User login
No advantage for full-term aspirin in preventing preterm preeclampsia
Stopping aspirin at 24-28 weeks of gestation has no disadvantage, compared with continuing aspirin full term, for preventing preterm preeclampsia in women at high risk of preeclampsia who have a normal fms-like tyrosine kinase 1 to placental growth factor (sFlt-1:PlGF) ratio, a randomized controlled trial has found.
The findings were published online in JAMA.
Editorialists advise careful consideration
However, in an accompanying editorial, Ukachi N. Emeruwa, MD, MPH, with the division of maternal fetal medicine, department of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and colleagues noted that the questions surrounding continuing or discontinuing aspirin in this high-risk population need further consideration.
They added that the results from this study – conducted in nine maternity hospitals across Spain – are hard to translate for the U.S. population.
In this study, Manel Mendoza, PhD, with the maternal fetal medicine unit, department of obstetrics, at the Universitat Autònoma de Barcelona, and colleagues compared the two approaches because of the potential to mitigate peripartum bleeding by discontinuing aspirin before full term (37 weeks’ gestation) and by an accurate selection of women in the first trimester at higher risk of preeclampsia.
Aspirin cuts preterm preeclampsia by 62% in women at high risk
While aspirin might be associated with an increased risk of peripartum bleeding, aspirin has been proven to reduce the incidence of preterm preeclampsia by 62% in pregnant women at high risk of preeclampsia.
In the multicenter, open-label, randomized, phase 3, noninferiority trial, pregnant women who had a high risk of preeclampsia during the first-trimester screening and an sFlt-1:PlGF ratio of 38 or less at 24-28 weeks’ gestation were recruited between Aug. 20, 2019, and Sept. 15, 2021. Of those, 936 were analyzed (473 in the intervention group [stopping aspirin] and 473 in the control group [continuing]).
Screening for risk of preterm preeclampsia included analyzing maternal factors, uterine artery pulsatility index, mean arterial pressure, serum pregnancy-associated plasma protein A, and placental growth factor. Follow-up was until delivery for all participants.
Incidence of preterm preeclampsia was 1.48% in the intervention group (discontinuing aspirin) and 1.73% in the control group (continuing aspirin until 36 weeks of gestation; absolute difference, –0.25%; 95% confidence interval, –1.86% to 1.36%), which indicates noninferiority for stopping aspirin. The bar for noninferiority was less than a 1.9% difference in preterm preeclampsia incidences between groups.
Researchers did find a higher incidence of minor antepartum bleeding in the group that continued aspirin (7.61% in the low-dose aspirin discontinuation group vs. 12.31% in the low-dose aspirin continuation group; absolute difference, –4.70; 95% CI, –8.53 to –0.87).
Differences in U.S. guidelines
Dr. Emeruwa and colleagues noted the study challenges a growing body of evidence favoring increasingly widespread use of low-dose aspirin in pregnancy.
They called the study “well designed and provocative,” but wrote that the findings are hard to interpret for a U.S. population. Some key differences in the U.S. preeclampsia prevention guidelines, compared with the practices of the study’s authors, included the reliance on clinical maternal factors in the United States for screening for low-dose aspirin prophylaxis as opposed to molecular biomarkers; a different aspirin dose prescribed in the United States (81 mg daily), compared with international societies (150 mg daily); and a lack of a recommendation in the United States to stop prophylactic low-dose aspirin at 36 weeks.
Dr. Emeruwa and colleagues also questioned the scope of the outcome measure used.
They wrote that limiting outcomes to preterm preeclampsia dims the effects of all types of preeclampsia on perinatal and maternal outcomes and that early-onset preeclampsia at less than 34 weeks “occurs in just 0.38% of pregnancies, while 3%-5% are affected by late-onset preeclampsia.”
‘Late-onset preeclampsia has a higher overall impact’
Dr. Emeruwa and colleagues wrote: “Though the odds of adverse perinatal and maternal outcomes are higher with preterm preeclampsia, due to its overall higher incidence, late-onset preeclampsia has a higher overall impact on perinatal and maternal morbidity and mortality.”
The study can inform future U.S. approaches, the editorialists wrote, and build on work already being done in the United States.
The study investigators used biophysical and molecular markers to more accurately assess risk for starting low-dose aspirin prophylaxis in the first trimester and applied a growing body of data showing the high negative predictive value of second-trimester biomarkers.
The editorialists noted that the U.S. Preventive Services Task Force recommendations would have captured “less than 50% of the at-risk population” that Dr. Mendoza’s team found eligible for low-dose aspirin.
Those factors, the editorialists wrote, point to the potential to improve guidelines for personalized preeclampsia management in pregnancy.
They concluded: “U.S. practitioners and professional societies should reconsider current risk assessment strategies, which are largely based on maternal factors, and evaluate whether incorporation of molecular biomarkers would improve maternal and fetal/neonatal outcomes.”
The study authors acknowledged that 92% of participants in the study were White, thus limiting generalizability.
The authors and editorialists reported no relevant financial relationships.
Stopping aspirin at 24-28 weeks of gestation has no disadvantage, compared with continuing aspirin full term, for preventing preterm preeclampsia in women at high risk of preeclampsia who have a normal fms-like tyrosine kinase 1 to placental growth factor (sFlt-1:PlGF) ratio, a randomized controlled trial has found.
The findings were published online in JAMA.
Editorialists advise careful consideration
However, in an accompanying editorial, Ukachi N. Emeruwa, MD, MPH, with the division of maternal fetal medicine, department of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and colleagues noted that the questions surrounding continuing or discontinuing aspirin in this high-risk population need further consideration.
They added that the results from this study – conducted in nine maternity hospitals across Spain – are hard to translate for the U.S. population.
In this study, Manel Mendoza, PhD, with the maternal fetal medicine unit, department of obstetrics, at the Universitat Autònoma de Barcelona, and colleagues compared the two approaches because of the potential to mitigate peripartum bleeding by discontinuing aspirin before full term (37 weeks’ gestation) and by an accurate selection of women in the first trimester at higher risk of preeclampsia.
Aspirin cuts preterm preeclampsia by 62% in women at high risk
While aspirin might be associated with an increased risk of peripartum bleeding, aspirin has been proven to reduce the incidence of preterm preeclampsia by 62% in pregnant women at high risk of preeclampsia.
In the multicenter, open-label, randomized, phase 3, noninferiority trial, pregnant women who had a high risk of preeclampsia during the first-trimester screening and an sFlt-1:PlGF ratio of 38 or less at 24-28 weeks’ gestation were recruited between Aug. 20, 2019, and Sept. 15, 2021. Of those, 936 were analyzed (473 in the intervention group [stopping aspirin] and 473 in the control group [continuing]).
Screening for risk of preterm preeclampsia included analyzing maternal factors, uterine artery pulsatility index, mean arterial pressure, serum pregnancy-associated plasma protein A, and placental growth factor. Follow-up was until delivery for all participants.
Incidence of preterm preeclampsia was 1.48% in the intervention group (discontinuing aspirin) and 1.73% in the control group (continuing aspirin until 36 weeks of gestation; absolute difference, –0.25%; 95% confidence interval, –1.86% to 1.36%), which indicates noninferiority for stopping aspirin. The bar for noninferiority was less than a 1.9% difference in preterm preeclampsia incidences between groups.
Researchers did find a higher incidence of minor antepartum bleeding in the group that continued aspirin (7.61% in the low-dose aspirin discontinuation group vs. 12.31% in the low-dose aspirin continuation group; absolute difference, –4.70; 95% CI, –8.53 to –0.87).
Differences in U.S. guidelines
Dr. Emeruwa and colleagues noted the study challenges a growing body of evidence favoring increasingly widespread use of low-dose aspirin in pregnancy.
They called the study “well designed and provocative,” but wrote that the findings are hard to interpret for a U.S. population. Some key differences in the U.S. preeclampsia prevention guidelines, compared with the practices of the study’s authors, included the reliance on clinical maternal factors in the United States for screening for low-dose aspirin prophylaxis as opposed to molecular biomarkers; a different aspirin dose prescribed in the United States (81 mg daily), compared with international societies (150 mg daily); and a lack of a recommendation in the United States to stop prophylactic low-dose aspirin at 36 weeks.
Dr. Emeruwa and colleagues also questioned the scope of the outcome measure used.
They wrote that limiting outcomes to preterm preeclampsia dims the effects of all types of preeclampsia on perinatal and maternal outcomes and that early-onset preeclampsia at less than 34 weeks “occurs in just 0.38% of pregnancies, while 3%-5% are affected by late-onset preeclampsia.”
‘Late-onset preeclampsia has a higher overall impact’
Dr. Emeruwa and colleagues wrote: “Though the odds of adverse perinatal and maternal outcomes are higher with preterm preeclampsia, due to its overall higher incidence, late-onset preeclampsia has a higher overall impact on perinatal and maternal morbidity and mortality.”
The study can inform future U.S. approaches, the editorialists wrote, and build on work already being done in the United States.
The study investigators used biophysical and molecular markers to more accurately assess risk for starting low-dose aspirin prophylaxis in the first trimester and applied a growing body of data showing the high negative predictive value of second-trimester biomarkers.
The editorialists noted that the U.S. Preventive Services Task Force recommendations would have captured “less than 50% of the at-risk population” that Dr. Mendoza’s team found eligible for low-dose aspirin.
Those factors, the editorialists wrote, point to the potential to improve guidelines for personalized preeclampsia management in pregnancy.
They concluded: “U.S. practitioners and professional societies should reconsider current risk assessment strategies, which are largely based on maternal factors, and evaluate whether incorporation of molecular biomarkers would improve maternal and fetal/neonatal outcomes.”
The study authors acknowledged that 92% of participants in the study were White, thus limiting generalizability.
The authors and editorialists reported no relevant financial relationships.
Stopping aspirin at 24-28 weeks of gestation has no disadvantage, compared with continuing aspirin full term, for preventing preterm preeclampsia in women at high risk of preeclampsia who have a normal fms-like tyrosine kinase 1 to placental growth factor (sFlt-1:PlGF) ratio, a randomized controlled trial has found.
The findings were published online in JAMA.
Editorialists advise careful consideration
However, in an accompanying editorial, Ukachi N. Emeruwa, MD, MPH, with the division of maternal fetal medicine, department of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and colleagues noted that the questions surrounding continuing or discontinuing aspirin in this high-risk population need further consideration.
They added that the results from this study – conducted in nine maternity hospitals across Spain – are hard to translate for the U.S. population.
In this study, Manel Mendoza, PhD, with the maternal fetal medicine unit, department of obstetrics, at the Universitat Autònoma de Barcelona, and colleagues compared the two approaches because of the potential to mitigate peripartum bleeding by discontinuing aspirin before full term (37 weeks’ gestation) and by an accurate selection of women in the first trimester at higher risk of preeclampsia.
Aspirin cuts preterm preeclampsia by 62% in women at high risk
While aspirin might be associated with an increased risk of peripartum bleeding, aspirin has been proven to reduce the incidence of preterm preeclampsia by 62% in pregnant women at high risk of preeclampsia.
In the multicenter, open-label, randomized, phase 3, noninferiority trial, pregnant women who had a high risk of preeclampsia during the first-trimester screening and an sFlt-1:PlGF ratio of 38 or less at 24-28 weeks’ gestation were recruited between Aug. 20, 2019, and Sept. 15, 2021. Of those, 936 were analyzed (473 in the intervention group [stopping aspirin] and 473 in the control group [continuing]).
Screening for risk of preterm preeclampsia included analyzing maternal factors, uterine artery pulsatility index, mean arterial pressure, serum pregnancy-associated plasma protein A, and placental growth factor. Follow-up was until delivery for all participants.
Incidence of preterm preeclampsia was 1.48% in the intervention group (discontinuing aspirin) and 1.73% in the control group (continuing aspirin until 36 weeks of gestation; absolute difference, –0.25%; 95% confidence interval, –1.86% to 1.36%), which indicates noninferiority for stopping aspirin. The bar for noninferiority was less than a 1.9% difference in preterm preeclampsia incidences between groups.
Researchers did find a higher incidence of minor antepartum bleeding in the group that continued aspirin (7.61% in the low-dose aspirin discontinuation group vs. 12.31% in the low-dose aspirin continuation group; absolute difference, –4.70; 95% CI, –8.53 to –0.87).
Differences in U.S. guidelines
Dr. Emeruwa and colleagues noted the study challenges a growing body of evidence favoring increasingly widespread use of low-dose aspirin in pregnancy.
They called the study “well designed and provocative,” but wrote that the findings are hard to interpret for a U.S. population. Some key differences in the U.S. preeclampsia prevention guidelines, compared with the practices of the study’s authors, included the reliance on clinical maternal factors in the United States for screening for low-dose aspirin prophylaxis as opposed to molecular biomarkers; a different aspirin dose prescribed in the United States (81 mg daily), compared with international societies (150 mg daily); and a lack of a recommendation in the United States to stop prophylactic low-dose aspirin at 36 weeks.
Dr. Emeruwa and colleagues also questioned the scope of the outcome measure used.
They wrote that limiting outcomes to preterm preeclampsia dims the effects of all types of preeclampsia on perinatal and maternal outcomes and that early-onset preeclampsia at less than 34 weeks “occurs in just 0.38% of pregnancies, while 3%-5% are affected by late-onset preeclampsia.”
‘Late-onset preeclampsia has a higher overall impact’
Dr. Emeruwa and colleagues wrote: “Though the odds of adverse perinatal and maternal outcomes are higher with preterm preeclampsia, due to its overall higher incidence, late-onset preeclampsia has a higher overall impact on perinatal and maternal morbidity and mortality.”
The study can inform future U.S. approaches, the editorialists wrote, and build on work already being done in the United States.
The study investigators used biophysical and molecular markers to more accurately assess risk for starting low-dose aspirin prophylaxis in the first trimester and applied a growing body of data showing the high negative predictive value of second-trimester biomarkers.
The editorialists noted that the U.S. Preventive Services Task Force recommendations would have captured “less than 50% of the at-risk population” that Dr. Mendoza’s team found eligible for low-dose aspirin.
Those factors, the editorialists wrote, point to the potential to improve guidelines for personalized preeclampsia management in pregnancy.
They concluded: “U.S. practitioners and professional societies should reconsider current risk assessment strategies, which are largely based on maternal factors, and evaluate whether incorporation of molecular biomarkers would improve maternal and fetal/neonatal outcomes.”
The study authors acknowledged that 92% of participants in the study were White, thus limiting generalizability.
The authors and editorialists reported no relevant financial relationships.
FROM JAMA
NICU use up, birth weights down in babies of mothers with HCV
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
FROM THE PREGNANCY MEETING
Scientists create ‘vagina on a chip’: What to know
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
FROM MICROBIOME
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
Magnesium sulfate shown to reduce risk of cerebral palsy in premature babies
A program to increase the use of magnesium sulfate to reduce the risk of cerebral palsy is effective, say researchers. Giving magnesium sulfate to women at risk of premature birth can reduce the risk of a child having cerebral palsy by a third, and costs just £1 per dose.
However, the authors of the new observational study, published in Archives of Disease in Childhood – Fetal and Neonatal Edition, pointed out that in 2017 only around two-thirds (64%) of eligible women were being given magnesium sulfate in England, Scotland, and Wales, with “wide regional variations.”
To address this, in 2014 the PReCePT (Preventing Cerebral Palsy in Pre Term labor) quality improvement toolkit was developed by both parents and staff with the aim of supporting all maternity units in England to improve maternity staff awareness and increase the use of magnesium sulfate in mothers at risk of giving birth at 30 weeks’ gestation or under. PReCePT provided practical tools and training to support hospital staff to give magnesium sulfate to eligible mothers.
The pilot study in 2015, which involved five maternity units, found an increase in uptake from 21% to 88% associated with the PReCePT approach. Subsequently, in 2018, NHS England funded the National PReCePT Programme, which scaled up the intervention for national roll-out and provided the PReCePT quality toolkit – which includes preterm labor proforma, staff training presentations, parent information leaflet, posters for the unit, and a learning log – to each maternity unit.
Improvement ‘over and above’ expectation
For the first evaluation of a U.K. universally implemented national perinatal quality improvement program to increase administration of an evidence-based drug, researchers, led by University of Bristol, England, set out to evaluate the effectiveness and cost-effectiveness of the National PReCePT Programme in increasing use of magnesium sulfate in preterm births.
Using data from the U.K. National Neonatal Research Database for the year before and the year after PReCePT was implemented in maternity units in England, the researchers performed a before-and-after study that involved 137 maternity units within NHS England. Participants were babies born at 30 weeks’ gestation or under admitted to neonatal units in England, and the main outcome measure was magnesium sulfate uptake before and after the implementation of the National PReCePT Programme. In addition, implementation and lifetime costs were estimated.
During the first year, post implementation of the program, uptake increased by an average of 6.3 percentage points (to 83.1%) across all maternity units in England, which the authors explained was “over and above” the increase that would be expected over time as the practice spread organically. The researchers also found that after adjusting for variations in when maternity units started the program, the increase in use of magnesium sulfate was 9.5 percentage points. “By May 2020, on average 86.4% of eligible mothers were receiving magnesium sulfate,” they said.
Professor John Macleod, NIHR ARC West Director, professor in clinical epidemiology and primary care, University of Bristol, and principal investigator of the evaluation, said: “Our in-depth analysis has been able to demonstrate that the PReCePT program is both effective and cost-effective. The program has increased uptake of magnesium sulfate, which we know is a cost-effective medicine to prevent cerebral palsy, much more quickly than we could have otherwise expected.”
From a societal and lifetime perspective, the health gains and cost savings associated with the National PReCePT Programme generated a “net monetary benefit of £866 per preterm baby,” with the probability of the program being cost-effective being “greater than 95%,” the authors highlighted.
The researchers also estimated that the program’s first year could be associated with a lifetime saving to society of £3 million – which accounts for the costs of the program, of administering the treatment, of cerebral palsy to society over a lifetime, and the associated health gains of avoiding cases. “This is across all the extra babies the program helped get access to the treatment during the first year,” they said.
The authors highlighted that in the five pilot sites, the improved use of magnesium sulfate has been “sustained over the years” since PReCePT was implemented. As the program costs were mostly in the first year of implementation, longer-term national analysis may show that PReCePT is “even more cost-effective over a longer period,” they postulated.
Accelerate uptake
Uptake of new evidence or guidelines is often “slow” due to practical barriers, lack of knowledge, and need for behavior change, and can “take decades to become embedded” in perinatal clinical practice, expressed the authors, which in turn comes at a “high clinical and economic cost.”
Karen Luyt, professor in neonatal medicine, University of Bristol, said: “The PReCePT national quality improvement program demonstrates that a collaborative and coordinated perinatal implementation program supporting every hospital in England can accelerate the uptake of new evidence-based treatments into routine practice, enabling equitable health benefits to babies and ultimately reductions in lifetime societal costs.”
The authors said the PReCePT model “may serve as a blueprint for future interventions to improve perinatal care.”
Professor Lucy Chappell, chief executive officer of the National Institute for Health and Care Research, said: “This important study shows the impact of taking a promising intervention that had been shown to work in a research setting and scaling it up across the country. Giving magnesium sulfate to prevent cerebral palsy in premature babies is a simple, inexpensive intervention that can make such a difference to families and the health service.”
Prof. Macleod added: “We are pleased to have played a part in helping get this cheap yet effective treatment to more babies.”
This work was jointly funded by the National Institute for Health and Care Research Applied Research Collaboration West and the AHSN Network funded by NHS England. The Health Foundation funded the health economics evaluation. The authors declare that the study management group has no competing financial, professional, or personal interests that might have influenced the study design or conduct.
A version of this article first appeared on Medscape UK.
A program to increase the use of magnesium sulfate to reduce the risk of cerebral palsy is effective, say researchers. Giving magnesium sulfate to women at risk of premature birth can reduce the risk of a child having cerebral palsy by a third, and costs just £1 per dose.
However, the authors of the new observational study, published in Archives of Disease in Childhood – Fetal and Neonatal Edition, pointed out that in 2017 only around two-thirds (64%) of eligible women were being given magnesium sulfate in England, Scotland, and Wales, with “wide regional variations.”
To address this, in 2014 the PReCePT (Preventing Cerebral Palsy in Pre Term labor) quality improvement toolkit was developed by both parents and staff with the aim of supporting all maternity units in England to improve maternity staff awareness and increase the use of magnesium sulfate in mothers at risk of giving birth at 30 weeks’ gestation or under. PReCePT provided practical tools and training to support hospital staff to give magnesium sulfate to eligible mothers.
The pilot study in 2015, which involved five maternity units, found an increase in uptake from 21% to 88% associated with the PReCePT approach. Subsequently, in 2018, NHS England funded the National PReCePT Programme, which scaled up the intervention for national roll-out and provided the PReCePT quality toolkit – which includes preterm labor proforma, staff training presentations, parent information leaflet, posters for the unit, and a learning log – to each maternity unit.
Improvement ‘over and above’ expectation
For the first evaluation of a U.K. universally implemented national perinatal quality improvement program to increase administration of an evidence-based drug, researchers, led by University of Bristol, England, set out to evaluate the effectiveness and cost-effectiveness of the National PReCePT Programme in increasing use of magnesium sulfate in preterm births.
Using data from the U.K. National Neonatal Research Database for the year before and the year after PReCePT was implemented in maternity units in England, the researchers performed a before-and-after study that involved 137 maternity units within NHS England. Participants were babies born at 30 weeks’ gestation or under admitted to neonatal units in England, and the main outcome measure was magnesium sulfate uptake before and after the implementation of the National PReCePT Programme. In addition, implementation and lifetime costs were estimated.
During the first year, post implementation of the program, uptake increased by an average of 6.3 percentage points (to 83.1%) across all maternity units in England, which the authors explained was “over and above” the increase that would be expected over time as the practice spread organically. The researchers also found that after adjusting for variations in when maternity units started the program, the increase in use of magnesium sulfate was 9.5 percentage points. “By May 2020, on average 86.4% of eligible mothers were receiving magnesium sulfate,” they said.
Professor John Macleod, NIHR ARC West Director, professor in clinical epidemiology and primary care, University of Bristol, and principal investigator of the evaluation, said: “Our in-depth analysis has been able to demonstrate that the PReCePT program is both effective and cost-effective. The program has increased uptake of magnesium sulfate, which we know is a cost-effective medicine to prevent cerebral palsy, much more quickly than we could have otherwise expected.”
From a societal and lifetime perspective, the health gains and cost savings associated with the National PReCePT Programme generated a “net monetary benefit of £866 per preterm baby,” with the probability of the program being cost-effective being “greater than 95%,” the authors highlighted.
The researchers also estimated that the program’s first year could be associated with a lifetime saving to society of £3 million – which accounts for the costs of the program, of administering the treatment, of cerebral palsy to society over a lifetime, and the associated health gains of avoiding cases. “This is across all the extra babies the program helped get access to the treatment during the first year,” they said.
The authors highlighted that in the five pilot sites, the improved use of magnesium sulfate has been “sustained over the years” since PReCePT was implemented. As the program costs were mostly in the first year of implementation, longer-term national analysis may show that PReCePT is “even more cost-effective over a longer period,” they postulated.
Accelerate uptake
Uptake of new evidence or guidelines is often “slow” due to practical barriers, lack of knowledge, and need for behavior change, and can “take decades to become embedded” in perinatal clinical practice, expressed the authors, which in turn comes at a “high clinical and economic cost.”
Karen Luyt, professor in neonatal medicine, University of Bristol, said: “The PReCePT national quality improvement program demonstrates that a collaborative and coordinated perinatal implementation program supporting every hospital in England can accelerate the uptake of new evidence-based treatments into routine practice, enabling equitable health benefits to babies and ultimately reductions in lifetime societal costs.”
The authors said the PReCePT model “may serve as a blueprint for future interventions to improve perinatal care.”
Professor Lucy Chappell, chief executive officer of the National Institute for Health and Care Research, said: “This important study shows the impact of taking a promising intervention that had been shown to work in a research setting and scaling it up across the country. Giving magnesium sulfate to prevent cerebral palsy in premature babies is a simple, inexpensive intervention that can make such a difference to families and the health service.”
Prof. Macleod added: “We are pleased to have played a part in helping get this cheap yet effective treatment to more babies.”
This work was jointly funded by the National Institute for Health and Care Research Applied Research Collaboration West and the AHSN Network funded by NHS England. The Health Foundation funded the health economics evaluation. The authors declare that the study management group has no competing financial, professional, or personal interests that might have influenced the study design or conduct.
A version of this article first appeared on Medscape UK.
A program to increase the use of magnesium sulfate to reduce the risk of cerebral palsy is effective, say researchers. Giving magnesium sulfate to women at risk of premature birth can reduce the risk of a child having cerebral palsy by a third, and costs just £1 per dose.
However, the authors of the new observational study, published in Archives of Disease in Childhood – Fetal and Neonatal Edition, pointed out that in 2017 only around two-thirds (64%) of eligible women were being given magnesium sulfate in England, Scotland, and Wales, with “wide regional variations.”
To address this, in 2014 the PReCePT (Preventing Cerebral Palsy in Pre Term labor) quality improvement toolkit was developed by both parents and staff with the aim of supporting all maternity units in England to improve maternity staff awareness and increase the use of magnesium sulfate in mothers at risk of giving birth at 30 weeks’ gestation or under. PReCePT provided practical tools and training to support hospital staff to give magnesium sulfate to eligible mothers.
The pilot study in 2015, which involved five maternity units, found an increase in uptake from 21% to 88% associated with the PReCePT approach. Subsequently, in 2018, NHS England funded the National PReCePT Programme, which scaled up the intervention for national roll-out and provided the PReCePT quality toolkit – which includes preterm labor proforma, staff training presentations, parent information leaflet, posters for the unit, and a learning log – to each maternity unit.
Improvement ‘over and above’ expectation
For the first evaluation of a U.K. universally implemented national perinatal quality improvement program to increase administration of an evidence-based drug, researchers, led by University of Bristol, England, set out to evaluate the effectiveness and cost-effectiveness of the National PReCePT Programme in increasing use of magnesium sulfate in preterm births.
Using data from the U.K. National Neonatal Research Database for the year before and the year after PReCePT was implemented in maternity units in England, the researchers performed a before-and-after study that involved 137 maternity units within NHS England. Participants were babies born at 30 weeks’ gestation or under admitted to neonatal units in England, and the main outcome measure was magnesium sulfate uptake before and after the implementation of the National PReCePT Programme. In addition, implementation and lifetime costs were estimated.
During the first year, post implementation of the program, uptake increased by an average of 6.3 percentage points (to 83.1%) across all maternity units in England, which the authors explained was “over and above” the increase that would be expected over time as the practice spread organically. The researchers also found that after adjusting for variations in when maternity units started the program, the increase in use of magnesium sulfate was 9.5 percentage points. “By May 2020, on average 86.4% of eligible mothers were receiving magnesium sulfate,” they said.
Professor John Macleod, NIHR ARC West Director, professor in clinical epidemiology and primary care, University of Bristol, and principal investigator of the evaluation, said: “Our in-depth analysis has been able to demonstrate that the PReCePT program is both effective and cost-effective. The program has increased uptake of magnesium sulfate, which we know is a cost-effective medicine to prevent cerebral palsy, much more quickly than we could have otherwise expected.”
From a societal and lifetime perspective, the health gains and cost savings associated with the National PReCePT Programme generated a “net monetary benefit of £866 per preterm baby,” with the probability of the program being cost-effective being “greater than 95%,” the authors highlighted.
The researchers also estimated that the program’s first year could be associated with a lifetime saving to society of £3 million – which accounts for the costs of the program, of administering the treatment, of cerebral palsy to society over a lifetime, and the associated health gains of avoiding cases. “This is across all the extra babies the program helped get access to the treatment during the first year,” they said.
The authors highlighted that in the five pilot sites, the improved use of magnesium sulfate has been “sustained over the years” since PReCePT was implemented. As the program costs were mostly in the first year of implementation, longer-term national analysis may show that PReCePT is “even more cost-effective over a longer period,” they postulated.
Accelerate uptake
Uptake of new evidence or guidelines is often “slow” due to practical barriers, lack of knowledge, and need for behavior change, and can “take decades to become embedded” in perinatal clinical practice, expressed the authors, which in turn comes at a “high clinical and economic cost.”
Karen Luyt, professor in neonatal medicine, University of Bristol, said: “The PReCePT national quality improvement program demonstrates that a collaborative and coordinated perinatal implementation program supporting every hospital in England can accelerate the uptake of new evidence-based treatments into routine practice, enabling equitable health benefits to babies and ultimately reductions in lifetime societal costs.”
The authors said the PReCePT model “may serve as a blueprint for future interventions to improve perinatal care.”
Professor Lucy Chappell, chief executive officer of the National Institute for Health and Care Research, said: “This important study shows the impact of taking a promising intervention that had been shown to work in a research setting and scaling it up across the country. Giving magnesium sulfate to prevent cerebral palsy in premature babies is a simple, inexpensive intervention that can make such a difference to families and the health service.”
Prof. Macleod added: “We are pleased to have played a part in helping get this cheap yet effective treatment to more babies.”
This work was jointly funded by the National Institute for Health and Care Research Applied Research Collaboration West and the AHSN Network funded by NHS England. The Health Foundation funded the health economics evaluation. The authors declare that the study management group has no competing financial, professional, or personal interests that might have influenced the study design or conduct.
A version of this article first appeared on Medscape UK.
Mediterranean diet linked with fewer pregnancy complications
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
Decoding mechanisms of diabetic embryopathy suggests therapeutic targets
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.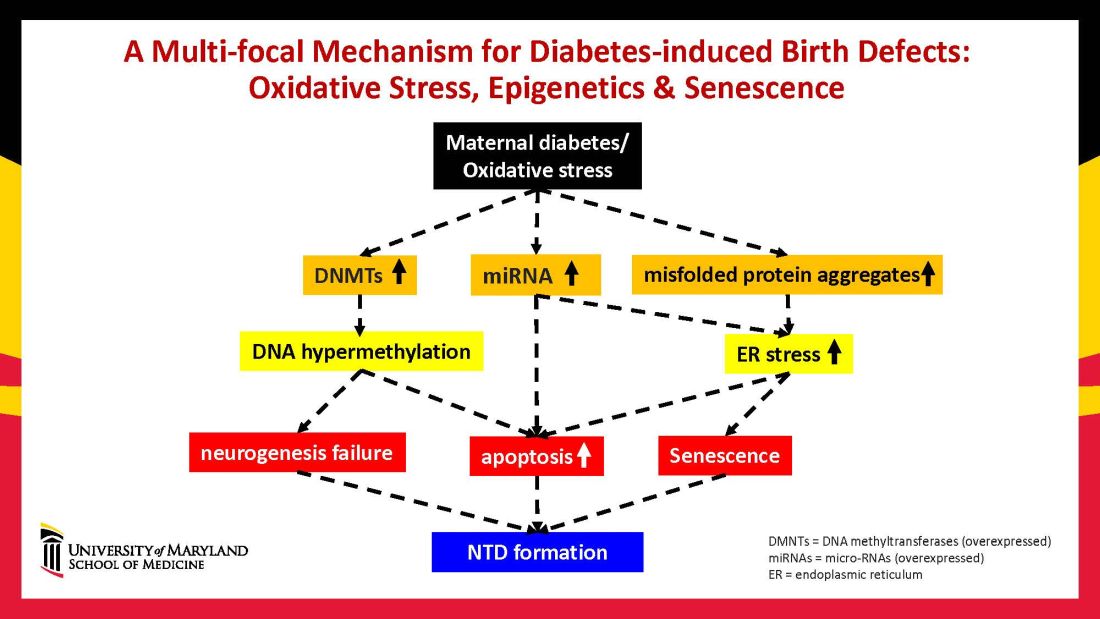
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.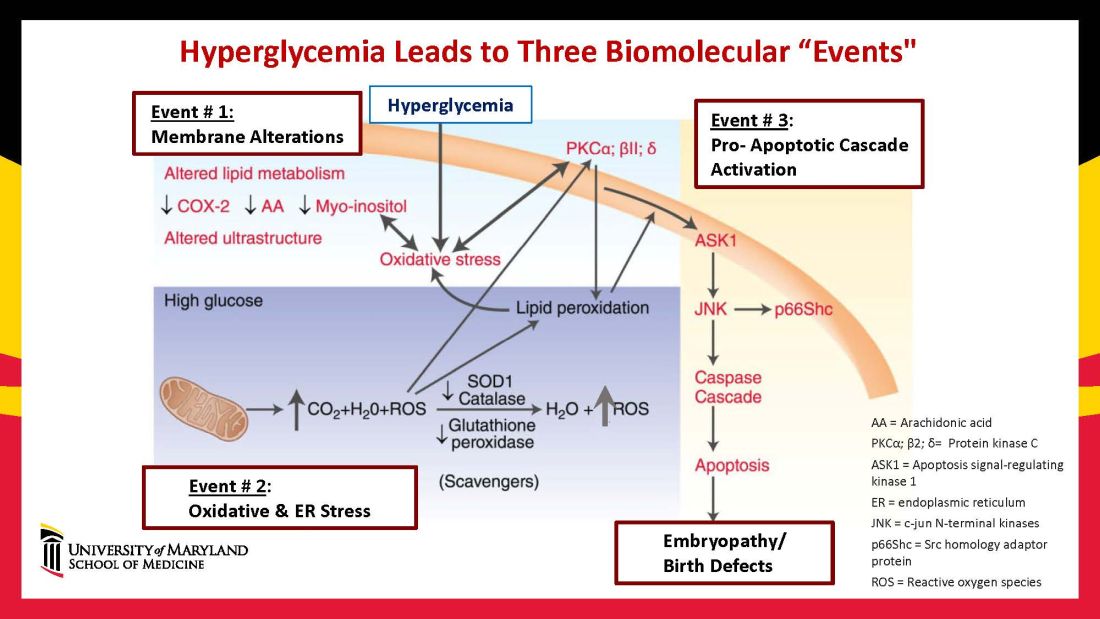
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9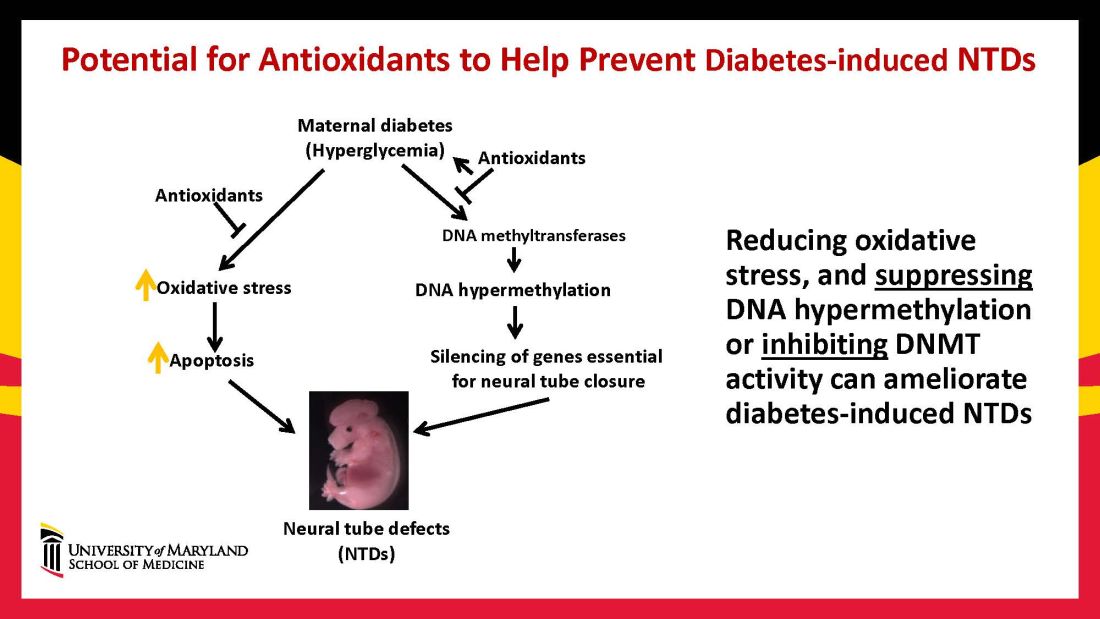
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
New electrodes made of sugar more effectively monitor mom’s health
A new type of electrode made from sugar could help doctors and researchers more effectively monitor contractions during preterm labor, a condition that precedes almost half of preterm births and is the leading cause of U.S. neonatal deaths.
The sensors, developed by engineers at the McKelvey School of Engineering at Washington University, St. Louis, could help us understand why some patients experience preterm labor, improve medical interventions, and save lives. In the experiment, the researchers built an array of the new electrodes and successfully tested it on a pregnant person in a lab.
The goal is a home-monitoring belt that is comfortable enough for patients to wear and accurate enough to be clinically useful. Built off a framework of sugar and conductive polymers, the thin electrodes have a sponge-like quality that allows them to hold more gel than standard electrodes, measure for 3 hours instead of 1, and resist artifacts created by patient movement. When tested on a pregnant woman, the new electrodes picked up clean signals even when the patient moved, said electrical engineer and article co-author Chuan Wang, PhD.
There is current technology that exists to monitor and map contractions during early labor, but the tests require hundreds of wire electrodes. Patients must sit still for half an hour while the electrodes are applied, then remain immobile for the test itself, which has a high sensitivity to movement.
“It’s very uncomfortable. In the clinical setting, the recording typically lasts for 15 minutes to half an hour. During that time, doctors want the patient to be still,” said Dr. Wang. “If the patient has to move, it’s going to introduce some artifacts, which is going to ruin the imaging process.”
Dr. Wang and colleagues wanted to develop an inexpensive new electrode that would be more comfortable for patients to wear for longer periods of time, yet sensitive enough to detect electrical signals in the body during preterm labor.
To do this, they used sugar structures to create a pliable electrode with a spongy structure. The new electrodes have micropores that hold conductive gel, increasing the amount of electrified surface area touching the skin.
“With the porous structure, we are effectively increasing the area by many, many times,” Dr. Wang said. “Because all those voids also contact the skin, increasing the contact area can boost the strength of the signal.”
With conventional electrodes, the gel dries quickly on the flat surface, causing signal quality to plummet. But the new electrodes can be used for “many hours” before drying out, according to Dr. Wang.
Additionally, the soft material of the new electrode acts “like a buffer” that absorbs motion and prevents the electrode from sliding around, according to Dr. Wang. That means patients can move while wearing the spongy electrodes without disturbing the recording of electrical signals in the body.
From sugar cube to spongy electrode
To create the new electrode, the researchers began by molding sugar into an electrode-shaped template. The template was then dipped into a liquid polymer, which oozed in between the grains of sugar. Next, the template underwent oven curing, emerging as a solid yet spongy structure. Hot water was then applied to dissolve the sugar.
The sugar structure is useful here because of the negative space around the grains, which is filled by the polymer – and then because of the negative space left when the sugar dissolves.
“When the sugar grains are removed, that’s where the pores are located,” Dr. Wang explained.
The sponge surface was then converted from hydrophobic to hydrophilic, thanks to an oxygen plasma treatment. Next, the sponge was blanketed in a layer of conductive polymer – a liquid that Dr. Wang likens to black ink – transforming it into an electrode. (Without the oxygen plasma step, the sponge wouldn’t have absorbed the conductive material.) After another oven-curing session, the device was affixed with wires and ready to be used.
The researchers are continuing to refine the concept and hope to develop a wireless wearable device with many spongy electrodes that record signals simultaneously – and that patients can use at home.
In addition to monitoring maternal and fetal health during labor, the researchers say the belt-like device could be used for other types of imaging and diagnosis.
“Depending on the scenario, different signals can be recorded,” Dr. Wang said. “It could be an EMG for a pregnant woman, or an ECG for an athlete or a patient with chronic cardiovascular disease that needs monitoring.”
This work was funded by the Bill & Melinda Gates Foundation (INV-005417, INV-035476). The authors acknowledge the Washington University in St. Louis Institute of Materials Science and Engineering for the use of instruments and staff assistance.
A version of this article first appeared on Medscape.com.
A new type of electrode made from sugar could help doctors and researchers more effectively monitor contractions during preterm labor, a condition that precedes almost half of preterm births and is the leading cause of U.S. neonatal deaths.
The sensors, developed by engineers at the McKelvey School of Engineering at Washington University, St. Louis, could help us understand why some patients experience preterm labor, improve medical interventions, and save lives. In the experiment, the researchers built an array of the new electrodes and successfully tested it on a pregnant person in a lab.
The goal is a home-monitoring belt that is comfortable enough for patients to wear and accurate enough to be clinically useful. Built off a framework of sugar and conductive polymers, the thin electrodes have a sponge-like quality that allows them to hold more gel than standard electrodes, measure for 3 hours instead of 1, and resist artifacts created by patient movement. When tested on a pregnant woman, the new electrodes picked up clean signals even when the patient moved, said electrical engineer and article co-author Chuan Wang, PhD.
There is current technology that exists to monitor and map contractions during early labor, but the tests require hundreds of wire electrodes. Patients must sit still for half an hour while the electrodes are applied, then remain immobile for the test itself, which has a high sensitivity to movement.
“It’s very uncomfortable. In the clinical setting, the recording typically lasts for 15 minutes to half an hour. During that time, doctors want the patient to be still,” said Dr. Wang. “If the patient has to move, it’s going to introduce some artifacts, which is going to ruin the imaging process.”
Dr. Wang and colleagues wanted to develop an inexpensive new electrode that would be more comfortable for patients to wear for longer periods of time, yet sensitive enough to detect electrical signals in the body during preterm labor.
To do this, they used sugar structures to create a pliable electrode with a spongy structure. The new electrodes have micropores that hold conductive gel, increasing the amount of electrified surface area touching the skin.
“With the porous structure, we are effectively increasing the area by many, many times,” Dr. Wang said. “Because all those voids also contact the skin, increasing the contact area can boost the strength of the signal.”
With conventional electrodes, the gel dries quickly on the flat surface, causing signal quality to plummet. But the new electrodes can be used for “many hours” before drying out, according to Dr. Wang.
Additionally, the soft material of the new electrode acts “like a buffer” that absorbs motion and prevents the electrode from sliding around, according to Dr. Wang. That means patients can move while wearing the spongy electrodes without disturbing the recording of electrical signals in the body.
From sugar cube to spongy electrode
To create the new electrode, the researchers began by molding sugar into an electrode-shaped template. The template was then dipped into a liquid polymer, which oozed in between the grains of sugar. Next, the template underwent oven curing, emerging as a solid yet spongy structure. Hot water was then applied to dissolve the sugar.
The sugar structure is useful here because of the negative space around the grains, which is filled by the polymer – and then because of the negative space left when the sugar dissolves.
“When the sugar grains are removed, that’s where the pores are located,” Dr. Wang explained.
The sponge surface was then converted from hydrophobic to hydrophilic, thanks to an oxygen plasma treatment. Next, the sponge was blanketed in a layer of conductive polymer – a liquid that Dr. Wang likens to black ink – transforming it into an electrode. (Without the oxygen plasma step, the sponge wouldn’t have absorbed the conductive material.) After another oven-curing session, the device was affixed with wires and ready to be used.
The researchers are continuing to refine the concept and hope to develop a wireless wearable device with many spongy electrodes that record signals simultaneously – and that patients can use at home.
In addition to monitoring maternal and fetal health during labor, the researchers say the belt-like device could be used for other types of imaging and diagnosis.
“Depending on the scenario, different signals can be recorded,” Dr. Wang said. “It could be an EMG for a pregnant woman, or an ECG for an athlete or a patient with chronic cardiovascular disease that needs monitoring.”
This work was funded by the Bill & Melinda Gates Foundation (INV-005417, INV-035476). The authors acknowledge the Washington University in St. Louis Institute of Materials Science and Engineering for the use of instruments and staff assistance.
A version of this article first appeared on Medscape.com.
A new type of electrode made from sugar could help doctors and researchers more effectively monitor contractions during preterm labor, a condition that precedes almost half of preterm births and is the leading cause of U.S. neonatal deaths.
The sensors, developed by engineers at the McKelvey School of Engineering at Washington University, St. Louis, could help us understand why some patients experience preterm labor, improve medical interventions, and save lives. In the experiment, the researchers built an array of the new electrodes and successfully tested it on a pregnant person in a lab.
The goal is a home-monitoring belt that is comfortable enough for patients to wear and accurate enough to be clinically useful. Built off a framework of sugar and conductive polymers, the thin electrodes have a sponge-like quality that allows them to hold more gel than standard electrodes, measure for 3 hours instead of 1, and resist artifacts created by patient movement. When tested on a pregnant woman, the new electrodes picked up clean signals even when the patient moved, said electrical engineer and article co-author Chuan Wang, PhD.
There is current technology that exists to monitor and map contractions during early labor, but the tests require hundreds of wire electrodes. Patients must sit still for half an hour while the electrodes are applied, then remain immobile for the test itself, which has a high sensitivity to movement.
“It’s very uncomfortable. In the clinical setting, the recording typically lasts for 15 minutes to half an hour. During that time, doctors want the patient to be still,” said Dr. Wang. “If the patient has to move, it’s going to introduce some artifacts, which is going to ruin the imaging process.”
Dr. Wang and colleagues wanted to develop an inexpensive new electrode that would be more comfortable for patients to wear for longer periods of time, yet sensitive enough to detect electrical signals in the body during preterm labor.
To do this, they used sugar structures to create a pliable electrode with a spongy structure. The new electrodes have micropores that hold conductive gel, increasing the amount of electrified surface area touching the skin.
“With the porous structure, we are effectively increasing the area by many, many times,” Dr. Wang said. “Because all those voids also contact the skin, increasing the contact area can boost the strength of the signal.”
With conventional electrodes, the gel dries quickly on the flat surface, causing signal quality to plummet. But the new electrodes can be used for “many hours” before drying out, according to Dr. Wang.
Additionally, the soft material of the new electrode acts “like a buffer” that absorbs motion and prevents the electrode from sliding around, according to Dr. Wang. That means patients can move while wearing the spongy electrodes without disturbing the recording of electrical signals in the body.
From sugar cube to spongy electrode
To create the new electrode, the researchers began by molding sugar into an electrode-shaped template. The template was then dipped into a liquid polymer, which oozed in between the grains of sugar. Next, the template underwent oven curing, emerging as a solid yet spongy structure. Hot water was then applied to dissolve the sugar.
The sugar structure is useful here because of the negative space around the grains, which is filled by the polymer – and then because of the negative space left when the sugar dissolves.
“When the sugar grains are removed, that’s where the pores are located,” Dr. Wang explained.
The sponge surface was then converted from hydrophobic to hydrophilic, thanks to an oxygen plasma treatment. Next, the sponge was blanketed in a layer of conductive polymer – a liquid that Dr. Wang likens to black ink – transforming it into an electrode. (Without the oxygen plasma step, the sponge wouldn’t have absorbed the conductive material.) After another oven-curing session, the device was affixed with wires and ready to be used.
The researchers are continuing to refine the concept and hope to develop a wireless wearable device with many spongy electrodes that record signals simultaneously – and that patients can use at home.
In addition to monitoring maternal and fetal health during labor, the researchers say the belt-like device could be used for other types of imaging and diagnosis.
“Depending on the scenario, different signals can be recorded,” Dr. Wang said. “It could be an EMG for a pregnant woman, or an ECG for an athlete or a patient with chronic cardiovascular disease that needs monitoring.”
This work was funded by the Bill & Melinda Gates Foundation (INV-005417, INV-035476). The authors acknowledge the Washington University in St. Louis Institute of Materials Science and Engineering for the use of instruments and staff assistance.
A version of this article first appeared on Medscape.com.
Shopping voucher incentives ‘doubles smoking quit rate in pregnancy’
Offering shopping vouchers to pregnant women as an incentive to quit smoking showed promising results, a study found, despite most participants relapsing after giving birth.
Rewarding pregnant women with up to £400 to spend on Main Street, in addition to usual support, more than doubled the proportion who were still smoke-free late in their pregnancy, and could save the National Health Service money in the long term, according to the research, published in the BMJ, led by the University of Glasgow and the University of York, England.
Although the proportion of women in the United Kingdom who smoke during pregnancy has halved over the past 20 years, those who still do are more reluctant to engage with cessation services.
Interventions using financial incentives were pioneered in the United States, but there is a lack of evidence for how effective they might be in the United Kingdom.
Vouchers linked to passing saliva tests
The phase 3 Cessation in Pregnancy Incentives Trial was based on an earlier feasibility study in Glasgow and involved 941 pregnant women aged 16 or older, with a mean age of 27.9 years when they were recruited, from seven stop-smoking services in Scotland, Northern Ireland, and England between January 2018 and April 2020. Participants self-reported that they smoked at least one cigarette a week.
The cohort was randomised into two groups: a control group who received usual stop smoking support that included the offer of counselling by trained workers combined with free nicotine-replacement therapy, and an intervention group who were given the same interventional support plus targets to receive LoveToShop vouchers.
Although vouchers to the value of £400 were on offer, earning them depended on successfully reaching four milestones. They received a first £50 voucher for engaging with stop-smoking services and setting a quit date and further vouchers for being declared smoke-free by biochemical verification at specific time points in the pregnancy.
Factors including the mother’s age, years of smoking, income, use of nicotine-replacement therapy and e-cigarettes, timing of birth, and birth weight were taken into account.
The study found that 71% of the participants in the incentive group engaged with stop-smoking services and set a quit date, compared with 64% in the control group. By late pregnancy, 126 participants (27%) of the 471 in the intervention group were smoke-free, compared with 58 (12%) of the 470 in the control group.
Most women in the trial went back to smoking
However, abstinence rates measured 6 months after giving birth were low in both groups: 6% in the intervention group vs. 4% in the control group.
The researchers also reported no significant differences in birth weight between the two groups.
Overall, the birth weight of babies from 443 intervention participants and 450 controls showed no significant difference between groups (average 3.18 kg vs. 3.13 kg).
The researchers did find a clinically important but not significant 10% increase in birth weight in the subset of participants who adhered with their treatment allocation, but they said further analysis is needed to better understand the relevance of this finding.
Severity of preterm birth was similar between groups, and all serious adverse events, such as miscarriages and stillbirths, were considered unrelated to the intervention.
The researchers acknowledged some limitations to their investigation, including that only 23% of women screened by stop-smoking services were enrolled, and that almost all participants were White. Also, the onset of COVID-19 disrupted some of the trial processes.
However, they concluded that their trial “supports implementation advocated in NICE [National Institute for Health and Care Excellence] guidelines by showing an effective, cost-effective, and generalisable pragmatic bolt-on U.K. format for incentive payments” to reduce smoking rates in pregnancy.
In a linked editorial, Daniel Kotz from the Heinrich Heine University, Düsseldorf, Germany, and Jasper Been from University Medical Center, Rotterdam, the Netherlands, pointed out that “partners of most pregnant women who smoke are also smokers,” which needed to be addressed. However, they wrote: “The medical community now has good evidence supporting effective tools, such as financial incentives, to reduce the health burden associated with tobacco smoking during pregnancy. These tools should be implemented wherever possible to protect and improve the health of women, their children, and their families.”
The trial was funded by Cancer Research UK; Chief Scientist Office, Scottish Government; HSC Public Health Agency Northern Ireland; Health and Social Care R&D Division NI Opportunity-Led Research Award; Chest Heart and Stroke Northern Ireland; Scottish Cot Death Trust; and Lullaby Trust 272. The authors declare no competing interests.
A version of this article first appeared on MedscapeUK.
Offering shopping vouchers to pregnant women as an incentive to quit smoking showed promising results, a study found, despite most participants relapsing after giving birth.
Rewarding pregnant women with up to £400 to spend on Main Street, in addition to usual support, more than doubled the proportion who were still smoke-free late in their pregnancy, and could save the National Health Service money in the long term, according to the research, published in the BMJ, led by the University of Glasgow and the University of York, England.
Although the proportion of women in the United Kingdom who smoke during pregnancy has halved over the past 20 years, those who still do are more reluctant to engage with cessation services.
Interventions using financial incentives were pioneered in the United States, but there is a lack of evidence for how effective they might be in the United Kingdom.
Vouchers linked to passing saliva tests
The phase 3 Cessation in Pregnancy Incentives Trial was based on an earlier feasibility study in Glasgow and involved 941 pregnant women aged 16 or older, with a mean age of 27.9 years when they were recruited, from seven stop-smoking services in Scotland, Northern Ireland, and England between January 2018 and April 2020. Participants self-reported that they smoked at least one cigarette a week.
The cohort was randomised into two groups: a control group who received usual stop smoking support that included the offer of counselling by trained workers combined with free nicotine-replacement therapy, and an intervention group who were given the same interventional support plus targets to receive LoveToShop vouchers.
Although vouchers to the value of £400 were on offer, earning them depended on successfully reaching four milestones. They received a first £50 voucher for engaging with stop-smoking services and setting a quit date and further vouchers for being declared smoke-free by biochemical verification at specific time points in the pregnancy.
Factors including the mother’s age, years of smoking, income, use of nicotine-replacement therapy and e-cigarettes, timing of birth, and birth weight were taken into account.
The study found that 71% of the participants in the incentive group engaged with stop-smoking services and set a quit date, compared with 64% in the control group. By late pregnancy, 126 participants (27%) of the 471 in the intervention group were smoke-free, compared with 58 (12%) of the 470 in the control group.
Most women in the trial went back to smoking
However, abstinence rates measured 6 months after giving birth were low in both groups: 6% in the intervention group vs. 4% in the control group.
The researchers also reported no significant differences in birth weight between the two groups.
Overall, the birth weight of babies from 443 intervention participants and 450 controls showed no significant difference between groups (average 3.18 kg vs. 3.13 kg).
The researchers did find a clinically important but not significant 10% increase in birth weight in the subset of participants who adhered with their treatment allocation, but they said further analysis is needed to better understand the relevance of this finding.
Severity of preterm birth was similar between groups, and all serious adverse events, such as miscarriages and stillbirths, were considered unrelated to the intervention.
The researchers acknowledged some limitations to their investigation, including that only 23% of women screened by stop-smoking services were enrolled, and that almost all participants were White. Also, the onset of COVID-19 disrupted some of the trial processes.
However, they concluded that their trial “supports implementation advocated in NICE [National Institute for Health and Care Excellence] guidelines by showing an effective, cost-effective, and generalisable pragmatic bolt-on U.K. format for incentive payments” to reduce smoking rates in pregnancy.
In a linked editorial, Daniel Kotz from the Heinrich Heine University, Düsseldorf, Germany, and Jasper Been from University Medical Center, Rotterdam, the Netherlands, pointed out that “partners of most pregnant women who smoke are also smokers,” which needed to be addressed. However, they wrote: “The medical community now has good evidence supporting effective tools, such as financial incentives, to reduce the health burden associated with tobacco smoking during pregnancy. These tools should be implemented wherever possible to protect and improve the health of women, their children, and their families.”
The trial was funded by Cancer Research UK; Chief Scientist Office, Scottish Government; HSC Public Health Agency Northern Ireland; Health and Social Care R&D Division NI Opportunity-Led Research Award; Chest Heart and Stroke Northern Ireland; Scottish Cot Death Trust; and Lullaby Trust 272. The authors declare no competing interests.
A version of this article first appeared on MedscapeUK.
Offering shopping vouchers to pregnant women as an incentive to quit smoking showed promising results, a study found, despite most participants relapsing after giving birth.
Rewarding pregnant women with up to £400 to spend on Main Street, in addition to usual support, more than doubled the proportion who were still smoke-free late in their pregnancy, and could save the National Health Service money in the long term, according to the research, published in the BMJ, led by the University of Glasgow and the University of York, England.
Although the proportion of women in the United Kingdom who smoke during pregnancy has halved over the past 20 years, those who still do are more reluctant to engage with cessation services.
Interventions using financial incentives were pioneered in the United States, but there is a lack of evidence for how effective they might be in the United Kingdom.
Vouchers linked to passing saliva tests
The phase 3 Cessation in Pregnancy Incentives Trial was based on an earlier feasibility study in Glasgow and involved 941 pregnant women aged 16 or older, with a mean age of 27.9 years when they were recruited, from seven stop-smoking services in Scotland, Northern Ireland, and England between January 2018 and April 2020. Participants self-reported that they smoked at least one cigarette a week.
The cohort was randomised into two groups: a control group who received usual stop smoking support that included the offer of counselling by trained workers combined with free nicotine-replacement therapy, and an intervention group who were given the same interventional support plus targets to receive LoveToShop vouchers.
Although vouchers to the value of £400 were on offer, earning them depended on successfully reaching four milestones. They received a first £50 voucher for engaging with stop-smoking services and setting a quit date and further vouchers for being declared smoke-free by biochemical verification at specific time points in the pregnancy.
Factors including the mother’s age, years of smoking, income, use of nicotine-replacement therapy and e-cigarettes, timing of birth, and birth weight were taken into account.
The study found that 71% of the participants in the incentive group engaged with stop-smoking services and set a quit date, compared with 64% in the control group. By late pregnancy, 126 participants (27%) of the 471 in the intervention group were smoke-free, compared with 58 (12%) of the 470 in the control group.
Most women in the trial went back to smoking
However, abstinence rates measured 6 months after giving birth were low in both groups: 6% in the intervention group vs. 4% in the control group.
The researchers also reported no significant differences in birth weight between the two groups.
Overall, the birth weight of babies from 443 intervention participants and 450 controls showed no significant difference between groups (average 3.18 kg vs. 3.13 kg).
The researchers did find a clinically important but not significant 10% increase in birth weight in the subset of participants who adhered with their treatment allocation, but they said further analysis is needed to better understand the relevance of this finding.
Severity of preterm birth was similar between groups, and all serious adverse events, such as miscarriages and stillbirths, were considered unrelated to the intervention.
The researchers acknowledged some limitations to their investigation, including that only 23% of women screened by stop-smoking services were enrolled, and that almost all participants were White. Also, the onset of COVID-19 disrupted some of the trial processes.
However, they concluded that their trial “supports implementation advocated in NICE [National Institute for Health and Care Excellence] guidelines by showing an effective, cost-effective, and generalisable pragmatic bolt-on U.K. format for incentive payments” to reduce smoking rates in pregnancy.
In a linked editorial, Daniel Kotz from the Heinrich Heine University, Düsseldorf, Germany, and Jasper Been from University Medical Center, Rotterdam, the Netherlands, pointed out that “partners of most pregnant women who smoke are also smokers,” which needed to be addressed. However, they wrote: “The medical community now has good evidence supporting effective tools, such as financial incentives, to reduce the health burden associated with tobacco smoking during pregnancy. These tools should be implemented wherever possible to protect and improve the health of women, their children, and their families.”
The trial was funded by Cancer Research UK; Chief Scientist Office, Scottish Government; HSC Public Health Agency Northern Ireland; Health and Social Care R&D Division NI Opportunity-Led Research Award; Chest Heart and Stroke Northern Ireland; Scottish Cot Death Trust; and Lullaby Trust 272. The authors declare no competing interests.
A version of this article first appeared on MedscapeUK.
FROM BMJ
FDA panel recommends withdrawal of Makena for preterm birth
A federal advisory panel recommended the United States withdraw from the market an injection given to women at risk for giving birth prematurely. Many of its members argued this step is needed to allow further testing to see if this drug actually works.
The Food and Drug Administration has been seeking to pull the approval of hydroxyprogesterone caproate (17P) injection (Makena, Covis) since 2020, after the drug failed to show a benefit in the PROLONG study. This study was meant as a confirmatory trial for the accelerated approval the FDA granted Makena in 2011 based on promising results from an earlier small study, known as the Meis trial. The manufacturer, Covis, contends that the flaws in the PROLONG study made Makena appear ineffective.
The FDA asked its Obstetrics, Reproductive and Urologic Drugs Advisory Committee to review the evidence gathered to date on Makena at a hearing that ran from Oct. 17 to Oct. 19. At the conclusion, the FDA asked the committee to vote on whether the agency should allow Makena to remain on the market while an appropriate confirmatory study is designed and conducted.
The vote was 14-1 against this plan.
There needs to be another study as a “tiebreaker” to determine which of the previous Makena trials was correct, said FDA panelist Michael K. Lindsay, MD, MPH, who is also director of the division of maternal-fetal medicine for Grady and Emory University Hospital Midtown, Atlanta.
“I think there needs to be another trial,” Dr. Lindsay said. “If you can do the trial without the medication being FDA approved, then I am supportive of that.”
Members of the FDA panel noted the difficulties that would ensue if Covis attempted further study of Makena with the drug still approved, including difficulties in recruiting patients. Indeed, there were delays in recruiting patients for the PROLONG trial in part because Makena was perceived as the standard of care for pregnant women who had a prior spontaneous preterm birth. That led to efforts to enroll patients outside of the United States, particularly in Eastern European countries.
Panelist Cassandra E. Henderson, MD, of the New York-based Garden OB/GYN practice, was the dissenter in the 14-1 vote.
Withdrawing the approval of Makena may lead to increased use of pharmacy-compounded versions of this medicine, as women look for options to try to extend their pregnancies, she said.
“They may seek it in other ways and get something that we don’t have any control over, and we don’t know what the fetus may be exposed to,” Dr. Henderson said.
Dr. Henderson also said there should be greater discussion with patients about questions of potential “intergenerational risk” because of fetal exposure to the medicine. Covis could add a registry similar to the University of Chicago’s DES Program to its research program for Makena, she said.
Race-based argument
Covis has been fighting to keep the Makena approval by offering theories for why the PROLONG study failed to show a benefit for the drug.
Covis emphasizes the different racial make-up of patients in the two trials. Black women composed 59% of the Meis study population, compared with only 6.7% for the PROLONG study, Covis said in its briefing document for the hearing. The Luxembourg-based company also says that there may have been unreliable estimates of the gestational age in the PROLONG trial, which enrolled many subjects in Ukraine and Russia.
During deliberations among panelists on Oct. 19, Dr. Henderson emphasized a need to consider other factors that may have been involved and encouraged continued study of the drug in Black women. She dismissed the idea of a race-based difference being the explanation for the difference between the two trials, but instead stressed that race serves as a marker for inequities, which are known to increase risk for preterm birth.
“Targeting a population that is at risk, particularly Black women in the United States, may show something that would be beneficial” from Makena, Dr. Henderson said.
Other physicians have argued that this approach would actually put Black women and children at greater risk of an ineffective drug with potential side effects.
“The drug is not proven to work so keeping it on the market to be injected into Black women to see what subgroups it might work in essentially amounts to experimentation,” said Adam Urato, MD, chief of maternal-fetal medicine at MetroWest Medical Center in Framingham, Mass., during the public comment session of the hearing.
The vote marks the second time that the FDA’s advisers on reproductive health have told the agency that the evidence gathered on the drug does not support its use. An advisory committee also cast votes against the drug at a 2019 meeting.
The rate of preterm birth in Black women in 2020 was 14.4%, significantly higher than the rate of preterm birth in White or Hispanic women, 9.1% and 9.8%, respectively, according to the Centers for Disease Control and Prevention. The potential for harm to children from premature birth led the FDA to clear Makena through the accelerated approval pathway, said Patrizia Cavazzoni, MD, the director of FDA’s Center for Drug Evaluation and Research, in the opening session of the hearing.
“We once thought Makena was likely to be part of the answer to that problem,” Dr. Cavazzoni said. “Unfortunately we no longer do, based on the evidence available.”
A federal advisory panel recommended the United States withdraw from the market an injection given to women at risk for giving birth prematurely. Many of its members argued this step is needed to allow further testing to see if this drug actually works.
The Food and Drug Administration has been seeking to pull the approval of hydroxyprogesterone caproate (17P) injection (Makena, Covis) since 2020, after the drug failed to show a benefit in the PROLONG study. This study was meant as a confirmatory trial for the accelerated approval the FDA granted Makena in 2011 based on promising results from an earlier small study, known as the Meis trial. The manufacturer, Covis, contends that the flaws in the PROLONG study made Makena appear ineffective.
The FDA asked its Obstetrics, Reproductive and Urologic Drugs Advisory Committee to review the evidence gathered to date on Makena at a hearing that ran from Oct. 17 to Oct. 19. At the conclusion, the FDA asked the committee to vote on whether the agency should allow Makena to remain on the market while an appropriate confirmatory study is designed and conducted.
The vote was 14-1 against this plan.
There needs to be another study as a “tiebreaker” to determine which of the previous Makena trials was correct, said FDA panelist Michael K. Lindsay, MD, MPH, who is also director of the division of maternal-fetal medicine for Grady and Emory University Hospital Midtown, Atlanta.
“I think there needs to be another trial,” Dr. Lindsay said. “If you can do the trial without the medication being FDA approved, then I am supportive of that.”
Members of the FDA panel noted the difficulties that would ensue if Covis attempted further study of Makena with the drug still approved, including difficulties in recruiting patients. Indeed, there were delays in recruiting patients for the PROLONG trial in part because Makena was perceived as the standard of care for pregnant women who had a prior spontaneous preterm birth. That led to efforts to enroll patients outside of the United States, particularly in Eastern European countries.
Panelist Cassandra E. Henderson, MD, of the New York-based Garden OB/GYN practice, was the dissenter in the 14-1 vote.
Withdrawing the approval of Makena may lead to increased use of pharmacy-compounded versions of this medicine, as women look for options to try to extend their pregnancies, she said.
“They may seek it in other ways and get something that we don’t have any control over, and we don’t know what the fetus may be exposed to,” Dr. Henderson said.
Dr. Henderson also said there should be greater discussion with patients about questions of potential “intergenerational risk” because of fetal exposure to the medicine. Covis could add a registry similar to the University of Chicago’s DES Program to its research program for Makena, she said.
Race-based argument
Covis has been fighting to keep the Makena approval by offering theories for why the PROLONG study failed to show a benefit for the drug.
Covis emphasizes the different racial make-up of patients in the two trials. Black women composed 59% of the Meis study population, compared with only 6.7% for the PROLONG study, Covis said in its briefing document for the hearing. The Luxembourg-based company also says that there may have been unreliable estimates of the gestational age in the PROLONG trial, which enrolled many subjects in Ukraine and Russia.
During deliberations among panelists on Oct. 19, Dr. Henderson emphasized a need to consider other factors that may have been involved and encouraged continued study of the drug in Black women. She dismissed the idea of a race-based difference being the explanation for the difference between the two trials, but instead stressed that race serves as a marker for inequities, which are known to increase risk for preterm birth.
“Targeting a population that is at risk, particularly Black women in the United States, may show something that would be beneficial” from Makena, Dr. Henderson said.
Other physicians have argued that this approach would actually put Black women and children at greater risk of an ineffective drug with potential side effects.
“The drug is not proven to work so keeping it on the market to be injected into Black women to see what subgroups it might work in essentially amounts to experimentation,” said Adam Urato, MD, chief of maternal-fetal medicine at MetroWest Medical Center in Framingham, Mass., during the public comment session of the hearing.
The vote marks the second time that the FDA’s advisers on reproductive health have told the agency that the evidence gathered on the drug does not support its use. An advisory committee also cast votes against the drug at a 2019 meeting.
The rate of preterm birth in Black women in 2020 was 14.4%, significantly higher than the rate of preterm birth in White or Hispanic women, 9.1% and 9.8%, respectively, according to the Centers for Disease Control and Prevention. The potential for harm to children from premature birth led the FDA to clear Makena through the accelerated approval pathway, said Patrizia Cavazzoni, MD, the director of FDA’s Center for Drug Evaluation and Research, in the opening session of the hearing.
“We once thought Makena was likely to be part of the answer to that problem,” Dr. Cavazzoni said. “Unfortunately we no longer do, based on the evidence available.”
A federal advisory panel recommended the United States withdraw from the market an injection given to women at risk for giving birth prematurely. Many of its members argued this step is needed to allow further testing to see if this drug actually works.
The Food and Drug Administration has been seeking to pull the approval of hydroxyprogesterone caproate (17P) injection (Makena, Covis) since 2020, after the drug failed to show a benefit in the PROLONG study. This study was meant as a confirmatory trial for the accelerated approval the FDA granted Makena in 2011 based on promising results from an earlier small study, known as the Meis trial. The manufacturer, Covis, contends that the flaws in the PROLONG study made Makena appear ineffective.
The FDA asked its Obstetrics, Reproductive and Urologic Drugs Advisory Committee to review the evidence gathered to date on Makena at a hearing that ran from Oct. 17 to Oct. 19. At the conclusion, the FDA asked the committee to vote on whether the agency should allow Makena to remain on the market while an appropriate confirmatory study is designed and conducted.
The vote was 14-1 against this plan.
There needs to be another study as a “tiebreaker” to determine which of the previous Makena trials was correct, said FDA panelist Michael K. Lindsay, MD, MPH, who is also director of the division of maternal-fetal medicine for Grady and Emory University Hospital Midtown, Atlanta.
“I think there needs to be another trial,” Dr. Lindsay said. “If you can do the trial without the medication being FDA approved, then I am supportive of that.”
Members of the FDA panel noted the difficulties that would ensue if Covis attempted further study of Makena with the drug still approved, including difficulties in recruiting patients. Indeed, there were delays in recruiting patients for the PROLONG trial in part because Makena was perceived as the standard of care for pregnant women who had a prior spontaneous preterm birth. That led to efforts to enroll patients outside of the United States, particularly in Eastern European countries.
Panelist Cassandra E. Henderson, MD, of the New York-based Garden OB/GYN practice, was the dissenter in the 14-1 vote.
Withdrawing the approval of Makena may lead to increased use of pharmacy-compounded versions of this medicine, as women look for options to try to extend their pregnancies, she said.
“They may seek it in other ways and get something that we don’t have any control over, and we don’t know what the fetus may be exposed to,” Dr. Henderson said.
Dr. Henderson also said there should be greater discussion with patients about questions of potential “intergenerational risk” because of fetal exposure to the medicine. Covis could add a registry similar to the University of Chicago’s DES Program to its research program for Makena, she said.
Race-based argument
Covis has been fighting to keep the Makena approval by offering theories for why the PROLONG study failed to show a benefit for the drug.
Covis emphasizes the different racial make-up of patients in the two trials. Black women composed 59% of the Meis study population, compared with only 6.7% for the PROLONG study, Covis said in its briefing document for the hearing. The Luxembourg-based company also says that there may have been unreliable estimates of the gestational age in the PROLONG trial, which enrolled many subjects in Ukraine and Russia.
During deliberations among panelists on Oct. 19, Dr. Henderson emphasized a need to consider other factors that may have been involved and encouraged continued study of the drug in Black women. She dismissed the idea of a race-based difference being the explanation for the difference between the two trials, but instead stressed that race serves as a marker for inequities, which are known to increase risk for preterm birth.
“Targeting a population that is at risk, particularly Black women in the United States, may show something that would be beneficial” from Makena, Dr. Henderson said.
Other physicians have argued that this approach would actually put Black women and children at greater risk of an ineffective drug with potential side effects.
“The drug is not proven to work so keeping it on the market to be injected into Black women to see what subgroups it might work in essentially amounts to experimentation,” said Adam Urato, MD, chief of maternal-fetal medicine at MetroWest Medical Center in Framingham, Mass., during the public comment session of the hearing.
The vote marks the second time that the FDA’s advisers on reproductive health have told the agency that the evidence gathered on the drug does not support its use. An advisory committee also cast votes against the drug at a 2019 meeting.
The rate of preterm birth in Black women in 2020 was 14.4%, significantly higher than the rate of preterm birth in White or Hispanic women, 9.1% and 9.8%, respectively, according to the Centers for Disease Control and Prevention. The potential for harm to children from premature birth led the FDA to clear Makena through the accelerated approval pathway, said Patrizia Cavazzoni, MD, the director of FDA’s Center for Drug Evaluation and Research, in the opening session of the hearing.
“We once thought Makena was likely to be part of the answer to that problem,” Dr. Cavazzoni said. “Unfortunately we no longer do, based on the evidence available.”


