User login
Tropical travelers’ top dermatologic infestations
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
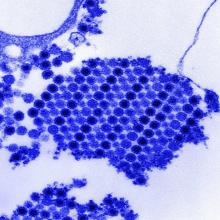
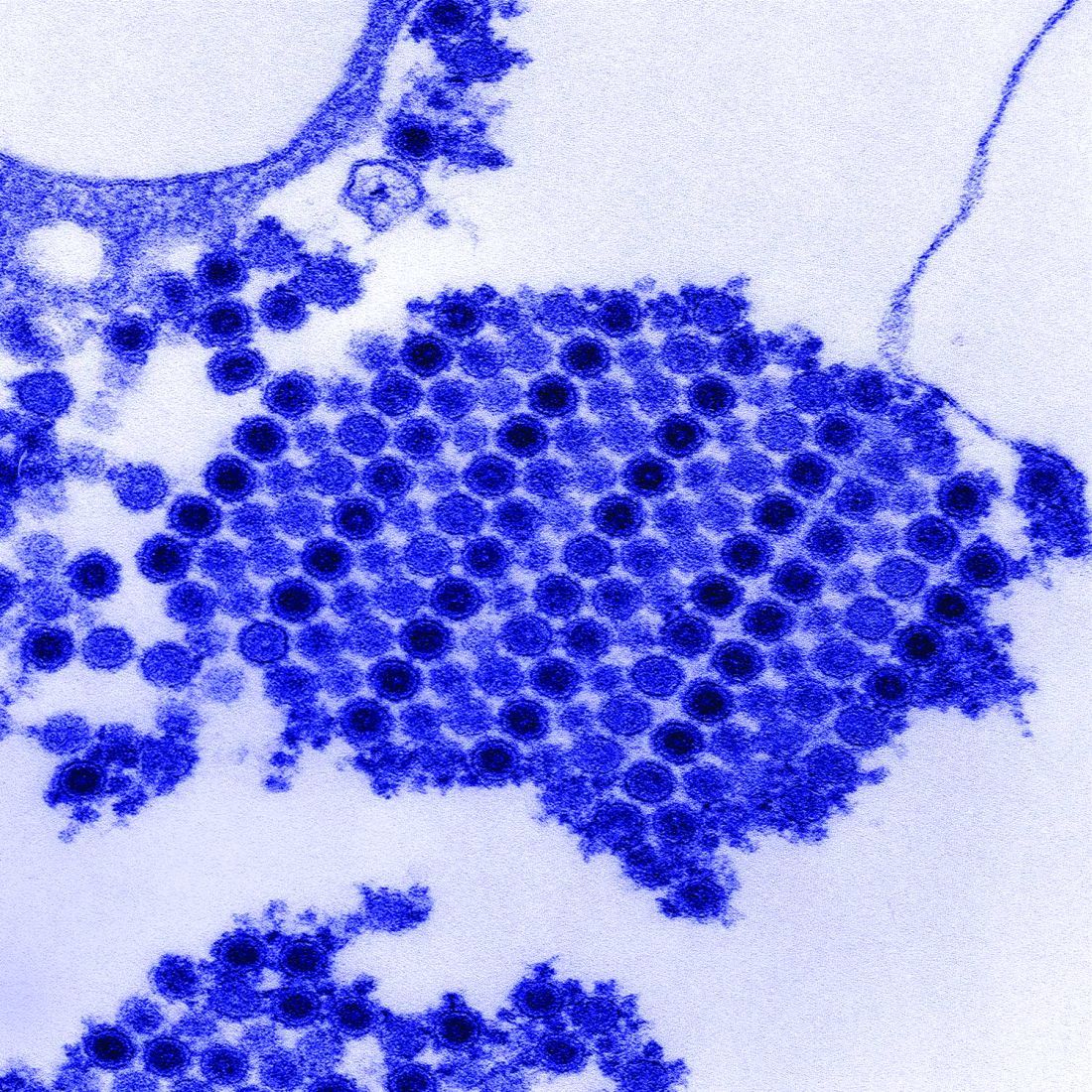
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans
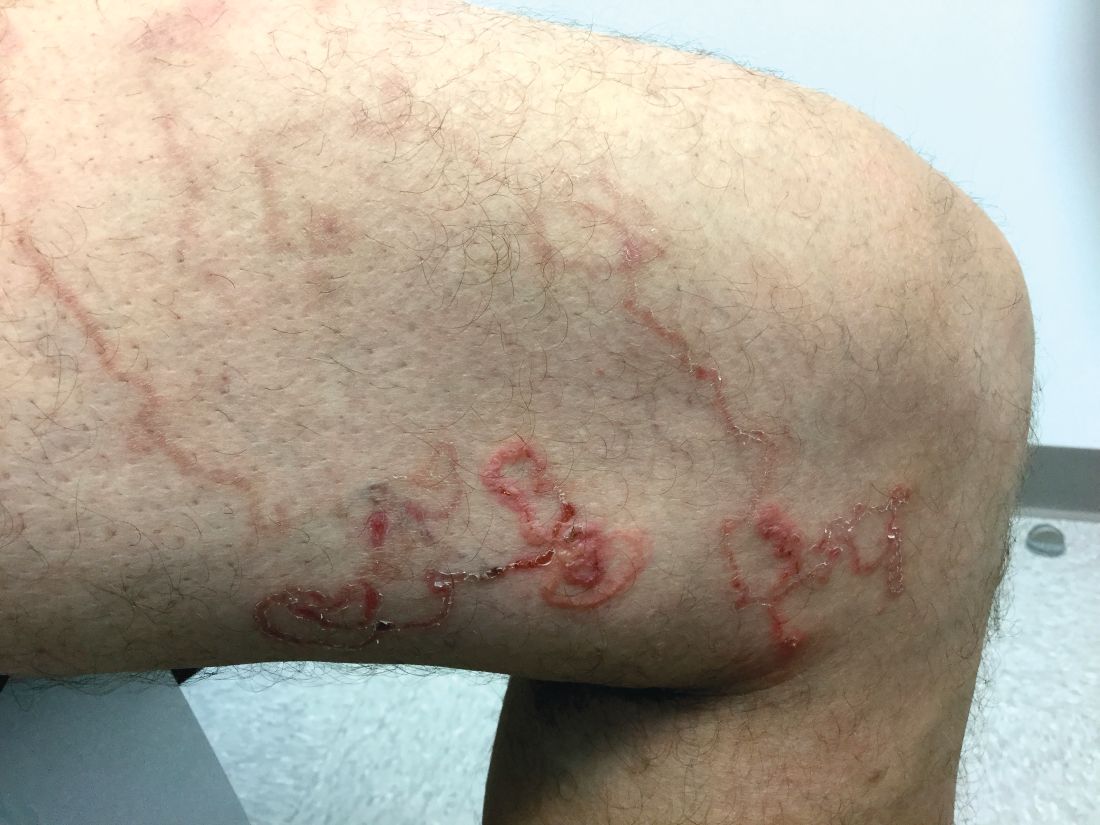
This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Dengue antibodies may reduce Zika infection risk
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
FROM SCIENCE
Key clinical point: Higher dengue antibody titers are associated with a lower risk of Zika virus infection.
Major finding: The highest tertile of dengue antibody titers was associated with a 44% reduction in the risk of Zika seropositivity.
Study details: A prospective cohort study of 1,453 residents in Salvador, Brazil.
Disclosures: The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
Source: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Single-dose tafenoquine appears to prevent malaria relapse
Single-dose tafenoquine therapy safely reduces the risk of Plasmodium vivax relapse in patients with normal glucose-6-phosphate dehydrogenase (G6PD) activity, according to the results of two phase 3, double-blind, randomized controlled trials.
Findings from both studies were published in two separate reports in the New England Journal of Medicine.
In the first study, the Dose and Efficacy Trial Evaluating Chloroquine and Tafenoquine in Vivax Elimination (DETECTIVE), the risk of P. vivax recurrence was approximately 70% lower with tafenoquine versus placebo, wrote Marcus V.G. Lacerda, MD, of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado in Manaus, Brazil, and his colleagues.
The study included 522 patients with confirmed P. vivax infection from Peru, Brazil, Ethiopia, Cambodia, Thailand, and the Philippines. Patients received 3 days of chloroquine therapy (600 mg on days 1 and 2, and 300 mg on day 3) and were randomly assigned on a 2:1:1 basis to receive a single 300-mg dose of tafenoquine on day 1 or 2, primaquine once daily for 14 days, or placebo.
Since primaquine and tafenoquine can cause clinically significant hemolysis in individuals with G6PD deficiency, the study included only patients with normal G6PD activity.
In the intention-to-treat analysis, 62% of tafenoquine recipients were free from P. vivax recurrence (95% confidence interval [CI], 55%-69%) at 6 months, as were 70% of primaquine recipients (95% CI, 60%-77%) and 28% of placebo recipients (95% CI, 20%-37%). Compared with placebo, the reduction in risk of recurrence was 70% with tafenoquine (hazard ratio [HR], 0.30; 95% CI, 0.22-0.40; P less than .001) and 74% with primaquine.
Declines in hemoglobin levels were greatest in the tafenoquine group but were not associated with symptomatic anemia and resolved without intervention, the investigators wrote.
In addition to the quantitative G6PD test, the investigators also evaluated a qualitative test, which “failed to identify 16 patients most at risk for hemolysis,” they reported. “If tafenoquine use is expanded, adoption of reliable quantitative point-of-care G6PD tests will be needed; such tests are not currently available but are in development.”
In the second study, Global Assessment of Tafenoquine Hemolytic Risk (GATHER), Alejandro Llanos-Cuentas, MD, of Universidad Peruana Cayetano Heredia, Lima, Peru, and his colleagues enrolled 251 patients with confirmed P. vivax infection from Peru, Brazil, Columbia, Vietnam, and Thailand. They attempted to recruit women with moderate G6PD levels, but only one participant met this criterion – all others had normal G6PD activity.
Patients received 3-day course of chloroquine and were randomly assigned on a 2:1 basis to either tafenoquine or primaquine at the same doses as in the DETECTIVE trial.
At 6 months, 2% (95% CI, 1%-6%) of tafenoquine recipients and 1% (95% CI, 0.2%-6%) of primaquine recipients had decreased hemoglobin levels, but none consequently needed treatment. The medications also caused a similar degree and time course of hemoglobin decrease, the investigators noted.
A meta-analysis of GATHER and DETECTIVE confirmed that tafenoquine more often led to a decreased hemoglobin level (4% versus 1.5% with primaquine). Tafenoquine also did not meet prespecified criteria for noninferiority compared with primaquine, with respective 6-month recurrence rates of 67% versus 73%.
However, GATHER deployed “extensive” support to help patients adhere to the 15-day primaquine course, Dr. Llanos-Cuentas and his colleagues wrote. “Without such interventions, adherence to primaquine has been reported to be as low as 24% in Southeast Asia, with a corresponding attenuation of efficacy.”
GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having no conflicts of interest.
SOURCES: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
The studies show that tafenoquine reduces the risk of Plasmodium vivax recurrence in patients with quantitatively confirmed normal glucose-6-phosphate dehydrogenase (G6PD) activity, Nicholas J. White, FRS, wrote in an accompanying editorial.
But the need for this test and current prescribing restrictions will “limit the potential deployment of tafenoquine, at least in the immediate future,” he said. He praised the developers of tafenoquine “for persevering with this potentially valuable antimalarial drug, despite the difficulties,” but cautioned that it’s too soon to conclude that tafenoquine can be used safely and routinely on a large scale “and thus fulfill its promise as a radical improvement in the treatment of malaria.”
Currently, tafenoquine may not be used during pregnancy, lactation, or in patients younger than 16 years, Dr. White noted. Tafenoquine, like primaquine, causes dose-dependent hemolysis in patients with G6PD deficiency, but unlike primaquine, it is given as a single large dose. Hence, pretreatment quantitative G6PD testing is necessary. Point-of-care quantitative G6PD tests have been developed but await extensive field testing, Dr. White said.
Dr. White is with Mahidol University, Bangkok, Thailand, and University of Oxford, England. He reported having no financial disclosures. These comments are from his accompanying editorial ( N Engl J Med. 2019;380:285-6 ).
The studies show that tafenoquine reduces the risk of Plasmodium vivax recurrence in patients with quantitatively confirmed normal glucose-6-phosphate dehydrogenase (G6PD) activity, Nicholas J. White, FRS, wrote in an accompanying editorial.
But the need for this test and current prescribing restrictions will “limit the potential deployment of tafenoquine, at least in the immediate future,” he said. He praised the developers of tafenoquine “for persevering with this potentially valuable antimalarial drug, despite the difficulties,” but cautioned that it’s too soon to conclude that tafenoquine can be used safely and routinely on a large scale “and thus fulfill its promise as a radical improvement in the treatment of malaria.”
Currently, tafenoquine may not be used during pregnancy, lactation, or in patients younger than 16 years, Dr. White noted. Tafenoquine, like primaquine, causes dose-dependent hemolysis in patients with G6PD deficiency, but unlike primaquine, it is given as a single large dose. Hence, pretreatment quantitative G6PD testing is necessary. Point-of-care quantitative G6PD tests have been developed but await extensive field testing, Dr. White said.
Dr. White is with Mahidol University, Bangkok, Thailand, and University of Oxford, England. He reported having no financial disclosures. These comments are from his accompanying editorial ( N Engl J Med. 2019;380:285-6 ).
The studies show that tafenoquine reduces the risk of Plasmodium vivax recurrence in patients with quantitatively confirmed normal glucose-6-phosphate dehydrogenase (G6PD) activity, Nicholas J. White, FRS, wrote in an accompanying editorial.
But the need for this test and current prescribing restrictions will “limit the potential deployment of tafenoquine, at least in the immediate future,” he said. He praised the developers of tafenoquine “for persevering with this potentially valuable antimalarial drug, despite the difficulties,” but cautioned that it’s too soon to conclude that tafenoquine can be used safely and routinely on a large scale “and thus fulfill its promise as a radical improvement in the treatment of malaria.”
Currently, tafenoquine may not be used during pregnancy, lactation, or in patients younger than 16 years, Dr. White noted. Tafenoquine, like primaquine, causes dose-dependent hemolysis in patients with G6PD deficiency, but unlike primaquine, it is given as a single large dose. Hence, pretreatment quantitative G6PD testing is necessary. Point-of-care quantitative G6PD tests have been developed but await extensive field testing, Dr. White said.
Dr. White is with Mahidol University, Bangkok, Thailand, and University of Oxford, England. He reported having no financial disclosures. These comments are from his accompanying editorial ( N Engl J Med. 2019;380:285-6 ).
Single-dose tafenoquine therapy safely reduces the risk of Plasmodium vivax relapse in patients with normal glucose-6-phosphate dehydrogenase (G6PD) activity, according to the results of two phase 3, double-blind, randomized controlled trials.
Findings from both studies were published in two separate reports in the New England Journal of Medicine.
In the first study, the Dose and Efficacy Trial Evaluating Chloroquine and Tafenoquine in Vivax Elimination (DETECTIVE), the risk of P. vivax recurrence was approximately 70% lower with tafenoquine versus placebo, wrote Marcus V.G. Lacerda, MD, of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado in Manaus, Brazil, and his colleagues.
The study included 522 patients with confirmed P. vivax infection from Peru, Brazil, Ethiopia, Cambodia, Thailand, and the Philippines. Patients received 3 days of chloroquine therapy (600 mg on days 1 and 2, and 300 mg on day 3) and were randomly assigned on a 2:1:1 basis to receive a single 300-mg dose of tafenoquine on day 1 or 2, primaquine once daily for 14 days, or placebo.
Since primaquine and tafenoquine can cause clinically significant hemolysis in individuals with G6PD deficiency, the study included only patients with normal G6PD activity.
In the intention-to-treat analysis, 62% of tafenoquine recipients were free from P. vivax recurrence (95% confidence interval [CI], 55%-69%) at 6 months, as were 70% of primaquine recipients (95% CI, 60%-77%) and 28% of placebo recipients (95% CI, 20%-37%). Compared with placebo, the reduction in risk of recurrence was 70% with tafenoquine (hazard ratio [HR], 0.30; 95% CI, 0.22-0.40; P less than .001) and 74% with primaquine.
Declines in hemoglobin levels were greatest in the tafenoquine group but were not associated with symptomatic anemia and resolved without intervention, the investigators wrote.
In addition to the quantitative G6PD test, the investigators also evaluated a qualitative test, which “failed to identify 16 patients most at risk for hemolysis,” they reported. “If tafenoquine use is expanded, adoption of reliable quantitative point-of-care G6PD tests will be needed; such tests are not currently available but are in development.”
In the second study, Global Assessment of Tafenoquine Hemolytic Risk (GATHER), Alejandro Llanos-Cuentas, MD, of Universidad Peruana Cayetano Heredia, Lima, Peru, and his colleagues enrolled 251 patients with confirmed P. vivax infection from Peru, Brazil, Columbia, Vietnam, and Thailand. They attempted to recruit women with moderate G6PD levels, but only one participant met this criterion – all others had normal G6PD activity.
Patients received 3-day course of chloroquine and were randomly assigned on a 2:1 basis to either tafenoquine or primaquine at the same doses as in the DETECTIVE trial.
At 6 months, 2% (95% CI, 1%-6%) of tafenoquine recipients and 1% (95% CI, 0.2%-6%) of primaquine recipients had decreased hemoglobin levels, but none consequently needed treatment. The medications also caused a similar degree and time course of hemoglobin decrease, the investigators noted.
A meta-analysis of GATHER and DETECTIVE confirmed that tafenoquine more often led to a decreased hemoglobin level (4% versus 1.5% with primaquine). Tafenoquine also did not meet prespecified criteria for noninferiority compared with primaquine, with respective 6-month recurrence rates of 67% versus 73%.
However, GATHER deployed “extensive” support to help patients adhere to the 15-day primaquine course, Dr. Llanos-Cuentas and his colleagues wrote. “Without such interventions, adherence to primaquine has been reported to be as low as 24% in Southeast Asia, with a corresponding attenuation of efficacy.”
GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having no conflicts of interest.
SOURCES: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
Single-dose tafenoquine therapy safely reduces the risk of Plasmodium vivax relapse in patients with normal glucose-6-phosphate dehydrogenase (G6PD) activity, according to the results of two phase 3, double-blind, randomized controlled trials.
Findings from both studies were published in two separate reports in the New England Journal of Medicine.
In the first study, the Dose and Efficacy Trial Evaluating Chloroquine and Tafenoquine in Vivax Elimination (DETECTIVE), the risk of P. vivax recurrence was approximately 70% lower with tafenoquine versus placebo, wrote Marcus V.G. Lacerda, MD, of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado in Manaus, Brazil, and his colleagues.
The study included 522 patients with confirmed P. vivax infection from Peru, Brazil, Ethiopia, Cambodia, Thailand, and the Philippines. Patients received 3 days of chloroquine therapy (600 mg on days 1 and 2, and 300 mg on day 3) and were randomly assigned on a 2:1:1 basis to receive a single 300-mg dose of tafenoquine on day 1 or 2, primaquine once daily for 14 days, or placebo.
Since primaquine and tafenoquine can cause clinically significant hemolysis in individuals with G6PD deficiency, the study included only patients with normal G6PD activity.
In the intention-to-treat analysis, 62% of tafenoquine recipients were free from P. vivax recurrence (95% confidence interval [CI], 55%-69%) at 6 months, as were 70% of primaquine recipients (95% CI, 60%-77%) and 28% of placebo recipients (95% CI, 20%-37%). Compared with placebo, the reduction in risk of recurrence was 70% with tafenoquine (hazard ratio [HR], 0.30; 95% CI, 0.22-0.40; P less than .001) and 74% with primaquine.
Declines in hemoglobin levels were greatest in the tafenoquine group but were not associated with symptomatic anemia and resolved without intervention, the investigators wrote.
In addition to the quantitative G6PD test, the investigators also evaluated a qualitative test, which “failed to identify 16 patients most at risk for hemolysis,” they reported. “If tafenoquine use is expanded, adoption of reliable quantitative point-of-care G6PD tests will be needed; such tests are not currently available but are in development.”
In the second study, Global Assessment of Tafenoquine Hemolytic Risk (GATHER), Alejandro Llanos-Cuentas, MD, of Universidad Peruana Cayetano Heredia, Lima, Peru, and his colleagues enrolled 251 patients with confirmed P. vivax infection from Peru, Brazil, Columbia, Vietnam, and Thailand. They attempted to recruit women with moderate G6PD levels, but only one participant met this criterion – all others had normal G6PD activity.
Patients received 3-day course of chloroquine and were randomly assigned on a 2:1 basis to either tafenoquine or primaquine at the same doses as in the DETECTIVE trial.
At 6 months, 2% (95% CI, 1%-6%) of tafenoquine recipients and 1% (95% CI, 0.2%-6%) of primaquine recipients had decreased hemoglobin levels, but none consequently needed treatment. The medications also caused a similar degree and time course of hemoglobin decrease, the investigators noted.
A meta-analysis of GATHER and DETECTIVE confirmed that tafenoquine more often led to a decreased hemoglobin level (4% versus 1.5% with primaquine). Tafenoquine also did not meet prespecified criteria for noninferiority compared with primaquine, with respective 6-month recurrence rates of 67% versus 73%.
However, GATHER deployed “extensive” support to help patients adhere to the 15-day primaquine course, Dr. Llanos-Cuentas and his colleagues wrote. “Without such interventions, adherence to primaquine has been reported to be as low as 24% in Southeast Asia, with a corresponding attenuation of efficacy.”
GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having no conflicts of interest.
SOURCES: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: In DETECTIVE, 6-month rates of freedom from recurrence from Plasmodium vivax infection were 62% with tafenoquine, 70% with primaquine, and 28% with placebo.
Study details: Two randomized, phase 3, double-blind controlled trials of patients with confirmed P. vivax infection (DETECTIVE and GATHER) and without deficient G6PD activity.
Disclosures: GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having financial disclosures.
Source: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
Puppy bite at yoga retreat leads to rabies death: Prompts health warning
Individuals going to rabies-endemic countries should have a pretrip consultation with a travel health specialist, say authors of a case report describing the death of a woman who sustained a bite from a rabid puppy during a 2017 yoga retreat in rural India.
Preexposure prophylaxis is warranted, especially for individuals expected to be in those countries for long durations, those planning to go to remote areas, or if they plan activities that may put them at risk for rabies exposure, the authors wrote in Morbidity and Mortality Weekly Report.
“In the case of the yoga retreat tour, given the extended length of the tour and the rural and community activities involved, pretravel rabies vaccination should have been considered,” said Julia Murphy, DVM, a veterinarian with the Virginia Department of Health, and her coauthors in the recently published report.
The case also underscores the importance of prompt rabies diagnosis, according to Dr. Murphy and her colleagues: 250 health care workers were assessed for exposure to the patient, 72 (29%) of whom were advised to initiate postexposure prophylaxis and were treated at a cost of nearly a quarter million dollars.
The Virginia woman described in the case report was aged 65 years and had no preexisting health conditions. She had spent more than 2 months on a yoga retreat tour in India and was bitten by a puppy near her hotel in Rishikesh in northern India, according to results of a public health investigation.
That retreat ended on April 7, 2017, according to the report, and on May 3, 2017, the woman started to have pain and paresthesia in her right arm during gardening.
On May 6, she sought care at an urgent care facility, resulting in a diagnosis of carpal tunnel syndrome and a prescription for an NSAID.
The next day, she was evaluated at a hospital for anxiety, insomnia, shortness of breath, and difficulty swallowing water and was given lorazepam for a presumed panic attack. She was discharged and, in her car, experienced claustrophobia and shortness of breath. She returned to the hospital’s ED, received more lorazepam, and was again discharged.
The day after that, she was transported by ambulance to another hospital with increased anxiety, shortness of breath, chest discomfort, and progressive paresthesia; she was found to have elevated cardiac enzymes and underwent emergency cardiac catheterization, which revealed normal arteries.
That evening, the patient became “progressively agitated and combative,” according to the report, and was found to be gasping for air while trying to drink water. When family were questioned about animal exposures, the woman’s husband indicated that she had been bitten on the right hand by a puppy during the yoga retreat, about 6 weeks before the symptoms started.
Once a diagnosis of rabies was confirmed, the woman was started on aggressive treatment but eventually died, according to Dr. Murphy and her coauthors, which made this patient the ninth person in the United States to die from rabies exposure while overseas since 2008. Canine rabies has been eliminated in the United States because of the strict vaccination laws.
“These events underscore the importance of obtaining a thorough pretravel health consultation, particularly when visiting countries with high incidence of emerging or zoonotic pathogens, to ensure awareness of health risks and appropriate pretravel and postexposure health care actions,” they concluded in their report.
Dr. Murphy and her coauthors reported no potential conflicts of interest related to the case report.
SOURCE: Murphy J et al. MMWR Morb Mortal Wkly Rep. 2019 Jan 4;67(5152):1410-4.
Individuals going to rabies-endemic countries should have a pretrip consultation with a travel health specialist, say authors of a case report describing the death of a woman who sustained a bite from a rabid puppy during a 2017 yoga retreat in rural India.
Preexposure prophylaxis is warranted, especially for individuals expected to be in those countries for long durations, those planning to go to remote areas, or if they plan activities that may put them at risk for rabies exposure, the authors wrote in Morbidity and Mortality Weekly Report.
“In the case of the yoga retreat tour, given the extended length of the tour and the rural and community activities involved, pretravel rabies vaccination should have been considered,” said Julia Murphy, DVM, a veterinarian with the Virginia Department of Health, and her coauthors in the recently published report.
The case also underscores the importance of prompt rabies diagnosis, according to Dr. Murphy and her colleagues: 250 health care workers were assessed for exposure to the patient, 72 (29%) of whom were advised to initiate postexposure prophylaxis and were treated at a cost of nearly a quarter million dollars.
The Virginia woman described in the case report was aged 65 years and had no preexisting health conditions. She had spent more than 2 months on a yoga retreat tour in India and was bitten by a puppy near her hotel in Rishikesh in northern India, according to results of a public health investigation.
That retreat ended on April 7, 2017, according to the report, and on May 3, 2017, the woman started to have pain and paresthesia in her right arm during gardening.
On May 6, she sought care at an urgent care facility, resulting in a diagnosis of carpal tunnel syndrome and a prescription for an NSAID.
The next day, she was evaluated at a hospital for anxiety, insomnia, shortness of breath, and difficulty swallowing water and was given lorazepam for a presumed panic attack. She was discharged and, in her car, experienced claustrophobia and shortness of breath. She returned to the hospital’s ED, received more lorazepam, and was again discharged.
The day after that, she was transported by ambulance to another hospital with increased anxiety, shortness of breath, chest discomfort, and progressive paresthesia; she was found to have elevated cardiac enzymes and underwent emergency cardiac catheterization, which revealed normal arteries.
That evening, the patient became “progressively agitated and combative,” according to the report, and was found to be gasping for air while trying to drink water. When family were questioned about animal exposures, the woman’s husband indicated that she had been bitten on the right hand by a puppy during the yoga retreat, about 6 weeks before the symptoms started.
Once a diagnosis of rabies was confirmed, the woman was started on aggressive treatment but eventually died, according to Dr. Murphy and her coauthors, which made this patient the ninth person in the United States to die from rabies exposure while overseas since 2008. Canine rabies has been eliminated in the United States because of the strict vaccination laws.
“These events underscore the importance of obtaining a thorough pretravel health consultation, particularly when visiting countries with high incidence of emerging or zoonotic pathogens, to ensure awareness of health risks and appropriate pretravel and postexposure health care actions,” they concluded in their report.
Dr. Murphy and her coauthors reported no potential conflicts of interest related to the case report.
SOURCE: Murphy J et al. MMWR Morb Mortal Wkly Rep. 2019 Jan 4;67(5152):1410-4.
Individuals going to rabies-endemic countries should have a pretrip consultation with a travel health specialist, say authors of a case report describing the death of a woman who sustained a bite from a rabid puppy during a 2017 yoga retreat in rural India.
Preexposure prophylaxis is warranted, especially for individuals expected to be in those countries for long durations, those planning to go to remote areas, or if they plan activities that may put them at risk for rabies exposure, the authors wrote in Morbidity and Mortality Weekly Report.
“In the case of the yoga retreat tour, given the extended length of the tour and the rural and community activities involved, pretravel rabies vaccination should have been considered,” said Julia Murphy, DVM, a veterinarian with the Virginia Department of Health, and her coauthors in the recently published report.
The case also underscores the importance of prompt rabies diagnosis, according to Dr. Murphy and her colleagues: 250 health care workers were assessed for exposure to the patient, 72 (29%) of whom were advised to initiate postexposure prophylaxis and were treated at a cost of nearly a quarter million dollars.
The Virginia woman described in the case report was aged 65 years and had no preexisting health conditions. She had spent more than 2 months on a yoga retreat tour in India and was bitten by a puppy near her hotel in Rishikesh in northern India, according to results of a public health investigation.
That retreat ended on April 7, 2017, according to the report, and on May 3, 2017, the woman started to have pain and paresthesia in her right arm during gardening.
On May 6, she sought care at an urgent care facility, resulting in a diagnosis of carpal tunnel syndrome and a prescription for an NSAID.
The next day, she was evaluated at a hospital for anxiety, insomnia, shortness of breath, and difficulty swallowing water and was given lorazepam for a presumed panic attack. She was discharged and, in her car, experienced claustrophobia and shortness of breath. She returned to the hospital’s ED, received more lorazepam, and was again discharged.
The day after that, she was transported by ambulance to another hospital with increased anxiety, shortness of breath, chest discomfort, and progressive paresthesia; she was found to have elevated cardiac enzymes and underwent emergency cardiac catheterization, which revealed normal arteries.
That evening, the patient became “progressively agitated and combative,” according to the report, and was found to be gasping for air while trying to drink water. When family were questioned about animal exposures, the woman’s husband indicated that she had been bitten on the right hand by a puppy during the yoga retreat, about 6 weeks before the symptoms started.
Once a diagnosis of rabies was confirmed, the woman was started on aggressive treatment but eventually died, according to Dr. Murphy and her coauthors, which made this patient the ninth person in the United States to die from rabies exposure while overseas since 2008. Canine rabies has been eliminated in the United States because of the strict vaccination laws.
“These events underscore the importance of obtaining a thorough pretravel health consultation, particularly when visiting countries with high incidence of emerging or zoonotic pathogens, to ensure awareness of health risks and appropriate pretravel and postexposure health care actions,” they concluded in their report.
Dr. Murphy and her coauthors reported no potential conflicts of interest related to the case report.
SOURCE: Murphy J et al. MMWR Morb Mortal Wkly Rep. 2019 Jan 4;67(5152):1410-4.
FROM MMWR
Key clinical point: depending on length of trip, location, and activities involved.
Major finding: A Virginia woman died after sustaining a bite from a rabid puppy during a 2017 yoga retreat in rural India.
Study details: Case report including details of the 65-year-old woman’s trip, rabies exposure, symptoms, diagnosis, and eventual death.
Disclosures: Authors reported no potential conflicts of interest.
Source: Murphy J et al. MMWR Morb Mortal Wkly Rep. 2019 Jan 4;67(5152):1410-4.
Methotrexate relieves pain of Chikungunya-associated arthritis
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
FROM JOURNAL OF CLINICAL RHEUMATOLOGY
Key clinical point:
Major finding: On a 10-point visual analog scale, the pain reduction from baseline on methotrexate at 8 weeks was 4.5 (P less than .0001).
Study details: Retrospective observational study.
Disclosures: The authors declared no potential conflicts of interest.
Source: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943
FDA approves rifamycin for treatment of traveler’s diarrhea
The Food and Drug Administration has approved rifamycin (Aemcolo) for the treatment of traveler’s diarrhea caused by noninvasive strains of Escherichia coli.
FDA approval was based on results of three clinical trials. The efficacy of rifamycin was shown in a trial of 264 adults with traveler’s diarrhea in Guatemala and Mexico. Compared with placebo, rifamycin significantly reduced symptoms of the condition. The safety of rifamycin was illustrated in a pair of studies including 619 adults with traveler’s diarrhea who took rifamycin orally for 3-4 days. The most common adverse events were headache and constipation.
Traveler’s diarrhea is the most common travel-related illness, affecting 10%-40% of travelers. It can be caused by a multitude of pathogens, but bacteria from food or water is the most common source. High-risk areas include much of Asia, the Middle East, Mexico, Central and South America, and Africa.
Rifamycin was not effective in patients with diarrhea complicated by fever and/or bloody stool or in diarrhea caused by a pathogen other than E. coli.
“Travelers’ diarrhea affects millions of people each year, and having treatment options for this condition can help reduce symptoms of the condition,” Edward Cox, MD, MPH, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has approved rifamycin (Aemcolo) for the treatment of traveler’s diarrhea caused by noninvasive strains of Escherichia coli.
FDA approval was based on results of three clinical trials. The efficacy of rifamycin was shown in a trial of 264 adults with traveler’s diarrhea in Guatemala and Mexico. Compared with placebo, rifamycin significantly reduced symptoms of the condition. The safety of rifamycin was illustrated in a pair of studies including 619 adults with traveler’s diarrhea who took rifamycin orally for 3-4 days. The most common adverse events were headache and constipation.
Traveler’s diarrhea is the most common travel-related illness, affecting 10%-40% of travelers. It can be caused by a multitude of pathogens, but bacteria from food or water is the most common source. High-risk areas include much of Asia, the Middle East, Mexico, Central and South America, and Africa.
Rifamycin was not effective in patients with diarrhea complicated by fever and/or bloody stool or in diarrhea caused by a pathogen other than E. coli.
“Travelers’ diarrhea affects millions of people each year, and having treatment options for this condition can help reduce symptoms of the condition,” Edward Cox, MD, MPH, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has approved rifamycin (Aemcolo) for the treatment of traveler’s diarrhea caused by noninvasive strains of Escherichia coli.
FDA approval was based on results of three clinical trials. The efficacy of rifamycin was shown in a trial of 264 adults with traveler’s diarrhea in Guatemala and Mexico. Compared with placebo, rifamycin significantly reduced symptoms of the condition. The safety of rifamycin was illustrated in a pair of studies including 619 adults with traveler’s diarrhea who took rifamycin orally for 3-4 days. The most common adverse events were headache and constipation.
Traveler’s diarrhea is the most common travel-related illness, affecting 10%-40% of travelers. It can be caused by a multitude of pathogens, but bacteria from food or water is the most common source. High-risk areas include much of Asia, the Middle East, Mexico, Central and South America, and Africa.
Rifamycin was not effective in patients with diarrhea complicated by fever and/or bloody stool or in diarrhea caused by a pathogen other than E. coli.
“Travelers’ diarrhea affects millions of people each year, and having treatment options for this condition can help reduce symptoms of the condition,” Edward Cox, MD, MPH, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
FDA authorizes emergency use of rapid fingerstick test for Ebola
The Food and Drug Administration has issued an emergency use authorization (EUA) for the DPP Ebola Antigen System, a rapid, single-use test for the detection of Ebola virus.
The DPP Ebola Antigen System can provide rapid results in locations where health care providers lack access to authorized Ebola virus nucleic acid tests, which are highly sensitive but require an adequately equipped laboratory setting. The new system is authorized to use blood specimens from capillary whole blood, ethylenediaminetetraacetic acid (EDTA) venous whole blood, and EDTA plasma. It is to be used in individuals with signs and symptoms of Ebola virus disease, in addition to other risk factors, such as living in an area with high Ebola virus prevalence or having had contact with people showing signs or symptoms of the disease.
The system is the second Ebola rapid antigen fingerstick test made available through the EUA, but it is the first to use a portable, battery-operated reader, allowing for easier use in remote areas where patients are likely to be treated.
The FDA noted that a negative result from the DPP Ebola Antigen system does not necessarily indicate a negative diagnosis and should not be taken authoritatively, especially in individuals displaying signs and systems of Ebola virus disease.
“This EUA is part of the agency’s ongoing efforts to help mitigate potential, future threats by making medical products that have the potential to prevent, diagnosis, or treat available as quickly as possible. We’re committed to helping the people of the DRC [Democratic Republic of the Congo] effectively confront and end the current Ebola outbreak. By authorizing the first fingerstick test with a portable reader, we hope to better arm health care providers in the field to more quickly detect the virus in patients and improve patient outcomes,” FDA Commissioner Scott Gottlieb, MD, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has issued an emergency use authorization (EUA) for the DPP Ebola Antigen System, a rapid, single-use test for the detection of Ebola virus.
The DPP Ebola Antigen System can provide rapid results in locations where health care providers lack access to authorized Ebola virus nucleic acid tests, which are highly sensitive but require an adequately equipped laboratory setting. The new system is authorized to use blood specimens from capillary whole blood, ethylenediaminetetraacetic acid (EDTA) venous whole blood, and EDTA plasma. It is to be used in individuals with signs and symptoms of Ebola virus disease, in addition to other risk factors, such as living in an area with high Ebola virus prevalence or having had contact with people showing signs or symptoms of the disease.
The system is the second Ebola rapid antigen fingerstick test made available through the EUA, but it is the first to use a portable, battery-operated reader, allowing for easier use in remote areas where patients are likely to be treated.
The FDA noted that a negative result from the DPP Ebola Antigen system does not necessarily indicate a negative diagnosis and should not be taken authoritatively, especially in individuals displaying signs and systems of Ebola virus disease.
“This EUA is part of the agency’s ongoing efforts to help mitigate potential, future threats by making medical products that have the potential to prevent, diagnosis, or treat available as quickly as possible. We’re committed to helping the people of the DRC [Democratic Republic of the Congo] effectively confront and end the current Ebola outbreak. By authorizing the first fingerstick test with a portable reader, we hope to better arm health care providers in the field to more quickly detect the virus in patients and improve patient outcomes,” FDA Commissioner Scott Gottlieb, MD, said in the press release.
Find the full press release on the FDA website.
The Food and Drug Administration has issued an emergency use authorization (EUA) for the DPP Ebola Antigen System, a rapid, single-use test for the detection of Ebola virus.
The DPP Ebola Antigen System can provide rapid results in locations where health care providers lack access to authorized Ebola virus nucleic acid tests, which are highly sensitive but require an adequately equipped laboratory setting. The new system is authorized to use blood specimens from capillary whole blood, ethylenediaminetetraacetic acid (EDTA) venous whole blood, and EDTA plasma. It is to be used in individuals with signs and symptoms of Ebola virus disease, in addition to other risk factors, such as living in an area with high Ebola virus prevalence or having had contact with people showing signs or symptoms of the disease.
The system is the second Ebola rapid antigen fingerstick test made available through the EUA, but it is the first to use a portable, battery-operated reader, allowing for easier use in remote areas where patients are likely to be treated.
The FDA noted that a negative result from the DPP Ebola Antigen system does not necessarily indicate a negative diagnosis and should not be taken authoritatively, especially in individuals displaying signs and systems of Ebola virus disease.
“This EUA is part of the agency’s ongoing efforts to help mitigate potential, future threats by making medical products that have the potential to prevent, diagnosis, or treat available as quickly as possible. We’re committed to helping the people of the DRC [Democratic Republic of the Congo] effectively confront and end the current Ebola outbreak. By authorizing the first fingerstick test with a portable reader, we hope to better arm health care providers in the field to more quickly detect the virus in patients and improve patient outcomes,” FDA Commissioner Scott Gottlieb, MD, said in the press release.
Find the full press release on the FDA website.
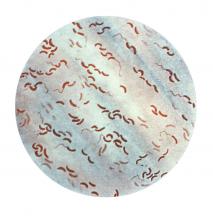
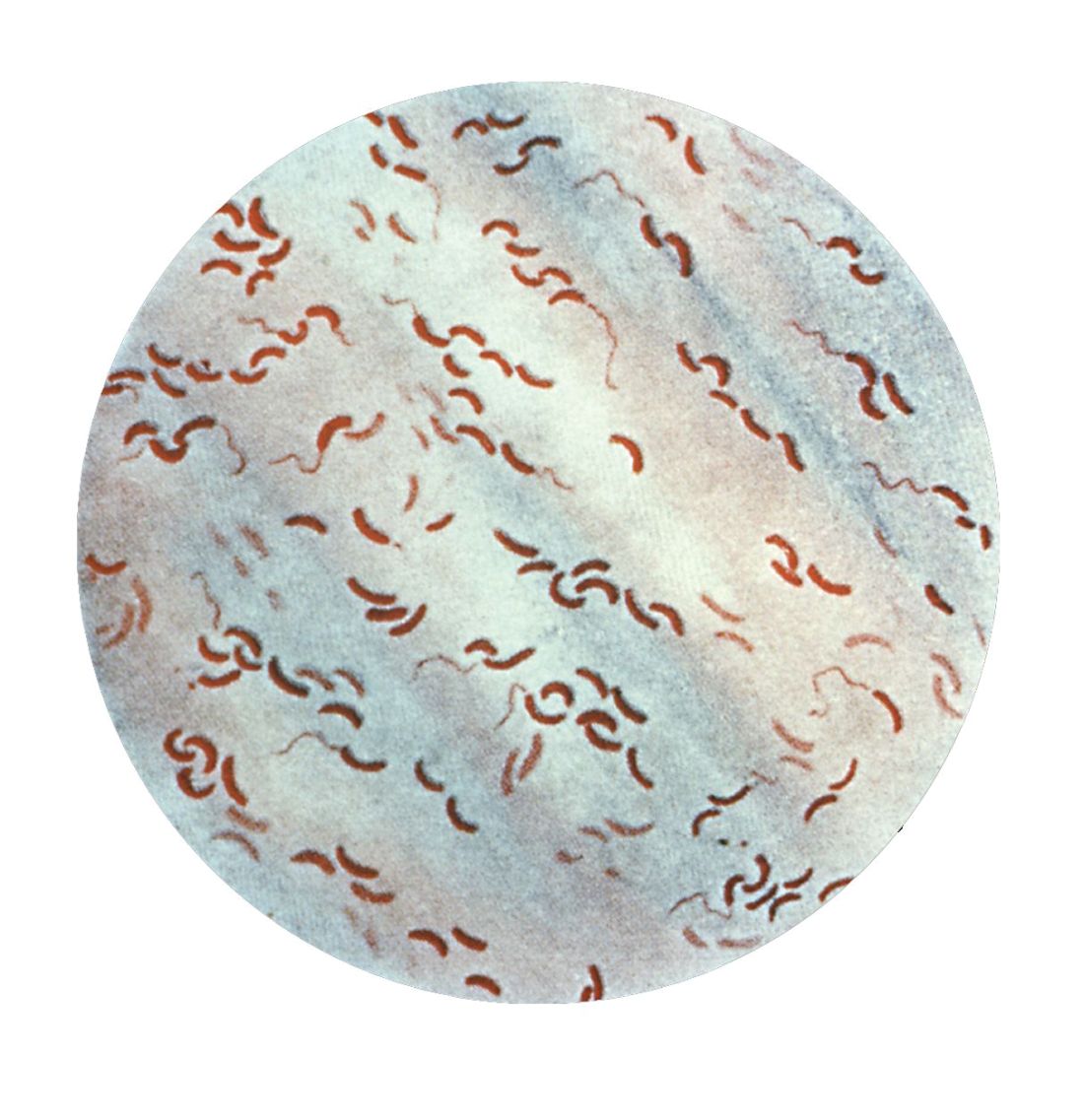
Cholera, bacteriophage in an epic evolutionary struggle
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
REPORTING FROM IDWEEK 2018
Key clinical point: Bacteriophages place selective pressure on bacteria that may influence the emergence of new pathogenic strains.
Major finding: Bacteriophages turn on their own CRISPR system to target cholera defensive genes.
Study details: Genetic analysis of an antibiotic resistant strain and attacking bacteriophage strains.
Disclosures: The National Institutes of Health and the Chan Zuckerberg Biohub provided research funding.
Source: Seed K et al. ID Week 2018. Abstract 954.
Arginine deficiency implicated in novel hemorrhagic fever fatality
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
koakes@mdedge.com
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
koakes@mdedge.com
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
koakes@mdedge.com
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Low arginine bioavailability was associated with increased risk for severe fever with thrombocytopenia syndrome (SFTS) fatality.
Major finding: Arginine bioavailability had an area under the receiver operating curve of 0.713 for predicting fatality.
Study details: A prospective cohort metabolomics study of 242 serum samples from patients with and without SFTS and a randomized, double-blind, placebo-controlled clinical trial of 113 patients given intravenous arginine supplementation or vehicle alone, in conjunction with supportive care.
Disclosures: The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
Source: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
UN aims to eradicate TB by 2030
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.
On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
FROM A WORLD HEALTH ORGANIZATION PRESS CONFERENCE