User login
How to use lofexidine for quick opioid withdrawal
SAN DIEGO – Lofexidine (Lucemyra), the new kid on the block in the United States for opioid withdrawal, can help patients get through the process in a few days, instead of a week or more, according to Thomas Kosten, MD, a psychiatry professor and director of the division of addictions at Baylor College of Medicine, Houston.
Lofexidine relieves symptom withdrawal and has significant advantages over clonidine, a similar drug, including easier dosing and no orthostatic hypertension.
In a video interview at the annual Psych Congress, Dr. Kosten went into the nuts and bolts of how to use lofexidine with buprenorphine and naltrexone – plus benzodiazepines when needed – to help people safely go through withdrawal and in just a few days.
Once chronic pain patients are off opioids, the next question is what to do for their pain. In a presentation before the interview, Dr. Kosten said he favors tricyclic antidepressants, especially desipramine because it has the fewest side effects. The effect size with tricyclic antidepressants is larger than with gabapentin and other options. They take a few weeks to kick in, however, so he’s thinking about a unique approach: using ketamine – either infusions or the new nasal spray esketamine (Spravato) – to tide people over in the meantime. It’s becoming well known that ketamine works amazingly fast for depression and suicidality, and there is emerging support that it might do the same for chronic pain. Dr. Kosten is a consultant for US Worldmeds, maker of lofexidine.
SAN DIEGO – Lofexidine (Lucemyra), the new kid on the block in the United States for opioid withdrawal, can help patients get through the process in a few days, instead of a week or more, according to Thomas Kosten, MD, a psychiatry professor and director of the division of addictions at Baylor College of Medicine, Houston.
Lofexidine relieves symptom withdrawal and has significant advantages over clonidine, a similar drug, including easier dosing and no orthostatic hypertension.
In a video interview at the annual Psych Congress, Dr. Kosten went into the nuts and bolts of how to use lofexidine with buprenorphine and naltrexone – plus benzodiazepines when needed – to help people safely go through withdrawal and in just a few days.
Once chronic pain patients are off opioids, the next question is what to do for their pain. In a presentation before the interview, Dr. Kosten said he favors tricyclic antidepressants, especially desipramine because it has the fewest side effects. The effect size with tricyclic antidepressants is larger than with gabapentin and other options. They take a few weeks to kick in, however, so he’s thinking about a unique approach: using ketamine – either infusions or the new nasal spray esketamine (Spravato) – to tide people over in the meantime. It’s becoming well known that ketamine works amazingly fast for depression and suicidality, and there is emerging support that it might do the same for chronic pain. Dr. Kosten is a consultant for US Worldmeds, maker of lofexidine.
SAN DIEGO – Lofexidine (Lucemyra), the new kid on the block in the United States for opioid withdrawal, can help patients get through the process in a few days, instead of a week or more, according to Thomas Kosten, MD, a psychiatry professor and director of the division of addictions at Baylor College of Medicine, Houston.
Lofexidine relieves symptom withdrawal and has significant advantages over clonidine, a similar drug, including easier dosing and no orthostatic hypertension.
In a video interview at the annual Psych Congress, Dr. Kosten went into the nuts and bolts of how to use lofexidine with buprenorphine and naltrexone – plus benzodiazepines when needed – to help people safely go through withdrawal and in just a few days.
Once chronic pain patients are off opioids, the next question is what to do for their pain. In a presentation before the interview, Dr. Kosten said he favors tricyclic antidepressants, especially desipramine because it has the fewest side effects. The effect size with tricyclic antidepressants is larger than with gabapentin and other options. They take a few weeks to kick in, however, so he’s thinking about a unique approach: using ketamine – either infusions or the new nasal spray esketamine (Spravato) – to tide people over in the meantime. It’s becoming well known that ketamine works amazingly fast for depression and suicidality, and there is emerging support that it might do the same for chronic pain. Dr. Kosten is a consultant for US Worldmeds, maker of lofexidine.
REPORTING FROM PSYCH CONGRESS 2019
Buprenorphine merits more attention for treatment of opioid use disorder
SAN DIEGO – Prescribing buprenorphine for the treatment of opioid use disorder requires strict discernment on the part of clinicians, Arwen Podesta, MD, said at the annual Psych Congress.
She encouraged clinicians to be prepared for a visit from the Drug Enforcement Administration, understand the unique properties of buprenorphine, and make sure that patients grasp the importance of sublingual administration.
Research shows that only 5% of physicians are allowed to prescribe buprenorphine – an opioid – by way of a DEA waiver, Dr. Podesta said. About half do not prescribe the drug. Barriers to prescribing buprenorphine include factors such as low reimbursement and untrained support staff, said Dr. Podesta, a board-certified psychiatrist who subspecializes in addiction medicine and practices in New Orleans.
But she noted that the Substance Abuse and Mental Health Services Administration has recommended that medication-assisted therapy (MAT) – methadone, buprenorphine, and naltrexone – be considered in all patients with opioid use disorder. The drugs are safe and effective when used correctly, the federal agency has said.
Remember, Dr. Podesta said, that “patients taking MAT are considered to be in recovery.” In the big picture, she added, “we have to improve access to care because we have so many people who don’t have access to treatment.”
Getting permission from the DEA to prescribe buprenorphine – a schedule III controlled substance – comes with a price, Dr. Podesta said. “We have special scrutiny from the DEA,” she said. They come in and want to see your records. It sounds very punitive, although it’s their jobs.”
The best approach is to document that you know what you’re doing, she said. “It’s your job to educate them about why you’re using buprenorphine and produce the records to show that.”
Being aware of buprenorphine’s unique properties is important, she said. The drug is safer on the overdose front than are other opioids, Dr. Podesta said, but it can be very dangerous in patients without opioid tolerance. According to the DEA, as an analgesic, buprenorphine is 20-30 times more potent than morphine. Also, like morphine, patients who take buprenorphine are likely to experience euphoria, papillary restriction, and respiratory depression and sedation.
The buprenorphine/naloxone formulation is preferred to treat opioid use disorder, she noted.
The reason that naloxone, which treats opioid overdoses, is part of the drug combo is because as an add-on, it reduces the risk that buprenorphine will be crushed and snorted for an opioid high, she said. Those who take the combo drug via that method could end up with sudden and nasty withdrawal symptoms.
When the drug combo is administered sublingually, the idea is that the “good stuff” (buprenorphine) is absorbed in the mouth, while the “bad stuff” (naloxone) is harmlessly absorbed in the gut, Dr. Podesta said. This happens because the drugs are absorbed differently.
But patients can mistakenly trigger symptoms of withdrawal if, for example, they put the combo drug on their tongue and then go to sleep. “That’s a peril,” she said, and it’s important to make sure patients know what to do – and what not to do.
Dr. Podesta emphasized the importance of choosing language related to patients with addictions carefully and respectfully.
“We have stigma,” she said. “We have been saying that patients are ‘dirty’ or ‘clean,’ and if they’re ‘clean,’ they’re the opposite of ‘dirty.’
She also suggested that clinicians drop the use of the word “contract” to describe treatment agreements between patients and clinicians. “Call it an ‘agreement,’ ” she said. “It seems more mutual and less punitive or risky for the patient to sign, especially when they’re in a precarious comfort zone.”
And consider that even the words “substance abuse” can be misleading, she said. “Many [patients] are taking the medications that the doctor prescribed and following instructions to the letter.”
Dr. Podesta disclosed consulting with Kaleo, Pear Therapeutics, and JayMac, and serving on the speakers bureau of Alkermes, Orexo, and US WorldMeds. She is the author of “Hooked: A Concise Guide to the Underlying Mechanics of Addiction and Treatment for Patients, Families, and Providers” (Dog Ear Publishing, 2016).
SAN DIEGO – Prescribing buprenorphine for the treatment of opioid use disorder requires strict discernment on the part of clinicians, Arwen Podesta, MD, said at the annual Psych Congress.
She encouraged clinicians to be prepared for a visit from the Drug Enforcement Administration, understand the unique properties of buprenorphine, and make sure that patients grasp the importance of sublingual administration.
Research shows that only 5% of physicians are allowed to prescribe buprenorphine – an opioid – by way of a DEA waiver, Dr. Podesta said. About half do not prescribe the drug. Barriers to prescribing buprenorphine include factors such as low reimbursement and untrained support staff, said Dr. Podesta, a board-certified psychiatrist who subspecializes in addiction medicine and practices in New Orleans.
But she noted that the Substance Abuse and Mental Health Services Administration has recommended that medication-assisted therapy (MAT) – methadone, buprenorphine, and naltrexone – be considered in all patients with opioid use disorder. The drugs are safe and effective when used correctly, the federal agency has said.
Remember, Dr. Podesta said, that “patients taking MAT are considered to be in recovery.” In the big picture, she added, “we have to improve access to care because we have so many people who don’t have access to treatment.”
Getting permission from the DEA to prescribe buprenorphine – a schedule III controlled substance – comes with a price, Dr. Podesta said. “We have special scrutiny from the DEA,” she said. They come in and want to see your records. It sounds very punitive, although it’s their jobs.”
The best approach is to document that you know what you’re doing, she said. “It’s your job to educate them about why you’re using buprenorphine and produce the records to show that.”
Being aware of buprenorphine’s unique properties is important, she said. The drug is safer on the overdose front than are other opioids, Dr. Podesta said, but it can be very dangerous in patients without opioid tolerance. According to the DEA, as an analgesic, buprenorphine is 20-30 times more potent than morphine. Also, like morphine, patients who take buprenorphine are likely to experience euphoria, papillary restriction, and respiratory depression and sedation.
The buprenorphine/naloxone formulation is preferred to treat opioid use disorder, she noted.
The reason that naloxone, which treats opioid overdoses, is part of the drug combo is because as an add-on, it reduces the risk that buprenorphine will be crushed and snorted for an opioid high, she said. Those who take the combo drug via that method could end up with sudden and nasty withdrawal symptoms.
When the drug combo is administered sublingually, the idea is that the “good stuff” (buprenorphine) is absorbed in the mouth, while the “bad stuff” (naloxone) is harmlessly absorbed in the gut, Dr. Podesta said. This happens because the drugs are absorbed differently.
But patients can mistakenly trigger symptoms of withdrawal if, for example, they put the combo drug on their tongue and then go to sleep. “That’s a peril,” she said, and it’s important to make sure patients know what to do – and what not to do.
Dr. Podesta emphasized the importance of choosing language related to patients with addictions carefully and respectfully.
“We have stigma,” she said. “We have been saying that patients are ‘dirty’ or ‘clean,’ and if they’re ‘clean,’ they’re the opposite of ‘dirty.’
She also suggested that clinicians drop the use of the word “contract” to describe treatment agreements between patients and clinicians. “Call it an ‘agreement,’ ” she said. “It seems more mutual and less punitive or risky for the patient to sign, especially when they’re in a precarious comfort zone.”
And consider that even the words “substance abuse” can be misleading, she said. “Many [patients] are taking the medications that the doctor prescribed and following instructions to the letter.”
Dr. Podesta disclosed consulting with Kaleo, Pear Therapeutics, and JayMac, and serving on the speakers bureau of Alkermes, Orexo, and US WorldMeds. She is the author of “Hooked: A Concise Guide to the Underlying Mechanics of Addiction and Treatment for Patients, Families, and Providers” (Dog Ear Publishing, 2016).
SAN DIEGO – Prescribing buprenorphine for the treatment of opioid use disorder requires strict discernment on the part of clinicians, Arwen Podesta, MD, said at the annual Psych Congress.
She encouraged clinicians to be prepared for a visit from the Drug Enforcement Administration, understand the unique properties of buprenorphine, and make sure that patients grasp the importance of sublingual administration.
Research shows that only 5% of physicians are allowed to prescribe buprenorphine – an opioid – by way of a DEA waiver, Dr. Podesta said. About half do not prescribe the drug. Barriers to prescribing buprenorphine include factors such as low reimbursement and untrained support staff, said Dr. Podesta, a board-certified psychiatrist who subspecializes in addiction medicine and practices in New Orleans.
But she noted that the Substance Abuse and Mental Health Services Administration has recommended that medication-assisted therapy (MAT) – methadone, buprenorphine, and naltrexone – be considered in all patients with opioid use disorder. The drugs are safe and effective when used correctly, the federal agency has said.
Remember, Dr. Podesta said, that “patients taking MAT are considered to be in recovery.” In the big picture, she added, “we have to improve access to care because we have so many people who don’t have access to treatment.”
Getting permission from the DEA to prescribe buprenorphine – a schedule III controlled substance – comes with a price, Dr. Podesta said. “We have special scrutiny from the DEA,” she said. They come in and want to see your records. It sounds very punitive, although it’s their jobs.”
The best approach is to document that you know what you’re doing, she said. “It’s your job to educate them about why you’re using buprenorphine and produce the records to show that.”
Being aware of buprenorphine’s unique properties is important, she said. The drug is safer on the overdose front than are other opioids, Dr. Podesta said, but it can be very dangerous in patients without opioid tolerance. According to the DEA, as an analgesic, buprenorphine is 20-30 times more potent than morphine. Also, like morphine, patients who take buprenorphine are likely to experience euphoria, papillary restriction, and respiratory depression and sedation.
The buprenorphine/naloxone formulation is preferred to treat opioid use disorder, she noted.
The reason that naloxone, which treats opioid overdoses, is part of the drug combo is because as an add-on, it reduces the risk that buprenorphine will be crushed and snorted for an opioid high, she said. Those who take the combo drug via that method could end up with sudden and nasty withdrawal symptoms.
When the drug combo is administered sublingually, the idea is that the “good stuff” (buprenorphine) is absorbed in the mouth, while the “bad stuff” (naloxone) is harmlessly absorbed in the gut, Dr. Podesta said. This happens because the drugs are absorbed differently.
But patients can mistakenly trigger symptoms of withdrawal if, for example, they put the combo drug on their tongue and then go to sleep. “That’s a peril,” she said, and it’s important to make sure patients know what to do – and what not to do.
Dr. Podesta emphasized the importance of choosing language related to patients with addictions carefully and respectfully.
“We have stigma,” she said. “We have been saying that patients are ‘dirty’ or ‘clean,’ and if they’re ‘clean,’ they’re the opposite of ‘dirty.’
She also suggested that clinicians drop the use of the word “contract” to describe treatment agreements between patients and clinicians. “Call it an ‘agreement,’ ” she said. “It seems more mutual and less punitive or risky for the patient to sign, especially when they’re in a precarious comfort zone.”
And consider that even the words “substance abuse” can be misleading, she said. “Many [patients] are taking the medications that the doctor prescribed and following instructions to the letter.”
Dr. Podesta disclosed consulting with Kaleo, Pear Therapeutics, and JayMac, and serving on the speakers bureau of Alkermes, Orexo, and US WorldMeds. She is the author of “Hooked: A Concise Guide to the Underlying Mechanics of Addiction and Treatment for Patients, Families, and Providers” (Dog Ear Publishing, 2016).
REPORTING FROM PSYCH CONGRESS 2019
Long-term opioid use more common in hidradenitis suppurativa
, in a retrospective cohort study.
“These results suggest that periodic assessment of pain and screening for long-term opioid use may be warranted, particularly among patients who are older, who smoke tobacco, or who have depression and other medical comorbidities,” wrote the authors of the study (JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610).
Researchers led by Sarah Reddy, BA, of the Zucker School of Medicine at Hofstra/ Northwell, New Hyde Park, N.Y., used data from a health-care database that represents an estimated 17% of the U.S. population. They focused on opioid-naive adults who were in the database for at least 3 years from 2008-2018 and monitored whether they began opioid use and then maintained use for at least 1 year.
Nearly 829,000 patients were in the control group, and 22,277 were in the HS group. The mean age of those with HS was 41 years, 76% were women, and 59% were white.
Over 1 year, the crude incidence of long-term opioid use among HS patients who were opioid naive was 0.33%, compared with 0.14% of controls (P less than .001).
An analysis, adjusted for potential confounding factors, found that compared with controls, those with HS were more likely to develop long-term opioid use (odds ratio [OR], 1.53, 95% confidence interval, 1.20-1.95; P less than .001). In the adjusted analysis, long-term opioid use was increased among those in the HS group who had ever smoked tobacco (OR, 3.64, 95% CI, 2.06-6.41; P less than .001), compared with patients with HS who had never smoked; and those who had a history of depression (OR, 1.97, 95% CI, 1.21-3.19; P = .006), compared with HS patients who had not had depression.
The risk of long-term opioid use among those with HS increased by 2% with each additional year in age.
In addition, 5% of patients with HS and long-term opioid use were diagnosed with opioid use disorder over the study period. “Sex, race/ethnicity, disease duration, established dermatologic care, alcohol abuse, and nonopioid substance abuse were not associated with increased risk of long-term opioid use among patients with HS,” the authors wrote.
Emphasizing that these results “should not further stigmatize” people with HS, they said, “our hope is that the medical community, including dermatologists, will further embrace and engage in an integrated care plan that comprehensively supports the needs of patients with HS, including pain management.”
Future research, they added, “should include evaluating the association between disease severity and risk of opioid use, the role of disease-modifying therapies in reducing opioid use, and the development of effective and appropriate multimodal pain management strategies for HS.”
An educational grant to a study author from AbbVie partially funded the study. No other study funding was reported. Ms. Reddy had no disclosures; one author disclosed having received grants and personal fees from AbbVie and UCB during the study.
SOURCE: Reddy S et al. JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610.
, in a retrospective cohort study.
“These results suggest that periodic assessment of pain and screening for long-term opioid use may be warranted, particularly among patients who are older, who smoke tobacco, or who have depression and other medical comorbidities,” wrote the authors of the study (JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610).
Researchers led by Sarah Reddy, BA, of the Zucker School of Medicine at Hofstra/ Northwell, New Hyde Park, N.Y., used data from a health-care database that represents an estimated 17% of the U.S. population. They focused on opioid-naive adults who were in the database for at least 3 years from 2008-2018 and monitored whether they began opioid use and then maintained use for at least 1 year.
Nearly 829,000 patients were in the control group, and 22,277 were in the HS group. The mean age of those with HS was 41 years, 76% were women, and 59% were white.
Over 1 year, the crude incidence of long-term opioid use among HS patients who were opioid naive was 0.33%, compared with 0.14% of controls (P less than .001).
An analysis, adjusted for potential confounding factors, found that compared with controls, those with HS were more likely to develop long-term opioid use (odds ratio [OR], 1.53, 95% confidence interval, 1.20-1.95; P less than .001). In the adjusted analysis, long-term opioid use was increased among those in the HS group who had ever smoked tobacco (OR, 3.64, 95% CI, 2.06-6.41; P less than .001), compared with patients with HS who had never smoked; and those who had a history of depression (OR, 1.97, 95% CI, 1.21-3.19; P = .006), compared with HS patients who had not had depression.
The risk of long-term opioid use among those with HS increased by 2% with each additional year in age.
In addition, 5% of patients with HS and long-term opioid use were diagnosed with opioid use disorder over the study period. “Sex, race/ethnicity, disease duration, established dermatologic care, alcohol abuse, and nonopioid substance abuse were not associated with increased risk of long-term opioid use among patients with HS,” the authors wrote.
Emphasizing that these results “should not further stigmatize” people with HS, they said, “our hope is that the medical community, including dermatologists, will further embrace and engage in an integrated care plan that comprehensively supports the needs of patients with HS, including pain management.”
Future research, they added, “should include evaluating the association between disease severity and risk of opioid use, the role of disease-modifying therapies in reducing opioid use, and the development of effective and appropriate multimodal pain management strategies for HS.”
An educational grant to a study author from AbbVie partially funded the study. No other study funding was reported. Ms. Reddy had no disclosures; one author disclosed having received grants and personal fees from AbbVie and UCB during the study.
SOURCE: Reddy S et al. JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610.
, in a retrospective cohort study.
“These results suggest that periodic assessment of pain and screening for long-term opioid use may be warranted, particularly among patients who are older, who smoke tobacco, or who have depression and other medical comorbidities,” wrote the authors of the study (JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610).
Researchers led by Sarah Reddy, BA, of the Zucker School of Medicine at Hofstra/ Northwell, New Hyde Park, N.Y., used data from a health-care database that represents an estimated 17% of the U.S. population. They focused on opioid-naive adults who were in the database for at least 3 years from 2008-2018 and monitored whether they began opioid use and then maintained use for at least 1 year.
Nearly 829,000 patients were in the control group, and 22,277 were in the HS group. The mean age of those with HS was 41 years, 76% were women, and 59% were white.
Over 1 year, the crude incidence of long-term opioid use among HS patients who were opioid naive was 0.33%, compared with 0.14% of controls (P less than .001).
An analysis, adjusted for potential confounding factors, found that compared with controls, those with HS were more likely to develop long-term opioid use (odds ratio [OR], 1.53, 95% confidence interval, 1.20-1.95; P less than .001). In the adjusted analysis, long-term opioid use was increased among those in the HS group who had ever smoked tobacco (OR, 3.64, 95% CI, 2.06-6.41; P less than .001), compared with patients with HS who had never smoked; and those who had a history of depression (OR, 1.97, 95% CI, 1.21-3.19; P = .006), compared with HS patients who had not had depression.
The risk of long-term opioid use among those with HS increased by 2% with each additional year in age.
In addition, 5% of patients with HS and long-term opioid use were diagnosed with opioid use disorder over the study period. “Sex, race/ethnicity, disease duration, established dermatologic care, alcohol abuse, and nonopioid substance abuse were not associated with increased risk of long-term opioid use among patients with HS,” the authors wrote.
Emphasizing that these results “should not further stigmatize” people with HS, they said, “our hope is that the medical community, including dermatologists, will further embrace and engage in an integrated care plan that comprehensively supports the needs of patients with HS, including pain management.”
Future research, they added, “should include evaluating the association between disease severity and risk of opioid use, the role of disease-modifying therapies in reducing opioid use, and the development of effective and appropriate multimodal pain management strategies for HS.”
An educational grant to a study author from AbbVie partially funded the study. No other study funding was reported. Ms. Reddy had no disclosures; one author disclosed having received grants and personal fees from AbbVie and UCB during the study.
SOURCE: Reddy S et al. JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610.
FROM JAMA DERMATOLOGY
Assessing decisional capacity in patients with substance use disorders
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Drug abuse–linked infective endocarditis spiking in U.S.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point:
Major finding: Incidence of drug abuse–associated IC increased from 48 cases/100,000 in 2002 to 79/100,000 in 2016.
Study details: A retrospective cohort study identifying about a more than 950,000 cases of IC from the National Inpatient Sample registry.
Disclosures: None.
Source: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
CDC, SAMHSA commit $1.8 billion to combat opioid crisis
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
Our EHRs have a drug problem
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
USPSTF draft guidance calls for drug use screening
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
FROM THE USPSTF
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).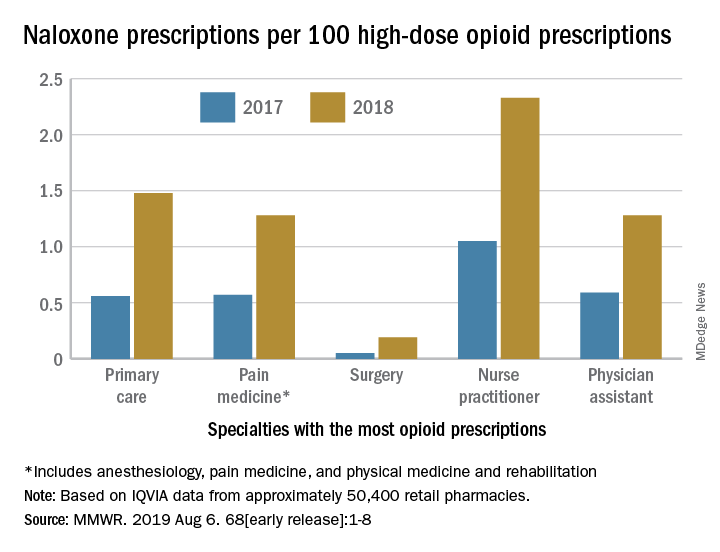
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Pharmacist stigma a barrier to rural buprenorphine access
SAN ANTONIO – Most attention paid to barriers for medication-assisted treatment of opioid use disorder has focused on prescribers and patients, but pharmacists are “a neglected link in the chain,” according to Hannah Cooper, ScD, an assistant professor of behavioral sciences and health education at Emory University, Atlanta.
“Pharmacy-based dispensing of buprenorphine is one of the medication’s major advances over methadone,” Dr. Cooper told attendees at the annual meeting of the College on Problems of Drug Dependence. Yet, early interviews she and her colleagues conducted with rural Kentucky pharmacist colleagues in the CARE2HOPE study “revealed that pharmacy-level barriers might also curtail access to buprenorphine.”
Little research has examined those barriers, but Dr. Cooper noted. Further, anecdotal evidence has suggested that wholesaler concerns about Drug Enforcement Administration restrictions on dispensing buprenorphine has caused shortages at pharmacies.
Dr. Cooper and colleagues, therefore, designed a qualitative study aimed at learning about pharmacists’ attitudes and dispensing practices related to buprenorphine. They also looked at whether DEA limits actually exist on dispensing the drug. They interviewed 14 pharmacists operating 15 pharmacies across all 12 counties in two rural Kentucky health districts. Eleven of the pharmacists worked in independent pharmacies; the others worked at chains. Six pharmacies dispensed more than 100 buprenorphine prescriptions a month, five dispensed only several dozen a month, and four refused to dispense it at all.
Perceptions of federal restrictions
“Variations in buprenorphine dispensing did not solely reflect underlying variations in local need or prescribing practices,” Dr. Cooper said. At 12 of the 15 pharmacies, limits on buprenorphine resulted from a perceived DEA “cap” on dispensing the drug or “because of distrust in buprenorphine itself, its prescribers and its patients.”
The perceived cap from the DEA was shrouded in uncertainty: 10 of the pharmacists said the DEA capped the percentage of controlled substances pharmacists could dispense that were opioids, yet the pharmacists did not know what that percentage was.
Five of those interviewed said the cap often significantly cut short how many buprenorphine prescriptions they would dispense. Since they did not know how much the cap was, they internally set arbitrary limits, such as dispensing two prescriptions per day, to avoid risk of the DEA investigating their pharmacy.
Yet, those limits could not meet patient demand, so several pharmacists rationed buprenorphine only to local residents or long-term customers, causing additional problems. That practice strained relationships with prescribers, who then had to call multiple pharmacies to find one that would dispense the drug to new patients. It also put pharmacy staff at risk when a rejection angered a customer and “undermined local recovery efforts,” Dr. Cooper said.
Five other pharmacists, however, did not ration their buprenorphine and did not worry about exceeding the DEA cap.
No numerical cap appears to exist, but DEA regulations and the SUPPORT for Patients and Communities Act do require internal opioid surveillance systems at wholesalers that flag suspicious orders of controlled substances, including buprenorphine. And they enforce it: An $80 million settlement in 2013 resulted from the DEA’s charge that Walgreens distribution centers did not report suspicious drug orders.
Stigma among some pharmacists
Six of the pharmacists had low trust in buprenorphine and in those who prescribed it and used it, Dr. Cooper reported. Three would not dispense the drug at all, and two would not take new buprenorphine patients.
One such pharmacist told researchers: “It is supposed to be the drug to help them [recover.] They want Suboxone worse than they do the hydrocodone. … It’s not what it’s designed to be.”
Those pharmacists also reported believing that malpractice was common among prescribers, who, for example, did not provide required counseling to patients or did not quickly wean them off buprenorphine. The pharmacists perceived the physicians prescribing buprenorphine as doing so only to make more money, just as they had done by prescribing opioids in the first place.
Those pharmacists also believed the patients themselves sold buprenorphine to make money and that opioid use disorder was a choice. They told researchers that dispensing buprenorphine would bring more drug users to their stores and subsequently hurt business.
Yet, those beliefs were not universal among the pharmacists. Eight believed buprenorphine was an appropriate opioid use disorder treatment and had positive attitudes toward patients. Unlike those who viewed the disorder as a choice, those pharmacists saw it as a disease and viewed the patients admirably for their commitment to recovery.
Though a small, qualitative study, those findings suggest a need to more closely examine how pharmacies affect access to medication to treat opioid use disorder, Dr. Cooper said.
“In an epicenter of the U.S. opioid epidemic, policies and stigma curtail access to buprenorphine,” she told attendees. “DEA regulations, the SUPPORT Act, and related lawsuits have led wholesalers to develop proprietary caps that force some pharmacists to ration the number of buprenorphine prescriptions they filled.” Some pharmacists will not dispense the drug at all, while others “limited dispensing to known or local patients and prescribers, a practice that pharmacists recognized hurt patients who had to travel far to reach prescribers.”
The research was funded by the National Institutes of Health through CARE2HOPE, Rural Health Project, and the Emory Center for AIDS Research. The authors reported no disclosures.
SAN ANTONIO – Most attention paid to barriers for medication-assisted treatment of opioid use disorder has focused on prescribers and patients, but pharmacists are “a neglected link in the chain,” according to Hannah Cooper, ScD, an assistant professor of behavioral sciences and health education at Emory University, Atlanta.
“Pharmacy-based dispensing of buprenorphine is one of the medication’s major advances over methadone,” Dr. Cooper told attendees at the annual meeting of the College on Problems of Drug Dependence. Yet, early interviews she and her colleagues conducted with rural Kentucky pharmacist colleagues in the CARE2HOPE study “revealed that pharmacy-level barriers might also curtail access to buprenorphine.”
Little research has examined those barriers, but Dr. Cooper noted. Further, anecdotal evidence has suggested that wholesaler concerns about Drug Enforcement Administration restrictions on dispensing buprenorphine has caused shortages at pharmacies.
Dr. Cooper and colleagues, therefore, designed a qualitative study aimed at learning about pharmacists’ attitudes and dispensing practices related to buprenorphine. They also looked at whether DEA limits actually exist on dispensing the drug. They interviewed 14 pharmacists operating 15 pharmacies across all 12 counties in two rural Kentucky health districts. Eleven of the pharmacists worked in independent pharmacies; the others worked at chains. Six pharmacies dispensed more than 100 buprenorphine prescriptions a month, five dispensed only several dozen a month, and four refused to dispense it at all.
Perceptions of federal restrictions
“Variations in buprenorphine dispensing did not solely reflect underlying variations in local need or prescribing practices,” Dr. Cooper said. At 12 of the 15 pharmacies, limits on buprenorphine resulted from a perceived DEA “cap” on dispensing the drug or “because of distrust in buprenorphine itself, its prescribers and its patients.”
The perceived cap from the DEA was shrouded in uncertainty: 10 of the pharmacists said the DEA capped the percentage of controlled substances pharmacists could dispense that were opioids, yet the pharmacists did not know what that percentage was.
Five of those interviewed said the cap often significantly cut short how many buprenorphine prescriptions they would dispense. Since they did not know how much the cap was, they internally set arbitrary limits, such as dispensing two prescriptions per day, to avoid risk of the DEA investigating their pharmacy.
Yet, those limits could not meet patient demand, so several pharmacists rationed buprenorphine only to local residents or long-term customers, causing additional problems. That practice strained relationships with prescribers, who then had to call multiple pharmacies to find one that would dispense the drug to new patients. It also put pharmacy staff at risk when a rejection angered a customer and “undermined local recovery efforts,” Dr. Cooper said.
Five other pharmacists, however, did not ration their buprenorphine and did not worry about exceeding the DEA cap.
No numerical cap appears to exist, but DEA regulations and the SUPPORT for Patients and Communities Act do require internal opioid surveillance systems at wholesalers that flag suspicious orders of controlled substances, including buprenorphine. And they enforce it: An $80 million settlement in 2013 resulted from the DEA’s charge that Walgreens distribution centers did not report suspicious drug orders.
Stigma among some pharmacists
Six of the pharmacists had low trust in buprenorphine and in those who prescribed it and used it, Dr. Cooper reported. Three would not dispense the drug at all, and two would not take new buprenorphine patients.
One such pharmacist told researchers: “It is supposed to be the drug to help them [recover.] They want Suboxone worse than they do the hydrocodone. … It’s not what it’s designed to be.”
Those pharmacists also reported believing that malpractice was common among prescribers, who, for example, did not provide required counseling to patients or did not quickly wean them off buprenorphine. The pharmacists perceived the physicians prescribing buprenorphine as doing so only to make more money, just as they had done by prescribing opioids in the first place.
Those pharmacists also believed the patients themselves sold buprenorphine to make money and that opioid use disorder was a choice. They told researchers that dispensing buprenorphine would bring more drug users to their stores and subsequently hurt business.
Yet, those beliefs were not universal among the pharmacists. Eight believed buprenorphine was an appropriate opioid use disorder treatment and had positive attitudes toward patients. Unlike those who viewed the disorder as a choice, those pharmacists saw it as a disease and viewed the patients admirably for their commitment to recovery.
Though a small, qualitative study, those findings suggest a need to more closely examine how pharmacies affect access to medication to treat opioid use disorder, Dr. Cooper said.
“In an epicenter of the U.S. opioid epidemic, policies and stigma curtail access to buprenorphine,” she told attendees. “DEA regulations, the SUPPORT Act, and related lawsuits have led wholesalers to develop proprietary caps that force some pharmacists to ration the number of buprenorphine prescriptions they filled.” Some pharmacists will not dispense the drug at all, while others “limited dispensing to known or local patients and prescribers, a practice that pharmacists recognized hurt patients who had to travel far to reach prescribers.”
The research was funded by the National Institutes of Health through CARE2HOPE, Rural Health Project, and the Emory Center for AIDS Research. The authors reported no disclosures.
SAN ANTONIO – Most attention paid to barriers for medication-assisted treatment of opioid use disorder has focused on prescribers and patients, but pharmacists are “a neglected link in the chain,” according to Hannah Cooper, ScD, an assistant professor of behavioral sciences and health education at Emory University, Atlanta.
“Pharmacy-based dispensing of buprenorphine is one of the medication’s major advances over methadone,” Dr. Cooper told attendees at the annual meeting of the College on Problems of Drug Dependence. Yet, early interviews she and her colleagues conducted with rural Kentucky pharmacist colleagues in the CARE2HOPE study “revealed that pharmacy-level barriers might also curtail access to buprenorphine.”
Little research has examined those barriers, but Dr. Cooper noted. Further, anecdotal evidence has suggested that wholesaler concerns about Drug Enforcement Administration restrictions on dispensing buprenorphine has caused shortages at pharmacies.
Dr. Cooper and colleagues, therefore, designed a qualitative study aimed at learning about pharmacists’ attitudes and dispensing practices related to buprenorphine. They also looked at whether DEA limits actually exist on dispensing the drug. They interviewed 14 pharmacists operating 15 pharmacies across all 12 counties in two rural Kentucky health districts. Eleven of the pharmacists worked in independent pharmacies; the others worked at chains. Six pharmacies dispensed more than 100 buprenorphine prescriptions a month, five dispensed only several dozen a month, and four refused to dispense it at all.
Perceptions of federal restrictions
“Variations in buprenorphine dispensing did not solely reflect underlying variations in local need or prescribing practices,” Dr. Cooper said. At 12 of the 15 pharmacies, limits on buprenorphine resulted from a perceived DEA “cap” on dispensing the drug or “because of distrust in buprenorphine itself, its prescribers and its patients.”
The perceived cap from the DEA was shrouded in uncertainty: 10 of the pharmacists said the DEA capped the percentage of controlled substances pharmacists could dispense that were opioids, yet the pharmacists did not know what that percentage was.
Five of those interviewed said the cap often significantly cut short how many buprenorphine prescriptions they would dispense. Since they did not know how much the cap was, they internally set arbitrary limits, such as dispensing two prescriptions per day, to avoid risk of the DEA investigating their pharmacy.
Yet, those limits could not meet patient demand, so several pharmacists rationed buprenorphine only to local residents or long-term customers, causing additional problems. That practice strained relationships with prescribers, who then had to call multiple pharmacies to find one that would dispense the drug to new patients. It also put pharmacy staff at risk when a rejection angered a customer and “undermined local recovery efforts,” Dr. Cooper said.
Five other pharmacists, however, did not ration their buprenorphine and did not worry about exceeding the DEA cap.
No numerical cap appears to exist, but DEA regulations and the SUPPORT for Patients and Communities Act do require internal opioid surveillance systems at wholesalers that flag suspicious orders of controlled substances, including buprenorphine. And they enforce it: An $80 million settlement in 2013 resulted from the DEA’s charge that Walgreens distribution centers did not report suspicious drug orders.
Stigma among some pharmacists
Six of the pharmacists had low trust in buprenorphine and in those who prescribed it and used it, Dr. Cooper reported. Three would not dispense the drug at all, and two would not take new buprenorphine patients.
One such pharmacist told researchers: “It is supposed to be the drug to help them [recover.] They want Suboxone worse than they do the hydrocodone. … It’s not what it’s designed to be.”
Those pharmacists also reported believing that malpractice was common among prescribers, who, for example, did not provide required counseling to patients or did not quickly wean them off buprenorphine. The pharmacists perceived the physicians prescribing buprenorphine as doing so only to make more money, just as they had done by prescribing opioids in the first place.
Those pharmacists also believed the patients themselves sold buprenorphine to make money and that opioid use disorder was a choice. They told researchers that dispensing buprenorphine would bring more drug users to their stores and subsequently hurt business.
Yet, those beliefs were not universal among the pharmacists. Eight believed buprenorphine was an appropriate opioid use disorder treatment and had positive attitudes toward patients. Unlike those who viewed the disorder as a choice, those pharmacists saw it as a disease and viewed the patients admirably for their commitment to recovery.
Though a small, qualitative study, those findings suggest a need to more closely examine how pharmacies affect access to medication to treat opioid use disorder, Dr. Cooper said.
“In an epicenter of the U.S. opioid epidemic, policies and stigma curtail access to buprenorphine,” she told attendees. “DEA regulations, the SUPPORT Act, and related lawsuits have led wholesalers to develop proprietary caps that force some pharmacists to ration the number of buprenorphine prescriptions they filled.” Some pharmacists will not dispense the drug at all, while others “limited dispensing to known or local patients and prescribers, a practice that pharmacists recognized hurt patients who had to travel far to reach prescribers.”
The research was funded by the National Institutes of Health through CARE2HOPE, Rural Health Project, and the Emory Center for AIDS Research. The authors reported no disclosures.
REPORTING FROM CPDD 2019










