User login
Nordic walking bests other workouts on functional outcome in CVD
Nordic walking was significantly better at improving functional capacity than were moderate- to vigorous-intensity continuous training and high-intensity interval training (HIIT) in a single-center randomized controlled trial.
Participants who did Nordic walking saw better improvements in functional capacity, measured via the 6-minute walk test distances, than did individuals doing either of the other exercise strategies (interaction effect, P = .010).
From baseline to 26 weeks, the average changes in 6-minute walk test distance were 55.6 m and 59.9 m for moderate- to vigorous-intensity continuous training and HIIT, respectively, but 94.2 m in the Nordic walking group, reported Tasuku Terada, PhD, University of Ottawa Heart Institute, Ontario, and colleagues.
Previous research looked at these results at the end of a 12-week supervised exercise intervention and showed that although all three strategies were safe and had positive effects on physical and mental health in these patients, Nordic walking had a better effect in raising the 6-minute walk test scores than did moderate- to vigorous-intensity continuous training and HIIT, the researchers noted.
“This study is a follow-up on the previous study to show that Nordic walking had greater sustained effects even after the observation phase,” from 12 to 26 weeks, Dr. Terada said in an interview.
“Exercise is a medicine to improve the health of patients, but unfortunately, sometimes it is not as often utilized,” Dr. Terada told this news organization.
Giving patients additional exercise modalities is beneficial because not everyone likes HIIT workouts or long continuous walking, Dr. Terada said. “So, if that’s the case, we can recommend Nordic walking as another type of exercise and expect a similar or good impact in functional capacity.”
The results were published online in the Canadian Journal of Cardiology.
“I think it honestly supports the idea that, as many other studies show, physical activity and exercise improve functional capacity no matter how you measure it and have beneficial effects on mental health and quality of life and particularly depression as well,” Carl “Chip” Lavie, MD, University of Queensland, New Orleans, who coauthored an editorial accompanying the publication, said in an interview.
“Clinicians need to get patients to do the type of exercise that they are going to do. A lot of people ask what’s the best exercise, and the best exercise is one that the person is going to do,” Dr. Lavie said.
Nordic walking is an enhanced form of walking that engages the upper and lower body musculatures, noted Dr. Lavie.
“With regard to Nordic walking, I think that now adds an additional option that many people wouldn’t have thought about. For many of the patients that have issues that are musculoskeletal, issues with posture, gait, or balance, using the poles can be a way to allow them to walk much better and increase their speed, and as they do that, they become fitter,” Dr. Lavie continued.
Moreover, these findings support the use of Nordic walking in cardiac rehabilitation programs, the editorialists noted.
Cardiac rehabilitation
The study examined patients with coronary artery disease who underwent cardiac revascularization. They were then referred by their physicians to cardiac rehabilitation.
Participants were randomly assigned to one of the following intervention groups: Nordic walking (n = 30), moderate- to vigorous-intensity continuous training (n = 27), and HIIT (n = 29) for a 12-week period. There was then an additional 14-week observation period after the exercise program. Mean age was 60 years across the intervention groups.
The research team analyzed the extent of participants’ depression with Beck Depression Inventory–II, quality of life with Short Form–36 and HeartQoL, and functional capacity with a 6-minute walk test. They assessed functional capacity, depression, and quality of life at baseline, 12 weeks, and 26 weeks.
Using linear mixed models with extended measures, the study authors evaluated sustained effects, which were between week 12 and week 26, and prolonged effects, which were between baseline and week 26.
From baseline to 26 weeks, participants saw significantly better outcomes in quality of life, depression symptoms, and 6-minute walk test (P < .05).
Physical quality of life and 6-minute walk test distance rose significantly between weeks 12 and 26 (P < .05).
Notably, at week 26, all training groups achieved the minimal clinical threshold difference of 54 m, although participants in the Nordic walking cohort demonstrated significantly greater improvement in outcomes.
Other data indicated the following:
- From baseline to week 12, physical activity levels rose significantly, and this improvement was sustained through the observation period.
- During the observation period, mental component summary significantly declined while physical component summary outcomes improved.
- After completion of cardiac rehabilitation, functional capacity continued to increase significantly.
- Moderate- to vigorous-intensity continuous training, HIIT, and Nordic walking had positive and significant prolonged effects on depression symptoms and general and disease-specific quality of life, with no differences in the extent of improvements between exercise types.
Some limitations of the study include the fact that women comprised a small portion of the study group, which limits the generalizability of these data, the cohort was recruited from a single medical facility, and there was a short follow-up time, the researchers noted.
“Further research is warranted to investigate the efficacy and integration of Nordic walking into home-based exercise after supervised cardiac rehabilitation for maintenance of physical and mental health,” the editorialists concluded.
Dr. Terada, Dr. Lavie, and Dr. Taylor reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nordic walking was significantly better at improving functional capacity than were moderate- to vigorous-intensity continuous training and high-intensity interval training (HIIT) in a single-center randomized controlled trial.
Participants who did Nordic walking saw better improvements in functional capacity, measured via the 6-minute walk test distances, than did individuals doing either of the other exercise strategies (interaction effect, P = .010).
From baseline to 26 weeks, the average changes in 6-minute walk test distance were 55.6 m and 59.9 m for moderate- to vigorous-intensity continuous training and HIIT, respectively, but 94.2 m in the Nordic walking group, reported Tasuku Terada, PhD, University of Ottawa Heart Institute, Ontario, and colleagues.
Previous research looked at these results at the end of a 12-week supervised exercise intervention and showed that although all three strategies were safe and had positive effects on physical and mental health in these patients, Nordic walking had a better effect in raising the 6-minute walk test scores than did moderate- to vigorous-intensity continuous training and HIIT, the researchers noted.
“This study is a follow-up on the previous study to show that Nordic walking had greater sustained effects even after the observation phase,” from 12 to 26 weeks, Dr. Terada said in an interview.
“Exercise is a medicine to improve the health of patients, but unfortunately, sometimes it is not as often utilized,” Dr. Terada told this news organization.
Giving patients additional exercise modalities is beneficial because not everyone likes HIIT workouts or long continuous walking, Dr. Terada said. “So, if that’s the case, we can recommend Nordic walking as another type of exercise and expect a similar or good impact in functional capacity.”
The results were published online in the Canadian Journal of Cardiology.
“I think it honestly supports the idea that, as many other studies show, physical activity and exercise improve functional capacity no matter how you measure it and have beneficial effects on mental health and quality of life and particularly depression as well,” Carl “Chip” Lavie, MD, University of Queensland, New Orleans, who coauthored an editorial accompanying the publication, said in an interview.
“Clinicians need to get patients to do the type of exercise that they are going to do. A lot of people ask what’s the best exercise, and the best exercise is one that the person is going to do,” Dr. Lavie said.
Nordic walking is an enhanced form of walking that engages the upper and lower body musculatures, noted Dr. Lavie.
“With regard to Nordic walking, I think that now adds an additional option that many people wouldn’t have thought about. For many of the patients that have issues that are musculoskeletal, issues with posture, gait, or balance, using the poles can be a way to allow them to walk much better and increase their speed, and as they do that, they become fitter,” Dr. Lavie continued.
Moreover, these findings support the use of Nordic walking in cardiac rehabilitation programs, the editorialists noted.
Cardiac rehabilitation
The study examined patients with coronary artery disease who underwent cardiac revascularization. They were then referred by their physicians to cardiac rehabilitation.
Participants were randomly assigned to one of the following intervention groups: Nordic walking (n = 30), moderate- to vigorous-intensity continuous training (n = 27), and HIIT (n = 29) for a 12-week period. There was then an additional 14-week observation period after the exercise program. Mean age was 60 years across the intervention groups.
The research team analyzed the extent of participants’ depression with Beck Depression Inventory–II, quality of life with Short Form–36 and HeartQoL, and functional capacity with a 6-minute walk test. They assessed functional capacity, depression, and quality of life at baseline, 12 weeks, and 26 weeks.
Using linear mixed models with extended measures, the study authors evaluated sustained effects, which were between week 12 and week 26, and prolonged effects, which were between baseline and week 26.
From baseline to 26 weeks, participants saw significantly better outcomes in quality of life, depression symptoms, and 6-minute walk test (P < .05).
Physical quality of life and 6-minute walk test distance rose significantly between weeks 12 and 26 (P < .05).
Notably, at week 26, all training groups achieved the minimal clinical threshold difference of 54 m, although participants in the Nordic walking cohort demonstrated significantly greater improvement in outcomes.
Other data indicated the following:
- From baseline to week 12, physical activity levels rose significantly, and this improvement was sustained through the observation period.
- During the observation period, mental component summary significantly declined while physical component summary outcomes improved.
- After completion of cardiac rehabilitation, functional capacity continued to increase significantly.
- Moderate- to vigorous-intensity continuous training, HIIT, and Nordic walking had positive and significant prolonged effects on depression symptoms and general and disease-specific quality of life, with no differences in the extent of improvements between exercise types.
Some limitations of the study include the fact that women comprised a small portion of the study group, which limits the generalizability of these data, the cohort was recruited from a single medical facility, and there was a short follow-up time, the researchers noted.
“Further research is warranted to investigate the efficacy and integration of Nordic walking into home-based exercise after supervised cardiac rehabilitation for maintenance of physical and mental health,” the editorialists concluded.
Dr. Terada, Dr. Lavie, and Dr. Taylor reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nordic walking was significantly better at improving functional capacity than were moderate- to vigorous-intensity continuous training and high-intensity interval training (HIIT) in a single-center randomized controlled trial.
Participants who did Nordic walking saw better improvements in functional capacity, measured via the 6-minute walk test distances, than did individuals doing either of the other exercise strategies (interaction effect, P = .010).
From baseline to 26 weeks, the average changes in 6-minute walk test distance were 55.6 m and 59.9 m for moderate- to vigorous-intensity continuous training and HIIT, respectively, but 94.2 m in the Nordic walking group, reported Tasuku Terada, PhD, University of Ottawa Heart Institute, Ontario, and colleagues.
Previous research looked at these results at the end of a 12-week supervised exercise intervention and showed that although all three strategies were safe and had positive effects on physical and mental health in these patients, Nordic walking had a better effect in raising the 6-minute walk test scores than did moderate- to vigorous-intensity continuous training and HIIT, the researchers noted.
“This study is a follow-up on the previous study to show that Nordic walking had greater sustained effects even after the observation phase,” from 12 to 26 weeks, Dr. Terada said in an interview.
“Exercise is a medicine to improve the health of patients, but unfortunately, sometimes it is not as often utilized,” Dr. Terada told this news organization.
Giving patients additional exercise modalities is beneficial because not everyone likes HIIT workouts or long continuous walking, Dr. Terada said. “So, if that’s the case, we can recommend Nordic walking as another type of exercise and expect a similar or good impact in functional capacity.”
The results were published online in the Canadian Journal of Cardiology.
“I think it honestly supports the idea that, as many other studies show, physical activity and exercise improve functional capacity no matter how you measure it and have beneficial effects on mental health and quality of life and particularly depression as well,” Carl “Chip” Lavie, MD, University of Queensland, New Orleans, who coauthored an editorial accompanying the publication, said in an interview.
“Clinicians need to get patients to do the type of exercise that they are going to do. A lot of people ask what’s the best exercise, and the best exercise is one that the person is going to do,” Dr. Lavie said.
Nordic walking is an enhanced form of walking that engages the upper and lower body musculatures, noted Dr. Lavie.
“With regard to Nordic walking, I think that now adds an additional option that many people wouldn’t have thought about. For many of the patients that have issues that are musculoskeletal, issues with posture, gait, or balance, using the poles can be a way to allow them to walk much better and increase their speed, and as they do that, they become fitter,” Dr. Lavie continued.
Moreover, these findings support the use of Nordic walking in cardiac rehabilitation programs, the editorialists noted.
Cardiac rehabilitation
The study examined patients with coronary artery disease who underwent cardiac revascularization. They were then referred by their physicians to cardiac rehabilitation.
Participants were randomly assigned to one of the following intervention groups: Nordic walking (n = 30), moderate- to vigorous-intensity continuous training (n = 27), and HIIT (n = 29) for a 12-week period. There was then an additional 14-week observation period after the exercise program. Mean age was 60 years across the intervention groups.
The research team analyzed the extent of participants’ depression with Beck Depression Inventory–II, quality of life with Short Form–36 and HeartQoL, and functional capacity with a 6-minute walk test. They assessed functional capacity, depression, and quality of life at baseline, 12 weeks, and 26 weeks.
Using linear mixed models with extended measures, the study authors evaluated sustained effects, which were between week 12 and week 26, and prolonged effects, which were between baseline and week 26.
From baseline to 26 weeks, participants saw significantly better outcomes in quality of life, depression symptoms, and 6-minute walk test (P < .05).
Physical quality of life and 6-minute walk test distance rose significantly between weeks 12 and 26 (P < .05).
Notably, at week 26, all training groups achieved the minimal clinical threshold difference of 54 m, although participants in the Nordic walking cohort demonstrated significantly greater improvement in outcomes.
Other data indicated the following:
- From baseline to week 12, physical activity levels rose significantly, and this improvement was sustained through the observation period.
- During the observation period, mental component summary significantly declined while physical component summary outcomes improved.
- After completion of cardiac rehabilitation, functional capacity continued to increase significantly.
- Moderate- to vigorous-intensity continuous training, HIIT, and Nordic walking had positive and significant prolonged effects on depression symptoms and general and disease-specific quality of life, with no differences in the extent of improvements between exercise types.
Some limitations of the study include the fact that women comprised a small portion of the study group, which limits the generalizability of these data, the cohort was recruited from a single medical facility, and there was a short follow-up time, the researchers noted.
“Further research is warranted to investigate the efficacy and integration of Nordic walking into home-based exercise after supervised cardiac rehabilitation for maintenance of physical and mental health,” the editorialists concluded.
Dr. Terada, Dr. Lavie, and Dr. Taylor reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
Early cardiac rehab as effective as later start after sternotomy
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Women benefit but lag behind in intracoronary imaging in PCI
A real-world analysis reveals that women are consistently less likely to undergo intracoronary imaging as part of percutaneous coronary intervention (PCI), even though it benefits both sexes equally.
Results from nearly all PCIs performed in England and Wales between 2006 and 2019 showed the absolute rate of intracoronary imaging with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was 5% lower in the later study years among women at 14.5%, compared with 19.6% in men (P < .001).
After adjustment, female sex was an independent predictor of lower intracoronary imaging use (odds ratio, 0.93; 95% confidence interval, 0.91-0.96), according to the study, published in JACC: Cardiovascular Interventions.
“One of the thoughts I had when we were running this analysis was, well, maybe the indications for that imaging, as recommended by guidelines, are less common in women,” Mamas Mamas, MD, told this news organization. “So what we did was to look at just cases where imaging is recommended by the EAPCI [European Association of Percutaneous Coronary Intervention].”
Again, the use of intracoronary imaging was consistently lower among women than among men for all of the following EAPCI-recommended indications:
- Acute coronary syndrome: 11.6% vs. 12.3% (P < .01).
- Stent thrombosis: 30.9% vs. 34.9% (P < .01).
- Long lesions: 13.1% vs. 16.3% (P < .01).
- Chronic total occlusions: 16.2% vs. 18.3% (P < .01).
- Left main stem PCI: 55.1% vs. 57.5% (P < .01).
- In-stent restenosis: 28.0% vs. 30.7%.
- Calcified lesions: 36.6% vs. 40.1% (P < .01).
- Renal disease: 17.4% vs. 19.5% (P < .01).
As to what might be driving the lower use, Dr. Mamas dismissed the argument that women undergo much simpler PCI, which wouldn’t benefit from imaging. Women do have smaller coronary arteries, however, and there is a belief that it’s easier to eyeball the size of vessels that are smaller rather than larger.
“I’m not convinced that’s entirely true,” he said. “I don’t have a good answer for you, I’m afraid. I don’t really know why we’re seeing it. I just think it’s one of those disparities that is important to highlight.”
Central to this belief is that the benefits of intracoronary imaging were found to be similar in men and women. Intracoronary imaging was associated with lower adjusted odds of in-hospital mortality (OR, 0.56; 95% CI, 0.48-0.64) and major adverse cardiac and cerebrovascular events (OR, 0.83; 95% CI, 0.76-0.91) in women and men (OR, 0.48; 95% CI, 0.44-0.53 and OR, 0.75; 95% CI, 0.71-0.80, respectively), compared with nonimaging groups.
“This really should be a call to arms, particularly given that we show this disparity persists, even in guideline-recommended cases where we should be using it,” said Dr. Mamas, from the Keele (England) Cardiovascular Research Group, Keele University, and Royal Stoke University Hospital, Stoke-on-Trent, England.
“Actually, I would argue that we should be using more imaging in women than men anyway because many of the presentations for acute coronary syndromes in women, like spontaneous coronary artery dissection or MINOCA [MI with nonobstructive coronary arteries], you often need intracoronary imaging to make that kind of diagnosis,” he observed.
Getting worse, not better
Previous studies have shown that women are less likely than men in acute coronary syndromes to receive the transradial approach and P2Y12 inhibitors, but none have specifically looked at intracoronary imaging, Dr. Mamas said.
To fill the gap, the researchers drew on data from 994,478 patients in the British Cardiovascular Intervention Society registry, of whom, 8.4% of 738,616 men and 7.9% of 255,862 women received intracoronary imaging.
Women in the imaging group were older, more likely to be an ethnic minority, and more likely to undergo PCI for non–ST-segment elevation MI than their male counterparts.
One of the more surprising findings was that rates of IVUS and OCT were superimposable between the sexes at the start of the study but quickly diverged starting in around 2012, when the technology took off, Dr. Mamas said. In the most recent data, use was about 3% lower in women overall and rising to 6% in those with stable angina.
“Whilst the disparities between men and women are significant, the bigger question is why are we using so little imaging in guideline-recommended cases where there is a benefit?” he said.
Possible actionable items, he suggested, include providing older physicians who didn’t have access to intracoronary imaging during their training with opportunities in their cath lab or with industry sponsors to increase their skills and confidence. Intracoronary imaging use could also be routinely captured in U.S. and European PCI registries and used as a quality metric.
“In left main, you see a massive difference between centers, and that’s the kind of data that drives discussion,” Dr. Mamas said. “If we start reporting quality metrics, such as radial use, intracoronary imaging, P2Y12 inhibitors by center, then you’ve got something to benchmark centers against.”
Nathaniel Smilowitz, MD, an interventional cardiologist at New York Langone Health, who was not associated with the study, said that it’s troubling to see that the utilization intravascular imaging is so low, despite randomized trials and large meta-analyses showing a mortality benefit associated with its use in PCI.
“Even among men, only 19.6% in the later years were getting intravascular imaging performed to guide their coronary intervention, so one out of five,” he said. “There are opportunities to improve.”
Dr. Smilowitz said he’s also perplexed as to why adoption would be lower in women but that the findings echo those in other domains where women receive less intensive cardiovascular therapy.
“There’s no biological, really plausible, mechanism as to why the need for intravascular imaging would be lower and, particularly, because they showed in stent thrombosis, for example, where intravascular imaging is tremendously important, there were still sex differences,” he said. “So even with clear indications for imaging, women just received the optimal therapy less often than men. It’s disappointing.”
Dr. Smilowitz agreed that there may be a need to incorporate intravascular imaging into metrics, which are reported back to physicians, potentially even for comparisons with peers or regional rates to incentivize physicians to improve uptake.
“As a society, we’ve been quite slow to integrate intravascular imaging to guide PCI and we can do better,” he said.
A version of this article first appeared on Medscape.com.
A real-world analysis reveals that women are consistently less likely to undergo intracoronary imaging as part of percutaneous coronary intervention (PCI), even though it benefits both sexes equally.
Results from nearly all PCIs performed in England and Wales between 2006 and 2019 showed the absolute rate of intracoronary imaging with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was 5% lower in the later study years among women at 14.5%, compared with 19.6% in men (P < .001).
After adjustment, female sex was an independent predictor of lower intracoronary imaging use (odds ratio, 0.93; 95% confidence interval, 0.91-0.96), according to the study, published in JACC: Cardiovascular Interventions.
“One of the thoughts I had when we were running this analysis was, well, maybe the indications for that imaging, as recommended by guidelines, are less common in women,” Mamas Mamas, MD, told this news organization. “So what we did was to look at just cases where imaging is recommended by the EAPCI [European Association of Percutaneous Coronary Intervention].”
Again, the use of intracoronary imaging was consistently lower among women than among men for all of the following EAPCI-recommended indications:
- Acute coronary syndrome: 11.6% vs. 12.3% (P < .01).
- Stent thrombosis: 30.9% vs. 34.9% (P < .01).
- Long lesions: 13.1% vs. 16.3% (P < .01).
- Chronic total occlusions: 16.2% vs. 18.3% (P < .01).
- Left main stem PCI: 55.1% vs. 57.5% (P < .01).
- In-stent restenosis: 28.0% vs. 30.7%.
- Calcified lesions: 36.6% vs. 40.1% (P < .01).
- Renal disease: 17.4% vs. 19.5% (P < .01).
As to what might be driving the lower use, Dr. Mamas dismissed the argument that women undergo much simpler PCI, which wouldn’t benefit from imaging. Women do have smaller coronary arteries, however, and there is a belief that it’s easier to eyeball the size of vessels that are smaller rather than larger.
“I’m not convinced that’s entirely true,” he said. “I don’t have a good answer for you, I’m afraid. I don’t really know why we’re seeing it. I just think it’s one of those disparities that is important to highlight.”
Central to this belief is that the benefits of intracoronary imaging were found to be similar in men and women. Intracoronary imaging was associated with lower adjusted odds of in-hospital mortality (OR, 0.56; 95% CI, 0.48-0.64) and major adverse cardiac and cerebrovascular events (OR, 0.83; 95% CI, 0.76-0.91) in women and men (OR, 0.48; 95% CI, 0.44-0.53 and OR, 0.75; 95% CI, 0.71-0.80, respectively), compared with nonimaging groups.
“This really should be a call to arms, particularly given that we show this disparity persists, even in guideline-recommended cases where we should be using it,” said Dr. Mamas, from the Keele (England) Cardiovascular Research Group, Keele University, and Royal Stoke University Hospital, Stoke-on-Trent, England.
“Actually, I would argue that we should be using more imaging in women than men anyway because many of the presentations for acute coronary syndromes in women, like spontaneous coronary artery dissection or MINOCA [MI with nonobstructive coronary arteries], you often need intracoronary imaging to make that kind of diagnosis,” he observed.
Getting worse, not better
Previous studies have shown that women are less likely than men in acute coronary syndromes to receive the transradial approach and P2Y12 inhibitors, but none have specifically looked at intracoronary imaging, Dr. Mamas said.
To fill the gap, the researchers drew on data from 994,478 patients in the British Cardiovascular Intervention Society registry, of whom, 8.4% of 738,616 men and 7.9% of 255,862 women received intracoronary imaging.
Women in the imaging group were older, more likely to be an ethnic minority, and more likely to undergo PCI for non–ST-segment elevation MI than their male counterparts.
One of the more surprising findings was that rates of IVUS and OCT were superimposable between the sexes at the start of the study but quickly diverged starting in around 2012, when the technology took off, Dr. Mamas said. In the most recent data, use was about 3% lower in women overall and rising to 6% in those with stable angina.
“Whilst the disparities between men and women are significant, the bigger question is why are we using so little imaging in guideline-recommended cases where there is a benefit?” he said.
Possible actionable items, he suggested, include providing older physicians who didn’t have access to intracoronary imaging during their training with opportunities in their cath lab or with industry sponsors to increase their skills and confidence. Intracoronary imaging use could also be routinely captured in U.S. and European PCI registries and used as a quality metric.
“In left main, you see a massive difference between centers, and that’s the kind of data that drives discussion,” Dr. Mamas said. “If we start reporting quality metrics, such as radial use, intracoronary imaging, P2Y12 inhibitors by center, then you’ve got something to benchmark centers against.”
Nathaniel Smilowitz, MD, an interventional cardiologist at New York Langone Health, who was not associated with the study, said that it’s troubling to see that the utilization intravascular imaging is so low, despite randomized trials and large meta-analyses showing a mortality benefit associated with its use in PCI.
“Even among men, only 19.6% in the later years were getting intravascular imaging performed to guide their coronary intervention, so one out of five,” he said. “There are opportunities to improve.”
Dr. Smilowitz said he’s also perplexed as to why adoption would be lower in women but that the findings echo those in other domains where women receive less intensive cardiovascular therapy.
“There’s no biological, really plausible, mechanism as to why the need for intravascular imaging would be lower and, particularly, because they showed in stent thrombosis, for example, where intravascular imaging is tremendously important, there were still sex differences,” he said. “So even with clear indications for imaging, women just received the optimal therapy less often than men. It’s disappointing.”
Dr. Smilowitz agreed that there may be a need to incorporate intravascular imaging into metrics, which are reported back to physicians, potentially even for comparisons with peers or regional rates to incentivize physicians to improve uptake.
“As a society, we’ve been quite slow to integrate intravascular imaging to guide PCI and we can do better,” he said.
A version of this article first appeared on Medscape.com.
A real-world analysis reveals that women are consistently less likely to undergo intracoronary imaging as part of percutaneous coronary intervention (PCI), even though it benefits both sexes equally.
Results from nearly all PCIs performed in England and Wales between 2006 and 2019 showed the absolute rate of intracoronary imaging with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was 5% lower in the later study years among women at 14.5%, compared with 19.6% in men (P < .001).
After adjustment, female sex was an independent predictor of lower intracoronary imaging use (odds ratio, 0.93; 95% confidence interval, 0.91-0.96), according to the study, published in JACC: Cardiovascular Interventions.
“One of the thoughts I had when we were running this analysis was, well, maybe the indications for that imaging, as recommended by guidelines, are less common in women,” Mamas Mamas, MD, told this news organization. “So what we did was to look at just cases where imaging is recommended by the EAPCI [European Association of Percutaneous Coronary Intervention].”
Again, the use of intracoronary imaging was consistently lower among women than among men for all of the following EAPCI-recommended indications:
- Acute coronary syndrome: 11.6% vs. 12.3% (P < .01).
- Stent thrombosis: 30.9% vs. 34.9% (P < .01).
- Long lesions: 13.1% vs. 16.3% (P < .01).
- Chronic total occlusions: 16.2% vs. 18.3% (P < .01).
- Left main stem PCI: 55.1% vs. 57.5% (P < .01).
- In-stent restenosis: 28.0% vs. 30.7%.
- Calcified lesions: 36.6% vs. 40.1% (P < .01).
- Renal disease: 17.4% vs. 19.5% (P < .01).
As to what might be driving the lower use, Dr. Mamas dismissed the argument that women undergo much simpler PCI, which wouldn’t benefit from imaging. Women do have smaller coronary arteries, however, and there is a belief that it’s easier to eyeball the size of vessels that are smaller rather than larger.
“I’m not convinced that’s entirely true,” he said. “I don’t have a good answer for you, I’m afraid. I don’t really know why we’re seeing it. I just think it’s one of those disparities that is important to highlight.”
Central to this belief is that the benefits of intracoronary imaging were found to be similar in men and women. Intracoronary imaging was associated with lower adjusted odds of in-hospital mortality (OR, 0.56; 95% CI, 0.48-0.64) and major adverse cardiac and cerebrovascular events (OR, 0.83; 95% CI, 0.76-0.91) in women and men (OR, 0.48; 95% CI, 0.44-0.53 and OR, 0.75; 95% CI, 0.71-0.80, respectively), compared with nonimaging groups.
“This really should be a call to arms, particularly given that we show this disparity persists, even in guideline-recommended cases where we should be using it,” said Dr. Mamas, from the Keele (England) Cardiovascular Research Group, Keele University, and Royal Stoke University Hospital, Stoke-on-Trent, England.
“Actually, I would argue that we should be using more imaging in women than men anyway because many of the presentations for acute coronary syndromes in women, like spontaneous coronary artery dissection or MINOCA [MI with nonobstructive coronary arteries], you often need intracoronary imaging to make that kind of diagnosis,” he observed.
Getting worse, not better
Previous studies have shown that women are less likely than men in acute coronary syndromes to receive the transradial approach and P2Y12 inhibitors, but none have specifically looked at intracoronary imaging, Dr. Mamas said.
To fill the gap, the researchers drew on data from 994,478 patients in the British Cardiovascular Intervention Society registry, of whom, 8.4% of 738,616 men and 7.9% of 255,862 women received intracoronary imaging.
Women in the imaging group were older, more likely to be an ethnic minority, and more likely to undergo PCI for non–ST-segment elevation MI than their male counterparts.
One of the more surprising findings was that rates of IVUS and OCT were superimposable between the sexes at the start of the study but quickly diverged starting in around 2012, when the technology took off, Dr. Mamas said. In the most recent data, use was about 3% lower in women overall and rising to 6% in those with stable angina.
“Whilst the disparities between men and women are significant, the bigger question is why are we using so little imaging in guideline-recommended cases where there is a benefit?” he said.
Possible actionable items, he suggested, include providing older physicians who didn’t have access to intracoronary imaging during their training with opportunities in their cath lab or with industry sponsors to increase their skills and confidence. Intracoronary imaging use could also be routinely captured in U.S. and European PCI registries and used as a quality metric.
“In left main, you see a massive difference between centers, and that’s the kind of data that drives discussion,” Dr. Mamas said. “If we start reporting quality metrics, such as radial use, intracoronary imaging, P2Y12 inhibitors by center, then you’ve got something to benchmark centers against.”
Nathaniel Smilowitz, MD, an interventional cardiologist at New York Langone Health, who was not associated with the study, said that it’s troubling to see that the utilization intravascular imaging is so low, despite randomized trials and large meta-analyses showing a mortality benefit associated with its use in PCI.
“Even among men, only 19.6% in the later years were getting intravascular imaging performed to guide their coronary intervention, so one out of five,” he said. “There are opportunities to improve.”
Dr. Smilowitz said he’s also perplexed as to why adoption would be lower in women but that the findings echo those in other domains where women receive less intensive cardiovascular therapy.
“There’s no biological, really plausible, mechanism as to why the need for intravascular imaging would be lower and, particularly, because they showed in stent thrombosis, for example, where intravascular imaging is tremendously important, there were still sex differences,” he said. “So even with clear indications for imaging, women just received the optimal therapy less often than men. It’s disappointing.”
Dr. Smilowitz agreed that there may be a need to incorporate intravascular imaging into metrics, which are reported back to physicians, potentially even for comparisons with peers or regional rates to incentivize physicians to improve uptake.
“As a society, we’ve been quite slow to integrate intravascular imaging to guide PCI and we can do better,” he said.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
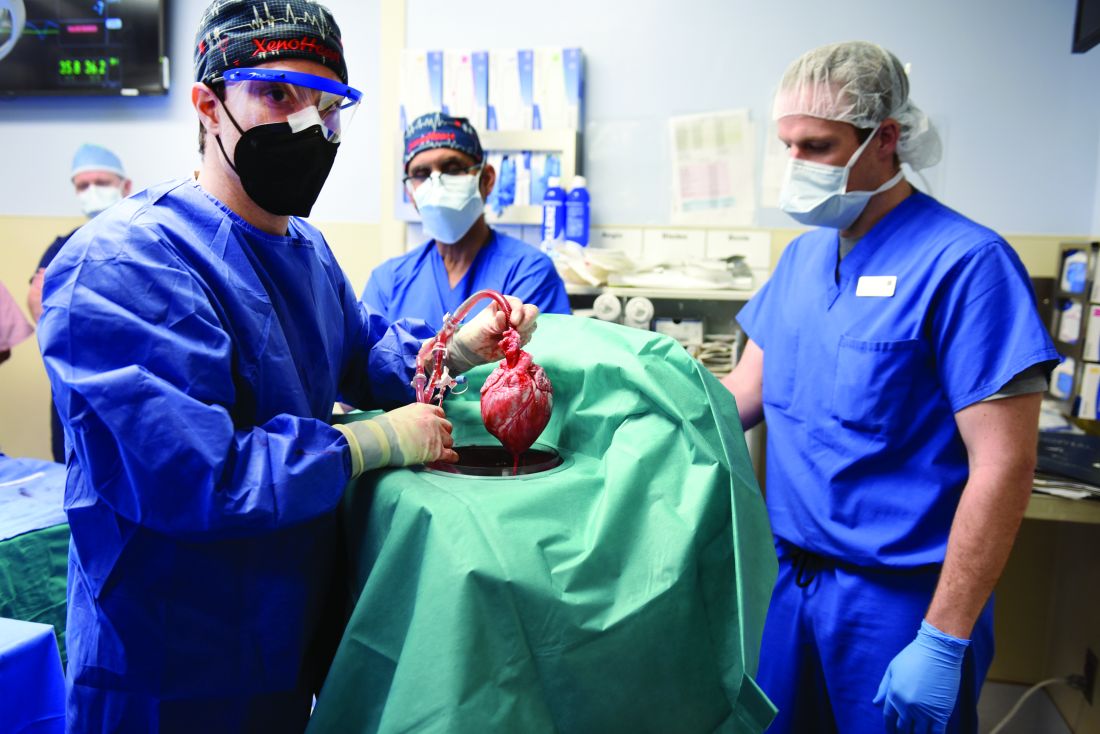
Pig-heart transplant case published with new details, insights
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
Class I recall for Medtronic’s HeartWare HVAD batteries
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to rs.cfqfca@medtronic.com.
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to rs.cfqfca@medtronic.com.
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to rs.cfqfca@medtronic.com.
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Meta-analysis points to safety of acetylcholine coronary testing
Provocation testing with intracoronary acetylcholine is safe, particularly among Western patients, suggests a large systematic review that underscores the importance of functional coronary angiography to diagnose epicardial or microvascular spasm.
The results, derived from more than 12,000 patients in 16 studies, showed a 0.5% risk of major complications, defined as death, ventricular tachycardia/ventricular fibrillation, myocardial infarction, and shock requiring resuscitation.
Ventricular tachycardia/fibrillation were the most common events and mainly reported from two Japanese studies. There were no deaths.
Exploratory subgroup analyses revealed significantly fewer major complications in Western populations (0.0%; P for heterogeneity = .938), compared with Asian populations (2.3%; P for heterogeneity < .001).
The pooled positive vasospasm rate was also lower in Western versus Asian studies (37.9% vs. 50.7%; P for between-group heterogeneity = .010), as reported by the Microvascular Network in the Journal of the American College of Cardiology.
“If you look at the data between Asian studies versus others, mainly European or U.S. studies, primarily in Caucasian populations, it’s like zero percent history of major complications. So, it sounds extremely safe to do this testing in Caucasian populations,” Yuhei Kobayashi, MD, NewYork-Presbyterian Brooklyn Methodist Hospital, Weill Cornell Medicine, said.
Safety will need to be assessed in African Americans and other racial/ethnic groups, but “it makes us think we should end up testing more in the United States,” he told this news organization.
Intracoronary acetylcholine testing is daily practice in Japan but is limited in the United States and Europe to a few specialized centers due to safety concerns. Three deaths were reported in 1980 with intravenous ergonovine testing, whereas the safety of acetylcholine protocols has been studied largely in single-center retrospective studies, typically in Asian populations.
Growing recognition of myocardial infarction with nonobstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA), however, is changing the landscape. In recent U.S. and European guidelines, intracoronary acetylcholine testing is indicated as a class 2a recommendation in MINOCA/INOCA.
“More and more institutions in Europe and the United States are starting to do acetylcholine testing, because now we know that chest pain isn’t necessarily coming from the blocked arteries,” Dr. Kobayashi said. “There are functional abnormalities, including coronary spasm, and if we diagnose it, we have appropriate medical regimens for this kind of disease.”
First safety meta-analysis
The present review and meta-analysis included 12,585 participants in 16 studies through November 2021. Of these, 63% were conducted in Western countries, and most were prospective studies published over the past decade in patients with MINOCA or INOCA.
Ten studies used the contemporary diagnostic criteria for epicardial spasm of at least 90% reduction in coronary diameter. Acetylcholine was administered into the left coronary artery at up to 100 mcg and 200 mcg in seven and six studies, respectively, and was used in the other three studies to assess endothelial function with a slower infusion of up to 36.4 mcg.
Major complications were significantly higher in studies following the contemporary diagnostic cutoff than in those using a lower cutoff of at least 75% diameter reduction (1.0% vs. 0.0%; P for between-group heterogeneity < .001).
The incidence of major complications was 0.2% with the slower infusion of up to 36.4 mcg, 0.8% with a maximum dose of 100 mcg, and 0.3% with a maximum dose of 200 mcg. The positive vasospasm rate was similar with the latter two protocols, at 46.3% and 41.4%, respectively.
Minor complications occurred in 3.3% of patients but were not detailed. They can include paroxysmal atrial fibrillation, ventricular ectopic beats, transient hypotension, and bradycardia requiring intervention.
As with major complications, minor complications were lower in studies using noncontemporary versus contemporary diagnostic cutoffs for epicardial spasm (1.8% vs. 4.7%) and in Western versus Asian populations (2.6% vs. 9.4%). Minor complications were similar between protocols with maximum doses of 100 mcg and 200 mcg (3.6% vs. 3.8%).
Dr. Kobayashi suggested that several factors may explain the racial differences, including previously reported smooth muscle hyperresponsiveness to provocation stimuli in Japanese patients and the inclusion of a wide range of patients in Japanese studies, such as those with obstructive coronary disease.
Japanese studies also used sequential acetylcholine injection into both the right and left coronaries, a faster injection speed of 20 seconds, and upfront placement of a temporary pacing catheter in case of acetylcholine-induced bradycardia, particularly with right coronary injection.
Although the protocol is largely settled in Japan, he said, provocation protocols need to be standardized because “depending on the country and depending on the institution, people are doing totally different things.”
A big step forward
Commenting on the study, C. Noel Bairey Merz, MD, from Cedars Sinai, Los Angeles, said it has “widespread relevance” because half of all coronary angiograms done invasively in the United States for suspected ischemia find no obstructive coronary disease. Left untreated, however, MINOCA has a 2.5% annual event rate, and a quarter of that is death.
“This is a big step forward with likely equal opportunity to improve women and men’s ischemic heart disease,” she said.
On the other hand, all studies were conducted at centers of excellence, so safety will need to be carefully watched as testing rolls out to more community care, Dr. Merz said. “And it always needs to be underscored that this is done by an interventional cardiologist because they’re familiar with wires that can dissect arteries, and they’re familiar with minor complications that could turn into major, if someone didn’t act appropriately.”
Dr. Merz also called for unifying protocols and the need to raise awareness within the general cardiology community to ask interventionalists for acetylcholine spasm testing. Randomized controlled data from within the WISE study and the CorMica study showed that diagnostic certainty leads to greater therapeutic certainty. “You do a much better job about who and how to treat,” she said.
There are also three ongoing randomized controlled trials – WARRIOR, MINOCA-BAT, and iCorMica – in the INOCA and MINOCA populations testing different treatment strategies for hard clinical outcomes like death and myocardial infarction.
“So in addition to this publication being guideline-forming for diagnosis, we anticipate in the next several years to have clinical trial evidence about therapeutics, again, for formulation of class 1 guidelines,” Dr. Merz said.
John Beltrame, BMBS, PhD, University of Adelaide, Australia, said the meta-analysis shows that intracoronary acetylcholine spasm testing is safe and should prompt greater adoption of invasive functional angiography.
Interventionalists are quite happy to do fractional flow reserve using intravenous adenosine to assess coronary microvascular dysfunction, he said, and “what we think is that functional angiography should test both – both the spasm as well as the microvasculature – and that will give us a clear direction because the treatments are slightly different when you’re treating the large arteries as compared to the microscopic arteries. It’s an important thing.”
Dr. Beltrame and colleagues further detail the benefits of comprehensive invasive functional angiography over structural angiography in a related editorial.
He also noted that the Coronary Vasomotion Disorders International Study Group published international diagnostic criteria for microvascular angina and that several protocols for acetylcholine spasm testing are in the works, including one from Australia. Australian investigators are also organizing an accreditation program for those performing the test.
“The protocol itself is relatively straightforward, but it’s not merely picking up a manual and following the instructions,” Dr. Beltrame said. “Just the same as when you train someone in angioplasty, you don’t just go out and do it. You need to develop some experience in it and so should be proctored.”
Dr. Kobayashi reported consulting agreements with Abbott Vascular. Coauthor disclosures are listed in the paper. Dr. Beltrame and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Provocation testing with intracoronary acetylcholine is safe, particularly among Western patients, suggests a large systematic review that underscores the importance of functional coronary angiography to diagnose epicardial or microvascular spasm.
The results, derived from more than 12,000 patients in 16 studies, showed a 0.5% risk of major complications, defined as death, ventricular tachycardia/ventricular fibrillation, myocardial infarction, and shock requiring resuscitation.
Ventricular tachycardia/fibrillation were the most common events and mainly reported from two Japanese studies. There were no deaths.
Exploratory subgroup analyses revealed significantly fewer major complications in Western populations (0.0%; P for heterogeneity = .938), compared with Asian populations (2.3%; P for heterogeneity < .001).
The pooled positive vasospasm rate was also lower in Western versus Asian studies (37.9% vs. 50.7%; P for between-group heterogeneity = .010), as reported by the Microvascular Network in the Journal of the American College of Cardiology.
“If you look at the data between Asian studies versus others, mainly European or U.S. studies, primarily in Caucasian populations, it’s like zero percent history of major complications. So, it sounds extremely safe to do this testing in Caucasian populations,” Yuhei Kobayashi, MD, NewYork-Presbyterian Brooklyn Methodist Hospital, Weill Cornell Medicine, said.
Safety will need to be assessed in African Americans and other racial/ethnic groups, but “it makes us think we should end up testing more in the United States,” he told this news organization.
Intracoronary acetylcholine testing is daily practice in Japan but is limited in the United States and Europe to a few specialized centers due to safety concerns. Three deaths were reported in 1980 with intravenous ergonovine testing, whereas the safety of acetylcholine protocols has been studied largely in single-center retrospective studies, typically in Asian populations.
Growing recognition of myocardial infarction with nonobstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA), however, is changing the landscape. In recent U.S. and European guidelines, intracoronary acetylcholine testing is indicated as a class 2a recommendation in MINOCA/INOCA.
“More and more institutions in Europe and the United States are starting to do acetylcholine testing, because now we know that chest pain isn’t necessarily coming from the blocked arteries,” Dr. Kobayashi said. “There are functional abnormalities, including coronary spasm, and if we diagnose it, we have appropriate medical regimens for this kind of disease.”
First safety meta-analysis
The present review and meta-analysis included 12,585 participants in 16 studies through November 2021. Of these, 63% were conducted in Western countries, and most were prospective studies published over the past decade in patients with MINOCA or INOCA.
Ten studies used the contemporary diagnostic criteria for epicardial spasm of at least 90% reduction in coronary diameter. Acetylcholine was administered into the left coronary artery at up to 100 mcg and 200 mcg in seven and six studies, respectively, and was used in the other three studies to assess endothelial function with a slower infusion of up to 36.4 mcg.
Major complications were significantly higher in studies following the contemporary diagnostic cutoff than in those using a lower cutoff of at least 75% diameter reduction (1.0% vs. 0.0%; P for between-group heterogeneity < .001).
The incidence of major complications was 0.2% with the slower infusion of up to 36.4 mcg, 0.8% with a maximum dose of 100 mcg, and 0.3% with a maximum dose of 200 mcg. The positive vasospasm rate was similar with the latter two protocols, at 46.3% and 41.4%, respectively.
Minor complications occurred in 3.3% of patients but were not detailed. They can include paroxysmal atrial fibrillation, ventricular ectopic beats, transient hypotension, and bradycardia requiring intervention.
As with major complications, minor complications were lower in studies using noncontemporary versus contemporary diagnostic cutoffs for epicardial spasm (1.8% vs. 4.7%) and in Western versus Asian populations (2.6% vs. 9.4%). Minor complications were similar between protocols with maximum doses of 100 mcg and 200 mcg (3.6% vs. 3.8%).
Dr. Kobayashi suggested that several factors may explain the racial differences, including previously reported smooth muscle hyperresponsiveness to provocation stimuli in Japanese patients and the inclusion of a wide range of patients in Japanese studies, such as those with obstructive coronary disease.
Japanese studies also used sequential acetylcholine injection into both the right and left coronaries, a faster injection speed of 20 seconds, and upfront placement of a temporary pacing catheter in case of acetylcholine-induced bradycardia, particularly with right coronary injection.
Although the protocol is largely settled in Japan, he said, provocation protocols need to be standardized because “depending on the country and depending on the institution, people are doing totally different things.”
A big step forward
Commenting on the study, C. Noel Bairey Merz, MD, from Cedars Sinai, Los Angeles, said it has “widespread relevance” because half of all coronary angiograms done invasively in the United States for suspected ischemia find no obstructive coronary disease. Left untreated, however, MINOCA has a 2.5% annual event rate, and a quarter of that is death.
“This is a big step forward with likely equal opportunity to improve women and men’s ischemic heart disease,” she said.
On the other hand, all studies were conducted at centers of excellence, so safety will need to be carefully watched as testing rolls out to more community care, Dr. Merz said. “And it always needs to be underscored that this is done by an interventional cardiologist because they’re familiar with wires that can dissect arteries, and they’re familiar with minor complications that could turn into major, if someone didn’t act appropriately.”
Dr. Merz also called for unifying protocols and the need to raise awareness within the general cardiology community to ask interventionalists for acetylcholine spasm testing. Randomized controlled data from within the WISE study and the CorMica study showed that diagnostic certainty leads to greater therapeutic certainty. “You do a much better job about who and how to treat,” she said.
There are also three ongoing randomized controlled trials – WARRIOR, MINOCA-BAT, and iCorMica – in the INOCA and MINOCA populations testing different treatment strategies for hard clinical outcomes like death and myocardial infarction.
“So in addition to this publication being guideline-forming for diagnosis, we anticipate in the next several years to have clinical trial evidence about therapeutics, again, for formulation of class 1 guidelines,” Dr. Merz said.
John Beltrame, BMBS, PhD, University of Adelaide, Australia, said the meta-analysis shows that intracoronary acetylcholine spasm testing is safe and should prompt greater adoption of invasive functional angiography.
Interventionalists are quite happy to do fractional flow reserve using intravenous adenosine to assess coronary microvascular dysfunction, he said, and “what we think is that functional angiography should test both – both the spasm as well as the microvasculature – and that will give us a clear direction because the treatments are slightly different when you’re treating the large arteries as compared to the microscopic arteries. It’s an important thing.”
Dr. Beltrame and colleagues further detail the benefits of comprehensive invasive functional angiography over structural angiography in a related editorial.
He also noted that the Coronary Vasomotion Disorders International Study Group published international diagnostic criteria for microvascular angina and that several protocols for acetylcholine spasm testing are in the works, including one from Australia. Australian investigators are also organizing an accreditation program for those performing the test.
“The protocol itself is relatively straightforward, but it’s not merely picking up a manual and following the instructions,” Dr. Beltrame said. “Just the same as when you train someone in angioplasty, you don’t just go out and do it. You need to develop some experience in it and so should be proctored.”
Dr. Kobayashi reported consulting agreements with Abbott Vascular. Coauthor disclosures are listed in the paper. Dr. Beltrame and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Provocation testing with intracoronary acetylcholine is safe, particularly among Western patients, suggests a large systematic review that underscores the importance of functional coronary angiography to diagnose epicardial or microvascular spasm.
The results, derived from more than 12,000 patients in 16 studies, showed a 0.5% risk of major complications, defined as death, ventricular tachycardia/ventricular fibrillation, myocardial infarction, and shock requiring resuscitation.
Ventricular tachycardia/fibrillation were the most common events and mainly reported from two Japanese studies. There were no deaths.
Exploratory subgroup analyses revealed significantly fewer major complications in Western populations (0.0%; P for heterogeneity = .938), compared with Asian populations (2.3%; P for heterogeneity < .001).
The pooled positive vasospasm rate was also lower in Western versus Asian studies (37.9% vs. 50.7%; P for between-group heterogeneity = .010), as reported by the Microvascular Network in the Journal of the American College of Cardiology.
“If you look at the data between Asian studies versus others, mainly European or U.S. studies, primarily in Caucasian populations, it’s like zero percent history of major complications. So, it sounds extremely safe to do this testing in Caucasian populations,” Yuhei Kobayashi, MD, NewYork-Presbyterian Brooklyn Methodist Hospital, Weill Cornell Medicine, said.
Safety will need to be assessed in African Americans and other racial/ethnic groups, but “it makes us think we should end up testing more in the United States,” he told this news organization.
Intracoronary acetylcholine testing is daily practice in Japan but is limited in the United States and Europe to a few specialized centers due to safety concerns. Three deaths were reported in 1980 with intravenous ergonovine testing, whereas the safety of acetylcholine protocols has been studied largely in single-center retrospective studies, typically in Asian populations.
Growing recognition of myocardial infarction with nonobstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA), however, is changing the landscape. In recent U.S. and European guidelines, intracoronary acetylcholine testing is indicated as a class 2a recommendation in MINOCA/INOCA.
“More and more institutions in Europe and the United States are starting to do acetylcholine testing, because now we know that chest pain isn’t necessarily coming from the blocked arteries,” Dr. Kobayashi said. “There are functional abnormalities, including coronary spasm, and if we diagnose it, we have appropriate medical regimens for this kind of disease.”
First safety meta-analysis
The present review and meta-analysis included 12,585 participants in 16 studies through November 2021. Of these, 63% were conducted in Western countries, and most were prospective studies published over the past decade in patients with MINOCA or INOCA.
Ten studies used the contemporary diagnostic criteria for epicardial spasm of at least 90% reduction in coronary diameter. Acetylcholine was administered into the left coronary artery at up to 100 mcg and 200 mcg in seven and six studies, respectively, and was used in the other three studies to assess endothelial function with a slower infusion of up to 36.4 mcg.
Major complications were significantly higher in studies following the contemporary diagnostic cutoff than in those using a lower cutoff of at least 75% diameter reduction (1.0% vs. 0.0%; P for between-group heterogeneity < .001).
The incidence of major complications was 0.2% with the slower infusion of up to 36.4 mcg, 0.8% with a maximum dose of 100 mcg, and 0.3% with a maximum dose of 200 mcg. The positive vasospasm rate was similar with the latter two protocols, at 46.3% and 41.4%, respectively.
Minor complications occurred in 3.3% of patients but were not detailed. They can include paroxysmal atrial fibrillation, ventricular ectopic beats, transient hypotension, and bradycardia requiring intervention.
As with major complications, minor complications were lower in studies using noncontemporary versus contemporary diagnostic cutoffs for epicardial spasm (1.8% vs. 4.7%) and in Western versus Asian populations (2.6% vs. 9.4%). Minor complications were similar between protocols with maximum doses of 100 mcg and 200 mcg (3.6% vs. 3.8%).
Dr. Kobayashi suggested that several factors may explain the racial differences, including previously reported smooth muscle hyperresponsiveness to provocation stimuli in Japanese patients and the inclusion of a wide range of patients in Japanese studies, such as those with obstructive coronary disease.
Japanese studies also used sequential acetylcholine injection into both the right and left coronaries, a faster injection speed of 20 seconds, and upfront placement of a temporary pacing catheter in case of acetylcholine-induced bradycardia, particularly with right coronary injection.
Although the protocol is largely settled in Japan, he said, provocation protocols need to be standardized because “depending on the country and depending on the institution, people are doing totally different things.”
A big step forward
Commenting on the study, C. Noel Bairey Merz, MD, from Cedars Sinai, Los Angeles, said it has “widespread relevance” because half of all coronary angiograms done invasively in the United States for suspected ischemia find no obstructive coronary disease. Left untreated, however, MINOCA has a 2.5% annual event rate, and a quarter of that is death.
“This is a big step forward with likely equal opportunity to improve women and men’s ischemic heart disease,” she said.
On the other hand, all studies were conducted at centers of excellence, so safety will need to be carefully watched as testing rolls out to more community care, Dr. Merz said. “And it always needs to be underscored that this is done by an interventional cardiologist because they’re familiar with wires that can dissect arteries, and they’re familiar with minor complications that could turn into major, if someone didn’t act appropriately.”
Dr. Merz also called for unifying protocols and the need to raise awareness within the general cardiology community to ask interventionalists for acetylcholine spasm testing. Randomized controlled data from within the WISE study and the CorMica study showed that diagnostic certainty leads to greater therapeutic certainty. “You do a much better job about who and how to treat,” she said.
There are also three ongoing randomized controlled trials – WARRIOR, MINOCA-BAT, and iCorMica – in the INOCA and MINOCA populations testing different treatment strategies for hard clinical outcomes like death and myocardial infarction.
“So in addition to this publication being guideline-forming for diagnosis, we anticipate in the next several years to have clinical trial evidence about therapeutics, again, for formulation of class 1 guidelines,” Dr. Merz said.
John Beltrame, BMBS, PhD, University of Adelaide, Australia, said the meta-analysis shows that intracoronary acetylcholine spasm testing is safe and should prompt greater adoption of invasive functional angiography.
Interventionalists are quite happy to do fractional flow reserve using intravenous adenosine to assess coronary microvascular dysfunction, he said, and “what we think is that functional angiography should test both – both the spasm as well as the microvasculature – and that will give us a clear direction because the treatments are slightly different when you’re treating the large arteries as compared to the microscopic arteries. It’s an important thing.”
Dr. Beltrame and colleagues further detail the benefits of comprehensive invasive functional angiography over structural angiography in a related editorial.
He also noted that the Coronary Vasomotion Disorders International Study Group published international diagnostic criteria for microvascular angina and that several protocols for acetylcholine spasm testing are in the works, including one from Australia. Australian investigators are also organizing an accreditation program for those performing the test.
“The protocol itself is relatively straightforward, but it’s not merely picking up a manual and following the instructions,” Dr. Beltrame said. “Just the same as when you train someone in angioplasty, you don’t just go out and do it. You need to develop some experience in it and so should be proctored.”
Dr. Kobayashi reported consulting agreements with Abbott Vascular. Coauthor disclosures are listed in the paper. Dr. Beltrame and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cause of death in pig heart recipient: New clues
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
Emergency angiography for cardiac arrest without ST elevation?
Patients successfully resuscitated after an out-of-hospital cardiac arrest who did not have ST-segment elevation on their electrocardiogram did not benefit from emergency coronary angiography in a new randomized clinical trial.
In the EMERGE trial, a strategy of emergency coronary angiography was not found to be better than a strategy of delayed coronary angiography with respect to the 180-day survival rate with no or minimal neurologic sequelae.
The authors note that, although the study was underpowered, the results are consistent with previously published studies and do not support routine emergency coronary angiography in survivors of out-of-hospital cardiac arrest without ST elevation.
But senior author, Christian Spaulding, MD, PhD, European Hospital Georges Pompidou, Paris, believes some such patients may still benefit from emergency angiography.
“Most patients who have been resuscitated after out of hospital cardiac arrest will have neurological damage, which will be the primary cause of death,” Dr. Spaulding told this news organization. “It will not make any difference to these patients if they have a coronary lesion treated. So, going forward, I think we need to look for patients who are likely not to have a high degree of neurological damage and who could still benefit from early angiography.”
The EMERGE study was published online in JAMA Cardiology.
In patients who have suffered an out-of-hospital cardiac arrest with no obvious noncardiac cause such as trauma, it is believed that the cardiac arrest is caused by coronary occlusions, and emergency angiography may be able to improve survival in these patients, Dr. Spaulding explained.
In about one-third of such patients, the ECG before hospitalization shows ST elevation, and in this group, there is a high probability (around 70%-80%) that there is going to be a coronary occlusion, so these patients are usually taken directly to emergency angiography.
But, in the other two-thirds of patients, there is no ST elevation on the ECG, and in these patients the chances of finding a coronary occlusion are lower (around 25%-35%).
The EMERGE trial was conducted in this latter group without ST elevation.
For the study, which was conducted in 22 French centers, 279 such patients (mean age, 64 years) were randomized to either emergency or delayed (48-96 hours) coronary angiography. The mean time delay between randomization and coronary angiography was 0.6 hours in the emergency group and 55.1 hours in the delayed group.
The primary outcome was the 180-day survival rate with minimal neurological damage, defined as Cerebral Performance Category of 2 or less. This occurred in 34.1% of the emergency angiography group and 30.7% of the delayed angiography group (hazard ratio, 0.87; 95% confidence interval, 0.65-1.15; P = .32).
There was also no difference in the overall survival rate at 180 days (36.2% vs. 33.3%; HR, 0.86; P = .31) and in secondary outcomes between the two groups.
Dr. Spaulding noted that three other randomized trials in a similar patient population have all shown similar results, with no difference in survival found between patients who have emergency coronary angiography as soon as they are admitted to hospital and those in whom angiography was not performed until a couple of days later.
However, several registry studies in a total of more than 6,000 patients have suggested a benefit of immediate angiography in these patients. “So, there is some disconnect here,” he said.
Dr. Spaulding believes the reason for this disconnect may be that the registry studies may have included patients with less neurological damage so more likely to survive and to benefit from having coronary lesions treated promptly.
“Paramedics sometimes make a judgment on which patients may have minimal neurological damage, and this may affect the choice of hospital a patient is taken to, and then the emergency department doctor may again assess whether a patient should go for immediate angiography or not. So, patients in these registry studies who received emergency angiography were likely already preselected to some extent,” he suggested.
In contrast, the randomized trials have accepted all patients, so there were probably more with neurological damage. “In our trial, almost 70% of patients were in asystole. These are the ones who [are] the most likely to have neurological damage,” he pointed out.
“Because there was such a striking difference in the registry studies, I think there is a group of patients [who] will benefit from immediate emergency coronary angiography, but we have to work out how to select these patients,” he commented.
Dr. Spaulding noted that a recent registry study published in JACC: Cardiovascular Interventions used a score known as MIRACLE2, (which takes into account various factors including age of patient and type of rhythm on ECG) and the degree of cardiogenic shock on arrival at hospital as measured by the SCAI shock score to define a potential cohort of patients at low risk for neurologic injury who benefit most from immediate coronary angiography.
“In my practice at present, I would advise the emergency team that a young patient who had had resuscitation started quickly, had been defibrillated early, and got to hospital quickly should go for an immediate coronary angiogram. It can’t do any harm, and there may be a benefit in such patients,” Dr. Spaulding added.The EMERGE study was supported in part by Assistance Publique–Hôpitaux de Paris and the French Ministry of Health, through the national Programme Hospitalier de Recherche Clinique. Dr. Spaulding reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients successfully resuscitated after an out-of-hospital cardiac arrest who did not have ST-segment elevation on their electrocardiogram did not benefit from emergency coronary angiography in a new randomized clinical trial.
In the EMERGE trial, a strategy of emergency coronary angiography was not found to be better than a strategy of delayed coronary angiography with respect to the 180-day survival rate with no or minimal neurologic sequelae.
The authors note that, although the study was underpowered, the results are consistent with previously published studies and do not support routine emergency coronary angiography in survivors of out-of-hospital cardiac arrest without ST elevation.
But senior author, Christian Spaulding, MD, PhD, European Hospital Georges Pompidou, Paris, believes some such patients may still benefit from emergency angiography.
“Most patients who have been resuscitated after out of hospital cardiac arrest will have neurological damage, which will be the primary cause of death,” Dr. Spaulding told this news organization. “It will not make any difference to these patients if they have a coronary lesion treated. So, going forward, I think we need to look for patients who are likely not to have a high degree of neurological damage and who could still benefit from early angiography.”
The EMERGE study was published online in JAMA Cardiology.
In patients who have suffered an out-of-hospital cardiac arrest with no obvious noncardiac cause such as trauma, it is believed that the cardiac arrest is caused by coronary occlusions, and emergency angiography may be able to improve survival in these patients, Dr. Spaulding explained.
In about one-third of such patients, the ECG before hospitalization shows ST elevation, and in this group, there is a high probability (around 70%-80%) that there is going to be a coronary occlusion, so these patients are usually taken directly to emergency angiography.
But, in the other two-thirds of patients, there is no ST elevation on the ECG, and in these patients the chances of finding a coronary occlusion are lower (around 25%-35%).
The EMERGE trial was conducted in this latter group without ST elevation.
For the study, which was conducted in 22 French centers, 279 such patients (mean age, 64 years) were randomized to either emergency or delayed (48-96 hours) coronary angiography. The mean time delay between randomization and coronary angiography was 0.6 hours in the emergency group and 55.1 hours in the delayed group.
The primary outcome was the 180-day survival rate with minimal neurological damage, defined as Cerebral Performance Category of 2 or less. This occurred in 34.1% of the emergency angiography group and 30.7% of the delayed angiography group (hazard ratio, 0.87; 95% confidence interval, 0.65-1.15; P = .32).
There was also no difference in the overall survival rate at 180 days (36.2% vs. 33.3%; HR, 0.86; P = .31) and in secondary outcomes between the two groups.
Dr. Spaulding noted that three other randomized trials in a similar patient population have all shown similar results, with no difference in survival found between patients who have emergency coronary angiography as soon as they are admitted to hospital and those in whom angiography was not performed until a couple of days later.
However, several registry studies in a total of more than 6,000 patients have suggested a benefit of immediate angiography in these patients. “So, there is some disconnect here,” he said.
Dr. Spaulding believes the reason for this disconnect may be that the registry studies may have included patients with less neurological damage so more likely to survive and to benefit from having coronary lesions treated promptly.
“Paramedics sometimes make a judgment on which patients may have minimal neurological damage, and this may affect the choice of hospital a patient is taken to, and then the emergency department doctor may again assess whether a patient should go for immediate angiography or not. So, patients in these registry studies who received emergency angiography were likely already preselected to some extent,” he suggested.
In contrast, the randomized trials have accepted all patients, so there were probably more with neurological damage. “In our trial, almost 70% of patients were in asystole. These are the ones who [are] the most likely to have neurological damage,” he pointed out.
“Because there was such a striking difference in the registry studies, I think there is a group of patients [who] will benefit from immediate emergency coronary angiography, but we have to work out how to select these patients,” he commented.
Dr. Spaulding noted that a recent registry study published in JACC: Cardiovascular Interventions used a score known as MIRACLE2, (which takes into account various factors including age of patient and type of rhythm on ECG) and the degree of cardiogenic shock on arrival at hospital as measured by the SCAI shock score to define a potential cohort of patients at low risk for neurologic injury who benefit most from immediate coronary angiography.
“In my practice at present, I would advise the emergency team that a young patient who had had resuscitation started quickly, had been defibrillated early, and got to hospital quickly should go for an immediate coronary angiogram. It can’t do any harm, and there may be a benefit in such patients,” Dr. Spaulding added.The EMERGE study was supported in part by Assistance Publique–Hôpitaux de Paris and the French Ministry of Health, through the national Programme Hospitalier de Recherche Clinique. Dr. Spaulding reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients successfully resuscitated after an out-of-hospital cardiac arrest who did not have ST-segment elevation on their electrocardiogram did not benefit from emergency coronary angiography in a new randomized clinical trial.
In the EMERGE trial, a strategy of emergency coronary angiography was not found to be better than a strategy of delayed coronary angiography with respect to the 180-day survival rate with no or minimal neurologic sequelae.
The authors note that, although the study was underpowered, the results are consistent with previously published studies and do not support routine emergency coronary angiography in survivors of out-of-hospital cardiac arrest without ST elevation.
But senior author, Christian Spaulding, MD, PhD, European Hospital Georges Pompidou, Paris, believes some such patients may still benefit from emergency angiography.
“Most patients who have been resuscitated after out of hospital cardiac arrest will have neurological damage, which will be the primary cause of death,” Dr. Spaulding told this news organization. “It will not make any difference to these patients if they have a coronary lesion treated. So, going forward, I think we need to look for patients who are likely not to have a high degree of neurological damage and who could still benefit from early angiography.”
The EMERGE study was published online in JAMA Cardiology.
In patients who have suffered an out-of-hospital cardiac arrest with no obvious noncardiac cause such as trauma, it is believed that the cardiac arrest is caused by coronary occlusions, and emergency angiography may be able to improve survival in these patients, Dr. Spaulding explained.
In about one-third of such patients, the ECG before hospitalization shows ST elevation, and in this group, there is a high probability (around 70%-80%) that there is going to be a coronary occlusion, so these patients are usually taken directly to emergency angiography.
But, in the other two-thirds of patients, there is no ST elevation on the ECG, and in these patients the chances of finding a coronary occlusion are lower (around 25%-35%).
The EMERGE trial was conducted in this latter group without ST elevation.
For the study, which was conducted in 22 French centers, 279 such patients (mean age, 64 years) were randomized to either emergency or delayed (48-96 hours) coronary angiography. The mean time delay between randomization and coronary angiography was 0.6 hours in the emergency group and 55.1 hours in the delayed group.
The primary outcome was the 180-day survival rate with minimal neurological damage, defined as Cerebral Performance Category of 2 or less. This occurred in 34.1% of the emergency angiography group and 30.7% of the delayed angiography group (hazard ratio, 0.87; 95% confidence interval, 0.65-1.15; P = .32).
There was also no difference in the overall survival rate at 180 days (36.2% vs. 33.3%; HR, 0.86; P = .31) and in secondary outcomes between the two groups.
Dr. Spaulding noted that three other randomized trials in a similar patient population have all shown similar results, with no difference in survival found between patients who have emergency coronary angiography as soon as they are admitted to hospital and those in whom angiography was not performed until a couple of days later.
However, several registry studies in a total of more than 6,000 patients have suggested a benefit of immediate angiography in these patients. “So, there is some disconnect here,” he said.
Dr. Spaulding believes the reason for this disconnect may be that the registry studies may have included patients with less neurological damage so more likely to survive and to benefit from having coronary lesions treated promptly.
“Paramedics sometimes make a judgment on which patients may have minimal neurological damage, and this may affect the choice of hospital a patient is taken to, and then the emergency department doctor may again assess whether a patient should go for immediate angiography or not. So, patients in these registry studies who received emergency angiography were likely already preselected to some extent,” he suggested.
In contrast, the randomized trials have accepted all patients, so there were probably more with neurological damage. “In our trial, almost 70% of patients were in asystole. These are the ones who [are] the most likely to have neurological damage,” he pointed out.
“Because there was such a striking difference in the registry studies, I think there is a group of patients [who] will benefit from immediate emergency coronary angiography, but we have to work out how to select these patients,” he commented.
Dr. Spaulding noted that a recent registry study published in JACC: Cardiovascular Interventions used a score known as MIRACLE2, (which takes into account various factors including age of patient and type of rhythm on ECG) and the degree of cardiogenic shock on arrival at hospital as measured by the SCAI shock score to define a potential cohort of patients at low risk for neurologic injury who benefit most from immediate coronary angiography.
“In my practice at present, I would advise the emergency team that a young patient who had had resuscitation started quickly, had been defibrillated early, and got to hospital quickly should go for an immediate coronary angiogram. It can’t do any harm, and there may be a benefit in such patients,” Dr. Spaulding added.The EMERGE study was supported in part by Assistance Publique–Hôpitaux de Paris and the French Ministry of Health, through the national Programme Hospitalier de Recherche Clinique. Dr. Spaulding reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guideline for in-hospital care of diabetes says use CGMs
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022