User login
Second infection hikes long COVID risk: Expert Q&A
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
People with long COVID don’t show signs of brain damage
A pair of new studies published about long COVID have shed more light on the sometimes-disabling condition that affects millions of people in the United States.
Scientists worldwide have been working to understand the wide-ranging condition, from risk factors to causes to potential treatments.
In the first study, 31 adults underwent lumbar puncture and blood draws to look for changes in their immune systems and also to look for changes in the nerve cells that could affect transmission of signals to the brain.
Among the participants, 25 people had neurocognitive symptoms of long COVID, such as memory loss or attention problems. Six participants had fully recovered from COVID, and 17 people had never had COVID.
Those who had COVID were diagnosed between March 2020 and May 2021. Their fluid samples were drawn at least three months after their first symptoms.
The results were published in the Journal of Infectious Diseases.
According to a summary of the study from the University of Gothenburg (Sweden), where the researchers work, “there were no significant differences between the groups when analyzing blood and cerebrospinal fluid for immune activation or brain injury markers. The findings thus suggest that post-COVID condition is not the result of ongoing infection, immune activation, or brain damage.”
In the second study, Norwegian researchers compared the likelihood of having 17 different long COVID symptoms based on whether a person had been infected with COVID. The analysis included 53,846 people who were diagnosed with COVID between February 2020 and February 2021, as well as more than 485,000 people who were not infected. Most people had not been vaccinated against COVID-19 during the time of the study.
The results were published in the journal BMC Infectious Diseases. Study results showed that people who had COVID were more than twice as likely to experience shortness of breath or fatigue. They were also more likely to experience memory loss or headache compared to people who never had COVID. Researchers only looked at symptoms that occurred at least three months after a COVID diagnosis.
They also found that hospitalization increased the risk for experiencing long COVID symptoms of shortness of breath, fatigue, and memory loss.
The authors noted that a limitation of their study was that, often, not all symptoms reported during a visit with a general practice medical provider are recorded in Norway, which could have affected the results.
A version of this article appeared on Medscape.com.
A pair of new studies published about long COVID have shed more light on the sometimes-disabling condition that affects millions of people in the United States.
Scientists worldwide have been working to understand the wide-ranging condition, from risk factors to causes to potential treatments.
In the first study, 31 adults underwent lumbar puncture and blood draws to look for changes in their immune systems and also to look for changes in the nerve cells that could affect transmission of signals to the brain.
Among the participants, 25 people had neurocognitive symptoms of long COVID, such as memory loss or attention problems. Six participants had fully recovered from COVID, and 17 people had never had COVID.
Those who had COVID were diagnosed between March 2020 and May 2021. Their fluid samples were drawn at least three months after their first symptoms.
The results were published in the Journal of Infectious Diseases.
According to a summary of the study from the University of Gothenburg (Sweden), where the researchers work, “there were no significant differences between the groups when analyzing blood and cerebrospinal fluid for immune activation or brain injury markers. The findings thus suggest that post-COVID condition is not the result of ongoing infection, immune activation, or brain damage.”
In the second study, Norwegian researchers compared the likelihood of having 17 different long COVID symptoms based on whether a person had been infected with COVID. The analysis included 53,846 people who were diagnosed with COVID between February 2020 and February 2021, as well as more than 485,000 people who were not infected. Most people had not been vaccinated against COVID-19 during the time of the study.
The results were published in the journal BMC Infectious Diseases. Study results showed that people who had COVID were more than twice as likely to experience shortness of breath or fatigue. They were also more likely to experience memory loss or headache compared to people who never had COVID. Researchers only looked at symptoms that occurred at least three months after a COVID diagnosis.
They also found that hospitalization increased the risk for experiencing long COVID symptoms of shortness of breath, fatigue, and memory loss.
The authors noted that a limitation of their study was that, often, not all symptoms reported during a visit with a general practice medical provider are recorded in Norway, which could have affected the results.
A version of this article appeared on Medscape.com.
A pair of new studies published about long COVID have shed more light on the sometimes-disabling condition that affects millions of people in the United States.
Scientists worldwide have been working to understand the wide-ranging condition, from risk factors to causes to potential treatments.
In the first study, 31 adults underwent lumbar puncture and blood draws to look for changes in their immune systems and also to look for changes in the nerve cells that could affect transmission of signals to the brain.
Among the participants, 25 people had neurocognitive symptoms of long COVID, such as memory loss or attention problems. Six participants had fully recovered from COVID, and 17 people had never had COVID.
Those who had COVID were diagnosed between March 2020 and May 2021. Their fluid samples were drawn at least three months after their first symptoms.
The results were published in the Journal of Infectious Diseases.
According to a summary of the study from the University of Gothenburg (Sweden), where the researchers work, “there were no significant differences between the groups when analyzing blood and cerebrospinal fluid for immune activation or brain injury markers. The findings thus suggest that post-COVID condition is not the result of ongoing infection, immune activation, or brain damage.”
In the second study, Norwegian researchers compared the likelihood of having 17 different long COVID symptoms based on whether a person had been infected with COVID. The analysis included 53,846 people who were diagnosed with COVID between February 2020 and February 2021, as well as more than 485,000 people who were not infected. Most people had not been vaccinated against COVID-19 during the time of the study.
The results were published in the journal BMC Infectious Diseases. Study results showed that people who had COVID were more than twice as likely to experience shortness of breath or fatigue. They were also more likely to experience memory loss or headache compared to people who never had COVID. Researchers only looked at symptoms that occurred at least three months after a COVID diagnosis.
They also found that hospitalization increased the risk for experiencing long COVID symptoms of shortness of breath, fatigue, and memory loss.
The authors noted that a limitation of their study was that, often, not all symptoms reported during a visit with a general practice medical provider are recorded in Norway, which could have affected the results.
A version of this article appeared on Medscape.com.
Nirmatrelvir-ritonavir ineffective at reducing most post-COVID conditions
TOPLINE:
Thromboembolic events are the exception.
METHODOLOGY:
- A retrospective study of 9,593 veterans older than 65 years examined the impact of nirmatrelvir-ritonavir in comparison with no treatment on post–COVID-19 conditions (PCCs).
- Researchers coded 31 conditions, including those that fell into cardiac, pulmonary, renal, thromboembolic, gastrointestinal, neurologic, mental health, musculoskeletal, and endocrine categories.
- The incidence of PCCs was analyzed 31-180 days after treatment.
TAKEAWAY:
- The combined incidence of venous thromboembolism and pulmonary embolism was reduced among patients given nirmatrelvir-ritonavir.
- No statistically significant reduction of other conditions was found.
- Results differ from the conclusions of a smaller study that found that the incidence of 10 of 13 PCCs was lower.
IN PRACTICE:
“Our results suggest that considerations about PCCs may not be an important factor in COVID-19 treatment decisions,” the authors write.
SOURCE:
The study was funded by the Department of Veterans Affairs and was published online in Annals of Internal Medicine. George Ioannou, MD, director of hepatology at the VA Puget Sound Health Care System in Seattle, led the study.
LIMITATIONS:
A large number of outcomes were observed, so it’s possible that the association between treatment with nirmatrelvir-ritonavir and reduced incidence of thromboembolic events occurred by chance.
Data on COVID-19 treatments and PCCs may be incomplete. The long-term effects of PCCs may not have been fully captured by the ICD-10, which was used for diagnosis codes.
Electronic health records did not accurately capture the symptom burden or the date symptoms began. Patients in the treatment arm may have had more symptoms than matched control persons who were not treated.
DISCLOSURES:
The authors reported relationships with the Korean Diabetes Association, the American Diabetes Association, the International Society for the Diabetic Foot, Quality Insights, Brown University, and the Society for Women in Urology, among others.
A version of this article appeared on Medscape.com.
TOPLINE:
Thromboembolic events are the exception.
METHODOLOGY:
- A retrospective study of 9,593 veterans older than 65 years examined the impact of nirmatrelvir-ritonavir in comparison with no treatment on post–COVID-19 conditions (PCCs).
- Researchers coded 31 conditions, including those that fell into cardiac, pulmonary, renal, thromboembolic, gastrointestinal, neurologic, mental health, musculoskeletal, and endocrine categories.
- The incidence of PCCs was analyzed 31-180 days after treatment.
TAKEAWAY:
- The combined incidence of venous thromboembolism and pulmonary embolism was reduced among patients given nirmatrelvir-ritonavir.
- No statistically significant reduction of other conditions was found.
- Results differ from the conclusions of a smaller study that found that the incidence of 10 of 13 PCCs was lower.
IN PRACTICE:
“Our results suggest that considerations about PCCs may not be an important factor in COVID-19 treatment decisions,” the authors write.
SOURCE:
The study was funded by the Department of Veterans Affairs and was published online in Annals of Internal Medicine. George Ioannou, MD, director of hepatology at the VA Puget Sound Health Care System in Seattle, led the study.
LIMITATIONS:
A large number of outcomes were observed, so it’s possible that the association between treatment with nirmatrelvir-ritonavir and reduced incidence of thromboembolic events occurred by chance.
Data on COVID-19 treatments and PCCs may be incomplete. The long-term effects of PCCs may not have been fully captured by the ICD-10, which was used for diagnosis codes.
Electronic health records did not accurately capture the symptom burden or the date symptoms began. Patients in the treatment arm may have had more symptoms than matched control persons who were not treated.
DISCLOSURES:
The authors reported relationships with the Korean Diabetes Association, the American Diabetes Association, the International Society for the Diabetic Foot, Quality Insights, Brown University, and the Society for Women in Urology, among others.
A version of this article appeared on Medscape.com.
TOPLINE:
Thromboembolic events are the exception.
METHODOLOGY:
- A retrospective study of 9,593 veterans older than 65 years examined the impact of nirmatrelvir-ritonavir in comparison with no treatment on post–COVID-19 conditions (PCCs).
- Researchers coded 31 conditions, including those that fell into cardiac, pulmonary, renal, thromboembolic, gastrointestinal, neurologic, mental health, musculoskeletal, and endocrine categories.
- The incidence of PCCs was analyzed 31-180 days after treatment.
TAKEAWAY:
- The combined incidence of venous thromboembolism and pulmonary embolism was reduced among patients given nirmatrelvir-ritonavir.
- No statistically significant reduction of other conditions was found.
- Results differ from the conclusions of a smaller study that found that the incidence of 10 of 13 PCCs was lower.
IN PRACTICE:
“Our results suggest that considerations about PCCs may not be an important factor in COVID-19 treatment decisions,” the authors write.
SOURCE:
The study was funded by the Department of Veterans Affairs and was published online in Annals of Internal Medicine. George Ioannou, MD, director of hepatology at the VA Puget Sound Health Care System in Seattle, led the study.
LIMITATIONS:
A large number of outcomes were observed, so it’s possible that the association between treatment with nirmatrelvir-ritonavir and reduced incidence of thromboembolic events occurred by chance.
Data on COVID-19 treatments and PCCs may be incomplete. The long-term effects of PCCs may not have been fully captured by the ICD-10, which was used for diagnosis codes.
Electronic health records did not accurately capture the symptom burden or the date symptoms began. Patients in the treatment arm may have had more symptoms than matched control persons who were not treated.
DISCLOSURES:
The authors reported relationships with the Korean Diabetes Association, the American Diabetes Association, the International Society for the Diabetic Foot, Quality Insights, Brown University, and the Society for Women in Urology, among others.
A version of this article appeared on Medscape.com.
Long COVID: Advocating for Patients and Implementing Effective Techniques
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
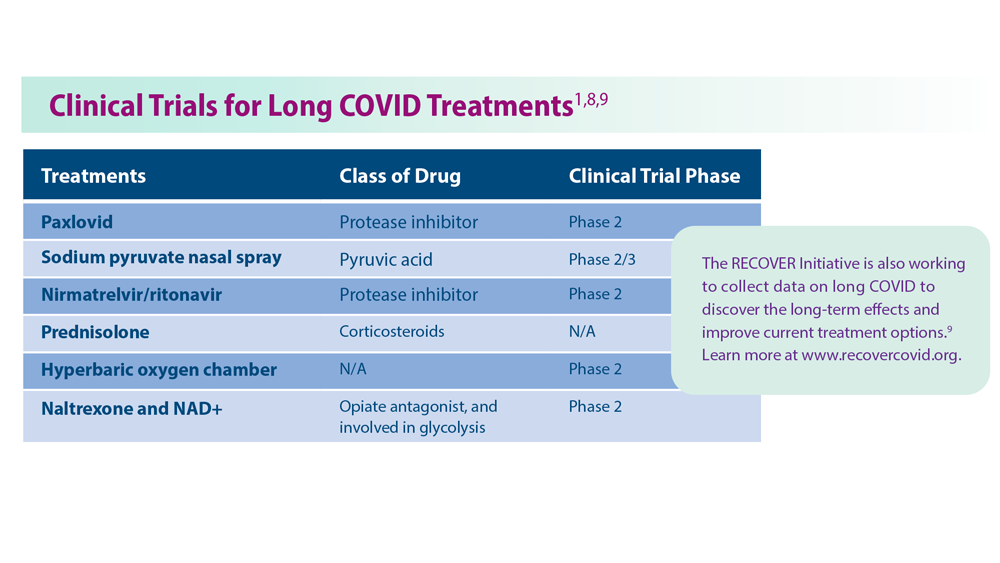
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
People with long COVID have specific blood biomarkers, study says
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
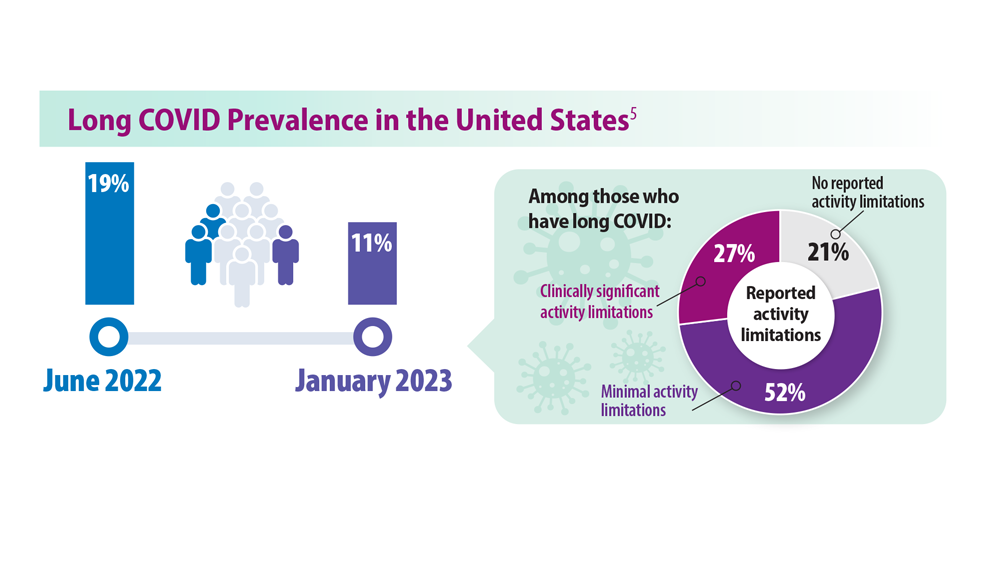
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
Creatine may improve key long COVID symptoms: Small study
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
FROM FOOD, SCIENCE & NUTRITION
One in five doctors with long COVID can no longer work: Survey
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Mepolizumab improves asthma after 1 year despite comorbidities
Adults with asthma who were newly prescribed mepolizumab showed significant improvement in symptoms after 1 year regardless of comorbidities, based on data from 822 individuals.
Comorbidities including chronic rhinosinusitis with polyps (CRSwNP), gastroesophageal reflux disease GERD), anxiety and depression, and chronic obstructive pulmonary disorder (COPD) are common in patients with severe asthma and add to the disease burden, wrote Mark C. Liu, MD, of Johns Hopkins University, Baltimore, and colleagues.
“Some comorbidities, such as CRSwNP, share pathophysiological mechanisms with severe asthma, with interleukin-5 (IL-5),” and treatments targeting IL-5 could improve outcomes, they said.
In the real-world REALITI-A study, mepolizumab, a humanized monoclonal antibody that targets IL-5, significantly reduced asthma exacerbation and oral corticosteroid use in severe asthma patients, they said.
To assess the impact of mepolizumab on patients with comorbidities, the researchers conducted a post hoc analysis of 822 adults with severe asthma, including 321 with CRSwNP, 309 with GERD, 203 with depression/anxiety, and 81 with COPD. The findings were published in the Journal of Allergy and Clinical Immunology: In Practice.
The main outcomes were the rate of clinically significant asthma exacerbations (CSEs) between the 12 months before and after mepolizumab initiation, and the changes from baseline in the daily maintenance use of oral corticosteroids (OCS).
Across all comorbidities, the rate of CSEs decreased significantly from the pretreatment period to the follow-up period, from 4.28 events per year to 1.23 events per year.
“A numerically greater reduction in the rate of CSEs was reported for patients with versus without CRSwNP, whereas the reverse was reported for patients with versus without COPD and depression/anxiety, although the confidence intervals were large for the with COPD subgroup,” the researchers wrote.
The median maintenance dose of oral corticosteroids decreased by at least 50% across all comorbidities after mepolizumab treatment; patients with CRSwNP had the greatest reduction (83%).
In addition, scores on the Asthma Control Questionnaire–5 decreased by at least 0.63 points, and least squared (LS) mean changes in forced expiratory volume per second (FEV1) increased from baseline across all comorbidities after mepolizumab treatment by at least 74 mL.
Although patients with versus without CRSwNP had greater improvements, patients without GERD, depression/anxiety, and COPD had greater improvements than did those without the respective conditions with the exception of greater FEV1 improvement in patients with vs. without COPD.
“Patients with severe asthma and comorbid CRSwNP are recognized as having a high disease burden, as demonstrated by more frequent exacerbations,” the researchers wrote in their discussion. “Mepolizumab may serve to reduce the disease burden of this high-risk group by targeting the common pathophysiological pathway of IL-5 and eosinophilic-driven inflammation because it has proven clinical benefits in treating asthma and CRSwNP separately and together,” and the current study findings support the use of mepolizumab for this population in particular, they said.
The findings were limited by several factors including the incomplete data for voluntary assessments, the post hoc design and relatively small numbers of patients in various subgroups, notably COPD, and the potential inaccurate diagnosis of COPD, the researchers noted.
“Nevertheless, because the amount of improvement in each outcome following mepolizumab treatment differed depending on the comorbidity in question, our findings highlight the impact that comorbidities and their prevalence and severity have on outcomes,” and the overall success of mepolizumab across clinical characteristics and comorbidities supports the generalizability of the findings to the larger population of adults with severe asthma, they concluded.
The study was supported by GlaxoSmithKline. Dr. Liu disclosed research funding from GSK, Boehringer Ingelheim, and Gossamer Bio, and participation on advisory boards for AstraZeneca, GSK, and Gossamer Bio.
Adults with asthma who were newly prescribed mepolizumab showed significant improvement in symptoms after 1 year regardless of comorbidities, based on data from 822 individuals.
Comorbidities including chronic rhinosinusitis with polyps (CRSwNP), gastroesophageal reflux disease GERD), anxiety and depression, and chronic obstructive pulmonary disorder (COPD) are common in patients with severe asthma and add to the disease burden, wrote Mark C. Liu, MD, of Johns Hopkins University, Baltimore, and colleagues.
“Some comorbidities, such as CRSwNP, share pathophysiological mechanisms with severe asthma, with interleukin-5 (IL-5),” and treatments targeting IL-5 could improve outcomes, they said.
In the real-world REALITI-A study, mepolizumab, a humanized monoclonal antibody that targets IL-5, significantly reduced asthma exacerbation and oral corticosteroid use in severe asthma patients, they said.
To assess the impact of mepolizumab on patients with comorbidities, the researchers conducted a post hoc analysis of 822 adults with severe asthma, including 321 with CRSwNP, 309 with GERD, 203 with depression/anxiety, and 81 with COPD. The findings were published in the Journal of Allergy and Clinical Immunology: In Practice.
The main outcomes were the rate of clinically significant asthma exacerbations (CSEs) between the 12 months before and after mepolizumab initiation, and the changes from baseline in the daily maintenance use of oral corticosteroids (OCS).
Across all comorbidities, the rate of CSEs decreased significantly from the pretreatment period to the follow-up period, from 4.28 events per year to 1.23 events per year.
“A numerically greater reduction in the rate of CSEs was reported for patients with versus without CRSwNP, whereas the reverse was reported for patients with versus without COPD and depression/anxiety, although the confidence intervals were large for the with COPD subgroup,” the researchers wrote.
The median maintenance dose of oral corticosteroids decreased by at least 50% across all comorbidities after mepolizumab treatment; patients with CRSwNP had the greatest reduction (83%).
In addition, scores on the Asthma Control Questionnaire–5 decreased by at least 0.63 points, and least squared (LS) mean changes in forced expiratory volume per second (FEV1) increased from baseline across all comorbidities after mepolizumab treatment by at least 74 mL.
Although patients with versus without CRSwNP had greater improvements, patients without GERD, depression/anxiety, and COPD had greater improvements than did those without the respective conditions with the exception of greater FEV1 improvement in patients with vs. without COPD.
“Patients with severe asthma and comorbid CRSwNP are recognized as having a high disease burden, as demonstrated by more frequent exacerbations,” the researchers wrote in their discussion. “Mepolizumab may serve to reduce the disease burden of this high-risk group by targeting the common pathophysiological pathway of IL-5 and eosinophilic-driven inflammation because it has proven clinical benefits in treating asthma and CRSwNP separately and together,” and the current study findings support the use of mepolizumab for this population in particular, they said.
The findings were limited by several factors including the incomplete data for voluntary assessments, the post hoc design and relatively small numbers of patients in various subgroups, notably COPD, and the potential inaccurate diagnosis of COPD, the researchers noted.
“Nevertheless, because the amount of improvement in each outcome following mepolizumab treatment differed depending on the comorbidity in question, our findings highlight the impact that comorbidities and their prevalence and severity have on outcomes,” and the overall success of mepolizumab across clinical characteristics and comorbidities supports the generalizability of the findings to the larger population of adults with severe asthma, they concluded.
The study was supported by GlaxoSmithKline. Dr. Liu disclosed research funding from GSK, Boehringer Ingelheim, and Gossamer Bio, and participation on advisory boards for AstraZeneca, GSK, and Gossamer Bio.
Adults with asthma who were newly prescribed mepolizumab showed significant improvement in symptoms after 1 year regardless of comorbidities, based on data from 822 individuals.
Comorbidities including chronic rhinosinusitis with polyps (CRSwNP), gastroesophageal reflux disease GERD), anxiety and depression, and chronic obstructive pulmonary disorder (COPD) are common in patients with severe asthma and add to the disease burden, wrote Mark C. Liu, MD, of Johns Hopkins University, Baltimore, and colleagues.
“Some comorbidities, such as CRSwNP, share pathophysiological mechanisms with severe asthma, with interleukin-5 (IL-5),” and treatments targeting IL-5 could improve outcomes, they said.
In the real-world REALITI-A study, mepolizumab, a humanized monoclonal antibody that targets IL-5, significantly reduced asthma exacerbation and oral corticosteroid use in severe asthma patients, they said.
To assess the impact of mepolizumab on patients with comorbidities, the researchers conducted a post hoc analysis of 822 adults with severe asthma, including 321 with CRSwNP, 309 with GERD, 203 with depression/anxiety, and 81 with COPD. The findings were published in the Journal of Allergy and Clinical Immunology: In Practice.
The main outcomes were the rate of clinically significant asthma exacerbations (CSEs) between the 12 months before and after mepolizumab initiation, and the changes from baseline in the daily maintenance use of oral corticosteroids (OCS).
Across all comorbidities, the rate of CSEs decreased significantly from the pretreatment period to the follow-up period, from 4.28 events per year to 1.23 events per year.
“A numerically greater reduction in the rate of CSEs was reported for patients with versus without CRSwNP, whereas the reverse was reported for patients with versus without COPD and depression/anxiety, although the confidence intervals were large for the with COPD subgroup,” the researchers wrote.
The median maintenance dose of oral corticosteroids decreased by at least 50% across all comorbidities after mepolizumab treatment; patients with CRSwNP had the greatest reduction (83%).
In addition, scores on the Asthma Control Questionnaire–5 decreased by at least 0.63 points, and least squared (LS) mean changes in forced expiratory volume per second (FEV1) increased from baseline across all comorbidities after mepolizumab treatment by at least 74 mL.
Although patients with versus without CRSwNP had greater improvements, patients without GERD, depression/anxiety, and COPD had greater improvements than did those without the respective conditions with the exception of greater FEV1 improvement in patients with vs. without COPD.
“Patients with severe asthma and comorbid CRSwNP are recognized as having a high disease burden, as demonstrated by more frequent exacerbations,” the researchers wrote in their discussion. “Mepolizumab may serve to reduce the disease burden of this high-risk group by targeting the common pathophysiological pathway of IL-5 and eosinophilic-driven inflammation because it has proven clinical benefits in treating asthma and CRSwNP separately and together,” and the current study findings support the use of mepolizumab for this population in particular, they said.
The findings were limited by several factors including the incomplete data for voluntary assessments, the post hoc design and relatively small numbers of patients in various subgroups, notably COPD, and the potential inaccurate diagnosis of COPD, the researchers noted.
“Nevertheless, because the amount of improvement in each outcome following mepolizumab treatment differed depending on the comorbidity in question, our findings highlight the impact that comorbidities and their prevalence and severity have on outcomes,” and the overall success of mepolizumab across clinical characteristics and comorbidities supports the generalizability of the findings to the larger population of adults with severe asthma, they concluded.
The study was supported by GlaxoSmithKline. Dr. Liu disclosed research funding from GSK, Boehringer Ingelheim, and Gossamer Bio, and participation on advisory boards for AstraZeneca, GSK, and Gossamer Bio.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Severe COVID may cause long-term cellular changes: Study
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
FROM CELL