User login
Postmenopausal women: Walk farther and faster to reduce heart failure risk
Brisk walking for at least 40 minutes two or three times a week reduced the risk of heart failure by approximately 25% in postmenopausal women, according to data from more that 89,000 participants in the Women’s Health Initiative.
The benefits of walking are well understood, said Somwail Rasla, MD, of Saint Vincent Hospital in Worcester, Mass., but he and his colleagues focused for the first time on how the speed, frequency, and duration of walking affected health in older women who may be less likely to visit a gym or engage in a formal exercise program.
The researchers followed the women, aged 50-79 years, for approximately 10 years.
Overall, the risk of heart failure was 20%-25% less for women who walked at least twice a week than it was for women who walked less frequently. In addition, women who walked for at least 40 minutes per walk had a 21%-25% lower heart failure risk than did those who walked less than 40 minutes per walk.
Pace mattered as well, Dr. Rasla pointed out. Women walking at an average pace and a fast pace had, respectively, 26% and 38% lower heart failure risk, compared with women who walked at a casual pace.
The researchers measured the women’s energy expenditure using the Metabolic Equivalent of Task (MET), a value calculated using the women’s self-reports of their walking frequency, duration, and speed. The results were similar across different age groups, ethnicities, and baseline body weight, which suggests the findings can be generalized to apply to most women. “I think we could give the same advice [about walking] to women up to age 79,” said Dr. Rasla.
The findings were limited by the use of self-reports, Dr. Rasla noted. However, the results suggest that walking can be a valuable and accessible form of exercise for older women, he said.
The Women’s Health Initiative is sponsored by the National Institutes of Health. The investigators reported no relevant conflicts of interest.
SOURCE: Rasla S et al. ACC 18, Poster 1315M-03.
Brisk walking for at least 40 minutes two or three times a week reduced the risk of heart failure by approximately 25% in postmenopausal women, according to data from more that 89,000 participants in the Women’s Health Initiative.
The benefits of walking are well understood, said Somwail Rasla, MD, of Saint Vincent Hospital in Worcester, Mass., but he and his colleagues focused for the first time on how the speed, frequency, and duration of walking affected health in older women who may be less likely to visit a gym or engage in a formal exercise program.
The researchers followed the women, aged 50-79 years, for approximately 10 years.
Overall, the risk of heart failure was 20%-25% less for women who walked at least twice a week than it was for women who walked less frequently. In addition, women who walked for at least 40 minutes per walk had a 21%-25% lower heart failure risk than did those who walked less than 40 minutes per walk.
Pace mattered as well, Dr. Rasla pointed out. Women walking at an average pace and a fast pace had, respectively, 26% and 38% lower heart failure risk, compared with women who walked at a casual pace.
The researchers measured the women’s energy expenditure using the Metabolic Equivalent of Task (MET), a value calculated using the women’s self-reports of their walking frequency, duration, and speed. The results were similar across different age groups, ethnicities, and baseline body weight, which suggests the findings can be generalized to apply to most women. “I think we could give the same advice [about walking] to women up to age 79,” said Dr. Rasla.
The findings were limited by the use of self-reports, Dr. Rasla noted. However, the results suggest that walking can be a valuable and accessible form of exercise for older women, he said.
The Women’s Health Initiative is sponsored by the National Institutes of Health. The investigators reported no relevant conflicts of interest.
SOURCE: Rasla S et al. ACC 18, Poster 1315M-03.
Brisk walking for at least 40 minutes two or three times a week reduced the risk of heart failure by approximately 25% in postmenopausal women, according to data from more that 89,000 participants in the Women’s Health Initiative.
The benefits of walking are well understood, said Somwail Rasla, MD, of Saint Vincent Hospital in Worcester, Mass., but he and his colleagues focused for the first time on how the speed, frequency, and duration of walking affected health in older women who may be less likely to visit a gym or engage in a formal exercise program.
The researchers followed the women, aged 50-79 years, for approximately 10 years.
Overall, the risk of heart failure was 20%-25% less for women who walked at least twice a week than it was for women who walked less frequently. In addition, women who walked for at least 40 minutes per walk had a 21%-25% lower heart failure risk than did those who walked less than 40 minutes per walk.
Pace mattered as well, Dr. Rasla pointed out. Women walking at an average pace and a fast pace had, respectively, 26% and 38% lower heart failure risk, compared with women who walked at a casual pace.
The researchers measured the women’s energy expenditure using the Metabolic Equivalent of Task (MET), a value calculated using the women’s self-reports of their walking frequency, duration, and speed. The results were similar across different age groups, ethnicities, and baseline body weight, which suggests the findings can be generalized to apply to most women. “I think we could give the same advice [about walking] to women up to age 79,” said Dr. Rasla.
The findings were limited by the use of self-reports, Dr. Rasla noted. However, the results suggest that walking can be a valuable and accessible form of exercise for older women, he said.
The Women’s Health Initiative is sponsored by the National Institutes of Health. The investigators reported no relevant conflicts of interest.
SOURCE: Rasla S et al. ACC 18, Poster 1315M-03.
FROM ACC18
Key clinical point: Urge older female patients to walk briskly at least twice a week.
Major finding: Patients with a fast pace had a 38% lower risk of heart failure.
Study details: A long-term, national observational study of 89,270 women.
Disclosures: The Women’s Health Initiative is sponsored by the National Institutes of Health. The investigators reported no relevant conflicts of interest.
Source: Rasla S et al. ACC 18, Poster 1315M-03.
ACC Day 2: Late-Breaking Clinical Trial highlights
Of the late-breaking clinical trials to be presented on Sunday, March 11, at the annual meeting of the American College of Cardiology in Orlando, the meeting’s vice chair, Andrew Kates, MD, highlighted two in a press briefing: ARTEMIS and MOMENTUM 3.
ARTEMIS
The investigators of this unique trial sought to determine whether reducing patients’ cost burden for their medication would improve adherence and, in turn, improve patient outcomes.
Specifically, ARTEMIS (Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) evaluated whether eliminating copayments for patients after acute MI could optimize antiplatelet therapy selection and long-term adherence, as well as patient outcomes and overall cost of care following acute MI.
Dr. Kates, professor of medicine, Washington University, St. Louis, said ARTEMIS would go far in answering the question, “If costs are lowered, are guidelines followed?”
Tracy Wang, MD, of Duke University, Durham, N.C., will present the results on Sunday in a joint Late-Breaking Clinical Trial session with JAMA, being held at 8 a.m.-9 a.m. in the Main Tent (Hall C).
MOMENTUM 3
Last year, HeartMate 3, a fully magnetically levitated centrifugal-flow circulatory pump, showed better outcomes at 6 months than did HEARTMATE 2, an axial-flow pump, in the primary results of MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3).
Most impressive was the complete elimination of pump thrombosis in the patients with the smaller HeartMate 3 device. On Sunday, the 2-year results will reveal whether longer-term use of the novel technology will carry similar benefits, Dr. Kates said.
MOMENTUM 3 will be presented at the third Late-Breaking Clinical Trials session, at 10:45-11:45 a.m., in the Main Tent (Hall C) by Mandeep R. Mehra, MD, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Of the late-breaking clinical trials to be presented on Sunday, March 11, at the annual meeting of the American College of Cardiology in Orlando, the meeting’s vice chair, Andrew Kates, MD, highlighted two in a press briefing: ARTEMIS and MOMENTUM 3.
ARTEMIS
The investigators of this unique trial sought to determine whether reducing patients’ cost burden for their medication would improve adherence and, in turn, improve patient outcomes.
Specifically, ARTEMIS (Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) evaluated whether eliminating copayments for patients after acute MI could optimize antiplatelet therapy selection and long-term adherence, as well as patient outcomes and overall cost of care following acute MI.
Dr. Kates, professor of medicine, Washington University, St. Louis, said ARTEMIS would go far in answering the question, “If costs are lowered, are guidelines followed?”
Tracy Wang, MD, of Duke University, Durham, N.C., will present the results on Sunday in a joint Late-Breaking Clinical Trial session with JAMA, being held at 8 a.m.-9 a.m. in the Main Tent (Hall C).
MOMENTUM 3
Last year, HeartMate 3, a fully magnetically levitated centrifugal-flow circulatory pump, showed better outcomes at 6 months than did HEARTMATE 2, an axial-flow pump, in the primary results of MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3).
Most impressive was the complete elimination of pump thrombosis in the patients with the smaller HeartMate 3 device. On Sunday, the 2-year results will reveal whether longer-term use of the novel technology will carry similar benefits, Dr. Kates said.
MOMENTUM 3 will be presented at the third Late-Breaking Clinical Trials session, at 10:45-11:45 a.m., in the Main Tent (Hall C) by Mandeep R. Mehra, MD, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Of the late-breaking clinical trials to be presented on Sunday, March 11, at the annual meeting of the American College of Cardiology in Orlando, the meeting’s vice chair, Andrew Kates, MD, highlighted two in a press briefing: ARTEMIS and MOMENTUM 3.
ARTEMIS
The investigators of this unique trial sought to determine whether reducing patients’ cost burden for their medication would improve adherence and, in turn, improve patient outcomes.
Specifically, ARTEMIS (Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) evaluated whether eliminating copayments for patients after acute MI could optimize antiplatelet therapy selection and long-term adherence, as well as patient outcomes and overall cost of care following acute MI.
Dr. Kates, professor of medicine, Washington University, St. Louis, said ARTEMIS would go far in answering the question, “If costs are lowered, are guidelines followed?”
Tracy Wang, MD, of Duke University, Durham, N.C., will present the results on Sunday in a joint Late-Breaking Clinical Trial session with JAMA, being held at 8 a.m.-9 a.m. in the Main Tent (Hall C).
MOMENTUM 3
Last year, HeartMate 3, a fully magnetically levitated centrifugal-flow circulatory pump, showed better outcomes at 6 months than did HEARTMATE 2, an axial-flow pump, in the primary results of MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3).
Most impressive was the complete elimination of pump thrombosis in the patients with the smaller HeartMate 3 device. On Sunday, the 2-year results will reveal whether longer-term use of the novel technology will carry similar benefits, Dr. Kates said.
MOMENTUM 3 will be presented at the third Late-Breaking Clinical Trials session, at 10:45-11:45 a.m., in the Main Tent (Hall C) by Mandeep R. Mehra, MD, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Mild cognitive impairment rises in heart patients with comorbidities
ANAHEIM, CALIF. – Across the spectrum of cardiovascular disease, the more comorbid conditions a patient has, the higher the likelihood of mild cognitive impairment, Jocasta Ball, PhD, reported at the American Heart Association scientific sessions.
Indeed, her cross-sectional analysis of baseline data on 2,161 participants in five randomized controlled trials of nurse-led chronic disease management in cardiovascular disease (CVD) showed that for every 1-unit increase in the age-adjusted Charlson Comorbidity Index, the likelihood of mild cognitive impairment (MCI) jumped by 19%.
This novel observation has important clinical implications: “MCI is becoming increasingly recognized as exerting a powerful and negative impact on the risk, management, and prognosis of CVD patients,” explained Dr. Ball of the Baker Heart and Diabetes Institute in Melbourne. “Because MCI undermines a patient’s ability to comply with medical treatment and adds to patient complexity, it is critical [to identify] higher-risk individuals who require closer surveillance and improved early intervention.”
She added that the findings open up a whole new field of research aimed at developing new interventions to help patients with CVD and MCI stay on track with their heart disease treatment program.
The 2,161 subjects, mean age 70 years and two-thirds male, ranged across the full spectrum of cardiovascular disease, from mild to severe. All were screened for MCI by completing the Montreal Cognitive Assessment, or MoCA. A MoCA score below 26 out of a possible 30 is defined as MCI.
Forty-seven percent of subjects had MCI. They were older, with a mean age of 73 years versus 67 years; were more likely to have a history of stroke, by a margin of 20% versus 12%; had a 52% prevalence of atrial fibrillation versus 44%; and had a 50% prevalence of heart failure versus 39% in subjects with normal cognition. In addition, 48% of the MCI group screened positive for depressive symptoms versus 37% of those without MCI, and 28% of patients with MCI had type 2 diabetes, compared with 22% of those without MCI. Renal disease was also significantly more prevalent in the MCI group, by a margin of 21% versus 14%.
In a multivariate regression analysis, the strongest predictors of MCI in patients across the spectrum of CVD were current smoking, with a 2.5-fold increased risk compared with that of nonsmokers, and atrial fibrillation, with a 1.3-fold increased risk.
Dr. Ball reported having no financial conflicts regarding her study.
SOURCE: Ball J. et al. AHA 2017, Abstract 16240.
ANAHEIM, CALIF. – Across the spectrum of cardiovascular disease, the more comorbid conditions a patient has, the higher the likelihood of mild cognitive impairment, Jocasta Ball, PhD, reported at the American Heart Association scientific sessions.
Indeed, her cross-sectional analysis of baseline data on 2,161 participants in five randomized controlled trials of nurse-led chronic disease management in cardiovascular disease (CVD) showed that for every 1-unit increase in the age-adjusted Charlson Comorbidity Index, the likelihood of mild cognitive impairment (MCI) jumped by 19%.
This novel observation has important clinical implications: “MCI is becoming increasingly recognized as exerting a powerful and negative impact on the risk, management, and prognosis of CVD patients,” explained Dr. Ball of the Baker Heart and Diabetes Institute in Melbourne. “Because MCI undermines a patient’s ability to comply with medical treatment and adds to patient complexity, it is critical [to identify] higher-risk individuals who require closer surveillance and improved early intervention.”
She added that the findings open up a whole new field of research aimed at developing new interventions to help patients with CVD and MCI stay on track with their heart disease treatment program.
The 2,161 subjects, mean age 70 years and two-thirds male, ranged across the full spectrum of cardiovascular disease, from mild to severe. All were screened for MCI by completing the Montreal Cognitive Assessment, or MoCA. A MoCA score below 26 out of a possible 30 is defined as MCI.
Forty-seven percent of subjects had MCI. They were older, with a mean age of 73 years versus 67 years; were more likely to have a history of stroke, by a margin of 20% versus 12%; had a 52% prevalence of atrial fibrillation versus 44%; and had a 50% prevalence of heart failure versus 39% in subjects with normal cognition. In addition, 48% of the MCI group screened positive for depressive symptoms versus 37% of those without MCI, and 28% of patients with MCI had type 2 diabetes, compared with 22% of those without MCI. Renal disease was also significantly more prevalent in the MCI group, by a margin of 21% versus 14%.
In a multivariate regression analysis, the strongest predictors of MCI in patients across the spectrum of CVD were current smoking, with a 2.5-fold increased risk compared with that of nonsmokers, and atrial fibrillation, with a 1.3-fold increased risk.
Dr. Ball reported having no financial conflicts regarding her study.
SOURCE: Ball J. et al. AHA 2017, Abstract 16240.
ANAHEIM, CALIF. – Across the spectrum of cardiovascular disease, the more comorbid conditions a patient has, the higher the likelihood of mild cognitive impairment, Jocasta Ball, PhD, reported at the American Heart Association scientific sessions.
Indeed, her cross-sectional analysis of baseline data on 2,161 participants in five randomized controlled trials of nurse-led chronic disease management in cardiovascular disease (CVD) showed that for every 1-unit increase in the age-adjusted Charlson Comorbidity Index, the likelihood of mild cognitive impairment (MCI) jumped by 19%.
This novel observation has important clinical implications: “MCI is becoming increasingly recognized as exerting a powerful and negative impact on the risk, management, and prognosis of CVD patients,” explained Dr. Ball of the Baker Heart and Diabetes Institute in Melbourne. “Because MCI undermines a patient’s ability to comply with medical treatment and adds to patient complexity, it is critical [to identify] higher-risk individuals who require closer surveillance and improved early intervention.”
She added that the findings open up a whole new field of research aimed at developing new interventions to help patients with CVD and MCI stay on track with their heart disease treatment program.
The 2,161 subjects, mean age 70 years and two-thirds male, ranged across the full spectrum of cardiovascular disease, from mild to severe. All were screened for MCI by completing the Montreal Cognitive Assessment, or MoCA. A MoCA score below 26 out of a possible 30 is defined as MCI.
Forty-seven percent of subjects had MCI. They were older, with a mean age of 73 years versus 67 years; were more likely to have a history of stroke, by a margin of 20% versus 12%; had a 52% prevalence of atrial fibrillation versus 44%; and had a 50% prevalence of heart failure versus 39% in subjects with normal cognition. In addition, 48% of the MCI group screened positive for depressive symptoms versus 37% of those without MCI, and 28% of patients with MCI had type 2 diabetes, compared with 22% of those without MCI. Renal disease was also significantly more prevalent in the MCI group, by a margin of 21% versus 14%.
In a multivariate regression analysis, the strongest predictors of MCI in patients across the spectrum of CVD were current smoking, with a 2.5-fold increased risk compared with that of nonsmokers, and atrial fibrillation, with a 1.3-fold increased risk.
Dr. Ball reported having no financial conflicts regarding her study.
SOURCE: Ball J. et al. AHA 2017, Abstract 16240.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The more comorbid conditions a patient with cardiovascular disease has, the greater the likelihood of mild cognitive impairment becomes.
Major finding: For each 1-unit increase in the Charlson Comorbidity Index, the likelihood of prevalent mild cognitive impairment rose by 19%.
Study details: This cross-sectional study assessed the association between mild cognitive impairment and Charlson Comorbidity Index score in 2,161 patients with cardiovascular disease of varied degrees of severity.
Disclosures: The presenter reported having no financial conflicts regarding her study.
Source: Ball J et al. AHA 2017, Abstract 16240
200 cardiovascular drugs now in development
, according to Pharmaceutical Research and Manufacturers of America.
One of those 42 treatments is “a nonviral gene therapy that targets a tissue repair and regeneration pathway in the body,” PhRMA noted. A total of 200 medicines, including those for lipid disorders and hypertension, were either in clinical trials or undergoing regulatory review as of Jan. 24, 2018, the group reported. Some treatments are being considered for more than one condition.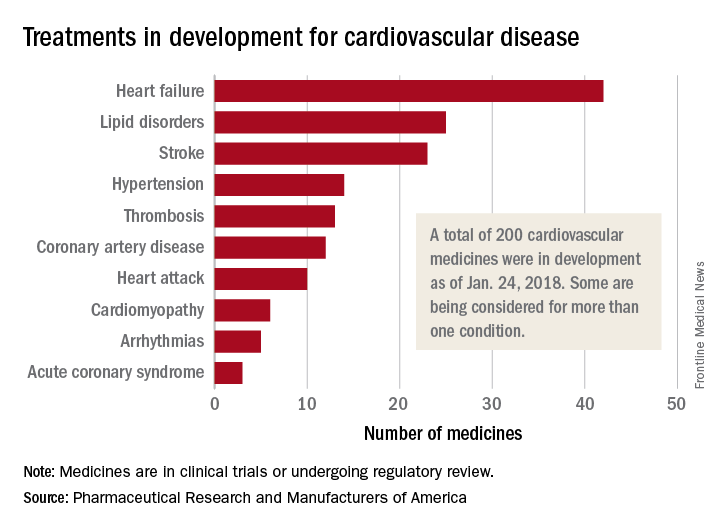
, according to Pharmaceutical Research and Manufacturers of America.
One of those 42 treatments is “a nonviral gene therapy that targets a tissue repair and regeneration pathway in the body,” PhRMA noted. A total of 200 medicines, including those for lipid disorders and hypertension, were either in clinical trials or undergoing regulatory review as of Jan. 24, 2018, the group reported. Some treatments are being considered for more than one condition.
, according to Pharmaceutical Research and Manufacturers of America.
One of those 42 treatments is “a nonviral gene therapy that targets a tissue repair and regeneration pathway in the body,” PhRMA noted. A total of 200 medicines, including those for lipid disorders and hypertension, were either in clinical trials or undergoing regulatory review as of Jan. 24, 2018, the group reported. Some treatments are being considered for more than one condition.
Abruptio placenta brings increased cardiovascular risk – and soon
ANAHEIM, CALIF. – Women who experience placental abruption are at significantly increased risk for multiple forms of cardiovascular disease beginning within the first few years after their pregnancy complication, according to a study of more than 1.6 million California women.
While gestational hypertension, preeclampsia, and fetal growth restriction have previously all been shown to be associated with increased risk of incident cardiovascular disease, this huge California study provides the first strong epidemiologic evidence that placental abruption is as well. Prior studies looking at the issue have been underpowered, Michael J. Healey, MD, said at the American Heart Association scientific sessions.
“Our hypothesis is that there might be some type of shared mechanism, probably involving microvascular dysfunction, that explains the relationships we see between these pregnancy complications and increased near-term risk of cardiovascular disease,” he explained in an interview.
Dr. Healy, a hospitalist attached to the heart failure service at the University of California, San Francisco, presented a retrospective study of a multiethnic cohort comprising 1,614,950 parous women aged 15-50 years who participated in the California Healthcare Cost and Utility Project during 2005-2009. Placental abruption occurred in 15,057 of them at a mean age of 29.2 years.
During a median 4.9 years of follow-up, women who experienced abruptio placenta were at 6% increased risk for heart failure, 11% greater risk for MI, 8% increased risk for hypertensive urgency, and 2% greater risk for myocardial infarction with no obstructive atherosclerosis (MINOCA) in an age- and race-adjusted analysis. All of these were statistically significant differences.
Of note, however, in a multivariate analysis fully adjusted for standard cardiovascular risk factors, as well as hypercoagulability, preterm birth, grand multiparity, and insurance status, placental abruption was independently associated with a 2.14-fold risk of MINOCA, but it was no longer linked to significantly increased risks of the other cardiovascular events.
The implication is that the increased risk of these other forms of cardiovascular disease is mediated through the women’s increased prevalence of the traditional cardiovascular risk factors, whereas a novel mechanism – most likely microvascular dysfunction – underlies the association between placental abruption and MINOCA, according to Dr. Healy.
He plans to extend this research by taking a look at the relationship between placental abruption and the various subtypes of MINOCA, including coronary dissection, vasospasm, thrombophilia disorders, and stress cardiomyopathy, in order to examine whether the increased risk posed by placental abruption is concentrated in certain forms of MINOCA. Data on MINOCA subtypes were recorded as part of the California project.
He reported having no financial conflicts of interest regarding his study.
ANAHEIM, CALIF. – Women who experience placental abruption are at significantly increased risk for multiple forms of cardiovascular disease beginning within the first few years after their pregnancy complication, according to a study of more than 1.6 million California women.
While gestational hypertension, preeclampsia, and fetal growth restriction have previously all been shown to be associated with increased risk of incident cardiovascular disease, this huge California study provides the first strong epidemiologic evidence that placental abruption is as well. Prior studies looking at the issue have been underpowered, Michael J. Healey, MD, said at the American Heart Association scientific sessions.
“Our hypothesis is that there might be some type of shared mechanism, probably involving microvascular dysfunction, that explains the relationships we see between these pregnancy complications and increased near-term risk of cardiovascular disease,” he explained in an interview.
Dr. Healy, a hospitalist attached to the heart failure service at the University of California, San Francisco, presented a retrospective study of a multiethnic cohort comprising 1,614,950 parous women aged 15-50 years who participated in the California Healthcare Cost and Utility Project during 2005-2009. Placental abruption occurred in 15,057 of them at a mean age of 29.2 years.
During a median 4.9 years of follow-up, women who experienced abruptio placenta were at 6% increased risk for heart failure, 11% greater risk for MI, 8% increased risk for hypertensive urgency, and 2% greater risk for myocardial infarction with no obstructive atherosclerosis (MINOCA) in an age- and race-adjusted analysis. All of these were statistically significant differences.
Of note, however, in a multivariate analysis fully adjusted for standard cardiovascular risk factors, as well as hypercoagulability, preterm birth, grand multiparity, and insurance status, placental abruption was independently associated with a 2.14-fold risk of MINOCA, but it was no longer linked to significantly increased risks of the other cardiovascular events.
The implication is that the increased risk of these other forms of cardiovascular disease is mediated through the women’s increased prevalence of the traditional cardiovascular risk factors, whereas a novel mechanism – most likely microvascular dysfunction – underlies the association between placental abruption and MINOCA, according to Dr. Healy.
He plans to extend this research by taking a look at the relationship between placental abruption and the various subtypes of MINOCA, including coronary dissection, vasospasm, thrombophilia disorders, and stress cardiomyopathy, in order to examine whether the increased risk posed by placental abruption is concentrated in certain forms of MINOCA. Data on MINOCA subtypes were recorded as part of the California project.
He reported having no financial conflicts of interest regarding his study.
ANAHEIM, CALIF. – Women who experience placental abruption are at significantly increased risk for multiple forms of cardiovascular disease beginning within the first few years after their pregnancy complication, according to a study of more than 1.6 million California women.
While gestational hypertension, preeclampsia, and fetal growth restriction have previously all been shown to be associated with increased risk of incident cardiovascular disease, this huge California study provides the first strong epidemiologic evidence that placental abruption is as well. Prior studies looking at the issue have been underpowered, Michael J. Healey, MD, said at the American Heart Association scientific sessions.
“Our hypothesis is that there might be some type of shared mechanism, probably involving microvascular dysfunction, that explains the relationships we see between these pregnancy complications and increased near-term risk of cardiovascular disease,” he explained in an interview.
Dr. Healy, a hospitalist attached to the heart failure service at the University of California, San Francisco, presented a retrospective study of a multiethnic cohort comprising 1,614,950 parous women aged 15-50 years who participated in the California Healthcare Cost and Utility Project during 2005-2009. Placental abruption occurred in 15,057 of them at a mean age of 29.2 years.
During a median 4.9 years of follow-up, women who experienced abruptio placenta were at 6% increased risk for heart failure, 11% greater risk for MI, 8% increased risk for hypertensive urgency, and 2% greater risk for myocardial infarction with no obstructive atherosclerosis (MINOCA) in an age- and race-adjusted analysis. All of these were statistically significant differences.
Of note, however, in a multivariate analysis fully adjusted for standard cardiovascular risk factors, as well as hypercoagulability, preterm birth, grand multiparity, and insurance status, placental abruption was independently associated with a 2.14-fold risk of MINOCA, but it was no longer linked to significantly increased risks of the other cardiovascular events.
The implication is that the increased risk of these other forms of cardiovascular disease is mediated through the women’s increased prevalence of the traditional cardiovascular risk factors, whereas a novel mechanism – most likely microvascular dysfunction – underlies the association between placental abruption and MINOCA, according to Dr. Healy.
He plans to extend this research by taking a look at the relationship between placental abruption and the various subtypes of MINOCA, including coronary dissection, vasospasm, thrombophilia disorders, and stress cardiomyopathy, in order to examine whether the increased risk posed by placental abruption is concentrated in certain forms of MINOCA. Data on MINOCA subtypes were recorded as part of the California project.
He reported having no financial conflicts of interest regarding his study.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Placental abruption is associated with increased risk of maternal cardiovascular events within a few years after delivery.
Major finding: Placental abruption was independently associated with a 2.14-fold increased risk of myocardial infarction with no obstructive atherosclerosis during a median 4.9 years of follow-up.
Study details: This was a retrospective study of more than 1.6 million parous women enrolled in the California Healthcare Cost and Utilization Project, including 15,057 with placental abruption.
Disclosures: The study presenter reported having no financial conflicts of interest.
Overweight and obese individuals face greater cardiovascular morbidity
.
In a report published online Feb. 28 in JAMA Cardiology, researchers presented an analysis of pooled data from 190,672 participants and 3.2 million person-years of follow-up in 10 prospective cohort studies, including the Framingham Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Atherosclerosis Risk in Communities Study.
Incident cardiovascular events occurred in 37% of overweight middle-aged men and 28% of overweight middle-aged women. In obese middle-aged men and women, those figures were 47% and 39%, respectively, and in the morbidly obese they were 65% and 48%. By comparison, incident cardiovascular events occurred in 32% of middle-aged men of normal BMI, and 22% of women.
Across all the studies, there were 7,136 fatal or nonfatal myocardial infarctions, 3,733 fatal or nonfatal strokes, 4,614 diagnoses of heart failure, and 13,457 cardiovascular disease events during 856,532 person-years of follow-up in middle-aged adults.
After adjustment for age, ethnicity, and smoking status, the competing hazard ratios for experiencing a cardiovascular disease event compared to a noncardiovascular disease death were greater in the higher-BMI categories, and greatest among morbidly obese middle-aged men and women, largely because of a greater proportion of coronary heart disease and heart failure events.
“In addition, greater all-cause mortality in higher-BMI categories occurred at the expense of a greater proportion of deaths from cardiovascular causes in middle-aged men and women who are overweight and obese,” wrote Sadiya S. Khan, MD, MSc, of Northwestern University, Chicago, and her coauthors.
The research suggested that for each increasing unit of BMI in middle-aged men and women, the adjusted competing hazard ratios of incident cardiovascular disease events increased by a significant 5%.
The study found that middle-aged men in the normal and overweight BMI group enjoyed more years free from cardiovascular disease than did obese middle-aged men. In middle-aged women, those who were in the normal BMI range had significantly more years lived free of cardiovascular disease than did overweight or obese women.
The incidence of cardiovascular disease was significantly delayed by an average of 7.5 years in middle-aged men of normal BMI and 7.1 years in middle-aged women of normal BMI, compared with those with morbid obesity.
In terms of longevity, men and women with normal BMI lived on average 5.6 years and 2 years longer, respectively, than did men and women with morbid obesity.
“The results of this study build on prior research from the Cardiovascular Disease Lifetime Risk Pooling Project highlighting marked differences in lifetime risks of CVD and further highlight the importance of consideration of BMI as a risk factor for diminished healthy longevity and greater overall CVD morbidity and mortality,” the authors wrote.
The study was intended to address recent controversy over the health implications of overweight, with some evidence suggesting that overweight individuals have all-cause mortality similar to or lower than that of normal-weight groups.
“While we do observe evidence of the well-described overweight and obesity paradox, in which heavier individuals appear to live longer on average after diagnosis of CVD compared with individuals with normal BMI, our data when following up individuals prior to the onset of CVD indicate that this occurs because of a trend toward earlier onset of disease in individuals who are overweight and obese,” they wrote.
The study did not account for change in BMI over the course of follow-up, nor did it use data on fat distribution or the degree of visceral adiposity, the researchers noted.
“Additional important outcomes of obesity-related morbidity, such as atrial fibrillation, sleep-disordered breathing, and chronic liver disease, were not ascertained routinely in our cohort studies, and we likely underestimated the overall comorbidity burden of excess weight.”
The National Heart, Lung, and Blood Institute supported the study. No conflicts of interest were declared.
SOURCE: Khan SS et al. JAMA Cardiol. 2018 Feb 28. doi: 10.1001/jamacardio.2018.0022.
.
In a report published online Feb. 28 in JAMA Cardiology, researchers presented an analysis of pooled data from 190,672 participants and 3.2 million person-years of follow-up in 10 prospective cohort studies, including the Framingham Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Atherosclerosis Risk in Communities Study.
Incident cardiovascular events occurred in 37% of overweight middle-aged men and 28% of overweight middle-aged women. In obese middle-aged men and women, those figures were 47% and 39%, respectively, and in the morbidly obese they were 65% and 48%. By comparison, incident cardiovascular events occurred in 32% of middle-aged men of normal BMI, and 22% of women.
Across all the studies, there were 7,136 fatal or nonfatal myocardial infarctions, 3,733 fatal or nonfatal strokes, 4,614 diagnoses of heart failure, and 13,457 cardiovascular disease events during 856,532 person-years of follow-up in middle-aged adults.
After adjustment for age, ethnicity, and smoking status, the competing hazard ratios for experiencing a cardiovascular disease event compared to a noncardiovascular disease death were greater in the higher-BMI categories, and greatest among morbidly obese middle-aged men and women, largely because of a greater proportion of coronary heart disease and heart failure events.
“In addition, greater all-cause mortality in higher-BMI categories occurred at the expense of a greater proportion of deaths from cardiovascular causes in middle-aged men and women who are overweight and obese,” wrote Sadiya S. Khan, MD, MSc, of Northwestern University, Chicago, and her coauthors.
The research suggested that for each increasing unit of BMI in middle-aged men and women, the adjusted competing hazard ratios of incident cardiovascular disease events increased by a significant 5%.
The study found that middle-aged men in the normal and overweight BMI group enjoyed more years free from cardiovascular disease than did obese middle-aged men. In middle-aged women, those who were in the normal BMI range had significantly more years lived free of cardiovascular disease than did overweight or obese women.
The incidence of cardiovascular disease was significantly delayed by an average of 7.5 years in middle-aged men of normal BMI and 7.1 years in middle-aged women of normal BMI, compared with those with morbid obesity.
In terms of longevity, men and women with normal BMI lived on average 5.6 years and 2 years longer, respectively, than did men and women with morbid obesity.
“The results of this study build on prior research from the Cardiovascular Disease Lifetime Risk Pooling Project highlighting marked differences in lifetime risks of CVD and further highlight the importance of consideration of BMI as a risk factor for diminished healthy longevity and greater overall CVD morbidity and mortality,” the authors wrote.
The study was intended to address recent controversy over the health implications of overweight, with some evidence suggesting that overweight individuals have all-cause mortality similar to or lower than that of normal-weight groups.
“While we do observe evidence of the well-described overweight and obesity paradox, in which heavier individuals appear to live longer on average after diagnosis of CVD compared with individuals with normal BMI, our data when following up individuals prior to the onset of CVD indicate that this occurs because of a trend toward earlier onset of disease in individuals who are overweight and obese,” they wrote.
The study did not account for change in BMI over the course of follow-up, nor did it use data on fat distribution or the degree of visceral adiposity, the researchers noted.
“Additional important outcomes of obesity-related morbidity, such as atrial fibrillation, sleep-disordered breathing, and chronic liver disease, were not ascertained routinely in our cohort studies, and we likely underestimated the overall comorbidity burden of excess weight.”
The National Heart, Lung, and Blood Institute supported the study. No conflicts of interest were declared.
SOURCE: Khan SS et al. JAMA Cardiol. 2018 Feb 28. doi: 10.1001/jamacardio.2018.0022.
.
In a report published online Feb. 28 in JAMA Cardiology, researchers presented an analysis of pooled data from 190,672 participants and 3.2 million person-years of follow-up in 10 prospective cohort studies, including the Framingham Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Atherosclerosis Risk in Communities Study.
Incident cardiovascular events occurred in 37% of overweight middle-aged men and 28% of overweight middle-aged women. In obese middle-aged men and women, those figures were 47% and 39%, respectively, and in the morbidly obese they were 65% and 48%. By comparison, incident cardiovascular events occurred in 32% of middle-aged men of normal BMI, and 22% of women.
Across all the studies, there were 7,136 fatal or nonfatal myocardial infarctions, 3,733 fatal or nonfatal strokes, 4,614 diagnoses of heart failure, and 13,457 cardiovascular disease events during 856,532 person-years of follow-up in middle-aged adults.
After adjustment for age, ethnicity, and smoking status, the competing hazard ratios for experiencing a cardiovascular disease event compared to a noncardiovascular disease death were greater in the higher-BMI categories, and greatest among morbidly obese middle-aged men and women, largely because of a greater proportion of coronary heart disease and heart failure events.
“In addition, greater all-cause mortality in higher-BMI categories occurred at the expense of a greater proportion of deaths from cardiovascular causes in middle-aged men and women who are overweight and obese,” wrote Sadiya S. Khan, MD, MSc, of Northwestern University, Chicago, and her coauthors.
The research suggested that for each increasing unit of BMI in middle-aged men and women, the adjusted competing hazard ratios of incident cardiovascular disease events increased by a significant 5%.
The study found that middle-aged men in the normal and overweight BMI group enjoyed more years free from cardiovascular disease than did obese middle-aged men. In middle-aged women, those who were in the normal BMI range had significantly more years lived free of cardiovascular disease than did overweight or obese women.
The incidence of cardiovascular disease was significantly delayed by an average of 7.5 years in middle-aged men of normal BMI and 7.1 years in middle-aged women of normal BMI, compared with those with morbid obesity.
In terms of longevity, men and women with normal BMI lived on average 5.6 years and 2 years longer, respectively, than did men and women with morbid obesity.
“The results of this study build on prior research from the Cardiovascular Disease Lifetime Risk Pooling Project highlighting marked differences in lifetime risks of CVD and further highlight the importance of consideration of BMI as a risk factor for diminished healthy longevity and greater overall CVD morbidity and mortality,” the authors wrote.
The study was intended to address recent controversy over the health implications of overweight, with some evidence suggesting that overweight individuals have all-cause mortality similar to or lower than that of normal-weight groups.
“While we do observe evidence of the well-described overweight and obesity paradox, in which heavier individuals appear to live longer on average after diagnosis of CVD compared with individuals with normal BMI, our data when following up individuals prior to the onset of CVD indicate that this occurs because of a trend toward earlier onset of disease in individuals who are overweight and obese,” they wrote.
The study did not account for change in BMI over the course of follow-up, nor did it use data on fat distribution or the degree of visceral adiposity, the researchers noted.
“Additional important outcomes of obesity-related morbidity, such as atrial fibrillation, sleep-disordered breathing, and chronic liver disease, were not ascertained routinely in our cohort studies, and we likely underestimated the overall comorbidity burden of excess weight.”
The National Heart, Lung, and Blood Institute supported the study. No conflicts of interest were declared.
SOURCE: Khan SS et al. JAMA Cardiol. 2018 Feb 28. doi: 10.1001/jamacardio.2018.0022.
FROM JAMA CARDIOLOGY
Key clinical point: Obese individuals have shorter life spans and spend significantly more time dealing with the burden of cardiovascular morbidity than do normal-weight individuals.
Major finding: Overweight and obese middle-aged individuals have a significantly higher incidence of cardiovascular events and mortality compared with normal-weight middle-aged individuals.
Data source: Analysis of pooled data from 190,672 participants and 3.2 million person-years of follow-up in 10 prospective cohort studies.
Disclosures: The National Heart, Lung, and Blood Institute supported the study. No conflicts of interest were declared.
Source: Khan SS et al. JAMA Cardiol. 2018 Feb 28. doi: 10.1001/jamacardio.2018.0022
Viremic suppression linked to decreased MACE rate in patients with HCV-cirrhosis
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
FROM AMERICAN HEART JOURNAL
Key clinical point: Predictive factors for MACE in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin.
Major finding: Achieving viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis.
Study details: A study at 35 French centers of 878 patients with HCV-related cirrhosis.
Disclosures: The authors reported having no conflicts of interest.
Source: Cacoub, P et al. Am Heart J. 2018;198:4-17.
AHA: Heart health helps optimize breast cancer outcomes
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
FROM CIRCULATION
Learn ‘four Ds’ approach to heart failure in diabetes
LOS ANGELES – , such as lung cancer, because diabetes makes the pathophysiology of heart failure worse, according to Mark Kearney, MD.
“Diabetes amplifies the neurohormonal response to heart failure, so it drives progressive heart failure and increases the risk for sudden death,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “As left ventricular function goes down, patients with diabetes have heightened activation of the renin angiotensin system. They have increased left ventricular hypertrophy, and they have increased sympathetic nervous system activation.”
Dr. Kearney, a cardiologist who directs the Leeds Institute of Cardiovascular & Metabolic Medicine at Leeds (England) University, offered a “four Ds” framework that clinicians can use to improve prognosis in these patients.
1. Use a loop diuretic to control symptoms. “If the patient has fluid retention, it’s important to get them out of congestive cardiac syndrome as quickly as possible,” he said.
2. Disease modification with beta-blockers and ACE inhibitors, to the maximal dose tolerated. “These are still the mainstays of treatment for patients with heart failure, and they’re even more important in patients with diabetes and heart failure,” he said.
3. Consider device therapy, including defibrillators and resynchronization therapy.4. Optimize diabetes management.
“If you keep these things in your mind as you see a patient with heart failure and diabetes, it will give you a guide to approaching these patients,” said Dr. Kearney, who is also research lead for heart failure services at the University of Leeds. “When I see patients, I’ll check for edema right away. If they have it, I’ll increase the diuretic, and I’ll think about the different steps in the treatment pathway.”
He described heart failure due to systolic dysfunction as a reduction in cardiac output that doesn’t meet the demands of the body, the endpoint of a whole range of insults to the left ventricle.
“The most common cause today is ischemic heart disease; it used to be hypertension,” he said. “People over 40 have a one in five chance of developing heart failure, so it’s really important for all of us to improve outcomes in this terrible syndrome.”
According to a study of nearly 2,000 unselected patients conducted by Dr. Kearney and his associates, those with diabetes and heart failure are more likely to have ischemia, compared with those who have heart failure and no diabetes (75% vs. 58%, respectively), lower hemoglobin (13 g/dL vs. 13.7 g/dL), and worse renal function (eGFR of 51 mL/min per 1.732 m2 vs 57 mL/min per 1.732 m2) (Diab Vasc Dis Res. 2013 Jul;10[4]:330-6).
He added that type 2 diabetes is a sudden death risk equivalent to patients with ischemic heart disease and left ventricular systolic dysfunction (Heart 2016 May 15;102[10]:735-40).
“So, if you have diabetes and the U.K. National Institute for Health and Care Excellence guidelines indication for a defibrillator, your risk of sudden death in 5 years is probably 50%,” Dr. Kearney said. “The best treatment in this case is a prophylactic defibrillator.”
ACE inhibitors are used to protect these patients against cardiac myocyte cell death and vasoconstriction, while beta-blockers are used to protect against the activation of the sympathetic nervous system.
“Often, patients don’t like taking beta-blockers because they say they make them feel tired – when in fact they don’t realize it’s their heart failure that’s making them feel tired,” Dr. Kearney said.
He and his associates examined the effect of different drugs doses on all-cause mortality at 5 years. They found that, among patients with heart failure, reduced ejection fraction, and no diabetes, ramipril conferred a 3% improvement in mortality per milligram. At the same time, the mortality among patients with diabetes and heart failure who did not receive a beta-blocker was about 7%.
However, the absolute gain from beta-blocker use in patients with diabetes and heart failure was three times that of patients without diabetes.
“So, every milligram you increase the dose by, there’s an associated improvement in risk,” Dr. Kearney said. “Over 5 years, comparing the lowest beta-blocker dose to the highest beta-blocker dose, it was 1 year of life gained. So, when I see my patients and they ask about side effects of beta-blockers, I now say to them, ‘The side effects actually make you live longer.’”
He concluded his remarks by noting that while he is not a diabetes expert, it’s clear that diabetes is intimately linked to the pathophysiology of heart failure.
“If you’re insulin resistant, you have hypertension, hyperglycemia, you have inflammation and bone marrow dysfunction – all of which can exacerbate left ventricle dysfunction,” he said. “You have a syndrome in which you have cardiac dysfunction and metabolic dysfunction that conspire to lead to worsening of left ventricular dysfunction.”
Dr. Kearney disclosed that he has been a speaker for Merck.
LOS ANGELES – , such as lung cancer, because diabetes makes the pathophysiology of heart failure worse, according to Mark Kearney, MD.
“Diabetes amplifies the neurohormonal response to heart failure, so it drives progressive heart failure and increases the risk for sudden death,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “As left ventricular function goes down, patients with diabetes have heightened activation of the renin angiotensin system. They have increased left ventricular hypertrophy, and they have increased sympathetic nervous system activation.”
Dr. Kearney, a cardiologist who directs the Leeds Institute of Cardiovascular & Metabolic Medicine at Leeds (England) University, offered a “four Ds” framework that clinicians can use to improve prognosis in these patients.
1. Use a loop diuretic to control symptoms. “If the patient has fluid retention, it’s important to get them out of congestive cardiac syndrome as quickly as possible,” he said.
2. Disease modification with beta-blockers and ACE inhibitors, to the maximal dose tolerated. “These are still the mainstays of treatment for patients with heart failure, and they’re even more important in patients with diabetes and heart failure,” he said.
3. Consider device therapy, including defibrillators and resynchronization therapy.4. Optimize diabetes management.
“If you keep these things in your mind as you see a patient with heart failure and diabetes, it will give you a guide to approaching these patients,” said Dr. Kearney, who is also research lead for heart failure services at the University of Leeds. “When I see patients, I’ll check for edema right away. If they have it, I’ll increase the diuretic, and I’ll think about the different steps in the treatment pathway.”
He described heart failure due to systolic dysfunction as a reduction in cardiac output that doesn’t meet the demands of the body, the endpoint of a whole range of insults to the left ventricle.
“The most common cause today is ischemic heart disease; it used to be hypertension,” he said. “People over 40 have a one in five chance of developing heart failure, so it’s really important for all of us to improve outcomes in this terrible syndrome.”
According to a study of nearly 2,000 unselected patients conducted by Dr. Kearney and his associates, those with diabetes and heart failure are more likely to have ischemia, compared with those who have heart failure and no diabetes (75% vs. 58%, respectively), lower hemoglobin (13 g/dL vs. 13.7 g/dL), and worse renal function (eGFR of 51 mL/min per 1.732 m2 vs 57 mL/min per 1.732 m2) (Diab Vasc Dis Res. 2013 Jul;10[4]:330-6).
He added that type 2 diabetes is a sudden death risk equivalent to patients with ischemic heart disease and left ventricular systolic dysfunction (Heart 2016 May 15;102[10]:735-40).
“So, if you have diabetes and the U.K. National Institute for Health and Care Excellence guidelines indication for a defibrillator, your risk of sudden death in 5 years is probably 50%,” Dr. Kearney said. “The best treatment in this case is a prophylactic defibrillator.”
ACE inhibitors are used to protect these patients against cardiac myocyte cell death and vasoconstriction, while beta-blockers are used to protect against the activation of the sympathetic nervous system.
“Often, patients don’t like taking beta-blockers because they say they make them feel tired – when in fact they don’t realize it’s their heart failure that’s making them feel tired,” Dr. Kearney said.
He and his associates examined the effect of different drugs doses on all-cause mortality at 5 years. They found that, among patients with heart failure, reduced ejection fraction, and no diabetes, ramipril conferred a 3% improvement in mortality per milligram. At the same time, the mortality among patients with diabetes and heart failure who did not receive a beta-blocker was about 7%.
However, the absolute gain from beta-blocker use in patients with diabetes and heart failure was three times that of patients without diabetes.
“So, every milligram you increase the dose by, there’s an associated improvement in risk,” Dr. Kearney said. “Over 5 years, comparing the lowest beta-blocker dose to the highest beta-blocker dose, it was 1 year of life gained. So, when I see my patients and they ask about side effects of beta-blockers, I now say to them, ‘The side effects actually make you live longer.’”
He concluded his remarks by noting that while he is not a diabetes expert, it’s clear that diabetes is intimately linked to the pathophysiology of heart failure.
“If you’re insulin resistant, you have hypertension, hyperglycemia, you have inflammation and bone marrow dysfunction – all of which can exacerbate left ventricle dysfunction,” he said. “You have a syndrome in which you have cardiac dysfunction and metabolic dysfunction that conspire to lead to worsening of left ventricular dysfunction.”
Dr. Kearney disclosed that he has been a speaker for Merck.
LOS ANGELES – , such as lung cancer, because diabetes makes the pathophysiology of heart failure worse, according to Mark Kearney, MD.
“Diabetes amplifies the neurohormonal response to heart failure, so it drives progressive heart failure and increases the risk for sudden death,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “As left ventricular function goes down, patients with diabetes have heightened activation of the renin angiotensin system. They have increased left ventricular hypertrophy, and they have increased sympathetic nervous system activation.”
Dr. Kearney, a cardiologist who directs the Leeds Institute of Cardiovascular & Metabolic Medicine at Leeds (England) University, offered a “four Ds” framework that clinicians can use to improve prognosis in these patients.
1. Use a loop diuretic to control symptoms. “If the patient has fluid retention, it’s important to get them out of congestive cardiac syndrome as quickly as possible,” he said.
2. Disease modification with beta-blockers and ACE inhibitors, to the maximal dose tolerated. “These are still the mainstays of treatment for patients with heart failure, and they’re even more important in patients with diabetes and heart failure,” he said.
3. Consider device therapy, including defibrillators and resynchronization therapy.4. Optimize diabetes management.
“If you keep these things in your mind as you see a patient with heart failure and diabetes, it will give you a guide to approaching these patients,” said Dr. Kearney, who is also research lead for heart failure services at the University of Leeds. “When I see patients, I’ll check for edema right away. If they have it, I’ll increase the diuretic, and I’ll think about the different steps in the treatment pathway.”
He described heart failure due to systolic dysfunction as a reduction in cardiac output that doesn’t meet the demands of the body, the endpoint of a whole range of insults to the left ventricle.
“The most common cause today is ischemic heart disease; it used to be hypertension,” he said. “People over 40 have a one in five chance of developing heart failure, so it’s really important for all of us to improve outcomes in this terrible syndrome.”
According to a study of nearly 2,000 unselected patients conducted by Dr. Kearney and his associates, those with diabetes and heart failure are more likely to have ischemia, compared with those who have heart failure and no diabetes (75% vs. 58%, respectively), lower hemoglobin (13 g/dL vs. 13.7 g/dL), and worse renal function (eGFR of 51 mL/min per 1.732 m2 vs 57 mL/min per 1.732 m2) (Diab Vasc Dis Res. 2013 Jul;10[4]:330-6).
He added that type 2 diabetes is a sudden death risk equivalent to patients with ischemic heart disease and left ventricular systolic dysfunction (Heart 2016 May 15;102[10]:735-40).
“So, if you have diabetes and the U.K. National Institute for Health and Care Excellence guidelines indication for a defibrillator, your risk of sudden death in 5 years is probably 50%,” Dr. Kearney said. “The best treatment in this case is a prophylactic defibrillator.”
ACE inhibitors are used to protect these patients against cardiac myocyte cell death and vasoconstriction, while beta-blockers are used to protect against the activation of the sympathetic nervous system.
“Often, patients don’t like taking beta-blockers because they say they make them feel tired – when in fact they don’t realize it’s their heart failure that’s making them feel tired,” Dr. Kearney said.
He and his associates examined the effect of different drugs doses on all-cause mortality at 5 years. They found that, among patients with heart failure, reduced ejection fraction, and no diabetes, ramipril conferred a 3% improvement in mortality per milligram. At the same time, the mortality among patients with diabetes and heart failure who did not receive a beta-blocker was about 7%.
However, the absolute gain from beta-blocker use in patients with diabetes and heart failure was three times that of patients without diabetes.
“So, every milligram you increase the dose by, there’s an associated improvement in risk,” Dr. Kearney said. “Over 5 years, comparing the lowest beta-blocker dose to the highest beta-blocker dose, it was 1 year of life gained. So, when I see my patients and they ask about side effects of beta-blockers, I now say to them, ‘The side effects actually make you live longer.’”
He concluded his remarks by noting that while he is not a diabetes expert, it’s clear that diabetes is intimately linked to the pathophysiology of heart failure.
“If you’re insulin resistant, you have hypertension, hyperglycemia, you have inflammation and bone marrow dysfunction – all of which can exacerbate left ventricle dysfunction,” he said. “You have a syndrome in which you have cardiac dysfunction and metabolic dysfunction that conspire to lead to worsening of left ventricular dysfunction.”
Dr. Kearney disclosed that he has been a speaker for Merck.
EXPERT ANALYSIS FROM WCIRDC 2017
Isolated severe tricuspid regurgitation: An emerging disease
SNOWMASS, COLO. – that’s treatable, provided affected patients are referred for surgery before the clinical course progresses to intractable right heart disease with cirrhosis and liver failure, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass.
“This is an emerging disease that you’re all going to see in your practices this year. You’ve got to know what to do with these patients. Get to them early,” urged Dr. Nishimura, professor of cardiovascular sciences and hypertension at the Mayo Clinic in Rochester, Minn.
The medical textbooks don’t discuss isolated severe tricuspid regurgitation (ISTR) or its etiology. ISTR is a disorder of progressive right ventricular dilation and dysfunction whose etiology involves either longstanding atrial fibrillation or valvular disruption due to interference from a crossing lead of a permanent pacemaker or implantable cardioverter defibrillator.
“This is something different. These patients have a normal left heart and left heart valves and normal pressures, with no pulmonary hypertension. So it doesn’t fit into any of the textbook categories of tricuspid regurgitation,” the cardiologist said.
Moreover, the current American College of Cardiology/American Heart Association guidelines on valvular heart disease don’t address ISTR, either. Physicians who attempt to apply the guidelines in deciding when to refer a patient with ISTR for surgery will oftentimes find they’ve waited too long and the patient has started to develop end-stage disease, according to Dr. Nishimura.
And he should know: He was lead author of the current ACC/AHA guidelines (J Am Coll Cardiol. 2014 Jun 10;63[22]:2438-88).
Decades ago, Eugene Braunwald, MD, of Harvard Medical School, Boston, famously called the tricuspid valve “the forgotten valve.” The history of the Snowmass winter cardiology conference bears that out. During 2007-2017, the conference featured an average of 5.4 sessions per year on aortic valve disease, 4.5 sessions per year on mitral valve disease, and not a single session on tricuspid valve disease. But the tricuspid valve is forgotten no longer, Dr. Nishimura emphasized.
How ISTR presents
The affected patient has a history of either longstanding atrial fibrillation or a permanent pacemaker or ICD.
“This is something that 4 or 5 years ago people said didn’t exist. Our pacemaker people told me, ‘Nah, you can never get tricuspid regurgitation from our leads.’ Now it’s one of the leading causes of tricuspid regurgitation going to operation,” said Dr. Nishimura.
The presenting symptoms of ISTR are typically ascites, edema, and shortness of breath.
“Why should patients with a right heart problem get dyspnea? It turns out that when the right ventricle dilates it pushes the septum in, so the effective operative compliance of the left ventricle decreases and you actually see the pulmonary artery wedge pressure go up,” he explained.
On physical examination, the patient will have elevated jugular venous pressure with large V waves.
“This is a clue that something is going on. The patient will have neck veins jumping up to her ear lobes. The ear lobes are going to wiggle with every heart beat – boom, boom, boom. If you see that, you start to figure out what’s going on. You need nothing else,” Dr. Nishimura said.
The patient will likely also have a pulsatile enlarged liver and, even though this is valve disease, a murmur that’s either soft or inaudible.
Echocardiographic diagnosis
Echocardiography will show a dilated right ventricle and right atrium, a dilated inferior vena cava, and a normal left ventricle with no pulmonary hypertension. The classic sign of ISTR on continuous wave Doppler echocardiography is a dagger-shaped tricuspid regurgitation peak velocity signal of less than 2.5 meters/sec, which indicates the absence of pulmonary hypertension. This dagger shape occurs because the right atrial pressure equalizes the right ventricular pressure.
It’s also important to point the echo probe at the hepatic veins to spot another echocardiographic hallmark of ISTR: systolic reversal.
A thorough echo exam makes hemodynamic catheterization unnecessary in these patients, Dr. Nishimura added.
When to refer for tricuspid valve repair or replacement
The clinical course of ISTR is progressive, often rapidly so. It starts with elevated jugular venous pressure, then comes fatigue and shortness of breath, moving on to ascites and edema, then finally cirrhosis and renal failure. It’s a vicious cycle in which tricuspid regurgitation begets annular dilation, which causes chordal stretching and worsening tricuspid regurgitation, leading to further annular dilation.
Patients typically aren’t referred for surgery – and may not even present to a physician – until they’ve already developed end-stage disease. That’s probably why the outcomes of surgery for ISTR are so poor. Dr. Nishimura was senior investigator of a recent retrospective study of national trends and outcomes for ISTR surgery based on the National Inpatient Sample. The number of operations increased by 250% during a recent 10-year period, but the surgery is still rare: 290 operations in 2004, climbing to 780 nationwide in 2013.
In-hospital mortality remained steady over time at 8.8%, far higher than rates of in-hospital mortality for surgery for aortic and mitral valve disease, which today stand at 1%-2% or less. The adjusted risk of in-hospital mortality for tricuspid valve replacement in patients with ISTR was 1.9-fold greater than for valve repair (J Am Coll Cardiol. 2017 Dec 19;70[24]:2953-60).
“I think the reason the operative risk of valve surgery for ISTR is so high is that we’re waiting until patients have end-stage disease,” Dr. Nishimura said.
Indeed, he recommends referral for surgery as soon as the echocardiographic diagnosis of ISTR is made in a patient with huge neck veins.
“This will probably take the operative risk down by going to a time when the right ventricle can still recover,” he added.
In a patient with ISTR and pacemaker or defibrillator leads crossing the valve, tricuspid valve repair or replacement should be accompanied by exteriorization of the leads.
Dr. Nishimura reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – that’s treatable, provided affected patients are referred for surgery before the clinical course progresses to intractable right heart disease with cirrhosis and liver failure, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass.
“This is an emerging disease that you’re all going to see in your practices this year. You’ve got to know what to do with these patients. Get to them early,” urged Dr. Nishimura, professor of cardiovascular sciences and hypertension at the Mayo Clinic in Rochester, Minn.
The medical textbooks don’t discuss isolated severe tricuspid regurgitation (ISTR) or its etiology. ISTR is a disorder of progressive right ventricular dilation and dysfunction whose etiology involves either longstanding atrial fibrillation or valvular disruption due to interference from a crossing lead of a permanent pacemaker or implantable cardioverter defibrillator.
“This is something different. These patients have a normal left heart and left heart valves and normal pressures, with no pulmonary hypertension. So it doesn’t fit into any of the textbook categories of tricuspid regurgitation,” the cardiologist said.
Moreover, the current American College of Cardiology/American Heart Association guidelines on valvular heart disease don’t address ISTR, either. Physicians who attempt to apply the guidelines in deciding when to refer a patient with ISTR for surgery will oftentimes find they’ve waited too long and the patient has started to develop end-stage disease, according to Dr. Nishimura.
And he should know: He was lead author of the current ACC/AHA guidelines (J Am Coll Cardiol. 2014 Jun 10;63[22]:2438-88).
Decades ago, Eugene Braunwald, MD, of Harvard Medical School, Boston, famously called the tricuspid valve “the forgotten valve.” The history of the Snowmass winter cardiology conference bears that out. During 2007-2017, the conference featured an average of 5.4 sessions per year on aortic valve disease, 4.5 sessions per year on mitral valve disease, and not a single session on tricuspid valve disease. But the tricuspid valve is forgotten no longer, Dr. Nishimura emphasized.
How ISTR presents
The affected patient has a history of either longstanding atrial fibrillation or a permanent pacemaker or ICD.
“This is something that 4 or 5 years ago people said didn’t exist. Our pacemaker people told me, ‘Nah, you can never get tricuspid regurgitation from our leads.’ Now it’s one of the leading causes of tricuspid regurgitation going to operation,” said Dr. Nishimura.
The presenting symptoms of ISTR are typically ascites, edema, and shortness of breath.
“Why should patients with a right heart problem get dyspnea? It turns out that when the right ventricle dilates it pushes the septum in, so the effective operative compliance of the left ventricle decreases and you actually see the pulmonary artery wedge pressure go up,” he explained.
On physical examination, the patient will have elevated jugular venous pressure with large V waves.
“This is a clue that something is going on. The patient will have neck veins jumping up to her ear lobes. The ear lobes are going to wiggle with every heart beat – boom, boom, boom. If you see that, you start to figure out what’s going on. You need nothing else,” Dr. Nishimura said.
The patient will likely also have a pulsatile enlarged liver and, even though this is valve disease, a murmur that’s either soft or inaudible.
Echocardiographic diagnosis
Echocardiography will show a dilated right ventricle and right atrium, a dilated inferior vena cava, and a normal left ventricle with no pulmonary hypertension. The classic sign of ISTR on continuous wave Doppler echocardiography is a dagger-shaped tricuspid regurgitation peak velocity signal of less than 2.5 meters/sec, which indicates the absence of pulmonary hypertension. This dagger shape occurs because the right atrial pressure equalizes the right ventricular pressure.
It’s also important to point the echo probe at the hepatic veins to spot another echocardiographic hallmark of ISTR: systolic reversal.
A thorough echo exam makes hemodynamic catheterization unnecessary in these patients, Dr. Nishimura added.
When to refer for tricuspid valve repair or replacement
The clinical course of ISTR is progressive, often rapidly so. It starts with elevated jugular venous pressure, then comes fatigue and shortness of breath, moving on to ascites and edema, then finally cirrhosis and renal failure. It’s a vicious cycle in which tricuspid regurgitation begets annular dilation, which causes chordal stretching and worsening tricuspid regurgitation, leading to further annular dilation.
Patients typically aren’t referred for surgery – and may not even present to a physician – until they’ve already developed end-stage disease. That’s probably why the outcomes of surgery for ISTR are so poor. Dr. Nishimura was senior investigator of a recent retrospective study of national trends and outcomes for ISTR surgery based on the National Inpatient Sample. The number of operations increased by 250% during a recent 10-year period, but the surgery is still rare: 290 operations in 2004, climbing to 780 nationwide in 2013.
In-hospital mortality remained steady over time at 8.8%, far higher than rates of in-hospital mortality for surgery for aortic and mitral valve disease, which today stand at 1%-2% or less. The adjusted risk of in-hospital mortality for tricuspid valve replacement in patients with ISTR was 1.9-fold greater than for valve repair (J Am Coll Cardiol. 2017 Dec 19;70[24]:2953-60).
“I think the reason the operative risk of valve surgery for ISTR is so high is that we’re waiting until patients have end-stage disease,” Dr. Nishimura said.
Indeed, he recommends referral for surgery as soon as the echocardiographic diagnosis of ISTR is made in a patient with huge neck veins.
“This will probably take the operative risk down by going to a time when the right ventricle can still recover,” he added.
In a patient with ISTR and pacemaker or defibrillator leads crossing the valve, tricuspid valve repair or replacement should be accompanied by exteriorization of the leads.
Dr. Nishimura reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – that’s treatable, provided affected patients are referred for surgery before the clinical course progresses to intractable right heart disease with cirrhosis and liver failure, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass.
“This is an emerging disease that you’re all going to see in your practices this year. You’ve got to know what to do with these patients. Get to them early,” urged Dr. Nishimura, professor of cardiovascular sciences and hypertension at the Mayo Clinic in Rochester, Minn.
The medical textbooks don’t discuss isolated severe tricuspid regurgitation (ISTR) or its etiology. ISTR is a disorder of progressive right ventricular dilation and dysfunction whose etiology involves either longstanding atrial fibrillation or valvular disruption due to interference from a crossing lead of a permanent pacemaker or implantable cardioverter defibrillator.
“This is something different. These patients have a normal left heart and left heart valves and normal pressures, with no pulmonary hypertension. So it doesn’t fit into any of the textbook categories of tricuspid regurgitation,” the cardiologist said.
Moreover, the current American College of Cardiology/American Heart Association guidelines on valvular heart disease don’t address ISTR, either. Physicians who attempt to apply the guidelines in deciding when to refer a patient with ISTR for surgery will oftentimes find they’ve waited too long and the patient has started to develop end-stage disease, according to Dr. Nishimura.
And he should know: He was lead author of the current ACC/AHA guidelines (J Am Coll Cardiol. 2014 Jun 10;63[22]:2438-88).
Decades ago, Eugene Braunwald, MD, of Harvard Medical School, Boston, famously called the tricuspid valve “the forgotten valve.” The history of the Snowmass winter cardiology conference bears that out. During 2007-2017, the conference featured an average of 5.4 sessions per year on aortic valve disease, 4.5 sessions per year on mitral valve disease, and not a single session on tricuspid valve disease. But the tricuspid valve is forgotten no longer, Dr. Nishimura emphasized.
How ISTR presents
The affected patient has a history of either longstanding atrial fibrillation or a permanent pacemaker or ICD.
“This is something that 4 or 5 years ago people said didn’t exist. Our pacemaker people told me, ‘Nah, you can never get tricuspid regurgitation from our leads.’ Now it’s one of the leading causes of tricuspid regurgitation going to operation,” said Dr. Nishimura.
The presenting symptoms of ISTR are typically ascites, edema, and shortness of breath.
“Why should patients with a right heart problem get dyspnea? It turns out that when the right ventricle dilates it pushes the septum in, so the effective operative compliance of the left ventricle decreases and you actually see the pulmonary artery wedge pressure go up,” he explained.
On physical examination, the patient will have elevated jugular venous pressure with large V waves.
“This is a clue that something is going on. The patient will have neck veins jumping up to her ear lobes. The ear lobes are going to wiggle with every heart beat – boom, boom, boom. If you see that, you start to figure out what’s going on. You need nothing else,” Dr. Nishimura said.
The patient will likely also have a pulsatile enlarged liver and, even though this is valve disease, a murmur that’s either soft or inaudible.
Echocardiographic diagnosis
Echocardiography will show a dilated right ventricle and right atrium, a dilated inferior vena cava, and a normal left ventricle with no pulmonary hypertension. The classic sign of ISTR on continuous wave Doppler echocardiography is a dagger-shaped tricuspid regurgitation peak velocity signal of less than 2.5 meters/sec, which indicates the absence of pulmonary hypertension. This dagger shape occurs because the right atrial pressure equalizes the right ventricular pressure.
It’s also important to point the echo probe at the hepatic veins to spot another echocardiographic hallmark of ISTR: systolic reversal.
A thorough echo exam makes hemodynamic catheterization unnecessary in these patients, Dr. Nishimura added.
When to refer for tricuspid valve repair or replacement
The clinical course of ISTR is progressive, often rapidly so. It starts with elevated jugular venous pressure, then comes fatigue and shortness of breath, moving on to ascites and edema, then finally cirrhosis and renal failure. It’s a vicious cycle in which tricuspid regurgitation begets annular dilation, which causes chordal stretching and worsening tricuspid regurgitation, leading to further annular dilation.
Patients typically aren’t referred for surgery – and may not even present to a physician – until they’ve already developed end-stage disease. That’s probably why the outcomes of surgery for ISTR are so poor. Dr. Nishimura was senior investigator of a recent retrospective study of national trends and outcomes for ISTR surgery based on the National Inpatient Sample. The number of operations increased by 250% during a recent 10-year period, but the surgery is still rare: 290 operations in 2004, climbing to 780 nationwide in 2013.
In-hospital mortality remained steady over time at 8.8%, far higher than rates of in-hospital mortality for surgery for aortic and mitral valve disease, which today stand at 1%-2% or less. The adjusted risk of in-hospital mortality for tricuspid valve replacement in patients with ISTR was 1.9-fold greater than for valve repair (J Am Coll Cardiol. 2017 Dec 19;70[24]:2953-60).
“I think the reason the operative risk of valve surgery for ISTR is so high is that we’re waiting until patients have end-stage disease,” Dr. Nishimura said.
Indeed, he recommends referral for surgery as soon as the echocardiographic diagnosis of ISTR is made in a patient with huge neck veins.
“This will probably take the operative risk down by going to a time when the right ventricle can still recover,” he added.
In a patient with ISTR and pacemaker or defibrillator leads crossing the valve, tricuspid valve repair or replacement should be accompanied by exteriorization of the leads.
Dr. Nishimura reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS