R. Zile, MD, said at the annual scientific sessions of the Heart Rhythm Society. He estimated that the device is appropriate for perhaps a third or more of patients with heart failure with reduced ejection fraction (HFrEF), specifically patients with New York Heart Association functional class III disease who are not candidates for treatment with cardiac resynchronization therapy (CRT) and with a blood level of N-terminal pro–brain natriuretic peptide (NT-proBNP) of less than 1,600 pg/mL, a cutoff that excludes patients with very severe class III HFrEF and focuses on those who benefited in the study.
“To our knowledge, this is the first successful pivotal trial of device-based neuromodulation therapy in HFrEF patients,” said Dr. Zile, professor of medicine at the Medical University of South Carolina in Charleston. “We think that BAT fills an unmet need” in a large number of HFrEF patients. He stressed that the placement of the single, 2-mm, unilateral electrode on the baroreceptor-containing carotid sinus is an “extremely safe and simple” surgery. The electrode attaches to a small, subcutaneously placed generator.
Dr. Zile attributed the treatment’s success, in contrast to a prior, failed attempt to treat HFrEF by vagus nerve stimulation (J Am Coll Cardiol. 2016 Jul 12;68[2]:147-56) to BAT’s action via the patient’s brain, which processes the afferent signal it receives from stimulation to in turn inhibit sympathetic activation and upregulate parasympathetic innervation, with both actions benefiting HFrEF patients. “The integrated autonomic balance is the real difference with this device,” he said. Other helpful effects from BAT are reduced heart rate, reduced cardiac remodeling, increased vasodilation, a decrease in elevated blood pressure, increased diuresis, and a drop in renin secretion. The pivotal trial built on findings from a phase 2 study (JACC Heart Fail. 2015 Jun;3[6]:487-96).
The BeAT-HF (Barostim Neo - Baroreflex Activation Therapy for Heart Failure) trial enrolled patients with class III HFrEF with a left ventricular ejection fraction of 35% or less and a 6-minute walk distance of 150-400 m, who were ineligible for CRT, on optimal medical therapy, and who had an elevated NT-proBNP level. After the study randomized 271 patients to either BAT or ongoing medical therapy only (without use of a sham procedure or sham BAT), the results showed a statistically significant benefit for three of the four primary endpoints. Patients treated with BAT for 6 months had statistically significant and clinically meaningful improvements in their quality of life scores as measured on the Minnesota Living With Heart Failure Questionnaire, in their function as measured by the 6-min walk distance, and in the treatment’s safety, based on the combined rate of major adverse neurological and cardiovascular events, which occurred in 6% of patients treated with BAT, which was significantly better than the study’s prespecified performance goal of 15%.
However, for the fourth primary endpoint – reduction in blood levels of NT-proBNP – the BAT-treated patients showed no significant improvement, compared with the controls. The design of BeAT-HF called for consultation with the Food and Drug Administration in such a situation, and further data analysis showed that the problem may have been that some enrolled patients entered with extremely elevated levels of this biomarker. The agency authorized an added protocol that randomized 102 additional patients that matched the initial cohort but had a requirement for an NT-proBNP level of less than 1,600 pg/mL. The 6-month outcomes of these patients were combined with the previously determined outcomes for 162 of the original 271 patients who entered with NT-proBNP levels within the specified limit, producing a total, final study group of 264 patients, of whom 120 received BAT and completed 6-month follow-up, and 125 received medical therapy only and had 6-month follow-up. These patients averaged about 63 years of age, and 20% were women. On average they were on four heart failure medications, and more than three-quarters also had an implanted cardiac device.
The results from an analysis of this cohort showed a statistically significant, 25% relative reduction in blood levels of NT-proBNP in the BAT patients, compared with the controls, and it also confirmed statistically significant and meaningful improvements in quality of life and function on BAT, compared with controls. The 14-point average improvement in the quality of life score in BAT patients, compared with the controls, on the Minnesota Living With Heart Failure Questionnaire was nearly triple the point improvement that’s considered clinically meaningful and hence was “very convincing” about the treatment’s efficacy, noted Dr. Zile, who is also director of cardiology at the VA Medical Center in Charleston. The 25% drop in average NT-proBNP levels “predicts a marked reduction in morbidity and mortality.” He added that researchers have developed a percutaneous, transcatheter method for placing the carotid electrode that will soon undergo clinical testing.
These results “reconfirm the safety of BAT,” but are limited by a relatively short follow-up of 6 months, no data on survival benefit, and by not having echocardiographic data on possible cardiac remodeling, commented Sanjeev Saksena, MD, medical director of the Electrophysiology Research Foundation in Warren, N.J.
BeAT-HF was sponsored by CVRx, the company developing the baroreflex activation device. Dr. Zile has been a consultant to CVRx and to Abbott, AstraZeneca, Bayer, Bristol-Myers Squibb, Lilly, Merck, and Novartis. Dr. Saksena had no disclosures.
SOURCE: Zile MR. Heart Rhythm 2019, Abstract, S-LBCT01-04.
SAN FRANCISCO – Baroreflex activation therapy met all four of its primary endpoints in its U.S. pivotal trial of 264 patients with advanced heart failure with reduced ejection fraction who were ineligible for cardiac resynchronization therapy.
The results showed that ongoing baroreflex activation therapy (BAT) via a single, stimulating electrode surgically placed on a patient’s carotid artery led to statistically significant and clinically meaningful improvements in quality of life and functional capacity while also reducing the level of a biomarker of heart failure severity in patients already on guideline-directed medical therapy, Michael R. Zile, MD, said at the annual scientific sessions of the Heart Rhythm Society. He estimated that the device is appropriate for perhaps a third or more of patients with heart failure with reduced ejection fraction (HFrEF), specifically patients with New York Heart Association functional class III disease who are not candidates for treatment with cardiac resynchronization therapy (CRT) and with a blood level of N-terminal pro–brain natriuretic peptide (NT-proBNP) of less than 1,600 pg/mL, a cutoff that excludes patients with very severe class III HFrEF and focuses on those who benefited in the study.
“To our knowledge, this is the first successful pivotal trial of device-based neuromodulation therapy in HFrEF patients,” said Dr. Zile, professor of medicine at the Medical University of South Carolina in Charleston. “We think that BAT fills an unmet need” in a large number of HFrEF patients. He stressed that the placement of the single, 2-mm, unilateral electrode on the baroreceptor-containing carotid sinus is an “extremely safe and simple” surgery. The electrode attaches to a small, subcutaneously placed generator.
Dr. Zile attributed the treatment’s success, in contrast to a prior, failed attempt to treat HFrEF by vagus nerve stimulation (J Am Coll Cardiol. 2016 Jul 12;68[2]:147-56) to BAT’s action via the patient’s brain, which processes the afferent signal it receives from stimulation to in turn inhibit sympathetic activation and upregulate parasympathetic innervation, with both actions benefiting HFrEF patients. “The integrated autonomic balance is the real difference with this device,” he said. Other helpful effects from BAT are reduced heart rate, reduced cardiac remodeling, increased vasodilation, a decrease in elevated blood pressure, increased diuresis, and a drop in renin secretion. The pivotal trial built on findings from a phase 2 study (JACC Heart Fail. 2015 Jun;3[6]:487-96).
The BeAT-HF (Barostim Neo - Baroreflex Activation Therapy for Heart Failure) trial enrolled patients with class III HFrEF with a left ventricular ejection fraction of 35% or less and a 6-minute walk distance of 150-400 m, who were ineligible for CRT, on optimal medical therapy, and who had an elevated NT-proBNP level. After the study randomized 271 patients to either BAT or ongoing medical therapy only (without use of a sham procedure or sham BAT), the results showed a statistically significant benefit for three of the four primary endpoints. Patients treated with BAT for 6 months had statistically significant and clinically meaningful improvements in their quality of life scores as measured on the Minnesota Living With Heart Failure Questionnaire, in their function as measured by the 6-min walk distance, and in the treatment’s safety, based on the combined rate of major adverse neurological and cardiovascular events, which occurred in 6% of patients treated with BAT, which was significantly better than the study’s prespecified performance goal of 15%.
However, for the fourth primary endpoint – reduction in blood levels of NT-proBNP – the BAT-treated patients showed no significant improvement, compared with the controls. The design of BeAT-HF called for consultation with the Food and Drug Administration in such a situation, and further data analysis showed that the problem may have been that some enrolled patients entered with extremely elevated levels of this biomarker. The agency authorized an added protocol that randomized 102 additional patients that matched the initial cohort but had a requirement for an NT-proBNP level of less than 1,600 pg/mL. The 6-month outcomes of these patients were combined with the previously determined outcomes for 162 of the original 271 patients who entered with NT-proBNP levels within the specified limit, producing a total, final study group of 264 patients, of whom 120 received BAT and completed 6-month follow-up, and 125 received medical therapy only and had 6-month follow-up. These patients averaged about 63 years of age, and 20% were women. On average they were on four heart failure medications, and more than three-quarters also had an implanted cardiac device.
The results from an analysis of this cohort showed a statistically significant, 25% relative reduction in blood levels of NT-proBNP in the BAT patients, compared with the controls, and it also confirmed statistically significant and meaningful improvements in quality of life and function on BAT, compared with controls. The 14-point average improvement in the quality of life score in BAT patients, compared with the controls, on the Minnesota Living With Heart Failure Questionnaire was nearly triple the point improvement that’s considered clinically meaningful and hence was “very convincing” about the treatment’s efficacy, noted Dr. Zile, who is also director of cardiology at the VA Medical Center in Charleston. The 25% drop in average NT-proBNP levels “predicts a marked reduction in morbidity and mortality.” He added that researchers have developed a percutaneous, transcatheter method for placing the carotid electrode that will soon undergo clinical testing.
These results “reconfirm the safety of BAT,” but are limited by a relatively short follow-up of 6 months, no data on survival benefit, and by not having echocardiographic data on possible cardiac remodeling, commented Sanjeev Saksena, MD, medical director of the Electrophysiology Research Foundation in Warren, N.J.
BeAT-HF was sponsored by CVRx, the company developing the baroreflex activation device. Dr. Zile has been a consultant to CVRx and to Abbott, AstraZeneca, Bayer, Bristol-Myers Squibb, Lilly, Merck, and Novartis. Dr. Saksena had no disclosures.
SOURCE: Zile MR. Heart Rhythm 2019, Abstract, S-LBCT01-04.
REPORTING FROM HEART RHYTHM 2019
MADIT-CHIC: CRT aids patients with chemotherapy-induced cardiomyopathy
SAN FRANCISCO – Patients with cardiomyopathy secondary to cancer chemotherapy who qualified for cardiac resynchronization therapy (CRT) by having a wide QRS interval showed a virtually uniform, positive response to this treatment in a multicenter study with 30 patients.
This is the first time this therapy has been prospectively assessed in this patient population.
The results “show for the first time that patients with chemotherapy-induced cardiomyopathy [CHIC] who meet criteria for CRT show significant improvement in left ventricular function and clinical symptoms in the short term” during follow-up of 6 months, Jagmeet P. Singh, MD, said at the annual scientific sessions of the Heart Rhythm Society.
Dr. Singh acknowledged that, with 30 patients, the study was small, uncontrolled, had a brief follow-up of 6 months, and was highly selective. It took collaborating investigators at 12 U.S. centers more than 3.5 years to find the 30 participating patients, who had to meet very specific criteria designed to identify true CHIC. Nonetheless, Dr. Singh considered the results convincing enough to shift practice.
Based on the results, “I would certainly feel comfortable using CRT in patients with CHIC,” said Dr. Singh, associate chief of cardiology at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston. “If a patient has CHIC with a wide QRS interval and evidence for a conduction defect on their ECG, they are a great candidate for CRT. The results highlight that there is a cohort of patients who develop cardiomyopathy after chemotherapy, and these patients are often written off” and until now have generally received little follow-up for their potential development of cardiomyopathy. Dr. Singh expressed hope that the recent emergence of cardio-oncology as a subspecialty will focus attention on CHIC patients.
The MADIT-CHIC (Multicenter Automatic Defibrillator Implantation Trial – Chemotherapy-Induced Cardiomyopathy) study enrolled patients with a history of exposure to a cancer chemotherapy regimen known to cause cardiomyopathy who had no history of heart failure prior to the chemotherapy. All patients had developed clinically apparent heart failure (New York Heart Association functional class II, III, or IV) at least 6 months after completing chemotherapy, had no other apparent cause of the cardiomyopathy as ascertained by a cardio-oncologist, and were on guideline-directed medical therapy. Enrolled patients also had to have a class I or II indication for CRT, with a left ventricular ejection fraction of 35% or less, a QRS interval of at least 120 milliseconds, sinus rhythm and left bundle branch block, or no left bundle branch block and a QRS of at least 150 milliseconds.
Just over three-quarters of the patients had received an anthracycline drug, and 73% had a history of breast cancer, 20% a history of leukemia or lymphoma, and 7% had a history of sarcoma. The patients averaged 64 years of age, and 87% were women. CRT placement occurred 18-256 months after the end of chemotherapy, with a median of 188 months.
The study’s primary endpoint was the change in left ventricular ejection fraction after 6 months, which increased from an average of 28% at baseline to 39% at follow-up, a statistically significant change. Ejection fraction increased in 29 of the 30 patients, with one patient showing a flat response to CRT. Cardiac function and geometry significantly improved by seven other measures, including left ventricular mass and left atrial volume, and the improved ejection fraction was consistent across several subgroup analyses. Patients’ NYHA functional class improved by at least one level in 41% of patients, and 83% of the patients stopped showing clinical features of heart failure after 6 months on CRT.
MADIT-CHIC received funding from Boston Scientific. Dr. Singh has been a consultant to Abbott, Back Beat, Biotronik, Boston Scientific, EBR, Impulse Dynamics, Medtronic, Microport, St. Jude, and Toray, and he has received research support from Abbott and Boston Scientific.
SOURCE: Singh JP et al. HRS 2019, Abstract S-LBCT02-04.
No guideline currently addresses using cardiac resynchronization therapy to treat chemotherapy-induced cardiomyopathy. The findings from MADIT-CHIC showed a striking benefit from treatment with cardiac resynchronization therapy of a magnitude we would expect to see in patients with nonischemic cardiomyopathy. Patients showed improvements in all measures of cardiac performance.
It appears that CHIC can take as long as decades to appear in a patient, but we now need to have a high level of suspicion for this complication. We need to come up with better ways to monitor development of CHIC in patients who have received cancer chemotherapy so that we can give eligible patients this beneficial treatment. We can be optimistic about the potential for benefit from CRT in these patients.
Kenneth A. Ellenbogen, MD , is chief of cardiology and a professor of medicine at Virginia Commonwealth University in Richmond, Va. He has been a consultant to Boston Scientific, Medtronic, and St. Jude; he has received honoraria from Biotronik; and he has received research funding from Boston Scientific and Medtronic. He made these comments as the designated discussant for the MADIT-CHIC report.
No guideline currently addresses using cardiac resynchronization therapy to treat chemotherapy-induced cardiomyopathy. The findings from MADIT-CHIC showed a striking benefit from treatment with cardiac resynchronization therapy of a magnitude we would expect to see in patients with nonischemic cardiomyopathy. Patients showed improvements in all measures of cardiac performance.
It appears that CHIC can take as long as decades to appear in a patient, but we now need to have a high level of suspicion for this complication. We need to come up with better ways to monitor development of CHIC in patients who have received cancer chemotherapy so that we can give eligible patients this beneficial treatment. We can be optimistic about the potential for benefit from CRT in these patients.
Kenneth A. Ellenbogen, MD , is chief of cardiology and a professor of medicine at Virginia Commonwealth University in Richmond, Va. He has been a consultant to Boston Scientific, Medtronic, and St. Jude; he has received honoraria from Biotronik; and he has received research funding from Boston Scientific and Medtronic. He made these comments as the designated discussant for the MADIT-CHIC report.
No guideline currently addresses using cardiac resynchronization therapy to treat chemotherapy-induced cardiomyopathy. The findings from MADIT-CHIC showed a striking benefit from treatment with cardiac resynchronization therapy of a magnitude we would expect to see in patients with nonischemic cardiomyopathy. Patients showed improvements in all measures of cardiac performance.
It appears that CHIC can take as long as decades to appear in a patient, but we now need to have a high level of suspicion for this complication. We need to come up with better ways to monitor development of CHIC in patients who have received cancer chemotherapy so that we can give eligible patients this beneficial treatment. We can be optimistic about the potential for benefit from CRT in these patients.
Kenneth A. Ellenbogen, MD , is chief of cardiology and a professor of medicine at Virginia Commonwealth University in Richmond, Va. He has been a consultant to Boston Scientific, Medtronic, and St. Jude; he has received honoraria from Biotronik; and he has received research funding from Boston Scientific and Medtronic. He made these comments as the designated discussant for the MADIT-CHIC report.
SAN FRANCISCO – Patients with cardiomyopathy secondary to cancer chemotherapy who qualified for cardiac resynchronization therapy (CRT) by having a wide QRS interval showed a virtually uniform, positive response to this treatment in a multicenter study with 30 patients.
This is the first time this therapy has been prospectively assessed in this patient population.
The results “show for the first time that patients with chemotherapy-induced cardiomyopathy [CHIC] who meet criteria for CRT show significant improvement in left ventricular function and clinical symptoms in the short term” during follow-up of 6 months, Jagmeet P. Singh, MD, said at the annual scientific sessions of the Heart Rhythm Society.
Dr. Singh acknowledged that, with 30 patients, the study was small, uncontrolled, had a brief follow-up of 6 months, and was highly selective. It took collaborating investigators at 12 U.S. centers more than 3.5 years to find the 30 participating patients, who had to meet very specific criteria designed to identify true CHIC. Nonetheless, Dr. Singh considered the results convincing enough to shift practice.
Based on the results, “I would certainly feel comfortable using CRT in patients with CHIC,” said Dr. Singh, associate chief of cardiology at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston. “If a patient has CHIC with a wide QRS interval and evidence for a conduction defect on their ECG, they are a great candidate for CRT. The results highlight that there is a cohort of patients who develop cardiomyopathy after chemotherapy, and these patients are often written off” and until now have generally received little follow-up for their potential development of cardiomyopathy. Dr. Singh expressed hope that the recent emergence of cardio-oncology as a subspecialty will focus attention on CHIC patients.
The MADIT-CHIC (Multicenter Automatic Defibrillator Implantation Trial – Chemotherapy-Induced Cardiomyopathy) study enrolled patients with a history of exposure to a cancer chemotherapy regimen known to cause cardiomyopathy who had no history of heart failure prior to the chemotherapy. All patients had developed clinically apparent heart failure (New York Heart Association functional class II, III, or IV) at least 6 months after completing chemotherapy, had no other apparent cause of the cardiomyopathy as ascertained by a cardio-oncologist, and were on guideline-directed medical therapy. Enrolled patients also had to have a class I or II indication for CRT, with a left ventricular ejection fraction of 35% or less, a QRS interval of at least 120 milliseconds, sinus rhythm and left bundle branch block, or no left bundle branch block and a QRS of at least 150 milliseconds.
Just over three-quarters of the patients had received an anthracycline drug, and 73% had a history of breast cancer, 20% a history of leukemia or lymphoma, and 7% had a history of sarcoma. The patients averaged 64 years of age, and 87% were women. CRT placement occurred 18-256 months after the end of chemotherapy, with a median of 188 months.
The study’s primary endpoint was the change in left ventricular ejection fraction after 6 months, which increased from an average of 28% at baseline to 39% at follow-up, a statistically significant change. Ejection fraction increased in 29 of the 30 patients, with one patient showing a flat response to CRT. Cardiac function and geometry significantly improved by seven other measures, including left ventricular mass and left atrial volume, and the improved ejection fraction was consistent across several subgroup analyses. Patients’ NYHA functional class improved by at least one level in 41% of patients, and 83% of the patients stopped showing clinical features of heart failure after 6 months on CRT.
MADIT-CHIC received funding from Boston Scientific. Dr. Singh has been a consultant to Abbott, Back Beat, Biotronik, Boston Scientific, EBR, Impulse Dynamics, Medtronic, Microport, St. Jude, and Toray, and he has received research support from Abbott and Boston Scientific.
SOURCE: Singh JP et al. HRS 2019, Abstract S-LBCT02-04.
SAN FRANCISCO – Patients with cardiomyopathy secondary to cancer chemotherapy who qualified for cardiac resynchronization therapy (CRT) by having a wide QRS interval showed a virtually uniform, positive response to this treatment in a multicenter study with 30 patients.
This is the first time this therapy has been prospectively assessed in this patient population.
The results “show for the first time that patients with chemotherapy-induced cardiomyopathy [CHIC] who meet criteria for CRT show significant improvement in left ventricular function and clinical symptoms in the short term” during follow-up of 6 months, Jagmeet P. Singh, MD, said at the annual scientific sessions of the Heart Rhythm Society.
Dr. Singh acknowledged that, with 30 patients, the study was small, uncontrolled, had a brief follow-up of 6 months, and was highly selective. It took collaborating investigators at 12 U.S. centers more than 3.5 years to find the 30 participating patients, who had to meet very specific criteria designed to identify true CHIC. Nonetheless, Dr. Singh considered the results convincing enough to shift practice.
Based on the results, “I would certainly feel comfortable using CRT in patients with CHIC,” said Dr. Singh, associate chief of cardiology at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston. “If a patient has CHIC with a wide QRS interval and evidence for a conduction defect on their ECG, they are a great candidate for CRT. The results highlight that there is a cohort of patients who develop cardiomyopathy after chemotherapy, and these patients are often written off” and until now have generally received little follow-up for their potential development of cardiomyopathy. Dr. Singh expressed hope that the recent emergence of cardio-oncology as a subspecialty will focus attention on CHIC patients.
The MADIT-CHIC (Multicenter Automatic Defibrillator Implantation Trial – Chemotherapy-Induced Cardiomyopathy) study enrolled patients with a history of exposure to a cancer chemotherapy regimen known to cause cardiomyopathy who had no history of heart failure prior to the chemotherapy. All patients had developed clinically apparent heart failure (New York Heart Association functional class II, III, or IV) at least 6 months after completing chemotherapy, had no other apparent cause of the cardiomyopathy as ascertained by a cardio-oncologist, and were on guideline-directed medical therapy. Enrolled patients also had to have a class I or II indication for CRT, with a left ventricular ejection fraction of 35% or less, a QRS interval of at least 120 milliseconds, sinus rhythm and left bundle branch block, or no left bundle branch block and a QRS of at least 150 milliseconds.
Just over three-quarters of the patients had received an anthracycline drug, and 73% had a history of breast cancer, 20% a history of leukemia or lymphoma, and 7% had a history of sarcoma. The patients averaged 64 years of age, and 87% were women. CRT placement occurred 18-256 months after the end of chemotherapy, with a median of 188 months.
The study’s primary endpoint was the change in left ventricular ejection fraction after 6 months, which increased from an average of 28% at baseline to 39% at follow-up, a statistically significant change. Ejection fraction increased in 29 of the 30 patients, with one patient showing a flat response to CRT. Cardiac function and geometry significantly improved by seven other measures, including left ventricular mass and left atrial volume, and the improved ejection fraction was consistent across several subgroup analyses. Patients’ NYHA functional class improved by at least one level in 41% of patients, and 83% of the patients stopped showing clinical features of heart failure after 6 months on CRT.
MADIT-CHIC received funding from Boston Scientific. Dr. Singh has been a consultant to Abbott, Back Beat, Biotronik, Boston Scientific, EBR, Impulse Dynamics, Medtronic, Microport, St. Jude, and Toray, and he has received research support from Abbott and Boston Scientific.
SOURCE: Singh JP et al. HRS 2019, Abstract S-LBCT02-04.
REPORTING FROM HEART RHYTHM 2019