User login
Should people who play sports pay higher medical insurance premiums?
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re anywhere near Seattle, anywhere near Florida, or anywhere where it might be not oppressively hot outside but encouraging some people who might want to go out and get a little exercise, you’ve undoubtedly seen or heard of pickleball.
This took off, I think, out of Bainbridge Island, Wash. It was meant as a gentlemanly game where people didn’t exert themselves too much. The joke is you could play it while holding a drink in one hand. It’s gotten more popular and more competitive. It’s kind of a miniature version of tennis, with a smaller court, a plastic ball, and a wooden paddle. The ball can go back and forth rapidly, but you’re always playing doubles and it doesn’t take as much energy, exertion, and, if you will, fitness as a game like singles tennis.
The upside is it’s gotten many people outdoors getting some exercise and socializing. That’s all to the good. But a recent study suggested that there are about $500 million worth of injuries coming into the health care system associated with pickleball. There have been leg sprains, broken bones, people getting hit in the eye, hamstring pulls, and many other problems. I’ve been told that many of the spectators who show up for pickleball matches are there with a cast or have some kind of a wrap on because they were injured.
Well, many people have argued in the past about what we are going to do about health care costs. Some suggest if you voluntarily incur health care damage, you ought to pay for that yourself and you ought to have a big copay.
If you decide you’re going to do cross-country skiing or downhill skiing and you injure yourself, you chose to do it, so you pay. If you’re not going to maintain your weight, you’re going to smoke, or you’re going to ride around without a helmet, that’s your choice. You ought to pay.
I think the pickleball example is really a good challenge to these views. You obviously want people to go out and get some exercise. Here, we’re talking about a population that’s a little older and oftentimes doesn’t get out there as much as doctors would like to get the exercise that’s still important that they need, and yet it does incur injuries and problems.
My suggestion would be to make the game a little safer. Let’s try to encourage people to warm up more before they get out there and jump out of the car and engage in their pickleball battles. Goggles might be important to prevent the eye injuries in a game that’s played up close. Maybe we want to make sure that people look out for one another out there. If they think they’re getting dehydrated or tired, they should say, “Let’s sit down.”
I’m not willing to put a tax or a copay on the pickleball players of America. I know they choose to do it. It’s got an upside and benefits, as many things like skiing and other behaviors that have some risk do, but I think we want to be encouraging, not discouraging, of it.
I don’t like a society where anybody who tries to do something that takes risk winds up bearing extra cost for doing that. I understand that that gets people irritated when it comes to dangerous, hyper-risky behavior like smoking and not wearing a motorcycle helmet. I think the way to engage is not to call out the sinner or to try and punish those who are trying to do things that bring them enjoyment, reward, or in some of these cases, physical fitness, but to try to make things safer and try to gradually improve and get rid of the risk side to capture the full benefit side.
I’m not sure I’ve come up with all the best ways to make pickleball safer, but I think that’s where our thinking in health care should go. My view is to get out there and play pickleball. If you do pull your hamstring, raise my insurance premium a little bit. I’ll help to pay for it. Better you get some enjoyment and some exercise.
I get the downside, but come on, folks, we ought to be, as a community, somewhat supportive of the fun and recreation that our fellow citizens engage in.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center. He disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); and as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Hepatic presentations of celiac disease
Liver biopsy findings may include variable degrees of steatosis, inflammation, and fibrosis.
In one case we have seen, the patient presented with unexplained ascites and features suggestive of Budd-Chiari syndrome. The serum ascites albumin gradient was 2.3 with a total protein of 0.8 g/dL, and albumin 0.5 g/dL, with an ascitic WBC count of 88/mm3.
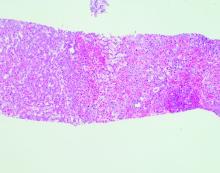
Echocardiography showed an ejection fraction of 80%. Transjugular liver biopsy revealed a normal hepatic venous pressure gradient but marked sinusoidal dilatation and congestion with hepatocyte atrophy and focal necrosis suggestive of vascular outlet obstruction (Figure 1).
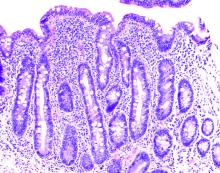
Hepatic venography, however, showed no evidence of Budd-Chiari syndrome. When seen in consultation, pertinent observations included Irish ancestry, a history of occasional diarrhea, short stature, osteoporosis, and an atrophic spleen on computed tomography. An IgA transglutaminase antibody was positive, and a small-bowel biopsy confirmed celiac disease (Figure 2).
On a gluten-free diet, the patient’s symptoms resolved, with clinical and laboratory abnormalities returning to normal. She lived another 20 years before dying of primary pulmonary hypertension. Recognition of an unusual hepatic manifestation of celiac disease led to effective management.
Dr. Friedman is the Anton R. Fried, MD, Chair of the department of medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University School of Medicine, all in Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the Miller School of Medicine, University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
Previously published in Gastro Hep Advances. 2023. doi: 10.1016/j.gastha.2023.03.018.
Liver biopsy findings may include variable degrees of steatosis, inflammation, and fibrosis.
In one case we have seen, the patient presented with unexplained ascites and features suggestive of Budd-Chiari syndrome. The serum ascites albumin gradient was 2.3 with a total protein of 0.8 g/dL, and albumin 0.5 g/dL, with an ascitic WBC count of 88/mm3.
Echocardiography showed an ejection fraction of 80%. Transjugular liver biopsy revealed a normal hepatic venous pressure gradient but marked sinusoidal dilatation and congestion with hepatocyte atrophy and focal necrosis suggestive of vascular outlet obstruction (Figure 1).
Hepatic venography, however, showed no evidence of Budd-Chiari syndrome. When seen in consultation, pertinent observations included Irish ancestry, a history of occasional diarrhea, short stature, osteoporosis, and an atrophic spleen on computed tomography. An IgA transglutaminase antibody was positive, and a small-bowel biopsy confirmed celiac disease (Figure 2).
On a gluten-free diet, the patient’s symptoms resolved, with clinical and laboratory abnormalities returning to normal. She lived another 20 years before dying of primary pulmonary hypertension. Recognition of an unusual hepatic manifestation of celiac disease led to effective management.
Dr. Friedman is the Anton R. Fried, MD, Chair of the department of medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University School of Medicine, all in Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the Miller School of Medicine, University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
Previously published in Gastro Hep Advances. 2023. doi: 10.1016/j.gastha.2023.03.018.
Liver biopsy findings may include variable degrees of steatosis, inflammation, and fibrosis.
In one case we have seen, the patient presented with unexplained ascites and features suggestive of Budd-Chiari syndrome. The serum ascites albumin gradient was 2.3 with a total protein of 0.8 g/dL, and albumin 0.5 g/dL, with an ascitic WBC count of 88/mm3.
Echocardiography showed an ejection fraction of 80%. Transjugular liver biopsy revealed a normal hepatic venous pressure gradient but marked sinusoidal dilatation and congestion with hepatocyte atrophy and focal necrosis suggestive of vascular outlet obstruction (Figure 1).
Hepatic venography, however, showed no evidence of Budd-Chiari syndrome. When seen in consultation, pertinent observations included Irish ancestry, a history of occasional diarrhea, short stature, osteoporosis, and an atrophic spleen on computed tomography. An IgA transglutaminase antibody was positive, and a small-bowel biopsy confirmed celiac disease (Figure 2).
On a gluten-free diet, the patient’s symptoms resolved, with clinical and laboratory abnormalities returning to normal. She lived another 20 years before dying of primary pulmonary hypertension. Recognition of an unusual hepatic manifestation of celiac disease led to effective management.
Dr. Friedman is the Anton R. Fried, MD, Chair of the department of medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University School of Medicine, all in Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the Miller School of Medicine, University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
Previously published in Gastro Hep Advances. 2023. doi: 10.1016/j.gastha.2023.03.018.
Barbie has an anxiety disorder
And it’s a great time to be a therapist
The Barbie movie is generating a lot of feelings, ranging from praise to vitriol. However one feels about the movie, let’s all pause and reflect for a moment on the fact that the number-one grossing film of 2023 is about our childhood doll trying to treat her anxiety disorder.
“Life imitates art more than art imitates life.” So said Oscar Wilde in 1889.
When my adult daughter, a childhood Barbie enthusiast, asked me to see the film, we put on pink and went. Twice. Little did I know that it would stir up so many thoughts and feelings. The one I want to share is how blessed I feel at this moment in time to be a mental health care provider! No longer is mental health something to be whispered about at the water cooler; instead, even Barbie is suffering. And with all the controversy in the press about the movie, no one seems at all surprised by this storyline.
I was raised by two child psychiatrists and have been practicing as an adult psychiatrist since 1991. The start of the pandemic was the most difficult time of my career, as almost every patient was struggling simultaneously, as was I. Three long years later, we are gradually emerging from our shared trauma. How ironic, now with the opportunity to go back to work, I have elected to maintain the majority of my practice online from home. It seems that most patients and providers prefer this mode of treatment, with a full 90 percent of practitioners saying they are using a hybrid model.
As mental health professionals, we know that anywhere from 3% to 49% of those experiencing trauma will develop posttraumatic stress disorder (PTSD), and we have been trained to treat them.
But what happens when an entire global population is exposed simultaneously to trauma? Historians and social scientists refer to such events by many different names, such as: Singularity, Black Swan Event, and Tipping Point. These events are incredibly rare, and afterwards everything is different. These global traumas always lead to massive change.
I think we are at that tipping point. This is the singularity. This is our Black Swan Event. Within a 3-year span, we have experienced the following:
- A global traumatic event (COVID-19).
- A sudden and seemingly permanent shift from office to remote video meetings mostly from home.
- Upending of traditional fundamentals of the stock market as the game literally stopped in January 2021.
- Rapid and widespread availability of Artificial Intelligence.
- The first generation to be fully raised on the Internet and social media (Gen Z) is now entering the workforce.
- Ongoing war in Ukraine.
That’s already an overwhelming list, and I could go on, but let’s get back to Barbie’s anxiety disorder.
The awareness about and acceptance of mental health issues has never been higher. The access to treatment never greater. There are now more online therapy options than ever. Treatment options have dramatically expanded in recent years, from Transcranial Magnetic Stimulation (TMS) to ketamine centers and psychedelics, as well as more mainstream options such as dialectical behavior therapy (DBT), cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs), and so many more.
What is particularly unique about this moment is the direct access to care. Self-help books abound with many making it to the New York Times bestseller list. YouTube is loaded with fantastic content on overcoming many mental health issues, although one should be careful with selecting reliable sources. Apps like HeadSpace and Calm are being downloaded by millions of people around the globe. Investors provided a record-breaking $1.5 billion to mental health startups in 2020 alone.
For most practitioners, our phones have been ringing off the hook since 2020. Applications to psychology, psychiatric residency, social work, and counseling degree programs are on the rise, with workforce shortages expected to continue for decades. Psychological expertise has been embraced by businesses especially for DEI (diversity, equity, and inclusion). Mental health experts are the most asked-for experts through media request services. Elite athletes are talking openly about bringing us on their teams.
In this unique moment, when everything seems set to transform into something else, it is time for mental health professionals to exert some agency and influence over where mental health will go from here. I think the next frontier for mental health specialists is to figure out how to speak collectively and help guide society.
Neil Howe, in his sweeping book “The Fourth Turning is Here,” says we have another 10 years in this “Millennial Crisis” phase. He calls this our “winter,” and it remains to be seen how we will emerge from our current challenges. I think we can make a difference.
If the Barbie movie is indeed a canary in the coal mine, I see positive trends ahead as we move past some of the societal and structural issues facing us, and work together to create a more open and egalitarian society. We must find creative solutions that will solve truly massive problems threatening our well-being and perhaps even our existence.
I am so grateful to be able to continue to practice and share my thoughts with you here from my home office, and I hope you can take a break and see this movie, which is not only entertaining but also thought- and emotion-provoking.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
And it’s a great time to be a therapist
And it’s a great time to be a therapist
The Barbie movie is generating a lot of feelings, ranging from praise to vitriol. However one feels about the movie, let’s all pause and reflect for a moment on the fact that the number-one grossing film of 2023 is about our childhood doll trying to treat her anxiety disorder.
“Life imitates art more than art imitates life.” So said Oscar Wilde in 1889.
When my adult daughter, a childhood Barbie enthusiast, asked me to see the film, we put on pink and went. Twice. Little did I know that it would stir up so many thoughts and feelings. The one I want to share is how blessed I feel at this moment in time to be a mental health care provider! No longer is mental health something to be whispered about at the water cooler; instead, even Barbie is suffering. And with all the controversy in the press about the movie, no one seems at all surprised by this storyline.
I was raised by two child psychiatrists and have been practicing as an adult psychiatrist since 1991. The start of the pandemic was the most difficult time of my career, as almost every patient was struggling simultaneously, as was I. Three long years later, we are gradually emerging from our shared trauma. How ironic, now with the opportunity to go back to work, I have elected to maintain the majority of my practice online from home. It seems that most patients and providers prefer this mode of treatment, with a full 90 percent of practitioners saying they are using a hybrid model.
As mental health professionals, we know that anywhere from 3% to 49% of those experiencing trauma will develop posttraumatic stress disorder (PTSD), and we have been trained to treat them.
But what happens when an entire global population is exposed simultaneously to trauma? Historians and social scientists refer to such events by many different names, such as: Singularity, Black Swan Event, and Tipping Point. These events are incredibly rare, and afterwards everything is different. These global traumas always lead to massive change.
I think we are at that tipping point. This is the singularity. This is our Black Swan Event. Within a 3-year span, we have experienced the following:
- A global traumatic event (COVID-19).
- A sudden and seemingly permanent shift from office to remote video meetings mostly from home.
- Upending of traditional fundamentals of the stock market as the game literally stopped in January 2021.
- Rapid and widespread availability of Artificial Intelligence.
- The first generation to be fully raised on the Internet and social media (Gen Z) is now entering the workforce.
- Ongoing war in Ukraine.
That’s already an overwhelming list, and I could go on, but let’s get back to Barbie’s anxiety disorder.
The awareness about and acceptance of mental health issues has never been higher. The access to treatment never greater. There are now more online therapy options than ever. Treatment options have dramatically expanded in recent years, from Transcranial Magnetic Stimulation (TMS) to ketamine centers and psychedelics, as well as more mainstream options such as dialectical behavior therapy (DBT), cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs), and so many more.
What is particularly unique about this moment is the direct access to care. Self-help books abound with many making it to the New York Times bestseller list. YouTube is loaded with fantastic content on overcoming many mental health issues, although one should be careful with selecting reliable sources. Apps like HeadSpace and Calm are being downloaded by millions of people around the globe. Investors provided a record-breaking $1.5 billion to mental health startups in 2020 alone.
For most practitioners, our phones have been ringing off the hook since 2020. Applications to psychology, psychiatric residency, social work, and counseling degree programs are on the rise, with workforce shortages expected to continue for decades. Psychological expertise has been embraced by businesses especially for DEI (diversity, equity, and inclusion). Mental health experts are the most asked-for experts through media request services. Elite athletes are talking openly about bringing us on their teams.
In this unique moment, when everything seems set to transform into something else, it is time for mental health professionals to exert some agency and influence over where mental health will go from here. I think the next frontier for mental health specialists is to figure out how to speak collectively and help guide society.
Neil Howe, in his sweeping book “The Fourth Turning is Here,” says we have another 10 years in this “Millennial Crisis” phase. He calls this our “winter,” and it remains to be seen how we will emerge from our current challenges. I think we can make a difference.
If the Barbie movie is indeed a canary in the coal mine, I see positive trends ahead as we move past some of the societal and structural issues facing us, and work together to create a more open and egalitarian society. We must find creative solutions that will solve truly massive problems threatening our well-being and perhaps even our existence.
I am so grateful to be able to continue to practice and share my thoughts with you here from my home office, and I hope you can take a break and see this movie, which is not only entertaining but also thought- and emotion-provoking.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
The Barbie movie is generating a lot of feelings, ranging from praise to vitriol. However one feels about the movie, let’s all pause and reflect for a moment on the fact that the number-one grossing film of 2023 is about our childhood doll trying to treat her anxiety disorder.
“Life imitates art more than art imitates life.” So said Oscar Wilde in 1889.
When my adult daughter, a childhood Barbie enthusiast, asked me to see the film, we put on pink and went. Twice. Little did I know that it would stir up so many thoughts and feelings. The one I want to share is how blessed I feel at this moment in time to be a mental health care provider! No longer is mental health something to be whispered about at the water cooler; instead, even Barbie is suffering. And with all the controversy in the press about the movie, no one seems at all surprised by this storyline.
I was raised by two child psychiatrists and have been practicing as an adult psychiatrist since 1991. The start of the pandemic was the most difficult time of my career, as almost every patient was struggling simultaneously, as was I. Three long years later, we are gradually emerging from our shared trauma. How ironic, now with the opportunity to go back to work, I have elected to maintain the majority of my practice online from home. It seems that most patients and providers prefer this mode of treatment, with a full 90 percent of practitioners saying they are using a hybrid model.
As mental health professionals, we know that anywhere from 3% to 49% of those experiencing trauma will develop posttraumatic stress disorder (PTSD), and we have been trained to treat them.
But what happens when an entire global population is exposed simultaneously to trauma? Historians and social scientists refer to such events by many different names, such as: Singularity, Black Swan Event, and Tipping Point. These events are incredibly rare, and afterwards everything is different. These global traumas always lead to massive change.
I think we are at that tipping point. This is the singularity. This is our Black Swan Event. Within a 3-year span, we have experienced the following:
- A global traumatic event (COVID-19).
- A sudden and seemingly permanent shift from office to remote video meetings mostly from home.
- Upending of traditional fundamentals of the stock market as the game literally stopped in January 2021.
- Rapid and widespread availability of Artificial Intelligence.
- The first generation to be fully raised on the Internet and social media (Gen Z) is now entering the workforce.
- Ongoing war in Ukraine.
That’s already an overwhelming list, and I could go on, but let’s get back to Barbie’s anxiety disorder.
The awareness about and acceptance of mental health issues has never been higher. The access to treatment never greater. There are now more online therapy options than ever. Treatment options have dramatically expanded in recent years, from Transcranial Magnetic Stimulation (TMS) to ketamine centers and psychedelics, as well as more mainstream options such as dialectical behavior therapy (DBT), cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs), and so many more.
What is particularly unique about this moment is the direct access to care. Self-help books abound with many making it to the New York Times bestseller list. YouTube is loaded with fantastic content on overcoming many mental health issues, although one should be careful with selecting reliable sources. Apps like HeadSpace and Calm are being downloaded by millions of people around the globe. Investors provided a record-breaking $1.5 billion to mental health startups in 2020 alone.
For most practitioners, our phones have been ringing off the hook since 2020. Applications to psychology, psychiatric residency, social work, and counseling degree programs are on the rise, with workforce shortages expected to continue for decades. Psychological expertise has been embraced by businesses especially for DEI (diversity, equity, and inclusion). Mental health experts are the most asked-for experts through media request services. Elite athletes are talking openly about bringing us on their teams.
In this unique moment, when everything seems set to transform into something else, it is time for mental health professionals to exert some agency and influence over where mental health will go from here. I think the next frontier for mental health specialists is to figure out how to speak collectively and help guide society.
Neil Howe, in his sweeping book “The Fourth Turning is Here,” says we have another 10 years in this “Millennial Crisis” phase. He calls this our “winter,” and it remains to be seen how we will emerge from our current challenges. I think we can make a difference.
If the Barbie movie is indeed a canary in the coal mine, I see positive trends ahead as we move past some of the societal and structural issues facing us, and work together to create a more open and egalitarian society. We must find creative solutions that will solve truly massive problems threatening our well-being and perhaps even our existence.
I am so grateful to be able to continue to practice and share my thoughts with you here from my home office, and I hope you can take a break and see this movie, which is not only entertaining but also thought- and emotion-provoking.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
The new normal in body temperature
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.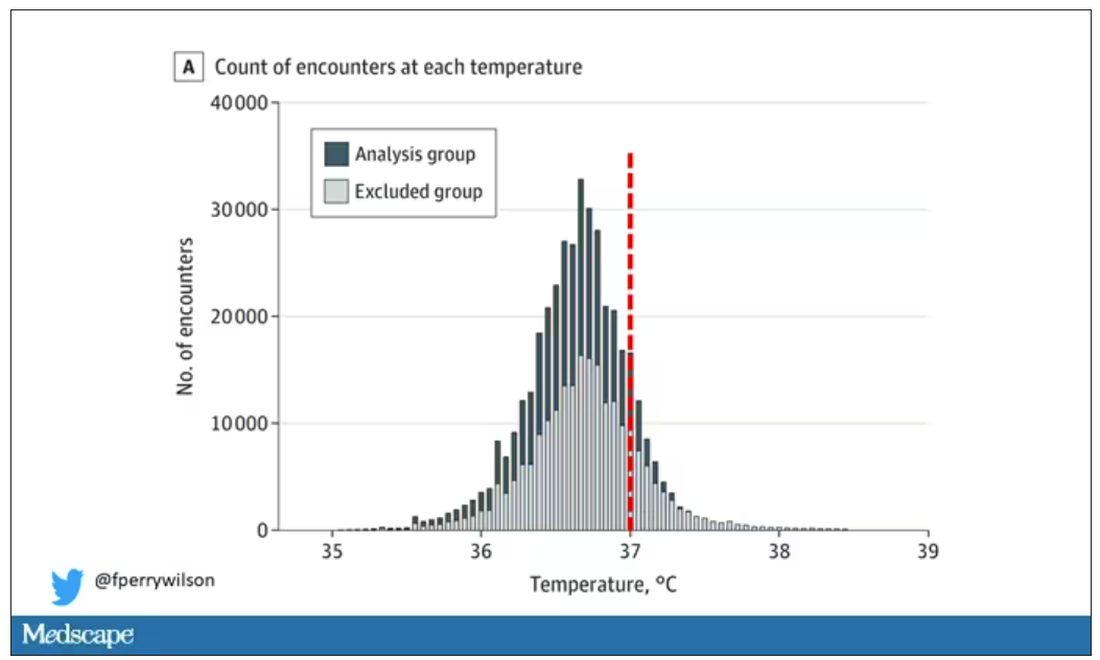
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.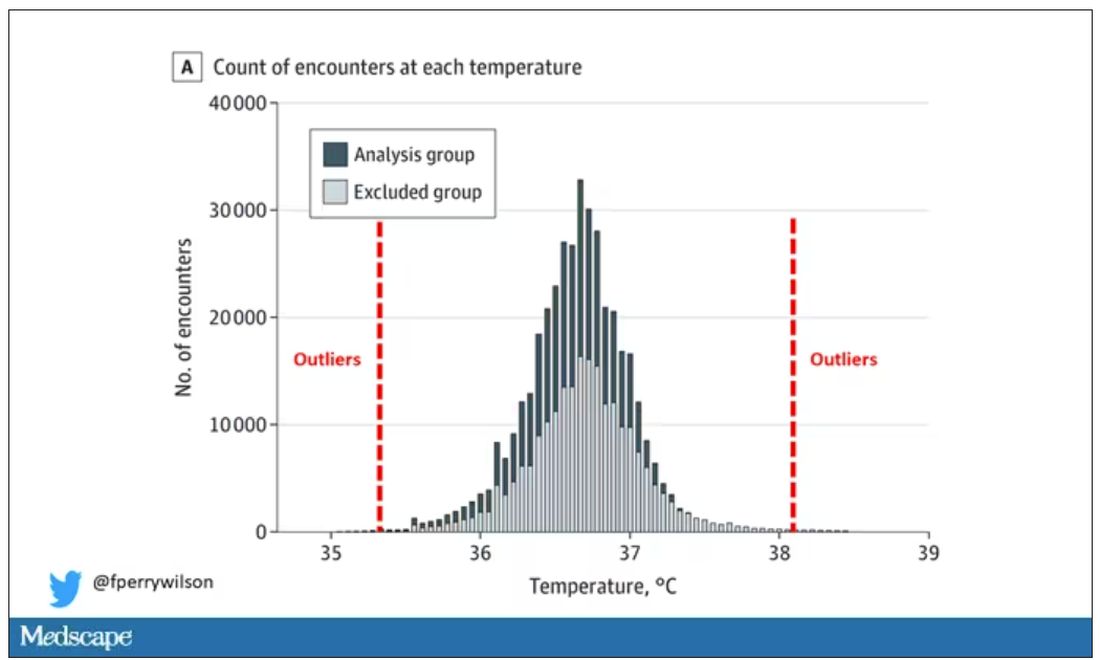
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.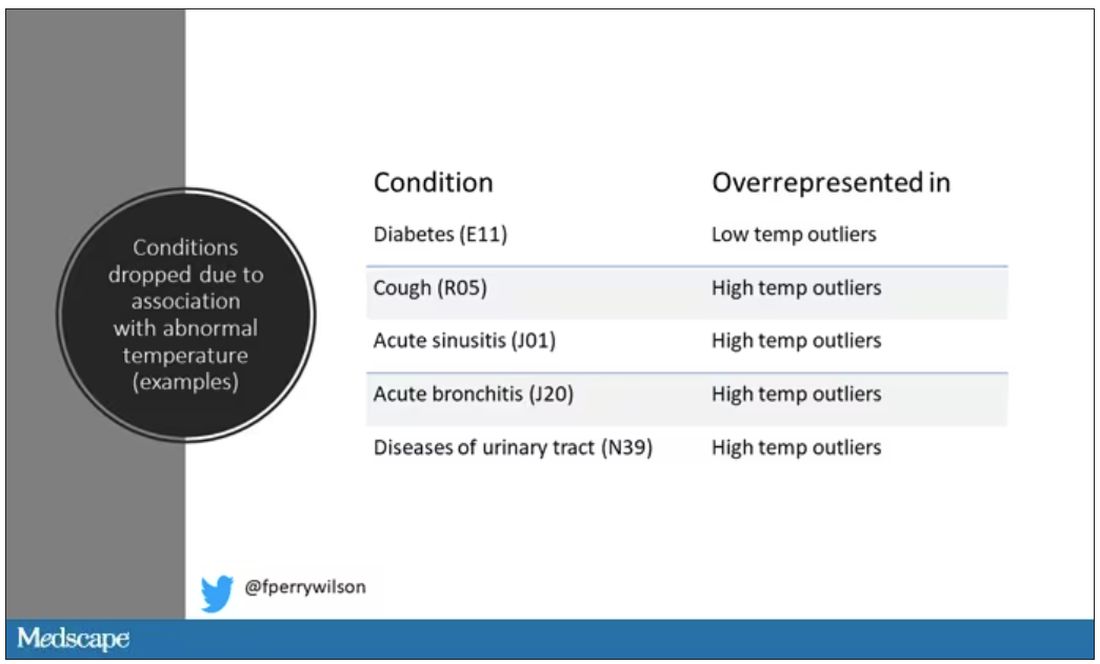
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.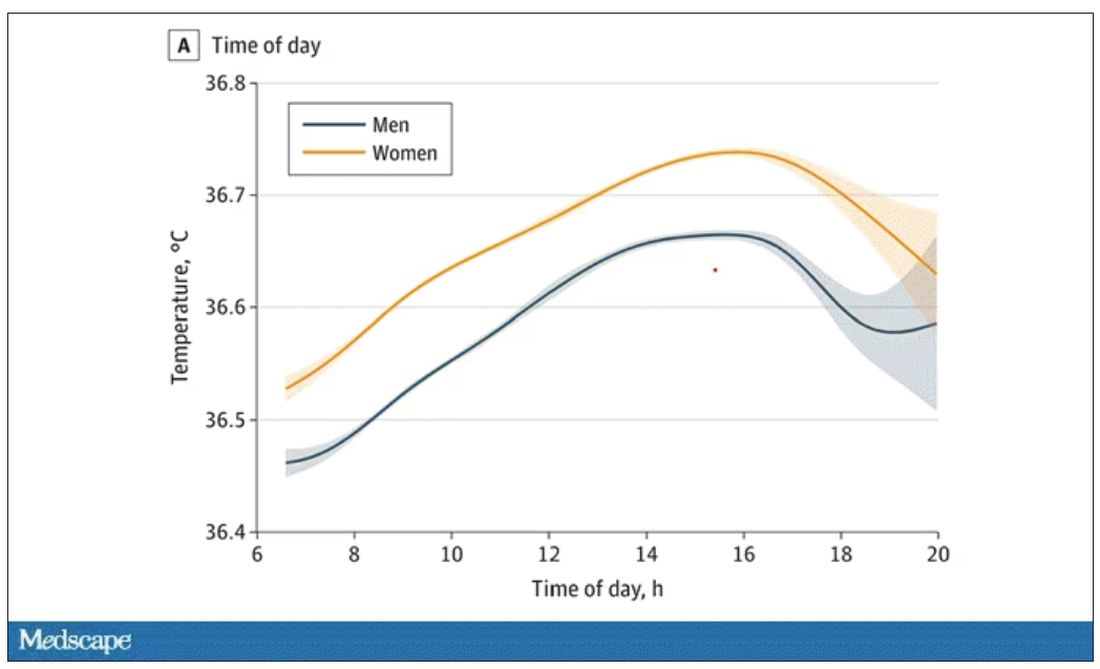
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.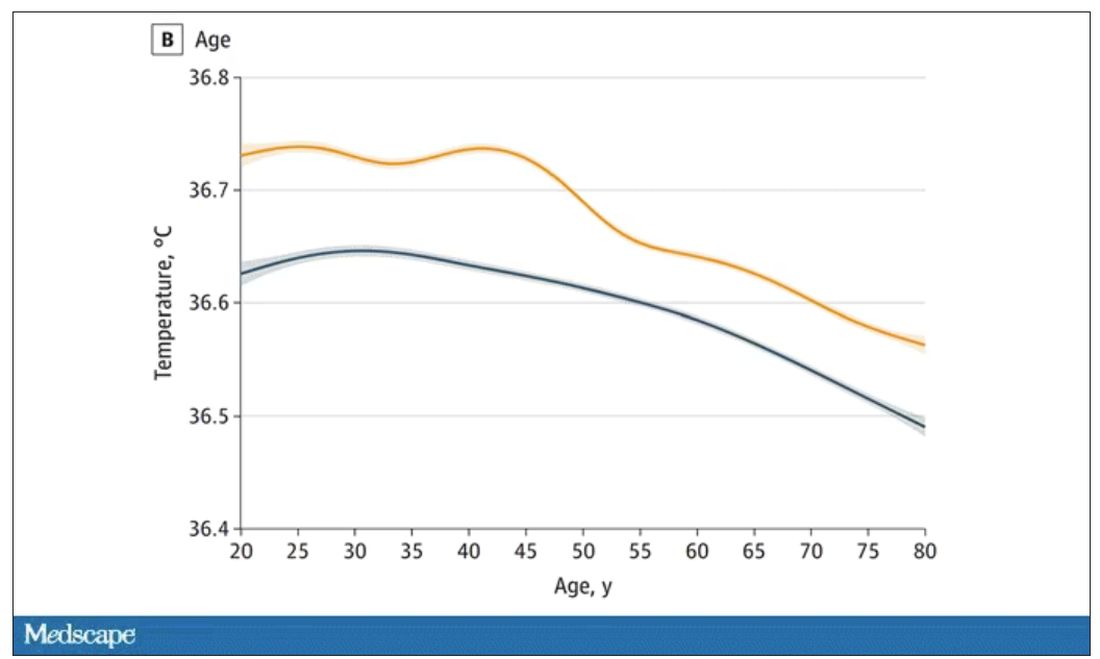
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.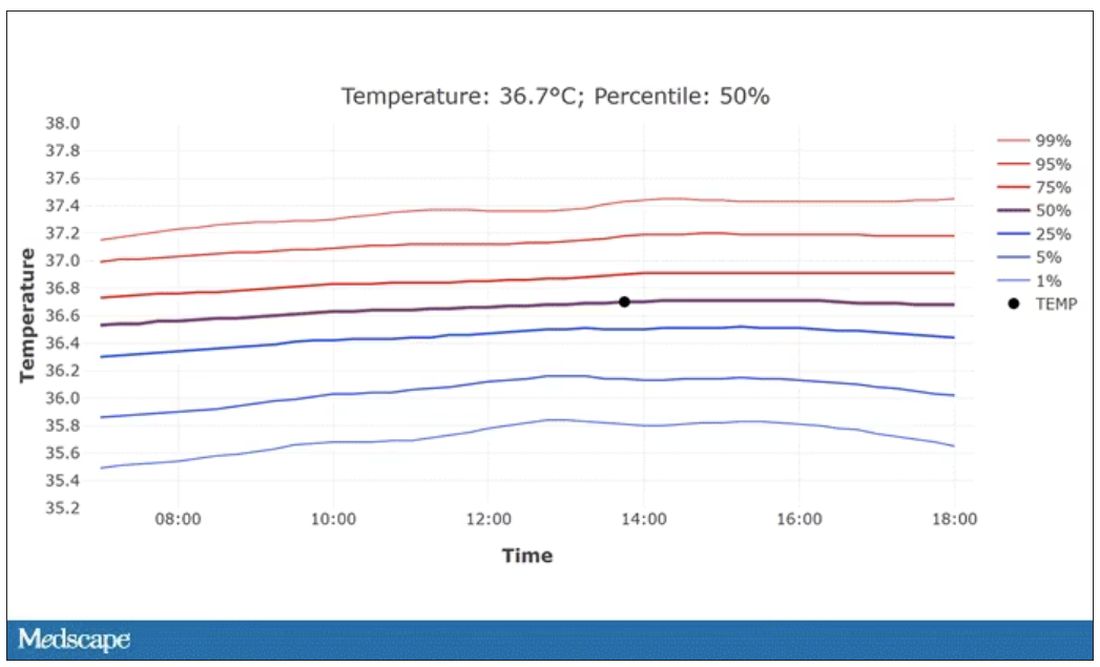
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
The cult of the suicide risk assessment
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
IQ and concussion recovery
Pediatric concussion is one of those rare phenomena in which we may be witnessing its emergence and clarification in a generation. When I was serving as the game doctor for our local high school football team in the 1970s, I and many other physicians had a very simplistic view of concussion. If the patient never lost conscious and had a reasonably intact short-term memory, we didn’t seriously entertain concussion as a diagnosis. “What’s the score and who is the president?” Were my favorite screening questions.
Obviously, we were underdiagnosing and mismanaging concussion. In part thanks to some high-profile athletes who suffered multiple concussions and eventually chronic traumatic encephalopathy (CTE) physicians began to realize that they should be looking more closely at children who sustained a head injury. The diagnostic criteria were expanded to include any injury that even temporarily effected brain function.
With the new appreciation for the risk of multiple concussions, the focus broadened to include the question of when is it safe for the athlete to return to competition. What signs or symptoms can the patient offer us so we can be sure his or her brain is sufficiently recovered? Here we stepped off into a deep abyss of ignorance. Fortunately, it became obvious fairly quickly that imaging studies weren’t going to help us, as they were invariably normal or at least didn’t tell us anything that wasn’t obvious on a physical exam.
If the patient had a headache, complained of dizziness, or manifested amnesia, monitoring the patient was fairly straightforward. But, in the absence of symptoms and no obvious way to determine the pace of recovery of an organ we couldn’t visualize, clinicians were pulling criteria and time tables out of thin air. Guessing that the concussed brain was in some ways like a torn muscle or overstretched tendon, “brain rest” was often suggested. So no TV, no reading, and certainly none of the cerebral challenging activity of school. Fortunately, we don’t hear much about the notion of brain rest anymore and there is at least one study that suggests that patients kept home from school recover more slowly.
But . Sometimes they describe headache or dizziness but often they complain of a vague mental unwellness. “Brain fog,” a term that has emerged in the wake of the COVID pandemic, might be an apt descriptor. Management of these slow recoverers has been a challenge.
However, two recent articles in the journal Pediatrics may provide some clarity and offer guidance in their management. In a study coming from the psychology department at Georgia State University, researchers reported that they have been able to find “no evidence of clinical meaningful differences in IQ after pediatric concussion.” In their words there is “strong evidence against reduced intelligence in the first few weeks to month after pediatric concussion.”
While their findings may simply toss the IQ onto the pile of worthless measures of healing, a companion commentary by Talin Babikian, PhD, a psychologist at the Semel Institute for Neuroscience and Human Behavior at UCLA, provides a more nuanced interpretation. He writes that if we are looking for an explanation when a patient’s recovery is taking longer than we might expect we need to look beyond some structural damage. Maybe the patient has a previously undiagnosed premorbid condition effecting his or her intellectual, cognitive, or learning abilities. Could the stall in improvement be the result of other symptoms? Here fatigue and sleep deprivation may be the culprits. Could some underlying emotional factor such as anxiety or depression be the problem? For example, I have seen patients whose fear of re-injury has prevented their return to full function. And, finally, the patient may be avoiding a “nonpreferred or challenging situation” unrelated to the injury.
In other words, the concussion may simply be the most obvious rip in a fabric that was already frayed and under stress. This kind of broad holistic (a word I usually like to avoid) thinking may be what is lacking as we struggle to understand other mysterious and chronic conditions such as Lyme disease and chronic fatigue syndrome.
While these two papers help provide some clarity in the management of pediatric concussion, what they fail to address is the bigger question of the relationship between head injury and CTE. The answers to that conundrum are enshrouded in a mix of politics and publicity that I doubt will clear in the near future.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Pediatric concussion is one of those rare phenomena in which we may be witnessing its emergence and clarification in a generation. When I was serving as the game doctor for our local high school football team in the 1970s, I and many other physicians had a very simplistic view of concussion. If the patient never lost conscious and had a reasonably intact short-term memory, we didn’t seriously entertain concussion as a diagnosis. “What’s the score and who is the president?” Were my favorite screening questions.
Obviously, we were underdiagnosing and mismanaging concussion. In part thanks to some high-profile athletes who suffered multiple concussions and eventually chronic traumatic encephalopathy (CTE) physicians began to realize that they should be looking more closely at children who sustained a head injury. The diagnostic criteria were expanded to include any injury that even temporarily effected brain function.
With the new appreciation for the risk of multiple concussions, the focus broadened to include the question of when is it safe for the athlete to return to competition. What signs or symptoms can the patient offer us so we can be sure his or her brain is sufficiently recovered? Here we stepped off into a deep abyss of ignorance. Fortunately, it became obvious fairly quickly that imaging studies weren’t going to help us, as they were invariably normal or at least didn’t tell us anything that wasn’t obvious on a physical exam.
If the patient had a headache, complained of dizziness, or manifested amnesia, monitoring the patient was fairly straightforward. But, in the absence of symptoms and no obvious way to determine the pace of recovery of an organ we couldn’t visualize, clinicians were pulling criteria and time tables out of thin air. Guessing that the concussed brain was in some ways like a torn muscle or overstretched tendon, “brain rest” was often suggested. So no TV, no reading, and certainly none of the cerebral challenging activity of school. Fortunately, we don’t hear much about the notion of brain rest anymore and there is at least one study that suggests that patients kept home from school recover more slowly.
But . Sometimes they describe headache or dizziness but often they complain of a vague mental unwellness. “Brain fog,” a term that has emerged in the wake of the COVID pandemic, might be an apt descriptor. Management of these slow recoverers has been a challenge.
However, two recent articles in the journal Pediatrics may provide some clarity and offer guidance in their management. In a study coming from the psychology department at Georgia State University, researchers reported that they have been able to find “no evidence of clinical meaningful differences in IQ after pediatric concussion.” In their words there is “strong evidence against reduced intelligence in the first few weeks to month after pediatric concussion.”
While their findings may simply toss the IQ onto the pile of worthless measures of healing, a companion commentary by Talin Babikian, PhD, a psychologist at the Semel Institute for Neuroscience and Human Behavior at UCLA, provides a more nuanced interpretation. He writes that if we are looking for an explanation when a patient’s recovery is taking longer than we might expect we need to look beyond some structural damage. Maybe the patient has a previously undiagnosed premorbid condition effecting his or her intellectual, cognitive, or learning abilities. Could the stall in improvement be the result of other symptoms? Here fatigue and sleep deprivation may be the culprits. Could some underlying emotional factor such as anxiety or depression be the problem? For example, I have seen patients whose fear of re-injury has prevented their return to full function. And, finally, the patient may be avoiding a “nonpreferred or challenging situation” unrelated to the injury.
In other words, the concussion may simply be the most obvious rip in a fabric that was already frayed and under stress. This kind of broad holistic (a word I usually like to avoid) thinking may be what is lacking as we struggle to understand other mysterious and chronic conditions such as Lyme disease and chronic fatigue syndrome.
While these two papers help provide some clarity in the management of pediatric concussion, what they fail to address is the bigger question of the relationship between head injury and CTE. The answers to that conundrum are enshrouded in a mix of politics and publicity that I doubt will clear in the near future.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Pediatric concussion is one of those rare phenomena in which we may be witnessing its emergence and clarification in a generation. When I was serving as the game doctor for our local high school football team in the 1970s, I and many other physicians had a very simplistic view of concussion. If the patient never lost conscious and had a reasonably intact short-term memory, we didn’t seriously entertain concussion as a diagnosis. “What’s the score and who is the president?” Were my favorite screening questions.
Obviously, we were underdiagnosing and mismanaging concussion. In part thanks to some high-profile athletes who suffered multiple concussions and eventually chronic traumatic encephalopathy (CTE) physicians began to realize that they should be looking more closely at children who sustained a head injury. The diagnostic criteria were expanded to include any injury that even temporarily effected brain function.
With the new appreciation for the risk of multiple concussions, the focus broadened to include the question of when is it safe for the athlete to return to competition. What signs or symptoms can the patient offer us so we can be sure his or her brain is sufficiently recovered? Here we stepped off into a deep abyss of ignorance. Fortunately, it became obvious fairly quickly that imaging studies weren’t going to help us, as they were invariably normal or at least didn’t tell us anything that wasn’t obvious on a physical exam.
If the patient had a headache, complained of dizziness, or manifested amnesia, monitoring the patient was fairly straightforward. But, in the absence of symptoms and no obvious way to determine the pace of recovery of an organ we couldn’t visualize, clinicians were pulling criteria and time tables out of thin air. Guessing that the concussed brain was in some ways like a torn muscle or overstretched tendon, “brain rest” was often suggested. So no TV, no reading, and certainly none of the cerebral challenging activity of school. Fortunately, we don’t hear much about the notion of brain rest anymore and there is at least one study that suggests that patients kept home from school recover more slowly.
But . Sometimes they describe headache or dizziness but often they complain of a vague mental unwellness. “Brain fog,” a term that has emerged in the wake of the COVID pandemic, might be an apt descriptor. Management of these slow recoverers has been a challenge.
However, two recent articles in the journal Pediatrics may provide some clarity and offer guidance in their management. In a study coming from the psychology department at Georgia State University, researchers reported that they have been able to find “no evidence of clinical meaningful differences in IQ after pediatric concussion.” In their words there is “strong evidence against reduced intelligence in the first few weeks to month after pediatric concussion.”
While their findings may simply toss the IQ onto the pile of worthless measures of healing, a companion commentary by Talin Babikian, PhD, a psychologist at the Semel Institute for Neuroscience and Human Behavior at UCLA, provides a more nuanced interpretation. He writes that if we are looking for an explanation when a patient’s recovery is taking longer than we might expect we need to look beyond some structural damage. Maybe the patient has a previously undiagnosed premorbid condition effecting his or her intellectual, cognitive, or learning abilities. Could the stall in improvement be the result of other symptoms? Here fatigue and sleep deprivation may be the culprits. Could some underlying emotional factor such as anxiety or depression be the problem? For example, I have seen patients whose fear of re-injury has prevented their return to full function. And, finally, the patient may be avoiding a “nonpreferred or challenging situation” unrelated to the injury.
In other words, the concussion may simply be the most obvious rip in a fabric that was already frayed and under stress. This kind of broad holistic (a word I usually like to avoid) thinking may be what is lacking as we struggle to understand other mysterious and chronic conditions such as Lyme disease and chronic fatigue syndrome.
While these two papers help provide some clarity in the management of pediatric concussion, what they fail to address is the bigger question of the relationship between head injury and CTE. The answers to that conundrum are enshrouded in a mix of politics and publicity that I doubt will clear in the near future.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Can a decrease in dopamine lead to binge eating?
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When does a bicarb drip make sense?
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
The magic of music
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The most important study from ESC: FRAIL-AF
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.