User login
Zika virus syndrome may adversely affect children normocephalic at birth
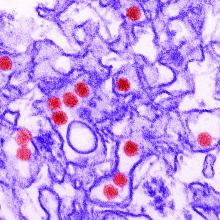
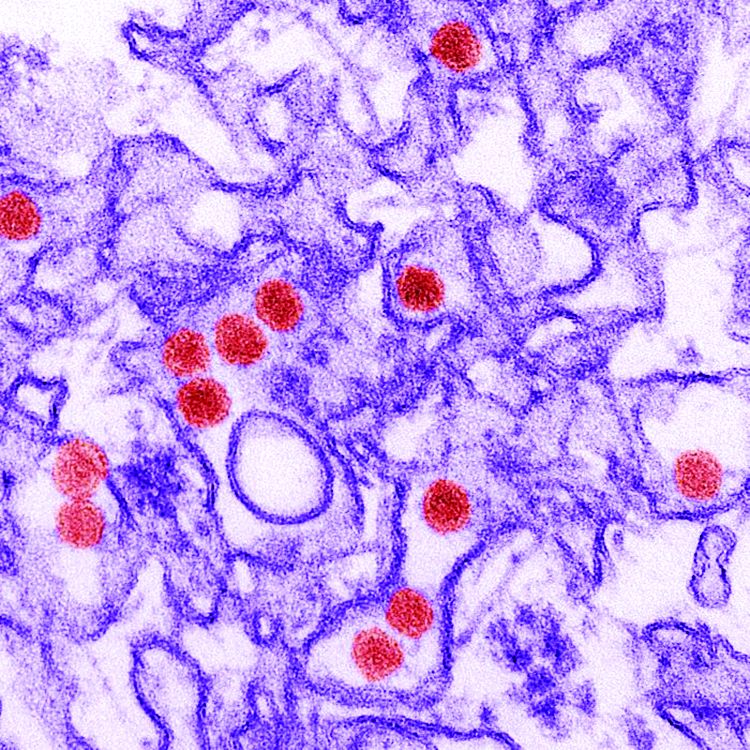
Microcephaly may be the hallmark of congenital Zika virus syndrome, but neurologic abnormalities also are common in normocephalic children exposed to the virus in utero, according to data from a large pediatric referral center in Rio de Janeiro.
The retrospective analysis demonstrated that there is a “spectrum of clinical manifestations” in children with congenital Zika virus syndrome, including those who “had initially been perceived as developing normally based on [head circumference],” Jessica S. Cranston, a medical student at the University of California, Los Angeles, and associates wrote in JAMA Network Open.
Previous studies have described the poor clinical outcomes in Zika virus–exposed infants with microcephaly, but the current analysis evaluated head circumference (HC) as a continuous variable and stratified outcomes according to the presence or absence of microcephaly, they explained.
In the cohort of 215 children referred to Instituto Fernandes Figueira who had laboratory-confirmed antenatal Zika virus exposure, 53 had microcephaly (cephalic perimeter z score of less than –2 standard deviations) and 162 were normocephalic, the investigators said.
The children were evaluated monthly for the first 6 months of life and then every 3 months. Neurodevelopmental evaluation with the Bayley Scales of Infant and Toddler Development, Third Edition, between 6 months and 3 years of age showed that all of those with microcephaly had abnormal neuromotor findings. All but two of the children with microcephaly had abnormal neuroimaging results, and 38 (72%) had failure to thrive, they reported.
Among the children with normocephaly at birth, 68% had abnormal neurologic findings, including hyperreflexia (27%), abnormal tone (39%), and other congenital neuromotor signs (42%). Results of neuroimaging results, primarily in the form of transfontanelle ultrasonography, were abnormal in 29% of children with normocephaly.
“Infants with a larger birth HC, within the normocephalic range (±2 SDs), had higher overall neurodevelopmental scores on the Bayley-III assessment,” Sarah B. Mulkey, MD, PhD, said in an invited commentary, “whereas infants with a smaller birth HC within the normocephalic range had lower scores in the domains of cognitive and language functions.”
If HC measurements could be combined with early neurologic data such as the results of neuroimaging or a neurological exam, she suggested, it might provide “a practical tool to help determine risk for adverse clinical outcomes in a [Zika virus–]exposed infant at birth that can be widely used in a variety of follow-up settings.”
In nutritional assessments performed for 143 children with normocephaly, 51% had failure to thrive “because of neurologic repercussions leading to poor feeding,” Ms. Cranston and associates wrote, adding that 15 of the 73 (21%) infants with normocephaly and failure to thrive developed secondary microcephaly.
Altogether, 17 of the 162 (10.5%) children with normocephaly developed microcephaly during the follow-up, with the reverse – microcephaly resolving in infants who were microcephalic at birth – occurring in 4 of the 53 (7.5%) affected infants, indicating that “head circumference was not static,” they said.
“The trajectory of head growth is critical,” said Dr. Mulkey of the Prenatal Pediatrics Institute at Children’s National Hospital in Washington. “The neurologic outcome of a child who develops postnatal microcephaly would be very concerning compared with an infant who is born with normocephaly and maintains a steady HC percentile over time.”
HC is just one piece of the puzzle, however, since children with Zika virus syndrome may exhibit “a variety of manifestations and outcomes.” This lack of certainty suggests that “careful monitoring and evaluation of children with suspected exposure is essential for ensuring early detection of possible disabilities and referral to interventional services,” the investigators wrote.
The findings of this study “are both highly statistically significant and clinically significant,”said Kevin T. Powell, MD, PhD, a pediatric hospitalist and clinical ethics consultant living in St. Louis who was not associated with the study.
“While outcomes at birth are dichotomized into those with and without microcephaly, the developmental outcomes measured at 3 years of age are on a spectrum. ... Those with microcephaly tend to be more severely affected, but many infants with small but normal-sized heads are also mild to moderately impacted. The flip side is that 64% of infected babies ended up with average or better development” based on Bayley-III evaluations, said Dr. Powell, who is a member of the Pediatric News editorial advisory board.
The study was funded by grants from the National Institute of Allergy and Infectious Diseases, the National Eye Institute, and the Thrasher Foundation and by awards from Brazil’s National Council of Scientific and Technological Development; Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro. Individual investigators received fees and grants from these and other organizations.
Dr. Mulkey received a contract from the Centers for Disease Control and Prevention for technical expertise for Zika virus studies and received support for Zika studies from the Thrasher Research Fund. Dr. Powell had no relevant financial disclosures.
SOURCE: Cranston JS et al. JAMA Netw Open. 2020 July 7;3(7):e209303.
Microcephaly may be the hallmark of congenital Zika virus syndrome, but neurologic abnormalities also are common in normocephalic children exposed to the virus in utero, according to data from a large pediatric referral center in Rio de Janeiro.
The retrospective analysis demonstrated that there is a “spectrum of clinical manifestations” in children with congenital Zika virus syndrome, including those who “had initially been perceived as developing normally based on [head circumference],” Jessica S. Cranston, a medical student at the University of California, Los Angeles, and associates wrote in JAMA Network Open.
Previous studies have described the poor clinical outcomes in Zika virus–exposed infants with microcephaly, but the current analysis evaluated head circumference (HC) as a continuous variable and stratified outcomes according to the presence or absence of microcephaly, they explained.
In the cohort of 215 children referred to Instituto Fernandes Figueira who had laboratory-confirmed antenatal Zika virus exposure, 53 had microcephaly (cephalic perimeter z score of less than –2 standard deviations) and 162 were normocephalic, the investigators said.
The children were evaluated monthly for the first 6 months of life and then every 3 months. Neurodevelopmental evaluation with the Bayley Scales of Infant and Toddler Development, Third Edition, between 6 months and 3 years of age showed that all of those with microcephaly had abnormal neuromotor findings. All but two of the children with microcephaly had abnormal neuroimaging results, and 38 (72%) had failure to thrive, they reported.
Among the children with normocephaly at birth, 68% had abnormal neurologic findings, including hyperreflexia (27%), abnormal tone (39%), and other congenital neuromotor signs (42%). Results of neuroimaging results, primarily in the form of transfontanelle ultrasonography, were abnormal in 29% of children with normocephaly.
“Infants with a larger birth HC, within the normocephalic range (±2 SDs), had higher overall neurodevelopmental scores on the Bayley-III assessment,” Sarah B. Mulkey, MD, PhD, said in an invited commentary, “whereas infants with a smaller birth HC within the normocephalic range had lower scores in the domains of cognitive and language functions.”
If HC measurements could be combined with early neurologic data such as the results of neuroimaging or a neurological exam, she suggested, it might provide “a practical tool to help determine risk for adverse clinical outcomes in a [Zika virus–]exposed infant at birth that can be widely used in a variety of follow-up settings.”
In nutritional assessments performed for 143 children with normocephaly, 51% had failure to thrive “because of neurologic repercussions leading to poor feeding,” Ms. Cranston and associates wrote, adding that 15 of the 73 (21%) infants with normocephaly and failure to thrive developed secondary microcephaly.
Altogether, 17 of the 162 (10.5%) children with normocephaly developed microcephaly during the follow-up, with the reverse – microcephaly resolving in infants who were microcephalic at birth – occurring in 4 of the 53 (7.5%) affected infants, indicating that “head circumference was not static,” they said.
“The trajectory of head growth is critical,” said Dr. Mulkey of the Prenatal Pediatrics Institute at Children’s National Hospital in Washington. “The neurologic outcome of a child who develops postnatal microcephaly would be very concerning compared with an infant who is born with normocephaly and maintains a steady HC percentile over time.”
HC is just one piece of the puzzle, however, since children with Zika virus syndrome may exhibit “a variety of manifestations and outcomes.” This lack of certainty suggests that “careful monitoring and evaluation of children with suspected exposure is essential for ensuring early detection of possible disabilities and referral to interventional services,” the investigators wrote.
The findings of this study “are both highly statistically significant and clinically significant,”said Kevin T. Powell, MD, PhD, a pediatric hospitalist and clinical ethics consultant living in St. Louis who was not associated with the study.
“While outcomes at birth are dichotomized into those with and without microcephaly, the developmental outcomes measured at 3 years of age are on a spectrum. ... Those with microcephaly tend to be more severely affected, but many infants with small but normal-sized heads are also mild to moderately impacted. The flip side is that 64% of infected babies ended up with average or better development” based on Bayley-III evaluations, said Dr. Powell, who is a member of the Pediatric News editorial advisory board.
The study was funded by grants from the National Institute of Allergy and Infectious Diseases, the National Eye Institute, and the Thrasher Foundation and by awards from Brazil’s National Council of Scientific and Technological Development; Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro. Individual investigators received fees and grants from these and other organizations.
Dr. Mulkey received a contract from the Centers for Disease Control and Prevention for technical expertise for Zika virus studies and received support for Zika studies from the Thrasher Research Fund. Dr. Powell had no relevant financial disclosures.
SOURCE: Cranston JS et al. JAMA Netw Open. 2020 July 7;3(7):e209303.
Microcephaly may be the hallmark of congenital Zika virus syndrome, but neurologic abnormalities also are common in normocephalic children exposed to the virus in utero, according to data from a large pediatric referral center in Rio de Janeiro.
The retrospective analysis demonstrated that there is a “spectrum of clinical manifestations” in children with congenital Zika virus syndrome, including those who “had initially been perceived as developing normally based on [head circumference],” Jessica S. Cranston, a medical student at the University of California, Los Angeles, and associates wrote in JAMA Network Open.
Previous studies have described the poor clinical outcomes in Zika virus–exposed infants with microcephaly, but the current analysis evaluated head circumference (HC) as a continuous variable and stratified outcomes according to the presence or absence of microcephaly, they explained.
In the cohort of 215 children referred to Instituto Fernandes Figueira who had laboratory-confirmed antenatal Zika virus exposure, 53 had microcephaly (cephalic perimeter z score of less than –2 standard deviations) and 162 were normocephalic, the investigators said.
The children were evaluated monthly for the first 6 months of life and then every 3 months. Neurodevelopmental evaluation with the Bayley Scales of Infant and Toddler Development, Third Edition, between 6 months and 3 years of age showed that all of those with microcephaly had abnormal neuromotor findings. All but two of the children with microcephaly had abnormal neuroimaging results, and 38 (72%) had failure to thrive, they reported.
Among the children with normocephaly at birth, 68% had abnormal neurologic findings, including hyperreflexia (27%), abnormal tone (39%), and other congenital neuromotor signs (42%). Results of neuroimaging results, primarily in the form of transfontanelle ultrasonography, were abnormal in 29% of children with normocephaly.
“Infants with a larger birth HC, within the normocephalic range (±2 SDs), had higher overall neurodevelopmental scores on the Bayley-III assessment,” Sarah B. Mulkey, MD, PhD, said in an invited commentary, “whereas infants with a smaller birth HC within the normocephalic range had lower scores in the domains of cognitive and language functions.”
If HC measurements could be combined with early neurologic data such as the results of neuroimaging or a neurological exam, she suggested, it might provide “a practical tool to help determine risk for adverse clinical outcomes in a [Zika virus–]exposed infant at birth that can be widely used in a variety of follow-up settings.”
In nutritional assessments performed for 143 children with normocephaly, 51% had failure to thrive “because of neurologic repercussions leading to poor feeding,” Ms. Cranston and associates wrote, adding that 15 of the 73 (21%) infants with normocephaly and failure to thrive developed secondary microcephaly.
Altogether, 17 of the 162 (10.5%) children with normocephaly developed microcephaly during the follow-up, with the reverse – microcephaly resolving in infants who were microcephalic at birth – occurring in 4 of the 53 (7.5%) affected infants, indicating that “head circumference was not static,” they said.
“The trajectory of head growth is critical,” said Dr. Mulkey of the Prenatal Pediatrics Institute at Children’s National Hospital in Washington. “The neurologic outcome of a child who develops postnatal microcephaly would be very concerning compared with an infant who is born with normocephaly and maintains a steady HC percentile over time.”
HC is just one piece of the puzzle, however, since children with Zika virus syndrome may exhibit “a variety of manifestations and outcomes.” This lack of certainty suggests that “careful monitoring and evaluation of children with suspected exposure is essential for ensuring early detection of possible disabilities and referral to interventional services,” the investigators wrote.
The findings of this study “are both highly statistically significant and clinically significant,”said Kevin T. Powell, MD, PhD, a pediatric hospitalist and clinical ethics consultant living in St. Louis who was not associated with the study.
“While outcomes at birth are dichotomized into those with and without microcephaly, the developmental outcomes measured at 3 years of age are on a spectrum. ... Those with microcephaly tend to be more severely affected, but many infants with small but normal-sized heads are also mild to moderately impacted. The flip side is that 64% of infected babies ended up with average or better development” based on Bayley-III evaluations, said Dr. Powell, who is a member of the Pediatric News editorial advisory board.
The study was funded by grants from the National Institute of Allergy and Infectious Diseases, the National Eye Institute, and the Thrasher Foundation and by awards from Brazil’s National Council of Scientific and Technological Development; Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro. Individual investigators received fees and grants from these and other organizations.
Dr. Mulkey received a contract from the Centers for Disease Control and Prevention for technical expertise for Zika virus studies and received support for Zika studies from the Thrasher Research Fund. Dr. Powell had no relevant financial disclosures.
SOURCE: Cranston JS et al. JAMA Netw Open. 2020 July 7;3(7):e209303.
FROM JAMA NETWORK OPEN
Declines in infant mortality tempered by disparities
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Liposomal bupivacaine excreted in breast milk, but levels appear safe
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
FROM OBSTETRICS & GYNECOLOGY
In-hospital formula feeding more than doubles odds of early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
FROM PEDIATRICS
COVID-19 may increase risk of preterm birth and cesarean delivery
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
FROM OBSTETRICS & GYNECOLOGY
Extremely preterm infants fare better with corticosteroid and magnesium combo
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
FROM OBSTETRICS & GYNECOLOGY
Vitamin D intake among U.S. infants has not improved, despite guidance
with breastfed infants less likely to have adequate levels than formula-fed infants, according to results of a study.
The American Association of Pediatrics has recommended since 2008 that breastfeeding babies under 1 year of age receive 400 IU of vitamin D supplementation daily, usually in the form of drops, to prevent rickets. For formula-fed infants, the AAP recommends that infants be fed one liter of formula daily, as formulas must contain 400 IU of vitamin D per liter.
A study looking at caregiver-reported dietary data through 2012 suggested that the guideline was having little impact, with only 27% of U.S. infants considered to be getting adequate vitamin D. The same researchers have now updated those findings with data through 2016 to report virtually no improvement over time. For their research, published in Pediatrics, Alan E. Simon, MD, of the National Institutes of Health in Rockville, Md., and Katherine A. Ahrens, PhD, of the University of Southern Maine in Portland, analyzed data for 1,435 infants aged 0-11 months. All data were recorded during 2009-2016 as part of the ongoing National Health and Nutrition Examination Survey (NHANES).
Overall, 27% of infants in the study were considered likely to meet the guidelines. Among nonbreastfeeding infants, 31% were deemed to have adequate levels, compared with 21% of breastfeeding infants (P less than .01).
Parents’ income and education affected infants’ likelihood of meeting guidelines. Breastfeeding infants in families with incomes above 400% of the federal poverty level were twice as likely to meet guidelines (31% vs. 14%-16% for lower income brackets, P less than .05). Babies from families whose head of household had a college degree had a 26% likelihood of having enough vitamin D, compared with less than 11% of those in whose parents had less than a high school education (P less than .05). Babies from families with private insurance also had a better chance of meeting guidelines, compared with those with public insurance (24% vs. 13%; P less than .05).
Ethnicity was seen as affecting vitamin D intake only insofar as some groups had more formula use than breastfeeding. The only ethnic or racial subgroup in the study that saw more than 40% of infants likely to meet guidelines was nonbreastfeeding infants of Asian, American Indian, Native Hawaiian or Pacific Islander, or multiracial parentage, with 46% considered to have adequate vitamin D levels. This group makes up 6% of the infant population in the United States.
“Reasons for low rates of meeting guidelines in the United States and little improvement over time are not fully known,” Dr. Simon and Dr. Ahrens wrote in their analysis. “One factor may be that the impact of low vitamin D in infancy is not highly visible to physicians because rickets is an uncommon diagnosis in the United States.” They noted that recent studies from Canada, where public health officials have done more to promote supplementation, have shown rates of adequate vitamin D in breastfeeding babies to be as high as 90%.
The researchers listed among limitations of their study the fact that the data source, NHANES, captured nutrition information only for the previous 24 hours; that it relied on parental report, and did not confirm serum levels of vitamin D; and that it was possible that cow’s milk – which is not recommended before age 1 but frequently given to older infants anyway – could be a hidden source of vitamin D that was not taken into consideration.
In an editorial comment, Jaspreet Loyal, MD, and Annette Cameron, MD, of Yale University in New Haven, Conn., faulted “a combination of inconsistent prescribing by clinicians and poor adherence to the use of a supplement by parents of infants … further complicated by a lack of awareness of the consequences of vitamin D deficiency in infants among the public” for the low adherence to guidelines in the United States, compared with other countries.
Also, the editorialists noted, the dropper used to administer liquid supplements has been associated with “inconsistent precision” and concerns about infants gagging on the liquid. More research is needed to better understand “prescribing patterns, barriers to adherence by parents of infants, and alternate strategies for vitamin D supplementation to inform novel public health programs in the United States,” they wrote.
The National Institutes of Health funded the study, and Dr. Ahrens is supported by a faculty development grant from the Maine Economic Improvement Fund. The researchers declared no conflicts of interest. Dr. Loyal and Dr. Cameron disclosed no funding and no relevant financial disclosures.
SOURCE: Simon AE and Ahrens KA. Pediatrics 2020 May. doi: 10.1542/peds.2019-3574; Loyal J and Cameron A. Pediatrics. 2020 May. doi: 10.1542/peds.2020-0504.
with breastfed infants less likely to have adequate levels than formula-fed infants, according to results of a study.
The American Association of Pediatrics has recommended since 2008 that breastfeeding babies under 1 year of age receive 400 IU of vitamin D supplementation daily, usually in the form of drops, to prevent rickets. For formula-fed infants, the AAP recommends that infants be fed one liter of formula daily, as formulas must contain 400 IU of vitamin D per liter.
A study looking at caregiver-reported dietary data through 2012 suggested that the guideline was having little impact, with only 27% of U.S. infants considered to be getting adequate vitamin D. The same researchers have now updated those findings with data through 2016 to report virtually no improvement over time. For their research, published in Pediatrics, Alan E. Simon, MD, of the National Institutes of Health in Rockville, Md., and Katherine A. Ahrens, PhD, of the University of Southern Maine in Portland, analyzed data for 1,435 infants aged 0-11 months. All data were recorded during 2009-2016 as part of the ongoing National Health and Nutrition Examination Survey (NHANES).
Overall, 27% of infants in the study were considered likely to meet the guidelines. Among nonbreastfeeding infants, 31% were deemed to have adequate levels, compared with 21% of breastfeeding infants (P less than .01).
Parents’ income and education affected infants’ likelihood of meeting guidelines. Breastfeeding infants in families with incomes above 400% of the federal poverty level were twice as likely to meet guidelines (31% vs. 14%-16% for lower income brackets, P less than .05). Babies from families whose head of household had a college degree had a 26% likelihood of having enough vitamin D, compared with less than 11% of those in whose parents had less than a high school education (P less than .05). Babies from families with private insurance also had a better chance of meeting guidelines, compared with those with public insurance (24% vs. 13%; P less than .05).
Ethnicity was seen as affecting vitamin D intake only insofar as some groups had more formula use than breastfeeding. The only ethnic or racial subgroup in the study that saw more than 40% of infants likely to meet guidelines was nonbreastfeeding infants of Asian, American Indian, Native Hawaiian or Pacific Islander, or multiracial parentage, with 46% considered to have adequate vitamin D levels. This group makes up 6% of the infant population in the United States.
“Reasons for low rates of meeting guidelines in the United States and little improvement over time are not fully known,” Dr. Simon and Dr. Ahrens wrote in their analysis. “One factor may be that the impact of low vitamin D in infancy is not highly visible to physicians because rickets is an uncommon diagnosis in the United States.” They noted that recent studies from Canada, where public health officials have done more to promote supplementation, have shown rates of adequate vitamin D in breastfeeding babies to be as high as 90%.
The researchers listed among limitations of their study the fact that the data source, NHANES, captured nutrition information only for the previous 24 hours; that it relied on parental report, and did not confirm serum levels of vitamin D; and that it was possible that cow’s milk – which is not recommended before age 1 but frequently given to older infants anyway – could be a hidden source of vitamin D that was not taken into consideration.
In an editorial comment, Jaspreet Loyal, MD, and Annette Cameron, MD, of Yale University in New Haven, Conn., faulted “a combination of inconsistent prescribing by clinicians and poor adherence to the use of a supplement by parents of infants … further complicated by a lack of awareness of the consequences of vitamin D deficiency in infants among the public” for the low adherence to guidelines in the United States, compared with other countries.
Also, the editorialists noted, the dropper used to administer liquid supplements has been associated with “inconsistent precision” and concerns about infants gagging on the liquid. More research is needed to better understand “prescribing patterns, barriers to adherence by parents of infants, and alternate strategies for vitamin D supplementation to inform novel public health programs in the United States,” they wrote.
The National Institutes of Health funded the study, and Dr. Ahrens is supported by a faculty development grant from the Maine Economic Improvement Fund. The researchers declared no conflicts of interest. Dr. Loyal and Dr. Cameron disclosed no funding and no relevant financial disclosures.
SOURCE: Simon AE and Ahrens KA. Pediatrics 2020 May. doi: 10.1542/peds.2019-3574; Loyal J and Cameron A. Pediatrics. 2020 May. doi: 10.1542/peds.2020-0504.
with breastfed infants less likely to have adequate levels than formula-fed infants, according to results of a study.
The American Association of Pediatrics has recommended since 2008 that breastfeeding babies under 1 year of age receive 400 IU of vitamin D supplementation daily, usually in the form of drops, to prevent rickets. For formula-fed infants, the AAP recommends that infants be fed one liter of formula daily, as formulas must contain 400 IU of vitamin D per liter.
A study looking at caregiver-reported dietary data through 2012 suggested that the guideline was having little impact, with only 27% of U.S. infants considered to be getting adequate vitamin D. The same researchers have now updated those findings with data through 2016 to report virtually no improvement over time. For their research, published in Pediatrics, Alan E. Simon, MD, of the National Institutes of Health in Rockville, Md., and Katherine A. Ahrens, PhD, of the University of Southern Maine in Portland, analyzed data for 1,435 infants aged 0-11 months. All data were recorded during 2009-2016 as part of the ongoing National Health and Nutrition Examination Survey (NHANES).
Overall, 27% of infants in the study were considered likely to meet the guidelines. Among nonbreastfeeding infants, 31% were deemed to have adequate levels, compared with 21% of breastfeeding infants (P less than .01).
Parents’ income and education affected infants’ likelihood of meeting guidelines. Breastfeeding infants in families with incomes above 400% of the federal poverty level were twice as likely to meet guidelines (31% vs. 14%-16% for lower income brackets, P less than .05). Babies from families whose head of household had a college degree had a 26% likelihood of having enough vitamin D, compared with less than 11% of those in whose parents had less than a high school education (P less than .05). Babies from families with private insurance also had a better chance of meeting guidelines, compared with those with public insurance (24% vs. 13%; P less than .05).
Ethnicity was seen as affecting vitamin D intake only insofar as some groups had more formula use than breastfeeding. The only ethnic or racial subgroup in the study that saw more than 40% of infants likely to meet guidelines was nonbreastfeeding infants of Asian, American Indian, Native Hawaiian or Pacific Islander, or multiracial parentage, with 46% considered to have adequate vitamin D levels. This group makes up 6% of the infant population in the United States.
“Reasons for low rates of meeting guidelines in the United States and little improvement over time are not fully known,” Dr. Simon and Dr. Ahrens wrote in their analysis. “One factor may be that the impact of low vitamin D in infancy is not highly visible to physicians because rickets is an uncommon diagnosis in the United States.” They noted that recent studies from Canada, where public health officials have done more to promote supplementation, have shown rates of adequate vitamin D in breastfeeding babies to be as high as 90%.
The researchers listed among limitations of their study the fact that the data source, NHANES, captured nutrition information only for the previous 24 hours; that it relied on parental report, and did not confirm serum levels of vitamin D; and that it was possible that cow’s milk – which is not recommended before age 1 but frequently given to older infants anyway – could be a hidden source of vitamin D that was not taken into consideration.
In an editorial comment, Jaspreet Loyal, MD, and Annette Cameron, MD, of Yale University in New Haven, Conn., faulted “a combination of inconsistent prescribing by clinicians and poor adherence to the use of a supplement by parents of infants … further complicated by a lack of awareness of the consequences of vitamin D deficiency in infants among the public” for the low adherence to guidelines in the United States, compared with other countries.
Also, the editorialists noted, the dropper used to administer liquid supplements has been associated with “inconsistent precision” and concerns about infants gagging on the liquid. More research is needed to better understand “prescribing patterns, barriers to adherence by parents of infants, and alternate strategies for vitamin D supplementation to inform novel public health programs in the United States,” they wrote.
The National Institutes of Health funded the study, and Dr. Ahrens is supported by a faculty development grant from the Maine Economic Improvement Fund. The researchers declared no conflicts of interest. Dr. Loyal and Dr. Cameron disclosed no funding and no relevant financial disclosures.
SOURCE: Simon AE and Ahrens KA. Pediatrics 2020 May. doi: 10.1542/peds.2019-3574; Loyal J and Cameron A. Pediatrics. 2020 May. doi: 10.1542/peds.2020-0504.
FROM PEDIATRICS
Progress report: Elimination of neonatal tetanus
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
FROM MMWR
AAP issues guidance on managing infants born to mothers with COVID-19
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
Reports suggest possible in utero transmission of novel coronavirus 2019
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
FROM JAMA