User login
‘Gift That Keeps Giving’: The Impact of GLP-1 in Asthma
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
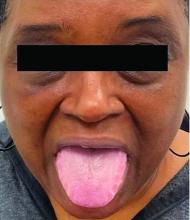
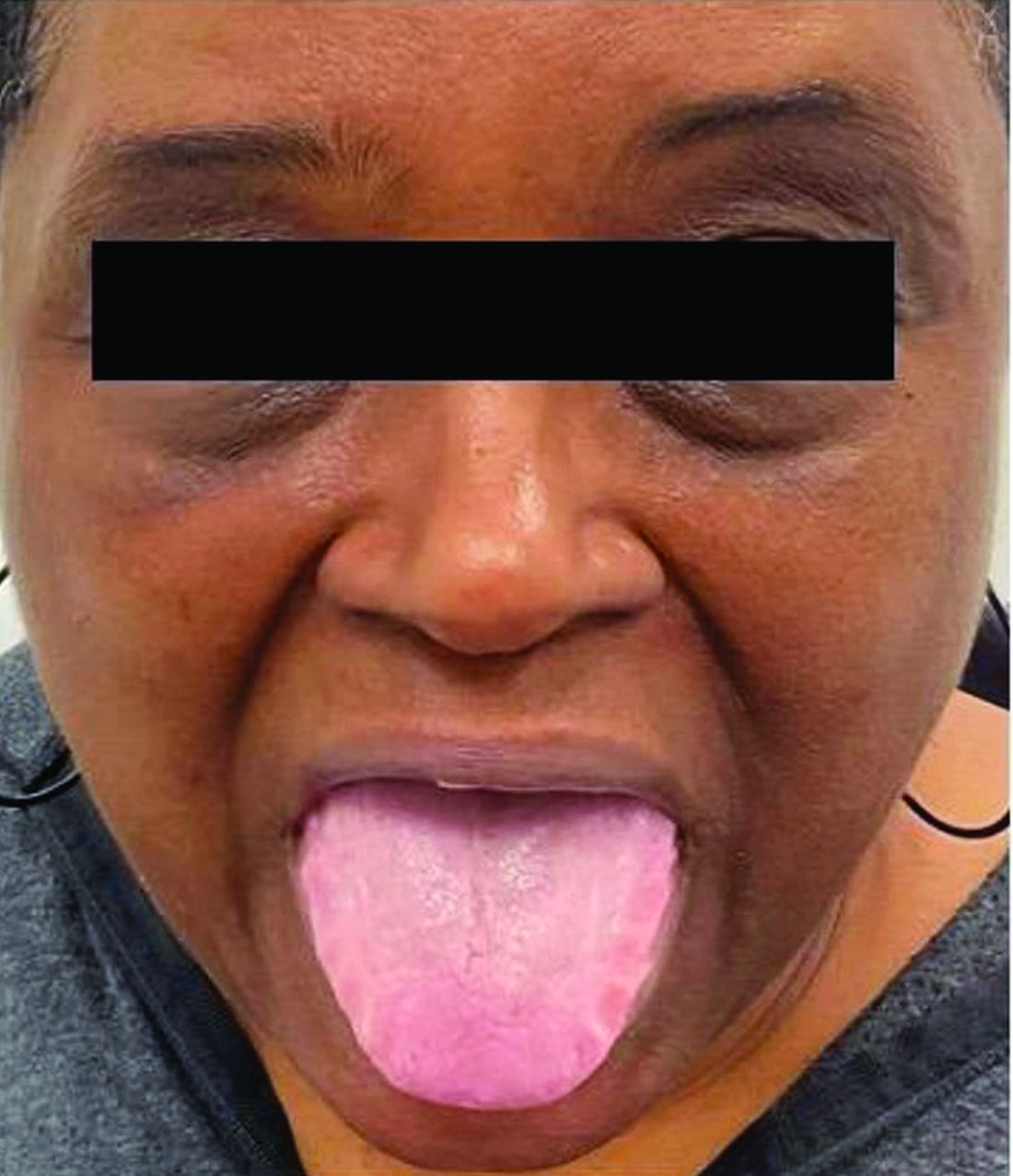
A 62-year-old Black female presented with an epidermal inclusion cyst on her left upper back
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
Low HPV Vaccination in the United States Is a Public Health ‘Failure’
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
It’s Never Too Late to Convince Patients to Quit Smoking
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Beers Criteria Update for Inappropriate Medication Use in Older Adults
Primary care physicians know the complexities of treating older patients, from increased complications from medications and procedures to comorbidities stemming from having multiple medical conditions. The Beers Criteria were established by the American Geriatrics Society as a guide for physicians about medications that may possess more risks than benefits in older patients, specifically those aged 65 years and older.
There are approximately 100 medications on the list. Criteria used to establish the list include medications to avoid over the age of 65 in an outpatient setting, medications to avoid in certain medical conditions, medications to avoid that may interact with other medications, medications to avoid with renal impairment, and medications to avoid where harmful side effects outweigh the possible benefits. The American Geriatrics Society updates the list as new published evidence becomes available.
The latest updates to the Beers Criteria include several medications commonly used in primary care. Regarding anticoagulation, warfarin should be avoided as initial therapy and apixaban should be used in patients with reduced renal function. These guidelines looked particularly at antithrombotic medications because of new evidence arising in nonvalvular atrial fibrillation and venous thromboembolism. In addition to the previous recommendations, the use of aspirin is no longer recommended in older adults.
The latest guidelines also make recommendations regarding certain diabetic medications as well as combinations to avoid. The Beers Criteria now place all sulfonylureas in the class to avoid, and not just the long-acting formulations as was recommended in the previous guidelines. If a sulfonylurea is necessary, use of a short-acting one is advised. Several other classes of medications were addressed and doctors practicing primary care medicine should be aware of these guidelines, especially as the population continues to age.
Overall, these guidelines are a great resource for treating patients aged 65 and older. It is important to keep in mind that they look at a whole population of patients and it is not patient specific. As primary care doctors, we know many of our patients don’t fit into the textbook box. While these guidelines consider the dangers of a certain medication, sometimes the benefits do outweigh the risks at the patient-specific level.
As doctors, we are trained to weigh the risks and benefits when prescribing any medication to our patients. These guidelines shouldn’t be approached as a do or don’t list but should be considered in the overall plan when prescribing for our patients. Sometimes, these medications can be used with careful observation by the prescribing physician. When they are utilized, we need to make the patient aware of specific side effects and what to watch out for. We need to make these decisions together with our patients and their caregivers.
For example, we all know how agonizing taking care of an older dementia patient can be, and sometimes there is nothing left to try except one of the medications on the list.
An additional practical point not considered in the guidelines is real-world use. Often, certain medications are not covered by a patient’s insurance company. The cost can be prohibitive to use the recommended agent. We are left in the middle to go off script with a medication that the patient may be able to access easily or keep pushing for the most appropriate medication for the patient. Unfortunately, in our current healthcare climate, prior authorizations can sometimes take weeks to obtain (or to be denied). For most of the conditions we treat in our older patients, it is not safe to leave them without any medication while we fight this prior authorizations war.
Our older patients often have multiple specialists as well. Each of these specialists may be prescribing different medications. It is imperative that we know all the medications a patient is taking so that we may look for potentially dangerous drug interactions. Many patients don’t remember the names of all their medications, nor do they realize that many classes of medications are “little white pills.” Asking them to bring their pill bottles to every visit can be a great help in searching out interactions.
That being said, the Beers Criteria do an excellent job reviewing the latest evidence and developing guidelines. As primary care physicians, we have never been busier and having someone do the research and set it forth so clearly is a great tool. We should be aware of the Beers Criteria and the medications and interactions listed there.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no conflicts of interest.
Primary care physicians know the complexities of treating older patients, from increased complications from medications and procedures to comorbidities stemming from having multiple medical conditions. The Beers Criteria were established by the American Geriatrics Society as a guide for physicians about medications that may possess more risks than benefits in older patients, specifically those aged 65 years and older.
There are approximately 100 medications on the list. Criteria used to establish the list include medications to avoid over the age of 65 in an outpatient setting, medications to avoid in certain medical conditions, medications to avoid that may interact with other medications, medications to avoid with renal impairment, and medications to avoid where harmful side effects outweigh the possible benefits. The American Geriatrics Society updates the list as new published evidence becomes available.
The latest updates to the Beers Criteria include several medications commonly used in primary care. Regarding anticoagulation, warfarin should be avoided as initial therapy and apixaban should be used in patients with reduced renal function. These guidelines looked particularly at antithrombotic medications because of new evidence arising in nonvalvular atrial fibrillation and venous thromboembolism. In addition to the previous recommendations, the use of aspirin is no longer recommended in older adults.
The latest guidelines also make recommendations regarding certain diabetic medications as well as combinations to avoid. The Beers Criteria now place all sulfonylureas in the class to avoid, and not just the long-acting formulations as was recommended in the previous guidelines. If a sulfonylurea is necessary, use of a short-acting one is advised. Several other classes of medications were addressed and doctors practicing primary care medicine should be aware of these guidelines, especially as the population continues to age.
Overall, these guidelines are a great resource for treating patients aged 65 and older. It is important to keep in mind that they look at a whole population of patients and it is not patient specific. As primary care doctors, we know many of our patients don’t fit into the textbook box. While these guidelines consider the dangers of a certain medication, sometimes the benefits do outweigh the risks at the patient-specific level.
As doctors, we are trained to weigh the risks and benefits when prescribing any medication to our patients. These guidelines shouldn’t be approached as a do or don’t list but should be considered in the overall plan when prescribing for our patients. Sometimes, these medications can be used with careful observation by the prescribing physician. When they are utilized, we need to make the patient aware of specific side effects and what to watch out for. We need to make these decisions together with our patients and their caregivers.
For example, we all know how agonizing taking care of an older dementia patient can be, and sometimes there is nothing left to try except one of the medications on the list.
An additional practical point not considered in the guidelines is real-world use. Often, certain medications are not covered by a patient’s insurance company. The cost can be prohibitive to use the recommended agent. We are left in the middle to go off script with a medication that the patient may be able to access easily or keep pushing for the most appropriate medication for the patient. Unfortunately, in our current healthcare climate, prior authorizations can sometimes take weeks to obtain (or to be denied). For most of the conditions we treat in our older patients, it is not safe to leave them without any medication while we fight this prior authorizations war.
Our older patients often have multiple specialists as well. Each of these specialists may be prescribing different medications. It is imperative that we know all the medications a patient is taking so that we may look for potentially dangerous drug interactions. Many patients don’t remember the names of all their medications, nor do they realize that many classes of medications are “little white pills.” Asking them to bring their pill bottles to every visit can be a great help in searching out interactions.
That being said, the Beers Criteria do an excellent job reviewing the latest evidence and developing guidelines. As primary care physicians, we have never been busier and having someone do the research and set it forth so clearly is a great tool. We should be aware of the Beers Criteria and the medications and interactions listed there.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no conflicts of interest.
Primary care physicians know the complexities of treating older patients, from increased complications from medications and procedures to comorbidities stemming from having multiple medical conditions. The Beers Criteria were established by the American Geriatrics Society as a guide for physicians about medications that may possess more risks than benefits in older patients, specifically those aged 65 years and older.
There are approximately 100 medications on the list. Criteria used to establish the list include medications to avoid over the age of 65 in an outpatient setting, medications to avoid in certain medical conditions, medications to avoid that may interact with other medications, medications to avoid with renal impairment, and medications to avoid where harmful side effects outweigh the possible benefits. The American Geriatrics Society updates the list as new published evidence becomes available.
The latest updates to the Beers Criteria include several medications commonly used in primary care. Regarding anticoagulation, warfarin should be avoided as initial therapy and apixaban should be used in patients with reduced renal function. These guidelines looked particularly at antithrombotic medications because of new evidence arising in nonvalvular atrial fibrillation and venous thromboembolism. In addition to the previous recommendations, the use of aspirin is no longer recommended in older adults.
The latest guidelines also make recommendations regarding certain diabetic medications as well as combinations to avoid. The Beers Criteria now place all sulfonylureas in the class to avoid, and not just the long-acting formulations as was recommended in the previous guidelines. If a sulfonylurea is necessary, use of a short-acting one is advised. Several other classes of medications were addressed and doctors practicing primary care medicine should be aware of these guidelines, especially as the population continues to age.
Overall, these guidelines are a great resource for treating patients aged 65 and older. It is important to keep in mind that they look at a whole population of patients and it is not patient specific. As primary care doctors, we know many of our patients don’t fit into the textbook box. While these guidelines consider the dangers of a certain medication, sometimes the benefits do outweigh the risks at the patient-specific level.
As doctors, we are trained to weigh the risks and benefits when prescribing any medication to our patients. These guidelines shouldn’t be approached as a do or don’t list but should be considered in the overall plan when prescribing for our patients. Sometimes, these medications can be used with careful observation by the prescribing physician. When they are utilized, we need to make the patient aware of specific side effects and what to watch out for. We need to make these decisions together with our patients and their caregivers.
For example, we all know how agonizing taking care of an older dementia patient can be, and sometimes there is nothing left to try except one of the medications on the list.
An additional practical point not considered in the guidelines is real-world use. Often, certain medications are not covered by a patient’s insurance company. The cost can be prohibitive to use the recommended agent. We are left in the middle to go off script with a medication that the patient may be able to access easily or keep pushing for the most appropriate medication for the patient. Unfortunately, in our current healthcare climate, prior authorizations can sometimes take weeks to obtain (or to be denied). For most of the conditions we treat in our older patients, it is not safe to leave them without any medication while we fight this prior authorizations war.
Our older patients often have multiple specialists as well. Each of these specialists may be prescribing different medications. It is imperative that we know all the medications a patient is taking so that we may look for potentially dangerous drug interactions. Many patients don’t remember the names of all their medications, nor do they realize that many classes of medications are “little white pills.” Asking them to bring their pill bottles to every visit can be a great help in searching out interactions.
That being said, the Beers Criteria do an excellent job reviewing the latest evidence and developing guidelines. As primary care physicians, we have never been busier and having someone do the research and set it forth so clearly is a great tool. We should be aware of the Beers Criteria and the medications and interactions listed there.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no conflicts of interest.
A Checklist for Compounded Semaglutide or Tirzepatide
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
What You Need to Know About Oropouche Virus Disease
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
E-Bikes: The Good ... and the Ugly
Bicycles have been woven into my life since I first straddled a hand-me-down with a fan belt drive when I was 3. At age 12 my friend Ricky and I took a 250 mile–plus 2-night adventure on our 3-speed “English” style bikes. We still marvel that our parents let us do it when neither cell phones nor GPS existed.
I have always bike commuted to work, including the years when that involved a perilous navigation into Boston from the suburbs. In our mid-50s my wife and I biked from Washington state back here to Maine with another couple unsupported. We continue to do at least one self-guided cycle tour out of the country each year.
Not surprisingly, I keep a close eye on what’s happening in the bicycle market. For decades the trends have shifted back and forth between sleek road models and beefier off-roaders. There have been boom years here and there for the dealers and manufacturers, but nothing like what the bike industry is experiencing now with the arrival of e-bikes on the market. Driven primarily by electrification, micromobility ridership (which includes conventional bikes and scooters) has grown more than 50-fold over the last 10 years. Projections suggest the market’s value will be $300 billion by 2030.
It doesn’t take an MBA with a major in marketing to understand the broad appeal of electrification. Most adults have ridden a bicycle as children, but several decades of gap years has left many of them with a level of fitness that makes pedaling against the wind or up any incline difficult and unappealing. An e-bike can put even the least fitness conscious back in the saddle and open the options for outdoor recreation they haven’t dreamed of since childhood.
In large part the people flocking to e-bikes are retiree’s who thought they were “over the hill.” They are having so much fun they don’t care if the Lycra-clad “serious” cyclists notice the battery bulge in the frame on their e-bikes. Another group of e-bike adopters are motivated by the “greenness” of a fossil-fuel–free electric powered transportation which, with minimal compromise, can be used as they would a car around town and for longer commutes than they would have considered on a purely pedal-powered bicycle.
Unfortunately, there is a growing group of younger e-bike riders who are motivated and uninhibited by the potential that the power boost of a small electric motor can provide. And here is where the ugliness begins to intrude on what was otherwise a beautiful and expanding landscape. However, it is the young who are, not surprisingly, drawn to the speed, and with any vehicle – motorized or conventional – as speed increases so does the frequency and seriousness of accidents.
The term e-bike covers a broad range of vehicles, from those designated class 1, which require pedaling and are limited to 20 miles per hour, to class 3, which may have a throttle and unmodified can hit 28 mph. Class 2 bikes have a throttle that will allow the rider to reach 20 mph without pedaling. Modifying any class of e-bike can substantially increase its speed, but this is more common in classes 2 and 3. As an example, some very fast micromobiles are considered unclassified e-bikes and avoid being labeled motorcycles simply because they have pedals.
One has to give some credit to the e-bike industry for eventually adopting this classification system. But, we must give the rest of us, including parents and public safety officials, a failing grade for doing a poor job of translating these scores into enforceable regulations to protect both riders and pedestrians from serious injury.
On the governmental side only a little more than half of US states have used the three category classification to craft their regulations. Many jurisdictions have failed to differentiate between streets, sidewalks, and trails. Regulations vary from state to state, and many states leave it up to local communities. From my experience chairing our town’s Bicycle and Pedestrian Advisory Committee, I can tell you that even “progressive” communities are struggling to decide who can ride what where. The result has been that people of all ages, but mostly adolescents, are traveling on busy streets and sidewalks at speeds that put themselves and pedestrians at risk.
On the parental side of the problem are families that have either allowed or enabled their children to ride class 2 and 3 e-bikes without proper safety equipment or consideration for the safety of the rest of the community. Currently, this is not much of a problem here in Maine thanks to the weather and the high price of e-bikes. However, I frequently visit an affluent community in the San Francisco Bay Area, where it is not uncommon to see middle school children speeding along well in excess of 20 mph.
Unfortunately this is another example, like television and cell phone, in which our society has been unable to keep up with technology by molding the behavior of our children and/or creating enforceable rules that allow us to reap the benefits of new discoveries while minimizing the collateral damage that can accompany them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Bicycles have been woven into my life since I first straddled a hand-me-down with a fan belt drive when I was 3. At age 12 my friend Ricky and I took a 250 mile–plus 2-night adventure on our 3-speed “English” style bikes. We still marvel that our parents let us do it when neither cell phones nor GPS existed.
I have always bike commuted to work, including the years when that involved a perilous navigation into Boston from the suburbs. In our mid-50s my wife and I biked from Washington state back here to Maine with another couple unsupported. We continue to do at least one self-guided cycle tour out of the country each year.
Not surprisingly, I keep a close eye on what’s happening in the bicycle market. For decades the trends have shifted back and forth between sleek road models and beefier off-roaders. There have been boom years here and there for the dealers and manufacturers, but nothing like what the bike industry is experiencing now with the arrival of e-bikes on the market. Driven primarily by electrification, micromobility ridership (which includes conventional bikes and scooters) has grown more than 50-fold over the last 10 years. Projections suggest the market’s value will be $300 billion by 2030.
It doesn’t take an MBA with a major in marketing to understand the broad appeal of electrification. Most adults have ridden a bicycle as children, but several decades of gap years has left many of them with a level of fitness that makes pedaling against the wind or up any incline difficult and unappealing. An e-bike can put even the least fitness conscious back in the saddle and open the options for outdoor recreation they haven’t dreamed of since childhood.
In large part the people flocking to e-bikes are retiree’s who thought they were “over the hill.” They are having so much fun they don’t care if the Lycra-clad “serious” cyclists notice the battery bulge in the frame on their e-bikes. Another group of e-bike adopters are motivated by the “greenness” of a fossil-fuel–free electric powered transportation which, with minimal compromise, can be used as they would a car around town and for longer commutes than they would have considered on a purely pedal-powered bicycle.
Unfortunately, there is a growing group of younger e-bike riders who are motivated and uninhibited by the potential that the power boost of a small electric motor can provide. And here is where the ugliness begins to intrude on what was otherwise a beautiful and expanding landscape. However, it is the young who are, not surprisingly, drawn to the speed, and with any vehicle – motorized or conventional – as speed increases so does the frequency and seriousness of accidents.
The term e-bike covers a broad range of vehicles, from those designated class 1, which require pedaling and are limited to 20 miles per hour, to class 3, which may have a throttle and unmodified can hit 28 mph. Class 2 bikes have a throttle that will allow the rider to reach 20 mph without pedaling. Modifying any class of e-bike can substantially increase its speed, but this is more common in classes 2 and 3. As an example, some very fast micromobiles are considered unclassified e-bikes and avoid being labeled motorcycles simply because they have pedals.
One has to give some credit to the e-bike industry for eventually adopting this classification system. But, we must give the rest of us, including parents and public safety officials, a failing grade for doing a poor job of translating these scores into enforceable regulations to protect both riders and pedestrians from serious injury.
On the governmental side only a little more than half of US states have used the three category classification to craft their regulations. Many jurisdictions have failed to differentiate between streets, sidewalks, and trails. Regulations vary from state to state, and many states leave it up to local communities. From my experience chairing our town’s Bicycle and Pedestrian Advisory Committee, I can tell you that even “progressive” communities are struggling to decide who can ride what where. The result has been that people of all ages, but mostly adolescents, are traveling on busy streets and sidewalks at speeds that put themselves and pedestrians at risk.
On the parental side of the problem are families that have either allowed or enabled their children to ride class 2 and 3 e-bikes without proper safety equipment or consideration for the safety of the rest of the community. Currently, this is not much of a problem here in Maine thanks to the weather and the high price of e-bikes. However, I frequently visit an affluent community in the San Francisco Bay Area, where it is not uncommon to see middle school children speeding along well in excess of 20 mph.
Unfortunately this is another example, like television and cell phone, in which our society has been unable to keep up with technology by molding the behavior of our children and/or creating enforceable rules that allow us to reap the benefits of new discoveries while minimizing the collateral damage that can accompany them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Bicycles have been woven into my life since I first straddled a hand-me-down with a fan belt drive when I was 3. At age 12 my friend Ricky and I took a 250 mile–plus 2-night adventure on our 3-speed “English” style bikes. We still marvel that our parents let us do it when neither cell phones nor GPS existed.
I have always bike commuted to work, including the years when that involved a perilous navigation into Boston from the suburbs. In our mid-50s my wife and I biked from Washington state back here to Maine with another couple unsupported. We continue to do at least one self-guided cycle tour out of the country each year.
Not surprisingly, I keep a close eye on what’s happening in the bicycle market. For decades the trends have shifted back and forth between sleek road models and beefier off-roaders. There have been boom years here and there for the dealers and manufacturers, but nothing like what the bike industry is experiencing now with the arrival of e-bikes on the market. Driven primarily by electrification, micromobility ridership (which includes conventional bikes and scooters) has grown more than 50-fold over the last 10 years. Projections suggest the market’s value will be $300 billion by 2030.
It doesn’t take an MBA with a major in marketing to understand the broad appeal of electrification. Most adults have ridden a bicycle as children, but several decades of gap years has left many of them with a level of fitness that makes pedaling against the wind or up any incline difficult and unappealing. An e-bike can put even the least fitness conscious back in the saddle and open the options for outdoor recreation they haven’t dreamed of since childhood.
In large part the people flocking to e-bikes are retiree’s who thought they were “over the hill.” They are having so much fun they don’t care if the Lycra-clad “serious” cyclists notice the battery bulge in the frame on their e-bikes. Another group of e-bike adopters are motivated by the “greenness” of a fossil-fuel–free electric powered transportation which, with minimal compromise, can be used as they would a car around town and for longer commutes than they would have considered on a purely pedal-powered bicycle.
Unfortunately, there is a growing group of younger e-bike riders who are motivated and uninhibited by the potential that the power boost of a small electric motor can provide. And here is where the ugliness begins to intrude on what was otherwise a beautiful and expanding landscape. However, it is the young who are, not surprisingly, drawn to the speed, and with any vehicle – motorized or conventional – as speed increases so does the frequency and seriousness of accidents.
The term e-bike covers a broad range of vehicles, from those designated class 1, which require pedaling and are limited to 20 miles per hour, to class 3, which may have a throttle and unmodified can hit 28 mph. Class 2 bikes have a throttle that will allow the rider to reach 20 mph without pedaling. Modifying any class of e-bike can substantially increase its speed, but this is more common in classes 2 and 3. As an example, some very fast micromobiles are considered unclassified e-bikes and avoid being labeled motorcycles simply because they have pedals.
One has to give some credit to the e-bike industry for eventually adopting this classification system. But, we must give the rest of us, including parents and public safety officials, a failing grade for doing a poor job of translating these scores into enforceable regulations to protect both riders and pedestrians from serious injury.
On the governmental side only a little more than half of US states have used the three category classification to craft their regulations. Many jurisdictions have failed to differentiate between streets, sidewalks, and trails. Regulations vary from state to state, and many states leave it up to local communities. From my experience chairing our town’s Bicycle and Pedestrian Advisory Committee, I can tell you that even “progressive” communities are struggling to decide who can ride what where. The result has been that people of all ages, but mostly adolescents, are traveling on busy streets and sidewalks at speeds that put themselves and pedestrians at risk.
On the parental side of the problem are families that have either allowed or enabled their children to ride class 2 and 3 e-bikes without proper safety equipment or consideration for the safety of the rest of the community. Currently, this is not much of a problem here in Maine thanks to the weather and the high price of e-bikes. However, I frequently visit an affluent community in the San Francisco Bay Area, where it is not uncommon to see middle school children speeding along well in excess of 20 mph.
Unfortunately this is another example, like television and cell phone, in which our society has been unable to keep up with technology by molding the behavior of our children and/or creating enforceable rules that allow us to reap the benefits of new discoveries while minimizing the collateral damage that can accompany them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Viral Season 2024-2025: Try for An Ounce of Prevention
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
Doctor I-Don’t-Know
Many, many years ago there was a Thanksgiving when as I was just beginning to earn a reputation in my wife’s family. There were no place cards on the table and the usual hovering and jockeying seats was well underway. From behind me I heard one of my young nieces pipe up: “I want to sit next to Doctor I-don’t-know.”
After a few words of negotiation we were all settled in our places and ready to enjoy our meal. It took only a few seconds of introspection for me to grasp how I had received that moniker, which some physicians might consider disrespectful.
I was the only physician within several generations of that family and, as such, my in-laws thought it only appropriate to ask me medical questions. They courteously seemed to avoid personal questions about their own health and were particularly careful not to roll up their sleeves or unbutton their shirts to show me a lesion or a recently acquired surgical scar. No, my wife’s family members were curious. They wanted answers to deeper questions, the hard science so to speak. “How does aspirin work?” was a typical and painful example. Maybe pharmacologists today have better answers but 40 years ago I’m not so sure; I certainly didn’t know back then and would reply, “I don’t know.” Probably for the third or fourth time that day.
Usually I genuinely didn’t know the answer. However, sometimes my answer was going to be so different from the beliefs and biases of my inquisitor that, in the interest of expediency, “I don’t know” seemed the most appropriate response.
If you were reading Letters from Maine 25 years ago, that scenario might sound familiar. I have chosen to pull it out of the archives as a jumping-off point for a consideration of the unfortunate example some of us set when the COVID pandemic threw a tsunami of unknowns at us. Too many physician-“experts” were afraid to say, “I don’t know.” Instead, and maybe because, they themselves were afraid that the patients couldn’t handle the truth that none of us in the profession knew the correct answers. When so many initial pronouncements proved incorrect, it was too late to undo the damage that had been done to the community’s trust in the rest of us.
It turns out that my in-laws were not the only folks who thought of me as Doctor I-don’t-know. One of the perks of remaining in the same community after one retires is that encounters with former patients and their parents happen frequently. On more than one occasion a parent has thanked me for admitting my ignorance. Some have even claimed that my candid approach was what they remembered most fondly. And, that quality increased their trust when I finally provided an answer.
There is an art to delivering “I don’t know.” Thirty years ago I would excuse myself and tell the family I was going to my office to pull a book off the shelf or call a previous mentor. Now one only needs to ask Dr. Google. No need to leave the room. If appropriate, the provider can swing the computer screen so that the patient can share in the search for the answer.
That strategy only works when the provider merely needs to update or expand his/her knowledge. However, there are those difficult situations when no one could know the answer given the current parameters of the patient’s situation. More lab work might be needed. It may be too early in the trajectory of the patient’s illness for the illnesses signs and symptoms to declare themselves.
In these situations “I don’t know” must be followed by a “but.” It is what comes after that “but” and how it is delivered that can convert the provider’s admission of ignorance into a demonstration of his or her character. Is he/she a caring person trying to understand the patient’s concerns? Willing to enter into a cooperative relationship as together they search for the cause and hopefully for a cure for the patient’s currently mysterious illness?
I recently read about a physician who is encouraging medical educators to incorporate more discussions of “humility” and its role in patient care into the medical school and postgraduate training curricula. He feels, as do I, that
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Many, many years ago there was a Thanksgiving when as I was just beginning to earn a reputation in my wife’s family. There were no place cards on the table and the usual hovering and jockeying seats was well underway. From behind me I heard one of my young nieces pipe up: “I want to sit next to Doctor I-don’t-know.”
After a few words of negotiation we were all settled in our places and ready to enjoy our meal. It took only a few seconds of introspection for me to grasp how I had received that moniker, which some physicians might consider disrespectful.
I was the only physician within several generations of that family and, as such, my in-laws thought it only appropriate to ask me medical questions. They courteously seemed to avoid personal questions about their own health and were particularly careful not to roll up their sleeves or unbutton their shirts to show me a lesion or a recently acquired surgical scar. No, my wife’s family members were curious. They wanted answers to deeper questions, the hard science so to speak. “How does aspirin work?” was a typical and painful example. Maybe pharmacologists today have better answers but 40 years ago I’m not so sure; I certainly didn’t know back then and would reply, “I don’t know.” Probably for the third or fourth time that day.
Usually I genuinely didn’t know the answer. However, sometimes my answer was going to be so different from the beliefs and biases of my inquisitor that, in the interest of expediency, “I don’t know” seemed the most appropriate response.
If you were reading Letters from Maine 25 years ago, that scenario might sound familiar. I have chosen to pull it out of the archives as a jumping-off point for a consideration of the unfortunate example some of us set when the COVID pandemic threw a tsunami of unknowns at us. Too many physician-“experts” were afraid to say, “I don’t know.” Instead, and maybe because, they themselves were afraid that the patients couldn’t handle the truth that none of us in the profession knew the correct answers. When so many initial pronouncements proved incorrect, it was too late to undo the damage that had been done to the community’s trust in the rest of us.
It turns out that my in-laws were not the only folks who thought of me as Doctor I-don’t-know. One of the perks of remaining in the same community after one retires is that encounters with former patients and their parents happen frequently. On more than one occasion a parent has thanked me for admitting my ignorance. Some have even claimed that my candid approach was what they remembered most fondly. And, that quality increased their trust when I finally provided an answer.
There is an art to delivering “I don’t know.” Thirty years ago I would excuse myself and tell the family I was going to my office to pull a book off the shelf or call a previous mentor. Now one only needs to ask Dr. Google. No need to leave the room. If appropriate, the provider can swing the computer screen so that the patient can share in the search for the answer.
That strategy only works when the provider merely needs to update or expand his/her knowledge. However, there are those difficult situations when no one could know the answer given the current parameters of the patient’s situation. More lab work might be needed. It may be too early in the trajectory of the patient’s illness for the illnesses signs and symptoms to declare themselves.
In these situations “I don’t know” must be followed by a “but.” It is what comes after that “but” and how it is delivered that can convert the provider’s admission of ignorance into a demonstration of his or her character. Is he/she a caring person trying to understand the patient’s concerns? Willing to enter into a cooperative relationship as together they search for the cause and hopefully for a cure for the patient’s currently mysterious illness?
I recently read about a physician who is encouraging medical educators to incorporate more discussions of “humility” and its role in patient care into the medical school and postgraduate training curricula. He feels, as do I, that
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Many, many years ago there was a Thanksgiving when as I was just beginning to earn a reputation in my wife’s family. There were no place cards on the table and the usual hovering and jockeying seats was well underway. From behind me I heard one of my young nieces pipe up: “I want to sit next to Doctor I-don’t-know.”
After a few words of negotiation we were all settled in our places and ready to enjoy our meal. It took only a few seconds of introspection for me to grasp how I had received that moniker, which some physicians might consider disrespectful.
I was the only physician within several generations of that family and, as such, my in-laws thought it only appropriate to ask me medical questions. They courteously seemed to avoid personal questions about their own health and were particularly careful not to roll up their sleeves or unbutton their shirts to show me a lesion or a recently acquired surgical scar. No, my wife’s family members were curious. They wanted answers to deeper questions, the hard science so to speak. “How does aspirin work?” was a typical and painful example. Maybe pharmacologists today have better answers but 40 years ago I’m not so sure; I certainly didn’t know back then and would reply, “I don’t know.” Probably for the third or fourth time that day.
Usually I genuinely didn’t know the answer. However, sometimes my answer was going to be so different from the beliefs and biases of my inquisitor that, in the interest of expediency, “I don’t know” seemed the most appropriate response.
If you were reading Letters from Maine 25 years ago, that scenario might sound familiar. I have chosen to pull it out of the archives as a jumping-off point for a consideration of the unfortunate example some of us set when the COVID pandemic threw a tsunami of unknowns at us. Too many physician-“experts” were afraid to say, “I don’t know.” Instead, and maybe because, they themselves were afraid that the patients couldn’t handle the truth that none of us in the profession knew the correct answers. When so many initial pronouncements proved incorrect, it was too late to undo the damage that had been done to the community’s trust in the rest of us.
It turns out that my in-laws were not the only folks who thought of me as Doctor I-don’t-know. One of the perks of remaining in the same community after one retires is that encounters with former patients and their parents happen frequently. On more than one occasion a parent has thanked me for admitting my ignorance. Some have even claimed that my candid approach was what they remembered most fondly. And, that quality increased their trust when I finally provided an answer.
There is an art to delivering “I don’t know.” Thirty years ago I would excuse myself and tell the family I was going to my office to pull a book off the shelf or call a previous mentor. Now one only needs to ask Dr. Google. No need to leave the room. If appropriate, the provider can swing the computer screen so that the patient can share in the search for the answer.
That strategy only works when the provider merely needs to update or expand his/her knowledge. However, there are those difficult situations when no one could know the answer given the current parameters of the patient’s situation. More lab work might be needed. It may be too early in the trajectory of the patient’s illness for the illnesses signs and symptoms to declare themselves.
In these situations “I don’t know” must be followed by a “but.” It is what comes after that “but” and how it is delivered that can convert the provider’s admission of ignorance into a demonstration of his or her character. Is he/she a caring person trying to understand the patient’s concerns? Willing to enter into a cooperative relationship as together they search for the cause and hopefully for a cure for the patient’s currently mysterious illness?
I recently read about a physician who is encouraging medical educators to incorporate more discussions of “humility” and its role in patient care into the medical school and postgraduate training curricula. He feels, as do I, that
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.