User login
Cannabis and schizophrenia: A complex relationship
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Symptoms of psychosis and OCD in a patient with postpartum depression
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.
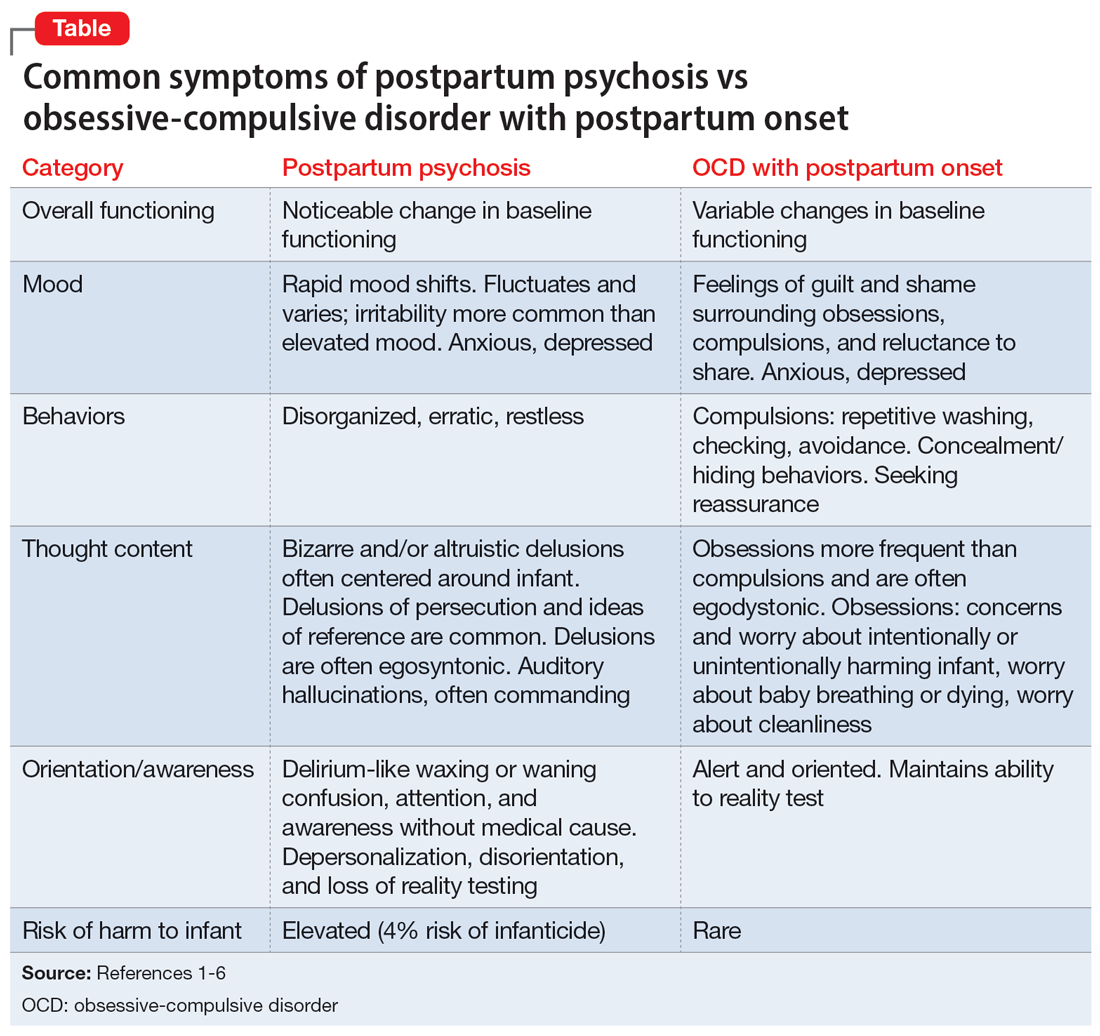
Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.
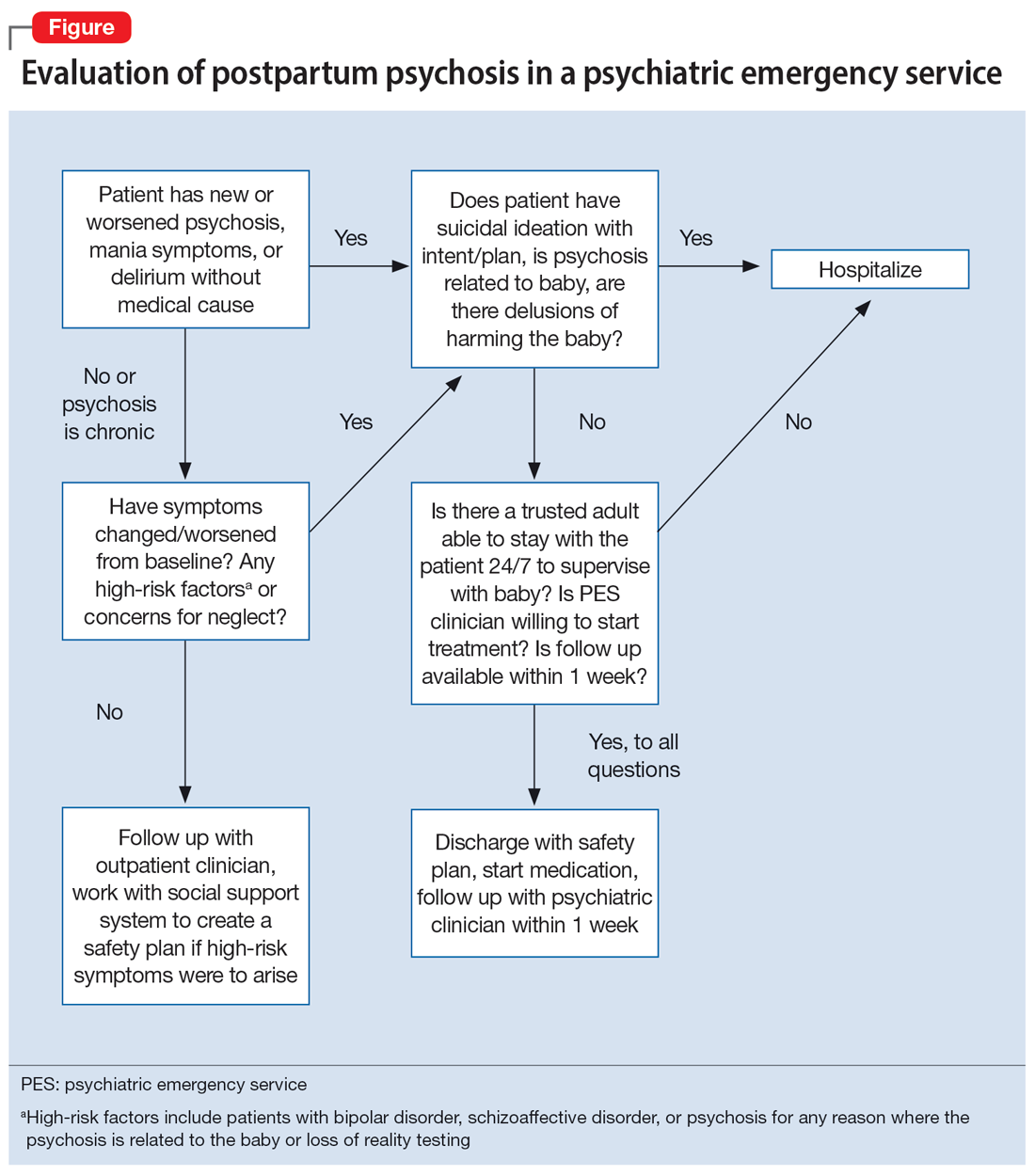
If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
Black psychiatric inpatients more likely to be restrained and for longer
TOPLINE:
, new research suggests.
METHODOLOGY:
- The study, part of a larger retrospective chart review of inpatient psychiatric electronic medical records (EMRs), included 29,739 adolescents (aged 12-17 years) and adults admitted because of severe and disruptive psychiatric illness or concerns about self-harm.
- A restraint event was defined as a physician-ordered physical or mechanical hold in which patients are unable to move their limbs, body, or head or are given medication to restrict their movement.
- Researchers used scores on the Dynamic Appraisal of Situational Aggression (DASA) at admission to assess risk for aggression among high-risk psychiatric inpatients (scores ranged from a low of 0 to a high of 7).
- From restraint event data extracted from the EMR, researchers investigated whether restraint frequency or duration was affected by “adultification,” a form of racial bias in which adolescents are perceived as being older than their actual age and are treated accordingly.
TAKEAWAY:
- Of the entire cohort, 1867 (6.3%) experienced a restraint event, and 27,872 (93.7%) did not.
- Compared with White patients, restraint was 85% more likely among Black patients (adjusted odds ratio, 1.85; P < .001) and 36% more likely among multiracial patients (aOR, 1.36; P = .006), which researchers suggest may reflect systemic racism within psychiatry and medicine, as well as an implicit or learned perception that people of color are more aggressive and dangerous.
- Lower DASA score at admission (P = .001), shorter length of stay (P < .001), adult age (P = .001), female sex (P = .042), and Black race, compared with White race (P = .001), were significantly associated with longer restraint duration, which may serve as a proxy for psychiatric symptom severity.
- Neither age group alone (adolescent or adult) nor the interaction of race and age group was significantly associated with experiencing a restraint event, suggesting no evidence of “adultification.”
IN PRACTICE:
It’s important to raise awareness about racial differences in restraint events in inpatient psychiatric settings, the authors write, adding that addressing overcrowding and investing in bias assessment and restraint education may reduce bias in the care of agitated patients and the use of restraints.
SOURCE:
The study was carried out by Sonali Singal, BS, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., and colleagues. It was published online in Psychiatric Services.
LIMITATIONS:
The variables analyzed in the study were limited by the retrospective chart review and by the available EMR data, which may have contained entry errors. Although the investigators used DASA scores to control for differences in aggression, they could not control for symptom severity. The study could not examine the impact of race on seclusion (involuntary confinement), a variable often examined in tandem with restraint, because there were too few such events. The analysis also did not control for substance use disorder, which can influence a patient’s behavior and be related to restraint use.
DISCLOSURES:
Ms. Singal reported no relevant financial relationships. The original article has disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- The study, part of a larger retrospective chart review of inpatient psychiatric electronic medical records (EMRs), included 29,739 adolescents (aged 12-17 years) and adults admitted because of severe and disruptive psychiatric illness or concerns about self-harm.
- A restraint event was defined as a physician-ordered physical or mechanical hold in which patients are unable to move their limbs, body, or head or are given medication to restrict their movement.
- Researchers used scores on the Dynamic Appraisal of Situational Aggression (DASA) at admission to assess risk for aggression among high-risk psychiatric inpatients (scores ranged from a low of 0 to a high of 7).
- From restraint event data extracted from the EMR, researchers investigated whether restraint frequency or duration was affected by “adultification,” a form of racial bias in which adolescents are perceived as being older than their actual age and are treated accordingly.
TAKEAWAY:
- Of the entire cohort, 1867 (6.3%) experienced a restraint event, and 27,872 (93.7%) did not.
- Compared with White patients, restraint was 85% more likely among Black patients (adjusted odds ratio, 1.85; P < .001) and 36% more likely among multiracial patients (aOR, 1.36; P = .006), which researchers suggest may reflect systemic racism within psychiatry and medicine, as well as an implicit or learned perception that people of color are more aggressive and dangerous.
- Lower DASA score at admission (P = .001), shorter length of stay (P < .001), adult age (P = .001), female sex (P = .042), and Black race, compared with White race (P = .001), were significantly associated with longer restraint duration, which may serve as a proxy for psychiatric symptom severity.
- Neither age group alone (adolescent or adult) nor the interaction of race and age group was significantly associated with experiencing a restraint event, suggesting no evidence of “adultification.”
IN PRACTICE:
It’s important to raise awareness about racial differences in restraint events in inpatient psychiatric settings, the authors write, adding that addressing overcrowding and investing in bias assessment and restraint education may reduce bias in the care of agitated patients and the use of restraints.
SOURCE:
The study was carried out by Sonali Singal, BS, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., and colleagues. It was published online in Psychiatric Services.
LIMITATIONS:
The variables analyzed in the study were limited by the retrospective chart review and by the available EMR data, which may have contained entry errors. Although the investigators used DASA scores to control for differences in aggression, they could not control for symptom severity. The study could not examine the impact of race on seclusion (involuntary confinement), a variable often examined in tandem with restraint, because there were too few such events. The analysis also did not control for substance use disorder, which can influence a patient’s behavior and be related to restraint use.
DISCLOSURES:
Ms. Singal reported no relevant financial relationships. The original article has disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- The study, part of a larger retrospective chart review of inpatient psychiatric electronic medical records (EMRs), included 29,739 adolescents (aged 12-17 years) and adults admitted because of severe and disruptive psychiatric illness or concerns about self-harm.
- A restraint event was defined as a physician-ordered physical or mechanical hold in which patients are unable to move their limbs, body, or head or are given medication to restrict their movement.
- Researchers used scores on the Dynamic Appraisal of Situational Aggression (DASA) at admission to assess risk for aggression among high-risk psychiatric inpatients (scores ranged from a low of 0 to a high of 7).
- From restraint event data extracted from the EMR, researchers investigated whether restraint frequency or duration was affected by “adultification,” a form of racial bias in which adolescents are perceived as being older than their actual age and are treated accordingly.
TAKEAWAY:
- Of the entire cohort, 1867 (6.3%) experienced a restraint event, and 27,872 (93.7%) did not.
- Compared with White patients, restraint was 85% more likely among Black patients (adjusted odds ratio, 1.85; P < .001) and 36% more likely among multiracial patients (aOR, 1.36; P = .006), which researchers suggest may reflect systemic racism within psychiatry and medicine, as well as an implicit or learned perception that people of color are more aggressive and dangerous.
- Lower DASA score at admission (P = .001), shorter length of stay (P < .001), adult age (P = .001), female sex (P = .042), and Black race, compared with White race (P = .001), were significantly associated with longer restraint duration, which may serve as a proxy for psychiatric symptom severity.
- Neither age group alone (adolescent or adult) nor the interaction of race and age group was significantly associated with experiencing a restraint event, suggesting no evidence of “adultification.”
IN PRACTICE:
It’s important to raise awareness about racial differences in restraint events in inpatient psychiatric settings, the authors write, adding that addressing overcrowding and investing in bias assessment and restraint education may reduce bias in the care of agitated patients and the use of restraints.
SOURCE:
The study was carried out by Sonali Singal, BS, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., and colleagues. It was published online in Psychiatric Services.
LIMITATIONS:
The variables analyzed in the study were limited by the retrospective chart review and by the available EMR data, which may have contained entry errors. Although the investigators used DASA scores to control for differences in aggression, they could not control for symptom severity. The study could not examine the impact of race on seclusion (involuntary confinement), a variable often examined in tandem with restraint, because there were too few such events. The analysis also did not control for substance use disorder, which can influence a patient’s behavior and be related to restraint use.
DISCLOSURES:
Ms. Singal reported no relevant financial relationships. The original article has disclosures of other authors.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES
Managing psychotropic-induced hyperhidrosis
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.
The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.
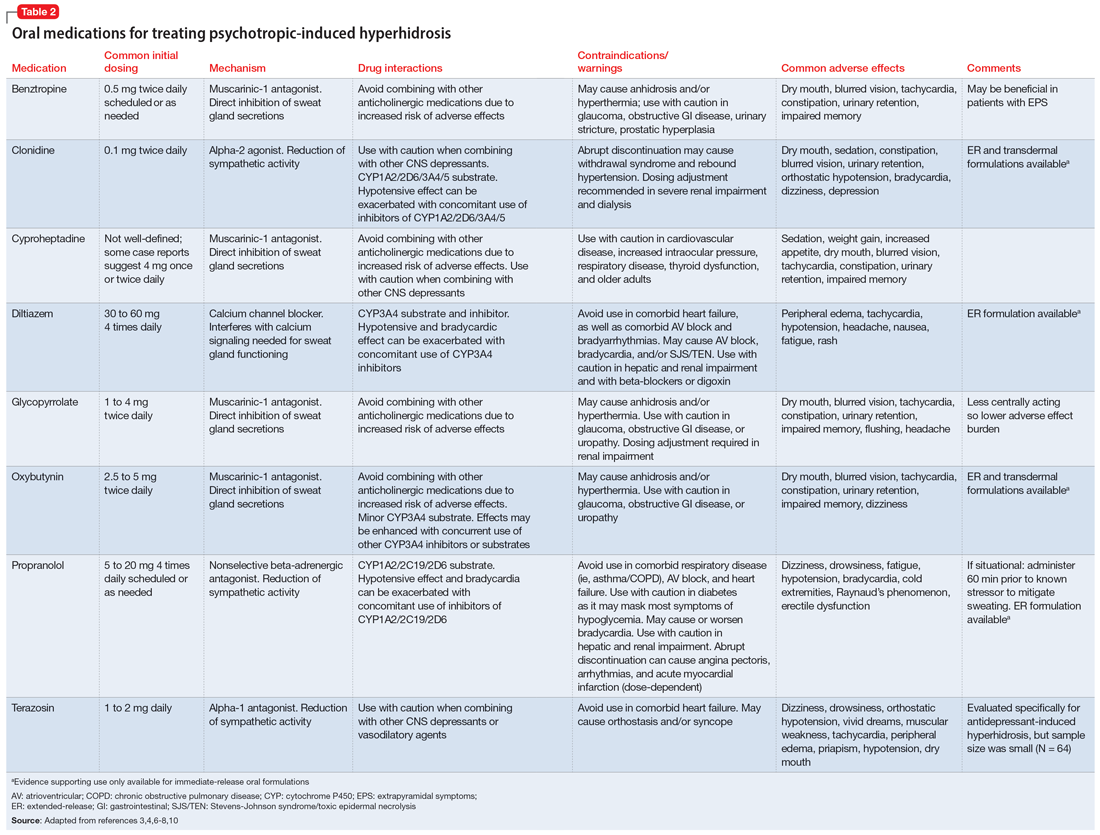
Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Interviewing a patient experiencing psychosis
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
Substance-induced psychosis tied to schizophrenia risk
TOPLINE:
Three years after an initial ER visit, 18.5% of those with substance-induced psychosis were diagnosed with an SSD. Cannabis-induced psychosis was associated with the greatest risk.
METHODOLOGY:
- In this retrospective, population-based cohort study, investigators evaluated the risk of transition to a diagnosis of SSD for individuals with an ER visit for substance use versus the general population.
- Investigators at The Ottawa Hospital and the Institute for Clinical Evaluative Sciences, both in Ontario, analyzed data from six linked databases containing health information on nearly 10 million Ontario residents aged 14-65 years eligible for medical coverage.
- Investigators collected the health data between January 2008 and March 2022 on residents with substance use–related ER visits with, and without, psychosis.
TAKEAWAY:
- There were nearly 408,000 individuals with an ER visit for substance use, of which 13,800 (3.4%) of the visits were for substance-induced psychosis.
- Individuals with substance-induced psychosis were at a 163-fold (age- and sex-adjusted hazard ratio, 163.2; 95% confidence interval, 156.1-170.5) increased risk of transitioning to an SSD, relative to the general population (3-year risk, 18.5% vs. 0.1%).
- Individuals with an ER visit for substance use without psychosis had a lower relative risk of transitioning (aHR, 9.8; 95% CI, 9.5-10.2; 3-year risk, 1.4%) but incurred more than three times the absolute number of transitions (9,969 vs. 3,029).
- ER visits related to cannabis use had the highest transition risk among visits with psychosis (aHR, 241.6; 95% CI, 225.5-258.9) and the third-highest risk among visits without psychosis (aHR, 14.3; 95% CI, 13.5-15.2).
- Younger age and male sex were associated with a higher risk of transition, and the risk of male sex was greater in younger, compared with older, individuals particularly for cannabis use.
IN PRACTICE:
“Primary prevention efforts aimed at reducing substance use and substance use disorders could substantially reduce the population-level burden of chronic psychoses,” the investigators write. “Our findings also highlight the need for targeted secondary prevention providing early intervention and reducing substance use in the highest-risk groups, which may delay or prevent transition to schizophrenia spectrum disorders.”
SOURCE:
Daniel T. Myran, MD, MPH, of the Ottawa Hospital Research Institute, led the study, which was funded by the Canadian Institutes of Health Research and the University of Ottawa department of family medicine. The study was published online in JAMA Psychiatry.
LIMITATIONS:
Investigators did not have access to detailed data on substance-related outpatient visits or patterns of substance use, which could provide additional prognostic information.
DISCLOSURES:
Dr. Myran reported receiving grants from the Canadian Institutes of Health Research during the conduct of the study. Dr. Solmi reported receiving honoraria for participation on advisory boards or presentations from AbbVie, Angelini, Lundbeck, and Otsuka outside the submitted work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Three years after an initial ER visit, 18.5% of those with substance-induced psychosis were diagnosed with an SSD. Cannabis-induced psychosis was associated with the greatest risk.
METHODOLOGY:
- In this retrospective, population-based cohort study, investigators evaluated the risk of transition to a diagnosis of SSD for individuals with an ER visit for substance use versus the general population.
- Investigators at The Ottawa Hospital and the Institute for Clinical Evaluative Sciences, both in Ontario, analyzed data from six linked databases containing health information on nearly 10 million Ontario residents aged 14-65 years eligible for medical coverage.
- Investigators collected the health data between January 2008 and March 2022 on residents with substance use–related ER visits with, and without, psychosis.
TAKEAWAY:
- There were nearly 408,000 individuals with an ER visit for substance use, of which 13,800 (3.4%) of the visits were for substance-induced psychosis.
- Individuals with substance-induced psychosis were at a 163-fold (age- and sex-adjusted hazard ratio, 163.2; 95% confidence interval, 156.1-170.5) increased risk of transitioning to an SSD, relative to the general population (3-year risk, 18.5% vs. 0.1%).
- Individuals with an ER visit for substance use without psychosis had a lower relative risk of transitioning (aHR, 9.8; 95% CI, 9.5-10.2; 3-year risk, 1.4%) but incurred more than three times the absolute number of transitions (9,969 vs. 3,029).
- ER visits related to cannabis use had the highest transition risk among visits with psychosis (aHR, 241.6; 95% CI, 225.5-258.9) and the third-highest risk among visits without psychosis (aHR, 14.3; 95% CI, 13.5-15.2).
- Younger age and male sex were associated with a higher risk of transition, and the risk of male sex was greater in younger, compared with older, individuals particularly for cannabis use.
IN PRACTICE:
“Primary prevention efforts aimed at reducing substance use and substance use disorders could substantially reduce the population-level burden of chronic psychoses,” the investigators write. “Our findings also highlight the need for targeted secondary prevention providing early intervention and reducing substance use in the highest-risk groups, which may delay or prevent transition to schizophrenia spectrum disorders.”
SOURCE:
Daniel T. Myran, MD, MPH, of the Ottawa Hospital Research Institute, led the study, which was funded by the Canadian Institutes of Health Research and the University of Ottawa department of family medicine. The study was published online in JAMA Psychiatry.
LIMITATIONS:
Investigators did not have access to detailed data on substance-related outpatient visits or patterns of substance use, which could provide additional prognostic information.
DISCLOSURES:
Dr. Myran reported receiving grants from the Canadian Institutes of Health Research during the conduct of the study. Dr. Solmi reported receiving honoraria for participation on advisory boards or presentations from AbbVie, Angelini, Lundbeck, and Otsuka outside the submitted work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Three years after an initial ER visit, 18.5% of those with substance-induced psychosis were diagnosed with an SSD. Cannabis-induced psychosis was associated with the greatest risk.
METHODOLOGY:
- In this retrospective, population-based cohort study, investigators evaluated the risk of transition to a diagnosis of SSD for individuals with an ER visit for substance use versus the general population.
- Investigators at The Ottawa Hospital and the Institute for Clinical Evaluative Sciences, both in Ontario, analyzed data from six linked databases containing health information on nearly 10 million Ontario residents aged 14-65 years eligible for medical coverage.
- Investigators collected the health data between January 2008 and March 2022 on residents with substance use–related ER visits with, and without, psychosis.
TAKEAWAY:
- There were nearly 408,000 individuals with an ER visit for substance use, of which 13,800 (3.4%) of the visits were for substance-induced psychosis.
- Individuals with substance-induced psychosis were at a 163-fold (age- and sex-adjusted hazard ratio, 163.2; 95% confidence interval, 156.1-170.5) increased risk of transitioning to an SSD, relative to the general population (3-year risk, 18.5% vs. 0.1%).
- Individuals with an ER visit for substance use without psychosis had a lower relative risk of transitioning (aHR, 9.8; 95% CI, 9.5-10.2; 3-year risk, 1.4%) but incurred more than three times the absolute number of transitions (9,969 vs. 3,029).
- ER visits related to cannabis use had the highest transition risk among visits with psychosis (aHR, 241.6; 95% CI, 225.5-258.9) and the third-highest risk among visits without psychosis (aHR, 14.3; 95% CI, 13.5-15.2).
- Younger age and male sex were associated with a higher risk of transition, and the risk of male sex was greater in younger, compared with older, individuals particularly for cannabis use.
IN PRACTICE:
“Primary prevention efforts aimed at reducing substance use and substance use disorders could substantially reduce the population-level burden of chronic psychoses,” the investigators write. “Our findings also highlight the need for targeted secondary prevention providing early intervention and reducing substance use in the highest-risk groups, which may delay or prevent transition to schizophrenia spectrum disorders.”
SOURCE:
Daniel T. Myran, MD, MPH, of the Ottawa Hospital Research Institute, led the study, which was funded by the Canadian Institutes of Health Research and the University of Ottawa department of family medicine. The study was published online in JAMA Psychiatry.
LIMITATIONS:
Investigators did not have access to detailed data on substance-related outpatient visits or patterns of substance use, which could provide additional prognostic information.
DISCLOSURES:
Dr. Myran reported receiving grants from the Canadian Institutes of Health Research during the conduct of the study. Dr. Solmi reported receiving honoraria for participation on advisory boards or presentations from AbbVie, Angelini, Lundbeck, and Otsuka outside the submitted work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
New insight into genetic link between schizophrenia and CVD
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Auditory hallucinations in a patient who is hearing impaired
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.
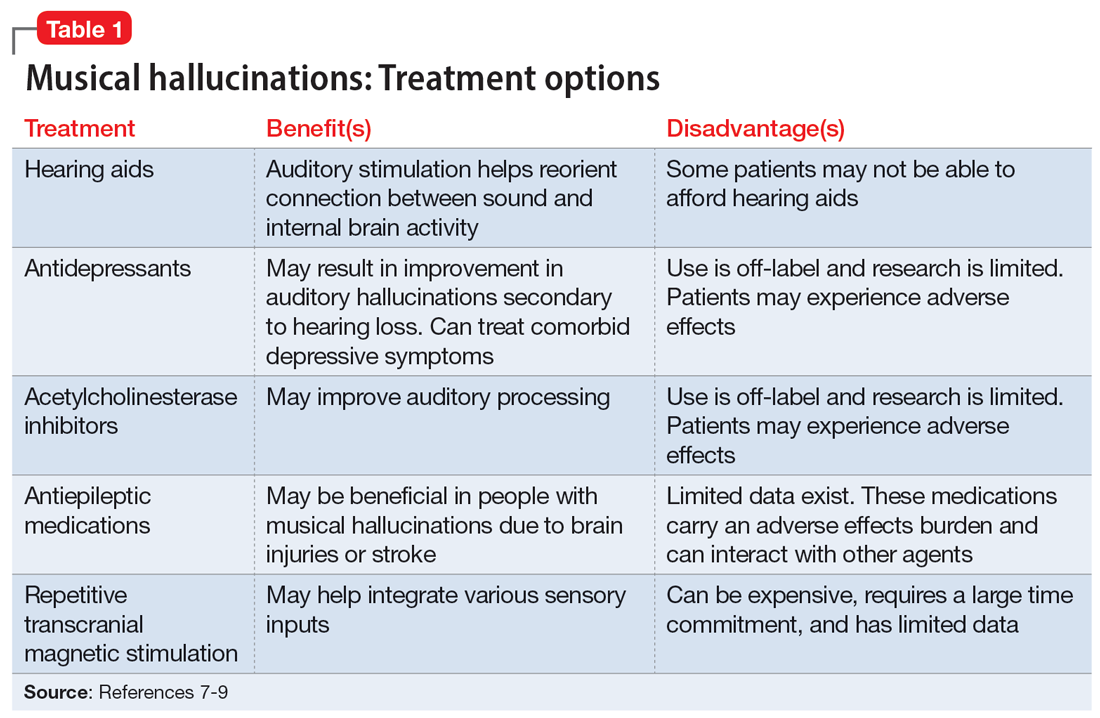
Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11
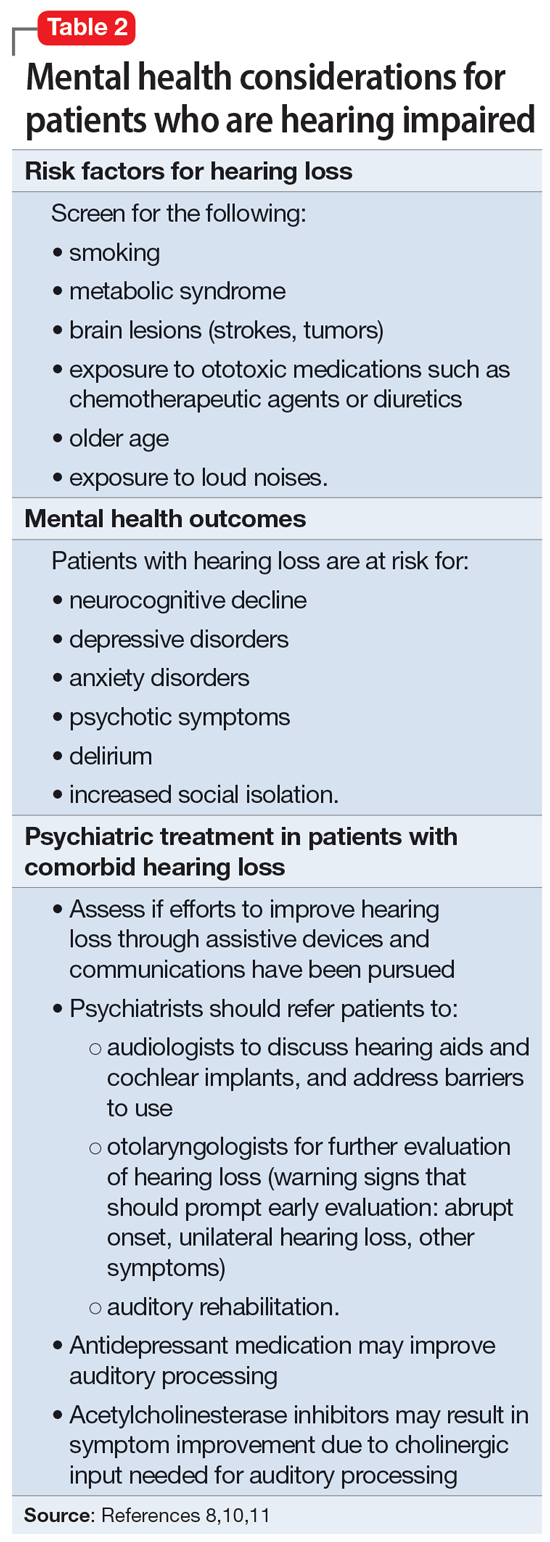
Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11
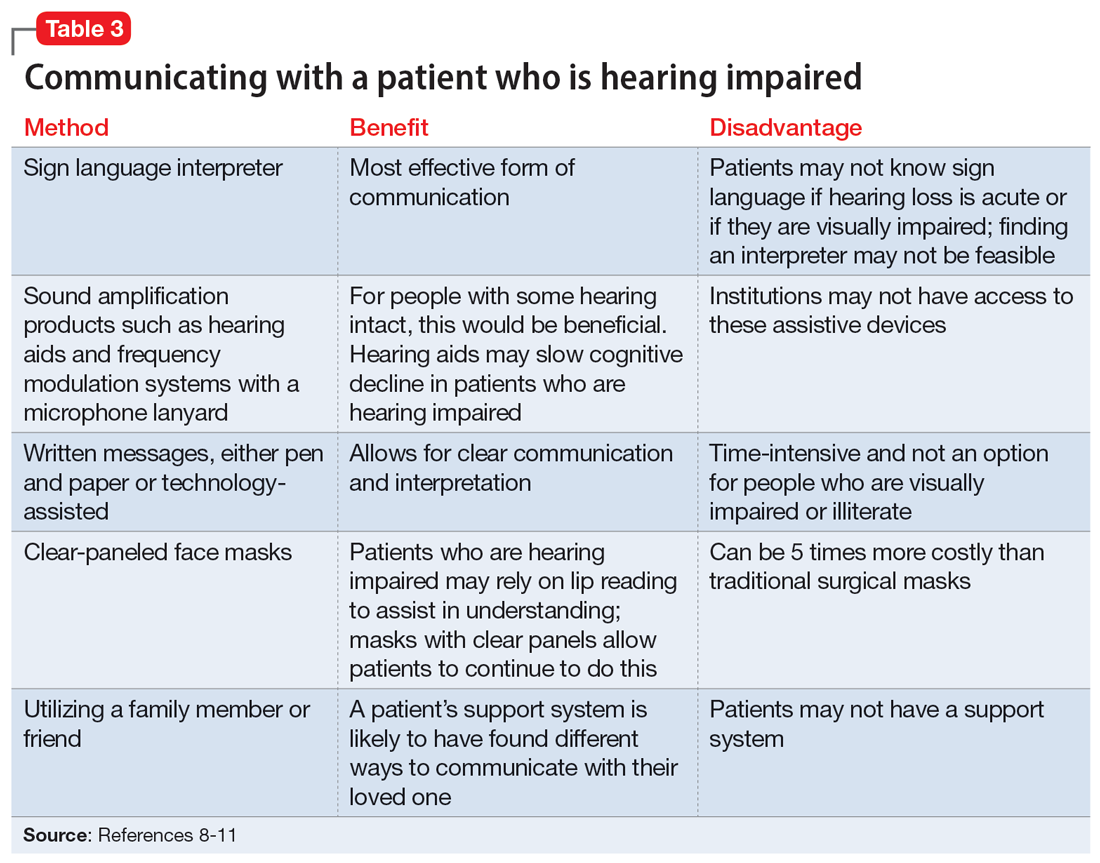
OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
A street medicine view of tobacco use in patients with schizophrenia
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
Neuropsychiatric aspects of Parkinson’s disease: Practical considerations
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).
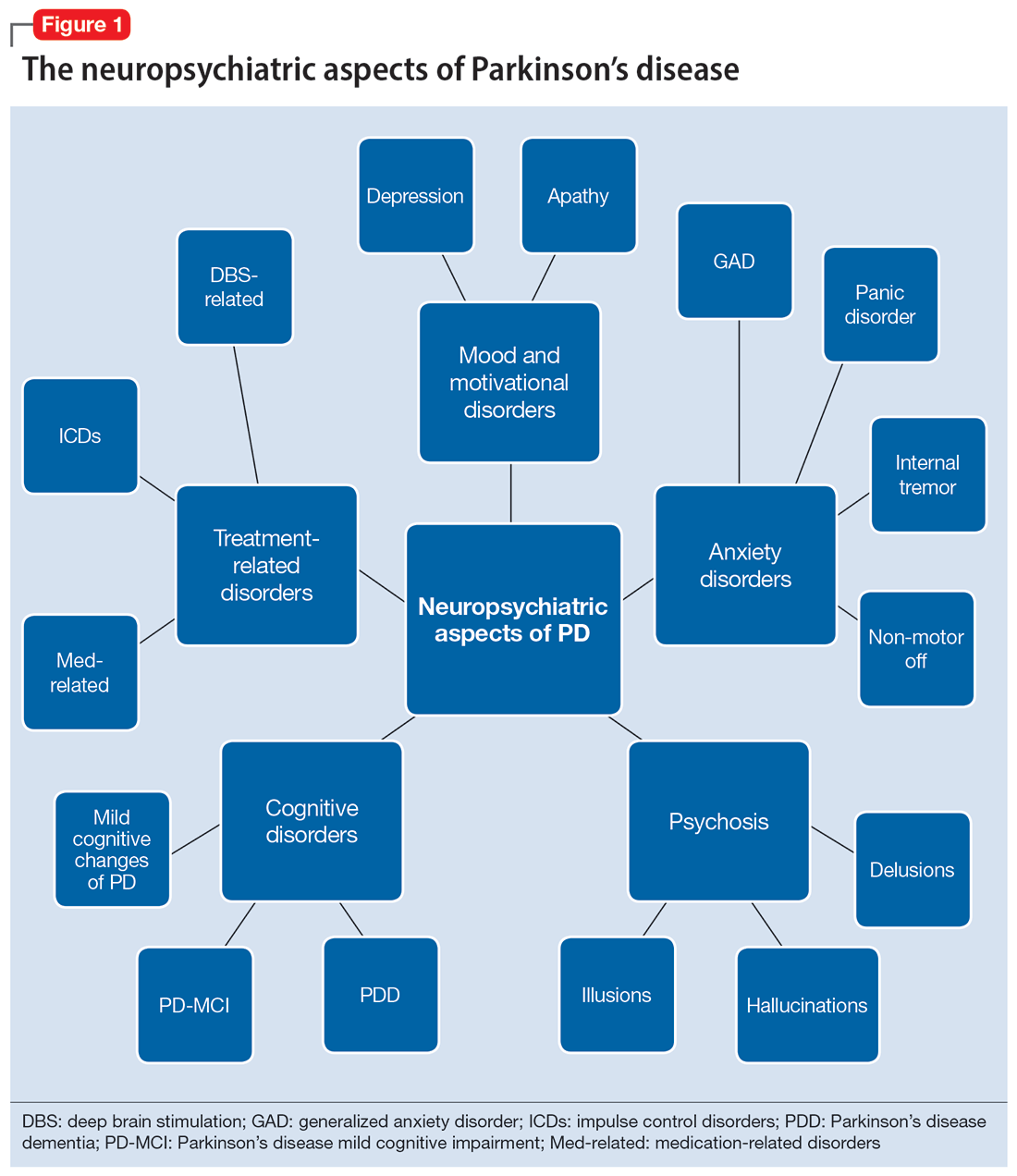
Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32
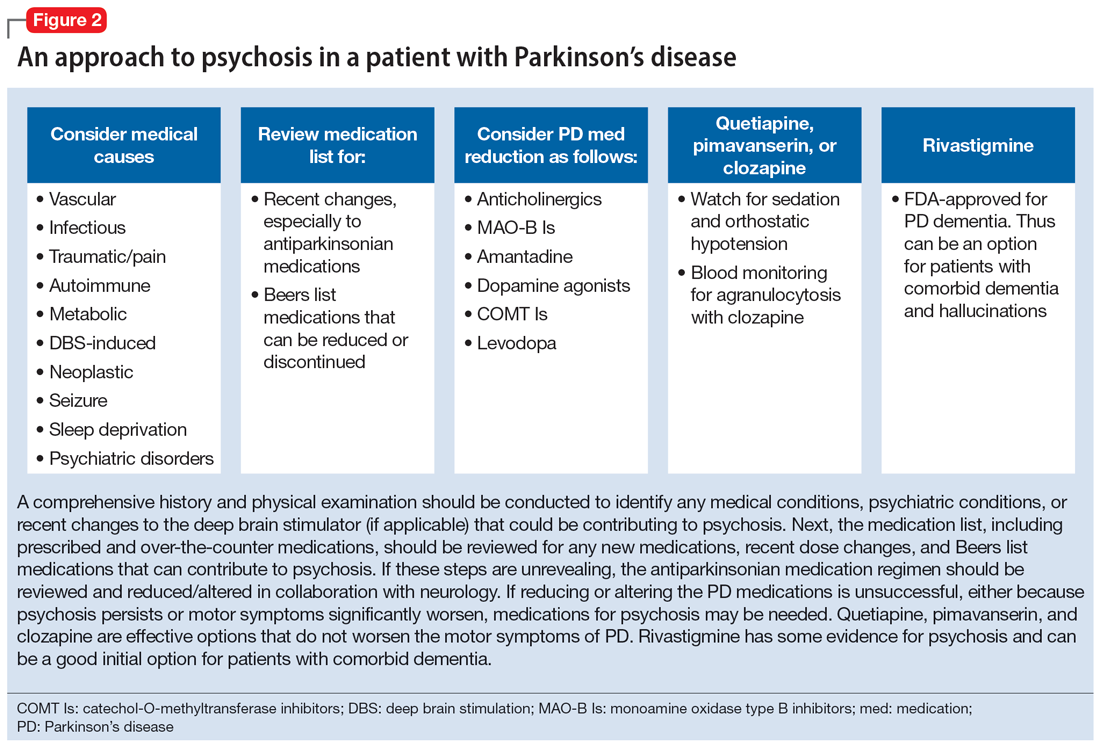
Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36
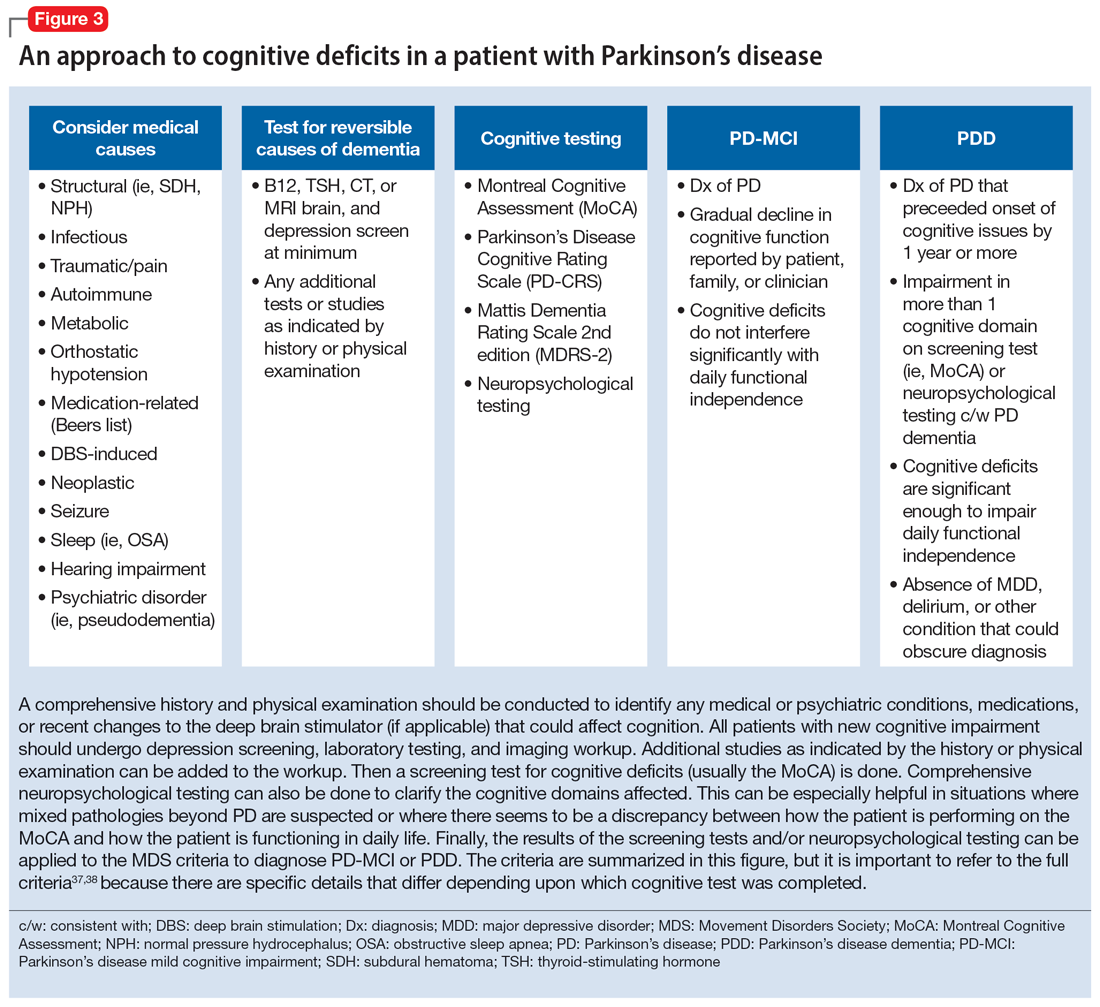
Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42
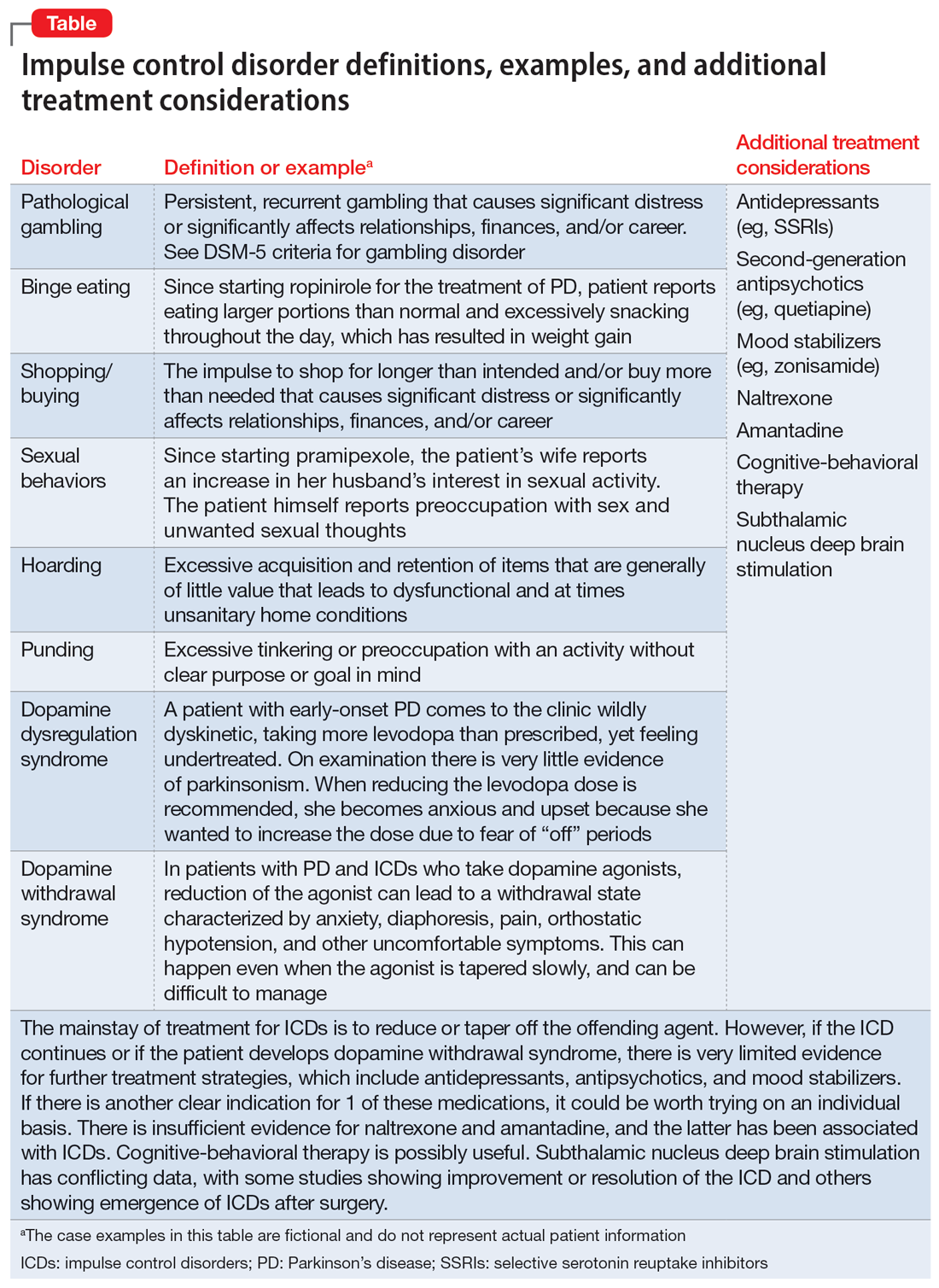
The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.