User login
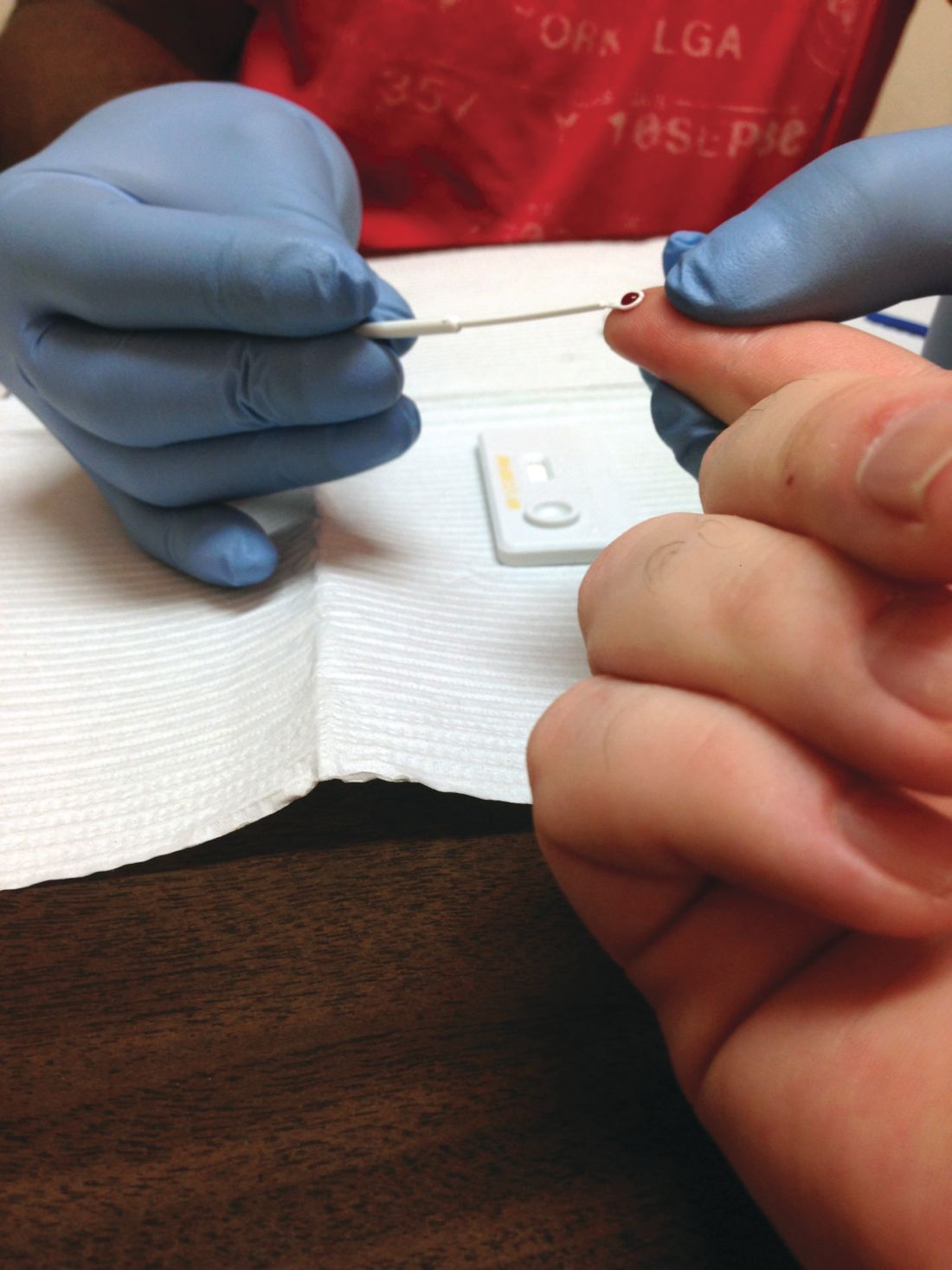
More than half of U.S. adults have never received HIV screening
Despite a 2006 recommendation by the Centers for Disease Control and Prevention recommending universal screening for HIV, less than half of U.S. adults have ever been tested for the disease, a new study found.
“HIV screening is a critical entry point to a range of HIV prevention and treatment options. For persons at ongoing risk for HIV infection exposure, annual screening also offers the opportunity to discuss options to reduce risk, including HIV preexposure prophylaxis,” Marc A. Pitasi, MPH, and investigators from the CDC wrote in the Morbidity and Mortality Weekly Report.
The CDC investigators analyzed data collected from the Behavioral Risk Factor Surveillance System in 2016-2017 to assess the percentage of adults screened for HIV ever and in the past year across the entirety of the United States, in 50 local jurisdictions where the majority of new U.S. HIV cases occur, and in seven states with a disproportionately high rate of HIV in rural populations.
The rate of ever testing was 38.9% across the entire country, 46.9% in the 50 local jurisdictions, and 35.5% in the seven states. The percentage of adults who had undergone HIV screening in the past year was significantly smaller at 10.1% across the entire country, 14.5% in the 50 local jurisdictions, and 9.3% in the seven states. This improved to 29.2%, 34.3%, and 26.2%, respectively, in adults who were tested in the past year who were also at risk for the disease.
The rate of ever testing varied widely among the 50 jurisdictions, ranging from 36.5% in Maricopa County, Ariz., to 70.7% in Washington, D.C. However, in addition to D.C., only Baltimore, Bronx County (N.Y.), and New York County reached or exceeded 60% coverage. Bronx County also had the highest rate of HIV screening in the past year at 31.3%, while Alameda County, Calif., had the lowest rate at 8.1%.
Of the seven states (Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina), Mississippi had the highest overall ever-testing rate at 40.2%, and Oklahoma had the lowest at 29.7%. People living in rural areas in those states were less likely to have ever been tested (32.1%) than those living in urban areas (37.2%). The difference between rural and urban areas increased for people at risk for HIV who had been screened in the past year, with 18.4% and 29.0%, respectively, reporting undergoing screening.
“These data provide a baseline from which to measure changes in screening in these jurisdictions and other parts of the United States over time. To achieve national goals and end the HIV epidemic in the United States, innovative and novel screening approaches might be needed to reach segments of the population that have never been tested for HIV,” the investigators concluded.
The investigators reported no conflicts of interest.
SOURCE: Pitasi MA et al. MMWR Morb Mortal Wkly Rep. 2019;68:561-7.
Despite a 2006 recommendation by the Centers for Disease Control and Prevention recommending universal screening for HIV, less than half of U.S. adults have ever been tested for the disease, a new study found.
“HIV screening is a critical entry point to a range of HIV prevention and treatment options. For persons at ongoing risk for HIV infection exposure, annual screening also offers the opportunity to discuss options to reduce risk, including HIV preexposure prophylaxis,” Marc A. Pitasi, MPH, and investigators from the CDC wrote in the Morbidity and Mortality Weekly Report.
The CDC investigators analyzed data collected from the Behavioral Risk Factor Surveillance System in 2016-2017 to assess the percentage of adults screened for HIV ever and in the past year across the entirety of the United States, in 50 local jurisdictions where the majority of new U.S. HIV cases occur, and in seven states with a disproportionately high rate of HIV in rural populations.
The rate of ever testing was 38.9% across the entire country, 46.9% in the 50 local jurisdictions, and 35.5% in the seven states. The percentage of adults who had undergone HIV screening in the past year was significantly smaller at 10.1% across the entire country, 14.5% in the 50 local jurisdictions, and 9.3% in the seven states. This improved to 29.2%, 34.3%, and 26.2%, respectively, in adults who were tested in the past year who were also at risk for the disease.
The rate of ever testing varied widely among the 50 jurisdictions, ranging from 36.5% in Maricopa County, Ariz., to 70.7% in Washington, D.C. However, in addition to D.C., only Baltimore, Bronx County (N.Y.), and New York County reached or exceeded 60% coverage. Bronx County also had the highest rate of HIV screening in the past year at 31.3%, while Alameda County, Calif., had the lowest rate at 8.1%.
Of the seven states (Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina), Mississippi had the highest overall ever-testing rate at 40.2%, and Oklahoma had the lowest at 29.7%. People living in rural areas in those states were less likely to have ever been tested (32.1%) than those living in urban areas (37.2%). The difference between rural and urban areas increased for people at risk for HIV who had been screened in the past year, with 18.4% and 29.0%, respectively, reporting undergoing screening.
“These data provide a baseline from which to measure changes in screening in these jurisdictions and other parts of the United States over time. To achieve national goals and end the HIV epidemic in the United States, innovative and novel screening approaches might be needed to reach segments of the population that have never been tested for HIV,” the investigators concluded.
The investigators reported no conflicts of interest.
SOURCE: Pitasi MA et al. MMWR Morb Mortal Wkly Rep. 2019;68:561-7.
Despite a 2006 recommendation by the Centers for Disease Control and Prevention recommending universal screening for HIV, less than half of U.S. adults have ever been tested for the disease, a new study found.
“HIV screening is a critical entry point to a range of HIV prevention and treatment options. For persons at ongoing risk for HIV infection exposure, annual screening also offers the opportunity to discuss options to reduce risk, including HIV preexposure prophylaxis,” Marc A. Pitasi, MPH, and investigators from the CDC wrote in the Morbidity and Mortality Weekly Report.
The CDC investigators analyzed data collected from the Behavioral Risk Factor Surveillance System in 2016-2017 to assess the percentage of adults screened for HIV ever and in the past year across the entirety of the United States, in 50 local jurisdictions where the majority of new U.S. HIV cases occur, and in seven states with a disproportionately high rate of HIV in rural populations.
The rate of ever testing was 38.9% across the entire country, 46.9% in the 50 local jurisdictions, and 35.5% in the seven states. The percentage of adults who had undergone HIV screening in the past year was significantly smaller at 10.1% across the entire country, 14.5% in the 50 local jurisdictions, and 9.3% in the seven states. This improved to 29.2%, 34.3%, and 26.2%, respectively, in adults who were tested in the past year who were also at risk for the disease.
The rate of ever testing varied widely among the 50 jurisdictions, ranging from 36.5% in Maricopa County, Ariz., to 70.7% in Washington, D.C. However, in addition to D.C., only Baltimore, Bronx County (N.Y.), and New York County reached or exceeded 60% coverage. Bronx County also had the highest rate of HIV screening in the past year at 31.3%, while Alameda County, Calif., had the lowest rate at 8.1%.
Of the seven states (Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina), Mississippi had the highest overall ever-testing rate at 40.2%, and Oklahoma had the lowest at 29.7%. People living in rural areas in those states were less likely to have ever been tested (32.1%) than those living in urban areas (37.2%). The difference between rural and urban areas increased for people at risk for HIV who had been screened in the past year, with 18.4% and 29.0%, respectively, reporting undergoing screening.
“These data provide a baseline from which to measure changes in screening in these jurisdictions and other parts of the United States over time. To achieve national goals and end the HIV epidemic in the United States, innovative and novel screening approaches might be needed to reach segments of the population that have never been tested for HIV,” the investigators concluded.
The investigators reported no conflicts of interest.
SOURCE: Pitasi MA et al. MMWR Morb Mortal Wkly Rep. 2019;68:561-7.
FROM MMWR
USPSTF reaffirms HIV screening recommendations
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
FROM JAMA
USPSTF recommends PrEP combo for adults at high risk of HIV infection
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
FROM JAMA
Consider measles vaccine booster in HIV-positive patients
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Babesiosis HIV
According to the CDC, the number of reported tickborne diseases more than doubled between 2004-2016 and accounted for > 60% of all reported mosquito-borne, tickborne, and fleaborne disease cases. Which is why it is important to keep an eye out for anyone who has a history of being in a tick-promoting environment. Clinicians from Lehigh Valley Health Network Pocono and Geisinger Commonwealth School of Medicine, both in East Stroudsburg, Pennsylvania, report on a patient whose diagnosis turned on that fact.
The patient, a 71-year-old man, had fever, weakness, headaches, near syncope, and nausea for 4 days. He also had not been eating well.
A complete blood count showed pancytopenia with an excess of band cells, an indicator of inflammation and infection. The patient’s aspartate transaminase levels were elevated. The diagnostic dilemma centered on these findings: Serology tests for HIV 1 and 2 were positive, and a peripheral blood smear showed 0.5% parasitemia consistent with Babesia microti. Both babesiosis and HIV were among the possible diagnoses. Two important factors the clinicians had to consider: The patient had recently been bitten by ticks and was homosexual.
The clinicians note that a variety of infections can lead to false-positive HIV serology, such as malaria, Mycobacterium tuberculosis or Rickettsia species, influenza and hepatitis B vaccinations. Moreover, the Ixodes tick, the same vector that transmits Borrelia burgdorferi, which causes Lyme disease, also transmits B microti. Conversely, HIV infection can exacerbate Lyme disease or babesiosis.
The tests showing B microti were the clincher for the clinicians, who started treatment with fluids, atovaquone, and azithromycin. The patient recovered completely. Repeat HIV serology was negative.
The authors of the report note that babesiosis can be a life-threatening infection in patients with reduced immunity. It is possible that, like malaria and HIV serologies, Babesia and HIV serologies cross-react, the clinicians say. Thus, it is important to screen for both in both infections.
This is the first case, to the clinician’s knowledge, of HIV associated with active babesiosis
According to the CDC, the number of reported tickborne diseases more than doubled between 2004-2016 and accounted for > 60% of all reported mosquito-borne, tickborne, and fleaborne disease cases. Which is why it is important to keep an eye out for anyone who has a history of being in a tick-promoting environment. Clinicians from Lehigh Valley Health Network Pocono and Geisinger Commonwealth School of Medicine, both in East Stroudsburg, Pennsylvania, report on a patient whose diagnosis turned on that fact.
The patient, a 71-year-old man, had fever, weakness, headaches, near syncope, and nausea for 4 days. He also had not been eating well.
A complete blood count showed pancytopenia with an excess of band cells, an indicator of inflammation and infection. The patient’s aspartate transaminase levels were elevated. The diagnostic dilemma centered on these findings: Serology tests for HIV 1 and 2 were positive, and a peripheral blood smear showed 0.5% parasitemia consistent with Babesia microti. Both babesiosis and HIV were among the possible diagnoses. Two important factors the clinicians had to consider: The patient had recently been bitten by ticks and was homosexual.
The clinicians note that a variety of infections can lead to false-positive HIV serology, such as malaria, Mycobacterium tuberculosis or Rickettsia species, influenza and hepatitis B vaccinations. Moreover, the Ixodes tick, the same vector that transmits Borrelia burgdorferi, which causes Lyme disease, also transmits B microti. Conversely, HIV infection can exacerbate Lyme disease or babesiosis.
The tests showing B microti were the clincher for the clinicians, who started treatment with fluids, atovaquone, and azithromycin. The patient recovered completely. Repeat HIV serology was negative.
The authors of the report note that babesiosis can be a life-threatening infection in patients with reduced immunity. It is possible that, like malaria and HIV serologies, Babesia and HIV serologies cross-react, the clinicians say. Thus, it is important to screen for both in both infections.
This is the first case, to the clinician’s knowledge, of HIV associated with active babesiosis
According to the CDC, the number of reported tickborne diseases more than doubled between 2004-2016 and accounted for > 60% of all reported mosquito-borne, tickborne, and fleaborne disease cases. Which is why it is important to keep an eye out for anyone who has a history of being in a tick-promoting environment. Clinicians from Lehigh Valley Health Network Pocono and Geisinger Commonwealth School of Medicine, both in East Stroudsburg, Pennsylvania, report on a patient whose diagnosis turned on that fact.
The patient, a 71-year-old man, had fever, weakness, headaches, near syncope, and nausea for 4 days. He also had not been eating well.
A complete blood count showed pancytopenia with an excess of band cells, an indicator of inflammation and infection. The patient’s aspartate transaminase levels were elevated. The diagnostic dilemma centered on these findings: Serology tests for HIV 1 and 2 were positive, and a peripheral blood smear showed 0.5% parasitemia consistent with Babesia microti. Both babesiosis and HIV were among the possible diagnoses. Two important factors the clinicians had to consider: The patient had recently been bitten by ticks and was homosexual.
The clinicians note that a variety of infections can lead to false-positive HIV serology, such as malaria, Mycobacterium tuberculosis or Rickettsia species, influenza and hepatitis B vaccinations. Moreover, the Ixodes tick, the same vector that transmits Borrelia burgdorferi, which causes Lyme disease, also transmits B microti. Conversely, HIV infection can exacerbate Lyme disease or babesiosis.
The tests showing B microti were the clincher for the clinicians, who started treatment with fluids, atovaquone, and azithromycin. The patient recovered completely. Repeat HIV serology was negative.
The authors of the report note that babesiosis can be a life-threatening infection in patients with reduced immunity. It is possible that, like malaria and HIV serologies, Babesia and HIV serologies cross-react, the clinicians say. Thus, it is important to screen for both in both infections.
This is the first case, to the clinician’s knowledge, of HIV associated with active babesiosis
Zero HIV transmission rate when viral load suppressed
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
FROM THE LANCET
ICYMI: Anti-CD4 antibody maintains viral suppression in HIV patients post ART
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Dolutegravir passes late pregnancy test
SEATTLE – In HIV-positive pregnant women who had not previously been on antiretroviral therapy (ART), initiation of treatment with dolutegravir during the third trimester led to lower viral loads at delivery and reduced HIV transmission to offspring compared to treatment with efavirenz. The result comes from a study conducted in South Africa and Uganda. Both drugs were combined with two nucleoside reverse transcriptase inhibitors (NRTIs).
ART-naive women who start ART during the third trimester often do not achieve sufficient reduction of viral load, and the researchers believed that integrase inhibitors like dolutegravir may lead to greater success because they can lead to viral clearance faster than other classes of drugs, which aren’t powerful enough to get viral loads to undetectable levels by the time of delivery. In fact, women who start therapy in the third trimester are at a 600% higher risk of transmitting the virus to offspring, and infant mortality is doubled in the first year of life, according to Saye Khoo, MD, professor of molecular and clinical pharmacology at the University of Liverpool (England).
“Clearly the viral load and undetectable viral load at the time of delivery is the best proxy for the lowest risk of mother to child transmission,” Dr. Khoo said at the Conference on Retroviruses & Opportunistic Infections. “This study clearly showed that there were big differences between dolutegravir and efavirenz. Viral load was undetectable in 74% in the dolutegravir group compared to 43% in efavirenz, which was highly significant.”
In the open-label DolPHIN-2 study, 268 women were randomized after week 28 of their pregnancy (median, 31 weeks) to receive dolutegravir or efavirenz plus two NRTIs. The dolutegravir group was more likely to achieve viral load less than 50 copies/mL at delivery (74% vs. 43%; adjusted risk ratio, 1.66; 95% confidence interval, 1.32-2.09). The trend occurred across subanalyses that included baseline viral load, baseline CD4 count, and gestation at initiation. 93% of the women in the dolutegravir group had a viral load less than 1,000 copies/mL, compared with 83% in the efavirenz group (RR, 1.11; 95% CI, 1.00-1.23).
There were no differences between the two groups with respect to frequency of severe adverse events, organ class of severe adverse events, gestational age at delivery, births earlier than 34 weeks, or births earlier than 37 weeks.
Four stillbirths occurred in the dolutegravir arm, and none in the efavirenz group. “Each of these was examined in great detail, as you might imagine. There were clear explanations for the cause of death, and it was related to maternal infection in two cases and obstetric complications in the other two,” said Dr. Khoo. There were no observations of neural tube defects – a concern that has been raised in the use of dolutegravir at the time of conception.
“I think we did see clear differences in virologic response in the arms, and so that would argue that dolutegravir certainly could be considered in these high-risk scenarios,” said Dr. Khoo.
The study was funded by the University of Liverpool. Dr. Khoo reported consulting for and receiving research funding from ViiV.
SOURCE: Khoo S et al. CROI 2019, Abstract 40 LB.
SEATTLE – In HIV-positive pregnant women who had not previously been on antiretroviral therapy (ART), initiation of treatment with dolutegravir during the third trimester led to lower viral loads at delivery and reduced HIV transmission to offspring compared to treatment with efavirenz. The result comes from a study conducted in South Africa and Uganda. Both drugs were combined with two nucleoside reverse transcriptase inhibitors (NRTIs).
ART-naive women who start ART during the third trimester often do not achieve sufficient reduction of viral load, and the researchers believed that integrase inhibitors like dolutegravir may lead to greater success because they can lead to viral clearance faster than other classes of drugs, which aren’t powerful enough to get viral loads to undetectable levels by the time of delivery. In fact, women who start therapy in the third trimester are at a 600% higher risk of transmitting the virus to offspring, and infant mortality is doubled in the first year of life, according to Saye Khoo, MD, professor of molecular and clinical pharmacology at the University of Liverpool (England).
“Clearly the viral load and undetectable viral load at the time of delivery is the best proxy for the lowest risk of mother to child transmission,” Dr. Khoo said at the Conference on Retroviruses & Opportunistic Infections. “This study clearly showed that there were big differences between dolutegravir and efavirenz. Viral load was undetectable in 74% in the dolutegravir group compared to 43% in efavirenz, which was highly significant.”
In the open-label DolPHIN-2 study, 268 women were randomized after week 28 of their pregnancy (median, 31 weeks) to receive dolutegravir or efavirenz plus two NRTIs. The dolutegravir group was more likely to achieve viral load less than 50 copies/mL at delivery (74% vs. 43%; adjusted risk ratio, 1.66; 95% confidence interval, 1.32-2.09). The trend occurred across subanalyses that included baseline viral load, baseline CD4 count, and gestation at initiation. 93% of the women in the dolutegravir group had a viral load less than 1,000 copies/mL, compared with 83% in the efavirenz group (RR, 1.11; 95% CI, 1.00-1.23).
There were no differences between the two groups with respect to frequency of severe adverse events, organ class of severe adverse events, gestational age at delivery, births earlier than 34 weeks, or births earlier than 37 weeks.
Four stillbirths occurred in the dolutegravir arm, and none in the efavirenz group. “Each of these was examined in great detail, as you might imagine. There were clear explanations for the cause of death, and it was related to maternal infection in two cases and obstetric complications in the other two,” said Dr. Khoo. There were no observations of neural tube defects – a concern that has been raised in the use of dolutegravir at the time of conception.
“I think we did see clear differences in virologic response in the arms, and so that would argue that dolutegravir certainly could be considered in these high-risk scenarios,” said Dr. Khoo.
The study was funded by the University of Liverpool. Dr. Khoo reported consulting for and receiving research funding from ViiV.
SOURCE: Khoo S et al. CROI 2019, Abstract 40 LB.
SEATTLE – In HIV-positive pregnant women who had not previously been on antiretroviral therapy (ART), initiation of treatment with dolutegravir during the third trimester led to lower viral loads at delivery and reduced HIV transmission to offspring compared to treatment with efavirenz. The result comes from a study conducted in South Africa and Uganda. Both drugs were combined with two nucleoside reverse transcriptase inhibitors (NRTIs).
ART-naive women who start ART during the third trimester often do not achieve sufficient reduction of viral load, and the researchers believed that integrase inhibitors like dolutegravir may lead to greater success because they can lead to viral clearance faster than other classes of drugs, which aren’t powerful enough to get viral loads to undetectable levels by the time of delivery. In fact, women who start therapy in the third trimester are at a 600% higher risk of transmitting the virus to offspring, and infant mortality is doubled in the first year of life, according to Saye Khoo, MD, professor of molecular and clinical pharmacology at the University of Liverpool (England).
“Clearly the viral load and undetectable viral load at the time of delivery is the best proxy for the lowest risk of mother to child transmission,” Dr. Khoo said at the Conference on Retroviruses & Opportunistic Infections. “This study clearly showed that there were big differences between dolutegravir and efavirenz. Viral load was undetectable in 74% in the dolutegravir group compared to 43% in efavirenz, which was highly significant.”
In the open-label DolPHIN-2 study, 268 women were randomized after week 28 of their pregnancy (median, 31 weeks) to receive dolutegravir or efavirenz plus two NRTIs. The dolutegravir group was more likely to achieve viral load less than 50 copies/mL at delivery (74% vs. 43%; adjusted risk ratio, 1.66; 95% confidence interval, 1.32-2.09). The trend occurred across subanalyses that included baseline viral load, baseline CD4 count, and gestation at initiation. 93% of the women in the dolutegravir group had a viral load less than 1,000 copies/mL, compared with 83% in the efavirenz group (RR, 1.11; 95% CI, 1.00-1.23).
There were no differences between the two groups with respect to frequency of severe adverse events, organ class of severe adverse events, gestational age at delivery, births earlier than 34 weeks, or births earlier than 37 weeks.
Four stillbirths occurred in the dolutegravir arm, and none in the efavirenz group. “Each of these was examined in great detail, as you might imagine. There were clear explanations for the cause of death, and it was related to maternal infection in two cases and obstetric complications in the other two,” said Dr. Khoo. There were no observations of neural tube defects – a concern that has been raised in the use of dolutegravir at the time of conception.
“I think we did see clear differences in virologic response in the arms, and so that would argue that dolutegravir certainly could be considered in these high-risk scenarios,” said Dr. Khoo.
The study was funded by the University of Liverpool. Dr. Khoo reported consulting for and receiving research funding from ViiV.
SOURCE: Khoo S et al. CROI 2019, Abstract 40 LB.
REPORTING FROM CROI 2019
Weight gain highest with integrase inhibitors for HIV
SEATTLE – Weight gain in HIV treatment is highest with integrase inhibitors, especially dolutegravir (Tivicay), followed by raltegravir (Isentress), according to a review of thousands of North American patients during their first 5 years of therapy.
People “starting integrase inhibitors are gaining significantly more weight than ART [antiretroviral therapy] naive patients starting NNRTIs [nonnucleoside reverse transcriptase inhibitors]. They are also gaining more weight than ART patients starting PIs [protease inhibitors],” however, the differences do not always reach statistical significance, said lead investigator Kassem Bourgi, MD, an infectious disease fellow at Vanderbilt University, Nashville, Tenn.
“This is clinically important as” integrase inhibitor-based “regimens are now recommended first line,” and, as people with HIV live normal lifespans, they “are at increasing risk for obesity, metabolic comorbidities, and cardiovascular disease,” just like the rest of the population, he said.
Dr. Bourgi’s presentation was one of several at the Conference on Retroviruses and Opportunistic Infections tackling the issue of ART-induced obesity. There was great interest in the topic, and his study wasn’t the only one to find an increased risk of excess weight gain with integrase inhibitors.
“This is a real-world issue, and this is what we are seeing in our patients; they are dealing with this every day,” explained moderator Jane O’Halloran, MD, when asked about the impressive audience turnout for the ART obesity session.
Dr. Bourgi’s team turned to the North American AIDS Cohort Collaboration on Research and Design, which includes data on HIV patients throughout Canada and the United States, to compare weight gain outcomes among 24,001 people who started HIV treatment from 2007-2016; 49% started on NNRTI-based regimens, 31% on PIs, and 20% on integrase inhibitors. Elvitegravir (Vitekta) was most commonly used in the integrase group (45%), followed by raltegravir (35%), and dolutegravir (20%).
At 2 and 5 years, patients started on integrase inhibitors gained 4.9 and 6 kg, respectively, over their predicted weight, compared with 3.3 and 4.3 kg for NNRTIs, and 4.4 and 5.1 kg for PIs, adjusted for age, sex, race, cohort site, HIV acquisition mode, ART initiation year, and baseline weight, HIV-1 RNA, and CD4 cell count.
At 2 years among the integrase group, weight gain was 6 kg for dolutegravir, 4.9 kg for raltegravir, and 3.8 kg for elvitegravir; the weight gain for those on elvitegravir was comparable to that for those on PIs.
“Patients who started dolutegravir or raltegravir gained significantly more weight by year 2 compared with those who started elvitegravir,” Dr. Bourgi noted.
The mechanisms for the differences are unknown.
Integrase inhibitors are very well tolerated, so it could be that once on them, people feel so much better that, as one audience member put it, they just do “what everyone else does”: overeat. Dolutegravir also is associated with psychiatric symptoms, so maybe people on it are more likely to be taking psychiatric drugs that pack on the weight. Perhaps it’s something peculiar to the drugs. More work is needed on the issue, Dr. O’Halloran said.
Most of the subjects were men, and 38% were men who have sex with men. The median age at ART initiation was 42 years, and median weight body mass index 25 kg/m2. The median CD4 cell count was 303 cells/mm3.
The work was funded by Gilead, through an investigator sponsored grant. Dr. Bourgi didn’t say he had any disclosures.
SOURCE: Bourgi K et al. CROI 2019, Abstract 670.
SEATTLE – Weight gain in HIV treatment is highest with integrase inhibitors, especially dolutegravir (Tivicay), followed by raltegravir (Isentress), according to a review of thousands of North American patients during their first 5 years of therapy.
People “starting integrase inhibitors are gaining significantly more weight than ART [antiretroviral therapy] naive patients starting NNRTIs [nonnucleoside reverse transcriptase inhibitors]. They are also gaining more weight than ART patients starting PIs [protease inhibitors],” however, the differences do not always reach statistical significance, said lead investigator Kassem Bourgi, MD, an infectious disease fellow at Vanderbilt University, Nashville, Tenn.
“This is clinically important as” integrase inhibitor-based “regimens are now recommended first line,” and, as people with HIV live normal lifespans, they “are at increasing risk for obesity, metabolic comorbidities, and cardiovascular disease,” just like the rest of the population, he said.
Dr. Bourgi’s presentation was one of several at the Conference on Retroviruses and Opportunistic Infections tackling the issue of ART-induced obesity. There was great interest in the topic, and his study wasn’t the only one to find an increased risk of excess weight gain with integrase inhibitors.
“This is a real-world issue, and this is what we are seeing in our patients; they are dealing with this every day,” explained moderator Jane O’Halloran, MD, when asked about the impressive audience turnout for the ART obesity session.
Dr. Bourgi’s team turned to the North American AIDS Cohort Collaboration on Research and Design, which includes data on HIV patients throughout Canada and the United States, to compare weight gain outcomes among 24,001 people who started HIV treatment from 2007-2016; 49% started on NNRTI-based regimens, 31% on PIs, and 20% on integrase inhibitors. Elvitegravir (Vitekta) was most commonly used in the integrase group (45%), followed by raltegravir (35%), and dolutegravir (20%).
At 2 and 5 years, patients started on integrase inhibitors gained 4.9 and 6 kg, respectively, over their predicted weight, compared with 3.3 and 4.3 kg for NNRTIs, and 4.4 and 5.1 kg for PIs, adjusted for age, sex, race, cohort site, HIV acquisition mode, ART initiation year, and baseline weight, HIV-1 RNA, and CD4 cell count.
At 2 years among the integrase group, weight gain was 6 kg for dolutegravir, 4.9 kg for raltegravir, and 3.8 kg for elvitegravir; the weight gain for those on elvitegravir was comparable to that for those on PIs.
“Patients who started dolutegravir or raltegravir gained significantly more weight by year 2 compared with those who started elvitegravir,” Dr. Bourgi noted.
The mechanisms for the differences are unknown.
Integrase inhibitors are very well tolerated, so it could be that once on them, people feel so much better that, as one audience member put it, they just do “what everyone else does”: overeat. Dolutegravir also is associated with psychiatric symptoms, so maybe people on it are more likely to be taking psychiatric drugs that pack on the weight. Perhaps it’s something peculiar to the drugs. More work is needed on the issue, Dr. O’Halloran said.
Most of the subjects were men, and 38% were men who have sex with men. The median age at ART initiation was 42 years, and median weight body mass index 25 kg/m2. The median CD4 cell count was 303 cells/mm3.
The work was funded by Gilead, through an investigator sponsored grant. Dr. Bourgi didn’t say he had any disclosures.
SOURCE: Bourgi K et al. CROI 2019, Abstract 670.
SEATTLE – Weight gain in HIV treatment is highest with integrase inhibitors, especially dolutegravir (Tivicay), followed by raltegravir (Isentress), according to a review of thousands of North American patients during their first 5 years of therapy.
People “starting integrase inhibitors are gaining significantly more weight than ART [antiretroviral therapy] naive patients starting NNRTIs [nonnucleoside reverse transcriptase inhibitors]. They are also gaining more weight than ART patients starting PIs [protease inhibitors],” however, the differences do not always reach statistical significance, said lead investigator Kassem Bourgi, MD, an infectious disease fellow at Vanderbilt University, Nashville, Tenn.
“This is clinically important as” integrase inhibitor-based “regimens are now recommended first line,” and, as people with HIV live normal lifespans, they “are at increasing risk for obesity, metabolic comorbidities, and cardiovascular disease,” just like the rest of the population, he said.
Dr. Bourgi’s presentation was one of several at the Conference on Retroviruses and Opportunistic Infections tackling the issue of ART-induced obesity. There was great interest in the topic, and his study wasn’t the only one to find an increased risk of excess weight gain with integrase inhibitors.
“This is a real-world issue, and this is what we are seeing in our patients; they are dealing with this every day,” explained moderator Jane O’Halloran, MD, when asked about the impressive audience turnout for the ART obesity session.
Dr. Bourgi’s team turned to the North American AIDS Cohort Collaboration on Research and Design, which includes data on HIV patients throughout Canada and the United States, to compare weight gain outcomes among 24,001 people who started HIV treatment from 2007-2016; 49% started on NNRTI-based regimens, 31% on PIs, and 20% on integrase inhibitors. Elvitegravir (Vitekta) was most commonly used in the integrase group (45%), followed by raltegravir (35%), and dolutegravir (20%).
At 2 and 5 years, patients started on integrase inhibitors gained 4.9 and 6 kg, respectively, over their predicted weight, compared with 3.3 and 4.3 kg for NNRTIs, and 4.4 and 5.1 kg for PIs, adjusted for age, sex, race, cohort site, HIV acquisition mode, ART initiation year, and baseline weight, HIV-1 RNA, and CD4 cell count.
At 2 years among the integrase group, weight gain was 6 kg for dolutegravir, 4.9 kg for raltegravir, and 3.8 kg for elvitegravir; the weight gain for those on elvitegravir was comparable to that for those on PIs.
“Patients who started dolutegravir or raltegravir gained significantly more weight by year 2 compared with those who started elvitegravir,” Dr. Bourgi noted.
The mechanisms for the differences are unknown.
Integrase inhibitors are very well tolerated, so it could be that once on them, people feel so much better that, as one audience member put it, they just do “what everyone else does”: overeat. Dolutegravir also is associated with psychiatric symptoms, so maybe people on it are more likely to be taking psychiatric drugs that pack on the weight. Perhaps it’s something peculiar to the drugs. More work is needed on the issue, Dr. O’Halloran said.
Most of the subjects were men, and 38% were men who have sex with men. The median age at ART initiation was 42 years, and median weight body mass index 25 kg/m2. The median CD4 cell count was 303 cells/mm3.
The work was funded by Gilead, through an investigator sponsored grant. Dr. Bourgi didn’t say he had any disclosures.
SOURCE: Bourgi K et al. CROI 2019, Abstract 670.
REPORTING FROM CROI 2019
FDA approves first two-drug tablet for HIV
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
mlesney@mdedge.com
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
mlesney@mdedge.com
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
mlesney@mdedge.com