User login
For OUD patients, ‘a lot of work to be done’
Most Americans who need medication-assisted treatment not getting it
LAS VEGAS – For Karen J. Hartwell, MD, few things in her clinical work bring more reward than providing medication-assisted treatment (MAT) to patients with opioid use disorder.
or to see a depression remit on your selection of an antidepressant,” she said at an annual psychopharmacology update held by the Nevada Psychiatric Association. “We know that medication-assisted treatment is underused and, sadly, relapse rates remain high.”
According to the Centers for Disease Control and Prevention, there were 70,237 drug-related overdose deaths in 2017 – 47,600 from prescription and illicit opioids. “This is being driven predominately by fentanyl and other high-potency synthetic opioids, followed by prescription opioids and heroin,” said Dr. Hartwell, an associate professor in the addiction sciences division in the department of psychiatry and behavioral sciences at the Medical University of South Carolina, Charleston.
There were an estimated 2 million Americans with an opioid use disorder (OUD) in 2018, she said, and more than 10 million misused prescription opioids. At the same time, prescriptions for opioids have dropped to lowest level in 10 years from a peak in 2012 of 81.3 prescriptions per 100 persons to 58.7 prescriptions per 100 persons in 2017 – total of more than 191 million scripts. “There is a decline in the number of opioid prescriptions, but there is still a lot of diversion, and there are some prescription ‘hot spots’ in the Southeast,” Dr. Hartwell said. “Heroin is a very low cost, and we’re wrestling with the issue of fentanyl.”
To complicate matters, most Americans with opioid use disorder are not in treatment. “In many people, the disorder is never diagnosed, and even fewer engage in care,” she said. “There are challenges with treatment retention, and even fewer achieve remission. There’s a lot of work to be done. One of which is the availability of medication-assisted treatment.”
Dr. Hartwell said that she knows of physician colleagues who have obtained a waiver to prescribe buprenorphine but have yet to prescribe it. “Some people may prefer to avoid the dance [of buprenorphine prescribing],” she said. “I’m here to advise you to dance.” Clinicians can learn about MAT waiver training opportunities by visiting the website of the Providers Clinical Support System, a program funded by the Substance Abuse and Mental Health Services Administration (SAMHSA).
Another option is to join a telementoring session on the topic facilitated by Project ECHO, or Extension for Community Healthcare Outcomes, which is being used by the University of New Mexico, Albuquerque. The goal of this model is to break down the walls between specialty and primary care by linking experts at an academic “hub” with primary care doctors and nurses in nearby communities.
“Our Project ECHO at the Medical University of South Carolina is twice a month on Fridays,” Dr. Hartwell said. “The first half is a case. The second half is a didactic [session], and you get a free hour of CME.”
The most common drugs used for medication-assisted treatment of opioid disorder are buprenorphine (a partial agonist), naltrexone (an antagonist), and methadone (a full agonist). Methadone retention generally is better than buprenorphine or naltrexone. The recommended treatment duration is 6-12 months, yet many studies demonstrate that many only stay on treatment for 30-60 days.
“You want to keep patients on treatment as long as they benefit from the medication,” Dr. Hartwell said. One large study of Medicaid claims data found that the risk of acute care service use and overdose were high following buprenorphine discontinuation, regardless of treatment duration. Superior outcomes became significant with treatment duration beyond 15 months, although rates of the primary adverse outcomes remained high (Am J Psychiatry. 2020 Feb 1;177[2]:117-24). About 5% of patients across all cohorts experienced one or more medically treated overdoses.
“One thing I don’t want is for people to drop out of treatment and not come back to see me,” Dr. Hartwell said. “This is a time for us to use our shared decision-making skills. I like to use the Tapering Readiness Inventory, a list of 16 questions. It asks such things as ‘Are you able to cope with difficult situations without using?’ and ‘Do you have all of the [drug] paraphernalia out of the house?’ We then have a discussion. If the patient decides to go ahead and do a taper, I always leave the door open. So, as that taper persists and someone says, ‘I’m starting to think about using, Doctor,’ I’ll put them back on [buprenorphine]. Or, if they come off the drug and they find themselves at risk of relapsing, they come back in and see me.”
There’s also some evidence that contingency management might be helpful, both in terms of opioid negative urines, and retention and treatment. Meanwhile, extended-release forms of buprenorphine are emerging.
In 2017, the Food and Drug Administration approved Sublocade, the first once-monthly injectable buprenorphine product for the treatment of moderate-to-severe OUD in adult patients who have initiated treatment with a transmucosal buprenorphine-containing product. “The recommendations are that you have about a 7-day lead-in of sublingual buprenorphine, and then 2 months of a 300-mg IV injection,” Dr. Hartwell said. “This is followed by either 100-mg injections monthly or 300-mg maintenance in select cases. There is some pain at the injection site. Some clinicians are getting around this by using a little bit of lidocaine prior to giving the injection.”
Another product, Brixadi, is an extended-release weekly (8 mg, 16 mg, 24 mg, 32 mg) and monthly (64 mg, 96 mg, 128 mg) buprenorphine injection used for the treatment of moderate to severe OUD. It is expected to be available in December 2020.
In 2016, the FDA approved Probuphine, the first buprenorphine implant for the maintenance treatment of opioid dependence. Probuphine is designed to provide a constant, low-level dose of buprenorphine for 6 months in patients who are already stable on low to moderate doses of other forms of buprenorphine, as part of a complete treatment program. “The 6-month duration kind of takes the issue of adherence off the table,” Dr. Hartwell said. “The caveat with this is that you have to be stable on 8 mg of buprenorphine per day or less. The majority of my patients require much higher doses.”
Dr. Hartwell reported having no relevant disclosures.
Most Americans who need medication-assisted treatment not getting it
Most Americans who need medication-assisted treatment not getting it
LAS VEGAS – For Karen J. Hartwell, MD, few things in her clinical work bring more reward than providing medication-assisted treatment (MAT) to patients with opioid use disorder.
or to see a depression remit on your selection of an antidepressant,” she said at an annual psychopharmacology update held by the Nevada Psychiatric Association. “We know that medication-assisted treatment is underused and, sadly, relapse rates remain high.”
According to the Centers for Disease Control and Prevention, there were 70,237 drug-related overdose deaths in 2017 – 47,600 from prescription and illicit opioids. “This is being driven predominately by fentanyl and other high-potency synthetic opioids, followed by prescription opioids and heroin,” said Dr. Hartwell, an associate professor in the addiction sciences division in the department of psychiatry and behavioral sciences at the Medical University of South Carolina, Charleston.
There were an estimated 2 million Americans with an opioid use disorder (OUD) in 2018, she said, and more than 10 million misused prescription opioids. At the same time, prescriptions for opioids have dropped to lowest level in 10 years from a peak in 2012 of 81.3 prescriptions per 100 persons to 58.7 prescriptions per 100 persons in 2017 – total of more than 191 million scripts. “There is a decline in the number of opioid prescriptions, but there is still a lot of diversion, and there are some prescription ‘hot spots’ in the Southeast,” Dr. Hartwell said. “Heroin is a very low cost, and we’re wrestling with the issue of fentanyl.”
To complicate matters, most Americans with opioid use disorder are not in treatment. “In many people, the disorder is never diagnosed, and even fewer engage in care,” she said. “There are challenges with treatment retention, and even fewer achieve remission. There’s a lot of work to be done. One of which is the availability of medication-assisted treatment.”
Dr. Hartwell said that she knows of physician colleagues who have obtained a waiver to prescribe buprenorphine but have yet to prescribe it. “Some people may prefer to avoid the dance [of buprenorphine prescribing],” she said. “I’m here to advise you to dance.” Clinicians can learn about MAT waiver training opportunities by visiting the website of the Providers Clinical Support System, a program funded by the Substance Abuse and Mental Health Services Administration (SAMHSA).
Another option is to join a telementoring session on the topic facilitated by Project ECHO, or Extension for Community Healthcare Outcomes, which is being used by the University of New Mexico, Albuquerque. The goal of this model is to break down the walls between specialty and primary care by linking experts at an academic “hub” with primary care doctors and nurses in nearby communities.
“Our Project ECHO at the Medical University of South Carolina is twice a month on Fridays,” Dr. Hartwell said. “The first half is a case. The second half is a didactic [session], and you get a free hour of CME.”
The most common drugs used for medication-assisted treatment of opioid disorder are buprenorphine (a partial agonist), naltrexone (an antagonist), and methadone (a full agonist). Methadone retention generally is better than buprenorphine or naltrexone. The recommended treatment duration is 6-12 months, yet many studies demonstrate that many only stay on treatment for 30-60 days.
“You want to keep patients on treatment as long as they benefit from the medication,” Dr. Hartwell said. One large study of Medicaid claims data found that the risk of acute care service use and overdose were high following buprenorphine discontinuation, regardless of treatment duration. Superior outcomes became significant with treatment duration beyond 15 months, although rates of the primary adverse outcomes remained high (Am J Psychiatry. 2020 Feb 1;177[2]:117-24). About 5% of patients across all cohorts experienced one or more medically treated overdoses.
“One thing I don’t want is for people to drop out of treatment and not come back to see me,” Dr. Hartwell said. “This is a time for us to use our shared decision-making skills. I like to use the Tapering Readiness Inventory, a list of 16 questions. It asks such things as ‘Are you able to cope with difficult situations without using?’ and ‘Do you have all of the [drug] paraphernalia out of the house?’ We then have a discussion. If the patient decides to go ahead and do a taper, I always leave the door open. So, as that taper persists and someone says, ‘I’m starting to think about using, Doctor,’ I’ll put them back on [buprenorphine]. Or, if they come off the drug and they find themselves at risk of relapsing, they come back in and see me.”
There’s also some evidence that contingency management might be helpful, both in terms of opioid negative urines, and retention and treatment. Meanwhile, extended-release forms of buprenorphine are emerging.
In 2017, the Food and Drug Administration approved Sublocade, the first once-monthly injectable buprenorphine product for the treatment of moderate-to-severe OUD in adult patients who have initiated treatment with a transmucosal buprenorphine-containing product. “The recommendations are that you have about a 7-day lead-in of sublingual buprenorphine, and then 2 months of a 300-mg IV injection,” Dr. Hartwell said. “This is followed by either 100-mg injections monthly or 300-mg maintenance in select cases. There is some pain at the injection site. Some clinicians are getting around this by using a little bit of lidocaine prior to giving the injection.”
Another product, Brixadi, is an extended-release weekly (8 mg, 16 mg, 24 mg, 32 mg) and monthly (64 mg, 96 mg, 128 mg) buprenorphine injection used for the treatment of moderate to severe OUD. It is expected to be available in December 2020.
In 2016, the FDA approved Probuphine, the first buprenorphine implant for the maintenance treatment of opioid dependence. Probuphine is designed to provide a constant, low-level dose of buprenorphine for 6 months in patients who are already stable on low to moderate doses of other forms of buprenorphine, as part of a complete treatment program. “The 6-month duration kind of takes the issue of adherence off the table,” Dr. Hartwell said. “The caveat with this is that you have to be stable on 8 mg of buprenorphine per day or less. The majority of my patients require much higher doses.”
Dr. Hartwell reported having no relevant disclosures.
LAS VEGAS – For Karen J. Hartwell, MD, few things in her clinical work bring more reward than providing medication-assisted treatment (MAT) to patients with opioid use disorder.
or to see a depression remit on your selection of an antidepressant,” she said at an annual psychopharmacology update held by the Nevada Psychiatric Association. “We know that medication-assisted treatment is underused and, sadly, relapse rates remain high.”
According to the Centers for Disease Control and Prevention, there were 70,237 drug-related overdose deaths in 2017 – 47,600 from prescription and illicit opioids. “This is being driven predominately by fentanyl and other high-potency synthetic opioids, followed by prescription opioids and heroin,” said Dr. Hartwell, an associate professor in the addiction sciences division in the department of psychiatry and behavioral sciences at the Medical University of South Carolina, Charleston.
There were an estimated 2 million Americans with an opioid use disorder (OUD) in 2018, she said, and more than 10 million misused prescription opioids. At the same time, prescriptions for opioids have dropped to lowest level in 10 years from a peak in 2012 of 81.3 prescriptions per 100 persons to 58.7 prescriptions per 100 persons in 2017 – total of more than 191 million scripts. “There is a decline in the number of opioid prescriptions, but there is still a lot of diversion, and there are some prescription ‘hot spots’ in the Southeast,” Dr. Hartwell said. “Heroin is a very low cost, and we’re wrestling with the issue of fentanyl.”
To complicate matters, most Americans with opioid use disorder are not in treatment. “In many people, the disorder is never diagnosed, and even fewer engage in care,” she said. “There are challenges with treatment retention, and even fewer achieve remission. There’s a lot of work to be done. One of which is the availability of medication-assisted treatment.”
Dr. Hartwell said that she knows of physician colleagues who have obtained a waiver to prescribe buprenorphine but have yet to prescribe it. “Some people may prefer to avoid the dance [of buprenorphine prescribing],” she said. “I’m here to advise you to dance.” Clinicians can learn about MAT waiver training opportunities by visiting the website of the Providers Clinical Support System, a program funded by the Substance Abuse and Mental Health Services Administration (SAMHSA).
Another option is to join a telementoring session on the topic facilitated by Project ECHO, or Extension for Community Healthcare Outcomes, which is being used by the University of New Mexico, Albuquerque. The goal of this model is to break down the walls between specialty and primary care by linking experts at an academic “hub” with primary care doctors and nurses in nearby communities.
“Our Project ECHO at the Medical University of South Carolina is twice a month on Fridays,” Dr. Hartwell said. “The first half is a case. The second half is a didactic [session], and you get a free hour of CME.”
The most common drugs used for medication-assisted treatment of opioid disorder are buprenorphine (a partial agonist), naltrexone (an antagonist), and methadone (a full agonist). Methadone retention generally is better than buprenorphine or naltrexone. The recommended treatment duration is 6-12 months, yet many studies demonstrate that many only stay on treatment for 30-60 days.
“You want to keep patients on treatment as long as they benefit from the medication,” Dr. Hartwell said. One large study of Medicaid claims data found that the risk of acute care service use and overdose were high following buprenorphine discontinuation, regardless of treatment duration. Superior outcomes became significant with treatment duration beyond 15 months, although rates of the primary adverse outcomes remained high (Am J Psychiatry. 2020 Feb 1;177[2]:117-24). About 5% of patients across all cohorts experienced one or more medically treated overdoses.
“One thing I don’t want is for people to drop out of treatment and not come back to see me,” Dr. Hartwell said. “This is a time for us to use our shared decision-making skills. I like to use the Tapering Readiness Inventory, a list of 16 questions. It asks such things as ‘Are you able to cope with difficult situations without using?’ and ‘Do you have all of the [drug] paraphernalia out of the house?’ We then have a discussion. If the patient decides to go ahead and do a taper, I always leave the door open. So, as that taper persists and someone says, ‘I’m starting to think about using, Doctor,’ I’ll put them back on [buprenorphine]. Or, if they come off the drug and they find themselves at risk of relapsing, they come back in and see me.”
There’s also some evidence that contingency management might be helpful, both in terms of opioid negative urines, and retention and treatment. Meanwhile, extended-release forms of buprenorphine are emerging.
In 2017, the Food and Drug Administration approved Sublocade, the first once-monthly injectable buprenorphine product for the treatment of moderate-to-severe OUD in adult patients who have initiated treatment with a transmucosal buprenorphine-containing product. “The recommendations are that you have about a 7-day lead-in of sublingual buprenorphine, and then 2 months of a 300-mg IV injection,” Dr. Hartwell said. “This is followed by either 100-mg injections monthly or 300-mg maintenance in select cases. There is some pain at the injection site. Some clinicians are getting around this by using a little bit of lidocaine prior to giving the injection.”
Another product, Brixadi, is an extended-release weekly (8 mg, 16 mg, 24 mg, 32 mg) and monthly (64 mg, 96 mg, 128 mg) buprenorphine injection used for the treatment of moderate to severe OUD. It is expected to be available in December 2020.
In 2016, the FDA approved Probuphine, the first buprenorphine implant for the maintenance treatment of opioid dependence. Probuphine is designed to provide a constant, low-level dose of buprenorphine for 6 months in patients who are already stable on low to moderate doses of other forms of buprenorphine, as part of a complete treatment program. “The 6-month duration kind of takes the issue of adherence off the table,” Dr. Hartwell said. “The caveat with this is that you have to be stable on 8 mg of buprenorphine per day or less. The majority of my patients require much higher doses.”
Dr. Hartwell reported having no relevant disclosures.
REPORTING FROM NPA 2020
U.S. heroin use: Good news, bad news?
U.S. rates of heroin use, heroin use disorder, and heroin injections all increased overall among adults during a recent 17-year period, but rates have plateaued, new research shows.
Although on the face of it this may seem like good news, investigators at the Substance Abuse and Mental Health Services Administration (SAMHSA) note that the plateau in heroin use may simply reflect a switch to fentanyl.
“The recent leveling off of heroin use might reflect shifts from heroin to illicit fentanyl-related compounds,” wrote the investigators, led by Beth Han, MD, PhD, MPH.
The study was published online Feb. 11 as a research letter in JAMA (2020;323[6]:568-71).
National data
For the study, researchers collected data from a nationally representative group of adults aged 18 years or older who participated in the 2002-2018 National Survey on Drug Use and Health (NSDUH).
The analysis included 800,500 respondents during the study period. The mean age of respondents was 34.5 years, and 53.2% were women.
Results showed that the reported past-year prevalence of heroin use increased from 0.17% in 2002 to 0.32% in 2018 (average annual percentage change [AAPC], 5.6; 95% confidence interval [CI], 1.0-10.5; P = .02). During 2002-2016, the APC was 7.6 (95% CI, 6.3-9.0; P less than .001) but then plateaued during 2016-2018 (APC, –7.1; 95% CI, –36.9 to 36.7; P = .69).
The prevalence of heroin use disorder increased from 0.10% in 2002 to 0.21% in 2018 (AAPC, 6.0; 95% CI, 3.2-8.8; P less than .001). The rate remained stable during 2002-2008, increased during 2008-2015, then plateaued during 2015-2018.
The prevalence of heroin injections increased from 0.09% in 2002 to 0.17% in 2018 (AAPC, 6.9; 95% CI, 5.7-8.0; P less than .001), although there was a dip from the previous year. This rate increased during the study period among both men and women, those aged 35-49 years, non-Hispanic whites, and those residing in the Northeast or West regions.
For individuals up to age 25 years and those living in the Midwest, the heroin injection rate stopped increasing and plateaued, but there was an overall increase during the study period.
In 2018, the rate of past-year heroin injection was highest in those in the Northeast, those up to age 49 years, men, and non-Hispanic whites.
More infectious disease testing
Prevalence of heroin injection did not increase among adults who used heroin or who had heroin use disorder. This, the researchers note, “suggests that increases in heroin injection are related to overall increases in heroin use rather than increases in the propensity to inject.”
Future research should examine differences in heroin injection trends across subgroups, the authors wrote.
The researchers advocate for expanding HIV and hepatitis testing and treatment, the provision of sterile syringes, and use of Food and Drug Administration–approved medications for opioid use disorders, particularly among populations at greatest risk – adults in the Northeast, those aged 18-49 years, men, and non-Hispanic whites.
“In parallel, interventions to prevent opioid misuse and opioid use disorder are needed to avert further increases in injection drug use,” they noted.
A limitation of the study was that the NSDUH excludes jail and prison populations and homeless people not in living shelters. In addition, the NSDUH is subject to recall bias.
The study was jointly sponsored by SAMHSA and the National Institute on Drug Abuse of the National Institutes of Health. One author reports owning stock in General Electric Co, 3M Co, and Pfizer Inc.
A version of this article first appeared on Medscape.com.
U.S. rates of heroin use, heroin use disorder, and heroin injections all increased overall among adults during a recent 17-year period, but rates have plateaued, new research shows.
Although on the face of it this may seem like good news, investigators at the Substance Abuse and Mental Health Services Administration (SAMHSA) note that the plateau in heroin use may simply reflect a switch to fentanyl.
“The recent leveling off of heroin use might reflect shifts from heroin to illicit fentanyl-related compounds,” wrote the investigators, led by Beth Han, MD, PhD, MPH.
The study was published online Feb. 11 as a research letter in JAMA (2020;323[6]:568-71).
National data
For the study, researchers collected data from a nationally representative group of adults aged 18 years or older who participated in the 2002-2018 National Survey on Drug Use and Health (NSDUH).
The analysis included 800,500 respondents during the study period. The mean age of respondents was 34.5 years, and 53.2% were women.
Results showed that the reported past-year prevalence of heroin use increased from 0.17% in 2002 to 0.32% in 2018 (average annual percentage change [AAPC], 5.6; 95% confidence interval [CI], 1.0-10.5; P = .02). During 2002-2016, the APC was 7.6 (95% CI, 6.3-9.0; P less than .001) but then plateaued during 2016-2018 (APC, –7.1; 95% CI, –36.9 to 36.7; P = .69).
The prevalence of heroin use disorder increased from 0.10% in 2002 to 0.21% in 2018 (AAPC, 6.0; 95% CI, 3.2-8.8; P less than .001). The rate remained stable during 2002-2008, increased during 2008-2015, then plateaued during 2015-2018.
The prevalence of heroin injections increased from 0.09% in 2002 to 0.17% in 2018 (AAPC, 6.9; 95% CI, 5.7-8.0; P less than .001), although there was a dip from the previous year. This rate increased during the study period among both men and women, those aged 35-49 years, non-Hispanic whites, and those residing in the Northeast or West regions.
For individuals up to age 25 years and those living in the Midwest, the heroin injection rate stopped increasing and plateaued, but there was an overall increase during the study period.
In 2018, the rate of past-year heroin injection was highest in those in the Northeast, those up to age 49 years, men, and non-Hispanic whites.
More infectious disease testing
Prevalence of heroin injection did not increase among adults who used heroin or who had heroin use disorder. This, the researchers note, “suggests that increases in heroin injection are related to overall increases in heroin use rather than increases in the propensity to inject.”
Future research should examine differences in heroin injection trends across subgroups, the authors wrote.
The researchers advocate for expanding HIV and hepatitis testing and treatment, the provision of sterile syringes, and use of Food and Drug Administration–approved medications for opioid use disorders, particularly among populations at greatest risk – adults in the Northeast, those aged 18-49 years, men, and non-Hispanic whites.
“In parallel, interventions to prevent opioid misuse and opioid use disorder are needed to avert further increases in injection drug use,” they noted.
A limitation of the study was that the NSDUH excludes jail and prison populations and homeless people not in living shelters. In addition, the NSDUH is subject to recall bias.
The study was jointly sponsored by SAMHSA and the National Institute on Drug Abuse of the National Institutes of Health. One author reports owning stock in General Electric Co, 3M Co, and Pfizer Inc.
A version of this article first appeared on Medscape.com.
U.S. rates of heroin use, heroin use disorder, and heroin injections all increased overall among adults during a recent 17-year period, but rates have plateaued, new research shows.
Although on the face of it this may seem like good news, investigators at the Substance Abuse and Mental Health Services Administration (SAMHSA) note that the plateau in heroin use may simply reflect a switch to fentanyl.
“The recent leveling off of heroin use might reflect shifts from heroin to illicit fentanyl-related compounds,” wrote the investigators, led by Beth Han, MD, PhD, MPH.
The study was published online Feb. 11 as a research letter in JAMA (2020;323[6]:568-71).
National data
For the study, researchers collected data from a nationally representative group of adults aged 18 years or older who participated in the 2002-2018 National Survey on Drug Use and Health (NSDUH).
The analysis included 800,500 respondents during the study period. The mean age of respondents was 34.5 years, and 53.2% were women.
Results showed that the reported past-year prevalence of heroin use increased from 0.17% in 2002 to 0.32% in 2018 (average annual percentage change [AAPC], 5.6; 95% confidence interval [CI], 1.0-10.5; P = .02). During 2002-2016, the APC was 7.6 (95% CI, 6.3-9.0; P less than .001) but then plateaued during 2016-2018 (APC, –7.1; 95% CI, –36.9 to 36.7; P = .69).
The prevalence of heroin use disorder increased from 0.10% in 2002 to 0.21% in 2018 (AAPC, 6.0; 95% CI, 3.2-8.8; P less than .001). The rate remained stable during 2002-2008, increased during 2008-2015, then plateaued during 2015-2018.
The prevalence of heroin injections increased from 0.09% in 2002 to 0.17% in 2018 (AAPC, 6.9; 95% CI, 5.7-8.0; P less than .001), although there was a dip from the previous year. This rate increased during the study period among both men and women, those aged 35-49 years, non-Hispanic whites, and those residing in the Northeast or West regions.
For individuals up to age 25 years and those living in the Midwest, the heroin injection rate stopped increasing and plateaued, but there was an overall increase during the study period.
In 2018, the rate of past-year heroin injection was highest in those in the Northeast, those up to age 49 years, men, and non-Hispanic whites.
More infectious disease testing
Prevalence of heroin injection did not increase among adults who used heroin or who had heroin use disorder. This, the researchers note, “suggests that increases in heroin injection are related to overall increases in heroin use rather than increases in the propensity to inject.”
Future research should examine differences in heroin injection trends across subgroups, the authors wrote.
The researchers advocate for expanding HIV and hepatitis testing and treatment, the provision of sterile syringes, and use of Food and Drug Administration–approved medications for opioid use disorders, particularly among populations at greatest risk – adults in the Northeast, those aged 18-49 years, men, and non-Hispanic whites.
“In parallel, interventions to prevent opioid misuse and opioid use disorder are needed to avert further increases in injection drug use,” they noted.
A limitation of the study was that the NSDUH excludes jail and prison populations and homeless people not in living shelters. In addition, the NSDUH is subject to recall bias.
The study was jointly sponsored by SAMHSA and the National Institute on Drug Abuse of the National Institutes of Health. One author reports owning stock in General Electric Co, 3M Co, and Pfizer Inc.
A version of this article first appeared on Medscape.com.
Patients remain satisfied despite reduced use of opioids post partum
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
REPORTING FROM THE PREGNANCY MEETING
Opioid use disorder in adolescents: An overview
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
CDC: Opioid prescribing and use rates down since 2010
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
FROM MMWR SURVEILLANCE SUMMARIES
Rural treatment of opioid use disorder increasingly driven by nonphysician workforce
Nurse practitioners and physician assistants, rather than physicians, are the clinicians who have boosted capacity for buprenorphine prescribing in rural America, according to a study in a rural health–focused issue of the journal Health Affairs.
In the face of an ongoing crisis of opioid use disorder, and associated overdoses and deaths that have spared no sector of the U.S. population, the federal government expanded its waiver program for buprenorphine prescribing in 2017. The waiver expansion allows nurse practitioners (NPs) and physician assistants (PAs) – along with clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives – to use the drug for medication-assisted treatment (MAT) for opioid use disorder after completing 24 hours of mandated training; physicians are required to complete 8 hours of training to receive their waiver.
From 2016 to 2019, capacity for MAT in rural areas increased, with the number of clinicians with buprenorphine waivers more than doubling. Of the newly waivered prescribers accounting for this 111% increase, more than half were NPs and PAs.
In many areas, NPs and PAs led the way forward, wrote the study’s lead author Michael L. Barnett, MD, and coauthors, noting in the abstract accompanying the paper that “NPs and PAs accounted for more than half of this increase and were the first waivered clinicians in 285 rural counties with 5.7 million residents.” Overall, the proportion of people living in a county without a waivered clinician has decreased by 36% since NPs and PAs were permitted to obtain waivers.
SAMHSA data identifies trends
In an in-depth interview, Dr. Barnett, an internal medicine physician and health services researcher at the Harvard School of Public Health, Boston, said the issue today is “not so much continuing to dissect the risks and benefits of opioids as a treatment for pain, but more trying to address the current overdose crisis, and the fact that our patient treatment infrastructure is woefully inadequate for the magnitude of the problem that we face.”
Dr. Barnett’s chief intention for this study, he said, was to generate information that will drive policy to implement effective opioid treatment. He’d always been interested in models of care delivery that move beyond seeing just the physician-patient dyad.
“There are a whole range of nonphysician providers that are probably better at providing many different types of care – things that physicians aren’t necessarily that well trained to do,” he said.
Expansion of buprenorphine waivers to NPs and PAs, said Dr. Barnett, presented “a very interesting opportunity to see: How does a nonphysician workforce respond to a new practice opportunity, to really be engaged in areas that many physicians really were neglecting?”
The researchers used information drawn from what Dr. Barnett characterized as a “gold-standard” dataset maintained by the federal Substance Abuse and Mental Health Services Administration. They found that, by March 2019, 52% of U.S. rural residents lived in counties with at least one NP or PA holding a buprenorphine waiver, though there was wide geographic variation: Every county in Maine and New Hampshire had waivered NPs or PAs, but in Tennessee, just 3 of 95 counties had an NP or PA with a waiver.
Scope-of-practice regulations matter
The scope of practice permitted NPs and PAs varies by state, and Dr. Barnett and coauthors also looked to see whether broader scope of practice meant that more advanced practice clinicians were getting buprenorphine waivers. This did appear to be the case: In an analysis that dichotomized scope of practice into “broad” and “restricted,” states with broader practice scope saw twice as many waivered NPs per 100,000 rural residents as those with restrictive practice scope. This association was not seen for PAs, but Dr. Barnett pointed out that PAs are less likely overall to work in primary care.
This, he added, is where scope of practice starts to matter. “A lot of states are still bickering about scope of practice. We show in our paper the clear relationship between scope of practice and the degree to which providers are able to take up these waivers. We can’t prove causality, but I think it’s not a big stretch to think that these policies are playing a big role. I hope we’re working to try to advance that conversation.”
Helping address the unmet need for evidence-based treatment of opioid use disorder, he said, “is one of the more important examples, because doctors have been leaving rural areas in droves. We are lucky that there is a workforce of NPs that still seem to recognize the market opportunity; rural areas still need providers, and they have been willing to fill the gap.”
Waivered NPs or PAs can apply for an expanded waiver, permitting expansion of the buprenorphine panel from 30 to 100 patients after 1 year of holding their initial waiver. Physicians may apply for a waiver to treat up to 275 patients.
Effect on quality of care
The evidence doesn’t support big worries about quality of care, he said. “We don’t have any data on this in the clinical context of addiction, but all of the data that are out there in terms of evaluating the quality of care and level of care being offered by NPs and PAs versus primary care doctors – the types of things that we think of as within the scope of NP and PA practice typically – have shown that they are the same.” Dr. Barnett acknowledged that “there are a little bit of mixed results here and there in one direction or another, but largely, the care being delivered is much more the same than different.”
In addressing the opioid crisis as in the rest of medicine, it’s a mistake not to include this sector of the health care workforce when policies are being crafted, said Dr. Barnett. “People who are making policy and aren’t familiar with the workforce in rural areas could miss the boat. ... NPs aren’t just an asterisk to the workforce – they are an essential part of delivering medicine, just as much as physicians are.”
Dr. Barnett said that, in his estimation, “a lot of protectionist myths get physicians worked up around increased scope of practice for NPs.” However, “The truth is that there’s enough health care spending to go around for everybody and there’s plenty of work to go around.”
Dr. Barnett acknowledged that the current study captured only prescribing capacity, and not actual prescription volume. But, based on some preliminary data, “my sense is that NPs and PAs who acquire waivers are more likely to be prescribing to a larger number of patients proportionately than MDs.” He wasn’t surprised to see this, since the many more hours of training required for NPs and PAs to acquire a waiver means they’re likely to be committed to using the waiver in practice.
Stepping back to look at the bigger picture, Dr. Barnett remarked that, “taking a look at the waiver requirement, a part of me feels that it’s a bit of an anachronistic regulation, anyway – it’s really hard to justify clinically or ethically versus other things that we do.” The waiver program he said, is “a regulation barrier whose time should be limited. ... I’m hoping that the waiver disappears soon.”
Prescribing issues will linger beyond any future abolition of the waiver program, since many clinicians will still not be comfortable prescribing medication for MAT of opioid use disorder, said Dr. Barnett. “It’ll be a lot of the same stigma and structural barriers that were in place prior to the waiver.”
Dr. Barnett reported that he has been retained as an expert witness for plaintiffs in lawsuits against opioid manufacturers. The study was partly funded by the National Institutes of Health.
SOURCE: Barnett ML et al. Health Aff. 2019 Jan;38(12):2048-56.
Nurse practitioners and physician assistants, rather than physicians, are the clinicians who have boosted capacity for buprenorphine prescribing in rural America, according to a study in a rural health–focused issue of the journal Health Affairs.
In the face of an ongoing crisis of opioid use disorder, and associated overdoses and deaths that have spared no sector of the U.S. population, the federal government expanded its waiver program for buprenorphine prescribing in 2017. The waiver expansion allows nurse practitioners (NPs) and physician assistants (PAs) – along with clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives – to use the drug for medication-assisted treatment (MAT) for opioid use disorder after completing 24 hours of mandated training; physicians are required to complete 8 hours of training to receive their waiver.
From 2016 to 2019, capacity for MAT in rural areas increased, with the number of clinicians with buprenorphine waivers more than doubling. Of the newly waivered prescribers accounting for this 111% increase, more than half were NPs and PAs.
In many areas, NPs and PAs led the way forward, wrote the study’s lead author Michael L. Barnett, MD, and coauthors, noting in the abstract accompanying the paper that “NPs and PAs accounted for more than half of this increase and were the first waivered clinicians in 285 rural counties with 5.7 million residents.” Overall, the proportion of people living in a county without a waivered clinician has decreased by 36% since NPs and PAs were permitted to obtain waivers.
SAMHSA data identifies trends
In an in-depth interview, Dr. Barnett, an internal medicine physician and health services researcher at the Harvard School of Public Health, Boston, said the issue today is “not so much continuing to dissect the risks and benefits of opioids as a treatment for pain, but more trying to address the current overdose crisis, and the fact that our patient treatment infrastructure is woefully inadequate for the magnitude of the problem that we face.”
Dr. Barnett’s chief intention for this study, he said, was to generate information that will drive policy to implement effective opioid treatment. He’d always been interested in models of care delivery that move beyond seeing just the physician-patient dyad.
“There are a whole range of nonphysician providers that are probably better at providing many different types of care – things that physicians aren’t necessarily that well trained to do,” he said.
Expansion of buprenorphine waivers to NPs and PAs, said Dr. Barnett, presented “a very interesting opportunity to see: How does a nonphysician workforce respond to a new practice opportunity, to really be engaged in areas that many physicians really were neglecting?”
The researchers used information drawn from what Dr. Barnett characterized as a “gold-standard” dataset maintained by the federal Substance Abuse and Mental Health Services Administration. They found that, by March 2019, 52% of U.S. rural residents lived in counties with at least one NP or PA holding a buprenorphine waiver, though there was wide geographic variation: Every county in Maine and New Hampshire had waivered NPs or PAs, but in Tennessee, just 3 of 95 counties had an NP or PA with a waiver.
Scope-of-practice regulations matter
The scope of practice permitted NPs and PAs varies by state, and Dr. Barnett and coauthors also looked to see whether broader scope of practice meant that more advanced practice clinicians were getting buprenorphine waivers. This did appear to be the case: In an analysis that dichotomized scope of practice into “broad” and “restricted,” states with broader practice scope saw twice as many waivered NPs per 100,000 rural residents as those with restrictive practice scope. This association was not seen for PAs, but Dr. Barnett pointed out that PAs are less likely overall to work in primary care.
This, he added, is where scope of practice starts to matter. “A lot of states are still bickering about scope of practice. We show in our paper the clear relationship between scope of practice and the degree to which providers are able to take up these waivers. We can’t prove causality, but I think it’s not a big stretch to think that these policies are playing a big role. I hope we’re working to try to advance that conversation.”
Helping address the unmet need for evidence-based treatment of opioid use disorder, he said, “is one of the more important examples, because doctors have been leaving rural areas in droves. We are lucky that there is a workforce of NPs that still seem to recognize the market opportunity; rural areas still need providers, and they have been willing to fill the gap.”
Waivered NPs or PAs can apply for an expanded waiver, permitting expansion of the buprenorphine panel from 30 to 100 patients after 1 year of holding their initial waiver. Physicians may apply for a waiver to treat up to 275 patients.
Effect on quality of care
The evidence doesn’t support big worries about quality of care, he said. “We don’t have any data on this in the clinical context of addiction, but all of the data that are out there in terms of evaluating the quality of care and level of care being offered by NPs and PAs versus primary care doctors – the types of things that we think of as within the scope of NP and PA practice typically – have shown that they are the same.” Dr. Barnett acknowledged that “there are a little bit of mixed results here and there in one direction or another, but largely, the care being delivered is much more the same than different.”
In addressing the opioid crisis as in the rest of medicine, it’s a mistake not to include this sector of the health care workforce when policies are being crafted, said Dr. Barnett. “People who are making policy and aren’t familiar with the workforce in rural areas could miss the boat. ... NPs aren’t just an asterisk to the workforce – they are an essential part of delivering medicine, just as much as physicians are.”
Dr. Barnett said that, in his estimation, “a lot of protectionist myths get physicians worked up around increased scope of practice for NPs.” However, “The truth is that there’s enough health care spending to go around for everybody and there’s plenty of work to go around.”
Dr. Barnett acknowledged that the current study captured only prescribing capacity, and not actual prescription volume. But, based on some preliminary data, “my sense is that NPs and PAs who acquire waivers are more likely to be prescribing to a larger number of patients proportionately than MDs.” He wasn’t surprised to see this, since the many more hours of training required for NPs and PAs to acquire a waiver means they’re likely to be committed to using the waiver in practice.
Stepping back to look at the bigger picture, Dr. Barnett remarked that, “taking a look at the waiver requirement, a part of me feels that it’s a bit of an anachronistic regulation, anyway – it’s really hard to justify clinically or ethically versus other things that we do.” The waiver program he said, is “a regulation barrier whose time should be limited. ... I’m hoping that the waiver disappears soon.”
Prescribing issues will linger beyond any future abolition of the waiver program, since many clinicians will still not be comfortable prescribing medication for MAT of opioid use disorder, said Dr. Barnett. “It’ll be a lot of the same stigma and structural barriers that were in place prior to the waiver.”
Dr. Barnett reported that he has been retained as an expert witness for plaintiffs in lawsuits against opioid manufacturers. The study was partly funded by the National Institutes of Health.
SOURCE: Barnett ML et al. Health Aff. 2019 Jan;38(12):2048-56.
Nurse practitioners and physician assistants, rather than physicians, are the clinicians who have boosted capacity for buprenorphine prescribing in rural America, according to a study in a rural health–focused issue of the journal Health Affairs.
In the face of an ongoing crisis of opioid use disorder, and associated overdoses and deaths that have spared no sector of the U.S. population, the federal government expanded its waiver program for buprenorphine prescribing in 2017. The waiver expansion allows nurse practitioners (NPs) and physician assistants (PAs) – along with clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives – to use the drug for medication-assisted treatment (MAT) for opioid use disorder after completing 24 hours of mandated training; physicians are required to complete 8 hours of training to receive their waiver.
From 2016 to 2019, capacity for MAT in rural areas increased, with the number of clinicians with buprenorphine waivers more than doubling. Of the newly waivered prescribers accounting for this 111% increase, more than half were NPs and PAs.
In many areas, NPs and PAs led the way forward, wrote the study’s lead author Michael L. Barnett, MD, and coauthors, noting in the abstract accompanying the paper that “NPs and PAs accounted for more than half of this increase and were the first waivered clinicians in 285 rural counties with 5.7 million residents.” Overall, the proportion of people living in a county without a waivered clinician has decreased by 36% since NPs and PAs were permitted to obtain waivers.
SAMHSA data identifies trends
In an in-depth interview, Dr. Barnett, an internal medicine physician and health services researcher at the Harvard School of Public Health, Boston, said the issue today is “not so much continuing to dissect the risks and benefits of opioids as a treatment for pain, but more trying to address the current overdose crisis, and the fact that our patient treatment infrastructure is woefully inadequate for the magnitude of the problem that we face.”
Dr. Barnett’s chief intention for this study, he said, was to generate information that will drive policy to implement effective opioid treatment. He’d always been interested in models of care delivery that move beyond seeing just the physician-patient dyad.
“There are a whole range of nonphysician providers that are probably better at providing many different types of care – things that physicians aren’t necessarily that well trained to do,” he said.
Expansion of buprenorphine waivers to NPs and PAs, said Dr. Barnett, presented “a very interesting opportunity to see: How does a nonphysician workforce respond to a new practice opportunity, to really be engaged in areas that many physicians really were neglecting?”
The researchers used information drawn from what Dr. Barnett characterized as a “gold-standard” dataset maintained by the federal Substance Abuse and Mental Health Services Administration. They found that, by March 2019, 52% of U.S. rural residents lived in counties with at least one NP or PA holding a buprenorphine waiver, though there was wide geographic variation: Every county in Maine and New Hampshire had waivered NPs or PAs, but in Tennessee, just 3 of 95 counties had an NP or PA with a waiver.
Scope-of-practice regulations matter
The scope of practice permitted NPs and PAs varies by state, and Dr. Barnett and coauthors also looked to see whether broader scope of practice meant that more advanced practice clinicians were getting buprenorphine waivers. This did appear to be the case: In an analysis that dichotomized scope of practice into “broad” and “restricted,” states with broader practice scope saw twice as many waivered NPs per 100,000 rural residents as those with restrictive practice scope. This association was not seen for PAs, but Dr. Barnett pointed out that PAs are less likely overall to work in primary care.
This, he added, is where scope of practice starts to matter. “A lot of states are still bickering about scope of practice. We show in our paper the clear relationship between scope of practice and the degree to which providers are able to take up these waivers. We can’t prove causality, but I think it’s not a big stretch to think that these policies are playing a big role. I hope we’re working to try to advance that conversation.”
Helping address the unmet need for evidence-based treatment of opioid use disorder, he said, “is one of the more important examples, because doctors have been leaving rural areas in droves. We are lucky that there is a workforce of NPs that still seem to recognize the market opportunity; rural areas still need providers, and they have been willing to fill the gap.”
Waivered NPs or PAs can apply for an expanded waiver, permitting expansion of the buprenorphine panel from 30 to 100 patients after 1 year of holding their initial waiver. Physicians may apply for a waiver to treat up to 275 patients.
Effect on quality of care
The evidence doesn’t support big worries about quality of care, he said. “We don’t have any data on this in the clinical context of addiction, but all of the data that are out there in terms of evaluating the quality of care and level of care being offered by NPs and PAs versus primary care doctors – the types of things that we think of as within the scope of NP and PA practice typically – have shown that they are the same.” Dr. Barnett acknowledged that “there are a little bit of mixed results here and there in one direction or another, but largely, the care being delivered is much more the same than different.”
In addressing the opioid crisis as in the rest of medicine, it’s a mistake not to include this sector of the health care workforce when policies are being crafted, said Dr. Barnett. “People who are making policy and aren’t familiar with the workforce in rural areas could miss the boat. ... NPs aren’t just an asterisk to the workforce – they are an essential part of delivering medicine, just as much as physicians are.”
Dr. Barnett said that, in his estimation, “a lot of protectionist myths get physicians worked up around increased scope of practice for NPs.” However, “The truth is that there’s enough health care spending to go around for everybody and there’s plenty of work to go around.”
Dr. Barnett acknowledged that the current study captured only prescribing capacity, and not actual prescription volume. But, based on some preliminary data, “my sense is that NPs and PAs who acquire waivers are more likely to be prescribing to a larger number of patients proportionately than MDs.” He wasn’t surprised to see this, since the many more hours of training required for NPs and PAs to acquire a waiver means they’re likely to be committed to using the waiver in practice.
Stepping back to look at the bigger picture, Dr. Barnett remarked that, “taking a look at the waiver requirement, a part of me feels that it’s a bit of an anachronistic regulation, anyway – it’s really hard to justify clinically or ethically versus other things that we do.” The waiver program he said, is “a regulation barrier whose time should be limited. ... I’m hoping that the waiver disappears soon.”
Prescribing issues will linger beyond any future abolition of the waiver program, since many clinicians will still not be comfortable prescribing medication for MAT of opioid use disorder, said Dr. Barnett. “It’ll be a lot of the same stigma and structural barriers that were in place prior to the waiver.”
Dr. Barnett reported that he has been retained as an expert witness for plaintiffs in lawsuits against opioid manufacturers. The study was partly funded by the National Institutes of Health.
SOURCE: Barnett ML et al. Health Aff. 2019 Jan;38(12):2048-56.
FROM HEALTH AFFAIRS
IBD: Inpatient opioids linked with outpatient use
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Patients with inflammatory bowel disease (IBD) who receive opioids while hospitalized are three times as likely to be prescribed opioids after discharge.
Major finding: Patients who were given intravenous opioids while hospitalized were three times as likely to receive a postdischarge opioid prescription, compared with patients who did not receive inpatient intravenous opioids (odds ratio, 3.3).
Study details: A retrospective cohort study involving 862 patients with inflammatory bowel disease.
Disclosures: The investigators disclosed relationships Abbott, Gilead, Romark, and others.
Source: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
Adding cannabinoids to opioids doesn’t improve cancer pain control
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
FROM BMJ SUPPORTIVE & PALLIATIVE CARE
We can achieve opioid-free analgesia after childbirth: Stop prescribing opioids after vaginal delivery and reduce their use after cesarean
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)
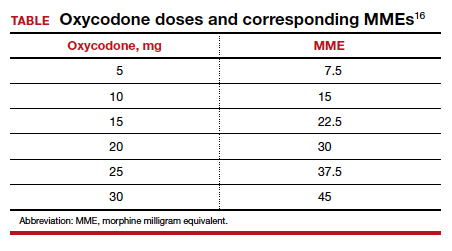
A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)

A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)

A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
Lofexidine: An option for treating opioid withdrawal
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.