User login
Long COVID and Blame Hunting
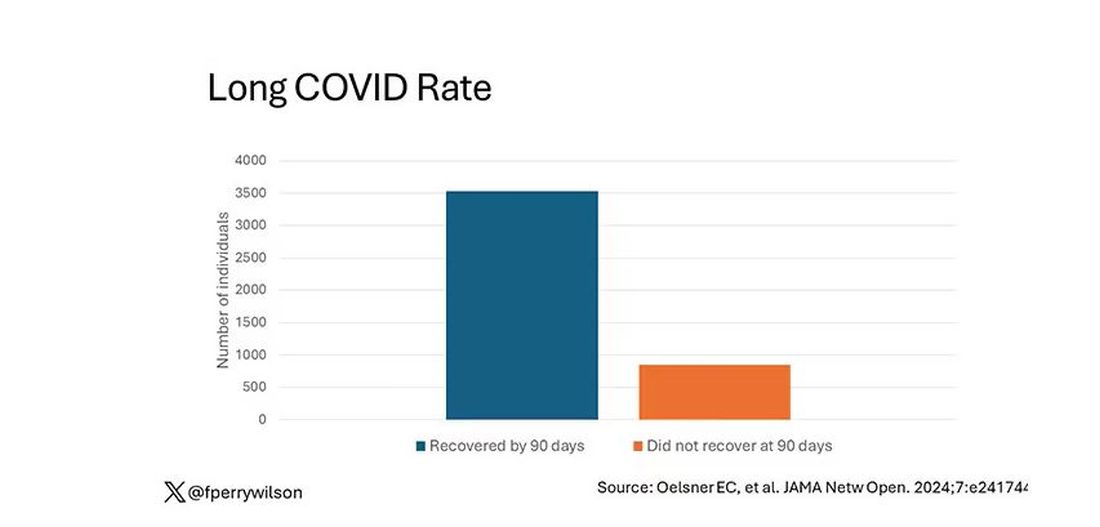
I suspect that many of you have seen or read about a recent study regarding the “long COVID” enigma. The investigators surveyed the records of more than 4000 pediatric patients who had been infected and nearly 1400 who had not. The researchers then developed models in which 14 symptoms were more common in previous SARS-CoV2–infected individuals in all age groups, compared with the uninfected. There were four additional symptoms in children only and three additional symptoms in the adolescents.
Using these data, the investigators created research indices that “correlated with poor overall health and quality of life” and emphasized “neurocognitive, pain, and gastrointestinal symptoms in school-age children” and a “change or loss in smell or taste, pain, and fatigue/malaise-related symptoms in adolescents.”
So now thanks to these investigators we have research indices for characterizing PASC (post-acute sequelae of SARS-CoV-2, aka. long COVID). What should we to do with them? I’m not sure these results move us any further if our goal is finding something to help patients who believe, or have been told, that they have long COVID.
Even to a non-statistician like myself there appear to be some problems with this study. In an editorial accompanying this study, Suchitra Rao, MBBS, MSCS in the Department of Pediatrics, University of Colorado School of Medicine, Aurora, noted the study has the potential for ascertainment bias. For example, the researchers’ subject recruitment procedure resulted in a higher “proportion of neurocognitive/behavioral manifestations” may have skewed the results.
Also, some of the patient evaluations were not done at a consistent interval after the initial infection, which could result in recall bias. And, more importantly, because there were no baseline measurements to determine preinfection status, the investigators had no way of determining to what degree the patients’ underlying conditions may have reflected the quality of life scores.
Although I wouldn’t consider it a bias, I wonder if the investigators have a preconceived vision of what long COVID is going to look like once it is better understood. The fact that they undertook this project suggests that they believe the truth about the phenomenon will be discoverable using data based on collections of vague symptoms.
Or, do the researchers share my vision of long COVID that if it exists it will be something akin to the burst of Parkinson’s disease seen decades later in survivors of the 1918-1920 flu pandemic. Or, maybe it is something like post-polio syndrome, in which survivors in childhood develop atrophy and muscle weakness as they age. Do the researchers believe that COVID survivors are harboring some remnant of SARS-CoV-2 or its genome inside their bodies ticking like a time bomb ready to surface in the future? Think shingles.
I suspect that there are some folks who may or not share my ticking time bomb vision, but who, like me, wonder if there is really such a thing as long COVID – at least one in the form characterized by the work of these investigators. Unfortunately, the $1 billion the National Institutes of Health has invested in the Researching COVID to Enhance Recovery (RECOVER) initiative is not going to discover delayed sequelae until time is ready to tell us. What researchers are looking at now is a collection of patients, some who were not well to begin with but now describe a collection of vague symptoms, some of which are unique to COVID, but most are not. The loss of taste and smell being the one notable and important exception.
It is easy to understand why patients and their physicians would like to have a diagnosis like “long COVID” to at least validate their symptoms that up until now have eluded explanation or remedy. Not surprisingly, they may feel that, if researchers can’t find a cure, let’s at least have something we can lay the blame on.
A major flaw in this current attempt to characterize long COVID is the lack of a true control group. Yes, the subjects the researchers labeled as “uninfected” lived contemporaneously with the patients unfortunate enough to have acquired the virus. However, this illness was mysterious from its first appearance, continued to be more frightening as we struggled to learn more about it, and was clumsily managed in a way that turned our way of life upside down. This was particularly true for school-age children. It unmasked previously unsuspected underlying conditions and quickly acquired a poorly documented reputation for having a “long” variety.
Of course the “uninfected” also lived through these same tumultuous times. But knowing that you harbored, and may still harbor, this mysterious invader moves the infected and their families into a whole new level of concern and anxiety the rest of us who were more fortunate don’t share.
We must not ignore the fact that patients and their caregivers may receive some comfort when they have something to blame for their symptoms. However, we must shift our focus away from blame hunting, which up to this point has been fruitless. Instead, Each patient should be treated as an individual and not part of a group with similar symptoms cobbled together with data acquired under a cloud of bias.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect that many of you have seen or read about a recent study regarding the “long COVID” enigma. The investigators surveyed the records of more than 4000 pediatric patients who had been infected and nearly 1400 who had not. The researchers then developed models in which 14 symptoms were more common in previous SARS-CoV2–infected individuals in all age groups, compared with the uninfected. There were four additional symptoms in children only and three additional symptoms in the adolescents.
Using these data, the investigators created research indices that “correlated with poor overall health and quality of life” and emphasized “neurocognitive, pain, and gastrointestinal symptoms in school-age children” and a “change or loss in smell or taste, pain, and fatigue/malaise-related symptoms in adolescents.”
So now thanks to these investigators we have research indices for characterizing PASC (post-acute sequelae of SARS-CoV-2, aka. long COVID). What should we to do with them? I’m not sure these results move us any further if our goal is finding something to help patients who believe, or have been told, that they have long COVID.
Even to a non-statistician like myself there appear to be some problems with this study. In an editorial accompanying this study, Suchitra Rao, MBBS, MSCS in the Department of Pediatrics, University of Colorado School of Medicine, Aurora, noted the study has the potential for ascertainment bias. For example, the researchers’ subject recruitment procedure resulted in a higher “proportion of neurocognitive/behavioral manifestations” may have skewed the results.
Also, some of the patient evaluations were not done at a consistent interval after the initial infection, which could result in recall bias. And, more importantly, because there were no baseline measurements to determine preinfection status, the investigators had no way of determining to what degree the patients’ underlying conditions may have reflected the quality of life scores.
Although I wouldn’t consider it a bias, I wonder if the investigators have a preconceived vision of what long COVID is going to look like once it is better understood. The fact that they undertook this project suggests that they believe the truth about the phenomenon will be discoverable using data based on collections of vague symptoms.
Or, do the researchers share my vision of long COVID that if it exists it will be something akin to the burst of Parkinson’s disease seen decades later in survivors of the 1918-1920 flu pandemic. Or, maybe it is something like post-polio syndrome, in which survivors in childhood develop atrophy and muscle weakness as they age. Do the researchers believe that COVID survivors are harboring some remnant of SARS-CoV-2 or its genome inside their bodies ticking like a time bomb ready to surface in the future? Think shingles.
I suspect that there are some folks who may or not share my ticking time bomb vision, but who, like me, wonder if there is really such a thing as long COVID – at least one in the form characterized by the work of these investigators. Unfortunately, the $1 billion the National Institutes of Health has invested in the Researching COVID to Enhance Recovery (RECOVER) initiative is not going to discover delayed sequelae until time is ready to tell us. What researchers are looking at now is a collection of patients, some who were not well to begin with but now describe a collection of vague symptoms, some of which are unique to COVID, but most are not. The loss of taste and smell being the one notable and important exception.
It is easy to understand why patients and their physicians would like to have a diagnosis like “long COVID” to at least validate their symptoms that up until now have eluded explanation or remedy. Not surprisingly, they may feel that, if researchers can’t find a cure, let’s at least have something we can lay the blame on.
A major flaw in this current attempt to characterize long COVID is the lack of a true control group. Yes, the subjects the researchers labeled as “uninfected” lived contemporaneously with the patients unfortunate enough to have acquired the virus. However, this illness was mysterious from its first appearance, continued to be more frightening as we struggled to learn more about it, and was clumsily managed in a way that turned our way of life upside down. This was particularly true for school-age children. It unmasked previously unsuspected underlying conditions and quickly acquired a poorly documented reputation for having a “long” variety.
Of course the “uninfected” also lived through these same tumultuous times. But knowing that you harbored, and may still harbor, this mysterious invader moves the infected and their families into a whole new level of concern and anxiety the rest of us who were more fortunate don’t share.
We must not ignore the fact that patients and their caregivers may receive some comfort when they have something to blame for their symptoms. However, we must shift our focus away from blame hunting, which up to this point has been fruitless. Instead, Each patient should be treated as an individual and not part of a group with similar symptoms cobbled together with data acquired under a cloud of bias.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect that many of you have seen or read about a recent study regarding the “long COVID” enigma. The investigators surveyed the records of more than 4000 pediatric patients who had been infected and nearly 1400 who had not. The researchers then developed models in which 14 symptoms were more common in previous SARS-CoV2–infected individuals in all age groups, compared with the uninfected. There were four additional symptoms in children only and three additional symptoms in the adolescents.
Using these data, the investigators created research indices that “correlated with poor overall health and quality of life” and emphasized “neurocognitive, pain, and gastrointestinal symptoms in school-age children” and a “change or loss in smell or taste, pain, and fatigue/malaise-related symptoms in adolescents.”
So now thanks to these investigators we have research indices for characterizing PASC (post-acute sequelae of SARS-CoV-2, aka. long COVID). What should we to do with them? I’m not sure these results move us any further if our goal is finding something to help patients who believe, or have been told, that they have long COVID.
Even to a non-statistician like myself there appear to be some problems with this study. In an editorial accompanying this study, Suchitra Rao, MBBS, MSCS in the Department of Pediatrics, University of Colorado School of Medicine, Aurora, noted the study has the potential for ascertainment bias. For example, the researchers’ subject recruitment procedure resulted in a higher “proportion of neurocognitive/behavioral manifestations” may have skewed the results.
Also, some of the patient evaluations were not done at a consistent interval after the initial infection, which could result in recall bias. And, more importantly, because there were no baseline measurements to determine preinfection status, the investigators had no way of determining to what degree the patients’ underlying conditions may have reflected the quality of life scores.
Although I wouldn’t consider it a bias, I wonder if the investigators have a preconceived vision of what long COVID is going to look like once it is better understood. The fact that they undertook this project suggests that they believe the truth about the phenomenon will be discoverable using data based on collections of vague symptoms.
Or, do the researchers share my vision of long COVID that if it exists it will be something akin to the burst of Parkinson’s disease seen decades later in survivors of the 1918-1920 flu pandemic. Or, maybe it is something like post-polio syndrome, in which survivors in childhood develop atrophy and muscle weakness as they age. Do the researchers believe that COVID survivors are harboring some remnant of SARS-CoV-2 or its genome inside their bodies ticking like a time bomb ready to surface in the future? Think shingles.
I suspect that there are some folks who may or not share my ticking time bomb vision, but who, like me, wonder if there is really such a thing as long COVID – at least one in the form characterized by the work of these investigators. Unfortunately, the $1 billion the National Institutes of Health has invested in the Researching COVID to Enhance Recovery (RECOVER) initiative is not going to discover delayed sequelae until time is ready to tell us. What researchers are looking at now is a collection of patients, some who were not well to begin with but now describe a collection of vague symptoms, some of which are unique to COVID, but most are not. The loss of taste and smell being the one notable and important exception.
It is easy to understand why patients and their physicians would like to have a diagnosis like “long COVID” to at least validate their symptoms that up until now have eluded explanation or remedy. Not surprisingly, they may feel that, if researchers can’t find a cure, let’s at least have something we can lay the blame on.
A major flaw in this current attempt to characterize long COVID is the lack of a true control group. Yes, the subjects the researchers labeled as “uninfected” lived contemporaneously with the patients unfortunate enough to have acquired the virus. However, this illness was mysterious from its first appearance, continued to be more frightening as we struggled to learn more about it, and was clumsily managed in a way that turned our way of life upside down. This was particularly true for school-age children. It unmasked previously unsuspected underlying conditions and quickly acquired a poorly documented reputation for having a “long” variety.
Of course the “uninfected” also lived through these same tumultuous times. But knowing that you harbored, and may still harbor, this mysterious invader moves the infected and their families into a whole new level of concern and anxiety the rest of us who were more fortunate don’t share.
We must not ignore the fact that patients and their caregivers may receive some comfort when they have something to blame for their symptoms. However, we must shift our focus away from blame hunting, which up to this point has been fruitless. Instead, Each patient should be treated as an individual and not part of a group with similar symptoms cobbled together with data acquired under a cloud of bias.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Severe COVID-19 Tied to Increased Risk for Mental Illness
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Paxlovid, Supplements May Improve Long COVID Symptoms
Paxlovid, an antiviral approved in 2023 to treat acute infections of COVID-19, is showing great potential as a new treatment for long COVID and may be the most promising experimental therapy now being studied for treating the condition.
New research offers strong evidence that Paxlovid provides significant benefits for COVID-19 patients who are at high risk for severe or prolonged disease, particularly older adults and those who are immunocompromised, said Lisa Sanders, MD, medical director of Yale’s Long COVID Multidisciplinary Care Center, New Haven, Connecticut.
“We all know that long COVID is a disease smorgasbord of illnesses that have been somehow triggered by COVID. So, the question is, are there some types of these disorders that can respond to Paxlovid?” Dr. Sanders said.
Some patients have also benefited from supplements such as N-acetyl cysteine (NAC), as well as vitamins B, C, D and alpha lipoic acid, in which the risks are low and there are potential benefits, Dr. Sanders said.
A study published in 2023 by JAMA Internal Medicine reviewed the charts of nearly 300,000 veterans with severe acute COVID infections. The study found that Paxlovid treatment reduced the likelihood of developing long COVID. But a more recent study at Stanford University, Palo Alto, California — the STOP-PASC trial— did not find Paxlovid improved symptoms when given to 155 patients who had already recovered from acute infection. Participants with long COVID symptoms — and who had on average recovered from acute infection around 16 months earlier — were given a 15-day course of Paxlovid. Common symptoms like fog, fatigue, and cardiovascular or gastrointestinal symptoms did not improve.
However, long COVID likely has multiple drivers. Viral persistence may still be at play for a subset of patients. This means that, despite the fact that patients recover from acute infection, hidden reservoirs of SARS-CoV-2 are still present in the body, possibly bringing on long COVID symptoms. Which means Paxlovid may help some long COVID patients but not others, Dr. Sanders explained. That’s why research needs to continue to identify the best cases for Paxlovid’s use and to identify other treatments for those who do not benefit from Paxlovid.
The PAX LC trial at Yale suggests there may not be a one-size-fits-all treatment for the condition, but a range of factors that may determine the best therapy for individual patients. Led by Yale School of Medicine’s Harlan Krumholz, MD, and Akiko Iwasaki, PhD, the study tested the effects of Paxlovid overall and was designed to determine who is most likely to benefit from antiviral treatment and gain further understanding of the immune response in long COVID. Results should be reported soon.
“This acknowledges one line of thinking that long COVID is caused by viral persistence,” Dr. Sanders said. “Do these people have hidden reservoirs of the virus? The question is, are there people who seem to respond [to Paxlovid]? And if so, what characterizes these people?”
Low-Risk, High-Reward Supplements
Some of Dr. Sanders’ colleagues at Yale are focusing on long COVID’s neurological symptoms and neuropathogenesis. There’s evidence showing these symptoms — notably brain fog — can be treated with supplements.
In 2022, a Yale study by Arman Fesharaki-Zadeh, MD, PhD, found promise in treating brain fog through a combination supplement of NAC and guanfacine — the latter developed by Yale neuroscientist, Amy Arnsten, PhD.
The two published their study in Neuroimmunology Reports in November 2023. NAC is available over the counter and patients can get a prescription for guanfacine off label from their physician. Guanfacine is approved to treat high blood pressure by decreasing heart rate and relaxing blood vessels. But it’s also been shown to treat attention-deficit/hyperactivity disorder (ADHD) and other cognitive issues.
Though NAC can treat respiratory problems, it’s also commonly used to treat postconcussion symptoms. Dr. Fesharaki-Zadeh found that it helps treat brain fog, increases energy, and improves memory. When paired with guanfacine, substantial benefits were reported, such as better multitasking abilities and markedly improved organizational skills.
Dr. Sanders is now using NAC and guanfacine for patients in her clinic.
‘Mitochondrial Enhancement’ Through Vitamins
Dr. Sanders has also used a combination of alpha lipoic acid and vitamin C, and a combo of B vitamins that make up what’s called a “mitochondrial enhancement regimen.”
To treat a very common symptom like fatigue, Dr. Sanders prefers supplement combinations over other drugs like Modafinil or Adderall.
Modafinil is a central nervous system stimulant used to reduce extreme sleepiness caused by narcolepsy or other sleep disorders. Adderall is an amphetamine also used to treat narcolepsy as well as ADHD. Both work on your sleep and alertness, but long COVID affects the whole body, causing a physical fatigue similar to postexertional malaise (PEM) that isn’t remedied by those kinds of drugs, as studies suggest what’s involved in PEM is mitochondria, Dr. Sanders said.
PEM is a worsening of symptoms that occurs after minimal physical or mental exertion. These are activities that should be well tolerated, but PEM causes extreme fatigue and flu-like symptoms. It’s become a hallmark symptom of long COVID after having already been a key diagnostic factor in myalgic encephalomyelitis/chronic fatigue syndrome.
As Dr. Sanders noted in her long COVID blog, which tracks the latest research and treatment options for doctors who treat long COVID patients, previous studies have shown low vitamin D levels may not only increase the risk for severe COVID-19 but delay recovery from long COVID. Those without long COVID had higher levels of vitamin D, compared with long COVID patients. Vitamin D is known to boost the immune system.
Dr. Sanders found that those with vitamin D deficiencies are most likely to benefit from this approach. For people who don’t have sufficient sun exposure, which prompts the production of vitamin D, she says supplementation with 1000 IUs of vitamin D3 daily is enough for most adults.
Research is also currently being underway on the use of the diabetes drug metformin in people with acute COVID infections to determine if it may reduce the likelihood of developing long COVID. In a recent long COVID clinical trial, early outpatient COVID-19 treatment with metformin decreased the subsequent risk for long COVID by 41.3% during 10-month follow-up.
Other New Treatments Under Study
Dr. Sanders believes the foundation for many of long COVID’s symptoms could be neurological.
“I think that long COVID is probably a neurologic disorder,” Dr. Sanders said.
Lindsey McAlpine, MD, director of the Yale Medicine NeuroCovid Clinic, is focusing on neuropsychiatric long COVID and the causes of neurologic post-acute sequelae of SARS-CoV-2 infection (neuro-PASC). Symptoms of neuro-PASC include cognitive impairment, headaches, and dizziness.
“Lindsey is trying to see which parts of the brain are involved and see if there are phenotypes of brain abnormalities that match up with clinical abnormalities,” Dr. Sanders said.
The National Institute of Neurological Disorders and Stroke recently awarded her a 5-year K23 grant to support her ongoing study, “Magnetic Resonance Imaging Biomarkers of Post-COVID-19 Cerebral Microvascular Dysfunction.”
Utilizing advanced MRI techniques to identify microvascular dysfunction biomarkers in the brain, Dr. McAlpine hopes to unearth and better understand the pathophysiology behind neurological issues post COVID.
Many of Dr. McAlpine’s patients with cognitive symptoms have responded well to NAC and guanfacine.
Still, the hope is that her brain-imaging studies will bear fruit that leads to a better understanding of long COVID and new treatment methods.
A version of this article first appeared on Medscape.com.
Paxlovid, an antiviral approved in 2023 to treat acute infections of COVID-19, is showing great potential as a new treatment for long COVID and may be the most promising experimental therapy now being studied for treating the condition.
New research offers strong evidence that Paxlovid provides significant benefits for COVID-19 patients who are at high risk for severe or prolonged disease, particularly older adults and those who are immunocompromised, said Lisa Sanders, MD, medical director of Yale’s Long COVID Multidisciplinary Care Center, New Haven, Connecticut.
“We all know that long COVID is a disease smorgasbord of illnesses that have been somehow triggered by COVID. So, the question is, are there some types of these disorders that can respond to Paxlovid?” Dr. Sanders said.
Some patients have also benefited from supplements such as N-acetyl cysteine (NAC), as well as vitamins B, C, D and alpha lipoic acid, in which the risks are low and there are potential benefits, Dr. Sanders said.
A study published in 2023 by JAMA Internal Medicine reviewed the charts of nearly 300,000 veterans with severe acute COVID infections. The study found that Paxlovid treatment reduced the likelihood of developing long COVID. But a more recent study at Stanford University, Palo Alto, California — the STOP-PASC trial— did not find Paxlovid improved symptoms when given to 155 patients who had already recovered from acute infection. Participants with long COVID symptoms — and who had on average recovered from acute infection around 16 months earlier — were given a 15-day course of Paxlovid. Common symptoms like fog, fatigue, and cardiovascular or gastrointestinal symptoms did not improve.
However, long COVID likely has multiple drivers. Viral persistence may still be at play for a subset of patients. This means that, despite the fact that patients recover from acute infection, hidden reservoirs of SARS-CoV-2 are still present in the body, possibly bringing on long COVID symptoms. Which means Paxlovid may help some long COVID patients but not others, Dr. Sanders explained. That’s why research needs to continue to identify the best cases for Paxlovid’s use and to identify other treatments for those who do not benefit from Paxlovid.
The PAX LC trial at Yale suggests there may not be a one-size-fits-all treatment for the condition, but a range of factors that may determine the best therapy for individual patients. Led by Yale School of Medicine’s Harlan Krumholz, MD, and Akiko Iwasaki, PhD, the study tested the effects of Paxlovid overall and was designed to determine who is most likely to benefit from antiviral treatment and gain further understanding of the immune response in long COVID. Results should be reported soon.
“This acknowledges one line of thinking that long COVID is caused by viral persistence,” Dr. Sanders said. “Do these people have hidden reservoirs of the virus? The question is, are there people who seem to respond [to Paxlovid]? And if so, what characterizes these people?”
Low-Risk, High-Reward Supplements
Some of Dr. Sanders’ colleagues at Yale are focusing on long COVID’s neurological symptoms and neuropathogenesis. There’s evidence showing these symptoms — notably brain fog — can be treated with supplements.
In 2022, a Yale study by Arman Fesharaki-Zadeh, MD, PhD, found promise in treating brain fog through a combination supplement of NAC and guanfacine — the latter developed by Yale neuroscientist, Amy Arnsten, PhD.
The two published their study in Neuroimmunology Reports in November 2023. NAC is available over the counter and patients can get a prescription for guanfacine off label from their physician. Guanfacine is approved to treat high blood pressure by decreasing heart rate and relaxing blood vessels. But it’s also been shown to treat attention-deficit/hyperactivity disorder (ADHD) and other cognitive issues.
Though NAC can treat respiratory problems, it’s also commonly used to treat postconcussion symptoms. Dr. Fesharaki-Zadeh found that it helps treat brain fog, increases energy, and improves memory. When paired with guanfacine, substantial benefits were reported, such as better multitasking abilities and markedly improved organizational skills.
Dr. Sanders is now using NAC and guanfacine for patients in her clinic.
‘Mitochondrial Enhancement’ Through Vitamins
Dr. Sanders has also used a combination of alpha lipoic acid and vitamin C, and a combo of B vitamins that make up what’s called a “mitochondrial enhancement regimen.”
To treat a very common symptom like fatigue, Dr. Sanders prefers supplement combinations over other drugs like Modafinil or Adderall.
Modafinil is a central nervous system stimulant used to reduce extreme sleepiness caused by narcolepsy or other sleep disorders. Adderall is an amphetamine also used to treat narcolepsy as well as ADHD. Both work on your sleep and alertness, but long COVID affects the whole body, causing a physical fatigue similar to postexertional malaise (PEM) that isn’t remedied by those kinds of drugs, as studies suggest what’s involved in PEM is mitochondria, Dr. Sanders said.
PEM is a worsening of symptoms that occurs after minimal physical or mental exertion. These are activities that should be well tolerated, but PEM causes extreme fatigue and flu-like symptoms. It’s become a hallmark symptom of long COVID after having already been a key diagnostic factor in myalgic encephalomyelitis/chronic fatigue syndrome.
As Dr. Sanders noted in her long COVID blog, which tracks the latest research and treatment options for doctors who treat long COVID patients, previous studies have shown low vitamin D levels may not only increase the risk for severe COVID-19 but delay recovery from long COVID. Those without long COVID had higher levels of vitamin D, compared with long COVID patients. Vitamin D is known to boost the immune system.
Dr. Sanders found that those with vitamin D deficiencies are most likely to benefit from this approach. For people who don’t have sufficient sun exposure, which prompts the production of vitamin D, she says supplementation with 1000 IUs of vitamin D3 daily is enough for most adults.
Research is also currently being underway on the use of the diabetes drug metformin in people with acute COVID infections to determine if it may reduce the likelihood of developing long COVID. In a recent long COVID clinical trial, early outpatient COVID-19 treatment with metformin decreased the subsequent risk for long COVID by 41.3% during 10-month follow-up.
Other New Treatments Under Study
Dr. Sanders believes the foundation for many of long COVID’s symptoms could be neurological.
“I think that long COVID is probably a neurologic disorder,” Dr. Sanders said.
Lindsey McAlpine, MD, director of the Yale Medicine NeuroCovid Clinic, is focusing on neuropsychiatric long COVID and the causes of neurologic post-acute sequelae of SARS-CoV-2 infection (neuro-PASC). Symptoms of neuro-PASC include cognitive impairment, headaches, and dizziness.
“Lindsey is trying to see which parts of the brain are involved and see if there are phenotypes of brain abnormalities that match up with clinical abnormalities,” Dr. Sanders said.
The National Institute of Neurological Disorders and Stroke recently awarded her a 5-year K23 grant to support her ongoing study, “Magnetic Resonance Imaging Biomarkers of Post-COVID-19 Cerebral Microvascular Dysfunction.”
Utilizing advanced MRI techniques to identify microvascular dysfunction biomarkers in the brain, Dr. McAlpine hopes to unearth and better understand the pathophysiology behind neurological issues post COVID.
Many of Dr. McAlpine’s patients with cognitive symptoms have responded well to NAC and guanfacine.
Still, the hope is that her brain-imaging studies will bear fruit that leads to a better understanding of long COVID and new treatment methods.
A version of this article first appeared on Medscape.com.
Paxlovid, an antiviral approved in 2023 to treat acute infections of COVID-19, is showing great potential as a new treatment for long COVID and may be the most promising experimental therapy now being studied for treating the condition.
New research offers strong evidence that Paxlovid provides significant benefits for COVID-19 patients who are at high risk for severe or prolonged disease, particularly older adults and those who are immunocompromised, said Lisa Sanders, MD, medical director of Yale’s Long COVID Multidisciplinary Care Center, New Haven, Connecticut.
“We all know that long COVID is a disease smorgasbord of illnesses that have been somehow triggered by COVID. So, the question is, are there some types of these disorders that can respond to Paxlovid?” Dr. Sanders said.
Some patients have also benefited from supplements such as N-acetyl cysteine (NAC), as well as vitamins B, C, D and alpha lipoic acid, in which the risks are low and there are potential benefits, Dr. Sanders said.
A study published in 2023 by JAMA Internal Medicine reviewed the charts of nearly 300,000 veterans with severe acute COVID infections. The study found that Paxlovid treatment reduced the likelihood of developing long COVID. But a more recent study at Stanford University, Palo Alto, California — the STOP-PASC trial— did not find Paxlovid improved symptoms when given to 155 patients who had already recovered from acute infection. Participants with long COVID symptoms — and who had on average recovered from acute infection around 16 months earlier — were given a 15-day course of Paxlovid. Common symptoms like fog, fatigue, and cardiovascular or gastrointestinal symptoms did not improve.
However, long COVID likely has multiple drivers. Viral persistence may still be at play for a subset of patients. This means that, despite the fact that patients recover from acute infection, hidden reservoirs of SARS-CoV-2 are still present in the body, possibly bringing on long COVID symptoms. Which means Paxlovid may help some long COVID patients but not others, Dr. Sanders explained. That’s why research needs to continue to identify the best cases for Paxlovid’s use and to identify other treatments for those who do not benefit from Paxlovid.
The PAX LC trial at Yale suggests there may not be a one-size-fits-all treatment for the condition, but a range of factors that may determine the best therapy for individual patients. Led by Yale School of Medicine’s Harlan Krumholz, MD, and Akiko Iwasaki, PhD, the study tested the effects of Paxlovid overall and was designed to determine who is most likely to benefit from antiviral treatment and gain further understanding of the immune response in long COVID. Results should be reported soon.
“This acknowledges one line of thinking that long COVID is caused by viral persistence,” Dr. Sanders said. “Do these people have hidden reservoirs of the virus? The question is, are there people who seem to respond [to Paxlovid]? And if so, what characterizes these people?”
Low-Risk, High-Reward Supplements
Some of Dr. Sanders’ colleagues at Yale are focusing on long COVID’s neurological symptoms and neuropathogenesis. There’s evidence showing these symptoms — notably brain fog — can be treated with supplements.
In 2022, a Yale study by Arman Fesharaki-Zadeh, MD, PhD, found promise in treating brain fog through a combination supplement of NAC and guanfacine — the latter developed by Yale neuroscientist, Amy Arnsten, PhD.
The two published their study in Neuroimmunology Reports in November 2023. NAC is available over the counter and patients can get a prescription for guanfacine off label from their physician. Guanfacine is approved to treat high blood pressure by decreasing heart rate and relaxing blood vessels. But it’s also been shown to treat attention-deficit/hyperactivity disorder (ADHD) and other cognitive issues.
Though NAC can treat respiratory problems, it’s also commonly used to treat postconcussion symptoms. Dr. Fesharaki-Zadeh found that it helps treat brain fog, increases energy, and improves memory. When paired with guanfacine, substantial benefits were reported, such as better multitasking abilities and markedly improved organizational skills.
Dr. Sanders is now using NAC and guanfacine for patients in her clinic.
‘Mitochondrial Enhancement’ Through Vitamins
Dr. Sanders has also used a combination of alpha lipoic acid and vitamin C, and a combo of B vitamins that make up what’s called a “mitochondrial enhancement regimen.”
To treat a very common symptom like fatigue, Dr. Sanders prefers supplement combinations over other drugs like Modafinil or Adderall.
Modafinil is a central nervous system stimulant used to reduce extreme sleepiness caused by narcolepsy or other sleep disorders. Adderall is an amphetamine also used to treat narcolepsy as well as ADHD. Both work on your sleep and alertness, but long COVID affects the whole body, causing a physical fatigue similar to postexertional malaise (PEM) that isn’t remedied by those kinds of drugs, as studies suggest what’s involved in PEM is mitochondria, Dr. Sanders said.
PEM is a worsening of symptoms that occurs after minimal physical or mental exertion. These are activities that should be well tolerated, but PEM causes extreme fatigue and flu-like symptoms. It’s become a hallmark symptom of long COVID after having already been a key diagnostic factor in myalgic encephalomyelitis/chronic fatigue syndrome.
As Dr. Sanders noted in her long COVID blog, which tracks the latest research and treatment options for doctors who treat long COVID patients, previous studies have shown low vitamin D levels may not only increase the risk for severe COVID-19 but delay recovery from long COVID. Those without long COVID had higher levels of vitamin D, compared with long COVID patients. Vitamin D is known to boost the immune system.
Dr. Sanders found that those with vitamin D deficiencies are most likely to benefit from this approach. For people who don’t have sufficient sun exposure, which prompts the production of vitamin D, she says supplementation with 1000 IUs of vitamin D3 daily is enough for most adults.
Research is also currently being underway on the use of the diabetes drug metformin in people with acute COVID infections to determine if it may reduce the likelihood of developing long COVID. In a recent long COVID clinical trial, early outpatient COVID-19 treatment with metformin decreased the subsequent risk for long COVID by 41.3% during 10-month follow-up.
Other New Treatments Under Study
Dr. Sanders believes the foundation for many of long COVID’s symptoms could be neurological.
“I think that long COVID is probably a neurologic disorder,” Dr. Sanders said.
Lindsey McAlpine, MD, director of the Yale Medicine NeuroCovid Clinic, is focusing on neuropsychiatric long COVID and the causes of neurologic post-acute sequelae of SARS-CoV-2 infection (neuro-PASC). Symptoms of neuro-PASC include cognitive impairment, headaches, and dizziness.
“Lindsey is trying to see which parts of the brain are involved and see if there are phenotypes of brain abnormalities that match up with clinical abnormalities,” Dr. Sanders said.
The National Institute of Neurological Disorders and Stroke recently awarded her a 5-year K23 grant to support her ongoing study, “Magnetic Resonance Imaging Biomarkers of Post-COVID-19 Cerebral Microvascular Dysfunction.”
Utilizing advanced MRI techniques to identify microvascular dysfunction biomarkers in the brain, Dr. McAlpine hopes to unearth and better understand the pathophysiology behind neurological issues post COVID.
Many of Dr. McAlpine’s patients with cognitive symptoms have responded well to NAC and guanfacine.
Still, the hope is that her brain-imaging studies will bear fruit that leads to a better understanding of long COVID and new treatment methods.
A version of this article first appeared on Medscape.com.
Disruptive Sleep Linked to Increased Susceptibility to COVID-19
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Scientist Aims to Unravel Long COVID’s Neurologic Impacts
Neurologic symptoms of long COVID are vast, common, hard to treat, disabling, and can mimic dozens of other syndromes, with some symptoms as serious as those seen in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and postural orthostatic tachycardia syndrome (POTS).
Now, recent evidence has suggested long COVID is primarily an autonomic nervous system disorder.
Their lives may never be the same.
Lindsay S. McAlpine, MD, a specialist in the neurologic sequelae of COVID-19 at the Yale School of Medicine and director of the Yale NeuroCOVID Clinic, New Haven, Connecticut, treats patients who struggle with neurologic symptoms even after disease recovery.
“Some people have the brain fog and the shortness of breath; some have the palpitations and the headaches ... it’s kind of a mix and match,” she said.
Dr. McAlpine’s research has been slowly building up into what could bring about a significant breakthrough in treating some of the most misunderstood and difficult-to-treat symptoms of long COVID.
The Effect of Vascular Inflammation on Long COVID
The National Institute of Neurological Disorders and Stroke recently awarded her a 5-year K23 grant to support her ongoing study, “Magnetic Resonance Imaging Biomarkers of Post-COVID-19 Cerebral Microvascular Dysfunction.”
Using advanced MRI techniques to identify microvascular dysfunction biomarkers in the brain, McAlpine hopes to unearth and better understand the pathophysiology behind neurologic issues post-COVID.
Dr. McAlpine said, “What we’re seeing is that there’s a unique signature of vascular inflammation in long COVID that is distinct from acute COVID. And it has to do with endothelial apathy and platelet dysfunction.”
She’s also looking into whether microvascular dysfunction could increase one’s risk for small vessel disease. Her research is quantitatively building an overall pathophysiology piece by piece.
“We’re quantifying cognitive dysfunction and using objective testing ... a very rigorous 3-hour protocol to really identify the patterns of weakness until we find deficits in memory working and declarative memory, deficits in executive functioning, and others. Those are the three pieces that I’m trying to piece together: The MRI, the blood work, and the cognitive testing,” she said.
Ultimately, Dr. McAlpine believes long COVID will eventually be classified as a peripheral autonomic disorder. The damage being wrought to the whole body also damages the brain’s vasculature, and Dr. McAlpine’s MRI techniques probe at this connection.
“Some of my MRI techniques are dependent on the very subtle changes in blood flow to different regions in response to demand. Brain fog has been a key symptom of POTS and ME/CFS. And it’s now a key symptom of long COVID ... what I’m looking at in some of my studies is how and in which parts of the brain are affected by this,” she said.
Dr. McAlpine’s interest in COVID’s effect on our nervous system goes back all the way to the first wave of patients with COVID, where she noticed an unusually high incidence of ischemic stroke.
“We recognized that COVID really has a huge impact on the vessels ... there’s quite a bit of vascular inflammation. In terms of neurology, we were seeing quite a bit of ischemic stroke, which is unusual,” she said.
Patients don’t normally present with stroke while infected with a virus. Seeking answers, she conducted a stroke study in patients with acute COVID and found profound endotheliopathy — damage to key cells in the lining of blood vessels — leading to a cascade of dysfunction and clotting.
A Constellation of Neuropsychiatric Symptoms
In early June, Dr. McAlpine gave a presentation of her research at the Demystifying Long COVID North American Conference 2024 in Boston. She’s been hard at work in extrapolating the causes of neuropsychiatric long COVID, a tangled web of symptoms seen in patients with long COVID that range from cognitive dysfunction to headaches, neuropathy, mental health, and the aforementioned dysautonomia.
Amid the sea of neurologic long COVID symptoms, she said “symptoms that are mixing and matching are very similar. So, I wanted to specifically look at a symptom that I could definitely isolate to the brain, and that is brain fog and cognitive dysfunction and impairment.”
In September 2021, the journal Translational Psychiatry published a study titled “Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions.”
Going back all the way to the first cases of COVID in March 2020, the initial symptoms most patients complained of during an acute viral infection were around the respiratory system. Yet delirium, confusion, and neurocognitive disorders were also reported, puzzling experts and inciting a well-founded fear among many.
Even worse, after recovery, these neuropsychiatric symptoms persisted. The study found that coronavirus was able to invade the central nervous system through blood vessels and neuronal retrograde pathways, leading to brain injury and dysfunction of the cardiorespiratory center in the brainstem.
The study concluded by reporting that neuroimaging and neurochemical evidence indicated neuroimmune dysfunction and brain injury in severe patients with COVID-19. Suggested treatments included immunosuppressive therapies, vaccines to target the coronavirus’ spike protein, and pharmacological agents to improve endothelial integrity.
But there was still much that was unknown, and the study’s authors stressed the need for multidisciplinary research going forward.
How Immune Dysfunction Plays a Role
Similarly, Dr. McAlpine and her research team are still trying to sift their way through this opaque web to see why long COVID can cause autoimmune flare-ups.
In a study published in April, Dr. McAlpine and others found that small fiber neuropathy (SFN) after COVID is autoimmune-mediated and a dysfunction of the immune system.
Notably, they found that SFN could be a key pathologic finding in long COVID. SFN before the pandemic had been linked to ME/CFS and POTS, and the basic hypothesis revolved around an inflammatory immune response during a viral illness that may lead to immune dysregulation (dysimmunity) and damage to small fiber nerves.
But much still remains to be answered.
“We’ve seen quite a bit of that, but we still haven’t figured it out,” Dr. McAlpine said. “My big question is, how is this autonomic dysfunction related to the immune dysfunction, and how is that related to the vascular inflammation? There’s quite a bit of overlap in individuals with autoimmune disease and those who go on to develop this long COVID,” she added.
Still, a large portion of patients with long COVID don’t show autoimmune dysfunction, and those patients lack common biomarkers for an autoimmune condition.
“When we look at the spinal fluid in those individuals [with multiple sclerosis or a neuroinfectious disease], there’s inflammation going on ... the white blood cell count is elevated, the protein is elevated, the antibodies, the bands are elevated. I’ve been seeing long COVID patients now for 4 years, and their presentation is so distinctly different compared to my individuals that I see my patients with MS, or a neuroinfectious disease,” she said.
The mechanisms behind how all of this is interlaced remain unclear, and there may not be a one-size-fits-all treatment or definite pathogenesis for everyone.
“It’s that intersection of the immune system and the vessel wall ... Next is to figure out what do we treat, what are the targets, all of that, but there’s so many different presentations, and everybody has kind of a unique case,” she said.
How Physician Can Treat Common Symptoms Now
Though a cure for symptoms still eludes the scientific community, recent evidence has suggested that a combination of N-acetyl cysteine (NAC) and guanfacine has been successful in easing neurologic symptoms.
In November 2023, Arman Fesharaki-Zadeh, MD, PhD, a Yale Medicine behavioral neurologist and neuropsychiatrist, published a small study in Neuroimmunology Reports with his colleague, Yale neuroscientist Amy Arnsten, PhD. The two researchers showed how among 12 patients given 600 mg NAC daily, along with 1 mg guanfacine (increased to 2 mg after a month if well-tolerated), eight demonstrated improved cognitive abilities.
In patients who stayed on guanfacine + NAC, improved working memory, concentration, and executive functions were seen.
Also, they resumed their normal work schedule. Interruption and inability to work has been a significant factor in the lower quality-of-life long COVID patients experience.
Placebo-controlled trials will be needed going forward, but their small study has established safety and could open up a larger study in the future. For the moment, NAC can be gotten over the counter, and patients could get a prescription off-label from their doctor.
Dr. McAlpine has seen this combination work well for her own patients at Yale’s NeuroCOVID clinic.
Additionally, lifestyle practices such as quitting tobacco, increased exercise, exercising the mind, lowering alcohol intake, and even vitamin D supplementation (1000-2000 IU daily) could prove beneficial in tamping down persistent brain fog.
Vitamin D supports brain and nerve function through its reduction of brain aging biomarkers, regulating genes important for brain function, activating and deactivating enzymes important for neurotransmitter synthesis, and supporting neuronal growth and survival.
A version of this article first appeared on Medscape.com.
Neurologic symptoms of long COVID are vast, common, hard to treat, disabling, and can mimic dozens of other syndromes, with some symptoms as serious as those seen in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and postural orthostatic tachycardia syndrome (POTS).
Now, recent evidence has suggested long COVID is primarily an autonomic nervous system disorder.
Their lives may never be the same.
Lindsay S. McAlpine, MD, a specialist in the neurologic sequelae of COVID-19 at the Yale School of Medicine and director of the Yale NeuroCOVID Clinic, New Haven, Connecticut, treats patients who struggle with neurologic symptoms even after disease recovery.
“Some people have the brain fog and the shortness of breath; some have the palpitations and the headaches ... it’s kind of a mix and match,” she said.
Dr. McAlpine’s research has been slowly building up into what could bring about a significant breakthrough in treating some of the most misunderstood and difficult-to-treat symptoms of long COVID.
The Effect of Vascular Inflammation on Long COVID
The National Institute of Neurological Disorders and Stroke recently awarded her a 5-year K23 grant to support her ongoing study, “Magnetic Resonance Imaging Biomarkers of Post-COVID-19 Cerebral Microvascular Dysfunction.”
Using advanced MRI techniques to identify microvascular dysfunction biomarkers in the brain, McAlpine hopes to unearth and better understand the pathophysiology behind neurologic issues post-COVID.
Dr. McAlpine said, “What we’re seeing is that there’s a unique signature of vascular inflammation in long COVID that is distinct from acute COVID. And it has to do with endothelial apathy and platelet dysfunction.”
She’s also looking into whether microvascular dysfunction could increase one’s risk for small vessel disease. Her research is quantitatively building an overall pathophysiology piece by piece.
“We’re quantifying cognitive dysfunction and using objective testing ... a very rigorous 3-hour protocol to really identify the patterns of weakness until we find deficits in memory working and declarative memory, deficits in executive functioning, and others. Those are the three pieces that I’m trying to piece together: The MRI, the blood work, and the cognitive testing,” she said.
Ultimately, Dr. McAlpine believes long COVID will eventually be classified as a peripheral autonomic disorder. The damage being wrought to the whole body also damages the brain’s vasculature, and Dr. McAlpine’s MRI techniques probe at this connection.
“Some of my MRI techniques are dependent on the very subtle changes in blood flow to different regions in response to demand. Brain fog has been a key symptom of POTS and ME/CFS. And it’s now a key symptom of long COVID ... what I’m looking at in some of my studies is how and in which parts of the brain are affected by this,” she said.
Dr. McAlpine’s interest in COVID’s effect on our nervous system goes back all the way to the first wave of patients with COVID, where she noticed an unusually high incidence of ischemic stroke.
“We recognized that COVID really has a huge impact on the vessels ... there’s quite a bit of vascular inflammation. In terms of neurology, we were seeing quite a bit of ischemic stroke, which is unusual,” she said.
Patients don’t normally present with stroke while infected with a virus. Seeking answers, she conducted a stroke study in patients with acute COVID and found profound endotheliopathy — damage to key cells in the lining of blood vessels — leading to a cascade of dysfunction and clotting.
A Constellation of Neuropsychiatric Symptoms
In early June, Dr. McAlpine gave a presentation of her research at the Demystifying Long COVID North American Conference 2024 in Boston. She’s been hard at work in extrapolating the causes of neuropsychiatric long COVID, a tangled web of symptoms seen in patients with long COVID that range from cognitive dysfunction to headaches, neuropathy, mental health, and the aforementioned dysautonomia.
Amid the sea of neurologic long COVID symptoms, she said “symptoms that are mixing and matching are very similar. So, I wanted to specifically look at a symptom that I could definitely isolate to the brain, and that is brain fog and cognitive dysfunction and impairment.”
In September 2021, the journal Translational Psychiatry published a study titled “Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions.”
Going back all the way to the first cases of COVID in March 2020, the initial symptoms most patients complained of during an acute viral infection were around the respiratory system. Yet delirium, confusion, and neurocognitive disorders were also reported, puzzling experts and inciting a well-founded fear among many.
Even worse, after recovery, these neuropsychiatric symptoms persisted. The study found that coronavirus was able to invade the central nervous system through blood vessels and neuronal retrograde pathways, leading to brain injury and dysfunction of the cardiorespiratory center in the brainstem.
The study concluded by reporting that neuroimaging and neurochemical evidence indicated neuroimmune dysfunction and brain injury in severe patients with COVID-19. Suggested treatments included immunosuppressive therapies, vaccines to target the coronavirus’ spike protein, and pharmacological agents to improve endothelial integrity.
But there was still much that was unknown, and the study’s authors stressed the need for multidisciplinary research going forward.
How Immune Dysfunction Plays a Role
Similarly, Dr. McAlpine and her research team are still trying to sift their way through this opaque web to see why long COVID can cause autoimmune flare-ups.
In a study published in April, Dr. McAlpine and others found that small fiber neuropathy (SFN) after COVID is autoimmune-mediated and a dysfunction of the immune system.
Notably, they found that SFN could be a key pathologic finding in long COVID. SFN before the pandemic had been linked to ME/CFS and POTS, and the basic hypothesis revolved around an inflammatory immune response during a viral illness that may lead to immune dysregulation (dysimmunity) and damage to small fiber nerves.
But much still remains to be answered.
“We’ve seen quite a bit of that, but we still haven’t figured it out,” Dr. McAlpine said. “My big question is, how is this autonomic dysfunction related to the immune dysfunction, and how is that related to the vascular inflammation? There’s quite a bit of overlap in individuals with autoimmune disease and those who go on to develop this long COVID,” she added.
Still, a large portion of patients with long COVID don’t show autoimmune dysfunction, and those patients lack common biomarkers for an autoimmune condition.
“When we look at the spinal fluid in those individuals [with multiple sclerosis or a neuroinfectious disease], there’s inflammation going on ... the white blood cell count is elevated, the protein is elevated, the antibodies, the bands are elevated. I’ve been seeing long COVID patients now for 4 years, and their presentation is so distinctly different compared to my individuals that I see my patients with MS, or a neuroinfectious disease,” she said.
The mechanisms behind how all of this is interlaced remain unclear, and there may not be a one-size-fits-all treatment or definite pathogenesis for everyone.
“It’s that intersection of the immune system and the vessel wall ... Next is to figure out what do we treat, what are the targets, all of that, but there’s so many different presentations, and everybody has kind of a unique case,” she said.
How Physician Can Treat Common Symptoms Now
Though a cure for symptoms still eludes the scientific community, recent evidence has suggested that a combination of N-acetyl cysteine (NAC) and guanfacine has been successful in easing neurologic symptoms.
In November 2023, Arman Fesharaki-Zadeh, MD, PhD, a Yale Medicine behavioral neurologist and neuropsychiatrist, published a small study in Neuroimmunology Reports with his colleague, Yale neuroscientist Amy Arnsten, PhD. The two researchers showed how among 12 patients given 600 mg NAC daily, along with 1 mg guanfacine (increased to 2 mg after a month if well-tolerated), eight demonstrated improved cognitive abilities.
In patients who stayed on guanfacine + NAC, improved working memory, concentration, and executive functions were seen.
Also, they resumed their normal work schedule. Interruption and inability to work has been a significant factor in the lower quality-of-life long COVID patients experience.
Placebo-controlled trials will be needed going forward, but their small study has established safety and could open up a larger study in the future. For the moment, NAC can be gotten over the counter, and patients could get a prescription off-label from their doctor.
Dr. McAlpine has seen this combination work well for her own patients at Yale’s NeuroCOVID clinic.
Additionally, lifestyle practices such as quitting tobacco, increased exercise, exercising the mind, lowering alcohol intake, and even vitamin D supplementation (1000-2000 IU daily) could prove beneficial in tamping down persistent brain fog.
Vitamin D supports brain and nerve function through its reduction of brain aging biomarkers, regulating genes important for brain function, activating and deactivating enzymes important for neurotransmitter synthesis, and supporting neuronal growth and survival.
A version of this article first appeared on Medscape.com.
Neurologic symptoms of long COVID are vast, common, hard to treat, disabling, and can mimic dozens of other syndromes, with some symptoms as serious as those seen in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and postural orthostatic tachycardia syndrome (POTS).
Now, recent evidence has suggested long COVID is primarily an autonomic nervous system disorder.
Their lives may never be the same.
Lindsay S. McAlpine, MD, a specialist in the neurologic sequelae of COVID-19 at the Yale School of Medicine and director of the Yale NeuroCOVID Clinic, New Haven, Connecticut, treats patients who struggle with neurologic symptoms even after disease recovery.
“Some people have the brain fog and the shortness of breath; some have the palpitations and the headaches ... it’s kind of a mix and match,” she said.
Dr. McAlpine’s research has been slowly building up into what could bring about a significant breakthrough in treating some of the most misunderstood and difficult-to-treat symptoms of long COVID.
The Effect of Vascular Inflammation on Long COVID
The National Institute of Neurological Disorders and Stroke recently awarded her a 5-year K23 grant to support her ongoing study, “Magnetic Resonance Imaging Biomarkers of Post-COVID-19 Cerebral Microvascular Dysfunction.”
Using advanced MRI techniques to identify microvascular dysfunction biomarkers in the brain, McAlpine hopes to unearth and better understand the pathophysiology behind neurologic issues post-COVID.
Dr. McAlpine said, “What we’re seeing is that there’s a unique signature of vascular inflammation in long COVID that is distinct from acute COVID. And it has to do with endothelial apathy and platelet dysfunction.”
She’s also looking into whether microvascular dysfunction could increase one’s risk for small vessel disease. Her research is quantitatively building an overall pathophysiology piece by piece.
“We’re quantifying cognitive dysfunction and using objective testing ... a very rigorous 3-hour protocol to really identify the patterns of weakness until we find deficits in memory working and declarative memory, deficits in executive functioning, and others. Those are the three pieces that I’m trying to piece together: The MRI, the blood work, and the cognitive testing,” she said.
Ultimately, Dr. McAlpine believes long COVID will eventually be classified as a peripheral autonomic disorder. The damage being wrought to the whole body also damages the brain’s vasculature, and Dr. McAlpine’s MRI techniques probe at this connection.
“Some of my MRI techniques are dependent on the very subtle changes in blood flow to different regions in response to demand. Brain fog has been a key symptom of POTS and ME/CFS. And it’s now a key symptom of long COVID ... what I’m looking at in some of my studies is how and in which parts of the brain are affected by this,” she said.
Dr. McAlpine’s interest in COVID’s effect on our nervous system goes back all the way to the first wave of patients with COVID, where she noticed an unusually high incidence of ischemic stroke.
“We recognized that COVID really has a huge impact on the vessels ... there’s quite a bit of vascular inflammation. In terms of neurology, we were seeing quite a bit of ischemic stroke, which is unusual,” she said.
Patients don’t normally present with stroke while infected with a virus. Seeking answers, she conducted a stroke study in patients with acute COVID and found profound endotheliopathy — damage to key cells in the lining of blood vessels — leading to a cascade of dysfunction and clotting.
A Constellation of Neuropsychiatric Symptoms
In early June, Dr. McAlpine gave a presentation of her research at the Demystifying Long COVID North American Conference 2024 in Boston. She’s been hard at work in extrapolating the causes of neuropsychiatric long COVID, a tangled web of symptoms seen in patients with long COVID that range from cognitive dysfunction to headaches, neuropathy, mental health, and the aforementioned dysautonomia.
Amid the sea of neurologic long COVID symptoms, she said “symptoms that are mixing and matching are very similar. So, I wanted to specifically look at a symptom that I could definitely isolate to the brain, and that is brain fog and cognitive dysfunction and impairment.”
In September 2021, the journal Translational Psychiatry published a study titled “Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions.”
Going back all the way to the first cases of COVID in March 2020, the initial symptoms most patients complained of during an acute viral infection were around the respiratory system. Yet delirium, confusion, and neurocognitive disorders were also reported, puzzling experts and inciting a well-founded fear among many.
Even worse, after recovery, these neuropsychiatric symptoms persisted. The study found that coronavirus was able to invade the central nervous system through blood vessels and neuronal retrograde pathways, leading to brain injury and dysfunction of the cardiorespiratory center in the brainstem.
The study concluded by reporting that neuroimaging and neurochemical evidence indicated neuroimmune dysfunction and brain injury in severe patients with COVID-19. Suggested treatments included immunosuppressive therapies, vaccines to target the coronavirus’ spike protein, and pharmacological agents to improve endothelial integrity.
But there was still much that was unknown, and the study’s authors stressed the need for multidisciplinary research going forward.
How Immune Dysfunction Plays a Role
Similarly, Dr. McAlpine and her research team are still trying to sift their way through this opaque web to see why long COVID can cause autoimmune flare-ups.
In a study published in April, Dr. McAlpine and others found that small fiber neuropathy (SFN) after COVID is autoimmune-mediated and a dysfunction of the immune system.
Notably, they found that SFN could be a key pathologic finding in long COVID. SFN before the pandemic had been linked to ME/CFS and POTS, and the basic hypothesis revolved around an inflammatory immune response during a viral illness that may lead to immune dysregulation (dysimmunity) and damage to small fiber nerves.
But much still remains to be answered.
“We’ve seen quite a bit of that, but we still haven’t figured it out,” Dr. McAlpine said. “My big question is, how is this autonomic dysfunction related to the immune dysfunction, and how is that related to the vascular inflammation? There’s quite a bit of overlap in individuals with autoimmune disease and those who go on to develop this long COVID,” she added.
Still, a large portion of patients with long COVID don’t show autoimmune dysfunction, and those patients lack common biomarkers for an autoimmune condition.
“When we look at the spinal fluid in those individuals [with multiple sclerosis or a neuroinfectious disease], there’s inflammation going on ... the white blood cell count is elevated, the protein is elevated, the antibodies, the bands are elevated. I’ve been seeing long COVID patients now for 4 years, and their presentation is so distinctly different compared to my individuals that I see my patients with MS, or a neuroinfectious disease,” she said.
The mechanisms behind how all of this is interlaced remain unclear, and there may not be a one-size-fits-all treatment or definite pathogenesis for everyone.
“It’s that intersection of the immune system and the vessel wall ... Next is to figure out what do we treat, what are the targets, all of that, but there’s so many different presentations, and everybody has kind of a unique case,” she said.
How Physician Can Treat Common Symptoms Now
Though a cure for symptoms still eludes the scientific community, recent evidence has suggested that a combination of N-acetyl cysteine (NAC) and guanfacine has been successful in easing neurologic symptoms.
In November 2023, Arman Fesharaki-Zadeh, MD, PhD, a Yale Medicine behavioral neurologist and neuropsychiatrist, published a small study in Neuroimmunology Reports with his colleague, Yale neuroscientist Amy Arnsten, PhD. The two researchers showed how among 12 patients given 600 mg NAC daily, along with 1 mg guanfacine (increased to 2 mg after a month if well-tolerated), eight demonstrated improved cognitive abilities.
In patients who stayed on guanfacine + NAC, improved working memory, concentration, and executive functions were seen.
Also, they resumed their normal work schedule. Interruption and inability to work has been a significant factor in the lower quality-of-life long COVID patients experience.
Placebo-controlled trials will be needed going forward, but their small study has established safety and could open up a larger study in the future. For the moment, NAC can be gotten over the counter, and patients could get a prescription off-label from their doctor.
Dr. McAlpine has seen this combination work well for her own patients at Yale’s NeuroCOVID clinic.
Additionally, lifestyle practices such as quitting tobacco, increased exercise, exercising the mind, lowering alcohol intake, and even vitamin D supplementation (1000-2000 IU daily) could prove beneficial in tamping down persistent brain fog.
Vitamin D supports brain and nerve function through its reduction of brain aging biomarkers, regulating genes important for brain function, activating and deactivating enzymes important for neurotransmitter synthesis, and supporting neuronal growth and survival.
A version of this article first appeared on Medscape.com.
Long COVID & Chronic Fatigue: The Similarities are Uncanny
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
Almost 10% of Infected Pregnant People Develop Long COVID
Almost 1 in 10 pregnant people infected with COVID-19 end up developing long COVID, according to a study published in Obstetrics & Gynecology.
Researchers at University of Utah Health looked at the medical records of more than 1500 people who got COVID-19 while pregnant and checked their self-reported symptoms at least 6 months after infection, according to a news release from the school.
The scientists discovered that 9.3% of those people reported long COVID symptoms, such as fatigue and issues in their gut.
To make sure those long COVID symptoms were not actually symptoms of pregnancy, the research team did a second analysis of people who reported symptoms more than 12 weeks after giving birth. The risk of long COVID was about the same as in the first analysis.
“It was surprising to me that the prevalence was that high,” Torri D. Metz, MD, vice chair for research of obstetrics and gynecology at the school and co-leader of the study, said in the release. “This is something that does continue to affect otherwise reasonably healthy and young populations.”
The school said this is the first study to look at long COVID risks in pregnant people. Previous research found other dangers for pregnant people who get COVID, such as a higher chance of hospitalization or death, or complications such as preterm birth.
In the general population, research shows that 10%-20% of people who get COVID develop long COVID.
Dr. Metz said healthcare providers need to remain alert about long COVID, including in pregnant people.
“We need to have this on our radar as we’re seeing patients. It’s something we really don’t want to miss. And we want to get people referred to appropriate specialists who treat long COVID,” she said.
A version of this article first appeared on WebMD.com.
Almost 1 in 10 pregnant people infected with COVID-19 end up developing long COVID, according to a study published in Obstetrics & Gynecology.
Researchers at University of Utah Health looked at the medical records of more than 1500 people who got COVID-19 while pregnant and checked their self-reported symptoms at least 6 months after infection, according to a news release from the school.
The scientists discovered that 9.3% of those people reported long COVID symptoms, such as fatigue and issues in their gut.
To make sure those long COVID symptoms were not actually symptoms of pregnancy, the research team did a second analysis of people who reported symptoms more than 12 weeks after giving birth. The risk of long COVID was about the same as in the first analysis.
“It was surprising to me that the prevalence was that high,” Torri D. Metz, MD, vice chair for research of obstetrics and gynecology at the school and co-leader of the study, said in the release. “This is something that does continue to affect otherwise reasonably healthy and young populations.”
The school said this is the first study to look at long COVID risks in pregnant people. Previous research found other dangers for pregnant people who get COVID, such as a higher chance of hospitalization or death, or complications such as preterm birth.
In the general population, research shows that 10%-20% of people who get COVID develop long COVID.
Dr. Metz said healthcare providers need to remain alert about long COVID, including in pregnant people.
“We need to have this on our radar as we’re seeing patients. It’s something we really don’t want to miss. And we want to get people referred to appropriate specialists who treat long COVID,” she said.
A version of this article first appeared on WebMD.com.
Almost 1 in 10 pregnant people infected with COVID-19 end up developing long COVID, according to a study published in Obstetrics & Gynecology.
Researchers at University of Utah Health looked at the medical records of more than 1500 people who got COVID-19 while pregnant and checked their self-reported symptoms at least 6 months after infection, according to a news release from the school.
The scientists discovered that 9.3% of those people reported long COVID symptoms, such as fatigue and issues in their gut.
To make sure those long COVID symptoms were not actually symptoms of pregnancy, the research team did a second analysis of people who reported symptoms more than 12 weeks after giving birth. The risk of long COVID was about the same as in the first analysis.
“It was surprising to me that the prevalence was that high,” Torri D. Metz, MD, vice chair for research of obstetrics and gynecology at the school and co-leader of the study, said in the release. “This is something that does continue to affect otherwise reasonably healthy and young populations.”
The school said this is the first study to look at long COVID risks in pregnant people. Previous research found other dangers for pregnant people who get COVID, such as a higher chance of hospitalization or death, or complications such as preterm birth.
In the general population, research shows that 10%-20% of people who get COVID develop long COVID.
Dr. Metz said healthcare providers need to remain alert about long COVID, including in pregnant people.
“We need to have this on our radar as we’re seeing patients. It’s something we really don’t want to miss. And we want to get people referred to appropriate specialists who treat long COVID,” she said.
A version of this article first appeared on WebMD.com.
FROM OBSTETRICS & GYNECOLOGY
Cold or Flu Virus May Trigger Relapse of Long COVID
researchers have found.
In some cases, they may be experiencing what researchers call viral interference, something also experienced by people with HIV and other infections associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Clinical studies on the issue are limited, but patients, doctors, and researchers report many people who previously had long COVID have developed recurring symptoms after consequent viral infections.
Viral persistence — where bits of virus linger in the body — and viral reactivation remain two of the leading suspects for Yale researchers. Viral activation occurs when the immune system responds to an infection by triggering a dormant virus.
Anecdotally, these flare-ups occur more commonly in patients with long COVID with autonomic dysfunction — severe dizziness when standing up — and other symptoms of ME/CFS, said Alba Azola, MD, a Johns Hopkins Medicine rehabilitation specialist in Baltimore, Maryland, who works with patients with long COVID and other “fatiguing illnesses.”
At last count, about 18% of those surveyed by the Centers for Disease Control and Prevention said they had experienced long COVID. Nearly 60% of those surveyed said they had contracted COVID-19 at least once.
Dr. Azola said that very afternoon she had seen a patient with the flu and a recurrence of previous long COVID symptoms. Not much data exist about cases like this.
“I can’t say there is a specific study looking at this, but anecdotally, we see it all the time,” Dr. Azola said.
She has not seen completely different symptoms; more commonly, she sees a flare-up of previously existing symptoms.
David Putrino, PhD, is director of rehabilitation innovation for the Mount Sinai Health System in New York City. He treats and studies patients with long COVID and echoes what others have seen.
Patients can “recover (or feel recovered) from long COVID until the next immune challenge — another COVID infection, flu infection, pregnancy, food poisoning (all examples we have seen in the clinic) — and experience a significant flare-up of your initial COVID infection,” he said.
“Relapse” is a better term than reinfection, said Jeffrey Parsonnet, MD, an infectious diseases specialist and director of the Dartmouth Hitchcock Post-Acute COVID Syndrome Clinic, Lebanon, New Hampshire.
“We see patients who had COVID-19 followed by long COVID who then get better — either completely or mostly better. Then they’ve gotten COVID again, and this is followed by recurrence of long COVID symptoms,” he said.
“Every patient looks different in terms of what gets better and how quickly. And again, some patients are not better (or even minimally so) after a couple of years,” he said.
Patients Tell Their Stories
On the COVID-19 Long Haulers Support Facebook group, many of the 100,000 followers ask about viral reactivation. Delainne “Laney” Bond, RN, who has battled postinfection chronic illness herself, runs the Facebook group. From what she sees, “each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing long COVID or experiencing worse long COVID. Multiple infections can lead to progressive health complications.”
The posts on her site include many queries about reinfections. A post from December included nearly 80 comments with people describing the full range of symptoms. Some stories relayed how the reinfection symptoms were short lived. Some report returning to their baseline — not completely symptom free but improved.
Doctors and patients say long COVID comes and goes — relapsing-remitting — and shares many features with other complex multisystem chronic conditions, according to a new National Academy of Sciences report. Those include ME/CFS and the Epstein-Barr virus.
As far as how to treat, Dr. Putrino is one of the clinical researchers testing antivirals. One is Paxlovid; the others are drugs developed for the AIDS virus.
“A plausible mechanism for long COVID is persistence of the SARS-CoV-2 virus in tissue and/or the reactivation of latent pathogens,” according to an explanation of the research on the PolyBio Institute website, which is involved with the research.
In the meantime, “long COVID appears to be a chronic condition with few patients achieving full remission,” according to a new Academy of Sciences report. The report concludes that long COVID recovery can plateau at 6-12 months. They also note that 18%-22% of people who have long COVID symptoms at 5 months are still ill at 1 year.
A version of this article first appeared on Medscape.com.
researchers have found.
In some cases, they may be experiencing what researchers call viral interference, something also experienced by people with HIV and other infections associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Clinical studies on the issue are limited, but patients, doctors, and researchers report many people who previously had long COVID have developed recurring symptoms after consequent viral infections.
Viral persistence — where bits of virus linger in the body — and viral reactivation remain two of the leading suspects for Yale researchers. Viral activation occurs when the immune system responds to an infection by triggering a dormant virus.
Anecdotally, these flare-ups occur more commonly in patients with long COVID with autonomic dysfunction — severe dizziness when standing up — and other symptoms of ME/CFS, said Alba Azola, MD, a Johns Hopkins Medicine rehabilitation specialist in Baltimore, Maryland, who works with patients with long COVID and other “fatiguing illnesses.”
At last count, about 18% of those surveyed by the Centers for Disease Control and Prevention said they had experienced long COVID. Nearly 60% of those surveyed said they had contracted COVID-19 at least once.
Dr. Azola said that very afternoon she had seen a patient with the flu and a recurrence of previous long COVID symptoms. Not much data exist about cases like this.
“I can’t say there is a specific study looking at this, but anecdotally, we see it all the time,” Dr. Azola said.
She has not seen completely different symptoms; more commonly, she sees a flare-up of previously existing symptoms.
David Putrino, PhD, is director of rehabilitation innovation for the Mount Sinai Health System in New York City. He treats and studies patients with long COVID and echoes what others have seen.
Patients can “recover (or feel recovered) from long COVID until the next immune challenge — another COVID infection, flu infection, pregnancy, food poisoning (all examples we have seen in the clinic) — and experience a significant flare-up of your initial COVID infection,” he said.
“Relapse” is a better term than reinfection, said Jeffrey Parsonnet, MD, an infectious diseases specialist and director of the Dartmouth Hitchcock Post-Acute COVID Syndrome Clinic, Lebanon, New Hampshire.
“We see patients who had COVID-19 followed by long COVID who then get better — either completely or mostly better. Then they’ve gotten COVID again, and this is followed by recurrence of long COVID symptoms,” he said.
“Every patient looks different in terms of what gets better and how quickly. And again, some patients are not better (or even minimally so) after a couple of years,” he said.
Patients Tell Their Stories
On the COVID-19 Long Haulers Support Facebook group, many of the 100,000 followers ask about viral reactivation. Delainne “Laney” Bond, RN, who has battled postinfection chronic illness herself, runs the Facebook group. From what she sees, “each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing long COVID or experiencing worse long COVID. Multiple infections can lead to progressive health complications.”
The posts on her site include many queries about reinfections. A post from December included nearly 80 comments with people describing the full range of symptoms. Some stories relayed how the reinfection symptoms were short lived. Some report returning to their baseline — not completely symptom free but improved.
Doctors and patients say long COVID comes and goes — relapsing-remitting — and shares many features with other complex multisystem chronic conditions, according to a new National Academy of Sciences report. Those include ME/CFS and the Epstein-Barr virus.
As far as how to treat, Dr. Putrino is one of the clinical researchers testing antivirals. One is Paxlovid; the others are drugs developed for the AIDS virus.
“A plausible mechanism for long COVID is persistence of the SARS-CoV-2 virus in tissue and/or the reactivation of latent pathogens,” according to an explanation of the research on the PolyBio Institute website, which is involved with the research.
In the meantime, “long COVID appears to be a chronic condition with few patients achieving full remission,” according to a new Academy of Sciences report. The report concludes that long COVID recovery can plateau at 6-12 months. They also note that 18%-22% of people who have long COVID symptoms at 5 months are still ill at 1 year.
A version of this article first appeared on Medscape.com.
researchers have found.
In some cases, they may be experiencing what researchers call viral interference, something also experienced by people with HIV and other infections associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Clinical studies on the issue are limited, but patients, doctors, and researchers report many people who previously had long COVID have developed recurring symptoms after consequent viral infections.
Viral persistence — where bits of virus linger in the body — and viral reactivation remain two of the leading suspects for Yale researchers. Viral activation occurs when the immune system responds to an infection by triggering a dormant virus.
Anecdotally, these flare-ups occur more commonly in patients with long COVID with autonomic dysfunction — severe dizziness when standing up — and other symptoms of ME/CFS, said Alba Azola, MD, a Johns Hopkins Medicine rehabilitation specialist in Baltimore, Maryland, who works with patients with long COVID and other “fatiguing illnesses.”
At last count, about 18% of those surveyed by the Centers for Disease Control and Prevention said they had experienced long COVID. Nearly 60% of those surveyed said they had contracted COVID-19 at least once.
Dr. Azola said that very afternoon she had seen a patient with the flu and a recurrence of previous long COVID symptoms. Not much data exist about cases like this.
“I can’t say there is a specific study looking at this, but anecdotally, we see it all the time,” Dr. Azola said.
She has not seen completely different symptoms; more commonly, she sees a flare-up of previously existing symptoms.
David Putrino, PhD, is director of rehabilitation innovation for the Mount Sinai Health System in New York City. He treats and studies patients with long COVID and echoes what others have seen.
Patients can “recover (or feel recovered) from long COVID until the next immune challenge — another COVID infection, flu infection, pregnancy, food poisoning (all examples we have seen in the clinic) — and experience a significant flare-up of your initial COVID infection,” he said.
“Relapse” is a better term than reinfection, said Jeffrey Parsonnet, MD, an infectious diseases specialist and director of the Dartmouth Hitchcock Post-Acute COVID Syndrome Clinic, Lebanon, New Hampshire.
“We see patients who had COVID-19 followed by long COVID who then get better — either completely or mostly better. Then they’ve gotten COVID again, and this is followed by recurrence of long COVID symptoms,” he said.
“Every patient looks different in terms of what gets better and how quickly. And again, some patients are not better (or even minimally so) after a couple of years,” he said.
Patients Tell Their Stories
On the COVID-19 Long Haulers Support Facebook group, many of the 100,000 followers ask about viral reactivation. Delainne “Laney” Bond, RN, who has battled postinfection chronic illness herself, runs the Facebook group. From what she sees, “each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing long COVID or experiencing worse long COVID. Multiple infections can lead to progressive health complications.”
The posts on her site include many queries about reinfections. A post from December included nearly 80 comments with people describing the full range of symptoms. Some stories relayed how the reinfection symptoms were short lived. Some report returning to their baseline — not completely symptom free but improved.
Doctors and patients say long COVID comes and goes — relapsing-remitting — and shares many features with other complex multisystem chronic conditions, according to a new National Academy of Sciences report. Those include ME/CFS and the Epstein-Barr virus.
As far as how to treat, Dr. Putrino is one of the clinical researchers testing antivirals. One is Paxlovid; the others are drugs developed for the AIDS virus.
“A plausible mechanism for long COVID is persistence of the SARS-CoV-2 virus in tissue and/or the reactivation of latent pathogens,” according to an explanation of the research on the PolyBio Institute website, which is involved with the research.
In the meantime, “long COVID appears to be a chronic condition with few patients achieving full remission,” according to a new Academy of Sciences report. The report concludes that long COVID recovery can plateau at 6-12 months. They also note that 18%-22% of people who have long COVID symptoms at 5 months are still ill at 1 year.
A version of this article first appeared on Medscape.com.
Long COVID Can’t Be Solved Until We Decide What It Is
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.
Recovery times were longer for smokers, those with diabetes, and those who were obese.
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here.
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.
Recovery times were longer for smokers, those with diabetes, and those who were obese.
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here.
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.
Recovery times were longer for smokers, those with diabetes, and those who were obese.
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here.
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
National Academies Issue New Broad Definition of Long COVID
A new broadly inclusive definition of long COVID from the National Academies of Sciences, Engineering, and Medicine (NASEM) has been developed with the aim of improving consistency, documentation, and treatment for both adults and children.
According to the 2024 NASEM definition of long COVID issued on June 11, 2024, “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
People with long COVID may present with one or more of a long list of symptoms, such as shortness of breath, rapid heartbeat, extreme fatigue, post-exertional malaise, or sleep disturbance and with single or multiple diagnosable conditions, including interstitial lung disease, arrhythmias, postural orthostatic tachycardia syndrome (POTS), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), diabetes, or autoimmune disorders. The condition can exacerbate preexisting health conditions or present as new ones.
The definition does not require laboratory confirmation or other proof of initial infection. Long COVID can follow SARS-CoV-2 infection of any severity, including asymptomatic infections, whether or not they were initially recognized.
Several working definitions and terms for long COVID had previously been proposed, including those from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention, but no common definition or terminology had been established.
The new definition was developed at the request of the Administration for Strategic Preparedness and Response and the Office of the Assistant Secretary for Health (OASH). It was written by a multi-stakeholder panel convened by NASEM, which recommended that the new definition be universally adopted by the federal government, clinical societies and associations, public health practitioners, clinicians, payers, the drug industry, and others using the term long COVID.
Recent surveys suggest that approximately 7% of Americans have experienced or are experiencing long COVID. “It’s millions of people,” panel chair Harvey V. Fineberg, MD, president of the Gordon and Betty Moore Foundation, told this news organization.
The new definition “does not erase the problem of clinical judgment ... But we think this definition has the real advantage of elevating to the clinician’s mind the real likelihood in the current environment of prevalence of this virus that a presenting patient’s strange symptoms are both real and maybe related as an expression of long COVID,” Dr. Fineberg noted.
One way this new definition differs from previous ones such as WHO’s, he said, is “they talk about a diagnosis of exclusion. One of the important points in our definition is that other diagnosable conditions like ME/CFS or POTS can be part of the picture of long COVID. They are not alternative. They are, in fact, an expression of long COVID.”
Indeed, the NASEM report also introduces the term infection-associated chronic condition (IACC). This was important, Dr. Fineberg said, “because it’s the larger family of conditions of which long COVID is a part. It emphasizes a relatedness of long COVID to other conditions that can follow from a variety of infections. We also adopted the term ‘disease state’ to convey the seriousness and reality of this condition in the lives of patients.”
Comments on New Definition
In a statement provided to this news organization, Lucinda Bateman, MD, and Brayden Yellman, MD, co-medical directors of the Bateman-Horne Center in Salt Lake City, said that “describing long COVID as an IACC ... not only meets the NASEM goal of allowing clinicians, researchers, and public health officials to meaningfully identify and serve all persons who suffer illness or disability in the wake of a SARS-CoV-2 infection, but also draws direct comparison to other known IACC’s (such as ME/CFS, post-treatment Lyme, POTS) that have been plaguing many for decades.”
Dr. Fineberg noted another important aspect of the NASEM report: “Our definition includes an explicit statement on equity, explaining that long COVID can affect anyone, young and old, different races, different ages, different sexes, different genders, different orientations, different socioeconomic conditions ... This does not mean that every single person is at equal risk. There are risk factors, but the important point is the universal nature of this as a condition.”
Two clinical directors of long COVID programs who were contacted by this news organization praised the new definition. Zijian Chen, MD, director of Mount Sinai’s Center for Post-COVID Care, New York, said that it’s “very similar to the definition that we have used for our clinical practice since 2020. It is very important that the broad definition helps to be inclusive of all patients that may be affected. The inclusion of children as a consideration is important as well, since there is routinely less focus on children because they tend to have less disease frequency ... The creation of a unified definition helps both with clinical practice and research.”
Nisha Viswanathan, MD, director of the long COVID program at the University of California, Los Angeles, said: “I think they left it intentionally broad for the medical practitioner to not necessarily use the definition to rule out individuals, but to perhaps use more of a clinical gestalt to help rule in this diagnosis ... I think this definition is providing clarity to health care providers on what exactly would be falling under the long-COVID diagnosis header.”
Dr. Viswanathan also said that she anticipates this definition to help patients make their case in filing disability claims. “Because long COVID has not previously had a good fleshed-out definition, it was very easy for disability providers to reject claims for patients who continue to have symptoms ... I actually think this might help our patients ultimately in their attempt to be able to have the ability to care for themselves when they’re disabled enough to not be able to work.”
Written into the report is the expectation that the definition “will evolve as new evidence emerges and the understanding of long COVID matures.” The writing committee calls for reexamination in “no more than 3 years.” Factors that would prompt a reevaluation could include improved testing methods, discovery of medical factors and/or biomarkers that distinguish long COVID from other conditions, and new treatments.
Meanwhile, Dr. Fineberg told this news organization, “If this definition adds to the readiness, awareness, openness, and response to the patient with long COVID, it will have done its job.”
Dr. Fineberg, Dr. Bateman, Dr. Yellman, Dr. Viswanathan, and Dr. Chen have no relevant disclosures.
A version of this article appeared on Medscape.com.
A new broadly inclusive definition of long COVID from the National Academies of Sciences, Engineering, and Medicine (NASEM) has been developed with the aim of improving consistency, documentation, and treatment for both adults and children.
According to the 2024 NASEM definition of long COVID issued on June 11, 2024, “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
People with long COVID may present with one or more of a long list of symptoms, such as shortness of breath, rapid heartbeat, extreme fatigue, post-exertional malaise, or sleep disturbance and with single or multiple diagnosable conditions, including interstitial lung disease, arrhythmias, postural orthostatic tachycardia syndrome (POTS), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), diabetes, or autoimmune disorders. The condition can exacerbate preexisting health conditions or present as new ones.
The definition does not require laboratory confirmation or other proof of initial infection. Long COVID can follow SARS-CoV-2 infection of any severity, including asymptomatic infections, whether or not they were initially recognized.
Several working definitions and terms for long COVID had previously been proposed, including those from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention, but no common definition or terminology had been established.
The new definition was developed at the request of the Administration for Strategic Preparedness and Response and the Office of the Assistant Secretary for Health (OASH). It was written by a multi-stakeholder panel convened by NASEM, which recommended that the new definition be universally adopted by the federal government, clinical societies and associations, public health practitioners, clinicians, payers, the drug industry, and others using the term long COVID.
Recent surveys suggest that approximately 7% of Americans have experienced or are experiencing long COVID. “It’s millions of people,” panel chair Harvey V. Fineberg, MD, president of the Gordon and Betty Moore Foundation, told this news organization.
The new definition “does not erase the problem of clinical judgment ... But we think this definition has the real advantage of elevating to the clinician’s mind the real likelihood in the current environment of prevalence of this virus that a presenting patient’s strange symptoms are both real and maybe related as an expression of long COVID,” Dr. Fineberg noted.
One way this new definition differs from previous ones such as WHO’s, he said, is “they talk about a diagnosis of exclusion. One of the important points in our definition is that other diagnosable conditions like ME/CFS or POTS can be part of the picture of long COVID. They are not alternative. They are, in fact, an expression of long COVID.”
Indeed, the NASEM report also introduces the term infection-associated chronic condition (IACC). This was important, Dr. Fineberg said, “because it’s the larger family of conditions of which long COVID is a part. It emphasizes a relatedness of long COVID to other conditions that can follow from a variety of infections. We also adopted the term ‘disease state’ to convey the seriousness and reality of this condition in the lives of patients.”
Comments on New Definition
In a statement provided to this news organization, Lucinda Bateman, MD, and Brayden Yellman, MD, co-medical directors of the Bateman-Horne Center in Salt Lake City, said that “describing long COVID as an IACC ... not only meets the NASEM goal of allowing clinicians, researchers, and public health officials to meaningfully identify and serve all persons who suffer illness or disability in the wake of a SARS-CoV-2 infection, but also draws direct comparison to other known IACC’s (such as ME/CFS, post-treatment Lyme, POTS) that have been plaguing many for decades.”
Dr. Fineberg noted another important aspect of the NASEM report: “Our definition includes an explicit statement on equity, explaining that long COVID can affect anyone, young and old, different races, different ages, different sexes, different genders, different orientations, different socioeconomic conditions ... This does not mean that every single person is at equal risk. There are risk factors, but the important point is the universal nature of this as a condition.”
Two clinical directors of long COVID programs who were contacted by this news organization praised the new definition. Zijian Chen, MD, director of Mount Sinai’s Center for Post-COVID Care, New York, said that it’s “very similar to the definition that we have used for our clinical practice since 2020. It is very important that the broad definition helps to be inclusive of all patients that may be affected. The inclusion of children as a consideration is important as well, since there is routinely less focus on children because they tend to have less disease frequency ... The creation of a unified definition helps both with clinical practice and research.”
Nisha Viswanathan, MD, director of the long COVID program at the University of California, Los Angeles, said: “I think they left it intentionally broad for the medical practitioner to not necessarily use the definition to rule out individuals, but to perhaps use more of a clinical gestalt to help rule in this diagnosis ... I think this definition is providing clarity to health care providers on what exactly would be falling under the long-COVID diagnosis header.”
Dr. Viswanathan also said that she anticipates this definition to help patients make their case in filing disability claims. “Because long COVID has not previously had a good fleshed-out definition, it was very easy for disability providers to reject claims for patients who continue to have symptoms ... I actually think this might help our patients ultimately in their attempt to be able to have the ability to care for themselves when they’re disabled enough to not be able to work.”
Written into the report is the expectation that the definition “will evolve as new evidence emerges and the understanding of long COVID matures.” The writing committee calls for reexamination in “no more than 3 years.” Factors that would prompt a reevaluation could include improved testing methods, discovery of medical factors and/or biomarkers that distinguish long COVID from other conditions, and new treatments.
Meanwhile, Dr. Fineberg told this news organization, “If this definition adds to the readiness, awareness, openness, and response to the patient with long COVID, it will have done its job.”
Dr. Fineberg, Dr. Bateman, Dr. Yellman, Dr. Viswanathan, and Dr. Chen have no relevant disclosures.
A version of this article appeared on Medscape.com.
A new broadly inclusive definition of long COVID from the National Academies of Sciences, Engineering, and Medicine (NASEM) has been developed with the aim of improving consistency, documentation, and treatment for both adults and children.
According to the 2024 NASEM definition of long COVID issued on June 11, 2024, “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
People with long COVID may present with one or more of a long list of symptoms, such as shortness of breath, rapid heartbeat, extreme fatigue, post-exertional malaise, or sleep disturbance and with single or multiple diagnosable conditions, including interstitial lung disease, arrhythmias, postural orthostatic tachycardia syndrome (POTS), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), diabetes, or autoimmune disorders. The condition can exacerbate preexisting health conditions or present as new ones.
The definition does not require laboratory confirmation or other proof of initial infection. Long COVID can follow SARS-CoV-2 infection of any severity, including asymptomatic infections, whether or not they were initially recognized.
Several working definitions and terms for long COVID had previously been proposed, including those from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention, but no common definition or terminology had been established.
The new definition was developed at the request of the Administration for Strategic Preparedness and Response and the Office of the Assistant Secretary for Health (OASH). It was written by a multi-stakeholder panel convened by NASEM, which recommended that the new definition be universally adopted by the federal government, clinical societies and associations, public health practitioners, clinicians, payers, the drug industry, and others using the term long COVID.
Recent surveys suggest that approximately 7% of Americans have experienced or are experiencing long COVID. “It’s millions of people,” panel chair Harvey V. Fineberg, MD, president of the Gordon and Betty Moore Foundation, told this news organization.
The new definition “does not erase the problem of clinical judgment ... But we think this definition has the real advantage of elevating to the clinician’s mind the real likelihood in the current environment of prevalence of this virus that a presenting patient’s strange symptoms are both real and maybe related as an expression of long COVID,” Dr. Fineberg noted.
One way this new definition differs from previous ones such as WHO’s, he said, is “they talk about a diagnosis of exclusion. One of the important points in our definition is that other diagnosable conditions like ME/CFS or POTS can be part of the picture of long COVID. They are not alternative. They are, in fact, an expression of long COVID.”
Indeed, the NASEM report also introduces the term infection-associated chronic condition (IACC). This was important, Dr. Fineberg said, “because it’s the larger family of conditions of which long COVID is a part. It emphasizes a relatedness of long COVID to other conditions that can follow from a variety of infections. We also adopted the term ‘disease state’ to convey the seriousness and reality of this condition in the lives of patients.”
Comments on New Definition
In a statement provided to this news organization, Lucinda Bateman, MD, and Brayden Yellman, MD, co-medical directors of the Bateman-Horne Center in Salt Lake City, said that “describing long COVID as an IACC ... not only meets the NASEM goal of allowing clinicians, researchers, and public health officials to meaningfully identify and serve all persons who suffer illness or disability in the wake of a SARS-CoV-2 infection, but also draws direct comparison to other known IACC’s (such as ME/CFS, post-treatment Lyme, POTS) that have been plaguing many for decades.”
Dr. Fineberg noted another important aspect of the NASEM report: “Our definition includes an explicit statement on equity, explaining that long COVID can affect anyone, young and old, different races, different ages, different sexes, different genders, different orientations, different socioeconomic conditions ... This does not mean that every single person is at equal risk. There are risk factors, but the important point is the universal nature of this as a condition.”
Two clinical directors of long COVID programs who were contacted by this news organization praised the new definition. Zijian Chen, MD, director of Mount Sinai’s Center for Post-COVID Care, New York, said that it’s “very similar to the definition that we have used for our clinical practice since 2020. It is very important that the broad definition helps to be inclusive of all patients that may be affected. The inclusion of children as a consideration is important as well, since there is routinely less focus on children because they tend to have less disease frequency ... The creation of a unified definition helps both with clinical practice and research.”
Nisha Viswanathan, MD, director of the long COVID program at the University of California, Los Angeles, said: “I think they left it intentionally broad for the medical practitioner to not necessarily use the definition to rule out individuals, but to perhaps use more of a clinical gestalt to help rule in this diagnosis ... I think this definition is providing clarity to health care providers on what exactly would be falling under the long-COVID diagnosis header.”
Dr. Viswanathan also said that she anticipates this definition to help patients make their case in filing disability claims. “Because long COVID has not previously had a good fleshed-out definition, it was very easy for disability providers to reject claims for patients who continue to have symptoms ... I actually think this might help our patients ultimately in their attempt to be able to have the ability to care for themselves when they’re disabled enough to not be able to work.”
Written into the report is the expectation that the definition “will evolve as new evidence emerges and the understanding of long COVID matures.” The writing committee calls for reexamination in “no more than 3 years.” Factors that would prompt a reevaluation could include improved testing methods, discovery of medical factors and/or biomarkers that distinguish long COVID from other conditions, and new treatments.
Meanwhile, Dr. Fineberg told this news organization, “If this definition adds to the readiness, awareness, openness, and response to the patient with long COVID, it will have done its job.”
Dr. Fineberg, Dr. Bateman, Dr. Yellman, Dr. Viswanathan, and Dr. Chen have no relevant disclosures.
A version of this article appeared on Medscape.com.