User login
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
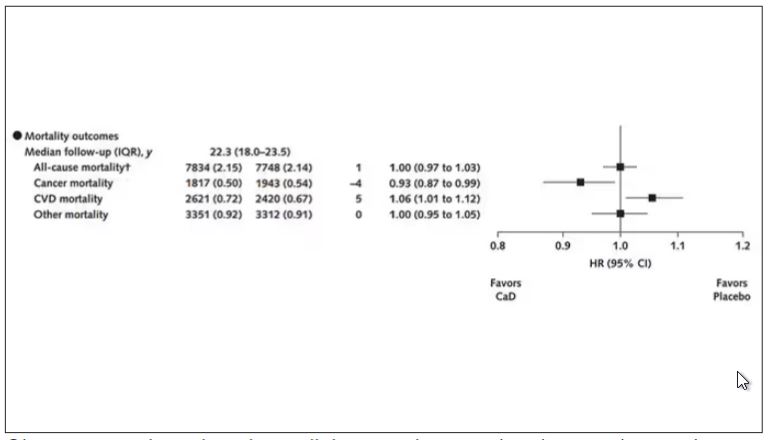
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
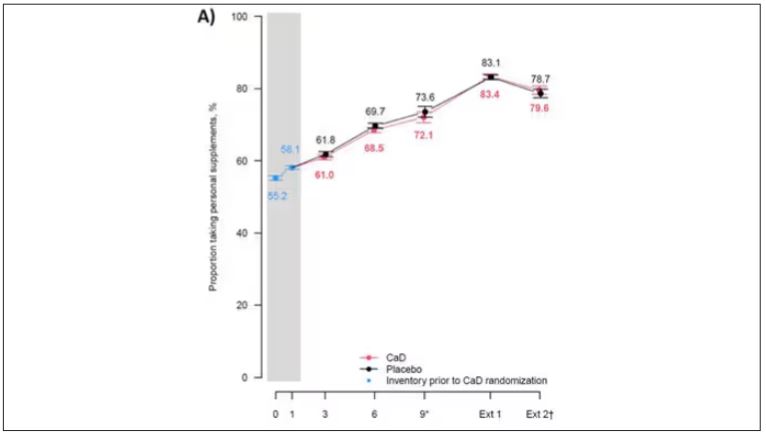
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
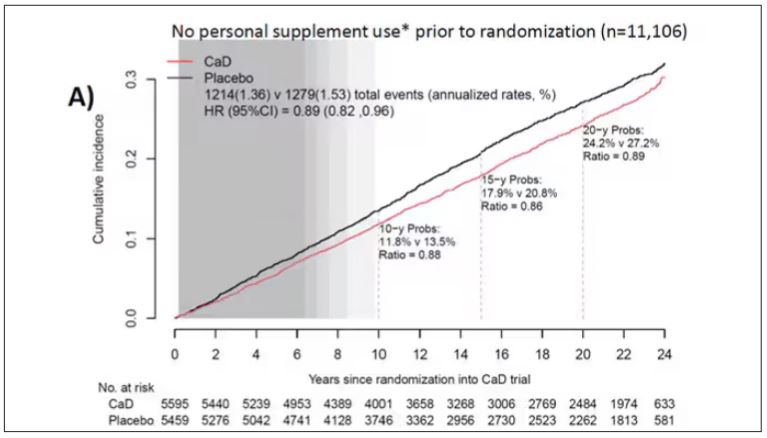
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
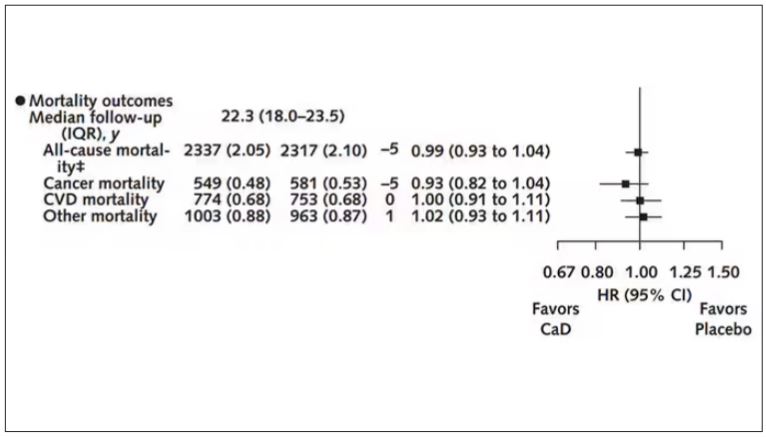
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
Survival Advantage of Adjuvant IO ‘Big News’ in Renal Cancer
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
Attrition in Youth Sports
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
COVID-19 Is a Very Weird Virus
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.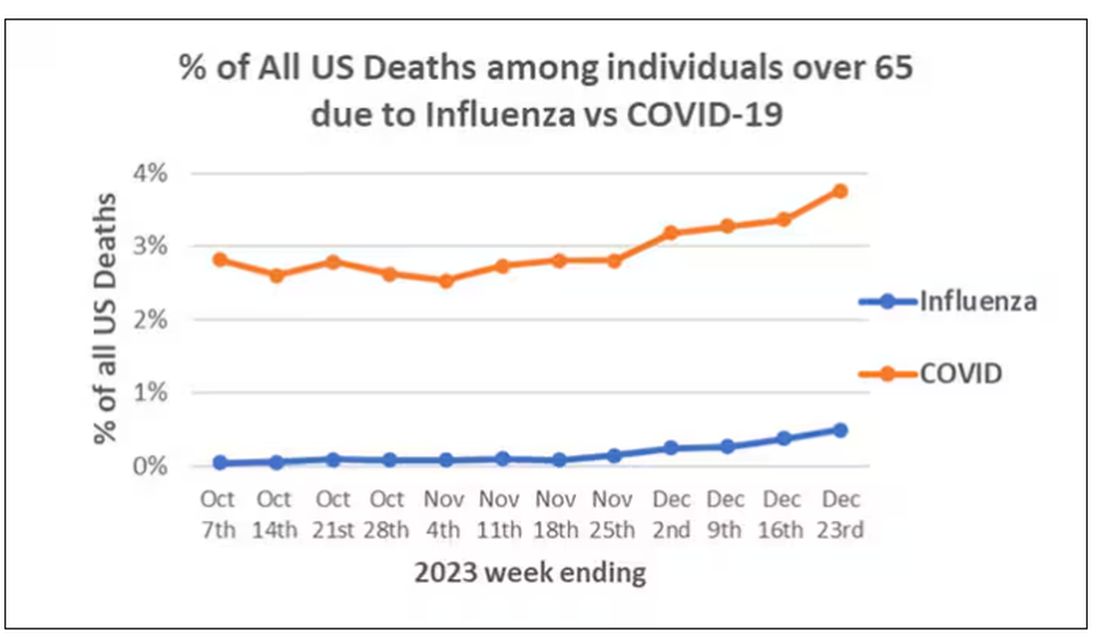
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)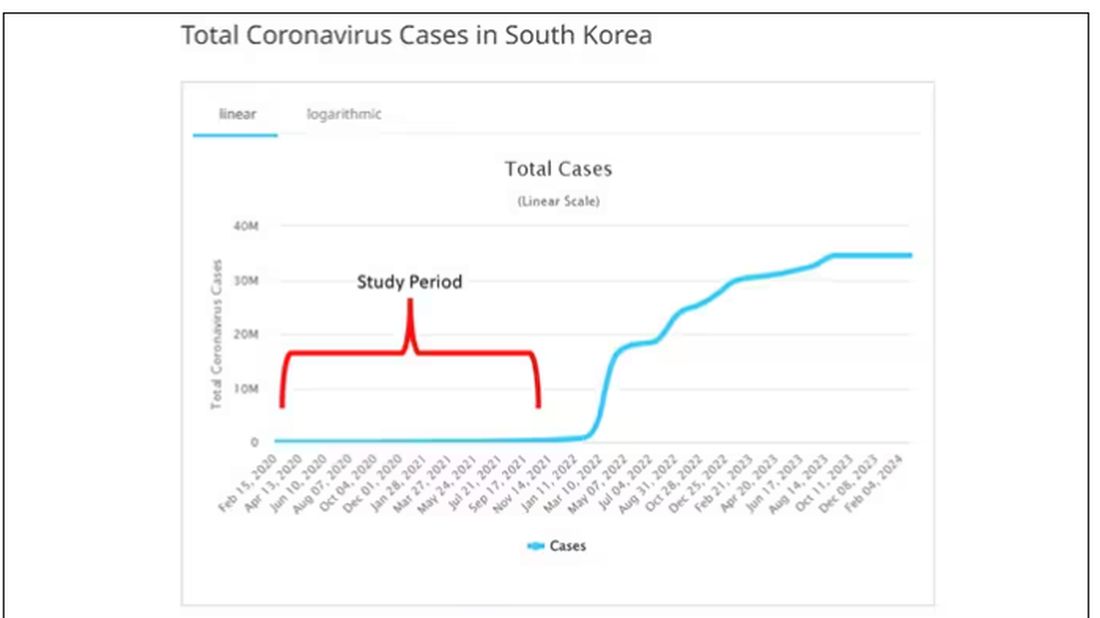
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.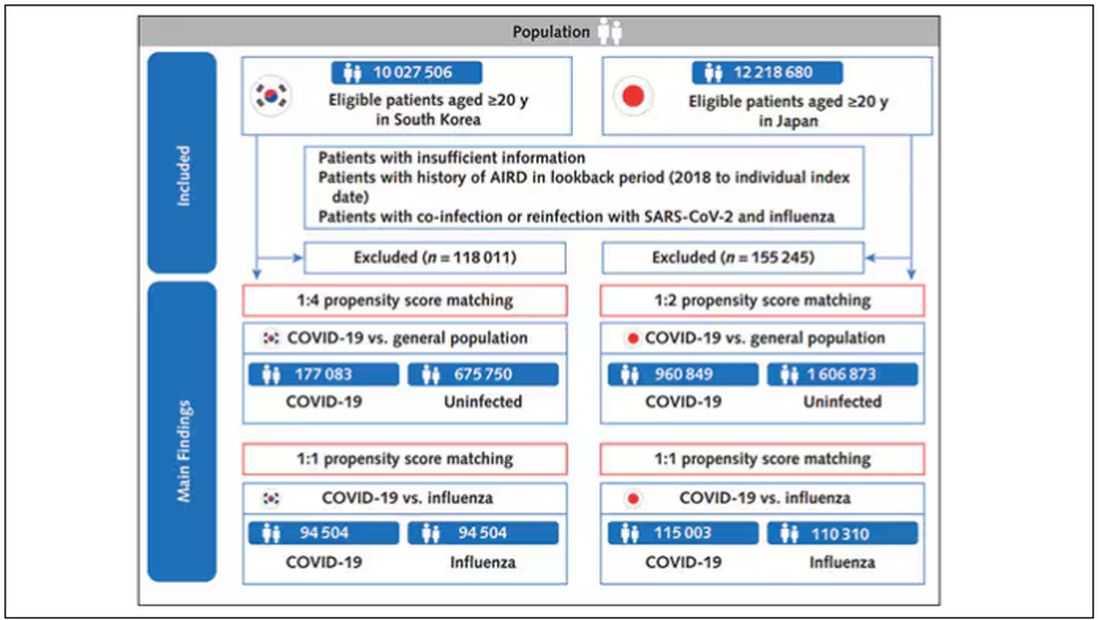
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).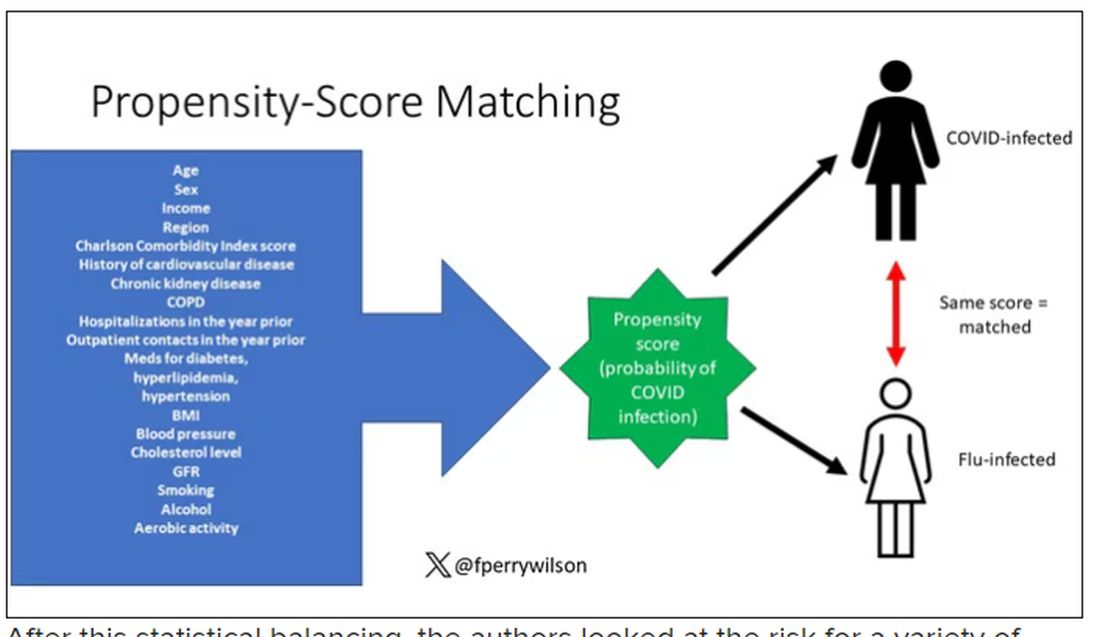
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.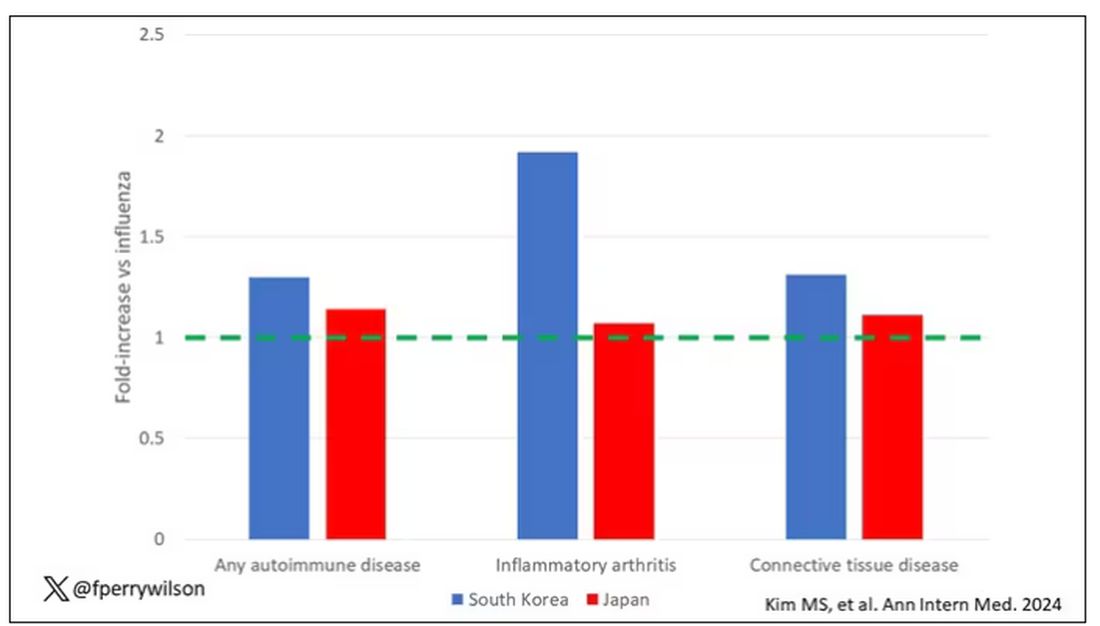
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.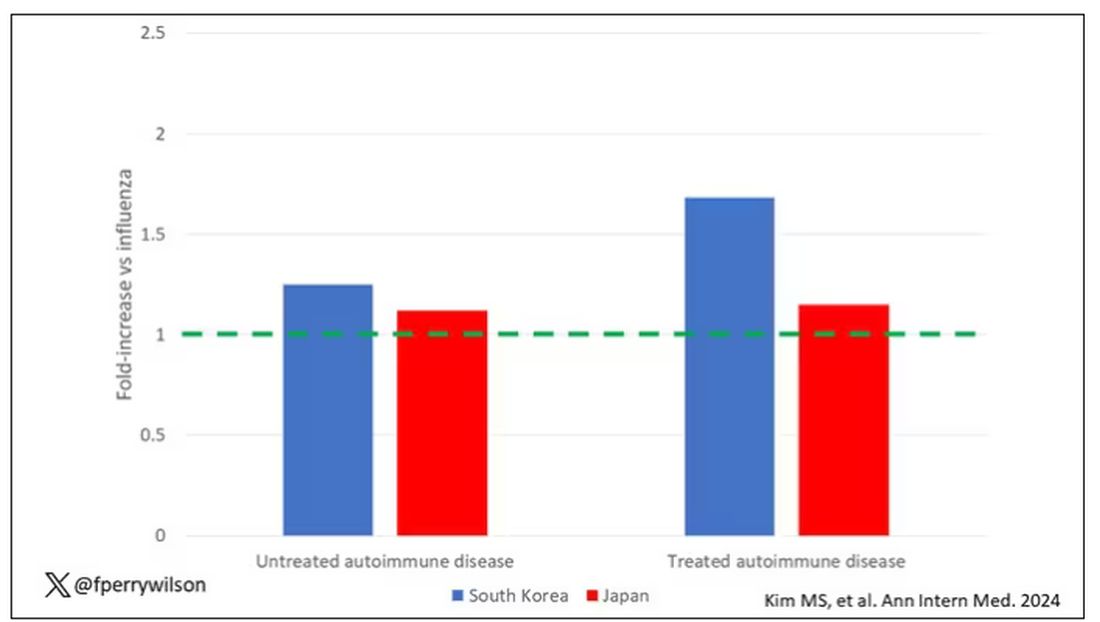
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.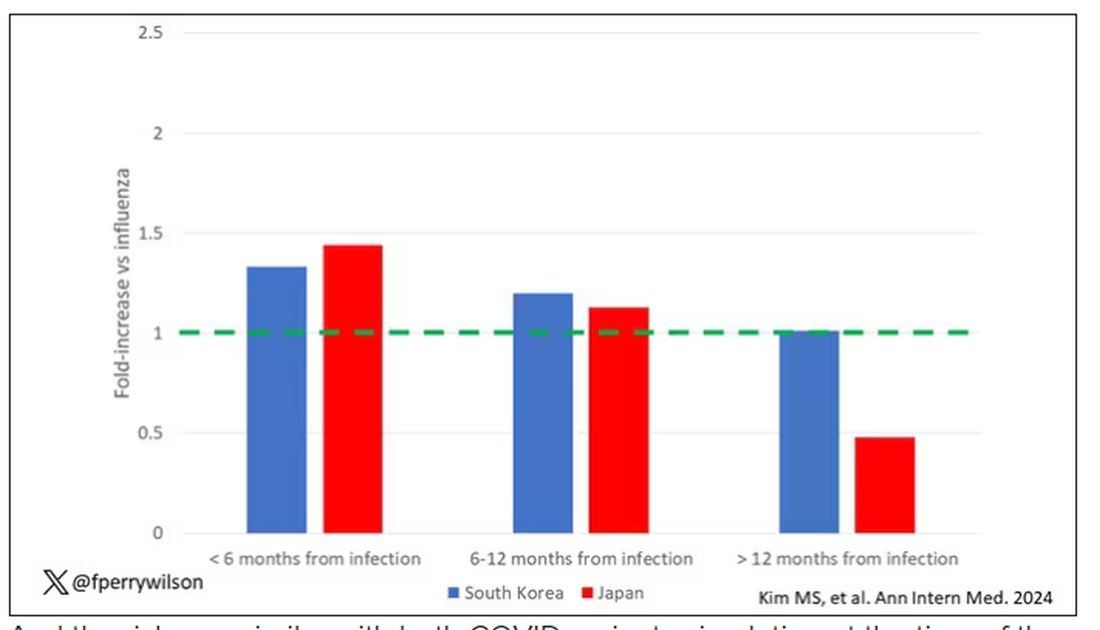
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?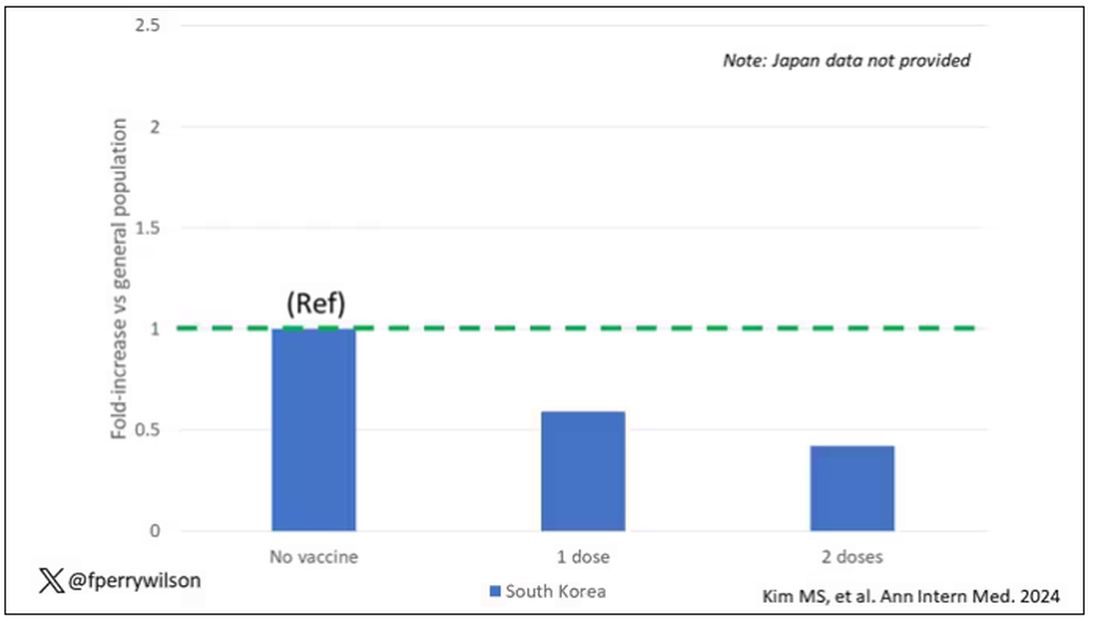
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
What’s Changed in Asthma Treatment? Quite a Bit
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
The Small Office Vibe
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
My first civilian job after finishing my training was as an associate and eventually a partner of a pediatrician whose office was in a wing of his large 19th-century home. The pediatrician in the neighboring town had his office in a small house next to his home. This model of small one- or two -provider offices in or nearby their homes was replicated up and down the coast. After 7 years, the 12-minute drive from my home to the office became intolerable and I asked to dissolve what was otherwise a successful partnership. I opened a one-provider office with a 6-minute bike ride commute and my wife served as the billing clerk and bookkeeper.
Those next 10 years of solo practice were the most rewarding, both economically and professionally. Eventually faced with the need to add another provider, I reluctantly joined a recently formed group of primary care physicians who, like me, had been running one- or two-provider offices often with spouses as support staff — basically Mom and Pop operations. However, the group was gradually absorbed by increasingly larger entities and our practices that once were as individual as our personalities became homogenized. Neither my patients nor I liked the new feel of the office.
Still pining for that small office vibe, I continue to wonder if it could be scaled up and adapted to today’s healthcare realities. I recently read a New York Times article describing how a pediatrician has launched such a practice model into the uncharted waters of Greater New York City.
At age 34, Dr. Michel Cohen, a Moroccan-French émigré, opened his storefront pediatric practice in 1994. The upper story housed his loft apartment. A self-described “hippie doctor,” Dr. Cohen developed a following based on his book on parenting and publicity surrounding his role in the even more popular book on French-style parenting titled Bringing up Bebe by Pamela Druckerman. By 2009 his practice had grown to three small storefront offices. However, they weren’t sufficiently profitable. He decided to shun the distractions of his celebrity practice trappings and instead focus on growth, hoping that the gravitas associated with even more office locations would allow him to offer better service and improve the bottom line. Sort of an “economy of scale” notion applied to the small office setting.
He now has 48 offices having added 12 new sites last year with 5 more planned for next year. These are all one- or two-physician installations with two exam rooms per provider. The offices are bright and colorful, focused on appealing to a child’s taste. The furniture is blond wood, most of it based on Dr. Cohen’s designs and in some cases handmade. Current staffing is 112 physicians and nurse practitioners and volume exceeds 100,000 visits per year.
The volume has allowed the practice to add a user-friendly patient portal and offer an after-hours call-in option. The larger volume means that staffing can be more easily adjusted to illness and vacations. The goal is to have the practitioners become identified with their sites and the patients assigned to them whenever possible. Uniformity in office designs allow a provider filling in from another site to easily find supplies and function within a familiar system.
While the sites have generally served upscale gentrified neighborhoods, the practice has recently expanded to less affluent areas and accepts Medicaid. Dr. Cohen’s dream is to expand his network nationally as a nonprofit in which low-income sites would be subsidized by the more profitable offices. A previous attempt at expansion with two offices in Southern California did not work out because the time zone difference didn’t mesh well with the Internet portal.
Wanting to hear a firsthand account from a family on how the Tribeca Pediatric system works, I contacted a neighbor who has recently moved his young family here to Brunswick. His impression was generally positive. He gave high marks to the patient portal for the ability to get school and camp forms and vaccination records quickly. Appointments made electronically was a plus, although the after-hours response time sometimes took an hour or two. He would have preferred to see their assigned provider for a higher percentage of visits, but this seems to be a common complaint even in systems with the greatest availability. Care was dispensed efficiently but didn’t seem to be overly rushed.
In the NY Times article there is one complaint by a former provider who felt she was getting burned out by the system and leaned on 10 minutes for sick visits and 20 minutes for well visits. Personally, I don’t see this as a problem. The length of a visit and the quality of the care are not always related. Given good support services and an efficiently run office, those slot guidelines seem very reasonable, realizing that a skilled clinician must have already learned to adjust his or her pace to the realities of the patient mix. However, as the pediatric sick population has leaned more toward behavioral and mental health problems, a primary care practice should be offering some option for these patients either in-house or with reliable referral relationships. Although the NY Times article doesn’t provide any numbers, it does mention that the providers are generally young and there is some turnover, possibly as providers use the practice as a “stepping stone.”
To some extent Dr. Cohen’s success seems to be the result of his real estate acumen and business sense. Because the majority of recent medical school graduates enter the work force with a substantial debt, it is difficult to imagine that a young physician would have Dr. Cohen’s entrepreneurial passion. However, clearly his success, at least in the short term, demonstrates that there is a substantial percentage of both patients and providers who prefer small personalized offices if given the option. It will be interesting to see if and how Tribeca Pediatrics expands and whether any of the larger existing networks attempt to imitate it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Unexpectedly Helpful Effects of Drugs Used For Other Reasons
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
Shining a Light on Colorectal Cancer
For more than two decades, March has been designated Colorectal Cancer Awareness Month. This annual observance serves as a reminder to spread the word in our local and national communities regarding the value of colorectal cancer screening and prevention. CRC prevention through screening and surveillance is a core part of our practice as gastroenterologists and plays a critical role in improving outcomes and reducing mortality from the second leading cause of cancer deaths in the US.
While we have made great strides in increasing awareness among patients of the need for screening, overall screening rates remain well below our national target of 80% and significant disparities in screening persist. By disseminating key information about risk factors, promoting early detection through evidence-based screening, continuing to improve access to care by reducing financial and other barriers, and educating patients about available screening options that best fit their needs and preferences, we can continue to move the needle in improving overall screening rates and optimizing outcomes.
In this month’s issue of GIHN, we feature an excellent narrative review by Dr. Samir Gupta and colleagues describing the phenomenon of “birth cohort CRC,” which is thought to explain recent changes in CRC epidemiology, including rising incidence of early-onset colorectal cancer. We also highlight a timely study out of Kaiser Permanente investigating how best to communicate with patients with prior low-risk adenomas regarding updated colonoscopy intervals given recent guideline changes extending surveillance intervals from 5 to 7-10 years. This question is particularly relevant to resource-constrained healthcare settings, where proactive de-implementation of outdated surveillance intervals could improve access for other patients with more immediate need.
In our March Member Spotlight, we feature Dr. Andy Tau of Austin Gastroenterology, who shares important insights regarding his career as a GI hospitalist, a growing area of GI practice. Finally, in this month’s Perspectives column, Drs. Michael Weinstein of Capital Digestive Care and Paul Berggreen of GI Alliance provide powerful contrasting perspectives highlighting the pros and cons of private equity in GI and how to evaluate if it’s right for your practice. I found it to be a particularly fascinating read!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
For more than two decades, March has been designated Colorectal Cancer Awareness Month. This annual observance serves as a reminder to spread the word in our local and national communities regarding the value of colorectal cancer screening and prevention. CRC prevention through screening and surveillance is a core part of our practice as gastroenterologists and plays a critical role in improving outcomes and reducing mortality from the second leading cause of cancer deaths in the US.
While we have made great strides in increasing awareness among patients of the need for screening, overall screening rates remain well below our national target of 80% and significant disparities in screening persist. By disseminating key information about risk factors, promoting early detection through evidence-based screening, continuing to improve access to care by reducing financial and other barriers, and educating patients about available screening options that best fit their needs and preferences, we can continue to move the needle in improving overall screening rates and optimizing outcomes.
In this month’s issue of GIHN, we feature an excellent narrative review by Dr. Samir Gupta and colleagues describing the phenomenon of “birth cohort CRC,” which is thought to explain recent changes in CRC epidemiology, including rising incidence of early-onset colorectal cancer. We also highlight a timely study out of Kaiser Permanente investigating how best to communicate with patients with prior low-risk adenomas regarding updated colonoscopy intervals given recent guideline changes extending surveillance intervals from 5 to 7-10 years. This question is particularly relevant to resource-constrained healthcare settings, where proactive de-implementation of outdated surveillance intervals could improve access for other patients with more immediate need.
In our March Member Spotlight, we feature Dr. Andy Tau of Austin Gastroenterology, who shares important insights regarding his career as a GI hospitalist, a growing area of GI practice. Finally, in this month’s Perspectives column, Drs. Michael Weinstein of Capital Digestive Care and Paul Berggreen of GI Alliance provide powerful contrasting perspectives highlighting the pros and cons of private equity in GI and how to evaluate if it’s right for your practice. I found it to be a particularly fascinating read!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
For more than two decades, March has been designated Colorectal Cancer Awareness Month. This annual observance serves as a reminder to spread the word in our local and national communities regarding the value of colorectal cancer screening and prevention. CRC prevention through screening and surveillance is a core part of our practice as gastroenterologists and plays a critical role in improving outcomes and reducing mortality from the second leading cause of cancer deaths in the US.
While we have made great strides in increasing awareness among patients of the need for screening, overall screening rates remain well below our national target of 80% and significant disparities in screening persist. By disseminating key information about risk factors, promoting early detection through evidence-based screening, continuing to improve access to care by reducing financial and other barriers, and educating patients about available screening options that best fit their needs and preferences, we can continue to move the needle in improving overall screening rates and optimizing outcomes.
In this month’s issue of GIHN, we feature an excellent narrative review by Dr. Samir Gupta and colleagues describing the phenomenon of “birth cohort CRC,” which is thought to explain recent changes in CRC epidemiology, including rising incidence of early-onset colorectal cancer. We also highlight a timely study out of Kaiser Permanente investigating how best to communicate with patients with prior low-risk adenomas regarding updated colonoscopy intervals given recent guideline changes extending surveillance intervals from 5 to 7-10 years. This question is particularly relevant to resource-constrained healthcare settings, where proactive de-implementation of outdated surveillance intervals could improve access for other patients with more immediate need.
In our March Member Spotlight, we feature Dr. Andy Tau of Austin Gastroenterology, who shares important insights regarding his career as a GI hospitalist, a growing area of GI practice. Finally, in this month’s Perspectives column, Drs. Michael Weinstein of Capital Digestive Care and Paul Berggreen of GI Alliance provide powerful contrasting perspectives highlighting the pros and cons of private equity in GI and how to evaluate if it’s right for your practice. I found it to be a particularly fascinating read!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Private Equity in GI
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
‘Less is More’ in Myeloma
Among those that intrigue me most are the pioneering “less is more” trials that challenged conventional practices and remain relevant today. One such trial was inspired by a patient’s dissatisfaction with high doses of dexamethasone and its side effects.
Unlike the prevailing norm of frequent high doses, this trial compared a steroid dose administered weekly (as opposed to doses given several days a week). Lo and behold, the lower steroid dosage was associated with significantly better survival rates. At 1-year follow-up, 96% of patients in the lower-dose group were alive, compared with 87% in the higher-dose group.
Another noteworthy “less-is more” trial that I love, spearheaded by an Italian team, also focused on steroid dosage. This trial investigated discontinuing dexamethasone after nine cycles, along with reducing the dose of lenalidomide, versus maintaining long-term treatment without reductions. The findings revealed comparable progression-free survival with reduced toxicity, highlighting the potential benefits of this less-is-more approach.
While these trials are inspirational, a closer examination of myeloma trial history, especially those that led to regulatory approvals, reveals a preponderance of “add-on” trials. You add a potentially effective drug to an existing backbone, and you get an improvement in an outcome such as response rate (shrinking cancer) or duration of remission or progression free survival (amount of time alive and in remission).
Such trials have led to an abundance of effective options. But these same trials have almost always been a comparison of three drugs versus two drugs, and almost never three drugs versus three. And the drugs are often given continuously, especially the “newer” added drug, without a break. As a result, we are left completely unsure of how to sequence our drugs, and whether a finite course of the new drug would be equivalent to administering that new drug forever.
This problem is not unique to myeloma. Yet it is very apparent in myeloma, because we have been lucky to have so many good drugs (or at least “potentially” good drugs) that make it to phase 3 trials.
Unfortunately, the landscape of clinical trials is heavily influenced by the pharmaceutical industry, with limited funding available from alternative sources. As a result, there is a scarcity of trials exploring “less is more” approaches, despite their potential to optimize treatment outcomes and quality of life.
Even government-funded trials run by cooperative groups require industry buy-in or are run by people who have very close contacts and conflicts of interest with industry. We need so many more of these less-is-more trials, but we have limited means to fund them.
These are the kinds of discussions I have with my patients daily. We grapple with questions about the necessity of lifelong (or any) maintenance therapy or the feasibility of treatment breaks for patients with stable disease. While we strive to provide the best care possible, the lack of definitive data often leaves us making tough decisions in the clinic.
I am grateful to those who are working tirelessly to facilitate trials that prioritize quality of life and “less is more” approaches. Your efforts are invaluable. Looking forward, I aspire to contribute to this important work.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
Among those that intrigue me most are the pioneering “less is more” trials that challenged conventional practices and remain relevant today. One such trial was inspired by a patient’s dissatisfaction with high doses of dexamethasone and its side effects.
Unlike the prevailing norm of frequent high doses, this trial compared a steroid dose administered weekly (as opposed to doses given several days a week). Lo and behold, the lower steroid dosage was associated with significantly better survival rates. At 1-year follow-up, 96% of patients in the lower-dose group were alive, compared with 87% in the higher-dose group.
Another noteworthy “less-is more” trial that I love, spearheaded by an Italian team, also focused on steroid dosage. This trial investigated discontinuing dexamethasone after nine cycles, along with reducing the dose of lenalidomide, versus maintaining long-term treatment without reductions. The findings revealed comparable progression-free survival with reduced toxicity, highlighting the potential benefits of this less-is-more approach.
While these trials are inspirational, a closer examination of myeloma trial history, especially those that led to regulatory approvals, reveals a preponderance of “add-on” trials. You add a potentially effective drug to an existing backbone, and you get an improvement in an outcome such as response rate (shrinking cancer) or duration of remission or progression free survival (amount of time alive and in remission).
Such trials have led to an abundance of effective options. But these same trials have almost always been a comparison of three drugs versus two drugs, and almost never three drugs versus three. And the drugs are often given continuously, especially the “newer” added drug, without a break. As a result, we are left completely unsure of how to sequence our drugs, and whether a finite course of the new drug would be equivalent to administering that new drug forever.
This problem is not unique to myeloma. Yet it is very apparent in myeloma, because we have been lucky to have so many good drugs (or at least “potentially” good drugs) that make it to phase 3 trials.
Unfortunately, the landscape of clinical trials is heavily influenced by the pharmaceutical industry, with limited funding available from alternative sources. As a result, there is a scarcity of trials exploring “less is more” approaches, despite their potential to optimize treatment outcomes and quality of life.
Even government-funded trials run by cooperative groups require industry buy-in or are run by people who have very close contacts and conflicts of interest with industry. We need so many more of these less-is-more trials, but we have limited means to fund them.
These are the kinds of discussions I have with my patients daily. We grapple with questions about the necessity of lifelong (or any) maintenance therapy or the feasibility of treatment breaks for patients with stable disease. While we strive to provide the best care possible, the lack of definitive data often leaves us making tough decisions in the clinic.
I am grateful to those who are working tirelessly to facilitate trials that prioritize quality of life and “less is more” approaches. Your efforts are invaluable. Looking forward, I aspire to contribute to this important work.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
Among those that intrigue me most are the pioneering “less is more” trials that challenged conventional practices and remain relevant today. One such trial was inspired by a patient’s dissatisfaction with high doses of dexamethasone and its side effects.
Unlike the prevailing norm of frequent high doses, this trial compared a steroid dose administered weekly (as opposed to doses given several days a week). Lo and behold, the lower steroid dosage was associated with significantly better survival rates. At 1-year follow-up, 96% of patients in the lower-dose group were alive, compared with 87% in the higher-dose group.
Another noteworthy “less-is more” trial that I love, spearheaded by an Italian team, also focused on steroid dosage. This trial investigated discontinuing dexamethasone after nine cycles, along with reducing the dose of lenalidomide, versus maintaining long-term treatment without reductions. The findings revealed comparable progression-free survival with reduced toxicity, highlighting the potential benefits of this less-is-more approach.
While these trials are inspirational, a closer examination of myeloma trial history, especially those that led to regulatory approvals, reveals a preponderance of “add-on” trials. You add a potentially effective drug to an existing backbone, and you get an improvement in an outcome such as response rate (shrinking cancer) or duration of remission or progression free survival (amount of time alive and in remission).
Such trials have led to an abundance of effective options. But these same trials have almost always been a comparison of three drugs versus two drugs, and almost never three drugs versus three. And the drugs are often given continuously, especially the “newer” added drug, without a break. As a result, we are left completely unsure of how to sequence our drugs, and whether a finite course of the new drug would be equivalent to administering that new drug forever.
This problem is not unique to myeloma. Yet it is very apparent in myeloma, because we have been lucky to have so many good drugs (or at least “potentially” good drugs) that make it to phase 3 trials.
Unfortunately, the landscape of clinical trials is heavily influenced by the pharmaceutical industry, with limited funding available from alternative sources. As a result, there is a scarcity of trials exploring “less is more” approaches, despite their potential to optimize treatment outcomes and quality of life.
Even government-funded trials run by cooperative groups require industry buy-in or are run by people who have very close contacts and conflicts of interest with industry. We need so many more of these less-is-more trials, but we have limited means to fund them.
These are the kinds of discussions I have with my patients daily. We grapple with questions about the necessity of lifelong (or any) maintenance therapy or the feasibility of treatment breaks for patients with stable disease. While we strive to provide the best care possible, the lack of definitive data often leaves us making tough decisions in the clinic.
I am grateful to those who are working tirelessly to facilitate trials that prioritize quality of life and “less is more” approaches. Your efforts are invaluable. Looking forward, I aspire to contribute to this important work.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.