User login
When burnout is moral injury
Several years have passed since I stood among a cohort of eager medical students wearing regalia that signaled a new beginning. Four years of grueling study culminated in a cacophony of unified voices, each reciting a pledge that I had longed to take since early adolescence. Together we celebrated, triumphant despite innumerable exams and various iterations of the Socratic method – all under the guise of assessing knowledge while in truth seeking to insidiously erode the crowd of prospective physicians. Yet our anxiety and uncertainty melted away as names were called, hands firmly clasped, and tassels transposed. For a moment in time, we stood on the precipice of victory, enthusiastic albeit oblivious of the tremendous obstacles that loomed ahead.
Wistfully I reminisce about the unequivocal joy that abounds within the protective shield of naiveté. Specifically, I think about that time when the edict of medicine and the art of being a physician felt congruent. Yet, reality is fickle and often supersedes expectation. Occasionally my thoughts drift to the early days of residency – a time during which the emotional weight of caring for vulnerable patients while learning to master my chosen specialty felt woefully insurmountable. I recall wading blindly through each rotation attempting to emulate the competent and compassionate care so effortlessly demonstrated by senior physicians as they moved through the health care system with apparent ease. They stepped fluidly, as I watched in awe through rose-tinted glasses.
As months passed into years, my perception cleared. What I initially viewed as graceful patient care belied a complex tapestry of health care workers often pressured into arduous decisions, not necessarily in service of a well-constructed treatment plan. Gradually, formidable barriers emerged, guidelines and restrictions embedded within a confining path that suffocated those who dared to cross it. As a result, a field built on the foundations of autonomy, benevolence, and nonmaleficence was slowly engulfed by a system fraught with contrivances. Amid such stressors, physical and psychological health grows tenuous. Classically, this overwhelming feeling of distress is recognized as burnout. Studies reformulated this malady to that which was first described in Vietnam war veterans, a condition known as “moral injury.”
The impact of burnout
To explain the development – and explore the complexities – of moral injury, we must return to 1975 when the term burnout was initially formulated by Herbert Freudenberger, PhD, a psychologist renowned for his work in substance use disorders, psychoanalysis, and clinical education.1 Dr. Freudenberger’s studies noted incidences of heightened emotional and physical distress in his colleagues working in substance abuse and other clinics. He sought to define these experiences as well as understand his own battle with malaise, apathy, and frustration.1 Ultimately, Dr. Freudenberger described burnout as “Becoming exhausted by making excessive demands on energy, strength, or resources in the workplace.”2 Although it characteristically overlaps with depression and anxiety, burnout is conceptualized as a separate entity specifically forged within a context of perfectionism, integrity, and self-sacrifice.2 Such qualities are integral in health care and, as a result, physicians are particularly vulnerable.
Since Dr. Freudenberger published “Burnout: The High Cost of Achievement” in 1980, immense research has assisted in not only identifying critical factors that contribute to its development but also the detrimental effects it has on physiological health.3 These include exhaustion from poor work conditions and extreme commitment to employee responsibilities that in turn precipitate mood destabilization and impaired work performance.3 Furthermore, research has also demonstrated that burnout triggers alterations in neural circuitry via the prefrontal cortex and the amygdala, structures critical for emotional regulation.4 To combat the ill effects of burnout while maintaining productivity and maximizing profit, several high-profile corporations instituted changes focusing on self-care, wellness, benefits, and incentives. Although these modifications are effective in decreasing the rate of employee turnover, such strategies are not easily transferable to health care. In fact, the rate of physician burnout has steadily increased over the past two decades as the business of medicine shifts towards longer hours, decreased reimbursement rates, and inexhaustible insurance stipulations.2,5 Consequently, occupational dissatisfaction increases the risk of cynicism, frustration with patients, internalization of failure, and likelihood of early retirement.5 Moreover, burnout may also fracture interpersonal relationships as well as precipitate errors, negative patient outcomes, malpractice, and development of severe mental health conditions associated with high morbidity and mortality.5,8
Although the concept of burnout is critical in understanding the side effects of stereotypical workplace culture, critics of the concept bemoan a suggestion of individual blame.6,8 In essence, they argue that burnout is explained as a side effect of toxic workplace conditions, but covertly represents a lack of resilience, motivation, and ambition to thrive in a physically or emotionally taxing occupational setting.6,8 Thus, the responsibility of acclimation lies upon the impacted individuals rather than the employer. For this reason, many strategies to ameliorate burnout are focused on the individual, including meditation, wellness retreats, creating or adjusting self-care regimens, or in some cases psychotherapy and psychopharmacology.6 Whereas burnout may respond (at least partially) to such interventions, without altering the causal factors, it is unlikely to remit. This is especially the case in health care, where systemic constraints lie beyond the control of an individual physician. Rather than promoting or specifically relying upon personal improvement and recovery, amendments are needed on multiple levels to affect meaningful change.
Moral injury
Similar to burnout, moral injury was not initially conceived within the scope of health care. In the 1990s Jonathan Shay, MD, PhD, identified veterans presenting with symptoms mimicking PTSD that failed to respond to standard, well established and efficacious treatments.9-11 With further analysis he determined that veterans who demonstrated minimal improvement reported similar histories of guilt, shame, and disgust following perceived injustices enacted or abetted by immoral leaders.10,11 Ultimately Shay identified three components of moral injury: 1. A betrayal of what is morally right; 2. By someone who holds legitimate priority; 3. In a high stakes situation.10
This definition was further modified in 2007 by Brett Linz, PhD, and colleagues as: “Perpetuating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”10,11 By expanding this description to include distress experienced by physicians and health care workers, Wendy Dean and Simon Talbot (in 2018 and 2019 respectively) explored how the health care system leads practitioners to deliver what they identify as substandard treatment.6-8 This results in disillusionment and lays the foundation for ethical and moral dilemmas in clinicians.
Themes of moral injury are repeatedly cited in various surveys and studies as a cause for occupational dissatisfaction. As physicians and other health care professionals reel from the aftermath of COVID-19, the effects of reconfiguring medicine into a business-oriented framework are glaringly conspicuous. Vast hospital nursing shortages, high patient census exacerbated by the political misuse and polarization of science, and insufficient availability of psychiatric beds, have culminated in a deluge of psychological strain in emergency medical physicians. Furthermore, pressure from administrators, mandated patient satisfaction measures, tedious electronic medical record systems, and copious licensing and certification requirements, contribute to physician distress as they attempt to navigate a system that challenges the vows which they swore to uphold.8 Because the cost of pursuing a medical degree frequently necessitates acquisition of loans that, without a physician income, may be difficult to repay,9 many doctors feel trapped within a seemingly endless cycle of misgiving that contributes to emotional exhaustion, pessimism, and low morale.
In my next series of The Myth of the Superdoctor columns, we will explore various factors that potentiate risk of moral injury. From medical school and residency training to corporate infrastructure and insurance obstacles, I will seek to discern and deliberate strategies for repair and rehabilitation. It is my hope that together we will illuminate the myriad complexities within the business of medicine, and become advocates and harbingers of change not only for physicians and health care workers but also for the sake of our patients and their families.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
References
1. King N. When a Psychologist Succumbed to Stress, He Coined The Term Burnout. 2016 Dec 8. NPR: All Things Considered.
2. Maslach C and Leiter MP. World Psychiatry. 2016 Jun;15(2):103-11. doi: 10.1002/wps.20311.
3. InformedHealth.org and Institute for Quality and Efficiency in Health Care. Depression: What is burnout?. https://www.ncbi.nlm.nih.gov/books/NBK279286/.
4. Michel A. Burnout and the Brain. Observer. 2016 Jan 29. https://www.psychologicalscience.org/observer/burnout-and-the-brain.
5. Patel RS et al. Behav Sci. 2018;8(11):98. doi:10.3390/bs8110098.
6. Dean W and Talbot S. Physicians aren’t ‘burning out.’ They’re suffering from moral injury. Stat. 2018 Jul 26. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/.
7. Dean W and Talbot S. Moral injury and burnout in medicine: A year of lessons learned. Stat. 2019 Jul 26. https://www.statnews.com/2019/07/26/moral-injury-burnout-medicine-lessons-learned/.
8. Dean W et al. Reframing Clinician Distress: Moral Injury Not Burnout. Fed Pract. 2019 Sep; 36(9):400-2. https://www.mdedge.com/fedprac/article/207458/mental-health/reframing-clinician-distress-moral-injury-not-burnout.
9. Bailey M. Beyond Burnout: Docs Decry ‘Moral Injury’ From Financial Pressures of Health Care. KHN. 2020 Feb 4. https://khn.org/news/beyond-burnout-docs-decry-moral-injury-from-financial-pressures-of-health-care/.
10. Litz B et al. Clin Psychol Rev. 2009 Dec;29(8):695-706. doi: 10.1016/j.cpr.2009.07.003.
11. Norman S and Maguen S. Moral Injury. PTSD: National Center for PTSD. https://www.ptsd.va.gov/professional/treat/cooccurring/moral_injury.asp.
Several years have passed since I stood among a cohort of eager medical students wearing regalia that signaled a new beginning. Four years of grueling study culminated in a cacophony of unified voices, each reciting a pledge that I had longed to take since early adolescence. Together we celebrated, triumphant despite innumerable exams and various iterations of the Socratic method – all under the guise of assessing knowledge while in truth seeking to insidiously erode the crowd of prospective physicians. Yet our anxiety and uncertainty melted away as names were called, hands firmly clasped, and tassels transposed. For a moment in time, we stood on the precipice of victory, enthusiastic albeit oblivious of the tremendous obstacles that loomed ahead.
Wistfully I reminisce about the unequivocal joy that abounds within the protective shield of naiveté. Specifically, I think about that time when the edict of medicine and the art of being a physician felt congruent. Yet, reality is fickle and often supersedes expectation. Occasionally my thoughts drift to the early days of residency – a time during which the emotional weight of caring for vulnerable patients while learning to master my chosen specialty felt woefully insurmountable. I recall wading blindly through each rotation attempting to emulate the competent and compassionate care so effortlessly demonstrated by senior physicians as they moved through the health care system with apparent ease. They stepped fluidly, as I watched in awe through rose-tinted glasses.
As months passed into years, my perception cleared. What I initially viewed as graceful patient care belied a complex tapestry of health care workers often pressured into arduous decisions, not necessarily in service of a well-constructed treatment plan. Gradually, formidable barriers emerged, guidelines and restrictions embedded within a confining path that suffocated those who dared to cross it. As a result, a field built on the foundations of autonomy, benevolence, and nonmaleficence was slowly engulfed by a system fraught with contrivances. Amid such stressors, physical and psychological health grows tenuous. Classically, this overwhelming feeling of distress is recognized as burnout. Studies reformulated this malady to that which was first described in Vietnam war veterans, a condition known as “moral injury.”
The impact of burnout
To explain the development – and explore the complexities – of moral injury, we must return to 1975 when the term burnout was initially formulated by Herbert Freudenberger, PhD, a psychologist renowned for his work in substance use disorders, psychoanalysis, and clinical education.1 Dr. Freudenberger’s studies noted incidences of heightened emotional and physical distress in his colleagues working in substance abuse and other clinics. He sought to define these experiences as well as understand his own battle with malaise, apathy, and frustration.1 Ultimately, Dr. Freudenberger described burnout as “Becoming exhausted by making excessive demands on energy, strength, or resources in the workplace.”2 Although it characteristically overlaps with depression and anxiety, burnout is conceptualized as a separate entity specifically forged within a context of perfectionism, integrity, and self-sacrifice.2 Such qualities are integral in health care and, as a result, physicians are particularly vulnerable.
Since Dr. Freudenberger published “Burnout: The High Cost of Achievement” in 1980, immense research has assisted in not only identifying critical factors that contribute to its development but also the detrimental effects it has on physiological health.3 These include exhaustion from poor work conditions and extreme commitment to employee responsibilities that in turn precipitate mood destabilization and impaired work performance.3 Furthermore, research has also demonstrated that burnout triggers alterations in neural circuitry via the prefrontal cortex and the amygdala, structures critical for emotional regulation.4 To combat the ill effects of burnout while maintaining productivity and maximizing profit, several high-profile corporations instituted changes focusing on self-care, wellness, benefits, and incentives. Although these modifications are effective in decreasing the rate of employee turnover, such strategies are not easily transferable to health care. In fact, the rate of physician burnout has steadily increased over the past two decades as the business of medicine shifts towards longer hours, decreased reimbursement rates, and inexhaustible insurance stipulations.2,5 Consequently, occupational dissatisfaction increases the risk of cynicism, frustration with patients, internalization of failure, and likelihood of early retirement.5 Moreover, burnout may also fracture interpersonal relationships as well as precipitate errors, negative patient outcomes, malpractice, and development of severe mental health conditions associated with high morbidity and mortality.5,8
Although the concept of burnout is critical in understanding the side effects of stereotypical workplace culture, critics of the concept bemoan a suggestion of individual blame.6,8 In essence, they argue that burnout is explained as a side effect of toxic workplace conditions, but covertly represents a lack of resilience, motivation, and ambition to thrive in a physically or emotionally taxing occupational setting.6,8 Thus, the responsibility of acclimation lies upon the impacted individuals rather than the employer. For this reason, many strategies to ameliorate burnout are focused on the individual, including meditation, wellness retreats, creating or adjusting self-care regimens, or in some cases psychotherapy and psychopharmacology.6 Whereas burnout may respond (at least partially) to such interventions, without altering the causal factors, it is unlikely to remit. This is especially the case in health care, where systemic constraints lie beyond the control of an individual physician. Rather than promoting or specifically relying upon personal improvement and recovery, amendments are needed on multiple levels to affect meaningful change.
Moral injury
Similar to burnout, moral injury was not initially conceived within the scope of health care. In the 1990s Jonathan Shay, MD, PhD, identified veterans presenting with symptoms mimicking PTSD that failed to respond to standard, well established and efficacious treatments.9-11 With further analysis he determined that veterans who demonstrated minimal improvement reported similar histories of guilt, shame, and disgust following perceived injustices enacted or abetted by immoral leaders.10,11 Ultimately Shay identified three components of moral injury: 1. A betrayal of what is morally right; 2. By someone who holds legitimate priority; 3. In a high stakes situation.10
This definition was further modified in 2007 by Brett Linz, PhD, and colleagues as: “Perpetuating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”10,11 By expanding this description to include distress experienced by physicians and health care workers, Wendy Dean and Simon Talbot (in 2018 and 2019 respectively) explored how the health care system leads practitioners to deliver what they identify as substandard treatment.6-8 This results in disillusionment and lays the foundation for ethical and moral dilemmas in clinicians.
Themes of moral injury are repeatedly cited in various surveys and studies as a cause for occupational dissatisfaction. As physicians and other health care professionals reel from the aftermath of COVID-19, the effects of reconfiguring medicine into a business-oriented framework are glaringly conspicuous. Vast hospital nursing shortages, high patient census exacerbated by the political misuse and polarization of science, and insufficient availability of psychiatric beds, have culminated in a deluge of psychological strain in emergency medical physicians. Furthermore, pressure from administrators, mandated patient satisfaction measures, tedious electronic medical record systems, and copious licensing and certification requirements, contribute to physician distress as they attempt to navigate a system that challenges the vows which they swore to uphold.8 Because the cost of pursuing a medical degree frequently necessitates acquisition of loans that, without a physician income, may be difficult to repay,9 many doctors feel trapped within a seemingly endless cycle of misgiving that contributes to emotional exhaustion, pessimism, and low morale.
In my next series of The Myth of the Superdoctor columns, we will explore various factors that potentiate risk of moral injury. From medical school and residency training to corporate infrastructure and insurance obstacles, I will seek to discern and deliberate strategies for repair and rehabilitation. It is my hope that together we will illuminate the myriad complexities within the business of medicine, and become advocates and harbingers of change not only for physicians and health care workers but also for the sake of our patients and their families.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
References
1. King N. When a Psychologist Succumbed to Stress, He Coined The Term Burnout. 2016 Dec 8. NPR: All Things Considered.
2. Maslach C and Leiter MP. World Psychiatry. 2016 Jun;15(2):103-11. doi: 10.1002/wps.20311.
3. InformedHealth.org and Institute for Quality and Efficiency in Health Care. Depression: What is burnout?. https://www.ncbi.nlm.nih.gov/books/NBK279286/.
4. Michel A. Burnout and the Brain. Observer. 2016 Jan 29. https://www.psychologicalscience.org/observer/burnout-and-the-brain.
5. Patel RS et al. Behav Sci. 2018;8(11):98. doi:10.3390/bs8110098.
6. Dean W and Talbot S. Physicians aren’t ‘burning out.’ They’re suffering from moral injury. Stat. 2018 Jul 26. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/.
7. Dean W and Talbot S. Moral injury and burnout in medicine: A year of lessons learned. Stat. 2019 Jul 26. https://www.statnews.com/2019/07/26/moral-injury-burnout-medicine-lessons-learned/.
8. Dean W et al. Reframing Clinician Distress: Moral Injury Not Burnout. Fed Pract. 2019 Sep; 36(9):400-2. https://www.mdedge.com/fedprac/article/207458/mental-health/reframing-clinician-distress-moral-injury-not-burnout.
9. Bailey M. Beyond Burnout: Docs Decry ‘Moral Injury’ From Financial Pressures of Health Care. KHN. 2020 Feb 4. https://khn.org/news/beyond-burnout-docs-decry-moral-injury-from-financial-pressures-of-health-care/.
10. Litz B et al. Clin Psychol Rev. 2009 Dec;29(8):695-706. doi: 10.1016/j.cpr.2009.07.003.
11. Norman S and Maguen S. Moral Injury. PTSD: National Center for PTSD. https://www.ptsd.va.gov/professional/treat/cooccurring/moral_injury.asp.
Several years have passed since I stood among a cohort of eager medical students wearing regalia that signaled a new beginning. Four years of grueling study culminated in a cacophony of unified voices, each reciting a pledge that I had longed to take since early adolescence. Together we celebrated, triumphant despite innumerable exams and various iterations of the Socratic method – all under the guise of assessing knowledge while in truth seeking to insidiously erode the crowd of prospective physicians. Yet our anxiety and uncertainty melted away as names were called, hands firmly clasped, and tassels transposed. For a moment in time, we stood on the precipice of victory, enthusiastic albeit oblivious of the tremendous obstacles that loomed ahead.
Wistfully I reminisce about the unequivocal joy that abounds within the protective shield of naiveté. Specifically, I think about that time when the edict of medicine and the art of being a physician felt congruent. Yet, reality is fickle and often supersedes expectation. Occasionally my thoughts drift to the early days of residency – a time during which the emotional weight of caring for vulnerable patients while learning to master my chosen specialty felt woefully insurmountable. I recall wading blindly through each rotation attempting to emulate the competent and compassionate care so effortlessly demonstrated by senior physicians as they moved through the health care system with apparent ease. They stepped fluidly, as I watched in awe through rose-tinted glasses.
As months passed into years, my perception cleared. What I initially viewed as graceful patient care belied a complex tapestry of health care workers often pressured into arduous decisions, not necessarily in service of a well-constructed treatment plan. Gradually, formidable barriers emerged, guidelines and restrictions embedded within a confining path that suffocated those who dared to cross it. As a result, a field built on the foundations of autonomy, benevolence, and nonmaleficence was slowly engulfed by a system fraught with contrivances. Amid such stressors, physical and psychological health grows tenuous. Classically, this overwhelming feeling of distress is recognized as burnout. Studies reformulated this malady to that which was first described in Vietnam war veterans, a condition known as “moral injury.”
The impact of burnout
To explain the development – and explore the complexities – of moral injury, we must return to 1975 when the term burnout was initially formulated by Herbert Freudenberger, PhD, a psychologist renowned for his work in substance use disorders, psychoanalysis, and clinical education.1 Dr. Freudenberger’s studies noted incidences of heightened emotional and physical distress in his colleagues working in substance abuse and other clinics. He sought to define these experiences as well as understand his own battle with malaise, apathy, and frustration.1 Ultimately, Dr. Freudenberger described burnout as “Becoming exhausted by making excessive demands on energy, strength, or resources in the workplace.”2 Although it characteristically overlaps with depression and anxiety, burnout is conceptualized as a separate entity specifically forged within a context of perfectionism, integrity, and self-sacrifice.2 Such qualities are integral in health care and, as a result, physicians are particularly vulnerable.
Since Dr. Freudenberger published “Burnout: The High Cost of Achievement” in 1980, immense research has assisted in not only identifying critical factors that contribute to its development but also the detrimental effects it has on physiological health.3 These include exhaustion from poor work conditions and extreme commitment to employee responsibilities that in turn precipitate mood destabilization and impaired work performance.3 Furthermore, research has also demonstrated that burnout triggers alterations in neural circuitry via the prefrontal cortex and the amygdala, structures critical for emotional regulation.4 To combat the ill effects of burnout while maintaining productivity and maximizing profit, several high-profile corporations instituted changes focusing on self-care, wellness, benefits, and incentives. Although these modifications are effective in decreasing the rate of employee turnover, such strategies are not easily transferable to health care. In fact, the rate of physician burnout has steadily increased over the past two decades as the business of medicine shifts towards longer hours, decreased reimbursement rates, and inexhaustible insurance stipulations.2,5 Consequently, occupational dissatisfaction increases the risk of cynicism, frustration with patients, internalization of failure, and likelihood of early retirement.5 Moreover, burnout may also fracture interpersonal relationships as well as precipitate errors, negative patient outcomes, malpractice, and development of severe mental health conditions associated with high morbidity and mortality.5,8
Although the concept of burnout is critical in understanding the side effects of stereotypical workplace culture, critics of the concept bemoan a suggestion of individual blame.6,8 In essence, they argue that burnout is explained as a side effect of toxic workplace conditions, but covertly represents a lack of resilience, motivation, and ambition to thrive in a physically or emotionally taxing occupational setting.6,8 Thus, the responsibility of acclimation lies upon the impacted individuals rather than the employer. For this reason, many strategies to ameliorate burnout are focused on the individual, including meditation, wellness retreats, creating or adjusting self-care regimens, or in some cases psychotherapy and psychopharmacology.6 Whereas burnout may respond (at least partially) to such interventions, without altering the causal factors, it is unlikely to remit. This is especially the case in health care, where systemic constraints lie beyond the control of an individual physician. Rather than promoting or specifically relying upon personal improvement and recovery, amendments are needed on multiple levels to affect meaningful change.
Moral injury
Similar to burnout, moral injury was not initially conceived within the scope of health care. In the 1990s Jonathan Shay, MD, PhD, identified veterans presenting with symptoms mimicking PTSD that failed to respond to standard, well established and efficacious treatments.9-11 With further analysis he determined that veterans who demonstrated minimal improvement reported similar histories of guilt, shame, and disgust following perceived injustices enacted or abetted by immoral leaders.10,11 Ultimately Shay identified three components of moral injury: 1. A betrayal of what is morally right; 2. By someone who holds legitimate priority; 3. In a high stakes situation.10
This definition was further modified in 2007 by Brett Linz, PhD, and colleagues as: “Perpetuating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”10,11 By expanding this description to include distress experienced by physicians and health care workers, Wendy Dean and Simon Talbot (in 2018 and 2019 respectively) explored how the health care system leads practitioners to deliver what they identify as substandard treatment.6-8 This results in disillusionment and lays the foundation for ethical and moral dilemmas in clinicians.
Themes of moral injury are repeatedly cited in various surveys and studies as a cause for occupational dissatisfaction. As physicians and other health care professionals reel from the aftermath of COVID-19, the effects of reconfiguring medicine into a business-oriented framework are glaringly conspicuous. Vast hospital nursing shortages, high patient census exacerbated by the political misuse and polarization of science, and insufficient availability of psychiatric beds, have culminated in a deluge of psychological strain in emergency medical physicians. Furthermore, pressure from administrators, mandated patient satisfaction measures, tedious electronic medical record systems, and copious licensing and certification requirements, contribute to physician distress as they attempt to navigate a system that challenges the vows which they swore to uphold.8 Because the cost of pursuing a medical degree frequently necessitates acquisition of loans that, without a physician income, may be difficult to repay,9 many doctors feel trapped within a seemingly endless cycle of misgiving that contributes to emotional exhaustion, pessimism, and low morale.
In my next series of The Myth of the Superdoctor columns, we will explore various factors that potentiate risk of moral injury. From medical school and residency training to corporate infrastructure and insurance obstacles, I will seek to discern and deliberate strategies for repair and rehabilitation. It is my hope that together we will illuminate the myriad complexities within the business of medicine, and become advocates and harbingers of change not only for physicians and health care workers but also for the sake of our patients and their families.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
References
1. King N. When a Psychologist Succumbed to Stress, He Coined The Term Burnout. 2016 Dec 8. NPR: All Things Considered.
2. Maslach C and Leiter MP. World Psychiatry. 2016 Jun;15(2):103-11. doi: 10.1002/wps.20311.
3. InformedHealth.org and Institute for Quality and Efficiency in Health Care. Depression: What is burnout?. https://www.ncbi.nlm.nih.gov/books/NBK279286/.
4. Michel A. Burnout and the Brain. Observer. 2016 Jan 29. https://www.psychologicalscience.org/observer/burnout-and-the-brain.
5. Patel RS et al. Behav Sci. 2018;8(11):98. doi:10.3390/bs8110098.
6. Dean W and Talbot S. Physicians aren’t ‘burning out.’ They’re suffering from moral injury. Stat. 2018 Jul 26. https://www.statnews.com/2018/07/26/physicians-not-burning-out-they-are-suffering-moral-injury/.
7. Dean W and Talbot S. Moral injury and burnout in medicine: A year of lessons learned. Stat. 2019 Jul 26. https://www.statnews.com/2019/07/26/moral-injury-burnout-medicine-lessons-learned/.
8. Dean W et al. Reframing Clinician Distress: Moral Injury Not Burnout. Fed Pract. 2019 Sep; 36(9):400-2. https://www.mdedge.com/fedprac/article/207458/mental-health/reframing-clinician-distress-moral-injury-not-burnout.
9. Bailey M. Beyond Burnout: Docs Decry ‘Moral Injury’ From Financial Pressures of Health Care. KHN. 2020 Feb 4. https://khn.org/news/beyond-burnout-docs-decry-moral-injury-from-financial-pressures-of-health-care/.
10. Litz B et al. Clin Psychol Rev. 2009 Dec;29(8):695-706. doi: 10.1016/j.cpr.2009.07.003.
11. Norman S and Maguen S. Moral Injury. PTSD: National Center for PTSD. https://www.ptsd.va.gov/professional/treat/cooccurring/moral_injury.asp.
Caregiver Support in a Case of Posttraumatic Stress Disorder and Lewy Body Dementia
Caregiving for a person with dementia in the community can be extremely difficult work. Much of this work falls on unpaid or informal caregivers. Sixty-three percent of older adults with dementia depend completely on unpaid caregivers, and an additional 26% receive some combination of paid and unpaid support, together comprising nearly 90% of the more than 3 million older Americans with dementia.1 In-home care is preferable for these patients. For veterans, the Caregiver Support Program (CSP) is the only US Department of Veterans Affairs (VA) program that exclusively supports caregivers. Although the CSP is not a nursing home diversion or cost savings program, successfully enabling at-home living in lieu of facility living also has the potential to reduce overall cost of care, and most importantly, to enable veterans who desire it to age at home.2,3
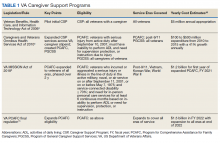
The CSP has 2 unique programs for caregivers of eligible veterans. The Program of General Caregiver Support Services (PGCSS) provides resources, education, and support to caregivers of all veterans enrolled in the Veterans Health Administration (VHA). The Program of Comprehensive Assistance for Family Caregivers (PCAFC) provides education and training, access to health care insurance if eligible, mental health counseling, access to a monthly caregiver stipend, enhanced respite care, wellness contacts, and travel compensation for VA health care appointments (Table 1).4,5
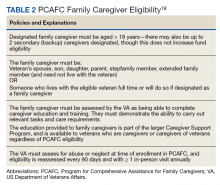
Patients undergo a rigorous assessment and highly specialized and individualized clinical decision-making process to confirm the service is appropriate for the patient. PCAFC was restructured and expanded on October 1, 2020.6 Currently, veterans who incurred or aggravated a serious injury (defined by a single or combined service-connection rating of ≥ 70%) in active military service before May 8, 1975, or after September 10, 2001, are eligible for PCAFC.6 Most notably, these changes opened eligibility in the PCAFC to caregivers of veterans from the Vietnam, Korean, and World War II eras of conflict and veterans with dependence in activities of daily living (ADL) due to a wider variety of illnesses, including dementia.6 The PCAFC is set to further expand to caregivers of otherwise eligible veterans of all eras of service on October 1, 2022, 2 years after the initial expansion, as laid out in the 2018 VA MISSION Act.6 Additional information on the history of the PGCSS and PCAFC and eligibility criteria for veterans and their family caregivers can be found in Tables 2 and 3.
Posttraumatic stress disorder (PTSD) and cognitive impairment are 2 common causes of disability among veterans who receive VHA care. Among older veterans, rates of lifetime development of PTSD reach up to 30%.7 Dementia diagnosis is also more common in older veterans compared with age-matched civilians.8 Furthermore, a prior diagnosis of PTSD has been associated with nearly a 2-fold increase in risk of development of dementia in older age.7 These conditions are also linked to high degrees of service connection. PTSD is the third most prevalent service-connected disability for veterans receiving compensation and cognitive limitation is the third most prevalent category of service-connected disability among veterans.9
We present a case of a Vietnam-era veteran with a history of combat exposure and service-connected PTSD, and a later diagnosis of Lewy body dementia (LBD). Through combination of VHA geriatric services, the CSP, and the expanded PCAFC, the veteran’s primary family caregiver received the materials, support, and financial resources necessary to enable at-home living for the veteran, despite his illness and later complications.
Case Presentation
A male combat veteran presented to his primary care practitioner (PCP) with concerns of several years of progressive changes in gait, forgetfulness, and a gradual decline in the ability to live independently without assistance. At that time, his medical history was notable for PTSD (50% service connection), which had been diagnosed over a decade prior (but for which the veteran had refused medication or therapy on multiple occasions, stating he preferred to “breathe through” his intrusive symptom flare-ups), localized prostate cancer with a radical prostatectomy (100% service connection), multiple kidney stones with persistent left ureteral inflammation, and arteriosclerotic heart disease (10% service connection). A Saint Louis University Mental Status Exam (SLUMS) performed by the PCP was notable for a score of 9/30, in the dementia range. A computed tomography of the brain demonstrated scattered foci of hypoattenuation attributable to normal aging without any other pathology noted.
The veteran was referred to the Cognitive Care clinic, a local longitudinal multidisciplinary dementia care clinic, along with his spouse/caregiver. Cognitive testing was performed by a licensed clinical psychologist in the clinic and was notable for a Mini-Mental State Exam (MMSE) score of 18/30, also in the dementia range, and a more robust neuropsychiatric battery demonstrated borderline intact memory and language function but impairments in executive function and visuospatial skills. The patient’s clinical history included functional loss over time, with total dependence in instrumental activities of daily living (IADL), or tasks necessary to be fully independent or manage a household, including inability to manage finances, and some need for assistance in ADL, or personal care tasks such as dressing or grooming, including bathing. Physical examination was notable for bradykinesia, a shuffling gait, and rare episodes of speaking to someone who was not in the room, thought to be due to mild nondistressing hallucinations.
A diagnosis of LBD was made. At time of diagnosis, the patient met criteria for probable dementia with Lewy bodies, with 2 of 4 core clinical features (hallucinations and Parkinsonism), and multiple supportive features (gait disturbance, sensory disturbance, and altered mood).10,11 The veteran continued to develop more supportive features for diagnosis of LBD over time, including evidence of autonomic instability.
The veteran and his caregiver were educated on his diagnosis, and longitudinal support was offered. The veteran was no longer driving, and due to the severity of his symptoms, the importance of driving cessation was reinforced by the care team. Over the course of the next year, his illness progressed, with more frequent behaviors and psychological symptoms of dementia (BPSD). He began to exhibit nighttime wandering throughout the house and became more anxious and restless during the day. He lost the ability to make his own health care decisions, and his spouse became his activated health care power of attorney (HCPOA). His BPSD became more disruptive to daily life and was accompanied by a change in the character of his hallucinations, with prior nondistressing visions of other people being replaced with visions of war, burning bodies, and violence, much of it related to combat experiences in Vietnam. The BPSD began to include hiding behind furniture, running out of the house, and shouting and crying in response to hallucinations. At times, his BPSD became violent, lashing out in fear against his hallucinations and caregiver.
The veteran’s change in BPSD was concerning for a new baseline, rather than being clearly related to an underlying unmet physical need, such as pain, hunger, sleep, or discomfort. Multiple hospital admissions during that year involved IV hydration and treatment for urinary tract infections (UTI) for several days of inpatient stay at a time, but these behaviors persisted despite infection treatment and hydration. The patient’s changes in BPSD were thought to be secondary to uncovered and intensified PTSD in the setting of progressive dementia. Due to the clear danger the patient posed to himself and others, potential treatment options for these PTSD-related hallucinations were discussed with his caregiver. The caregiver shared that the patient’s BPSD and hallucinations were so distressing that “he would never want to live like this,” and that things had progressed to the point that “he has no quality of life.”
Oral aripiprazole 2 mg twice daily was prescribed after the risks of infection, cardiac complications, and exacerbation of movement disorder symptoms, such as increased stiffness and falls, were discussed with the caregiver. The caregiver was employed and relied on continued employment for income, but the patient could not be safely left alone. As the patient and his caregiver had reached a crisis point and living at home no longer appeared to be safe, the patient was referred to a VA-contracted skilled nursing facility (SNF) for long-term care. The patient’s caregiver was also referred to CSP for support during this transition. Due to the patient’s level of service connection and personal needs, as well as the patient and caregiver’s preference for the veteran to remain in his home, they were evaluated for the PCAFC for enhanced support to enable home as an alternative to facility living, should the patient respond to the antipsychotic therapy sufficiently, which was evaluated on a regular basis.
After several months, the patient’s BPSD had improved significantly, and he was no longer experiencing distressing hallucinations. However, his mobility also declined, and he became fully dependent in most ADL, including transfers, hygiene, and toileting. Due to the COVID-19 pandemic, visitation was limited, which was difficult for both the patient and his caregiver. The veteran and caregiver were approved for PCAFC due to the veteran’s combination of service-connected illnesses > 70%, dependence for most ADLs, and need for continuous supervision. A transfer home from the SNF was arranged.
The PCAFC allowed the veteran’s caregiver and family members to provide in-home full-time caregiving, as an alternative to facility placement. The caregiver received a variety of support, including access to peer support, instruction on ways to assist in his toileting, hygiene, and transfers, and a caregiving stipend. In addition to offsetting lost wages, the stipend also helped offset the cost of care supplies which were not provided or were not readily available from the VA, which at the time included the patient’s preferred nutritional supplement and some supplies for personal care.
The veteran’s care needs continued to escalate. A fall at home resulted in a hip fracture, which was treated with surgical pinning. Postfracture physical therapy in a facility was considered, but ultimately was provided at home. The patient also experienced multiple UTIs and resulting delirium, with accompanying agitation and hallucinations. These episodes improved with IV antibiotics and hydration during short hospital stays. Ultimately, a computed tomography demonstrated overflow incontinence likely related to urologic damage from prior kidney stones and stent placement was recommended.
Visiting skilled nurses for the patient’s area were difficult to coordinate but were eventually arranged. The patient continued residing in his home with the support of his caregiver, the PCAFC, and the local VA medical center geriatric and transitional care services. The patient was also referred to the palliative care outpatient specialty clinic for discussion of goals of care and assistance with advance care planning as his illness progressed. Mental health and geriatric psychiatry consult teams were considered for this case but not utilized.
Discussion
Older adult Americans are at high risk of poor financial wellbeing, with nearly one quarter of Americans aged > 62 years experiencing financial insecurity.12 Even in this case with health care provided by the VA, successful in-home care was challenging and required a dedicated live-in caregiver, care coordination resources, and financial support. As part of its mission of caring for veterans, the VA has instituted CSP, whose mission is to promote the health and well-being of family caregivers through education, support, and services.
PCAFC offers enhanced clinical support for caregivers of eligible veterans who are seriously injured. This includes resources, education, support, financial stipends, health insurance (if eligible), and beneficiary travel (if eligible) to primary caregivers of eligible veterans. PCAFC was originally reserved for veterans who had onset of service-related disability after September 11, 2001, with an associated personal care need. In this population, PCAFC demonstrated an increased usage of clinical resources, likely related to increased ease in accessing care.13
The cohort of post-9/11 veterans is very different from the cohort of veterans and their caregivers who may now qualify for the PCAFC after its October 2020 expansion. Veterans from the Vietnam, Korean, and World War II eras of conflict have rates of service-connected disability 2 to 3 times higher than those of post-9/11 era veterans and are at greater risk for dementia.9 Veterans aged ≥ 75 years who have service connection also report higher rates of difficulty with independent living and self-care compared with their younger peers.9 Since dementia and PTSD are common causes of service connection and disability it is likely that a significant proportion of older veterans will be eligible to apply for the newly expanded PCAFC.
To be eligible for PCAFC, a veteran must have a service-connected disability rating of ≥ 70% and must need in-person care services for ≥ 6 continuous months, based on either an inability to perform an ADL, or a need for supervision, protection, or instruction.
It is not clear how CSP use or increased access to PCAFC will impact costs. However, the PCAFC monthly stipend is scaled to the median wage of a home health aide and to the location of the caregiver, which is considerably less than the cost of recurrent hospitalization or a year of facility-level care.15 The CSP may eventually be a successful long-term investment in cost savings. In order to ensure the process for PCAFC approval is uniform and prompt as the program expands, CSP has restructured, increasing the number of employees, improving the patient review process, and expanding staff training.16 The VA plans to continually re-assess CSP using the infrastructure of the Caregiver Record Management Application as it continues to expand.17
Conclusions
Dementia and PTSD commonly coexist and are a significant source of disability in the service-connected veteran population. This case brings attention to the recent expansion of PCAFC, which now has the potential to support eligible veterans from the World War II, Korean, and Vietnam-era conflicts, in whom these illnesses are more common. In this case, in-home care was preferred by the veteran and primary caregiver but would not have been possible without a complex intervention. There is still more research to be done on the best way to meet the needs of older adults with dementia, the impact of in-home care, and the system-wide implications of PCAFC, especially as the program grows. However, in-home care is preferable to SNF living for many veterans and caregivers, and CSP will continue to be an essential element of providing care for this population.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. The authors would also like to thank the members of the Veterans Affairs Central Office and National Caregiver Support Program Office, including Elyse Kaplan, Melinda Hogue, Beth Wolfsohn, Colleen Richardson, and Timothy Jobin, for their thorough review of the work and edits to ensure accurate program description.
1. Chi W, Graf E, Hughes L, et al. Community-dwelling older adults with dementia and their caregivers: key indicators from the National Health and Aging Trends study. Published January 29, 2019. Accessed February 16, 2022. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//186501/DemChartbook.pdf
2. Rapaport P, Burton A, Leverton M, et al. “I just keep thinking that I don’t want to rely on people.” A qualitative study of how people living with dementia achieve and maintain independence at home: stakeholder perspectives. BMC Geriatr. 2020;20(1):1-11. doi:10.1186/s12877-019-1406-6
3. Miller EA, Gidmark S, Gadbois E, Rudolph JL, Intrator O. Nursing home referral within the Veterans Health Administration: practice variation by payment source and facility type. Res Aging. 2018;40(7):687-711. doi:10.1177/0164027517730383
4. Veterans Benefits, Health Care, and Information Technology Act of 2006, Pub L No. 109-461, 120 Stat. 3403.
5. Caregivers and Veterans Omnibus Health Services Act of 2010, Pub L No. 111-163, 115 Stat 552.
6. VA MISSION Act of 2018. 38 CFR § 17.
7. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
8. Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20(4):245-253. doi:10.1159/000087345
9. Holder, KA. The Disability of Veterans. Social, Economic, and Housing Statistics Division, US Census Bureau; 2014. Accessed February 9, 2022. https://www.census.gov/content/dam/Census/library/working-papers/2016/demo/Holder-2016-01.pdf
10. Sanford AM. Lewy body dementia. Clin Geriatr Med. 2018;34(4):603-615. doi:10.1016/j.cger.2018.06.007
11. Armstrong MJ. Lewy body dementias. Continuum (Minneap Minn). 2019;25(1):128-146. doi:10.1212/CON.0000000000000685
12. Bureau of Consumer Financial Protection. Financial well-being of older Americans. Published December 2018. Accessed February 17, 2022. https://files.consumerfinance.gov/f/documents/bcfp_financial-well-being-older-americans_report.pdf
13. Van Houtven CH, Smith VA, Stechuchak KM, et al. Comprehensive support for family caregivers: impact on veteran health care utilization and costs. Med Care Res Rev. 2019;76(1):89-114. doi:10.1177/1077558717697015
14. Boland L, Légaré F, Perez MMB, et al. Impact of home care versus alternative locations of care on elder health outcomes: an overview of systematic reviews. BMC Geriatr. 2017;17(1):20. doi:10.1186/s12877-016-0395-y
15. Program of Comprehensive Assistance for Family Caregivers Improvements and Amendments Under the VA MISSION Act of 2018. 85 FR § 13356.
16. Extension of Program of Comprehensive Assistance for Family Caregivers Eligibility for Legacy Participants and Legacy Applicants. 86 FR § 52614.
17. US Department of Veterans Affairs, 2020. Certification of the Implementation of the Caregiver Records Management Application (CARMA). 85 FR § 63358.
18. Sussman, JS. Department of Veterans Affairs: Caregiver Support. Congressional Research Service Report No. R46282. Published March 24, 2020. Accessed February 16, 2022. https://www.everycrsreport.com/files/20200324_R46282_656f1e8338af12a2a676c471be3b3c13b2fcb0bb.pdf
19. US Department of Veterans Affairs. Veterans Affairs Program of Comprehensive Assistance for Family Caregivers Eligibility Criteria Fact Sheet. Washington, DC: U.S. Department of Veterans Affairs; 2020. Accessed February 9, 2022. https://www.caregiver.va.gov/pdfs/MissionAct/EligibilityCriteriaFactsheet_Chapter2_Launch_Approved_Final_100120.pdf
Caregiving for a person with dementia in the community can be extremely difficult work. Much of this work falls on unpaid or informal caregivers. Sixty-three percent of older adults with dementia depend completely on unpaid caregivers, and an additional 26% receive some combination of paid and unpaid support, together comprising nearly 90% of the more than 3 million older Americans with dementia.1 In-home care is preferable for these patients. For veterans, the Caregiver Support Program (CSP) is the only US Department of Veterans Affairs (VA) program that exclusively supports caregivers. Although the CSP is not a nursing home diversion or cost savings program, successfully enabling at-home living in lieu of facility living also has the potential to reduce overall cost of care, and most importantly, to enable veterans who desire it to age at home.2,3
The CSP has 2 unique programs for caregivers of eligible veterans. The Program of General Caregiver Support Services (PGCSS) provides resources, education, and support to caregivers of all veterans enrolled in the Veterans Health Administration (VHA). The Program of Comprehensive Assistance for Family Caregivers (PCAFC) provides education and training, access to health care insurance if eligible, mental health counseling, access to a monthly caregiver stipend, enhanced respite care, wellness contacts, and travel compensation for VA health care appointments (Table 1).4,5
Patients undergo a rigorous assessment and highly specialized and individualized clinical decision-making process to confirm the service is appropriate for the patient. PCAFC was restructured and expanded on October 1, 2020.6 Currently, veterans who incurred or aggravated a serious injury (defined by a single or combined service-connection rating of ≥ 70%) in active military service before May 8, 1975, or after September 10, 2001, are eligible for PCAFC.6 Most notably, these changes opened eligibility in the PCAFC to caregivers of veterans from the Vietnam, Korean, and World War II eras of conflict and veterans with dependence in activities of daily living (ADL) due to a wider variety of illnesses, including dementia.6 The PCAFC is set to further expand to caregivers of otherwise eligible veterans of all eras of service on October 1, 2022, 2 years after the initial expansion, as laid out in the 2018 VA MISSION Act.6 Additional information on the history of the PGCSS and PCAFC and eligibility criteria for veterans and their family caregivers can be found in Tables 2 and 3.
Posttraumatic stress disorder (PTSD) and cognitive impairment are 2 common causes of disability among veterans who receive VHA care. Among older veterans, rates of lifetime development of PTSD reach up to 30%.7 Dementia diagnosis is also more common in older veterans compared with age-matched civilians.8 Furthermore, a prior diagnosis of PTSD has been associated with nearly a 2-fold increase in risk of development of dementia in older age.7 These conditions are also linked to high degrees of service connection. PTSD is the third most prevalent service-connected disability for veterans receiving compensation and cognitive limitation is the third most prevalent category of service-connected disability among veterans.9
We present a case of a Vietnam-era veteran with a history of combat exposure and service-connected PTSD, and a later diagnosis of Lewy body dementia (LBD). Through combination of VHA geriatric services, the CSP, and the expanded PCAFC, the veteran’s primary family caregiver received the materials, support, and financial resources necessary to enable at-home living for the veteran, despite his illness and later complications.
Case Presentation
A male combat veteran presented to his primary care practitioner (PCP) with concerns of several years of progressive changes in gait, forgetfulness, and a gradual decline in the ability to live independently without assistance. At that time, his medical history was notable for PTSD (50% service connection), which had been diagnosed over a decade prior (but for which the veteran had refused medication or therapy on multiple occasions, stating he preferred to “breathe through” his intrusive symptom flare-ups), localized prostate cancer with a radical prostatectomy (100% service connection), multiple kidney stones with persistent left ureteral inflammation, and arteriosclerotic heart disease (10% service connection). A Saint Louis University Mental Status Exam (SLUMS) performed by the PCP was notable for a score of 9/30, in the dementia range. A computed tomography of the brain demonstrated scattered foci of hypoattenuation attributable to normal aging without any other pathology noted.
The veteran was referred to the Cognitive Care clinic, a local longitudinal multidisciplinary dementia care clinic, along with his spouse/caregiver. Cognitive testing was performed by a licensed clinical psychologist in the clinic and was notable for a Mini-Mental State Exam (MMSE) score of 18/30, also in the dementia range, and a more robust neuropsychiatric battery demonstrated borderline intact memory and language function but impairments in executive function and visuospatial skills. The patient’s clinical history included functional loss over time, with total dependence in instrumental activities of daily living (IADL), or tasks necessary to be fully independent or manage a household, including inability to manage finances, and some need for assistance in ADL, or personal care tasks such as dressing or grooming, including bathing. Physical examination was notable for bradykinesia, a shuffling gait, and rare episodes of speaking to someone who was not in the room, thought to be due to mild nondistressing hallucinations.
A diagnosis of LBD was made. At time of diagnosis, the patient met criteria for probable dementia with Lewy bodies, with 2 of 4 core clinical features (hallucinations and Parkinsonism), and multiple supportive features (gait disturbance, sensory disturbance, and altered mood).10,11 The veteran continued to develop more supportive features for diagnosis of LBD over time, including evidence of autonomic instability.
The veteran and his caregiver were educated on his diagnosis, and longitudinal support was offered. The veteran was no longer driving, and due to the severity of his symptoms, the importance of driving cessation was reinforced by the care team. Over the course of the next year, his illness progressed, with more frequent behaviors and psychological symptoms of dementia (BPSD). He began to exhibit nighttime wandering throughout the house and became more anxious and restless during the day. He lost the ability to make his own health care decisions, and his spouse became his activated health care power of attorney (HCPOA). His BPSD became more disruptive to daily life and was accompanied by a change in the character of his hallucinations, with prior nondistressing visions of other people being replaced with visions of war, burning bodies, and violence, much of it related to combat experiences in Vietnam. The BPSD began to include hiding behind furniture, running out of the house, and shouting and crying in response to hallucinations. At times, his BPSD became violent, lashing out in fear against his hallucinations and caregiver.
The veteran’s change in BPSD was concerning for a new baseline, rather than being clearly related to an underlying unmet physical need, such as pain, hunger, sleep, or discomfort. Multiple hospital admissions during that year involved IV hydration and treatment for urinary tract infections (UTI) for several days of inpatient stay at a time, but these behaviors persisted despite infection treatment and hydration. The patient’s changes in BPSD were thought to be secondary to uncovered and intensified PTSD in the setting of progressive dementia. Due to the clear danger the patient posed to himself and others, potential treatment options for these PTSD-related hallucinations were discussed with his caregiver. The caregiver shared that the patient’s BPSD and hallucinations were so distressing that “he would never want to live like this,” and that things had progressed to the point that “he has no quality of life.”
Oral aripiprazole 2 mg twice daily was prescribed after the risks of infection, cardiac complications, and exacerbation of movement disorder symptoms, such as increased stiffness and falls, were discussed with the caregiver. The caregiver was employed and relied on continued employment for income, but the patient could not be safely left alone. As the patient and his caregiver had reached a crisis point and living at home no longer appeared to be safe, the patient was referred to a VA-contracted skilled nursing facility (SNF) for long-term care. The patient’s caregiver was also referred to CSP for support during this transition. Due to the patient’s level of service connection and personal needs, as well as the patient and caregiver’s preference for the veteran to remain in his home, they were evaluated for the PCAFC for enhanced support to enable home as an alternative to facility living, should the patient respond to the antipsychotic therapy sufficiently, which was evaluated on a regular basis.
After several months, the patient’s BPSD had improved significantly, and he was no longer experiencing distressing hallucinations. However, his mobility also declined, and he became fully dependent in most ADL, including transfers, hygiene, and toileting. Due to the COVID-19 pandemic, visitation was limited, which was difficult for both the patient and his caregiver. The veteran and caregiver were approved for PCAFC due to the veteran’s combination of service-connected illnesses > 70%, dependence for most ADLs, and need for continuous supervision. A transfer home from the SNF was arranged.
The PCAFC allowed the veteran’s caregiver and family members to provide in-home full-time caregiving, as an alternative to facility placement. The caregiver received a variety of support, including access to peer support, instruction on ways to assist in his toileting, hygiene, and transfers, and a caregiving stipend. In addition to offsetting lost wages, the stipend also helped offset the cost of care supplies which were not provided or were not readily available from the VA, which at the time included the patient’s preferred nutritional supplement and some supplies for personal care.
The veteran’s care needs continued to escalate. A fall at home resulted in a hip fracture, which was treated with surgical pinning. Postfracture physical therapy in a facility was considered, but ultimately was provided at home. The patient also experienced multiple UTIs and resulting delirium, with accompanying agitation and hallucinations. These episodes improved with IV antibiotics and hydration during short hospital stays. Ultimately, a computed tomography demonstrated overflow incontinence likely related to urologic damage from prior kidney stones and stent placement was recommended.
Visiting skilled nurses for the patient’s area were difficult to coordinate but were eventually arranged. The patient continued residing in his home with the support of his caregiver, the PCAFC, and the local VA medical center geriatric and transitional care services. The patient was also referred to the palliative care outpatient specialty clinic for discussion of goals of care and assistance with advance care planning as his illness progressed. Mental health and geriatric psychiatry consult teams were considered for this case but not utilized.
Discussion
Older adult Americans are at high risk of poor financial wellbeing, with nearly one quarter of Americans aged > 62 years experiencing financial insecurity.12 Even in this case with health care provided by the VA, successful in-home care was challenging and required a dedicated live-in caregiver, care coordination resources, and financial support. As part of its mission of caring for veterans, the VA has instituted CSP, whose mission is to promote the health and well-being of family caregivers through education, support, and services.
PCAFC offers enhanced clinical support for caregivers of eligible veterans who are seriously injured. This includes resources, education, support, financial stipends, health insurance (if eligible), and beneficiary travel (if eligible) to primary caregivers of eligible veterans. PCAFC was originally reserved for veterans who had onset of service-related disability after September 11, 2001, with an associated personal care need. In this population, PCAFC demonstrated an increased usage of clinical resources, likely related to increased ease in accessing care.13
The cohort of post-9/11 veterans is very different from the cohort of veterans and their caregivers who may now qualify for the PCAFC after its October 2020 expansion. Veterans from the Vietnam, Korean, and World War II eras of conflict have rates of service-connected disability 2 to 3 times higher than those of post-9/11 era veterans and are at greater risk for dementia.9 Veterans aged ≥ 75 years who have service connection also report higher rates of difficulty with independent living and self-care compared with their younger peers.9 Since dementia and PTSD are common causes of service connection and disability it is likely that a significant proportion of older veterans will be eligible to apply for the newly expanded PCAFC.
To be eligible for PCAFC, a veteran must have a service-connected disability rating of ≥ 70% and must need in-person care services for ≥ 6 continuous months, based on either an inability to perform an ADL, or a need for supervision, protection, or instruction.
It is not clear how CSP use or increased access to PCAFC will impact costs. However, the PCAFC monthly stipend is scaled to the median wage of a home health aide and to the location of the caregiver, which is considerably less than the cost of recurrent hospitalization or a year of facility-level care.15 The CSP may eventually be a successful long-term investment in cost savings. In order to ensure the process for PCAFC approval is uniform and prompt as the program expands, CSP has restructured, increasing the number of employees, improving the patient review process, and expanding staff training.16 The VA plans to continually re-assess CSP using the infrastructure of the Caregiver Record Management Application as it continues to expand.17
Conclusions
Dementia and PTSD commonly coexist and are a significant source of disability in the service-connected veteran population. This case brings attention to the recent expansion of PCAFC, which now has the potential to support eligible veterans from the World War II, Korean, and Vietnam-era conflicts, in whom these illnesses are more common. In this case, in-home care was preferred by the veteran and primary caregiver but would not have been possible without a complex intervention. There is still more research to be done on the best way to meet the needs of older adults with dementia, the impact of in-home care, and the system-wide implications of PCAFC, especially as the program grows. However, in-home care is preferable to SNF living for many veterans and caregivers, and CSP will continue to be an essential element of providing care for this population.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. The authors would also like to thank the members of the Veterans Affairs Central Office and National Caregiver Support Program Office, including Elyse Kaplan, Melinda Hogue, Beth Wolfsohn, Colleen Richardson, and Timothy Jobin, for their thorough review of the work and edits to ensure accurate program description.
Caregiving for a person with dementia in the community can be extremely difficult work. Much of this work falls on unpaid or informal caregivers. Sixty-three percent of older adults with dementia depend completely on unpaid caregivers, and an additional 26% receive some combination of paid and unpaid support, together comprising nearly 90% of the more than 3 million older Americans with dementia.1 In-home care is preferable for these patients. For veterans, the Caregiver Support Program (CSP) is the only US Department of Veterans Affairs (VA) program that exclusively supports caregivers. Although the CSP is not a nursing home diversion or cost savings program, successfully enabling at-home living in lieu of facility living also has the potential to reduce overall cost of care, and most importantly, to enable veterans who desire it to age at home.2,3
The CSP has 2 unique programs for caregivers of eligible veterans. The Program of General Caregiver Support Services (PGCSS) provides resources, education, and support to caregivers of all veterans enrolled in the Veterans Health Administration (VHA). The Program of Comprehensive Assistance for Family Caregivers (PCAFC) provides education and training, access to health care insurance if eligible, mental health counseling, access to a monthly caregiver stipend, enhanced respite care, wellness contacts, and travel compensation for VA health care appointments (Table 1).4,5
Patients undergo a rigorous assessment and highly specialized and individualized clinical decision-making process to confirm the service is appropriate for the patient. PCAFC was restructured and expanded on October 1, 2020.6 Currently, veterans who incurred or aggravated a serious injury (defined by a single or combined service-connection rating of ≥ 70%) in active military service before May 8, 1975, or after September 10, 2001, are eligible for PCAFC.6 Most notably, these changes opened eligibility in the PCAFC to caregivers of veterans from the Vietnam, Korean, and World War II eras of conflict and veterans with dependence in activities of daily living (ADL) due to a wider variety of illnesses, including dementia.6 The PCAFC is set to further expand to caregivers of otherwise eligible veterans of all eras of service on October 1, 2022, 2 years after the initial expansion, as laid out in the 2018 VA MISSION Act.6 Additional information on the history of the PGCSS and PCAFC and eligibility criteria for veterans and their family caregivers can be found in Tables 2 and 3.
Posttraumatic stress disorder (PTSD) and cognitive impairment are 2 common causes of disability among veterans who receive VHA care. Among older veterans, rates of lifetime development of PTSD reach up to 30%.7 Dementia diagnosis is also more common in older veterans compared with age-matched civilians.8 Furthermore, a prior diagnosis of PTSD has been associated with nearly a 2-fold increase in risk of development of dementia in older age.7 These conditions are also linked to high degrees of service connection. PTSD is the third most prevalent service-connected disability for veterans receiving compensation and cognitive limitation is the third most prevalent category of service-connected disability among veterans.9
We present a case of a Vietnam-era veteran with a history of combat exposure and service-connected PTSD, and a later diagnosis of Lewy body dementia (LBD). Through combination of VHA geriatric services, the CSP, and the expanded PCAFC, the veteran’s primary family caregiver received the materials, support, and financial resources necessary to enable at-home living for the veteran, despite his illness and later complications.
Case Presentation
A male combat veteran presented to his primary care practitioner (PCP) with concerns of several years of progressive changes in gait, forgetfulness, and a gradual decline in the ability to live independently without assistance. At that time, his medical history was notable for PTSD (50% service connection), which had been diagnosed over a decade prior (but for which the veteran had refused medication or therapy on multiple occasions, stating he preferred to “breathe through” his intrusive symptom flare-ups), localized prostate cancer with a radical prostatectomy (100% service connection), multiple kidney stones with persistent left ureteral inflammation, and arteriosclerotic heart disease (10% service connection). A Saint Louis University Mental Status Exam (SLUMS) performed by the PCP was notable for a score of 9/30, in the dementia range. A computed tomography of the brain demonstrated scattered foci of hypoattenuation attributable to normal aging without any other pathology noted.
The veteran was referred to the Cognitive Care clinic, a local longitudinal multidisciplinary dementia care clinic, along with his spouse/caregiver. Cognitive testing was performed by a licensed clinical psychologist in the clinic and was notable for a Mini-Mental State Exam (MMSE) score of 18/30, also in the dementia range, and a more robust neuropsychiatric battery demonstrated borderline intact memory and language function but impairments in executive function and visuospatial skills. The patient’s clinical history included functional loss over time, with total dependence in instrumental activities of daily living (IADL), or tasks necessary to be fully independent or manage a household, including inability to manage finances, and some need for assistance in ADL, or personal care tasks such as dressing or grooming, including bathing. Physical examination was notable for bradykinesia, a shuffling gait, and rare episodes of speaking to someone who was not in the room, thought to be due to mild nondistressing hallucinations.
A diagnosis of LBD was made. At time of diagnosis, the patient met criteria for probable dementia with Lewy bodies, with 2 of 4 core clinical features (hallucinations and Parkinsonism), and multiple supportive features (gait disturbance, sensory disturbance, and altered mood).10,11 The veteran continued to develop more supportive features for diagnosis of LBD over time, including evidence of autonomic instability.
The veteran and his caregiver were educated on his diagnosis, and longitudinal support was offered. The veteran was no longer driving, and due to the severity of his symptoms, the importance of driving cessation was reinforced by the care team. Over the course of the next year, his illness progressed, with more frequent behaviors and psychological symptoms of dementia (BPSD). He began to exhibit nighttime wandering throughout the house and became more anxious and restless during the day. He lost the ability to make his own health care decisions, and his spouse became his activated health care power of attorney (HCPOA). His BPSD became more disruptive to daily life and was accompanied by a change in the character of his hallucinations, with prior nondistressing visions of other people being replaced with visions of war, burning bodies, and violence, much of it related to combat experiences in Vietnam. The BPSD began to include hiding behind furniture, running out of the house, and shouting and crying in response to hallucinations. At times, his BPSD became violent, lashing out in fear against his hallucinations and caregiver.
The veteran’s change in BPSD was concerning for a new baseline, rather than being clearly related to an underlying unmet physical need, such as pain, hunger, sleep, or discomfort. Multiple hospital admissions during that year involved IV hydration and treatment for urinary tract infections (UTI) for several days of inpatient stay at a time, but these behaviors persisted despite infection treatment and hydration. The patient’s changes in BPSD were thought to be secondary to uncovered and intensified PTSD in the setting of progressive dementia. Due to the clear danger the patient posed to himself and others, potential treatment options for these PTSD-related hallucinations were discussed with his caregiver. The caregiver shared that the patient’s BPSD and hallucinations were so distressing that “he would never want to live like this,” and that things had progressed to the point that “he has no quality of life.”
Oral aripiprazole 2 mg twice daily was prescribed after the risks of infection, cardiac complications, and exacerbation of movement disorder symptoms, such as increased stiffness and falls, were discussed with the caregiver. The caregiver was employed and relied on continued employment for income, but the patient could not be safely left alone. As the patient and his caregiver had reached a crisis point and living at home no longer appeared to be safe, the patient was referred to a VA-contracted skilled nursing facility (SNF) for long-term care. The patient’s caregiver was also referred to CSP for support during this transition. Due to the patient’s level of service connection and personal needs, as well as the patient and caregiver’s preference for the veteran to remain in his home, they were evaluated for the PCAFC for enhanced support to enable home as an alternative to facility living, should the patient respond to the antipsychotic therapy sufficiently, which was evaluated on a regular basis.
After several months, the patient’s BPSD had improved significantly, and he was no longer experiencing distressing hallucinations. However, his mobility also declined, and he became fully dependent in most ADL, including transfers, hygiene, and toileting. Due to the COVID-19 pandemic, visitation was limited, which was difficult for both the patient and his caregiver. The veteran and caregiver were approved for PCAFC due to the veteran’s combination of service-connected illnesses > 70%, dependence for most ADLs, and need for continuous supervision. A transfer home from the SNF was arranged.
The PCAFC allowed the veteran’s caregiver and family members to provide in-home full-time caregiving, as an alternative to facility placement. The caregiver received a variety of support, including access to peer support, instruction on ways to assist in his toileting, hygiene, and transfers, and a caregiving stipend. In addition to offsetting lost wages, the stipend also helped offset the cost of care supplies which were not provided or were not readily available from the VA, which at the time included the patient’s preferred nutritional supplement and some supplies for personal care.
The veteran’s care needs continued to escalate. A fall at home resulted in a hip fracture, which was treated with surgical pinning. Postfracture physical therapy in a facility was considered, but ultimately was provided at home. The patient also experienced multiple UTIs and resulting delirium, with accompanying agitation and hallucinations. These episodes improved with IV antibiotics and hydration during short hospital stays. Ultimately, a computed tomography demonstrated overflow incontinence likely related to urologic damage from prior kidney stones and stent placement was recommended.
Visiting skilled nurses for the patient’s area were difficult to coordinate but were eventually arranged. The patient continued residing in his home with the support of his caregiver, the PCAFC, and the local VA medical center geriatric and transitional care services. The patient was also referred to the palliative care outpatient specialty clinic for discussion of goals of care and assistance with advance care planning as his illness progressed. Mental health and geriatric psychiatry consult teams were considered for this case but not utilized.
Discussion
Older adult Americans are at high risk of poor financial wellbeing, with nearly one quarter of Americans aged > 62 years experiencing financial insecurity.12 Even in this case with health care provided by the VA, successful in-home care was challenging and required a dedicated live-in caregiver, care coordination resources, and financial support. As part of its mission of caring for veterans, the VA has instituted CSP, whose mission is to promote the health and well-being of family caregivers through education, support, and services.
PCAFC offers enhanced clinical support for caregivers of eligible veterans who are seriously injured. This includes resources, education, support, financial stipends, health insurance (if eligible), and beneficiary travel (if eligible) to primary caregivers of eligible veterans. PCAFC was originally reserved for veterans who had onset of service-related disability after September 11, 2001, with an associated personal care need. In this population, PCAFC demonstrated an increased usage of clinical resources, likely related to increased ease in accessing care.13
The cohort of post-9/11 veterans is very different from the cohort of veterans and their caregivers who may now qualify for the PCAFC after its October 2020 expansion. Veterans from the Vietnam, Korean, and World War II eras of conflict have rates of service-connected disability 2 to 3 times higher than those of post-9/11 era veterans and are at greater risk for dementia.9 Veterans aged ≥ 75 years who have service connection also report higher rates of difficulty with independent living and self-care compared with their younger peers.9 Since dementia and PTSD are common causes of service connection and disability it is likely that a significant proportion of older veterans will be eligible to apply for the newly expanded PCAFC.
To be eligible for PCAFC, a veteran must have a service-connected disability rating of ≥ 70% and must need in-person care services for ≥ 6 continuous months, based on either an inability to perform an ADL, or a need for supervision, protection, or instruction.
It is not clear how CSP use or increased access to PCAFC will impact costs. However, the PCAFC monthly stipend is scaled to the median wage of a home health aide and to the location of the caregiver, which is considerably less than the cost of recurrent hospitalization or a year of facility-level care.15 The CSP may eventually be a successful long-term investment in cost savings. In order to ensure the process for PCAFC approval is uniform and prompt as the program expands, CSP has restructured, increasing the number of employees, improving the patient review process, and expanding staff training.16 The VA plans to continually re-assess CSP using the infrastructure of the Caregiver Record Management Application as it continues to expand.17
Conclusions
Dementia and PTSD commonly coexist and are a significant source of disability in the service-connected veteran population. This case brings attention to the recent expansion of PCAFC, which now has the potential to support eligible veterans from the World War II, Korean, and Vietnam-era conflicts, in whom these illnesses are more common. In this case, in-home care was preferred by the veteran and primary caregiver but would not have been possible without a complex intervention. There is still more research to be done on the best way to meet the needs of older adults with dementia, the impact of in-home care, and the system-wide implications of PCAFC, especially as the program grows. However, in-home care is preferable to SNF living for many veterans and caregivers, and CSP will continue to be an essential element of providing care for this population.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. The authors would also like to thank the members of the Veterans Affairs Central Office and National Caregiver Support Program Office, including Elyse Kaplan, Melinda Hogue, Beth Wolfsohn, Colleen Richardson, and Timothy Jobin, for their thorough review of the work and edits to ensure accurate program description.
1. Chi W, Graf E, Hughes L, et al. Community-dwelling older adults with dementia and their caregivers: key indicators from the National Health and Aging Trends study. Published January 29, 2019. Accessed February 16, 2022. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//186501/DemChartbook.pdf
2. Rapaport P, Burton A, Leverton M, et al. “I just keep thinking that I don’t want to rely on people.” A qualitative study of how people living with dementia achieve and maintain independence at home: stakeholder perspectives. BMC Geriatr. 2020;20(1):1-11. doi:10.1186/s12877-019-1406-6
3. Miller EA, Gidmark S, Gadbois E, Rudolph JL, Intrator O. Nursing home referral within the Veterans Health Administration: practice variation by payment source and facility type. Res Aging. 2018;40(7):687-711. doi:10.1177/0164027517730383
4. Veterans Benefits, Health Care, and Information Technology Act of 2006, Pub L No. 109-461, 120 Stat. 3403.
5. Caregivers and Veterans Omnibus Health Services Act of 2010, Pub L No. 111-163, 115 Stat 552.
6. VA MISSION Act of 2018. 38 CFR § 17.
7. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
8. Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20(4):245-253. doi:10.1159/000087345
9. Holder, KA. The Disability of Veterans. Social, Economic, and Housing Statistics Division, US Census Bureau; 2014. Accessed February 9, 2022. https://www.census.gov/content/dam/Census/library/working-papers/2016/demo/Holder-2016-01.pdf
10. Sanford AM. Lewy body dementia. Clin Geriatr Med. 2018;34(4):603-615. doi:10.1016/j.cger.2018.06.007
11. Armstrong MJ. Lewy body dementias. Continuum (Minneap Minn). 2019;25(1):128-146. doi:10.1212/CON.0000000000000685
12. Bureau of Consumer Financial Protection. Financial well-being of older Americans. Published December 2018. Accessed February 17, 2022. https://files.consumerfinance.gov/f/documents/bcfp_financial-well-being-older-americans_report.pdf
13. Van Houtven CH, Smith VA, Stechuchak KM, et al. Comprehensive support for family caregivers: impact on veteran health care utilization and costs. Med Care Res Rev. 2019;76(1):89-114. doi:10.1177/1077558717697015
14. Boland L, Légaré F, Perez MMB, et al. Impact of home care versus alternative locations of care on elder health outcomes: an overview of systematic reviews. BMC Geriatr. 2017;17(1):20. doi:10.1186/s12877-016-0395-y
15. Program of Comprehensive Assistance for Family Caregivers Improvements and Amendments Under the VA MISSION Act of 2018. 85 FR § 13356.
16. Extension of Program of Comprehensive Assistance for Family Caregivers Eligibility for Legacy Participants and Legacy Applicants. 86 FR § 52614.
17. US Department of Veterans Affairs, 2020. Certification of the Implementation of the Caregiver Records Management Application (CARMA). 85 FR § 63358.
18. Sussman, JS. Department of Veterans Affairs: Caregiver Support. Congressional Research Service Report No. R46282. Published March 24, 2020. Accessed February 16, 2022. https://www.everycrsreport.com/files/20200324_R46282_656f1e8338af12a2a676c471be3b3c13b2fcb0bb.pdf
19. US Department of Veterans Affairs. Veterans Affairs Program of Comprehensive Assistance for Family Caregivers Eligibility Criteria Fact Sheet. Washington, DC: U.S. Department of Veterans Affairs; 2020. Accessed February 9, 2022. https://www.caregiver.va.gov/pdfs/MissionAct/EligibilityCriteriaFactsheet_Chapter2_Launch_Approved_Final_100120.pdf
1. Chi W, Graf E, Hughes L, et al. Community-dwelling older adults with dementia and their caregivers: key indicators from the National Health and Aging Trends study. Published January 29, 2019. Accessed February 16, 2022. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//186501/DemChartbook.pdf
2. Rapaport P, Burton A, Leverton M, et al. “I just keep thinking that I don’t want to rely on people.” A qualitative study of how people living with dementia achieve and maintain independence at home: stakeholder perspectives. BMC Geriatr. 2020;20(1):1-11. doi:10.1186/s12877-019-1406-6
3. Miller EA, Gidmark S, Gadbois E, Rudolph JL, Intrator O. Nursing home referral within the Veterans Health Administration: practice variation by payment source and facility type. Res Aging. 2018;40(7):687-711. doi:10.1177/0164027517730383
4. Veterans Benefits, Health Care, and Information Technology Act of 2006, Pub L No. 109-461, 120 Stat. 3403.
5. Caregivers and Veterans Omnibus Health Services Act of 2010, Pub L No. 111-163, 115 Stat 552.
6. VA MISSION Act of 2018. 38 CFR § 17.
7. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
8. Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20(4):245-253. doi:10.1159/000087345
9. Holder, KA. The Disability of Veterans. Social, Economic, and Housing Statistics Division, US Census Bureau; 2014. Accessed February 9, 2022. https://www.census.gov/content/dam/Census/library/working-papers/2016/demo/Holder-2016-01.pdf
10. Sanford AM. Lewy body dementia. Clin Geriatr Med. 2018;34(4):603-615. doi:10.1016/j.cger.2018.06.007
11. Armstrong MJ. Lewy body dementias. Continuum (Minneap Minn). 2019;25(1):128-146. doi:10.1212/CON.0000000000000685
12. Bureau of Consumer Financial Protection. Financial well-being of older Americans. Published December 2018. Accessed February 17, 2022. https://files.consumerfinance.gov/f/documents/bcfp_financial-well-being-older-americans_report.pdf
13. Van Houtven CH, Smith VA, Stechuchak KM, et al. Comprehensive support for family caregivers: impact on veteran health care utilization and costs. Med Care Res Rev. 2019;76(1):89-114. doi:10.1177/1077558717697015
14. Boland L, Légaré F, Perez MMB, et al. Impact of home care versus alternative locations of care on elder health outcomes: an overview of systematic reviews. BMC Geriatr. 2017;17(1):20. doi:10.1186/s12877-016-0395-y
15. Program of Comprehensive Assistance for Family Caregivers Improvements and Amendments Under the VA MISSION Act of 2018. 85 FR § 13356.
16. Extension of Program of Comprehensive Assistance for Family Caregivers Eligibility for Legacy Participants and Legacy Applicants. 86 FR § 52614.
17. US Department of Veterans Affairs, 2020. Certification of the Implementation of the Caregiver Records Management Application (CARMA). 85 FR § 63358.
18. Sussman, JS. Department of Veterans Affairs: Caregiver Support. Congressional Research Service Report No. R46282. Published March 24, 2020. Accessed February 16, 2022. https://www.everycrsreport.com/files/20200324_R46282_656f1e8338af12a2a676c471be3b3c13b2fcb0bb.pdf
19. US Department of Veterans Affairs. Veterans Affairs Program of Comprehensive Assistance for Family Caregivers Eligibility Criteria Fact Sheet. Washington, DC: U.S. Department of Veterans Affairs; 2020. Accessed February 9, 2022. https://www.caregiver.va.gov/pdfs/MissionAct/EligibilityCriteriaFactsheet_Chapter2_Launch_Approved_Final_100120.pdf
Psychodynamic factors in psychotropic prescribing
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
Medical noncompliance and patient resistance to treatment are frequent problems in medical practice. According to an older report by the US Office of Inspector General, approximately 125,000 people die each year in the United States because they do not take their medication properly.1 The World Health Organization reported that 10% to 25% of hospital and nursing home admissions are a result of patient noncompliance.2 In addition, approximately 50% of prescriptions filled for chronic diseases in developed nations are not taken correctly, and up to 40% of patients do not adhere to their treatment regimens.2 Among psychiatric patients, noncompliance with medications and other treatments ranges from 25% to 75%.3
In recent years, combining pharmacotherapy with psychodynamic psychotherapy has become a fairly common form of psychiatric practice. A main reason for combining these treatments is that a patient with severe psychiatric symptoms may be unable to engage in self-reflective insightful therapy until those symptoms are substantially relieved with pharmacotherapy. The efficacy of combined pharmacotherapy/psychotherapy may also be more than additive and result in a therapeutic alliance that is greater than the sum of the 2 individual treatments.4 Establishing a therapeutic alliance is critical to successful treatment, but this alliance can be distorted by the needs and expectations of both the patient and the clinician.
A psychodynamic understanding of the patient and the therapeutic alliance can facilitate combined treatment in several ways. It can lead to better communication, which in turn can lead to a realistic discussion of a patient’s fears and worries about any medications they have been prescribed. A dynamically aware clinician may better understand what the symptoms mean to the patient. Such clinicians will not only be able to explain the value of a medication, its target symptoms, and the rationale for taking it, but will also be able to discuss the psychological significance of the medication, along with its medical and biological significance.5
This article briefly reviews the therapeutic alliance and the influence of transference (the emotional reactions of the patient towards the clinician),6 countertransference (the emotional reactions of the clinician towards the patient),6 and patient resistance/nonadherence to treatment on the failure or success of pharmacotherapy. We provide case examples to illustrate how these psychodynamic factors can be at play in prescribing.
The therapeutic alliance
The therapeutic alliance is a rational agreement or contract between a patient and the clinician; it is a cornerstone of treatment in medicine.6 Its basic premise is that the patient’s rational expectation that their physician is appropriately qualified, will perform a suitable evaluation, and will prescribe relevant treatment is matched by the physician’s expectation that the patient will do their best to comply with treatment recommendations. For this to succeed, the contract needs to be straightforward, and there needs to be no covert agenda. A covert agenda may be in the form of unrealistic expectations and wishes rooted in insecure experiences in childhood by either party. A patient under stress may react to the physician with mistrust, excessive demands, and noncompliance. A physician under stress may react to a patient by becoming authoritative or indecisive, or by overmedicating or underprescribing.
Transference
Transference is a phenomenon whereby a patient’s feelings and attitudes are unconsciously transferred from a person or situation in the past to the clinician or treatment in the present.6 For example, a patient who is scared of a serious illness may adopt a helpless, childlike role and project an omnipotent, parentlike quality on the clinician (positive transference) that may be unrealistic. Positive transference may underlie a placebo response to medication in which a patient’s response is too quick or too complete, and it may be a way of unconsciously pleasing an authoritative parent figure from childhood. On the other hand, a patient may unconsciously view their physician as a controlling parent (negative transference) and react angrily or rebelliously. A patient’s flirtatious behavior toward their physician may be a form of transference from unresolved sexual trauma during childhood. However, not all patient reactions should be considered transference; a patient may be appropriately thankful and deferential, or irritated and questioning, depending on the clinician’s demeanor and treatment approach.
Countertransference
Countertransference is the response elicited in the physician by a patient’s appearance and behaviors, or by a patient’s transference projections.6 This response can be positive or negative and includes both feelings and associated thoughts related to the physician’s past experiences. For example, a physician in the emergency department may get angry with a patient with an alcohol use disorder because of the physician’s negative experiences with an alcoholic parent during childhood. On the other hand, a physician raised by a compulsive mother may order unnecessary tests on a demanding older female patient. Or, a clinician raised by a sheltering parent may react to a hapless and dependent patient by spending excessive time with them or providing additional medication samples. However, not all clinician reactions are countertransference. For example, a physician’s empathic or stoic demeanor may be an appropriate emotional response to a patient’s diagnosis such as cancer.
Continue to: Patient resistance/nonadherence
Patient resistance/nonadherence
In 1920, Freud conceptualized the psychodynamic factors in patient resistance to treatment and theorized that many patients were unconsciously reluctant to give up their symptoms or were driven, for transference reasons, to resist the physician.7 This same concept may underlie patient resistance to pharmacotherapy. When symptoms constitute an important defense mechanism, patients are likely to resist medication effects until they have developed more mature defenses or more effective ways of coping.8 Even when patients do not resist symptom relief, they may still resist the physician’s choice of treatment due to negative transference. Such patients often negotiate the type of medication, dose, timing of the dose, and start date as a way of trying to “keep control” of a “doctor they don’t quite trust.”8 They may manage their own medication regimen by taking more or less than the prescribed dose. This resistance might lead to a “nocebo” effect in which a medication trial fails not because of its ineffectiveness but instead from the unconscious mind influencing the patient’s body to resist. Nonadherence to treatment may occur in patients who have attachment difficulties that make it difficult for them to trust anyone as a result of negative childhood experiences.9 Clinicians need to recognize the dynamics of power struggles, control, and trust. A warm, collaborative and cooperative stance is likely to be more beneficial than an authoritative and detached approach.10
The following 3 case examples illustrate how psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and the outcomes of pharmacotherapy.
CASE 1
Mr. A, age 63, has posttraumatic stress disorder originating from his father’s death by a self-inflicted gunshot wound when Mr. A was 19, and later from the symbolic loss of his mother when she remarried. He reported vivid memories of his father sexually assaulting his mother when he was 6. This fostered a protective nature in him for his mother, as well as for his 3 younger siblings. After his father’s suicide, Mr. A had to take on a paternal role for his 3 siblings. He often feels he grew up too quickly, and resents this. He feels his mother betrayed him when she got remarried. Mr. A attempts suicide, is admitted to a local hospital, and then follows up at a university hospital outpatient psychiatry clinic.
At the clinic, Mr. A begins psychodynamic psychotherapy with a female resident physician. They establish a good rapport. Mr. A begins working through his past traumas and looks forward to his therapy sessions. The physician views this as positive transference, perhaps because her personality style and appearance are similar to that of Mr. A’s mother. She also often notes a positive countertransference during sessions; Mr. A seemingly reminds her of her father in personality and appearance. Perhaps due to this positive transference/positive countertransference dynamic, Mr. A feels comfortable with having his medication regimen simplified after years of unsuccessful medication trials and a course of electroconvulsive therapy. His regimen soon consists of only a selective serotonin reuptake inhibitor and a glutamate modulator as an adjunct for anxiety. Psychotherapy sessions remain the mainstay of his treatment plan. Mr. A’s mood and anxiety improve significantly over a short time.
CASE 2
Ms. G, age 24, is admitted to a partial hospitalization program (PHP). Her diagnoses include seasonal affective disorder, anxiety, and attention-deficit/hyperactivity disorder (ADHD); she might have a genetic disposition to bipolar disorder. Ms. G recently had attempted suicide and was discharged from an inpatient unit. She is a middle child and was raised by emotionally and verbally abusive parents in a tumultuous household. Her father rarely kept a job for more than a few months, displayed rage, and lacked empathy. Ms. G feels unloved by her mother and says that her mother is emotionally unstable. Upon admission to the PHP, Ms. G is quick to question the credentials of every staff member she meets, and suggests the abuse and lack of trust she had experienced during her formative years have made her aggressive and paranoid.
Continue to: Since her teens...
Since her teens, Ms. G had received treatment for ADHD with various stimulant and nonstimulant medications that were prescribed by an outpatient psychiatrist. During her sophomore year of college, she was also prescribed medications for depression and anxiety. Ms. G speaks very highly of and praises the skill of her previous psychiatrist while voicing concerns about having to see new clinicians in the PHP. She had recently seen a therapist who moved out of state after a few sessions. Ms. G has abandonment fears and appears to react with anger toward new clinicians.
A negative transference towards Ms. G’s treatment team and the PHP as a whole are evident during the first week. She skips most group therapy sessions and criticizes the clinicians’ skills and training as ineffective. When her psychiatrist recommends changes in medication, she initially argues. She eventually agrees to take a new medication but soon reports intolerable adverse effects, which suggests negative transference toward the psychiatrist as an authority figure, and toward the medication as an extension of the psychiatrist. The treatment team also interprets this as nocebo effect. Ms. G engages in “splitting” by complaining about her psychiatrist to her therapist. The psychiatrist resents having been belittled. Ms. G demands to see a different psychiatrist, and when her demands are not met, she discharges herself from the PHP against medical advice. The treatment team interprets Ms. G’s resistance to treatment to have resulted from poor attachment during childhood and subsequent negative transference.
CASE 3
Ms. U, age 60, is seen at a local mental health center and diagnosed with major depressive disorder, likely resulting from grief and loss from her husband’s recent death. She was raised by her single mother and mostly absent father. Ms. U is a homemaker and had been married for more than 30 years. She participates in weekly psychotherapy with a young male psychiatrist, who prescribes an antidepressant. Ms. U is eager to please and makes every effort to be the perfect patient: she is always early for her appointments, takes her medications as prescribed, and frequently expresses her respect and appreciation for her psychiatrist. Within a few weeks, Ms. U’s depressive symptoms rapidly improve.
Ms. U is a talented and avid knit and crochet expert. At an appointment soon before Christmas, she gives her psychiatrist a pair of socks she knitted. While the gift is of little monetary value, the psychiatrist interprets this as part of transference, but the intimate nature of the gift makes him uncomfortable. He and Ms. U discuss this at length, which reveals definite transference as Ms. U says the psychiatrist perhaps reminds her of her husband, who also had brown skin. It is also apparent that Ms. U’s tendency to please perhaps comes from the lack of having a father figure, which her husband had fulfilled. The psychiatrist believes that Ms. U’s rapid response may be a placebo effect from positive transference. Upon further reflection, the psychiatrist realizes that Ms. U is a motherly figure to him, and that positive countertransference is at play in that he could not turn down the gift and had looked forward to the therapy sessions with her.
Bottom Line
Even clinicians who do not provide psychodynamic psychotherapy can use an awareness of psychodynamic factors to improve treatment. Psychodynamic factors such as transference and countertransference can influence the therapeutic alliance, treatment decisions, and patient outcomes. Patients’ experiences and difficulties with attachment during childhood should be recognized and addressed as part of pharmacotherapy.
Related Resources
- Neumann M, Silva N, Opler DJ. ARISE to supportive psychotherapy. Current Psychiatry. 2021;20(5):48-49. doi:10.12788/cp.0123
- Mintz D. Psychodynamic psychopharmacology. Psychiatric Times. 2011;28(9). https://www.psychiatrictimes.com/view/psychodynamic-psychopharmacology
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
1. Office of Inspector General, Office of Evaluation and Inspections. Medication Regimens: Causes of Noncompliance. 1990. Accessed April 13, 2022. https://oig.hhs.gov/oei/reports/oei-04-89-89121.pdf
2. World Health Organization. Adherence to Long Term Therapies: Evidence for Action. World Health Organization; 2003.
3. Powell AD. The medication life. J Psychother Pract Res. 2001;10(4):217-222.
4. Wright JH, Hollifield M. Combining pharmacotherapy and psychotherapy. Psychiatric Annals. 2006;36(5):302-305.
5. Summers RF, Barber JP. Psychodynamic Therapy: A Guide to Evidence-Based Practice. Guilford Press; 2013:265-290.
6. Hughes P, Kerr I. Transference and countertransference in communication between doctor and patient. Advances in Psychiatric Treatment. 2000;6(1):57-64.
7. Freud S. Resistance and suppression. In: Freud S. A General Introduction to Psychoanalysis. Boni and Liveright Publishers; 1920:248-261.
8. Vlastelica M. Psychodynamic approach as a creative factor in psychopharmacotherapy. Psychiatr Danub. 2013;25(3):316-319.
9. Alfonso CA. Understanding the psychodynamics of nonadherence. Psychiatric Times. 2011;28(5). Accessed April 13, 2022. https://www.psychiatrictimes.com/view/understanding-psychodynamics-nonadherence
10. Wallin DJ. Attachment in Psychotherapy. Guilford Press; 2007.
The woman who kept passing out
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
How can we help refugees with PTSD?
This article was originally published in Italian on Univadis .
The arrival of Ukrainian refugees in Western Europe, and especially the arrival of women and children coming from the cities most affected by the attacks, has made local medical services the first point of contact for the diagnosis and care of the psychological effects of war.
Many studies demonstrate the high prevalence of posttraumatic stress disorder (PTSD), depression, and anxiety among refugees. For example, a study from 2019 on refugee mental health offers a disturbing epidemiologic insight: Ten percent of refugees escaping conflict in Nepal showed signs of PTSD, 27.5% suffered from depression, and 22.9% suffered from anxiety. The rate of depression surpasses 90% in all studies carried out on survivors of torture.
Official guidelines
Posttraumatic stress is a form of mental disorder that manifests after experiencing highly traumatic events. Defined and studied in the United States, especially in veterans of the Vietnam War, and subsequently reviewed in relation to more recent conflicts, PTSD may appear in people of all ages. It also can occur in family members, witnesses, and aid workers involved in traumatic events. PTSD may come from repeated exposure to episodes of violence and degradation.
Because PTSD is a complex mental disorder derived from multiple factors, both personal and environmental, a diagnosis is never straightforward. It is generally indicated as “a condition of acute stress which manifests after exposure to a traumatic event.”
Among the most common symptoms of war-related trauma, according to experts, are the onset of flashbacks of troublesome memories, intrusive trauma-related thoughts, panic attacks, insomnia and night terrors, and social avoidance. In children, elements of regression can be observed, such as a need to sleep next to their parents.
Research performed directly on different areas of the brain has demonstrated that people affected by PTSD produce abnormal levels of hormones that are involved in responding to stress and fear. The area of the brain responsible for this response is the amygdala, which activates during moments of fear and produces natural pain-relieving molecules. In people with PTSD, the production of these molecules can carry on long after the event is over, causing a change in emotional state. Furthermore, levels of neurotransmitters that reach the hippocampus are also altered, which influences memory and learning capability. These same alterations concerning neurotransmitter levels are the basis for sudden flashbacks.
People with PTSD are subject to a change in blood flow to the brain and structural changes to brain tissue.
Social factors
“A factor that worsens the mental condition of Ukrainian refugees is the speed in which they have passed from a normal life, similar to that of many other Western countries, to a state of war, death, and injury,” writes Arash Javanbakht, MD, associate professor of psychiatry at Wayne State University in Detroit, and an expert in PTSD in war refugees.
“Furthermore, they are experiencing an awful feeling of injustice, as the democracy and freedom that they have fought so hard to have has been put at risk, and they don’t feel sufficiently supported by their allies.”
As of this writing, the World Health Organization has estimated that there are 3.6 million Ukrainian refugees. This population already has experienced war and its psychological consequences. A study from 2019 assessed the prevalence of PTSD (27%) and depression (21%) among the 1.5 million Ukrainians who had to leave their homes after the first Russian invasion in 2014 and the rebellion of predominantly Russian regions.
Children are particularly at risk of developing PTSD, as seen from studies conducted on Syrian refugees. They have a roughly 70% chance of developing separation anxiety, a condition that many workers and volunteers have experienced firsthand recently. Some children do not accept being separated from their parents even to allow them to go to the bathroom or wash themselves, which also aggravates stress levels among adults. Infant trauma increases the risk of developing physical or mental disorders during adulthood, including depression, chronic pain, heart disorders, and diabetes.
War-related trauma entails transmissible epigenetic alterations, as shown by studies on the transmissibility of trauma on a biological level.
Diagnosis and treatment
People with PTSD have difficulty controlling their emotions, resulting in irritability, sudden rage or emotional confusion, depression and anxiety, and insomnia. They also are determined to avoid any actions that remind them of the traumatic event. Another common symptom is a sense of shame, as a result of having survived or not having been able to save others.
Physical symptoms include chest pain, dizziness, gastrointestinal problems, migraine, and a weakened immune system. A diagnosis of PTSD can be made when, in accordance with the National Institute of Mental Health (NIMH), the patient presents with the characteristic symptoms for more than a month after the event that caused the symptoms occurred.
The NIMH highlights that a diagnosis cannot always be made in a systematic way. In many cases, patients with PTSD are treated for the physical symptoms only, without any consideration for the overall picture.
The American Psychiatric Association (APA) has created a detailed list of PTSD symptoms. According to the APA, these symptoms usually appear within 3 months of the trauma, even if stress may appear later. Symptoms are arranged into the following three well-defined categories:
- Intrusive memories. People with PTSD suffer from sudden, vivid memories that are accompanied by painful emotions and a feeling of reliving the trauma. At times, this experience is so strong that the person involved feels as though the traumatic event is repeating itself.
- Avoidance and numbing. The individual seeks to avoid contact with anyone or anything that brings back memories of the trauma. Initially, the person experiences an emotional state of disinterest and detachment, reducing the capacity for emotional interaction and resulting in participation only in simple, routine activities. A lack of emotional processing causes an accumulation of anxiety and tension, which can become a chronic condition, leading to depression. At the same time, people frequently experience a sense of shame.
- Hyperarousal and hypervigilance. People behave as if they are constantly threatened. They react in a sudden, violent way, are unable to concentrate, and have problems with their memory. At times, they use alcohol or other drugs to alleviate pain. People with PTSD may lose control of their lives and therefore be at risk for suicidal behavior.
Why do some people pass unscathed through traumatic situations, whereas others carry the scars forever? There is a correlation with the severity of the trauma, but also with biological and genetic factors, as well as with previous experiences that contribute to increasing an individual’s resilience. Another key element is the rapid and effective treatment of symptoms, which also relates to personal and financial security.
It is not a coincidence that the first guidelines that clinicians follow when treating a traumatized patient aren’t strictly medical. It is necessary to guarantee the financial security of a refugee, but also the security of the few valuable items they have with them (such as keepsakes and pets). Clinicians are advised to facilitate contact with any of the patient’s family members located elsewhere whenever possible. It is appropriate to use relaxation techniques that are compatible with the patient’s cultural approach. Clinicians also check for the most common conditions in the refugee’s population of origin. It is advisable constantly to check for trauma-related symptoms and to listen to the patient’s story. Caregivers should be allowed to stay close to their children and should be provided sufficient information, but not an overwhelming amount.
There isn’t a consensus on how to treat people with PTSD. The possibility that PTSD can be resolved even without specific treatments has not been excluded, if the affected person is cared for and helped within a family and community setting, and if the person’s personal condition allows for this. However, in general, some form of treatment is beneficial before symptoms become chronic.
Pharmacologic and psychological treatment may be implemented. For the latter, the NIMH and the APA suggest that good results can be obtained from cognitive behavioral therapy, where the patient learns to manage his or her anxiety and depression and amend dangerous behaviors, such as the dismissal of his or her own emotions. According to these organizations, group therapy and other forms of psychotherapy have provided good results. The indicated duration of treatment is generally 6-12 weeks, even if this duration strongly depends on the individual’s condition, with subsequent periodic follow-ups. The involvement of the patient’s family and community is important.
The National Center for PTSD in Washington (run by the U.S. Department of Veterans Affairs) has highlighted the importance of a detailed case-by-case assessment to put in place a precise therapy plan. If patients should continue to find themselves in a state of crisis, for example during a war or in cases of domestic violence, working toward removing the cause of stress is first necessary before beginning treatment.
An important aspect is making the victim aware of the disorder. Treatment should therefore begin after the patient and family have been informed about the possibility of PTSD and the way in which it develops. Recognizing the symptoms over the following weeks and working quickly to manage and treat them significantly affects treatment success.
A version of this article first appeared on Medscape.com.
This article was originally published in Italian on Univadis .
The arrival of Ukrainian refugees in Western Europe, and especially the arrival of women and children coming from the cities most affected by the attacks, has made local medical services the first point of contact for the diagnosis and care of the psychological effects of war.
Many studies demonstrate the high prevalence of posttraumatic stress disorder (PTSD), depression, and anxiety among refugees. For example, a study from 2019 on refugee mental health offers a disturbing epidemiologic insight: Ten percent of refugees escaping conflict in Nepal showed signs of PTSD, 27.5% suffered from depression, and 22.9% suffered from anxiety. The rate of depression surpasses 90% in all studies carried out on survivors of torture.
Official guidelines
Posttraumatic stress is a form of mental disorder that manifests after experiencing highly traumatic events. Defined and studied in the United States, especially in veterans of the Vietnam War, and subsequently reviewed in relation to more recent conflicts, PTSD may appear in people of all ages. It also can occur in family members, witnesses, and aid workers involved in traumatic events. PTSD may come from repeated exposure to episodes of violence and degradation.
Because PTSD is a complex mental disorder derived from multiple factors, both personal and environmental, a diagnosis is never straightforward. It is generally indicated as “a condition of acute stress which manifests after exposure to a traumatic event.”
Among the most common symptoms of war-related trauma, according to experts, are the onset of flashbacks of troublesome memories, intrusive trauma-related thoughts, panic attacks, insomnia and night terrors, and social avoidance. In children, elements of regression can be observed, such as a need to sleep next to their parents.
Research performed directly on different areas of the brain has demonstrated that people affected by PTSD produce abnormal levels of hormones that are involved in responding to stress and fear. The area of the brain responsible for this response is the amygdala, which activates during moments of fear and produces natural pain-relieving molecules. In people with PTSD, the production of these molecules can carry on long after the event is over, causing a change in emotional state. Furthermore, levels of neurotransmitters that reach the hippocampus are also altered, which influences memory and learning capability. These same alterations concerning neurotransmitter levels are the basis for sudden flashbacks.
People with PTSD are subject to a change in blood flow to the brain and structural changes to brain tissue.
Social factors
“A factor that worsens the mental condition of Ukrainian refugees is the speed in which they have passed from a normal life, similar to that of many other Western countries, to a state of war, death, and injury,” writes Arash Javanbakht, MD, associate professor of psychiatry at Wayne State University in Detroit, and an expert in PTSD in war refugees.
“Furthermore, they are experiencing an awful feeling of injustice, as the democracy and freedom that they have fought so hard to have has been put at risk, and they don’t feel sufficiently supported by their allies.”
As of this writing, the World Health Organization has estimated that there are 3.6 million Ukrainian refugees. This population already has experienced war and its psychological consequences. A study from 2019 assessed the prevalence of PTSD (27%) and depression (21%) among the 1.5 million Ukrainians who had to leave their homes after the first Russian invasion in 2014 and the rebellion of predominantly Russian regions.
Children are particularly at risk of developing PTSD, as seen from studies conducted on Syrian refugees. They have a roughly 70% chance of developing separation anxiety, a condition that many workers and volunteers have experienced firsthand recently. Some children do not accept being separated from their parents even to allow them to go to the bathroom or wash themselves, which also aggravates stress levels among adults. Infant trauma increases the risk of developing physical or mental disorders during adulthood, including depression, chronic pain, heart disorders, and diabetes.
War-related trauma entails transmissible epigenetic alterations, as shown by studies on the transmissibility of trauma on a biological level.
Diagnosis and treatment
People with PTSD have difficulty controlling their emotions, resulting in irritability, sudden rage or emotional confusion, depression and anxiety, and insomnia. They also are determined to avoid any actions that remind them of the traumatic event. Another common symptom is a sense of shame, as a result of having survived or not having been able to save others.
Physical symptoms include chest pain, dizziness, gastrointestinal problems, migraine, and a weakened immune system. A diagnosis of PTSD can be made when, in accordance with the National Institute of Mental Health (NIMH), the patient presents with the characteristic symptoms for more than a month after the event that caused the symptoms occurred.
The NIMH highlights that a diagnosis cannot always be made in a systematic way. In many cases, patients with PTSD are treated for the physical symptoms only, without any consideration for the overall picture.
The American Psychiatric Association (APA) has created a detailed list of PTSD symptoms. According to the APA, these symptoms usually appear within 3 months of the trauma, even if stress may appear later. Symptoms are arranged into the following three well-defined categories:
- Intrusive memories. People with PTSD suffer from sudden, vivid memories that are accompanied by painful emotions and a feeling of reliving the trauma. At times, this experience is so strong that the person involved feels as though the traumatic event is repeating itself.
- Avoidance and numbing. The individual seeks to avoid contact with anyone or anything that brings back memories of the trauma. Initially, the person experiences an emotional state of disinterest and detachment, reducing the capacity for emotional interaction and resulting in participation only in simple, routine activities. A lack of emotional processing causes an accumulation of anxiety and tension, which can become a chronic condition, leading to depression. At the same time, people frequently experience a sense of shame.
- Hyperarousal and hypervigilance. People behave as if they are constantly threatened. They react in a sudden, violent way, are unable to concentrate, and have problems with their memory. At times, they use alcohol or other drugs to alleviate pain. People with PTSD may lose control of their lives and therefore be at risk for suicidal behavior.
Why do some people pass unscathed through traumatic situations, whereas others carry the scars forever? There is a correlation with the severity of the trauma, but also with biological and genetic factors, as well as with previous experiences that contribute to increasing an individual’s resilience. Another key element is the rapid and effective treatment of symptoms, which also relates to personal and financial security.
It is not a coincidence that the first guidelines that clinicians follow when treating a traumatized patient aren’t strictly medical. It is necessary to guarantee the financial security of a refugee, but also the security of the few valuable items they have with them (such as keepsakes and pets). Clinicians are advised to facilitate contact with any of the patient’s family members located elsewhere whenever possible. It is appropriate to use relaxation techniques that are compatible with the patient’s cultural approach. Clinicians also check for the most common conditions in the refugee’s population of origin. It is advisable constantly to check for trauma-related symptoms and to listen to the patient’s story. Caregivers should be allowed to stay close to their children and should be provided sufficient information, but not an overwhelming amount.
There isn’t a consensus on how to treat people with PTSD. The possibility that PTSD can be resolved even without specific treatments has not been excluded, if the affected person is cared for and helped within a family and community setting, and if the person’s personal condition allows for this. However, in general, some form of treatment is beneficial before symptoms become chronic.
Pharmacologic and psychological treatment may be implemented. For the latter, the NIMH and the APA suggest that good results can be obtained from cognitive behavioral therapy, where the patient learns to manage his or her anxiety and depression and amend dangerous behaviors, such as the dismissal of his or her own emotions. According to these organizations, group therapy and other forms of psychotherapy have provided good results. The indicated duration of treatment is generally 6-12 weeks, even if this duration strongly depends on the individual’s condition, with subsequent periodic follow-ups. The involvement of the patient’s family and community is important.
The National Center for PTSD in Washington (run by the U.S. Department of Veterans Affairs) has highlighted the importance of a detailed case-by-case assessment to put in place a precise therapy plan. If patients should continue to find themselves in a state of crisis, for example during a war or in cases of domestic violence, working toward removing the cause of stress is first necessary before beginning treatment.
An important aspect is making the victim aware of the disorder. Treatment should therefore begin after the patient and family have been informed about the possibility of PTSD and the way in which it develops. Recognizing the symptoms over the following weeks and working quickly to manage and treat them significantly affects treatment success.
A version of this article first appeared on Medscape.com.
This article was originally published in Italian on Univadis .
The arrival of Ukrainian refugees in Western Europe, and especially the arrival of women and children coming from the cities most affected by the attacks, has made local medical services the first point of contact for the diagnosis and care of the psychological effects of war.
Many studies demonstrate the high prevalence of posttraumatic stress disorder (PTSD), depression, and anxiety among refugees. For example, a study from 2019 on refugee mental health offers a disturbing epidemiologic insight: Ten percent of refugees escaping conflict in Nepal showed signs of PTSD, 27.5% suffered from depression, and 22.9% suffered from anxiety. The rate of depression surpasses 90% in all studies carried out on survivors of torture.
Official guidelines
Posttraumatic stress is a form of mental disorder that manifests after experiencing highly traumatic events. Defined and studied in the United States, especially in veterans of the Vietnam War, and subsequently reviewed in relation to more recent conflicts, PTSD may appear in people of all ages. It also can occur in family members, witnesses, and aid workers involved in traumatic events. PTSD may come from repeated exposure to episodes of violence and degradation.
Because PTSD is a complex mental disorder derived from multiple factors, both personal and environmental, a diagnosis is never straightforward. It is generally indicated as “a condition of acute stress which manifests after exposure to a traumatic event.”
Among the most common symptoms of war-related trauma, according to experts, are the onset of flashbacks of troublesome memories, intrusive trauma-related thoughts, panic attacks, insomnia and night terrors, and social avoidance. In children, elements of regression can be observed, such as a need to sleep next to their parents.
Research performed directly on different areas of the brain has demonstrated that people affected by PTSD produce abnormal levels of hormones that are involved in responding to stress and fear. The area of the brain responsible for this response is the amygdala, which activates during moments of fear and produces natural pain-relieving molecules. In people with PTSD, the production of these molecules can carry on long after the event is over, causing a change in emotional state. Furthermore, levels of neurotransmitters that reach the hippocampus are also altered, which influences memory and learning capability. These same alterations concerning neurotransmitter levels are the basis for sudden flashbacks.
People with PTSD are subject to a change in blood flow to the brain and structural changes to brain tissue.
Social factors
“A factor that worsens the mental condition of Ukrainian refugees is the speed in which they have passed from a normal life, similar to that of many other Western countries, to a state of war, death, and injury,” writes Arash Javanbakht, MD, associate professor of psychiatry at Wayne State University in Detroit, and an expert in PTSD in war refugees.
“Furthermore, they are experiencing an awful feeling of injustice, as the democracy and freedom that they have fought so hard to have has been put at risk, and they don’t feel sufficiently supported by their allies.”
As of this writing, the World Health Organization has estimated that there are 3.6 million Ukrainian refugees. This population already has experienced war and its psychological consequences. A study from 2019 assessed the prevalence of PTSD (27%) and depression (21%) among the 1.5 million Ukrainians who had to leave their homes after the first Russian invasion in 2014 and the rebellion of predominantly Russian regions.
Children are particularly at risk of developing PTSD, as seen from studies conducted on Syrian refugees. They have a roughly 70% chance of developing separation anxiety, a condition that many workers and volunteers have experienced firsthand recently. Some children do not accept being separated from their parents even to allow them to go to the bathroom or wash themselves, which also aggravates stress levels among adults. Infant trauma increases the risk of developing physical or mental disorders during adulthood, including depression, chronic pain, heart disorders, and diabetes.
War-related trauma entails transmissible epigenetic alterations, as shown by studies on the transmissibility of trauma on a biological level.
Diagnosis and treatment
People with PTSD have difficulty controlling their emotions, resulting in irritability, sudden rage or emotional confusion, depression and anxiety, and insomnia. They also are determined to avoid any actions that remind them of the traumatic event. Another common symptom is a sense of shame, as a result of having survived or not having been able to save others.
Physical symptoms include chest pain, dizziness, gastrointestinal problems, migraine, and a weakened immune system. A diagnosis of PTSD can be made when, in accordance with the National Institute of Mental Health (NIMH), the patient presents with the characteristic symptoms for more than a month after the event that caused the symptoms occurred.
The NIMH highlights that a diagnosis cannot always be made in a systematic way. In many cases, patients with PTSD are treated for the physical symptoms only, without any consideration for the overall picture.
The American Psychiatric Association (APA) has created a detailed list of PTSD symptoms. According to the APA, these symptoms usually appear within 3 months of the trauma, even if stress may appear later. Symptoms are arranged into the following three well-defined categories:
- Intrusive memories. People with PTSD suffer from sudden, vivid memories that are accompanied by painful emotions and a feeling of reliving the trauma. At times, this experience is so strong that the person involved feels as though the traumatic event is repeating itself.
- Avoidance and numbing. The individual seeks to avoid contact with anyone or anything that brings back memories of the trauma. Initially, the person experiences an emotional state of disinterest and detachment, reducing the capacity for emotional interaction and resulting in participation only in simple, routine activities. A lack of emotional processing causes an accumulation of anxiety and tension, which can become a chronic condition, leading to depression. At the same time, people frequently experience a sense of shame.
- Hyperarousal and hypervigilance. People behave as if they are constantly threatened. They react in a sudden, violent way, are unable to concentrate, and have problems with their memory. At times, they use alcohol or other drugs to alleviate pain. People with PTSD may lose control of their lives and therefore be at risk for suicidal behavior.
Why do some people pass unscathed through traumatic situations, whereas others carry the scars forever? There is a correlation with the severity of the trauma, but also with biological and genetic factors, as well as with previous experiences that contribute to increasing an individual’s resilience. Another key element is the rapid and effective treatment of symptoms, which also relates to personal and financial security.
It is not a coincidence that the first guidelines that clinicians follow when treating a traumatized patient aren’t strictly medical. It is necessary to guarantee the financial security of a refugee, but also the security of the few valuable items they have with them (such as keepsakes and pets). Clinicians are advised to facilitate contact with any of the patient’s family members located elsewhere whenever possible. It is appropriate to use relaxation techniques that are compatible with the patient’s cultural approach. Clinicians also check for the most common conditions in the refugee’s population of origin. It is advisable constantly to check for trauma-related symptoms and to listen to the patient’s story. Caregivers should be allowed to stay close to their children and should be provided sufficient information, but not an overwhelming amount.
There isn’t a consensus on how to treat people with PTSD. The possibility that PTSD can be resolved even without specific treatments has not been excluded, if the affected person is cared for and helped within a family and community setting, and if the person’s personal condition allows for this. However, in general, some form of treatment is beneficial before symptoms become chronic.
Pharmacologic and psychological treatment may be implemented. For the latter, the NIMH and the APA suggest that good results can be obtained from cognitive behavioral therapy, where the patient learns to manage his or her anxiety and depression and amend dangerous behaviors, such as the dismissal of his or her own emotions. According to these organizations, group therapy and other forms of psychotherapy have provided good results. The indicated duration of treatment is generally 6-12 weeks, even if this duration strongly depends on the individual’s condition, with subsequent periodic follow-ups. The involvement of the patient’s family and community is important.
The National Center for PTSD in Washington (run by the U.S. Department of Veterans Affairs) has highlighted the importance of a detailed case-by-case assessment to put in place a precise therapy plan. If patients should continue to find themselves in a state of crisis, for example during a war or in cases of domestic violence, working toward removing the cause of stress is first necessary before beginning treatment.
An important aspect is making the victim aware of the disorder. Treatment should therefore begin after the patient and family have been informed about the possibility of PTSD and the way in which it develops. Recognizing the symptoms over the following weeks and working quickly to manage and treat them significantly affects treatment success.
A version of this article first appeared on Medscape.com.
Mental illness tied to COVID-19 breakthrough infection
“Psychiatric disorders remained significantly associated with incident breakthrough infections above and beyond sociodemographic and medical factors, suggesting that mental health is important to consider in conjunction with other risk factors,” wrote the investigators, led by Aoife O’Donovan, PhD, University of California, San Francisco.
Individuals with psychiatric disorders “should be prioritized for booster vaccinations and other critical preventive efforts, including increased SARS-CoV-2 screening, public health campaigns, or COVID-19 discussions during clinical care,” they added.
The study was published online in JAMA Network Open.
Elderly most vulnerable
The researchers reviewed the records of 263,697 veterans who were fully vaccinated against COVID-19.
Just over a half (51.4%) had one or more psychiatric diagnoses within the last 5 years and 14.8% developed breakthrough COVID-19 infections, confirmed by a positive SARS-CoV-2 test.
Psychiatric diagnoses among the veterans included depression, posttraumatic stress, anxiety, adjustment disorder, substance use disorder, bipolar disorder, psychosis, ADHD, dissociation, and eating disorders.
In the overall sample, a history of any psychiatric disorder was associated with a 7% higher incidence of breakthrough COVID-19 infection in models adjusted for potential confounders (adjusted relative risk, 1.07; 95% confidence interval, 1.05-1.09) and a 3% higher incidence in models additionally adjusted for underlying medical comorbidities and smoking (aRR, 1.03; 95% CI, 1.01-1.05).
Most psychiatric disorders were associated with a higher incidence of breakthrough infection, with the highest relative risk observed for substance use disorders (aRR, 1.16; 95% CI, 1.12 -1.21) and adjustment disorder (aRR, 1.13; 95% CI, 1.10-1.16) in fully adjusted models.
Older vaccinated veterans with psychiatric illnesses appear to be most vulnerable to COVID-19 reinfection.
In veterans aged 65 and older, all psychiatric disorders were associated with an increased incidence of breakthrough infection, with increases in the incidence rate ranging from 3% to 24% in fully adjusted models.
In the younger veterans, in contrast, only anxiety, adjustment, and substance use disorders were associated with an increased incidence of breakthrough infection in fully adjusted models.
Psychotic disorders were associated with a 10% lower incidence of breakthrough infection among younger veterans, perhaps because of greater social isolation, the researchers said.
Risky behavior or impaired immunity?
“Although some of the larger observed effect sizes are compelling at an individual level, even the relatively modest effect sizes may have a large effect at the population level when considering the high prevalence of psychiatric disorders and the global reach and scale of the pandemic,” Dr. O’Donovan and colleagues wrote.
They noted that psychiatric disorders, including depression, schizophrenia, and bipolar disorders, have been associated with impaired cellular immunity and blunted response to vaccines. Therefore, it’s possible that those with psychiatric disorders have poorer responses to COVID-19 vaccination.
It’s also possible that immunity following vaccination wanes more quickly or more strongly in people with psychiatric disorders and they could have less protection against new variants, they added.
Patients with psychiatric disorders could be more apt to engage in risky behaviors for contracting COVID-19, which could also increase the risk for breakthrough infection, they said.
The study was supported by a UCSF Department of Psychiatry Rapid Award and UCSF Faculty Resource Fund Award. Dr. O’Donovan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Psychiatric disorders remained significantly associated with incident breakthrough infections above and beyond sociodemographic and medical factors, suggesting that mental health is important to consider in conjunction with other risk factors,” wrote the investigators, led by Aoife O’Donovan, PhD, University of California, San Francisco.
Individuals with psychiatric disorders “should be prioritized for booster vaccinations and other critical preventive efforts, including increased SARS-CoV-2 screening, public health campaigns, or COVID-19 discussions during clinical care,” they added.
The study was published online in JAMA Network Open.
Elderly most vulnerable
The researchers reviewed the records of 263,697 veterans who were fully vaccinated against COVID-19.
Just over a half (51.4%) had one or more psychiatric diagnoses within the last 5 years and 14.8% developed breakthrough COVID-19 infections, confirmed by a positive SARS-CoV-2 test.
Psychiatric diagnoses among the veterans included depression, posttraumatic stress, anxiety, adjustment disorder, substance use disorder, bipolar disorder, psychosis, ADHD, dissociation, and eating disorders.
In the overall sample, a history of any psychiatric disorder was associated with a 7% higher incidence of breakthrough COVID-19 infection in models adjusted for potential confounders (adjusted relative risk, 1.07; 95% confidence interval, 1.05-1.09) and a 3% higher incidence in models additionally adjusted for underlying medical comorbidities and smoking (aRR, 1.03; 95% CI, 1.01-1.05).
Most psychiatric disorders were associated with a higher incidence of breakthrough infection, with the highest relative risk observed for substance use disorders (aRR, 1.16; 95% CI, 1.12 -1.21) and adjustment disorder (aRR, 1.13; 95% CI, 1.10-1.16) in fully adjusted models.
Older vaccinated veterans with psychiatric illnesses appear to be most vulnerable to COVID-19 reinfection.
In veterans aged 65 and older, all psychiatric disorders were associated with an increased incidence of breakthrough infection, with increases in the incidence rate ranging from 3% to 24% in fully adjusted models.
In the younger veterans, in contrast, only anxiety, adjustment, and substance use disorders were associated with an increased incidence of breakthrough infection in fully adjusted models.
Psychotic disorders were associated with a 10% lower incidence of breakthrough infection among younger veterans, perhaps because of greater social isolation, the researchers said.
Risky behavior or impaired immunity?
“Although some of the larger observed effect sizes are compelling at an individual level, even the relatively modest effect sizes may have a large effect at the population level when considering the high prevalence of psychiatric disorders and the global reach and scale of the pandemic,” Dr. O’Donovan and colleagues wrote.
They noted that psychiatric disorders, including depression, schizophrenia, and bipolar disorders, have been associated with impaired cellular immunity and blunted response to vaccines. Therefore, it’s possible that those with psychiatric disorders have poorer responses to COVID-19 vaccination.
It’s also possible that immunity following vaccination wanes more quickly or more strongly in people with psychiatric disorders and they could have less protection against new variants, they added.
Patients with psychiatric disorders could be more apt to engage in risky behaviors for contracting COVID-19, which could also increase the risk for breakthrough infection, they said.
The study was supported by a UCSF Department of Psychiatry Rapid Award and UCSF Faculty Resource Fund Award. Dr. O’Donovan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Psychiatric disorders remained significantly associated with incident breakthrough infections above and beyond sociodemographic and medical factors, suggesting that mental health is important to consider in conjunction with other risk factors,” wrote the investigators, led by Aoife O’Donovan, PhD, University of California, San Francisco.
Individuals with psychiatric disorders “should be prioritized for booster vaccinations and other critical preventive efforts, including increased SARS-CoV-2 screening, public health campaigns, or COVID-19 discussions during clinical care,” they added.
The study was published online in JAMA Network Open.
Elderly most vulnerable
The researchers reviewed the records of 263,697 veterans who were fully vaccinated against COVID-19.
Just over a half (51.4%) had one or more psychiatric diagnoses within the last 5 years and 14.8% developed breakthrough COVID-19 infections, confirmed by a positive SARS-CoV-2 test.
Psychiatric diagnoses among the veterans included depression, posttraumatic stress, anxiety, adjustment disorder, substance use disorder, bipolar disorder, psychosis, ADHD, dissociation, and eating disorders.
In the overall sample, a history of any psychiatric disorder was associated with a 7% higher incidence of breakthrough COVID-19 infection in models adjusted for potential confounders (adjusted relative risk, 1.07; 95% confidence interval, 1.05-1.09) and a 3% higher incidence in models additionally adjusted for underlying medical comorbidities and smoking (aRR, 1.03; 95% CI, 1.01-1.05).
Most psychiatric disorders were associated with a higher incidence of breakthrough infection, with the highest relative risk observed for substance use disorders (aRR, 1.16; 95% CI, 1.12 -1.21) and adjustment disorder (aRR, 1.13; 95% CI, 1.10-1.16) in fully adjusted models.
Older vaccinated veterans with psychiatric illnesses appear to be most vulnerable to COVID-19 reinfection.
In veterans aged 65 and older, all psychiatric disorders were associated with an increased incidence of breakthrough infection, with increases in the incidence rate ranging from 3% to 24% in fully adjusted models.
In the younger veterans, in contrast, only anxiety, adjustment, and substance use disorders were associated with an increased incidence of breakthrough infection in fully adjusted models.
Psychotic disorders were associated with a 10% lower incidence of breakthrough infection among younger veterans, perhaps because of greater social isolation, the researchers said.
Risky behavior or impaired immunity?
“Although some of the larger observed effect sizes are compelling at an individual level, even the relatively modest effect sizes may have a large effect at the population level when considering the high prevalence of psychiatric disorders and the global reach and scale of the pandemic,” Dr. O’Donovan and colleagues wrote.
They noted that psychiatric disorders, including depression, schizophrenia, and bipolar disorders, have been associated with impaired cellular immunity and blunted response to vaccines. Therefore, it’s possible that those with psychiatric disorders have poorer responses to COVID-19 vaccination.
It’s also possible that immunity following vaccination wanes more quickly or more strongly in people with psychiatric disorders and they could have less protection against new variants, they added.
Patients with psychiatric disorders could be more apt to engage in risky behaviors for contracting COVID-19, which could also increase the risk for breakthrough infection, they said.
The study was supported by a UCSF Department of Psychiatry Rapid Award and UCSF Faculty Resource Fund Award. Dr. O’Donovan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Ukraine and PTSD: How psychiatry can help
The war in Ukraine is resulting in a devastating loss of life, catastrophic injuries, and physical destruction. But the war also will take an enormous mental health toll on millions of people, resulting in what I think will lead to an epidemic of posttraumatic stress disorder.
Think about the horrors that Ukrainians are experiencing. Millions of Ukrainians have been displaced to locations inside and outside of the country. People are being forced to leave behind family members, neighbors, and their pets and homes. In one recent news report, a Ukrainian woman who left Kyiv for Belgium reported having dreams in which she heard explosions. Smells, sounds, and even colors can trigger intrusive memories and a host of other problems. The mind can barely comprehend the scope of this human crisis.
Ukrainian soldiers are witnessing horrors that are unspeakable. Doctors, emergency service workers, and other medical professionals in Ukraine are being exposed to the catastrophe on a large scale. Children and youth are among the most affected victims, and it is difficult to predict the impact all of this upheaval is having on them.
The most important question for those of us who treat mental illness is “how will we help devastated people suffering from extreme trauma tied to death, dying, severe injuries, and torture by the invading soldiers?”
I have been treating patients with PTSD for many years. In my lifetime, the devastation in Ukraine will translate into what I expect will be the first overwhelming mass epidemic of PTSD – at least that I can recall. Yes, surely PTSD occurred during and after the Holocaust in the World War II era, but at that time, the mental health profession was not equipped to recognize it – even though the disorder most certainly existed. Even in ancient times, an Assyrian text from Mesopotamia (currently Iraq) described what we would define as PTSD symptoms in soldiers, such as sleep disturbances, flashbacks, and “low mood,” according to a 2014 article in the journal Early Science and Medicine.
The DSM-5 describes numerous criteria for PTSD mainly centering on trauma exposing a person to actual or threatened death, serious injury, or a variety of assaults, including direct exposure or witnessing the event. However, in my clinical experience, I’ve seen lesser events leading to PTSD. Much depends on how each individual processes what is occurring or has occurred.
What appears to be clear is that some key aspects of PTSD according to the DSM-5 – such as trauma-related thoughts or feelings, or trauma-related reminders, as well as nightmares and flashbacks – are likely occurring among Ukrainians. In addition, hypervigilance and exaggerated startle response seem to be key components of PTSD whether or not the cause is a major event or what one would perceive as less traumatic or dramatic.
I’ve certainly seen PTSD secondary to a hospitalization, especially in care involving ICUs or cardiac care units. In addition, I’ve had the occasion to note PTSD signs and symptoms after financial loss or divorce, situations in which some clinicians would never believe PTSD would occur, and would often diagnose as anxiety or depression. For me, again from a clinical point of view, it’s always been critical to assess how individuals process the event or events around them.
We know that there is already a shortage of mental health clinicians across the globe. This means that, in light of the hundreds of thousands – possibly millions – of Ukrainians affected by PTSD, a one-to-one approach will not do. For those Ukrainians who are able to find safe havens, I believe that PTSD symptoms can be debilitating, and the mental health community needs to begin putting supports in place now to address this trauma.
Specifically, proven cognitive-behavioral therapy (CBT) and guided imagery should be used to begin helping some of these people recover from the unbelievable trauma of war. For some, medication management might be helpful in those experiencing nightmares combined with anxiety and depression. But the main approach and first line of care should be CBT and guided imagery.
PTSD symptoms can make people feel like they are losing control, and prevent them from rebuilding their lives. We must do all we can in the mental health community to destigmatize care and develop support services to get ahead of this crisis. Only through medical, psychiatric, and health care organizations banding together using modern technology can the large number of people psychologically affected by this ongoing crisis be helped and saved.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The war in Ukraine is resulting in a devastating loss of life, catastrophic injuries, and physical destruction. But the war also will take an enormous mental health toll on millions of people, resulting in what I think will lead to an epidemic of posttraumatic stress disorder.
Think about the horrors that Ukrainians are experiencing. Millions of Ukrainians have been displaced to locations inside and outside of the country. People are being forced to leave behind family members, neighbors, and their pets and homes. In one recent news report, a Ukrainian woman who left Kyiv for Belgium reported having dreams in which she heard explosions. Smells, sounds, and even colors can trigger intrusive memories and a host of other problems. The mind can barely comprehend the scope of this human crisis.
Ukrainian soldiers are witnessing horrors that are unspeakable. Doctors, emergency service workers, and other medical professionals in Ukraine are being exposed to the catastrophe on a large scale. Children and youth are among the most affected victims, and it is difficult to predict the impact all of this upheaval is having on them.
The most important question for those of us who treat mental illness is “how will we help devastated people suffering from extreme trauma tied to death, dying, severe injuries, and torture by the invading soldiers?”
I have been treating patients with PTSD for many years. In my lifetime, the devastation in Ukraine will translate into what I expect will be the first overwhelming mass epidemic of PTSD – at least that I can recall. Yes, surely PTSD occurred during and after the Holocaust in the World War II era, but at that time, the mental health profession was not equipped to recognize it – even though the disorder most certainly existed. Even in ancient times, an Assyrian text from Mesopotamia (currently Iraq) described what we would define as PTSD symptoms in soldiers, such as sleep disturbances, flashbacks, and “low mood,” according to a 2014 article in the journal Early Science and Medicine.
The DSM-5 describes numerous criteria for PTSD mainly centering on trauma exposing a person to actual or threatened death, serious injury, or a variety of assaults, including direct exposure or witnessing the event. However, in my clinical experience, I’ve seen lesser events leading to PTSD. Much depends on how each individual processes what is occurring or has occurred.
What appears to be clear is that some key aspects of PTSD according to the DSM-5 – such as trauma-related thoughts or feelings, or trauma-related reminders, as well as nightmares and flashbacks – are likely occurring among Ukrainians. In addition, hypervigilance and exaggerated startle response seem to be key components of PTSD whether or not the cause is a major event or what one would perceive as less traumatic or dramatic.
I’ve certainly seen PTSD secondary to a hospitalization, especially in care involving ICUs or cardiac care units. In addition, I’ve had the occasion to note PTSD signs and symptoms after financial loss or divorce, situations in which some clinicians would never believe PTSD would occur, and would often diagnose as anxiety or depression. For me, again from a clinical point of view, it’s always been critical to assess how individuals process the event or events around them.
We know that there is already a shortage of mental health clinicians across the globe. This means that, in light of the hundreds of thousands – possibly millions – of Ukrainians affected by PTSD, a one-to-one approach will not do. For those Ukrainians who are able to find safe havens, I believe that PTSD symptoms can be debilitating, and the mental health community needs to begin putting supports in place now to address this trauma.
Specifically, proven cognitive-behavioral therapy (CBT) and guided imagery should be used to begin helping some of these people recover from the unbelievable trauma of war. For some, medication management might be helpful in those experiencing nightmares combined with anxiety and depression. But the main approach and first line of care should be CBT and guided imagery.
PTSD symptoms can make people feel like they are losing control, and prevent them from rebuilding their lives. We must do all we can in the mental health community to destigmatize care and develop support services to get ahead of this crisis. Only through medical, psychiatric, and health care organizations banding together using modern technology can the large number of people psychologically affected by this ongoing crisis be helped and saved.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The war in Ukraine is resulting in a devastating loss of life, catastrophic injuries, and physical destruction. But the war also will take an enormous mental health toll on millions of people, resulting in what I think will lead to an epidemic of posttraumatic stress disorder.
Think about the horrors that Ukrainians are experiencing. Millions of Ukrainians have been displaced to locations inside and outside of the country. People are being forced to leave behind family members, neighbors, and their pets and homes. In one recent news report, a Ukrainian woman who left Kyiv for Belgium reported having dreams in which she heard explosions. Smells, sounds, and even colors can trigger intrusive memories and a host of other problems. The mind can barely comprehend the scope of this human crisis.
Ukrainian soldiers are witnessing horrors that are unspeakable. Doctors, emergency service workers, and other medical professionals in Ukraine are being exposed to the catastrophe on a large scale. Children and youth are among the most affected victims, and it is difficult to predict the impact all of this upheaval is having on them.
The most important question for those of us who treat mental illness is “how will we help devastated people suffering from extreme trauma tied to death, dying, severe injuries, and torture by the invading soldiers?”
I have been treating patients with PTSD for many years. In my lifetime, the devastation in Ukraine will translate into what I expect will be the first overwhelming mass epidemic of PTSD – at least that I can recall. Yes, surely PTSD occurred during and after the Holocaust in the World War II era, but at that time, the mental health profession was not equipped to recognize it – even though the disorder most certainly existed. Even in ancient times, an Assyrian text from Mesopotamia (currently Iraq) described what we would define as PTSD symptoms in soldiers, such as sleep disturbances, flashbacks, and “low mood,” according to a 2014 article in the journal Early Science and Medicine.
The DSM-5 describes numerous criteria for PTSD mainly centering on trauma exposing a person to actual or threatened death, serious injury, or a variety of assaults, including direct exposure or witnessing the event. However, in my clinical experience, I’ve seen lesser events leading to PTSD. Much depends on how each individual processes what is occurring or has occurred.
What appears to be clear is that some key aspects of PTSD according to the DSM-5 – such as trauma-related thoughts or feelings, or trauma-related reminders, as well as nightmares and flashbacks – are likely occurring among Ukrainians. In addition, hypervigilance and exaggerated startle response seem to be key components of PTSD whether or not the cause is a major event or what one would perceive as less traumatic or dramatic.
I’ve certainly seen PTSD secondary to a hospitalization, especially in care involving ICUs or cardiac care units. In addition, I’ve had the occasion to note PTSD signs and symptoms after financial loss or divorce, situations in which some clinicians would never believe PTSD would occur, and would often diagnose as anxiety or depression. For me, again from a clinical point of view, it’s always been critical to assess how individuals process the event or events around them.
We know that there is already a shortage of mental health clinicians across the globe. This means that, in light of the hundreds of thousands – possibly millions – of Ukrainians affected by PTSD, a one-to-one approach will not do. For those Ukrainians who are able to find safe havens, I believe that PTSD symptoms can be debilitating, and the mental health community needs to begin putting supports in place now to address this trauma.
Specifically, proven cognitive-behavioral therapy (CBT) and guided imagery should be used to begin helping some of these people recover from the unbelievable trauma of war. For some, medication management might be helpful in those experiencing nightmares combined with anxiety and depression. But the main approach and first line of care should be CBT and guided imagery.
PTSD symptoms can make people feel like they are losing control, and prevent them from rebuilding their lives. We must do all we can in the mental health community to destigmatize care and develop support services to get ahead of this crisis. Only through medical, psychiatric, and health care organizations banding together using modern technology can the large number of people psychologically affected by this ongoing crisis be helped and saved.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Diagnosing PTSD: Heart rate variability may help
, according to a study published in Frontiers in Psychiatry.
It is estimated that between 8% and 15% of clinically recognized pregnancies and up to 30% of all pregnancies result in miscarriage – a loss that can be devastating for everyone. There are limited data on the strength of the association between perinatal loss and subsequent common mental health disorders, such as anxiety, depression, and PTSD. The prevalence of PTSD among this group is still unknown, and one of the factors that contribute to the absence of data is that diagnostic evaluation is subjective.
To address this issue, researchers from Anhembi Morumbi University (UAM) in São José dos Campos, Brazil, along with teams in the United States and United Arab Emirates (UAE), investigated biomarkers for the severity of PTSD. The hope is that the research will enable psychiatrists to assess women who experience pregnancy loss more objectively. Study author Ovidiu Constantin Baltatu, MD, PhD, a professor at Brazil’s UAM and the UAE’s Khalifa University, spoke to this news organization about the study.
Under the guidance of Dr. Baltatu, psychologist Cláudia de Faria Cardoso carried out the research as part of her studies in biomedical engineering at UAM. Fifty-three women were recruited; the average age of the cohort was 33 years. All participants had a history of at least one perinatal loss. Pregnancy loss intervals ranged from less than 40 days to more than 6 months.
Participants completed a clinical interview and a questionnaire; PTSD symptoms were assessed on the basis of criteria in the DSM-5. The instrument used for the assessment was the Brazilian version of the Post-traumatic Stress Disorder Checklist (PCL-5). In addition, to evaluate general autonomic dysfunction, patients completed the Composite Autonomic Symptom Score 31 (COMPASS-31) questionnaire.
HRV was assessed during a deep breathing test using an HRV scanner system with wireless electrocardiography that enabled real-time data analysis and visualization. The investigators examined the following HRV measures: standard deviation (SD) of normal R-R wave intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent normal R wave intervals, and the number of all R-R intervals in which the change in consecutive normal sinus intervals exceeds 50 ms divided by the total number of R-R intervals measured.
Of the 53 participants, 25 had been diagnosed with pregnancy loss–induced PTSD. The results indicated a significant association between PCL-5 scores and HRV indices. The SDNN index effectively distinguished between patients with PTSD and those without.
To Dr. Baltatu, HRV indices reflect dysfunction of the autonomic nervous system (ANS), one of the major neural pathways activated by stress.
Although the deep breathing test has been around for a long time, it’s not widely used in current clinical practice, he said. According to him, maximum and minimum heart rates during breathing at six cycles per minute can typically be used to calculate the inspiratory-to-expiratory ratio, thus providing an indication of ANS function. “Our group introduced the study of HRV during deep breathing test, which is a step forward,” he said.
The methodology used by the team was well received by the participants. “With the deep breathing test, the women were able to look at a screen and see real-time graphics displaying the stress that they were experiencing after having suffered trauma. This visualization of objective measures was perceived as an improved care,” said Dr. Baltatu.
In general, HRV provides a more objective means of diagnosing PTSD. “Normally, PTSD is assessed through a questionnaire and an interview with psychologists,” said Dr. Baltatu. The subjectivity of the assessment is one of the main factors associated with the underdiagnosis of this condition, he explained.
It is important to remember that other factors, such as a lack of awareness about the problem, also hinder the diagnosis of PTSD in this population, Dr. Baltatu added. Women who have had a miscarriage often don’t think that their symptoms may result from PTSD. This fact highlights why it is so important that hospitals have a clinical psychologist on staff. In addition, Dr. Baltatu pointed out that a woman who experiences a pregnancy loss usually has negative memories of the hospital and is therefore reluctant to reach out for professional help. “In our study, all psychological care and assessments took place outside of a hospital setting, which the participants seemed to appreciate,” he emphasized.
Dr. Baltatu and his team are conducting follow-up research. The preliminary results indicate that the biomarkers identified in the study are promising in the assessment of patients’ clinical progress. This finding may reflect the fact that the HRV indices have proven useful not only in diagnosing but also in monitoring women in treatment, because they are able to identify which patients are responding better to treatment.
A version of this article first appeared on Medscape.com.
, according to a study published in Frontiers in Psychiatry.
It is estimated that between 8% and 15% of clinically recognized pregnancies and up to 30% of all pregnancies result in miscarriage – a loss that can be devastating for everyone. There are limited data on the strength of the association between perinatal loss and subsequent common mental health disorders, such as anxiety, depression, and PTSD. The prevalence of PTSD among this group is still unknown, and one of the factors that contribute to the absence of data is that diagnostic evaluation is subjective.
To address this issue, researchers from Anhembi Morumbi University (UAM) in São José dos Campos, Brazil, along with teams in the United States and United Arab Emirates (UAE), investigated biomarkers for the severity of PTSD. The hope is that the research will enable psychiatrists to assess women who experience pregnancy loss more objectively. Study author Ovidiu Constantin Baltatu, MD, PhD, a professor at Brazil’s UAM and the UAE’s Khalifa University, spoke to this news organization about the study.
Under the guidance of Dr. Baltatu, psychologist Cláudia de Faria Cardoso carried out the research as part of her studies in biomedical engineering at UAM. Fifty-three women were recruited; the average age of the cohort was 33 years. All participants had a history of at least one perinatal loss. Pregnancy loss intervals ranged from less than 40 days to more than 6 months.
Participants completed a clinical interview and a questionnaire; PTSD symptoms were assessed on the basis of criteria in the DSM-5. The instrument used for the assessment was the Brazilian version of the Post-traumatic Stress Disorder Checklist (PCL-5). In addition, to evaluate general autonomic dysfunction, patients completed the Composite Autonomic Symptom Score 31 (COMPASS-31) questionnaire.
HRV was assessed during a deep breathing test using an HRV scanner system with wireless electrocardiography that enabled real-time data analysis and visualization. The investigators examined the following HRV measures: standard deviation (SD) of normal R-R wave intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent normal R wave intervals, and the number of all R-R intervals in which the change in consecutive normal sinus intervals exceeds 50 ms divided by the total number of R-R intervals measured.
Of the 53 participants, 25 had been diagnosed with pregnancy loss–induced PTSD. The results indicated a significant association between PCL-5 scores and HRV indices. The SDNN index effectively distinguished between patients with PTSD and those without.
To Dr. Baltatu, HRV indices reflect dysfunction of the autonomic nervous system (ANS), one of the major neural pathways activated by stress.
Although the deep breathing test has been around for a long time, it’s not widely used in current clinical practice, he said. According to him, maximum and minimum heart rates during breathing at six cycles per minute can typically be used to calculate the inspiratory-to-expiratory ratio, thus providing an indication of ANS function. “Our group introduced the study of HRV during deep breathing test, which is a step forward,” he said.
The methodology used by the team was well received by the participants. “With the deep breathing test, the women were able to look at a screen and see real-time graphics displaying the stress that they were experiencing after having suffered trauma. This visualization of objective measures was perceived as an improved care,” said Dr. Baltatu.
In general, HRV provides a more objective means of diagnosing PTSD. “Normally, PTSD is assessed through a questionnaire and an interview with psychologists,” said Dr. Baltatu. The subjectivity of the assessment is one of the main factors associated with the underdiagnosis of this condition, he explained.
It is important to remember that other factors, such as a lack of awareness about the problem, also hinder the diagnosis of PTSD in this population, Dr. Baltatu added. Women who have had a miscarriage often don’t think that their symptoms may result from PTSD. This fact highlights why it is so important that hospitals have a clinical psychologist on staff. In addition, Dr. Baltatu pointed out that a woman who experiences a pregnancy loss usually has negative memories of the hospital and is therefore reluctant to reach out for professional help. “In our study, all psychological care and assessments took place outside of a hospital setting, which the participants seemed to appreciate,” he emphasized.
Dr. Baltatu and his team are conducting follow-up research. The preliminary results indicate that the biomarkers identified in the study are promising in the assessment of patients’ clinical progress. This finding may reflect the fact that the HRV indices have proven useful not only in diagnosing but also in monitoring women in treatment, because they are able to identify which patients are responding better to treatment.
A version of this article first appeared on Medscape.com.
, according to a study published in Frontiers in Psychiatry.
It is estimated that between 8% and 15% of clinically recognized pregnancies and up to 30% of all pregnancies result in miscarriage – a loss that can be devastating for everyone. There are limited data on the strength of the association between perinatal loss and subsequent common mental health disorders, such as anxiety, depression, and PTSD. The prevalence of PTSD among this group is still unknown, and one of the factors that contribute to the absence of data is that diagnostic evaluation is subjective.
To address this issue, researchers from Anhembi Morumbi University (UAM) in São José dos Campos, Brazil, along with teams in the United States and United Arab Emirates (UAE), investigated biomarkers for the severity of PTSD. The hope is that the research will enable psychiatrists to assess women who experience pregnancy loss more objectively. Study author Ovidiu Constantin Baltatu, MD, PhD, a professor at Brazil’s UAM and the UAE’s Khalifa University, spoke to this news organization about the study.
Under the guidance of Dr. Baltatu, psychologist Cláudia de Faria Cardoso carried out the research as part of her studies in biomedical engineering at UAM. Fifty-three women were recruited; the average age of the cohort was 33 years. All participants had a history of at least one perinatal loss. Pregnancy loss intervals ranged from less than 40 days to more than 6 months.
Participants completed a clinical interview and a questionnaire; PTSD symptoms were assessed on the basis of criteria in the DSM-5. The instrument used for the assessment was the Brazilian version of the Post-traumatic Stress Disorder Checklist (PCL-5). In addition, to evaluate general autonomic dysfunction, patients completed the Composite Autonomic Symptom Score 31 (COMPASS-31) questionnaire.
HRV was assessed during a deep breathing test using an HRV scanner system with wireless electrocardiography that enabled real-time data analysis and visualization. The investigators examined the following HRV measures: standard deviation (SD) of normal R-R wave intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent normal R wave intervals, and the number of all R-R intervals in which the change in consecutive normal sinus intervals exceeds 50 ms divided by the total number of R-R intervals measured.
Of the 53 participants, 25 had been diagnosed with pregnancy loss–induced PTSD. The results indicated a significant association between PCL-5 scores and HRV indices. The SDNN index effectively distinguished between patients with PTSD and those without.
To Dr. Baltatu, HRV indices reflect dysfunction of the autonomic nervous system (ANS), one of the major neural pathways activated by stress.
Although the deep breathing test has been around for a long time, it’s not widely used in current clinical practice, he said. According to him, maximum and minimum heart rates during breathing at six cycles per minute can typically be used to calculate the inspiratory-to-expiratory ratio, thus providing an indication of ANS function. “Our group introduced the study of HRV during deep breathing test, which is a step forward,” he said.
The methodology used by the team was well received by the participants. “With the deep breathing test, the women were able to look at a screen and see real-time graphics displaying the stress that they were experiencing after having suffered trauma. This visualization of objective measures was perceived as an improved care,” said Dr. Baltatu.
In general, HRV provides a more objective means of diagnosing PTSD. “Normally, PTSD is assessed through a questionnaire and an interview with psychologists,” said Dr. Baltatu. The subjectivity of the assessment is one of the main factors associated with the underdiagnosis of this condition, he explained.
It is important to remember that other factors, such as a lack of awareness about the problem, also hinder the diagnosis of PTSD in this population, Dr. Baltatu added. Women who have had a miscarriage often don’t think that their symptoms may result from PTSD. This fact highlights why it is so important that hospitals have a clinical psychologist on staff. In addition, Dr. Baltatu pointed out that a woman who experiences a pregnancy loss usually has negative memories of the hospital and is therefore reluctant to reach out for professional help. “In our study, all psychological care and assessments took place outside of a hospital setting, which the participants seemed to appreciate,” he emphasized.
Dr. Baltatu and his team are conducting follow-up research. The preliminary results indicate that the biomarkers identified in the study are promising in the assessment of patients’ clinical progress. This finding may reflect the fact that the HRV indices have proven useful not only in diagnosing but also in monitoring women in treatment, because they are able to identify which patients are responding better to treatment.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PSYCHIATRY
Exercise to Reduce Posttraumatic Stress Disorder Symptoms in Veterans
Physical exercise offers preventative and therapeutic benefits for a range of chronic health conditions, including cardiovascular disease, type 2 diabetes mellitus, Alzheimer disease, and depression.1,2 Exercise has been well studied for its antidepressant effects, its ability to reduce risk of aging-related dementia, and favorable effects on a range of cognitive functions.2 Lesser evidence exists regarding the impact of exercise on other mental health concerns. Therefore, an accurate understanding of whether physical exercise may ameliorate other conditions is important.
A small meta-analysis by Rosenbaum and colleagues found that exercise interventions were superior to control conditions for symptom reduction in study participants with posttraumatic stress disorder (PTSD).3 This meta-analysis included 4 randomized clinical trials representing 200 cases. The trial included a variety of physical activities (eg, yoga, aerobic, and strength-building exercises) and control conditions, and participants recruited from online, community, inpatient, and outpatient settings. The standardized mean difference (SMD) produced by the analysis indicated a small-to-medium effect (Hedges g, -0.35), with the authors reporting no evidence of publication bias, although an assessment of potential bias associated with individual trial design characteristics was not conducted. Of note, a meta-analysis by Watts and colleagues found that effect sizes for PTSD treatments tend to be smaller in veteran populations.4 Therefore, how much the mean effect size estimate in the study is applicable to veterans with PTSD is unknown.3
Veterans represent a unique subpopulation in which PTSD is common, although no meta-analysis yet published has synthesized the effects of exercise interventions from trials of veterans with PTSD.5 A recent systematic review by Whitworth and Ciccolo concluded that exercise may be associated with reduced risk of PTSD, a briefer course of PTSD symptoms, and/or reduced sleep- and depression-related difficulties.6 However, that review primarily included observational, cross-sectional, and qualitative works. No trials included in our meta-analysis were included in that review.6
Evidence-based psychotherapies like cognitive processing therapy and prolonged exposure have been shown to be effective for treating PTSD in veterans; however, these modalities are accompanied by high rates of dropout (eg, 40-60%), thereby limiting their clinical utility.7 The use of complementary and alternative approaches for treatment in the United States has increased in recent years, and exercise represents an important complementary treatment option.8 In a study by Baldwin and colleagues, nearly 50% of veterans reported using complementary or alternative approaches, and veterans with PTSD were among those likely to use such approaches.9 However, current studies of the effects of exercise interventions on PTSD symptom reduction are mostly small and varied, making determinations difficult regarding the potential utility of exercise for treating this condition in veterans.
Literature Search
No previous research has synthesized the literature on the effects of exercise on PTSD in the veteran population. The current meta-analysis aims to provide a synthesis of systematically selected studies on this topic to determine whether exercise-based interventions are effective at reducing veterans’ symptoms of PTSD. Our hypothesis was that, when used as a primary or adjuvant intervention for PTSD, physical exercise would be associated with a reduction of PTSD symptom scale scores. We planned a priori to produce separate estimates for single-arm and multi-arm trials. We also wanted to conduct a careful risk of bias assessment—or evaluation of study features that may have systematically influenced results—for included trials, not only to provide context for interpretation of results, but also to inform suggestions for research to advance this field of inquiry.10
Methods
This study was preregistered on PROSPERO and followed PRISMA guidelines for meta-analyses and systematic reviews.11 Supplementary materials, such as the PRISMA checklist, study data, and funnel plots, are available online (doi.org/10.6084/m9.figshare.c.5618437.v1). Conference abstracts were omitted due to a lack of necessary information. We decided early in the planning process to include both randomized and single-arm trials, expecting the number of completed studies in the area of exercise for PTSD symptom reduction in veterans, and particularly randomized trials of such, would be relatively small.
Studies were included if they met the following criteria: (1) the study was a single- or multi-arm interventional trial; (2) participants were veterans; (3) participants had a current diagnosis of PTSD or exhibited subthreshold PTSD symptoms, as established by authors of the individual studies and supported by a structured clinical interview, semistructured interview, or elevated scores on PTSD symptom self-report measures; (4) the study included an intervention in which exercise (physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of physical fitness or health is an objective) was the primary component; (5) PTSD symptom severity was by a clinician-rated or self-report measure; and (6) the study was published in a peer-reviewed journal.12 Studies were excluded if means, standard deviations, and sample sizes were not available or the full text of the study was not available in English.
The systematic review was conducted using PubMed, PsycINFO, and Cochrane Library databases, from the earliest record to February 2021. The following search phrase was used, without additional limits, to acquire a list of potential studies: (“PTSD” or “post-traumatic stress disorder” or “posttraumatic stress disorder” or “post traumatic stress disorder”) and (“veteran” or “veterans”) and (“exercise” or “aerobic” or “activity” or “physical activity”). The references of identified publications also were searched for additional studies. Then, study titles and abstracts were evaluated and finally, full texts were evaluated to determine study inclusion. All screening, study selection, and risk of bias and data extraction activities were performed by 2 independent reviewers (DR and MJ) with disagreements resolved through discussion and consensus (Figure 1). A list of studies excluded during full-text review and rationales can be viewed online (doi.org/10.6084/m9.figshare.c.5618437.v1).
Data Collection
Data were extracted from included studies using custom forms and included the following information based on PRISMA guidelines: (1) study design characteristics; (2) intervention details; and (3) PTSD outcome information.11 PTSD symptom severity was the primary outcome of interest. Outcome data were included if they were derived from a measure of PTSD symptoms—equivalency across measures was assumed for meta-analyses. Potential study bias for each outcome was evaluated using the ROBINS-I and Cochrane Collaboration’s RoB 2 tools for single-arm and multi-arm trials, respectively.13,14 These tools evaluate domains related to the design, conduct, and analysis of studies that are associated with bias (ie, systematic error in findings, such as under- or overestimation of results).10 Examples include how well authors performed and concealed randomization procedures, addressed missing data, and measured study outcomes.13,14 The risk of bias (eg, low, moderate, serious) associated with each domain is rated and, based on the domain ratings, each study is then given an overall rating regarding how much risk influences bias.13,14 Broadly, lower risk of bias corresponds to higher confidence in the validity of results.
Finally, 4 authors (associated with 2 single- and 2 multi-arm studies) were contacted and asked to provide further information. Data for 1 additional multi-arm study were obtained from these communications and included in the final study selection.15 These authors were also asked for information about any unpublished works of which they were aware, although no additional works were identified.
Statistical Analyses
Analyses were performed with R Studio R 3.6.0 software.16 An SMD (also known as Hedges g) was calculated for each study outcome: for single-arm trials, this was the SMD between pre- and postintervention scores, whereas for multi-arm trials, this was the SMD between postintervention outcome scores across groups. CIs for each SMD were calculated using a standard normal distribution. Combined SMDs were estimated separately for single- and multi-arm studies, using random-effects meta-analyses. In order to include multiple relevant outcomes from a single trial (ie, for studies using multiple PTSD symptom measures), robust variance estimation was used.17 Precision was used to weight SMDs.
Correlations between pre- and postintervention scores were not available for 1 single-arm study.18 A correlation coefficient of 0.8 was imputed to calculate the standard error of the of the SMDs for the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist (PCL), as this value is consistent with past findings regarding the test-retest reliability of these measures.19-22 A sensitivity analysis, using several alternative correlational values, revealed that the choice of correlation coefficient did not impact the overall results of the meta-analysis.
I2 was used to evaluate between-study heterogeneity. Values of I2 > 25%, 50%, and 75% were selected to reflect low, moderate, and high heterogeneity, respectively, in accordance with guidelines described by Higgins and colleagues.23 Potential publication bias was assessed via funnel plot and Egger test.24 Finally, although collection of depressive symptom scores was proposed as a secondary outcome in the study protocol, such data were available only for 1 multi-arm study. As a result, this outcome was not evaluated.
Results
Six studies with 101 total participants were included in the single-arm analyses (Table 1).18,25-29 Participants consisted of veterans with chronic pain, post-9/11 veterans, female veterans of childbearing age, veterans with a history of trauma therapy, and other veterans. Types of exercise included moderate aerobic exercise and yoga. PTSD symptom measures included the CAPS and the PCL (PCL-5 or PCL-M versions). Reported financial sources for included studies included federal grant funding, nonprofit material support, outside organization support, use of US Department of Veterans Affairs (VA) resources, and no reported financial support.
With respect to individual studies, Shivakumar and colleagues found that completion of an aerobic exercise program was associated with reduced scores on 2 different PTSD symptom scales (PCL and CAPS) in 16 women veterans.18 A trauma-informed yoga intervention study with 18 participants by Cushing and colleagues demonstrated veteran participation to be associated with large reductions in PTSD, anxiety, and depression scale scores.25 In a study with 34 veterans, Chopin and colleagues found that a trauma-informed yoga intervention was associated with a statistically significant reduction in PTSD symptoms, as did a study by Zaccari and colleagues with 17 veterans.26,29 Justice and Brems also found some evidence that trauma-informed yoga interventions helped PTSD symptoms in a small sample of 4 veterans, although these results were not quantitatively analyzed.27 In contrast, a small pilot study (n = 12) by Staples and colleagues testing a biweekly, 6-week yoga program did not show a significant effect on PTSD symptoms.28
Three studies with 217 total veteran participants were included in the multi-arm analyses (Table 2).15,30,31 As all multi-arm trials incorporated randomization, they will be referred to as randomized controlled trials (RCTs). On contact, Davis and colleagues provided veteran-specific results for their trial; as such, our data differ from those within the published article.15 Participants from all included studies were veterans currently experiencing symptoms of PTSD. Types of exercise included yoga and combined methods (eg, aerobic and strength training).15,30,31 PTSD symptom measures included the CAPS or the PCL-5.15,30,31 Reported financial sources for included studies included federal grant funding, as well as nonprofit support, private donations, and VA and Department of Defense resources.
Davis and colleagues conducted a recently concluded RCT with > 130 veteran participants and found that a novel manualized yoga program was superior to an attention control in reducing PTSD symptom scale scores for veterans.15 Goldstein and colleagues found that a program consisting of both aerobic and resistance exercises reduced PTSD symptoms to a greater extent than a waitlist control condition, with 47 veterans randomized in this trial.30 Likewise, Hall and colleagues conducted a pilot RCT in which an intervention that integrated exercise and cognitive behavioral techniques was compared to a waitlist control condition.31 For the 48 veterans included in the analyses, the authors reported greater PTSD symptom reduction associated with integrated exercise than that of the control condition; however, the study was not powered to detect statistically significant differences between groups.
Bias Assessment
Results for the risk of bias assessments can be viewed in Tables 3 and 4. For single-arm studies, overall risk of bias was serious for all included trials. Serious risk of bias was found in 2 domains: confounding, due to a lack of accounting for potential preexisting baseline trends (eg, regression to the mean) that could have impacted study results; and measurement, due to the use of a self-report symptom measure (PCL) or CAPS with unblinded assessors. Multiple studies also showed moderate risk in the missing data domain due to participant dropout without appropriate analytic methods to address potential bias.
For RCTs, overall risk of bias ranged from some concerns to high risk. High risk of bias was found in 1 domain, measurement of outcome, due to use of a self-report symptom measure (PCL) with unblinded groups.31 The other 2 studies all had some concern of bias in at least 1 of the following domains: randomization, missing data, and measurement of outcome.
Pooled Standardized Mean Differences
Meta-analytic results can be viewed in Figure 2. The pooled SMD for the 6 single-arm studies was -0.60 (df = 4.41, 95% CI, -1.08 to -0.12, P = .03), indicating a statistically significant reduction in PTSD symptoms over the course of an exercise intervention. Combining SMDs for the 3 included RCTs revealed a pooled SMD of -0.40 (df = 1.57, 95% CI, -0.86 to 0.06, P = .06), indicating that exercise did not result in a statistically significant reduction in PTSD symptoms compared with control conditions.
Publication Bias and Heterogeneity
Visual inspection funnel plots and Egger test did not suggest the presence of publication bias for RCTs (t = 1.21, df = 2, P = .35) or single-arm studies (t = -0.36, df = 5, P = .73).
Single-arm studies displayed a high degree of heterogeneity (I2 = 81.5%). Including sample size or exercise duration as variables in meta-regressions did not reduce heterogeneity (I2 = 85.2% and I2 = 83.8%, respectively). Performing a subgroup analysis only on studies using yoga as an intervention also did not reduce heterogeneity (I2 = 79.2%). Due to the small number of studies, no further exploration of heterogeneity was conducted on single-arm studies. RCTs did not display any heterogeneity (I2 = 0%).
Discussion
Our report represents an early synthesis of the first prospective studies of physical exercise interventions for PTSD in veterans. Results from meta-analyses of 6 single-arm studies (101 participants) and 3 RCTs (217 participants) provide early evidence that exercise may reduce PTSD symptoms in veterans. Yoga was the most common form of exercise used in single-arm studies, whereas RCTs used a wider range of interventions. The pooled SMD of -0.60 for single-arm longitudinal studies suggest a medium decrease in PTSD symptoms for veterans who engage in exercise interventions. Analysis of the RCTs supported this finding, with a pooled SMD of -0.40 reflecting a small-to-medium effect of exercise on PTSD symptoms over control conditions, although this result did not achieve statistical significance. Of note, while the nonsignificant finding for RCTs may have been due to insufficient power caused by the limited number of included studies, possibly exercise was not more efficacious than were the control conditions.
Although RCTs represented a variety of exercise types, PTSD symptom measures, and veteran subgroups, statistical results were not indicative of heterogeneity. However, only the largest and most comprehensive study of exercise for PTSD in veterans to date by Davis and colleagues had a statistically significant SMD.15 Of note, one of the other 2 RCTs displayed an SMD of a similar magnitude, but this study had a much smaller sample size and was underpowered to detect significance.30 Additionally, risk of bias assessments for single-arm studies and RCTs revealed study characteristics that suggest possible inflation of absolute effect sizes for individual studies. Therefore, the pooled SMDs we report are interpretable but may exceed the true effect of exercise for PTSD symptom reduction in veterans.
Based on results of our analyses, it is reasonable, albeit preliminary, to conclude that exercise interventions may result in reduced PTSD symptoms among veterans. At the very least, these findings support the continued investigation of such interventions for veterans. Given the unique and salubrious characteristics of physical exercise, such results, if supported by further research, suggest that exercise-based interventions may be particularly valuable within the trauma treatment realm. For example, exercise can be less expensive and more convenient than attending traditional treatment, and for veterans reluctant to engage in standard treatment approaches such as psychiatric and psychosocial modalities, complementary approaches entailing exercise may be viewed as particularly acceptable or enjoyable.32 In addition to possibly reducing PTSD symptoms, exercise is a well-established treatment for conditions commonly comorbid with PTSD, including depression, anxiety disorders, cognitive difficulties, and certain chronic pain conditions.6 As such, exercise represents a holistic treatment option that has the potential to augment standard PTSD care.
Limitations
The present study has several important limitations. First, few studies were found that met the broad eligibility criteria and those that did often had a small sample size. Besides highlighting a gap in the extant research, the limited studies available for meta-analysis means that caution must be taken when interpreting results. Fortunately, this issue will likely resolve once additional studies investigating the impact of exercise on PTSD symptoms in veterans are available for synthesis.
Relatedly, the included study interventions varied considerably, both in the types of exercise used and the characteristics of the exercises (eg, frequency, duration, and intensity), which is relevant as different exercise modalities are associated with differential physical effects.33 Including such a mixture of exercises may have given an incomplete picture of their potential therapeutic effects. Also, none of the RCTs compared exercise against first-line treatments for PTSD, such as prolonged exposure or cognitive processing therapy, which would have provided further insight into the role exercise could play in clinical settings.7
Another limitation is the elevated risk of bias found in most studies, particularly present in the longitudinal single-arm studies, all of which were rated at serious risk. For instance, no single-arm study controlled for preexisting baseline trends: without such (and lacking a comparison control group like in RCTs), it is possible that the observed effects were due to extraneous factors, rather than the exercise intervention. Although not as severe, the multi-arm RCTs also displayed at least moderate risk of bias. Therefore, SMDs may have been overestimated for each group of studies.
Finally, the results of the single-arm meta-analysis displayed high statistical heterogeneity, reducing the generalizability of the results. One possible cause of this heterogeneity may have been the yoga interventions, as a separate analysis removing the only nonyoga study did not reduce heterogeneity. This result was surprising, as the included yoga interventions seemed similar across studies. While the presence of high heterogeneity does require some caution when applying these results to outside interventions, the present study made use of random-effects meta-analysis, a technique that incorporates study heterogeneity into the statistical model, thereby strengthening the findings compared with that of a traditional fixed-effects approach.10
Future Steps
Several future steps are warranted to improve knowledge of exercise as a treatment for PTSD in veterans and in the general population. With current meta-analyses limited to small numbers of studies, additional studies of the efficacy of exercise for treating PTSD could help in several ways. A larger pool of studies would enable future meta-analyses to explore related questions, such as those regarding the impact of exercise on quality of life or depressive symptom reduction among veterans with PTSD. A greater number of studies also would enable meta-analysts to explore potentially critical moderators. For example, the duration, frequency, or type of exercise may moderate the effect of exercise on PTSD symptom reduction. Moderators related to patient or study design characteristics also should be explored in future studies.
Future work also should evaluate the impact that specific features of exercise regimens have on PTSD. Knowing whether the type or structure of exercise affects its clinical use would be invaluable in developing and implementing efficient exercise-based interventions. For example, if facilitated exercise was found to be significantly more effective at reducing PTSD symptoms than exercise completed independently, the development of exercise intervention programs in the VA and other facilities that commonly treat PTSD may be warranted. Additionally, it may be useful to identify specific mechanisms through which exercise reduces PTSD symptoms. For example, in addition to its beneficial biological effects, exercise also promotes psychological health through behavioral activation and alterations within reinforcement/reward systems, suggesting that exercise regularity may be more important than intensity.34,35 Understanding which mechanisms contribute most to change will aid in the development of more efficient interventions.
Given that veterans are demonstrating considerable interest in complementary and alternative PTSD treatments, it is critical that researchers focus on high-quality randomized tests of these interventions. Therefore, in addition to greater quality of exercise intervention studies, future efforts should be focused on RCTs that are designed in such a way as to limit potential introduction of bias. For example, assessment data should be completed by blinded assessors using standardized measures, and analyses should account for missing data and unequal participant attrition between groups. Ideally, pre-intervention trends across multiple baseline datapoints also would be collected in single-arm studies to avoid confounding related to regression to the mean. It is also recommended that future meta-analyses use risk of bias assessments and consider how the results of such assessments may impact the interpretation of results.
Conclusions
Findings from both single-arm studies and RCTs suggest possible benefit of exercise on PTSD symptom reduction, although confirmation of findings is needed. No study found increased symptoms following exercise intervention. Thus, it is reasonable to consider physical exercise, such as yoga, as an adjunct, whole-health consistent treatment. HCPs working with veterans with past traumatic experiences should consider incorporating exercise into patient care. Enhanced educational efforts emphasizing the psychotherapeutic impact of exercise may also have value for the veteran population. Furthermore, the current risk of bias assessments highlights the need for additional high-quality RCTs evaluating the specific impact of exercise on PTSD symptom reduction in veterans. In particular, this field of inquiry would benefit from larger samples and design characteristics to reduce bias (eg, blinding when possible, use of CAPS vs only self-report symptom measures, reducing problematic attrition, corrections for missing data, etc).
Acknowledgments
This research is the result of work supported with resources and the use of facilities at the VA Eastern Kansas Healthcare System (Dwight D. Eisenhower VA Medical Center). It was also supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, as well as the Rocky Mountain Mental Illness Research, Education, and Clinical Center. Since Dr. Reis and Dr. Gaddy are employees of the US Government and contributed to this manuscript as part of their official duties, the work is not subject to US copyright. This study was preregistered on PROSPERO (https://www.crd.york.ac.uk/prospero/; ID: CRD42020153419).
1. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity—a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi:10.1186/1471-2458-13-813
2. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592. doi:10.1037/a0021769
3. Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of posttraumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230(2):130-136. doi:10.1016/j.psychres.2015.10.017
4. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-550. doi:10.4088/JCP.12r08225
5. Tanielian T, Jaycox L, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; 2008
6. Whitworth JW, Ciccolo JT. Exercise and post-traumatic stress disorder in military veterans: a systematic review. Mil Med. 2016;181(9):953-960. doi:10.7205/MILMED-D-15-00488
7. Rutt BT, Oehlert ME, Krieshok TS, Lichtenberg JW. Effectiveness of cognitive processing therapy and prolonged exposure in the Department of Veterans Affairs. Psychol Rep. 2018;121(2):282-302. doi:10.1177/0033294117727746
8. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
9. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: Implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704. doi:10.1001/archinte.162.15.1697
10. Higgins JPT, Thomas J, Chanlder J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane; 2021.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
12. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131.
13. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919
14. Sterne JAC, Savovic´ J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
15. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
16. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2019.
17. Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods .2015;20(3):375-393. doi:10.1037/met0000011
18. Shivakumar G, Anderson EH, Surís AM, North CS. Exercise for PTSD in women veterans: a proof-of-concept study. Mil Med. 2017;182(11):e1809-e1814. doi:10.7205/MILMED-D-16-00440
19. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
20. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669-673. doi:10.1016/0005-7967(96)00033-2
21. Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician- Administered PTSD Scale for DSM-5 (CAPS- 5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383-395.doi:10.1037/pas0000486
22. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596-606. doi:10.1002/da.20837
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi:10.1136/bmj.315.7109.629
25. Cushing RE, Braun KL, Alden CISW, Katz AR. Military- tailored yoga for veterans with post-traumatic stress disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. Justice L, Brems C. Bridging body and mind: case series of a 10-week trauma-informed yoga protocol for veterans. Int J Yoga Therap. 2019;29(1):65-79. doi:10.17761/D-17-2019-00029
28. Staples JK, Hamilton MF, Uddo M. A yoga program for the symptoms of post-traumatic stress disorder in veterans. Mil Med. 2013;178(8):854-860. doi:10.7205/MILMED-D-12-00536
29. Zaccari B, Callahan ML, Storzbach D, McFarlane N, Hudson R, Loftis JM. Yoga for veterans with PTSD: Cognitive functioning, mental health, and salivary cortisol. Psychol Trauma. 2020;12(8):913-917. doi:10.1037/tra0000909
30. Goldstein LA, Mehling WE, Metzler TJ, et al. Veterans Group Exercise: A randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345-352. doi:10.1016/j.jad.2017.11.002
31. Hall KS, Morey MC, Bosworth HB, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2020;43(4):648-659. doi:10.1007/s10865-019-00073-w
32. Gaddy MA. Implementation of an integrative medicine treatment program at a Veterans Health Administration residential mental health facility. Psychol Serv. 2018;15(4):503- 509. doi:10.1037/ser0000189
33. Werner CM, Hecksteden A, Morsch A, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34- 46. doi:10.1093/eurheartj/ehy585
34. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi:10.1098/rsfs.2014.0040
35. Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45-62. doi:10.1146/annurev-med-060619-022943.
Physical exercise offers preventative and therapeutic benefits for a range of chronic health conditions, including cardiovascular disease, type 2 diabetes mellitus, Alzheimer disease, and depression.1,2 Exercise has been well studied for its antidepressant effects, its ability to reduce risk of aging-related dementia, and favorable effects on a range of cognitive functions.2 Lesser evidence exists regarding the impact of exercise on other mental health concerns. Therefore, an accurate understanding of whether physical exercise may ameliorate other conditions is important.
A small meta-analysis by Rosenbaum and colleagues found that exercise interventions were superior to control conditions for symptom reduction in study participants with posttraumatic stress disorder (PTSD).3 This meta-analysis included 4 randomized clinical trials representing 200 cases. The trial included a variety of physical activities (eg, yoga, aerobic, and strength-building exercises) and control conditions, and participants recruited from online, community, inpatient, and outpatient settings. The standardized mean difference (SMD) produced by the analysis indicated a small-to-medium effect (Hedges g, -0.35), with the authors reporting no evidence of publication bias, although an assessment of potential bias associated with individual trial design characteristics was not conducted. Of note, a meta-analysis by Watts and colleagues found that effect sizes for PTSD treatments tend to be smaller in veteran populations.4 Therefore, how much the mean effect size estimate in the study is applicable to veterans with PTSD is unknown.3
Veterans represent a unique subpopulation in which PTSD is common, although no meta-analysis yet published has synthesized the effects of exercise interventions from trials of veterans with PTSD.5 A recent systematic review by Whitworth and Ciccolo concluded that exercise may be associated with reduced risk of PTSD, a briefer course of PTSD symptoms, and/or reduced sleep- and depression-related difficulties.6 However, that review primarily included observational, cross-sectional, and qualitative works. No trials included in our meta-analysis were included in that review.6
Evidence-based psychotherapies like cognitive processing therapy and prolonged exposure have been shown to be effective for treating PTSD in veterans; however, these modalities are accompanied by high rates of dropout (eg, 40-60%), thereby limiting their clinical utility.7 The use of complementary and alternative approaches for treatment in the United States has increased in recent years, and exercise represents an important complementary treatment option.8 In a study by Baldwin and colleagues, nearly 50% of veterans reported using complementary or alternative approaches, and veterans with PTSD were among those likely to use such approaches.9 However, current studies of the effects of exercise interventions on PTSD symptom reduction are mostly small and varied, making determinations difficult regarding the potential utility of exercise for treating this condition in veterans.
Literature Search
No previous research has synthesized the literature on the effects of exercise on PTSD in the veteran population. The current meta-analysis aims to provide a synthesis of systematically selected studies on this topic to determine whether exercise-based interventions are effective at reducing veterans’ symptoms of PTSD. Our hypothesis was that, when used as a primary or adjuvant intervention for PTSD, physical exercise would be associated with a reduction of PTSD symptom scale scores. We planned a priori to produce separate estimates for single-arm and multi-arm trials. We also wanted to conduct a careful risk of bias assessment—or evaluation of study features that may have systematically influenced results—for included trials, not only to provide context for interpretation of results, but also to inform suggestions for research to advance this field of inquiry.10
Methods
This study was preregistered on PROSPERO and followed PRISMA guidelines for meta-analyses and systematic reviews.11 Supplementary materials, such as the PRISMA checklist, study data, and funnel plots, are available online (doi.org/10.6084/m9.figshare.c.5618437.v1). Conference abstracts were omitted due to a lack of necessary information. We decided early in the planning process to include both randomized and single-arm trials, expecting the number of completed studies in the area of exercise for PTSD symptom reduction in veterans, and particularly randomized trials of such, would be relatively small.
Studies were included if they met the following criteria: (1) the study was a single- or multi-arm interventional trial; (2) participants were veterans; (3) participants had a current diagnosis of PTSD or exhibited subthreshold PTSD symptoms, as established by authors of the individual studies and supported by a structured clinical interview, semistructured interview, or elevated scores on PTSD symptom self-report measures; (4) the study included an intervention in which exercise (physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of physical fitness or health is an objective) was the primary component; (5) PTSD symptom severity was by a clinician-rated or self-report measure; and (6) the study was published in a peer-reviewed journal.12 Studies were excluded if means, standard deviations, and sample sizes were not available or the full text of the study was not available in English.
The systematic review was conducted using PubMed, PsycINFO, and Cochrane Library databases, from the earliest record to February 2021. The following search phrase was used, without additional limits, to acquire a list of potential studies: (“PTSD” or “post-traumatic stress disorder” or “posttraumatic stress disorder” or “post traumatic stress disorder”) and (“veteran” or “veterans”) and (“exercise” or “aerobic” or “activity” or “physical activity”). The references of identified publications also were searched for additional studies. Then, study titles and abstracts were evaluated and finally, full texts were evaluated to determine study inclusion. All screening, study selection, and risk of bias and data extraction activities were performed by 2 independent reviewers (DR and MJ) with disagreements resolved through discussion and consensus (Figure 1). A list of studies excluded during full-text review and rationales can be viewed online (doi.org/10.6084/m9.figshare.c.5618437.v1).
Data Collection
Data were extracted from included studies using custom forms and included the following information based on PRISMA guidelines: (1) study design characteristics; (2) intervention details; and (3) PTSD outcome information.11 PTSD symptom severity was the primary outcome of interest. Outcome data were included if they were derived from a measure of PTSD symptoms—equivalency across measures was assumed for meta-analyses. Potential study bias for each outcome was evaluated using the ROBINS-I and Cochrane Collaboration’s RoB 2 tools for single-arm and multi-arm trials, respectively.13,14 These tools evaluate domains related to the design, conduct, and analysis of studies that are associated with bias (ie, systematic error in findings, such as under- or overestimation of results).10 Examples include how well authors performed and concealed randomization procedures, addressed missing data, and measured study outcomes.13,14 The risk of bias (eg, low, moderate, serious) associated with each domain is rated and, based on the domain ratings, each study is then given an overall rating regarding how much risk influences bias.13,14 Broadly, lower risk of bias corresponds to higher confidence in the validity of results.
Finally, 4 authors (associated with 2 single- and 2 multi-arm studies) were contacted and asked to provide further information. Data for 1 additional multi-arm study were obtained from these communications and included in the final study selection.15 These authors were also asked for information about any unpublished works of which they were aware, although no additional works were identified.
Statistical Analyses
Analyses were performed with R Studio R 3.6.0 software.16 An SMD (also known as Hedges g) was calculated for each study outcome: for single-arm trials, this was the SMD between pre- and postintervention scores, whereas for multi-arm trials, this was the SMD between postintervention outcome scores across groups. CIs for each SMD were calculated using a standard normal distribution. Combined SMDs were estimated separately for single- and multi-arm studies, using random-effects meta-analyses. In order to include multiple relevant outcomes from a single trial (ie, for studies using multiple PTSD symptom measures), robust variance estimation was used.17 Precision was used to weight SMDs.
Correlations between pre- and postintervention scores were not available for 1 single-arm study.18 A correlation coefficient of 0.8 was imputed to calculate the standard error of the of the SMDs for the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist (PCL), as this value is consistent with past findings regarding the test-retest reliability of these measures.19-22 A sensitivity analysis, using several alternative correlational values, revealed that the choice of correlation coefficient did not impact the overall results of the meta-analysis.
I2 was used to evaluate between-study heterogeneity. Values of I2 > 25%, 50%, and 75% were selected to reflect low, moderate, and high heterogeneity, respectively, in accordance with guidelines described by Higgins and colleagues.23 Potential publication bias was assessed via funnel plot and Egger test.24 Finally, although collection of depressive symptom scores was proposed as a secondary outcome in the study protocol, such data were available only for 1 multi-arm study. As a result, this outcome was not evaluated.
Results
Six studies with 101 total participants were included in the single-arm analyses (Table 1).18,25-29 Participants consisted of veterans with chronic pain, post-9/11 veterans, female veterans of childbearing age, veterans with a history of trauma therapy, and other veterans. Types of exercise included moderate aerobic exercise and yoga. PTSD symptom measures included the CAPS and the PCL (PCL-5 or PCL-M versions). Reported financial sources for included studies included federal grant funding, nonprofit material support, outside organization support, use of US Department of Veterans Affairs (VA) resources, and no reported financial support.
With respect to individual studies, Shivakumar and colleagues found that completion of an aerobic exercise program was associated with reduced scores on 2 different PTSD symptom scales (PCL and CAPS) in 16 women veterans.18 A trauma-informed yoga intervention study with 18 participants by Cushing and colleagues demonstrated veteran participation to be associated with large reductions in PTSD, anxiety, and depression scale scores.25 In a study with 34 veterans, Chopin and colleagues found that a trauma-informed yoga intervention was associated with a statistically significant reduction in PTSD symptoms, as did a study by Zaccari and colleagues with 17 veterans.26,29 Justice and Brems also found some evidence that trauma-informed yoga interventions helped PTSD symptoms in a small sample of 4 veterans, although these results were not quantitatively analyzed.27 In contrast, a small pilot study (n = 12) by Staples and colleagues testing a biweekly, 6-week yoga program did not show a significant effect on PTSD symptoms.28
Three studies with 217 total veteran participants were included in the multi-arm analyses (Table 2).15,30,31 As all multi-arm trials incorporated randomization, they will be referred to as randomized controlled trials (RCTs). On contact, Davis and colleagues provided veteran-specific results for their trial; as such, our data differ from those within the published article.15 Participants from all included studies were veterans currently experiencing symptoms of PTSD. Types of exercise included yoga and combined methods (eg, aerobic and strength training).15,30,31 PTSD symptom measures included the CAPS or the PCL-5.15,30,31 Reported financial sources for included studies included federal grant funding, as well as nonprofit support, private donations, and VA and Department of Defense resources.
Davis and colleagues conducted a recently concluded RCT with > 130 veteran participants and found that a novel manualized yoga program was superior to an attention control in reducing PTSD symptom scale scores for veterans.15 Goldstein and colleagues found that a program consisting of both aerobic and resistance exercises reduced PTSD symptoms to a greater extent than a waitlist control condition, with 47 veterans randomized in this trial.30 Likewise, Hall and colleagues conducted a pilot RCT in which an intervention that integrated exercise and cognitive behavioral techniques was compared to a waitlist control condition.31 For the 48 veterans included in the analyses, the authors reported greater PTSD symptom reduction associated with integrated exercise than that of the control condition; however, the study was not powered to detect statistically significant differences between groups.
Bias Assessment
Results for the risk of bias assessments can be viewed in Tables 3 and 4. For single-arm studies, overall risk of bias was serious for all included trials. Serious risk of bias was found in 2 domains: confounding, due to a lack of accounting for potential preexisting baseline trends (eg, regression to the mean) that could have impacted study results; and measurement, due to the use of a self-report symptom measure (PCL) or CAPS with unblinded assessors. Multiple studies also showed moderate risk in the missing data domain due to participant dropout without appropriate analytic methods to address potential bias.
For RCTs, overall risk of bias ranged from some concerns to high risk. High risk of bias was found in 1 domain, measurement of outcome, due to use of a self-report symptom measure (PCL) with unblinded groups.31 The other 2 studies all had some concern of bias in at least 1 of the following domains: randomization, missing data, and measurement of outcome.
Pooled Standardized Mean Differences
Meta-analytic results can be viewed in Figure 2. The pooled SMD for the 6 single-arm studies was -0.60 (df = 4.41, 95% CI, -1.08 to -0.12, P = .03), indicating a statistically significant reduction in PTSD symptoms over the course of an exercise intervention. Combining SMDs for the 3 included RCTs revealed a pooled SMD of -0.40 (df = 1.57, 95% CI, -0.86 to 0.06, P = .06), indicating that exercise did not result in a statistically significant reduction in PTSD symptoms compared with control conditions.
Publication Bias and Heterogeneity
Visual inspection funnel plots and Egger test did not suggest the presence of publication bias for RCTs (t = 1.21, df = 2, P = .35) or single-arm studies (t = -0.36, df = 5, P = .73).
Single-arm studies displayed a high degree of heterogeneity (I2 = 81.5%). Including sample size or exercise duration as variables in meta-regressions did not reduce heterogeneity (I2 = 85.2% and I2 = 83.8%, respectively). Performing a subgroup analysis only on studies using yoga as an intervention also did not reduce heterogeneity (I2 = 79.2%). Due to the small number of studies, no further exploration of heterogeneity was conducted on single-arm studies. RCTs did not display any heterogeneity (I2 = 0%).
Discussion
Our report represents an early synthesis of the first prospective studies of physical exercise interventions for PTSD in veterans. Results from meta-analyses of 6 single-arm studies (101 participants) and 3 RCTs (217 participants) provide early evidence that exercise may reduce PTSD symptoms in veterans. Yoga was the most common form of exercise used in single-arm studies, whereas RCTs used a wider range of interventions. The pooled SMD of -0.60 for single-arm longitudinal studies suggest a medium decrease in PTSD symptoms for veterans who engage in exercise interventions. Analysis of the RCTs supported this finding, with a pooled SMD of -0.40 reflecting a small-to-medium effect of exercise on PTSD symptoms over control conditions, although this result did not achieve statistical significance. Of note, while the nonsignificant finding for RCTs may have been due to insufficient power caused by the limited number of included studies, possibly exercise was not more efficacious than were the control conditions.
Although RCTs represented a variety of exercise types, PTSD symptom measures, and veteran subgroups, statistical results were not indicative of heterogeneity. However, only the largest and most comprehensive study of exercise for PTSD in veterans to date by Davis and colleagues had a statistically significant SMD.15 Of note, one of the other 2 RCTs displayed an SMD of a similar magnitude, but this study had a much smaller sample size and was underpowered to detect significance.30 Additionally, risk of bias assessments for single-arm studies and RCTs revealed study characteristics that suggest possible inflation of absolute effect sizes for individual studies. Therefore, the pooled SMDs we report are interpretable but may exceed the true effect of exercise for PTSD symptom reduction in veterans.
Based on results of our analyses, it is reasonable, albeit preliminary, to conclude that exercise interventions may result in reduced PTSD symptoms among veterans. At the very least, these findings support the continued investigation of such interventions for veterans. Given the unique and salubrious characteristics of physical exercise, such results, if supported by further research, suggest that exercise-based interventions may be particularly valuable within the trauma treatment realm. For example, exercise can be less expensive and more convenient than attending traditional treatment, and for veterans reluctant to engage in standard treatment approaches such as psychiatric and psychosocial modalities, complementary approaches entailing exercise may be viewed as particularly acceptable or enjoyable.32 In addition to possibly reducing PTSD symptoms, exercise is a well-established treatment for conditions commonly comorbid with PTSD, including depression, anxiety disorders, cognitive difficulties, and certain chronic pain conditions.6 As such, exercise represents a holistic treatment option that has the potential to augment standard PTSD care.
Limitations
The present study has several important limitations. First, few studies were found that met the broad eligibility criteria and those that did often had a small sample size. Besides highlighting a gap in the extant research, the limited studies available for meta-analysis means that caution must be taken when interpreting results. Fortunately, this issue will likely resolve once additional studies investigating the impact of exercise on PTSD symptoms in veterans are available for synthesis.
Relatedly, the included study interventions varied considerably, both in the types of exercise used and the characteristics of the exercises (eg, frequency, duration, and intensity), which is relevant as different exercise modalities are associated with differential physical effects.33 Including such a mixture of exercises may have given an incomplete picture of their potential therapeutic effects. Also, none of the RCTs compared exercise against first-line treatments for PTSD, such as prolonged exposure or cognitive processing therapy, which would have provided further insight into the role exercise could play in clinical settings.7
Another limitation is the elevated risk of bias found in most studies, particularly present in the longitudinal single-arm studies, all of which were rated at serious risk. For instance, no single-arm study controlled for preexisting baseline trends: without such (and lacking a comparison control group like in RCTs), it is possible that the observed effects were due to extraneous factors, rather than the exercise intervention. Although not as severe, the multi-arm RCTs also displayed at least moderate risk of bias. Therefore, SMDs may have been overestimated for each group of studies.
Finally, the results of the single-arm meta-analysis displayed high statistical heterogeneity, reducing the generalizability of the results. One possible cause of this heterogeneity may have been the yoga interventions, as a separate analysis removing the only nonyoga study did not reduce heterogeneity. This result was surprising, as the included yoga interventions seemed similar across studies. While the presence of high heterogeneity does require some caution when applying these results to outside interventions, the present study made use of random-effects meta-analysis, a technique that incorporates study heterogeneity into the statistical model, thereby strengthening the findings compared with that of a traditional fixed-effects approach.10
Future Steps
Several future steps are warranted to improve knowledge of exercise as a treatment for PTSD in veterans and in the general population. With current meta-analyses limited to small numbers of studies, additional studies of the efficacy of exercise for treating PTSD could help in several ways. A larger pool of studies would enable future meta-analyses to explore related questions, such as those regarding the impact of exercise on quality of life or depressive symptom reduction among veterans with PTSD. A greater number of studies also would enable meta-analysts to explore potentially critical moderators. For example, the duration, frequency, or type of exercise may moderate the effect of exercise on PTSD symptom reduction. Moderators related to patient or study design characteristics also should be explored in future studies.
Future work also should evaluate the impact that specific features of exercise regimens have on PTSD. Knowing whether the type or structure of exercise affects its clinical use would be invaluable in developing and implementing efficient exercise-based interventions. For example, if facilitated exercise was found to be significantly more effective at reducing PTSD symptoms than exercise completed independently, the development of exercise intervention programs in the VA and other facilities that commonly treat PTSD may be warranted. Additionally, it may be useful to identify specific mechanisms through which exercise reduces PTSD symptoms. For example, in addition to its beneficial biological effects, exercise also promotes psychological health through behavioral activation and alterations within reinforcement/reward systems, suggesting that exercise regularity may be more important than intensity.34,35 Understanding which mechanisms contribute most to change will aid in the development of more efficient interventions.
Given that veterans are demonstrating considerable interest in complementary and alternative PTSD treatments, it is critical that researchers focus on high-quality randomized tests of these interventions. Therefore, in addition to greater quality of exercise intervention studies, future efforts should be focused on RCTs that are designed in such a way as to limit potential introduction of bias. For example, assessment data should be completed by blinded assessors using standardized measures, and analyses should account for missing data and unequal participant attrition between groups. Ideally, pre-intervention trends across multiple baseline datapoints also would be collected in single-arm studies to avoid confounding related to regression to the mean. It is also recommended that future meta-analyses use risk of bias assessments and consider how the results of such assessments may impact the interpretation of results.
Conclusions
Findings from both single-arm studies and RCTs suggest possible benefit of exercise on PTSD symptom reduction, although confirmation of findings is needed. No study found increased symptoms following exercise intervention. Thus, it is reasonable to consider physical exercise, such as yoga, as an adjunct, whole-health consistent treatment. HCPs working with veterans with past traumatic experiences should consider incorporating exercise into patient care. Enhanced educational efforts emphasizing the psychotherapeutic impact of exercise may also have value for the veteran population. Furthermore, the current risk of bias assessments highlights the need for additional high-quality RCTs evaluating the specific impact of exercise on PTSD symptom reduction in veterans. In particular, this field of inquiry would benefit from larger samples and design characteristics to reduce bias (eg, blinding when possible, use of CAPS vs only self-report symptom measures, reducing problematic attrition, corrections for missing data, etc).
Acknowledgments
This research is the result of work supported with resources and the use of facilities at the VA Eastern Kansas Healthcare System (Dwight D. Eisenhower VA Medical Center). It was also supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, as well as the Rocky Mountain Mental Illness Research, Education, and Clinical Center. Since Dr. Reis and Dr. Gaddy are employees of the US Government and contributed to this manuscript as part of their official duties, the work is not subject to US copyright. This study was preregistered on PROSPERO (https://www.crd.york.ac.uk/prospero/; ID: CRD42020153419).
Physical exercise offers preventative and therapeutic benefits for a range of chronic health conditions, including cardiovascular disease, type 2 diabetes mellitus, Alzheimer disease, and depression.1,2 Exercise has been well studied for its antidepressant effects, its ability to reduce risk of aging-related dementia, and favorable effects on a range of cognitive functions.2 Lesser evidence exists regarding the impact of exercise on other mental health concerns. Therefore, an accurate understanding of whether physical exercise may ameliorate other conditions is important.
A small meta-analysis by Rosenbaum and colleagues found that exercise interventions were superior to control conditions for symptom reduction in study participants with posttraumatic stress disorder (PTSD).3 This meta-analysis included 4 randomized clinical trials representing 200 cases. The trial included a variety of physical activities (eg, yoga, aerobic, and strength-building exercises) and control conditions, and participants recruited from online, community, inpatient, and outpatient settings. The standardized mean difference (SMD) produced by the analysis indicated a small-to-medium effect (Hedges g, -0.35), with the authors reporting no evidence of publication bias, although an assessment of potential bias associated with individual trial design characteristics was not conducted. Of note, a meta-analysis by Watts and colleagues found that effect sizes for PTSD treatments tend to be smaller in veteran populations.4 Therefore, how much the mean effect size estimate in the study is applicable to veterans with PTSD is unknown.3
Veterans represent a unique subpopulation in which PTSD is common, although no meta-analysis yet published has synthesized the effects of exercise interventions from trials of veterans with PTSD.5 A recent systematic review by Whitworth and Ciccolo concluded that exercise may be associated with reduced risk of PTSD, a briefer course of PTSD symptoms, and/or reduced sleep- and depression-related difficulties.6 However, that review primarily included observational, cross-sectional, and qualitative works. No trials included in our meta-analysis were included in that review.6
Evidence-based psychotherapies like cognitive processing therapy and prolonged exposure have been shown to be effective for treating PTSD in veterans; however, these modalities are accompanied by high rates of dropout (eg, 40-60%), thereby limiting their clinical utility.7 The use of complementary and alternative approaches for treatment in the United States has increased in recent years, and exercise represents an important complementary treatment option.8 In a study by Baldwin and colleagues, nearly 50% of veterans reported using complementary or alternative approaches, and veterans with PTSD were among those likely to use such approaches.9 However, current studies of the effects of exercise interventions on PTSD symptom reduction are mostly small and varied, making determinations difficult regarding the potential utility of exercise for treating this condition in veterans.
Literature Search
No previous research has synthesized the literature on the effects of exercise on PTSD in the veteran population. The current meta-analysis aims to provide a synthesis of systematically selected studies on this topic to determine whether exercise-based interventions are effective at reducing veterans’ symptoms of PTSD. Our hypothesis was that, when used as a primary or adjuvant intervention for PTSD, physical exercise would be associated with a reduction of PTSD symptom scale scores. We planned a priori to produce separate estimates for single-arm and multi-arm trials. We also wanted to conduct a careful risk of bias assessment—or evaluation of study features that may have systematically influenced results—for included trials, not only to provide context for interpretation of results, but also to inform suggestions for research to advance this field of inquiry.10
Methods
This study was preregistered on PROSPERO and followed PRISMA guidelines for meta-analyses and systematic reviews.11 Supplementary materials, such as the PRISMA checklist, study data, and funnel plots, are available online (doi.org/10.6084/m9.figshare.c.5618437.v1). Conference abstracts were omitted due to a lack of necessary information. We decided early in the planning process to include both randomized and single-arm trials, expecting the number of completed studies in the area of exercise for PTSD symptom reduction in veterans, and particularly randomized trials of such, would be relatively small.
Studies were included if they met the following criteria: (1) the study was a single- or multi-arm interventional trial; (2) participants were veterans; (3) participants had a current diagnosis of PTSD or exhibited subthreshold PTSD symptoms, as established by authors of the individual studies and supported by a structured clinical interview, semistructured interview, or elevated scores on PTSD symptom self-report measures; (4) the study included an intervention in which exercise (physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of physical fitness or health is an objective) was the primary component; (5) PTSD symptom severity was by a clinician-rated or self-report measure; and (6) the study was published in a peer-reviewed journal.12 Studies were excluded if means, standard deviations, and sample sizes were not available or the full text of the study was not available in English.
The systematic review was conducted using PubMed, PsycINFO, and Cochrane Library databases, from the earliest record to February 2021. The following search phrase was used, without additional limits, to acquire a list of potential studies: (“PTSD” or “post-traumatic stress disorder” or “posttraumatic stress disorder” or “post traumatic stress disorder”) and (“veteran” or “veterans”) and (“exercise” or “aerobic” or “activity” or “physical activity”). The references of identified publications also were searched for additional studies. Then, study titles and abstracts were evaluated and finally, full texts were evaluated to determine study inclusion. All screening, study selection, and risk of bias and data extraction activities were performed by 2 independent reviewers (DR and MJ) with disagreements resolved through discussion and consensus (Figure 1). A list of studies excluded during full-text review and rationales can be viewed online (doi.org/10.6084/m9.figshare.c.5618437.v1).
Data Collection
Data were extracted from included studies using custom forms and included the following information based on PRISMA guidelines: (1) study design characteristics; (2) intervention details; and (3) PTSD outcome information.11 PTSD symptom severity was the primary outcome of interest. Outcome data were included if they were derived from a measure of PTSD symptoms—equivalency across measures was assumed for meta-analyses. Potential study bias for each outcome was evaluated using the ROBINS-I and Cochrane Collaboration’s RoB 2 tools for single-arm and multi-arm trials, respectively.13,14 These tools evaluate domains related to the design, conduct, and analysis of studies that are associated with bias (ie, systematic error in findings, such as under- or overestimation of results).10 Examples include how well authors performed and concealed randomization procedures, addressed missing data, and measured study outcomes.13,14 The risk of bias (eg, low, moderate, serious) associated with each domain is rated and, based on the domain ratings, each study is then given an overall rating regarding how much risk influences bias.13,14 Broadly, lower risk of bias corresponds to higher confidence in the validity of results.
Finally, 4 authors (associated with 2 single- and 2 multi-arm studies) were contacted and asked to provide further information. Data for 1 additional multi-arm study were obtained from these communications and included in the final study selection.15 These authors were also asked for information about any unpublished works of which they were aware, although no additional works were identified.
Statistical Analyses
Analyses were performed with R Studio R 3.6.0 software.16 An SMD (also known as Hedges g) was calculated for each study outcome: for single-arm trials, this was the SMD between pre- and postintervention scores, whereas for multi-arm trials, this was the SMD between postintervention outcome scores across groups. CIs for each SMD were calculated using a standard normal distribution. Combined SMDs were estimated separately for single- and multi-arm studies, using random-effects meta-analyses. In order to include multiple relevant outcomes from a single trial (ie, for studies using multiple PTSD symptom measures), robust variance estimation was used.17 Precision was used to weight SMDs.
Correlations between pre- and postintervention scores were not available for 1 single-arm study.18 A correlation coefficient of 0.8 was imputed to calculate the standard error of the of the SMDs for the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist (PCL), as this value is consistent with past findings regarding the test-retest reliability of these measures.19-22 A sensitivity analysis, using several alternative correlational values, revealed that the choice of correlation coefficient did not impact the overall results of the meta-analysis.
I2 was used to evaluate between-study heterogeneity. Values of I2 > 25%, 50%, and 75% were selected to reflect low, moderate, and high heterogeneity, respectively, in accordance with guidelines described by Higgins and colleagues.23 Potential publication bias was assessed via funnel plot and Egger test.24 Finally, although collection of depressive symptom scores was proposed as a secondary outcome in the study protocol, such data were available only for 1 multi-arm study. As a result, this outcome was not evaluated.
Results
Six studies with 101 total participants were included in the single-arm analyses (Table 1).18,25-29 Participants consisted of veterans with chronic pain, post-9/11 veterans, female veterans of childbearing age, veterans with a history of trauma therapy, and other veterans. Types of exercise included moderate aerobic exercise and yoga. PTSD symptom measures included the CAPS and the PCL (PCL-5 or PCL-M versions). Reported financial sources for included studies included federal grant funding, nonprofit material support, outside organization support, use of US Department of Veterans Affairs (VA) resources, and no reported financial support.
With respect to individual studies, Shivakumar and colleagues found that completion of an aerobic exercise program was associated with reduced scores on 2 different PTSD symptom scales (PCL and CAPS) in 16 women veterans.18 A trauma-informed yoga intervention study with 18 participants by Cushing and colleagues demonstrated veteran participation to be associated with large reductions in PTSD, anxiety, and depression scale scores.25 In a study with 34 veterans, Chopin and colleagues found that a trauma-informed yoga intervention was associated with a statistically significant reduction in PTSD symptoms, as did a study by Zaccari and colleagues with 17 veterans.26,29 Justice and Brems also found some evidence that trauma-informed yoga interventions helped PTSD symptoms in a small sample of 4 veterans, although these results were not quantitatively analyzed.27 In contrast, a small pilot study (n = 12) by Staples and colleagues testing a biweekly, 6-week yoga program did not show a significant effect on PTSD symptoms.28
Three studies with 217 total veteran participants were included in the multi-arm analyses (Table 2).15,30,31 As all multi-arm trials incorporated randomization, they will be referred to as randomized controlled trials (RCTs). On contact, Davis and colleagues provided veteran-specific results for their trial; as such, our data differ from those within the published article.15 Participants from all included studies were veterans currently experiencing symptoms of PTSD. Types of exercise included yoga and combined methods (eg, aerobic and strength training).15,30,31 PTSD symptom measures included the CAPS or the PCL-5.15,30,31 Reported financial sources for included studies included federal grant funding, as well as nonprofit support, private donations, and VA and Department of Defense resources.
Davis and colleagues conducted a recently concluded RCT with > 130 veteran participants and found that a novel manualized yoga program was superior to an attention control in reducing PTSD symptom scale scores for veterans.15 Goldstein and colleagues found that a program consisting of both aerobic and resistance exercises reduced PTSD symptoms to a greater extent than a waitlist control condition, with 47 veterans randomized in this trial.30 Likewise, Hall and colleagues conducted a pilot RCT in which an intervention that integrated exercise and cognitive behavioral techniques was compared to a waitlist control condition.31 For the 48 veterans included in the analyses, the authors reported greater PTSD symptom reduction associated with integrated exercise than that of the control condition; however, the study was not powered to detect statistically significant differences between groups.
Bias Assessment
Results for the risk of bias assessments can be viewed in Tables 3 and 4. For single-arm studies, overall risk of bias was serious for all included trials. Serious risk of bias was found in 2 domains: confounding, due to a lack of accounting for potential preexisting baseline trends (eg, regression to the mean) that could have impacted study results; and measurement, due to the use of a self-report symptom measure (PCL) or CAPS with unblinded assessors. Multiple studies also showed moderate risk in the missing data domain due to participant dropout without appropriate analytic methods to address potential bias.
For RCTs, overall risk of bias ranged from some concerns to high risk. High risk of bias was found in 1 domain, measurement of outcome, due to use of a self-report symptom measure (PCL) with unblinded groups.31 The other 2 studies all had some concern of bias in at least 1 of the following domains: randomization, missing data, and measurement of outcome.
Pooled Standardized Mean Differences
Meta-analytic results can be viewed in Figure 2. The pooled SMD for the 6 single-arm studies was -0.60 (df = 4.41, 95% CI, -1.08 to -0.12, P = .03), indicating a statistically significant reduction in PTSD symptoms over the course of an exercise intervention. Combining SMDs for the 3 included RCTs revealed a pooled SMD of -0.40 (df = 1.57, 95% CI, -0.86 to 0.06, P = .06), indicating that exercise did not result in a statistically significant reduction in PTSD symptoms compared with control conditions.
Publication Bias and Heterogeneity
Visual inspection funnel plots and Egger test did not suggest the presence of publication bias for RCTs (t = 1.21, df = 2, P = .35) or single-arm studies (t = -0.36, df = 5, P = .73).
Single-arm studies displayed a high degree of heterogeneity (I2 = 81.5%). Including sample size or exercise duration as variables in meta-regressions did not reduce heterogeneity (I2 = 85.2% and I2 = 83.8%, respectively). Performing a subgroup analysis only on studies using yoga as an intervention also did not reduce heterogeneity (I2 = 79.2%). Due to the small number of studies, no further exploration of heterogeneity was conducted on single-arm studies. RCTs did not display any heterogeneity (I2 = 0%).
Discussion
Our report represents an early synthesis of the first prospective studies of physical exercise interventions for PTSD in veterans. Results from meta-analyses of 6 single-arm studies (101 participants) and 3 RCTs (217 participants) provide early evidence that exercise may reduce PTSD symptoms in veterans. Yoga was the most common form of exercise used in single-arm studies, whereas RCTs used a wider range of interventions. The pooled SMD of -0.60 for single-arm longitudinal studies suggest a medium decrease in PTSD symptoms for veterans who engage in exercise interventions. Analysis of the RCTs supported this finding, with a pooled SMD of -0.40 reflecting a small-to-medium effect of exercise on PTSD symptoms over control conditions, although this result did not achieve statistical significance. Of note, while the nonsignificant finding for RCTs may have been due to insufficient power caused by the limited number of included studies, possibly exercise was not more efficacious than were the control conditions.
Although RCTs represented a variety of exercise types, PTSD symptom measures, and veteran subgroups, statistical results were not indicative of heterogeneity. However, only the largest and most comprehensive study of exercise for PTSD in veterans to date by Davis and colleagues had a statistically significant SMD.15 Of note, one of the other 2 RCTs displayed an SMD of a similar magnitude, but this study had a much smaller sample size and was underpowered to detect significance.30 Additionally, risk of bias assessments for single-arm studies and RCTs revealed study characteristics that suggest possible inflation of absolute effect sizes for individual studies. Therefore, the pooled SMDs we report are interpretable but may exceed the true effect of exercise for PTSD symptom reduction in veterans.
Based on results of our analyses, it is reasonable, albeit preliminary, to conclude that exercise interventions may result in reduced PTSD symptoms among veterans. At the very least, these findings support the continued investigation of such interventions for veterans. Given the unique and salubrious characteristics of physical exercise, such results, if supported by further research, suggest that exercise-based interventions may be particularly valuable within the trauma treatment realm. For example, exercise can be less expensive and more convenient than attending traditional treatment, and for veterans reluctant to engage in standard treatment approaches such as psychiatric and psychosocial modalities, complementary approaches entailing exercise may be viewed as particularly acceptable or enjoyable.32 In addition to possibly reducing PTSD symptoms, exercise is a well-established treatment for conditions commonly comorbid with PTSD, including depression, anxiety disorders, cognitive difficulties, and certain chronic pain conditions.6 As such, exercise represents a holistic treatment option that has the potential to augment standard PTSD care.
Limitations
The present study has several important limitations. First, few studies were found that met the broad eligibility criteria and those that did often had a small sample size. Besides highlighting a gap in the extant research, the limited studies available for meta-analysis means that caution must be taken when interpreting results. Fortunately, this issue will likely resolve once additional studies investigating the impact of exercise on PTSD symptoms in veterans are available for synthesis.
Relatedly, the included study interventions varied considerably, both in the types of exercise used and the characteristics of the exercises (eg, frequency, duration, and intensity), which is relevant as different exercise modalities are associated with differential physical effects.33 Including such a mixture of exercises may have given an incomplete picture of their potential therapeutic effects. Also, none of the RCTs compared exercise against first-line treatments for PTSD, such as prolonged exposure or cognitive processing therapy, which would have provided further insight into the role exercise could play in clinical settings.7
Another limitation is the elevated risk of bias found in most studies, particularly present in the longitudinal single-arm studies, all of which were rated at serious risk. For instance, no single-arm study controlled for preexisting baseline trends: without such (and lacking a comparison control group like in RCTs), it is possible that the observed effects were due to extraneous factors, rather than the exercise intervention. Although not as severe, the multi-arm RCTs also displayed at least moderate risk of bias. Therefore, SMDs may have been overestimated for each group of studies.
Finally, the results of the single-arm meta-analysis displayed high statistical heterogeneity, reducing the generalizability of the results. One possible cause of this heterogeneity may have been the yoga interventions, as a separate analysis removing the only nonyoga study did not reduce heterogeneity. This result was surprising, as the included yoga interventions seemed similar across studies. While the presence of high heterogeneity does require some caution when applying these results to outside interventions, the present study made use of random-effects meta-analysis, a technique that incorporates study heterogeneity into the statistical model, thereby strengthening the findings compared with that of a traditional fixed-effects approach.10
Future Steps
Several future steps are warranted to improve knowledge of exercise as a treatment for PTSD in veterans and in the general population. With current meta-analyses limited to small numbers of studies, additional studies of the efficacy of exercise for treating PTSD could help in several ways. A larger pool of studies would enable future meta-analyses to explore related questions, such as those regarding the impact of exercise on quality of life or depressive symptom reduction among veterans with PTSD. A greater number of studies also would enable meta-analysts to explore potentially critical moderators. For example, the duration, frequency, or type of exercise may moderate the effect of exercise on PTSD symptom reduction. Moderators related to patient or study design characteristics also should be explored in future studies.
Future work also should evaluate the impact that specific features of exercise regimens have on PTSD. Knowing whether the type or structure of exercise affects its clinical use would be invaluable in developing and implementing efficient exercise-based interventions. For example, if facilitated exercise was found to be significantly more effective at reducing PTSD symptoms than exercise completed independently, the development of exercise intervention programs in the VA and other facilities that commonly treat PTSD may be warranted. Additionally, it may be useful to identify specific mechanisms through which exercise reduces PTSD symptoms. For example, in addition to its beneficial biological effects, exercise also promotes psychological health through behavioral activation and alterations within reinforcement/reward systems, suggesting that exercise regularity may be more important than intensity.34,35 Understanding which mechanisms contribute most to change will aid in the development of more efficient interventions.
Given that veterans are demonstrating considerable interest in complementary and alternative PTSD treatments, it is critical that researchers focus on high-quality randomized tests of these interventions. Therefore, in addition to greater quality of exercise intervention studies, future efforts should be focused on RCTs that are designed in such a way as to limit potential introduction of bias. For example, assessment data should be completed by blinded assessors using standardized measures, and analyses should account for missing data and unequal participant attrition between groups. Ideally, pre-intervention trends across multiple baseline datapoints also would be collected in single-arm studies to avoid confounding related to regression to the mean. It is also recommended that future meta-analyses use risk of bias assessments and consider how the results of such assessments may impact the interpretation of results.
Conclusions
Findings from both single-arm studies and RCTs suggest possible benefit of exercise on PTSD symptom reduction, although confirmation of findings is needed. No study found increased symptoms following exercise intervention. Thus, it is reasonable to consider physical exercise, such as yoga, as an adjunct, whole-health consistent treatment. HCPs working with veterans with past traumatic experiences should consider incorporating exercise into patient care. Enhanced educational efforts emphasizing the psychotherapeutic impact of exercise may also have value for the veteran population. Furthermore, the current risk of bias assessments highlights the need for additional high-quality RCTs evaluating the specific impact of exercise on PTSD symptom reduction in veterans. In particular, this field of inquiry would benefit from larger samples and design characteristics to reduce bias (eg, blinding when possible, use of CAPS vs only self-report symptom measures, reducing problematic attrition, corrections for missing data, etc).
Acknowledgments
This research is the result of work supported with resources and the use of facilities at the VA Eastern Kansas Healthcare System (Dwight D. Eisenhower VA Medical Center). It was also supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, as well as the Rocky Mountain Mental Illness Research, Education, and Clinical Center. Since Dr. Reis and Dr. Gaddy are employees of the US Government and contributed to this manuscript as part of their official duties, the work is not subject to US copyright. This study was preregistered on PROSPERO (https://www.crd.york.ac.uk/prospero/; ID: CRD42020153419).
1. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity—a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi:10.1186/1471-2458-13-813
2. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592. doi:10.1037/a0021769
3. Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of posttraumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230(2):130-136. doi:10.1016/j.psychres.2015.10.017
4. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-550. doi:10.4088/JCP.12r08225
5. Tanielian T, Jaycox L, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; 2008
6. Whitworth JW, Ciccolo JT. Exercise and post-traumatic stress disorder in military veterans: a systematic review. Mil Med. 2016;181(9):953-960. doi:10.7205/MILMED-D-15-00488
7. Rutt BT, Oehlert ME, Krieshok TS, Lichtenberg JW. Effectiveness of cognitive processing therapy and prolonged exposure in the Department of Veterans Affairs. Psychol Rep. 2018;121(2):282-302. doi:10.1177/0033294117727746
8. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
9. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: Implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704. doi:10.1001/archinte.162.15.1697
10. Higgins JPT, Thomas J, Chanlder J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane; 2021.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
12. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131.
13. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919
14. Sterne JAC, Savovic´ J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
15. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
16. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2019.
17. Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods .2015;20(3):375-393. doi:10.1037/met0000011
18. Shivakumar G, Anderson EH, Surís AM, North CS. Exercise for PTSD in women veterans: a proof-of-concept study. Mil Med. 2017;182(11):e1809-e1814. doi:10.7205/MILMED-D-16-00440
19. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
20. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669-673. doi:10.1016/0005-7967(96)00033-2
21. Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician- Administered PTSD Scale for DSM-5 (CAPS- 5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383-395.doi:10.1037/pas0000486
22. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596-606. doi:10.1002/da.20837
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi:10.1136/bmj.315.7109.629
25. Cushing RE, Braun KL, Alden CISW, Katz AR. Military- tailored yoga for veterans with post-traumatic stress disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. Justice L, Brems C. Bridging body and mind: case series of a 10-week trauma-informed yoga protocol for veterans. Int J Yoga Therap. 2019;29(1):65-79. doi:10.17761/D-17-2019-00029
28. Staples JK, Hamilton MF, Uddo M. A yoga program for the symptoms of post-traumatic stress disorder in veterans. Mil Med. 2013;178(8):854-860. doi:10.7205/MILMED-D-12-00536
29. Zaccari B, Callahan ML, Storzbach D, McFarlane N, Hudson R, Loftis JM. Yoga for veterans with PTSD: Cognitive functioning, mental health, and salivary cortisol. Psychol Trauma. 2020;12(8):913-917. doi:10.1037/tra0000909
30. Goldstein LA, Mehling WE, Metzler TJ, et al. Veterans Group Exercise: A randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345-352. doi:10.1016/j.jad.2017.11.002
31. Hall KS, Morey MC, Bosworth HB, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2020;43(4):648-659. doi:10.1007/s10865-019-00073-w
32. Gaddy MA. Implementation of an integrative medicine treatment program at a Veterans Health Administration residential mental health facility. Psychol Serv. 2018;15(4):503- 509. doi:10.1037/ser0000189
33. Werner CM, Hecksteden A, Morsch A, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34- 46. doi:10.1093/eurheartj/ehy585
34. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi:10.1098/rsfs.2014.0040
35. Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45-62. doi:10.1146/annurev-med-060619-022943.
1. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity—a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi:10.1186/1471-2458-13-813
2. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592. doi:10.1037/a0021769
3. Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of posttraumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230(2):130-136. doi:10.1016/j.psychres.2015.10.017
4. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-550. doi:10.4088/JCP.12r08225
5. Tanielian T, Jaycox L, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; 2008
6. Whitworth JW, Ciccolo JT. Exercise and post-traumatic stress disorder in military veterans: a systematic review. Mil Med. 2016;181(9):953-960. doi:10.7205/MILMED-D-15-00488
7. Rutt BT, Oehlert ME, Krieshok TS, Lichtenberg JW. Effectiveness of cognitive processing therapy and prolonged exposure in the Department of Veterans Affairs. Psychol Rep. 2018;121(2):282-302. doi:10.1177/0033294117727746
8. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
9. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: Implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704. doi:10.1001/archinte.162.15.1697
10. Higgins JPT, Thomas J, Chanlder J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane; 2021.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
12. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131.
13. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919
14. Sterne JAC, Savovic´ J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
15. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
16. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2019.
17. Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods .2015;20(3):375-393. doi:10.1037/met0000011
18. Shivakumar G, Anderson EH, Surís AM, North CS. Exercise for PTSD in women veterans: a proof-of-concept study. Mil Med. 2017;182(11):e1809-e1814. doi:10.7205/MILMED-D-16-00440
19. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
20. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669-673. doi:10.1016/0005-7967(96)00033-2
21. Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician- Administered PTSD Scale for DSM-5 (CAPS- 5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383-395.doi:10.1037/pas0000486
22. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596-606. doi:10.1002/da.20837
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi:10.1136/bmj.315.7109.629
25. Cushing RE, Braun KL, Alden CISW, Katz AR. Military- tailored yoga for veterans with post-traumatic stress disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. Justice L, Brems C. Bridging body and mind: case series of a 10-week trauma-informed yoga protocol for veterans. Int J Yoga Therap. 2019;29(1):65-79. doi:10.17761/D-17-2019-00029
28. Staples JK, Hamilton MF, Uddo M. A yoga program for the symptoms of post-traumatic stress disorder in veterans. Mil Med. 2013;178(8):854-860. doi:10.7205/MILMED-D-12-00536
29. Zaccari B, Callahan ML, Storzbach D, McFarlane N, Hudson R, Loftis JM. Yoga for veterans with PTSD: Cognitive functioning, mental health, and salivary cortisol. Psychol Trauma. 2020;12(8):913-917. doi:10.1037/tra0000909
30. Goldstein LA, Mehling WE, Metzler TJ, et al. Veterans Group Exercise: A randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345-352. doi:10.1016/j.jad.2017.11.002
31. Hall KS, Morey MC, Bosworth HB, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2020;43(4):648-659. doi:10.1007/s10865-019-00073-w
32. Gaddy MA. Implementation of an integrative medicine treatment program at a Veterans Health Administration residential mental health facility. Psychol Serv. 2018;15(4):503- 509. doi:10.1037/ser0000189
33. Werner CM, Hecksteden A, Morsch A, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34- 46. doi:10.1093/eurheartj/ehy585
34. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi:10.1098/rsfs.2014.0040
35. Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45-62. doi:10.1146/annurev-med-060619-022943.
I STEP: Recognizing cognitive distortions in posttraumatic stress disorder
Evidence-based cognitive-behavioral therapies for posttraumatic stress disorder (PTSD) may employ cognitive restructuring. This psychotherapeutic technique entails recognizing and correcting maladaptive, inaccurate thoughts that perpetuate illness.1 For example, a clinician helps a patient recognize that the negative thought “Nobody loves me” following a romantic breakup is an overgeneralization. The patient is taught to self-correct this to “While my ex-girlfriend doesn’t love me, others do. It only feels like nobody loves me.”2
We introduce the acronym I STEP to help clinicians recognize several common distorted thoughts in PTSD. These tend to occur within stereotyped themes in PTSD,3 as outlined and illustrated below. Recognizing distorted thoughts in these patients will help clinicians understand and address psychological distress following trauma.
Intimacy/In-touch. Intimacy involves comfort in relationships, including but not limited to sexual intimacy. This requires being in touch emotionally with self and others. In trauma involving loss, fear of further loss may impair intimacy with others. Difficulty with self-intimacy impairs commitment to life’s goals and prompts unhelpful avoidance behaviors, such as difficulty being alone or self-injurious use of drugs or alcohol. Comfort in spending some portion of time alone with one’s thoughts and emotions is a life skill necessary to attain optimum function. Patients who are unable to tolerate their own emotions without constant company might have excessive anxiety when social supports are otherwise occupied. Such patients might seek excessive and repeated reassurance rather than learning to tolerate their own emotions and thoughts. They would then find it difficult to engage successfully in solo activities.
Safety. After trauma, patients may view themselves and others as unsafe, and may overestimate risk. For example, a pedestrian who is struck by a vehicle may believe that crossing a street will again result in getting hit by a car without appreciating that people frequently cross streets without injury or that crossing cautiously is an essential life skill. Parents who have suffered from trauma may unduly believe that their children are in danger when engaging in an activity generally considered to be safe. This may create challenges in parenting and impede their children’s ability to develop a sense of independence.
Trust. Trauma victims may unfairly blame themselves, leading them to mistrust their own judgment. Such patients may have difficulty making decisions confidently and independently, such as choosing a job or a romantic partner. When traumatized by another person or people, it can be difficult to maintain positive views of others or to accept others’ positive behaviors as genuine. For example, a common reaction following rape may be a generalized mistrust of all men.
Esteem. Patients’ self-esteem may suffer following trauma due to irrational self-blame or believing the “just world hypothesis”—the idea that bad things only happen to bad people. For example, a patient who suffers an assault by an acquaintance might think “I must be stupid if I couldn’t figure out that my friend was dangerous.”
Power. Traumatic events usually occur outside of one’s control. Survivors of trauma may lose confidence in their ability to control any aspect of their lives. Conversely, they may attempt to gain control of all of life’s circumstances, including those that are beyond anyone’s control. Control can be applied to emotions, behaviors, or events. A driver struck by a vehicle may think “I can’t control other drivers, so I have no power to control my safety while driving,” and hence give up driving. While there are things that are beyond our control, this extreme thought ignores things that we can control, such as wearing seatbelts or having the vehicle’s brakes regularly serviced.
1. Wenzel A. Basic strategies of cognitive behavioral therapy. Psychiatr Clin North Am. 2017;40(4):597-609.
2. Beck J. Cognitive behavioral therapy: basics and beyond. 2nd ed. The Guilford Press; 2011.
3. Resick PA, Monson CM, Chard KM. Cognitive processing therapy for PTSD. A comprehensive manual. The Guilford Press; 2017.
Evidence-based cognitive-behavioral therapies for posttraumatic stress disorder (PTSD) may employ cognitive restructuring. This psychotherapeutic technique entails recognizing and correcting maladaptive, inaccurate thoughts that perpetuate illness.1 For example, a clinician helps a patient recognize that the negative thought “Nobody loves me” following a romantic breakup is an overgeneralization. The patient is taught to self-correct this to “While my ex-girlfriend doesn’t love me, others do. It only feels like nobody loves me.”2
We introduce the acronym I STEP to help clinicians recognize several common distorted thoughts in PTSD. These tend to occur within stereotyped themes in PTSD,3 as outlined and illustrated below. Recognizing distorted thoughts in these patients will help clinicians understand and address psychological distress following trauma.
Intimacy/In-touch. Intimacy involves comfort in relationships, including but not limited to sexual intimacy. This requires being in touch emotionally with self and others. In trauma involving loss, fear of further loss may impair intimacy with others. Difficulty with self-intimacy impairs commitment to life’s goals and prompts unhelpful avoidance behaviors, such as difficulty being alone or self-injurious use of drugs or alcohol. Comfort in spending some portion of time alone with one’s thoughts and emotions is a life skill necessary to attain optimum function. Patients who are unable to tolerate their own emotions without constant company might have excessive anxiety when social supports are otherwise occupied. Such patients might seek excessive and repeated reassurance rather than learning to tolerate their own emotions and thoughts. They would then find it difficult to engage successfully in solo activities.
Safety. After trauma, patients may view themselves and others as unsafe, and may overestimate risk. For example, a pedestrian who is struck by a vehicle may believe that crossing a street will again result in getting hit by a car without appreciating that people frequently cross streets without injury or that crossing cautiously is an essential life skill. Parents who have suffered from trauma may unduly believe that their children are in danger when engaging in an activity generally considered to be safe. This may create challenges in parenting and impede their children’s ability to develop a sense of independence.
Trust. Trauma victims may unfairly blame themselves, leading them to mistrust their own judgment. Such patients may have difficulty making decisions confidently and independently, such as choosing a job or a romantic partner. When traumatized by another person or people, it can be difficult to maintain positive views of others or to accept others’ positive behaviors as genuine. For example, a common reaction following rape may be a generalized mistrust of all men.
Esteem. Patients’ self-esteem may suffer following trauma due to irrational self-blame or believing the “just world hypothesis”—the idea that bad things only happen to bad people. For example, a patient who suffers an assault by an acquaintance might think “I must be stupid if I couldn’t figure out that my friend was dangerous.”
Power. Traumatic events usually occur outside of one’s control. Survivors of trauma may lose confidence in their ability to control any aspect of their lives. Conversely, they may attempt to gain control of all of life’s circumstances, including those that are beyond anyone’s control. Control can be applied to emotions, behaviors, or events. A driver struck by a vehicle may think “I can’t control other drivers, so I have no power to control my safety while driving,” and hence give up driving. While there are things that are beyond our control, this extreme thought ignores things that we can control, such as wearing seatbelts or having the vehicle’s brakes regularly serviced.
Evidence-based cognitive-behavioral therapies for posttraumatic stress disorder (PTSD) may employ cognitive restructuring. This psychotherapeutic technique entails recognizing and correcting maladaptive, inaccurate thoughts that perpetuate illness.1 For example, a clinician helps a patient recognize that the negative thought “Nobody loves me” following a romantic breakup is an overgeneralization. The patient is taught to self-correct this to “While my ex-girlfriend doesn’t love me, others do. It only feels like nobody loves me.”2
We introduce the acronym I STEP to help clinicians recognize several common distorted thoughts in PTSD. These tend to occur within stereotyped themes in PTSD,3 as outlined and illustrated below. Recognizing distorted thoughts in these patients will help clinicians understand and address psychological distress following trauma.
Intimacy/In-touch. Intimacy involves comfort in relationships, including but not limited to sexual intimacy. This requires being in touch emotionally with self and others. In trauma involving loss, fear of further loss may impair intimacy with others. Difficulty with self-intimacy impairs commitment to life’s goals and prompts unhelpful avoidance behaviors, such as difficulty being alone or self-injurious use of drugs or alcohol. Comfort in spending some portion of time alone with one’s thoughts and emotions is a life skill necessary to attain optimum function. Patients who are unable to tolerate their own emotions without constant company might have excessive anxiety when social supports are otherwise occupied. Such patients might seek excessive and repeated reassurance rather than learning to tolerate their own emotions and thoughts. They would then find it difficult to engage successfully in solo activities.
Safety. After trauma, patients may view themselves and others as unsafe, and may overestimate risk. For example, a pedestrian who is struck by a vehicle may believe that crossing a street will again result in getting hit by a car without appreciating that people frequently cross streets without injury or that crossing cautiously is an essential life skill. Parents who have suffered from trauma may unduly believe that their children are in danger when engaging in an activity generally considered to be safe. This may create challenges in parenting and impede their children’s ability to develop a sense of independence.
Trust. Trauma victims may unfairly blame themselves, leading them to mistrust their own judgment. Such patients may have difficulty making decisions confidently and independently, such as choosing a job or a romantic partner. When traumatized by another person or people, it can be difficult to maintain positive views of others or to accept others’ positive behaviors as genuine. For example, a common reaction following rape may be a generalized mistrust of all men.
Esteem. Patients’ self-esteem may suffer following trauma due to irrational self-blame or believing the “just world hypothesis”—the idea that bad things only happen to bad people. For example, a patient who suffers an assault by an acquaintance might think “I must be stupid if I couldn’t figure out that my friend was dangerous.”
Power. Traumatic events usually occur outside of one’s control. Survivors of trauma may lose confidence in their ability to control any aspect of their lives. Conversely, they may attempt to gain control of all of life’s circumstances, including those that are beyond anyone’s control. Control can be applied to emotions, behaviors, or events. A driver struck by a vehicle may think “I can’t control other drivers, so I have no power to control my safety while driving,” and hence give up driving. While there are things that are beyond our control, this extreme thought ignores things that we can control, such as wearing seatbelts or having the vehicle’s brakes regularly serviced.
1. Wenzel A. Basic strategies of cognitive behavioral therapy. Psychiatr Clin North Am. 2017;40(4):597-609.
2. Beck J. Cognitive behavioral therapy: basics and beyond. 2nd ed. The Guilford Press; 2011.
3. Resick PA, Monson CM, Chard KM. Cognitive processing therapy for PTSD. A comprehensive manual. The Guilford Press; 2017.
1. Wenzel A. Basic strategies of cognitive behavioral therapy. Psychiatr Clin North Am. 2017;40(4):597-609.
2. Beck J. Cognitive behavioral therapy: basics and beyond. 2nd ed. The Guilford Press; 2011.
3. Resick PA, Monson CM, Chard KM. Cognitive processing therapy for PTSD. A comprehensive manual. The Guilford Press; 2017.