User login
In addiction, abusive partners can wreak havoc
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
Gender-based violence could be driver of opioid epidemic, expert suggests
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
REPORTING FROM AAAP 2019
Survey: Cancer-related pain, opioid use up since 2018
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Depression linked to persistent opioid use after hysterectomy
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Pediatricians uniquely qualified to treat adolescents with opioid use disorder
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Opioid reduction works after minimally invasive gynecologic surgery
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Fentanyl-related deaths show strong regional pattern
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.
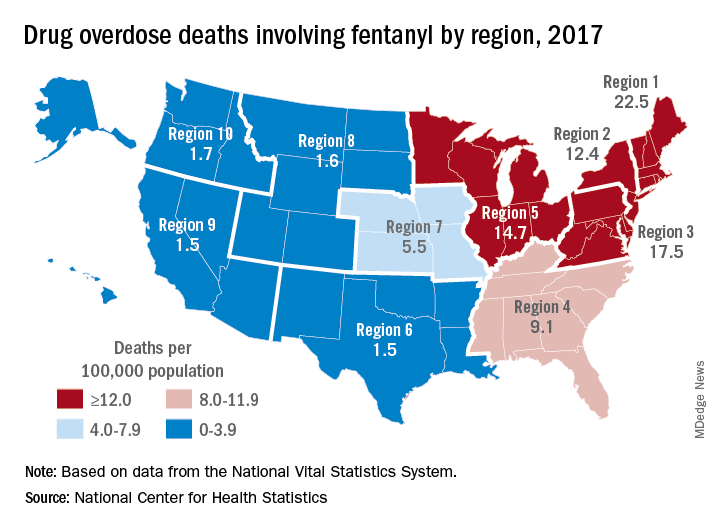
Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.

Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.

Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Cancer pain management inadequate in opioid-saturated areas
Patients with cancer who live in regions with high levels of opioid misuse may be undertreated for pain, according to investigators who studied opioid prescription patterns and cancer incidence in rural southwest Virginia.
Among 4,324 patients with cancer, only 22.16% were prescribed a Controlled Schedule II (C-II) prescription opioid medication at least 3 times in 1 year, from prescribers likely to be treating cancer pain. More than 60% of patients never received a C-II opioid prescription, reported Virginia T. LeBaron, PhD, of the University of Virginia School of Nursing in Charlottesville, and colleagues.
“A clearer view of geographic patterns and predictors of both POM [prescription opioid medication] prescribing and potential harms can inform targeted interventions and policy initiatives that achieve a balanced approach to POMs – ensuring access for patients in need while reducing risk to both patients and communities. Our research makes an important contribution by exploring how the current ‘opioid epidemic’ relates to rural patients with cancer,” they wrote. Their report is in Journal of Oncology Practice.
The investigators studied the confluence of disproportionately high cancer mortality rates and opioid fatality rates in rural southwest Virginia, in the heart of Appalachia.
They conducted a longitudinal, exploratory secondary analysis of data from the Commonwealth of Virginia All Payer Claims database to look at opioid prescribing patterns and explore whether concerns about opioid misuse could result in undertreatment of pain in cancer patients.
They looked at prescribing patterns at the patient, provider, and insurance claim levels, predictors of opioid prescription frequency, opioid-related harms and patterns related to opioid prescribing, cancer incidence, and fatalities.
They identified 4,324 patients with cancer, 958 of whom (22.16%) received a C-II opioid at least three times in any study year. The majority of patients were in the 45-64 age range, and approximately 88% were diagnosed with solid malignancies, with breast cancer and lung cancer being the most frequent diagnoses.
As noted, more than 60% of patients never received a C-II prescription.
“The large percentages of cancer patients never prescribed a C-II are concerning for a number of reasons, especially when we consider the results per year,” the investigators wrote. “First, the ‘no C-II’ patients remain over 80% of the total sample, each year, even after accounting for the upscheduling (from C-III to C-II) of commonly-prescribed hydrocodone products in 2014. Second, anecdotal data and emerging empirical evidence demonstrate that patients with legitimate pain needs, including patients with cancer, experience significant difficulty accessing POMs.”
They noted that regulations regarding opioid prescriptions have become increasingly strict since the end date of their analysis in 2015, suggesting that the number of patients with cancer who are not receiving C-II opioids today may be even higher.
They also pointed to evidence of prescription practices suggesting suboptimal pain management or potential patient harm, such as frequent prescription of opioid-acetaminophen combinations that are dose-limited due to acetaminophen toxicity; coprescription of opioids and benzodiazepines, which is not recommended under current prescribing guidelines; and infrequent use of deterrent formulations of C-II opioids such as crush-resistant tablets.
The study was supported by the University of Virginia Cancer Center, Cancer Control & Population Health Division and the Virginia Tobacco Region Revitalization Commission. The authors reported having no disclaimers or conflicts of interest.
SOURCE: LeBaron VT et al. J Oncol Pract. 2019 Nov. 4. doi: 10.1200/JOP.19.00149.
Patients with cancer who live in regions with high levels of opioid misuse may be undertreated for pain, according to investigators who studied opioid prescription patterns and cancer incidence in rural southwest Virginia.
Among 4,324 patients with cancer, only 22.16% were prescribed a Controlled Schedule II (C-II) prescription opioid medication at least 3 times in 1 year, from prescribers likely to be treating cancer pain. More than 60% of patients never received a C-II opioid prescription, reported Virginia T. LeBaron, PhD, of the University of Virginia School of Nursing in Charlottesville, and colleagues.
“A clearer view of geographic patterns and predictors of both POM [prescription opioid medication] prescribing and potential harms can inform targeted interventions and policy initiatives that achieve a balanced approach to POMs – ensuring access for patients in need while reducing risk to both patients and communities. Our research makes an important contribution by exploring how the current ‘opioid epidemic’ relates to rural patients with cancer,” they wrote. Their report is in Journal of Oncology Practice.
The investigators studied the confluence of disproportionately high cancer mortality rates and opioid fatality rates in rural southwest Virginia, in the heart of Appalachia.
They conducted a longitudinal, exploratory secondary analysis of data from the Commonwealth of Virginia All Payer Claims database to look at opioid prescribing patterns and explore whether concerns about opioid misuse could result in undertreatment of pain in cancer patients.
They looked at prescribing patterns at the patient, provider, and insurance claim levels, predictors of opioid prescription frequency, opioid-related harms and patterns related to opioid prescribing, cancer incidence, and fatalities.
They identified 4,324 patients with cancer, 958 of whom (22.16%) received a C-II opioid at least three times in any study year. The majority of patients were in the 45-64 age range, and approximately 88% were diagnosed with solid malignancies, with breast cancer and lung cancer being the most frequent diagnoses.
As noted, more than 60% of patients never received a C-II prescription.
“The large percentages of cancer patients never prescribed a C-II are concerning for a number of reasons, especially when we consider the results per year,” the investigators wrote. “First, the ‘no C-II’ patients remain over 80% of the total sample, each year, even after accounting for the upscheduling (from C-III to C-II) of commonly-prescribed hydrocodone products in 2014. Second, anecdotal data and emerging empirical evidence demonstrate that patients with legitimate pain needs, including patients with cancer, experience significant difficulty accessing POMs.”
They noted that regulations regarding opioid prescriptions have become increasingly strict since the end date of their analysis in 2015, suggesting that the number of patients with cancer who are not receiving C-II opioids today may be even higher.
They also pointed to evidence of prescription practices suggesting suboptimal pain management or potential patient harm, such as frequent prescription of opioid-acetaminophen combinations that are dose-limited due to acetaminophen toxicity; coprescription of opioids and benzodiazepines, which is not recommended under current prescribing guidelines; and infrequent use of deterrent formulations of C-II opioids such as crush-resistant tablets.
The study was supported by the University of Virginia Cancer Center, Cancer Control & Population Health Division and the Virginia Tobacco Region Revitalization Commission. The authors reported having no disclaimers or conflicts of interest.
SOURCE: LeBaron VT et al. J Oncol Pract. 2019 Nov. 4. doi: 10.1200/JOP.19.00149.
Patients with cancer who live in regions with high levels of opioid misuse may be undertreated for pain, according to investigators who studied opioid prescription patterns and cancer incidence in rural southwest Virginia.
Among 4,324 patients with cancer, only 22.16% were prescribed a Controlled Schedule II (C-II) prescription opioid medication at least 3 times in 1 year, from prescribers likely to be treating cancer pain. More than 60% of patients never received a C-II opioid prescription, reported Virginia T. LeBaron, PhD, of the University of Virginia School of Nursing in Charlottesville, and colleagues.
“A clearer view of geographic patterns and predictors of both POM [prescription opioid medication] prescribing and potential harms can inform targeted interventions and policy initiatives that achieve a balanced approach to POMs – ensuring access for patients in need while reducing risk to both patients and communities. Our research makes an important contribution by exploring how the current ‘opioid epidemic’ relates to rural patients with cancer,” they wrote. Their report is in Journal of Oncology Practice.
The investigators studied the confluence of disproportionately high cancer mortality rates and opioid fatality rates in rural southwest Virginia, in the heart of Appalachia.
They conducted a longitudinal, exploratory secondary analysis of data from the Commonwealth of Virginia All Payer Claims database to look at opioid prescribing patterns and explore whether concerns about opioid misuse could result in undertreatment of pain in cancer patients.
They looked at prescribing patterns at the patient, provider, and insurance claim levels, predictors of opioid prescription frequency, opioid-related harms and patterns related to opioid prescribing, cancer incidence, and fatalities.
They identified 4,324 patients with cancer, 958 of whom (22.16%) received a C-II opioid at least three times in any study year. The majority of patients were in the 45-64 age range, and approximately 88% were diagnosed with solid malignancies, with breast cancer and lung cancer being the most frequent diagnoses.
As noted, more than 60% of patients never received a C-II prescription.
“The large percentages of cancer patients never prescribed a C-II are concerning for a number of reasons, especially when we consider the results per year,” the investigators wrote. “First, the ‘no C-II’ patients remain over 80% of the total sample, each year, even after accounting for the upscheduling (from C-III to C-II) of commonly-prescribed hydrocodone products in 2014. Second, anecdotal data and emerging empirical evidence demonstrate that patients with legitimate pain needs, including patients with cancer, experience significant difficulty accessing POMs.”
They noted that regulations regarding opioid prescriptions have become increasingly strict since the end date of their analysis in 2015, suggesting that the number of patients with cancer who are not receiving C-II opioids today may be even higher.
They also pointed to evidence of prescription practices suggesting suboptimal pain management or potential patient harm, such as frequent prescription of opioid-acetaminophen combinations that are dose-limited due to acetaminophen toxicity; coprescription of opioids and benzodiazepines, which is not recommended under current prescribing guidelines; and infrequent use of deterrent formulations of C-II opioids such as crush-resistant tablets.
The study was supported by the University of Virginia Cancer Center, Cancer Control & Population Health Division and the Virginia Tobacco Region Revitalization Commission. The authors reported having no disclaimers or conflicts of interest.
SOURCE: LeBaron VT et al. J Oncol Pract. 2019 Nov. 4. doi: 10.1200/JOP.19.00149.
FROM JOURNAL OF ONCOLOGY PRACTICE
Opioids, benzodiazepines carry greater risk of COPD-related hospitalization
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Clinical interventions for global drug use need updating
Public health approach requires greater emphasis on harms, benefits of substance use.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Public health approach requires greater emphasis on harms, benefits of substance use.
Public health approach requires greater emphasis on harms, benefits of substance use.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
FROM THE LANCET
Key clinical point: People with drug use disorders around the world need evidence-based and nonjudgmental clinical care.
Major finding: Many interventions aimed at reducing the harm of illicit drug use are not informed by evidence.
Study details: Series of four papers reviewing the evidence on cannabinoids, opioids, new psychoactive substances, and stimulants.
Disclosures: The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
Sources: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Drug crisis continues to evolve beyond opioids
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.
“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.